User login
Opioid use disorder in pregnancy: A strategy for using methadone
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
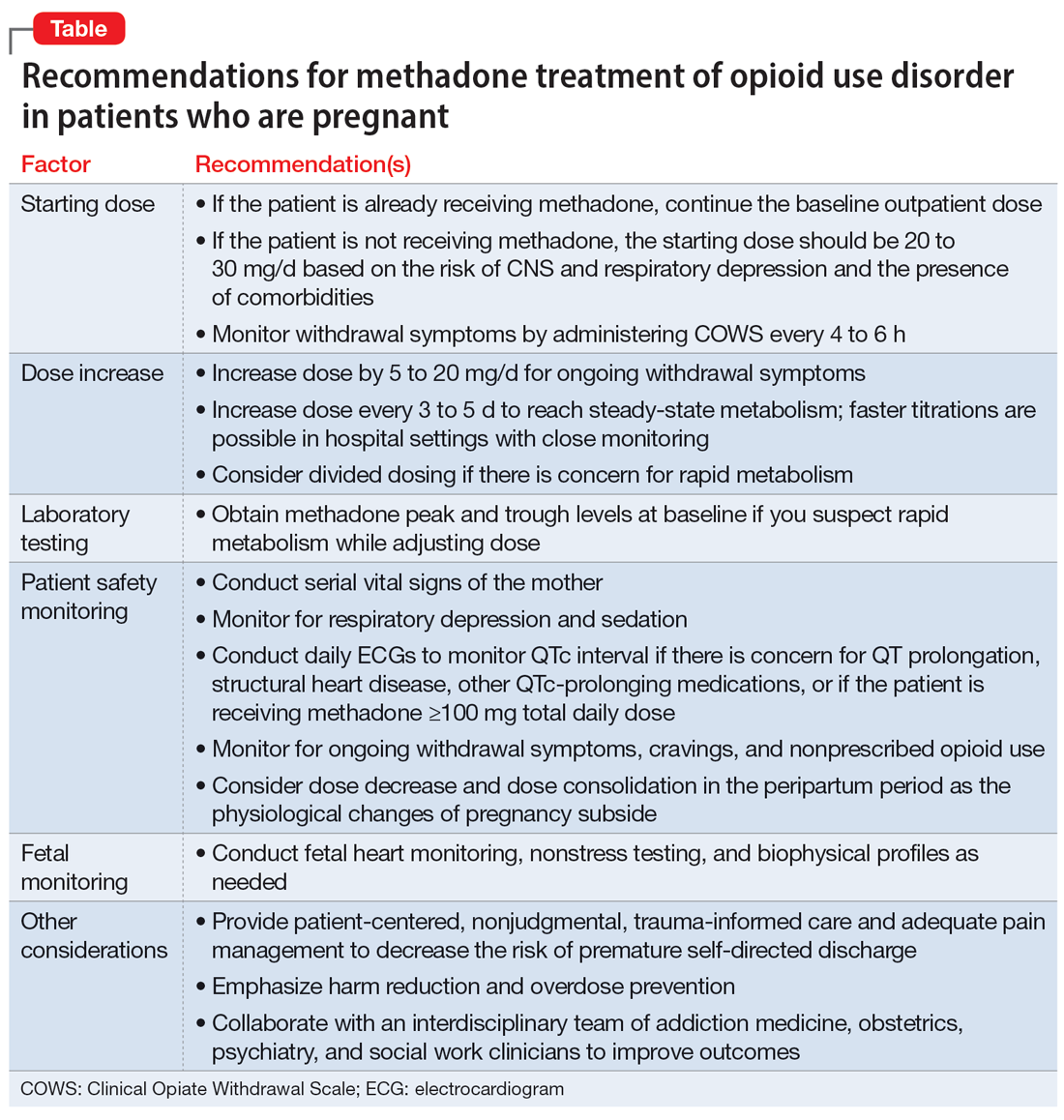
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.
Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.

Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.

Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
Product Update
LUTECH LT-300 HD FOR COLPOSCOPY
Background. In March 1924, the colposcope was introduced to evaluate the portio of the cervix by Hans Hinselmann in Germany after years of work with the famous lens manufacturer Leitz.1 Although its adoption as a standard tool for evaluating lower genital tract neoplasia was protracted, today it sits as a cornerstone technology in gynecology, and every ObGyn provider has been trained to perform colposcopic exams that include visualizing the cervix, vagina, and vulva as well as taking biopsies. In December 2000, after 75 years of glass lens technology, Welch-Allyn (Skaneateles Falls, New York) introduced the first video colposcope, shepherding the field into the 21st century with only limited traction. Now, Lutech is entering the fray hoping to further nudge traditionalists into the digital age.
Design/Functionality. The Lutech LT-300 HD works off of a Sony Exmor CMOS (complementary metaloxide semiconductor) camera with 2.13 megapixels to provide high-definition optical magnification of 1-30X illuminated by a circular cool LED array that offers 3000 lx of white light with an adjustable green filter to allow for contrast at working distances between 5.1 and 15.7 inches. The colposcope comes with either a vertical stand or a swing arm stand and has both HDMI and USB 3.0 video output so that the system can be attached to either a stand-alone monitor or a computer (not included). The colposcope also comes in a standard definition configuration (LT-300 SD), but I did not trial that model because the price difference did not seem to justify the potentially lower resolution.
In my experience with its use, the Lutech LT-300 HD was pretty excellent. Being a man and a doctor, I refused the online training session that comes free with the colposcope, assuming I could figure it out on my own. My assumption was mostly true, but there were definitely some tips and tricks that would have made my life easier had I not been so stiff-necked. That said, the biggest adjustment is getting used to looking at a screen and not having to look through eyepieces. The picture output is great and, as a patient (or student) teaching tool, it is phenomenal. Also, because it is digital, the image capture features allow for image importation into notes (although it is clunky and requires work arounds when using Epic).
Innovation. From an innovation point of view, I am not sure that Lutech re-invented fire since, in essence, the LT-300 HD is a modified CMOS video camera. But the company did do a nice job bringing together a lot of existing technologies into a highly functional product. I would love to see better integration with some of the larger electronic medical records (EMRs), but I suspect the barriers lie with the EMR companies rather than with Lutech, so I am giving them a pass on that front.
Summary. At its core, a colposcope is simply a tool with which to obtain a magnified view of the cervix, vagina, and/or vulva. Prior to advent and proliferation of CMOS camera technology, the most readily available means of accomplishing this was to employ glass lenses. But that was then, and this is now; CMOS technology is just better, cheaper, and more versatile. I no longer turn my head to look over my shoulder while backing up my car—I use the back-up camera. My Kodak instamatic has given way to my iPhone. And now, my incredibly heavy, unwieldy Leisegang colposcope has been replaced by a light-weight camera on a stand that I can easily move from room to room. I won’t lie, though,…it still seems weird to not look through eyepieces and work the focus knobs, but I am happy with the change. My patients can now see what I am looking at and better understand their diagnosis (if they want), and my notes are prettier. Onward march of progress.
Reference
1. Fusco E, Padula F, Mancini E, et al. History of colposcopy: a brief biography of Hinselmann. J Prenat Med. 2008;2:19-23.
Continue to: DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH...
DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH
The single-use DTR Medical Cervical Rotating Biopsy Punch from Innovia Medical (Swansea, United Kingdom) “works great” and “is reasonably cost-effective to replace reusables.”
Background. Integral to every colposcopic examination is the potential need to biopsy abnormal appearing tissues. To accomplish this latter task, numerous punch-style biopsy devices have been developed in a variety of jaw shapes and styles, crafted from materials ranging from stainless steel to titanium to ceramic, with the ultimate goal the same—get a piece of tissue from the cervix as easily as possible.
Design/Functionality. DTR Medical Cervical Rotating Biopsy Punch is a single-use sterile device that comes packaged as 10 per box. It features Kevorkian-style “stronger than Titanium” jaws that yield a 3.0 mm x 7.5 mm sample attached to a metal shaft that can rotate 360°. The shaft inserts into a lightweight plastic pistol-grip style handle. From tip to handle, the device measures 36.5 cm (14.125 in).
In my experience with its use, the DTR Medical Cervical Rotating Biopsy Punch performed flawlessly. Its relatively low-profile jaws allowed for unobstructed access to biopsy sites and the ability to rotate the jaws was a big plus. The “stronger than Titanium” jaws consistently yielded the exact biopsies I wanted, like a knife going through butter.
Innovation. From an innovation standpoint, the DTR Medical Cervical Rotating Biopsy Punch is more of an engineering “duh” than “wow,” but it works great so who cares that it’s not a fusion reactor. That said, the innovative part from Innovia Medical is their ability to make such a high-quality biopsy device and sell it at a price that makes it reasonably cost-effective to replace reusables.
Summary. Whether it is a Tischler, Kevorkian, or Burke tip, the real question before any gynecologist uses the cervical biopsy device she/he/they has in her/his/ their hand is, will it cut? Because all reusable surgical instruments are in fact reusable, those edges that are designed to cut invariably become dull with reuse. And, unless they are meticulously maintained and routinely sharpened (spoiler alert, they never are), providers are not infrequently chagrinned by the gnawing rather than cutting that these instruments deliver. Thinking back, I could not remember the last time I had made an incision with a surgical scalpel blade that had previously been used then sharpened and re-sterilized. Then I did remember…never. Reflecting on this, I wondered why I was doing this with my cervical biopsy devices. While I really do not like the environmental waste created by single-use devices, reusable instruments that require re-processing do have an environmental impact and a significant cost. Considering this, I do not think that environmental reasons are enough of a barrier to justify using dull biopsy tools if it can be done cost-effectively with a minimal carbon footprint. All-in-all, I like this product, and I plan to use it. ●
LUTECH LT-300 HD FOR COLPOSCOPY
Background. In March 1924, the colposcope was introduced to evaluate the portio of the cervix by Hans Hinselmann in Germany after years of work with the famous lens manufacturer Leitz.1 Although its adoption as a standard tool for evaluating lower genital tract neoplasia was protracted, today it sits as a cornerstone technology in gynecology, and every ObGyn provider has been trained to perform colposcopic exams that include visualizing the cervix, vagina, and vulva as well as taking biopsies. In December 2000, after 75 years of glass lens technology, Welch-Allyn (Skaneateles Falls, New York) introduced the first video colposcope, shepherding the field into the 21st century with only limited traction. Now, Lutech is entering the fray hoping to further nudge traditionalists into the digital age.
Design/Functionality. The Lutech LT-300 HD works off of a Sony Exmor CMOS (complementary metaloxide semiconductor) camera with 2.13 megapixels to provide high-definition optical magnification of 1-30X illuminated by a circular cool LED array that offers 3000 lx of white light with an adjustable green filter to allow for contrast at working distances between 5.1 and 15.7 inches. The colposcope comes with either a vertical stand or a swing arm stand and has both HDMI and USB 3.0 video output so that the system can be attached to either a stand-alone monitor or a computer (not included). The colposcope also comes in a standard definition configuration (LT-300 SD), but I did not trial that model because the price difference did not seem to justify the potentially lower resolution.
In my experience with its use, the Lutech LT-300 HD was pretty excellent. Being a man and a doctor, I refused the online training session that comes free with the colposcope, assuming I could figure it out on my own. My assumption was mostly true, but there were definitely some tips and tricks that would have made my life easier had I not been so stiff-necked. That said, the biggest adjustment is getting used to looking at a screen and not having to look through eyepieces. The picture output is great and, as a patient (or student) teaching tool, it is phenomenal. Also, because it is digital, the image capture features allow for image importation into notes (although it is clunky and requires work arounds when using Epic).
Innovation. From an innovation point of view, I am not sure that Lutech re-invented fire since, in essence, the LT-300 HD is a modified CMOS video camera. But the company did do a nice job bringing together a lot of existing technologies into a highly functional product. I would love to see better integration with some of the larger electronic medical records (EMRs), but I suspect the barriers lie with the EMR companies rather than with Lutech, so I am giving them a pass on that front.
Summary. At its core, a colposcope is simply a tool with which to obtain a magnified view of the cervix, vagina, and/or vulva. Prior to advent and proliferation of CMOS camera technology, the most readily available means of accomplishing this was to employ glass lenses. But that was then, and this is now; CMOS technology is just better, cheaper, and more versatile. I no longer turn my head to look over my shoulder while backing up my car—I use the back-up camera. My Kodak instamatic has given way to my iPhone. And now, my incredibly heavy, unwieldy Leisegang colposcope has been replaced by a light-weight camera on a stand that I can easily move from room to room. I won’t lie, though,…it still seems weird to not look through eyepieces and work the focus knobs, but I am happy with the change. My patients can now see what I am looking at and better understand their diagnosis (if they want), and my notes are prettier. Onward march of progress.
Reference
1. Fusco E, Padula F, Mancini E, et al. History of colposcopy: a brief biography of Hinselmann. J Prenat Med. 2008;2:19-23.
Continue to: DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH...
DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH
The single-use DTR Medical Cervical Rotating Biopsy Punch from Innovia Medical (Swansea, United Kingdom) “works great” and “is reasonably cost-effective to replace reusables.”
Background. Integral to every colposcopic examination is the potential need to biopsy abnormal appearing tissues. To accomplish this latter task, numerous punch-style biopsy devices have been developed in a variety of jaw shapes and styles, crafted from materials ranging from stainless steel to titanium to ceramic, with the ultimate goal the same—get a piece of tissue from the cervix as easily as possible.
Design/Functionality. DTR Medical Cervical Rotating Biopsy Punch is a single-use sterile device that comes packaged as 10 per box. It features Kevorkian-style “stronger than Titanium” jaws that yield a 3.0 mm x 7.5 mm sample attached to a metal shaft that can rotate 360°. The shaft inserts into a lightweight plastic pistol-grip style handle. From tip to handle, the device measures 36.5 cm (14.125 in).
In my experience with its use, the DTR Medical Cervical Rotating Biopsy Punch performed flawlessly. Its relatively low-profile jaws allowed for unobstructed access to biopsy sites and the ability to rotate the jaws was a big plus. The “stronger than Titanium” jaws consistently yielded the exact biopsies I wanted, like a knife going through butter.
Innovation. From an innovation standpoint, the DTR Medical Cervical Rotating Biopsy Punch is more of an engineering “duh” than “wow,” but it works great so who cares that it’s not a fusion reactor. That said, the innovative part from Innovia Medical is their ability to make such a high-quality biopsy device and sell it at a price that makes it reasonably cost-effective to replace reusables.
Summary. Whether it is a Tischler, Kevorkian, or Burke tip, the real question before any gynecologist uses the cervical biopsy device she/he/they has in her/his/ their hand is, will it cut? Because all reusable surgical instruments are in fact reusable, those edges that are designed to cut invariably become dull with reuse. And, unless they are meticulously maintained and routinely sharpened (spoiler alert, they never are), providers are not infrequently chagrinned by the gnawing rather than cutting that these instruments deliver. Thinking back, I could not remember the last time I had made an incision with a surgical scalpel blade that had previously been used then sharpened and re-sterilized. Then I did remember…never. Reflecting on this, I wondered why I was doing this with my cervical biopsy devices. While I really do not like the environmental waste created by single-use devices, reusable instruments that require re-processing do have an environmental impact and a significant cost. Considering this, I do not think that environmental reasons are enough of a barrier to justify using dull biopsy tools if it can be done cost-effectively with a minimal carbon footprint. All-in-all, I like this product, and I plan to use it. ●
LUTECH LT-300 HD FOR COLPOSCOPY
Background. In March 1924, the colposcope was introduced to evaluate the portio of the cervix by Hans Hinselmann in Germany after years of work with the famous lens manufacturer Leitz.1 Although its adoption as a standard tool for evaluating lower genital tract neoplasia was protracted, today it sits as a cornerstone technology in gynecology, and every ObGyn provider has been trained to perform colposcopic exams that include visualizing the cervix, vagina, and vulva as well as taking biopsies. In December 2000, after 75 years of glass lens technology, Welch-Allyn (Skaneateles Falls, New York) introduced the first video colposcope, shepherding the field into the 21st century with only limited traction. Now, Lutech is entering the fray hoping to further nudge traditionalists into the digital age.
Design/Functionality. The Lutech LT-300 HD works off of a Sony Exmor CMOS (complementary metaloxide semiconductor) camera with 2.13 megapixels to provide high-definition optical magnification of 1-30X illuminated by a circular cool LED array that offers 3000 lx of white light with an adjustable green filter to allow for contrast at working distances between 5.1 and 15.7 inches. The colposcope comes with either a vertical stand or a swing arm stand and has both HDMI and USB 3.0 video output so that the system can be attached to either a stand-alone monitor or a computer (not included). The colposcope also comes in a standard definition configuration (LT-300 SD), but I did not trial that model because the price difference did not seem to justify the potentially lower resolution.
In my experience with its use, the Lutech LT-300 HD was pretty excellent. Being a man and a doctor, I refused the online training session that comes free with the colposcope, assuming I could figure it out on my own. My assumption was mostly true, but there were definitely some tips and tricks that would have made my life easier had I not been so stiff-necked. That said, the biggest adjustment is getting used to looking at a screen and not having to look through eyepieces. The picture output is great and, as a patient (or student) teaching tool, it is phenomenal. Also, because it is digital, the image capture features allow for image importation into notes (although it is clunky and requires work arounds when using Epic).
Innovation. From an innovation point of view, I am not sure that Lutech re-invented fire since, in essence, the LT-300 HD is a modified CMOS video camera. But the company did do a nice job bringing together a lot of existing technologies into a highly functional product. I would love to see better integration with some of the larger electronic medical records (EMRs), but I suspect the barriers lie with the EMR companies rather than with Lutech, so I am giving them a pass on that front.
Summary. At its core, a colposcope is simply a tool with which to obtain a magnified view of the cervix, vagina, and/or vulva. Prior to advent and proliferation of CMOS camera technology, the most readily available means of accomplishing this was to employ glass lenses. But that was then, and this is now; CMOS technology is just better, cheaper, and more versatile. I no longer turn my head to look over my shoulder while backing up my car—I use the back-up camera. My Kodak instamatic has given way to my iPhone. And now, my incredibly heavy, unwieldy Leisegang colposcope has been replaced by a light-weight camera on a stand that I can easily move from room to room. I won’t lie, though,…it still seems weird to not look through eyepieces and work the focus knobs, but I am happy with the change. My patients can now see what I am looking at and better understand their diagnosis (if they want), and my notes are prettier. Onward march of progress.
Reference
1. Fusco E, Padula F, Mancini E, et al. History of colposcopy: a brief biography of Hinselmann. J Prenat Med. 2008;2:19-23.
Continue to: DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH...
DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH
The single-use DTR Medical Cervical Rotating Biopsy Punch from Innovia Medical (Swansea, United Kingdom) “works great” and “is reasonably cost-effective to replace reusables.”
Background. Integral to every colposcopic examination is the potential need to biopsy abnormal appearing tissues. To accomplish this latter task, numerous punch-style biopsy devices have been developed in a variety of jaw shapes and styles, crafted from materials ranging from stainless steel to titanium to ceramic, with the ultimate goal the same—get a piece of tissue from the cervix as easily as possible.
Design/Functionality. DTR Medical Cervical Rotating Biopsy Punch is a single-use sterile device that comes packaged as 10 per box. It features Kevorkian-style “stronger than Titanium” jaws that yield a 3.0 mm x 7.5 mm sample attached to a metal shaft that can rotate 360°. The shaft inserts into a lightweight plastic pistol-grip style handle. From tip to handle, the device measures 36.5 cm (14.125 in).
In my experience with its use, the DTR Medical Cervical Rotating Biopsy Punch performed flawlessly. Its relatively low-profile jaws allowed for unobstructed access to biopsy sites and the ability to rotate the jaws was a big plus. The “stronger than Titanium” jaws consistently yielded the exact biopsies I wanted, like a knife going through butter.
Innovation. From an innovation standpoint, the DTR Medical Cervical Rotating Biopsy Punch is more of an engineering “duh” than “wow,” but it works great so who cares that it’s not a fusion reactor. That said, the innovative part from Innovia Medical is their ability to make such a high-quality biopsy device and sell it at a price that makes it reasonably cost-effective to replace reusables.
Summary. Whether it is a Tischler, Kevorkian, or Burke tip, the real question before any gynecologist uses the cervical biopsy device she/he/they has in her/his/ their hand is, will it cut? Because all reusable surgical instruments are in fact reusable, those edges that are designed to cut invariably become dull with reuse. And, unless they are meticulously maintained and routinely sharpened (spoiler alert, they never are), providers are not infrequently chagrinned by the gnawing rather than cutting that these instruments deliver. Thinking back, I could not remember the last time I had made an incision with a surgical scalpel blade that had previously been used then sharpened and re-sterilized. Then I did remember…never. Reflecting on this, I wondered why I was doing this with my cervical biopsy devices. While I really do not like the environmental waste created by single-use devices, reusable instruments that require re-processing do have an environmental impact and a significant cost. Considering this, I do not think that environmental reasons are enough of a barrier to justify using dull biopsy tools if it can be done cost-effectively with a minimal carbon footprint. All-in-all, I like this product, and I plan to use it. ●
Delirious mania: Presentation, pathogenesis, and management
Delirious mania is a syndrome characterized by the acute onset of severe hyperactivity, psychosis, catatonia, and intermittent confusion. While there have been growing reports of this phenomenon over the last 2 decades, it remains poorly recognized and understood.1,2 There is no widely accepted nosology for delirious mania and the condition is absent from DSM-5, which magnifies the difficulties in making a timely diagnosis and initiating appropriate treatment. Delayed diagnosis and treatment may result in a detrimental outcome.2,3 Delirious mania has also been labeled as lethal catatonia, specific febrile delirium, hyperactive or exhaustive mania, and Bell’s mania.2,4,5 The characterization and diagnosis of this condition have a long and inconsistent history (Box1,6-11).
Box
Delirious mania was originally recognized in 1849 by Luther Bell in McLean Hospital after he observed 40 cases that were uniquely distinct from 1,700 other cases from 1836 to 1849.6 He described these patients as being suddenly confused, demonstrating unprovoked combativeness, remarkable decreased need for sleep, excessive motor restlessness, extreme fearfulness, and certain physiological signs, including rapid pulse and sweating. Bell was limited to the psychiatric treatment of his time, which largely was confined to physical restraints. Approximately three-fourths of these patients died.6
Following Bell’s report, this syndrome remained unexplored and rarely described. Some researchers postulated that the development of confusion was a natural progression of late-phase mania in close to 20% of patients.7 However, this did not account for the rapid onset of symptoms as well as certain unexplained movement abnormalities. In 1980, Bond8 presented 3 cases that were similar in nature to Bell’s depiction: acute onset with extraordinary irritability, withdrawal, delirium, and mania.
For the next 2 decades, delirious mania was seldom reported in the literature. The term was often reserved to illustrate when a patient had nothing more than mania with features of delirium.9
By 1996, catatonia became better recognized in its wide array of symptomology and diagnostic scales.10,11 In 1999, in addition to the sudden onset of excitement, paranoia, grandiosity, and disorientation, Fink1 reported catatonic signs including negativism, stereotypy, posturing, grimacing, and echo phenomena in patients with delirious mania. He identified its sensitive response to electroconvulsive therapy.
Delirious mania continues to be met with incertitude in clinical practice, and numerous inconsistencies have been reported in the literature. For example, some cases that have been reported as delirious mania had more evidence of primary delirium due to another medical condition or primary mania.12,13 Other cases have demonstrated swift improvement of symptoms after monotherapy with antipsychotics without a trial of benzodiazepines or electroconvulsive therapy (ECT); the exclusion of a sudden onset questions the validity of the diagnosis and promotes the use of less efficacious treatments.14,15 Other reports have confirmed that the diagnosis is missed when certain symptoms are more predominant, such as a thought disorder (acute schizophrenia), grandiosity and delusional ideation (bipolar disorder [BD]), and less commonly assessed catatonic signs (ambitendency, automatic obedience). These symptoms are mistakenly attributed to the respective disease.1,16 This especially holds true when delirious mania is initially diagnosed as a primary psychosis, which leads to the administration of antipsychotics.17 Other cases have reported that delirious mania was resistant to treatment, but ECT was never pursued.18
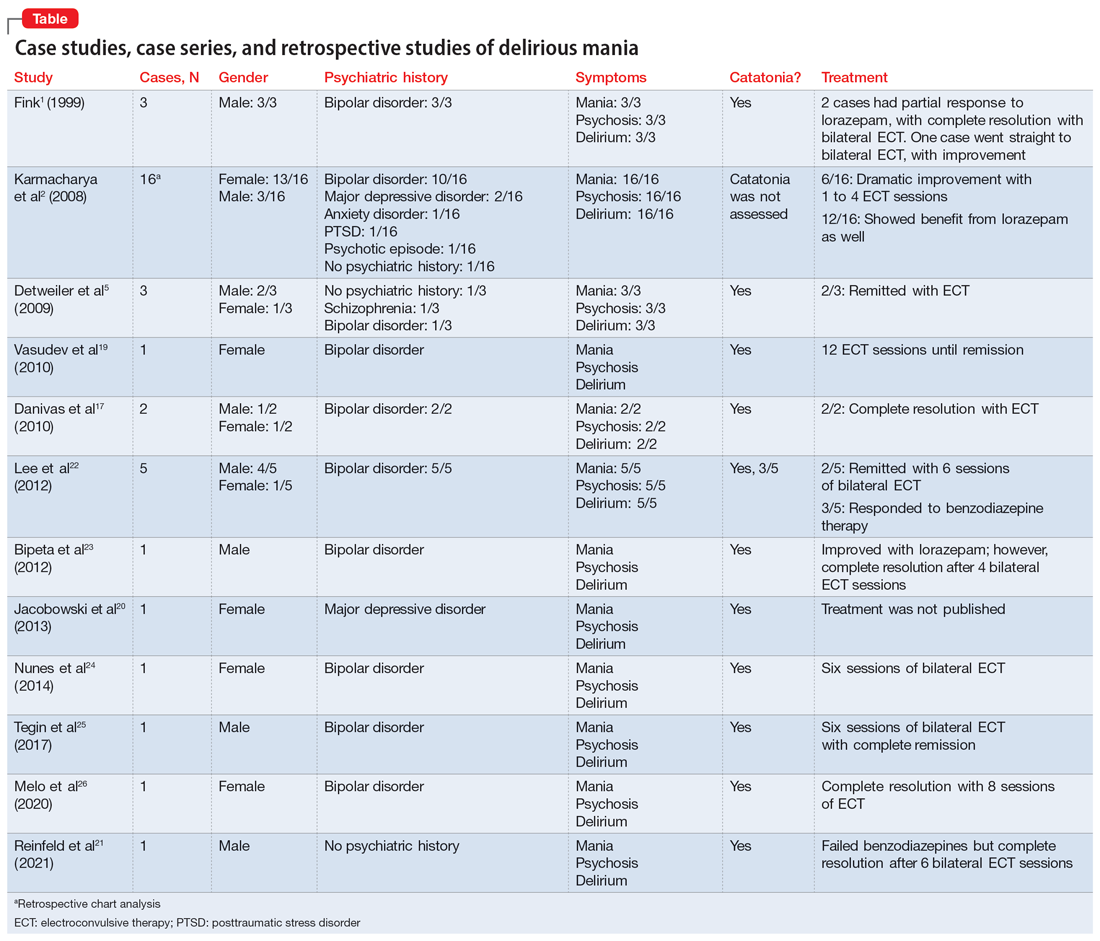
In this review, we provide a more comprehensive perspective of the clinical presentation, pathogenesis, and management of delirious mania. We searched PubMed and Google Scholar using the keywords “delirious mania,” “delirious mania AND catatonia,” or “manic delirium.” Most articles we found were case reports, case series, or retrospective chart reviews. There were no systematic reviews, meta analyses, or randomized control trials (RCTs). The 12 articles included in this review consist of 7 individual case reports, 4 case series, and 1 retrospective chart review that describe a total of 36 cases (Table1,2,5,17,19-26).
Clinical presentation: What to look for
Patients with delirious mania typically develop symptoms extremely rapidly. In virtually all published literature, symptoms were reported to emerge within hours to days and consisted of severe forms of mania, psychosis, and delirium; 100% of the cases in our review had these symptoms. Commonly reported symptoms were:
- intense excitement
- emotional lability
- grandiose delusions
- profound insomnia
- pressured and rapid speech
- auditory and visual hallucinations
- hypersexuality
- thought disorganization.
Exquisite paranoia can also result in violent aggression (and may require the use of physical restraints). Patients may confine themselves to very small spaces (such as a closet) in response to the intense paranoia. Impairments in various neurocognitive domains—including inability to focus; disorientation; language and visuospatial disturbances; difficulty with shifting and sustaining attention; and short-term memory impairments—have been reported. Patients often cannot recall the events during the episode.1,2,5,27,28
Catatonia has been closely associated with delirious mania.29 Features of excited catatonia—such as excessive motor activity, negativism, grimacing, posturing, echolalia, echopraxia, stereotypy, automatic obedience, verbigeration, combativeness, impulsivity, and rigidity—typically accompany delirious mania.1,5,10,19,27
In addition to these symptoms, patients may engage in specific behaviors. They may exhibit inappropriate toileting such as smearing feces on walls or in bags, fecal or urinary incontinence, disrobing or running naked in public places, or pouring liquid on the floor or on one’s head.1,2
Continue to: Of the 36 cases...
Of the 36 cases reported in the literature we reviewed, 20 (55%) were female. Most patients had an underlining psychiatric condition, including BD (72%), major depressive disorder (8%), and schizophrenia (2%). Three patients had no psychiatric history.
Physical examination
On initial presentation, a patient with delirious mania may be dehydrated, with dry mucous membranes, pale conjunctiva, tongue dryness, and poor skin turgor.28,30 Due to excessive motor activity, diaphoresis with tachycardia, fluctuating blood pressure, and fever may be present.31
Certain basic cognitive tasks should be assessed to determine the patient’s orientation to place, date, and time. Assess if the patient can recall recent events, names of objects, or perform serial 7s; clock drawing capabilities also should be ascertained.1,2,5 A Mini-Mental State Examination is useful.32
The Bush-Francis Catatonia Rating Scale should be used to elicit features of catatonia, such as waxy flexibility, negativism, gegenhalten, mitgehen, catalepsy, ambitendency, automatic obedience, and grasp reflex.10
Laboratory findings are nonspecific
Although no specific laboratory findings are associated with delirious mania, bloodwork and imaging are routinely investigated, especially if delirium characteristics are most striking. A complete blood count, chemistries, hepatic panel, thyroid functioning, blood and/or urine cultures, creatinine phosphokinase (CPK), and urinalysis can be ordered. Head imaging such as MRI and CT to rule out intracranial pathology are typically performed.19 However, the diagnosis of delirious mania is based on the presence of the phenotypic features, by verification of catatonia, and by the responsiveness to the treatment delivered.29
Continue to: Pathogenisis: Several hypotheses
Pathogenesis: Several hypotheses
The pathogenesis of delirious mania is not well understood. There are several postulations but no salient theory. Most patients with delirious mania have an underlying systemic medical or psychiatric condition.
Mood disorders. Patients with BD or schizoaffective disorder are especially susceptible to delirious mania. The percentage of manic patients who present with delirious mania varies by study. One study suggested approximately 19% have features of the phenomenon,33 while others estimated 15% to 25%.34 Elias et al35 calculated that 15% of patients with mania succumb to manic exhaustion; from this it can be reasonably concluded that these were cases of misdiagnosed delirious mania.
Delirium hypothesis. Patients with delirious mania typically have features of delirium, including fluctuation of consciousness, disorientation, and/or poor sleep-wake cycle.36 During rapid eye movement (REM) and non-REM sleep, memory circuits are fortified. When there is a substantial loss of REM and non-REM sleep, these circuits become faulty, even after 1 night. Pathological brain waves on EEG reflect the inability to reinforce the memory circuits. Patients with these waves may develop hallucinations, bizarre delusions, and altered sensorium. ECT reduces the pathological slow wave morphologies, thus restoring the synaptic maintenance and correcting the incompetent circuitry. This can explain the robust and rapid response of ECT in a patient with delirious mania.37,38
Neurotransmitter hypothesis. It has been shown that in patients with delirious mania there is dysregulation of dopamine transport, which leads to dopamine overflow in the synapse. In contrast to a drug effect (ie, cocaine or methamphetamine) that acts by inhibiting dopamine reuptake, dopamine overflow in delirious mania is caused by the loss of dopamine transporter regulation. This results in a dysfunctional dopaminergic state that precipitates an acute state of delirium and agitation.39,40
Serotonin plays a role in mood disorders, including mania and depression.41,42 More specifically, serotonin has been implicated in impulsivity and aggression as shown by reduced levels of CSF 5-hydroxyindoleacetic acid (5-HIAA) and depletion of 5-hydroxytryptophan (5-HTP).43
Continue to: Alterations in gamma-aminobutyric acid (GABA) transmission...
Alterations in gamma-aminobutyric acid (GABA) transmission are known to occur in delirium and catatonia. In delirium, GABA signaling is increased, which disrupts the circadian rhythm and melatonin release, thus impairing the sleep-wake cycle.44 Deficiencies in acetylcholine and melatonin are seen as well as excess of other neurotransmitters, including norepinephrine and glutamate.45 Conversely, in catatonia, functional imaging studies found decreased GABA-A binding in orbitofrontal, prefrontal, parietal, and motor cortical regions.46 A study analyzing 10 catatonic patients found decreased density of GABA-A receptors in the left sensorimotor cortex compared to psychiatric and healthy controls.47
Other neurotransmitters, such as glutamate, at the N-methyl-D-aspartate receptors (NMDAR) have been hypothesized to be hyperactive, causing downstream dysregulation of GABA functioning.48 However, the exact connection between delirious mania and all these receptors and neurotransmitters remains unknown.
Encephalitis hypothesis. The relationship between delirious mania and autoimmune encephalitis suggests delirious mania has etiologies other than a primary psychiatric illness. In a 2020 retrospective study49 that analyzed 79 patients with anti-NMDAR encephalitis, 25.3% met criteria for delirious mania, and 95% of these patients had catatonic features. Dalmau et al50 found that in many cases, patients tend to respond to ECT; in a cases series of 3 patients, 2 responded to benzodiazepines.
COVID-19 hypothesis. The SARS-CoV-2 virion has been associated with many neuropsychiatric complications, including mood, psychotic, and neurocognitive disorders.51,52 There also have been cases of COVID-19–induced catatonia.53-55 One case of delirious mania in a patient with COVID-19 has been reported.21 The general mechanism has been proposed to be related to the stimulation of the proinflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, which the virus produces in large quantities.56 These cytokines have been linked to psychosis and other psychiatric disorders.57 The patient with COVID-19–induced delirious mania had elevated inflammatory markers, including erythrocyte sedimentation rate, C-reactive protein, ferritin, and D-dimer, which supports a proinflammatory state. This patient had a complete resolution of symptoms with ECT.21
Management: Benzodiazepines and ECT
A step-by-step algorithm for managing delirious mania is outlined in the Figure. Regardless of the underlining etiology, management of delirious mania consists of benzodiazepines (lorazepam and diazepam); prompt use of ECT, particularly for patients who do not improve with large doses of lorazepam; or (if applicable) continued treatment of the underlining medical condition, which does not preclude the use of benzodiazepines or ECT. Recent reports27,58 have described details for using ECT for delirious mania, highlighting the use of high-energy dosing, bilateral electrode placement, and frequent sessions.
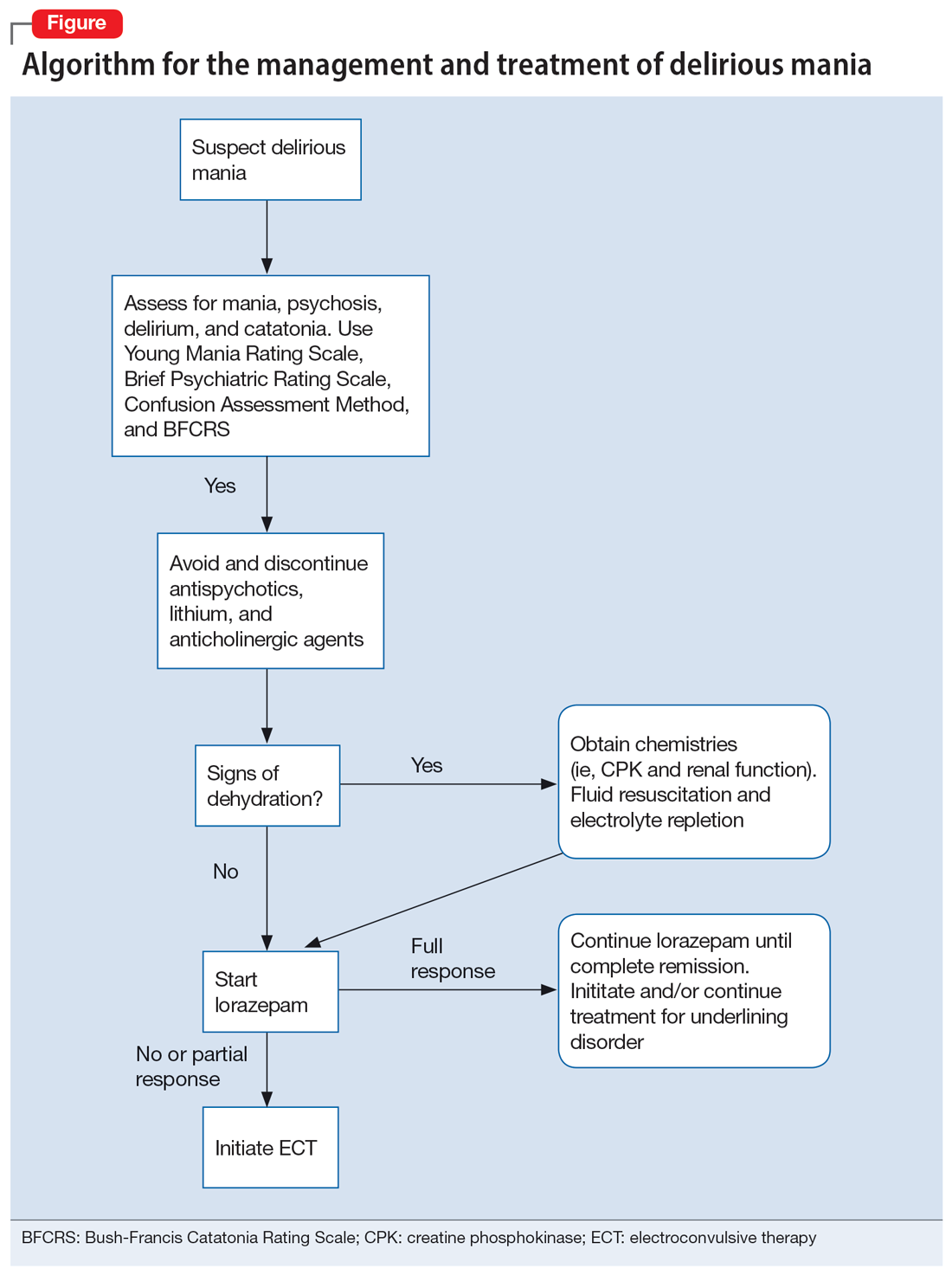
Continue to: Knowing which medications...
Knowing which medications to avoid is as important as knowing which agents to administer. Be vigilant in avoiding high-potency antipsychotics, as these medications can worsen extrapyramidal symptoms and may precipitate seizures or neuroleptic malignant syndrome (NMS).28 Anticholinergic agents should also be avoided because they worsen confusion. Although lithium is effective in BD, in delirious mania, high doses of lithium and haloperidol may cause severe encephalopathic syndromes, with symptoms that can include lethargy, tremors, cerebellar dysfunction, and worsened confusion; it may also cause widespread and irreversible brain damage.59
Due to long periods of hyperactivity, withdrawal, and diaphoresis, patients with delirious mania may be severely dehydrated with metabolic derangements, including elevated CPK due to rhabdomyolysis from prolonged exertion, IM antipsychotics, or rigidity. To prevent acute renal failure, this must be immediately addressed with rapid fluid resuscitation and electrolyte repletion.61
Benzodiazepines. The rapid use of lorazepam should be initiated when delirious mania is suspected. Doses of 6 to 20 mg have been reported to be effective if tolerated.5,20 Typically, high-dose lorazepam will not have the sedative effect that would normally occur in a patient who does not have delirious mania.2 Lorazepam should be titrated until full resolution of symptoms. Doses up to 30 mg have been reported as effective and tolerable.62 In our literature review, 50% of patients (18/36) responded or partially responded to lorazepam. However, only 3 case reports documented a complete remission with lorazepam, and many patients needed ECT for remission of symptoms.
ECT is generally reserved for patients who are not helped by benzodiazepine therapy, which is estimated to be up to 20%.5 ECT is highly effective in delirious mania, with remission rates ranging from 80% to 100%.1 ECT is also effective in acute nondelirious mania (comparable to depression); however, it is only used in a small minority of cases (0.2% to 12%).35 In our review, 58% of cases (21/36) reported using ECT, and in all cases it resulted in complete remission.
A dramatic improvement can be seen even after a single ECT session, though most patients show improvement after 4 sessions or 3 to 7 days.1,2,5 In our review, most patients received 4 to 12 sessions until achieving complete remission.
Continue to: No RCTs have evaluated...
No RCTs have evaluated ECT electrode placement in patients with delirious mania. However, several RCTs have investigated electrode placement in patients with acute nondelirious mania. Hiremani et al63 found that bitemporal placement had a more rapid response rate than bifrontal placement, but there was no overall difference in response rate. Barekatain et al64 found no difference between these 2 bilateral approaches. Many of the delirious mania cases report using a bilateral placement (including 42% of the ECT cases in our review) due to the emergent need for rapid relief of symptoms, which is especially necessary if the patient is experiencing hemodynamic instability, excessive violence, risk for self-harm, worsening delirium, or resistance to lorazepam.
Prognosis: Often fatal if left untreated
Patients with delirious mania are at high risk to progress to a more severe form of NMS or malignant catatonia. Therefore, high-potency antipsychotics should be avoided; mortality can be elevated from 60% without antipsychotics to 78% with antipsychotics.4 Some researchers estimate 75% to 78% of cases of delirious mania can be fatal if left untreated.3,6
Bottom Line
Delirious mania is routinely mistaken for more conventional manic or psychotic disorders. Clinicians need to be able to rapidly recognize the symptoms of this syndrome, which include mania, psychosis, delirium, and possible catatonia, so they can avoid administering toxic agents and instead initiate effective treatments such as benzodiazepines and electroconvulsive therapy.
Related Resources
- Arsan C, Baker C, Wong J, et al. Delirious mania: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2021;23(1):20f02744. doi:10.4088/PCC.20f02744
- Lamba G, Kennedy EA, Vu CP. Case report: ECT for delirious mania. Clinical Psychiatry News. December 14, 2021. https://www.mdedge.com/psychiatry/article/249909/bipolar-disorder/case-report-ect-delirious-mania
Drug Brand Names
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
1. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
2. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
3. Friedman RS, Mufson MJ, Eisenberg TD, et al. Medically and psychiatrically ill: the challenge of delirious mania. Harv Rev Psychiatry. 2003;11(2):91-98.
4. Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry. 1986;143(11):1374-1381.
5. Detweiler MB, Mehra A, Rowell T, et al. Delirious mania and malignant catatonia: a report of 3 cases and review. Psychiatr Q. 2009;80(1):23-40.
6. Bell L. On a form of disease resembling some advanced stages of mania and fever. American Journal of Insanity. 1849;6(2):97-127.
7. Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Arch Gen Psychiatry. 1973;28(2):221-228.
8. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
9. Hutchinson G, David A. Manic pseudo-delirium - two case reports. Behav Neurol. 1997;10(1):21-23.
10. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
11. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
12. Cordeiro CR, Saraiva R, Côrte-Real B, et al. When the bell rings: clinical features of Bell’s mania. Prim Care Companion CNS Disord. 2020;22(2):19l02511. doi:10.4088/PCC.19l02511
13. Yeo LX, Kuo TC, Hu KC, et al. Lurasidone-induced delirious mania. Am J Ther. 2019;26(6):e786-e787.
14. Jung WY, Lee BD. Quetiapine treatment for delirious mania in a military soldier. Prim Care Companion J Clin Psychiatry. 2010;12(2):PCC.09l00830. doi:10.4088/PCC.09l00830yel
15. Wahid N, Chin G, Turner AH, et al. Clinical response of clozapine as a treatment for delirious mania. Ment Illn. 2017;9(2):7182. doi:10.4081/mi.2017.7182
16. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
17. Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
18. O’Callaghan N, McDonald C, Hallahan B. Delirious mania intractable to treatment. Ir J Psychol Med. 2016;33(2):129-132.
19. Vasudev K, Grunze H. What works for delirious catatonic mania? BMJ Case Rep. 2010;2010:bcr0220102713. doi:10.1136/bcr.02.2010.2713
20. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
21. Reinfeld S, Yacoub A. A case of delirious mania induced by COVID-19 treated with electroconvulsive therapy. J ECT. 2021;37(4):e38-e39.
22. Lee BS, Huang SS, Hsu WY, et al. Clinical features of delirious mania: a series of five cases and a brief literature review. BMC Psychiatry. 2012;12:65. doi:10.1186/1471-244X-12-65
23. Bipeta R, Khan MA. Delirious mania: can we get away with this concept? A case report and review of the literature. Case Rep Psychiatry. 2012;2012:720354. doi:10.1155/2012/720354
24. Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
25. Tegin C, Kalayil G, Lippmann S. Electroconvulsive therapy and delirious catatonic mania. J ECT. 2017;33(4):e33-e34.
26. Melo AL, Serra M. Delirious mania and catatonia. Bipolar Disord. 2020;22(6):647-649.
27. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
28. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
29. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 1:I8-I13.
30. Vivanti A, Harvey K, Ash S, et al. Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008;47(3):340-355.
31. Ware MR, Feller DB, Hall KL. Neuroleptic malignant syndrome: diagnosis and management. Prim Care Companion CNS Disord. 2018;20(1):17r02185. doi:10.4088/PCC.17r0218
32. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
33. Taylor MA, Abrams R. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry. 1973;29(4):520-522.
34. Klerman GL. The spectrum of mania. Compr Psychiatry. 1981;22(1):11-20.
35. Elias A, Thomas N, Sackeim HA. Electroconvulsive therapy in mania: a review of 80 years of clinical experience. Am J Psychiatry. 2021;178(3):229-239.
36. Thom RP, Levy-Carrick NC, Bui M, et al. Delirium. Am J Psychiatry. 2019;176(10):785-793.
37. Charlton BG, Kavanau JL. Delirium and psychotic symptoms--an integrative model. Med Hypotheses. 2002;58(1):24-27.
38. Kramp P, Bolwig TG. Electroconvulsive therapy in acute delirious states. Compr Psychiatry. 1981;22(4):368-371.
39. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
40. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
41. Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59 Suppl 14:11-14.
42. Shiah IS, Yatham LN. Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disord. 2000;2(2):77-92.
43. Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42-58.
44. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
45. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222.
46. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
47. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445-450.
48. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380.
49. Restrepo-Martínez M, Chacón-González J, Bayliss L, et al. Delirious mania as a neuropsychiatric presentation in patients with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics. 2020;61(1):64-69.
50. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
51. Steardo L Jr, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10(1):261.
52. Iqbal Y, Al Abdulla MA, Albrahim S, et al. Psychiatric presentation of patients with acute SARS-CoV-2 infection: a retrospective review of 50 consecutive patients seen by a consultation-liaison psychiatry team. BJPsych Open. 2020;6(5):e109.
53. Gouse BM, Spears WE, Nieves Archibald A, et al. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529-530.
54. Caan MP, Lim CT, Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61(5):556-560.
55. Zain SM, Muthukanagaraj P, Rahman N. Excited catatonia - a delayed neuropsychiatric complication of COVID-19 infection. Cureus. 2021;13(3):e13891.
56. Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: a review. J Infect Public Health. 2020;13(11):1619-1629.
57. Radhakrishnan R, Kaser M, Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr Bull. 2017;43(4):693-697.
58. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
59. Cohen WJ, Cohen NH. Lithium carbonate, haloperidol, and irreversible brain damage. JAMA. 1974;230(9):1283-1287.
60. Davis MJ, de Nesnera A, Folks DG. Confused and nearly naked after going on spending sprees. Current Psychiatry. 2014;13(7):56-62.
61. Stanley M, Chippa V, Aeddula NR, et al. Rhabdomyolysis. StatPearls Publishing; 2021.
62. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173-1177.
63. Hiremani RM, Thirthalli J, Tharayil BS, et al. Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord. 2008;10(6):701-707.
64. Barekatain M, Jahangard L, Haghighi M, et al. Bifrontal versus bitemporal electroconvulsive therapy in severe manic patients. J ECT. 2008;24(3):199-202.
Delirious mania is a syndrome characterized by the acute onset of severe hyperactivity, psychosis, catatonia, and intermittent confusion. While there have been growing reports of this phenomenon over the last 2 decades, it remains poorly recognized and understood.1,2 There is no widely accepted nosology for delirious mania and the condition is absent from DSM-5, which magnifies the difficulties in making a timely diagnosis and initiating appropriate treatment. Delayed diagnosis and treatment may result in a detrimental outcome.2,3 Delirious mania has also been labeled as lethal catatonia, specific febrile delirium, hyperactive or exhaustive mania, and Bell’s mania.2,4,5 The characterization and diagnosis of this condition have a long and inconsistent history (Box1,6-11).
Box
Delirious mania was originally recognized in 1849 by Luther Bell in McLean Hospital after he observed 40 cases that were uniquely distinct from 1,700 other cases from 1836 to 1849.6 He described these patients as being suddenly confused, demonstrating unprovoked combativeness, remarkable decreased need for sleep, excessive motor restlessness, extreme fearfulness, and certain physiological signs, including rapid pulse and sweating. Bell was limited to the psychiatric treatment of his time, which largely was confined to physical restraints. Approximately three-fourths of these patients died.6
Following Bell’s report, this syndrome remained unexplored and rarely described. Some researchers postulated that the development of confusion was a natural progression of late-phase mania in close to 20% of patients.7 However, this did not account for the rapid onset of symptoms as well as certain unexplained movement abnormalities. In 1980, Bond8 presented 3 cases that were similar in nature to Bell’s depiction: acute onset with extraordinary irritability, withdrawal, delirium, and mania.
For the next 2 decades, delirious mania was seldom reported in the literature. The term was often reserved to illustrate when a patient had nothing more than mania with features of delirium.9
By 1996, catatonia became better recognized in its wide array of symptomology and diagnostic scales.10,11 In 1999, in addition to the sudden onset of excitement, paranoia, grandiosity, and disorientation, Fink1 reported catatonic signs including negativism, stereotypy, posturing, grimacing, and echo phenomena in patients with delirious mania. He identified its sensitive response to electroconvulsive therapy.
Delirious mania continues to be met with incertitude in clinical practice, and numerous inconsistencies have been reported in the literature. For example, some cases that have been reported as delirious mania had more evidence of primary delirium due to another medical condition or primary mania.12,13 Other cases have demonstrated swift improvement of symptoms after monotherapy with antipsychotics without a trial of benzodiazepines or electroconvulsive therapy (ECT); the exclusion of a sudden onset questions the validity of the diagnosis and promotes the use of less efficacious treatments.14,15 Other reports have confirmed that the diagnosis is missed when certain symptoms are more predominant, such as a thought disorder (acute schizophrenia), grandiosity and delusional ideation (bipolar disorder [BD]), and less commonly assessed catatonic signs (ambitendency, automatic obedience). These symptoms are mistakenly attributed to the respective disease.1,16 This especially holds true when delirious mania is initially diagnosed as a primary psychosis, which leads to the administration of antipsychotics.17 Other cases have reported that delirious mania was resistant to treatment, but ECT was never pursued.18
In this review, we provide a more comprehensive perspective of the clinical presentation, pathogenesis, and management of delirious mania. We searched PubMed and Google Scholar using the keywords “delirious mania,” “delirious mania AND catatonia,” or “manic delirium.” Most articles we found were case reports, case series, or retrospective chart reviews. There were no systematic reviews, meta analyses, or randomized control trials (RCTs). The 12 articles included in this review consist of 7 individual case reports, 4 case series, and 1 retrospective chart review that describe a total of 36 cases (Table1,2,5,17,19-26).

