User login
Treatment of opioid use disorder in hospitalized patients
An opportunity for impact
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.
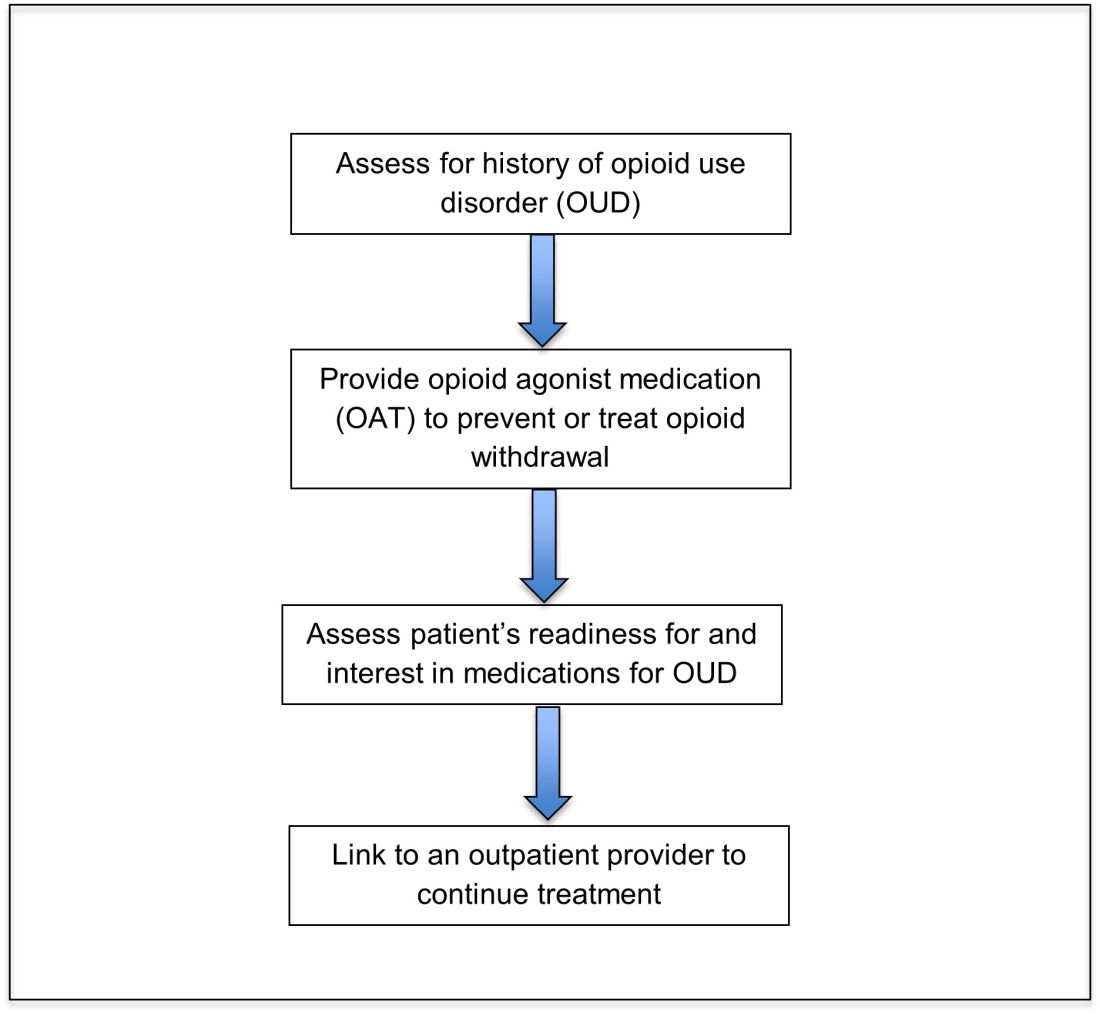
Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.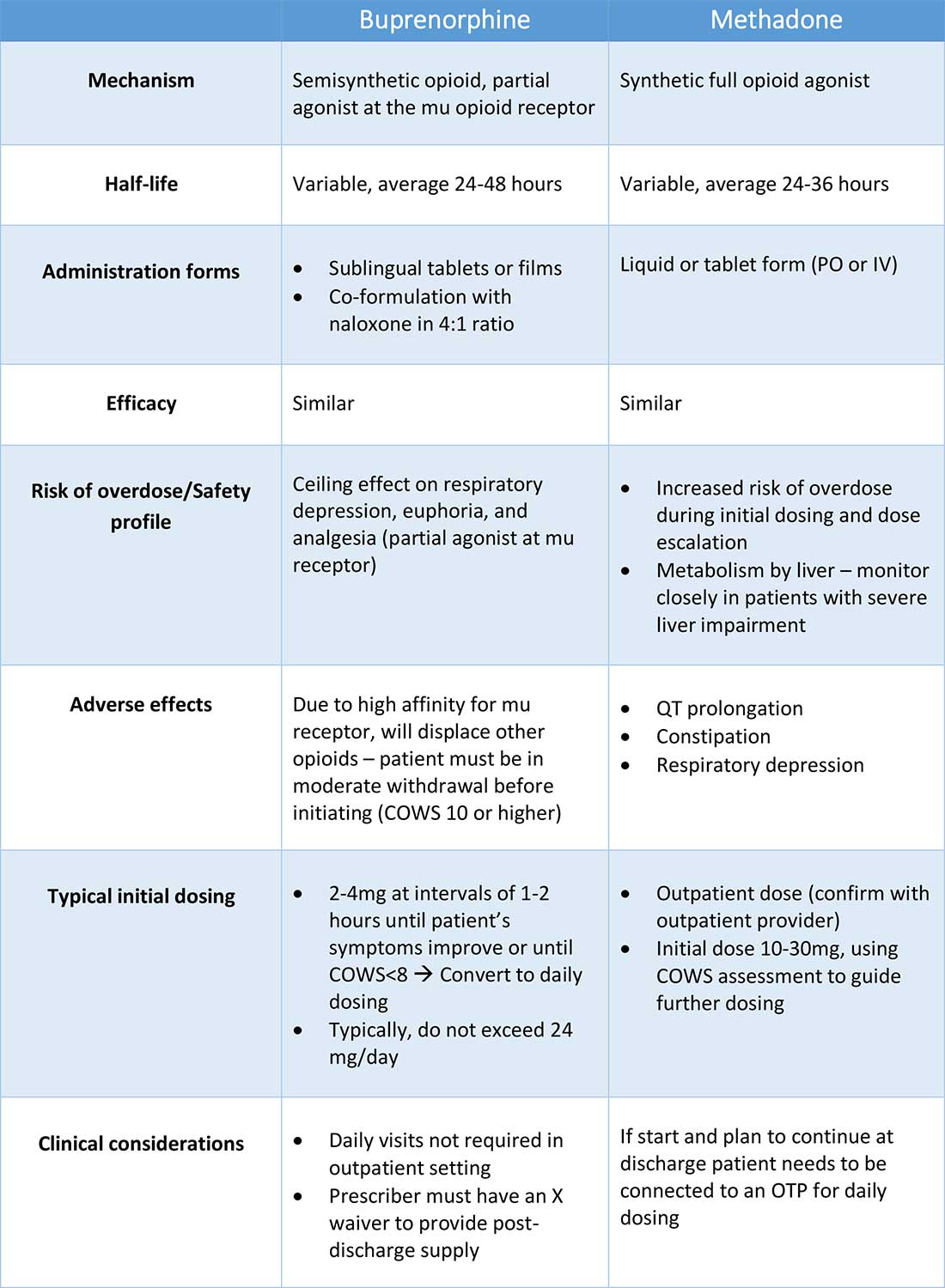
Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
An opportunity for impact
An opportunity for impact
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.

Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.
Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.

Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.
Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
A case-based framework for de-escalating conflict
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Does morning discharge really improve hospital throughput?
‘Perennial debate’ likely to be reignited
A recent study published in the Journal of Hospital Medicine examined patient discharges from hospitals in Ontario, Canada, to determine if morning discharges were associated with positive outcomes. Some hospitalist programs have embraced discharge before noon (DBN) initiatives like those studied in the article.1 Unfortunately, the researchers concluded that the Canadian DBNs did not positively impact hospital length of stay, readmissions, or mortality rates.
DBN has been a quality improvement target for hospitals hoping to improve throughput and free up scarce beds, while promoting patient safety by encouraging discharge as soon as patients are ready to leave. Yet other researchers have questioned its actual impact on quality metrics. One author called DBN’s purported impact an “urban legend,”2 while a JHM editorial accompanying the Ontario study noted, “Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others.”3
Might DBN be an artificial target that doesn’t actually enhance throughput, but leads instead to unintended consequences, such as patients being held over for an additional night in the hospital, rather than being discharged when they are ready to go on the afternoon before, in order to boost DBN rates? A perennial debate in hospital medicine is likely to be reignited by the new findings.
‘No significant overall association’
Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creation of deadlines for discharge orders, the new study’s authors noted. Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust.
The Canadian researchers retrospectively reviewed all patient admissions to general internal medicine services (GIMs) – largely similar to hospital medicine services in the United States – at seven hospitals in Toronto and Mississauga over a 7-year period ending Oct. 31, 2017, counting all of these patients who were discharged alive between 8 a.m. and noon. DBN averaged 19% of total live discharges across the diverse hospitals, with their diverse discharge practices.
But they found no significant overall association between morning discharge and hospital or emergency department length of stay. “Our findings suggest that increasing the number of morning discharges alone is unlikely to substantially improve patient throughput in GIM, but further research is needed to determine the effectiveness of specific interventions,” they concluded.
“We used a very narrow lens, looking specifically at throughput for the hospitals and emergency departments and whether DBN makes it more efficient,” said corresponding author Amol Verma, MD, MPhil, FRCPC, clinician-scientist at St. Michael’s Hospital, University of Toronto, in a recent interview. “What we found was that, on days when more patients are discharged in the morning, patients do not flow more quickly through the hospital. That suggests that increasing morning discharges is unlikely to make a difference.”
