User login
Clinical Progress Note: Rhythm Control for Patients With Atrial Fibrillation
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
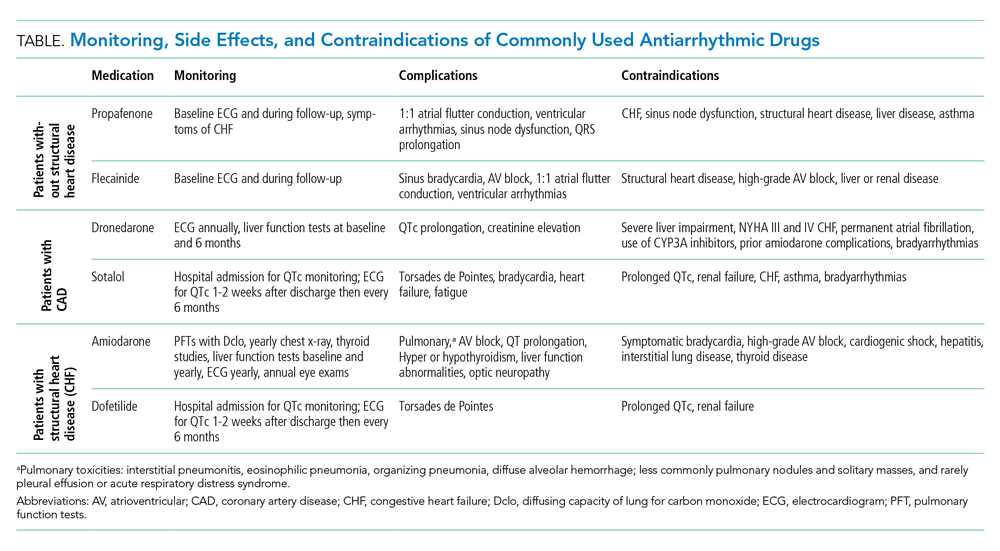
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3
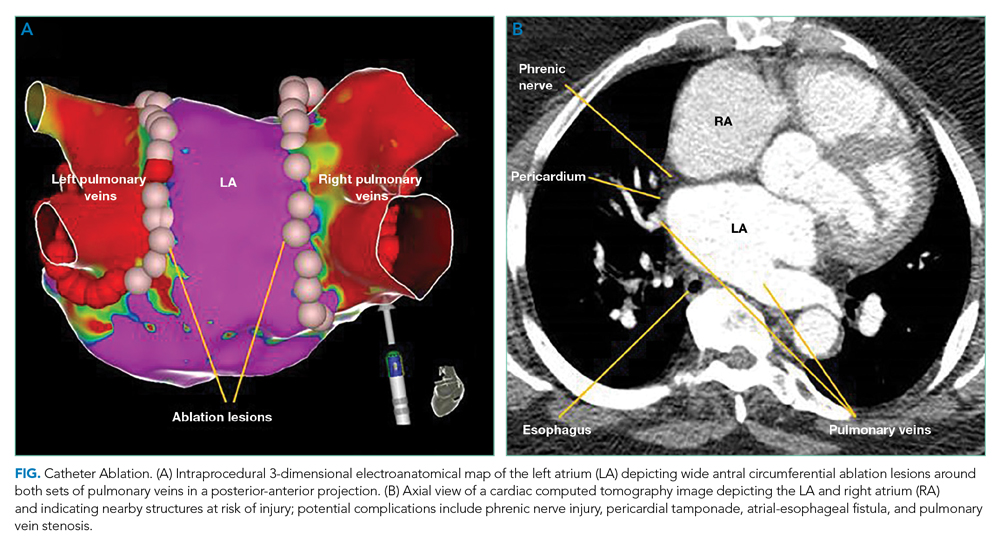
Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7
Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3

Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7

Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3

Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7

Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
© 2021 Society of Hospital Medicine
Things We Do For No Reason™: Ultrasonography After an Initial Negative CT in Patients Presenting With Acute Abdominal or Pelvic Pain
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
A 70-year-old woman presented to the emergency department (ED) with diffuse abdominal pain, nausea, and vomiting with normal liver function tests and lipase. Computed tomography (CT) of the abdomen and pelvis with intravenous contrast revealed no acute intraabdominal pathology except for an incidentally noted, mildly enlarged but nondistended gallbladder without evident cholelithiasis, pericholecystic fluid, or gallbladder wall edema. The hospitalist orders an abdominal ultrasound to evaluate for acute biliary pathology potentially missed by CT.
Why You Might Consider Ordering an Abdominal Ultrasound After a Negative CT
Guidelines and expert opinion recommend an “ultrasound-first” approach when patients present with right upper quadrant (RUQ) abdominal pain or pelvic pain of suspected gynecologic origin.1-3 When evaluating suspected biliary disease, experts recommend beginning with ultrasonography based on the speed of obtaining results, absence of radiation exposure, reduced cost, and good diagnostic accuracy.1 Ultrasound has superior sensitivity, of 98%,4 in identifying radiolucent gallstones, compared to CT’s 79% sensitivity.5 Ultrasonography also differentiates gallbladder sludge from cholelithiasis, evaluates the extrahepatic and intrahepatic bile ducts, and can identify alternate causes of RUQ pain.1,3 Since ultrasound has important advantages, a negative initial CT may lead the clinician to consider an ultrasound to evaluate for gallbladder diseases.
Additionally, ultrasound provides improved anatomic detail of pelvic structures when diagnosing endometrial or ovarian pathology2 and improves diagnostic accuracy when the initial CT reveals an abnormal pelvic finding (eg, defining an enlarged ovary on CT as ovarian torsion, a cyst, or an adnexal mass).6 While CT excludes emergent surgical diagnoses, ultrasound may add value in elucidating a cause of the pain, even when urgent surgical management is not necessary.7
Many providers believe that a CT lacks sensitivity for acute biliary or pelvic pathology and will order an ultrasound to avoid missing an important diagnosis.7 Within 6 months at a single center, clinicians ordered 614 abdominal ultrasounds within 72 hours of an abdominal CT; 227 of these orders were to evaluate the gallbladder. Clinicians documented a discussion with a radiologist in only 19% of cases.8
Why Ordering an Ultrasound After a Negative CT Is Unnecessary
While ultrasound is more sensitive for detecting gallstones, the data do not indicate that it is more sensitive than CT for detecting acute cholecystitis. Abdominal ultrasound has a sensitivity for the diagnosis of acute cholecystitis of 81%, with a specificity of 83%,9 while CT has a comparable 85% to 94%9,10 sensitivity and specificity ranging from 59% to 99%.9,11 A recent study using more stringent radiographic criteria (two or more abnormal features) for diagnosing acute cholecystitis found ultrasound and CT had near equivalent sensitivities of 61% and 55%, respectively.12 Even with these stringent criteria, CT had a negative predictive value of 90% and approached 95% when applying a less strict (one feature) criterion.12 As a result, an abdominal ultrasound will rarely diagnose cholecystitis after a normal CT.
A 2020 study evaluated the diagnostic yield and clinical impact of ordering an abdominal or pelvic ultrasound within 24 hours of a negative abdominal CT.7
As with abdominal CT and ultrasound, the recommendation for an initial pelvic ultrasound when evaluating female pelvic pain also stems from the reduced cost, absence of radiation exposure, and superior anatomic visualization of the pelvic organs when compared with pelvic CT.2,13 However, as with the results of studies investigating the use of abdominal ultrasound after negative CT, a study of pelvic ultrasound after a negative CT revealed that only 4/126 (3.2%) follow-up ultrasounds had an abnormal finding not identified on CT.13 Pelvic ultrasound found four endometrial abnormalities that did not alter acute management.13 Notably, in 58% of the cases, the indication for ordering the subsequent ultrasound was “rule out ovarian torsion.” However, CT almost always finds a morphologically abnormal ovary in the case of torsion.6 One study and literature review found that all 28 patients studied and all 85 patients from previous studies with proven ovarian torsion had either an adnexal mass or an enlarged ovary on pelvic CT.6 Harfouch et al found that 0 out of 199 pelvic ultrasounds ordered after a negative CT revealed acute surgical pathology, but pelvic ultrasound did identify nonsurgical uterine and ovarian abnormalities.7 In conclusion, when clinicians order CT as the first study to diagnose acute, surgical biliary or gynecologic causes of pain, follow-up ultrasound has a low probability of affecting diagnosis or management if the CT is normal.
When You Should Consider Ultrasound After CT
The previous discussion only applies if hospitalists order an ultrasound within 24 to 48 hours of the initial CT. Time and clinical course are critical diagnostic tools during an admission for abdominal pain. Consider pelvic or abdominal ultrasound based on guideline recommendations if a patient develops new or evolving RUQ or pelvic pain.1,2 The rationale for obtaining the initial negative CT may no longer apply, and the clinician must consider the changing characteristics of the patient’s symptoms. For example, initial CT imaging may miss cholelithiasis in a patient presenting for biliary colic. Under observation, the patient may develop acute cholecystitis, potentially requiring an abdominal ultrasound. Also, the data for pelvic ultrasound apply to a normal CT of the abdomen and pelvis. Ultrasound may help to further evaluate indeterminate findings present on initial CT or if recommended by radiology.
What You Should Do Instead
When the hospitalist assumes care for a patient with abdominal pain and a negative CT, appropriate next steps include taking time to reexamine the differential diagnosis, repeating the history and physical, and communicating directly with a radiologist. These steps ensure the highest diagnostic yield and the lowest cost and help prevent diagnostic error arising from anchoring on the initial negative ED evaluation. Prior research demonstrates that the initial history alone can lead to the correct diagnosis in up to 76% of cases of abdominal pain.14 If repeat evaluation determines that additional imaging is necessary, the American College of Radiology provides evidence-based guidelines to help clinicians determine the correct imaging test based on the clinical situation (Appendix Table).1,2 For example, an equivocal ultrasound or CT exam with continued suspicion for acute cholecystitis or an alternate diagnosis, such as acalculous cholecystitis or choledocholithiasis, merits alternative tests with improved sensitivity and specificity profiles (Tc 99 m hepatobiliary iminodiacetic acid scan, also known as cholescintigraphy, for cholecystitis and acalculous cholecystitis, or magnetic resonance cholangiopancreatography for choledocholithiasis).1
Remember to communicate with the radiologist to rule out “can’t miss” diagnoses, increase mutual understanding of the radiographic test characteristics for specific disease processes, and improve the radiologist’s understanding of the patient’s history and clinical question.15 Collaboration with the radiologist can also determine the need for follow-up imaging and its timing. One single-center study found that surgeons’ diagnostic impression and management changed in 35/100 (35%) cases after an in-person review with the radiologist.15 Observing patients in the hospital with a nondiagnostic initial evaluation but concerning clinical features often allows for either a trial of cure or for the disease process to “declare itself.”14 This allows clinicians to target additional testing to a specific diagnosis and avoid reflexive ordering of additional radiographic studies.
Recommendations
- Order an ultrasound for initial imaging of RUQ and female pelvic pain.
- Do not reflexively order an ultrasound within 24 to 48 hours of a negative CT scan to pursue biliary or pelvic pathology.
- Only order repeat abdominal imaging if clinical circumstances evolve or discussions with a radiologist conclude it will answer a more specific diagnostic question.
