User login
Autonomous AI Outperforms Humans in Optical Diagnosis of Colorectal Polyps
, while providing greater alignment with pathology-based surveillance intervals, based on a randomized controlled trial.
These findings suggest that autonomous AI may one day replace histologic assessment of diminutive polyps, reported lead author Roupen Djinbachian, MD, of the Montreal University Hospital Research Center, Montreal, Quebec, Canada, and colleagues.Optical diagnosis of diminutive colorectal polyps has been proposed as a cost-effective alternative to histologic diagnosis, but its implementation in general clinical practice has been hindered by endoscopists’ concerns about incorrect diagnoses, the investigators wrote in Gastroenterology.“AI-based systems (CADx) have been proposed as a solution to these barriers to implementation, with studies showing high adherence to Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds when using AI-H,” they wrote. “However, the efficacy and safety of autonomous AI-based diagnostic platforms have not yet been evaluated.”
To address this knowledge gap, Dr. Djinbachian and colleagues conducted a randomized controlled noninferiority trial involving 467 patients, all of whom underwent elective colonoscopies at a single academic institution.
Participants were randomly assigned to one of two groups. The first group received an optical diagnosis of diminutive (1-5 mm) colorectal polyps using an autonomous AI-based CADx system without any human input. The second group had diagnoses performed by endoscopists who used AI-H to make their optical diagnoses.
The primary outcome was the accuracy of optical diagnosis compared with the gold standard of histologic evaluation. Secondarily, the investigators explored associations between pathology-based surveillance intervals and various measures of accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
The results showed that the accuracy of optical diagnosis for diminutive polyps was similar between the two groups, supporting noninferiority. Autonomous AI achieved an accuracy rate of 77.2%, while the AI-H group had an accuracy of 72.1%, which was not statistically significant (P = .86).
But when it came to pathology-based surveillance intervals, autonomous AI showed a clear advantage; the autonomous AI system achieved a 91.5% agreement rate, compared with 82.1% for the AI-H group (P = .016).
“These findings indicate that autonomous AI not only matches but also surpasses AI-H in accuracy for determining surveillance intervals,” the investigators wrote, noting that this finding highlights the “complexities of human interaction with AI modules where human intervention could lead to worse outcomes.”
Further analysis revealed that the sensitivity of autonomous AI for identifying adenomas was 84.8%, slightly higher than the 83.6% sensitivity of the AI-H group. Specificity was 64.4% for autonomous AI vs 63.8% for AI-H. While PPV was higher in the autonomous AI group (85.6%), compared with the AI-H group (78.6%), NPV was lower for autonomous AI than AI-H (63.0% vs 71.0%).
Dr. Djinbachian and colleagues suggested that future research should focus on larger, multicenter trials to validate these findings and further explore the integration of autonomous AI systems in clinical practice. They also noted that improving AI algorithms to accurately diagnose sessile serrated lesions could enhance the overall effectiveness of AI-based optical diagnosis.
“The performance of autonomous AI in accurately diagnosing diminutive polyps and determining appropriate surveillance intervals suggests that it could play a crucial role in streamlining colorectal cancer screening processes, reducing the burden on pathologists, and potentially lowering healthcare costs,” the investigators concluded.The study was supported by Fujifilm, which had no role in the study design or data analysis. Dr. von Renteln reported additional research funding from Vantage and Fujifilm.
In the era of computer vision for endoscopy and colonoscopy, current paradigms rely on AI as a co-pilot or second observer, with the physician serving as the final arbiter in procedure-related decision-making. This study by Djinbachian and Haumesser et al brings up the interesting wrinkle of autonomous AI as a potentially superior (or noninferior) option in narrow, task-specific use cases.
In this study, human input from the endoscopist after CADx diagnosis led to lower agreement between the AI-predicted diagnosis and corresponding surveillance intervals; human oversight more often incorrectly changed the resultant diagnosis and led to shorter than recommended surveillance intervals.
This study offers a small but very important update to the growing body of literature on CADx in colonoscopy. So far, prospective validation of CADx compared with the human eye for in-situ diagnosis of polyps has provided mixed results. This study is one of the first to examine the potential role of “automatic” CADx without additional human input and sheds light on the importance of the AI-human hybrid in medical care. How do the ways in which humans interact with the user interface and output of AI lead to changes in outcome? How can we optimize the AI-human interaction in order to provide optimal results?
[[{"fid":"301890","view_mode":"medstat_image_flush_left","fields":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Dr. Jeremy R. Glissen Brown, Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina","field_file_image_credit[und][0][value]":"Duke University Medical Center","field_file_image_caption[und][0][value]":"Dr. Jeremy R. Glissen Brown"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Dr. Jeremy R. Glissen Brown, Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina","field_file_image_credit[und][0][value]":"Duke University Medical Center","field_file_image_caption[und][0][value]":"Dr. Jeremy R. Glissen Brown"}},"attributes":{"alt":"Dr. Jeremy R. Glissen Brown, Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina","class":"media-element file-medstat-image-flush-left","data-delta":"1"}}]]In this case, the suggestion is that less is more when it comes to human interference with optical diagnosis, but further research is needed on how to best optimize this important relationship as well as how AI might (or might not) support diagnose-and-leave and diagnose-and-discard strategies in the United States and worldwide.
Jeremy R. Glissen Brown is an assistant professor in the Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina. He has served as a consultant for Medtronic and Olympus, and on the advisory board for Odin Vision.
In the era of computer vision for endoscopy and colonoscopy, current paradigms rely on AI as a co-pilot or second observer, with the physician serving as the final arbiter in procedure-related decision-making. This study by Djinbachian and Haumesser et al brings up the interesting wrinkle of autonomous AI as a potentially superior (or noninferior) option in narrow, task-specific use cases.
In this study, human input from the endoscopist after CADx diagnosis led to lower agreement between the AI-predicted diagnosis and corresponding surveillance intervals; human oversight more often incorrectly changed the resultant diagnosis and led to shorter than recommended surveillance intervals.
This study offers a small but very important update to the growing body of literature on CADx in colonoscopy. So far, prospective validation of CADx compared with the human eye for in-situ diagnosis of polyps has provided mixed results. This study is one of the first to examine the potential role of “automatic” CADx without additional human input and sheds light on the importance of the AI-human hybrid in medical care. How do the ways in which humans interact with the user interface and output of AI lead to changes in outcome? How can we optimize the AI-human interaction in order to provide optimal results?
[[{"fid":"301890","view_mode":"medstat_image_flush_left","fields":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Dr. Jeremy R. Glissen Brown, Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina","field_file_image_credit[und][0][value]":"Duke University Medical Center","field_file_image_caption[und][0][value]":"Dr. Jeremy R. Glissen Brown"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Dr. Jeremy R. Glissen Brown, Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina","field_file_image_credit[und][0][value]":"Duke University Medical Center","field_file_image_caption[und][0][value]":"Dr. Jeremy R. Glissen Brown"}},"attributes":{"alt":"Dr. Jeremy R. Glissen Brown, Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina","class":"media-element file-medstat-image-flush-left","data-delta":"1"}}]]In this case, the suggestion is that less is more when it comes to human interference with optical diagnosis, but further research is needed on how to best optimize this important relationship as well as how AI might (or might not) support diagnose-and-leave and diagnose-and-discard strategies in the United States and worldwide.
Jeremy R. Glissen Brown is an assistant professor in the Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina. He has served as a consultant for Medtronic and Olympus, and on the advisory board for Odin Vision.
In the era of computer vision for endoscopy and colonoscopy, current paradigms rely on AI as a co-pilot or second observer, with the physician serving as the final arbiter in procedure-related decision-making. This study by Djinbachian and Haumesser et al brings up the interesting wrinkle of autonomous AI as a potentially superior (or noninferior) option in narrow, task-specific use cases.
In this study, human input from the endoscopist after CADx diagnosis led to lower agreement between the AI-predicted diagnosis and corresponding surveillance intervals; human oversight more often incorrectly changed the resultant diagnosis and led to shorter than recommended surveillance intervals.
This study offers a small but very important update to the growing body of literature on CADx in colonoscopy. So far, prospective validation of CADx compared with the human eye for in-situ diagnosis of polyps has provided mixed results. This study is one of the first to examine the potential role of “automatic” CADx without additional human input and sheds light on the importance of the AI-human hybrid in medical care. How do the ways in which humans interact with the user interface and output of AI lead to changes in outcome? How can we optimize the AI-human interaction in order to provide optimal results?
[[{"fid":"301890","view_mode":"medstat_image_flush_left","fields":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Dr. Jeremy R. Glissen Brown, Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina","field_file_image_credit[und][0][value]":"Duke University Medical Center","field_file_image_caption[und][0][value]":"Dr. Jeremy R. Glissen Brown"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Dr. Jeremy R. Glissen Brown, Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina","field_file_image_credit[und][0][value]":"Duke University Medical Center","field_file_image_caption[und][0][value]":"Dr. Jeremy R. Glissen Brown"}},"attributes":{"alt":"Dr. Jeremy R. Glissen Brown, Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina","class":"media-element file-medstat-image-flush-left","data-delta":"1"}}]]In this case, the suggestion is that less is more when it comes to human interference with optical diagnosis, but further research is needed on how to best optimize this important relationship as well as how AI might (or might not) support diagnose-and-leave and diagnose-and-discard strategies in the United States and worldwide.
Jeremy R. Glissen Brown is an assistant professor in the Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina. He has served as a consultant for Medtronic and Olympus, and on the advisory board for Odin Vision.
, while providing greater alignment with pathology-based surveillance intervals, based on a randomized controlled trial.
These findings suggest that autonomous AI may one day replace histologic assessment of diminutive polyps, reported lead author Roupen Djinbachian, MD, of the Montreal University Hospital Research Center, Montreal, Quebec, Canada, and colleagues.Optical diagnosis of diminutive colorectal polyps has been proposed as a cost-effective alternative to histologic diagnosis, but its implementation in general clinical practice has been hindered by endoscopists’ concerns about incorrect diagnoses, the investigators wrote in Gastroenterology.“AI-based systems (CADx) have been proposed as a solution to these barriers to implementation, with studies showing high adherence to Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds when using AI-H,” they wrote. “However, the efficacy and safety of autonomous AI-based diagnostic platforms have not yet been evaluated.”
To address this knowledge gap, Dr. Djinbachian and colleagues conducted a randomized controlled noninferiority trial involving 467 patients, all of whom underwent elective colonoscopies at a single academic institution.
Participants were randomly assigned to one of two groups. The first group received an optical diagnosis of diminutive (1-5 mm) colorectal polyps using an autonomous AI-based CADx system without any human input. The second group had diagnoses performed by endoscopists who used AI-H to make their optical diagnoses.
The primary outcome was the accuracy of optical diagnosis compared with the gold standard of histologic evaluation. Secondarily, the investigators explored associations between pathology-based surveillance intervals and various measures of accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
The results showed that the accuracy of optical diagnosis for diminutive polyps was similar between the two groups, supporting noninferiority. Autonomous AI achieved an accuracy rate of 77.2%, while the AI-H group had an accuracy of 72.1%, which was not statistically significant (P = .86).
But when it came to pathology-based surveillance intervals, autonomous AI showed a clear advantage; the autonomous AI system achieved a 91.5% agreement rate, compared with 82.1% for the AI-H group (P = .016).
“These findings indicate that autonomous AI not only matches but also surpasses AI-H in accuracy for determining surveillance intervals,” the investigators wrote, noting that this finding highlights the “complexities of human interaction with AI modules where human intervention could lead to worse outcomes.”
Further analysis revealed that the sensitivity of autonomous AI for identifying adenomas was 84.8%, slightly higher than the 83.6% sensitivity of the AI-H group. Specificity was 64.4% for autonomous AI vs 63.8% for AI-H. While PPV was higher in the autonomous AI group (85.6%), compared with the AI-H group (78.6%), NPV was lower for autonomous AI than AI-H (63.0% vs 71.0%).
Dr. Djinbachian and colleagues suggested that future research should focus on larger, multicenter trials to validate these findings and further explore the integration of autonomous AI systems in clinical practice. They also noted that improving AI algorithms to accurately diagnose sessile serrated lesions could enhance the overall effectiveness of AI-based optical diagnosis.
“The performance of autonomous AI in accurately diagnosing diminutive polyps and determining appropriate surveillance intervals suggests that it could play a crucial role in streamlining colorectal cancer screening processes, reducing the burden on pathologists, and potentially lowering healthcare costs,” the investigators concluded.The study was supported by Fujifilm, which had no role in the study design or data analysis. Dr. von Renteln reported additional research funding from Vantage and Fujifilm.
, while providing greater alignment with pathology-based surveillance intervals, based on a randomized controlled trial.
These findings suggest that autonomous AI may one day replace histologic assessment of diminutive polyps, reported lead author Roupen Djinbachian, MD, of the Montreal University Hospital Research Center, Montreal, Quebec, Canada, and colleagues.Optical diagnosis of diminutive colorectal polyps has been proposed as a cost-effective alternative to histologic diagnosis, but its implementation in general clinical practice has been hindered by endoscopists’ concerns about incorrect diagnoses, the investigators wrote in Gastroenterology.“AI-based systems (CADx) have been proposed as a solution to these barriers to implementation, with studies showing high adherence to Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds when using AI-H,” they wrote. “However, the efficacy and safety of autonomous AI-based diagnostic platforms have not yet been evaluated.”
To address this knowledge gap, Dr. Djinbachian and colleagues conducted a randomized controlled noninferiority trial involving 467 patients, all of whom underwent elective colonoscopies at a single academic institution.
Participants were randomly assigned to one of two groups. The first group received an optical diagnosis of diminutive (1-5 mm) colorectal polyps using an autonomous AI-based CADx system without any human input. The second group had diagnoses performed by endoscopists who used AI-H to make their optical diagnoses.
The primary outcome was the accuracy of optical diagnosis compared with the gold standard of histologic evaluation. Secondarily, the investigators explored associations between pathology-based surveillance intervals and various measures of accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
The results showed that the accuracy of optical diagnosis for diminutive polyps was similar between the two groups, supporting noninferiority. Autonomous AI achieved an accuracy rate of 77.2%, while the AI-H group had an accuracy of 72.1%, which was not statistically significant (P = .86).
But when it came to pathology-based surveillance intervals, autonomous AI showed a clear advantage; the autonomous AI system achieved a 91.5% agreement rate, compared with 82.1% for the AI-H group (P = .016).
“These findings indicate that autonomous AI not only matches but also surpasses AI-H in accuracy for determining surveillance intervals,” the investigators wrote, noting that this finding highlights the “complexities of human interaction with AI modules where human intervention could lead to worse outcomes.”
Further analysis revealed that the sensitivity of autonomous AI for identifying adenomas was 84.8%, slightly higher than the 83.6% sensitivity of the AI-H group. Specificity was 64.4% for autonomous AI vs 63.8% for AI-H. While PPV was higher in the autonomous AI group (85.6%), compared with the AI-H group (78.6%), NPV was lower for autonomous AI than AI-H (63.0% vs 71.0%).
Dr. Djinbachian and colleagues suggested that future research should focus on larger, multicenter trials to validate these findings and further explore the integration of autonomous AI systems in clinical practice. They also noted that improving AI algorithms to accurately diagnose sessile serrated lesions could enhance the overall effectiveness of AI-based optical diagnosis.
“The performance of autonomous AI in accurately diagnosing diminutive polyps and determining appropriate surveillance intervals suggests that it could play a crucial role in streamlining colorectal cancer screening processes, reducing the burden on pathologists, and potentially lowering healthcare costs,” the investigators concluded.The study was supported by Fujifilm, which had no role in the study design or data analysis. Dr. von Renteln reported additional research funding from Vantage and Fujifilm.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>168264</fileName> <TBEID>0C050575.SIG</TBEID> <TBUniqueIdentifier>MD_0C050575</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname>Gastro_Djinbachian_AI</storyname> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240611T140656</QCDate> <firstPublished>20240611T150325</firstPublished> <LastPublished>20240611T150325</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240611T150325</CMSDate> <articleSource>FROM GASTROENTEROLOGY</articleSource> <facebookInfo/> <meetingNumber/> <byline>Will Pass</byline> <bylineText>WILL PASS</bylineText> <bylineFull>WILL PASS</bylineFull> <bylineTitleText>MDedge News</bylineTitleText> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Autonomous artificial intelligence (AI) can achieve similar accuracy to AI-assisted humans (AI-H) in the optical diagnosis of diminutive colorectal polyps</metaDescription> <articlePDF/> <teaserImage>301890</teaserImage> <teaser>Autonomous AI may one day replace histologic assessment of diminutive polyps.</teaser> <title>Autonomous AI Outperforms Humans in Optical Diagnosis of Colorectal Polyps</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>gih</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">17</term> </publications> <sections> <term canonical="true">69</term> <term>27970</term> <term>39313</term> </sections> <topics> <term canonical="true">39702</term> <term>344</term> <term>345</term> </topics> <links> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/240129e5.jpg</altRep> <description role="drol:caption">Dr. Jeremy R. Glissen Brown</description> <description role="drol:credit">Duke University Medical Center</description> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Autonomous AI Outperforms Humans in Optical Diagnosis of Colorectal Polyps</title> <deck/> </itemMeta> <itemContent> <p><span class="tag metaDescription">Autonomous artificial intelligence (AI) can achieve similar accuracy to AI-assisted humans (AI-H) in the optical diagnosis of diminutive colorectal polyps</span>, while providing greater alignment with pathology-based surveillance intervals, based on a randomized controlled trial.</p> <p>These findings suggest that autonomous AI may one day replace histologic assessment of diminutive polyps, reported lead author <a href="https://www.researchgate.net/profile/Roupen-Djinbachian">Roupen Djinbachian, MD</a>, of the Montreal University Hospital Research Center, Montreal, Quebec, Canada, and colleagues.Optical diagnosis of diminutive colorectal polyps has been proposed as a cost-effective alternative to histologic diagnosis, but its implementation in general clinical practice has been hindered by endoscopists’ concerns about incorrect diagnoses, the investigators wrote in<strong> </strong><em><a href="https://www.gastrojournal.org/article/S0016-5085(24)00131-8/fulltext">Gastroenterology</a></em>.“AI-based systems (CADx) have been proposed as a solution to these barriers to implementation, with studies showing high adherence to Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds when using AI-H,” they wrote. “However, the efficacy and safety of autonomous AI-based diagnostic platforms have not yet been evaluated.”<br/><br/>To address this knowledge gap, Dr. Djinbachian and colleagues conducted a randomized controlled noninferiority trial involving 467 patients, all of whom underwent elective colonoscopies at a single academic institution.<br/><br/>Participants were randomly assigned to one of two groups. The first group received an optical diagnosis of diminutive (1-5 mm) colorectal polyps using an autonomous AI-based CADx system without any human input. The second group had diagnoses performed by endoscopists who used AI-H to make their optical diagnoses.<br/><br/>The primary outcome was the accuracy of optical diagnosis compared with the gold standard of histologic evaluation. Secondarily, the investigators explored associations between pathology-based surveillance intervals and various measures of accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).<br/><br/>The results showed that the accuracy of optical diagnosis for diminutive polyps was similar between the two groups, supporting noninferiority. Autonomous AI achieved an accuracy rate of 77.2%, while the AI-H group had an accuracy of 72.1%, which was not statistically significant (<em>P</em> = .86). <br/><br/>But when it came to pathology-based surveillance intervals, autonomous AI showed a clear advantage; the autonomous AI system achieved a 91.5% agreement rate, compared with 82.1% for the AI-H group (<em>P</em> = .016).<br/><br/>“These findings indicate that autonomous AI not only matches but also surpasses AI-H in accuracy for determining surveillance intervals,” the investigators wrote, noting that this finding highlights the “complexities of human interaction with AI modules where human intervention could lead to worse outcomes.”<br/><br/>Further analysis revealed that the sensitivity of autonomous AI for identifying adenomas was 84.8%, slightly higher than the 83.6% sensitivity of the AI-H group. Specificity was 64.4% for autonomous AI vs 63.8% for AI-H. While PPV was higher in the autonomous AI group (85.6%), compared with the AI-H group (78.6%), NPV was lower for autonomous AI than AI-H (63.0% vs 71.0%).<br/><br/>Dr. Djinbachian and colleagues suggested that future research should focus on larger, multicenter trials to validate these findings and further explore the integration of autonomous AI systems in clinical practice. They also noted that improving AI algorithms to accurately diagnose sessile serrated lesions could enhance the overall effectiveness of AI-based optical diagnosis.<br/><br/>“The performance of autonomous AI in accurately diagnosing diminutive polyps and determining appropriate surveillance intervals suggests that it could play a crucial role in streamlining colorectal cancer screening processes, reducing the burden on pathologists, and potentially lowering healthcare costs,” the investigators concluded.The study was supported by Fujifilm, which had no role in the study design or data analysis. Dr. von Renteln reported additional research funding from Vantage and Fujifilm.<span class="end"/></p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>views</itemRole> <itemClass>text</itemClass> <title>‘Automatic’ CADx in Colonoscopy</title> <deck/> </itemMeta> <itemContent> <p>In the era of computer vision for endoscopy and colonoscopy, current paradigms rely on AI as a co-pilot or second observer, with the physician serving as the final arbiter in procedure-related decision-making. This study by Djinbachian and Haumesser et al brings up the interesting wrinkle of autonomous AI as a potentially superior (or noninferior) option in narrow, task-specific use cases.</p> <p>In this study, human input from the endoscopist after CADx diagnosis led to lower agreement between the AI-predicted diagnosis and corresponding surveillance intervals; human oversight more often incorrectly changed the resultant diagnosis and led to shorter than recommended surveillance intervals.<br/><br/>This study offers a small but very important update to the growing body of literature on CADx in colonoscopy. So far, prospective validation of CADx compared with the human eye for in-situ diagnosis of polyps has provided mixed results. This study is one of the first to examine the potential role of “automatic” CADx without additional human input and sheds light on the importance of the AI-human hybrid in medical care. How do the ways in which humans interact with the user interface and output of AI lead to changes in outcome? How can we optimize the AI-human interaction in order to provide optimal results?<br/><br/>[[{"fid":"301890","view_mode":"medstat_image_flush_left","fields":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Dr. Jeremy R. Glissen Brown, Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina","field_file_image_credit[und][0][value]":"Duke University Medical Center","field_file_image_caption[und][0][value]":"Dr. Jeremy R. Glissen Brown"},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_left"}}]]In this case, the suggestion is that less is more when it comes to human interference with optical diagnosis, but further research is needed on how to best optimize this important relationship as well as how AI might (or might not) support diagnose-and-leave and diagnose-and-discard strategies in the United States and worldwide.</p> <p><em> <em>Jeremy R. Glissen Brown is an assistant professor in the Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina. He has served as a consultant for Medtronic and Olympus, and on the advisory board for Odin Vision.</em> </em></p> </itemContent> </newsItem> </itemSet></root>
FROM GASTROENTEROLOGY
Gastroenterology Data Trends 2024

