User login
Improving Colorectal Cancer Screening via Mailed Fecal Immunochemical Testing in a Veterans Affairs Health System
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
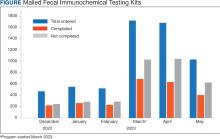
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
GLP-1 RAs Reduce Early-Onset CRC Risk in Patients With Type 2 Diabetes
PHILADELPHIA — according to the results of a retrospective study.
“This is the first large study to investigate the impact of GLP-1 RA use on EO-CRC risk,” principal investigator Temitope Olasehinde, MD, resident physician at the University Hospitals Cleveland Medical Center, Case Western Reserve University in Cleveland, Ohio, said in an interview.
The results indicate the GLP-1 RAs have a potentially protective role to play in combating EO-CRC, the incidence of which is notably rising in younger adults, with a corresponding increase in associated mortality.
Previous studies investigating the link between GLP-1 RAs and CRC did not capture patients aged younger than 50 years; thus, it was unknown if these results could be extrapolated to a younger age group, said Olasehinde.
The researcher presented the findings at the annual meeting of the American College of Gastroenterology.
Retrospective Database Analysis
Olasehinde and colleagues analyzed data from TriNetX, a large federated deidentified health research network, to identify patients (age ≤ 49 years) with diagnosed T2D subsequently prescribed antidiabetic medications who had not received a prior diagnosis of CRC. Additionally, patients were stratified on the basis of first-time GLP-1 RA use.
They identified 2,025,034 drug-naive patients with T2D; of these, 284,685 were subsequently prescribed GLP-1 RAs, and 1,740,349 remained in the non–GLP-1 RA cohort. Following propensity score matching, there were 86,186 patients in each cohort.
Patients who received GLP-1 RAs had significantly lower odds of developing EO-CRC than those who received non–GLP-1 RAs (0.6% vs 0.9%; P < .001; odds ratio [OR], 0.61; 95% CI, 0.54-068).
Furthermore, a sub-analysis revealed that patients who were obese and taking GLP-1 RAs had significantly lower odds of developing EO-CRC than patients who were obese but not taking GLP-1 RAs (0.7% vs 1.1%; P < .001; OR, 0.58; 95% CI, 0.50-067).
A Proposed Protective Effect
Although GLP-1 RAs are indicated for the treatment of T2D and obesity, recent evidence suggests that they may play a role in reducing the risk for CRC as well. This protective effect may be produced not only by addressing T2D and obesity — both important risk factors for CRC — but also via cellular mechanisms, Olasehinde noted.
“GLP-1 receptors are widely expressed throughout the gastrointestinal tract, with various effects on tissues in the stomach, small intestine, and colon,” she explained. Specifically, activation of these receptors in the proximal and distal colon promotes the release of “important factors that protect and facilitate healing of the intestinal epithelium” and “regulate the gut microbiome.”
This is particularly relevant in EO-CRC, she added, given its greater association with T2D and obesity, both factors that “have been shown to create dysbiosis in the gut microbiome and low-grade inflammation via release of free radicals/inflammatory cytokines.”
These results provide more evidence that EO-CRC “is clinically and molecularly distinct from late-onset colorectal cancer,” which is important for both clinicians and patients to understand, said Olasehinde.
“It is imperative that we are all aware of the specific signs and symptoms this population presents with and the implications of this diagnosis in younger age groups,” she added. “Patients should continue making informed dietary and lifestyle modifications/choices to help reduce the burden of EO-CRC.”
Hypothesis-Generating Results
Aasma Shaukat, MD, MPH, who was not affiliated with the research, called the results promising but — at this stage — primarily useful for stimulating future research.
"We do need more studies such as this to generate hypotheses that can be studied prospectively," Shaukat, professor of medicine and population health, and director of GI Outcomes Research at NYU Langone Health in New York City, told Medscape Medical News.
She referred to another study, published in JAMA Oncology, that also used the TriNetX research network, which showed that GLP-1 RAs were associated with reduced CRC risk in drug-naive patients with T2D.
Shaukat also noted that the current analysis has limitations that should be considered. "The study is retrospective, and confounding is a possibility,” she said.
“How the groups that did and did not receive GLP-1 RAs differ in other risk factors that could be the drivers of the cancers is not known. Whether cancers were detected through screening or symptoms, stage, and other features that may differ are not known. Finally, since we don’t know who did or did not have colonoscopy, undiagnosed cancers are not known," she explained.
Shaukat, who was the lead author of the ACG 2021 Colorectal Cancer Screening Guidelines, added that the field would benefit from studies providing "biological plausibility information, such as animal studies to understand how GLP-1 RAs may modulate risk of colon cancer; other population-based cohort studies on the incidence of colon cancer among GLP-1 RA users and non-users; and prospective trials on chemoprevention."
The study had no specific funding. Olasehinde reported no relevant financial relationships. Shaukat reported serving as a consultant for Freenome, Medtronic, and Motus GI, as well as an advisory board member for Iterative Scopes Inc.
A version of this article appeared on Medscape.com.
PHILADELPHIA — according to the results of a retrospective study.
“This is the first large study to investigate the impact of GLP-1 RA use on EO-CRC risk,” principal investigator Temitope Olasehinde, MD, resident physician at the University Hospitals Cleveland Medical Center, Case Western Reserve University in Cleveland, Ohio, said in an interview.
The results indicate the GLP-1 RAs have a potentially protective role to play in combating EO-CRC, the incidence of which is notably rising in younger adults, with a corresponding increase in associated mortality.
Previous studies investigating the link between GLP-1 RAs and CRC did not capture patients aged younger than 50 years; thus, it was unknown if these results could be extrapolated to a younger age group, said Olasehinde.
The researcher presented the findings at the annual meeting of the American College of Gastroenterology.
Retrospective Database Analysis
Olasehinde and colleagues analyzed data from TriNetX, a large federated deidentified health research network, to identify patients (age ≤ 49 years) with diagnosed T2D subsequently prescribed antidiabetic medications who had not received a prior diagnosis of CRC. Additionally, patients were stratified on the basis of first-time GLP-1 RA use.
They identified 2,025,034 drug-naive patients with T2D; of these, 284,685 were subsequently prescribed GLP-1 RAs, and 1,740,349 remained in the non–GLP-1 RA cohort. Following propensity score matching, there were 86,186 patients in each cohort.
Patients who received GLP-1 RAs had significantly lower odds of developing EO-CRC than those who received non–GLP-1 RAs (0.6% vs 0.9%; P < .001; odds ratio [OR], 0.61; 95% CI, 0.54-068).
Furthermore, a sub-analysis revealed that patients who were obese and taking GLP-1 RAs had significantly lower odds of developing EO-CRC than patients who were obese but not taking GLP-1 RAs (0.7% vs 1.1%; P < .001; OR, 0.58; 95% CI, 0.50-067).
A Proposed Protective Effect
Although GLP-1 RAs are indicated for the treatment of T2D and obesity, recent evidence suggests that they may play a role in reducing the risk for CRC as well. This protective effect may be produced not only by addressing T2D and obesity — both important risk factors for CRC — but also via cellular mechanisms, Olasehinde noted.
“GLP-1 receptors are widely expressed throughout the gastrointestinal tract, with various effects on tissues in the stomach, small intestine, and colon,” she explained. Specifically, activation of these receptors in the proximal and distal colon promotes the release of “important factors that protect and facilitate healing of the intestinal epithelium” and “regulate the gut microbiome.”
This is particularly relevant in EO-CRC, she added, given its greater association with T2D and obesity, both factors that “have been shown to create dysbiosis in the gut microbiome and low-grade inflammation via release of free radicals/inflammatory cytokines.”
These results provide more evidence that EO-CRC “is clinically and molecularly distinct from late-onset colorectal cancer,” which is important for both clinicians and patients to understand, said Olasehinde.
“It is imperative that we are all aware of the specific signs and symptoms this population presents with and the implications of this diagnosis in younger age groups,” she added. “Patients should continue making informed dietary and lifestyle modifications/choices to help reduce the burden of EO-CRC.”
Hypothesis-Generating Results
Aasma Shaukat, MD, MPH, who was not affiliated with the research, called the results promising but — at this stage — primarily useful for stimulating future research.
"We do need more studies such as this to generate hypotheses that can be studied prospectively," Shaukat, professor of medicine and population health, and director of GI Outcomes Research at NYU Langone Health in New York City, told Medscape Medical News.
She referred to another study, published in JAMA Oncology, that also used the TriNetX research network, which showed that GLP-1 RAs were associated with reduced CRC risk in drug-naive patients with T2D.
Shaukat also noted that the current analysis has limitations that should be considered. "The study is retrospective, and confounding is a possibility,” she said.
“How the groups that did and did not receive GLP-1 RAs differ in other risk factors that could be the drivers of the cancers is not known. Whether cancers were detected through screening or symptoms, stage, and other features that may differ are not known. Finally, since we don’t know who did or did not have colonoscopy, undiagnosed cancers are not known," she explained.
Shaukat, who was the lead author of the ACG 2021 Colorectal Cancer Screening Guidelines, added that the field would benefit from studies providing "biological plausibility information, such as animal studies to understand how GLP-1 RAs may modulate risk of colon cancer; other population-based cohort studies on the incidence of colon cancer among GLP-1 RA users and non-users; and prospective trials on chemoprevention."
The study had no specific funding. Olasehinde reported no relevant financial relationships. Shaukat reported serving as a consultant for Freenome, Medtronic, and Motus GI, as well as an advisory board member for Iterative Scopes Inc.
A version of this article appeared on Medscape.com.
PHILADELPHIA — according to the results of a retrospective study.
“This is the first large study to investigate the impact of GLP-1 RA use on EO-CRC risk,” principal investigator Temitope Olasehinde, MD, resident physician at the University Hospitals Cleveland Medical Center, Case Western Reserve University in Cleveland, Ohio, said in an interview.
The results indicate the GLP-1 RAs have a potentially protective role to play in combating EO-CRC, the incidence of which is notably rising in younger adults, with a corresponding increase in associated mortality.
Previous studies investigating the link between GLP-1 RAs and CRC did not capture patients aged younger than 50 years; thus, it was unknown if these results could be extrapolated to a younger age group, said Olasehinde.
The researcher presented the findings at the annual meeting of the American College of Gastroenterology.
Retrospective Database Analysis
Olasehinde and colleagues analyzed data from TriNetX, a large federated deidentified health research network, to identify patients (age ≤ 49 years) with diagnosed T2D subsequently prescribed antidiabetic medications who had not received a prior diagnosis of CRC. Additionally, patients were stratified on the basis of first-time GLP-1 RA use.
They identified 2,025,034 drug-naive patients with T2D; of these, 284,685 were subsequently prescribed GLP-1 RAs, and 1,740,349 remained in the non–GLP-1 RA cohort. Following propensity score matching, there were 86,186 patients in each cohort.
Patients who received GLP-1 RAs had significantly lower odds of developing EO-CRC than those who received non–GLP-1 RAs (0.6% vs 0.9%; P < .001; odds ratio [OR], 0.61; 95% CI, 0.54-068).
Furthermore, a sub-analysis revealed that patients who were obese and taking GLP-1 RAs had significantly lower odds of developing EO-CRC than patients who were obese but not taking GLP-1 RAs (0.7% vs 1.1%; P < .001; OR, 0.58; 95% CI, 0.50-067).
A Proposed Protective Effect
Although GLP-1 RAs are indicated for the treatment of T2D and obesity, recent evidence suggests that they may play a role in reducing the risk for CRC as well. This protective effect may be produced not only by addressing T2D and obesity — both important risk factors for CRC — but also via cellular mechanisms, Olasehinde noted.
“GLP-1 receptors are widely expressed throughout the gastrointestinal tract, with various effects on tissues in the stomach, small intestine, and colon,” she explained. Specifically, activation of these receptors in the proximal and distal colon promotes the release of “important factors that protect and facilitate healing of the intestinal epithelium” and “regulate the gut microbiome.”
This is particularly relevant in EO-CRC, she added, given its greater association with T2D and obesity, both factors that “have been shown to create dysbiosis in the gut microbiome and low-grade inflammation via release of free radicals/inflammatory cytokines.”
These results provide more evidence that EO-CRC “is clinically and molecularly distinct from late-onset colorectal cancer,” which is important for both clinicians and patients to understand, said Olasehinde.
“It is imperative that we are all aware of the specific signs and symptoms this population presents with and the implications of this diagnosis in younger age groups,” she added. “Patients should continue making informed dietary and lifestyle modifications/choices to help reduce the burden of EO-CRC.”
Hypothesis-Generating Results
Aasma Shaukat, MD, MPH, who was not affiliated with the research, called the results promising but — at this stage — primarily useful for stimulating future research.