Conclusion
In our clinical scenario involving a patient with diffuse abdominal pain and a negative CT, the hospitalist should reevaluate the history, exam, and differential diagnosis before pursuing further diagnostic imaging. Based on the evidence presented, CT has similar diagnostic accuracy to ultrasound for biliary and gynecologic pathologies necessitating urgent surgical management (eg, acute cholecystitis, ovarian torsion), and a follow-up ultrasound adds little. If the utility of imaging remains in question, hospitalist consultation with a radiologist can clarify whether prior imaging answered the clinical question and the diagnostic utility of repeat abdominal imaging. With thoughtful reevaluation of the history and physical, and communication with radiology, hospitalists can reduce unnecessary, low-yield imaging and reduce healthcare costs when evaluating patients with abdominal pain.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Expert Panel on Gastrointestinal Imaging; Peterson CM, McNamara MM, Kamel IR, et al. ACR Appropriateness Criteria® Right Upper Quadrant Pain. J Am Coll Radiol. 2019;16(5S):S235-S243. https://doi.org/10.1016/j.jacr.2019.02.013
2. Bhosale PR, Javitt MC, Atri M, et al. ACR Appropriateness Criteria® Acute Pelvic Pain in the Reproductive Age Group. Ultrasound Q. 2016;32(2):108-115. https://doi.org/10.1097/RUQ.0000000000000200
3. Revzin MV, Scoutt LM, Garner JG, Moore CL. Right upper quadrant pain: ultrasound first! J Ultrasound Med. 2017;36(10):1975-1985. https://doi.org/10.1002/jum.14274
4. Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med. 1980;302(23):1277-1279. https://doi.org/10.1056/NEJM198006053022303
5. Barakos JA, Ralls PW, Lapin SA, et al. Cholelithiasis: evaluation with CT. Radiology. 1987;162(2):415-418. https://doi.org/10.1148/radiology.162.2.3797654
6. Moore C, Meyers AB, Capotasto J, Bokhari J. Prevalence of abnormal CT findings in patients with proven ovarian torsion and a proposed triage schema. Emerg Radiol. 2009;16(2):115-120. https://doi.org/10.1007/s10140-008-0754-x
7. Harfouch N, Stern J, Chowdhary V, et al. Utility of ultrasound after a negative CT abdomen and pelvis in the emergency department. Clin Imaging. 2020;68:29-35. https://doi.org/10.1016/j.clinimag.2020.06.007
8. Adenaw N, Wen J, Pahwa AK, Sheth S, Johnson PT. Decreasing duplicative imaging: inpatient and emergency medicine abdominal ultrasound within 72 hours of abdominal CT. J Am Coll Radiol. 2020;17(5):590-596. https://doi.org/10.1016/j.jacr.2020.03.010
9. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720. https://doi.org/10.1148/radiol.12111561
10. Wertz JR, Lopez JM, Olson D, Thompson WM. Comparing the diagnostic accuracy of ultrasound and CT in evaluating acute cholecystitis. AJR Am J Roentgenol. 2018;211(2):W92-W97. https://doi.org/10.2214/AJR.17.18884
11. Bennett GL, Rusinek H, Lisi V, et al. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178(2):275-281. https://doi.org/10.2214/ajr.178.2.1780275
12. Hiatt KD, Ou JJ, Childs DD. Role of ultrasound and CT in the workup of right upper quadrant pain in adults in the emergency department: a retrospective review of more than 2800 cases. AJR Am J Roentgenol. 2020;214(6):1305-1310. https://doi.org/10.2214/AJR.19.22188
13. Gao Y, Lee K, Camacho M. Utility of pelvic ultrasound following negative abdominal and pelvic CT in the emergency room. Clin Radiol. 2013;68(11):e586-e592. https://doi.org/10.1016/j.crad.2013.05.101
14. Natesan S, Lee J, Volkamer H, Thoureen T. Evidence-based medicine approach to abdominal pain. Emerg Med Clin North Am. 2016;34(2):165-190. https://doi.org/10.1016/j.emc.2015.12.008.
15. Dickerson EC, Alam HB, Brown RK, Stojanovska J, Davenport MS; Michigan Radiology Quality Collaborative. In-person communication between radiologists and acute care surgeons leads to significant alterations in surgical decision making. J Am Coll Radiol. 2016;13(8):943-949. https://doi.org/10.1016/j.jacr.2016.02.005
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
A 70-year-old woman presented to the emergency department (ED) with diffuse abdominal pain, nausea, and vomiting with normal liver function tests and lipase. Computed tomography (CT) of the abdomen and pelvis with intravenous contrast revealed no acute intraabdominal pathology except for an incidentally noted, mildly enlarged but nondistended gallbladder without evident cholelithiasis, pericholecystic fluid, or gallbladder wall edema. The hospitalist orders an abdominal ultrasound to evaluate for acute biliary pathology potentially missed by CT.
Why You Might Consider Ordering an Abdominal Ultrasound After a Negative CT
Guidelines and expert opinion recommend an “ultrasound-first” approach when patients present with right upper quadrant (RUQ) abdominal pain or pelvic pain of suspected gynecologic origin.1-3 When evaluating suspected biliary disease, experts recommend beginning with ultrasonography based on the speed of obtaining results, absence of radiation exposure, reduced cost, and good diagnostic accuracy.1 Ultrasound has superior sensitivity, of 98%,4 in identifying radiolucent gallstones, compared to CT’s 79% sensitivity.5 Ultrasonography also differentiates gallbladder sludge from cholelithiasis, evaluates the extrahepatic and intrahepatic bile ducts, and can identify alternate causes of RUQ pain.1,3 Since ultrasound has important advantages, a negative initial CT may lead the clinician to consider an ultrasound to evaluate for gallbladder diseases.
Additionally, ultrasound provides improved anatomic detail of pelvic structures when diagnosing endometrial or ovarian pathology2 and improves diagnostic accuracy when the initial CT reveals an abnormal pelvic finding (eg, defining an enlarged ovary on CT as ovarian torsion, a cyst, or an adnexal mass).6 While CT excludes emergent surgical diagnoses, ultrasound may add value in elucidating a cause of the pain, even when urgent surgical management is not necessary.7
Many providers believe that a CT lacks sensitivity for acute biliary or pelvic pathology and will order an ultrasound to avoid missing an important diagnosis.7 Within 6 months at a single center, clinicians ordered 614 abdominal ultrasounds within 72 hours of an abdominal CT; 227 of these orders were to evaluate the gallbladder. Clinicians documented a discussion with a radiologist in only 19% of cases.8
Why Ordering an Ultrasound After a Negative CT Is Unnecessary
While ultrasound is more sensitive for detecting gallstones, the data do not indicate that it is more sensitive than CT for detecting acute cholecystitis. Abdominal ultrasound has a sensitivity for the diagnosis of acute cholecystitis of 81%, with a specificity of 83%,9 while CT has a comparable 85% to 94%9,10 sensitivity and specificity ranging from 59% to 99%.9,11 A recent study using more stringent radiographic criteria (two or more abnormal features) for diagnosing acute cholecystitis found ultrasound and CT had near equivalent sensitivities of 61% and 55%, respectively.12 Even with these stringent criteria, CT had a negative predictive value of 90% and approached 95% when applying a less strict (one feature) criterion.12 As a result, an abdominal ultrasound will rarely diagnose cholecystitis after a normal CT.
A 2020 study evaluated the diagnostic yield and clinical impact of ordering an abdominal or pelvic ultrasound within 24 hours of a negative abdominal CT.7
As with abdominal CT and ultrasound, the recommendation for an initial pelvic ultrasound when evaluating female pelvic pain also stems from the reduced cost, absence of radiation exposure, and superior anatomic visualization of the pelvic organs when compared with pelvic CT.2,13 However, as with the results of studies investigating the use of abdominal ultrasound after negative CT, a study of pelvic ultrasound after a negative CT revealed that only 4/126 (3.2%) follow-up ultrasounds had an abnormal finding not identified on CT.13 Pelvic ultrasound found four endometrial abnormalities that did not alter acute management.13 Notably, in 58% of the cases, the indication for ordering the subsequent ultrasound was “rule out ovarian torsion.” However, CT almost always finds a morphologically abnormal ovary in the case of torsion.6 One study and literature review found that all 28 patients studied and all 85 patients from previous studies with proven ovarian torsion had either an adnexal mass or an enlarged ovary on pelvic CT.6 Harfouch et al found that 0 out of 199 pelvic ultrasounds ordered after a negative CT revealed acute surgical pathology, but pelvic ultrasound did identify nonsurgical uterine and ovarian abnormalities.7 In conclusion, when clinicians order CT as the first study to diagnose acute, surgical biliary or gynecologic causes of pain, follow-up ultrasound has a low probability of affecting diagnosis or management if the CT is normal.
When You Should Consider Ultrasound After CT
The previous discussion only applies if hospitalists order an ultrasound within 24 to 48 hours of the initial CT. Time and clinical course are critical diagnostic tools during an admission for abdominal pain. Consider pelvic or abdominal ultrasound based on guideline recommendations if a patient develops new or evolving RUQ or pelvic pain.1,2 The rationale for obtaining the initial negative CT may no longer apply, and the clinician must consider the changing characteristics of the patient’s symptoms. For example, initial CT imaging may miss cholelithiasis in a patient presenting for biliary colic. Under observation, the patient may develop acute cholecystitis, potentially requiring an abdominal ultrasound. Also, the data for pelvic ultrasound apply to a normal CT of the abdomen and pelvis. Ultrasound may help to further evaluate indeterminate findings present on initial CT or if recommended by radiology.
What You Should Do Instead
When the hospitalist assumes care for a patient with abdominal pain and a negative CT, appropriate next steps include taking time to reexamine the differential diagnosis, repeating the history and physical, and communicating directly with a radiologist. These steps ensure the highest diagnostic yield and the lowest cost and help prevent diagnostic error arising from anchoring on the initial negative ED evaluation. Prior research demonstrates that the initial history alone can lead to the correct diagnosis in up to 76% of cases of abdominal pain.14 If repeat evaluation determines that additional imaging is necessary, the American College of Radiology provides evidence-based guidelines to help clinicians determine the correct imaging test based on the clinical situation (Appendix Table).1,2 For example, an equivocal ultrasound or CT exam with continued suspicion for acute cholecystitis or an alternate diagnosis, such as acalculous cholecystitis or choledocholithiasis, merits alternative tests with improved sensitivity and specificity profiles (Tc 99 m hepatobiliary iminodiacetic acid scan, also known as cholescintigraphy, for cholecystitis and acalculous cholecystitis, or magnetic resonance cholangiopancreatography for choledocholithiasis).1
Remember to communicate with the radiologist to rule out “can’t miss” diagnoses, increase mutual understanding of the radiographic test characteristics for specific disease processes, and improve the radiologist’s understanding of the patient’s history and clinical question.15 Collaboration with the radiologist can also determine the need for follow-up imaging and its timing. One single-center study found that surgeons’ diagnostic impression and management changed in 35/100 (35%) cases after an in-person review with the radiologist.15 Observing patients in the hospital with a nondiagnostic initial evaluation but concerning clinical features often allows for either a trial of cure or for the disease process to “declare itself.”14 This allows clinicians to target additional testing to a specific diagnosis and avoid reflexive ordering of additional radiographic studies.
Recommendations
- Order an ultrasound for initial imaging of RUQ and female pelvic pain.
- Do not reflexively order an ultrasound within 24 to 48 hours of a negative CT scan to pursue biliary or pelvic pathology.
- Only order repeat abdominal imaging if clinical circumstances evolve or discussions with a radiologist conclude it will answer a more specific diagnostic question.
Conclusion
In our clinical scenario involving a patient with diffuse abdominal pain and a negative CT, the hospitalist should reevaluate the history, exam, and differential diagnosis before pursuing further diagnostic imaging. Based on the evidence presented, CT has similar diagnostic accuracy to ultrasound for biliary and gynecologic pathologies necessitating urgent surgical management (eg, acute cholecystitis, ovarian torsion), and a follow-up ultrasound adds little. If the utility of imaging remains in question, hospitalist consultation with a radiologist can clarify whether prior imaging answered the clinical question and the diagnostic utility of repeat abdominal imaging. With thoughtful reevaluation of the history and physical, and communication with radiology, hospitalists can reduce unnecessary, low-yield imaging and reduce healthcare costs when evaluating patients with abdominal pain.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
A 70-year-old woman presented to the emergency department (ED) with diffuse abdominal pain, nausea, and vomiting with normal liver function tests and lipase. Computed tomography (CT) of the abdomen and pelvis with intravenous contrast revealed no acute intraabdominal pathology except for an incidentally noted, mildly enlarged but nondistended gallbladder without evident cholelithiasis, pericholecystic fluid, or gallbladder wall edema. The hospitalist orders an abdominal ultrasound to evaluate for acute biliary pathology potentially missed by CT.
Why You Might Consider Ordering an Abdominal Ultrasound After a Negative CT
Guidelines and expert opinion recommend an “ultrasound-first” approach when patients present with right upper quadrant (RUQ) abdominal pain or pelvic pain of suspected gynecologic origin.1-3 When evaluating suspected biliary disease, experts recommend beginning with ultrasonography based on the speed of obtaining results, absence of radiation exposure, reduced cost, and good diagnostic accuracy.1 Ultrasound has superior sensitivity, of 98%,4 in identifying radiolucent gallstones, compared to CT’s 79% sensitivity.5 Ultrasonography also differentiates gallbladder sludge from cholelithiasis, evaluates the extrahepatic and intrahepatic bile ducts, and can identify alternate causes of RUQ pain.1,3 Since ultrasound has important advantages, a negative initial CT may lead the clinician to consider an ultrasound to evaluate for gallbladder diseases.
