User login
Ready for post-acute care?
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
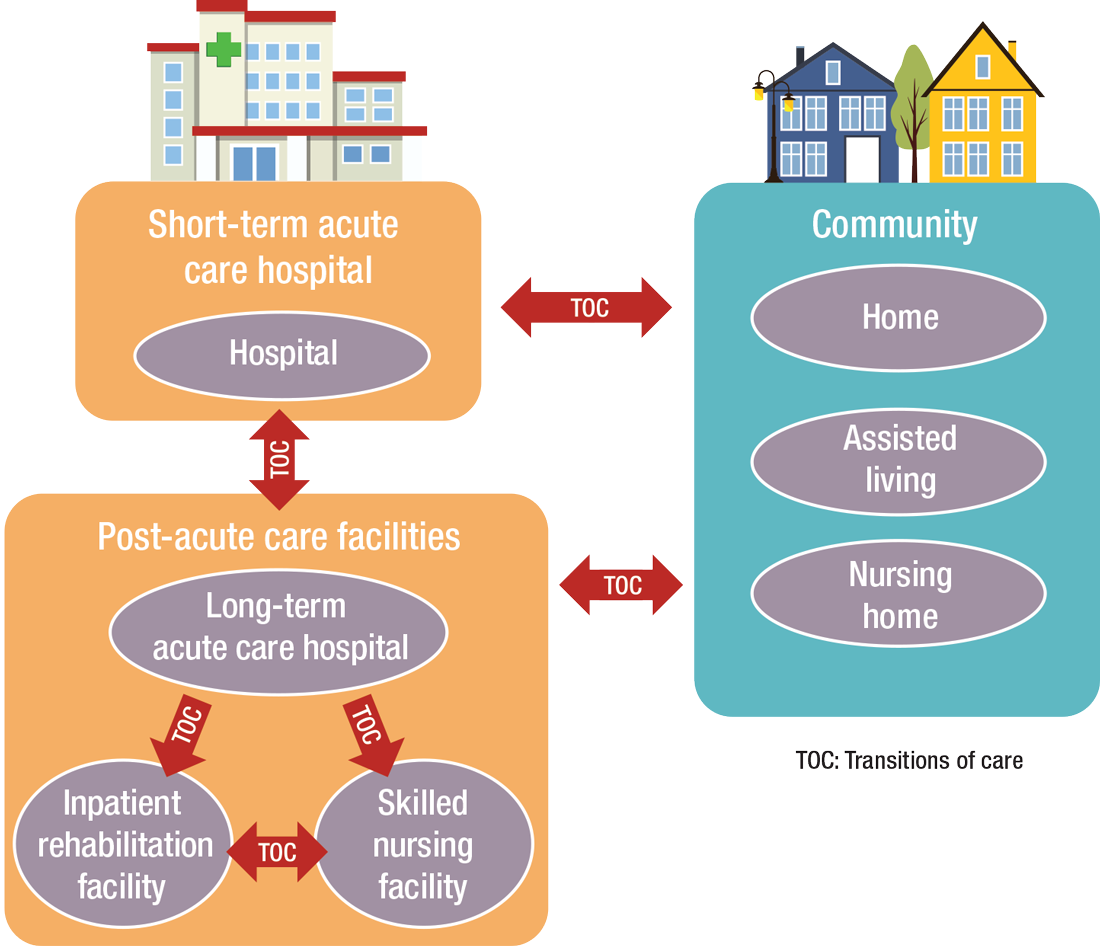
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
The cost of care, and other PAC facts and figures
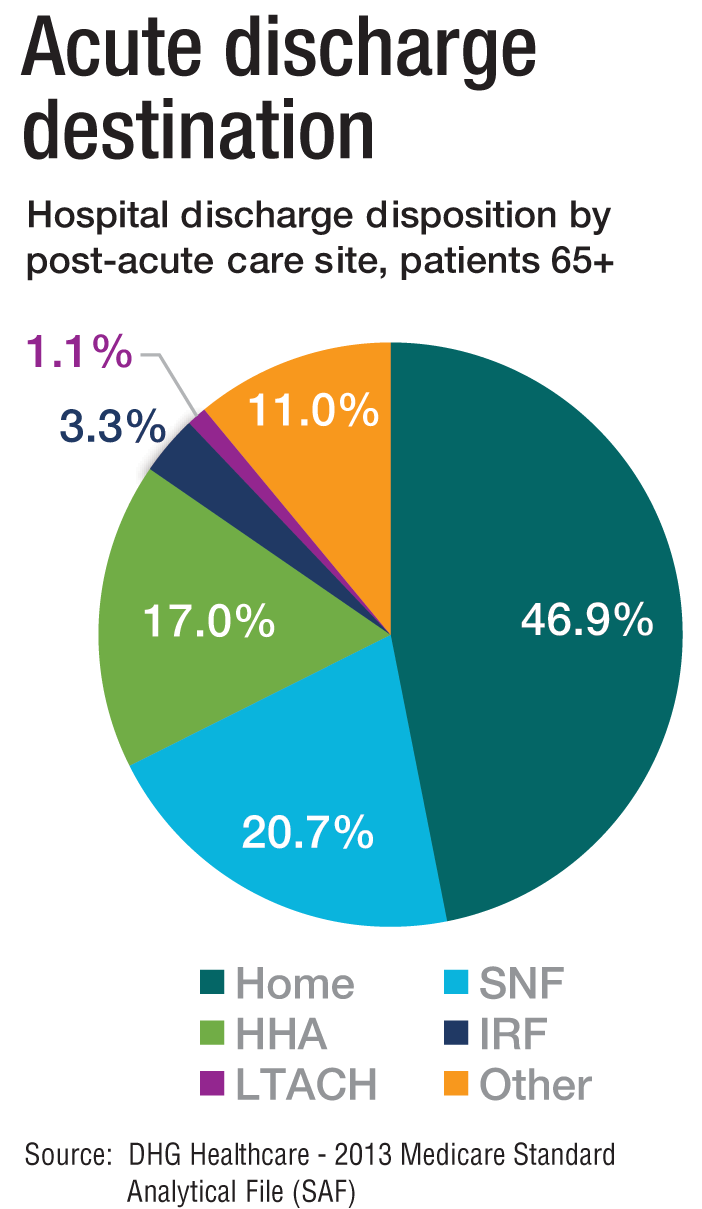
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
Registered Dieticians Sparse in VA Cancer Care
Veterans Health Administration cancer centers are lacking registered dieticians (RDs), and patients are more likely to be diagnosed with malnutrition when they are on staff, according to a new study.
The average number of full-time RDs across 13 cancer centers was just 1 per 1,065 patients, advanced practice oncology dietitian Katherine Petersen, MS, RDN, CSO, of the Phoenix VA Health Care System, reported at the AVAHO annual meeting.
However, patients treated by RDs were more likely to be diagnosed with malnutrition (odds ratio [OR], 2.9, 95% CI, 1.6-5.1). And patients were more likely to maintain weight if their clinic had a higher ratio of RDs to oncologists (OR, 1.6 for each 10% increase in ratio, 95% CI, 2.0-127.5).
Petersen told Federal Practitioner that dieticians came up with the idea for the study after attending AVAHO meetings. “A lot of the questions we were getting from physicians and other providers were: How do we get dietitians in our clinic?”
There is currently no standard staffing model for dieticians in oncology centers, Petersen said, and they are not reimbursed through Medicare or Medicaid. “We thought, ‘What do we add to the cancer center by having adequate staffing levels and seeing cancer patients?’ We designed a study to try and get to the heart of that.”
Petersen and her team focused on malnutrition. Nutrition impairment impacts an estimated 40% to 80% of patients with gastrointestinal, head and neck, pancreas, and colorectal cancer at diagnosis, she said.
Petersen discussed the published evidence that outlines how physicians recognize malnutrition at a lower rate than RDs. Dietary counseling from an RD is linked to better nutritional outcomes, physical function, and quality of life.
The study authors examined 2016 and 2017 VA registry data and reviewed charts of 681 veterans treated by 207 oncologists. Oncology clinics had a mean of 0.5 full-time equivalent (FTE) RD. The mean ratio of full-time RDs to oncologists was 1 per 48.5 and ranged from 1 per 4 to 1 per 850.
“It's almost like somebody randomly assigned [RDs] to cancer centers, and it has nothing to do with how many patients are seen in that particular center,” Petersen said. “Some clinics only have .1 or .2 FTEs assigned, and that may be a larger cancer center where they have maybe 85 cancer oncology providers, which includes surgical, medical, and radiation oncology and trainees.”
Why would a clinic have a .1 FTE RD, which suggests someone may be working 4 hours a week? In this kind of situation, an RD may cover a variety of areas and only work in cancer care when they receive a referral, Petersen said.
“That is just vastly underserving veterans,” she said. “You're missing so many veterans whom you could help with preventative care if you're only getting patients referred based on consults.”
As for the findings regarding higher RD staffing and higher detection of malnutrition, the study text notes “there was not a ‘high enough’ level of RD staffing at which we stopped seeing this trend. This is probably because – at least at the time of this study – no VA cancer center was adequately staffed for nutrition.”
Petersen hopes the findings will convince VA cancer center leadership to boost better patient outcomes by prioritizing the hiring of RDs.
Katherine Petersen, MS, RDN, CSO has no disclosures.
Veterans Health Administration cancer centers are lacking registered dieticians (RDs), and patients are more likely to be diagnosed with malnutrition when they are on staff, according to a new study.
The average number of full-time RDs across 13 cancer centers was just 1 per 1,065 patients, advanced practice oncology dietitian Katherine Petersen, MS, RDN, CSO, of the Phoenix VA Health Care System, reported at the AVAHO annual meeting.
However, patients treated by RDs were more likely to be diagnosed with malnutrition (odds ratio [OR], 2.9, 95% CI, 1.6-5.1). And patients were more likely to maintain weight if their clinic had a higher ratio of RDs to oncologists (OR, 1.6 for each 10% increase in ratio, 95% CI, 2.0-127.5).
Petersen told Federal Practitioner that dieticians came up with the idea for the study after attending AVAHO meetings. “A lot of the questions we were getting from physicians and other providers were: How do we get dietitians in our clinic?”
There is currently no standard staffing model for dieticians in oncology centers, Petersen said, and they are not reimbursed through Medicare or Medicaid. “We thought, ‘What do we add to the cancer center by having adequate staffing levels and seeing cancer patients?’ We designed a study to try and get to the heart of that.”
Petersen and her team focused on malnutrition. Nutrition impairment impacts an estimated 40% to 80% of patients with gastrointestinal, head and neck, pancreas, and colorectal cancer at diagnosis, she said.
Petersen discussed the published evidence that outlines how physicians recognize malnutrition at a lower rate than RDs. Dietary counseling from an RD is linked to better nutritional outcomes, physical function, and quality of life.
The study authors examined 2016 and 2017 VA registry data and reviewed charts of 681 veterans treated by 207 oncologists. Oncology clinics had a mean of 0.5 full-time equivalent (FTE) RD. The mean ratio of full-time RDs to oncologists was 1 per 48.5 and ranged from 1 per 4 to 1 per 850.
“It's almost like somebody randomly assigned [RDs] to cancer centers, and it has nothing to do with how many patients are seen in that particular center,” Petersen said. “Some clinics only have .1 or .2 FTEs assigned, and that may be a larger cancer center where they have maybe 85 cancer oncology providers, which includes surgical, medical, and radiation oncology and trainees.”
Why would a clinic have a .1 FTE RD, which suggests someone may be working 4 hours a week? In this kind of situation, an RD may cover a variety of areas and only work in cancer care when they receive a referral, Petersen said.
“That is just vastly underserving veterans,” she said. “You're missing so many veterans whom you could help with preventative care if you're only getting patients referred based on consults.”
As for the findings regarding higher RD staffing and higher detection of malnutrition, the study text notes “there was not a ‘high enough’ level of RD staffing at which we stopped seeing this trend. This is probably because – at least at the time of this study – no VA cancer center was adequately staffed for nutrition.”
Petersen hopes the findings will convince VA cancer center leadership to boost better patient outcomes by prioritizing the hiring of RDs.
Katherine Petersen, MS, RDN, CSO has no disclosures.
Veterans Health Administration cancer centers are lacking registered dieticians (RDs), and patients are more likely to be diagnosed with malnutrition when they are on staff, according to a new study.
The average number of full-time RDs across 13 cancer centers was just 1 per 1,065 patients, advanced practice oncology dietitian Katherine Petersen, MS, RDN, CSO, of the Phoenix VA Health Care System, reported at the AVAHO annual meeting.
However, patients treated by RDs were more likely to be diagnosed with malnutrition (odds ratio [OR], 2.9, 95% CI, 1.6-5.1). And patients were more likely to maintain weight if their clinic had a higher ratio of RDs to oncologists (OR, 1.6 for each 10% increase in ratio, 95% CI, 2.0-127.5).
Petersen told Federal Practitioner that dieticians came up with the idea for the study after attending AVAHO meetings. “A lot of the questions we were getting from physicians and other providers were: How do we get dietitians in our clinic?”
There is currently no standard staffing model for dieticians in oncology centers, Petersen said, and they are not reimbursed through Medicare or Medicaid. “We thought, ‘What do we add to the cancer center by having adequate staffing levels and seeing cancer patients?’ We designed a study to try and get to the heart of that.”
Petersen and her team focused on malnutrition. Nutrition impairment impacts an estimated 40% to 80% of patients with gastrointestinal, head and neck, pancreas, and colorectal cancer at diagnosis, she said.
Petersen discussed the published evidence that outlines how physicians recognize malnutrition at a lower rate than RDs. Dietary counseling from an RD is linked to better nutritional outcomes, physical function, and quality of life.
The study authors examined 2016 and 2017 VA registry data and reviewed charts of 681 veterans treated by 207 oncologists. Oncology clinics had a mean of 0.5 full-time equivalent (FTE) RD. The mean ratio of full-time RDs to oncologists was 1 per 48.5 and ranged from 1 per 4 to 1 per 850.