"We do need more studies such as this to generate hypotheses that can be studied prospectively," Shaukat, professor of medicine and population health, and director of GI Outcomes Research at NYU Langone Health in New York City, told Medscape Medical News.
She referred to another study, published in JAMA Oncology, that also used the TriNetX research network, which showed that GLP-1 RAs were associated with reduced CRC risk in drug-naive patients with T2D.
Shaukat also noted that the current analysis has limitations that should be considered. "The study is retrospective, and confounding is a possibility,” she said.
“How the groups that did and did not receive GLP-1 RAs differ in other risk factors that could be the drivers of the cancers is not known. Whether cancers were detected through screening or symptoms, stage, and other features that may differ are not known. Finally, since we don’t know who did or did not have colonoscopy, undiagnosed cancers are not known," she explained.
Shaukat, who was the lead author of the ACG 2021 Colorectal Cancer Screening Guidelines, added that the field would benefit from studies providing "biological plausibility information, such as animal studies to understand how GLP-1 RAs may modulate risk of colon cancer; other population-based cohort studies on the incidence of colon cancer among GLP-1 RA users and non-users; and prospective trials on chemoprevention."
The study had no specific funding. Olasehinde reported no relevant financial relationships. Shaukat reported serving as a consultant for Freenome, Medtronic, and Motus GI, as well as an advisory board member for Iterative Scopes Inc.
A version of this article appeared on Medscape.com.
FROM ACG 2024
FIT Completion and Yield Similar in Younger and Older Adults
, a new study has found.
The study also found a similar low 3% rate of CRC detected at colonoscopy in both the younger and older adults.
“Our study suggests that adults ages 45-49 have a colorectal cancer risk that is similar to what we see in adults age 50,” senior author Jeffrey K. Lee, MD, MPH, gastroenterologist and research scientist at Kaiser Permanente Northern California Division of Research (DOR) in Oakland, California, said in a news release.
“The low number of cancers we found also provides support for initially offering younger adults a non-invasive test, like FIT, to determine which patients would benefit from a colonoscopy,” Lee noted.
Timely and Important Question
“This study addresses a timely and important clinical question, namely, is FIT an acceptable screening modality in patients aged 45-49,” Ziad F. Gellad, MD, MPH, AGAF, professor of medicine, Duke University Medical Center, Durham, North Carolina, who was not involved in the study, said in an interview.
“The finding that FIT completion and yield in younger patients is similar to those aged 50 and above is good news because it supports the use of this screening modality in the younger cohort,” said Gellad, section chief, gastroenterology, Durham VA Health Care System.
The study was published online in Annals of Internal Medicine.
In 2021, the US Preventive Services Task Force lowered the age to start CRC screening from 50 to 45 years, in response to studies showing an increased rate of CRC in adults aged 45-49 years.
The decision to start CRC screening at age 45 was made based on modeling studies, which are dependent on assumptions, co-first author Theodore R. Levin, MD, who is also a gastroenterologist and research scientist at Kaiser Permanente DOR, said in an interview.
“We thought it was important to collect real-world data on the experience of screening in this age group. We had no basis to know whether younger people would take up screening or if the yield of screening would be sufficiently high to warrant starting screening in this age group,” said Levin.
The researchers compared FIT screening completion and outcomes in 213,928 patients aged 45-49 years and 53,804 patients aged 50 years who received a FIT kit for the first time. The patients were from Kaiser Permanente Northern California, Washington, and Colorado.
Overall, FIT completion rates were slightly higher in the younger adults than in the 50-year-olds (38.9% vs 37.5%; adjusted risk ratio [aRR], 1.05), although the younger patients from Colorado were substantially less apt to complete a FIT (30.7% vs 40.2%; aRR, 0.77).
In the overall 45- to 49-year age group, 3.6% of adults had a positive FIT result, only slightly lower than the 4% positivity rate in the 50-year age group (aRR, 0.91).
About two thirds of adults in both groups who had a positive FIT result went on to have a colonoscopy within 3 months of receiving the test result.
Adenoma detection during colonoscopy was slightly lower in the younger than in the older group (58.8% vs 67.7%; aRR, 0.88). However, yields were similar for adenoma with advanced histology (13.2% vs 15.9%; aRR, 0.86), polyp with high-grade dysplasia (3.4% vs 5.1%; aRR, 0.68), sessile serrated lesion (10.3% vs 11.7%; aRR, 0.92), and CRC (2.8% vs 2.7%; aRR, 1.10).
FIT First Fits With Younger Adults’ Busy Lives
“Overall, people under 50 have lower incidence of cancer than people in their 50s, 60s, and 70s. However, if you do a test like FIT first, you can improve the yield of colonoscopy, which is a much more efficient strategy,” Levin said.
He noted that younger people are the least likely to be screened.
“They are busy with work and family responsibilities and may not realize that they are at risk for CRC. It is important to offer them a test that is easy to perform and does not require them to miss a day of work or arrange for a driver. They should be offered an option to screen with a stool-based test as an easy way to fit CRC screening into their busy lives,” Levin said.
Gellad said the study also highlights the limitations of FIT, “namely, that the low uptake and suboptimal colonoscopy follow-up of positive tests, also extend into the lower age group.”
Additionally, Gellad said he hopes other large systems will replicate this study to address the generalizability of these findings outside the Kaiser system.
The study was funded by the Kaiser Permanente Sydney R. Garfield Memorial Fund. Disclosures for study authors are available with the original article. Gellad consulted for Merck & Co. and Novo Nordisk and is a co-founder of Higgs Boson, Inc.
A version of this article appeared on Medscape.com.
, a new study has found.
The study also found a similar low 3% rate of CRC detected at colonoscopy in both the younger and older adults.
“Our study suggests that adults ages 45-49 have a colorectal cancer risk that is similar to what we see in adults age 50,” senior author Jeffrey K. Lee, MD, MPH, gastroenterologist and research scientist at Kaiser Permanente Northern California Division of Research (DOR) in Oakland, California, said in a news release.
“The low number of cancers we found also provides support for initially offering younger adults a non-invasive test, like FIT, to determine which patients would benefit from a colonoscopy,” Lee noted.
Timely and Important Question
“This study addresses a timely and important clinical question, namely, is FIT an acceptable screening modality in patients aged 45-49,” Ziad F. Gellad, MD, MPH, AGAF, professor of medicine, Duke University Medical Center, Durham, North Carolina, who was not involved in the study, said in an interview.
“The finding that FIT completion and yield in younger patients is similar to those aged 50 and above is good news because it supports the use of this screening modality in the younger cohort,” said Gellad, section chief, gastroenterology, Durham VA Health Care System.
The study was published online in Annals of Internal Medicine.
In 2021, the US Preventive Services Task Force lowered the age to start CRC screening from 50 to 45 years, in response to studies showing an increased rate of CRC in adults aged 45-49 years.
The decision to start CRC screening at age 45 was made based on modeling studies, which are dependent on assumptions, co-first author Theodore R. Levin, MD, who is also a gastroenterologist and research scientist at Kaiser Permanente DOR, said in an interview.
“We thought it was important to collect real-world data on the experience of screening in this age group. We had no basis to know whether younger people would take up screening or if the yield of screening would be sufficiently high to warrant starting screening in this age group,” said Levin.
The researchers compared FIT screening completion and outcomes in 213,928 patients aged 45-49 years and 53,804 patients aged 50 years who received a FIT kit for the first time. The patients were from Kaiser Permanente Northern California, Washington, and Colorado.
Overall, FIT completion rates were slightly higher in the younger adults than in the 50-year-olds (38.9% vs 37.5%; adjusted risk ratio [aRR], 1.05), although the younger patients from Colorado were substantially less apt to complete a FIT (30.7% vs 40.2%; aRR, 0.77).
In the overall 45- to 49-year age group, 3.6% of adults had a positive FIT result, only slightly lower than the 4% positivity rate in the 50-year age group (aRR, 0.91).
About two thirds of adults in both groups who had a positive FIT result went on to have a colonoscopy within 3 months of receiving the test result.
Adenoma detection during colonoscopy was slightly lower in the younger than in the older group (58.8% vs 67.7%; aRR, 0.88). However, yields were similar for adenoma with advanced histology (13.2% vs 15.9%; aRR, 0.86), polyp with high-grade dysplasia (3.4% vs 5.1%; aRR, 0.68), sessile serrated lesion (10.3% vs 11.7%; aRR, 0.92), and CRC (2.8% vs 2.7%; aRR, 1.10).
FIT First Fits With Younger Adults’ Busy Lives
“Overall, people under 50 have lower incidence of cancer than people in their 50s, 60s, and 70s. However, if you do a test like FIT first, you can improve the yield of colonoscopy, which is a much more efficient strategy,” Levin said.
He noted that younger people are the least likely to be screened.
“They are busy with work and family responsibilities and may not realize that they are at risk for CRC. It is important to offer them a test that is easy to perform and does not require them to miss a day of work or arrange for a driver. They should be offered an option to screen with a stool-based test as an easy way to fit CRC screening into their busy lives,” Levin said.
Gellad said the study also highlights the limitations of FIT, “namely, that the low uptake and suboptimal colonoscopy follow-up of positive tests, also extend into the lower age group.”
Additionally, Gellad said he hopes other large systems will replicate this study to address the generalizability of these findings outside the Kaiser system.
The study was funded by the Kaiser Permanente Sydney R. Garfield Memorial Fund. Disclosures for study authors are available with the original article. Gellad consulted for Merck & Co. and Novo Nordisk and is a co-founder of Higgs Boson, Inc.
A version of this article appeared on Medscape.com.
, a new study has found.
The study also found a similar low 3% rate of CRC detected at colonoscopy in both the younger and older adults.
“Our study suggests that adults ages 45-49 have a colorectal cancer risk that is similar to what we see in adults age 50,” senior author Jeffrey K. Lee, MD, MPH, gastroenterologist and research scientist at Kaiser Permanente Northern California Division of Research (DOR) in Oakland, California, said in a news release.
“The low number of cancers we found also provides support for initially offering younger adults a non-invasive test, like FIT, to determine which patients would benefit from a colonoscopy,” Lee noted.
Timely and Important Question
“This study addresses a timely and important clinical question, namely, is FIT an acceptable screening modality in patients aged 45-49,” Ziad F. Gellad, MD, MPH, AGAF, professor of medicine, Duke University Medical Center, Durham, North Carolina, who was not involved in the study, said in an interview.
“The finding that FIT completion and yield in younger patients is similar to those aged 50 and above is good news because it supports the use of this screening modality in the younger cohort,” said Gellad, section chief, gastroenterology, Durham VA Health Care System.
The study was published online in Annals of Internal Medicine.
In 2021, the US Preventive Services Task Force lowered the age to start CRC screening from 50 to 45 years, in response to studies showing an increased rate of CRC in adults aged 45-49 years.
The decision to start CRC screening at age 45 was made based on modeling studies, which are dependent on assumptions, co-first author Theodore R. Levin, MD, who is also a gastroenterologist and research scientist at Kaiser Permanente DOR, said in an interview.
“We thought it was important to collect real-world data on the experience of screening in this age group. We had no basis to know whether younger people would take up screening or if the yield of screening would be sufficiently high to warrant starting screening in this age group,” said Levin.
The researchers compared FIT screening completion and outcomes in 213,928 patients aged 45-49 years and 53,804 patients aged 50 years who received a FIT kit for the first time. The patients were from Kaiser Permanente Northern California, Washington, and Colorado.
Overall, FIT completion rates were slightly higher in the younger adults than in the 50-year-olds (38.9% vs 37.5%; adjusted risk ratio [aRR], 1.05), although the younger patients from Colorado were substantially less apt to complete a FIT (30.7% vs 40.2%; aRR, 0.77).
In the overall 45- to 49-year age group, 3.6% of adults had a positive FIT result, only slightly lower than the 4% positivity rate in the 50-year age group (aRR, 0.91).
About two thirds of adults in both groups who had a positive FIT result went on to have a colonoscopy within 3 months of receiving the test result.
Adenoma detection during colonoscopy was slightly lower in the younger than in the older group (58.8% vs 67.7%; aRR, 0.88). However, yields were similar for adenoma with advanced histology (13.2% vs 15.9%; aRR, 0.86), polyp with high-grade dysplasia (3.4% vs 5.1%; aRR, 0.68), sessile serrated lesion (10.3% vs 11.7%; aRR, 0.92), and CRC (2.8% vs 2.7%; aRR, 1.10).
FIT First Fits With Younger Adults’ Busy Lives
“Overall, people under 50 have lower incidence of cancer than people in their 50s, 60s, and 70s. However, if you do a test like FIT first, you can improve the yield of colonoscopy, which is a much more efficient strategy,” Levin said.
He noted that younger people are the least likely to be screened.
