User login
An Official Publication of the Society of Hospital Medicine
Copyright © by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-5606


Clinical Progress Note: Rhythm Control for Patients With Atrial Fibrillation
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
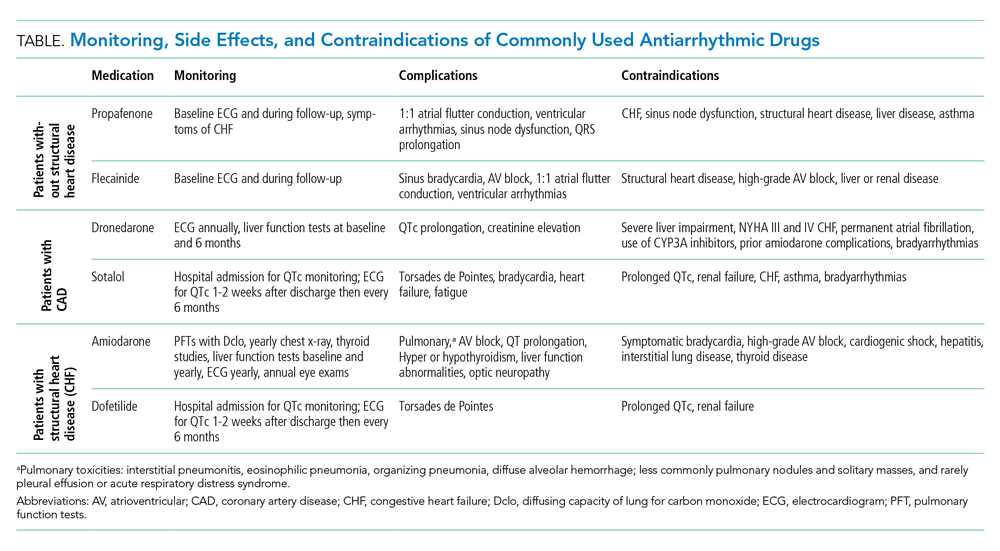
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3
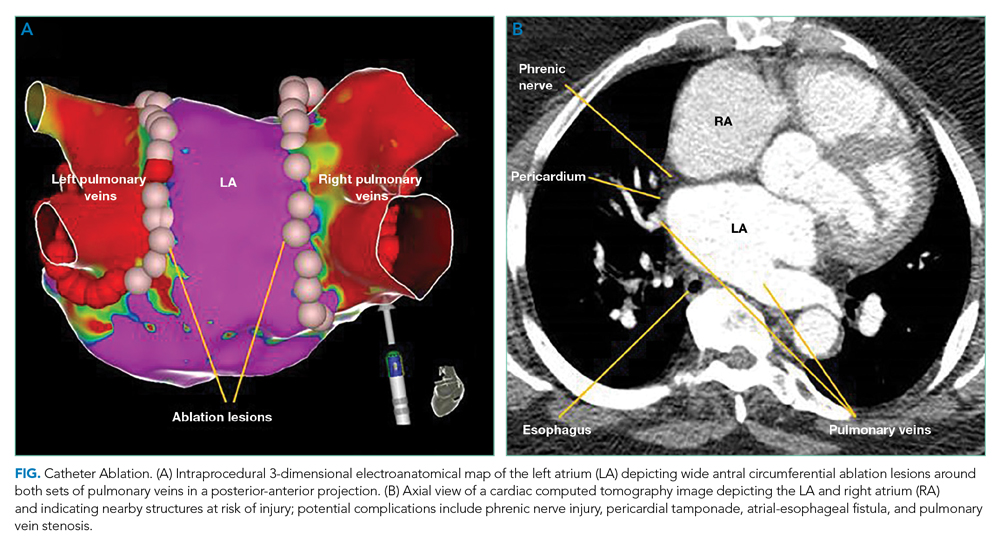
Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7
Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3

Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7

Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3

Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7

Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
© 2021 Society of Hospital Medicine
Beyond a Purple Journal: Improving Hospital-Based Addiction Care
Rosa* was one of my first patients as an intern rotating at the county hospital. Her marriage had disintegrated years earlier. To cope with depression, she hid a daily ritual of orange juice and vodka from her children. She worked as a cashier, until nausea and fatigue overwhelmed her.
The first time I met her she sat on the gurney: petite, tanned, and pregnant. Then I saw her yellow eyes and revised: temporal wasting, jaundiced, and swollen with ascites. Rosa didn’t know that alcohol could cause liver disease. Without insurance or access to primary care, her untreated alcohol use disorder (AUD) and depression had snowballed for years.
Midway through my intern year, I’d taken care of many people with AUD. However, I’d barely learned anything about it as a medical student, though we’d spent weeks studying esoteric diseases, that now––9 years after medical school––I still have not encountered.
Among the 28.3 million individuals in the United States with AUD, only 1% receive medication treatment.1 In the United States, unhealthy alcohol use accounts for more than 95,000 deaths each year.2 This number likely under-captures alcohol-related mortality and is higher now given recent reports of increasing alcohol-related deaths and prevalence of unhealthy alcohol use, especially among women, younger age groups, and marginalized populations.3-5
Rosa had alcohol-related hepatitis, which can cause severe inflammation and liver failure and quickly lead to death. As her liver failure progressed, I asked the gastroenterologists, “What other treatments can we offer? Is she a liver transplant candidate?” “Nothing” and “No” they answered.
Later, I emailed the hepatologist and transplant surgeon begging them to reevaluate her transplantation candidacy, but they told me there was no exception to the institution’s 6-month sobriety rule.
Maintaining a 6-month sobriety period is not an evidence-based criterion for transplantation. However, 50% of transplant centers do not perform transplantation prior to 6 months of alcohol abstinence for alcohol-related hepatitis due to concern for return to drinking after transplant.6 This practice may promote bias in patient selection for transplantation. A recent study found that individuals with alcohol-related liver disease transplanted before 6 months of abstinence had similar rates of survival and return to drinking compared to those who abstained from alcohol for 6 months and participated in AUD treatment before transplantation.7
There are other liver transplant practices that result in inequities for individuals with substance use disorders (SUD). Some liver transplant centers consider being on a medication for opioid use disorder a contraindication for transplantation—even if the individual is in recovery and abstaining from substances.8 Others mandate that individuals with alcohol-related liver disease attend Alcoholics Anonymous (AA) meetings prior to transplant. While mutual help groups, including AA, may benefit some individuals, different approaches work for different people.9 Other psychosocial interventions (eg, cognitive-behavioral therapy, contingency management, and residential treatment) and medications also help individuals reduce or stop drinking. Some meet their goals without any treatment. Addiction care works best when it respects autonomy and meets individuals where they are by allowing them to decide among options.
While organ allocations are a crystalized example of inequities in addiction care, they are also ethically complex. Many individuals—with and without SUD—die on waiting lists and must meet stringent transplantation criteria. However, we can at least remove the unnecessary biases that compound inequities in care people with SUD already face.
As Rosa’s liver succumbed, her kidneys failed too, and she required dialysis. She sensed what was coming. “I want everything…for now. I need to take care of my children.” I, too, wanted Rosa to live and see her youngest start kindergarten.
A few days before her discharge, I walked to the pharmacy and bought a purple journal. In a rare moment, I found Rosa alone in her room, without her ex-husband, sister, and mother, who rarely left her bedside. Together, we called AA and explored whether she could start participating in phone meetings from the hospital. I explained that one way to document a commitment to sobriety, as the transplant center’s rules dictated, was to attend and document AA meetings in this notebook. “In 5 months, you will be a liver transplant candidate,” I remember saying, wishing it to fruition.
I became Rosa’s primary care physician and saw her in clinic. Over the next few weeks, her skin took on an ashen tone. Sleep escaped her and her thoughts and speech blurred. Her walk slowed and she needed a wheelchair. The quiet fierceness that had defined her dissipated as encephalopathy took over. But until our last visit, she brought her purple journal, tracking the AA meetings she’d attended. Dialysis became intolerable, but not before Rosa made care arrangements for her girls. When that happened, she stopped dialysis and went to Mexico, where she died in her sleep after saying good-bye to her father.
Earlier access to healthcare and effective depression and AUD treatment could have saved Rosa’s life. While it was too late for her, as hospitalists we care for many others with substance-related complications and may miss opportunities to discuss and offer evidence-based addiction treatment. For example, we initiate the most up-to-date management for a patient’s gastrointestinal bleed but may leave the alcohol discussion for someone else. It is similar for other SUD: we treat cellulitis, epidural abscesses, bacteremia, chronic obstructive pulmonary disease, heart failure exacerbations, and other complications of SUD without addressing the root cause of the hospitalization—other than to prescribe abstinence from substance use or, at our worst, scold individuals for continuing to use.
But what can we offer? Most healthcare professionals still do not receive addiction education during training. Without tools, we enact temporizing measures, until patients return to the hospital or die.
In addition to increasing alcohol-related morbidity, there have also been increases in drug-related overdoses, fueled by COVID-19, synthetic opioids like fentanyl, and stimulants.10 In the 12-month period ending April 2021, more than 100,000 individuals died of drug-related overdoses, the highest number of deaths ever recorded in a year.11 Despite this, most healthcare systems remain unequipped to provide addiction services during hospitalization due to inadequate training, stigma, and lack of systems-based care.
Hospitalists and healthcare systems cannot be bystanders amid our worsening addiction crisis. We must empower clinicians with addiction education and ensure health systems offer evidence-based SUD services.
Educational efforts can close the knowledge gaps for both medical students and hospitalists. Medical schools should include foundational curricular content in screening, assessing, diagnosing, and treating SUD in alignment with standards set by the Liaison Committee on Medical Education, which accredits US medical schools. Residency programs can offer educational conferences, cased-based discussions, and addiction medicine rotations. Hospitalists can participate in educational didactics and review evidence-based addiction guidelines.12,13 While the focus here is on hospitalists, clinicians across practice settings and specialties will encounter patients with SUD, and all need to be well-versed in the diagnosis and treatment of addiction given the all-hands-on deck approach necessary amidst our worsening addiction crisis.
With one in nine hospitalizations involving individuals with SUD, and this number quickly rising, and with an annual cost to US hospitals of $13.2 billion, healthcare system leaders must invest in addiction care.14,15 Hospital-based addiction services could pay for themselves and save healthcare systems money while improving the patient and clinician experience.16One way to implement hospital-based addiction care is through an addiction consult team (ACT).17 While ACT compositions vary, most are interprofessional, offer evidence-based addiction treatment, and connect patients to community care.18 Our hospital’s ACT has nurses, patient navigators, and physicians who assess, diagnose, and treat SUD, and arrange follow-up addiction care.19 In addition to caring for individual patients, our ACT has led systems change. For example, we created order sets to guide clinicians, added medications to our hospital formulary to ensure access to evidence-based addiction treatment, and partnered with community stakeholders to streamline care transitions and access to psychosocial and medication treatment. Our team also worked with hospital leadership, nursing, and a syringe service program to integrate hospital harm reduction education and supply provision. Additionally, we are building capacity among staff, trainees, and clinicians through education and systems changes.
In hospitals without an ACT, leadership can finance SUD champions and integrate them into policy-level decision-making to implement best practices in addiction care and lead hospital-wide educational efforts. This will transform hospital culture and improve care as all clinicians develop essential addiction skills.
Addiction champions and ACTs could also advocate for equitable practices for patients with SUD to reduce the stigma that both prevents patients from seeking care and results in self-discharges.20 For example, with interprofessional support, we revised our in-hospital substance use policy. It previously entailed hospital security responding to substance use concerns, which unintentionally harmed patients and perpetuated stigma. Our revised policy ensures we offer medications for cravings and withdrawal, adequate pain management, and other services that address patients’ reasons for in-hospital substance use.
With the increasing prevalence of SUD among hospitalized patients, escalating substance-related deaths, rising healthcare costs, and the impact of addiction on health and well-being, addiction care, including ACTs and champions, must be adequately funded. However, sustainable financing remains a challenge.18
Caring for Rosa and others with SUD sparked my desire to learn about addiction, obtain addiction medicine board certification as a practicing hospitalist, and create an ACT that offers evidence-based addiction treatment. While much remains to be done, by collaborating with addiction champions and engaging hospital leadership, we have transformed our hospital’s approach to substance use care.
With the knowledge and resources I now have as an addiction medicine physician, I reimagine the possibilities for patients like Rosa.
Rosa died when living was possible.
*Name has been changed for patient privacy.
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. HHS Publication No. PEP21-07-01-003, NSDUH Series H-56. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Accessed December 1, 2021. www.samhsa.gov/data/
2. Centers for Disease Control and Prevention. Alcohol and public health: alcohol-related disease impact (ARDI) application, 2013. Average for United States 2006–2010 alcohol-attributable deaths due to excessive alcohol use. Accessed December 1, 2021. www.cdc.gov/ARDI
3. Spillane S, Shiels MS, Best AF, et al. Trends in alcohol-induced deaths in the United States, 2000-2016. JAMA Netw Open. 2020;3(2):e1921451. https://doi.org/ 10.1001/jamanetworkopen.2019.21451
4. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911-923. https://doi.org/10.1001/jamapsychiatry.2017.2161 https://doi.org/10.1001/jamapsychiatry.2017.2161
5. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the covid-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. https://doi.org/10.1001/jamanetworkopen.2020.22942
6. Bangaru S, Pedersen MR, Macconmara MP, Singal AG, Mufti AR. Survey of liver transplantation practices for severe acute alcoholic hepatitis. Liver Transpl. 2018;24(10):1357-1362. https://doi.org/10.1002/lt.25285
7. Herrick-Reynolds KM, Punchhi G, Greenberg RS, et al. Evaluation of early vs standard liver transplant for alcohol-associated liver disease. JAMA Surg. 2021;156(11):1026-1034. https://doi.org/10.1001/jamasurg.2021.3748
8. Fleming JN, Lai JC, Te HS, Said A, Spengler EK, Rogal SS. Opioid and opioid substitution therapy in liver transplant candidates: A survey of center policies and practices. Clin Transplant. 2017;31(12):e13119. https://doi.org/10.1111/ctr.13119
9. Klimas J, Fairgrieve C, Tobin H, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev. 2018;12(12):CD009269. https://doi.org/10.1002/14651858.CD009269.pub4
10. Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202–207. https://doi.org/10.15585/mmwr.mm7006a4
11. Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. Accessed November 18, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
12. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
13. California Bridge Program. Tools: Treat substance use disorders from the acute care setting. Accessed August 20, 2021. https://cabridge.org/tools
14. Peterson C, Li M, Xu L, Mikosz CA, Luo F. Assessment of annual cost of substance use disorder in US hospitals. JAMA Netw Open. 2021;4(3):e210242. https://doi.org/10.1001/jamanetworkopen.2021.0242
15. Suen LW, Makam AN, Snyder HR, et al. National prevalence of alcohol and other substance use disorders among emergency department visits and hospitalizations: NHAMCS 2014-2018. J Gen Intern Med. 2021;13:1-9. https://doi.org/10.1007/s11606-021-07069-w
16. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: Qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993
17. Priest KC, McCarty D. Making the business case for an addiction medicine consult service: a qualitative analysis. BMC Health Services Research. 2019;19(1):822. https://doi.org/10.1186/s12913-019-4670-4
18. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496
19. Martin M, Snyder HR, Coffa D, et al. Time to ACT: launching an Addiction Care Team (ACT) in an urban safety-net health system. BMJ Open Qual. 2021;10(1):e001111. https://doi.org/10.1136/bmjoq-2020-001111
20. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: A qualitative study. Subst Abus. 2020;41(4):519-525. https://doi.org/10.1080/08897077.2019.1671942
Rosa* was one of my first patients as an intern rotating at the county hospital. Her marriage had disintegrated years earlier. To cope with depression, she hid a daily ritual of orange juice and vodka from her children. She worked as a cashier, until nausea and fatigue overwhelmed her.
The first time I met her she sat on the gurney: petite, tanned, and pregnant. Then I saw her yellow eyes and revised: temporal wasting, jaundiced, and swollen with ascites. Rosa didn’t know that alcohol could cause liver disease. Without insurance or access to primary care, her untreated alcohol use disorder (AUD) and depression had snowballed for years.
Midway through my intern year, I’d taken care of many people with AUD. However, I’d barely learned anything about it as a medical student, though we’d spent weeks studying esoteric diseases, that now––9 years after medical school––I still have not encountered.
Among the 28.3 million individuals in the United States with AUD, only 1% receive medication treatment.1 In the United States, unhealthy alcohol use accounts for more than 95,000 deaths each year.2 This number likely under-captures alcohol-related mortality and is higher now given recent reports of increasing alcohol-related deaths and prevalence of unhealthy alcohol use, especially among women, younger age groups, and marginalized populations.3-5
Rosa had alcohol-related hepatitis, which can cause severe inflammation and liver failure and quickly lead to death. As her liver failure progressed, I asked the gastroenterologists, “What other treatments can we offer? Is she a liver transplant candidate?” “Nothing” and “No” they answered.
Later, I emailed the hepatologist and transplant surgeon begging them to reevaluate her transplantation candidacy, but they told me there was no exception to the institution’s 6-month sobriety rule.
Maintaining a 6-month sobriety period is not an evidence-based criterion for transplantation. However, 50% of transplant centers do not perform transplantation prior to 6 months of alcohol abstinence for alcohol-related hepatitis due to concern for return to drinking after transplant.6 This practice may promote bias in patient selection for transplantation. A recent study found that individuals with alcohol-related liver disease transplanted before 6 months of abstinence had similar rates of survival and return to drinking compared to those who abstained from alcohol for 6 months and participated in AUD treatment before transplantation.7
There are other liver transplant practices that result in inequities for individuals with substance use disorders (SUD). Some liver transplant centers consider being on a medication for opioid use disorder a contraindication for transplantation—even if the individual is in recovery and abstaining from substances.8 Others mandate that individuals with alcohol-related liver disease attend Alcoholics Anonymous (AA) meetings prior to transplant. While mutual help groups, including AA, may benefit some individuals, different approaches work for different people.9 Other psychosocial interventions (eg, cognitive-behavioral therapy, contingency management, and residential treatment) and medications also help individuals reduce or stop drinking. Some meet their goals without any treatment. Addiction care works best when it respects autonomy and meets individuals where they are by allowing them to decide among options.
While organ allocations are a crystalized example of inequities in addiction care, they are also ethically complex. Many individuals—with and without SUD—die on waiting lists and must meet stringent transplantation criteria. However, we can at least remove the unnecessary biases that compound inequities in care people with SUD already face.
As Rosa’s liver succumbed, her kidneys failed too, and she required dialysis. She sensed what was coming. “I want everything…for now. I need to take care of my children.” I, too, wanted Rosa to live and see her youngest start kindergarten.
A few days before her discharge, I walked to the pharmacy and bought a purple journal. In a rare moment, I found Rosa alone in her room, without her ex-husband, sister, and mother, who rarely left her bedside. Together, we called AA and explored whether she could start participating in phone meetings from the hospital. I explained that one way to document a commitment to sobriety, as the transplant center’s rules dictated, was to attend and document AA meetings in this notebook. “In 5 months, you will be a liver transplant candidate,” I remember saying, wishing it to fruition.
I became Rosa’s primary care physician and saw her in clinic. Over the next few weeks, her skin took on an ashen tone. Sleep escaped her and her thoughts and speech blurred. Her walk slowed and she needed a wheelchair. The quiet fierceness that had defined her dissipated as encephalopathy took over. But until our last visit, she brought her purple journal, tracking the AA meetings she’d attended. Dialysis became intolerable, but not before Rosa made care arrangements for her girls. When that happened, she stopped dialysis and went to Mexico, where she died in her sleep after saying good-bye to her father.
Earlier access to healthcare and effective depression and AUD treatment could have saved Rosa’s life. While it was too late for her, as hospitalists we care for many others with substance-related complications and may miss opportunities to discuss and offer evidence-based addiction treatment. For example, we initiate the most up-to-date management for a patient’s gastrointestinal bleed but may leave the alcohol discussion for someone else. It is similar for other SUD: we treat cellulitis, epidural abscesses, bacteremia, chronic obstructive pulmonary disease, heart failure exacerbations, and other complications of SUD without addressing the root cause of the hospitalization—other than to prescribe abstinence from substance use or, at our worst, scold individuals for continuing to use.
But what can we offer? Most healthcare professionals still do not receive addiction education during training. Without tools, we enact temporizing measures, until patients return to the hospital or die.
In addition to increasing alcohol-related morbidity, there have also been increases in drug-related overdoses, fueled by COVID-19, synthetic opioids like fentanyl, and stimulants.10 In the 12-month period ending April 2021, more than 100,000 individuals died of drug-related overdoses, the highest number of deaths ever recorded in a year.11 Despite this, most healthcare systems remain unequipped to provide addiction services during hospitalization due to inadequate training, stigma, and lack of systems-based care.
Hospitalists and healthcare systems cannot be bystanders amid our worsening addiction crisis. We must empower clinicians with addiction education and ensure health systems offer evidence-based SUD services.
Educational efforts can close the knowledge gaps for both medical students and hospitalists. Medical schools should include foundational curricular content in screening, assessing, diagnosing, and treating SUD in alignment with standards set by the Liaison Committee on Medical Education, which accredits US medical schools. Residency programs can offer educational conferences, cased-based discussions, and addiction medicine rotations. Hospitalists can participate in educational didactics and review evidence-based addiction guidelines.12,13 While the focus here is on hospitalists, clinicians across practice settings and specialties will encounter patients with SUD, and all need to be well-versed in the diagnosis and treatment of addiction given the all-hands-on deck approach necessary amidst our worsening addiction crisis.
With one in nine hospitalizations involving individuals with SUD, and this number quickly rising, and with an annual cost to US hospitals of $13.2 billion, healthcare system leaders must invest in addiction care.14,15 Hospital-based addiction services could pay for themselves and save healthcare systems money while improving the patient and clinician experience.16One way to implement hospital-based addiction care is through an addiction consult team (ACT).17 While ACT compositions vary, most are interprofessional, offer evidence-based addiction treatment, and connect patients to community care.18 Our hospital’s ACT has nurses, patient navigators, and physicians who assess, diagnose, and treat SUD, and arrange follow-up addiction care.19 In addition to caring for individual patients, our ACT has led systems change. For example, we created order sets to guide clinicians, added medications to our hospital formulary to ensure access to evidence-based addiction treatment, and partnered with community stakeholders to streamline care transitions and access to psychosocial and medication treatment. Our team also worked with hospital leadership, nursing, and a syringe service program to integrate hospital harm reduction education and supply provision. Additionally, we are building capacity among staff, trainees, and clinicians through education and systems changes.
In hospitals without an ACT, leadership can finance SUD champions and integrate them into policy-level decision-making to implement best practices in addiction care and lead hospital-wide educational efforts. This will transform hospital culture and improve care as all clinicians develop essential addiction skills.
Addiction champions and ACTs could also advocate for equitable practices for patients with SUD to reduce the stigma that both prevents patients from seeking care and results in self-discharges.20 For example, with interprofessional support, we revised our in-hospital substance use policy. It previously entailed hospital security responding to substance use concerns, which unintentionally harmed patients and perpetuated stigma. Our revised policy ensures we offer medications for cravings and withdrawal, adequate pain management, and other services that address patients’ reasons for in-hospital substance use.
