User login
2023 Update on obstetrics
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
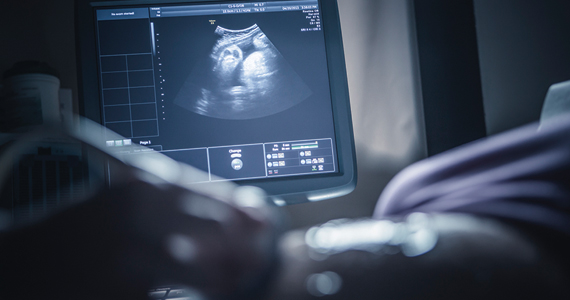
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
2022 Update on obstetrics
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.