User login
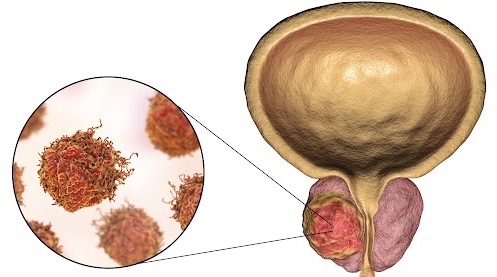
Transgender Women and Prostate Cancer: It’s Complicated
The Veterans Health Administration (VHA) provides care for about 10,000 transgender women, and clinicians must understand their distinctive needs for prostate cancer screening, a urologist told cancer specialists during a presentation at the 2024 annual meeting of the Association of VA Hematology/Oncology in Atlanta.
Even if they’ve undergone gender reassignment surgery, “all transgender women still have a prostate, so therefore they remain at risk of prostate cancer and could still be considered for prostate cancer screening,” said Farnoosh Nik-Ahd, MD, a resident physician at the University of California San Francisco. However, “clinicians and patients may not be aware of prostate cancer risk, so that they may not think [of screening] transgender women.”
Nik-Ahd also noted another complication: The results of prostate screening tests may be misleading in this population.
Transgender women were born biologically male but now identify as female. These individuals may have undergone gender reassignment surgery to remove male genitalia, but the procedures do not remove the prostate. They also might be taking estrogen therapy. “Prostate cancer is a hormonally driven cancer, and the exact impact of gender-affirming hormones on prostate cancer risk and development is unknown,” Nik-Ahd said.
In a 2023 study in JAMA, Nik-Ahd and colleagues identified 155 cases of prostate cancer in transgender women within the VHA (about 14 cases per year) from 2000 to 2022. Of these patients, 116 had never used estrogen, while 17 had used it previously and 22 used it at diagnosis.
The median age of patients was 61 years, 88% identified as White, and the median prostate-specific antigen (PSA) was 6.8 ng/mL. “Given estimates of 10,000 transgender women in the US Department of Veterans Affairs, 33 cases per year would be expected. Instead, only about 14 per year were observed,” the researchers wrote. “Lower rates may stem from less PSA screening owing to barriers including lack of prostate cancer risk awareness or stigma, the suppressive effects of estrogen on prostate cancer development, or prostate cancers being missed in transgender women because of misinterpretation of ‘normal’ PSA levels among those receiving gender-affirming hormone therapies.”
In the presentation, Nik-Ahd said, “PSA density, which is a marker of prostate cancer aggressiveness, was highest in transgender women who were actively on estrogen.”
She noted, “the existing thyrotropin reference ranges, which is what we use to interpret PSA values, are all based on data from cisgender men.” The ranges would be expected to be far lower in transgender women who are taking estrogen, potentially throwing off screening tests, she said, and “ultimately missing clinically significant prostate cancer.”
In the larger picture, there are no specific guidelines about PSA screening in transgender women, she said.
A recent study published in JAMA by Nik-Ahd and colleagues examined PSA levels in 210 transgender women (mean age 60 years) treated within the VHA from 2000 to 2023. All were aged 40 to 80 years, had received estrogen for at least 6 months (mean duration 4.7 years), and didn’t have prostate cancer diagnoses.
“Median (IQR) PSA was 0.02 (0-0.2) ng/mL and the 95th percentile value was 0.6 ng/mL,” the report found. “PSAs were undetectable in 36% of patients (23% and 49% of PSAs in patients without and with orchiectomy, respectively).”
The researchers write that “the historic cut point of 4 ng/mL, often used as a threshold for further evaluation, is likely far too high a threshold for this population.”
Nik-Ahd noted, “clinicians should interpret PSA values in transgender women on estrogen with extreme caution. In this population, normal might actually not be normal, and a value that is considered normal might be very abnormal for somebody who is on estrogen. If you're unsure of whether a PSA value is appropriate for a transgender woman on estrogen, refer that patient to a urologist so they can undergo further evaluation.”
Farnoosh Nik-Ahd discloses consulting for Janssen.
The Veterans Health Administration (VHA) provides care for about 10,000 transgender women, and clinicians must understand their distinctive needs for prostate cancer screening, a urologist told cancer specialists during a presentation at the 2024 annual meeting of the Association of VA Hematology/Oncology in Atlanta.
Even if they’ve undergone gender reassignment surgery, “all transgender women still have a prostate, so therefore they remain at risk of prostate cancer and could still be considered for prostate cancer screening,” said Farnoosh Nik-Ahd, MD, a resident physician at the University of California San Francisco. However, “clinicians and patients may not be aware of prostate cancer risk, so that they may not think [of screening] transgender women.”
Nik-Ahd also noted another complication: The results of prostate screening tests may be misleading in this population.
Transgender women were born biologically male but now identify as female. These individuals may have undergone gender reassignment surgery to remove male genitalia, but the procedures do not remove the prostate. They also might be taking estrogen therapy. “Prostate cancer is a hormonally driven cancer, and the exact impact of gender-affirming hormones on prostate cancer risk and development is unknown,” Nik-Ahd said.
In a 2023 study in JAMA, Nik-Ahd and colleagues identified 155 cases of prostate cancer in transgender women within the VHA (about 14 cases per year) from 2000 to 2022. Of these patients, 116 had never used estrogen, while 17 had used it previously and 22 used it at diagnosis.
The median age of patients was 61 years, 88% identified as White, and the median prostate-specific antigen (PSA) was 6.8 ng/mL. “Given estimates of 10,000 transgender women in the US Department of Veterans Affairs, 33 cases per year would be expected. Instead, only about 14 per year were observed,” the researchers wrote. “Lower rates may stem from less PSA screening owing to barriers including lack of prostate cancer risk awareness or stigma, the suppressive effects of estrogen on prostate cancer development, or prostate cancers being missed in transgender women because of misinterpretation of ‘normal’ PSA levels among those receiving gender-affirming hormone therapies.”
In the presentation, Nik-Ahd said, “PSA density, which is a marker of prostate cancer aggressiveness, was highest in transgender women who were actively on estrogen.”
She noted, “the existing thyrotropin reference ranges, which is what we use to interpret PSA values, are all based on data from cisgender men.” The ranges would be expected to be far lower in transgender women who are taking estrogen, potentially throwing off screening tests, she said, and “ultimately missing clinically significant prostate cancer.”
In the larger picture, there are no specific guidelines about PSA screening in transgender women, she said.
A recent study published in JAMA by Nik-Ahd and colleagues examined PSA levels in 210 transgender women (mean age 60 years) treated within the VHA from 2000 to 2023. All were aged 40 to 80 years, had received estrogen for at least 6 months (mean duration 4.7 years), and didn’t have prostate cancer diagnoses.
“Median (IQR) PSA was 0.02 (0-0.2) ng/mL and the 95th percentile value was 0.6 ng/mL,” the report found. “PSAs were undetectable in 36% of patients (23% and 49% of PSAs in patients without and with orchiectomy, respectively).”
The researchers write that “the historic cut point of 4 ng/mL, often used as a threshold for further evaluation, is likely far too high a threshold for this population.”
Nik-Ahd noted, “clinicians should interpret PSA values in transgender women on estrogen with extreme caution. In this population, normal might actually not be normal, and a value that is considered normal might be very abnormal for somebody who is on estrogen. If you're unsure of whether a PSA value is appropriate for a transgender woman on estrogen, refer that patient to a urologist so they can undergo further evaluation.”
Farnoosh Nik-Ahd discloses consulting for Janssen.
The Veterans Health Administration (VHA) provides care for about 10,000 transgender women, and clinicians must understand their distinctive needs for prostate cancer screening, a urologist told cancer specialists during a presentation at the 2024 annual meeting of the Association of VA Hematology/Oncology in Atlanta.
Even if they’ve undergone gender reassignment surgery, “all transgender women still have a prostate, so therefore they remain at risk of prostate cancer and could still be considered for prostate cancer screening,” said Farnoosh Nik-Ahd, MD, a resident physician at the University of California San Francisco. However, “clinicians and patients may not be aware of prostate cancer risk, so that they may not think [of screening] transgender women.”
Nik-Ahd also noted another complication: The results of prostate screening tests may be misleading in this population.
Transgender women were born biologically male but now identify as female. These individuals may have undergone gender reassignment surgery to remove male genitalia, but the procedures do not remove the prostate. They also might be taking estrogen therapy. “Prostate cancer is a hormonally driven cancer, and the exact impact of gender-affirming hormones on prostate cancer risk and development is unknown,” Nik-Ahd said.
In a 2023 study in JAMA, Nik-Ahd and colleagues identified 155 cases of prostate cancer in transgender women within the VHA (about 14 cases per year) from 2000 to 2022. Of these patients, 116 had never used estrogen, while 17 had used it previously and 22 used it at diagnosis.
The median age of patients was 61 years, 88% identified as White, and the median prostate-specific antigen (PSA) was 6.8 ng/mL. “Given estimates of 10,000 transgender women in the US Department of Veterans Affairs, 33 cases per year would be expected. Instead, only about 14 per year were observed,” the researchers wrote. “Lower rates may stem from less PSA screening owing to barriers including lack of prostate cancer risk awareness or stigma, the suppressive effects of estrogen on prostate cancer development, or prostate cancers being missed in transgender women because of misinterpretation of ‘normal’ PSA levels among those receiving gender-affirming hormone therapies.”
In the presentation, Nik-Ahd said, “PSA density, which is a marker of prostate cancer aggressiveness, was highest in transgender women who were actively on estrogen.”
She noted, “the existing thyrotropin reference ranges, which is what we use to interpret PSA values, are all based on data from cisgender men.” The ranges would be expected to be far lower in transgender women who are taking estrogen, potentially throwing off screening tests, she said, and “ultimately missing clinically significant prostate cancer.”
In the larger picture, there are no specific guidelines about PSA screening in transgender women, she said.
A recent study published in JAMA by Nik-Ahd and colleagues examined PSA levels in 210 transgender women (mean age 60 years) treated within the VHA from 2000 to 2023. All were aged 40 to 80 years, had received estrogen for at least 6 months (mean duration 4.7 years), and didn’t have prostate cancer diagnoses.
“Median (IQR) PSA was 0.02 (0-0.2) ng/mL and the 95th percentile value was 0.6 ng/mL,” the report found. “PSAs were undetectable in 36% of patients (23% and 49% of PSAs in patients without and with orchiectomy, respectively).”
The researchers write that “the historic cut point of 4 ng/mL, often used as a threshold for further evaluation, is likely far too high a threshold for this population.”
Nik-Ahd noted, “clinicians should interpret PSA values in transgender women on estrogen with extreme caution. In this population, normal might actually not be normal, and a value that is considered normal might be very abnormal for somebody who is on estrogen. If you're unsure of whether a PSA value is appropriate for a transgender woman on estrogen, refer that patient to a urologist so they can undergo further evaluation.”
Farnoosh Nik-Ahd discloses consulting for Janssen.
VA Choice Bill Defeated in the House
A U.S. House of Representatives appropriation to fund the Veterans Choice Program surprisingly went down to defeat on Monday. The VA Choice Program is set to run out of money in September, and VA officials have been calling for Congress to provide additional funding for the program. Republican leaders, hoping to expedite the bill’s passage and thinking that it was not controversial, submitted the bill in a process that required the votes of two-thirds of the representatives. The 219-186 vote fell well short of the necessary two-thirds, and voting fell largely along party lines.
Many veterans service organizations (VSOs) were critical of the bill and called on the House to make substantial changes to it. Seven VSOs signed a joint statement calling for the bill’s defeat. “As organizations who represent and support the interests of America’s 21 million veterans, and in fulfillment of our mandate to ensure that the men and women who served are able to receive the health care and benefits they need and deserve, we are calling on Members of Congress to defeat the House vote on unacceptable choice funding legislation (S. 114, with amendments),” the statement read.
