User login
For uterine-sparing fibroid treatment, consider laparoscopic ultrasound-guided radiofrequency ablation
CASE: WOMAN WITH HEAVY BLEEDING HOPES TO AVOID HYSTERECTOMY
L.W. is a 44-year-old woman (G2P2) with a 2-year history of menorrhagia and severe dysmenorrhea but no intermenstrual spotting or bleeding. She reports that she has tried to control her symptoms using hormonal methods, without success.
Examination reveals that she has an irregular, nontender uterus 8 weeks in size. Transvaginal ultrasonography shows two deep, prominent, intramural fibroids. The first is 2 cm by 3 cm in size in the left lateral uterus, adjacent to the endometrial stripe. The second fibroid is 3 cm by 4 cm in the fundal region. Sonohysterography reveals no intracavitary fibroids, although the left lateral myoma has distorted the endometrial cavity.
The patient is seeking removal of her fibroids but would like to preserve her uterus, if at all possible.
What options would you offer her?
Uterine fibroids are common in premenopausal women, affecting 70% to 80% of the population, especially women of African descent.1 Although the majority of women with fibroids are asymptomatic, approximately 30% report pelvic pressure, dyspareunia, dysmenorrhea, or abnormal uterine bleeding.2
Our patients are increasingly aware of uterine-sparing treatments for symptomatic fibroids.3,4 Women seek conservative procedures to avoid the risks and extended recovery times commonly associated with major surgery. Current laparoscopic techniques for removal of uterine fibroids can be complex and require advanced surgical skills. Deep intramural fibroids may not always be visible or readily accessible, making laparoscopic removal a more invasive and challenging approach for many gynecologic surgeons.
A significant recent advancement in minimally invasive gynecologic treatment of fibroids is the Acessa System (Halt Medical, Brentwood, California). This laparoscopic system involves the outpatient use of ultrasound-guided radiofrequency volumetric thermal ablation, or RFVTA.
In this article, I outline the development of this option and step you through its technique. I also review the outcomes data that have been published to date.
How the procedure evolved
In the 1980s, Donnez and Nisolle developed a method of laparoscopic Nd:YAG laser treatment of uterine fibroids, commonly referred to as myolysis.5 Later, Goldfarb developed a laparoscopic bipolar needle technique that coagulated and occluded blood vessels at the periphery of the fibroid.6 However, myolysis led to the formation of significant adhesions to the small bowel or omentum, or both, and was abandoned by the surgical community.
Other fibroid treatments have been developed, such as laparoscopic myomectomy, uterine artery ligation, uterine artery embolization (alone or as adjuvant treatment), and high-intensity focused ultrasound. Although these procedures have made a significant contribution to the minimally invasive treatment of fibroids, the need for reintervention can be substantial, as high as 29% at 1 to 10 years of follow-up in some reports.7–17
Related article: Ins and outs of straight-stick myomectomy James Robinson, MD, MD, and Gaby Moawad, MD (September 2012)
Radiofrequency needle ablation has been used successfully in the treatment and destruction of liver and kidney tumors for years. Lee envisioned use of the technology to treat uterine fibroids. He developed a technique using a retractable multiarray radiofrequency needle (Starburst XL, RITA Medical Systems, Fremont, California) that is inserted directly into the fibroid. In 2002, he reported the first use of intraperitoneal ultrasound for needle guidance in the laparoscopic ablation of 197 myomas in 52 symptomatic women who had declined hysterectomy and myomectomy.18 He found that a significant proportion of women experienced resolution of their symptoms, including heavy menstrual bleeding, dysmenorrhea, dyspareunia, and pelvic pressure, by 3 months, with continued improvement at 12 months.
Because he was unhappy with aspects of the RITA needle that prevented accurate and consistent ablation of fibroids, he developed other devices that would lead to RFVTA and the Acessa System. This system allows the gynecologic surgeon to target and treat deep intramural fibroids that may not be readily visible via conventional laparoscopy.
Between the time of Lee’s first report and his subsequent refinement of the device and procedure, several international fibroid radiofrequency ablation procedures emerged, including those of Bergamini, Milic, Ghezzi, and Carrafiello.19–22 These investigators published results in small cohorts and described significant improvement in patient-reported Uterine Fibroid Symptom and Quality-of-Life (UFS-QOL) scores as well as a reduction in myoma volume.23 Their studies provided evidence that RFA is a safe and effective uterine-sparing treatment of symptomatic uterine fibroids for selected patients.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
How it works
RFVTA uses laparoscopy and laparoscopic ultrasound to guide placement of a needle electrode with a deployable array. Earlier international studies of radiofrequency ablation18–22 and ongoing studies of RFVTA23–26 have demonstrated the procedure’s therapeutic potential. Acessa was recently cleared (November 2012) by the US Food and Drug Administration (FDA).
RFVTA technique, step by step
1. Begin with a standard 5-mm laparoscopic infraumbilical port for the camera and video laparoscope (See VIDEO).
2. Place a 12-mm port in the midline, suprapubically at the level of the uterus, for insertion of the laparoscopic ultrasound probe. Now it is possible to map the uterus and plan an approach to destroy the fibroids.
3. Once you have determined your approach, insert the handpiece containing the radiofrequency needle through the abdominal wall under laparoscopic visualization.
4. Place the needle into the targeted fibroid using both laparoscopic and ultrasound guidance. Depending on the size of the fibroid, the needle array can be deployed to the maximum diameter necessary to effect destruction.
5. Engage the radiofrequency generator (FIGURE 1), utilizing the timing function for optimal destruction of the fibroid, based on the appropriate algorithm. The fibroid then will be ablated and destroyed without damage to the surrounding healthy myometrium.
