User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Ready for post-acute care?
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
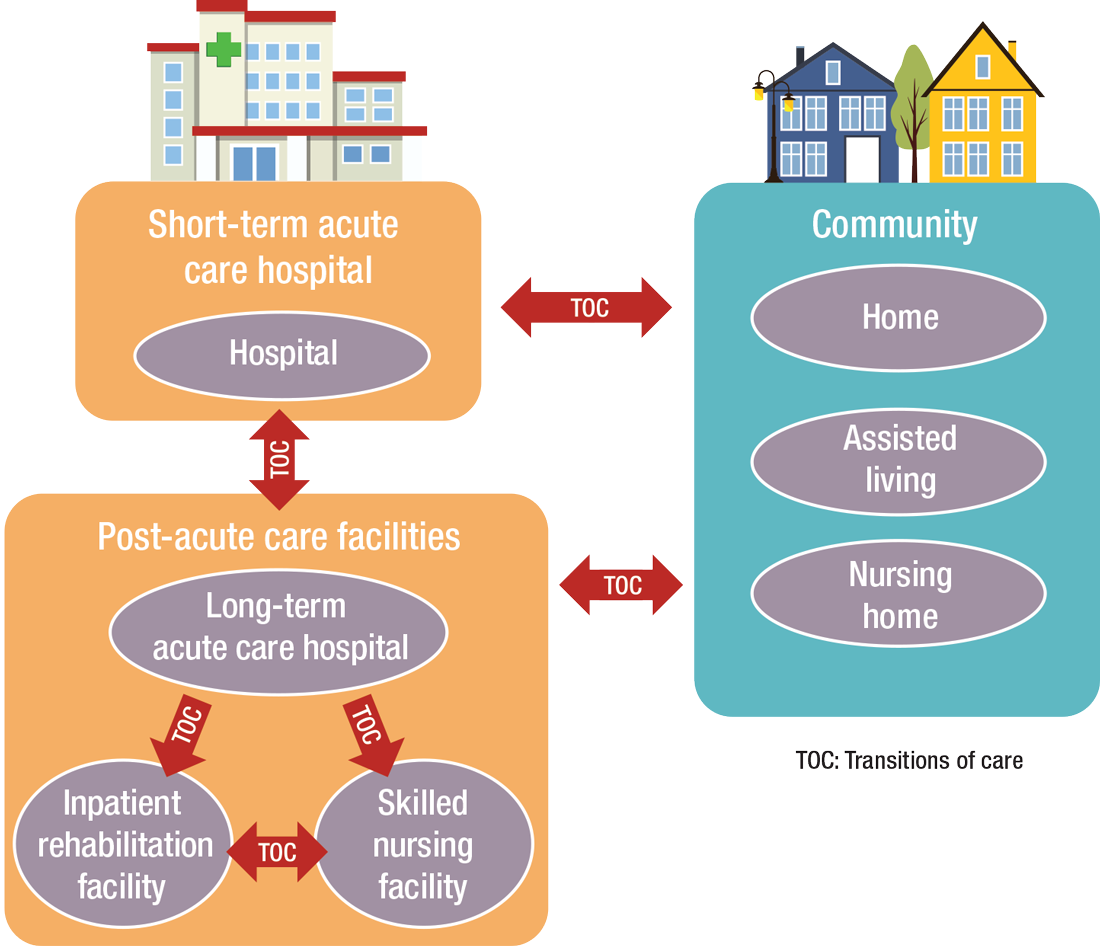
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
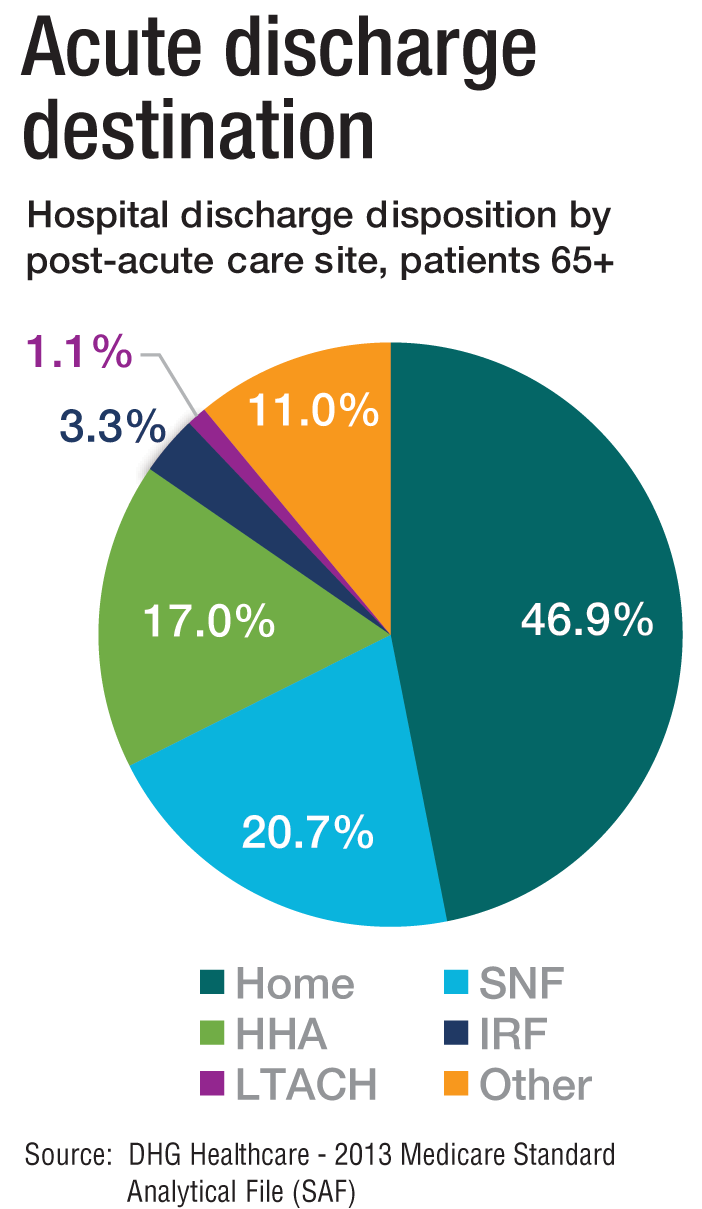
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
Children and COVID: Weekly cases resume their climb
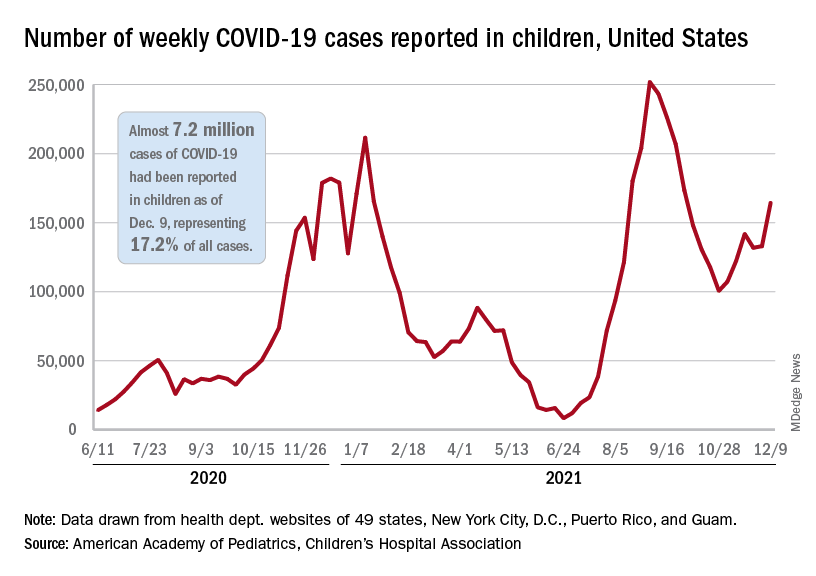
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
Epilepsy linked to 1.5-fold higher COVID-19 mortality in hospital
according to a new study presented at the annual meeting of the American Epilepsy Society. While the findings are preliminary and not yet adjusted for various confounders, the authors say they are a warning sign that patients with epilepsy may face higher risks.
“These findings suggest that epilepsy may be a pre-existing condition that places patients at increased risk for death if hospitalized with a COVID-19 infection. It may offer neurologists guidance when counseling patients on critical preventative measures such as masking, social distancing, and most importantly, vaccination,” lead author Claire Ufongene, a student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
According to Ms. Ufongene, there’s sparse data about COVID-19 outcomes in patients with epilepsy, although she highlighted a 2021 meta-analysis of 13 studies that found a higher risk of severity (odds ratio, 1.69; 95% confidence interval, 1.11-2.59, P = .010) and mortality (OR, 1.71; 95% CI, 1.14-2.56, P = .010).
For the new study, researchers retrospectively tracked identified 334 patients with epilepsy and COVID-19 and 9,499 other patients with COVID-19 from March 15, 2020, to May 17, 2021. All were treated at hospitals within the New York–based Icahn School of Medicine at Mount Sinai.
The groups of patients with and without epilepsy were similar in some ways: 45% and 46%, respectively, were female (P = .674), and their ages were similar (average, 62 years and 65 years, respectively; P = .02). Racial makeup was also similar (non-Hispanic groups made up 27.8% of those with epilepsy and 24.5% of those without; the difference was not statistically significant).
“In addition, more of those with epilepsy were English speaking [83.2% vs. 77.9%] and had Medicaid insurance [50.9% vs. 38.9%], while fewer of those with epilepsy had private insurance [16.2% vs. 25.5%] or were Spanish speaking [14.0% vs. 9.3%],” study coauthor Nathalie Jette, MD, MSc, a neurologist at Icahn School of Medicine at Mount Sinai, said in an interview.
In terms of outcomes, patients with epilepsy were much more likely to need ventilator support (37.7% vs. 14.3%; P < .001), to be admitted to the ICU (39.2% vs. 17.7%; P < .001), and to die in the hospital (29.6% vs. 19.9%; P < .001).
“Most patients we follow in our practices with epilepsy who experienced COVID-19 in general have had symptoms similar to the general population,” Dr. Jette said. “There are rare instances where COVID-19 can result in an exacerbation of seizures in some with pre-existing epilepsy. This is not surprising as infections in particular can decrease the seizure threshold and result in breakthrough seizures in people living with epilepsy.”
