User login
Diagnosing and Classifying Anemia in Adult Primary Care
CE/CME No: CR-1708
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the importance of diagnosing the type of anemia in order to provide appropriate treatment.
• Describe how the complete blood count and its indices are used to initially determine if an anemia is microcytic, normocytic, or macrocytic.
• List the more common causes of microcytic, normocytic, and macrocytic anemia.
• Discuss addictional laboratory tests that may be used to further assess the cause of anemia.
FACULTY
Jean O’Neil is an Assistant Professor and Coordinator of the Adult Gerontology Acute Care Nurse Practitioner Program in the Patricia A. Chin School of Nursing at California State University, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2018.
Article begins on next page >>
Anemia affects more than 3 million people in the United States, making it a common problem in primary care practices. Once anemia is detected, clinicians must define the type and identify its underlying cause prior to initiating treatment. In most cases, the cause can be determined using information from the patient history, physical exam, and complete blood count.
Anemia is commonly identified during routine physical exams and laboratory testing.1-3 However, treating anemia can present a challenge for the primary care provider if the immediate cause is not apparent. Iron deficiency is a leading cause of anemia, but simply prescribing an iron supplement without determining the type or the cause of the anemia is not appropriate. Anemia that is misdiagnosed or goes untreated can be associated with a worse prognosis, as well as increased health care costs.4
Primary care providers often manage patients with common types of anemia and refer patients with severe or complex anemia to specialists for further testing and treatment. The most commonly used and cost-effective diagnostic tool for anemia is the complete blood count (CBC).2-6 The CBC provides details that can help the provider determine the type of anemia present, which in turn guides proper diagnostic testing and treatment.
EPIDEMIOLOGY
Anemia involves a reduction in the number of circulating red blood cells, the blood hemoglobin content, or the hematocrit, which leads to impaired delivery of oxygen to the body. Anemia affects more than 2 billion people worldwide, with iron deficiency the most common cause.7 Other leading nutritional causes of anemia include vitamin B12 and folate deficiency.4,7 Approximately 3 to 4 million Americans have anemia in some form, and it affects about 6.6% of men and 12.4% of women.5,8 The prevalence of anemia increases with age. Approximately 11% of men and 10% of women ages 65 or older have anemia, and in men ages 85 or older, prevalence of 20% to 44% has been reported.1,4 Anemia is present in about 3.5% of patients with chronic disease, but only 15% of them receive treatment.4
PATHOPHYSIOLOGY
Blood is composed of water-based plasma (54%), white blood cells and platelets (1%), and red blood cells (45%).5 Hemoglobin, the primary protein of the red blood cell, binds oxygen from the lungs and transports it to the rest of the body. Oxygen is then exchanged for carbon dioxide, which is carried back to the lungs to be exhaled.
Hemoglobin is made up of four globin chains, each containing an iron ion held in a porphyrin ring known as a heme group.5 When the body detects low tissue oxygen, the endothelial cells in the kidneys secrete the hormone erythropoietin (EPO), which stimulates the bone marrow to increase red cell production.5 This feedback loop can be interrupted by renal failure or chronic disease.4 In addition, bone marrow cannot produce enough red blood cells if there are insufficient levels of iron, amino acids, protein, carbohydrates, lipids, folate, and vitamin B12.5 Toxins (eg, lead), some types of cancer (eg, lymphoma), or even common infections (eg, pneumonia) can suppress the bone marrow, causing anemia. The more severe the anemia, the more likely oxygen transport will be compromised and organ failure will ensue.
Mutations affecting the genes that encode the globin chains within hemoglobin can cause one of the more than 600 known hemoglobinopathies (genetic defects of hemoglobin structure), such as sickle cell disease and thalassemias.5,9 While it is important to identify and treat patients with hemoglobinopathies, most anemias have other causes, such as iron deficiency, chronic disease, bone marrow defects, B12 deficiency, renal failure, medications, alcoholism, pregnancy, nutritional intake problems, gastrointestinal malabsorption, and active or recent history of blood loss.5,10
CLINICAL PRESENTATION
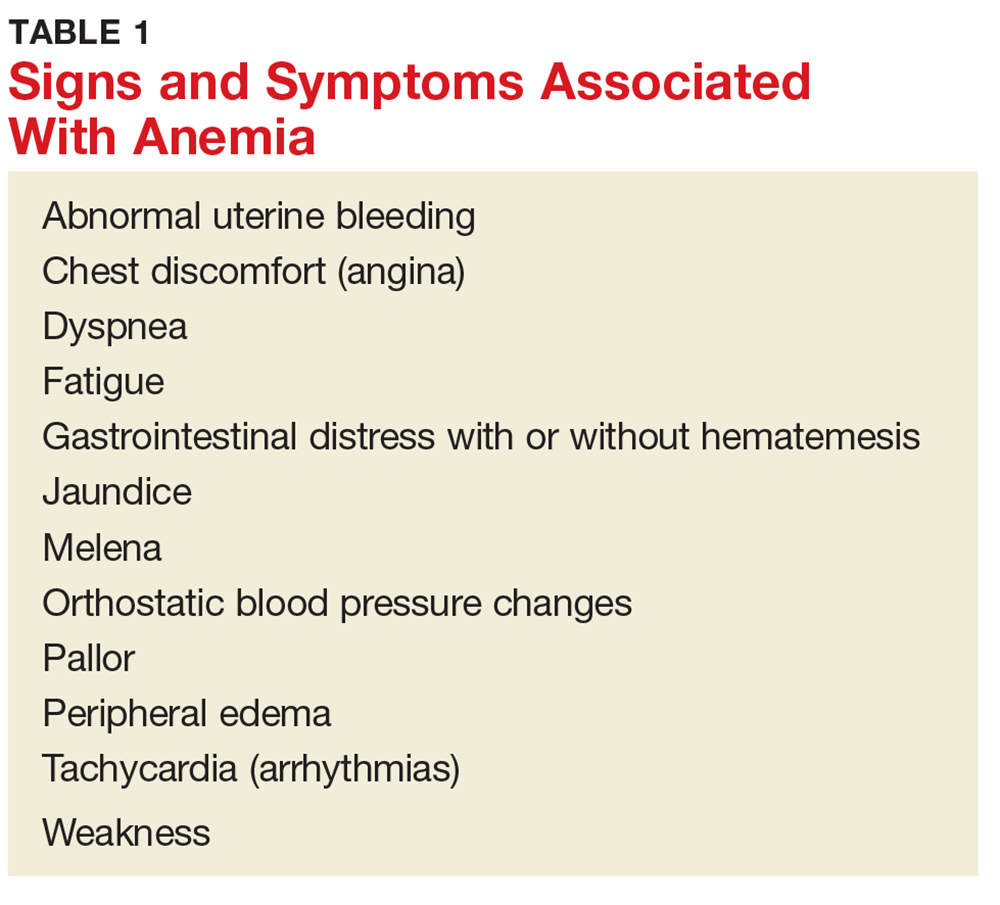
There are several signs and symptoms that should lead the primary care provider to suspect anemia (see Table 1).5,6 The severity of these symptoms can vary from mild to very serious. Severe anemia can lead to organ failure and death. However, most patients with anemia are asymptomatic, and anemia is typically detected incidentally during laboratory testing.1,2
Once anemia is confirmed, the evaluation focuses on diagnosing its underlying cause. It should include a thorough patient history and review of systems to ascertain whether the patient has symptoms such as increased fatigue, palpitations, gastrointestinal distress, weakness, or dizziness.
If the provider has access to past CBC results, a comparison of the current and previous results will help determine whether the anemia is acute or chronic. Anemia caused by acute conditions, such as a suspicion of blood loss or bone marrow suppression, must be attended to immediately. A patient with chronic anemia should be carefully monitored and may need follow-up for ongoing treatment. While a provider has more time to work up a patient with chronic anemia, the causes may not be as straightforward.
DIAGNOSIS AND CLASSIFICATION
Anemia in adults is defined as hemoglobin less than 13 g/dL in males and 12 g/dL in females.6 The hemoglobin is part of the complete blood cell report, which also includes the white blood cell count (WBC), red blood cell count (RBC), hematocrit, platelet count, and indices.