What does DBN really mean?
The semantics of DBN deserve further exploration. Is DBN about the actual hour of discharge, or the time when the hospitalist signs a discharge order – which may be well before the patient actually gets a wheelchair ride down to the hospital’s front doors? And if DBN is an organized program promoting morning discharges, how is it incentivized or otherwise rewarded?
Other factors, such as arrival of medications from the pharmacy or results from clinical tests, access to an ambulance if needed, transport to the front door, and bed cleaning will impact how quickly a doctor’s discharge orders get acted upon – and how quickly the newly emptied bed is available for the next occupant.
The clinician’s views on discharge practices may diverge from hospital administrator or health system perspectives, with its imperatives for efficient throughput in order to bring in more patients, Dr. Verma said. The hospitalist is also concerned about whether the patient feels ready to go home. “We can all agree that patients should leave the hospital as soon as they are medically able to do so,” he said. Longer hospital stays are associated with increased rates of hospital-acquired infections and other iatrogenic complications.
But there is not agreement on the components of a safe discharge – or on the other dimensions of effective patient flow and transitions of care. How do we optimize treatments initiated in the hospital? Does the patient need one more CAT scan? And what about the concerns of patient-centered care? Does the patient have a caregiver able to help them when they get home? There is a lot of uncertainty, Dr. Verma said. “These kinds of decisions have to get made many times every day by hospitalists,” he noted.
“We find ourselves trying to mirror the ebbs and flows of the emergency department with what’s happening in the hospital,” said Venkat Gundareddy, MBBS, MPH, associate director of the division of hospital medicine at Johns Hopkins Medicine in Baltimore. “The majority of hospital discharges happen during business hours, but the emergency department doesn’t stop admitting overnight, thus creating a throughput challenge.” Discharges are also based on clinical outcomes and on patients transferring to other facilities that prefer patients to arrive earlier in the day.
“Hospitalists may not fully appreciate these dynamics, because we’re siloed on our units,” Dr. Gundareddy said. “There is a subset of patients who would fit the bill for early discharge, but other patients come into the hospital with greater complexities, and a need for more coordination. Their discharges are harder to predict, although it gets clearer as their care progresses.”
The hospitals included in the Ontario study are at 90% -100% capacity, so their flexibility is constrained and throughput is a critical issue, Dr. Verma said. “But if you start with the target of more efficient throughput, there is no logical or practical reason to assume that discharge before noon would help. If we believe someone is ready for discharge based on physiologic changes, their response to treatment, and the conclusion of medical investigations, none of these conform to the clock. It’s equally likely the patient achieves them in the afternoon or evening.”
Other views on morning discharge
An alternative perspective comes from New York University’s Langone Medical Center, which has published positive results, including earlier subsequent arrivals to the inpatient unit from the emergency department, from increasing its hospital’s DBN rate.4
The hospital has continued to encourage morning discharges, which have consistently run 35%-40% or more of total discharges on two acute inpatient units at Langone’s Tisch Hospital. A previous study described the multidisciplinary intervention that resulted in a statistically significant increase in DBN – from 11% to 38% in the first 13 months – while significantly reducing high-frequency admission peaks.5
“We’ve been doing DBN for a number of years,” said Benjamin Wertheimer, MD, a hospitalist at Langone Medical Center and one of the studies’ authors. It is an achievable – and sustainable – goal. “Many hospitals around the country have problems with the flow of patients. Many hospitals are full – even before accounting for the COVID pandemic.” There is good evidence that, for a patient who no longer requires hospitalization, getting them out as early as possible, with a safe plan for their discharge, is a good thing, he said. “We see DBN as an important operational metric.”
If the necessary work is done correctly on the afternoon before the discharge, then a DBN approach can push communication, coordination, and advance planning, Dr Wertheimer said. Otherwise, essential discharge tasks may lag until the last minute. “We try to put the pieces in place the day before through a better planned process. But it should never be that DBN takes precedence over when the patient is safely ready to go,” he said.
“Our true measure of success would be how well we are preparing, communicating, putting safe plans into place,” he added. “DBN does not in and of itself answer all the safety and quality concerns. We set priorities around specific quality targets. DBN is just one of our operational and safety measures.”
The DBN intervention at Langone started with a multidisciplinary kickoff event in which all team members received education on its importance, a clear description of roles in the DBN process, and a corresponding checklist of daily responsibilities. The checklist was utilized at newly implemented afternoon interdisciplinary rounds, scripted to identify next-day DBNs, and make sure everything is in place for them, he explained.
“We provide daily feedback to floor staff on the DBN percentage, celebrate success, and offer real-time opportunities for case review,” Dr. Wertheimer said. “We have been careful about how we message this goal. Quality and safety come first, and we want to be prepared for discharge in advance of when the patient is ready.”
A boost for discharges
Mark Williams, MD, MHM, recently appointed chief of hospital medicine at Washington University School of Medicine in St. Louis, and a principal investigator for Project BOOST (Better Outcomes by Optimizing Safe Transitions), SHM’s quality improvement mentoring initiative aimed at helping hospitals improve care transitions, said that debates about DBN have gone on for a long time in hospital medicine.
“Around 2002, consultants told the CEO of a community hospital affiliated with Emory Healthcare that if our hospitalists could discharge patients before noon it would improve throughput,” he recalled. The consultants came from the hospitality industry, where DBN is easier to achieve.
But in hospital medicine, he said, “We use the whole day of the discharge in delivering care. I said to the CEO, ‘I can get you 100% discharge before noon – I’ll just hold the patients overnight,’” he explained. “In our initial experience, we pushed DBN up to about 10% -15%, and it opened up a few beds, which rapidly filled.”
Project BOOST encouraged the goal of getting patients ready to go out as soon as they were clinically ready, but did not advocate specifically for DBN, Dr. Williams said. “The problem is that hospital throughput starts to gum up when occupancy goes over 80% or 90%, and many academic medical centers regularly reach occupancy rates greater than 100%, particularly in the afternoon.” The deluge of patients includes transfers from other hospitals, postsurgical patients, and admissions from the emergency department.
“Boarding in the ED is a real issue,” he said. “Right now, it’s a crisis of overoccupancy, and the problem is that the pipeline is pouring patients into the system faster than they can be discharged.”
Dr. Williams believes there needs to be bigger thinking about these issues. Could hospitals, health systems, and hospitalists practice more preventive medicine so that some of these patients don’t need to come to the hospital? “Can you better address high blood pressure to prevent strokes and make sure patients with heart disease risk factors are enrolled in exercise and nutrition programs? What about access to healthy foods and the other social determinants of health? What if we provided adequate, consistent housing and transportation to medical visits?” he wondered.
Hospital at home programs may also offer some relief, he said. “If suddenly there weren’t so many emergency room visits by patients who need to get admitted, we’d have enough beds in the hospital.”
A more holistic view
John Nelson, MD, MHM, hospital medicine pioneer and management consultant, has been studying hospital throughput and policies to improve it for a long time. His 2010 column in The Hospitalist, “The Earlier the Better,” said attaching a financial incentive for hospitalists to discharge patients by a preset hour has produced mixed results.6 But Dr. Nelson offered some easy steps hospitalists can take to maximize earlier discharges, including to write “probable discharge tomorrow” as an order in the patient’s medical record.
The afternoon before a planned discharge, the hospitalist could talk to a patient’s family members about the discharge plan and order any outstanding tests to be done that evening to be ready for morning rounds – which he suggested should start by 7:00 a.m. The hospitalist could dictate the discharge summary the afternoon before. Even if a discharge can’t proceed as planned, the time isn’t necessarily wasted.
In a recent interview, Dr. Nelson noted that the movement to reduce average length of stay in the hospital has complicated the discharge picture by reducing a hospital’s flexibility. But he added that it’s still worth tracking and collecting data on discharge times, and to keep the conversation going. “Just don’t lose sight of the real goal, which is not DBN but optimal length-of-stay management,” he said.
Dr. Gundareddy said that, as his group has dealt with these issues, some steps have emerged to help manage discharges and throughput. “We didn’t have case management and social work services over the weekend, but when we added that support, it changed how our Mondays went.”
He encourages hospitalists to focus on the actual processes that create bottlenecks preventing throughput. “A good example of effective restructuring is lab testing. It’s amazing to think that you could have lab test results available for 7:00 a.m. rounds. There are areas that deserve more attention and more research regarding DBN. What is the impact of discharge before noon programs on the patients who aren’t being planned for discharge that day? Do they get neglected? I feel that happens sometimes.”
The COVID pandemic has further complicated these questions, Dr. Gundareddy said. “Early on in the pandemic, we were unsure how things were going with discharges, since all of the focus was on the COVID crisis. A lot of outpatient and surgical services came to a standstill, and there weren’t enough of the right kinds of beds for COVID patients. It was hard to align staff appropriately with the new clinical goals and to train them during the crisis.” Now, patients who delayed care during the pandemic are turning up at the hospital with greater acuity.
As with all incentives, DBN can have unintended consequences – especially if you monetize the practice, Dr. Verma said. “Most hospitalists are already working so hard – making so many decisions every day. These incentives could push decisions that aren’t in anybody’s best interests.”
Various groups have created comprehensive packages of protocols for improving transitions of care, he said. Organized programs to maximize efficiency of transitions and patient flow, including Project BOOST and Project RED (Re-Engineered Discharge) at Boston University Medical Center, are important sources of tools and resources. “But we should stop flogging hospitalists to discharge patients before noon,” Dr. Verma said, “Discharge is more complex than that. Instead, we should work to improve discharges in more holistic ways.”