In this issue:
- Eosinophilic Gastrointestinal Diseases: Beyond EoE
Nirmala Gonsalves, MD, AGAF, FACG - The Changing Face of IBD: Beyond the Western World
Gilaad G. Kaplan, MD, MPH, AGAF; Paulo Kotze, MD, MS, PhD; Siew C. Ng, MBBS, PhD, AGAF - Role of Non-invasive Biomarkers in the Evaluation and Management of MASLD
Julia J. Wattacheril, MD, MPH - The Emerging Role of Liquid Biopsy in the Diagnosis and Management of CRC
David Lieberman, MD, AGAF - Cannabinoids and Digestive Disorders
Jami A. Kinnucan, MD, AGAF, FACG - AI and Machine Learning in IBD: Promising Applications and Remaining Challenges
Shirley Cohen-Mekelburg, MD, MS - Simulation-Based Training in Endoscopy: Benefits and Challenges
Richa Shukla, MD - Fluid Management in Acute Pancreatitis
Jorge D. Machicado, MD, MPH

In this issue:
- Eosinophilic Gastrointestinal Diseases: Beyond EoE
Nirmala Gonsalves, MD, AGAF, FACG - The Changing Face of IBD: Beyond the Western World
Gilaad G. Kaplan, MD, MPH, AGAF; Paulo Kotze, MD, MS, PhD; Siew C. Ng, MBBS, PhD, AGAF - Role of Non-invasive Biomarkers in the Evaluation and Management of MASLD
Julia J. Wattacheril, MD, MPH - The Emerging Role of Liquid Biopsy in the Diagnosis and Management of CRC
David Lieberman, MD, AGAF - Cannabinoids and Digestive Disorders
Jami A. Kinnucan, MD, AGAF, FACG - AI and Machine Learning in IBD: Promising Applications and Remaining Challenges
Shirley Cohen-Mekelburg, MD, MS - Simulation-Based Training in Endoscopy: Benefits and Challenges
Richa Shukla, MD - Fluid Management in Acute Pancreatitis
Jorge D. Machicado, MD, MPH

In this issue:
- Eosinophilic Gastrointestinal Diseases: Beyond EoE
Nirmala Gonsalves, MD, AGAF, FACG - The Changing Face of IBD: Beyond the Western World
Gilaad G. Kaplan, MD, MPH, AGAF; Paulo Kotze, MD, MS, PhD; Siew C. Ng, MBBS, PhD, AGAF - Role of Non-invasive Biomarkers in the Evaluation and Management of MASLD
Julia J. Wattacheril, MD, MPH - The Emerging Role of Liquid Biopsy in the Diagnosis and Management of CRC
David Lieberman, MD, AGAF - Cannabinoids and Digestive Disorders
Jami A. Kinnucan, MD, AGAF, FACG - AI and Machine Learning in IBD: Promising Applications and Remaining Challenges
Shirley Cohen-Mekelburg, MD, MS - Simulation-Based Training in Endoscopy: Benefits and Challenges
Richa Shukla, MD - Fluid Management in Acute Pancreatitis
Jorge D. Machicado, MD, MPH
The Emerging Role of Liquid Biopsy in the Diagnosis and Management of CRC
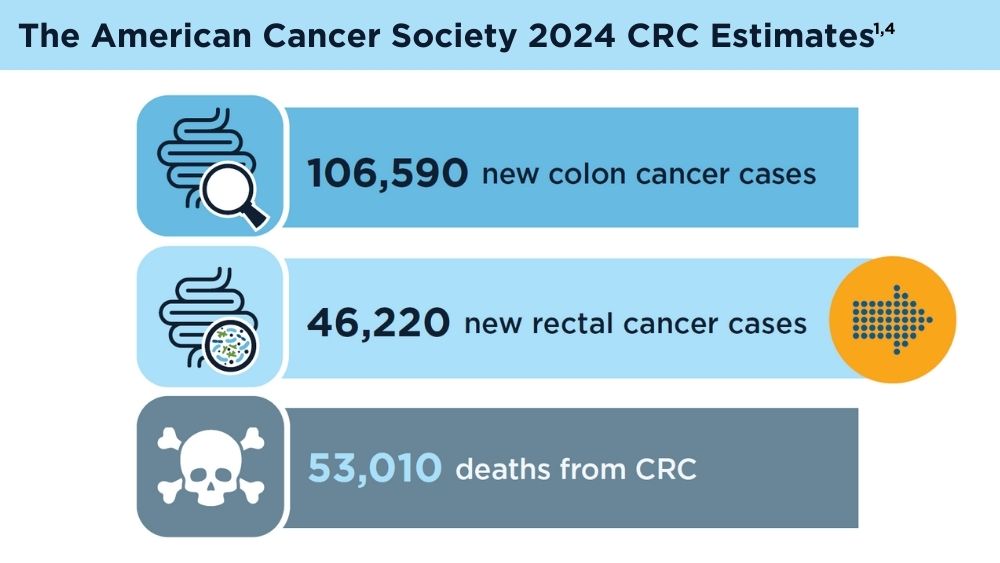
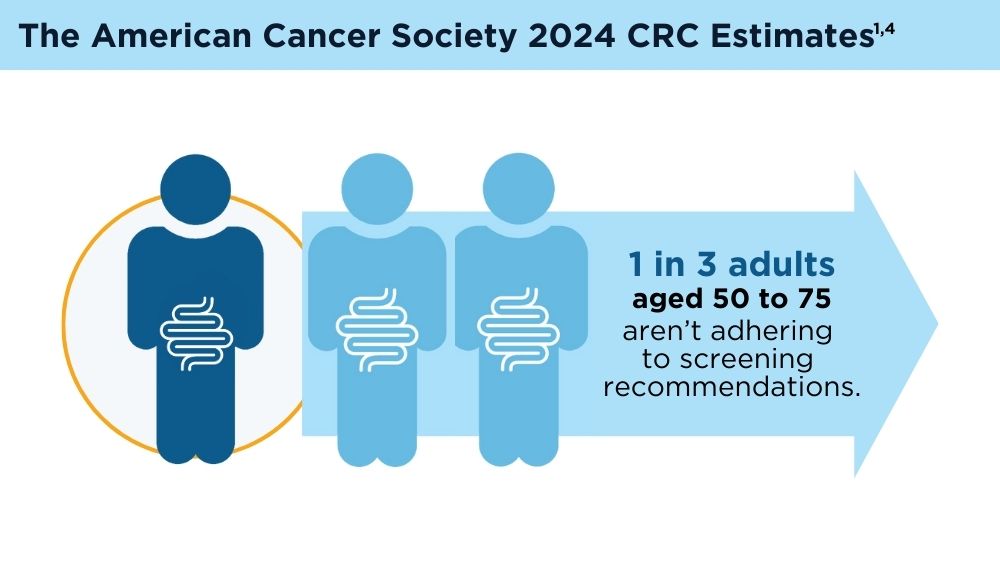
Key statistics for colorectal cancer. American Cancer Society. Revised January 13, 2023. Accessed November 30, 2023. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
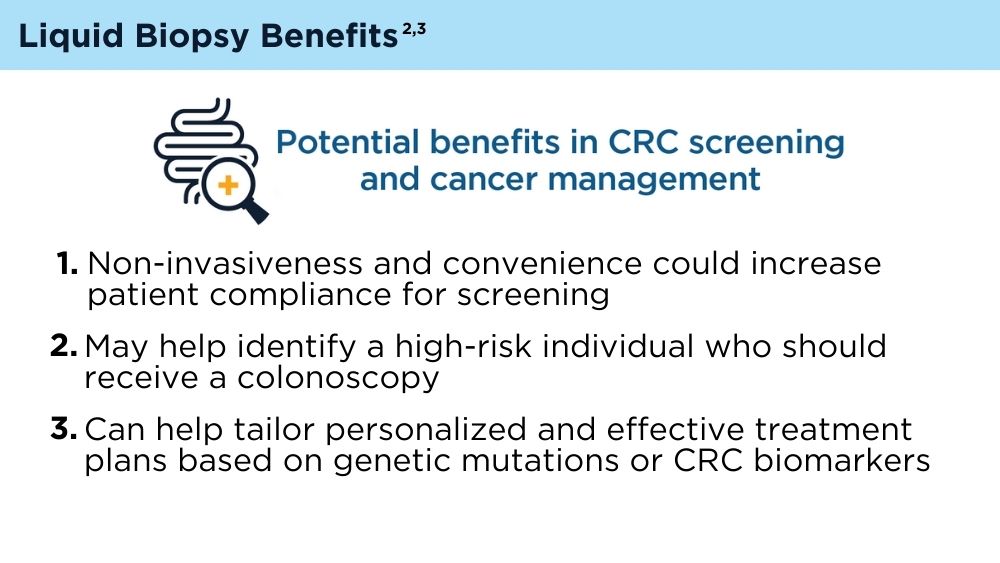
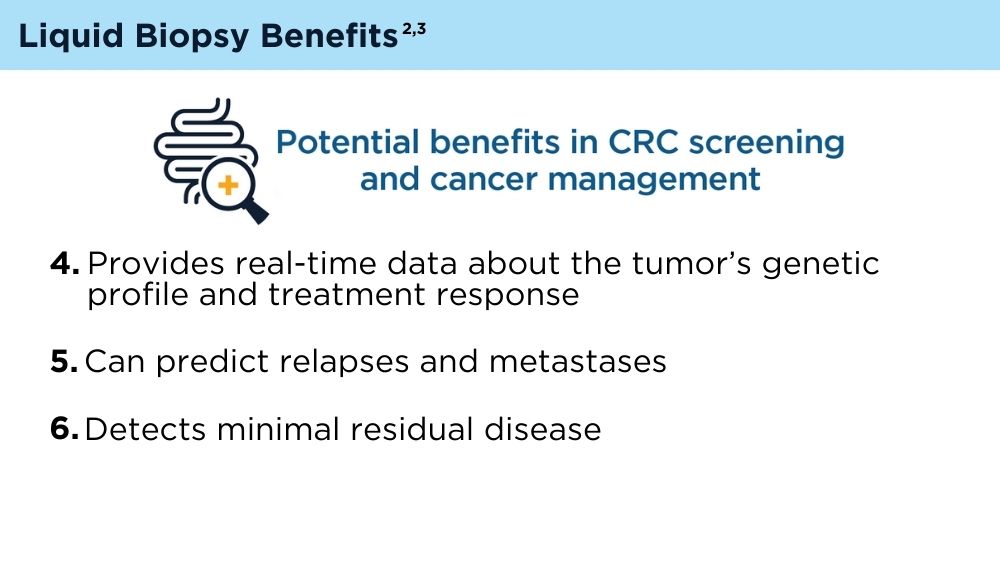
Mazouji O, Ouhajjou A, Incitti R, Mansour H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front Cell Dev Biol. 2021;9:660924. doi:10.3389/fcell.2021.660924
Vacante M, Ciuni R, Basile F, Biondi A. The liquid biopsy in the management of colorectal cancer: an overview. Biomedicines. 2020;8(9):308. doi:10.3390/biomedicines8090308
American Cancer Society. Colorectal cancer facts & figures 2020-2022. Published 2022. Accessed November 30, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
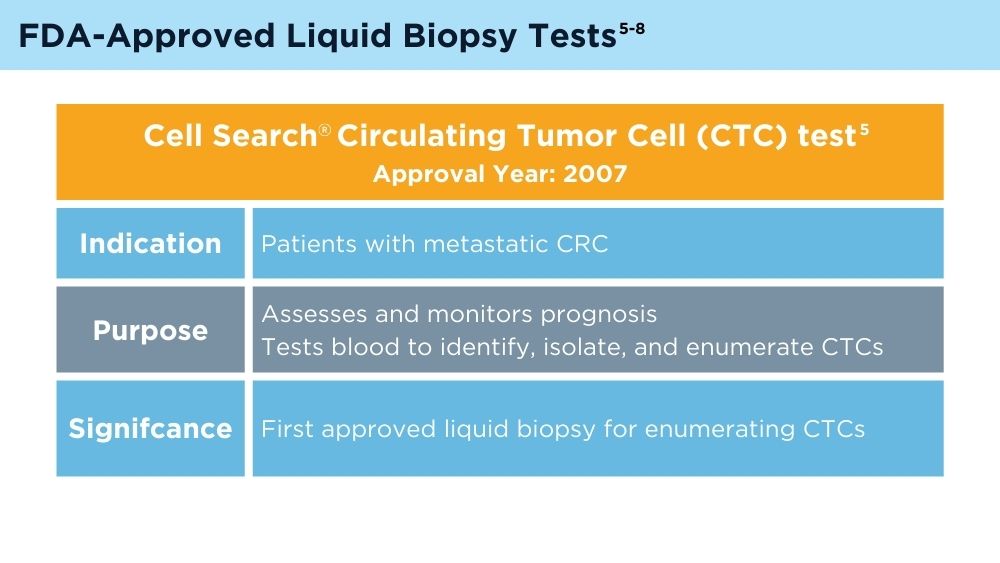
Johnson & Johnson. FDA clears Cellsearch™ circulating tumor cell test [news release]. Published February 27, 2008. Accessed November 30, 2023. https://johnsonandjohnson.gcs-web.com/news-releases/news-release-details/fda-clears-cellsearchtm-circulating-tumor-cell-test
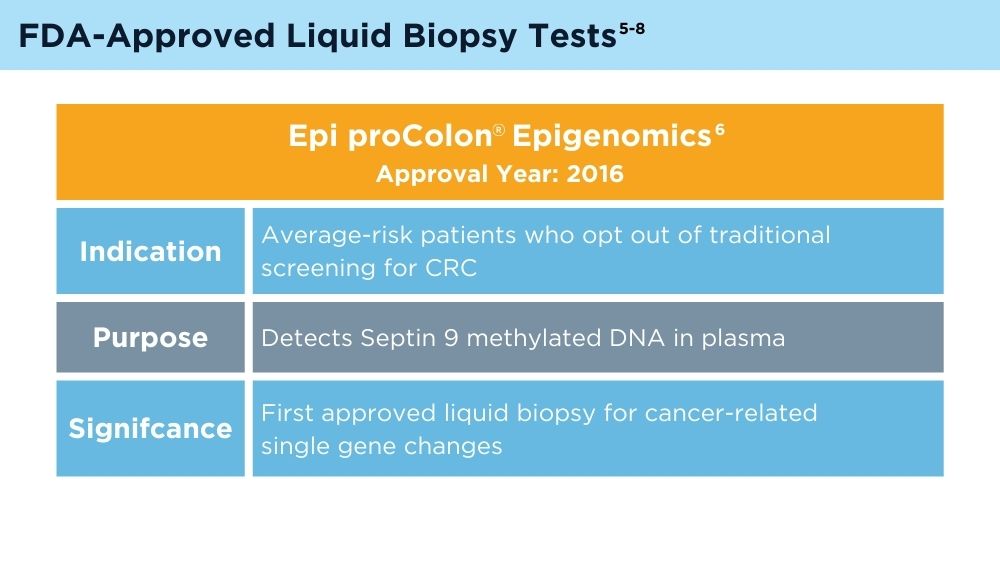
US Food and Drug Administration. Summary of safety and effectiveness data, Epi proColon®. PMA number P130001. Published April 12, 2016. Accessed November 30, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130001b.pdf
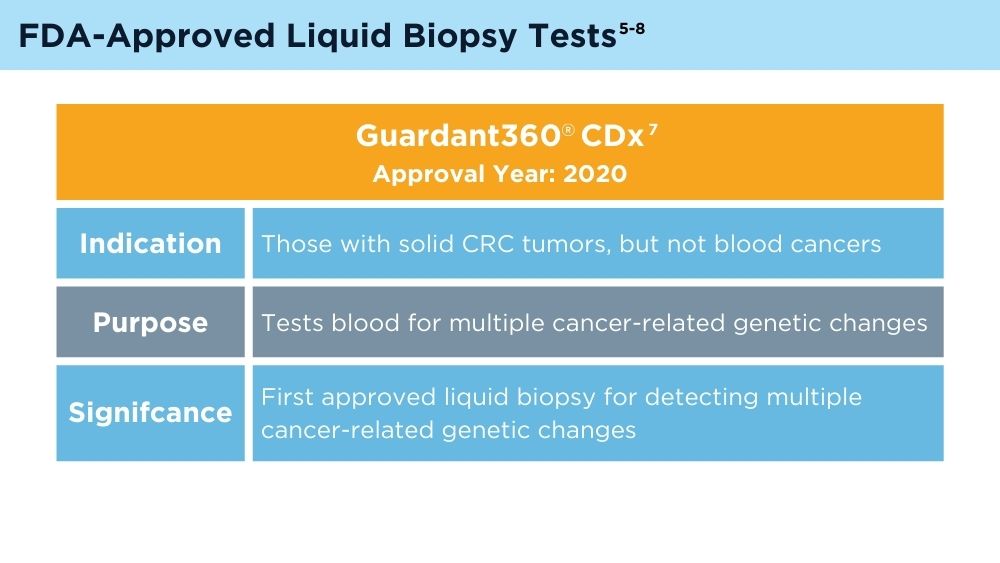
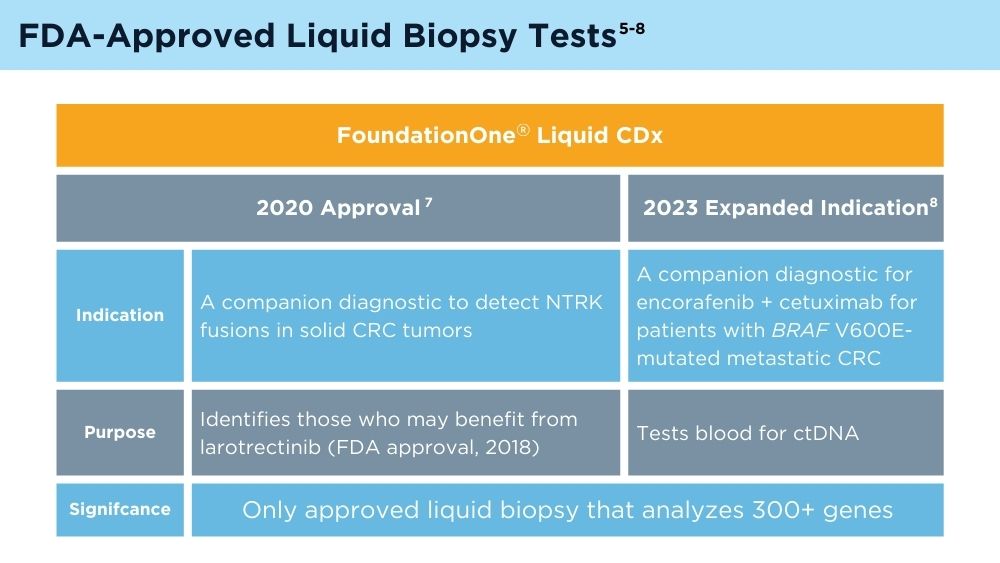
FDA approves blood tests that can help guide cancer treatment. National Institutes of Health, National Cancer Institute. Published October 15, 2020. Accessed November 30, 2023. https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy
Foundation Medicine. US Food and Drug Administration (FDA) approves FoundationOne®LiquidCDx as a companion diagnostic for Pfizer’s BRAFTOVI® (encorafenib) in combination with cetuximab to identify patients with BRAF V600E alterations in metastatic colorectal cancer [press release]. Published June 10, 2023. Accessed November 30, 2023. https://www.foundationmedicine.com/press-releases/f9b285eb-db6d-4f61-856c-3f1edb803937
Key statistics for colorectal cancer. American Cancer Society. Revised January 13, 2023. Accessed November 30, 2023. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
Mazouji O, Ouhajjou A, Incitti R, Mansour H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front Cell Dev Biol. 2021;9:660924. doi:10.3389/fcell.2021.660924
Vacante M, Ciuni R, Basile F, Biondi A. The liquid biopsy in the management of colorectal cancer: an overview. Biomedicines. 2020;8(9):308. doi:10.3390/biomedicines8090308
American Cancer Society. Colorectal cancer facts & figures 2020-2022. Published 2022. Accessed November 30, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
Johnson & Johnson. FDA clears Cellsearch™ circulating tumor cell test [news release]. Published February 27, 2008. Accessed November 30, 2023. https://johnsonandjohnson.gcs-web.com/news-releases/news-release-details/fda-clears-cellsearchtm-circulating-tumor-cell-test
US Food and Drug Administration. Summary of safety and effectiveness data, Epi proColon®. PMA number P130001. Published April 12, 2016. Accessed November 30, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130001b.pdf
FDA approves blood tests that can help guide cancer treatment. National Institutes of Health, National Cancer Institute. Published October 15, 2020. Accessed November 30, 2023. https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy
Foundation Medicine. US Food and Drug Administration (FDA) approves FoundationOne®LiquidCDx as a companion diagnostic for Pfizer’s BRAFTOVI® (encorafenib) in combination with cetuximab to identify patients with BRAF V600E alterations in metastatic colorectal cancer [press release]. Published June 10, 2023. Accessed November 30, 2023. https://www.foundationmedicine.com/press-releases/f9b285eb-db6d-4f61-856c-3f1edb803937
Key statistics for colorectal cancer. American Cancer Society. Revised January 13, 2023. Accessed November 30, 2023. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
Mazouji O, Ouhajjou A, Incitti R, Mansour H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front Cell Dev Biol. 2021;9:660924. doi:10.3389/fcell.2021.660924
Vacante M, Ciuni R, Basile F, Biondi A. The liquid biopsy in the management of colorectal cancer: an overview. Biomedicines. 2020;8(9):308. doi:10.3390/biomedicines8090308
American Cancer Society. Colorectal cancer facts & figures 2020-2022. Published 2022. Accessed November 30, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
Johnson & Johnson. FDA clears Cellsearch™ circulating tumor cell test [news release]. Published February 27, 2008. Accessed November 30, 2023. https://johnsonandjohnson.gcs-web.com/news-releases/news-release-details/fda-clears-cellsearchtm-circulating-tumor-cell-test
US Food and Drug Administration. Summary of safety and effectiveness data, Epi proColon®. PMA number P130001. Published April 12, 2016. Accessed November 30, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130001b.pdf
FDA approves blood tests that can help guide cancer treatment. National Institutes of Health, National Cancer Institute. Published October 15, 2020. Accessed November 30, 2023. https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy
Foundation Medicine. US Food and Drug Administration (FDA) approves FoundationOne®LiquidCDx as a companion diagnostic for Pfizer’s BRAFTOVI® (encorafenib) in combination with cetuximab to identify patients with BRAF V600E alterations in metastatic colorectal cancer [press release]. Published June 10, 2023. Accessed November 30, 2023. https://www.foundationmedicine.com/press-releases/f9b285eb-db6d-4f61-856c-3f1edb803937
Colorectal Cancer Is Spiking Among Some Young Americans
WASHINGTON — Despite encouraging drops in overall colorectal cancer rates in the past two decades, one group stands out as an exception: Americans younger than 45.
, according to new research presented at the annual Digestive Disease Week®.
As high as those percentages appear, the number of people affected at these ages remains small compared with rates in Americans 45 and older, said Loren Laine, MD, AGAF, professor of medicine (digestive diseases) at Yale School of Medicine, who co-moderated a news briefing discussing the research.