Clinical presentation: What to look for
Patients with delirious mania typically develop symptoms extremely rapidly. In virtually all published literature, symptoms were reported to emerge within hours to days and consisted of severe forms of mania, psychosis, and delirium; 100% of the cases in our review had these symptoms. Commonly reported symptoms were:
- intense excitement
- emotional lability
- grandiose delusions
- profound insomnia
- pressured and rapid speech
- auditory and visual hallucinations
- hypersexuality
- thought disorganization.
Exquisite paranoia can also result in violent aggression (and may require the use of physical restraints). Patients may confine themselves to very small spaces (such as a closet) in response to the intense paranoia. Impairments in various neurocognitive domains—including inability to focus; disorientation; language and visuospatial disturbances; difficulty with shifting and sustaining attention; and short-term memory impairments—have been reported. Patients often cannot recall the events during the episode.1,2,5,27,28
Catatonia has been closely associated with delirious mania.29 Features of excited catatonia—such as excessive motor activity, negativism, grimacing, posturing, echolalia, echopraxia, stereotypy, automatic obedience, verbigeration, combativeness, impulsivity, and rigidity—typically accompany delirious mania.1,5,10,19,27
In addition to these symptoms, patients may engage in specific behaviors. They may exhibit inappropriate toileting such as smearing feces on walls or in bags, fecal or urinary incontinence, disrobing or running naked in public places, or pouring liquid on the floor or on one’s head.1,2
Continue to: Of the 36 cases...
Of the 36 cases reported in the literature we reviewed, 20 (55%) were female. Most patients had an underlining psychiatric condition, including BD (72%), major depressive disorder (8%), and schizophrenia (2%). Three patients had no psychiatric history.
Physical examination
On initial presentation, a patient with delirious mania may be dehydrated, with dry mucous membranes, pale conjunctiva, tongue dryness, and poor skin turgor.28,30 Due to excessive motor activity, diaphoresis with tachycardia, fluctuating blood pressure, and fever may be present.31
Certain basic cognitive tasks should be assessed to determine the patient’s orientation to place, date, and time. Assess if the patient can recall recent events, names of objects, or perform serial 7s; clock drawing capabilities also should be ascertained.1,2,5 A Mini-Mental State Examination is useful.32
The Bush-Francis Catatonia Rating Scale should be used to elicit features of catatonia, such as waxy flexibility, negativism, gegenhalten, mitgehen, catalepsy, ambitendency, automatic obedience, and grasp reflex.10
Laboratory findings are nonspecific
Although no specific laboratory findings are associated with delirious mania, bloodwork and imaging are routinely investigated, especially if delirium characteristics are most striking. A complete blood count, chemistries, hepatic panel, thyroid functioning, blood and/or urine cultures, creatinine phosphokinase (CPK), and urinalysis can be ordered. Head imaging such as MRI and CT to rule out intracranial pathology are typically performed.19 However, the diagnosis of delirious mania is based on the presence of the phenotypic features, by verification of catatonia, and by the responsiveness to the treatment delivered.29
Continue to: Pathogenisis: Several hypotheses
Pathogenesis: Several hypotheses
The pathogenesis of delirious mania is not well understood. There are several postulations but no salient theory. Most patients with delirious mania have an underlying systemic medical or psychiatric condition.
Mood disorders. Patients with BD or schizoaffective disorder are especially susceptible to delirious mania. The percentage of manic patients who present with delirious mania varies by study. One study suggested approximately 19% have features of the phenomenon,33 while others estimated 15% to 25%.34 Elias et al35 calculated that 15% of patients with mania succumb to manic exhaustion; from this it can be reasonably concluded that these were cases of misdiagnosed delirious mania.
Delirium hypothesis. Patients with delirious mania typically have features of delirium, including fluctuation of consciousness, disorientation, and/or poor sleep-wake cycle.36 During rapid eye movement (REM) and non-REM sleep, memory circuits are fortified. When there is a substantial loss of REM and non-REM sleep, these circuits become faulty, even after 1 night. Pathological brain waves on EEG reflect the inability to reinforce the memory circuits. Patients with these waves may develop hallucinations, bizarre delusions, and altered sensorium. ECT reduces the pathological slow wave morphologies, thus restoring the synaptic maintenance and correcting the incompetent circuitry. This can explain the robust and rapid response of ECT in a patient with delirious mania.37,38
Neurotransmitter hypothesis. It has been shown that in patients with delirious mania there is dysregulation of dopamine transport, which leads to dopamine overflow in the synapse. In contrast to a drug effect (ie, cocaine or methamphetamine) that acts by inhibiting dopamine reuptake, dopamine overflow in delirious mania is caused by the loss of dopamine transporter regulation. This results in a dysfunctional dopaminergic state that precipitates an acute state of delirium and agitation.39,40
Serotonin plays a role in mood disorders, including mania and depression.41,42 More specifically, serotonin has been implicated in impulsivity and aggression as shown by reduced levels of CSF 5-hydroxyindoleacetic acid (5-HIAA) and depletion of 5-hydroxytryptophan (5-HTP).43
Continue to: Alterations in gamma-aminobutyric acid (GABA) transmission...
Alterations in gamma-aminobutyric acid (GABA) transmission are known to occur in delirium and catatonia. In delirium, GABA signaling is increased, which disrupts the circadian rhythm and melatonin release, thus impairing the sleep-wake cycle.44 Deficiencies in acetylcholine and melatonin are seen as well as excess of other neurotransmitters, including norepinephrine and glutamate.45 Conversely, in catatonia, functional imaging studies found decreased GABA-A binding in orbitofrontal, prefrontal, parietal, and motor cortical regions.46 A study analyzing 10 catatonic patients found decreased density of GABA-A receptors in the left sensorimotor cortex compared to psychiatric and healthy controls.47
Other neurotransmitters, such as glutamate, at the N-methyl-D-aspartate receptors (NMDAR) have been hypothesized to be hyperactive, causing downstream dysregulation of GABA functioning.48 However, the exact connection between delirious mania and all these receptors and neurotransmitters remains unknown.
Encephalitis hypothesis. The relationship between delirious mania and autoimmune encephalitis suggests delirious mania has etiologies other than a primary psychiatric illness. In a 2020 retrospective study49 that analyzed 79 patients with anti-NMDAR encephalitis, 25.3% met criteria for delirious mania, and 95% of these patients had catatonic features. Dalmau et al50 found that in many cases, patients tend to respond to ECT; in a cases series of 3 patients, 2 responded to benzodiazepines.
COVID-19 hypothesis. The SARS-CoV-2 virion has been associated with many neuropsychiatric complications, including mood, psychotic, and neurocognitive disorders.51,52 There also have been cases of COVID-19–induced catatonia.53-55 One case of delirious mania in a patient with COVID-19 has been reported.21 The general mechanism has been proposed to be related to the stimulation of the proinflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, which the virus produces in large quantities.56 These cytokines have been linked to psychosis and other psychiatric disorders.57 The patient with COVID-19–induced delirious mania had elevated inflammatory markers, including erythrocyte sedimentation rate, C-reactive protein, ferritin, and D-dimer, which supports a proinflammatory state. This patient had a complete resolution of symptoms with ECT.21
Management: Benzodiazepines and ECT
A step-by-step algorithm for managing delirious mania is outlined in the Figure. Regardless of the underlining etiology, management of delirious mania consists of benzodiazepines (lorazepam and diazepam); prompt use of ECT, particularly for patients who do not improve with large doses of lorazepam; or (if applicable) continued treatment of the underlining medical condition, which does not preclude the use of benzodiazepines or ECT. Recent reports27,58 have described details for using ECT for delirious mania, highlighting the use of high-energy dosing, bilateral electrode placement, and frequent sessions.