With the increasing prevalence of SUD among hospitalized patients, escalating substance-related deaths, rising healthcare costs, and the impact of addiction on health and well-being, addiction care, including ACTs and champions, must be adequately funded. However, sustainable financing remains a challenge.18
Caring for Rosa and others with SUD sparked my desire to learn about addiction, obtain addiction medicine board certification as a practicing hospitalist, and create an ACT that offers evidence-based addiction treatment. While much remains to be done, by collaborating with addiction champions and engaging hospital leadership, we have transformed our hospital’s approach to substance use care.
With the knowledge and resources I now have as an addiction medicine physician, I reimagine the possibilities for patients like Rosa.
Rosa died when living was possible.
*Name has been changed for patient privacy.
Rosa* was one of my first patients as an intern rotating at the county hospital. Her marriage had disintegrated years earlier. To cope with depression, she hid a daily ritual of orange juice and vodka from her children. She worked as a cashier, until nausea and fatigue overwhelmed her.
The first time I met her she sat on the gurney: petite, tanned, and pregnant. Then I saw her yellow eyes and revised: temporal wasting, jaundiced, and swollen with ascites. Rosa didn’t know that alcohol could cause liver disease. Without insurance or access to primary care, her untreated alcohol use disorder (AUD) and depression had snowballed for years.
Midway through my intern year, I’d taken care of many people with AUD. However, I’d barely learned anything about it as a medical student, though we’d spent weeks studying esoteric diseases, that now––9 years after medical school––I still have not encountered.
Among the 28.3 million individuals in the United States with AUD, only 1% receive medication treatment.1 In the United States, unhealthy alcohol use accounts for more than 95,000 deaths each year.2 This number likely under-captures alcohol-related mortality and is higher now given recent reports of increasing alcohol-related deaths and prevalence of unhealthy alcohol use, especially among women, younger age groups, and marginalized populations.3-5
Rosa had alcohol-related hepatitis, which can cause severe inflammation and liver failure and quickly lead to death. As her liver failure progressed, I asked the gastroenterologists, “What other treatments can we offer? Is she a liver transplant candidate?” “Nothing” and “No” they answered.
Later, I emailed the hepatologist and transplant surgeon begging them to reevaluate her transplantation candidacy, but they told me there was no exception to the institution’s 6-month sobriety rule.
Maintaining a 6-month sobriety period is not an evidence-based criterion for transplantation. However, 50% of transplant centers do not perform transplantation prior to 6 months of alcohol abstinence for alcohol-related hepatitis due to concern for return to drinking after transplant.6 This practice may promote bias in patient selection for transplantation. A recent study found that individuals with alcohol-related liver disease transplanted before 6 months of abstinence had similar rates of survival and return to drinking compared to those who abstained from alcohol for 6 months and participated in AUD treatment before transplantation.7
There are other liver transplant practices that result in inequities for individuals with substance use disorders (SUD). Some liver transplant centers consider being on a medication for opioid use disorder a contraindication for transplantation—even if the individual is in recovery and abstaining from substances.8 Others mandate that individuals with alcohol-related liver disease attend Alcoholics Anonymous (AA) meetings prior to transplant. While mutual help groups, including AA, may benefit some individuals, different approaches work for different people.9 Other psychosocial interventions (eg, cognitive-behavioral therapy, contingency management, and residential treatment) and medications also help individuals reduce or stop drinking. Some meet their goals without any treatment. Addiction care works best when it respects autonomy and meets individuals where they are by allowing them to decide among options.
While organ allocations are a crystalized example of inequities in addiction care, they are also ethically complex. Many individuals—with and without SUD—die on waiting lists and must meet stringent transplantation criteria. However, we can at least remove the unnecessary biases that compound inequities in care people with SUD already face.
As Rosa’s liver succumbed, her kidneys failed too, and she required dialysis. She sensed what was coming. “I want everything…for now. I need to take care of my children.” I, too, wanted Rosa to live and see her youngest start kindergarten.
A few days before her discharge, I walked to the pharmacy and bought a purple journal. In a rare moment, I found Rosa alone in her room, without her ex-husband, sister, and mother, who rarely left her bedside. Together, we called AA and explored whether she could start participating in phone meetings from the hospital. I explained that one way to document a commitment to sobriety, as the transplant center’s rules dictated, was to attend and document AA meetings in this notebook. “In 5 months, you will be a liver transplant candidate,” I remember saying, wishing it to fruition.
I became Rosa’s primary care physician and saw her in clinic. Over the next few weeks, her skin took on an ashen tone. Sleep escaped her and her thoughts and speech blurred. Her walk slowed and she needed a wheelchair. The quiet fierceness that had defined her dissipated as encephalopathy took over. But until our last visit, she brought her purple journal, tracking the AA meetings she’d attended. Dialysis became intolerable, but not before Rosa made care arrangements for her girls. When that happened, she stopped dialysis and went to Mexico, where she died in her sleep after saying good-bye to her father.
Earlier access to healthcare and effective depression and AUD treatment could have saved Rosa’s life. While it was too late for her, as hospitalists we care for many others with substance-related complications and may miss opportunities to discuss and offer evidence-based addiction treatment. For example, we initiate the most up-to-date management for a patient’s gastrointestinal bleed but may leave the alcohol discussion for someone else. It is similar for other SUD: we treat cellulitis, epidural abscesses, bacteremia, chronic obstructive pulmonary disease, heart failure exacerbations, and other complications of SUD without addressing the root cause of the hospitalization—other than to prescribe abstinence from substance use or, at our worst, scold individuals for continuing to use.
But what can we offer? Most healthcare professionals still do not receive addiction education during training. Without tools, we enact temporizing measures, until patients return to the hospital or die.
In addition to increasing alcohol-related morbidity, there have also been increases in drug-related overdoses, fueled by COVID-19, synthetic opioids like fentanyl, and stimulants.10 In the 12-month period ending April 2021, more than 100,000 individuals died of drug-related overdoses, the highest number of deaths ever recorded in a year.11 Despite this, most healthcare systems remain unequipped to provide addiction services during hospitalization due to inadequate training, stigma, and lack of systems-based care.
Hospitalists and healthcare systems cannot be bystanders amid our worsening addiction crisis. We must empower clinicians with addiction education and ensure health systems offer evidence-based SUD services.
Educational efforts can close the knowledge gaps for both medical students and hospitalists. Medical schools should include foundational curricular content in screening, assessing, diagnosing, and treating SUD in alignment with standards set by the Liaison Committee on Medical Education, which accredits US medical schools. Residency programs can offer educational conferences, cased-based discussions, and addiction medicine rotations. Hospitalists can participate in educational didactics and review evidence-based addiction guidelines.12,13 While the focus here is on hospitalists, clinicians across practice settings and specialties will encounter patients with SUD, and all need to be well-versed in the diagnosis and treatment of addiction given the all-hands-on deck approach necessary amidst our worsening addiction crisis.
With one in nine hospitalizations involving individuals with SUD, and this number quickly rising, and with an annual cost to US hospitals of $13.2 billion, healthcare system leaders must invest in addiction care.14,15 Hospital-based addiction services could pay for themselves and save healthcare systems money while improving the patient and clinician experience.16One way to implement hospital-based addiction care is through an addiction consult team (ACT).17 While ACT compositions vary, most are interprofessional, offer evidence-based addiction treatment, and connect patients to community care.18 Our hospital’s ACT has nurses, patient navigators, and physicians who assess, diagnose, and treat SUD, and arrange follow-up addiction care.19 In addition to caring for individual patients, our ACT has led systems change. For example, we created order sets to guide clinicians, added medications to our hospital formulary to ensure access to evidence-based addiction treatment, and partnered with community stakeholders to streamline care transitions and access to psychosocial and medication treatment. Our team also worked with hospital leadership, nursing, and a syringe service program to integrate hospital harm reduction education and supply provision. Additionally, we are building capacity among staff, trainees, and clinicians through education and systems changes.
In hospitals without an ACT, leadership can finance SUD champions and integrate them into policy-level decision-making to implement best practices in addiction care and lead hospital-wide educational efforts. This will transform hospital culture and improve care as all clinicians develop essential addiction skills.
Addiction champions and ACTs could also advocate for equitable practices for patients with SUD to reduce the stigma that both prevents patients from seeking care and results in self-discharges.20 For example, with interprofessional support, we revised our in-hospital substance use policy. It previously entailed hospital security responding to substance use concerns, which unintentionally harmed patients and perpetuated stigma. Our revised policy ensures we offer medications for cravings and withdrawal, adequate pain management, and other services that address patients’ reasons for in-hospital substance use.
With the increasing prevalence of SUD among hospitalized patients, escalating substance-related deaths, rising healthcare costs, and the impact of addiction on health and well-being, addiction care, including ACTs and champions, must be adequately funded. However, sustainable financing remains a challenge.18
Caring for Rosa and others with SUD sparked my desire to learn about addiction, obtain addiction medicine board certification as a practicing hospitalist, and create an ACT that offers evidence-based addiction treatment. While much remains to be done, by collaborating with addiction champions and engaging hospital leadership, we have transformed our hospital’s approach to substance use care.
With the knowledge and resources I now have as an addiction medicine physician, I reimagine the possibilities for patients like Rosa.
Rosa died when living was possible.
*Name has been changed for patient privacy.
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. HHS Publication No. PEP21-07-01-003, NSDUH Series H-56. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Accessed December 1, 2021. www.samhsa.gov/data/
2. Centers for Disease Control and Prevention. Alcohol and public health: alcohol-related disease impact (ARDI) application, 2013. Average for United States 2006–2010 alcohol-attributable deaths due to excessive alcohol use. Accessed December 1, 2021. www.cdc.gov/ARDI
3. Spillane S, Shiels MS, Best AF, et al. Trends in alcohol-induced deaths in the United States, 2000-2016. JAMA Netw Open. 2020;3(2):e1921451. https://doi.org/ 10.1001/jamanetworkopen.2019.21451
4. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911-923. https://doi.org/10.1001/jamapsychiatry.2017.2161 https://doi.org/10.1001/jamapsychiatry.2017.2161
5. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the covid-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. https://doi.org/10.1001/jamanetworkopen.2020.22942
6. Bangaru S, Pedersen MR, Macconmara MP, Singal AG, Mufti AR. Survey of liver transplantation practices for severe acute alcoholic hepatitis. Liver Transpl. 2018;24(10):1357-1362. https://doi.org/10.1002/lt.25285
7. Herrick-Reynolds KM, Punchhi G, Greenberg RS, et al. Evaluation of early vs standard liver transplant for alcohol-associated liver disease. JAMA Surg. 2021;156(11):1026-1034. https://doi.org/10.1001/jamasurg.2021.3748
8. Fleming JN, Lai JC, Te HS, Said A, Spengler EK, Rogal SS. Opioid and opioid substitution therapy in liver transplant candidates: A survey of center policies and practices. Clin Transplant. 2017;31(12):e13119. https://doi.org/10.1111/ctr.13119
9. Klimas J, Fairgrieve C, Tobin H, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev. 2018;12(12):CD009269. https://doi.org/10.1002/14651858.CD009269.pub4
10. Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202–207. https://doi.org/10.15585/mmwr.mm7006a4
11. Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. Accessed November 18, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
12. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
13. California Bridge Program. Tools: Treat substance use disorders from the acute care setting. Accessed August 20, 2021. https://cabridge.org/tools
14. Peterson C, Li M, Xu L, Mikosz CA, Luo F. Assessment of annual cost of substance use disorder in US hospitals. JAMA Netw Open. 2021;4(3):e210242. https://doi.org/10.1001/jamanetworkopen.2021.0242
15. Suen LW, Makam AN, Snyder HR, et al. National prevalence of alcohol and other substance use disorders among emergency department visits and hospitalizations: NHAMCS 2014-2018. J Gen Intern Med. 2021;13:1-9. https://doi.org/10.1007/s11606-021-07069-w
16. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: Qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993
17. Priest KC, McCarty D. Making the business case for an addiction medicine consult service: a qualitative analysis. BMC Health Services Research. 2019;19(1):822. https://doi.org/10.1186/s12913-019-4670-4
18. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496
19. Martin M, Snyder HR, Coffa D, et al. Time to ACT: launching an Addiction Care Team (ACT) in an urban safety-net health system. BMJ Open Qual. 2021;10(1):e001111. https://doi.org/10.1136/bmjoq-2020-001111
20. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: A qualitative study. Subst Abus. 2020;41(4):519-525. https://doi.org/10.1080/08897077.2019.1671942
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. HHS Publication No. PEP21-07-01-003, NSDUH Series H-56. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Accessed December 1, 2021. www.samhsa.gov/data/
2. Centers for Disease Control and Prevention. Alcohol and public health: alcohol-related disease impact (ARDI) application, 2013. Average for United States 2006–2010 alcohol-attributable deaths due to excessive alcohol use. Accessed December 1, 2021. www.cdc.gov/ARDI
3. Spillane S, Shiels MS, Best AF, et al. Trends in alcohol-induced deaths in the United States, 2000-2016. JAMA Netw Open. 2020;3(2):e1921451. https://doi.org/ 10.1001/jamanetworkopen.2019.21451
4. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911-923. https://doi.org/10.1001/jamapsychiatry.2017.2161 https://doi.org/10.1001/jamapsychiatry.2017.2161
5. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the covid-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. https://doi.org/10.1001/jamanetworkopen.2020.22942
6. Bangaru S, Pedersen MR, Macconmara MP, Singal AG, Mufti AR. Survey of liver transplantation practices for severe acute alcoholic hepatitis. Liver Transpl. 2018;24(10):1357-1362. https://doi.org/10.1002/lt.25285
7. Herrick-Reynolds KM, Punchhi G, Greenberg RS, et al. Evaluation of early vs standard liver transplant for alcohol-associated liver disease. JAMA Surg. 2021;156(11):1026-1034. https://doi.org/10.1001/jamasurg.2021.3748
8. Fleming JN, Lai JC, Te HS, Said A, Spengler EK, Rogal SS. Opioid and opioid substitution therapy in liver transplant candidates: A survey of center policies and practices. Clin Transplant. 2017;31(12):e13119. https://doi.org/10.1111/ctr.13119
9. Klimas J, Fairgrieve C, Tobin H, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev. 2018;12(12):CD009269. https://doi.org/10.1002/14651858.CD009269.pub4
10. Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202–207. https://doi.org/10.15585/mmwr.mm7006a4
11. Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. Accessed November 18, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
12. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
13. California Bridge Program. Tools: Treat substance use disorders from the acute care setting. Accessed August 20, 2021. https://cabridge.org/tools
14. Peterson C, Li M, Xu L, Mikosz CA, Luo F. Assessment of annual cost of substance use disorder in US hospitals. JAMA Netw Open. 2021;4(3):e210242. https://doi.org/10.1001/jamanetworkopen.2021.0242
15. Suen LW, Makam AN, Snyder HR, et al. National prevalence of alcohol and other substance use disorders among emergency department visits and hospitalizations: NHAMCS 2014-2018. J Gen Intern Med. 2021;13:1-9. https://doi.org/10.1007/s11606-021-07069-w
16. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: Qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993
17. Priest KC, McCarty D. Making the business case for an addiction medicine consult service: a qualitative analysis. BMC Health Services Research. 2019;19(1):822. https://doi.org/10.1186/s12913-019-4670-4
18. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496
19. Martin M, Snyder HR, Coffa D, et al. Time to ACT: launching an Addiction Care Team (ACT) in an urban safety-net health system. BMJ Open Qual. 2021;10(1):e001111. https://doi.org/10.1136/bmjoq-2020-001111
20. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: A qualitative study. Subst Abus. 2020;41(4):519-525. https://doi.org/10.1080/08897077.2019.1671942
© 2021 Society of Hospital Medicine
The Kids Are Not Alright
“...but it all started to get worse during the pandemic.”
As the patient’s† door closed, I (JS) thought about what his father had shared: his 12-year-old son had experienced a slow decline in his mental health since March 2020. There had been a gradual loss of all the things his son needed for psychological well-being: school went virtual and extracurricular activities ceased, and with them went any sense of routine, normalcy, or authentic opportunities to socialize. His feelings of isolation and depression culminated in an attempt to end his own life. My mind shifted to other patients under our care: an 8-year-old with behavioral outbursts intensifying after school-based therapy ended, a 13-year-old who became suicidal from isolation and virtual bullying. These children’s families sought emergent care because they no longer had the resources to care for their children at home. My team left each of these rooms heartbroken, unsure of exactly what to say and aware of the limitations of our current healthcare system.
Before and during the COVID-19 pandemic, many pediatric providers have had similar experiences caring for countless patients who are “boarding”—awaiting transfer to a psychiatric facility for their primary acute psychiatric issue, initially in the emergency room, often for 5 days or more,1 then ultimately admitted to a general medical floor if an appropriate psychiatric bed is still not available.2 Unfortunately, just as parents have run out of resources to care for their children’s psychiatric needs, so too is our medical system lacking in resources to provide the acute care these children need in general hospitals.
This mental health crisis began before the COVID-19 pandemic3 but has only worsened in the wake of its resulting social isolation. During the pandemic, suicide hotlines had a 1000% increase in call volumes.4 COVID-19–induced bed closures simultaneously worsened an existing critical bed shortage5,6 and led to an increase in the average length of stay (LOS) for patients boarding in the emergency department (ED).7 In the state of Massachusetts, for example, psychiatric patients awaiting inpatient beds boarded for more than 10,000 hours in January 2021—more than ever before, and up approximately 4000 hours since January 2017.6 For pediatric patients, the average wait time is now 59 hours.6 In the first 6 months of the pandemic, 39% of children presenting to EDs for mental health complaints ended up boarding, which is an astounding figure and is unfortunately 7% higher than in 2019.8 Even these staggering numbers do not capture the full range of experiences, as many statistics do not account for time spent on inpatient units by patients who do not receive a bed placement after waiting hours to several days in the ED.
Shortages of space, as well as an underfunded and understaffed mental health workforce, lead to these prolonged, often traumatic boarding periods in hospitals designed to care for acute medical, rather than acute psychiatric, conditions. Patients awaiting psychiatric placement are waiting in settings that are chaotic, inconsistent, and lacking in privacy. A patient in the throes of psychosis or suicidality needs a therapeutic milieu, not one that interrupts their daily routine,2 disconnects them from their existing support networks, and is punctuated by the incessant clangs of bedside monitors and the hubbub of code teams. These environments are not therapeutic3 for young infants with fevers, let alone for teenagers battling suicidality and eating disorders. In fact, for these reasons, we suspect that many of our patients’ inpatient ”behavioral escalations” are in fact triggered by their hospital environment, which may contribute to the 300% increase in the number of pharmacological restraints used during mental health visits in the ED over the past 10 years.9
None of us imagined when we chose to pursue pediatrics a that significant—and at times predominant—portion of our training would encompass caring for patients with acute mental health concerns. And although we did not anticipate this crisis, we have now been tasked with managing it. Throughout the day, when we are called to see our patients with primarily psychiatric pathology, we are often at war with ourselves. We weigh forming deeply meaningful relationships with these patients against the potential of unintentionally retraumatizing them or forming bonds that will be abruptly severed when patients are transferred to a psychiatric facility, which often occurs with barely a few hours’ notice. Moreover, many healthcare workers have training ill-suited to meet the needs of these patients. Just as emergency physicians can diagnose appendicitis but rely on surgeons to provide timely surgical treatment, general pediatricians identify psychiatric crises but rely on psychiatrists for ideal treatment plans. And almost daily, we are called to an “escalating” patient and arrive minutes into a stressful situation that others expect us to extinguish expeditiously. Along with nursing colleagues and the behavioral response team, we enact the treatment plan laid out by our psychiatry colleagues and wonder whether there is a better way.
We propose the following changes to create a more ideal health system (Table). We acknowledge that each health system has unique resources, challenges, and patient populations. Thus, our recommendations are not comprehensive and are largely based on experiences within our own institutions and state, but they encompass many domains that impact and are affected by child and adolescent mental healthcare in the United States, ranging from program- and hospital-level innovation to community and legislative action.
UPSTREAM PREVENTION
Like all good health system designs, we recommend prioritizing prevention. This would entail funding programs and legislation such as H.R. 3180, the RISE from Trauma Act, and H.R. 8544, the STRONG Support for Children Act of 2020 (both currently under consideration in the US House of Representatives) that support early childhood development and prevent adverse childhood experiences and trauma, averting mental health diagnoses such as depression and attention-deficit/hyperactivity disorder before they begin.10
OUTPATIENT AND COMMUNITY RESOURCES
We recognize that schools and general pediatricians have far more exposure to children at risk for mental health crises than do subspecialists. Thus, we urge an equitable increase in access to mental healthcare in the community so that patients needing assistance are screened and diagnosed earlier in their illness, allowing for secondary prevention of worsening mental health disorders. We support increased funding for programs such as the Massachusetts Child Psychiatry Access Program, which allows primary care doctors to consult psychiatrists in real time, closing the gap between a primary care visit and specialty follow-up. Telehealth services will be key to improving access for patients themselves and to allow pediatricians to consult with mental health professionals to initiate care prior to specialist availability. We envision that strengthening school-based behavioral health resources will also help prevent ED visits. Behavioral healthcare should be integrated into schools and community centers while police presence is simultaneously reduced, as there is evidence of an increased likelihood of juvenile justice involvement for children with disabilities and mental health needs.11,12
WORKFORCE DEVELOPMENT AND TRAINING
Ensuring access necessitates increasing the capacity of our psychiatric workforce by encouraging graduates to pursue mental health occupations with concrete financial incentives such as loan repayment and training grants. We thus support legislation such as H.R. 6597, the Mental Health Professionals Workforce Shortage Loan Repayment Act of 2018 (currently under consideration in the US House of Representatives). This may also improve recruitment and retention of individuals who are underrepresented in medicine, one step in helping ensure children have access to linguistically appropriate and culturally sensitive care. Residency programs and hospital systems should expand their training and education to identify and stabilize patients in mental health in extremis through culturally sensitive curricula focused on behavioral de-escalation techniques, trauma-informed care, and psychopharmacology. Our own residency program created a 2-week mental health rotation13 that includes rotating with outpatient mental health providers and our hospital’s behavioral response team, a group of trauma-informed responders for behavioral emergencies. Similar training should be available for nursing and other allied health professionals, who are often the first responders to behavioral escalations.13
INSTITUTIONAL DEVELOPMENT AND CLINICAL PRACTICES
Ideally, patients requiring higher-intensity psychiatric care would be referred to specialized pediatric behavioral health urgent care centers so their conditions can be adequately evaluated and addressed by staff trained in psychiatric management and in therapeutic environments. We believe all providers caring for children with mental health needs should be trained in basic, but core, behavioral health and de-escalation competencies, including specialized training for children with comorbid medical and neurodevelopmental diagnoses, such as autism. These centers should have specific beds for young children and those with developmental or complex care needs, and services should be available in numerous languages and levels of health literacy to allow all families to participate in their child’s care. At the same time, even nonpsychiatric EDs and inpatient units should commit resources to developing a maximally therapeutic environment, including allowing adjunctive services such as child life services, group therapy, and pet and music therapy, and create environments that support, rather than disrupt, normal routines.
