User login
Solo Vs McDoctors Inc.
STAT News recently ran a series on UnitedHealthcare (UHC) and its growing physician empire. This includes the corporation pressuring its employed physicians to see more patients, work weekends, upcode visits, add in diagnoses that will increase reimbursement, yadda, yadda, yadda.
For legal disclaimer purposes, I’m not saying UHC did any of this, nor am I saying they didn’t. But the series on STAT is worth reading.
Reading the articles brings back memories of the last time I was an employed physician, 24 years ago. I didn’t have people telling me to upcode visits, but I do remember hearing terms such as “dollars per physician per square foot” bandied about concerning my performance. At least back then no one was going to yell at me about a 1-star online review from a disgruntled patient.
After a little over 2 years I’d had enough and went solo.
I have no desire at this point to go back to that. I certainly make a lot less money than my employed counterparts, but I also have time and a degree of peace, which are worth something.
I’m not paying for anyone else’s overhead. I don’t slack off, but at least I know what I’m working for, and where the money is going when I write out a check. I can work my schedule around having to take my dog to the vet, or pick a kid up at the airport, or whatever.
I can spend more time with the patients who need it. Isn’t that part of why I’m here?
Wearing Hawaiian shirts and shorts to the office everyday is also a plus (at least I think so).
It surprises me that more physicians aren’t willing to go into solo or small group practice. The big advantage is freedom, only needing to pay the overhead and your salary, and cover for others when needed.
The downside is financial. Like our hunting and gathering ancestors, you eat what you kill. If there’s a shortfall in cash flow, I’m the one who doesn’t get paid. It’s always good to have a line of credit available to fall back on in a pinch.
I can see why it’s daunting. Coming out of training you have loans to pay off. You may have a young family, and your first mortgage. You sure don’t want to take out another loan to start a private practice. The security of a guaranteed paycheck and no start-up costs is attractive. I was there, too, and I also took the first job I was offered back then.
There’s also the fear of suddenly working without a net for the first time in your career. It’s reassuring to get some added experience while being able to bounce a challenging case off another doctor. (I still do that, too, and always will.)
But no one tells me to upcode visits or add diagnostic codes just to get more money. Patients don’t call in panicked that they have an ICD-10 code for a condition no one told them they had.
At the end of the day I can tell the guy in the mirror that I’m doing my best.
Medicine has changed a lot over time ... but being a doctor hasn’t. The spark that led us all here is still there, somewhere, I hope. Go back and read Neighbor Rosicky by Willa Cather, and The Doctor Stories by William Carlos Williams.
In an age when technology is moving us forward, I think the practice of medicine should move backward, away from McDoctors Inc. A small, even solo, medical practice isn’t incompatible with the shiny toys of 2024 medicine. You can make good patient care happen with both.
I freely admit that it’s not for everyone.
But I wish more people would see it as a realistic option, and take the road less traveled.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
STAT News recently ran a series on UnitedHealthcare (UHC) and its growing physician empire. This includes the corporation pressuring its employed physicians to see more patients, work weekends, upcode visits, add in diagnoses that will increase reimbursement, yadda, yadda, yadda.
For legal disclaimer purposes, I’m not saying UHC did any of this, nor am I saying they didn’t. But the series on STAT is worth reading.
Reading the articles brings back memories of the last time I was an employed physician, 24 years ago. I didn’t have people telling me to upcode visits, but I do remember hearing terms such as “dollars per physician per square foot” bandied about concerning my performance. At least back then no one was going to yell at me about a 1-star online review from a disgruntled patient.
After a little over 2 years I’d had enough and went solo.
I have no desire at this point to go back to that. I certainly make a lot less money than my employed counterparts, but I also have time and a degree of peace, which are worth something.
I’m not paying for anyone else’s overhead. I don’t slack off, but at least I know what I’m working for, and where the money is going when I write out a check. I can work my schedule around having to take my dog to the vet, or pick a kid up at the airport, or whatever.
I can spend more time with the patients who need it. Isn’t that part of why I’m here?
Wearing Hawaiian shirts and shorts to the office everyday is also a plus (at least I think so).
It surprises me that more physicians aren’t willing to go into solo or small group practice. The big advantage is freedom, only needing to pay the overhead and your salary, and cover for others when needed.
The downside is financial. Like our hunting and gathering ancestors, you eat what you kill. If there’s a shortfall in cash flow, I’m the one who doesn’t get paid. It’s always good to have a line of credit available to fall back on in a pinch.
I can see why it’s daunting. Coming out of training you have loans to pay off. You may have a young family, and your first mortgage. You sure don’t want to take out another loan to start a private practice. The security of a guaranteed paycheck and no start-up costs is attractive. I was there, too, and I also took the first job I was offered back then.
There’s also the fear of suddenly working without a net for the first time in your career. It’s reassuring to get some added experience while being able to bounce a challenging case off another doctor. (I still do that, too, and always will.)
But no one tells me to upcode visits or add diagnostic codes just to get more money. Patients don’t call in panicked that they have an ICD-10 code for a condition no one told them they had.
At the end of the day I can tell the guy in the mirror that I’m doing my best.
Medicine has changed a lot over time ... but being a doctor hasn’t. The spark that led us all here is still there, somewhere, I hope. Go back and read Neighbor Rosicky by Willa Cather, and The Doctor Stories by William Carlos Williams.
In an age when technology is moving us forward, I think the practice of medicine should move backward, away from McDoctors Inc. A small, even solo, medical practice isn’t incompatible with the shiny toys of 2024 medicine. You can make good patient care happen with both.
I freely admit that it’s not for everyone.
But I wish more people would see it as a realistic option, and take the road less traveled.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
STAT News recently ran a series on UnitedHealthcare (UHC) and its growing physician empire. This includes the corporation pressuring its employed physicians to see more patients, work weekends, upcode visits, add in diagnoses that will increase reimbursement, yadda, yadda, yadda.
For legal disclaimer purposes, I’m not saying UHC did any of this, nor am I saying they didn’t. But the series on STAT is worth reading.
Reading the articles brings back memories of the last time I was an employed physician, 24 years ago. I didn’t have people telling me to upcode visits, but I do remember hearing terms such as “dollars per physician per square foot” bandied about concerning my performance. At least back then no one was going to yell at me about a 1-star online review from a disgruntled patient.
After a little over 2 years I’d had enough and went solo.
I have no desire at this point to go back to that. I certainly make a lot less money than my employed counterparts, but I also have time and a degree of peace, which are worth something.
I’m not paying for anyone else’s overhead. I don’t slack off, but at least I know what I’m working for, and where the money is going when I write out a check. I can work my schedule around having to take my dog to the vet, or pick a kid up at the airport, or whatever.
I can spend more time with the patients who need it. Isn’t that part of why I’m here?
Wearing Hawaiian shirts and shorts to the office everyday is also a plus (at least I think so).
It surprises me that more physicians aren’t willing to go into solo or small group practice. The big advantage is freedom, only needing to pay the overhead and your salary, and cover for others when needed.
The downside is financial. Like our hunting and gathering ancestors, you eat what you kill. If there’s a shortfall in cash flow, I’m the one who doesn’t get paid. It’s always good to have a line of credit available to fall back on in a pinch.
I can see why it’s daunting. Coming out of training you have loans to pay off. You may have a young family, and your first mortgage. You sure don’t want to take out another loan to start a private practice. The security of a guaranteed paycheck and no start-up costs is attractive. I was there, too, and I also took the first job I was offered back then.
There’s also the fear of suddenly working without a net for the first time in your career. It’s reassuring to get some added experience while being able to bounce a challenging case off another doctor. (I still do that, too, and always will.)
But no one tells me to upcode visits or add diagnostic codes just to get more money. Patients don’t call in panicked that they have an ICD-10 code for a condition no one told them they had.
At the end of the day I can tell the guy in the mirror that I’m doing my best.
Medicine has changed a lot over time ... but being a doctor hasn’t. The spark that led us all here is still there, somewhere, I hope. Go back and read Neighbor Rosicky by Willa Cather, and The Doctor Stories by William Carlos Williams.
In an age when technology is moving us forward, I think the practice of medicine should move backward, away from McDoctors Inc. A small, even solo, medical practice isn’t incompatible with the shiny toys of 2024 medicine. You can make good patient care happen with both.
I freely admit that it’s not for everyone.
But I wish more people would see it as a realistic option, and take the road less traveled.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Nutrition and Medical Education
How comfortable are you giving nutritional advice to your patients? When you offer it are you basing your advice on something you learned during medical school or your training? Was it included in a course devoted to nutrition? Did you learn it later as part of continuing medical education course (CME)? Or was it just something you just picked up from your experience seeing patients (osmosis)? It is very unlikely that a significant portion, or any part for that matter, of your medical training was devoted to nutrition. It certainly wasn’t during my training.
I recently read an interview with Emily M. Broad Leib, JD, faculty director of the Harvard School Center for Health Law and Policy Innovation, Cambridge, Massachusetts, who would like to correct that deficiency. She feels doctors need to know more about food and that acquiring that knowledge should be a significant component of their formal training.
In the interview, Leib said that “roughly 86% of physicians report they do not feel adequately trained to answer basic questions on diet or nutrition.” She also notes that while “72% of entering medical students report they believe food is important to health” less than 50% retained this belief after graduation.
Leib and associates feel they have recently reached a milestone in their efforts to include nutrition in the mainstream of medical education this fall by publishing a paper that demonstrates “consensus on doctor-approved nutritional standard for medical schools and residency programs.”
36 Recommended Competencies
Curious about what these nutrition experts chose to include in medical training, I decided to drill down into the list of 36 consensus-driven competencies they had agreed upon.
It was an interesting voyage into a forest of redundancies, many of which can be boiled down to having the student demonstrate that he/she understands that what we eat is important to our health and that there is a complex web of relationships connecting our society to the food consume.
Some of the recommended competencies I found make perfect sense. For example the student/trainee should be able to take a diet and food history and be able to interpret lab values and anthropometric measurements and be able to discuss the patient’s weight and diet with sensitivity while keeping in mind his/her own biases about food.
Some other recommendations are more problematic, for example, “performs a comprehensive nutrition-focused physical examination” or “demonstrates knowledge of how to create culinary nutrition SMART [Specific, Measurable, Achievable, Relevant, and Time-Bound] goals for personal use and for patient care” or “provides brief counseling interventions to help patients decrease visceral adiposity or reduce the risk of metabolic syndrome.” Including competencies like these demonstrates a lack of understanding of the time restraints and realities of a primary care physician’s life and training.
Instead of simply reinforcing the prospective physician’s preexisting assumption that food and health are entwined and discussing when and how to consult a nutrition expert, these 36 competencies seem to be an attempt to create fast-tracked part-time dietitians and nutrition advocates out of medical students and trainees who already believe that nutrition is important for health but also have a very full plate of clinical responsibilities ahead of them.
The study that Leib quotes — that 72% of medical students believed food was important in health while after graduation only 50% of agreed — doesn’t necessarily mean that professors are preaching that food was unimportant. It is more likely by the end of medical school the students have seen that food must share the spotlight with numerous other factors that influence their patients’ health.
‘A More Appropriate Focus’
In my experience, diet and lifestyle counseling done well is extremely time consuming and best done by people for whom that is their specialty. A more appropriate focus for a list of nutritional competencies for physicians in training would be for the student to achieve an understanding of when and how to consult a dietitian and then how to support and evaluate the dietitian’s recommendations to the patient.
Finally, I don’t think we can ignore a serious public relations problem that hangs like a cloud over the nutrition advocacy community. It is the same one that casts a shadow on the medical community as well. It is a common perception among the lay public that nutritionists (and physicians) are always changing their recommendations when it comes to food. What is believable? Just think about eggs, red wine, or introducing peanuts to infants, to name just a few. And what about the food pyramids that seem to have been rebuilt every several years? The problem is compounded when some “credentialed” nutritionists and physicians continue to make dietary pronouncements with only a shred of evidence or poorly documented anecdotal observations.
The first of the 36 competencies I reviewed reads: “Provide evidence-based, culturally sensitive nutrition and food recommendations for the prevention and treatment of disease.” When it comes to nutrition the “evidence” can be tough to come by. The natural experiments in which individuals and populations had extremely limited access to a certain nutrients (eg, scurvy) don’t occur very often. Animal studies don’t always extrapolate to humans. And, observational studies concerning diet often have co-factors that are difficult to control and must run over time courses that can tax even the most patient researchers.
I certainly applaud Leib and associates for promoting their primary goal of including more about of the relationship between food and health in the medical school and trainee curriculum. But I must voice a caution to be careful to keep it truly evidence-based and in a format that acknowledges the realities of the life and education of a primary care provider.
The best nutritional advice I ever received in my training was from an older pediatric professor who suggested that a healthy diet consisted of everything in moderation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
How comfortable are you giving nutritional advice to your patients? When you offer it are you basing your advice on something you learned during medical school or your training? Was it included in a course devoted to nutrition? Did you learn it later as part of continuing medical education course (CME)? Or was it just something you just picked up from your experience seeing patients (osmosis)? It is very unlikely that a significant portion, or any part for that matter, of your medical training was devoted to nutrition. It certainly wasn’t during my training.
I recently read an interview with Emily M. Broad Leib, JD, faculty director of the Harvard School Center for Health Law and Policy Innovation, Cambridge, Massachusetts, who would like to correct that deficiency. She feels doctors need to know more about food and that acquiring that knowledge should be a significant component of their formal training.
In the interview, Leib said that “roughly 86% of physicians report they do not feel adequately trained to answer basic questions on diet or nutrition.” She also notes that while “72% of entering medical students report they believe food is important to health” less than 50% retained this belief after graduation.
Leib and associates feel they have recently reached a milestone in their efforts to include nutrition in the mainstream of medical education this fall by publishing a paper that demonstrates “consensus on doctor-approved nutritional standard for medical schools and residency programs.”
36 Recommended Competencies
Curious about what these nutrition experts chose to include in medical training, I decided to drill down into the list of 36 consensus-driven competencies they had agreed upon.
It was an interesting voyage into a forest of redundancies, many of which can be boiled down to having the student demonstrate that he/she understands that what we eat is important to our health and that there is a complex web of relationships connecting our society to the food consume.
Some of the recommended competencies I found make perfect sense. For example the student/trainee should be able to take a diet and food history and be able to interpret lab values and anthropometric measurements and be able to discuss the patient’s weight and diet with sensitivity while keeping in mind his/her own biases about food.
Some other recommendations are more problematic, for example, “performs a comprehensive nutrition-focused physical examination” or “demonstrates knowledge of how to create culinary nutrition SMART [Specific, Measurable, Achievable, Relevant, and Time-Bound] goals for personal use and for patient care” or “provides brief counseling interventions to help patients decrease visceral adiposity or reduce the risk of metabolic syndrome.” Including competencies like these demonstrates a lack of understanding of the time restraints and realities of a primary care physician’s life and training.
Instead of simply reinforcing the prospective physician’s preexisting assumption that food and health are entwined and discussing when and how to consult a nutrition expert, these 36 competencies seem to be an attempt to create fast-tracked part-time dietitians and nutrition advocates out of medical students and trainees who already believe that nutrition is important for health but also have a very full plate of clinical responsibilities ahead of them.
The study that Leib quotes — that 72% of medical students believed food was important in health while after graduation only 50% of agreed — doesn’t necessarily mean that professors are preaching that food was unimportant. It is more likely by the end of medical school the students have seen that food must share the spotlight with numerous other factors that influence their patients’ health.
‘A More Appropriate Focus’
In my experience, diet and lifestyle counseling done well is extremely time consuming and best done by people for whom that is their specialty. A more appropriate focus for a list of nutritional competencies for physicians in training would be for the student to achieve an understanding of when and how to consult a dietitian and then how to support and evaluate the dietitian’s recommendations to the patient.
Finally, I don’t think we can ignore a serious public relations problem that hangs like a cloud over the nutrition advocacy community. It is the same one that casts a shadow on the medical community as well. It is a common perception among the lay public that nutritionists (and physicians) are always changing their recommendations when it comes to food. What is believable? Just think about eggs, red wine, or introducing peanuts to infants, to name just a few. And what about the food pyramids that seem to have been rebuilt every several years? The problem is compounded when some “credentialed” nutritionists and physicians continue to make dietary pronouncements with only a shred of evidence or poorly documented anecdotal observations.
The first of the 36 competencies I reviewed reads: “Provide evidence-based, culturally sensitive nutrition and food recommendations for the prevention and treatment of disease.” When it comes to nutrition the “evidence” can be tough to come by. The natural experiments in which individuals and populations had extremely limited access to a certain nutrients (eg, scurvy) don’t occur very often. Animal studies don’t always extrapolate to humans. And, observational studies concerning diet often have co-factors that are difficult to control and must run over time courses that can tax even the most patient researchers.
I certainly applaud Leib and associates for promoting their primary goal of including more about of the relationship between food and health in the medical school and trainee curriculum. But I must voice a caution to be careful to keep it truly evidence-based and in a format that acknowledges the realities of the life and education of a primary care provider.
