User login
Opioid Utilization and Perception of Pain Control in Hospitalized Patients: A Cross-Sectional Study of 11 Sites in 8 Countries
Since 2000, the United States has seen a marked increase in opioid prescribing1-3 and opioid-related complications, including overdoses, hospitalizations, and deaths.2,4,5 A study from 2015 showed that more than one-third of the US civilian noninstitutionalized population reported receiving an opioid prescription in the prior year, with 12.5% reporting misuse, and, of those, 16.7% reported a prescription use disorder.6 While there has been a slight decrease in opioid prescriptions in the US since 2012, rates of opioid prescribing in 2015 were three times higher than in 1999 and approximately four times higher than in Europe in 2015.3,7
Pain is commonly reported by hospitalized patients,8,9 and opioids are often a mainstay of treatment;9,10 however, treatment with opioids can have a number of adverse outcomes.2,10,11 Short-term exposure to opioids can lead to long-term use,12-16 and patients on opioids are at an increased risk for subsequent hospitalization and longer inpatient lengths of stay.5
Physician prescribing practices for opioids and patient expectations for pain control vary as a function of geographic region and culture,10,12,17,18 and pain is influenced by the cultural context in which it occurs.17,19-22 Treatment of pain may also be affected by limited access to or restrictions on selected medications, as well as by cultural biases.23 Whether these variations in the treatment of pain are reflected in patients’ satisfaction with pain control is uncertain.
We sought to compare the inpatient analgesic prescribing practices and patients’ perceptions of pain control for medical patients in four teaching hospitals in the US and in seven teaching hospitals in seven other countries.
METHODS
Study Design
We utilized a cross-sectional, observational design. The study was approved by the Institutional Review Boards at all participating sites.
Setting
The study was conducted at 11 academic hospitals in eight countries from October 8, 2013 to August 31, 2015. Sites in the US included Denver Health in Denver, Colorado; the University of Colorado Hospital in Aurora, Colorado; Hennepin Healthcare in Minneapolis, Minnesota; and Legacy Health in Portland, Oregon. Sites outside the US included McMaster University in Hamilton, Ontario, Canada; Hospital de la Santa Creu i Sant Pau, Universitat Autonòma de Barcelona in Barcelona, Spain; the University of Study of Milan and the University Ospedale “Luigi Sacco” in Milan, Italy, the National Taiwan University Hospital, in Taipei, Taiwan, the University of Ulsan College of Medicine, Asan Medical Center, in Seoul, Korea, the Imperial College, Chelsea and Westminster Hospital, in London, United Kingdom and Dunedin Hospital, Dunedin, New Zealand.
Inclusion and Exclusion Criteria
We included patients 18-89 years of age (20-89 in Taiwan because patients under 20 years of age in this country are a restricted group with respect to participating in research), admitted to an internal medicine service from the Emergency Department or Urgent Care clinic with an acute illness for a minimum of 24 hours (with time zero defined as the time care was initiated in the Emergency Department or Urgent Care Clinic), who reported pain at some time during the first 24-36 hours of their hospitalization and who provided informed consent. In the US, “admission” included both observation and inpatient status. We limited the patient population to those admitted via emergency departments and urgent care clinics in order to enroll similar patient populations across sites.
Scheduled admissions, patients transferred from an outside facility, patients admitted directly from a clinic, and those receiving care in intensive care units were excluded. We also excluded patients who were incarcerated, pregnant, those who received major surgery within the previous 14 days, those with a known diagnosis of active cancer, and those who were receiving palliative or hospice care. Patients receiving care from an investigator in the study at the time of enrollment were not eligible due to the potential conflict of interest.
Patient Screening
Primary teams were contacted to determine if any patients on their service might meet the criteria for inclusion in the study on preselected study days chosen on the basis of the research team’s availability. Identified patients were then screened to establish if they met the eligibility criteria. Patients were asked directly if they had experienced pain during their preadmission evaluation or during their hospitalization.
Data Collection
All patients were hospitalized at the time they gave consent and when data were collected. Data were collected via interviews with patients, as well as through chart review. We recorded patients’ age, gender, race, admitting diagnosis(es), length of stay, psychiatric illness, illicit drug use, whether they reported receiving opioid analgesics at the time of hospitalization, whether they were prescribed opioids and/or nonopioid analgesics during their hospitalization, the median and maximum doses of opioids prescribed and dispensed, and whether they were discharged on opioids. The question of illicit drug use was asked of all patients with the exception of those hospitalized in South Korea due to potential legal implications.
Opioid prescribing and receipt of opioids was recorded based upon current provider orders and medication administration records, respectively. Perception of and satisfaction with pain control was assessed with the American Pain Society Patient Outcome Questionnaire–Modified (APS-POQ-Modified).24,25 Versions of this survey have been validated in English as well as in other languages and cultures.26-28 Because hospitalization practices could differ across hospitals and in different countries, we compared patients’ severity of illness by using Charlson comorbidity scores. Consent forms and the APS-POQ were translated into each country’s primary language according to established processes.29 The survey was filled out by having site investigators read questions aloud and by use of a large-font visual analog scale to aid patients’ verbal responses.
Data were collected and managed using a secure, web-based application electronic data capture tool (Research Electronic Data Capture [REDCap], Nashville, Tennessee), hosted at Denver Health.30
Study Size
Preliminary data from the internal medicine units at our institution suggested that 40% of patients without cancer received opioid analgesics during their hospitalization. Assuming 90% power to detect an absolute difference in the proportion of inpatient medical patients who are receiving opioid analgesics during their hospital stay of 17%, a two-sided type 1 error rate of 0.05, six hospitals in the US, and nine hospitals from all other countries, we calculated an initial sample size of 150 patients per site. This sample size was considered feasible for enrollment in a busy inpatient clinical setting. Study end points were to either reach the goal number of patients (150 per site) or the predetermined study end date, whichever came first.
Data Analysis
We generated means with standard deviations (SDs) and medians with interquartile ranges (IQRs) for normally and nonnormally distributed continuous variables, respectively, and frequencies for categorical variables. We used linear mixed modeling for the analysis of continuous variables. For binary outcomes, our data were fitted to a generalized linear mixed model with logit as the link function and a binary distribution. For ordinal variables, specifically patient-reported satisfaction with pain control and the opinion statements, the data were fitted to a generalized linear mixed model with a cumulative logit link and a multinomial distribution. Hospital was included as a random effect in all models to account for patients cared for in the same hospital.
Country of origin, dichotomized as US or non-US, was the independent variable of interest for all models. An interaction term for exposure to opioids prior to admission and country was entered into all models to explore whether differences in the effect of country existed for patients who reported taking opioids prior to admission and those who did not.
The models for the frequency with which analgesics were given, doses of opioids given during hospitalization and at discharge, patient-reported pain score, and patient-reported satisfaction with pain control were adjusted for (1) age, (2) gender, (3) Charlson Comorbidity Index, (4) length of stay, (5) history of illicit drug use, (6) history of psychiatric illness, (7) daily dose in morphine milligram equivalents (MME) for opioids prior to admission, (8) average pain score, and (9) hospital. The patient-reported satisfaction with pain control model was also adjusted for whether or not opioids were given to the patient during their hospitalization. P < .05 was considered to indicate significance. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, North Carolina). We reported data on medications that were prescribed and dispensed (as opposed to just prescribed and not necessarily given). Opioids prescribed at discharge represented the total possible opioids that could be given based upon the order/prescription (eg, oxycodone 5 mg every 6 hours as needed for pain would be counted as 20 mg/24 hours maximum possible dose followed by conversion to MME).
Missing Data
When there were missing data, a query was sent to sites to verify if the data were retrievable. If retrievable, the data were then entered. Data were missing in 5% and 2% of patients who did or did not report taking an opioid prior to admission, respectively. If a variable was included in a specific statistical test, then subjects with missing data were excluded from that analysis (ie, complete case analysis).
RESULTS
We approached 1,309 eligible patients, of which 981 provided informed consent, for a response rate of 75%; 503 from the US and 478 patients from other countries (Figure). In unadjusted analyses, we found no significant differences between US and non-US patients in age (mean age 51, SD 15 vs 59, SD 19; P = .30), race, ethnicity, or Charlson comorbidity index scores (median 2, IQR 1-3 vs 3, IQR 1-4; P = .45). US patients had shorter lengths of stay (median 3 days, IQR 2-4 vs 6 days, IQR 3-11; P = .04), a more frequent history of illicit drug use (33% vs 6%; P = .003), a higher frequency of psychiatric illness (27% vs 8%; P < .0001), and more were receiving opioid analgesics prior to admission (38% vs 17%; P = .007) than those hospitalized in other countries (Table 1, Appendix 1). The primary admitting diagnoses for all patients in the study are listed in Appendix 2. Opioid prescribing practices across the individual sites are shown in Appendix 3.
Patients Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found that more patients in the US were given opioids during their hospitalization and in higher doses than patients from other countries and more were prescribed opioids at discharge. Fewer patients in the US were dispensed nonopioid analgesics during their hospitalization than patients from other countries, but this difference was not significant (Table 2). Appendix 4 shows the types of nonopioid pain medications prescribed in the US and other countries.
After adjustment for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys. We found no significant difference in satisfaction with pain control between patients from the US and other countries in the models, regardless of whether we included average pain score or opioid receipt during hospitalization in the model (Table 3).
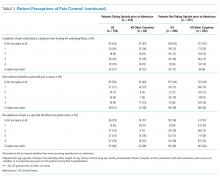
In unadjusted analyses, compared with patients hospitalized in other countries, more patients in the US stated that they would like a stronger dose of analgesic if they were still in pain, though the difference was nonsignificant, and US patients were more likely to agree with the statement that people become addicted to pain medication easily and less likely to agree with the statement that it is easier to endure pain than deal with the side effects of pain medications (Table 3).
Patients Not Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found no significant difference in the proportion of US patients provided with nonopioid pain medications during their hospitalization compared with patients in other countries, but a greater percentage of US patients were given opioids during their hospitalization and at discharge and in higher doses (Table 2).
After adjusting for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys and greater pain severity in the 24-36 hours prior to completing the survey than patients from other countries, but we found no difference in patient satisfaction with pain control (Table 3). After we included the average pain score and whether or not opioids were given to the patient during their hospitalization in this model, patients in the US were more likely to report a higher level of satisfaction with pain control than patients in all other countries (P = .001).
In unadjusted analyses, compared with patients hospitalized in other countries, those in the US were less likely to agree with the statement that good patients avoid talking about pain (Table 3).
Patient Satisfaction and Opioid Receipt
Among patients cared for in the US, after controlling for the average pain score, we did not find a significant association between receiving opioids while in the hospital and satisfaction with pain control for patients who either did or did not endorse taking opioids prior to admission (P = .38 and P = .24, respectively). Among patients cared for in all other countries, after controlling for the average pain score, we found a significant association between receiving opioids while in the hospital and a lower level of satisfaction with pain control for patients who reported taking opioids prior to admission (P = .02) but not for patients who did not report taking opioids prior to admission (P = .08).
DISCUSSION
Compared with patients hospitalized in other countries, a greater percentage of those hospitalized in the US were prescribed opioid analgesics both during hospitalization and at the time of discharge, even after adjustment for pain severity. In addition, patients hospitalized in the US reported greater pain severity at the time they completed their pain surveys and in the 24 to 36 hours prior to completing the survey than patients from other countries. In this sample, satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Our study also suggests that opioid receipt did not lead to improved patient satisfaction with pain control.
The frequency with which we observed opioid analgesics being prescribed during hospitalization in US hospitals (79%) was higher than the 51% of patients who received opioids reported by Herzig and colleagues.10 Patients in our study had a higher prevalence of illicit drug abuse and psychiatric illness, and our study only included patients who reported pain at some point during their hospitalization. We also studied prescribing practices through analysis of provider orders and medication administration records at the time the patient was hospitalized.
While we observed that physicians in the US more frequently prescribed opioid analgesics during hospitalizations than physicians working in other countries, we also observed that patients in the US reported higher levels of pain during their hospitalization. After adjusting for a number of variables, including pain severity, however, we still found that opioids were more commonly prescribed during hospitalizations by physicians working in the US sites studied than by physicians in the non-US sites.
Opioid prescribing practices varied across the sites sampled in our study. While the US sites, Taiwan, and Korea tended to be heavier utilizers of opioids during hospitalization, there were notable differences in discharge prescribing of opioids, with the US sites more commonly prescribing opioids and higher MME for patients who did not report taking opioids prior to their hospitalization (Appendix 3). A sensitivity analysis was conducted excluding South Korea from modeling, given that patients there were not asked about illicit opioid use. There were no important changes in the magnitude or direction of the results.
Our study supports previous studies indicating that there are cultural and societal differences when it comes to the experience of pain and the expectations around pain control.17,20-22,31 Much of the focus on reducing opioid utilization has been on provider practices32 and on prescription drug monitoring programs.33 Our findings suggest that another area of focus that may be important in mitigating the opioid epidemic is patient expectations of pain control.
Our study has a number of strengths. First, we included 11 hospitals from eight different countries. Second, we believe this is the first study to assess opioid prescribing and dispensing practices during hospitalization as well as at the time of discharge. Third, patient perceptions of pain control were assessed in conjunction with analgesic prescribing and were assessed during hospitalization. Fourth, we had high response rates for patient participation in our study. Fifth, we found much larger differences in opioid prescribing than anticipated, and thus, while we did not achieve the sample size originally planned for either the number of hospitals or patients enrolled per hospital, we were sufficiently powered. This is likely secondary to the fact that the population we studied was one that specifically reported pain, resulting in the larger differences seen.
Our study also had a number of limitations. First, the prescribing practices in countries other than the US are represented by only one hospital per country and, in some countries, by limited numbers of patients. While we studied four sites in the US, we did not have a site in the Northeast, a region previously shown to have lower prescribing rates.10 Additionally, patient samples for the US sites compared with the sites in other countries varied considerably with respect to ethnicity. While some studies in US patients have shown that opioid prescribing may vary based on race/ethnicity,34 we are uncertain as to how this might impact a study that crosses multiple countries. We also had a low number of patients receiving opioids prior to hospitalization for several of the non-US countries, which reduced the power to detect differences in this subgroup. Previous research has shown that there are wide variations in prescribing practices even within countries;10,12,18 therefore, caution should be taken when generalizing our findings. Second, we assessed analgesic prescribing patterns and pain control during the first 24 to 36 hours of hospitalization and did not consider hospital days beyond this timeframe with the exception of noting what medications were prescribed at discharge. We chose this methodology in an attempt to eliminate as many differences that might exist in the duration of hospitalization across many countries. Third, investigators in the study administered the survey, and respondents may have been affected by social desirability bias in how the survey questions were answered. Because investigators were not a part of the care team of any study patients, we believe this to be unlikely. Fourth, our study was conducted from October 8, 2013 to August 31, 2015 and the opioid epidemic is dynamic. Accordingly, our data may not reflect current opioid prescribing practices or patients’ current beliefs regarding pain control. Fifth, we did not collect demographic data on the patients who did not participate and could not look for systematic differences between participants and nonparticipants. Sixth, we relied on patients to self-report whether they were taking opioids prior to hospitalization or using illicit drugs. Seventh, we found comorbid mental health conditions to be more frequent in the US population studied. Previous work has shown regional variation in mental health conditions,35,36 which could have affected our findings. To account for this, our models included psychiatric illness.
CONCLUSIONS
Our data suggest that physicians in the US may prescribe opioids more frequently during patients’ hospitalizations and at discharge than their colleagues in other countries. We also found that patient satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Although the small number of hospitals included in our sample coupled with the small sample size in some of the non-US countries limits the generalizability of our findings, the data suggest that reducing the opioid epidemic in the US may require addressing patients’ expectations regarding pain control in addition to providers’ inpatient analgesic prescribing patterns.
Disclosures
The authors report no conflicts of interest.
Funding
The authors report no funding source for this work.
1. Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70-78. https://doi.org/10.1001/jama.2007.64.
2. Herzig SJ. Growing concerns regarding long-term opioid use: the hospitalization hazard. J Hosp Med. 2015;10(7):469-470. https://doi.org/10.1002/jhm.2369.
3. Guy GP Jr, Zhang K, Bohm MK, et al. Vital Signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. https://doi.org/10.15585/mmwr.mm6626a4.
4. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
5. Liang Y, Turner BJ. National cohort study of opioid analgesic dose and risk of future hospitalization. J Hosp Med. 2015;10(7):425-431. https://doi.org/10.1002/jhm.2350.
6. Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. https://doi.org/10.7326/M17-0865.
7. Schuchat A, Houry D, Guy GP, Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426. https://doi.org/10.1001/jama.2017.8913.
8. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. https://doi.org/10.1016/j.pmn.2008.02.001.
9. Gupta A, Daigle S, Mojica J, Hurley RW. Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157-164. https://doi.org/10.2147/JPR.S7903.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
11. Kanjanarat P, Winterstein AG, Johns TE, et al. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750-1759. https://doi.org/10.1093/ajhp/60.17.1750.
12. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. https://doi.org/10.1001/jamainternmed.2016.2737.
13. Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and risk factors for progression From short-term to episodic or long-term opioid prescribing: A population-based study. Mayo Clin Proc. 2015;90(7):850-856. https://doi.org/10.1016/j.mayocp.2015.04.012.
14. Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. https://doi.org/10.1001/archinternmed.2011.1827.
15. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. https://doi.org/10.1056/NEJMsa1610524.
16. Calcaterra SL, Scarbro S, Hull ML, et al. Prediction of future chronic opioid use Among hospitalized patients. J Gen Intern Med. 2018;33(6):898-905. https://doi.org/10.1007/s11606-018-4335-8.
17. Callister LC. Cultural influences on pain perceptions and behaviors. Home Health Care Manag Pract. 2003;15(3):207-211. https://doi.org/10.1177/1084822302250687.
18. Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines--United States, 2012. J Saf Res. 2014;63(26):563-568. https://doi.org/10.1016/j.jsr.2014.09.001.
19. Callister LC, Khalaf I, Semenic S, Kartchner R, Vehvilainen-Julkunen K. The pain of childbirth: perceptions of culturally diverse women. Pain Manag Nurs. 2003;4(4):145-154. https://doi.org/10.1016/S1524-9042(03)00028-6.
20. Moore R, Brødsgaard I, Mao TK, Miller ML, Dworkin SF. Perceived need for local anesthesia in tooth drilling among Anglo-Americans, Chinese, and Scandinavians. Anesth Prog. 1998;45(1):22-28.
21. Kankkunen PM, Vehviläinen-Julkunen KM, Pietilä AM, et al. A tale of two countries: comparison of the perceptions of analgesics among Finnish and American parents. Pain Manag Nurs. 2008;9(3):113-119. https://doi.org/10.1016/j.pmn.2007.12.003.
22. Hanoch Y, Katsikopoulos KV, Gummerum M, Brass EP. American and German students’ knowledge, perceptions, and behaviors with respect to over-the-counter pain relievers. Health Psychol. 2007;26(6):802-806. https://doi.org/10.1037/0278-6133.26.6.802.
23. Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N. Availability and utilization of opioids for pain management: global issues. Ochsner J. 2014;14(2):208-215.
24. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274(23):1874-1880.
25. McNeill JA, Sherwood GD, Starck PL, Thompson CJ. Assessing clinical outcomes: patient satisfaction with pain management. J Pain Symptom Manag. 1998;16(1):29-40. https://doi.org/10.1016/S0885-3924(98)00034-7.
26. Ferrari R, Novello C, Catania G, Visentin M. Patients’ satisfaction with pain management: the Italian version of the Patient Outcome Questionnaire of the American Pain Society. Recenti Prog Med. 2010;101(7–8):283-288.
27. Malouf J, Andión O, Torrubia R, Cañellas M, Baños JE. A survey of perceptions with pain management in Spanish inpatients. J Pain Symptom Manag. 2006;32(4):361-371. https://doi.org/10.1016/j.jpainsymman.2006.05.006.
28. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. https://doi.org/10.1016/j.jpain.2010.02.012.
29. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-3191. https://doi.org/10.1097/00007632-200012150-00014.
30. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
31. Duman F. After surgery in Germany, I wanted Vicodin, not herbal tea. New York Times. January 27, 2018. https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed November 6, 2018.
32. Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109-118. https://doi.org/10.5055/jom.2016.0322. 33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
34. Friedman J, Kim D, Schneberk T, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2018.6721.
35. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43(2):476-493. https://doi.org/10.1093/ije/dyu038.
36. Simon GE, Goldberg DP, Von Korff M, Ustün TB. Understanding cross-national differences in depression prevalence. Psychol Med. 2002;32(4):585-594. https://doi.org/10.1017/S0033291702005457.
Since 2000, the United States has seen a marked increase in opioid prescribing1-3 and opioid-related complications, including overdoses, hospitalizations, and deaths.2,4,5 A study from 2015 showed that more than one-third of the US civilian noninstitutionalized population reported receiving an opioid prescription in the prior year, with 12.5% reporting misuse, and, of those, 16.7% reported a prescription use disorder.6 While there has been a slight decrease in opioid prescriptions in the US since 2012, rates of opioid prescribing in 2015 were three times higher than in 1999 and approximately four times higher than in Europe in 2015.3,7
Pain is commonly reported by hospitalized patients,8,9 and opioids are often a mainstay of treatment;9,10 however, treatment with opioids can have a number of adverse outcomes.2,10,11 Short-term exposure to opioids can lead to long-term use,12-16 and patients on opioids are at an increased risk for subsequent hospitalization and longer inpatient lengths of stay.5
Physician prescribing practices for opioids and patient expectations for pain control vary as a function of geographic region and culture,10,12,17,18 and pain is influenced by the cultural context in which it occurs.17,19-22 Treatment of pain may also be affected by limited access to or restrictions on selected medications, as well as by cultural biases.23 Whether these variations in the treatment of pain are reflected in patients’ satisfaction with pain control is uncertain.
We sought to compare the inpatient analgesic prescribing practices and patients’ perceptions of pain control for medical patients in four teaching hospitals in the US and in seven teaching hospitals in seven other countries.
METHODS
Study Design
We utilized a cross-sectional, observational design. The study was approved by the Institutional Review Boards at all participating sites.
Setting
The study was conducted at 11 academic hospitals in eight countries from October 8, 2013 to August 31, 2015. Sites in the US included Denver Health in Denver, Colorado; the University of Colorado Hospital in Aurora, Colorado; Hennepin Healthcare in Minneapolis, Minnesota; and Legacy Health in Portland, Oregon. Sites outside the US included McMaster University in Hamilton, Ontario, Canada; Hospital de la Santa Creu i Sant Pau, Universitat Autonòma de Barcelona in Barcelona, Spain; the University of Study of Milan and the University Ospedale “Luigi Sacco” in Milan, Italy, the National Taiwan University Hospital, in Taipei, Taiwan, the University of Ulsan College of Medicine, Asan Medical Center, in Seoul, Korea, the Imperial College, Chelsea and Westminster Hospital, in London, United Kingdom and Dunedin Hospital, Dunedin, New Zealand.
Inclusion and Exclusion Criteria
We included patients 18-89 years of age (20-89 in Taiwan because patients under 20 years of age in this country are a restricted group with respect to participating in research), admitted to an internal medicine service from the Emergency Department or Urgent Care clinic with an acute illness for a minimum of 24 hours (with time zero defined as the time care was initiated in the Emergency Department or Urgent Care Clinic), who reported pain at some time during the first 24-36 hours of their hospitalization and who provided informed consent. In the US, “admission” included both observation and inpatient status. We limited the patient population to those admitted via emergency departments and urgent care clinics in order to enroll similar patient populations across sites.
Scheduled admissions, patients transferred from an outside facility, patients admitted directly from a clinic, and those receiving care in intensive care units were excluded. We also excluded patients who were incarcerated, pregnant, those who received major surgery within the previous 14 days, those with a known diagnosis of active cancer, and those who were receiving palliative or hospice care. Patients receiving care from an investigator in the study at the time of enrollment were not eligible due to the potential conflict of interest.
Patient Screening
Primary teams were contacted to determine if any patients on their service might meet the criteria for inclusion in the study on preselected study days chosen on the basis of the research team’s availability. Identified patients were then screened to establish if they met the eligibility criteria. Patients were asked directly if they had experienced pain during their preadmission evaluation or during their hospitalization.
Data Collection
All patients were hospitalized at the time they gave consent and when data were collected. Data were collected via interviews with patients, as well as through chart review. We recorded patients’ age, gender, race, admitting diagnosis(es), length of stay, psychiatric illness, illicit drug use, whether they reported receiving opioid analgesics at the time of hospitalization, whether they were prescribed opioids and/or nonopioid analgesics during their hospitalization, the median and maximum doses of opioids prescribed and dispensed, and whether they were discharged on opioids. The question of illicit drug use was asked of all patients with the exception of those hospitalized in South Korea due to potential legal implications.
Opioid prescribing and receipt of opioids was recorded based upon current provider orders and medication administration records, respectively. Perception of and satisfaction with pain control was assessed with the American Pain Society Patient Outcome Questionnaire–Modified (APS-POQ-Modified).24,25 Versions of this survey have been validated in English as well as in other languages and cultures.26-28 Because hospitalization practices could differ across hospitals and in different countries, we compared patients’ severity of illness by using Charlson comorbidity scores. Consent forms and the APS-POQ were translated into each country’s primary language according to established processes.29 The survey was filled out by having site investigators read questions aloud and by use of a large-font visual analog scale to aid patients’ verbal responses.
Data were collected and managed using a secure, web-based application electronic data capture tool (Research Electronic Data Capture [REDCap], Nashville, Tennessee), hosted at Denver Health.30
Study Size
Preliminary data from the internal medicine units at our institution suggested that 40% of patients without cancer received opioid analgesics during their hospitalization. Assuming 90% power to detect an absolute difference in the proportion of inpatient medical patients who are receiving opioid analgesics during their hospital stay of 17%, a two-sided type 1 error rate of 0.05, six hospitals in the US, and nine hospitals from all other countries, we calculated an initial sample size of 150 patients per site. This sample size was considered feasible for enrollment in a busy inpatient clinical setting. Study end points were to either reach the goal number of patients (150 per site) or the predetermined study end date, whichever came first.
Data Analysis
We generated means with standard deviations (SDs) and medians with interquartile ranges (IQRs) for normally and nonnormally distributed continuous variables, respectively, and frequencies for categorical variables. We used linear mixed modeling for the analysis of continuous variables. For binary outcomes, our data were fitted to a generalized linear mixed model with logit as the link function and a binary distribution. For ordinal variables, specifically patient-reported satisfaction with pain control and the opinion statements, the data were fitted to a generalized linear mixed model with a cumulative logit link and a multinomial distribution. Hospital was included as a random effect in all models to account for patients cared for in the same hospital.
Country of origin, dichotomized as US or non-US, was the independent variable of interest for all models. An interaction term for exposure to opioids prior to admission and country was entered into all models to explore whether differences in the effect of country existed for patients who reported taking opioids prior to admission and those who did not.
The models for the frequency with which analgesics were given, doses of opioids given during hospitalization and at discharge, patient-reported pain score, and patient-reported satisfaction with pain control were adjusted for (1) age, (2) gender, (3) Charlson Comorbidity Index, (4) length of stay, (5) history of illicit drug use, (6) history of psychiatric illness, (7) daily dose in morphine milligram equivalents (MME) for opioids prior to admission, (8) average pain score, and (9) hospital. The patient-reported satisfaction with pain control model was also adjusted for whether or not opioids were given to the patient during their hospitalization. P < .05 was considered to indicate significance. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, North Carolina). We reported data on medications that were prescribed and dispensed (as opposed to just prescribed and not necessarily given). Opioids prescribed at discharge represented the total possible opioids that could be given based upon the order/prescription (eg, oxycodone 5 mg every 6 hours as needed for pain would be counted as 20 mg/24 hours maximum possible dose followed by conversion to MME).
Missing Data
When there were missing data, a query was sent to sites to verify if the data were retrievable. If retrievable, the data were then entered. Data were missing in 5% and 2% of patients who did or did not report taking an opioid prior to admission, respectively. If a variable was included in a specific statistical test, then subjects with missing data were excluded from that analysis (ie, complete case analysis).
RESULTS
We approached 1,309 eligible patients, of which 981 provided informed consent, for a response rate of 75%; 503 from the US and 478 patients from other countries (Figure). In unadjusted analyses, we found no significant differences between US and non-US patients in age (mean age 51, SD 15 vs 59, SD 19; P = .30), race, ethnicity, or Charlson comorbidity index scores (median 2, IQR 1-3 vs 3, IQR 1-4; P = .45). US patients had shorter lengths of stay (median 3 days, IQR 2-4 vs 6 days, IQR 3-11; P = .04), a more frequent history of illicit drug use (33% vs 6%; P = .003), a higher frequency of psychiatric illness (27% vs 8%; P < .0001), and more were receiving opioid analgesics prior to admission (38% vs 17%; P = .007) than those hospitalized in other countries (Table 1, Appendix 1). The primary admitting diagnoses for all patients in the study are listed in Appendix 2. Opioid prescribing practices across the individual sites are shown in Appendix 3.
Patients Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found that more patients in the US were given opioids during their hospitalization and in higher doses than patients from other countries and more were prescribed opioids at discharge. Fewer patients in the US were dispensed nonopioid analgesics during their hospitalization than patients from other countries, but this difference was not significant (Table 2). Appendix 4 shows the types of nonopioid pain medications prescribed in the US and other countries.
After adjustment for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys. We found no significant difference in satisfaction with pain control between patients from the US and other countries in the models, regardless of whether we included average pain score or opioid receipt during hospitalization in the model (Table 3).
In unadjusted analyses, compared with patients hospitalized in other countries, more patients in the US stated that they would like a stronger dose of analgesic if they were still in pain, though the difference was nonsignificant, and US patients were more likely to agree with the statement that people become addicted to pain medication easily and less likely to agree with the statement that it is easier to endure pain than deal with the side effects of pain medications (Table 3).
Patients Not Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found no significant difference in the proportion of US patients provided with nonopioid pain medications during their hospitalization compared with patients in other countries, but a greater percentage of US patients were given opioids during their hospitalization and at discharge and in higher doses (Table 2).
After adjusting for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys and greater pain severity in the 24-36 hours prior to completing the survey than patients from other countries, but we found no difference in patient satisfaction with pain control (Table 3). After we included the average pain score and whether or not opioids were given to the patient during their hospitalization in this model, patients in the US were more likely to report a higher level of satisfaction with pain control than patients in all other countries (P = .001).
In unadjusted analyses, compared with patients hospitalized in other countries, those in the US were less likely to agree with the statement that good patients avoid talking about pain (Table 3).
Patient Satisfaction and Opioid Receipt
Among patients cared for in the US, after controlling for the average pain score, we did not find a significant association between receiving opioids while in the hospital and satisfaction with pain control for patients who either did or did not endorse taking opioids prior to admission (P = .38 and P = .24, respectively). Among patients cared for in all other countries, after controlling for the average pain score, we found a significant association between receiving opioids while in the hospital and a lower level of satisfaction with pain control for patients who reported taking opioids prior to admission (P = .02) but not for patients who did not report taking opioids prior to admission (P = .08).
DISCUSSION
Compared with patients hospitalized in other countries, a greater percentage of those hospitalized in the US were prescribed opioid analgesics both during hospitalization and at the time of discharge, even after adjustment for pain severity. In addition, patients hospitalized in the US reported greater pain severity at the time they completed their pain surveys and in the 24 to 36 hours prior to completing the survey than patients from other countries. In this sample, satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Our study also suggests that opioid receipt did not lead to improved patient satisfaction with pain control.
The frequency with which we observed opioid analgesics being prescribed during hospitalization in US hospitals (79%) was higher than the 51% of patients who received opioids reported by Herzig and colleagues.10 Patients in our study had a higher prevalence of illicit drug abuse and psychiatric illness, and our study only included patients who reported pain at some point during their hospitalization. We also studied prescribing practices through analysis of provider orders and medication administration records at the time the patient was hospitalized.
While we observed that physicians in the US more frequently prescribed opioid analgesics during hospitalizations than physicians working in other countries, we also observed that patients in the US reported higher levels of pain during their hospitalization. After adjusting for a number of variables, including pain severity, however, we still found that opioids were more commonly prescribed during hospitalizations by physicians working in the US sites studied than by physicians in the non-US sites.