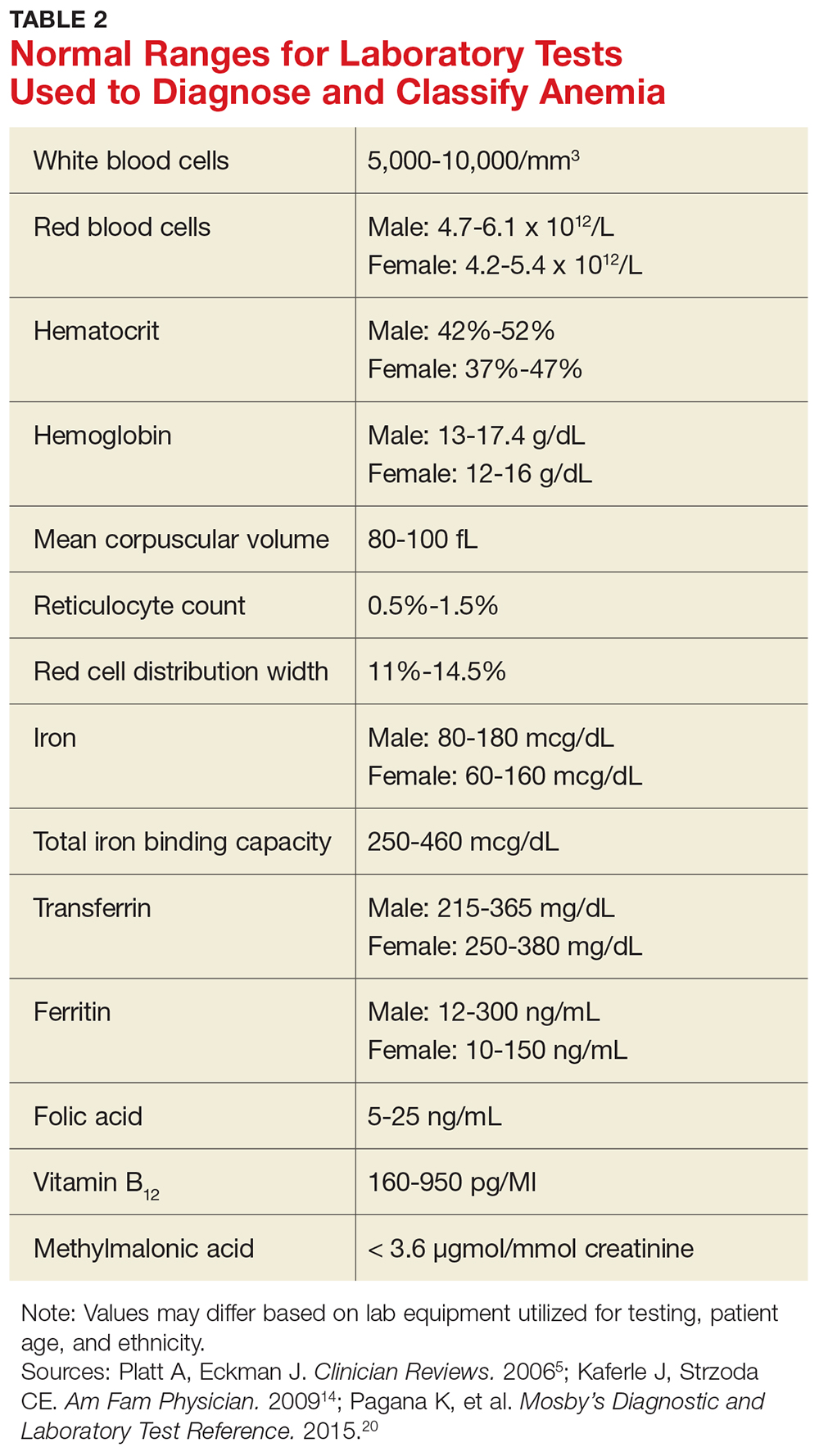
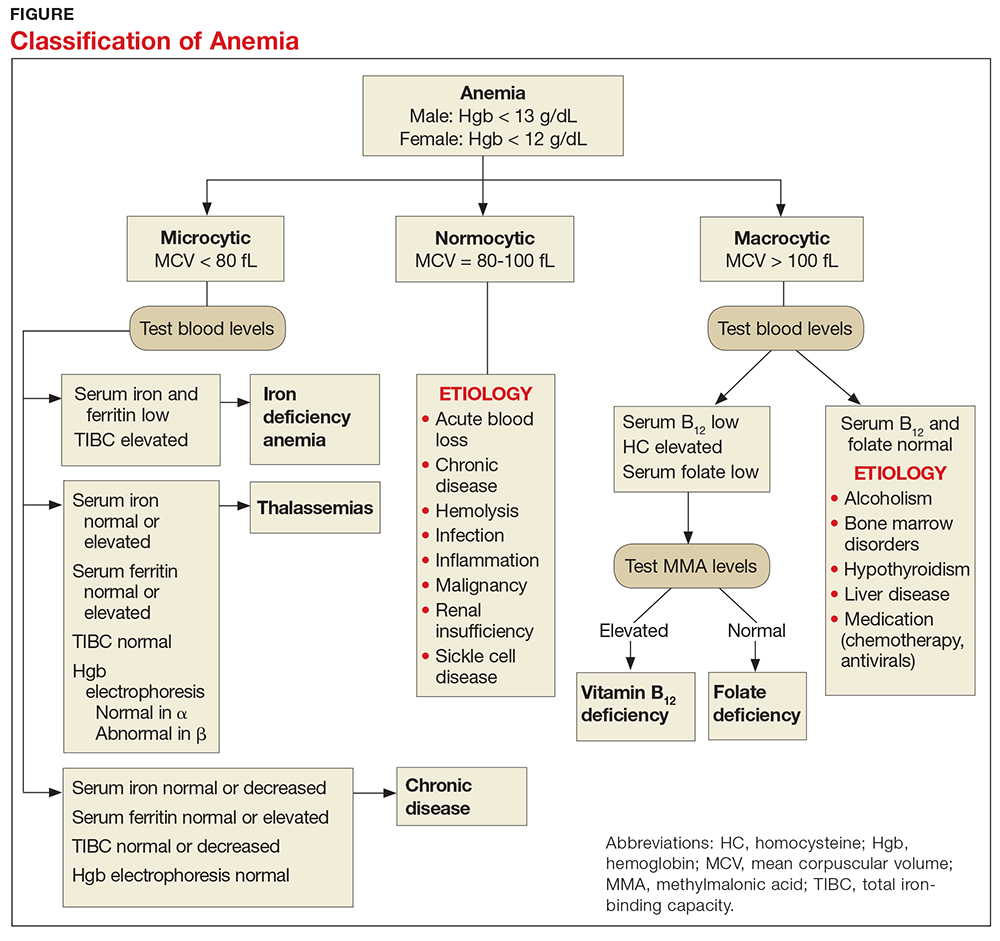
When investigating the underlying cause of anemia, the most useful parts of the CBC are the hemoglobin and the mean corpuscular volume (MCV; see Table 2).6,10 The MCV is the average volume of red cells in a specimen. This parameter is used to classify the anemia as microcytic (MCV < 80 fL), normocytic (MCV 80-100 fL), or macrocytic (MCV > 100 fL), which helps to narrow the differential diagnosis and guide any further testing (see Figure).5,6,10
It is important to note that the normal ranges of the CBC parameters differ based on race, with persons of African ancestry having lower normal hemoglobin levels than persons of Caucasian ancestry.10 In addition, laboratories may have slightly different normal values for the CBC based on the equipment they utilize. Therefore, providers must follow their laboratory’s parameters, as well as adjust for the patient’s gender, age, and ethnicity.10
Microcytic Anemia
Iron deficiency
In microcytic anemia, the RBCs are smaller than average (MCV < 80 fL), as well as hypochromic due to lack of hemoglobin.9 Iron deficiency is the most common cause of microcytic anemia worldwide.11,12 Therefore, when a patient has microcytic anemia, a serum ferritin needs to be ordered. Further testing of total iron-binding capacity (TIBC), transferrin saturation, serum iron, and serum receptor levels may be helpful if the ferritin level is between 46-99 ng/mL and anemia due to iron deficiency is not confirmed (see Table 2).12
In iron deficiency anemia, serum ferritin and serum iron levels are low due to lack of iron, but serum TIBC is high.6 The elevated TIBC reflects increased synthesis of transferrin by the liver as it attempts to compensate for the patient’s low serum iron level.9 Since iron levels are controlled by absorption rather than excretion, iron is essentially only depleted from the body through blood loss.12 Therefore, an adult patient who is iron deficient has lost more iron through blood loss than was replaced through nutritional intake and gastrointestinal absorption. In children, increased growth-related iron requirements combined with poor nutritional intake of iron-rich foods is an additional mechanism for iron deficiency.11
Iron def
If the nutritional problem is corrected or the source of bleeding is controlled, treatment with oral or intravenous iron supplements should result in improved serum hemoglobin and reticulocyte counts.13 In the primary care setting, ferrous sulfate 325 mg, which provides 65 mg of elemental iron per tablet, orally three times daily is recommended for adults.13 This gives the patient the recommended dose of approximately 200 mg of elemental iron. Repeat hemoglobin and iron studies should be conducted again in three to six months.12,13
If the patient’s iron deficiency anemia does not improve after oral iron therapy, there may be a source of blood loss the provider missed or a problem with malabsorption of iron, which can be seen in those who have undergone gastric bypass surgery or who have inflammatory bowel disease.13 Such patients should be referred to a specialist, such as a gastroenterologist, for further evaluation.