Additionally, ultrasound provides improved anatomic detail of pelvic structures when diagnosing endometrial or ovarian pathology2 and improves diagnostic accuracy when the initial CT reveals an abnormal pelvic finding (eg, defining an enlarged ovary on CT as ovarian torsion, a cyst, or an adnexal mass).6 While CT excludes emergent surgical diagnoses, ultrasound may add value in elucidating a cause of the pain, even when urgent surgical management is not necessary.7
Many providers believe that a CT lacks sensitivity for acute biliary or pelvic pathology and will order an ultrasound to avoid missing an important diagnosis.7 Within 6 months at a single center, clinicians ordered 614 abdominal ultrasounds within 72 hours of an abdominal CT; 227 of these orders were to evaluate the gallbladder. Clinicians documented a discussion with a radiologist in only 19% of cases.8
Why Ordering an Ultrasound After a Negative CT Is Unnecessary
While ultrasound is more sensitive for detecting gallstones, the data do not indicate that it is more sensitive than CT for detecting acute cholecystitis. Abdominal ultrasound has a sensitivity for the diagnosis of acute cholecystitis of 81%, with a specificity of 83%,9 while CT has a comparable 85% to 94%9,10 sensitivity and specificity ranging from 59% to 99%.9,11 A recent study using more stringent radiographic criteria (two or more abnormal features) for diagnosing acute cholecystitis found ultrasound and CT had near equivalent sensitivities of 61% and 55%, respectively.12 Even with these stringent criteria, CT had a negative predictive value of 90% and approached 95% when applying a less strict (one feature) criterion.12 As a result, an abdominal ultrasound will rarely diagnose cholecystitis after a normal CT.
A 2020 study evaluated the diagnostic yield and clinical impact of ordering an abdominal or pelvic ultrasound within 24 hours of a negative abdominal CT.7
As with abdominal CT and ultrasound, the recommendation for an initial pelvic ultrasound when evaluating female pelvic pain also stems from the reduced cost, absence of radiation exposure, and superior anatomic visualization of the pelvic organs when compared with pelvic CT.2,13 However, as with the results of studies investigating the use of abdominal ultrasound after negative CT, a study of pelvic ultrasound after a negative CT revealed that only 4/126 (3.2%) follow-up ultrasounds had an abnormal finding not identified on CT.13 Pelvic ultrasound found four endometrial abnormalities that did not alter acute management.13 Notably, in 58% of the cases, the indication for ordering the subsequent ultrasound was “rule out ovarian torsion.” However, CT almost always finds a morphologically abnormal ovary in the case of torsion.6 One study and literature review found that all 28 patients studied and all 85 patients from previous studies with proven ovarian torsion had either an adnexal mass or an enlarged ovary on pelvic CT.6 Harfouch et al found that 0 out of 199 pelvic ultrasounds ordered after a negative CT revealed acute surgical pathology, but pelvic ultrasound did identify nonsurgical uterine and ovarian abnormalities.7 In conclusion, when clinicians order CT as the first study to diagnose acute, surgical biliary or gynecologic causes of pain, follow-up ultrasound has a low probability of affecting diagnosis or management if the CT is normal.
When You Should Consider Ultrasound After CT
The previous discussion only applies if hospitalists order an ultrasound within 24 to 48 hours of the initial CT. Time and clinical course are critical diagnostic tools during an admission for abdominal pain. Consider pelvic or abdominal ultrasound based on guideline recommendations if a patient develops new or evolving RUQ or pelvic pain.1,2 The rationale for obtaining the initial negative CT may no longer apply, and the clinician must consider the changing characteristics of the patient’s symptoms. For example, initial CT imaging may miss cholelithiasis in a patient presenting for biliary colic. Under observation, the patient may develop acute cholecystitis, potentially requiring an abdominal ultrasound. Also, the data for pelvic ultrasound apply to a normal CT of the abdomen and pelvis. Ultrasound may help to further evaluate indeterminate findings present on initial CT or if recommended by radiology.
What You Should Do Instead
When the hospitalist assumes care for a patient with abdominal pain and a negative CT, appropriate next steps include taking time to reexamine the differential diagnosis, repeating the history and physical, and communicating directly with a radiologist. These steps ensure the highest diagnostic yield and the lowest cost and help prevent diagnostic error arising from anchoring on the initial negative ED evaluation. Prior research demonstrates that the initial history alone can lead to the correct diagnosis in up to 76% of cases of abdominal pain.14 If repeat evaluation determines that additional imaging is necessary, the American College of Radiology provides evidence-based guidelines to help clinicians determine the correct imaging test based on the clinical situation (Appendix Table).1,2 For example, an equivocal ultrasound or CT exam with continued suspicion for acute cholecystitis or an alternate diagnosis, such as acalculous cholecystitis or choledocholithiasis, merits alternative tests with improved sensitivity and specificity profiles (Tc 99 m hepatobiliary iminodiacetic acid scan, also known as cholescintigraphy, for cholecystitis and acalculous cholecystitis, or magnetic resonance cholangiopancreatography for choledocholithiasis).1
Remember to communicate with the radiologist to rule out “can’t miss” diagnoses, increase mutual understanding of the radiographic test characteristics for specific disease processes, and improve the radiologist’s understanding of the patient’s history and clinical question.15 Collaboration with the radiologist can also determine the need for follow-up imaging and its timing. One single-center study found that surgeons’ diagnostic impression and management changed in 35/100 (35%) cases after an in-person review with the radiologist.15 Observing patients in the hospital with a nondiagnostic initial evaluation but concerning clinical features often allows for either a trial of cure or for the disease process to “declare itself.”14 This allows clinicians to target additional testing to a specific diagnosis and avoid reflexive ordering of additional radiographic studies.
Recommendations
- Order an ultrasound for initial imaging of RUQ and female pelvic pain.
- Do not reflexively order an ultrasound within 24 to 48 hours of a negative CT scan to pursue biliary or pelvic pathology.
- Only order repeat abdominal imaging if clinical circumstances evolve or discussions with a radiologist conclude it will answer a more specific diagnostic question.
Conclusion
In our clinical scenario involving a patient with diffuse abdominal pain and a negative CT, the hospitalist should reevaluate the history, exam, and differential diagnosis before pursuing further diagnostic imaging. Based on the evidence presented, CT has similar diagnostic accuracy to ultrasound for biliary and gynecologic pathologies necessitating urgent surgical management (eg, acute cholecystitis, ovarian torsion), and a follow-up ultrasound adds little. If the utility of imaging remains in question, hospitalist consultation with a radiologist can clarify whether prior imaging answered the clinical question and the diagnostic utility of repeat abdominal imaging. With thoughtful reevaluation of the history and physical, and communication with radiology, hospitalists can reduce unnecessary, low-yield imaging and reduce healthcare costs when evaluating patients with abdominal pain.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Expert Panel on Gastrointestinal Imaging; Peterson CM, McNamara MM, Kamel IR, et al. ACR Appropriateness Criteria® Right Upper Quadrant Pain. J Am Coll Radiol. 2019;16(5S):S235-S243. https://doi.org/10.1016/j.jacr.2019.02.013
2. Bhosale PR, Javitt MC, Atri M, et al. ACR Appropriateness Criteria® Acute Pelvic Pain in the Reproductive Age Group. Ultrasound Q. 2016;32(2):108-115. https://doi.org/10.1097/RUQ.0000000000000200
3. Revzin MV, Scoutt LM, Garner JG, Moore CL. Right upper quadrant pain: ultrasound first! J Ultrasound Med. 2017;36(10):1975-1985. https://doi.org/10.1002/jum.14274
4. Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med. 1980;302(23):1277-1279. https://doi.org/10.1056/NEJM198006053022303
5. Barakos JA, Ralls PW, Lapin SA, et al. Cholelithiasis: evaluation with CT. Radiology. 1987;162(2):415-418. https://doi.org/10.1148/radiology.162.2.3797654
6. Moore C, Meyers AB, Capotasto J, Bokhari J. Prevalence of abnormal CT findings in patients with proven ovarian torsion and a proposed triage schema. Emerg Radiol. 2009;16(2):115-120. https://doi.org/10.1007/s10140-008-0754-x
7. Harfouch N, Stern J, Chowdhary V, et al. Utility of ultrasound after a negative CT abdomen and pelvis in the emergency department. Clin Imaging. 2020;68:29-35. https://doi.org/10.1016/j.clinimag.2020.06.007
8. Adenaw N, Wen J, Pahwa AK, Sheth S, Johnson PT. Decreasing duplicative imaging: inpatient and emergency medicine abdominal ultrasound within 72 hours of abdominal CT. J Am Coll Radiol. 2020;17(5):590-596. https://doi.org/10.1016/j.jacr.2020.03.010
9. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720. https://doi.org/10.1148/radiol.12111561
10. Wertz JR, Lopez JM, Olson D, Thompson WM. Comparing the diagnostic accuracy of ultrasound and CT in evaluating acute cholecystitis. AJR Am J Roentgenol. 2018;211(2):W92-W97. https://doi.org/10.2214/AJR.17.18884
11. Bennett GL, Rusinek H, Lisi V, et al. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178(2):275-281. https://doi.org/10.2214/ajr.178.2.1780275
12. Hiatt KD, Ou JJ, Childs DD. Role of ultrasound and CT in the workup of right upper quadrant pain in adults in the emergency department: a retrospective review of more than 2800 cases. AJR Am J Roentgenol. 2020;214(6):1305-1310. https://doi.org/10.2214/AJR.19.22188
13. Gao Y, Lee K, Camacho M. Utility of pelvic ultrasound following negative abdominal and pelvic CT in the emergency room. Clin Radiol. 2013;68(11):e586-e592. https://doi.org/10.1016/j.crad.2013.05.101
14. Natesan S, Lee J, Volkamer H, Thoureen T. Evidence-based medicine approach to abdominal pain. Emerg Med Clin North Am. 2016;34(2):165-190. https://doi.org/10.1016/j.emc.2015.12.008.
15. Dickerson EC, Alam HB, Brown RK, Stojanovska J, Davenport MS; Michigan Radiology Quality Collaborative. In-person communication between radiologists and acute care surgeons leads to significant alterations in surgical decision making. J Am Coll Radiol. 2016;13(8):943-949. https://doi.org/10.1016/j.jacr.2016.02.005
1. Expert Panel on Gastrointestinal Imaging; Peterson CM, McNamara MM, Kamel IR, et al. ACR Appropriateness Criteria® Right Upper Quadrant Pain. J Am Coll Radiol. 2019;16(5S):S235-S243. https://doi.org/10.1016/j.jacr.2019.02.013
2. Bhosale PR, Javitt MC, Atri M, et al. ACR Appropriateness Criteria® Acute Pelvic Pain in the Reproductive Age Group. Ultrasound Q. 2016;32(2):108-115. https://doi.org/10.1097/RUQ.0000000000000200
3. Revzin MV, Scoutt LM, Garner JG, Moore CL. Right upper quadrant pain: ultrasound first! J Ultrasound Med. 2017;36(10):1975-1985. https://doi.org/10.1002/jum.14274
4. Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med. 1980;302(23):1277-1279. https://doi.org/10.1056/NEJM198006053022303
5. Barakos JA, Ralls PW, Lapin SA, et al. Cholelithiasis: evaluation with CT. Radiology. 1987;162(2):415-418. https://doi.org/10.1148/radiology.162.2.3797654
6. Moore C, Meyers AB, Capotasto J, Bokhari J. Prevalence of abnormal CT findings in patients with proven ovarian torsion and a proposed triage schema. Emerg Radiol. 2009;16(2):115-120. https://doi.org/10.1007/s10140-008-0754-x
7. Harfouch N, Stern J, Chowdhary V, et al. Utility of ultrasound after a negative CT abdomen and pelvis in the emergency department. Clin Imaging. 2020;68:29-35. https://doi.org/10.1016/j.clinimag.2020.06.007
8. Adenaw N, Wen J, Pahwa AK, Sheth S, Johnson PT. Decreasing duplicative imaging: inpatient and emergency medicine abdominal ultrasound within 72 hours of abdominal CT. J Am Coll Radiol. 2020;17(5):590-596. https://doi.org/10.1016/j.jacr.2020.03.010
9. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720. https://doi.org/10.1148/radiol.12111561
10. Wertz JR, Lopez JM, Olson D, Thompson WM. Comparing the diagnostic accuracy of ultrasound and CT in evaluating acute cholecystitis. AJR Am J Roentgenol. 2018;211(2):W92-W97. https://doi.org/10.2214/AJR.17.18884
11. Bennett GL, Rusinek H, Lisi V, et al. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178(2):275-281. https://doi.org/10.2214/ajr.178.2.1780275
12. Hiatt KD, Ou JJ, Childs DD. Role of ultrasound and CT in the workup of right upper quadrant pain in adults in the emergency department: a retrospective review of more than 2800 cases. AJR Am J Roentgenol. 2020;214(6):1305-1310. https://doi.org/10.2214/AJR.19.22188
13. Gao Y, Lee K, Camacho M. Utility of pelvic ultrasound following negative abdominal and pelvic CT in the emergency room. Clin Radiol. 2013;68(11):e586-e592. https://doi.org/10.1016/j.crad.2013.05.101
14. Natesan S, Lee J, Volkamer H, Thoureen T. Evidence-based medicine approach to abdominal pain. Emerg Med Clin North Am. 2016;34(2):165-190. https://doi.org/10.1016/j.emc.2015.12.008.