“They are busy with work and family responsibilities and may not realize that they are at risk for CRC. It is important to offer them a test that is easy to perform and does not require them to miss a day of work or arrange for a driver. They should be offered an option to screen with a stool-based test as an easy way to fit CRC screening into their busy lives,” Levin said.
Gellad said the study also highlights the limitations of FIT, “namely, that the low uptake and suboptimal colonoscopy follow-up of positive tests, also extend into the lower age group.”
Additionally, Gellad said he hopes other large systems will replicate this study to address the generalizability of these findings outside the Kaiser system.
The study was funded by the Kaiser Permanente Sydney R. Garfield Memorial Fund. Disclosures for study authors are available with the original article. Gellad consulted for Merck & Co. and Novo Nordisk and is a co-founder of Higgs Boson, Inc.
A version of this article appeared on Medscape.com.
When It Comes to Polyp Diagnosis With CADx, Location Matters
VIENNA —
In particular, the diagnostic performance of CADx for polyps showed significantly lower specificity in the proximal colon than in the distal colon.
“While current CADx systems are suitable for use in the distal colon, they should not be employed for diagnosing polyps in the proximal colon until new, higher performing systems are developed specifically for these lesions,” said study lead Tommy Rizkala, MD, Endoscopy Unit, IRCCS Humanitas Clinical and Research Center, Rozzano, Italy.
The “main strength” of the review is that the researchers contacted each study author for more specific information and were therefore able to divide the data into the proximal colon and the rectosigmoid colon, he explained.
“This is the first paper that has really collected these data. Most papers provide data for the entire colon or just for the rectosigmoid colon,” said Rizkala, who presented the findings at the United European Gastroenterology (UEG) Week 2024.
The study was also recently published in Clinical Gastroenterology and Hepatology.
Optical diagnosis enables real-time histologic predictions of polyps 5 mm or smaller during colonoscopy, offering potential clinical and cost-saving benefits. Two optical diagnostic strategies are used for polyps in this size range based on location: A leave-in-situ strategy (applied only in the rectosigmoid colon when there is high confidence of non-neoplastic polyps) and a resect-and-discard strategy (applied only in the whole colon when there is high confidence of neoplastic polyps upon optical diagnosis).
Rizkala carried out a review of studies that evaluated the performance of real-time CADx alone — independent of endoscopist judgment — for predicting the histology of colorectal polyps 5 mm or smaller. The primary endpoints were CADx sensitivity and specificity in the proximal colon (the portion extending from the descending colon to the cecum) and the distal colon (limited to the rectosigmoid region). Secondary outcomes were the negative predictive value (NPV), positive predictive value (PPV), and accuracy of the CADx alone in the proximal colon and the distal colon.
Lower Specificity in the Proximal Colon
An analysis of data based on 7782 polyps ≤ 5 mm from 11 studies found specificity values of 0.62 (95% CI, 0.52-0.71) and 0.85 (95% CI, 0.75-0.92) for the proximal and distal regions of the colon, respectively, with a risk ratio (RR) of 0.74 (95% CI, 0.72-0.84), meaning that CADx accuracy was significantly lower in the proximal colon than in the distal colon.
“According to the optical diagnosis strategy, we can use the leave-in-situ approach for the distal colon because the performance is adequate, but for the rest of the colon, CADx requires further enhancement,” Rizkala said.
Sensitivity values were 0.89 (95% CI, 0.83-0.93) and 0.87 (95% CI, 0.80-0.92) for the proximal and distal regions, respectively, with an RR of 1.00 (95% CI, 0.97-1.03).
Regarding the secondary outcomes, the NPV was 0.64 vs 0.93 for the proximal vs distal colon, with an RR of 0.71 (95% CI, 0.64-0.79), and accuracy was 0.81 vs 0.86, with an RR of 0.95 (95% CI, 0.91-0.99).
With the higher prevalence of neoplastic lesions in the proximal colon than in the distal colon, a lower NPV was observed in the proximal colon, Rizkala noted.
The PPV was 0.87 vs 0.76 for the proximal vs distal colon, with an RR of 1.11 (95% CI, 1.06-1.17), so the two parts of the colon were comparable, he reported.
In the future, CADx systems should focus on using lesions from the proximal colon to train more accurately because currently CADx systems are trained on the available endoscopic data in which most of those polyps are from the rectosigmoid colon, Rizkala said.
We would also “like manufacturers of CADx systems to provide public access to data balanced between the proximal and distal regions of the colon,” he added.
Diagnosis More Challenging Than Detection With CADx
Commenting on the study, comoderator David G. Graham, MD, consultant gastroenterologist at University College London Hospital in England, remarked: “The key questions here relate to why are these systems underperforming in the proximal colon, and how can we improve this?”
Are these results “due to the very different appearance of adenomas in the distal colon vs the proximal colon on CADx (which is not what we see as endoscopists but seems to be what the systems are seeing), or is it due to a different characterization of polyps,” that is, more sessile serrated lesions in the proximal colon than in the distal colon, he asked.
Also commenting on the study was Raf Bisschops, MD, head of endoscopy at KU Leuven in Belgium. He remarked that the review underscores the fact that optical diagnosis by artificial intelligence is a more challenging task than detection.
It is “not entirely clear” what would explain the difference in performance of CADx between the distal colon and proximal colon, he said. It can’t be excluded that the inclusion of different CADx systems, some of which clearly underperformed, may account for the difference.
He went on to suggest that the differences might be down to location beyond proximal and distal.
“The difference in performance between the right and left colon is also interesting, since recent insights in the molecular and morphological features of hyperplastic polyps indicates that there are different classes with more goblet cell–rich hyperplastic polyps in the right colon, and more microvesicular hyperplastic polyps in the left.”
These have “distinct microscopic and endoscopic appearances” that could account for a difference in performance of a CADx system if not included in the training and validation sets, he explained.
Rizkala and Graham reported no relevant disclosures. Bisschops reported receiving research grants and speaker fees from Medtronic, Fujifilm, and Pentax.
A version of this article first appeared on Medscape.com.
VIENNA —
In particular, the diagnostic performance of CADx for polyps showed significantly lower specificity in the proximal colon than in the distal colon.
“While current CADx systems are suitable for use in the distal colon, they should not be employed for diagnosing polyps in the proximal colon until new, higher performing systems are developed specifically for these lesions,” said study lead Tommy Rizkala, MD, Endoscopy Unit, IRCCS Humanitas Clinical and Research Center, Rozzano, Italy.
The “main strength” of the review is that the researchers contacted each study author for more specific information and were therefore able to divide the data into the proximal colon and the rectosigmoid colon, he explained.
“This is the first paper that has really collected these data. Most papers provide data for the entire colon or just for the rectosigmoid colon,” said Rizkala, who presented the findings at the United European Gastroenterology (UEG) Week 2024.
The study was also recently published in Clinical Gastroenterology and Hepatology.
Optical diagnosis enables real-time histologic predictions of polyps 5 mm or smaller during colonoscopy, offering potential clinical and cost-saving benefits. Two optical diagnostic strategies are used for polyps in this size range based on location: A leave-in-situ strategy (applied only in the rectosigmoid colon when there is high confidence of non-neoplastic polyps) and a resect-and-discard strategy (applied only in the whole colon when there is high confidence of neoplastic polyps upon optical diagnosis).
Rizkala carried out a review of studies that evaluated the performance of real-time CADx alone — independent of endoscopist judgment — for predicting the histology of colorectal polyps 5 mm or smaller. The primary endpoints were CADx sensitivity and specificity in the proximal colon (the portion extending from the descending colon to the cecum) and the distal colon (limited to the rectosigmoid region). Secondary outcomes were the negative predictive value (NPV), positive predictive value (PPV), and accuracy of the CADx alone in the proximal colon and the distal colon.
Lower Specificity in the Proximal Colon
An analysis of data based on 7782 polyps ≤ 5 mm from 11 studies found specificity values of 0.62 (95% CI, 0.52-0.71) and 0.85 (95% CI, 0.75-0.92) for the proximal and distal regions of the colon, respectively, with a risk ratio (RR) of 0.74 (95% CI, 0.72-0.84), meaning that CADx accuracy was significantly lower in the proximal colon than in the distal colon.
“According to the optical diagnosis strategy, we can use the leave-in-situ approach for the distal colon because the performance is adequate, but for the rest of the colon, CADx requires further enhancement,” Rizkala said.
Sensitivity values were 0.89 (95% CI, 0.83-0.93) and 0.87 (95% CI, 0.80-0.92) for the proximal and distal regions, respectively, with an RR of 1.00 (95% CI, 0.97-1.03).
Regarding the secondary outcomes, the NPV was 0.64 vs 0.93 for the proximal vs distal colon, with an RR of 0.71 (95% CI, 0.64-0.79), and accuracy was 0.81 vs 0.86, with an RR of 0.95 (95% CI, 0.91-0.99).
With the higher prevalence of neoplastic lesions in the proximal colon than in the distal colon, a lower NPV was observed in the proximal colon, Rizkala noted.
The PPV was 0.87 vs 0.76 for the proximal vs distal colon, with an RR of 1.11 (95% CI, 1.06-1.17), so the two parts of the colon were comparable, he reported.
In the future, CADx systems should focus on using lesions from the proximal colon to train more accurately because currently CADx systems are trained on the available endoscopic data in which most of those polyps are from the rectosigmoid colon, Rizkala said.
We would also “like manufacturers of CADx systems to provide public access to data balanced between the proximal and distal regions of the colon,” he added.
Diagnosis More Challenging Than Detection With CADx
Commenting on the study, comoderator David G. Graham, MD, consultant gastroenterologist at University College London Hospital in England, remarked: “The key questions here relate to why are these systems underperforming in the proximal colon, and how can we improve this?”
Are these results “due to the very different appearance of adenomas in the distal colon vs the proximal colon on CADx (which is not what we see as endoscopists but seems to be what the systems are seeing), or is it due to a different characterization of polyps,” that is, more sessile serrated lesions in the proximal colon than in the distal colon, he asked.
Also commenting on the study was Raf Bisschops, MD, head of endoscopy at KU Leuven in Belgium. He remarked that the review underscores the fact that optical diagnosis by artificial intelligence is a more challenging task than detection.
It is “not entirely clear” what would explain the difference in performance of CADx between the distal colon and proximal colon, he said. It can’t be excluded that the inclusion of different CADx systems, some of which clearly underperformed, may account for the difference.
He went on to suggest that the differences might be down to location beyond proximal and distal.
“The difference in performance between the right and left colon is also interesting, since recent insights in the molecular and morphological features of hyperplastic polyps indicates that there are different classes with more goblet cell–rich hyperplastic polyps in the right colon, and more microvesicular hyperplastic polyps in the left.”
These have “distinct microscopic and endoscopic appearances” that could account for a difference in performance of a CADx system if not included in the training and validation sets, he explained.
Rizkala and Graham reported no relevant disclosures. Bisschops reported receiving research grants and speaker fees from Medtronic, Fujifilm, and Pentax.
A version of this article first appeared on Medscape.com.
VIENNA —
In particular, the diagnostic performance of CADx for polyps showed significantly lower specificity in the proximal colon than in the distal colon.
“While current CADx systems are suitable for use in the distal colon, they should not be employed for diagnosing polyps in the proximal colon until new, higher performing systems are developed specifically for these lesions,” said study lead Tommy Rizkala, MD, Endoscopy Unit, IRCCS Humanitas Clinical and Research Center, Rozzano, Italy.
The “main strength” of the review is that the researchers contacted each study author for more specific information and were therefore able to divide the data into the proximal colon and the rectosigmoid colon, he explained.
“This is the first paper that has really collected these data. Most papers provide data for the entire colon or just for the rectosigmoid colon,” said Rizkala, who presented the findings at the United European Gastroenterology (UEG) Week 2024.
The study was also recently published in Clinical Gastroenterology and Hepatology.
Optical diagnosis enables real-time histologic predictions of polyps 5 mm or smaller during colonoscopy, offering potential clinical and cost-saving benefits. Two optical diagnostic strategies are used for polyps in this size range based on location: A leave-in-situ strategy (applied only in the rectosigmoid colon when there is high confidence of non-neoplastic polyps) and a resect-and-discard strategy (applied only in the whole colon when there is high confidence of neoplastic polyps upon optical diagnosis).
Rizkala carried out a review of studies that evaluated the performance of real-time CADx alone — independent of endoscopist judgment — for predicting the histology of colorectal polyps 5 mm or smaller. The primary endpoints were CADx sensitivity and specificity in the proximal colon (the portion extending from the descending colon to the cecum) and the distal colon (limited to the rectosigmoid region). Secondary outcomes were the negative predictive value (NPV), positive predictive value (PPV), and accuracy of the CADx alone in the proximal colon and the distal colon.