AMVETS, Disabled American Veterans , Military Officers Association of America, Military Order of the Purple Heart, Veterans of Foreign Wars, Vietnam Veterans of America, and Wounded Warrior Project all signed on to the statement. The chief complaint was that the legislation “includes funding only for the ‘choice’ program which provides additional community care options, but makes no investment in VA and uses ‘savings’ from other veterans benefits or services to ‘pay’ for the ‘choice’ program.”
The bill would have allocated $2 billion for the Veterans Choice Program, taken funding for veteran housing loan fees, and would reduce the pensions for some veterans living in nursing facilities that also could be paid for under the Medicaid program.
The fate of the bill and funding for the Veterans Choice Program remains unclear. Senate and House veterans committees seem to be far apart on how to fund the program and for efforts to make more substantive changes to the program. Although House Republicans eventually may be able to pass a bill without Democrats, in the Senate, they will need the support of at least a handful of Democrats to move the bill to the President’s desk.
A U.S. House of Representatives appropriation to fund the Veterans Choice Program surprisingly went down to defeat on Monday. The VA Choice Program is set to run out of money in September, and VA officials have been calling for Congress to provide additional funding for the program. Republican leaders, hoping to expedite the bill’s passage and thinking that it was not controversial, submitted the bill in a process that required the votes of two-thirds of the representatives. The 219-186 vote fell well short of the necessary two-thirds, and voting fell largely along party lines.
Many veterans service organizations (VSOs) were critical of the bill and called on the House to make substantial changes to it. Seven VSOs signed a joint statement calling for the bill’s defeat. “As organizations who represent and support the interests of America’s 21 million veterans, and in fulfillment of our mandate to ensure that the men and women who served are able to receive the health care and benefits they need and deserve, we are calling on Members of Congress to defeat the House vote on unacceptable choice funding legislation (S. 114, with amendments),” the statement read.
AMVETS, Disabled American Veterans , Military Officers Association of America, Military Order of the Purple Heart, Veterans of Foreign Wars, Vietnam Veterans of America, and Wounded Warrior Project all signed on to the statement. The chief complaint was that the legislation “includes funding only for the ‘choice’ program which provides additional community care options, but makes no investment in VA and uses ‘savings’ from other veterans benefits or services to ‘pay’ for the ‘choice’ program.”
The bill would have allocated $2 billion for the Veterans Choice Program, taken funding for veteran housing loan fees, and would reduce the pensions for some veterans living in nursing facilities that also could be paid for under the Medicaid program.
The fate of the bill and funding for the Veterans Choice Program remains unclear. Senate and House veterans committees seem to be far apart on how to fund the program and for efforts to make more substantive changes to the program. Although House Republicans eventually may be able to pass a bill without Democrats, in the Senate, they will need the support of at least a handful of Democrats to move the bill to the President’s desk.
A U.S. House of Representatives appropriation to fund the Veterans Choice Program surprisingly went down to defeat on Monday. The VA Choice Program is set to run out of money in September, and VA officials have been calling for Congress to provide additional funding for the program. Republican leaders, hoping to expedite the bill’s passage and thinking that it was not controversial, submitted the bill in a process that required the votes of two-thirds of the representatives. The 219-186 vote fell well short of the necessary two-thirds, and voting fell largely along party lines.
Many veterans service organizations (VSOs) were critical of the bill and called on the House to make substantial changes to it. Seven VSOs signed a joint statement calling for the bill’s defeat. “As organizations who represent and support the interests of America’s 21 million veterans, and in fulfillment of our mandate to ensure that the men and women who served are able to receive the health care and benefits they need and deserve, we are calling on Members of Congress to defeat the House vote on unacceptable choice funding legislation (S. 114, with amendments),” the statement read.
AMVETS, Disabled American Veterans , Military Officers Association of America, Military Order of the Purple Heart, Veterans of Foreign Wars, Vietnam Veterans of America, and Wounded Warrior Project all signed on to the statement. The chief complaint was that the legislation “includes funding only for the ‘choice’ program which provides additional community care options, but makes no investment in VA and uses ‘savings’ from other veterans benefits or services to ‘pay’ for the ‘choice’ program.”
The bill would have allocated $2 billion for the Veterans Choice Program, taken funding for veteran housing loan fees, and would reduce the pensions for some veterans living in nursing facilities that also could be paid for under the Medicaid program.
The fate of the bill and funding for the Veterans Choice Program remains unclear. Senate and House veterans committees seem to be far apart on how to fund the program and for efforts to make more substantive changes to the program. Although House Republicans eventually may be able to pass a bill without Democrats, in the Senate, they will need the support of at least a handful of Democrats to move the bill to the President’s desk.
VA Awards Grants to Support Adaptive Sports
The US Department of Veterans Affairs (VA) is awarding $15.9 million in grants to fund adaptive sports, recreational activities, and equine therapy for > 15,000 veterans and service members living with disabilities.
Marine Corps veteran Jataya Taylor — who competed in wheelchair fencing at the 2024 Paralympics — experienced mental health symptoms until she began participating in adaptive sports through an organization supported by the VA Adaptive Sports Grant Program.
“Getting involved in adaptive sports was a saving grace for me,” Taylor said. “Participating in these programs got me on the bike to start with, then got me climbing, and eventually it became an important part of my mental health to participate. I found my people. I found my new network of friends.”
Adaptive sports, which are customized to fit the needs of veterans with disabilities, include paralympic sports, archery, cycling, skiing, hunting, rock climbing, and sky diving. Mike Gooler, another Marine Corps veteran, praised the Adaptive Sports Center’s facilities in Crested Butte, Colorado, calling it “nothing short of amazing.”
“[S]ki therapy has been instrumental in helping me navigate through my experiences and injuries,” Gooler said. “Skiing provides me with sense of freedom and empowerment … and having my family by my side, witnessing my progress and sharing the joy of skiing, was truly special.”
The grant program is facilitated and managed by the National Veterans Sports Programs and Special Events Office and will provide grants to 91 national, regional, and community-based programs for fiscal year 2024 across all 50 states, the District of Columbia, Guam, and Puerto Rico.
“These grants give veterans life-changing opportunities,” Secretary of VA Denis McDonough said. “We know adaptive sports and recreational activities can be transformational for veterans living with disabilities, improving their overall physical and mental health, and also giving them important community with fellow heroes who served.”
Information about the awardees and details of the program are available at www.va.gov/adaptivesports and on Facebook at Sports4Vets.
The US Department of Veterans Affairs (VA) is awarding $15.9 million in grants to fund adaptive sports, recreational activities, and equine therapy for > 15,000 veterans and service members living with disabilities.
Marine Corps veteran Jataya Taylor — who competed in wheelchair fencing at the 2024 Paralympics — experienced mental health symptoms until she began participating in adaptive sports through an organization supported by the VA Adaptive Sports Grant Program.
“Getting involved in adaptive sports was a saving grace for me,” Taylor said. “Participating in these programs got me on the bike to start with, then got me climbing, and eventually it became an important part of my mental health to participate. I found my people. I found my new network of friends.”
Adaptive sports, which are customized to fit the needs of veterans with disabilities, include paralympic sports, archery, cycling, skiing, hunting, rock climbing, and sky diving. Mike Gooler, another Marine Corps veteran, praised the Adaptive Sports Center’s facilities in Crested Butte, Colorado, calling it “nothing short of amazing.”
“[S]ki therapy has been instrumental in helping me navigate through my experiences and injuries,” Gooler said. “Skiing provides me with sense of freedom and empowerment … and having my family by my side, witnessing my progress and sharing the joy of skiing, was truly special.”
The grant program is facilitated and managed by the National Veterans Sports Programs and Special Events Office and will provide grants to 91 national, regional, and community-based programs for fiscal year 2024 across all 50 states, the District of Columbia, Guam, and Puerto Rico.
“These grants give veterans life-changing opportunities,” Secretary of VA Denis McDonough said. “We know adaptive sports and recreational activities can be transformational for veterans living with disabilities, improving their overall physical and mental health, and also giving them important community with fellow heroes who served.”
Information about the awardees and details of the program are available at www.va.gov/adaptivesports and on Facebook at Sports4Vets.
The US Department of Veterans Affairs (VA) is awarding $15.9 million in grants to fund adaptive sports, recreational activities, and equine therapy for > 15,000 veterans and service members living with disabilities.
Marine Corps veteran Jataya Taylor — who competed in wheelchair fencing at the 2024 Paralympics — experienced mental health symptoms until she began participating in adaptive sports through an organization supported by the VA Adaptive Sports Grant Program.
“Getting involved in adaptive sports was a saving grace for me,” Taylor said. “Participating in these programs got me on the bike to start with, then got me climbing, and eventually it became an important part of my mental health to participate. I found my people. I found my new network of friends.”
Adaptive sports, which are customized to fit the needs of veterans with disabilities, include paralympic sports, archery, cycling, skiing, hunting, rock climbing, and sky diving. Mike Gooler, another Marine Corps veteran, praised the Adaptive Sports Center’s facilities in Crested Butte, Colorado, calling it “nothing short of amazing.”
“[S]ki therapy has been instrumental in helping me navigate through my experiences and injuries,” Gooler said. “Skiing provides me with sense of freedom and empowerment … and having my family by my side, witnessing my progress and sharing the joy of skiing, was truly special.”
The grant program is facilitated and managed by the National Veterans Sports Programs and Special Events Office and will provide grants to 91 national, regional, and community-based programs for fiscal year 2024 across all 50 states, the District of Columbia, Guam, and Puerto Rico.
“These grants give veterans life-changing opportunities,” Secretary of VA Denis McDonough said. “We know adaptive sports and recreational activities can be transformational for veterans living with disabilities, improving their overall physical and mental health, and also giving them important community with fellow heroes who served.”
Information about the awardees and details of the program are available at www.va.gov/adaptivesports and on Facebook at Sports4Vets.
New mRNA Vaccine May Shield Against C difficile Infections
A group of researchers from the University of Pennsylvania, Philadelphia, has developed a messenger RNA (mRNA) vaccine, delivered via lipid nanoparticles (LNPs) — the same type as the COVID-19 vaccine produced by Moderna and Pfizer — targeting Clostridioides difficile (formerly Clostridium difficile). According to the authors, the results of their preclinical study, published in Science, demonstrated this technology as a promising platform for C difficile vaccine development and could be the starting point for curbing intestinal infections that, in their most severe forms (pseudomembranous colitis, toxic megacolon), can be fatal.
An Increasingly Pressing Issue
C difficile is the leading cause of infectious diarrhea acquired in healthcare settings.
A 2019 study reported a global incidence of C difficile infections at 2.2 per 1000 hospital admissions per year and 3.5 per 10,000 patient-days per year.
The Vaccine Candidate
Vaccine candidates tested so far have used toxoids or recombinant proteins targeting the combined repetitive oligopeptide (CROP) or receptor-binding domain (RBD) of the two primary C difficile toxins, TcdA and TcdB. The US researchers are now exploring the mRNA-LNP vaccine approach to target multiple antigens simultaneously. They developed a bivalent vaccine (including the CROP and RBD domains of both toxins) and a trivalent vaccine (with an additional virulence factor, the metalloprotease Pro-Pro endopeptidase-1).
Mice vaccinated with the bivalent and trivalent vaccines produced immunoglobulin G antibody titers two to four times higher than those elicited by recombinant protein with an adjuvant. The vaccination stimulated the proliferation of follicular T helper cells and the antigen-specific response of B lymphocytes, laying the foundation for a strong and long-lasting humoral response. The vaccines were also immunogenic in hamsters.
Vaccinated mice not only survived a toxin dose five times higher than the 100% lethal dose but also demonstrated the vaccine’s protective effect through serum transfer; unvaccinated mice given serum from vaccinated mice survived the lethal challenge. More importantly, when exposed to a lethal dose of the bacterium itself, all vaccinated mice survived.
To demonstrate the vaccine’s efficacy in patients with a history of C difficile infection and high recurrence risk — ideal candidates for vaccination — the researchers vaccinated mice that had previously survived a sublethal infection. Six months after the initial infection and vaccination, these mice remained protected against mortality when reexposed to the bacterium.