References
1. Kirubarajan A et al. Morning discharges and patient length of stay in inpatient general internal medicine. J Hosp Med. 2021 Jun;16(6):333-8. doi: 10.12788/jhm.3605.
2. Shine D. Discharge before noon: An urban legend. Am J Med. 2015 May;128(5):445-6. doi:10.1016/j.amjmed.2014.12.011.
3. Zorian A et al. Discharge by noon: Toward a better understanding of benefits and costs. J Hosp Med. 2021 Jun;16(6):384. doi: 10.12788/jhm.3613.
4. Wertheimer B et al. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015 Oct;10(10):664-9. doi: 10.1002/jhm.2412.
5. Wertheimer B et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014 Apr;9(4):210-4. doi: 10.1002/jhm.2154.
6. Nelson J. The earlier, the better. The Hospitalist. 2010 May.
‘Perennial debate’ likely to be reignited
‘Perennial debate’ likely to be reignited
A recent study published in the Journal of Hospital Medicine examined patient discharges from hospitals in Ontario, Canada, to determine if morning discharges were associated with positive outcomes. Some hospitalist programs have embraced discharge before noon (DBN) initiatives like those studied in the article.1 Unfortunately, the researchers concluded that the Canadian DBNs did not positively impact hospital length of stay, readmissions, or mortality rates.
DBN has been a quality improvement target for hospitals hoping to improve throughput and free up scarce beds, while promoting patient safety by encouraging discharge as soon as patients are ready to leave. Yet other researchers have questioned its actual impact on quality metrics. One author called DBN’s purported impact an “urban legend,”2 while a JHM editorial accompanying the Ontario study noted, “Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others.”3
Might DBN be an artificial target that doesn’t actually enhance throughput, but leads instead to unintended consequences, such as patients being held over for an additional night in the hospital, rather than being discharged when they are ready to go on the afternoon before, in order to boost DBN rates? A perennial debate in hospital medicine is likely to be reignited by the new findings.
‘No significant overall association’
Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creation of deadlines for discharge orders, the new study’s authors noted. Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust.
The Canadian researchers retrospectively reviewed all patient admissions to general internal medicine services (GIMs) – largely similar to hospital medicine services in the United States – at seven hospitals in Toronto and Mississauga over a 7-year period ending Oct. 31, 2017, counting all of these patients who were discharged alive between 8 a.m. and noon. DBN averaged 19% of total live discharges across the diverse hospitals, with their diverse discharge practices.
But they found no significant overall association between morning discharge and hospital or emergency department length of stay. “Our findings suggest that increasing the number of morning discharges alone is unlikely to substantially improve patient throughput in GIM, but further research is needed to determine the effectiveness of specific interventions,” they concluded.
“We used a very narrow lens, looking specifically at throughput for the hospitals and emergency departments and whether DBN makes it more efficient,” said corresponding author Amol Verma, MD, MPhil, FRCPC, clinician-scientist at St. Michael’s Hospital, University of Toronto, in a recent interview. “What we found was that, on days when more patients are discharged in the morning, patients do not flow more quickly through the hospital. That suggests that increasing morning discharges is unlikely to make a difference.”
What does DBN really mean?
The semantics of DBN deserve further exploration. Is DBN about the actual hour of discharge, or the time when the hospitalist signs a discharge order – which may be well before the patient actually gets a wheelchair ride down to the hospital’s front doors? And if DBN is an organized program promoting morning discharges, how is it incentivized or otherwise rewarded?
Other factors, such as arrival of medications from the pharmacy or results from clinical tests, access to an ambulance if needed, transport to the front door, and bed cleaning will impact how quickly a doctor’s discharge orders get acted upon – and how quickly the newly emptied bed is available for the next occupant.
The clinician’s views on discharge practices may diverge from hospital administrator or health system perspectives, with its imperatives for efficient throughput in order to bring in more patients, Dr. Verma said. The hospitalist is also concerned about whether the patient feels ready to go home. “We can all agree that patients should leave the hospital as soon as they are medically able to do so,” he said. Longer hospital stays are associated with increased rates of hospital-acquired infections and other iatrogenic complications.
But there is not agreement on the components of a safe discharge – or on the other dimensions of effective patient flow and transitions of care. How do we optimize treatments initiated in the hospital? Does the patient need one more CAT scan? And what about the concerns of patient-centered care? Does the patient have a caregiver able to help them when they get home? There is a lot of uncertainty, Dr. Verma said. “These kinds of decisions have to get made many times every day by hospitalists,” he noted.
“We find ourselves trying to mirror the ebbs and flows of the emergency department with what’s happening in the hospital,” said Venkat Gundareddy, MBBS, MPH, associate director of the division of hospital medicine at Johns Hopkins Medicine in Baltimore. “The majority of hospital discharges happen during business hours, but the emergency department doesn’t stop admitting overnight, thus creating a throughput challenge.” Discharges are also based on clinical outcomes and on patients transferring to other facilities that prefer patients to arrive earlier in the day.
“Hospitalists may not fully appreciate these dynamics, because we’re siloed on our units,” Dr. Gundareddy said. “There is a subset of patients who would fit the bill for early discharge, but other patients come into the hospital with greater complexities, and a need for more coordination. Their discharges are harder to predict, although it gets clearer as their care progresses.”
The hospitals included in the Ontario study are at 90% -100% capacity, so their flexibility is constrained and throughput is a critical issue, Dr. Verma said. “But if you start with the target of more efficient throughput, there is no logical or practical reason to assume that discharge before noon would help. If we believe someone is ready for discharge based on physiologic changes, their response to treatment, and the conclusion of medical investigations, none of these conform to the clock. It’s equally likely the patient achieves them in the afternoon or evening.”
Other views on morning discharge
An alternative perspective comes from New York University’s Langone Medical Center, which has published positive results, including earlier subsequent arrivals to the inpatient unit from the emergency department, from increasing its hospital’s DBN rate.4
The hospital has continued to encourage morning discharges, which have consistently run 35%-40% or more of total discharges on two acute inpatient units at Langone’s Tisch Hospital. A previous study described the multidisciplinary intervention that resulted in a statistically significant increase in DBN – from 11% to 38% in the first 13 months – while significantly reducing high-frequency admission peaks.5
“We’ve been doing DBN for a number of years,” said Benjamin Wertheimer, MD, a hospitalist at Langone Medical Center and one of the studies’ authors. It is an achievable – and sustainable – goal. “Many hospitals around the country have problems with the flow of patients. Many hospitals are full – even before accounting for the COVID pandemic.” There is good evidence that, for a patient who no longer requires hospitalization, getting them out as early as possible, with a safe plan for their discharge, is a good thing, he said. “We see DBN as an important operational metric.”
If the necessary work is done correctly on the afternoon before the discharge, then a DBN approach can push communication, coordination, and advance planning, Dr Wertheimer said. Otherwise, essential discharge tasks may lag until the last minute. “We try to put the pieces in place the day before through a better planned process. But it should never be that DBN takes precedence over when the patient is safely ready to go,” he said.
“Our true measure of success would be how well we are preparing, communicating, putting safe plans into place,” he added. “DBN does not in and of itself answer all the safety and quality concerns. We set priorities around specific quality targets. DBN is just one of our operational and safety measures.”
The DBN intervention at Langone started with a multidisciplinary kickoff event in which all team members received education on its importance, a clear description of roles in the DBN process, and a corresponding checklist of daily responsibilities. The checklist was utilized at newly implemented afternoon interdisciplinary rounds, scripted to identify next-day DBNs, and make sure everything is in place for them, he explained.
“We provide daily feedback to floor staff on the DBN percentage, celebrate success, and offer real-time opportunities for case review,” Dr. Wertheimer said. “We have been careful about how we message this goal. Quality and safety come first, and we want to be prepared for discharge in advance of when the patient is ready.”
A boost for discharges
Mark Williams, MD, MHM, recently appointed chief of hospital medicine at Washington University School of Medicine in St. Louis, and a principal investigator for Project BOOST (Better Outcomes by Optimizing Safe Transitions), SHM’s quality improvement mentoring initiative aimed at helping hospitals improve care transitions, said that debates about DBN have gone on for a long time in hospital medicine.
“Around 2002, consultants told the CEO of a community hospital affiliated with Emory Healthcare that if our hospitalists could discharge patients before noon it would improve throughput,” he recalled. The consultants came from the hospitality industry, where DBN is easier to achieve.
But in hospital medicine, he said, “We use the whole day of the discharge in delivering care. I said to the CEO, ‘I can get you 100% discharge before noon – I’ll just hold the patients overnight,’” he explained. “In our initial experience, we pushed DBN up to about 10% -15%, and it opened up a few beds, which rapidly filled.”
Project BOOST encouraged the goal of getting patients ready to go out as soon as they were clinically ready, but did not advocate specifically for DBN, Dr. Williams said. “The problem is that hospital throughput starts to gum up when occupancy goes over 80% or 90%, and many academic medical centers regularly reach occupancy rates greater than 100%, particularly in the afternoon.” The deluge of patients includes transfers from other hospitals, postsurgical patients, and admissions from the emergency department.
“Boarding in the ED is a real issue,” he said. “Right now, it’s a crisis of overoccupancy, and the problem is that the pipeline is pouring patients into the system faster than they can be discharged.”
Dr. Williams believes there needs to be bigger thinking about these issues. Could hospitals, health systems, and hospitalists practice more preventive medicine so that some of these patients don’t need to come to the hospital? “Can you better address high blood pressure to prevent strokes and make sure patients with heart disease risk factors are enrolled in exercise and nutrition programs? What about access to healthy foods and the other social determinants of health? What if we provided adequate, consistent housing and transportation to medical visits?” he wondered.
Hospital at home programs may also offer some relief, he said. “If suddenly there weren’t so many emergency room visits by patients who need to get admitted, we’d have enough beds in the hospital.”