The best nutritional advice I ever received in my training was from an older pediatric professor who suggested that a healthy diet consisted of everything in moderation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
How comfortable are you giving nutritional advice to your patients? When you offer it are you basing your advice on something you learned during medical school or your training? Was it included in a course devoted to nutrition? Did you learn it later as part of continuing medical education course (CME)? Or was it just something you just picked up from your experience seeing patients (osmosis)? It is very unlikely that a significant portion, or any part for that matter, of your medical training was devoted to nutrition. It certainly wasn’t during my training.
I recently read an interview with Emily M. Broad Leib, JD, faculty director of the Harvard School Center for Health Law and Policy Innovation, Cambridge, Massachusetts, who would like to correct that deficiency. She feels doctors need to know more about food and that acquiring that knowledge should be a significant component of their formal training.
In the interview, Leib said that “roughly 86% of physicians report they do not feel adequately trained to answer basic questions on diet or nutrition.” She also notes that while “72% of entering medical students report they believe food is important to health” less than 50% retained this belief after graduation.
Leib and associates feel they have recently reached a milestone in their efforts to include nutrition in the mainstream of medical education this fall by publishing a paper that demonstrates “consensus on doctor-approved nutritional standard for medical schools and residency programs.”
36 Recommended Competencies
Curious about what these nutrition experts chose to include in medical training, I decided to drill down into the list of 36 consensus-driven competencies they had agreed upon.
It was an interesting voyage into a forest of redundancies, many of which can be boiled down to having the student demonstrate that he/she understands that what we eat is important to our health and that there is a complex web of relationships connecting our society to the food consume.
Some of the recommended competencies I found make perfect sense. For example the student/trainee should be able to take a diet and food history and be able to interpret lab values and anthropometric measurements and be able to discuss the patient’s weight and diet with sensitivity while keeping in mind his/her own biases about food.
Some other recommendations are more problematic, for example, “performs a comprehensive nutrition-focused physical examination” or “demonstrates knowledge of how to create culinary nutrition SMART [Specific, Measurable, Achievable, Relevant, and Time-Bound] goals for personal use and for patient care” or “provides brief counseling interventions to help patients decrease visceral adiposity or reduce the risk of metabolic syndrome.” Including competencies like these demonstrates a lack of understanding of the time restraints and realities of a primary care physician’s life and training.
Instead of simply reinforcing the prospective physician’s preexisting assumption that food and health are entwined and discussing when and how to consult a nutrition expert, these 36 competencies seem to be an attempt to create fast-tracked part-time dietitians and nutrition advocates out of medical students and trainees who already believe that nutrition is important for health but also have a very full plate of clinical responsibilities ahead of them.
The study that Leib quotes — that 72% of medical students believed food was important in health while after graduation only 50% of agreed — doesn’t necessarily mean that professors are preaching that food was unimportant. It is more likely by the end of medical school the students have seen that food must share the spotlight with numerous other factors that influence their patients’ health.
‘A More Appropriate Focus’
In my experience, diet and lifestyle counseling done well is extremely time consuming and best done by people for whom that is their specialty. A more appropriate focus for a list of nutritional competencies for physicians in training would be for the student to achieve an understanding of when and how to consult a dietitian and then how to support and evaluate the dietitian’s recommendations to the patient.
Finally, I don’t think we can ignore a serious public relations problem that hangs like a cloud over the nutrition advocacy community. It is the same one that casts a shadow on the medical community as well. It is a common perception among the lay public that nutritionists (and physicians) are always changing their recommendations when it comes to food. What is believable? Just think about eggs, red wine, or introducing peanuts to infants, to name just a few. And what about the food pyramids that seem to have been rebuilt every several years? The problem is compounded when some “credentialed” nutritionists and physicians continue to make dietary pronouncements with only a shred of evidence or poorly documented anecdotal observations.
The first of the 36 competencies I reviewed reads: “Provide evidence-based, culturally sensitive nutrition and food recommendations for the prevention and treatment of disease.” When it comes to nutrition the “evidence” can be tough to come by. The natural experiments in which individuals and populations had extremely limited access to a certain nutrients (eg, scurvy) don’t occur very often. Animal studies don’t always extrapolate to humans. And, observational studies concerning diet often have co-factors that are difficult to control and must run over time courses that can tax even the most patient researchers.
I certainly applaud Leib and associates for promoting their primary goal of including more about of the relationship between food and health in the medical school and trainee curriculum. But I must voice a caution to be careful to keep it truly evidence-based and in a format that acknowledges the realities of the life and education of a primary care provider.
The best nutritional advice I ever received in my training was from an older pediatric professor who suggested that a healthy diet consisted of everything in moderation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
On Second Thought: Aspirin for Primary Prevention — What We Really Know
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Screening Options for Rare Malignancies
Dear colleagues,
As gastroenterologists and endoscopists, we spend significant time preventing and diagnosing GI malignancies.
For instance, is it worthwhile screening for pancreatic cancer, and, if so, how should this be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher risk populations, such as primary sclerosing cholangitis? And what about the costs, financial and otherwise, associated with screening?
In this issue of Perspectives, Dr. Darshan Kothari and Dr. Daniel Bernstein discuss their approach to pancreatic cancer screening, including who is eligible, the preferred screening modalities, and the barriers to screening. In the accompanying perspective, Dr. Aparna Goel and Dr. Judah Kupferman focus on cholangiocarcinoma screening, identifying high-risk populations and discussing some of the concerns with screening, necessitating shared decision-making.
We welcome your thoughts on this issue. Share with us on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
An Approach to Pancreatic Cancer Screening
BY DANIEL A. BERNSTEIN, MD, AND DARSHAN KOTHARI, MD
Pancreatic cancer carries a dismal prognosis, now accounting for the third-most cancer-related mortality in the United States. A small proportion of patients are diagnosed at a local stage of disease, with over half found to have metastatic disease at presentation. Given the low overall incidence and lifetime risk in the general population, population-based screening is not justified.
About 10% of cases of pancreas cancer are associated with germ-line mutations and/or with a strong family history of pancreatic cancer. Several academic societies and expert committees now recommend regular screening for pancreatic cancer in patients who are considered high-risk individuals, as they carry a fivefold relative risk for pancreatic cancer. Moreover, studies suggest that screening has the potential to identify early-stage resectable disease and decrease mortality in this patient population.
Patients who benefit from pancreatic cancer screening are those who carry an increased lifetime risk (in excess of 5%) of pancreatic cancer. High-risk individuals include those with germ-line mutations and/or those with a family history of pancreatic cancer in first-degree relatives. Consensus guidelines by the International Cancer of the Pancreas Screening Consortium and the American Society for Gastrointestinal Endoscopy provide medical centers with detailed recommendations on who and when to start screening.
High-risk individuals fall into three categories:
- Patients with high-risk germline mutations including: familial atypical multiple mole melanoma syndrome (CDKN2A), hereditary breast and ovarian cancer syndromes (BRCA1, BRCA2, and PALB2), Peutz-Jeghers syndrome (STK11), and hereditary pancreatitis (PRSS1 and SPINK1)
- Patients with low- to moderate-risk germ-line mutations with at least one first-degree relative with pancreatic cancer: Lynch Syndrome (particularly MLH1 mutation), ataxia-telangiectasia (ATM), or Li-Fraumeni syndrome (p53)
- Patients with one first-degree relative with pancreatic cancer who in turn has one first-degree relative with pancreatic cancer (eg, a patient’s mother and maternal aunt or a patient’s father and patient’s sister)
Consistent with established guidelines, we recommend screening for high-risk patients beginning at age 50, or 10 years before the youngest age at which pancreas cancer was diagnosed in an affected relative. Screening is recommended earlier in patients with particularly high risk: at age 40 for patients with CDKN2A and STKI11 mutations and age 40 for patients with PRSS1 mutation or 20 years after the first attack of acute pancreatitis. For patients with a strong family history of pancreas cancer, we recommend comprehensive evaluation by a certified genetic counselor at a high-volume cancer center.
In practice, patients at our institution who are identified as high risk based on the above criteria are referred for an initial consultation at our pancreas center. In most cases, this should occur no sooner than 5 years prior to the recommended starting age for screening. All patients who are identified as high risk should be screened annually for diabetes given the growing evidence base supporting an association between new-onset diabetes and pancreatic cancer.
After an initial visit and discussion of the risks and benefits of screening, most screening protocols start with a baseline endoscopic ultrasound (EUS) and contrast-enhanced magnetic resonance abdomen with magnetic resonance cholangiopancreatography (MRI/MRCP), which will be repeated annually or sooner as the clinical condition warrants. A sooner-interval EUS should be considered for patients already undergoing screening who are newly found to have diabetes.
At our institution, we start with an in-person clinic evaluation followed by EUS. Thereafter, patients undergo MRI/MRCP (synchronized with a same-day clinic visit) alternating with EUS every 6 months to ensure patients are seen twice a year, though there is no specific data to support this approach. Non-diabetics also undergo yearly diabetes screening which will trigger an EUS if patients become diabetic.
We engage in shared decision-making with our high-risk individuals undergoing pancreatic cancer screening and at each visit we review their concurrent medical conditions and suitability to continue screening. We consider discontinuing screening after age 75, at the onset of any life-limiting illness, or after a discussion of risks and benefits if comorbidities lead to a substantial deterioration in a patient’s overall health status.
While a growing body of evidence exists to support the application of pancreatic cancer screening in high-risk individuals, this preventive service remains underutilized. Recent analysis of the screening cohort at our institution showed a demographically homogeneous group of mostly highly educated, high-income White females. These findings are consistent with the patient cohorts described in other pancreatic cancer screening programs and represent only a fraction of people who would qualify for pancreatic cancer screening.
A survey of patients undergoing screening at our institution identified cost, travel, and time associated with pancreatic cancer screening to be frequent challenges to participation. Further studies are needed to fully explore the barriers and psychological burden of pancreas cancer screening in high-risk individuals, and to identify ways to enrich the cohort of patients undergoing screening. This may involve novel methods to identify family members of patients with a new diagnosis of pancreas cancer and increasing health literacy around pancreatic cancer screening among patients and providers.
Pancreatic cancer screening has the potential to identify early-stage disease in patients who are at high risk because of germ-line mutations and/or family history. We recommend that patients engage in pancreatic cancer screening at high-volume centers with well-supported oncology, genetics, and research infrastructure.
Dr. Bernstein is a gastroenterology fellow at Duke University School of Medicine, Durham, North Carolina. Dr. Kothari is an associate professor of medicine in gastroenterology and hepatology at Duke University School of Medicine.
Screening for Cholangiocarcinoma
BY JUDAH KUPFERMAN, MD, AND APARNA GOEL, MD
Cholangiocarcinoma is a rare but aggressive cancer of the bile ducts that poses many diagnostic challenges. Approximately 3% of gastrointestinal cancers are attributed to cholangiocarcinoma, and while the annual incidence of disease in the United States is about 1.26 per 100,000 people, the incidence of intrahepatic disease has been rising considerably.1,2 Screening for cholangiocarcinoma is reserved for high-risk individuals — such as those with primary sclerosing cholangitis (PSC), secondary sclerosing cholangitis (SSC), and biliary tract disorders such as choledochal cysts or Caroli’s disease. The goal is to balance the benefits of early diagnosis with the costs and risks associated with screening, particularly given the limitations of available tools like MRI with cholangiopancreatography (MRCP), which has a sensitivity of 70%-85%. In general, we recommend annual cholangiocarcinoma screening for high-risk individuals with MRI and MRCP as well as with cancer antigen (CA) 19-9. .
Screening in Patients with Primary Sclerosing Cholangitis
The lifetime risk of cholangiocarcinoma in patients with PSC is 10%-15% with an annual risk of 0.5%-1.5%. In our experience, this is often the most feared complication for PSC patients, even more so than the risk of liver transplantation. We recommend annual MRI with MRCP in addition to CA 19-9 for patients with PSC in the first decade of their diagnosis, as most cancers are diagnosed during this period. If a patient’s imaging has remained stable for over a decade and there is minimal hepatic fibrosis, we discuss the option of reducing screening frequency to every 2 years to minimize costs and exposure to MRI contrast risks.
If MRI reveals a concerning new large duct stricture, we will evaluate this with an endoscopic retrograde cholangiopancreatography (ERCP), as differentiating benign and malignant strictures is quite challenging with MRI. We generally recommend ERCP with brush cytology and fluorescence in situ hybridization to improve diagnostic yield. Depending on imaging findings and location of the new large duct stricture, we may consider cholangioscopy during ERCP for direct visualization of the bile duct and directed tissue biopsies. Unfortunately, even in young, asymptomatic patients who undergo regular screening, cholangiocarcinoma is frequently diagnosed at an advanced stage.
Screening in Patients with Secondary Sclerosing Cholangitis
Patients with SSC may develop cholangiocarcinoma because of chronic inflammatory and fibrotic processes, such as IgG4-associated cholangiopathy, sarcoidosis, ischemic cholangiopathy, cystic fibrosis, recurrent pyogenic cholangitis, severe sepsis (as recently seen from SARS-CoV-2), surgical complications, or other etiologies. When the condition is reversible, such as with IgG4-associated cholangiopathy, cancer screening may not be necessary. However, when irreversible damage occurs, the cancer risk increases, though it varies by disease type and severity. In most cases, we recommend routine screening for cholangiocarcinoma with MRI and CA 19-9 in this population.
Screening in Patients with Biliary Tract Disorders
Biliary tract disorders such as choledochal cysts and Caroli’s disease also harbor an increased risk of cholangiocarcinoma. Choledochal cysts are congenital cystic dilations of the bile duct that have a 10%-30% lifetime risk of malignant transformation to cholangiocarcinoma. Surgical intervention to remove the cyst is often recommended because of this high risk. However, some patients may be unable or unwilling to undergo this surgery or they may have residual cysts. We recommend ongoing screening with MRI and CA 19-9 for these patients. Similarly, Caroli’s disease is a congenital disease associated with intrahepatic and extrahepatic bile duct cysts and associated with a 5%-15% lifetime risk of cholangiocarcinoma. MRI with MRCP and CA 19-9 should be performed routinely for patients with Caroli’s disease and syndrome.
Risks and Challenges in Cholangiocarcinoma Screening
While MRI with MRCP is the gold standard for cholangiocarcinoma screening, its limitations must be carefully considered. One growing concern is the potential for gadolinium retention in the brain, bones, or skin following repeated MRI scans. Though the long-term effects of gadolinium retention are not fully understood, we factor this into screening decisions, particularly for younger patients who may undergo decades of regular imaging.
MRI is not always feasible for certain patients, including those with metal implants, on hemodialysis, or with severe allergic reactions. In such cases, CT or ultrasound may serve as alternatives, though with lower sensitivity for detecting cholangiocarcinoma. Additionally, claustrophobia during MRI can be addressed with sedation, but this underscores the importance of shared decision-making.
From our perspective, cholangiocarcinoma screening in high-risk patients is crucial but not without challenges. Our current screening methods, while essential, are far from perfect, often missing early cancers or leading to unnecessary interventions. Because of these limitations, the window for treatment of localized disease can easily be missed. In our practice, we tailor screening strategies to each patient’s specific needs, weighing the potential benefits against the risks, costs, and the inherent uncertainty of early detection tools. We believe it is essential to involve patients in this decision-making process to provide a balanced, individualized approach that considers both clinical evidence and the personal preferences of each person.
Dr. Kupferman is a gastroenterology fellow at Stanford University School of Medicine in California. Dr. Goel is a transplant hepatologist and a clinical associate professor in gastroenterology & hepatology at Stanford.
References
1. Vithayathil M and Khan SA. J Hepatol. 2022 Dec. doi: 10.1016/j.jhep.2022.07.022.
2. Patel N and Benipal B. Cureus. 2019 Jan. doi: 10.7759/cureus.3962.
Dear colleagues,
As gastroenterologists and endoscopists, we spend significant time preventing and diagnosing GI malignancies.
For instance, is it worthwhile screening for pancreatic cancer, and, if so, how should this be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher risk populations, such as primary sclerosing cholangitis? And what about the costs, financial and otherwise, associated with screening?
In this issue of Perspectives, Dr. Darshan Kothari and Dr. Daniel Bernstein discuss their approach to pancreatic cancer screening, including who is eligible, the preferred screening modalities, and the barriers to screening. In the accompanying perspective, Dr. Aparna Goel and Dr. Judah Kupferman focus on cholangiocarcinoma screening, identifying high-risk populations and discussing some of the concerns with screening, necessitating shared decision-making.
We welcome your thoughts on this issue. Share with us on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
An Approach to Pancreatic Cancer Screening
BY DANIEL A. BERNSTEIN, MD, AND DARSHAN KOTHARI, MD
Pancreatic cancer carries a dismal prognosis, now accounting for the third-most cancer-related mortality in the United States. A small proportion of patients are diagnosed at a local stage of disease, with over half found to have metastatic disease at presentation. Given the low overall incidence and lifetime risk in the general population, population-based screening is not justified.
