User login
Effect of Multidisciplinary Transitional Pain Service on Health Care Use and Costs Following Orthopedic Surgery
Opioid use disorder (OUD) is a significant cause of morbidity, mortality, and health care costs in the US.1,2 Surgery can be the inciting cause for exposure to an opioid; as many as 23% of patients develop chronic OUD following surgery.3,4 Patients with a history of substance use, mood disorders, anxiety, or previous chronic opioid use (COU) are at risk for relapse, dose escalation, and poor pain control after high-risk surgery, such as orthopedic joint procedures.5 Recently focus has been on identifying high-risk patients before orthopedic joint surgery and implementing evidence-based strategies that reduce the postoperative incidence of COU.
A transitional pain service (TPS) has been shown to reduce COU for high-risk surgical patients in different health care settings.6-9 The TPS model bundles multiple interventions that can be applied to patients at high risk for COU within a health care system. This includes individually tailored programs for preoperative education or pain management planning, use of multimodal analgesia (including regional or neuraxial techniques or nonopioid systemic medications), application of nonpharmacologic modalities (such as cognitive-based intervention), and a coordinated approach to postdischarge instructions and transitions of care. These interventions are coordinated by a multidisciplinary clinical service consisting of anesthesiologists and advanced practice clinicians with specialization in acute pain management and opioid tapering, nurse care coordinators, and psychologists with expertise in cognitive behavioral therapy.
TPS has been shown to reduce the incidence of COU for patients undergoing orthopedic joint surgery, but its impact on health care use and costs is unknown.6-9 The TPS intervention is resource intensive and increases the use of health care for preoperative education or pain management, which may increase the burden of costs. However, reducing long-term COU may reduce the use of health care for COU- and OUD-related complications, leading to cost savings. This study evaluated whether the TPS intervention influenced health care use and cost for inpatient, outpatient, or pharmacy services during the year following orthopedic joint surgery compared with that of the standard pain management care for procedures that place patients at high risk for COU. We used a difference-in-differences (DID) analysis to estimate this intervention effect, using multivariable regression models that controlled for unobserved time trends and cohort characteristics.
METHODS
This was a quasi-experimental study of patients who underwent orthopedic joint surgery and associated procedures at high risk for COU at the Veterans Affairs Salt Lake City Healthcare System (VASLCHS) between January 2016 through April 2020. The pre-TPS period between January 2016 through December 2017 was compared with the post-TPS period between January 2018 to September 2019. The control patient cohort was selected from 5 geographically diverse VA health care systems throughout the US: Eastern Colorado, Central Plains (Nebraska), White River Junction (Vermont), North Florida/South Georgia, and Portland (Oregon). By sampling health care costs from VA medical centers (VAMCs) across these different regions, our control group was generalizable to veterans receiving orthopedic joint surgery across the US. This study used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a repository of nearly all clinical and administrative data found in electronic health records for VA-provided care and fee-basis care paid for by the VA.10 All data were hosted and analyzed in the VA Informatics and Computing Infrastructure (VINCI) workspace. The University of Utah Institutional Review Board and the VASLCHS Office of Research and Development approved the protocol for this study.
TPS Intervention
The VASLCHS TPS has already been described in detail elsewhere.6,7 Briefly, patients at high risk for COU at the VASLCHS were enrolled in the TPS program before surgery for total knee, hip, or shoulder arthroplasty or rotator cuff procedures. The TPS service consists of an anesthesiologist and advanced practice clinician with specialization in acute pain management and opioid tapering, a psychologist with expertise in cognitive behavioral therapy, and 3 nurse care coordinators. These TPS practitioners work together to provide preoperative education, including setting expectations regarding postoperative pain, recommending nonopioid pain management strategies, and providing guidance regarding the appropriate use of opioids for surgical pain. Individual pain plans were developed and implemented for the perioperative period. After surgery, the TPS provided recommendations and support for nonopioid pain therapies and opioid tapers. Patients were followed by the TPS team for at least 12 months after surgery. At a minimum, the goals set by TPS included cessation of all opioid use for prior nonopioid users (NOU) by 90 days after surgery and the return to baseline opioid use or lower for prior COU patients by 90 days after surgery. The TPS also encouraged and supported opioid tapering among COU patients to reduce or completely stop opioid use after surgery.
Patient Cohorts
Veterans having primary or revision total knee, hip, or shoulder arthroplasty or rotator cuff repair between January 1, 2016, and September 30, 2019, at the aforementioned VAMCs were included in the study. Patients who had any hospitalization within 90 days pre- or postindex surgery or who died within 8 months after surgery were excluded from analysis. Patients who had multiple surgeries during the index inpatient visit or within 90 days after the index surgery also were excluded. Comorbid conditions for mental health and substance use were identified using the International Classification of Diseases, 10th revision Clinical Modification (ICD-10) codes or 9th revision equivalent grouped by Clinical Classifications Software Refined (CCS-R).11 Preoperative exposure to clinically relevant pharmacotherapy (ie, agents associated with prolonged opioid use and nonopioid adjuvants) was captured using VA outpatient prescription records (eAppendix 1).
Outcome Variables
Outcome variables included health care use and costs during 1-year pre- and postperiods from the date of surgery. VA health care costs for outpatient, inpatient, and pharmacy services for direct patient care were collected from the Managerial Cost Accounting System, an activity-based cost allocation system that generates estimates of the cost of individual VA hospital stays, health care encounters, and medications. Health care use was defined as the number of encounters for each visit type in the Managerial Cost Accounting System. All costs were adjusted to 2019 US dollars, using the Personal Consumption Expenditures price index for health care services.15
A set of sociodemographic variables including sex, age at surgery, race and ethnicity, rurality, military branch (Army, Air Force, Marine Corps, Navy, and other), and service connectivity were included as covariates in our regression models.
Statistical Analyses
Descriptive analyses were used to evaluate differences in baseline patient sociodemographic and clinical characteristics between pre- and postperiods for TPS intervention and control cohorts using 2-sample t tests for continuous variables and χ2 tests for categorical variables. We summarized unadjusted health care use and costs for outpatient, inpatient, and pharmacy visits and compared the pre- and postintervention periods using the Mann-Whitney test. Both mean (SD) and median (IQR) were considered, reflecting the skewed distribution of the outcome variables.