Lower Specificity in the Proximal Colon
An analysis of data based on 7782 polyps ≤ 5 mm from 11 studies found specificity values of 0.62 (95% CI, 0.52-0.71) and 0.85 (95% CI, 0.75-0.92) for the proximal and distal regions of the colon, respectively, with a risk ratio (RR) of 0.74 (95% CI, 0.72-0.84), meaning that CADx accuracy was significantly lower in the proximal colon than in the distal colon.
“According to the optical diagnosis strategy, we can use the leave-in-situ approach for the distal colon because the performance is adequate, but for the rest of the colon, CADx requires further enhancement,” Rizkala said.
Sensitivity values were 0.89 (95% CI, 0.83-0.93) and 0.87 (95% CI, 0.80-0.92) for the proximal and distal regions, respectively, with an RR of 1.00 (95% CI, 0.97-1.03).
Regarding the secondary outcomes, the NPV was 0.64 vs 0.93 for the proximal vs distal colon, with an RR of 0.71 (95% CI, 0.64-0.79), and accuracy was 0.81 vs 0.86, with an RR of 0.95 (95% CI, 0.91-0.99).
With the higher prevalence of neoplastic lesions in the proximal colon than in the distal colon, a lower NPV was observed in the proximal colon, Rizkala noted.
The PPV was 0.87 vs 0.76 for the proximal vs distal colon, with an RR of 1.11 (95% CI, 1.06-1.17), so the two parts of the colon were comparable, he reported.
In the future, CADx systems should focus on using lesions from the proximal colon to train more accurately because currently CADx systems are trained on the available endoscopic data in which most of those polyps are from the rectosigmoid colon, Rizkala said.
We would also “like manufacturers of CADx systems to provide public access to data balanced between the proximal and distal regions of the colon,” he added.
Diagnosis More Challenging Than Detection With CADx
Commenting on the study, comoderator David G. Graham, MD, consultant gastroenterologist at University College London Hospital in England, remarked: “The key questions here relate to why are these systems underperforming in the proximal colon, and how can we improve this?”
Are these results “due to the very different appearance of adenomas in the distal colon vs the proximal colon on CADx (which is not what we see as endoscopists but seems to be what the systems are seeing), or is it due to a different characterization of polyps,” that is, more sessile serrated lesions in the proximal colon than in the distal colon, he asked.
Also commenting on the study was Raf Bisschops, MD, head of endoscopy at KU Leuven in Belgium. He remarked that the review underscores the fact that optical diagnosis by artificial intelligence is a more challenging task than detection.
It is “not entirely clear” what would explain the difference in performance of CADx between the distal colon and proximal colon, he said. It can’t be excluded that the inclusion of different CADx systems, some of which clearly underperformed, may account for the difference.
He went on to suggest that the differences might be down to location beyond proximal and distal.
“The difference in performance between the right and left colon is also interesting, since recent insights in the molecular and morphological features of hyperplastic polyps indicates that there are different classes with more goblet cell–rich hyperplastic polyps in the right colon, and more microvesicular hyperplastic polyps in the left.”
These have “distinct microscopic and endoscopic appearances” that could account for a difference in performance of a CADx system if not included in the training and validation sets, he explained.
Rizkala and Graham reported no relevant disclosures. Bisschops reported receiving research grants and speaker fees from Medtronic, Fujifilm, and Pentax.
A version of this article first appeared on Medscape.com.
FROM UEG 2024
Mortality Rates From Early-Onset CRC Have Risen Considerably Over Last 2 Decades
PHILADELPHIA — according to a new analysis of the two largest US mortality databases.
Data from the Centers for Disease Control and Prevention’s National Center of Health Statistics (NCHS) and the Surveillance, Epidemiology, and End Results (SEER) databases provide yet more evidence of the increasing prevalence of EO-CRC, which is defined as a diagnosis of CRC in patients younger than age 50 years.
Furthermore, the researchers reported that increased mortality occurred across all patients included in the study (aged 20-54) regardless of tumor stage at diagnosis.
These findings “prompt tailoring further efforts toward raising awareness of colorectal cancer symptoms and keeping a low clinical suspicion in younger patients presenting with anemia, gastrointestinal bleeding, or change in bowel habits,” Yazan Abboud, MD, internal medicine PGY-3, assistant chief resident, and chair of resident research at Rutgers New Jersey Medical School, Newark, said in an interview.
Abboud presented the findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Analyzing NCHS and SEER
Rising rates of EO-CRC had prompted US medical societies to recommend reducing the screening age to 45 years. The US Preventive Services Task Force officially lowered it to this age in 2021. This shift is supported by real-world evidence, which shows that earlier screening leads to a significantly reduced risk for colorectal cancer. However, because colorectal cancer cases are decreasing overall in older adults, there is considerable interest in discovering why young adults are experiencing a paradoxical uptick in EO-CRC, and what impact this is having on associated mortality.
Abboud and colleagues collected age-adjusted mortality rates for EO-CRC between 2000 and 2022 from the NCHS database. In addition, stage-specific incidence-based mortality rates between 2004-2020 were obtained from the SEER 22 database. The NCHS database covers approximately 100% of the US population, whereas the SEER 22 database, which is included within the NCHS, covers 42%.
The researchers divided patients into two cohorts based on age (20-44 years and 45-54 years) and tumor stage at diagnosis (early stage and late stage), and compared the annual percentage change (APC) and the average APC between the two groups. They also assessed trends for the entire cohort of patients aged 20-54 years.
In the NCHS database, there were 147,026 deaths in total across all ages studied resulting from EO-CRC, of which 27% (39,746) occurred in those 20-44 years of age. Although associated mortality rates decreased between 2000-2005 in all ages studied (APC, –1.56), they increased from 2005-2022 (APC, 0.87).
In the cohort aged 45-54 years, mortality decreased between 2000-2005 and increased thereafter, whereas in the cohort aged 20-44 years mortality increased steadily for the entire follow-up duration of 2000 to 2022 (APC, 0.93). A comparison of the age cohorts confirmed that those aged 20-44 years had a greater increase in mortality (average APC, 0.85; P < .001).
In the SEER 22 database, there were 4652 deaths in those with early-stage tumors across all age groups studied (average APC, 12.17). Mortality increased in patients aged 45-54 years (average APC, 11.52) with early-stage tumors, but there were insufficient numbers in those aged 20-44 years to determine this outcome.
There were 42,120 deaths in those with late-stage tumors across all age groups (average APC, 10.05) in the SEER 22 database. And increased mortality was observed in those with late-stage tumors in both age cohorts: 45-54 years (average APC, 9.58) and 20-44 years (average APC, 11.06).
“When evaluating the SEER database and stratifying the tumors by stage at diagnosis, we demonstrated increasing mortality of early-onset colorectal cancer in both early- and late-stage tumors on average over the study period,” Abboud said.
Identifying At-Risk Patients
In a comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia School of Medicine in Norfolk, said the findings speak to the need for evidence-based means of identifying younger individuals at a higher risk of EO-CRC.
“I suspect many of younger patients with CRC had their cancer detected when it was more advanced due to delayed presentation and diagnostic testing,” said Johnson, who was not involved in the study.
But it would be interesting to evaluate if the cancers in the cohort aged 20-44 years were more aggressive biologically or if these patients were dismissive of early signs or symptoms, he said.
Younger patients may dismiss “alarm” features that indicate CRC testing, said Johnson. “In particular, overt bleeding and iron deficiency need a focused evaluation in these younger cohorts.”
“Future research is needed to investigate the role of neoadjuvant chemotherapy in younger patients with early-stage colorectal cancer and evaluate patients’ outcomes,” Abboud added.
The study had no specific funding. Abboud reported no relevant financial relationships. Johnson reported serving as an adviser to ISOTHRIVE. He is also on the Medscape Gastroenterology editorial board.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a new analysis of the two largest US mortality databases.
Data from the Centers for Disease Control and Prevention’s National Center of Health Statistics (NCHS) and the Surveillance, Epidemiology, and End Results (SEER) databases provide yet more evidence of the increasing prevalence of EO-CRC, which is defined as a diagnosis of CRC in patients younger than age 50 years.
Furthermore, the researchers reported that increased mortality occurred across all patients included in the study (aged 20-54) regardless of tumor stage at diagnosis.
These findings “prompt tailoring further efforts toward raising awareness of colorectal cancer symptoms and keeping a low clinical suspicion in younger patients presenting with anemia, gastrointestinal bleeding, or change in bowel habits,” Yazan Abboud, MD, internal medicine PGY-3, assistant chief resident, and chair of resident research at Rutgers New Jersey Medical School, Newark, said in an interview.
Abboud presented the findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Analyzing NCHS and SEER
Rising rates of EO-CRC had prompted US medical societies to recommend reducing the screening age to 45 years. The US Preventive Services Task Force officially lowered it to this age in 2021. This shift is supported by real-world evidence, which shows that earlier screening leads to a significantly reduced risk for colorectal cancer. However, because colorectal cancer cases are decreasing overall in older adults, there is considerable interest in discovering why young adults are experiencing a paradoxical uptick in EO-CRC, and what impact this is having on associated mortality.
Abboud and colleagues collected age-adjusted mortality rates for EO-CRC between 2000 and 2022 from the NCHS database. In addition, stage-specific incidence-based mortality rates between 2004-2020 were obtained from the SEER 22 database. The NCHS database covers approximately 100% of the US population, whereas the SEER 22 database, which is included within the NCHS, covers 42%.
The researchers divided patients into two cohorts based on age (20-44 years and 45-54 years) and tumor stage at diagnosis (early stage and late stage), and compared the annual percentage change (APC) and the average APC between the two groups. They also assessed trends for the entire cohort of patients aged 20-54 years.
In the NCHS database, there were 147,026 deaths in total across all ages studied resulting from EO-CRC, of which 27% (39,746) occurred in those 20-44 years of age. Although associated mortality rates decreased between 2000-2005 in all ages studied (APC, –1.56), they increased from 2005-2022 (APC, 0.87).
In the cohort aged 45-54 years, mortality decreased between 2000-2005 and increased thereafter, whereas in the cohort aged 20-44 years mortality increased steadily for the entire follow-up duration of 2000 to 2022 (APC, 0.93). A comparison of the age cohorts confirmed that those aged 20-44 years had a greater increase in mortality (average APC, 0.85; P < .001).
In the SEER 22 database, there were 4652 deaths in those with early-stage tumors across all age groups studied (average APC, 12.17). Mortality increased in patients aged 45-54 years (average APC, 11.52) with early-stage tumors, but there were insufficient numbers in those aged 20-44 years to determine this outcome.
There were 42,120 deaths in those with late-stage tumors across all age groups (average APC, 10.05) in the SEER 22 database. And increased mortality was observed in those with late-stage tumors in both age cohorts: 45-54 years (average APC, 9.58) and 20-44 years (average APC, 11.06).
“When evaluating the SEER database and stratifying the tumors by stage at diagnosis, we demonstrated increasing mortality of early-onset colorectal cancer in both early- and late-stage tumors on average over the study period,” Abboud said.
Identifying At-Risk Patients
In a comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia School of Medicine in Norfolk, said the findings speak to the need for evidence-based means of identifying younger individuals at a higher risk of EO-CRC.
“I suspect many of younger patients with CRC had their cancer detected when it was more advanced due to delayed presentation and diagnostic testing,” said Johnson, who was not involved in the study.
But it would be interesting to evaluate if the cancers in the cohort aged 20-44 years were more aggressive biologically or if these patients were dismissive of early signs or symptoms, he said.
Younger patients may dismiss “alarm” features that indicate CRC testing, said Johnson. “In particular, overt bleeding and iron deficiency need a focused evaluation in these younger cohorts.”
“Future research is needed to investigate the role of neoadjuvant chemotherapy in younger patients with early-stage colorectal cancer and evaluate patients’ outcomes,” Abboud added.
The study had no specific funding. Abboud reported no relevant financial relationships. Johnson reported serving as an adviser to ISOTHRIVE. He is also on the Medscape Gastroenterology editorial board.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a new analysis of the two largest US mortality databases.
Data from the Centers for Disease Control and Prevention’s National Center of Health Statistics (NCHS) and the Surveillance, Epidemiology, and End Results (SEER) databases provide yet more evidence of the increasing prevalence of EO-CRC, which is defined as a diagnosis of CRC in patients younger than age 50 years.
Furthermore, the researchers reported that increased mortality occurred across all patients included in the study (aged 20-54) regardless of tumor stage at diagnosis.
These findings “prompt tailoring further efforts toward raising awareness of colorectal cancer symptoms and keeping a low clinical suspicion in younger patients presenting with anemia, gastrointestinal bleeding, or change in bowel habits,” Yazan Abboud, MD, internal medicine PGY-3, assistant chief resident, and chair of resident research at Rutgers New Jersey Medical School, Newark, said in an interview.