“The trends are alarming [but] the actual numbers of colorectal cancer cases among children and teens are not high enough to suggest widespread screening,” agreed lead investigator Islam Mohamed, MD, an internal medicine resident at the University of Missouri-Kansas City.

For example, 1 out of every 333,000 15- to-19-year-olds developed colorectal cancer in 1999. Colorectal cancer became more common by 2020, when 1 out of every 77,000 teens developed it.
At the same time, the number of cases in young adults 20 to 24 increased from less than 1 to 2 per 100,000 in 2020.
Even if the risk is relatively low in terms of absolute numbers, experts are keeping an eye on why the rates are increasing. It’s also about raising awareness. If someone younger than 45 experiences colorectal cancer symptoms like blood in their stool, stomach pain, changes in bowel habits, or others, they should seek medical attention, Dr. Laine said.
“If you have symptoms like rectal bleeding, you shouldn’t take it lightly. It’s still pretty unlikely that they’re going to have colon cancer ... but obviously you should still not totally dismiss it,” Dr. Laine said.
“Colorectal cancer is no longer considered just a disease of the elderly population,” Dr. Mohamed said during the briefing. “It’s important that the public is aware of signs and symptoms of colorectal cancer.”
Dr. Mohamed and colleagues studied colorectal cancer cases using numbers from the CDC Wonder Database, a central database of public health information. They calculated increases by comparing rates in 1999 to 2020.
Colorectal cancer is a major cause of cancer-related death in the United States. It currently ranks third in terms of new cases and cancer-related deaths once some skin cancers are excluded, American Cancer Society data indicates.
Some Risk Factors Can Be Changed
The colorectal cancer rates in younger people “have been consistently rising. It might be related to the environmental factors, lifestyle factors, and genetic factors as well,” Dr. Mohamed said. “It also might mean that we are doing better. Maybe we’re screening patients more, and maybe we’re doing a greater job of picking patients who are at high risk of colorectal cancer in the younger population.”
“Adopting a healthy lifestyle would be a great approach to curb the rising incidence of colorectal cancer as we saw metabolic syndrome is a big [factor],” Dr. Mohamed added. Patients should be encouraged to maintain a balanced diet, engage in regular physical activity, and maybe limit alcohol consumption, he said.
On the other hand, up to one third of early-onset colorectal cancer cases are linked to factors that cannot be changed. A family history of colorectal cancer, presence of inflammatory bowel disease, and certain types of cancers linked to genetic mutations are examples. “When you think about it, most of those young people [with colorectal cancer] probably have genetic syndromes,” Dr. Laine said. “The big issue is, frankly, finding better ways to identify families that have genetic syndromes. That’s probably the biggest message.”
Risk Varied by Age
In addition to the increases in the 15- to 19-year-old and 20- to 24-year-old groups, the rates in 2020 compared with 1999 showed a
- 68% increase for ages 25 to 29.
- 71% increase for ages 30 to 34.
- 58% increase for ages 35 to 39.
- 45% increase for ages 40 to 44.
“These findings all emphasize the urgent needs for public awareness and personalized screening approaches,” Dr. Mohamed said, “particularly among younger populations who had the most substantial increase in colorectal cancer incidence we observed.”
The US Preventive Services Task Force lowered the recommended age for colorectal cancer screening from 50 to 45 in 2021. Dr. Mohamed suggested more targeted screening for people under 45 at higher risk.
“I think also staying informed about the rising incidence and the latest research and recommendations in terms of colorectal cancer prevention and screening will be really, really helpful.”
A version of this article appeared on WebMD Health News.
WASHINGTON — Despite encouraging drops in overall colorectal cancer rates in the past two decades, one group stands out as an exception: Americans younger than 45.
, according to new research presented at the annual Digestive Disease Week®.
As high as those percentages appear, the number of people affected at these ages remains small compared with rates in Americans 45 and older, said Loren Laine, MD, AGAF, professor of medicine (digestive diseases) at Yale School of Medicine, who co-moderated a news briefing discussing the research.

“The trends are alarming [but] the actual numbers of colorectal cancer cases among children and teens are not high enough to suggest widespread screening,” agreed lead investigator Islam Mohamed, MD, an internal medicine resident at the University of Missouri-Kansas City.

For example, 1 out of every 333,000 15- to-19-year-olds developed colorectal cancer in 1999. Colorectal cancer became more common by 2020, when 1 out of every 77,000 teens developed it.
At the same time, the number of cases in young adults 20 to 24 increased from less than 1 to 2 per 100,000 in 2020.
Even if the risk is relatively low in terms of absolute numbers, experts are keeping an eye on why the rates are increasing. It’s also about raising awareness. If someone younger than 45 experiences colorectal cancer symptoms like blood in their stool, stomach pain, changes in bowel habits, or others, they should seek medical attention, Dr. Laine said.
“If you have symptoms like rectal bleeding, you shouldn’t take it lightly. It’s still pretty unlikely that they’re going to have colon cancer ... but obviously you should still not totally dismiss it,” Dr. Laine said.
“Colorectal cancer is no longer considered just a disease of the elderly population,” Dr. Mohamed said during the briefing. “It’s important that the public is aware of signs and symptoms of colorectal cancer.”
Dr. Mohamed and colleagues studied colorectal cancer cases using numbers from the CDC Wonder Database, a central database of public health information. They calculated increases by comparing rates in 1999 to 2020.
Colorectal cancer is a major cause of cancer-related death in the United States. It currently ranks third in terms of new cases and cancer-related deaths once some skin cancers are excluded, American Cancer Society data indicates.
Some Risk Factors Can Be Changed
The colorectal cancer rates in younger people “have been consistently rising. It might be related to the environmental factors, lifestyle factors, and genetic factors as well,” Dr. Mohamed said. “It also might mean that we are doing better. Maybe we’re screening patients more, and maybe we’re doing a greater job of picking patients who are at high risk of colorectal cancer in the younger population.”
“Adopting a healthy lifestyle would be a great approach to curb the rising incidence of colorectal cancer as we saw metabolic syndrome is a big [factor],” Dr. Mohamed added. Patients should be encouraged to maintain a balanced diet, engage in regular physical activity, and maybe limit alcohol consumption, he said.
On the other hand, up to one third of early-onset colorectal cancer cases are linked to factors that cannot be changed. A family history of colorectal cancer, presence of inflammatory bowel disease, and certain types of cancers linked to genetic mutations are examples. “When you think about it, most of those young people [with colorectal cancer] probably have genetic syndromes,” Dr. Laine said. “The big issue is, frankly, finding better ways to identify families that have genetic syndromes. That’s probably the biggest message.”
Risk Varied by Age
In addition to the increases in the 15- to 19-year-old and 20- to 24-year-old groups, the rates in 2020 compared with 1999 showed a
- 68% increase for ages 25 to 29.
- 71% increase for ages 30 to 34.
- 58% increase for ages 35 to 39.
- 45% increase for ages 40 to 44.
“These findings all emphasize the urgent needs for public awareness and personalized screening approaches,” Dr. Mohamed said, “particularly among younger populations who had the most substantial increase in colorectal cancer incidence we observed.”
The US Preventive Services Task Force lowered the recommended age for colorectal cancer screening from 50 to 45 in 2021. Dr. Mohamed suggested more targeted screening for people under 45 at higher risk.
“I think also staying informed about the rising incidence and the latest research and recommendations in terms of colorectal cancer prevention and screening will be really, really helpful.”
A version of this article appeared on WebMD Health News.
WASHINGTON — Despite encouraging drops in overall colorectal cancer rates in the past two decades, one group stands out as an exception: Americans younger than 45.
, according to new research presented at the annual Digestive Disease Week®.
As high as those percentages appear, the number of people affected at these ages remains small compared with rates in Americans 45 and older, said Loren Laine, MD, AGAF, professor of medicine (digestive diseases) at Yale School of Medicine, who co-moderated a news briefing discussing the research.

“The trends are alarming [but] the actual numbers of colorectal cancer cases among children and teens are not high enough to suggest widespread screening,” agreed lead investigator Islam Mohamed, MD, an internal medicine resident at the University of Missouri-Kansas City.