We used a DID approach to assess the intervention effect while minimizing confounding from the nonrandom sample. The DID approach controls for unobserved differences between VAMCs that are related to both the intervention and outcomes while controlling for trends over time that could affect outcomes across clinics. To implement the DID approach, we included 3 key independent variables in our regression models: (1) an indicator for whether the observation occurred in the postintervention period; (2) an indicator for whether the patient was exposed to the TPS intervention; and (3) the interaction between these 2 variables.
For cost outcomes, we used multivariable generalized linear models with a log link and a Poisson or Υ family. We analyzed inpatient costs using a 2-part generalized linear model because only 17% to 20% of patients had ≥ 1 inpatient visit. We used multivariable negative binomial regression for health care use outcomes. Demographic and clinical covariates described earlier were included in the regression models to control for differences in the composition of patient groups and clinics that could lead to confounding bias.
RESULTS
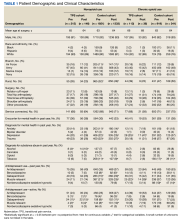
Of the 4954 patients included in our study cohort, 3545 (71.6%) were in the NOU group and 1409 (28.4%) were in the COU group. Among the NOU cohort, 361 patients were in the intervention group and 3184 in the control group. Among the COU cohort, 149 patients were in the intervention group and 1260 in the control group (Table 1). Most patients were male, White race, with a mean (SD) age of 64 (11) years. The most common orthopedic procedure was total knee arthroplasty, followed by total hip arthroplasty. Among both NOU and COU cohorts, patients’ characteristics were similar between the pre- and postintervention period among either TPS or control cohort.
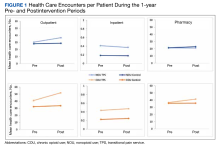
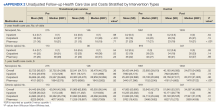
Figures 1 and 2 and eAppendix 2 depict unadjusted per-person average outpatient, inpatient, and pharmacy visits and costs incurred during the 1-year pre- and postintervention periods for the NOU and COU cohorts. Average total health care follow-up costs ranged from $40,000 to $53,000 for NOU and from $47,000 to $82,000 for COU cohort. Cost for outpatient visits accounted for about 70% of the average total costs, followed by costs for inpatient visits of about 20%, and costs for pharmacy for the remaining.
For the NOU cohort, the number of health care encounters remained fairly stable between periods except for the outpatient visits among the TPS group. The TPS group experienced an increase in mean outpatient visits in the postperiod: 30 vs 37 visits (23%) (
Table 2 summarizes the results from the multivariable DID analyses for the outpatient, inpatient, and pharmacy visit and cost outcomes. Here, the estimated effect of the TPS intervention is the coefficient from the interaction between the postintervention and TPS exposure indicator variables. This coefficient was calculated as the difference in the outcome before and after the TPS intervention among the TPS group minus the difference in the outcome before and after the TPS intervention among the control group. For the NOU cohort, TPS was associated with an increase in the use of outpatient health care (mean [SD] increase of 6.9 [2] visits; P < .001) after the surgery with no statistically significant effect on outpatient costs (mean [SD] increase of $2787 [$3749]; P = .55). There was no statistically significant effect of TPS on the use of inpatient visits or pharmacy, but a decrease in costs for inpatient visits among those who had at least 1 inpatient visit (mean [SD] decrease of $12,170 [$6100]; P = .02). For the COU cohort, TPS had no statistically significant impact on the use of outpatient, inpatient, or pharmacy or the corresponding costs.
DISCUSSION
TPS is a multidisciplinary approach to perioperative pain management that has been shown to reduce both the quantity and duration of opioid use among orthopedic surgery patients.6,7 Although the cost burden of providing TPS services to prevent COU is borne by the individual health care system, it is unclear whether this expense is offset by lower long-term medical costs and health care use for COU- and OUD-related complications. In this study focused on a veteran population undergoing orthopedic joint procedures, a DID analysis of cost and health care use showed that TPS, which has been shown to reduce COU for high-risk surgical patients, can be implemented without increasing the overall costs to the VA health care system during the 1 year following surgery, even with increased outpatient visits. For NOU patients, there was no difference in outpatient visit costs or pharmacy costs over 12 months after surgery, although there was a significant reduction in subsequent inpatient costs over the same period. Further, there was no difference in outpatient, inpatient, or pharmacy costs after surgery for COU patients. These findings suggest that TPS can be a cost-effective approach to reduce opioid use among patients undergoing orthopedic joint surgery in VAMCs.
The costs of managing COU after surgery are substantial. Prior reports have shown that adjusted total health care costs are 1.6 to 2.5 times higher for previously NOU patients with new COU after major surgery than those for such patients without persistent use.16 The 1-year costs associated with new COU in this prior study ranged between $7944 and $17,702 after inpatient surgery and between $5598 and $12,834 after outpatient index surgery, depending on the payer, which are in line with the cost differences found in our current study. Another report among patients with COU following orthopedic joint replacement showed that they had higher use of inpatient, emergency department, and ambulance/paramedic services in the 12 months following their surgery than did those without persistent use.17 Although these results highlight the impact that COU plays in driving increased costs after major surgery, there have been limited studies focused on interventions that can neutralize the costs associated with opioid misuse after surgery. To our knowledge, our study is the first analysis to show the impact of using an intervention such as TPS to reduce postoperative opioid use on health care use and cost.
Although a rigorous and comprehensive return on investment analysis was beyond the scope of this analysis, these results may have several implications for other health care systems and hospitals that wish to invest in a multidisciplinary perioperative pain management program such as TPS but may be reluctant due to the upfront investment. First, the increased number of patient follow-up visits needed during TPS seems to be more than offset by the reduction in opioid use and associated complications that may occur after surgery. Second, TPS did not seem to be associated with an increase in overall health care costs during the 1-year follow-up period. Together, these results indicate that the return on investment for a TPS approach to perioperative pain management in which optimal patient-centered outcomes are achieved without increasing long-term costs to a health care system may be positive.