“It's almost like somebody randomly assigned [RDs] to cancer centers, and it has nothing to do with how many patients are seen in that particular center,” Petersen said. “Some clinics only have .1 or .2 FTEs assigned, and that may be a larger cancer center where they have maybe 85 cancer oncology providers, which includes surgical, medical, and radiation oncology and trainees.”
Why would a clinic have a .1 FTE RD, which suggests someone may be working 4 hours a week? In this kind of situation, an RD may cover a variety of areas and only work in cancer care when they receive a referral, Petersen said.
“That is just vastly underserving veterans,” she said. “You're missing so many veterans whom you could help with preventative care if you're only getting patients referred based on consults.”
As for the findings regarding higher RD staffing and higher detection of malnutrition, the study text notes “there was not a ‘high enough’ level of RD staffing at which we stopped seeing this trend. This is probably because – at least at the time of this study – no VA cancer center was adequately staffed for nutrition.”
Petersen hopes the findings will convince VA cancer center leadership to boost better patient outcomes by prioritizing the hiring of RDs.
Katherine Petersen, MS, RDN, CSO has no disclosures.
Age-Friendly Health Systems Transformation: A Whole Person Approach to Support the Well-Being of Older AdultsAge-Friendly Health Systems Transformation: A Whole Person Approach to Support the Well-Being of Older Adults
The COVID-19 pandemic established a new normal for health care delivery, with leaders rethinking core practices to survive and thrive in a changing environment and improve the health and well-being of patients. The Veterans Health Administration (VHA) is embracing a shift in focus from “what is the matter” to “what really matters” to address pre- and postpandemic challenges through a whole health approach.1 Initially conceptualized by the VHA in 2011, whole health “is an approach to health care that empowers and equips people to take charge of their health and well-being so that they can live their life to the fullest.”1 Whole health integrates evidence-based complementary and integrative health (CIH) therapies to manage pain; this includes acupuncture, meditation, tai chi, yoga, massage therapy, guided imagery, biofeedback, and clinical hypnosis.1 The VHA now recognizes well-being as a core value, helping clinicians respond to emerging challenges related to the social determinants of health (eg, access to health care, physical activity, and healthy foods) and guiding health care decision making.1,2
Well-being through empowerment—elements of whole health and Age-Friendly Health Systems (AFHS)—encourages health care institutions to work with employees, patients, and other stakeholders to address global challenges, clinician burnout, and social issues faced by their communities. This approach focuses on life’s purpose and meaning for individuals and inspires leaders to engage with patients, staff, and communities in new, impactful ways by focusing on wellbeing and wholeness rather than illness and disease. Having a higher sense of purpose is associated with lower all-cause mortality, reduced risk of specific diseases, better health behaviors, greater use of preventive services, and fewer hospital days of care.3
This article describes how AFHS supports the well-being of older adults and aligns with the whole health model of care. It also outlines the VHA investment to transform health care to be more person-centered by documenting what matters in the electronic health record (EHR).
AGE-FRIENDLY CARE
Given that nearly half of veterans enrolled in the VHA are aged ≥ 65 years, there is an increased need to identify models of care to support this aging population.4 This is especially critical because older veterans often have multiple chronic conditions and complex care needs that benefit from a whole person approach. The AFHS movement aims to provide evidence-based care aligned with what matters to older adults and provides a mechanism for transforming care to meet the needs of older veterans. This includes addressing age-related health concerns while promoting optimal health outcomes and quality of life. AFHS follows the 4Ms framework: what matters, medication, mentation, and mobility.5 The 4Ms serve as a guide for the health care of older adults in any setting, where each “M” is assessed and acted on to support what matters.5 Since 2020, > 390 teams have developed a plan to implement the 4Ms at 156 VHA facilities, demonstrating the VHA commitment to transforming health care for veterans.6
When VHA teams join the AFHS movement, they may also engage older veterans in a whole health system (WHS) (Figure). While AFHS is designed to improve care for patients aged ≥ 65 years, it also complements whole health, a person-centered approach available to all veterans enrolled in the VHA. Through the WHS and AFHS, veterans are empowered and equipped to take charge of their health and well-being through conversations about their unique goals, preferences, and health priorities.4 Clinicians are challenged to assess what matters by asking questions like, “What brings you joy?” and, “How can we help you meet your health goals?”1,5 These questions shift the conversation from disease-based treatment and enable clinicians to better understand the veteran as a person.1,5
For whole health and AFHS, conversations about what matters are anchored in the veteran’s goals and preferences, especially those facing a significant health change (ie, a new diagnosis or treatment decision).5,7 Together, the veteran’s goals and priorities serve as the foundation for developing person-centered care plans that often go beyond conventional medical treatments to address the physical, mental, emotional, and social aspects of health.
SYSTEM-WIDE DIRECTIVE
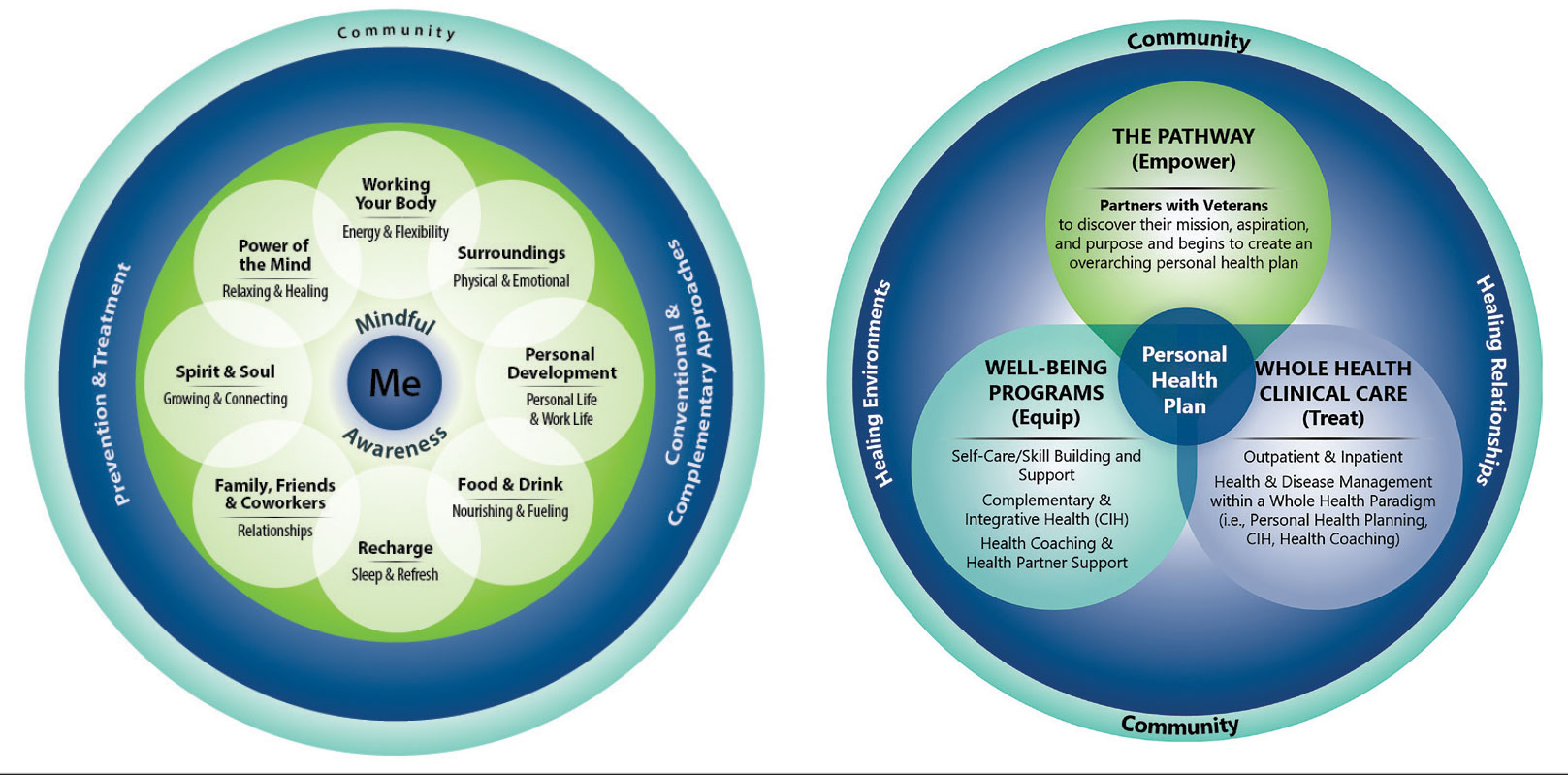
The WHS enhances AFHS discussions about what matters to veterans by adding a system-level lens for conceptualizing health care delivery by leveraging the 3 components of WHS: the “pathway,” well-being programs, and whole health clinical care.
The Pathway
Discovering what matters, or the veteran’s “mission, aspiration, and purpose,” begins with the WHS pathway. When stepping into the pathway, veterans begin completing a personal health inventory, or “walking the circle of health,” which encourages self-reflection that focuses on components of their life that can influence health and well-being.1,8 The circle of health offers a visual representation of the 4 most important aspects of health and well-being: First, “Me” at the center as an individual who is the expert on their life, values, goals, and priorities. Only the individual can know what really matters through mindful awareness and what works for their life. Second, self-care consists of 8 areas that impact health and wellbeing: working your body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind. Third, professional care consists of prevention, conventional care, and complementary care. Finally, the community that supports the individual.
Well-Being Programs
VHA provides WHS programs that support veterans in building self-care skills and improving their quality of life, often through integrative care clinics that offer coaching and CIH therapies. For example, a veteran who prioritizes mobility when seeking care at an integrative care clinic will not only receive conventional medical treatment for their physical symptoms but may also be offered CIH therapies depending on their goals. The veteran may set a daily mobility goal with their care team that supports what matters, incorporating CIH approaches, such as yoga and tai chi into the care plan.5 These holistic approaches for moving the body can help alleviate physical symptoms, reduce stress, improve mindful awareness, and provide opportunities for self-discovery and growth, thus promote overall well-being
Whole Health Clinical Care
AFHS and the 4Ms embody the clinical care component of the WHS. Because what matters is the driver of the 4Ms, every action taken by the care team supports wellbeing and quality of life by promoting independence, connection, and support, and addressing external factors, such as social determinants of health. At a minimum, well-being includes “functioning well: the experience of positive emotions such as happiness and contentment as well as the development of one’s potential, having some control over one’s life, having a sense of purpose, and experiencing positive relationships.”9 From a system perspective, the VHA has begun to normalize focusing on what matters to veterans, using an interprofessional approach, one of the first steps to implementing AFHS.
As the programs expand, AFHS teams can learn from whole health well-being programs and increase the capacity for self-care in older veterans. Learning about the key elements included in the circle of health helps clinicians understand each veteran’s perceived strengths and weaknesses to support their self-care. From there, teams can act on the 4Ms and connect older veterans with the most appropriate programs and services at their facility, ensuring continuum of care.
DOCUMENTATION
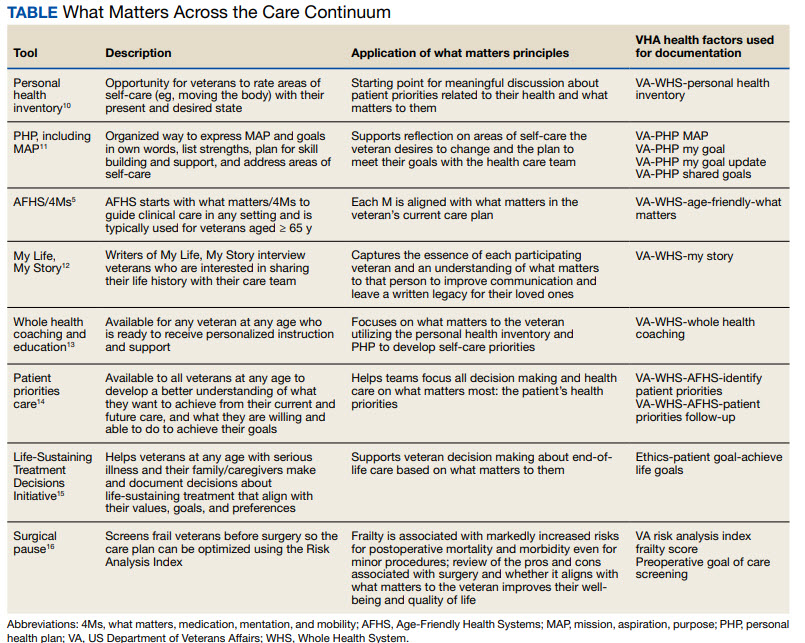
The VHA leverages several tools and evidence-based practices to assess and act on what matters for veterans of all ages (Table).5,10-16 The VHA EHR and associated dashboards contain a wealth of information about whole health and AFHS implementation, scale up, and spread. A national AFHS 4Ms note template contains standardized data elements called health factors, which provide a mechanism for monitoring 4Ms care via its related dashboard. This template was developed by an interprofessional workgroup of VHA staff and underwent a thorough human factors engineering review and testing process prior to its release. Although teams continue to personalize care based on what matters to the veteran, data from the standardized 4Ms note template and dashboard provide a way to establish consistent, equitable care across multiple care settings.17
Between January 2022 and December 2023, > 612,000 participants aged ≥ 65 years identified what matters to them through 1.35 million assessments. During that period, > 36,000 veterans aged ≥ 65 years participated in AFHS and had what matters conversations documented. A personalized health plan was completed by 585,270 veterans for a total of 1.1 million assessments.11 Whole health coaching has been documented for > 57,000 veterans with > 200,000 assessments completed.13 In fiscal year 2023, a total of 1,802,131 veterans participated in whole health.
When teams share information about what matters to the veteran in a clinicianfacing format in the EHR, this helps ensure that the VHA honors veteran preferences throughout transitions of care and across all phases of health care. Although the EHR captures data on what matters, measurement of the overall impact on veteran and health system outcomes is essential. Further evaluation and ongoing education are needed to ensure clinicians are accurately and efficiently capturing the care provided by completing the appropriate EHR. Additional challenges include identifying ways to balance the documentation burden, while ensuring notes include valuable patient-centered information to guide care. EHR tools and templates have helped to unlock important insights on health care delivery in the VHA; however, health systems must consider how these clinical practices support the overall well-being of patients. How leaders empower frontline clinicians in any care setting to use these data to drive meaningful change is also important.