Loss of seizure control
How might epilepsy be related to worse outcomes in COVID-19? Andrew Wilner, MD, a neurologist and internist at University of Tennessee Health Science Center, Memphis, who’s familiar with the study findings, said COVID-19 itself may not worsen epilepsy. “Evidence to suggest that COVID-19 directly affects the central nervous system is extremely limited. As such, one would not expect that a COVID-19 infection would cause epilepsy or exacerbate epilepsy,” he said. “However, patients with epilepsy who suffer from infections may be predisposed to decreased seizure control. Consequently, it would not be surprising if patients with epilepsy who also had COVID-19 had loss of seizure control and even status epilepticus, which could adversely affect their hospital course. However, there are no data on this potential phenomenon.”
Dr. Wilner suspected that comorbidities explain the higher mortality in patients with epilepsy. “The findings are probably most useful in that they call attention to the fact that epilepsy patients are more vulnerable to a host of comorbidities and resultant poorer outcomes due to any acute illness.”
As for treatment, Dr. Wilner urged colleagues to make sure that hospitalized patients with epilepsy “continue to receive their antiepileptic medications, which they may no longer be able to take orally. They may need to be switched temporarily to an intravenous formulation.”
In an interview, Selim Benbadis, MD, a neurologist from the University of South Florida, Tampa, suggested that antiseizure medications may play a role in the COVID-19 disease course because they can reduce the efficacy of other medications, although he noted that drug treatments for COVID-19 were limited early on. He recommended that neurologists “avoid old enzyme-inducing seizure medications, as is generally recommended.”
No study funding is reported. The study authors and Dr. Benbadis reported no relevant disclosures. Dr. Wilner is a medical adviser for the epilepsy disease management program for CVS/Health.
according to a new study presented at the annual meeting of the American Epilepsy Society. While the findings are preliminary and not yet adjusted for various confounders, the authors say they are a warning sign that patients with epilepsy may face higher risks.
“These findings suggest that epilepsy may be a pre-existing condition that places patients at increased risk for death if hospitalized with a COVID-19 infection. It may offer neurologists guidance when counseling patients on critical preventative measures such as masking, social distancing, and most importantly, vaccination,” lead author Claire Ufongene, a student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
According to Ms. Ufongene, there’s sparse data about COVID-19 outcomes in patients with epilepsy, although she highlighted a 2021 meta-analysis of 13 studies that found a higher risk of severity (odds ratio, 1.69; 95% confidence interval, 1.11-2.59, P = .010) and mortality (OR, 1.71; 95% CI, 1.14-2.56, P = .010).
For the new study, researchers retrospectively tracked identified 334 patients with epilepsy and COVID-19 and 9,499 other patients with COVID-19 from March 15, 2020, to May 17, 2021. All were treated at hospitals within the New York–based Icahn School of Medicine at Mount Sinai.
The groups of patients with and without epilepsy were similar in some ways: 45% and 46%, respectively, were female (P = .674), and their ages were similar (average, 62 years and 65 years, respectively; P = .02). Racial makeup was also similar (non-Hispanic groups made up 27.8% of those with epilepsy and 24.5% of those without; the difference was not statistically significant).
“In addition, more of those with epilepsy were English speaking [83.2% vs. 77.9%] and had Medicaid insurance [50.9% vs. 38.9%], while fewer of those with epilepsy had private insurance [16.2% vs. 25.5%] or were Spanish speaking [14.0% vs. 9.3%],” study coauthor Nathalie Jette, MD, MSc, a neurologist at Icahn School of Medicine at Mount Sinai, said in an interview.
In terms of outcomes, patients with epilepsy were much more likely to need ventilator support (37.7% vs. 14.3%; P < .001), to be admitted to the ICU (39.2% vs. 17.7%; P < .001), and to die in the hospital (29.6% vs. 19.9%; P < .001).
“Most patients we follow in our practices with epilepsy who experienced COVID-19 in general have had symptoms similar to the general population,” Dr. Jette said. “There are rare instances where COVID-19 can result in an exacerbation of seizures in some with pre-existing epilepsy. This is not surprising as infections in particular can decrease the seizure threshold and result in breakthrough seizures in people living with epilepsy.”
Loss of seizure control
How might epilepsy be related to worse outcomes in COVID-19? Andrew Wilner, MD, a neurologist and internist at University of Tennessee Health Science Center, Memphis, who’s familiar with the study findings, said COVID-19 itself may not worsen epilepsy. “Evidence to suggest that COVID-19 directly affects the central nervous system is extremely limited. As such, one would not expect that a COVID-19 infection would cause epilepsy or exacerbate epilepsy,” he said. “However, patients with epilepsy who suffer from infections may be predisposed to decreased seizure control. Consequently, it would not be surprising if patients with epilepsy who also had COVID-19 had loss of seizure control and even status epilepticus, which could adversely affect their hospital course. However, there are no data on this potential phenomenon.”
Dr. Wilner suspected that comorbidities explain the higher mortality in patients with epilepsy. “The findings are probably most useful in that they call attention to the fact that epilepsy patients are more vulnerable to a host of comorbidities and resultant poorer outcomes due to any acute illness.”
As for treatment, Dr. Wilner urged colleagues to make sure that hospitalized patients with epilepsy “continue to receive their antiepileptic medications, which they may no longer be able to take orally. They may need to be switched temporarily to an intravenous formulation.”
In an interview, Selim Benbadis, MD, a neurologist from the University of South Florida, Tampa, suggested that antiseizure medications may play a role in the COVID-19 disease course because they can reduce the efficacy of other medications, although he noted that drug treatments for COVID-19 were limited early on. He recommended that neurologists “avoid old enzyme-inducing seizure medications, as is generally recommended.”
No study funding is reported. The study authors and Dr. Benbadis reported no relevant disclosures. Dr. Wilner is a medical adviser for the epilepsy disease management program for CVS/Health.
according to a new study presented at the annual meeting of the American Epilepsy Society. While the findings are preliminary and not yet adjusted for various confounders, the authors say they are a warning sign that patients with epilepsy may face higher risks.
“These findings suggest that epilepsy may be a pre-existing condition that places patients at increased risk for death if hospitalized with a COVID-19 infection. It may offer neurologists guidance when counseling patients on critical preventative measures such as masking, social distancing, and most importantly, vaccination,” lead author Claire Ufongene, a student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
According to Ms. Ufongene, there’s sparse data about COVID-19 outcomes in patients with epilepsy, although she highlighted a 2021 meta-analysis of 13 studies that found a higher risk of severity (odds ratio, 1.69; 95% confidence interval, 1.11-2.59, P = .010) and mortality (OR, 1.71; 95% CI, 1.14-2.56, P = .010).
For the new study, researchers retrospectively tracked identified 334 patients with epilepsy and COVID-19 and 9,499 other patients with COVID-19 from March 15, 2020, to May 17, 2021. All were treated at hospitals within the New York–based Icahn School of Medicine at Mount Sinai.
The groups of patients with and without epilepsy were similar in some ways: 45% and 46%, respectively, were female (P = .674), and their ages were similar (average, 62 years and 65 years, respectively; P = .02). Racial makeup was also similar (non-Hispanic groups made up 27.8% of those with epilepsy and 24.5% of those without; the difference was not statistically significant).
“In addition, more of those with epilepsy were English speaking [83.2% vs. 77.9%] and had Medicaid insurance [50.9% vs. 38.9%], while fewer of those with epilepsy had private insurance [16.2% vs. 25.5%] or were Spanish speaking [14.0% vs. 9.3%],” study coauthor Nathalie Jette, MD, MSc, a neurologist at Icahn School of Medicine at Mount Sinai, said in an interview.
In terms of outcomes, patients with epilepsy were much more likely to need ventilator support (37.7% vs. 14.3%; P < .001), to be admitted to the ICU (39.2% vs. 17.7%; P < .001), and to die in the hospital (29.6% vs. 19.9%; P < .001).
“Most patients we follow in our practices with epilepsy who experienced COVID-19 in general have had symptoms similar to the general population,” Dr. Jette said. “There are rare instances where COVID-19 can result in an exacerbation of seizures in some with pre-existing epilepsy. This is not surprising as infections in particular can decrease the seizure threshold and result in breakthrough seizures in people living with epilepsy.”
Loss of seizure control
How might epilepsy be related to worse outcomes in COVID-19? Andrew Wilner, MD, a neurologist and internist at University of Tennessee Health Science Center, Memphis, who’s familiar with the study findings, said COVID-19 itself may not worsen epilepsy. “Evidence to suggest that COVID-19 directly affects the central nervous system is extremely limited. As such, one would not expect that a COVID-19 infection would cause epilepsy or exacerbate epilepsy,” he said. “However, patients with epilepsy who suffer from infections may be predisposed to decreased seizure control. Consequently, it would not be surprising if patients with epilepsy who also had COVID-19 had loss of seizure control and even status epilepticus, which could adversely affect their hospital course. However, there are no data on this potential phenomenon.”
Dr. Wilner suspected that comorbidities explain the higher mortality in patients with epilepsy. “The findings are probably most useful in that they call attention to the fact that epilepsy patients are more vulnerable to a host of comorbidities and resultant poorer outcomes due to any acute illness.”
As for treatment, Dr. Wilner urged colleagues to make sure that hospitalized patients with epilepsy “continue to receive their antiepileptic medications, which they may no longer be able to take orally. They may need to be switched temporarily to an intravenous formulation.”
In an interview, Selim Benbadis, MD, a neurologist from the University of South Florida, Tampa, suggested that antiseizure medications may play a role in the COVID-19 disease course because they can reduce the efficacy of other medications, although he noted that drug treatments for COVID-19 were limited early on. He recommended that neurologists “avoid old enzyme-inducing seizure medications, as is generally recommended.”
No study funding is reported. The study authors and Dr. Benbadis reported no relevant disclosures. Dr. Wilner is a medical adviser for the epilepsy disease management program for CVS/Health.
FROM AES 2021
Treatment of opioid use disorder in hospitalized patients
An opportunity for impact
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.