For example, 1 out of every 333,000 15- to-19-year-olds developed colorectal cancer in 1999. Colorectal cancer became more common by 2020, when 1 out of every 77,000 teens developed it.
At the same time, the number of cases in young adults 20 to 24 increased from less than 1 to 2 per 100,000 in 2020.
Even if the risk is relatively low in terms of absolute numbers, experts are keeping an eye on why the rates are increasing. It’s also about raising awareness. If someone younger than 45 experiences colorectal cancer symptoms like blood in their stool, stomach pain, changes in bowel habits, or others, they should seek medical attention, Dr. Laine said.
“If you have symptoms like rectal bleeding, you shouldn’t take it lightly. It’s still pretty unlikely that they’re going to have colon cancer ... but obviously you should still not totally dismiss it,” Dr. Laine said.
“Colorectal cancer is no longer considered just a disease of the elderly population,” Dr. Mohamed said during the briefing. “It’s important that the public is aware of signs and symptoms of colorectal cancer.”
Dr. Mohamed and colleagues studied colorectal cancer cases using numbers from the CDC Wonder Database, a central database of public health information. They calculated increases by comparing rates in 1999 to 2020.
Colorectal cancer is a major cause of cancer-related death in the United States. It currently ranks third in terms of new cases and cancer-related deaths once some skin cancers are excluded, American Cancer Society data indicates.
Some Risk Factors Can Be Changed
The colorectal cancer rates in younger people “have been consistently rising. It might be related to the environmental factors, lifestyle factors, and genetic factors as well,” Dr. Mohamed said. “It also might mean that we are doing better. Maybe we’re screening patients more, and maybe we’re doing a greater job of picking patients who are at high risk of colorectal cancer in the younger population.”
“Adopting a healthy lifestyle would be a great approach to curb the rising incidence of colorectal cancer as we saw metabolic syndrome is a big [factor],” Dr. Mohamed added. Patients should be encouraged to maintain a balanced diet, engage in regular physical activity, and maybe limit alcohol consumption, he said.
On the other hand, up to one third of early-onset colorectal cancer cases are linked to factors that cannot be changed. A family history of colorectal cancer, presence of inflammatory bowel disease, and certain types of cancers linked to genetic mutations are examples. “When you think about it, most of those young people [with colorectal cancer] probably have genetic syndromes,” Dr. Laine said. “The big issue is, frankly, finding better ways to identify families that have genetic syndromes. That’s probably the biggest message.”
Risk Varied by Age
In addition to the increases in the 15- to 19-year-old and 20- to 24-year-old groups, the rates in 2020 compared with 1999 showed a
- 68% increase for ages 25 to 29.
- 71% increase for ages 30 to 34.
- 58% increase for ages 35 to 39.
- 45% increase for ages 40 to 44.
“These findings all emphasize the urgent needs for public awareness and personalized screening approaches,” Dr. Mohamed said, “particularly among younger populations who had the most substantial increase in colorectal cancer incidence we observed.”
The US Preventive Services Task Force lowered the recommended age for colorectal cancer screening from 50 to 45 in 2021. Dr. Mohamed suggested more targeted screening for people under 45 at higher risk.
“I think also staying informed about the rising incidence and the latest research and recommendations in terms of colorectal cancer prevention and screening will be really, really helpful.”
A version of this article appeared on WebMD Health News.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>168093</fileName> <TBEID>0C050213.SIG</TBEID> <TBUniqueIdentifier>MD_0C050213</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240531T145634</QCDate> <firstPublished>20240531T151840</firstPublished> <LastPublished>20240531T151840</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240531T151840</CMSDate> <articleSource>FROM DDW 2024</articleSource> <facebookInfo/> <meetingNumber>3042-24</meetingNumber> <byline>Damian McNamara, MA</byline> <bylineText>DAMIAN MCNAMARA, MA</bylineText> <bylineFull>DAMIAN MCNAMARA, MA</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Colorectal cancer cases increased 333% among 15- to 19-year-olds and 185% among 20- to 24-year-olds from 1999 to 2020</metaDescription> <articlePDF/> <teaserImage>301485</teaserImage> <teaser>Up to one third of early-onset colorectal cancer cases are linked to factors that cannot be changed.</teaser> <title>Colorectal Cancer Is Spiking Among Some Young Americans</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>gih</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">17</term> <term>21</term> <term>15</term> <term>31</term> </publications> <sections> <term canonical="true">53</term> <term>39313</term> </sections> <topics> <term canonical="true">344</term> <term>213</term> <term>263</term> <term>67020</term> </topics> <links> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/2401295e.jpg</altRep> <description role="drol:caption">Dr. Loren Laine</description> <description role="drol:credit"/> </link> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/240129c7.jpg</altRep> <description role="drol:caption">Dr. Islam Mohamed</description> <description role="drol:credit"/> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Colorectal Cancer Is Spiking Among Some Young Americans</title> <deck/> </itemMeta> <itemContent> <p>WASHINGTON — Despite encouraging drops in overall colorectal cancer rates in the past two decades, one group stands out as an exception: Americans younger than 45. </p> <p><span class="tag metaDescription">Colorectal cancer cases increased 333% among 15- to 19-year-olds and 185% among 20- to 24-year-olds from 1999 to 2020</span>, according to new research presented at the annual Digestive Disease Week<sup>®</sup>. <br/><br/>As high as those percentages appear, the number of people affected at these ages remains small compared with rates in Americans 45 and older, said Loren Laine, MD, AGAF, professor of medicine (digestive diseases) at Yale School of Medicine, who co-moderated a news briefing discussing the research.[[{"fid":"301485","view_mode":"medstat_image_flush_left","fields":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Dr. Loren Laine, chief of the section of digestive diseases, internal medicine, and medical chief, digestive health, Yale School of Medicine, New Haven, Connecticut","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][value]":"Dr. Loren Laine"},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_left"}}]]<br/><br/>“The trends are alarming [but] the actual numbers of colorectal cancer cases among children and teens are not high enough to suggest widespread screening,” agreed lead investigator Islam Mohamed, MD, an internal medicine resident at the University of Missouri-Kansas City.[[{"fid":"301707","view_mode":"medstat_image_flush_right","fields":{"format":"medstat_image_flush_right","field_file_image_alt_text[und][0][value]":"Dr. Islam Mohamed, internal medicine resident at the University of Missouri-Kansas City","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][value]":"Dr. Islam Mohamed"},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_right"}}]]<br/><br/>For example, 1 out of every 333,000 15- to-19-year-olds developed colorectal cancer in 1999. Colorectal cancer became more common by 2020, when 1 out of every 77,000 teens developed it.<br/><br/>At the same time, the number of cases in young adults 20 to 24 increased from less than 1 to 2 per 100,000 in 2020. <br/><br/>Even if the risk is relatively low in terms of absolute numbers, experts are keeping an eye on why the rates are increasing. It’s also about raising awareness. If someone younger than 45 experiences colorectal cancer symptoms like blood in their stool, stomach pain, changes in bowel habits, or others, they should seek medical attention, Dr. Laine said.<br/><br/>“If you have symptoms like rectal bleeding, you shouldn’t take it lightly. It’s still pretty unlikely that they’re going to have colon cancer ... but obviously you should still not totally dismiss it,” Dr. Laine said. <br/><br/>“Colorectal cancer is no longer considered just a disease of the elderly population,” Dr. Mohamed said during the briefing. “It’s important that the public is aware of signs and symptoms of colorectal cancer.”<br/><br/>Dr. Mohamed and colleagues studied colorectal cancer cases using numbers from the <a href="https://wonder.cdc.gov/">CDC Wonder Database</a>, a central database of public health information. They calculated increases by comparing rates in 1999 to 2020. <br/><br/>Colorectal cancer is a major cause of cancer-related death in the United States. It currently <a href="https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html">ranks third</a> in terms of new cases and cancer-related deaths once some skin cancers are excluded, American Cancer Society data indicates.<br/><br/></p> <h2>Some Risk Factors Can Be Changed</h2> <p>The colorectal cancer rates in younger people “have been consistently rising. It might be related to the environmental factors, lifestyle factors, and genetic factors as well,” Dr. Mohamed said. “It also might mean that we are doing better. Maybe we’re screening patients more, and maybe we’re doing a greater job of picking patients who are at high risk of colorectal cancer in the younger population.” </p> <p>“Adopting a healthy lifestyle would be a great approach to curb the rising incidence of colorectal cancer as we saw metabolic syndrome is a big [factor],” Dr. Mohamed added. Patients should be encouraged to maintain a balanced diet, engage in regular physical activity, and maybe limit alcohol consumption, he said.<br/><br/>On the other hand, up to one third of early-onset colorectal cancer cases are linked to factors that cannot be changed. A family history of colorectal cancer, presence of inflammatory bowel disease, and certain types of cancers linked to genetic mutations are examples. “When you think about it, most of those young people [with colorectal cancer] probably have genetic syndromes,” Dr. Laine said. “The big issue is, frankly, finding better ways to identify families that have genetic syndromes. That’s probably the biggest message.”<br/><br/></p> <h2>Risk Varied by Age</h2> <p>In addition to the increases in the 15- to 19-year-old and 20- to 24-year-old groups, the rates in 2020 compared with 1999 showed a</p> <ul class="body"> <li>68% increase for ages 25 to 29.</li> <li>71% increase for ages 30 to 34.</li> <li>58% increase for ages 35 to 39.</li> <li>45% increase for ages 40 to 44.</li> </ul> <p>“These findings all emphasize the urgent needs for public awareness and personalized screening approaches,” Dr. Mohamed said, “particularly among younger populations who had the most substantial increase in colorectal cancer incidence we observed.”</p> <p>The US Preventive Services Task Force lowered the recommended age for colorectal cancer screening <a href="https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening">from 50 to 45</a> in 2021. Dr. Mohamed suggested more targeted screening for people under 45 at higher risk. <br/><br/>“I think also staying informed about the rising incidence and the latest research and recommendations in terms of colorectal cancer prevention and screening will be really, really helpful.”<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.webmd.com/colorectal-cancer/news/20240515/behind-the-spike-in-colorectal-cancer-cases">WebMD Health News</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM DDW 2024
Mailed Outreach for CRC Screening Appeals Across Races and Ethnicities
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
In a comparison of four outreach approaches, sending a FIT kit to people between the ages of 45 and 49 via mail garnered better response rates than opt-in strategies to participate in FIT, inviting them to undergo colonoscopy, or asking them to choose between FIT or colonoscopy. At the same time, when given a choice between colonoscopy and FIT, colonoscopy was preferred across all racial and ethnic groups.
“It is well known that colorectal cancer is the second-leading cause of cancer-related deaths in the United States. The good news is that for the past several decades, we’ve seen a decline in colorectal cancer incidence and mortality in ages 50 and above. However, there has been a recent rise in incidence and mortality in people younger than 50,” said lead author Rebecca Ekeanyanwu, a third-year medical student at Meharry Medical College School of Medicine in Nashville, Tennessee. She was awarded the 2024 AGA Institute Council Healthcare Disparities Research Award for the top oral presentation for research in racial and ethnic health care disparities.
CRC incidence, screening rates, and mortality also vary by race and ethnicity, with higher incidence and mortality rates seen among non-Hispanic Black patients, more late-stage diagnoses among Hispanic patients, and lower screening rates among Asian patients.
“There’s no formal guidance on how to screen the population under age 50,” she said. “With the disparities in race and ethnicity, it remains unclear what would be the best population health strategy to optimize colorectal screening participation in young minorities.”
Ms. Ekeanyanwu and colleagues conducted a subanalysis of a 2022 randomized controlled trial at the University of California, Los Angeles, that looked at screening strategies for average-risk patients between ages 45 and 49. The study population included patients who were assigned to a primary care provider in the UCLA Health system and had active electronic portal use and excluded those with a personal or family history of adenoma or CRC, history of IBD or gastrointestinal cancer, and a prior FIT or colonoscopy.
In this study, the research team focused on the completion of any CRC screening at 26 weeks, stratified by race and ethnicity. They included four outreach scenarios: FIT invitation, colonoscopy invitation, a choice between FIT or colonoscopy invitation, or a default mailed FIT kit, which served as the control and typically is sent to UCLA patients overdue for screening among ages 50 and older. The researchers sent letters via US Postal Service and the online patient portal, as well as two texts about CRC screening.
Among 20,509 patients, 8918 were White (43.5%), 2757 were Hispanic (13.4%), 2613 were Asian (12.7%), and 797 were Black (3.9%).
The overall screening participation rate was 18.6%, with the lowest percentage among Black participants at 16.7% and the highest among Asian participants at 23.8%. These numbers varied significantly from the 20% seen among both White and Hispanic participants.
The default mailed outreach approach had the highest uptake with higher screening rates, at 26.2% overall, and had the highest participation in each racial and ethnic group. The rates were 28.7% among White patients, 20.1% among Black patients, 27.5% among Hispanic patients, and 31% among Asian patients.
Participation was lowest among the colonoscopy invitation group — as well as for White (14.8%), Hispanic (16%), and Asian (19.3%) patients. Among Black patients, participation was lowest in the FIT invitation group (12.8%).
Notably, in the choice group, more participants chose colonoscopy above FIT — across all racial and ethnic groups — at 12.1% versus 5.6% overall. In addition, among both FIT groups, there was significant crossover to colonoscopy, with about 7%-14% among the racial and ethnic groups preferring colonoscopy.
Ms. Ekeanyanwu noted the study may be limited by variations in sample size by race and ethnicity, as well as the socioeconomic status of typical patients at UCLA, who tend to fall in middle class and affluent groups. Demographic and socioeconomic factors may play a part in patients’ decision to get screened, she noted.
Patient participation in the digital portal may affect response rates as well, said Benjamin Lebwohl, MD, AGAF, an associate professor of medicine and epidemiology at Columbia University Medical Center, New York, who moderated the DDW session titled Reducing the Burden of GI Cancers Through Early Interventions.

“At least at my institution, we have a large number of such patients [not on the digital portal] who tend to be of lower socioeconomic status and tend to be at higher risk of not getting screened,” Dr. Lebwohl said. It would be important to consider “those who might need this intervention the most.”
Ms. Ekeanyanwu declared no relevant disclosures.
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
In a comparison of four outreach approaches, sending a FIT kit to people between the ages of 45 and 49 via mail garnered better response rates than opt-in strategies to participate in FIT, inviting them to undergo colonoscopy, or asking them to choose between FIT or colonoscopy. At the same time, when given a choice between colonoscopy and FIT, colonoscopy was preferred across all racial and ethnic groups.
“It is well known that colorectal cancer is the second-leading cause of cancer-related deaths in the United States. The good news is that for the past several decades, we’ve seen a decline in colorectal cancer incidence and mortality in ages 50 and above. However, there has been a recent rise in incidence and mortality in people younger than 50,” said lead author Rebecca Ekeanyanwu, a third-year medical student at Meharry Medical College School of Medicine in Nashville, Tennessee. She was awarded the 2024 AGA Institute Council Healthcare Disparities Research Award for the top oral presentation for research in racial and ethnic health care disparities.
CRC incidence, screening rates, and mortality also vary by race and ethnicity, with higher incidence and mortality rates seen among non-Hispanic Black patients, more late-stage diagnoses among Hispanic patients, and lower screening rates among Asian patients.
“There’s no formal guidance on how to screen the population under age 50,” she said. “With the disparities in race and ethnicity, it remains unclear what would be the best population health strategy to optimize colorectal screening participation in young minorities.”
Ms. Ekeanyanwu and colleagues conducted a subanalysis of a 2022 randomized controlled trial at the University of California, Los Angeles, that looked at screening strategies for average-risk patients between ages 45 and 49. The study population included patients who were assigned to a primary care provider in the UCLA Health system and had active electronic portal use and excluded those with a personal or family history of adenoma or CRC, history of IBD or gastrointestinal cancer, and a prior FIT or colonoscopy.
In this study, the research team focused on the completion of any CRC screening at 26 weeks, stratified by race and ethnicity. They included four outreach scenarios: FIT invitation, colonoscopy invitation, a choice between FIT or colonoscopy invitation, or a default mailed FIT kit, which served as the control and typically is sent to UCLA patients overdue for screening among ages 50 and older. The researchers sent letters via US Postal Service and the online patient portal, as well as two texts about CRC screening.
Among 20,509 patients, 8918 were White (43.5%), 2757 were Hispanic (13.4%), 2613 were Asian (12.7%), and 797 were Black (3.9%).
The overall screening participation rate was 18.6%, with the lowest percentage among Black participants at 16.7% and the highest among Asian participants at 23.8%. These numbers varied significantly from the 20% seen among both White and Hispanic participants.
The default mailed outreach approach had the highest uptake with higher screening rates, at 26.2% overall, and had the highest participation in each racial and ethnic group. The rates were 28.7% among White patients, 20.1% among Black patients, 27.5% among Hispanic patients, and 31% among Asian patients.
Participation was lowest among the colonoscopy invitation group — as well as for White (14.8%), Hispanic (16%), and Asian (19.3%) patients. Among Black patients, participation was lowest in the FIT invitation group (12.8%).
Notably, in the choice group, more participants chose colonoscopy above FIT — across all racial and ethnic groups — at 12.1% versus 5.6% overall. In addition, among both FIT groups, there was significant crossover to colonoscopy, with about 7%-14% among the racial and ethnic groups preferring colonoscopy.
Ms. Ekeanyanwu noted the study may be limited by variations in sample size by race and ethnicity, as well as the socioeconomic status of typical patients at UCLA, who tend to fall in middle class and affluent groups. Demographic and socioeconomic factors may play a part in patients’ decision to get screened, she noted.
Patient participation in the digital portal may affect response rates as well, said Benjamin Lebwohl, MD, AGAF, an associate professor of medicine and epidemiology at Columbia University Medical Center, New York, who moderated the DDW session titled Reducing the Burden of GI Cancers Through Early Interventions.

“At least at my institution, we have a large number of such patients [not on the digital portal] who tend to be of lower socioeconomic status and tend to be at higher risk of not getting screened,” Dr. Lebwohl said. It would be important to consider “those who might need this intervention the most.”
Ms. Ekeanyanwu declared no relevant disclosures.
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
In a comparison of four outreach approaches, sending a FIT kit to people between the ages of 45 and 49 via mail garnered better response rates than opt-in strategies to participate in FIT, inviting them to undergo colonoscopy, or asking them to choose between FIT or colonoscopy. At the same time, when given a choice between colonoscopy and FIT, colonoscopy was preferred across all racial and ethnic groups.
“It is well known that colorectal cancer is the second-leading cause of cancer-related deaths in the United States. The good news is that for the past several decades, we’ve seen a decline in colorectal cancer incidence and mortality in ages 50 and above. However, there has been a recent rise in incidence and mortality in people younger than 50,” said lead author Rebecca Ekeanyanwu, a third-year medical student at Meharry Medical College School of Medicine in Nashville, Tennessee. She was awarded the 2024 AGA Institute Council Healthcare Disparities Research Award for the top oral presentation for research in racial and ethnic health care disparities.
CRC incidence, screening rates, and mortality also vary by race and ethnicity, with higher incidence and mortality rates seen among non-Hispanic Black patients, more late-stage diagnoses among Hispanic patients, and lower screening rates among Asian patients.
“There’s no formal guidance on how to screen the population under age 50,” she said. “With the disparities in race and ethnicity, it remains unclear what would be the best population health strategy to optimize colorectal screening participation in young minorities.”
Ms. Ekeanyanwu and colleagues conducted a subanalysis of a 2022 randomized controlled trial at the University of California, Los Angeles, that looked at screening strategies for average-risk patients between ages 45 and 49. The study population included patients who were assigned to a primary care provider in the UCLA Health system and had active electronic portal use and excluded those with a personal or family history of adenoma or CRC, history of IBD or gastrointestinal cancer, and a prior FIT or colonoscopy.
In this study, the research team focused on the completion of any CRC screening at 26 weeks, stratified by race and ethnicity. They included four outreach scenarios: FIT invitation, colonoscopy invitation, a choice between FIT or colonoscopy invitation, or a default mailed FIT kit, which served as the control and typically is sent to UCLA patients overdue for screening among ages 50 and older. The researchers sent letters via US Postal Service and the online patient portal, as well as two texts about CRC screening.
Among 20,509 patients, 8918 were White (43.5%), 2757 were Hispanic (13.4%), 2613 were Asian (12.7%), and 797 were Black (3.9%).
The overall screening participation rate was 18.6%, with the lowest percentage among Black participants at 16.7% and the highest among Asian participants at 23.8%. These numbers varied significantly from the 20% seen among both White and Hispanic participants.
The default mailed outreach approach had the highest uptake with higher screening rates, at 26.2% overall, and had the highest participation in each racial and ethnic group. The rates were 28.7% among White patients, 20.1% among Black patients, 27.5% among Hispanic patients, and 31% among Asian patients.
Participation was lowest among the colonoscopy invitation group — as well as for White (14.8%), Hispanic (16%), and Asian (19.3%) patients. Among Black patients, participation was lowest in the FIT invitation group (12.8%).
Notably, in the choice group, more participants chose colonoscopy above FIT — across all racial and ethnic groups — at 12.1% versus 5.6% overall. In addition, among both FIT groups, there was significant crossover to colonoscopy, with about 7%-14% among the racial and ethnic groups preferring colonoscopy.
Ms. Ekeanyanwu noted the study may be limited by variations in sample size by race and ethnicity, as well as the socioeconomic status of typical patients at UCLA, who tend to fall in middle class and affluent groups. Demographic and socioeconomic factors may play a part in patients’ decision to get screened, she noted.
Patient participation in the digital portal may affect response rates as well, said Benjamin Lebwohl, MD, AGAF, an associate professor of medicine and epidemiology at Columbia University Medical Center, New York, who moderated the DDW session titled Reducing the Burden of GI Cancers Through Early Interventions.

