User login
When clozapine may be right for your patient, and how to initiate therapy
Clozapine for schizophrenia: Life-threatening or life-saving treatment?
Researchers in Finland surprised psychiatrists this year by announcing that clozapine “seems to be associated with a substantially lower mortality than any other antipsychotic.”1 This finding also surprised the researchers, who expected their 11-year study to link long-term use of second-generation (“atypical”) antipsychotics with increased mortality in patients with schizophrenia. Instead they found longer lives in patients who used antipsychotics (and particularly clozapine), compared with no antipsychotic use.
This study’s findings do not change clozapine’s association with potentially fatal agranulocytosis as well as weight gain, metabolic abnormalities, and other adverse effects. Clozapine also is difficult to administer ( Box 1 ),2 and patients must be enrolled in FDA-mandated registries (see Related Resources ). These obstacles might discourage you from offering clozapine to patients who could benefit from it ( Box 2 ).3-5
Why bother considering clozapine? Because recent data on decreased mortality, decreased suicidality, and control of aggressive behavior make clozapine a compelling choice for many patients. Careful attention to clozapine’s adverse effect profile is necessary, but you can manage these risks with appropriate monitoring.
Because of clozapine’s risk for leukopenia and agranulocytosis, frequent white blood cell count (WBC) monitoring is required. The risk of drug-induced blood dyscrasias has been shown to decrease over time, however, from 0.70/1,000 patient-years in the second 6 months of treatment to 0.39/1,000 patient-years after the first year.2
To start clozapine treatment, FDA guidelines require that the patient’s WBC must be at least 3,500 mm3, and the absolute neutrophil count (ANC) must be at least 2,000 mm3. For the first 6 months, patients receiving clozapine must have a weekly blood test for WBC and ANC.
The dispensing pharmacist must see the blood work result prior to releasing the drug to the patient. The blood draw date must be within the previous 7 days for the pharmacist to dispense a 1-week supply of clozapine.
Decreased monitoring over time. After 6 months of continuous therapy with clozapine without any interruptions because of a low WBC and/or ANC—defined as WBC 3 and/or ANC 3 or increased monitoring (when WBC 3 and/or ANC 3)—the patient’s blood monitoring may be done every 14 days and a 2-week supply of clozapine can be dispensed.
After 12 months of continuous clozapine therapy—6 months of continuous weekly monitoring, then 6 months of continuous biweekly monitoring—without any interruptions or increased monitoring, the patient may have blood monitoring done every 4 weeks and can receive a 4-week supply of clozapine.
One advantage of these monitoring requirements is that the increased frequency of visits can be used to foster greater patient engagement with treatment and promote a therapeutic alliance. Peer-led clozapine support groups, available in some communities, can facilitate adherence to monitoring requirements.
Clozapine was approved in the United States in 1989 for severely ill patients with schizophrenia who had not responded adequately to standard drug treatment. In 2002 it received an indication for patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for re-experiencing suicidal behavior, based on history and recent clinical state.
Off-label, clozapine has been commonly used for refractory bipolar disorder. Since 1998, it has been available in generic formulations and in a proprietary orally-disintegrating tablet formulation.
Dosing. The recommended target clozapine dosage is 300 to 450 mg/d. If an adequate response is not achieved, obtaining a plasma level might be helpful.3 Plasma levels ≥350 ng/mL constitute an optimal clozapine trial.
Not a ‘last resort.’ American Psychiatric Association treatment guidelines for schizophrenia state: “Because of clozapine’s superior efficacy, a trial of clozapine should be considered for a patient with a clinically inadequate response to antipsychotic treatment or for a patient with suicidal ideation or behavior. Besides clozapine, there are limited options for the many patients who have severe and significant residual symptoms even after antipsychotic monotherapy has been optimized, and none have proven benefits.”4
As additional evidence accumulates—including benefits regarding mortality and aggression—clozapine’s advantages support its clinical use earlier than as a “last resort.” In institutional settings, clozapine use has increased with the availability of additional data, such as from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE).
In New York State Office of Mental Health hospitals, clozapine use increased from 20.6% of prescriptions in 2005 to 24.9% in 2007, compared with the other CATIE medications (olanzapine, quetiapine, risperidone, ziprasidone) and haloperidol.5 Whether clozapine use will increase in outpatient settings remains to be seen.
Potential for longer life?
The population-based, cohort study from Finland demonstrated that—contrary to popular belief—the introduction of atypical antipsychotics during the 1990s did not adversely affect mortality of patients with schizophrenia, at least in Finland.1
This study made specific drug comparisons and used perphenazine as the reference drug. The lowest risk for mortality was observed with clozapine, which showed a 26% relative advantage compared with perphenazine. Clozapine’s advantage was statistically significant when compared with all other antipsychotics tested.
The authors suggested provocatively that restrictions on clozapine use as a second- or third-line agent should be reassessed. A few caveats, however, might affect how one interprets this study or applies its findings to clinical practice:
- The main comparisons were for patients receiving outpatient antipsychotic monotherapy. No information was available about antipsychotics used during inhospital treatment.
- Only the most frequently used atypical antipsychotics (clozapine, olanzapine, oral risperidone, and quetiapine) or the most frequently prescribed first-generation antipsychotics (oral perphenazine, thioridazine, and oral haloperidol) were assessed individually.
- Data about patients’ marital status, diagnoses of substance abuse, socioeconomic status, and other social variables were not available.
- Not all antipsychotics were available throughout the study (quetiapine was the newest and available for the shortest time).
- The study population consisted of patients of all ages, including those under 20 and over 70 years of age. Although the number of deaths and mortality rates increased with age, causes of mortality may differ when younger and older persons are compared. A data supplement to the study—available at www.thelancet.com—contains information about odds ratios by age and other factors.
Recommendation. Consider clozapine earlier than as a “last resort” in the disease course of patients with schizophrenia. At the very least, routinely present clozapine to patients and their families as a possible treatment option.
Antiaggressive properties
Case series and retrospective studies have provided insights into clozapine’s antiaggressive properties, but the strongest evidence comes from a 12-week, double-blind, randomized trial that specifically enrolled patients with violent behavior.6 Clozapine, olanzapine, and haloperidol were directly compared in the treatment of assaults and other aggressive behaviors by physically assaultive in patients with schizophrenia and schizoaffective disorder:
- The Modified Overt Aggression Scale (MOAS) physical aggression score measured the number and severity of assaults.
- The Positive and Negative Syndrome Scale (PANSS) was used to assess psychiatric symptoms.
Recommendation. Offer clozapine as an option for patients with schizophrenia or schizoaffective disorder and persistent aggressive behavior. Another antipsychotic might not be “good enough.”
Reduced risk of suicidality
The International Suicide Prevention Trial (InterSePT) was a multicenter, randomized, 2-year clinical study that compared the risk for suicidal behavior in patients treated with clozapine vs olanzapine.7 Enrolled were 980 patients with schizophrenia or schizoaffective disorder who were considered at high risk for suicide because of past suicide attempts or current suicidal ideation. Approximately one-quarter had not responded adequately to previous treatment.
All patients were seen weekly for 6 months, then biweekly for 18 months. The weekly or biweekly contact required to monitor for clozapine-associated agranulocytosis was matched with a similar visit schedule for olanzapine-treated patients, during which clinicians obtained vital signs. Primary endpoints included suicide attempts (including death), hospitalization to prevent suicide, and a rating of “much worsening of suicidality” from baseline. Blinded raters, including an independent suicide monitoring board, determined when patients achieved endpoint criteria.
Patients receiving clozapine showed significantly less suicidal behavior than those treated with olanzapine (a 24% relative advantage in the hazard ratio for suicide attempts or hospitalizations to prevent suicide). Fewer patients in the clozapine group:
- attempted suicide (34 vs 55)
- required hospitalization (82 vs 107) or rescue interventions to prevent suicide (118 vs 155)
- required concomitant treatment with antidepressants (221 vs 258) or anxiolytics/soporifics (301 vs 331).
More deaths from suicide occurred in the clozapine group than the olanzapine group, but the numbers were small (5 vs 3) and the difference between clozapine and olanzapine on this outcome was not statistically significant (P=.73).
Recommendation. Clozapine is a first-line treatment for patients with schizophrenia or schizoaffective disorder who exhibit suicidal behavior. This is reflected in the drug’s product labeling.
Superior symptom management
CATIE findings. Phase 2 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) showed clozapine to be more effective than other atypical antipsychotics, as measured by time to all-cause discontinuation.8 Patients in this phase of CATIE had discontinued another atypical antipsychotic in phase 1, principally because of lack of adequate efficacy. In phase 2, they were re-randomized to receive open-label clozapine or double-blinded risperidone, olanzapine, or quetiapine.
Only 90 patients were included in the time-to-discontinuation analysis, yet the greater amount of time that patients remained on clozapine (median 10.5 months) compared with quetiapine (median 3.3 months) or risperidone (median 2.8 months) was statistically significant. Time to discontinuation because of inadequate therapeutic effect also was significantly longer for clozapine than for olanzapine, quetiapine, or risperidone.
The NNT for the outcome of all-cause discontinuation for clozapine was 4 compared with risperidone and 3 compared with quetiapine. This means for every 4 or 3 patients randomly assigned to clozapine instead of risperidone or quetiapine, respectively, 1 additional patient successfully completed the CATIE trial on the original phase 2 medication.9 The NNT for clozapine vs olanzapine was 7, indicating a respectable effect size difference that might have been statistically significant if the sample size had been larger.
Meta-analyses support CATIE results. Clozapine’s greater efficacy (and effectiveness) compared with other antipsychotics as demonstrated in CATIE is supported by 2 meta-analyses:
- A systematic review of clinical trials between January 1953 and May 2002 found clozapine’s effect size in reducing symptoms for patients with schizophrenia was greater than that of any other antipsychotic.10
- In a similar but more recent meta-analysis of 150 double-blind, mostly short-term studies totaling 21,533 participants, clozapine showed the largest effect size when atypical antipsychotics were compared with first-generation antipsychotics.11
Caveats about clozapine
First-episode schizophrenia. Clozapine has been shown to be more effective than chlorpromazine in terms of time to remission and maintenance of remission for treatment-naïve patients with first-episode schizophrenia.13 Even so, most clinicians probably would not consider clozapine as a first-line treatment for an uncomplicated first-episode patient because of concerns about agranulocytosis. When genetic testing becomes available to determine individual risk for agranulocytosis, perhaps clozapine will be used earlier in the disease course.14
The patient’s ethnicity may influence the risk of adverse effects, as observed in the study examining clozapine’s antiaggressive effect;6 African-American patients receiving antipsychotics—and particularly clozapine—may be more likely to develop metabolic abnormalities than patients in other ethnic groups.15 Carefully monitor all patients receiving clozapine for metabolic adverse effects, and be prepared to institute remediative psychosocial, lifestyle, and adjunctive medication interventions, such as statins.
16
Table
Common adverse effects of clozapine
| Adverse effect | Frequency* |
|---|---|
| Hypersalivation | 31% to 48% |
| Drowsiness/sedation/somnolence | 39% to 46% |
| Weight increase | 31% |
| Tachycardia | 25% |
| Dizziness/vertigo | 19% to 27% |
| Constipation | 14% to 25% |
| Seizures | 5% (can be higher with doses approaching 900 mg/d); slow titration needed |
| *Pooled data from clinical trials reporting percentage of patients taking clozapine who experienced adverse effects | |
| Source: Prescribing information for Clozaril® brand clozapine tablets. Available at: http://www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf. Accessed October 27, 2009 | |
Adjunctive treatments. Patients with a low baseline white blood cell count (WBC) and/or absolute neutrophil count (ANC) may benefit from adjunctive lithium treatment to increase their WBC, as demonstrated in case reports.18
When no other alternatives were clinically feasible, chronic treatment with granulocyte colony-stimulating factor (filgrastim) has been used successfully for some patients whose clozapine course was interrupted because of a low WBC and/or ANC.19
- Clozapine product information (as revised July 2009): www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf.
- Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
- Teva: www.clozapineregistry.com.
- Clozaril: www.clozarilcare.com.
- Caraco: www.caracoclozapine.com.
- FazaClo: www.fazacloregistry.com.
- Mylan: www.mylan-clozapine.com.
- Chlorpromazine • Thorazine
- Clozapine • Clozaril, FazaClo
- Filgrastim • Neupogen
- Haloperidol • Haldol
- Lithium • Lithobid, others
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca, Avanir Pharmaceuticals, Azur Pharma Inc., Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Pfizer Inc., Schering-Plough Corporation, and Vanda Pharmaceuticals. No writing assistance or external financial support was utilized in the preparation of this review article.
1. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627 [online-only data supplement available with the article at ].
2. Schulte PFJ. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Ann Pharmacother. 2006;40:683-688.
3. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harvard Rev Psychiatry. 2002;10:280-291.
4. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
5. Citrome L, Jaffe A, Martello D, et al. Did CATIE influence antipsychotic use? Psychiatr Serv. 2008;59(5):476.-
6. Krakowski MI, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
7. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.Erratum in: Arch Gen Psychiatry. 2003;60(7):735.
8. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
9. Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
10. Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60(6):553-564.
11. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
12. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152-163.
13. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
14. Opgen-Rhein C, Dettling M. Clozapine-induced agranulocytosis and its genetic determinants. Pharmacogenomics. 2008;9(8):1101-1111.
15. Krakowski M, Czobor P, Citrome L. Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double-blind clozapine, olanzapine or haloperidol. Schizophr Res. 2009;110(1-3):95-102.
16. Citrome L, Vreeland B. Schizophrenia, obesity, and antipsychotic medications: what can we do? Postgrad Med. 2008;120(2):18-33.
17. Annamraju S, Sheitman B, Saik S, et al. Early recognition of clozapine-induced myocarditis. J Clin Psychopharmacol. 2007;27(5):479-483.
18. Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009;9(1):55-71.
19. Mathewson KA, Lindenmayer JP. Clozapine and granulocyte colony-stimulating factor: potential for long-term combination treatment for clozapine-induced neutropenia. J Clin Psycho pharmacol. 2007;27(6):714-715.
Researchers in Finland surprised psychiatrists this year by announcing that clozapine “seems to be associated with a substantially lower mortality than any other antipsychotic.”1 This finding also surprised the researchers, who expected their 11-year study to link long-term use of second-generation (“atypical”) antipsychotics with increased mortality in patients with schizophrenia. Instead they found longer lives in patients who used antipsychotics (and particularly clozapine), compared with no antipsychotic use.
This study’s findings do not change clozapine’s association with potentially fatal agranulocytosis as well as weight gain, metabolic abnormalities, and other adverse effects. Clozapine also is difficult to administer ( Box 1 ),2 and patients must be enrolled in FDA-mandated registries (see Related Resources ). These obstacles might discourage you from offering clozapine to patients who could benefit from it ( Box 2 ).3-5
Why bother considering clozapine? Because recent data on decreased mortality, decreased suicidality, and control of aggressive behavior make clozapine a compelling choice for many patients. Careful attention to clozapine’s adverse effect profile is necessary, but you can manage these risks with appropriate monitoring.
Because of clozapine’s risk for leukopenia and agranulocytosis, frequent white blood cell count (WBC) monitoring is required. The risk of drug-induced blood dyscrasias has been shown to decrease over time, however, from 0.70/1,000 patient-years in the second 6 months of treatment to 0.39/1,000 patient-years after the first year.2
To start clozapine treatment, FDA guidelines require that the patient’s WBC must be at least 3,500 mm3, and the absolute neutrophil count (ANC) must be at least 2,000 mm3. For the first 6 months, patients receiving clozapine must have a weekly blood test for WBC and ANC.
The dispensing pharmacist must see the blood work result prior to releasing the drug to the patient. The blood draw date must be within the previous 7 days for the pharmacist to dispense a 1-week supply of clozapine.
Decreased monitoring over time. After 6 months of continuous therapy with clozapine without any interruptions because of a low WBC and/or ANC—defined as WBC 3 and/or ANC 3 or increased monitoring (when WBC 3 and/or ANC 3)—the patient’s blood monitoring may be done every 14 days and a 2-week supply of clozapine can be dispensed.
After 12 months of continuous clozapine therapy—6 months of continuous weekly monitoring, then 6 months of continuous biweekly monitoring—without any interruptions or increased monitoring, the patient may have blood monitoring done every 4 weeks and can receive a 4-week supply of clozapine.
One advantage of these monitoring requirements is that the increased frequency of visits can be used to foster greater patient engagement with treatment and promote a therapeutic alliance. Peer-led clozapine support groups, available in some communities, can facilitate adherence to monitoring requirements.
Clozapine was approved in the United States in 1989 for severely ill patients with schizophrenia who had not responded adequately to standard drug treatment. In 2002 it received an indication for patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for re-experiencing suicidal behavior, based on history and recent clinical state.
Off-label, clozapine has been commonly used for refractory bipolar disorder. Since 1998, it has been available in generic formulations and in a proprietary orally-disintegrating tablet formulation.
Dosing. The recommended target clozapine dosage is 300 to 450 mg/d. If an adequate response is not achieved, obtaining a plasma level might be helpful.3 Plasma levels ≥350 ng/mL constitute an optimal clozapine trial.
Not a ‘last resort.’ American Psychiatric Association treatment guidelines for schizophrenia state: “Because of clozapine’s superior efficacy, a trial of clozapine should be considered for a patient with a clinically inadequate response to antipsychotic treatment or for a patient with suicidal ideation or behavior. Besides clozapine, there are limited options for the many patients who have severe and significant residual symptoms even after antipsychotic monotherapy has been optimized, and none have proven benefits.”4
As additional evidence accumulates—including benefits regarding mortality and aggression—clozapine’s advantages support its clinical use earlier than as a “last resort.” In institutional settings, clozapine use has increased with the availability of additional data, such as from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE).
In New York State Office of Mental Health hospitals, clozapine use increased from 20.6% of prescriptions in 2005 to 24.9% in 2007, compared with the other CATIE medications (olanzapine, quetiapine, risperidone, ziprasidone) and haloperidol.5 Whether clozapine use will increase in outpatient settings remains to be seen.
Potential for longer life?
The population-based, cohort study from Finland demonstrated that—contrary to popular belief—the introduction of atypical antipsychotics during the 1990s did not adversely affect mortality of patients with schizophrenia, at least in Finland.1
This study made specific drug comparisons and used perphenazine as the reference drug. The lowest risk for mortality was observed with clozapine, which showed a 26% relative advantage compared with perphenazine. Clozapine’s advantage was statistically significant when compared with all other antipsychotics tested.
The authors suggested provocatively that restrictions on clozapine use as a second- or third-line agent should be reassessed. A few caveats, however, might affect how one interprets this study or applies its findings to clinical practice:
- The main comparisons were for patients receiving outpatient antipsychotic monotherapy. No information was available about antipsychotics used during inhospital treatment.
- Only the most frequently used atypical antipsychotics (clozapine, olanzapine, oral risperidone, and quetiapine) or the most frequently prescribed first-generation antipsychotics (oral perphenazine, thioridazine, and oral haloperidol) were assessed individually.
- Data about patients’ marital status, diagnoses of substance abuse, socioeconomic status, and other social variables were not available.
- Not all antipsychotics were available throughout the study (quetiapine was the newest and available for the shortest time).
- The study population consisted of patients of all ages, including those under 20 and over 70 years of age. Although the number of deaths and mortality rates increased with age, causes of mortality may differ when younger and older persons are compared. A data supplement to the study—available at www.thelancet.com—contains information about odds ratios by age and other factors.
Recommendation. Consider clozapine earlier than as a “last resort” in the disease course of patients with schizophrenia. At the very least, routinely present clozapine to patients and their families as a possible treatment option.
Antiaggressive properties
Case series and retrospective studies have provided insights into clozapine’s antiaggressive properties, but the strongest evidence comes from a 12-week, double-blind, randomized trial that specifically enrolled patients with violent behavior.6 Clozapine, olanzapine, and haloperidol were directly compared in the treatment of assaults and other aggressive behaviors by physically assaultive in patients with schizophrenia and schizoaffective disorder:
- The Modified Overt Aggression Scale (MOAS) physical aggression score measured the number and severity of assaults.
- The Positive and Negative Syndrome Scale (PANSS) was used to assess psychiatric symptoms.
Recommendation. Offer clozapine as an option for patients with schizophrenia or schizoaffective disorder and persistent aggressive behavior. Another antipsychotic might not be “good enough.”
Reduced risk of suicidality
The International Suicide Prevention Trial (InterSePT) was a multicenter, randomized, 2-year clinical study that compared the risk for suicidal behavior in patients treated with clozapine vs olanzapine.7 Enrolled were 980 patients with schizophrenia or schizoaffective disorder who were considered at high risk for suicide because of past suicide attempts or current suicidal ideation. Approximately one-quarter had not responded adequately to previous treatment.
All patients were seen weekly for 6 months, then biweekly for 18 months. The weekly or biweekly contact required to monitor for clozapine-associated agranulocytosis was matched with a similar visit schedule for olanzapine-treated patients, during which clinicians obtained vital signs. Primary endpoints included suicide attempts (including death), hospitalization to prevent suicide, and a rating of “much worsening of suicidality” from baseline. Blinded raters, including an independent suicide monitoring board, determined when patients achieved endpoint criteria.
Patients receiving clozapine showed significantly less suicidal behavior than those treated with olanzapine (a 24% relative advantage in the hazard ratio for suicide attempts or hospitalizations to prevent suicide). Fewer patients in the clozapine group:
- attempted suicide (34 vs 55)
- required hospitalization (82 vs 107) or rescue interventions to prevent suicide (118 vs 155)
- required concomitant treatment with antidepressants (221 vs 258) or anxiolytics/soporifics (301 vs 331).
More deaths from suicide occurred in the clozapine group than the olanzapine group, but the numbers were small (5 vs 3) and the difference between clozapine and olanzapine on this outcome was not statistically significant (P=.73).
Recommendation. Clozapine is a first-line treatment for patients with schizophrenia or schizoaffective disorder who exhibit suicidal behavior. This is reflected in the drug’s product labeling.
Superior symptom management
CATIE findings. Phase 2 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) showed clozapine to be more effective than other atypical antipsychotics, as measured by time to all-cause discontinuation.8 Patients in this phase of CATIE had discontinued another atypical antipsychotic in phase 1, principally because of lack of adequate efficacy. In phase 2, they were re-randomized to receive open-label clozapine or double-blinded risperidone, olanzapine, or quetiapine.
Only 90 patients were included in the time-to-discontinuation analysis, yet the greater amount of time that patients remained on clozapine (median 10.5 months) compared with quetiapine (median 3.3 months) or risperidone (median 2.8 months) was statistically significant. Time to discontinuation because of inadequate therapeutic effect also was significantly longer for clozapine than for olanzapine, quetiapine, or risperidone.
The NNT for the outcome of all-cause discontinuation for clozapine was 4 compared with risperidone and 3 compared with quetiapine. This means for every 4 or 3 patients randomly assigned to clozapine instead of risperidone or quetiapine, respectively, 1 additional patient successfully completed the CATIE trial on the original phase 2 medication.9 The NNT for clozapine vs olanzapine was 7, indicating a respectable effect size difference that might have been statistically significant if the sample size had been larger.
Meta-analyses support CATIE results. Clozapine’s greater efficacy (and effectiveness) compared with other antipsychotics as demonstrated in CATIE is supported by 2 meta-analyses:
- A systematic review of clinical trials between January 1953 and May 2002 found clozapine’s effect size in reducing symptoms for patients with schizophrenia was greater than that of any other antipsychotic.10
- In a similar but more recent meta-analysis of 150 double-blind, mostly short-term studies totaling 21,533 participants, clozapine showed the largest effect size when atypical antipsychotics were compared with first-generation antipsychotics.11
Caveats about clozapine
First-episode schizophrenia. Clozapine has been shown to be more effective than chlorpromazine in terms of time to remission and maintenance of remission for treatment-naïve patients with first-episode schizophrenia.13 Even so, most clinicians probably would not consider clozapine as a first-line treatment for an uncomplicated first-episode patient because of concerns about agranulocytosis. When genetic testing becomes available to determine individual risk for agranulocytosis, perhaps clozapine will be used earlier in the disease course.14
The patient’s ethnicity may influence the risk of adverse effects, as observed in the study examining clozapine’s antiaggressive effect;6 African-American patients receiving antipsychotics—and particularly clozapine—may be more likely to develop metabolic abnormalities than patients in other ethnic groups.15 Carefully monitor all patients receiving clozapine for metabolic adverse effects, and be prepared to institute remediative psychosocial, lifestyle, and adjunctive medication interventions, such as statins.
16
Table
Common adverse effects of clozapine
| Adverse effect | Frequency* |
|---|---|
| Hypersalivation | 31% to 48% |
| Drowsiness/sedation/somnolence | 39% to 46% |
| Weight increase | 31% |
| Tachycardia | 25% |
| Dizziness/vertigo | 19% to 27% |
| Constipation | 14% to 25% |
| Seizures | 5% (can be higher with doses approaching 900 mg/d); slow titration needed |
| *Pooled data from clinical trials reporting percentage of patients taking clozapine who experienced adverse effects | |
| Source: Prescribing information for Clozaril® brand clozapine tablets. Available at: http://www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf. Accessed October 27, 2009 | |
Adjunctive treatments. Patients with a low baseline white blood cell count (WBC) and/or absolute neutrophil count (ANC) may benefit from adjunctive lithium treatment to increase their WBC, as demonstrated in case reports.18
When no other alternatives were clinically feasible, chronic treatment with granulocyte colony-stimulating factor (filgrastim) has been used successfully for some patients whose clozapine course was interrupted because of a low WBC and/or ANC.19
- Clozapine product information (as revised July 2009): www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf.
- Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
- Teva: www.clozapineregistry.com.
- Clozaril: www.clozarilcare.com.
- Caraco: www.caracoclozapine.com.
- FazaClo: www.fazacloregistry.com.
- Mylan: www.mylan-clozapine.com.
- Chlorpromazine • Thorazine
- Clozapine • Clozaril, FazaClo
- Filgrastim • Neupogen
- Haloperidol • Haldol
- Lithium • Lithobid, others
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca, Avanir Pharmaceuticals, Azur Pharma Inc., Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Pfizer Inc., Schering-Plough Corporation, and Vanda Pharmaceuticals. No writing assistance or external financial support was utilized in the preparation of this review article.
Researchers in Finland surprised psychiatrists this year by announcing that clozapine “seems to be associated with a substantially lower mortality than any other antipsychotic.”1 This finding also surprised the researchers, who expected their 11-year study to link long-term use of second-generation (“atypical”) antipsychotics with increased mortality in patients with schizophrenia. Instead they found longer lives in patients who used antipsychotics (and particularly clozapine), compared with no antipsychotic use.
This study’s findings do not change clozapine’s association with potentially fatal agranulocytosis as well as weight gain, metabolic abnormalities, and other adverse effects. Clozapine also is difficult to administer ( Box 1 ),2 and patients must be enrolled in FDA-mandated registries (see Related Resources ). These obstacles might discourage you from offering clozapine to patients who could benefit from it ( Box 2 ).3-5
Why bother considering clozapine? Because recent data on decreased mortality, decreased suicidality, and control of aggressive behavior make clozapine a compelling choice for many patients. Careful attention to clozapine’s adverse effect profile is necessary, but you can manage these risks with appropriate monitoring.
Because of clozapine’s risk for leukopenia and agranulocytosis, frequent white blood cell count (WBC) monitoring is required. The risk of drug-induced blood dyscrasias has been shown to decrease over time, however, from 0.70/1,000 patient-years in the second 6 months of treatment to 0.39/1,000 patient-years after the first year.2
To start clozapine treatment, FDA guidelines require that the patient’s WBC must be at least 3,500 mm3, and the absolute neutrophil count (ANC) must be at least 2,000 mm3. For the first 6 months, patients receiving clozapine must have a weekly blood test for WBC and ANC.
The dispensing pharmacist must see the blood work result prior to releasing the drug to the patient. The blood draw date must be within the previous 7 days for the pharmacist to dispense a 1-week supply of clozapine.
Decreased monitoring over time. After 6 months of continuous therapy with clozapine without any interruptions because of a low WBC and/or ANC—defined as WBC 3 and/or ANC 3 or increased monitoring (when WBC 3 and/or ANC 3)—the patient’s blood monitoring may be done every 14 days and a 2-week supply of clozapine can be dispensed.
After 12 months of continuous clozapine therapy—6 months of continuous weekly monitoring, then 6 months of continuous biweekly monitoring—without any interruptions or increased monitoring, the patient may have blood monitoring done every 4 weeks and can receive a 4-week supply of clozapine.
One advantage of these monitoring requirements is that the increased frequency of visits can be used to foster greater patient engagement with treatment and promote a therapeutic alliance. Peer-led clozapine support groups, available in some communities, can facilitate adherence to monitoring requirements.
Clozapine was approved in the United States in 1989 for severely ill patients with schizophrenia who had not responded adequately to standard drug treatment. In 2002 it received an indication for patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for re-experiencing suicidal behavior, based on history and recent clinical state.
Off-label, clozapine has been commonly used for refractory bipolar disorder. Since 1998, it has been available in generic formulations and in a proprietary orally-disintegrating tablet formulation.
Dosing. The recommended target clozapine dosage is 300 to 450 mg/d. If an adequate response is not achieved, obtaining a plasma level might be helpful.3 Plasma levels ≥350 ng/mL constitute an optimal clozapine trial.
Not a ‘last resort.’ American Psychiatric Association treatment guidelines for schizophrenia state: “Because of clozapine’s superior efficacy, a trial of clozapine should be considered for a patient with a clinically inadequate response to antipsychotic treatment or for a patient with suicidal ideation or behavior. Besides clozapine, there are limited options for the many patients who have severe and significant residual symptoms even after antipsychotic monotherapy has been optimized, and none have proven benefits.”4
As additional evidence accumulates—including benefits regarding mortality and aggression—clozapine’s advantages support its clinical use earlier than as a “last resort.” In institutional settings, clozapine use has increased with the availability of additional data, such as from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE).