Continue to: Knowing which medications...
Knowing which medications to avoid is as important as knowing which agents to administer. Be vigilant in avoiding high-potency antipsychotics, as these medications can worsen extrapyramidal symptoms and may precipitate seizures or neuroleptic malignant syndrome (NMS).28 Anticholinergic agents should also be avoided because they worsen confusion. Although lithium is effective in BD, in delirious mania, high doses of lithium and haloperidol may cause severe encephalopathic syndromes, with symptoms that can include lethargy, tremors, cerebellar dysfunction, and worsened confusion; it may also cause widespread and irreversible brain damage.59
Due to long periods of hyperactivity, withdrawal, and diaphoresis, patients with delirious mania may be severely dehydrated with metabolic derangements, including elevated CPK due to rhabdomyolysis from prolonged exertion, IM antipsychotics, or rigidity. To prevent acute renal failure, this must be immediately addressed with rapid fluid resuscitation and electrolyte repletion.61
Benzodiazepines. The rapid use of lorazepam should be initiated when delirious mania is suspected. Doses of 6 to 20 mg have been reported to be effective if tolerated.5,20 Typically, high-dose lorazepam will not have the sedative effect that would normally occur in a patient who does not have delirious mania.2 Lorazepam should be titrated until full resolution of symptoms. Doses up to 30 mg have been reported as effective and tolerable.62 In our literature review, 50% of patients (18/36) responded or partially responded to lorazepam. However, only 3 case reports documented a complete remission with lorazepam, and many patients needed ECT for remission of symptoms.
ECT is generally reserved for patients who are not helped by benzodiazepine therapy, which is estimated to be up to 20%.5 ECT is highly effective in delirious mania, with remission rates ranging from 80% to 100%.1 ECT is also effective in acute nondelirious mania (comparable to depression); however, it is only used in a small minority of cases (0.2% to 12%).35 In our review, 58% of cases (21/36) reported using ECT, and in all cases it resulted in complete remission.
A dramatic improvement can be seen even after a single ECT session, though most patients show improvement after 4 sessions or 3 to 7 days.1,2,5 In our review, most patients received 4 to 12 sessions until achieving complete remission.
Continue to: No RCTs have evaluated...
No RCTs have evaluated ECT electrode placement in patients with delirious mania. However, several RCTs have investigated electrode placement in patients with acute nondelirious mania. Hiremani et al63 found that bitemporal placement had a more rapid response rate than bifrontal placement, but there was no overall difference in response rate. Barekatain et al64 found no difference between these 2 bilateral approaches. Many of the delirious mania cases report using a bilateral placement (including 42% of the ECT cases in our review) due to the emergent need for rapid relief of symptoms, which is especially necessary if the patient is experiencing hemodynamic instability, excessive violence, risk for self-harm, worsening delirium, or resistance to lorazepam.
Prognosis: Often fatal if left untreated
Patients with delirious mania are at high risk to progress to a more severe form of NMS or malignant catatonia. Therefore, high-potency antipsychotics should be avoided; mortality can be elevated from 60% without antipsychotics to 78% with antipsychotics.4 Some researchers estimate 75% to 78% of cases of delirious mania can be fatal if left untreated.3,6
Bottom Line
Delirious mania is routinely mistaken for more conventional manic or psychotic disorders. Clinicians need to be able to rapidly recognize the symptoms of this syndrome, which include mania, psychosis, delirium, and possible catatonia, so they can avoid administering toxic agents and instead initiate effective treatments such as benzodiazepines and electroconvulsive therapy.
Related Resources
- Arsan C, Baker C, Wong J, et al. Delirious mania: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2021;23(1):20f02744. doi:10.4088/PCC.20f02744
- Lamba G, Kennedy EA, Vu CP. Case report: ECT for delirious mania. Clinical Psychiatry News. December 14, 2021. https://www.mdedge.com/psychiatry/article/249909/bipolar-disorder/case-report-ect-delirious-mania
Drug Brand Names
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Delirious mania is a syndrome characterized by the acute onset of severe hyperactivity, psychosis, catatonia, and intermittent confusion. While there have been growing reports of this phenomenon over the last 2 decades, it remains poorly recognized and understood.1,2 There is no widely accepted nosology for delirious mania and the condition is absent from DSM-5, which magnifies the difficulties in making a timely diagnosis and initiating appropriate treatment. Delayed diagnosis and treatment may result in a detrimental outcome.2,3 Delirious mania has also been labeled as lethal catatonia, specific febrile delirium, hyperactive or exhaustive mania, and Bell’s mania.2,4,5 The characterization and diagnosis of this condition have a long and inconsistent history (Box1,6-11).
Box
Delirious mania was originally recognized in 1849 by Luther Bell in McLean Hospital after he observed 40 cases that were uniquely distinct from 1,700 other cases from 1836 to 1849.6 He described these patients as being suddenly confused, demonstrating unprovoked combativeness, remarkable decreased need for sleep, excessive motor restlessness, extreme fearfulness, and certain physiological signs, including rapid pulse and sweating. Bell was limited to the psychiatric treatment of his time, which largely was confined to physical restraints. Approximately three-fourths of these patients died.6
Following Bell’s report, this syndrome remained unexplored and rarely described. Some researchers postulated that the development of confusion was a natural progression of late-phase mania in close to 20% of patients.7 However, this did not account for the rapid onset of symptoms as well as certain unexplained movement abnormalities. In 1980, Bond8 presented 3 cases that were similar in nature to Bell’s depiction: acute onset with extraordinary irritability, withdrawal, delirium, and mania.
For the next 2 decades, delirious mania was seldom reported in the literature. The term was often reserved to illustrate when a patient had nothing more than mania with features of delirium.9
By 1996, catatonia became better recognized in its wide array of symptomology and diagnostic scales.10,11 In 1999, in addition to the sudden onset of excitement, paranoia, grandiosity, and disorientation, Fink1 reported catatonic signs including negativism, stereotypy, posturing, grimacing, and echo phenomena in patients with delirious mania. He identified its sensitive response to electroconvulsive therapy.
Delirious mania continues to be met with incertitude in clinical practice, and numerous inconsistencies have been reported in the literature. For example, some cases that have been reported as delirious mania had more evidence of primary delirium due to another medical condition or primary mania.12,13 Other cases have demonstrated swift improvement of symptoms after monotherapy with antipsychotics without a trial of benzodiazepines or electroconvulsive therapy (ECT); the exclusion of a sudden onset questions the validity of the diagnosis and promotes the use of less efficacious treatments.14,15 Other reports have confirmed that the diagnosis is missed when certain symptoms are more predominant, such as a thought disorder (acute schizophrenia), grandiosity and delusional ideation (bipolar disorder [BD]), and less commonly assessed catatonic signs (ambitendency, automatic obedience). These symptoms are mistakenly attributed to the respective disease.1,16 This especially holds true when delirious mania is initially diagnosed as a primary psychosis, which leads to the administration of antipsychotics.17 Other cases have reported that delirious mania was resistant to treatment, but ECT was never pursued.18
In this review, we provide a more comprehensive perspective of the clinical presentation, pathogenesis, and management of delirious mania. We searched PubMed and Google Scholar using the keywords “delirious mania,” “delirious mania AND catatonia,” or “manic delirium.” Most articles we found were case reports, case series, or retrospective chart reviews. There were no systematic reviews, meta analyses, or randomized control trials (RCTs). The 12 articles included in this review consist of 7 individual case reports, 4 case series, and 1 retrospective chart review that describe a total of 36 cases (Table1,2,5,17,19-26).

Clinical presentation: What to look for
Patients with delirious mania typically develop symptoms extremely rapidly. In virtually all published literature, symptoms were reported to emerge within hours to days and consisted of severe forms of mania, psychosis, and delirium; 100% of the cases in our review had these symptoms. Commonly reported symptoms were:
- intense excitement
- emotional lability
- grandiose delusions
- profound insomnia
- pressured and rapid speech
- auditory and visual hallucinations
- hypersexuality
- thought disorganization.
Exquisite paranoia can also result in violent aggression (and may require the use of physical restraints). Patients may confine themselves to very small spaces (such as a closet) in response to the intense paranoia. Impairments in various neurocognitive domains—including inability to focus; disorientation; language and visuospatial disturbances; difficulty with shifting and sustaining attention; and short-term memory impairments—have been reported. Patients often cannot recall the events during the episode.1,2,5,27,28
Catatonia has been closely associated with delirious mania.29 Features of excited catatonia—such as excessive motor activity, negativism, grimacing, posturing, echolalia, echopraxia, stereotypy, automatic obedience, verbigeration, combativeness, impulsivity, and rigidity—typically accompany delirious mania.1,5,10,19,27
In addition to these symptoms, patients may engage in specific behaviors. They may exhibit inappropriate toileting such as smearing feces on walls or in bags, fecal or urinary incontinence, disrobing or running naked in public places, or pouring liquid on the floor or on one’s head.1,2
Continue to: Of the 36 cases...
Of the 36 cases reported in the literature we reviewed, 20 (55%) were female. Most patients had an underlining psychiatric condition, including BD (72%), major depressive disorder (8%), and schizophrenia (2%). Three patients had no psychiatric history.
Physical examination
On initial presentation, a patient with delirious mania may be dehydrated, with dry mucous membranes, pale conjunctiva, tongue dryness, and poor skin turgor.28,30 Due to excessive motor activity, diaphoresis with tachycardia, fluctuating blood pressure, and fever may be present.31
Certain basic cognitive tasks should be assessed to determine the patient’s orientation to place, date, and time. Assess if the patient can recall recent events, names of objects, or perform serial 7s; clock drawing capabilities also should be ascertained.1,2,5 A Mini-Mental State Examination is useful.32
The Bush-Francis Catatonia Rating Scale should be used to elicit features of catatonia, such as waxy flexibility, negativism, gegenhalten, mitgehen, catalepsy, ambitendency, automatic obedience, and grasp reflex.10
Laboratory findings are nonspecific
Although no specific laboratory findings are associated with delirious mania, bloodwork and imaging are routinely investigated, especially if delirium characteristics are most striking. A complete blood count, chemistries, hepatic panel, thyroid functioning, blood and/or urine cultures, creatinine phosphokinase (CPK), and urinalysis can be ordered. Head imaging such as MRI and CT to rule out intracranial pathology are typically performed.19 However, the diagnosis of delirious mania is based on the presence of the phenotypic features, by verification of catatonia, and by the responsiveness to the treatment delivered.29
Continue to: Pathogenisis: Several hypotheses
Pathogenesis: Several hypotheses
The pathogenesis of delirious mania is not well understood. There are several postulations but no salient theory. Most patients with delirious mania have an underlying systemic medical or psychiatric condition.
Mood disorders. Patients with BD or schizoaffective disorder are especially susceptible to delirious mania. The percentage of manic patients who present with delirious mania varies by study. One study suggested approximately 19% have features of the phenomenon,33 while others estimated 15% to 25%.34 Elias et al35 calculated that 15% of patients with mania succumb to manic exhaustion; from this it can be reasonably concluded that these were cases of misdiagnosed delirious mania.
Delirium hypothesis. Patients with delirious mania typically have features of delirium, including fluctuation of consciousness, disorientation, and/or poor sleep-wake cycle.36 During rapid eye movement (REM) and non-REM sleep, memory circuits are fortified. When there is a substantial loss of REM and non-REM sleep, these circuits become faulty, even after 1 night. Pathological brain waves on EEG reflect the inability to reinforce the memory circuits. Patients with these waves may develop hallucinations, bizarre delusions, and altered sensorium. ECT reduces the pathological slow wave morphologies, thus restoring the synaptic maintenance and correcting the incompetent circuitry. This can explain the robust and rapid response of ECT in a patient with delirious mania.37,38
Neurotransmitter hypothesis. It has been shown that in patients with delirious mania there is dysregulation of dopamine transport, which leads to dopamine overflow in the synapse. In contrast to a drug effect (ie, cocaine or methamphetamine) that acts by inhibiting dopamine reuptake, dopamine overflow in delirious mania is caused by the loss of dopamine transporter regulation. This results in a dysfunctional dopaminergic state that precipitates an acute state of delirium and agitation.39,40
Serotonin plays a role in mood disorders, including mania and depression.41,42 More specifically, serotonin has been implicated in impulsivity and aggression as shown by reduced levels of CSF 5-hydroxyindoleacetic acid (5-HIAA) and depletion of 5-hydroxytryptophan (5-HTP).43
Continue to: Alterations in gamma-aminobutyric acid (GABA) transmission...
Alterations in gamma-aminobutyric acid (GABA) transmission are known to occur in delirium and catatonia. In delirium, GABA signaling is increased, which disrupts the circadian rhythm and melatonin release, thus impairing the sleep-wake cycle.44 Deficiencies in acetylcholine and melatonin are seen as well as excess of other neurotransmitters, including norepinephrine and glutamate.45 Conversely, in catatonia, functional imaging studies found decreased GABA-A binding in orbitofrontal, prefrontal, parietal, and motor cortical regions.46 A study analyzing 10 catatonic patients found decreased density of GABA-A receptors in the left sensorimotor cortex compared to psychiatric and healthy controls.47
Other neurotransmitters, such as glutamate, at the N-methyl-D-aspartate receptors (NMDAR) have been hypothesized to be hyperactive, causing downstream dysregulation of GABA functioning.48 However, the exact connection between delirious mania and all these receptors and neurotransmitters remains unknown.
Encephalitis hypothesis. The relationship between delirious mania and autoimmune encephalitis suggests delirious mania has etiologies other than a primary psychiatric illness. In a 2020 retrospective study49 that analyzed 79 patients with anti-NMDAR encephalitis, 25.3% met criteria for delirious mania, and 95% of these patients had catatonic features. Dalmau et al50 found that in many cases, patients tend to respond to ECT; in a cases series of 3 patients, 2 responded to benzodiazepines.
COVID-19 hypothesis. The SARS-CoV-2 virion has been associated with many neuropsychiatric complications, including mood, psychotic, and neurocognitive disorders.51,52 There also have been cases of COVID-19–induced catatonia.53-55 One case of delirious mania in a patient with COVID-19 has been reported.21 The general mechanism has been proposed to be related to the stimulation of the proinflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, which the virus produces in large quantities.56 These cytokines have been linked to psychosis and other psychiatric disorders.57 The patient with COVID-19–induced delirious mania had elevated inflammatory markers, including erythrocyte sedimentation rate, C-reactive protein, ferritin, and D-dimer, which supports a proinflammatory state. This patient had a complete resolution of symptoms with ECT.21
Management: Benzodiazepines and ECT
A step-by-step algorithm for managing delirious mania is outlined in the Figure. Regardless of the underlining etiology, management of delirious mania consists of benzodiazepines (lorazepam and diazepam); prompt use of ECT, particularly for patients who do not improve with large doses of lorazepam; or (if applicable) continued treatment of the underlining medical condition, which does not preclude the use of benzodiazepines or ECT. Recent reports27,58 have described details for using ECT for delirious mania, highlighting the use of high-energy dosing, bilateral electrode placement, and frequent sessions.