Opioid prescribing practices varied across the sites sampled in our study. While the US sites, Taiwan, and Korea tended to be heavier utilizers of opioids during hospitalization, there were notable differences in discharge prescribing of opioids, with the US sites more commonly prescribing opioids and higher MME for patients who did not report taking opioids prior to their hospitalization (Appendix 3). A sensitivity analysis was conducted excluding South Korea from modeling, given that patients there were not asked about illicit opioid use. There were no important changes in the magnitude or direction of the results.
Our study supports previous studies indicating that there are cultural and societal differences when it comes to the experience of pain and the expectations around pain control.17,20-22,31 Much of the focus on reducing opioid utilization has been on provider practices32 and on prescription drug monitoring programs.33 Our findings suggest that another area of focus that may be important in mitigating the opioid epidemic is patient expectations of pain control.
Our study has a number of strengths. First, we included 11 hospitals from eight different countries. Second, we believe this is the first study to assess opioid prescribing and dispensing practices during hospitalization as well as at the time of discharge. Third, patient perceptions of pain control were assessed in conjunction with analgesic prescribing and were assessed during hospitalization. Fourth, we had high response rates for patient participation in our study. Fifth, we found much larger differences in opioid prescribing than anticipated, and thus, while we did not achieve the sample size originally planned for either the number of hospitals or patients enrolled per hospital, we were sufficiently powered. This is likely secondary to the fact that the population we studied was one that specifically reported pain, resulting in the larger differences seen.
Our study also had a number of limitations. First, the prescribing practices in countries other than the US are represented by only one hospital per country and, in some countries, by limited numbers of patients. While we studied four sites in the US, we did not have a site in the Northeast, a region previously shown to have lower prescribing rates.10 Additionally, patient samples for the US sites compared with the sites in other countries varied considerably with respect to ethnicity. While some studies in US patients have shown that opioid prescribing may vary based on race/ethnicity,34 we are uncertain as to how this might impact a study that crosses multiple countries. We also had a low number of patients receiving opioids prior to hospitalization for several of the non-US countries, which reduced the power to detect differences in this subgroup. Previous research has shown that there are wide variations in prescribing practices even within countries;10,12,18 therefore, caution should be taken when generalizing our findings. Second, we assessed analgesic prescribing patterns and pain control during the first 24 to 36 hours of hospitalization and did not consider hospital days beyond this timeframe with the exception of noting what medications were prescribed at discharge. We chose this methodology in an attempt to eliminate as many differences that might exist in the duration of hospitalization across many countries. Third, investigators in the study administered the survey, and respondents may have been affected by social desirability bias in how the survey questions were answered. Because investigators were not a part of the care team of any study patients, we believe this to be unlikely. Fourth, our study was conducted from October 8, 2013 to August 31, 2015 and the opioid epidemic is dynamic. Accordingly, our data may not reflect current opioid prescribing practices or patients’ current beliefs regarding pain control. Fifth, we did not collect demographic data on the patients who did not participate and could not look for systematic differences between participants and nonparticipants. Sixth, we relied on patients to self-report whether they were taking opioids prior to hospitalization or using illicit drugs. Seventh, we found comorbid mental health conditions to be more frequent in the US population studied. Previous work has shown regional variation in mental health conditions,35,36 which could have affected our findings. To account for this, our models included psychiatric illness.
CONCLUSIONS
Our data suggest that physicians in the US may prescribe opioids more frequently during patients’ hospitalizations and at discharge than their colleagues in other countries. We also found that patient satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Although the small number of hospitals included in our sample coupled with the small sample size in some of the non-US countries limits the generalizability of our findings, the data suggest that reducing the opioid epidemic in the US may require addressing patients’ expectations regarding pain control in addition to providers’ inpatient analgesic prescribing patterns.
Disclosures
The authors report no conflicts of interest.
Funding
The authors report no funding source for this work.
Since 2000, the United States has seen a marked increase in opioid prescribing1-3 and opioid-related complications, including overdoses, hospitalizations, and deaths.2,4,5 A study from 2015 showed that more than one-third of the US civilian noninstitutionalized population reported receiving an opioid prescription in the prior year, with 12.5% reporting misuse, and, of those, 16.7% reported a prescription use disorder.6 While there has been a slight decrease in opioid prescriptions in the US since 2012, rates of opioid prescribing in 2015 were three times higher than in 1999 and approximately four times higher than in Europe in 2015.3,7
Pain is commonly reported by hospitalized patients,8,9 and opioids are often a mainstay of treatment;9,10 however, treatment with opioids can have a number of adverse outcomes.2,10,11 Short-term exposure to opioids can lead to long-term use,12-16 and patients on opioids are at an increased risk for subsequent hospitalization and longer inpatient lengths of stay.5
Physician prescribing practices for opioids and patient expectations for pain control vary as a function of geographic region and culture,10,12,17,18 and pain is influenced by the cultural context in which it occurs.17,19-22 Treatment of pain may also be affected by limited access to or restrictions on selected medications, as well as by cultural biases.23 Whether these variations in the treatment of pain are reflected in patients’ satisfaction with pain control is uncertain.
We sought to compare the inpatient analgesic prescribing practices and patients’ perceptions of pain control for medical patients in four teaching hospitals in the US and in seven teaching hospitals in seven other countries.
METHODS
Study Design
We utilized a cross-sectional, observational design. The study was approved by the Institutional Review Boards at all participating sites.
Setting
The study was conducted at 11 academic hospitals in eight countries from October 8, 2013 to August 31, 2015. Sites in the US included Denver Health in Denver, Colorado; the University of Colorado Hospital in Aurora, Colorado; Hennepin Healthcare in Minneapolis, Minnesota; and Legacy Health in Portland, Oregon. Sites outside the US included McMaster University in Hamilton, Ontario, Canada; Hospital de la Santa Creu i Sant Pau, Universitat Autonòma de Barcelona in Barcelona, Spain; the University of Study of Milan and the University Ospedale “Luigi Sacco” in Milan, Italy, the National Taiwan University Hospital, in Taipei, Taiwan, the University of Ulsan College of Medicine, Asan Medical Center, in Seoul, Korea, the Imperial College, Chelsea and Westminster Hospital, in London, United Kingdom and Dunedin Hospital, Dunedin, New Zealand.
Inclusion and Exclusion Criteria
We included patients 18-89 years of age (20-89 in Taiwan because patients under 20 years of age in this country are a restricted group with respect to participating in research), admitted to an internal medicine service from the Emergency Department or Urgent Care clinic with an acute illness for a minimum of 24 hours (with time zero defined as the time care was initiated in the Emergency Department or Urgent Care Clinic), who reported pain at some time during the first 24-36 hours of their hospitalization and who provided informed consent. In the US, “admission” included both observation and inpatient status. We limited the patient population to those admitted via emergency departments and urgent care clinics in order to enroll similar patient populations across sites.
Scheduled admissions, patients transferred from an outside facility, patients admitted directly from a clinic, and those receiving care in intensive care units were excluded. We also excluded patients who were incarcerated, pregnant, those who received major surgery within the previous 14 days, those with a known diagnosis of active cancer, and those who were receiving palliative or hospice care. Patients receiving care from an investigator in the study at the time of enrollment were not eligible due to the potential conflict of interest.
Patient Screening
Primary teams were contacted to determine if any patients on their service might meet the criteria for inclusion in the study on preselected study days chosen on the basis of the research team’s availability. Identified patients were then screened to establish if they met the eligibility criteria. Patients were asked directly if they had experienced pain during their preadmission evaluation or during their hospitalization.
Data Collection
All patients were hospitalized at the time they gave consent and when data were collected. Data were collected via interviews with patients, as well as through chart review. We recorded patients’ age, gender, race, admitting diagnosis(es), length of stay, psychiatric illness, illicit drug use, whether they reported receiving opioid analgesics at the time of hospitalization, whether they were prescribed opioids and/or nonopioid analgesics during their hospitalization, the median and maximum doses of opioids prescribed and dispensed, and whether they were discharged on opioids. The question of illicit drug use was asked of all patients with the exception of those hospitalized in South Korea due to potential legal implications.
Opioid prescribing and receipt of opioids was recorded based upon current provider orders and medication administration records, respectively. Perception of and satisfaction with pain control was assessed with the American Pain Society Patient Outcome Questionnaire–Modified (APS-POQ-Modified).24,25 Versions of this survey have been validated in English as well as in other languages and cultures.26-28 Because hospitalization practices could differ across hospitals and in different countries, we compared patients’ severity of illness by using Charlson comorbidity scores. Consent forms and the APS-POQ were translated into each country’s primary language according to established processes.29 The survey was filled out by having site investigators read questions aloud and by use of a large-font visual analog scale to aid patients’ verbal responses.
Data were collected and managed using a secure, web-based application electronic data capture tool (Research Electronic Data Capture [REDCap], Nashville, Tennessee), hosted at Denver Health.30
Study Size
Preliminary data from the internal medicine units at our institution suggested that 40% of patients without cancer received opioid analgesics during their hospitalization. Assuming 90% power to detect an absolute difference in the proportion of inpatient medical patients who are receiving opioid analgesics during their hospital stay of 17%, a two-sided type 1 error rate of 0.05, six hospitals in the US, and nine hospitals from all other countries, we calculated an initial sample size of 150 patients per site. This sample size was considered feasible for enrollment in a busy inpatient clinical setting. Study end points were to either reach the goal number of patients (150 per site) or the predetermined study end date, whichever came first.
Data Analysis
We generated means with standard deviations (SDs) and medians with interquartile ranges (IQRs) for normally and nonnormally distributed continuous variables, respectively, and frequencies for categorical variables. We used linear mixed modeling for the analysis of continuous variables. For binary outcomes, our data were fitted to a generalized linear mixed model with logit as the link function and a binary distribution. For ordinal variables, specifically patient-reported satisfaction with pain control and the opinion statements, the data were fitted to a generalized linear mixed model with a cumulative logit link and a multinomial distribution. Hospital was included as a random effect in all models to account for patients cared for in the same hospital.
Country of origin, dichotomized as US or non-US, was the independent variable of interest for all models. An interaction term for exposure to opioids prior to admission and country was entered into all models to explore whether differences in the effect of country existed for patients who reported taking opioids prior to admission and those who did not.
The models for the frequency with which analgesics were given, doses of opioids given during hospitalization and at discharge, patient-reported pain score, and patient-reported satisfaction with pain control were adjusted for (1) age, (2) gender, (3) Charlson Comorbidity Index, (4) length of stay, (5) history of illicit drug use, (6) history of psychiatric illness, (7) daily dose in morphine milligram equivalents (MME) for opioids prior to admission, (8) average pain score, and (9) hospital. The patient-reported satisfaction with pain control model was also adjusted for whether or not opioids were given to the patient during their hospitalization. P < .05 was considered to indicate significance. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, North Carolina). We reported data on medications that were prescribed and dispensed (as opposed to just prescribed and not necessarily given). Opioids prescribed at discharge represented the total possible opioids that could be given based upon the order/prescription (eg, oxycodone 5 mg every 6 hours as needed for pain would be counted as 20 mg/24 hours maximum possible dose followed by conversion to MME).
Missing Data
When there were missing data, a query was sent to sites to verify if the data were retrievable. If retrievable, the data were then entered. Data were missing in 5% and 2% of patients who did or did not report taking an opioid prior to admission, respectively. If a variable was included in a specific statistical test, then subjects with missing data were excluded from that analysis (ie, complete case analysis).
RESULTS
We approached 1,309 eligible patients, of which 981 provided informed consent, for a response rate of 75%; 503 from the US and 478 patients from other countries (Figure). In unadjusted analyses, we found no significant differences between US and non-US patients in age (mean age 51, SD 15 vs 59, SD 19; P = .30), race, ethnicity, or Charlson comorbidity index scores (median 2, IQR 1-3 vs 3, IQR 1-4; P = .45). US patients had shorter lengths of stay (median 3 days, IQR 2-4 vs 6 days, IQR 3-11; P = .04), a more frequent history of illicit drug use (33% vs 6%; P = .003), a higher frequency of psychiatric illness (27% vs 8%; P < .0001), and more were receiving opioid analgesics prior to admission (38% vs 17%; P = .007) than those hospitalized in other countries (Table 1, Appendix 1). The primary admitting diagnoses for all patients in the study are listed in Appendix 2. Opioid prescribing practices across the individual sites are shown in Appendix 3.
Patients Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found that more patients in the US were given opioids during their hospitalization and in higher doses than patients from other countries and more were prescribed opioids at discharge. Fewer patients in the US were dispensed nonopioid analgesics during their hospitalization than patients from other countries, but this difference was not significant (Table 2). Appendix 4 shows the types of nonopioid pain medications prescribed in the US and other countries.
After adjustment for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys. We found no significant difference in satisfaction with pain control between patients from the US and other countries in the models, regardless of whether we included average pain score or opioid receipt during hospitalization in the model (Table 3).
In unadjusted analyses, compared with patients hospitalized in other countries, more patients in the US stated that they would like a stronger dose of analgesic if they were still in pain, though the difference was nonsignificant, and US patients were more likely to agree with the statement that people become addicted to pain medication easily and less likely to agree with the statement that it is easier to endure pain than deal with the side effects of pain medications (Table 3).
Patients Not Taking Opioids Prior to Admission
After adjusting for relevant covariates, we found no significant difference in the proportion of US patients provided with nonopioid pain medications during their hospitalization compared with patients in other countries, but a greater percentage of US patients were given opioids during their hospitalization and at discharge and in higher doses (Table 2).
After adjusting for relevant covariates, US patients reported greater pain severity at the time they completed their pain surveys and greater pain severity in the 24-36 hours prior to completing the survey than patients from other countries, but we found no difference in patient satisfaction with pain control (Table 3). After we included the average pain score and whether or not opioids were given to the patient during their hospitalization in this model, patients in the US were more likely to report a higher level of satisfaction with pain control than patients in all other countries (P = .001).
In unadjusted analyses, compared with patients hospitalized in other countries, those in the US were less likely to agree with the statement that good patients avoid talking about pain (Table 3).
Patient Satisfaction and Opioid Receipt
Among patients cared for in the US, after controlling for the average pain score, we did not find a significant association between receiving opioids while in the hospital and satisfaction with pain control for patients who either did or did not endorse taking opioids prior to admission (P = .38 and P = .24, respectively). Among patients cared for in all other countries, after controlling for the average pain score, we found a significant association between receiving opioids while in the hospital and a lower level of satisfaction with pain control for patients who reported taking opioids prior to admission (P = .02) but not for patients who did not report taking opioids prior to admission (P = .08).
DISCUSSION
Compared with patients hospitalized in other countries, a greater percentage of those hospitalized in the US were prescribed opioid analgesics both during hospitalization and at the time of discharge, even after adjustment for pain severity. In addition, patients hospitalized in the US reported greater pain severity at the time they completed their pain surveys and in the 24 to 36 hours prior to completing the survey than patients from other countries. In this sample, satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Our study also suggests that opioid receipt did not lead to improved patient satisfaction with pain control.
The frequency with which we observed opioid analgesics being prescribed during hospitalization in US hospitals (79%) was higher than the 51% of patients who received opioids reported by Herzig and colleagues.10 Patients in our study had a higher prevalence of illicit drug abuse and psychiatric illness, and our study only included patients who reported pain at some point during their hospitalization. We also studied prescribing practices through analysis of provider orders and medication administration records at the time the patient was hospitalized.
While we observed that physicians in the US more frequently prescribed opioid analgesics during hospitalizations than physicians working in other countries, we also observed that patients in the US reported higher levels of pain during their hospitalization. After adjusting for a number of variables, including pain severity, however, we still found that opioids were more commonly prescribed during hospitalizations by physicians working in the US sites studied than by physicians in the non-US sites.
Opioid prescribing practices varied across the sites sampled in our study. While the US sites, Taiwan, and Korea tended to be heavier utilizers of opioids during hospitalization, there were notable differences in discharge prescribing of opioids, with the US sites more commonly prescribing opioids and higher MME for patients who did not report taking opioids prior to their hospitalization (Appendix 3). A sensitivity analysis was conducted excluding South Korea from modeling, given that patients there were not asked about illicit opioid use. There were no important changes in the magnitude or direction of the results.
Our study supports previous studies indicating that there are cultural and societal differences when it comes to the experience of pain and the expectations around pain control.17,20-22,31 Much of the focus on reducing opioid utilization has been on provider practices32 and on prescription drug monitoring programs.33 Our findings suggest that another area of focus that may be important in mitigating the opioid epidemic is patient expectations of pain control.
