User login
Dx Across the Skin Color Spectrum: Longitudinal Melanonychia
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Longitudinal Melanonychia
THE COMPARISON
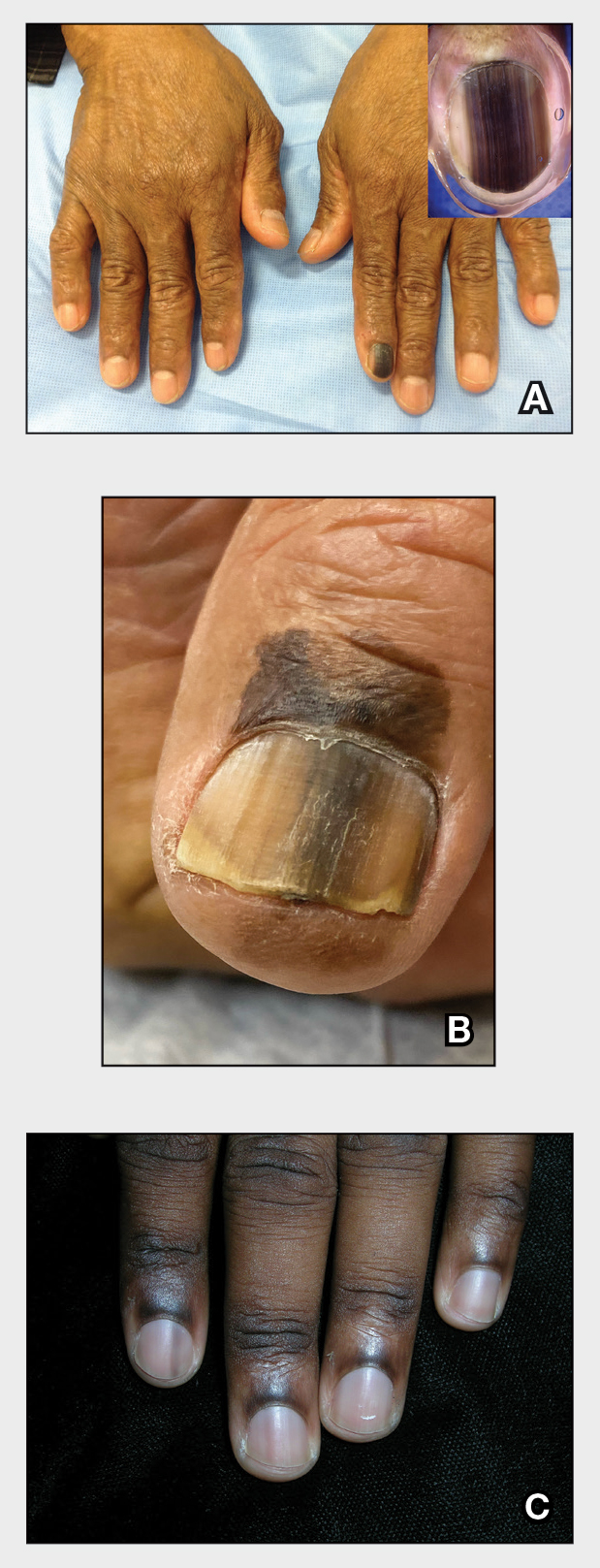
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
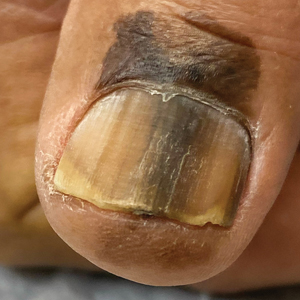
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009

THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.

THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Allergic contact dermatitis
THE COMPARISON
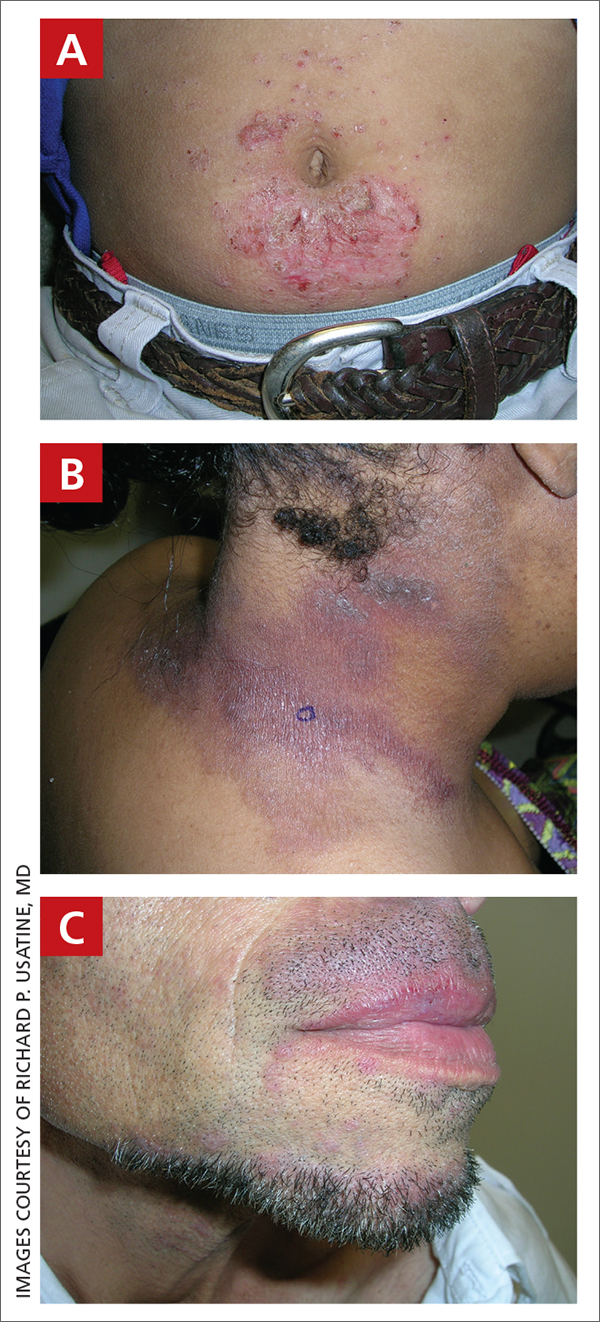
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
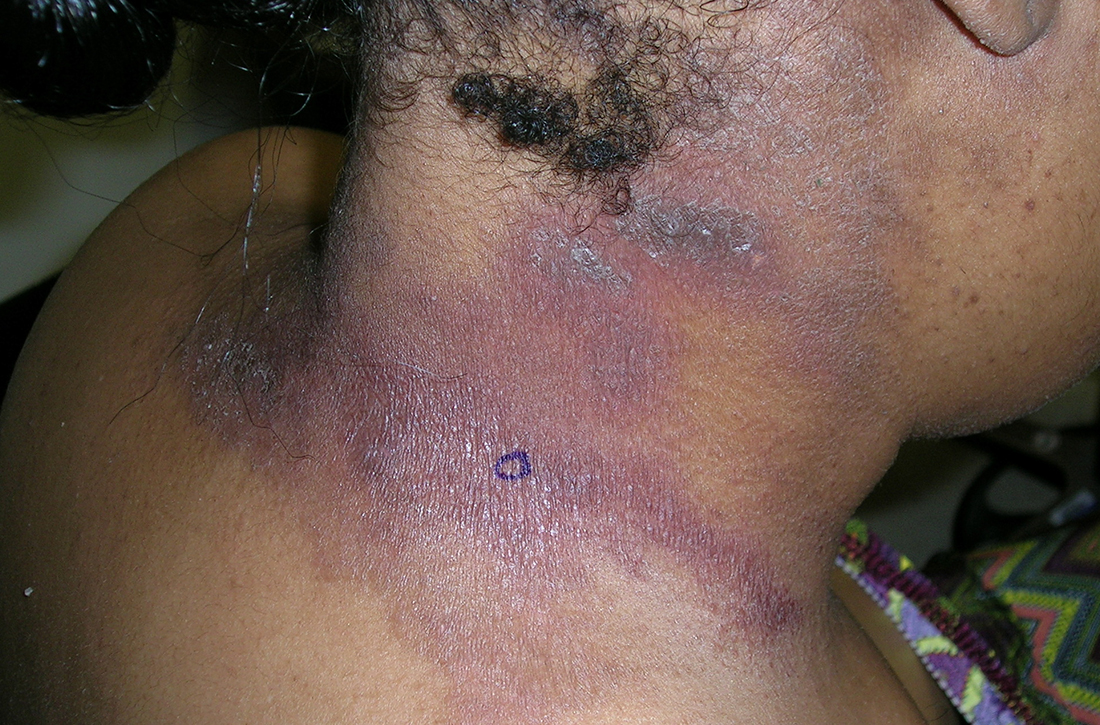
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas of the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to 1 or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is re-exposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 ACD is a challenge to manage, as complete avoidance of the allergen may not be possible.8
Continue to: The underrepresentation of patients...
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%-23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area, including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N = 19,457); 92.9% of these patients were White and only 7.1% were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
ACD is more common in women, with nickel being the most frequently identified allergen (FIGURE A).10 Personal care products often are linked to ACD (FIGURE B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD; a common component of hair dye) (FIGURE C).12
There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative; 9.1% vs 2.6%) compared to White men.13
Continue to: Ethnicity and cultural practices...
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. ACD due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on Day 1 and covered. Then, on Day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around Days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Continue to: Furthermore, Scott et al...
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N = 1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children ages 0-12 years) were significantly lower than for other groups when ACD was suspected (P < .0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23 The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi: 10.12788/cutis.0342
4. Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi: 10.1016/j.jaci.2022.02.002
5. Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi: 10.1007/s11882-023- 01070-5
6. Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi: 10.1111/j.1365-2133.2005.06415.x
7. Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi: 10.1007/s40257-017-0340-7
8. Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi: 10.1016/ j.phrs.2020.105282
9. Nielsen NH, Menne T. The relationship between IgE‐mediatedand cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi: 10.1111/j.1365-2133.1996.tb06967.x
10. Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi: 10.1111/cod.13119
11. DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292. doi: 10.1097/ DER.0000000000000220
12. Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi: 10.1016/j jaad.2020.10.003
13. Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi: 10.1053/ajcd.2001.20110
14. DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi: 10.1067/mjd.2002.120792
15. Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi: 10.12788/cutis.0292
16. Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi: 10.1111/ pde.14578
17. Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. Stat- Pearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. www.ncbi.nlm.nih.gov/books/NBK459230/
18. Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi: 10.1016/j.jaad.2018.08.049
19. Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi: 10.1097/DER.0000000000000581
20. Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi: 10.1016/j.jaad.2021.09.022
21. Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi: 10.1016/j.jaad.2022.08.041
22. Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi: 10.1016/j.jaad.2022.11.031
23. Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas of the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to 1 or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is re-exposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 ACD is a challenge to manage, as complete avoidance of the allergen may not be possible.8
Continue to: The underrepresentation of patients...
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%-23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area, including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N = 19,457); 92.9% of these patients were White and only 7.1% were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
ACD is more common in women, with nickel being the most frequently identified allergen (FIGURE A).10 Personal care products often are linked to ACD (FIGURE B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD; a common component of hair dye) (FIGURE C).12

There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative; 9.1% vs 2.6%) compared to White men.13
Continue to: Ethnicity and cultural practices...
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. ACD due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on Day 1 and covered. Then, on Day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around Days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Continue to: Furthermore, Scott et al...