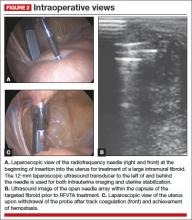
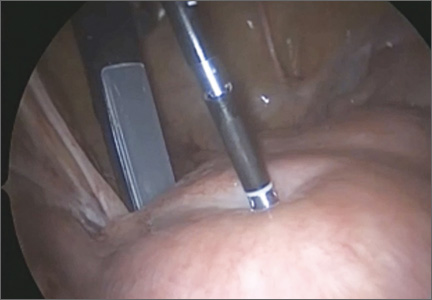
The Acessa radiofrequency generator and accessories are designed to deliver monopolar radiofrequency energy to tissue through the handheld electrical probe. The procedure is monitored by real-time feedback to and from the generator via each of the thermocouples at the tips of the seven-needle array (FIGURE 1). In addition, laparoscopic ultrasound-guided visualization permits monitoring of needle placement within the fibroid capsule (FIGURES 2A and 2B) and confirmation of hemostasis upon removal of the probe tip (FIGURE 2C).
RFVTA treats a wide range of fibroids
The Acessa System is used to treat fibroids of any location and type, with the exception of pedunculated or Type 0 myomas, in which case laparoscopic or hysteroscopic myomectomy is recommended. Calcified fibroids pose no challenges. A single radiofrequency needle generally is used to treat multiple fibroids, with ablation zones ranging in diameter from 1.0 to 6.7 cm. Large, irregularly shaped fibroids may require multiple ablation zones during the same procedure.
Final steps
6. Once the treatment is complete, as confirmed by informatics transmitted by the generator, retract the needle array, set the generator to coagulation mode to coagulate the needle track during withdrawal of the probe, and confirm hemostasis (FIGURE 2C).
7. After completion of all fibroid destruction, remove the disposable radiofrequency probe and close the laparoscopic and ultrasound port sites using standard procedures.
The patient can be discharged the same day and generally is able to return to normal activities within 3 to 5 days.
Postoperative analgesia usually consists solely of nonsteroidal anti-inflammatory agents.
A look at outcomes data
Garza and colleagues published the first feasibility report in 2011.24 They described the results of laparoscopic, ultrasound-guided RFVTA for the treatment of symptomatic uterine fibroids in Mexico using an Acessa predecessor. The 31 women enrolled in the study desired uterine preservation. Of these participants, 19 had completed 12 months of follow-up by the time the report was submitted for publication. Their responses to the UFS-QOL questionnaire indicated that their symptoms had decreased significantly in severity from baseline to 12 months, and their quality-of-life scores also improved significantly (P <.001). Mean uterine volume also declined significantly.
Robles and colleagues set out to confirm these findings using the investigational Halt Ablation System. They conducted a prospective, single-center, open-label clinical trial in Guatemala.25 They found dramatic improvement in symptom severity and health-related quality of life from baseline to 12 months. Mean uterine volume also decreased by 23.6% during this period. In addition, the severity and duration of menses declined.
Acessa is currently not approved for women seeking future childbearing. However, several successful pregnancies have been reported following the procedure.24,26–28 Theoretically, because it causes less damage to and disruption of healthy myometrium, Acessa may prove to be preferable to myomectomy. Head-to-head studies to evaluate this premise are ongoing.
Latest findings confirm widespread improvement of symptoms
The most recent and detailed reports of safety and efficacy of laparoscopic, ultrasound-guided RFVTA at 12 and 24 months were published by teams led by Chudnoff26 and Guido27 earlier this year. These investigations involved 135 women who reported moderate to severe heavy menstrual bleeding (160 mL–500 mL by alkaline hematin analysis). Clinically significant reduction in menstrual blood loss was achieved in 67.7% of participants at 12 months of follow-up.29
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Galen and colleagues performed a retrospective analysis of these same patients, demonstrating that RFVTA of intramural fibroids without submucosal components leads to a clinically and statistically significant reduction in menstrual blood loss.30
Patients generally began these trials with high symptom-severity scores and low health-related quality of life, yet all achieved significant improvement over time, as their UFS-QOL scores indicate (TABLE). The majority of patients (96%) were treated on an outpatient basis. As measured by contrast-enhanced magnetic resonance imaging, total mean uterine volume decreased by 24.3% at 12 months, and total mean fibroid volume decreased by 45.1% during the same interval. Ninety-four percent of the women reported that the treatment was effective in eliminating their symptoms at 12 months, and 98% said they would recommend the procedure to a friend having the same health problems.
Complication rates were low
Complications included a postoperative pelvic abscess and four other minor events. The latter were treated at the time of RFVTA or resolved without additional procedures. The reintervention rate was 0.7% (1/135 patients).26
Guido and colleagues reported 24-month outcomes for the 124 women entering the second year of the Halt study and affirmed the findings of Chudnoff’s team.27 The 112 patient-reported UFS-QOL scores at 24 months were virtually identical to those reported at 12 months. Six surgical reinterventions (6 of 124 patients, or 4.8%) for fibroid-related bleeding were reported between 12 and 24 months.
280 women have been treated so far
As of September 2013, 280 women have been treated under five international study protocols using the Halt Acessa System, and approximately two-thirds have been followed for more than 12 months, with excellent results. Two of the five clinical trials are under way in Germany and Canada:
- The Laparoscopic Uterine-Sparing Techniques Outcomes and Reinterventions (LUSTOR) trial in Germany is a prospective, randomized, single-center study comparing outcomes of RFVTA and laparoscopic myomectomy. Fifty women were enrolled and treated and will be followed for as long as 60 months.
- The Treatment Results of Uterine-Sparing Technologies (TRUST) trial is a prospective, multicenter, randomized study in Canada that compares direct and indirect costs associated with RFVTA, laparoscopic and abdominal myomectomy, and uterine artery embolization. Some US sites also will participate. As many as 200 women will be treated and followed for as long as 60 months.
RFVTA offers several benefits
Although hysterectomy provides a definitive “cure” to fibroid-related symptoms, it is not preferred by many patients and generally is more costly, with an increased risk of complications, a longer hospital stay, longer recovery (6–8 weeks), longer disability and more time off from work, and potential long-term sequelae.