Limitations
This study has several limitations. First, this was a quasi-experimental observational study, and the associations we identified between intervention and outcomes should not be assumed to demonstrate causality. Although our DID analysis controlled for an array of demographic and clinical characteristics, differences in medical costs and health care use between the 2 cohorts might be driven by unobserved confounding variables.
Our study also was limited to veterans who received medical care at the VA, and results may not be generalizable to other non-VA health care systems or to veterans with Medicare insurance who have dual benefits. While our finding on health care use and costs may be incomplete because of the uncaptured health care use outside the VA, our DID analysis helped reduce unobserved bias because the absence of data outside of VA care applies to both TPS and control groups. Further, the total costs of operating a TPS program at any given institution will depend on the size of the hospital and volume of surgical patients who meet criteria for enrollment. However, the relative differences in health care use and costs may be extrapolated to patients undergoing orthopedic surgery in other types of academic and community-based health care systems.
Furthermore, this analysis focused primarily on COU and NOU patients undergoing orthopedic joint surgery. While this represents a high-risk population for OUD, the costs and health care use associated with delivering the TPS intervention to other types of surgical procedures may be significantly different. All costs in this analysis were based on 2019 estimates and do not account for the potential inflation over the past several years. Nonmonetary costs to the patient and per-person average total intervention costs were not included in the study. However, we assumed that costs associated with TPS and standard of care would have increased to an equivalent degree over the same period. Further, the average cost of TPS per patient (approximately $900) is relatively small compared with the average annual costs during 1-year pre- and postoperative periods and was not expected to have a significant effect on the analysis.
Conclusions
We found that the significant reduction in COU seen in previous studies following the implementation of TPS was not accompanied by increased health care costs.6,7 When considering the other costs of long-term opioid use, such as abuse potential, overdose, death, and increased disability, implementation of a TPS service has the potential to improve patient quality of life while reducing other health-related costs. Health care systems should consider the implementation of similar multidisciplinary approaches to perioperative pain management to improve outcomes after orthopedic joint surgery and other high-risk procedures.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
2. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906. doi:10.1097/MLR.0000000000000625
3. Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265(4):722-727. doi:10.1097/SLA.0000000000001780
4. Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957, e3. doi:10.1016/j.jhsa.2016.07.113
5. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi:10.1001/jamasurg.2017.0504
6. Buys MJ, Bayless K, Romesser J, et al. Multidisciplinary transitional pain service for the veteran population. Fed Pract. 2020;37(10):472-478. doi:10.12788/fp.0053
7. Buys MJ, Bayless K, Romesser J, et al. Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 2020;45(11):847-852. doi:10.1136/rapm-2020-101797
8. Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag. 2015;5(2):97-105. doi:10.2217/pmt.15.7
9. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/JPR.S91924
10. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
11. Agency for Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR). Updated December 9, 2022. Accessed October 30, 2023. www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
12. Mosher HJ, Richardson KK, Lund BC. The 1-year treatment course of new opioid recipients in Veterans Health Administration. Pain Med. 2016;17(7):1282-1291. doi:10.1093/pm/pnw058
13. Hadlandsmyth K, Mosher HJ, Vander Weg MW, O’Shea AM, McCoy KD, Lund BC. Utility of accumulated opioid supply days and individual patient factors in predicting probability of transitioning to long-term opioid use: an observational study in the Veterans Health Administration. Pharmacol Res Perspect. 2020;8(2):e00571. doi:10.1002/prp2.571
14. Pagé MG, Kudrina I, Zomahoun HTV, et al. Relative frequency and risk factors for long-term opioid therapy following surgery and trauma among adults: a systematic review protocol. Syst Rev. 2018;7(1):97. doi:10.1186/s13643-018-0760-3
15. US. Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product. Accessed October 30, 2023. https://apps.bea.gov/iTable/?reqid=19&step=3&isuri=1&nipa_table_list=64&categories=survey
16. Brummett CM, Evans-Shields J, England C, et al. Increased health care costs associated with new persistent opioid use after major surgery in opioid-naive patients. J Manag Care Spec Pharm. 2021;27(6):760-771. doi:10.18553/jmcp.2021.20507
17. Gold LS, Strassels SA, Hansen RN. Health care costs and utilization in patients receiving prescriptions for long-acting opioids for acute postsurgical pain. Clin J Pain. 2016;32(9):747-754. doi:10.1097/ajp.0000000000000322
Opioid use disorder (OUD) is a significant cause of morbidity, mortality, and health care costs in the US.1,2 Surgery can be the inciting cause for exposure to an opioid; as many as 23% of patients develop chronic OUD following surgery.3,4 Patients with a history of substance use, mood disorders, anxiety, or previous chronic opioid use (COU) are at risk for relapse, dose escalation, and poor pain control after high-risk surgery, such as orthopedic joint procedures.5 Recently focus has been on identifying high-risk patients before orthopedic joint surgery and implementing evidence-based strategies that reduce the postoperative incidence of COU.
A transitional pain service (TPS) has been shown to reduce COU for high-risk surgical patients in different health care settings.6-9 The TPS model bundles multiple interventions that can be applied to patients at high risk for COU within a health care system. This includes individually tailored programs for preoperative education or pain management planning, use of multimodal analgesia (including regional or neuraxial techniques or nonopioid systemic medications), application of nonpharmacologic modalities (such as cognitive-based intervention), and a coordinated approach to postdischarge instructions and transitions of care. These interventions are coordinated by a multidisciplinary clinical service consisting of anesthesiologists and advanced practice clinicians with specialization in acute pain management and opioid tapering, nurse care coordinators, and psychologists with expertise in cognitive behavioral therapy.
TPS has been shown to reduce the incidence of COU for patients undergoing orthopedic joint surgery, but its impact on health care use and costs is unknown.6-9 The TPS intervention is resource intensive and increases the use of health care for preoperative education or pain management, which may increase the burden of costs. However, reducing long-term COU may reduce the use of health care for COU- and OUD-related complications, leading to cost savings. This study evaluated whether the TPS intervention influenced health care use and cost for inpatient, outpatient, or pharmacy services during the year following orthopedic joint surgery compared with that of the standard pain management care for procedures that place patients at high risk for COU. We used a difference-in-differences (DID) analysis to estimate this intervention effect, using multivariable regression models that controlled for unobserved time trends and cohort characteristics.