In New York State Office of Mental Health hospitals, clozapine use increased from 20.6% of prescriptions in 2005 to 24.9% in 2007, compared with the other CATIE medications (olanzapine, quetiapine, risperidone, ziprasidone) and haloperidol.5 Whether clozapine use will increase in outpatient settings remains to be seen.
Potential for longer life?
The population-based, cohort study from Finland demonstrated that—contrary to popular belief—the introduction of atypical antipsychotics during the 1990s did not adversely affect mortality of patients with schizophrenia, at least in Finland.1
This study made specific drug comparisons and used perphenazine as the reference drug. The lowest risk for mortality was observed with clozapine, which showed a 26% relative advantage compared with perphenazine. Clozapine’s advantage was statistically significant when compared with all other antipsychotics tested.
The authors suggested provocatively that restrictions on clozapine use as a second- or third-line agent should be reassessed. A few caveats, however, might affect how one interprets this study or applies its findings to clinical practice:
- The main comparisons were for patients receiving outpatient antipsychotic monotherapy. No information was available about antipsychotics used during inhospital treatment.
- Only the most frequently used atypical antipsychotics (clozapine, olanzapine, oral risperidone, and quetiapine) or the most frequently prescribed first-generation antipsychotics (oral perphenazine, thioridazine, and oral haloperidol) were assessed individually.
- Data about patients’ marital status, diagnoses of substance abuse, socioeconomic status, and other social variables were not available.
- Not all antipsychotics were available throughout the study (quetiapine was the newest and available for the shortest time).
- The study population consisted of patients of all ages, including those under 20 and over 70 years of age. Although the number of deaths and mortality rates increased with age, causes of mortality may differ when younger and older persons are compared. A data supplement to the study—available at www.thelancet.com—contains information about odds ratios by age and other factors.
Recommendation. Consider clozapine earlier than as a “last resort” in the disease course of patients with schizophrenia. At the very least, routinely present clozapine to patients and their families as a possible treatment option.
Antiaggressive properties
Case series and retrospective studies have provided insights into clozapine’s antiaggressive properties, but the strongest evidence comes from a 12-week, double-blind, randomized trial that specifically enrolled patients with violent behavior.6 Clozapine, olanzapine, and haloperidol were directly compared in the treatment of assaults and other aggressive behaviors by physically assaultive in patients with schizophrenia and schizoaffective disorder:
- The Modified Overt Aggression Scale (MOAS) physical aggression score measured the number and severity of assaults.
- The Positive and Negative Syndrome Scale (PANSS) was used to assess psychiatric symptoms.
Recommendation. Offer clozapine as an option for patients with schizophrenia or schizoaffective disorder and persistent aggressive behavior. Another antipsychotic might not be “good enough.”
Reduced risk of suicidality
The International Suicide Prevention Trial (InterSePT) was a multicenter, randomized, 2-year clinical study that compared the risk for suicidal behavior in patients treated with clozapine vs olanzapine.7 Enrolled were 980 patients with schizophrenia or schizoaffective disorder who were considered at high risk for suicide because of past suicide attempts or current suicidal ideation. Approximately one-quarter had not responded adequately to previous treatment.
All patients were seen weekly for 6 months, then biweekly for 18 months. The weekly or biweekly contact required to monitor for clozapine-associated agranulocytosis was matched with a similar visit schedule for olanzapine-treated patients, during which clinicians obtained vital signs. Primary endpoints included suicide attempts (including death), hospitalization to prevent suicide, and a rating of “much worsening of suicidality” from baseline. Blinded raters, including an independent suicide monitoring board, determined when patients achieved endpoint criteria.
Patients receiving clozapine showed significantly less suicidal behavior than those treated with olanzapine (a 24% relative advantage in the hazard ratio for suicide attempts or hospitalizations to prevent suicide). Fewer patients in the clozapine group:
- attempted suicide (34 vs 55)
- required hospitalization (82 vs 107) or rescue interventions to prevent suicide (118 vs 155)
- required concomitant treatment with antidepressants (221 vs 258) or anxiolytics/soporifics (301 vs 331).
More deaths from suicide occurred in the clozapine group than the olanzapine group, but the numbers were small (5 vs 3) and the difference between clozapine and olanzapine on this outcome was not statistically significant (P=.73).
Recommendation. Clozapine is a first-line treatment for patients with schizophrenia or schizoaffective disorder who exhibit suicidal behavior. This is reflected in the drug’s product labeling.
Superior symptom management
CATIE findings. Phase 2 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) showed clozapine to be more effective than other atypical antipsychotics, as measured by time to all-cause discontinuation.8 Patients in this phase of CATIE had discontinued another atypical antipsychotic in phase 1, principally because of lack of adequate efficacy. In phase 2, they were re-randomized to receive open-label clozapine or double-blinded risperidone, olanzapine, or quetiapine.
Only 90 patients were included in the time-to-discontinuation analysis, yet the greater amount of time that patients remained on clozapine (median 10.5 months) compared with quetiapine (median 3.3 months) or risperidone (median 2.8 months) was statistically significant. Time to discontinuation because of inadequate therapeutic effect also was significantly longer for clozapine than for olanzapine, quetiapine, or risperidone.
The NNT for the outcome of all-cause discontinuation for clozapine was 4 compared with risperidone and 3 compared with quetiapine. This means for every 4 or 3 patients randomly assigned to clozapine instead of risperidone or quetiapine, respectively, 1 additional patient successfully completed the CATIE trial on the original phase 2 medication.9 The NNT for clozapine vs olanzapine was 7, indicating a respectable effect size difference that might have been statistically significant if the sample size had been larger.
Meta-analyses support CATIE results. Clozapine’s greater efficacy (and effectiveness) compared with other antipsychotics as demonstrated in CATIE is supported by 2 meta-analyses:
- A systematic review of clinical trials between January 1953 and May 2002 found clozapine’s effect size in reducing symptoms for patients with schizophrenia was greater than that of any other antipsychotic.10
- In a similar but more recent meta-analysis of 150 double-blind, mostly short-term studies totaling 21,533 participants, clozapine showed the largest effect size when atypical antipsychotics were compared with first-generation antipsychotics.11
Caveats about clozapine
First-episode schizophrenia. Clozapine has been shown to be more effective than chlorpromazine in terms of time to remission and maintenance of remission for treatment-naïve patients with first-episode schizophrenia.13 Even so, most clinicians probably would not consider clozapine as a first-line treatment for an uncomplicated first-episode patient because of concerns about agranulocytosis. When genetic testing becomes available to determine individual risk for agranulocytosis, perhaps clozapine will be used earlier in the disease course.14
The patient’s ethnicity may influence the risk of adverse effects, as observed in the study examining clozapine’s antiaggressive effect;6 African-American patients receiving antipsychotics—and particularly clozapine—may be more likely to develop metabolic abnormalities than patients in other ethnic groups.15 Carefully monitor all patients receiving clozapine for metabolic adverse effects, and be prepared to institute remediative psychosocial, lifestyle, and adjunctive medication interventions, such as statins.
16
Table
Common adverse effects of clozapine
| Adverse effect | Frequency* |
|---|---|
| Hypersalivation | 31% to 48% |
| Drowsiness/sedation/somnolence | 39% to 46% |
| Weight increase | 31% |
| Tachycardia | 25% |
| Dizziness/vertigo | 19% to 27% |
| Constipation | 14% to 25% |
| Seizures | 5% (can be higher with doses approaching 900 mg/d); slow titration needed |
| *Pooled data from clinical trials reporting percentage of patients taking clozapine who experienced adverse effects | |
| Source: Prescribing information for Clozaril® brand clozapine tablets. Available at: http://www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf. Accessed October 27, 2009 | |
Adjunctive treatments. Patients with a low baseline white blood cell count (WBC) and/or absolute neutrophil count (ANC) may benefit from adjunctive lithium treatment to increase their WBC, as demonstrated in case reports.18
When no other alternatives were clinically feasible, chronic treatment with granulocyte colony-stimulating factor (filgrastim) has been used successfully for some patients whose clozapine course was interrupted because of a low WBC and/or ANC.19
- Clozapine product information (as revised July 2009): www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf.
- Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
- Teva: www.clozapineregistry.com.
- Clozaril: www.clozarilcare.com.
- Caraco: www.caracoclozapine.com.
- FazaClo: www.fazacloregistry.com.
- Mylan: www.mylan-clozapine.com.
- Chlorpromazine • Thorazine
- Clozapine • Clozaril, FazaClo
- Filgrastim • Neupogen
- Haloperidol • Haldol
- Lithium • Lithobid, others
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca, Avanir Pharmaceuticals, Azur Pharma Inc., Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Pfizer Inc., Schering-Plough Corporation, and Vanda Pharmaceuticals. No writing assistance or external financial support was utilized in the preparation of this review article.
1. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627 [online-only data supplement available with the article at ].
2. Schulte PFJ. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Ann Pharmacother. 2006;40:683-688.
3. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harvard Rev Psychiatry. 2002;10:280-291.
4. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
5. Citrome L, Jaffe A, Martello D, et al. Did CATIE influence antipsychotic use? Psychiatr Serv. 2008;59(5):476.-
6. Krakowski MI, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
7. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.Erratum in: Arch Gen Psychiatry. 2003;60(7):735.
8. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
9. Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
10. Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60(6):553-564.
11. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
12. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152-163.
13. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
14. Opgen-Rhein C, Dettling M. Clozapine-induced agranulocytosis and its genetic determinants. Pharmacogenomics. 2008;9(8):1101-1111.
15. Krakowski M, Czobor P, Citrome L. Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double-blind clozapine, olanzapine or haloperidol. Schizophr Res. 2009;110(1-3):95-102.
16. Citrome L, Vreeland B. Schizophrenia, obesity, and antipsychotic medications: what can we do? Postgrad Med. 2008;120(2):18-33.
17. Annamraju S, Sheitman B, Saik S, et al. Early recognition of clozapine-induced myocarditis. J Clin Psychopharmacol. 2007;27(5):479-483.
18. Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009;9(1):55-71.
19. Mathewson KA, Lindenmayer JP. Clozapine and granulocyte colony-stimulating factor: potential for long-term combination treatment for clozapine-induced neutropenia. J Clin Psycho pharmacol. 2007;27(6):714-715.
1. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627 [online-only data supplement available with the article at ].
2. Schulte PFJ. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Ann Pharmacother. 2006;40:683-688.
3. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harvard Rev Psychiatry. 2002;10:280-291.
4. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
5. Citrome L, Jaffe A, Martello D, et al. Did CATIE influence antipsychotic use? Psychiatr Serv. 2008;59(5):476.-
6. Krakowski MI, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
7. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.Erratum in: Arch Gen Psychiatry. 2003;60(7):735.
8. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
9. Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
10. Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60(6):553-564.
11. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
12. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152-163.
13. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
14. Opgen-Rhein C, Dettling M. Clozapine-induced agranulocytosis and its genetic determinants. Pharmacogenomics. 2008;9(8):1101-1111.
15. Krakowski M, Czobor P, Citrome L. Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double-blind clozapine, olanzapine or haloperidol. Schizophr Res. 2009;110(1-3):95-102.
16. Citrome L, Vreeland B. Schizophrenia, obesity, and antipsychotic medications: what can we do? Postgrad Med. 2008;120(2):18-33.
17. Annamraju S, Sheitman B, Saik S, et al. Early recognition of clozapine-induced myocarditis. J Clin Psychopharmacol. 2007;27(5):479-483.
18. Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009;9(1):55-71.
19. Mathewson KA, Lindenmayer JP. Clozapine and granulocyte colony-stimulating factor: potential for long-term combination treatment for clozapine-induced neutropenia. J Clin Psycho pharmacol. 2007;27(6):714-715.
Can you interpret confidence intervals? It’s not that difficult
Number needed to treat (NNT) is a measure of clinical effect that has been called medicine’s “secret stat”(Box 1).1,2 By itself, however, the NNT provides no information about whether a trial result is probably true (statistical significance). If a NNT is statistically significant, the confidence interval (CI) can tell you the range of numbers within which the truth probably lies.
In the March 2007 issue of Current Psychiatry, we described how to use NNT to interpret and apply research data in daily practice.3 In this article, we explain the “secrets” of NNT and CI by providing sample calculations and several figures for visual learning. For illustration, we analyze data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) in schizophrenia, this time focusing on phase 2E—the efficacy pathway in which patients were randomly assigned to open-label clozapine or a double-blinded second-generation antipsychotic (SGA).4
Confidence intervals: Is the NNT statistically significant?
To find out a NNT’s statistical significance, you can examine the CI. A 95% CI means that the truth lies between the interval’s lower and upper bounds with a 95% probability.
Calculating CI. Although formulas to calculate the CI appear complicated,5 they are easily inserted into a Microsoft Excel-brand spreadsheet. Useful alternatives are online calculators (seeRelated Resources), which can be downloaded to your hand-held device or pocket PC.
Time magazine recently declared NNT as medicine’s “secret stat.”1 NNT allows us to place a number on how often we would see a difference between 2 interventions.
In a handbook on essentials of evidence-based clinical practice, Guyatt et al2 define NNT as “the number of patients who must receive an intervention of therapy during a specific period of time to prevent 1 adverse outcome or produce 1 positive outcome.”
If a difference in therapeutic outcome is seen once in every 5 patients treated with 1 intervention vs another (NNT of 5), it will likely influence day-to-day practice. However, if a therapeutic difference occurs in 1 of every 100 patients (NNT of 100), the difference between 2 treatments is not usually of great concern (except, for example, in assessing immunization against a rare but very dangerous illness).
A 95% CI of 5 to 15 means we are dealing with a NNT that with 95% probability falls between 5 and 15. However, if the NNT is not statistically significant, it becomes more difficult to describe the CI.6 A non-statistically significant NNT would have a CI that includes a negative number and a positive number: When comparing intervention A with intervention B, A might be better than B or B might be better than A. One bound of the CI may be a NNT of 10 and the other may be –10. It would be tempting to describe the CI as –10 to 10, but this would be misleading.
Attributable risk. NNT is calculated by taking the reciprocal of the difference between 2 rates for a particular outcome (Box 2). This difference is known as the attributable risk (AR). We can calculate a 95% CI for the AR, and the AR is considered statistically significant if both ends of the 95% CI are positive or both ends are negative.
If the 95% CI includes zero, then the AR is considered not statistically significant.
An AR value of zero means the rates of the outcome of interest are the same for the 2 interventions (there is no difference). Translating this to NNT would mean that no matter how many patients you treat with 1 intervention versus the other, you will not see a difference on the outcome of interest. The NNT would be “infinite” (represented by the symbol “∞”). Mathematically, if we tried to calculate the NNT when AR was zero, we would be trying to calculate the reciprocal of zero.
CI in CATIE’s efficacy phase
What do NNT and CI calculations tell us about data from clinical trials such as CATIE for schizophrenia? In CATIE, 1,493 patients were randomly assigned to 1 of 5 antipsychotics—perphenazine, olanzapine, quetiapine, risperidone, or ziprasidone—for up to 18 months. Patients who received an SGA and discontinued phase 1 before 18 months could participate in phase 2:
- Those who discontinued because of poor symptom control were expected to enter the efficacy arm (2E) and receive open-label clozapine (n = 49) or an SGA not taken in phase 1 (n = 50).
- Those who discontinued phase 1 because of poor tolerability (n = 444) were expected to enter the tolerability arm (2T), and receive an SGA they had not taken in phase 1.
The investigator could choose which arm a patient entered, but many more patients entered 2T than 2E (perhaps because they were reluctant to enter a pathway in which they might receive clozapine). Those in phase 2E who were randomly assigned to clozapine knew they were receiving clozapine and that clozapine was a treatment for patients who did not have successful outcomes with other antipsychotic(s). This design may have influenced whether or not patients remained in the study.
In phase 2E, time until treatment discontinuation for any reason was statistically significantly longer for clozapine (median 10.5 months) than for quetiapine (median 3.3 months) or risperidone (median 2.8 months) but not statistically significantly longer than for olanzapine (median 2.7 months).
What is the NNT for an outcome for drug A versus drug B?
fA = frequency of outcome for drug A
fB = frequency of outcome for drug B
Attributable risk (AR) = fA-fB
NNT = 1/AR
(By convention, we round up the NNT to the next higher whole number.)
For example, let’s say drugs A and B are used to treat depression, and they result in 6-week response rates of 55% and 75%, respectively. The NNT to see a difference between drug B and drug A in terms of responders at 6 weeks can be calculated as follows:
- Difference in response rates = 0.75-0.55 = 0.20
- NNT = 1/0.20 = 5
What happens if response rates are reversed?
- Difference in response rates = 0.55–0.75 = -0.20
- NNT = 1/(–0.20) = -5
Here the NNT is –5, meaning a disadvantage for drug B, or a number needed to harm (NNH) of +5
What happens if response rates are identical?
- Difference in response rates = 0.75-0.75 = 0
- NNT = 1/0 = "infinity" (∞)
A NNT of 8 means it would take an infinite number of patients on drug A vs drug B to see a difference (in other words, no difference). This is by definition the "weakest" possible effect size.
What happens if the response rate is 100% for one intervention and zero for the other?
- Difference in response rates = 1.00–0 = 1.00
- NNT = 1/1 = 1
Theoretically, this is the "strongest" possible effect size.
Thus all possible values of NNT range from 1 to ∞, or –1 to –∞ it is not possible for a NNT to be zero.
Time to discontinuation because of inadequate therapeutic effect was significantly longer for clozapine than for olanzapine, quetiapine, or risperi-done.4 These statements give us the rank order of the tested medications’ performance and some idea of the size of the differences. We do not know, however, how often these differences will affect day-to-day patient treatment.
The question becomes “how many patients do I need to treat with clozapine instead of [olanzapine, quetiapine, or risperidone] before I see 1 extra success (defined as remaining on the medication)?” Similar questions can be asked about other outcomes, such as adverse events. NNT can convert the study results to a common language: numbers of patients.
Advantages for clozapine. NNTs for outcomes in CATIE phase 2E are shown in the Table. From the conventional analysis,4 we knew that patients randomly assigned to clozapine were more likely to stay on clozapine than patients assigned to other SGAs. The NNT comparing clozapine with quetiapine is 3, which means for every 3 patients treated with clozapine instead of quetiapine, 1 extra patient remained on the drug. A NNT of 3 is a medium to large effect size,7 similar to that seen when antidepressant treatment is compared with placebo in terms of reducing depressive symptoms by at least 50% among patients with major depressive disorder.8
The NNT comparing clozapine with risperidone was 4 and that for olanzapine was 7. The difference in all-cause discontinuation between clozapine and olanzapine was not statistically significant, however, perhaps because of a small sample size. The effectiveness analysis included
only 45 patients assigned to clozapine, 14 to quetiapine, 14 to risperidone, and 17 to olanzapine—far fewer than the 183 to 333 subjects in each arm in the phase-1 effectiveness analyses.9
Disadvantages for clozapine can be seen as “negative” NNT values in the Table. A negative NNT can be interpreted as a number needed to harm (NNH).
Tolerability. Discontinuation because of poor tolerability showed a disadvantage when clozapine was compared with risperidone, with a NNT of –9 (in other words, a NNH of 9). This means that for every 9 patients receiving clozapine instead of risperidone, 1 extra patient would discontinue because of poor tolerability.
Anticholinergic effects. Another statistically significant disadvantage is seen when clozapine was compared with olanzapine on the occurrence of urinary hesitancy, dry mouth, or constipation, with a NNT for clozapine of –5 (NNH 5). The comparison of clozapine with risperidone on this outcome, which yielded a NNT of –8, was not statistically significant. Clozapine vs quetiapine on this measure also was not statistically significant but showed an advantage for clozapine (disadvantage for quetiapine), with a NNT of 4.
Sialorrhea is a common adverse event attributed to clozapine. Here the NNTs for clozapine compared with olanzapine, risperidone, and quetiapine were –5, –5, and –4, respectively. The comparison with risperidone was not statistically significant.
Table
Using NNTs to compare clozapine’s effects in CATIE phase 2E
| Comparison | Clozapine vs olanzapine | Clozapine vs risperidone | Clozapine vs quetiapine |
|---|---|---|---|
| All cause discontinuation | 7 | 4* | 3* |
| Discontinuation because of poor efficacy | 5 | 4* | 4* |
| Discontinuation because of poor tolerability | –20 | –9* | 10 |
| Urinary hesitancy, dry mouth, constipation | –5* | –8 | 4 |
| Sialorrhea | –5* | –5 | –4* |
| *Statistically significant p<0.05 | |||
Interpreting the CI
The CI width is affected by the variability of the estimate and the sample size, not the true population effect size. This means that a larger sample size might decrease the CI width. Sometimes, narrowing the CI width will change a nonsignificant result to statistically significant. When researchers design a study, a large sample size helps minimize the chance of not finding a statistically significant difference if a true difference exists.
A CI that includes ∞ indicates a NNT that is not statistically significant, but low CI boundaries (close to 1 or –1) can suggest potentially important results and the need for more studies to provide additional data. The study might have been “under-powered” with an inadequate sample size.
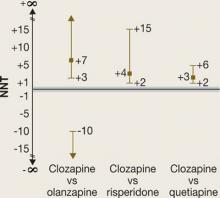
NNTs for all-cause discontinuation and their CIs when comparing clozapine with olanzapine, risperidone, or quetiapine in CATIE phase 2E are shown in Figure 1. The figure’s y-axis is centered on zero, but because a NNT must fall between 1 and ∞ (or –1 to –∞), we “grayed out” the interval around zero.
CI is easy to interpret for a statistically significant NNT. For NNT values that are not statistically significant, the CI contains 2 ranges of numbers. For the comparison of clozapine vs olanzapine, the 2 ranges are 3 to ∞ and –10 to –∞. The NNT of 7 falls within the range of 3 to ∞, but the 95% confidence interval also includes the range of –10 to –∞.
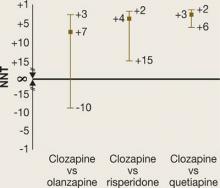
It may be easier to visualize and understand the CI by reformatting the figure so that it is centered on ∞ (Figure 2). Any CI that “crosses” ∞ represents a result that is not statistically significant. In Figure 1 and Figure 2 we also can examine the “width” of the CI. The comparison of clozapine vs quetiapine yields a NNT with a narrower CI than the comparison of clozapine vs risperidone. A narrow CI implies greater precision of our estimate of NNT and potentially its clinical importance.
Figure 1
CATIE Phase 2E: What was the advantage for clozapine?
NNTs for all-cause discontinuation and 95% confidence intervals in comparing clozapine with other SGAs. The y-axis is centered on zero, but because a NNT must fall between 1 and infinity (∞) (or –1 to –∞), the interval around zero is ‘grayed out.’
Figure 2
CATIE Phase 2E: What was the advantage for clozapine (revised)?
NNTs for all-cause discontinuation and 95% confidence intervals (CI) in comparing clozapine with other SGAs. Figure 2 shows Figure 1 reformatted to center on infinity (∞). Any CI that ‘crosses’ ∞ represents a result that is not statistically significant.Related Resources
- Confidence interval calculator. www.cebm.utoronto.ca/practise/ca/statscal.
- Guyatt G, Rennie D. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago, IL: AMA Press; 2001.
- Straus SE, Richardson WS, Glasziou P, et al. Evidence-based medicine: how to practice and teach EBM. 3rd ed. Edinburgh, UK: Elsevier/Churchill Livingstone; 2005.
Drug brand names
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca Pharmaceuticals, Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceutica, Jazz Pharmaceuticals, and Pfizer Inc.
1. Lemonick MD. Medicine’s secret stat. Time. February 15, 2007. Available at: http://www.time.com/time/printout/0,8816,1590464,00.html. Accessed February 20, 2007.
2. Guyatt G, Cook D, Devereaux PJ, et al. Therapy. In: Guyatt G, Rennie D, eds. Users’ guides to the medical literature, chapter 1B1. Chicago, IL: AMA Press, 2002.
3. Citrome L. Dissecting clinical trials with ‘number needed to treat.’ Current Psychiatry. 2007;6(3):66-71.
4. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-10.
5. Citrome L, Stroup TS. Schizophrenia, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract. 2006;60(8):933-40.
6. Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309-12.
7. Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990-6.
8. Pinson L, Gray GE. Number needed to treat: an underused measure of treatment effect. Psychiatr Serv. 2003;54(2):145-6,154.
9. Lieberman JA, Stroup TS, McEvoy JP, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-23.
Number needed to treat (NNT) is a measure of clinical effect that has been called medicine’s “secret stat”(Box 1).1,2 By itself, however, the NNT provides no information about whether a trial result is probably true (statistical significance). If a NNT is statistically significant, the confidence interval (CI) can tell you the range of numbers within which the truth probably lies.
In the March 2007 issue of Current Psychiatry, we described how to use NNT to interpret and apply research data in daily practice.3 In this article, we explain the “secrets” of NNT and CI by providing sample calculations and several figures for visual learning. For illustration, we analyze data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) in schizophrenia, this time focusing on phase 2E—the efficacy pathway in which patients were randomly assigned to open-label clozapine or a double-blinded second-generation antipsychotic (SGA).4
Confidence intervals: Is the NNT statistically significant?
To find out a NNT’s statistical significance, you can examine the CI. A 95% CI means that the truth lies between the interval’s lower and upper bounds with a 95% probability.
Calculating CI. Although formulas to calculate the CI appear complicated,5 they are easily inserted into a Microsoft Excel-brand spreadsheet. Useful alternatives are online calculators (seeRelated Resources), which can be downloaded to your hand-held device or pocket PC.
Time magazine recently declared NNT as medicine’s “secret stat.”1 NNT allows us to place a number on how often we would see a difference between 2 interventions.
In a handbook on essentials of evidence-based clinical practice, Guyatt et al2 define NNT as “the number of patients who must receive an intervention of therapy during a specific period of time to prevent 1 adverse outcome or produce 1 positive outcome.”
If a difference in therapeutic outcome is seen once in every 5 patients treated with 1 intervention vs another (NNT of 5), it will likely influence day-to-day practice. However, if a therapeutic difference occurs in 1 of every 100 patients (NNT of 100), the difference between 2 treatments is not usually of great concern (except, for example, in assessing immunization against a rare but very dangerous illness).
A 95% CI of 5 to 15 means we are dealing with a NNT that with 95% probability falls between 5 and 15. However, if the NNT is not statistically significant, it becomes more difficult to describe the CI.6 A non-statistically significant NNT would have a CI that includes a negative number and a positive number: When comparing intervention A with intervention B, A might be better than B or B might be better than A. One bound of the CI may be a NNT of 10 and the other may be –10. It would be tempting to describe the CI as –10 to 10, but this would be misleading.
Attributable risk. NNT is calculated by taking the reciprocal of the difference between 2 rates for a particular outcome (Box 2). This difference is known as the attributable risk (AR). We can calculate a 95% CI for the AR, and the AR is considered statistically significant if both ends of the 95% CI are positive or both ends are negative.
If the 95% CI includes zero, then the AR is considered not statistically significant.
An AR value of zero means the rates of the outcome of interest are the same for the 2 interventions (there is no difference). Translating this to NNT would mean that no matter how many patients you treat with 1 intervention versus the other, you will not see a difference on the outcome of interest. The NNT would be “infinite” (represented by the symbol “∞”). Mathematically, if we tried to calculate the NNT when AR was zero, we would be trying to calculate the reciprocal of zero.
CI in CATIE’s efficacy phase
What do NNT and CI calculations tell us about data from clinical trials such as CATIE for schizophrenia? In CATIE, 1,493 patients were randomly assigned to 1 of 5 antipsychotics—perphenazine, olanzapine, quetiapine, risperidone, or ziprasidone—for up to 18 months. Patients who received an SGA and discontinued phase 1 before 18 months could participate in phase 2:
- Those who discontinued because of poor symptom control were expected to enter the efficacy arm (2E) and receive open-label clozapine (n = 49) or an SGA not taken in phase 1 (n = 50).
- Those who discontinued phase 1 because of poor tolerability (n = 444) were expected to enter the tolerability arm (2T), and receive an SGA they had not taken in phase 1.
The investigator could choose which arm a patient entered, but many more patients entered 2T than 2E (perhaps because they were reluctant to enter a pathway in which they might receive clozapine). Those in phase 2E who were randomly assigned to clozapine knew they were receiving clozapine and that clozapine was a treatment for patients who did not have successful outcomes with other antipsychotic(s). This design may have influenced whether or not patients remained in the study.
In phase 2E, time until treatment discontinuation for any reason was statistically significantly longer for clozapine (median 10.5 months) than for quetiapine (median 3.3 months) or risperidone (median 2.8 months) but not statistically significantly longer than for olanzapine (median 2.7 months).
What is the NNT for an outcome for drug A versus drug B?
fA = frequency of outcome for drug A
fB = frequency of outcome for drug B
Attributable risk (AR) = fA-fB
NNT = 1/AR
(By convention, we round up the NNT to the next higher whole number.)
For example, let’s say drugs A and B are used to treat depression, and they result in 6-week response rates of 55% and 75%, respectively. The NNT to see a difference between drug B and drug A in terms of responders at 6 weeks can be calculated as follows:
- Difference in response rates = 0.75-0.55 = 0.20
- NNT = 1/0.20 = 5
What happens if response rates are reversed?
- Difference in response rates = 0.55–0.75 = -0.20
- NNT = 1/(–0.20) = -5
Here the NNT is –5, meaning a disadvantage for drug B, or a number needed to harm (NNH) of +5
What happens if response rates are identical?
- Difference in response rates = 0.75-0.75 = 0
- NNT = 1/0 = "infinity" (∞)
A NNT of 8 means it would take an infinite number of patients on drug A vs drug B to see a difference (in other words, no difference). This is by definition the "weakest" possible effect size.
What happens if the response rate is 100% for one intervention and zero for the other?
- Difference in response rates = 1.00–0 = 1.00
- NNT = 1/1 = 1
Theoretically, this is the "strongest" possible effect size.
Thus all possible values of NNT range from 1 to ∞, or –1 to –∞ it is not possible for a NNT to be zero.