15. Dickerson EC, Alam HB, Brown RK, Stojanovska J, Davenport MS; Michigan Radiology Quality Collaborative. In-person communication between radiologists and acute care surgeons leads to significant alterations in surgical decision making. J Am Coll Radiol. 2016;13(8):943-949. https://doi.org/10.1016/j.jacr.2016.02.005
© 2021 Society of Hospital Medicine
Opioid Utilization and Perception of Pain Control in Hospitalized Patients: A Cross-Sectional Study of 11 Sites in 8 Countries
Since 2000, the United States has seen a marked increase in opioid prescribing1-3 and opioid-related complications, including overdoses, hospitalizations, and deaths.2,4,5 A study from 2015 showed that more than one-third of the US civilian noninstitutionalized population reported receiving an opioid prescription in the prior year, with 12.5% reporting misuse, and, of those, 16.7% reported a prescription use disorder.6 While there has been a slight decrease in opioid prescriptions in the US since 2012, rates of opioid prescribing in 2015 were three times higher than in 1999 and approximately four times higher than in Europe in 2015.3,7
Pain is commonly reported by hospitalized patients,8,9 and opioids are often a mainstay of treatment;9,10 however, treatment with opioids can have a number of adverse outcomes.2,10,11 Short-term exposure to opioids can lead to long-term use,12-16 and patients on opioids are at an increased risk for subsequent hospitalization and longer inpatient lengths of stay.5
Physician prescribing practices for opioids and patient expectations for pain control vary as a function of geographic region and culture,10,12,17,18 and pain is influenced by the cultural context in which it occurs.17,19-22 Treatment of pain may also be affected by limited access to or restrictions on selected medications, as well as by cultural biases.23 Whether these variations in the treatment of pain are reflected in patients’ satisfaction with pain control is uncertain.
We sought to compare the inpatient analgesic prescribing practices and patients’ perceptions of pain control for medical patients in four teaching hospitals in the US and in seven teaching hospitals in seven other countries.
METHODS
Study Design
We utilized a cross-sectional, observational design. The study was approved by the Institutional Review Boards at all participating sites.
Setting
The study was conducted at 11 academic hospitals in eight countries from October 8, 2013 to August 31, 2015. Sites in the US included Denver Health in Denver, Colorado; the University of Colorado Hospital in Aurora, Colorado; Hennepin Healthcare in Minneapolis, Minnesota; and Legacy Health in Portland, Oregon. Sites outside the US included McMaster University in Hamilton, Ontario, Canada; Hospital de la Santa Creu i Sant Pau, Universitat Autonòma de Barcelona in Barcelona, Spain; the University of Study of Milan and the University Ospedale “Luigi Sacco” in Milan, Italy, the National Taiwan University Hospital, in Taipei, Taiwan, the University of Ulsan College of Medicine, Asan Medical Center, in Seoul, Korea, the Imperial College, Chelsea and Westminster Hospital, in London, United Kingdom and Dunedin Hospital, Dunedin, New Zealand.
Inclusion and Exclusion Criteria
We included patients 18-89 years of age (20-89 in Taiwan because patients under 20 years of age in this country are a restricted group with respect to participating in research), admitted to an internal medicine service from the Emergency Department or Urgent Care clinic with an acute illness for a minimum of 24 hours (with time zero defined as the time care was initiated in the Emergency Department or Urgent Care Clinic), who reported pain at some time during the first 24-36 hours of their hospitalization and who provided informed consent. In the US, “admission” included both observation and inpatient status. We limited the patient population to those admitted via emergency departments and urgent care clinics in order to enroll similar patient populations across sites.
Scheduled admissions, patients transferred from an outside facility, patients admitted directly from a clinic, and those receiving care in intensive care units were excluded. We also excluded patients who were incarcerated, pregnant, those who received major surgery within the previous 14 days, those with a known diagnosis of active cancer, and those who were receiving palliative or hospice care. Patients receiving care from an investigator in the study at the time of enrollment were not eligible due to the potential conflict of interest.
Patient Screening
Primary teams were contacted to determine if any patients on their service might meet the criteria for inclusion in the study on preselected study days chosen on the basis of the research team’s availability. Identified patients were then screened to establish if they met the eligibility criteria. Patients were asked directly if they had experienced pain during their preadmission evaluation or during their hospitalization.
Data Collection
All patients were hospitalized at the time they gave consent and when data were collected. Data were collected via interviews with patients, as well as through chart review. We recorded patients’ age, gender, race, admitting diagnosis(es), length of stay, psychiatric illness, illicit drug use, whether they reported receiving opioid analgesics at the time of hospitalization, whether they were prescribed opioids and/or nonopioid analgesics during their hospitalization, the median and maximum doses of opioids prescribed and dispensed, and whether they were discharged on opioids. The question of illicit drug use was asked of all patients with the exception of those hospitalized in South Korea due to potential legal implications.
Opioid prescribing and receipt of opioids was recorded based upon current provider orders and medication administration records, respectively. Perception of and satisfaction with pain control was assessed with the American Pain Society Patient Outcome Questionnaire–Modified (APS-POQ-Modified).24,25 Versions of this survey have been validated in English as well as in other languages and cultures.26-28 Because hospitalization practices could differ across hospitals and in different countries, we compared patients’ severity of illness by using Charlson comorbidity scores. Consent forms and the APS-POQ were translated into each country’s primary language according to established processes.29 The survey was filled out by having site investigators read questions aloud and by use of a large-font visual analog scale to aid patients’ verbal responses.
Data were collected and managed using a secure, web-based application electronic data capture tool (Research Electronic Data Capture [REDCap], Nashville, Tennessee), hosted at Denver Health.30
Study Size
Preliminary data from the internal medicine units at our institution suggested that 40% of patients without cancer received opioid analgesics during their hospitalization. Assuming 90% power to detect an absolute difference in the proportion of inpatient medical patients who are receiving opioid analgesics during their hospital stay of 17%, a two-sided type 1 error rate of 0.05, six hospitals in the US, and nine hospitals from all other countries, we calculated an initial sample size of 150 patients per site. This sample size was considered feasible for enrollment in a busy inpatient clinical setting. Study end points were to either reach the goal number of patients (150 per site) or the predetermined study end date, whichever came first.
Data Analysis
We generated means with standard deviations (SDs) and medians with interquartile ranges (IQRs) for normally and nonnormally distributed continuous variables, respectively, and frequencies for categorical variables. We used linear mixed modeling for the analysis of continuous variables. For binary outcomes, our data were fitted to a generalized linear mixed model with logit as the link function and a binary distribution. For ordinal variables, specifically patient-reported satisfaction with pain control and the opinion statements, the data were fitted to a generalized linear mixed model with a cumulative logit link and a multinomial distribution. Hospital was included as a random effect in all models to account for patients cared for in the same hospital.
Country of origin, dichotomized as US or non-US, was the independent variable of interest for all models. An interaction term for exposure to opioids prior to admission and country was entered into all models to explore whether differences in the effect of country existed for patients who reported taking opioids prior to admission and those who did not.
The models for the frequency with which analgesics were given, doses of opioids given during hospitalization and at discharge, patient-reported pain score, and patient-reported satisfaction with pain control were adjusted for (1) age, (2) gender, (3) Charlson Comorbidity Index, (4) length of stay, (5) history of illicit drug use, (6) history of psychiatric illness, (7) daily dose in morphine milligram equivalents (MME) for opioids prior to admission, (8) average pain score, and (9) hospital. The patient-reported satisfaction with pain control model was also adjusted for whether or not opioids were given to the patient during their hospitalization. P < .05 was considered to indicate significance. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, North Carolina). We reported data on medications that were prescribed and dispensed (as opposed to just prescribed and not necessarily given). Opioids prescribed at discharge represented the total possible opioids that could be given based upon the order/prescription (eg, oxycodone 5 mg every 6 hours as needed for pain would be counted as 20 mg/24 hours maximum possible dose followed by conversion to MME).
Missing Data
When there were missing data, a query was sent to sites to verify if the data were retrievable. If retrievable, the data were then entered. Data were missing in 5% and 2% of patients who did or did not report taking an opioid prior to admission, respectively. If a variable was included in a specific statistical test, then subjects with missing data were excluded from that analysis (ie, complete case analysis).
RESULTS
We approached 1,309 eligible patients, of which 981 provided informed consent, for a response rate of 75%; 503 from the US and 478 patients from other countries (Figure). In unadjusted analyses, we found no significant differences between US and non-US patients in age (mean age 51, SD 15 vs 59, SD 19; P = .30), race, ethnicity, or Charlson comorbidity index scores (median 2, IQR 1-3 vs 3, IQR 1-4; P = .45). US patients had shorter lengths of stay (median 3 days, IQR 2-4 vs 6 days, IQR 3-11; P = .04), a more frequent history of illicit drug use (33% vs 6%; P = .003), a higher frequency of psychiatric illness (27% vs 8%; P < .0001), and more were receiving opioid analgesics prior to admission (38% vs 17%; P = .007) than those hospitalized in other countries (Table 1, Appendix 1). The primary admitting diagnoses for all patients in the study are listed in Appendix 2. Opioid prescribing practices across the individual sites are shown in Appendix 3.
Patients Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found that more patients in the US were given opioids during their hospitalization and in higher doses than patients from other countries and more were prescribed opioids at discharge. Fewer patients in the US were dispensed nonopioid analgesics during their hospitalization than patients from other countries, but this difference was not significant (Table 2). Appendix 4 shows the types of nonopioid pain medications prescribed in the US and other countries.
After adjustment for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys. We found no significant difference in satisfaction with pain control between patients from the US and other countries in the models, regardless of whether we included average pain score or opioid receipt during hospitalization in the model (Table 3).
In unadjusted analyses, compared with patients hospitalized in other countries, more patients in the US stated that they would like a stronger dose of analgesic if they were still in pain, though the difference was nonsignificant, and US patients were more likely to agree with the statement that people become addicted to pain medication easily and less likely to agree with the statement that it is easier to endure pain than deal with the side effects of pain medications (Table 3).
Patients Not Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found no significant difference in the proportion of US patients provided with nonopioid pain medications during their hospitalization compared with patients in other countries, but a greater percentage of US patients were given opioids during their hospitalization and at discharge and in higher doses (Table 2).
After adjusting for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys and greater pain severity in the 24-36 hours prior to completing the survey than patients from other countries, but we found no difference in patient satisfaction with pain control (Table 3). After we included the average pain score and whether or not opioids were given to the patient during their hospitalization in this model, patients in the US were more likely to report a higher level of satisfaction with pain control than patients in all other countries (P = .001).
In unadjusted analyses, compared with patients hospitalized in other countries, those in the US were less likely to agree with the statement that good patients avoid talking about pain (Table 3).
Patient Satisfaction and Opioid Receipt
Among patients cared for in the US, after controlling for the average pain score, we did not find a significant association between receiving opioids while in the hospital and satisfaction with pain control for patients who either did or did not endorse taking opioids prior to admission (P = .38 and P = .24, respectively). Among patients cared for in all other countries, after controlling for the average pain score, we found a significant association between receiving opioids while in the hospital and a lower level of satisfaction with pain control for patients who reported taking opioids prior to admission (P = .02) but not for patients who did not report taking opioids prior to admission (P = .08).
DISCUSSION
Compared with patients hospitalized in other countries, a greater percentage of those hospitalized in the US were prescribed opioid analgesics both during hospitalization and at the time of discharge, even after adjustment for pain severity. In addition, patients hospitalized in the US reported greater pain severity at the time they completed their pain surveys and in the 24 to 36 hours prior to completing the survey than patients from other countries. In this sample, satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Our study also suggests that opioid receipt did not lead to improved patient satisfaction with pain control.
The frequency with which we observed opioid analgesics being prescribed during hospitalization in US hospitals (79%) was higher than the 51% of patients who received opioids reported by Herzig and colleagues.10 Patients in our study had a higher prevalence of illicit drug abuse and psychiatric illness, and our study only included patients who reported pain at some point during their hospitalization. We also studied prescribing practices through analysis of provider orders and medication administration records at the time the patient was hospitalized.