Thalassemia
Microcytic anemia with normal or elevated serum iron and normal-to-increased serum ferritin can be seen in patients with a type of thalassemia (see Figure).2 Thalassemias are inherited blood disorders that reduce hemoglobin production, leading to microcytosis; they are more common in those of Mediterranean, African, and Southeast Asian descent.2 Red cells in patients with a form of thalassemia are usually very small (microcytic) and have normal or elevated red cell distribution width (RDW).10
Moderate and severe forms of thalassemia can cause anemia. However, thalassemia syndromes that can cause severe (transfusion-dependent) anemia are usually diagnosed in childhood.9 Patients with one of the minor forms of thalassemia typically need minimal to no treatment.5 A patient with significant anemia suspicious for thalassemia should undergo hemoglobin electrophoresis testing to confirm the diagnosis and to determine the type of thalassemia.2 Typically, hemoglobin electrophoresis is normal in α thalassemia and is abnormal in ß thalassemia, as well as other forms of thalassemia. Referral to a hematologist for interpretation of these results and for further evaluation is appropriate.10
Chronic disease
If the patient has microcytic anemia and is not iron deficient or does not have thalassemia, then anemia related to a chronic disease should be considered.5 In such cases, the provider should order a reticulocyte count, which reveals how the bone marrow is responding to the anemia.5 Reticulocytes are immature red cells that have just been released from the bone marrow into the blood stream. The bone marrow increases the release of these cells in response to anemia.6
Any condition that stimulates reticulocyte production or prevents the bone marrow from producing reticulocytes will result in abnormal values (see Table 3). A normal reticulocyte count, expressed as the reticulocyte production index, is between 0.5% and 1.5%.5 The reticulocyte count is low in iron deficiency anemia and diseases that lead to decreased bone marrow production.5,6 Bone marrow suppression can occur in the context of chronic disease, infection, or inflammation. Malignancies are a less common cause for chronic disease microcytic anemia.6
If the cause of the decreased reticulocyte count is iron deficiency anemia, then treatment with iron supplementation should result in an increased reticulocyte count within one week.13 The primary care provider works in conjunction with the specialist to monitor the patient’s anemia when it is due to chronic disease or malignancy.
MACROCYTIC ANEMIA
In macrocytic anemia, the RBCs are larger than normal (MCV > 100 fL). This form of anemia is usually caused by vitamin B12 and folate deficiency, but it can also result from alcoholism, certain medications (eg, chemotherapy, antivirals), bone marrow disorders (eg, leukemia), and liver disease (eg, cirrhosis; see Figure).5,14 Common medications that can cause macrocytosis include the antiseizure drug phenytoin, the antibiotics trimethoprim/sulfamethoxazole and nitrofurantoin, the disease-modifying antirheumatic drug sulfasalazine, and immunosuppressants such as azathioprine.14,15 Antiviral agents, such as reverse transcriptase inhibitors (eg, zidovudine) used to treat HIV infection, can also cause macrocytosis with or without anemia.6,14
Macrocytic anemias caused by low serum levels of B12 and folate usually reflect problems with gastrointestinal malabsorption. For example, gastric bypass or Crohn disease can lead to malabsorption of vitamin B12 and increase a patient’s risk for macrocytic anemia.13
Vitamin B12 deficiency occurs in patients with pernicious anemia because they are missing intrinsic factor, which is necessary to facilitate B12 absorption in the ileum.10 Low vitamin B12 and folate levels also can result from inadequate dietary intake, although this is rare in the United States due to mandatory fortification of certain foods. A diet low in fresh vegetables is the leading cause of folate deficiency. While folate deficiency related to poor nutritional intake can be seen in all age groups, vitamin B12 deficiency more frequently affects the elderly or persons following a strict vegan diet.14
In addition to the fatigue and pallor associated with macrocytic anemia, patients with vitamin B12 deficiency may also have a smooth tongue, peripheral neuropathy, and edema.5,14 Severe vitamin B12 deficiency can lead to subacute combined degeneration of the spinal cord, with demyelination of the dorsal and lateral columns most often occurring in the cervical and thoracic regions.16,17 This spinal cord degeneration can cause paresthesia, muscle spasticity, and ataxia.16
When there is a macrocytic anemia, but the B12 or folate level is only borderline low, additional tests should be performed to help distinguish between B12 and folate deficiency. Both B12 and folate deficiencies can cause elevated homocysteine levels.13 Clinically significant B12 deficiency causes elevation of methylmalonic acid (MMA), whereas folate deficiency does not.13,14 Elevation of MMA can be very sensitive for B12 deficiency but lacks specificity in certain situations, such as pregnancy, renal insufficiency, and advanced age.13,14
Treatment of vitamin B12 and folate deficiencies with supplementation prevents progression of the disease, and has the potential to relieve most of the symptoms. Oral, sublingual, or parenteral vitamin B12 or oral folate supplements can be started in the primary care setting once the provider has identified whether the patient is B12 deficient, folate deficient, or both.
The vitamin B12 dose used for deficiency-induced macrocytic anemia depends on the cause—for example, a temporary condition such as pregnancy versus a lifelong disorder such as pernicious anemia.13 The usual oral dosing regimen is 2 mg/d; if intramuscular injections are used, 50 to 100 mcg are given daily for a week, followed by weekly injections for a month, and then monthly injections of 1 mg for life, if necessary.13 Bone marrow response to supplemental B12 is very rapid, with increased reticulocyte counts seen within four or five days.13
The usual dose for oral folic acid is 1 mg/d as needed.13 Folic acid can be given for folate deficiency only if the vitamin B12 level is normal. Giving folate to a patient with untreated vitamin B12 deficiency can potentially worsen subacute combined degeneration of the spinal cord.13,16
NORMOCYTIC ANEMIA
In normocytic anemia, the hemoglobin is low but the MCV is normal (see Figure).1 The history and physical exam should provide clues about whether the underlying cause of the anemia requires emergent (eg, active bleeding) or nonemergent (eg, anemia of chronic disease) management. Some of the causes of normocytic anemia are active bleeding, pregnancy, malnutrition, renal failure, chronic disease, hemolytic disorders, hypersplenism, congenital disorders, endocrine disorders, infection, and primary bone marrow disorders.1,5 Expanded plasma volume, as seen in pregnancy and overhydration, can also cause normocytic anemia.5 If gastrointestinal bleeding is suspected or the patient reports dark, tarry stools consistent with melena, fecal occult blood testing should be done. A positive result strongly supports gastrointestinal bleeding as the cause of the anemia.18
The reticulocyte count can also be helpful in identifying the cause of this type of anemia. A normocytic anemia with a normal reticulocyte and normal RDW count is usually related to chronic disease.1,10 For example, chronic kidney disease (CKD) is associated with decreased EPO production due to impaired renal function, which leads to reduced erythropoiesis. Decreased EPO prevents the bone marrow from making red blood cells, resulting in anemia. However, a normocytic anemia with an elevated reticulocyte count points to bleeding or hemolysis, as the reticulosis shows that the bone marrow is increasing red cell production to make up for the lost red cells.5
Additional diagnostic laboratory testing for patients with normocytic anemia may involve, for example, creatinine and blood urea nitrogen for patients with CKD, prothrombin time with an INR and liver function tests for patients with liver disease, and urine human chorionic gonadotropin if pregnancy is suspected.
For patients with an infection that is causing severe hemolysis (eg, sepsis due to a ß-hemolytic streptococcal infection), blood cultures should be drawn.5 If red blood cell destruction due to an artificial cardiac valve or an autoimmune disorder is suspected as the cause of the anemia, a hematology consult is needed.1 Anemia caused by disseminated intravascular coagulation or thrombotic thrombocytopenic purpura resulting in hemolysis are usually emergent conditions that require immediate intervention, including hospitalization and management by a hematologist.1
PATIENT EDUCATION
Patients and any accompanying family members should be educated about the signs and symptoms of anemia, the diagnostic testing and treatment regimens specific to their anemia, and medication compliance issues.