About 10% of cases of pancreas cancer are associated with germ-line mutations and/or with a strong family history of pancreatic cancer. Several academic societies and expert committees now recommend regular screening for pancreatic cancer in patients who are considered high-risk individuals, as they carry a fivefold relative risk for pancreatic cancer. Moreover, studies suggest that screening has the potential to identify early-stage resectable disease and decrease mortality in this patient population.
Patients who benefit from pancreatic cancer screening are those who carry an increased lifetime risk (in excess of 5%) of pancreatic cancer. High-risk individuals include those with germ-line mutations and/or those with a family history of pancreatic cancer in first-degree relatives. Consensus guidelines by the International Cancer of the Pancreas Screening Consortium and the American Society for Gastrointestinal Endoscopy provide medical centers with detailed recommendations on who and when to start screening.
High-risk individuals fall into three categories:
- Patients with high-risk germline mutations including: familial atypical multiple mole melanoma syndrome (CDKN2A), hereditary breast and ovarian cancer syndromes (BRCA1, BRCA2, and PALB2), Peutz-Jeghers syndrome (STK11), and hereditary pancreatitis (PRSS1 and SPINK1)
- Patients with low- to moderate-risk germ-line mutations with at least one first-degree relative with pancreatic cancer: Lynch Syndrome (particularly MLH1 mutation), ataxia-telangiectasia (ATM), or Li-Fraumeni syndrome (p53)
- Patients with one first-degree relative with pancreatic cancer who in turn has one first-degree relative with pancreatic cancer (eg, a patient’s mother and maternal aunt or a patient’s father and patient’s sister)
Consistent with established guidelines, we recommend screening for high-risk patients beginning at age 50, or 10 years before the youngest age at which pancreas cancer was diagnosed in an affected relative. Screening is recommended earlier in patients with particularly high risk: at age 40 for patients with CDKN2A and STKI11 mutations and age 40 for patients with PRSS1 mutation or 20 years after the first attack of acute pancreatitis. For patients with a strong family history of pancreas cancer, we recommend comprehensive evaluation by a certified genetic counselor at a high-volume cancer center.
In practice, patients at our institution who are identified as high risk based on the above criteria are referred for an initial consultation at our pancreas center. In most cases, this should occur no sooner than 5 years prior to the recommended starting age for screening. All patients who are identified as high risk should be screened annually for diabetes given the growing evidence base supporting an association between new-onset diabetes and pancreatic cancer.
After an initial visit and discussion of the risks and benefits of screening, most screening protocols start with a baseline endoscopic ultrasound (EUS) and contrast-enhanced magnetic resonance abdomen with magnetic resonance cholangiopancreatography (MRI/MRCP), which will be repeated annually or sooner as the clinical condition warrants. A sooner-interval EUS should be considered for patients already undergoing screening who are newly found to have diabetes.
At our institution, we start with an in-person clinic evaluation followed by EUS. Thereafter, patients undergo MRI/MRCP (synchronized with a same-day clinic visit) alternating with EUS every 6 months to ensure patients are seen twice a year, though there is no specific data to support this approach. Non-diabetics also undergo yearly diabetes screening which will trigger an EUS if patients become diabetic.
We engage in shared decision-making with our high-risk individuals undergoing pancreatic cancer screening and at each visit we review their concurrent medical conditions and suitability to continue screening. We consider discontinuing screening after age 75, at the onset of any life-limiting illness, or after a discussion of risks and benefits if comorbidities lead to a substantial deterioration in a patient’s overall health status.
While a growing body of evidence exists to support the application of pancreatic cancer screening in high-risk individuals, this preventive service remains underutilized. Recent analysis of the screening cohort at our institution showed a demographically homogeneous group of mostly highly educated, high-income White females. These findings are consistent with the patient cohorts described in other pancreatic cancer screening programs and represent only a fraction of people who would qualify for pancreatic cancer screening.
A survey of patients undergoing screening at our institution identified cost, travel, and time associated with pancreatic cancer screening to be frequent challenges to participation. Further studies are needed to fully explore the barriers and psychological burden of pancreas cancer screening in high-risk individuals, and to identify ways to enrich the cohort of patients undergoing screening. This may involve novel methods to identify family members of patients with a new diagnosis of pancreas cancer and increasing health literacy around pancreatic cancer screening among patients and providers.
Pancreatic cancer screening has the potential to identify early-stage disease in patients who are at high risk because of germ-line mutations and/or family history. We recommend that patients engage in pancreatic cancer screening at high-volume centers with well-supported oncology, genetics, and research infrastructure.
Dr. Bernstein is a gastroenterology fellow at Duke University School of Medicine, Durham, North Carolina. Dr. Kothari is an associate professor of medicine in gastroenterology and hepatology at Duke University School of Medicine.
Screening for Cholangiocarcinoma
BY JUDAH KUPFERMAN, MD, AND APARNA GOEL, MD
Cholangiocarcinoma is a rare but aggressive cancer of the bile ducts that poses many diagnostic challenges. Approximately 3% of gastrointestinal cancers are attributed to cholangiocarcinoma, and while the annual incidence of disease in the United States is about 1.26 per 100,000 people, the incidence of intrahepatic disease has been rising considerably.1,2 Screening for cholangiocarcinoma is reserved for high-risk individuals — such as those with primary sclerosing cholangitis (PSC), secondary sclerosing cholangitis (SSC), and biliary tract disorders such as choledochal cysts or Caroli’s disease. The goal is to balance the benefits of early diagnosis with the costs and risks associated with screening, particularly given the limitations of available tools like MRI with cholangiopancreatography (MRCP), which has a sensitivity of 70%-85%. In general, we recommend annual cholangiocarcinoma screening for high-risk individuals with MRI and MRCP as well as with cancer antigen (CA) 19-9. .
Screening in Patients with Primary Sclerosing Cholangitis
The lifetime risk of cholangiocarcinoma in patients with PSC is 10%-15% with an annual risk of 0.5%-1.5%. In our experience, this is often the most feared complication for PSC patients, even more so than the risk of liver transplantation. We recommend annual MRI with MRCP in addition to CA 19-9 for patients with PSC in the first decade of their diagnosis, as most cancers are diagnosed during this period. If a patient’s imaging has remained stable for over a decade and there is minimal hepatic fibrosis, we discuss the option of reducing screening frequency to every 2 years to minimize costs and exposure to MRI contrast risks.
If MRI reveals a concerning new large duct stricture, we will evaluate this with an endoscopic retrograde cholangiopancreatography (ERCP), as differentiating benign and malignant strictures is quite challenging with MRI. We generally recommend ERCP with brush cytology and fluorescence in situ hybridization to improve diagnostic yield. Depending on imaging findings and location of the new large duct stricture, we may consider cholangioscopy during ERCP for direct visualization of the bile duct and directed tissue biopsies. Unfortunately, even in young, asymptomatic patients who undergo regular screening, cholangiocarcinoma is frequently diagnosed at an advanced stage.
Screening in Patients with Secondary Sclerosing Cholangitis
Patients with SSC may develop cholangiocarcinoma because of chronic inflammatory and fibrotic processes, such as IgG4-associated cholangiopathy, sarcoidosis, ischemic cholangiopathy, cystic fibrosis, recurrent pyogenic cholangitis, severe sepsis (as recently seen from SARS-CoV-2), surgical complications, or other etiologies. When the condition is reversible, such as with IgG4-associated cholangiopathy, cancer screening may not be necessary. However, when irreversible damage occurs, the cancer risk increases, though it varies by disease type and severity. In most cases, we recommend routine screening for cholangiocarcinoma with MRI and CA 19-9 in this population.
Screening in Patients with Biliary Tract Disorders
Biliary tract disorders such as choledochal cysts and Caroli’s disease also harbor an increased risk of cholangiocarcinoma. Choledochal cysts are congenital cystic dilations of the bile duct that have a 10%-30% lifetime risk of malignant transformation to cholangiocarcinoma. Surgical intervention to remove the cyst is often recommended because of this high risk. However, some patients may be unable or unwilling to undergo this surgery or they may have residual cysts. We recommend ongoing screening with MRI and CA 19-9 for these patients. Similarly, Caroli’s disease is a congenital disease associated with intrahepatic and extrahepatic bile duct cysts and associated with a 5%-15% lifetime risk of cholangiocarcinoma. MRI with MRCP and CA 19-9 should be performed routinely for patients with Caroli’s disease and syndrome.
Risks and Challenges in Cholangiocarcinoma Screening
While MRI with MRCP is the gold standard for cholangiocarcinoma screening, its limitations must be carefully considered. One growing concern is the potential for gadolinium retention in the brain, bones, or skin following repeated MRI scans. Though the long-term effects of gadolinium retention are not fully understood, we factor this into screening decisions, particularly for younger patients who may undergo decades of regular imaging.
MRI is not always feasible for certain patients, including those with metal implants, on hemodialysis, or with severe allergic reactions. In such cases, CT or ultrasound may serve as alternatives, though with lower sensitivity for detecting cholangiocarcinoma. Additionally, claustrophobia during MRI can be addressed with sedation, but this underscores the importance of shared decision-making.
From our perspective, cholangiocarcinoma screening in high-risk patients is crucial but not without challenges. Our current screening methods, while essential, are far from perfect, often missing early cancers or leading to unnecessary interventions. Because of these limitations, the window for treatment of localized disease can easily be missed. In our practice, we tailor screening strategies to each patient’s specific needs, weighing the potential benefits against the risks, costs, and the inherent uncertainty of early detection tools. We believe it is essential to involve patients in this decision-making process to provide a balanced, individualized approach that considers both clinical evidence and the personal preferences of each person.
Dr. Kupferman is a gastroenterology fellow at Stanford University School of Medicine in California. Dr. Goel is a transplant hepatologist and a clinical associate professor in gastroenterology & hepatology at Stanford.
References
1. Vithayathil M and Khan SA. J Hepatol. 2022 Dec. doi: 10.1016/j.jhep.2022.07.022.
2. Patel N and Benipal B. Cureus. 2019 Jan. doi: 10.7759/cureus.3962.
Dear colleagues,
As gastroenterologists and endoscopists, we spend significant time preventing and diagnosing GI malignancies.
For instance, is it worthwhile screening for pancreatic cancer, and, if so, how should this be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher risk populations, such as primary sclerosing cholangitis? And what about the costs, financial and otherwise, associated with screening?
In this issue of Perspectives, Dr. Darshan Kothari and Dr. Daniel Bernstein discuss their approach to pancreatic cancer screening, including who is eligible, the preferred screening modalities, and the barriers to screening. In the accompanying perspective, Dr. Aparna Goel and Dr. Judah Kupferman focus on cholangiocarcinoma screening, identifying high-risk populations and discussing some of the concerns with screening, necessitating shared decision-making.
We welcome your thoughts on this issue. Share with us on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
An Approach to Pancreatic Cancer Screening
BY DANIEL A. BERNSTEIN, MD, AND DARSHAN KOTHARI, MD
Pancreatic cancer carries a dismal prognosis, now accounting for the third-most cancer-related mortality in the United States. A small proportion of patients are diagnosed at a local stage of disease, with over half found to have metastatic disease at presentation. Given the low overall incidence and lifetime risk in the general population, population-based screening is not justified.
About 10% of cases of pancreas cancer are associated with germ-line mutations and/or with a strong family history of pancreatic cancer. Several academic societies and expert committees now recommend regular screening for pancreatic cancer in patients who are considered high-risk individuals, as they carry a fivefold relative risk for pancreatic cancer. Moreover, studies suggest that screening has the potential to identify early-stage resectable disease and decrease mortality in this patient population.
Patients who benefit from pancreatic cancer screening are those who carry an increased lifetime risk (in excess of 5%) of pancreatic cancer. High-risk individuals include those with germ-line mutations and/or those with a family history of pancreatic cancer in first-degree relatives. Consensus guidelines by the International Cancer of the Pancreas Screening Consortium and the American Society for Gastrointestinal Endoscopy provide medical centers with detailed recommendations on who and when to start screening.
High-risk individuals fall into three categories:
- Patients with high-risk germline mutations including: familial atypical multiple mole melanoma syndrome (CDKN2A), hereditary breast and ovarian cancer syndromes (BRCA1, BRCA2, and PALB2), Peutz-Jeghers syndrome (STK11), and hereditary pancreatitis (PRSS1 and SPINK1)
- Patients with low- to moderate-risk germ-line mutations with at least one first-degree relative with pancreatic cancer: Lynch Syndrome (particularly MLH1 mutation), ataxia-telangiectasia (ATM), or Li-Fraumeni syndrome (p53)
- Patients with one first-degree relative with pancreatic cancer who in turn has one first-degree relative with pancreatic cancer (eg, a patient’s mother and maternal aunt or a patient’s father and patient’s sister)
Consistent with established guidelines, we recommend screening for high-risk patients beginning at age 50, or 10 years before the youngest age at which pancreas cancer was diagnosed in an affected relative. Screening is recommended earlier in patients with particularly high risk: at age 40 for patients with CDKN2A and STKI11 mutations and age 40 for patients with PRSS1 mutation or 20 years after the first attack of acute pancreatitis. For patients with a strong family history of pancreas cancer, we recommend comprehensive evaluation by a certified genetic counselor at a high-volume cancer center.
In practice, patients at our institution who are identified as high risk based on the above criteria are referred for an initial consultation at our pancreas center. In most cases, this should occur no sooner than 5 years prior to the recommended starting age for screening. All patients who are identified as high risk should be screened annually for diabetes given the growing evidence base supporting an association between new-onset diabetes and pancreatic cancer.
After an initial visit and discussion of the risks and benefits of screening, most screening protocols start with a baseline endoscopic ultrasound (EUS) and contrast-enhanced magnetic resonance abdomen with magnetic resonance cholangiopancreatography (MRI/MRCP), which will be repeated annually or sooner as the clinical condition warrants. A sooner-interval EUS should be considered for patients already undergoing screening who are newly found to have diabetes.
At our institution, we start with an in-person clinic evaluation followed by EUS. Thereafter, patients undergo MRI/MRCP (synchronized with a same-day clinic visit) alternating with EUS every 6 months to ensure patients are seen twice a year, though there is no specific data to support this approach. Non-diabetics also undergo yearly diabetes screening which will trigger an EUS if patients become diabetic.
We engage in shared decision-making with our high-risk individuals undergoing pancreatic cancer screening and at each visit we review their concurrent medical conditions and suitability to continue screening. We consider discontinuing screening after age 75, at the onset of any life-limiting illness, or after a discussion of risks and benefits if comorbidities lead to a substantial deterioration in a patient’s overall health status.
While a growing body of evidence exists to support the application of pancreatic cancer screening in high-risk individuals, this preventive service remains underutilized. Recent analysis of the screening cohort at our institution showed a demographically homogeneous group of mostly highly educated, high-income White females. These findings are consistent with the patient cohorts described in other pancreatic cancer screening programs and represent only a fraction of people who would qualify for pancreatic cancer screening.
A survey of patients undergoing screening at our institution identified cost, travel, and time associated with pancreatic cancer screening to be frequent challenges to participation. Further studies are needed to fully explore the barriers and psychological burden of pancreas cancer screening in high-risk individuals, and to identify ways to enrich the cohort of patients undergoing screening. This may involve novel methods to identify family members of patients with a new diagnosis of pancreas cancer and increasing health literacy around pancreatic cancer screening among patients and providers.
Pancreatic cancer screening has the potential to identify early-stage disease in patients who are at high risk because of germ-line mutations and/or family history. We recommend that patients engage in pancreatic cancer screening at high-volume centers with well-supported oncology, genetics, and research infrastructure.
Dr. Bernstein is a gastroenterology fellow at Duke University School of Medicine, Durham, North Carolina. Dr. Kothari is an associate professor of medicine in gastroenterology and hepatology at Duke University School of Medicine.
Screening for Cholangiocarcinoma
BY JUDAH KUPFERMAN, MD, AND APARNA GOEL, MD
Cholangiocarcinoma is a rare but aggressive cancer of the bile ducts that poses many diagnostic challenges. Approximately 3% of gastrointestinal cancers are attributed to cholangiocarcinoma, and while the annual incidence of disease in the United States is about 1.26 per 100,000 people, the incidence of intrahepatic disease has been rising considerably.1,2 Screening for cholangiocarcinoma is reserved for high-risk individuals — such as those with primary sclerosing cholangitis (PSC), secondary sclerosing cholangitis (SSC), and biliary tract disorders such as choledochal cysts or Caroli’s disease. The goal is to balance the benefits of early diagnosis with the costs and risks associated with screening, particularly given the limitations of available tools like MRI with cholangiopancreatography (MRCP), which has a sensitivity of 70%-85%. In general, we recommend annual cholangiocarcinoma screening for high-risk individuals with MRI and MRCP as well as with cancer antigen (CA) 19-9. .
Screening in Patients with Primary Sclerosing Cholangitis
The lifetime risk of cholangiocarcinoma in patients with PSC is 10%-15% with an annual risk of 0.5%-1.5%. In our experience, this is often the most feared complication for PSC patients, even more so than the risk of liver transplantation. We recommend annual MRI with MRCP in addition to CA 19-9 for patients with PSC in the first decade of their diagnosis, as most cancers are diagnosed during this period. If a patient’s imaging has remained stable for over a decade and there is minimal hepatic fibrosis, we discuss the option of reducing screening frequency to every 2 years to minimize costs and exposure to MRI contrast risks.