While we observed that physicians in the US more frequently prescribed opioid analgesics during hospitalizations than physicians working in other countries, we also observed that patients in the US reported higher levels of pain during their hospitalization. After adjusting for a number of variables, including pain severity, however, we still found that opioids were more commonly prescribed during hospitalizations by physicians working in the US sites studied than by physicians in the non-US sites.
Opioid prescribing practices varied across the sites sampled in our study. While the US sites, Taiwan, and Korea tended to be heavier utilizers of opioids during hospitalization, there were notable differences in discharge prescribing of opioids, with the US sites more commonly prescribing opioids and higher MME for patients who did not report taking opioids prior to their hospitalization (Appendix 3). A sensitivity analysis was conducted excluding South Korea from modeling, given that patients there were not asked about illicit opioid use. There were no important changes in the magnitude or direction of the results.
Our study supports previous studies indicating that there are cultural and societal differences when it comes to the experience of pain and the expectations around pain control.17,20-22,31 Much of the focus on reducing opioid utilization has been on provider practices32 and on prescription drug monitoring programs.33 Our findings suggest that another area of focus that may be important in mitigating the opioid epidemic is patient expectations of pain control.
Our study has a number of strengths. First, we included 11 hospitals from eight different countries. Second, we believe this is the first study to assess opioid prescribing and dispensing practices during hospitalization as well as at the time of discharge. Third, patient perceptions of pain control were assessed in conjunction with analgesic prescribing and were assessed during hospitalization. Fourth, we had high response rates for patient participation in our study. Fifth, we found much larger differences in opioid prescribing than anticipated, and thus, while we did not achieve the sample size originally planned for either the number of hospitals or patients enrolled per hospital, we were sufficiently powered. This is likely secondary to the fact that the population we studied was one that specifically reported pain, resulting in the larger differences seen.
Our study also had a number of limitations. First, the prescribing practices in countries other than the US are represented by only one hospital per country and, in some countries, by limited numbers of patients. While we studied four sites in the US, we did not have a site in the Northeast, a region previously shown to have lower prescribing rates.10 Additionally, patient samples for the US sites compared with the sites in other countries varied considerably with respect to ethnicity. While some studies in US patients have shown that opioid prescribing may vary based on race/ethnicity,34 we are uncertain as to how this might impact a study that crosses multiple countries. We also had a low number of patients receiving opioids prior to hospitalization for several of the non-US countries, which reduced the power to detect differences in this subgroup. Previous research has shown that there are wide variations in prescribing practices even within countries;10,12,18 therefore, caution should be taken when generalizing our findings. Second, we assessed analgesic prescribing patterns and pain control during the first 24 to 36 hours of hospitalization and did not consider hospital days beyond this timeframe with the exception of noting what medications were prescribed at discharge. We chose this methodology in an attempt to eliminate as many differences that might exist in the duration of hospitalization across many countries. Third, investigators in the study administered the survey, and respondents may have been affected by social desirability bias in how the survey questions were answered. Because investigators were not a part of the care team of any study patients, we believe this to be unlikely. Fourth, our study was conducted from October 8, 2013 to August 31, 2015 and the opioid epidemic is dynamic. Accordingly, our data may not reflect current opioid prescribing practices or patients’ current beliefs regarding pain control. Fifth, we did not collect demographic data on the patients who did not participate and could not look for systematic differences between participants and nonparticipants. Sixth, we relied on patients to self-report whether they were taking opioids prior to hospitalization or using illicit drugs. Seventh, we found comorbid mental health conditions to be more frequent in the US population studied. Previous work has shown regional variation in mental health conditions,35,36 which could have affected our findings. To account for this, our models included psychiatric illness.
CONCLUSIONS
Our data suggest that physicians in the US may prescribe opioids more frequently during patients’ hospitalizations and at discharge than their colleagues in other countries. We also found that patient satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Although the small number of hospitals included in our sample coupled with the small sample size in some of the non-US countries limits the generalizability of our findings, the data suggest that reducing the opioid epidemic in the US may require addressing patients’ expectations regarding pain control in addition to providers’ inpatient analgesic prescribing patterns.
Disclosures
The authors report no conflicts of interest.
Funding
The authors report no funding source for this work.
1. Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70-78. https://doi.org/10.1001/jama.2007.64.
2. Herzig SJ. Growing concerns regarding long-term opioid use: the hospitalization hazard. J Hosp Med. 2015;10(7):469-470. https://doi.org/10.1002/jhm.2369.
3. Guy GP Jr, Zhang K, Bohm MK, et al. Vital Signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. https://doi.org/10.15585/mmwr.mm6626a4.
4. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
5. Liang Y, Turner BJ. National cohort study of opioid analgesic dose and risk of future hospitalization. J Hosp Med. 2015;10(7):425-431. https://doi.org/10.1002/jhm.2350.
6. Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. https://doi.org/10.7326/M17-0865.
7. Schuchat A, Houry D, Guy GP, Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426. https://doi.org/10.1001/jama.2017.8913.
8. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. https://doi.org/10.1016/j.pmn.2008.02.001.
9. Gupta A, Daigle S, Mojica J, Hurley RW. Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157-164. https://doi.org/10.2147/JPR.S7903.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
11. Kanjanarat P, Winterstein AG, Johns TE, et al. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750-1759. https://doi.org/10.1093/ajhp/60.17.1750.
12. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. https://doi.org/10.1001/jamainternmed.2016.2737.
13. Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and risk factors for progression From short-term to episodic or long-term opioid prescribing: A population-based study. Mayo Clin Proc. 2015;90(7):850-856. https://doi.org/10.1016/j.mayocp.2015.04.012.
14. Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. https://doi.org/10.1001/archinternmed.2011.1827.
15. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. https://doi.org/10.1056/NEJMsa1610524.
16. Calcaterra SL, Scarbro S, Hull ML, et al. Prediction of future chronic opioid use Among hospitalized patients. J Gen Intern Med. 2018;33(6):898-905. https://doi.org/10.1007/s11606-018-4335-8.
17. Callister LC. Cultural influences on pain perceptions and behaviors. Home Health Care Manag Pract. 2003;15(3):207-211. https://doi.org/10.1177/1084822302250687.
18. Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines--United States, 2012. J Saf Res. 2014;63(26):563-568. https://doi.org/10.1016/j.jsr.2014.09.001.
19. Callister LC, Khalaf I, Semenic S, Kartchner R, Vehvilainen-Julkunen K. The pain of childbirth: perceptions of culturally diverse women. Pain Manag Nurs. 2003;4(4):145-154. https://doi.org/10.1016/S1524-9042(03)00028-6.
20. Moore R, Brødsgaard I, Mao TK, Miller ML, Dworkin SF. Perceived need for local anesthesia in tooth drilling among Anglo-Americans, Chinese, and Scandinavians. Anesth Prog. 1998;45(1):22-28.
21. Kankkunen PM, Vehviläinen-Julkunen KM, Pietilä AM, et al. A tale of two countries: comparison of the perceptions of analgesics among Finnish and American parents. Pain Manag Nurs. 2008;9(3):113-119. https://doi.org/10.1016/j.pmn.2007.12.003.
22. Hanoch Y, Katsikopoulos KV, Gummerum M, Brass EP. American and German students’ knowledge, perceptions, and behaviors with respect to over-the-counter pain relievers. Health Psychol. 2007;26(6):802-806. https://doi.org/10.1037/0278-6133.26.6.802.
23. Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N. Availability and utilization of opioids for pain management: global issues. Ochsner J. 2014;14(2):208-215.
24. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274(23):1874-1880.
25. McNeill JA, Sherwood GD, Starck PL, Thompson CJ. Assessing clinical outcomes: patient satisfaction with pain management. J Pain Symptom Manag. 1998;16(1):29-40. https://doi.org/10.1016/S0885-3924(98)00034-7.
26. Ferrari R, Novello C, Catania G, Visentin M. Patients’ satisfaction with pain management: the Italian version of the Patient Outcome Questionnaire of the American Pain Society. Recenti Prog Med. 2010;101(7–8):283-288.
27. Malouf J, Andión O, Torrubia R, Cañellas M, Baños JE. A survey of perceptions with pain management in Spanish inpatients. J Pain Symptom Manag. 2006;32(4):361-371. https://doi.org/10.1016/j.jpainsymman.2006.05.006.
28. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. https://doi.org/10.1016/j.jpain.2010.02.012.
29. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-3191. https://doi.org/10.1097/00007632-200012150-00014.
30. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
31. Duman F. After surgery in Germany, I wanted Vicodin, not herbal tea. New York Times. January 27, 2018. https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed November 6, 2018.
32. Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109-118. https://doi.org/10.5055/jom.2016.0322. 33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
34. Friedman J, Kim D, Schneberk T, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2018.6721.
35. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43(2):476-493. https://doi.org/10.1093/ije/dyu038.
36. Simon GE, Goldberg DP, Von Korff M, Ustün TB. Understanding cross-national differences in depression prevalence. Psychol Med. 2002;32(4):585-594. https://doi.org/10.1017/S0033291702005457.
Since 2000, the United States has seen a marked increase in opioid prescribing1-3 and opioid-related complications, including overdoses, hospitalizations, and deaths.2,4,5 A study from 2015 showed that more than one-third of the US civilian noninstitutionalized population reported receiving an opioid prescription in the prior year, with 12.5% reporting misuse, and, of those, 16.7% reported a prescription use disorder.6 While there has been a slight decrease in opioid prescriptions in the US since 2012, rates of opioid prescribing in 2015 were three times higher than in 1999 and approximately four times higher than in Europe in 2015.3,7
Pain is commonly reported by hospitalized patients,8,9 and opioids are often a mainstay of treatment;9,10 however, treatment with opioids can have a number of adverse outcomes.2,10,11 Short-term exposure to opioids can lead to long-term use,12-16 and patients on opioids are at an increased risk for subsequent hospitalization and longer inpatient lengths of stay.5
Physician prescribing practices for opioids and patient expectations for pain control vary as a function of geographic region and culture,10,12,17,18 and pain is influenced by the cultural context in which it occurs.17,19-22 Treatment of pain may also be affected by limited access to or restrictions on selected medications, as well as by cultural biases.23 Whether these variations in the treatment of pain are reflected in patients’ satisfaction with pain control is uncertain.
We sought to compare the inpatient analgesic prescribing practices and patients’ perceptions of pain control for medical patients in four teaching hospitals in the US and in seven teaching hospitals in seven other countries.
METHODS
Study Design
We utilized a cross-sectional, observational design. The study was approved by the Institutional Review Boards at all participating sites.
Setting
The study was conducted at 11 academic hospitals in eight countries from October 8, 2013 to August 31, 2015. Sites in the US included Denver Health in Denver, Colorado; the University of Colorado Hospital in Aurora, Colorado; Hennepin Healthcare in Minneapolis, Minnesota; and Legacy Health in Portland, Oregon. Sites outside the US included McMaster University in Hamilton, Ontario, Canada; Hospital de la Santa Creu i Sant Pau, Universitat Autonòma de Barcelona in Barcelona, Spain; the University of Study of Milan and the University Ospedale “Luigi Sacco” in Milan, Italy, the National Taiwan University Hospital, in Taipei, Taiwan, the University of Ulsan College of Medicine, Asan Medical Center, in Seoul, Korea, the Imperial College, Chelsea and Westminster Hospital, in London, United Kingdom and Dunedin Hospital, Dunedin, New Zealand.
Inclusion and Exclusion Criteria
We included patients 18-89 years of age (20-89 in Taiwan because patients under 20 years of age in this country are a restricted group with respect to participating in research), admitted to an internal medicine service from the Emergency Department or Urgent Care clinic with an acute illness for a minimum of 24 hours (with time zero defined as the time care was initiated in the Emergency Department or Urgent Care Clinic), who reported pain at some time during the first 24-36 hours of their hospitalization and who provided informed consent. In the US, “admission” included both observation and inpatient status. We limited the patient population to those admitted via emergency departments and urgent care clinics in order to enroll similar patient populations across sites.
Scheduled admissions, patients transferred from an outside facility, patients admitted directly from a clinic, and those receiving care in intensive care units were excluded. We also excluded patients who were incarcerated, pregnant, those who received major surgery within the previous 14 days, those with a known diagnosis of active cancer, and those who were receiving palliative or hospice care. Patients receiving care from an investigator in the study at the time of enrollment were not eligible due to the potential conflict of interest.