“At least at my institution, we have a large number of such patients [not on the digital portal] who tend to be of lower socioeconomic status and tend to be at higher risk of not getting screened,” Dr. Lebwohl said. It would be important to consider “those who might need this intervention the most.”
Ms. Ekeanyanwu declared no relevant disclosures.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>168250</fileName> <TBEID>0C0505A6.SIG</TBEID> <TBUniqueIdentifier>MD_0C0505A6</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname>DDW mtg story</storyname> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240531T115122</QCDate> <firstPublished>20240531T120803</firstPublished> <LastPublished>20240531T120803</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240531T120803</CMSDate> <articleSource>FROM DDW 2024</articleSource> <facebookInfo/> <meetingNumber>3042-24</meetingNumber> <byline>Carolyn Crist</byline> <bylineText>CAROLYN CRIST</bylineText> <bylineFull>CAROLYN CRIST</bylineFull> <bylineTitleText>MDedge News</bylineTitleText> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Mailing outreach notices for colonoscopies or fecal immunochemical test (FIT) kits may be a great way to increase colorectal cancer (CRC) screening in younger a</metaDescription> <articlePDF/> <teaserImage>301700</teaserImage> <teaser>More participants chose colonoscopy above FIT — across all racial and ethnic groups — at 12.1% versus 5.6% overall.</teaser> <title>Mailed Outreach for CRC Screening Appeals Across Races and Ethnicities</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>gih</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">17</term> <term>21</term> <term>15</term> <term>31</term> </publications> <sections> <term canonical="true">53</term> <term>39313</term> </sections> <topics> <term canonical="true">344</term> <term>213</term> <term>263</term> <term>280</term> <term>66772</term> <term>67020</term> </topics> <links> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/240129c6.jpg</altRep> <description role="drol:caption">Ms. Rebecca Ekeanyanwu</description> <description role="drol:credit"/> </link> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/2400c9da.jpg</altRep> <description role="drol:caption">Dr. Benjamin Lebwohl</description> <description role="drol:credit"/> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Mailed Outreach for CRC Screening Appeals Across Races and Ethnicities</title> <deck/> </itemMeta> <itemContent> <p><span class="dateline">WASHINGTON —</span> <span class="tag metaDescription">Mailing outreach notices for colonoscopies or fecal immunochemical test (FIT) kits may be a great way to increase colorectal cancer (CRC) screening in younger adults</span>, according to a study presented at the annual Digestive Disease Week<sup>®</sup> (DDW).</p> <p>In a comparison of four outreach approaches, sending a FIT kit to people between the ages of 45 and 49 via mail garnered better response rates than opt-in strategies to participate in FIT, inviting them to undergo colonoscopy, or asking them to choose between FIT or colonoscopy. At the same time, when given a choice between colonoscopy and FIT, colonoscopy was preferred across all racial and ethnic groups.<br/><br/>“It is well known that colorectal cancer is the second-leading cause of cancer-related deaths in the United States. The good news is that for the past several decades, we’ve seen a decline in colorectal cancer incidence and mortality in ages 50 and above. However, there has been a recent rise in incidence and mortality in people younger than 50,” said lead author Rebecca Ekeanyanwu, a third-year medical student at Meharry Medical College School of Medicine in Nashville, Tennessee. She was awarded the <span class="Hyperlink"><a href="https://gastro.org/news/three-award-winning-abstracts-to-see-at-ddw/">2024 AGA Institute Council Healthcare Disparities Research Award</a></span> for the top oral presentation for research in racial and ethnic health care disparities.[[{"fid":"301700","view_mode":"medstat_image_flush_right","fields":{"format":"medstat_image_flush_right","field_file_image_alt_text[und][0][value]":"Ms. Rebecca Ekeanyanwu, third-year medical student at Meharry Medical College School of Medicine in Nashville, Tennessee","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][value]":"Ms. Rebecca Ekeanyanwu"},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_right"}}]]<br/><br/>CRC incidence, screening rates, and mortality also vary by race and ethnicity, with higher incidence and mortality rates seen among non-Hispanic Black patients, more late-stage diagnoses among Hispanic patients, and lower screening rates among Asian patients. <br/><br/>“There’s no formal guidance on how to screen the population under age 50,” she said. “With the disparities in race and ethnicity, it remains unclear what would be the best population health strategy to optimize colorectal screening participation in young minorities.”<br/><br/>Ms. Ekeanyanwu and colleagues conducted a subanalysis of a 2022 randomized controlled trial at the University of California, Los Angeles, that looked at screening strategies for average-risk patients between ages 45 and 49. The study population included patients who were assigned to a primary care provider in the UCLA Health system and had active electronic portal use and excluded those with a personal or family history of adenoma or CRC, history of IBD or gastrointestinal cancer, and a prior FIT or colonoscopy.<br/><br/>In this study, the research team focused on the completion of any CRC screening at 26 weeks, stratified by race and ethnicity. They included four outreach scenarios: FIT invitation, colonoscopy invitation, a choice between FIT or colonoscopy invitation, or a default mailed FIT kit, which served as the control and typically is sent to UCLA patients overdue for screening among ages 50 and older. The researchers sent letters via US Postal Service and the online patient portal, as well as two texts about CRC screening.<br/><br/>Among 20,509 patients, 8918 were White (43.5%), 2757 were Hispanic (13.4%), 2613 were Asian (12.7%), and 797 were Black (3.9%).<br/><br/>The overall screening participation rate was 18.6%, with the lowest percentage among Black participants at 16.7% and the highest among Asian participants at 23.8%. These numbers varied significantly from the 20% seen among both White and Hispanic participants.<br/><br/>The default mailed outreach approach had the highest uptake with higher screening rates, at 26.2% overall, and had the highest participation in each racial and ethnic group. The rates were 28.7% among White patients, 20.1% among Black patients, 27.5% among Hispanic patients, and 31% among Asian patients.<br/><br/>Participation was lowest among the colonoscopy invitation group — as well as for White (14.8%), Hispanic (16%), and Asian (19.3%) patients. Among Black patients, participation was lowest in the FIT invitation group (12.8%).<br/><br/>Notably, in the choice group, more participants chose colonoscopy above FIT — across all racial and ethnic groups — at 12.1% versus 5.6% overall. In addition, among both FIT groups, there was significant crossover to colonoscopy, with about 7%-14% among the racial and ethnic groups preferring colonoscopy.<br/><br/>Ms. Ekeanyanwu noted the study may be limited by variations in sample size by race and ethnicity, as well as the socioeconomic status of typical patients at UCLA, who tend to fall in middle class and affluent groups. Demographic and socioeconomic factors may play a part in patients’ decision to get screened, she noted. <br/><br/>Patient participation in the digital portal may affect response rates as well, said Benjamin Lebwohl, MD, AGAF, an associate professor of medicine and epidemiology at Columbia University Medical Center, New York, who moderated the DDW session titled Reducing the Burden of GI Cancers Through Early Interventions.[[{"fid":"250239","view_mode":"medstat_image_flush_right","fields":{"format":"medstat_image_flush_right","field_file_image_alt_text[und][0][value]":"Dr. Benjamin Lebwohl, Columbia University, New York","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][value]":"Dr. Benjamin Lebwohl"},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_right"}}]]<br/><br/>“At least at my institution, we have a large number of such patients [not on the digital portal] who tend to be of lower socioeconomic status and tend to be at higher risk of not getting screened,” Dr. Lebwohl said. It would be important to consider “those who might need this intervention the most.”<br/><br/>Ms. Ekeanyanwu declared no relevant disclosures.<span class="end"/></p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM DDW 2024
FDA OKs First Multitarget Stool RNA Test for CRC Screening
ColoSense, which had breakthrough device designation by the FDA, detects colorectal neoplasia–associated RNA markers and the presence of occult hemoglobin in human stool.
A positive ColoSense test result may indicate the presence of CRC, advanced adenomas, or serrated precancerous lesions and should be followed by a colonoscopy, the company said in a news release.
The FDA approval was based on results of the CRC-PREVENT trial, which evaluated the ColoSense mt-sRNA test in a diverse group of adults undergoing colonoscopy. 
The mt-sRNA test results were compared with the colonoscopy results.
Among all average-risk individuals, the sensitivity of the mt-sRNA test was 93% for CRC, 100% for early (stage I) CRC, and 45% for advanced adenomas. In a subgroup of those aged 45-49 years, the sensitivity was 100% for CRC and 44% for advanced adenomas.
The trial results were presented last year at the American College of Gastroenterology annual meeting and simultaneously published in JAMA .
CRC is the second deadliest cancer in the United States, and adherence rates to recommended colonoscopies as a screening modality have remained consistently low at roughly 60%.
Cases of CRC are also rising among people younger than age 50 years, leading the United States Preventive Services Task Force to recommend initiation of CRC screening at age 45 years.
“The growing number of adults diagnosed with colorectal cancer underscores the urgent need for innovative approaches in screening. It’s essential to eliminate obstacles and broaden the availability of screening methods for healthcare providers and patients,” Anjee Davis, president of Fight CRC, said in the news release.
“We hope that introducing new FDA-approved diagnostic tools, including stool-based tests like ColoSense, will help to advance access and increase screening rates, ultimately reducing the impact of late-stage colorectal cancer diagnoses,” Ms. Davis said.
The company plans to make ColoSense available in the United States later this year or early in 2025.
A version of this article appeared on Medscape.com.
ColoSense, which had breakthrough device designation by the FDA, detects colorectal neoplasia–associated RNA markers and the presence of occult hemoglobin in human stool.
A positive ColoSense test result may indicate the presence of CRC, advanced adenomas, or serrated precancerous lesions and should be followed by a colonoscopy, the company said in a news release.
The FDA approval was based on results of the CRC-PREVENT trial, which evaluated the ColoSense mt-sRNA test in a diverse group of adults undergoing colonoscopy. 
The mt-sRNA test results were compared with the colonoscopy results.
Among all average-risk individuals, the sensitivity of the mt-sRNA test was 93% for CRC, 100% for early (stage I) CRC, and 45% for advanced adenomas. In a subgroup of those aged 45-49 years, the sensitivity was 100% for CRC and 44% for advanced adenomas.
The trial results were presented last year at the American College of Gastroenterology annual meeting and simultaneously published in JAMA .
CRC is the second deadliest cancer in the United States, and adherence rates to recommended colonoscopies as a screening modality have remained consistently low at roughly 60%.
Cases of CRC are also rising among people younger than age 50 years, leading the United States Preventive Services Task Force to recommend initiation of CRC screening at age 45 years.
“The growing number of adults diagnosed with colorectal cancer underscores the urgent need for innovative approaches in screening. It’s essential to eliminate obstacles and broaden the availability of screening methods for healthcare providers and patients,” Anjee Davis, president of Fight CRC, said in the news release.
“We hope that introducing new FDA-approved diagnostic tools, including stool-based tests like ColoSense, will help to advance access and increase screening rates, ultimately reducing the impact of late-stage colorectal cancer diagnoses,” Ms. Davis said.
The company plans to make ColoSense available in the United States later this year or early in 2025.
A version of this article appeared on Medscape.com.
ColoSense, which had breakthrough device designation by the FDA, detects colorectal neoplasia–associated RNA markers and the presence of occult hemoglobin in human stool.
A positive ColoSense test result may indicate the presence of CRC, advanced adenomas, or serrated precancerous lesions and should be followed by a colonoscopy, the company said in a news release.
The FDA approval was based on results of the CRC-PREVENT trial, which evaluated the ColoSense mt-sRNA test in a diverse group of adults undergoing colonoscopy. 
The mt-sRNA test results were compared with the colonoscopy results.
Among all average-risk individuals, the sensitivity of the mt-sRNA test was 93% for CRC, 100% for early (stage I) CRC, and 45% for advanced adenomas. In a subgroup of those aged 45-49 years, the sensitivity was 100% for CRC and 44% for advanced adenomas.
The trial results were presented last year at the American College of Gastroenterology annual meeting and simultaneously published in JAMA .
CRC is the second deadliest cancer in the United States, and adherence rates to recommended colonoscopies as a screening modality have remained consistently low at roughly 60%.
Cases of CRC are also rising among people younger than age 50 years, leading the United States Preventive Services Task Force to recommend initiation of CRC screening at age 45 years.
“The growing number of adults diagnosed with colorectal cancer underscores the urgent need for innovative approaches in screening. It’s essential to eliminate obstacles and broaden the availability of screening methods for healthcare providers and patients,” Anjee Davis, president of Fight CRC, said in the news release.
“We hope that introducing new FDA-approved diagnostic tools, including stool-based tests like ColoSense, will help to advance access and increase screening rates, ultimately reducing the impact of late-stage colorectal cancer diagnoses,” Ms. Davis said.
The company plans to make ColoSense available in the United States later this year or early in 2025.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167987</fileName> <TBEID>0C04FFED.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FFED</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240515T111314</QCDate> <firstPublished>20240515T113135</firstPublished> <LastPublished>20240515T113135</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240515T113134</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Megan Brooks</byline> <bylineText>MEGAN BROOKS</bylineText> <bylineFull>MEGAN BROOKS</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>The US Food and Drug Administration (FDA) has approved ColoSense (Geneoscopy, Inc), a multitarget stool RNA (mt-sRNA) test for colorectal cancer (CRC) screening</metaDescription> <articlePDF/> <teaserImage>247577</teaserImage> <teaser>The FDA approval was based on results of the CRC-PREVENT trial.</teaser> <title>FDA OKs First Multitarget Stool RNA Test for CRC Screening</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>gih</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">17</term> <term>15</term> <term>21</term> <term>31</term> </publications> <sections> <term canonical="true">27979</term> <term>39313</term> </sections> <topics> <term canonical="true">344</term> <term>263</term> <term>213</term> <term>280</term> <term>67020</term> </topics> <links> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/2400c51f.jpg</altRep> <description role="drol:caption"/> <description role="drol:credit">Olivier Le Moal/Getty Images</description> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>FDA OKs First Multitarget Stool RNA Test for CRC Screening</title> <deck/> </itemMeta> <itemContent> <p> <span class="tag metaDescription">The US Food and Drug Administration (FDA) has approved ColoSense (Geneoscopy, Inc), a multitarget stool RNA (mt-sRNA) test for <span class="Hyperlink"><a href="https://emedicine.medscape.com/article/2500006-overview">colorectal cancer</a></span> (CRC) screening in adults aged 45 years or older who are at average risk for CRC.</span> </p> <p>ColoSense, which had breakthrough device designation by the FDA, detects colorectal neoplasia–associated RNA markers and the presence of occult hemoglobin in human stool. <br/><br/>A positive ColoSense test result may indicate the presence of CRC, advanced adenomas, or serrated precancerous lesions and should be followed by a <span class="Hyperlink"><a href="https://emedicine.medscape.com/article/1819350-overview">colonoscopy</a></span>, the company said in a <span class="Hyperlink"><a href="https://www.geneoscopy.com/fda-approves-colosense-geneoscopys-noninvasive-multi-target-stool-rna-mtrna-colorectal-cancer-screening-test/">news release</a></span>. <br/><br/>The FDA approval was based on results of the CRC-PREVENT trial, which evaluated the ColoSense mt-sRNA test in a diverse group of adults undergoing colonoscopy. <br/><br/>[[{"fid":"247577","view_mode":"medstat_image_flush_right","fields":{"format":"medstat_image_flush_right","field_file_image_alt_text[und][0][value]":"A stamp saying &quot;FDA approved.&quot;","field_file_image_credit[und][0][value]":"Olivier Le Moal/Getty Images","field_file_image_caption[und][0][value]":""},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_right"}}]]The mt-sRNA test results were compared with the colonoscopy results.<br/><br/>Among all average-risk individuals, the sensitivity of the mt-sRNA test was 93% for CRC, 100% for early (stage I) CRC, and 45% for advanced adenomas. In a subgroup of those aged 45-49 years, the sensitivity was 100% for CRC and 44% for advanced adenomas.<br/><br/>The trial <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/997609">results</a></span> were presented last year at the American College of Gastroenterology annual meeting and simultaneously <span class="Hyperlink"><a href="https://jamanetwork.com/journals/jama/fullarticle/2811133">published</a></span> in <em>JAMA</em> .<br/><br/>CRC is the second deadliest cancer in the United States, and adherence rates to recommended colonoscopies as a screening modality have remained consistently low at roughly 60%. <br/><br/>Cases of CRC are also rising among people younger than age 50 years, leading the United States Preventive Services Task Force to recommend initiation of CRC screening at age 45 years.<br/><br/>“The growing number of adults diagnosed with colorectal cancer underscores the urgent need for innovative approaches in screening. It’s essential to eliminate obstacles and broaden the availability of screening methods for healthcare providers and patients,” Anjee Davis, president of Fight CRC, said in the news release. <br/><br/>“We hope that introducing new FDA-approved diagnostic tools, including stool-based tests like ColoSense, will help to advance access and increase screening rates, ultimately reducing the impact of late-stage colorectal cancer diagnoses,” Ms. Davis said. <br/><br/>The company plans to make ColoSense available in the United States later this year or early in 2025.<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/fda-oks-first-multitarget-stool-rna-test-crc-screening-2024a10008tu">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Oral Microbiome Test Could Detect Gastric Cancer Earlier
WASHINGTON, DC –
Researchers found distinct bacterial composition differences in patient samples that point to the potential for oral microbial signatures to be used as biomarkers for assessing gastric cancer risk.
“Too many patients are being diagnosed too late. There are no formal screening guidelines for gastric cancer, and more than half of patients with gastric cancer do not receive a diagnosis until their cancer is already at an advanced stage,” said Shruthi Reddy Perati, MD, a general surgery resident at Rutgers University Robert Wood Johnson School of Medicine in New Brunswick, New Jersey.
Detecting gastric cancer now generally requires an invasive procedure, such as endoscopy. Therefore, a noninvasive “swish and spit” test could be more accessible and allow for more widespread screening, Dr. Perati said at a May 8 press briefing during which her research (Abstract 949) was previewed for Digestive Disease Week® (DDW).
Gastric cancer, also known as stomach cancer, is the fourth most common cause of cancer-related death in the world. The United States can expect 26,890 new cases and 10,880 deaths from this type of cancer in 2024, the American Cancer Society estimates.
Microbial Signatures Found
Dr. Perati and colleagues collected oral rinse samples from 98 patients: 30 known to have gastric cancer , 30 with precancerous gastric conditions (pre–gastric cancer), and 38 control participants without pre-gastric or gastric cancer. Sixty-two percent were women, 32% were Hispanic, 31% had diabetes, and 18% were smokers.
The researchers analyzed the samples for alpha and beta diversity and conducted differential analysis using the framework called analysis of compositions of microbiomes.
They found distinct differences between the oral microbiomes of the healthy group and those of the groups with gastric cancer and pre–gastric cancer. In addition, the microbiomes of participants with cancer and of those with precancerous conditions were similar.
The results suggest that the microbiome changes may occur as soon as the stomach environment starts to undergo changes that can eventually turn into cancer.
“The oral microbiome may serve as a window into the composition of the stomach environment,” Dr. Perati said.
The investigators created a screening model to detect the most relevant 13 bacterial genera that differed between the control group and the gastric cancer and pre–gastric cancer groups. The tenfold cross-validation model demonstrated good ability to discriminate using bacteria alone (area under the curve [AUC], 0.74) and was further improved with the addition of clinical variables, including demographics and comorbidities (AUC, 0.91), the researchers noted.
As the investigators noted, the model’s performance improved with the addition of clinical variables, said Loren Laine, MD, professor of medicine (digestive diseases) at Yale School of Medicine and chair of DDW 2024.
An AUC of 0.74 using bacteria alone, which increased to 0.91 by adding demographics and comorbidities, “[is] starting to be really meaningful,” Dr. Laine said.
Further studies should evaluate the test’s sensitivity and specificity, Dr. Laine added.
Additional Considerations
The microbiome can vary between people and within the same individual over time. Probiotics, antibiotics, and diet can lead to changes in the microbiome, Dr. Perati said.
When asked how these changes could affect the accuracy of an oral rinse test, Dr. Perati said “it’s known that, in general, dietary modifications can have an impact on the diversity and the prevalence of certain bacteria throughout the GI tract.”
Though variance is expected, we’re hoping to see that the differences in the microbiome composition between the malignant groups and the control groups are more significant than those lower-level background changes due to dietary modifications, for example, she added.
The research is in its early days, and the results need to be validated in a larger study, Dr. Perati said.
Ninety-eight patients is “still a very small number,” said Dr. Laine, who co-moderated the press briefing. “More research is needed.”
Still, the study “has huge implications that could eventually lead to the development of noninvasive and accessible early screening for gastric cancer,” she said.
Dr. Perati and Dr. Laine reported no relevant financial relationships. The study was independently supported.
A version of this article appeared on Medscape.com.
WASHINGTON, DC –
Researchers found distinct bacterial composition differences in patient samples that point to the potential for oral microbial signatures to be used as biomarkers for assessing gastric cancer risk.
“Too many patients are being diagnosed too late. There are no formal screening guidelines for gastric cancer, and more than half of patients with gastric cancer do not receive a diagnosis until their cancer is already at an advanced stage,” said Shruthi Reddy Perati, MD, a general surgery resident at Rutgers University Robert Wood Johnson School of Medicine in New Brunswick, New Jersey.
Detecting gastric cancer now generally requires an invasive procedure, such as endoscopy. Therefore, a noninvasive “swish and spit” test could be more accessible and allow for more widespread screening, Dr. Perati said at a May 8 press briefing during which her research (Abstract 949) was previewed for Digestive Disease Week® (DDW).
Gastric cancer, also known as stomach cancer, is the fourth most common cause of cancer-related death in the world. The United States can expect 26,890 new cases and 10,880 deaths from this type of cancer in 2024, the American Cancer Society estimates.
Microbial Signatures Found
Dr. Perati and colleagues collected oral rinse samples from 98 patients: 30 known to have gastric cancer , 30 with precancerous gastric conditions (pre–gastric cancer), and 38 control participants without pre-gastric or gastric cancer. Sixty-two percent were women, 32% were Hispanic, 31% had diabetes, and 18% were smokers.
The researchers analyzed the samples for alpha and beta diversity and conducted differential analysis using the framework called analysis of compositions of microbiomes.
They found distinct differences between the oral microbiomes of the healthy group and those of the groups with gastric cancer and pre–gastric cancer. In addition, the microbiomes of participants with cancer and of those with precancerous conditions were similar.
The results suggest that the microbiome changes may occur as soon as the stomach environment starts to undergo changes that can eventually turn into cancer.
“The oral microbiome may serve as a window into the composition of the stomach environment,” Dr. Perati said.
The investigators created a screening model to detect the most relevant 13 bacterial genera that differed between the control group and the gastric cancer and pre–gastric cancer groups. The tenfold cross-validation model demonstrated good ability to discriminate using bacteria alone (area under the curve [AUC], 0.74) and was further improved with the addition of clinical variables, including demographics and comorbidities (AUC, 0.91), the researchers noted.
As the investigators noted, the model’s performance improved with the addition of clinical variables, said Loren Laine, MD, professor of medicine (digestive diseases) at Yale School of Medicine and chair of DDW 2024.
An AUC of 0.74 using bacteria alone, which increased to 0.91 by adding demographics and comorbidities, “[is] starting to be really meaningful,” Dr. Laine said.
Further studies should evaluate the test’s sensitivity and specificity, Dr. Laine added.
Additional Considerations
The microbiome can vary between people and within the same individual over time. Probiotics, antibiotics, and diet can lead to changes in the microbiome, Dr. Perati said.
When asked how these changes could affect the accuracy of an oral rinse test, Dr. Perati said “it’s known that, in general, dietary modifications can have an impact on the diversity and the prevalence of certain bacteria throughout the GI tract.”
Though variance is expected, we’re hoping to see that the differences in the microbiome composition between the malignant groups and the control groups are more significant than those lower-level background changes due to dietary modifications, for example, she added.
The research is in its early days, and the results need to be validated in a larger study, Dr. Perati said.
Ninety-eight patients is “still a very small number,” said Dr. Laine, who co-moderated the press briefing. “More research is needed.”
Still, the study “has huge implications that could eventually lead to the development of noninvasive and accessible early screening for gastric cancer,” she said.
Dr. Perati and Dr. Laine reported no relevant financial relationships. The study was independently supported.
A version of this article appeared on Medscape.com.
WASHINGTON, DC –
Researchers found distinct bacterial composition differences in patient samples that point to the potential for oral microbial signatures to be used as biomarkers for assessing gastric cancer risk.
“Too many patients are being diagnosed too late. There are no formal screening guidelines for gastric cancer, and more than half of patients with gastric cancer do not receive a diagnosis until their cancer is already at an advanced stage,” said Shruthi Reddy Perati, MD, a general surgery resident at Rutgers University Robert Wood Johnson School of Medicine in New Brunswick, New Jersey.
Detecting gastric cancer now generally requires an invasive procedure, such as endoscopy. Therefore, a noninvasive “swish and spit” test could be more accessible and allow for more widespread screening, Dr. Perati said at a May 8 press briefing during which her research (Abstract 949) was previewed for Digestive Disease Week® (DDW).
Gastric cancer, also known as stomach cancer, is the fourth most common cause of cancer-related death in the world. The United States can expect 26,890 new cases and 10,880 deaths from this type of cancer in 2024, the American Cancer Society estimates.
Microbial Signatures Found
Dr. Perati and colleagues collected oral rinse samples from 98 patients: 30 known to have gastric cancer , 30 with precancerous gastric conditions (pre–gastric cancer), and 38 control participants without pre-gastric or gastric cancer. Sixty-two percent were women, 32% were Hispanic, 31% had diabetes, and 18% were smokers.
The researchers analyzed the samples for alpha and beta diversity and conducted differential analysis using the framework called analysis of compositions of microbiomes.
They found distinct differences between the oral microbiomes of the healthy group and those of the groups with gastric cancer and pre–gastric cancer. In addition, the microbiomes of participants with cancer and of those with precancerous conditions were similar.
The results suggest that the microbiome changes may occur as soon as the stomach environment starts to undergo changes that can eventually turn into cancer.
“The oral microbiome may serve as a window into the composition of the stomach environment,” Dr. Perati said.
The investigators created a screening model to detect the most relevant 13 bacterial genera that differed between the control group and the gastric cancer and pre–gastric cancer groups. The tenfold cross-validation model demonstrated good ability to discriminate using bacteria alone (area under the curve [AUC], 0.74) and was further improved with the addition of clinical variables, including demographics and comorbidities (AUC, 0.91), the researchers noted.
As the investigators noted, the model’s performance improved with the addition of clinical variables, said Loren Laine, MD, professor of medicine (digestive diseases) at Yale School of Medicine and chair of DDW 2024.
An AUC of 0.74 using bacteria alone, which increased to 0.91 by adding demographics and comorbidities, “[is] starting to be really meaningful,” Dr. Laine said.
Further studies should evaluate the test’s sensitivity and specificity, Dr. Laine added.
Additional Considerations
The microbiome can vary between people and within the same individual over time. Probiotics, antibiotics, and diet can lead to changes in the microbiome, Dr. Perati said.
When asked how these changes could affect the accuracy of an oral rinse test, Dr. Perati said “it’s known that, in general, dietary modifications can have an impact on the diversity and the prevalence of certain bacteria throughout the GI tract.”
Though variance is expected, we’re hoping to see that the differences in the microbiome composition between the malignant groups and the control groups are more significant than those lower-level background changes due to dietary modifications, for example, she added.
The research is in its early days, and the results need to be validated in a larger study, Dr. Perati said.
Ninety-eight patients is “still a very small number,” said Dr. Laine, who co-moderated the press briefing. “More research is needed.”
Still, the study “has huge implications that could eventually lead to the development of noninvasive and accessible early screening for gastric cancer,” she said.
Dr. Perati and Dr. Laine reported no relevant financial relationships. The study was independently supported.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>168059</fileName> <TBEID>0C05011B.SIG</TBEID> <TBUniqueIdentifier>MD_0C05011B</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240514T092338</QCDate> <firstPublished>20240514T093128</firstPublished> <LastPublished>20240514T093128</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240514T093128</CMSDate> <articleSource>AT DDW 2024</articleSource> <facebookInfo/> <meetingNumber>3042-24</meetingNumber> <byline>Damian McNamara</byline> <bylineText>DAMIAN MCNAMARA, MA</bylineText> <bylineFull>DAMIAN MCNAMARA, MA</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>A mouth rinse used to identify oral microbiome composition could serve as an early-detection tool for gastric cancer, new evidence suggests.</metaDescription> <articlePDF/> <teaserImage/> <teaser>A noninvasive “swish and spit” test could be more accessible and allow for more widespread screening.</teaser> <title>Oral Microbiome Test Could Detect Gastric Cancer Earlier</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>gih</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">17</term> <term>31</term> <term>21</term> <term>15</term> </publications> <sections> <term canonical="true">53</term> <term>39313</term> </sections> <topics> <term canonical="true">344</term> <term>213</term> <term>67020</term> <term>280</term> <term>263</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Oral Microbiome Test Could Detect Gastric Cancer Earlier</title> <deck/> </itemMeta> <itemContent> <p>WASHINGTON, DC – <span class="tag metaDescription">A mouth rinse used to identify oral microbiome composition could serve as an early-detection tool for gastric cancer, new evidence suggests.</span></p> <p>Researchers found distinct bacterial composition differences in patient samples that point to the potential for oral microbial signatures to be used as biomarkers for assessing gastric cancer risk. <br/><br/>“Too many patients are being diagnosed too late. There are no formal screening guidelines for gastric cancer, and more than half of patients with gastric cancer do not receive a diagnosis until their cancer is already at an advanced stage,” said Shruthi Reddy Perati, MD, a general surgery resident at Rutgers University Robert Wood Johnson School of Medicine in New Brunswick, New Jersey.<br/><br/>Detecting gastric cancer now generally requires an invasive procedure, such as endoscopy. Therefore, a noninvasive “swish and spit” test could be more accessible and allow for more widespread screening, Dr. Perati said at a May 8 press briefing during which her research (Abstract 949) was previewed for Digestive Disease Week<sup>®</sup> (DDW).<br/><br/>Gastric cancer, also known as stomach cancer, is the <a href="https://reference.medscape.com/slideshow/gastric-cancer-6009811">fourth most common cause</a> of cancer-related death in the world. The United States can expect 26,890 new cases and 10,880 deaths from this type of cancer in 2024, the American Cancer Society <a href="https://www.cancer.org/cancer/types/stomach-cancer/about/key-statistics.html">estimates</a>.<br/><br/></p> <h2>Microbial Signatures Found</h2> <p>Dr. Perati and colleagues collected oral rinse samples from 98 patients: 30 known to have gastric cancer , 30 with precancerous gastric conditions (pre–gastric cancer), and 38 control participants without pre-gastric or gastric cancer. Sixty-two percent were women, 32% were Hispanic, 31% had diabetes, and 18% were smokers.</p> <p>The researchers analyzed the samples for alpha and beta diversity and conducted differential analysis using the framework called analysis of compositions of microbiomes.<br/><br/>They found distinct differences between the oral microbiomes of the healthy group and those of the groups with gastric cancer and pre–gastric cancer. In addition, the microbiomes of participants with cancer and of those with precancerous conditions were similar.<br/><br/>The results suggest that the microbiome changes may occur as soon as the stomach environment starts to undergo changes that can eventually turn into cancer.<br/><br/>“The oral microbiome may serve as a window into the composition of the stomach environment,” Dr. Perati said.<br/><br/>The investigators created a screening model to detect the most relevant 13 bacterial genera that differed between the control group and the gastric cancer and pre–gastric cancer groups. The tenfold cross-validation model demonstrated good ability to discriminate using bacteria alone (area under the curve [AUC], 0.74) and was further improved with the addition of clinical variables, including demographics and comorbidities (AUC, 0.91), the researchers noted.<br/><br/>As the investigators noted, the model’s performance improved with the addition of clinical variables, said Loren Laine, MD, professor of medicine (digestive diseases) at Yale School of Medicine and chair of DDW 2024. <br/><br/>An AUC of 0.74 using bacteria alone, which increased to 0.91 by adding demographics and comorbidities, “[is] starting to be really meaningful,” Dr. Laine said.<br/><br/>Further studies should evaluate the test’s sensitivity and specificity, Dr. Laine added.<br/><br/></p> <h2>Additional Considerations</h2> <p>The microbiome can vary between people and within the same individual over time. Probiotics, antibiotics, and diet can lead to changes in the microbiome, Dr. Perati said.</p> <p>When asked how these changes could affect the accuracy of an oral rinse test, Dr. Perati said “it’s known that, in general, dietary modifications can have an impact on the diversity and the prevalence of certain bacteria throughout the GI tract.”<br/><br/>Though variance is expected, we’re hoping to see that the differences in the microbiome composition between the malignant groups and the control groups are more significant than those lower-level background changes due to dietary modifications, for example, she added.<br/><br/>The research is in its early days, and the results need to be validated in a larger study, Dr. Perati said.<br/><br/>Ninety-eight patients is “still a very small number,” said Dr. Laine, who co-moderated the press briefing. “More research is needed.”<br/><br/>Still, the study “has huge implications that could eventually lead to the development of noninvasive and accessible early screening for gastric cancer,” she said.<br/><br/>Dr. Perati and Dr. Laine reported no relevant financial relationships. The study was independently supported.<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/oral-microbiome-test-could-detect-gastric-cancer-earlier-2024a100090j">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
AT DDW 2024
May 2024 – ICYMI
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167942</fileName> <TBEID>0C04FEDB.SIG</TBEID> <TBUniqueIdentifier>MD_0C04FEDB</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240503T160409</QCDate> <firstPublished>20240503T162424</firstPublished> <LastPublished>20240503T162424</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240503T162424</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>(None)</byline> <bylineText/> <bylineFull/> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>In case you missed it: Recent studies published in the journals of the American Gastroenterology Association.</metaDescription> <articlePDF/> <teaserImage/> <teaser> <span class="tag metaDescription">In case you missed it: Recent studies published in the journals of the American Gastroenterology Association.</span> </teaser> <title>May 2024 – ICYMI</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>gih</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">17</term> </publications> <sections> <term>47431</term> <term canonical="true">46646</term> </sections> <topics> <term canonical="true">347</term> <term>344</term> <term>345</term> <term>39703</term> <term>39702</term> <term>343</term> <term>346</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>May 2024 – ICYMI</title> <deck/> </itemMeta> <itemContent> <h2>Gastroenterology</h2> <p> <strong>January 2024</strong> </p> <p>Hirano I, et al; ASCENT WORKING GROUP. <span class="Hyperlink"><a href="https://doi.org/10.1053/j.gastro.2023.09.004">Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis</a></span>. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.<br/><br/><br/><br/>Åkerström JH, et al. <span class="Hyperlink"><a href="https://doi.org/10.1053/j.gastro.2023.08.050">Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus</a></span>. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.<br/><br/><br/><br/>Barnes EL, et al; AGA Clinical Guidelines Committee. <span class="Hyperlink"><a href="https://doi.org/10.1053/j.gastro.2023.10.015">AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders</a></span>. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.</p> <p><strong>February 2024</strong><br/><br/>Yoo HW, et al. <span class="Hyperlink"><a href="https://doi.org/10.1053/j.gastro.2023.10.013">Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study</a></span>. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.<br/><br/><br/><br/>Yang J, et al. <span class="Hyperlink"><a href="https://doi.org/10.1053/j.gastro.2023.10.012">High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice</a></span>. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.<br/><br/><br/><br/>Young E, et al. <span class="Hyperlink"><a href="https://doi.org/10.1053/j.gastro.2023.10.008">Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial</a></span>. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.<br/><br/></p> <h2>Clinical Gastroenterology and Hepatology</h2> <p><strong>January 2024</strong><br/><br/>Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. <span class="Hyperlink"><a href="https://doi.org/10.1016/j.cgh.2023.03.035">Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk</a></span>. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.</p> <p><br/><br/>Reddy CA, et al. <span class="Hyperlink"><a href="https://doi.org/10.1016/j.cgh.2023.06.013">Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders</a></span>. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.<br/><br/>Thiruvengadam NR, et al. <span class="Hyperlink"><a href="https://doi.org/10.1016/j.cgh.2023.05.028">The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis</a></span>. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.</p> <p><strong>February 2024</strong><br/><br/>Goodoory VC, et al. <span class="Hyperlink"><a href="https://doi.org/10.1016/j.cgh.2023.02.014">Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome</a></span>. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.<br/><br/>Brenner DM, et al. <span class="Hyperlink"><a href="https://doi.org/10.1016/j.cgh.2023.09.013">Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome</a></span>. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.<br/><br/></p> <h2>Techniques and Innovations in Gastrointestinal Endoscopy</h2> <p><strong>January 2024</strong><br/><br/>Ramirez PR, et al. <span class="Hyperlink"><a href="https://doi.org/10.1016/j.tige.2023.10.001">Gaps and Improvement Opportunities in Post-Colonoscopy Communication</a></span>. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.</p> <p><br/><br/>Gonzaga ER, et al. <span class="Hyperlink"><a href="https://doi.org/10.1016/j.tige.2023.09.002">Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis</a></span>. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.<br/><br/><br/><br/>Wang D, et al. <span class="Hyperlink"><a href="https://doi.org/10.1016/j.tige.2023.10.003">Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis</a></span>. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.<br/><br/></p> <h2>Gastro Hep Advances</h2> <p><strong>January 2024</strong><br/><br/>Adeniran E, et al. <span class="Hyperlink"><a href="https://doi.org/10.1016/j.gastha.2023.08.017">Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention</a></span>. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.</p> <p><br/><br/>Alkhouri N, et al. <span class="Hyperlink"><a href="https://doi.org/10.1016/j.gastha.2023.08.019">A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease</a></span>. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.</p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Late-Stage Incidence Rates Support CRC Screening From Age 45
, a cross-sectional study of stage-stratified CRC found.
It is well known that CRC is becoming more prevalent generally in the under 50-year population, but stage-related analyses have not been done.
Staging analysis in this age group is important, however, as an increasing burden of advance-staged disease would provide further evidence for earlier screening initiation, wrote Eric M. Montminy, MD, a gastroenterologist at John H. Stroger Hospital of County Cook, Chicago, Illinois, and colleagues in JAMA Network Open.

