User login
2016 Update on pelvic floor dysfunction
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
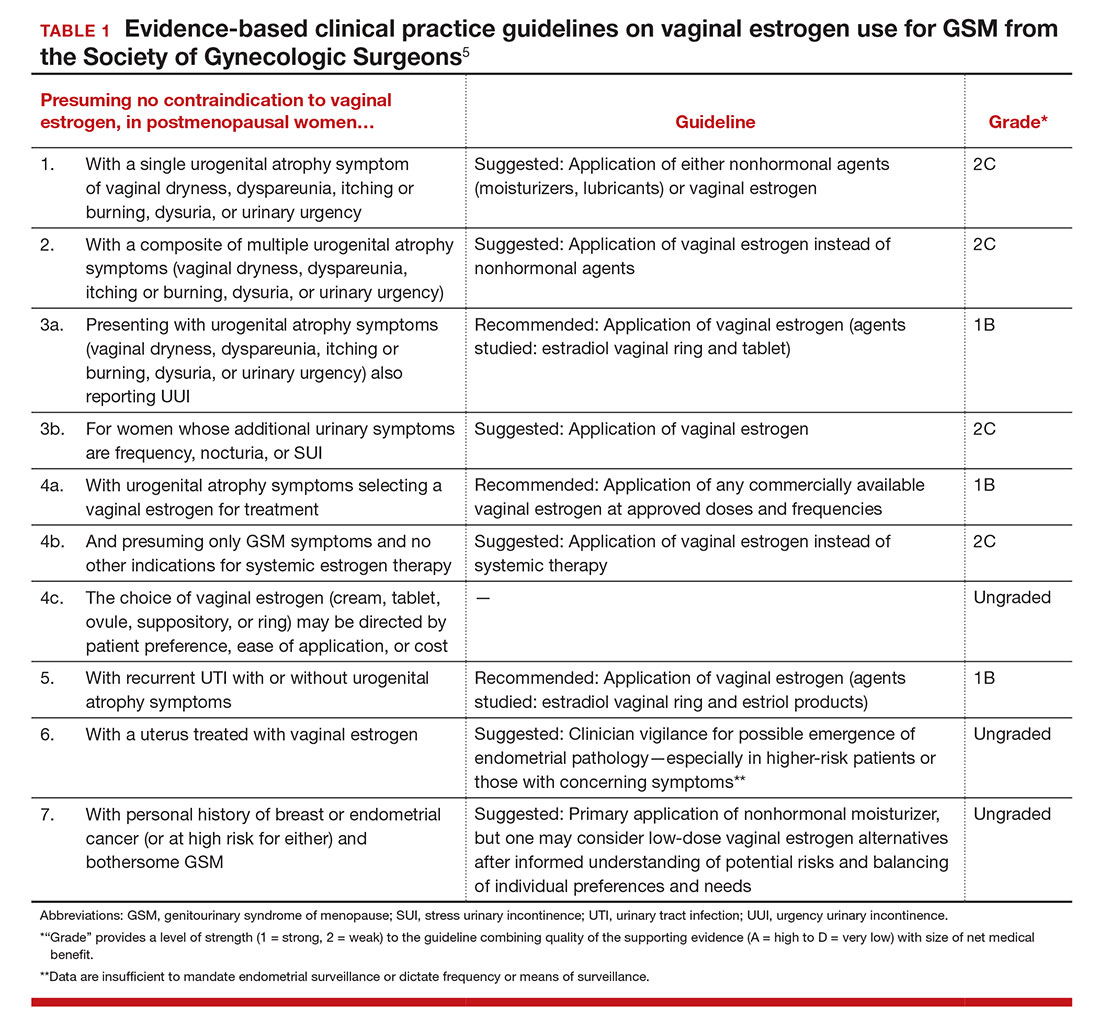
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6

Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6


Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6


Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
In this Article
- Low-dose estrogen vaginal cream
- Ospemifene therapy
- Fractional CO2 laser treatment