HEALTH SYSTEMS REFORM AND ADVOCACY
Underpinning all the above innovations are changes to our healthcare payment system and provider networks, including the need for insurance coverage and payment parity for behavioral health, to ensure care is not only accessible but affordable. Additionally, for durable change, we need more than just education—we need coalition building and advocacy. Many organizations, including the American Academy of Pediatrics and the Children’s Hospital Association, have begun this work, which we must all continue.14 Bringing in diverse partners, including health systems, providers, educators, hospital administrators, payors, elected officials, and communities, will prioritize children’s needs and create a more ideal pediatric behavioral healthcare system.15
The COVID-19 pandemic has highlighted the dire need for comprehensive mental healthcare in the United States, a need that existed before the pandemic and will persist in a more fragile state long after it ends. Our hope is that the pandemic serves as the catalyst necessary to promote the magnitude of investments and stakeholder buy-in necessary to improve pediatric mental health and engender a radical redesign of our behavioral healthcare system. Our patients are counting on us to act. Together, we can build a system that ensures that the kids will be alright.
†Patient details have been changed for patient privacy.
Acknowledgments
The authors thank Joanna Perdomo, MD, Amara Azubuike, JD, and Josh Greenberg, JD, for reading and providing feedback on earlier versions of this work.
1. “This is a crisis”: mom whose son has boarded 33 days for psych bed calls for state action. WBUR News. Updated March 2, 2021. Accessed August 4, 2021. www.wbur.org/news/2021/02/26/mental-health-boarding-hospitals
2. Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813-824. https://doi.org/10.1016/S2215-0366(20)30307-2
3. Nash KA, Zima BT, Rothenberg C, et al. Prolonged emergency department length of stay for US pediatric mental health visits (2005-2015). Pediatrics. 2021;147(5):e2020030692. https://doi.org/10.1542/peds.2020-030692
4. Cloutier RL, Marshaall R. A dangerous pandemic pair: Covid19 and adolescent mental health emergencies. Am J Emerg Med. 2021;46:776-777. https://doi.org/10.1016/j.ajem.2020.09.008
5. Schoenberg S. Lack of mental health beds means long ER waits. CommonWealth Magazine. April 15, 2021. Accessed August 5, 2021. https://commonwealthmagazine.org/health-care/lack-of-mental-health-beds-means-long-er-waits/
6. Jolicoeur L, Mullins L. Mass. physicians call on state to address ER “boarding” of patients awaiting admission. WBUR News. Updated February 3, 2021. Accessed August 5, 2021. www.wbur.org/news/2021/02/02/emergency-department-er-inpatient-beds-boarding
7. Krass P, Dalton E, Doupnik SK, Esposito J. US pediatric emergency department visits for mental health conditions during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4):e218533. https://doi.org/10.1001/jamanetworkopen.2021.8533
8. Impact of COVID-19 on the Massachusetts Health Care System: Interim Report. Massachusetts Health Policy Commission. April 2021. Accessed September 25, 2021. www.mass.gov/doc/impact-of-covid-19-on-the-massachusetts-health-care-system-interim-report/download
9. Foster AA, Porter JJ, Monuteaux MC, Hoffmann JA, Hudgins JD. Pharmacologic restraint use during mental health visits in pediatric emergency departments. J Pediatr. 2021;236:276-283.e2. https://doi.org/10.1016/j.jpeds.2021.03.027
10. Brown NM, Brown SN, Briggs RD, Germán M, Belamarich PF, Oyeku SO. Associations between adverse childhood experiences and ADHD diagnosis and severity. Acad Pediatr. 2017;17(4):349-355. https://doi.org/10.1016/j.acap.2016.08.013
11. Harper K, Ryberg R, Temkin D. Black students and students with disabilities remain more likely to receive out-of-school suspensions, despite overall declines. Child Trends. April 29, 2019. Accessed August 5, 2021. www.childtrends.org/publications/black-students-disabilities-out-of-school-suspensions
12. Whitaker A, Torres-Guillén S, Morton M, et al. Cops and no counselors: how the lack of school mental health staff is harming students. American Civil Liberties Union. Accessed August 6, 2021. www.aclu.org/report/cops-and-no-counselors
13. Education. Boston Combined Residence Program. Accessed August 5, 2021. https://msbcrp.wpengine.com/program/education/
14. American Academy of Pediatrics. Interim guidance on supporting the emotional and behavioral health needs of children, adolescents, and families during the COVID-19 pandemic. Updated July 28, 2021. Accessed August 5, 2021. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-on-supporting-the-emotional-and-behavioral-health-needs-of-children-adolescents-and-families-during-the-covid-19-pandemic/
15. Advocacy. Children’s Mental Health Campaign. Accessed August 4, 2021. https://childrensmentalhealthcampaign.org/advocacy
“...but it all started to get worse during the pandemic.”
As the patient’s† door closed, I (JS) thought about what his father had shared: his 12-year-old son had experienced a slow decline in his mental health since March 2020. There had been a gradual loss of all the things his son needed for psychological well-being: school went virtual and extracurricular activities ceased, and with them went any sense of routine, normalcy, or authentic opportunities to socialize. His feelings of isolation and depression culminated in an attempt to end his own life. My mind shifted to other patients under our care: an 8-year-old with behavioral outbursts intensifying after school-based therapy ended, a 13-year-old who became suicidal from isolation and virtual bullying. These children’s families sought emergent care because they no longer had the resources to care for their children at home. My team left each of these rooms heartbroken, unsure of exactly what to say and aware of the limitations of our current healthcare system.
Before and during the COVID-19 pandemic, many pediatric providers have had similar experiences caring for countless patients who are “boarding”—awaiting transfer to a psychiatric facility for their primary acute psychiatric issue, initially in the emergency room, often for 5 days or more,1 then ultimately admitted to a general medical floor if an appropriate psychiatric bed is still not available.2 Unfortunately, just as parents have run out of resources to care for their children’s psychiatric needs, so too is our medical system lacking in resources to provide the acute care these children need in general hospitals.
This mental health crisis began before the COVID-19 pandemic3 but has only worsened in the wake of its resulting social isolation. During the pandemic, suicide hotlines had a 1000% increase in call volumes.4 COVID-19–induced bed closures simultaneously worsened an existing critical bed shortage5,6 and led to an increase in the average length of stay (LOS) for patients boarding in the emergency department (ED).7 In the state of Massachusetts, for example, psychiatric patients awaiting inpatient beds boarded for more than 10,000 hours in January 2021—more than ever before, and up approximately 4000 hours since January 2017.6 For pediatric patients, the average wait time is now 59 hours.6 In the first 6 months of the pandemic, 39% of children presenting to EDs for mental health complaints ended up boarding, which is an astounding figure and is unfortunately 7% higher than in 2019.8 Even these staggering numbers do not capture the full range of experiences, as many statistics do not account for time spent on inpatient units by patients who do not receive a bed placement after waiting hours to several days in the ED.
Shortages of space, as well as an underfunded and understaffed mental health workforce, lead to these prolonged, often traumatic boarding periods in hospitals designed to care for acute medical, rather than acute psychiatric, conditions. Patients awaiting psychiatric placement are waiting in settings that are chaotic, inconsistent, and lacking in privacy. A patient in the throes of psychosis or suicidality needs a therapeutic milieu, not one that interrupts their daily routine,2 disconnects them from their existing support networks, and is punctuated by the incessant clangs of bedside monitors and the hubbub of code teams. These environments are not therapeutic3 for young infants with fevers, let alone for teenagers battling suicidality and eating disorders. In fact, for these reasons, we suspect that many of our patients’ inpatient ”behavioral escalations” are in fact triggered by their hospital environment, which may contribute to the 300% increase in the number of pharmacological restraints used during mental health visits in the ED over the past 10 years.9
None of us imagined when we chose to pursue pediatrics a that significant—and at times predominant—portion of our training would encompass caring for patients with acute mental health concerns. And although we did not anticipate this crisis, we have now been tasked with managing it. Throughout the day, when we are called to see our patients with primarily psychiatric pathology, we are often at war with ourselves. We weigh forming deeply meaningful relationships with these patients against the potential of unintentionally retraumatizing them or forming bonds that will be abruptly severed when patients are transferred to a psychiatric facility, which often occurs with barely a few hours’ notice. Moreover, many healthcare workers have training ill-suited to meet the needs of these patients. Just as emergency physicians can diagnose appendicitis but rely on surgeons to provide timely surgical treatment, general pediatricians identify psychiatric crises but rely on psychiatrists for ideal treatment plans. And almost daily, we are called to an “escalating” patient and arrive minutes into a stressful situation that others expect us to extinguish expeditiously. Along with nursing colleagues and the behavioral response team, we enact the treatment plan laid out by our psychiatry colleagues and wonder whether there is a better way.
We propose the following changes to create a more ideal health system (Table). We acknowledge that each health system has unique resources, challenges, and patient populations. Thus, our recommendations are not comprehensive and are largely based on experiences within our own institutions and state, but they encompass many domains that impact and are affected by child and adolescent mental healthcare in the United States, ranging from program- and hospital-level innovation to community and legislative action.
UPSTREAM PREVENTION
Like all good health system designs, we recommend prioritizing prevention. This would entail funding programs and legislation such as H.R. 3180, the RISE from Trauma Act, and H.R. 8544, the STRONG Support for Children Act of 2020 (both currently under consideration in the US House of Representatives) that support early childhood development and prevent adverse childhood experiences and trauma, averting mental health diagnoses such as depression and attention-deficit/hyperactivity disorder before they begin.10
OUTPATIENT AND COMMUNITY RESOURCES
We recognize that schools and general pediatricians have far more exposure to children at risk for mental health crises than do subspecialists. Thus, we urge an equitable increase in access to mental healthcare in the community so that patients needing assistance are screened and diagnosed earlier in their illness, allowing for secondary prevention of worsening mental health disorders. We support increased funding for programs such as the Massachusetts Child Psychiatry Access Program, which allows primary care doctors to consult psychiatrists in real time, closing the gap between a primary care visit and specialty follow-up. Telehealth services will be key to improving access for patients themselves and to allow pediatricians to consult with mental health professionals to initiate care prior to specialist availability. We envision that strengthening school-based behavioral health resources will also help prevent ED visits. Behavioral healthcare should be integrated into schools and community centers while police presence is simultaneously reduced, as there is evidence of an increased likelihood of juvenile justice involvement for children with disabilities and mental health needs.11,12
WORKFORCE DEVELOPMENT AND TRAINING
Ensuring access necessitates increasing the capacity of our psychiatric workforce by encouraging graduates to pursue mental health occupations with concrete financial incentives such as loan repayment and training grants. We thus support legislation such as H.R. 6597, the Mental Health Professionals Workforce Shortage Loan Repayment Act of 2018 (currently under consideration in the US House of Representatives). This may also improve recruitment and retention of individuals who are underrepresented in medicine, one step in helping ensure children have access to linguistically appropriate and culturally sensitive care. Residency programs and hospital systems should expand their training and education to identify and stabilize patients in mental health in extremis through culturally sensitive curricula focused on behavioral de-escalation techniques, trauma-informed care, and psychopharmacology. Our own residency program created a 2-week mental health rotation13 that includes rotating with outpatient mental health providers and our hospital’s behavioral response team, a group of trauma-informed responders for behavioral emergencies. Similar training should be available for nursing and other allied health professionals, who are often the first responders to behavioral escalations.13
INSTITUTIONAL DEVELOPMENT AND CLINICAL PRACTICES
Ideally, patients requiring higher-intensity psychiatric care would be referred to specialized pediatric behavioral health urgent care centers so their conditions can be adequately evaluated and addressed by staff trained in psychiatric management and in therapeutic environments. We believe all providers caring for children with mental health needs should be trained in basic, but core, behavioral health and de-escalation competencies, including specialized training for children with comorbid medical and neurodevelopmental diagnoses, such as autism. These centers should have specific beds for young children and those with developmental or complex care needs, and services should be available in numerous languages and levels of health literacy to allow all families to participate in their child’s care. At the same time, even nonpsychiatric EDs and inpatient units should commit resources to developing a maximally therapeutic environment, including allowing adjunctive services such as child life services, group therapy, and pet and music therapy, and create environments that support, rather than disrupt, normal routines.
HEALTH SYSTEMS REFORM AND ADVOCACY
Underpinning all the above innovations are changes to our healthcare payment system and provider networks, including the need for insurance coverage and payment parity for behavioral health, to ensure care is not only accessible but affordable. Additionally, for durable change, we need more than just education—we need coalition building and advocacy. Many organizations, including the American Academy of Pediatrics and the Children’s Hospital Association, have begun this work, which we must all continue.14 Bringing in diverse partners, including health systems, providers, educators, hospital administrators, payors, elected officials, and communities, will prioritize children’s needs and create a more ideal pediatric behavioral healthcare system.15
The COVID-19 pandemic has highlighted the dire need for comprehensive mental healthcare in the United States, a need that existed before the pandemic and will persist in a more fragile state long after it ends. Our hope is that the pandemic serves as the catalyst necessary to promote the magnitude of investments and stakeholder buy-in necessary to improve pediatric mental health and engender a radical redesign of our behavioral healthcare system. Our patients are counting on us to act. Together, we can build a system that ensures that the kids will be alright.
†Patient details have been changed for patient privacy.
Acknowledgments
The authors thank Joanna Perdomo, MD, Amara Azubuike, JD, and Josh Greenberg, JD, for reading and providing feedback on earlier versions of this work.
“...but it all started to get worse during the pandemic.”
As the patient’s† door closed, I (JS) thought about what his father had shared: his 12-year-old son had experienced a slow decline in his mental health since March 2020. There had been a gradual loss of all the things his son needed for psychological well-being: school went virtual and extracurricular activities ceased, and with them went any sense of routine, normalcy, or authentic opportunities to socialize. His feelings of isolation and depression culminated in an attempt to end his own life. My mind shifted to other patients under our care: an 8-year-old with behavioral outbursts intensifying after school-based therapy ended, a 13-year-old who became suicidal from isolation and virtual bullying. These children’s families sought emergent care because they no longer had the resources to care for their children at home. My team left each of these rooms heartbroken, unsure of exactly what to say and aware of the limitations of our current healthcare system.
Before and during the COVID-19 pandemic, many pediatric providers have had similar experiences caring for countless patients who are “boarding”—awaiting transfer to a psychiatric facility for their primary acute psychiatric issue, initially in the emergency room, often for 5 days or more,1 then ultimately admitted to a general medical floor if an appropriate psychiatric bed is still not available.2 Unfortunately, just as parents have run out of resources to care for their children’s psychiatric needs, so too is our medical system lacking in resources to provide the acute care these children need in general hospitals.
This mental health crisis began before the COVID-19 pandemic3 but has only worsened in the wake of its resulting social isolation. During the pandemic, suicide hotlines had a 1000% increase in call volumes.4 COVID-19–induced bed closures simultaneously worsened an existing critical bed shortage5,6 and led to an increase in the average length of stay (LOS) for patients boarding in the emergency department (ED).7 In the state of Massachusetts, for example, psychiatric patients awaiting inpatient beds boarded for more than 10,000 hours in January 2021—more than ever before, and up approximately 4000 hours since January 2017.6 For pediatric patients, the average wait time is now 59 hours.6 In the first 6 months of the pandemic, 39% of children presenting to EDs for mental health complaints ended up boarding, which is an astounding figure and is unfortunately 7% higher than in 2019.8 Even these staggering numbers do not capture the full range of experiences, as many statistics do not account for time spent on inpatient units by patients who do not receive a bed placement after waiting hours to several days in the ED.
Shortages of space, as well as an underfunded and understaffed mental health workforce, lead to these prolonged, often traumatic boarding periods in hospitals designed to care for acute medical, rather than acute psychiatric, conditions. Patients awaiting psychiatric placement are waiting in settings that are chaotic, inconsistent, and lacking in privacy. A patient in the throes of psychosis or suicidality needs a therapeutic milieu, not one that interrupts their daily routine,2 disconnects them from their existing support networks, and is punctuated by the incessant clangs of bedside monitors and the hubbub of code teams. These environments are not therapeutic3 for young infants with fevers, let alone for teenagers battling suicidality and eating disorders. In fact, for these reasons, we suspect that many of our patients’ inpatient ”behavioral escalations” are in fact triggered by their hospital environment, which may contribute to the 300% increase in the number of pharmacological restraints used during mental health visits in the ED over the past 10 years.9
None of us imagined when we chose to pursue pediatrics a that significant—and at times predominant—portion of our training would encompass caring for patients with acute mental health concerns. And although we did not anticipate this crisis, we have now been tasked with managing it. Throughout the day, when we are called to see our patients with primarily psychiatric pathology, we are often at war with ourselves. We weigh forming deeply meaningful relationships with these patients against the potential of unintentionally retraumatizing them or forming bonds that will be abruptly severed when patients are transferred to a psychiatric facility, which often occurs with barely a few hours’ notice. Moreover, many healthcare workers have training ill-suited to meet the needs of these patients. Just as emergency physicians can diagnose appendicitis but rely on surgeons to provide timely surgical treatment, general pediatricians identify psychiatric crises but rely on psychiatrists for ideal treatment plans. And almost daily, we are called to an “escalating” patient and arrive minutes into a stressful situation that others expect us to extinguish expeditiously. Along with nursing colleagues and the behavioral response team, we enact the treatment plan laid out by our psychiatry colleagues and wonder whether there is a better way.
We propose the following changes to create a more ideal health system (Table). We acknowledge that each health system has unique resources, challenges, and patient populations. Thus, our recommendations are not comprehensive and are largely based on experiences within our own institutions and state, but they encompass many domains that impact and are affected by child and adolescent mental healthcare in the United States, ranging from program- and hospital-level innovation to community and legislative action.
UPSTREAM PREVENTION
Like all good health system designs, we recommend prioritizing prevention. This would entail funding programs and legislation such as H.R. 3180, the RISE from Trauma Act, and H.R. 8544, the STRONG Support for Children Act of 2020 (both currently under consideration in the US House of Representatives) that support early childhood development and prevent adverse childhood experiences and trauma, averting mental health diagnoses such as depression and attention-deficit/hyperactivity disorder before they begin.10
OUTPATIENT AND COMMUNITY RESOURCES
We recognize that schools and general pediatricians have far more exposure to children at risk for mental health crises than do subspecialists. Thus, we urge an equitable increase in access to mental healthcare in the community so that patients needing assistance are screened and diagnosed earlier in their illness, allowing for secondary prevention of worsening mental health disorders. We support increased funding for programs such as the Massachusetts Child Psychiatry Access Program, which allows primary care doctors to consult psychiatrists in real time, closing the gap between a primary care visit and specialty follow-up. Telehealth services will be key to improving access for patients themselves and to allow pediatricians to consult with mental health professionals to initiate care prior to specialist availability. We envision that strengthening school-based behavioral health resources will also help prevent ED visits. Behavioral healthcare should be integrated into schools and community centers while police presence is simultaneously reduced, as there is evidence of an increased likelihood of juvenile justice involvement for children with disabilities and mental health needs.11,12
WORKFORCE DEVELOPMENT AND TRAINING
Ensuring access necessitates increasing the capacity of our psychiatric workforce by encouraging graduates to pursue mental health occupations with concrete financial incentives such as loan repayment and training grants. We thus support legislation such as H.R. 6597, the Mental Health Professionals Workforce Shortage Loan Repayment Act of 2018 (currently under consideration in the US House of Representatives). This may also improve recruitment and retention of individuals who are underrepresented in medicine, one step in helping ensure children have access to linguistically appropriate and culturally sensitive care. Residency programs and hospital systems should expand their training and education to identify and stabilize patients in mental health in extremis through culturally sensitive curricula focused on behavioral de-escalation techniques, trauma-informed care, and psychopharmacology. Our own residency program created a 2-week mental health rotation13 that includes rotating with outpatient mental health providers and our hospital’s behavioral response team, a group of trauma-informed responders for behavioral emergencies. Similar training should be available for nursing and other allied health professionals, who are often the first responders to behavioral escalations.13
INSTITUTIONAL DEVELOPMENT AND CLINICAL PRACTICES
Ideally, patients requiring higher-intensity psychiatric care would be referred to specialized pediatric behavioral health urgent care centers so their conditions can be adequately evaluated and addressed by staff trained in psychiatric management and in therapeutic environments. We believe all providers caring for children with mental health needs should be trained in basic, but core, behavioral health and de-escalation competencies, including specialized training for children with comorbid medical and neurodevelopmental diagnoses, such as autism. These centers should have specific beds for young children and those with developmental or complex care needs, and services should be available in numerous languages and levels of health literacy to allow all families to participate in their child’s care. At the same time, even nonpsychiatric EDs and inpatient units should commit resources to developing a maximally therapeutic environment, including allowing adjunctive services such as child life services, group therapy, and pet and music therapy, and create environments that support, rather than disrupt, normal routines.
HEALTH SYSTEMS REFORM AND ADVOCACY
Underpinning all the above innovations are changes to our healthcare payment system and provider networks, including the need for insurance coverage and payment parity for behavioral health, to ensure care is not only accessible but affordable. Additionally, for durable change, we need more than just education—we need coalition building and advocacy. Many organizations, including the American Academy of Pediatrics and the Children’s Hospital Association, have begun this work, which we must all continue.14 Bringing in diverse partners, including health systems, providers, educators, hospital administrators, payors, elected officials, and communities, will prioritize children’s needs and create a more ideal pediatric behavioral healthcare system.15
The COVID-19 pandemic has highlighted the dire need for comprehensive mental healthcare in the United States, a need that existed before the pandemic and will persist in a more fragile state long after it ends. Our hope is that the pandemic serves as the catalyst necessary to promote the magnitude of investments and stakeholder buy-in necessary to improve pediatric mental health and engender a radical redesign of our behavioral healthcare system. Our patients are counting on us to act. Together, we can build a system that ensures that the kids will be alright.
†Patient details have been changed for patient privacy.
Acknowledgments
The authors thank Joanna Perdomo, MD, Amara Azubuike, JD, and Josh Greenberg, JD, for reading and providing feedback on earlier versions of this work.