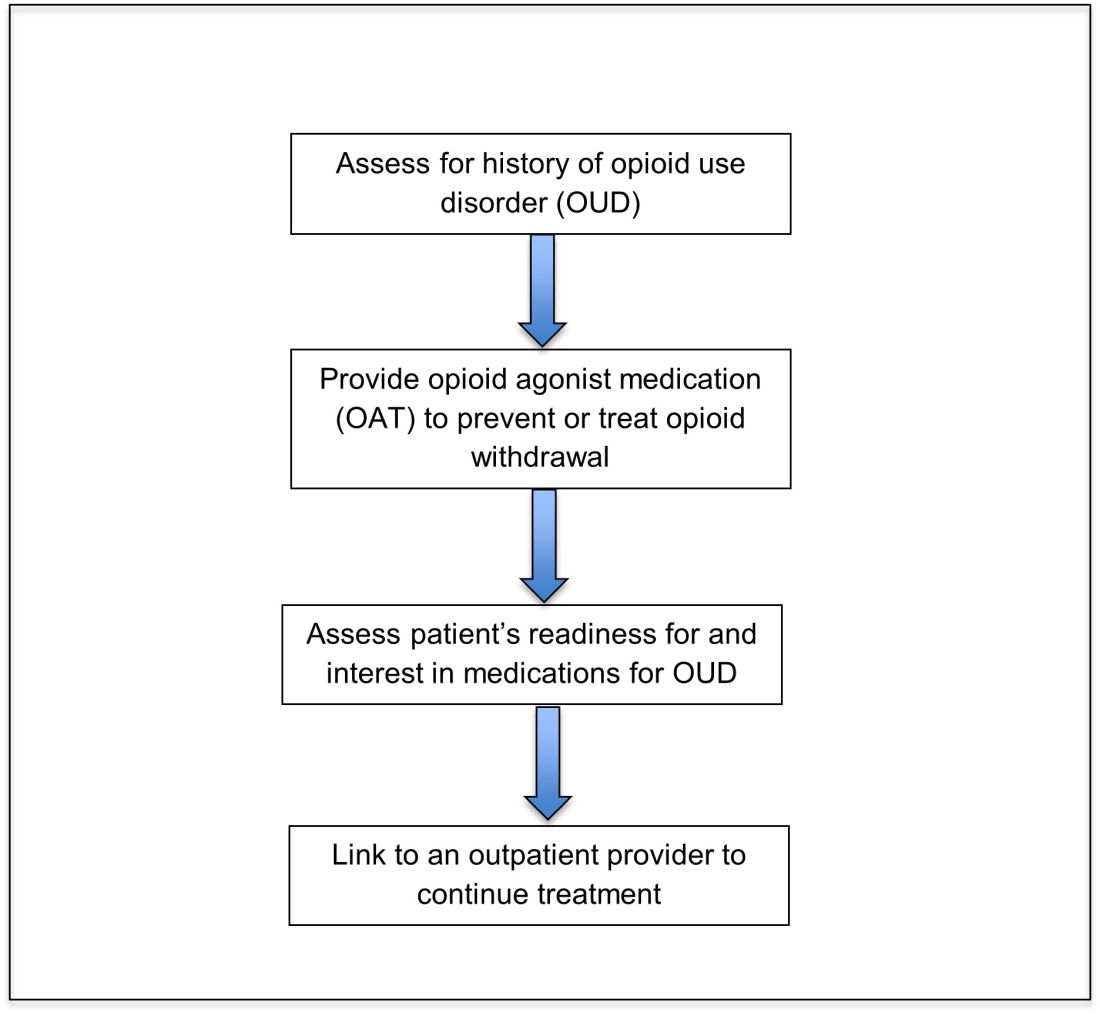
Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.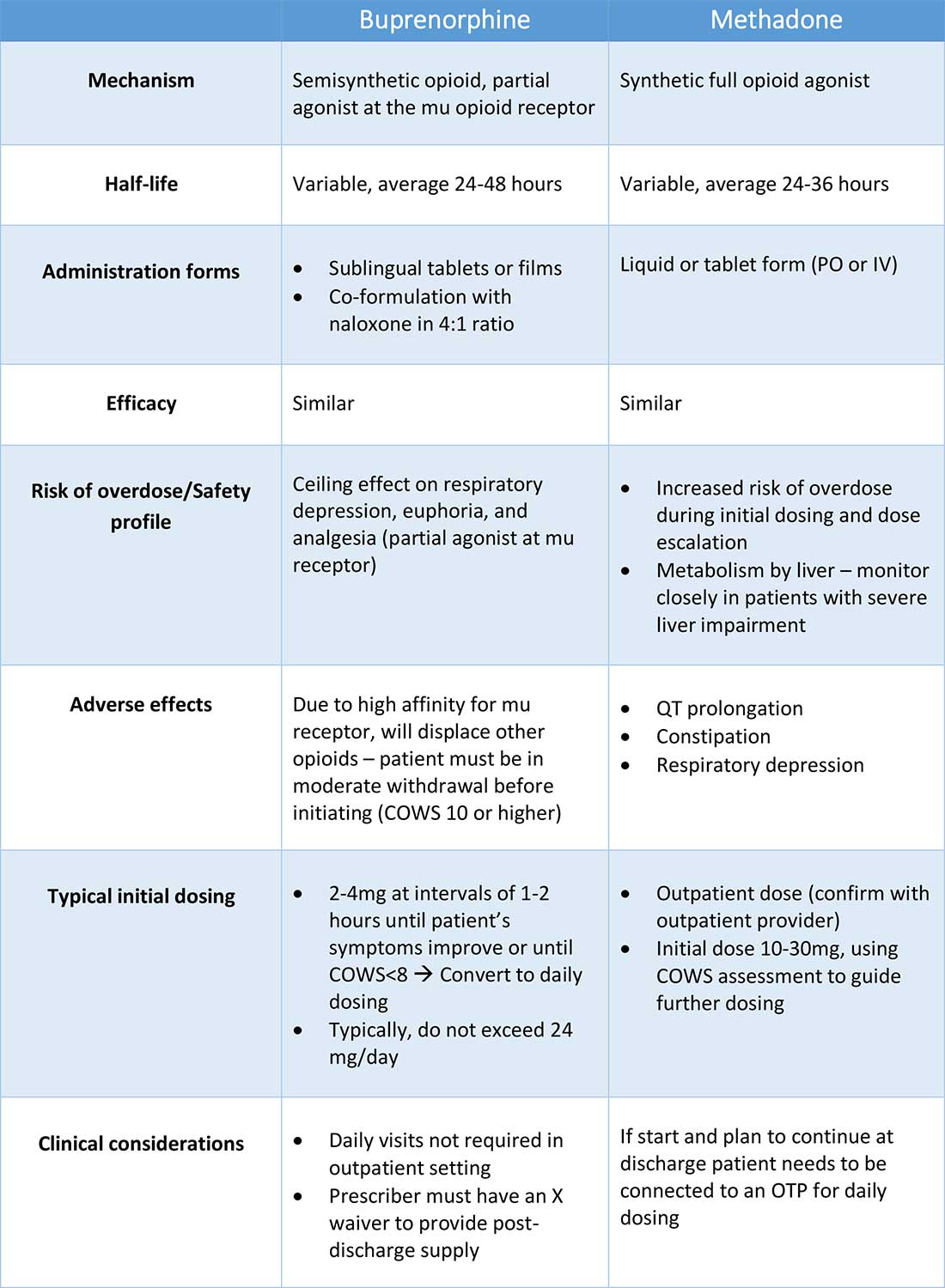
Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
An opportunity for impact
An opportunity for impact
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.

Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.
Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.

Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.
Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
A case-based framework for de-escalating conflict
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
D-dimer thresholds rule out PE in meta-analysis
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
FROM THE ANNALS OF INTERNAL MEDICINE
Does morning discharge really improve hospital throughput?
‘Perennial debate’ likely to be reignited
A recent study published in the Journal of Hospital Medicine examined patient discharges from hospitals in Ontario, Canada, to determine if morning discharges were associated with positive outcomes. Some hospitalist programs have embraced discharge before noon (DBN) initiatives like those studied in the article.1 Unfortunately, the researchers concluded that the Canadian DBNs did not positively impact hospital length of stay, readmissions, or mortality rates.
DBN has been a quality improvement target for hospitals hoping to improve throughput and free up scarce beds, while promoting patient safety by encouraging discharge as soon as patients are ready to leave. Yet other researchers have questioned its actual impact on quality metrics. One author called DBN’s purported impact an “urban legend,”2 while a JHM editorial accompanying the Ontario study noted, “Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others.”3
Might DBN be an artificial target that doesn’t actually enhance throughput, but leads instead to unintended consequences, such as patients being held over for an additional night in the hospital, rather than being discharged when they are ready to go on the afternoon before, in order to boost DBN rates? A perennial debate in hospital medicine is likely to be reignited by the new findings.
‘No significant overall association’
Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creation of deadlines for discharge orders, the new study’s authors noted. Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust.
The Canadian researchers retrospectively reviewed all patient admissions to general internal medicine services (GIMs) – largely similar to hospital medicine services in the United States – at seven hospitals in Toronto and Mississauga over a 7-year period ending Oct. 31, 2017, counting all of these patients who were discharged alive between 8 a.m. and noon. DBN averaged 19% of total live discharges across the diverse hospitals, with their diverse discharge practices.
But they found no significant overall association between morning discharge and hospital or emergency department length of stay. “Our findings suggest that increasing the number of morning discharges alone is unlikely to substantially improve patient throughput in GIM, but further research is needed to determine the effectiveness of specific interventions,” they concluded.
“We used a very narrow lens, looking specifically at throughput for the hospitals and emergency departments and whether DBN makes it more efficient,” said corresponding author Amol Verma, MD, MPhil, FRCPC, clinician-scientist at St. Michael’s Hospital, University of Toronto, in a recent interview. “What we found was that, on days when more patients are discharged in the morning, patients do not flow more quickly through the hospital. That suggests that increasing morning discharges is unlikely to make a difference.”
What does DBN really mean?
The semantics of DBN deserve further exploration. Is DBN about the actual hour of discharge, or the time when the hospitalist signs a discharge order – which may be well before the patient actually gets a wheelchair ride down to the hospital’s front doors? And if DBN is an organized program promoting morning discharges, how is it incentivized or otherwise rewarded?
Other factors, such as arrival of medications from the pharmacy or results from clinical tests, access to an ambulance if needed, transport to the front door, and bed cleaning will impact how quickly a doctor’s discharge orders get acted upon – and how quickly the newly emptied bed is available for the next occupant.