TRANSFORMING VHA CARE DELIVERY
In Achieving Whole Health: A New Approach for Veterans and the Nation, the National Academy of Science proposes a framework for the transformation of health care institutions to provide better whole health to veterans.3 Transformation requires change in entire systems and leaders who mobilize people “for participation in the process of change, encouraging a sense of collective identity and collective efficacy, which in turn brings stronger feelings of self-worth and self-efficacy,” and an enhanced sense of meaningfulness in their work and lives.18
Shifting health care approaches to equipping and empowering veterans and employees with whole health and AFHS resources is transformational and requires radically different assumptions and approaches that cannot be realized through traditional approaches. This change requires robust and multifaceted cultural transformation spanning all levels of the organization. Whole health and AFHS are facilitating this transformation by supporting documentation and data needs, tracking outcomes across settings, and accelerating spread to new facilities and care settings nationwide to support older veterans in improving their health and well-being.
Whole health and AFHS are complementary approaches to care that can work to empower veterans (as well as caregivers and clinicians) to align services with what matters most to veterans. Lessons such as standardizing person-centered assessments of what matters, creating supportive structures to better align care with veterans’ priorities, and identifying meaningful veteran and system-level outcomes to help sustain transformational change can be applied from whole health to AFHS. Together these programs have the potential to enhance overall health outcomes and quality of life for veterans.
- Kligler B, Hyde J, Gantt C, Bokhour B. The Whole Health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?” Med Care. 2022;60(5):387-391. doi:10.1097/MLR.0000000000001706
- Centers for Disease Control and Prevention. Social determinants of health (SDOH) at CDC. January 17, 2024. Accessed September 12, 2024. https://www.cdc.gov/public-health-gateway/php/about/social-determinants-of-health.html
- National Academies of Sciences, Engineering, and Medicine. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023. Accessed September 9, 2024. doi:10.17226/26854
- Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58 Suppl 1(Suppl 1):5-8. doi:10.1111/1475-6773.14110
- Laderman M, Jackson C, Little K, Duong T, Pelton L. “What Matters” to older adults? A toolkit for health systems to design better care with older adults. Institute for Healthcare Improvement; 2019. Accessed September 9, 2024. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Documents/IHI_Age_Friendly_What_Matters_to_Older_Adults_Toolkit.pdf
- U.S. Department of Veterans Affairs. Age-Friendly Health Systems. Updated September 4, 2024. Accessed September 9, 2024. https://marketplace.va.gov/innovations/age-friendly-health-systems
- Brown TT, Hurley VB, Rodriguez HP, et al. Shared dec i s i o n - m a k i n g l o w e r s m e d i c a l e x p e n d i t u re s a n d the effect is amplified in racially-ethnically concordant relationships. Med Care. 2023;61(8):528-535. doi:10.1097/MLR.0000000000001881
- Kligler B. Whole Health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Ruggeri K, Garcia-Garzon E, Maguire Á, Matz S, Huppert FA. Well-being is more than happiness and life satisfaction: a multidimensional analysis of 21 countries. Health Qual Life Outcomes. 2020;18(1):192. doi:10.1186/s12955-020-01423-y
- U.S. Department of Veterans Affairs. Personal Health Inventory. Updated May 2022. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/docs/PHI-long-May22-fillable-508.pdf doi:10.1177/2164957X221077214
- Veterans Health Administration. Personal Health Plan. Updated March 2019. Accessed September 9, 2024. https:// www.va.gov/WHOLEHEALTH/docs/PersonalHealthPlan_508_03-2019.pdf
- Veterans Health Administration. Whole Health: My Life, My Story. Updated March 20, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/mylifemystory/index.asp
- U.S. Department of Veterans Affairs. Whole Health Library: Whole Health for Skill Building. Updated April 17, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTHLIBRARY/courses/whole-health-skill-building.asp
- U.S. Department of Veterans Affairs. Making Decisions: Current Care Planning. Updated May 21, 2024. Accessed September 9, 2024. https://www.va.gov/geriatrics/pages/making_decisions.asp
- U.S. Department of Veterans Affairs. Life-Sustaining Treatment Decisions Initiative (LSTDI). Updated March 2024. Accessed September 12, 2024. https://marketplace.va.gov/innovations/life-sustaining-treatment-decisions-initiative
- U.S. Department of Veterans Affairs. Center for Health Equity Research and Promotion: Surgical Pause Saving Veterans Lives. Updated September 22, 2021. Accessed September 9, 2024. https://www.cherp.research.va.gov/features/Surgical_Pause_Saving_Veterans_Lives.asp
- Munro S, Church K, Berner C, et al. Implementation of an agefriendly template in the Veterans Health Administration electronic health record. J Inform Nurs. 2023;8(3):6-11.
- Burns JM. Transforming Leadership: A New Pursuit of Happiness. Grove Press; 2003.
- US Department of Veterans Affairs, Veterans Health Administration. Whole Health: Circle of Health Overview. Updated May 20, 2024. Accessed September 12, 2024. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
The COVID-19 pandemic established a new normal for health care delivery, with leaders rethinking core practices to survive and thrive in a changing environment and improve the health and well-being of patients. The Veterans Health Administration (VHA) is embracing a shift in focus from “what is the matter” to “what really matters” to address pre- and postpandemic challenges through a whole health approach.1 Initially conceptualized by the VHA in 2011, whole health “is an approach to health care that empowers and equips people to take charge of their health and well-being so that they can live their life to the fullest.”1 Whole health integrates evidence-based complementary and integrative health (CIH) therapies to manage pain; this includes acupuncture, meditation, tai chi, yoga, massage therapy, guided imagery, biofeedback, and clinical hypnosis.1 The VHA now recognizes well-being as a core value, helping clinicians respond to emerging challenges related to the social determinants of health (eg, access to health care, physical activity, and healthy foods) and guiding health care decision making.1,2
Well-being through empowerment—elements of whole health and Age-Friendly Health Systems (AFHS)—encourages health care institutions to work with employees, patients, and other stakeholders to address global challenges, clinician burnout, and social issues faced by their communities. This approach focuses on life’s purpose and meaning for individuals and inspires leaders to engage with patients, staff, and communities in new, impactful ways by focusing on wellbeing and wholeness rather than illness and disease. Having a higher sense of purpose is associated with lower all-cause mortality, reduced risk of specific diseases, better health behaviors, greater use of preventive services, and fewer hospital days of care.3
This article describes how AFHS supports the well-being of older adults and aligns with the whole health model of care. It also outlines the VHA investment to transform health care to be more person-centered by documenting what matters in the electronic health record (EHR).
AGE-FRIENDLY CARE
Given that nearly half of veterans enrolled in the VHA are aged ≥ 65 years, there is an increased need to identify models of care to support this aging population.4 This is especially critical because older veterans often have multiple chronic conditions and complex care needs that benefit from a whole person approach. The AFHS movement aims to provide evidence-based care aligned with what matters to older adults and provides a mechanism for transforming care to meet the needs of older veterans. This includes addressing age-related health concerns while promoting optimal health outcomes and quality of life. AFHS follows the 4Ms framework: what matters, medication, mentation, and mobility.5 The 4Ms serve as a guide for the health care of older adults in any setting, where each “M” is assessed and acted on to support what matters.5 Since 2020, > 390 teams have developed a plan to implement the 4Ms at 156 VHA facilities, demonstrating the VHA commitment to transforming health care for veterans.6
When VHA teams join the AFHS movement, they may also engage older veterans in a whole health system (WHS) (Figure). While AFHS is designed to improve care for patients aged ≥ 65 years, it also complements whole health, a person-centered approach available to all veterans enrolled in the VHA. Through the WHS and AFHS, veterans are empowered and equipped to take charge of their health and well-being through conversations about their unique goals, preferences, and health priorities.4 Clinicians are challenged to assess what matters by asking questions like, “What brings you joy?” and, “How can we help you meet your health goals?”1,5 These questions shift the conversation from disease-based treatment and enable clinicians to better understand the veteran as a person.1,5

For whole health and AFHS, conversations about what matters are anchored in the veteran’s goals and preferences, especially those facing a significant health change (ie, a new diagnosis or treatment decision).5,7 Together, the veteran’s goals and priorities serve as the foundation for developing person-centered care plans that often go beyond conventional medical treatments to address the physical, mental, emotional, and social aspects of health.
SYSTEM-WIDE DIRECTIVE
The WHS enhances AFHS discussions about what matters to veterans by adding a system-level lens for conceptualizing health care delivery by leveraging the 3 components of WHS: the “pathway,” well-being programs, and whole health clinical care.
The Pathway
Discovering what matters, or the veteran’s “mission, aspiration, and purpose,” begins with the WHS pathway. When stepping into the pathway, veterans begin completing a personal health inventory, or “walking the circle of health,” which encourages self-reflection that focuses on components of their life that can influence health and well-being.1,8 The circle of health offers a visual representation of the 4 most important aspects of health and well-being: First, “Me” at the center as an individual who is the expert on their life, values, goals, and priorities. Only the individual can know what really matters through mindful awareness and what works for their life. Second, self-care consists of 8 areas that impact health and wellbeing: working your body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind. Third, professional care consists of prevention, conventional care, and complementary care. Finally, the community that supports the individual.
Well-Being Programs
VHA provides WHS programs that support veterans in building self-care skills and improving their quality of life, often through integrative care clinics that offer coaching and CIH therapies. For example, a veteran who prioritizes mobility when seeking care at an integrative care clinic will not only receive conventional medical treatment for their physical symptoms but may also be offered CIH therapies depending on their goals. The veteran may set a daily mobility goal with their care team that supports what matters, incorporating CIH approaches, such as yoga and tai chi into the care plan.5 These holistic approaches for moving the body can help alleviate physical symptoms, reduce stress, improve mindful awareness, and provide opportunities for self-discovery and growth, thus promote overall well-being
Whole Health Clinical Care
AFHS and the 4Ms embody the clinical care component of the WHS. Because what matters is the driver of the 4Ms, every action taken by the care team supports wellbeing and quality of life by promoting independence, connection, and support, and addressing external factors, such as social determinants of health. At a minimum, well-being includes “functioning well: the experience of positive emotions such as happiness and contentment as well as the development of one’s potential, having some control over one’s life, having a sense of purpose, and experiencing positive relationships.”9 From a system perspective, the VHA has begun to normalize focusing on what matters to veterans, using an interprofessional approach, one of the first steps to implementing AFHS.
As the programs expand, AFHS teams can learn from whole health well-being programs and increase the capacity for self-care in older veterans. Learning about the key elements included in the circle of health helps clinicians understand each veteran’s perceived strengths and weaknesses to support their self-care. From there, teams can act on the 4Ms and connect older veterans with the most appropriate programs and services at their facility, ensuring continuum of care.
DOCUMENTATION
The VHA leverages several tools and evidence-based practices to assess and act on what matters for veterans of all ages (Table).5,10-16 The VHA EHR and associated dashboards contain a wealth of information about whole health and AFHS implementation, scale up, and spread. A national AFHS 4Ms note template contains standardized data elements called health factors, which provide a mechanism for monitoring 4Ms care via its related dashboard. This template was developed by an interprofessional workgroup of VHA staff and underwent a thorough human factors engineering review and testing process prior to its release. Although teams continue to personalize care based on what matters to the veteran, data from the standardized 4Ms note template and dashboard provide a way to establish consistent, equitable care across multiple care settings.17

Between January 2022 and December 2023, > 612,000 participants aged ≥ 65 years identified what matters to them through 1.35 million assessments. During that period, > 36,000 veterans aged ≥ 65 years participated in AFHS and had what matters conversations documented. A personalized health plan was completed by 585,270 veterans for a total of 1.1 million assessments.11 Whole health coaching has been documented for > 57,000 veterans with > 200,000 assessments completed.13 In fiscal year 2023, a total of 1,802,131 veterans participated in whole health.
When teams share information about what matters to the veteran in a clinicianfacing format in the EHR, this helps ensure that the VHA honors veteran preferences throughout transitions of care and across all phases of health care. Although the EHR captures data on what matters, measurement of the overall impact on veteran and health system outcomes is essential. Further evaluation and ongoing education are needed to ensure clinicians are accurately and efficiently capturing the care provided by completing the appropriate EHR. Additional challenges include identifying ways to balance the documentation burden, while ensuring notes include valuable patient-centered information to guide care. EHR tools and templates have helped to unlock important insights on health care delivery in the VHA; however, health systems must consider how these clinical practices support the overall well-being of patients. How leaders empower frontline clinicians in any care setting to use these data to drive meaningful change is also important.
TRANSFORMING VHA CARE DELIVERY
In Achieving Whole Health: A New Approach for Veterans and the Nation, the National Academy of Science proposes a framework for the transformation of health care institutions to provide better whole health to veterans.3 Transformation requires change in entire systems and leaders who mobilize people “for participation in the process of change, encouraging a sense of collective identity and collective efficacy, which in turn brings stronger feelings of self-worth and self-efficacy,” and an enhanced sense of meaningfulness in their work and lives.18
Shifting health care approaches to equipping and empowering veterans and employees with whole health and AFHS resources is transformational and requires radically different assumptions and approaches that cannot be realized through traditional approaches. This change requires robust and multifaceted cultural transformation spanning all levels of the organization. Whole health and AFHS are facilitating this transformation by supporting documentation and data needs, tracking outcomes across settings, and accelerating spread to new facilities and care settings nationwide to support older veterans in improving their health and well-being.
Whole health and AFHS are complementary approaches to care that can work to empower veterans (as well as caregivers and clinicians) to align services with what matters most to veterans. Lessons such as standardizing person-centered assessments of what matters, creating supportive structures to better align care with veterans’ priorities, and identifying meaningful veteran and system-level outcomes to help sustain transformational change can be applied from whole health to AFHS. Together these programs have the potential to enhance overall health outcomes and quality of life for veterans.