Time to discontinuation because of inadequate therapeutic effect was significantly longer for clozapine than for olanzapine, quetiapine, or risperi-done.4 These statements give us the rank order of the tested medications’ performance and some idea of the size of the differences. We do not know, however, how often these differences will affect day-to-day patient treatment.
The question becomes “how many patients do I need to treat with clozapine instead of [olanzapine, quetiapine, or risperidone] before I see 1 extra success (defined as remaining on the medication)?” Similar questions can be asked about other outcomes, such as adverse events. NNT can convert the study results to a common language: numbers of patients.
Advantages for clozapine. NNTs for outcomes in CATIE phase 2E are shown in the Table. From the conventional analysis,4 we knew that patients randomly assigned to clozapine were more likely to stay on clozapine than patients assigned to other SGAs. The NNT comparing clozapine with quetiapine is 3, which means for every 3 patients treated with clozapine instead of quetiapine, 1 extra patient remained on the drug. A NNT of 3 is a medium to large effect size,7 similar to that seen when antidepressant treatment is compared with placebo in terms of reducing depressive symptoms by at least 50% among patients with major depressive disorder.8
The NNT comparing clozapine with risperidone was 4 and that for olanzapine was 7. The difference in all-cause discontinuation between clozapine and olanzapine was not statistically significant, however, perhaps because of a small sample size. The effectiveness analysis included
only 45 patients assigned to clozapine, 14 to quetiapine, 14 to risperidone, and 17 to olanzapine—far fewer than the 183 to 333 subjects in each arm in the phase-1 effectiveness analyses.9
Disadvantages for clozapine can be seen as “negative” NNT values in the Table. A negative NNT can be interpreted as a number needed to harm (NNH).
Tolerability. Discontinuation because of poor tolerability showed a disadvantage when clozapine was compared with risperidone, with a NNT of –9 (in other words, a NNH of 9). This means that for every 9 patients receiving clozapine instead of risperidone, 1 extra patient would discontinue because of poor tolerability.
Anticholinergic effects. Another statistically significant disadvantage is seen when clozapine was compared with olanzapine on the occurrence of urinary hesitancy, dry mouth, or constipation, with a NNT for clozapine of –5 (NNH 5). The comparison of clozapine with risperidone on this outcome, which yielded a NNT of –8, was not statistically significant. Clozapine vs quetiapine on this measure also was not statistically significant but showed an advantage for clozapine (disadvantage for quetiapine), with a NNT of 4.
Sialorrhea is a common adverse event attributed to clozapine. Here the NNTs for clozapine compared with olanzapine, risperidone, and quetiapine were –5, –5, and –4, respectively. The comparison with risperidone was not statistically significant.
Table
Using NNTs to compare clozapine’s effects in CATIE phase 2E
| Comparison | Clozapine vs olanzapine | Clozapine vs risperidone | Clozapine vs quetiapine |
|---|---|---|---|
| All cause discontinuation | 7 | 4* | 3* |
| Discontinuation because of poor efficacy | 5 | 4* | 4* |
| Discontinuation because of poor tolerability | –20 | –9* | 10 |
| Urinary hesitancy, dry mouth, constipation | –5* | –8 | 4 |
| Sialorrhea | –5* | –5 | –4* |
| *Statistically significant p<0.05 | |||
Interpreting the CI
The CI width is affected by the variability of the estimate and the sample size, not the true population effect size. This means that a larger sample size might decrease the CI width. Sometimes, narrowing the CI width will change a nonsignificant result to statistically significant. When researchers design a study, a large sample size helps minimize the chance of not finding a statistically significant difference if a true difference exists.
A CI that includes ∞ indicates a NNT that is not statistically significant, but low CI boundaries (close to 1 or –1) can suggest potentially important results and the need for more studies to provide additional data. The study might have been “under-powered” with an inadequate sample size.
NNTs for all-cause discontinuation and their CIs when comparing clozapine with olanzapine, risperidone, or quetiapine in CATIE phase 2E are shown in Figure 1. The figure’s y-axis is centered on zero, but because a NNT must fall between 1 and ∞ (or –1 to –∞), we “grayed out” the interval around zero.
CI is easy to interpret for a statistically significant NNT. For NNT values that are not statistically significant, the CI contains 2 ranges of numbers. For the comparison of clozapine vs olanzapine, the 2 ranges are 3 to ∞ and –10 to –∞. The NNT of 7 falls within the range of 3 to ∞, but the 95% confidence interval also includes the range of –10 to –∞.
It may be easier to visualize and understand the CI by reformatting the figure so that it is centered on ∞ (Figure 2). Any CI that “crosses” ∞ represents a result that is not statistically significant. In Figure 1 and Figure 2 we also can examine the “width” of the CI. The comparison of clozapine vs quetiapine yields a NNT with a narrower CI than the comparison of clozapine vs risperidone. A narrow CI implies greater precision of our estimate of NNT and potentially its clinical importance.
Figure 1
CATIE Phase 2E: What was the advantage for clozapine?
NNTs for all-cause discontinuation and 95% confidence intervals in comparing clozapine with other SGAs. The y-axis is centered on zero, but because a NNT must fall between 1 and infinity (∞) (or –1 to –∞), the interval around zero is ‘grayed out.’
Figure 2
CATIE Phase 2E: What was the advantage for clozapine (revised)?
NNTs for all-cause discontinuation and 95% confidence intervals (CI) in comparing clozapine with other SGAs. Figure 2 shows Figure 1 reformatted to center on infinity (∞). Any CI that ‘crosses’ ∞ represents a result that is not statistically significant.Related Resources
- Confidence interval calculator. www.cebm.utoronto.ca/practise/ca/statscal.
- Guyatt G, Rennie D. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago, IL: AMA Press; 2001.
- Straus SE, Richardson WS, Glasziou P, et al. Evidence-based medicine: how to practice and teach EBM. 3rd ed. Edinburgh, UK: Elsevier/Churchill Livingstone; 2005.
Drug brand names
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca Pharmaceuticals, Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceutica, Jazz Pharmaceuticals, and Pfizer Inc.
Number needed to treat (NNT) is a measure of clinical effect that has been called medicine’s “secret stat”(Box 1).1,2 By itself, however, the NNT provides no information about whether a trial result is probably true (statistical significance). If a NNT is statistically significant, the confidence interval (CI) can tell you the range of numbers within which the truth probably lies.
In the March 2007 issue of Current Psychiatry, we described how to use NNT to interpret and apply research data in daily practice.3 In this article, we explain the “secrets” of NNT and CI by providing sample calculations and several figures for visual learning. For illustration, we analyze data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) in schizophrenia, this time focusing on phase 2E—the efficacy pathway in which patients were randomly assigned to open-label clozapine or a double-blinded second-generation antipsychotic (SGA).4
Confidence intervals: Is the NNT statistically significant?
To find out a NNT’s statistical significance, you can examine the CI. A 95% CI means that the truth lies between the interval’s lower and upper bounds with a 95% probability.
Calculating CI. Although formulas to calculate the CI appear complicated,5 they are easily inserted into a Microsoft Excel-brand spreadsheet. Useful alternatives are online calculators (seeRelated Resources), which can be downloaded to your hand-held device or pocket PC.
Time magazine recently declared NNT as medicine’s “secret stat.”1 NNT allows us to place a number on how often we would see a difference between 2 interventions.
In a handbook on essentials of evidence-based clinical practice, Guyatt et al2 define NNT as “the number of patients who must receive an intervention of therapy during a specific period of time to prevent 1 adverse outcome or produce 1 positive outcome.”
If a difference in therapeutic outcome is seen once in every 5 patients treated with 1 intervention vs another (NNT of 5), it will likely influence day-to-day practice. However, if a therapeutic difference occurs in 1 of every 100 patients (NNT of 100), the difference between 2 treatments is not usually of great concern (except, for example, in assessing immunization against a rare but very dangerous illness).
A 95% CI of 5 to 15 means we are dealing with a NNT that with 95% probability falls between 5 and 15. However, if the NNT is not statistically significant, it becomes more difficult to describe the CI.6 A non-statistically significant NNT would have a CI that includes a negative number and a positive number: When comparing intervention A with intervention B, A might be better than B or B might be better than A. One bound of the CI may be a NNT of 10 and the other may be –10. It would be tempting to describe the CI as –10 to 10, but this would be misleading.
Attributable risk. NNT is calculated by taking the reciprocal of the difference between 2 rates for a particular outcome (Box 2). This difference is known as the attributable risk (AR). We can calculate a 95% CI for the AR, and the AR is considered statistically significant if both ends of the 95% CI are positive or both ends are negative.
If the 95% CI includes zero, then the AR is considered not statistically significant.
An AR value of zero means the rates of the outcome of interest are the same for the 2 interventions (there is no difference). Translating this to NNT would mean that no matter how many patients you treat with 1 intervention versus the other, you will not see a difference on the outcome of interest. The NNT would be “infinite” (represented by the symbol “∞”). Mathematically, if we tried to calculate the NNT when AR was zero, we would be trying to calculate the reciprocal of zero.
CI in CATIE’s efficacy phase
What do NNT and CI calculations tell us about data from clinical trials such as CATIE for schizophrenia? In CATIE, 1,493 patients were randomly assigned to 1 of 5 antipsychotics—perphenazine, olanzapine, quetiapine, risperidone, or ziprasidone—for up to 18 months. Patients who received an SGA and discontinued phase 1 before 18 months could participate in phase 2:
- Those who discontinued because of poor symptom control were expected to enter the efficacy arm (2E) and receive open-label clozapine (n = 49) or an SGA not taken in phase 1 (n = 50).
- Those who discontinued phase 1 because of poor tolerability (n = 444) were expected to enter the tolerability arm (2T), and receive an SGA they had not taken in phase 1.
The investigator could choose which arm a patient entered, but many more patients entered 2T than 2E (perhaps because they were reluctant to enter a pathway in which they might receive clozapine). Those in phase 2E who were randomly assigned to clozapine knew they were receiving clozapine and that clozapine was a treatment for patients who did not have successful outcomes with other antipsychotic(s). This design may have influenced whether or not patients remained in the study.
In phase 2E, time until treatment discontinuation for any reason was statistically significantly longer for clozapine (median 10.5 months) than for quetiapine (median 3.3 months) or risperidone (median 2.8 months) but not statistically significantly longer than for olanzapine (median 2.7 months).
What is the NNT for an outcome for drug A versus drug B?
fA = frequency of outcome for drug A
fB = frequency of outcome for drug B
Attributable risk (AR) = fA-fB
NNT = 1/AR
(By convention, we round up the NNT to the next higher whole number.)
For example, let’s say drugs A and B are used to treat depression, and they result in 6-week response rates of 55% and 75%, respectively. The NNT to see a difference between drug B and drug A in terms of responders at 6 weeks can be calculated as follows:
- Difference in response rates = 0.75-0.55 = 0.20
- NNT = 1/0.20 = 5
What happens if response rates are reversed?
- Difference in response rates = 0.55–0.75 = -0.20
- NNT = 1/(–0.20) = -5
Here the NNT is –5, meaning a disadvantage for drug B, or a number needed to harm (NNH) of +5
What happens if response rates are identical?
- Difference in response rates = 0.75-0.75 = 0
- NNT = 1/0 = "infinity" (∞)
A NNT of 8 means it would take an infinite number of patients on drug A vs drug B to see a difference (in other words, no difference). This is by definition the "weakest" possible effect size.
What happens if the response rate is 100% for one intervention and zero for the other?
- Difference in response rates = 1.00–0 = 1.00
- NNT = 1/1 = 1
Theoretically, this is the "strongest" possible effect size.
Thus all possible values of NNT range from 1 to ∞, or –1 to –∞ it is not possible for a NNT to be zero.
Time to discontinuation because of inadequate therapeutic effect was significantly longer for clozapine than for olanzapine, quetiapine, or risperi-done.4 These statements give us the rank order of the tested medications’ performance and some idea of the size of the differences. We do not know, however, how often these differences will affect day-to-day patient treatment.
The question becomes “how many patients do I need to treat with clozapine instead of [olanzapine, quetiapine, or risperidone] before I see 1 extra success (defined as remaining on the medication)?” Similar questions can be asked about other outcomes, such as adverse events. NNT can convert the study results to a common language: numbers of patients.
Advantages for clozapine. NNTs for outcomes in CATIE phase 2E are shown in the Table. From the conventional analysis,4 we knew that patients randomly assigned to clozapine were more likely to stay on clozapine than patients assigned to other SGAs. The NNT comparing clozapine with quetiapine is 3, which means for every 3 patients treated with clozapine instead of quetiapine, 1 extra patient remained on the drug. A NNT of 3 is a medium to large effect size,7 similar to that seen when antidepressant treatment is compared with placebo in terms of reducing depressive symptoms by at least 50% among patients with major depressive disorder.8
The NNT comparing clozapine with risperidone was 4 and that for olanzapine was 7. The difference in all-cause discontinuation between clozapine and olanzapine was not statistically significant, however, perhaps because of a small sample size. The effectiveness analysis included
only 45 patients assigned to clozapine, 14 to quetiapine, 14 to risperidone, and 17 to olanzapine—far fewer than the 183 to 333 subjects in each arm in the phase-1 effectiveness analyses.9
Disadvantages for clozapine can be seen as “negative” NNT values in the Table. A negative NNT can be interpreted as a number needed to harm (NNH).
Tolerability. Discontinuation because of poor tolerability showed a disadvantage when clozapine was compared with risperidone, with a NNT of –9 (in other words, a NNH of 9). This means that for every 9 patients receiving clozapine instead of risperidone, 1 extra patient would discontinue because of poor tolerability.
Anticholinergic effects. Another statistically significant disadvantage is seen when clozapine was compared with olanzapine on the occurrence of urinary hesitancy, dry mouth, or constipation, with a NNT for clozapine of –5 (NNH 5). The comparison of clozapine with risperidone on this outcome, which yielded a NNT of –8, was not statistically significant. Clozapine vs quetiapine on this measure also was not statistically significant but showed an advantage for clozapine (disadvantage for quetiapine), with a NNT of 4.
Sialorrhea is a common adverse event attributed to clozapine. Here the NNTs for clozapine compared with olanzapine, risperidone, and quetiapine were –5, –5, and –4, respectively. The comparison with risperidone was not statistically significant.
Table
Using NNTs to compare clozapine’s effects in CATIE phase 2E
| Comparison | Clozapine vs olanzapine | Clozapine vs risperidone | Clozapine vs quetiapine |
|---|---|---|---|
| All cause discontinuation | 7 | 4* | 3* |
| Discontinuation because of poor efficacy | 5 | 4* | 4* |
| Discontinuation because of poor tolerability | –20 | –9* | 10 |
| Urinary hesitancy, dry mouth, constipation | –5* | –8 | 4 |
| Sialorrhea | –5* | –5 | –4* |
| *Statistically significant p<0.05 | |||
Interpreting the CI
The CI width is affected by the variability of the estimate and the sample size, not the true population effect size. This means that a larger sample size might decrease the CI width. Sometimes, narrowing the CI width will change a nonsignificant result to statistically significant. When researchers design a study, a large sample size helps minimize the chance of not finding a statistically significant difference if a true difference exists.
A CI that includes ∞ indicates a NNT that is not statistically significant, but low CI boundaries (close to 1 or –1) can suggest potentially important results and the need for more studies to provide additional data. The study might have been “under-powered” with an inadequate sample size.
NNTs for all-cause discontinuation and their CIs when comparing clozapine with olanzapine, risperidone, or quetiapine in CATIE phase 2E are shown in Figure 1. The figure’s y-axis is centered on zero, but because a NNT must fall between 1 and ∞ (or –1 to –∞), we “grayed out” the interval around zero.
CI is easy to interpret for a statistically significant NNT. For NNT values that are not statistically significant, the CI contains 2 ranges of numbers. For the comparison of clozapine vs olanzapine, the 2 ranges are 3 to ∞ and –10 to –∞. The NNT of 7 falls within the range of 3 to ∞, but the 95% confidence interval also includes the range of –10 to –∞.
It may be easier to visualize and understand the CI by reformatting the figure so that it is centered on ∞ (Figure 2). Any CI that “crosses” ∞ represents a result that is not statistically significant. In Figure 1 and Figure 2 we also can examine the “width” of the CI. The comparison of clozapine vs quetiapine yields a NNT with a narrower CI than the comparison of clozapine vs risperidone. A narrow CI implies greater precision of our estimate of NNT and potentially its clinical importance.
Figure 1
CATIE Phase 2E: What was the advantage for clozapine?
NNTs for all-cause discontinuation and 95% confidence intervals in comparing clozapine with other SGAs. The y-axis is centered on zero, but because a NNT must fall between 1 and infinity (∞) (or –1 to –∞), the interval around zero is ‘grayed out.’
Figure 2
CATIE Phase 2E: What was the advantage for clozapine (revised)?
NNTs for all-cause discontinuation and 95% confidence intervals (CI) in comparing clozapine with other SGAs. Figure 2 shows Figure 1 reformatted to center on infinity (∞). Any CI that ‘crosses’ ∞ represents a result that is not statistically significant.Related Resources
- Confidence interval calculator. www.cebm.utoronto.ca/practise/ca/statscal.
- Guyatt G, Rennie D. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago, IL: AMA Press; 2001.
- Straus SE, Richardson WS, Glasziou P, et al. Evidence-based medicine: how to practice and teach EBM. 3rd ed. Edinburgh, UK: Elsevier/Churchill Livingstone; 2005.
Drug brand names
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Citrome is a consultant for, has received honoraria from, or has conducted clinical research supported by Abbott Laboratories, AstraZeneca Pharmaceuticals, Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Forest Research Institute, GlaxoSmithKline, Janssen Pharmaceutica, Jazz Pharmaceuticals, and Pfizer Inc.
1. Lemonick MD. Medicine’s secret stat. Time. February 15, 2007. Available at: http://www.time.com/time/printout/0,8816,1590464,00.html. Accessed February 20, 2007.
2. Guyatt G, Cook D, Devereaux PJ, et al. Therapy. In: Guyatt G, Rennie D, eds. Users’ guides to the medical literature, chapter 1B1. Chicago, IL: AMA Press, 2002.
3. Citrome L. Dissecting clinical trials with ‘number needed to treat.’ Current Psychiatry. 2007;6(3):66-71.
4. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-10.
5. Citrome L, Stroup TS. Schizophrenia, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract. 2006;60(8):933-40.
6. Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309-12.
7. Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990-6.
8. Pinson L, Gray GE. Number needed to treat: an underused measure of treatment effect. Psychiatr Serv. 2003;54(2):145-6,154.
9. Lieberman JA, Stroup TS, McEvoy JP, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-23.
1. Lemonick MD. Medicine’s secret stat. Time. February 15, 2007. Available at: http://www.time.com/time/printout/0,8816,1590464,00.html. Accessed February 20, 2007.
2. Guyatt G, Cook D, Devereaux PJ, et al. Therapy. In: Guyatt G, Rennie D, eds. Users’ guides to the medical literature, chapter 1B1. Chicago, IL: AMA Press, 2002.
3. Citrome L. Dissecting clinical trials with ‘number needed to treat.’ Current Psychiatry. 2007;6(3):66-71.
4. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-10.
5. Citrome L, Stroup TS. Schizophrenia, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract. 2006;60(8):933-40.
6. Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309-12.
7. Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990-6.
8. Pinson L, Gray GE. Number needed to treat: an underused measure of treatment effect. Psychiatr Serv. 2003;54(2):145-6,154.
9. Lieberman JA, Stroup TS, McEvoy JP, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-23.
Dissecting clinical trials with ‘number needed to treat’
Clinical trials produce a mountain of data that can be difficult to interpret and apply to clinical practice. When reading about studies such as the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) for schizophrenia, you may wonder:
- How large is the effect being measured?
- Is it clinically important?
- Are we dealing with a result that may be statistically significant but irrelevant for day-to-day patient care?
Number needed to treat (NNT) and number needed to harm (NNH)—two tools of evidence-based medicine (EBM, Box 11,2)—can help answer these questions. This article shows how to calculate NNT and NNH, then applies these tools to published results from CATIE phases 1 and 2.
Evidence-based medicine (EBM) is a process by which a clinician extracts information from the medical literature and applies it in day-to-day patient treatment. Gray and Pinson1 summarize EBM’s 5 steps as:
- formulate the question
- search for answers
- appraise the evidence
- apply the results
- assess the outcome.
This is not a trivial task. To help clinicians, EBM pioneers such as Gordon Guyatt, MD, MSc, and Drummond Rennie, MD, have published useful, readable, short reviews of EBM methods in the “Users’ Guides to the Medical Literature” in the Journal of the American Medical Association.2
Internet resources also are available, including:
- Centre for Evidence-Based Medicine, University of Toronto. www.cebm.utoronto.ca
- Eskind Biomedical Library, Vanderbilt University. Evidence-based knowledge portal. www.mc.vanderbilt.edu/biolib/ebmportal/login.html
- Hayward Medical Communications. Evidence-Based Medicine: What is…? series. www.evidence-based-medicine.co.uk/What_is_series.html.
What is nnt?
NNT helps us gauge effect size—or clinical significance. It is different from knowing if a clinical trial result is statistically significant.
NNT allows us to place a number on how often we can expect to see a difference between two interventions. If we see a therapeutic difference once every 100 patients (an NNT of 100), the difference between two treatments is not of great concern under most circumstances. But if a difference in outcome is seen once in every 5 patients being treated with one intervention versus another (an NNT of5), the result will likely influence day-to-day practice. Together with calculating a confidence interval (Box 2),3 the NNT can help you judge the clinical significance of a statistically significant result.
Calculating number needed to treat (NNT) or number needed to harm (NNH) does not tell you whether the result is statistically significant. You can find out by examining a range of values called the confidence interval (CI).
An NNT with a 95% CI means that the truth probably lies between the lower and upper bounds of the interval with a probability of 95%. A 95% CI with an NNT of 5 to 15 means we have an NNT that with 95% certainty falls between 5 and 15.
Formulas can be used to calculate CIs.3 One useful online calculator is available at: www.cebm.utoronto.ca/practise/ca/statscal.
Sometimes the lower bound of a CI is a negative number and the upper bound is a positive number (such as –10 to +10). This occurs when the result is not statistically significant. Having a negative number and a positive number in the CI means when comparing intervention A to intervention B, intervention A might be better than B, or B might be better than A. We could not conclude that a difference exists between the two interventions.
Calculating nnt and nnh
NNT and NNH are easy to calculate:
- First determine the difference between the frequencies of the outcome of interest for two interventions.
- Then calculate the reciprocal of this difference.
- Difference in response rates=0.75–0.55=0.20
- NNT=1/0.20=5.
Interpreting the importance of NNT values is easy, too. The smaller the NNT, the larger the clinical difference between interventions; the larger the NNT, the smaller the difference.
- An NNT of 100 or more usually means little difference exists between interventions for the outcome of interest.
- An NNT of 2 would be hugely important and is rarely encountered.
Example. We can calculate the NNT (actually, NNH) for risk of new-onset diabetes mellitus attributable to second-generation antipsychotics (SGAs), using data from a study that compared diabetes rates in patients given SGAs versus conventional antipsychotics.4 Differences in new-onset diabetes rates across ≤25 months were 2.03%, 0.80%, 0.63%, and 0.05% for clozapine, quetiapine, olanzapine, and risperidone, respectively, versus first-generation antipsychotics (FGAs).
The NNH for clozapine compared with FGAs is 1/0.0203=49. This means you would need to treat 49 patients with clozapine instead of an FGA for up to 25 months to encounter 1 extra case of new-onset diabetes mellitus. NNH calculations for quetiapine, olanzapine, and risperidone compared with FGAs would be 125, 159, and 2,000, respectively.
Applying nnt and nnh to catie
An ongoing controversy in schizophrenia treatment is the relative merit of using the more-expensive SGAs versus FGAs. The National Institute of Mental Health-funded CATIE study addressed this issue.5-7
In CATIE phase 1, which was double-blinded, 1,493 patients with schizophrenia were randomly assigned to 1 of 5 antipsychotics—perphenazine, olanzapine, quetiapine, risperidone, or ziprasidone—for up to 18 months. Patients who discontinued phase 1 before 18 months could participate in phase 2, where 543 patients were randomly assigned to 1 of 5 SGAs that they did not receive in phase 1. Those who prematurely discontinued phase 2 were offered open-label treatment with one or two antipsychotics. When they enrolled, patients were told these switches were possible.
Nearly one-half of all patients who enrolled finished 18 months of follow-up. What resulted, however, was a morass of percentages and p values that were misinterpreted by various parties—including The New York Times, which published an article headlined, “Little difference found in schizophrenia drugs.”8 We can apply NNT and NNH to the CATIE study results, however, and discover that:
- important differences do exist between the drugs tested
- these differences are clinically and statistically significant.3
- lack of efficacy
- poor tolerability
- patient decision.
- NNT=1/(difference in discontinuation rates)=1/(0.82 - 0.64)=1/0.18=5.6. By convention, we round up to the next whole number, in this case 6. This means that for every 6 patients randomized to olanzapine treatment, 1 extra patient completed phase 1 on his or her initially initial medication, compared with patients randomized to quetiapine treatment.
In measuring the number of hospitalizations for exacerbation of schizophrenia symptoms per total person-year of exposure, NNT ranged from 3 to 7 in favor of olanzapine compared with the other antipsychotics. This means that for every 3 to 7 patients treated with olanzapine versus another antipsychotic, 1 hospitalization was avoided.
Tolerability. Calculating NNH can show how often you could expect specific tolerability outcomes when comparing medications. In CATIE, differences in tolerability emerged among the medications, and each antipsychotic had a unique profile of relative strengths and weaknesses that can be expressed in NNT and NNH. For example, in CATIE phase 1:
- For every 5 to 8 patients treated with olanzapine compared to other antipsychotics, 1 additional patient gained >7% in body weight (NNH is 5 to 8; not corrected for duration of exposure to the medication)
- For every 13 to 18 patients treated with olanzapine versus another antipsychotic, 1 additional patient discontinued because of weight gain or metabolic effects.
Potential pitfalls
Different studies can provide different estimates of outcomes such as response, remission, hospitalization, or adverse events. Two studies of the risk of new-onset diabetes with antipsychotics demonstrate that these differences can be difficult to interpret, particularly when populations and study designs differ.
- A Department of Veterans Affairs study of data on 56,849 patients4 produced an NNH of 159 when olanzapine was compared with conventional antipsychotics, meaning 1 extra case of new-onset diabetes was encountered for every 159 patients treated with olanzapine compared to conventional antipsychotics.
- In the CATIE study,5 examining new prescriptions of antidiabetic agents yields an NNH of 61 when olanzapine is compared with perphenazine, meaning that 1 extra case of a new prescription of an antidiabetic agent was encountered for every 61 patients treated with olanzapine versus perphenazine.
NNT and NNH are best calculated from well-controlled clinical trials. However, the underlying study design and potential biases may affect how NNT and NNH apply to clinical practice. A more complete discussion of the CATIE NNT and NNH secondary analysis can be found elsewhere,3 but issues to consider include the impact of differential switching9 and the possible effects of dosages.10
Related resources
- Guyatt G, Rennie D. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago: AMA Press; 2001.
- Straus SE, Richardson WS, Glasziou P, et al. Evidence-based medicine: how to practice and teach EBM, 3rd ed. Edinburgh, UK: Elsevier/Churchill Livingstone; 2005.
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Citrome receives research support from AstraZeneca Pharmaceuticals, Barr Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer. He is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Jazz Pharmaceuticals, and Pfizer, and a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Eli Lilly and Company, and Pfizer.
1. Gray GE, Pinson LA. Evidence-based medicine and psychiatric practice. Psychiatr Q 2003;74(4):387-99.
2. Guyatt GH, Rennie D. Users’ guides to the medical literature [editorial]. JAMA 1993;270(17):2096-7.
3. Citrome L, Stroup TS. Schizophrenia, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract 2006;60(8):933-40.
4. Leslie DL, Rosenheck RA. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am J Psychiatry 2004;161(9):1709-11.
5. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
6. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163(4):600-10.
7. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163(4):611-22.
8. Carey B. Little difference found in schizophrenia drugs. The New York Times. September 20, 2005.
9. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163(12):2090-5.
10. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harv Rev Psychiatry 2002;10(5):280-91.
Clinical trials produce a mountain of data that can be difficult to interpret and apply to clinical practice. When reading about studies such as the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) for schizophrenia, you may wonder:
- How large is the effect being measured?
- Is it clinically important?
- Are we dealing with a result that may be statistically significant but irrelevant for day-to-day patient care?
Number needed to treat (NNT) and number needed to harm (NNH)—two tools of evidence-based medicine (EBM, Box 11,2)—can help answer these questions. This article shows how to calculate NNT and NNH, then applies these tools to published results from CATIE phases 1 and 2.
Evidence-based medicine (EBM) is a process by which a clinician extracts information from the medical literature and applies it in day-to-day patient treatment. Gray and Pinson1 summarize EBM’s 5 steps as:
- formulate the question
- search for answers
- appraise the evidence
- apply the results
- assess the outcome.
This is not a trivial task. To help clinicians, EBM pioneers such as Gordon Guyatt, MD, MSc, and Drummond Rennie, MD, have published useful, readable, short reviews of EBM methods in the “Users’ Guides to the Medical Literature” in the Journal of the American Medical Association.2
Internet resources also are available, including:
- Centre for Evidence-Based Medicine, University of Toronto. www.cebm.utoronto.ca
- Eskind Biomedical Library, Vanderbilt University. Evidence-based knowledge portal. www.mc.vanderbilt.edu/biolib/ebmportal/login.html
- Hayward Medical Communications. Evidence-Based Medicine: What is…? series. www.evidence-based-medicine.co.uk/What_is_series.html.
What is nnt?
NNT helps us gauge effect size—or clinical significance. It is different from knowing if a clinical trial result is statistically significant.
NNT allows us to place a number on how often we can expect to see a difference between two interventions. If we see a therapeutic difference once every 100 patients (an NNT of 100), the difference between two treatments is not of great concern under most circumstances. But if a difference in outcome is seen once in every 5 patients being treated with one intervention versus another (an NNT of5), the result will likely influence day-to-day practice. Together with calculating a confidence interval (Box 2),3 the NNT can help you judge the clinical significance of a statistically significant result.