The clinician’s views on discharge practices may diverge from hospital administrator or health system perspectives, with its imperatives for efficient throughput in order to bring in more patients, Dr. Verma said. The hospitalist is also concerned about whether the patient feels ready to go home. “We can all agree that patients should leave the hospital as soon as they are medically able to do so,” he said. Longer hospital stays are associated with increased rates of hospital-acquired infections and other iatrogenic complications.
But there is not agreement on the components of a safe discharge – or on the other dimensions of effective patient flow and transitions of care. How do we optimize treatments initiated in the hospital? Does the patient need one more CAT scan? And what about the concerns of patient-centered care? Does the patient have a caregiver able to help them when they get home? There is a lot of uncertainty, Dr. Verma said. “These kinds of decisions have to get made many times every day by hospitalists,” he noted.
“We find ourselves trying to mirror the ebbs and flows of the emergency department with what’s happening in the hospital,” said Venkat Gundareddy, MBBS, MPH, associate director of the division of hospital medicine at Johns Hopkins Medicine in Baltimore. “The majority of hospital discharges happen during business hours, but the emergency department doesn’t stop admitting overnight, thus creating a throughput challenge.” Discharges are also based on clinical outcomes and on patients transferring to other facilities that prefer patients to arrive earlier in the day.
“Hospitalists may not fully appreciate these dynamics, because we’re siloed on our units,” Dr. Gundareddy said. “There is a subset of patients who would fit the bill for early discharge, but other patients come into the hospital with greater complexities, and a need for more coordination. Their discharges are harder to predict, although it gets clearer as their care progresses.”
The hospitals included in the Ontario study are at 90% -100% capacity, so their flexibility is constrained and throughput is a critical issue, Dr. Verma said. “But if you start with the target of more efficient throughput, there is no logical or practical reason to assume that discharge before noon would help. If we believe someone is ready for discharge based on physiologic changes, their response to treatment, and the conclusion of medical investigations, none of these conform to the clock. It’s equally likely the patient achieves them in the afternoon or evening.”
Other views on morning discharge
An alternative perspective comes from New York University’s Langone Medical Center, which has published positive results, including earlier subsequent arrivals to the inpatient unit from the emergency department, from increasing its hospital’s DBN rate.4
The hospital has continued to encourage morning discharges, which have consistently run 35%-40% or more of total discharges on two acute inpatient units at Langone’s Tisch Hospital. A previous study described the multidisciplinary intervention that resulted in a statistically significant increase in DBN – from 11% to 38% in the first 13 months – while significantly reducing high-frequency admission peaks.5
“We’ve been doing DBN for a number of years,” said Benjamin Wertheimer, MD, a hospitalist at Langone Medical Center and one of the studies’ authors. It is an achievable – and sustainable – goal. “Many hospitals around the country have problems with the flow of patients. Many hospitals are full – even before accounting for the COVID pandemic.” There is good evidence that, for a patient who no longer requires hospitalization, getting them out as early as possible, with a safe plan for their discharge, is a good thing, he said. “We see DBN as an important operational metric.”
If the necessary work is done correctly on the afternoon before the discharge, then a DBN approach can push communication, coordination, and advance planning, Dr Wertheimer said. Otherwise, essential discharge tasks may lag until the last minute. “We try to put the pieces in place the day before through a better planned process. But it should never be that DBN takes precedence over when the patient is safely ready to go,” he said.
“Our true measure of success would be how well we are preparing, communicating, putting safe plans into place,” he added. “DBN does not in and of itself answer all the safety and quality concerns. We set priorities around specific quality targets. DBN is just one of our operational and safety measures.”
The DBN intervention at Langone started with a multidisciplinary kickoff event in which all team members received education on its importance, a clear description of roles in the DBN process, and a corresponding checklist of daily responsibilities. The checklist was utilized at newly implemented afternoon interdisciplinary rounds, scripted to identify next-day DBNs, and make sure everything is in place for them, he explained.
“We provide daily feedback to floor staff on the DBN percentage, celebrate success, and offer real-time opportunities for case review,” Dr. Wertheimer said. “We have been careful about how we message this goal. Quality and safety come first, and we want to be prepared for discharge in advance of when the patient is ready.”
A boost for discharges
Mark Williams, MD, MHM, recently appointed chief of hospital medicine at Washington University School of Medicine in St. Louis, and a principal investigator for Project BOOST (Better Outcomes by Optimizing Safe Transitions), SHM’s quality improvement mentoring initiative aimed at helping hospitals improve care transitions, said that debates about DBN have gone on for a long time in hospital medicine.
“Around 2002, consultants told the CEO of a community hospital affiliated with Emory Healthcare that if our hospitalists could discharge patients before noon it would improve throughput,” he recalled. The consultants came from the hospitality industry, where DBN is easier to achieve.
But in hospital medicine, he said, “We use the whole day of the discharge in delivering care. I said to the CEO, ‘I can get you 100% discharge before noon – I’ll just hold the patients overnight,’” he explained. “In our initial experience, we pushed DBN up to about 10% -15%, and it opened up a few beds, which rapidly filled.”
Project BOOST encouraged the goal of getting patients ready to go out as soon as they were clinically ready, but did not advocate specifically for DBN, Dr. Williams said. “The problem is that hospital throughput starts to gum up when occupancy goes over 80% or 90%, and many academic medical centers regularly reach occupancy rates greater than 100%, particularly in the afternoon.” The deluge of patients includes transfers from other hospitals, postsurgical patients, and admissions from the emergency department.
“Boarding in the ED is a real issue,” he said. “Right now, it’s a crisis of overoccupancy, and the problem is that the pipeline is pouring patients into the system faster than they can be discharged.”
Dr. Williams believes there needs to be bigger thinking about these issues. Could hospitals, health systems, and hospitalists practice more preventive medicine so that some of these patients don’t need to come to the hospital? “Can you better address high blood pressure to prevent strokes and make sure patients with heart disease risk factors are enrolled in exercise and nutrition programs? What about access to healthy foods and the other social determinants of health? What if we provided adequate, consistent housing and transportation to medical visits?” he wondered.
Hospital at home programs may also offer some relief, he said. “If suddenly there weren’t so many emergency room visits by patients who need to get admitted, we’d have enough beds in the hospital.”
A more holistic view
John Nelson, MD, MHM, hospital medicine pioneer and management consultant, has been studying hospital throughput and policies to improve it for a long time. His 2010 column in The Hospitalist, “The Earlier the Better,” said attaching a financial incentive for hospitalists to discharge patients by a preset hour has produced mixed results.6 But Dr. Nelson offered some easy steps hospitalists can take to maximize earlier discharges, including to write “probable discharge tomorrow” as an order in the patient’s medical record.
The afternoon before a planned discharge, the hospitalist could talk to a patient’s family members about the discharge plan and order any outstanding tests to be done that evening to be ready for morning rounds – which he suggested should start by 7:00 a.m. The hospitalist could dictate the discharge summary the afternoon before. Even if a discharge can’t proceed as planned, the time isn’t necessarily wasted.
In a recent interview, Dr. Nelson noted that the movement to reduce average length of stay in the hospital has complicated the discharge picture by reducing a hospital’s flexibility. But he added that it’s still worth tracking and collecting data on discharge times, and to keep the conversation going. “Just don’t lose sight of the real goal, which is not DBN but optimal length-of-stay management,” he said.
Dr. Gundareddy said that, as his group has dealt with these issues, some steps have emerged to help manage discharges and throughput. “We didn’t have case management and social work services over the weekend, but when we added that support, it changed how our Mondays went.”
He encourages hospitalists to focus on the actual processes that create bottlenecks preventing throughput. “A good example of effective restructuring is lab testing. It’s amazing to think that you could have lab test results available for 7:00 a.m. rounds. There are areas that deserve more attention and more research regarding DBN. What is the impact of discharge before noon programs on the patients who aren’t being planned for discharge that day? Do they get neglected? I feel that happens sometimes.”
The COVID pandemic has further complicated these questions, Dr. Gundareddy said. “Early on in the pandemic, we were unsure how things were going with discharges, since all of the focus was on the COVID crisis. A lot of outpatient and surgical services came to a standstill, and there weren’t enough of the right kinds of beds for COVID patients. It was hard to align staff appropriately with the new clinical goals and to train them during the crisis.” Now, patients who delayed care during the pandemic are turning up at the hospital with greater acuity.
As with all incentives, DBN can have unintended consequences – especially if you monetize the practice, Dr. Verma said. “Most hospitalists are already working so hard – making so many decisions every day. These incentives could push decisions that aren’t in anybody’s best interests.”
Various groups have created comprehensive packages of protocols for improving transitions of care, he said. Organized programs to maximize efficiency of transitions and patient flow, including Project BOOST and Project RED (Re-Engineered Discharge) at Boston University Medical Center, are important sources of tools and resources. “But we should stop flogging hospitalists to discharge patients before noon,” Dr. Verma said, “Discharge is more complex than that. Instead, we should work to improve discharges in more holistic ways.”
References
1. Kirubarajan A et al. Morning discharges and patient length of stay in inpatient general internal medicine. J Hosp Med. 2021 Jun;16(6):333-8. doi: 10.12788/jhm.3605.
2. Shine D. Discharge before noon: An urban legend. Am J Med. 2015 May;128(5):445-6. doi:10.1016/j.amjmed.2014.12.011.
3. Zorian A et al. Discharge by noon: Toward a better understanding of benefits and costs. J Hosp Med. 2021 Jun;16(6):384. doi: 10.12788/jhm.3613.
4. Wertheimer B et al. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015 Oct;10(10):664-9. doi: 10.1002/jhm.2412.
5. Wertheimer B et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014 Apr;9(4):210-4. doi: 10.1002/jhm.2154.
6. Nelson J. The earlier, the better. The Hospitalist. 2010 May.
‘Perennial debate’ likely to be reignited
‘Perennial debate’ likely to be reignited
A recent study published in the Journal of Hospital Medicine examined patient discharges from hospitals in Ontario, Canada, to determine if morning discharges were associated with positive outcomes. Some hospitalist programs have embraced discharge before noon (DBN) initiatives like those studied in the article.1 Unfortunately, the researchers concluded that the Canadian DBNs did not positively impact hospital length of stay, readmissions, or mortality rates.