Additionally, a quadrivalent vaccine that included an immunogen targeting C difficile spores — key agents in transmission — also proved effective. Low levels of bacteria and toxins in the feces of mice vaccinated in this way suggested that spore vaccination could limit initial colonization.
In tests with nonhuman primates, two doses of the vaccines targeting either the vegetative form or the spores elicited strong immune responses against bacterial toxins and virulence factors. Human trials may indeed be on the horizon.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
A group of researchers from the University of Pennsylvania, Philadelphia, has developed a messenger RNA (mRNA) vaccine, delivered via lipid nanoparticles (LNPs) — the same type as the COVID-19 vaccine produced by Moderna and Pfizer — targeting Clostridioides difficile (formerly Clostridium difficile). According to the authors, the results of their preclinical study, published in Science, demonstrated this technology as a promising platform for C difficile vaccine development and could be the starting point for curbing intestinal infections that, in their most severe forms (pseudomembranous colitis, toxic megacolon), can be fatal.
An Increasingly Pressing Issue
C difficile is the leading cause of infectious diarrhea acquired in healthcare settings.
A 2019 study reported a global incidence of C difficile infections at 2.2 per 1000 hospital admissions per year and 3.5 per 10,000 patient-days per year.
The Vaccine Candidate
Vaccine candidates tested so far have used toxoids or recombinant proteins targeting the combined repetitive oligopeptide (CROP) or receptor-binding domain (RBD) of the two primary C difficile toxins, TcdA and TcdB. The US researchers are now exploring the mRNA-LNP vaccine approach to target multiple antigens simultaneously. They developed a bivalent vaccine (including the CROP and RBD domains of both toxins) and a trivalent vaccine (with an additional virulence factor, the metalloprotease Pro-Pro endopeptidase-1).
Mice vaccinated with the bivalent and trivalent vaccines produced immunoglobulin G antibody titers two to four times higher than those elicited by recombinant protein with an adjuvant. The vaccination stimulated the proliferation of follicular T helper cells and the antigen-specific response of B lymphocytes, laying the foundation for a strong and long-lasting humoral response. The vaccines were also immunogenic in hamsters.
Vaccinated mice not only survived a toxin dose five times higher than the 100% lethal dose but also demonstrated the vaccine’s protective effect through serum transfer; unvaccinated mice given serum from vaccinated mice survived the lethal challenge. More importantly, when exposed to a lethal dose of the bacterium itself, all vaccinated mice survived.
To demonstrate the vaccine’s efficacy in patients with a history of C difficile infection and high recurrence risk — ideal candidates for vaccination — the researchers vaccinated mice that had previously survived a sublethal infection. Six months after the initial infection and vaccination, these mice remained protected against mortality when reexposed to the bacterium.
Additionally, a quadrivalent vaccine that included an immunogen targeting C difficile spores — key agents in transmission — also proved effective. Low levels of bacteria and toxins in the feces of mice vaccinated in this way suggested that spore vaccination could limit initial colonization.
In tests with nonhuman primates, two doses of the vaccines targeting either the vegetative form or the spores elicited strong immune responses against bacterial toxins and virulence factors. Human trials may indeed be on the horizon.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
A group of researchers from the University of Pennsylvania, Philadelphia, has developed a messenger RNA (mRNA) vaccine, delivered via lipid nanoparticles (LNPs) — the same type as the COVID-19 vaccine produced by Moderna and Pfizer — targeting Clostridioides difficile (formerly Clostridium difficile). According to the authors, the results of their preclinical study, published in Science, demonstrated this technology as a promising platform for C difficile vaccine development and could be the starting point for curbing intestinal infections that, in their most severe forms (pseudomembranous colitis, toxic megacolon), can be fatal.
An Increasingly Pressing Issue
C difficile is the leading cause of infectious diarrhea acquired in healthcare settings.
A 2019 study reported a global incidence of C difficile infections at 2.2 per 1000 hospital admissions per year and 3.5 per 10,000 patient-days per year.
The Vaccine Candidate
Vaccine candidates tested so far have used toxoids or recombinant proteins targeting the combined repetitive oligopeptide (CROP) or receptor-binding domain (RBD) of the two primary C difficile toxins, TcdA and TcdB. The US researchers are now exploring the mRNA-LNP vaccine approach to target multiple antigens simultaneously. They developed a bivalent vaccine (including the CROP and RBD domains of both toxins) and a trivalent vaccine (with an additional virulence factor, the metalloprotease Pro-Pro endopeptidase-1).
Mice vaccinated with the bivalent and trivalent vaccines produced immunoglobulin G antibody titers two to four times higher than those elicited by recombinant protein with an adjuvant. The vaccination stimulated the proliferation of follicular T helper cells and the antigen-specific response of B lymphocytes, laying the foundation for a strong and long-lasting humoral response. The vaccines were also immunogenic in hamsters.
Vaccinated mice not only survived a toxin dose five times higher than the 100% lethal dose but also demonstrated the vaccine’s protective effect through serum transfer; unvaccinated mice given serum from vaccinated mice survived the lethal challenge. More importantly, when exposed to a lethal dose of the bacterium itself, all vaccinated mice survived.
To demonstrate the vaccine’s efficacy in patients with a history of C difficile infection and high recurrence risk — ideal candidates for vaccination — the researchers vaccinated mice that had previously survived a sublethal infection. Six months after the initial infection and vaccination, these mice remained protected against mortality when reexposed to the bacterium.
Additionally, a quadrivalent vaccine that included an immunogen targeting C difficile spores — key agents in transmission — also proved effective. Low levels of bacteria and toxins in the feces of mice vaccinated in this way suggested that spore vaccination could limit initial colonization.
In tests with nonhuman primates, two doses of the vaccines targeting either the vegetative form or the spores elicited strong immune responses against bacterial toxins and virulence factors. Human trials may indeed be on the horizon.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Disc Degeneration in Chronic Low Back Pain: Can Stem Cells Help?
TOPLINE:
Allogeneic bone marrow–derived mesenchymal stromal cells (BM-MSCs) are safe but do not show efficacy in treating intervertebral disc degeneration (IDD) in patients with chronic low back pain.
METHODOLOGY:
- The RESPINE trial assessed the efficacy and safety of a single intradiscal injection of allogeneic BM-MSCs in the treatment of chronic low back pain caused by single-level IDD.
- Overall, 114 patients (mean age, 40.9 years; 35% women) with IDD-associated chronic low back pain that was persistent for 3 months or more despite conventional medical therapy and without previous surgery, were recruited across four European countries from April 2018 to April 2021 and randomly assigned to receive either intradiscal injections of allogeneic BM-MSCs (n = 58) or sham injections (n = 56).
- The first co-primary endpoint was the rate of response to BM-MSC injections at 12 months after treatment, defined as improvement of at least 20% or 20 mm in the Visual Analog Scale for pain or improvement of at least 20% in the Oswestry Disability Index for functional status.
- The secondary co-primary endpoint was structural efficacy, based on disc fluid content measured by quantitative T2 MRI between baseline and month 12.
TAKEAWAY:
- At 12 months post-intervention, 74% of patients in the BM-MSC group were classified as responders compared with 68.8% in the placebo group. However, the difference between the groups was not statistically significant.
- The probability of being a responder was higher in the BM-MSC group than in the sham group; however, the findings did not reach statistical significance.
- The average change in disc fluid content, indicative of disc regeneration, from baseline to 12 months was 37.9% in the BM-MSC group and 41.7% in the placebo group, with no significant difference between the groups.
- The incidence of adverse events and serious adverse events was not significantly different between the treatment groups.
IN PRACTICE:
“BM-MSC represents a promising opportunity for the biological treatment of IDD, but only high-quality randomized controlled trials, comparing it to standard care, can determine whether it is a truly effective alternative to spine fusion or disc replacement,” the authors wrote.
SOURCE:
The study was led by Yves-Marie Pers, MD, PhD, Clinical Immunology and Osteoarticular Diseases Therapeutic Unit, CHRU Lapeyronie, Montpellier, France. It was published online on October 11, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
MRI results were collected from only 55 patients across both trial arms, which may have affected the statistical power of the findings. Although patients were monitored for up to 24 months, the long-term efficacy and safety of BM-MSC therapy for IDD may not have been fully captured. Selection bias could not be excluded because of the difficulty in accurately identifying patients with chronic low back pain caused by single-level IDD.
DISCLOSURES:
The study was funded by the European Union’s Horizon 2020 Research and Innovation Programme. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Allogeneic bone marrow–derived mesenchymal stromal cells (BM-MSCs) are safe but do not show efficacy in treating intervertebral disc degeneration (IDD) in patients with chronic low back pain.
METHODOLOGY:
- The RESPINE trial assessed the efficacy and safety of a single intradiscal injection of allogeneic BM-MSCs in the treatment of chronic low back pain caused by single-level IDD.
- Overall, 114 patients (mean age, 40.9 years; 35% women) with IDD-associated chronic low back pain that was persistent for 3 months or more despite conventional medical therapy and without previous surgery, were recruited across four European countries from April 2018 to April 2021 and randomly assigned to receive either intradiscal injections of allogeneic BM-MSCs (n = 58) or sham injections (n = 56).
- The first co-primary endpoint was the rate of response to BM-MSC injections at 12 months after treatment, defined as improvement of at least 20% or 20 mm in the Visual Analog Scale for pain or improvement of at least 20% in the Oswestry Disability Index for functional status.
- The secondary co-primary endpoint was structural efficacy, based on disc fluid content measured by quantitative T2 MRI between baseline and month 12.
TAKEAWAY:
- At 12 months post-intervention, 74% of patients in the BM-MSC group were classified as responders compared with 68.8% in the placebo group. However, the difference between the groups was not statistically significant.
- The probability of being a responder was higher in the BM-MSC group than in the sham group; however, the findings did not reach statistical significance.
- The average change in disc fluid content, indicative of disc regeneration, from baseline to 12 months was 37.9% in the BM-MSC group and 41.7% in the placebo group, with no significant difference between the groups.
- The incidence of adverse events and serious adverse events was not significantly different between the treatment groups.
IN PRACTICE:
“BM-MSC represents a promising opportunity for the biological treatment of IDD, but only high-quality randomized controlled trials, comparing it to standard care, can determine whether it is a truly effective alternative to spine fusion or disc replacement,” the authors wrote.
SOURCE:
The study was led by Yves-Marie Pers, MD, PhD, Clinical Immunology and Osteoarticular Diseases Therapeutic Unit, CHRU Lapeyronie, Montpellier, France. It was published online on October 11, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
MRI results were collected from only 55 patients across both trial arms, which may have affected the statistical power of the findings. Although patients were monitored for up to 24 months, the long-term efficacy and safety of BM-MSC therapy for IDD may not have been fully captured. Selection bias could not be excluded because of the difficulty in accurately identifying patients with chronic low back pain caused by single-level IDD.
DISCLOSURES:
The study was funded by the European Union’s Horizon 2020 Research and Innovation Programme. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Allogeneic bone marrow–derived mesenchymal stromal cells (BM-MSCs) are safe but do not show efficacy in treating intervertebral disc degeneration (IDD) in patients with chronic low back pain.
METHODOLOGY:
- The RESPINE trial assessed the efficacy and safety of a single intradiscal injection of allogeneic BM-MSCs in the treatment of chronic low back pain caused by single-level IDD.
- Overall, 114 patients (mean age, 40.9 years; 35% women) with IDD-associated chronic low back pain that was persistent for 3 months or more despite conventional medical therapy and without previous surgery, were recruited across four European countries from April 2018 to April 2021 and randomly assigned to receive either intradiscal injections of allogeneic BM-MSCs (n = 58) or sham injections (n = 56).
- The first co-primary endpoint was the rate of response to BM-MSC injections at 12 months after treatment, defined as improvement of at least 20% or 20 mm in the Visual Analog Scale for pain or improvement of at least 20% in the Oswestry Disability Index for functional status.