The United States Preventive Services Task Force (USPSTF) has recommended that average-risk screening begin at 45 years of age, as do the American Gastroenterological Association and other GI societies, although the American College of Physicians last year published clinical guidance recommending 50 years as the age to start screening for CRC for patients with average risk.
“Patients aged 46-49 may become confused on which guideline to follow, similar to confusion occurring with prior breast cancer screening changes,” Dr. Montminy said in an interview. “We wanted to demonstrate incidence rates with stage stratification to help clarify the incidence trends in this age group. Stage stratification is a key because it provides insight into the relationship between time and cancer incidence, ie, is screening finding early cancer or not?”
A 2020 study in JAMA Network Open demonstrated a 46.1% increase in CRC incidence rates (IRs) in persons aged 49-50 years. This steep increase is consistent with the presence of a large preexisting and undetected case burden.
“Our results demonstrate that adults aged 46-49 years, who are between now-conflicting guidelines on whether to start screening at age 45 or 50 years, have an increasing burden of more advanced-stage CRC and thus may be at an increased risk if screening is not initiated at age 45 years,” Dr. Montminy’s group wrote.
Using incidence data per 100,000 population from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry, the investigators observed the following IRs for early-onset CRC in the age group of 46-49 years:
- Distant adenocarcinoma IRs increased faster than other stages: annual percentage change (APC), 2.2 (95% CI, 1.8-2.6).
- Regional IRs also significantly increased: APC, 1.3 (95% CI, 0.8-1.7).
- Absolute regional IRs of CRC in the age bracket of 46-49 years are similar to total pancreatic cancer IRs in all ages and all stages combined (13.2 of 100,000) over similar years. When distant IRs for CRC are included with regional IRs, those for IRs for CRC are double those for pancreatic cancer of all stages combined.
- The only decrease was seen in localized IRs: APC, -0.6 (95% CI, -1 to -0.2).
“My best advice for clinicians is to provide the facts from the data to patients so they can make an informed health decision,” Dr. Montminy said. “This includes taking an appropriate personal and family history and having the patient factor this aspect into their decision on when and how they want to perform colon cancer screening.”
His institution adheres to the USPSTF recommendation of initiation of CRC screening at age 45 years.
Findings From 2000 to 2020
During 2000-2020 period, 26,887 CRCs were diagnosed in adults aged 46-49 years (54.5% in men).
As of 2020, the localized adenocarcinoma IR decreased to 7.7 of 100,000, but regional adenocarcinoma IR increased to 13.4 of 100,000 and distant adenocarcinoma IR increased to 9.0 of 100,000.
Regional adenocarcinoma IR remained the highest of all stages in 2000-2020. From 2014 to 2020, distant IRs became similar to localized IRs, except in 2017 when distant IRs were significantly higher than localized.
Why the CRC Uptick?
“It remains an enigma at this time as to why we’re seeing this shift,” Dr. Montminy said, noting that etiologies from the colonic microbiome to cellphones have been postulated. “To date, no theory has substantially provided causality. But whatever the source is, it is affecting Western countries in unison with data demonstrating a birth cohort effect as well,” he added. “We additionally know, based on the current epidemiologic data, that current screening practices are failing, and a unified discussion must occur in order to prevent young patients from developing advanced colon cancer.”