A more holistic view
John Nelson, MD, MHM, hospital medicine pioneer and management consultant, has been studying hospital throughput and policies to improve it for a long time. His 2010 column in The Hospitalist, “The Earlier the Better,” said attaching a financial incentive for hospitalists to discharge patients by a preset hour has produced mixed results.6 But Dr. Nelson offered some easy steps hospitalists can take to maximize earlier discharges, including to write “probable discharge tomorrow” as an order in the patient’s medical record.
The afternoon before a planned discharge, the hospitalist could talk to a patient’s family members about the discharge plan and order any outstanding tests to be done that evening to be ready for morning rounds – which he suggested should start by 7:00 a.m. The hospitalist could dictate the discharge summary the afternoon before. Even if a discharge can’t proceed as planned, the time isn’t necessarily wasted.
In a recent interview, Dr. Nelson noted that the movement to reduce average length of stay in the hospital has complicated the discharge picture by reducing a hospital’s flexibility. But he added that it’s still worth tracking and collecting data on discharge times, and to keep the conversation going. “Just don’t lose sight of the real goal, which is not DBN but optimal length-of-stay management,” he said.
Dr. Gundareddy said that, as his group has dealt with these issues, some steps have emerged to help manage discharges and throughput. “We didn’t have case management and social work services over the weekend, but when we added that support, it changed how our Mondays went.”
He encourages hospitalists to focus on the actual processes that create bottlenecks preventing throughput. “A good example of effective restructuring is lab testing. It’s amazing to think that you could have lab test results available for 7:00 a.m. rounds. There are areas that deserve more attention and more research regarding DBN. What is the impact of discharge before noon programs on the patients who aren’t being planned for discharge that day? Do they get neglected? I feel that happens sometimes.”
The COVID pandemic has further complicated these questions, Dr. Gundareddy said. “Early on in the pandemic, we were unsure how things were going with discharges, since all of the focus was on the COVID crisis. A lot of outpatient and surgical services came to a standstill, and there weren’t enough of the right kinds of beds for COVID patients. It was hard to align staff appropriately with the new clinical goals and to train them during the crisis.” Now, patients who delayed care during the pandemic are turning up at the hospital with greater acuity.
As with all incentives, DBN can have unintended consequences – especially if you monetize the practice, Dr. Verma said. “Most hospitalists are already working so hard – making so many decisions every day. These incentives could push decisions that aren’t in anybody’s best interests.”
Various groups have created comprehensive packages of protocols for improving transitions of care, he said. Organized programs to maximize efficiency of transitions and patient flow, including Project BOOST and Project RED (Re-Engineered Discharge) at Boston University Medical Center, are important sources of tools and resources. “But we should stop flogging hospitalists to discharge patients before noon,” Dr. Verma said, “Discharge is more complex than that. Instead, we should work to improve discharges in more holistic ways.”
References
1. Kirubarajan A et al. Morning discharges and patient length of stay in inpatient general internal medicine. J Hosp Med. 2021 Jun;16(6):333-8. doi: 10.12788/jhm.3605.
2. Shine D. Discharge before noon: An urban legend. Am J Med. 2015 May;128(5):445-6. doi:10.1016/j.amjmed.2014.12.011.
3. Zorian A et al. Discharge by noon: Toward a better understanding of benefits and costs. J Hosp Med. 2021 Jun;16(6):384. doi: 10.12788/jhm.3613.
4. Wertheimer B et al. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015 Oct;10(10):664-9. doi: 10.1002/jhm.2412.
5. Wertheimer B et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014 Apr;9(4):210-4. doi: 10.1002/jhm.2154.
6. Nelson J. The earlier, the better. The Hospitalist. 2010 May.
A recent study published in the Journal of Hospital Medicine examined patient discharges from hospitals in Ontario, Canada, to determine if morning discharges were associated with positive outcomes. Some hospitalist programs have embraced discharge before noon (DBN) initiatives like those studied in the article.1 Unfortunately, the researchers concluded that the Canadian DBNs did not positively impact hospital length of stay, readmissions, or mortality rates.
DBN has been a quality improvement target for hospitals hoping to improve throughput and free up scarce beds, while promoting patient safety by encouraging discharge as soon as patients are ready to leave. Yet other researchers have questioned its actual impact on quality metrics. One author called DBN’s purported impact an “urban legend,”2 while a JHM editorial accompanying the Ontario study noted, “Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others.”3
Might DBN be an artificial target that doesn’t actually enhance throughput, but leads instead to unintended consequences, such as patients being held over for an additional night in the hospital, rather than being discharged when they are ready to go on the afternoon before, in order to boost DBN rates? A perennial debate in hospital medicine is likely to be reignited by the new findings.
‘No significant overall association’
Quality improvement initiatives targeting morning discharges have included stakeholder meetings, incentives programs, discharge-centered breakfast programs, and creation of deadlines for discharge orders, the new study’s authors noted. Although these initiatives have gained support, critics have suggested that their supporting evidence is not robust.
The Canadian researchers retrospectively reviewed all patient admissions to general internal medicine services (GIMs) – largely similar to hospital medicine services in the United States – at seven hospitals in Toronto and Mississauga over a 7-year period ending Oct. 31, 2017, counting all of these patients who were discharged alive between 8 a.m. and noon. DBN averaged 19% of total live discharges across the diverse hospitals, with their diverse discharge practices.
But they found no significant overall association between morning discharge and hospital or emergency department length of stay. “Our findings suggest that increasing the number of morning discharges alone is unlikely to substantially improve patient throughput in GIM, but further research is needed to determine the effectiveness of specific interventions,” they concluded.
“We used a very narrow lens, looking specifically at throughput for the hospitals and emergency departments and whether DBN makes it more efficient,” said corresponding author Amol Verma, MD, MPhil, FRCPC, clinician-scientist at St. Michael’s Hospital, University of Toronto, in a recent interview. “What we found was that, on days when more patients are discharged in the morning, patients do not flow more quickly through the hospital. That suggests that increasing morning discharges is unlikely to make a difference.”
What does DBN really mean?
The semantics of DBN deserve further exploration. Is DBN about the actual hour of discharge, or the time when the hospitalist signs a discharge order – which may be well before the patient actually gets a wheelchair ride down to the hospital’s front doors? And if DBN is an organized program promoting morning discharges, how is it incentivized or otherwise rewarded?
Other factors, such as arrival of medications from the pharmacy or results from clinical tests, access to an ambulance if needed, transport to the front door, and bed cleaning will impact how quickly a doctor’s discharge orders get acted upon – and how quickly the newly emptied bed is available for the next occupant.
The clinician’s views on discharge practices may diverge from hospital administrator or health system perspectives, with its imperatives for efficient throughput in order to bring in more patients, Dr. Verma said. The hospitalist is also concerned about whether the patient feels ready to go home. “We can all agree that patients should leave the hospital as soon as they are medically able to do so,” he said. Longer hospital stays are associated with increased rates of hospital-acquired infections and other iatrogenic complications.
But there is not agreement on the components of a safe discharge – or on the other dimensions of effective patient flow and transitions of care. How do we optimize treatments initiated in the hospital? Does the patient need one more CAT scan? And what about the concerns of patient-centered care? Does the patient have a caregiver able to help them when they get home? There is a lot of uncertainty, Dr. Verma said. “These kinds of decisions have to get made many times every day by hospitalists,” he noted.
“We find ourselves trying to mirror the ebbs and flows of the emergency department with what’s happening in the hospital,” said Venkat Gundareddy, MBBS, MPH, associate director of the division of hospital medicine at Johns Hopkins Medicine in Baltimore. “The majority of hospital discharges happen during business hours, but the emergency department doesn’t stop admitting overnight, thus creating a throughput challenge.” Discharges are also based on clinical outcomes and on patients transferring to other facilities that prefer patients to arrive earlier in the day.
“Hospitalists may not fully appreciate these dynamics, because we’re siloed on our units,” Dr. Gundareddy said. “There is a subset of patients who would fit the bill for early discharge, but other patients come into the hospital with greater complexities, and a need for more coordination. Their discharges are harder to predict, although it gets clearer as their care progresses.”
The hospitals included in the Ontario study are at 90% -100% capacity, so their flexibility is constrained and throughput is a critical issue, Dr. Verma said. “But if you start with the target of more efficient throughput, there is no logical or practical reason to assume that discharge before noon would help. If we believe someone is ready for discharge based on physiologic changes, their response to treatment, and the conclusion of medical investigations, none of these conform to the clock. It’s equally likely the patient achieves them in the afternoon or evening.”
Other views on morning discharge
An alternative perspective comes from New York University’s Langone Medical Center, which has published positive results, including earlier subsequent arrivals to the inpatient unit from the emergency department, from increasing its hospital’s DBN rate.4
The hospital has continued to encourage morning discharges, which have consistently run 35%-40% or more of total discharges on two acute inpatient units at Langone’s Tisch Hospital. A previous study described the multidisciplinary intervention that resulted in a statistically significant increase in DBN – from 11% to 38% in the first 13 months – while significantly reducing high-frequency admission peaks.5
“We’ve been doing DBN for a number of years,” said Benjamin Wertheimer, MD, a hospitalist at Langone Medical Center and one of the studies’ authors. It is an achievable – and sustainable – goal. “Many hospitals around the country have problems with the flow of patients. Many hospitals are full – even before accounting for the COVID pandemic.” There is good evidence that, for a patient who no longer requires hospitalization, getting them out as early as possible, with a safe plan for their discharge, is a good thing, he said. “We see DBN as an important operational metric.”
If the necessary work is done correctly on the afternoon before the discharge, then a DBN approach can push communication, coordination, and advance planning, Dr Wertheimer said. Otherwise, essential discharge tasks may lag until the last minute. “We try to put the pieces in place the day before through a better planned process. But it should never be that DBN takes precedence over when the patient is safely ready to go,” he said.
