User login
When Would a Metal-Backed Component Become Cost-Effective Over an All-Polyethylene Tibia in Total Knee Arthroplasty?
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
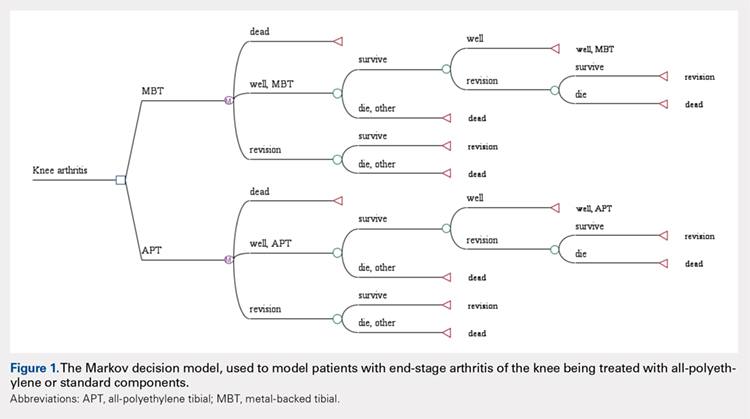
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.
The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
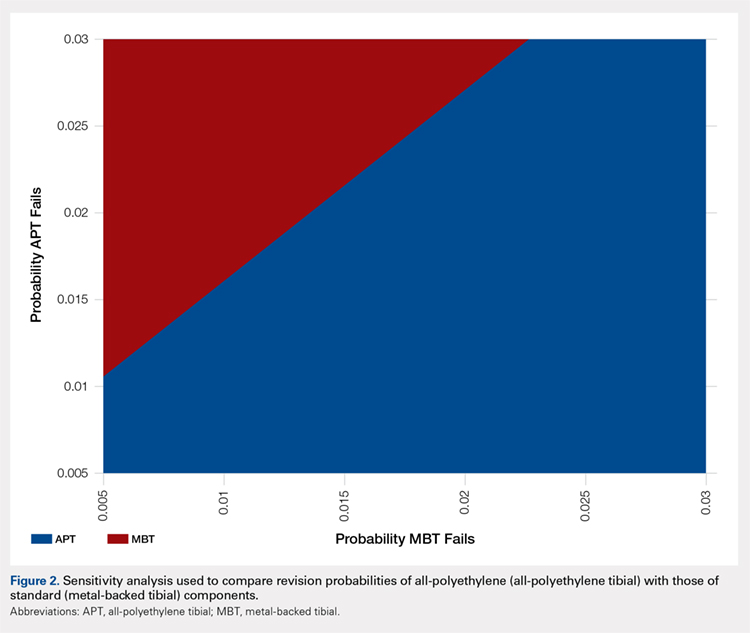
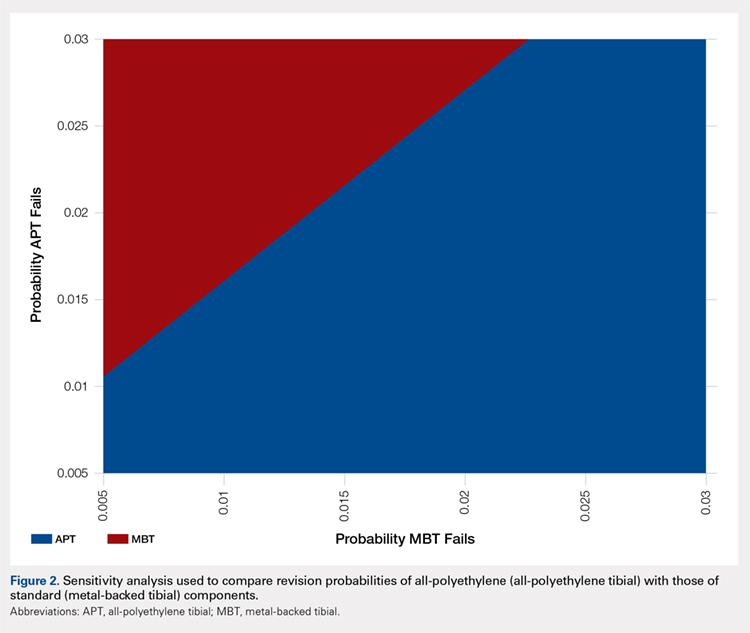
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.
DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.

The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.

DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.

The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.

DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
TAKE-HOME POINTS
- APT components have been shown to be cost-effective when compared to MBT designs in TKA.
- Revision rates would have to be substantially lower in MBT to afford a cost advantage over APT components.
- Given that only a small percentage of surgeons routinely use APT components, factors other than cost-effectiveness must influence the choice of implant.
- Surgeons may find that APT components are more technically demanding to use and they do not allow for modular stems or augmentations.
- Institutional cost data is known to vary widely among institutions, and our conclusions regarding comparable revision rates would change with different cost inputs.