User login
Guidance for the Clinical Management of Thirdhand Smoke Exposure in the Child Health Care Setting
From the Center for Child and Adolescent Health Research and Policy, Division of General Academic Pediatrics, Massachusetts General Hospital for Children, and the Tobacco Research and Treatment Center, Massachusetts General Hospital, Boston, MA.
Abstract
- Objective: To explain the concept of thirdhand smoke and how it can be used to protect the health of children and improve delivery of tobacco control interventions for parents in the child health care setting.
- Methods: Review of the literature and descriptive report.
- Results: The thirdhand smoke concept has been used in the CEASE intervention to improve the delivery of tobacco control counseling and services to parents. Materials and techniques have been developed for the child health care setting that use the concept of thirdhand smoke. Scientific findings demonstrate that thirdhand smoke exposure is harmful and establishes the need for clinicians to communicate the cessation imperative: the only way to protect non-smoking household members from thirdhand smoke is for all household smokers to quit smoking completely. As the scientific knowledge of thirdhand smoke increases, advocates will likely rely on it to encourage completely smoke-free places.
- Conclusion: Recent scientific studies on thirdhand smoke are impelling further research on the topic, spurring the creation of tobacco control policies to protect people from thridhand smoke and stimulating improvements to the delivery of tobacco control counseling and services to parents in child health care settings.
Key words: thirdhand smoke; smoking; tobacco; indoor air quality; smoking cessation; pediatrics.
While “thirdhand smoke” may be a relatively new term, it is rooted in an old concept—the particulate matter and residue from tobacco smoke left behind after tobacco is burned. In 1953, Dr. Ernest Wynder and his colleagues from the Washington University School of Medicine in St. Louis showed that condensate made from the residue of cigarette smoke causes cancer [1]. This residue left behind by burning cigarettes is now known as thirdhand smoke [2]. Dr. Wynder used acetone to rinse the leftover tobacco smoke residue from a smoking chamber where he had burned cigarettes. He then painted the solution of acetone and thirdhand smoke residue onto the backs of mice. The results of Dr. Wynder’s study demonstrated that exposed mice developed cancerous skin lesions, whereas mice exposed to the acetone alone did not display skin lesions. Dr. Wynder sounded an alarm bell in his manuscript when he wrote, “Such studies, in view of the corollary clinical data relating smoking to various types of cancer, appear urgent. They may result not only in furthering our knowledge of carcinogenesis, but in promoting some practical aspects of cancer prevention [1].”
Decades of research has been conducted since Dr. Wynder’s discovery to definitively conclude that smoking tobacco and exposure to secondhand tobacco smoke is harmful to human health. It is estimated that 480,000 annual premature deaths in the United States alone are attributable to smoking and exposure to secondhand smoke [3]. The World Health Organization estimates that worldwide tobacco use is responsible for more than 7 million deaths per year, with 890,000 of those deaths caused by secondhand smoke exposure of nonsmokers [4]. Epidemiological evidence of the harm posed by tobacco has spurred the U.S Surgeon General to conclude that there is no risk-free level of exposure to tobacco smoke [5]. Despite the overwhelming evidence implicating tobacco as the cause of an unprecedented amount of disease resulting from the use of a consumer product, only recently has a dedicated research agenda been pursued to study what Dr. Wynder urgently called for back in 1953: further exploration of the health effects of thirdhand tobacco smoke.
The term "thirdhand smoke" was first coined in 2006 by researchers with the Clinical Effort Against Secondhand Smoke Exposure (CEASE) program at Massachusetts General Hospital in Boston [6], and recent research has begun to shed considerable light on the topic. In 2011, a research consortium of scientists funded by the Tobacco-Related Disease Research Program [7] in California was set up to conduct pioneering research on the characterization, exposure and health effects of thirdhand tobacco smoke [8]. Research findings from this consortium and other scientists from around the world are quickly expanding and disseminating knowledge on this important topic.
While the research on thirdhand smoke is ongoing, this paper summarizes the current literature most relevant to the pediatric population and outlines clinical and policy recommendations to protect children and families from the harms of exposure to thirdhand smoke.
What Is Thirdhand Smoke and How Is It Different from Secondhand Smoke?
Thirdhand smoke is a result of combusted tobacco, most often from smoking cigarettes, pipes, cigars, or cigarillos. Thirdhand smoke remains on surfaces and in dust for a longtime after smoking happens, reacts with oxidants and other compounds to form secondary pollutants, and is re-emitted as a gas and/or resuspended when particles are disturbed and go back into the air where they can be inhaled [9]. One dramatic example of how thirdhand smoke can remain on surfaces long after secondhand smoke dissipates was discovered on the ornate constellation ceiling in the main concourse of the Grand Central Terminal in New York City. According to Sam Roberts, a correspondent for the New York Times and the author of a book about the historic train station, the dark residue that accumulated on the concourse ceiling over decades and was originally believed to be the result of soot from train engines was primarily residue from tobacco smoke [10–12]. It wasn’t until a restoration in the 1990s when workers scrubbed the tar and nicotine residue from the ceiling could the elaborate design of the zodiac signs and constellations be seen again [13]. A similar process takes place inside homes, where smoke residue accumulates on surfaces such as walls and ceilings after smoking happens. Owners of homes that have been previously smoked in are faced with unanswered questions about how to clean up the toxic substances left behind.
When tobacco is smoked, the particulates contained in secondhand smoke settle on surfaces; this contamination is absorbed deep into materials such as hair, clothes, carpeting, furniture, and wallboard [9,14]. After depositing onto surfaces, the chemicals undergo an aging process, which changes the chemical structure of the smoke pollutants. The nicotine in thirdhand smoke residue reacts with common indoor air pollutants, such as nitrous acid and ozone, to form hazardous substances. When the nicotine present in thirdhand smoke reacts with nitrous acid, it forms carcinogenic tobacco-specific nitrosamines such as NNK and NNN [15–17]. Nicotine also reacts with ozone to form additional harmful ultrafine particles that can embed deep within the lungs when inhaled [18]. As thirdhand smoke ages, it becomes more toxic [15]. The aged particles then undergo a process called “off-gassing,” in which gas is continuously re-emitted from these surfaces back into the air [19]. This process of off-gassing occurs long after cigarettes have been smoked indoors [19,20]. Thirdhand smoke particles can also be inhaled when they get resuspended into the air after contaminated surfaces are disturbed [21].
Common practices employed by smokers, like smoking in different rooms, using fans to diffuse the smoke, or opening windows, do not prevent the formation and inhalation of thirdhand smoke by people living or visiting these indoor spaces [22]. Environments with potential thirdhand smoke exposure include homes of smokers [23], apartments and homes previously occupied by smokers [24], multiunit housing where smoking is permitted [25], automobiles that have been smoked in [26], hotel rooms where smoking is permitted [27], and other indoor places where smoking has occurred.
Research Supports Having Completely Smoke-Free Environments
Recent research has shown that exposure to thirdhand smoke is harmful. These findings, many of which are described below, offer strong support in favor of advocating for environments free of thirdhand smoke contamination for families and children.
Genetic Damage from Thirdhand Smoke Exposure
In 2013, researchers from the Lawrence Berkeley National Laboratory were the first to demonstrate that thirdhand smoke causes significant genetic damage to human cells [28]. Using in vitro assays, the researchers showed that thirdhand smoke is a cause of harm to human DNA in the form of strand breaks and oxidative damage, which leads to mutations that can cause cancer. The researches also specifically tested the effect of NNA, a tobacco-specific nitrosamine that is commonly found in thirdhand smoke but not in secondhand smoke, on human cell cultures and found that it caused significant damage to DNA [28].
Children Show Elevated Biomarkers of Thirdhand Smoke Exposure in Their Urine and Hair Samples
In 2004, Matt and colleagues described how they collected household dust samples from living rooms and infants’ bedrooms [23]. Their research demonstrated that nicotine accumulated on the living room and infants’ bedroom surfaces of the homes belonging to smokers. Significantly higher amounts of urine cotinine, a biomarker for exposure to nicotine, were detected among infants who lived in homes where smoking happens inside compared to homes where smokers go outside to smoke [23]. As well, a study published in 2017 that measured the presence of hand nicotine on children of smokers who presented to the emergency room for an illness possibly related to tobacco smoke exposure detected hand nicotine on the hands of each child who participated in this pilot study. The researchers found a positive correlation between the amount of nicotine found on children’s hands and the amount of cotinine, a biomarker for nicotine exposure, detected in the children’s saliva [29].
Children Are Exposed to Higher Ratios of Thirdhand Smoke than Adults
In 2009, researchers discovered that the thirdhand smoke ratio of tobacco-specific nitrosamines to nicotine increases during the aging process [9]. Biomarkers measured in the urine can now be used to estimate the degree to which people have been exposed to secondhand or thirdhand smoke based on the ratio of the thirdhand smoke biomarker NNK and nicotine. Toddlers who live with adults who smoke have higher NNK/nicotine ratios, suggesting that they are exposed to a higher ratio of thirdhand smoke compared to secondhand smoke than adults [30]. Young children are likely exposed to higher ratios of thirdhand smoke as they spend more time on the floor, where thirdhand smoke accumulates. They frequently put their hands and other objects into their mouths. Young children breathe faster than adults, increasing their inhalation exposure and also have thinner skin, making dermal absorption more efficient [9].
Modeling Excess Cancer Risk
A 2014 United Kingdom study used official sources of toxicological data about chemicals detected in thirdhand smoke–contaminated homes to assess excess cancer risk posed from thirdhand smoke [17]. Using dust samples collected from homes where a smoker lived, they estimate that the median lifetime excess cancer risk from the exposure to all the nitrosamines present in thirdhand smoke is 9.6 additional cancer cases per 100,000 children exposed and could be as high as 1 excess cancer case per 1000 children exposed. The researchers concluded that young children aged 1 to 6 are at an especially increased risk for cancer because of their frequent contact with surfaces contaminated with thirdhand smoke and their ingestion of the particulate matter that settles on surfaces after smoking takes place [17].
Infants in Health Care Facilities Are Exposed to Thirdhand Smoke
Researchers have observed biomarkers confirming thirdhand smoke exposure in the urine of infants in the NICU. Found in incubators and cribs, particulates are likely being deposited in the NICU from visitors who have thirdhand smoke on their clothing, skin, and hair [31].
Animal Studies Link Thirdhand Smoke Exposure to Common Human Disease
Mice exposed to thirdhand smoke under conditions meant to simulate levels similar to human exposure are pre-diabetic, are at higher risk of developing metabolic syndrome, have inflammatory markers in the lungs that increase the risk for asthma, show slow wound healing, develop nonalcoholic fatty liver disease, and become behaviorally hyperactive [32]. Another recent study published in 2017 showed that mice exposed to thirdhand smoke after birth weighed less than mice not exposed to thirdhand smoke. Additionally, mice exposed to thirdhand smoke early in life showed changes in white blood cell counts that persisted into adulthood [9,33].
Summary
In summary, recent research makes a compelling case for invoking the precautionary principle to ensure that children avoid exposures to thirdhand smoke in their homes, cars, and healthcare settings. Studies reveal that:
- children live in homes where thirdhand smoke is present and this exposure is detectable in their bodies [23]
- concentrations of thirdhand smoke exposure observed in children are disproportionately higher than adults [30]
- chemicals present in thirdhand smoke cause damage to DNA [28]
- thirdhand smoke contains carcinogens that put exposed children at increased risk of cancer [17]
- thirdhand smoke is being detected within medical settings [34] and in the bodies of medically-vulnerable children [29], and
- animal studies have linked exposure to thirdhand smoke to a number of adverse health conditions commonly seen in today’s pediatric population such as metabolic syndrome, prediabetes, asthma, hyperactivity [32] and low birth weight [33].
Using the Thirdhand Smoke Concept in Clinical Practice
The clinical setting is an ideal place to address thirdhand smoke with families as a component of a comprehensive tobacco control strategy.
The Cessation Imperative—A Novel Motivational Message Prompted by Thirdhand Smoke
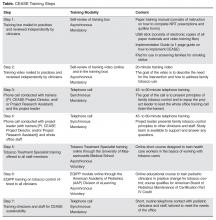
While there are potentially many ways to address thirdhand smoke exposure with families, the CEASE program has been used in the primary care setting to train child health care clinicians and office staff to address second- and thirdhand smoke. The training also educates clinicians on providing cessation counseling and resources to families with the goal of helping all family members become tobacco free, as well as to helping families keep completely smoke-free homes and cars [35,36]. The concept of thirdhand smoke creates what we have coined the cessation imperative [36]. The cessation imperative is based on the notion that the only way to protect non-smoking family and household members from thirdhand smoke is for all household smokers to quit smoking completely. Smoking, even when not in the presence of children, can expose others to toxic contaminates that settle on the surfaces of the home, the car as well as to the skin, hair, and clothing of family members who smoke. A discussion with parents about eliminating only secondhand smoke exposure for children does not adequately address how continued smoking, even when children are not present, can be harmful. The thirdhand smoke concept can be presented early, making it an efficient way to advocate for completely smoke-free families.
Thirdhand Smoke Counseling Helps Clinicians Achieve Key Tobacco Control Goals
The American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP) recommend that health care providers deliver advice to parents regarding establishing smoke-free homes and cars and provide information about how their smoking adversely affects their children’s health [37,38]. It is AAP and AAFP policy that health care providers provide tobacco dependence treatment and referral to cessation services to help adult family members quit smoking [38,39]. Successfully integrating counseling around the topic of thirdhand smoke into existing smoking cessation service delivery is possible. The CEASE research and implementation team developed and disseminated educational content to clinicians about thirdhand smoke through AAP courses delivered online [40] as well as made presentations to clinicians at AAP-sponsored training sessions. Thirdhand smoke messaging has been included in the CEASE practice trainings so that participating clinicians in pediatric offices are equipped to engage parents on this topic. Further information about these educational resources and opportunities can be obtained from the AAP Julius B. Richmond Center of Excellence website [41] and from the Massachusetts General Hospital CEASE program’s website [42].
Counseling parents about thirdhand smoke can help assist parents with their smoking in the critical context of their child’s care. Most parents see their child’s health care clinician more often than their own [43]. Increasing the number of pediatric clinical encounters where parental smoking is addressed while also increasing the effectiveness of these clinical encounters by increasing parents’ motivation to protect their children from tobacco smoke exposure are important goals. The topic of thirdhand smoke is a novel concept that clinicians can use to engage with parents around their smoking in a new way. Recent research conducted by the CEASE team suggests that counseling parents in the pediatric setting about thirdhand smoke can be useful in helping achieve tobacco control goals with families. Parent’s belief about thirdhand smoke is associated with the likelihood the parent will take concrete steps to protect their child. Parents who believe thirdhand smoke is harmful are more likely to protect their children from exposure by adopting strictly enforced smoke-free home and car rules [44]. Parents who changed their thirdhand smoke beliefs over the course of a year to believing that thirdhand smoke is harmful were more likely to try to quit smoking [44].
Child health care clinicians are effective at influencing parents’ beliefs about the potential harm thirdhand smoke poses to their children. Parents who received advice from pediatricians to quit smoking or to adopt smoke-free home or policies were more likely to believe that thirdhand smoke was harmful to the health of children [45]. Fathers (as compared with mothers) and parents who smoked more cigarettes each day were less likely to accept that thirdhand smoke is harmful to children [45]. Conversely, delivering effective educational messages and counseling around the topic of thirdhand smoke to parents may help promote smoke-free rules and acceptance of cessation assistance.
Protect Patients from Thirdhand Smoke Risks
All health care settings should be completely smoke-free. Smoking bans help protect all families and children from second and thirdhand smoke exposure. It is especially important for medically vulnerable children to visit facilities free from all forms of tobacco smoke contamination. CEASE trainings encourage practices to implement a zone of wellness on the grounds of the healthcare facility by completely banning smoking. The CEASE implementation team also trains practice leaders to reach out to all staff that use tobacco and offer resources and support for quitting. Having a non-smoking staff sets a great example for families who visit the healthcare facility, and reduces the likelihood of bringing thirdhand smoke contaminates into the facility. Creating a policy that addresses thirdhand smoke exposure is a concrete step that health care organizations can take to protect patients.
Thirdhand Smoke Resources Developed and/or Used by the CEASE Program
The CEASE program has developed and/or identified a number of clinical resources to educate parents and clinicians about thirdhand smoke. These free resources can enhance awareness of thirdhand smoke and help promote the use of the thirdhand smoke concept in clinical practice.
- Posters with messages designed to educate parents about thirdhand smoke to encourage receipt of cessation resources were created for use in waiting areas and exam rooms of child health care practices. A poster for clinical practice (Figure 1) can be downloaded and printed from the CEASE program website [42].
- Health education handouts that directly address thirdhand smoke exposure are available. The handouts can be taken home to family members who are not present at the visit and contain the telephone number for the tobacco quitline service, which connects smokers in the United States with free telephone support for smoking cessation. Handouts for clinical practice can be downloaded and printed from the CEASE program website. Figure 2 shows a handout that encourages parents to keep a smoke-free car by pointing out that tobacco smoke stays in the car long after the cigarette is out.
- Videos about thirdhand smoke can be viewed by parents while in child health care offices or shared on practice websites or social media platforms. The CEASE program encourages practices to distribute videos about thirdhand smoke to introduce parents to the concept of thirdhand smoke and to encourage parents to engage in a discussion with their child’s clinicians about ways to limit thirdhand smoke exposure. Suitable videos for parental viewing include the 2 listed below, which highlight information from the Thirdhand Smoke Research Consortium.
-University of California Riverside https://youtu.be/i1rhqRy-2e8
-San Diego State University https://youtu.be/rqzi-9sXLdU - Letters for landlords and management companies were created to stress the importance of providing a smoke-free living environment for children. The letters are meant to be signed by the child’s health care provider. The letters state that eliminating smoking in their buildings would result in landlords that “Pay less for cleaning and turnover fees.” Landlord letter templates can be downloaded and printed from the CEASE program website [42].
- Educational content for child health care clinicians about thirdhand smoke and how to counsel parents is included in the American Academy of Pediatrics Education in Quality Improvement for Pediatric Practice (EQIPP) online course entitled “Eliminating Tobacco” Use and Exposure to Secondhand Smoke. A section devoted to educating clinicians on the topic of thirdhand smoke is presented in this course. The course can be accessed through the AAP website and it qualifies for American Board of Pediatrics maintenance of certification part IV credit [40].
The CEASE team has worked with mass media outlets to communicate the messages about thirdhand smoke to build public awareness. The Today Show helped to popularize the concept of thirdhand smoke in 2009 after a paper published in the journal Pediatrics linked thirdhand smoke beliefs to home smoking bans [2].
Systems Approaches to Reduce Thirdhand Smoke Exposure
Public Policy Approaches
A clear policy agenda can help people protect their families from exposure to thirdhand smoke [46]. Policy approaches that have worked for lead, asbestos, and radon are examples of common household contaminants that are regulated using different mechanisms in an effort to protect the public health [46]. Strengths and weaknesses in each of these different approaches should be carefully considered when developing a comprehensive policy agenda to address thirdhand smoke. Recently, research on the health effects of thirdhand smoke spurred the passage of California legislative bill AB 1819 that “prohibits smoking tobacco at all times in the homes of licensed family child care homes and in areas where children are present [47].” As well, a recent US Department of Housing and Urban Development rule was finalized that requires all public housing agencies to implement a smoke-free policy by 30 July 2018 [48]. Smoke-free housing protects occupants from both secondhand and thirdhand smoke exposure. Pediatricians and other child health care professionals are well positioned to advocate for legislative actions that protect children from harmful exposures to thirdhand smoke.