The Acessa procedure not only is an outpatient, minimally invasive, uterine-sparing fibroid treatment option, it permits access to and ablation of almost all fibroids (with the exception of penduculated or Type 0 myomas). The data to date suggest that RFVTA provides many benefits, including a decline in symptom severity and improved health-related quality of life, as well as good cosmesis, quick recovery, and a rapid return to full activity.
There is a learning curve for RFVTA with Acessa—as with any new procedure. If physicians already perform ultrasound imaging, the learning process is shorter. Physicians interested in Acessa and furthering their education and training in laparoscopic RFVTA should consult the manufacturer’s professional education division through the company Web site at http://www.haltmedical.com.
CASE: RESOLVED
After she is counseled about the various uterine-sparing options for treatment, L.W. chooses to undergo laparoscopic, ultrasound-guided RFVTA using the Acessa System. The procedure is performed successfully, with the two fibroids destroyed using the appropriate algorithms, which took into account the size of the fibroids and the deployed needle array. Two additional 1-cm fibroids were found during the procedure and ablated. The patient was discharged home shortly after the procedure, with a nonsteroidal anti-inflammatory drug given for pain relief.
We want to hear from you! Tell us what you think.
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107.
- Katz VL, Lentz G, Lobo RA, Gershenson D, eds. Comprehensive Gynecology. 5th ed. Philadelphia, PA: Elsevier Mosby Saunders; 2007.
- Rosen P. The endangered uterus. More Magazine Web site. http://www.more.com/health/wellness/endangered-uterus. December 2008/January 2009. Accessed October 16, 2013.
- Tulandi T, Levy BS. So You’re Having a Hysterectomy? Hoboken, NJ: John Wiley & Sons; 2004.
- Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Human Reprod Update. 2000;6(6):609–613.
- Goldfarb HA. Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc. 1995;2(2):175–179.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Goodwin SC, Spies JB, Worthington-Kirsch R, et al; Fibroid Registry for Outcomes Data (FIBROID) Registry Steering Committee and Core Site Investigators. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22–33.
- Yoo EH, Lee PI, Huh C-Y, et al. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–370.
- Huang JY, Kafy S, Dugas A, Valenti D, Tulandi T. Failure of uterine fibroid embolization. Fertil Steril. 2006;85(1):30–35.
- Kroncke TJ, Gauruder-Burmester A, Scheurig C, et al. Transarterial embolization for uterine fibroids: clinical success rate and results of magnetic resonance imaging [in German]. Rofo. 2005;177(1):89–98.
- Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85.
- Munoz JL, Jimenez JS, Hernandez C, et al. Hysteroscopic myomectomy: our experience and review. JSLS. 2003;7(1):39–48.
- Reed SD, Newton KM, Thompson LB, McCrummen BA, Warolin AK. The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt). 2006;15(9):1046–1052.
- Spies JB, Bruno J, Czeyda-Pommersheim F, et al. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106(5 Pt 1):933–939.
- Lee BB. Radiofrequency ablation of uterine leiomyomata: a new minimally invasive hysterectomy alternative. Obstet Gynecol. 2002;99(4Suppl):9S.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192(3):768–773.
- Milic A, Asch MR, Hawrylyshyn PA, et al. Laparoscopic ultrasound-guided radiofrequency ablation of uterine fibroids. Cardiovasc Intervent Radiol. 2006;29(4):694–698.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21(11):2081–2085.
- Carrafiello G, Recaldini C, Fontana F, et al. Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol. 2010;33(1):113–119.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomymata. Obstet Gynecol. 2002;99(2):290–300.
- Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 ablation system. J Minim Invasive Gynecol. 2011;18(3):364–371.
- Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric ablation of uterine myomas with 12 months of follow-up. Int J Gynecol Obstet. 2013;120(1):65–69.
- Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075–1082.
- Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Quality Life Outcomes. 2013;11(139):1–8.
- Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid. J Reprod Med. 2012;57:159–163.
- Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study [published online ahead of print September 6, 2013]. J Minim Invasive Gynecol. doi: 10.1016/j.jmig.2013.05.007.
- Lukes AS, Muse K, Richter HE, Moore KA, Patrick DL. Estimating a meaningful reduction in menstrual blood loss for women with heavy bleeding. Curr Med Res Opinion. 2010;26(11):2673–2678.
CASE: WOMAN WITH HEAVY BLEEDING HOPES TO AVOID HYSTERECTOMY
L.W. is a 44-year-old woman (G2P2) with a 2-year history of menorrhagia and severe dysmenorrhea but no intermenstrual spotting or bleeding. She reports that she has tried to control her symptoms using hormonal methods, without success.
Examination reveals that she has an irregular, nontender uterus 8 weeks in size. Transvaginal ultrasonography shows two deep, prominent, intramural fibroids. The first is 2 cm by 3 cm in size in the left lateral uterus, adjacent to the endometrial stripe. The second fibroid is 3 cm by 4 cm in the fundal region. Sonohysterography reveals no intracavitary fibroids, although the left lateral myoma has distorted the endometrial cavity.
The patient is seeking removal of her fibroids but would like to preserve her uterus, if at all possible.
What options would you offer her?
Uterine fibroids are common in premenopausal women, affecting 70% to 80% of the population, especially women of African descent.1 Although the majority of women with fibroids are asymptomatic, approximately 30% report pelvic pressure, dyspareunia, dysmenorrhea, or abnormal uterine bleeding.2
Our patients are increasingly aware of uterine-sparing treatments for symptomatic fibroids.3,4 Women seek conservative procedures to avoid the risks and extended recovery times commonly associated with major surgery. Current laparoscopic techniques for removal of uterine fibroids can be complex and require advanced surgical skills. Deep intramural fibroids may not always be visible or readily accessible, making laparoscopic removal a more invasive and challenging approach for many gynecologic surgeons.