DBN has been a quality improvement target for hospitals hoping to improve throughput and free up scarce beds, while promoting patient safety by encouraging discharge as soon as patients are ready to leave. Yet other researchers have questioned its actual impact on quality metrics. One author called DBN’s purported impact an “urban legend,”2 while a JHM editorial accompanying the Ontario study noted, “Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others.”3
Might DBN be an artificial target that doesn’t actually enhance throughput, but leads instead to unintended consequences, such as patients being held over for an additional night in the hospital, rather than being discharged when they are ready to go on the afternoon before, in order to boost DBN rates? A perennial debate in hospital medicine is likely to be reignited by the new findings.
‘No significant overall association’
Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creation of deadlines for discharge orders, the new study’s authors noted. Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust.
The Canadian researchers retrospectively reviewed all patient admissions to general internal medicine services (GIMs) – largely similar to hospital medicine services in the United States – at seven hospitals in Toronto and Mississauga over a 7-year period ending Oct. 31, 2017, counting all of these patients who were discharged alive between 8 a.m. and noon. DBN averaged 19% of total live discharges across the diverse hospitals, with their diverse discharge practices.
But they found no significant overall association between morning discharge and hospital or emergency department length of stay. “Our findings suggest that increasing the number of morning discharges alone is unlikely to substantially improve patient throughput in GIM, but further research is needed to determine the effectiveness of specific interventions,” they concluded.
“We used a very narrow lens, looking specifically at throughput for the hospitals and emergency departments and whether DBN makes it more efficient,” said corresponding author Amol Verma, MD, MPhil, FRCPC, clinician-scientist at St. Michael’s Hospital, University of Toronto, in a recent interview. “What we found was that, on days when more patients are discharged in the morning, patients do not flow more quickly through the hospital. That suggests that increasing morning discharges is unlikely to make a difference.”
What does DBN really mean?
The semantics of DBN deserve further exploration. Is DBN about the actual hour of discharge, or the time when the hospitalist signs a discharge order – which may be well before the patient actually gets a wheelchair ride down to the hospital’s front doors? And if DBN is an organized program promoting morning discharges, how is it incentivized or otherwise rewarded?
Other factors, such as arrival of medications from the pharmacy or results from clinical tests, access to an ambulance if needed, transport to the front door, and bed cleaning will impact how quickly a doctor’s discharge orders get acted upon – and how quickly the newly emptied bed is available for the next occupant.
The clinician’s views on discharge practices may diverge from hospital administrator or health system perspectives, with its imperatives for efficient throughput in order to bring in more patients, Dr. Verma said. The hospitalist is also concerned about whether the patient feels ready to go home. “We can all agree that patients should leave the hospital as soon as they are medically able to do so,” he said. Longer hospital stays are associated with increased rates of hospital-acquired infections and other iatrogenic complications.
But there is not agreement on the components of a safe discharge – or on the other dimensions of effective patient flow and transitions of care. How do we optimize treatments initiated in the hospital? Does the patient need one more CAT scan? And what about the concerns of patient-centered care? Does the patient have a caregiver able to help them when they get home? There is a lot of uncertainty, Dr. Verma said. “These kinds of decisions have to get made many times every day by hospitalists,” he noted.
“We find ourselves trying to mirror the ebbs and flows of the emergency department with what’s happening in the hospital,” said Venkat Gundareddy, MBBS, MPH, associate director of the division of hospital medicine at Johns Hopkins Medicine in Baltimore. “The majority of hospital discharges happen during business hours, but the emergency department doesn’t stop admitting overnight, thus creating a throughput challenge.” Discharges are also based on clinical outcomes and on patients transferring to other facilities that prefer patients to arrive earlier in the day.
“Hospitalists may not fully appreciate these dynamics, because we’re siloed on our units,” Dr. Gundareddy said. “There is a subset of patients who would fit the bill for early discharge, but other patients come into the hospital with greater complexities, and a need for more coordination. Their discharges are harder to predict, although it gets clearer as their care progresses.”
The hospitals included in the Ontario study are at 90% -100% capacity, so their flexibility is constrained and throughput is a critical issue, Dr. Verma said. “But if you start with the target of more efficient throughput, there is no logical or practical reason to assume that discharge before noon would help. If we believe someone is ready for discharge based on physiologic changes, their response to treatment, and the conclusion of medical investigations, none of these conform to the clock. It’s equally likely the patient achieves them in the afternoon or evening.”
Other views on morning discharge
An alternative perspective comes from New York University’s Langone Medical Center, which has published positive results, including earlier subsequent arrivals to the inpatient unit from the emergency department, from increasing its hospital’s DBN rate.4
The hospital has continued to encourage morning discharges, which have consistently run 35%-40% or more of total discharges on two acute inpatient units at Langone’s Tisch Hospital. A previous study described the multidisciplinary intervention that resulted in a statistically significant increase in DBN – from 11% to 38% in the first 13 months – while significantly reducing high-frequency admission peaks.5
“We’ve been doing DBN for a number of years,” said Benjamin Wertheimer, MD, a hospitalist at Langone Medical Center and one of the studies’ authors. It is an achievable – and sustainable – goal. “Many hospitals around the country have problems with the flow of patients. Many hospitals are full – even before accounting for the COVID pandemic.” There is good evidence that, for a patient who no longer requires hospitalization, getting them out as early as possible, with a safe plan for their discharge, is a good thing, he said. “We see DBN as an important operational metric.”
If the necessary work is done correctly on the afternoon before the discharge, then a DBN approach can push communication, coordination, and advance planning, Dr Wertheimer said. Otherwise, essential discharge tasks may lag until the last minute. “We try to put the pieces in place the day before through a better planned process. But it should never be that DBN takes precedence over when the patient is safely ready to go,” he said.
“Our true measure of success would be how well we are preparing, communicating, putting safe plans into place,” he added. “DBN does not in and of itself answer all the safety and quality concerns. We set priorities around specific quality targets. DBN is just one of our operational and safety measures.”
The DBN intervention at Langone started with a multidisciplinary kickoff event in which all team members received education on its importance, a clear description of roles in the DBN process, and a corresponding checklist of daily responsibilities. The checklist was utilized at newly implemented afternoon interdisciplinary rounds, scripted to identify next-day DBNs, and make sure everything is in place for them, he explained.
“We provide daily feedback to floor staff on the DBN percentage, celebrate success, and offer real-time opportunities for case review,” Dr. Wertheimer said. “We have been careful about how we message this goal. Quality and safety come first, and we want to be prepared for discharge in advance of when the patient is ready.”
A boost for discharges
Mark Williams, MD, MHM, recently appointed chief of hospital medicine at Washington University School of Medicine in St. Louis, and a principal investigator for Project BOOST (Better Outcomes by Optimizing Safe Transitions), SHM’s quality improvement mentoring initiative aimed at helping hospitals improve care transitions, said that debates about DBN have gone on for a long time in hospital medicine.
“Around 2002, consultants told the CEO of a community hospital affiliated with Emory Healthcare that if our hospitalists could discharge patients before noon it would improve throughput,” he recalled. The consultants came from the hospitality industry, where DBN is easier to achieve.
But in hospital medicine, he said, “We use the whole day of the discharge in delivering care. I said to the CEO, ‘I can get you 100% discharge before noon – I’ll just hold the patients overnight,’” he explained. “In our initial experience, we pushed DBN up to about 10% -15%, and it opened up a few beds, which rapidly filled.”
Project BOOST encouraged the goal of getting patients ready to go out as soon as they were clinically ready, but did not advocate specifically for DBN, Dr. Williams said. “The problem is that hospital throughput starts to gum up when occupancy goes over 80% or 90%, and many academic medical centers regularly reach occupancy rates greater than 100%, particularly in the afternoon.” The deluge of patients includes transfers from other hospitals, postsurgical patients, and admissions from the emergency department.
“Boarding in the ED is a real issue,” he said. “Right now, it’s a crisis of overoccupancy, and the problem is that the pipeline is pouring patients into the system faster than they can be discharged.”
Dr. Williams believes there needs to be bigger thinking about these issues. Could hospitals, health systems, and hospitalists practice more preventive medicine so that some of these patients don’t need to come to the hospital? “Can you better address high blood pressure to prevent strokes and make sure patients with heart disease risk factors are enrolled in exercise and nutrition programs? What about access to healthy foods and the other social determinants of health? What if we provided adequate, consistent housing and transportation to medical visits?” he wondered.
Hospital at home programs may also offer some relief, he said. “If suddenly there weren’t so many emergency room visits by patients who need to get admitted, we’d have enough beds in the hospital.”
A more holistic view
John Nelson, MD, MHM, hospital medicine pioneer and management consultant, has been studying hospital throughput and policies to improve it for a long time. His 2010 column in The Hospitalist, “The Earlier the Better,” said attaching a financial incentive for hospitalists to discharge patients by a preset hour has produced mixed results.6 But Dr. Nelson offered some easy steps hospitalists can take to maximize earlier discharges, including to write “probable discharge tomorrow” as an order in the patient’s medical record.
The afternoon before a planned discharge, the hospitalist could talk to a patient’s family members about the discharge plan and order any outstanding tests to be done that evening to be ready for morning rounds – which he suggested should start by 7:00 a.m. The hospitalist could dictate the discharge summary the afternoon before. Even if a discharge can’t proceed as planned, the time isn’t necessarily wasted.
In a recent interview, Dr. Nelson noted that the movement to reduce average length of stay in the hospital has complicated the discharge picture by reducing a hospital’s flexibility. But he added that it’s still worth tracking and collecting data on discharge times, and to keep the conversation going. “Just don’t lose sight of the real goal, which is not DBN but optimal length-of-stay management,” he said.
Dr. Gundareddy said that, as his group has dealt with these issues, some steps have emerged to help manage discharges and throughput. “We didn’t have case management and social work services over the weekend, but when we added that support, it changed how our Mondays went.”
He encourages hospitalists to focus on the actual processes that create bottlenecks preventing throughput. “A good example of effective restructuring is lab testing. It’s amazing to think that you could have lab test results available for 7:00 a.m. rounds. There are areas that deserve more attention and more research regarding DBN. What is the impact of discharge before noon programs on the patients who aren’t being planned for discharge that day? Do they get neglected? I feel that happens sometimes.”