Practice Change in Child Health Care Settings
Designing health care systems to screen for tobacco smoke exposure and to provide evidence-based cessation resources for all smokers is one of the best ways to reduce exposures to thirdhand smoke. Preventing thirdhand smoke exposure can work as novel messaging to promote tobacco cessation programs. Developing electronic medical record systems that allow for documentation of the smoking status of household members and whether or not homes and cars are completely smokefree can be particularly helpful tools for child health care providers when addressing thirdhand smoke with families. Good documentation about smoke-free homes and cars can enhance follow-up discussions with families as they work towards reducing thirdhand smoke exposures.
Summary
The thirdhand smoke concept has been used to improve delivery of tobacco control counseling and services for parents in the child health care context. Free materials are available that utilize thirdhand smoke messaging. As the science of thirdhand smoke matures, it will increasingly be used to help promote completely smoke-free places. The existing research on thirdhand smoke establishes the need for clinicians to communicate the cessation imperative. By using it, clinicians can help all smokers and non-smokers understand that there is no way to smoke tobacco without exposing friends and family.
Corresponding author: Jeremy E. Drehmer, MPH, 125 Nashua St., Suite 860, Boston, MA 02114, jdrehmer@ mgh.harvard.edu.
Financial disclosures: None
1. Wynder EL, Graham EA, Croninger AB, et al. Experimental production of carcinoma with cigarette tar experimental production of carcinoma with cigarette tar. 1953;36:855–64.
2. Winickoff JP, Friebely J, Tanski SE, et al. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics 2009;123:e74–9.
3. US Department of Health and Human Services. The health consequences of smoking- 50 years of progress: a report of the Surgeon General, Executive Summary. 2014.
4. World Health Organization. Tobacco fact sheet [Internet]. [cited 2017 Aug 15]. Available at www.who.int/mediacentre/factsheets/fs339/en/.
5. U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta (GA); 2006.
6. Winickoff J, Friebely J, Tanski S, et al. Beliefs about the health effects of third-hand smoke predict home and car smoking bans. In: Poster presented at the 2006 Pediatric Academic Societies Meeting. San Francisco, CA; 2006.
7. Tobacco-Related Disease Research Program [Internet]. Accessed 2017 Jul 7 at www.trdrp.org.
8. Matt GE, Quintana PJ, Destaillats H, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect 2011;119:1218–26.
9. Jacob P, Benowitz NL, Destaillats H, et al. Thirdhand smoke: new evidence, challenges, and future directions. Chem Res Toxicol 2017;30:270–94.
10. Roberts S, Hamill P. Grand Central: how a train station transformed America. Grand Central Publishing; 2013.
11. Sachs S. From gritty depot, a glittery destination; refurbished Grand Central terminal, worthy of its name, is reopened. New York Times 1998 Oct 2.
12. Grand Central: an engine of scientific innovation [Internet]. National Public Radio - Talk of the Nation; 2013. Available at www.npr.org/templates/transcript/transcript.php?storyId=175054273.
13. Lueck TJ. Work starts 100 feet above Grand Central commuters. New York Times 1996 Sep 20.
14. Van Loy MD, Nazaroff WW, Daisey JM. Nicotine as a marker for environmental tobacco smoke: implications of sorption on indoor surface materials. J Air Waste Manag Assoc 1998;48:959–68.
15. Sleiman M, Gundel LA, Pankow JF, et al. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc Natl Acad Sci U S A 2010;107:6576–81.
16. Xue J, Yang S, Seng S. Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers (Basel) 2014;6:1138–56.
17. Ramirez N, Ozel MZ, Lewis AC, et al. Exposure to nitrosamines in thirdhand tobacco smoke increases cancer risk in non-smokers. Environ Int 2014;71:139–47.
18. Destaillats H, Singer BC, Lee SK, Gundel LA. Effect of ozone on nicotine desorption from model surfaces: evidence for heterogeneous chemistry. Environ Sci Technol 2006;40:1799–805.
19. Singer BC, Hodgson AT, Guevarra KS, et al. Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Env Sci Technol 2002;36:846–53.
20. Singer BC, Hodgson AT, Nazaroff WW. Gas-phase organics in environmental tobacco smoke: 2. Exposure-relevant emission factors and indirect exposures from habitual smoking. Atmos Environ 2003;37:5551–61.
21. Becquemin MH, Bertholon JF, Bentayeb M, et al. Third-hand smoking: indoor measurements of concentration and sizes of cigarette smoke particles after resuspension. Tob Control 2010;19:347–8.
22. Centers for Disease Control and Prevention [Internet]. How can we protect our children from secondhand smoke: a parent’s guide. Accessed 2017 Aug 15 at www.cdc.gov/tobacco/basic_information/secondhand_smoke/protect_children/pdfs/protect_children_guide.pdf.
23. Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control 2004;13:29–37.
24. Matt GE, Quintana PJE, Zakarian JM, et al. When smokers move out and non-smokers move in: residential thirdhand smoke pollution and exposure. Tob Control 2011;20:e1.
25. Kraev TA, Adamkiewicz G, Hammond SK, Spengler JD. Indoor concentrations of nicotine in low-income, multi-unit housing: associations with smoking behaviours and housing characteristics. Tob Control 2009;18:438–44.
26. Matt GE, Quintana PJE, Hovell MF, et al. Residual tobacco smoke pollution in used cars for sale: air, dust, and surfaces. Nicotine Tob Res 2008;10:1467–75.
27. Matt GE, Quintana PJE, Fortmann AL, et al. Thirdhand smoke and exposure in California hotels: non-smoking rooms fail to protect non-smoking hotel guests from tobacco smoke exposure. Tob Control 2014;23:264–72.
28. Hang B, Sarker AH, Havel C, et al. Thirdhand smoke causes DNA damage in human cells. Mutagenesis 2013;28:381–91.
29. Mahabee-Gittens EM, Merianos AL, Matt GE. Preliminary evidence that high levels of nicotine on children’s hands may contribute to overall tobacco smoke exposure. Tob Control 2017 Mar 30.
30. Hovell MF, Zakarian JM, Matt GE, et al. Counseling to reduce children’s secondhand smoke exposure and help parents quit smoking: a controlled trial. Nicotine Tob Res 2009;11:1383–94.
31. Northrup TF, Khan AM, Jacob 3rd P, et al. Thirdhand smoke contamination in hospital settings: assessing exposure risk for vulnerable paediatric patients. Tob Control 2016; 25: 619–23.
32. Martins-Green M, Adhami N, Frankos M, et al. Cigarette smoke toxins deposited on surfaces: Implications for human health. PLoS One 2014;9:1–12.
33. Hang B, Snijders AM, Huang Y, et al. Early exposure to thirdhand cigarette smoke affects body mass and the development of immunity in mice. Sci Rep 2017;7:41915.
34. Northrup TF, Matt GE, Hovell MF, et al. Thirdhand smoke in the homes of medically fragile children: Assessing the impact of indoor smoking levels and smoking bans. Nicotine Tob Res 2016;18:1290–8.
35. Marbin JN, Purdy CN, Klaas K, et al. The Clinical Effort against Secondhand Smoke Exposure (CEASE) California: implementing a pediatric clinical intervention to reduce secondhand smoke exposure. Clin Pediatr (Phila) 2016;1(3).
36. Winickoff JP, Hipple B, Drehmer J, et al. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention: A decade of lessons learned. J Clin Outcomes Manag 2012;19:414–9.
37. Farber HJ, Groner J, Walley S, Nelson K. Protecting children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:e1439–67.
38. American Academy of Family Physicians [Internet]. AAFP policies. Tobacco use, prevention, and cessation. Accessed 2017 Aug 29 at www.aafp.org/about/policies/all/tobacco-smoking.html.
39. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:1008–17.
40. Drehmer J, Hipple B, Murphy S, Winickoff JP. EQIPP: Eliminating tobacco use and exposure to secondhand smoke [online course] PediaLink [Internet]. American Academy of Pediatrics. 2014. Available at bit.ly/eliminate-tobacco-responsive.
41. The American Academy of Pediatrics Julius B. Richmond Center of Excellence [Internet]. Accessed 2017 Aug 9 at www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Richmond-Center/Pages/default.aspx.
42. Clinical Effort Against Secondhand Smoke Exposure [Internet]. Accessed at www.massgeneral.org/ceasetobacco/.
43. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132:109–17.
44. Drehmer JE, Ossip DJ, Nabi-Burza E, et al. Thirdhand smoke beliefs of parents. Pediatrics 2014;133:e850–6.
45. Drehmer JE, Ossip DJ, Rigotti NA, et al. Pediatrician interventions and thirdhand smoke beliefs of parents. Am J Prev Med 2012;43:533–6.
46. Samet JM, Chanson D, Wipfli H. The challenges of limiting exposure to THS in vulnerable populations. Curr Environ Health Rep 2015;2:215–25.
47. Thirdhand Smoke Research Consortium [Internet]. Accessed 2017 Aug 15 at www.trdrp.org/highlights-news-events/thirdhand-smoke-consortium.html.
48. Office of the Federal Register (US) [Internet]. Rule instituting smoke-free public housing. 2016. Available at www.federalregister.gov/documents/2016/12/05/2016-28986/instituting-smoke-free-public-housing.
From the Center for Child and Adolescent Health Research and Policy, Division of General Academic Pediatrics, Massachusetts General Hospital for Children, and the Tobacco Research and Treatment Center, Massachusetts General Hospital, Boston, MA.
Abstract
- Objective: To explain the concept of thirdhand smoke and how it can be used to protect the health of children and improve delivery of tobacco control interventions for parents in the child health care setting.
- Methods: Review of the literature and descriptive report.
- Results: The thirdhand smoke concept has been used in the CEASE intervention to improve the delivery of tobacco control counseling and services to parents. Materials and techniques have been developed for the child health care setting that use the concept of thirdhand smoke. Scientific findings demonstrate that thirdhand smoke exposure is harmful and establishes the need for clinicians to communicate the cessation imperative: the only way to protect non-smoking household members from thirdhand smoke is for all household smokers to quit smoking completely. As the scientific knowledge of thirdhand smoke increases, advocates will likely rely on it to encourage completely smoke-free places.
- Conclusion: Recent scientific studies on thirdhand smoke are impelling further research on the topic, spurring the creation of tobacco control policies to protect people from thridhand smoke and stimulating improvements to the delivery of tobacco control counseling and services to parents in child health care settings.
Key words: thirdhand smoke; smoking; tobacco; indoor air quality; smoking cessation; pediatrics.
While “thirdhand smoke” may be a relatively new term, it is rooted in an old concept—the particulate matter and residue from tobacco smoke left behind after tobacco is burned. In 1953, Dr. Ernest Wynder and his colleagues from the Washington University School of Medicine in St. Louis showed that condensate made from the residue of cigarette smoke causes cancer [1]. This residue left behind by burning cigarettes is now known as thirdhand smoke [2]. Dr. Wynder used acetone to rinse the leftover tobacco smoke residue from a smoking chamber where he had burned cigarettes. He then painted the solution of acetone and thirdhand smoke residue onto the backs of mice. The results of Dr. Wynder’s study demonstrated that exposed mice developed cancerous skin lesions, whereas mice exposed to the acetone alone did not display skin lesions. Dr. Wynder sounded an alarm bell in his manuscript when he wrote, “Such studies, in view of the corollary clinical data relating smoking to various types of cancer, appear urgent. They may result not only in furthering our knowledge of carcinogenesis, but in promoting some practical aspects of cancer prevention [1].”
Decades of research has been conducted since Dr. Wynder’s discovery to definitively conclude that smoking tobacco and exposure to secondhand tobacco smoke is harmful to human health. It is estimated that 480,000 annual premature deaths in the United States alone are attributable to smoking and exposure to secondhand smoke [3]. The World Health Organization estimates that worldwide tobacco use is responsible for more than 7 million deaths per year, with 890,000 of those deaths caused by secondhand smoke exposure of nonsmokers [4]. Epidemiological evidence of the harm posed by tobacco has spurred the U.S Surgeon General to conclude that there is no risk-free level of exposure to tobacco smoke [5]. Despite the overwhelming evidence implicating tobacco as the cause of an unprecedented amount of disease resulting from the use of a consumer product, only recently has a dedicated research agenda been pursued to study what Dr. Wynder urgently called for back in 1953: further exploration of the health effects of thirdhand tobacco smoke.
The term "thirdhand smoke" was first coined in 2006 by researchers with the Clinical Effort Against Secondhand Smoke Exposure (CEASE) program at Massachusetts General Hospital in Boston [6], and recent research has begun to shed considerable light on the topic. In 2011, a research consortium of scientists funded by the Tobacco-Related Disease Research Program [7] in California was set up to conduct pioneering research on the characterization, exposure and health effects of thirdhand tobacco smoke [8]. Research findings from this consortium and other scientists from around the world are quickly expanding and disseminating knowledge on this important topic.
While the research on thirdhand smoke is ongoing, this paper summarizes the current literature most relevant to the pediatric population and outlines clinical and policy recommendations to protect children and families from the harms of exposure to thirdhand smoke.
What Is Thirdhand Smoke and How Is It Different from Secondhand Smoke?
Thirdhand smoke is a result of combusted tobacco, most often from smoking cigarettes, pipes, cigars, or cigarillos. Thirdhand smoke remains on surfaces and in dust for a longtime after smoking happens, reacts with oxidants and other compounds to form secondary pollutants, and is re-emitted as a gas and/or resuspended when particles are disturbed and go back into the air where they can be inhaled [9]. One dramatic example of how thirdhand smoke can remain on surfaces long after secondhand smoke dissipates was discovered on the ornate constellation ceiling in the main concourse of the Grand Central Terminal in New York City. According to Sam Roberts, a correspondent for the New York Times and the author of a book about the historic train station, the dark residue that accumulated on the concourse ceiling over decades and was originally believed to be the result of soot from train engines was primarily residue from tobacco smoke [10–12]. It wasn’t until a restoration in the 1990s when workers scrubbed the tar and nicotine residue from the ceiling could the elaborate design of the zodiac signs and constellations be seen again [13]. A similar process takes place inside homes, where smoke residue accumulates on surfaces such as walls and ceilings after smoking happens. Owners of homes that have been previously smoked in are faced with unanswered questions about how to clean up the toxic substances left behind.
When tobacco is smoked, the particulates contained in secondhand smoke settle on surfaces; this contamination is absorbed deep into materials such as hair, clothes, carpeting, furniture, and wallboard [9,14]. After depositing onto surfaces, the chemicals undergo an aging process, which changes the chemical structure of the smoke pollutants. The nicotine in thirdhand smoke residue reacts with common indoor air pollutants, such as nitrous acid and ozone, to form hazardous substances. When the nicotine present in thirdhand smoke reacts with nitrous acid, it forms carcinogenic tobacco-specific nitrosamines such as NNK and NNN [15–17]. Nicotine also reacts with ozone to form additional harmful ultrafine particles that can embed deep within the lungs when inhaled [18]. As thirdhand smoke ages, it becomes more toxic [15]. The aged particles then undergo a process called “off-gassing,” in which gas is continuously re-emitted from these surfaces back into the air [19]. This process of off-gassing occurs long after cigarettes have been smoked indoors [19,20]. Thirdhand smoke particles can also be inhaled when they get resuspended into the air after contaminated surfaces are disturbed [21].
Common practices employed by smokers, like smoking in different rooms, using fans to diffuse the smoke, or opening windows, do not prevent the formation and inhalation of thirdhand smoke by people living or visiting these indoor spaces [22]. Environments with potential thirdhand smoke exposure include homes of smokers [23], apartments and homes previously occupied by smokers [24], multiunit housing where smoking is permitted [25], automobiles that have been smoked in [26], hotel rooms where smoking is permitted [27], and other indoor places where smoking has occurred.
Research Supports Having Completely Smoke-Free Environments
Recent research has shown that exposure to thirdhand smoke is harmful. These findings, many of which are described below, offer strong support in favor of advocating for environments free of thirdhand smoke contamination for families and children.
Genetic Damage from Thirdhand Smoke Exposure
In 2013, researchers from the Lawrence Berkeley National Laboratory were the first to demonstrate that thirdhand smoke causes significant genetic damage to human cells [28]. Using in vitro assays, the researchers showed that thirdhand smoke is a cause of harm to human DNA in the form of strand breaks and oxidative damage, which leads to mutations that can cause cancer. The researches also specifically tested the effect of NNA, a tobacco-specific nitrosamine that is commonly found in thirdhand smoke but not in secondhand smoke, on human cell cultures and found that it caused significant damage to DNA [28].
Children Show Elevated Biomarkers of Thirdhand Smoke Exposure in Their Urine and Hair Samples
In 2004, Matt and colleagues described how they collected household dust samples from living rooms and infants’ bedrooms [23]. Their research demonstrated that nicotine accumulated on the living room and infants’ bedroom surfaces of the homes belonging to smokers. Significantly higher amounts of urine cotinine, a biomarker for exposure to nicotine, were detected among infants who lived in homes where smoking happens inside compared to homes where smokers go outside to smoke [23]. As well, a study published in 2017 that measured the presence of hand nicotine on children of smokers who presented to the emergency room for an illness possibly related to tobacco smoke exposure detected hand nicotine on the hands of each child who participated in this pilot study. The researchers found a positive correlation between the amount of nicotine found on children’s hands and the amount of cotinine, a biomarker for nicotine exposure, detected in the children’s saliva [29].
Children Are Exposed to Higher Ratios of Thirdhand Smoke than Adults
In 2009, researchers discovered that the thirdhand smoke ratio of tobacco-specific nitrosamines to nicotine increases during the aging process [9]. Biomarkers measured in the urine can now be used to estimate the degree to which people have been exposed to secondhand or thirdhand smoke based on the ratio of the thirdhand smoke biomarker NNK and nicotine. Toddlers who live with adults who smoke have higher NNK/nicotine ratios, suggesting that they are exposed to a higher ratio of thirdhand smoke compared to secondhand smoke than adults [30]. Young children are likely exposed to higher ratios of thirdhand smoke as they spend more time on the floor, where thirdhand smoke accumulates. They frequently put their hands and other objects into their mouths. Young children breathe faster than adults, increasing their inhalation exposure and also have thinner skin, making dermal absorption more efficient [9].
Modeling Excess Cancer Risk
A 2014 United Kingdom study used official sources of toxicological data about chemicals detected in thirdhand smoke–contaminated homes to assess excess cancer risk posed from thirdhand smoke [17]. Using dust samples collected from homes where a smoker lived, they estimate that the median lifetime excess cancer risk from the exposure to all the nitrosamines present in thirdhand smoke is 9.6 additional cancer cases per 100,000 children exposed and could be as high as 1 excess cancer case per 1000 children exposed. The researchers concluded that young children aged 1 to 6 are at an especially increased risk for cancer because of their frequent contact with surfaces contaminated with thirdhand smoke and their ingestion of the particulate matter that settles on surfaces after smoking takes place [17].