Our study has a number of strengths. First, we included 11 hospitals from eight different countries. Second, we believe this is the first study to assess opioid prescribing and dispensing practices during hospitalization as well as at the time of discharge. Third, patient perceptions of pain control were assessed in conjunction with analgesic prescribing and were assessed during hospitalization. Fourth, we had high response rates for patient participation in our study. Fifth, we found much larger differences in opioid prescribing than anticipated, and thus, while we did not achieve the sample size originally planned for either the number of hospitals or patients enrolled per hospital, we were sufficiently powered. This is likely secondary to the fact that the population we studied was one that specifically reported pain, resulting in the larger differences seen.
Our study also had a number of limitations. First, the prescribing practices in countries other than the US are represented by only one hospital per country and, in some countries, by limited numbers of patients. While we studied four sites in the US, we did not have a site in the Northeast, a region previously shown to have lower prescribing rates.10 Additionally, patient samples for the US sites compared with the sites in other countries varied considerably with respect to ethnicity. While some studies in US patients have shown that opioid prescribing may vary based on race/ethnicity,34 we are uncertain as to how this might impact a study that crosses multiple countries. We also had a low number of patients receiving opioids prior to hospitalization for several of the non-US countries, which reduced the power to detect differences in this subgroup. Previous research has shown that there are wide variations in prescribing practices even within countries;10,12,18 therefore, caution should be taken when generalizing our findings. Second, we assessed analgesic prescribing patterns and pain control during the first 24 to 36 hours of hospitalization and did not consider hospital days beyond this timeframe with the exception of noting what medications were prescribed at discharge. We chose this methodology in an attempt to eliminate as many differences that might exist in the duration of hospitalization across many countries. Third, investigators in the study administered the survey, and respondents may have been affected by social desirability bias in how the survey questions were answered. Because investigators were not a part of the care team of any study patients, we believe this to be unlikely. Fourth, our study was conducted from October 8, 2013 to August 31, 2015 and the opioid epidemic is dynamic. Accordingly, our data may not reflect current opioid prescribing practices or patients’ current beliefs regarding pain control. Fifth, we did not collect demographic data on the patients who did not participate and could not look for systematic differences between participants and nonparticipants. Sixth, we relied on patients to self-report whether they were taking opioids prior to hospitalization or using illicit drugs. Seventh, we found comorbid mental health conditions to be more frequent in the US population studied. Previous work has shown regional variation in mental health conditions,35,36 which could have affected our findings. To account for this, our models included psychiatric illness.
CONCLUSIONS
Our data suggest that physicians in the US may prescribe opioids more frequently during patients’ hospitalizations and at discharge than their colleagues in other countries. We also found that patient satisfaction, beliefs, and expectations about pain control differed between patients in the US and other sites. Although the small number of hospitals included in our sample coupled with the small sample size in some of the non-US countries limits the generalizability of our findings, the data suggest that reducing the opioid epidemic in the US may require addressing patients’ expectations regarding pain control in addition to providers’ inpatient analgesic prescribing patterns.
Disclosures
The authors report no conflicts of interest.
Funding
The authors report no funding source for this work.
1. Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70-78. https://doi.org/10.1001/jama.2007.64.
2. Herzig SJ. Growing concerns regarding long-term opioid use: the hospitalization hazard. J Hosp Med. 2015;10(7):469-470. https://doi.org/10.1002/jhm.2369.
3. Guy GP Jr, Zhang K, Bohm MK, et al. Vital Signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. https://doi.org/10.15585/mmwr.mm6626a4.
4. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
5. Liang Y, Turner BJ. National cohort study of opioid analgesic dose and risk of future hospitalization. J Hosp Med. 2015;10(7):425-431. https://doi.org/10.1002/jhm.2350.
6. Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. https://doi.org/10.7326/M17-0865.
7. Schuchat A, Houry D, Guy GP, Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426. https://doi.org/10.1001/jama.2017.8913.
8. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. https://doi.org/10.1016/j.pmn.2008.02.001.
9. Gupta A, Daigle S, Mojica J, Hurley RW. Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157-164. https://doi.org/10.2147/JPR.S7903.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
11. Kanjanarat P, Winterstein AG, Johns TE, et al. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750-1759. https://doi.org/10.1093/ajhp/60.17.1750.
12. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. https://doi.org/10.1001/jamainternmed.2016.2737.
13. Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and risk factors for progression From short-term to episodic or long-term opioid prescribing: A population-based study. Mayo Clin Proc. 2015;90(7):850-856. https://doi.org/10.1016/j.mayocp.2015.04.012.
14. Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. https://doi.org/10.1001/archinternmed.2011.1827.
15. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. https://doi.org/10.1056/NEJMsa1610524.
16. Calcaterra SL, Scarbro S, Hull ML, et al. Prediction of future chronic opioid use Among hospitalized patients. J Gen Intern Med. 2018;33(6):898-905. https://doi.org/10.1007/s11606-018-4335-8.
17. Callister LC. Cultural influences on pain perceptions and behaviors. Home Health Care Manag Pract. 2003;15(3):207-211. https://doi.org/10.1177/1084822302250687.
18. Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines--United States, 2012. J Saf Res. 2014;63(26):563-568. https://doi.org/10.1016/j.jsr.2014.09.001.
19. Callister LC, Khalaf I, Semenic S, Kartchner R, Vehvilainen-Julkunen K. The pain of childbirth: perceptions of culturally diverse women. Pain Manag Nurs. 2003;4(4):145-154. https://doi.org/10.1016/S1524-9042(03)00028-6.
20. Moore R, Brødsgaard I, Mao TK, Miller ML, Dworkin SF. Perceived need for local anesthesia in tooth drilling among Anglo-Americans, Chinese, and Scandinavians. Anesth Prog. 1998;45(1):22-28.
21. Kankkunen PM, Vehviläinen-Julkunen KM, Pietilä AM, et al. A tale of two countries: comparison of the perceptions of analgesics among Finnish and American parents. Pain Manag Nurs. 2008;9(3):113-119. https://doi.org/10.1016/j.pmn.2007.12.003.
22. Hanoch Y, Katsikopoulos KV, Gummerum M, Brass EP. American and German students’ knowledge, perceptions, and behaviors with respect to over-the-counter pain relievers. Health Psychol. 2007;26(6):802-806. https://doi.org/10.1037/0278-6133.26.6.802.
23. Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N. Availability and utilization of opioids for pain management: global issues. Ochsner J. 2014;14(2):208-215.
24. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274(23):1874-1880.
25. McNeill JA, Sherwood GD, Starck PL, Thompson CJ. Assessing clinical outcomes: patient satisfaction with pain management. J Pain Symptom Manag. 1998;16(1):29-40. https://doi.org/10.1016/S0885-3924(98)00034-7.
26. Ferrari R, Novello C, Catania G, Visentin M. Patients’ satisfaction with pain management: the Italian version of the Patient Outcome Questionnaire of the American Pain Society. Recenti Prog Med. 2010;101(7–8):283-288.
27. Malouf J, Andión O, Torrubia R, Cañellas M, Baños JE. A survey of perceptions with pain management in Spanish inpatients. J Pain Symptom Manag. 2006;32(4):361-371. https://doi.org/10.1016/j.jpainsymman.2006.05.006.
28. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. https://doi.org/10.1016/j.jpain.2010.02.012.
29. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-3191. https://doi.org/10.1097/00007632-200012150-00014.
30. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
31. Duman F. After surgery in Germany, I wanted Vicodin, not herbal tea. New York Times. January 27, 2018. https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed November 6, 2018.
32. Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109-118. https://doi.org/10.5055/jom.2016.0322. 33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
34. Friedman J, Kim D, Schneberk T, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2018.6721.
35. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43(2):476-493. https://doi.org/10.1093/ije/dyu038.
36. Simon GE, Goldberg DP, Von Korff M, Ustün TB. Understanding cross-national differences in depression prevalence. Psychol Med. 2002;32(4):585-594. https://doi.org/10.1017/S0033291702005457.
1. Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70-78. https://doi.org/10.1001/jama.2007.64.
2. Herzig SJ. Growing concerns regarding long-term opioid use: the hospitalization hazard. J Hosp Med. 2015;10(7):469-470. https://doi.org/10.1002/jhm.2369.
3. Guy GP Jr, Zhang K, Bohm MK, et al. Vital Signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. https://doi.org/10.15585/mmwr.mm6626a4.
4. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
5. Liang Y, Turner BJ. National cohort study of opioid analgesic dose and risk of future hospitalization. J Hosp Med. 2015;10(7):425-431. https://doi.org/10.1002/jhm.2350.
6. Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293-301. https://doi.org/10.7326/M17-0865.
7. Schuchat A, Houry D, Guy GP, Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426. https://doi.org/10.1001/jama.2017.8913.
8. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. https://doi.org/10.1016/j.pmn.2008.02.001.
9. Gupta A, Daigle S, Mojica J, Hurley RW. Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157-164. https://doi.org/10.2147/JPR.S7903.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
11. Kanjanarat P, Winterstein AG, Johns TE, et al. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60(17):1750-1759. https://doi.org/10.1093/ajhp/60.17.1750.
12. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. https://doi.org/10.1001/jamainternmed.2016.2737.
13. Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and risk factors for progression From short-term to episodic or long-term opioid prescribing: A population-based study. Mayo Clin Proc. 2015;90(7):850-856. https://doi.org/10.1016/j.mayocp.2015.04.012.
14. Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. https://doi.org/10.1001/archinternmed.2011.1827.
15. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. https://doi.org/10.1056/NEJMsa1610524.
16. Calcaterra SL, Scarbro S, Hull ML, et al. Prediction of future chronic opioid use Among hospitalized patients. J Gen Intern Med. 2018;33(6):898-905. https://doi.org/10.1007/s11606-018-4335-8.
17. Callister LC. Cultural influences on pain perceptions and behaviors. Home Health Care Manag Pract. 2003;15(3):207-211. https://doi.org/10.1177/1084822302250687.
18. Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines--United States, 2012. J Saf Res. 2014;63(26):563-568. https://doi.org/10.1016/j.jsr.2014.09.001.
19. Callister LC, Khalaf I, Semenic S, Kartchner R, Vehvilainen-Julkunen K. The pain of childbirth: perceptions of culturally diverse women. Pain Manag Nurs. 2003;4(4):145-154. https://doi.org/10.1016/S1524-9042(03)00028-6.
20. Moore R, Brødsgaard I, Mao TK, Miller ML, Dworkin SF. Perceived need for local anesthesia in tooth drilling among Anglo-Americans, Chinese, and Scandinavians. Anesth Prog. 1998;45(1):22-28.
21. Kankkunen PM, Vehviläinen-Julkunen KM, Pietilä AM, et al. A tale of two countries: comparison of the perceptions of analgesics among Finnish and American parents. Pain Manag Nurs. 2008;9(3):113-119. https://doi.org/10.1016/j.pmn.2007.12.003.
22. Hanoch Y, Katsikopoulos KV, Gummerum M, Brass EP. American and German students’ knowledge, perceptions, and behaviors with respect to over-the-counter pain relievers. Health Psychol. 2007;26(6):802-806. https://doi.org/10.1037/0278-6133.26.6.802.
23. Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N. Availability and utilization of opioids for pain management: global issues. Ochsner J. 2014;14(2):208-215.
24. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274(23):1874-1880.
25. McNeill JA, Sherwood GD, Starck PL, Thompson CJ. Assessing clinical outcomes: patient satisfaction with pain management. J Pain Symptom Manag. 1998;16(1):29-40. https://doi.org/10.1016/S0885-3924(98)00034-7.
26. Ferrari R, Novello C, Catania G, Visentin M. Patients’ satisfaction with pain management: the Italian version of the Patient Outcome Questionnaire of the American Pain Society. Recenti Prog Med. 2010;101(7–8):283-288.
27. Malouf J, Andión O, Torrubia R, Cañellas M, Baños JE. A survey of perceptions with pain management in Spanish inpatients. J Pain Symptom Manag. 2006;32(4):361-371. https://doi.org/10.1016/j.jpainsymman.2006.05.006.
28. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. https://doi.org/10.1016/j.jpain.2010.02.012.
29. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-3191. https://doi.org/10.1097/00007632-200012150-00014.
30. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
31. Duman F. After surgery in Germany, I wanted Vicodin, not herbal tea. New York Times. January 27, 2018. https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed November 6, 2018.
32. Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag. 2016;12(2):109-118. https://doi.org/10.5055/jom.2016.0322. 33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
33. Bao Y, Wen K, Johnson P, et al. Assessing the impact of state policies for prescription drug monitoring programs on high-risk opioid prescriptions. Health Aff (Millwood). 2018;37(10):1596-1604. https://doi.org/10.1377/hlthaff.2018.0512.
34. Friedman J, Kim D, Schneberk T, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2018.6721.
35. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43(2):476-493. https://doi.org/10.1093/ije/dyu038.
36. Simon GE, Goldberg DP, Von Korff M, Ustün TB. Understanding cross-national differences in depression prevalence. Psychol Med. 2002;32(4):585-594. https://doi.org/10.1017/S0033291702005457.
© 2019 Society of Hospital Medicine
In the Literature: Research You Need to Know
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability.Am J Med. 2012;125(5):505-511.
For more physician reviews of recent HM-relevant literature, visit our website.
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability.Am J Med. 2012;125(5):505-511.
For more physician reviews of recent HM-relevant literature, visit our website.
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability.Am J Med. 2012;125(5):505-511.
For more physician reviews of recent HM-relevant literature, visit our website.
ITL: Physician Reviews of HM-Relevant Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prediction tool for neurological outcomes after in-hospital cardiac arrest
- Radiation exposure in integrated healthcare systems, 1996-2010
- Postoperative troponin predicts 30-day mortality
- Clinical prediction model of mortality in acute heart failure
- Indwelling pleural catheter vs. talc pleurodesis via chest tube
- Early surgery for high-risk, native-valve endocarditis patients
- Risk factors after ED visit for syncope
- Acute hyperglycemia in CAP patients
- Hospital delirium associated with cognitive decline, institutionalization, and death
- Seven-day ciprofloxacin effective against acute pyelonephritis
- Advance directives in community patients with heart failure
- Chlorhexidine bathing effective against CVC-associated bloodstream infections
- Simulation training improves lumbar puncture skills
- PCP referrals to hospitals and publicly reported data
- Medication reconciliation best practices
Prediction Tool Validated for Prognosticating Favorable Neurological Outcome after In-Hospital Cardiac Arrest
Clinical question: Does the Cardiac Arrest Survival Post Resuscitation In-Hospital (CASPRI) score accurately predict favorable neurological outcomes?
Background: Previous cardiac arrest prediction models have been focused on survival to discharge without consideration of neurological status and have not been translated into valid bedside prognostication tools. Neurologic prognosis can assist patients, families, and physicians in decisions about continued goals of care post-arrest.
Study design: Retrospective cohort study.
Setting: Acute-care hospitals.
Synopsis: Using the Get with the Guidelines Resuscitation Registry, 551 hospitals identified 42,957 patients who were successfully resuscitated from an in-hospital cardiac arrest from January 2000 to October 2009. Researchers developed a simple prediction tool for favorable neurological outcomes (defined as “no” or “moderate” neurological disability) at discharge. The 11 predictors used to calculate the CASPRI score are age; time to defibrillation; pre-arrest neurological status; hospital location; duration of resuscitation; and pre-arrest comorbidities: mechanical ventilation, renal insufficiency, hepatic insufficiency, sepsis, malignancy,
and hypotension.
Rates of favorable neurological outcome were similar between derivation cohort (24.6%) and validation cohort (24.5%). The model had excellent discrimination with a C score of 0.80. Probability of favorable neurological survival ranged from 70.7% in the top decile of patients (CASPRI <10) and 2.8% in bottom decile (CASPRI ≥ 28).
This tool is not generalizable to patients with out-of-hospital arrest or undergoing therapeutic hypothermia.
Bottom line: CASPRI is a simple bedside tool validated to estimate probability of favorable neurological outcome after in-hospital cardiac arrest.
Citation: Chan PS, Spertus JA, Krumholz HA, et al. A validated prediction tool for initial survivors in in-hospital cardiac arrest. Arch Intern Med. 2012;172(12):947-953.