The COVID-19 pandemic established a new normal for health care delivery, with leaders rethinking core practices to survive and thrive in a changing environment and improve the health and well-being of patients. The Veterans Health Administration (VHA) is embracing a shift in focus from “what is the matter” to “what really matters” to address pre- and postpandemic challenges through a whole health approach.1 Initially conceptualized by the VHA in 2011, whole health “is an approach to health care that empowers and equips people to take charge of their health and well-being so that they can live their life to the fullest.”1 Whole health integrates evidence-based complementary and integrative health (CIH) therapies to manage pain; this includes acupuncture, meditation, tai chi, yoga, massage therapy, guided imagery, biofeedback, and clinical hypnosis.1 The VHA now recognizes well-being as a core value, helping clinicians respond to emerging challenges related to the social determinants of health (eg, access to health care, physical activity, and healthy foods) and guiding health care decision making.1,2
Well-being through empowerment—elements of whole health and Age-Friendly Health Systems (AFHS)—encourages health care institutions to work with employees, patients, and other stakeholders to address global challenges, clinician burnout, and social issues faced by their communities. This approach focuses on life’s purpose and meaning for individuals and inspires leaders to engage with patients, staff, and communities in new, impactful ways by focusing on wellbeing and wholeness rather than illness and disease. Having a higher sense of purpose is associated with lower all-cause mortality, reduced risk of specific diseases, better health behaviors, greater use of preventive services, and fewer hospital days of care.3
This article describes how AFHS supports the well-being of older adults and aligns with the whole health model of care. It also outlines the VHA investment to transform health care to be more person-centered by documenting what matters in the electronic health record (EHR).
AGE-FRIENDLY CARE
Given that nearly half of veterans enrolled in the VHA are aged ≥ 65 years, there is an increased need to identify models of care to support this aging population.4 This is especially critical because older veterans often have multiple chronic conditions and complex care needs that benefit from a whole person approach. The AFHS movement aims to provide evidence-based care aligned with what matters to older adults and provides a mechanism for transforming care to meet the needs of older veterans. This includes addressing age-related health concerns while promoting optimal health outcomes and quality of life. AFHS follows the 4Ms framework: what matters, medication, mentation, and mobility.5 The 4Ms serve as a guide for the health care of older adults in any setting, where each “M” is assessed and acted on to support what matters.5 Since 2020, > 390 teams have developed a plan to implement the 4Ms at 156 VHA facilities, demonstrating the VHA commitment to transforming health care for veterans.6
When VHA teams join the AFHS movement, they may also engage older veterans in a whole health system (WHS) (Figure). While AFHS is designed to improve care for patients aged ≥ 65 years, it also complements whole health, a person-centered approach available to all veterans enrolled in the VHA. Through the WHS and AFHS, veterans are empowered and equipped to take charge of their health and well-being through conversations about their unique goals, preferences, and health priorities.4 Clinicians are challenged to assess what matters by asking questions like, “What brings you joy?” and, “How can we help you meet your health goals?”1,5 These questions shift the conversation from disease-based treatment and enable clinicians to better understand the veteran as a person.1,5

For whole health and AFHS, conversations about what matters are anchored in the veteran’s goals and preferences, especially those facing a significant health change (ie, a new diagnosis or treatment decision).5,7 Together, the veteran’s goals and priorities serve as the foundation for developing person-centered care plans that often go beyond conventional medical treatments to address the physical, mental, emotional, and social aspects of health.
SYSTEM-WIDE DIRECTIVE
The WHS enhances AFHS discussions about what matters to veterans by adding a system-level lens for conceptualizing health care delivery by leveraging the 3 components of WHS: the “pathway,” well-being programs, and whole health clinical care.
The Pathway
Discovering what matters, or the veteran’s “mission, aspiration, and purpose,” begins with the WHS pathway. When stepping into the pathway, veterans begin completing a personal health inventory, or “walking the circle of health,” which encourages self-reflection that focuses on components of their life that can influence health and well-being.1,8 The circle of health offers a visual representation of the 4 most important aspects of health and well-being: First, “Me” at the center as an individual who is the expert on their life, values, goals, and priorities. Only the individual can know what really matters through mindful awareness and what works for their life. Second, self-care consists of 8 areas that impact health and wellbeing: working your body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind. Third, professional care consists of prevention, conventional care, and complementary care. Finally, the community that supports the individual.
Well-Being Programs
VHA provides WHS programs that support veterans in building self-care skills and improving their quality of life, often through integrative care clinics that offer coaching and CIH therapies. For example, a veteran who prioritizes mobility when seeking care at an integrative care clinic will not only receive conventional medical treatment for their physical symptoms but may also be offered CIH therapies depending on their goals. The veteran may set a daily mobility goal with their care team that supports what matters, incorporating CIH approaches, such as yoga and tai chi into the care plan.5 These holistic approaches for moving the body can help alleviate physical symptoms, reduce stress, improve mindful awareness, and provide opportunities for self-discovery and growth, thus promote overall well-being
Whole Health Clinical Care
AFHS and the 4Ms embody the clinical care component of the WHS. Because what matters is the driver of the 4Ms, every action taken by the care team supports wellbeing and quality of life by promoting independence, connection, and support, and addressing external factors, such as social determinants of health. At a minimum, well-being includes “functioning well: the experience of positive emotions such as happiness and contentment as well as the development of one’s potential, having some control over one’s life, having a sense of purpose, and experiencing positive relationships.”9 From a system perspective, the VHA has begun to normalize focusing on what matters to veterans, using an interprofessional approach, one of the first steps to implementing AFHS.
As the programs expand, AFHS teams can learn from whole health well-being programs and increase the capacity for self-care in older veterans. Learning about the key elements included in the circle of health helps clinicians understand each veteran’s perceived strengths and weaknesses to support their self-care. From there, teams can act on the 4Ms and connect older veterans with the most appropriate programs and services at their facility, ensuring continuum of care.
DOCUMENTATION
The VHA leverages several tools and evidence-based practices to assess and act on what matters for veterans of all ages (Table).5,10-16 The VHA EHR and associated dashboards contain a wealth of information about whole health and AFHS implementation, scale up, and spread. A national AFHS 4Ms note template contains standardized data elements called health factors, which provide a mechanism for monitoring 4Ms care via its related dashboard. This template was developed by an interprofessional workgroup of VHA staff and underwent a thorough human factors engineering review and testing process prior to its release. Although teams continue to personalize care based on what matters to the veteran, data from the standardized 4Ms note template and dashboard provide a way to establish consistent, equitable care across multiple care settings.17

Between January 2022 and December 2023, > 612,000 participants aged ≥ 65 years identified what matters to them through 1.35 million assessments. During that period, > 36,000 veterans aged ≥ 65 years participated in AFHS and had what matters conversations documented. A personalized health plan was completed by 585,270 veterans for a total of 1.1 million assessments.11 Whole health coaching has been documented for > 57,000 veterans with > 200,000 assessments completed.13 In fiscal year 2023, a total of 1,802,131 veterans participated in whole health.
When teams share information about what matters to the veteran in a clinicianfacing format in the EHR, this helps ensure that the VHA honors veteran preferences throughout transitions of care and across all phases of health care. Although the EHR captures data on what matters, measurement of the overall impact on veteran and health system outcomes is essential. Further evaluation and ongoing education are needed to ensure clinicians are accurately and efficiently capturing the care provided by completing the appropriate EHR. Additional challenges include identifying ways to balance the documentation burden, while ensuring notes include valuable patient-centered information to guide care. EHR tools and templates have helped to unlock important insights on health care delivery in the VHA; however, health systems must consider how these clinical practices support the overall well-being of patients. How leaders empower frontline clinicians in any care setting to use these data to drive meaningful change is also important.
TRANSFORMING VHA CARE DELIVERY
In Achieving Whole Health: A New Approach for Veterans and the Nation, the National Academy of Science proposes a framework for the transformation of health care institutions to provide better whole health to veterans.3 Transformation requires change in entire systems and leaders who mobilize people “for participation in the process of change, encouraging a sense of collective identity and collective efficacy, which in turn brings stronger feelings of self-worth and self-efficacy,” and an enhanced sense of meaningfulness in their work and lives.18
Shifting health care approaches to equipping and empowering veterans and employees with whole health and AFHS resources is transformational and requires radically different assumptions and approaches that cannot be realized through traditional approaches. This change requires robust and multifaceted cultural transformation spanning all levels of the organization. Whole health and AFHS are facilitating this transformation by supporting documentation and data needs, tracking outcomes across settings, and accelerating spread to new facilities and care settings nationwide to support older veterans in improving their health and well-being.
Whole health and AFHS are complementary approaches to care that can work to empower veterans (as well as caregivers and clinicians) to align services with what matters most to veterans. Lessons such as standardizing person-centered assessments of what matters, creating supportive structures to better align care with veterans’ priorities, and identifying meaningful veteran and system-level outcomes to help sustain transformational change can be applied from whole health to AFHS. Together these programs have the potential to enhance overall health outcomes and quality of life for veterans.
- Kligler B, Hyde J, Gantt C, Bokhour B. The Whole Health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?” Med Care. 2022;60(5):387-391. doi:10.1097/MLR.0000000000001706
- Centers for Disease Control and Prevention. Social determinants of health (SDOH) at CDC. January 17, 2024. Accessed September 12, 2024. https://www.cdc.gov/public-health-gateway/php/about/social-determinants-of-health.html
- National Academies of Sciences, Engineering, and Medicine. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023. Accessed September 9, 2024. doi:10.17226/26854
- Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58 Suppl 1(Suppl 1):5-8. doi:10.1111/1475-6773.14110
- Laderman M, Jackson C, Little K, Duong T, Pelton L. “What Matters” to older adults? A toolkit for health systems to design better care with older adults. Institute for Healthcare Improvement; 2019. Accessed September 9, 2024. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Documents/IHI_Age_Friendly_What_Matters_to_Older_Adults_Toolkit.pdf
- U.S. Department of Veterans Affairs. Age-Friendly Health Systems. Updated September 4, 2024. Accessed September 9, 2024. https://marketplace.va.gov/innovations/age-friendly-health-systems
- Brown TT, Hurley VB, Rodriguez HP, et al. Shared dec i s i o n - m a k i n g l o w e r s m e d i c a l e x p e n d i t u re s a n d the effect is amplified in racially-ethnically concordant relationships. Med Care. 2023;61(8):528-535. doi:10.1097/MLR.0000000000001881
- Kligler B. Whole Health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Ruggeri K, Garcia-Garzon E, Maguire Á, Matz S, Huppert FA. Well-being is more than happiness and life satisfaction: a multidimensional analysis of 21 countries. Health Qual Life Outcomes. 2020;18(1):192. doi:10.1186/s12955-020-01423-y
- U.S. Department of Veterans Affairs. Personal Health Inventory. Updated May 2022. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/docs/PHI-long-May22-fillable-508.pdf doi:10.1177/2164957X221077214
- Veterans Health Administration. Personal Health Plan. Updated March 2019. Accessed September 9, 2024. https:// www.va.gov/WHOLEHEALTH/docs/PersonalHealthPlan_508_03-2019.pdf
- Veterans Health Administration. Whole Health: My Life, My Story. Updated March 20, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/mylifemystory/index.asp
- U.S. Department of Veterans Affairs. Whole Health Library: Whole Health for Skill Building. Updated April 17, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTHLIBRARY/courses/whole-health-skill-building.asp
- U.S. Department of Veterans Affairs. Making Decisions: Current Care Planning. Updated May 21, 2024. Accessed September 9, 2024. https://www.va.gov/geriatrics/pages/making_decisions.asp
- U.S. Department of Veterans Affairs. Life-Sustaining Treatment Decisions Initiative (LSTDI). Updated March 2024. Accessed September 12, 2024. https://marketplace.va.gov/innovations/life-sustaining-treatment-decisions-initiative
- U.S. Department of Veterans Affairs. Center for Health Equity Research and Promotion: Surgical Pause Saving Veterans Lives. Updated September 22, 2021. Accessed September 9, 2024. https://www.cherp.research.va.gov/features/Surgical_Pause_Saving_Veterans_Lives.asp
- Munro S, Church K, Berner C, et al. Implementation of an agefriendly template in the Veterans Health Administration electronic health record. J Inform Nurs. 2023;8(3):6-11.
- Burns JM. Transforming Leadership: A New Pursuit of Happiness. Grove Press; 2003.
- US Department of Veterans Affairs, Veterans Health Administration. Whole Health: Circle of Health Overview. Updated May 20, 2024. Accessed September 12, 2024. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
- Kligler B, Hyde J, Gantt C, Bokhour B. The Whole Health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?” Med Care. 2022;60(5):387-391. doi:10.1097/MLR.0000000000001706
- Centers for Disease Control and Prevention. Social determinants of health (SDOH) at CDC. January 17, 2024. Accessed September 12, 2024. https://www.cdc.gov/public-health-gateway/php/about/social-determinants-of-health.html
- National Academies of Sciences, Engineering, and Medicine. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023. Accessed September 9, 2024. doi:10.17226/26854
- Church K, Munro S, Shaughnessy M, Clancy C. Age-friendly health systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58 Suppl 1(Suppl 1):5-8. doi:10.1111/1475-6773.14110
- Laderman M, Jackson C, Little K, Duong T, Pelton L. “What Matters” to older adults? A toolkit for health systems to design better care with older adults. Institute for Healthcare Improvement; 2019. Accessed September 9, 2024. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Documents/IHI_Age_Friendly_What_Matters_to_Older_Adults_Toolkit.pdf
- U.S. Department of Veterans Affairs. Age-Friendly Health Systems. Updated September 4, 2024. Accessed September 9, 2024. https://marketplace.va.gov/innovations/age-friendly-health-systems
- Brown TT, Hurley VB, Rodriguez HP, et al. Shared dec i s i o n - m a k i n g l o w e r s m e d i c a l e x p e n d i t u re s a n d the effect is amplified in racially-ethnically concordant relationships. Med Care. 2023;61(8):528-535. doi:10.1097/MLR.0000000000001881
- Kligler B. Whole Health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Ruggeri K, Garcia-Garzon E, Maguire Á, Matz S, Huppert FA. Well-being is more than happiness and life satisfaction: a multidimensional analysis of 21 countries. Health Qual Life Outcomes. 2020;18(1):192. doi:10.1186/s12955-020-01423-y
- U.S. Department of Veterans Affairs. Personal Health Inventory. Updated May 2022. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/docs/PHI-long-May22-fillable-508.pdf doi:10.1177/2164957X221077214
- Veterans Health Administration. Personal Health Plan. Updated March 2019. Accessed September 9, 2024. https:// www.va.gov/WHOLEHEALTH/docs/PersonalHealthPlan_508_03-2019.pdf
- Veterans Health Administration. Whole Health: My Life, My Story. Updated March 20, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTH/mylifemystory/index.asp
- U.S. Department of Veterans Affairs. Whole Health Library: Whole Health for Skill Building. Updated April 17, 2024. Accessed September 9, 2024. https://www.va.gov/WHOLEHEALTHLIBRARY/courses/whole-health-skill-building.asp
- U.S. Department of Veterans Affairs. Making Decisions: Current Care Planning. Updated May 21, 2024. Accessed September 9, 2024. https://www.va.gov/geriatrics/pages/making_decisions.asp
- U.S. Department of Veterans Affairs. Life-Sustaining Treatment Decisions Initiative (LSTDI). Updated March 2024. Accessed September 12, 2024. https://marketplace.va.gov/innovations/life-sustaining-treatment-decisions-initiative
- U.S. Department of Veterans Affairs. Center for Health Equity Research and Promotion: Surgical Pause Saving Veterans Lives. Updated September 22, 2021. Accessed September 9, 2024. https://www.cherp.research.va.gov/features/Surgical_Pause_Saving_Veterans_Lives.asp
- Munro S, Church K, Berner C, et al. Implementation of an agefriendly template in the Veterans Health Administration electronic health record. J Inform Nurs. 2023;8(3):6-11.