Infants in Health Care Facilities Are Exposed to Thirdhand Smoke
Researchers have observed biomarkers confirming thirdhand smoke exposure in the urine of infants in the NICU. Found in incubators and cribs, particulates are likely being deposited in the NICU from visitors who have thirdhand smoke on their clothing, skin, and hair [31].
Animal Studies Link Thirdhand Smoke Exposure to Common Human Disease
Mice exposed to thirdhand smoke under conditions meant to simulate levels similar to human exposure are pre-diabetic, are at higher risk of developing metabolic syndrome, have inflammatory markers in the lungs that increase the risk for asthma, show slow wound healing, develop nonalcoholic fatty liver disease, and become behaviorally hyperactive [32]. Another recent study published in 2017 showed that mice exposed to thirdhand smoke after birth weighed less than mice not exposed to thirdhand smoke. Additionally, mice exposed to thirdhand smoke early in life showed changes in white blood cell counts that persisted into adulthood [9,33].
Summary
In summary, recent research makes a compelling case for invoking the precautionary principle to ensure that children avoid exposures to thirdhand smoke in their homes, cars, and healthcare settings. Studies reveal that:
- children live in homes where thirdhand smoke is present and this exposure is detectable in their bodies [23]
- concentrations of thirdhand smoke exposure observed in children are disproportionately higher than adults [30]
- chemicals present in thirdhand smoke cause damage to DNA [28]
- thirdhand smoke contains carcinogens that put exposed children at increased risk of cancer [17]
- thirdhand smoke is being detected within medical settings [34] and in the bodies of medically-vulnerable children [29], and
- animal studies have linked exposure to thirdhand smoke to a number of adverse health conditions commonly seen in today’s pediatric population such as metabolic syndrome, prediabetes, asthma, hyperactivity [32] and low birth weight [33].
Using the Thirdhand Smoke Concept in Clinical Practice
The clinical setting is an ideal place to address thirdhand smoke with families as a component of a comprehensive tobacco control strategy.
The Cessation Imperative—A Novel Motivational Message Prompted by Thirdhand Smoke
While there are potentially many ways to address thirdhand smoke exposure with families, the CEASE program has been used in the primary care setting to train child health care clinicians and office staff to address second- and thirdhand smoke. The training also educates clinicians on providing cessation counseling and resources to families with the goal of helping all family members become tobacco free, as well as to helping families keep completely smoke-free homes and cars [35,36]. The concept of thirdhand smoke creates what we have coined the cessation imperative [36]. The cessation imperative is based on the notion that the only way to protect non-smoking family and household members from thirdhand smoke is for all household smokers to quit smoking completely. Smoking, even when not in the presence of children, can expose others to toxic contaminates that settle on the surfaces of the home, the car as well as to the skin, hair, and clothing of family members who smoke. A discussion with parents about eliminating only secondhand smoke exposure for children does not adequately address how continued smoking, even when children are not present, can be harmful. The thirdhand smoke concept can be presented early, making it an efficient way to advocate for completely smoke-free families.
Thirdhand Smoke Counseling Helps Clinicians Achieve Key Tobacco Control Goals
The American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP) recommend that health care providers deliver advice to parents regarding establishing smoke-free homes and cars and provide information about how their smoking adversely affects their children’s health [37,38]. It is AAP and AAFP policy that health care providers provide tobacco dependence treatment and referral to cessation services to help adult family members quit smoking [38,39]. Successfully integrating counseling around the topic of thirdhand smoke into existing smoking cessation service delivery is possible. The CEASE research and implementation team developed and disseminated educational content to clinicians about thirdhand smoke through AAP courses delivered online [40] as well as made presentations to clinicians at AAP-sponsored training sessions. Thirdhand smoke messaging has been included in the CEASE practice trainings so that participating clinicians in pediatric offices are equipped to engage parents on this topic. Further information about these educational resources and opportunities can be obtained from the AAP Julius B. Richmond Center of Excellence website [41] and from the Massachusetts General Hospital CEASE program’s website [42].
Counseling parents about thirdhand smoke can help assist parents with their smoking in the critical context of their child’s care. Most parents see their child’s health care clinician more often than their own [43]. Increasing the number of pediatric clinical encounters where parental smoking is addressed while also increasing the effectiveness of these clinical encounters by increasing parents’ motivation to protect their children from tobacco smoke exposure are important goals. The topic of thirdhand smoke is a novel concept that clinicians can use to engage with parents around their smoking in a new way. Recent research conducted by the CEASE team suggests that counseling parents in the pediatric setting about thirdhand smoke can be useful in helping achieve tobacco control goals with families. Parent’s belief about thirdhand smoke is associated with the likelihood the parent will take concrete steps to protect their child. Parents who believe thirdhand smoke is harmful are more likely to protect their children from exposure by adopting strictly enforced smoke-free home and car rules [44]. Parents who changed their thirdhand smoke beliefs over the course of a year to believing that thirdhand smoke is harmful were more likely to try to quit smoking [44].
Child health care clinicians are effective at influencing parents’ beliefs about the potential harm thirdhand smoke poses to their children. Parents who received advice from pediatricians to quit smoking or to adopt smoke-free home or policies were more likely to believe that thirdhand smoke was harmful to the health of children [45]. Fathers (as compared with mothers) and parents who smoked more cigarettes each day were less likely to accept that thirdhand smoke is harmful to children [45]. Conversely, delivering effective educational messages and counseling around the topic of thirdhand smoke to parents may help promote smoke-free rules and acceptance of cessation assistance.
Protect Patients from Thirdhand Smoke Risks
All health care settings should be completely smoke-free. Smoking bans help protect all families and children from second and thirdhand smoke exposure. It is especially important for medically vulnerable children to visit facilities free from all forms of tobacco smoke contamination. CEASE trainings encourage practices to implement a zone of wellness on the grounds of the healthcare facility by completely banning smoking. The CEASE implementation team also trains practice leaders to reach out to all staff that use tobacco and offer resources and support for quitting. Having a non-smoking staff sets a great example for families who visit the healthcare facility, and reduces the likelihood of bringing thirdhand smoke contaminates into the facility. Creating a policy that addresses thirdhand smoke exposure is a concrete step that health care organizations can take to protect patients.
Thirdhand Smoke Resources Developed and/or Used by the CEASE Program
The CEASE program has developed and/or identified a number of clinical resources to educate parents and clinicians about thirdhand smoke. These free resources can enhance awareness of thirdhand smoke and help promote the use of the thirdhand smoke concept in clinical practice.
- Posters with messages designed to educate parents about thirdhand smoke to encourage receipt of cessation resources were created for use in waiting areas and exam rooms of child health care practices. A poster for clinical practice (Figure 1) can be downloaded and printed from the CEASE program website [42].
- Health education handouts that directly address thirdhand smoke exposure are available. The handouts can be taken home to family members who are not present at the visit and contain the telephone number for the tobacco quitline service, which connects smokers in the United States with free telephone support for smoking cessation. Handouts for clinical practice can be downloaded and printed from the CEASE program website. Figure 2 shows a handout that encourages parents to keep a smoke-free car by pointing out that tobacco smoke stays in the car long after the cigarette is out.
- Videos about thirdhand smoke can be viewed by parents while in child health care offices or shared on practice websites or social media platforms. The CEASE program encourages practices to distribute videos about thirdhand smoke to introduce parents to the concept of thirdhand smoke and to encourage parents to engage in a discussion with their child’s clinicians about ways to limit thirdhand smoke exposure. Suitable videos for parental viewing include the 2 listed below, which highlight information from the Thirdhand Smoke Research Consortium.
-University of California Riverside https://youtu.be/i1rhqRy-2e8
-San Diego State University https://youtu.be/rqzi-9sXLdU - Letters for landlords and management companies were created to stress the importance of providing a smoke-free living environment for children. The letters are meant to be signed by the child’s health care provider. The letters state that eliminating smoking in their buildings would result in landlords that “Pay less for cleaning and turnover fees.” Landlord letter templates can be downloaded and printed from the CEASE program website [42].
- Educational content for child health care clinicians about thirdhand smoke and how to counsel parents is included in the American Academy of Pediatrics Education in Quality Improvement for Pediatric Practice (EQIPP) online course entitled “Eliminating Tobacco” Use and Exposure to Secondhand Smoke. A section devoted to educating clinicians on the topic of thirdhand smoke is presented in this course. The course can be accessed through the AAP website and it qualifies for American Board of Pediatrics maintenance of certification part IV credit [40].
The CEASE team has worked with mass media outlets to communicate the messages about thirdhand smoke to build public awareness. The Today Show helped to popularize the concept of thirdhand smoke in 2009 after a paper published in the journal Pediatrics linked thirdhand smoke beliefs to home smoking bans [2].
Systems Approaches to Reduce Thirdhand Smoke Exposure
Public Policy Approaches
A clear policy agenda can help people protect their families from exposure to thirdhand smoke [46]. Policy approaches that have worked for lead, asbestos, and radon are examples of common household contaminants that are regulated using different mechanisms in an effort to protect the public health [46]. Strengths and weaknesses in each of these different approaches should be carefully considered when developing a comprehensive policy agenda to address thirdhand smoke. Recently, research on the health effects of thirdhand smoke spurred the passage of California legislative bill AB 1819 that “prohibits smoking tobacco at all times in the homes of licensed family child care homes and in areas where children are present [47].” As well, a recent US Department of Housing and Urban Development rule was finalized that requires all public housing agencies to implement a smoke-free policy by 30 July 2018 [48]. Smoke-free housing protects occupants from both secondhand and thirdhand smoke exposure. Pediatricians and other child health care professionals are well positioned to advocate for legislative actions that protect children from harmful exposures to thirdhand smoke.
Practice Change in Child Health Care Settings
Designing health care systems to screen for tobacco smoke exposure and to provide evidence-based cessation resources for all smokers is one of the best ways to reduce exposures to thirdhand smoke. Preventing thirdhand smoke exposure can work as novel messaging to promote tobacco cessation programs. Developing electronic medical record systems that allow for documentation of the smoking status of household members and whether or not homes and cars are completely smokefree can be particularly helpful tools for child health care providers when addressing thirdhand smoke with families. Good documentation about smoke-free homes and cars can enhance follow-up discussions with families as they work towards reducing thirdhand smoke exposures.
Summary
The thirdhand smoke concept has been used to improve delivery of tobacco control counseling and services for parents in the child health care context. Free materials are available that utilize thirdhand smoke messaging. As the science of thirdhand smoke matures, it will increasingly be used to help promote completely smoke-free places. The existing research on thirdhand smoke establishes the need for clinicians to communicate the cessation imperative. By using it, clinicians can help all smokers and non-smokers understand that there is no way to smoke tobacco without exposing friends and family.
Corresponding author: Jeremy E. Drehmer, MPH, 125 Nashua St., Suite 860, Boston, MA 02114, jdrehmer@ mgh.harvard.edu.
Financial disclosures: None
From the Center for Child and Adolescent Health Research and Policy, Division of General Academic Pediatrics, Massachusetts General Hospital for Children, and the Tobacco Research and Treatment Center, Massachusetts General Hospital, Boston, MA.
Abstract
- Objective: To explain the concept of thirdhand smoke and how it can be used to protect the health of children and improve delivery of tobacco control interventions for parents in the child health care setting.
- Methods: Review of the literature and descriptive report.
- Results: The thirdhand smoke concept has been used in the CEASE intervention to improve the delivery of tobacco control counseling and services to parents. Materials and techniques have been developed for the child health care setting that use the concept of thirdhand smoke. Scientific findings demonstrate that thirdhand smoke exposure is harmful and establishes the need for clinicians to communicate the cessation imperative: the only way to protect non-smoking household members from thirdhand smoke is for all household smokers to quit smoking completely. As the scientific knowledge of thirdhand smoke increases, advocates will likely rely on it to encourage completely smoke-free places.
- Conclusion: Recent scientific studies on thirdhand smoke are impelling further research on the topic, spurring the creation of tobacco control policies to protect people from thridhand smoke and stimulating improvements to the delivery of tobacco control counseling and services to parents in child health care settings.
Key words: thirdhand smoke; smoking; tobacco; indoor air quality; smoking cessation; pediatrics.
While “thirdhand smoke” may be a relatively new term, it is rooted in an old concept—the particulate matter and residue from tobacco smoke left behind after tobacco is burned. In 1953, Dr. Ernest Wynder and his colleagues from the Washington University School of Medicine in St. Louis showed that condensate made from the residue of cigarette smoke causes cancer [1]. This residue left behind by burning cigarettes is now known as thirdhand smoke [2]. Dr. Wynder used acetone to rinse the leftover tobacco smoke residue from a smoking chamber where he had burned cigarettes. He then painted the solution of acetone and thirdhand smoke residue onto the backs of mice. The results of Dr. Wynder’s study demonstrated that exposed mice developed cancerous skin lesions, whereas mice exposed to the acetone alone did not display skin lesions. Dr. Wynder sounded an alarm bell in his manuscript when he wrote, “Such studies, in view of the corollary clinical data relating smoking to various types of cancer, appear urgent. They may result not only in furthering our knowledge of carcinogenesis, but in promoting some practical aspects of cancer prevention [1].”
Decades of research has been conducted since Dr. Wynder’s discovery to definitively conclude that smoking tobacco and exposure to secondhand tobacco smoke is harmful to human health. It is estimated that 480,000 annual premature deaths in the United States alone are attributable to smoking and exposure to secondhand smoke [3]. The World Health Organization estimates that worldwide tobacco use is responsible for more than 7 million deaths per year, with 890,000 of those deaths caused by secondhand smoke exposure of nonsmokers [4]. Epidemiological evidence of the harm posed by tobacco has spurred the U.S Surgeon General to conclude that there is no risk-free level of exposure to tobacco smoke [5]. Despite the overwhelming evidence implicating tobacco as the cause of an unprecedented amount of disease resulting from the use of a consumer product, only recently has a dedicated research agenda been pursued to study what Dr. Wynder urgently called for back in 1953: further exploration of the health effects of thirdhand tobacco smoke.
The term "thirdhand smoke" was first coined in 2006 by researchers with the Clinical Effort Against Secondhand Smoke Exposure (CEASE) program at Massachusetts General Hospital in Boston [6], and recent research has begun to shed considerable light on the topic. In 2011, a research consortium of scientists funded by the Tobacco-Related Disease Research Program [7] in California was set up to conduct pioneering research on the characterization, exposure and health effects of thirdhand tobacco smoke [8]. Research findings from this consortium and other scientists from around the world are quickly expanding and disseminating knowledge on this important topic.
While the research on thirdhand smoke is ongoing, this paper summarizes the current literature most relevant to the pediatric population and outlines clinical and policy recommendations to protect children and families from the harms of exposure to thirdhand smoke.
What Is Thirdhand Smoke and How Is It Different from Secondhand Smoke?
Thirdhand smoke is a result of combusted tobacco, most often from smoking cigarettes, pipes, cigars, or cigarillos. Thirdhand smoke remains on surfaces and in dust for a longtime after smoking happens, reacts with oxidants and other compounds to form secondary pollutants, and is re-emitted as a gas and/or resuspended when particles are disturbed and go back into the air where they can be inhaled [9]. One dramatic example of how thirdhand smoke can remain on surfaces long after secondhand smoke dissipates was discovered on the ornate constellation ceiling in the main concourse of the Grand Central Terminal in New York City. According to Sam Roberts, a correspondent for the New York Times and the author of a book about the historic train station, the dark residue that accumulated on the concourse ceiling over decades and was originally believed to be the result of soot from train engines was primarily residue from tobacco smoke [10–12]. It wasn’t until a restoration in the 1990s when workers scrubbed the tar and nicotine residue from the ceiling could the elaborate design of the zodiac signs and constellations be seen again [13]. A similar process takes place inside homes, where smoke residue accumulates on surfaces such as walls and ceilings after smoking happens. Owners of homes that have been previously smoked in are faced with unanswered questions about how to clean up the toxic substances left behind.
When tobacco is smoked, the particulates contained in secondhand smoke settle on surfaces; this contamination is absorbed deep into materials such as hair, clothes, carpeting, furniture, and wallboard [9,14]. After depositing onto surfaces, the chemicals undergo an aging process, which changes the chemical structure of the smoke pollutants. The nicotine in thirdhand smoke residue reacts with common indoor air pollutants, such as nitrous acid and ozone, to form hazardous substances. When the nicotine present in thirdhand smoke reacts with nitrous acid, it forms carcinogenic tobacco-specific nitrosamines such as NNK and NNN [15–17]. Nicotine also reacts with ozone to form additional harmful ultrafine particles that can embed deep within the lungs when inhaled [18]. As thirdhand smoke ages, it becomes more toxic [15]. The aged particles then undergo a process called “off-gassing,” in which gas is continuously re-emitted from these surfaces back into the air [19]. This process of off-gassing occurs long after cigarettes have been smoked indoors [19,20]. Thirdhand smoke particles can also be inhaled when they get resuspended into the air after contaminated surfaces are disturbed [21].
Common practices employed by smokers, like smoking in different rooms, using fans to diffuse the smoke, or opening windows, do not prevent the formation and inhalation of thirdhand smoke by people living or visiting these indoor spaces [22]. Environments with potential thirdhand smoke exposure include homes of smokers [23], apartments and homes previously occupied by smokers [24], multiunit housing where smoking is permitted [25], automobiles that have been smoked in [26], hotel rooms where smoking is permitted [27], and other indoor places where smoking has occurred.
Research Supports Having Completely Smoke-Free Environments
Recent research has shown that exposure to thirdhand smoke is harmful. These findings, many of which are described below, offer strong support in favor of advocating for environments free of thirdhand smoke contamination for families and children.
Genetic Damage from Thirdhand Smoke Exposure
In 2013, researchers from the Lawrence Berkeley National Laboratory were the first to demonstrate that thirdhand smoke causes significant genetic damage to human cells [28]. Using in vitro assays, the researchers showed that thirdhand smoke is a cause of harm to human DNA in the form of strand breaks and oxidative damage, which leads to mutations that can cause cancer. The researches also specifically tested the effect of NNA, a tobacco-specific nitrosamine that is commonly found in thirdhand smoke but not in secondhand smoke, on human cell cultures and found that it caused significant damage to DNA [28].
Children Show Elevated Biomarkers of Thirdhand Smoke Exposure in Their Urine and Hair Samples
In 2004, Matt and colleagues described how they collected household dust samples from living rooms and infants’ bedrooms [23]. Their research demonstrated that nicotine accumulated on the living room and infants’ bedroom surfaces of the homes belonging to smokers. Significantly higher amounts of urine cotinine, a biomarker for exposure to nicotine, were detected among infants who lived in homes where smoking happens inside compared to homes where smokers go outside to smoke [23]. As well, a study published in 2017 that measured the presence of hand nicotine on children of smokers who presented to the emergency room for an illness possibly related to tobacco smoke exposure detected hand nicotine on the hands of each child who participated in this pilot study. The researchers found a positive correlation between the amount of nicotine found on children’s hands and the amount of cotinine, a biomarker for nicotine exposure, detected in the children’s saliva [29].