A significant recent advancement in minimally invasive gynecologic treatment of fibroids is the Acessa System (Halt Medical, Brentwood, California). This laparoscopic system involves the outpatient use of ultrasound-guided radiofrequency volumetric thermal ablation, or RFVTA.
In this article, I outline the development of this option and step you through its technique. I also review the outcomes data that have been published to date.
How the procedure evolved
In the 1980s, Donnez and Nisolle developed a method of laparoscopic Nd:YAG laser treatment of uterine fibroids, commonly referred to as myolysis.5 Later, Goldfarb developed a laparoscopic bipolar needle technique that coagulated and occluded blood vessels at the periphery of the fibroid.6 However, myolysis led to the formation of significant adhesions to the small bowel or omentum, or both, and was abandoned by the surgical community.
Other fibroid treatments have been developed, such as laparoscopic myomectomy, uterine artery ligation, uterine artery embolization (alone or as adjuvant treatment), and high-intensity focused ultrasound. Although these procedures have made a significant contribution to the minimally invasive treatment of fibroids, the need for reintervention can be substantial, as high as 29% at 1 to 10 years of follow-up in some reports.7–17
Related article: Ins and outs of straight-stick myomectomy James Robinson, MD, MD, and Gaby Moawad, MD (September 2012)
Radiofrequency needle ablation has been used successfully in the treatment and destruction of liver and kidney tumors for years. Lee envisioned use of the technology to treat uterine fibroids. He developed a technique using a retractable multiarray radiofrequency needle (Starburst XL, RITA Medical Systems, Fremont, California) that is inserted directly into the fibroid. In 2002, he reported the first use of intraperitoneal ultrasound for needle guidance in the laparoscopic ablation of 197 myomas in 52 symptomatic women who had declined hysterectomy and myomectomy.18 He found that a significant proportion of women experienced resolution of their symptoms, including heavy menstrual bleeding, dysmenorrhea, dyspareunia, and pelvic pressure, by 3 months, with continued improvement at 12 months.
Because he was unhappy with aspects of the RITA needle that prevented accurate and consistent ablation of fibroids, he developed other devices that would lead to RFVTA and the Acessa System. This system allows the gynecologic surgeon to target and treat deep intramural fibroids that may not be readily visible via conventional laparoscopy.
Between the time of Lee’s first report and his subsequent refinement of the device and procedure, several international fibroid radiofrequency ablation procedures emerged, including those of Bergamini, Milic, Ghezzi, and Carrafiello.19–22 These investigators published results in small cohorts and described significant improvement in patient-reported Uterine Fibroid Symptom and Quality-of-Life (UFS-QOL) scores as well as a reduction in myoma volume.23 Their studies provided evidence that RFA is a safe and effective uterine-sparing treatment of symptomatic uterine fibroids for selected patients.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
How it works
RFVTA uses laparoscopy and laparoscopic ultrasound to guide placement of a needle electrode with a deployable array. Earlier international studies of radiofrequency ablation18–22 and ongoing studies of RFVTA23–26 have demonstrated the procedure’s therapeutic potential. Acessa was recently cleared (November 2012) by the US Food and Drug Administration (FDA).
RFVTA technique, step by step
1. Begin with a standard 5-mm laparoscopic infraumbilical port for the camera and video laparoscope (See VIDEO).
2. Place a 12-mm port in the midline, suprapubically at the level of the uterus, for insertion of the laparoscopic ultrasound probe. Now it is possible to map the uterus and plan an approach to destroy the fibroids.
3. Once you have determined your approach, insert the handpiece containing the radiofrequency needle through the abdominal wall under laparoscopic visualization.
4. Place the needle into the targeted fibroid using both laparoscopic and ultrasound guidance. Depending on the size of the fibroid, the needle array can be deployed to the maximum diameter necessary to effect destruction.
5. Engage the radiofrequency generator (FIGURE 1), utilizing the timing function for optimal destruction of the fibroid, based on the appropriate algorithm. The fibroid then will be ablated and destroyed without damage to the surrounding healthy myometrium.
The Acessa radiofrequency generator and accessories are designed to deliver monopolar radiofrequency energy to tissue through the handheld electrical probe. The procedure is monitored by real-time feedback to and from the generator via each of the thermocouples at the tips of the seven-needle array (FIGURE 1). In addition, laparoscopic ultrasound-guided visualization permits monitoring of needle placement within the fibroid capsule (FIGURES 2A and 2B) and confirmation of hemostasis upon removal of the probe tip (FIGURE 2C).
RFVTA treats a wide range of fibroids
The Acessa System is used to treat fibroids of any location and type, with the exception of pedunculated or Type 0 myomas, in which case laparoscopic or hysteroscopic myomectomy is recommended. Calcified fibroids pose no challenges. A single radiofrequency needle generally is used to treat multiple fibroids, with ablation zones ranging in diameter from 1.0 to 6.7 cm. Large, irregularly shaped fibroids may require multiple ablation zones during the same procedure.
Final steps
6. Once the treatment is complete, as confirmed by informatics transmitted by the generator, retract the needle array, set the generator to coagulation mode to coagulate the needle track during withdrawal of the probe, and confirm hemostasis (FIGURE 2C).
7. After completion of all fibroid destruction, remove the disposable radiofrequency probe and close the laparoscopic and ultrasound port sites using standard procedures.
The patient can be discharged the same day and generally is able to return to normal activities within 3 to 5 days.
Postoperative analgesia usually consists solely of nonsteroidal anti-inflammatory agents.
A look at outcomes data
Garza and colleagues published the first feasibility report in 2011.24 They described the results of laparoscopic, ultrasound-guided RFVTA for the treatment of symptomatic uterine fibroids in Mexico using an Acessa predecessor. The 31 women enrolled in the study desired uterine preservation. Of these participants, 19 had completed 12 months of follow-up by the time the report was submitted for publication. Their responses to the UFS-QOL questionnaire indicated that their symptoms had decreased significantly in severity from baseline to 12 months, and their quality-of-life scores also improved significantly (P <.001). Mean uterine volume also declined significantly.