1. “This is a crisis”: mom whose son has boarded 33 days for psych bed calls for state action. WBUR News. Updated March 2, 2021. Accessed August 4, 2021. www.wbur.org/news/2021/02/26/mental-health-boarding-hospitals
2. Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813-824. https://doi.org/10.1016/S2215-0366(20)30307-2
3. Nash KA, Zima BT, Rothenberg C, et al. Prolonged emergency department length of stay for US pediatric mental health visits (2005-2015). Pediatrics. 2021;147(5):e2020030692. https://doi.org/10.1542/peds.2020-030692
4. Cloutier RL, Marshaall R. A dangerous pandemic pair: Covid19 and adolescent mental health emergencies. Am J Emerg Med. 2021;46:776-777. https://doi.org/10.1016/j.ajem.2020.09.008
5. Schoenberg S. Lack of mental health beds means long ER waits. CommonWealth Magazine. April 15, 2021. Accessed August 5, 2021. https://commonwealthmagazine.org/health-care/lack-of-mental-health-beds-means-long-er-waits/
6. Jolicoeur L, Mullins L. Mass. physicians call on state to address ER “boarding” of patients awaiting admission. WBUR News. Updated February 3, 2021. Accessed August 5, 2021. www.wbur.org/news/2021/02/02/emergency-department-er-inpatient-beds-boarding
7. Krass P, Dalton E, Doupnik SK, Esposito J. US pediatric emergency department visits for mental health conditions during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4):e218533. https://doi.org/10.1001/jamanetworkopen.2021.8533
8. Impact of COVID-19 on the Massachusetts Health Care System: Interim Report. Massachusetts Health Policy Commission. April 2021. Accessed September 25, 2021. www.mass.gov/doc/impact-of-covid-19-on-the-massachusetts-health-care-system-interim-report/download
9. Foster AA, Porter JJ, Monuteaux MC, Hoffmann JA, Hudgins JD. Pharmacologic restraint use during mental health visits in pediatric emergency departments. J Pediatr. 2021;236:276-283.e2. https://doi.org/10.1016/j.jpeds.2021.03.027
10. Brown NM, Brown SN, Briggs RD, Germán M, Belamarich PF, Oyeku SO. Associations between adverse childhood experiences and ADHD diagnosis and severity. Acad Pediatr. 2017;17(4):349-355. https://doi.org/10.1016/j.acap.2016.08.013
11. Harper K, Ryberg R, Temkin D. Black students and students with disabilities remain more likely to receive out-of-school suspensions, despite overall declines. Child Trends. April 29, 2019. Accessed August 5, 2021. www.childtrends.org/publications/black-students-disabilities-out-of-school-suspensions
12. Whitaker A, Torres-Guillén S, Morton M, et al. Cops and no counselors: how the lack of school mental health staff is harming students. American Civil Liberties Union. Accessed August 6, 2021. www.aclu.org/report/cops-and-no-counselors
13. Education. Boston Combined Residence Program. Accessed August 5, 2021. https://msbcrp.wpengine.com/program/education/
14. American Academy of Pediatrics. Interim guidance on supporting the emotional and behavioral health needs of children, adolescents, and families during the COVID-19 pandemic. Updated July 28, 2021. Accessed August 5, 2021. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-on-supporting-the-emotional-and-behavioral-health-needs-of-children-adolescents-and-families-during-the-covid-19-pandemic/
15. Advocacy. Children’s Mental Health Campaign. Accessed August 4, 2021. https://childrensmentalhealthcampaign.org/advocacy
1. “This is a crisis”: mom whose son has boarded 33 days for psych bed calls for state action. WBUR News. Updated March 2, 2021. Accessed August 4, 2021. www.wbur.org/news/2021/02/26/mental-health-boarding-hospitals
2. Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813-824. https://doi.org/10.1016/S2215-0366(20)30307-2
3. Nash KA, Zima BT, Rothenberg C, et al. Prolonged emergency department length of stay for US pediatric mental health visits (2005-2015). Pediatrics. 2021;147(5):e2020030692. https://doi.org/10.1542/peds.2020-030692
4. Cloutier RL, Marshaall R. A dangerous pandemic pair: Covid19 and adolescent mental health emergencies. Am J Emerg Med. 2021;46:776-777. https://doi.org/10.1016/j.ajem.2020.09.008
5. Schoenberg S. Lack of mental health beds means long ER waits. CommonWealth Magazine. April 15, 2021. Accessed August 5, 2021. https://commonwealthmagazine.org/health-care/lack-of-mental-health-beds-means-long-er-waits/
6. Jolicoeur L, Mullins L. Mass. physicians call on state to address ER “boarding” of patients awaiting admission. WBUR News. Updated February 3, 2021. Accessed August 5, 2021. www.wbur.org/news/2021/02/02/emergency-department-er-inpatient-beds-boarding
7. Krass P, Dalton E, Doupnik SK, Esposito J. US pediatric emergency department visits for mental health conditions during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4):e218533. https://doi.org/10.1001/jamanetworkopen.2021.8533
8. Impact of COVID-19 on the Massachusetts Health Care System: Interim Report. Massachusetts Health Policy Commission. April 2021. Accessed September 25, 2021. www.mass.gov/doc/impact-of-covid-19-on-the-massachusetts-health-care-system-interim-report/download
9. Foster AA, Porter JJ, Monuteaux MC, Hoffmann JA, Hudgins JD. Pharmacologic restraint use during mental health visits in pediatric emergency departments. J Pediatr. 2021;236:276-283.e2. https://doi.org/10.1016/j.jpeds.2021.03.027
10. Brown NM, Brown SN, Briggs RD, Germán M, Belamarich PF, Oyeku SO. Associations between adverse childhood experiences and ADHD diagnosis and severity. Acad Pediatr. 2017;17(4):349-355. https://doi.org/10.1016/j.acap.2016.08.013
11. Harper K, Ryberg R, Temkin D. Black students and students with disabilities remain more likely to receive out-of-school suspensions, despite overall declines. Child Trends. April 29, 2019. Accessed August 5, 2021. www.childtrends.org/publications/black-students-disabilities-out-of-school-suspensions
12. Whitaker A, Torres-Guillén S, Morton M, et al. Cops and no counselors: how the lack of school mental health staff is harming students. American Civil Liberties Union. Accessed August 6, 2021. www.aclu.org/report/cops-and-no-counselors
13. Education. Boston Combined Residence Program. Accessed August 5, 2021. https://msbcrp.wpengine.com/program/education/
14. American Academy of Pediatrics. Interim guidance on supporting the emotional and behavioral health needs of children, adolescents, and families during the COVID-19 pandemic. Updated July 28, 2021. Accessed August 5, 2021. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-on-supporting-the-emotional-and-behavioral-health-needs-of-children-adolescents-and-families-during-the-covid-19-pandemic/
15. Advocacy. Children’s Mental Health Campaign. Accessed August 4, 2021. https://childrensmentalhealthcampaign.org/advocacy
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Discontinuing Urate-Lowering Therapy on Admission
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: 2020 American Society of Addiction Medicine Clinical Practice Guideline on Alcohol Withdrawal Management
Alcohol is the most common substance implicated in hospitalizations for substance use disorders,1 and as a result, hospitalists commonly diagnose and manage alcohol withdrawal syndrome (AWS) in the inpatient medical setting. The 2020 guidelines of the American Society of Addiction Medicine (ASAM) provide updated recommendations for the diagnosis, monitoring, and treatment of patients hospitalized with AWS, which we have condensed to emphasize key changes from the last update2 and clarify ongoing areas of uncertainty.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnosis
Recommendation 1. All inpatients who have used alcohol recently or regularly should be risk-stratified for AWS, regardless of whether or not they have suggestive symptoms (recommendations I.3, I.4, I.5, II.10). The Alcohol Use Disorders Identification Test-(Piccinelli) Consumption (AUDIT-PC) identifies patients at risk for AWS, and the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) identifies those at risk for severe or complicated AWS, which includes seizures and alcohol withdrawal delirium (formerly delirium tremens). The guideline emphasizes use of these tools rather than simply initiating Clinical Institute Withdrawal Assessment for Alcohol, revised (CIWA-Ar) monitoring on all such patients to diagnose AWS, as CIWA-Ar was developed for monitoring response to treatment, not diagnosis (recommendation I.6).
Treating Mild/Moderate or Uncomplicated AWS
Recommendation 2. Because of their proven track record of reducing the incidence of seizure and alcohol withdrawal delirium, benzodiazepines remain the recommended first-line therapy (recommendations V.13, V.16). Symptom-triggered administration of benzodiazepines (via CIWA-Ar) is recommended over fixed-dose administration because the former is associated with shorter length of stay and lower cumulative benzodiazepine administration3,4 (recommendation V.23). Patients with mild AWS who are at low risk for severe or complicated withdrawal should be monitored for up to 36 hours for the development of worsening symptoms (recommendation V.1). For patients with high CIWA-Ar scores or who are at increased risk for severe or complicated AWS, frequent administration of moderate to high doses of a long-acting benzodiazepine early in AWS treatment (a practice called frontloading) is recommended to quickly control symptoms and prevent clinical worsening. This approach has been shown to reduce the incidence of seizures and alcohol withdrawal delirium (recommendations V.14, V.19, V.24).
Carbamazepine or gabapentin may be used in mild or moderate AWS if benzodiazepines are contraindicated; however, neither agent is recommended as first-line therapy because a clear reduction in seizure and withdrawal delirium has not been established (recommendation V.16). Alpha-2 agonists (eg, clonidine, dexmedetomidine) may be used to treat persistent autonomic hyperactivity or anxiety when these are not adequately controlled by benzodiazepines alone (recommendation V.36).
Treating Severe or Complicated AWS
Recommendation 3. The guideline defines severe AWS as withdrawal with severe signs and symptoms, and complicated AWS as withdrawal accompanied by seizures or delirium (Appendix Table5). The development of complications warrants prompt treatment. Patients who experience seizure should receive a fast-acting benzodiazepine (eg, intravenous [IV] diazepam or lorazepam) (recommendation VI.4). Patients with withdrawal delirium should receive a benzodiazepine (preferably parenterally) dosed to achieve light sedation. Clinicians should be prepared for the possibility that large doses may be required and to monitor patients for oversedation and respiratory depression (recommendations VI.13, VI.17). Antipsychotics may be used as adjuncts when withdrawal delirium or other symptoms, such as hallucinosis, are not adequately controlled by benzodiazepines alone, but should not be used as monotherapy (recommendation VI.20). The guideline emphasizes that alpha-2 agonists should not be used to treat withdrawal delirium (recommendation VI.21), but they may be used as adjuncts for resistant alcohol withdrawal in the intensive care unit (ICU) (recommendations VI.27, VI.29). Phenobarbital is an acceptable alternative to benzodiazepines for severe withdrawal (recommendation V.17); however, the guideline recommends that clinicians should be experienced in its use.
Treating Wernicke Encephalopathy
Recommendation 4. Thiamine should be administered to prevent Wernicke encephalopathy (WE), with parenteral formulations recommended in patients with malnutrition, severe/complicated withdrawal, or requiring ICU-level care (recommendations V.7, V.8). In particular, all patients admitted to an ICU for AWS should receive thiamine, as diagnosis of WE is often difficult in this population. Although there is no consensus on the required dose of thiamine to treat WE, 100 mg IV or intramuscularly (IM) daily for 3 to 5 days is commonly administered (recommendation V.7). Because of a lack of evidence of harm, thiamine may be given before, after, or concurrently with glucose or dextrose (recommendation V.7). The guideline does not make a specific recommendation regarding how to risk-stratify patients for WE.
Treating Underlying Alcohol Use Disorder
Recommendation 5. Hospitalization for AWS is an important opportunity to engage patients in treatment for alcohol use disorder (AUD), including pharmacotherapy and connection with outpatient providers (recommendation V.12). The guideline emphasizes that treatment for AUD should be initiated concomitantly with AWS management whenever possible but does not make recommendations regarding specific pharmacotherapies.
CRITIQUE
This guideline was authored by a committee of emergency medicine physicians, psychiatrists, and internists using the Department of Veterans Affairs/Department of Defense guidelines and the RAND/UCLA appropriateness method to combine the scientific literature with expert opinion. The result is a series of recommendations for physicians, physician assistants, nurse practitioners, and pharmacists that are not rated by strength; an assessment of the quality of the supporting evidence is available in an appendix. Four of the nine guideline committee members reported significant financial relationships with industry and other entities relevant to these guidelines.
Despite concern about oversedation from phenobarbital raised in small case series,6 observational studies comparing phenobarbital with benzodiazepines suggest phenobarbital has similar efficacy for treating AWS and that oversedation is rare.7-9 Large randomized controlled trials in this area are lacking; however, at least one small randomized controlled trial10 among patients with AWS presenting to emergency departments supports the safety and efficacy of phenobarbital when used in combination with benzodiazepines. Given the growing body of evidence supporting the safety of phenobarbital, we believe a stronger recommendation for use in patients presenting with alcohol withdrawal delirium or treatment-resistant alcohol withdrawal is warranted. The guidelines also suggest that only “experienced clinicians” use phenobarbital for AWS, which may suppress appropriate use. Nationally, phenobarbital use for AWS remains low.11Finally, although the guideline recommends initiation of treatment for AUD, specific recommendations for pharmacotherapy are not provided. Three medications currently have approval from the US Food and Drug Administration for treatment of AUD: acamprosate, naltrexone, and disulfiram. Large randomized controlled trials support the safety and efficacy of acamprosate and naltrexone, with or without counselling, in the treatment of AUD,12 and disulfiram may be appropriate for selected highly motivated patients. We believe more specific recommendations to assist in choosing among these options would be useful.
AREAS IN NEED OF FUTURE STUDY
More data are needed on the safety and efficacy of phenobarbital in patients with AWS, as well as comparative effectiveness against benzodiazepines. Recruitment is ongoing for a single clinical trial comparing the effect of phenobarbital and lorazepam on length of stay among patients in the ICU with AWS (NCT04156464); to date, no randomized trials of phenobarbital have been conducted in medical inpatients with AWS. In addition, gaps in the literature exist regarding benzodiazepine selection, and head-to-head comparisons of symptom-triggered usage of different benzodiazepines are lacking.
1. Heslin KC, Elixhauser A, Steiner CA. Hospitalizations involving mental and substance use disorders among adults, 2012. HCUP Statistical Brief #191. June 2015. Accessed November 17, 2021. www.hcup-us.ahrq.gov/reports/statbriefs/sb191-Hospitalization-Mental-Substance-Use-Disorders-2012.pdf
2. Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405-1412. https://doi.org/10.1001/archinte.164.13.1405
3. Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272(7):519-523.
4. Daeppen J-B, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. https://doi.org/10.1001/archinte.162.10.1117
5. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. J Addict Med. 2020;14(3S suppl):1-72. https://doi.org/10.1097/ADM.0000000000000668
6. Oks M, Cleven KL, Healy L, et al. The safety and utility of phenobarbital use for the treatment of severe alcohol withdrawal syndrome in the medical intensive care unit. J Intensive Care Med. 2020;35(9):844-850. https://doi.org/10.1177/0885066618783947
7. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357. https://doi.org/10.1111/j.1360-0443.1989.tb00737.x
8. Ibarra Jr F. Single dose phenobarbital in addition to symptom-triggered lorazepam in alcohol withdrawal. Am J Emerg Med. 2020;38(2):178-181. https://doi.org/10.1016/j.ajem.2019.01.053
9. Nisavic M, Nejad SH, Isenberg BM, et al. Use of phenobarbital in alcohol withdrawal management–a retrospective comparison study of phenobarbital and benzodiazepines for acute alcohol withdrawal management in general medical patients. Psychosomatics. 2019;60(5):458-467. https://doi.org/10.1016/j.psym.2019.02.002
10. Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med. 2013;44(3):592-598.e2. https://doi.org/10.1016/j.jemermed.2012.07.056
11. Gupta N, Emerman CL. Trends in the management of inpatients with alcohol withdrawal syndrome. Addict Disord Their Treat. 2021;20(1):29-32. https://doi.org/10.1097/ADT.0000000000000203
12. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017. https://doi.org/10.1001/jama.295.17.2003
Alcohol is the most common substance implicated in hospitalizations for substance use disorders,1 and as a result, hospitalists commonly diagnose and manage alcohol withdrawal syndrome (AWS) in the inpatient medical setting. The 2020 guidelines of the American Society of Addiction Medicine (ASAM) provide updated recommendations for the diagnosis, monitoring, and treatment of patients hospitalized with AWS, which we have condensed to emphasize key changes from the last update2 and clarify ongoing areas of uncertainty.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnosis
Recommendation 1. All inpatients who have used alcohol recently or regularly should be risk-stratified for AWS, regardless of whether or not they have suggestive symptoms (recommendations I.3, I.4, I.5, II.10). The Alcohol Use Disorders Identification Test-(Piccinelli) Consumption (AUDIT-PC) identifies patients at risk for AWS, and the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) identifies those at risk for severe or complicated AWS, which includes seizures and alcohol withdrawal delirium (formerly delirium tremens). The guideline emphasizes use of these tools rather than simply initiating Clinical Institute Withdrawal Assessment for Alcohol, revised (CIWA-Ar) monitoring on all such patients to diagnose AWS, as CIWA-Ar was developed for monitoring response to treatment, not diagnosis (recommendation I.6).
Treating Mild/Moderate or Uncomplicated AWS
Recommendation 2. Because of their proven track record of reducing the incidence of seizure and alcohol withdrawal delirium, benzodiazepines remain the recommended first-line therapy (recommendations V.13, V.16). Symptom-triggered administration of benzodiazepines (via CIWA-Ar) is recommended over fixed-dose administration because the former is associated with shorter length of stay and lower cumulative benzodiazepine administration3,4 (recommendation V.23). Patients with mild AWS who are at low risk for severe or complicated withdrawal should be monitored for up to 36 hours for the development of worsening symptoms (recommendation V.1). For patients with high CIWA-Ar scores or who are at increased risk for severe or complicated AWS, frequent administration of moderate to high doses of a long-acting benzodiazepine early in AWS treatment (a practice called frontloading) is recommended to quickly control symptoms and prevent clinical worsening. This approach has been shown to reduce the incidence of seizures and alcohol withdrawal delirium (recommendations V.14, V.19, V.24).
Carbamazepine or gabapentin may be used in mild or moderate AWS if benzodiazepines are contraindicated; however, neither agent is recommended as first-line therapy because a clear reduction in seizure and withdrawal delirium has not been established (recommendation V.16). Alpha-2 agonists (eg, clonidine, dexmedetomidine) may be used to treat persistent autonomic hyperactivity or anxiety when these are not adequately controlled by benzodiazepines alone (recommendation V.36).
Treating Severe or Complicated AWS
Recommendation 3. The guideline defines severe AWS as withdrawal with severe signs and symptoms, and complicated AWS as withdrawal accompanied by seizures or delirium (Appendix Table5). The development of complications warrants prompt treatment. Patients who experience seizure should receive a fast-acting benzodiazepine (eg, intravenous [IV] diazepam or lorazepam) (recommendation VI.4). Patients with withdrawal delirium should receive a benzodiazepine (preferably parenterally) dosed to achieve light sedation. Clinicians should be prepared for the possibility that large doses may be required and to monitor patients for oversedation and respiratory depression (recommendations VI.13, VI.17). Antipsychotics may be used as adjuncts when withdrawal delirium or other symptoms, such as hallucinosis, are not adequately controlled by benzodiazepines alone, but should not be used as monotherapy (recommendation VI.20). The guideline emphasizes that alpha-2 agonists should not be used to treat withdrawal delirium (recommendation VI.21), but they may be used as adjuncts for resistant alcohol withdrawal in the intensive care unit (ICU) (recommendations VI.27, VI.29). Phenobarbital is an acceptable alternative to benzodiazepines for severe withdrawal (recommendation V.17); however, the guideline recommends that clinicians should be experienced in its use.
Treating Wernicke Encephalopathy
Recommendation 4. Thiamine should be administered to prevent Wernicke encephalopathy (WE), with parenteral formulations recommended in patients with malnutrition, severe/complicated withdrawal, or requiring ICU-level care (recommendations V.7, V.8). In particular, all patients admitted to an ICU for AWS should receive thiamine, as diagnosis of WE is often difficult in this population. Although there is no consensus on the required dose of thiamine to treat WE, 100 mg IV or intramuscularly (IM) daily for 3 to 5 days is commonly administered (recommendation V.7). Because of a lack of evidence of harm, thiamine may be given before, after, or concurrently with glucose or dextrose (recommendation V.7). The guideline does not make a specific recommendation regarding how to risk-stratify patients for WE.
Treating Underlying Alcohol Use Disorder
Recommendation 5. Hospitalization for AWS is an important opportunity to engage patients in treatment for alcohol use disorder (AUD), including pharmacotherapy and connection with outpatient providers (recommendation V.12). The guideline emphasizes that treatment for AUD should be initiated concomitantly with AWS management whenever possible but does not make recommendations regarding specific pharmacotherapies.
CRITIQUE
This guideline was authored by a committee of emergency medicine physicians, psychiatrists, and internists using the Department of Veterans Affairs/Department of Defense guidelines and the RAND/UCLA appropriateness method to combine the scientific literature with expert opinion. The result is a series of recommendations for physicians, physician assistants, nurse practitioners, and pharmacists that are not rated by strength; an assessment of the quality of the supporting evidence is available in an appendix. Four of the nine guideline committee members reported significant financial relationships with industry and other entities relevant to these guidelines.
Despite concern about oversedation from phenobarbital raised in small case series,6 observational studies comparing phenobarbital with benzodiazepines suggest phenobarbital has similar efficacy for treating AWS and that oversedation is rare.7-9 Large randomized controlled trials in this area are lacking; however, at least one small randomized controlled trial10 among patients with AWS presenting to emergency departments supports the safety and efficacy of phenobarbital when used in combination with benzodiazepines. Given the growing body of evidence supporting the safety of phenobarbital, we believe a stronger recommendation for use in patients presenting with alcohol withdrawal delirium or treatment-resistant alcohol withdrawal is warranted. The guidelines also suggest that only “experienced clinicians” use phenobarbital for AWS, which may suppress appropriate use. Nationally, phenobarbital use for AWS remains low.11Finally, although the guideline recommends initiation of treatment for AUD, specific recommendations for pharmacotherapy are not provided. Three medications currently have approval from the US Food and Drug Administration for treatment of AUD: acamprosate, naltrexone, and disulfiram. Large randomized controlled trials support the safety and efficacy of acamprosate and naltrexone, with or without counselling, in the treatment of AUD,12 and disulfiram may be appropriate for selected highly motivated patients. We believe more specific recommendations to assist in choosing among these options would be useful.
AREAS IN NEED OF FUTURE STUDY
More data are needed on the safety and efficacy of phenobarbital in patients with AWS, as well as comparative effectiveness against benzodiazepines. Recruitment is ongoing for a single clinical trial comparing the effect of phenobarbital and lorazepam on length of stay among patients in the ICU with AWS (NCT04156464); to date, no randomized trials of phenobarbital have been conducted in medical inpatients with AWS. In addition, gaps in the literature exist regarding benzodiazepine selection, and head-to-head comparisons of symptom-triggered usage of different benzodiazepines are lacking.
Alcohol is the most common substance implicated in hospitalizations for substance use disorders,1 and as a result, hospitalists commonly diagnose and manage alcohol withdrawal syndrome (AWS) in the inpatient medical setting. The 2020 guidelines of the American Society of Addiction Medicine (ASAM) provide updated recommendations for the diagnosis, monitoring, and treatment of patients hospitalized with AWS, which we have condensed to emphasize key changes from the last update2 and clarify ongoing areas of uncertainty.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnosis
Recommendation 1. All inpatients who have used alcohol recently or regularly should be risk-stratified for AWS, regardless of whether or not they have suggestive symptoms (recommendations I.3, I.4, I.5, II.10). The Alcohol Use Disorders Identification Test-(Piccinelli) Consumption (AUDIT-PC) identifies patients at risk for AWS, and the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) identifies those at risk for severe or complicated AWS, which includes seizures and alcohol withdrawal delirium (formerly delirium tremens). The guideline emphasizes use of these tools rather than simply initiating Clinical Institute Withdrawal Assessment for Alcohol, revised (CIWA-Ar) monitoring on all such patients to diagnose AWS, as CIWA-Ar was developed for monitoring response to treatment, not diagnosis (recommendation I.6).