- The secondary co-primary endpoint was structural efficacy, based on disc fluid content measured by quantitative T2 MRI between baseline and month 12.
TAKEAWAY:
- At 12 months post-intervention, 74% of patients in the BM-MSC group were classified as responders compared with 68.8% in the placebo group. However, the difference between the groups was not statistically significant.
- The probability of being a responder was higher in the BM-MSC group than in the sham group; however, the findings did not reach statistical significance.
- The average change in disc fluid content, indicative of disc regeneration, from baseline to 12 months was 37.9% in the BM-MSC group and 41.7% in the placebo group, with no significant difference between the groups.
- The incidence of adverse events and serious adverse events was not significantly different between the treatment groups.
IN PRACTICE:
“BM-MSC represents a promising opportunity for the biological treatment of IDD, but only high-quality randomized controlled trials, comparing it to standard care, can determine whether it is a truly effective alternative to spine fusion or disc replacement,” the authors wrote.
SOURCE:
The study was led by Yves-Marie Pers, MD, PhD, Clinical Immunology and Osteoarticular Diseases Therapeutic Unit, CHRU Lapeyronie, Montpellier, France. It was published online on October 11, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
MRI results were collected from only 55 patients across both trial arms, which may have affected the statistical power of the findings. Although patients were monitored for up to 24 months, the long-term efficacy and safety of BM-MSC therapy for IDD may not have been fully captured. Selection bias could not be excluded because of the difficulty in accurately identifying patients with chronic low back pain caused by single-level IDD.
DISCLOSURES:
The study was funded by the European Union’s Horizon 2020 Research and Innovation Programme. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Bipartisan Bill to Provide Free Gun Lockboxes to Veterans
About 7 of every 10 veterans who die by suicide involve the use of a firearm. A reason for this high rate is access, as half of veterans report owning ≥ 1 personal firearms. Of those individuals, more than half report storing firearms loaded and/or unsecured and one-third of veterans who store their firearms loaded and unlocked do not own a lockbox or safe.
Suicide death prevention has improved as firearms have become more difficult to obtain. That’s why Navy veteran Rep. Chris Deluzio (D-PA), former FBI Special Agent and federal prosecutor Rep. Brian Fitzpatrick (R-PA), and Rep. Greg Landsman (D-OH) have teamed up to introduce the Saving Our Veterans Lives Act of 2024. Under the proposed act, any veteran would be able to get a free lockbox from the US Department of Veterans Affairs (VA).
Suicidal crises can be brief. According to the VA, if a person experiencing a suicidal crisis can’t access the method they planned to use, they generally do not seek out other lethal means. Lockboxes are a way of “putting space between thought and trigger,” the VA said.
The VA Suicide Prevention Program distributes free firearm cable locks to any veteran who requests one. However, many veterans favor lockboxes and safes to secure their guns. A VA pilot program offers free lockboxes to veterans enrolled in the Veterans Health Administration who are at an elevated risk for suicide. The program is set to launch in late 2024 and is a collaboration between the Rocky Mountain Mental Illness Research, Education, and Clinical Center for Suicide Prevention, VA National Prosthetics Service, and VA Office of Suicide Prevention.
The proposed bill would make the lockboxes (which typically cost between $25 and $350) free to any veteran, regardless of VA enrollment status or diagnosis. It ensures “sufficient funding for many tens of thousands of lockboxes to be distributed.” The bill would also direct the VA to create a public education campaign on the availability of lockboxes and the importance of secure firearm storage in suicide prevention.
“The alarming and tragic reality is that our veterans face a suicide rate 57% higher than that of civilians,” Rep. Fitzpatrick said. “This commonsense, bipartisan initiative is more than a solution—it's a lifeline.”
The representatives report that the bill has been endorsed by an “unprecedented” number of organizations, including the National Shooting Sports Foundation, Disabled American Veterans, The American Legion, GIFFORDS, Everytown for Gun Safety, Brady, American Psychological Association, American Foundation for Suicide Prevention, and Association of VA Psychologist Leaders.
“Did you know that in some cases only 10 minutes elapse between an individual having suicidal ideation and acting?” American Legion National Commander James LaCoursiere said in the representatives’ press release. “The Saving Our Veterans Lives Act is an important part of preventing suicide as it will provide veterans with the information and means to securely store their firearms to prevent suicide, while still protecting their Second Amendment rights. The Legion commends Rep. Deluzio and his team for bringing this bill forward and for their continued dedication to the welfare of our nation’s veterans.”
"I hear colleagues all the time talk about veteran suicide," Rep. Deluzio said in an interview with Military.com. "It is a problem in my community. It's a problem across the country. Let's take action. This is a chance where we can do it that I think can cut through the politics that normally divide us on these [gun] issues. And I think the coalition supporting the bill tells you, we've got a path to pass it."
About 7 of every 10 veterans who die by suicide involve the use of a firearm. A reason for this high rate is access, as half of veterans report owning ≥ 1 personal firearms. Of those individuals, more than half report storing firearms loaded and/or unsecured and one-third of veterans who store their firearms loaded and unlocked do not own a lockbox or safe.
Suicide death prevention has improved as firearms have become more difficult to obtain. That’s why Navy veteran Rep. Chris Deluzio (D-PA), former FBI Special Agent and federal prosecutor Rep. Brian Fitzpatrick (R-PA), and Rep. Greg Landsman (D-OH) have teamed up to introduce the Saving Our Veterans Lives Act of 2024. Under the proposed act, any veteran would be able to get a free lockbox from the US Department of Veterans Affairs (VA).
Suicidal crises can be brief. According to the VA, if a person experiencing a suicidal crisis can’t access the method they planned to use, they generally do not seek out other lethal means. Lockboxes are a way of “putting space between thought and trigger,” the VA said.
The VA Suicide Prevention Program distributes free firearm cable locks to any veteran who requests one. However, many veterans favor lockboxes and safes to secure their guns. A VA pilot program offers free lockboxes to veterans enrolled in the Veterans Health Administration who are at an elevated risk for suicide. The program is set to launch in late 2024 and is a collaboration between the Rocky Mountain Mental Illness Research, Education, and Clinical Center for Suicide Prevention, VA National Prosthetics Service, and VA Office of Suicide Prevention.
The proposed bill would make the lockboxes (which typically cost between $25 and $350) free to any veteran, regardless of VA enrollment status or diagnosis. It ensures “sufficient funding for many tens of thousands of lockboxes to be distributed.” The bill would also direct the VA to create a public education campaign on the availability of lockboxes and the importance of secure firearm storage in suicide prevention.
“The alarming and tragic reality is that our veterans face a suicide rate 57% higher than that of civilians,” Rep. Fitzpatrick said. “This commonsense, bipartisan initiative is more than a solution—it's a lifeline.”
The representatives report that the bill has been endorsed by an “unprecedented” number of organizations, including the National Shooting Sports Foundation, Disabled American Veterans, The American Legion, GIFFORDS, Everytown for Gun Safety, Brady, American Psychological Association, American Foundation for Suicide Prevention, and Association of VA Psychologist Leaders.
“Did you know that in some cases only 10 minutes elapse between an individual having suicidal ideation and acting?” American Legion National Commander James LaCoursiere said in the representatives’ press release. “The Saving Our Veterans Lives Act is an important part of preventing suicide as it will provide veterans with the information and means to securely store their firearms to prevent suicide, while still protecting their Second Amendment rights. The Legion commends Rep. Deluzio and his team for bringing this bill forward and for their continued dedication to the welfare of our nation’s veterans.”
"I hear colleagues all the time talk about veteran suicide," Rep. Deluzio said in an interview with Military.com. "It is a problem in my community. It's a problem across the country. Let's take action. This is a chance where we can do it that I think can cut through the politics that normally divide us on these [gun] issues. And I think the coalition supporting the bill tells you, we've got a path to pass it."
About 7 of every 10 veterans who die by suicide involve the use of a firearm. A reason for this high rate is access, as half of veterans report owning ≥ 1 personal firearms. Of those individuals, more than half report storing firearms loaded and/or unsecured and one-third of veterans who store their firearms loaded and unlocked do not own a lockbox or safe.
Suicide death prevention has improved as firearms have become more difficult to obtain. That’s why Navy veteran Rep. Chris Deluzio (D-PA), former FBI Special Agent and federal prosecutor Rep. Brian Fitzpatrick (R-PA), and Rep. Greg Landsman (D-OH) have teamed up to introduce the Saving Our Veterans Lives Act of 2024. Under the proposed act, any veteran would be able to get a free lockbox from the US Department of Veterans Affairs (VA).
Suicidal crises can be brief. According to the VA, if a person experiencing a suicidal crisis can’t access the method they planned to use, they generally do not seek out other lethal means. Lockboxes are a way of “putting space between thought and trigger,” the VA said.
The VA Suicide Prevention Program distributes free firearm cable locks to any veteran who requests one. However, many veterans favor lockboxes and safes to secure their guns. A VA pilot program offers free lockboxes to veterans enrolled in the Veterans Health Administration who are at an elevated risk for suicide. The program is set to launch in late 2024 and is a collaboration between the Rocky Mountain Mental Illness Research, Education, and Clinical Center for Suicide Prevention, VA National Prosthetics Service, and VA Office of Suicide Prevention.
The proposed bill would make the lockboxes (which typically cost between $25 and $350) free to any veteran, regardless of VA enrollment status or diagnosis. It ensures “sufficient funding for many tens of thousands of lockboxes to be distributed.” The bill would also direct the VA to create a public education campaign on the availability of lockboxes and the importance of secure firearm storage in suicide prevention.
“The alarming and tragic reality is that our veterans face a suicide rate 57% higher than that of civilians,” Rep. Fitzpatrick said. “This commonsense, bipartisan initiative is more than a solution—it's a lifeline.”
The representatives report that the bill has been endorsed by an “unprecedented” number of organizations, including the National Shooting Sports Foundation, Disabled American Veterans, The American Legion, GIFFORDS, Everytown for Gun Safety, Brady, American Psychological Association, American Foundation for Suicide Prevention, and Association of VA Psychologist Leaders.
“Did you know that in some cases only 10 minutes elapse between an individual having suicidal ideation and acting?” American Legion National Commander James LaCoursiere said in the representatives’ press release. “The Saving Our Veterans Lives Act is an important part of preventing suicide as it will provide veterans with the information and means to securely store their firearms to prevent suicide, while still protecting their Second Amendment rights. The Legion commends Rep. Deluzio and his team for bringing this bill forward and for their continued dedication to the welfare of our nation’s veterans.”
"I hear colleagues all the time talk about veteran suicide," Rep. Deluzio said in an interview with Military.com. "It is a problem in my community. It's a problem across the country. Let's take action. This is a chance where we can do it that I think can cut through the politics that normally divide us on these [gun] issues. And I think the coalition supporting the bill tells you, we've got a path to pass it."
Veterans Affairs Hailed as a ‘Bright Spot’ in ALS Care
SAVANNAH, GEORGIA — , said one expert.
In a plenary address at the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024, Ileana Howard, MD, medical co-director of the ALS Center of Excellence at VA Puget Sound in Seattle, said the recently released National Academies report “Living with ALS” cited the Veterans Administration as “a bright spot in the landscape of ALS care due to its interdisciplinary, holistic, and proactive approach to care.”
Since the early 2000s and the publication of several studies linking active military service with ALS, the US Department of Veterans Affairs (VA) has opened an ALS registry, a tissue and brain biobank, and in 2008, granted 100% presumptive service connection to any individual who served more than 90 days of active duty and was later diagnosed with ALS, she said.
“We now serve approximately 4000 veterans with ALS across the system, and we count 47 full interdisciplinary clinics within VA across the nation, with ALS coordinators designated for all 170 VA facilities, regardless of whether they had an ALS clinic or not, to serve as a navigator for patients and their families, to identify the closest ALS clinic that could meet their needs.”
Multidisciplinary vs Interdisciplinary
Howard emphasized that transdisciplinary collaboration is essential for maintaining an effective system. She pointed out that the term “multidisciplinary” is outdated, referring to teams that work independently but in parallel on the same issue.