Continue to: Knowing which medications...
Knowing which medications to avoid is as important as knowing which agents to administer. Be vigilant in avoiding high-potency antipsychotics, as these medications can worsen extrapyramidal symptoms and may precipitate seizures or neuroleptic malignant syndrome (NMS).28 Anticholinergic agents should also be avoided because they worsen confusion. Although lithium is effective in BD, in delirious mania, high doses of lithium and haloperidol may cause severe encephalopathic syndromes, with symptoms that can include lethargy, tremors, cerebellar dysfunction, and worsened confusion; it may also cause widespread and irreversible brain damage.59
Due to long periods of hyperactivity, withdrawal, and diaphoresis, patients with delirious mania may be severely dehydrated with metabolic derangements, including elevated CPK due to rhabdomyolysis from prolonged exertion, IM antipsychotics, or rigidity. To prevent acute renal failure, this must be immediately addressed with rapid fluid resuscitation and electrolyte repletion.61
Benzodiazepines. The rapid use of lorazepam should be initiated when delirious mania is suspected. Doses of 6 to 20 mg have been reported to be effective if tolerated.5,20 Typically, high-dose lorazepam will not have the sedative effect that would normally occur in a patient who does not have delirious mania.2 Lorazepam should be titrated until full resolution of symptoms. Doses up to 30 mg have been reported as effective and tolerable.62 In our literature review, 50% of patients (18/36) responded or partially responded to lorazepam. However, only 3 case reports documented a complete remission with lorazepam, and many patients needed ECT for remission of symptoms.
ECT is generally reserved for patients who are not helped by benzodiazepine therapy, which is estimated to be up to 20%.5 ECT is highly effective in delirious mania, with remission rates ranging from 80% to 100%.1 ECT is also effective in acute nondelirious mania (comparable to depression); however, it is only used in a small minority of cases (0.2% to 12%).35 In our review, 58% of cases (21/36) reported using ECT, and in all cases it resulted in complete remission.
A dramatic improvement can be seen even after a single ECT session, though most patients show improvement after 4 sessions or 3 to 7 days.1,2,5 In our review, most patients received 4 to 12 sessions until achieving complete remission.
Continue to: No RCTs have evaluated...
No RCTs have evaluated ECT electrode placement in patients with delirious mania. However, several RCTs have investigated electrode placement in patients with acute nondelirious mania. Hiremani et al63 found that bitemporal placement had a more rapid response rate than bifrontal placement, but there was no overall difference in response rate. Barekatain et al64 found no difference between these 2 bilateral approaches. Many of the delirious mania cases report using a bilateral placement (including 42% of the ECT cases in our review) due to the emergent need for rapid relief of symptoms, which is especially necessary if the patient is experiencing hemodynamic instability, excessive violence, risk for self-harm, worsening delirium, or resistance to lorazepam.
Prognosis: Often fatal if left untreated
Patients with delirious mania are at high risk to progress to a more severe form of NMS or malignant catatonia. Therefore, high-potency antipsychotics should be avoided; mortality can be elevated from 60% without antipsychotics to 78% with antipsychotics.4 Some researchers estimate 75% to 78% of cases of delirious mania can be fatal if left untreated.3,6
Bottom Line
Delirious mania is routinely mistaken for more conventional manic or psychotic disorders. Clinicians need to be able to rapidly recognize the symptoms of this syndrome, which include mania, psychosis, delirium, and possible catatonia, so they can avoid administering toxic agents and instead initiate effective treatments such as benzodiazepines and electroconvulsive therapy.
Related Resources
- Arsan C, Baker C, Wong J, et al. Delirious mania: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2021;23(1):20f02744. doi:10.4088/PCC.20f02744
- Lamba G, Kennedy EA, Vu CP. Case report: ECT for delirious mania. Clinical Psychiatry News. December 14, 2021. https://www.mdedge.com/psychiatry/article/249909/bipolar-disorder/case-report-ect-delirious-mania
Drug Brand Names
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
1. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
2. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
3. Friedman RS, Mufson MJ, Eisenberg TD, et al. Medically and psychiatrically ill: the challenge of delirious mania. Harv Rev Psychiatry. 2003;11(2):91-98.
4. Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry. 1986;143(11):1374-1381.
5. Detweiler MB, Mehra A, Rowell T, et al. Delirious mania and malignant catatonia: a report of 3 cases and review. Psychiatr Q. 2009;80(1):23-40.
6. Bell L. On a form of disease resembling some advanced stages of mania and fever. American Journal of Insanity. 1849;6(2):97-127.
7. Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Arch Gen Psychiatry. 1973;28(2):221-228.
8. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
9. Hutchinson G, David A. Manic pseudo-delirium - two case reports. Behav Neurol. 1997;10(1):21-23.
10. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
11. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
12. Cordeiro CR, Saraiva R, Côrte-Real B, et al. When the bell rings: clinical features of Bell’s mania. Prim Care Companion CNS Disord. 2020;22(2):19l02511. doi:10.4088/PCC.19l02511
13. Yeo LX, Kuo TC, Hu KC, et al. Lurasidone-induced delirious mania. Am J Ther. 2019;26(6):e786-e787.
14. Jung WY, Lee BD. Quetiapine treatment for delirious mania in a military soldier. Prim Care Companion J Clin Psychiatry. 2010;12(2):PCC.09l00830. doi:10.4088/PCC.09l00830yel
15. Wahid N, Chin G, Turner AH, et al. Clinical response of clozapine as a treatment for delirious mania. Ment Illn. 2017;9(2):7182. doi:10.4081/mi.2017.7182
16. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
17. Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
18. O’Callaghan N, McDonald C, Hallahan B. Delirious mania intractable to treatment. Ir J Psychol Med. 2016;33(2):129-132.
19. Vasudev K, Grunze H. What works for delirious catatonic mania? BMJ Case Rep. 2010;2010:bcr0220102713. doi:10.1136/bcr.02.2010.2713
20. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
21. Reinfeld S, Yacoub A. A case of delirious mania induced by COVID-19 treated with electroconvulsive therapy. J ECT. 2021;37(4):e38-e39.
22. Lee BS, Huang SS, Hsu WY, et al. Clinical features of delirious mania: a series of five cases and a brief literature review. BMC Psychiatry. 2012;12:65. doi:10.1186/1471-244X-12-65
23. Bipeta R, Khan MA. Delirious mania: can we get away with this concept? A case report and review of the literature. Case Rep Psychiatry. 2012;2012:720354. doi:10.1155/2012/720354
24. Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
25. Tegin C, Kalayil G, Lippmann S. Electroconvulsive therapy and delirious catatonic mania. J ECT. 2017;33(4):e33-e34.
26. Melo AL, Serra M. Delirious mania and catatonia. Bipolar Disord. 2020;22(6):647-649.
27. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
28. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
29. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 1:I8-I13.
30. Vivanti A, Harvey K, Ash S, et al. Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008;47(3):340-355.
31. Ware MR, Feller DB, Hall KL. Neuroleptic malignant syndrome: diagnosis and management. Prim Care Companion CNS Disord. 2018;20(1):17r02185. doi:10.4088/PCC.17r0218
32. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
33. Taylor MA, Abrams R. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry. 1973;29(4):520-522.
34. Klerman GL. The spectrum of mania. Compr Psychiatry. 1981;22(1):11-20.
35. Elias A, Thomas N, Sackeim HA. Electroconvulsive therapy in mania: a review of 80 years of clinical experience. Am J Psychiatry. 2021;178(3):229-239.
36. Thom RP, Levy-Carrick NC, Bui M, et al. Delirium. Am J Psychiatry. 2019;176(10):785-793.
37. Charlton BG, Kavanau JL. Delirium and psychotic symptoms--an integrative model. Med Hypotheses. 2002;58(1):24-27.
38. Kramp P, Bolwig TG. Electroconvulsive therapy in acute delirious states. Compr Psychiatry. 1981;22(4):368-371.
39. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
40. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
41. Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59 Suppl 14:11-14.
42. Shiah IS, Yatham LN. Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disord. 2000;2(2):77-92.
43. Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42-58.
44. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
45. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222.
46. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
47. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445-450.
48. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380.
49. Restrepo-Martínez M, Chacón-González J, Bayliss L, et al. Delirious mania as a neuropsychiatric presentation in patients with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics. 2020;61(1):64-69.
50. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
51. Steardo L Jr, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10(1):261.
52. Iqbal Y, Al Abdulla MA, Albrahim S, et al. Psychiatric presentation of patients with acute SARS-CoV-2 infection: a retrospective review of 50 consecutive patients seen by a consultation-liaison psychiatry team. BJPsych Open. 2020;6(5):e109.
53. Gouse BM, Spears WE, Nieves Archibald A, et al. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529-530.
54. Caan MP, Lim CT, Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61(5):556-560.
55. Zain SM, Muthukanagaraj P, Rahman N. Excited catatonia - a delayed neuropsychiatric complication of COVID-19 infection. Cureus. 2021;13(3):e13891.
56. Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: a review. J Infect Public Health. 2020;13(11):1619-1629.
57. Radhakrishnan R, Kaser M, Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr Bull. 2017;43(4):693-697.
58. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
59. Cohen WJ, Cohen NH. Lithium carbonate, haloperidol, and irreversible brain damage. JAMA. 1974;230(9):1283-1287.
60. Davis MJ, de Nesnera A, Folks DG. Confused and nearly naked after going on spending sprees. Current Psychiatry. 2014;13(7):56-62.
61. Stanley M, Chippa V, Aeddula NR, et al. Rhabdomyolysis. StatPearls Publishing; 2021.
62. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173-1177.
63. Hiremani RM, Thirthalli J, Tharayil BS, et al. Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord. 2008;10(6):701-707.
64. Barekatain M, Jahangard L, Haghighi M, et al. Bifrontal versus bitemporal electroconvulsive therapy in severe manic patients. J ECT. 2008;24(3):199-202.
1. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
2. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
3. Friedman RS, Mufson MJ, Eisenberg TD, et al. Medically and psychiatrically ill: the challenge of delirious mania. Harv Rev Psychiatry. 2003;11(2):91-98.
4. Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry. 1986;143(11):1374-1381.
5. Detweiler MB, Mehra A, Rowell T, et al. Delirious mania and malignant catatonia: a report of 3 cases and review. Psychiatr Q. 2009;80(1):23-40.
6. Bell L. On a form of disease resembling some advanced stages of mania and fever. American Journal of Insanity. 1849;6(2):97-127.
7. Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Arch Gen Psychiatry. 1973;28(2):221-228.
8. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
9. Hutchinson G, David A. Manic pseudo-delirium - two case reports. Behav Neurol. 1997;10(1):21-23.
10. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
11. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
12. Cordeiro CR, Saraiva R, Côrte-Real B, et al. When the bell rings: clinical features of Bell’s mania. Prim Care Companion CNS Disord. 2020;22(2):19l02511. doi:10.4088/PCC.19l02511
13. Yeo LX, Kuo TC, Hu KC, et al. Lurasidone-induced delirious mania. Am J Ther. 2019;26(6):e786-e787.
14. Jung WY, Lee BD. Quetiapine treatment for delirious mania in a military soldier. Prim Care Companion J Clin Psychiatry. 2010;12(2):PCC.09l00830. doi:10.4088/PCC.09l00830yel
15. Wahid N, Chin G, Turner AH, et al. Clinical response of clozapine as a treatment for delirious mania. Ment Illn. 2017;9(2):7182. doi:10.4081/mi.2017.7182
16. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
17. Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
18. O’Callaghan N, McDonald C, Hallahan B. Delirious mania intractable to treatment. Ir J Psychol Med. 2016;33(2):129-132.
19. Vasudev K, Grunze H. What works for delirious catatonic mania? BMJ Case Rep. 2010;2010:bcr0220102713. doi:10.1136/bcr.02.2010.2713
20. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
21. Reinfeld S, Yacoub A. A case of delirious mania induced by COVID-19 treated with electroconvulsive therapy. J ECT. 2021;37(4):e38-e39.
22. Lee BS, Huang SS, Hsu WY, et al. Clinical features of delirious mania: a series of five cases and a brief literature review. BMC Psychiatry. 2012;12:65. doi:10.1186/1471-244X-12-65
23. Bipeta R, Khan MA. Delirious mania: can we get away with this concept? A case report and review of the literature. Case Rep Psychiatry. 2012;2012:720354. doi:10.1155/2012/720354
24. Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
25. Tegin C, Kalayil G, Lippmann S. Electroconvulsive therapy and delirious catatonic mania. J ECT. 2017;33(4):e33-e34.
26. Melo AL, Serra M. Delirious mania and catatonia. Bipolar Disord. 2020;22(6):647-649.
27. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
28. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
29. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 1:I8-I13.
30. Vivanti A, Harvey K, Ash S, et al. Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008;47(3):340-355.
31. Ware MR, Feller DB, Hall KL. Neuroleptic malignant syndrome: diagnosis and management. Prim Care Companion CNS Disord. 2018;20(1):17r02185. doi:10.4088/PCC.17r0218
32. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
33. Taylor MA, Abrams R. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry. 1973;29(4):520-522.
34. Klerman GL. The spectrum of mania. Compr Psychiatry. 1981;22(1):11-20.
35. Elias A, Thomas N, Sackeim HA. Electroconvulsive therapy in mania: a review of 80 years of clinical experience. Am J Psychiatry. 2021;178(3):229-239.
36. Thom RP, Levy-Carrick NC, Bui M, et al. Delirium. Am J Psychiatry. 2019;176(10):785-793.
37. Charlton BG, Kavanau JL. Delirium and psychotic symptoms--an integrative model. Med Hypotheses. 2002;58(1):24-27.
38. Kramp P, Bolwig TG. Electroconvulsive therapy in acute delirious states. Compr Psychiatry. 1981;22(4):368-371.
39. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
40. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
41. Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59 Suppl 14:11-14.
42. Shiah IS, Yatham LN. Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disord. 2000;2(2):77-92.
43. Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42-58.
44. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
45. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222.
46. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
47. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445-450.
48. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380.
49. Restrepo-Martínez M, Chacón-González J, Bayliss L, et al. Delirious mania as a neuropsychiatric presentation in patients with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics. 2020;61(1):64-69.
50. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
51. Steardo L Jr, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10(1):261.
52. Iqbal Y, Al Abdulla MA, Albrahim S, et al. Psychiatric presentation of patients with acute SARS-CoV-2 infection: a retrospective review of 50 consecutive patients seen by a consultation-liaison psychiatry team. BJPsych Open. 2020;6(5):e109.
53. Gouse BM, Spears WE, Nieves Archibald A, et al. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529-530.
54. Caan MP, Lim CT, Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61(5):556-560.
55. Zain SM, Muthukanagaraj P, Rahman N. Excited catatonia - a delayed neuropsychiatric complication of COVID-19 infection. Cureus. 2021;13(3):e13891.
56. Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: a review. J Infect Public Health. 2020;13(11):1619-1629.
57. Radhakrishnan R, Kaser M, Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr Bull. 2017;43(4):693-697.
58. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
59. Cohen WJ, Cohen NH. Lithium carbonate, haloperidol, and irreversible brain damage. JAMA. 1974;230(9):1283-1287.
60. Davis MJ, de Nesnera A, Folks DG. Confused and nearly naked after going on spending sprees. Current Psychiatry. 2014;13(7):56-62.
61. Stanley M, Chippa V, Aeddula NR, et al. Rhabdomyolysis. StatPearls Publishing; 2021.
62. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173-1177.
63. Hiremani RM, Thirthalli J, Tharayil BS, et al. Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord. 2008;10(6):701-707.
64. Barekatain M, Jahangard L, Haghighi M, et al. Bifrontal versus bitemporal electroconvulsive therapy in severe manic patients. J ECT. 2008;24(3):199-202.
Dear patients: Letters psychiatrists should and should not write
After several months of difficulty living in her current apartment complex, Ms. M asks you as her psychiatrist to write a letter to the management company requesting she be moved to an apartment on the opposite side of the maintenance closet because the noise aggravates her posttraumatic stress disorder. What should you consider when asked to write such a letter?
Psychiatric practice often extends beyond the treatment of mental illness to include addressing patients’ social well-being. Psychiatrists commonly inquire about a patient’s social situation to understand the impact of these environmental factors. Similarly, psychiatric illness may affect a patient’s ability to work or fulfill responsibilities. As a result, patients may ask their psychiatrists for assistance by requesting letters that address various aspects of their social well-being.1 These communications may address an array of topics, from a patient’s readiness to return to work to their ability to pay child support. This article focuses on the role psychiatrists have in writing patient-requested letters across a variety of topics, including the consideration of potential legal liability and ethical implications.
Types of letters
The categories of letters patients request can be divided into 2 groups. The first is comprised of letters relating to the patient’s medical needs (Table 12,3). These address the patient’s ability to work (eg, medical leave, return to work, or accommodations) or travel (eg, ability to drive or use public transportation), or need for specific medical treatment (ie, gender-affirming care or cannabis use in specific settings). The second group relates to legal requests such as excusal from jury duty, emotional support animals, or any other letter used specifically for legal purposes (in civil or criminal cases) (Table 21,4-6).
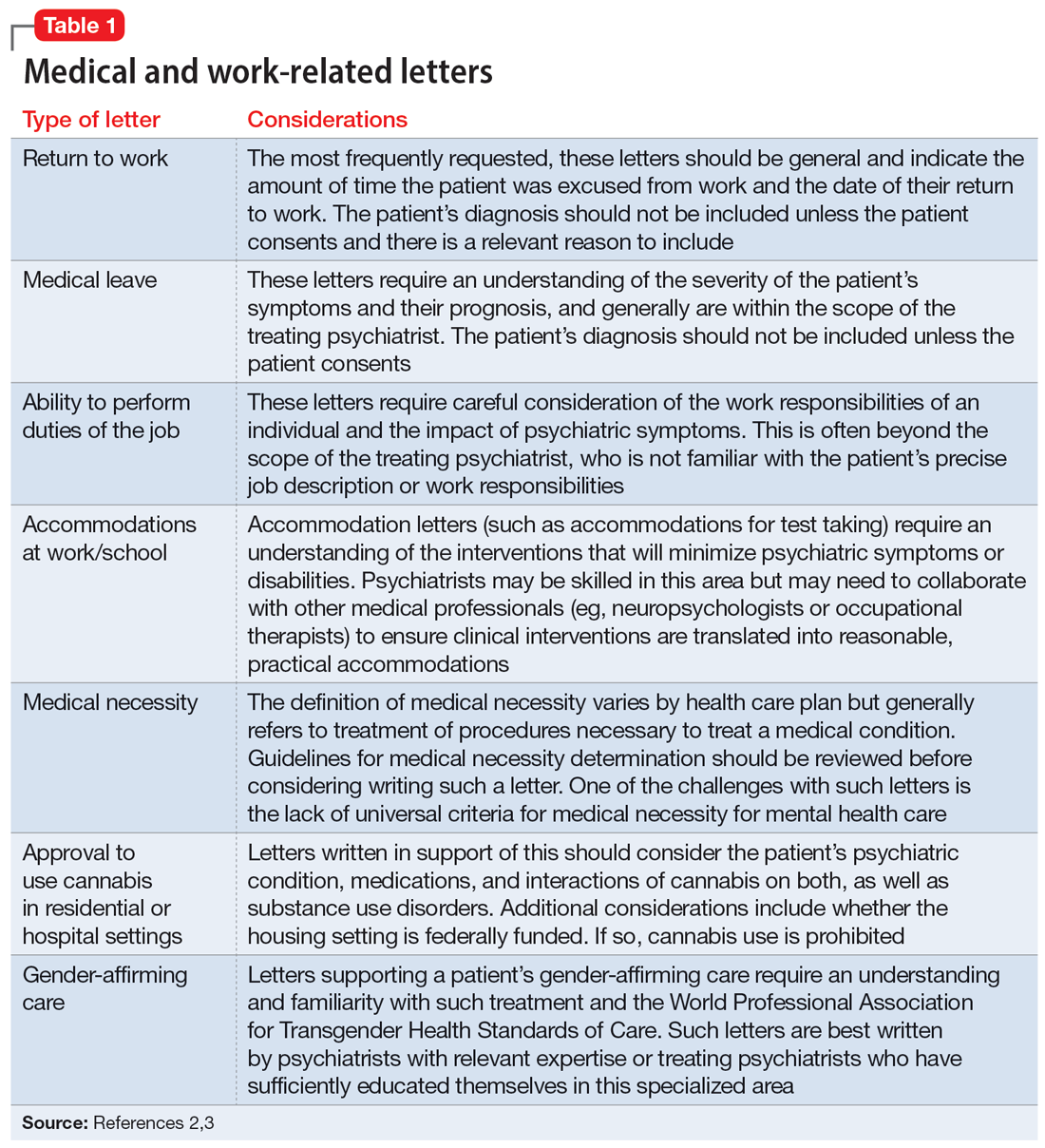
The decision to write a letter on behalf of a patient should be based on whether you have sufficient knowledge to answer the referral question, and whether the requested evaluation fits within your role as the treating psychiatrist. Many requests fall short of the first condition. For example, a request to opine about an individual’s ability to perform their job duties requires specific knowledge and careful consideration of the patient’s work responsibilities, knowledge of the impact of their psychiatric symptoms, and specialized knowledge about interventions that would ameliorate symptoms in the specialized work setting. Most psychiatrists are not sufficiently familiar with a specific workplace to provide opinions regarding reasonable accommodations.
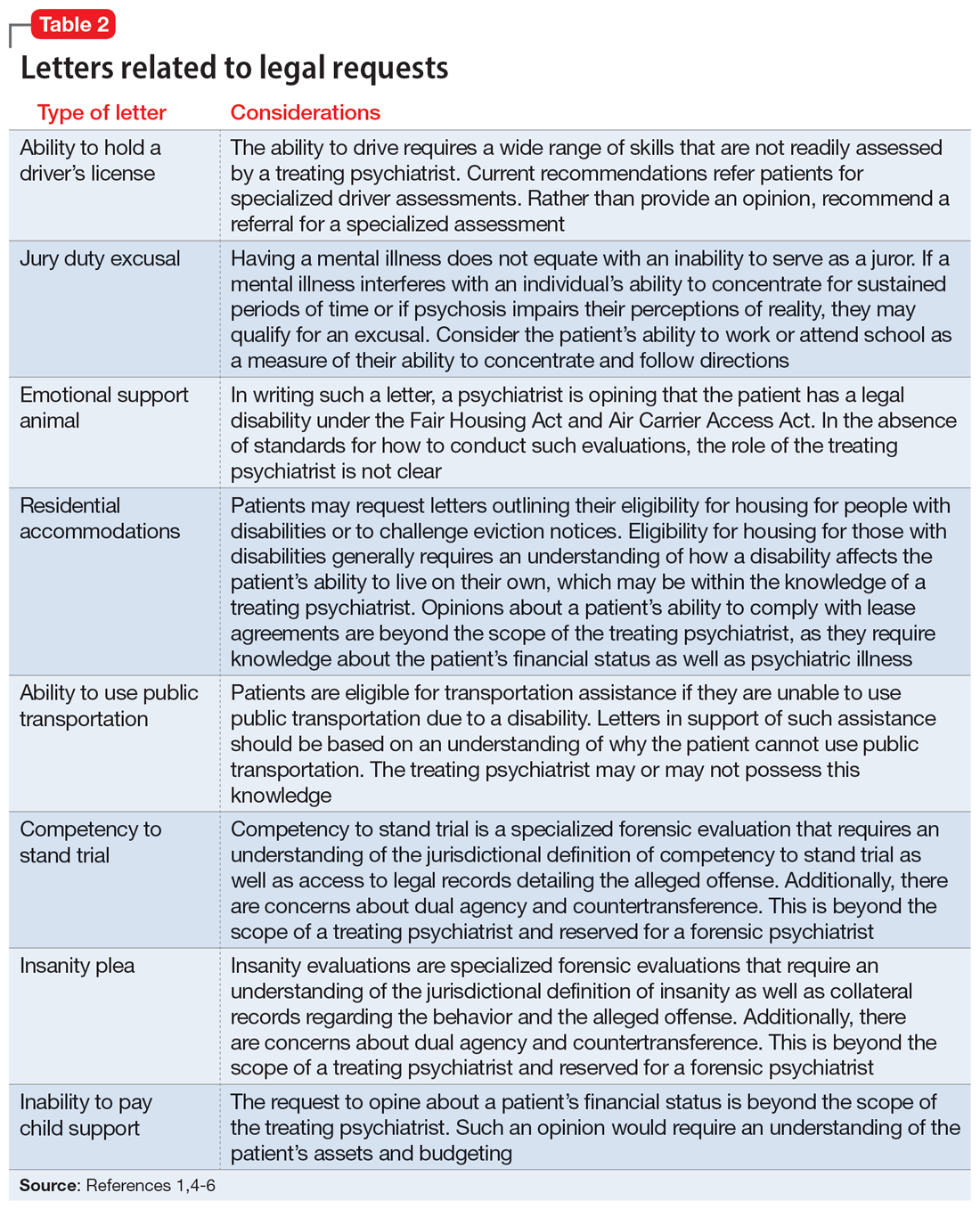
The second condition refers to the role and responsibilities of the psychiatrist. Many letter requests are clearly within the scope of the clinical psychiatrist, such as a medical leave note due to a psychiatric decompensation or a jury duty excusal due to an unstable mental state. Other letters reach beyond the role of the general or treating psychiatrist, such as opinions about suitable housing or a patient’s competency to stand trial.
Components of letters
The decision to write or not to write a letter should be discussed with the patient. Identify the reasons for and against letter writing. If you decide to write a letter, the letter should have the following basic framework (Figure): the identity of the person who requested the letter, the referral question, and an answer to the referral question with a clear rationale. Describe the patient’s psychiatric diagnosis using DSM criteria. Any limitations to the answer should be identified. The letter should not go beyond the referral question and should not include information that was not requested. It also should be preserved in the medical record.
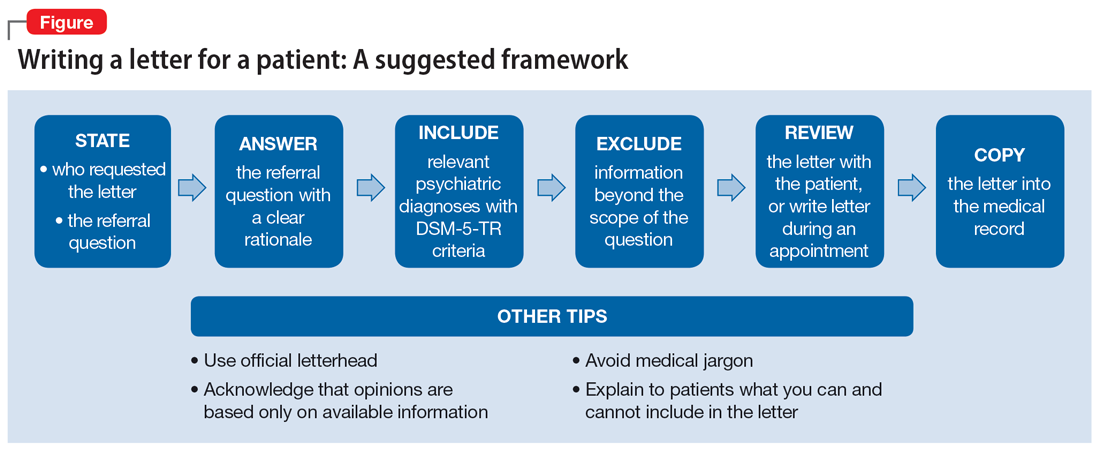
It is recommended to write or review the letter in the presence of the patient to discuss the contents of the letter and what the psychiatrist can or cannot write. As in forensic reports, conclusory statements are not helpful. Provide descriptive information instead of relying on psychiatric jargon, and a rationale for the opinion as opposed to stating an opinion as fact. In the letter, you must acknowledge that your opinion is based upon information provided by the patient (and the patient’s family, when accurate) and as a result, is not fully objective.
Continue to: Liability and dual agency
Liability and dual agency
Psychiatrists are familiar with clinical situations in which a duty to the patient is mitigated or superseded by a duty to a third party. As the Tarasoff court famously stated, “the protective privilege ends where the public peril begins.”7
To be liable to either a patient or a third party means to be “bound or obliged in law or equity; responsible; chargeable; answerable; compellable to make satisfaction, compensation, or restitution.”8 Liabilities related to clinical treatment are well-established; medical students learn the fundamentals before ever treating a patient, and physicians carry malpractice insurance throughout their careers.
Less well-established is the liability a treating psychiatrist owes a third party when forming an opinion that impacts both their patient and the third party (eg, an employer when writing a return-to-work letter, or a disability insurer when qualifying a patient for disability benefits). The American Academy of Psychiatry and the Law discourages treating psychiatrists from performing these types of evaluations of their patients based on the inherent conflict of serving as a dual agent, or acting both as an advocate for the patient and as an independent evaluator striving for objectivity.9 However, such requests commonly arise, and some may be unavoidable.
Dual-agency situations subject the treating psychiatrist to avenues of legal action arising from the patient-doctor relationship as well as the forensic evaluator relationship. If a letter is written during a clinical treatment, all duties owed to the patient continue to apply, and the relevant benchmarks of local statutes and principle of a standard of care are relevant. It is conceivable that a patient could bring a negligence lawsuit based on a standard of care allegation (eg, that writing certain types of letters is so ordinary that failure to write them would fall below the standard of care). Confidentiality is also of the utmost importance,10 and you should obtain a written release of information from the patient before releasing any letter with privileged information about the patient.11 Additional relevant legal causes of action the patient could include are torts such as defamation of character, invasion of privacy, breach of contract, and intentional infliction of emotional distress. There is limited case law supporting patients’ rights to sue psychiatrists for defamation.10
A psychiatrist writing a letter to a third party may also subject themselves to avenues of legal action occurring outside the physician-patient relationship. Importantly, damages resulting from these breaches would not be covered by your malpractice insurance. Extreme cases involve allegations of fraud or perjury, which could be pursued in criminal court. If a psychiatrist intentionally deceives a third party for the purpose of obtaining some benefit for the patient, this is clear grounds for civil or criminal action. Fraud is defined as “a false representation of a matter of fact, whether by words or by conduct, by false or misleading allegations, or by concealment of that which should have been disclosed, which deceives and is intended to deceive another so that he shall act upon it to his legal injury.”8 Negligence can also be grounds for liability if a third party suffers injury or loss. Although the liability is clearer if the third party retains an independent psychiatrist rather than soliciting an opinion from a patient’s treating psychiatrist, both parties are subject to the claim of negligence.10
Continue to: There are some important protections...
There are some important protections that limit psychiatrists’ good-faith opinions from litigation. The primary one is the “professional medical judgment rule,” which shields physicians from the consequences of erroneous opinions so long as the examination was competent, complete, and performed in an ordinary fashion.10 In some cases, psychiatrists writing a letter or report for a government agency may also qualify for quasi-judicial immunity or witness immunity, but case law shows significant variation in when and how these privileges apply and whether such privileges would be applied to a clinical psychiatrist in the context of a traditional physician-patient relationship.12 In general, these privileges are not absolute and may not be sufficiently well-established to discourage a plaintiff from filing suit or prompt early judicial dismissal of a case.
Like all aspects of practicing medicine, letter writing is subject to scrutiny and accountability. Think carefully about your obligations and the potential consequences of writing or not writing a letter to a third party.
Ethical considerations
The decision to write a letter for a patient must be carefully considered from multiple angles.6 In addition to liability concerns, various ethical considerations also arise. Guided by the principles of beneficence, nonmaleficence, autonomy, and justice,13 we recommend the following approaches.
Maintain objectivity
During letter writing, a conflict of interest may arise between your allegiance to the patient and the imperative to provide accurate information.14-16 If the conflict is overwhelming, the most appropriate approach is to recuse yourself from the case and refer the patient to a third party. When electing to write a letter, you accept the responsibility to provide an objective assessment of the relevant situation. This promotes a just outcome and may also serve to promote the patient’s or society’s well-being.
Encourage activity and overall function
Evidence suggests that participation in multiple aspects of life promotes positive health outcomes.17,18 As a physician, it is your duty to promote health and support and facilitate accommodations that allow patients to participate and flourish in society. By the same logic, when approached by patients with a request for letters in support of reduced activity, you should consider not only the benefits but also the potential detriments of such disruptions. This may entail recommending temporary restrictions or modifications, as appropriate.
Continue to: Think beyond the patient
Think beyond the patient
Letter writing, particularly when recommending accommodations, can have implications beyond the patient.16 Such letters may cause unintended societal harm. For example, others may have to assume additional responsibilities; competitive goods (eg, housing) may be rendered to the patient rather than to a person with greater needs; and workplace safety could be compromised due to absence. Consider not only the individual patient but also possible public health and societal effects of letter writing.
Deciding not to write
From an ethical perspective, a physician cannot be compelled to write a letter if such an undertaking violates a stronger moral obligation. An example of this is if writing a letter could cause significant harm to the patient or society, or if writing a letter might compromise a physician’s professionalism.19 When you elect to not write a letter, the ethical principles of autonomy and truth telling dictate that you must inform your patients of this choice.6 You should also provide an explanation to the patient as long as such information would not cause undue psychological or physical harm.20,21
Schedule time to write letters
Some physicians implement policies that all letters are to be completed during scheduled appointments. Others designate administrative time to complete requested letters. Finally, some physicians flexibly complete such requests between appointments or during other undedicated time slots. Any of these approaches are justifiable, though some urgent requests may require more immediate attention outside of appointments. Some physicians may choose to bill for the letter writing if completed outside an appointment and the patient is treated in private practice. Whatever your policy, inform patients of it at the beginning of care and remind them when appropriate, such as before completing a letter that may be billed.
Manage uncertainty
Always strive for objectivity in letter writing. However, some requests inherently hinge on subjective reports and assessments. For example, a patient may request an excuse letter due to feeling unwell. In the absence of objective findings, what should you do? We advise the following.
Acquire collateral information. Adequate information is essential when making any medical recommendation. The same is true for writing letters. With the patient’s permission, you may need to contact relevant parties to better understand the circumstance or activity about which you are being asked to write a letter. For example, a patient may request leave from work due to injury. If the specific parameters of the work impeded by the injury are unclear to you, refrain from writing the letter and explain the rationale to the patient.
Continue to: Integrate prior knowledge of the patient
Integrate prior knowledge of the patient. No letter writing request exists in a vacuum. If you know the patient, the letter should be contextualized within the patient’s prior behaviors.
Stay within your scope
Given the various dilemmas and challenges, you may want to consider whether some letter writing is out of your professional scope.14-16 One solution would be to leave such requests to other entities (eg, requiring employers to retain medical personnel with specialized skills in occupational evaluations) and make such recommendations to patients. Regardless, physicians should think carefully about their professional boundaries and scope regarding letter requests and adopt and implement a consistent standard for all patients.
Regarding the letter requested by Ms. M, you should consider whether the appeal is consistent with the patient’s psychiatric illness. You should also consider whether you have sufficient knowledge about the patient’s living environment to support their claim. Such a letter should be written only if you understand both considerations. Regardless of your decision, you should explain your rationale to the patient.
Bottom Line
Patients may ask their psychiatrists to write letters that address aspects of their social well-being. However, psychiatrists must be alert to requests that are outside their scope of practice or ethically or legally fraught. Carefully consider whether writing a letter is appropriate and if not, discuss with the patient the reasons you cannot write such a letter and any recommended alternative avenues to address their request.
Related Resources
- Riese A. Writing letters for transgender patients undergoing medical transition. Current Psychiatry. 2021;20(8):51-52. doi:10.12788/cp.0159
- Joshi KG. Service animals and emotional support animals: should you write that letter? Current Psychiatry. 2021;20(11):16-19,24. doi:10.12788/cp.0183
1. West S, Friedman SH. To be or not to be: treating psychiatrist and expert witness. Psychiatric Times. 2007;24(6). Accessed March 14, 2023. https://www.psychiatrictimes.com/view/be-or-not-be-treating-psychiatrist-and-expert-witness
2. Knoepflmacher D. ‘Medical necessity’ in psychiatry: whose definition is it anyway? Psychiatric News. 2016;51(18):12-14. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2016.9b14
3. Lampe JR. Recent developments in marijuana law (LSB10859). Congressional Research Service. 2022. Accessed October 25, 2023. https://crsreports.congress.gov/product/pdf/LSB/LSB10859/2
4. Brunnauer A, Buschert V, Segmiller F, et al. Mobility behaviour and driving status of patients with mental disorders – an exploratory study. Int J Psychiatry Clin Pract. 2016;20(1):40-46. doi:10.3109/13651501.2015.1089293
5. Chiu CW, Law CK, Cheng AS. Driver assessment service for people with mental illness. Hong Kong J Occup Ther. 2019;32(2):77-83. doi:10.1177/1569186119886773
6. Joshi KG. Service animals and emotional support animals: should you write that letter? Current Psychiatry. 2021;20(11):16-19. doi:10.12788/cp.0183
7. Tarasoff v Regents of University of California, 17 Cal 3d 425, 551 P2d 334, 131 Cal. Rptr. 14 (Cal 1976).
8. Black HC. Liability. Black’s Law Dictionary. Revised 4th ed. West Publishing; 1975:1060.
9. American Academy of Psychiatry and the Law. Ethics guidelines for the practice of forensic psychiatry. 2005. Accessed March 15, 2023. https://www.aapl.org/ethics.htm
10. Gold LH, Davidson JE. Do you understand your risk? Liability and third-party evaluations in civil litigation. J Am Acad Psychiatry Law. 2007;35(2):200-210.
11. Schouten R. Approach to the patient seeking disability benefits. In: Stern TA, Herman JB, Slavin PL, eds. The MGH Guide to Psychiatry in Primary Care. McGraw Hill; 1998:121-126.
12. Appelbaum PS. Law and psychiatry: liability for forensic evaluations: a word of caution. Psychiatr Serv. 2001;52(7):885-886. doi:10.1176/appi.ps.52.7.885
13. Varkey B. Principles of clinical ethics and their application to practice. Med Princ Pract. 2021;30(1):17-28. doi:10.1159/000509119
14. Mayhew HE, Nordlund DJ. Absenteeism certification: the physician’s role. J Fam Pract. 1988;26(6):651-655.
15. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260. doi:10.1037/pro0000083
16. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518. doi:1-.29158/JAAPL.200047-20
17. Strully KW. Job loss and health in the U.S. labor market. Demography. 2009;46(2):221-246. doi:10.1353/dem.0.0050
18. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59(6):e125-131. doi:10.1097/JOM.0000000000001055
19. Munyaradzi M. Critical reflections on the principle of beneficence in biomedicine. Pan Afr Med J. 2012;11:29.
20. Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 7th ed. Oxford University Press; 2012.
21. Gold M. Is honesty always the best policy? Ethical aspects of truth telling. Intern Med J. 2004;34(9-10):578-580. doi:10.1111/j.1445-5994.2004.00673.x
After several months of difficulty living in her current apartment complex, Ms. M asks you as her psychiatrist to write a letter to the management company requesting she be moved to an apartment on the opposite side of the maintenance closet because the noise aggravates her posttraumatic stress disorder. What should you consider when asked to write such a letter?
Psychiatric practice often extends beyond the treatment of mental illness to include addressing patients’ social well-being. Psychiatrists commonly inquire about a patient’s social situation to understand the impact of these environmental factors. Similarly, psychiatric illness may affect a patient’s ability to work or fulfill responsibilities. As a result, patients may ask their psychiatrists for assistance by requesting letters that address various aspects of their social well-being.1 These communications may address an array of topics, from a patient’s readiness to return to work to their ability to pay child support. This article focuses on the role psychiatrists have in writing patient-requested letters across a variety of topics, including the consideration of potential legal liability and ethical implications.
Types of letters
The categories of letters patients request can be divided into 2 groups. The first is comprised of letters relating to the patient’s medical needs (Table 12,3). These address the patient’s ability to work (eg, medical leave, return to work, or accommodations) or travel (eg, ability to drive or use public transportation), or need for specific medical treatment (ie, gender-affirming care or cannabis use in specific settings). The second group relates to legal requests such as excusal from jury duty, emotional support animals, or any other letter used specifically for legal purposes (in civil or criminal cases) (Table 21,4-6).