Patient Screening
Primary teams were contacted to determine if any patients on their service might meet the criteria for inclusion in the study on preselected study days chosen on the basis of the research team’s availability. Identified patients were then screened to establish if they met the eligibility criteria. Patients were asked directly if they had experienced pain during their preadmission evaluation or during their hospitalization.
Data Collection
All patients were hospitalized at the time they gave consent and when data were collected. Data were collected via interviews with patients, as well as through chart review. We recorded patients’ age, gender, race, admitting diagnosis(es), length of stay, psychiatric illness, illicit drug use, whether they reported receiving opioid analgesics at the time of hospitalization, whether they were prescribed opioids and/or nonopioid analgesics during their hospitalization, the median and maximum doses of opioids prescribed and dispensed, and whether they were discharged on opioids. The question of illicit drug use was asked of all patients with the exception of those hospitalized in South Korea due to potential legal implications.
Opioid prescribing and receipt of opioids was recorded based upon current provider orders and medication administration records, respectively. Perception of and satisfaction with pain control was assessed with the American Pain Society Patient Outcome Questionnaire–Modified (APS-POQ-Modified).24,25 Versions of this survey have been validated in English as well as in other languages and cultures.26-28 Because hospitalization practices could differ across hospitals and in different countries, we compared patients’ severity of illness by using Charlson comorbidity scores. Consent forms and the APS-POQ were translated into each country’s primary language according to established processes.29 The survey was filled out by having site investigators read questions aloud and by use of a large-font visual analog scale to aid patients’ verbal responses.
Data were collected and managed using a secure, web-based application electronic data capture tool (Research Electronic Data Capture [REDCap], Nashville, Tennessee), hosted at Denver Health.30
Study Size
Preliminary data from the internal medicine units at our institution suggested that 40% of patients without cancer received opioid analgesics during their hospitalization. Assuming 90% power to detect an absolute difference in the proportion of inpatient medical patients who are receiving opioid analgesics during their hospital stay of 17%, a two-sided type 1 error rate of 0.05, six hospitals in the US, and nine hospitals from all other countries, we calculated an initial sample size of 150 patients per site. This sample size was considered feasible for enrollment in a busy inpatient clinical setting. Study end points were to either reach the goal number of patients (150 per site) or the predetermined study end date, whichever came first.
Data Analysis
We generated means with standard deviations (SDs) and medians with interquartile ranges (IQRs) for normally and nonnormally distributed continuous variables, respectively, and frequencies for categorical variables. We used linear mixed modeling for the analysis of continuous variables. For binary outcomes, our data were fitted to a generalized linear mixed model with logit as the link function and a binary distribution. For ordinal variables, specifically patient-reported satisfaction with pain control and the opinion statements, the data were fitted to a generalized linear mixed model with a cumulative logit link and a multinomial distribution. Hospital was included as a random effect in all models to account for patients cared for in the same hospital.
Country of origin, dichotomized as US or non-US, was the independent variable of interest for all models. An interaction term for exposure to opioids prior to admission and country was entered into all models to explore whether differences in the effect of country existed for patients who reported taking opioids prior to admission and those who did not.
The models for the frequency with which analgesics were given, doses of opioids given during hospitalization and at discharge, patient-reported pain score, and patient-reported satisfaction with pain control were adjusted for (1) age, (2) gender, (3) Charlson Comorbidity Index, (4) length of stay, (5) history of illicit drug use, (6) history of psychiatric illness, (7) daily dose in morphine milligram equivalents (MME) for opioids prior to admission, (8) average pain score, and (9) hospital. The patient-reported satisfaction with pain control model was also adjusted for whether or not opioids were given to the patient during their hospitalization. P < .05 was considered to indicate significance. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, North Carolina). We reported data on medications that were prescribed and dispensed (as opposed to just prescribed and not necessarily given). Opioids prescribed at discharge represented the total possible opioids that could be given based upon the order/prescription (eg, oxycodone 5 mg every 6 hours as needed for pain would be counted as 20 mg/24 hours maximum possible dose followed by conversion to MME).
Missing Data
When there were missing data, a query was sent to sites to verify if the data were retrievable. If retrievable, the data were then entered. Data were missing in 5% and 2% of patients who did or did not report taking an opioid prior to admission, respectively. If a variable was included in a specific statistical test, then subjects with missing data were excluded from that analysis (ie, complete case analysis).
RESULTS
We approached 1,309 eligible patients, of which 981 provided informed consent, for a response rate of 75%; 503 from the US and 478 patients from other countries (Figure). In unadjusted analyses, we found no significant differences between US and non-US patients in age (mean age 51, SD 15 vs 59, SD 19; P = .30), race, ethnicity, or Charlson comorbidity index scores (median 2, IQR 1-3 vs 3, IQR 1-4; P = .45). US patients had shorter lengths of stay (median 3 days, IQR 2-4 vs 6 days, IQR 3-11; P = .04), a more frequent history of illicit drug use (33% vs 6%; P = .003), a higher frequency of psychiatric illness (27% vs 8%; P < .0001), and more were receiving opioid analgesics prior to admission (38% vs 17%; P = .007) than those hospitalized in other countries (Table 1, Appendix 1). The primary admitting diagnoses for all patients in the study are listed in Appendix 2. Opioid prescribing practices across the individual sites are shown in Appendix 3.
Patients Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found that more patients in the US were given opioids during their hospitalization and in higher doses than patients from other countries and more were prescribed opioids at discharge. Fewer patients in the US were dispensed nonopioid analgesics during their hospitalization than patients from other countries, but this difference was not significant (Table 2). Appendix 4 shows the types of nonopioid pain medications prescribed in the US and other countries.
After adjustment for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys. We found no significant difference in satisfaction with pain control between patients from the US and other countries in the models, regardless of whether we included average pain score or opioid receipt during hospitalization in the model (Table 3).
In unadjusted analyses, compared with patients hospitalized in other countries, more patients in the US stated that they would like a stronger dose of analgesic if they were still in pain, though the difference was nonsignificant, and US patients were more likely to agree with the statement that people become addicted to pain medication easily and less likely to agree with the statement that it is easier to endure pain than deal with the side effects of pain medications (Table 3).
Patients Not Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found no significant difference in the proportion of US patients provided with nonopioid pain medications during their hospitalization compared with patients in other countries, but a greater percentage of US patients were given opioids during their hospitalization and at discharge and in higher doses (Table 2).
After adjusting for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys and greater pain severity in the 24-36 hours prior to completing the survey than patients from other countries, but we found no difference in patient satisfaction with pain control (Table 3). After we included the average pain score and whether or not opioids were given to the patient during their hospitalization in this model, patients in the US were more likely to report a higher level of satisfaction with pain control than patients in all other countries (P = .001).
In unadjusted analyses, compared with patients hospitalized in other countries, those in the US were less likely to agree with the statement that good patients avoid talking about pain (Table 3).
Patient Satisfaction and Opioid Receipt
Among patients cared for in the US, after controlling for the average pain score, we did not find a significant association between receiving opioids while in the hospital and satisfaction with pain control for patients who either did or did not endorse taking opioids prior to admission (P = .38 and P = .24, respectively). Among patients cared for in all other countries, after controlling for the average pain score, we found a significant association between receiving opioids while in the hospital and a lower level of satisfaction with pain control for patients who reported taking opioids prior to admission (P = .02) but not for patients who did not report taking opioids prior to admission (P = .08).
DISCUSSION
Compared with patients hospitalized in other countries, a greater percentage of those hospitalized in the US were prescribed opioid analgesics both during hospitalization and at the time of discharge, even after adjustment for pain severity. In addition, patients hospitalized in the US reported greater pain severity at the time they completed their pain surveys and in the 24 to 36 hours prior to completing the survey than patients from other countries. In this sample, satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Our study also suggests that opioid receipt did not lead to improved patient satisfaction with pain control.
The frequency with which we observed opioid analgesics being prescribed during hospitalization in US hospitals (79%) was higher than the 51% of patients who received opioids reported by Herzig and colleagues.10 Patients in our study had a higher prevalence of illicit drug abuse and psychiatric illness, and our study only included patients who reported pain at some point during their hospitalization. We also studied prescribing practices through analysis of provider orders and medication administration records at the time the patient was hospitalized.
While we observed that physicians in the US more frequently prescribed opioid analgesics during hospitalizations than physicians working in other countries, we also observed that patients in the US reported higher levels of pain during their hospitalization. After adjusting for a number of variables, including pain severity, however, we still found that opioids were more commonly prescribed during hospitalizations by physicians working in the US sites studied than by physicians in the non-US sites.
Opioid prescribing practices varied across the sites sampled in our study. While the US sites, Taiwan, and Korea tended to be heavier utilizers of opioids during hospitalization, there were notable differences in discharge prescribing of opioids, with the US sites more commonly prescribing opioids and higher MME for patients who did not report taking opioids prior to their hospitalization (Appendix 3). A sensitivity analysis was conducted excluding South Korea from modeling, given that patients there were not asked about illicit opioid use. There were no important changes in the magnitude or direction of the results.
Our study supports previous studies indicating that there are cultural and societal differences when it comes to the experience of pain and the expectations around pain control.17,20-22,31 Much of the focus on reducing opioid utilization has been on provider practices32 and on prescription drug monitoring programs.33 Our findings suggest that another area of focus that may be important in mitigating the opioid epidemic is patient expectations of pain control.
Our study has a number of strengths. First, we included 11 hospitals from eight different countries. Second, we believe this is the first study to assess opioid prescribing and dispensing practices during hospitalization as well as at the time of discharge. Third, patient perceptions of pain control were assessed in conjunction with analgesic prescribing and were assessed during hospitalization. Fourth, we had high response rates for patient participation in our study. Fifth, we found much larger differences in opioid prescribing than anticipated, and thus, while we did not achieve the sample size originally planned for either the number of hospitals or patients enrolled per hospital, we were sufficiently powered. This is likely secondary to the fact that the population we studied was one that specifically reported pain, resulting in the larger differences seen.
Our study also had a number of limitations. First, the prescribing practices in countries other than the US are represented by only one hospital per country and, in some countries, by limited numbers of patients. While we studied four sites in the US, we did not have a site in the Northeast, a region previously shown to have lower prescribing rates.10 Additionally, patient samples for the US sites compared with the sites in other countries varied considerably with respect to ethnicity. While some studies in US patients have shown that opioid prescribing may vary based on race/ethnicity,34 we are uncertain as to how this might impact a study that crosses multiple countries. We also had a low number of patients receiving opioids prior to hospitalization for several of the non-US countries, which reduced the power to detect differences in this subgroup. Previous research has shown that there are wide variations in prescribing practices even within countries;10,12,18 therefore, caution should be taken when generalizing our findings. Second, we assessed analgesic prescribing patterns and pain control during the first 24 to 36 hours of hospitalization and did not consider hospital days beyond this timeframe with the exception of noting what medications were prescribed at discharge. We chose this methodology in an attempt to eliminate as many differences that might exist in the duration of hospitalization across many countries. Third, investigators in the study administered the survey, and respondents may have been affected by social desirability bias in how the survey questions were answered. Because investigators were not a part of the care team of any study patients, we believe this to be unlikely. Fourth, our study was conducted from October 8, 2013 to August 31, 2015 and the opioid epidemic is dynamic. Accordingly, our data may not reflect current opioid prescribing practices or patients’ current beliefs regarding pain control. Fifth, we did not collect demographic data on the patients who did not participate and could not look for systematic differences between participants and nonparticipants. Sixth, we relied on patients to self-report whether they were taking opioids prior to hospitalization or using illicit drugs. Seventh, we found comorbid mental health conditions to be more frequent in the US population studied. Previous work has shown regional variation in mental health conditions,35,36 which could have affected our findings. To account for this, our models included psychiatric illness.
CONCLUSIONS
Our data suggest that physicians in the US may prescribe opioids more frequently during patients’ hospitalizations and at discharge than their colleagues in other countries. We also found that patient satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Although the small number of hospitals included in our sample coupled with the small sample size in some of the non-US countries limits the generalizability of our findings, the data suggest that reducing the opioid epidemic in the US may require addressing patients’ expectations regarding pain control in addition to providers’ inpatient analgesic prescribing patterns.
Disclosures
The authors report no conflicts of interest.
Funding
The authors report no funding source for this work.