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N = 1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children ages 0-12 years) were significantly lower than for other groups when ACD was suspected (P < .0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23 The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas of the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to 1 or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is re-exposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 ACD is a challenge to manage, as complete avoidance of the allergen may not be possible.8
Continue to: The underrepresentation of patients...
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%-23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area, including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N = 19,457); 92.9% of these patients were White and only 7.1% were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
ACD is more common in women, with nickel being the most frequently identified allergen (FIGURE A).10 Personal care products often are linked to ACD (FIGURE B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD; a common component of hair dye) (FIGURE C).12

There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative; 9.1% vs 2.6%) compared to White men.13
Continue to: Ethnicity and cultural practices...
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. ACD due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on Day 1 and covered. Then, on Day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around Days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Continue to: Furthermore, Scott et al...
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N = 1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children ages 0-12 years) were significantly lower than for other groups when ACD was suspected (P < .0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23 The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi: 10.12788/cutis.0342
4. Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi: 10.1016/j.jaci.2022.02.002
5. Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi: 10.1007/s11882-023- 01070-5
6. Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi: 10.1111/j.1365-2133.2005.06415.x
7. Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi: 10.1007/s40257-017-0340-7
8. Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi: 10.1016/ j.phrs.2020.105282
9. Nielsen NH, Menne T. The relationship between IgE‐mediatedand cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi: 10.1111/j.1365-2133.1996.tb06967.x
10. Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi: 10.1111/cod.13119
11. DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292. doi: 10.1097/ DER.0000000000000220
12. Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi: 10.1016/j jaad.2020.10.003
13. Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi: 10.1053/ajcd.2001.20110
14. DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi: 10.1067/mjd.2002.120792
15. Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi: 10.12788/cutis.0292
16. Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi: 10.1111/ pde.14578
17. Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. Stat- Pearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. www.ncbi.nlm.nih.gov/books/NBK459230/
18. Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi: 10.1016/j.jaad.2018.08.049
19. Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi: 10.1097/DER.0000000000000581
20. Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi: 10.1016/j.jaad.2021.09.022
21. Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi: 10.1016/j.jaad.2022.08.041
22. Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi: 10.1016/j.jaad.2022.11.031
23. Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi: 10.12788/cutis.0342
4. Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi: 10.1016/j.jaci.2022.02.002
5. Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi: 10.1007/s11882-023- 01070-5
6. Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi: 10.1111/j.1365-2133.2005.06415.x
7. Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi: 10.1007/s40257-017-0340-7
8. Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi: 10.1016/ j.phrs.2020.105282
9. Nielsen NH, Menne T. The relationship between IgE‐mediatedand cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi: 10.1111/j.1365-2133.1996.tb06967.x
10. Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi: 10.1111/cod.13119
11. DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292. doi: 10.1097/ DER.0000000000000220
12. Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi: 10.1016/j jaad.2020.10.003
13. Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi: 10.1053/ajcd.2001.20110
14. DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi: 10.1067/mjd.2002.120792
15. Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi: 10.12788/cutis.0292
16. Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi: 10.1111/ pde.14578
17. Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. Stat- Pearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. www.ncbi.nlm.nih.gov/books/NBK459230/
18. Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi: 10.1016/j.jaad.2018.08.049
19. Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi: 10.1097/DER.0000000000000581
20. Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi: 10.1016/j.jaad.2021.09.022
21. Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi: 10.1016/j.jaad.2022.08.041
22. Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi: 10.1016/j.jaad.2022.11.031
23. Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
Allergic Contact Dermatitis
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas on the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
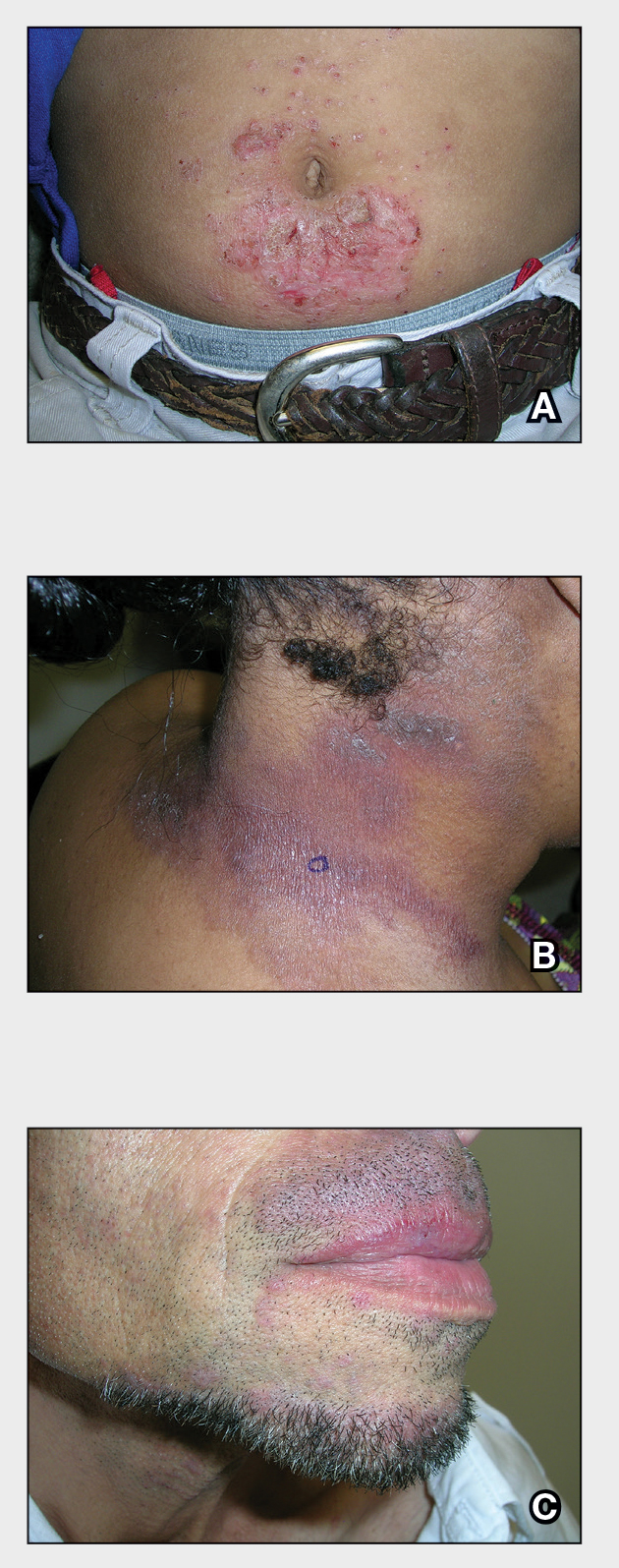
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to one or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is reexposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 Allergic contact dermatitis is a challenge to manage, as complete avoidance of the allergen may not be possible.8
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%- 23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N=19,457); 17,803 (92.9%) of these patients were White and only 1360 (7.1%) were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
Allergic contact dermatitis is more common in women, with nickel being the most frequently identified allergen (Figure, A).10 Personal care products often are linked to ACD (Figure, B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD)(a common component of hair dye) (Figure, C).12
There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative) (9.1% vs 2.6%) compared to White men.13
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. Allergic contact dermatitis due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on day 1 and covered. Then, on day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N=1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children aged 0–12 years) were significantly lower than for other groups when ACD was suspected (P<.0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23
The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74: 1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi:10.1016/j.jaci.2022.02.002
- Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi:10.1007/s11882-023-01070-5
- Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi:10.1111/j .1365-2133.2005.06415.x
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Nielsen NH, Menne T. The relationship between IgE‐mediated and cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi:10.1111/j.1365-2133.1996.tb06967.x
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998- 2006. Dermatitis. 2016;27:288-292. doi:10.1097/DER.0000000000000220
- Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi:10.1016/j.jaad.2020.10.003
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi:10.1053/ajcd.2001.20110
- DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi:10.1067/mjd.2002.120792
- Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi:10.12788/cutis.0292
- Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi:10.1111/pde.14578
- Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. StatPearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459230/
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi:10.1016/j.jaad.2018.08.049
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi:10.1097 /DER.0000000000000581
- Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi:10.1016/j.jaad.2021.09.022
- Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi:10.1016/j.jaad.2022.08.041
- Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi:10.1016/j.jaad.2022.11.031
- Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas on the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).

Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to one or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is reexposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 Allergic contact dermatitis is a challenge to manage, as complete avoidance of the allergen may not be possible.8
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%- 23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N=19,457); 17,803 (92.9%) of these patients were White and only 1360 (7.1%) were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
Allergic contact dermatitis is more common in women, with nickel being the most frequently identified allergen (Figure, A).10 Personal care products often are linked to ACD (Figure, B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD)(a common component of hair dye) (Figure, C).12
There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative) (9.1% vs 2.6%) compared to White men.13
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. Allergic contact dermatitis due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on day 1 and covered. Then, on day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N=1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children aged 0–12 years) were significantly lower than for other groups when ACD was suspected (P<.0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23
The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas on the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).

Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to one or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is reexposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 Allergic contact dermatitis is a challenge to manage, as complete avoidance of the allergen may not be possible.8
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%- 23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N=19,457); 17,803 (92.9%) of these patients were White and only 1360 (7.1%) were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
Allergic contact dermatitis is more common in women, with nickel being the most frequently identified allergen (Figure, A).10 Personal care products often are linked to ACD (Figure, B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD)(a common component of hair dye) (Figure, C).12
There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative) (9.1% vs 2.6%) compared to White men.13
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. Allergic contact dermatitis due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on day 1 and covered. Then, on day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N=1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children aged 0–12 years) were significantly lower than for other groups when ACD was suspected (P<.0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23
The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74: 1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi:10.1016/j.jaci.2022.02.002
- Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi:10.1007/s11882-023-01070-5
- Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi:10.1111/j .1365-2133.2005.06415.x
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Nielsen NH, Menne T. The relationship between IgE‐mediated and cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi:10.1111/j.1365-2133.1996.tb06967.x
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998- 2006. Dermatitis. 2016;27:288-292. doi:10.1097/DER.0000000000000220
- Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi:10.1016/j.jaad.2020.10.003
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi:10.1053/ajcd.2001.20110
- DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi:10.1067/mjd.2002.120792
- Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi:10.12788/cutis.0292
- Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi:10.1111/pde.14578
- Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. StatPearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459230/
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi:10.1016/j.jaad.2018.08.049
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi:10.1097 /DER.0000000000000581
- Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi:10.1016/j.jaad.2021.09.022
- Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi:10.1016/j.jaad.2022.08.041
- Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi:10.1016/j.jaad.2022.11.031
- Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74: 1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi:10.1016/j.jaci.2022.02.002
- Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi:10.1007/s11882-023-01070-5
- Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi:10.1111/j .1365-2133.2005.06415.x
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Nielsen NH, Menne T. The relationship between IgE‐mediated and cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi:10.1111/j.1365-2133.1996.tb06967.x
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998- 2006. Dermatitis. 2016;27:288-292. doi:10.1097/DER.0000000000000220
- Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi:10.1016/j.jaad.2020.10.003
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi:10.1053/ajcd.2001.20110
- DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi:10.1067/mjd.2002.120792
- Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi:10.12788/cutis.0292
- Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi:10.1111/pde.14578
- Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. StatPearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459230/
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi:10.1016/j.jaad.2018.08.049
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi:10.1097 /DER.0000000000000581
- Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi:10.1016/j.jaad.2021.09.022
- Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi:10.1016/j.jaad.2022.08.041
- Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi:10.1016/j.jaad.2022.11.031
- Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.