Children Are Exposed to Higher Ratios of Thirdhand Smoke than Adults
In 2009, researchers discovered that the thirdhand smoke ratio of tobacco-specific nitrosamines to nicotine increases during the aging process [9]. Biomarkers measured in the urine can now be used to estimate the degree to which people have been exposed to secondhand or thirdhand smoke based on the ratio of the thirdhand smoke biomarker NNK and nicotine. Toddlers who live with adults who smoke have higher NNK/nicotine ratios, suggesting that they are exposed to a higher ratio of thirdhand smoke compared to secondhand smoke than adults [30]. Young children are likely exposed to higher ratios of thirdhand smoke as they spend more time on the floor, where thirdhand smoke accumulates. They frequently put their hands and other objects into their mouths. Young children breathe faster than adults, increasing their inhalation exposure and also have thinner skin, making dermal absorption more efficient [9].
Modeling Excess Cancer Risk
A 2014 United Kingdom study used official sources of toxicological data about chemicals detected in thirdhand smoke–contaminated homes to assess excess cancer risk posed from thirdhand smoke [17]. Using dust samples collected from homes where a smoker lived, they estimate that the median lifetime excess cancer risk from the exposure to all the nitrosamines present in thirdhand smoke is 9.6 additional cancer cases per 100,000 children exposed and could be as high as 1 excess cancer case per 1000 children exposed. The researchers concluded that young children aged 1 to 6 are at an especially increased risk for cancer because of their frequent contact with surfaces contaminated with thirdhand smoke and their ingestion of the particulate matter that settles on surfaces after smoking takes place [17].
Infants in Health Care Facilities Are Exposed to Thirdhand Smoke
Researchers have observed biomarkers confirming thirdhand smoke exposure in the urine of infants in the NICU. Found in incubators and cribs, particulates are likely being deposited in the NICU from visitors who have thirdhand smoke on their clothing, skin, and hair [31].
Animal Studies Link Thirdhand Smoke Exposure to Common Human Disease
Mice exposed to thirdhand smoke under conditions meant to simulate levels similar to human exposure are pre-diabetic, are at higher risk of developing metabolic syndrome, have inflammatory markers in the lungs that increase the risk for asthma, show slow wound healing, develop nonalcoholic fatty liver disease, and become behaviorally hyperactive [32]. Another recent study published in 2017 showed that mice exposed to thirdhand smoke after birth weighed less than mice not exposed to thirdhand smoke. Additionally, mice exposed to thirdhand smoke early in life showed changes in white blood cell counts that persisted into adulthood [9,33].
Summary
In summary, recent research makes a compelling case for invoking the precautionary principle to ensure that children avoid exposures to thirdhand smoke in their homes, cars, and healthcare settings. Studies reveal that:
- children live in homes where thirdhand smoke is present and this exposure is detectable in their bodies [23]
- concentrations of thirdhand smoke exposure observed in children are disproportionately higher than adults [30]
- chemicals present in thirdhand smoke cause damage to DNA [28]
- thirdhand smoke contains carcinogens that put exposed children at increased risk of cancer [17]
- thirdhand smoke is being detected within medical settings [34] and in the bodies of medically-vulnerable children [29], and
- animal studies have linked exposure to thirdhand smoke to a number of adverse health conditions commonly seen in today’s pediatric population such as metabolic syndrome, prediabetes, asthma, hyperactivity [32] and low birth weight [33].
Using the Thirdhand Smoke Concept in Clinical Practice
The clinical setting is an ideal place to address thirdhand smoke with families as a component of a comprehensive tobacco control strategy.
The Cessation Imperative—A Novel Motivational Message Prompted by Thirdhand Smoke
While there are potentially many ways to address thirdhand smoke exposure with families, the CEASE program has been used in the primary care setting to train child health care clinicians and office staff to address second- and thirdhand smoke. The training also educates clinicians on providing cessation counseling and resources to families with the goal of helping all family members become tobacco free, as well as to helping families keep completely smoke-free homes and cars [35,36]. The concept of thirdhand smoke creates what we have coined the cessation imperative [36]. The cessation imperative is based on the notion that the only way to protect non-smoking family and household members from thirdhand smoke is for all household smokers to quit smoking completely. Smoking, even when not in the presence of children, can expose others to toxic contaminates that settle on the surfaces of the home, the car as well as to the skin, hair, and clothing of family members who smoke. A discussion with parents about eliminating only secondhand smoke exposure for children does not adequately address how continued smoking, even when children are not present, can be harmful. The thirdhand smoke concept can be presented early, making it an efficient way to advocate for completely smoke-free families.
Thirdhand Smoke Counseling Helps Clinicians Achieve Key Tobacco Control Goals
The American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP) recommend that health care providers deliver advice to parents regarding establishing smoke-free homes and cars and provide information about how their smoking adversely affects their children’s health [37,38]. It is AAP and AAFP policy that health care providers provide tobacco dependence treatment and referral to cessation services to help adult family members quit smoking [38,39]. Successfully integrating counseling around the topic of thirdhand smoke into existing smoking cessation service delivery is possible. The CEASE research and implementation team developed and disseminated educational content to clinicians about thirdhand smoke through AAP courses delivered online [40] as well as made presentations to clinicians at AAP-sponsored training sessions. Thirdhand smoke messaging has been included in the CEASE practice trainings so that participating clinicians in pediatric offices are equipped to engage parents on this topic. Further information about these educational resources and opportunities can be obtained from the AAP Julius B. Richmond Center of Excellence website [41] and from the Massachusetts General Hospital CEASE program’s website [42].
Counseling parents about thirdhand smoke can help assist parents with their smoking in the critical context of their child’s care. Most parents see their child’s health care clinician more often than their own [43]. Increasing the number of pediatric clinical encounters where parental smoking is addressed while also increasing the effectiveness of these clinical encounters by increasing parents’ motivation to protect their children from tobacco smoke exposure are important goals. The topic of thirdhand smoke is a novel concept that clinicians can use to engage with parents around their smoking in a new way. Recent research conducted by the CEASE team suggests that counseling parents in the pediatric setting about thirdhand smoke can be useful in helping achieve tobacco control goals with families. Parent’s belief about thirdhand smoke is associated with the likelihood the parent will take concrete steps to protect their child. Parents who believe thirdhand smoke is harmful are more likely to protect their children from exposure by adopting strictly enforced smoke-free home and car rules [44]. Parents who changed their thirdhand smoke beliefs over the course of a year to believing that thirdhand smoke is harmful were more likely to try to quit smoking [44].
Child health care clinicians are effective at influencing parents’ beliefs about the potential harm thirdhand smoke poses to their children. Parents who received advice from pediatricians to quit smoking or to adopt smoke-free home or policies were more likely to believe that thirdhand smoke was harmful to the health of children [45]. Fathers (as compared with mothers) and parents who smoked more cigarettes each day were less likely to accept that thirdhand smoke is harmful to children [45]. Conversely, delivering effective educational messages and counseling around the topic of thirdhand smoke to parents may help promote smoke-free rules and acceptance of cessation assistance.
Protect Patients from Thirdhand Smoke Risks
All health care settings should be completely smoke-free. Smoking bans help protect all families and children from second and thirdhand smoke exposure. It is especially important for medically vulnerable children to visit facilities free from all forms of tobacco smoke contamination. CEASE trainings encourage practices to implement a zone of wellness on the grounds of the healthcare facility by completely banning smoking. The CEASE implementation team also trains practice leaders to reach out to all staff that use tobacco and offer resources and support for quitting. Having a non-smoking staff sets a great example for families who visit the healthcare facility, and reduces the likelihood of bringing thirdhand smoke contaminates into the facility. Creating a policy that addresses thirdhand smoke exposure is a concrete step that health care organizations can take to protect patients.
Thirdhand Smoke Resources Developed and/or Used by the CEASE Program
The CEASE program has developed and/or identified a number of clinical resources to educate parents and clinicians about thirdhand smoke. These free resources can enhance awareness of thirdhand smoke and help promote the use of the thirdhand smoke concept in clinical practice.
- Posters with messages designed to educate parents about thirdhand smoke to encourage receipt of cessation resources were created for use in waiting areas and exam rooms of child health care practices. A poster for clinical practice (Figure 1) can be downloaded and printed from the CEASE program website [42].
- Health education handouts that directly address thirdhand smoke exposure are available. The handouts can be taken home to family members who are not present at the visit and contain the telephone number for the tobacco quitline service, which connects smokers in the United States with free telephone support for smoking cessation. Handouts for clinical practice can be downloaded and printed from the CEASE program website. Figure 2 shows a handout that encourages parents to keep a smoke-free car by pointing out that tobacco smoke stays in the car long after the cigarette is out.
- Videos about thirdhand smoke can be viewed by parents while in child health care offices or shared on practice websites or social media platforms. The CEASE program encourages practices to distribute videos about thirdhand smoke to introduce parents to the concept of thirdhand smoke and to encourage parents to engage in a discussion with their child’s clinicians about ways to limit thirdhand smoke exposure. Suitable videos for parental viewing include the 2 listed below, which highlight information from the Thirdhand Smoke Research Consortium.
-University of California Riverside https://youtu.be/i1rhqRy-2e8
-San Diego State University https://youtu.be/rqzi-9sXLdU - Letters for landlords and management companies were created to stress the importance of providing a smoke-free living environment for children. The letters are meant to be signed by the child’s health care provider. The letters state that eliminating smoking in their buildings would result in landlords that “Pay less for cleaning and turnover fees.” Landlord letter templates can be downloaded and printed from the CEASE program website [42].
- Educational content for child health care clinicians about thirdhand smoke and how to counsel parents is included in the American Academy of Pediatrics Education in Quality Improvement for Pediatric Practice (EQIPP) online course entitled “Eliminating Tobacco” Use and Exposure to Secondhand Smoke. A section devoted to educating clinicians on the topic of thirdhand smoke is presented in this course. The course can be accessed through the AAP website and it qualifies for American Board of Pediatrics maintenance of certification part IV credit [40].
The CEASE team has worked with mass media outlets to communicate the messages about thirdhand smoke to build public awareness. The Today Show helped to popularize the concept of thirdhand smoke in 2009 after a paper published in the journal Pediatrics linked thirdhand smoke beliefs to home smoking bans [2].
Systems Approaches to Reduce Thirdhand Smoke Exposure
Public Policy Approaches
A clear policy agenda can help people protect their families from exposure to thirdhand smoke [46]. Policy approaches that have worked for lead, asbestos, and radon are examples of common household contaminants that are regulated using different mechanisms in an effort to protect the public health [46]. Strengths and weaknesses in each of these different approaches should be carefully considered when developing a comprehensive policy agenda to address thirdhand smoke. Recently, research on the health effects of thirdhand smoke spurred the passage of California legislative bill AB 1819 that “prohibits smoking tobacco at all times in the homes of licensed family child care homes and in areas where children are present [47].” As well, a recent US Department of Housing and Urban Development rule was finalized that requires all public housing agencies to implement a smoke-free policy by 30 July 2018 [48]. Smoke-free housing protects occupants from both secondhand and thirdhand smoke exposure. Pediatricians and other child health care professionals are well positioned to advocate for legislative actions that protect children from harmful exposures to thirdhand smoke.
Practice Change in Child Health Care Settings
Designing health care systems to screen for tobacco smoke exposure and to provide evidence-based cessation resources for all smokers is one of the best ways to reduce exposures to thirdhand smoke. Preventing thirdhand smoke exposure can work as novel messaging to promote tobacco cessation programs. Developing electronic medical record systems that allow for documentation of the smoking status of household members and whether or not homes and cars are completely smokefree can be particularly helpful tools for child health care providers when addressing thirdhand smoke with families. Good documentation about smoke-free homes and cars can enhance follow-up discussions with families as they work towards reducing thirdhand smoke exposures.
Summary
The thirdhand smoke concept has been used to improve delivery of tobacco control counseling and services for parents in the child health care context. Free materials are available that utilize thirdhand smoke messaging. As the science of thirdhand smoke matures, it will increasingly be used to help promote completely smoke-free places. The existing research on thirdhand smoke establishes the need for clinicians to communicate the cessation imperative. By using it, clinicians can help all smokers and non-smokers understand that there is no way to smoke tobacco without exposing friends and family.
Corresponding author: Jeremy E. Drehmer, MPH, 125 Nashua St., Suite 860, Boston, MA 02114, jdrehmer@ mgh.harvard.edu.
Financial disclosures: None
1. Wynder EL, Graham EA, Croninger AB, et al. Experimental production of carcinoma with cigarette tar experimental production of carcinoma with cigarette tar. 1953;36:855–64.
2. Winickoff JP, Friebely J, Tanski SE, et al. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics 2009;123:e74–9.
3. US Department of Health and Human Services. The health consequences of smoking- 50 years of progress: a report of the Surgeon General, Executive Summary. 2014.
4. World Health Organization. Tobacco fact sheet [Internet]. [cited 2017 Aug 15]. Available at www.who.int/mediacentre/factsheets/fs339/en/.
5. U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta (GA); 2006.
6. Winickoff J, Friebely J, Tanski S, et al. Beliefs about the health effects of third-hand smoke predict home and car smoking bans. In: Poster presented at the 2006 Pediatric Academic Societies Meeting. San Francisco, CA; 2006.
7. Tobacco-Related Disease Research Program [Internet]. Accessed 2017 Jul 7 at www.trdrp.org.
8. Matt GE, Quintana PJ, Destaillats H, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect 2011;119:1218–26.
9. Jacob P, Benowitz NL, Destaillats H, et al. Thirdhand smoke: new evidence, challenges, and future directions. Chem Res Toxicol 2017;30:270–94.
10. Roberts S, Hamill P. Grand Central: how a train station transformed America. Grand Central Publishing; 2013.
11. Sachs S. From gritty depot, a glittery destination; refurbished Grand Central terminal, worthy of its name, is reopened. New York Times 1998 Oct 2.
12. Grand Central: an engine of scientific innovation [Internet]. National Public Radio - Talk of the Nation; 2013. Available at www.npr.org/templates/transcript/transcript.php?storyId=175054273.
13. Lueck TJ. Work starts 100 feet above Grand Central commuters. New York Times 1996 Sep 20.
14. Van Loy MD, Nazaroff WW, Daisey JM. Nicotine as a marker for environmental tobacco smoke: implications of sorption on indoor surface materials. J Air Waste Manag Assoc 1998;48:959–68.
15. Sleiman M, Gundel LA, Pankow JF, et al. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc Natl Acad Sci U S A 2010;107:6576–81.
16. Xue J, Yang S, Seng S. Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers (Basel) 2014;6:1138–56.
17. Ramirez N, Ozel MZ, Lewis AC, et al. Exposure to nitrosamines in thirdhand tobacco smoke increases cancer risk in non-smokers. Environ Int 2014;71:139–47.
18. Destaillats H, Singer BC, Lee SK, Gundel LA. Effect of ozone on nicotine desorption from model surfaces: evidence for heterogeneous chemistry. Environ Sci Technol 2006;40:1799–805.
19. Singer BC, Hodgson AT, Guevarra KS, et al. Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Env Sci Technol 2002;36:846–53.
20. Singer BC, Hodgson AT, Nazaroff WW. Gas-phase organics in environmental tobacco smoke: 2. Exposure-relevant emission factors and indirect exposures from habitual smoking. Atmos Environ 2003;37:5551–61.
21. Becquemin MH, Bertholon JF, Bentayeb M, et al. Third-hand smoking: indoor measurements of concentration and sizes of cigarette smoke particles after resuspension. Tob Control 2010;19:347–8.
22. Centers for Disease Control and Prevention [Internet]. How can we protect our children from secondhand smoke: a parent’s guide. Accessed 2017 Aug 15 at www.cdc.gov/tobacco/basic_information/secondhand_smoke/protect_children/pdfs/protect_children_guide.pdf.
23. Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control 2004;13:29–37.
24. Matt GE, Quintana PJE, Zakarian JM, et al. When smokers move out and non-smokers move in: residential thirdhand smoke pollution and exposure. Tob Control 2011;20:e1.
25. Kraev TA, Adamkiewicz G, Hammond SK, Spengler JD. Indoor concentrations of nicotine in low-income, multi-unit housing: associations with smoking behaviours and housing characteristics. Tob Control 2009;18:438–44.
26. Matt GE, Quintana PJE, Hovell MF, et al. Residual tobacco smoke pollution in used cars for sale: air, dust, and surfaces. Nicotine Tob Res 2008;10:1467–75.
27. Matt GE, Quintana PJE, Fortmann AL, et al. Thirdhand smoke and exposure in California hotels: non-smoking rooms fail to protect non-smoking hotel guests from tobacco smoke exposure. Tob Control 2014;23:264–72.
28. Hang B, Sarker AH, Havel C, et al. Thirdhand smoke causes DNA damage in human cells. Mutagenesis 2013;28:381–91.
29. Mahabee-Gittens EM, Merianos AL, Matt GE. Preliminary evidence that high levels of nicotine on children’s hands may contribute to overall tobacco smoke exposure. Tob Control 2017 Mar 30.
30. Hovell MF, Zakarian JM, Matt GE, et al. Counseling to reduce children’s secondhand smoke exposure and help parents quit smoking: a controlled trial. Nicotine Tob Res 2009;11:1383–94.
31. Northrup TF, Khan AM, Jacob 3rd P, et al. Thirdhand smoke contamination in hospital settings: assessing exposure risk for vulnerable paediatric patients. Tob Control 2016; 25: 619–23.
32. Martins-Green M, Adhami N, Frankos M, et al. Cigarette smoke toxins deposited on surfaces: Implications for human health. PLoS One 2014;9:1–12.
33. Hang B, Snijders AM, Huang Y, et al. Early exposure to thirdhand cigarette smoke affects body mass and the development of immunity in mice. Sci Rep 2017;7:41915.
34. Northrup TF, Matt GE, Hovell MF, et al. Thirdhand smoke in the homes of medically fragile children: Assessing the impact of indoor smoking levels and smoking bans. Nicotine Tob Res 2016;18:1290–8.
35. Marbin JN, Purdy CN, Klaas K, et al. The Clinical Effort against Secondhand Smoke Exposure (CEASE) California: implementing a pediatric clinical intervention to reduce secondhand smoke exposure. Clin Pediatr (Phila) 2016;1(3).
36. Winickoff JP, Hipple B, Drehmer J, et al. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention: A decade of lessons learned. J Clin Outcomes Manag 2012;19:414–9.
37. Farber HJ, Groner J, Walley S, Nelson K. Protecting children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:e1439–67.
38. American Academy of Family Physicians [Internet]. AAFP policies. Tobacco use, prevention, and cessation. Accessed 2017 Aug 29 at www.aafp.org/about/policies/all/tobacco-smoking.html.
39. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:1008–17.
40. Drehmer J, Hipple B, Murphy S, Winickoff JP. EQIPP: Eliminating tobacco use and exposure to secondhand smoke [online course] PediaLink [Internet]. American Academy of Pediatrics. 2014. Available at bit.ly/eliminate-tobacco-responsive.
41. The American Academy of Pediatrics Julius B. Richmond Center of Excellence [Internet]. Accessed 2017 Aug 9 at www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Richmond-Center/Pages/default.aspx.
42. Clinical Effort Against Secondhand Smoke Exposure [Internet]. Accessed at www.massgeneral.org/ceasetobacco/.
43. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132:109–17.