Abboud presented the findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Analyzing NCHS and SEER
Rising rates of EO-CRC had prompted US medical societies to recommend reducing the screening age to 45 years. The US Preventive Services Task Force officially lowered it to this age in 2021. This shift is supported by real-world evidence, which shows that earlier screening leads to a significantly reduced risk for colorectal cancer. However, because colorectal cancer cases are decreasing overall in older adults, there is considerable interest in discovering why young adults are experiencing a paradoxical uptick in EO-CRC, and what impact this is having on associated mortality.
Abboud and colleagues collected age-adjusted mortality rates for EO-CRC between 2000 and 2022 from the NCHS database. In addition, stage-specific incidence-based mortality rates between 2004-2020 were obtained from the SEER 22 database. The NCHS database covers approximately 100% of the US population, whereas the SEER 22 database, which is included within the NCHS, covers 42%.
The researchers divided patients into two cohorts based on age (20-44 years and 45-54 years) and tumor stage at diagnosis (early stage and late stage), and compared the annual percentage change (APC) and the average APC between the two groups. They also assessed trends for the entire cohort of patients aged 20-54 years.
In the NCHS database, there were 147,026 deaths in total across all ages studied resulting from EO-CRC, of which 27% (39,746) occurred in those 20-44 years of age. Although associated mortality rates decreased between 2000-2005 in all ages studied (APC, –1.56), they increased from 2005-2022 (APC, 0.87).
In the cohort aged 45-54 years, mortality decreased between 2000-2005 and increased thereafter, whereas in the cohort aged 20-44 years mortality increased steadily for the entire follow-up duration of 2000 to 2022 (APC, 0.93). A comparison of the age cohorts confirmed that those aged 20-44 years had a greater increase in mortality (average APC, 0.85; P < .001).
In the SEER 22 database, there were 4652 deaths in those with early-stage tumors across all age groups studied (average APC, 12.17). Mortality increased in patients aged 45-54 years (average APC, 11.52) with early-stage tumors, but there were insufficient numbers in those aged 20-44 years to determine this outcome.
There were 42,120 deaths in those with late-stage tumors across all age groups (average APC, 10.05) in the SEER 22 database. And increased mortality was observed in those with late-stage tumors in both age cohorts: 45-54 years (average APC, 9.58) and 20-44 years (average APC, 11.06).
“When evaluating the SEER database and stratifying the tumors by stage at diagnosis, we demonstrated increasing mortality of early-onset colorectal cancer in both early- and late-stage tumors on average over the study period,” Abboud said.
Identifying At-Risk Patients
In a comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia School of Medicine in Norfolk, said the findings speak to the need for evidence-based means of identifying younger individuals at a higher risk of EO-CRC.
“I suspect many of younger patients with CRC had their cancer detected when it was more advanced due to delayed presentation and diagnostic testing,” said Johnson, who was not involved in the study.
But it would be interesting to evaluate if the cancers in the cohort aged 20-44 years were more aggressive biologically or if these patients were dismissive of early signs or symptoms, he said.
Younger patients may dismiss “alarm” features that indicate CRC testing, said Johnson. “In particular, overt bleeding and iron deficiency need a focused evaluation in these younger cohorts.”
“Future research is needed to investigate the role of neoadjuvant chemotherapy in younger patients with early-stage colorectal cancer and evaluate patients’ outcomes,” Abboud added.
The study had no specific funding. Abboud reported no relevant financial relationships. Johnson reported serving as an adviser to ISOTHRIVE. He is also on the Medscape Gastroenterology editorial board.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Molecular Stool Testing Could Cut Post-Polypectomy Colonoscopies by 15%-41%
It might also reduce colonoscopies by an estimated 15%-41%.
The greatest reduction would likely be achieved by annual FIT-based surveillance, especially with FIT FOB-Gold at a threshold of at least 32 µg/g feces, according to findings from the Dutch MOCCAS study published in Gastroenterology.
In this cross-sectional observational study, the multitarget DNA test outperformed FIT for detecting advanced precursor lesions, especially serrated polyps. According to long-term-impact mathematical modeling, however, DNA-based surveillance would be more costly than colonoscopy surveillance, whereas FIT would save costs.
“With the worldwide implementation of FIT-based screening programs, following a positive test, many more people enter surveillance programs after polypectomy. This results in an increased pressure on the colonoscopy capacity and healthcare budgets,” lead author Beatriz Carvalho, PhD, a molecular biologist in the Department of Pathology of the Netherlands Cancer Institute in Amsterdam, said in an interview.
A noninvasive strategy could ease the surveillance burden on healthcare resources and be more palatable to patients. Post-polypectomy guidelines have already been relaxed to allow less intensive surveillance.
“Our working hypothesis was that although the sensitivity of a singular molecular test to detect CRC or advanced adenomas is lower than that of colonoscopy, repeating molecular stool testing would yield similar detection rates as colonoscopy-based surveillance. And our hypothesis was confirmed,” Carvalho said.
The results of the MOCCAS study align with those of other studies that found that FIT could be safely applied as a triage test in post-polypectomy surveillance and could safely extend the interval of surveillance colonoscopy. “But these studies did not include a long-term impact analysis,” she said. “The next step is to run a prospective interventional study to validate the MOCCAS findings.”
Offering an outsider’s perspective on the findings, Uri Ladabaum, MD, director of the Gastrointestinal Cancer Prevention Program and a professor of medicine at Stanford University School of Medicine in Palo Alto, California, said the real-world results on lesion detection and the multi-year-horizon modeling performed are provocative and point to the potential to base post-polypectomy surveillance on stool tests.
He cautioned, however, that the proposed paradigm requires the ability to deploy FIT-based surveillance with broad flexibility in relation to hemoglobin-detection thresholds and testing interval, depending on the specific FIT that is chosen, with the possibility these may differ by setting based on the characteristics of the population and the relevant epidemiology.
“Such flexibility may or may not be technically feasible in all settings — for instance, in the current US regulatory context, it would be challenging to implement FIT-based testing at newly adjusted detection thresholds,” he said.
Nevertheless, the study provides a strong rationale for a real-world study of FIT-based surveillance, he added. “The choice of specific FIT and detection threshold will be critical. Multiple rounds of FIT-based surveillance, that is, years of prospective surveillance, will be needed to constitute a properly designed comparison with surveillance colonoscopy.”
Study Details
The cross-sectional observational study included individuals aged 50-75 years who provided stool samples for the DNA test and two FITs. Test accuracy was calculated for all surveillance indications.
For the post-polypectomy indication only, which is the most common and associated with a relatively low CRC risk, the long-term impact of stool-based surveillance was evaluated with the Adenoma and Serrated Pathway to Colorectal Cancer model. Stool-based strategies were simulated to tune each test’s positivity threshold to obtain strategies that are at least as effective as colonoscopy surveillance.
A total of 3453 individuals had results for all stool tests and colonoscopy; among them, 2226 had previously undergone polypectomy, 1003 had a history of CRC, and 224 had a familial risk.
Areas under the receiver operating characteristic curve for advanced neoplasia were as follows:
- 0.72 (95% CI, 0.69-0.75) for the multitarget stool DNA test
- 0.61 (95% CI, 0.58-0.64) for the FIT OC-SENSOR
- 0.59 (95% CI, 0.56-0.61) for the FIT FOB-Gold
Stool-based surveillance was estimated to be at least as effective as colonoscopy surveillance and required 5.6 to 9.5 stool tests over a person’s lifetime. DNA-based surveillance was more costly than colonoscopy surveillance, whereas FIT-based surveillance saved costs.
“These findings provide a basis to embark on a prospective intervention study to assess the clinical utility of FIT as an alternative to colonoscopy surveillance in a post-polypectomy CRC surveillance population,” the authors wrote.
In the United States, Ladabaum said, it would likely be possible to find FIT-based strategies that closely approximate or match surveillance colonoscopy — “if we could deploy FIT with the required flexibility, for example, by adjusting the threshold and if the reference surveillance standard were somewhat relaxed compared with current guidelines.”
He worries, however, that if FIT for screening and FIT for surveillance were optimized at different hemoglobin detection thresholds, “there could be confusion and room for error in real-world clinical implementation.”
The authors called for research to increase understanding of the mechanisms underlying progression from adenomas to malignancy over time, which may yield better biomarkers to improve stool test accuracy.
This study was funded by the Alpe d’HuZes charity and the Dutch Cancer Society. Exact Sciences provided test equipment and performed multitarget stool DNA test analysis. Sentinel Diagnostics provided equipment and reagents.
Carvalho and Veerle M. H. Coupé, PhD, disclosed several patents pending and/or issued. Other coauthors disclosed multiple financial relationships with private companies, including Exact Sciences and Sentinel, for research support, travel, board membership, advisory or speaker fees, consulting, employment, stock ownership, or patents.
Ladabaum disclosed no competing interests relevant to his comments.
A version of this article appeared on Medscape.com.
It might also reduce colonoscopies by an estimated 15%-41%.
The greatest reduction would likely be achieved by annual FIT-based surveillance, especially with FIT FOB-Gold at a threshold of at least 32 µg/g feces, according to findings from the Dutch MOCCAS study published in Gastroenterology.
In this cross-sectional observational study, the multitarget DNA test outperformed FIT for detecting advanced precursor lesions, especially serrated polyps. According to long-term-impact mathematical modeling, however, DNA-based surveillance would be more costly than colonoscopy surveillance, whereas FIT would save costs.
“With the worldwide implementation of FIT-based screening programs, following a positive test, many more people enter surveillance programs after polypectomy. This results in an increased pressure on the colonoscopy capacity and healthcare budgets,” lead author Beatriz Carvalho, PhD, a molecular biologist in the Department of Pathology of the Netherlands Cancer Institute in Amsterdam, said in an interview.
A noninvasive strategy could ease the surveillance burden on healthcare resources and be more palatable to patients. Post-polypectomy guidelines have already been relaxed to allow less intensive surveillance.
“Our working hypothesis was that although the sensitivity of a singular molecular test to detect CRC or advanced adenomas is lower than that of colonoscopy, repeating molecular stool testing would yield similar detection rates as colonoscopy-based surveillance. And our hypothesis was confirmed,” Carvalho said.
The results of the MOCCAS study align with those of other studies that found that FIT could be safely applied as a triage test in post-polypectomy surveillance and could safely extend the interval of surveillance colonoscopy. “But these studies did not include a long-term impact analysis,” she said. “The next step is to run a prospective interventional study to validate the MOCCAS findings.”
Offering an outsider’s perspective on the findings, Uri Ladabaum, MD, director of the Gastrointestinal Cancer Prevention Program and a professor of medicine at Stanford University School of Medicine in Palo Alto, California, said the real-world results on lesion detection and the multi-year-horizon modeling performed are provocative and point to the potential to base post-polypectomy surveillance on stool tests.
He cautioned, however, that the proposed paradigm requires the ability to deploy FIT-based surveillance with broad flexibility in relation to hemoglobin-detection thresholds and testing interval, depending on the specific FIT that is chosen, with the possibility these may differ by setting based on the characteristics of the population and the relevant epidemiology.
“Such flexibility may or may not be technically feasible in all settings — for instance, in the current US regulatory context, it would be challenging to implement FIT-based testing at newly adjusted detection thresholds,” he said.
Nevertheless, the study provides a strong rationale for a real-world study of FIT-based surveillance, he added. “The choice of specific FIT and detection threshold will be critical. Multiple rounds of FIT-based surveillance, that is, years of prospective surveillance, will be needed to constitute a properly designed comparison with surveillance colonoscopy.”
Study Details
The cross-sectional observational study included individuals aged 50-75 years who provided stool samples for the DNA test and two FITs. Test accuracy was calculated for all surveillance indications.
For the post-polypectomy indication only, which is the most common and associated with a relatively low CRC risk, the long-term impact of stool-based surveillance was evaluated with the Adenoma and Serrated Pathway to Colorectal Cancer model. Stool-based strategies were simulated to tune each test’s positivity threshold to obtain strategies that are at least as effective as colonoscopy surveillance.
A total of 3453 individuals had results for all stool tests and colonoscopy; among them, 2226 had previously undergone polypectomy, 1003 had a history of CRC, and 224 had a familial risk.
Areas under the receiver operating characteristic curve for advanced neoplasia were as follows:
- 0.72 (95% CI, 0.69-0.75) for the multitarget stool DNA test
- 0.61 (95% CI, 0.58-0.64) for the FIT OC-SENSOR
- 0.59 (95% CI, 0.56-0.61) for the FIT FOB-Gold
Stool-based surveillance was estimated to be at least as effective as colonoscopy surveillance and required 5.6 to 9.5 stool tests over a person’s lifetime. DNA-based surveillance was more costly than colonoscopy surveillance, whereas FIT-based surveillance saved costs.
