User login
2021 Update on obstetrics
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
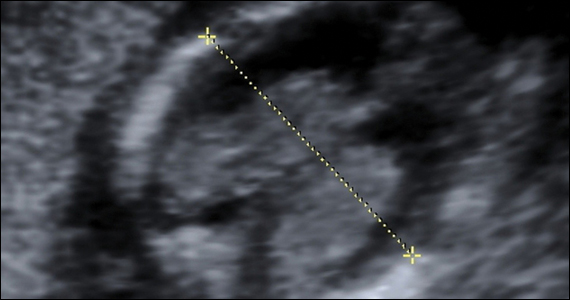
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.