The decision to write a letter on behalf of a patient should be based on whether you have sufficient knowledge to answer the referral question, and whether the requested evaluation fits within your role as the treating psychiatrist. Many requests fall short of the first condition. For example, a request to opine about an individual’s ability to perform their job duties requires specific knowledge and careful consideration of the patient’s work responsibilities, knowledge of the impact of their psychiatric symptoms, and specialized knowledge about interventions that would ameliorate symptoms in the specialized work setting. Most psychiatrists are not sufficiently familiar with a specific workplace to provide opinions regarding reasonable accommodations.

The second condition refers to the role and responsibilities of the psychiatrist. Many letter requests are clearly within the scope of the clinical psychiatrist, such as a medical leave note due to a psychiatric decompensation or a jury duty excusal due to an unstable mental state. Other letters reach beyond the role of the general or treating psychiatrist, such as opinions about suitable housing or a patient’s competency to stand trial.
Components of letters
The decision to write or not to write a letter should be discussed with the patient. Identify the reasons for and against letter writing. If you decide to write a letter, the letter should have the following basic framework (Figure): the identity of the person who requested the letter, the referral question, and an answer to the referral question with a clear rationale. Describe the patient’s psychiatric diagnosis using DSM criteria. Any limitations to the answer should be identified. The letter should not go beyond the referral question and should not include information that was not requested. It also should be preserved in the medical record.

It is recommended to write or review the letter in the presence of the patient to discuss the contents of the letter and what the psychiatrist can or cannot write. As in forensic reports, conclusory statements are not helpful. Provide descriptive information instead of relying on psychiatric jargon, and a rationale for the opinion as opposed to stating an opinion as fact. In the letter, you must acknowledge that your opinion is based upon information provided by the patient (and the patient’s family, when accurate) and as a result, is not fully objective.
Continue to: Liability and dual agency
Liability and dual agency
Psychiatrists are familiar with clinical situations in which a duty to the patient is mitigated or superseded by a duty to a third party. As the Tarasoff court famously stated, “the protective privilege ends where the public peril begins.”7
To be liable to either a patient or a third party means to be “bound or obliged in law or equity; responsible; chargeable; answerable; compellable to make satisfaction, compensation, or restitution.”8 Liabilities related to clinical treatment are well-established; medical students learn the fundamentals before ever treating a patient, and physicians carry malpractice insurance throughout their careers.
Less well-established is the liability a treating psychiatrist owes a third party when forming an opinion that impacts both their patient and the third party (eg, an employer when writing a return-to-work letter, or a disability insurer when qualifying a patient for disability benefits). The American Academy of Psychiatry and the Law discourages treating psychiatrists from performing these types of evaluations of their patients based on the inherent conflict of serving as a dual agent, or acting both as an advocate for the patient and as an independent evaluator striving for objectivity.9 However, such requests commonly arise, and some may be unavoidable.
Dual-agency situations subject the treating psychiatrist to avenues of legal action arising from the patient-doctor relationship as well as the forensic evaluator relationship. If a letter is written during a clinical treatment, all duties owed to the patient continue to apply, and the relevant benchmarks of local statutes and principle of a standard of care are relevant. It is conceivable that a patient could bring a negligence lawsuit based on a standard of care allegation (eg, that writing certain types of letters is so ordinary that failure to write them would fall below the standard of care). Confidentiality is also of the utmost importance,10 and you should obtain a written release of information from the patient before releasing any letter with privileged information about the patient.11 Additional relevant legal causes of action the patient could include are torts such as defamation of character, invasion of privacy, breach of contract, and intentional infliction of emotional distress. There is limited case law supporting patients’ rights to sue psychiatrists for defamation.10
A psychiatrist writing a letter to a third party may also subject themselves to avenues of legal action occurring outside the physician-patient relationship. Importantly, damages resulting from these breaches would not be covered by your malpractice insurance. Extreme cases involve allegations of fraud or perjury, which could be pursued in criminal court. If a psychiatrist intentionally deceives a third party for the purpose of obtaining some benefit for the patient, this is clear grounds for civil or criminal action. Fraud is defined as “a false representation of a matter of fact, whether by words or by conduct, by false or misleading allegations, or by concealment of that which should have been disclosed, which deceives and is intended to deceive another so that he shall act upon it to his legal injury.”8 Negligence can also be grounds for liability if a third party suffers injury or loss. Although the liability is clearer if the third party retains an independent psychiatrist rather than soliciting an opinion from a patient’s treating psychiatrist, both parties are subject to the claim of negligence.10
Continue to: There are some important protections...
There are some important protections that limit psychiatrists’ good-faith opinions from litigation. The primary one is the “professional medical judgment rule,” which shields physicians from the consequences of erroneous opinions so long as the examination was competent, complete, and performed in an ordinary fashion.10 In some cases, psychiatrists writing a letter or report for a government agency may also qualify for quasi-judicial immunity or witness immunity, but case law shows significant variation in when and how these privileges apply and whether such privileges would be applied to a clinical psychiatrist in the context of a traditional physician-patient relationship.12 In general, these privileges are not absolute and may not be sufficiently well-established to discourage a plaintiff from filing suit or prompt early judicial dismissal of a case.
Like all aspects of practicing medicine, letter writing is subject to scrutiny and accountability. Think carefully about your obligations and the potential consequences of writing or not writing a letter to a third party.
Ethical considerations
The decision to write a letter for a patient must be carefully considered from multiple angles.6 In addition to liability concerns, various ethical considerations also arise. Guided by the principles of beneficence, nonmaleficence, autonomy, and justice,13 we recommend the following approaches.
Maintain objectivity
During letter writing, a conflict of interest may arise between your allegiance to the patient and the imperative to provide accurate information.14-16 If the conflict is overwhelming, the most appropriate approach is to recuse yourself from the case and refer the patient to a third party. When electing to write a letter, you accept the responsibility to provide an objective assessment of the relevant situation. This promotes a just outcome and may also serve to promote the patient’s or society’s well-being.
Encourage activity and overall function
Evidence suggests that participation in multiple aspects of life promotes positive health outcomes.17,18 As a physician, it is your duty to promote health and support and facilitate accommodations that allow patients to participate and flourish in society. By the same logic, when approached by patients with a request for letters in support of reduced activity, you should consider not only the benefits but also the potential detriments of such disruptions. This may entail recommending temporary restrictions or modifications, as appropriate.
Continue to: Think beyond the patient
Think beyond the patient
Letter writing, particularly when recommending accommodations, can have implications beyond the patient.16 Such letters may cause unintended societal harm. For example, others may have to assume additional responsibilities; competitive goods (eg, housing) may be rendered to the patient rather than to a person with greater needs; and workplace safety could be compromised due to absence. Consider not only the individual patient but also possible public health and societal effects of letter writing.
Deciding not to write
From an ethical perspective, a physician cannot be compelled to write a letter if such an undertaking violates a stronger moral obligation. An example of this is if writing a letter could cause significant harm to the patient or society, or if writing a letter might compromise a physician’s professionalism.19 When you elect to not write a letter, the ethical principles of autonomy and truth telling dictate that you must inform your patients of this choice.6 You should also provide an explanation to the patient as long as such information would not cause undue psychological or physical harm.20,21
Schedule time to write letters
Some physicians implement policies that all letters are to be completed during scheduled appointments. Others designate administrative time to complete requested letters. Finally, some physicians flexibly complete such requests between appointments or during other undedicated time slots. Any of these approaches are justifiable, though some urgent requests may require more immediate attention outside of appointments. Some physicians may choose to bill for the letter writing if completed outside an appointment and the patient is treated in private practice. Whatever your policy, inform patients of it at the beginning of care and remind them when appropriate, such as before completing a letter that may be billed.
Manage uncertainty
Always strive for objectivity in letter writing. However, some requests inherently hinge on subjective reports and assessments. For example, a patient may request an excuse letter due to feeling unwell. In the absence of objective findings, what should you do? We advise the following.
Acquire collateral information. Adequate information is essential when making any medical recommendation. The same is true for writing letters. With the patient’s permission, you may need to contact relevant parties to better understand the circumstance or activity about which you are being asked to write a letter. For example, a patient may request leave from work due to injury. If the specific parameters of the work impeded by the injury are unclear to you, refrain from writing the letter and explain the rationale to the patient.
Continue to: Integrate prior knowledge of the patient
Integrate prior knowledge of the patient. No letter writing request exists in a vacuum. If you know the patient, the letter should be contextualized within the patient’s prior behaviors.
Stay within your scope
Given the various dilemmas and challenges, you may want to consider whether some letter writing is out of your professional scope.14-16 One solution would be to leave such requests to other entities (eg, requiring employers to retain medical personnel with specialized skills in occupational evaluations) and make such recommendations to patients. Regardless, physicians should think carefully about their professional boundaries and scope regarding letter requests and adopt and implement a consistent standard for all patients.
Regarding the letter requested by Ms. M, you should consider whether the appeal is consistent with the patient’s psychiatric illness. You should also consider whether you have sufficient knowledge about the patient’s living environment to support their claim. Such a letter should be written only if you understand both considerations. Regardless of your decision, you should explain your rationale to the patient.
Bottom Line
Patients may ask their psychiatrists to write letters that address aspects of their social well-being. However, psychiatrists must be alert to requests that are outside their scope of practice or ethically or legally fraught. Carefully consider whether writing a letter is appropriate and if not, discuss with the patient the reasons you cannot write such a letter and any recommended alternative avenues to address their request.
Related Resources
- Riese A. Writing letters for transgender patients undergoing medical transition. Current Psychiatry. 2021;20(8):51-52. doi:10.12788/cp.0159
- Joshi KG. Service animals and emotional support animals: should you write that letter? Current Psychiatry. 2021;20(11):16-19,24. doi:10.12788/cp.0183
After several months of difficulty living in her current apartment complex, Ms. M asks you as her psychiatrist to write a letter to the management company requesting she be moved to an apartment on the opposite side of the maintenance closet because the noise aggravates her posttraumatic stress disorder. What should you consider when asked to write such a letter?
Psychiatric practice often extends beyond the treatment of mental illness to include addressing patients’ social well-being. Psychiatrists commonly inquire about a patient’s social situation to understand the impact of these environmental factors. Similarly, psychiatric illness may affect a patient’s ability to work or fulfill responsibilities. As a result, patients may ask their psychiatrists for assistance by requesting letters that address various aspects of their social well-being.1 These communications may address an array of topics, from a patient’s readiness to return to work to their ability to pay child support. This article focuses on the role psychiatrists have in writing patient-requested letters across a variety of topics, including the consideration of potential legal liability and ethical implications.
Types of letters
The categories of letters patients request can be divided into 2 groups. The first is comprised of letters relating to the patient’s medical needs (Table 12,3). These address the patient’s ability to work (eg, medical leave, return to work, or accommodations) or travel (eg, ability to drive or use public transportation), or need for specific medical treatment (ie, gender-affirming care or cannabis use in specific settings). The second group relates to legal requests such as excusal from jury duty, emotional support animals, or any other letter used specifically for legal purposes (in civil or criminal cases) (Table 21,4-6).

The decision to write a letter on behalf of a patient should be based on whether you have sufficient knowledge to answer the referral question, and whether the requested evaluation fits within your role as the treating psychiatrist. Many requests fall short of the first condition. For example, a request to opine about an individual’s ability to perform their job duties requires specific knowledge and careful consideration of the patient’s work responsibilities, knowledge of the impact of their psychiatric symptoms, and specialized knowledge about interventions that would ameliorate symptoms in the specialized work setting. Most psychiatrists are not sufficiently familiar with a specific workplace to provide opinions regarding reasonable accommodations.

The second condition refers to the role and responsibilities of the psychiatrist. Many letter requests are clearly within the scope of the clinical psychiatrist, such as a medical leave note due to a psychiatric decompensation or a jury duty excusal due to an unstable mental state. Other letters reach beyond the role of the general or treating psychiatrist, such as opinions about suitable housing or a patient’s competency to stand trial.
Components of letters
The decision to write or not to write a letter should be discussed with the patient. Identify the reasons for and against letter writing. If you decide to write a letter, the letter should have the following basic framework (Figure): the identity of the person who requested the letter, the referral question, and an answer to the referral question with a clear rationale. Describe the patient’s psychiatric diagnosis using DSM criteria. Any limitations to the answer should be identified. The letter should not go beyond the referral question and should not include information that was not requested. It also should be preserved in the medical record.