Calculating number needed to treat (NNT) or number needed to harm (NNH) does not tell you whether the result is statistically significant. You can find out by examining a range of values called the confidence interval (CI).
An NNT with a 95% CI means that the truth probably lies between the lower and upper bounds of the interval with a probability of 95%. A 95% CI with an NNT of 5 to 15 means we have an NNT that with 95% certainty falls between 5 and 15.
Formulas can be used to calculate CIs.3 One useful online calculator is available at: www.cebm.utoronto.ca/practise/ca/statscal.
Sometimes the lower bound of a CI is a negative number and the upper bound is a positive number (such as –10 to +10). This occurs when the result is not statistically significant. Having a negative number and a positive number in the CI means when comparing intervention A to intervention B, intervention A might be better than B, or B might be better than A. We could not conclude that a difference exists between the two interventions.
Calculating nnt and nnh
NNT and NNH are easy to calculate:
- First determine the difference between the frequencies of the outcome of interest for two interventions.
- Then calculate the reciprocal of this difference.
- Difference in response rates=0.75–0.55=0.20
- NNT=1/0.20=5.
Interpreting the importance of NNT values is easy, too. The smaller the NNT, the larger the clinical difference between interventions; the larger the NNT, the smaller the difference.
- An NNT of 100 or more usually means little difference exists between interventions for the outcome of interest.
- An NNT of 2 would be hugely important and is rarely encountered.
Example. We can calculate the NNT (actually, NNH) for risk of new-onset diabetes mellitus attributable to second-generation antipsychotics (SGAs), using data from a study that compared diabetes rates in patients given SGAs versus conventional antipsychotics.4 Differences in new-onset diabetes rates across ≤25 months were 2.03%, 0.80%, 0.63%, and 0.05% for clozapine, quetiapine, olanzapine, and risperidone, respectively, versus first-generation antipsychotics (FGAs).
The NNH for clozapine compared with FGAs is 1/0.0203=49. This means you would need to treat 49 patients with clozapine instead of an FGA for up to 25 months to encounter 1 extra case of new-onset diabetes mellitus. NNH calculations for quetiapine, olanzapine, and risperidone compared with FGAs would be 125, 159, and 2,000, respectively.
Applying nnt and nnh to catie
An ongoing controversy in schizophrenia treatment is the relative merit of using the more-expensive SGAs versus FGAs. The National Institute of Mental Health-funded CATIE study addressed this issue.5-7
In CATIE phase 1, which was double-blinded, 1,493 patients with schizophrenia were randomly assigned to 1 of 5 antipsychotics—perphenazine, olanzapine, quetiapine, risperidone, or ziprasidone—for up to 18 months. Patients who discontinued phase 1 before 18 months could participate in phase 2, where 543 patients were randomly assigned to 1 of 5 SGAs that they did not receive in phase 1. Those who prematurely discontinued phase 2 were offered open-label treatment with one or two antipsychotics. When they enrolled, patients were told these switches were possible.
Nearly one-half of all patients who enrolled finished 18 months of follow-up. What resulted, however, was a morass of percentages and p values that were misinterpreted by various parties—including The New York Times, which published an article headlined, “Little difference found in schizophrenia drugs.”8 We can apply NNT and NNH to the CATIE study results, however, and discover that:
- important differences do exist between the drugs tested
- these differences are clinically and statistically significant.3
- lack of efficacy
- poor tolerability
- patient decision.
- NNT=1/(difference in discontinuation rates)=1/(0.82 - 0.64)=1/0.18=5.6. By convention, we round up to the next whole number, in this case 6. This means that for every 6 patients randomized to olanzapine treatment, 1 extra patient completed phase 1 on his or her initially initial medication, compared with patients randomized to quetiapine treatment.
In measuring the number of hospitalizations for exacerbation of schizophrenia symptoms per total person-year of exposure, NNT ranged from 3 to 7 in favor of olanzapine compared with the other antipsychotics. This means that for every 3 to 7 patients treated with olanzapine versus another antipsychotic, 1 hospitalization was avoided.
Tolerability. Calculating NNH can show how often you could expect specific tolerability outcomes when comparing medications. In CATIE, differences in tolerability emerged among the medications, and each antipsychotic had a unique profile of relative strengths and weaknesses that can be expressed in NNT and NNH. For example, in CATIE phase 1:
- For every 5 to 8 patients treated with olanzapine compared to other antipsychotics, 1 additional patient gained >7% in body weight (NNH is 5 to 8; not corrected for duration of exposure to the medication)
- For every 13 to 18 patients treated with olanzapine versus another antipsychotic, 1 additional patient discontinued because of weight gain or metabolic effects.
Potential pitfalls
Different studies can provide different estimates of outcomes such as response, remission, hospitalization, or adverse events. Two studies of the risk of new-onset diabetes with antipsychotics demonstrate that these differences can be difficult to interpret, particularly when populations and study designs differ.
- A Department of Veterans Affairs study of data on 56,849 patients4 produced an NNH of 159 when olanzapine was compared with conventional antipsychotics, meaning 1 extra case of new-onset diabetes was encountered for every 159 patients treated with olanzapine compared to conventional antipsychotics.
- In the CATIE study,5 examining new prescriptions of antidiabetic agents yields an NNH of 61 when olanzapine is compared with perphenazine, meaning that 1 extra case of a new prescription of an antidiabetic agent was encountered for every 61 patients treated with olanzapine versus perphenazine.
NNT and NNH are best calculated from well-controlled clinical trials. However, the underlying study design and potential biases may affect how NNT and NNH apply to clinical practice. A more complete discussion of the CATIE NNT and NNH secondary analysis can be found elsewhere,3 but issues to consider include the impact of differential switching9 and the possible effects of dosages.10
Related resources
- Guyatt G, Rennie D. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago: AMA Press; 2001.
- Straus SE, Richardson WS, Glasziou P, et al. Evidence-based medicine: how to practice and teach EBM, 3rd ed. Edinburgh, UK: Elsevier/Churchill Livingstone; 2005.
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Citrome receives research support from AstraZeneca Pharmaceuticals, Barr Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer. He is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Jazz Pharmaceuticals, and Pfizer, and a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Eli Lilly and Company, and Pfizer.
Clinical trials produce a mountain of data that can be difficult to interpret and apply to clinical practice. When reading about studies such as the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) for schizophrenia, you may wonder:
- How large is the effect being measured?
- Is it clinically important?
- Are we dealing with a result that may be statistically significant but irrelevant for day-to-day patient care?
Number needed to treat (NNT) and number needed to harm (NNH)—two tools of evidence-based medicine (EBM, Box 11,2)—can help answer these questions. This article shows how to calculate NNT and NNH, then applies these tools to published results from CATIE phases 1 and 2.
Evidence-based medicine (EBM) is a process by which a clinician extracts information from the medical literature and applies it in day-to-day patient treatment. Gray and Pinson1 summarize EBM’s 5 steps as:
- formulate the question
- search for answers
- appraise the evidence
- apply the results
- assess the outcome.
This is not a trivial task. To help clinicians, EBM pioneers such as Gordon Guyatt, MD, MSc, and Drummond Rennie, MD, have published useful, readable, short reviews of EBM methods in the “Users’ Guides to the Medical Literature” in the Journal of the American Medical Association.2
Internet resources also are available, including:
- Centre for Evidence-Based Medicine, University of Toronto. www.cebm.utoronto.ca
- Eskind Biomedical Library, Vanderbilt University. Evidence-based knowledge portal. www.mc.vanderbilt.edu/biolib/ebmportal/login.html
- Hayward Medical Communications. Evidence-Based Medicine: What is…? series. www.evidence-based-medicine.co.uk/What_is_series.html.
What is nnt?
NNT helps us gauge effect size—or clinical significance. It is different from knowing if a clinical trial result is statistically significant.
NNT allows us to place a number on how often we can expect to see a difference between two interventions. If we see a therapeutic difference once every 100 patients (an NNT of 100), the difference between two treatments is not of great concern under most circumstances. But if a difference in outcome is seen once in every 5 patients being treated with one intervention versus another (an NNT of5), the result will likely influence day-to-day practice. Together with calculating a confidence interval (Box 2),3 the NNT can help you judge the clinical significance of a statistically significant result.
Calculating number needed to treat (NNT) or number needed to harm (NNH) does not tell you whether the result is statistically significant. You can find out by examining a range of values called the confidence interval (CI).
An NNT with a 95% CI means that the truth probably lies between the lower and upper bounds of the interval with a probability of 95%. A 95% CI with an NNT of 5 to 15 means we have an NNT that with 95% certainty falls between 5 and 15.
Formulas can be used to calculate CIs.3 One useful online calculator is available at: www.cebm.utoronto.ca/practise/ca/statscal.
Sometimes the lower bound of a CI is a negative number and the upper bound is a positive number (such as –10 to +10). This occurs when the result is not statistically significant. Having a negative number and a positive number in the CI means when comparing intervention A to intervention B, intervention A might be better than B, or B might be better than A. We could not conclude that a difference exists between the two interventions.
Calculating nnt and nnh
NNT and NNH are easy to calculate:
- First determine the difference between the frequencies of the outcome of interest for two interventions.
- Then calculate the reciprocal of this difference.
- Difference in response rates=0.75–0.55=0.20
- NNT=1/0.20=5.
Interpreting the importance of NNT values is easy, too. The smaller the NNT, the larger the clinical difference between interventions; the larger the NNT, the smaller the difference.
- An NNT of 100 or more usually means little difference exists between interventions for the outcome of interest.
- An NNT of 2 would be hugely important and is rarely encountered.
Example. We can calculate the NNT (actually, NNH) for risk of new-onset diabetes mellitus attributable to second-generation antipsychotics (SGAs), using data from a study that compared diabetes rates in patients given SGAs versus conventional antipsychotics.4 Differences in new-onset diabetes rates across ≤25 months were 2.03%, 0.80%, 0.63%, and 0.05% for clozapine, quetiapine, olanzapine, and risperidone, respectively, versus first-generation antipsychotics (FGAs).
The NNH for clozapine compared with FGAs is 1/0.0203=49. This means you would need to treat 49 patients with clozapine instead of an FGA for up to 25 months to encounter 1 extra case of new-onset diabetes mellitus. NNH calculations for quetiapine, olanzapine, and risperidone compared with FGAs would be 125, 159, and 2,000, respectively.
Applying nnt and nnh to catie
An ongoing controversy in schizophrenia treatment is the relative merit of using the more-expensive SGAs versus FGAs. The National Institute of Mental Health-funded CATIE study addressed this issue.5-7
In CATIE phase 1, which was double-blinded, 1,493 patients with schizophrenia were randomly assigned to 1 of 5 antipsychotics—perphenazine, olanzapine, quetiapine, risperidone, or ziprasidone—for up to 18 months. Patients who discontinued phase 1 before 18 months could participate in phase 2, where 543 patients were randomly assigned to 1 of 5 SGAs that they did not receive in phase 1. Those who prematurely discontinued phase 2 were offered open-label treatment with one or two antipsychotics. When they enrolled, patients were told these switches were possible.
Nearly one-half of all patients who enrolled finished 18 months of follow-up. What resulted, however, was a morass of percentages and p values that were misinterpreted by various parties—including The New York Times, which published an article headlined, “Little difference found in schizophrenia drugs.”8 We can apply NNT and NNH to the CATIE study results, however, and discover that:
- important differences do exist between the drugs tested
- these differences are clinically and statistically significant.3
- lack of efficacy
- poor tolerability
- patient decision.
- NNT=1/(difference in discontinuation rates)=1/(0.82 - 0.64)=1/0.18=5.6. By convention, we round up to the next whole number, in this case 6. This means that for every 6 patients randomized to olanzapine treatment, 1 extra patient completed phase 1 on his or her initially initial medication, compared with patients randomized to quetiapine treatment.
In measuring the number of hospitalizations for exacerbation of schizophrenia symptoms per total person-year of exposure, NNT ranged from 3 to 7 in favor of olanzapine compared with the other antipsychotics. This means that for every 3 to 7 patients treated with olanzapine versus another antipsychotic, 1 hospitalization was avoided.
Tolerability. Calculating NNH can show how often you could expect specific tolerability outcomes when comparing medications. In CATIE, differences in tolerability emerged among the medications, and each antipsychotic had a unique profile of relative strengths and weaknesses that can be expressed in NNT and NNH. For example, in CATIE phase 1:
- For every 5 to 8 patients treated with olanzapine compared to other antipsychotics, 1 additional patient gained >7% in body weight (NNH is 5 to 8; not corrected for duration of exposure to the medication)
- For every 13 to 18 patients treated with olanzapine versus another antipsychotic, 1 additional patient discontinued because of weight gain or metabolic effects.
Potential pitfalls
Different studies can provide different estimates of outcomes such as response, remission, hospitalization, or adverse events. Two studies of the risk of new-onset diabetes with antipsychotics demonstrate that these differences can be difficult to interpret, particularly when populations and study designs differ.
- A Department of Veterans Affairs study of data on 56,849 patients4 produced an NNH of 159 when olanzapine was compared with conventional antipsychotics, meaning 1 extra case of new-onset diabetes was encountered for every 159 patients treated with olanzapine compared to conventional antipsychotics.
- In the CATIE study,5 examining new prescriptions of antidiabetic agents yields an NNH of 61 when olanzapine is compared with perphenazine, meaning that 1 extra case of a new prescription of an antidiabetic agent was encountered for every 61 patients treated with olanzapine versus perphenazine.
NNT and NNH are best calculated from well-controlled clinical trials. However, the underlying study design and potential biases may affect how NNT and NNH apply to clinical practice. A more complete discussion of the CATIE NNT and NNH secondary analysis can be found elsewhere,3 but issues to consider include the impact of differential switching9 and the possible effects of dosages.10
Related resources
- Guyatt G, Rennie D. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago: AMA Press; 2001.
- Straus SE, Richardson WS, Glasziou P, et al. Evidence-based medicine: how to practice and teach EBM, 3rd ed. Edinburgh, UK: Elsevier/Churchill Livingstone; 2005.
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Citrome receives research support from AstraZeneca Pharmaceuticals, Barr Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer. He is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Jazz Pharmaceuticals, and Pfizer, and a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Eli Lilly and Company, and Pfizer.
1. Gray GE, Pinson LA. Evidence-based medicine and psychiatric practice. Psychiatr Q 2003;74(4):387-99.
2. Guyatt GH, Rennie D. Users’ guides to the medical literature [editorial]. JAMA 1993;270(17):2096-7.
3. Citrome L, Stroup TS. Schizophrenia, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract 2006;60(8):933-40.
4. Leslie DL, Rosenheck RA. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am J Psychiatry 2004;161(9):1709-11.
5. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
6. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163(4):600-10.
7. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163(4):611-22.
8. Carey B. Little difference found in schizophrenia drugs. The New York Times. September 20, 2005.
9. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163(12):2090-5.
10. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harv Rev Psychiatry 2002;10(5):280-91.
1. Gray GE, Pinson LA. Evidence-based medicine and psychiatric practice. Psychiatr Q 2003;74(4):387-99.
2. Guyatt GH, Rennie D. Users’ guides to the medical literature [editorial]. JAMA 1993;270(17):2096-7.
3. Citrome L, Stroup TS. Schizophrenia, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract 2006;60(8):933-40.
4. Leslie DL, Rosenheck RA. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am J Psychiatry 2004;161(9):1709-11.
5. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
6. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163(4):600-10.
7. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163(4):611-22.
8. Carey B. Little difference found in schizophrenia drugs. The New York Times. September 20, 2005.
9. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163(12):2090-5.
10. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harv Rev Psychiatry 2002;10(5):280-91.
Antipsychotics for patients without psychosis?
Controlled clinical trial results can help you make two prescribing decisions:
- Is an antipsychotic the right choice for this patient?
- If yes, which agent?
Prescribing antipsychotics off-label can be worthwhile when a patient gets better, but even then two worries remain:
- Most uses of antipsychotics for nonpsychotic illness are not evidence-based.
- This practice may expose clinicians to liability if the patient gets worse.
Consider the use of second-generation antipsychotics (SGAs) to manage acute behaviors in patients with dementia. The FDA ordered a black box warning in 2005 that SGAs may increase mortality risk in older patients. In October, the Clinical Antipsychotic Trials of Intervention Effectiveness-Alzheimer’s Disease (CATIE-AD) reported that SGAs’ side effects offset their benefits when compared with placebo (see Will CATIE-AD change dementia treatment?).1
What do you do when FDA-approved drugs fail to help your patient with dementia, unipolar depression, anxiety disorders, or other nonpsychotic symptoms, and SGAs may be the next consideration? The answers lie in managing side effects and knowing which antipsychotic uses are supported by data from controlled clinical trials, which we review here.
- FGAs showed efficacy for nonpsychotic disorders
- SGAs are associated with a lower risk of EPS and tardive dyskinesia at therapeutic dosages, compared with FGAs
- Many patients fail to respond adequately to medications approved for their illnesses
- Evidence on SGAs’ efficacy in nonpsychotic disorders has grown substantially in the past 10 years.
EPS: extrapyramidal symptoms
FGA: first-generation antipsychotic
SGA: second-generation antipsychotic
Prescribing considerations
For a variety of reasons (Box 1), SGAs have rapidly assumed a major role in treating nonpsychotic disorders. Thirty-one percent of psychotropics are dispensed off-label,2 and Buckley3 reported in a 3-state survey that 70% of SGA prescriptions were written for indications other than schizophrenia.
Using antipsychotics for nonpsychotic symptoms is a longstanding clinical practice. In schizophrenia patients, antipsychotics have been shown to improve psychotic and nonpsychotic symptoms: agitation, violence, negative symptoms, social isolation, depression, suicidality, anxiety, insomnia, poor appetite, compulsions, cognition, smoking, alcohol and drug use, polydipsia, tardive dyskinesia, and tardive dystonia. Some clinicians may view these reports as evidence that antipsychotics might relieve these symptoms in patients with nonpsychotic disorders as well, but the issue is more complicated than that (Box 2).4
Caveats. SGAs do offer clinicians unique tools; no other class of psychotropics can claim efficacy in psychotic disorders, bipolar disorder, depression, and other disorders we describe in this review. On the other hand:
- Although some SGAs are approved for bipolar disorder and one was recently approved to treat irritability in autism (Table 1), most SGA uses in nonpsychotic disorders are off-label and supported by few—if any—large, randomized, controlled trials.
- Antipsychotics can cause the very symptoms they relieve, including depression, obsessive-compulsive disorder (OCD), anxiety, poorer cognition, agitation, mania, insomnia, and abnormal movements.
- Few controlled studies have compared SGAs to usual first-line treatments; most have evaluated SGAs as adjuncts to other psychotropics—such as serotonin reuptake inhibitors (SRIs)—for patients with treatment-resistant disorders.
- Published head-to-head studies have rarely compared the efficacy of various SGAs in treating nonpsychotic disorders.
- Long-term safety studies of SGAs for nonpsychotic indications have not been done.
Table 1
Bipolar and other nonpsychotic indications FDA-approved for SGAs
| SGA | Bipolar mania | Bipolar depression | Bipolar maintenance | Other |
|---|---|---|---|---|
| Aripiprazole | Acute mania or mixed episodes | Bipolar I disorder, most recent episode manic or mixed | ||
| Clozapine | Risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorders | |||
| Olanzapine | Acute mania or mixed episodes; monotherapy or with lithium or valproate for manic episodes | Bipolar disorder maintenance monotherapy | ||
| Olanzapine/fluoxetine combination | Bipolar depressive episodes | |||
| Quetiapine | Acute manic episodes; monotherapy or with lithium or valproate | Bipolar depressive episodes | ||
| Risperidone | Acute mania or mixed episodes; monotherapy or with lithium or valproate | Irritability in autism | ||
| Ziprasidone | Acute manic or mixed episodes | |||
| SGA: second-generation antipsychotic (oral forms) | ||||
Prescribing decisions. SGA’s potential adverse effects complicate clinical decision-making. First you must decide whether to use an SGA for your patient with a nonpsychotic disorder.
Second-generation antipsychotics (SGAs) show efficacy in so many psychotic and nonpsychotic disorders that a specific therapeutic action for each disorder is highly doubtful. One might ask, then: What do they improve, and how do they do it?
The complete answer is beyond current understanding, unfortunately. We do know, however, that SGAs have not shown efficacy for treating nonpsychotic disorders that first-generation antipsychotics (FGAs) did not show—except perhaps for maintenance treatment in bipolar disorder.
Calming action. The major clinical action of SGAs appears to be in calming patients, which also was the first therapeutic effect attributed to the FGA chlorpromazine. This calming effect would explain SGAs’ efficacy in treating agitation, aggressiveness, anxiety, and possibly mania. Other clinical effects specific to psychosis and possibly to depression are possible.
Receptor-blocking action. SGAs’ D2 and 5-HT2A receptor-blocking activity may explain much of the drugs’ therapeutic effect. However, if SGAs’ effect on nonpsychotic symptoms derives from their action on nondopaminergic receptors, then individual SGAs would vary widely in efficacy and pure dopaminergic agents such as amisulpride would be ineffective.
SGAs also bind at other receptor sites, and the clinical importance of this may vary from patient to patient, drug to drug, and dose to dose.4
Knowing, for example, that antipsychotics have been shown to increase mortality and cerebrovascular events in older patients might make you less likely to prescribe an SGA for a patient with dementia-related agitation. No other pharmacologic treatment has shown clear efficacy for these patients, however, so other factors are important to consider, including:
- patient history and clinical characteristics
- potential side effects
- individual therapeutic response to previous medications.
Table 2
SGA uses in nonpsychotic disorders supported by evidence
from published double-blind clinical trials*
| SGA | Unipolar depression | OCD | Anxiety disorders | Dementia | Developmental disorders | Borderline personality disorder |
|---|---|---|---|---|---|---|
| Aripiprazole | Yes | |||||
| Clozapine | ||||||
| Olanzapine | Yes | Yes | Yes | Yes | Yes | |
| Quetiapine | Yes | |||||
| Risperidone | Yes | Yes | Yes | Yes | ||
| Ziprasidone | ||||||
| * Not including studies of bipolar disorder | ||||||
| OCD: obsessive-compulsive disorder | ||||||
| SGA: second-generation antipsychotic | ||||||
Dementia
Most Alzheimer’s patients—75% to 90%—experience behavioral problems during this progressive dementia. Double-blind studies have found risperidone (mean dosage ~1 mg/d) and olanzapine (mean dosage 5 to 10 mg/d) effective in reducing agitation and aggression, even in nonpsychotic patients with Alzheimer’s disease or vascular dementia.5,6 Quetiapine, ≤100 mg/d, was not more effective than placebo in reducing agitation.7 One study comparing IM olanzapine with IM lorazepam and placebo in acute agitation found both active treatments more effective than placebo.8
CATIE-AD—sponsored by the National Institute of Mental Health—compared olanzapine, risperidone, and quetiapine with placebo in 421 outpatients with behavioral symptoms such as psychosis, agitation, or aggressiveness.1 No significant differences were seen in overall effectiveness (measured as discontinuation for any cause9), although patients receiving olanzapine (mean dosage 5.5 mg/d) or risperidone (mean dosage 1 mg/d) had lower discontinuation rates for lack of efficacy than those receiving placebo.
Unfortunately, the results of the first phase of CATIE-AD provide no clear guidance on the therapeutic strategy to use in dementia. Its findings do suggest two secondary conclusions, however, about using SGAs for patients with dementia:
- Because quetiapine, mean dosage 56.5 mg/d, was not more effective than placebo on any measures, consider higher dosages when using this agent.
- Close attention to preventing and treating SGAs’ side effects is the key to effectively treating agitation and psychosis in dementia.
Recommendation. Nonpharmacologic interventions are an important part of treating behavioral problems in patients with dementia.13,14 Antipsychotics—particularly SGAs—have shown efficacy for psychosis and agitation in these patients and remain the first therapeutic option. The CATIE-AD investigators recommend that clinicians evaluate potential risks and benefits of pharmacotherapy and discuss these with patients and caregivers.1 Also:
- Consider which SGAs have the lowest risk of causing side effects for an individual patient.
- Start with low dosages and increase as needed, based on efficacy and tolerability.
Bipolar disorder
Acute mania. Five SGAs—aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone—are FDA-approved for acute mania (Table 1). Large double-blind studies supporting this indication show that SGAs have efficacy in treating mania as monotherapy and in combination with lithium or divalproex.15 These clinical trials included patients who were not psychotic at baseline.
Antipsychotic dosages in these studies were within the ranges used in schizophrenia treatment studies. Combining an SGA with lithium or divalproex generally yields greater reductions in mania rating scale scores, higher response rates, and higher remission rates than using lithium or divalproex alone. No published study has compared SGAs with each other in mania, but differences in efficacy among these compounds are likely to be small.16
Bipolar depression. SGAs’ efficacy in bipolar depression has been evaluated in double-blind studies, and quetiapine and the olanzapine/fluoxetine combination are FDA-approved for this indication.
Olanzapine plus fluoxetine was more effective in improving depressive symptoms than olanzapine alone in a double-blind study of 833 adults with depressive symptoms of bipolar I disorder, as measured by Montgomery-Åsburg Depression Rating Scale (MADRS) scores. Olanzapine alone was more effective than placebo. Mean dosages were olanzapine, 7.4 mg/d, and fluoxetine, 39.3 mg/d, in combination therapy and olanzapine, 9.7 mg/d, as monotherapy.
MADRS scores indicated that combination therapy—but not olanzapine alone—improved core depressive symptoms such as sadness, lassitude, inability to feel, and pessimistic thoughts.17
Quetiapine. A double-blind, placebo-controlled trial (BOLDER I) evaluated quetiapine in 542 outpatients experiencing a major depressive episode associated with bipolar I or II disorder. After 8 weeks, quetiapine at 300 or 600 mg/d was more effective than placebo in reducing depressive symptoms, as measured by MADRS score changes.
Response rates were 58% with quetiapine and 36% with placebo; remission rates were 53% with quetiapine and 28% with placebo. Most symptoms, including core depression items, improved significantly with quetiapine, compared with placebo.18 Results of a second double-blind study (BOLDER II) have been presented at conferences but have not been fully published.
Risperidone. A smaller double-blind study compared risperidone plus placebo, paroxetine plus placebo, and risperidone plus paroxetine in 30 patients in the depressed phase of bipolar I or II disorder. Patients continued taking mood stabilizers during the study. After 12 weeks, depressive symptoms improved significantly in all three groups, with no significant differences.19
Maintenance therapy. Olanzapine and aripiprazole are FDA-approved for maintenance therapy in bipolar disorder (Table 1).
Unipolar depression
FGAs have shown efficacy in depression in multiple controlled studies.20 SGAs have been evaluated mostly as add-on therapies in antidepressant-resistant depression.
Olanzapine. Shelton et al21 compared olanzapine monotherapy, fluoxetine monotherapy, and combined treatment in 34 nonpsychotic, treatment-resistant depressed subjects. Olanzapine plus fluoxetine was more effective than either agent alone. A subsequent double-blind study, however, showed similar efficacy after 8 weeks among the three treatments and nortriptyline monotherapy. Patients in the double-blind trial appeared to respond more rapidly to combined treatment than to the monotherapies.22
Risperidone. A multiphase study of the efficacy of risperidone augmentation in treatment-resistant major depression began when 489 outpatients (2% with psychotic symptoms) received open-label citalopram, 20 to 60 mg/d. After 4 to 6 weeks, 386 nonresponders entered the augmentation phase with open-label risperidone, 0.25 to 2 mg/d. After 4 to 6 weeks of combination therapy, 241 (63%) patients whose symptoms resolved entered a double-blind discontinuation phase, in which they were randomly assigned to augmentation with risperidone or placebo, while on citalopram.
Median time to relapse during the double-blind phase was 102 days with risperidone augmentation and 85 days with placebo—not a statistically significant difference. Relapse rates after 24 weeks were 53.3% and 54.6%, respectively.23 This study showed that the improvement observed after adding risperidone was not sustained over time.
Quetiapine. In a prospective single-blind study, paroxetine augmented with quetiapine, 200 mg/d, was compared to paroxetine alone in major depression with anxiety.24 Combination therapy was more effective in improving anxiety and depression symptoms.
Others. Open-label, add-on studies indicate that aripiprazole and ziprasidone can improve treatment-resistant depression.25-27
Anxiety disorders
OCD. SGAs also have been investigated as augmentation therapy for patients with OCD resistant to SRIs. A single-blind study of 27 patients found adjunctive quetiapine more effective than placebo in improving OCD symptoms.28 SGAs were more effective than placebo as augmentation therapy to SRIs for treatment-refractory OCD in double-blind, placebo-controlled studies using mean dosages of:
In other trials:
- A small double-blind study of patients with social anxiety disorder found olanzapine monotherapy more effective than placebo.34
- Low-dose risperidone (mean dosage 1.1 mg/d) improved core symptoms of generalized anxiety disorder in a 5-week, double-blind, placebo-controlled trial.35
- Some authors have reported clinical improvement of panic disorder with olanzapine augmentation.36
Developmental disorders
Antipsychotics represent one-third of all filled psychotropic prescriptions for individuals with pervasive developmental disorders (PDD).37 Haloperidol and thioridazine are the only two FDA-approved FGAs for severe behavioral problems in PDD (and for hyperactivity with conduct disorders). Recently, risperidone received FDA approval for the treatment of irritability associated with autistic disorder in children.
Risperidone—the most-studied SGA in the PDD population—has shown efficacy in autism and in PDD not otherwise specified. Risperidone at dosages >3 mg/d improved repetitive behavior and aggression in adult patients.38
In children with autism, risperidone can improve tantrums, aggression, and self-injury. In a study of risperidone’s effect on autism’s core symptoms, the authors reported improvements in repetitive and stereotyped behavior but not in social relatedness or verbal communication.39
Double-blind studies have shown positive effects on aggression and behavioral disturbances in children with conduct disorder, oppositional defiant disorder, and other disruptive disorders, developmentally delayed adolescents, and mentally retarded subjects of various ages.40-42 Children and adolescents appear to be more sensitive than adults to risperidone’s side effects such as weight gain, EPS, and pancreatitis.