For instance, patients who abuse alcohol often have both vitamin B12 and folate deficiencies. If the macrocytosis is caused by alcohol intake, then the provider should educate the patient on the importance of alcohol abstention, as well as refer the patient for rehabilitation and psychologic counseling, as needed. These patients can sometimes recover from macrocytic anemia simply by stopping alcohol intake and improving their nutrition.19 Patients with microcytosis due to iron deficiency anemia should be advised about the importance of good nutrition and compliance with iron supplementation.
Repeat CBCs and a follow-up patient history and physical exam will help the provider assess whether the anemia is resolving. Individualized plans that target the specific type of anemia identified, as well as its underlying cause, are key to successful treatment.
CONCLUSION
When managing a patient with anemia, providers must define the type of anemia present and identify its underlying cause before starting treatment. Clues from the patient’s history, physical exam, and CBC can help isolate the cause of anemia. The MCV is the most helpful of the red blood cell indices because it allows the provider to classify the anemia as microcytic, macrocytic, or normocytic.
In cases in which the anemia is acute or severe—or in which the patient remains anemic even after being treated by the primary care provider—referral to a specialist is appropriate.
1. Brill JR, Baumgardner D. Normocytic anemia. Am Fam Physician. 2000;62(10):2255-2263.
2. Van Vranken M. Evaluation of microcytosis. Am Fam Physician. 2010;82(9):1117-1122.
3. National Institutes of Health/National Heart, Lung, and Blood Institute. How is anemia diagnosed? www.nhlbi.nih.gov/health/health-topics/topics/anemia/diagnosis. Accessed April 28, 2017.
4. Smith RE Jr. The clinical and economic burden of anemia. Am J Manag Care. 2010;16(3):S59-S66.
5. Platt A, Eckman J. Diagnosing anemia. Clinician Reviews. 2006;16(2):44-50.
6. Karnath B. Anemia in the adult patient. Hosp Physician. 2004;40(10):32-36.
7. World Health Organization. Micronutrient deficiencies. www.who.int/nutrition/topics/ida/en/#. Accessed April 29, 2017.
8. US Department of Health and Human Services, Office of Women’s Health. Iron-deficiency anemia. www.womenshealth.gov/publications/our-publications/fact-sheet/anemia.html#a. Accessed April 29, 2017.
9. DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(14): 1324-1331.
10. Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923-936.
11. Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015; 372(19):1832-1843.
12. Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75(5):671-678.
13. Rodgers GP, Young N. The Bethesda Handbook of Clinical Hematology. 3rd ed. Baltimore: Lippincott Williams and Wilkins; 2013:1-21.
14. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
15. Hesdorffer CS, Longo DL. Drug-induced megaloblastic anemia. N Engl J Med. 2015;373(17):1649-1658.
16. Vasconcelos OM, Poehm EH, McCarter RJ, et al. Potential outcome factors in subacute combined degeneration. J Gen Intern Med. 2006;21(10):1063-1068.
17. Vide AT, Marques AM, Costa JD. MRI findings in subacute combined degeneration of the spinal cord in a patient with restricted diet. Neurol Sci. 2011;33(3):711-713.
18. Kessenich CR, Cronin K. Fecal occult blood testing in older adult patients with anemia. Nurse Pract. 2013;38(1):6-8.
19. Imashuku S, Kudo N, Kaneda S. Spontaneous resolution of macrocytic anemia: old disease revisited. J Blood Med. 2012;3:45-47.
20. Pagana K, Pagana T, Pagana T. Mosby’s Diagnostic and Laboratory Test Reference. 12th ed. St. Louis, MO: Elsevier Mosby; 2015: 497-501, 785-791, 805-806.
CE/CME No: CR-1708
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the importance of diagnosing the type of anemia in order to provide appropriate treatment.
• Describe how the complete blood count and its indices are used to initially determine if an anemia is microcytic, normocytic, or macrocytic.
• List the more common causes of microcytic, normocytic, and macrocytic anemia.
• Discuss addictional laboratory tests that may be used to further assess the cause of anemia.
FACULTY
Jean O’Neil is an Assistant Professor and Coordinator of the Adult Gerontology Acute Care Nurse Practitioner Program in the Patricia A. Chin School of Nursing at California State University, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2018.
Article begins on next page >>
Anemia affects more than 3 million people in the United States, making it a common problem in primary care practices. Once anemia is detected, clinicians must define the type and identify its underlying cause prior to initiating treatment. In most cases, the cause can be determined using information from the patient history, physical exam, and complete blood count.
Anemia is commonly identified during routine physical exams and laboratory testing.1-3 However, treating anemia can present a challenge for the primary care provider if the immediate cause is not apparent. Iron deficiency is a leading cause of anemia, but simply prescribing an iron supplement without determining the type or the cause of the anemia is not appropriate. Anemia that is misdiagnosed or goes untreated can be associated with a worse prognosis, as well as increased health care costs.4
Primary care providers often manage patients with common types of anemia and refer patients with severe or complex anemia to specialists for further testing and treatment. The most commonly used and cost-effective diagnostic tool for anemia is the complete blood count (CBC).2-6 The CBC provides details that can help the provider determine the type of anemia present, which in turn guides proper diagnostic testing and treatment.
EPIDEMIOLOGY
Anemia involves a reduction in the number of circulating red blood cells, the blood hemoglobin content, or the hematocrit, which leads to impaired delivery of oxygen to the body. Anemia affects more than 2 billion people worldwide, with iron deficiency the most common cause.7 Other leading nutritional causes of anemia include vitamin B12 and folate deficiency.4,7 Approximately 3 to 4 million Americans have anemia in some form, and it affects about 6.6% of men and 12.4% of women.5,8 The prevalence of anemia increases with age. Approximately 11% of men and 10% of women ages 65 or older have anemia, and in men ages 85 or older, prevalence of 20% to 44% has been reported.1,4 Anemia is present in about 3.5% of patients with chronic disease, but only 15% of them receive treatment.4
PATHOPHYSIOLOGY
Blood is composed of water-based plasma (54%), white blood cells and platelets (1%), and red blood cells (45%).5 Hemoglobin, the primary protein of the red blood cell, binds oxygen from the lungs and transports it to the rest of the body. Oxygen is then exchanged for carbon dioxide, which is carried back to the lungs to be exhaled.
Hemoglobin is made up of four globin chains, each containing an iron ion held in a porphyrin ring known as a heme group.5 When the body detects low tissue oxygen, the endothelial cells in the kidneys secrete the hormone erythropoietin (EPO), which stimulates the bone marrow to increase red cell production.5 This feedback loop can be interrupted by renal failure or chronic disease.4 In addition, bone marrow cannot produce enough red blood cells if there are insufficient levels of iron, amino acids, protein, carbohydrates, lipids, folate, and vitamin B12.5 Toxins (eg, lead), some types of cancer (eg, lymphoma), or even common infections (eg, pneumonia) can suppress the bone marrow, causing anemia. The more severe the anemia, the more likely oxygen transport will be compromised and organ failure will ensue.
Mutations affecting the genes that encode the globin chains within hemoglobin can cause one of the more than 600 known hemoglobinopathies (genetic defects of hemoglobin structure), such as sickle cell disease and thalassemias.5,9 While it is important to identify and treat patients with hemoglobinopathies, most anemias have other causes, such as iron deficiency, chronic disease, bone marrow defects, B12 deficiency, renal failure, medications, alcoholism, pregnancy, nutritional intake problems, gastrointestinal malabsorption, and active or recent history of blood loss.5,10
CLINICAL PRESENTATION
There are several signs and symptoms that should lead the primary care provider to suspect anemia (see Table 1).5,6 The severity of these symptoms can vary from mild to very serious. Severe anemia can lead to organ failure and death. However, most patients with anemia are asymptomatic, and anemia is typically detected incidentally during laboratory testing.1,2
Once anemia is confirmed, the evaluation focuses on diagnosing its underlying cause. It should include a thorough patient history and review of systems to ascertain whether the patient has symptoms such as increased fatigue, palpitations, gastrointestinal distress, weakness, or dizziness.
If the provider has access to past CBC results, a comparison of the current and previous results will help determine whether the anemia is acute or chronic. Anemia caused by acute conditions, such as a suspicion of blood loss or bone marrow suppression, must be attended to immediately. A patient with chronic anemia should be carefully monitored and may need follow-up for ongoing treatment. While a provider has more time to work up a patient with chronic anemia, the causes may not be as straightforward.
