User login
Management and Prevention of Intraoperative Acetabular Fracture in Primary Total Hip Arthroplasty
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
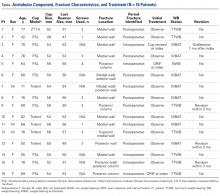
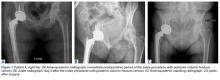
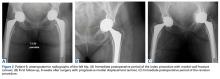
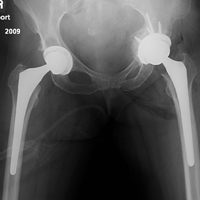
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.