In contrast, interdisciplinary teams integrate their assessments into a cohesive plan of care, whereas transdisciplinary teams take it further by combining both their assessments and care plans, allowing for greater intentional overlap.
The VA’s ALS handbook lists approximately 20 essential clinicians for a VA ALS clinic, including recreation therapists, assistive technology specialists, and veteran benefit service officers to assist with disability benefits application, among others, she said.
Essential to this collaboration is “role release,” which deliberately blurs the boundaries between disciplines. “The future of our specialty hinges on effective and selfless collaboration,” she said.
Howard encouraged ALS healthcare providers to move away from outdated terminology rooted in hierarchical team models and to break down silos that no longer benefit either the patients or the care teams.
She noted that while teamwork can enhance patient outcomes and overall health, it has also been associated with better health among healthcare providers. It’s well-known, she said, that neurologists and physiatrists are among the specialties with the highest burnout rates, and ALS teams, in particular, experience significant stress and burnout.
Better Together
A recent Canadian study on resiliency and burnout in ALS clinics surveyed a wide range of practitioners within ALS centers and found respondents drew resiliency through relationships with patients and colleagues, and that there was a strongly expressed desire for increased resources, team building/debriefing, and formal training in emotional exhaustion and burnout.
“A consistent theme was the lack of adequate allied health support (nursing, social work, occupational therapy) to address the complex needs of patients,” said the report’s senior author Kerri Lynn Schellenberg, MD, medical director of the ALS/Motor Neuron Diseases clinic and associate professor at the University of Saskatchewan College of Medicine in Saskatoon, Saskatchewan, Canada.
“The majority of participants felt they would benefit from more consistent team building exercises and debriefing,” noted the authors.
Schellenberg agreed, emphasizing that care teams perform best when there is mutual appreciation and support among members. By learning from one another and reaching consensus together, the care plan benefits from the collective expertise of the team. “We are stronger together,” she said.
Howard and Schellenberg reported no disclosures.
A version of this article appeared on Medscape.com.
SAVANNAH, GEORGIA — , said one expert.
In a plenary address at the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024, Ileana Howard, MD, medical co-director of the ALS Center of Excellence at VA Puget Sound in Seattle, said the recently released National Academies report “Living with ALS” cited the Veterans Administration as “a bright spot in the landscape of ALS care due to its interdisciplinary, holistic, and proactive approach to care.”
Since the early 2000s and the publication of several studies linking active military service with ALS, the US Department of Veterans Affairs (VA) has opened an ALS registry, a tissue and brain biobank, and in 2008, granted 100% presumptive service connection to any individual who served more than 90 days of active duty and was later diagnosed with ALS, she said.
“We now serve approximately 4000 veterans with ALS across the system, and we count 47 full interdisciplinary clinics within VA across the nation, with ALS coordinators designated for all 170 VA facilities, regardless of whether they had an ALS clinic or not, to serve as a navigator for patients and their families, to identify the closest ALS clinic that could meet their needs.”
Multidisciplinary vs Interdisciplinary
Howard emphasized that transdisciplinary collaboration is essential for maintaining an effective system. She pointed out that the term “multidisciplinary” is outdated, referring to teams that work independently but in parallel on the same issue.
In contrast, interdisciplinary teams integrate their assessments into a cohesive plan of care, whereas transdisciplinary teams take it further by combining both their assessments and care plans, allowing for greater intentional overlap.
The VA’s ALS handbook lists approximately 20 essential clinicians for a VA ALS clinic, including recreation therapists, assistive technology specialists, and veteran benefit service officers to assist with disability benefits application, among others, she said.
Essential to this collaboration is “role release,” which deliberately blurs the boundaries between disciplines. “The future of our specialty hinges on effective and selfless collaboration,” she said.
Howard encouraged ALS healthcare providers to move away from outdated terminology rooted in hierarchical team models and to break down silos that no longer benefit either the patients or the care teams.
She noted that while teamwork can enhance patient outcomes and overall health, it has also been associated with better health among healthcare providers. It’s well-known, she said, that neurologists and physiatrists are among the specialties with the highest burnout rates, and ALS teams, in particular, experience significant stress and burnout.
Better Together
A recent Canadian study on resiliency and burnout in ALS clinics surveyed a wide range of practitioners within ALS centers and found respondents drew resiliency through relationships with patients and colleagues, and that there was a strongly expressed desire for increased resources, team building/debriefing, and formal training in emotional exhaustion and burnout.
“A consistent theme was the lack of adequate allied health support (nursing, social work, occupational therapy) to address the complex needs of patients,” said the report’s senior author Kerri Lynn Schellenberg, MD, medical director of the ALS/Motor Neuron Diseases clinic and associate professor at the University of Saskatchewan College of Medicine in Saskatoon, Saskatchewan, Canada.
“The majority of participants felt they would benefit from more consistent team building exercises and debriefing,” noted the authors.
Schellenberg agreed, emphasizing that care teams perform best when there is mutual appreciation and support among members. By learning from one another and reaching consensus together, the care plan benefits from the collective expertise of the team. “We are stronger together,” she said.
Howard and Schellenberg reported no disclosures.
A version of this article appeared on Medscape.com.
SAVANNAH, GEORGIA — , said one expert.
In a plenary address at the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024, Ileana Howard, MD, medical co-director of the ALS Center of Excellence at VA Puget Sound in Seattle, said the recently released National Academies report “Living with ALS” cited the Veterans Administration as “a bright spot in the landscape of ALS care due to its interdisciplinary, holistic, and proactive approach to care.”
Since the early 2000s and the publication of several studies linking active military service with ALS, the US Department of Veterans Affairs (VA) has opened an ALS registry, a tissue and brain biobank, and in 2008, granted 100% presumptive service connection to any individual who served more than 90 days of active duty and was later diagnosed with ALS, she said.
“We now serve approximately 4000 veterans with ALS across the system, and we count 47 full interdisciplinary clinics within VA across the nation, with ALS coordinators designated for all 170 VA facilities, regardless of whether they had an ALS clinic or not, to serve as a navigator for patients and their families, to identify the closest ALS clinic that could meet their needs.”
Multidisciplinary vs Interdisciplinary
Howard emphasized that transdisciplinary collaboration is essential for maintaining an effective system. She pointed out that the term “multidisciplinary” is outdated, referring to teams that work independently but in parallel on the same issue.
In contrast, interdisciplinary teams integrate their assessments into a cohesive plan of care, whereas transdisciplinary teams take it further by combining both their assessments and care plans, allowing for greater intentional overlap.
The VA’s ALS handbook lists approximately 20 essential clinicians for a VA ALS clinic, including recreation therapists, assistive technology specialists, and veteran benefit service officers to assist with disability benefits application, among others, she said.
Essential to this collaboration is “role release,” which deliberately blurs the boundaries between disciplines. “The future of our specialty hinges on effective and selfless collaboration,” she said.
Howard encouraged ALS healthcare providers to move away from outdated terminology rooted in hierarchical team models and to break down silos that no longer benefit either the patients or the care teams.
She noted that while teamwork can enhance patient outcomes and overall health, it has also been associated with better health among healthcare providers. It’s well-known, she said, that neurologists and physiatrists are among the specialties with the highest burnout rates, and ALS teams, in particular, experience significant stress and burnout.
Better Together
A recent Canadian study on resiliency and burnout in ALS clinics surveyed a wide range of practitioners within ALS centers and found respondents drew resiliency through relationships with patients and colleagues, and that there was a strongly expressed desire for increased resources, team building/debriefing, and formal training in emotional exhaustion and burnout.
“A consistent theme was the lack of adequate allied health support (nursing, social work, occupational therapy) to address the complex needs of patients,” said the report’s senior author Kerri Lynn Schellenberg, MD, medical director of the ALS/Motor Neuron Diseases clinic and associate professor at the University of Saskatchewan College of Medicine in Saskatoon, Saskatchewan, Canada.
“The majority of participants felt they would benefit from more consistent team building exercises and debriefing,” noted the authors.
Schellenberg agreed, emphasizing that care teams perform best when there is mutual appreciation and support among members. By learning from one another and reaching consensus together, the care plan benefits from the collective expertise of the team. “We are stronger together,” she said.
Howard and Schellenberg reported no disclosures.
A version of this article appeared on Medscape.com.
FROM AANEM 2024
ACIP Recommends Pneumococcal Vaccine for Adults 50 Years or Older
The US Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) now recommends a pneumococcal conjugate vaccine (PCV) for all PCV-naive adults aged 50 years or older. The new recommendation, which passed with an ACIP member vote of 14 for and one against, expands the current age-based recommendations, which include children younger than 5 years and adults older than 65 years, as well as adults aged 19-64 years with underlying conditions or risk factors who have not received a PCV and certain adults who have received PCV13 but not PCV20.
The decision was based in part on economic analyses of the use of PCV in adults aged 50-64 years in the United States. Miwako Kobayashi, MD, presented the summary of the Pneumococcal Vaccines Work Group’s interpretation of the evidence and the proposed recommendation in a meeting of the ACIP on October 23, 2024, when the ACIP voting occurred.
Data from the CDC show an increase in the relative burden of pneumococcal disease in adults aged 50-64 years based in part on the success of the pediatric PCV program, she said.
Health equity was another main factor in the Work Group’s decision to recommend vaccination for adults aged 50 years or older. “Disparities in pneumococcal vaccine coverage by race and ethnicity exist for both age-based and risk-based indications,” Kobayashi noted in her presentation. The Work Group acknowledged that the overall effect of a vaccine recommendation on health equity is complex, but the majority agreed that the update would improve health equity by increasing vaccine coverage for those with known or unknown risk factors and providing protection at an earlier age when some populations already experience elevated disease rates, she said.
As for safety, the Work Group concluded that the undesirable anticipated effects of PCVs are minimal, despite the potential signal for Guillain-Barré Syndrome, and the CDC and US Food and Drug Administration will continue to monitor post-licensure safety of PCVs.
Support Not Universal
A majority of the ACIP Pneumococcal Vaccines Work Group supported the approved option, but agreed that a future booster dose may be needed, Work Group Chair James Loehr, MD, said in his introductory presentation.
Overall, key uncertainties remain, including indirect effects of new pediatric pneumococcal vaccines on adults, data on the duration of protection with adult vaccinations, and the impact new higher-valency vaccines have on adults, several of which are in development, Loehr said.
A new 21-valent PCV, known as PCV 21, was approved by the FDA for adults aged 18 years or older in June 2024, said Loehr. “PCV21 is not PCV20 with one additional serotype” and provides additional protection, he emphasized. The Work Group examined models involving PCV21 and the existing PCV20. However, a majority of the Work Group agreed that having age-based recommendations based on vaccine product would be more challenging to implement and that insurance coverage may be a factor given the recent approval of PCV21. Therefore, the proposal submitted to the full ACIP was not for a specific PCV.
Notably, Loehr said that, although as Work Group Chair he was tasked with making the motion in favor of the recommendation, he voted against it as a voting member because of his strong opinion that only the PCV21 vaccine is needed for vaccine-naive adults aged 50 or older. “I think that PCV21 is a better vaccine that targets more serotypes,” he said during the discussion. Data presented at the February 2024 ACIP meeting showed more than 80% coverage vs less than 60% coverage for invasive pneumococcal disease with PCV21 vs PCV20 among adults aged 65 years or older and those aged 19-64 years with a risk-based indication, Loehr said.
A version of this article appeared on Medscape.com.
The US Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) now recommends a pneumococcal conjugate vaccine (PCV) for all PCV-naive adults aged 50 years or older. The new recommendation, which passed with an ACIP member vote of 14 for and one against, expands the current age-based recommendations, which include children younger than 5 years and adults older than 65 years, as well as adults aged 19-64 years with underlying conditions or risk factors who have not received a PCV and certain adults who have received PCV13 but not PCV20.