Personality disorders
Antipsychotics have been recommended for paranoid ideas and psychotic-like symptoms in borderline personality disorder and in paranoid personality disorder.43
Olanzapine. A 24-week, double-blind study found low-dose olanzapine (mean dosage 5.3 mg/d) more effective than placebo for anxiety, interpersonal sensitivity, paranoia, and anger/hostility in women with borderline personality disorder.44
In another double-blind study, 12 weeks of olanzapine therapy (mean 6.9 mg/d) was more effective than placebo for inappropriate anger in borderline personality disorder, as measured by a modified Clinical Global Impression scale.45
Others. Anger and hostility improved more with aripiprazole, 15 mg/d, than with placebo in an 8-week double-blind study of patients with borderline personality disorder.46 Quetiapine, risperidone, ziprasidone, and clozapine have shown efficacy in open-label studies and case reports.
Related resources
- Boos J. Off label use–label off use? Ann Oncol 2003;14(1):1-5.
- Blum RS. Legal considerations in off-label medication prescribing. Arch Intern Med 2002;162(15):1777-9.
- Jeste DV, Dolder CR. Treatment of non-schizophrenic disorders: focus on atypical antipsychotics. J Psychiatr Res 2004;38(1):73-103.
- Food and Drug Administration. Searchable catalog of FDA-approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda.
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Nortriptyline • Pamelor, Aventyl
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Trémeau receives grant/research support from Eli Lilly and Company.
Dr. Citrome receives grant/research support from AstraZeneca, Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Janssen Research Foundation, and Pfizer; is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Jazz Pharmaceuticals, and GlaxoSmithKline; and is a speaker for Abbott Laboratories, AstraZeneca, Eli Lilly and Company, and Pfizer.
1. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med 2006;166:1021-6.
3. Buckley PF. New antipsychotic agents: emerging clinical profiles. J Clin Psychiatry 1999;60(suppl 1):12-7.
4. Shayegan DK, Stahl SM. Atypical antipsychotics: matching receptor profile to individual patient’s clinical profile. CNS Spectr 2004;9(10 suppl 11):6-14.
5. Jeste DV, Dolder CR, Nayak GV, Salzman C. Atypical antipsychotics in elderly patients with dementia or schizophrenia: review of recent literature. Harv Rev Psychiatry 2005;13(6):340-51.
6. Carson S, McDonagh MS, Peterson K. A systematic review of the efficacy and safety of atypical antipsychotics in patients with psychological and behavioral symptoms of dementia. J Am Geriatr Soc 2006;54(2):354-61.
7. Ballard C, Margallo-Lana M, Juszczak E, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ 2005;330(7496):874.-
8. Meehan KM, Wang H, David SR, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26(4):494-504.
9. Schneider LS, Tariot PN, Lyketsos CG, et al. National Institute of Mental Health Antipsychotic Trials of Intervention Effectiveness (CATIE). Alzheimer disease trial methodology. Am J Geriatr Psychiatry 2001;9:346-60.
10. Kennedy J, Deberdt W, Siegal A, et al. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer’s dementia. Int J Geriatr Psychiatry 2005;20(11):1020-7.
11. U.S. Food and Drug Administration. Center for Drug Evaluation and Research. FDA public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Cder/drug/advisory/antipsychotics.htm. Accessed October 17, 2006.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353(22):2335-41.
13. Rabins P, Bland W, Bright-Long L, et al. from the Work Group on Alzheimer’s disease and related dementias. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias of late life. American Psychiatric Association Practice Guideline 1997. Available at http://www.psych.org/psych_pract/ treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
14. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease: a random control trial. JAMA 1996;276:1725-31.
15. Citrome L, Goldberg JF, Stahl SM. Toward convergence in the medication treatment of bipolar disorder and schizophrenia. Harv Rev Psychiatry 2005;13(1):28-42.
16. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry 2006;67(4):509-16.
17. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60(11):1079-88.
18. Calabrese JR, Keck PE, Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005;162(7):1351-60.
19. Shelton RC, Stahl SM. Risperidone and paroxetine given singly and in combination for bipolar depression. J Clin Psychiatry 2004;65(12):1715-9.
20. Robertson MM, Trimble MR. Major tranquillisers used as antidepressants. A review. J Affect Disord 1982;4(3):173-93.
21. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158(1):131-4.
22. Shelton RC, Williamson DJ, Corya SA, et al. Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 2005;66(10):1289-97.
23. Rapaport MH, Gharabawi GM, Canuso CM, et al. Effects of risperidone augmentation in patients with treatment-resistant depression: results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology 2006;31(11):2505-13.
24. Yargic LI, Corapcioglu A, Kocabasoglu N, et al. A prospective randomized single-blind, multicenter trial comparing the efficacy and safety of paroxetine with and without quetiapine therapy in depression associated with anxiety. Int J Psychiatry Clin Pract 2004;8:205-11.
25. Papakostas GI, Petersen TJ, Kinrys G, et al. Aripiprazole augmentation of selective serotonin reuptake inhibitors for treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66(10):1326-30.
26. Papakostas GI, Petersen TJ, Nierenberg AA, et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry 2004;65(2):217-21.
27. Simon JS, Nemeroff CB. Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry 2005;66(10):1216-20.
28. Atmaca M, Kuloglu M, Tezcan E, Gecici O. Quetiapine augmentation in patients with treatment resistant obsessive-compulsive disorder: a single-blind, placebo-controlled study. Int Clin Psychopharmacol 2002;17(3):115-9.
29. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000;57(8):794-801.
30. Bystritsky A, Ackerman DL, Rosen RM, et al. Augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder using adjunctive olanzapine: a placebo-controlled trial. J Clin Psychiatry 2004;65(4):565-8.
31. Denys D, de Geus F, van Megen HJ, Westenberg HG. A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. J Clin Psychiatry 2004;65(8):1040-8.
32. Bartzokis G, Lu PH, Turner J, et al. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry 2005;57(5):474-9.
33. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002;159(10):1777-9.
34. Barnett SD, Kramer ML, Casat CD, et al. Efficacy of olanzapine in social anxiety disorder: a pilot study. J Psychopharmacol 2002;16(4):365-8.
35. Brawman-Mintzer O, Knapp RG, Nietert PJ. Adjunctive risperidone in generalized anxiety disorder: a double-blind, placebo-controlled study. J Clin Psychiatry 2005;66(10):1321-5.
36. Khaldi S, Kornreich C, Dan B, Pelc I. Usefulness of olanzapine in refractory panic attacks. J Clin Psychopharmacol 2003;23(1):100-1.
37. Lott IT, McGregor M, Engelman L, et al. Longitudinal prescribing patterns for psychoactive medications in community-based individuals with developmental disabilities: utilization of pharmacy records. J Intellect Disabil Res 2004;48(Pt 6):563-71.
38. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 1998;55(7):633-41.
39. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162(6):1142-8.
40. Vanden Borre R, Vermote R, Buttiens M, et al. Risperidone as addon therapy in behavioural disturbances in mental retardation: a double-blind placebo-controlled cross-over study. Acta Psychiatr Scand 1993;87(3):167-71.
41. Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, Melman CT. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry 2001;62(4):239-48.
42. Snyder R, Turgay A, Aman M, et al; Risperidone Conduct Study Group. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry 2002;41(9):1026-36.
43. Oldham JM, Gabbard GO, Goin MK, et al, from the workgroup on borderline personality disorder. Practice guideline for the treatment of patients with borderline personality disorder. American Psychiatric Association Practice Guideline 2001. Available at http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
44. Zanarini MC, Frankenburg FR. Olanzapine treatment of female borderline personality disorder patients: a double-blind, placebo-controlled pilot study. J Clin Psychiatry 2001;62(11):849-54.
45. Bogenschutz MP, George Nurnberg H. Olanzapine versus placebo in the treatment of borderline personality disorder. J Clin Psychiatry 2004;65(1):104-9.
46. Nickel MK, Muehlbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry 2006;163(5):833-8.
Controlled clinical trial results can help you make two prescribing decisions:
- Is an antipsychotic the right choice for this patient?
- If yes, which agent?
Prescribing antipsychotics off-label can be worthwhile when a patient gets better, but even then two worries remain:
- Most uses of antipsychotics for nonpsychotic illness are not evidence-based.
- This practice may expose clinicians to liability if the patient gets worse.
Consider the use of second-generation antipsychotics (SGAs) to manage acute behaviors in patients with dementia. The FDA ordered a black box warning in 2005 that SGAs may increase mortality risk in older patients. In October, the Clinical Antipsychotic Trials of Intervention Effectiveness-Alzheimer’s Disease (CATIE-AD) reported that SGAs’ side effects offset their benefits when compared with placebo (see Will CATIE-AD change dementia treatment?).1
What do you do when FDA-approved drugs fail to help your patient with dementia, unipolar depression, anxiety disorders, or other nonpsychotic symptoms, and SGAs may be the next consideration? The answers lie in managing side effects and knowing which antipsychotic uses are supported by data from controlled clinical trials, which we review here.
- FGAs showed efficacy for nonpsychotic disorders
- SGAs are associated with a lower risk of EPS and tardive dyskinesia at therapeutic dosages, compared with FGAs
- Many patients fail to respond adequately to medications approved for their illnesses
- Evidence on SGAs’ efficacy in nonpsychotic disorders has grown substantially in the past 10 years.
EPS: extrapyramidal symptoms
FGA: first-generation antipsychotic
SGA: second-generation antipsychotic
Prescribing considerations
For a variety of reasons (Box 1), SGAs have rapidly assumed a major role in treating nonpsychotic disorders. Thirty-one percent of psychotropics are dispensed off-label,2 and Buckley3 reported in a 3-state survey that 70% of SGA prescriptions were written for indications other than schizophrenia.
Using antipsychotics for nonpsychotic symptoms is a longstanding clinical practice. In schizophrenia patients, antipsychotics have been shown to improve psychotic and nonpsychotic symptoms: agitation, violence, negative symptoms, social isolation, depression, suicidality, anxiety, insomnia, poor appetite, compulsions, cognition, smoking, alcohol and drug use, polydipsia, tardive dyskinesia, and tardive dystonia. Some clinicians may view these reports as evidence that antipsychotics might relieve these symptoms in patients with nonpsychotic disorders as well, but the issue is more complicated than that (Box 2).4
Caveats. SGAs do offer clinicians unique tools; no other class of psychotropics can claim efficacy in psychotic disorders, bipolar disorder, depression, and other disorders we describe in this review. On the other hand:
- Although some SGAs are approved for bipolar disorder and one was recently approved to treat irritability in autism (Table 1), most SGA uses in nonpsychotic disorders are off-label and supported by few—if any—large, randomized, controlled trials.
- Antipsychotics can cause the very symptoms they relieve, including depression, obsessive-compulsive disorder (OCD), anxiety, poorer cognition, agitation, mania, insomnia, and abnormal movements.
- Few controlled studies have compared SGAs to usual first-line treatments; most have evaluated SGAs as adjuncts to other psychotropics—such as serotonin reuptake inhibitors (SRIs)—for patients with treatment-resistant disorders.
- Published head-to-head studies have rarely compared the efficacy of various SGAs in treating nonpsychotic disorders.
- Long-term safety studies of SGAs for nonpsychotic indications have not been done.
Table 1
Bipolar and other nonpsychotic indications FDA-approved for SGAs
| SGA | Bipolar mania | Bipolar depression | Bipolar maintenance | Other |
|---|---|---|---|---|
| Aripiprazole | Acute mania or mixed episodes | Bipolar I disorder, most recent episode manic or mixed | ||
| Clozapine | Risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorders | |||
| Olanzapine | Acute mania or mixed episodes; monotherapy or with lithium or valproate for manic episodes | Bipolar disorder maintenance monotherapy | ||
| Olanzapine/fluoxetine combination | Bipolar depressive episodes | |||
| Quetiapine | Acute manic episodes; monotherapy or with lithium or valproate | Bipolar depressive episodes | ||
| Risperidone | Acute mania or mixed episodes; monotherapy or with lithium or valproate | Irritability in autism | ||
| Ziprasidone | Acute manic or mixed episodes | |||
| SGA: second-generation antipsychotic (oral forms) | ||||
Prescribing decisions. SGA’s potential adverse effects complicate clinical decision-making. First you must decide whether to use an SGA for your patient with a nonpsychotic disorder.
Second-generation antipsychotics (SGAs) show efficacy in so many psychotic and nonpsychotic disorders that a specific therapeutic action for each disorder is highly doubtful. One might ask, then: What do they improve, and how do they do it?
The complete answer is beyond current understanding, unfortunately. We do know, however, that SGAs have not shown efficacy for treating nonpsychotic disorders that first-generation antipsychotics (FGAs) did not show—except perhaps for maintenance treatment in bipolar disorder.
Calming action. The major clinical action of SGAs appears to be in calming patients, which also was the first therapeutic effect attributed to the FGA chlorpromazine. This calming effect would explain SGAs’ efficacy in treating agitation, aggressiveness, anxiety, and possibly mania. Other clinical effects specific to psychosis and possibly to depression are possible.
Receptor-blocking action. SGAs’ D2 and 5-HT2A receptor-blocking activity may explain much of the drugs’ therapeutic effect. However, if SGAs’ effect on nonpsychotic symptoms derives from their action on nondopaminergic receptors, then individual SGAs would vary widely in efficacy and pure dopaminergic agents such as amisulpride would be ineffective.
SGAs also bind at other receptor sites, and the clinical importance of this may vary from patient to patient, drug to drug, and dose to dose.4
Knowing, for example, that antipsychotics have been shown to increase mortality and cerebrovascular events in older patients might make you less likely to prescribe an SGA for a patient with dementia-related agitation. No other pharmacologic treatment has shown clear efficacy for these patients, however, so other factors are important to consider, including:
- patient history and clinical characteristics
- potential side effects
- individual therapeutic response to previous medications.
Table 2
SGA uses in nonpsychotic disorders supported by evidence
from published double-blind clinical trials*
| SGA | Unipolar depression | OCD | Anxiety disorders | Dementia | Developmental disorders | Borderline personality disorder |
|---|---|---|---|---|---|---|
| Aripiprazole | Yes | |||||
| Clozapine | ||||||
| Olanzapine | Yes | Yes | Yes | Yes | Yes | |
| Quetiapine | Yes | |||||
| Risperidone | Yes | Yes | Yes | Yes | ||
| Ziprasidone | ||||||
| * Not including studies of bipolar disorder | ||||||
| OCD: obsessive-compulsive disorder | ||||||
| SGA: second-generation antipsychotic | ||||||
Dementia
Most Alzheimer’s patients—75% to 90%—experience behavioral problems during this progressive dementia. Double-blind studies have found risperidone (mean dosage ~1 mg/d) and olanzapine (mean dosage 5 to 10 mg/d) effective in reducing agitation and aggression, even in nonpsychotic patients with Alzheimer’s disease or vascular dementia.5,6 Quetiapine, ≤100 mg/d, was not more effective than placebo in reducing agitation.7 One study comparing IM olanzapine with IM lorazepam and placebo in acute agitation found both active treatments more effective than placebo.8
CATIE-AD—sponsored by the National Institute of Mental Health—compared olanzapine, risperidone, and quetiapine with placebo in 421 outpatients with behavioral symptoms such as psychosis, agitation, or aggressiveness.1 No significant differences were seen in overall effectiveness (measured as discontinuation for any cause9), although patients receiving olanzapine (mean dosage 5.5 mg/d) or risperidone (mean dosage 1 mg/d) had lower discontinuation rates for lack of efficacy than those receiving placebo.
Unfortunately, the results of the first phase of CATIE-AD provide no clear guidance on the therapeutic strategy to use in dementia. Its findings do suggest two secondary conclusions, however, about using SGAs for patients with dementia:
- Because quetiapine, mean dosage 56.5 mg/d, was not more effective than placebo on any measures, consider higher dosages when using this agent.
- Close attention to preventing and treating SGAs’ side effects is the key to effectively treating agitation and psychosis in dementia.
Recommendation. Nonpharmacologic interventions are an important part of treating behavioral problems in patients with dementia.13,14 Antipsychotics—particularly SGAs—have shown efficacy for psychosis and agitation in these patients and remain the first therapeutic option. The CATIE-AD investigators recommend that clinicians evaluate potential risks and benefits of pharmacotherapy and discuss these with patients and caregivers.1 Also:
- Consider which SGAs have the lowest risk of causing side effects for an individual patient.
- Start with low dosages and increase as needed, based on efficacy and tolerability.
Bipolar disorder
Acute mania. Five SGAs—aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone—are FDA-approved for acute mania (Table 1). Large double-blind studies supporting this indication show that SGAs have efficacy in treating mania as monotherapy and in combination with lithium or divalproex.15 These clinical trials included patients who were not psychotic at baseline.
Antipsychotic dosages in these studies were within the ranges used in schizophrenia treatment studies. Combining an SGA with lithium or divalproex generally yields greater reductions in mania rating scale scores, higher response rates, and higher remission rates than using lithium or divalproex alone. No published study has compared SGAs with each other in mania, but differences in efficacy among these compounds are likely to be small.16
Bipolar depression. SGAs’ efficacy in bipolar depression has been evaluated in double-blind studies, and quetiapine and the olanzapine/fluoxetine combination are FDA-approved for this indication.
Olanzapine plus fluoxetine was more effective in improving depressive symptoms than olanzapine alone in a double-blind study of 833 adults with depressive symptoms of bipolar I disorder, as measured by Montgomery-Åsburg Depression Rating Scale (MADRS) scores. Olanzapine alone was more effective than placebo. Mean dosages were olanzapine, 7.4 mg/d, and fluoxetine, 39.3 mg/d, in combination therapy and olanzapine, 9.7 mg/d, as monotherapy.
MADRS scores indicated that combination therapy—but not olanzapine alone—improved core depressive symptoms such as sadness, lassitude, inability to feel, and pessimistic thoughts.17
Quetiapine. A double-blind, placebo-controlled trial (BOLDER I) evaluated quetiapine in 542 outpatients experiencing a major depressive episode associated with bipolar I or II disorder. After 8 weeks, quetiapine at 300 or 600 mg/d was more effective than placebo in reducing depressive symptoms, as measured by MADRS score changes.
Response rates were 58% with quetiapine and 36% with placebo; remission rates were 53% with quetiapine and 28% with placebo. Most symptoms, including core depression items, improved significantly with quetiapine, compared with placebo.18 Results of a second double-blind study (BOLDER II) have been presented at conferences but have not been fully published.
Risperidone. A smaller double-blind study compared risperidone plus placebo, paroxetine plus placebo, and risperidone plus paroxetine in 30 patients in the depressed phase of bipolar I or II disorder. Patients continued taking mood stabilizers during the study. After 12 weeks, depressive symptoms improved significantly in all three groups, with no significant differences.19
Maintenance therapy. Olanzapine and aripiprazole are FDA-approved for maintenance therapy in bipolar disorder (Table 1).
Unipolar depression
FGAs have shown efficacy in depression in multiple controlled studies.20 SGAs have been evaluated mostly as add-on therapies in antidepressant-resistant depression.
Olanzapine. Shelton et al21 compared olanzapine monotherapy, fluoxetine monotherapy, and combined treatment in 34 nonpsychotic, treatment-resistant depressed subjects. Olanzapine plus fluoxetine was more effective than either agent alone. A subsequent double-blind study, however, showed similar efficacy after 8 weeks among the three treatments and nortriptyline monotherapy. Patients in the double-blind trial appeared to respond more rapidly to combined treatment than to the monotherapies.22
Risperidone. A multiphase study of the efficacy of risperidone augmentation in treatment-resistant major depression began when 489 outpatients (2% with psychotic symptoms) received open-label citalopram, 20 to 60 mg/d. After 4 to 6 weeks, 386 nonresponders entered the augmentation phase with open-label risperidone, 0.25 to 2 mg/d. After 4 to 6 weeks of combination therapy, 241 (63%) patients whose symptoms resolved entered a double-blind discontinuation phase, in which they were randomly assigned to augmentation with risperidone or placebo, while on citalopram.
Median time to relapse during the double-blind phase was 102 days with risperidone augmentation and 85 days with placebo—not a statistically significant difference. Relapse rates after 24 weeks were 53.3% and 54.6%, respectively.23 This study showed that the improvement observed after adding risperidone was not sustained over time.
Quetiapine. In a prospective single-blind study, paroxetine augmented with quetiapine, 200 mg/d, was compared to paroxetine alone in major depression with anxiety.24 Combination therapy was more effective in improving anxiety and depression symptoms.
Others. Open-label, add-on studies indicate that aripiprazole and ziprasidone can improve treatment-resistant depression.25-27
Anxiety disorders
OCD. SGAs also have been investigated as augmentation therapy for patients with OCD resistant to SRIs. A single-blind study of 27 patients found adjunctive quetiapine more effective than placebo in improving OCD symptoms.28 SGAs were more effective than placebo as augmentation therapy to SRIs for treatment-refractory OCD in double-blind, placebo-controlled studies using mean dosages of:
In other trials:
- A small double-blind study of patients with social anxiety disorder found olanzapine monotherapy more effective than placebo.34
- Low-dose risperidone (mean dosage 1.1 mg/d) improved core symptoms of generalized anxiety disorder in a 5-week, double-blind, placebo-controlled trial.35
- Some authors have reported clinical improvement of panic disorder with olanzapine augmentation.36
Developmental disorders
Antipsychotics represent one-third of all filled psychotropic prescriptions for individuals with pervasive developmental disorders (PDD).37 Haloperidol and thioridazine are the only two FDA-approved FGAs for severe behavioral problems in PDD (and for hyperactivity with conduct disorders). Recently, risperidone received FDA approval for the treatment of irritability associated with autistic disorder in children.
Risperidone—the most-studied SGA in the PDD population—has shown efficacy in autism and in PDD not otherwise specified. Risperidone at dosages >3 mg/d improved repetitive behavior and aggression in adult patients.38
In children with autism, risperidone can improve tantrums, aggression, and self-injury. In a study of risperidone’s effect on autism’s core symptoms, the authors reported improvements in repetitive and stereotyped behavior but not in social relatedness or verbal communication.39
Double-blind studies have shown positive effects on aggression and behavioral disturbances in children with conduct disorder, oppositional defiant disorder, and other disruptive disorders, developmentally delayed adolescents, and mentally retarded subjects of various ages.40-42 Children and adolescents appear to be more sensitive than adults to risperidone’s side effects such as weight gain, EPS, and pancreatitis.
Personality disorders
Antipsychotics have been recommended for paranoid ideas and psychotic-like symptoms in borderline personality disorder and in paranoid personality disorder.43
Olanzapine. A 24-week, double-blind study found low-dose olanzapine (mean dosage 5.3 mg/d) more effective than placebo for anxiety, interpersonal sensitivity, paranoia, and anger/hostility in women with borderline personality disorder.44
In another double-blind study, 12 weeks of olanzapine therapy (mean 6.9 mg/d) was more effective than placebo for inappropriate anger in borderline personality disorder, as measured by a modified Clinical Global Impression scale.45
Others. Anger and hostility improved more with aripiprazole, 15 mg/d, than with placebo in an 8-week double-blind study of patients with borderline personality disorder.46 Quetiapine, risperidone, ziprasidone, and clozapine have shown efficacy in open-label studies and case reports.
Related resources
- Boos J. Off label use–label off use? Ann Oncol 2003;14(1):1-5.
- Blum RS. Legal considerations in off-label medication prescribing. Arch Intern Med 2002;162(15):1777-9.
- Jeste DV, Dolder CR. Treatment of non-schizophrenic disorders: focus on atypical antipsychotics. J Psychiatr Res 2004;38(1):73-103.
- Food and Drug Administration. Searchable catalog of FDA-approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda.
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Nortriptyline • Pamelor, Aventyl
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Trémeau receives grant/research support from Eli Lilly and Company.
Dr. Citrome receives grant/research support from AstraZeneca, Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Janssen Research Foundation, and Pfizer; is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Jazz Pharmaceuticals, and GlaxoSmithKline; and is a speaker for Abbott Laboratories, AstraZeneca, Eli Lilly and Company, and Pfizer.
Controlled clinical trial results can help you make two prescribing decisions:
- Is an antipsychotic the right choice for this patient?
- If yes, which agent?
Prescribing antipsychotics off-label can be worthwhile when a patient gets better, but even then two worries remain:
- Most uses of antipsychotics for nonpsychotic illness are not evidence-based.
- This practice may expose clinicians to liability if the patient gets worse.
Consider the use of second-generation antipsychotics (SGAs) to manage acute behaviors in patients with dementia. The FDA ordered a black box warning in 2005 that SGAs may increase mortality risk in older patients. In October, the Clinical Antipsychotic Trials of Intervention Effectiveness-Alzheimer’s Disease (CATIE-AD) reported that SGAs’ side effects offset their benefits when compared with placebo (see Will CATIE-AD change dementia treatment?).1
What do you do when FDA-approved drugs fail to help your patient with dementia, unipolar depression, anxiety disorders, or other nonpsychotic symptoms, and SGAs may be the next consideration? The answers lie in managing side effects and knowing which antipsychotic uses are supported by data from controlled clinical trials, which we review here.
- FGAs showed efficacy for nonpsychotic disorders
- SGAs are associated with a lower risk of EPS and tardive dyskinesia at therapeutic dosages, compared with FGAs
- Many patients fail to respond adequately to medications approved for their illnesses
- Evidence on SGAs’ efficacy in nonpsychotic disorders has grown substantially in the past 10 years.
EPS: extrapyramidal symptoms
FGA: first-generation antipsychotic
SGA: second-generation antipsychotic
Prescribing considerations
For a variety of reasons (Box 1), SGAs have rapidly assumed a major role in treating nonpsychotic disorders. Thirty-one percent of psychotropics are dispensed off-label,2 and Buckley3 reported in a 3-state survey that 70% of SGA prescriptions were written for indications other than schizophrenia.
Using antipsychotics for nonpsychotic symptoms is a longstanding clinical practice. In schizophrenia patients, antipsychotics have been shown to improve psychotic and nonpsychotic symptoms: agitation, violence, negative symptoms, social isolation, depression, suicidality, anxiety, insomnia, poor appetite, compulsions, cognition, smoking, alcohol and drug use, polydipsia, tardive dyskinesia, and tardive dystonia. Some clinicians may view these reports as evidence that antipsychotics might relieve these symptoms in patients with nonpsychotic disorders as well, but the issue is more complicated than that (Box 2).4
Caveats. SGAs do offer clinicians unique tools; no other class of psychotropics can claim efficacy in psychotic disorders, bipolar disorder, depression, and other disorders we describe in this review. On the other hand:
- Although some SGAs are approved for bipolar disorder and one was recently approved to treat irritability in autism (Table 1), most SGA uses in nonpsychotic disorders are off-label and supported by few—if any—large, randomized, controlled trials.
- Antipsychotics can cause the very symptoms they relieve, including depression, obsessive-compulsive disorder (OCD), anxiety, poorer cognition, agitation, mania, insomnia, and abnormal movements.
- Few controlled studies have compared SGAs to usual first-line treatments; most have evaluated SGAs as adjuncts to other psychotropics—such as serotonin reuptake inhibitors (SRIs)—for patients with treatment-resistant disorders.
- Published head-to-head studies have rarely compared the efficacy of various SGAs in treating nonpsychotic disorders.
- Long-term safety studies of SGAs for nonpsychotic indications have not been done.
Table 1
Bipolar and other nonpsychotic indications FDA-approved for SGAs
| SGA | Bipolar mania | Bipolar depression | Bipolar maintenance | Other |
|---|---|---|---|---|
| Aripiprazole | Acute mania or mixed episodes | Bipolar I disorder, most recent episode manic or mixed | ||
| Clozapine | Risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorders | |||
| Olanzapine | Acute mania or mixed episodes; monotherapy or with lithium or valproate for manic episodes | Bipolar disorder maintenance monotherapy | ||
| Olanzapine/fluoxetine combination | Bipolar depressive episodes | |||
| Quetiapine | Acute manic episodes; monotherapy or with lithium or valproate | Bipolar depressive episodes | ||
| Risperidone | Acute mania or mixed episodes; monotherapy or with lithium or valproate | Irritability in autism | ||
| Ziprasidone | Acute manic or mixed episodes | |||
| SGA: second-generation antipsychotic (oral forms) | ||||
Prescribing decisions. SGA’s potential adverse effects complicate clinical decision-making. First you must decide whether to use an SGA for your patient with a nonpsychotic disorder.
Second-generation antipsychotics (SGAs) show efficacy in so many psychotic and nonpsychotic disorders that a specific therapeutic action for each disorder is highly doubtful. One might ask, then: What do they improve, and how do they do it?
The complete answer is beyond current understanding, unfortunately. We do know, however, that SGAs have not shown efficacy for treating nonpsychotic disorders that first-generation antipsychotics (FGAs) did not show—except perhaps for maintenance treatment in bipolar disorder.
Calming action. The major clinical action of SGAs appears to be in calming patients, which also was the first therapeutic effect attributed to the FGA chlorpromazine. This calming effect would explain SGAs’ efficacy in treating agitation, aggressiveness, anxiety, and possibly mania. Other clinical effects specific to psychosis and possibly to depression are possible.
Receptor-blocking action. SGAs’ D2 and 5-HT2A receptor-blocking activity may explain much of the drugs’ therapeutic effect. However, if SGAs’ effect on nonpsychotic symptoms derives from their action on nondopaminergic receptors, then individual SGAs would vary widely in efficacy and pure dopaminergic agents such as amisulpride would be ineffective.
SGAs also bind at other receptor sites, and the clinical importance of this may vary from patient to patient, drug to drug, and dose to dose.4
Knowing, for example, that antipsychotics have been shown to increase mortality and cerebrovascular events in older patients might make you less likely to prescribe an SGA for a patient with dementia-related agitation. No other pharmacologic treatment has shown clear efficacy for these patients, however, so other factors are important to consider, including:
- patient history and clinical characteristics
- potential side effects
- individual therapeutic response to previous medications.