If MRI reveals a concerning new large duct stricture, we will evaluate this with an endoscopic retrograde cholangiopancreatography (ERCP), as differentiating benign and malignant strictures is quite challenging with MRI. We generally recommend ERCP with brush cytology and fluorescence in situ hybridization to improve diagnostic yield. Depending on imaging findings and location of the new large duct stricture, we may consider cholangioscopy during ERCP for direct visualization of the bile duct and directed tissue biopsies. Unfortunately, even in young, asymptomatic patients who undergo regular screening, cholangiocarcinoma is frequently diagnosed at an advanced stage.
Screening in Patients with Secondary Sclerosing Cholangitis
Patients with SSC may develop cholangiocarcinoma because of chronic inflammatory and fibrotic processes, such as IgG4-associated cholangiopathy, sarcoidosis, ischemic cholangiopathy, cystic fibrosis, recurrent pyogenic cholangitis, severe sepsis (as recently seen from SARS-CoV-2), surgical complications, or other etiologies. When the condition is reversible, such as with IgG4-associated cholangiopathy, cancer screening may not be necessary. However, when irreversible damage occurs, the cancer risk increases, though it varies by disease type and severity. In most cases, we recommend routine screening for cholangiocarcinoma with MRI and CA 19-9 in this population.
Screening in Patients with Biliary Tract Disorders
Biliary tract disorders such as choledochal cysts and Caroli’s disease also harbor an increased risk of cholangiocarcinoma. Choledochal cysts are congenital cystic dilations of the bile duct that have a 10%-30% lifetime risk of malignant transformation to cholangiocarcinoma. Surgical intervention to remove the cyst is often recommended because of this high risk. However, some patients may be unable or unwilling to undergo this surgery or they may have residual cysts. We recommend ongoing screening with MRI and CA 19-9 for these patients. Similarly, Caroli’s disease is a congenital disease associated with intrahepatic and extrahepatic bile duct cysts and associated with a 5%-15% lifetime risk of cholangiocarcinoma. MRI with MRCP and CA 19-9 should be performed routinely for patients with Caroli’s disease and syndrome.
Risks and Challenges in Cholangiocarcinoma Screening
While MRI with MRCP is the gold standard for cholangiocarcinoma screening, its limitations must be carefully considered. One growing concern is the potential for gadolinium retention in the brain, bones, or skin following repeated MRI scans. Though the long-term effects of gadolinium retention are not fully understood, we factor this into screening decisions, particularly for younger patients who may undergo decades of regular imaging.
MRI is not always feasible for certain patients, including those with metal implants, on hemodialysis, or with severe allergic reactions. In such cases, CT or ultrasound may serve as alternatives, though with lower sensitivity for detecting cholangiocarcinoma. Additionally, claustrophobia during MRI can be addressed with sedation, but this underscores the importance of shared decision-making.
From our perspective, cholangiocarcinoma screening in high-risk patients is crucial but not without challenges. Our current screening methods, while essential, are far from perfect, often missing early cancers or leading to unnecessary interventions. Because of these limitations, the window for treatment of localized disease can easily be missed. In our practice, we tailor screening strategies to each patient’s specific needs, weighing the potential benefits against the risks, costs, and the inherent uncertainty of early detection tools. We believe it is essential to involve patients in this decision-making process to provide a balanced, individualized approach that considers both clinical evidence and the personal preferences of each person.
Dr. Kupferman is a gastroenterology fellow at Stanford University School of Medicine in California. Dr. Goel is a transplant hepatologist and a clinical associate professor in gastroenterology & hepatology at Stanford.
References
1. Vithayathil M and Khan SA. J Hepatol. 2022 Dec. doi: 10.1016/j.jhep.2022.07.022.
2. Patel N and Benipal B. Cureus. 2019 Jan. doi: 10.7759/cureus.3962.
Obesity: A Social Vulnerability
Sometime in the last year or 2 I wrote that, despite my considerable reservations, I had finally come to the conclusion that the American Medical Association’s decision to designate obesity as a disease was appropriate. My rationalization was that the disease label would open more opportunities for funding obesity treatments. However, the explosive growth and popularity of glucagon-like peptide 1 (GLP-1) agonists over the last year has had me rethinking my decision to suppress my long-held reservations about the disease designation.
So, if it’s not a disease, then what should we call it? How do we explain its surge in high-income countries that began in the 1980s? While there are still some folks who see obesity as a character flaw, I think you and I as healthcare providers have difficulty explaining the increase prevalence of obesity as either global breakdown of willpower or a widespread genetic shift as the result of burst of radiation from solar flares.
However, if we want to continue our search and finger-pointing we need to have a better definition of exactly what obesity is. If we’re going to continue calling it a disease we have done a pretty sloppy job of creating diagnostic criteria. To be honest, we aren’t doing such a hot job with “long COVID” either.
A recent article in the New York Times makes it clear that I’m not the only physician who is feeling uncomfortable with this lack of diagnostic specificity.
We know that using body mass index (BMI) as a criteria is imprecise. There are healthy individuals with elevated BMIs and there are others who are carrying an unhealthy amount of fat who have normal BMIs. And, there are individuals who have what might appear to be an excess amount of fat who are fit and healthy by other criteria.
Some investigators feel that a set of measurements that includes a waist and/or hip measurement may be a more accurate way of determining visceral adipose tissue. However, this body roundness index (BRI) currently relies on a tape measurement. Until the technique can be preformed by an inexpensive and readily available scanner, the BRI cannot be considered a practical tool for determining obesity.
Dr. Francisco Rubino, the chair of metabolic and bariatric surgery at Kings College in London, England, has been quoted as saying that, “if one defines a disease inaccurately, everything that stems from that – from diagnosis to treatment to policies – will be distorted and biased.”
Denmark has been forced to relabel obesity as a risk factor because the disease designation was stressing the financial viability of their healthcare system as more and more patients were being prescribe GLP-1 agonists, sometimes off label. A rationing strategy was resulting in suboptimal treatment of a significant portion of the obese population.
Spearheaded by Dr. Rubino, a Lancet Commission composed of physicians has tasked itself to define an “evidence-based diagnosis for obesity. Instead of relying on a single metric such as the BMI or BRI, diagnosing “clinical obesity” would involve a broad array of observations including a history, physical examination, standard laboratory and additional testing, “naming signs and symptoms, organ by organ, tissue by tissue, with plausible mechanisms for each one.” In other words, treating each patient as an individual using evidence-based criteria to make a diagnosis. While likely to be time consuming, this strategy feels like a more scientific approach. I suspect once clinical obesity is more rigorously defined it could be divided into several subtypes. For example, there would be a few conditions that were genetic; Prader-Willi syndrome being the best known.
However, I think the Lancet Commission’s strategy will find that the majority of individuals who make up this half-century global surge have become clinically obese because they have been unable to adapt to the obeseogenic forces in our society, which include diet, autocentricity, and attractive sedentary forms of entertainment, to name just three.
In some cases these unfortunate individuals are more vulnerable because there were born into an economically disadvantaged situation. In other scenarios a lack of foresight and/or political will may have left individuals with no other choice but to rely on automobiles to get around. Still others may find themselves living in a nutritional desert because all of the grocery stores have closed.
I recently encountered a descriptor in a story about the Federal Emergency Management Agency which could easily be adapted to describe this large and growing subtype of individuals with clinical obesity. “Social vulnerability” is measure of how well a community can withstand external stressors that impact human health. For example, the emergency management folks are thinking in terms of natural disaster such as hurricanes, floods, and tornadoes and are asking how well a given community can meet the challenges one would create.
But, the term social vulnerability can easily be applied to individuals living in a society in which unhealthy food is abundant, an infrastructure that discourages or outright prevents non-motorized travel, and the temptation of sedentary entertainment options is unavoidable. Fortunately, not every citizen living in an obesogenic society becomes obese. What factors have protected the non-obese individuals from these obeseogenic stressors? What are the characteristics of the unfortunate “vulnerables” living in the same society who end up being obese?
It is time to shift our focus away from a poorly defined disease model to one in which we begin looking at our society to find out why we have so many socially vulnerable individuals. The toll of obesity as it is currently defined is many order of magnitudes greater than any natural disaster. We have become communities that can no longer withstand the its obesogenic stressors many of which we have created and/or allowed to accumulate over the last century.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Sometime in the last year or 2 I wrote that, despite my considerable reservations, I had finally come to the conclusion that the American Medical Association’s decision to designate obesity as a disease was appropriate. My rationalization was that the disease label would open more opportunities for funding obesity treatments. However, the explosive growth and popularity of glucagon-like peptide 1 (GLP-1) agonists over the last year has had me rethinking my decision to suppress my long-held reservations about the disease designation.
So, if it’s not a disease, then what should we call it? How do we explain its surge in high-income countries that began in the 1980s? While there are still some folks who see obesity as a character flaw, I think you and I as healthcare providers have difficulty explaining the increase prevalence of obesity as either global breakdown of willpower or a widespread genetic shift as the result of burst of radiation from solar flares.
However, if we want to continue our search and finger-pointing we need to have a better definition of exactly what obesity is. If we’re going to continue calling it a disease we have done a pretty sloppy job of creating diagnostic criteria. To be honest, we aren’t doing such a hot job with “long COVID” either.
A recent article in the New York Times makes it clear that I’m not the only physician who is feeling uncomfortable with this lack of diagnostic specificity.
We know that using body mass index (BMI) as a criteria is imprecise. There are healthy individuals with elevated BMIs and there are others who are carrying an unhealthy amount of fat who have normal BMIs. And, there are individuals who have what might appear to be an excess amount of fat who are fit and healthy by other criteria.
Some investigators feel that a set of measurements that includes a waist and/or hip measurement may be a more accurate way of determining visceral adipose tissue. However, this body roundness index (BRI) currently relies on a tape measurement. Until the technique can be preformed by an inexpensive and readily available scanner, the BRI cannot be considered a practical tool for determining obesity.
Dr. Francisco Rubino, the chair of metabolic and bariatric surgery at Kings College in London, England, has been quoted as saying that, “if one defines a disease inaccurately, everything that stems from that – from diagnosis to treatment to policies – will be distorted and biased.”
Denmark has been forced to relabel obesity as a risk factor because the disease designation was stressing the financial viability of their healthcare system as more and more patients were being prescribe GLP-1 agonists, sometimes off label. A rationing strategy was resulting in suboptimal treatment of a significant portion of the obese population.
Spearheaded by Dr. Rubino, a Lancet Commission composed of physicians has tasked itself to define an “evidence-based diagnosis for obesity. Instead of relying on a single metric such as the BMI or BRI, diagnosing “clinical obesity” would involve a broad array of observations including a history, physical examination, standard laboratory and additional testing, “naming signs and symptoms, organ by organ, tissue by tissue, with plausible mechanisms for each one.” In other words, treating each patient as an individual using evidence-based criteria to make a diagnosis. While likely to be time consuming, this strategy feels like a more scientific approach. I suspect once clinical obesity is more rigorously defined it could be divided into several subtypes. For example, there would be a few conditions that were genetic; Prader-Willi syndrome being the best known.
However, I think the Lancet Commission’s strategy will find that the majority of individuals who make up this half-century global surge have become clinically obese because they have been unable to adapt to the obeseogenic forces in our society, which include diet, autocentricity, and attractive sedentary forms of entertainment, to name just three.
In some cases these unfortunate individuals are more vulnerable because there were born into an economically disadvantaged situation. In other scenarios a lack of foresight and/or political will may have left individuals with no other choice but to rely on automobiles to get around. Still others may find themselves living in a nutritional desert because all of the grocery stores have closed.
I recently encountered a descriptor in a story about the Federal Emergency Management Agency which could easily be adapted to describe this large and growing subtype of individuals with clinical obesity. “Social vulnerability” is measure of how well a community can withstand external stressors that impact human health. For example, the emergency management folks are thinking in terms of natural disaster such as hurricanes, floods, and tornadoes and are asking how well a given community can meet the challenges one would create.
But, the term social vulnerability can easily be applied to individuals living in a society in which unhealthy food is abundant, an infrastructure that discourages or outright prevents non-motorized travel, and the temptation of sedentary entertainment options is unavoidable. Fortunately, not every citizen living in an obesogenic society becomes obese. What factors have protected the non-obese individuals from these obeseogenic stressors? What are the characteristics of the unfortunate “vulnerables” living in the same society who end up being obese?
It is time to shift our focus away from a poorly defined disease model to one in which we begin looking at our society to find out why we have so many socially vulnerable individuals. The toll of obesity as it is currently defined is many order of magnitudes greater than any natural disaster. We have become communities that can no longer withstand the its obesogenic stressors many of which we have created and/or allowed to accumulate over the last century.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Sometime in the last year or 2 I wrote that, despite my considerable reservations, I had finally come to the conclusion that the American Medical Association’s decision to designate obesity as a disease was appropriate. My rationalization was that the disease label would open more opportunities for funding obesity treatments. However, the explosive growth and popularity of glucagon-like peptide 1 (GLP-1) agonists over the last year has had me rethinking my decision to suppress my long-held reservations about the disease designation.
So, if it’s not a disease, then what should we call it? How do we explain its surge in high-income countries that began in the 1980s? While there are still some folks who see obesity as a character flaw, I think you and I as healthcare providers have difficulty explaining the increase prevalence of obesity as either global breakdown of willpower or a widespread genetic shift as the result of burst of radiation from solar flares.
However, if we want to continue our search and finger-pointing we need to have a better definition of exactly what obesity is. If we’re going to continue calling it a disease we have done a pretty sloppy job of creating diagnostic criteria. To be honest, we aren’t doing such a hot job with “long COVID” either.
A recent article in the New York Times makes it clear that I’m not the only physician who is feeling uncomfortable with this lack of diagnostic specificity.
We know that using body mass index (BMI) as a criteria is imprecise. There are healthy individuals with elevated BMIs and there are others who are carrying an unhealthy amount of fat who have normal BMIs. And, there are individuals who have what might appear to be an excess amount of fat who are fit and healthy by other criteria.
Some investigators feel that a set of measurements that includes a waist and/or hip measurement may be a more accurate way of determining visceral adipose tissue. However, this body roundness index (BRI) currently relies on a tape measurement. Until the technique can be preformed by an inexpensive and readily available scanner, the BRI cannot be considered a practical tool for determining obesity.
Dr. Francisco Rubino, the chair of metabolic and bariatric surgery at Kings College in London, England, has been quoted as saying that, “if one defines a disease inaccurately, everything that stems from that – from diagnosis to treatment to policies – will be distorted and biased.”
Denmark has been forced to relabel obesity as a risk factor because the disease designation was stressing the financial viability of their healthcare system as more and more patients were being prescribe GLP-1 agonists, sometimes off label. A rationing strategy was resulting in suboptimal treatment of a significant portion of the obese population.
Spearheaded by Dr. Rubino, a Lancet Commission composed of physicians has tasked itself to define an “evidence-based diagnosis for obesity. Instead of relying on a single metric such as the BMI or BRI, diagnosing “clinical obesity” would involve a broad array of observations including a history, physical examination, standard laboratory and additional testing, “naming signs and symptoms, organ by organ, tissue by tissue, with plausible mechanisms for each one.” In other words, treating each patient as an individual using evidence-based criteria to make a diagnosis. While likely to be time consuming, this strategy feels like a more scientific approach. I suspect once clinical obesity is more rigorously defined it could be divided into several subtypes. For example, there would be a few conditions that were genetic; Prader-Willi syndrome being the best known.
However, I think the Lancet Commission’s strategy will find that the majority of individuals who make up this half-century global surge have become clinically obese because they have been unable to adapt to the obeseogenic forces in our society, which include diet, autocentricity, and attractive sedentary forms of entertainment, to name just three.
In some cases these unfortunate individuals are more vulnerable because there were born into an economically disadvantaged situation. In other scenarios a lack of foresight and/or political will may have left individuals with no other choice but to rely on automobiles to get around. Still others may find themselves living in a nutritional desert because all of the grocery stores have closed.
I recently encountered a descriptor in a story about the Federal Emergency Management Agency which could easily be adapted to describe this large and growing subtype of individuals with clinical obesity. “Social vulnerability” is measure of how well a community can withstand external stressors that impact human health. For example, the emergency management folks are thinking in terms of natural disaster such as hurricanes, floods, and tornadoes and are asking how well a given community can meet the challenges one would create.
But, the term social vulnerability can easily be applied to individuals living in a society in which unhealthy food is abundant, an infrastructure that discourages or outright prevents non-motorized travel, and the temptation of sedentary entertainment options is unavoidable. Fortunately, not every citizen living in an obesogenic society becomes obese. What factors have protected the non-obese individuals from these obeseogenic stressors? What are the characteristics of the unfortunate “vulnerables” living in the same society who end up being obese?