Treating Mild/Moderate or Uncomplicated AWS
Recommendation 2. Because of their proven track record of reducing the incidence of seizure and alcohol withdrawal delirium, benzodiazepines remain the recommended first-line therapy (recommendations V.13, V.16). Symptom-triggered administration of benzodiazepines (via CIWA-Ar) is recommended over fixed-dose administration because the former is associated with shorter length of stay and lower cumulative benzodiazepine administration3,4 (recommendation V.23). Patients with mild AWS who are at low risk for severe or complicated withdrawal should be monitored for up to 36 hours for the development of worsening symptoms (recommendation V.1). For patients with high CIWA-Ar scores or who are at increased risk for severe or complicated AWS, frequent administration of moderate to high doses of a long-acting benzodiazepine early in AWS treatment (a practice called frontloading) is recommended to quickly control symptoms and prevent clinical worsening. This approach has been shown to reduce the incidence of seizures and alcohol withdrawal delirium (recommendations V.14, V.19, V.24).
Carbamazepine or gabapentin may be used in mild or moderate AWS if benzodiazepines are contraindicated; however, neither agent is recommended as first-line therapy because a clear reduction in seizure and withdrawal delirium has not been established (recommendation V.16). Alpha-2 agonists (eg, clonidine, dexmedetomidine) may be used to treat persistent autonomic hyperactivity or anxiety when these are not adequately controlled by benzodiazepines alone (recommendation V.36).
Treating Severe or Complicated AWS
Recommendation 3. The guideline defines severe AWS as withdrawal with severe signs and symptoms, and complicated AWS as withdrawal accompanied by seizures or delirium (Appendix Table5). The development of complications warrants prompt treatment. Patients who experience seizure should receive a fast-acting benzodiazepine (eg, intravenous [IV] diazepam or lorazepam) (recommendation VI.4). Patients with withdrawal delirium should receive a benzodiazepine (preferably parenterally) dosed to achieve light sedation. Clinicians should be prepared for the possibility that large doses may be required and to monitor patients for oversedation and respiratory depression (recommendations VI.13, VI.17). Antipsychotics may be used as adjuncts when withdrawal delirium or other symptoms, such as hallucinosis, are not adequately controlled by benzodiazepines alone, but should not be used as monotherapy (recommendation VI.20). The guideline emphasizes that alpha-2 agonists should not be used to treat withdrawal delirium (recommendation VI.21), but they may be used as adjuncts for resistant alcohol withdrawal in the intensive care unit (ICU) (recommendations VI.27, VI.29). Phenobarbital is an acceptable alternative to benzodiazepines for severe withdrawal (recommendation V.17); however, the guideline recommends that clinicians should be experienced in its use.
Treating Wernicke Encephalopathy
Recommendation 4. Thiamine should be administered to prevent Wernicke encephalopathy (WE), with parenteral formulations recommended in patients with malnutrition, severe/complicated withdrawal, or requiring ICU-level care (recommendations V.7, V.8). In particular, all patients admitted to an ICU for AWS should receive thiamine, as diagnosis of WE is often difficult in this population. Although there is no consensus on the required dose of thiamine to treat WE, 100 mg IV or intramuscularly (IM) daily for 3 to 5 days is commonly administered (recommendation V.7). Because of a lack of evidence of harm, thiamine may be given before, after, or concurrently with glucose or dextrose (recommendation V.7). The guideline does not make a specific recommendation regarding how to risk-stratify patients for WE.
Treating Underlying Alcohol Use Disorder
Recommendation 5. Hospitalization for AWS is an important opportunity to engage patients in treatment for alcohol use disorder (AUD), including pharmacotherapy and connection with outpatient providers (recommendation V.12). The guideline emphasizes that treatment for AUD should be initiated concomitantly with AWS management whenever possible but does not make recommendations regarding specific pharmacotherapies.
CRITIQUE
This guideline was authored by a committee of emergency medicine physicians, psychiatrists, and internists using the Department of Veterans Affairs/Department of Defense guidelines and the RAND/UCLA appropriateness method to combine the scientific literature with expert opinion. The result is a series of recommendations for physicians, physician assistants, nurse practitioners, and pharmacists that are not rated by strength; an assessment of the quality of the supporting evidence is available in an appendix. Four of the nine guideline committee members reported significant financial relationships with industry and other entities relevant to these guidelines.
Despite concern about oversedation from phenobarbital raised in small case series,6 observational studies comparing phenobarbital with benzodiazepines suggest phenobarbital has similar efficacy for treating AWS and that oversedation is rare.7-9 Large randomized controlled trials in this area are lacking; however, at least one small randomized controlled trial10 among patients with AWS presenting to emergency departments supports the safety and efficacy of phenobarbital when used in combination with benzodiazepines. Given the growing body of evidence supporting the safety of phenobarbital, we believe a stronger recommendation for use in patients presenting with alcohol withdrawal delirium or treatment-resistant alcohol withdrawal is warranted. The guidelines also suggest that only “experienced clinicians” use phenobarbital for AWS, which may suppress appropriate use. Nationally, phenobarbital use for AWS remains low.11Finally, although the guideline recommends initiation of treatment for AUD, specific recommendations for pharmacotherapy are not provided. Three medications currently have approval from the US Food and Drug Administration for treatment of AUD: acamprosate, naltrexone, and disulfiram. Large randomized controlled trials support the safety and efficacy of acamprosate and naltrexone, with or without counselling, in the treatment of AUD,12 and disulfiram may be appropriate for selected highly motivated patients. We believe more specific recommendations to assist in choosing among these options would be useful.
AREAS IN NEED OF FUTURE STUDY
More data are needed on the safety and efficacy of phenobarbital in patients with AWS, as well as comparative effectiveness against benzodiazepines. Recruitment is ongoing for a single clinical trial comparing the effect of phenobarbital and lorazepam on length of stay among patients in the ICU with AWS (NCT04156464); to date, no randomized trials of phenobarbital have been conducted in medical inpatients with AWS. In addition, gaps in the literature exist regarding benzodiazepine selection, and head-to-head comparisons of symptom-triggered usage of different benzodiazepines are lacking.
1. Heslin KC, Elixhauser A, Steiner CA. Hospitalizations involving mental and substance use disorders among adults, 2012. HCUP Statistical Brief #191. June 2015. Accessed November 17, 2021. www.hcup-us.ahrq.gov/reports/statbriefs/sb191-Hospitalization-Mental-Substance-Use-Disorders-2012.pdf
2. Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405-1412. https://doi.org/10.1001/archinte.164.13.1405
3. Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272(7):519-523.
4. Daeppen J-B, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. https://doi.org/10.1001/archinte.162.10.1117
5. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. J Addict Med. 2020;14(3S suppl):1-72. https://doi.org/10.1097/ADM.0000000000000668
6. Oks M, Cleven KL, Healy L, et al. The safety and utility of phenobarbital use for the treatment of severe alcohol withdrawal syndrome in the medical intensive care unit. J Intensive Care Med. 2020;35(9):844-850. https://doi.org/10.1177/0885066618783947
7. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357. https://doi.org/10.1111/j.1360-0443.1989.tb00737.x
8. Ibarra Jr F. Single dose phenobarbital in addition to symptom-triggered lorazepam in alcohol withdrawal. Am J Emerg Med. 2020;38(2):178-181. https://doi.org/10.1016/j.ajem.2019.01.053
9. Nisavic M, Nejad SH, Isenberg BM, et al. Use of phenobarbital in alcohol withdrawal management–a retrospective comparison study of phenobarbital and benzodiazepines for acute alcohol withdrawal management in general medical patients. Psychosomatics. 2019;60(5):458-467. https://doi.org/10.1016/j.psym.2019.02.002
10. Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med. 2013;44(3):592-598.e2. https://doi.org/10.1016/j.jemermed.2012.07.056
11. Gupta N, Emerman CL. Trends in the management of inpatients with alcohol withdrawal syndrome. Addict Disord Their Treat. 2021;20(1):29-32. https://doi.org/10.1097/ADT.0000000000000203
12. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017. https://doi.org/10.1001/jama.295.17.2003
1. Heslin KC, Elixhauser A, Steiner CA. Hospitalizations involving mental and substance use disorders among adults, 2012. HCUP Statistical Brief #191. June 2015. Accessed November 17, 2021. www.hcup-us.ahrq.gov/reports/statbriefs/sb191-Hospitalization-Mental-Substance-Use-Disorders-2012.pdf
2. Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405-1412. https://doi.org/10.1001/archinte.164.13.1405
3. Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272(7):519-523.
4. Daeppen J-B, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. https://doi.org/10.1001/archinte.162.10.1117
5. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. J Addict Med. 2020;14(3S suppl):1-72. https://doi.org/10.1097/ADM.0000000000000668
6. Oks M, Cleven KL, Healy L, et al. The safety and utility of phenobarbital use for the treatment of severe alcohol withdrawal syndrome in the medical intensive care unit. J Intensive Care Med. 2020;35(9):844-850. https://doi.org/10.1177/0885066618783947
7. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357. https://doi.org/10.1111/j.1360-0443.1989.tb00737.x
8. Ibarra Jr F. Single dose phenobarbital in addition to symptom-triggered lorazepam in alcohol withdrawal. Am J Emerg Med. 2020;38(2):178-181. https://doi.org/10.1016/j.ajem.2019.01.053
9. Nisavic M, Nejad SH, Isenberg BM, et al. Use of phenobarbital in alcohol withdrawal management–a retrospective comparison study of phenobarbital and benzodiazepines for acute alcohol withdrawal management in general medical patients. Psychosomatics. 2019;60(5):458-467. https://doi.org/10.1016/j.psym.2019.02.002
10. Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med. 2013;44(3):592-598.e2. https://doi.org/10.1016/j.jemermed.2012.07.056
11. Gupta N, Emerman CL. Trends in the management of inpatients with alcohol withdrawal syndrome. Addict Disord Their Treat. 2021;20(1):29-32. https://doi.org/10.1097/ADT.0000000000000203
12. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017. https://doi.org/10.1001/jama.295.17.2003
© 2021 Society of Hospital Medicine
Leadership & Professional Development: Relational Leadership—It’s Not About You
“Lead me, follow me, or get the hell out of my way.”
—George Patton
The concept of leadership is often viewed through the lens of the individual. Terms such as “born leader” are canon in our lexicon, and motivational images are common, frequently paired with a singular majestic animal on a mountain peak, meant to inspire awe in the value of the individual leader. This mindset can be problematically reductive, suggesting that leadership is binary and mutually exclusive: we are either leaders or followers. The terminology can also be pejorative, as few are likely to populate a curriculum vitae with examples of being a great follower.
Leadership can instead be regarded as a role rather than a personality trait or superpower. Many of us inhabit multiple leadership roles in our professional lives. Whether participating on a committee, designing an educational curriculum, overseeing a clinical service line, or supervising learners as ward teaching attending, we function as leaders in the context of our relationships with other members of the numerous cohorts within which we work. As leaders, we must consider our relationships to others in a group as opposed to our intrinsic personalities.1
The following pearls can help operationalize relational leadership concepts2,3:
We are not alone. In any given leadership role, we must understand with whom we work (and often depend upon) and what we need to do to allow others to help us succeed. When entering a leadership role with a new group, it is important to assess the interests and skill sets of the rest of the team by either formal or informal means. Many are used to doing so on the first day of attending on a new ward service, but this concept also applies to other roles, such as chairing a new committee.
Work with individuals and groups whose knowledge, experience, skills, and/or attitudes are complementary to our own. This is not as easy as it sounds; when hiring individuals or assembling groups, we tend to gravitate to those like ourselves. Seeking different opinions and styles can be valuable, and promoting diversity, inclusion, and equity is paramount. To do so, we must make efforts to understand our own personal strengths and limitations, ideally supplemented with observation and feedback from a trusted mentor or coach. Taking an honest look at our preconceptions and assumptions is crucial. Consider how we view other silos with which we interact and the presuppositions we make, such as the “typical” surgeon or emergency department practitioner.
Recognize and publicly share shortcomings. Transparency about our limitations allows us to build relationships that are more effective and impactful. A leader who meaningfully reveals a weakness for which they need other group members to contribute specific expertise can allow team members to feel more connected or engaged with that leader or group by shifting from interpreting an ask as “Do this task” to the more empowering “I need your help.”
Leadership can be effectively conceptualized as a relational skill, fulfilled by various roles in our professional lives. Collaboration, introspection, and transparency are essential to becoming a successful leader.
Acknowledgments
The author gratefully acknowledges Dr David Berg for his invaluable mentorship as well as the core faculty of the SHM-SGIM Academic Hospitalist Academy 2.0 for their support and encouragement.
1. Wood M, Dibben M. Leadership as a relational process. Process Studies. 2015;44(1): 24-47. https://doi.org/10.5840/process20154412
2. Berg DN. Resurrecting the muse: followership in organizations. In: Klein EB, Gabelnick E, Herr R, eds. Psychodynamics of Leadership. Psychosocial Press; 1998.
3. Berg DN, Bradley EH. Leadership: Rhetoric vs. Reality. 2015. Accessed September 22, 2021. https://www.youtube.com/watch?v=77IwJ8wXaM8
“Lead me, follow me, or get the hell out of my way.”
—George Patton
The concept of leadership is often viewed through the lens of the individual. Terms such as “born leader” are canon in our lexicon, and motivational images are common, frequently paired with a singular majestic animal on a mountain peak, meant to inspire awe in the value of the individual leader. This mindset can be problematically reductive, suggesting that leadership is binary and mutually exclusive: we are either leaders or followers. The terminology can also be pejorative, as few are likely to populate a curriculum vitae with examples of being a great follower.
Leadership can instead be regarded as a role rather than a personality trait or superpower. Many of us inhabit multiple leadership roles in our professional lives. Whether participating on a committee, designing an educational curriculum, overseeing a clinical service line, or supervising learners as ward teaching attending, we function as leaders in the context of our relationships with other members of the numerous cohorts within which we work. As leaders, we must consider our relationships to others in a group as opposed to our intrinsic personalities.1
The following pearls can help operationalize relational leadership concepts2,3:
We are not alone. In any given leadership role, we must understand with whom we work (and often depend upon) and what we need to do to allow others to help us succeed. When entering a leadership role with a new group, it is important to assess the interests and skill sets of the rest of the team by either formal or informal means. Many are used to doing so on the first day of attending on a new ward service, but this concept also applies to other roles, such as chairing a new committee.
Work with individuals and groups whose knowledge, experience, skills, and/or attitudes are complementary to our own. This is not as easy as it sounds; when hiring individuals or assembling groups, we tend to gravitate to those like ourselves. Seeking different opinions and styles can be valuable, and promoting diversity, inclusion, and equity is paramount. To do so, we must make efforts to understand our own personal strengths and limitations, ideally supplemented with observation and feedback from a trusted mentor or coach. Taking an honest look at our preconceptions and assumptions is crucial. Consider how we view other silos with which we interact and the presuppositions we make, such as the “typical” surgeon or emergency department practitioner.
Recognize and publicly share shortcomings. Transparency about our limitations allows us to build relationships that are more effective and impactful. A leader who meaningfully reveals a weakness for which they need other group members to contribute specific expertise can allow team members to feel more connected or engaged with that leader or group by shifting from interpreting an ask as “Do this task” to the more empowering “I need your help.”
Leadership can be effectively conceptualized as a relational skill, fulfilled by various roles in our professional lives. Collaboration, introspection, and transparency are essential to becoming a successful leader.
Acknowledgments
The author gratefully acknowledges Dr David Berg for his invaluable mentorship as well as the core faculty of the SHM-SGIM Academic Hospitalist Academy 2.0 for their support and encouragement.
“Lead me, follow me, or get the hell out of my way.”
—George Patton
The concept of leadership is often viewed through the lens of the individual. Terms such as “born leader” are canon in our lexicon, and motivational images are common, frequently paired with a singular majestic animal on a mountain peak, meant to inspire awe in the value of the individual leader. This mindset can be problematically reductive, suggesting that leadership is binary and mutually exclusive: we are either leaders or followers. The terminology can also be pejorative, as few are likely to populate a curriculum vitae with examples of being a great follower.
Leadership can instead be regarded as a role rather than a personality trait or superpower. Many of us inhabit multiple leadership roles in our professional lives. Whether participating on a committee, designing an educational curriculum, overseeing a clinical service line, or supervising learners as ward teaching attending, we function as leaders in the context of our relationships with other members of the numerous cohorts within which we work. As leaders, we must consider our relationships to others in a group as opposed to our intrinsic personalities.1
The following pearls can help operationalize relational leadership concepts2,3:
We are not alone. In any given leadership role, we must understand with whom we work (and often depend upon) and what we need to do to allow others to help us succeed. When entering a leadership role with a new group, it is important to assess the interests and skill sets of the rest of the team by either formal or informal means. Many are used to doing so on the first day of attending on a new ward service, but this concept also applies to other roles, such as chairing a new committee.
Work with individuals and groups whose knowledge, experience, skills, and/or attitudes are complementary to our own. This is not as easy as it sounds; when hiring individuals or assembling groups, we tend to gravitate to those like ourselves. Seeking different opinions and styles can be valuable, and promoting diversity, inclusion, and equity is paramount. To do so, we must make efforts to understand our own personal strengths and limitations, ideally supplemented with observation and feedback from a trusted mentor or coach. Taking an honest look at our preconceptions and assumptions is crucial. Consider how we view other silos with which we interact and the presuppositions we make, such as the “typical” surgeon or emergency department practitioner.
Recognize and publicly share shortcomings. Transparency about our limitations allows us to build relationships that are more effective and impactful. A leader who meaningfully reveals a weakness for which they need other group members to contribute specific expertise can allow team members to feel more connected or engaged with that leader or group by shifting from interpreting an ask as “Do this task” to the more empowering “I need your help.”
Leadership can be effectively conceptualized as a relational skill, fulfilled by various roles in our professional lives. Collaboration, introspection, and transparency are essential to becoming a successful leader.
Acknowledgments
The author gratefully acknowledges Dr David Berg for his invaluable mentorship as well as the core faculty of the SHM-SGIM Academic Hospitalist Academy 2.0 for their support and encouragement.
1. Wood M, Dibben M. Leadership as a relational process. Process Studies. 2015;44(1): 24-47. https://doi.org/10.5840/process20154412
2. Berg DN. Resurrecting the muse: followership in organizations. In: Klein EB, Gabelnick E, Herr R, eds. Psychodynamics of Leadership. Psychosocial Press; 1998.
3. Berg DN, Bradley EH. Leadership: Rhetoric vs. Reality. 2015. Accessed September 22, 2021. https://www.youtube.com/watch?v=77IwJ8wXaM8
1. Wood M, Dibben M. Leadership as a relational process. Process Studies. 2015;44(1): 24-47. https://doi.org/10.5840/process20154412
2. Berg DN. Resurrecting the muse: followership in organizations. In: Klein EB, Gabelnick E, Herr R, eds. Psychodynamics of Leadership. Psychosocial Press; 1998.
3. Berg DN, Bradley EH. Leadership: Rhetoric vs. Reality. 2015. Accessed September 22, 2021. https://www.youtube.com/watch?v=77IwJ8wXaM8
Simulation-Based Training in Medical Education: Immediate Growth or Cautious Optimism?
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
Techniques and Technologies to Improve Peripheral Intravenous Catheter Outcomes in Pediatric Patients: Systematic Review and Meta-Analysis
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.
Study Characteristics
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.
Study Quality
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).
Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).
Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.
Study Characteristics
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.

Study Quality
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).
Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).
Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.
Study Characteristics
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.

Study Quality
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).
Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).
Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
© 2021 Society of Hospital Medicine
Responsibilities and Interests of Pediatricians Practicing Hospital Medicine in the United States
As one of the youngest fields of pediatric practice in the United States, pediatric hospital medicine (PHM) has grown rapidly over the past 2 decades. Approximately 10% of recent graduates from pediatric residency programs in the United States have entered PHM, with two-thirds reporting an intention to remain as hospitalists long term.1,2
In October 2016, the American Board of Medical Specialties (ABMS) approved a petition for PHM to become the newest pediatric subspecialty.3 The application for subspeciality status, led by the Joint Council of Pediatric Hospital Medicine, articulated that subspecialty certification would more clearly define subspecialty hospitalists’ scope of practice, create a “new and larger cadre” of quality improvement (QI) experts, and strengthen opportunities for professional development related to child health safety within healthcare systems.4 Approximately 1500 pediatric hospitalists sat for the first PHM board-certification exam in November 2019, illustrating broad interest and commitment to this subspecialty.5
Characterizing the current responsibilities, practice settings, and professional interests of pediatric hospitalists is critical to understanding the continued development of the field. However, the most recent national survey of pediatric hospitalists’ roles and responsibilities was conducted more than a decade ago, and shared definitions of what constitutes PHM across institutions are lacking.6 Furthermore, studies suggest wide variability in PHM workload.7-9 We therefore aimed to describe the characteristics, responsibilities, and practice settings of pediatricians who reported practicing PHM in the United States and determine how exclusive PHM practice, compared with PHM practice in combination with primary or subspecialty care, was associated with professional responsibilities and interests. We hypothesized that those reporting exclusive PHM practice would be more likely to report interest in QI leadership and intention to take the PHM certifying exam than those practicing PHM in combination with primary or subspecialty care.
METHODS
Participants and Survey
Pediatricians enrolling in the American Board of Pediatrics (ABP) Maintenance of Certification (MOC) program in 2017 and 2018 were asked to complete a voluntary survey about their professional roles and scope of practice (Appendix Methods). The survey, offered to all MOC enrollees, included a hospital medicine module administered to those reporting PHM practice, given the ABP’s interest in characterizing PHM roles, responsibilities, practice settings, and interests in QI. Respondents were excluded if they were practicing outside of the United States, if they were unemployed or in a volunteer position, or if they were in fellowship training.
To ascertain areas of clinical practice, respondents were provided with a list of clinical practice areas and asked, “In which of the following areas are you practicing?” Those selecting “hospital medicine” were classified as self-identified hospitalists (hereafter, “hospitalists”). Given variation across institutions in physician roles and responsibilities, we stratified hospitalists into three groups: (1) exclusive PHM practice, representing those who reported PHM as their only area of practice; (2) PHM in combination with general pediatrics, representing those who reported practicing PHM and general pediatrics; and (3) PHM in combination with other subspecialties, representing those who reported practicing PHM in addition to one or more subspecialties. Respondents who reported practicing hospital medicine, general pediatrics, and another subspecialty were classified in the subspecialty group. The ABP’s institutional review board of record deemed the survey exempt from human subjects review.