DIAGNOSIS AND CLASSIFICATION
Anemia in adults is defined as hemoglobin less than 13 g/dL in males and 12 g/dL in females.6 The hemoglobin is part of the complete blood cell report, which also includes the white blood cell count (WBC), red blood cell count (RBC), hematocrit, platelet count, and indices.
When investigating the underlying cause of anemia, the most useful parts of the CBC are the hemoglobin and the mean corpuscular volume (MCV; see Table 2).6,10 The MCV is the average volume of red cells in a specimen. This parameter is used to classify the anemia as microcytic (MCV < 80 fL), normocytic (MCV 80-100 fL), or macrocytic (MCV > 100 fL), which helps to narrow the differential diagnosis and guide any further testing (see Figure).5,6,10
It is important to note that the normal ranges of the CBC parameters differ based on race, with persons of African ancestry having lower normal hemoglobin levels than persons of Caucasian ancestry.10 In addition, laboratories may have slightly different normal values for the CBC based on the equipment they utilize. Therefore, providers must follow their laboratory’s parameters, as well as adjust for the patient’s gender, age, and ethnicity.10
Microcytic Anemia
Iron deficiency
In microcytic anemia, the RBCs are smaller than average (MCV < 80 fL), as well as hypochromic due to lack of hemoglobin.9 Iron deficiency is the most common cause of microcytic anemia worldwide.11,12 Therefore, when a patient has microcytic anemia, a serum ferritin needs to be ordered. Further testing of total iron-binding capacity (TIBC), transferrin saturation, serum iron, and serum receptor levels may be helpful if the ferritin level is between 46-99 ng/mL and anemia due to iron deficiency is not confirmed (see Table 2).12
In iron deficiency anemia, serum ferritin and serum iron levels are low due to lack of iron, but serum TIBC is high.6 The elevated TIBC reflects increased synthesis of transferrin by the liver as it attempts to compensate for the patient’s low serum iron level.9 Since iron levels are controlled by absorption rather than excretion, iron is essentially only depleted from the body through blood loss.12 Therefore, an adult patient who is iron deficient has lost more iron through blood loss than was replaced through nutritional intake and gastrointestinal absorption. In children, increased growth-related iron requirements combined with poor nutritional intake of iron-rich foods is an additional mechanism for iron deficiency.11
Iron def
If the nutritional problem is corrected or the source of bleeding is controlled, treatment with oral or intravenous iron supplements should result in improved serum hemoglobin and reticulocyte counts.13 In the primary care setting, ferrous sulfate 325 mg, which provides 65 mg of elemental iron per tablet, orally three times daily is recommended for adults.13 This gives the patient the recommended dose of approximately 200 mg of elemental iron. Repeat hemoglobin and iron studies should be conducted again in three to six months.12,13
If the patient’s iron deficiency anemia does not improve after oral iron therapy, there may be a source of blood loss the provider missed or a problem with malabsorption of iron, which can be seen in those who have undergone gastric bypass surgery or who have inflammatory bowel disease.13 Such patients should be referred to a specialist, such as a gastroenterologist, for further evaluation.
Thalassemia
Microcytic anemia with normal or elevated serum iron and normal-to-increased serum ferritin can be seen in patients with a type of thalassemia (see Figure).2 Thalassemias are inherited blood disorders that reduce hemoglobin production, leading to microcytosis; they are more common in those of Mediterranean, African, and Southeast Asian descent.2 Red cells in patients with a form of thalassemia are usually very small (microcytic) and have normal or elevated red cell distribution width (RDW).10
Moderate and severe forms of thalassemia can cause anemia. However, thalassemia syndromes that can cause severe (transfusion-dependent) anemia are usually diagnosed in childhood.9 Patients with one of the minor forms of thalassemia typically need minimal to no treatment.5 A patient with significant anemia suspicious for thalassemia should undergo hemoglobin electrophoresis testing to confirm the diagnosis and to determine the type of thalassemia.2 Typically, hemoglobin electrophoresis is normal in α thalassemia and is abnormal in ß thalassemia, as well as other forms of thalassemia. Referral to a hematologist for interpretation of these results and for further evaluation is appropriate.10
Chronic disease
If the patient has microcytic anemia and is not iron deficient or does not have thalassemia, then anemia related to a chronic disease should be considered.5 In such cases, the provider should order a reticulocyte count, which reveals how the bone marrow is responding to the anemia.5 Reticulocytes are immature red cells that have just been released from the bone marrow into the blood stream. The bone marrow increases the release of these cells in response to anemia.6
Any condition that stimulates reticulocyte production or prevents the bone marrow from producing reticulocytes will result in abnormal values (see Table 3). A normal reticulocyte count, expressed as the reticulocyte production index, is between 0.5% and 1.5%.5 The reticulocyte count is low in iron deficiency anemia and diseases that lead to decreased bone marrow production.5,6 Bone marrow suppression can occur in the context of chronic disease, infection, or inflammation. Malignancies are a less common cause for chronic disease microcytic anemia.6
If the cause of the decreased reticulocyte count is iron deficiency anemia, then treatment with iron supplementation should result in an increased reticulocyte count within one week.13 The primary care provider works in conjunction with the specialist to monitor the patient’s anemia when it is due to chronic disease or malignancy.
MACROCYTIC ANEMIA
In macrocytic anemia, the RBCs are larger than normal (MCV > 100 fL). This form of anemia is usually caused by vitamin B12 and folate deficiency, but it can also result from alcoholism, certain medications (eg, chemotherapy, antivirals), bone marrow disorders (eg, leukemia), and liver disease (eg, cirrhosis; see Figure).5,14 Common medications that can cause macrocytosis include the antiseizure drug phenytoin, the antibiotics trimethoprim/sulfamethoxazole and nitrofurantoin, the disease-modifying antirheumatic drug sulfasalazine, and immunosuppressants such as azathioprine.14,15 Antiviral agents, such as reverse transcriptase inhibitors (eg, zidovudine) used to treat HIV infection, can also cause macrocytosis with or without anemia.6,14
Macrocytic anemias caused by low serum levels of B12 and folate usually reflect problems with gastrointestinal malabsorption. For example, gastric bypass or Crohn disease can lead to malabsorption of vitamin B12 and increase a patient’s risk for macrocytic anemia.13
Vitamin B12 deficiency occurs in patients with pernicious anemia because they are missing intrinsic factor, which is necessary to facilitate B12 absorption in the ileum.10 Low vitamin B12 and folate levels also can result from inadequate dietary intake, although this is rare in the United States due to mandatory fortification of certain foods. A diet low in fresh vegetables is the leading cause of folate deficiency. While folate deficiency related to poor nutritional intake can be seen in all age groups, vitamin B12 deficiency more frequently affects the elderly or persons following a strict vegan diet.14
In addition to the fatigue and pallor associated with macrocytic anemia, patients with vitamin B12 deficiency may also have a smooth tongue, peripheral neuropathy, and edema.5,14 Severe vitamin B12 deficiency can lead to subacute combined degeneration of the spinal cord, with demyelination of the dorsal and lateral columns most often occurring in the cervical and thoracic regions.16,17 This spinal cord degeneration can cause paresthesia, muscle spasticity, and ataxia.16
When there is a macrocytic anemia, but the B12 or folate level is only borderline low, additional tests should be performed to help distinguish between B12 and folate deficiency. Both B12 and folate deficiencies can cause elevated homocysteine levels.13 Clinically significant B12 deficiency causes elevation of methylmalonic acid (MMA), whereas folate deficiency does not.13,14 Elevation of MMA can be very sensitive for B12 deficiency but lacks specificity in certain situations, such as pregnancy, renal insufficiency, and advanced age.13,14
Treatment of vitamin B12 and folate deficiencies with supplementation prevents progression of the disease, and has the potential to relieve most of the symptoms. Oral, sublingual, or parenteral vitamin B12 or oral folate supplements can be started in the primary care setting once the provider has identified whether the patient is B12 deficient, folate deficient, or both.