Increased Use of Radiologic Imaging and Associated Radiation Exposure in Integrated Healthcare Systems, 1996-2010
Clinical question: How much has imaging utilization and associated radiation exposure increased over 15 years in integrated healthcare systems independent of financial incentives in a fee-for-service system?
Background: Use of diagnostic imaging has increased significantly within fee-for-service healthcare models. The associated radiation exposure has increased the risk of radiation-induced malignancies. Little is known about the pattern of imaging use in integrated healthcare systems without the financial incentives seen in other models of care.
Study design: Retrospective cohort study.
Setting: Six integrated healthcare systems in the U.S.
Synopsis: The number of diagnostic imaging studies performed and estimated radiation exposure were determined from analysis of electronic medical records from member patients enrolled in health systems in the HMO Research Network from 1996 to 2010. Annual increases in use of advanced diagnostics were noted in CT (7.8% annual growth), MRI (10%), ultrasound (3.9%), and PET (57%) studies.
Increased CT use over the 15-year study period resulted in increased radiation exposure, doubling mean per capita effective dose (1.2 mSv to 2.3 mSv), as well as those receiving high exposure (1.2% to 2.5%) and very high exposure (0.6% to 1.4%).
The increased imaging use and radiation exposure among HMO enrollees was similar to that of fee-for-service Medicare patients in previous studies.
Bottom line: There is a significant increase in use of diagnostic imaging studies and associated radiation exposure among integrated healthcare system enrollees from 1996 to 2010, similar to patients in fee-for-service health plans.
Citation: Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409.
Postoperative Troponin Predicts 30-Day Mortality
Clinical question: Does postoperative peak troponin level predict 30-day mortality in patients undergoing noncardiac surgery?
Background: The use of postoperative peak troponin levels in predicting 30-day mortality for patients undergoing noncardiac surgery has not been studied extensively. Identifying patients at high risk for death following noncardiac surgery could facilitate appropriate postoperative care and improve survival.
Study design: Prospective cohort study.
Setting: International university and nonuniversity hospitals.
Synopsis: The Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study is a large, international, multicenter, prospective cohort study designed to evaluate the major complications of noncardiac surgery. More than 15,100 patients ages 45 and older requiring at least an overnight hospitalization were enrolled following noncardiac surgery.
Peak troponin measurements during the first three postoperative days of 0.01 ng/ml or less, 0.02 ng/ml, 0.03 ng/ml to 0.29 ng/ml, and 0.3 ng/ml or greater had 30-day mortality rates of 1.0%, 4.0%, 9.3%, and 16.9%, respectively.
This study demonstrates the sensitivity of troponin measurement for predicting postoperative 30-day mortality in patients undergoing noncardiac surgery. The study does not address interventions based on an increased postoperative troponin level. Future studies might investigate postoperative modifiable risk factors.
Bottom line: Postoperative peak troponin level predicts 30-day mortality in patients undergoing noncardiac surgery.
Citation: Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
Clinical Prediction Model of Mortality in Acute Heart Failure
Clinical question: Can a clinical prediction model accurately risk-stratify patients presenting to the ED with acute heart failure?
Background: Accurately prognosticating mortality is essential when determining whether to hospitalize or discharge patients presenting to the ED with acute heart failure. Evidence-based clinical prediction models enable physicians to risk-stratify patients and optimize care.
Study design: Retrospective cohort study.
Setting: Multicenter study of 86 hospitals in Ontario, Canada.
Synopsis: Data collected from 12,591 patients who presented to EDs with acute heart failure in Ontario were analyzed. A clinical prediction model of seven-day mortality of discharged and hospitalized patients was derived and validated. The Emergency Heart Failure Mortality Risk Grade (EHMRG) found an increased mortality based on higher triage heart rate, lower triage systolic blood pressure, initial oxygen saturation, and elevated troponin levels. This model uses readily available data collected in ED visits. The high-risk EHMRG score predicted about 8% seven-day mortality versus 0.3% in the low-risk score.
This model was not applied to chronic heart failure, did not utilize left ventricular function, and does not differentiate between systolic and diastolic heart failure.
Bottom line: The Emergency Heart Failure Mortality Risk Grade predicts seven-day mortality in acute heart failure in the emergent setting.
Citation: Lee DS, Stitt A, Austin PC, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156(11):767-775.
Indwelling Pleural Catheter Is as Effective as Talc Pleurodesis Via Chest Tube in Relieving Dyspnea in Patients with Malignant Pleural Effusion
Clinical question: Is indwelling pleural catheter (IPC) as effective as chest tube and talc pleurodesis (talc) in improving dyspnea from malignant pleural effusion in patients who had no previous pleurodesis?
Background: Despite guidelines recommending chest tube insertion with pleurodesis as a first-line treatment for symptom palliation from malignant pleural effusion, there has been no randomized trial comparing indwelling pleural catheter with chest tube and talc pleurodesis.
Study design: Open-label, randomized controlled trial.
Setting: Seven hospitals in the United Kingdom.
Synopsis: One hundred six patients with malignant pleural effusion were randomized to undergo either IPC or talc treatment, and their daily mean dyspnea was measured. There was a clinically significant improvement of dyspnea in both IPC and talc groups over the first 42 days of the trial, without any significant difference in dyspnea between the two groups. After six months, researchers found a clinically significant decrease in dyspnea in the IPC group compared with the talc group. Chest pain and global quality of life were improved and were similar in both groups throughout the trial period. Length of hospital stay was significantly shorter in the IPC group compared with the talc group, but more patients in the IPC group experienced adverse events.
Bottom line: Indwelling pleural catheter is as effective as talc pleurodesis in reliving dyspnea from malignant pleural effusion; however, IPC is associated with increased adverse events despite shorter length of hospital stay.
Citation: Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383-2389.
Early Surgery Better than Conventional Treatment in High-Risk Native-Valve Endocarditis
Clinical question: Is early cardiac surgery better than conventional treatment for patients with left-sided, native-valve, infective endocarditis?
Background: Although guidelines strongly recommend early surgery for patients with infective endocarditis and congestive heart failure, the timing of surgery for patients with large vegetations and high risk of embolism without heart failure symptoms remains controversial.
Study design: Prospective, randomized trial.
Setting: Two medical centers in South Korea.
Synopsis: Seventy-six patients with left-sided, native-valve, infective endocarditis with a high risk of embolism (defined as vegetation with a diameter greater than 10 mm or severe mitral or aortic valve disease) were randomized to undergo early surgery (within 48 hours of enrollment) or conventional treatment (antibiotic therapy and surgery only if complications required urgent surgery). The primary outcome of composite in-hospital death or
clinical embolic events within six weeks of the trial occurred in only one patient in the early surgery group, compared with nine patients in the conventional group (hazard ratio 0.10, 95% CI, 0.01-0.82, P=0.03).
There was no difference in all-cause mortality at six months between the two groups, but the rate of composite endpoint of death from any cause, embolic events, or recurrence of infective endocarditis at six months was significantly lower in the early surgery group compared with the conventional group.
Bottom line: Early cardiac surgery for patients with left-sided, native-valve infective endocarditis with a high risk of embolism significantly improved the composite outcome of all-cause mortality, embolic events, or recurrence of endocarditis compared with the conventional therapy.
Citation: Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366(26):2466-2473.
Risk Factors for Short-Term Mortality after Emergency Department Visit for Syncope
Clinical question: What are the risk factors for short-term mortality after an ED evaluation for syncope or near-syncope?
Background: Syncope accounts for 1% to 2% of all ED visits and an equal number of hospital admissions. The risk of death after an ED visit for syncope is poorly understood, resulting in frequent hospital admissions.
Study design: Retrospective cohort study.
Setting: EDs in Southern California.
Synopsis: Authors evaluated 23,951 ED visits resulting in syncope as sole primary diagnosis. Age was identified as the most significant risk factor for short-term mortality. Cumulative survival data revealed that more than 1% of patients 60 or older died by 30 days. There were 215 deaths (2.84%) in patients hospitalized from the ED and 66 deaths (0.45%) among patients not hospitalized.
Pre-existing comorbidities significantly associated with increased mortality included heart failure (HR=14.3 in ages 18-53; HR=3.09 in ages 60-79; HR=2.34 in ages 80-plus), diabetes (HR=1.49), seizure (HR=1.65), dementia (HR=1.41), and a recent prior visit for syncope (HR=1.86). The risk of death by 30 days was less than 0.2% in patients under 60 without heart failure and more than 2.5% in patients of all ages with heart failure.
Bottom line: After an ED visit for syncope, patients with a history of heart failure and patients 60 and older have a significantly increased risk of short-term mortality.
Citation: Derose SF, Gabayan GZ, Chiu VY, Sun BC. Patterns and preexisting risk factors of 30-day mortality after a primary discharge diagnosis of syncope or near syncope. Acad Emerg Med. 2012;19(5):488-496.
Acute Hyperglycemia Associated with Increased Mortality in Community-Acquired Pneumonia
Clinical question: In patients admitted to the hospital for community-acquired pneumonia, is serum glucose level on admission associated with mortality?
Background: Some retrospective studies have shown an association between alterations in serum glucose levels or pre-existing diabetes and higher mortality due to infections, while other studies have shown no clear association.
Study design: Multicenter, prospective cohort study.
Setting: Hospitals and private practices in Germany, Switzerland, and Austria.
Synopsis: Prospective data from 6,891 patients were included in the analysis. Patients without diabetes and normal serum glucose levels had the lowest mortality after 90 days. Patients without diabetes but with mild acute hyperglycemia (108 mg/dL to 198 mg/dL) had a significantly increased risk of death at 90 days (HR 1.56), and patients without diabetes but with more severe acute hyperglycemia (over 252 mg/dL) had an even higher risk of death at 90 days (HR 2.37).
The 90-day mortality rate was significantly higher in patients with pre-existing diabetes (HR 2.47), although this was not affected by serum glucose levels on admission.
Bottom line: Acute hyperglycemia, as well as pre-existing diabetes, was associated with an increased risk of 90-day mortality in patients with community acquired pneumonia.
Citation: Lepper PM, Ott S, Nüesch E, et al. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ. 2012;344:e3397.
Hospital Delirium Associated with Cognitive Decline, Institutionalization, and Death
Clinical question: What is the risk of subsequent cognitive decline, institutionalization, or death due to delirium in patients with dementia?
Background: Patients suffering delirium during hospitalization can suffer additional cognitive decline. Whether this is due to additional damage from the delirium state or reflects pre-existing cognitive vulnerability remains uncertain.
Study design: Prospective analysis of a cohort of Alzheimer’s patients.
Setting: Massachusetts community-based disease registry.
Synopsis: The analysis compared nonhospitalized individuals to patients hospitalized with, and without, delirium. In 771 individuals with dementia, at least one adverse outcome (including cognitive decline, institutionalization, or death) occurred in 32% of those not hospitalized, 55% of those hospitalized without delirium, and 79% of those hospitalized with delirium. Even after adjusting for confounders, hospitalization increased the risk for each of the adverse outcomes; the highest risk was in those with delirium.
Among hospitalized patients, the authors estimated 1 in 5 cases of cognitive decline, 1 in 7 institutionalizations, and 1 in 16 deaths were attributable to delirium. Some of the attributed risk could be the result of residual confounding from unmeasured variables, limiting conclusions of causality. Despite these limitations, this study supports the hypothesis that delirium prevention measures could improve important patient outcomes.
Bottom line: Hospitalization is associated with high rates of adverse outcomes in elderly patients with dementia, the worst of which occurs in those who experience delirium.
Citation: Fong TG, Jones RN, Marcantonio ER, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Int Med. 2012;156:848-856.
In Acute Pyelonephritis, a Seven-Day Course of Ciprofloxacin is Effective in Obtaining Clinical Cure
Clinical question: What is the efficacy of ciprofloxacin for seven days compared with 14 days in women with community-acquired acute pyelonephritis?
Background: Community-acquired acute pyelonephritis is a common and sometimes serious infection in women. In an era of increasing antibiotic resistance worldwide, it is prudent to reduce antibiotic utilization. There are limited controlled trials to assess the optimum duration of antibiotic treatment for this common infection.
Study design: Prospective, randomized, double-blind, noninferiority trial.
Setting: Twenty-one infectious-disease centers in Sweden.
Synopsis: Researchers randomly assigned 284 women 18 or older with a presumptive diagnosis of acute pyelonephritis to ciprofloxacin treatment for seven or 14 days. The primary endpoint was clinical and bacteriological cure 10 to 14 days after the completion of the treatment regimen. Short-term clinical cure occurred in 97% of the patients treated for seven days and 96% treated for 14 days. Long-term follow-up showed cumulative efficacy of 93% in each group. Both regimens were well tolerated.
Patients in this study had a low occurrence of complicated (9%) and recurrent (13%) infections. Whether short courses of antibiotics are effective in more complicated infections cannot be ascertained from this study. Also, the high cure rate obtained with a seven-day course of ciprofloxacin should not be extrapolated to other classes of antibiotics. Fluoroquinolones, such as ciprofloxacin, are recommended as first-line agents for empiric oral treatment of acute pyelonephritis if the resistance rate of the uropathogens remains lower than 10%; however, there is growing evidence that E. coli strains are becoming increasingly resistant to ciprofloxacin, limiting its usefulness.
Bottom line: Acute pyelonephritis in women can be treated successfully and safely with a seven-day course of ciprofloxacin, in areas with low ciprofloxacin resistance.
Citation: Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomized, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380:484-490.
Advance Directives in Community Patients with Heart Failure
Clinical question: How prevalent are advance directives in heart-failure patients, and does a completed advance directive decrease end-of-life resource use (hospitalizations, ICU admissions, mechanical ventilation)?
Background: Heart failure is a common chronic and fatal disease. End-of-life care in heart-failure patients is associated with extremely high healthcare utilization. Heart failure guidelines recommend completing advance directives in all patients.
Study design: Population-based longitudinal cohort study.
Setting: Rochester Epidemiology Project in Olmstead County, Minn.
Synopsis: Investigators enrolled 608 patients presenting with heart failure between October 2007 and October 2011. At the time of enrollment, only 41% of the patients had existing advance directives. Independent predictors of advance directive completion included older age, history of malignancy, and renal dysfunction.
After a mean follow-up of 1.8 years, 164 patients (27%) had died. Among those patients, 106 had an advance directive (64.6%) at time of death—75 had an advance directive at the time of enrollment and another 31 completed an advance directive after enrollment.
Twenty-five patients (23.6%) specified DNR/DNI and another 39 (36.8%) denoted limitations on aggressiveness of care if death was imminent. Among the patients who died, 88 (53.7%) were hospitalized in the last month of their life and 50 (30.5%) died in the hospital. There was no difference in hospitalizations between those with an advance directive specifying limits and those who did not specify limits (OR 1.26, 95% CI 0.64-2.48). However, those with an advance directive specifying limits were less frequently mechanically ventilated (OR 0.26, 95% CI 0.06-0.88), and there was a trend toward them being less frequently admitted into the ICU (OR 0.45, 95% CI 0.16-1.29).
Bottom line: Less than half of community patients with heart failure had an advance directive, and many of these failed to address end-of-life decisions. Patients with an advance directive that specified limits in care were less likely to receive mechanical ventilation.
Citation: Dunlay SM, Swetz KM, Mueller PS, Roger VL. Advance directives in community patients with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5:283-289.
Chlorhexidine Bathing Associated with Significant, Sustainable Reductions in Central-Venous-Catheter-Associated Bloodstream Infection
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511.
Simulation Training Improves Lumbar Puncture Skills
Clinical question: What effect does simulation have on lumbar puncture (LP) skills of PGY1 internal-medicine (IM) residents compared with PGY2-4 neurology residents who have not received simulation training?
Background: LPs are common procedures. The American College of General Medical Education does not define competency; neither do the internal-medicine (IM) or neurology board certifications. Simulation can improve skills in many areas but has not been well studied in LPs.
Study design: Pre-test-post-test.
Setting: Northwestern University’s Feinberg School of Medicine in Chicago.
Synopsis: The intervention group included 58 PGY1 IM residents, while the control group was 49 PGY2-to-PGY4 neurology residents. The pre-test consisted of a 21-point checklist. IM residents watched a three-hour video, performed LPs on simulators, and received feedback. The post-test was a clinical skills examination using the checklist. If this exam was failed, the participant practiced and was retested. Neurology residents completed the pre-test and demonstrated an LP using the simulator.