The decision was based in part on economic analyses of the use of PCV in adults aged 50-64 years in the United States. Miwako Kobayashi, MD, presented the summary of the Pneumococcal Vaccines Work Group’s interpretation of the evidence and the proposed recommendation in a meeting of the ACIP on October 23, 2024, when the ACIP voting occurred.
Data from the CDC show an increase in the relative burden of pneumococcal disease in adults aged 50-64 years based in part on the success of the pediatric PCV program, she said.
Health equity was another main factor in the Work Group’s decision to recommend vaccination for adults aged 50 years or older. “Disparities in pneumococcal vaccine coverage by race and ethnicity exist for both age-based and risk-based indications,” Kobayashi noted in her presentation. The Work Group acknowledged that the overall effect of a vaccine recommendation on health equity is complex, but the majority agreed that the update would improve health equity by increasing vaccine coverage for those with known or unknown risk factors and providing protection at an earlier age when some populations already experience elevated disease rates, she said.
As for safety, the Work Group concluded that the undesirable anticipated effects of PCVs are minimal, despite the potential signal for Guillain-Barré Syndrome, and the CDC and US Food and Drug Administration will continue to monitor post-licensure safety of PCVs.
Support Not Universal
A majority of the ACIP Pneumococcal Vaccines Work Group supported the approved option, but agreed that a future booster dose may be needed, Work Group Chair James Loehr, MD, said in his introductory presentation.
Overall, key uncertainties remain, including indirect effects of new pediatric pneumococcal vaccines on adults, data on the duration of protection with adult vaccinations, and the impact new higher-valency vaccines have on adults, several of which are in development, Loehr said.
A new 21-valent PCV, known as PCV 21, was approved by the FDA for adults aged 18 years or older in June 2024, said Loehr. “PCV21 is not PCV20 with one additional serotype” and provides additional protection, he emphasized. The Work Group examined models involving PCV21 and the existing PCV20. However, a majority of the Work Group agreed that having age-based recommendations based on vaccine product would be more challenging to implement and that insurance coverage may be a factor given the recent approval of PCV21. Therefore, the proposal submitted to the full ACIP was not for a specific PCV.
Notably, Loehr said that, although as Work Group Chair he was tasked with making the motion in favor of the recommendation, he voted against it as a voting member because of his strong opinion that only the PCV21 vaccine is needed for vaccine-naive adults aged 50 or older. “I think that PCV21 is a better vaccine that targets more serotypes,” he said during the discussion. Data presented at the February 2024 ACIP meeting showed more than 80% coverage vs less than 60% coverage for invasive pneumococcal disease with PCV21 vs PCV20 among adults aged 65 years or older and those aged 19-64 years with a risk-based indication, Loehr said.
A version of this article appeared on Medscape.com.
The US Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) now recommends a pneumococcal conjugate vaccine (PCV) for all PCV-naive adults aged 50 years or older. The new recommendation, which passed with an ACIP member vote of 14 for and one against, expands the current age-based recommendations, which include children younger than 5 years and adults older than 65 years, as well as adults aged 19-64 years with underlying conditions or risk factors who have not received a PCV and certain adults who have received PCV13 but not PCV20.
The decision was based in part on economic analyses of the use of PCV in adults aged 50-64 years in the United States. Miwako Kobayashi, MD, presented the summary of the Pneumococcal Vaccines Work Group’s interpretation of the evidence and the proposed recommendation in a meeting of the ACIP on October 23, 2024, when the ACIP voting occurred.
Data from the CDC show an increase in the relative burden of pneumococcal disease in adults aged 50-64 years based in part on the success of the pediatric PCV program, she said.
Health equity was another main factor in the Work Group’s decision to recommend vaccination for adults aged 50 years or older. “Disparities in pneumococcal vaccine coverage by race and ethnicity exist for both age-based and risk-based indications,” Kobayashi noted in her presentation. The Work Group acknowledged that the overall effect of a vaccine recommendation on health equity is complex, but the majority agreed that the update would improve health equity by increasing vaccine coverage for those with known or unknown risk factors and providing protection at an earlier age when some populations already experience elevated disease rates, she said.
As for safety, the Work Group concluded that the undesirable anticipated effects of PCVs are minimal, despite the potential signal for Guillain-Barré Syndrome, and the CDC and US Food and Drug Administration will continue to monitor post-licensure safety of PCVs.
Support Not Universal
A majority of the ACIP Pneumococcal Vaccines Work Group supported the approved option, but agreed that a future booster dose may be needed, Work Group Chair James Loehr, MD, said in his introductory presentation.
Overall, key uncertainties remain, including indirect effects of new pediatric pneumococcal vaccines on adults, data on the duration of protection with adult vaccinations, and the impact new higher-valency vaccines have on adults, several of which are in development, Loehr said.
A new 21-valent PCV, known as PCV 21, was approved by the FDA for adults aged 18 years or older in June 2024, said Loehr. “PCV21 is not PCV20 with one additional serotype” and provides additional protection, he emphasized. The Work Group examined models involving PCV21 and the existing PCV20. However, a majority of the Work Group agreed that having age-based recommendations based on vaccine product would be more challenging to implement and that insurance coverage may be a factor given the recent approval of PCV21. Therefore, the proposal submitted to the full ACIP was not for a specific PCV.
Notably, Loehr said that, although as Work Group Chair he was tasked with making the motion in favor of the recommendation, he voted against it as a voting member because of his strong opinion that only the PCV21 vaccine is needed for vaccine-naive adults aged 50 or older. “I think that PCV21 is a better vaccine that targets more serotypes,” he said during the discussion. Data presented at the February 2024 ACIP meeting showed more than 80% coverage vs less than 60% coverage for invasive pneumococcal disease with PCV21 vs PCV20 among adults aged 65 years or older and those aged 19-64 years with a risk-based indication, Loehr said.
A version of this article appeared on Medscape.com.
Cannabis in Cancer: What Oncologists and Patients Should Know
first, and oncologists may be hesitant to broach the topic with their patients.
Updated guidelines from the American Society of Clinical Oncology (ASCO) on the use of cannabis and cannabinoids in adults with cancer stress that it’s an important conversation to have.
According to the ASCO expert panel, access to and use of cannabis alongside cancer care have outpaced the science on evidence-based indications, and overall high-quality data on the effects of cannabis during cancer care are lacking. While several observational studies support cannabis use to help ease chemotherapy-related nausea and vomiting, the literature remains more divided on other potential benefits, such as alleviating cancer pain and sleep problems, and some evidence points to potential downsides of cannabis use.
Oncologists should “absolutely talk to patients” about cannabis, Brooke Worster, MD, medical director for the Master of Science in Medical Cannabis Science & Business program at Thomas Jefferson University, Philadelphia, told Medscape Medical News.
“Patients are interested, and they are going to find access to information. As a medical professional, it’s our job to help guide them through these spaces in a safe, nonjudgmental way.”
But, Worster noted, oncologists don’t have to be experts on cannabis to begin the conversation with patients.
So, “let yourself off the hook,” Worster urged.
Plus, avoiding the conversation won’t stop patients from using cannabis. In a recent study, Worster and her colleagues found that nearly one third of patients at 12 National Cancer Institute-designated cancer centers had used cannabis since their diagnosis — most often for sleep disturbance, pain, stress, and anxiety. Most (60%) felt somewhat or extremely comfortable talking to their healthcare provider about it, but only 21.5% said they had done so. Even fewer — about 10% — had talked to their treating oncologist.
Because patients may not discuss cannabis use, it’s especially important for oncologists to open up a line of communication, said Worster, also the enterprise director of supportive oncology at the Thomas Jefferson University.
Evidence on Cannabis During Cancer Care
A substantial proportion of people with cancer believe cannabis can help manage cancer-related symptoms.
In Worster’s recent survey study, regardless of whether patients had used cannabis, almost 90% of those surveyed reported a perceived benefit. Although 65% also reported perceived risks for cannabis use, including difficulty concentrating, lung damage, and impaired memory, the perceived benefits outweighed the risks.
Despite generally positive perceptions, the overall literature on the benefits of cannabis in patients with cancer paints a less clear picture.
The ASCO guidelines, which were based on 13 systematic reviews and five additional primary studies, reported that cannabis can improve refractory, chemotherapy-induced nausea or vomiting when added to guideline-concordant antiemetic regimens, but that there is no clear evidence of benefit or harm for other supportive care outcomes.
The “certainty of evidence for most outcomes was low or very low,” the ASCO authors wrote.
The ASCO experts explained that, outside the context of a clinical trial, the evidence is not sufficient to recommend cannabis or cannabinoids for managing cancer pain, sleep issues, appetite loss, or anxiety and depression. For these outcomes, some studies indicate a benefit, while others don’t.
Real-world data from a large registry study, for instance, have indicated that medical cannabis is “a safe and effective complementary treatment for pain relief in patients with cancer.” However, a 2020 meta-analysis found that, in studies with a low risk for bias, adding cannabinoids to opioids did not reduce cancer pain in adults with advanced cancer.
There can be downsides to cannabis use, too. In one recent study, some patients reported feeling worse physically and psychologically compared with those who didn’t use cannabis. Another study found that oral cannabis was associated with “bothersome” side effects, including sedation, dizziness, and transient anxiety.
The ASCO guidelines also made it clear that cannabis or cannabinoids should not be used as cancer-directed treatment, outside of a clinical trial.
Talking to Patients About Cannabis
Given the level of evidence and patient interest in cannabis, it is important for oncologists to raise the topic of cannabis use with their patients.
To help inform decision-making and approaches to care, the ASCO guidelines suggest that oncologists can guide care themselves or direct patients to appropriate “unbiased, evidence-based” resources. For those who use cannabis or cannabinoids outside of evidence-based indications or clinician recommendations, it’s important to explore patients’ goals, educate them, and try to minimize harm.
One strategy for broaching the topic, Worster suggested, is to simply ask patients if they have tried or considered trying cannabis to control symptoms like nausea and vomiting, loss of appetite, or cancer pain.
The conversation with patients should then include an overview of the potential benefits and potential risks for cannabis use as well as risk reduction strategies, Worster noted.
But “approach it in an open and nonjudgmental frame of mind,” she said. “Just have a conversation.”
Discussing the formulation and concentration of tetrahydrocannabinol (THC) and cannabidiol (CBD) in products matters as well.
Will the product be inhaled, ingested, or topical? Inhaled cannabis is not ideal but is sometimes what patients have access to, Worster explained. Inhaled formulations tend to have faster onset, which might be preferable for treating chemotherapy-related nausea and vomiting, whereas edible formulations may take a while to start working.
It’s also important to warn patients about taking too much, she said, explaining that inhaling THC at higher doses can increase the risk for cardiovascular effects, anxiety, paranoia, panic, and psychosis.
CBD, on the other hand, is anti-inflammatory, but early data suggest it may blunt immune responses in high doses and should be used cautiously by patients receiving immunotherapy.
Worster noted that as laws change and the science advances, new cannabis products and formulations will emerge, as will artificial intelligence tools for helping to guide patients and clinicians in optimal use of cannabis for cancer care. State websites are a particularly helpful tool for providing state-specific medical education related to cannabis laws and use, as well, she said.
The bottom line, she said, is that talking to patients about the ins and outs of cannabis use “really matters.”
Worster disclosed that she is a medical consultant for EO Care.
A version of this article appeared on Medscape.com.
first, and oncologists may be hesitant to broach the topic with their patients.
Updated guidelines from the American Society of Clinical Oncology (ASCO) on the use of cannabis and cannabinoids in adults with cancer stress that it’s an important conversation to have.
According to the ASCO expert panel, access to and use of cannabis alongside cancer care have outpaced the science on evidence-based indications, and overall high-quality data on the effects of cannabis during cancer care are lacking. While several observational studies support cannabis use to help ease chemotherapy-related nausea and vomiting, the literature remains more divided on other potential benefits, such as alleviating cancer pain and sleep problems, and some evidence points to potential downsides of cannabis use.
Oncologists should “absolutely talk to patients” about cannabis, Brooke Worster, MD, medical director for the Master of Science in Medical Cannabis Science & Business program at Thomas Jefferson University, Philadelphia, told Medscape Medical News.