The COVID pandemic has further complicated these questions, Dr. Gundareddy said. “Early on in the pandemic, we were unsure how things were going with discharges, since all of the focus was on the COVID crisis. A lot of outpatient and surgical services came to a standstill, and there weren’t enough of the right kinds of beds for COVID patients. It was hard to align staff appropriately with the new clinical goals and to train them during the crisis.” Now, patients who delayed care during the pandemic are turning up at the hospital with greater acuity.
As with all incentives, DBN can have unintended consequences – especially if you monetize the practice, Dr. Verma said. “Most hospitalists are already working so hard – making so many decisions every day. These incentives could push decisions that aren’t in anybody’s best interests.”
Various groups have created comprehensive packages of protocols for improving transitions of care, he said. Organized programs to maximize efficiency of transitions and patient flow, including Project BOOST and Project RED (Re-Engineered Discharge) at Boston University Medical Center, are important sources of tools and resources. “But we should stop flogging hospitalists to discharge patients before noon,” Dr. Verma said, “Discharge is more complex than that. Instead, we should work to improve discharges in more holistic ways.”
References
1. Kirubarajan A et al. Morning discharges and patient length of stay in inpatient general internal medicine. J Hosp Med. 2021 Jun;16(6):333-8. doi: 10.12788/jhm.3605.
2. Shine D. Discharge before noon: An urban legend. Am J Med. 2015 May;128(5):445-6. doi:10.1016/j.amjmed.2014.12.011.
3. Zorian A et al. Discharge by noon: Toward a better understanding of benefits and costs. J Hosp Med. 2021 Jun;16(6):384. doi: 10.12788/jhm.3613.
4. Wertheimer B et al. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015 Oct;10(10):664-9. doi: 10.1002/jhm.2412.
5. Wertheimer B et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014 Apr;9(4):210-4. doi: 10.1002/jhm.2154.
6. Nelson J. The earlier, the better. The Hospitalist. 2010 May.
A recent study published in the Journal of Hospital Medicine examined patient discharges from hospitals in Ontario, Canada, to determine if morning discharges were associated with positive outcomes. Some hospitalist programs have embraced discharge before noon (DBN) initiatives like those studied in the article.1 Unfortunately, the researchers concluded that the Canadian DBNs did not positively impact hospital length of stay, readmissions, or mortality rates.
DBN has been a quality improvement target for hospitals hoping to improve throughput and free up scarce beds, while promoting patient safety by encouraging discharge as soon as patients are ready to leave. Yet other researchers have questioned its actual impact on quality metrics. One author called DBN’s purported impact an “urban legend,”2 while a JHM editorial accompanying the Ontario study noted, “Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others.”3
Might DBN be an artificial target that doesn’t actually enhance throughput, but leads instead to unintended consequences, such as patients being held over for an additional night in the hospital, rather than being discharged when they are ready to go on the afternoon before, in order to boost DBN rates? A perennial debate in hospital medicine is likely to be reignited by the new findings.
‘No significant overall association’
Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creation of deadlines for discharge orders, the new study’s authors noted. Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust.
The Canadian researchers retrospectively reviewed all patient admissions to general internal medicine services (GIMs) – largely similar to hospital medicine services in the United States – at seven hospitals in Toronto and Mississauga over a 7-year period ending Oct. 31, 2017, counting all of these patients who were discharged alive between 8 a.m. and noon. DBN averaged 19% of total live discharges across the diverse hospitals, with their diverse discharge practices.
But they found no significant overall association between morning discharge and hospital or emergency department length of stay. “Our findings suggest that increasing the number of morning discharges alone is unlikely to substantially improve patient throughput in GIM, but further research is needed to determine the effectiveness of specific interventions,” they concluded.
“We used a very narrow lens, looking specifically at throughput for the hospitals and emergency departments and whether DBN makes it more efficient,” said corresponding author Amol Verma, MD, MPhil, FRCPC, clinician-scientist at St. Michael’s Hospital, University of Toronto, in a recent interview. “What we found was that, on days when more patients are discharged in the morning, patients do not flow more quickly through the hospital. That suggests that increasing morning discharges is unlikely to make a difference.”
What does DBN really mean?
The semantics of DBN deserve further exploration. Is DBN about the actual hour of discharge, or the time when the hospitalist signs a discharge order – which may be well before the patient actually gets a wheelchair ride down to the hospital’s front doors? And if DBN is an organized program promoting morning discharges, how is it incentivized or otherwise rewarded?
Other factors, such as arrival of medications from the pharmacy or results from clinical tests, access to an ambulance if needed, transport to the front door, and bed cleaning will impact how quickly a doctor’s discharge orders get acted upon – and how quickly the newly emptied bed is available for the next occupant.
The clinician’s views on discharge practices may diverge from hospital administrator or health system perspectives, with its imperatives for efficient throughput in order to bring in more patients, Dr. Verma said. The hospitalist is also concerned about whether the patient feels ready to go home. “We can all agree that patients should leave the hospital as soon as they are medically able to do so,” he said. Longer hospital stays are associated with increased rates of hospital-acquired infections and other iatrogenic complications.
But there is not agreement on the components of a safe discharge – or on the other dimensions of effective patient flow and transitions of care. How do we optimize treatments initiated in the hospital? Does the patient need one more CAT scan? And what about the concerns of patient-centered care? Does the patient have a caregiver able to help them when they get home? There is a lot of uncertainty, Dr. Verma said. “These kinds of decisions have to get made many times every day by hospitalists,” he noted.
“We find ourselves trying to mirror the ebbs and flows of the emergency department with what’s happening in the hospital,” said Venkat Gundareddy, MBBS, MPH, associate director of the division of hospital medicine at Johns Hopkins Medicine in Baltimore. “The majority of hospital discharges happen during business hours, but the emergency department doesn’t stop admitting overnight, thus creating a throughput challenge.” Discharges are also based on clinical outcomes and on patients transferring to other facilities that prefer patients to arrive earlier in the day.
“Hospitalists may not fully appreciate these dynamics, because we’re siloed on our units,” Dr. Gundareddy said. “There is a subset of patients who would fit the bill for early discharge, but other patients come into the hospital with greater complexities, and a need for more coordination. Their discharges are harder to predict, although it gets clearer as their care progresses.”
The hospitals included in the Ontario study are at 90% -100% capacity, so their flexibility is constrained and throughput is a critical issue, Dr. Verma said. “But if you start with the target of more efficient throughput, there is no logical or practical reason to assume that discharge before noon would help. If we believe someone is ready for discharge based on physiologic changes, their response to treatment, and the conclusion of medical investigations, none of these conform to the clock. It’s equally likely the patient achieves them in the afternoon or evening.”
Other views on morning discharge
An alternative perspective comes from New York University’s Langone Medical Center, which has published positive results, including earlier subsequent arrivals to the inpatient unit from the emergency department, from increasing its hospital’s DBN rate.4
The hospital has continued to encourage morning discharges, which have consistently run 35%-40% or more of total discharges on two acute inpatient units at Langone’s Tisch Hospital. A previous study described the multidisciplinary intervention that resulted in a statistically significant increase in DBN – from 11% to 38% in the first 13 months – while significantly reducing high-frequency admission peaks.5
“We’ve been doing DBN for a number of years,” said Benjamin Wertheimer, MD, a hospitalist at Langone Medical Center and one of the studies’ authors. It is an achievable – and sustainable – goal. “Many hospitals around the country have problems with the flow of patients. Many hospitals are full – even before accounting for the COVID pandemic.” There is good evidence that, for a patient who no longer requires hospitalization, getting them out as early as possible, with a safe plan for their discharge, is a good thing, he said. “We see DBN as an important operational metric.”
If the necessary work is done correctly on the afternoon before the discharge, then a DBN approach can push communication, coordination, and advance planning, Dr Wertheimer said. Otherwise, essential discharge tasks may lag until the last minute. “We try to put the pieces in place the day before through a better planned process. But it should never be that DBN takes precedence over when the patient is safely ready to go,” he said.
“Our true measure of success would be how well we are preparing, communicating, putting safe plans into place,” he added. “DBN does not in and of itself answer all the safety and quality concerns. We set priorities around specific quality targets. DBN is just one of our operational and safety measures.”
The DBN intervention at Langone started with a multidisciplinary kickoff event in which all team members received education on its importance, a clear description of roles in the DBN process, and a corresponding checklist of daily responsibilities. The checklist was utilized at newly implemented afternoon interdisciplinary rounds, scripted to identify next-day DBNs, and make sure everything is in place for them, he explained.
“We provide daily feedback to floor staff on the DBN percentage, celebrate success, and offer real-time opportunities for case review,” Dr. Wertheimer said. “We have been careful about how we message this goal. Quality and safety come first, and we want to be prepared for discharge in advance of when the patient is ready.”
A boost for discharges
Mark Williams, MD, MHM, recently appointed chief of hospital medicine at Washington University School of Medicine in St. Louis, and a principal investigator for Project BOOST (Better Outcomes by Optimizing Safe Transitions), SHM’s quality improvement mentoring initiative aimed at helping hospitals improve care transitions, said that debates about DBN have gone on for a long time in hospital medicine.
“Around 2002, consultants told the CEO of a community hospital affiliated with Emory Healthcare that if our hospitalists could discharge patients before noon it would improve throughput,” he recalled. The consultants came from the hospitality industry, where DBN is easier to achieve.
But in hospital medicine, he said, “We use the whole day of the discharge in delivering care. I said to the CEO, ‘I can get you 100% discharge before noon – I’ll just hold the patients overnight,’” he explained. “In our initial experience, we pushed DBN up to about 10% -15%, and it opened up a few beds, which rapidly filled.”
Project BOOST encouraged the goal of getting patients ready to go out as soon as they were clinically ready, but did not advocate specifically for DBN, Dr. Williams said. “The problem is that hospital throughput starts to gum up when occupancy goes over 80% or 90%, and many academic medical centers regularly reach occupancy rates greater than 100%, particularly in the afternoon.” The deluge of patients includes transfers from other hospitals, postsurgical patients, and admissions from the emergency department.
“Boarding in the ED is a real issue,” he said. “Right now, it’s a crisis of overoccupancy, and the problem is that the pipeline is pouring patients into the system faster than they can be discharged.”