It is recommended to write or review the letter in the presence of the patient to discuss the contents of the letter and what the psychiatrist can or cannot write. As in forensic reports, conclusory statements are not helpful. Provide descriptive information instead of relying on psychiatric jargon, and a rationale for the opinion as opposed to stating an opinion as fact. In the letter, you must acknowledge that your opinion is based upon information provided by the patient (and the patient’s family, when accurate) and as a result, is not fully objective.
Continue to: Liability and dual agency
Liability and dual agency
Psychiatrists are familiar with clinical situations in which a duty to the patient is mitigated or superseded by a duty to a third party. As the Tarasoff court famously stated, “the protective privilege ends where the public peril begins.”7
To be liable to either a patient or a third party means to be “bound or obliged in law or equity; responsible; chargeable; answerable; compellable to make satisfaction, compensation, or restitution.”8 Liabilities related to clinical treatment are well-established; medical students learn the fundamentals before ever treating a patient, and physicians carry malpractice insurance throughout their careers.
Less well-established is the liability a treating psychiatrist owes a third party when forming an opinion that impacts both their patient and the third party (eg, an employer when writing a return-to-work letter, or a disability insurer when qualifying a patient for disability benefits). The American Academy of Psychiatry and the Law discourages treating psychiatrists from performing these types of evaluations of their patients based on the inherent conflict of serving as a dual agent, or acting both as an advocate for the patient and as an independent evaluator striving for objectivity.9 However, such requests commonly arise, and some may be unavoidable.
Dual-agency situations subject the treating psychiatrist to avenues of legal action arising from the patient-doctor relationship as well as the forensic evaluator relationship. If a letter is written during a clinical treatment, all duties owed to the patient continue to apply, and the relevant benchmarks of local statutes and principle of a standard of care are relevant. It is conceivable that a patient could bring a negligence lawsuit based on a standard of care allegation (eg, that writing certain types of letters is so ordinary that failure to write them would fall below the standard of care). Confidentiality is also of the utmost importance,10 and you should obtain a written release of information from the patient before releasing any letter with privileged information about the patient.11 Additional relevant legal causes of action the patient could include are torts such as defamation of character, invasion of privacy, breach of contract, and intentional infliction of emotional distress. There is limited case law supporting patients’ rights to sue psychiatrists for defamation.10
A psychiatrist writing a letter to a third party may also subject themselves to avenues of legal action occurring outside the physician-patient relationship. Importantly, damages resulting from these breaches would not be covered by your malpractice insurance. Extreme cases involve allegations of fraud or perjury, which could be pursued in criminal court. If a psychiatrist intentionally deceives a third party for the purpose of obtaining some benefit for the patient, this is clear grounds for civil or criminal action. Fraud is defined as “a false representation of a matter of fact, whether by words or by conduct, by false or misleading allegations, or by concealment of that which should have been disclosed, which deceives and is intended to deceive another so that he shall act upon it to his legal injury.”8 Negligence can also be grounds for liability if a third party suffers injury or loss. Although the liability is clearer if the third party retains an independent psychiatrist rather than soliciting an opinion from a patient’s treating psychiatrist, both parties are subject to the claim of negligence.10
Continue to: There are some important protections...
There are some important protections that limit psychiatrists’ good-faith opinions from litigation. The primary one is the “professional medical judgment rule,” which shields physicians from the consequences of erroneous opinions so long as the examination was competent, complete, and performed in an ordinary fashion.10 In some cases, psychiatrists writing a letter or report for a government agency may also qualify for quasi-judicial immunity or witness immunity, but case law shows significant variation in when and how these privileges apply and whether such privileges would be applied to a clinical psychiatrist in the context of a traditional physician-patient relationship.12 In general, these privileges are not absolute and may not be sufficiently well-established to discourage a plaintiff from filing suit or prompt early judicial dismissal of a case.
Like all aspects of practicing medicine, letter writing is subject to scrutiny and accountability. Think carefully about your obligations and the potential consequences of writing or not writing a letter to a third party.
Ethical considerations
The decision to write a letter for a patient must be carefully considered from multiple angles.6 In addition to liability concerns, various ethical considerations also arise. Guided by the principles of beneficence, nonmaleficence, autonomy, and justice,13 we recommend the following approaches.
Maintain objectivity
During letter writing, a conflict of interest may arise between your allegiance to the patient and the imperative to provide accurate information.14-16 If the conflict is overwhelming, the most appropriate approach is to recuse yourself from the case and refer the patient to a third party. When electing to write a letter, you accept the responsibility to provide an objective assessment of the relevant situation. This promotes a just outcome and may also serve to promote the patient’s or society’s well-being.
Encourage activity and overall function
Evidence suggests that participation in multiple aspects of life promotes positive health outcomes.17,18 As a physician, it is your duty to promote health and support and facilitate accommodations that allow patients to participate and flourish in society. By the same logic, when approached by patients with a request for letters in support of reduced activity, you should consider not only the benefits but also the potential detriments of such disruptions. This may entail recommending temporary restrictions or modifications, as appropriate.
Continue to: Think beyond the patient
Think beyond the patient
Letter writing, particularly when recommending accommodations, can have implications beyond the patient.16 Such letters may cause unintended societal harm. For example, others may have to assume additional responsibilities; competitive goods (eg, housing) may be rendered to the patient rather than to a person with greater needs; and workplace safety could be compromised due to absence. Consider not only the individual patient but also possible public health and societal effects of letter writing.
Deciding not to write
From an ethical perspective, a physician cannot be compelled to write a letter if such an undertaking violates a stronger moral obligation. An example of this is if writing a letter could cause significant harm to the patient or society, or if writing a letter might compromise a physician’s professionalism.19 When you elect to not write a letter, the ethical principles of autonomy and truth telling dictate that you must inform your patients of this choice.6 You should also provide an explanation to the patient as long as such information would not cause undue psychological or physical harm.20,21
Schedule time to write letters
Some physicians implement policies that all letters are to be completed during scheduled appointments. Others designate administrative time to complete requested letters. Finally, some physicians flexibly complete such requests between appointments or during other undedicated time slots. Any of these approaches are justifiable, though some urgent requests may require more immediate attention outside of appointments. Some physicians may choose to bill for the letter writing if completed outside an appointment and the patient is treated in private practice. Whatever your policy, inform patients of it at the beginning of care and remind them when appropriate, such as before completing a letter that may be billed.
Manage uncertainty
Always strive for objectivity in letter writing. However, some requests inherently hinge on subjective reports and assessments. For example, a patient may request an excuse letter due to feeling unwell. In the absence of objective findings, what should you do? We advise the following.
Acquire collateral information. Adequate information is essential when making any medical recommendation. The same is true for writing letters. With the patient’s permission, you may need to contact relevant parties to better understand the circumstance or activity about which you are being asked to write a letter. For example, a patient may request leave from work due to injury. If the specific parameters of the work impeded by the injury are unclear to you, refrain from writing the letter and explain the rationale to the patient.
Continue to: Integrate prior knowledge of the patient
Integrate prior knowledge of the patient. No letter writing request exists in a vacuum. If you know the patient, the letter should be contextualized within the patient’s prior behaviors.
Stay within your scope
Given the various dilemmas and challenges, you may want to consider whether some letter writing is out of your professional scope.14-16 One solution would be to leave such requests to other entities (eg, requiring employers to retain medical personnel with specialized skills in occupational evaluations) and make such recommendations to patients. Regardless, physicians should think carefully about their professional boundaries and scope regarding letter requests and adopt and implement a consistent standard for all patients.
Regarding the letter requested by Ms. M, you should consider whether the appeal is consistent with the patient’s psychiatric illness. You should also consider whether you have sufficient knowledge about the patient’s living environment to support their claim. Such a letter should be written only if you understand both considerations. Regardless of your decision, you should explain your rationale to the patient.
Bottom Line
Patients may ask their psychiatrists to write letters that address aspects of their social well-being. However, psychiatrists must be alert to requests that are outside their scope of practice or ethically or legally fraught. Carefully consider whether writing a letter is appropriate and if not, discuss with the patient the reasons you cannot write such a letter and any recommended alternative avenues to address their request.
Related Resources
- Riese A. Writing letters for transgender patients undergoing medical transition. Current Psychiatry. 2021;20(8):51-52. doi:10.12788/cp.0159
- Joshi KG. Service animals and emotional support animals: should you write that letter? Current Psychiatry. 2021;20(11):16-19,24. doi:10.12788/cp.0183
1. West S, Friedman SH. To be or not to be: treating psychiatrist and expert witness. Psychiatric Times. 2007;24(6). Accessed March 14, 2023. https://www.psychiatrictimes.com/view/be-or-not-be-treating-psychiatrist-and-expert-witness
2. Knoepflmacher D. ‘Medical necessity’ in psychiatry: whose definition is it anyway? Psychiatric News. 2016;51(18):12-14. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2016.9b14
3. Lampe JR. Recent developments in marijuana law (LSB10859). Congressional Research Service. 2022. Accessed October 25, 2023. https://crsreports.congress.gov/product/pdf/LSB/LSB10859/2
4. Brunnauer A, Buschert V, Segmiller F, et al. Mobility behaviour and driving status of patients with mental disorders – an exploratory study. Int J Psychiatry Clin Pract. 2016;20(1):40-46. doi:10.3109/13651501.2015.1089293
5. Chiu CW, Law CK, Cheng AS. Driver assessment service for people with mental illness. Hong Kong J Occup Ther. 2019;32(2):77-83. doi:10.1177/1569186119886773
6. Joshi KG. Service animals and emotional support animals: should you write that letter? Current Psychiatry. 2021;20(11):16-19. doi:10.12788/cp.0183
7. Tarasoff v Regents of University of California, 17 Cal 3d 425, 551 P2d 334, 131 Cal. Rptr. 14 (Cal 1976).
8. Black HC. Liability. Black’s Law Dictionary. Revised 4th ed. West Publishing; 1975:1060.
9. American Academy of Psychiatry and the Law. Ethics guidelines for the practice of forensic psychiatry. 2005. Accessed March 15, 2023. https://www.aapl.org/ethics.htm
10. Gold LH, Davidson JE. Do you understand your risk? Liability and third-party evaluations in civil litigation. J Am Acad Psychiatry Law. 2007;35(2):200-210.
11. Schouten R. Approach to the patient seeking disability benefits. In: Stern TA, Herman JB, Slavin PL, eds. The MGH Guide to Psychiatry in Primary Care. McGraw Hill; 1998:121-126.
12. Appelbaum PS. Law and psychiatry: liability for forensic evaluations: a word of caution. Psychiatr Serv. 2001;52(7):885-886. doi:10.1176/appi.ps.52.7.885
13. Varkey B. Principles of clinical ethics and their application to practice. Med Princ Pract. 2021;30(1):17-28. doi:10.1159/000509119
14. Mayhew HE, Nordlund DJ. Absenteeism certification: the physician’s role. J Fam Pract. 1988;26(6):651-655.
15. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260. doi:10.1037/pro0000083
16. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518. doi:1-.29158/JAAPL.200047-20
17. Strully KW. Job loss and health in the U.S. labor market. Demography. 2009;46(2):221-246. doi:10.1353/dem.0.0050
18. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59(6):e125-131. doi:10.1097/JOM.0000000000001055
19. Munyaradzi M. Critical reflections on the principle of beneficence in biomedicine. Pan Afr Med J. 2012;11:29.
20. Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 7th ed. Oxford University Press; 2012.
21. Gold M. Is honesty always the best policy? Ethical aspects of truth telling. Intern Med J. 2004;34(9-10):578-580. doi:10.1111/j.1445-5994.2004.00673.x
1. West S, Friedman SH. To be or not to be: treating psychiatrist and expert witness. Psychiatric Times. 2007;24(6). Accessed March 14, 2023. https://www.psychiatrictimes.com/view/be-or-not-be-treating-psychiatrist-and-expert-witness
2. Knoepflmacher D. ‘Medical necessity’ in psychiatry: whose definition is it anyway? Psychiatric News. 2016;51(18):12-14. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2016.9b14
3. Lampe JR. Recent developments in marijuana law (LSB10859). Congressional Research Service. 2022. Accessed October 25, 2023. https://crsreports.congress.gov/product/pdf/LSB/LSB10859/2
4. Brunnauer A, Buschert V, Segmiller F, et al. Mobility behaviour and driving status of patients with mental disorders – an exploratory study. Int J Psychiatry Clin Pract. 2016;20(1):40-46. doi:10.3109/13651501.2015.1089293
5. Chiu CW, Law CK, Cheng AS. Driver assessment service for people with mental illness. Hong Kong J Occup Ther. 2019;32(2):77-83. doi:10.1177/1569186119886773
6. Joshi KG. Service animals and emotional support animals: should you write that letter? Current Psychiatry. 2021;20(11):16-19. doi:10.12788/cp.0183
7. Tarasoff v Regents of University of California, 17 Cal 3d 425, 551 P2d 334, 131 Cal. Rptr. 14 (Cal 1976).
8. Black HC. Liability. Black’s Law Dictionary. Revised 4th ed. West Publishing; 1975:1060.
9. American Academy of Psychiatry and the Law. Ethics guidelines for the practice of forensic psychiatry. 2005. Accessed March 15, 2023. https://www.aapl.org/ethics.htm
10. Gold LH, Davidson JE. Do you understand your risk? Liability and third-party evaluations in civil litigation. J Am Acad Psychiatry Law. 2007;35(2):200-210.
11. Schouten R. Approach to the patient seeking disability benefits. In: Stern TA, Herman JB, Slavin PL, eds. The MGH Guide to Psychiatry in Primary Care. McGraw Hill; 1998:121-126.
12. Appelbaum PS. Law and psychiatry: liability for forensic evaluations: a word of caution. Psychiatr Serv. 2001;52(7):885-886. doi:10.1176/appi.ps.52.7.885
13. Varkey B. Principles of clinical ethics and their application to practice. Med Princ Pract. 2021;30(1):17-28. doi:10.1159/000509119
14. Mayhew HE, Nordlund DJ. Absenteeism certification: the physician’s role. J Fam Pract. 1988;26(6):651-655.
15. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260. doi:10.1037/pro0000083
16. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518. doi:1-.29158/JAAPL.200047-20
17. Strully KW. Job loss and health in the U.S. labor market. Demography. 2009;46(2):221-246. doi:10.1353/dem.0.0050
18. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59(6):e125-131. doi:10.1097/JOM.0000000000001055
19. Munyaradzi M. Critical reflections on the principle of beneficence in biomedicine. Pan Afr Med J. 2012;11:29.
20. Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 7th ed. Oxford University Press; 2012.
21. Gold M. Is honesty always the best policy? Ethical aspects of truth telling. Intern Med J. 2004;34(9-10):578-580. doi:10.1111/j.1445-5994.2004.00673.x
Cannabis and schizophrenia: A complex relationship
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
Treating chronic insomnia: An alternating medication strategy
Patients with chronic insomnia that does not improve with nonpharmacologic techniques often develop tolerance to sedative medications (benzodiazepines) prescribed for nightly use. When nonbenzodiazepine medications are used, tachyphylaxis can develop and these medications no longer initiate or maintain sleep. Strategies that alternate between these 2 types of agents are simple to follow and may allow patients to maintain sensitivity to both types of medications. In this article, I review the types, causes, evaluation, and treatment of insomnia; describe an alternating medication strategy to help patients avoid developing tolerance/tachyphylaxis; and present 3 fictional case vignettes to illustrate this approach.
A common, troubling condition
Insomnia is a common problem among psychiatric patients. Approximately 30% to 50% of adults experience occasional, short-term (<3 months) insomnia, and 5% to 10% experience chronic (≥3 months) insomnia,1 with associated negative impacts on health and quality of life. Insomnia is sometimes primary and may have a hereditary component, but more often is associated with medical, neurologic, or psychiatric disorders.
Patterns of insomnia include difficulty falling asleep (initial or sleep-onset insomnia), remaining asleep (middle or sleep-maintenance insomnia), or falling back asleep after early awakening (late or sleep-offset insomnia). Sleep-onset insomnia correlates with high levels of anxiety and worrying, but once asleep, patients usually stay asleep. Sleep-maintenance problems involve multiple awakenings after falling asleep and taking hours to fall back to sleep. These patients experience inadequate sleep when they must wake up early for school or work. Early-awakening patients report feeling wide awake by 4 to 5
Caffeine is an important consideration for patients with sleep difficulties. Its use is widespread in much of the world, whether ingested as coffee, tea, in soft drinks, or in “energy” drinks that may contain as much as 200 mg of caffeine (twice the amount in a typical cup of brewed coffee). Caffeine may also be ingested as an ingredient of medications for headache or migraine. While some individuals maintain that they can fall asleep easily after drinking caffeinated coffee, many may not recognize the amount of caffeine they consume and its negative impact on sleep.2 Author Michael Pollan stopped use of all caffeine and reported on the surprising positive effect on his sleep.3
Patients with mood, anxiety, or psychotic disorders are likely to experience insomnia intermittently or chronically, and insomnia predisposes some individuals to develop mood and anxiety symptoms.4 Patients with insomnia often experience anxiety focused on a fear of not getting adequate sleep, which creates a vicious cycle in which hyperarousal associated with fear of not sleeping complicates other causes of insomnia. A patient’s chronotype (preference for the time of day in which they carry out activities vs sleeping) also may play a role in sleep difficulties (Box5).
Box
Chronotypes—the expression of circadian rhythmicity in an individual—have been studied extensively.5 Psychiatrists may encounter patients who sleep most of the day and stay awake at night, those who sleep up to 20 hours per day, and those who sleep <4 hours in 24 hours. Patients typically know which category they fall into. The early bird typically is awake by 6 or 7 am, remains alert through most of the day, and feels sleepy by 10 pm. The usual diurnal variation in cortisol, with peaks at 7 am and 7 pm and nadirs at 1 pm and 1 am, correspond with the early bird’s habits.
Night owls typically report feeling exhausted and irritable in the early morning; prefer to sleep past noon; feel energized around dark, when they can do their best studying, concentrating, etc; and do not feel sleepy until early morning. While this night owl pattern is a natural variation and not necessarily associated with psychiatric illness, patients with mood disorders frequently have chaotic sleep patterns that may not conform to a pattern. Night owls maintain the same diurnal pattern of cortisol secretion as early birds.
Certain medications may contribute to insomnia, particularly stimulants. It is important to understand and explain to patients the time frame during which immediate-release or extended-release (ER) stimulants are active, which varies in individuals depending on liver enzyme activity. Other commonly used psychotropic medications—including bupropion, modafinil, armodafinil, atomoxetine, amphetamine salts, and methylphenidate—may interfere with sleep if used later in the day.6
Patients typically do not mention their use of alcohol and/or marijuana unless asked. Those who are binge drinkers or alcohol-dependent may expect alcohol to help them fall asleep, but usually find their sleep is disrupted and difficult to maintain. Patients may use marijuana to help them sleep, particularly marijuana high in tetrahydrocannabinol (THC). While it may help with sleep initiation, THC can disrupt sleep maintenance. Cannabidiol does not have intrinsic sedating effects and may even interfere with sleep.7,8
Continue to: Women may be more likely...
Women may be more likely than men to experience insomnia.9 The onset of menopause can bring hot flashes that interfere with sleep.
Women with a history of mood disorders are more likely to have a history of premenstrual dysphoric disorder, postpartum depression, and unusual responses to oral contraceptives.10 These women are more likely to report problems with mood, energy, and sleep at perimenopause. Treatment with estrogen replacement may be an option for women without risk factors, such as clotting disorders, smoking history, or a personal or family history of breast or uterine cancer. For many who are not candidates for or who refuse estrogen replacement, use of a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor at low doses may help with vasomotor symptoms but not with insomnia.
Insomnia symptoms typically increase with age.11 When sleep is adequate early in life but becomes a problem in midlife, an individual’s eating habits, obesity, and lack of exercise may be contributing factors. The typical American diet includes highly refined carbohydrates with excess salt; such foods are often readily available to the exclusion of healthy options. Overweight and obese patients may insist they eat a healthy diet with 3 meals per day, but a careful history often uncovers nighttime binge eating. Nighttime binge eating is rarely reported. This not only maintains obesity, but also interferes with sleep, since patients stay up late to avoid discovery by family members.12 This lack of sleep can lead to an endless loop because insufficient sleep is a risk factor for obesity.13
Evaluating sleep difficulties
New patient evaluations should include a careful history beginning with childhood, including personal early childhood history and family psychiatric history. Patients often report the onset of sleep difficulty and anxiety during childhood, which should raise further questions about aspects of mood regulation from early life such as concentration, energy, motivation, appetite, and academic performance. While many children and adolescents are diagnosed with attention-deficit/hyperactivity disorder due to concentration problems that cause difficulties at school, be aware this might be part of a syndrome related to mood regulation.14 Unexpected responses to an SSRI—such as agitation, euphoria, or an immediate response with the first dose—should also raise suspicion of a mood disorder. Once the underlying mood disorder is stabilized, many patients report improved sleep.15
If a patient reports having difficulty falling and remaining asleep but is not sure if there is a pattern, keeping a sleep diary can help. Further questioning may uncover the cause. Does the patient have spontaneous jerks of lower extremities (restless leg syndrome) that interfere with falling asleep or wake them up? Have they noticed problems with dreams/nightmares that wake them, which could be associated with posttraumatic stress, anxiety, or depression? Have they been told by a partner that they act out dreams or are seemingly awake but not responsive, which could point to REM sleep behavior disorder or early Parkinson’s disease? Referral to a sleep laboratory and a neurologist can help establish the correct diagnosis and point to appropriate treatment.16-18
Treatment options
Several cognitive-behavioral techniques, including cognitive-behavioral therapy for insomnia (CBT-I), yogic breathing, progressive relaxation, mindfulness meditation, and sleep hygiene techniques may help considerably,19,20 but insomnia often remains difficult to treat. Pharmacotherapy is not necessarily more effective than nonpharmacologic approaches. Both options require the patient to take initiative to either find nonpharmacologic approaches or discuss the problem with a physician and agree to take medication.21 A trial comparing CBT-I to sedatives or the combination of CBT-I plus sedatives found higher rates of sleep with CBT-I for 3 months, after which improvement fluctuated; the combination showed sustained improvement for the entire 6-month trial.22 CBT-I has also been shown to be as effective with patients who do not have psychiatric illness as for those who are depressed, anxious, or stressed.23 However, behavioral techniques that require regular practice may be difficult for individuals to maintain, particularly when they are depressed or anxious.
Continue to: Clinicians should understand...
Clinicians should understand the distinctions among the various types of pharmacotherapy for insomnia. Sedative-hypnotics include medications with varying half-lives and metabolic pathways. Short-acting benzodiazepines such as triazolam or alprazolam and the “z-drugs” zolpidem or zaleplon may help initiate sleep in patients with sleep-onset insomnia. Longer-acting benzodiazepines such as diazepam, clonazepam, or temazepam and the z-drug eszopiclone may also help with sleep maintenance.23 Based on my clinical experience, individual patients may respond better to 1 type of medication over another, or even to different agents within the same class of sedative-hypnotics.
Some clinicians prescribe nonbenzodiazepine medications for sleep, such as doxepin (which is FDA-approved for treating insomnia) or off-label trazodone, mirtazapine, or quetiapine. Their antihistaminic properties confer sedating effects. Virtually all over-the-counter (OTC) medications for insomnia are antihistaminic. These OTC medications are not designed to treat insomnia, and the optimal dosage to maintain sleep without daytime sedation must be determined by trial and error. Sedating nonbenzodiazepine medications may be slowly absorbed if taken at bedtime (depending on whether they are taken with or without food) and cause daytime sedation and cognitive slowness in patients with sleep-onset and maintenance insomnia who must wake up early. Starting trazodone at 50 to 75 mg may cause slow metabolizers to wake up with considerable sedation, while fast metabolizers might never feel soundly asleep.24
Patients with mood and anxiety disorders that complicate insomnia are often prescribed second-generation antipsychotics such as quetiapine, lurasidone, or olanzapine, which are sedating as well as mood-stabilizing. These approaches require careful attention to titrating doses and timing their use.
Problems with pharmacotherapy
When either benzodiazepines or nonbenzodiazepine medications are used on a long-standing, nightly basis, they often stop working well. It is not unusual that after days to weeks of taking a benzodiazepine, patients find they no longer stay asleep but can’t fall asleep if they don’t take them. Once tolerance develops, the individual experiences pharmacologic withdrawal with an inability to fall asleep or stay asleep. The medication becomes necessary but ineffective, and many patients increase their use to higher doses to fall asleep, and sometimes in early morning to maintain sleep. This leads to negative effects on cognition, coordination/balance, and mood during the day, especially in older patients.
Nonbenzodiazepine sedating medications do not lead to pharmacologic tolerance but do lead to tachyphylaxis as the CNS attempts to downregulate sedation to keep the organism safe. For some patients, this happens quickly, within a matter of days.25 Others increase doses to stay asleep. For example, a patient with a starting dose of trazodone 75 mg/d might increase the dosage to 300 mg/d. While trazodone is approved in doses of 300 to 600 mg as an antidepressant, it is preferable to keep doses lower when used only for sedation.
Continue to: An alternating medication strategy
An alternating medication strategy
Alternating between medications from different classes can help patients avoid developing tolerance with benzodiazepines or tachyphylaxis as occurs with antihistaminic medications. It can be effective for patients with primary insomnia as well as for those whose sleep problems are associated with mood or anxiety disorders. Patients typically maintain sensitivity to any form of pharmacologic sedation for several nights without loss of effect but need to take a break to maintain the sedation effect. For example, in 1 case study, a 30-year-old female who rapidly developed tachyphylaxis to the sedative action of mirtazapine experienced a return of the medication’s sedative effects after taking a 3-day break.25
To initiate an alternating strategy, the clinician must first help the patient establish a sedating dose of 2 medications from different classes, such as trazodone and zolpidem, and then instruct the patient to use each for 2 to 3 consecutive nights on an alternating basis. Patients can use calendars or pillboxes to avoid confusion about which medication to take on a given night. In many cases, this approach can work indefinitely.
The following 3 case vignettes illustrate how this alternating medication strategy can work.
CASE 1
Mr. B, age 58, is a married salesman whose territory includes 3 states. He drives from client to client from Monday through Thursday each week, staying overnight in hotels. He is comfortable talking to clients, has a close and supportive relationship with his wife, and enjoys socializing with friends. Mr. B has a high level of trait anxiety and perfectionism and is proud of his sales record throughout his career, but this leads to insomnia during his nights on the road, and often on Sunday night as he starts anticipating the week ahead. Mr. B denies having a depressed mood or cognitive problems. When on vacation with his wife he has no trouble sleeping. He has no psychiatric family history or any substantial medical problems. He simply wishes that he could sleep on work nights.
We set up an alternating medication approach. Mr. B takes trazodone 100 mg on the first night and 150 mg on the second and third nights. He then takes triazolam 0.25 mg for 2 nights; previously, he had found that zolpidem did not work as well for maintaining sleep. He can sleep adequately for the 2 weekend nights, then restarts the alternating pattern. Mr. B has done well with this regimen for >10 years.
Continue to: CASE 2
CASE 2
Ms. C, age 60, is widowed and has a successful career as a corporate attorney. She has been anxious since early childhood and has had trouble falling asleep for much of her life. Once she falls asleep on her sofa—often between 1 and 2
Ms. C denies having depression, but experienced appropriate grief related to her husband’s illness and death from metastatic cancer 3 years ago. At the time, her internist prescribed escitalopram and zolpidem; escitalopram caused greater agitation and distress, so she stopped it after 10 days. Zolpidem 10 mg/d allowed her to sleep but she worried about taking it because her mother had long-standing sedative dependence. Ms. C lives alone, but her adult children live nearby, and she has a strong support system that includes colleagues at her firm, friends at her book club, and a support group for partners of cancer patients.
Ms. C tries trazodone, starting with 50 mg, but reports feeling agitated rather than sleepy and has cognitive fogginess in the morning. She is switched to quetiapine 50 mg, which she tolerates well and allows her to sleep soundly. To avoid developing tachyphylaxis with quetiapine, she takes eszopiclone 3 mg for 2 nights, alternating with quetiapine for 3 nights. This strategy allows her to reliably fall asleep by 11
CASE 3
Ms. D, age 55, is married with a long-standing diagnosis of generalized anxiety disorder (GAD), panic disorder, and depression so severe she is unable to work as a preschool teacher. She notes that past clinicians have prescribed a wide array of antidepressants and benzodiazepines but she remains anxious, agitated, and unable to sleep. She worries constantly about running out of benzodiazepines, which are “the only medication that helps me.” At the time of evaluation, her medications are venlafaxine ER 150 mg/d, lorazepam 1 mg 3 times daily and 2 mg at bedtime, and buspirone 15 mg 3 times daily, which she admits to not taking. She is overweight and does not exercise. She spends her days snacking and watching television. She can’t settle down enough to read and feels overwhelmed most of the time. Her adult children won’t allow her to babysit their young children because she dozes during the day.
Ms. D has a strong family history of psychiatric illness, including a father with bipolar I disorder and alcohol use disorder and a sister with schizoaffective disorder. Ms. D has never felt overtly manic, but has spent most of her life feeling depressed, anxious, and hopeless, and at times she has wished she was dead. She has had poor responses to many antidepressants, with transient euphoria followed by more anxiety.
Continue to: Rather than major depressive disorder...
Rather than major depressive disorder or GAD, Ms. D’s symptoms better meet the criteria for bipolar II disorder. She agrees to a slow taper of venlafaxine and a slow increase of divalproex, starting with 125 mg each evening. While taking venlafaxine 75 mg/d and divalproex 375 mg/d, she experiences distinct improvement in anxiety and agitation, which further improve after venlafaxine is stopped and divalproex is increased to 750 mg in the evening. She finds that she forgets daytime doses of lorazepam but depends on it to fall asleep. While taking quetiapine 50 mg and lorazepam 1 mg at bedtime, Ms. D reports sleeping soundly and feeling alert in the morning. Over several weeks, she tapers lorazepam slowly by 0.5 mg every 2 weeks. She finds she needs a higher dose of quetiapine to stay asleep, eventually requiring 400 mg each night. Ms. D says overall she feels better but is distressed because she has gained 25 lbs since starting divalproex and quetiapine.
To avoid further increases in quetiapine and maintain its sedating effect, Ms. D is switched to an alternating schedule of clonazepam 1.5 mg for 2 nights and quetiapine 300 mg for 3 nights. She agrees to begin exercising by walking in her neighborhood daily, and gradually increases this to 1 hour per day. After starting to exercise regularly, she finds she is oversedated by quetiapine at night, so she is gradually decreased to a dose of 150 mg, while still alternating with clonazepam 1.5 mg. Ms. D loses most of the weight she had gained and begins volunteering as a reading coach in the elementary school in her neighborhood.
Bottom Line
Patients with chronic insomnia can often maintain adequate sedation without developing tolerance to benzodiazepines or tachyphylaxis with nonsedating agents by using 2 sleep medications that have different mechanisms of action on an alternating schedule.
Related Resources
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2): 307-349. doi:10.5664/jcsm.6470
- Muppavarapu K, Muthukanagaraj M, Saeed SA. Cognitive-behavioral therapy for insomnia: a review of 8 studies. Current Psychiatry. 2020;19(9):40-46. doi:10.12788/cp.0040
Drug Brand Names
Alprazolam • Xanax
Armodafinil • Nuvigil
Atomoxetine • Strattera
Bupropion • Wellbutrin
Clonazepam • Klonopin
Diazepam • Valium
Divalproex • Depakote
Doxepin • Sinequan
Escitalopram • Lexapro
Eszopiclone • Lunesta
Lorazepam • Ativan
Lurasidone • Latuda
Methylphenidate • Concerta
Mirtazapine • Remeron
Modafinil • Provigil
Olanzapine • Zyprexa
Quetiapine • Seroquel
Temazepam • Restoril
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Zaleplon • Sonata
Zolpidem • Ambien
1. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
2. Drake C, Roehrs T, Shambroom J, et al. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195-1200.
3. Pollan M. Caffeine: How Coffee and Tea Created the Modern World. 2023; Audible Audiobooks.
4. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021:17:2549-2566.
5. Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405-415.
6. Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509-517.
7. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):23.
8. Monti JM, Pandi-Perumal SR. Clinical management of sleep and sleep disorders with cannabis and cannabinoids: implications to practicing psychiatrists. Clin Neuropharmacol. 2022;45(2):27-31.
9. Dockray S, Steptoe A. Chronotype and diurnal cortisol profile in working women: differences between work and leisure days. Psychoneuroendocrinology. 2011;36(5):649-655.
10. Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102-S108.
11. Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):59-66.
12. Stunkard A. Eating disorders and obesity. Psychiatr Clin North Am. 2011; 34(4):765-771.
13. Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. 2016;29(6):409-412.
14. Gillberg C, Gillberg IC, Rasmussen P, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004; 1(Suppl 1):i80-i92.
15. Goldberg JF, Nierenberg AA, Iosifescu DV. Wrestling with antidepressant use in bipolar disorder: the ongoing debate. J Clin Psychiatry. 2021;82(1):19. doi:10.4088/JCP.19ac13181
16. Baltzan M, Yao C, Rizzo D, et al. Dream enactment behavior: review for the clinician. J Clin Sleep Med. 2020;16(11):1949-1969.
17. Barone DA. Dream enactment behavior—a real nightmare: a review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J Clin Sleep Med. 2020;16(11):1943-1948.
18. Figorilli M, Meloni M, Lanza G, et al. Considering REM sleep behavior disorder in the management of Parkinson’s disease. Nat Sci Sleep. 2023;15:333-352.
19. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281-297.
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139.
21. Lu M, Zhang Y, Zhang J, et al. Comparative effectiveness of digital cognitive behavioral therapy vs. medication therapy among patients with insomnia. JAMA Network Open. 2023;6(4):e237597.
22. Sweetman A, McEvoy RD, Catcheside PG, et al. Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2021;17(3):545-554.
23. O’Brien CP. Benzodiazepine use, abuse and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28-33.
24. Wichniak A, Wierzbicka AE, Jarema M. Treatment of insomnia - effect of trazodone and hypnotics on sleep. Psychiatr Pol. 2021;55(4):743-755.
25. Papazisis G, Siafis S, Tzachanis D. Tachyphylaxis to the sedative action of mirtazapine. Am J Case Rep. 2018;19:410-412.
Patients with chronic insomnia that does not improve with nonpharmacologic techniques often develop tolerance to sedative medications (benzodiazepines) prescribed for nightly use. When nonbenzodiazepine medications are used, tachyphylaxis can develop and these medications no longer initiate or maintain sleep. Strategies that alternate between these 2 types of agents are simple to follow and may allow patients to maintain sensitivity to both types of medications. In this article, I review the types, causes, evaluation, and treatment of insomnia; describe an alternating medication strategy to help patients avoid developing tolerance/tachyphylaxis; and present 3 fictional case vignettes to illustrate this approach.
A common, troubling condition
Insomnia is a common problem among psychiatric patients. Approximately 30% to 50% of adults experience occasional, short-term (<3 months) insomnia, and 5% to 10% experience chronic (≥3 months) insomnia,1 with associated negative impacts on health and quality of life. Insomnia is sometimes primary and may have a hereditary component, but more often is associated with medical, neurologic, or psychiatric disorders.
Patterns of insomnia include difficulty falling asleep (initial or sleep-onset insomnia), remaining asleep (middle or sleep-maintenance insomnia), or falling back asleep after early awakening (late or sleep-offset insomnia). Sleep-onset insomnia correlates with high levels of anxiety and worrying, but once asleep, patients usually stay asleep. Sleep-maintenance problems involve multiple awakenings after falling asleep and taking hours to fall back to sleep. These patients experience inadequate sleep when they must wake up early for school or work. Early-awakening patients report feeling wide awake by 4 to 5
Caffeine is an important consideration for patients with sleep difficulties. Its use is widespread in much of the world, whether ingested as coffee, tea, in soft drinks, or in “energy” drinks that may contain as much as 200 mg of caffeine (twice the amount in a typical cup of brewed coffee). Caffeine may also be ingested as an ingredient of medications for headache or migraine. While some individuals maintain that they can fall asleep easily after drinking caffeinated coffee, many may not recognize the amount of caffeine they consume and its negative impact on sleep.2 Author Michael Pollan stopped use of all caffeine and reported on the surprising positive effect on his sleep.3
Patients with mood, anxiety, or psychotic disorders are likely to experience insomnia intermittently or chronically, and insomnia predisposes some individuals to develop mood and anxiety symptoms.4 Patients with insomnia often experience anxiety focused on a fear of not getting adequate sleep, which creates a vicious cycle in which hyperarousal associated with fear of not sleeping complicates other causes of insomnia. A patient’s chronotype (preference for the time of day in which they carry out activities vs sleeping) also may play a role in sleep difficulties (Box5).