- Burns JM. Transforming Leadership: A New Pursuit of Happiness. Grove Press; 2003.
- US Department of Veterans Affairs, Veterans Health Administration. Whole Health: Circle of Health Overview. Updated May 20, 2024. Accessed September 12, 2024. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
Impact of Stewardship Assistance Pilot Program for Veterans on Adherence and Persistence to Oral mCRPC Therapies
Background
Given the poor prognosis of patients with metastatic castration-resistant prostate cancer (mCRPC), interventions aimed at increasing adherence to oral treatments have the potential to improve patient outcomes. This study evaluates the impact of a patient stewardship assistance pilot program (stewardship program) on the adherence and persistence to oral treatments among patients with mCRPC at VA medical centers (VAMCs).
Methods
A non-randomized controlled study design and data from the VA Corporate Data Warehouse were used. The study included patients treated with an oral mCRPC therapy (i.e., abiraterone acetate or enzalutamide) between 08/2018 and 12/2019. Patients participating in the stewardship program formed the intervention arm and patients not participating the controls. Control patients were selected and matched 1:3 based on age, race and index year. The index date was the date of initiation of abiraterone acetate or enzalutamide. Outcomes included persistence (no gap >60 days of supply) and adherence (proportion of days covered [PDC] ≥80%) to oral mCRPC treatment post-index. Persistence and adherence were compared between the two arms using a Cox proportional hazard model and logistic regression model, respectively, adjusted for baseline characteristics.
Results
The study included 108 intervention patients (mean age: 74.6, 19.4% Black or African American, 44.4% from South, mean Quan-CCI: 6.7) and 324 control patients (mean age: 74.6, 19.4% Black or African American, 31.5% from South, mean Quan-CCI: 6.2). There was no statistically significant difference in persistence between the intervention and control arms (hazard ratio [95% confidence interval]: 0.84 [0.66-1.10], p-value: 0.211), with respective median times to discontinuation of 18 and 19 months. Over the first 12 months post-index, the proportion of adherent patients was not significantly different between the intervention arm and the control arm (50.6% vs. 50.9%; odds ratio [95% confidence interval]: 1.05 [0.80-1.38], p-value: 0.729).
Conclusions
In this racially diverse study of patients treated at VAMCs, high levels of persistence and adherence to oral mCRPC therapy were observed. The absence of any significant difference in adherence and persistence from the study intervention suggests that a stewardship assistance program aimed at improving adherence and persistence of patients with mCRPC may not be required at VAMCs.
Background
Given the poor prognosis of patients with metastatic castration-resistant prostate cancer (mCRPC), interventions aimed at increasing adherence to oral treatments have the potential to improve patient outcomes. This study evaluates the impact of a patient stewardship assistance pilot program (stewardship program) on the adherence and persistence to oral treatments among patients with mCRPC at VA medical centers (VAMCs).
Methods
A non-randomized controlled study design and data from the VA Corporate Data Warehouse were used. The study included patients treated with an oral mCRPC therapy (i.e., abiraterone acetate or enzalutamide) between 08/2018 and 12/2019. Patients participating in the stewardship program formed the intervention arm and patients not participating the controls. Control patients were selected and matched 1:3 based on age, race and index year. The index date was the date of initiation of abiraterone acetate or enzalutamide. Outcomes included persistence (no gap >60 days of supply) and adherence (proportion of days covered [PDC] ≥80%) to oral mCRPC treatment post-index. Persistence and adherence were compared between the two arms using a Cox proportional hazard model and logistic regression model, respectively, adjusted for baseline characteristics.
Results
The study included 108 intervention patients (mean age: 74.6, 19.4% Black or African American, 44.4% from South, mean Quan-CCI: 6.7) and 324 control patients (mean age: 74.6, 19.4% Black or African American, 31.5% from South, mean Quan-CCI: 6.2). There was no statistically significant difference in persistence between the intervention and control arms (hazard ratio [95% confidence interval]: 0.84 [0.66-1.10], p-value: 0.211), with respective median times to discontinuation of 18 and 19 months. Over the first 12 months post-index, the proportion of adherent patients was not significantly different between the intervention arm and the control arm (50.6% vs. 50.9%; odds ratio [95% confidence interval]: 1.05 [0.80-1.38], p-value: 0.729).
Conclusions
In this racially diverse study of patients treated at VAMCs, high levels of persistence and adherence to oral mCRPC therapy were observed. The absence of any significant difference in adherence and persistence from the study intervention suggests that a stewardship assistance program aimed at improving adherence and persistence of patients with mCRPC may not be required at VAMCs.
Background
Given the poor prognosis of patients with metastatic castration-resistant prostate cancer (mCRPC), interventions aimed at increasing adherence to oral treatments have the potential to improve patient outcomes. This study evaluates the impact of a patient stewardship assistance pilot program (stewardship program) on the adherence and persistence to oral treatments among patients with mCRPC at VA medical centers (VAMCs).
Methods
A non-randomized controlled study design and data from the VA Corporate Data Warehouse were used. The study included patients treated with an oral mCRPC therapy (i.e., abiraterone acetate or enzalutamide) between 08/2018 and 12/2019. Patients participating in the stewardship program formed the intervention arm and patients not participating the controls. Control patients were selected and matched 1:3 based on age, race and index year. The index date was the date of initiation of abiraterone acetate or enzalutamide. Outcomes included persistence (no gap >60 days of supply) and adherence (proportion of days covered [PDC] ≥80%) to oral mCRPC treatment post-index. Persistence and adherence were compared between the two arms using a Cox proportional hazard model and logistic regression model, respectively, adjusted for baseline characteristics.
Results
The study included 108 intervention patients (mean age: 74.6, 19.4% Black or African American, 44.4% from South, mean Quan-CCI: 6.7) and 324 control patients (mean age: 74.6, 19.4% Black or African American, 31.5% from South, mean Quan-CCI: 6.2). There was no statistically significant difference in persistence between the intervention and control arms (hazard ratio [95% confidence interval]: 0.84 [0.66-1.10], p-value: 0.211), with respective median times to discontinuation of 18 and 19 months. Over the first 12 months post-index, the proportion of adherent patients was not significantly different between the intervention arm and the control arm (50.6% vs. 50.9%; odds ratio [95% confidence interval]: 1.05 [0.80-1.38], p-value: 0.729).
Conclusions
In this racially diverse study of patients treated at VAMCs, high levels of persistence and adherence to oral mCRPC therapy were observed. The absence of any significant difference in adherence and persistence from the study intervention suggests that a stewardship assistance program aimed at improving adherence and persistence of patients with mCRPC may not be required at VAMCs.
Act Fast With Traction Alopecia to Avoid Permanent Hair Loss
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Dx Across the Skin Color Spectrum: Longitudinal Melanonychia
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Age-Friendly Health Systems and Meeting the Principles of High Reliability Organizations in the VHA
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDEprogram for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDEprogram for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDEprogram for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
Who Gets to Determine Whether Home Is “Unsafe” at the End of Life?
Sometimes a patient at the end of life (EOL) just wants to go home. We recently treated such a patient, “Joe,” a 66-year-old veteran with end-stage chronic obstructive pulmonary disorder (COPD), severe hearing loss, and heavy alcohol use. A neighbor brought Joe to the hospital when he developed a urinary tract infection. Before hospitalization, Joe spent his days in bed. His neighbor was his designated health care agent (HCA) and caregiver, dropping off meals and bringing Joe to medical appointments. Joe had no other social support. In the hospital, Joe could not participate in physical therapy (PT) evaluations due to severe dyspnea on exertion. He was recommended for home PT, a home health aide, and home nursing, but Joe declined these services out of concern for encroachment on his independence. Given his heavy alcohol use, limited support, and functional limitations, the hospitalist team felt that Joe would be best served in a skilled nursing facility. As the palliative care team, we were consulted and felt that he was eligible for hospice. Joe simply wanted to go home.
Many patients like Joe experience functional decline at EOL, leading to increased care needs and transitions between sites of care.1 Some hospitalized patients at EOL want to transition directly to home, but due to their limited functioning and social support, discharge home may be deemed unsafe by health care professionals (HCPs). Clinicians then face the difficult balancing act of honoring patient wishes and avoiding a bad outcome. For patients at EOL, issues of capacity and risk become particularly salient. Furthermore, the unique structure of the US Department of Veterans Affairs (VA) health system and the psychosocial needs of some veterans add additional considerations for complex EOL discharges.2
End-of-life Decision Making
While patients may express strong preferences regarding their health care, their decision-making ability may worsen as they approach EOL. Contributing factors include older age, effects of hospitalization, treatment adverse effects, and comorbidities, including cognitive impairment. Studies of terminally ill patients show high rates of impaired decisional capacity.3,4 It is critical to assess capacity as part of discharge planning. Even when patients have the capacity, families and caregivers have an important voice, since they are often instrumental in maintaining patients at home.
Defining Risk
Determining whether a discharge is risky or unsafe is highly subjective, with differing opinions among clinicians and between patients and clinicians.5-7 In a qualitative study by Coombs and colleagues, HCPs tended toward a risk-averse approach to discharge decisions, sometimes favoring discharge to care facilities despite patient preferences.6 This approach also reflects pressures from the health care system to decrease the length of stay and reduce readmissions, important metrics for patient care and cost containment. However, keeping patients hospitalized or in nursing facilities does not completely mitigate risks (eg, falls) and carries other hazards (eg, nosocomial infections), as highlighted during the COVID-19 pandemic.7,8 The prospect of malpractice lawsuits and HCP moral distress about perceived risky home situations can also understandably affect decision making.
At the same time, risk calculation changes depending on the patient’s clinical status and priorities. Coombs and colleagues found that in contrast to clinicians, patients nearing EOL are willing to accept increasing risks and suboptimal living conditions to remain at home.6 What may be intolerable for a younger, healthier patient with a long life expectancy may be acceptable for someone who is approaching EOL. In our framework, a risky home discharge at EOL is considered one in which other adverse events, such as falls or inadequate symptom management, are likely.
Ethical Considerations
Unsafe discharges are challenging in part because some of the pillars of medical ethics can conflict. Prior articles have analyzed the ethical concerns of unsafe discharges in detail.9-11 Briefly, when patients wish to return home against initial medical recommendations, treatment teams may focus on the principles of beneficence and nonmaleficence, as exemplified by the desire to minimize harm, and justice, in which clinicians consider resource allocation and risks that a home discharge poses to family members, caregivers, and home health professionals. However, autonomy is important to consider as well. The concept of dignity of risk highlights the imperative to respect others’ decisions even when they increase the chance of harm, particularly given the overall shift in medicine from paternalism to shared decision making.12 Accommodating patient choice in how and where health care is received allows patients to regain some control over their lives, thereby enhancing their quality of life and promoting patient dignity, especially in their remaining days.13
Discharge Risk Framework
Our risk assessment framework helps clinicians more objectively identify factors that increase or decrease risk, inform discharge planning, partner with patients and families, give patients a prominent role in EOL decisions, and mitigate the risk of a bad outcome. This concept has been used in psychiatry, in which formal suicide assessment includes identifying risk factors and protective factors to estimate suicide risk and determine interventions.14 Similar to suicide risk estimation, this framework is based on clinical judgment rather than a specific calculation.
While this framework serves as a guide for determining and mitigating risk, we encourage teams to consider legal or ethical consultations in challenging cases, such as those in which patients lack both capacity and an involved HCA.
Step 1: Determine the patient’s capacity regarding disposition planning. Patients at EOL are at a higher risk of impaired decision-making capabilities; therefore, capacity evaluation is a critical step.
Step 2: Identify risk factors and protective factors for discharge home. Risk factors are intrinsic and extrinsic factors that increase risk such as functional or sensory impairments. Protective factors are intrinsic and extrinsic factors that decrease risk, including a good understanding of illness and consistent connection with the health care system (Table 1).
Step 3: Determine discharge to home risk level based on identified risk factors and protective factors. Patients may be at low, moderate, or high risk of having an adverse event, such as a fall or inadequate symptom control (Table 2).
Step 4: Identify risk mitigation strategies. These should be tailored to the patient based on the factors identified in Step 2. Examples include home nursing and therapy, mental health treatment, a medical alert system, and frequent contact between the patient and health care team.