“Patients are interested, and they are going to find access to information. As a medical professional, it’s our job to help guide them through these spaces in a safe, nonjudgmental way.”
But, Worster noted, oncologists don’t have to be experts on cannabis to begin the conversation with patients.
So, “let yourself off the hook,” Worster urged.
Plus, avoiding the conversation won’t stop patients from using cannabis. In a recent study, Worster and her colleagues found that nearly one third of patients at 12 National Cancer Institute-designated cancer centers had used cannabis since their diagnosis — most often for sleep disturbance, pain, stress, and anxiety. Most (60%) felt somewhat or extremely comfortable talking to their healthcare provider about it, but only 21.5% said they had done so. Even fewer — about 10% — had talked to their treating oncologist.
Because patients may not discuss cannabis use, it’s especially important for oncologists to open up a line of communication, said Worster, also the enterprise director of supportive oncology at the Thomas Jefferson University.
Evidence on Cannabis During Cancer Care
A substantial proportion of people with cancer believe cannabis can help manage cancer-related symptoms.
In Worster’s recent survey study, regardless of whether patients had used cannabis, almost 90% of those surveyed reported a perceived benefit. Although 65% also reported perceived risks for cannabis use, including difficulty concentrating, lung damage, and impaired memory, the perceived benefits outweighed the risks.
Despite generally positive perceptions, the overall literature on the benefits of cannabis in patients with cancer paints a less clear picture.
The ASCO guidelines, which were based on 13 systematic reviews and five additional primary studies, reported that cannabis can improve refractory, chemotherapy-induced nausea or vomiting when added to guideline-concordant antiemetic regimens, but that there is no clear evidence of benefit or harm for other supportive care outcomes.
The “certainty of evidence for most outcomes was low or very low,” the ASCO authors wrote.
The ASCO experts explained that, outside the context of a clinical trial, the evidence is not sufficient to recommend cannabis or cannabinoids for managing cancer pain, sleep issues, appetite loss, or anxiety and depression. For these outcomes, some studies indicate a benefit, while others don’t.
Real-world data from a large registry study, for instance, have indicated that medical cannabis is “a safe and effective complementary treatment for pain relief in patients with cancer.” However, a 2020 meta-analysis found that, in studies with a low risk for bias, adding cannabinoids to opioids did not reduce cancer pain in adults with advanced cancer.
There can be downsides to cannabis use, too. In one recent study, some patients reported feeling worse physically and psychologically compared with those who didn’t use cannabis. Another study found that oral cannabis was associated with “bothersome” side effects, including sedation, dizziness, and transient anxiety.
The ASCO guidelines also made it clear that cannabis or cannabinoids should not be used as cancer-directed treatment, outside of a clinical trial.
Talking to Patients About Cannabis
Given the level of evidence and patient interest in cannabis, it is important for oncologists to raise the topic of cannabis use with their patients.
To help inform decision-making and approaches to care, the ASCO guidelines suggest that oncologists can guide care themselves or direct patients to appropriate “unbiased, evidence-based” resources. For those who use cannabis or cannabinoids outside of evidence-based indications or clinician recommendations, it’s important to explore patients’ goals, educate them, and try to minimize harm.
One strategy for broaching the topic, Worster suggested, is to simply ask patients if they have tried or considered trying cannabis to control symptoms like nausea and vomiting, loss of appetite, or cancer pain.
The conversation with patients should then include an overview of the potential benefits and potential risks for cannabis use as well as risk reduction strategies, Worster noted.
But “approach it in an open and nonjudgmental frame of mind,” she said. “Just have a conversation.”
Discussing the formulation and concentration of tetrahydrocannabinol (THC) and cannabidiol (CBD) in products matters as well.
Will the product be inhaled, ingested, or topical? Inhaled cannabis is not ideal but is sometimes what patients have access to, Worster explained. Inhaled formulations tend to have faster onset, which might be preferable for treating chemotherapy-related nausea and vomiting, whereas edible formulations may take a while to start working.
It’s also important to warn patients about taking too much, she said, explaining that inhaling THC at higher doses can increase the risk for cardiovascular effects, anxiety, paranoia, panic, and psychosis.
CBD, on the other hand, is anti-inflammatory, but early data suggest it may blunt immune responses in high doses and should be used cautiously by patients receiving immunotherapy.
Worster noted that as laws change and the science advances, new cannabis products and formulations will emerge, as will artificial intelligence tools for helping to guide patients and clinicians in optimal use of cannabis for cancer care. State websites are a particularly helpful tool for providing state-specific medical education related to cannabis laws and use, as well, she said.
The bottom line, she said, is that talking to patients about the ins and outs of cannabis use “really matters.”
Worster disclosed that she is a medical consultant for EO Care.
A version of this article appeared on Medscape.com.
first, and oncologists may be hesitant to broach the topic with their patients.
Updated guidelines from the American Society of Clinical Oncology (ASCO) on the use of cannabis and cannabinoids in adults with cancer stress that it’s an important conversation to have.
According to the ASCO expert panel, access to and use of cannabis alongside cancer care have outpaced the science on evidence-based indications, and overall high-quality data on the effects of cannabis during cancer care are lacking. While several observational studies support cannabis use to help ease chemotherapy-related nausea and vomiting, the literature remains more divided on other potential benefits, such as alleviating cancer pain and sleep problems, and some evidence points to potential downsides of cannabis use.
Oncologists should “absolutely talk to patients” about cannabis, Brooke Worster, MD, medical director for the Master of Science in Medical Cannabis Science & Business program at Thomas Jefferson University, Philadelphia, told Medscape Medical News.
“Patients are interested, and they are going to find access to information. As a medical professional, it’s our job to help guide them through these spaces in a safe, nonjudgmental way.”
But, Worster noted, oncologists don’t have to be experts on cannabis to begin the conversation with patients.
So, “let yourself off the hook,” Worster urged.
Plus, avoiding the conversation won’t stop patients from using cannabis. In a recent study, Worster and her colleagues found that nearly one third of patients at 12 National Cancer Institute-designated cancer centers had used cannabis since their diagnosis — most often for sleep disturbance, pain, stress, and anxiety. Most (60%) felt somewhat or extremely comfortable talking to their healthcare provider about it, but only 21.5% said they had done so. Even fewer — about 10% — had talked to their treating oncologist.
Because patients may not discuss cannabis use, it’s especially important for oncologists to open up a line of communication, said Worster, also the enterprise director of supportive oncology at the Thomas Jefferson University.
Evidence on Cannabis During Cancer Care
A substantial proportion of people with cancer believe cannabis can help manage cancer-related symptoms.
In Worster’s recent survey study, regardless of whether patients had used cannabis, almost 90% of those surveyed reported a perceived benefit. Although 65% also reported perceived risks for cannabis use, including difficulty concentrating, lung damage, and impaired memory, the perceived benefits outweighed the risks.
Despite generally positive perceptions, the overall literature on the benefits of cannabis in patients with cancer paints a less clear picture.
The ASCO guidelines, which were based on 13 systematic reviews and five additional primary studies, reported that cannabis can improve refractory, chemotherapy-induced nausea or vomiting when added to guideline-concordant antiemetic regimens, but that there is no clear evidence of benefit or harm for other supportive care outcomes.
The “certainty of evidence for most outcomes was low or very low,” the ASCO authors wrote.
The ASCO experts explained that, outside the context of a clinical trial, the evidence is not sufficient to recommend cannabis or cannabinoids for managing cancer pain, sleep issues, appetite loss, or anxiety and depression. For these outcomes, some studies indicate a benefit, while others don’t.
Real-world data from a large registry study, for instance, have indicated that medical cannabis is “a safe and effective complementary treatment for pain relief in patients with cancer.” However, a 2020 meta-analysis found that, in studies with a low risk for bias, adding cannabinoids to opioids did not reduce cancer pain in adults with advanced cancer.
There can be downsides to cannabis use, too. In one recent study, some patients reported feeling worse physically and psychologically compared with those who didn’t use cannabis. Another study found that oral cannabis was associated with “bothersome” side effects, including sedation, dizziness, and transient anxiety.
The ASCO guidelines also made it clear that cannabis or cannabinoids should not be used as cancer-directed treatment, outside of a clinical trial.
Talking to Patients About Cannabis
Given the level of evidence and patient interest in cannabis, it is important for oncologists to raise the topic of cannabis use with their patients.
To help inform decision-making and approaches to care, the ASCO guidelines suggest that oncologists can guide care themselves or direct patients to appropriate “unbiased, evidence-based” resources. For those who use cannabis or cannabinoids outside of evidence-based indications or clinician recommendations, it’s important to explore patients’ goals, educate them, and try to minimize harm.
One strategy for broaching the topic, Worster suggested, is to simply ask patients if they have tried or considered trying cannabis to control symptoms like nausea and vomiting, loss of appetite, or cancer pain.
The conversation with patients should then include an overview of the potential benefits and potential risks for cannabis use as well as risk reduction strategies, Worster noted.
But “approach it in an open and nonjudgmental frame of mind,” she said. “Just have a conversation.”
Discussing the formulation and concentration of tetrahydrocannabinol (THC) and cannabidiol (CBD) in products matters as well.
Will the product be inhaled, ingested, or topical? Inhaled cannabis is not ideal but is sometimes what patients have access to, Worster explained. Inhaled formulations tend to have faster onset, which might be preferable for treating chemotherapy-related nausea and vomiting, whereas edible formulations may take a while to start working.
It’s also important to warn patients about taking too much, she said, explaining that inhaling THC at higher doses can increase the risk for cardiovascular effects, anxiety, paranoia, panic, and psychosis.
CBD, on the other hand, is anti-inflammatory, but early data suggest it may blunt immune responses in high doses and should be used cautiously by patients receiving immunotherapy.
Worster noted that as laws change and the science advances, new cannabis products and formulations will emerge, as will artificial intelligence tools for helping to guide patients and clinicians in optimal use of cannabis for cancer care. State websites are a particularly helpful tool for providing state-specific medical education related to cannabis laws and use, as well, she said.
The bottom line, she said, is that talking to patients about the ins and outs of cannabis use “really matters.”
Worster disclosed that she is a medical consultant for EO Care.
A version of this article appeared on Medscape.com.
Groups With Highest Unmet Need for PrEP Highlighted in Analysis
LOS ANGELES — Use of preexposure prophylaxis (PrEP) to prevent HIV is increasing overall, but both the rate of increase for starting PrEP and the rate of unmet need differ widely by demographic group, according to new data from a large study.
An analysis by Li Tao, MD, MS, PhD, director of real-world evidence at Gilead Sciences, and colleagues looked at statistical trends from 2019 to 2023 and found that Black, Hispanic, and Medicaid-insured populations continue to lack equitable access to PrEP.
Among the findings were that most new PrEP users were men with HIV risk factors who are commercially insured and live in predominantly non-Hispanic White areas (53% in 2019 and 43% in 2023). For comparison, men living in predominantly Black or Hispanic neighborhoods, or who are insured by Medicaid, saw lower proportions of PrEP use (16% in 2019 and 17% in 2023) despite higher annual increases in PrEP use (11% per year) and higher unmet needs.
Half a Million Real-World Participants
Tao presented her team’s findings at the Infectious Disease Week (IDWeek) 2024 Annual Meeting. The study included “more than half a million real-world PrEP users over the past 5 years,” she said.
The group with the lowest growth in initiation of PrEP in the study period (an annual percentage increase of 2%) and the lowest unmet need included men with HIV risk factors, who were using commercial insurance and living in White-dominant neighborhoods.
HIV risk factors included diagnosis of any sexually transmitted disease, contact with and exposure to communicable diseases, high-risk sexual behavior, contact with a hypodermic needle, long-term prophylaxis, HIV prevention counseling, and HIV screening.
Other men with HIV risk factors (those who were commercially insured, living in Black/Hispanic neighborhoods, or those on Medicaid across all neighborhoods) had a moderate increase in PrEP initiation (an annual percentage increase of 11%-16%) and higher unmet needs.