Offering his perspective on the findings, Joshua Meyer, MD, vice chair of translational research in the Department of Radiation Oncology at Fox Chase Cancer Center in Philadelphia, said the findings reinforce the practice of offering screening to average-risk individuals starting at age 45 years, the threshold at his institution. “There are previously published data demonstrating an increase in advanced stage at the time of screening initiation, and these data support that,” said Dr. Meyer, who was not involved in the present analysis.
More research needs to be done, he continued, not just on optimal age but also on the effect of multiple other factors impacting risk. “These may include family history and genetic risk as well as the role of blood- and stool-based screening assays in an integrated strategy to screen for colorectal cancer.”
There are multiple screening tests, and while colonoscopy, the gold standard, is very safe, it is not completely without risks, Dr. Meyer added. “And the question of the appropriate allocation of limited societal resources continues to be discussed on a broader level and largely explains the difference between the two guidelines.”
This study received no specific funding. Co-author Jordan J. Karlitz, MD, reported personal fees from GRAIL (senior medical director) and an equity position from Gastro Girl/GI On Demand outside f the submitted work. Dr. Meyer disclosed no conflicts of interest relevant to his comments.
, a cross-sectional study of stage-stratified CRC found.
It is well known that CRC is becoming more prevalent generally in the under 50-year population, but stage-related analyses have not been done.
Staging analysis in this age group is important, however, as an increasing burden of advance-staged disease would provide further evidence for earlier screening initiation, wrote Eric M. Montminy, MD, a gastroenterologist at John H. Stroger Hospital of County Cook, Chicago, Illinois, and colleagues in JAMA Network Open.

The United States Preventive Services Task Force (USPSTF) has recommended that average-risk screening begin at 45 years of age, as do the American Gastroenterological Association and other GI societies, although the American College of Physicians last year published clinical guidance recommending 50 years as the age to start screening for CRC for patients with average risk.
“Patients aged 46-49 may become confused on which guideline to follow, similar to confusion occurring with prior breast cancer screening changes,” Dr. Montminy said in an interview. “We wanted to demonstrate incidence rates with stage stratification to help clarify the incidence trends in this age group. Stage stratification is a key because it provides insight into the relationship between time and cancer incidence, ie, is screening finding early cancer or not?”
A 2020 study in JAMA Network Open demonstrated a 46.1% increase in CRC incidence rates (IRs) in persons aged 49-50 years. This steep increase is consistent with the presence of a large preexisting and undetected case burden.
“Our results demonstrate that adults aged 46-49 years, who are between now-conflicting guidelines on whether to start screening at age 45 or 50 years, have an increasing burden of more advanced-stage CRC and thus may be at an increased risk if screening is not initiated at age 45 years,” Dr. Montminy’s group wrote.
Using incidence data per 100,000 population from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry, the investigators observed the following IRs for early-onset CRC in the age group of 46-49 years:
- Distant adenocarcinoma IRs increased faster than other stages: annual percentage change (APC), 2.2 (95% CI, 1.8-2.6).
- Regional IRs also significantly increased: APC, 1.3 (95% CI, 0.8-1.7).
- Absolute regional IRs of CRC in the age bracket of 46-49 years are similar to total pancreatic cancer IRs in all ages and all stages combined (13.2 of 100,000) over similar years. When distant IRs for CRC are included with regional IRs, those for IRs for CRC are double those for pancreatic cancer of all stages combined.
- The only decrease was seen in localized IRs: APC, -0.6 (95% CI, -1 to -0.2).
“My best advice for clinicians is to provide the facts from the data to patients so they can make an informed health decision,” Dr. Montminy said. “This includes taking an appropriate personal and family history and having the patient factor this aspect into their decision on when and how they want to perform colon cancer screening.”
His institution adheres to the USPSTF recommendation of initiation of CRC screening at age 45 years.
Findings From 2000 to 2020
During 2000-2020 period, 26,887 CRCs were diagnosed in adults aged 46-49 years (54.5% in men).
As of 2020, the localized adenocarcinoma IR decreased to 7.7 of 100,000, but regional adenocarcinoma IR increased to 13.4 of 100,000 and distant adenocarcinoma IR increased to 9.0 of 100,000.
Regional adenocarcinoma IR remained the highest of all stages in 2000-2020. From 2014 to 2020, distant IRs became similar to localized IRs, except in 2017 when distant IRs were significantly higher than localized.
Why the CRC Uptick?
“It remains an enigma at this time as to why we’re seeing this shift,” Dr. Montminy said, noting that etiologies from the colonic microbiome to cellphones have been postulated. “To date, no theory has substantially provided causality. But whatever the source is, it is affecting Western countries in unison with data demonstrating a birth cohort effect as well,” he added. “We additionally know, based on the current epidemiologic data, that current screening practices are failing, and a unified discussion must occur in order to prevent young patients from developing advanced colon cancer.”

Offering his perspective on the findings, Joshua Meyer, MD, vice chair of translational research in the Department of Radiation Oncology at Fox Chase Cancer Center in Philadelphia, said the findings reinforce the practice of offering screening to average-risk individuals starting at age 45 years, the threshold at his institution. “There are previously published data demonstrating an increase in advanced stage at the time of screening initiation, and these data support that,” said Dr. Meyer, who was not involved in the present analysis.
More research needs to be done, he continued, not just on optimal age but also on the effect of multiple other factors impacting risk. “These may include family history and genetic risk as well as the role of blood- and stool-based screening assays in an integrated strategy to screen for colorectal cancer.”
There are multiple screening tests, and while colonoscopy, the gold standard, is very safe, it is not completely without risks, Dr. Meyer added. “And the question of the appropriate allocation of limited societal resources continues to be discussed on a broader level and largely explains the difference between the two guidelines.”
This study received no specific funding. Co-author Jordan J. Karlitz, MD, reported personal fees from GRAIL (senior medical director) and an equity position from Gastro Girl/GI On Demand outside f the submitted work. Dr. Meyer disclosed no conflicts of interest relevant to his comments.
, a cross-sectional study of stage-stratified CRC found.
It is well known that CRC is becoming more prevalent generally in the under 50-year population, but stage-related analyses have not been done.
Staging analysis in this age group is important, however, as an increasing burden of advance-staged disease would provide further evidence for earlier screening initiation, wrote Eric M. Montminy, MD, a gastroenterologist at John H. Stroger Hospital of County Cook, Chicago, Illinois, and colleagues in JAMA Network Open.

The United States Preventive Services Task Force (USPSTF) has recommended that average-risk screening begin at 45 years of age, as do the American Gastroenterological Association and other GI societies, although the American College of Physicians last year published clinical guidance recommending 50 years as the age to start screening for CRC for patients with average risk.
“Patients aged 46-49 may become confused on which guideline to follow, similar to confusion occurring with prior breast cancer screening changes,” Dr. Montminy said in an interview. “We wanted to demonstrate incidence rates with stage stratification to help clarify the incidence trends in this age group. Stage stratification is a key because it provides insight into the relationship between time and cancer incidence, ie, is screening finding early cancer or not?”
A 2020 study in JAMA Network Open demonstrated a 46.1% increase in CRC incidence rates (IRs) in persons aged 49-50 years. This steep increase is consistent with the presence of a large preexisting and undetected case burden.
“Our results demonstrate that adults aged 46-49 years, who are between now-conflicting guidelines on whether to start screening at age 45 or 50 years, have an increasing burden of more advanced-stage CRC and thus may be at an increased risk if screening is not initiated at age 45 years,” Dr. Montminy’s group wrote.
Using incidence data per 100,000 population from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry, the investigators observed the following IRs for early-onset CRC in the age group of 46-49 years:
- Distant adenocarcinoma IRs increased faster than other stages: annual percentage change (APC), 2.2 (95% CI, 1.8-2.6).
- Regional IRs also significantly increased: APC, 1.3 (95% CI, 0.8-1.7).
- Absolute regional IRs of CRC in the age bracket of 46-49 years are similar to total pancreatic cancer IRs in all ages and all stages combined (13.2 of 100,000) over similar years. When distant IRs for CRC are included with regional IRs, those for IRs for CRC are double those for pancreatic cancer of all stages combined.
- The only decrease was seen in localized IRs: APC, -0.6 (95% CI, -1 to -0.2).
“My best advice for clinicians is to provide the facts from the data to patients so they can make an informed health decision,” Dr. Montminy said. “This includes taking an appropriate personal and family history and having the patient factor this aspect into their decision on when and how they want to perform colon cancer screening.”
His institution adheres to the USPSTF recommendation of initiation of CRC screening at age 45 years.
Findings From 2000 to 2020
During 2000-2020 period, 26,887 CRCs were diagnosed in adults aged 46-49 years (54.5% in men).
As of 2020, the localized adenocarcinoma IR decreased to 7.7 of 100,000, but regional adenocarcinoma IR increased to 13.4 of 100,000 and distant adenocarcinoma IR increased to 9.0 of 100,000.
Regional adenocarcinoma IR remained the highest of all stages in 2000-2020. From 2014 to 2020, distant IRs became similar to localized IRs, except in 2017 when distant IRs were significantly higher than localized.
Why the CRC Uptick?
“It remains an enigma at this time as to why we’re seeing this shift,” Dr. Montminy said, noting that etiologies from the colonic microbiome to cellphones have been postulated. “To date, no theory has substantially provided causality. But whatever the source is, it is affecting Western countries in unison with data demonstrating a birth cohort effect as well,” he added. “We additionally know, based on the current epidemiologic data, that current screening practices are failing, and a unified discussion must occur in order to prevent young patients from developing advanced colon cancer.”