Table 2
SGA uses in nonpsychotic disorders supported by evidence
from published double-blind clinical trials*
| SGA | Unipolar depression | OCD | Anxiety disorders | Dementia | Developmental disorders | Borderline personality disorder |
|---|---|---|---|---|---|---|
| Aripiprazole | Yes | |||||
| Clozapine | ||||||
| Olanzapine | Yes | Yes | Yes | Yes | Yes | |
| Quetiapine | Yes | |||||
| Risperidone | Yes | Yes | Yes | Yes | ||
| Ziprasidone | ||||||
| * Not including studies of bipolar disorder | ||||||
| OCD: obsessive-compulsive disorder | ||||||
| SGA: second-generation antipsychotic | ||||||
Dementia
Most Alzheimer’s patients—75% to 90%—experience behavioral problems during this progressive dementia. Double-blind studies have found risperidone (mean dosage ~1 mg/d) and olanzapine (mean dosage 5 to 10 mg/d) effective in reducing agitation and aggression, even in nonpsychotic patients with Alzheimer’s disease or vascular dementia.5,6 Quetiapine, ≤100 mg/d, was not more effective than placebo in reducing agitation.7 One study comparing IM olanzapine with IM lorazepam and placebo in acute agitation found both active treatments more effective than placebo.8
CATIE-AD—sponsored by the National Institute of Mental Health—compared olanzapine, risperidone, and quetiapine with placebo in 421 outpatients with behavioral symptoms such as psychosis, agitation, or aggressiveness.1 No significant differences were seen in overall effectiveness (measured as discontinuation for any cause9), although patients receiving olanzapine (mean dosage 5.5 mg/d) or risperidone (mean dosage 1 mg/d) had lower discontinuation rates for lack of efficacy than those receiving placebo.
Unfortunately, the results of the first phase of CATIE-AD provide no clear guidance on the therapeutic strategy to use in dementia. Its findings do suggest two secondary conclusions, however, about using SGAs for patients with dementia:
- Because quetiapine, mean dosage 56.5 mg/d, was not more effective than placebo on any measures, consider higher dosages when using this agent.
- Close attention to preventing and treating SGAs’ side effects is the key to effectively treating agitation and psychosis in dementia.
Recommendation. Nonpharmacologic interventions are an important part of treating behavioral problems in patients with dementia.13,14 Antipsychotics—particularly SGAs—have shown efficacy for psychosis and agitation in these patients and remain the first therapeutic option. The CATIE-AD investigators recommend that clinicians evaluate potential risks and benefits of pharmacotherapy and discuss these with patients and caregivers.1 Also:
- Consider which SGAs have the lowest risk of causing side effects for an individual patient.
- Start with low dosages and increase as needed, based on efficacy and tolerability.
Bipolar disorder
Acute mania. Five SGAs—aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone—are FDA-approved for acute mania (Table 1). Large double-blind studies supporting this indication show that SGAs have efficacy in treating mania as monotherapy and in combination with lithium or divalproex.15 These clinical trials included patients who were not psychotic at baseline.
Antipsychotic dosages in these studies were within the ranges used in schizophrenia treatment studies. Combining an SGA with lithium or divalproex generally yields greater reductions in mania rating scale scores, higher response rates, and higher remission rates than using lithium or divalproex alone. No published study has compared SGAs with each other in mania, but differences in efficacy among these compounds are likely to be small.16
Bipolar depression. SGAs’ efficacy in bipolar depression has been evaluated in double-blind studies, and quetiapine and the olanzapine/fluoxetine combination are FDA-approved for this indication.
Olanzapine plus fluoxetine was more effective in improving depressive symptoms than olanzapine alone in a double-blind study of 833 adults with depressive symptoms of bipolar I disorder, as measured by Montgomery-Åsburg Depression Rating Scale (MADRS) scores. Olanzapine alone was more effective than placebo. Mean dosages were olanzapine, 7.4 mg/d, and fluoxetine, 39.3 mg/d, in combination therapy and olanzapine, 9.7 mg/d, as monotherapy.
MADRS scores indicated that combination therapy—but not olanzapine alone—improved core depressive symptoms such as sadness, lassitude, inability to feel, and pessimistic thoughts.17
Quetiapine. A double-blind, placebo-controlled trial (BOLDER I) evaluated quetiapine in 542 outpatients experiencing a major depressive episode associated with bipolar I or II disorder. After 8 weeks, quetiapine at 300 or 600 mg/d was more effective than placebo in reducing depressive symptoms, as measured by MADRS score changes.
Response rates were 58% with quetiapine and 36% with placebo; remission rates were 53% with quetiapine and 28% with placebo. Most symptoms, including core depression items, improved significantly with quetiapine, compared with placebo.18 Results of a second double-blind study (BOLDER II) have been presented at conferences but have not been fully published.
Risperidone. A smaller double-blind study compared risperidone plus placebo, paroxetine plus placebo, and risperidone plus paroxetine in 30 patients in the depressed phase of bipolar I or II disorder. Patients continued taking mood stabilizers during the study. After 12 weeks, depressive symptoms improved significantly in all three groups, with no significant differences.19
Maintenance therapy. Olanzapine and aripiprazole are FDA-approved for maintenance therapy in bipolar disorder (Table 1).
Unipolar depression
FGAs have shown efficacy in depression in multiple controlled studies.20 SGAs have been evaluated mostly as add-on therapies in antidepressant-resistant depression.
Olanzapine. Shelton et al21 compared olanzapine monotherapy, fluoxetine monotherapy, and combined treatment in 34 nonpsychotic, treatment-resistant depressed subjects. Olanzapine plus fluoxetine was more effective than either agent alone. A subsequent double-blind study, however, showed similar efficacy after 8 weeks among the three treatments and nortriptyline monotherapy. Patients in the double-blind trial appeared to respond more rapidly to combined treatment than to the monotherapies.22
Risperidone. A multiphase study of the efficacy of risperidone augmentation in treatment-resistant major depression began when 489 outpatients (2% with psychotic symptoms) received open-label citalopram, 20 to 60 mg/d. After 4 to 6 weeks, 386 nonresponders entered the augmentation phase with open-label risperidone, 0.25 to 2 mg/d. After 4 to 6 weeks of combination therapy, 241 (63%) patients whose symptoms resolved entered a double-blind discontinuation phase, in which they were randomly assigned to augmentation with risperidone or placebo, while on citalopram.
Median time to relapse during the double-blind phase was 102 days with risperidone augmentation and 85 days with placebo—not a statistically significant difference. Relapse rates after 24 weeks were 53.3% and 54.6%, respectively.23 This study showed that the improvement observed after adding risperidone was not sustained over time.
Quetiapine. In a prospective single-blind study, paroxetine augmented with quetiapine, 200 mg/d, was compared to paroxetine alone in major depression with anxiety.24 Combination therapy was more effective in improving anxiety and depression symptoms.
Others. Open-label, add-on studies indicate that aripiprazole and ziprasidone can improve treatment-resistant depression.25-27
Anxiety disorders
OCD. SGAs also have been investigated as augmentation therapy for patients with OCD resistant to SRIs. A single-blind study of 27 patients found adjunctive quetiapine more effective than placebo in improving OCD symptoms.28 SGAs were more effective than placebo as augmentation therapy to SRIs for treatment-refractory OCD in double-blind, placebo-controlled studies using mean dosages of:
In other trials:
- A small double-blind study of patients with social anxiety disorder found olanzapine monotherapy more effective than placebo.34
- Low-dose risperidone (mean dosage 1.1 mg/d) improved core symptoms of generalized anxiety disorder in a 5-week, double-blind, placebo-controlled trial.35
- Some authors have reported clinical improvement of panic disorder with olanzapine augmentation.36
Developmental disorders
Antipsychotics represent one-third of all filled psychotropic prescriptions for individuals with pervasive developmental disorders (PDD).37 Haloperidol and thioridazine are the only two FDA-approved FGAs for severe behavioral problems in PDD (and for hyperactivity with conduct disorders). Recently, risperidone received FDA approval for the treatment of irritability associated with autistic disorder in children.
Risperidone—the most-studied SGA in the PDD population—has shown efficacy in autism and in PDD not otherwise specified. Risperidone at dosages >3 mg/d improved repetitive behavior and aggression in adult patients.38
In children with autism, risperidone can improve tantrums, aggression, and self-injury. In a study of risperidone’s effect on autism’s core symptoms, the authors reported improvements in repetitive and stereotyped behavior but not in social relatedness or verbal communication.39
Double-blind studies have shown positive effects on aggression and behavioral disturbances in children with conduct disorder, oppositional defiant disorder, and other disruptive disorders, developmentally delayed adolescents, and mentally retarded subjects of various ages.40-42 Children and adolescents appear to be more sensitive than adults to risperidone’s side effects such as weight gain, EPS, and pancreatitis.
Personality disorders
Antipsychotics have been recommended for paranoid ideas and psychotic-like symptoms in borderline personality disorder and in paranoid personality disorder.43
Olanzapine. A 24-week, double-blind study found low-dose olanzapine (mean dosage 5.3 mg/d) more effective than placebo for anxiety, interpersonal sensitivity, paranoia, and anger/hostility in women with borderline personality disorder.44
In another double-blind study, 12 weeks of olanzapine therapy (mean 6.9 mg/d) was more effective than placebo for inappropriate anger in borderline personality disorder, as measured by a modified Clinical Global Impression scale.45
Others. Anger and hostility improved more with aripiprazole, 15 mg/d, than with placebo in an 8-week double-blind study of patients with borderline personality disorder.46 Quetiapine, risperidone, ziprasidone, and clozapine have shown efficacy in open-label studies and case reports.
Related resources
- Boos J. Off label use–label off use? Ann Oncol 2003;14(1):1-5.
- Blum RS. Legal considerations in off-label medication prescribing. Arch Intern Med 2002;162(15):1777-9.
- Jeste DV, Dolder CR. Treatment of non-schizophrenic disorders: focus on atypical antipsychotics. J Psychiatr Res 2004;38(1):73-103.
- Food and Drug Administration. Searchable catalog of FDA-approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda.
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Nortriptyline • Pamelor, Aventyl
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Trémeau receives grant/research support from Eli Lilly and Company.
Dr. Citrome receives grant/research support from AstraZeneca, Barr Laboratories, Bristol-Myers Squibb, Eli Lilly and Company, Janssen Research Foundation, and Pfizer; is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Jazz Pharmaceuticals, and GlaxoSmithKline; and is a speaker for Abbott Laboratories, AstraZeneca, Eli Lilly and Company, and Pfizer.
1. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med 2006;166:1021-6.
3. Buckley PF. New antipsychotic agents: emerging clinical profiles. J Clin Psychiatry 1999;60(suppl 1):12-7.
4. Shayegan DK, Stahl SM. Atypical antipsychotics: matching receptor profile to individual patient’s clinical profile. CNS Spectr 2004;9(10 suppl 11):6-14.
5. Jeste DV, Dolder CR, Nayak GV, Salzman C. Atypical antipsychotics in elderly patients with dementia or schizophrenia: review of recent literature. Harv Rev Psychiatry 2005;13(6):340-51.
6. Carson S, McDonagh MS, Peterson K. A systematic review of the efficacy and safety of atypical antipsychotics in patients with psychological and behavioral symptoms of dementia. J Am Geriatr Soc 2006;54(2):354-61.
7. Ballard C, Margallo-Lana M, Juszczak E, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ 2005;330(7496):874.-
8. Meehan KM, Wang H, David SR, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26(4):494-504.
9. Schneider LS, Tariot PN, Lyketsos CG, et al. National Institute of Mental Health Antipsychotic Trials of Intervention Effectiveness (CATIE). Alzheimer disease trial methodology. Am J Geriatr Psychiatry 2001;9:346-60.
10. Kennedy J, Deberdt W, Siegal A, et al. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer’s dementia. Int J Geriatr Psychiatry 2005;20(11):1020-7.
11. U.S. Food and Drug Administration. Center for Drug Evaluation and Research. FDA public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Cder/drug/advisory/antipsychotics.htm. Accessed October 17, 2006.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353(22):2335-41.
13. Rabins P, Bland W, Bright-Long L, et al. from the Work Group on Alzheimer’s disease and related dementias. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias of late life. American Psychiatric Association Practice Guideline 1997. Available at http://www.psych.org/psych_pract/ treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
14. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease: a random control trial. JAMA 1996;276:1725-31.
15. Citrome L, Goldberg JF, Stahl SM. Toward convergence in the medication treatment of bipolar disorder and schizophrenia. Harv Rev Psychiatry 2005;13(1):28-42.
16. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry 2006;67(4):509-16.
17. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60(11):1079-88.
18. Calabrese JR, Keck PE, Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005;162(7):1351-60.
19. Shelton RC, Stahl SM. Risperidone and paroxetine given singly and in combination for bipolar depression. J Clin Psychiatry 2004;65(12):1715-9.
20. Robertson MM, Trimble MR. Major tranquillisers used as antidepressants. A review. J Affect Disord 1982;4(3):173-93.
21. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158(1):131-4.
22. Shelton RC, Williamson DJ, Corya SA, et al. Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 2005;66(10):1289-97.
23. Rapaport MH, Gharabawi GM, Canuso CM, et al. Effects of risperidone augmentation in patients with treatment-resistant depression: results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology 2006;31(11):2505-13.
24. Yargic LI, Corapcioglu A, Kocabasoglu N, et al. A prospective randomized single-blind, multicenter trial comparing the efficacy and safety of paroxetine with and without quetiapine therapy in depression associated with anxiety. Int J Psychiatry Clin Pract 2004;8:205-11.
25. Papakostas GI, Petersen TJ, Kinrys G, et al. Aripiprazole augmentation of selective serotonin reuptake inhibitors for treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66(10):1326-30.
26. Papakostas GI, Petersen TJ, Nierenberg AA, et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry 2004;65(2):217-21.
27. Simon JS, Nemeroff CB. Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry 2005;66(10):1216-20.
28. Atmaca M, Kuloglu M, Tezcan E, Gecici O. Quetiapine augmentation in patients with treatment resistant obsessive-compulsive disorder: a single-blind, placebo-controlled study. Int Clin Psychopharmacol 2002;17(3):115-9.
29. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000;57(8):794-801.
30. Bystritsky A, Ackerman DL, Rosen RM, et al. Augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder using adjunctive olanzapine: a placebo-controlled trial. J Clin Psychiatry 2004;65(4):565-8.
31. Denys D, de Geus F, van Megen HJ, Westenberg HG. A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. J Clin Psychiatry 2004;65(8):1040-8.
32. Bartzokis G, Lu PH, Turner J, et al. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry 2005;57(5):474-9.
33. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002;159(10):1777-9.
34. Barnett SD, Kramer ML, Casat CD, et al. Efficacy of olanzapine in social anxiety disorder: a pilot study. J Psychopharmacol 2002;16(4):365-8.
35. Brawman-Mintzer O, Knapp RG, Nietert PJ. Adjunctive risperidone in generalized anxiety disorder: a double-blind, placebo-controlled study. J Clin Psychiatry 2005;66(10):1321-5.
36. Khaldi S, Kornreich C, Dan B, Pelc I. Usefulness of olanzapine in refractory panic attacks. J Clin Psychopharmacol 2003;23(1):100-1.
37. Lott IT, McGregor M, Engelman L, et al. Longitudinal prescribing patterns for psychoactive medications in community-based individuals with developmental disabilities: utilization of pharmacy records. J Intellect Disabil Res 2004;48(Pt 6):563-71.
38. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 1998;55(7):633-41.
39. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162(6):1142-8.
40. Vanden Borre R, Vermote R, Buttiens M, et al. Risperidone as addon therapy in behavioural disturbances in mental retardation: a double-blind placebo-controlled cross-over study. Acta Psychiatr Scand 1993;87(3):167-71.
41. Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, Melman CT. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry 2001;62(4):239-48.
42. Snyder R, Turgay A, Aman M, et al; Risperidone Conduct Study Group. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry 2002;41(9):1026-36.
43. Oldham JM, Gabbard GO, Goin MK, et al, from the workgroup on borderline personality disorder. Practice guideline for the treatment of patients with borderline personality disorder. American Psychiatric Association Practice Guideline 2001. Available at http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
44. Zanarini MC, Frankenburg FR. Olanzapine treatment of female borderline personality disorder patients: a double-blind, placebo-controlled pilot study. J Clin Psychiatry 2001;62(11):849-54.
45. Bogenschutz MP, George Nurnberg H. Olanzapine versus placebo in the treatment of borderline personality disorder. J Clin Psychiatry 2004;65(1):104-9.
46. Nickel MK, Muehlbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry 2006;163(5):833-8.
1. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38.
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med 2006;166:1021-6.
3. Buckley PF. New antipsychotic agents: emerging clinical profiles. J Clin Psychiatry 1999;60(suppl 1):12-7.
4. Shayegan DK, Stahl SM. Atypical antipsychotics: matching receptor profile to individual patient’s clinical profile. CNS Spectr 2004;9(10 suppl 11):6-14.
5. Jeste DV, Dolder CR, Nayak GV, Salzman C. Atypical antipsychotics in elderly patients with dementia or schizophrenia: review of recent literature. Harv Rev Psychiatry 2005;13(6):340-51.
6. Carson S, McDonagh MS, Peterson K. A systematic review of the efficacy and safety of atypical antipsychotics in patients with psychological and behavioral symptoms of dementia. J Am Geriatr Soc 2006;54(2):354-61.
7. Ballard C, Margallo-Lana M, Juszczak E, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ 2005;330(7496):874.-
8. Meehan KM, Wang H, David SR, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26(4):494-504.
9. Schneider LS, Tariot PN, Lyketsos CG, et al. National Institute of Mental Health Antipsychotic Trials of Intervention Effectiveness (CATIE). Alzheimer disease trial methodology. Am J Geriatr Psychiatry 2001;9:346-60.
10. Kennedy J, Deberdt W, Siegal A, et al. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer’s dementia. Int J Geriatr Psychiatry 2005;20(11):1020-7.
11. U.S. Food and Drug Administration. Center for Drug Evaluation and Research. FDA public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Cder/drug/advisory/antipsychotics.htm. Accessed October 17, 2006.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353(22):2335-41.
13. Rabins P, Bland W, Bright-Long L, et al. from the Work Group on Alzheimer’s disease and related dementias. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias of late life. American Psychiatric Association Practice Guideline 1997. Available at http://www.psych.org/psych_pract/ treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
14. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease: a random control trial. JAMA 1996;276:1725-31.
15. Citrome L, Goldberg JF, Stahl SM. Toward convergence in the medication treatment of bipolar disorder and schizophrenia. Harv Rev Psychiatry 2005;13(1):28-42.
16. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry 2006;67(4):509-16.
17. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60(11):1079-88.
18. Calabrese JR, Keck PE, Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005;162(7):1351-60.
19. Shelton RC, Stahl SM. Risperidone and paroxetine given singly and in combination for bipolar depression. J Clin Psychiatry 2004;65(12):1715-9.
20. Robertson MM, Trimble MR. Major tranquillisers used as antidepressants. A review. J Affect Disord 1982;4(3):173-93.
21. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158(1):131-4.
22. Shelton RC, Williamson DJ, Corya SA, et al. Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 2005;66(10):1289-97.
23. Rapaport MH, Gharabawi GM, Canuso CM, et al. Effects of risperidone augmentation in patients with treatment-resistant depression: results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology 2006;31(11):2505-13.
24. Yargic LI, Corapcioglu A, Kocabasoglu N, et al. A prospective randomized single-blind, multicenter trial comparing the efficacy and safety of paroxetine with and without quetiapine therapy in depression associated with anxiety. Int J Psychiatry Clin Pract 2004;8:205-11.
25. Papakostas GI, Petersen TJ, Kinrys G, et al. Aripiprazole augmentation of selective serotonin reuptake inhibitors for treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66(10):1326-30.
26. Papakostas GI, Petersen TJ, Nierenberg AA, et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry 2004;65(2):217-21.
27. Simon JS, Nemeroff CB. Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry 2005;66(10):1216-20.
28. Atmaca M, Kuloglu M, Tezcan E, Gecici O. Quetiapine augmentation in patients with treatment resistant obsessive-compulsive disorder: a single-blind, placebo-controlled study. Int Clin Psychopharmacol 2002;17(3):115-9.
29. McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2000;57(8):794-801.
30. Bystritsky A, Ackerman DL, Rosen RM, et al. Augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder using adjunctive olanzapine: a placebo-controlled trial. J Clin Psychiatry 2004;65(4):565-8.
31. Denys D, de Geus F, van Megen HJ, Westenberg HG. A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. J Clin Psychiatry 2004;65(8):1040-8.
32. Bartzokis G, Lu PH, Turner J, et al. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry 2005;57(5):474-9.
33. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002;159(10):1777-9.
34. Barnett SD, Kramer ML, Casat CD, et al. Efficacy of olanzapine in social anxiety disorder: a pilot study. J Psychopharmacol 2002;16(4):365-8.
35. Brawman-Mintzer O, Knapp RG, Nietert PJ. Adjunctive risperidone in generalized anxiety disorder: a double-blind, placebo-controlled study. J Clin Psychiatry 2005;66(10):1321-5.
36. Khaldi S, Kornreich C, Dan B, Pelc I. Usefulness of olanzapine in refractory panic attacks. J Clin Psychopharmacol 2003;23(1):100-1.
37. Lott IT, McGregor M, Engelman L, et al. Longitudinal prescribing patterns for psychoactive medications in community-based individuals with developmental disabilities: utilization of pharmacy records. J Intellect Disabil Res 2004;48(Pt 6):563-71.
38. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 1998;55(7):633-41.
39. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162(6):1142-8.
40. Vanden Borre R, Vermote R, Buttiens M, et al. Risperidone as addon therapy in behavioural disturbances in mental retardation: a double-blind placebo-controlled cross-over study. Acta Psychiatr Scand 1993;87(3):167-71.
41. Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, Melman CT. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry 2001;62(4):239-48.
42. Snyder R, Turgay A, Aman M, et al; Risperidone Conduct Study Group. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry 2002;41(9):1026-36.
43. Oldham JM, Gabbard GO, Goin MK, et al, from the workgroup on borderline personality disorder. Practice guideline for the treatment of patients with borderline personality disorder. American Psychiatric Association Practice Guideline 2001. Available at http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed November 15, 2006.
44. Zanarini MC, Frankenburg FR. Olanzapine treatment of female borderline personality disorder patients: a double-blind, placebo-controlled pilot study. J Clin Psychiatry 2001;62(11):849-54.
45. Bogenschutz MP, George Nurnberg H. Olanzapine versus placebo in the treatment of borderline personality disorder. J Clin Psychiatry 2004;65(1):104-9.
46. Nickel MK, Muehlbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry 2006;163(5):833-8.
Treatment-resistant schizophrenia: What role for mood stabilizers?
In patients with treatment-resistant schizophrenia, lithium and anticonvulsants have become such common adjuncts to antipsychotics that 50% of inpatients may be receiving them.1 Evidence supporting this practice is mixed:
- Initial case reports and open-label studies that showed benefit have not always been followed by randomized clinical trials.
- Lack of clear benefit also has been described (Table 1).
This article examines the extent of this prescribing pattern, its evidence base, and mechanisms of action that may help explain why some adjunctive mood stabilizers are more effective than others for treatment-resistant schizophrenia.
EXTENT OF USE
For 5 years, the rate at which inpatients with schizophrenia received adjunctive mood stabilizers has held steady at approximately 50% in New York State Office of Mental Health (NYSOMH) facilities (Figure1). These facilities provide intermediate and long-term care for the seriously, persistently mentally ill. Adjunctive mood stabilizers might not be used as often for outpatients or for inpatients treated in short-stay facilities.
Table 1
Evidence for adjunctive use of lithium or anticonvulsants for treating schizophrenia
| Agent | Case reports and open studies | Randomized, double-blind trials | Benefit? |
|---|---|---|---|
| Lithium | Yes (many) | Yes (several, +/-) | Probably not |
| Carbamazepine | Yes (many) | Yes (several; small total sample) | Limited |
| Valproate | Yes (many) | Yes | Yes |
| Gabapentin | Yes (very few, +/-) | None | Probably not |
| Lamotrigine | Yes (few, +/-) | Yes (2, +) | Yes |
| Topiramate | Yes (very few, +/-) | Yes (1, +/-) | Probably not |
| Oxcarbazepine | Yes (very few, +/-) | None | Probably not |
| + = positive results | |||
| - = negative results | |||
| +/- = both positive and negative results | |||
Valproate is the most commonly used anticonvulsant, with one out of three patients with schizophrenia receiving it. Adjunctive gabapentin use is declining, probably because of inadequate efficacy—as will be discussed later. Use of adjunctive lamotrigine is expected to increase as more data become available on its usefulness in treatment-resistant schizophrenia.
Clinicians are using substantial dosages of adjunctive mood stabilizers. During first-quarter 2004, average daily dosages for 4,788 NYSOMH patients (80% with schizophrenia or schizoaffective disorder) receiving antipsychotics were:
- valproate, 1639 mg (n = 1921)
- gabapentin, 1524 mg (n = 303)
- oxcarbazepine, 1226 mg (n = 201)
- carbamazepine, 908 mg (n = 112)
- lithium, 894 mg (n = 715)
- topiramate, 234 mg (n = 269)
- lamotrigine, 204 mg (n = 231).2
Mood-stabilizer combinations were also used. Approximately one-half of patients receiving adjunctive mood stabilizers—with the exception of valproate—were receiving more than one. In patients receiving valproate, the rate of mood-stabilizer co-prescribing was about 25%.2
WHAT IS THE EVIDENCE?
Evidence supporting the use of adjunctive lithium or anticonvulsants to treat schizophrenia varies in quality and quantity (Table 1). Case reports and open studies offer the weakest evidence but can spur double-blind, randomized clinical trials (RCTs). Unfortunately, RCTs are not often done, and the published studies usually suffer from methodologic flaws such as:
- inadequate number of subjects (insufficient statistical power to detect differences)
- lack of control of confounds such as mood symptoms (seen when studies include patients with schizoaffective disorder)
- inadequate duration
- inappropriate target populations (patients with acute exacerbations of schizophrenia instead of persistent symptoms in treatment-resistant schizophrenia).
Because controlled trials of the use of adjunctive mood stabilizers are relatively scarce, clinical practice has transcended clinical research. Clinicians need effective regimens for treatment-resistant schizophrenia, and mood-stabilizer augmentation helps some patients.
Lithium is perhaps the best-known mood stabilizer. Although early studies showed adjunctive lithium useful in treating schizophrenia, later and better-designed trials did not. The authors of a recent meta-analysis of randomized clinical trials (n = 611 in 20 studies) concluded that despite some evidence supporting the efficacy of lithium augmentation, overall results were inconclusive. A large trial would be required to detect a small benefit in patients with schizophrenia who lack affective symptoms.3
Carbamazepine use among patients with schizophrenia is declining, primarily because this drug induces its own metabolism and can require frequent dose adjustments. Adjunctive carbamazepine has been used to manage persistent aggressive behavior in patients with schizophrenia and schizoaffective disorder. Evidence comes primarily from small trials or case reports (Table 2),4-8 but results of a larger clinical trial (n = 162) by Okuma et al6 are also available
In the Okuma report—a double-blind, placebo-controlled trial of carbamazepine in patients with DSM-III schizophrenia or schizoaffective disorder—carbamazepine did not significantly improve patients’ total Brief Psychiatric Rating Scale (BPRS) scores. Compared with placebo, however, some benefit with carbamazepine did emerge in measures of suspiciousness, uncooperativeness, and excitement.
A systematic review and meta-analysis (n = 283 in 10 studies) detected a trend toward reduced psychopathology with carbamazepine augmentation for schizophrenia. BPRS scores declined by 20% and 35% in the six trials (n = 147) for which data were available (P = 0.08 and 0.09, respectively).9 Because the double-blind trial by Okuma et al6 was not randomized, it was not included in this meta-analysis.9
Figure 1 10-year trend in use of adjunctive mood stabilizers for schizophrenia
Valproate. Among the anticonvulsants, the greatest body of evidence supports the use of valproate in patients with schizophrenia,10 although a recent meta-analysis (n = 378 in 5 studies) indicates inconsistent beneficial effects.11
Initial double-blind, RCTs of adjunctive valproate in patients with schizophrenia were limited in size and failed to show benefit with adjunctive valproate12-15 (Table 2). A more recent study16 showed that adjunctive valproate affects acute psychotic symptoms rather than mood. This study, however, did not answer whether adjunctive valproate would help patients with persistent symptoms of schizophrenia.
Table 2
Double-blind studies of adjunctive carbamazepine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Neppe (1983) | 11 | 42 | Crossover | Mixed, 8 with schizophrenia | “Overall clinical rating” improved |
| Dose et al (1987) | 22 | 28 | HAL + CBZ vs HAL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS |
| Okuma et al (1989) | 162 | 28 | NL + CBZ vs NL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS; possible improvement in suspiciousness, uncooperativeness, and excitement |
| Nachshoni et al (1994) | 28 | 49 | NL + CBZ vs NL + placebo | “Residual schizophrenia with negative symptoms” | No difference on BPRS or SANS |
| Simhandl et al (1996) | 42 | 42 | NL + CBZ vs NL + lithium vs NL + placebo | Schizophrenia(treatment- nonresponsive) | No difference on BPRS; CGI improved from baseline in groups receiving CBZ and lithium |
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| CBZ = carbamazepine | |||||
| HAL = haloperidol | |||||
| NL = neuroleptic (first-generation antipsychotic) | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In this large (n = 249), multicenter, randomized, double-blind trial, hospitalized patients with an acute exacerbation of schizophrenia received olanzapine or risperidone plus divalproex or placebo for 28 days. Patients with schizoaffective disorder and treatment-resistant schizophrenia were excluded.
By day 6, dosages reached 6 mg/d for risperidone and 15 mg/d for olanzapine. Divalproex was started at 15 mg/kg and titrated to a maximum of 30 mg/kg by day 14. Mean divalproex dosage was approximately 2300 mg/d (mean plasma level approximately 100 mg/mL).