Step 5: Meet with inpatient and outpatient HCP teams. Meetings should include the primary care professional (PCP) or relevant subspecialist, such as an oncologist for patients with cancer. For veterans receiving care solely at a local VA medical center, this can be easier to facilitate, but for veterans who receive care through both VA and non-VA systems, this step may require additional coordination. We also recommend including interdisciplinary team members, such as social workers, case managers, and the relevant home care or hospice agency. Certain agencies may decline admission if they perceive increased risk, such as no 24-hour care, perceived self-neglect, and limited instrumental support. During this meeting, HCPs discuss risk mitigation strategies identified in Step 4 and create a plan to propose to patients and families.
Step 6: Meet with patient, HCA, and family members. In addition to sharing information about prognosis, assessing caregiver capabilities and burden can guide conversations about discharge. The discharge plan should be determined through shared decision making.11 If the patient lacks capacity regarding disposition planning, this should be shared with the HCA. However, even when patients lack capacity, it is important to continue to engage them to understand their goals and preferences.
Step 7: Maximize risk mitigation strategies. If a moderate- or high-risk discharge is requested, the health care team should maximize risk mitigation strategies. For low-risk discharges, risk mitigation strategies can still promote safety, especially since risk increases as patients progress toward EOL. In some instances, patients, their HCAs, or caregivers may decline all risk mitigation strategies despite best efforts to communicate and negotiate options. In such circumstances, we recommend discussing the case with the outpatient team for a warm handoff. HCPs should also document all efforts (helpful from a legal standpoint as well as for the patient’s future treatment teams) and respect the decision to discharge home.
Applying the Framework
Our patient Joe provides a good illustration of how to implement this EOL framework. He was deemed to have the capacity to make decisions regarding discharge (Step 1). We determined his risk factors and protective factors for discharge (Step 2). His poor functional status, limited instrumental support, heavy alcohol use, rejection of home services, and communication barriers due to severe hearing impairment all increased his risk. Protective factors included an appreciation of functional limitations, intact cognition, and an involved HCA. Based on his limited instrumental support and poor function but good insight into limitations, discharge home was deemed to be of moderate risk (Step 3). Although risk factors such as alcohol use and severe hearing impairment could have raised his level to high risk, we felt that his involved HCA maintained him in the moderate-risk category.
We worked with the hospitalist team, PT, and audiology to identify multiple risk mitigation strategies: frequent phone calls between the HCA and outpatient palliative care team, home PT to improve transfers from bed to bedside commode, home nursing services either through a routine agency or hospice, and hearing aids for better communication (Steps 4 and 5). We then proposed these strategies to Joe and his HCA (Step 6). Due to concerns about infringement on his independence, Joe declined all home services but agreed to twice-daily check-ins by his HCA, frequent communication between his HCA and our team, and new hearing aids.
Joe returned home with the agreed-upon risk mitigation strategies in place (Step 7). Despite clinicians’ original reservations about sending Joe home without formal services, his HCA maintained close contact with our team, noting that Joe remained stable and happy to be at home in the months following discharge.
Conclusions
Fortunately, VA HCPs operate in an integrated health care system with access to psychological, social, and at-home medical support that can help mitigate risks. Still, we have benefitted from having a tool to help us evaluate risk systematically. Even if patients, families, and HCPs disagree on ideal discharge plans, this tool helps clinicians approach discharges methodically while maintaining open communication and partnership with patients. In doing so, our framework reflects the shift in medical culture from a patriarchal approach to shared decision-making practices regarding all aspects of medical care. Furthermore, we hope that this can help reduce clinician moral distress stemming from these challenging cases.
Future research on best practices for discharge risk assessment and optimizing home safety are needed. We also hope to evaluate the impact and effectiveness of our framework through interviews with key stakeholders. For Joe and other veterans like him, where to spend their final days may be the last important decision they make in life, and our framework allows for their voices to be better heard throughout the decision-making process.
Acknowledgments
We thank Brooke Lifland, MD, for her theoretical contributions to the concept behind this paper.
1. Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press (US); March 19, 2015.
2. Casarett D, Pickard A, Amos Bailey F, et al. Important aspects of end-of-life care among veterans: implications for measurement and quality improvement. J Pain Symptom Manage. 2008;35(2):115-125. doi:10.1016/j.jpainsymman.2007.03.008
3. Kolva E, Rosenfeld B, Brescia R, Comfort C. Assessing decision-making capacity at end of life. Gen Hosp Psychiatry. 2014;36(4):392-397. doi:10.1016/j.genhosppsych.2014.02.013
4. Kolva E, Rosenfeld B, Saracino R. Assessing the decision-making capacity of terminally ill patients with cancer. Am J Geriatr Psychiatry. 2018;26(5):523-531. doi:10.1016/j.jagp.2017.11.012
5. Macmillan MS. Hospital staff’s perceptions of risk associated with the discharge of elderly people from acute hospital care. J Adv Nurs. 1994;19(2):249-256. doi:10.1111/j.1365-2648.1994.tb01078.x
6. Coombs MA, Parker R, de Vries K. Managing risk during care transitions when approaching end of life: A qualitative study of patients’ and health care professionals’ decision making. Palliat Med. 2017;31(7):617-624. doi:10.1177/0269216316673476
7. Hyslop B. ‘Not safe for discharge’? Words, values, and person-centred care. Age Ageing. 2020;49(3):334-336. doi:10.1093/ageing/afz170
8. Goodacre S. Safe discharge: an irrational, unhelpful and unachievable concept. Emerg Med J. 2006;23(10):753-755. doi:10.1136/emj.2006.037903
9. Swidler RN, Seastrum T, Shelton W. Difficult hospital inpatient discharge decisions: ethical, legal and clinical practice issues. Am J Bioeth. 2007;7(3):23-28. doi:10.1080/15265160601171739
10. Hill J, Filer W. Safety and ethical considerations in discharging patients to suboptimal living situations. AMA J Ethics. 2015;17(6):506-510. Published 2015 Jun 1. doi:10.1001/journalofethics.2015.17.6.ecas2-1506
11. West JC. What is an ethically informed approach to managing patient safety risk during discharge planning?. AMA J Ethics. 2020;22(11):E919-E923. Published 2020 Nov 1. doi:10.1001/amajethics.2020.919
12. Mukherjee D. Discharge decisions and the dignity of risk. Hastings Cent Rep. 2015;45(3):7-8. doi:10.1002/hast.441
13. Wheatley VJ, Baker JI. “Please, I want to go home”: ethical issues raised when considering choice of place of care in palliative care. Postgrad Med J. 2007;83(984):643-648. doi:10.1136/pgmj.2007.058487
14. Work Group on Suicidal Behaviors. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(suppl 11):1-60.
Sometimes a patient at the end of life (EOL) just wants to go home. We recently treated such a patient, “Joe,” a 66-year-old veteran with end-stage chronic obstructive pulmonary disorder (COPD), severe hearing loss, and heavy alcohol use. A neighbor brought Joe to the hospital when he developed a urinary tract infection. Before hospitalization, Joe spent his days in bed. His neighbor was his designated health care agent (HCA) and caregiver, dropping off meals and bringing Joe to medical appointments. Joe had no other social support. In the hospital, Joe could not participate in physical therapy (PT) evaluations due to severe dyspnea on exertion. He was recommended for home PT, a home health aide, and home nursing, but Joe declined these services out of concern for encroachment on his independence. Given his heavy alcohol use, limited support, and functional limitations, the hospitalist team felt that Joe would be best served in a skilled nursing facility. As the palliative care team, we were consulted and felt that he was eligible for hospice. Joe simply wanted to go home.
Many patients like Joe experience functional decline at EOL, leading to increased care needs and transitions between sites of care.1 Some hospitalized patients at EOL want to transition directly to home, but due to their limited functioning and social support, discharge home may be deemed unsafe by health care professionals (HCPs). Clinicians then face the difficult balancing act of honoring patient wishes and avoiding a bad outcome. For patients at EOL, issues of capacity and risk become particularly salient. Furthermore, the unique structure of the US Department of Veterans Affairs (VA) health system and the psychosocial needs of some veterans add additional considerations for complex EOL discharges.2
End-of-life Decision Making
While patients may express strong preferences regarding their health care, their decision-making ability may worsen as they approach EOL. Contributing factors include older age, effects of hospitalization, treatment adverse effects, and comorbidities, including cognitive impairment. Studies of terminally ill patients show high rates of impaired decisional capacity.3,4 It is critical to assess capacity as part of discharge planning. Even when patients have the capacity, families and caregivers have an important voice, since they are often instrumental in maintaining patients at home.
Defining Risk
Determining whether a discharge is risky or unsafe is highly subjective, with differing opinions among clinicians and between patients and clinicians.5-7 In a qualitative study by Coombs and colleagues, HCPs tended toward a risk-averse approach to discharge decisions, sometimes favoring discharge to care facilities despite patient preferences.6 This approach also reflects pressures from the health care system to decrease the length of stay and reduce readmissions, important metrics for patient care and cost containment. However, keeping patients hospitalized or in nursing facilities does not completely mitigate risks (eg, falls) and carries other hazards (eg, nosocomial infections), as highlighted during the COVID-19 pandemic.7,8 The prospect of malpractice lawsuits and HCP moral distress about perceived risky home situations can also understandably affect decision making.
At the same time, risk calculation changes depending on the patient’s clinical status and priorities. Coombs and colleagues found that in contrast to clinicians, patients nearing EOL are willing to accept increasing risks and suboptimal living conditions to remain at home.6 What may be intolerable for a younger, healthier patient with a long life expectancy may be acceptable for someone who is approaching EOL. In our framework, a risky home discharge at EOL is considered one in which other adverse events, such as falls or inadequate symptom management, are likely.
Ethical Considerations
Unsafe discharges are challenging in part because some of the pillars of medical ethics can conflict. Prior articles have analyzed the ethical concerns of unsafe discharges in detail.9-11 Briefly, when patients wish to return home against initial medical recommendations, treatment teams may focus on the principles of beneficence and nonmaleficence, as exemplified by the desire to minimize harm, and justice, in which clinicians consider resource allocation and risks that a home discharge poses to family members, caregivers, and home health professionals. However, autonomy is important to consider as well. The concept of dignity of risk highlights the imperative to respect others’ decisions even when they increase the chance of harm, particularly given the overall shift in medicine from paternalism to shared decision making.12 Accommodating patient choice in how and where health care is received allows patients to regain some control over their lives, thereby enhancing their quality of life and promoting patient dignity, especially in their remaining days.13
Discharge Risk Framework
Our risk assessment framework helps clinicians more objectively identify factors that increase or decrease risk, inform discharge planning, partner with patients and families, give patients a prominent role in EOL decisions, and mitigate the risk of a bad outcome. This concept has been used in psychiatry, in which formal suicide assessment includes identifying risk factors and protective factors to estimate suicide risk and determine interventions.14 Similar to suicide risk estimation, this framework is based on clinical judgment rather than a specific calculation.
While this framework serves as a guide for determining and mitigating risk, we encourage teams to consider legal or ethical consultations in challenging cases, such as those in which patients lack both capacity and an involved HCA.
Step 1: Determine the patient’s capacity regarding disposition planning. Patients at EOL are at a higher risk of impaired decision-making capabilities; therefore, capacity evaluation is a critical step.
Step 2: Identify risk factors and protective factors for discharge home. Risk factors are intrinsic and extrinsic factors that increase risk such as functional or sensory impairments. Protective factors are intrinsic and extrinsic factors that decrease risk, including a good understanding of illness and consistent connection with the health care system (Table 1).
Step 3: Determine discharge to home risk level based on identified risk factors and protective factors. Patients may be at low, moderate, or high risk of having an adverse event, such as a fall or inadequate symptom control (Table 2).
Step 4: Identify risk mitigation strategies. These should be tailored to the patient based on the factors identified in Step 2. Examples include home nursing and therapy, mental health treatment, a medical alert system, and frequent contact between the patient and health care team.
Step 5: Meet with inpatient and outpatient HCP teams. Meetings should include the primary care professional (PCP) or relevant subspecialist, such as an oncologist for patients with cancer. For veterans receiving care solely at a local VA medical center, this can be easier to facilitate, but for veterans who receive care through both VA and non-VA systems, this step may require additional coordination. We also recommend including interdisciplinary team members, such as social workers, case managers, and the relevant home care or hospice agency. Certain agencies may decline admission if they perceive increased risk, such as no 24-hour care, perceived self-neglect, and limited instrumental support. During this meeting, HCPs discuss risk mitigation strategies identified in Step 4 and create a plan to propose to patients and families.
Step 6: Meet with patient, HCA, and family members. In addition to sharing information about prognosis, assessing caregiver capabilities and burden can guide conversations about discharge. The discharge plan should be determined through shared decision making.11 If the patient lacks capacity regarding disposition planning, this should be shared with the HCA. However, even when patients lack capacity, it is important to continue to engage them to understand their goals and preferences.
Step 7: Maximize risk mitigation strategies. If a moderate- or high-risk discharge is requested, the health care team should maximize risk mitigation strategies. For low-risk discharges, risk mitigation strategies can still promote safety, especially since risk increases as patients progress toward EOL. In some instances, patients, their HCAs, or caregivers may decline all risk mitigation strategies despite best efforts to communicate and negotiate options. In such circumstances, we recommend discussing the case with the outpatient team for a warm handoff. HCPs should also document all efforts (helpful from a legal standpoint as well as for the patient’s future treatment teams) and respect the decision to discharge home.
Applying the Framework
Our patient Joe provides a good illustration of how to implement this EOL framework. He was deemed to have the capacity to make decisions regarding discharge (Step 1). We determined his risk factors and protective factors for discharge (Step 2). His poor functional status, limited instrumental support, heavy alcohol use, rejection of home services, and communication barriers due to severe hearing impairment all increased his risk. Protective factors included an appreciation of functional limitations, intact cognition, and an involved HCA. Based on his limited instrumental support and poor function but good insight into limitations, discharge home was deemed to be of moderate risk (Step 3). Although risk factors such as alcohol use and severe hearing impairment could have raised his level to high risk, we felt that his involved HCA maintained him in the moderate-risk category.