Since 2000, the United States has seen a marked increase in opioid prescribing1-3 and opioid-related complications, including overdoses, hospitalizations, and deaths.2,4,5 A study from 2015 showed that more than one-third of the US civilian noninstitutionalized population reported receiving an opioid prescription in the prior year, with 12.5% reporting misuse, and, of those, 16.7% reported a prescription use disorder.6 While there has been a slight decrease in opioid prescriptions in the US since 2012, rates of opioid prescribing in 2015 were three times higher than in 1999 and approximately four times higher than in Europe in 2015.3,7
Pain is commonly reported by hospitalized patients,8,9 and opioids are often a mainstay of treatment;9,10 however, treatment with opioids can have a number of adverse outcomes.2,10,11 Short-term exposure to opioids can lead to long-term use,12-16 and patients on opioids are at an increased risk for subsequent hospitalization and longer inpatient lengths of stay.5
Physician prescribing practices for opioids and patient expectations for pain control vary as a function of geographic region and culture,10,12,17,18 and pain is influenced by the cultural context in which it occurs.17,19-22 Treatment of pain may also be affected by limited access to or restrictions on selected medications, as well as by cultural biases.23 Whether these variations in the treatment of pain are reflected in patients’ satisfaction with pain control is uncertain.
We sought to compare the inpatient analgesic prescribing practices and patients’ perceptions of pain control for medical patients in four teaching hospitals in the US and in seven teaching hospitals in seven other countries.
METHODS
Study Design
We utilized a cross-sectional, observational design. The study was approved by the Institutional Review Boards at all participating sites.
Setting
The study was conducted at 11 academic hospitals in eight countries from October 8, 2013 to August 31, 2015. Sites in the US included Denver Health in Denver, Colorado; the University of Colorado Hospital in Aurora, Colorado; Hennepin Healthcare in Minneapolis, Minnesota; and Legacy Health in Portland, Oregon. Sites outside the US included McMaster University in Hamilton, Ontario, Canada; Hospital de la Santa Creu i Sant Pau, Universitat Autonòma de Barcelona in Barcelona, Spain; the University of Study of Milan and the University Ospedale “Luigi Sacco” in Milan, Italy, the National Taiwan University Hospital, in Taipei, Taiwan, the University of Ulsan College of Medicine, Asan Medical Center, in Seoul, Korea, the Imperial College, Chelsea and Westminster Hospital, in London, United Kingdom and Dunedin Hospital, Dunedin, New Zealand.
Inclusion and Exclusion Criteria
We included patients 18-89 years of age (20-89 in Taiwan because patients under 20 years of age in this country are a restricted group with respect to participating in research), admitted to an internal medicine service from the Emergency Department or Urgent Care clinic with an acute illness for a minimum of 24 hours (with time zero defined as the time care was initiated in the Emergency Department or Urgent Care Clinic), who reported pain at some time during the first 24-36 hours of their hospitalization and who provided informed consent. In the US, “admission” included both observation and inpatient status. We limited the patient population to those admitted via emergency departments and urgent care clinics in order to enroll similar patient populations across sites.
Scheduled admissions, patients transferred from an outside facility, patients admitted directly from a clinic, and those receiving care in intensive care units were excluded. We also excluded patients who were incarcerated, pregnant, those who received major surgery within the previous 14 days, those with a known diagnosis of active cancer, and those who were receiving palliative or hospice care. Patients receiving care from an investigator in the study at the time of enrollment were not eligible due to the potential conflict of interest.
Patient Screening
Primary teams were contacted to determine if any patients on their service might meet the criteria for inclusion in the study on preselected study days chosen on the basis of the research team’s availability. Identified patients were then screened to establish if they met the eligibility criteria. Patients were asked directly if they had experienced pain during their preadmission evaluation or during their hospitalization.
Data Collection
All patients were hospitalized at the time they gave consent and when data were collected. Data were collected via interviews with patients, as well as through chart review. We recorded patients’ age, gender, race, admitting diagnosis(es), length of stay, psychiatric illness, illicit drug use, whether they reported receiving opioid analgesics at the time of hospitalization, whether they were prescribed opioids and/or nonopioid analgesics during their hospitalization, the median and maximum doses of opioids prescribed and dispensed, and whether they were discharged on opioids. The question of illicit drug use was asked of all patients with the exception of those hospitalized in South Korea due to potential legal implications.
Opioid prescribing and receipt of opioids was recorded based upon current provider orders and medication administration records, respectively. Perception of and satisfaction with pain control was assessed with the American Pain Society Patient Outcome Questionnaire–Modified (APS-POQ-Modified).24,25 Versions of this survey have been validated in English as well as in other languages and cultures.26-28 Because hospitalization practices could differ across hospitals and in different countries, we compared patients’ severity of illness by using Charlson comorbidity scores. Consent forms and the APS-POQ were translated into each country’s primary language according to established processes.29 The survey was filled out by having site investigators read questions aloud and by use of a large-font visual analog scale to aid patients’ verbal responses.
Data were collected and managed using a secure, web-based application electronic data capture tool (Research Electronic Data Capture [REDCap], Nashville, Tennessee), hosted at Denver Health.30
Study Size
Preliminary data from the internal medicine units at our institution suggested that 40% of patients without cancer received opioid analgesics during their hospitalization. Assuming 90% power to detect an absolute difference in the proportion of inpatient medical patients who are receiving opioid analgesics during their hospital stay of 17%, a two-sided type 1 error rate of 0.05, six hospitals in the US, and nine hospitals from all other countries, we calculated an initial sample size of 150 patients per site. This sample size was considered feasible for enrollment in a busy inpatient clinical setting. Study end points were to either reach the goal number of patients (150 per site) or the predetermined study end date, whichever came first.
Data Analysis
We generated means with standard deviations (SDs) and medians with interquartile ranges (IQRs) for normally and nonnormally distributed continuous variables, respectively, and frequencies for categorical variables. We used linear mixed modeling for the analysis of continuous variables. For binary outcomes, our data were fitted to a generalized linear mixed model with logit as the link function and a binary distribution. For ordinal variables, specifically patient-reported satisfaction with pain control and the opinion statements, the data were fitted to a generalized linear mixed model with a cumulative logit link and a multinomial distribution. Hospital was included as a random effect in all models to account for patients cared for in the same hospital.
Country of origin, dichotomized as US or non-US, was the independent variable of interest for all models. An interaction term for exposure to opioids prior to admission and country was entered into all models to explore whether differences in the effect of country existed for patients who reported taking opioids prior to admission and those who did not.
The models for the frequency with which analgesics were given, doses of opioids given during hospitalization and at discharge, patient-reported pain score, and patient-reported satisfaction with pain control were adjusted for (1) age, (2) gender, (3) Charlson Comorbidity Index, (4) length of stay, (5) history of illicit drug use, (6) history of psychiatric illness, (7) daily dose in morphine milligram equivalents (MME) for opioids prior to admission, (8) average pain score, and (9) hospital. The patient-reported satisfaction with pain control model was also adjusted for whether or not opioids were given to the patient during their hospitalization. P < .05 was considered to indicate significance. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, North Carolina). We reported data on medications that were prescribed and dispensed (as opposed to just prescribed and not necessarily given). Opioids prescribed at discharge represented the total possible opioids that could be given based upon the order/prescription (eg, oxycodone 5 mg every 6 hours as needed for pain would be counted as 20 mg/24 hours maximum possible dose followed by conversion to MME).
Missing Data
When there were missing data, a query was sent to sites to verify if the data were retrievable. If retrievable, the data were then entered. Data were missing in 5% and 2% of patients who did or did not report taking an opioid prior to admission, respectively. If a variable was included in a specific statistical test, then subjects with missing data were excluded from that analysis (ie, complete case analysis).
RESULTS
We approached 1,309 eligible patients, of which 981 provided informed consent, for a response rate of 75%; 503 from the US and 478 patients from other countries (Figure). In unadjusted analyses, we found no significant differences between US and non-US patients in age (mean age 51, SD 15 vs 59, SD 19; P = .30), race, ethnicity, or Charlson comorbidity index scores (median 2, IQR 1-3 vs 3, IQR 1-4; P = .45). US patients had shorter lengths of stay (median 3 days, IQR 2-4 vs 6 days, IQR 3-11; P = .04), a more frequent history of illicit drug use (33% vs 6%; P = .003), a higher frequency of psychiatric illness (27% vs 8%; P < .0001), and more were receiving opioid analgesics prior to admission (38% vs 17%; P = .007) than those hospitalized in other countries (Table 1, Appendix 1). The primary admitting diagnoses for all patients in the study are listed in Appendix 2. Opioid prescribing practices across the individual sites are shown in Appendix 3.
Patients Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found that more patients in the US were given opioids during their hospitalization and in higher doses than patients from other countries and more were prescribed opioids at discharge. Fewer patients in the US were dispensed nonopioid analgesics during their hospitalization than patients from other countries, but this difference was not significant (Table 2). Appendix 4 shows the types of nonopioid pain medications prescribed in the US and other countries.
After adjustment for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys. We found no significant difference in satisfaction with pain control between patients from the US and other countries in the models, regardless of whether we included average pain score or opioid receipt during hospitalization in the model (Table 3).
In unadjusted analyses, compared with patients hospitalized in other countries, more patients in the US stated that they would like a stronger dose of analgesic if they were still in pain, though the difference was nonsignificant, and US patients were more likely to agree with the statement that people become addicted to pain medication easily and less likely to agree with the statement that it is easier to endure pain than deal with the side effects of pain medications (Table 3).
Patients Not Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found no significant difference in the proportion of US patients provided with nonopioid pain medications during their hospitalization compared with patients in other countries, but a greater percentage of US patients were given opioids during their hospitalization and at discharge and in higher doses (Table 2).
After adjusting for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys and greater pain severity in the 24-36 hours prior to completing the survey than patients from other countries, but we found no difference in patient satisfaction with pain control (Table 3). After we included the average pain score and whether or not opioids were given to the patient during their hospitalization in this model, patients in the US were more likely to report a higher level of satisfaction with pain control than patients in all other countries (P = .001).
In unadjusted analyses, compared with patients hospitalized in other countries, those in the US were less likely to agree with the statement that good patients avoid talking about pain (Table 3).
Patient Satisfaction and Opioid Receipt
Among patients cared for in the US, after controlling for the average pain score, we did not find a significant association between receiving opioids while in the hospital and satisfaction with pain control for patients who either did or did not endorse taking opioids prior to admission (P = .38 and P = .24, respectively). Among patients cared for in all other countries, after controlling for the average pain score, we found a significant association between receiving opioids while in the hospital and a lower level of satisfaction with pain control for patients who reported taking opioids prior to admission (P = .02) but not for patients who did not report taking opioids prior to admission (P = .08).
DISCUSSION
Compared with patients hospitalized in other countries, a greater percentage of those hospitalized in the US were prescribed opioid analgesics both during hospitalization and at the time of discharge, even after adjustment for pain severity. In addition, patients hospitalized in the US reported greater pain severity at the time they completed their pain surveys and in the 24 to 36 hours prior to completing the survey than patients from other countries. In this sample, satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Our study also suggests that opioid receipt did not lead to improved patient satisfaction with pain control.
The frequency with which we observed opioid analgesics being prescribed during hospitalization in US hospitals (79%) was higher than the 51% of patients who received opioids reported by Herzig and colleagues.10 Patients in our study had a higher prevalence of illicit drug abuse and psychiatric illness, and our study only included patients who reported pain at some point during their hospitalization. We also studied prescribing practices through analysis of provider orders and medication administration records at the time the patient was hospitalized.
While we observed that physicians in the US more frequently prescribed opioid analgesics during hospitalizations than physicians working in other countries, we also observed that patients in the US reported higher levels of pain during their hospitalization. After adjusting for a number of variables, including pain severity, however, we still found that opioids were more commonly prescribed during hospitalizations by physicians working in the US sites studied than by physicians in the non-US sites.
Opioid prescribing practices varied across the sites sampled in our study. While the US sites, Taiwan, and Korea tended to be heavier utilizers of opioids during hospitalization, there were notable differences in discharge prescribing of opioids, with the US sites more commonly prescribing opioids and higher MME for patients who did not report taking opioids prior to their hospitalization (Appendix 3). A sensitivity analysis was conducted excluding South Korea from modeling, given that patients there were not asked about illicit opioid use. There were no important changes in the magnitude or direction of the results.
Our study supports previous studies indicating that there are cultural and societal differences when it comes to the experience of pain and the expectations around pain control.17,20-22,31 Much of the focus on reducing opioid utilization has been on provider practices32 and on prescription drug monitoring programs.33 Our findings suggest that another area of focus that may be important in mitigating the opioid epidemic is patient expectations of pain control.