“These findings provide a basis to embark on a prospective intervention study to assess the clinical utility of FIT as an alternative to colonoscopy surveillance in a post-polypectomy CRC surveillance population,” the authors wrote.
In the United States, Ladabaum said, it would likely be possible to find FIT-based strategies that closely approximate or match surveillance colonoscopy — “if we could deploy FIT with the required flexibility, for example, by adjusting the threshold and if the reference surveillance standard were somewhat relaxed compared with current guidelines.”
He worries, however, that if FIT for screening and FIT for surveillance were optimized at different hemoglobin detection thresholds, “there could be confusion and room for error in real-world clinical implementation.”
The authors called for research to increase understanding of the mechanisms underlying progression from adenomas to malignancy over time, which may yield better biomarkers to improve stool test accuracy.
This study was funded by the Alpe d’HuZes charity and the Dutch Cancer Society. Exact Sciences provided test equipment and performed multitarget stool DNA test analysis. Sentinel Diagnostics provided equipment and reagents.
Carvalho and Veerle M. H. Coupé, PhD, disclosed several patents pending and/or issued. Other coauthors disclosed multiple financial relationships with private companies, including Exact Sciences and Sentinel, for research support, travel, board membership, advisory or speaker fees, consulting, employment, stock ownership, or patents.
Ladabaum disclosed no competing interests relevant to his comments.
A version of this article appeared on Medscape.com.
It might also reduce colonoscopies by an estimated 15%-41%.
The greatest reduction would likely be achieved by annual FIT-based surveillance, especially with FIT FOB-Gold at a threshold of at least 32 µg/g feces, according to findings from the Dutch MOCCAS study published in Gastroenterology.
In this cross-sectional observational study, the multitarget DNA test outperformed FIT for detecting advanced precursor lesions, especially serrated polyps. According to long-term-impact mathematical modeling, however, DNA-based surveillance would be more costly than colonoscopy surveillance, whereas FIT would save costs.
“With the worldwide implementation of FIT-based screening programs, following a positive test, many more people enter surveillance programs after polypectomy. This results in an increased pressure on the colonoscopy capacity and healthcare budgets,” lead author Beatriz Carvalho, PhD, a molecular biologist in the Department of Pathology of the Netherlands Cancer Institute in Amsterdam, said in an interview.
A noninvasive strategy could ease the surveillance burden on healthcare resources and be more palatable to patients. Post-polypectomy guidelines have already been relaxed to allow less intensive surveillance.
“Our working hypothesis was that although the sensitivity of a singular molecular test to detect CRC or advanced adenomas is lower than that of colonoscopy, repeating molecular stool testing would yield similar detection rates as colonoscopy-based surveillance. And our hypothesis was confirmed,” Carvalho said.
The results of the MOCCAS study align with those of other studies that found that FIT could be safely applied as a triage test in post-polypectomy surveillance and could safely extend the interval of surveillance colonoscopy. “But these studies did not include a long-term impact analysis,” she said. “The next step is to run a prospective interventional study to validate the MOCCAS findings.”
Offering an outsider’s perspective on the findings, Uri Ladabaum, MD, director of the Gastrointestinal Cancer Prevention Program and a professor of medicine at Stanford University School of Medicine in Palo Alto, California, said the real-world results on lesion detection and the multi-year-horizon modeling performed are provocative and point to the potential to base post-polypectomy surveillance on stool tests.
He cautioned, however, that the proposed paradigm requires the ability to deploy FIT-based surveillance with broad flexibility in relation to hemoglobin-detection thresholds and testing interval, depending on the specific FIT that is chosen, with the possibility these may differ by setting based on the characteristics of the population and the relevant epidemiology.
“Such flexibility may or may not be technically feasible in all settings — for instance, in the current US regulatory context, it would be challenging to implement FIT-based testing at newly adjusted detection thresholds,” he said.
Nevertheless, the study provides a strong rationale for a real-world study of FIT-based surveillance, he added. “The choice of specific FIT and detection threshold will be critical. Multiple rounds of FIT-based surveillance, that is, years of prospective surveillance, will be needed to constitute a properly designed comparison with surveillance colonoscopy.”
Study Details
The cross-sectional observational study included individuals aged 50-75 years who provided stool samples for the DNA test and two FITs. Test accuracy was calculated for all surveillance indications.
For the post-polypectomy indication only, which is the most common and associated with a relatively low CRC risk, the long-term impact of stool-based surveillance was evaluated with the Adenoma and Serrated Pathway to Colorectal Cancer model. Stool-based strategies were simulated to tune each test’s positivity threshold to obtain strategies that are at least as effective as colonoscopy surveillance.
A total of 3453 individuals had results for all stool tests and colonoscopy; among them, 2226 had previously undergone polypectomy, 1003 had a history of CRC, and 224 had a familial risk.
Areas under the receiver operating characteristic curve for advanced neoplasia were as follows:
- 0.72 (95% CI, 0.69-0.75) for the multitarget stool DNA test
- 0.61 (95% CI, 0.58-0.64) for the FIT OC-SENSOR
- 0.59 (95% CI, 0.56-0.61) for the FIT FOB-Gold
Stool-based surveillance was estimated to be at least as effective as colonoscopy surveillance and required 5.6 to 9.5 stool tests over a person’s lifetime. DNA-based surveillance was more costly than colonoscopy surveillance, whereas FIT-based surveillance saved costs.
“These findings provide a basis to embark on a prospective intervention study to assess the clinical utility of FIT as an alternative to colonoscopy surveillance in a post-polypectomy CRC surveillance population,” the authors wrote.
In the United States, Ladabaum said, it would likely be possible to find FIT-based strategies that closely approximate or match surveillance colonoscopy — “if we could deploy FIT with the required flexibility, for example, by adjusting the threshold and if the reference surveillance standard were somewhat relaxed compared with current guidelines.”
He worries, however, that if FIT for screening and FIT for surveillance were optimized at different hemoglobin detection thresholds, “there could be confusion and room for error in real-world clinical implementation.”
The authors called for research to increase understanding of the mechanisms underlying progression from adenomas to malignancy over time, which may yield better biomarkers to improve stool test accuracy.
This study was funded by the Alpe d’HuZes charity and the Dutch Cancer Society. Exact Sciences provided test equipment and performed multitarget stool DNA test analysis. Sentinel Diagnostics provided equipment and reagents.
Carvalho and Veerle M. H. Coupé, PhD, disclosed several patents pending and/or issued. Other coauthors disclosed multiple financial relationships with private companies, including Exact Sciences and Sentinel, for research support, travel, board membership, advisory or speaker fees, consulting, employment, stock ownership, or patents.
Ladabaum disclosed no competing interests relevant to his comments.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
True Benefit of Screening Colonoscopy for CRC Underestimated in NordICC
TOPLINE:
a new analysis found.
METHODOLOGY:
- The NordICC trial randomly assigned 85,179 adults aged 55-64 years in a 1:2 ratio to receive or not receive an invitation for a single screening colonoscopy and determined the risk for CRC diagnosis and death over 10-15 years of follow-up using cancer registries. After randomization, the trial excluded 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization.
- The trial found that CRC risk and associated mortality were lower in adults who had colonoscopy, though only modestly so, which generated considerable controversy.
- Because registration delays are a known concern with population-based cancer registries but the trial did not account for them, researchers on the current study postulated that delays might have led to an underestimation of the impact of colonoscopy on CRC risk.
- They estimated the magnitude of delayed reporting of CRC diagnosis to cancer registries by comparing the 221 exclusions with expected CRC diagnoses per year. They explored the impact that delays may have had on the results of the trial’s intention-to-screen analysis and adjusted per-protocol analysis.
TAKEAWAY:
- The trial’s post hoc exclusion of 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization suggests delays of 2-3 years in registration.
- With no assumed delay in cancer registration, the 10-year reported CRC risk difference was 0.22% in intention-to-screen and 0.38% in adjusted per-protocol analyses. With a mean delay in cancer registration of 2 years, the risk difference rose to 0.44% in the intention-to-screen analysis and 0.76% in the adjusted per-protocol analysis.
- Assuming no delay in cancer registration, the number needed to invite for screening colonoscopy and number needed to undergo the procedure to prevent 1 CRC diagnosis/death were 455 and 263, respectively. These numbers decreased to 227 and 132, respectively, with a 2-year reporting delay.
- Registration delays of 1, 2, or 3 years led to an underestimated risk for CRC by 25%, 50%, and 75%, respectively.
IN PRACTICE:
“Updated analyses ensuring complete 10- and 15-year follow-up will be crucial to derive the true reductions of CRC risk and mortality in the trial’s predefined interim and primary analysis. In the meantime, available estimates are to be interpreted with caution, as they likely severely underestimate true screening colonoscopy effects,” the authors concluded.
The lag in reporting found by the study raises questions about the time interval needed beyond the end of a study to assure its completeness, which varies across registries, wrote Chyke A. Doubeni, MD, MPH, Ohio State University Wexner Medical Center, Columbus, and colleagues in an invited commentary. “Publication guidelines should be strengthened to ensure affirmation of completeness and quality of cancer registries used for outcomes ascertainment to minimize uncertainties.”
SOURCE:
The study, with first author Hermann Brenner, MD, MPH, German Cancer Research Center, Heidelberg, was published online in JAMA Network Open, as was the invited commentary.
LIMITATIONS:
Exact quantification of and correction for registration delays were not possible.
DISCLOSURES:
The study was partially funded by the German Federal Ministry of Education and Research and German Cancer Aid. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a new analysis found.
METHODOLOGY:
- The NordICC trial randomly assigned 85,179 adults aged 55-64 years in a 1:2 ratio to receive or not receive an invitation for a single screening colonoscopy and determined the risk for CRC diagnosis and death over 10-15 years of follow-up using cancer registries. After randomization, the trial excluded 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization.
- The trial found that CRC risk and associated mortality were lower in adults who had colonoscopy, though only modestly so, which generated considerable controversy.
- Because registration delays are a known concern with population-based cancer registries but the trial did not account for them, researchers on the current study postulated that delays might have led to an underestimation of the impact of colonoscopy on CRC risk.
- They estimated the magnitude of delayed reporting of CRC diagnosis to cancer registries by comparing the 221 exclusions with expected CRC diagnoses per year. They explored the impact that delays may have had on the results of the trial’s intention-to-screen analysis and adjusted per-protocol analysis.
TAKEAWAY:
- The trial’s post hoc exclusion of 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization suggests delays of 2-3 years in registration.
- With no assumed delay in cancer registration, the 10-year reported CRC risk difference was 0.22% in intention-to-screen and 0.38% in adjusted per-protocol analyses. With a mean delay in cancer registration of 2 years, the risk difference rose to 0.44% in the intention-to-screen analysis and 0.76% in the adjusted per-protocol analysis.
- Assuming no delay in cancer registration, the number needed to invite for screening colonoscopy and number needed to undergo the procedure to prevent 1 CRC diagnosis/death were 455 and 263, respectively. These numbers decreased to 227 and 132, respectively, with a 2-year reporting delay.
- Registration delays of 1, 2, or 3 years led to an underestimated risk for CRC by 25%, 50%, and 75%, respectively.
IN PRACTICE:
“Updated analyses ensuring complete 10- and 15-year follow-up will be crucial to derive the true reductions of CRC risk and mortality in the trial’s predefined interim and primary analysis. In the meantime, available estimates are to be interpreted with caution, as they likely severely underestimate true screening colonoscopy effects,” the authors concluded.
The lag in reporting found by the study raises questions about the time interval needed beyond the end of a study to assure its completeness, which varies across registries, wrote Chyke A. Doubeni, MD, MPH, Ohio State University Wexner Medical Center, Columbus, and colleagues in an invited commentary. “Publication guidelines should be strengthened to ensure affirmation of completeness and quality of cancer registries used for outcomes ascertainment to minimize uncertainties.”
SOURCE:
The study, with first author Hermann Brenner, MD, MPH, German Cancer Research Center, Heidelberg, was published online in JAMA Network Open, as was the invited commentary.
LIMITATIONS:
Exact quantification of and correction for registration delays were not possible.
DISCLOSURES:
The study was partially funded by the German Federal Ministry of Education and Research and German Cancer Aid. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a new analysis found.
METHODOLOGY:
- The NordICC trial randomly assigned 85,179 adults aged 55-64 years in a 1:2 ratio to receive or not receive an invitation for a single screening colonoscopy and determined the risk for CRC diagnosis and death over 10-15 years of follow-up using cancer registries. After randomization, the trial excluded 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization.
- The trial found that CRC risk and associated mortality were lower in adults who had colonoscopy, though only modestly so, which generated considerable controversy.
- Because registration delays are a known concern with population-based cancer registries but the trial did not account for them, researchers on the current study postulated that delays might have led to an underestimation of the impact of colonoscopy on CRC risk.