It is time to shift our focus away from a poorly defined disease model to one in which we begin looking at our society to find out why we have so many socially vulnerable individuals. The toll of obesity as it is currently defined is many order of magnitudes greater than any natural disaster. We have become communities that can no longer withstand the its obesogenic stressors many of which we have created and/or allowed to accumulate over the last century.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Preventing Pediatric Migraine
I suspect you all have some experience with childhood migraine. It can mean a painful several hours for the patient, arriving often without warning, with recurrences spaced months or sometimes even years apart. It may be accompanied by vomiting, which in some cases overshadows the severity of the headache. It can result in lost days from school and ruin family activities. It can occur so infrequently that the family can’t recall accurately when the last episode happened. In some ways it is a different animal than the adult version.
Most of the pediatric patients with migraine I have seen have experienced attacks that were occurring so infrequently that the families and I seldom discussed medication as an option. Back then imipramine was the only choice. However, currently there are more than a half dozen medications and combinations that have been tried. Recently a review of 45 clinical trials of these medications was published in JAMA Network Open.
I will let you review for yourself the details of these Iranian investigators’ network meta-analysis, but the bottom line is that some medications were associated with a reduction in migraine frequency. Others were associated with headache intensity. “However, no treatments were associated with significant improvements in quality of life or reduction of the duration of migraine attacks.”
Obviously, this paper illustrates clearly that we have not yet discovered the medicinal magic bullet for pediatric migraine prophylaxis. This doesn’t surprise me. After listening to scores of families tell their migraine stories, it became apparent to me that there was often a pattern in which the child’s headache had arrived after a period of acute sleep deprivation. For example, a trip to an amusement park in which travel or excitement may have resulted in the child going to bed later and/or getting up earlier. By afternoon the child’s reserves of something (currently unknown) were depleted to a point that the headache and/or vomiting struck.
Because these episodes were often so infrequent, separated by months, that taking a history demonstrating a recurring pattern could take considerable patience on the part of the family and the provider, even for a physician like myself who believes that better sleep is the answer for everything. However, once I could convince a family of the connection between the sleep deprivation and the headaches, they could often recall other episodes in the past that substantiated my explanation.
In some cases there was no obvious history of acute sleep deprivation, or at least it was so subtle that even a history taker with a sleep obsession couldn’t detect it. However, in these cases I could usually elicit a history of chronic sleep deprivation. For example, falling asleep instantly on automobile rides, difficulty with waking in the morning, or unhealthy bedtime routines. With this underlying vulnerability of chronic sleep deprivation, a slightly more exciting or vigorous day was all that was necessary to trigger the headache.
For those of you who don’t share my contention that childhood migraine is usually the result of sleep deprivation, consider the similarity between an epileptic seizure, which can be triggered by fatigue. Both events are usually followed by a deep sleep from which the child wakes refreshed and symptom free.
I think it is interesting that this recent meta-analysis could find no benefit in the quality of life for any of the medications. The explanation may be that the child with migraine already had a somewhat diminished quality of life as a result of the sleep deprivation, either acute or chronic.
When speaking with parents of migraine sufferers, I would tell them that once the headache had started there was little I had to offer to forestall the inevitable pain and vomiting. Certainly not in the form of an oral medication. While many adults will have an aura that warns them of the headache onset, I have found that most children don’t describe an aura. It may be they simply lack the ability to express it. Occasionally an observant parent may detect pallor or a behavior change that indicates a migraine is beginning. On rare occasions a parent may be able to abort the attack by quickly getting the child to a quiet, dark, and calm environment.
Although this recent meta-analysis review of treatment options is discouraging, it may be providing a clue to effective prophylaxis. Some of the medications that decrease the frequency of the attacks may be doing so because they improve the patient’s sleep patterns. Those that decrease the intensity of the pain are probably working on pain pathway that is not specific to migraine.
Continuing a search for a prophylactic medication is a worthy goal, particularly for those patients in which their migraines are debilitating. However, based on my experience, enhanced by my bias, the safest and most effective prophylaxis results from increasing the family’s awareness of the role that sleep deprivation plays in the illness. Even when the family buys into the message and attempts to avoid situations that will tax their vulnerable children, parents will need to accept that sometimes stuff happens even though siblings and peers may be able to tolerate the situation. Spontaneous activities can converge on a day when for whatever reason the migraine-prone child is overtired and the headache and vomiting will erupt.
A lifestyle change is always preferable to a pharmacological intervention. However, that doesn’t mean it is always easy to achieve.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I suspect you all have some experience with childhood migraine. It can mean a painful several hours for the patient, arriving often without warning, with recurrences spaced months or sometimes even years apart. It may be accompanied by vomiting, which in some cases overshadows the severity of the headache. It can result in lost days from school and ruin family activities. It can occur so infrequently that the family can’t recall accurately when the last episode happened. In some ways it is a different animal than the adult version.
Most of the pediatric patients with migraine I have seen have experienced attacks that were occurring so infrequently that the families and I seldom discussed medication as an option. Back then imipramine was the only choice. However, currently there are more than a half dozen medications and combinations that have been tried. Recently a review of 45 clinical trials of these medications was published in JAMA Network Open.
I will let you review for yourself the details of these Iranian investigators’ network meta-analysis, but the bottom line is that some medications were associated with a reduction in migraine frequency. Others were associated with headache intensity. “However, no treatments were associated with significant improvements in quality of life or reduction of the duration of migraine attacks.”
Obviously, this paper illustrates clearly that we have not yet discovered the medicinal magic bullet for pediatric migraine prophylaxis. This doesn’t surprise me. After listening to scores of families tell their migraine stories, it became apparent to me that there was often a pattern in which the child’s headache had arrived after a period of acute sleep deprivation. For example, a trip to an amusement park in which travel or excitement may have resulted in the child going to bed later and/or getting up earlier. By afternoon the child’s reserves of something (currently unknown) were depleted to a point that the headache and/or vomiting struck.
Because these episodes were often so infrequent, separated by months, that taking a history demonstrating a recurring pattern could take considerable patience on the part of the family and the provider, even for a physician like myself who believes that better sleep is the answer for everything. However, once I could convince a family of the connection between the sleep deprivation and the headaches, they could often recall other episodes in the past that substantiated my explanation.
In some cases there was no obvious history of acute sleep deprivation, or at least it was so subtle that even a history taker with a sleep obsession couldn’t detect it. However, in these cases I could usually elicit a history of chronic sleep deprivation. For example, falling asleep instantly on automobile rides, difficulty with waking in the morning, or unhealthy bedtime routines. With this underlying vulnerability of chronic sleep deprivation, a slightly more exciting or vigorous day was all that was necessary to trigger the headache.
For those of you who don’t share my contention that childhood migraine is usually the result of sleep deprivation, consider the similarity between an epileptic seizure, which can be triggered by fatigue. Both events are usually followed by a deep sleep from which the child wakes refreshed and symptom free.
I think it is interesting that this recent meta-analysis could find no benefit in the quality of life for any of the medications. The explanation may be that the child with migraine already had a somewhat diminished quality of life as a result of the sleep deprivation, either acute or chronic.
When speaking with parents of migraine sufferers, I would tell them that once the headache had started there was little I had to offer to forestall the inevitable pain and vomiting. Certainly not in the form of an oral medication. While many adults will have an aura that warns them of the headache onset, I have found that most children don’t describe an aura. It may be they simply lack the ability to express it. Occasionally an observant parent may detect pallor or a behavior change that indicates a migraine is beginning. On rare occasions a parent may be able to abort the attack by quickly getting the child to a quiet, dark, and calm environment.
Although this recent meta-analysis review of treatment options is discouraging, it may be providing a clue to effective prophylaxis. Some of the medications that decrease the frequency of the attacks may be doing so because they improve the patient’s sleep patterns. Those that decrease the intensity of the pain are probably working on pain pathway that is not specific to migraine.
Continuing a search for a prophylactic medication is a worthy goal, particularly for those patients in which their migraines are debilitating. However, based on my experience, enhanced by my bias, the safest and most effective prophylaxis results from increasing the family’s awareness of the role that sleep deprivation plays in the illness. Even when the family buys into the message and attempts to avoid situations that will tax their vulnerable children, parents will need to accept that sometimes stuff happens even though siblings and peers may be able to tolerate the situation. Spontaneous activities can converge on a day when for whatever reason the migraine-prone child is overtired and the headache and vomiting will erupt.
A lifestyle change is always preferable to a pharmacological intervention. However, that doesn’t mean it is always easy to achieve.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I suspect you all have some experience with childhood migraine. It can mean a painful several hours for the patient, arriving often without warning, with recurrences spaced months or sometimes even years apart. It may be accompanied by vomiting, which in some cases overshadows the severity of the headache. It can result in lost days from school and ruin family activities. It can occur so infrequently that the family can’t recall accurately when the last episode happened. In some ways it is a different animal than the adult version.
Most of the pediatric patients with migraine I have seen have experienced attacks that were occurring so infrequently that the families and I seldom discussed medication as an option. Back then imipramine was the only choice. However, currently there are more than a half dozen medications and combinations that have been tried. Recently a review of 45 clinical trials of these medications was published in JAMA Network Open.
I will let you review for yourself the details of these Iranian investigators’ network meta-analysis, but the bottom line is that some medications were associated with a reduction in migraine frequency. Others were associated with headache intensity. “However, no treatments were associated with significant improvements in quality of life or reduction of the duration of migraine attacks.”
Obviously, this paper illustrates clearly that we have not yet discovered the medicinal magic bullet for pediatric migraine prophylaxis. This doesn’t surprise me. After listening to scores of families tell their migraine stories, it became apparent to me that there was often a pattern in which the child’s headache had arrived after a period of acute sleep deprivation. For example, a trip to an amusement park in which travel or excitement may have resulted in the child going to bed later and/or getting up earlier. By afternoon the child’s reserves of something (currently unknown) were depleted to a point that the headache and/or vomiting struck.
Because these episodes were often so infrequent, separated by months, that taking a history demonstrating a recurring pattern could take considerable patience on the part of the family and the provider, even for a physician like myself who believes that better sleep is the answer for everything. However, once I could convince a family of the connection between the sleep deprivation and the headaches, they could often recall other episodes in the past that substantiated my explanation.
In some cases there was no obvious history of acute sleep deprivation, or at least it was so subtle that even a history taker with a sleep obsession couldn’t detect it. However, in these cases I could usually elicit a history of chronic sleep deprivation. For example, falling asleep instantly on automobile rides, difficulty with waking in the morning, or unhealthy bedtime routines. With this underlying vulnerability of chronic sleep deprivation, a slightly more exciting or vigorous day was all that was necessary to trigger the headache.
For those of you who don’t share my contention that childhood migraine is usually the result of sleep deprivation, consider the similarity between an epileptic seizure, which can be triggered by fatigue. Both events are usually followed by a deep sleep from which the child wakes refreshed and symptom free.
I think it is interesting that this recent meta-analysis could find no benefit in the quality of life for any of the medications. The explanation may be that the child with migraine already had a somewhat diminished quality of life as a result of the sleep deprivation, either acute or chronic.
When speaking with parents of migraine sufferers, I would tell them that once the headache had started there was little I had to offer to forestall the inevitable pain and vomiting. Certainly not in the form of an oral medication. While many adults will have an aura that warns them of the headache onset, I have found that most children don’t describe an aura. It may be they simply lack the ability to express it. Occasionally an observant parent may detect pallor or a behavior change that indicates a migraine is beginning. On rare occasions a parent may be able to abort the attack by quickly getting the child to a quiet, dark, and calm environment.
Although this recent meta-analysis review of treatment options is discouraging, it may be providing a clue to effective prophylaxis. Some of the medications that decrease the frequency of the attacks may be doing so because they improve the patient’s sleep patterns. Those that decrease the intensity of the pain are probably working on pain pathway that is not specific to migraine.
Continuing a search for a prophylactic medication is a worthy goal, particularly for those patients in which their migraines are debilitating. However, based on my experience, enhanced by my bias, the safest and most effective prophylaxis results from increasing the family’s awareness of the role that sleep deprivation plays in the illness. Even when the family buys into the message and attempts to avoid situations that will tax their vulnerable children, parents will need to accept that sometimes stuff happens even though siblings and peers may be able to tolerate the situation. Spontaneous activities can converge on a day when for whatever reason the migraine-prone child is overtired and the headache and vomiting will erupt.
A lifestyle change is always preferable to a pharmacological intervention. However, that doesn’t mean it is always easy to achieve.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Just Call It ‘Chronic Rhinitis’ and Reach for These Treatments
This transcript has been edited for clarity.
Matthew F. Watto, MD: I’m here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about rhinitis?
Paul N. Williams, MD: I’m excited. It’s always the season to talk about rhinitis.
Watto: We had a great guest for this podcast, Rhinitis and Environmental Allergies with Dr. Olajumoke Fadugba from Penn Medicine. She’s an allergist and immunologist. One of her pet peeves is when people just call everything “allergic rhinitis” because we should be calling it “chronic rhinitis,” if it’s chronic. That’s an umbrella term, and there are many buckets underneath it that people could fall into.
When you’re taking a history, you have to figure out whether it’s perennial (meaning it happens year round) because certain things can cause that. Cat dander is around all the time, so people with cats might have sinus symptoms all year. Dust mites are another one, and it’s pretty hard to avoid those. Those are some perennial allergens.
Then there is allergic vs nonallergic rhinitis, which is something I hadn’t really put too much thought into.
Williams: I didn’t realize exactly how nuanced it got. Nonallergic rhinitis can still be seasonal because changes in temperature and humidity can trigger the rhinitis. And it matters what medications you use for what.
Watto: Here are some ways you can try to figure out if rhinitis is allergic or nonallergic. Ask the patient if they have itchy eyes and are sneezing a lot. That can be more of an allergic rhinitis, but both allergic and nonallergic rhinitis have the congestion, the rhinorrhea, so you can’t figure it out based on that alone.
Dr. Fadugba said that one clue that it might be nonallergic rhinitis is the age of onset. If the symptoms are later in onset (older age), then 30%-40% of rhinitis is nonallergic. If the patient has never had allergies and now all of a sudden they have new chronic sinus symptoms, it’s probably nonallergic rhinitis. It’s a diagnosis of exclusion.
I guess they need allergy testing?
Williams: If you want to make a definitive diagnosis, you need to rule it out. I suspect that you might be able to get away with some empirical treatment. If they get better, you can feel like a winner because getting booked in for allergy testing can be a little bit of a challenge.
Watto: The main treatment difference is that the oral antihistamines do not really seem to work for nonallergic rhinitis, but they can help with allergic rhinitis. Weirdly, the nasal antihistamines and nasal steroids do seem to work for both allergic and nonallergic rhinitis.
I don’t understand the mechanism there, but if you think someone might have nonallergic rhinitis, I wouldn’t go with the oral antihistamines as your first-line treatment. I would go with a nasal spray; you pretty much can’t go wrong with either an antihistamine or a steroid nasal spray.
Williams: We typically start with the nasal sprays. That’s kind of first-line for almost everybody, allergic or nonallergic. You’re probably going to start with an intranasal steroid, and then it’s kind of dealer’s choice what the patient can tolerate and afford. Sometimes you can get them covered by insurance, at least in my experience.
I will say that this is one of the medications — like nicotine patches and other things — where we as doctors don’t really counsel patients on how to use it appropriately. So with our expert, we revisited the idea of the patient pointing the nasal spray laterally, toward their ear basically, and not spraying toward their brain. There should not be a slurping sound afterward, because “if you taste it, you waste it,” as the allergists and immunologists say. It’s supposed to sit up there and not be swallowed immediately.
If your patient is sensitive to the floral flavor of some of the fluticasones (which I don’t mind so much as a user myself), then you can try mometasone or the other formulations. They are all roughly equivalent.
Speaking of medications, which medications can cause rhinitis? Any meds we commonly use in primary care?
Williams: Apparently the combined hormonal oral contraceptives can do it. Also the phosphodiesterase 5 (PDE-5) inhibitors. Drugs that cause vasodilation can also do it. Some of the antihypertensives. I’ve seen beta-blockers and angiotensin-converting enzyme (ACE) inhibitors listed specifically, and some of the medications for benign prostatic hyperplasia (BPH). So there are a couple of medications that you can think about as a potential cause of rhinitis, although my suspicion is not going to be as high as for some of the other causes.
Watto: We mentioned medication treatments for patients who are really bothered by rhinorrhea, and maybe they are already on a steroid or an antihistamine.
You can try nasal ipratropium for people that have really prominent rhinorrhea. Dr. Fadugba said that can work well, and it’s usually taken three or four times a day. I’ve had good success prescribing it for my patients. Another one that I have never prescribed, but that Dr. Fadugba said is available over the counter, is intranasal cromolyn — a mast cell stabilizer. She said it can be beneficial.
Let’s say I had a cat allergy and I was going to visit Paul. I could use the intranasal cromolyn ahead of time to reduce rhinitis when I’m around the cats.
Paul, what about montelukast? I never know what to do with that one.