Dr. Williams believes there needs to be bigger thinking about these issues. Could hospitals, health systems, and hospitalists practice more preventive medicine so that some of these patients don’t need to come to the hospital? “Can you better address high blood pressure to prevent strokes and make sure patients with heart disease risk factors are enrolled in exercise and nutrition programs? What about access to healthy foods and the other social determinants of health? What if we provided adequate, consistent housing and transportation to medical visits?” he wondered.
Hospital at home programs may also offer some relief, he said. “If suddenly there weren’t so many emergency room visits by patients who need to get admitted, we’d have enough beds in the hospital.”
A more holistic view
John Nelson, MD, MHM, hospital medicine pioneer and management consultant, has been studying hospital throughput and policies to improve it for a long time. His 2010 column in The Hospitalist, “The Earlier the Better,” said attaching a financial incentive for hospitalists to discharge patients by a preset hour has produced mixed results.6 But Dr. Nelson offered some easy steps hospitalists can take to maximize earlier discharges, including to write “probable discharge tomorrow” as an order in the patient’s medical record.
The afternoon before a planned discharge, the hospitalist could talk to a patient’s family members about the discharge plan and order any outstanding tests to be done that evening to be ready for morning rounds – which he suggested should start by 7:00 a.m. The hospitalist could dictate the discharge summary the afternoon before. Even if a discharge can’t proceed as planned, the time isn’t necessarily wasted.
In a recent interview, Dr. Nelson noted that the movement to reduce average length of stay in the hospital has complicated the discharge picture by reducing a hospital’s flexibility. But he added that it’s still worth tracking and collecting data on discharge times, and to keep the conversation going. “Just don’t lose sight of the real goal, which is not DBN but optimal length-of-stay management,” he said.
Dr. Gundareddy said that, as his group has dealt with these issues, some steps have emerged to help manage discharges and throughput. “We didn’t have case management and social work services over the weekend, but when we added that support, it changed how our Mondays went.”
He encourages hospitalists to focus on the actual processes that create bottlenecks preventing throughput. “A good example of effective restructuring is lab testing. It’s amazing to think that you could have lab test results available for 7:00 a.m. rounds. There are areas that deserve more attention and more research regarding DBN. What is the impact of discharge before noon programs on the patients who aren’t being planned for discharge that day? Do they get neglected? I feel that happens sometimes.”
The COVID pandemic has further complicated these questions, Dr. Gundareddy said. “Early on in the pandemic, we were unsure how things were going with discharges, since all of the focus was on the COVID crisis. A lot of outpatient and surgical services came to a standstill, and there weren’t enough of the right kinds of beds for COVID patients. It was hard to align staff appropriately with the new clinical goals and to train them during the crisis.” Now, patients who delayed care during the pandemic are turning up at the hospital with greater acuity.
As with all incentives, DBN can have unintended consequences – especially if you monetize the practice, Dr. Verma said. “Most hospitalists are already working so hard – making so many decisions every day. These incentives could push decisions that aren’t in anybody’s best interests.”
Various groups have created comprehensive packages of protocols for improving transitions of care, he said. Organized programs to maximize efficiency of transitions and patient flow, including Project BOOST and Project RED (Re-Engineered Discharge) at Boston University Medical Center, are important sources of tools and resources. “But we should stop flogging hospitalists to discharge patients before noon,” Dr. Verma said, “Discharge is more complex than that. Instead, we should work to improve discharges in more holistic ways.”
References
1. Kirubarajan A et al. Morning discharges and patient length of stay in inpatient general internal medicine. J Hosp Med. 2021 Jun;16(6):333-8. doi: 10.12788/jhm.3605.
2. Shine D. Discharge before noon: An urban legend. Am J Med. 2015 May;128(5):445-6. doi:10.1016/j.amjmed.2014.12.011.
3. Zorian A et al. Discharge by noon: Toward a better understanding of benefits and costs. J Hosp Med. 2021 Jun;16(6):384. doi: 10.12788/jhm.3613.
4. Wertheimer B et al. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015 Oct;10(10):664-9. doi: 10.1002/jhm.2412.
5. Wertheimer B et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014 Apr;9(4):210-4. doi: 10.1002/jhm.2154.
6. Nelson J. The earlier, the better. The Hospitalist. 2010 May.
Oral step-down therapy for infective endocarditis
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Reflecting on 2021, looking forward to 2022
This month marks the end of my first full calendar year as SHM CEO. Over the years, I have made it a habit to take time to reflect during the month of December, assessing the previous year by reviewing what went well and what could have gone better, and how I can grow and change to meet the needs of future challenges. This reflection sets the stage for my personal and professional “New Year” goals.
This year, 2021, is certainly a year deserving of reflection, and I believe 2022 (and beyond) will need ambitious goals made by dedicated leaders, hospitalists included. Here are my thoughts on what went well in 2021 and what I wish went better – from our greater society to our specialty, to SHM.
Society (as in the larger society)
What went well: Vaccines
There is a lot to be impressed with in 2021, and for me, at the top of that list are the COVID-19 vaccines. I realize the research for mRNA vaccines started more than 20 years ago, and the most successful mRNA vaccine companies have been around for more than a decade, but to roll out a COVID-19 vaccine in less than a year is still just incredible. To take a disease with a 2% mortality rate for someone like myself and effectively reduce that to near zero is something historians will be writing about for years to come.
What I wish went better: Open dialogue
I can’t remember when we stopped listening to each other, and by that, I mean listening to those who do not think exactly like ourselves. As a kid, I was taught to be careful about discussing topics at social events that could go sideways. That usually involved politics, money, or strong beliefs, but wow – now, that list is much longer. Talking about the weather used to be safe, but not anymore. If I were to show pictures of the recent flooding in Annapolis? There would almost certainly be a debate about climate change. At least we can agree on Ted Lasso as a safe topic.
Our specialty
What went well: Hospitalists are vital
There are many, many professions that deserve “hero” status for their part in taming this pandemic: nurses, doctors, emergency medical services, physical therapists, physician assistants, nurse practitioners, administrators, and more. But in the doctor category, hospitalists are at the top. Along with our emergency department and intensivist colleagues, hospitalists are one of the pillars of the inpatient response to COVID. More than 3.2 million COVID-19 hospitalizations have occurred, according to the Centers for Disease Control and Prevention, with numerous state dashboards showing three-quarters of those are cared for on general medical wards, the domain of hospitalists (for example, see my own state of Maryland’s COVID-19 dashboard: https://coronavirus.maryland.gov).
We’ve always had “two patients” – the patient in the bed and the health care system. Many hospitalists have helped their institutions by building COVID care teams, COVID wards, or in the case of Dr. Mindy Kantsiper, building an entire COVID field hospital in a convention center. Without hospitalists, both patients and the system that serves them would have fared much worse in this pandemic. Hospitalists are vital to patients and the health care system. The end. Period. End of story.
What I wish went better: Getting credit
As a profession, we need to be more deliberate about getting credit for the fantastic work we have done to care for COVID-19 patients, as well as inpatients in general. SHM can and must focus more on how to highlight the great work hospitalists have done and will continue to do. A greater understanding by the health care industry – as well as the general public – regarding the important role we play for patient care will help add autonomy in our profession, which in turn adds to resilience during these challenging times.
SHM
What went well: Membership grew
This is the one thing that we at SHM – and I personally – are most proud of. SHM is a membership society; it is the single most important metric for me personally. If physicians aren’t joining, then we are not meeting our core mission to provide value to hospitalists. My sense is the services SHM provides to hospitalists continue to be of value – even during these strenuous times of the pandemic when we had to be physically distant.
Whether it’s our Government Relations Department advocating for hospitalists in Washington, or the Journal of Hospital Medicine, or this very magazine, The Hospitalist, or SHM’s numerous educational offerings, chapter events, and SHM national meetings (Converge, Pediatric Hospital Medicine, Leadership Academies, Academic Hospitalist Academy, and more), SHM continues to provide hospitalists with vital tools to help you in your career.
This is also very much a two-way street. If you are reading this, know that without you, our members, our success would not be possible. Your passion and partnership drive us to innovate to meet your needs and those of the patients you serve every day. Thank you for your continued support and inspiration.
What could have gone better: Seeing more of you, in person
This is a tough one for me. Everything I worried about going wrong for SHM in 2021 never materialized. A year ago, my fears for SHM were that membership would shrink, finances would dry up, and the SHM staff would leave (by furlough or by choice). Thankfully, membership grew, our finances are in very good shape for any year, let alone a pandemic year, and the staff have remained at SHM and are engaged and dedicated! SHM even received a “Best Place to Work” award from the Philadelphia Business Journal.
Maybe the one regret I have is that we could not do more in-person events. But even there, I think we did better than most. We had some chapter meetings in person, and the October 2021 Leadership Academy hosted 110 hospitalist leaders, in person, at Amelia Island, Fla. That Leadership Academy went off without a hitch, and the early reviews are superb. I am very optimistic about 2022 in-person events!
Looking forward: 2022 and beyond
I have no illusions that 2022 is going to be easy. I know that the pandemic will not be gone (even though cases are falling nationwide as of this writing), that our nation will struggle with how to deal with polarization, and the workplace will continue to be redefined. Yet, I can’t help but be optimistic.
The pandemic will end eventually; all pandemics do. My hope is that young leaders will step forward to help our nation work through the divisive challenges, and some of those leaders will even be hospitalists! I also know that our profession is more vital than ever, for both patients and the health care system. We’re even getting ready to celebrate SHM’s 25th anniversary, and we can’t wait to revisit our humble beginnings while looking at the bright future of our society and our field.
I am working on my 2022 “New Year” goals, but you can be pretty sure they will revolve around making the world a better place, investing in people, and being ethical and transparent.
Dr. Howell is the CEO of the Society of Hospital Medicine.
This month marks the end of my first full calendar year as SHM CEO. Over the years, I have made it a habit to take time to reflect during the month of December, assessing the previous year by reviewing what went well and what could have gone better, and how I can grow and change to meet the needs of future challenges. This reflection sets the stage for my personal and professional “New Year” goals.
This year, 2021, is certainly a year deserving of reflection, and I believe 2022 (and beyond) will need ambitious goals made by dedicated leaders, hospitalists included. Here are my thoughts on what went well in 2021 and what I wish went better – from our greater society to our specialty, to SHM.