Box
Chronotypes—the expression of circadian rhythmicity in an individual—have been studied extensively.5 Psychiatrists may encounter patients who sleep most of the day and stay awake at night, those who sleep up to 20 hours per day, and those who sleep <4 hours in 24 hours. Patients typically know which category they fall into. The early bird typically is awake by 6 or 7 am, remains alert through most of the day, and feels sleepy by 10 pm. The usual diurnal variation in cortisol, with peaks at 7 am and 7 pm and nadirs at 1 pm and 1 am, correspond with the early bird’s habits.
Night owls typically report feeling exhausted and irritable in the early morning; prefer to sleep past noon; feel energized around dark, when they can do their best studying, concentrating, etc; and do not feel sleepy until early morning. While this night owl pattern is a natural variation and not necessarily associated with psychiatric illness, patients with mood disorders frequently have chaotic sleep patterns that may not conform to a pattern. Night owls maintain the same diurnal pattern of cortisol secretion as early birds.
Certain medications may contribute to insomnia, particularly stimulants. It is important to understand and explain to patients the time frame during which immediate-release or extended-release (ER) stimulants are active, which varies in individuals depending on liver enzyme activity. Other commonly used psychotropic medications—including bupropion, modafinil, armodafinil, atomoxetine, amphetamine salts, and methylphenidate—may interfere with sleep if used later in the day.6
Patients typically do not mention their use of alcohol and/or marijuana unless asked. Those who are binge drinkers or alcohol-dependent may expect alcohol to help them fall asleep, but usually find their sleep is disrupted and difficult to maintain. Patients may use marijuana to help them sleep, particularly marijuana high in tetrahydrocannabinol (THC). While it may help with sleep initiation, THC can disrupt sleep maintenance. Cannabidiol does not have intrinsic sedating effects and may even interfere with sleep.7,8
Continue to: Women may be more likely...
Women may be more likely than men to experience insomnia.9 The onset of menopause can bring hot flashes that interfere with sleep.
Women with a history of mood disorders are more likely to have a history of premenstrual dysphoric disorder, postpartum depression, and unusual responses to oral contraceptives.10 These women are more likely to report problems with mood, energy, and sleep at perimenopause. Treatment with estrogen replacement may be an option for women without risk factors, such as clotting disorders, smoking history, or a personal or family history of breast or uterine cancer. For many who are not candidates for or who refuse estrogen replacement, use of a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor at low doses may help with vasomotor symptoms but not with insomnia.
Insomnia symptoms typically increase with age.11 When sleep is adequate early in life but becomes a problem in midlife, an individual’s eating habits, obesity, and lack of exercise may be contributing factors. The typical American diet includes highly refined carbohydrates with excess salt; such foods are often readily available to the exclusion of healthy options. Overweight and obese patients may insist they eat a healthy diet with 3 meals per day, but a careful history often uncovers nighttime binge eating. Nighttime binge eating is rarely reported. This not only maintains obesity, but also interferes with sleep, since patients stay up late to avoid discovery by family members.12 This lack of sleep can lead to an endless loop because insufficient sleep is a risk factor for obesity.13
Evaluating sleep difficulties
New patient evaluations should include a careful history beginning with childhood, including personal early childhood history and family psychiatric history. Patients often report the onset of sleep difficulty and anxiety during childhood, which should raise further questions about aspects of mood regulation from early life such as concentration, energy, motivation, appetite, and academic performance. While many children and adolescents are diagnosed with attention-deficit/hyperactivity disorder due to concentration problems that cause difficulties at school, be aware this might be part of a syndrome related to mood regulation.14 Unexpected responses to an SSRI—such as agitation, euphoria, or an immediate response with the first dose—should also raise suspicion of a mood disorder. Once the underlying mood disorder is stabilized, many patients report improved sleep.15
If a patient reports having difficulty falling and remaining asleep but is not sure if there is a pattern, keeping a sleep diary can help. Further questioning may uncover the cause. Does the patient have spontaneous jerks of lower extremities (restless leg syndrome) that interfere with falling asleep or wake them up? Have they noticed problems with dreams/nightmares that wake them, which could be associated with posttraumatic stress, anxiety, or depression? Have they been told by a partner that they act out dreams or are seemingly awake but not responsive, which could point to REM sleep behavior disorder or early Parkinson’s disease? Referral to a sleep laboratory and a neurologist can help establish the correct diagnosis and point to appropriate treatment.16-18
Treatment options
Several cognitive-behavioral techniques, including cognitive-behavioral therapy for insomnia (CBT-I), yogic breathing, progressive relaxation, mindfulness meditation, and sleep hygiene techniques may help considerably,19,20 but insomnia often remains difficult to treat. Pharmacotherapy is not necessarily more effective than nonpharmacologic approaches. Both options require the patient to take initiative to either find nonpharmacologic approaches or discuss the problem with a physician and agree to take medication.21 A trial comparing CBT-I to sedatives or the combination of CBT-I plus sedatives found higher rates of sleep with CBT-I for 3 months, after which improvement fluctuated; the combination showed sustained improvement for the entire 6-month trial.22 CBT-I has also been shown to be as effective with patients who do not have psychiatric illness as for those who are depressed, anxious, or stressed.23 However, behavioral techniques that require regular practice may be difficult for individuals to maintain, particularly when they are depressed or anxious.
Continue to: Clinicians should understand...
Clinicians should understand the distinctions among the various types of pharmacotherapy for insomnia. Sedative-hypnotics include medications with varying half-lives and metabolic pathways. Short-acting benzodiazepines such as triazolam or alprazolam and the “z-drugs” zolpidem or zaleplon may help initiate sleep in patients with sleep-onset insomnia. Longer-acting benzodiazepines such as diazepam, clonazepam, or temazepam and the z-drug eszopiclone may also help with sleep maintenance.23 Based on my clinical experience, individual patients may respond better to 1 type of medication over another, or even to different agents within the same class of sedative-hypnotics.
Some clinicians prescribe nonbenzodiazepine medications for sleep, such as doxepin (which is FDA-approved for treating insomnia) or off-label trazodone, mirtazapine, or quetiapine. Their antihistaminic properties confer sedating effects. Virtually all over-the-counter (OTC) medications for insomnia are antihistaminic. These OTC medications are not designed to treat insomnia, and the optimal dosage to maintain sleep without daytime sedation must be determined by trial and error. Sedating nonbenzodiazepine medications may be slowly absorbed if taken at bedtime (depending on whether they are taken with or without food) and cause daytime sedation and cognitive slowness in patients with sleep-onset and maintenance insomnia who must wake up early. Starting trazodone at 50 to 75 mg may cause slow metabolizers to wake up with considerable sedation, while fast metabolizers might never feel soundly asleep.24
Patients with mood and anxiety disorders that complicate insomnia are often prescribed second-generation antipsychotics such as quetiapine, lurasidone, or olanzapine, which are sedating as well as mood-stabilizing. These approaches require careful attention to titrating doses and timing their use.
Problems with pharmacotherapy
When either benzodiazepines or nonbenzodiazepine medications are used on a long-standing, nightly basis, they often stop working well. It is not unusual that after days to weeks of taking a benzodiazepine, patients find they no longer stay asleep but can’t fall asleep if they don’t take them. Once tolerance develops, the individual experiences pharmacologic withdrawal with an inability to fall asleep or stay asleep. The medication becomes necessary but ineffective, and many patients increase their use to higher doses to fall asleep, and sometimes in early morning to maintain sleep. This leads to negative effects on cognition, coordination/balance, and mood during the day, especially in older patients.
Nonbenzodiazepine sedating medications do not lead to pharmacologic tolerance but do lead to tachyphylaxis as the CNS attempts to downregulate sedation to keep the organism safe. For some patients, this happens quickly, within a matter of days.25 Others increase doses to stay asleep. For example, a patient with a starting dose of trazodone 75 mg/d might increase the dosage to 300 mg/d. While trazodone is approved in doses of 300 to 600 mg as an antidepressant, it is preferable to keep doses lower when used only for sedation.
Continue to: An alternating medication strategy
An alternating medication strategy
Alternating between medications from different classes can help patients avoid developing tolerance with benzodiazepines or tachyphylaxis as occurs with antihistaminic medications. It can be effective for patients with primary insomnia as well as for those whose sleep problems are associated with mood or anxiety disorders. Patients typically maintain sensitivity to any form of pharmacologic sedation for several nights without loss of effect but need to take a break to maintain the sedation effect. For example, in 1 case study, a 30-year-old female who rapidly developed tachyphylaxis to the sedative action of mirtazapine experienced a return of the medication’s sedative effects after taking a 3-day break.25
To initiate an alternating strategy, the clinician must first help the patient establish a sedating dose of 2 medications from different classes, such as trazodone and zolpidem, and then instruct the patient to use each for 2 to 3 consecutive nights on an alternating basis. Patients can use calendars or pillboxes to avoid confusion about which medication to take on a given night. In many cases, this approach can work indefinitely.
The following 3 case vignettes illustrate how this alternating medication strategy can work.
CASE 1
Mr. B, age 58, is a married salesman whose territory includes 3 states. He drives from client to client from Monday through Thursday each week, staying overnight in hotels. He is comfortable talking to clients, has a close and supportive relationship with his wife, and enjoys socializing with friends. Mr. B has a high level of trait anxiety and perfectionism and is proud of his sales record throughout his career, but this leads to insomnia during his nights on the road, and often on Sunday night as he starts anticipating the week ahead. Mr. B denies having a depressed mood or cognitive problems. When on vacation with his wife he has no trouble sleeping. He has no psychiatric family history or any substantial medical problems. He simply wishes that he could sleep on work nights.
We set up an alternating medication approach. Mr. B takes trazodone 100 mg on the first night and 150 mg on the second and third nights. He then takes triazolam 0.25 mg for 2 nights; previously, he had found that zolpidem did not work as well for maintaining sleep. He can sleep adequately for the 2 weekend nights, then restarts the alternating pattern. Mr. B has done well with this regimen for >10 years.
Continue to: CASE 2
CASE 2
Ms. C, age 60, is widowed and has a successful career as a corporate attorney. She has been anxious since early childhood and has had trouble falling asleep for much of her life. Once she falls asleep on her sofa—often between 1 and 2
Ms. C denies having depression, but experienced appropriate grief related to her husband’s illness and death from metastatic cancer 3 years ago. At the time, her internist prescribed escitalopram and zolpidem; escitalopram caused greater agitation and distress, so she stopped it after 10 days. Zolpidem 10 mg/d allowed her to sleep but she worried about taking it because her mother had long-standing sedative dependence. Ms. C lives alone, but her adult children live nearby, and she has a strong support system that includes colleagues at her firm, friends at her book club, and a support group for partners of cancer patients.
Ms. C tries trazodone, starting with 50 mg, but reports feeling agitated rather than sleepy and has cognitive fogginess in the morning. She is switched to quetiapine 50 mg, which she tolerates well and allows her to sleep soundly. To avoid developing tachyphylaxis with quetiapine, she takes eszopiclone 3 mg for 2 nights, alternating with quetiapine for 3 nights. This strategy allows her to reliably fall asleep by 11
CASE 3
Ms. D, age 55, is married with a long-standing diagnosis of generalized anxiety disorder (GAD), panic disorder, and depression so severe she is unable to work as a preschool teacher. She notes that past clinicians have prescribed a wide array of antidepressants and benzodiazepines but she remains anxious, agitated, and unable to sleep. She worries constantly about running out of benzodiazepines, which are “the only medication that helps me.” At the time of evaluation, her medications are venlafaxine ER 150 mg/d, lorazepam 1 mg 3 times daily and 2 mg at bedtime, and buspirone 15 mg 3 times daily, which she admits to not taking. She is overweight and does not exercise. She spends her days snacking and watching television. She can’t settle down enough to read and feels overwhelmed most of the time. Her adult children won’t allow her to babysit their young children because she dozes during the day.
Ms. D has a strong family history of psychiatric illness, including a father with bipolar I disorder and alcohol use disorder and a sister with schizoaffective disorder. Ms. D has never felt overtly manic, but has spent most of her life feeling depressed, anxious, and hopeless, and at times she has wished she was dead. She has had poor responses to many antidepressants, with transient euphoria followed by more anxiety.
Continue to: Rather than major depressive disorder...
Rather than major depressive disorder or GAD, Ms. D’s symptoms better meet the criteria for bipolar II disorder. She agrees to a slow taper of venlafaxine and a slow increase of divalproex, starting with 125 mg each evening. While taking venlafaxine 75 mg/d and divalproex 375 mg/d, she experiences distinct improvement in anxiety and agitation, which further improve after venlafaxine is stopped and divalproex is increased to 750 mg in the evening. She finds that she forgets daytime doses of lorazepam but depends on it to fall asleep. While taking quetiapine 50 mg and lorazepam 1 mg at bedtime, Ms. D reports sleeping soundly and feeling alert in the morning. Over several weeks, she tapers lorazepam slowly by 0.5 mg every 2 weeks. She finds she needs a higher dose of quetiapine to stay asleep, eventually requiring 400 mg each night. Ms. D says overall she feels better but is distressed because she has gained 25 lbs since starting divalproex and quetiapine.
To avoid further increases in quetiapine and maintain its sedating effect, Ms. D is switched to an alternating schedule of clonazepam 1.5 mg for 2 nights and quetiapine 300 mg for 3 nights. She agrees to begin exercising by walking in her neighborhood daily, and gradually increases this to 1 hour per day. After starting to exercise regularly, she finds she is oversedated by quetiapine at night, so she is gradually decreased to a dose of 150 mg, while still alternating with clonazepam 1.5 mg. Ms. D loses most of the weight she had gained and begins volunteering as a reading coach in the elementary school in her neighborhood.
Bottom Line
Patients with chronic insomnia can often maintain adequate sedation without developing tolerance to benzodiazepines or tachyphylaxis with nonsedating agents by using 2 sleep medications that have different mechanisms of action on an alternating schedule.
Related Resources
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2): 307-349. doi:10.5664/jcsm.6470
- Muppavarapu K, Muthukanagaraj M, Saeed SA. Cognitive-behavioral therapy for insomnia: a review of 8 studies. Current Psychiatry. 2020;19(9):40-46. doi:10.12788/cp.0040
Drug Brand Names
Alprazolam • Xanax
Armodafinil • Nuvigil
Atomoxetine • Strattera
Bupropion • Wellbutrin
Clonazepam • Klonopin
Diazepam • Valium
Divalproex • Depakote
Doxepin • Sinequan
Escitalopram • Lexapro
Eszopiclone • Lunesta
Lorazepam • Ativan
Lurasidone • Latuda
Methylphenidate • Concerta
Mirtazapine • Remeron
Modafinil • Provigil
Olanzapine • Zyprexa
Quetiapine • Seroquel
Temazepam • Restoril
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Zaleplon • Sonata
Zolpidem • Ambien
Patients with chronic insomnia that does not improve with nonpharmacologic techniques often develop tolerance to sedative medications (benzodiazepines) prescribed for nightly use. When nonbenzodiazepine medications are used, tachyphylaxis can develop and these medications no longer initiate or maintain sleep. Strategies that alternate between these 2 types of agents are simple to follow and may allow patients to maintain sensitivity to both types of medications. In this article, I review the types, causes, evaluation, and treatment of insomnia; describe an alternating medication strategy to help patients avoid developing tolerance/tachyphylaxis; and present 3 fictional case vignettes to illustrate this approach.
A common, troubling condition
Insomnia is a common problem among psychiatric patients. Approximately 30% to 50% of adults experience occasional, short-term (<3 months) insomnia, and 5% to 10% experience chronic (≥3 months) insomnia,1 with associated negative impacts on health and quality of life. Insomnia is sometimes primary and may have a hereditary component, but more often is associated with medical, neurologic, or psychiatric disorders.
Patterns of insomnia include difficulty falling asleep (initial or sleep-onset insomnia), remaining asleep (middle or sleep-maintenance insomnia), or falling back asleep after early awakening (late or sleep-offset insomnia). Sleep-onset insomnia correlates with high levels of anxiety and worrying, but once asleep, patients usually stay asleep. Sleep-maintenance problems involve multiple awakenings after falling asleep and taking hours to fall back to sleep. These patients experience inadequate sleep when they must wake up early for school or work. Early-awakening patients report feeling wide awake by 4 to 5
Caffeine is an important consideration for patients with sleep difficulties. Its use is widespread in much of the world, whether ingested as coffee, tea, in soft drinks, or in “energy” drinks that may contain as much as 200 mg of caffeine (twice the amount in a typical cup of brewed coffee). Caffeine may also be ingested as an ingredient of medications for headache or migraine. While some individuals maintain that they can fall asleep easily after drinking caffeinated coffee, many may not recognize the amount of caffeine they consume and its negative impact on sleep.2 Author Michael Pollan stopped use of all caffeine and reported on the surprising positive effect on his sleep.3
Patients with mood, anxiety, or psychotic disorders are likely to experience insomnia intermittently or chronically, and insomnia predisposes some individuals to develop mood and anxiety symptoms.4 Patients with insomnia often experience anxiety focused on a fear of not getting adequate sleep, which creates a vicious cycle in which hyperarousal associated with fear of not sleeping complicates other causes of insomnia. A patient’s chronotype (preference for the time of day in which they carry out activities vs sleeping) also may play a role in sleep difficulties (Box5).
Box
Chronotypes—the expression of circadian rhythmicity in an individual—have been studied extensively.5 Psychiatrists may encounter patients who sleep most of the day and stay awake at night, those who sleep up to 20 hours per day, and those who sleep <4 hours in 24 hours. Patients typically know which category they fall into. The early bird typically is awake by 6 or 7 am, remains alert through most of the day, and feels sleepy by 10 pm. The usual diurnal variation in cortisol, with peaks at 7 am and 7 pm and nadirs at 1 pm and 1 am, correspond with the early bird’s habits.
Night owls typically report feeling exhausted and irritable in the early morning; prefer to sleep past noon; feel energized around dark, when they can do their best studying, concentrating, etc; and do not feel sleepy until early morning. While this night owl pattern is a natural variation and not necessarily associated with psychiatric illness, patients with mood disorders frequently have chaotic sleep patterns that may not conform to a pattern. Night owls maintain the same diurnal pattern of cortisol secretion as early birds.
Certain medications may contribute to insomnia, particularly stimulants. It is important to understand and explain to patients the time frame during which immediate-release or extended-release (ER) stimulants are active, which varies in individuals depending on liver enzyme activity. Other commonly used psychotropic medications—including bupropion, modafinil, armodafinil, atomoxetine, amphetamine salts, and methylphenidate—may interfere with sleep if used later in the day.6
Patients typically do not mention their use of alcohol and/or marijuana unless asked. Those who are binge drinkers or alcohol-dependent may expect alcohol to help them fall asleep, but usually find their sleep is disrupted and difficult to maintain. Patients may use marijuana to help them sleep, particularly marijuana high in tetrahydrocannabinol (THC). While it may help with sleep initiation, THC can disrupt sleep maintenance. Cannabidiol does not have intrinsic sedating effects and may even interfere with sleep.7,8
Continue to: Women may be more likely...
Women may be more likely than men to experience insomnia.9 The onset of menopause can bring hot flashes that interfere with sleep.
Women with a history of mood disorders are more likely to have a history of premenstrual dysphoric disorder, postpartum depression, and unusual responses to oral contraceptives.10 These women are more likely to report problems with mood, energy, and sleep at perimenopause. Treatment with estrogen replacement may be an option for women without risk factors, such as clotting disorders, smoking history, or a personal or family history of breast or uterine cancer. For many who are not candidates for or who refuse estrogen replacement, use of a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor at low doses may help with vasomotor symptoms but not with insomnia.
Insomnia symptoms typically increase with age.11 When sleep is adequate early in life but becomes a problem in midlife, an individual’s eating habits, obesity, and lack of exercise may be contributing factors. The typical American diet includes highly refined carbohydrates with excess salt; such foods are often readily available to the exclusion of healthy options. Overweight and obese patients may insist they eat a healthy diet with 3 meals per day, but a careful history often uncovers nighttime binge eating. Nighttime binge eating is rarely reported. This not only maintains obesity, but also interferes with sleep, since patients stay up late to avoid discovery by family members.12 This lack of sleep can lead to an endless loop because insufficient sleep is a risk factor for obesity.13
Evaluating sleep difficulties
New patient evaluations should include a careful history beginning with childhood, including personal early childhood history and family psychiatric history. Patients often report the onset of sleep difficulty and anxiety during childhood, which should raise further questions about aspects of mood regulation from early life such as concentration, energy, motivation, appetite, and academic performance. While many children and adolescents are diagnosed with attention-deficit/hyperactivity disorder due to concentration problems that cause difficulties at school, be aware this might be part of a syndrome related to mood regulation.14 Unexpected responses to an SSRI—such as agitation, euphoria, or an immediate response with the first dose—should also raise suspicion of a mood disorder. Once the underlying mood disorder is stabilized, many patients report improved sleep.15
If a patient reports having difficulty falling and remaining asleep but is not sure if there is a pattern, keeping a sleep diary can help. Further questioning may uncover the cause. Does the patient have spontaneous jerks of lower extremities (restless leg syndrome) that interfere with falling asleep or wake them up? Have they noticed problems with dreams/nightmares that wake them, which could be associated with posttraumatic stress, anxiety, or depression? Have they been told by a partner that they act out dreams or are seemingly awake but not responsive, which could point to REM sleep behavior disorder or early Parkinson’s disease? Referral to a sleep laboratory and a neurologist can help establish the correct diagnosis and point to appropriate treatment.16-18
Treatment options
Several cognitive-behavioral techniques, including cognitive-behavioral therapy for insomnia (CBT-I), yogic breathing, progressive relaxation, mindfulness meditation, and sleep hygiene techniques may help considerably,19,20 but insomnia often remains difficult to treat. Pharmacotherapy is not necessarily more effective than nonpharmacologic approaches. Both options require the patient to take initiative to either find nonpharmacologic approaches or discuss the problem with a physician and agree to take medication.21 A trial comparing CBT-I to sedatives or the combination of CBT-I plus sedatives found higher rates of sleep with CBT-I for 3 months, after which improvement fluctuated; the combination showed sustained improvement for the entire 6-month trial.22 CBT-I has also been shown to be as effective with patients who do not have psychiatric illness as for those who are depressed, anxious, or stressed.23 However, behavioral techniques that require regular practice may be difficult for individuals to maintain, particularly when they are depressed or anxious.
Continue to: Clinicians should understand...
Clinicians should understand the distinctions among the various types of pharmacotherapy for insomnia. Sedative-hypnotics include medications with varying half-lives and metabolic pathways. Short-acting benzodiazepines such as triazolam or alprazolam and the “z-drugs” zolpidem or zaleplon may help initiate sleep in patients with sleep-onset insomnia. Longer-acting benzodiazepines such as diazepam, clonazepam, or temazepam and the z-drug eszopiclone may also help with sleep maintenance.23 Based on my clinical experience, individual patients may respond better to 1 type of medication over another, or even to different agents within the same class of sedative-hypnotics.
Some clinicians prescribe nonbenzodiazepine medications for sleep, such as doxepin (which is FDA-approved for treating insomnia) or off-label trazodone, mirtazapine, or quetiapine. Their antihistaminic properties confer sedating effects. Virtually all over-the-counter (OTC) medications for insomnia are antihistaminic. These OTC medications are not designed to treat insomnia, and the optimal dosage to maintain sleep without daytime sedation must be determined by trial and error. Sedating nonbenzodiazepine medications may be slowly absorbed if taken at bedtime (depending on whether they are taken with or without food) and cause daytime sedation and cognitive slowness in patients with sleep-onset and maintenance insomnia who must wake up early. Starting trazodone at 50 to 75 mg may cause slow metabolizers to wake up with considerable sedation, while fast metabolizers might never feel soundly asleep.24
Patients with mood and anxiety disorders that complicate insomnia are often prescribed second-generation antipsychotics such as quetiapine, lurasidone, or olanzapine, which are sedating as well as mood-stabilizing. These approaches require careful attention to titrating doses and timing their use.
Problems with pharmacotherapy
When either benzodiazepines or nonbenzodiazepine medications are used on a long-standing, nightly basis, they often stop working well. It is not unusual that after days to weeks of taking a benzodiazepine, patients find they no longer stay asleep but can’t fall asleep if they don’t take them. Once tolerance develops, the individual experiences pharmacologic withdrawal with an inability to fall asleep or stay asleep. The medication becomes necessary but ineffective, and many patients increase their use to higher doses to fall asleep, and sometimes in early morning to maintain sleep. This leads to negative effects on cognition, coordination/balance, and mood during the day, especially in older patients.
Nonbenzodiazepine sedating medications do not lead to pharmacologic tolerance but do lead to tachyphylaxis as the CNS attempts to downregulate sedation to keep the organism safe. For some patients, this happens quickly, within a matter of days.25 Others increase doses to stay asleep. For example, a patient with a starting dose of trazodone 75 mg/d might increase the dosage to 300 mg/d. While trazodone is approved in doses of 300 to 600 mg as an antidepressant, it is preferable to keep doses lower when used only for sedation.
Continue to: An alternating medication strategy
An alternating medication strategy
Alternating between medications from different classes can help patients avoid developing tolerance with benzodiazepines or tachyphylaxis as occurs with antihistaminic medications. It can be effective for patients with primary insomnia as well as for those whose sleep problems are associated with mood or anxiety disorders. Patients typically maintain sensitivity to any form of pharmacologic sedation for several nights without loss of effect but need to take a break to maintain the sedation effect. For example, in 1 case study, a 30-year-old female who rapidly developed tachyphylaxis to the sedative action of mirtazapine experienced a return of the medication’s sedative effects after taking a 3-day break.25
To initiate an alternating strategy, the clinician must first help the patient establish a sedating dose of 2 medications from different classes, such as trazodone and zolpidem, and then instruct the patient to use each for 2 to 3 consecutive nights on an alternating basis. Patients can use calendars or pillboxes to avoid confusion about which medication to take on a given night. In many cases, this approach can work indefinitely.
The following 3 case vignettes illustrate how this alternating medication strategy can work.
CASE 1
Mr. B, age 58, is a married salesman whose territory includes 3 states. He drives from client to client from Monday through Thursday each week, staying overnight in hotels. He is comfortable talking to clients, has a close and supportive relationship with his wife, and enjoys socializing with friends. Mr. B has a high level of trait anxiety and perfectionism and is proud of his sales record throughout his career, but this leads to insomnia during his nights on the road, and often on Sunday night as he starts anticipating the week ahead. Mr. B denies having a depressed mood or cognitive problems. When on vacation with his wife he has no trouble sleeping. He has no psychiatric family history or any substantial medical problems. He simply wishes that he could sleep on work nights.
We set up an alternating medication approach. Mr. B takes trazodone 100 mg on the first night and 150 mg on the second and third nights. He then takes triazolam 0.25 mg for 2 nights; previously, he had found that zolpidem did not work as well for maintaining sleep. He can sleep adequately for the 2 weekend nights, then restarts the alternating pattern. Mr. B has done well with this regimen for >10 years.
Continue to: CASE 2
CASE 2
Ms. C, age 60, is widowed and has a successful career as a corporate attorney. She has been anxious since early childhood and has had trouble falling asleep for much of her life. Once she falls asleep on her sofa—often between 1 and 2
Ms. C denies having depression, but experienced appropriate grief related to her husband’s illness and death from metastatic cancer 3 years ago. At the time, her internist prescribed escitalopram and zolpidem; escitalopram caused greater agitation and distress, so she stopped it after 10 days. Zolpidem 10 mg/d allowed her to sleep but she worried about taking it because her mother had long-standing sedative dependence. Ms. C lives alone, but her adult children live nearby, and she has a strong support system that includes colleagues at her firm, friends at her book club, and a support group for partners of cancer patients.
Ms. C tries trazodone, starting with 50 mg, but reports feeling agitated rather than sleepy and has cognitive fogginess in the morning. She is switched to quetiapine 50 mg, which she tolerates well and allows her to sleep soundly. To avoid developing tachyphylaxis with quetiapine, she takes eszopiclone 3 mg for 2 nights, alternating with quetiapine for 3 nights. This strategy allows her to reliably fall asleep by 11
CASE 3
Ms. D, age 55, is married with a long-standing diagnosis of generalized anxiety disorder (GAD), panic disorder, and depression so severe she is unable to work as a preschool teacher. She notes that past clinicians have prescribed a wide array of antidepressants and benzodiazepines but she remains anxious, agitated, and unable to sleep. She worries constantly about running out of benzodiazepines, which are “the only medication that helps me.” At the time of evaluation, her medications are venlafaxine ER 150 mg/d, lorazepam 1 mg 3 times daily and 2 mg at bedtime, and buspirone 15 mg 3 times daily, which she admits to not taking. She is overweight and does not exercise. She spends her days snacking and watching television. She can’t settle down enough to read and feels overwhelmed most of the time. Her adult children won’t allow her to babysit their young children because she dozes during the day.
Ms. D has a strong family history of psychiatric illness, including a father with bipolar I disorder and alcohol use disorder and a sister with schizoaffective disorder. Ms. D has never felt overtly manic, but has spent most of her life feeling depressed, anxious, and hopeless, and at times she has wished she was dead. She has had poor responses to many antidepressants, with transient euphoria followed by more anxiety.
Continue to: Rather than major depressive disorder...
Rather than major depressive disorder or GAD, Ms. D’s symptoms better meet the criteria for bipolar II disorder. She agrees to a slow taper of venlafaxine and a slow increase of divalproex, starting with 125 mg each evening. While taking venlafaxine 75 mg/d and divalproex 375 mg/d, she experiences distinct improvement in anxiety and agitation, which further improve after venlafaxine is stopped and divalproex is increased to 750 mg in the evening. She finds that she forgets daytime doses of lorazepam but depends on it to fall asleep. While taking quetiapine 50 mg and lorazepam 1 mg at bedtime, Ms. D reports sleeping soundly and feeling alert in the morning. Over several weeks, she tapers lorazepam slowly by 0.5 mg every 2 weeks. She finds she needs a higher dose of quetiapine to stay asleep, eventually requiring 400 mg each night. Ms. D says overall she feels better but is distressed because she has gained 25 lbs since starting divalproex and quetiapine.
To avoid further increases in quetiapine and maintain its sedating effect, Ms. D is switched to an alternating schedule of clonazepam 1.5 mg for 2 nights and quetiapine 300 mg for 3 nights. She agrees to begin exercising by walking in her neighborhood daily, and gradually increases this to 1 hour per day. After starting to exercise regularly, she finds she is oversedated by quetiapine at night, so she is gradually decreased to a dose of 150 mg, while still alternating with clonazepam 1.5 mg. Ms. D loses most of the weight she had gained and begins volunteering as a reading coach in the elementary school in her neighborhood.
Bottom Line
Patients with chronic insomnia can often maintain adequate sedation without developing tolerance to benzodiazepines or tachyphylaxis with nonsedating agents by using 2 sleep medications that have different mechanisms of action on an alternating schedule.
Related Resources
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2): 307-349. doi:10.5664/jcsm.6470
- Muppavarapu K, Muthukanagaraj M, Saeed SA. Cognitive-behavioral therapy for insomnia: a review of 8 studies. Current Psychiatry. 2020;19(9):40-46. doi:10.12788/cp.0040
Drug Brand Names
Alprazolam • Xanax
Armodafinil • Nuvigil
Atomoxetine • Strattera
Bupropion • Wellbutrin
Clonazepam • Klonopin
Diazepam • Valium
Divalproex • Depakote
Doxepin • Sinequan
Escitalopram • Lexapro
Eszopiclone • Lunesta
Lorazepam • Ativan
Lurasidone • Latuda
Methylphenidate • Concerta
Mirtazapine • Remeron
Modafinil • Provigil
Olanzapine • Zyprexa
Quetiapine • Seroquel
Temazepam • Restoril
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Zaleplon • Sonata
Zolpidem • Ambien
1. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
2. Drake C, Roehrs T, Shambroom J, et al. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195-1200.
3. Pollan M. Caffeine: How Coffee and Tea Created the Modern World. 2023; Audible Audiobooks.
4. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021:17:2549-2566.
5. Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405-415.
6. Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509-517.
7. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):23.
8. Monti JM, Pandi-Perumal SR. Clinical management of sleep and sleep disorders with cannabis and cannabinoids: implications to practicing psychiatrists. Clin Neuropharmacol. 2022;45(2):27-31.
9. Dockray S, Steptoe A. Chronotype and diurnal cortisol profile in working women: differences between work and leisure days. Psychoneuroendocrinology. 2011;36(5):649-655.
10. Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102-S108.
11. Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):59-66.
12. Stunkard A. Eating disorders and obesity. Psychiatr Clin North Am. 2011; 34(4):765-771.
13. Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. 2016;29(6):409-412.
14. Gillberg C, Gillberg IC, Rasmussen P, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004; 1(Suppl 1):i80-i92.
15. Goldberg JF, Nierenberg AA, Iosifescu DV. Wrestling with antidepressant use in bipolar disorder: the ongoing debate. J Clin Psychiatry. 2021;82(1):19. doi:10.4088/JCP.19ac13181
16. Baltzan M, Yao C, Rizzo D, et al. Dream enactment behavior: review for the clinician. J Clin Sleep Med. 2020;16(11):1949-1969.
17. Barone DA. Dream enactment behavior—a real nightmare: a review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J Clin Sleep Med. 2020;16(11):1943-1948.
18. Figorilli M, Meloni M, Lanza G, et al. Considering REM sleep behavior disorder in the management of Parkinson’s disease. Nat Sci Sleep. 2023;15:333-352.
19. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281-297.
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139.
21. Lu M, Zhang Y, Zhang J, et al. Comparative effectiveness of digital cognitive behavioral therapy vs. medication therapy among patients with insomnia. JAMA Network Open. 2023;6(4):e237597.
22. Sweetman A, McEvoy RD, Catcheside PG, et al. Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2021;17(3):545-554.
23. O’Brien CP. Benzodiazepine use, abuse and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28-33.
24. Wichniak A, Wierzbicka AE, Jarema M. Treatment of insomnia - effect of trazodone and hypnotics on sleep. Psychiatr Pol. 2021;55(4):743-755.
25. Papazisis G, Siafis S, Tzachanis D. Tachyphylaxis to the sedative action of mirtazapine. Am J Case Rep. 2018;19:410-412.
1. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
2. Drake C, Roehrs T, Shambroom J, et al. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195-1200.
3. Pollan M. Caffeine: How Coffee and Tea Created the Modern World. 2023; Audible Audiobooks.
4. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021:17:2549-2566.
5. Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405-415.
6. Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509-517.
7. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):23.
8. Monti JM, Pandi-Perumal SR. Clinical management of sleep and sleep disorders with cannabis and cannabinoids: implications to practicing psychiatrists. Clin Neuropharmacol. 2022;45(2):27-31.
9. Dockray S, Steptoe A. Chronotype and diurnal cortisol profile in working women: differences between work and leisure days. Psychoneuroendocrinology. 2011;36(5):649-655.
10. Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102-S108.
11. Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):59-66.
12. Stunkard A. Eating disorders and obesity. Psychiatr Clin North Am. 2011; 34(4):765-771.
13. Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. 2016;29(6):409-412.
14. Gillberg C, Gillberg IC, Rasmussen P, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004; 1(Suppl 1):i80-i92.
15. Goldberg JF, Nierenberg AA, Iosifescu DV. Wrestling with antidepressant use in bipolar disorder: the ongoing debate. J Clin Psychiatry. 2021;82(1):19. doi:10.4088/JCP.19ac13181
16. Baltzan M, Yao C, Rizzo D, et al. Dream enactment behavior: review for the clinician. J Clin Sleep Med. 2020;16(11):1949-1969.
17. Barone DA. Dream enactment behavior—a real nightmare: a review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J Clin Sleep Med. 2020;16(11):1943-1948.
18. Figorilli M, Meloni M, Lanza G, et al. Considering REM sleep behavior disorder in the management of Parkinson’s disease. Nat Sci Sleep. 2023;15:333-352.
19. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281-297.
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139.
21. Lu M, Zhang Y, Zhang J, et al. Comparative effectiveness of digital cognitive behavioral therapy vs. medication therapy among patients with insomnia. JAMA Network Open. 2023;6(4):e237597.
22. Sweetman A, McEvoy RD, Catcheside PG, et al. Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2021;17(3):545-554.
23. O’Brien CP. Benzodiazepine use, abuse and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28-33.
24. Wichniak A, Wierzbicka AE, Jarema M. Treatment of insomnia - effect of trazodone and hypnotics on sleep. Psychiatr Pol. 2021;55(4):743-755.
25. Papazisis G, Siafis S, Tzachanis D. Tachyphylaxis to the sedative action of mirtazapine. Am J Case Rep. 2018;19:410-412.
Adult ADHD: 6 studies of nonpharmacologic interventions
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).
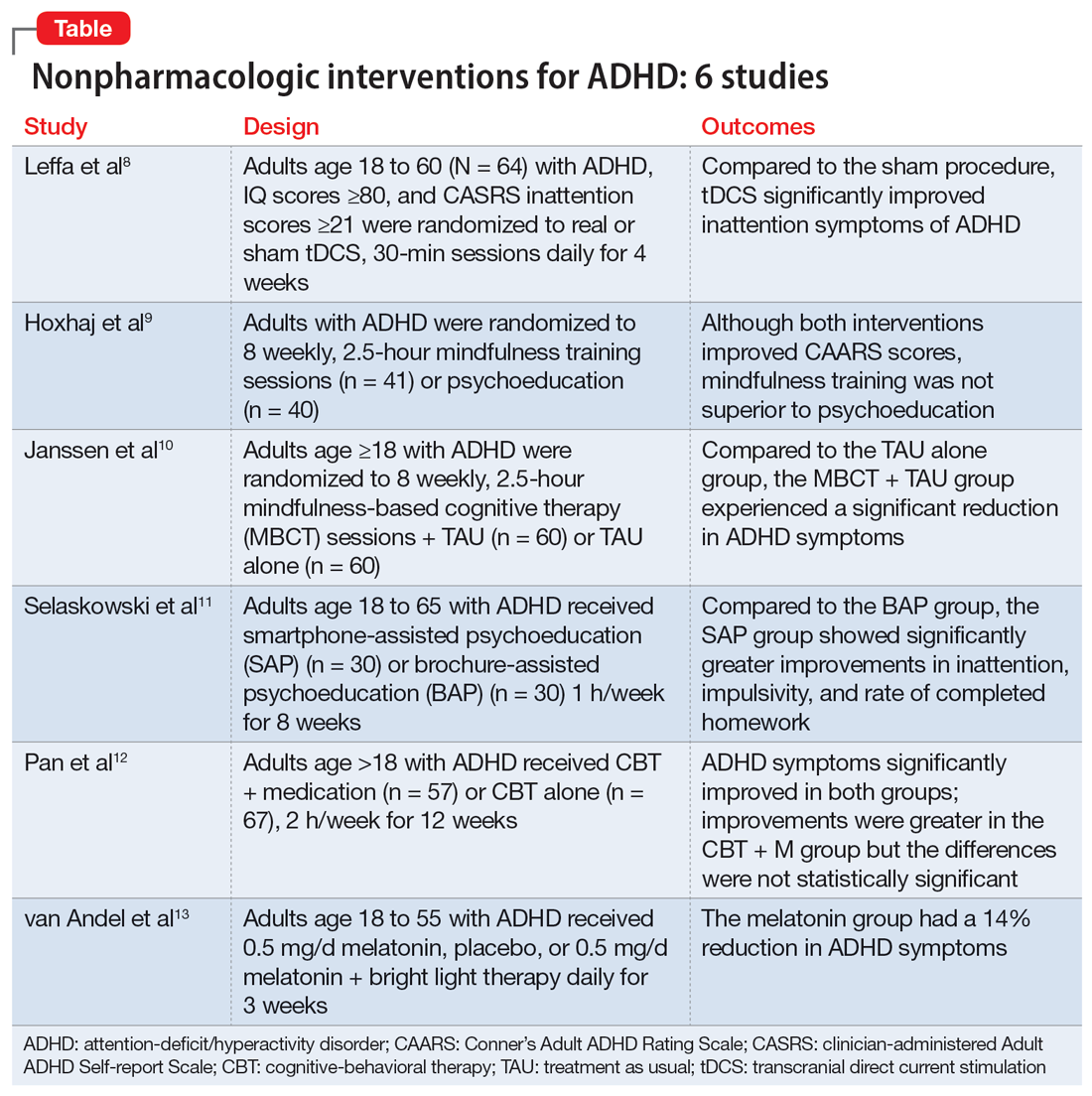
1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).