Positive and Negative Syndrome Scale (PANSS) total scores improved significantly in patients receiving adjunctive divalproex compared with those receiving antipsychotic monotherapy, and significant differences occurred as early as day 3. The major effect was seen on schizophrenia’s positive symptoms. A post-hoc analysis17 also showed greater reductions in hostility (as measured by the hostility item in the PANSS Positive Subscale) in patients receiving adjunctive divalproex compared with antipsychotic monotherapy. This effect was independent of the effect on positive symptoms or sedation.
A large, multi-site, 84-day acute schizophrenia RCT similar to the 28-day trial—but using extended-release divalproex—is being conducted. An extended-release preparation may be particularly helpful in encouraging medication adherence for patients taking complicated medication regimens.
Table 3
Double-blind studies of adjunctive valproate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Ko et al (1985) | 6 | 28 | Crossover | Neurolepticresistant patients with chronic schizophrenia, not exacerbation | No valproate effect noted |
| Fisk and York (1987) | 62 | 42 | Antipsychotic + valproate vs antipsychotic + placebo | Chronic psychosis and tardive dyskinesia | No differences in mental state and behavior, as measured by“ Krawiecka scale”* |
| Dose et al (1998) | 42 | 28 | HAL + valproate vs HAL + placebo | Acute, nonmanic schizophrenic or schizoaffective psychosis | No difference on BPRS; possible effect on “hostile belligerence” |
| Wassef et al (2000) | 12 | 21 | HAL + valproate vs HAL + placebo | Acute exacerbation of chronic schizophrenia | CGI and SANS scores improved significantly, but BPRS scores did not |
| Casey et al (2003) | 249 | 28 | RIS + valproate vs OLZ + valproate vs RIS + placebo vs OLZ + placebo | Acute exacerbation of schizophrenia | PANSS scores improved |
| * Krawiecka M, Goldberg D, Vaughan M. A standardized psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatr Scand 1977;55(4):299-308. | |||||
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| HAL = haloperidol | |||||
| OLZ = olanzapine | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| RIS = risperidone | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In a recent large (n = 10,262), retrospective, pharmacoepidemiologic analysis,18 valproate augmentation led to longer persistence of treatment than did the strategy of switching antipsychotics. Average valproate dosages were small, however (<425 mg/d), as were antipsychotic dosages (risperidone <1.7 mg/d, quetiapine <120 mg/d, and olanzapine <7.5 mg/d). Patients’ diagnostic categories were not available. One interpretation of this study is that valproate augmentation would be more successful than switching antipsychotics, assuming that treatment persistence can be viewed as a positive outcome.
Table 4
Double-blind studies of adjunctive lamotrigine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen et al (2003) | 34 | 84 | Crossover; clozapine with or without lamotrigine | Clozapine-resistant male inpatients with chronic schizophrenia, not exacerbation | BPRS, PANSS positive, and PANSS general psychopathology symptom scores improved Negative symptoms did not improve |
| Kremer et al (2004) | 38 | 70 | Antipsychotic* + lamotrigine vs antipsychotic* + placebo | Treatment- resistant inpatients with schizophrenia | Completers’ PANSS positive, general psychopathology and total symptom scores improved No difference in negative symptoms or total BPRS scores No difference with intent-to-treat analyses |
| n = number of patients | |||||
| * First- or second-generation antipsychotic | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
Lamotrigine is the only other anticonvulsant for which published, double-blind, randomized evidence of use in patients with schizophrenia is available (Table 4).19,20 Adjunctive lamotrigine may be effective in managing treatment-resistant schizophrenia, as was shown in a small (n = 34), double-blind, placebo-controlled, crossover trial.19 Hospitalized patients whose symptoms were inadequately controlled with clozapine monotherapy received lamotrigine, 200 mg/d, for up to 12 weeks. Adjunctive lamotrigine improved positive but not negative symptoms.
Similar results were seen in treatment-resistant inpatients with schizophrenia (n = 38) in a 10-week, double-blind, parallel group trial by Kremer et al.20 Adjunctive lamotrigine improved PANSS positive, general psychopathology, and total symptom scores in the 31 patients who completed the trial. No differences were seen, however, in negative symptoms, total BPRS scores, or in the intent-to-treat analysis. These results have spurred the launch of a large, multi-site, RCT of adjunctive lamotrigine in patients with schizophrenia who have responded inadequately to antipsychotics alone.
Topiramate, one of the few psychotropics associated with weight loss, has attracted interest as an adjunct to second-generation antipsychotics to address weight gain. Although case reports have shown benefit,21 one showed deterioration in both positive and negative symptoms when topiramate was added to second-generation antipsychotics.22
Table 5
Double-blind study of adjunctive topiramate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen (2004)* | 26 | 84 | Crossover; SGA plus topiramate or placebo | Treatment- resistant male inpatients with chronic schizophrenia | PANSS general scores improved No difference in total PANSS, PANSS positive, or PANSS negative scores |
| * 2004 Collegium Internationale Neuro-Psychopharmacologicum (CINP) presentation, and personal communication (6/22/04) | |||||
| n = number of patients | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| SGA = Second-generation antipsychotic (patients were taking clozapine, olanzapine, or quetiapine) | |||||
An unpublished, randomized, crossover trial compared second-generation antipsychotics plus topiramate or placebo in 26 male inpatients with chronic schizophrenia. With adjunctive topiramate, the authors found a statistically significant improvement in the PANSS general psychopathology subscale but not in PANSS total, positive subscale, or negative subscale scores (Table 5) (Tiihonen J, personal communication 6/22/04).
Other agents. Very little information—all uncontrolled—supports adjunctive use of gabapentin or oxcarbazepine for patients with schizophrenia.23-28 Of concern are reports of patients suffering worsening of psychosis with gabapentin25 or of dysphoria and irritability with oxcarbazepine (attributed to a pharmacokinetic interaction).26
Conclusion. More trials are needed to examine the use of adjunctive mood stabilizers in patients with schizophrenia—particularly in those with chronic symptoms. Although mood stabilizers are widely used in this population, important questions remain unanswered, including:
- characteristics of patients likely to require adjunctive treatment
- how long treatment should continue.
MECHANISMS OF ACTION
Unlike antipsychotics, mood stabilizers do not exert their therapeutic effects by acting directly on dopamine (D2) receptors. Differences in mechanism of action among the anticonvulsants may help explain why some—such as valproate and lamotrigine—have been useful for bipolar disorder or schizophrenia and others—such as gabapentin—have not.29
One possibility is that anticonvulsants that affect voltage-gated sodium channels—such as valproate, lamotrigine, carbamazepine and oxcarbazepine—may be most useful for patients with bipolar disorder or schizophrenia. On the other hand, agents that affect voltage-gated calcium channels—such as gabapentin—may be efficacious as anticonvulsants but not as efficacious for bipolar disorder or schizophrenia.
Ketter et al30 proposed an anticonvulsant classification system based on predominant psychotropic profiles:
- the “GABA-ergic” group predominantly potentiates the inhibitory neurotransmitter GABA, resulting in sedation, fatigue, cognitive slowing, and weight gain, as well as possible anxiolytic and antimanic effects
- the “anti-glutamate” group predominantly attenuates glutamate excitatory neurotransmission and is associated with activation, weight loss, and possibly anxiogenic and antidepressant effects.
In the GABA-ergic group are anticonvulsants such as barbiturates, benzodiazepines, valproate, gabapentin, tiagabine, and vigabatrin. The antiglutamate group includes agents such as felbamate and lamotrigine. A “mixed” category includes anticonvulsants with GABA-ergic and anti-glutaminergic actions such as topiramate, which has sedating and weight-loss properties.
Because GABA appears to modulate dopamine neurotransmission,31 this may explain valproate’s role as an adjunctive agent for schizophrenia. Similarly, mechanisms related to Nmethyl-D-aspartate (NMDA) and non-NMDA glutamate receptor function may explain lamotrigine’s usefulness in this setting.19,20
SUMMARY
Clinicians resort to combination therapies when monotherapies fail to adequately control symptoms or maintain response. Co-prescribing of anticonvulsants with antipsychotics for inpatients with schizophrenia is common practice in New York State and most likely elsewhere. In general, antipsychotics’ and mood stabilizers’ different—and perhaps complementary—mechanisms of action explain the synergism between them. Mechanisms of action also may explain why some anticonvulsants help in schizophrenia (or bipolar disorder) whereas others do not.
Evidence for using adjunctive anticonvulsants is variable. The strongest data support using valproate (and perhaps lamotrigine), followed by carbamazepine and then topiramate. Gabapentin and oxcarbazepine have only anecdotal evidence, some of it negative. Well-designed, randomized clinical trials with the appropriate populations are needed.
Related resources
- Stahl SM. Essential psychopharmacology of antipsychotics and mood stabilizers. New York: Cambridge University Press, 2002.
- Harvard Medical School Department of Psychiatry’s psychopharmacology algorithm project. Osser DN, Patterson RD. Consultant for the pharmacotherapy of schizophrenia. Available at http://mhc.com/Algorithms/. Accessed Nov. 5, 2004.
Drug brand names
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Lithobid, Eskalith
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Topiramate • Topamax
- Valproate (valproic acid, divalproex sodium) • Depakene, Depakote
Disclosure
Dr. Citrome receives research grants/contracts from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, and Pfizer Inc. He is a consultant to and/or speaker for Bristol-Myers Squibb Co., Eli Lilly & Co., Pfizer Inc., Abbott Laboratories, AstraZeneca Pharmaceuticals, and Novartis Pharmaceuticals Corp.
Acknowledgment
Adapted from Citrome L. “Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S. perspective” (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum (CINP), June 2004 [abstract in Int J Neuropsychopharmacol. 2004; 7(suppl 1):S69], and from Citrome L. “Mood-stabilizer use in schizophrenia: 1994-2002” (NR350) (poster). New York: American Psychiatric Association annual meeting, May 2004.
1. Citrome L, Jaffe A, Levine J. Datapoints - mood stabilizers: utilization trends in patients diagnosed with schizophrenia 1994-2001. Psychiatr Serv 2002;53(10):1212.-
2. Citrome L. Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S.perspective (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum(CINP), June 2004 [abstract in Int J Neuropsychopharmacol 2004;7(suppl 1):S69].
3. Leucht S, Kissling W, McGrath J. Lithium for schizophrenia revisited: a systematic review and meta-analysis of randomized clinical trials. J Clin Psychiatry 2004;65(2):177-86.
4. Neppe VM. Carbamazepine as adjunctive treatment in nonepileptic chronic inpatients with EEG temporal lobe abnormalities. J Clin Psychiatry 1983;44:326-31.
5. Dose M, Apelt S, Emrich HM. Carbamazepine as an adjunct of antipsychotic therapy. Psychiatry Res 1987;22:303-10.
6. Okuma T, Yamashita I, Takahashi R, et al. A double-blind study of adjunctive carbamazepine versus placebo on excited states of schizophrenic and schizoaffective disorders. Acta Psychiatr Scand 1989;80:250-9.
7. Nachshoni T, Levin Y, Levy A, et al. A double-blind trial of carbamazepine in negative symptom schizophrenia. Biol Psychiatry 1994;35(1):22-26.
8. Simhandl C, Meszaros K, Denk E, et al. Adjunctive carbamazepine or lithium carbonate in therapy-resistant chronic schizophrenia. Can J Psychiatry 1996;41(5):317.-
9. Leucht S, McGrath J, White P, et al. Carbamazepine augmentation for schizophrenia: how good is the evidence? J Clin Psychiatry 2002;63(3):218-24.
10. Citrome L. Schizophrenia and valproate. Psychopharmacol Bull 2003;37(suppl 2):74-88.
11. Basan A, Kissling W, Leucht S. Valproate as an adjunct to antipsychotics for schizophrenia: a systematic review of randomized trials. Schizophr Res 2004;70(1):33-7.
12. Ko GN, Korpi ER, Freed WJ, et al. Effect of valproic acid on behavior and plasma amino acid concentrations in chronic schizophrenia patients. Biol Psychiatry 1985;20:209-15.
13. Dose M, Hellweg R, Yassouridis A, et al. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry 1998;31(4):122-5.
14. Fisk GG, York SM. The effect of sodium valproate on tardive dyskinesia—revisited. Br J Psychiatry 1987;150:542-6.
15. Wassef AA, Dott SG, Harris A, et al. Randomized, placebo-controlled pilot study of divalproex sodium in the treatment of acute exacerbations of chronic schizophrenia. J Clin Psychopharmacol 2000;20(3):357-361.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacol 2003;28(1):182-92.
17. Citrome L, Casey DE, Daniel DG, et al. Effects of adjunctive valproate on hostility in patients with schizophrenia receiving olanzapine or risperidone: a double-blind multi-center study. Psychiatr Serv 2004;55(3):290-4.
18. Cramer JA, Sernyak M. Results of a naturalistic study of treatment options: switching atypical antipsychotic drugs or augmenting with valproate. Clin Ther 2004;26(6):905-14.
19. Tiihonen J, Hallikainen T, Ryynanen OP, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled trial. Biol Psychiatry 2003;54(11):1241-8.
20. Kremer I, Vass A, Gurelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry 2004;56(6):441-6.
21. Drapalski AL, Rosse RB, Peebles RR, et al. Topiramate improves deficit symptoms in a patient with schizophrenia when added to a stable regimen of antipsychotic medication. Clin Neuropharmacol 2001;24:290-4.
22. Millson RC, Owen JA, Lorberg GW, Tackaberry L. Topiramate for refractory schizophrenia. Am J Psychiatry 2002;159(4):675.-
23. Chouinard G, Beauclair L, Belanger MC. Gabapentin: long term antianxiety and hypnotic effects in psychiatric patients with comorbid anxiety-related disorders. Can J Psychiatry 1998;43:305.-
24. Megna JL, Devitt PJ, Sauro MD, Dewan MJ. Gabapentin’s effect on agitation in severely and persistently mentally ill patients. Ann Pharmacother 2002;35:12-16.
25. Jablonowski K, Margolese HC, Chouinard G. Gabapentin-induced paradoxical exacerbation of psychosis in a patient with schizophrenia. Can J Psychiatry 2002;47(10):975-6.
26. Baird P. The interactive metabolism effect of oxcarbazepine coadministered with tricyclic antidepressant therapy for OCD symptoms. J Clin Psychopharmacol 2003;23(4):419.-
27. Centorrino F, Albert MJ, Berry JM, et al. Oxcarbazepine: clinical experience with hospitalized psychiatric patients. Bipolar Disord 2003;5(5):370-4.
28. Leweke FM, Gerth CW, Koethe D, et al. Oxcarbazepine as an adjunct for schizophrenia. Am J Psychiatry 2004;161(6):1130-1.
29. Stahl SM. Psychopharmacology of anticonvulsants: do all anticonvulsants have the same mechanism of action? J Clin Psychiatry 2004;65(2):149-50.
30. Ketter TA, Wong PW. The emerging differential roles of GABAergic and antiglutaminergic agents in bipolar disorders. J Clin Psychiatry 2003;64(suppl 3):15-20.
31. Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol 2003;23(6):601-40.
In patients with treatment-resistant schizophrenia, lithium and anticonvulsants have become such common adjuncts to antipsychotics that 50% of inpatients may be receiving them.1 Evidence supporting this practice is mixed:
- Initial case reports and open-label studies that showed benefit have not always been followed by randomized clinical trials.
- Lack of clear benefit also has been described (Table 1).
This article examines the extent of this prescribing pattern, its evidence base, and mechanisms of action that may help explain why some adjunctive mood stabilizers are more effective than others for treatment-resistant schizophrenia.
EXTENT OF USE
For 5 years, the rate at which inpatients with schizophrenia received adjunctive mood stabilizers has held steady at approximately 50% in New York State Office of Mental Health (NYSOMH) facilities (Figure1). These facilities provide intermediate and long-term care for the seriously, persistently mentally ill. Adjunctive mood stabilizers might not be used as often for outpatients or for inpatients treated in short-stay facilities.
Table 1
Evidence for adjunctive use of lithium or anticonvulsants for treating schizophrenia
| Agent | Case reports and open studies | Randomized, double-blind trials | Benefit? |
|---|---|---|---|
| Lithium | Yes (many) | Yes (several, +/-) | Probably not |
| Carbamazepine | Yes (many) | Yes (several; small total sample) | Limited |
| Valproate | Yes (many) | Yes | Yes |
| Gabapentin | Yes (very few, +/-) | None | Probably not |
| Lamotrigine | Yes (few, +/-) | Yes (2, +) | Yes |
| Topiramate | Yes (very few, +/-) | Yes (1, +/-) | Probably not |
| Oxcarbazepine | Yes (very few, +/-) | None | Probably not |
| + = positive results | |||
| - = negative results | |||
| +/- = both positive and negative results | |||
Valproate is the most commonly used anticonvulsant, with one out of three patients with schizophrenia receiving it. Adjunctive gabapentin use is declining, probably because of inadequate efficacy—as will be discussed later. Use of adjunctive lamotrigine is expected to increase as more data become available on its usefulness in treatment-resistant schizophrenia.
Clinicians are using substantial dosages of adjunctive mood stabilizers. During first-quarter 2004, average daily dosages for 4,788 NYSOMH patients (80% with schizophrenia or schizoaffective disorder) receiving antipsychotics were:
- valproate, 1639 mg (n = 1921)
- gabapentin, 1524 mg (n = 303)
- oxcarbazepine, 1226 mg (n = 201)
- carbamazepine, 908 mg (n = 112)
- lithium, 894 mg (n = 715)
- topiramate, 234 mg (n = 269)
- lamotrigine, 204 mg (n = 231).2
Mood-stabilizer combinations were also used. Approximately one-half of patients receiving adjunctive mood stabilizers—with the exception of valproate—were receiving more than one. In patients receiving valproate, the rate of mood-stabilizer co-prescribing was about 25%.2
WHAT IS THE EVIDENCE?
Evidence supporting the use of adjunctive lithium or anticonvulsants to treat schizophrenia varies in quality and quantity (Table 1). Case reports and open studies offer the weakest evidence but can spur double-blind, randomized clinical trials (RCTs). Unfortunately, RCTs are not often done, and the published studies usually suffer from methodologic flaws such as:
- inadequate number of subjects (insufficient statistical power to detect differences)
- lack of control of confounds such as mood symptoms (seen when studies include patients with schizoaffective disorder)
- inadequate duration
- inappropriate target populations (patients with acute exacerbations of schizophrenia instead of persistent symptoms in treatment-resistant schizophrenia).
Because controlled trials of the use of adjunctive mood stabilizers are relatively scarce, clinical practice has transcended clinical research. Clinicians need effective regimens for treatment-resistant schizophrenia, and mood-stabilizer augmentation helps some patients.
Lithium is perhaps the best-known mood stabilizer. Although early studies showed adjunctive lithium useful in treating schizophrenia, later and better-designed trials did not. The authors of a recent meta-analysis of randomized clinical trials (n = 611 in 20 studies) concluded that despite some evidence supporting the efficacy of lithium augmentation, overall results were inconclusive. A large trial would be required to detect a small benefit in patients with schizophrenia who lack affective symptoms.3
Carbamazepine use among patients with schizophrenia is declining, primarily because this drug induces its own metabolism and can require frequent dose adjustments. Adjunctive carbamazepine has been used to manage persistent aggressive behavior in patients with schizophrenia and schizoaffective disorder. Evidence comes primarily from small trials or case reports (Table 2),4-8 but results of a larger clinical trial (n = 162) by Okuma et al6 are also available
In the Okuma report—a double-blind, placebo-controlled trial of carbamazepine in patients with DSM-III schizophrenia or schizoaffective disorder—carbamazepine did not significantly improve patients’ total Brief Psychiatric Rating Scale (BPRS) scores. Compared with placebo, however, some benefit with carbamazepine did emerge in measures of suspiciousness, uncooperativeness, and excitement.
A systematic review and meta-analysis (n = 283 in 10 studies) detected a trend toward reduced psychopathology with carbamazepine augmentation for schizophrenia. BPRS scores declined by 20% and 35% in the six trials (n = 147) for which data were available (P = 0.08 and 0.09, respectively).9 Because the double-blind trial by Okuma et al6 was not randomized, it was not included in this meta-analysis.9
Figure 1 10-year trend in use of adjunctive mood stabilizers for schizophrenia
Valproate. Among the anticonvulsants, the greatest body of evidence supports the use of valproate in patients with schizophrenia,10 although a recent meta-analysis (n = 378 in 5 studies) indicates inconsistent beneficial effects.11
Initial double-blind, RCTs of adjunctive valproate in patients with schizophrenia were limited in size and failed to show benefit with adjunctive valproate12-15 (Table 2). A more recent study16 showed that adjunctive valproate affects acute psychotic symptoms rather than mood. This study, however, did not answer whether adjunctive valproate would help patients with persistent symptoms of schizophrenia.
Table 2
Double-blind studies of adjunctive carbamazepine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Neppe (1983) | 11 | 42 | Crossover | Mixed, 8 with schizophrenia | “Overall clinical rating” improved |
| Dose et al (1987) | 22 | 28 | HAL + CBZ vs HAL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS |
| Okuma et al (1989) | 162 | 28 | NL + CBZ vs NL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS; possible improvement in suspiciousness, uncooperativeness, and excitement |
| Nachshoni et al (1994) | 28 | 49 | NL + CBZ vs NL + placebo | “Residual schizophrenia with negative symptoms” | No difference on BPRS or SANS |
| Simhandl et al (1996) | 42 | 42 | NL + CBZ vs NL + lithium vs NL + placebo | Schizophrenia(treatment- nonresponsive) | No difference on BPRS; CGI improved from baseline in groups receiving CBZ and lithium |
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| CBZ = carbamazepine | |||||
| HAL = haloperidol | |||||
| NL = neuroleptic (first-generation antipsychotic) | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In this large (n = 249), multicenter, randomized, double-blind trial, hospitalized patients with an acute exacerbation of schizophrenia received olanzapine or risperidone plus divalproex or placebo for 28 days. Patients with schizoaffective disorder and treatment-resistant schizophrenia were excluded.
By day 6, dosages reached 6 mg/d for risperidone and 15 mg/d for olanzapine. Divalproex was started at 15 mg/kg and titrated to a maximum of 30 mg/kg by day 14. Mean divalproex dosage was approximately 2300 mg/d (mean plasma level approximately 100 mg/mL).
Positive and Negative Syndrome Scale (PANSS) total scores improved significantly in patients receiving adjunctive divalproex compared with those receiving antipsychotic monotherapy, and significant differences occurred as early as day 3. The major effect was seen on schizophrenia’s positive symptoms. A post-hoc analysis17 also showed greater reductions in hostility (as measured by the hostility item in the PANSS Positive Subscale) in patients receiving adjunctive divalproex compared with antipsychotic monotherapy. This effect was independent of the effect on positive symptoms or sedation.
A large, multi-site, 84-day acute schizophrenia RCT similar to the 28-day trial—but using extended-release divalproex—is being conducted. An extended-release preparation may be particularly helpful in encouraging medication adherence for patients taking complicated medication regimens.
Table 3
Double-blind studies of adjunctive valproate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Ko et al (1985) | 6 | 28 | Crossover | Neurolepticresistant patients with chronic schizophrenia, not exacerbation | No valproate effect noted |
| Fisk and York (1987) | 62 | 42 | Antipsychotic + valproate vs antipsychotic + placebo | Chronic psychosis and tardive dyskinesia | No differences in mental state and behavior, as measured by“ Krawiecka scale”* |
| Dose et al (1998) | 42 | 28 | HAL + valproate vs HAL + placebo | Acute, nonmanic schizophrenic or schizoaffective psychosis | No difference on BPRS; possible effect on “hostile belligerence” |
| Wassef et al (2000) | 12 | 21 | HAL + valproate vs HAL + placebo | Acute exacerbation of chronic schizophrenia | CGI and SANS scores improved significantly, but BPRS scores did not |
| Casey et al (2003) | 249 | 28 | RIS + valproate vs OLZ + valproate vs RIS + placebo vs OLZ + placebo | Acute exacerbation of schizophrenia | PANSS scores improved |
| * Krawiecka M, Goldberg D, Vaughan M. A standardized psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatr Scand 1977;55(4):299-308. | |||||
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| HAL = haloperidol | |||||
| OLZ = olanzapine | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| RIS = risperidone | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In a recent large (n = 10,262), retrospective, pharmacoepidemiologic analysis,18 valproate augmentation led to longer persistence of treatment than did the strategy of switching antipsychotics. Average valproate dosages were small, however (<425 mg/d), as were antipsychotic dosages (risperidone <1.7 mg/d, quetiapine <120 mg/d, and olanzapine <7.5 mg/d). Patients’ diagnostic categories were not available. One interpretation of this study is that valproate augmentation would be more successful than switching antipsychotics, assuming that treatment persistence can be viewed as a positive outcome.
Table 4
Double-blind studies of adjunctive lamotrigine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen et al (2003) | 34 | 84 | Crossover; clozapine with or without lamotrigine | Clozapine-resistant male inpatients with chronic schizophrenia, not exacerbation | BPRS, PANSS positive, and PANSS general psychopathology symptom scores improved Negative symptoms did not improve |
| Kremer et al (2004) | 38 | 70 | Antipsychotic* + lamotrigine vs antipsychotic* + placebo | Treatment- resistant inpatients with schizophrenia | Completers’ PANSS positive, general psychopathology and total symptom scores improved No difference in negative symptoms or total BPRS scores No difference with intent-to-treat analyses |
| n = number of patients | |||||
| * First- or second-generation antipsychotic | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
Lamotrigine is the only other anticonvulsant for which published, double-blind, randomized evidence of use in patients with schizophrenia is available (Table 4).19,20 Adjunctive lamotrigine may be effective in managing treatment-resistant schizophrenia, as was shown in a small (n = 34), double-blind, placebo-controlled, crossover trial.19 Hospitalized patients whose symptoms were inadequately controlled with clozapine monotherapy received lamotrigine, 200 mg/d, for up to 12 weeks. Adjunctive lamotrigine improved positive but not negative symptoms.
Similar results were seen in treatment-resistant inpatients with schizophrenia (n = 38) in a 10-week, double-blind, parallel group trial by Kremer et al.20 Adjunctive lamotrigine improved PANSS positive, general psychopathology, and total symptom scores in the 31 patients who completed the trial. No differences were seen, however, in negative symptoms, total BPRS scores, or in the intent-to-treat analysis. These results have spurred the launch of a large, multi-site, RCT of adjunctive lamotrigine in patients with schizophrenia who have responded inadequately to antipsychotics alone.
Topiramate, one of the few psychotropics associated with weight loss, has attracted interest as an adjunct to second-generation antipsychotics to address weight gain. Although case reports have shown benefit,21 one showed deterioration in both positive and negative symptoms when topiramate was added to second-generation antipsychotics.22
Table 5
Double-blind study of adjunctive topiramate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen (2004)* | 26 | 84 | Crossover; SGA plus topiramate or placebo | Treatment- resistant male inpatients with chronic schizophrenia | PANSS general scores improved No difference in total PANSS, PANSS positive, or PANSS negative scores |
| * 2004 Collegium Internationale Neuro-Psychopharmacologicum (CINP) presentation, and personal communication (6/22/04) | |||||
| n = number of patients | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| SGA = Second-generation antipsychotic (patients were taking clozapine, olanzapine, or quetiapine) | |||||
An unpublished, randomized, crossover trial compared second-generation antipsychotics plus topiramate or placebo in 26 male inpatients with chronic schizophrenia. With adjunctive topiramate, the authors found a statistically significant improvement in the PANSS general psychopathology subscale but not in PANSS total, positive subscale, or negative subscale scores (Table 5) (Tiihonen J, personal communication 6/22/04).
Other agents. Very little information—all uncontrolled—supports adjunctive use of gabapentin or oxcarbazepine for patients with schizophrenia.23-28 Of concern are reports of patients suffering worsening of psychosis with gabapentin25 or of dysphoria and irritability with oxcarbazepine (attributed to a pharmacokinetic interaction).26
Conclusion. More trials are needed to examine the use of adjunctive mood stabilizers in patients with schizophrenia—particularly in those with chronic symptoms. Although mood stabilizers are widely used in this population, important questions remain unanswered, including:
- characteristics of patients likely to require adjunctive treatment
- how long treatment should continue.
MECHANISMS OF ACTION
Unlike antipsychotics, mood stabilizers do not exert their therapeutic effects by acting directly on dopamine (D2) receptors. Differences in mechanism of action among the anticonvulsants may help explain why some—such as valproate and lamotrigine—have been useful for bipolar disorder or schizophrenia and others—such as gabapentin—have not.29
One possibility is that anticonvulsants that affect voltage-gated sodium channels—such as valproate, lamotrigine, carbamazepine and oxcarbazepine—may be most useful for patients with bipolar disorder or schizophrenia. On the other hand, agents that affect voltage-gated calcium channels—such as gabapentin—may be efficacious as anticonvulsants but not as efficacious for bipolar disorder or schizophrenia.
Ketter et al30 proposed an anticonvulsant classification system based on predominant psychotropic profiles:
- the “GABA-ergic” group predominantly potentiates the inhibitory neurotransmitter GABA, resulting in sedation, fatigue, cognitive slowing, and weight gain, as well as possible anxiolytic and antimanic effects
- the “anti-glutamate” group predominantly attenuates glutamate excitatory neurotransmission and is associated with activation, weight loss, and possibly anxiogenic and antidepressant effects.
In the GABA-ergic group are anticonvulsants such as barbiturates, benzodiazepines, valproate, gabapentin, tiagabine, and vigabatrin. The antiglutamate group includes agents such as felbamate and lamotrigine. A “mixed” category includes anticonvulsants with GABA-ergic and anti-glutaminergic actions such as topiramate, which has sedating and weight-loss properties.