METHODS
This was a quasi-experimental study of patients who underwent orthopedic joint surgery and associated procedures at high risk for COU at the Veterans Affairs Salt Lake City Healthcare System (VASLCHS) between January 2016 through April 2020. The pre-TPS period between January 2016 through December 2017 was compared with the post-TPS period between January 2018 to September 2019. The control patient cohort was selected from 5 geographically diverse VA health care systems throughout the US: Eastern Colorado, Central Plains (Nebraska), White River Junction (Vermont), North Florida/South Georgia, and Portland (Oregon). By sampling health care costs from VA medical centers (VAMCs) across these different regions, our control group was generalizable to veterans receiving orthopedic joint surgery across the US. This study used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a repository of nearly all clinical and administrative data found in electronic health records for VA-provided care and fee-basis care paid for by the VA.10 All data were hosted and analyzed in the VA Informatics and Computing Infrastructure (VINCI) workspace. The University of Utah Institutional Review Board and the VASLCHS Office of Research and Development approved the protocol for this study.
TPS Intervention
The VASLCHS TPS has already been described in detail elsewhere.6,7 Briefly, patients at high risk for COU at the VASLCHS were enrolled in the TPS program before surgery for total knee, hip, or shoulder arthroplasty or rotator cuff procedures. The TPS service consists of an anesthesiologist and advanced practice clinician with specialization in acute pain management and opioid tapering, a psychologist with expertise in cognitive behavioral therapy, and 3 nurse care coordinators. These TPS practitioners work together to provide preoperative education, including setting expectations regarding postoperative pain, recommending nonopioid pain management strategies, and providing guidance regarding the appropriate use of opioids for surgical pain. Individual pain plans were developed and implemented for the perioperative period. After surgery, the TPS provided recommendations and support for nonopioid pain therapies and opioid tapers. Patients were followed by the TPS team for at least 12 months after surgery. At a minimum, the goals set by TPS included cessation of all opioid use for prior nonopioid users (NOU) by 90 days after surgery and the return to baseline opioid use or lower for prior COU patients by 90 days after surgery. The TPS also encouraged and supported opioid tapering among COU patients to reduce or completely stop opioid use after surgery.
Patient Cohorts
Veterans having primary or revision total knee, hip, or shoulder arthroplasty or rotator cuff repair between January 1, 2016, and September 30, 2019, at the aforementioned VAMCs were included in the study. Patients who had any hospitalization within 90 days pre- or postindex surgery or who died within 8 months after surgery were excluded from analysis. Patients who had multiple surgeries during the index inpatient visit or within 90 days after the index surgery also were excluded. Comorbid conditions for mental health and substance use were identified using the International Classification of Diseases, 10th revision Clinical Modification (ICD-10) codes or 9th revision equivalent grouped by Clinical Classifications Software Refined (CCS-R).11 Preoperative exposure to clinically relevant pharmacotherapy (ie, agents associated with prolonged opioid use and nonopioid adjuvants) was captured using VA outpatient prescription records (eAppendix 1).
Outcome Variables
Outcome variables included health care use and costs during 1-year pre- and postperiods from the date of surgery. VA health care costs for outpatient, inpatient, and pharmacy services for direct patient care were collected from the Managerial Cost Accounting System, an activity-based cost allocation system that generates estimates of the cost of individual VA hospital stays, health care encounters, and medications. Health care use was defined as the number of encounters for each visit type in the Managerial Cost Accounting System. All costs were adjusted to 2019 US dollars, using the Personal Consumption Expenditures price index for health care services.15
A set of sociodemographic variables including sex, age at surgery, race and ethnicity, rurality, military branch (Army, Air Force, Marine Corps, Navy, and other), and service connectivity were included as covariates in our regression models.
Statistical Analyses
Descriptive analyses were used to evaluate differences in baseline patient sociodemographic and clinical characteristics between pre- and postperiods for TPS intervention and control cohorts using 2-sample t tests for continuous variables and χ2 tests for categorical variables. We summarized unadjusted health care use and costs for outpatient, inpatient, and pharmacy visits and compared the pre- and postintervention periods using the Mann-Whitney test. Both mean (SD) and median (IQR) were considered, reflecting the skewed distribution of the outcome variables.
We used a DID approach to assess the intervention effect while minimizing confounding from the nonrandom sample. The DID approach controls for unobserved differences between VAMCs that are related to both the intervention and outcomes while controlling for trends over time that could affect outcomes across clinics. To implement the DID approach, we included 3 key independent variables in our regression models: (1) an indicator for whether the observation occurred in the postintervention period; (2) an indicator for whether the patient was exposed to the TPS intervention; and (3) the interaction between these 2 variables.
For cost outcomes, we used multivariable generalized linear models with a log link and a Poisson or Υ family. We analyzed inpatient costs using a 2-part generalized linear model because only 17% to 20% of patients had ≥ 1 inpatient visit. We used multivariable negative binomial regression for health care use outcomes. Demographic and clinical covariates described earlier were included in the regression models to control for differences in the composition of patient groups and clinics that could lead to confounding bias.
RESULTS
Of the 4954 patients included in our study cohort, 3545 (71.6%) were in the NOU group and 1409 (28.4%) were in the COU group. Among the NOU cohort, 361 patients were in the intervention group and 3184 in the control group. Among the COU cohort, 149 patients were in the intervention group and 1260 in the control group (Table 1). Most patients were male, White race, with a mean (SD) age of 64 (11) years. The most common orthopedic procedure was total knee arthroplasty, followed by total hip arthroplasty. Among both NOU and COU cohorts, patients’ characteristics were similar between the pre- and postintervention period among either TPS or control cohort.