Hospitalist Characteristics and Clinical Roles
To characterize respondents, we examined their age, gender, medical school location (American medical school or international medical school), and survey year (2017 or 2018). We also examined the following practice characteristics: US Census region, part-time versus full-time employment, academic appointment (yes or no), proportion of time spent providing direct and/or consultative patient care and fulfilling nonclinical responsibilities (research, administration, medical education, and QI), hospital setting (children’s hospital, community hospital, or mix of these hospital types), and work schedule type (shift schedule, on-service work in blocks, or a combination of shift and block schedules).
To examine variation in clinical roles, we determined the proportion of total direct and/or consultative clinical care that was spent in each of the following areas: (1) inpatient pediatric care, defined as inpatient general or subspecialty care in patients up to 21 years of age; (2) neonatal care, defined as labor and delivery, inpatient normal newborn care, and/or neonatal intensive care; (3) outpatient practice, defined as outpatient general or subspecialty care in patients up to 21 years of age; (4) emergency department care; and (5) other, which included pediatric intensive care as well inpatient adult care. Recognizing that scope of practice may differ at community hospitals and children’s hospitals, we stratified this analysis by practice setting (children’s hospital, community hospital).
Dependent Variables
We examined four dependent variables, two that were hypothesis driven and two that were exploratory. To test our hypothesis that respondents practicing PHM exclusively would be more likely to report interest in QI leadership or consultation (given the emphasis on QI in the ABMS application for subspecialty status), we examined the frequency with which respondents endorsed being “somewhat interested” or “very interested” in “serving as a leader or consultant for QI activities.” To test our hypothesis that respondents practicing PHM exclusively would be more likely to report plans to take the PHM certifying exam, we noted the frequency with which respondents reported “yes” to the question, “Do you plan to take a certifying exam in hospitalist medicine when it becomes available?” As an exploratory outcome, we examined satisfaction with allocation of professional time, available on the 2017 survey only; satisfaction was defined as an affirmative response to the question, “Is the allocation of your total professional time approximately what you wanted in your current position?” Finally, intention to maintain more than one ABP certification, also reported only in 2017 and examined as an exploratory outcome, was defined as a reported intention to maintain more than one ABP certification, including general pediatrics, PHM, or any other subspecialty.
Statistical Analysis
We used chi-square tests and analysis of variance as appropriate to examine differences in sociodemographic and professional characteristics among respondents who reported exclusive PHM practice, PHM in combination with general pediatrics, and PHM in combination with another subspecialty. To examine differences across the three PHM groups in their allocation of time to various clinical responsibilities (eg, inpatient care, newborn care), we used Kruskal-Wallis equality-of-population rank tests, stratifying by hospital type. We used multivariable logistic regression to identify associations between exclusive PHM practice and our four dependent variables, adjusting for the sociodemographic and professional characteristics described above. All analyses were conducted using Stata 15 (StataCorp LLC), using two-sided tests, and defining P < .05 as statistically significant.
RESULTS
Study Sample
Of the 19,763 pediatricians enrolling in MOC in 2017 and 2018, 13,839 responded the survey, representing a response rate of 70.0%. There were no significant differences between survey respondents and nonrespondents with respect to gender; differences between respondents and nonrespondents in age, medical school location, and initial year of ABP certification year were small (mean age, 48.1 years and 47.1 years, respectively [P < .01]; 77.0% of respondents were graduates of US medical schools compared with 73.7% of nonrespondents [P < .01]; mean certification year for respondents was 2003 compared with 2004 for nonrespondents [P < .01]). After applying the described exclusion criteria, 1662 of 12,665 respondents self-identified as hospitalists, reflecting 13.1% of the sample and the focus of this analysis (Appendix Figure).
Participant Characteristics and Areas of Practice
Of 1662 self-identified hospitalists, 881 (53.0%) also reported practicing general pediatrics, and 653 (39.3%) also reported practicing at least one subspecialty in addition to PHM. The most frequently reported additional subspecialty practice areas included: (1) neonatology (n = 155, 9.3%); (2) adolescent medicine (n = 138, 8.3%); (3) pediatric critical care (n = 89, 5.4%); (4) pediatric emergency medicine (n = 80, 4.8%); and (5) medicine-pediatrics (n = 30, 4.7%, asked only on the 2018 survey). When stratified into mutually exclusive groups, 491 respondents (29.5%) identified as practicing PHM exclusively, 518 (31.2%) identified as practicing PHM in combination with general pediatrics, and 653 (39.3%) identified as practicing PHM in combination with one or more other subspecialties.
Table 1 summarizes the characteristics of respondents in these three groups. Respondents reporting exclusive PHM practice were, on average, younger, more likely to be female, and more likely to be graduates of US medical schools than those reporting PHM in combination with general or subspecialty pediatrics. In total, approximately two-thirds of the sample (n = 1068, 64.3%) reported holding an academic appointment, including 72.9% (n = 358) of those reporting exclusive PHM practice compared with 56.9% (n = 295) of those also reporting general pediatrics and 63.6% (n = 415) of those also reporting subspecialty care (P < .001). Respondents who reported practicing PHM exclusively most frequently worked at children’s hospitals (64.6%, n = 317), compared with 40.0% (n = 207) and 42.1% (n = 275) of those practicing PHM in combination with general and subspecialty pediatrics, respectively (P < .001).
Clinical and Nonclinical Roles and Responsibilities
The majority of respondents reported that they spent >75% of their professional time in direct clinical or consultative care, including 62.1% (n = 305) of those reporting PHM exclusively and 77.8% (n = 403) and 66.6% (n = 435) of those reporting PHM with general and subspecialty pediatrics, respectively (P < .001). Overall, <10% reported spending less than 50% of their time proving direct patient care, including 11.2% (n = 55) of those reporting exclusive PHM practice, 11.2% (n = 73) reporting PHM in combination with a subspecialty, and 6% (n = 31) in combination with general pediatrics. The mean proportion of time spent in nonclinical roles was 22.4% (SD, 20.4%), and the mean proportions of time spent in any one area (administration, research, education, or QI) were all <10%.
The proportion of time allocated to inpatient pediatric care, neonatal care, emergency care, and outpatient pediatric care varied substantially across PHM practice groups and settings. Among respondents who practiced at children’s hospitals, the median percentage of clinical time dedicated to inpatient pediatric care was 66.5% (interquartile range [IQR], 15%-100%), with neonatal care being the second most common clinical practice area (Figure, part A; Appendix Table). At community hospitals, the percentage of clinical time dedicated to inpatient pediatric care was lower, with a median of 10% (IQR, 3%-40%) (Figure, part B). Among those reporting exclusive PHM practice, the median proportion of clinical time spent delivering inpatient pediatric care was 100% (IQR, 80%-100%) at children’s hospitals and 40% (IQR, 20%-85%) at community hospitals. At community hospitals, neonatal care accounted for a similar proportion of clinical time as inpatient pediatric care for these respondents (median, 40% [IQR, 0%-70%]). With the exception of emergency room care, we observed significant differences in how clinical time was allocated by respondents reporting exclusive PHM practice compared with those reporting PHM in combination with general or specialty care (all P values < .001, Appendix Table).
Professional Development Interests
Approximately two-thirds of respondents reported interest in QI leadership or consultation (Table 2), with those reporting exclusive PHM practice significantly more likely to report this (70.3% [n = 345] compared with 57.7% [n = 297] of those practicing PHM with general pediatrics and 66.3% [n = 431] of those practicing PHM with another subspecialty, P < .001). Similarly, 69% (n = 339) of respondents who reported exclusive PHM practice described an intention to take the PHM certifying examination, compared with 20.4% (n = 105) of those practicing PHM and general pediatrics and 17.7% (n = 115) of those practicing PHM and subspeciality pediatrics (P < .001). A total of 82.5% (n = 846) of respondents reported that they were satisfied with the allocation of their professional time; there were no significant differences between those reporting exclusive PHM practice and those reporting PHM in combination with general or subspecialty pediatrics. Of hospitalists reporting exclusive PHM practice, 67.8% (n = 166) reported an intention to maintain more than one ABP certification, compared with 22.1% (n = 78) of those practicing PHM and general pediatrics and 53.9% (n = 230) of those practicing PHM and subspecialty pediatrics (P < .001).
In multivariate regression analyses, hospitalists reporting exclusive PHM practice had significantly greater odds of reported interest in QI leadership or consultation (adjusted odds ratio [OR], 1.39; 95% CI, 1.09-1.79), intention to take the PHM certifying exam (adjusted OR, 7.10; 95% CI, 5.45-9.25), and intention to maintain more than one ABP certification (adjusted OR, 2.64; 95% CI, 1.89-3.68) than those practicing PHM in combination with general or subspecialty pediatrics (Table 3). There was no significant difference across the three groups in the satisfaction with the allocation of professional time.
DISCUSSION
In this national survey of pediatricians seeking MOC from the ABP, 13.1% reported that they practiced hospital medicine, with approximately one-third of these individuals reporting that they practiced PHM exclusively. The distribution of clinical and nonclinical responsibilities differed across those reporting exclusive PHM practice relative to those practicing PHM in combination with general or subspecialty pediatrics. Relative to hospitalists who reported practicing PHM in addition to general or subspecialty care, those reporting exclusive PHM practice were significantly more likely to report an interest in QI leadership or consultation, intention to sit for the PHM board-certification exam, and intention to maintain more than one ABP certification.
These findings offer insight into the evolution of PHM and have important implications for workforce planning. The last nationally representative analysis of the PHM workforce was conducted in 2006, at which time 73% of hospitalists reported working at children’s hospitals.6 In the current analysis, less than 50% of hospitalists reported practicing PHM at children’s hospitals only; 10% reported working at both children’s hospitals and community hospitals and 40% at community hospitals alone. This diffusion of PHM from children’s hospitals into community hospitals represents an important development in the field and aligns with the epidemiology of pediatric hospitalization.10 Pediatric hospitalists who practice at community hospitals experience unique challenges, including a relative paucity of pediatric-specific clinical resources, limited mentorship opportunities and resources for scholarly work, and limited access to data from which to prioritize QI interventions.11,12 Our findings also illustrate that the scope of practice for hospitalists differs at community hospitals relative to children’s hospitals. Although the PHM fellowship curriculum requires training at a community hospital, the requirement is limited to one 4-week block, which may not provide sufficient preparation for the unique clinical responsibilities in this setting.13,14
Relative to past analyses of PHM workforce roles and responsibilities, a substantially greater proportion of respondents in the current study reported clinical responsibility for neonatal care, including more than 40% of those self-reporting practicing PHM exclusively and almost three-quarters of those self-reporting PHM in conjunction with general pediatrics.6,15 Given that more than half of the six million US pediatric hospitalizations that occur each year represent birth hospitalizations,16 pediatric hospitalists’ responsibilities for newborn care are consistent with these patterns of hospital-based care. Expanding hospitalists’ responsibilities to provide newborn care has also been shown to improve the financial performance of PHM programs with relatively low pediatric volumes, which may further explain this finding, particularly at community hospitals.17,18 Interestingly, although emergency department care has also been demonstrated as a model to improve the financial stability of PHM programs, relatively few hospitalists reported this as an area of clinical responsibility.19,20 This finding contrasts with past analyses and may reflect how the scope of PHM clinical responsibilities has changed since these prior studies were conducted.6,15
Because PHM had not been recognized as a subspecialty prior to 2016, a national count of pediatric hospitalists is lacking. In this study, approximately one in eight pediatricians reported that they practiced PHM, but less than 4% of the survey sample reported practicing PHM exclusively. Based on these results, we estimate that of the 76,214 to 89,608 ABP-certified pediatricians currently practicing in the United States, between 9984 and 11,738 would self-identify as practicing PHM, with between 2945 and 3462 reporting exclusive PHM practice.
Hospitalists who reported practicing PHM exclusively were significantly more likely to report an interest in QI leadership or consultation and plans to take the PHM certifying exam. These findings are consistent with PHM’s focus on QI, as articulated in the application to the ABMS for subspecialty status as well as the PHM Core Competencies and fellowship curriculum.4,13,21,22 Despite past research questioning the sustainability of some community- and university-based PHM programs and wide variability in workload,7-9 more than 80% of hospitalists reported satisfaction with the allocation of their professional time, with no significant differences between respondents practicing PHM exclusively or in combination with general or subspecialty care.
This analysis should be interpreted in light of its strengths and limitations. Strengths of this work include its national focus, large sample size, and comprehensive characterization of respondents’ professional roles and characteristics. Study limitations include the fact that respondents were classified as hospitalists based on self-report; we were unable to ascertain if they were classified as hospitalists at their place of employment or if they met the ABP’s eligibility criteria to sit for the PHM subspecialty certifying exam.19 Additionally, respondents self-reported their allocations of clinical and nonclinical time, and we are unable to correlate this with actual work hours. Respondents’ reported interest in QI leadership or consultation may not be correlated with QI effort in practice; the mean time reportedly dedicated to QI activities was quite low. Additionally, two of our outcomes were available only for respondents who enrolled in MOC in 2017, and the proportion practicing medicine-pediatrics was available only in 2018. Although this analysis represents approximately 40% of all pediatricians enrolling in MOC (2 years of the 5-year MOC cycle), it may not be representative of pediatricians who are not certified by the ABP. Finally, our outcomes related to board certification examined interest and intentions; future study will be needed to determine how many pediatricians take the PHM exam and maintain certification.
In conclusion, the field of PHM has evolved considerably since its inception, with pediatric hospitalists reporting diverse clinical and nonclinical responsibilities. Hospitalists practicing PHM exclusively were more likely to report an interest in QI leadership and intent to sit for the PHM certifying exam than those practicing PHM in combination with general pediatrics or another specialty. Continuing to monitor the evolution of PHM roles and responsibilities over time and across settings will be important to support the professional development needs of the PHM workforce.
1. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001
3. The American Board of Pediatrics. ABMS approves pediatric hospital medicine certification. November 8, 2016. Accessed October 12, 2021. https://www.abp.org/news/abms-approves-pediatric-hospital-medicine-certification
4. American Board of Medical Specialities. Application for a new subspecialty certificate: pediatric hospital medicine.
5. American Board of Pediatrics. 2019 Annual Report. Accessed October 12, 2021. https://www.abp.org/sites/abp/files/pdf/annual-report-2019.pdf
6. Freed GL, Dunham KM, Research Advisory Committee of the American Board of Pediatrics. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186. https://doi.org/10.1002/jhm.458
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(11):682-685. https://doi.org/10.12788/jhm.3263
8. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977
9. Gosdin C, Simmons J, Yau C, Sucharew H, Carlson D, Paciorkowski N. Survey of academic pediatric hospitalist programs in the US: organizational, administrative, and financial factors. J Hosp Med. 2013;8(6):285-291. https://doi.org/10.1002/jhm.2020
10. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
11. Leary JC, Walsh KE, Morin RA, Schainker EG, Leyenaar JK. Quality and safety of pediatric inpatient care in community hospitals: a scoping review. J Hosp Med. 2019;14:694-703. https://doi.org/10.12788/jhm.3268
12. Leyenaar JK, Capra LA, O’Brien ER, Leslie LK, Mackie TI. Determinants of career satisfaction among pediatric hospitalists: a qualitative exploration. Acad Pediatr. 2014;14(4):361-368. https://doi.org/10.1016/j.acap.2014.03.015
13. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. July 1, 2021. Accessed October 4, 2021.https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/334_PediatricHospitalMedicine_2020.pdf?ver=2020-06-29-163350-910&ver=2020-06-29-163350-910
15. Freed GL, Brzoznowski K, Neighbors K, Lakhani I, American Board of Pediatrics, Research Advisory Committee. Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(1):33-39. https://doi.org/10.1542/peds.2007-0304
16. Moore B, Freeman W, Jiang H. Costs of Pediatric Hospital Stays, 2016. Healthcare Cost and Utilization Project Statistical Brief #250. Accessed October 25, 2021. https://www.ncbi.nlm.nih.gov/books/NBK547762/
17. Carlson DW, Fentzke KM, Dawson JG. Pediatric hospitalists: fill varied roles in the care of newborns. Pediatr Ann. 2003;32(12):802-810. https://doi.org/10.3928/0090-4481-20031201-09
18. Tieder JS, Migita DS, Cowan CA, Melzer SM. Newborn care by pediatric hospitalists in a community hospital: effect on physician productivity and financial performance. Arch Pediatr Adolesc Med. 2008;162(1):74-78. https://doi.org/10.1001/archpediatrics.2007.15
19. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01
20. Dudas RA, Monroe D, McColligan Borger M. Community pediatric hospitalists providing care in the emergency department: an analysis of physician productivity and financial performance. Pediatr Emerg Care. 2011;27(11):1099-1103. https://doi.org/10.1097/PEC.0b013e31823606f5
21. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343. https://doi.org/10.1002/jhm.843
22. Maniscalco J, Gage S, Teferi S, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
As one of the youngest fields of pediatric practice in the United States, pediatric hospital medicine (PHM) has grown rapidly over the past 2 decades. Approximately 10% of recent graduates from pediatric residency programs in the United States have entered PHM, with two-thirds reporting an intention to remain as hospitalists long term.1,2
In October 2016, the American Board of Medical Specialties (ABMS) approved a petition for PHM to become the newest pediatric subspecialty.3 The application for subspeciality status, led by the Joint Council of Pediatric Hospital Medicine, articulated that subspecialty certification would more clearly define subspecialty hospitalists’ scope of practice, create a “new and larger cadre” of quality improvement (QI) experts, and strengthen opportunities for professional development related to child health safety within healthcare systems.4 Approximately 1500 pediatric hospitalists sat for the first PHM board-certification exam in November 2019, illustrating broad interest and commitment to this subspecialty.5
Characterizing the current responsibilities, practice settings, and professional interests of pediatric hospitalists is critical to understanding the continued development of the field. However, the most recent national survey of pediatric hospitalists’ roles and responsibilities was conducted more than a decade ago, and shared definitions of what constitutes PHM across institutions are lacking.6 Furthermore, studies suggest wide variability in PHM workload.7-9 We therefore aimed to describe the characteristics, responsibilities, and practice settings of pediatricians who reported practicing PHM in the United States and determine how exclusive PHM practice, compared with PHM practice in combination with primary or subspecialty care, was associated with professional responsibilities and interests. We hypothesized that those reporting exclusive PHM practice would be more likely to report interest in QI leadership and intention to take the PHM certifying exam than those practicing PHM in combination with primary or subspecialty care.
METHODS
Participants and Survey
Pediatricians enrolling in the American Board of Pediatrics (ABP) Maintenance of Certification (MOC) program in 2017 and 2018 were asked to complete a voluntary survey about their professional roles and scope of practice (Appendix Methods). The survey, offered to all MOC enrollees, included a hospital medicine module administered to those reporting PHM practice, given the ABP’s interest in characterizing PHM roles, responsibilities, practice settings, and interests in QI. Respondents were excluded if they were practicing outside of the United States, if they were unemployed or in a volunteer position, or if they were in fellowship training.
To ascertain areas of clinical practice, respondents were provided with a list of clinical practice areas and asked, “In which of the following areas are you practicing?” Those selecting “hospital medicine” were classified as self-identified hospitalists (hereafter, “hospitalists”). Given variation across institutions in physician roles and responsibilities, we stratified hospitalists into three groups: (1) exclusive PHM practice, representing those who reported PHM as their only area of practice; (2) PHM in combination with general pediatrics, representing those who reported practicing PHM and general pediatrics; and (3) PHM in combination with other subspecialties, representing those who reported practicing PHM in addition to one or more subspecialties. Respondents who reported practicing hospital medicine, general pediatrics, and another subspecialty were classified in the subspecialty group. The ABP’s institutional review board of record deemed the survey exempt from human subjects review.
Hospitalist Characteristics and Clinical Roles
To characterize respondents, we examined their age, gender, medical school location (American medical school or international medical school), and survey year (2017 or 2018). We also examined the following practice characteristics: US Census region, part-time versus full-time employment, academic appointment (yes or no), proportion of time spent providing direct and/or consultative patient care and fulfilling nonclinical responsibilities (research, administration, medical education, and QI), hospital setting (children’s hospital, community hospital, or mix of these hospital types), and work schedule type (shift schedule, on-service work in blocks, or a combination of shift and block schedules).
To examine variation in clinical roles, we determined the proportion of total direct and/or consultative clinical care that was spent in each of the following areas: (1) inpatient pediatric care, defined as inpatient general or subspecialty care in patients up to 21 years of age; (2) neonatal care, defined as labor and delivery, inpatient normal newborn care, and/or neonatal intensive care; (3) outpatient practice, defined as outpatient general or subspecialty care in patients up to 21 years of age; (4) emergency department care; and (5) other, which included pediatric intensive care as well inpatient adult care. Recognizing that scope of practice may differ at community hospitals and children’s hospitals, we stratified this analysis by practice setting (children’s hospital, community hospital).
Dependent Variables
We examined four dependent variables, two that were hypothesis driven and two that were exploratory. To test our hypothesis that respondents practicing PHM exclusively would be more likely to report interest in QI leadership or consultation (given the emphasis on QI in the ABMS application for subspecialty status), we examined the frequency with which respondents endorsed being “somewhat interested” or “very interested” in “serving as a leader or consultant for QI activities.” To test our hypothesis that respondents practicing PHM exclusively would be more likely to report plans to take the PHM certifying exam, we noted the frequency with which respondents reported “yes” to the question, “Do you plan to take a certifying exam in hospitalist medicine when it becomes available?” As an exploratory outcome, we examined satisfaction with allocation of professional time, available on the 2017 survey only; satisfaction was defined as an affirmative response to the question, “Is the allocation of your total professional time approximately what you wanted in your current position?” Finally, intention to maintain more than one ABP certification, also reported only in 2017 and examined as an exploratory outcome, was defined as a reported intention to maintain more than one ABP certification, including general pediatrics, PHM, or any other subspecialty.
Statistical Analysis
We used chi-square tests and analysis of variance as appropriate to examine differences in sociodemographic and professional characteristics among respondents who reported exclusive PHM practice, PHM in combination with general pediatrics, and PHM in combination with another subspecialty. To examine differences across the three PHM groups in their allocation of time to various clinical responsibilities (eg, inpatient care, newborn care), we used Kruskal-Wallis equality-of-population rank tests, stratifying by hospital type. We used multivariable logistic regression to identify associations between exclusive PHM practice and our four dependent variables, adjusting for the sociodemographic and professional characteristics described above. All analyses were conducted using Stata 15 (StataCorp LLC), using two-sided tests, and defining P < .05 as statistically significant.
RESULTS
Study Sample
Of the 19,763 pediatricians enrolling in MOC in 2017 and 2018, 13,839 responded the survey, representing a response rate of 70.0%. There were no significant differences between survey respondents and nonrespondents with respect to gender; differences between respondents and nonrespondents in age, medical school location, and initial year of ABP certification year were small (mean age, 48.1 years and 47.1 years, respectively [P < .01]; 77.0% of respondents were graduates of US medical schools compared with 73.7% of nonrespondents [P < .01]; mean certification year for respondents was 2003 compared with 2004 for nonrespondents [P < .01]). After applying the described exclusion criteria, 1662 of 12,665 respondents self-identified as hospitalists, reflecting 13.1% of the sample and the focus of this analysis (Appendix Figure).