“Our true measure of success would be how well we are preparing, communicating, putting safe plans into place,” he added. “DBN does not in and of itself answer all the safety and quality concerns. We set priorities around specific quality targets. DBN is just one of our operational and safety measures.”
The DBN intervention at Langone started with a multidisciplinary kickoff event in which all team members received education on its importance, a clear description of roles in the DBN process, and a corresponding checklist of daily responsibilities. The checklist was utilized at newly implemented afternoon interdisciplinary rounds, scripted to identify next-day DBNs, and make sure everything is in place for them, he explained.
“We provide daily feedback to floor staff on the DBN percentage, celebrate success, and offer real-time opportunities for case review,” Dr. Wertheimer said. “We have been careful about how we message this goal. Quality and safety come first, and we want to be prepared for discharge in advance of when the patient is ready.”
A boost for discharges
Mark Williams, MD, MHM, recently appointed chief of hospital medicine at Washington University School of Medicine in St. Louis, and a principal investigator for Project BOOST (Better Outcomes by Optimizing Safe Transitions), SHM’s quality improvement mentoring initiative aimed at helping hospitals improve care transitions, said that debates about DBN have gone on for a long time in hospital medicine.
“Around 2002, consultants told the CEO of a community hospital affiliated with Emory Healthcare that if our hospitalists could discharge patients before noon it would improve throughput,” he recalled. The consultants came from the hospitality industry, where DBN is easier to achieve.
But in hospital medicine, he said, “We use the whole day of the discharge in delivering care. I said to the CEO, ‘I can get you 100% discharge before noon – I’ll just hold the patients overnight,’” he explained. “In our initial experience, we pushed DBN up to about 10% -15%, and it opened up a few beds, which rapidly filled.”
Project BOOST encouraged the goal of getting patients ready to go out as soon as they were clinically ready, but did not advocate specifically for DBN, Dr. Williams said. “The problem is that hospital throughput starts to gum up when occupancy goes over 80% or 90%, and many academic medical centers regularly reach occupancy rates greater than 100%, particularly in the afternoon.” The deluge of patients includes transfers from other hospitals, postsurgical patients, and admissions from the emergency department.
“Boarding in the ED is a real issue,” he said. “Right now, it’s a crisis of overoccupancy, and the problem is that the pipeline is pouring patients into the system faster than they can be discharged.”
Dr. Williams believes there needs to be bigger thinking about these issues. Could hospitals, health systems, and hospitalists practice more preventive medicine so that some of these patients don’t need to come to the hospital? “Can you better address high blood pressure to prevent strokes and make sure patients with heart disease risk factors are enrolled in exercise and nutrition programs? What about access to healthy foods and the other social determinants of health? What if we provided adequate, consistent housing and transportation to medical visits?” he wondered.
Hospital at home programs may also offer some relief, he said. “If suddenly there weren’t so many emergency room visits by patients who need to get admitted, we’d have enough beds in the hospital.”
A more holistic view
John Nelson, MD, MHM, hospital medicine pioneer and management consultant, has been studying hospital throughput and policies to improve it for a long time. His 2010 column in The Hospitalist, “The Earlier the Better,” said attaching a financial incentive for hospitalists to discharge patients by a preset hour has produced mixed results.6 But Dr. Nelson offered some easy steps hospitalists can take to maximize earlier discharges, including to write “probable discharge tomorrow” as an order in the patient’s medical record.
The afternoon before a planned discharge, the hospitalist could talk to a patient’s family members about the discharge plan and order any outstanding tests to be done that evening to be ready for morning rounds – which he suggested should start by 7:00 a.m. The hospitalist could dictate the discharge summary the afternoon before. Even if a discharge can’t proceed as planned, the time isn’t necessarily wasted.
In a recent interview, Dr. Nelson noted that the movement to reduce average length of stay in the hospital has complicated the discharge picture by reducing a hospital’s flexibility. But he added that it’s still worth tracking and collecting data on discharge times, and to keep the conversation going. “Just don’t lose sight of the real goal, which is not DBN but optimal length-of-stay management,” he said.
Dr. Gundareddy said that, as his group has dealt with these issues, some steps have emerged to help manage discharges and throughput. “We didn’t have case management and social work services over the weekend, but when we added that support, it changed how our Mondays went.”
He encourages hospitalists to focus on the actual processes that create bottlenecks preventing throughput. “A good example of effective restructuring is lab testing. It’s amazing to think that you could have lab test results available for 7:00 a.m. rounds. There are areas that deserve more attention and more research regarding DBN. What is the impact of discharge before noon programs on the patients who aren’t being planned for discharge that day? Do they get neglected? I feel that happens sometimes.”
The COVID pandemic has further complicated these questions, Dr. Gundareddy said. “Early on in the pandemic, we were unsure how things were going with discharges, since all of the focus was on the COVID crisis. A lot of outpatient and surgical services came to a standstill, and there weren’t enough of the right kinds of beds for COVID patients. It was hard to align staff appropriately with the new clinical goals and to train them during the crisis.” Now, patients who delayed care during the pandemic are turning up at the hospital with greater acuity.
As with all incentives, DBN can have unintended consequences – especially if you monetize the practice, Dr. Verma said. “Most hospitalists are already working so hard – making so many decisions every day. These incentives could push decisions that aren’t in anybody’s best interests.”
Various groups have created comprehensive packages of protocols for improving transitions of care, he said. Organized programs to maximize efficiency of transitions and patient flow, including Project BOOST and Project RED (Re-Engineered Discharge) at Boston University Medical Center, are important sources of tools and resources. “But we should stop flogging hospitalists to discharge patients before noon,” Dr. Verma said, “Discharge is more complex than that. Instead, we should work to improve discharges in more holistic ways.”
References
1. Kirubarajan A et al. Morning discharges and patient length of stay in inpatient general internal medicine. J Hosp Med. 2021 Jun;16(6):333-8. doi: 10.12788/jhm.3605.
2. Shine D. Discharge before noon: An urban legend. Am J Med. 2015 May;128(5):445-6. doi:10.1016/j.amjmed.2014.12.011.
3. Zorian A et al. Discharge by noon: Toward a better understanding of benefits and costs. J Hosp Med. 2021 Jun;16(6):384. doi: 10.12788/jhm.3613.
4. Wertheimer B et al. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015 Oct;10(10):664-9. doi: 10.1002/jhm.2412.
5. Wertheimer B et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014 Apr;9(4):210-4. doi: 10.1002/jhm.2154.
6. Nelson J. The earlier, the better. The Hospitalist. 2010 May.
Oral step-down therapy for infective endocarditis
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Reflecting on 2021, looking forward to 2022
This month marks the end of my first full calendar year as SHM CEO. Over the years, I have made it a habit to take time to reflect during the month of December, assessing the previous year by reviewing what went well and what could have gone better, and how I can grow and change to meet the needs of future challenges. This reflection sets the stage for my personal and professional “New Year” goals.
This year, 2021, is certainly a year deserving of reflection, and I believe 2022 (and beyond) will need ambitious goals made by dedicated leaders, hospitalists included. Here are my thoughts on what went well in 2021 and what I wish went better – from our greater society to our specialty, to SHM.
Society (as in the larger society)
What went well: Vaccines
There is a lot to be impressed with in 2021, and for me, at the top of that list are the COVID-19 vaccines. I realize the research for mRNA vaccines started more than 20 years ago, and the most successful mRNA vaccine companies have been around for more than a decade, but to roll out a COVID-19 vaccine in less than a year is still just incredible. To take a disease with a 2% mortality rate for someone like myself and effectively reduce that to near zero is something historians will be writing about for years to come.
What I wish went better: Open dialogue
I can’t remember when we stopped listening to each other, and by that, I mean listening to those who do not think exactly like ourselves. As a kid, I was taught to be careful about discussing topics at social events that could go sideways. That usually involved politics, money, or strong beliefs, but wow – now, that list is much longer. Talking about the weather used to be safe, but not anymore. If I were to show pictures of the recent flooding in Annapolis? There would almost certainly be a debate about climate change. At least we can agree on Ted Lasso as a safe topic.
Our specialty
What went well: Hospitalists are vital
There are many, many professions that deserve “hero” status for their part in taming this pandemic: nurses, doctors, emergency medical services, physical therapists, physician assistants, nurse practitioners, administrators, and more. But in the doctor category, hospitalists are at the top. Along with our emergency department and intensivist colleagues, hospitalists are one of the pillars of the inpatient response to COVID. More than 3.2 million COVID-19 hospitalizations have occurred, according to the Centers for Disease Control and Prevention, with numerous state dashboards showing three-quarters of those are cared for on general medical wards, the domain of hospitalists (for example, see my own state of Maryland’s COVID-19 dashboard: https://coronavirus.maryland.gov).
We’ve always had “two patients” – the patient in the bed and the health care system. Many hospitalists have helped their institutions by building COVID care teams, COVID wards, or in the case of Dr. Mindy Kantsiper, building an entire COVID field hospital in a convention center. Without hospitalists, both patients and the system that serves them would have fared much worse in this pandemic. Hospitalists are vital to patients and the health care system. The end. Period. End of story.
What I wish went better: Getting credit
As a profession, we need to be more deliberate about getting credit for the fantastic work we have done to care for COVID-19 patients, as well as inpatients in general. SHM can and must focus more on how to highlight the great work hospitalists have done and will continue to do. A greater understanding by the health care industry – as well as the general public – regarding the important role we play for patient care will help add autonomy in our profession, which in turn adds to resilience during these challenging times.
SHM
What went well: Membership grew
This is the one thing that we at SHM – and I personally – are most proud of. SHM is a membership society; it is the single most important metric for me personally. If physicians aren’t joining, then we are not meeting our core mission to provide value to hospitalists. My sense is the services SHM provides to hospitalists continue to be of value – even during these strenuous times of the pandemic when we had to be physically distant.