Figures 1 and 2 and eAppendix 2 depict unadjusted per-person average outpatient, inpatient, and pharmacy visits and costs incurred during the 1-year pre- and postintervention periods for the NOU and COU cohorts. Average total health care follow-up costs ranged from $40,000 to $53,000 for NOU and from $47,000 to $82,000 for COU cohort. Cost for outpatient visits accounted for about 70% of the average total costs, followed by costs for inpatient visits of about 20%, and costs for pharmacy for the remaining.
For the NOU cohort, the number of health care encounters remained fairly stable between periods except for the outpatient visits among the TPS group. The TPS group experienced an increase in mean outpatient visits in the postperiod: 30 vs 37 visits (23%) (
Table 2 summarizes the results from the multivariable DID analyses for the outpatient, inpatient, and pharmacy visit and cost outcomes. Here, the estimated effect of the TPS intervention is the coefficient from the interaction between the postintervention and TPS exposure indicator variables. This coefficient was calculated as the difference in the outcome before and after the TPS intervention among the TPS group minus the difference in the outcome before and after the TPS intervention among the control group. For the NOU cohort, TPS was associated with an increase in the use of outpatient health care (mean [SD] increase of 6.9 [2] visits; P < .001) after the surgery with no statistically significant effect on outpatient costs (mean [SD] increase of $2787 [$3749]; P = .55). There was no statistically significant effect of TPS on the use of inpatient visits or pharmacy, but a decrease in costs for inpatient visits among those who had at least 1 inpatient visit (mean [SD] decrease of $12,170 [$6100]; P = .02). For the COU cohort, TPS had no statistically significant impact on the use of outpatient, inpatient, or pharmacy or the corresponding costs.
DISCUSSION
TPS is a multidisciplinary approach to perioperative pain management that has been shown to reduce both the quantity and duration of opioid use among orthopedic surgery patients.6,7 Although the cost burden of providing TPS services to prevent COU is borne by the individual health care system, it is unclear whether this expense is offset by lower long-term medical costs and health care use for COU- and OUD-related complications. In this study focused on a veteran population undergoing orthopedic joint procedures, a DID analysis of cost and health care use showed that TPS, which has been shown to reduce COU for high-risk surgical patients, can be implemented without increasing the overall costs to the VA health care system during the 1 year following surgery, even with increased outpatient visits. For NOU patients, there was no difference in outpatient visit costs or pharmacy costs over 12 months after surgery, although there was a significant reduction in subsequent inpatient costs over the same period. Further, there was no difference in outpatient, inpatient, or pharmacy costs after surgery for COU patients. These findings suggest that TPS can be a cost-effective approach to reduce opioid use among patients undergoing orthopedic joint surgery in VAMCs.
The costs of managing COU after surgery are substantial. Prior reports have shown that adjusted total health care costs are 1.6 to 2.5 times higher for previously NOU patients with new COU after major surgery than those for such patients without persistent use.16 The 1-year costs associated with new COU in this prior study ranged between $7944 and $17,702 after inpatient surgery and between $5598 and $12,834 after outpatient index surgery, depending on the payer, which are in line with the cost differences found in our current study. Another report among patients with COU following orthopedic joint replacement showed that they had higher use of inpatient, emergency department, and ambulance/paramedic services in the 12 months following their surgery than did those without persistent use.17 Although these results highlight the impact that COU plays in driving increased costs after major surgery, there have been limited studies focused on interventions that can neutralize the costs associated with opioid misuse after surgery. To our knowledge, our study is the first analysis to show the impact of using an intervention such as TPS to reduce postoperative opioid use on health care use and cost.
Although a rigorous and comprehensive return on investment analysis was beyond the scope of this analysis, these results may have several implications for other health care systems and hospitals that wish to invest in a multidisciplinary perioperative pain management program such as TPS but may be reluctant due to the upfront investment. First, the increased number of patient follow-up visits needed during TPS seems to be more than offset by the reduction in opioid use and associated complications that may occur after surgery. Second, TPS did not seem to be associated with an increase in overall health care costs during the 1-year follow-up period. Together, these results indicate that the return on investment for a TPS approach to perioperative pain management in which optimal patient-centered outcomes are achieved without increasing long-term costs to a health care system may be positive.
Limitations
This study has several limitations. First, this was a quasi-experimental observational study, and the associations we identified between intervention and outcomes should not be assumed to demonstrate causality. Although our DID analysis controlled for an array of demographic and clinical characteristics, differences in medical costs and health care use between the 2 cohorts might be driven by unobserved confounding variables.
Our study also was limited to veterans who received medical care at the VA, and results may not be generalizable to other non-VA health care systems or to veterans with Medicare insurance who have dual benefits. While our finding on health care use and costs may be incomplete because of the uncaptured health care use outside the VA, our DID analysis helped reduce unobserved bias because the absence of data outside of VA care applies to both TPS and control groups. Further, the total costs of operating a TPS program at any given institution will depend on the size of the hospital and volume of surgical patients who meet criteria for enrollment. However, the relative differences in health care use and costs may be extrapolated to patients undergoing orthopedic surgery in other types of academic and community-based health care systems.
Furthermore, this analysis focused primarily on COU and NOU patients undergoing orthopedic joint surgery. While this represents a high-risk population for OUD, the costs and health care use associated with delivering the TPS intervention to other types of surgical procedures may be significantly different. All costs in this analysis were based on 2019 estimates and do not account for the potential inflation over the past several years. Nonmonetary costs to the patient and per-person average total intervention costs were not included in the study. However, we assumed that costs associated with TPS and standard of care would have increased to an equivalent degree over the same period. Further, the average cost of TPS per patient (approximately $900) is relatively small compared with the average annual costs during 1-year pre- and postoperative periods and was not expected to have a significant effect on the analysis.
Conclusions
We found that the significant reduction in COU seen in previous studies following the implementation of TPS was not accompanied by increased health care costs.6,7 When considering the other costs of long-term opioid use, such as abuse potential, overdose, death, and increased disability, implementation of a TPS service has the potential to improve patient quality of life while reducing other health-related costs. Health care systems should consider the implementation of similar multidisciplinary approaches to perioperative pain management to improve outcomes after orthopedic joint surgery and other high-risk procedures.
