User login
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
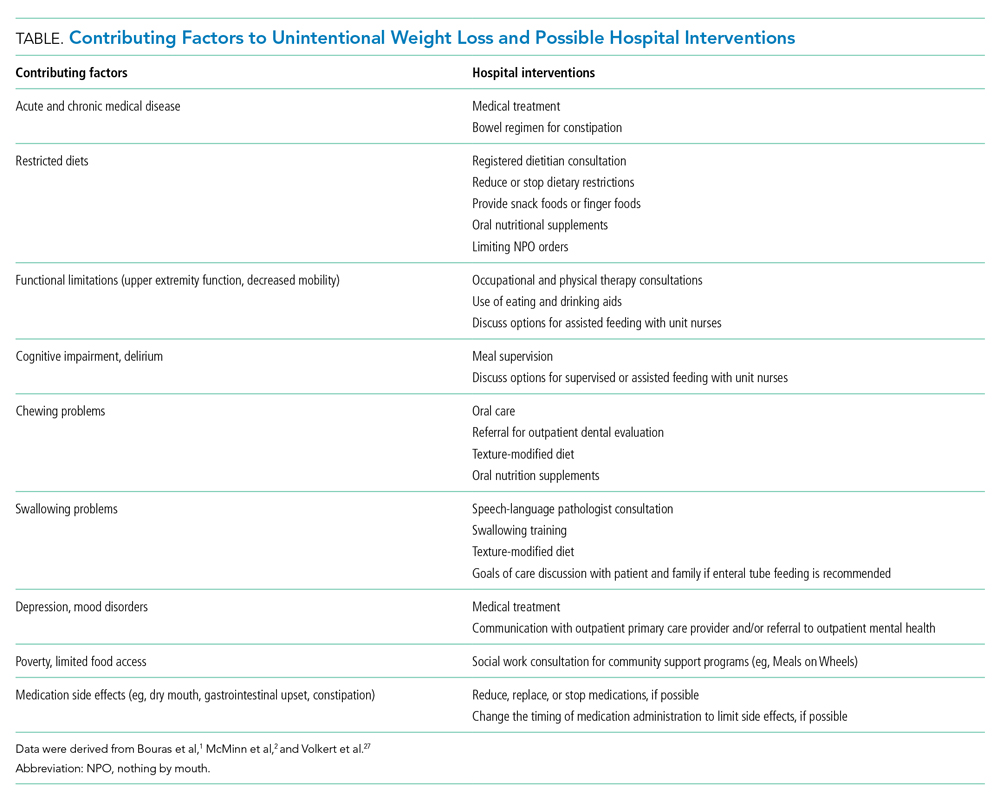
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
© 2021 Society of Hospital Medicine