We worked with the hospitalist team, PT, and audiology to identify multiple risk mitigation strategies: frequent phone calls between the HCA and outpatient palliative care team, home PT to improve transfers from bed to bedside commode, home nursing services either through a routine agency or hospice, and hearing aids for better communication (Steps 4 and 5). We then proposed these strategies to Joe and his HCA (Step 6). Due to concerns about infringement on his independence, Joe declined all home services but agreed to twice-daily check-ins by his HCA, frequent communication between his HCA and our team, and new hearing aids.
Joe returned home with the agreed-upon risk mitigation strategies in place (Step 7). Despite clinicians’ original reservations about sending Joe home without formal services, his HCA maintained close contact with our team, noting that Joe remained stable and happy to be at home in the months following discharge.
Conclusions
Fortunately, VA HCPs operate in an integrated health care system with access to psychological, social, and at-home medical support that can help mitigate risks. Still, we have benefitted from having a tool to help us evaluate risk systematically. Even if patients, families, and HCPs disagree on ideal discharge plans, this tool helps clinicians approach discharges methodically while maintaining open communication and partnership with patients. In doing so, our framework reflects the shift in medical culture from a patriarchal approach to shared decision-making practices regarding all aspects of medical care. Furthermore, we hope that this can help reduce clinician moral distress stemming from these challenging cases.
Future research on best practices for discharge risk assessment and optimizing home safety are needed. We also hope to evaluate the impact and effectiveness of our framework through interviews with key stakeholders. For Joe and other veterans like him, where to spend their final days may be the last important decision they make in life, and our framework allows for their voices to be better heard throughout the decision-making process.
Acknowledgments
We thank Brooke Lifland, MD, for her theoretical contributions to the concept behind this paper.
Sometimes a patient at the end of life (EOL) just wants to go home. We recently treated such a patient, “Joe,” a 66-year-old veteran with end-stage chronic obstructive pulmonary disorder (COPD), severe hearing loss, and heavy alcohol use. A neighbor brought Joe to the hospital when he developed a urinary tract infection. Before hospitalization, Joe spent his days in bed. His neighbor was his designated health care agent (HCA) and caregiver, dropping off meals and bringing Joe to medical appointments. Joe had no other social support. In the hospital, Joe could not participate in physical therapy (PT) evaluations due to severe dyspnea on exertion. He was recommended for home PT, a home health aide, and home nursing, but Joe declined these services out of concern for encroachment on his independence. Given his heavy alcohol use, limited support, and functional limitations, the hospitalist team felt that Joe would be best served in a skilled nursing facility. As the palliative care team, we were consulted and felt that he was eligible for hospice. Joe simply wanted to go home.
Many patients like Joe experience functional decline at EOL, leading to increased care needs and transitions between sites of care.1 Some hospitalized patients at EOL want to transition directly to home, but due to their limited functioning and social support, discharge home may be deemed unsafe by health care professionals (HCPs). Clinicians then face the difficult balancing act of honoring patient wishes and avoiding a bad outcome. For patients at EOL, issues of capacity and risk become particularly salient. Furthermore, the unique structure of the US Department of Veterans Affairs (VA) health system and the psychosocial needs of some veterans add additional considerations for complex EOL discharges.2
End-of-life Decision Making
While patients may express strong preferences regarding their health care, their decision-making ability may worsen as they approach EOL. Contributing factors include older age, effects of hospitalization, treatment adverse effects, and comorbidities, including cognitive impairment. Studies of terminally ill patients show high rates of impaired decisional capacity.3,4 It is critical to assess capacity as part of discharge planning. Even when patients have the capacity, families and caregivers have an important voice, since they are often instrumental in maintaining patients at home.
Defining Risk
Determining whether a discharge is risky or unsafe is highly subjective, with differing opinions among clinicians and between patients and clinicians.5-7 In a qualitative study by Coombs and colleagues, HCPs tended toward a risk-averse approach to discharge decisions, sometimes favoring discharge to care facilities despite patient preferences.6 This approach also reflects pressures from the health care system to decrease the length of stay and reduce readmissions, important metrics for patient care and cost containment. However, keeping patients hospitalized or in nursing facilities does not completely mitigate risks (eg, falls) and carries other hazards (eg, nosocomial infections), as highlighted during the COVID-19 pandemic.7,8 The prospect of malpractice lawsuits and HCP moral distress about perceived risky home situations can also understandably affect decision making.
At the same time, risk calculation changes depending on the patient’s clinical status and priorities. Coombs and colleagues found that in contrast to clinicians, patients nearing EOL are willing to accept increasing risks and suboptimal living conditions to remain at home.6 What may be intolerable for a younger, healthier patient with a long life expectancy may be acceptable for someone who is approaching EOL. In our framework, a risky home discharge at EOL is considered one in which other adverse events, such as falls or inadequate symptom management, are likely.
Ethical Considerations
Unsafe discharges are challenging in part because some of the pillars of medical ethics can conflict. Prior articles have analyzed the ethical concerns of unsafe discharges in detail.9-11 Briefly, when patients wish to return home against initial medical recommendations, treatment teams may focus on the principles of beneficence and nonmaleficence, as exemplified by the desire to minimize harm, and justice, in which clinicians consider resource allocation and risks that a home discharge poses to family members, caregivers, and home health professionals. However, autonomy is important to consider as well. The concept of dignity of risk highlights the imperative to respect others’ decisions even when they increase the chance of harm, particularly given the overall shift in medicine from paternalism to shared decision making.12 Accommodating patient choice in how and where health care is received allows patients to regain some control over their lives, thereby enhancing their quality of life and promoting patient dignity, especially in their remaining days.13
Discharge Risk Framework
Our risk assessment framework helps clinicians more objectively identify factors that increase or decrease risk, inform discharge planning, partner with patients and families, give patients a prominent role in EOL decisions, and mitigate the risk of a bad outcome. This concept has been used in psychiatry, in which formal suicide assessment includes identifying risk factors and protective factors to estimate suicide risk and determine interventions.14 Similar to suicide risk estimation, this framework is based on clinical judgment rather than a specific calculation.
While this framework serves as a guide for determining and mitigating risk, we encourage teams to consider legal or ethical consultations in challenging cases, such as those in which patients lack both capacity and an involved HCA.
Step 1: Determine the patient’s capacity regarding disposition planning. Patients at EOL are at a higher risk of impaired decision-making capabilities; therefore, capacity evaluation is a critical step.
Step 2: Identify risk factors and protective factors for discharge home. Risk factors are intrinsic and extrinsic factors that increase risk such as functional or sensory impairments. Protective factors are intrinsic and extrinsic factors that decrease risk, including a good understanding of illness and consistent connection with the health care system (Table 1).
Step 3: Determine discharge to home risk level based on identified risk factors and protective factors. Patients may be at low, moderate, or high risk of having an adverse event, such as a fall or inadequate symptom control (Table 2).
Step 4: Identify risk mitigation strategies. These should be tailored to the patient based on the factors identified in Step 2. Examples include home nursing and therapy, mental health treatment, a medical alert system, and frequent contact between the patient and health care team.
Step 5: Meet with inpatient and outpatient HCP teams. Meetings should include the primary care professional (PCP) or relevant subspecialist, such as an oncologist for patients with cancer. For veterans receiving care solely at a local VA medical center, this can be easier to facilitate, but for veterans who receive care through both VA and non-VA systems, this step may require additional coordination. We also recommend including interdisciplinary team members, such as social workers, case managers, and the relevant home care or hospice agency. Certain agencies may decline admission if they perceive increased risk, such as no 24-hour care, perceived self-neglect, and limited instrumental support. During this meeting, HCPs discuss risk mitigation strategies identified in Step 4 and create a plan to propose to patients and families.
Step 6: Meet with patient, HCA, and family members. In addition to sharing information about prognosis, assessing caregiver capabilities and burden can guide conversations about discharge. The discharge plan should be determined through shared decision making.11 If the patient lacks capacity regarding disposition planning, this should be shared with the HCA. However, even when patients lack capacity, it is important to continue to engage them to understand their goals and preferences.
Step 7: Maximize risk mitigation strategies. If a moderate- or high-risk discharge is requested, the health care team should maximize risk mitigation strategies. For low-risk discharges, risk mitigation strategies can still promote safety, especially since risk increases as patients progress toward EOL. In some instances, patients, their HCAs, or caregivers may decline all risk mitigation strategies despite best efforts to communicate and negotiate options. In such circumstances, we recommend discussing the case with the outpatient team for a warm handoff. HCPs should also document all efforts (helpful from a legal standpoint as well as for the patient’s future treatment teams) and respect the decision to discharge home.
Applying the Framework
Our patient Joe provides a good illustration of how to implement this EOL framework. He was deemed to have the capacity to make decisions regarding discharge (Step 1). We determined his risk factors and protective factors for discharge (Step 2). His poor functional status, limited instrumental support, heavy alcohol use, rejection of home services, and communication barriers due to severe hearing impairment all increased his risk. Protective factors included an appreciation of functional limitations, intact cognition, and an involved HCA. Based on his limited instrumental support and poor function but good insight into limitations, discharge home was deemed to be of moderate risk (Step 3). Although risk factors such as alcohol use and severe hearing impairment could have raised his level to high risk, we felt that his involved HCA maintained him in the moderate-risk category.
We worked with the hospitalist team, PT, and audiology to identify multiple risk mitigation strategies: frequent phone calls between the HCA and outpatient palliative care team, home PT to improve transfers from bed to bedside commode, home nursing services either through a routine agency or hospice, and hearing aids for better communication (Steps 4 and 5). We then proposed these strategies to Joe and his HCA (Step 6). Due to concerns about infringement on his independence, Joe declined all home services but agreed to twice-daily check-ins by his HCA, frequent communication between his HCA and our team, and new hearing aids.
Joe returned home with the agreed-upon risk mitigation strategies in place (Step 7). Despite clinicians’ original reservations about sending Joe home without formal services, his HCA maintained close contact with our team, noting that Joe remained stable and happy to be at home in the months following discharge.
Conclusions
Fortunately, VA HCPs operate in an integrated health care system with access to psychological, social, and at-home medical support that can help mitigate risks. Still, we have benefitted from having a tool to help us evaluate risk systematically. Even if patients, families, and HCPs disagree on ideal discharge plans, this tool helps clinicians approach discharges methodically while maintaining open communication and partnership with patients. In doing so, our framework reflects the shift in medical culture from a patriarchal approach to shared decision-making practices regarding all aspects of medical care. Furthermore, we hope that this can help reduce clinician moral distress stemming from these challenging cases.
Future research on best practices for discharge risk assessment and optimizing home safety are needed. We also hope to evaluate the impact and effectiveness of our framework through interviews with key stakeholders. For Joe and other veterans like him, where to spend their final days may be the last important decision they make in life, and our framework allows for their voices to be better heard throughout the decision-making process.
Acknowledgments
We thank Brooke Lifland, MD, for her theoretical contributions to the concept behind this paper.
1. Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press (US); March 19, 2015.
2. Casarett D, Pickard A, Amos Bailey F, et al. Important aspects of end-of-life care among veterans: implications for measurement and quality improvement. J Pain Symptom Manage. 2008;35(2):115-125. doi:10.1016/j.jpainsymman.2007.03.008
3. Kolva E, Rosenfeld B, Brescia R, Comfort C. Assessing decision-making capacity at end of life. Gen Hosp Psychiatry. 2014;36(4):392-397. doi:10.1016/j.genhosppsych.2014.02.013
4. Kolva E, Rosenfeld B, Saracino R. Assessing the decision-making capacity of terminally ill patients with cancer. Am J Geriatr Psychiatry. 2018;26(5):523-531. doi:10.1016/j.jagp.2017.11.012
5. Macmillan MS. Hospital staff’s perceptions of risk associated with the discharge of elderly people from acute hospital care. J Adv Nurs. 1994;19(2):249-256. doi:10.1111/j.1365-2648.1994.tb01078.x
6. Coombs MA, Parker R, de Vries K. Managing risk during care transitions when approaching end of life: A qualitative study of patients’ and health care professionals’ decision making. Palliat Med. 2017;31(7):617-624. doi:10.1177/0269216316673476
7. Hyslop B. ‘Not safe for discharge’? Words, values, and person-centred care. Age Ageing. 2020;49(3):334-336. doi:10.1093/ageing/afz170
8. Goodacre S. Safe discharge: an irrational, unhelpful and unachievable concept. Emerg Med J. 2006;23(10):753-755. doi:10.1136/emj.2006.037903
9. Swidler RN, Seastrum T, Shelton W. Difficult hospital inpatient discharge decisions: ethical, legal and clinical practice issues. Am J Bioeth. 2007;7(3):23-28. doi:10.1080/15265160601171739
10. Hill J, Filer W. Safety and ethical considerations in discharging patients to suboptimal living situations. AMA J Ethics. 2015;17(6):506-510. Published 2015 Jun 1. doi:10.1001/journalofethics.2015.17.6.ecas2-1506
11. West JC. What is an ethically informed approach to managing patient safety risk during discharge planning?. AMA J Ethics. 2020;22(11):E919-E923. Published 2020 Nov 1. doi:10.1001/amajethics.2020.919
12. Mukherjee D. Discharge decisions and the dignity of risk. Hastings Cent Rep. 2015;45(3):7-8. doi:10.1002/hast.441
13. Wheatley VJ, Baker JI. “Please, I want to go home”: ethical issues raised when considering choice of place of care in palliative care. Postgrad Med J. 2007;83(984):643-648. doi:10.1136/pgmj.2007.058487
14. Work Group on Suicidal Behaviors. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(suppl 11):1-60.
1. Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press (US); March 19, 2015.