Opioid use disorder (OUD) is a significant cause of morbidity, mortality, and health care costs in the US.1,2 Surgery can be the inciting cause for exposure to an opioid; as many as 23% of patients develop chronic OUD following surgery.3,4 Patients with a history of substance use, mood disorders, anxiety, or previous chronic opioid use (COU) are at risk for relapse, dose escalation, and poor pain control after high-risk surgery, such as orthopedic joint procedures.5 Recently focus has been on identifying high-risk patients before orthopedic joint surgery and implementing evidence-based strategies that reduce the postoperative incidence of COU.
A transitional pain service (TPS) has been shown to reduce COU for high-risk surgical patients in different health care settings.6-9 The TPS model bundles multiple interventions that can be applied to patients at high risk for COU within a health care system. This includes individually tailored programs for preoperative education or pain management planning, use of multimodal analgesia (including regional or neuraxial techniques or nonopioid systemic medications), application of nonpharmacologic modalities (such as cognitive-based intervention), and a coordinated approach to postdischarge instructions and transitions of care. These interventions are coordinated by a multidisciplinary clinical service consisting of anesthesiologists and advanced practice clinicians with specialization in acute pain management and opioid tapering, nurse care coordinators, and psychologists with expertise in cognitive behavioral therapy.
TPS has been shown to reduce the incidence of COU for patients undergoing orthopedic joint surgery, but its impact on health care use and costs is unknown.6-9 The TPS intervention is resource intensive and increases the use of health care for preoperative education or pain management, which may increase the burden of costs. However, reducing long-term COU may reduce the use of health care for COU- and OUD-related complications, leading to cost savings. This study evaluated whether the TPS intervention influenced health care use and cost for inpatient, outpatient, or pharmacy services during the year following orthopedic joint surgery compared with that of the standard pain management care for procedures that place patients at high risk for COU. We used a difference-in-differences (DID) analysis to estimate this intervention effect, using multivariable regression models that controlled for unobserved time trends and cohort characteristics.
METHODS
This was a quasi-experimental study of patients who underwent orthopedic joint surgery and associated procedures at high risk for COU at the Veterans Affairs Salt Lake City Healthcare System (VASLCHS) between January 2016 through April 2020. The pre-TPS period between January 2016 through December 2017 was compared with the post-TPS period between January 2018 to September 2019. The control patient cohort was selected from 5 geographically diverse VA health care systems throughout the US: Eastern Colorado, Central Plains (Nebraska), White River Junction (Vermont), North Florida/South Georgia, and Portland (Oregon). By sampling health care costs from VA medical centers (VAMCs) across these different regions, our control group was generalizable to veterans receiving orthopedic joint surgery across the US. This study used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a repository of nearly all clinical and administrative data found in electronic health records for VA-provided care and fee-basis care paid for by the VA.10 All data were hosted and analyzed in the VA Informatics and Computing Infrastructure (VINCI) workspace. The University of Utah Institutional Review Board and the VASLCHS Office of Research and Development approved the protocol for this study.
TPS Intervention
The VASLCHS TPS has already been described in detail elsewhere.6,7 Briefly, patients at high risk for COU at the VASLCHS were enrolled in the TPS program before surgery for total knee, hip, or shoulder arthroplasty or rotator cuff procedures. The TPS service consists of an anesthesiologist and advanced practice clinician with specialization in acute pain management and opioid tapering, a psychologist with expertise in cognitive behavioral therapy, and 3 nurse care coordinators. These TPS practitioners work together to provide preoperative education, including setting expectations regarding postoperative pain, recommending nonopioid pain management strategies, and providing guidance regarding the appropriate use of opioids for surgical pain. Individual pain plans were developed and implemented for the perioperative period. After surgery, the TPS provided recommendations and support for nonopioid pain therapies and opioid tapers. Patients were followed by the TPS team for at least 12 months after surgery. At a minimum, the goals set by TPS included cessation of all opioid use for prior nonopioid users (NOU) by 90 days after surgery and the return to baseline opioid use or lower for prior COU patients by 90 days after surgery. The TPS also encouraged and supported opioid tapering among COU patients to reduce or completely stop opioid use after surgery.
Patient Cohorts
Veterans having primary or revision total knee, hip, or shoulder arthroplasty or rotator cuff repair between January 1, 2016, and September 30, 2019, at the aforementioned VAMCs were included in the study. Patients who had any hospitalization within 90 days pre- or postindex surgery or who died within 8 months after surgery were excluded from analysis. Patients who had multiple surgeries during the index inpatient visit or within 90 days after the index surgery also were excluded. Comorbid conditions for mental health and substance use were identified using the International Classification of Diseases, 10th revision Clinical Modification (ICD-10) codes or 9th revision equivalent grouped by Clinical Classifications Software Refined (CCS-R).11 Preoperative exposure to clinically relevant pharmacotherapy (ie, agents associated with prolonged opioid use and nonopioid adjuvants) was captured using VA outpatient prescription records (eAppendix 1).
Outcome Variables
Outcome variables included health care use and costs during 1-year pre- and postperiods from the date of surgery. VA health care costs for outpatient, inpatient, and pharmacy services for direct patient care were collected from the Managerial Cost Accounting System, an activity-based cost allocation system that generates estimates of the cost of individual VA hospital stays, health care encounters, and medications. Health care use was defined as the number of encounters for each visit type in the Managerial Cost Accounting System. All costs were adjusted to 2019 US dollars, using the Personal Consumption Expenditures price index for health care services.15
A set of sociodemographic variables including sex, age at surgery, race and ethnicity, rurality, military branch (Army, Air Force, Marine Corps, Navy, and other), and service connectivity were included as covariates in our regression models.
Statistical Analyses
Descriptive analyses were used to evaluate differences in baseline patient sociodemographic and clinical characteristics between pre- and postperiods for TPS intervention and control cohorts using 2-sample t tests for continuous variables and χ2 tests for categorical variables. We summarized unadjusted health care use and costs for outpatient, inpatient, and pharmacy visits and compared the pre- and postintervention periods using the Mann-Whitney test. Both mean (SD) and median (IQR) were considered, reflecting the skewed distribution of the outcome variables.