44. Drehmer JE, Ossip DJ, Nabi-Burza E, et al. Thirdhand smoke beliefs of parents. Pediatrics 2014;133:e850–6.
45. Drehmer JE, Ossip DJ, Rigotti NA, et al. Pediatrician interventions and thirdhand smoke beliefs of parents. Am J Prev Med 2012;43:533–6.
46. Samet JM, Chanson D, Wipfli H. The challenges of limiting exposure to THS in vulnerable populations. Curr Environ Health Rep 2015;2:215–25.
47. Thirdhand Smoke Research Consortium [Internet]. Accessed 2017 Aug 15 at www.trdrp.org/highlights-news-events/thirdhand-smoke-consortium.html.
48. Office of the Federal Register (US) [Internet]. Rule instituting smoke-free public housing. 2016. Available at www.federalregister.gov/documents/2016/12/05/2016-28986/instituting-smoke-free-public-housing.
1. Wynder EL, Graham EA, Croninger AB, et al. Experimental production of carcinoma with cigarette tar experimental production of carcinoma with cigarette tar. 1953;36:855–64.
2. Winickoff JP, Friebely J, Tanski SE, et al. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics 2009;123:e74–9.
3. US Department of Health and Human Services. The health consequences of smoking- 50 years of progress: a report of the Surgeon General, Executive Summary. 2014.
4. World Health Organization. Tobacco fact sheet [Internet]. [cited 2017 Aug 15]. Available at www.who.int/mediacentre/factsheets/fs339/en/.
5. U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta (GA); 2006.
6. Winickoff J, Friebely J, Tanski S, et al. Beliefs about the health effects of third-hand smoke predict home and car smoking bans. In: Poster presented at the 2006 Pediatric Academic Societies Meeting. San Francisco, CA; 2006.
7. Tobacco-Related Disease Research Program [Internet]. Accessed 2017 Jul 7 at www.trdrp.org.
8. Matt GE, Quintana PJ, Destaillats H, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect 2011;119:1218–26.
9. Jacob P, Benowitz NL, Destaillats H, et al. Thirdhand smoke: new evidence, challenges, and future directions. Chem Res Toxicol 2017;30:270–94.
10. Roberts S, Hamill P. Grand Central: how a train station transformed America. Grand Central Publishing; 2013.
11. Sachs S. From gritty depot, a glittery destination; refurbished Grand Central terminal, worthy of its name, is reopened. New York Times 1998 Oct 2.
12. Grand Central: an engine of scientific innovation [Internet]. National Public Radio - Talk of the Nation; 2013. Available at www.npr.org/templates/transcript/transcript.php?storyId=175054273.
13. Lueck TJ. Work starts 100 feet above Grand Central commuters. New York Times 1996 Sep 20.
14. Van Loy MD, Nazaroff WW, Daisey JM. Nicotine as a marker for environmental tobacco smoke: implications of sorption on indoor surface materials. J Air Waste Manag Assoc 1998;48:959–68.
15. Sleiman M, Gundel LA, Pankow JF, et al. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc Natl Acad Sci U S A 2010;107:6576–81.
16. Xue J, Yang S, Seng S. Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers (Basel) 2014;6:1138–56.
17. Ramirez N, Ozel MZ, Lewis AC, et al. Exposure to nitrosamines in thirdhand tobacco smoke increases cancer risk in non-smokers. Environ Int 2014;71:139–47.
18. Destaillats H, Singer BC, Lee SK, Gundel LA. Effect of ozone on nicotine desorption from model surfaces: evidence for heterogeneous chemistry. Environ Sci Technol 2006;40:1799–805.
19. Singer BC, Hodgson AT, Guevarra KS, et al. Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Env Sci Technol 2002;36:846–53.
20. Singer BC, Hodgson AT, Nazaroff WW. Gas-phase organics in environmental tobacco smoke: 2. Exposure-relevant emission factors and indirect exposures from habitual smoking. Atmos Environ 2003;37:5551–61.
21. Becquemin MH, Bertholon JF, Bentayeb M, et al. Third-hand smoking: indoor measurements of concentration and sizes of cigarette smoke particles after resuspension. Tob Control 2010;19:347–8.
22. Centers for Disease Control and Prevention [Internet]. How can we protect our children from secondhand smoke: a parent’s guide. Accessed 2017 Aug 15 at www.cdc.gov/tobacco/basic_information/secondhand_smoke/protect_children/pdfs/protect_children_guide.pdf.
23. Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control 2004;13:29–37.
24. Matt GE, Quintana PJE, Zakarian JM, et al. When smokers move out and non-smokers move in: residential thirdhand smoke pollution and exposure. Tob Control 2011;20:e1.
25. Kraev TA, Adamkiewicz G, Hammond SK, Spengler JD. Indoor concentrations of nicotine in low-income, multi-unit housing: associations with smoking behaviours and housing characteristics. Tob Control 2009;18:438–44.
26. Matt GE, Quintana PJE, Hovell MF, et al. Residual tobacco smoke pollution in used cars for sale: air, dust, and surfaces. Nicotine Tob Res 2008;10:1467–75.
27. Matt GE, Quintana PJE, Fortmann AL, et al. Thirdhand smoke and exposure in California hotels: non-smoking rooms fail to protect non-smoking hotel guests from tobacco smoke exposure. Tob Control 2014;23:264–72.
28. Hang B, Sarker AH, Havel C, et al. Thirdhand smoke causes DNA damage in human cells. Mutagenesis 2013;28:381–91.
29. Mahabee-Gittens EM, Merianos AL, Matt GE. Preliminary evidence that high levels of nicotine on children’s hands may contribute to overall tobacco smoke exposure. Tob Control 2017 Mar 30.
30. Hovell MF, Zakarian JM, Matt GE, et al. Counseling to reduce children’s secondhand smoke exposure and help parents quit smoking: a controlled trial. Nicotine Tob Res 2009;11:1383–94.
31. Northrup TF, Khan AM, Jacob 3rd P, et al. Thirdhand smoke contamination in hospital settings: assessing exposure risk for vulnerable paediatric patients. Tob Control 2016; 25: 619–23.
32. Martins-Green M, Adhami N, Frankos M, et al. Cigarette smoke toxins deposited on surfaces: Implications for human health. PLoS One 2014;9:1–12.
33. Hang B, Snijders AM, Huang Y, et al. Early exposure to thirdhand cigarette smoke affects body mass and the development of immunity in mice. Sci Rep 2017;7:41915.
34. Northrup TF, Matt GE, Hovell MF, et al. Thirdhand smoke in the homes of medically fragile children: Assessing the impact of indoor smoking levels and smoking bans. Nicotine Tob Res 2016;18:1290–8.
35. Marbin JN, Purdy CN, Klaas K, et al. The Clinical Effort against Secondhand Smoke Exposure (CEASE) California: implementing a pediatric clinical intervention to reduce secondhand smoke exposure. Clin Pediatr (Phila) 2016;1(3).
36. Winickoff JP, Hipple B, Drehmer J, et al. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention: A decade of lessons learned. J Clin Outcomes Manag 2012;19:414–9.
37. Farber HJ, Groner J, Walley S, Nelson K. Protecting children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:e1439–67.
38. American Academy of Family Physicians [Internet]. AAFP policies. Tobacco use, prevention, and cessation. Accessed 2017 Aug 29 at www.aafp.org/about/policies/all/tobacco-smoking.html.
39. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:1008–17.
40. Drehmer J, Hipple B, Murphy S, Winickoff JP. EQIPP: Eliminating tobacco use and exposure to secondhand smoke [online course] PediaLink [Internet]. American Academy of Pediatrics. 2014. Available at bit.ly/eliminate-tobacco-responsive.
41. The American Academy of Pediatrics Julius B. Richmond Center of Excellence [Internet]. Accessed 2017 Aug 9 at www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Richmond-Center/Pages/default.aspx.
42. Clinical Effort Against Secondhand Smoke Exposure [Internet]. Accessed at www.massgeneral.org/ceasetobacco/.
43. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132:109–17.
44. Drehmer JE, Ossip DJ, Nabi-Burza E, et al. Thirdhand smoke beliefs of parents. Pediatrics 2014;133:e850–6.
45. Drehmer JE, Ossip DJ, Rigotti NA, et al. Pediatrician interventions and thirdhand smoke beliefs of parents. Am J Prev Med 2012;43:533–6.
46. Samet JM, Chanson D, Wipfli H. The challenges of limiting exposure to THS in vulnerable populations. Curr Environ Health Rep 2015;2:215–25.
47. Thirdhand Smoke Research Consortium [Internet]. Accessed 2017 Aug 15 at www.trdrp.org/highlights-news-events/thirdhand-smoke-consortium.html.
48. Office of the Federal Register (US) [Internet]. Rule instituting smoke-free public housing. 2016. Available at www.federalregister.gov/documents/2016/12/05/2016-28986/instituting-smoke-free-public-housing.
Clinician Telephone Training to Reduce Family Tobacco Use: Analysis of Transcribed Recordings
From the Massachusetts General Hospital for Children, Boston, MA (Walters, Drehmer, Nabi-Burza, Winickoff), the University of Rochester School of Medicine, Rochester, NY (Ossip), and the American Academy of Pediatrics Julius B. Richmond Center of Excellence, Elk Grove Village, IL (Whitmore, Gorzkowski). †Deceased 31 December 2015.
Abstract
- Background: Family tobacco use and exposure are significant threats to the health of children and their families. However, few pediatric clinicians address family tobacco use and exposure in a routine and effective manner. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice.
- Objective: To review the main considerations and questions that clinicians and office staff expressed during telephone training to participate in CEASE.
- Methods: This study was conducted in pediatric practices in 5 US states. Practices were recruited by the American Academy of Pediatrics (10 intervention, 10 control). Ten training calls were recorded and transcribed. The data was then coded inductively based on themes found in the transcripts.
- Results: The data revealed that clinicians and staff were concerned about prescribing, dosing, and insurance coverage of nicotine replacement therapy; motivation for and methods to help families become tobacco-free; and the impact of the intervention on practice operations.
- Conclusion: While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco-free, they expressed concerns that could threaten implementation of family tobacco control strategies.
The devastating health consequences of smoking and exposure to tobacco smoke have been well demonstrated. As declared in the 2006 Surgeon General’s Report, there is no safe level of exposure to tobacco [1]. Children are especially at risk for exposure to toxins and toxicants in tobacco smoke [1,2]. Exposure to tobacco smoke is associated with higher levels of asthma, increased risk of sudden infant death syndrome, increased rates of upper respiratory infections, and behavioral issues [3–5]. Recent research shows that over 70% of children in the United States have some level of exposure to tobacco smoke [6]; parents and other family members are commonly the cause of this exposure, especially in young children. Children and parents benefit when parents stop smoking; parent life expectancy increases by an average of 7 years [7], the risk of tobacco-related poor pregnancy outcomes is reduced, and future children are spared from exposure to tobacco smoke [8].
There is a growing movement to address tobacco use and exposure in the pediatric office setting; the 2015 American Academy of Pediatrics tobacco policy statement Clinical Practice Policy to Protect Children From Tobacco, Nicotine, and Tobacco Smoke recommends that pediatricians ask about children’s exposure to tobacco and address parental tobacco use by implementing office-wide systems to deliver advice, counseling, referral to cessation resources, and smoking cessation medication to smokers [9].
Despite significant risks of tobacco smoke exposure to children, we found in a previous paper that only 3.5% of parents in control practices received any tobacco control assistance [10]. Through a systematic and ongoing line of research, the Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice. The CEASE intervention has been successfully shown to train and equip pediatric officesfrom a distance to address family tobacco use within existing office systems [10–14]. An enhanced CEASE intervention is undergoing testing in pediatric practices in 5 US states.
One of the more innovative aspects of CEASE has been the use of training calls. In studies of CEASE, the peer-to-peer call was conducted by the principal investigator with the project leader at the practice using a train-the-trainer model. After the project leader was trained through the peer-to-peer call, the project leader then led the whole office training call, with the support of CEASE staff by phone. The training calls worked in conjunction with the other aspects of the training, as shown in the Table. The training calls for the practices provided a valuable research opportunity. We examined the concerns and issues that clinicians and office staff had about implementing an office-wide tobacco control program through a qualitative analysis of the call transcripts. This paper outlines the main considerations and questions that clinicians and office staff expressed during the training calls. Understanding the points of view of clinicians and staff will help researchers and clinical educators strengthen the design of tobacco control interventions.
Methods
Study Aims
The data for this paper were collected as part of a larger mixed-methods controlled trial. The overarching aims of the trial were to study implementation and sustainability of tobacco-control services delivered at the clinic level, to facilitate behavior change among parents and evaluate cost-per-quit among parents who smoke, and to study systems changes and the processes that affect them at the practice level. The study was conducted in 5 intervention and 5 control pediatric primary care practices in 5 states; this paper reports on data collected in intervention practices and focuses on understanding the systems changes and processes that are instituted when implementing a tobacco control program at the clinician and practice level.
Practice Recruitment and Eligibility
Practices were recruited through the American Academy of Pediatrics using direct emails, newsletter/listserv articles, phone calls to members, and in-person recruitment at national meetings. Eligible practices were located in a non–hospital-based setting, had an average patient flow of at least 50 patients per day, used an electronic medical record (EMR) system, and were matched in each state based on practice size and smoking rate. Interested practices also had to be willing to host a research assistant to collect exit interview data from parents. Practices were excluded if they took part in previous CEASE studies or were actively enrolling participants into other tobacco control research studies. Based on these criteria, 18 eligible practices from Indiana, North Carolina, Ohio, Tennessee, Michigan, and Virginia agreed to participate in the study. Of the 6 states, one state was chosen as a replacement state. Five practices from the remaining states were assigned to the intervention group, 5 to the control group, and 5 were assigned to the replacement group in case an intervention or control practice in their state withdrew from the study. Each intervention practice participated in a peer-to-peer training call and a whole office training call. Data analyzed in this paper was collected from all 10 intervention practice training calls.
Training Calls Data Collection
The peer-to-peer and whole office training calls were recorded and transcribed. Permission to record the calls was requested by the trainer (the principal investigator of the study) and given verbally by each person being trained. The training call recordings were then transcribed verbatim by a commercial service; the transcriptions were spot-checked for accuracy.
The transcripts were first read closely by the first author (BHW), then coded inductively into relevant themes that emerged from the calls. The inductive coding was guided by the questions and concerns that the clinicians raised during the training, as well as the ways in which the trainer addressed these concerns and tailored the training to the needs and interests of the pediatric clinicians [26]. The coding was reviewed and confirmed by the other study team members.
After the data were coded into themes, the coded data were analyzed by the first author using qualitative description. Qualitative description is a method of analyzing coded qualitative data by looking at the words and meanings expressed by respondents [27]. Through this method of analysis, we were able to understand what concerns the clinicians and staff voiced about aspects of the CEASE intervention.
Ethics
The study was approved institutional review boards at Massachusetts General Hospital, the AAP, and the health care practices that required local IRB approval. The quotes used in this paper have been anonymized and cleaned to remove any identifying information, such as location and names.
Peer-to-Peer Training Calls
The peer-to-peer training calls were conducted after training and study materials arrived. The project leader (a pediatrician in the practice who was interested in spearheading the CEASE intervention) was asked to watch the training video. Using an evidence-based, previously developed call script [28], the principal investigator trained the project leader in key aspects of addressing family tobacco use and exposure, such as using an electronic tablet screener survey to identify family members who smoke, exploring techniques for prescribing or recommending NRT, and identifying ways to connect family members to free tobacco cessation counseling and support services. On occasion, other staff from the pediatric office (eg, a nurse or office manager) joined the call.
The principal investigator presented information, clarified points in the video, explained the materials, and asked questions and elicited relevant experiences from the project leader. In addition to teaching the project leader about the tobacco control strategies used in CEASE, the peer-to-peer calls prepared the project leader to train the rest of their own practice clinicians and staff in the CEASE intervention.
Whole Office Training Calls
Each practice’s local project leader led the whole office training calls, but CEASE study staff were on the call to introduce themselves to office staff, answer any questions that staff may have raised that the project leader could not answer, give information about data collection, and to generally support the implementation of the CEASE intervention and research program. During this call, the project leader watched the video with the group and tailored the training for his or her practice, focusing on issues of relevance for patients and staff.
Training Calls as Research Data
As many practices struggle with research burden [29], finding innovative and unobtrusive methods of collecting data is especially useful for research teams and participating practices. During both calls, clinicians and staff were asked open-ended questions to learn about their concerns regarding intervention implementation, share their own experiences with tobacco and tobacco control, and explore practice-specific methods to address family smoking. CEASE staff used this opportunity to help practices tailor the intervention to the local setting, such as by offering quitline enrollment sheets in another language. Clinician and staff answers to open-ended questions provided qualitative data for this manuscript.
Results and Discussion
The research team used training call data to explore clinician and staff concerns and desires related to family-centered tobacco control. The most common themes were: (1) prescribing, dosing, and insurance coverage of NRT, (2) motivation for and methods to help families become tobacco-free, and (3) the impact of the CEASE intervention on the day-to-day operations of the practice.
Nicotine Replacement Therapy
Prescribing or recommending NRT is one of the best ways to help families become tobacco-free and is a crucial component of the CEASE intervention [30–32]. Through the telephone trainings, clinicians and staff were trained to prescribe NRT using pre-printed prescription sheets, presented information about the effectiveness of NRT for smoking cessation, and referred to an information sheet on NRT to answer other questions as needed.
During the calls, it became clear that the pediatric clinicians were interested in prescribing NRT to help smokers quit, but lacked the skills and knowledge to do so:
I’m writing all this down [about NRT], because I don’t know any of this. (IN peer-to-peer)
Is 4 mg the strongest the gum comes in? (NC whole office)
This lack of knowledge may be a barrier to prescribing NRT in the pediatric setting. A national survey revealed that while smoking parents would accept prescriptions for NRT from their child’s doctor, very few received a prescription [33]. The calls provided an opportunity to have clinicians’ questions about NRT be answered by a pediatric tobacco control expert.
Clinicians were interested in helping parents stop smoking with medication, but were worried about access to medication; one of the most common questions voiced was not about how or why to prescribe NRT but how to help low-income parents get NRT for free or low-cost.
Some people—they don’t have insurance, so, how much it costs, they need to know that. (TN peer-to-peer)
I just know I’ve got a bunch ... Obamacare doesn’t work down here, so—I’ve still got families who don’t have any insurance, and you’re like, “Oh, I was hoping you could get something,” and they’re like, “Well, we can’t.” I have a fair number of kids who—are on some type of insurance, but the parents don’t have any coverage for NRT. (VA peer-to-peer)
While NRT is covered under the Affordable Care Act, many states have not expanded their Medicaid coverage [34]; this leaves many low-income families without access to health insurance or to free or low-cost NRT. While NRT remains one of the best and most common smoking cessation tools [35] there was no way to reassure practices that parents would be able to obtain the prescribed NRT without guaranteed coverage. In a previous study, the cost of NRT was seen by smokers as a barrier to using NRT to quit smoking [32]. Clinicians’ concerns about the cost of NRT reveal an understanding of the needs and issues relevant to their patient population.
Motivation for and Methods to Help Families Become Tobacco-Free
Clinicians and office staff were motivated to help families become tobacco-free and were interested in various ways to do so. The motivation and interest were personal, clinical, and organizational, relating to the ways in which care in the pediatric office could be altered to address tobacco in a more systematic way.