Robles and colleagues set out to confirm these findings using the investigational Halt Ablation System. They conducted a prospective, single-center, open-label clinical trial in Guatemala.25 They found dramatic improvement in symptom severity and health-related quality of life from baseline to 12 months. Mean uterine volume also decreased by 23.6% during this period. In addition, the severity and duration of menses declined.
Acessa is currently not approved for women seeking future childbearing. However, several successful pregnancies have been reported following the procedure.24,26–28 Theoretically, because it causes less damage to and disruption of healthy myometrium, Acessa may prove to be preferable to myomectomy. Head-to-head studies to evaluate this premise are ongoing.
Latest findings confirm widespread improvement of symptoms
The most recent and detailed reports of safety and efficacy of laparoscopic, ultrasound-guided RFVTA at 12 and 24 months were published by teams led by Chudnoff26 and Guido27 earlier this year. These investigations involved 135 women who reported moderate to severe heavy menstrual bleeding (160 mL–500 mL by alkaline hematin analysis). Clinically significant reduction in menstrual blood loss was achieved in 67.7% of participants at 12 months of follow-up.29
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Galen and colleagues performed a retrospective analysis of these same patients, demonstrating that RFVTA of intramural fibroids without submucosal components leads to a clinically and statistically significant reduction in menstrual blood loss.30
Patients generally began these trials with high symptom-severity scores and low health-related quality of life, yet all achieved significant improvement over time, as their UFS-QOL scores indicate (TABLE). The majority of patients (96%) were treated on an outpatient basis. As measured by contrast-enhanced magnetic resonance imaging, total mean uterine volume decreased by 24.3% at 12 months, and total mean fibroid volume decreased by 45.1% during the same interval. Ninety-four percent of the women reported that the treatment was effective in eliminating their symptoms at 12 months, and 98% said they would recommend the procedure to a friend having the same health problems.
Complication rates were low
Complications included a postoperative pelvic abscess and four other minor events. The latter were treated at the time of RFVTA or resolved without additional procedures. The reintervention rate was 0.7% (1/135 patients).26
Guido and colleagues reported 24-month outcomes for the 124 women entering the second year of the Halt study and affirmed the findings of Chudnoff’s team.27 The 112 patient-reported UFS-QOL scores at 24 months were virtually identical to those reported at 12 months. Six surgical reinterventions (6 of 124 patients, or 4.8%) for fibroid-related bleeding were reported between 12 and 24 months.
280 women have been treated so far
As of September 2013, 280 women have been treated under five international study protocols using the Halt Acessa System, and approximately two-thirds have been followed for more than 12 months, with excellent results. Two of the five clinical trials are under way in Germany and Canada:
- The Laparoscopic Uterine-Sparing Techniques Outcomes and Reinterventions (LUSTOR) trial in Germany is a prospective, randomized, single-center study comparing outcomes of RFVTA and laparoscopic myomectomy. Fifty women were enrolled and treated and will be followed for as long as 60 months.
- The Treatment Results of Uterine-Sparing Technologies (TRUST) trial is a prospective, multicenter, randomized study in Canada that compares direct and indirect costs associated with RFVTA, laparoscopic and abdominal myomectomy, and uterine artery embolization. Some US sites also will participate. As many as 200 women will be treated and followed for as long as 60 months.
RFVTA offers several benefits
Although hysterectomy provides a definitive “cure” to fibroid-related symptoms, it is not preferred by many patients and generally is more costly, with an increased risk of complications, a longer hospital stay, longer recovery (6–8 weeks), longer disability and more time off from work, and potential long-term sequelae.
The Acessa procedure not only is an outpatient, minimally invasive, uterine-sparing fibroid treatment option, it permits access to and ablation of almost all fibroids (with the exception of penduculated or Type 0 myomas). The data to date suggest that RFVTA provides many benefits, including a decline in symptom severity and improved health-related quality of life, as well as good cosmesis, quick recovery, and a rapid return to full activity.
There is a learning curve for RFVTA with Acessa—as with any new procedure. If physicians already perform ultrasound imaging, the learning process is shorter. Physicians interested in Acessa and furthering their education and training in laparoscopic RFVTA should consult the manufacturer’s professional education division through the company Web site at http://www.haltmedical.com.
CASE: RESOLVED
After she is counseled about the various uterine-sparing options for treatment, L.W. chooses to undergo laparoscopic, ultrasound-guided RFVTA using the Acessa System. The procedure is performed successfully, with the two fibroids destroyed using the appropriate algorithms, which took into account the size of the fibroids and the deployed needle array. Two additional 1-cm fibroids were found during the procedure and ablated. The patient was discharged home shortly after the procedure, with a nonsteroidal anti-inflammatory drug given for pain relief.
We want to hear from you! Tell us what you think.
CASE: WOMAN WITH HEAVY BLEEDING HOPES TO AVOID HYSTERECTOMY
L.W. is a 44-year-old woman (G2P2) with a 2-year history of menorrhagia and severe dysmenorrhea but no intermenstrual spotting or bleeding. She reports that she has tried to control her symptoms using hormonal methods, without success.
Examination reveals that she has an irregular, nontender uterus 8 weeks in size. Transvaginal ultrasonography shows two deep, prominent, intramural fibroids. The first is 2 cm by 3 cm in size in the left lateral uterus, adjacent to the endometrial stripe. The second fibroid is 3 cm by 4 cm in the fundal region. Sonohysterography reveals no intracavitary fibroids, although the left lateral myoma has distorted the endometrial cavity.
The patient is seeking removal of her fibroids but would like to preserve her uterus, if at all possible.
What options would you offer her?