- They estimated the magnitude of delayed reporting of CRC diagnosis to cancer registries by comparing the 221 exclusions with expected CRC diagnoses per year. They explored the impact that delays may have had on the results of the trial’s intention-to-screen analysis and adjusted per-protocol analysis.
TAKEAWAY:
- The trial’s post hoc exclusion of 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization suggests delays of 2-3 years in registration.
- With no assumed delay in cancer registration, the 10-year reported CRC risk difference was 0.22% in intention-to-screen and 0.38% in adjusted per-protocol analyses. With a mean delay in cancer registration of 2 years, the risk difference rose to 0.44% in the intention-to-screen analysis and 0.76% in the adjusted per-protocol analysis.
- Assuming no delay in cancer registration, the number needed to invite for screening colonoscopy and number needed to undergo the procedure to prevent 1 CRC diagnosis/death were 455 and 263, respectively. These numbers decreased to 227 and 132, respectively, with a 2-year reporting delay.
- Registration delays of 1, 2, or 3 years led to an underestimated risk for CRC by 25%, 50%, and 75%, respectively.
IN PRACTICE:
“Updated analyses ensuring complete 10- and 15-year follow-up will be crucial to derive the true reductions of CRC risk and mortality in the trial’s predefined interim and primary analysis. In the meantime, available estimates are to be interpreted with caution, as they likely severely underestimate true screening colonoscopy effects,” the authors concluded.
The lag in reporting found by the study raises questions about the time interval needed beyond the end of a study to assure its completeness, which varies across registries, wrote Chyke A. Doubeni, MD, MPH, Ohio State University Wexner Medical Center, Columbus, and colleagues in an invited commentary. “Publication guidelines should be strengthened to ensure affirmation of completeness and quality of cancer registries used for outcomes ascertainment to minimize uncertainties.”
SOURCE:
The study, with first author Hermann Brenner, MD, MPH, German Cancer Research Center, Heidelberg, was published online in JAMA Network Open, as was the invited commentary.
LIMITATIONS:
Exact quantification of and correction for registration delays were not possible.
DISCLOSURES:
The study was partially funded by the German Federal Ministry of Education and Research and German Cancer Aid. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
MMR/MSI Testing for CRC Climbs, But Variations Persist
TOPLINE:
with testing rates differing by cancer stage, individual hospital, patient sex, race, and insurance status.
METHODOLOGY:
- In 2017, the National Comprehensive Cancer Network (NCCN) recommended universal testing for MMR and MSI among patients with CRC, but studies suggest that testing may still be underused.
- To assess trends and factors associated with MMR/MSI testing in the United States, researchers evaluated 834,797 patients diagnosed with stage I-IV CRC between 2012 and 2021 across 1366 Commission on Cancer–accredited hospitals in the National Cancer Database.
- The variability in MMR/MSI testing was assessed in relation to both patient and hospital-level factors.
- Overall, 70.7% patients had colon cancer, 7.3% had rectosigmoid cancer, and 22.0% had rectal cancer. The median patient age was 66 years; just over half (53%) were men, 81.8% were White, and 11.9% were Black.
TAKEAWAY:
- Overall, 43.9% patients underwent MMR/MSI testing, but testing rates increased more than threefold between 2012 and 2021 — from 22.7% to 71.5%. Still, testing rates varied depending on a range of factors.
- About 22% variability in MMR/MSI testing was attributed to hospital-level variations, with the best vs worst performing hospitals reporting testing rates of 90% vs 2%. This hospital-level variation may be caused by testing protocol differences at individual institutions, the authors said.
- The likelihood of undergoing MMR/MSI testing was lower in patients with stage IV vs stage I disease (adjusted odds ratio [aOR], 0.78) but higher in those with stage II (aOR, 1.53) and III (aOR, 1.40) disease.
- The likelihood of undergoing MMR/MSI testing was slightly lower for men than for women (aOR, 0.98) and for Black patients than for White patients (aOR, 0.97). Having a lower household income, public or no insurance (vs private insurance), or living a longer distance (more than 5 miles) from the treatment facility was also associated with lower odds of testing.
IN PRACTICE:
“This cohort study indicated that MMR/MSI testing increased markedly, suggesting increased NCCN guideline adherence,” the authors said. However, variations still exist by cancer stage, hospital, and patient factors. Implementing “widespread institution-level reflexive testing for every initial diagnostic biopsy” can improve testing rates and reduce disparities, the authors suggested.
SOURCE:
This study, led by Totadri Dhimal, MD, University of Rochester Medical Center in New York, was published online in JAMA Oncology.
LIMITATIONS:
The study lacked clinical granularity, and potential coding inaccuracies and incomplete data could have affected the interpretation and generalizability of the findings.
DISCLOSURES:
No funding information was provided for the study. One author reported receiving author royalties from UpToDate outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with testing rates differing by cancer stage, individual hospital, patient sex, race, and insurance status.
METHODOLOGY:
- In 2017, the National Comprehensive Cancer Network (NCCN) recommended universal testing for MMR and MSI among patients with CRC, but studies suggest that testing may still be underused.
- To assess trends and factors associated with MMR/MSI testing in the United States, researchers evaluated 834,797 patients diagnosed with stage I-IV CRC between 2012 and 2021 across 1366 Commission on Cancer–accredited hospitals in the National Cancer Database.
- The variability in MMR/MSI testing was assessed in relation to both patient and hospital-level factors.
- Overall, 70.7% patients had colon cancer, 7.3% had rectosigmoid cancer, and 22.0% had rectal cancer. The median patient age was 66 years; just over half (53%) were men, 81.8% were White, and 11.9% were Black.
TAKEAWAY:
- Overall, 43.9% patients underwent MMR/MSI testing, but testing rates increased more than threefold between 2012 and 2021 — from 22.7% to 71.5%. Still, testing rates varied depending on a range of factors.
- About 22% variability in MMR/MSI testing was attributed to hospital-level variations, with the best vs worst performing hospitals reporting testing rates of 90% vs 2%. This hospital-level variation may be caused by testing protocol differences at individual institutions, the authors said.
- The likelihood of undergoing MMR/MSI testing was lower in patients with stage IV vs stage I disease (adjusted odds ratio [aOR], 0.78) but higher in those with stage II (aOR, 1.53) and III (aOR, 1.40) disease.
- The likelihood of undergoing MMR/MSI testing was slightly lower for men than for women (aOR, 0.98) and for Black patients than for White patients (aOR, 0.97). Having a lower household income, public or no insurance (vs private insurance), or living a longer distance (more than 5 miles) from the treatment facility was also associated with lower odds of testing.
IN PRACTICE:
“This cohort study indicated that MMR/MSI testing increased markedly, suggesting increased NCCN guideline adherence,” the authors said. However, variations still exist by cancer stage, hospital, and patient factors. Implementing “widespread institution-level reflexive testing for every initial diagnostic biopsy” can improve testing rates and reduce disparities, the authors suggested.
SOURCE:
This study, led by Totadri Dhimal, MD, University of Rochester Medical Center in New York, was published online in JAMA Oncology.
LIMITATIONS:
The study lacked clinical granularity, and potential coding inaccuracies and incomplete data could have affected the interpretation and generalizability of the findings.
DISCLOSURES:
No funding information was provided for the study. One author reported receiving author royalties from UpToDate outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with testing rates differing by cancer stage, individual hospital, patient sex, race, and insurance status.
METHODOLOGY:
- In 2017, the National Comprehensive Cancer Network (NCCN) recommended universal testing for MMR and MSI among patients with CRC, but studies suggest that testing may still be underused.
- To assess trends and factors associated with MMR/MSI testing in the United States, researchers evaluated 834,797 patients diagnosed with stage I-IV CRC between 2012 and 2021 across 1366 Commission on Cancer–accredited hospitals in the National Cancer Database.
- The variability in MMR/MSI testing was assessed in relation to both patient and hospital-level factors.
- Overall, 70.7% patients had colon cancer, 7.3% had rectosigmoid cancer, and 22.0% had rectal cancer. The median patient age was 66 years; just over half (53%) were men, 81.8% were White, and 11.9% were Black.
TAKEAWAY:
- Overall, 43.9% patients underwent MMR/MSI testing, but testing rates increased more than threefold between 2012 and 2021 — from 22.7% to 71.5%. Still, testing rates varied depending on a range of factors.
- About 22% variability in MMR/MSI testing was attributed to hospital-level variations, with the best vs worst performing hospitals reporting testing rates of 90% vs 2%. This hospital-level variation may be caused by testing protocol differences at individual institutions, the authors said.
- The likelihood of undergoing MMR/MSI testing was lower in patients with stage IV vs stage I disease (adjusted odds ratio [aOR], 0.78) but higher in those with stage II (aOR, 1.53) and III (aOR, 1.40) disease.
- The likelihood of undergoing MMR/MSI testing was slightly lower for men than for women (aOR, 0.98) and for Black patients than for White patients (aOR, 0.97). Having a lower household income, public or no insurance (vs private insurance), or living a longer distance (more than 5 miles) from the treatment facility was also associated with lower odds of testing.
IN PRACTICE:
“This cohort study indicated that MMR/MSI testing increased markedly, suggesting increased NCCN guideline adherence,” the authors said. However, variations still exist by cancer stage, hospital, and patient factors. Implementing “widespread institution-level reflexive testing for every initial diagnostic biopsy” can improve testing rates and reduce disparities, the authors suggested.
SOURCE:
This study, led by Totadri Dhimal, MD, University of Rochester Medical Center in New York, was published online in JAMA Oncology.
LIMITATIONS:
The study lacked clinical granularity, and potential coding inaccuracies and incomplete data could have affected the interpretation and generalizability of the findings.
DISCLOSURES:
No funding information was provided for the study. One author reported receiving author royalties from UpToDate outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Are Targeted Drugs the Future in Colorectal Cancer?
This transcript has been edited for clarity.
Welcome back, everybody, from the European Society for Medical Oncology (ESMO) Congress in the wonderful city of Barcelona in Spain. I was coming from ESMO drenched in huge amounts of new data.
They looked at molecularly targeted drugs, some early-stage and a later-stage study in which there’s some evidence of promise.
She talked a little about the preliminary results from three trials suggesting some benefits, pretty marginal, of cetuximab plus irinotecan in patients who’d already had epidermal growth factor receptor (EGFR) receptor inhibitory treatment.
Amivantamab plus FOLFOX or FOLFIRI was also discussed. This is a bispecific antibody against EGFR and MET. Again, very early, but there are some potential marginal benefits coming through. She also discussed the results of a larger phase 3 randomized trial with an old friend, ramucirumab, the anti-angiogenic agent, in which the ramucirumab in combination with trifluridine-tipiracil failed to meet its primary endpoint of improving overall survival.
There were some interesting post hoc subgroup analyses showing potential benefits for women, left-sided tumors, and so on. She made an excellent presentation, which she summarized by saying that the future of colorectal cancer treatment lies in further defining molecularly targeted treatment.
Nobody would disagree with that. What is interesting, though, is that, if I were to use the analogy of mining, the more deeply we mine, perhaps the lower marginal the benefits are becoming. There’s no doubt that we’re understanding better the exquisite machinery of cell signaling. We understand that there’s redundancy, there’s repeatability, and the possibility of emergence of resistance can come quite quickly.
Although we can develop ever more precise molecularly targeted drugs, it does seem as if the clinical benefits of these, in some cases, are marginally small. I’d like to suggest that, in addition to Sara’s call for more molecularly targeted drugs, we should think about cellular targets.
We did a large amount of work (as have many others, of course) looking at the immune tumor microenvironment and trying to, in a way, separate and understand the contribution of the individual component cells — of which there are many, including cancer-associated fibroblasts, natural killer (NK) cells, whole hosts of different types of T-cell subsets, B cells, tumor-associated neutrophils, and so on — and how these interact together and of interact with the epithelial colorectal cancer cells.
We are collaborating with Patrick Soon-Shiong, a clever chap, who believes in combination immunotherapy, dissecting and understanding the individual role of these different cells, and coming up with cellular therapies or targeted therapies that either inhibit or stimulate some of the different cell components to be the way ahead for an immunologically cold tumor such as microsatellite-stable colorectal cancer.
For example, we’re looking at combinations of our histone deacetylase (HDAC) inhibitor, which switches on the machinery of antigen presentation, up-regulating major histocompatibility complex (MHC) class 1 and class 2, and some other of the molecules involved in antigen chopping and presentation; it’s like turning a microsatellite-stable immunologically cold tumor hot; an interleukin-15 superagonist that stimulates NK cells; and we’ve found a way to manipulate and reduce the number of Treg cells.
We have various approaches to reducing the microenvironment transforming growth factor beta and some of the downstream elements from that. We can look at combinatorial immunotherapy, but thinking at a cellular level and developing anticancer agents that either activate or inhibit these different cell components. I’d bring the two together.