Whether it’s our Government Relations Department advocating for hospitalists in Washington, or the Journal of Hospital Medicine, or this very magazine, The Hospitalist, or SHM’s numerous educational offerings, chapter events, and SHM national meetings (Converge, Pediatric Hospital Medicine, Leadership Academies, Academic Hospitalist Academy, and more), SHM continues to provide hospitalists with vital tools to help you in your career.
This is also very much a two-way street. If you are reading this, know that without you, our members, our success would not be possible. Your passion and partnership drive us to innovate to meet your needs and those of the patients you serve every day. Thank you for your continued support and inspiration.
What could have gone better: Seeing more of you, in person
This is a tough one for me. Everything I worried about going wrong for SHM in 2021 never materialized. A year ago, my fears for SHM were that membership would shrink, finances would dry up, and the SHM staff would leave (by furlough or by choice). Thankfully, membership grew, our finances are in very good shape for any year, let alone a pandemic year, and the staff have remained at SHM and are engaged and dedicated! SHM even received a “Best Place to Work” award from the Philadelphia Business Journal.
Maybe the one regret I have is that we could not do more in-person events. But even there, I think we did better than most. We had some chapter meetings in person, and the October 2021 Leadership Academy hosted 110 hospitalist leaders, in person, at Amelia Island, Fla. That Leadership Academy went off without a hitch, and the early reviews are superb. I am very optimistic about 2022 in-person events!
Looking forward: 2022 and beyond
I have no illusions that 2022 is going to be easy. I know that the pandemic will not be gone (even though cases are falling nationwide as of this writing), that our nation will struggle with how to deal with polarization, and the workplace will continue to be redefined. Yet, I can’t help but be optimistic.
The pandemic will end eventually; all pandemics do. My hope is that young leaders will step forward to help our nation work through the divisive challenges, and some of those leaders will even be hospitalists! I also know that our profession is more vital than ever, for both patients and the health care system. We’re even getting ready to celebrate SHM’s 25th anniversary, and we can’t wait to revisit our humble beginnings while looking at the bright future of our society and our field.
I am working on my 2022 “New Year” goals, but you can be pretty sure they will revolve around making the world a better place, investing in people, and being ethical and transparent.
Dr. Howell is the CEO of the Society of Hospital Medicine.
This month marks the end of my first full calendar year as SHM CEO. Over the years, I have made it a habit to take time to reflect during the month of December, assessing the previous year by reviewing what went well and what could have gone better, and how I can grow and change to meet the needs of future challenges. This reflection sets the stage for my personal and professional “New Year” goals.
This year, 2021, is certainly a year deserving of reflection, and I believe 2022 (and beyond) will need ambitious goals made by dedicated leaders, hospitalists included. Here are my thoughts on what went well in 2021 and what I wish went better – from our greater society to our specialty, to SHM.
Society (as in the larger society)
What went well: Vaccines
There is a lot to be impressed with in 2021, and for me, at the top of that list are the COVID-19 vaccines. I realize the research for mRNA vaccines started more than 20 years ago, and the most successful mRNA vaccine companies have been around for more than a decade, but to roll out a COVID-19 vaccine in less than a year is still just incredible. To take a disease with a 2% mortality rate for someone like myself and effectively reduce that to near zero is something historians will be writing about for years to come.
What I wish went better: Open dialogue
I can’t remember when we stopped listening to each other, and by that, I mean listening to those who do not think exactly like ourselves. As a kid, I was taught to be careful about discussing topics at social events that could go sideways. That usually involved politics, money, or strong beliefs, but wow – now, that list is much longer. Talking about the weather used to be safe, but not anymore. If I were to show pictures of the recent flooding in Annapolis? There would almost certainly be a debate about climate change. At least we can agree on Ted Lasso as a safe topic.
Our specialty
What went well: Hospitalists are vital
There are many, many professions that deserve “hero” status for their part in taming this pandemic: nurses, doctors, emergency medical services, physical therapists, physician assistants, nurse practitioners, administrators, and more. But in the doctor category, hospitalists are at the top. Along with our emergency department and intensivist colleagues, hospitalists are one of the pillars of the inpatient response to COVID. More than 3.2 million COVID-19 hospitalizations have occurred, according to the Centers for Disease Control and Prevention, with numerous state dashboards showing three-quarters of those are cared for on general medical wards, the domain of hospitalists (for example, see my own state of Maryland’s COVID-19 dashboard: https://coronavirus.maryland.gov).
We’ve always had “two patients” – the patient in the bed and the health care system. Many hospitalists have helped their institutions by building COVID care teams, COVID wards, or in the case of Dr. Mindy Kantsiper, building an entire COVID field hospital in a convention center. Without hospitalists, both patients and the system that serves them would have fared much worse in this pandemic. Hospitalists are vital to patients and the health care system. The end. Period. End of story.
What I wish went better: Getting credit
As a profession, we need to be more deliberate about getting credit for the fantastic work we have done to care for COVID-19 patients, as well as inpatients in general. SHM can and must focus more on how to highlight the great work hospitalists have done and will continue to do. A greater understanding by the health care industry – as well as the general public – regarding the important role we play for patient care will help add autonomy in our profession, which in turn adds to resilience during these challenging times.
SHM
What went well: Membership grew
This is the one thing that we at SHM – and I personally – are most proud of. SHM is a membership society; it is the single most important metric for me personally. If physicians aren’t joining, then we are not meeting our core mission to provide value to hospitalists. My sense is the services SHM provides to hospitalists continue to be of value – even during these strenuous times of the pandemic when we had to be physically distant.
Whether it’s our Government Relations Department advocating for hospitalists in Washington, or the Journal of Hospital Medicine, or this very magazine, The Hospitalist, or SHM’s numerous educational offerings, chapter events, and SHM national meetings (Converge, Pediatric Hospital Medicine, Leadership Academies, Academic Hospitalist Academy, and more), SHM continues to provide hospitalists with vital tools to help you in your career.
This is also very much a two-way street. If you are reading this, know that without you, our members, our success would not be possible. Your passion and partnership drive us to innovate to meet your needs and those of the patients you serve every day. Thank you for your continued support and inspiration.
What could have gone better: Seeing more of you, in person
This is a tough one for me. Everything I worried about going wrong for SHM in 2021 never materialized. A year ago, my fears for SHM were that membership would shrink, finances would dry up, and the SHM staff would leave (by furlough or by choice). Thankfully, membership grew, our finances are in very good shape for any year, let alone a pandemic year, and the staff have remained at SHM and are engaged and dedicated! SHM even received a “Best Place to Work” award from the Philadelphia Business Journal.
Maybe the one regret I have is that we could not do more in-person events. But even there, I think we did better than most. We had some chapter meetings in person, and the October 2021 Leadership Academy hosted 110 hospitalist leaders, in person, at Amelia Island, Fla. That Leadership Academy went off without a hitch, and the early reviews are superb. I am very optimistic about 2022 in-person events!
Looking forward: 2022 and beyond
I have no illusions that 2022 is going to be easy. I know that the pandemic will not be gone (even though cases are falling nationwide as of this writing), that our nation will struggle with how to deal with polarization, and the workplace will continue to be redefined. Yet, I can’t help but be optimistic.
The pandemic will end eventually; all pandemics do. My hope is that young leaders will step forward to help our nation work through the divisive challenges, and some of those leaders will even be hospitalists! I also know that our profession is more vital than ever, for both patients and the health care system. We’re even getting ready to celebrate SHM’s 25th anniversary, and we can’t wait to revisit our humble beginnings while looking at the bright future of our society and our field.
I am working on my 2022 “New Year” goals, but you can be pretty sure they will revolve around making the world a better place, investing in people, and being ethical and transparent.
Dr. Howell is the CEO of the Society of Hospital Medicine.
This month marks the end of my first full calendar year as SHM CEO. Over the years, I have made it a habit to take time to reflect during the month of December, assessing the previous year by reviewing what went well and what could have gone better, and how I can grow and change to meet the needs of future challenges. This reflection sets the stage for my personal and professional “New Year” goals.
This year, 2021, is certainly a year deserving of reflection, and I believe 2022 (and beyond) will need ambitious goals made by dedicated leaders, hospitalists included. Here are my thoughts on what went well in 2021 and what I wish went better – from our greater society to our specialty, to SHM.
Society (as in the larger society)
What went well: Vaccines
There is a lot to be impressed with in 2021, and for me, at the top of that list are the COVID-19 vaccines. I realize the research for mRNA vaccines started more than 20 years ago, and the most successful mRNA vaccine companies have been around for more than a decade, but to roll out a COVID-19 vaccine in less than a year is still just incredible. To take a disease with a 2% mortality rate for someone like myself and effectively reduce that to near zero is something historians will be writing about for years to come.
What I wish went better: Open dialogue
I can’t remember when we stopped listening to each other, and by that, I mean listening to those who do not think exactly like ourselves. As a kid, I was taught to be careful about discussing topics at social events that could go sideways. That usually involved politics, money, or strong beliefs, but wow – now, that list is much longer. Talking about the weather used to be safe, but not anymore. If I were to show pictures of the recent flooding in Annapolis? There would almost certainly be a debate about climate change. At least we can agree on Ted Lasso as a safe topic.
Our specialty
What went well: Hospitalists are vital
There are many, many professions that deserve “hero” status for their part in taming this pandemic: nurses, doctors, emergency medical services, physical therapists, physician assistants, nurse practitioners, administrators, and more. But in the doctor category, hospitalists are at the top. Along with our emergency department and intensivist colleagues, hospitalists are one of the pillars of the inpatient response to COVID. More than 3.2 million COVID-19 hospitalizations have occurred, according to the Centers for Disease Control and Prevention, with numerous state dashboards showing three-quarters of those are cared for on general medical wards, the domain of hospitalists (for example, see my own state of Maryland’s COVID-19 dashboard: https://coronavirus.maryland.gov).