Uterine fibroids are common in premenopausal women, affecting 70% to 80% of the population, especially women of African descent.1 Although the majority of women with fibroids are asymptomatic, approximately 30% report pelvic pressure, dyspareunia, dysmenorrhea, or abnormal uterine bleeding.2
Our patients are increasingly aware of uterine-sparing treatments for symptomatic fibroids.3,4 Women seek conservative procedures to avoid the risks and extended recovery times commonly associated with major surgery. Current laparoscopic techniques for removal of uterine fibroids can be complex and require advanced surgical skills. Deep intramural fibroids may not always be visible or readily accessible, making laparoscopic removal a more invasive and challenging approach for many gynecologic surgeons.
A significant recent advancement in minimally invasive gynecologic treatment of fibroids is the Acessa System (Halt Medical, Brentwood, California). This laparoscopic system involves the outpatient use of ultrasound-guided radiofrequency volumetric thermal ablation, or RFVTA.
In this article, I outline the development of this option and step you through its technique. I also review the outcomes data that have been published to date.
How the procedure evolved
In the 1980s, Donnez and Nisolle developed a method of laparoscopic Nd:YAG laser treatment of uterine fibroids, commonly referred to as myolysis.5 Later, Goldfarb developed a laparoscopic bipolar needle technique that coagulated and occluded blood vessels at the periphery of the fibroid.6 However, myolysis led to the formation of significant adhesions to the small bowel or omentum, or both, and was abandoned by the surgical community.
Other fibroid treatments have been developed, such as laparoscopic myomectomy, uterine artery ligation, uterine artery embolization (alone or as adjuvant treatment), and high-intensity focused ultrasound. Although these procedures have made a significant contribution to the minimally invasive treatment of fibroids, the need for reintervention can be substantial, as high as 29% at 1 to 10 years of follow-up in some reports.7–17
Related article: Ins and outs of straight-stick myomectomy James Robinson, MD, MD, and Gaby Moawad, MD (September 2012)
Radiofrequency needle ablation has been used successfully in the treatment and destruction of liver and kidney tumors for years. Lee envisioned use of the technology to treat uterine fibroids. He developed a technique using a retractable multiarray radiofrequency needle (Starburst XL, RITA Medical Systems, Fremont, California) that is inserted directly into the fibroid. In 2002, he reported the first use of intraperitoneal ultrasound for needle guidance in the laparoscopic ablation of 197 myomas in 52 symptomatic women who had declined hysterectomy and myomectomy.18 He found that a significant proportion of women experienced resolution of their symptoms, including heavy menstrual bleeding, dysmenorrhea, dyspareunia, and pelvic pressure, by 3 months, with continued improvement at 12 months.
Because he was unhappy with aspects of the RITA needle that prevented accurate and consistent ablation of fibroids, he developed other devices that would lead to RFVTA and the Acessa System. This system allows the gynecologic surgeon to target and treat deep intramural fibroids that may not be readily visible via conventional laparoscopy.
Between the time of Lee’s first report and his subsequent refinement of the device and procedure, several international fibroid radiofrequency ablation procedures emerged, including those of Bergamini, Milic, Ghezzi, and Carrafiello.19–22 These investigators published results in small cohorts and described significant improvement in patient-reported Uterine Fibroid Symptom and Quality-of-Life (UFS-QOL) scores as well as a reduction in myoma volume.23 Their studies provided evidence that RFA is a safe and effective uterine-sparing treatment of symptomatic uterine fibroids for selected patients.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
How it works
RFVTA uses laparoscopy and laparoscopic ultrasound to guide placement of a needle electrode with a deployable array. Earlier international studies of radiofrequency ablation18–22 and ongoing studies of RFVTA23–26 have demonstrated the procedure’s therapeutic potential. Acessa was recently cleared (November 2012) by the US Food and Drug Administration (FDA).
RFVTA technique, step by step
1. Begin with a standard 5-mm laparoscopic infraumbilical port for the camera and video laparoscope (See VIDEO).
2. Place a 12-mm port in the midline, suprapubically at the level of the uterus, for insertion of the laparoscopic ultrasound probe. Now it is possible to map the uterus and plan an approach to destroy the fibroids.
3. Once you have determined your approach, insert the handpiece containing the radiofrequency needle through the abdominal wall under laparoscopic visualization.
4. Place the needle into the targeted fibroid using both laparoscopic and ultrasound guidance. Depending on the size of the fibroid, the needle array can be deployed to the maximum diameter necessary to effect destruction.
5. Engage the radiofrequency generator (FIGURE 1), utilizing the timing function for optimal destruction of the fibroid, based on the appropriate algorithm. The fibroid then will be ablated and destroyed without damage to the surrounding healthy myometrium.
The Acessa radiofrequency generator and accessories are designed to deliver monopolar radiofrequency energy to tissue through the handheld electrical probe. The procedure is monitored by real-time feedback to and from the generator via each of the thermocouples at the tips of the seven-needle array (FIGURE 1). In addition, laparoscopic ultrasound-guided visualization permits monitoring of needle placement within the fibroid capsule (FIGURES 2A and 2B) and confirmation of hemostasis upon removal of the probe tip (FIGURE 2C).
RFVTA treats a wide range of fibroids
The Acessa System is used to treat fibroids of any location and type, with the exception of pedunculated or Type 0 myomas, in which case laparoscopic or hysteroscopic myomectomy is recommended. Calcified fibroids pose no challenges. A single radiofrequency needle generally is used to treat multiple fibroids, with ablation zones ranging in diameter from 1.0 to 6.7 cm. Large, irregularly shaped fibroids may require multiple ablation zones during the same procedure.
Final steps
6. Once the treatment is complete, as confirmed by informatics transmitted by the generator, retract the needle array, set the generator to coagulation mode to coagulate the needle track during withdrawal of the probe, and confirm hemostasis (FIGURE 2C).