1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).

1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
Neuropsychiatric aspects of Parkinson’s disease: Practical considerations
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).
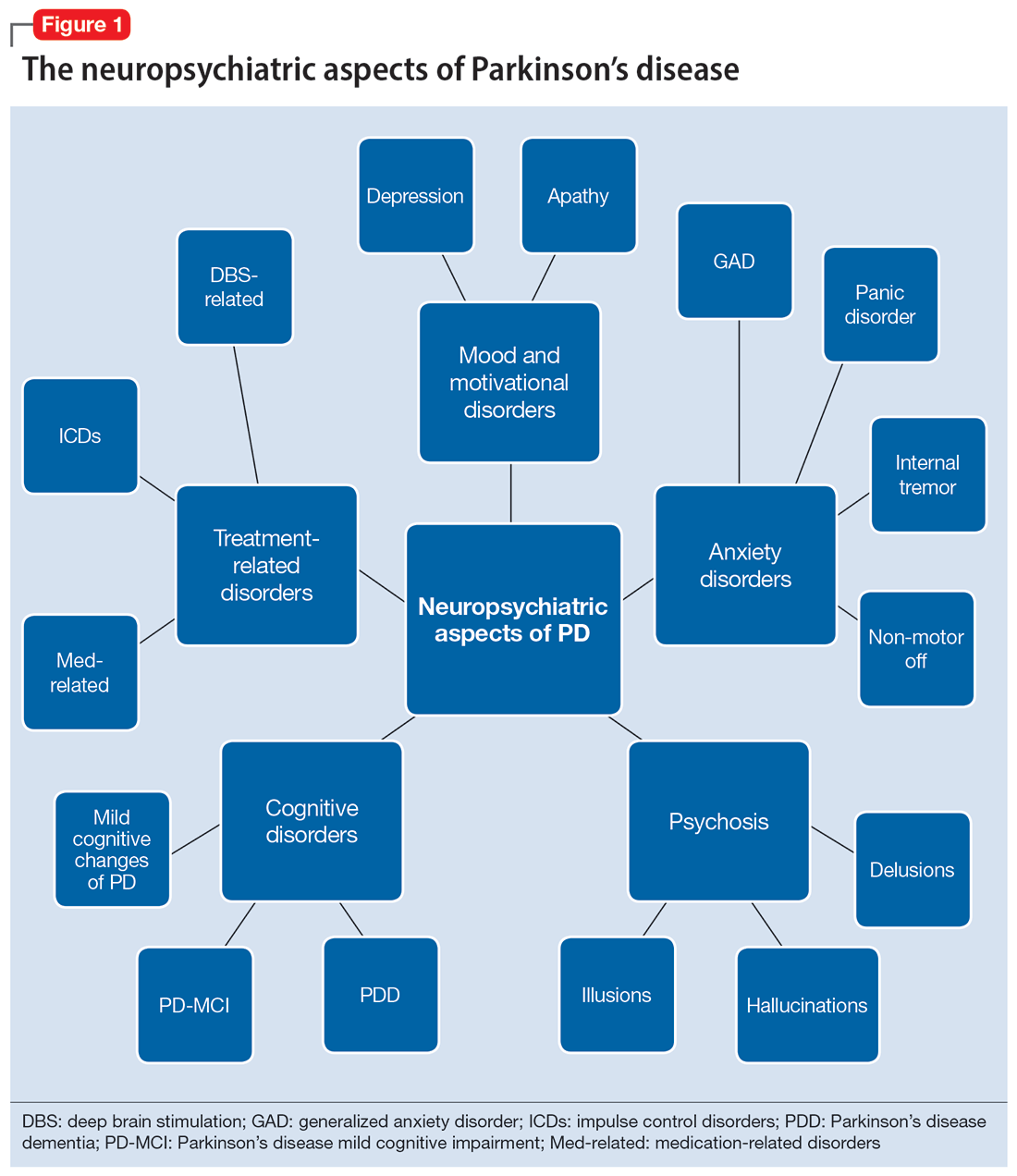
Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32
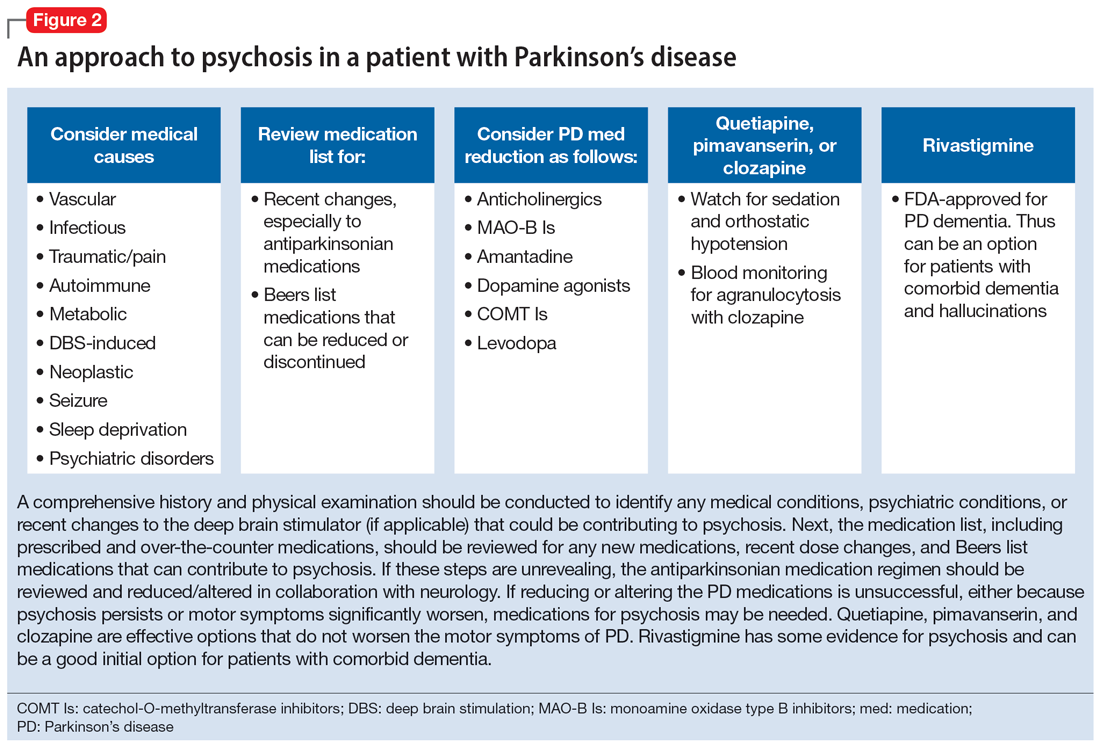
Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36
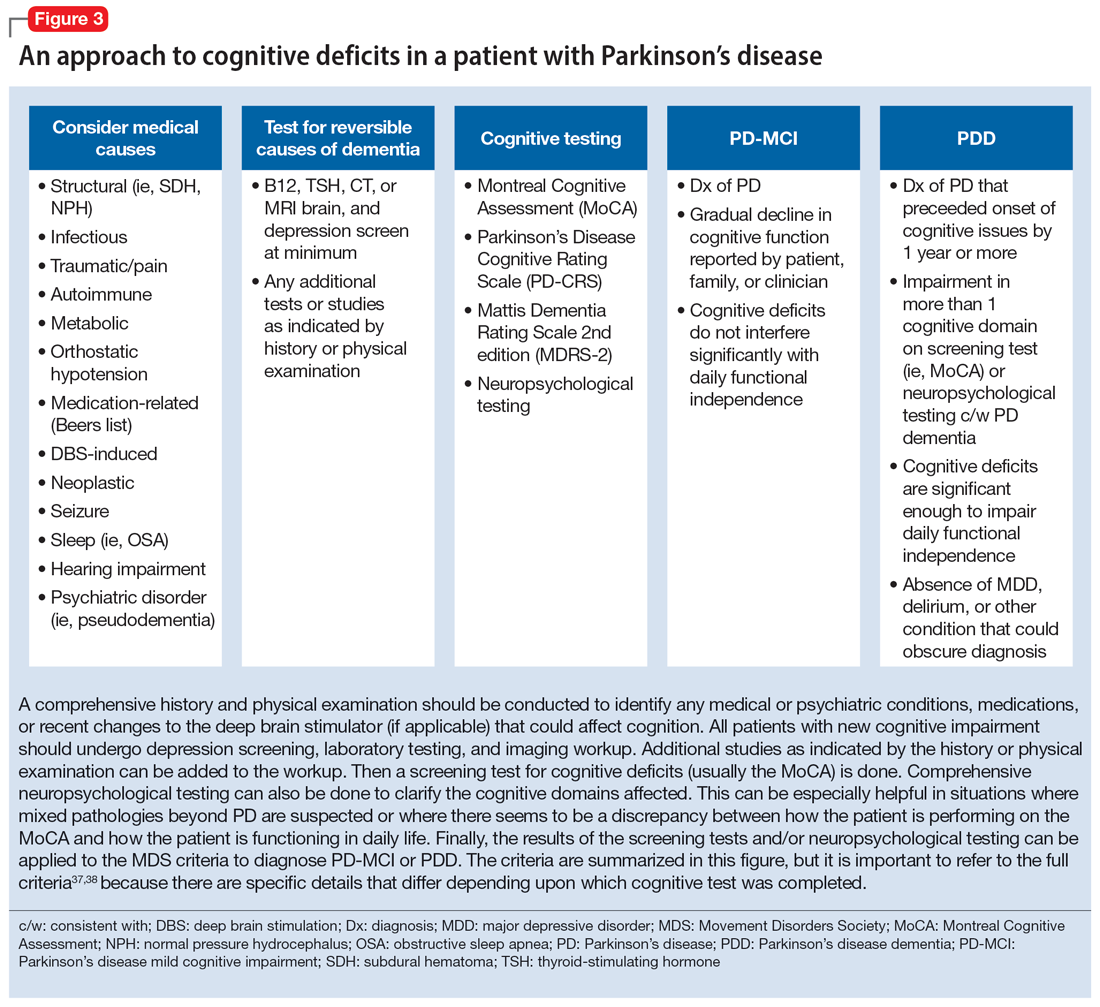
Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42
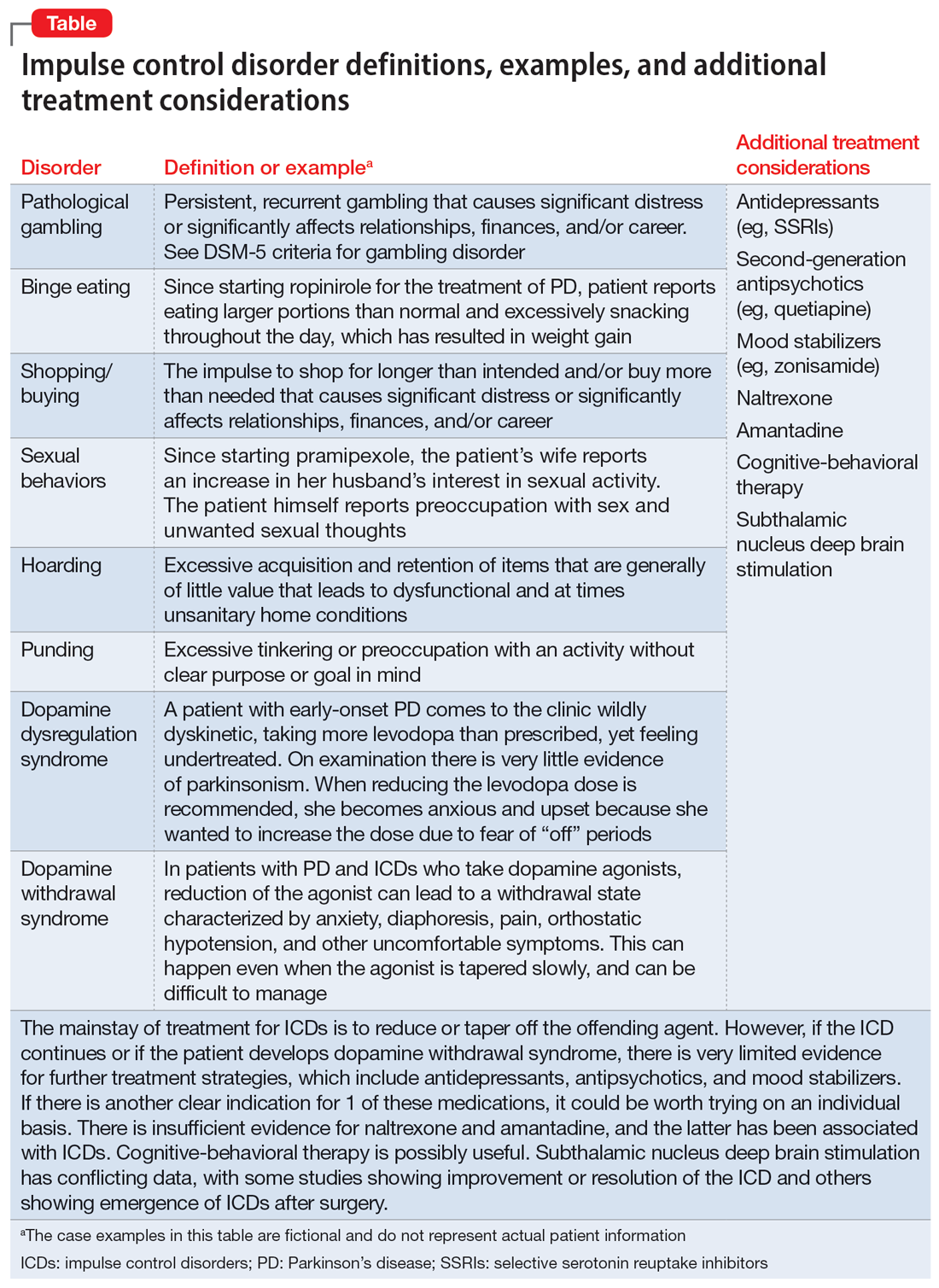
The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).

Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32

Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36

Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42

The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).

Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32

Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36

Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42

The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
ADHD in older adults: A closer look
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).
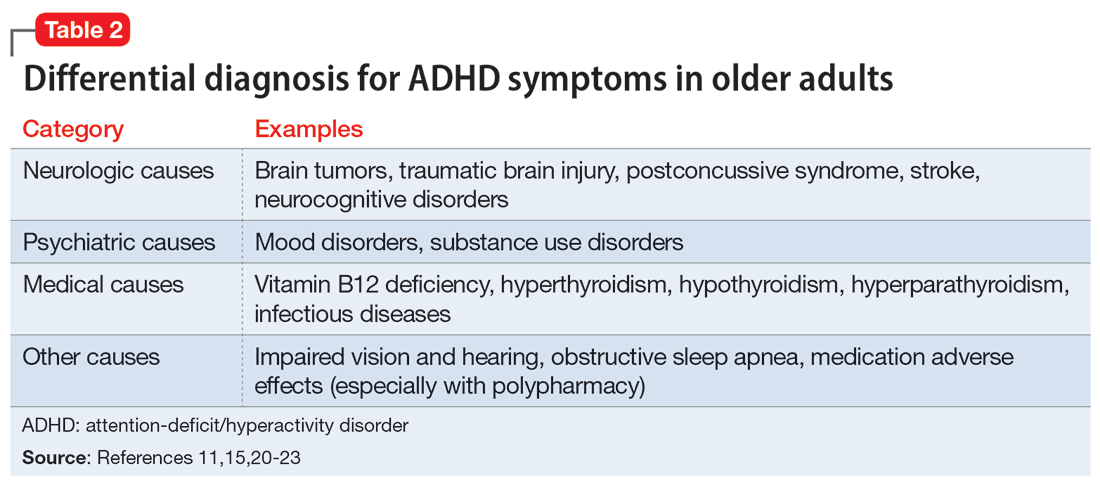
In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21
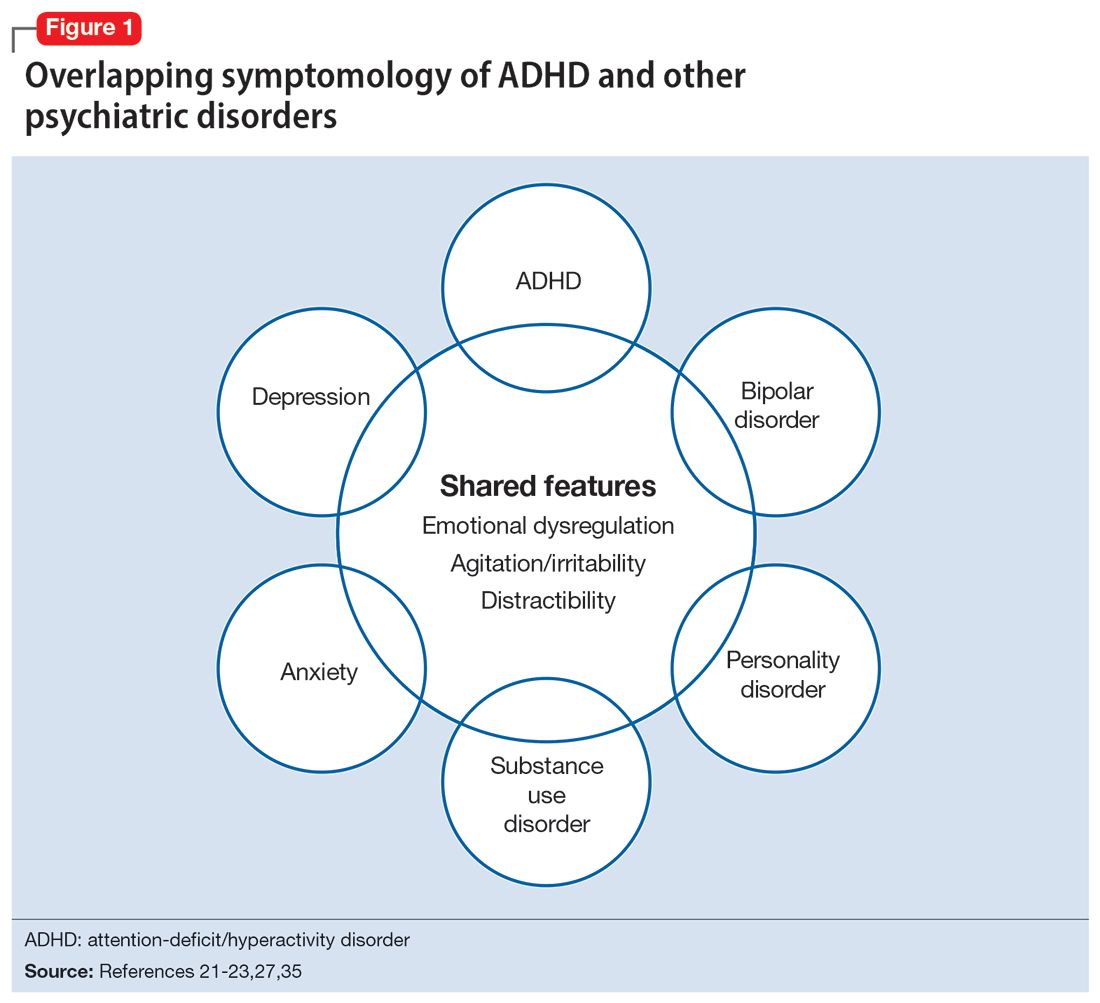
Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.
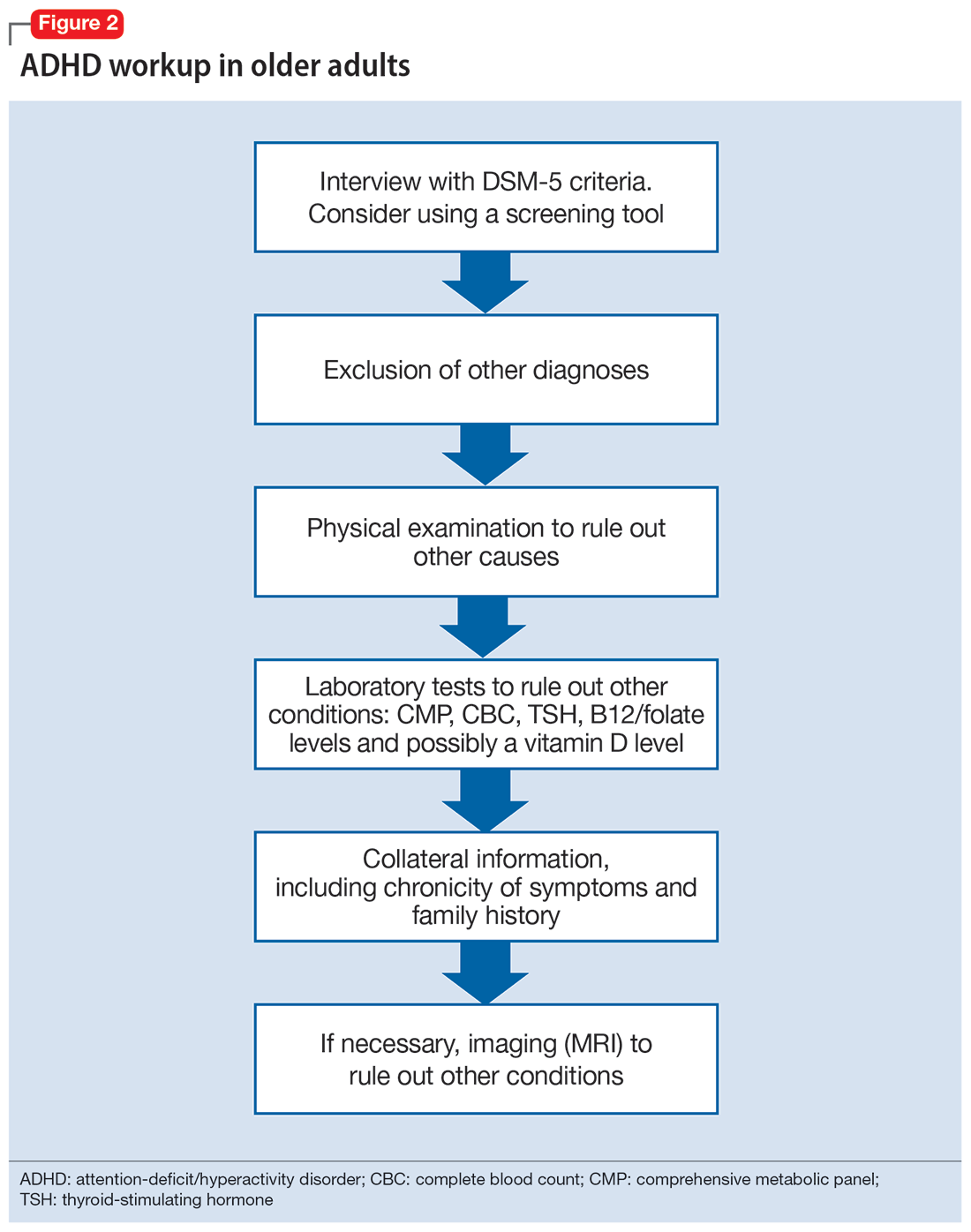
Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).

In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21

Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.

Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).

In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21

Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.

Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
Climate change and mental illness: What psychiatrists can do
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).
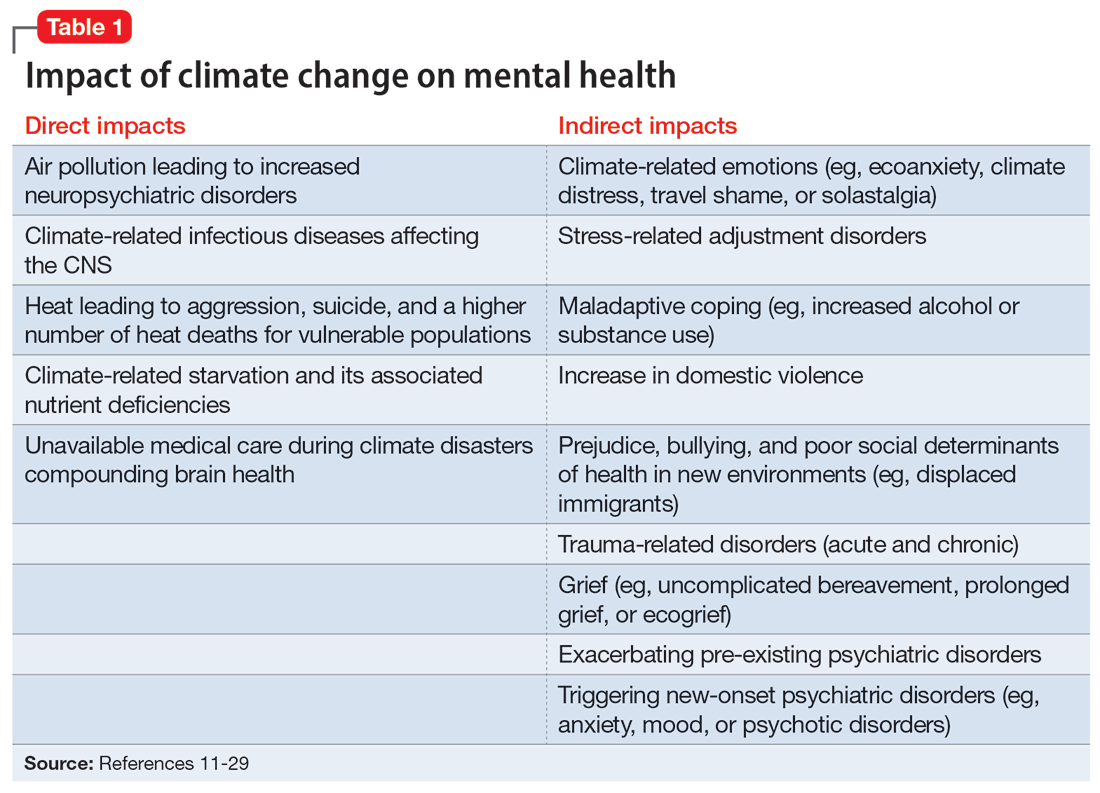
Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).
Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).

Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).

Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).

Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).

Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658