We’ve always had “two patients” – the patient in the bed and the health care system. Many hospitalists have helped their institutions by building COVID care teams, COVID wards, or in the case of Dr. Mindy Kantsiper, building an entire COVID field hospital in a convention center. Without hospitalists, both patients and the system that serves them would have fared much worse in this pandemic. Hospitalists are vital to patients and the health care system. The end. Period. End of story.
What I wish went better: Getting credit
As a profession, we need to be more deliberate about getting credit for the fantastic work we have done to care for COVID-19 patients, as well as inpatients in general. SHM can and must focus more on how to highlight the great work hospitalists have done and will continue to do. A greater understanding by the health care industry – as well as the general public – regarding the important role we play for patient care will help add autonomy in our profession, which in turn adds to resilience during these challenging times.
SHM
What went well: Membership grew
This is the one thing that we at SHM – and I personally – are most proud of. SHM is a membership society; it is the single most important metric for me personally. If physicians aren’t joining, then we are not meeting our core mission to provide value to hospitalists. My sense is the services SHM provides to hospitalists continue to be of value – even during these strenuous times of the pandemic when we had to be physically distant.
Whether it’s our Government Relations Department advocating for hospitalists in Washington, or the Journal of Hospital Medicine, or this very magazine, The Hospitalist, or SHM’s numerous educational offerings, chapter events, and SHM national meetings (Converge, Pediatric Hospital Medicine, Leadership Academies, Academic Hospitalist Academy, and more), SHM continues to provide hospitalists with vital tools to help you in your career.
This is also very much a two-way street. If you are reading this, know that without you, our members, our success would not be possible. Your passion and partnership drive us to innovate to meet your needs and those of the patients you serve every day. Thank you for your continued support and inspiration.
What could have gone better: Seeing more of you, in person
This is a tough one for me. Everything I worried about going wrong for SHM in 2021 never materialized. A year ago, my fears for SHM were that membership would shrink, finances would dry up, and the SHM staff would leave (by furlough or by choice). Thankfully, membership grew, our finances are in very good shape for any year, let alone a pandemic year, and the staff have remained at SHM and are engaged and dedicated! SHM even received a “Best Place to Work” award from the Philadelphia Business Journal.
Maybe the one regret I have is that we could not do more in-person events. But even there, I think we did better than most. We had some chapter meetings in person, and the October 2021 Leadership Academy hosted 110 hospitalist leaders, in person, at Amelia Island, Fla. That Leadership Academy went off without a hitch, and the early reviews are superb. I am very optimistic about 2022 in-person events!
Looking forward: 2022 and beyond
I have no illusions that 2022 is going to be easy. I know that the pandemic will not be gone (even though cases are falling nationwide as of this writing), that our nation will struggle with how to deal with polarization, and the workplace will continue to be redefined. Yet, I can’t help but be optimistic.
The pandemic will end eventually; all pandemics do. My hope is that young leaders will step forward to help our nation work through the divisive challenges, and some of those leaders will even be hospitalists! I also know that our profession is more vital than ever, for both patients and the health care system. We’re even getting ready to celebrate SHM’s 25th anniversary, and we can’t wait to revisit our humble beginnings while looking at the bright future of our society and our field.
I am working on my 2022 “New Year” goals, but you can be pretty sure they will revolve around making the world a better place, investing in people, and being ethical and transparent.
Dr. Howell is the CEO of the Society of Hospital Medicine.
Anticoagulant choice in antiphospholipid syndrome–associated thrombosis
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Chronically interrupted: The importance of communication with patient and family during the COVID-19 pandemic
Case narrative
A 35-year-old woman has worsening alcoholic cirrhosis and repeated admissions for ascites, hepato-renal syndrome, and alcoholic hepatitis. Upon recognition of her grave prognosis, we proceeded with a shared-management approach involving medicine, gastroenterology, social work, chaplaincy, and palliative care. When the team spoke with the patient’s health care proxy (HCP), family, and friends for collateral information and involvement in goals of care conversation, we realized that none were aware of her months-long decline and poor prognosis for recovery to hospital discharge.
Although several factors contributed to the disconnect between the patient and her support system, the obstacles were greatly exacerbated by profound changes in hospital protocol because of the COVID-19 pandemic. Physicians feel underprepared and challenged by prognostication and discussion of end of life during normal times; we believe COVID-19 has limited this essential physician role and led to tragic delays in effective communication and end of life planning.
Closing the loop
For patients with complex medical issues or those reaching end of life, effective communication within the health care system is critical. While inpatient teams often drive the plan, they care for their patients during a snapshot in time; contrarily, primary care providers and specialists often have established longitudinal relationships with their patients. Ergo, clinicians should communicate directly, and ideally with both patients and families, to achieve patient-centered and goal-concordant care.
For medically complex patients, PCPs tend to prefer verbal hand-offs. Timely and reliable communication between inpatient and outpatient providers has also been shown to prevent medical adverse events.1 Despite this, direct communication occurs infrequently.2 Given that hospitalists serve as primary inpatient providers for most general admissions, it is their responsibility to communicate with outpatient providers.
A multidisciplinary team redesigned the process by which PCPs were contacted following patient discharge. The transmission of information should ideally occur prior to discharge.3 Deficits in communication are extremely common and may negatively impact patient care, patient satisfaction, and patient safety.
Changes during the COVID-19 era
During the pandemic, patients have only one visitor per day, restricted visiting hours, and limited interactions with clinicians per implemented policies. Along with the increased burdens from personal protective equipment, remote hospital providers (social workers, case managers), and increased bureaucratic duties, COVID-19 has elucidated limitations in medical capacity and revealed the difficulties that clinicians face in communicating with patients and families, especially about serious illness.
Tasks include facilitating virtual goodbyes between dying patients and families, conducting family meetings via teleconference, and discussing patient care with specialists through virtual technologies.4 While these tasks are arguably more important during a global disaster, COVID-19 paradoxically restricts physical presence and severely hinders communication.5 Clinicians should continue to utilize core skills like building rapport, assessing patient/family perspectives and agenda, and using empathy.6 Patients tend to more frequently value functional outcomes while clinicians tend to default to treatment modalities.7 Additionally, goals of care and end of life discussions are associated with improved quality of life, fewer aggressive medical interventions near death, and even increased survival.
Given the limited resources and difficulties in communication during the pandemic, clinicians should place greater emphasis on values-based shared decision-making. Internet-based solutions are essential and widely used, and videoconferencing has been initiated at the institutional scale at many hospitals. Many clinicians with little experience are broadly implementing these technologies.7 Despite these technological innovations, issues still arise in how to communicate effectively in the hospital setting, and we must acknowledge that strategies require devices, Internet access, and technological literacy, highlighting disparities in access to quality health care.6 Conversations during the pandemic will require listening, empathy, responsive action, and the acknowledgment of the social determinants of health.7
Improving communication and transition of care
Multiple steps will be warranted to implement the safe transition process and improve communication. High-quality patient care encompasses careful review of medications, communication between inpatient and outpatient providers, and close follow-up at discharge. These steps serve to increase our reliance on patient compliance and the exchange of information about global progression of disease.
The quantitative and qualitative steps of transition of care should overcome disconnect between teams, specifically deficit areas regarding postdischarge communication, monitoring, and understanding of prognosis around the relevance to this era of COVID-19.
Dr. Haddad is a resident physician in the psychiatry residency program at Brigham and Women’s Hospital, Boston. Dr. Halporn is clinic director, Division of Adult Palliative Care, in the department of psychosocial oncology and palliative care, Dana-Farber Cancer Institute and Brigham and Women’s Hospital. Dr. Barkoudah is associate director of the Hospital Medicine Unit at Brigham and Women’s Hospital.
References
1. Goldman L et al. Passing the clinical baton: 6 principles to guide the hospitalist. Am J Med. 2001;111(9B):36S-39S. doi: 10.1016/s0002-9343(01)00968-8.
2. Kripalani S et al. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007 Feb 28;297(8):831-41. doi: 10.1001/jama.297.8.831.
3. Scotten M et al. Minding the gap: Interprofessional communication during inpatient and post discharge chasm care. Patient Educ Couns. 2015 Jul;98(7):895-900. doi: 10.1016/j.pec.2015.03.009.
4. Back A et al. Communication skills in the age of COVID-19. Ann Intern Med. 2020 Jun 2;172(11):759-60. doi: 10.7326/M20-1376.
5. Hart JL et al. Family-centered care during the COVID-19 era. J Pain Symptom Manage. 2020 Aug;60(2):e93-7. doi: 10.1016/j.jpainsymman.2020.04.017.
6. Rubinelli S et al. Implications of the current COVID-19 pandemic for communication in healthcare. Patient Educ Couns. 2020 Jun;103(6):1067-9. doi: 10.1016/j.pec.2020.04.021.
7. Simpson N et al. Don’t forget shared decision-making in the COVID-19 crisis. Intern Med J. 2020 Jun;50(6):761-3. doi: 10.1111/imj.14862.
Case narrative
A 35-year-old woman has worsening alcoholic cirrhosis and repeated admissions for ascites, hepato-renal syndrome, and alcoholic hepatitis. Upon recognition of her grave prognosis, we proceeded with a shared-management approach involving medicine, gastroenterology, social work, chaplaincy, and palliative care. When the team spoke with the patient’s health care proxy (HCP), family, and friends for collateral information and involvement in goals of care conversation, we realized that none were aware of her months-long decline and poor prognosis for recovery to hospital discharge.
Although several factors contributed to the disconnect between the patient and her support system, the obstacles were greatly exacerbated by profound changes in hospital protocol because of the COVID-19 pandemic. Physicians feel underprepared and challenged by prognostication and discussion of end of life during normal times; we believe COVID-19 has limited this essential physician role and led to tragic delays in effective communication and end of life planning.