2. Casarett D, Pickard A, Amos Bailey F, et al. Important aspects of end-of-life care among veterans: implications for measurement and quality improvement. J Pain Symptom Manage. 2008;35(2):115-125. doi:10.1016/j.jpainsymman.2007.03.008
3. Kolva E, Rosenfeld B, Brescia R, Comfort C. Assessing decision-making capacity at end of life. Gen Hosp Psychiatry. 2014;36(4):392-397. doi:10.1016/j.genhosppsych.2014.02.013
4. Kolva E, Rosenfeld B, Saracino R. Assessing the decision-making capacity of terminally ill patients with cancer. Am J Geriatr Psychiatry. 2018;26(5):523-531. doi:10.1016/j.jagp.2017.11.012
5. Macmillan MS. Hospital staff’s perceptions of risk associated with the discharge of elderly people from acute hospital care. J Adv Nurs. 1994;19(2):249-256. doi:10.1111/j.1365-2648.1994.tb01078.x
6. Coombs MA, Parker R, de Vries K. Managing risk during care transitions when approaching end of life: A qualitative study of patients’ and health care professionals’ decision making. Palliat Med. 2017;31(7):617-624. doi:10.1177/0269216316673476
7. Hyslop B. ‘Not safe for discharge’? Words, values, and person-centred care. Age Ageing. 2020;49(3):334-336. doi:10.1093/ageing/afz170
8. Goodacre S. Safe discharge: an irrational, unhelpful and unachievable concept. Emerg Med J. 2006;23(10):753-755. doi:10.1136/emj.2006.037903
9. Swidler RN, Seastrum T, Shelton W. Difficult hospital inpatient discharge decisions: ethical, legal and clinical practice issues. Am J Bioeth. 2007;7(3):23-28. doi:10.1080/15265160601171739
10. Hill J, Filer W. Safety and ethical considerations in discharging patients to suboptimal living situations. AMA J Ethics. 2015;17(6):506-510. Published 2015 Jun 1. doi:10.1001/journalofethics.2015.17.6.ecas2-1506
11. West JC. What is an ethically informed approach to managing patient safety risk during discharge planning?. AMA J Ethics. 2020;22(11):E919-E923. Published 2020 Nov 1. doi:10.1001/amajethics.2020.919
12. Mukherjee D. Discharge decisions and the dignity of risk. Hastings Cent Rep. 2015;45(3):7-8. doi:10.1002/hast.441
13. Wheatley VJ, Baker JI. “Please, I want to go home”: ethical issues raised when considering choice of place of care in palliative care. Postgrad Med J. 2007;83(984):643-648. doi:10.1136/pgmj.2007.058487
14. Work Group on Suicidal Behaviors. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(suppl 11):1-60.
Reducing Financial Toxicity Associated With Cancer Treatment New Mexico VAHCS Fisher House at its Finest!
PURPOSE
Reduce financial toxicity of housing costs associated with cancer treatment for rural Veterans.
BACKGROUND
Veterans diagnosed with cancer experience financial burdens associated with treatments: financial toxicities (FT). New Mexico (NM) an underserved and socioeconomically challenged state has one VA facility. Veterans commonly experience increased FT in the form of financial burdens related to travel distance, housing, and time off from work for caregivers as required to seek specialized care and cancer treatments. Travel pay and the Mission Act does little to alleviate this burden, and many still experience financial hardships.
METHODS
NMVAHCS Fisher House is reserved for families seeking housing accommodations during their loved one’s hospitalization. Surgical Service coordinated an additional plan to provide services for rural Veterans requiring 4-6 weeks of daily radiation therapy. Special accommodations were granted. Each case is reviewed via consult. Veteran requires an accompanying caregiver. Prior available Veteran discounted hotel rates averaged $96 per night. A 6-week course of shelter during radiation therapy could be $4,032.00, before taxes. No discounts or vouchers were available for meals, or other expenses.
RESULTS
Since FY23, 38 families seeking oncology care were welcomed into the Fisher House, reflecting a potential Veteran cost savings of $153,216.00 related to housing alone. Veterans also experienced cost saving related to food, as most meals were provided through community donations. Veteran satisfaction was improved, evidenced by Fisher House journal for families. Entries were heartwarming, with an outpouring of gratitude to the staff and VA for providing care and hospitality in a difficult time. Several Veterans stated they would not have been able to complete treatment without the Fisher House.
IMPLICATIONS
Although most Veterans have manageable associated out of pocket expenses with cancer treatments, many have associated extensive financial burdens related to receiving treatments. Even with the Mission Act, many live 4-6 hours from the closest oncology center providing radiation therapy, making a round trip for daily treatment up to 12 hours. Consideration in the reduction of travel time and housing expenses, can mean the difference of Veterans accepting treatments resulting in improved overall quality of life and survival outcomes.
PURPOSE
Reduce financial toxicity of housing costs associated with cancer treatment for rural Veterans.
BACKGROUND
Veterans diagnosed with cancer experience financial burdens associated with treatments: financial toxicities (FT). New Mexico (NM) an underserved and socioeconomically challenged state has one VA facility. Veterans commonly experience increased FT in the form of financial burdens related to travel distance, housing, and time off from work for caregivers as required to seek specialized care and cancer treatments. Travel pay and the Mission Act does little to alleviate this burden, and many still experience financial hardships.
METHODS
NMVAHCS Fisher House is reserved for families seeking housing accommodations during their loved one’s hospitalization. Surgical Service coordinated an additional plan to provide services for rural Veterans requiring 4-6 weeks of daily radiation therapy. Special accommodations were granted. Each case is reviewed via consult. Veteran requires an accompanying caregiver. Prior available Veteran discounted hotel rates averaged $96 per night. A 6-week course of shelter during radiation therapy could be $4,032.00, before taxes. No discounts or vouchers were available for meals, or other expenses.
RESULTS
Since FY23, 38 families seeking oncology care were welcomed into the Fisher House, reflecting a potential Veteran cost savings of $153,216.00 related to housing alone. Veterans also experienced cost saving related to food, as most meals were provided through community donations. Veteran satisfaction was improved, evidenced by Fisher House journal for families. Entries were heartwarming, with an outpouring of gratitude to the staff and VA for providing care and hospitality in a difficult time. Several Veterans stated they would not have been able to complete treatment without the Fisher House.
IMPLICATIONS
Although most Veterans have manageable associated out of pocket expenses with cancer treatments, many have associated extensive financial burdens related to receiving treatments. Even with the Mission Act, many live 4-6 hours from the closest oncology center providing radiation therapy, making a round trip for daily treatment up to 12 hours. Consideration in the reduction of travel time and housing expenses, can mean the difference of Veterans accepting treatments resulting in improved overall quality of life and survival outcomes.
PURPOSE
Reduce financial toxicity of housing costs associated with cancer treatment for rural Veterans.
BACKGROUND
Veterans diagnosed with cancer experience financial burdens associated with treatments: financial toxicities (FT). New Mexico (NM) an underserved and socioeconomically challenged state has one VA facility. Veterans commonly experience increased FT in the form of financial burdens related to travel distance, housing, and time off from work for caregivers as required to seek specialized care and cancer treatments. Travel pay and the Mission Act does little to alleviate this burden, and many still experience financial hardships.
METHODS
NMVAHCS Fisher House is reserved for families seeking housing accommodations during their loved one’s hospitalization. Surgical Service coordinated an additional plan to provide services for rural Veterans requiring 4-6 weeks of daily radiation therapy. Special accommodations were granted. Each case is reviewed via consult. Veteran requires an accompanying caregiver. Prior available Veteran discounted hotel rates averaged $96 per night. A 6-week course of shelter during radiation therapy could be $4,032.00, before taxes. No discounts or vouchers were available for meals, or other expenses.
RESULTS
Since FY23, 38 families seeking oncology care were welcomed into the Fisher House, reflecting a potential Veteran cost savings of $153,216.00 related to housing alone. Veterans also experienced cost saving related to food, as most meals were provided through community donations. Veteran satisfaction was improved, evidenced by Fisher House journal for families. Entries were heartwarming, with an outpouring of gratitude to the staff and VA for providing care and hospitality in a difficult time. Several Veterans stated they would not have been able to complete treatment without the Fisher House.
IMPLICATIONS
Although most Veterans have manageable associated out of pocket expenses with cancer treatments, many have associated extensive financial burdens related to receiving treatments. Even with the Mission Act, many live 4-6 hours from the closest oncology center providing radiation therapy, making a round trip for daily treatment up to 12 hours. Consideration in the reduction of travel time and housing expenses, can mean the difference of Veterans accepting treatments resulting in improved overall quality of life and survival outcomes.
Optimizing Health Literacy to Improve Veteran Satisfaction and Overall Surgical Outcomes
PURPOSE
To improve veteran surgical literacy, satisfaction, and overall outcomes.
BACKGROUND
For years, discharge education at the New Mexico VAHCS consisted of a fill-in templated non-specific and limited facility wide CPRS note written above an 8th grade reading level. Specific surgical instructions were not provided regarding drain/catheter/ostomy/wound care, activity and bathing instructions, and signs and symptoms to notify the provider. This resulted in post-discharge anxiety, provider calls, and avoidable re-admissions.
METHODS
Nurse Navigator/Patient Educator position was created and filled with intent to create discharge education database specific to diagnosis and procedure, 1:1 patient centered education, and direct access to subject matter expert. The Navigators collaborated with surgeons to develop concise post-operative, evidence- based education, which included easy to read diagrams, 8th grade reading level, and 14 font. Packets were approved through the VHEC/I committee for distribution and stored on the VA Intranet for afterhours ward access to ensure consistency.
RESULTS
28 educational packets were created for the most common surgeries completed to customize education to fit individual needs of the Veteran. Each packet contains basic information regarding the procedure and wound care, but is customizable to include specific drain, catheter, or ostomy teaching. The Navigators meet with the Veteran prior to surgery to develop trusting relationships and begin the education process. After surgery, they visit daily to reinforce education with teach back demonstrations and encourage self-care. Family members are included in education sessions and are provided time for questions. The Navigators ensure veterans do not leave the hospital without necessary equipment and medications. As a result, the NMVAHCS has experienced improvements in the Survey of Healthcare Experiences of Patients (SHEP) scores. Prior to improvements in the educational process, SHEP scores related to discharge education identified areas of concern. After hiring Nurse Navigators, SHEP scores for discharge information increased to 90.3%. General Surgery 14-day readmission rate improved (2.9% in FY 21 to 1.7% FY 22); and 30-day readmission rate improved (12.8% FY21 to 8.7% FY 22), despite increased operative volume.
IMPLICATIONS
Providing Veteran Centered Care with comprehensive education improves selfcare, patient satisfaction, and decreases avoidable readmissions.
PURPOSE
To improve veteran surgical literacy, satisfaction, and overall outcomes.
BACKGROUND
For years, discharge education at the New Mexico VAHCS consisted of a fill-in templated non-specific and limited facility wide CPRS note written above an 8th grade reading level. Specific surgical instructions were not provided regarding drain/catheter/ostomy/wound care, activity and bathing instructions, and signs and symptoms to notify the provider. This resulted in post-discharge anxiety, provider calls, and avoidable re-admissions.
METHODS
Nurse Navigator/Patient Educator position was created and filled with intent to create discharge education database specific to diagnosis and procedure, 1:1 patient centered education, and direct access to subject matter expert. The Navigators collaborated with surgeons to develop concise post-operative, evidence- based education, which included easy to read diagrams, 8th grade reading level, and 14 font. Packets were approved through the VHEC/I committee for distribution and stored on the VA Intranet for afterhours ward access to ensure consistency.
RESULTS
28 educational packets were created for the most common surgeries completed to customize education to fit individual needs of the Veteran. Each packet contains basic information regarding the procedure and wound care, but is customizable to include specific drain, catheter, or ostomy teaching. The Navigators meet with the Veteran prior to surgery to develop trusting relationships and begin the education process. After surgery, they visit daily to reinforce education with teach back demonstrations and encourage self-care. Family members are included in education sessions and are provided time for questions. The Navigators ensure veterans do not leave the hospital without necessary equipment and medications. As a result, the NMVAHCS has experienced improvements in the Survey of Healthcare Experiences of Patients (SHEP) scores. Prior to improvements in the educational process, SHEP scores related to discharge education identified areas of concern. After hiring Nurse Navigators, SHEP scores for discharge information increased to 90.3%. General Surgery 14-day readmission rate improved (2.9% in FY 21 to 1.7% FY 22); and 30-day readmission rate improved (12.8% FY21 to 8.7% FY 22), despite increased operative volume.
IMPLICATIONS
Providing Veteran Centered Care with comprehensive education improves selfcare, patient satisfaction, and decreases avoidable readmissions.
PURPOSE
To improve veteran surgical literacy, satisfaction, and overall outcomes.
BACKGROUND
For years, discharge education at the New Mexico VAHCS consisted of a fill-in templated non-specific and limited facility wide CPRS note written above an 8th grade reading level. Specific surgical instructions were not provided regarding drain/catheter/ostomy/wound care, activity and bathing instructions, and signs and symptoms to notify the provider. This resulted in post-discharge anxiety, provider calls, and avoidable re-admissions.
METHODS
Nurse Navigator/Patient Educator position was created and filled with intent to create discharge education database specific to diagnosis and procedure, 1:1 patient centered education, and direct access to subject matter expert. The Navigators collaborated with surgeons to develop concise post-operative, evidence- based education, which included easy to read diagrams, 8th grade reading level, and 14 font. Packets were approved through the VHEC/I committee for distribution and stored on the VA Intranet for afterhours ward access to ensure consistency.
RESULTS
28 educational packets were created for the most common surgeries completed to customize education to fit individual needs of the Veteran. Each packet contains basic information regarding the procedure and wound care, but is customizable to include specific drain, catheter, or ostomy teaching. The Navigators meet with the Veteran prior to surgery to develop trusting relationships and begin the education process. After surgery, they visit daily to reinforce education with teach back demonstrations and encourage self-care. Family members are included in education sessions and are provided time for questions. The Navigators ensure veterans do not leave the hospital without necessary equipment and medications. As a result, the NMVAHCS has experienced improvements in the Survey of Healthcare Experiences of Patients (SHEP) scores. Prior to improvements in the educational process, SHEP scores related to discharge education identified areas of concern. After hiring Nurse Navigators, SHEP scores for discharge information increased to 90.3%. General Surgery 14-day readmission rate improved (2.9% in FY 21 to 1.7% FY 22); and 30-day readmission rate improved (12.8% FY21 to 8.7% FY 22), despite increased operative volume.
IMPLICATIONS
Providing Veteran Centered Care with comprehensive education improves selfcare, patient satisfaction, and decreases avoidable readmissions.