Participant Characteristics and Areas of Practice
Of 1662 self-identified hospitalists, 881 (53.0%) also reported practicing general pediatrics, and 653 (39.3%) also reported practicing at least one subspecialty in addition to PHM. The most frequently reported additional subspecialty practice areas included: (1) neonatology (n = 155, 9.3%); (2) adolescent medicine (n = 138, 8.3%); (3) pediatric critical care (n = 89, 5.4%); (4) pediatric emergency medicine (n = 80, 4.8%); and (5) medicine-pediatrics (n = 30, 4.7%, asked only on the 2018 survey). When stratified into mutually exclusive groups, 491 respondents (29.5%) identified as practicing PHM exclusively, 518 (31.2%) identified as practicing PHM in combination with general pediatrics, and 653 (39.3%) identified as practicing PHM in combination with one or more other subspecialties.
Table 1 summarizes the characteristics of respondents in these three groups. Respondents reporting exclusive PHM practice were, on average, younger, more likely to be female, and more likely to be graduates of US medical schools than those reporting PHM in combination with general or subspecialty pediatrics. In total, approximately two-thirds of the sample (n = 1068, 64.3%) reported holding an academic appointment, including 72.9% (n = 358) of those reporting exclusive PHM practice compared with 56.9% (n = 295) of those also reporting general pediatrics and 63.6% (n = 415) of those also reporting subspecialty care (P < .001). Respondents who reported practicing PHM exclusively most frequently worked at children’s hospitals (64.6%, n = 317), compared with 40.0% (n = 207) and 42.1% (n = 275) of those practicing PHM in combination with general and subspecialty pediatrics, respectively (P < .001).
Clinical and Nonclinical Roles and Responsibilities
The majority of respondents reported that they spent >75% of their professional time in direct clinical or consultative care, including 62.1% (n = 305) of those reporting PHM exclusively and 77.8% (n = 403) and 66.6% (n = 435) of those reporting PHM with general and subspecialty pediatrics, respectively (P < .001). Overall, <10% reported spending less than 50% of their time proving direct patient care, including 11.2% (n = 55) of those reporting exclusive PHM practice, 11.2% (n = 73) reporting PHM in combination with a subspecialty, and 6% (n = 31) in combination with general pediatrics. The mean proportion of time spent in nonclinical roles was 22.4% (SD, 20.4%), and the mean proportions of time spent in any one area (administration, research, education, or QI) were all <10%.
The proportion of time allocated to inpatient pediatric care, neonatal care, emergency care, and outpatient pediatric care varied substantially across PHM practice groups and settings. Among respondents who practiced at children’s hospitals, the median percentage of clinical time dedicated to inpatient pediatric care was 66.5% (interquartile range [IQR], 15%-100%), with neonatal care being the second most common clinical practice area (Figure, part A; Appendix Table). At community hospitals, the percentage of clinical time dedicated to inpatient pediatric care was lower, with a median of 10% (IQR, 3%-40%) (Figure, part B). Among those reporting exclusive PHM practice, the median proportion of clinical time spent delivering inpatient pediatric care was 100% (IQR, 80%-100%) at children’s hospitals and 40% (IQR, 20%-85%) at community hospitals. At community hospitals, neonatal care accounted for a similar proportion of clinical time as inpatient pediatric care for these respondents (median, 40% [IQR, 0%-70%]). With the exception of emergency room care, we observed significant differences in how clinical time was allocated by respondents reporting exclusive PHM practice compared with those reporting PHM in combination with general or specialty care (all P values < .001, Appendix Table).
Professional Development Interests
Approximately two-thirds of respondents reported interest in QI leadership or consultation (Table 2), with those reporting exclusive PHM practice significantly more likely to report this (70.3% [n = 345] compared with 57.7% [n = 297] of those practicing PHM with general pediatrics and 66.3% [n = 431] of those practicing PHM with another subspecialty, P < .001). Similarly, 69% (n = 339) of respondents who reported exclusive PHM practice described an intention to take the PHM certifying examination, compared with 20.4% (n = 105) of those practicing PHM and general pediatrics and 17.7% (n = 115) of those practicing PHM and subspeciality pediatrics (P < .001). A total of 82.5% (n = 846) of respondents reported that they were satisfied with the allocation of their professional time; there were no significant differences between those reporting exclusive PHM practice and those reporting PHM in combination with general or subspecialty pediatrics. Of hospitalists reporting exclusive PHM practice, 67.8% (n = 166) reported an intention to maintain more than one ABP certification, compared with 22.1% (n = 78) of those practicing PHM and general pediatrics and 53.9% (n = 230) of those practicing PHM and subspecialty pediatrics (P < .001).
In multivariate regression analyses, hospitalists reporting exclusive PHM practice had significantly greater odds of reported interest in QI leadership or consultation (adjusted odds ratio [OR], 1.39; 95% CI, 1.09-1.79), intention to take the PHM certifying exam (adjusted OR, 7.10; 95% CI, 5.45-9.25), and intention to maintain more than one ABP certification (adjusted OR, 2.64; 95% CI, 1.89-3.68) than those practicing PHM in combination with general or subspecialty pediatrics (Table 3). There was no significant difference across the three groups in the satisfaction with the allocation of professional time.
DISCUSSION
In this national survey of pediatricians seeking MOC from the ABP, 13.1% reported that they practiced hospital medicine, with approximately one-third of these individuals reporting that they practiced PHM exclusively. The distribution of clinical and nonclinical responsibilities differed across those reporting exclusive PHM practice relative to those practicing PHM in combination with general or subspecialty pediatrics. Relative to hospitalists who reported practicing PHM in addition to general or subspecialty care, those reporting exclusive PHM practice were significantly more likely to report an interest in QI leadership or consultation, intention to sit for the PHM board-certification exam, and intention to maintain more than one ABP certification.
These findings offer insight into the evolution of PHM and have important implications for workforce planning. The last nationally representative analysis of the PHM workforce was conducted in 2006, at which time 73% of hospitalists reported working at children’s hospitals.6 In the current analysis, less than 50% of hospitalists reported practicing PHM at children’s hospitals only; 10% reported working at both children’s hospitals and community hospitals and 40% at community hospitals alone. This diffusion of PHM from children’s hospitals into community hospitals represents an important development in the field and aligns with the epidemiology of pediatric hospitalization.10 Pediatric hospitalists who practice at community hospitals experience unique challenges, including a relative paucity of pediatric-specific clinical resources, limited mentorship opportunities and resources for scholarly work, and limited access to data from which to prioritize QI interventions.11,12 Our findings also illustrate that the scope of practice for hospitalists differs at community hospitals relative to children’s hospitals. Although the PHM fellowship curriculum requires training at a community hospital, the requirement is limited to one 4-week block, which may not provide sufficient preparation for the unique clinical responsibilities in this setting.13,14
Relative to past analyses of PHM workforce roles and responsibilities, a substantially greater proportion of respondents in the current study reported clinical responsibility for neonatal care, including more than 40% of those self-reporting practicing PHM exclusively and almost three-quarters of those self-reporting PHM in conjunction with general pediatrics.6,15 Given that more than half of the six million US pediatric hospitalizations that occur each year represent birth hospitalizations,16 pediatric hospitalists’ responsibilities for newborn care are consistent with these patterns of hospital-based care. Expanding hospitalists’ responsibilities to provide newborn care has also been shown to improve the financial performance of PHM programs with relatively low pediatric volumes, which may further explain this finding, particularly at community hospitals.17,18 Interestingly, although emergency department care has also been demonstrated as a model to improve the financial stability of PHM programs, relatively few hospitalists reported this as an area of clinical responsibility.19,20 This finding contrasts with past analyses and may reflect how the scope of PHM clinical responsibilities has changed since these prior studies were conducted.6,15
Because PHM had not been recognized as a subspecialty prior to 2016, a national count of pediatric hospitalists is lacking. In this study, approximately one in eight pediatricians reported that they practiced PHM, but less than 4% of the survey sample reported practicing PHM exclusively. Based on these results, we estimate that of the 76,214 to 89,608 ABP-certified pediatricians currently practicing in the United States, between 9984 and 11,738 would self-identify as practicing PHM, with between 2945 and 3462 reporting exclusive PHM practice.
Hospitalists who reported practicing PHM exclusively were significantly more likely to report an interest in QI leadership or consultation and plans to take the PHM certifying exam. These findings are consistent with PHM’s focus on QI, as articulated in the application to the ABMS for subspecialty status as well as the PHM Core Competencies and fellowship curriculum.4,13,21,22 Despite past research questioning the sustainability of some community- and university-based PHM programs and wide variability in workload,7-9 more than 80% of hospitalists reported satisfaction with the allocation of their professional time, with no significant differences between respondents practicing PHM exclusively or in combination with general or subspecialty care.
This analysis should be interpreted in light of its strengths and limitations. Strengths of this work include its national focus, large sample size, and comprehensive characterization of respondents’ professional roles and characteristics. Study limitations include the fact that respondents were classified as hospitalists based on self-report; we were unable to ascertain if they were classified as hospitalists at their place of employment or if they met the ABP’s eligibility criteria to sit for the PHM subspecialty certifying exam.19 Additionally, respondents self-reported their allocations of clinical and nonclinical time, and we are unable to correlate this with actual work hours. Respondents’ reported interest in QI leadership or consultation may not be correlated with QI effort in practice; the mean time reportedly dedicated to QI activities was quite low. Additionally, two of our outcomes were available only for respondents who enrolled in MOC in 2017, and the proportion practicing medicine-pediatrics was available only in 2018. Although this analysis represents approximately 40% of all pediatricians enrolling in MOC (2 years of the 5-year MOC cycle), it may not be representative of pediatricians who are not certified by the ABP. Finally, our outcomes related to board certification examined interest and intentions; future study will be needed to determine how many pediatricians take the PHM exam and maintain certification.
In conclusion, the field of PHM has evolved considerably since its inception, with pediatric hospitalists reporting diverse clinical and nonclinical responsibilities. Hospitalists practicing PHM exclusively were more likely to report an interest in QI leadership and intent to sit for the PHM certifying exam than those practicing PHM in combination with general pediatrics or another specialty. Continuing to monitor the evolution of PHM roles and responsibilities over time and across settings will be important to support the professional development needs of the PHM workforce.
As one of the youngest fields of pediatric practice in the United States, pediatric hospital medicine (PHM) has grown rapidly over the past 2 decades. Approximately 10% of recent graduates from pediatric residency programs in the United States have entered PHM, with two-thirds reporting an intention to remain as hospitalists long term.1,2
In October 2016, the American Board of Medical Specialties (ABMS) approved a petition for PHM to become the newest pediatric subspecialty.3 The application for subspeciality status, led by the Joint Council of Pediatric Hospital Medicine, articulated that subspecialty certification would more clearly define subspecialty hospitalists’ scope of practice, create a “new and larger cadre” of quality improvement (QI) experts, and strengthen opportunities for professional development related to child health safety within healthcare systems.4 Approximately 1500 pediatric hospitalists sat for the first PHM board-certification exam in November 2019, illustrating broad interest and commitment to this subspecialty.5
Characterizing the current responsibilities, practice settings, and professional interests of pediatric hospitalists is critical to understanding the continued development of the field. However, the most recent national survey of pediatric hospitalists’ roles and responsibilities was conducted more than a decade ago, and shared definitions of what constitutes PHM across institutions are lacking.6 Furthermore, studies suggest wide variability in PHM workload.7-9 We therefore aimed to describe the characteristics, responsibilities, and practice settings of pediatricians who reported practicing PHM in the United States and determine how exclusive PHM practice, compared with PHM practice in combination with primary or subspecialty care, was associated with professional responsibilities and interests. We hypothesized that those reporting exclusive PHM practice would be more likely to report interest in QI leadership and intention to take the PHM certifying exam than those practicing PHM in combination with primary or subspecialty care.
METHODS
Participants and Survey
Pediatricians enrolling in the American Board of Pediatrics (ABP) Maintenance of Certification (MOC) program in 2017 and 2018 were asked to complete a voluntary survey about their professional roles and scope of practice (Appendix Methods). The survey, offered to all MOC enrollees, included a hospital medicine module administered to those reporting PHM practice, given the ABP’s interest in characterizing PHM roles, responsibilities, practice settings, and interests in QI. Respondents were excluded if they were practicing outside of the United States, if they were unemployed or in a volunteer position, or if they were in fellowship training.
To ascertain areas of clinical practice, respondents were provided with a list of clinical practice areas and asked, “In which of the following areas are you practicing?” Those selecting “hospital medicine” were classified as self-identified hospitalists (hereafter, “hospitalists”). Given variation across institutions in physician roles and responsibilities, we stratified hospitalists into three groups: (1) exclusive PHM practice, representing those who reported PHM as their only area of practice; (2) PHM in combination with general pediatrics, representing those who reported practicing PHM and general pediatrics; and (3) PHM in combination with other subspecialties, representing those who reported practicing PHM in addition to one or more subspecialties. Respondents who reported practicing hospital medicine, general pediatrics, and another subspecialty were classified in the subspecialty group. The ABP’s institutional review board of record deemed the survey exempt from human subjects review.
Hospitalist Characteristics and Clinical Roles
To characterize respondents, we examined their age, gender, medical school location (American medical school or international medical school), and survey year (2017 or 2018). We also examined the following practice characteristics: US Census region, part-time versus full-time employment, academic appointment (yes or no), proportion of time spent providing direct and/or consultative patient care and fulfilling nonclinical responsibilities (research, administration, medical education, and QI), hospital setting (children’s hospital, community hospital, or mix of these hospital types), and work schedule type (shift schedule, on-service work in blocks, or a combination of shift and block schedules).
To examine variation in clinical roles, we determined the proportion of total direct and/or consultative clinical care that was spent in each of the following areas: (1) inpatient pediatric care, defined as inpatient general or subspecialty care in patients up to 21 years of age; (2) neonatal care, defined as labor and delivery, inpatient normal newborn care, and/or neonatal intensive care; (3) outpatient practice, defined as outpatient general or subspecialty care in patients up to 21 years of age; (4) emergency department care; and (5) other, which included pediatric intensive care as well inpatient adult care. Recognizing that scope of practice may differ at community hospitals and children’s hospitals, we stratified this analysis by practice setting (children’s hospital, community hospital).
Dependent Variables
We examined four dependent variables, two that were hypothesis driven and two that were exploratory. To test our hypothesis that respondents practicing PHM exclusively would be more likely to report interest in QI leadership or consultation (given the emphasis on QI in the ABMS application for subspecialty status), we examined the frequency with which respondents endorsed being “somewhat interested” or “very interested” in “serving as a leader or consultant for QI activities.” To test our hypothesis that respondents practicing PHM exclusively would be more likely to report plans to take the PHM certifying exam, we noted the frequency with which respondents reported “yes” to the question, “Do you plan to take a certifying exam in hospitalist medicine when it becomes available?” As an exploratory outcome, we examined satisfaction with allocation of professional time, available on the 2017 survey only; satisfaction was defined as an affirmative response to the question, “Is the allocation of your total professional time approximately what you wanted in your current position?” Finally, intention to maintain more than one ABP certification, also reported only in 2017 and examined as an exploratory outcome, was defined as a reported intention to maintain more than one ABP certification, including general pediatrics, PHM, or any other subspecialty.
Statistical Analysis
We used chi-square tests and analysis of variance as appropriate to examine differences in sociodemographic and professional characteristics among respondents who reported exclusive PHM practice, PHM in combination with general pediatrics, and PHM in combination with another subspecialty. To examine differences across the three PHM groups in their allocation of time to various clinical responsibilities (eg, inpatient care, newborn care), we used Kruskal-Wallis equality-of-population rank tests, stratifying by hospital type. We used multivariable logistic regression to identify associations between exclusive PHM practice and our four dependent variables, adjusting for the sociodemographic and professional characteristics described above. All analyses were conducted using Stata 15 (StataCorp LLC), using two-sided tests, and defining P < .05 as statistically significant.
RESULTS
Study Sample
Of the 19,763 pediatricians enrolling in MOC in 2017 and 2018, 13,839 responded the survey, representing a response rate of 70.0%. There were no significant differences between survey respondents and nonrespondents with respect to gender; differences between respondents and nonrespondents in age, medical school location, and initial year of ABP certification year were small (mean age, 48.1 years and 47.1 years, respectively [P < .01]; 77.0% of respondents were graduates of US medical schools compared with 73.7% of nonrespondents [P < .01]; mean certification year for respondents was 2003 compared with 2004 for nonrespondents [P < .01]). After applying the described exclusion criteria, 1662 of 12,665 respondents self-identified as hospitalists, reflecting 13.1% of the sample and the focus of this analysis (Appendix Figure).
Participant Characteristics and Areas of Practice
Of 1662 self-identified hospitalists, 881 (53.0%) also reported practicing general pediatrics, and 653 (39.3%) also reported practicing at least one subspecialty in addition to PHM. The most frequently reported additional subspecialty practice areas included: (1) neonatology (n = 155, 9.3%); (2) adolescent medicine (n = 138, 8.3%); (3) pediatric critical care (n = 89, 5.4%); (4) pediatric emergency medicine (n = 80, 4.8%); and (5) medicine-pediatrics (n = 30, 4.7%, asked only on the 2018 survey). When stratified into mutually exclusive groups, 491 respondents (29.5%) identified as practicing PHM exclusively, 518 (31.2%) identified as practicing PHM in combination with general pediatrics, and 653 (39.3%) identified as practicing PHM in combination with one or more other subspecialties.
Table 1 summarizes the characteristics of respondents in these three groups. Respondents reporting exclusive PHM practice were, on average, younger, more likely to be female, and more likely to be graduates of US medical schools than those reporting PHM in combination with general or subspecialty pediatrics. In total, approximately two-thirds of the sample (n = 1068, 64.3%) reported holding an academic appointment, including 72.9% (n = 358) of those reporting exclusive PHM practice compared with 56.9% (n = 295) of those also reporting general pediatrics and 63.6% (n = 415) of those also reporting subspecialty care (P < .001). Respondents who reported practicing PHM exclusively most frequently worked at children’s hospitals (64.6%, n = 317), compared with 40.0% (n = 207) and 42.1% (n = 275) of those practicing PHM in combination with general and subspecialty pediatrics, respectively (P < .001).
Clinical and Nonclinical Roles and Responsibilities
The majority of respondents reported that they spent >75% of their professional time in direct clinical or consultative care, including 62.1% (n = 305) of those reporting PHM exclusively and 77.8% (n = 403) and 66.6% (n = 435) of those reporting PHM with general and subspecialty pediatrics, respectively (P < .001). Overall, <10% reported spending less than 50% of their time proving direct patient care, including 11.2% (n = 55) of those reporting exclusive PHM practice, 11.2% (n = 73) reporting PHM in combination with a subspecialty, and 6% (n = 31) in combination with general pediatrics. The mean proportion of time spent in nonclinical roles was 22.4% (SD, 20.4%), and the mean proportions of time spent in any one area (administration, research, education, or QI) were all <10%.
The proportion of time allocated to inpatient pediatric care, neonatal care, emergency care, and outpatient pediatric care varied substantially across PHM practice groups and settings. Among respondents who practiced at children’s hospitals, the median percentage of clinical time dedicated to inpatient pediatric care was 66.5% (interquartile range [IQR], 15%-100%), with neonatal care being the second most common clinical practice area (Figure, part A; Appendix Table). At community hospitals, the percentage of clinical time dedicated to inpatient pediatric care was lower, with a median of 10% (IQR, 3%-40%) (Figure, part B). Among those reporting exclusive PHM practice, the median proportion of clinical time spent delivering inpatient pediatric care was 100% (IQR, 80%-100%) at children’s hospitals and 40% (IQR, 20%-85%) at community hospitals. At community hospitals, neonatal care accounted for a similar proportion of clinical time as inpatient pediatric care for these respondents (median, 40% [IQR, 0%-70%]). With the exception of emergency room care, we observed significant differences in how clinical time was allocated by respondents reporting exclusive PHM practice compared with those reporting PHM in combination with general or specialty care (all P values < .001, Appendix Table).
Professional Development Interests
Approximately two-thirds of respondents reported interest in QI leadership or consultation (Table 2), with those reporting exclusive PHM practice significantly more likely to report this (70.3% [n = 345] compared with 57.7% [n = 297] of those practicing PHM with general pediatrics and 66.3% [n = 431] of those practicing PHM with another subspecialty, P < .001). Similarly, 69% (n = 339) of respondents who reported exclusive PHM practice described an intention to take the PHM certifying examination, compared with 20.4% (n = 105) of those practicing PHM and general pediatrics and 17.7% (n = 115) of those practicing PHM and subspeciality pediatrics (P < .001). A total of 82.5% (n = 846) of respondents reported that they were satisfied with the allocation of their professional time; there were no significant differences between those reporting exclusive PHM practice and those reporting PHM in combination with general or subspecialty pediatrics. Of hospitalists reporting exclusive PHM practice, 67.8% (n = 166) reported an intention to maintain more than one ABP certification, compared with 22.1% (n = 78) of those practicing PHM and general pediatrics and 53.9% (n = 230) of those practicing PHM and subspecialty pediatrics (P < .001).
In multivariate regression analyses, hospitalists reporting exclusive PHM practice had significantly greater odds of reported interest in QI leadership or consultation (adjusted odds ratio [OR], 1.39; 95% CI, 1.09-1.79), intention to take the PHM certifying exam (adjusted OR, 7.10; 95% CI, 5.45-9.25), and intention to maintain more than one ABP certification (adjusted OR, 2.64; 95% CI, 1.89-3.68) than those practicing PHM in combination with general or subspecialty pediatrics (Table 3). There was no significant difference across the three groups in the satisfaction with the allocation of professional time.
DISCUSSION
In this national survey of pediatricians seeking MOC from the ABP, 13.1% reported that they practiced hospital medicine, with approximately one-third of these individuals reporting that they practiced PHM exclusively. The distribution of clinical and nonclinical responsibilities differed across those reporting exclusive PHM practice relative to those practicing PHM in combination with general or subspecialty pediatrics. Relative to hospitalists who reported practicing PHM in addition to general or subspecialty care, those reporting exclusive PHM practice were significantly more likely to report an interest in QI leadership or consultation, intention to sit for the PHM board-certification exam, and intention to maintain more than one ABP certification.