Researchers gathered data on PrEP prescriptions and new HIV diagnoses (from 2019 to 2023) through the IQVIA pharmacy claims database. PrEP-to-need ratio (PNR) is the number of individuals using PrEP in a year divided by new HIV diagnoses in the previous year. It was calculated for subgroups defined by five PNR-associated factors: Sex, insurance, recorded HIV risk factors (identified by diagnosis or procedure codes), “Ending the HIV Epidemic” jurisdictions, and neighborhood race/ethnicity mix.
Disparities Persist
While PrEP use improved across all the groups studied in the 5 years, “disparities still persist and the need remains very significant,” Tao said. “It’s very crucial for guiding the future HIV prevention options.”
“Long-acting PrEP options may help to address some social determinants structural factors in HIV acquisition,” she added.
What Programs Are Helping?
Some guidelines and programs are helping increase uptake, Tao said.
The United States Preventive Services Task Force (USPSTF) guidelines “reinforce more accessible PrEP programs to individuals like zero-cost sharing or same-day dispensing,” Tao said in a press briefing. “Those kinds of policies are really effective. We can see that after the implementation of the USPSTF guidelines, the copay sharing is really decreasing and is coinciding with the HIV rates declining.”
The Medicaid coverage expansion in 40 states “has been really effective” in PrEP uptake, she added.
Colleen Kelley, MD, MPH, with the Division of Infectious Diseases at the Rollins School of Public Health, Emory University, in Atlanta, who was not part of the research, said there has been a slow but improving uptake of PrEP across the board in the United States, “but the issue is that the uptake has been inequitable.”
Large Study With Recent Data
“This is an extremely large study with very recent data,” Kelley said. “Additionally, they were able to couple (the uptake) with unmet need. People who are at higher risk of acquiring HIV or who live in high-risk areas for HIV should have greater access to PrEP. They have a greater need for PrEP. What we really need to do from an equity perspective is match the PrEP use with the PrEP need and we have not been successful in doing that.”
Kelley added that the finding that the group that had the highest unmet need for PrEP in the study also had no recorded HIV risk factors. “It’s an interesting time to start thinking about beyond risk factor coverage for PrEP,” she said.
Another issue, Kelley said, is that “people are using (PrEP) but they’re also stopping it. People will need to take PrEP many years for protection, but about half discontinue in the first 6-12 months.
“We need to look at how people will persist on PrEP over the long term. That’s the next frontier,” she said. “We hope the long-acting injectables will help overcome some of the PrEP fatigue. But some may just tire of taking medication repeatedly for an infection they don’t have,” she said.
The study was funded by Gilead Sciences. Tao is employed by and is a shareholder of Gilead Sciences. All relevant financial disclosures have been mitigated, according to the paper. Kelley has research grants to her institution from Gilead, Moderna, Novavax, ViiV, and Humanigen.
A version of this article first appeared on Medscape.com.
LOS ANGELES — Use of preexposure prophylaxis (PrEP) to prevent HIV is increasing overall, but both the rate of increase for starting PrEP and the rate of unmet need differ widely by demographic group, according to new data from a large study.
An analysis by Li Tao, MD, MS, PhD, director of real-world evidence at Gilead Sciences, and colleagues looked at statistical trends from 2019 to 2023 and found that Black, Hispanic, and Medicaid-insured populations continue to lack equitable access to PrEP.
Among the findings were that most new PrEP users were men with HIV risk factors who are commercially insured and live in predominantly non-Hispanic White areas (53% in 2019 and 43% in 2023). For comparison, men living in predominantly Black or Hispanic neighborhoods, or who are insured by Medicaid, saw lower proportions of PrEP use (16% in 2019 and 17% in 2023) despite higher annual increases in PrEP use (11% per year) and higher unmet needs.
Half a Million Real-World Participants
Tao presented her team’s findings at the Infectious Disease Week (IDWeek) 2024 Annual Meeting. The study included “more than half a million real-world PrEP users over the past 5 years,” she said.
The group with the lowest growth in initiation of PrEP in the study period (an annual percentage increase of 2%) and the lowest unmet need included men with HIV risk factors, who were using commercial insurance and living in White-dominant neighborhoods.
HIV risk factors included diagnosis of any sexually transmitted disease, contact with and exposure to communicable diseases, high-risk sexual behavior, contact with a hypodermic needle, long-term prophylaxis, HIV prevention counseling, and HIV screening.
Other men with HIV risk factors (those who were commercially insured, living in Black/Hispanic neighborhoods, or those on Medicaid across all neighborhoods) had a moderate increase in PrEP initiation (an annual percentage increase of 11%-16%) and higher unmet needs.
Researchers gathered data on PrEP prescriptions and new HIV diagnoses (from 2019 to 2023) through the IQVIA pharmacy claims database. PrEP-to-need ratio (PNR) is the number of individuals using PrEP in a year divided by new HIV diagnoses in the previous year. It was calculated for subgroups defined by five PNR-associated factors: Sex, insurance, recorded HIV risk factors (identified by diagnosis or procedure codes), “Ending the HIV Epidemic” jurisdictions, and neighborhood race/ethnicity mix.
Disparities Persist
While PrEP use improved across all the groups studied in the 5 years, “disparities still persist and the need remains very significant,” Tao said. “It’s very crucial for guiding the future HIV prevention options.”
“Long-acting PrEP options may help to address some social determinants structural factors in HIV acquisition,” she added.
What Programs Are Helping?
Some guidelines and programs are helping increase uptake, Tao said.
The United States Preventive Services Task Force (USPSTF) guidelines “reinforce more accessible PrEP programs to individuals like zero-cost sharing or same-day dispensing,” Tao said in a press briefing. “Those kinds of policies are really effective. We can see that after the implementation of the USPSTF guidelines, the copay sharing is really decreasing and is coinciding with the HIV rates declining.”
The Medicaid coverage expansion in 40 states “has been really effective” in PrEP uptake, she added.
Colleen Kelley, MD, MPH, with the Division of Infectious Diseases at the Rollins School of Public Health, Emory University, in Atlanta, who was not part of the research, said there has been a slow but improving uptake of PrEP across the board in the United States, “but the issue is that the uptake has been inequitable.”
Large Study With Recent Data
“This is an extremely large study with very recent data,” Kelley said. “Additionally, they were able to couple (the uptake) with unmet need. People who are at higher risk of acquiring HIV or who live in high-risk areas for HIV should have greater access to PrEP. They have a greater need for PrEP. What we really need to do from an equity perspective is match the PrEP use with the PrEP need and we have not been successful in doing that.”
Kelley added that the finding that the group that had the highest unmet need for PrEP in the study also had no recorded HIV risk factors. “It’s an interesting time to start thinking about beyond risk factor coverage for PrEP,” she said.
Another issue, Kelley said, is that “people are using (PrEP) but they’re also stopping it. People will need to take PrEP many years for protection, but about half discontinue in the first 6-12 months.
“We need to look at how people will persist on PrEP over the long term. That’s the next frontier,” she said. “We hope the long-acting injectables will help overcome some of the PrEP fatigue. But some may just tire of taking medication repeatedly for an infection they don’t have,” she said.
The study was funded by Gilead Sciences. Tao is employed by and is a shareholder of Gilead Sciences. All relevant financial disclosures have been mitigated, according to the paper. Kelley has research grants to her institution from Gilead, Moderna, Novavax, ViiV, and Humanigen.
A version of this article first appeared on Medscape.com.
LOS ANGELES — Use of preexposure prophylaxis (PrEP) to prevent HIV is increasing overall, but both the rate of increase for starting PrEP and the rate of unmet need differ widely by demographic group, according to new data from a large study.
An analysis by Li Tao, MD, MS, PhD, director of real-world evidence at Gilead Sciences, and colleagues looked at statistical trends from 2019 to 2023 and found that Black, Hispanic, and Medicaid-insured populations continue to lack equitable access to PrEP.
Among the findings were that most new PrEP users were men with HIV risk factors who are commercially insured and live in predominantly non-Hispanic White areas (53% in 2019 and 43% in 2023). For comparison, men living in predominantly Black or Hispanic neighborhoods, or who are insured by Medicaid, saw lower proportions of PrEP use (16% in 2019 and 17% in 2023) despite higher annual increases in PrEP use (11% per year) and higher unmet needs.
Half a Million Real-World Participants
Tao presented her team’s findings at the Infectious Disease Week (IDWeek) 2024 Annual Meeting. The study included “more than half a million real-world PrEP users over the past 5 years,” she said.
The group with the lowest growth in initiation of PrEP in the study period (an annual percentage increase of 2%) and the lowest unmet need included men with HIV risk factors, who were using commercial insurance and living in White-dominant neighborhoods.
HIV risk factors included diagnosis of any sexually transmitted disease, contact with and exposure to communicable diseases, high-risk sexual behavior, contact with a hypodermic needle, long-term prophylaxis, HIV prevention counseling, and HIV screening.
Other men with HIV risk factors (those who were commercially insured, living in Black/Hispanic neighborhoods, or those on Medicaid across all neighborhoods) had a moderate increase in PrEP initiation (an annual percentage increase of 11%-16%) and higher unmet needs.
Researchers gathered data on PrEP prescriptions and new HIV diagnoses (from 2019 to 2023) through the IQVIA pharmacy claims database. PrEP-to-need ratio (PNR) is the number of individuals using PrEP in a year divided by new HIV diagnoses in the previous year. It was calculated for subgroups defined by five PNR-associated factors: Sex, insurance, recorded HIV risk factors (identified by diagnosis or procedure codes), “Ending the HIV Epidemic” jurisdictions, and neighborhood race/ethnicity mix.
Disparities Persist
While PrEP use improved across all the groups studied in the 5 years, “disparities still persist and the need remains very significant,” Tao said. “It’s very crucial for guiding the future HIV prevention options.”
“Long-acting PrEP options may help to address some social determinants structural factors in HIV acquisition,” she added.
What Programs Are Helping?
Some guidelines and programs are helping increase uptake, Tao said.
The United States Preventive Services Task Force (USPSTF) guidelines “reinforce more accessible PrEP programs to individuals like zero-cost sharing or same-day dispensing,” Tao said in a press briefing. “Those kinds of policies are really effective. We can see that after the implementation of the USPSTF guidelines, the copay sharing is really decreasing and is coinciding with the HIV rates declining.”
The Medicaid coverage expansion in 40 states “has been really effective” in PrEP uptake, she added.
Colleen Kelley, MD, MPH, with the Division of Infectious Diseases at the Rollins School of Public Health, Emory University, in Atlanta, who was not part of the research, said there has been a slow but improving uptake of PrEP across the board in the United States, “but the issue is that the uptake has been inequitable.”
Large Study With Recent Data
“This is an extremely large study with very recent data,” Kelley said. “Additionally, they were able to couple (the uptake) with unmet need. People who are at higher risk of acquiring HIV or who live in high-risk areas for HIV should have greater access to PrEP. They have a greater need for PrEP. What we really need to do from an equity perspective is match the PrEP use with the PrEP need and we have not been successful in doing that.”
Kelley added that the finding that the group that had the highest unmet need for PrEP in the study also had no recorded HIV risk factors. “It’s an interesting time to start thinking about beyond risk factor coverage for PrEP,” she said.
Another issue, Kelley said, is that “people are using (PrEP) but they’re also stopping it. People will need to take PrEP many years for protection, but about half discontinue in the first 6-12 months.
“We need to look at how people will persist on PrEP over the long term. That’s the next frontier,” she said. “We hope the long-acting injectables will help overcome some of the PrEP fatigue. But some may just tire of taking medication repeatedly for an infection they don’t have,” she said.
The study was funded by Gilead Sciences. Tao is employed by and is a shareholder of Gilead Sciences. All relevant financial disclosures have been mitigated, according to the paper. Kelley has research grants to her institution from Gilead, Moderna, Novavax, ViiV, and Humanigen.
A version of this article first appeared on Medscape.com.
FROM IDWEEK 2024