7. After completion of all fibroid destruction, remove the disposable radiofrequency probe and close the laparoscopic and ultrasound port sites using standard procedures.
The patient can be discharged the same day and generally is able to return to normal activities within 3 to 5 days.
Postoperative analgesia usually consists solely of nonsteroidal anti-inflammatory agents.
A look at outcomes data
Garza and colleagues published the first feasibility report in 2011.24 They described the results of laparoscopic, ultrasound-guided RFVTA for the treatment of symptomatic uterine fibroids in Mexico using an Acessa predecessor. The 31 women enrolled in the study desired uterine preservation. Of these participants, 19 had completed 12 months of follow-up by the time the report was submitted for publication. Their responses to the UFS-QOL questionnaire indicated that their symptoms had decreased significantly in severity from baseline to 12 months, and their quality-of-life scores also improved significantly (P <.001). Mean uterine volume also declined significantly.
Robles and colleagues set out to confirm these findings using the investigational Halt Ablation System. They conducted a prospective, single-center, open-label clinical trial in Guatemala.25 They found dramatic improvement in symptom severity and health-related quality of life from baseline to 12 months. Mean uterine volume also decreased by 23.6% during this period. In addition, the severity and duration of menses declined.
Acessa is currently not approved for women seeking future childbearing. However, several successful pregnancies have been reported following the procedure.24,26–28 Theoretically, because it causes less damage to and disruption of healthy myometrium, Acessa may prove to be preferable to myomectomy. Head-to-head studies to evaluate this premise are ongoing.
Latest findings confirm widespread improvement of symptoms
The most recent and detailed reports of safety and efficacy of laparoscopic, ultrasound-guided RFVTA at 12 and 24 months were published by teams led by Chudnoff26 and Guido27 earlier this year. These investigations involved 135 women who reported moderate to severe heavy menstrual bleeding (160 mL–500 mL by alkaline hematin analysis). Clinically significant reduction in menstrual blood loss was achieved in 67.7% of participants at 12 months of follow-up.29
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Galen and colleagues performed a retrospective analysis of these same patients, demonstrating that RFVTA of intramural fibroids without submucosal components leads to a clinically and statistically significant reduction in menstrual blood loss.30
Patients generally began these trials with high symptom-severity scores and low health-related quality of life, yet all achieved significant improvement over time, as their UFS-QOL scores indicate (TABLE). The majority of patients (96%) were treated on an outpatient basis. As measured by contrast-enhanced magnetic resonance imaging, total mean uterine volume decreased by 24.3% at 12 months, and total mean fibroid volume decreased by 45.1% during the same interval. Ninety-four percent of the women reported that the treatment was effective in eliminating their symptoms at 12 months, and 98% said they would recommend the procedure to a friend having the same health problems.
Complication rates were low
Complications included a postoperative pelvic abscess and four other minor events. The latter were treated at the time of RFVTA or resolved without additional procedures. The reintervention rate was 0.7% (1/135 patients).26
Guido and colleagues reported 24-month outcomes for the 124 women entering the second year of the Halt study and affirmed the findings of Chudnoff’s team.27 The 112 patient-reported UFS-QOL scores at 24 months were virtually identical to those reported at 12 months. Six surgical reinterventions (6 of 124 patients, or 4.8%) for fibroid-related bleeding were reported between 12 and 24 months.
280 women have been treated so far
As of September 2013, 280 women have been treated under five international study protocols using the Halt Acessa System, and approximately two-thirds have been followed for more than 12 months, with excellent results. Two of the five clinical trials are under way in Germany and Canada:
- The Laparoscopic Uterine-Sparing Techniques Outcomes and Reinterventions (LUSTOR) trial in Germany is a prospective, randomized, single-center study comparing outcomes of RFVTA and laparoscopic myomectomy. Fifty women were enrolled and treated and will be followed for as long as 60 months.
- The Treatment Results of Uterine-Sparing Technologies (TRUST) trial is a prospective, multicenter, randomized study in Canada that compares direct and indirect costs associated with RFVTA, laparoscopic and abdominal myomectomy, and uterine artery embolization. Some US sites also will participate. As many as 200 women will be treated and followed for as long as 60 months.
RFVTA offers several benefits
Although hysterectomy provides a definitive “cure” to fibroid-related symptoms, it is not preferred by many patients and generally is more costly, with an increased risk of complications, a longer hospital stay, longer recovery (6–8 weeks), longer disability and more time off from work, and potential long-term sequelae.
The Acessa procedure not only is an outpatient, minimally invasive, uterine-sparing fibroid treatment option, it permits access to and ablation of almost all fibroids (with the exception of penduculated or Type 0 myomas). The data to date suggest that RFVTA provides many benefits, including a decline in symptom severity and improved health-related quality of life, as well as good cosmesis, quick recovery, and a rapid return to full activity.
There is a learning curve for RFVTA with Acessa—as with any new procedure. If physicians already perform ultrasound imaging, the learning process is shorter. Physicians interested in Acessa and furthering their education and training in laparoscopic RFVTA should consult the manufacturer’s professional education division through the company Web site at http://www.haltmedical.com.
CASE: RESOLVED
After she is counseled about the various uterine-sparing options for treatment, L.W. chooses to undergo laparoscopic, ultrasound-guided RFVTA using the Acessa System. The procedure is performed successfully, with the two fibroids destroyed using the appropriate algorithms, which took into account the size of the fibroids and the deployed needle array. Two additional 1-cm fibroids were found during the procedure and ablated. The patient was discharged home shortly after the procedure, with a nonsteroidal anti-inflammatory drug given for pain relief.
We want to hear from you! Tell us what you think.
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107.
- Katz VL, Lentz G, Lobo RA, Gershenson D, eds. Comprehensive Gynecology. 5th ed. Philadelphia, PA: Elsevier Mosby Saunders; 2007.