Society (as in the larger society)
What went well: Vaccines
There is a lot to be impressed with in 2021, and for me, at the top of that list are the COVID-19 vaccines. I realize the research for mRNA vaccines started more than 20 years ago, and the most successful mRNA vaccine companies have been around for more than a decade, but to roll out a COVID-19 vaccine in less than a year is still just incredible. To take a disease with a 2% mortality rate for someone like myself and effectively reduce that to near zero is something historians will be writing about for years to come.
What I wish went better: Open dialogue
I can’t remember when we stopped listening to each other, and by that, I mean listening to those who do not think exactly like ourselves. As a kid, I was taught to be careful about discussing topics at social events that could go sideways. That usually involved politics, money, or strong beliefs, but wow – now, that list is much longer. Talking about the weather used to be safe, but not anymore. If I were to show pictures of the recent flooding in Annapolis? There would almost certainly be a debate about climate change. At least we can agree on Ted Lasso as a safe topic.
Our specialty
What went well: Hospitalists are vital
There are many, many professions that deserve “hero” status for their part in taming this pandemic: nurses, doctors, emergency medical services, physical therapists, physician assistants, nurse practitioners, administrators, and more. But in the doctor category, hospitalists are at the top. Along with our emergency department and intensivist colleagues, hospitalists are one of the pillars of the inpatient response to COVID. More than 3.2 million COVID-19 hospitalizations have occurred, according to the Centers for Disease Control and Prevention, with numerous state dashboards showing three-quarters of those are cared for on general medical wards, the domain of hospitalists (for example, see my own state of Maryland’s COVID-19 dashboard: https://coronavirus.maryland.gov).
We’ve always had “two patients” – the patient in the bed and the health care system. Many hospitalists have helped their institutions by building COVID care teams, COVID wards, or in the case of Dr. Mindy Kantsiper, building an entire COVID field hospital in a convention center. Without hospitalists, both patients and the system that serves them would have fared much worse in this pandemic. Hospitalists are vital to patients and the health care system. The end. Period. End of story.
What I wish went better: Getting credit
As a profession, we need to be more deliberate about getting credit for the fantastic work we have done to care for COVID-19 patients, as well as inpatients in general. SHM can and must focus more on how to highlight the great work hospitalists have done and will continue to do. A greater understanding by the health care industry – as well as the general public – regarding the important role we play for patient care will help add autonomy in our profession, which in turn adds to resilience during these challenging times.
SHM
What went well: Membership grew
This is the one thing that we at SHM – and I personally – are most proud of. SHM is a membership society; it is the single most important metric for me personally. If physicians aren’t joining, then we are not meeting our core mission to provide value to hospitalists. My sense is the services SHM provides to hospitalists continue to be of value – even during these strenuous times of the pandemic when we had to be physically distant.
Whether it’s our Government Relations Department advocating for hospitalists in Washington, or the Journal of Hospital Medicine, or this very magazine, The Hospitalist, or SHM’s numerous educational offerings, chapter events, and SHM national meetings (Converge, Pediatric Hospital Medicine, Leadership Academies, Academic Hospitalist Academy, and more), SHM continues to provide hospitalists with vital tools to help you in your career.
This is also very much a two-way street. If you are reading this, know that without you, our members, our success would not be possible. Your passion and partnership drive us to innovate to meet your needs and those of the patients you serve every day. Thank you for your continued support and inspiration.
What could have gone better: Seeing more of you, in person
This is a tough one for me. Everything I worried about going wrong for SHM in 2021 never materialized. A year ago, my fears for SHM were that membership would shrink, finances would dry up, and the SHM staff would leave (by furlough or by choice). Thankfully, membership grew, our finances are in very good shape for any year, let alone a pandemic year, and the staff have remained at SHM and are engaged and dedicated! SHM even received a “Best Place to Work” award from the Philadelphia Business Journal.
Maybe the one regret I have is that we could not do more in-person events. But even there, I think we did better than most. We had some chapter meetings in person, and the October 2021 Leadership Academy hosted 110 hospitalist leaders, in person, at Amelia Island, Fla. That Leadership Academy went off without a hitch, and the early reviews are superb. I am very optimistic about 2022 in-person events!
Looking forward: 2022 and beyond
I have no illusions that 2022 is going to be easy. I know that the pandemic will not be gone (even though cases are falling nationwide as of this writing), that our nation will struggle with how to deal with polarization, and the workplace will continue to be redefined. Yet, I can’t help but be optimistic.
The pandemic will end eventually; all pandemics do. My hope is that young leaders will step forward to help our nation work through the divisive challenges, and some of those leaders will even be hospitalists! I also know that our profession is more vital than ever, for both patients and the health care system. We’re even getting ready to celebrate SHM’s 25th anniversary, and we can’t wait to revisit our humble beginnings while looking at the bright future of our society and our field.
I am working on my 2022 “New Year” goals, but you can be pretty sure they will revolve around making the world a better place, investing in people, and being ethical and transparent.
Dr. Howell is the CEO of the Society of Hospital Medicine.
This month marks the end of my first full calendar year as SHM CEO. Over the years, I have made it a habit to take time to reflect during the month of December, assessing the previous year by reviewing what went well and what could have gone better, and how I can grow and change to meet the needs of future challenges. This reflection sets the stage for my personal and professional “New Year” goals.
This year, 2021, is certainly a year deserving of reflection, and I believe 2022 (and beyond) will need ambitious goals made by dedicated leaders, hospitalists included. Here are my thoughts on what went well in 2021 and what I wish went better – from our greater society to our specialty, to SHM.
Society (as in the larger society)
What went well: Vaccines
There is a lot to be impressed with in 2021, and for me, at the top of that list are the COVID-19 vaccines. I realize the research for mRNA vaccines started more than 20 years ago, and the most successful mRNA vaccine companies have been around for more than a decade, but to roll out a COVID-19 vaccine in less than a year is still just incredible. To take a disease with a 2% mortality rate for someone like myself and effectively reduce that to near zero is something historians will be writing about for years to come.
What I wish went better: Open dialogue
I can’t remember when we stopped listening to each other, and by that, I mean listening to those who do not think exactly like ourselves. As a kid, I was taught to be careful about discussing topics at social events that could go sideways. That usually involved politics, money, or strong beliefs, but wow – now, that list is much longer. Talking about the weather used to be safe, but not anymore. If I were to show pictures of the recent flooding in Annapolis? There would almost certainly be a debate about climate change. At least we can agree on Ted Lasso as a safe topic.
Our specialty
What went well: Hospitalists are vital
There are many, many professions that deserve “hero” status for their part in taming this pandemic: nurses, doctors, emergency medical services, physical therapists, physician assistants, nurse practitioners, administrators, and more. But in the doctor category, hospitalists are at the top. Along with our emergency department and intensivist colleagues, hospitalists are one of the pillars of the inpatient response to COVID. More than 3.2 million COVID-19 hospitalizations have occurred, according to the Centers for Disease Control and Prevention, with numerous state dashboards showing three-quarters of those are cared for on general medical wards, the domain of hospitalists (for example, see my own state of Maryland’s COVID-19 dashboard: https://coronavirus.maryland.gov).
We’ve always had “two patients” – the patient in the bed and the health care system. Many hospitalists have helped their institutions by building COVID care teams, COVID wards, or in the case of Dr. Mindy Kantsiper, building an entire COVID field hospital in a convention center. Without hospitalists, both patients and the system that serves them would have fared much worse in this pandemic. Hospitalists are vital to patients and the health care system. The end. Period. End of story.
What I wish went better: Getting credit
As a profession, we need to be more deliberate about getting credit for the fantastic work we have done to care for COVID-19 patients, as well as inpatients in general. SHM can and must focus more on how to highlight the great work hospitalists have done and will continue to do. A greater understanding by the health care industry – as well as the general public – regarding the important role we play for patient care will help add autonomy in our profession, which in turn adds to resilience during these challenging times.
SHM
What went well: Membership grew
This is the one thing that we at SHM – and I personally – are most proud of. SHM is a membership society; it is the single most important metric for me personally. If physicians aren’t joining, then we are not meeting our core mission to provide value to hospitalists. My sense is the services SHM provides to hospitalists continue to be of value – even during these strenuous times of the pandemic when we had to be physically distant.
Whether it’s our Government Relations Department advocating for hospitalists in Washington, or the Journal of Hospital Medicine, or this very magazine, The Hospitalist, or SHM’s numerous educational offerings, chapter events, and SHM national meetings (Converge, Pediatric Hospital Medicine, Leadership Academies, Academic Hospitalist Academy, and more), SHM continues to provide hospitalists with vital tools to help you in your career.
This is also very much a two-way street. If you are reading this, know that without you, our members, our success would not be possible. Your passion and partnership drive us to innovate to meet your needs and those of the patients you serve every day. Thank you for your continued support and inspiration.
What could have gone better: Seeing more of you, in person
This is a tough one for me. Everything I worried about going wrong for SHM in 2021 never materialized. A year ago, my fears for SHM were that membership would shrink, finances would dry up, and the SHM staff would leave (by furlough or by choice). Thankfully, membership grew, our finances are in very good shape for any year, let alone a pandemic year, and the staff have remained at SHM and are engaged and dedicated! SHM even received a “Best Place to Work” award from the Philadelphia Business Journal.
Maybe the one regret I have is that we could not do more in-person events. But even there, I think we did better than most. We had some chapter meetings in person, and the October 2021 Leadership Academy hosted 110 hospitalist leaders, in person, at Amelia Island, Fla. That Leadership Academy went off without a hitch, and the early reviews are superb. I am very optimistic about 2022 in-person events!
Looking forward: 2022 and beyond
I have no illusions that 2022 is going to be easy. I know that the pandemic will not be gone (even though cases are falling nationwide as of this writing), that our nation will struggle with how to deal with polarization, and the workplace will continue to be redefined. Yet, I can’t help but be optimistic.
The pandemic will end eventually; all pandemics do. My hope is that young leaders will step forward to help our nation work through the divisive challenges, and some of those leaders will even be hospitalists! I also know that our profession is more vital than ever, for both patients and the health care system. We’re even getting ready to celebrate SHM’s 25th anniversary, and we can’t wait to revisit our humble beginnings while looking at the bright future of our society and our field.
I am working on my 2022 “New Year” goals, but you can be pretty sure they will revolve around making the world a better place, investing in people, and being ethical and transparent.
Dr. Howell is the CEO of the Society of Hospital Medicine.
Anticoagulant choice in antiphospholipid syndrome–associated thrombosis
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.