Motivation
The interest in smoking cessation stems from the desire to protect children from the harmful effects tobacco smoke and to prevent children themselves from taking up smoking:
We’d always talked about the smoking, and the parents finally quit. Probably not like I helped them—I just had been harping on them—but by that point the boy was smoking. When he was little he was like, “Oh, that’s nasty. I can’t believe my parents smoke.” Then by the time he was 14-15 and the parents actually did manage to quit, he was smoking, and I was like, “Ugh, really?” (VA peer-to-peer)
I totally understand the dire need for this project, in both the tobacco in the households, as well as the teenagers smoking. I heard one stat[istic], that one of our high schools had 80% of children using tobacco products… And that’s on my watch… I understand and I share the same passion that you do, for personal reasons, as well as reasons to help the whole community. (NC peer-to-peer)
Pediatricians saw themselves as responsible for protecting children’s health through reducing their tobacco smoke exposure, for working to prevent teen smoking, and for the overall health of their communities. Helping prevent childhood exposure to tobacco smoke and teen smoking initiation are crucial tasks for pediatricians; the 2015 AAP tobacco policy statement strongly recommends that pediatric offices include tobacco use prevention messages when talking to children and teens to help prevent smoking initiation, as well as helping families establish smoking bans for homes and cars [36]. By participating in the CEASE telephone trainings, clinicians and office staff were learning skills and tools to help them act on their motivation to protect families from the harms of tobacco.
Strategies
Pediatricians and office staff were interested in learning specific strategies and tools to help parents stop smoking. Practices wanted to know how and when to set a quit date with families, how to use services to help families become smoke-free, and how to tailor assistance to specific populations.
Yeah, we’re wondering about other languages, because we do have a large Hispanic patient population and a sizable group of folks that come from Saudi Arabia, and I know that some of them do smoke. (TN peer-to-peer)
Set[ting] a quit date for the patient —so how long we want to set the date? 6 months, 3 months, 1 year, 2 years, what? (TN peer-to-peer)
If you have a mom who lives with grandma and grandpa, the mom may not smoke but grandma and grandpa smoke, but they still live in that home… But anyone who comes in, we’re going to help. Does that sound right? (VA peer-to-peer)
By participating in the study, the clinicians and office staff were actively seeking to improve their knowledge of tobacco-related issues; past research has shown that pediatric residents saw lack of training in tobacco control as a key reason for inconsistent tobacco control outreach and intervention [37]. The training calls were an opportunity to gain information more specifically related to the pediatric practice’s population and office setting, building upon the other CEASE training materials. The training calls were also a chance for the CEASE research team to adapt strategies and tools to the practices, for example by providing materials that met the practices’ needs.
Impact of Intervention on Day-To-Day Operations
The training calls revealed that integrating CEASE into office workflows was a major concern. Integrating preventive services into routine office practice is a frequent concern of primary care providers [38–41]. These concerns about office flow reflect worries about financing [42] and benchmarking [43–45].
I think they’re going to have some of the same questions [that I initially had] in terms of how this might work with workflow. But as we’ve talked through all of this, I think we can make it work, and make it just sort of incorporated as part of our everyday questions that we ask. And it shouldn’t really slow things down. And I think that’ll be the main thing the providers would be focusing on is, how’s this going to impact me and all the other things I have to do in the course of a visit? This [phone call] answers a lot of questions I had in terms of that. (IN peer-to-peer)
As wait time was a performance measure for many of the practices, the clinicians and staff were hesitant to add any activities to check-in that might increase wait time.
I know, so especially, we’re trying to do a care team right now... don’t want them to spend too much time in the waiting room. (OH whole office)
During the calls, clinicians and office staff were asked to reflect on their practices and discuss ways that their practice would implement the CEASE intervention. This moment of reflection is a benefit of research participation, as it allows practices to improve the care they provide [46]. The calls allowed for on-the-spot tailoring of the intervention to meet the specific needs of the practice, an opportunity for the research staff and practice to work together to make the intervention fit their particular office situation and flow. Data collected from the training calls were also reviewed during the CEASE implementation process to support practices with specific concerns.
Strengths and Limitations
As these data were collected during training calls and subject to social desirability bias, the concerns raised may not be an exhaustive list of all concerns that clinicians and office staff had. However, the concerns that were raised by clinicians became a natural and essential part of the training process. As the practices’ initial concerns were identified early in the study, it was possible to address these concerns throughout the early implementation phases of CEASE. Transcribing calls and analyzing training call data as quickly as possible during the training phases of an intervention could prove beneficial for strengthening the implementation.
Dedicating the extra time and effort to record the training calls as a source of data formalized and strengthened the implementation process. By recording training calls, the study team was able to document the practices’ concerns and share them among the research team, including those who were not on training calls. This effort was a significant source of quality improvement data for the research team and helped ensure that we were responsive to the articulated needs of clinicians and practices.
Conclusion
The training call data revealed both the concerns as well as the interests of child health care clinicians in regard to addressing family tobacco use. While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco free, they expressed concerns that could threaten full implementation of family tobacco control strategies. These concerns and interests related to the coverage and affordability of NRT, integrating tobacco control strategies into the practice flow, and learning strategies to address family-wide tobacco use, such as helping grandparents quit smoking or addressing tobacco use with those who were not native English speakers. The concerns and interests of clinicians and office staff revealed that they were genuinely interested in learning ways to tailor strategies to address tobacco use for their practices and patient populations. By recording the training calls, the study team was better able to help them tailor the intervention to their practice, both during the calls and during subsequent implementation by providing new materials and additional information on subjects of concern to the practice. Carefully documenting training calls with health care practices are an ideal opportunity to collect information on issues that may impact full implementation of future interventions.
Corresponding author: Jonathan P. Winickoff, jwinickoff@mgh.harvard.edu
1. U.S. Department of Health and Human Services. The health consequences of involuntary tobacco smoke: a report of the Surgeon General. 2006.
2. Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol 2004;26:373–85.
3. Polanska K, Hanke W, Ronchetti R, et al. Environmental tobacco smoke exposure and children’s health. Acta Paediatr Suppl 2006;95:86–92.
4. American Academy of Pediatrics, Committee on Substance Abuse. Tobacco’s toll: implications for the pediatrician. Pediatrics 2001;107:794–8.
5. U.S. Department of Health and Human Services. Children and secondhand smoke exposure. Excerpts from the health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2007.
6. Wilson KM, Klein JD, Blumkin AK, et al. Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics 2011;127:85–92.
7. Taylor SM, Ross NA, Cummings KM, et al. Community intervention trial for smoking cessation (COMMIT): changes in community attitudes toward cigarette smoking. Health Educ Res 1998;13:109-22.
8. Winickoff JP, Healey EA, Regan S, et al. Using the postpartum hospital stay to address mothers’ and fathers’ smoking: the NEWS study. Pediatrics 2010;125:518–25.
9. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:1008–17.
10. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132:109–17.
11. Ossip DJ, Chang Y, Nabi-Burza E, et al. Strict smoke-free home policies among smoking parents in pediatric settings. Acad Pediatr 2013;13:517–23.
12. Winickoff JP, Park ER, Hipple BJ, et al. Clinical effort against secondhand smoke exposure: development of framework and intervention. Pediatrics 2008;122:e363–e75.
13. Nabi-Burza E, Winickoff JP, Finch S, Regan S. Triple tobacco screen: opportunity to help families become smokefree. Am J Prev Med 2013;45:728–31.
14. Winickoff JP. Pediatrician-led program increases provision of smoking cessation support, boosts quit rates among parents. Innovations in Medicine 2011. Accessed 24 Nov 2015 at https://innovations.ahrq.gov/profiles/pediatrician-led-program-increases-provision-smoking-cessation-support-boosts-quit-rates.
15. Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2000.
16. Winickoff JP, Nabi-Burza E, Chang Y, et al. Sustainability of a parental tobacco control intervention in pediatric practice. Pediatrics 2014;134:933-41.
17. Moore D, Aveyard P, Connock M, et al. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 2009;338:b1024.
18. Aveyard P, Wang D, Connock M, et al. Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: floating prolonged abstinence. Nicotine Tob Res 2009;11:475–80.
19. Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav 2011;36:764–8.
20.Curry SJ, Grothaus LC, McAfee T, Pabiniak C. Use and cost effectiveness of smoking-cessation services under four insurance plans in a health maintenance organization. N Engl J Med 1998;339:673–9.
21. Abroms LC, Ahuja M, Kodl Y, et al. Text2Quit: Results from a pilot test of a personalized, interactive mobile health smoking cessation program. J Health Commun 2012;17 Suppl 1:44-53.
22. Curry SJ, Ludman EJ, Graham E, et al. Pediatric-based smoking cessation intervention for low-income women: a randomized trial. Arch Pediatr Adolesc Med 2003;157:295–302.
23. Orleans CT, Schoenbach VJ, Wagner EH. Self-help quit smoking interventions: effects of self-help materials, social support materials, social support instructions and telephone counseling. J Consult Clin Psychol 1991;59:439–48.
24. An LC, Zhu SH, Nelson DB, et al. Benefits of telephone care over primary care for smoking cessation: a randomized trial. Arch Intern Med 2006;166:536–42.
25. Warner DO, Klesges RC, Dale LC, et al. Clinician-delivered intervention to facilitate tobacco quitline use by surgical patients. Anesthesiology 2011;114:847–55.
26. Creswell, JW. Qualitative inquiry and research design: choosing among five approaches. 2nd ed. Thousand Oaks, CA: Sage; 2007.
27. Sandelowski M. Focus on research methods: whatever happened to qualitative description. Res Nurs Health 2000;23:334–40.
28. Winickoff JP, Hipple B, Drehmer J, et al. The clinical effort against secondhand smoke exposure (CEASE) intervention: A decade of lessons learned. J Clin Outcomes Manag 2012;19:414–9.
29. Clark T, Sinclair R. The costs and benefits of acting as a research site. Evid Policy A J Res Debate Pract 2008;4:105–19.
30. Zhu S, Melcer T, Sun J. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med 2000;18:305–11.
31. Gilpin EA, Messer K, Pierce JP. Population effectiveness of pharmaceutical aids for smoking cessation: what is associated with increased success? Nicotine Tob Res 2006;8:661–9.
32. Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med 2005;28:119–22.
33. Winickoff JP, Tanski SE, McMillen RC, et al. Child health care clinicians’ use of medications to help parents quit smoking: a national parent survey. Pediatrics 2005;115:1013–7.
34. Kaiser Family Foundation. Status of state action on the medicaid expansion decision. Available at http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/.
35. U.S. Department of Health and Human Services. The health consequences of smoking- 50 years of progress: a report of the Surgeon General. 2014.
36. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Public policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:998–1007.
37. Collins BN, Levin KP, Bryant-Stephens T. Pediatricians’ practices and attitudes about environmental tobacco smoke and parental smoking. J Pediatr 2007;150:547–52.
38. Leininger LS, Finn L, Dickey L, et al. An office system for organizing preventive services: a report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems. Arch Fam Med 1996;5:108–15.
39. Swartz SH, Hays JT. Office-based intervention for tobacco dependence. Med Clin North Am 2004;88:1623–41.
40. Bordley WC, Margolis PA, Stuart J, et al. Improving preventive service delivery through office systems. Pediatrics 2001;108:E41.
41. Schoen C, Osborn R, Huynh PT, et al. On the front lines of care: primary care doctors’ office systems, experiences, and views in seven countries. Health Aff (Millwood) 25:w555–w71.
42. Rigotti NA, Quinn VP, Stevens VJ, et al. Tobacco-control policies in 11 leading managed care organizations: progress and challenges. Eff Clin Pract 2002;5:130–6.
43. Curry SJ. Organizational interventions to encourage guideline implementation. Chest 2000;118(2 Suppl):40S–6S.
44. Berg M, Meijerink Y, Gras M, et al. Feasibility first: developing public performance indicators on patient safety and clinical effectiveness for Dutch hospitals. Health Policy 2005;75:59–73.
45. Gandhi TK, Puopolo a L, Dasse P, et al. Obstacles to collaborative quality improvement: the case of ambulatory general medical care. Int J Qual Health Care 2000;12:115–23.
46. Mol A. Proving or improving: on health care research as a form of self-reflection. Qual Health Res 2006;16:405–14.
From the Massachusetts General Hospital for Children, Boston, MA (Walters, Drehmer, Nabi-Burza, Winickoff), the University of Rochester School of Medicine, Rochester, NY (Ossip), and the American Academy of Pediatrics Julius B. Richmond Center of Excellence, Elk Grove Village, IL (Whitmore, Gorzkowski). †Deceased 31 December 2015.
Abstract
- Background: Family tobacco use and exposure are significant threats to the health of children and their families. However, few pediatric clinicians address family tobacco use and exposure in a routine and effective manner. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice.
- Objective: To review the main considerations and questions that clinicians and office staff expressed during telephone training to participate in CEASE.
- Methods: This study was conducted in pediatric practices in 5 US states. Practices were recruited by the American Academy of Pediatrics (10 intervention, 10 control). Ten training calls were recorded and transcribed. The data was then coded inductively based on themes found in the transcripts.
- Results: The data revealed that clinicians and staff were concerned about prescribing, dosing, and insurance coverage of nicotine replacement therapy; motivation for and methods to help families become tobacco-free; and the impact of the intervention on practice operations.
- Conclusion: While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco-free, they expressed concerns that could threaten implementation of family tobacco control strategies.
The devastating health consequences of smoking and exposure to tobacco smoke have been well demonstrated. As declared in the 2006 Surgeon General’s Report, there is no safe level of exposure to tobacco [1]. Children are especially at risk for exposure to toxins and toxicants in tobacco smoke [1,2]. Exposure to tobacco smoke is associated with higher levels of asthma, increased risk of sudden infant death syndrome, increased rates of upper respiratory infections, and behavioral issues [3–5]. Recent research shows that over 70% of children in the United States have some level of exposure to tobacco smoke [6]; parents and other family members are commonly the cause of this exposure, especially in young children. Children and parents benefit when parents stop smoking; parent life expectancy increases by an average of 7 years [7], the risk of tobacco-related poor pregnancy outcomes is reduced, and future children are spared from exposure to tobacco smoke [8].
There is a growing movement to address tobacco use and exposure in the pediatric office setting; the 2015 American Academy of Pediatrics tobacco policy statement Clinical Practice Policy to Protect Children From Tobacco, Nicotine, and Tobacco Smoke recommends that pediatricians ask about children’s exposure to tobacco and address parental tobacco use by implementing office-wide systems to deliver advice, counseling, referral to cessation resources, and smoking cessation medication to smokers [9].
Despite significant risks of tobacco smoke exposure to children, we found in a previous paper that only 3.5% of parents in control practices received any tobacco control assistance [10]. Through a systematic and ongoing line of research, the Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice. The CEASE intervention has been successfully shown to train and equip pediatric officesfrom a distance to address family tobacco use within existing office systems [10–14]. An enhanced CEASE intervention is undergoing testing in pediatric practices in 5 US states.
One of the more innovative aspects of CEASE has been the use of training calls. In studies of CEASE, the peer-to-peer call was conducted by the principal investigator with the project leader at the practice using a train-the-trainer model. After the project leader was trained through the peer-to-peer call, the project leader then led the whole office training call, with the support of CEASE staff by phone. The training calls worked in conjunction with the other aspects of the training, as shown in the Table. The training calls for the practices provided a valuable research opportunity. We examined the concerns and issues that clinicians and office staff had about implementing an office-wide tobacco control program through a qualitative analysis of the call transcripts. This paper outlines the main considerations and questions that clinicians and office staff expressed during the training calls. Understanding the points of view of clinicians and staff will help researchers and clinical educators strengthen the design of tobacco control interventions.
Methods
Study Aims
The data for this paper were collected as part of a larger mixed-methods controlled trial. The overarching aims of the trial were to study implementation and sustainability of tobacco-control services delivered at the clinic level, to facilitate behavior change among parents and evaluate cost-per-quit among parents who smoke, and to study systems changes and the processes that affect them at the practice level. The study was conducted in 5 intervention and 5 control pediatric primary care practices in 5 states; this paper reports on data collected in intervention practices and focuses on understanding the systems changes and processes that are instituted when implementing a tobacco control program at the clinician and practice level.
Practice Recruitment and Eligibility
Practices were recruited through the American Academy of Pediatrics using direct emails, newsletter/listserv articles, phone calls to members, and in-person recruitment at national meetings. Eligible practices were located in a non–hospital-based setting, had an average patient flow of at least 50 patients per day, used an electronic medical record (EMR) system, and were matched in each state based on practice size and smoking rate. Interested practices also had to be willing to host a research assistant to collect exit interview data from parents. Practices were excluded if they took part in previous CEASE studies or were actively enrolling participants into other tobacco control research studies. Based on these criteria, 18 eligible practices from Indiana, North Carolina, Ohio, Tennessee, Michigan, and Virginia agreed to participate in the study. Of the 6 states, one state was chosen as a replacement state. Five practices from the remaining states were assigned to the intervention group, 5 to the control group, and 5 were assigned to the replacement group in case an intervention or control practice in their state withdrew from the study. Each intervention practice participated in a peer-to-peer training call and a whole office training call. Data analyzed in this paper was collected from all 10 intervention practice training calls.
Training Calls Data Collection
The peer-to-peer and whole office training calls were recorded and transcribed. Permission to record the calls was requested by the trainer (the principal investigator of the study) and given verbally by each person being trained. The training call recordings were then transcribed verbatim by a commercial service; the transcriptions were spot-checked for accuracy.
The transcripts were first read closely by the first author (BHW), then coded inductively into relevant themes that emerged from the calls. The inductive coding was guided by the questions and concerns that the clinicians raised during the training, as well as the ways in which the trainer addressed these concerns and tailored the training to the needs and interests of the pediatric clinicians [26]. The coding was reviewed and confirmed by the other study team members.
After the data were coded into themes, the coded data were analyzed by the first author using qualitative description. Qualitative description is a method of analyzing coded qualitative data by looking at the words and meanings expressed by respondents [27]. Through this method of analysis, we were able to understand what concerns the clinicians and staff voiced about aspects of the CEASE intervention.
Ethics
The study was approved institutional review boards at Massachusetts General Hospital, the AAP, and the health care practices that required local IRB approval. The quotes used in this paper have been anonymized and cleaned to remove any identifying information, such as location and names.
Peer-to-Peer Training Calls
The peer-to-peer training calls were conducted after training and study materials arrived. The project leader (a pediatrician in the practice who was interested in spearheading the CEASE intervention) was asked to watch the training video. Using an evidence-based, previously developed call script [28], the principal investigator trained the project leader in key aspects of addressing family tobacco use and exposure, such as using an electronic tablet screener survey to identify family members who smoke, exploring techniques for prescribing or recommending NRT, and identifying ways to connect family members to free tobacco cessation counseling and support services. On occasion, other staff from the pediatric office (eg, a nurse or office manager) joined the call.
The principal investigator presented information, clarified points in the video, explained the materials, and asked questions and elicited relevant experiences from the project leader. In addition to teaching the project leader about the tobacco control strategies used in CEASE, the peer-to-peer calls prepared the project leader to train the rest of their own practice clinicians and staff in the CEASE intervention.