Pre-test passing was achieved by only 2% of IM residents and 6% of neurology residents. Post-test passing was achieved by 95% of the IM residents on the first trial and 100% of IM residents after an hour of additional training. IM mean scores increased to 95.7% from 46.3%, while the mean score of neurology residents was 65.4%.
This study is limited by its single-center nature, as education is variable from center to center. The study evaluated the proficiency on simulators only, and it did not evaluate the proficiency of the participants on patients.
Bottom line: Simulation training improves lumbar puncture skills.
Citation: Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137.
Primary-Care Physicians Do Not Use Publicly Reported Data When Referring Patients to Hospitals
Clinical question: When referring patients with pneumonia to the hospital, what factors do primary-care physicians (PCPs) consider?
Background: Publicly reported data are widely available. Pneumonia has publicly reported quality measures and is a common reason for hospitalization. Fewer PCPs are attending in the hospital due to the hospitalist movement; therefore, PCPs refer patients to a hospital when the need arises.
Study design: Online survey.
Setting: PCPs within 10 miles of Springfield, Mass.
Synopsis: A total of 92 PCPs responded to the survey, which included presentation of a case regarding a patient with pneumonia. PCPs were asked the importance of multiple factors leading to their decision to refer to a hospital. Familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%) were considered to be very important to the PCPs that responded to the survey. Publicly reported data were very important to only 18% of respondents, and zero reported using publicly reported data when referring patients.
Importance of specific quality measures also was queried; antibiotics given within six hours of arrival (66%), appropriate choice of antibiotics (63%), and blood cultures prior to antibiotic administration (51%) were very important to respondents. Prestige, such as magnet status and U.S. News and World Report “Best Hospital” status, were deemed important by about 40% of PCPs.
Bottom line: Despite the availability of publicly reported data, PCPs do not use this information to refer patients to the hospital.
Citation: Morsi E, Lindenauer PK, Rothberg MB. Primary care physicians’ use of publicly reported quality data in hospital referral decisions. J Hosp Med. 2012;7(5):370-375.
What Works for Medication Reconciliation?
Clinical question: What are the most effective practices for medication reconciliation in the hospital setting?
Background: Medication discrepancies are common, occurring in as many as 70% of patients at hospital admission or discharge. Up to a third of these discrepancies have potential to cause patient harm, including prolonged hospital stays, ED visits, hospital recidivism, and use of other healthcare resources. Medication reconciliation (“med rec”) is a strategy for reducing these errors, though previous literature has not systematically reviewed best practices for hospital-based med rec.
Study design: Systematic review of literature.
Setting: Controlled studies from the U.S., Canada, Australia, New Zealand, Northern Ireland, United Kingdom, Belgium, Denmark, the Netherlands, and Sweden.
Synopsis: Investigators identified 26 controlled studies using a systematic search of English-language articles on med rec during inpatient hospitalizations published between Jan. 1, 1966, and Oct. 31, 2010. Fifteen studies reported on pharmacist-related interventions; six reported on technology-specific interventions; and five reported on other types of interventions, including staff education and use of standardized med-rec tools.
Analysis of these studies revealed that all of these interventions successfully decreased medication discrepancies and potential adverse drug events, but there was inconsistent benefit with regard to adverse drug events and healthcare utilization compared with usual care. The literature was most supportive of pharmacist-related interventions, including but not limited to comprehensive medication history at admission, med rec at discharge, patient counseling, discharge communication with outpatient providers, and post-discharge communication with the patient and post-hospital providers.
Bottom line: Successful med rec requires multiple interventions at various transitions of care and involves a variety of medical professionals. Patient-targeted interventions, including pharmacists, have the potential to decrease errors and adverse events.
Citation: Mueller S, Sponsler K, Kripalani S, Schnipper J. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prediction tool for neurological outcomes after in-hospital cardiac arrest
- Radiation exposure in integrated healthcare systems, 1996-2010
- Postoperative troponin predicts 30-day mortality
- Clinical prediction model of mortality in acute heart failure
- Indwelling pleural catheter vs. talc pleurodesis via chest tube
- Early surgery for high-risk, native-valve endocarditis patients
- Risk factors after ED visit for syncope
- Acute hyperglycemia in CAP patients
- Hospital delirium associated with cognitive decline, institutionalization, and death
- Seven-day ciprofloxacin effective against acute pyelonephritis
- Advance directives in community patients with heart failure
- Chlorhexidine bathing effective against CVC-associated bloodstream infections
- Simulation training improves lumbar puncture skills
- PCP referrals to hospitals and publicly reported data
- Medication reconciliation best practices
Prediction Tool Validated for Prognosticating Favorable Neurological Outcome after In-Hospital Cardiac Arrest
Clinical question: Does the Cardiac Arrest Survival Post Resuscitation In-Hospital (CASPRI) score accurately predict favorable neurological outcomes?
Background: Previous cardiac arrest prediction models have been focused on survival to discharge without consideration of neurological status and have not been translated into valid bedside prognostication tools. Neurologic prognosis can assist patients, families, and physicians in decisions about continued goals of care post-arrest.
Study design: Retrospective cohort study.
Setting: Acute-care hospitals.
Synopsis: Using the Get with the Guidelines Resuscitation Registry, 551 hospitals identified 42,957 patients who were successfully resuscitated from an in-hospital cardiac arrest from January 2000 to October 2009. Researchers developed a simple prediction tool for favorable neurological outcomes (defined as “no” or “moderate” neurological disability) at discharge. The 11 predictors used to calculate the CASPRI score are age; time to defibrillation; pre-arrest neurological status; hospital location; duration of resuscitation; and pre-arrest comorbidities: mechanical ventilation, renal insufficiency, hepatic insufficiency, sepsis, malignancy,
and hypotension.
Rates of favorable neurological outcome were similar between derivation cohort (24.6%) and validation cohort (24.5%). The model had excellent discrimination with a C score of 0.80. Probability of favorable neurological survival ranged from 70.7% in the top decile of patients (CASPRI <10) and 2.8% in bottom decile (CASPRI ≥ 28).
This tool is not generalizable to patients with out-of-hospital arrest or undergoing therapeutic hypothermia.
Bottom line: CASPRI is a simple bedside tool validated to estimate probability of favorable neurological outcome after in-hospital cardiac arrest.
Citation: Chan PS, Spertus JA, Krumholz HA, et al. A validated prediction tool for initial survivors in in-hospital cardiac arrest. Arch Intern Med. 2012;172(12):947-953.
Increased Use of Radiologic Imaging and Associated Radiation Exposure in Integrated Healthcare Systems, 1996-2010
Clinical question: How much has imaging utilization and associated radiation exposure increased over 15 years in integrated healthcare systems independent of financial incentives in a fee-for-service system?
Background: Use of diagnostic imaging has increased significantly within fee-for-service healthcare models. The associated radiation exposure has increased the risk of radiation-induced malignancies. Little is known about the pattern of imaging use in integrated healthcare systems without the financial incentives seen in other models of care.
Study design: Retrospective cohort study.
Setting: Six integrated healthcare systems in the U.S.
Synopsis: The number of diagnostic imaging studies performed and estimated radiation exposure were determined from analysis of electronic medical records from member patients enrolled in health systems in the HMO Research Network from 1996 to 2010. Annual increases in use of advanced diagnostics were noted in CT (7.8% annual growth), MRI (10%), ultrasound (3.9%), and PET (57%) studies.
Increased CT use over the 15-year study period resulted in increased radiation exposure, doubling mean per capita effective dose (1.2 mSv to 2.3 mSv), as well as those receiving high exposure (1.2% to 2.5%) and very high exposure (0.6% to 1.4%).
The increased imaging use and radiation exposure among HMO enrollees was similar to that of fee-for-service Medicare patients in previous studies.
Bottom line: There is a significant increase in use of diagnostic imaging studies and associated radiation exposure among integrated healthcare system enrollees from 1996 to 2010, similar to patients in fee-for-service health plans.
Citation: Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409.
Postoperative Troponin Predicts 30-Day Mortality
Clinical question: Does postoperative peak troponin level predict 30-day mortality in patients undergoing noncardiac surgery?
Background: The use of postoperative peak troponin levels in predicting 30-day mortality for patients undergoing noncardiac surgery has not been studied extensively. Identifying patients at high risk for death following noncardiac surgery could facilitate appropriate postoperative care and improve survival.
Study design: Prospective cohort study.
Setting: International university and nonuniversity hospitals.
Synopsis: The Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study is a large, international, multicenter, prospective cohort study designed to evaluate the major complications of noncardiac surgery. More than 15,100 patients ages 45 and older requiring at least an overnight hospitalization were enrolled following noncardiac surgery.
Peak troponin measurements during the first three postoperative days of 0.01 ng/ml or less, 0.02 ng/ml, 0.03 ng/ml to 0.29 ng/ml, and 0.3 ng/ml or greater had 30-day mortality rates of 1.0%, 4.0%, 9.3%, and 16.9%, respectively.
This study demonstrates the sensitivity of troponin measurement for predicting postoperative 30-day mortality in patients undergoing noncardiac surgery. The study does not address interventions based on an increased postoperative troponin level. Future studies might investigate postoperative modifiable risk factors.
Bottom line: Postoperative peak troponin level predicts 30-day mortality in patients undergoing noncardiac surgery.
Citation: Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
Clinical Prediction Model of Mortality in Acute Heart Failure
Clinical question: Can a clinical prediction model accurately risk-stratify patients presenting to the ED with acute heart failure?
Background: Accurately prognosticating mortality is essential when determining whether to hospitalize or discharge patients presenting to the ED with acute heart failure. Evidence-based clinical prediction models enable physicians to risk-stratify patients and optimize care.
Study design: Retrospective cohort study.
Setting: Multicenter study of 86 hospitals in Ontario, Canada.
Synopsis: Data collected from 12,591 patients who presented to EDs with acute heart failure in Ontario were analyzed. A clinical prediction model of seven-day mortality of discharged and hospitalized patients was derived and validated. The Emergency Heart Failure Mortality Risk Grade (EHMRG) found an increased mortality based on higher triage heart rate, lower triage systolic blood pressure, initial oxygen saturation, and elevated troponin levels. This model uses readily available data collected in ED visits. The high-risk EHMRG score predicted about 8% seven-day mortality versus 0.3% in the low-risk score.
This model was not applied to chronic heart failure, did not utilize left ventricular function, and does not differentiate between systolic and diastolic heart failure.
Bottom line: The Emergency Heart Failure Mortality Risk Grade predicts seven-day mortality in acute heart failure in the emergent setting.
Citation: Lee DS, Stitt A, Austin PC, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156(11):767-775.
Indwelling Pleural Catheter Is as Effective as Talc Pleurodesis Via Chest Tube in Relieving Dyspnea in Patients with Malignant Pleural Effusion
Clinical question: Is indwelling pleural catheter (IPC) as effective as chest tube and talc pleurodesis (talc) in improving dyspnea from malignant pleural effusion in patients who had no previous pleurodesis?
Background: Despite guidelines recommending chest tube insertion with pleurodesis as a first-line treatment for symptom palliation from malignant pleural effusion, there has been no randomized trial comparing indwelling pleural catheter with chest tube and talc pleurodesis.
Study design: Open-label, randomized controlled trial.
Setting: Seven hospitals in the United Kingdom.
Synopsis: One hundred six patients with malignant pleural effusion were randomized to undergo either IPC or talc treatment, and their daily mean dyspnea was measured. There was a clinically significant improvement of dyspnea in both IPC and talc groups over the first 42 days of the trial, without any significant difference in dyspnea between the two groups. After six months, researchers found a clinically significant decrease in dyspnea in the IPC group compared with the talc group. Chest pain and global quality of life were improved and were similar in both groups throughout the trial period. Length of hospital stay was significantly shorter in the IPC group compared with the talc group, but more patients in the IPC group experienced adverse events.
Bottom line: Indwelling pleural catheter is as effective as talc pleurodesis in reliving dyspnea from malignant pleural effusion; however, IPC is associated with increased adverse events despite shorter length of hospital stay.
Citation: Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383-2389.
Early Surgery Better than Conventional Treatment in High-Risk Native-Valve Endocarditis
Clinical question: Is early cardiac surgery better than conventional treatment for patients with left-sided, native-valve, infective endocarditis?
Background: Although guidelines strongly recommend early surgery for patients with infective endocarditis and congestive heart failure, the timing of surgery for patients with large vegetations and high risk of embolism without heart failure symptoms remains controversial.
Study design: Prospective, randomized trial.
Setting: Two medical centers in South Korea.
Synopsis: Seventy-six patients with left-sided, native-valve, infective endocarditis with a high risk of embolism (defined as vegetation with a diameter greater than 10 mm or severe mitral or aortic valve disease) were randomized to undergo early surgery (within 48 hours of enrollment) or conventional treatment (antibiotic therapy and surgery only if complications required urgent surgery). The primary outcome of composite in-hospital death or
clinical embolic events within six weeks of the trial occurred in only one patient in the early surgery group, compared with nine patients in the conventional group (hazard ratio 0.10, 95% CI, 0.01-0.82, P=0.03).
There was no difference in all-cause mortality at six months between the two groups, but the rate of composite endpoint of death from any cause, embolic events, or recurrence of infective endocarditis at six months was significantly lower in the early surgery group compared with the conventional group.
Bottom line: Early cardiac surgery for patients with left-sided, native-valve infective endocarditis with a high risk of embolism significantly improved the composite outcome of all-cause mortality, embolic events, or recurrence of endocarditis compared with the conventional therapy.
Citation: Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366(26):2466-2473.
Risk Factors for Short-Term Mortality after Emergency Department Visit for Syncope
Clinical question: What are the risk factors for short-term mortality after an ED evaluation for syncope or near-syncope?
Background: Syncope accounts for 1% to 2% of all ED visits and an equal number of hospital admissions. The risk of death after an ED visit for syncope is poorly understood, resulting in frequent hospital admissions.
Study design: Retrospective cohort study.
Setting: EDs in Southern California.
Synopsis: Authors evaluated 23,951 ED visits resulting in syncope as sole primary diagnosis. Age was identified as the most significant risk factor for short-term mortality. Cumulative survival data revealed that more than 1% of patients 60 or older died by 30 days. There were 215 deaths (2.84%) in patients hospitalized from the ED and 66 deaths (0.45%) among patients not hospitalized.
Pre-existing comorbidities significantly associated with increased mortality included heart failure (HR=14.3 in ages 18-53; HR=3.09 in ages 60-79; HR=2.34 in ages 80-plus), diabetes (HR=1.49), seizure (HR=1.65), dementia (HR=1.41), and a recent prior visit for syncope (HR=1.86). The risk of death by 30 days was less than 0.2% in patients under 60 without heart failure and more than 2.5% in patients of all ages with heart failure.
Bottom line: After an ED visit for syncope, patients with a history of heart failure and patients 60 and older have a significantly increased risk of short-term mortality.
Citation: Derose SF, Gabayan GZ, Chiu VY, Sun BC. Patterns and preexisting risk factors of 30-day mortality after a primary discharge diagnosis of syncope or near syncope. Acad Emerg Med. 2012;19(5):488-496.
Acute Hyperglycemia Associated with Increased Mortality in Community-Acquired Pneumonia
Clinical question: In patients admitted to the hospital for community-acquired pneumonia, is serum glucose level on admission associated with mortality?
Background: Some retrospective studies have shown an association between alterations in serum glucose levels or pre-existing diabetes and higher mortality due to infections, while other studies have shown no clear association.
Study design: Multicenter, prospective cohort study.
Setting: Hospitals and private practices in Germany, Switzerland, and Austria.
Synopsis: Prospective data from 6,891 patients were included in the analysis. Patients without diabetes and normal serum glucose levels had the lowest mortality after 90 days. Patients without diabetes but with mild acute hyperglycemia (108 mg/dL to 198 mg/dL) had a significantly increased risk of death at 90 days (HR 1.56), and patients without diabetes but with more severe acute hyperglycemia (over 252 mg/dL) had an even higher risk of death at 90 days (HR 2.37).
The 90-day mortality rate was significantly higher in patients with pre-existing diabetes (HR 2.47), although this was not affected by serum glucose levels on admission.
Bottom line: Acute hyperglycemia, as well as pre-existing diabetes, was associated with an increased risk of 90-day mortality in patients with community acquired pneumonia.
Citation: Lepper PM, Ott S, Nüesch E, et al. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ. 2012;344:e3397.