Offering his perspective on the findings, Joshua Meyer, MD, vice chair of translational research in the Department of Radiation Oncology at Fox Chase Cancer Center in Philadelphia, said the findings reinforce the practice of offering screening to average-risk individuals starting at age 45 years, the threshold at his institution. “There are previously published data demonstrating an increase in advanced stage at the time of screening initiation, and these data support that,” said Dr. Meyer, who was not involved in the present analysis.
More research needs to be done, he continued, not just on optimal age but also on the effect of multiple other factors impacting risk. “These may include family history and genetic risk as well as the role of blood- and stool-based screening assays in an integrated strategy to screen for colorectal cancer.”
There are multiple screening tests, and while colonoscopy, the gold standard, is very safe, it is not completely without risks, Dr. Meyer added. “And the question of the appropriate allocation of limited societal resources continues to be discussed on a broader level and largely explains the difference between the two guidelines.”
This study received no specific funding. Co-author Jordan J. Karlitz, MD, reported personal fees from GRAIL (senior medical director) and an equity position from Gastro Girl/GI On Demand outside f the submitted work. Dr. Meyer disclosed no conflicts of interest relevant to his comments.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167483</fileName> <TBEID>0C04F390.SIG</TBEID> <TBUniqueIdentifier>MD_0C04F390</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname>IRs up for advanced CRCs</storyname> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240426T182455</QCDate> <firstPublished>20240429T091047</firstPublished> <LastPublished>20240429T092924</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240429T091047</CMSDate> <articleSource>FROM JAMA NETWORK OPEN</articleSource> <facebookInfo/> <meetingNumber/> <byline>Diana Swift dianaswift@rogers.com</byline> <bylineText>DIANA SWIFT</bylineText> <bylineFull>DIANA SWIFT</bylineFull> <bylineTitleText>MDedge News</bylineTitleText> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>In the setting of conflicting national screening guidelines, the incidence of distant- and regional-stage colorectal adenocarcinoma (CRC) has been increasing in</metaDescription> <articlePDF/> <teaserImage/> <teaser>Stage stratification is key because it provides insight into the relationship between time and cancer incidence, said Dr. Eric Montminy.</teaser> <title>Late-Stage Incidence Rates Support CRC Screening From Age 45</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>3</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>gih</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">17</term> <term>21</term> <term>15</term> <term>31</term> </publications> <sections> <term canonical="true">27970</term> <term>39313</term> </sections> <topics> <term canonical="true">344</term> <term>213</term> <term>263</term> <term>67020</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Late-Stage Incidence Rates Support CRC Screening From Age 45</title> <deck/> </itemMeta> <itemContent> <p><span class="tag metaDescription">In the setting of conflicting national screening guidelines, the incidence of distant- and regional-stage colorectal adenocarcinoma (CRC) has been increasing in individuals aged 46-49 years</span>, a cross-sectional study of stage-stratified CRC found.</p> <p>It is well known that CRC is becoming more prevalent generally in the under 50-year population, but stage-related analyses have not been done.<br/><br/>Staging analysis in this age group is important, however, as an increasing burden of advance-staged disease would provide further evidence for earlier screening initiation, wrote Eric M. Montminy, MD, a gastroenterologist at John H. Stroger Hospital of County Cook, Chicago, Illinois, and colleagues <a href="https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2816222"><span class="Hyperlink">in</span><em> JAMA Network Open</em></a>.<br/><br/>The United States Preventive Services Task Force (USPSTF) has recommended that average-risk screening begin at 45 years of age, as do the American Gastroenterological Association and other GI societies, although the American College of Physicians last year published clinical guidance recommending 50 years as the age to start screening for CRC for patients with average risk.<br/><br/>“Patients aged 46-49 may become confused on which guideline to follow, similar to confusion occurring with prior breast cancer screening changes,” Dr. Montminy said in an interview. “We wanted to demonstrate incidence rates with stage stratification to help clarify the incidence trends in this age group. Stage stratification is a key because it provides insight into the relationship between time and cancer incidence, ie, is screening finding early cancer or not?”<br/><br/>A 2020 study <a href="https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2759846"><span class="Hyperlink">in </span><em>JAMA Network Open</em></a> demonstrated a 46.1% increase in CRC incidence rates (IRs) in persons aged 49-50 years. This steep increase is consistent with the presence of a large preexisting and undetected case burden.<br/><br/>“Our results demonstrate that adults aged 46-49 years, who are between now-conflicting guidelines on whether to start screening at age 45 or 50 years, have an increasing burden of more advanced-stage CRC and thus may be at an increased risk if screening is not initiated at age 45 years,” Dr. Montminy’s group wrote.<br/><br/>Using incidence data per 100,000 population from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry, the investigators observed the following IRs for early-onset CRC in the age group of 46-49 years:</p> <ul class="body"> <li>Distant adenocarcinoma IRs increased faster than other stages: annual percentage change (APC), 2.2 (95% CI, 1.8-2.6). </li> <li>Regional IRs also significantly increased: APC, 1.3 (95% CI, 0.8-1.7).</li> <li>Absolute regional IRs of CRC in the age bracket of 46-49 years are similar to total pancreatic cancer IRs in all ages and all stages combined (13.2 of 100,000) over similar years. When distant IRs for CRC are included with regional IRs, those for IRs for CRC are <span class="Hyperlink">double those for pancreatic cancer</span> of all stages combined.</li> <li>The only decrease was seen in localized IRs: APC, -0.6 (95% CI, -1 to -0.2).</li> </ul> <p>“My best advice for clinicians is to provide the facts from the data to patients so they can make an informed health decision,” Dr. Montminy said. “This includes taking an appropriate personal and family history and having the patient factor this aspect into their decision on when and how they want to perform colon cancer screening.”<br/><br/>His institution adheres to the USPSTF recommendation of initiation of CRC screening at age 45 years.<br/><br/></p> <h2>Findings From 2000 to 2020</h2> <p>During 2000-2020 period, 26,887 CRCs were diagnosed in adults aged 46-49 years (54.5% in men).</p> <p>As of 2020, the localized adenocarcinoma IR decreased to 7.7 of 100,000, but regional adenocarcinoma IR increased to 13.4 of 100,000 and distant adenocarcinoma IR increased to 9.0 of 100,000.<br/><br/>Regional adenocarcinoma IR remained the highest of all stages in 2000-2020. From 2014 to 2020, distant IRs became similar to localized IRs, except in 2017 when distant IRs were significantly higher than localized.<br/><br/></p> <h2>Why the CRC Uptick?</h2> <p>“It remains an enigma at this time as to why we’re seeing this shift,” Dr. Montminy said, noting that etiologies from the colonic microbiome to cellphones have been postulated. “To date, no theory has substantially provided causality. But whatever the source is, it is affecting Western countries in unison with data demonstrating a birth cohort effect as well,” he added. “We additionally know, based on the current epidemiologic data, that current screening practices are failing, and a unified discussion must occur in order to prevent young patients from developing advanced colon cancer.”</p> <p>Offering his perspective on the findings, Joshua Meyer, MD, vice chair of translational research in the Department of Radiation Oncology at Fox Chase Cancer Center in Philadelphia, said the findings reinforce the practice of offering screening to average-risk individuals starting at age 45 years, the threshold at his institution. “There are previously published data demonstrating an increase in advanced stage at the time of screening initiation, and these data support that,” said Dr. Meyer, who was not involved in the present analysis.<br/><br/>More research needs to be done, he continued, not just on optimal age but also on the effect of multiple other factors impacting risk. “These may include family history and genetic risk as well as the role of blood- and stool-based screening assays in an integrated strategy to screen for colorectal cancer.”<br/><br/>There are multiple screening tests, and while colonoscopy, the gold standard, is very safe, it is not completely without risks, Dr. Meyer added. “And the question of the appropriate allocation of limited societal resources continues to be discussed on a broader level and largely explains the difference between the two guidelines.”<br/><br/>This study received no specific funding. Co-author Jordan J. Karlitz, MD, reported personal fees from GRAIL (senior medical director) and an equity position from Gastro Girl/GI On Demand outside f the submitted work. Dr. Meyer disclosed no conflicts of interest relevant to his comments.<span class="end"/></p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM JAMA NETWORK OPEN
Liquid Biopsy Has Near-Perfect Accuracy for Early Pancreatic Cancer
the most common type of pancreatic cancer.
It is quite encouraging to know we have a blood test that could potentially find this disease early, said Ajay Goel, PhD, a molecular diagnostics specialist at City of Hope in Duarte, California, who presented the findings at the annual meeting of the American Association for Cancer Research (AACR).
Dr. Goel and colleagues developed a signature for pancreatic cancer based on microRNAs identified in the exomes shed from pancreatic cancers and cell-free DNA markers found in the blood of patients with the disease.
Their initial assay tested blood samples for this signature in a training cohort of 252 people in Japan, approximately 60% of whom had pancreatic cancer. The rest were healthy controls. The assay was then tested in validation cohorts of 400 subjects, half with pancreatic cancer and half controls, in China and South Korea.
In both the initial and validation tests, the microRNA assay had an accuracy of about 90% for stage I/II pancreatic cancer, already far better than commercially available assays.
In an additional validation cohort in the United States with 139 patients with pancreatic cancer and 193 controls at six centers across the country, the researchers found that adding carbohydrate antigen 19-9 — a well-known marker of pancreatic cancer — to the assay boosted the test’s accuracy to 97%.
The test performed the same whether the tumor was in the head or tail of the pancreas.
“We are very excited about this data,” said Dr. Goel.
The technology was recently licensed to Pharus Diagnostics for commercial development, which will likely include a prospective screening trial, he told this news organization.
Because pancreatic cancer is fairly uncommon, Dr. Goel did not anticipate the test being used for general screening but rather for screening high-risk patients such as those with newly diagnosed type 2 diabetes, a family history of pancreatic cancer, or predisposing genetic mutations.
“It should be a very inexpensive test; it doesn’t cost us much to do in the lab,” he added.
Study moderator Ryan Corcoran, MD, PhD, a gastrointestinal (GI) oncologist at Massachusetts General Hospital, Boston, saw the potential.
“As a GI oncologist, I know how lethal and hard to treat pancreatic cancer is,” he said. A test that could reliably detect pancreatic cancer early, with an acceptable false-positive rate, would be extremely useful.
“The cure rate is many, many times higher,” if we detect it before it has a chance to spread, he explained.
In the meantime, Dr. Goel said there’s more work to be done.
Almost 4,000 subjects have been enrolled in ongoing validation efforts, and efforts are underway to use the test to screen thousands of banked blood samples from the PLCO, a prospective cancer screening trial in healthy subjects.
The researchers also want to see if the test can distinguish benign pancreatic cysts from ones that turn cancerous.
The idea is to find the earliest possible signs of this disease to see if we can find it not “at the moment of clinical diagnosis, but possibly 6 months, 1 year, 2 years earlier” than with radiologic imaging, Dr. Goel said.
The work was funded by the National Cancer Institute and others. Dr. Goel is a consultant for Pharus Diagnostics and Cellomics. Dr. Corcoran is a consultant for, has grants from, and/or holds stock in numerous companies, including Pfizer, Novartis, Eli Lilly, and Revolution Medicines.
A version of this article appeared on Medscape.com.
the most common type of pancreatic cancer.
It is quite encouraging to know we have a blood test that could potentially find this disease early, said Ajay Goel, PhD, a molecular diagnostics specialist at City of Hope in Duarte, California, who presented the findings at the annual meeting of the American Association for Cancer Research (AACR).
Dr. Goel and colleagues developed a signature for pancreatic cancer based on microRNAs identified in the exomes shed from pancreatic cancers and cell-free DNA markers found in the blood of patients with the disease.
Their initial assay tested blood samples for this signature in a training cohort of 252 people in Japan, approximately 60% of whom had pancreatic cancer. The rest were healthy controls. The assay was then tested in validation cohorts of 400 subjects, half with pancreatic cancer and half controls, in China and South Korea.
In both the initial and validation tests, the microRNA assay had an accuracy of about 90% for stage I/II pancreatic cancer, already far better than commercially available assays.
In an additional validation cohort in the United States with 139 patients with pancreatic cancer and 193 controls at six centers across the country, the researchers found that adding carbohydrate antigen 19-9 — a well-known marker of pancreatic cancer — to the assay boosted the test’s accuracy to 97%.
The test performed the same whether the tumor was in the head or tail of the pancreas.
“We are very excited about this data,” said Dr. Goel.
The technology was recently licensed to Pharus Diagnostics for commercial development, which will likely include a prospective screening trial, he told this news organization.
Because pancreatic cancer is fairly uncommon, Dr. Goel did not anticipate the test being used for general screening but rather for screening high-risk patients such as those with newly diagnosed type 2 diabetes, a family history of pancreatic cancer, or predisposing genetic mutations.
“It should be a very inexpensive test; it doesn’t cost us much to do in the lab,” he added.
Study moderator Ryan Corcoran, MD, PhD, a gastrointestinal (GI) oncologist at Massachusetts General Hospital, Boston, saw the potential.
“As a GI oncologist, I know how lethal and hard to treat pancreatic cancer is,” he said. A test that could reliably detect pancreatic cancer early, with an acceptable false-positive rate, would be extremely useful.
“The cure rate is many, many times higher,” if we detect it before it has a chance to spread, he explained.
In the meantime, Dr. Goel said there’s more work to be done.
Almost 4,000 subjects have been enrolled in ongoing validation efforts, and efforts are underway to use the test to screen thousands of banked blood samples from the PLCO, a prospective cancer screening trial in healthy subjects.
The researchers also want to see if the test can distinguish benign pancreatic cysts from ones that turn cancerous.
The idea is to find the earliest possible signs of this disease to see if we can find it not “at the moment of clinical diagnosis, but possibly 6 months, 1 year, 2 years earlier” than with radiologic imaging, Dr. Goel said.
The work was funded by the National Cancer Institute and others. Dr. Goel is a consultant for Pharus Diagnostics and Cellomics. Dr. Corcoran is a consultant for, has grants from, and/or holds stock in numerous companies, including Pfizer, Novartis, Eli Lilly, and Revolution Medicines.
A version of this article appeared on Medscape.com.
the most common type of pancreatic cancer.
It is quite encouraging to know we have a blood test that could potentially find this disease early, said Ajay Goel, PhD, a molecular diagnostics specialist at City of Hope in Duarte, California, who presented the findings at the annual meeting of the American Association for Cancer Research (AACR).
Dr. Goel and colleagues developed a signature for pancreatic cancer based on microRNAs identified in the exomes shed from pancreatic cancers and cell-free DNA markers found in the blood of patients with the disease.
Their initial assay tested blood samples for this signature in a training cohort of 252 people in Japan, approximately 60% of whom had pancreatic cancer. The rest were healthy controls. The assay was then tested in validation cohorts of 400 subjects, half with pancreatic cancer and half controls, in China and South Korea.
In both the initial and validation tests, the microRNA assay had an accuracy of about 90% for stage I/II pancreatic cancer, already far better than commercially available assays.
In an additional validation cohort in the United States with 139 patients with pancreatic cancer and 193 controls at six centers across the country, the researchers found that adding carbohydrate antigen 19-9 — a well-known marker of pancreatic cancer — to the assay boosted the test’s accuracy to 97%.
The test performed the same whether the tumor was in the head or tail of the pancreas.
“We are very excited about this data,” said Dr. Goel.
The technology was recently licensed to Pharus Diagnostics for commercial development, which will likely include a prospective screening trial, he told this news organization.
Because pancreatic cancer is fairly uncommon, Dr. Goel did not anticipate the test being used for general screening but rather for screening high-risk patients such as those with newly diagnosed type 2 diabetes, a family history of pancreatic cancer, or predisposing genetic mutations.
“It should be a very inexpensive test; it doesn’t cost us much to do in the lab,” he added.
Study moderator Ryan Corcoran, MD, PhD, a gastrointestinal (GI) oncologist at Massachusetts General Hospital, Boston, saw the potential.
“As a GI oncologist, I know how lethal and hard to treat pancreatic cancer is,” he said. A test that could reliably detect pancreatic cancer early, with an acceptable false-positive rate, would be extremely useful.
“The cure rate is many, many times higher,” if we detect it before it has a chance to spread, he explained.
In the meantime, Dr. Goel said there’s more work to be done.
Almost 4,000 subjects have been enrolled in ongoing validation efforts, and efforts are underway to use the test to screen thousands of banked blood samples from the PLCO, a prospective cancer screening trial in healthy subjects.
The researchers also want to see if the test can distinguish benign pancreatic cysts from ones that turn cancerous.
The idea is to find the earliest possible signs of this disease to see if we can find it not “at the moment of clinical diagnosis, but possibly 6 months, 1 year, 2 years earlier” than with radiologic imaging, Dr. Goel said.
The work was funded by the National Cancer Institute and others. Dr. Goel is a consultant for Pharus Diagnostics and Cellomics. Dr. Corcoran is a consultant for, has grants from, and/or holds stock in numerous companies, including Pfizer, Novartis, Eli Lilly, and Revolution Medicines.
A version of this article appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>167647</fileName> <TBEID>0C04F827.SIG</TBEID> <TBUniqueIdentifier>MD_0C04F827</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20240411T132645</QCDate> <firstPublished>20240411T134005</firstPublished> <LastPublished>20240411T134005</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20240411T134005</CMSDate> <articleSource>FROM AACR 2024</articleSource> <facebookInfo/> <meetingNumber/> <byline>M. Alexander Otto, PA</byline> <bylineText>M. ALEXANDER OTTO, PA, MMS</bylineText> <bylineFull>M. ALEXANDER OTTO, PA, MMS</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>SAN DIEGO — A liquid biopsy assay that combines a microRNA signature and a well-known biomarker for pancreatic cancer has demonstrated an accuracy of 97% for de</metaDescription> <articlePDF/> <teaserImage/> <teaser>Researchers develop signature for pancreatic cancer based on microRNAs and cell-free DNA markers in the blood of patients with the disease.</teaser> <title>Liquid Biopsy Has Near-Perfect Accuracy for Early Pancreatic Cancer</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>endo</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>GIHOLD</publicationCode> <pubIssueName>January 2014</pubIssueName> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement/> </publicationData> </publications_g> <publications> <term canonical="true">31</term> <term>34</term> <term>15</term> <term>21</term> </publications> <sections> <term canonical="true">53</term> <term>39313</term> </sections> <topics> <term>270</term> <term>280</term> <term canonical="true">67020</term> <term>213</term> <term>205</term> <term>210</term> <term>277</term> <term>263</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Liquid Biopsy Has Near-Perfect Accuracy for Early Pancreatic Cancer</title> <deck/> </itemMeta> <itemContent> <p><span class="tag metaDescription"><span class="dateline">SAN DIEGO</span> — A liquid biopsy assay that combines a microRNA signature and a well-known biomarker for <span class="Hyperlink"><a href="https://emedicine.medscape.com/article/280605-overview">pancreatic cancer</a></span> has demonstrated an accuracy of 97% for detecting stage I/II pancreatic ductal adenocarcinoma,</span> the most common type of pancreatic cancer.</p> <p>It is quite encouraging to know we have a <span class="Hyperlink">blood test</span> that could potentially find this disease early, said <span class="Hyperlink"><a href="https://www.cityofhope.org/ajay-goel">Ajay Goel, PhD</a></span>, a molecular diagnostics specialist at City of Hope in Duarte, California, who presented the findings at the annual meeting of the <span class="Hyperlink"><a href="https://www.medscape.com/viewcollection/37452">American Association for Cancer Research (AACR)</a></span>.<br/><br/>Dr. Goel and colleagues developed a signature for pancreatic cancer based on microRNAs identified in the exomes shed from pancreatic cancers and cell-free DNA markers found in the blood of patients with the disease.<br/><br/>Their initial assay tested blood samples for this signature in a training cohort of 252 people in Japan, approximately 60% of whom had pancreatic cancer. The rest were healthy controls. The assay was then tested in validation cohorts of 400 subjects, half with pancreatic cancer and half controls, in China and South Korea.<br/><br/>In both the initial and validation tests, the microRNA assay had an accuracy of about 90% for stage I/II pancreatic cancer, already far better than commercially available assays.<br/><br/>In an additional validation cohort in the United States with 139 patients with pancreatic cancer and 193 controls at six centers across the country, the researchers found that adding carbohydrate antigen 19-9 — a well-known marker of pancreatic cancer — to the assay boosted the test’s accuracy to 97%.<br/><br/>The test performed the same whether the tumor was in the head or tail of the pancreas.<br/><br/>“We are very excited about this data,” said Dr. Goel.<br/><br/>The technology was recently licensed to <span class="Hyperlink"><a href="https://www.prnewswire.com/news-releases/pharus-diagnostics-signs-worldwide-exclusive-license-agreement-with-city-of-hope-for-novel-biomarkers-to-be-used-in-liquid-biopsy-screening-for-early-pancreatic-cancer-diagnosis-302062754.html">Pharus Diagnostics</a></span> for commercial development, which will likely include a prospective screening trial, he told this news organization.<br/><br/>Because pancreatic cancer is fairly uncommon, Dr. Goel did not anticipate the test being used for general screening but rather for screening high-risk patients such as those with newly diagnosed <span class="Hyperlink"><a href="https://emedicine.medscape.com/article/117853-overview">type 2 diabetes</a></span>, a family history of pancreatic cancer, or predisposing genetic mutations.<br/><br/>“It should be a very inexpensive test; it doesn’t cost us much to do in the lab,” he added.<br/><br/>Study moderator <span class="Hyperlink"><a href="https://www.massgeneral.org/doctors/23096/ryan-corcoran">Ryan Corcoran</a></span>, MD, PhD, a gastrointestinal (GI) oncologist at Massachusetts General Hospital, Boston, saw the potential.<br/><br/>“As a GI oncologist, I know how lethal and hard to treat pancreatic cancer is,” he said. A test that could reliably detect pancreatic cancer early, with an acceptable false-positive rate, would be extremely useful.<br/><br/>“The cure rate is many, many times higher,” if we detect it before it has a chance to spread, he explained.<br/><br/>In the meantime, Dr. Goel said there’s more work to be done.<br/><br/>Almost 4,000 subjects have been enrolled in ongoing validation efforts, and efforts are underway to use the test to screen thousands of banked blood samples from the <span class="Hyperlink"><a href="https://prevention.cancer.gov/major-programs/prostate-lung-colorectal-and-ovarian-cancer-screening-trial-plco">PLCO</a></span>, a prospective cancer screening trial in healthy subjects.<br/><br/>The researchers also want to see if the test can distinguish benign pancreatic cysts from ones that turn cancerous.<br/><br/>The idea is to find the earliest possible signs of this disease to see if we can find it not “at the moment of clinical diagnosis, but possibly 6 months, 1 year, 2 years earlier” than with radiologic imaging, Dr. Goel said.<br/><br/>The work was funded by the National Cancer Institute and others. Dr. Goel is a consultant for Pharus Diagnostics and Cellomics. Dr. Corcoran is a consultant for, has grants from, and/or holds stock in numerous companies, including Pfizer, Novartis, Eli Lilly, and Revolution Medicines.<span class="end"/></p> <p> <em>A version of this article appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/liquid-biopsy-has-near-perfect-accuracy-early-pancreatic-2024a10006ut">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM AACR 2024