Of course, the future has got to be better molecularly targeted drugs, but let’s think at a macro level as to how we can look at the different cellular interactions within the tumor microenvironment, and perhaps through that, come up with synergistic immunotherapeutic combinations.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and Professor of Cancer Medicine, Oxford Cancer Centre, both in England. He reported conflicts of interest with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Genomic Health, and Merck Serono.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back, everybody, from the European Society for Medical Oncology (ESMO) Congress in the wonderful city of Barcelona in Spain. I was coming from ESMO drenched in huge amounts of new data.
They looked at molecularly targeted drugs, some early-stage and a later-stage study in which there’s some evidence of promise.
She talked a little about the preliminary results from three trials suggesting some benefits, pretty marginal, of cetuximab plus irinotecan in patients who’d already had epidermal growth factor receptor (EGFR) receptor inhibitory treatment.
Amivantamab plus FOLFOX or FOLFIRI was also discussed. This is a bispecific antibody against EGFR and MET. Again, very early, but there are some potential marginal benefits coming through. She also discussed the results of a larger phase 3 randomized trial with an old friend, ramucirumab, the anti-angiogenic agent, in which the ramucirumab in combination with trifluridine-tipiracil failed to meet its primary endpoint of improving overall survival.
There were some interesting post hoc subgroup analyses showing potential benefits for women, left-sided tumors, and so on. She made an excellent presentation, which she summarized by saying that the future of colorectal cancer treatment lies in further defining molecularly targeted treatment.
Nobody would disagree with that. What is interesting, though, is that, if I were to use the analogy of mining, the more deeply we mine, perhaps the lower marginal the benefits are becoming. There’s no doubt that we’re understanding better the exquisite machinery of cell signaling. We understand that there’s redundancy, there’s repeatability, and the possibility of emergence of resistance can come quite quickly.
Although we can develop ever more precise molecularly targeted drugs, it does seem as if the clinical benefits of these, in some cases, are marginally small. I’d like to suggest that, in addition to Sara’s call for more molecularly targeted drugs, we should think about cellular targets.
We did a large amount of work (as have many others, of course) looking at the immune tumor microenvironment and trying to, in a way, separate and understand the contribution of the individual component cells — of which there are many, including cancer-associated fibroblasts, natural killer (NK) cells, whole hosts of different types of T-cell subsets, B cells, tumor-associated neutrophils, and so on — and how these interact together and of interact with the epithelial colorectal cancer cells.
We are collaborating with Patrick Soon-Shiong, a clever chap, who believes in combination immunotherapy, dissecting and understanding the individual role of these different cells, and coming up with cellular therapies or targeted therapies that either inhibit or stimulate some of the different cell components to be the way ahead for an immunologically cold tumor such as microsatellite-stable colorectal cancer.
For example, we’re looking at combinations of our histone deacetylase (HDAC) inhibitor, which switches on the machinery of antigen presentation, up-regulating major histocompatibility complex (MHC) class 1 and class 2, and some other of the molecules involved in antigen chopping and presentation; it’s like turning a microsatellite-stable immunologically cold tumor hot; an interleukin-15 superagonist that stimulates NK cells; and we’ve found a way to manipulate and reduce the number of Treg cells.
We have various approaches to reducing the microenvironment transforming growth factor beta and some of the downstream elements from that. We can look at combinatorial immunotherapy, but thinking at a cellular level and developing anticancer agents that either activate or inhibit these different cell components. I’d bring the two together.
Of course, the future has got to be better molecularly targeted drugs, but let’s think at a macro level as to how we can look at the different cellular interactions within the tumor microenvironment, and perhaps through that, come up with synergistic immunotherapeutic combinations.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and Professor of Cancer Medicine, Oxford Cancer Centre, both in England. He reported conflicts of interest with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Genomic Health, and Merck Serono.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back, everybody, from the European Society for Medical Oncology (ESMO) Congress in the wonderful city of Barcelona in Spain. I was coming from ESMO drenched in huge amounts of new data.
They looked at molecularly targeted drugs, some early-stage and a later-stage study in which there’s some evidence of promise.
She talked a little about the preliminary results from three trials suggesting some benefits, pretty marginal, of cetuximab plus irinotecan in patients who’d already had epidermal growth factor receptor (EGFR) receptor inhibitory treatment.
Amivantamab plus FOLFOX or FOLFIRI was also discussed. This is a bispecific antibody against EGFR and MET. Again, very early, but there are some potential marginal benefits coming through. She also discussed the results of a larger phase 3 randomized trial with an old friend, ramucirumab, the anti-angiogenic agent, in which the ramucirumab in combination with trifluridine-tipiracil failed to meet its primary endpoint of improving overall survival.
There were some interesting post hoc subgroup analyses showing potential benefits for women, left-sided tumors, and so on. She made an excellent presentation, which she summarized by saying that the future of colorectal cancer treatment lies in further defining molecularly targeted treatment.
Nobody would disagree with that. What is interesting, though, is that, if I were to use the analogy of mining, the more deeply we mine, perhaps the lower marginal the benefits are becoming. There’s no doubt that we’re understanding better the exquisite machinery of cell signaling. We understand that there’s redundancy, there’s repeatability, and the possibility of emergence of resistance can come quite quickly.
Although we can develop ever more precise molecularly targeted drugs, it does seem as if the clinical benefits of these, in some cases, are marginally small. I’d like to suggest that, in addition to Sara’s call for more molecularly targeted drugs, we should think about cellular targets.
We did a large amount of work (as have many others, of course) looking at the immune tumor microenvironment and trying to, in a way, separate and understand the contribution of the individual component cells — of which there are many, including cancer-associated fibroblasts, natural killer (NK) cells, whole hosts of different types of T-cell subsets, B cells, tumor-associated neutrophils, and so on — and how these interact together and of interact with the epithelial colorectal cancer cells.
We are collaborating with Patrick Soon-Shiong, a clever chap, who believes in combination immunotherapy, dissecting and understanding the individual role of these different cells, and coming up with cellular therapies or targeted therapies that either inhibit or stimulate some of the different cell components to be the way ahead for an immunologically cold tumor such as microsatellite-stable colorectal cancer.
For example, we’re looking at combinations of our histone deacetylase (HDAC) inhibitor, which switches on the machinery of antigen presentation, up-regulating major histocompatibility complex (MHC) class 1 and class 2, and some other of the molecules involved in antigen chopping and presentation; it’s like turning a microsatellite-stable immunologically cold tumor hot; an interleukin-15 superagonist that stimulates NK cells; and we’ve found a way to manipulate and reduce the number of Treg cells.
We have various approaches to reducing the microenvironment transforming growth factor beta and some of the downstream elements from that. We can look at combinatorial immunotherapy, but thinking at a cellular level and developing anticancer agents that either activate or inhibit these different cell components. I’d bring the two together.
Of course, the future has got to be better molecularly targeted drugs, but let’s think at a macro level as to how we can look at the different cellular interactions within the tumor microenvironment, and perhaps through that, come up with synergistic immunotherapeutic combinations.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and Professor of Cancer Medicine, Oxford Cancer Centre, both in England. He reported conflicts of interest with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Genomic Health, and Merck Serono.
A version of this article first appeared on Medscape.com.
FDA OKs Next-Gen Cologuard Test for CRC Screening
Developed in collaboration with Mayo Clinic, the company noted in the news release announcing its approval that this noninvasive test “raises the performance bar.”
The company says the enhanced sensitivity will help minimize unnecessary follow-up colonoscopy procedures by reducing the odds of a false-positive screening test.
Enhanced sample stability components also will give patients more time to return their sample to the lab.
Cologuard Plus tests for three novel methylated DNA markers and fecal hemoglobin.
The BLUE-C Study
The FDA’s approval was based on the results of the BLUE-C study involving more than 20,000 adults at average risk for CRC that compared the next-generation mt-sDNA test with a fecal immunochemical test (FIT) and colonoscopy.
According to the BLUE-C results, the sensitivities of Cologuard Plus were 95% for CRC and 43% for advanced precancerous lesions, at 94% specificity with no findings on colonoscopy.
The BLUE-C results also showed that the test significantly outperformed FIT for sensitivity for CRC overall, CRC stages I-III, high-grade dysplasia, and advanced precancerous lesions.
“To meaningfully improve outcomes in colorectal cancer, we must catch cancer early — when it is most treatable — and find advanced precancers, which can prevent cases of this cancer,” Thomas F. Imperiale, MD, AGAF, professor of medicine at the Indiana University School of Medicine and research scientist at the Regenstrief Institute, said in the news release.
“The high colorectal cancer sensitivity and specificity of the Cologuard Plus test gives me confidence in the test’s ability to do just that while simultaneously maintaining a low risk of false positives. This makes the Cologuard Plus test a strong option for first-line screening of average risk patients,” said Dr. Imperiale, who served as principal investigator of the BLUE-C study.
The company plans to launch Cologuard Plus in 2025.
They anticipate that it will be covered by Medicare and included in the United States Preventive Services Task Force (USPSTF) guidelines and within quality measures.
A version of this article first appeared on Medscape.com.
Developed in collaboration with Mayo Clinic, the company noted in the news release announcing its approval that this noninvasive test “raises the performance bar.”
The company says the enhanced sensitivity will help minimize unnecessary follow-up colonoscopy procedures by reducing the odds of a false-positive screening test.
Enhanced sample stability components also will give patients more time to return their sample to the lab.
Cologuard Plus tests for three novel methylated DNA markers and fecal hemoglobin.
The BLUE-C Study
The FDA’s approval was based on the results of the BLUE-C study involving more than 20,000 adults at average risk for CRC that compared the next-generation mt-sDNA test with a fecal immunochemical test (FIT) and colonoscopy.
According to the BLUE-C results, the sensitivities of Cologuard Plus were 95% for CRC and 43% for advanced precancerous lesions, at 94% specificity with no findings on colonoscopy.
The BLUE-C results also showed that the test significantly outperformed FIT for sensitivity for CRC overall, CRC stages I-III, high-grade dysplasia, and advanced precancerous lesions.
“To meaningfully improve outcomes in colorectal cancer, we must catch cancer early — when it is most treatable — and find advanced precancers, which can prevent cases of this cancer,” Thomas F. Imperiale, MD, AGAF, professor of medicine at the Indiana University School of Medicine and research scientist at the Regenstrief Institute, said in the news release.
“The high colorectal cancer sensitivity and specificity of the Cologuard Plus test gives me confidence in the test’s ability to do just that while simultaneously maintaining a low risk of false positives. This makes the Cologuard Plus test a strong option for first-line screening of average risk patients,” said Dr. Imperiale, who served as principal investigator of the BLUE-C study.
The company plans to launch Cologuard Plus in 2025.
They anticipate that it will be covered by Medicare and included in the United States Preventive Services Task Force (USPSTF) guidelines and within quality measures.
A version of this article first appeared on Medscape.com.
Developed in collaboration with Mayo Clinic, the company noted in the news release announcing its approval that this noninvasive test “raises the performance bar.”
The company says the enhanced sensitivity will help minimize unnecessary follow-up colonoscopy procedures by reducing the odds of a false-positive screening test.
Enhanced sample stability components also will give patients more time to return their sample to the lab.
Cologuard Plus tests for three novel methylated DNA markers and fecal hemoglobin.
The BLUE-C Study
The FDA’s approval was based on the results of the BLUE-C study involving more than 20,000 adults at average risk for CRC that compared the next-generation mt-sDNA test with a fecal immunochemical test (FIT) and colonoscopy.
According to the BLUE-C results, the sensitivities of Cologuard Plus were 95% for CRC and 43% for advanced precancerous lesions, at 94% specificity with no findings on colonoscopy.
The BLUE-C results also showed that the test significantly outperformed FIT for sensitivity for CRC overall, CRC stages I-III, high-grade dysplasia, and advanced precancerous lesions.
“To meaningfully improve outcomes in colorectal cancer, we must catch cancer early — when it is most treatable — and find advanced precancers, which can prevent cases of this cancer,” Thomas F. Imperiale, MD, AGAF, professor of medicine at the Indiana University School of Medicine and research scientist at the Regenstrief Institute, said in the news release.
“The high colorectal cancer sensitivity and specificity of the Cologuard Plus test gives me confidence in the test’s ability to do just that while simultaneously maintaining a low risk of false positives. This makes the Cologuard Plus test a strong option for first-line screening of average risk patients,” said Dr. Imperiale, who served as principal investigator of the BLUE-C study.
The company plans to launch Cologuard Plus in 2025.
They anticipate that it will be covered by Medicare and included in the United States Preventive Services Task Force (USPSTF) guidelines and within quality measures.
A version of this article first appeared on Medscape.com.