The vitamin B12 dose used for deficiency-induced macrocytic anemia depends on the cause—for example, a temporary condition such as pregnancy versus a lifelong disorder such as pernicious anemia.13 The usual oral dosing regimen is 2 mg/d; if intramuscular injections are used, 50 to 100 mcg are given daily for a week, followed by weekly injections for a month, and then monthly injections of 1 mg for life, if necessary.13 Bone marrow response to supplemental B12 is very rapid, with increased reticulocyte counts seen within four or five days.13
The usual dose for oral folic acid is 1 mg/d as needed.13 Folic acid can be given for folate deficiency only if the vitamin B12 level is normal. Giving folate to a patient with untreated vitamin B12 deficiency can potentially worsen subacute combined degeneration of the spinal cord.13,16
NORMOCYTIC ANEMIA
In normocytic anemia, the hemoglobin is low but the MCV is normal (see Figure).1 The history and physical exam should provide clues about whether the underlying cause of the anemia requires emergent (eg, active bleeding) or nonemergent (eg, anemia of chronic disease) management. Some of the causes of normocytic anemia are active bleeding, pregnancy, malnutrition, renal failure, chronic disease, hemolytic disorders, hypersplenism, congenital disorders, endocrine disorders, infection, and primary bone marrow disorders.1,5 Expanded plasma volume, as seen in pregnancy and overhydration, can also cause normocytic anemia.5 If gastrointestinal bleeding is suspected or the patient reports dark, tarry stools consistent with melena, fecal occult blood testing should be done. A positive result strongly supports gastrointestinal bleeding as the cause of the anemia.18
The reticulocyte count can also be helpful in identifying the cause of this type of anemia. A normocytic anemia with a normal reticulocyte and normal RDW count is usually related to chronic disease.1,10 For example, chronic kidney disease (CKD) is associated with decreased EPO production due to impaired renal function, which leads to reduced erythropoiesis. Decreased EPO prevents the bone marrow from making red blood cells, resulting in anemia. However, a normocytic anemia with an elevated reticulocyte count points to bleeding or hemolysis, as the reticulosis shows that the bone marrow is increasing red cell production to make up for the lost red cells.5
Additional diagnostic laboratory testing for patients with normocytic anemia may involve, for example, creatinine and blood urea nitrogen for patients with CKD, prothrombin time with an INR and liver function tests for patients with liver disease, and urine human chorionic gonadotropin if pregnancy is suspected.
For patients with an infection that is causing severe hemolysis (eg, sepsis due to a ß-hemolytic streptococcal infection), blood cultures should be drawn.5 If red blood cell destruction due to an artificial cardiac valve or an autoimmune disorder is suspected as the cause of the anemia, a hematology consult is needed.1 Anemia caused by disseminated intravascular coagulation or thrombotic thrombocytopenic purpura resulting in hemolysis are usually emergent conditions that require immediate intervention, including hospitalization and management by a hematologist.1
PATIENT EDUCATION
Patients and any accompanying family members should be educated about the signs and symptoms of anemia, the diagnostic testing and treatment regimens specific to their anemia, and medication compliance issues.
For instance, patients who abuse alcohol often have both vitamin B12 and folate deficiencies. If the macrocytosis is caused by alcohol intake, then the provider should educate the patient on the importance of alcohol abstention, as well as refer the patient for rehabilitation and psychologic counseling, as needed. These patients can sometimes recover from macrocytic anemia simply by stopping alcohol intake and improving their nutrition.19 Patients with microcytosis due to iron deficiency anemia should be advised about the importance of good nutrition and compliance with iron supplementation.
Repeat CBCs and a follow-up patient history and physical exam will help the provider assess whether the anemia is resolving. Individualized plans that target the specific type of anemia identified, as well as its underlying cause, are key to successful treatment.
CONCLUSION
When managing a patient with anemia, providers must define the type of anemia present and identify its underlying cause before starting treatment. Clues from the patient’s history, physical exam, and CBC can help isolate the cause of anemia. The MCV is the most helpful of the red blood cell indices because it allows the provider to classify the anemia as microcytic, macrocytic, or normocytic.
In cases in which the anemia is acute or severe—or in which the patient remains anemic even after being treated by the primary care provider—referral to a specialist is appropriate.
CE/CME No: CR-1708
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the importance of diagnosing the type of anemia in order to provide appropriate treatment.
• Describe how the complete blood count and its indices are used to initially determine if an anemia is microcytic, normocytic, or macrocytic.
• List the more common causes of microcytic, normocytic, and macrocytic anemia.
• Discuss addictional laboratory tests that may be used to further assess the cause of anemia.
FACULTY
Jean O’Neil is an Assistant Professor and Coordinator of the Adult Gerontology Acute Care Nurse Practitioner Program in the Patricia A. Chin School of Nursing at California State University, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2018.
Article begins on next page >>
Anemia affects more than 3 million people in the United States, making it a common problem in primary care practices. Once anemia is detected, clinicians must define the type and identify its underlying cause prior to initiating treatment. In most cases, the cause can be determined using information from the patient history, physical exam, and complete blood count.
Anemia is commonly identified during routine physical exams and laboratory testing.1-3 However, treating anemia can present a challenge for the primary care provider if the immediate cause is not apparent. Iron deficiency is a leading cause of anemia, but simply prescribing an iron supplement without determining the type or the cause of the anemia is not appropriate. Anemia that is misdiagnosed or goes untreated can be associated with a worse prognosis, as well as increased health care costs.4
Primary care providers often manage patients with common types of anemia and refer patients with severe or complex anemia to specialists for further testing and treatment. The most commonly used and cost-effective diagnostic tool for anemia is the complete blood count (CBC).2-6 The CBC provides details that can help the provider determine the type of anemia present, which in turn guides proper diagnostic testing and treatment.
EPIDEMIOLOGY
Anemia involves a reduction in the number of circulating red blood cells, the blood hemoglobin content, or the hematocrit, which leads to impaired delivery of oxygen to the body. Anemia affects more than 2 billion people worldwide, with iron deficiency the most common cause.7 Other leading nutritional causes of anemia include vitamin B12 and folate deficiency.4,7 Approximately 3 to 4 million Americans have anemia in some form, and it affects about 6.6% of men and 12.4% of women.5,8 The prevalence of anemia increases with age. Approximately 11% of men and 10% of women ages 65 or older have anemia, and in men ages 85 or older, prevalence of 20% to 44% has been reported.1,4 Anemia is present in about 3.5% of patients with chronic disease, but only 15% of them receive treatment.4
PATHOPHYSIOLOGY
Blood is composed of water-based plasma (54%), white blood cells and platelets (1%), and red blood cells (45%).5 Hemoglobin, the primary protein of the red blood cell, binds oxygen from the lungs and transports it to the rest of the body. Oxygen is then exchanged for carbon dioxide, which is carried back to the lungs to be exhaled.
Hemoglobin is made up of four globin chains, each containing an iron ion held in a porphyrin ring known as a heme group.5 When the body detects low tissue oxygen, the endothelial cells in the kidneys secrete the hormone erythropoietin (EPO), which stimulates the bone marrow to increase red cell production.5 This feedback loop can be interrupted by renal failure or chronic disease.4 In addition, bone marrow cannot produce enough red blood cells if there are insufficient levels of iron, amino acids, protein, carbohydrates, lipids, folate, and vitamin B12.5 Toxins (eg, lead), some types of cancer (eg, lymphoma), or even common infections (eg, pneumonia) can suppress the bone marrow, causing anemia. The more severe the anemia, the more likely oxygen transport will be compromised and organ failure will ensue.
Mutations affecting the genes that encode the globin chains within hemoglobin can cause one of the more than 600 known hemoglobinopathies (genetic defects of hemoglobin structure), such as sickle cell disease and thalassemias.5,9 While it is important to identify and treat patients with hemoglobinopathies, most anemias have other causes, such as iron deficiency, chronic disease, bone marrow defects, B12 deficiency, renal failure, medications, alcoholism, pregnancy, nutritional intake problems, gastrointestinal malabsorption, and active or recent history of blood loss.5,10
CLINICAL PRESENTATION
There are several signs and symptoms that should lead the primary care provider to suspect anemia (see Table 1).5,6 The severity of these symptoms can vary from mild to very serious. Severe anemia can lead to organ failure and death. However, most patients with anemia are asymptomatic, and anemia is typically detected incidentally during laboratory testing.1,2
Once anemia is confirmed, the evaluation focuses on diagnosing its underlying cause. It should include a thorough patient history and review of systems to ascertain whether the patient has symptoms such as increased fatigue, palpitations, gastrointestinal distress, weakness, or dizziness.
If the provider has access to past CBC results, a comparison of the current and previous results will help determine whether the anemia is acute or chronic. Anemia caused by acute conditions, such as a suspicion of blood loss or bone marrow suppression, must be attended to immediately. A patient with chronic anemia should be carefully monitored and may need follow-up for ongoing treatment. While a provider has more time to work up a patient with chronic anemia, the causes may not be as straightforward.