These findings offer insight into the evolution of PHM and have important implications for workforce planning. The last nationally representative analysis of the PHM workforce was conducted in 2006, at which time 73% of hospitalists reported working at children’s hospitals.6 In the current analysis, less than 50% of hospitalists reported practicing PHM at children’s hospitals only; 10% reported working at both children’s hospitals and community hospitals and 40% at community hospitals alone. This diffusion of PHM from children’s hospitals into community hospitals represents an important development in the field and aligns with the epidemiology of pediatric hospitalization.10 Pediatric hospitalists who practice at community hospitals experience unique challenges, including a relative paucity of pediatric-specific clinical resources, limited mentorship opportunities and resources for scholarly work, and limited access to data from which to prioritize QI interventions.11,12 Our findings also illustrate that the scope of practice for hospitalists differs at community hospitals relative to children’s hospitals. Although the PHM fellowship curriculum requires training at a community hospital, the requirement is limited to one 4-week block, which may not provide sufficient preparation for the unique clinical responsibilities in this setting.13,14
Relative to past analyses of PHM workforce roles and responsibilities, a substantially greater proportion of respondents in the current study reported clinical responsibility for neonatal care, including more than 40% of those self-reporting practicing PHM exclusively and almost three-quarters of those self-reporting PHM in conjunction with general pediatrics.6,15 Given that more than half of the six million US pediatric hospitalizations that occur each year represent birth hospitalizations,16 pediatric hospitalists’ responsibilities for newborn care are consistent with these patterns of hospital-based care. Expanding hospitalists’ responsibilities to provide newborn care has also been shown to improve the financial performance of PHM programs with relatively low pediatric volumes, which may further explain this finding, particularly at community hospitals.17,18 Interestingly, although emergency department care has also been demonstrated as a model to improve the financial stability of PHM programs, relatively few hospitalists reported this as an area of clinical responsibility.19,20 This finding contrasts with past analyses and may reflect how the scope of PHM clinical responsibilities has changed since these prior studies were conducted.6,15
Because PHM had not been recognized as a subspecialty prior to 2016, a national count of pediatric hospitalists is lacking. In this study, approximately one in eight pediatricians reported that they practiced PHM, but less than 4% of the survey sample reported practicing PHM exclusively. Based on these results, we estimate that of the 76,214 to 89,608 ABP-certified pediatricians currently practicing in the United States, between 9984 and 11,738 would self-identify as practicing PHM, with between 2945 and 3462 reporting exclusive PHM practice.
Hospitalists who reported practicing PHM exclusively were significantly more likely to report an interest in QI leadership or consultation and plans to take the PHM certifying exam. These findings are consistent with PHM’s focus on QI, as articulated in the application to the ABMS for subspecialty status as well as the PHM Core Competencies and fellowship curriculum.4,13,21,22 Despite past research questioning the sustainability of some community- and university-based PHM programs and wide variability in workload,7-9 more than 80% of hospitalists reported satisfaction with the allocation of their professional time, with no significant differences between respondents practicing PHM exclusively or in combination with general or subspecialty care.
This analysis should be interpreted in light of its strengths and limitations. Strengths of this work include its national focus, large sample size, and comprehensive characterization of respondents’ professional roles and characteristics. Study limitations include the fact that respondents were classified as hospitalists based on self-report; we were unable to ascertain if they were classified as hospitalists at their place of employment or if they met the ABP’s eligibility criteria to sit for the PHM subspecialty certifying exam.19 Additionally, respondents self-reported their allocations of clinical and nonclinical time, and we are unable to correlate this with actual work hours. Respondents’ reported interest in QI leadership or consultation may not be correlated with QI effort in practice; the mean time reportedly dedicated to QI activities was quite low. Additionally, two of our outcomes were available only for respondents who enrolled in MOC in 2017, and the proportion practicing medicine-pediatrics was available only in 2018. Although this analysis represents approximately 40% of all pediatricians enrolling in MOC (2 years of the 5-year MOC cycle), it may not be representative of pediatricians who are not certified by the ABP. Finally, our outcomes related to board certification examined interest and intentions; future study will be needed to determine how many pediatricians take the PHM exam and maintain certification.
In conclusion, the field of PHM has evolved considerably since its inception, with pediatric hospitalists reporting diverse clinical and nonclinical responsibilities. Hospitalists practicing PHM exclusively were more likely to report an interest in QI leadership and intent to sit for the PHM certifying exam than those practicing PHM in combination with general pediatrics or another specialty. Continuing to monitor the evolution of PHM roles and responsibilities over time and across settings will be important to support the professional development needs of the PHM workforce.
1. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001
3. The American Board of Pediatrics. ABMS approves pediatric hospital medicine certification. November 8, 2016. Accessed October 12, 2021. https://www.abp.org/news/abms-approves-pediatric-hospital-medicine-certification
4. American Board of Medical Specialities. Application for a new subspecialty certificate: pediatric hospital medicine.
5. American Board of Pediatrics. 2019 Annual Report. Accessed October 12, 2021. https://www.abp.org/sites/abp/files/pdf/annual-report-2019.pdf
6. Freed GL, Dunham KM, Research Advisory Committee of the American Board of Pediatrics. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186. https://doi.org/10.1002/jhm.458
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(11):682-685. https://doi.org/10.12788/jhm.3263
8. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977
9. Gosdin C, Simmons J, Yau C, Sucharew H, Carlson D, Paciorkowski N. Survey of academic pediatric hospitalist programs in the US: organizational, administrative, and financial factors. J Hosp Med. 2013;8(6):285-291. https://doi.org/10.1002/jhm.2020
10. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
11. Leary JC, Walsh KE, Morin RA, Schainker EG, Leyenaar JK. Quality and safety of pediatric inpatient care in community hospitals: a scoping review. J Hosp Med. 2019;14:694-703. https://doi.org/10.12788/jhm.3268
12. Leyenaar JK, Capra LA, O’Brien ER, Leslie LK, Mackie TI. Determinants of career satisfaction among pediatric hospitalists: a qualitative exploration. Acad Pediatr. 2014;14(4):361-368. https://doi.org/10.1016/j.acap.2014.03.015
13. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. July 1, 2021. Accessed October 4, 2021.https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/334_PediatricHospitalMedicine_2020.pdf?ver=2020-06-29-163350-910&ver=2020-06-29-163350-910
15. Freed GL, Brzoznowski K, Neighbors K, Lakhani I, American Board of Pediatrics, Research Advisory Committee. Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(1):33-39. https://doi.org/10.1542/peds.2007-0304
16. Moore B, Freeman W, Jiang H. Costs of Pediatric Hospital Stays, 2016. Healthcare Cost and Utilization Project Statistical Brief #250. Accessed October 25, 2021. https://www.ncbi.nlm.nih.gov/books/NBK547762/
17. Carlson DW, Fentzke KM, Dawson JG. Pediatric hospitalists: fill varied roles in the care of newborns. Pediatr Ann. 2003;32(12):802-810. https://doi.org/10.3928/0090-4481-20031201-09
18. Tieder JS, Migita DS, Cowan CA, Melzer SM. Newborn care by pediatric hospitalists in a community hospital: effect on physician productivity and financial performance. Arch Pediatr Adolesc Med. 2008;162(1):74-78. https://doi.org/10.1001/archpediatrics.2007.15
19. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01
20. Dudas RA, Monroe D, McColligan Borger M. Community pediatric hospitalists providing care in the emergency department: an analysis of physician productivity and financial performance. Pediatr Emerg Care. 2011;27(11):1099-1103. https://doi.org/10.1097/PEC.0b013e31823606f5
21. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343. https://doi.org/10.1002/jhm.843
22. Maniscalco J, Gage S, Teferi S, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
1. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001
3. The American Board of Pediatrics. ABMS approves pediatric hospital medicine certification. November 8, 2016. Accessed October 12, 2021. https://www.abp.org/news/abms-approves-pediatric-hospital-medicine-certification
4. American Board of Medical Specialities. Application for a new subspecialty certificate: pediatric hospital medicine.
5. American Board of Pediatrics. 2019 Annual Report. Accessed October 12, 2021. https://www.abp.org/sites/abp/files/pdf/annual-report-2019.pdf
6. Freed GL, Dunham KM, Research Advisory Committee of the American Board of Pediatrics. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186. https://doi.org/10.1002/jhm.458
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(11):682-685. https://doi.org/10.12788/jhm.3263
8. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977
9. Gosdin C, Simmons J, Yau C, Sucharew H, Carlson D, Paciorkowski N. Survey of academic pediatric hospitalist programs in the US: organizational, administrative, and financial factors. J Hosp Med. 2013;8(6):285-291. https://doi.org/10.1002/jhm.2020
10. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
11. Leary JC, Walsh KE, Morin RA, Schainker EG, Leyenaar JK. Quality and safety of pediatric inpatient care in community hospitals: a scoping review. J Hosp Med. 2019;14:694-703. https://doi.org/10.12788/jhm.3268
12. Leyenaar JK, Capra LA, O’Brien ER, Leslie LK, Mackie TI. Determinants of career satisfaction among pediatric hospitalists: a qualitative exploration. Acad Pediatr. 2014;14(4):361-368. https://doi.org/10.1016/j.acap.2014.03.015
13. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698
14. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. July 1, 2021. Accessed October 4, 2021.https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/334_PediatricHospitalMedicine_2020.pdf?ver=2020-06-29-163350-910&ver=2020-06-29-163350-910
15. Freed GL, Brzoznowski K, Neighbors K, Lakhani I, American Board of Pediatrics, Research Advisory Committee. Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(1):33-39. https://doi.org/10.1542/peds.2007-0304
16. Moore B, Freeman W, Jiang H. Costs of Pediatric Hospital Stays, 2016. Healthcare Cost and Utilization Project Statistical Brief #250. Accessed October 25, 2021. https://www.ncbi.nlm.nih.gov/books/NBK547762/
17. Carlson DW, Fentzke KM, Dawson JG. Pediatric hospitalists: fill varied roles in the care of newborns. Pediatr Ann. 2003;32(12):802-810. https://doi.org/10.3928/0090-4481-20031201-09
18. Tieder JS, Migita DS, Cowan CA, Melzer SM. Newborn care by pediatric hospitalists in a community hospital: effect on physician productivity and financial performance. Arch Pediatr Adolesc Med. 2008;162(1):74-78. https://doi.org/10.1001/archpediatrics.2007.15
19. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01
20. Dudas RA, Monroe D, McColligan Borger M. Community pediatric hospitalists providing care in the emergency department: an analysis of physician productivity and financial performance. Pediatr Emerg Care. 2011;27(11):1099-1103. https://doi.org/10.1097/PEC.0b013e31823606f5
21. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343. https://doi.org/10.1002/jhm.843
22. Maniscalco J, Gage S, Teferi S, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
© 2021 Society of Hospital Medicine
Pooled Testing for SARS-CoV-2 for Resource Conservation in the Hospital: A Dynamic Process
Pooled testing for SARS-CoV-2 has been proposed as a strategy to facilitate testing and conserve scarce laboratory resources in a variety of settings. Previously in the Journal of Hospital Medicine, we reported our initial experience with pooled testing in low-risk admitted patients from April 17, 2020, to May 11, 2020, at Saratoga Hospital, Saratoga Springs, New York.1 Early in the pandemic, when testing resources were critically short, pooling allowed us to meet our clinical goal of testing all admitted inpatients. We now present our subsequent experience to emphasize the dynamic nature of this strategy when used to offer testing while conserving resources within a hospital system.
From April 17, 2020, to December 10, 2020, pooled testing using the GeneXpert system (Cepheid) was performed as previously described on all patients admitted from the emergency department (ED) of Saratoga Hospital who met criteria for being at low risk for SARS-CoV-2 infection.1 During this period, we had a low community prevalence (<1%-2%). In our low-risk admitted patients, an overall positive rate of 0.5% allowed us to expand the pool size from our initial reported size of three samples to a maximum of five samples. As ED volumes changed, pool sizes could be adjusted by clinical leaders as supplies allowed the demands of throughput to be met. These adjustments were facilitated by regular discussion of aggregate testing results, pool size, patient-flow issues, and supply levels among our staff. In December 2020, we experienced a marked increase in community prevalence and hospital admissions. This surge ended our use of pooling and required us to test each admitted patient with a single cartridge, which fortunately had become available.
During our period of pooling, we tested 7755 low-risk patients using 1738 cartridges (1177 pools of five samples; 211 pools of four samples; 326 pools of three samples; and 24 pools of two samples). We had 39 positive pooled cartridges, which required the use of 174 additional single cartridges. The instructions for use of this system with single cartridges report a negative percent agreement (sensitivity) of 95.6% and a positive percent agreement (specificity) of 97.8% in the lab.2 We did not have any patients who tested negative in a pool subsequently turn positive during admission unless they had a known in-hospital exposure; however, our public health service alerted us to several patients with high-risk exposures who were excluded from pooling. Our pooling strategy resulted in use of 5843 fewer cartridges than if each test had been performed on a single patient. The total savings on cartridges was $225,000. Pooling did not directly increase staff costs, but required significant individual and organizational energy and commitment. At times, pooling could delay throughput of admitted patients from the ED to inpatient beds. The testing process often added 60 to 90 minutes to throughput time. During the night, waiting for admissions to create a pool could also cause delay. Close and ongoing communication among our ED, inpatient teams, nursing, and laboratory was required to minimize these negative effects.
Pooling can be an effective method of resource conservation in low-risk populations. The theoretical benefits of pooling have been calculated in various scenarios3 and recently comprehensively reviewed with emphasis on selecting the pooling method.4 Practically, pooling has been aptly described as a complex undertaking that should be one part of a broad approach to achieving various COVID-19 control goals.5 Our experience is that, in the hospital setting, it is a dynamic process that requires repeatedly balancing clinical goals, organizational realities, laboratory and mathematical parameters, and competing staff duties. The potential costs and benefits may change over time. We found success was highly dependent on our staff, who were highly motivated by strongly agreeing with our commitment to test all inpatients and our desire to maintain adequate supplies to accomplish this goal.
1. Mastrianni D, Falivena R, Brooks T, et al. Pooled testing for SARS-CoV-2 in hospitalized patients. J Hosp Med. 2020;15:538-539. https://doi.org/10.12788/jhm.3501
2. Xpert Xpress SARS-CoV-2. Instructions for use. Cepheid; 2020. Accessed October 7, 2021. https://www.cepheid.com/Package%20Insert%20Files/Xpert%20Xpress%20SARS-CoV-2%20Assay%20ENGLISH%20Package%20Insert%20302-3787%20Rev.%20B.pdf
3. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153(6):715-718. https://doi.org/10.1093/ajcp/aqaa064
4. Daniel EA, Esakialraj L BH, Anbalagan S, et al. Pooled testing strategies for SARS-CoV-2 diagnosis: a comprehensive review. Diagn Microbiol Infect Dis. 2021;101(2):115432. https://doi.org/10.1016/j.diagmicrobio.2021.115432
5. Schulte PA, Weissman DN, Luckhaupt SE, et al. Considerations for pooled testing of employees for SARS-CoV-2. J Occup Environ Med. 2021;63(1):1-9. https://doi.org/10.1097/JOM.0000000000002049
Pooled testing for SARS-CoV-2 has been proposed as a strategy to facilitate testing and conserve scarce laboratory resources in a variety of settings. Previously in the Journal of Hospital Medicine, we reported our initial experience with pooled testing in low-risk admitted patients from April 17, 2020, to May 11, 2020, at Saratoga Hospital, Saratoga Springs, New York.1 Early in the pandemic, when testing resources were critically short, pooling allowed us to meet our clinical goal of testing all admitted inpatients. We now present our subsequent experience to emphasize the dynamic nature of this strategy when used to offer testing while conserving resources within a hospital system.
From April 17, 2020, to December 10, 2020, pooled testing using the GeneXpert system (Cepheid) was performed as previously described on all patients admitted from the emergency department (ED) of Saratoga Hospital who met criteria for being at low risk for SARS-CoV-2 infection.1 During this period, we had a low community prevalence (<1%-2%). In our low-risk admitted patients, an overall positive rate of 0.5% allowed us to expand the pool size from our initial reported size of three samples to a maximum of five samples. As ED volumes changed, pool sizes could be adjusted by clinical leaders as supplies allowed the demands of throughput to be met. These adjustments were facilitated by regular discussion of aggregate testing results, pool size, patient-flow issues, and supply levels among our staff. In December 2020, we experienced a marked increase in community prevalence and hospital admissions. This surge ended our use of pooling and required us to test each admitted patient with a single cartridge, which fortunately had become available.
During our period of pooling, we tested 7755 low-risk patients using 1738 cartridges (1177 pools of five samples; 211 pools of four samples; 326 pools of three samples; and 24 pools of two samples). We had 39 positive pooled cartridges, which required the use of 174 additional single cartridges. The instructions for use of this system with single cartridges report a negative percent agreement (sensitivity) of 95.6% and a positive percent agreement (specificity) of 97.8% in the lab.2 We did not have any patients who tested negative in a pool subsequently turn positive during admission unless they had a known in-hospital exposure; however, our public health service alerted us to several patients with high-risk exposures who were excluded from pooling. Our pooling strategy resulted in use of 5843 fewer cartridges than if each test had been performed on a single patient. The total savings on cartridges was $225,000. Pooling did not directly increase staff costs, but required significant individual and organizational energy and commitment. At times, pooling could delay throughput of admitted patients from the ED to inpatient beds. The testing process often added 60 to 90 minutes to throughput time. During the night, waiting for admissions to create a pool could also cause delay. Close and ongoing communication among our ED, inpatient teams, nursing, and laboratory was required to minimize these negative effects.
Pooling can be an effective method of resource conservation in low-risk populations. The theoretical benefits of pooling have been calculated in various scenarios3 and recently comprehensively reviewed with emphasis on selecting the pooling method.4 Practically, pooling has been aptly described as a complex undertaking that should be one part of a broad approach to achieving various COVID-19 control goals.5 Our experience is that, in the hospital setting, it is a dynamic process that requires repeatedly balancing clinical goals, organizational realities, laboratory and mathematical parameters, and competing staff duties. The potential costs and benefits may change over time. We found success was highly dependent on our staff, who were highly motivated by strongly agreeing with our commitment to test all inpatients and our desire to maintain adequate supplies to accomplish this goal.
Pooled testing for SARS-CoV-2 has been proposed as a strategy to facilitate testing and conserve scarce laboratory resources in a variety of settings. Previously in the Journal of Hospital Medicine, we reported our initial experience with pooled testing in low-risk admitted patients from April 17, 2020, to May 11, 2020, at Saratoga Hospital, Saratoga Springs, New York.1 Early in the pandemic, when testing resources were critically short, pooling allowed us to meet our clinical goal of testing all admitted inpatients. We now present our subsequent experience to emphasize the dynamic nature of this strategy when used to offer testing while conserving resources within a hospital system.
From April 17, 2020, to December 10, 2020, pooled testing using the GeneXpert system (Cepheid) was performed as previously described on all patients admitted from the emergency department (ED) of Saratoga Hospital who met criteria for being at low risk for SARS-CoV-2 infection.1 During this period, we had a low community prevalence (<1%-2%). In our low-risk admitted patients, an overall positive rate of 0.5% allowed us to expand the pool size from our initial reported size of three samples to a maximum of five samples. As ED volumes changed, pool sizes could be adjusted by clinical leaders as supplies allowed the demands of throughput to be met. These adjustments were facilitated by regular discussion of aggregate testing results, pool size, patient-flow issues, and supply levels among our staff. In December 2020, we experienced a marked increase in community prevalence and hospital admissions. This surge ended our use of pooling and required us to test each admitted patient with a single cartridge, which fortunately had become available.
During our period of pooling, we tested 7755 low-risk patients using 1738 cartridges (1177 pools of five samples; 211 pools of four samples; 326 pools of three samples; and 24 pools of two samples). We had 39 positive pooled cartridges, which required the use of 174 additional single cartridges. The instructions for use of this system with single cartridges report a negative percent agreement (sensitivity) of 95.6% and a positive percent agreement (specificity) of 97.8% in the lab.2 We did not have any patients who tested negative in a pool subsequently turn positive during admission unless they had a known in-hospital exposure; however, our public health service alerted us to several patients with high-risk exposures who were excluded from pooling. Our pooling strategy resulted in use of 5843 fewer cartridges than if each test had been performed on a single patient. The total savings on cartridges was $225,000. Pooling did not directly increase staff costs, but required significant individual and organizational energy and commitment. At times, pooling could delay throughput of admitted patients from the ED to inpatient beds. The testing process often added 60 to 90 minutes to throughput time. During the night, waiting for admissions to create a pool could also cause delay. Close and ongoing communication among our ED, inpatient teams, nursing, and laboratory was required to minimize these negative effects.
Pooling can be an effective method of resource conservation in low-risk populations. The theoretical benefits of pooling have been calculated in various scenarios3 and recently comprehensively reviewed with emphasis on selecting the pooling method.4 Practically, pooling has been aptly described as a complex undertaking that should be one part of a broad approach to achieving various COVID-19 control goals.5 Our experience is that, in the hospital setting, it is a dynamic process that requires repeatedly balancing clinical goals, organizational realities, laboratory and mathematical parameters, and competing staff duties. The potential costs and benefits may change over time. We found success was highly dependent on our staff, who were highly motivated by strongly agreeing with our commitment to test all inpatients and our desire to maintain adequate supplies to accomplish this goal.
1. Mastrianni D, Falivena R, Brooks T, et al. Pooled testing for SARS-CoV-2 in hospitalized patients. J Hosp Med. 2020;15:538-539. https://doi.org/10.12788/jhm.3501
2. Xpert Xpress SARS-CoV-2. Instructions for use. Cepheid; 2020. Accessed October 7, 2021. https://www.cepheid.com/Package%20Insert%20Files/Xpert%20Xpress%20SARS-CoV-2%20Assay%20ENGLISH%20Package%20Insert%20302-3787%20Rev.%20B.pdf
3. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153(6):715-718. https://doi.org/10.1093/ajcp/aqaa064
4. Daniel EA, Esakialraj L BH, Anbalagan S, et al. Pooled testing strategies for SARS-CoV-2 diagnosis: a comprehensive review. Diagn Microbiol Infect Dis. 2021;101(2):115432. https://doi.org/10.1016/j.diagmicrobio.2021.115432
5. Schulte PA, Weissman DN, Luckhaupt SE, et al. Considerations for pooled testing of employees for SARS-CoV-2. J Occup Environ Med. 2021;63(1):1-9. https://doi.org/10.1097/JOM.0000000000002049
1. Mastrianni D, Falivena R, Brooks T, et al. Pooled testing for SARS-CoV-2 in hospitalized patients. J Hosp Med. 2020;15:538-539. https://doi.org/10.12788/jhm.3501
2. Xpert Xpress SARS-CoV-2. Instructions for use. Cepheid; 2020. Accessed October 7, 2021. https://www.cepheid.com/Package%20Insert%20Files/Xpert%20Xpress%20SARS-CoV-2%20Assay%20ENGLISH%20Package%20Insert%20302-3787%20Rev.%20B.pdf
3. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153(6):715-718. https://doi.org/10.1093/ajcp/aqaa064
4. Daniel EA, Esakialraj L BH, Anbalagan S, et al. Pooled testing strategies for SARS-CoV-2 diagnosis: a comprehensive review. Diagn Microbiol Infect Dis. 2021;101(2):115432. https://doi.org/10.1016/j.diagmicrobio.2021.115432
5. Schulte PA, Weissman DN, Luckhaupt SE, et al. Considerations for pooled testing of employees for SARS-CoV-2. J Occup Environ Med. 2021;63(1):1-9. https://doi.org/10.1097/JOM.0000000000002049
© 2021 Society of Hospital Medicine