Williams: I’ve seen it prescribed as a last-ditch attempt to fix chronic rhinitis. Dr. Fadugba said she only ever prescribes it for patients who have rhinitis symptoms and asthma and never just for chronic rhinitis because it doesn’t work. And also, there have been some new black-box warnings from the US Food and Drug Administration (FDA). So unless there’s a solid indication for it, montelukast is not something you should just prescribe to try to see if it will work. That’s probably not the right approach for this.
But if the patient has challenging control asthma, and as a component, challenging nasal symptoms as well, it might be a reasonable medication to try.
Watto: And finally, Paul, how does climate change possibly have anything to do with rhinitis?
Williams: I feel like I’m just seeing more and more of the stuff every year. I don’t know if I’m more sensitive to it or because I’m having more symptoms myself, but it turns out the prevalence actually is going up.
We’re seeing more of it in part because it’s getting hotter outside, which is in turn worsening the production of allergens and increasing the allergen exposure and the severity of the symptoms that go along with it. More people are having more severe disease because the world is changing as a result of the stuff that we do. So fix that. But also be mindful and expect to see even more of these problems as you move forward in your careers.
Watto: Dr. Fadugba gave us so many great tips. You can listen to the full podcast episode here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, disclosed ties with The Curbsiders.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: I’m here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about rhinitis?
Paul N. Williams, MD: I’m excited. It’s always the season to talk about rhinitis.
Watto: We had a great guest for this podcast, Rhinitis and Environmental Allergies with Dr. Olajumoke Fadugba from Penn Medicine. She’s an allergist and immunologist. One of her pet peeves is when people just call everything “allergic rhinitis” because we should be calling it “chronic rhinitis,” if it’s chronic. That’s an umbrella term, and there are many buckets underneath it that people could fall into.
When you’re taking a history, you have to figure out whether it’s perennial (meaning it happens year round) because certain things can cause that. Cat dander is around all the time, so people with cats might have sinus symptoms all year. Dust mites are another one, and it’s pretty hard to avoid those. Those are some perennial allergens.
Then there is allergic vs nonallergic rhinitis, which is something I hadn’t really put too much thought into.
Williams: I didn’t realize exactly how nuanced it got. Nonallergic rhinitis can still be seasonal because changes in temperature and humidity can trigger the rhinitis. And it matters what medications you use for what.
Watto: Here are some ways you can try to figure out if rhinitis is allergic or nonallergic. Ask the patient if they have itchy eyes and are sneezing a lot. That can be more of an allergic rhinitis, but both allergic and nonallergic rhinitis have the congestion, the rhinorrhea, so you can’t figure it out based on that alone.
Dr. Fadugba said that one clue that it might be nonallergic rhinitis is the age of onset. If the symptoms are later in onset (older age), then 30%-40% of rhinitis is nonallergic. If the patient has never had allergies and now all of a sudden they have new chronic sinus symptoms, it’s probably nonallergic rhinitis. It’s a diagnosis of exclusion.
I guess they need allergy testing?
Williams: If you want to make a definitive diagnosis, you need to rule it out. I suspect that you might be able to get away with some empirical treatment. If they get better, you can feel like a winner because getting booked in for allergy testing can be a little bit of a challenge.
Watto: The main treatment difference is that the oral antihistamines do not really seem to work for nonallergic rhinitis, but they can help with allergic rhinitis. Weirdly, the nasal antihistamines and nasal steroids do seem to work for both allergic and nonallergic rhinitis.
I don’t understand the mechanism there, but if you think someone might have nonallergic rhinitis, I wouldn’t go with the oral antihistamines as your first-line treatment. I would go with a nasal spray; you pretty much can’t go wrong with either an antihistamine or a steroid nasal spray.
Williams: We typically start with the nasal sprays. That’s kind of first-line for almost everybody, allergic or nonallergic. You’re probably going to start with an intranasal steroid, and then it’s kind of dealer’s choice what the patient can tolerate and afford. Sometimes you can get them covered by insurance, at least in my experience.
I will say that this is one of the medications — like nicotine patches and other things — where we as doctors don’t really counsel patients on how to use it appropriately. So with our expert, we revisited the idea of the patient pointing the nasal spray laterally, toward their ear basically, and not spraying toward their brain. There should not be a slurping sound afterward, because “if you taste it, you waste it,” as the allergists and immunologists say. It’s supposed to sit up there and not be swallowed immediately.
If your patient is sensitive to the floral flavor of some of the fluticasones (which I don’t mind so much as a user myself), then you can try mometasone or the other formulations. They are all roughly equivalent.
Speaking of medications, which medications can cause rhinitis? Any meds we commonly use in primary care?
Williams: Apparently the combined hormonal oral contraceptives can do it. Also the phosphodiesterase 5 (PDE-5) inhibitors. Drugs that cause vasodilation can also do it. Some of the antihypertensives. I’ve seen beta-blockers and angiotensin-converting enzyme (ACE) inhibitors listed specifically, and some of the medications for benign prostatic hyperplasia (BPH). So there are a couple of medications that you can think about as a potential cause of rhinitis, although my suspicion is not going to be as high as for some of the other causes.
Watto: We mentioned medication treatments for patients who are really bothered by rhinorrhea, and maybe they are already on a steroid or an antihistamine.
You can try nasal ipratropium for people that have really prominent rhinorrhea. Dr. Fadugba said that can work well, and it’s usually taken three or four times a day. I’ve had good success prescribing it for my patients. Another one that I have never prescribed, but that Dr. Fadugba said is available over the counter, is intranasal cromolyn — a mast cell stabilizer. She said it can be beneficial.
Let’s say I had a cat allergy and I was going to visit Paul. I could use the intranasal cromolyn ahead of time to reduce rhinitis when I’m around the cats.
Paul, what about montelukast? I never know what to do with that one.
Williams: I’ve seen it prescribed as a last-ditch attempt to fix chronic rhinitis. Dr. Fadugba said she only ever prescribes it for patients who have rhinitis symptoms and asthma and never just for chronic rhinitis because it doesn’t work. And also, there have been some new black-box warnings from the US Food and Drug Administration (FDA). So unless there’s a solid indication for it, montelukast is not something you should just prescribe to try to see if it will work. That’s probably not the right approach for this.
But if the patient has challenging control asthma, and as a component, challenging nasal symptoms as well, it might be a reasonable medication to try.
Watto: And finally, Paul, how does climate change possibly have anything to do with rhinitis?
Williams: I feel like I’m just seeing more and more of the stuff every year. I don’t know if I’m more sensitive to it or because I’m having more symptoms myself, but it turns out the prevalence actually is going up.
We’re seeing more of it in part because it’s getting hotter outside, which is in turn worsening the production of allergens and increasing the allergen exposure and the severity of the symptoms that go along with it. More people are having more severe disease because the world is changing as a result of the stuff that we do. So fix that. But also be mindful and expect to see even more of these problems as you move forward in your careers.
Watto: Dr. Fadugba gave us so many great tips. You can listen to the full podcast episode here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, disclosed ties with The Curbsiders.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: I’m here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about rhinitis?
Paul N. Williams, MD: I’m excited. It’s always the season to talk about rhinitis.
Watto: We had a great guest for this podcast, Rhinitis and Environmental Allergies with Dr. Olajumoke Fadugba from Penn Medicine. She’s an allergist and immunologist. One of her pet peeves is when people just call everything “allergic rhinitis” because we should be calling it “chronic rhinitis,” if it’s chronic. That’s an umbrella term, and there are many buckets underneath it that people could fall into.
When you’re taking a history, you have to figure out whether it’s perennial (meaning it happens year round) because certain things can cause that. Cat dander is around all the time, so people with cats might have sinus symptoms all year. Dust mites are another one, and it’s pretty hard to avoid those. Those are some perennial allergens.
Then there is allergic vs nonallergic rhinitis, which is something I hadn’t really put too much thought into.
Williams: I didn’t realize exactly how nuanced it got. Nonallergic rhinitis can still be seasonal because changes in temperature and humidity can trigger the rhinitis. And it matters what medications you use for what.
Watto: Here are some ways you can try to figure out if rhinitis is allergic or nonallergic. Ask the patient if they have itchy eyes and are sneezing a lot. That can be more of an allergic rhinitis, but both allergic and nonallergic rhinitis have the congestion, the rhinorrhea, so you can’t figure it out based on that alone.
Dr. Fadugba said that one clue that it might be nonallergic rhinitis is the age of onset. If the symptoms are later in onset (older age), then 30%-40% of rhinitis is nonallergic. If the patient has never had allergies and now all of a sudden they have new chronic sinus symptoms, it’s probably nonallergic rhinitis. It’s a diagnosis of exclusion.
I guess they need allergy testing?
Williams: If you want to make a definitive diagnosis, you need to rule it out. I suspect that you might be able to get away with some empirical treatment. If they get better, you can feel like a winner because getting booked in for allergy testing can be a little bit of a challenge.
Watto: The main treatment difference is that the oral antihistamines do not really seem to work for nonallergic rhinitis, but they can help with allergic rhinitis. Weirdly, the nasal antihistamines and nasal steroids do seem to work for both allergic and nonallergic rhinitis.
I don’t understand the mechanism there, but if you think someone might have nonallergic rhinitis, I wouldn’t go with the oral antihistamines as your first-line treatment. I would go with a nasal spray; you pretty much can’t go wrong with either an antihistamine or a steroid nasal spray.
Williams: We typically start with the nasal sprays. That’s kind of first-line for almost everybody, allergic or nonallergic. You’re probably going to start with an intranasal steroid, and then it’s kind of dealer’s choice what the patient can tolerate and afford. Sometimes you can get them covered by insurance, at least in my experience.
I will say that this is one of the medications — like nicotine patches and other things — where we as doctors don’t really counsel patients on how to use it appropriately. So with our expert, we revisited the idea of the patient pointing the nasal spray laterally, toward their ear basically, and not spraying toward their brain. There should not be a slurping sound afterward, because “if you taste it, you waste it,” as the allergists and immunologists say. It’s supposed to sit up there and not be swallowed immediately.
If your patient is sensitive to the floral flavor of some of the fluticasones (which I don’t mind so much as a user myself), then you can try mometasone or the other formulations. They are all roughly equivalent.
Speaking of medications, which medications can cause rhinitis? Any meds we commonly use in primary care?
Williams: Apparently the combined hormonal oral contraceptives can do it. Also the phosphodiesterase 5 (PDE-5) inhibitors. Drugs that cause vasodilation can also do it. Some of the antihypertensives. I’ve seen beta-blockers and angiotensin-converting enzyme (ACE) inhibitors listed specifically, and some of the medications for benign prostatic hyperplasia (BPH). So there are a couple of medications that you can think about as a potential cause of rhinitis, although my suspicion is not going to be as high as for some of the other causes.
Watto: We mentioned medication treatments for patients who are really bothered by rhinorrhea, and maybe they are already on a steroid or an antihistamine.
You can try nasal ipratropium for people that have really prominent rhinorrhea. Dr. Fadugba said that can work well, and it’s usually taken three or four times a day. I’ve had good success prescribing it for my patients. Another one that I have never prescribed, but that Dr. Fadugba said is available over the counter, is intranasal cromolyn — a mast cell stabilizer. She said it can be beneficial.
Let’s say I had a cat allergy and I was going to visit Paul. I could use the intranasal cromolyn ahead of time to reduce rhinitis when I’m around the cats.
Paul, what about montelukast? I never know what to do with that one.
Williams: I’ve seen it prescribed as a last-ditch attempt to fix chronic rhinitis. Dr. Fadugba said she only ever prescribes it for patients who have rhinitis symptoms and asthma and never just for chronic rhinitis because it doesn’t work. And also, there have been some new black-box warnings from the US Food and Drug Administration (FDA). So unless there’s a solid indication for it, montelukast is not something you should just prescribe to try to see if it will work. That’s probably not the right approach for this.
But if the patient has challenging control asthma, and as a component, challenging nasal symptoms as well, it might be a reasonable medication to try.
Watto: And finally, Paul, how does climate change possibly have anything to do with rhinitis?
Williams: I feel like I’m just seeing more and more of the stuff every year. I don’t know if I’m more sensitive to it or because I’m having more symptoms myself, but it turns out the prevalence actually is going up.
We’re seeing more of it in part because it’s getting hotter outside, which is in turn worsening the production of allergens and increasing the allergen exposure and the severity of the symptoms that go along with it. More people are having more severe disease because the world is changing as a result of the stuff that we do. So fix that. But also be mindful and expect to see even more of these problems as you move forward in your careers.
Watto: Dr. Fadugba gave us so many great tips. You can listen to the full podcast episode here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, disclosed ties with The Curbsiders.
A version of this article first appeared on Medscape.com.
Cardiovascular Disease 2050: No, GLP-1s Won’t Save the Day
This transcript has been edited for clarity .
Robert A. Harrington, MD: I’m here in London at the European Society of Cardiology meetings, at theheart.org | Medscape Cardiology booth, using the meetings as an opportunity to meet with colleagues to talk about recent things that they’ve been writing about.
Today I’m joined by a good friend and colleague, Dr. Dhruv Kazi from Beth Israel Deaconess in Boston. Thanks for joining us.
Dhruv S. Kazi, MD, MS: Thank you for having me.
Harrington: Dr. Kazi is an associate professor of medicine at Harvard Medical School. He’s also the associate director of the Smith Center, which is an outcomes research center at the Beth Israel Deaconess. Thanks for joining us.
Kazi: Excited to be here.
Harrington: The topic I think you know that I want to discuss is a really important paper. There are two papers. They’re part of the American Heart Association’s 100th anniversary celebration, if you will. Many of the papers looked back at where science taken us.
With your coauthor, Karen Joynt Maddox, your papers are looking forward. They’re about the burden of cardiovascular disease in 2050. One paper really focused on what I would call the clinical and public health issues. Yours is focused on the economics. Is that a good description?
Kazi: Perfect.
Harrington: Tell us what you, Karen, and the other writers set out to do. What were you asked to do?
Kazi: As you know, the American Heart Association is entering its second century. Part of this was an exercise to say, where will the country be in 2050, which is a long enough time horizon for us to start planning for the future.
We looked back and said, if prior trends remain the same, where will we be in 2050, accounting for changes in demographics, changes in the composition of the population, and knowing that some of the cardiovascular risk factors are getting worse?
Harrington: For me, what was really striking is that, when I first saw the title and read “2050,” I thought, Oh, that’s a long way away. Then as I started reading it, I realized that this is not so far away.
Kazi: Absolutely.
Harrington: If we’re going to make a difference, it might take us 25 years.
Kazi: Especially if we set ourselves ambitious goals, we›re going to have to dig deep. Business-as-usual is not going to get us there.
Harrington: No. What I think has happened is we›ve spent so much time taking care of acute illness. Case fatality rates are fantastic. I was actually making the comment yesterday to a colleague that when I was an intern, the 30-day death rate from acute myocardial infarction was about 20%.
Kazi: Oh, wow.
Harrington: Now it’s 5%. That’s a big difference in a career.
Trends in the Wrong Direction
Kazi: There are fundamental trends. The decline in case fatalities is a really positive development, and I would hope that, going forward, that would continue. Those are risk-adjusted death rates and what is happening is that risk is going up. This is a function of the fact that the US population is aging; 2030 will be the first year that all the baby boomers will be over the age of 65.
By the mid-2030s, we’ll have more adults over the age of 65 than kids. That aging of the population is going to increase risk. The second is — and this is a positive development — we are a more diverse population, but the populations that are minoritized have higher cardiovascular risk, for a variety of reasons.
As the population of Asian Americans increases and doubles, in fact, as the population of Hispanic Americans doubles, we’re going to see an increase in risk related to cardiovascular disease. The third is that, over the past decade, there are some risk factors that are going in the wrong direction.
Harrington: Let’s talk about that because that’s humbling. I’m involved, as you know, with the American Heart Association, as are you. Despite all the work on Life’s Simple 7 and now Life’s Essential 8, we still have some issues.
Kazi: The big ones that come to mind are hypertension, diabetes, and obesity, all of which are trending in the wrong direction. Hypertension, we were gaining traction; and then over the past decade, we’ve slipped again. As you know, national blood pressure control rates have declined in many populations.
Harrington: Rather substantially.
Kazi: Substantially so, which has implications, in particular, for stroke rates in the future and stroke rates in young adults in the future. Obesity is a problem that we have very little control over. We’re already at 40% on average, which means that some populations are already in the 60% range.
Harrington: We also have obesity in kids — the burden, I’ll call it, of obesity. It’s not that you become obese in your thirties or your forties; you›re becoming obese as a teenager or even younger.
Kazi: Exactly. Since the 1990s, obesity in US adults has doubled, but obesity in US children has quadrupled. It’s starting from a lower base, but it’s very much an escalating problem.
Harrington: Diabetes is tightly linked to it but not totally explained.
Kazi: Exactly. The increase in diabetes is largely driven by obesity, but it›s probably also driven by changes in diet and lifestyle that don›t go through obesity.
Harrington: Yeah, it’s interesting. I think I have this figure correctly. It used to be rare that you saw a child with type 2 diabetes or what we call type 2 diabetes.