DIAGNOSIS AND CLASSIFICATION
Anemia in adults is defined as hemoglobin less than 13 g/dL in males and 12 g/dL in females.6 The hemoglobin is part of the complete blood cell report, which also includes the white blood cell count (WBC), red blood cell count (RBC), hematocrit, platelet count, and indices.
When investigating the underlying cause of anemia, the most useful parts of the CBC are the hemoglobin and the mean corpuscular volume (MCV; see Table 2).6,10 The MCV is the average volume of red cells in a specimen. This parameter is used to classify the anemia as microcytic (MCV < 80 fL), normocytic (MCV 80-100 fL), or macrocytic (MCV > 100 fL), which helps to narrow the differential diagnosis and guide any further testing (see Figure).5,6,10
It is important to note that the normal ranges of the CBC parameters differ based on race, with persons of African ancestry having lower normal hemoglobin levels than persons of Caucasian ancestry.10 In addition, laboratories may have slightly different normal values for the CBC based on the equipment they utilize. Therefore, providers must follow their laboratory’s parameters, as well as adjust for the patient’s gender, age, and ethnicity.10
Microcytic Anemia
Iron deficiency
In microcytic anemia, the RBCs are smaller than average (MCV < 80 fL), as well as hypochromic due to lack of hemoglobin.9 Iron deficiency is the most common cause of microcytic anemia worldwide.11,12 Therefore, when a patient has microcytic anemia, a serum ferritin needs to be ordered. Further testing of total iron-binding capacity (TIBC), transferrin saturation, serum iron, and serum receptor levels may be helpful if the ferritin level is between 46-99 ng/mL and anemia due to iron deficiency is not confirmed (see Table 2).12
In iron deficiency anemia, serum ferritin and serum iron levels are low due to lack of iron, but serum TIBC is high.6 The elevated TIBC reflects increased synthesis of transferrin by the liver as it attempts to compensate for the patient’s low serum iron level.9 Since iron levels are controlled by absorption rather than excretion, iron is essentially only depleted from the body through blood loss.12 Therefore, an adult patient who is iron deficient has lost more iron through blood loss than was replaced through nutritional intake and gastrointestinal absorption. In children, increased growth-related iron requirements combined with poor nutritional intake of iron-rich foods is an additional mechanism for iron deficiency.11
Iron def
If the nutritional problem is corrected or the source of bleeding is controlled, treatment with oral or intravenous iron supplements should result in improved serum hemoglobin and reticulocyte counts.13 In the primary care setting, ferrous sulfate 325 mg, which provides 65 mg of elemental iron per tablet, orally three times daily is recommended for adults.13 This gives the patient the recommended dose of approximately 200 mg of elemental iron. Repeat hemoglobin and iron studies should be conducted again in three to six months.12,13
If the patient’s iron deficiency anemia does not improve after oral iron therapy, there may be a source of blood loss the provider missed or a problem with malabsorption of iron, which can be seen in those who have undergone gastric bypass surgery or who have inflammatory bowel disease.13 Such patients should be referred to a specialist, such as a gastroenterologist, for further evaluation.
Thalassemia
Microcytic anemia with normal or elevated serum iron and normal-to-increased serum ferritin can be seen in patients with a type of thalassemia (see Figure).2 Thalassemias are inherited blood disorders that reduce hemoglobin production, leading to microcytosis; they are more common in those of Mediterranean, African, and Southeast Asian descent.2 Red cells in patients with a form of thalassemia are usually very small (microcytic) and have normal or elevated red cell distribution width (RDW).10
Moderate and severe forms of thalassemia can cause anemia. However, thalassemia syndromes that can cause severe (transfusion-dependent) anemia are usually diagnosed in childhood.9 Patients with one of the minor forms of thalassemia typically need minimal to no treatment.5 A patient with significant anemia suspicious for thalassemia should undergo hemoglobin electrophoresis testing to confirm the diagnosis and to determine the type of thalassemia.2 Typically, hemoglobin electrophoresis is normal in α thalassemia and is abnormal in ß thalassemia, as well as other forms of thalassemia. Referral to a hematologist for interpretation of these results and for further evaluation is appropriate.10
Chronic disease
If the patient has microcytic anemia and is not iron deficient or does not have thalassemia, then anemia related to a chronic disease should be considered.5 In such cases, the provider should order a reticulocyte count, which reveals how the bone marrow is responding to the anemia.5 Reticulocytes are immature red cells that have just been released from the bone marrow into the blood stream. The bone marrow increases the release of these cells in response to anemia.6
Any condition that stimulates reticulocyte production or prevents the bone marrow from producing reticulocytes will result in abnormal values (see Table 3). A normal reticulocyte count, expressed as the reticulocyte production index, is between 0.5% and 1.5%.5 The reticulocyte count is low in iron deficiency anemia and diseases that lead to decreased bone marrow production.5,6 Bone marrow suppression can occur in the context of chronic disease, infection, or inflammation. Malignancies are a less common cause for chronic disease microcytic anemia.6
If the cause of the decreased reticulocyte count is iron deficiency anemia, then treatment with iron supplementation should result in an increased reticulocyte count within one week.13 The primary care provider works in conjunction with the specialist to monitor the patient’s anemia when it is due to chronic disease or malignancy.
MACROCYTIC ANEMIA
In macrocytic anemia, the RBCs are larger than normal (MCV > 100 fL). This form of anemia is usually caused by vitamin B12 and folate deficiency, but it can also result from alcoholism, certain medications (eg, chemotherapy, antivirals), bone marrow disorders (eg, leukemia), and liver disease (eg, cirrhosis; see Figure).5,14 Common medications that can cause macrocytosis include the antiseizure drug phenytoin, the antibiotics trimethoprim/sulfamethoxazole and nitrofurantoin, the disease-modifying antirheumatic drug sulfasalazine, and immunosuppressants such as azathioprine.14,15 Antiviral agents, such as reverse transcriptase inhibitors (eg, zidovudine) used to treat HIV infection, can also cause macrocytosis with or without anemia.6,14
Macrocytic anemias caused by low serum levels of B12 and folate usually reflect problems with gastrointestinal malabsorption. For example, gastric bypass or Crohn disease can lead to malabsorption of vitamin B12 and increase a patient’s risk for macrocytic anemia.13
Vitamin B12 deficiency occurs in patients with pernicious anemia because they are missing intrinsic factor, which is necessary to facilitate B12 absorption in the ileum.10 Low vitamin B12 and folate levels also can result from inadequate dietary intake, although this is rare in the United States due to mandatory fortification of certain foods. A diet low in fresh vegetables is the leading cause of folate deficiency. While folate deficiency related to poor nutritional intake can be seen in all age groups, vitamin B12 deficiency more frequently affects the elderly or persons following a strict vegan diet.14
In addition to the fatigue and pallor associated with macrocytic anemia, patients with vitamin B12 deficiency may also have a smooth tongue, peripheral neuropathy, and edema.5,14 Severe vitamin B12 deficiency can lead to subacute combined degeneration of the spinal cord, with demyelination of the dorsal and lateral columns most often occurring in the cervical and thoracic regions.16,17 This spinal cord degeneration can cause paresthesia, muscle spasticity, and ataxia.16
When there is a macrocytic anemia, but the B12 or folate level is only borderline low, additional tests should be performed to help distinguish between B12 and folate deficiency. Both B12 and folate deficiencies can cause elevated homocysteine levels.13 Clinically significant B12 deficiency causes elevation of methylmalonic acid (MMA), whereas folate deficiency does not.13,14 Elevation of MMA can be very sensitive for B12 deficiency but lacks specificity in certain situations, such as pregnancy, renal insufficiency, and advanced age.13,14
Treatment of vitamin B12 and folate deficiencies with supplementation prevents progression of the disease, and has the potential to relieve most of the symptoms. Oral, sublingual, or parenteral vitamin B12 or oral folate supplements can be started in the primary care setting once the provider has identified whether the patient is B12 deficient, folate deficient, or both.