Our study has a number of strengths. First, we included 11 hospitals from eight different countries. Second, we believe this is the first study to assess opioid prescribing and dispensing practices during hospitalization as well as at the time of discharge. Third, patient perceptions of pain control were assessed in conjunction with analgesic prescribing and were assessed during hospitalization. Fourth, we had high response rates for patient participation in our study. Fifth, we found much larger differences in opioid prescribing than anticipated, and thus, while we did not achieve the sample size originally planned for either the number of hospitals or patients enrolled per hospital, we were sufficiently powered. This is likely secondary to the fact that the population we studied was one that specifically reported pain, resulting in the larger differences seen.
Our study also had a number of limitations. First, the prescribing practices in countries other than the US are represented by only one hospital per country and, in some countries, by limited numbers of patients. While we studied four sites in the US, we did not have a site in the Northeast, a region previously shown to have lower prescribing rates.10 Additionally, patient samples for the US sites compared with the sites in other countries varied considerably with respect to ethnicity. While some studies in US patients have shown that opioid prescribing may vary based on race/ethnicity,34 we are uncertain as to how this might impact a study that crosses multiple countries. We also had a low number of patients receiving opioids prior to hospitalization for several of the non-US countries, which reduced the power to detect differences in this subgroup. Previous research has shown that there are wide variations in prescribing practices even within countries;10,12,18 therefore, caution should be taken when generalizing our findings. Second, we assessed analgesic prescribing patterns and pain control during the first 24 to 36 hours of hospitalization and did not consider hospital days beyond this timeframe with the exception of noting what medications were prescribed at discharge. We chose this methodology in an attempt to eliminate as many differences that might exist in the duration of hospitalization across many countries. Third, investigators in the study administered the survey, and respondents may have been affected by social desirability bias in how the survey questions were answered. Because investigators were not a part of the care team of any study patients, we believe this to be unlikely. Fourth, our study was conducted from October 8, 2013 to August 31, 2015 and the opioid epidemic is dynamic. Accordingly, our data may not reflect current opioid prescribing practices or patients’ current beliefs regarding pain control. Fifth, we did not collect demographic data on the patients who did not participate and could not look for systematic differences between participants and nonparticipants. Sixth, we relied on patients to self-report whether they were taking opioids prior to hospitalization or using illicit drugs. Seventh, we found comorbid mental health conditions to be more frequent in the US population studied. Previous work has shown regional variation in mental health conditions,35,36 which could have affected our findings. To account for this, our models included psychiatric illness.
CONCLUSIONS
Our data suggest that physicians in the US may prescribe opioids more frequently during patients’ hospitalizations and at discharge than their colleagues in other countries. We also found that patient satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Although the small number of hospitals included in our sample coupled with the small sample size in some of the non-US countries limits the generalizability of our findings, the data suggest that reducing the opioid epidemic in the US may require addressing patients’ expectations regarding pain control in addition to providers’ inpatient analgesic prescribing patterns.
Disclosures
The authors report no conflicts of interest.
Funding
The authors report no funding source for this work.
1. Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70-78. https://doi.org/10.1001/jama.2007.64.
2. Herzig SJ. Growing concerns regarding long-term opioid use: the hospitalization hazard. J Hosp Med. 2015;10(7):469-470. https://doi.org/10.1002/jhm.2369.
3. Guy GP Jr, Zhang K, Bohm MK, et al. Vital Signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. https://doi.org/10.15585/mmwr.mm6626a4.
4. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
5. Liang Y, Turner BJ. National cohort study of opioid analgesic dose and risk of future hospitalization. J Hosp Med. 2015;10(7):425-431. https://doi.org/10.1002/jhm.2350.
6. Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. https://doi.org/10.7326/M17-0865.
7. Schuchat A, Houry D, Guy GP, Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426. https://doi.org/10.1001/jama.2017.8913.
8. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. https://doi.org/10.1016/j.pmn.2008.02.001.
9. Gupta A, Daigle S, Mojica J, Hurley RW. Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157-164. https://doi.org/10.2147/JPR.S7903.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
11. Kanjanarat P, Winterstein AG, Johns TE, et al. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750-1759. https://doi.org/10.1093/ajhp/60.17.1750.
12. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. https://doi.org/10.1001/jamainternmed.2016.2737.
13. Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and risk factors for progression From short-term to episodic or long-term opioid prescribing: A population-based study. Mayo Clin Proc. 2015;90(7):850-856. https://doi.org/10.1016/j.mayocp.2015.04.012.
14. Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. https://doi.org/10.1001/archinternmed.2011.1827.
15. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. https://doi.org/10.1056/NEJMsa1610524.
16. Calcaterra SL, Scarbro S, Hull ML, et al. Prediction of future chronic opioid use Among hospitalized patients. J Gen Intern Med. 2018;33(6):898-905. https://doi.org/10.1007/s11606-018-4335-8.
17. Callister LC. Cultural influences on pain perceptions and behaviors. Home Health Care Manag Pract. 2003;15(3):207-211. https://doi.org/10.1177/1084822302250687.
18. Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines--United States, 2012. J Saf Res. 2014;63(26):563-568. https://doi.org/10.1016/j.jsr.2014.09.001.
19. Callister LC, Khalaf I, Semenic S, Kartchner R, Vehvilainen-Julkunen K. The pain of childbirth: perceptions of culturally diverse women. Pain Manag Nurs. 2003;4(4):145-154. https://doi.org/10.1016/S1524-9042(03)00028-6.
20. Moore R, Brødsgaard I, Mao TK, Miller ML, Dworkin SF. Perceived need for local anesthesia in tooth drilling among Anglo-Americans, Chinese, and Scandinavians. Anesth Prog. 1998;45(1):22-28.
21. Kankkunen PM, Vehviläinen-Julkunen KM, Pietilä AM, et al. A tale of two countries: comparison of the perceptions of analgesics among Finnish and American parents. Pain Manag Nurs. 2008;9(3):113-119. https://doi.org/10.1016/j.pmn.2007.12.003.
22. Hanoch Y, Katsikopoulos KV, Gummerum M, Brass EP. American and German students’ knowledge, perceptions, and behaviors with respect to over-the-counter pain relievers. Health Psychol. 2007;26(6):802-806. https://doi.org/10.1037/0278-6133.26.6.802.
23. Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N. Availability and utilization of opioids for pain management: global issues. Ochsner J. 2014;14(2):208-215.
24. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274(23):1874-1880.
25. McNeill JA, Sherwood GD, Starck PL, Thompson CJ. Assessing clinical outcomes: patient satisfaction with pain management. J Pain Symptom Manag. 1998;16(1):29-40. https://doi.org/10.1016/S0885-3924(98)00034-7.
26. Ferrari R, Novello C, Catania G, Visentin M. Patients’ satisfaction with pain management: the Italian version of the Patient Outcome Questionnaire of the American Pain Society. Recenti Prog Med. 2010;101(7–8):283-288.
27. Malouf J, Andión O, Torrubia R, Cañellas M, Baños JE. A survey of perceptions with pain management in Spanish inpatients. J Pain Symptom Manag. 2006;32(4):361-371. https://doi.org/10.1016/j.jpainsymman.2006.05.006.
28. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. https://doi.org/10.1016/j.jpain.2010.02.012.
29. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-3191. https://doi.org/10.1097/00007632-200012150-00014.
30. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
31. Duman F. After surgery in Germany, I wanted Vicodin, not herbal tea. New York Times. January 27, 2018. https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed November 6, 2018.
32. Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109-118. https://doi.org/10.5055/jom.2016.0322. 33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
34. Friedman J, Kim D, Schneberk T, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2018.6721.
35. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43(2):476-493. https://doi.org/10.1093/ije/dyu038.
36. Simon GE, Goldberg DP, Von Korff M, Ustün TB. Understanding cross-national differences in depression prevalence. Psychol Med. 2002;32(4):585-594. https://doi.org/10.1017/S0033291702005457.
1. Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70-78. https://doi.org/10.1001/jama.2007.64.
2. Herzig SJ. Growing concerns regarding long-term opioid use: the hospitalization hazard. J Hosp Med. 2015;10(7):469-470. https://doi.org/10.1002/jhm.2369.
3. Guy GP Jr, Zhang K, Bohm MK, et al. Vital Signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. https://doi.org/10.15585/mmwr.mm6626a4.
4. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
5. Liang Y, Turner BJ. National cohort study of opioid analgesic dose and risk of future hospitalization. J Hosp Med. 2015;10(7):425-431. https://doi.org/10.1002/jhm.2350.
6. Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. https://doi.org/10.7326/M17-0865.
7. Schuchat A, Houry D, Guy GP, Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426. https://doi.org/10.1001/jama.2017.8913.
8. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. https://doi.org/10.1016/j.pmn.2008.02.001.
9. Gupta A, Daigle S, Mojica J, Hurley RW. Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157-164. https://doi.org/10.2147/JPR.S7903.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
11. Kanjanarat P, Winterstein AG, Johns TE, et al. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750-1759. https://doi.org/10.1093/ajhp/60.17.1750.
12. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. https://doi.org/10.1001/jamainternmed.2016.2737.
13. Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and risk factors for progression From short-term to episodic or long-term opioid prescribing: A population-based study. Mayo Clin Proc. 2015;90(7):850-856. https://doi.org/10.1016/j.mayocp.2015.04.012.
14. Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. https://doi.org/10.1001/archinternmed.2011.1827.
15. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. https://doi.org/10.1056/NEJMsa1610524.
16. Calcaterra SL, Scarbro S, Hull ML, et al. Prediction of future chronic opioid use Among hospitalized patients. J Gen Intern Med. 2018;33(6):898-905. https://doi.org/10.1007/s11606-018-4335-8.
17. Callister LC. Cultural influences on pain perceptions and behaviors. Home Health Care Manag Pract. 2003;15(3):207-211. https://doi.org/10.1177/1084822302250687.
18. Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines--United States, 2012. J Saf Res. 2014;63(26):563-568. https://doi.org/10.1016/j.jsr.2014.09.001.
19. Callister LC, Khalaf I, Semenic S, Kartchner R, Vehvilainen-Julkunen K. The pain of childbirth: perceptions of culturally diverse women. Pain Manag Nurs. 2003;4(4):145-154. https://doi.org/10.1016/S1524-9042(03)00028-6.
20. Moore R, Brødsgaard I, Mao TK, Miller ML, Dworkin SF. Perceived need for local anesthesia in tooth drilling among Anglo-Americans, Chinese, and Scandinavians. Anesth Prog. 1998;45(1):22-28.
21. Kankkunen PM, Vehviläinen-Julkunen KM, Pietilä AM, et al. A tale of two countries: comparison of the perceptions of analgesics among Finnish and American parents. Pain Manag Nurs. 2008;9(3):113-119. https://doi.org/10.1016/j.pmn.2007.12.003.
22. Hanoch Y, Katsikopoulos KV, Gummerum M, Brass EP. American and German students’ knowledge, perceptions, and behaviors with respect to over-the-counter pain relievers. Health Psychol. 2007;26(6):802-806. https://doi.org/10.1037/0278-6133.26.6.802.
23. Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N. Availability and utilization of opioids for pain management: global issues. Ochsner J. 2014;14(2):208-215.
24. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274(23):1874-1880.
25. McNeill JA, Sherwood GD, Starck PL, Thompson CJ. Assessing clinical outcomes: patient satisfaction with pain management. J Pain Symptom Manag. 1998;16(1):29-40. https://doi.org/10.1016/S0885-3924(98)00034-7.
26. Ferrari R, Novello C, Catania G, Visentin M. Patients’ satisfaction with pain management: the Italian version of the Patient Outcome Questionnaire of the American Pain Society. Recenti Prog Med. 2010;101(7–8):283-288.
27. Malouf J, Andión O, Torrubia R, Cañellas M, Baños JE. A survey of perceptions with pain management in Spanish inpatients. J Pain Symptom Manag. 2006;32(4):361-371. https://doi.org/10.1016/j.jpainsymman.2006.05.006.
28. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. https://doi.org/10.1016/j.jpain.2010.02.012.
29. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-3191. https://doi.org/10.1097/00007632-200012150-00014.
30. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
31. Duman F. After surgery in Germany, I wanted Vicodin, not herbal tea. New York Times. January 27, 2018. https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed November 6, 2018.
32. Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109-118. https://doi.org/10.5055/jom.2016.0322. 33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
34. Friedman J, Kim D, Schneberk T, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2018.6721.
35. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43(2):476-493. https://doi.org/10.1093/ije/dyu038.
36. Simon GE, Goldberg DP, Von Korff M, Ustün TB. Understanding cross-national differences in depression prevalence. Psychol Med. 2002;32(4):585-594. https://doi.org/10.1017/S0033291702005457.
© 2019 Society of Hospital Medicine