Kazi: Yeah.
Harrington: Now, the vast majority of kids with diabetes have type 2 diabetes.
Kazi: In the adolescents/young adults age group, most of it is type 2.
Harrington: Diabetes going up, obesity up, hypertension not well controlled, smoking combustible cigarettes way down.
Kazi: Yeah.
Harrington: Cholesterol levels. I was surprised. Cholesterol looked better. You said — because I was at a meeting where somebody asked you — that’s not explained by treatment.
Kazi: No, it’s not, at least going back to the ‘70s, but likely even sooner. I think that can only be attributed to substantial dietary changes. We are consuming less fat and less trans-fat. It’s possible that those collectively are improving our cholesterol levels, possibly at the expense of our glucose levels, because we basically substituted fats in our diet with more carbs at a population level.
Cigarettes and Vaping
Harrington: Some things certainly trend in the right direction but others in a really difficult direction. It’s going to lead to pretty large changes in risk for coronary disease, atrial fibrillation, and heart failure.
Kazi: I want to go back to the tobacco point. There are definitely marked declines in tobacco, still tightly related to income in the country. You see much higher prevalence of tobacco use in lower-income populations, but it’s unclear to me where it’s going in kids. We know that combustible tobacco use is going down but e-cigarettes went up. What that leads to over the next 30 years is unclear to me.
Harrington: That is a really important comment that’s worth sidebarring. The vaping use has been a terrible epidemic among our high schoolers. What is that going to lead to? Is it going to lead to the use of combustible cigarettes and we’re going to see that go back up? It remains to be seen.
Kazi: Yes, it remains to be seen. Going back to your point about this change in risk factors and this change in demographics, both aging and becoming a more diverse population means that we have large increases in some healthcare conditions.
Coronary heart disease goes up some, there›s a big jump in stroke — nearly a doubling in stroke — which is related to hypertension, obesity, an aging population, and a more diverse population. There are changes in stroke in the young, and atrial fibrillation related to, again, hypertension. We’re seeing these projections, and with them come these pretty large projections in changes in healthcare spending.
Healthcare Spending Not Sustainable
Harrington: Big. I mean, it’s not sustainable. Give the audience the number — it’s pretty frightening.
Kazi: We’re talking about a quadrupling of healthcare costs related to cardiovascular disease over 25 years. We’ve gotten used to the narrative that healthcare in the US is expensive and drugs are expensive, but this is an enormous problem — an unsustainable problem, like you called it.
It’s a doubling as a proportion of the economy. I was looking this up this morning. If the US healthcare economy were its own economy, it would be the fourth largest economy in the world.
Harrington: Healthcare as it is today, is it 21% of our economy?
Kazi: It’s 17% now. If it were its own economy, it would be the fourth largest in the world. We are spending more on healthcare than all but two other countries’ total economies. It’s kind of crazy.
Harrington: We’re talking about a quadrupling.
Kazi: Within that, the cardiovascular piece is a big piece, and we›re talking about a quadrupling.
Harrington: That’s both direct and indirect costs.
Kazi: The quadrupling of costs is just the direct costs. Indirect costs, for the listeners, refer to costs unrelated to healthcare but changes in productivity, either because people are disabled and unable to participate fully in the workforce or they die early.
The productivity costs are also increased substantially as a result. If you look at both healthcare and productivity, that goes up threefold. These are very large changes.
Harrington: Let’s now get to what we can do about it. I made the comment to you when I first read the papers that I was very depressed. Then, after I went through my Kübler-Ross stages of depression, death, and dying, I came to acceptance.
What are we going to do about it? This is a focus on policy, but also a focus on how we deliver healthcare, how we think about healthcare, and how we develop drugs and devices.
The drug question is going to be the one the audience is thinking about. They say, well, what about GLP-1 agonists? Aren’t those going to save the day?
Kazi: Yes and no. I’ll say that, early in my career, I used to be very attracted to simple solutions to complex problems. I’ve come to realize that simple solutions are elegant, attractive, and wrong. We›re dealing with a very complex issue and I think we’re going to need a multipronged approach.
The way I think about it is that there was a group of people who are at very high risk today. How do we help those individuals? Then how do we help the future generation so that they’re not dealing with the projections that we’re talking about.
My colleague, Karen Joynt Maddox, who led one of the papers, as you mentioned, has an elegant line in the paper where she says projections are not destiny. These are things we can change.
Harrington: If nothing changes, this is what it’s going to look like.
Kazi: This is where we’re headed.
Harrington: We can change. We’ve got some time to change, but we don’t have forever.
Kazi: Yes, exactly. We picked the 25-year timeline instead of a “let’s plan for the next century” timeline because we want something concrete and actionable. It’s close enough to be meaningful but far enough to give us the runway we need to act.
Harrington: Give me two things from the policy perspective, because it’s mostly policy.
Kazi: There are policy and clinical interventions. From the policy perspective, if I had to list two things, one is expansion of access to care. As we talk about this big increase in the burden of disease and risk factors, if you have a large proportion of your population that has hypertension or diabetes, you’re going to have to expand access to care to ensure that people get treated so they can get access to this care before they develop the complications that we worry about, like stroke and heart disease, that are very expensive to treat downstream.
The second, more broadly related to access to care, is the access to medications that are effective. You bring up GLP-1s. I think we need a real strategy for how we can give people access to GLP-1s at a price that is affordable to individuals but also affordable to the health system, and to help them stay on the drugs.
GLP-1s are transformative in what they do for weight loss and for diabetes, but more than 50% of people who start one are off it at 12 months. There’s something fundamentally wrong about how we’re delivering GLP-1s today. It’s not just about the cost of the drugs but the support system people need to stay on.
Harrington: I’ve made the comment, in many forms now, that we know the drugs work. We have to figure out how to use them.
Kazi: Exactly, yes.
Harrington: Using them includes chronicity. This is a chronic condition. Some people can come off the drugs, but many can’t. We’re going to have to figure this out, and maybe the newer generations of drugs will help us address what people call the off-ramping. How are we going to do that? I think you’re spot-on. Those are critically important questions.
Kazi: As we looked at this modeling, I’ll tell you — I had a come-to-Jesus moment where I was like, there is no way to fix cardiovascular disease in the US without going through obesity and diabetes. We have to address obesity in the US. We can’t just treat our way out of it. Obesity is fundamentally a food problem and we’ve got to engage again with food policy in a meaningful way.
Harrington: As you know, with the American Heart Association, we›re doing a large amount of work now on food as medicine and food is medicine. We are trying to figure out what the levers are that we can pull to actually help people eat healthier diets.
Kazi: Yes. Rather than framing it as an individual choice that people are eating poorly, it’s, how do we make healthy diets the default in the environment?
Harrington: This is where you get to the children as well.
Kazi: Exactly.
Harrington: I could talk about this all day. I’ve had the benefit of reading the papers now a few times and talking to you on several occasions. Thank you for joining us.
Kazi: Thank you.
Dr. Harrington, Stephen and Suzanne Weiss Dean, Weill Cornell Medicine; Provost for Medical Affairs, Cornell University, New York, NY, disclosed ties with Baim Institute (DSMB); CSL (RCT Executive Committee); Janssen (RCT Char), NHLBI (RCT Executive Committee, DSMB Chair); PCORI (RCT Co-Chair); DCRI, Atropos Health; Bitterroot Bio; Bristol Myers Squibb; BridgeBio; Element Science; Edwards Lifesciences; Foresite Labs; Medscape/WebMD Board of Directors for: American Heart Association; College of the Holy Cross; and Cytokinetics. Dr. Kazi, Associate Director, Smith Center for Outcomes Research, Associate Professor, Department of Medicine (Cardiology), Harvard Medical School, Director, Department of Cardiac Critical Care Unit, Beth Israel Deaconess Medical Center, Boston, Massachusetts, has disclosed receiving a research grant from Boston Scientific (grant to examine the economics of stroke prevention).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity .
Robert A. Harrington, MD: I’m here in London at the European Society of Cardiology meetings, at theheart.org | Medscape Cardiology booth, using the meetings as an opportunity to meet with colleagues to talk about recent things that they’ve been writing about.
Today I’m joined by a good friend and colleague, Dr. Dhruv Kazi from Beth Israel Deaconess in Boston. Thanks for joining us.
Dhruv S. Kazi, MD, MS: Thank you for having me.
Harrington: Dr. Kazi is an associate professor of medicine at Harvard Medical School. He’s also the associate director of the Smith Center, which is an outcomes research center at the Beth Israel Deaconess. Thanks for joining us.
Kazi: Excited to be here.
Harrington: The topic I think you know that I want to discuss is a really important paper. There are two papers. They’re part of the American Heart Association’s 100th anniversary celebration, if you will. Many of the papers looked back at where science taken us.
With your coauthor, Karen Joynt Maddox, your papers are looking forward. They’re about the burden of cardiovascular disease in 2050. One paper really focused on what I would call the clinical and public health issues. Yours is focused on the economics. Is that a good description?
Kazi: Perfect.
Harrington: Tell us what you, Karen, and the other writers set out to do. What were you asked to do?
Kazi: As you know, the American Heart Association is entering its second century. Part of this was an exercise to say, where will the country be in 2050, which is a long enough time horizon for us to start planning for the future.
We looked back and said, if prior trends remain the same, where will we be in 2050, accounting for changes in demographics, changes in the composition of the population, and knowing that some of the cardiovascular risk factors are getting worse?
Harrington: For me, what was really striking is that, when I first saw the title and read “2050,” I thought, Oh, that’s a long way away. Then as I started reading it, I realized that this is not so far away.
Kazi: Absolutely.
Harrington: If we’re going to make a difference, it might take us 25 years.
Kazi: Especially if we set ourselves ambitious goals, we›re going to have to dig deep. Business-as-usual is not going to get us there.
Harrington: No. What I think has happened is we›ve spent so much time taking care of acute illness. Case fatality rates are fantastic. I was actually making the comment yesterday to a colleague that when I was an intern, the 30-day death rate from acute myocardial infarction was about 20%.
Kazi: Oh, wow.
Harrington: Now it’s 5%. That’s a big difference in a career.
Trends in the Wrong Direction
Kazi: There are fundamental trends. The decline in case fatalities is a really positive development, and I would hope that, going forward, that would continue. Those are risk-adjusted death rates and what is happening is that risk is going up. This is a function of the fact that the US population is aging; 2030 will be the first year that all the baby boomers will be over the age of 65.
By the mid-2030s, we’ll have more adults over the age of 65 than kids. That aging of the population is going to increase risk. The second is — and this is a positive development — we are a more diverse population, but the populations that are minoritized have higher cardiovascular risk, for a variety of reasons.
As the population of Asian Americans increases and doubles, in fact, as the population of Hispanic Americans doubles, we’re going to see an increase in risk related to cardiovascular disease. The third is that, over the past decade, there are some risk factors that are going in the wrong direction.
Harrington: Let’s talk about that because that’s humbling. I’m involved, as you know, with the American Heart Association, as are you. Despite all the work on Life’s Simple 7 and now Life’s Essential 8, we still have some issues.
Kazi: The big ones that come to mind are hypertension, diabetes, and obesity, all of which are trending in the wrong direction. Hypertension, we were gaining traction; and then over the past decade, we’ve slipped again. As you know, national blood pressure control rates have declined in many populations.
Harrington: Rather substantially.
Kazi: Substantially so, which has implications, in particular, for stroke rates in the future and stroke rates in young adults in the future. Obesity is a problem that we have very little control over. We’re already at 40% on average, which means that some populations are already in the 60% range.
Harrington: We also have obesity in kids — the burden, I’ll call it, of obesity. It’s not that you become obese in your thirties or your forties; you›re becoming obese as a teenager or even younger.
Kazi: Exactly. Since the 1990s, obesity in US adults has doubled, but obesity in US children has quadrupled. It’s starting from a lower base, but it’s very much an escalating problem.
Harrington: Diabetes is tightly linked to it but not totally explained.
Kazi: Exactly. The increase in diabetes is largely driven by obesity, but it›s probably also driven by changes in diet and lifestyle that don›t go through obesity.
Harrington: Yeah, it’s interesting. I think I have this figure correctly. It used to be rare that you saw a child with type 2 diabetes or what we call type 2 diabetes.
Kazi: Yeah.
Harrington: Now, the vast majority of kids with diabetes have type 2 diabetes.
Kazi: In the adolescents/young adults age group, most of it is type 2.
Harrington: Diabetes going up, obesity up, hypertension not well controlled, smoking combustible cigarettes way down.
Kazi: Yeah.
Harrington: Cholesterol levels. I was surprised. Cholesterol looked better. You said — because I was at a meeting where somebody asked you — that’s not explained by treatment.
Kazi: No, it’s not, at least going back to the ‘70s, but likely even sooner. I think that can only be attributed to substantial dietary changes. We are consuming less fat and less trans-fat. It’s possible that those collectively are improving our cholesterol levels, possibly at the expense of our glucose levels, because we basically substituted fats in our diet with more carbs at a population level.
Cigarettes and Vaping
Harrington: Some things certainly trend in the right direction but others in a really difficult direction. It’s going to lead to pretty large changes in risk for coronary disease, atrial fibrillation, and heart failure.
Kazi: I want to go back to the tobacco point. There are definitely marked declines in tobacco, still tightly related to income in the country. You see much higher prevalence of tobacco use in lower-income populations, but it’s unclear to me where it’s going in kids. We know that combustible tobacco use is going down but e-cigarettes went up. What that leads to over the next 30 years is unclear to me.
Harrington: That is a really important comment that’s worth sidebarring. The vaping use has been a terrible epidemic among our high schoolers. What is that going to lead to? Is it going to lead to the use of combustible cigarettes and we’re going to see that go back up? It remains to be seen.
Kazi: Yes, it remains to be seen. Going back to your point about this change in risk factors and this change in demographics, both aging and becoming a more diverse population means that we have large increases in some healthcare conditions.
Coronary heart disease goes up some, there›s a big jump in stroke — nearly a doubling in stroke — which is related to hypertension, obesity, an aging population, and a more diverse population. There are changes in stroke in the young, and atrial fibrillation related to, again, hypertension. We’re seeing these projections, and with them come these pretty large projections in changes in healthcare spending.
Healthcare Spending Not Sustainable
Harrington: Big. I mean, it’s not sustainable. Give the audience the number — it’s pretty frightening.
Kazi: We’re talking about a quadrupling of healthcare costs related to cardiovascular disease over 25 years. We’ve gotten used to the narrative that healthcare in the US is expensive and drugs are expensive, but this is an enormous problem — an unsustainable problem, like you called it.
It’s a doubling as a proportion of the economy. I was looking this up this morning. If the US healthcare economy were its own economy, it would be the fourth largest economy in the world.
Harrington: Healthcare as it is today, is it 21% of our economy?
Kazi: It’s 17% now. If it were its own economy, it would be the fourth largest in the world. We are spending more on healthcare than all but two other countries’ total economies. It’s kind of crazy.
Harrington: We’re talking about a quadrupling.
Kazi: Within that, the cardiovascular piece is a big piece, and we›re talking about a quadrupling.
Harrington: That’s both direct and indirect costs.
Kazi: The quadrupling of costs is just the direct costs. Indirect costs, for the listeners, refer to costs unrelated to healthcare but changes in productivity, either because people are disabled and unable to participate fully in the workforce or they die early.
The productivity costs are also increased substantially as a result. If you look at both healthcare and productivity, that goes up threefold. These are very large changes.
Harrington: Let’s now get to what we can do about it. I made the comment to you when I first read the papers that I was very depressed. Then, after I went through my Kübler-Ross stages of depression, death, and dying, I came to acceptance.
What are we going to do about it? This is a focus on policy, but also a focus on how we deliver healthcare, how we think about healthcare, and how we develop drugs and devices.
The drug question is going to be the one the audience is thinking about. They say, well, what about GLP-1 agonists? Aren’t those going to save the day?
Kazi: Yes and no. I’ll say that, early in my career, I used to be very attracted to simple solutions to complex problems. I’ve come to realize that simple solutions are elegant, attractive, and wrong. We›re dealing with a very complex issue and I think we’re going to need a multipronged approach.
The way I think about it is that there was a group of people who are at very high risk today. How do we help those individuals? Then how do we help the future generation so that they’re not dealing with the projections that we’re talking about.
My colleague, Karen Joynt Maddox, who led one of the papers, as you mentioned, has an elegant line in the paper where she says projections are not destiny. These are things we can change.
Harrington: If nothing changes, this is what it’s going to look like.
Kazi: This is where we’re headed.
Harrington: We can change. We’ve got some time to change, but we don’t have forever.
Kazi: Yes, exactly. We picked the 25-year timeline instead of a “let’s plan for the next century” timeline because we want something concrete and actionable. It’s close enough to be meaningful but far enough to give us the runway we need to act.
Harrington: Give me two things from the policy perspective, because it’s mostly policy.