The vitamin B12 dose used for deficiency-induced macrocytic anemia depends on the cause—for example, a temporary condition such as pregnancy versus a lifelong disorder such as pernicious anemia.13 The usual oral dosing regimen is 2 mg/d; if intramuscular injections are used, 50 to 100 mcg are given daily for a week, followed by weekly injections for a month, and then monthly injections of 1 mg for life, if necessary.13 Bone marrow response to supplemental B12 is very rapid, with increased reticulocyte counts seen within four or five days.13
The usual dose for oral folic acid is 1 mg/d as needed.13 Folic acid can be given for folate deficiency only if the vitamin B12 level is normal. Giving folate to a patient with untreated vitamin B12 deficiency can potentially worsen subacute combined degeneration of the spinal cord.13,16
NORMOCYTIC ANEMIA
In normocytic anemia, the hemoglobin is low but the MCV is normal (see Figure).1 The history and physical exam should provide clues about whether the underlying cause of the anemia requires emergent (eg, active bleeding) or nonemergent (eg, anemia of chronic disease) management. Some of the causes of normocytic anemia are active bleeding, pregnancy, malnutrition, renal failure, chronic disease, hemolytic disorders, hypersplenism, congenital disorders, endocrine disorders, infection, and primary bone marrow disorders.1,5 Expanded plasma volume, as seen in pregnancy and overhydration, can also cause normocytic anemia.5 If gastrointestinal bleeding is suspected or the patient reports dark, tarry stools consistent with melena, fecal occult blood testing should be done. A positive result strongly supports gastrointestinal bleeding as the cause of the anemia.18
The reticulocyte count can also be helpful in identifying the cause of this type of anemia. A normocytic anemia with a normal reticulocyte and normal RDW count is usually related to chronic disease.1,10 For example, chronic kidney disease (CKD) is associated with decreased EPO production due to impaired renal function, which leads to reduced erythropoiesis. Decreased EPO prevents the bone marrow from making red blood cells, resulting in anemia. However, a normocytic anemia with an elevated reticulocyte count points to bleeding or hemolysis, as the reticulosis shows that the bone marrow is increasing red cell production to make up for the lost red cells.5
Additional diagnostic laboratory testing for patients with normocytic anemia may involve, for example, creatinine and blood urea nitrogen for patients with CKD, prothrombin time with an INR and liver function tests for patients with liver disease, and urine human chorionic gonadotropin if pregnancy is suspected.
For patients with an infection that is causing severe hemolysis (eg, sepsis due to a ß-hemolytic streptococcal infection), blood cultures should be drawn.5 If red blood cell destruction due to an artificial cardiac valve or an autoimmune disorder is suspected as the cause of the anemia, a hematology consult is needed.1 Anemia caused by disseminated intravascular coagulation or thrombotic thrombocytopenic purpura resulting in hemolysis are usually emergent conditions that require immediate intervention, including hospitalization and management by a hematologist.1
PATIENT EDUCATION
Patients and any accompanying family members should be educated about the signs and symptoms of anemia, the diagnostic testing and treatment regimens specific to their anemia, and medication compliance issues.
For instance, patients who abuse alcohol often have both vitamin B12 and folate deficiencies. If the macrocytosis is caused by alcohol intake, then the provider should educate the patient on the importance of alcohol abstention, as well as refer the patient for rehabilitation and psychologic counseling, as needed. These patients can sometimes recover from macrocytic anemia simply by stopping alcohol intake and improving their nutrition.19 Patients with microcytosis due to iron deficiency anemia should be advised about the importance of good nutrition and compliance with iron supplementation.
Repeat CBCs and a follow-up patient history and physical exam will help the provider assess whether the anemia is resolving. Individualized plans that target the specific type of anemia identified, as well as its underlying cause, are key to successful treatment.
CONCLUSION
When managing a patient with anemia, providers must define the type of anemia present and identify its underlying cause before starting treatment. Clues from the patient’s history, physical exam, and CBC can help isolate the cause of anemia. The MCV is the most helpful of the red blood cell indices because it allows the provider to classify the anemia as microcytic, macrocytic, or normocytic.
In cases in which the anemia is acute or severe—or in which the patient remains anemic even after being treated by the primary care provider—referral to a specialist is appropriate.
1. Brill JR, Baumgardner D. Normocytic anemia. Am Fam Physician. 2000;62(10):2255-2263.
2. Van Vranken M. Evaluation of microcytosis. Am Fam Physician. 2010;82(9):1117-1122.
3. National Institutes of Health/National Heart, Lung, and Blood Institute. How is anemia diagnosed? www.nhlbi.nih.gov/health/health-topics/topics/anemia/diagnosis. Accessed April 28, 2017.
4. Smith RE Jr. The clinical and economic burden of anemia. Am J Manag Care. 2010;16(3):S59-S66.
5. Platt A, Eckman J. Diagnosing anemia. Clinician Reviews. 2006;16(2):44-50.
6. Karnath B. Anemia in the adult patient. Hosp Physician. 2004;40(10):32-36.
7. World Health Organization. Micronutrient deficiencies. www.who.int/nutrition/topics/ida/en/#. Accessed April 29, 2017.
8. US Department of Health and Human Services, Office of Women’s Health. Iron-deficiency anemia. www.womenshealth.gov/publications/our-publications/fact-sheet/anemia.html#a. Accessed April 29, 2017.
9. DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(14): 1324-1331.
10. Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923-936.
11. Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015; 372(19):1832-1843.
12. Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75(5):671-678.
13. Rodgers GP, Young N. The Bethesda Handbook of Clinical Hematology. 3rd ed. Baltimore: Lippincott Williams and Wilkins; 2013:1-21.
14. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
15. Hesdorffer CS, Longo DL. Drug-induced megaloblastic anemia. N Engl J Med. 2015;373(17):1649-1658.
16. Vasconcelos OM, Poehm EH, McCarter RJ, et al. Potential outcome factors in subacute combined degeneration. J Gen Intern Med. 2006;21(10):1063-1068.
17. Vide AT, Marques AM, Costa JD. MRI findings in subacute combined degeneration of the spinal cord in a patient with restricted diet. Neurol Sci. 2011;33(3):711-713.
18. Kessenich CR, Cronin K. Fecal occult blood testing in older adult patients with anemia. Nurse Pract. 2013;38(1):6-8.
19. Imashuku S, Kudo N, Kaneda S. Spontaneous resolution of macrocytic anemia: old disease revisited. J Blood Med. 2012;3:45-47.
20. Pagana K, Pagana T, Pagana T. Mosby’s Diagnostic and Laboratory Test Reference. 12th ed. St. Louis, MO: Elsevier Mosby; 2015: 497-501, 785-791, 805-806.
1. Brill JR, Baumgardner D. Normocytic anemia. Am Fam Physician. 2000;62(10):2255-2263.
2. Van Vranken M. Evaluation of microcytosis. Am Fam Physician. 2010;82(9):1117-1122.
3. National Institutes of Health/National Heart, Lung, and Blood Institute. How is anemia diagnosed? www.nhlbi.nih.gov/health/health-topics/topics/anemia/diagnosis. Accessed April 28, 2017.
4. Smith RE Jr. The clinical and economic burden of anemia. Am J Manag Care. 2010;16(3):S59-S66.
5. Platt A, Eckman J. Diagnosing anemia. Clinician Reviews. 2006;16(2):44-50.
6. Karnath B. Anemia in the adult patient. Hosp Physician. 2004;40(10):32-36.
7. World Health Organization. Micronutrient deficiencies. www.who.int/nutrition/topics/ida/en/#. Accessed April 29, 2017.
8. US Department of Health and Human Services, Office of Women’s Health. Iron-deficiency anemia. www.womenshealth.gov/publications/our-publications/fact-sheet/anemia.html#a. Accessed April 29, 2017.
9. DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(14): 1324-1331.
10. Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923-936.
11. Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015; 372(19):1832-1843.
12. Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75(5):671-678.
13. Rodgers GP, Young N. The Bethesda Handbook of Clinical Hematology. 3rd ed. Baltimore: Lippincott Williams and Wilkins; 2013:1-21.
14. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
15. Hesdorffer CS, Longo DL. Drug-induced megaloblastic anemia. N Engl J Med. 2015;373(17):1649-1658.
16. Vasconcelos OM, Poehm EH, McCarter RJ, et al. Potential outcome factors in subacute combined degeneration. J Gen Intern Med. 2006;21(10):1063-1068.
17. Vide AT, Marques AM, Costa JD. MRI findings in subacute combined degeneration of the spinal cord in a patient with restricted diet. Neurol Sci. 2011;33(3):711-713.
18. Kessenich CR, Cronin K. Fecal occult blood testing in older adult patients with anemia. Nurse Pract. 2013;38(1):6-8.
19. Imashuku S, Kudo N, Kaneda S. Spontaneous resolution of macrocytic anemia: old disease revisited. J Blood Med. 2012;3:45-47.
20. Pagana K, Pagana T, Pagana T. Mosby’s Diagnostic and Laboratory Test Reference. 12th ed. St. Louis, MO: Elsevier Mosby; 2015: 497-501, 785-791, 805-806.