Hospital Delirium Associated with Cognitive Decline, Institutionalization, and Death
Clinical question: What is the risk of subsequent cognitive decline, institutionalization, or death due to delirium in patients with dementia?
Background: Patients suffering delirium during hospitalization can suffer additional cognitive decline. Whether this is due to additional damage from the delirium state or reflects pre-existing cognitive vulnerability remains uncertain.
Study design: Prospective analysis of a cohort of Alzheimer’s patients.
Setting: Massachusetts community-based disease registry.
Synopsis: The analysis compared nonhospitalized individuals to patients hospitalized with, and without, delirium. In 771 individuals with dementia, at least one adverse outcome (including cognitive decline, institutionalization, or death) occurred in 32% of those not hospitalized, 55% of those hospitalized without delirium, and 79% of those hospitalized with delirium. Even after adjusting for confounders, hospitalization increased the risk for each of the adverse outcomes; the highest risk was in those with delirium.
Among hospitalized patients, the authors estimated 1 in 5 cases of cognitive decline, 1 in 7 institutionalizations, and 1 in 16 deaths were attributable to delirium. Some of the attributed risk could be the result of residual confounding from unmeasured variables, limiting conclusions of causality. Despite these limitations, this study supports the hypothesis that delirium prevention measures could improve important patient outcomes.
Bottom line: Hospitalization is associated with high rates of adverse outcomes in elderly patients with dementia, the worst of which occurs in those who experience delirium.
Citation: Fong TG, Jones RN, Marcantonio ER, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Int Med. 2012;156:848-856.
In Acute Pyelonephritis, a Seven-Day Course of Ciprofloxacin is Effective in Obtaining Clinical Cure
Clinical question: What is the efficacy of ciprofloxacin for seven days compared with 14 days in women with community-acquired acute pyelonephritis?
Background: Community-acquired acute pyelonephritis is a common and sometimes serious infection in women. In an era of increasing antibiotic resistance worldwide, it is prudent to reduce antibiotic utilization. There are limited controlled trials to assess the optimum duration of antibiotic treatment for this common infection.
Study design: Prospective, randomized, double-blind, noninferiority trial.
Setting: Twenty-one infectious-disease centers in Sweden.
Synopsis: Researchers randomly assigned 284 women 18 or older with a presumptive diagnosis of acute pyelonephritis to ciprofloxacin treatment for seven or 14 days. The primary endpoint was clinical and bacteriological cure 10 to 14 days after the completion of the treatment regimen. Short-term clinical cure occurred in 97% of the patients treated for seven days and 96% treated for 14 days. Long-term follow-up showed cumulative efficacy of 93% in each group. Both regimens were well tolerated.
Patients in this study had a low occurrence of complicated (9%) and recurrent (13%) infections. Whether short courses of antibiotics are effective in more complicated infections cannot be ascertained from this study. Also, the high cure rate obtained with a seven-day course of ciprofloxacin should not be extrapolated to other classes of antibiotics. Fluoroquinolones, such as ciprofloxacin, are recommended as first-line agents for empiric oral treatment of acute pyelonephritis if the resistance rate of the uropathogens remains lower than 10%; however, there is growing evidence that E. coli strains are becoming increasingly resistant to ciprofloxacin, limiting its usefulness.
Bottom line: Acute pyelonephritis in women can be treated successfully and safely with a seven-day course of ciprofloxacin, in areas with low ciprofloxacin resistance.
Citation: Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomized, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380:484-490.
Advance Directives in Community Patients with Heart Failure
Clinical question: How prevalent are advance directives in heart-failure patients, and does a completed advance directive decrease end-of-life resource use (hospitalizations, ICU admissions, mechanical ventilation)?
Background: Heart failure is a common chronic and fatal disease. End-of-life care in heart-failure patients is associated with extremely high healthcare utilization. Heart failure guidelines recommend completing advance directives in all patients.
Study design: Population-based longitudinal cohort study.
Setting: Rochester Epidemiology Project in Olmstead County, Minn.
Synopsis: Investigators enrolled 608 patients presenting with heart failure between October 2007 and October 2011. At the time of enrollment, only 41% of the patients had existing advance directives. Independent predictors of advance directive completion included older age, history of malignancy, and renal dysfunction.
After a mean follow-up of 1.8 years, 164 patients (27%) had died. Among those patients, 106 had an advance directive (64.6%) at time of death—75 had an advance directive at the time of enrollment and another 31 completed an advance directive after enrollment.
Twenty-five patients (23.6%) specified DNR/DNI and another 39 (36.8%) denoted limitations on aggressiveness of care if death was imminent. Among the patients who died, 88 (53.7%) were hospitalized in the last month of their life and 50 (30.5%) died in the hospital. There was no difference in hospitalizations between those with an advance directive specifying limits and those who did not specify limits (OR 1.26, 95% CI 0.64-2.48). However, those with an advance directive specifying limits were less frequently mechanically ventilated (OR 0.26, 95% CI 0.06-0.88), and there was a trend toward them being less frequently admitted into the ICU (OR 0.45, 95% CI 0.16-1.29).
Bottom line: Less than half of community patients with heart failure had an advance directive, and many of these failed to address end-of-life decisions. Patients with an advance directive that specified limits in care were less likely to receive mechanical ventilation.
Citation: Dunlay SM, Swetz KM, Mueller PS, Roger VL. Advance directives in community patients with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5:283-289.
Chlorhexidine Bathing Associated with Significant, Sustainable Reductions in Central-Venous-Catheter-Associated Bloodstream Infection
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511.
Simulation Training Improves Lumbar Puncture Skills
Clinical question: What effect does simulation have on lumbar puncture (LP) skills of PGY1 internal-medicine (IM) residents compared with PGY2-4 neurology residents who have not received simulation training?
Background: LPs are common procedures. The American College of General Medical Education does not define competency; neither do the internal-medicine (IM) or neurology board certifications. Simulation can improve skills in many areas but has not been well studied in LPs.
Study design: Pre-test-post-test.
Setting: Northwestern University’s Feinberg School of Medicine in Chicago.
Synopsis: The intervention group included 58 PGY1 IM residents, while the control group was 49 PGY2-to-PGY4 neurology residents. The pre-test consisted of a 21-point checklist. IM residents watched a three-hour video, performed LPs on simulators, and received feedback. The post-test was a clinical skills examination using the checklist. If this exam was failed, the participant practiced and was retested. Neurology residents completed the pre-test and demonstrated an LP using the simulator.
Pre-test passing was achieved by only 2% of IM residents and 6% of neurology residents. Post-test passing was achieved by 95% of the IM residents on the first trial and 100% of IM residents after an hour of additional training. IM mean scores increased to 95.7% from 46.3%, while the mean score of neurology residents was 65.4%.
This study is limited by its single-center nature, as education is variable from center to center. The study evaluated the proficiency on simulators only, and it did not evaluate the proficiency of the participants on patients.
Bottom line: Simulation training improves lumbar puncture skills.
Citation: Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137.
Primary-Care Physicians Do Not Use Publicly Reported Data When Referring Patients to Hospitals
Clinical question: When referring patients with pneumonia to the hospital, what factors do primary-care physicians (PCPs) consider?
Background: Publicly reported data are widely available. Pneumonia has publicly reported quality measures and is a common reason for hospitalization. Fewer PCPs are attending in the hospital due to the hospitalist movement; therefore, PCPs refer patients to a hospital when the need arises.
Study design: Online survey.
Setting: PCPs within 10 miles of Springfield, Mass.
Synopsis: A total of 92 PCPs responded to the survey, which included presentation of a case regarding a patient with pneumonia. PCPs were asked the importance of multiple factors leading to their decision to refer to a hospital. Familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%) were considered to be very important to the PCPs that responded to the survey. Publicly reported data were very important to only 18% of respondents, and zero reported using publicly reported data when referring patients.
Importance of specific quality measures also was queried; antibiotics given within six hours of arrival (66%), appropriate choice of antibiotics (63%), and blood cultures prior to antibiotic administration (51%) were very important to respondents. Prestige, such as magnet status and U.S. News and World Report “Best Hospital” status, were deemed important by about 40% of PCPs.
Bottom line: Despite the availability of publicly reported data, PCPs do not use this information to refer patients to the hospital.
Citation: Morsi E, Lindenauer PK, Rothberg MB. Primary care physicians’ use of publicly reported quality data in hospital referral decisions. J Hosp Med. 2012;7(5):370-375.
What Works for Medication Reconciliation?
Clinical question: What are the most effective practices for medication reconciliation in the hospital setting?
Background: Medication discrepancies are common, occurring in as many as 70% of patients at hospital admission or discharge. Up to a third of these discrepancies have potential to cause patient harm, including prolonged hospital stays, ED visits, hospital recidivism, and use of other healthcare resources. Medication reconciliation (“med rec”) is a strategy for reducing these errors, though previous literature has not systematically reviewed best practices for hospital-based med rec.
Study design: Systematic review of literature.
Setting: Controlled studies from the U.S., Canada, Australia, New Zealand, Northern Ireland, United Kingdom, Belgium, Denmark, the Netherlands, and Sweden.
Synopsis: Investigators identified 26 controlled studies using a systematic search of English-language articles on med rec during inpatient hospitalizations published between Jan. 1, 1966, and Oct. 31, 2010. Fifteen studies reported on pharmacist-related interventions; six reported on technology-specific interventions; and five reported on other types of interventions, including staff education and use of standardized med-rec tools.
Analysis of these studies revealed that all of these interventions successfully decreased medication discrepancies and potential adverse drug events, but there was inconsistent benefit with regard to adverse drug events and healthcare utilization compared with usual care. The literature was most supportive of pharmacist-related interventions, including but not limited to comprehensive medication history at admission, med rec at discharge, patient counseling, discharge communication with outpatient providers, and post-discharge communication with the patient and post-hospital providers.
Bottom line: Successful med rec requires multiple interventions at various transitions of care and involves a variety of medical professionals. Patient-targeted interventions, including pharmacists, have the potential to decrease errors and adverse events.
Citation: Mueller S, Sponsler K, Kripalani S, Schnipper J. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prediction tool for neurological outcomes after in-hospital cardiac arrest
- Radiation exposure in integrated healthcare systems, 1996-2010
- Postoperative troponin predicts 30-day mortality
- Clinical prediction model of mortality in acute heart failure
- Indwelling pleural catheter vs. talc pleurodesis via chest tube
- Early surgery for high-risk, native-valve endocarditis patients
- Risk factors after ED visit for syncope
- Acute hyperglycemia in CAP patients
- Hospital delirium associated with cognitive decline, institutionalization, and death
- Seven-day ciprofloxacin effective against acute pyelonephritis
- Advance directives in community patients with heart failure
- Chlorhexidine bathing effective against CVC-associated bloodstream infections
- Simulation training improves lumbar puncture skills
- PCP referrals to hospitals and publicly reported data
- Medication reconciliation best practices
Prediction Tool Validated for Prognosticating Favorable Neurological Outcome after In-Hospital Cardiac Arrest
Clinical question: Does the Cardiac Arrest Survival Post Resuscitation In-Hospital (CASPRI) score accurately predict favorable neurological outcomes?
Background: Previous cardiac arrest prediction models have been focused on survival to discharge without consideration of neurological status and have not been translated into valid bedside prognostication tools. Neurologic prognosis can assist patients, families, and physicians in decisions about continued goals of care post-arrest.
Study design: Retrospective cohort study.
Setting: Acute-care hospitals.
Synopsis: Using the Get with the Guidelines Resuscitation Registry, 551 hospitals identified 42,957 patients who were successfully resuscitated from an in-hospital cardiac arrest from January 2000 to October 2009. Researchers developed a simple prediction tool for favorable neurological outcomes (defined as “no” or “moderate” neurological disability) at discharge. The 11 predictors used to calculate the CASPRI score are age; time to defibrillation; pre-arrest neurological status; hospital location; duration of resuscitation; and pre-arrest comorbidities: mechanical ventilation, renal insufficiency, hepatic insufficiency, sepsis, malignancy,
and hypotension.
Rates of favorable neurological outcome were similar between derivation cohort (24.6%) and validation cohort (24.5%). The model had excellent discrimination with a C score of 0.80. Probability of favorable neurological survival ranged from 70.7% in the top decile of patients (CASPRI <10) and 2.8% in bottom decile (CASPRI ≥ 28).
This tool is not generalizable to patients with out-of-hospital arrest or undergoing therapeutic hypothermia.
Bottom line: CASPRI is a simple bedside tool validated to estimate probability of favorable neurological outcome after in-hospital cardiac arrest.
Citation: Chan PS, Spertus JA, Krumholz HA, et al. A validated prediction tool for initial survivors in in-hospital cardiac arrest. Arch Intern Med. 2012;172(12):947-953.
Increased Use of Radiologic Imaging and Associated Radiation Exposure in Integrated Healthcare Systems, 1996-2010
Clinical question: How much has imaging utilization and associated radiation exposure increased over 15 years in integrated healthcare systems independent of financial incentives in a fee-for-service system?
Background: Use of diagnostic imaging has increased significantly within fee-for-service healthcare models. The associated radiation exposure has increased the risk of radiation-induced malignancies. Little is known about the pattern of imaging use in integrated healthcare systems without the financial incentives seen in other models of care.
Study design: Retrospective cohort study.
Setting: Six integrated healthcare systems in the U.S.
Synopsis: The number of diagnostic imaging studies performed and estimated radiation exposure were determined from analysis of electronic medical records from member patients enrolled in health systems in the HMO Research Network from 1996 to 2010. Annual increases in use of advanced diagnostics were noted in CT (7.8% annual growth), MRI (10%), ultrasound (3.9%), and PET (57%) studies.
Increased CT use over the 15-year study period resulted in increased radiation exposure, doubling mean per capita effective dose (1.2 mSv to 2.3 mSv), as well as those receiving high exposure (1.2% to 2.5%) and very high exposure (0.6% to 1.4%).
The increased imaging use and radiation exposure among HMO enrollees was similar to that of fee-for-service Medicare patients in previous studies.
Bottom line: There is a significant increase in use of diagnostic imaging studies and associated radiation exposure among integrated healthcare system enrollees from 1996 to 2010, similar to patients in fee-for-service health plans.
Citation: Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409.
Postoperative Troponin Predicts 30-Day Mortality
Clinical question: Does postoperative peak troponin level predict 30-day mortality in patients undergoing noncardiac surgery?
Background: The use of postoperative peak troponin levels in predicting 30-day mortality for patients undergoing noncardiac surgery has not been studied extensively. Identifying patients at high risk for death following noncardiac surgery could facilitate appropriate postoperative care and improve survival.
Study design: Prospective cohort study.
Setting: International university and nonuniversity hospitals.
Synopsis: The Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study is a large, international, multicenter, prospective cohort study designed to evaluate the major complications of noncardiac surgery. More than 15,100 patients ages 45 and older requiring at least an overnight hospitalization were enrolled following noncardiac surgery.
Peak troponin measurements during the first three postoperative days of 0.01 ng/ml or less, 0.02 ng/ml, 0.03 ng/ml to 0.29 ng/ml, and 0.3 ng/ml or greater had 30-day mortality rates of 1.0%, 4.0%, 9.3%, and 16.9%, respectively.
This study demonstrates the sensitivity of troponin measurement for predicting postoperative 30-day mortality in patients undergoing noncardiac surgery. The study does not address interventions based on an increased postoperative troponin level. Future studies might investigate postoperative modifiable risk factors.
Bottom line: Postoperative peak troponin level predicts 30-day mortality in patients undergoing noncardiac surgery.
Citation: Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
Clinical Prediction Model of Mortality in Acute Heart Failure
Clinical question: Can a clinical prediction model accurately risk-stratify patients presenting to the ED with acute heart failure?
Background: Accurately prognosticating mortality is essential when determining whether to hospitalize or discharge patients presenting to the ED with acute heart failure. Evidence-based clinical prediction models enable physicians to risk-stratify patients and optimize care.
Study design: Retrospective cohort study.
Setting: Multicenter study of 86 hospitals in Ontario, Canada.
Synopsis: Data collected from 12,591 patients who presented to EDs with acute heart failure in Ontario were analyzed. A clinical prediction model of seven-day mortality of discharged and hospitalized patients was derived and validated. The Emergency Heart Failure Mortality Risk Grade (EHMRG) found an increased mortality based on higher triage heart rate, lower triage systolic blood pressure, initial oxygen saturation, and elevated troponin levels. This model uses readily available data collected in ED visits. The high-risk EHMRG score predicted about 8% seven-day mortality versus 0.3% in the low-risk score.