Whole Office Training Calls
Each practice’s local project leader led the whole office training calls, but CEASE study staff were on the call to introduce themselves to office staff, answer any questions that staff may have raised that the project leader could not answer, give information about data collection, and to generally support the implementation of the CEASE intervention and research program. During this call, the project leader watched the video with the group and tailored the training for his or her practice, focusing on issues of relevance for patients and staff.
Training Calls as Research Data
As many practices struggle with research burden [29], finding innovative and unobtrusive methods of collecting data is especially useful for research teams and participating practices. During both calls, clinicians and staff were asked open-ended questions to learn about their concerns regarding intervention implementation, share their own experiences with tobacco and tobacco control, and explore practice-specific methods to address family smoking. CEASE staff used this opportunity to help practices tailor the intervention to the local setting, such as by offering quitline enrollment sheets in another language. Clinician and staff answers to open-ended questions provided qualitative data for this manuscript.
Results and Discussion
The research team used training call data to explore clinician and staff concerns and desires related to family-centered tobacco control. The most common themes were: (1) prescribing, dosing, and insurance coverage of NRT, (2) motivation for and methods to help families become tobacco-free, and (3) the impact of the CEASE intervention on the day-to-day operations of the practice.
Nicotine Replacement Therapy
Prescribing or recommending NRT is one of the best ways to help families become tobacco-free and is a crucial component of the CEASE intervention [30–32]. Through the telephone trainings, clinicians and staff were trained to prescribe NRT using pre-printed prescription sheets, presented information about the effectiveness of NRT for smoking cessation, and referred to an information sheet on NRT to answer other questions as needed.
During the calls, it became clear that the pediatric clinicians were interested in prescribing NRT to help smokers quit, but lacked the skills and knowledge to do so:
I’m writing all this down [about NRT], because I don’t know any of this. (IN peer-to-peer)
Is 4 mg the strongest the gum comes in? (NC whole office)
This lack of knowledge may be a barrier to prescribing NRT in the pediatric setting. A national survey revealed that while smoking parents would accept prescriptions for NRT from their child’s doctor, very few received a prescription [33]. The calls provided an opportunity to have clinicians’ questions about NRT be answered by a pediatric tobacco control expert.
Clinicians were interested in helping parents stop smoking with medication, but were worried about access to medication; one of the most common questions voiced was not about how or why to prescribe NRT but how to help low-income parents get NRT for free or low-cost.
Some people—they don’t have insurance, so, how much it costs, they need to know that. (TN peer-to-peer)
I just know I’ve got a bunch ... Obamacare doesn’t work down here, so—I’ve still got families who don’t have any insurance, and you’re like, “Oh, I was hoping you could get something,” and they’re like, “Well, we can’t.” I have a fair number of kids who—are on some type of insurance, but the parents don’t have any coverage for NRT. (VA peer-to-peer)
While NRT is covered under the Affordable Care Act, many states have not expanded their Medicaid coverage [34]; this leaves many low-income families without access to health insurance or to free or low-cost NRT. While NRT remains one of the best and most common smoking cessation tools [35] there was no way to reassure practices that parents would be able to obtain the prescribed NRT without guaranteed coverage. In a previous study, the cost of NRT was seen by smokers as a barrier to using NRT to quit smoking [32]. Clinicians’ concerns about the cost of NRT reveal an understanding of the needs and issues relevant to their patient population.
Motivation for and Methods to Help Families Become Tobacco-Free
Clinicians and office staff were motivated to help families become tobacco-free and were interested in various ways to do so. The motivation and interest were personal, clinical, and organizational, relating to the ways in which care in the pediatric office could be altered to address tobacco in a more systematic way.
Motivation
The interest in smoking cessation stems from the desire to protect children from the harmful effects tobacco smoke and to prevent children themselves from taking up smoking:
We’d always talked about the smoking, and the parents finally quit. Probably not like I helped them—I just had been harping on them—but by that point the boy was smoking. When he was little he was like, “Oh, that’s nasty. I can’t believe my parents smoke.” Then by the time he was 14-15 and the parents actually did manage to quit, he was smoking, and I was like, “Ugh, really?” (VA peer-to-peer)
I totally understand the dire need for this project, in both the tobacco in the households, as well as the teenagers smoking. I heard one stat[istic], that one of our high schools had 80% of children using tobacco products… And that’s on my watch… I understand and I share the same passion that you do, for personal reasons, as well as reasons to help the whole community. (NC peer-to-peer)
Pediatricians saw themselves as responsible for protecting children’s health through reducing their tobacco smoke exposure, for working to prevent teen smoking, and for the overall health of their communities. Helping prevent childhood exposure to tobacco smoke and teen smoking initiation are crucial tasks for pediatricians; the 2015 AAP tobacco policy statement strongly recommends that pediatric offices include tobacco use prevention messages when talking to children and teens to help prevent smoking initiation, as well as helping families establish smoking bans for homes and cars [36]. By participating in the CEASE telephone trainings, clinicians and office staff were learning skills and tools to help them act on their motivation to protect families from the harms of tobacco.
Strategies
Pediatricians and office staff were interested in learning specific strategies and tools to help parents stop smoking. Practices wanted to know how and when to set a quit date with families, how to use services to help families become smoke-free, and how to tailor assistance to specific populations.
Yeah, we’re wondering about other languages, because we do have a large Hispanic patient population and a sizable group of folks that come from Saudi Arabia, and I know that some of them do smoke. (TN peer-to-peer)
Set[ting] a quit date for the patient —so how long we want to set the date? 6 months, 3 months, 1 year, 2 years, what? (TN peer-to-peer)
If you have a mom who lives with grandma and grandpa, the mom may not smoke but grandma and grandpa smoke, but they still live in that home… But anyone who comes in, we’re going to help. Does that sound right? (VA peer-to-peer)
By participating in the study, the clinicians and office staff were actively seeking to improve their knowledge of tobacco-related issues; past research has shown that pediatric residents saw lack of training in tobacco control as a key reason for inconsistent tobacco control outreach and intervention [37]. The training calls were an opportunity to gain information more specifically related to the pediatric practice’s population and office setting, building upon the other CEASE training materials. The training calls were also a chance for the CEASE research team to adapt strategies and tools to the practices, for example by providing materials that met the practices’ needs.
Impact of Intervention on Day-To-Day Operations
The training calls revealed that integrating CEASE into office workflows was a major concern. Integrating preventive services into routine office practice is a frequent concern of primary care providers [38–41]. These concerns about office flow reflect worries about financing [42] and benchmarking [43–45].
I think they’re going to have some of the same questions [that I initially had] in terms of how this might work with workflow. But as we’ve talked through all of this, I think we can make it work, and make it just sort of incorporated as part of our everyday questions that we ask. And it shouldn’t really slow things down. And I think that’ll be the main thing the providers would be focusing on is, how’s this going to impact me and all the other things I have to do in the course of a visit? This [phone call] answers a lot of questions I had in terms of that. (IN peer-to-peer)
As wait time was a performance measure for many of the practices, the clinicians and staff were hesitant to add any activities to check-in that might increase wait time.
I know, so especially, we’re trying to do a care team right now... don’t want them to spend too much time in the waiting room. (OH whole office)
During the calls, clinicians and office staff were asked to reflect on their practices and discuss ways that their practice would implement the CEASE intervention. This moment of reflection is a benefit of research participation, as it allows practices to improve the care they provide [46]. The calls allowed for on-the-spot tailoring of the intervention to meet the specific needs of the practice, an opportunity for the research staff and practice to work together to make the intervention fit their particular office situation and flow. Data collected from the training calls were also reviewed during the CEASE implementation process to support practices with specific concerns.
Strengths and Limitations
As these data were collected during training calls and subject to social desirability bias, the concerns raised may not be an exhaustive list of all concerns that clinicians and office staff had. However, the concerns that were raised by clinicians became a natural and essential part of the training process. As the practices’ initial concerns were identified early in the study, it was possible to address these concerns throughout the early implementation phases of CEASE. Transcribing calls and analyzing training call data as quickly as possible during the training phases of an intervention could prove beneficial for strengthening the implementation.
Dedicating the extra time and effort to record the training calls as a source of data formalized and strengthened the implementation process. By recording training calls, the study team was able to document the practices’ concerns and share them among the research team, including those who were not on training calls. This effort was a significant source of quality improvement data for the research team and helped ensure that we were responsive to the articulated needs of clinicians and practices.
Conclusion
The training call data revealed both the concerns as well as the interests of child health care clinicians in regard to addressing family tobacco use. While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco free, they expressed concerns that could threaten full implementation of family tobacco control strategies. These concerns and interests related to the coverage and affordability of NRT, integrating tobacco control strategies into the practice flow, and learning strategies to address family-wide tobacco use, such as helping grandparents quit smoking or addressing tobacco use with those who were not native English speakers. The concerns and interests of clinicians and office staff revealed that they were genuinely interested in learning ways to tailor strategies to address tobacco use for their practices and patient populations. By recording the training calls, the study team was better able to help them tailor the intervention to their practice, both during the calls and during subsequent implementation by providing new materials and additional information on subjects of concern to the practice. Carefully documenting training calls with health care practices are an ideal opportunity to collect information on issues that may impact full implementation of future interventions.
Corresponding author: Jonathan P. Winickoff, jwinickoff@mgh.harvard.edu
From the Massachusetts General Hospital for Children, Boston, MA (Walters, Drehmer, Nabi-Burza, Winickoff), the University of Rochester School of Medicine, Rochester, NY (Ossip), and the American Academy of Pediatrics Julius B. Richmond Center of Excellence, Elk Grove Village, IL (Whitmore, Gorzkowski). †Deceased 31 December 2015.
Abstract
- Background: Family tobacco use and exposure are significant threats to the health of children and their families. However, few pediatric clinicians address family tobacco use and exposure in a routine and effective manner. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice.
- Objective: To review the main considerations and questions that clinicians and office staff expressed during telephone training to participate in CEASE.
- Methods: This study was conducted in pediatric practices in 5 US states. Practices were recruited by the American Academy of Pediatrics (10 intervention, 10 control). Ten training calls were recorded and transcribed. The data was then coded inductively based on themes found in the transcripts.
- Results: The data revealed that clinicians and staff were concerned about prescribing, dosing, and insurance coverage of nicotine replacement therapy; motivation for and methods to help families become tobacco-free; and the impact of the intervention on practice operations.
- Conclusion: While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco-free, they expressed concerns that could threaten implementation of family tobacco control strategies.
The devastating health consequences of smoking and exposure to tobacco smoke have been well demonstrated. As declared in the 2006 Surgeon General’s Report, there is no safe level of exposure to tobacco [1]. Children are especially at risk for exposure to toxins and toxicants in tobacco smoke [1,2]. Exposure to tobacco smoke is associated with higher levels of asthma, increased risk of sudden infant death syndrome, increased rates of upper respiratory infections, and behavioral issues [3–5]. Recent research shows that over 70% of children in the United States have some level of exposure to tobacco smoke [6]; parents and other family members are commonly the cause of this exposure, especially in young children. Children and parents benefit when parents stop smoking; parent life expectancy increases by an average of 7 years [7], the risk of tobacco-related poor pregnancy outcomes is reduced, and future children are spared from exposure to tobacco smoke [8].
There is a growing movement to address tobacco use and exposure in the pediatric office setting; the 2015 American Academy of Pediatrics tobacco policy statement Clinical Practice Policy to Protect Children From Tobacco, Nicotine, and Tobacco Smoke recommends that pediatricians ask about children’s exposure to tobacco and address parental tobacco use by implementing office-wide systems to deliver advice, counseling, referral to cessation resources, and smoking cessation medication to smokers [9].
Despite significant risks of tobacco smoke exposure to children, we found in a previous paper that only 3.5% of parents in control practices received any tobacco control assistance [10]. Through a systematic and ongoing line of research, the Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice. The CEASE intervention has been successfully shown to train and equip pediatric officesfrom a distance to address family tobacco use within existing office systems [10–14]. An enhanced CEASE intervention is undergoing testing in pediatric practices in 5 US states.
One of the more innovative aspects of CEASE has been the use of training calls. In studies of CEASE, the peer-to-peer call was conducted by the principal investigator with the project leader at the practice using a train-the-trainer model. After the project leader was trained through the peer-to-peer call, the project leader then led the whole office training call, with the support of CEASE staff by phone. The training calls worked in conjunction with the other aspects of the training, as shown in the Table. The training calls for the practices provided a valuable research opportunity. We examined the concerns and issues that clinicians and office staff had about implementing an office-wide tobacco control program through a qualitative analysis of the call transcripts. This paper outlines the main considerations and questions that clinicians and office staff expressed during the training calls. Understanding the points of view of clinicians and staff will help researchers and clinical educators strengthen the design of tobacco control interventions.
Methods
Study Aims
The data for this paper were collected as part of a larger mixed-methods controlled trial. The overarching aims of the trial were to study implementation and sustainability of tobacco-control services delivered at the clinic level, to facilitate behavior change among parents and evaluate cost-per-quit among parents who smoke, and to study systems changes and the processes that affect them at the practice level. The study was conducted in 5 intervention and 5 control pediatric primary care practices in 5 states; this paper reports on data collected in intervention practices and focuses on understanding the systems changes and processes that are instituted when implementing a tobacco control program at the clinician and practice level.
Practice Recruitment and Eligibility
Practices were recruited through the American Academy of Pediatrics using direct emails, newsletter/listserv articles, phone calls to members, and in-person recruitment at national meetings. Eligible practices were located in a non–hospital-based setting, had an average patient flow of at least 50 patients per day, used an electronic medical record (EMR) system, and were matched in each state based on practice size and smoking rate. Interested practices also had to be willing to host a research assistant to collect exit interview data from parents. Practices were excluded if they took part in previous CEASE studies or were actively enrolling participants into other tobacco control research studies. Based on these criteria, 18 eligible practices from Indiana, North Carolina, Ohio, Tennessee, Michigan, and Virginia agreed to participate in the study. Of the 6 states, one state was chosen as a replacement state. Five practices from the remaining states were assigned to the intervention group, 5 to the control group, and 5 were assigned to the replacement group in case an intervention or control practice in their state withdrew from the study. Each intervention practice participated in a peer-to-peer training call and a whole office training call. Data analyzed in this paper was collected from all 10 intervention practice training calls.
Training Calls Data Collection
The peer-to-peer and whole office training calls were recorded and transcribed. Permission to record the calls was requested by the trainer (the principal investigator of the study) and given verbally by each person being trained. The training call recordings were then transcribed verbatim by a commercial service; the transcriptions were spot-checked for accuracy.
The transcripts were first read closely by the first author (BHW), then coded inductively into relevant themes that emerged from the calls. The inductive coding was guided by the questions and concerns that the clinicians raised during the training, as well as the ways in which the trainer addressed these concerns and tailored the training to the needs and interests of the pediatric clinicians [26]. The coding was reviewed and confirmed by the other study team members.
After the data were coded into themes, the coded data were analyzed by the first author using qualitative description. Qualitative description is a method of analyzing coded qualitative data by looking at the words and meanings expressed by respondents [27]. Through this method of analysis, we were able to understand what concerns the clinicians and staff voiced about aspects of the CEASE intervention.
Ethics
The study was approved institutional review boards at Massachusetts General Hospital, the AAP, and the health care practices that required local IRB approval. The quotes used in this paper have been anonymized and cleaned to remove any identifying information, such as location and names.
Peer-to-Peer Training Calls
The peer-to-peer training calls were conducted after training and study materials arrived. The project leader (a pediatrician in the practice who was interested in spearheading the CEASE intervention) was asked to watch the training video. Using an evidence-based, previously developed call script [28], the principal investigator trained the project leader in key aspects of addressing family tobacco use and exposure, such as using an electronic tablet screener survey to identify family members who smoke, exploring techniques for prescribing or recommending NRT, and identifying ways to connect family members to free tobacco cessation counseling and support services. On occasion, other staff from the pediatric office (eg, a nurse or office manager) joined the call.
The principal investigator presented information, clarified points in the video, explained the materials, and asked questions and elicited relevant experiences from the project leader. In addition to teaching the project leader about the tobacco control strategies used in CEASE, the peer-to-peer calls prepared the project leader to train the rest of their own practice clinicians and staff in the CEASE intervention.
Whole Office Training Calls
Each practice’s local project leader led the whole office training calls, but CEASE study staff were on the call to introduce themselves to office staff, answer any questions that staff may have raised that the project leader could not answer, give information about data collection, and to generally support the implementation of the CEASE intervention and research program. During this call, the project leader watched the video with the group and tailored the training for his or her practice, focusing on issues of relevance for patients and staff.
Training Calls as Research Data
As many practices struggle with research burden [29], finding innovative and unobtrusive methods of collecting data is especially useful for research teams and participating practices. During both calls, clinicians and staff were asked open-ended questions to learn about their concerns regarding intervention implementation, share their own experiences with tobacco and tobacco control, and explore practice-specific methods to address family smoking. CEASE staff used this opportunity to help practices tailor the intervention to the local setting, such as by offering quitline enrollment sheets in another language. Clinician and staff answers to open-ended questions provided qualitative data for this manuscript.
Results and Discussion
The research team used training call data to explore clinician and staff concerns and desires related to family-centered tobacco control. The most common themes were: (1) prescribing, dosing, and insurance coverage of NRT, (2) motivation for and methods to help families become tobacco-free, and (3) the impact of the CEASE intervention on the day-to-day operations of the practice.
Nicotine Replacement Therapy
Prescribing or recommending NRT is one of the best ways to help families become tobacco-free and is a crucial component of the CEASE intervention [30–32]. Through the telephone trainings, clinicians and staff were trained to prescribe NRT using pre-printed prescription sheets, presented information about the effectiveness of NRT for smoking cessation, and referred to an information sheet on NRT to answer other questions as needed.
During the calls, it became clear that the pediatric clinicians were interested in prescribing NRT to help smokers quit, but lacked the skills and knowledge to do so:
I’m writing all this down [about NRT], because I don’t know any of this. (IN peer-to-peer)
Is 4 mg the strongest the gum comes in? (NC whole office)
This lack of knowledge may be a barrier to prescribing NRT in the pediatric setting. A national survey revealed that while smoking parents would accept prescriptions for NRT from their child’s doctor, very few received a prescription [33]. The calls provided an opportunity to have clinicians’ questions about NRT be answered by a pediatric tobacco control expert.
Clinicians were interested in helping parents stop smoking with medication, but were worried about access to medication; one of the most common questions voiced was not about how or why to prescribe NRT but how to help low-income parents get NRT for free or low-cost.
Some people—they don’t have insurance, so, how much it costs, they need to know that. (TN peer-to-peer)
I just know I’ve got a bunch ... Obamacare doesn’t work down here, so—I’ve still got families who don’t have any insurance, and you’re like, “Oh, I was hoping you could get something,” and they’re like, “Well, we can’t.” I have a fair number of kids who—are on some type of insurance, but the parents don’t have any coverage for NRT. (VA peer-to-peer)
While NRT is covered under the Affordable Care Act, many states have not expanded their Medicaid coverage [34]; this leaves many low-income families without access to health insurance or to free or low-cost NRT. While NRT remains one of the best and most common smoking cessation tools [35] there was no way to reassure practices that parents would be able to obtain the prescribed NRT without guaranteed coverage. In a previous study, the cost of NRT was seen by smokers as a barrier to using NRT to quit smoking [32]. Clinicians’ concerns about the cost of NRT reveal an understanding of the needs and issues relevant to their patient population.