We used a DID approach to assess the intervention effect while minimizing confounding from the nonrandom sample. The DID approach controls for unobserved differences between VAMCs that are related to both the intervention and outcomes while controlling for trends over time that could affect outcomes across clinics. To implement the DID approach, we included 3 key independent variables in our regression models: (1) an indicator for whether the observation occurred in the postintervention period; (2) an indicator for whether the patient was exposed to the TPS intervention; and (3) the interaction between these 2 variables.
For cost outcomes, we used multivariable generalized linear models with a log link and a Poisson or Υ family. We analyzed inpatient costs using a 2-part generalized linear model because only 17% to 20% of patients had ≥ 1 inpatient visit. We used multivariable negative binomial regression for health care use outcomes. Demographic and clinical covariates described earlier were included in the regression models to control for differences in the composition of patient groups and clinics that could lead to confounding bias.
RESULTS
Of the 4954 patients included in our study cohort, 3545 (71.6%) were in the NOU group and 1409 (28.4%) were in the COU group. Among the NOU cohort, 361 patients were in the intervention group and 3184 in the control group. Among the COU cohort, 149 patients were in the intervention group and 1260 in the control group (Table 1). Most patients were male, White race, with a mean (SD) age of 64 (11) years. The most common orthopedic procedure was total knee arthroplasty, followed by total hip arthroplasty. Among both NOU and COU cohorts, patients’ characteristics were similar between the pre- and postintervention period among either TPS or control cohort.
Figures 1 and 2 and eAppendix 2 depict unadjusted per-person average outpatient, inpatient, and pharmacy visits and costs incurred during the 1-year pre- and postintervention periods for the NOU and COU cohorts. Average total health care follow-up costs ranged from $40,000 to $53,000 for NOU and from $47,000 to $82,000 for COU cohort. Cost for outpatient visits accounted for about 70% of the average total costs, followed by costs for inpatient visits of about 20%, and costs for pharmacy for the remaining.
For the NOU cohort, the number of health care encounters remained fairly stable between periods except for the outpatient visits among the TPS group. The TPS group experienced an increase in mean outpatient visits in the postperiod: 30 vs 37 visits (23%) (
Table 2 summarizes the results from the multivariable DID analyses for the outpatient, inpatient, and pharmacy visit and cost outcomes. Here, the estimated effect of the TPS intervention is the coefficient from the interaction between the postintervention and TPS exposure indicator variables. This coefficient was calculated as the difference in the outcome before and after the TPS intervention among the TPS group minus the difference in the outcome before and after the TPS intervention among the control group. For the NOU cohort, TPS was associated with an increase in the use of outpatient health care (mean [SD] increase of 6.9 [2] visits; P < .001) after the surgery with no statistically significant effect on outpatient costs (mean [SD] increase of $2787 [$3749]; P = .55). There was no statistically significant effect of TPS on the use of inpatient visits or pharmacy, but a decrease in costs for inpatient visits among those who had at least 1 inpatient visit (mean [SD] decrease of $12,170 [$6100]; P = .02). For the COU cohort, TPS had no statistically significant impact on the use of outpatient, inpatient, or pharmacy or the corresponding costs.
DISCUSSION
TPS is a multidisciplinary approach to perioperative pain management that has been shown to reduce both the quantity and duration of opioid use among orthopedic surgery patients.6,7 Although the cost burden of providing TPS services to prevent COU is borne by the individual health care system, it is unclear whether this expense is offset by lower long-term medical costs and health care use for COU- and OUD-related complications. In this study focused on a veteran population undergoing orthopedic joint procedures, a DID analysis of cost and health care use showed that TPS, which has been shown to reduce COU for high-risk surgical patients, can be implemented without increasing the overall costs to the VA health care system during the 1 year following surgery, even with increased outpatient visits. For NOU patients, there was no difference in outpatient visit costs or pharmacy costs over 12 months after surgery, although there was a significant reduction in subsequent inpatient costs over the same period. Further, there was no difference in outpatient, inpatient, or pharmacy costs after surgery for COU patients. These findings suggest that TPS can be a cost-effective approach to reduce opioid use among patients undergoing orthopedic joint surgery in VAMCs.
The costs of managing COU after surgery are substantial. Prior reports have shown that adjusted total health care costs are 1.6 to 2.5 times higher for previously NOU patients with new COU after major surgery than those for such patients without persistent use.16 The 1-year costs associated with new COU in this prior study ranged between $7944 and $17,702 after inpatient surgery and between $5598 and $12,834 after outpatient index surgery, depending on the payer, which are in line with the cost differences found in our current study. Another report among patients with COU following orthopedic joint replacement showed that they had higher use of inpatient, emergency department, and ambulance/paramedic services in the 12 months following their surgery than did those without persistent use.17 Although these results highlight the impact that COU plays in driving increased costs after major surgery, there have been limited studies focused on interventions that can neutralize the costs associated with opioid misuse after surgery. To our knowledge, our study is the first analysis to show the impact of using an intervention such as TPS to reduce postoperative opioid use on health care use and cost.
Although a rigorous and comprehensive return on investment analysis was beyond the scope of this analysis, these results may have several implications for other health care systems and hospitals that wish to invest in a multidisciplinary perioperative pain management program such as TPS but may be reluctant due to the upfront investment. First, the increased number of patient follow-up visits needed during TPS seems to be more than offset by the reduction in opioid use and associated complications that may occur after surgery. Second, TPS did not seem to be associated with an increase in overall health care costs during the 1-year follow-up period. Together, these results indicate that the return on investment for a TPS approach to perioperative pain management in which optimal patient-centered outcomes are achieved without increasing long-term costs to a health care system may be positive.
Limitations
This study has several limitations. First, this was a quasi-experimental observational study, and the associations we identified between intervention and outcomes should not be assumed to demonstrate causality. Although our DID analysis controlled for an array of demographic and clinical characteristics, differences in medical costs and health care use between the 2 cohorts might be driven by unobserved confounding variables.