Kazi: There are policy and clinical interventions. From the policy perspective, if I had to list two things, one is expansion of access to care. As we talk about this big increase in the burden of disease and risk factors, if you have a large proportion of your population that has hypertension or diabetes, you’re going to have to expand access to care to ensure that people get treated so they can get access to this care before they develop the complications that we worry about, like stroke and heart disease, that are very expensive to treat downstream.
The second, more broadly related to access to care, is the access to medications that are effective. You bring up GLP-1s. I think we need a real strategy for how we can give people access to GLP-1s at a price that is affordable to individuals but also affordable to the health system, and to help them stay on the drugs.
GLP-1s are transformative in what they do for weight loss and for diabetes, but more than 50% of people who start one are off it at 12 months. There’s something fundamentally wrong about how we’re delivering GLP-1s today. It’s not just about the cost of the drugs but the support system people need to stay on.
Harrington: I’ve made the comment, in many forms now, that we know the drugs work. We have to figure out how to use them.
Kazi: Exactly, yes.
Harrington: Using them includes chronicity. This is a chronic condition. Some people can come off the drugs, but many can’t. We’re going to have to figure this out, and maybe the newer generations of drugs will help us address what people call the off-ramping. How are we going to do that? I think you’re spot-on. Those are critically important questions.
Kazi: As we looked at this modeling, I’ll tell you — I had a come-to-Jesus moment where I was like, there is no way to fix cardiovascular disease in the US without going through obesity and diabetes. We have to address obesity in the US. We can’t just treat our way out of it. Obesity is fundamentally a food problem and we’ve got to engage again with food policy in a meaningful way.
Harrington: As you know, with the American Heart Association, we›re doing a large amount of work now on food as medicine and food is medicine. We are trying to figure out what the levers are that we can pull to actually help people eat healthier diets.
Kazi: Yes. Rather than framing it as an individual choice that people are eating poorly, it’s, how do we make healthy diets the default in the environment?
Harrington: This is where you get to the children as well.
Kazi: Exactly.
Harrington: I could talk about this all day. I’ve had the benefit of reading the papers now a few times and talking to you on several occasions. Thank you for joining us.
Kazi: Thank you.
Dr. Harrington, Stephen and Suzanne Weiss Dean, Weill Cornell Medicine; Provost for Medical Affairs, Cornell University, New York, NY, disclosed ties with Baim Institute (DSMB); CSL (RCT Executive Committee); Janssen (RCT Char), NHLBI (RCT Executive Committee, DSMB Chair); PCORI (RCT Co-Chair); DCRI, Atropos Health; Bitterroot Bio; Bristol Myers Squibb; BridgeBio; Element Science; Edwards Lifesciences; Foresite Labs; Medscape/WebMD Board of Directors for: American Heart Association; College of the Holy Cross; and Cytokinetics. Dr. Kazi, Associate Director, Smith Center for Outcomes Research, Associate Professor, Department of Medicine (Cardiology), Harvard Medical School, Director, Department of Cardiac Critical Care Unit, Beth Israel Deaconess Medical Center, Boston, Massachusetts, has disclosed receiving a research grant from Boston Scientific (grant to examine the economics of stroke prevention).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity .
Robert A. Harrington, MD: I’m here in London at the European Society of Cardiology meetings, at theheart.org | Medscape Cardiology booth, using the meetings as an opportunity to meet with colleagues to talk about recent things that they’ve been writing about.
Today I’m joined by a good friend and colleague, Dr. Dhruv Kazi from Beth Israel Deaconess in Boston. Thanks for joining us.
Dhruv S. Kazi, MD, MS: Thank you for having me.
Harrington: Dr. Kazi is an associate professor of medicine at Harvard Medical School. He’s also the associate director of the Smith Center, which is an outcomes research center at the Beth Israel Deaconess. Thanks for joining us.
Kazi: Excited to be here.
Harrington: The topic I think you know that I want to discuss is a really important paper. There are two papers. They’re part of the American Heart Association’s 100th anniversary celebration, if you will. Many of the papers looked back at where science taken us.
With your coauthor, Karen Joynt Maddox, your papers are looking forward. They’re about the burden of cardiovascular disease in 2050. One paper really focused on what I would call the clinical and public health issues. Yours is focused on the economics. Is that a good description?
Kazi: Perfect.
Harrington: Tell us what you, Karen, and the other writers set out to do. What were you asked to do?
Kazi: As you know, the American Heart Association is entering its second century. Part of this was an exercise to say, where will the country be in 2050, which is a long enough time horizon for us to start planning for the future.
We looked back and said, if prior trends remain the same, where will we be in 2050, accounting for changes in demographics, changes in the composition of the population, and knowing that some of the cardiovascular risk factors are getting worse?
Harrington: For me, what was really striking is that, when I first saw the title and read “2050,” I thought, Oh, that’s a long way away. Then as I started reading it, I realized that this is not so far away.
Kazi: Absolutely.
Harrington: If we’re going to make a difference, it might take us 25 years.
Kazi: Especially if we set ourselves ambitious goals, we›re going to have to dig deep. Business-as-usual is not going to get us there.
Harrington: No. What I think has happened is we›ve spent so much time taking care of acute illness. Case fatality rates are fantastic. I was actually making the comment yesterday to a colleague that when I was an intern, the 30-day death rate from acute myocardial infarction was about 20%.
Kazi: Oh, wow.
Harrington: Now it’s 5%. That’s a big difference in a career.
Trends in the Wrong Direction
Kazi: There are fundamental trends. The decline in case fatalities is a really positive development, and I would hope that, going forward, that would continue. Those are risk-adjusted death rates and what is happening is that risk is going up. This is a function of the fact that the US population is aging; 2030 will be the first year that all the baby boomers will be over the age of 65.
By the mid-2030s, we’ll have more adults over the age of 65 than kids. That aging of the population is going to increase risk. The second is — and this is a positive development — we are a more diverse population, but the populations that are minoritized have higher cardiovascular risk, for a variety of reasons.
As the population of Asian Americans increases and doubles, in fact, as the population of Hispanic Americans doubles, we’re going to see an increase in risk related to cardiovascular disease. The third is that, over the past decade, there are some risk factors that are going in the wrong direction.
Harrington: Let’s talk about that because that’s humbling. I’m involved, as you know, with the American Heart Association, as are you. Despite all the work on Life’s Simple 7 and now Life’s Essential 8, we still have some issues.
Kazi: The big ones that come to mind are hypertension, diabetes, and obesity, all of which are trending in the wrong direction. Hypertension, we were gaining traction; and then over the past decade, we’ve slipped again. As you know, national blood pressure control rates have declined in many populations.
Harrington: Rather substantially.
Kazi: Substantially so, which has implications, in particular, for stroke rates in the future and stroke rates in young adults in the future. Obesity is a problem that we have very little control over. We’re already at 40% on average, which means that some populations are already in the 60% range.
Harrington: We also have obesity in kids — the burden, I’ll call it, of obesity. It’s not that you become obese in your thirties or your forties; you›re becoming obese as a teenager or even younger.
Kazi: Exactly. Since the 1990s, obesity in US adults has doubled, but obesity in US children has quadrupled. It’s starting from a lower base, but it’s very much an escalating problem.
Harrington: Diabetes is tightly linked to it but not totally explained.
Kazi: Exactly. The increase in diabetes is largely driven by obesity, but it›s probably also driven by changes in diet and lifestyle that don›t go through obesity.
Harrington: Yeah, it’s interesting. I think I have this figure correctly. It used to be rare that you saw a child with type 2 diabetes or what we call type 2 diabetes.
Kazi: Yeah.
Harrington: Now, the vast majority of kids with diabetes have type 2 diabetes.
Kazi: In the adolescents/young adults age group, most of it is type 2.
Harrington: Diabetes going up, obesity up, hypertension not well controlled, smoking combustible cigarettes way down.
Kazi: Yeah.
Harrington: Cholesterol levels. I was surprised. Cholesterol looked better. You said — because I was at a meeting where somebody asked you — that’s not explained by treatment.
Kazi: No, it’s not, at least going back to the ‘70s, but likely even sooner. I think that can only be attributed to substantial dietary changes. We are consuming less fat and less trans-fat. It’s possible that those collectively are improving our cholesterol levels, possibly at the expense of our glucose levels, because we basically substituted fats in our diet with more carbs at a population level.
Cigarettes and Vaping
Harrington: Some things certainly trend in the right direction but others in a really difficult direction. It’s going to lead to pretty large changes in risk for coronary disease, atrial fibrillation, and heart failure.
Kazi: I want to go back to the tobacco point. There are definitely marked declines in tobacco, still tightly related to income in the country. You see much higher prevalence of tobacco use in lower-income populations, but it’s unclear to me where it’s going in kids. We know that combustible tobacco use is going down but e-cigarettes went up. What that leads to over the next 30 years is unclear to me.
Harrington: That is a really important comment that’s worth sidebarring. The vaping use has been a terrible epidemic among our high schoolers. What is that going to lead to? Is it going to lead to the use of combustible cigarettes and we’re going to see that go back up? It remains to be seen.
Kazi: Yes, it remains to be seen. Going back to your point about this change in risk factors and this change in demographics, both aging and becoming a more diverse population means that we have large increases in some healthcare conditions.
Coronary heart disease goes up some, there›s a big jump in stroke — nearly a doubling in stroke — which is related to hypertension, obesity, an aging population, and a more diverse population. There are changes in stroke in the young, and atrial fibrillation related to, again, hypertension. We’re seeing these projections, and with them come these pretty large projections in changes in healthcare spending.
Healthcare Spending Not Sustainable
Harrington: Big. I mean, it’s not sustainable. Give the audience the number — it’s pretty frightening.
Kazi: We’re talking about a quadrupling of healthcare costs related to cardiovascular disease over 25 years. We’ve gotten used to the narrative that healthcare in the US is expensive and drugs are expensive, but this is an enormous problem — an unsustainable problem, like you called it.
It’s a doubling as a proportion of the economy. I was looking this up this morning. If the US healthcare economy were its own economy, it would be the fourth largest economy in the world.
Harrington: Healthcare as it is today, is it 21% of our economy?
Kazi: It’s 17% now. If it were its own economy, it would be the fourth largest in the world. We are spending more on healthcare than all but two other countries’ total economies. It’s kind of crazy.
Harrington: We’re talking about a quadrupling.
Kazi: Within that, the cardiovascular piece is a big piece, and we›re talking about a quadrupling.
Harrington: That’s both direct and indirect costs.
Kazi: The quadrupling of costs is just the direct costs. Indirect costs, for the listeners, refer to costs unrelated to healthcare but changes in productivity, either because people are disabled and unable to participate fully in the workforce or they die early.
The productivity costs are also increased substantially as a result. If you look at both healthcare and productivity, that goes up threefold. These are very large changes.
Harrington: Let’s now get to what we can do about it. I made the comment to you when I first read the papers that I was very depressed. Then, after I went through my Kübler-Ross stages of depression, death, and dying, I came to acceptance.
What are we going to do about it? This is a focus on policy, but also a focus on how we deliver healthcare, how we think about healthcare, and how we develop drugs and devices.
The drug question is going to be the one the audience is thinking about. They say, well, what about GLP-1 agonists? Aren’t those going to save the day?
Kazi: Yes and no. I’ll say that, early in my career, I used to be very attracted to simple solutions to complex problems. I’ve come to realize that simple solutions are elegant, attractive, and wrong. We›re dealing with a very complex issue and I think we’re going to need a multipronged approach.
The way I think about it is that there was a group of people who are at very high risk today. How do we help those individuals? Then how do we help the future generation so that they’re not dealing with the projections that we’re talking about.
My colleague, Karen Joynt Maddox, who led one of the papers, as you mentioned, has an elegant line in the paper where she says projections are not destiny. These are things we can change.
Harrington: If nothing changes, this is what it’s going to look like.
Kazi: This is where we’re headed.
Harrington: We can change. We’ve got some time to change, but we don’t have forever.
Kazi: Yes, exactly. We picked the 25-year timeline instead of a “let’s plan for the next century” timeline because we want something concrete and actionable. It’s close enough to be meaningful but far enough to give us the runway we need to act.
Harrington: Give me two things from the policy perspective, because it’s mostly policy.
Kazi: There are policy and clinical interventions. From the policy perspective, if I had to list two things, one is expansion of access to care. As we talk about this big increase in the burden of disease and risk factors, if you have a large proportion of your population that has hypertension or diabetes, you’re going to have to expand access to care to ensure that people get treated so they can get access to this care before they develop the complications that we worry about, like stroke and heart disease, that are very expensive to treat downstream.
The second, more broadly related to access to care, is the access to medications that are effective. You bring up GLP-1s. I think we need a real strategy for how we can give people access to GLP-1s at a price that is affordable to individuals but also affordable to the health system, and to help them stay on the drugs.
GLP-1s are transformative in what they do for weight loss and for diabetes, but more than 50% of people who start one are off it at 12 months. There’s something fundamentally wrong about how we’re delivering GLP-1s today. It’s not just about the cost of the drugs but the support system people need to stay on.
Harrington: I’ve made the comment, in many forms now, that we know the drugs work. We have to figure out how to use them.
Kazi: Exactly, yes.
Harrington: Using them includes chronicity. This is a chronic condition. Some people can come off the drugs, but many can’t. We’re going to have to figure this out, and maybe the newer generations of drugs will help us address what people call the off-ramping. How are we going to do that? I think you’re spot-on. Those are critically important questions.
Kazi: As we looked at this modeling, I’ll tell you — I had a come-to-Jesus moment where I was like, there is no way to fix cardiovascular disease in the US without going through obesity and diabetes. We have to address obesity in the US. We can’t just treat our way out of it. Obesity is fundamentally a food problem and we’ve got to engage again with food policy in a meaningful way.
Harrington: As you know, with the American Heart Association, we›re doing a large amount of work now on food as medicine and food is medicine. We are trying to figure out what the levers are that we can pull to actually help people eat healthier diets.
Kazi: Yes. Rather than framing it as an individual choice that people are eating poorly, it’s, how do we make healthy diets the default in the environment?
Harrington: This is where you get to the children as well.
Kazi: Exactly.
Harrington: I could talk about this all day. I’ve had the benefit of reading the papers now a few times and talking to you on several occasions. Thank you for joining us.
Kazi: Thank you.
Dr. Harrington, Stephen and Suzanne Weiss Dean, Weill Cornell Medicine; Provost for Medical Affairs, Cornell University, New York, NY, disclosed ties with Baim Institute (DSMB); CSL (RCT Executive Committee); Janssen (RCT Char), NHLBI (RCT Executive Committee, DSMB Chair); PCORI (RCT Co-Chair); DCRI, Atropos Health; Bitterroot Bio; Bristol Myers Squibb; BridgeBio; Element Science; Edwards Lifesciences; Foresite Labs; Medscape/WebMD Board of Directors for: American Heart Association; College of the Holy Cross; and Cytokinetics. Dr. Kazi, Associate Director, Smith Center for Outcomes Research, Associate Professor, Department of Medicine (Cardiology), Harvard Medical School, Director, Department of Cardiac Critical Care Unit, Beth Israel Deaconess Medical Center, Boston, Massachusetts, has disclosed receiving a research grant from Boston Scientific (grant to examine the economics of stroke prevention).
A version of this article appeared on Medscape.com.
How Old Are You? Stand on One Leg and I’ll Tell You
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.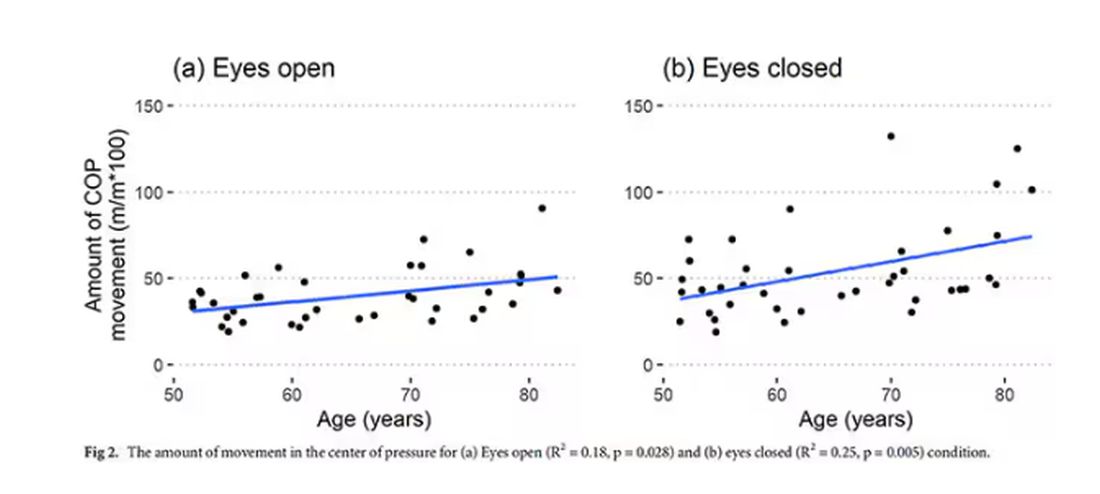
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
H pylori: ACG Guideline Advises New Approaches to Treatment
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.