Motivation for and Methods to Help Families Become Tobacco-Free
Clinicians and office staff were motivated to help families become tobacco-free and were interested in various ways to do so. The motivation and interest were personal, clinical, and organizational, relating to the ways in which care in the pediatric office could be altered to address tobacco in a more systematic way.
Motivation
The interest in smoking cessation stems from the desire to protect children from the harmful effects tobacco smoke and to prevent children themselves from taking up smoking:
We’d always talked about the smoking, and the parents finally quit. Probably not like I helped them—I just had been harping on them—but by that point the boy was smoking. When he was little he was like, “Oh, that’s nasty. I can’t believe my parents smoke.” Then by the time he was 14-15 and the parents actually did manage to quit, he was smoking, and I was like, “Ugh, really?” (VA peer-to-peer)
I totally understand the dire need for this project, in both the tobacco in the households, as well as the teenagers smoking. I heard one stat[istic], that one of our high schools had 80% of children using tobacco products… And that’s on my watch… I understand and I share the same passion that you do, for personal reasons, as well as reasons to help the whole community. (NC peer-to-peer)
Pediatricians saw themselves as responsible for protecting children’s health through reducing their tobacco smoke exposure, for working to prevent teen smoking, and for the overall health of their communities. Helping prevent childhood exposure to tobacco smoke and teen smoking initiation are crucial tasks for pediatricians; the 2015 AAP tobacco policy statement strongly recommends that pediatric offices include tobacco use prevention messages when talking to children and teens to help prevent smoking initiation, as well as helping families establish smoking bans for homes and cars [36]. By participating in the CEASE telephone trainings, clinicians and office staff were learning skills and tools to help them act on their motivation to protect families from the harms of tobacco.
Strategies
Pediatricians and office staff were interested in learning specific strategies and tools to help parents stop smoking. Practices wanted to know how and when to set a quit date with families, how to use services to help families become smoke-free, and how to tailor assistance to specific populations.
Yeah, we’re wondering about other languages, because we do have a large Hispanic patient population and a sizable group of folks that come from Saudi Arabia, and I know that some of them do smoke. (TN peer-to-peer)
Set[ting] a quit date for the patient —so how long we want to set the date? 6 months, 3 months, 1 year, 2 years, what? (TN peer-to-peer)
If you have a mom who lives with grandma and grandpa, the mom may not smoke but grandma and grandpa smoke, but they still live in that home… But anyone who comes in, we’re going to help. Does that sound right? (VA peer-to-peer)
By participating in the study, the clinicians and office staff were actively seeking to improve their knowledge of tobacco-related issues; past research has shown that pediatric residents saw lack of training in tobacco control as a key reason for inconsistent tobacco control outreach and intervention [37]. The training calls were an opportunity to gain information more specifically related to the pediatric practice’s population and office setting, building upon the other CEASE training materials. The training calls were also a chance for the CEASE research team to adapt strategies and tools to the practices, for example by providing materials that met the practices’ needs.
Impact of Intervention on Day-To-Day Operations
The training calls revealed that integrating CEASE into office workflows was a major concern. Integrating preventive services into routine office practice is a frequent concern of primary care providers [38–41]. These concerns about office flow reflect worries about financing [42] and benchmarking [43–45].
I think they’re going to have some of the same questions [that I initially had] in terms of how this might work with workflow. But as we’ve talked through all of this, I think we can make it work, and make it just sort of incorporated as part of our everyday questions that we ask. And it shouldn’t really slow things down. And I think that’ll be the main thing the providers would be focusing on is, how’s this going to impact me and all the other things I have to do in the course of a visit? This [phone call] answers a lot of questions I had in terms of that. (IN peer-to-peer)
As wait time was a performance measure for many of the practices, the clinicians and staff were hesitant to add any activities to check-in that might increase wait time.
I know, so especially, we’re trying to do a care team right now... don’t want them to spend too much time in the waiting room. (OH whole office)
During the calls, clinicians and office staff were asked to reflect on their practices and discuss ways that their practice would implement the CEASE intervention. This moment of reflection is a benefit of research participation, as it allows practices to improve the care they provide [46]. The calls allowed for on-the-spot tailoring of the intervention to meet the specific needs of the practice, an opportunity for the research staff and practice to work together to make the intervention fit their particular office situation and flow. Data collected from the training calls were also reviewed during the CEASE implementation process to support practices with specific concerns.
Strengths and Limitations
As these data were collected during training calls and subject to social desirability bias, the concerns raised may not be an exhaustive list of all concerns that clinicians and office staff had. However, the concerns that were raised by clinicians became a natural and essential part of the training process. As the practices’ initial concerns were identified early in the study, it was possible to address these concerns throughout the early implementation phases of CEASE. Transcribing calls and analyzing training call data as quickly as possible during the training phases of an intervention could prove beneficial for strengthening the implementation.
Dedicating the extra time and effort to record the training calls as a source of data formalized and strengthened the implementation process. By recording training calls, the study team was able to document the practices’ concerns and share them among the research team, including those who were not on training calls. This effort was a significant source of quality improvement data for the research team and helped ensure that we were responsive to the articulated needs of clinicians and practices.
Conclusion
The training call data revealed both the concerns as well as the interests of child health care clinicians in regard to addressing family tobacco use. While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco free, they expressed concerns that could threaten full implementation of family tobacco control strategies. These concerns and interests related to the coverage and affordability of NRT, integrating tobacco control strategies into the practice flow, and learning strategies to address family-wide tobacco use, such as helping grandparents quit smoking or addressing tobacco use with those who were not native English speakers. The concerns and interests of clinicians and office staff revealed that they were genuinely interested in learning ways to tailor strategies to address tobacco use for their practices and patient populations. By recording the training calls, the study team was better able to help them tailor the intervention to their practice, both during the calls and during subsequent implementation by providing new materials and additional information on subjects of concern to the practice. Carefully documenting training calls with health care practices are an ideal opportunity to collect information on issues that may impact full implementation of future interventions.
Corresponding author: Jonathan P. Winickoff, jwinickoff@mgh.harvard.edu
1. U.S. Department of Health and Human Services. The health consequences of involuntary tobacco smoke: a report of the Surgeon General. 2006.
2. Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol 2004;26:373–85.
3. Polanska K, Hanke W, Ronchetti R, et al. Environmental tobacco smoke exposure and children’s health. Acta Paediatr Suppl 2006;95:86–92.
4. American Academy of Pediatrics, Committee on Substance Abuse. Tobacco’s toll: implications for the pediatrician. Pediatrics 2001;107:794–8.
5. U.S. Department of Health and Human Services. Children and secondhand smoke exposure. Excerpts from the health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2007.
6. Wilson KM, Klein JD, Blumkin AK, et al. Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics 2011;127:85–92.
7. Taylor SM, Ross NA, Cummings KM, et al. Community intervention trial for smoking cessation (COMMIT): changes in community attitudes toward cigarette smoking. Health Educ Res 1998;13:109-22.
8. Winickoff JP, Healey EA, Regan S, et al. Using the postpartum hospital stay to address mothers’ and fathers’ smoking: the NEWS study. Pediatrics 2010;125:518–25.
9. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:1008–17.
10. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132:109–17.
11. Ossip DJ, Chang Y, Nabi-Burza E, et al. Strict smoke-free home policies among smoking parents in pediatric settings. Acad Pediatr 2013;13:517–23.
12. Winickoff JP, Park ER, Hipple BJ, et al. Clinical effort against secondhand smoke exposure: development of framework and intervention. Pediatrics 2008;122:e363–e75.
13. Nabi-Burza E, Winickoff JP, Finch S, Regan S. Triple tobacco screen: opportunity to help families become smokefree. Am J Prev Med 2013;45:728–31.
14. Winickoff JP. Pediatrician-led program increases provision of smoking cessation support, boosts quit rates among parents. Innovations in Medicine 2011. Accessed 24 Nov 2015 at https://innovations.ahrq.gov/profiles/pediatrician-led-program-increases-provision-smoking-cessation-support-boosts-quit-rates.
15. Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2000.
16. Winickoff JP, Nabi-Burza E, Chang Y, et al. Sustainability of a parental tobacco control intervention in pediatric practice. Pediatrics 2014;134:933-41.
17. Moore D, Aveyard P, Connock M, et al. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 2009;338:b1024.
18. Aveyard P, Wang D, Connock M, et al. Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: floating prolonged abstinence. Nicotine Tob Res 2009;11:475–80.
19. Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav 2011;36:764–8.
20.Curry SJ, Grothaus LC, McAfee T, Pabiniak C. Use and cost effectiveness of smoking-cessation services under four insurance plans in a health maintenance organization. N Engl J Med 1998;339:673–9.
21. Abroms LC, Ahuja M, Kodl Y, et al. Text2Quit: Results from a pilot test of a personalized, interactive mobile health smoking cessation program. J Health Commun 2012;17 Suppl 1:44-53.
22. Curry SJ, Ludman EJ, Graham E, et al. Pediatric-based smoking cessation intervention for low-income women: a randomized trial. Arch Pediatr Adolesc Med 2003;157:295–302.
23. Orleans CT, Schoenbach VJ, Wagner EH. Self-help quit smoking interventions: effects of self-help materials, social support materials, social support instructions and telephone counseling. J Consult Clin Psychol 1991;59:439–48.
24. An LC, Zhu SH, Nelson DB, et al. Benefits of telephone care over primary care for smoking cessation: a randomized trial. Arch Intern Med 2006;166:536–42.
25. Warner DO, Klesges RC, Dale LC, et al. Clinician-delivered intervention to facilitate tobacco quitline use by surgical patients. Anesthesiology 2011;114:847–55.
26. Creswell, JW. Qualitative inquiry and research design: choosing among five approaches. 2nd ed. Thousand Oaks, CA: Sage; 2007.
27. Sandelowski M. Focus on research methods: whatever happened to qualitative description. Res Nurs Health 2000;23:334–40.
28. Winickoff JP, Hipple B, Drehmer J, et al. The clinical effort against secondhand smoke exposure (CEASE) intervention: A decade of lessons learned. J Clin Outcomes Manag 2012;19:414–9.
29. Clark T, Sinclair R. The costs and benefits of acting as a research site. Evid Policy A J Res Debate Pract 2008;4:105–19.
30. Zhu S, Melcer T, Sun J. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med 2000;18:305–11.
31. Gilpin EA, Messer K, Pierce JP. Population effectiveness of pharmaceutical aids for smoking cessation: what is associated with increased success? Nicotine Tob Res 2006;8:661–9.
32. Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med 2005;28:119–22.
33. Winickoff JP, Tanski SE, McMillen RC, et al. Child health care clinicians’ use of medications to help parents quit smoking: a national parent survey. Pediatrics 2005;115:1013–7.
34. Kaiser Family Foundation. Status of state action on the medicaid expansion decision. Available at http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/.
35. U.S. Department of Health and Human Services. The health consequences of smoking- 50 years of progress: a report of the Surgeon General. 2014.
36. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Public policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:998–1007.
37. Collins BN, Levin KP, Bryant-Stephens T. Pediatricians’ practices and attitudes about environmental tobacco smoke and parental smoking. J Pediatr 2007;150:547–52.
38. Leininger LS, Finn L, Dickey L, et al. An office system for organizing preventive services: a report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems. Arch Fam Med 1996;5:108–15.
39. Swartz SH, Hays JT. Office-based intervention for tobacco dependence. Med Clin North Am 2004;88:1623–41.
40. Bordley WC, Margolis PA, Stuart J, et al. Improving preventive service delivery through office systems. Pediatrics 2001;108:E41.
41. Schoen C, Osborn R, Huynh PT, et al. On the front lines of care: primary care doctors’ office systems, experiences, and views in seven countries. Health Aff (Millwood) 25:w555–w71.
42. Rigotti NA, Quinn VP, Stevens VJ, et al. Tobacco-control policies in 11 leading managed care organizations: progress and challenges. Eff Clin Pract 2002;5:130–6.
43. Curry SJ. Organizational interventions to encourage guideline implementation. Chest 2000;118(2 Suppl):40S–6S.
44. Berg M, Meijerink Y, Gras M, et al. Feasibility first: developing public performance indicators on patient safety and clinical effectiveness for Dutch hospitals. Health Policy 2005;75:59–73.
45. Gandhi TK, Puopolo a L, Dasse P, et al. Obstacles to collaborative quality improvement: the case of ambulatory general medical care. Int J Qual Health Care 2000;12:115–23.
46. Mol A. Proving or improving: on health care research as a form of self-reflection. Qual Health Res 2006;16:405–14.
1. U.S. Department of Health and Human Services. The health consequences of involuntary tobacco smoke: a report of the Surgeon General. 2006.
2. Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol 2004;26:373–85.
3. Polanska K, Hanke W, Ronchetti R, et al. Environmental tobacco smoke exposure and children’s health. Acta Paediatr Suppl 2006;95:86–92.
4. American Academy of Pediatrics, Committee on Substance Abuse. Tobacco’s toll: implications for the pediatrician. Pediatrics 2001;107:794–8.
5. U.S. Department of Health and Human Services. Children and secondhand smoke exposure. Excerpts from the health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2007.
6. Wilson KM, Klein JD, Blumkin AK, et al. Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics 2011;127:85–92.
7. Taylor SM, Ross NA, Cummings KM, et al. Community intervention trial for smoking cessation (COMMIT): changes in community attitudes toward cigarette smoking. Health Educ Res 1998;13:109-22.
8. Winickoff JP, Healey EA, Regan S, et al. Using the postpartum hospital stay to address mothers’ and fathers’ smoking: the NEWS study. Pediatrics 2010;125:518–25.
9. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:1008–17.
10. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132:109–17.
11. Ossip DJ, Chang Y, Nabi-Burza E, et al. Strict smoke-free home policies among smoking parents in pediatric settings. Acad Pediatr 2013;13:517–23.
12. Winickoff JP, Park ER, Hipple BJ, et al. Clinical effort against secondhand smoke exposure: development of framework and intervention. Pediatrics 2008;122:e363–e75.
13. Nabi-Burza E, Winickoff JP, Finch S, Regan S. Triple tobacco screen: opportunity to help families become smokefree. Am J Prev Med 2013;45:728–31.
14. Winickoff JP. Pediatrician-led program increases provision of smoking cessation support, boosts quit rates among parents. Innovations in Medicine 2011. Accessed 24 Nov 2015 at https://innovations.ahrq.gov/profiles/pediatrician-led-program-increases-provision-smoking-cessation-support-boosts-quit-rates.
15. Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2000.
16. Winickoff JP, Nabi-Burza E, Chang Y, et al. Sustainability of a parental tobacco control intervention in pediatric practice. Pediatrics 2014;134:933-41.
17. Moore D, Aveyard P, Connock M, et al. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 2009;338:b1024.
18. Aveyard P, Wang D, Connock M, et al. Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: floating prolonged abstinence. Nicotine Tob Res 2009;11:475–80.
19. Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav 2011;36:764–8.
20.Curry SJ, Grothaus LC, McAfee T, Pabiniak C. Use and cost effectiveness of smoking-cessation services under four insurance plans in a health maintenance organization. N Engl J Med 1998;339:673–9.
21. Abroms LC, Ahuja M, Kodl Y, et al. Text2Quit: Results from a pilot test of a personalized, interactive mobile health smoking cessation program. J Health Commun 2012;17 Suppl 1:44-53.
22. Curry SJ, Ludman EJ, Graham E, et al. Pediatric-based smoking cessation intervention for low-income women: a randomized trial. Arch Pediatr Adolesc Med 2003;157:295–302.
23. Orleans CT, Schoenbach VJ, Wagner EH. Self-help quit smoking interventions: effects of self-help materials, social support materials, social support instructions and telephone counseling. J Consult Clin Psychol 1991;59:439–48.
24. An LC, Zhu SH, Nelson DB, et al. Benefits of telephone care over primary care for smoking cessation: a randomized trial. Arch Intern Med 2006;166:536–42.
25. Warner DO, Klesges RC, Dale LC, et al. Clinician-delivered intervention to facilitate tobacco quitline use by surgical patients. Anesthesiology 2011;114:847–55.
26. Creswell, JW. Qualitative inquiry and research design: choosing among five approaches. 2nd ed. Thousand Oaks, CA: Sage; 2007.
27. Sandelowski M. Focus on research methods: whatever happened to qualitative description. Res Nurs Health 2000;23:334–40.
28. Winickoff JP, Hipple B, Drehmer J, et al. The clinical effort against secondhand smoke exposure (CEASE) intervention: A decade of lessons learned. J Clin Outcomes Manag 2012;19:414–9.
29. Clark T, Sinclair R. The costs and benefits of acting as a research site. Evid Policy A J Res Debate Pract 2008;4:105–19.
30. Zhu S, Melcer T, Sun J. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med 2000;18:305–11.
31. Gilpin EA, Messer K, Pierce JP. Population effectiveness of pharmaceutical aids for smoking cessation: what is associated with increased success? Nicotine Tob Res 2006;8:661–9.
32. Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med 2005;28:119–22.
33. Winickoff JP, Tanski SE, McMillen RC, et al. Child health care clinicians’ use of medications to help parents quit smoking: a national parent survey. Pediatrics 2005;115:1013–7.
34. Kaiser Family Foundation. Status of state action on the medicaid expansion decision. Available at http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/.
35. U.S. Department of Health and Human Services. The health consequences of smoking- 50 years of progress: a report of the Surgeon General. 2014.
36. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Public policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:998–1007.
37. Collins BN, Levin KP, Bryant-Stephens T. Pediatricians’ practices and attitudes about environmental tobacco smoke and parental smoking. J Pediatr 2007;150:547–52.
38. Leininger LS, Finn L, Dickey L, et al. An office system for organizing preventive services: a report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems. Arch Fam Med 1996;5:108–15.
39. Swartz SH, Hays JT. Office-based intervention for tobacco dependence. Med Clin North Am 2004;88:1623–41.
40. Bordley WC, Margolis PA, Stuart J, et al. Improving preventive service delivery through office systems. Pediatrics 2001;108:E41.
41. Schoen C, Osborn R, Huynh PT, et al. On the front lines of care: primary care doctors’ office systems, experiences, and views in seven countries. Health Aff (Millwood) 25:w555–w71.
42. Rigotti NA, Quinn VP, Stevens VJ, et al. Tobacco-control policies in 11 leading managed care organizations: progress and challenges. Eff Clin Pract 2002;5:130–6.
43. Curry SJ. Organizational interventions to encourage guideline implementation. Chest 2000;118(2 Suppl):40S–6S.
44. Berg M, Meijerink Y, Gras M, et al. Feasibility first: developing public performance indicators on patient safety and clinical effectiveness for Dutch hospitals. Health Policy 2005;75:59–73.
45. Gandhi TK, Puopolo a L, Dasse P, et al. Obstacles to collaborative quality improvement: the case of ambulatory general medical care. Int J Qual Health Care 2000;12:115–23.
46. Mol A. Proving or improving: on health care research as a form of self-reflection. Qual Health Res 2006;16:405–14.