- Rosen P. The endangered uterus. More Magazine Web site. http://www.more.com/health/wellness/endangered-uterus. December 2008/January 2009. Accessed October 16, 2013.
- Tulandi T, Levy BS. So You’re Having a Hysterectomy? Hoboken, NJ: John Wiley & Sons; 2004.
- Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Human Reprod Update. 2000;6(6):609–613.
- Goldfarb HA. Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc. 1995;2(2):175–179.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Goodwin SC, Spies JB, Worthington-Kirsch R, et al; Fibroid Registry for Outcomes Data (FIBROID) Registry Steering Committee and Core Site Investigators. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22–33.
- Yoo EH, Lee PI, Huh C-Y, et al. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–370.
- Huang JY, Kafy S, Dugas A, Valenti D, Tulandi T. Failure of uterine fibroid embolization. Fertil Steril. 2006;85(1):30–35.
- Kroncke TJ, Gauruder-Burmester A, Scheurig C, et al. Transarterial embolization for uterine fibroids: clinical success rate and results of magnetic resonance imaging [in German]. Rofo. 2005;177(1):89–98.
- Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85.
- Munoz JL, Jimenez JS, Hernandez C, et al. Hysteroscopic myomectomy: our experience and review. JSLS. 2003;7(1):39–48.
- Reed SD, Newton KM, Thompson LB, McCrummen BA, Warolin AK. The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt). 2006;15(9):1046–1052.
- Spies JB, Bruno J, Czeyda-Pommersheim F, et al. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106(5 Pt 1):933–939.
- Lee BB. Radiofrequency ablation of uterine leiomyomata: a new minimally invasive hysterectomy alternative. Obstet Gynecol. 2002;99(4Suppl):9S.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192(3):768–773.
- Milic A, Asch MR, Hawrylyshyn PA, et al. Laparoscopic ultrasound-guided radiofrequency ablation of uterine fibroids. Cardiovasc Intervent Radiol. 2006;29(4):694–698.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21(11):2081–2085.
- Carrafiello G, Recaldini C, Fontana F, et al. Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol. 2010;33(1):113–119.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomymata. Obstet Gynecol. 2002;99(2):290–300.
- Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 ablation system. J Minim Invasive Gynecol. 2011;18(3):364–371.
- Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric ablation of uterine myomas with 12 months of follow-up. Int J Gynecol Obstet. 2013;120(1):65–69.
- Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075–1082.
- Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Quality Life Outcomes. 2013;11(139):1–8.
- Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid. J Reprod Med. 2012;57:159–163.
- Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study [published online ahead of print September 6, 2013]. J Minim Invasive Gynecol. doi: 10.1016/j.jmig.2013.05.007.
- Lukes AS, Muse K, Richter HE, Moore KA, Patrick DL. Estimating a meaningful reduction in menstrual blood loss for women with heavy bleeding. Curr Med Res Opinion. 2010;26(11):2673–2678.
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107.
- Katz VL, Lentz G, Lobo RA, Gershenson D, eds. Comprehensive Gynecology. 5th ed. Philadelphia, PA: Elsevier Mosby Saunders; 2007.
- Rosen P. The endangered uterus. More Magazine Web site. http://www.more.com/health/wellness/endangered-uterus. December 2008/January 2009. Accessed October 16, 2013.
- Tulandi T, Levy BS. So You’re Having a Hysterectomy? Hoboken, NJ: John Wiley & Sons; 2004.
- Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Human Reprod Update. 2000;6(6):609–613.
- Goldfarb HA. Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc. 1995;2(2):175–179.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Goodwin SC, Spies JB, Worthington-Kirsch R, et al; Fibroid Registry for Outcomes Data (FIBROID) Registry Steering Committee and Core Site Investigators. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22–33.
- Yoo EH, Lee PI, Huh C-Y, et al. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–370.
- Huang JY, Kafy S, Dugas A, Valenti D, Tulandi T. Failure of uterine fibroid embolization. Fertil Steril. 2006;85(1):30–35.
- Kroncke TJ, Gauruder-Burmester A, Scheurig C, et al. Transarterial embolization for uterine fibroids: clinical success rate and results of magnetic resonance imaging [in German]. Rofo. 2005;177(1):89–98.
- Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85.
- Munoz JL, Jimenez JS, Hernandez C, et al. Hysteroscopic myomectomy: our experience and review. JSLS. 2003;7(1):39–48.
- Reed SD, Newton KM, Thompson LB, McCrummen BA, Warolin AK. The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt). 2006;15(9):1046–1052.
- Spies JB, Bruno J, Czeyda-Pommersheim F, et al. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106(5 Pt 1):933–939.
- Lee BB. Radiofrequency ablation of uterine leiomyomata: a new minimally invasive hysterectomy alternative. Obstet Gynecol. 2002;99(4Suppl):9S.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192(3):768–773.
- Milic A, Asch MR, Hawrylyshyn PA, et al. Laparoscopic ultrasound-guided radiofrequency ablation of uterine fibroids. Cardiovasc Intervent Radiol. 2006;29(4):694–698.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21(11):2081–2085.
- Carrafiello G, Recaldini C, Fontana F, et al. Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol. 2010;33(1):113–119.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomymata. Obstet Gynecol. 2002;99(2):290–300.
- Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 ablation system. J Minim Invasive Gynecol. 2011;18(3):364–371.
- Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric ablation of uterine myomas with 12 months of follow-up. Int J Gynecol Obstet. 2013;120(1):65–69.
- Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075–1082.
- Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Quality Life Outcomes. 2013;11(139):1–8.
- Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid. J Reprod Med. 2012;57:159–163.
- Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study [published online ahead of print September 6, 2013]. J Minim Invasive Gynecol. doi: 10.1016/j.jmig.2013.05.007.
- Lukes AS, Muse K, Richter HE, Moore KA, Patrick DL. Estimating a meaningful reduction in menstrual blood loss for women with heavy bleeding. Curr Med Res Opinion. 2010;26(11):2673–2678.