This model was not applied to chronic heart failure, did not utilize left ventricular function, and does not differentiate between systolic and diastolic heart failure.
Bottom line: The Emergency Heart Failure Mortality Risk Grade predicts seven-day mortality in acute heart failure in the emergent setting.
Citation: Lee DS, Stitt A, Austin PC, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156(11):767-775.
Indwelling Pleural Catheter Is as Effective as Talc Pleurodesis Via Chest Tube in Relieving Dyspnea in Patients with Malignant Pleural Effusion
Clinical question: Is indwelling pleural catheter (IPC) as effective as chest tube and talc pleurodesis (talc) in improving dyspnea from malignant pleural effusion in patients who had no previous pleurodesis?
Background: Despite guidelines recommending chest tube insertion with pleurodesis as a first-line treatment for symptom palliation from malignant pleural effusion, there has been no randomized trial comparing indwelling pleural catheter with chest tube and talc pleurodesis.
Study design: Open-label, randomized controlled trial.
Setting: Seven hospitals in the United Kingdom.
Synopsis: One hundred six patients with malignant pleural effusion were randomized to undergo either IPC or talc treatment, and their daily mean dyspnea was measured. There was a clinically significant improvement of dyspnea in both IPC and talc groups over the first 42 days of the trial, without any significant difference in dyspnea between the two groups. After six months, researchers found a clinically significant decrease in dyspnea in the IPC group compared with the talc group. Chest pain and global quality of life were improved and were similar in both groups throughout the trial period. Length of hospital stay was significantly shorter in the IPC group compared with the talc group, but more patients in the IPC group experienced adverse events.
Bottom line: Indwelling pleural catheter is as effective as talc pleurodesis in reliving dyspnea from malignant pleural effusion; however, IPC is associated with increased adverse events despite shorter length of hospital stay.
Citation: Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383-2389.
Early Surgery Better than Conventional Treatment in High-Risk Native-Valve Endocarditis
Clinical question: Is early cardiac surgery better than conventional treatment for patients with left-sided, native-valve, infective endocarditis?
Background: Although guidelines strongly recommend early surgery for patients with infective endocarditis and congestive heart failure, the timing of surgery for patients with large vegetations and high risk of embolism without heart failure symptoms remains controversial.
Study design: Prospective, randomized trial.
Setting: Two medical centers in South Korea.
Synopsis: Seventy-six patients with left-sided, native-valve, infective endocarditis with a high risk of embolism (defined as vegetation with a diameter greater than 10 mm or severe mitral or aortic valve disease) were randomized to undergo early surgery (within 48 hours of enrollment) or conventional treatment (antibiotic therapy and surgery only if complications required urgent surgery). The primary outcome of composite in-hospital death or
clinical embolic events within six weeks of the trial occurred in only one patient in the early surgery group, compared with nine patients in the conventional group (hazard ratio 0.10, 95% CI, 0.01-0.82, P=0.03).
There was no difference in all-cause mortality at six months between the two groups, but the rate of composite endpoint of death from any cause, embolic events, or recurrence of infective endocarditis at six months was significantly lower in the early surgery group compared with the conventional group.
Bottom line: Early cardiac surgery for patients with left-sided, native-valve infective endocarditis with a high risk of embolism significantly improved the composite outcome of all-cause mortality, embolic events, or recurrence of endocarditis compared with the conventional therapy.
Citation: Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366(26):2466-2473.
Risk Factors for Short-Term Mortality after Emergency Department Visit for Syncope
Clinical question: What are the risk factors for short-term mortality after an ED evaluation for syncope or near-syncope?
Background: Syncope accounts for 1% to 2% of all ED visits and an equal number of hospital admissions. The risk of death after an ED visit for syncope is poorly understood, resulting in frequent hospital admissions.
Study design: Retrospective cohort study.
Setting: EDs in Southern California.
Synopsis: Authors evaluated 23,951 ED visits resulting in syncope as sole primary diagnosis. Age was identified as the most significant risk factor for short-term mortality. Cumulative survival data revealed that more than 1% of patients 60 or older died by 30 days. There were 215 deaths (2.84%) in patients hospitalized from the ED and 66 deaths (0.45%) among patients not hospitalized.
Pre-existing comorbidities significantly associated with increased mortality included heart failure (HR=14.3 in ages 18-53; HR=3.09 in ages 60-79; HR=2.34 in ages 80-plus), diabetes (HR=1.49), seizure (HR=1.65), dementia (HR=1.41), and a recent prior visit for syncope (HR=1.86). The risk of death by 30 days was less than 0.2% in patients under 60 without heart failure and more than 2.5% in patients of all ages with heart failure.
Bottom line: After an ED visit for syncope, patients with a history of heart failure and patients 60 and older have a significantly increased risk of short-term mortality.
Citation: Derose SF, Gabayan GZ, Chiu VY, Sun BC. Patterns and preexisting risk factors of 30-day mortality after a primary discharge diagnosis of syncope or near syncope. Acad Emerg Med. 2012;19(5):488-496.
Acute Hyperglycemia Associated with Increased Mortality in Community-Acquired Pneumonia
Clinical question: In patients admitted to the hospital for community-acquired pneumonia, is serum glucose level on admission associated with mortality?
Background: Some retrospective studies have shown an association between alterations in serum glucose levels or pre-existing diabetes and higher mortality due to infections, while other studies have shown no clear association.
Study design: Multicenter, prospective cohort study.
Setting: Hospitals and private practices in Germany, Switzerland, and Austria.
Synopsis: Prospective data from 6,891 patients were included in the analysis. Patients without diabetes and normal serum glucose levels had the lowest mortality after 90 days. Patients without diabetes but with mild acute hyperglycemia (108 mg/dL to 198 mg/dL) had a significantly increased risk of death at 90 days (HR 1.56), and patients without diabetes but with more severe acute hyperglycemia (over 252 mg/dL) had an even higher risk of death at 90 days (HR 2.37).
The 90-day mortality rate was significantly higher in patients with pre-existing diabetes (HR 2.47), although this was not affected by serum glucose levels on admission.
Bottom line: Acute hyperglycemia, as well as pre-existing diabetes, was associated with an increased risk of 90-day mortality in patients with community acquired pneumonia.
Citation: Lepper PM, Ott S, Nüesch E, et al. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ. 2012;344:e3397.
Hospital Delirium Associated with Cognitive Decline, Institutionalization, and Death
Clinical question: What is the risk of subsequent cognitive decline, institutionalization, or death due to delirium in patients with dementia?
Background: Patients suffering delirium during hospitalization can suffer additional cognitive decline. Whether this is due to additional damage from the delirium state or reflects pre-existing cognitive vulnerability remains uncertain.
Study design: Prospective analysis of a cohort of Alzheimer’s patients.
Setting: Massachusetts community-based disease registry.
Synopsis: The analysis compared nonhospitalized individuals to patients hospitalized with, and without, delirium. In 771 individuals with dementia, at least one adverse outcome (including cognitive decline, institutionalization, or death) occurred in 32% of those not hospitalized, 55% of those hospitalized without delirium, and 79% of those hospitalized with delirium. Even after adjusting for confounders, hospitalization increased the risk for each of the adverse outcomes; the highest risk was in those with delirium.
Among hospitalized patients, the authors estimated 1 in 5 cases of cognitive decline, 1 in 7 institutionalizations, and 1 in 16 deaths were attributable to delirium. Some of the attributed risk could be the result of residual confounding from unmeasured variables, limiting conclusions of causality. Despite these limitations, this study supports the hypothesis that delirium prevention measures could improve important patient outcomes.
Bottom line: Hospitalization is associated with high rates of adverse outcomes in elderly patients with dementia, the worst of which occurs in those who experience delirium.
Citation: Fong TG, Jones RN, Marcantonio ER, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Int Med. 2012;156:848-856.
In Acute Pyelonephritis, a Seven-Day Course of Ciprofloxacin is Effective in Obtaining Clinical Cure
Clinical question: What is the efficacy of ciprofloxacin for seven days compared with 14 days in women with community-acquired acute pyelonephritis?
Background: Community-acquired acute pyelonephritis is a common and sometimes serious infection in women. In an era of increasing antibiotic resistance worldwide, it is prudent to reduce antibiotic utilization. There are limited controlled trials to assess the optimum duration of antibiotic treatment for this common infection.
Study design: Prospective, randomized, double-blind, noninferiority trial.
Setting: Twenty-one infectious-disease centers in Sweden.
Synopsis: Researchers randomly assigned 284 women 18 or older with a presumptive diagnosis of acute pyelonephritis to ciprofloxacin treatment for seven or 14 days. The primary endpoint was clinical and bacteriological cure 10 to 14 days after the completion of the treatment regimen. Short-term clinical cure occurred in 97% of the patients treated for seven days and 96% treated for 14 days. Long-term follow-up showed cumulative efficacy of 93% in each group. Both regimens were well tolerated.
Patients in this study had a low occurrence of complicated (9%) and recurrent (13%) infections. Whether short courses of antibiotics are effective in more complicated infections cannot be ascertained from this study. Also, the high cure rate obtained with a seven-day course of ciprofloxacin should not be extrapolated to other classes of antibiotics. Fluoroquinolones, such as ciprofloxacin, are recommended as first-line agents for empiric oral treatment of acute pyelonephritis if the resistance rate of the uropathogens remains lower than 10%; however, there is growing evidence that E. coli strains are becoming increasingly resistant to ciprofloxacin, limiting its usefulness.
Bottom line: Acute pyelonephritis in women can be treated successfully and safely with a seven-day course of ciprofloxacin, in areas with low ciprofloxacin resistance.
Citation: Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomized, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380:484-490.
Advance Directives in Community Patients with Heart Failure
Clinical question: How prevalent are advance directives in heart-failure patients, and does a completed advance directive decrease end-of-life resource use (hospitalizations, ICU admissions, mechanical ventilation)?
Background: Heart failure is a common chronic and fatal disease. End-of-life care in heart-failure patients is associated with extremely high healthcare utilization. Heart failure guidelines recommend completing advance directives in all patients.
Study design: Population-based longitudinal cohort study.
Setting: Rochester Epidemiology Project in Olmstead County, Minn.
Synopsis: Investigators enrolled 608 patients presenting with heart failure between October 2007 and October 2011. At the time of enrollment, only 41% of the patients had existing advance directives. Independent predictors of advance directive completion included older age, history of malignancy, and renal dysfunction.
After a mean follow-up of 1.8 years, 164 patients (27%) had died. Among those patients, 106 had an advance directive (64.6%) at time of death—75 had an advance directive at the time of enrollment and another 31 completed an advance directive after enrollment.
Twenty-five patients (23.6%) specified DNR/DNI and another 39 (36.8%) denoted limitations on aggressiveness of care if death was imminent. Among the patients who died, 88 (53.7%) were hospitalized in the last month of their life and 50 (30.5%) died in the hospital. There was no difference in hospitalizations between those with an advance directive specifying limits and those who did not specify limits (OR 1.26, 95% CI 0.64-2.48). However, those with an advance directive specifying limits were less frequently mechanically ventilated (OR 0.26, 95% CI 0.06-0.88), and there was a trend toward them being less frequently admitted into the ICU (OR 0.45, 95% CI 0.16-1.29).
Bottom line: Less than half of community patients with heart failure had an advance directive, and many of these failed to address end-of-life decisions. Patients with an advance directive that specified limits in care were less likely to receive mechanical ventilation.
Citation: Dunlay SM, Swetz KM, Mueller PS, Roger VL. Advance directives in community patients with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5:283-289.
Chlorhexidine Bathing Associated with Significant, Sustainable Reductions in Central-Venous-Catheter-Associated Bloodstream Infection
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511.
Simulation Training Improves Lumbar Puncture Skills
Clinical question: What effect does simulation have on lumbar puncture (LP) skills of PGY1 internal-medicine (IM) residents compared with PGY2-4 neurology residents who have not received simulation training?
Background: LPs are common procedures. The American College of General Medical Education does not define competency; neither do the internal-medicine (IM) or neurology board certifications. Simulation can improve skills in many areas but has not been well studied in LPs.
Study design: Pre-test-post-test.
Setting: Northwestern University’s Feinberg School of Medicine in Chicago.
Synopsis: The intervention group included 58 PGY1 IM residents, while the control group was 49 PGY2-to-PGY4 neurology residents. The pre-test consisted of a 21-point checklist. IM residents watched a three-hour video, performed LPs on simulators, and received feedback. The post-test was a clinical skills examination using the checklist. If this exam was failed, the participant practiced and was retested. Neurology residents completed the pre-test and demonstrated an LP using the simulator.
Pre-test passing was achieved by only 2% of IM residents and 6% of neurology residents. Post-test passing was achieved by 95% of the IM residents on the first trial and 100% of IM residents after an hour of additional training. IM mean scores increased to 95.7% from 46.3%, while the mean score of neurology residents was 65.4%.
This study is limited by its single-center nature, as education is variable from center to center. The study evaluated the proficiency on simulators only, and it did not evaluate the proficiency of the participants on patients.
Bottom line: Simulation training improves lumbar puncture skills.
Citation: Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137.
Primary-Care Physicians Do Not Use Publicly Reported Data When Referring Patients to Hospitals
Clinical question: When referring patients with pneumonia to the hospital, what factors do primary-care physicians (PCPs) consider?
Background: Publicly reported data are widely available. Pneumonia has publicly reported quality measures and is a common reason for hospitalization. Fewer PCPs are attending in the hospital due to the hospitalist movement; therefore, PCPs refer patients to a hospital when the need arises.
Study design: Online survey.
Setting: PCPs within 10 miles of Springfield, Mass.
Synopsis: A total of 92 PCPs responded to the survey, which included presentation of a case regarding a patient with pneumonia. PCPs were asked the importance of multiple factors leading to their decision to refer to a hospital. Familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%) were considered to be very important to the PCPs that responded to the survey. Publicly reported data were very important to only 18% of respondents, and zero reported using publicly reported data when referring patients.
Importance of specific quality measures also was queried; antibiotics given within six hours of arrival (66%), appropriate choice of antibiotics (63%), and blood cultures prior to antibiotic administration (51%) were very important to respondents. Prestige, such as magnet status and U.S. News and World Report “Best Hospital” status, were deemed important by about 40% of PCPs.
Bottom line: Despite the availability of publicly reported data, PCPs do not use this information to refer patients to the hospital.
Citation: Morsi E, Lindenauer PK, Rothberg MB. Primary care physicians’ use of publicly reported quality data in hospital referral decisions. J Hosp Med. 2012;7(5):370-375.
What Works for Medication Reconciliation?
Clinical question: What are the most effective practices for medication reconciliation in the hospital setting?
Background: Medication discrepancies are common, occurring in as many as 70% of patients at hospital admission or discharge. Up to a third of these discrepancies have potential to cause patient harm, including prolonged hospital stays, ED visits, hospital recidivism, and use of other healthcare resources. Medication reconciliation (“med rec”) is a strategy for reducing these errors, though previous literature has not systematically reviewed best practices for hospital-based med rec.
Study design: Systematic review of literature.
Setting: Controlled studies from the U.S., Canada, Australia, New Zealand, Northern Ireland, United Kingdom, Belgium, Denmark, the Netherlands, and Sweden.
Synopsis: Investigators identified 26 controlled studies using a systematic search of English-language articles on med rec during inpatient hospitalizations published between Jan. 1, 1966, and Oct. 31, 2010. Fifteen studies reported on pharmacist-related interventions; six reported on technology-specific interventions; and five reported on other types of interventions, including staff education and use of standardized med-rec tools.
Analysis of these studies revealed that all of these interventions successfully decreased medication discrepancies and potential adverse drug events, but there was inconsistent benefit with regard to adverse drug events and healthcare utilization compared with usual care. The literature was most supportive of pharmacist-related interventions, including but not limited to comprehensive medication history at admission, med rec at discharge, patient counseling, discharge communication with outpatient providers, and post-discharge communication with the patient and post-hospital providers.
Bottom line: Successful med rec requires multiple interventions at various transitions of care and involves a variety of medical professionals. Patient-targeted interventions, including pharmacists, have the potential to decrease errors and adverse events.
Citation: Mueller S, Sponsler K, Kripalani S, Schnipper J. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069.