Because GABA appears to modulate dopamine neurotransmission,31 this may explain valproate’s role as an adjunctive agent for schizophrenia. Similarly, mechanisms related to Nmethyl-D-aspartate (NMDA) and non-NMDA glutamate receptor function may explain lamotrigine’s usefulness in this setting.19,20
SUMMARY
Clinicians resort to combination therapies when monotherapies fail to adequately control symptoms or maintain response. Co-prescribing of anticonvulsants with antipsychotics for inpatients with schizophrenia is common practice in New York State and most likely elsewhere. In general, antipsychotics’ and mood stabilizers’ different—and perhaps complementary—mechanisms of action explain the synergism between them. Mechanisms of action also may explain why some anticonvulsants help in schizophrenia (or bipolar disorder) whereas others do not.
Evidence for using adjunctive anticonvulsants is variable. The strongest data support using valproate (and perhaps lamotrigine), followed by carbamazepine and then topiramate. Gabapentin and oxcarbazepine have only anecdotal evidence, some of it negative. Well-designed, randomized clinical trials with the appropriate populations are needed.
Related resources
- Stahl SM. Essential psychopharmacology of antipsychotics and mood stabilizers. New York: Cambridge University Press, 2002.
- Harvard Medical School Department of Psychiatry’s psychopharmacology algorithm project. Osser DN, Patterson RD. Consultant for the pharmacotherapy of schizophrenia. Available at http://mhc.com/Algorithms/. Accessed Nov. 5, 2004.
Drug brand names
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Lithobid, Eskalith
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Topiramate • Topamax
- Valproate (valproic acid, divalproex sodium) • Depakene, Depakote
Disclosure
Dr. Citrome receives research grants/contracts from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, and Pfizer Inc. He is a consultant to and/or speaker for Bristol-Myers Squibb Co., Eli Lilly & Co., Pfizer Inc., Abbott Laboratories, AstraZeneca Pharmaceuticals, and Novartis Pharmaceuticals Corp.
Acknowledgment
Adapted from Citrome L. “Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S. perspective” (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum (CINP), June 2004 [abstract in Int J Neuropsychopharmacol. 2004; 7(suppl 1):S69], and from Citrome L. “Mood-stabilizer use in schizophrenia: 1994-2002” (NR350) (poster). New York: American Psychiatric Association annual meeting, May 2004.
In patients with treatment-resistant schizophrenia, lithium and anticonvulsants have become such common adjuncts to antipsychotics that 50% of inpatients may be receiving them.1 Evidence supporting this practice is mixed:
- Initial case reports and open-label studies that showed benefit have not always been followed by randomized clinical trials.
- Lack of clear benefit also has been described (Table 1).
This article examines the extent of this prescribing pattern, its evidence base, and mechanisms of action that may help explain why some adjunctive mood stabilizers are more effective than others for treatment-resistant schizophrenia.
EXTENT OF USE
For 5 years, the rate at which inpatients with schizophrenia received adjunctive mood stabilizers has held steady at approximately 50% in New York State Office of Mental Health (NYSOMH) facilities (Figure1). These facilities provide intermediate and long-term care for the seriously, persistently mentally ill. Adjunctive mood stabilizers might not be used as often for outpatients or for inpatients treated in short-stay facilities.
Table 1
Evidence for adjunctive use of lithium or anticonvulsants for treating schizophrenia
| Agent | Case reports and open studies | Randomized, double-blind trials | Benefit? |
|---|---|---|---|
| Lithium | Yes (many) | Yes (several, +/-) | Probably not |
| Carbamazepine | Yes (many) | Yes (several; small total sample) | Limited |
| Valproate | Yes (many) | Yes | Yes |
| Gabapentin | Yes (very few, +/-) | None | Probably not |
| Lamotrigine | Yes (few, +/-) | Yes (2, +) | Yes |
| Topiramate | Yes (very few, +/-) | Yes (1, +/-) | Probably not |
| Oxcarbazepine | Yes (very few, +/-) | None | Probably not |
| + = positive results | |||
| - = negative results | |||
| +/- = both positive and negative results | |||
Valproate is the most commonly used anticonvulsant, with one out of three patients with schizophrenia receiving it. Adjunctive gabapentin use is declining, probably because of inadequate efficacy—as will be discussed later. Use of adjunctive lamotrigine is expected to increase as more data become available on its usefulness in treatment-resistant schizophrenia.
Clinicians are using substantial dosages of adjunctive mood stabilizers. During first-quarter 2004, average daily dosages for 4,788 NYSOMH patients (80% with schizophrenia or schizoaffective disorder) receiving antipsychotics were:
- valproate, 1639 mg (n = 1921)
- gabapentin, 1524 mg (n = 303)
- oxcarbazepine, 1226 mg (n = 201)
- carbamazepine, 908 mg (n = 112)
- lithium, 894 mg (n = 715)
- topiramate, 234 mg (n = 269)
- lamotrigine, 204 mg (n = 231).2
Mood-stabilizer combinations were also used. Approximately one-half of patients receiving adjunctive mood stabilizers—with the exception of valproate—were receiving more than one. In patients receiving valproate, the rate of mood-stabilizer co-prescribing was about 25%.2
WHAT IS THE EVIDENCE?
Evidence supporting the use of adjunctive lithium or anticonvulsants to treat schizophrenia varies in quality and quantity (Table 1). Case reports and open studies offer the weakest evidence but can spur double-blind, randomized clinical trials (RCTs). Unfortunately, RCTs are not often done, and the published studies usually suffer from methodologic flaws such as:
- inadequate number of subjects (insufficient statistical power to detect differences)
- lack of control of confounds such as mood symptoms (seen when studies include patients with schizoaffective disorder)
- inadequate duration
- inappropriate target populations (patients with acute exacerbations of schizophrenia instead of persistent symptoms in treatment-resistant schizophrenia).
Because controlled trials of the use of adjunctive mood stabilizers are relatively scarce, clinical practice has transcended clinical research. Clinicians need effective regimens for treatment-resistant schizophrenia, and mood-stabilizer augmentation helps some patients.
Lithium is perhaps the best-known mood stabilizer. Although early studies showed adjunctive lithium useful in treating schizophrenia, later and better-designed trials did not. The authors of a recent meta-analysis of randomized clinical trials (n = 611 in 20 studies) concluded that despite some evidence supporting the efficacy of lithium augmentation, overall results were inconclusive. A large trial would be required to detect a small benefit in patients with schizophrenia who lack affective symptoms.3
Carbamazepine use among patients with schizophrenia is declining, primarily because this drug induces its own metabolism and can require frequent dose adjustments. Adjunctive carbamazepine has been used to manage persistent aggressive behavior in patients with schizophrenia and schizoaffective disorder. Evidence comes primarily from small trials or case reports (Table 2),4-8 but results of a larger clinical trial (n = 162) by Okuma et al6 are also available
In the Okuma report—a double-blind, placebo-controlled trial of carbamazepine in patients with DSM-III schizophrenia or schizoaffective disorder—carbamazepine did not significantly improve patients’ total Brief Psychiatric Rating Scale (BPRS) scores. Compared with placebo, however, some benefit with carbamazepine did emerge in measures of suspiciousness, uncooperativeness, and excitement.
A systematic review and meta-analysis (n = 283 in 10 studies) detected a trend toward reduced psychopathology with carbamazepine augmentation for schizophrenia. BPRS scores declined by 20% and 35% in the six trials (n = 147) for which data were available (P = 0.08 and 0.09, respectively).9 Because the double-blind trial by Okuma et al6 was not randomized, it was not included in this meta-analysis.9
Figure 1 10-year trend in use of adjunctive mood stabilizers for schizophrenia
Valproate. Among the anticonvulsants, the greatest body of evidence supports the use of valproate in patients with schizophrenia,10 although a recent meta-analysis (n = 378 in 5 studies) indicates inconsistent beneficial effects.11
Initial double-blind, RCTs of adjunctive valproate in patients with schizophrenia were limited in size and failed to show benefit with adjunctive valproate12-15 (Table 2). A more recent study16 showed that adjunctive valproate affects acute psychotic symptoms rather than mood. This study, however, did not answer whether adjunctive valproate would help patients with persistent symptoms of schizophrenia.
Table 2
Double-blind studies of adjunctive carbamazepine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Neppe (1983) | 11 | 42 | Crossover | Mixed, 8 with schizophrenia | “Overall clinical rating” improved |
| Dose et al (1987) | 22 | 28 | HAL + CBZ vs HAL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS |
| Okuma et al (1989) | 162 | 28 | NL + CBZ vs NL + placebo | Schizophrenia or schizoaffective disorder | No difference on BPRS; possible improvement in suspiciousness, uncooperativeness, and excitement |
| Nachshoni et al (1994) | 28 | 49 | NL + CBZ vs NL + placebo | “Residual schizophrenia with negative symptoms” | No difference on BPRS or SANS |
| Simhandl et al (1996) | 42 | 42 | NL + CBZ vs NL + lithium vs NL + placebo | Schizophrenia(treatment- nonresponsive) | No difference on BPRS; CGI improved from baseline in groups receiving CBZ and lithium |
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| CBZ = carbamazepine | |||||
| HAL = haloperidol | |||||
| NL = neuroleptic (first-generation antipsychotic) | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In this large (n = 249), multicenter, randomized, double-blind trial, hospitalized patients with an acute exacerbation of schizophrenia received olanzapine or risperidone plus divalproex or placebo for 28 days. Patients with schizoaffective disorder and treatment-resistant schizophrenia were excluded.
By day 6, dosages reached 6 mg/d for risperidone and 15 mg/d for olanzapine. Divalproex was started at 15 mg/kg and titrated to a maximum of 30 mg/kg by day 14. Mean divalproex dosage was approximately 2300 mg/d (mean plasma level approximately 100 mg/mL).
Positive and Negative Syndrome Scale (PANSS) total scores improved significantly in patients receiving adjunctive divalproex compared with those receiving antipsychotic monotherapy, and significant differences occurred as early as day 3. The major effect was seen on schizophrenia’s positive symptoms. A post-hoc analysis17 also showed greater reductions in hostility (as measured by the hostility item in the PANSS Positive Subscale) in patients receiving adjunctive divalproex compared with antipsychotic monotherapy. This effect was independent of the effect on positive symptoms or sedation.
A large, multi-site, 84-day acute schizophrenia RCT similar to the 28-day trial—but using extended-release divalproex—is being conducted. An extended-release preparation may be particularly helpful in encouraging medication adherence for patients taking complicated medication regimens.
Table 3
Double-blind studies of adjunctive valproate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Ko et al (1985) | 6 | 28 | Crossover | Neurolepticresistant patients with chronic schizophrenia, not exacerbation | No valproate effect noted |
| Fisk and York (1987) | 62 | 42 | Antipsychotic + valproate vs antipsychotic + placebo | Chronic psychosis and tardive dyskinesia | No differences in mental state and behavior, as measured by“ Krawiecka scale”* |
| Dose et al (1998) | 42 | 28 | HAL + valproate vs HAL + placebo | Acute, nonmanic schizophrenic or schizoaffective psychosis | No difference on BPRS; possible effect on “hostile belligerence” |
| Wassef et al (2000) | 12 | 21 | HAL + valproate vs HAL + placebo | Acute exacerbation of chronic schizophrenia | CGI and SANS scores improved significantly, but BPRS scores did not |
| Casey et al (2003) | 249 | 28 | RIS + valproate vs OLZ + valproate vs RIS + placebo vs OLZ + placebo | Acute exacerbation of schizophrenia | PANSS scores improved |
| * Krawiecka M, Goldberg D, Vaughan M. A standardized psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatr Scand 1977;55(4):299-308. | |||||
| n = number of subjects | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| CGI = Clinical Global Impression scale | |||||
| HAL = haloperidol | |||||
| OLZ = olanzapine | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| RIS = risperidone | |||||
| SANS = Scale for the Assessment of Negative Symptoms | |||||
In a recent large (n = 10,262), retrospective, pharmacoepidemiologic analysis,18 valproate augmentation led to longer persistence of treatment than did the strategy of switching antipsychotics. Average valproate dosages were small, however (<425 mg/d), as were antipsychotic dosages (risperidone <1.7 mg/d, quetiapine <120 mg/d, and olanzapine <7.5 mg/d). Patients’ diagnostic categories were not available. One interpretation of this study is that valproate augmentation would be more successful than switching antipsychotics, assuming that treatment persistence can be viewed as a positive outcome.
Table 4
Double-blind studies of adjunctive lamotrigine in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen et al (2003) | 34 | 84 | Crossover; clozapine with or without lamotrigine | Clozapine-resistant male inpatients with chronic schizophrenia, not exacerbation | BPRS, PANSS positive, and PANSS general psychopathology symptom scores improved Negative symptoms did not improve |
| Kremer et al (2004) | 38 | 70 | Antipsychotic* + lamotrigine vs antipsychotic* + placebo | Treatment- resistant inpatients with schizophrenia | Completers’ PANSS positive, general psychopathology and total symptom scores improved No difference in negative symptoms or total BPRS scores No difference with intent-to-treat analyses |
| n = number of patients | |||||
| * First- or second-generation antipsychotic | |||||
| BPRS = Brief Psychiatric Rating Scale | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
Lamotrigine is the only other anticonvulsant for which published, double-blind, randomized evidence of use in patients with schizophrenia is available (Table 4).19,20 Adjunctive lamotrigine may be effective in managing treatment-resistant schizophrenia, as was shown in a small (n = 34), double-blind, placebo-controlled, crossover trial.19 Hospitalized patients whose symptoms were inadequately controlled with clozapine monotherapy received lamotrigine, 200 mg/d, for up to 12 weeks. Adjunctive lamotrigine improved positive but not negative symptoms.
Similar results were seen in treatment-resistant inpatients with schizophrenia (n = 38) in a 10-week, double-blind, parallel group trial by Kremer et al.20 Adjunctive lamotrigine improved PANSS positive, general psychopathology, and total symptom scores in the 31 patients who completed the trial. No differences were seen, however, in negative symptoms, total BPRS scores, or in the intent-to-treat analysis. These results have spurred the launch of a large, multi-site, RCT of adjunctive lamotrigine in patients with schizophrenia who have responded inadequately to antipsychotics alone.
Topiramate, one of the few psychotropics associated with weight loss, has attracted interest as an adjunct to second-generation antipsychotics to address weight gain. Although case reports have shown benefit,21 one showed deterioration in both positive and negative symptoms when topiramate was added to second-generation antipsychotics.22
Table 5
Double-blind study of adjunctive topiramate in schizophrenia
| Author (yr) | n | Duration (days) | Design | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Tiihonen (2004)* | 26 | 84 | Crossover; SGA plus topiramate or placebo | Treatment- resistant male inpatients with chronic schizophrenia | PANSS general scores improved No difference in total PANSS, PANSS positive, or PANSS negative scores |
| * 2004 Collegium Internationale Neuro-Psychopharmacologicum (CINP) presentation, and personal communication (6/22/04) | |||||
| n = number of patients | |||||
| PANSS = Positive and Negative Syndrome Scale | |||||
| SGA = Second-generation antipsychotic (patients were taking clozapine, olanzapine, or quetiapine) | |||||
An unpublished, randomized, crossover trial compared second-generation antipsychotics plus topiramate or placebo in 26 male inpatients with chronic schizophrenia. With adjunctive topiramate, the authors found a statistically significant improvement in the PANSS general psychopathology subscale but not in PANSS total, positive subscale, or negative subscale scores (Table 5) (Tiihonen J, personal communication 6/22/04).
Other agents. Very little information—all uncontrolled—supports adjunctive use of gabapentin or oxcarbazepine for patients with schizophrenia.23-28 Of concern are reports of patients suffering worsening of psychosis with gabapentin25 or of dysphoria and irritability with oxcarbazepine (attributed to a pharmacokinetic interaction).26
Conclusion. More trials are needed to examine the use of adjunctive mood stabilizers in patients with schizophrenia—particularly in those with chronic symptoms. Although mood stabilizers are widely used in this population, important questions remain unanswered, including:
- characteristics of patients likely to require adjunctive treatment
- how long treatment should continue.
MECHANISMS OF ACTION
Unlike antipsychotics, mood stabilizers do not exert their therapeutic effects by acting directly on dopamine (D2) receptors. Differences in mechanism of action among the anticonvulsants may help explain why some—such as valproate and lamotrigine—have been useful for bipolar disorder or schizophrenia and others—such as gabapentin—have not.29
One possibility is that anticonvulsants that affect voltage-gated sodium channels—such as valproate, lamotrigine, carbamazepine and oxcarbazepine—may be most useful for patients with bipolar disorder or schizophrenia. On the other hand, agents that affect voltage-gated calcium channels—such as gabapentin—may be efficacious as anticonvulsants but not as efficacious for bipolar disorder or schizophrenia.
Ketter et al30 proposed an anticonvulsant classification system based on predominant psychotropic profiles:
- the “GABA-ergic” group predominantly potentiates the inhibitory neurotransmitter GABA, resulting in sedation, fatigue, cognitive slowing, and weight gain, as well as possible anxiolytic and antimanic effects
- the “anti-glutamate” group predominantly attenuates glutamate excitatory neurotransmission and is associated with activation, weight loss, and possibly anxiogenic and antidepressant effects.
In the GABA-ergic group are anticonvulsants such as barbiturates, benzodiazepines, valproate, gabapentin, tiagabine, and vigabatrin. The antiglutamate group includes agents such as felbamate and lamotrigine. A “mixed” category includes anticonvulsants with GABA-ergic and anti-glutaminergic actions such as topiramate, which has sedating and weight-loss properties.
Because GABA appears to modulate dopamine neurotransmission,31 this may explain valproate’s role as an adjunctive agent for schizophrenia. Similarly, mechanisms related to Nmethyl-D-aspartate (NMDA) and non-NMDA glutamate receptor function may explain lamotrigine’s usefulness in this setting.19,20
SUMMARY
Clinicians resort to combination therapies when monotherapies fail to adequately control symptoms or maintain response. Co-prescribing of anticonvulsants with antipsychotics for inpatients with schizophrenia is common practice in New York State and most likely elsewhere. In general, antipsychotics’ and mood stabilizers’ different—and perhaps complementary—mechanisms of action explain the synergism between them. Mechanisms of action also may explain why some anticonvulsants help in schizophrenia (or bipolar disorder) whereas others do not.
Evidence for using adjunctive anticonvulsants is variable. The strongest data support using valproate (and perhaps lamotrigine), followed by carbamazepine and then topiramate. Gabapentin and oxcarbazepine have only anecdotal evidence, some of it negative. Well-designed, randomized clinical trials with the appropriate populations are needed.
Related resources
- Stahl SM. Essential psychopharmacology of antipsychotics and mood stabilizers. New York: Cambridge University Press, 2002.
- Harvard Medical School Department of Psychiatry’s psychopharmacology algorithm project. Osser DN, Patterson RD. Consultant for the pharmacotherapy of schizophrenia. Available at http://mhc.com/Algorithms/. Accessed Nov. 5, 2004.
Drug brand names
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Lithobid, Eskalith
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Topiramate • Topamax
- Valproate (valproic acid, divalproex sodium) • Depakene, Depakote
Disclosure
Dr. Citrome receives research grants/contracts from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, and Pfizer Inc. He is a consultant to and/or speaker for Bristol-Myers Squibb Co., Eli Lilly & Co., Pfizer Inc., Abbott Laboratories, AstraZeneca Pharmaceuticals, and Novartis Pharmaceuticals Corp.
Acknowledgment
Adapted from Citrome L. “Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S. perspective” (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum (CINP), June 2004 [abstract in Int J Neuropsychopharmacol. 2004; 7(suppl 1):S69], and from Citrome L. “Mood-stabilizer use in schizophrenia: 1994-2002” (NR350) (poster). New York: American Psychiatric Association annual meeting, May 2004.
1. Citrome L, Jaffe A, Levine J. Datapoints - mood stabilizers: utilization trends in patients diagnosed with schizophrenia 1994-2001. Psychiatr Serv 2002;53(10):1212.-
2. Citrome L. Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S.perspective (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum(CINP), June 2004 [abstract in Int J Neuropsychopharmacol 2004;7(suppl 1):S69].
3. Leucht S, Kissling W, McGrath J. Lithium for schizophrenia revisited: a systematic review and meta-analysis of randomized clinical trials. J Clin Psychiatry 2004;65(2):177-86.
4. Neppe VM. Carbamazepine as adjunctive treatment in nonepileptic chronic inpatients with EEG temporal lobe abnormalities. J Clin Psychiatry 1983;44:326-31.
5. Dose M, Apelt S, Emrich HM. Carbamazepine as an adjunct of antipsychotic therapy. Psychiatry Res 1987;22:303-10.
6. Okuma T, Yamashita I, Takahashi R, et al. A double-blind study of adjunctive carbamazepine versus placebo on excited states of schizophrenic and schizoaffective disorders. Acta Psychiatr Scand 1989;80:250-9.
7. Nachshoni T, Levin Y, Levy A, et al. A double-blind trial of carbamazepine in negative symptom schizophrenia. Biol Psychiatry 1994;35(1):22-26.
8. Simhandl C, Meszaros K, Denk E, et al. Adjunctive carbamazepine or lithium carbonate in therapy-resistant chronic schizophrenia. Can J Psychiatry 1996;41(5):317.-
9. Leucht S, McGrath J, White P, et al. Carbamazepine augmentation for schizophrenia: how good is the evidence? J Clin Psychiatry 2002;63(3):218-24.
10. Citrome L. Schizophrenia and valproate. Psychopharmacol Bull 2003;37(suppl 2):74-88.
11. Basan A, Kissling W, Leucht S. Valproate as an adjunct to antipsychotics for schizophrenia: a systematic review of randomized trials. Schizophr Res 2004;70(1):33-7.
12. Ko GN, Korpi ER, Freed WJ, et al. Effect of valproic acid on behavior and plasma amino acid concentrations in chronic schizophrenia patients. Biol Psychiatry 1985;20:209-15.
13. Dose M, Hellweg R, Yassouridis A, et al. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry 1998;31(4):122-5.
14. Fisk GG, York SM. The effect of sodium valproate on tardive dyskinesia—revisited. Br J Psychiatry 1987;150:542-6.
15. Wassef AA, Dott SG, Harris A, et al. Randomized, placebo-controlled pilot study of divalproex sodium in the treatment of acute exacerbations of chronic schizophrenia. J Clin Psychopharmacol 2000;20(3):357-361.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacol 2003;28(1):182-92.
17. Citrome L, Casey DE, Daniel DG, et al. Effects of adjunctive valproate on hostility in patients with schizophrenia receiving olanzapine or risperidone: a double-blind multi-center study. Psychiatr Serv 2004;55(3):290-4.
18. Cramer JA, Sernyak M. Results of a naturalistic study of treatment options: switching atypical antipsychotic drugs or augmenting with valproate. Clin Ther 2004;26(6):905-14.
19. Tiihonen J, Hallikainen T, Ryynanen OP, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled trial. Biol Psychiatry 2003;54(11):1241-8.
20. Kremer I, Vass A, Gurelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry 2004;56(6):441-6.
21. Drapalski AL, Rosse RB, Peebles RR, et al. Topiramate improves deficit symptoms in a patient with schizophrenia when added to a stable regimen of antipsychotic medication. Clin Neuropharmacol 2001;24:290-4.
22. Millson RC, Owen JA, Lorberg GW, Tackaberry L. Topiramate for refractory schizophrenia. Am J Psychiatry 2002;159(4):675.-
23. Chouinard G, Beauclair L, Belanger MC. Gabapentin: long term antianxiety and hypnotic effects in psychiatric patients with comorbid anxiety-related disorders. Can J Psychiatry 1998;43:305.-
24. Megna JL, Devitt PJ, Sauro MD, Dewan MJ. Gabapentin’s effect on agitation in severely and persistently mentally ill patients. Ann Pharmacother 2002;35:12-16.
25. Jablonowski K, Margolese HC, Chouinard G. Gabapentin-induced paradoxical exacerbation of psychosis in a patient with schizophrenia. Can J Psychiatry 2002;47(10):975-6.
26. Baird P. The interactive metabolism effect of oxcarbazepine coadministered with tricyclic antidepressant therapy for OCD symptoms. J Clin Psychopharmacol 2003;23(4):419.-
27. Centorrino F, Albert MJ, Berry JM, et al. Oxcarbazepine: clinical experience with hospitalized psychiatric patients. Bipolar Disord 2003;5(5):370-4.
28. Leweke FM, Gerth CW, Koethe D, et al. Oxcarbazepine as an adjunct for schizophrenia. Am J Psychiatry 2004;161(6):1130-1.
29. Stahl SM. Psychopharmacology of anticonvulsants: do all anticonvulsants have the same mechanism of action? J Clin Psychiatry 2004;65(2):149-50.
30. Ketter TA, Wong PW. The emerging differential roles of GABAergic and antiglutaminergic agents in bipolar disorders. J Clin Psychiatry 2003;64(suppl 3):15-20.
31. Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol 2003;23(6):601-40.
1. Citrome L, Jaffe A, Levine J. Datapoints - mood stabilizers: utilization trends in patients diagnosed with schizophrenia 1994-2001. Psychiatr Serv 2002;53(10):1212.-
2. Citrome L. Antipsychotic polypharmacy versus augmentation with anticonvulsants: the U.S.perspective (presentation). Paris: Collegium Internationale Neuro-Psychopharmacologicum(CINP), June 2004 [abstract in Int J Neuropsychopharmacol 2004;7(suppl 1):S69].
3. Leucht S, Kissling W, McGrath J. Lithium for schizophrenia revisited: a systematic review and meta-analysis of randomized clinical trials. J Clin Psychiatry 2004;65(2):177-86.
4. Neppe VM. Carbamazepine as adjunctive treatment in nonepileptic chronic inpatients with EEG temporal lobe abnormalities. J Clin Psychiatry 1983;44:326-31.
5. Dose M, Apelt S, Emrich HM. Carbamazepine as an adjunct of antipsychotic therapy. Psychiatry Res 1987;22:303-10.
6. Okuma T, Yamashita I, Takahashi R, et al. A double-blind study of adjunctive carbamazepine versus placebo on excited states of schizophrenic and schizoaffective disorders. Acta Psychiatr Scand 1989;80:250-9.
7. Nachshoni T, Levin Y, Levy A, et al. A double-blind trial of carbamazepine in negative symptom schizophrenia. Biol Psychiatry 1994;35(1):22-26.
8. Simhandl C, Meszaros K, Denk E, et al. Adjunctive carbamazepine or lithium carbonate in therapy-resistant chronic schizophrenia. Can J Psychiatry 1996;41(5):317.-
9. Leucht S, McGrath J, White P, et al. Carbamazepine augmentation for schizophrenia: how good is the evidence? J Clin Psychiatry 2002;63(3):218-24.
10. Citrome L. Schizophrenia and valproate. Psychopharmacol Bull 2003;37(suppl 2):74-88.
11. Basan A, Kissling W, Leucht S. Valproate as an adjunct to antipsychotics for schizophrenia: a systematic review of randomized trials. Schizophr Res 2004;70(1):33-7.
12. Ko GN, Korpi ER, Freed WJ, et al. Effect of valproic acid on behavior and plasma amino acid concentrations in chronic schizophrenia patients. Biol Psychiatry 1985;20:209-15.
13. Dose M, Hellweg R, Yassouridis A, et al. Combined treatment of schizophrenic psychoses with haloperidol and valproate. Pharmacopsychiatry 1998;31(4):122-5.
14. Fisk GG, York SM. The effect of sodium valproate on tardive dyskinesia—revisited. Br J Psychiatry 1987;150:542-6.
15. Wassef AA, Dott SG, Harris A, et al. Randomized, placebo-controlled pilot study of divalproex sodium in the treatment of acute exacerbations of chronic schizophrenia. J Clin Psychopharmacol 2000;20(3):357-361.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacol 2003;28(1):182-92.
17. Citrome L, Casey DE, Daniel DG, et al. Effects of adjunctive valproate on hostility in patients with schizophrenia receiving olanzapine or risperidone: a double-blind multi-center study. Psychiatr Serv 2004;55(3):290-4.
18. Cramer JA, Sernyak M. Results of a naturalistic study of treatment options: switching atypical antipsychotic drugs or augmenting with valproate. Clin Ther 2004;26(6):905-14.
19. Tiihonen J, Hallikainen T, Ryynanen OP, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled trial. Biol Psychiatry 2003;54(11):1241-8.
20. Kremer I, Vass A, Gurelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry 2004;56(6):441-6.
21. Drapalski AL, Rosse RB, Peebles RR, et al. Topiramate improves deficit symptoms in a patient with schizophrenia when added to a stable regimen of antipsychotic medication. Clin Neuropharmacol 2001;24:290-4.
22. Millson RC, Owen JA, Lorberg GW, Tackaberry L. Topiramate for refractory schizophrenia. Am J Psychiatry 2002;159(4):675.-
23. Chouinard G, Beauclair L, Belanger MC. Gabapentin: long term antianxiety and hypnotic effects in psychiatric patients with comorbid anxiety-related disorders. Can J Psychiatry 1998;43:305.-
24. Megna JL, Devitt PJ, Sauro MD, Dewan MJ. Gabapentin’s effect on agitation in severely and persistently mentally ill patients. Ann Pharmacother 2002;35:12-16.
25. Jablonowski K, Margolese HC, Chouinard G. Gabapentin-induced paradoxical exacerbation of psychosis in a patient with schizophrenia. Can J Psychiatry 2002;47(10):975-6.
26. Baird P. The interactive metabolism effect of oxcarbazepine coadministered with tricyclic antidepressant therapy for OCD symptoms. J Clin Psychopharmacol 2003;23(4):419.-
27. Centorrino F, Albert MJ, Berry JM, et al. Oxcarbazepine: clinical experience with hospitalized psychiatric patients. Bipolar Disord 2003;5(5):370-4.
28. Leweke FM, Gerth CW, Koethe D, et al. Oxcarbazepine as an adjunct for schizophrenia. Am J Psychiatry 2004;161(6):1130-1.
29. Stahl SM. Psychopharmacology of anticonvulsants: do all anticonvulsants have the same mechanism of action? J Clin Psychiatry 2004;65(2):149-50.
30. Ketter TA, Wong PW. The emerging differential roles of GABAergic and antiglutaminergic agents in bipolar disorders. J Clin Psychiatry 2003;64(suppl 3):15-20.
31. Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol 2003;23(6):601-40.