Our study also was limited to veterans who received medical care at the VA, and results may not be generalizable to other non-VA health care systems or to veterans with Medicare insurance who have dual benefits. While our finding on health care use and costs may be incomplete because of the uncaptured health care use outside the VA, our DID analysis helped reduce unobserved bias because the absence of data outside of VA care applies to both TPS and control groups. Further, the total costs of operating a TPS program at any given institution will depend on the size of the hospital and volume of surgical patients who meet criteria for enrollment. However, the relative differences in health care use and costs may be extrapolated to patients undergoing orthopedic surgery in other types of academic and community-based health care systems.
Furthermore, this analysis focused primarily on COU and NOU patients undergoing orthopedic joint surgery. While this represents a high-risk population for OUD, the costs and health care use associated with delivering the TPS intervention to other types of surgical procedures may be significantly different. All costs in this analysis were based on 2019 estimates and do not account for the potential inflation over the past several years. Nonmonetary costs to the patient and per-person average total intervention costs were not included in the study. However, we assumed that costs associated with TPS and standard of care would have increased to an equivalent degree over the same period. Further, the average cost of TPS per patient (approximately $900) is relatively small compared with the average annual costs during 1-year pre- and postoperative periods and was not expected to have a significant effect on the analysis.
Conclusions
We found that the significant reduction in COU seen in previous studies following the implementation of TPS was not accompanied by increased health care costs.6,7 When considering the other costs of long-term opioid use, such as abuse potential, overdose, death, and increased disability, implementation of a TPS service has the potential to improve patient quality of life while reducing other health-related costs. Health care systems should consider the implementation of similar multidisciplinary approaches to perioperative pain management to improve outcomes after orthopedic joint surgery and other high-risk procedures.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
2. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906. doi:10.1097/MLR.0000000000000625
3. Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265(4):722-727. doi:10.1097/SLA.0000000000001780
4. Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957, e3. doi:10.1016/j.jhsa.2016.07.113
5. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi:10.1001/jamasurg.2017.0504
6. Buys MJ, Bayless K, Romesser J, et al. Multidisciplinary transitional pain service for the veteran population. Fed Pract. 2020;37(10):472-478. doi:10.12788/fp.0053
7. Buys MJ, Bayless K, Romesser J, et al. Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 2020;45(11):847-852. doi:10.1136/rapm-2020-101797
8. Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag. 2015;5(2):97-105. doi:10.2217/pmt.15.7
9. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/JPR.S91924
10. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
11. Agency for Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR). Updated December 9, 2022. Accessed October 30, 2023. www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
12. Mosher HJ, Richardson KK, Lund BC. The 1-year treatment course of new opioid recipients in Veterans Health Administration. Pain Med. 2016;17(7):1282-1291. doi:10.1093/pm/pnw058
13. Hadlandsmyth K, Mosher HJ, Vander Weg MW, O’Shea AM, McCoy KD, Lund BC. Utility of accumulated opioid supply days and individual patient factors in predicting probability of transitioning to long-term opioid use: an observational study in the Veterans Health Administration. Pharmacol Res Perspect. 2020;8(2):e00571. doi:10.1002/prp2.571
14. Pagé MG, Kudrina I, Zomahoun HTV, et al. Relative frequency and risk factors for long-term opioid therapy following surgery and trauma among adults: a systematic review protocol. Syst Rev. 2018;7(1):97. doi:10.1186/s13643-018-0760-3
15. US. Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product. Accessed October 30, 2023. https://apps.bea.gov/iTable/?reqid=19&step=3&isuri=1&nipa_table_list=64&categories=survey
16. Brummett CM, Evans-Shields J, England C, et al. Increased health care costs associated with new persistent opioid use after major surgery in opioid-naive patients. J Manag Care Spec Pharm. 2021;27(6):760-771. doi:10.18553/jmcp.2021.20507
17. Gold LS, Strassels SA, Hansen RN. Health care costs and utilization in patients receiving prescriptions for long-acting opioids for acute postsurgical pain. Clin J Pain. 2016;32(9):747-754. doi:10.1097/ajp.0000000000000322
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
2. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906. doi:10.1097/MLR.0000000000000625
3. Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265(4):722-727. doi:10.1097/SLA.0000000000001780
4. Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957, e3. doi:10.1016/j.jhsa.2016.07.113
5. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi:10.1001/jamasurg.2017.0504
6. Buys MJ, Bayless K, Romesser J, et al. Multidisciplinary transitional pain service for the veteran population. Fed Pract. 2020;37(10):472-478. doi:10.12788/fp.0053
7. Buys MJ, Bayless K, Romesser J, et al. Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 2020;45(11):847-852. doi:10.1136/rapm-2020-101797
8. Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag. 2015;5(2):97-105. doi:10.2217/pmt.15.7
9. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/JPR.S91924
10. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
11. Agency for Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR). Updated December 9, 2022. Accessed October 30, 2023. www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
12. Mosher HJ, Richardson KK, Lund BC. The 1-year treatment course of new opioid recipients in Veterans Health Administration. Pain Med. 2016;17(7):1282-1291. doi:10.1093/pm/pnw058
13. Hadlandsmyth K, Mosher HJ, Vander Weg MW, O’Shea AM, McCoy KD, Lund BC. Utility of accumulated opioid supply days and individual patient factors in predicting probability of transitioning to long-term opioid use: an observational study in the Veterans Health Administration. Pharmacol Res Perspect. 2020;8(2):e00571. doi:10.1002/prp2.571
14. Pagé MG, Kudrina I, Zomahoun HTV, et al. Relative frequency and risk factors for long-term opioid therapy following surgery and trauma among adults: a systematic review protocol. Syst Rev. 2018;7(1):97. doi:10.1186/s13643-018-0760-3
15. US. Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product. Accessed October 30, 2023. https://apps.bea.gov/iTable/?reqid=19&step=3&isuri=1&nipa_table_list=64&categories=survey
16. Brummett CM, Evans-Shields J, England C, et al. Increased health care costs associated with new persistent opioid use after major surgery in opioid-naive patients. J Manag Care Spec Pharm. 2021;27(6):760-771. doi:10.18553/jmcp.2021.20507
17. Gold LS, Strassels SA, Hansen RN. Health care costs and utilization in patients receiving prescriptions for long-acting opioids for acute postsurgical pain. Clin J Pain. 2016;32(9):747-754. doi:10.1097/ajp.0000000000000322