Closing the loop
For patients with complex medical issues or those reaching end of life, effective communication within the health care system is critical. While inpatient teams often drive the plan, they care for their patients during a snapshot in time; contrarily, primary care providers and specialists often have established longitudinal relationships with their patients. Ergo, clinicians should communicate directly, and ideally with both patients and families, to achieve patient-centered and goal-concordant care.
For medically complex patients, PCPs tend to prefer verbal hand-offs. Timely and reliable communication between inpatient and outpatient providers has also been shown to prevent medical adverse events.1 Despite this, direct communication occurs infrequently.2 Given that hospitalists serve as primary inpatient providers for most general admissions, it is their responsibility to communicate with outpatient providers.
A multidisciplinary team redesigned the process by which PCPs were contacted following patient discharge. The transmission of information should ideally occur prior to discharge.3 Deficits in communication are extremely common and may negatively impact patient care, patient satisfaction, and patient safety.
Changes during the COVID-19 era
During the pandemic, patients have only one visitor per day, restricted visiting hours, and limited interactions with clinicians per implemented policies. Along with the increased burdens from personal protective equipment, remote hospital providers (social workers, case managers), and increased bureaucratic duties, COVID-19 has elucidated limitations in medical capacity and revealed the difficulties that clinicians face in communicating with patients and families, especially about serious illness.
Tasks include facilitating virtual goodbyes between dying patients and families, conducting family meetings via teleconference, and discussing patient care with specialists through virtual technologies.4 While these tasks are arguably more important during a global disaster, COVID-19 paradoxically restricts physical presence and severely hinders communication.5 Clinicians should continue to utilize core skills like building rapport, assessing patient/family perspectives and agenda, and using empathy.6 Patients tend to more frequently value functional outcomes while clinicians tend to default to treatment modalities.7 Additionally, goals of care and end of life discussions are associated with improved quality of life, fewer aggressive medical interventions near death, and even increased survival.
Given the limited resources and difficulties in communication during the pandemic, clinicians should place greater emphasis on values-based shared decision-making. Internet-based solutions are essential and widely used, and videoconferencing has been initiated at the institutional scale at many hospitals. Many clinicians with little experience are broadly implementing these technologies.7 Despite these technological innovations, issues still arise in how to communicate effectively in the hospital setting, and we must acknowledge that strategies require devices, Internet access, and technological literacy, highlighting disparities in access to quality health care.6 Conversations during the pandemic will require listening, empathy, responsive action, and the acknowledgment of the social determinants of health.7
Improving communication and transition of care
Multiple steps will be warranted to implement the safe transition process and improve communication. High-quality patient care encompasses careful review of medications, communication between inpatient and outpatient providers, and close follow-up at discharge. These steps serve to increase our reliance on patient compliance and the exchange of information about global progression of disease.
The quantitative and qualitative steps of transition of care should overcome disconnect between teams, specifically deficit areas regarding postdischarge communication, monitoring, and understanding of prognosis around the relevance to this era of COVID-19.
Dr. Haddad is a resident physician in the psychiatry residency program at Brigham and Women’s Hospital, Boston. Dr. Halporn is clinic director, Division of Adult Palliative Care, in the department of psychosocial oncology and palliative care, Dana-Farber Cancer Institute and Brigham and Women’s Hospital. Dr. Barkoudah is associate director of the Hospital Medicine Unit at Brigham and Women’s Hospital.
References
1. Goldman L et al. Passing the clinical baton: 6 principles to guide the hospitalist. Am J Med. 2001;111(9B):36S-39S. doi: 10.1016/s0002-9343(01)00968-8.
2. Kripalani S et al. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007 Feb 28;297(8):831-41. doi: 10.1001/jama.297.8.831.
3. Scotten M et al. Minding the gap: Interprofessional communication during inpatient and post discharge chasm care. Patient Educ Couns. 2015 Jul;98(7):895-900. doi: 10.1016/j.pec.2015.03.009.
4. Back A et al. Communication skills in the age of COVID-19. Ann Intern Med. 2020 Jun 2;172(11):759-60. doi: 10.7326/M20-1376.
5. Hart JL et al. Family-centered care during the COVID-19 era. J Pain Symptom Manage. 2020 Aug;60(2):e93-7. doi: 10.1016/j.jpainsymman.2020.04.017.
6. Rubinelli S et al. Implications of the current COVID-19 pandemic for communication in healthcare. Patient Educ Couns. 2020 Jun;103(6):1067-9. doi: 10.1016/j.pec.2020.04.021.
7. Simpson N et al. Don’t forget shared decision-making in the COVID-19 crisis. Intern Med J. 2020 Jun;50(6):761-3. doi: 10.1111/imj.14862.
Case narrative
A 35-year-old woman has worsening alcoholic cirrhosis and repeated admissions for ascites, hepato-renal syndrome, and alcoholic hepatitis. Upon recognition of her grave prognosis, we proceeded with a shared-management approach involving medicine, gastroenterology, social work, chaplaincy, and palliative care. When the team spoke with the patient’s health care proxy (HCP), family, and friends for collateral information and involvement in goals of care conversation, we realized that none were aware of her months-long decline and poor prognosis for recovery to hospital discharge.
Although several factors contributed to the disconnect between the patient and her support system, the obstacles were greatly exacerbated by profound changes in hospital protocol because of the COVID-19 pandemic. Physicians feel underprepared and challenged by prognostication and discussion of end of life during normal times; we believe COVID-19 has limited this essential physician role and led to tragic delays in effective communication and end of life planning.
Closing the loop
For patients with complex medical issues or those reaching end of life, effective communication within the health care system is critical. While inpatient teams often drive the plan, they care for their patients during a snapshot in time; contrarily, primary care providers and specialists often have established longitudinal relationships with their patients. Ergo, clinicians should communicate directly, and ideally with both patients and families, to achieve patient-centered and goal-concordant care.
For medically complex patients, PCPs tend to prefer verbal hand-offs. Timely and reliable communication between inpatient and outpatient providers has also been shown to prevent medical adverse events.1 Despite this, direct communication occurs infrequently.2 Given that hospitalists serve as primary inpatient providers for most general admissions, it is their responsibility to communicate with outpatient providers.
A multidisciplinary team redesigned the process by which PCPs were contacted following patient discharge. The transmission of information should ideally occur prior to discharge.3 Deficits in communication are extremely common and may negatively impact patient care, patient satisfaction, and patient safety.
Changes during the COVID-19 era
During the pandemic, patients have only one visitor per day, restricted visiting hours, and limited interactions with clinicians per implemented policies. Along with the increased burdens from personal protective equipment, remote hospital providers (social workers, case managers), and increased bureaucratic duties, COVID-19 has elucidated limitations in medical capacity and revealed the difficulties that clinicians face in communicating with patients and families, especially about serious illness.
Tasks include facilitating virtual goodbyes between dying patients and families, conducting family meetings via teleconference, and discussing patient care with specialists through virtual technologies.4 While these tasks are arguably more important during a global disaster, COVID-19 paradoxically restricts physical presence and severely hinders communication.5 Clinicians should continue to utilize core skills like building rapport, assessing patient/family perspectives and agenda, and using empathy.6 Patients tend to more frequently value functional outcomes while clinicians tend to default to treatment modalities.7 Additionally, goals of care and end of life discussions are associated with improved quality of life, fewer aggressive medical interventions near death, and even increased survival.
Given the limited resources and difficulties in communication during the pandemic, clinicians should place greater emphasis on values-based shared decision-making. Internet-based solutions are essential and widely used, and videoconferencing has been initiated at the institutional scale at many hospitals. Many clinicians with little experience are broadly implementing these technologies.7 Despite these technological innovations, issues still arise in how to communicate effectively in the hospital setting, and we must acknowledge that strategies require devices, Internet access, and technological literacy, highlighting disparities in access to quality health care.6 Conversations during the pandemic will require listening, empathy, responsive action, and the acknowledgment of the social determinants of health.7
Improving communication and transition of care
Multiple steps will be warranted to implement the safe transition process and improve communication. High-quality patient care encompasses careful review of medications, communication between inpatient and outpatient providers, and close follow-up at discharge. These steps serve to increase our reliance on patient compliance and the exchange of information about global progression of disease.
The quantitative and qualitative steps of transition of care should overcome disconnect between teams, specifically deficit areas regarding postdischarge communication, monitoring, and understanding of prognosis around the relevance to this era of COVID-19.
Dr. Haddad is a resident physician in the psychiatry residency program at Brigham and Women’s Hospital, Boston. Dr. Halporn is clinic director, Division of Adult Palliative Care, in the department of psychosocial oncology and palliative care, Dana-Farber Cancer Institute and Brigham and Women’s Hospital. Dr. Barkoudah is associate director of the Hospital Medicine Unit at Brigham and Women’s Hospital.
References
1. Goldman L et al. Passing the clinical baton: 6 principles to guide the hospitalist. Am J Med. 2001;111(9B):36S-39S. doi: 10.1016/s0002-9343(01)00968-8.
2. Kripalani S et al. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007 Feb 28;297(8):831-41. doi: 10.1001/jama.297.8.831.
3. Scotten M et al. Minding the gap: Interprofessional communication during inpatient and post discharge chasm care. Patient Educ Couns. 2015 Jul;98(7):895-900. doi: 10.1016/j.pec.2015.03.009.
4. Back A et al. Communication skills in the age of COVID-19. Ann Intern Med. 2020 Jun 2;172(11):759-60. doi: 10.7326/M20-1376.
5. Hart JL et al. Family-centered care during the COVID-19 era. J Pain Symptom Manage. 2020 Aug;60(2):e93-7. doi: 10.1016/j.jpainsymman.2020.04.017.
6. Rubinelli S et al. Implications of the current COVID-19 pandemic for communication in healthcare. Patient Educ Couns. 2020 Jun;103(6):1067-9. doi: 10.1016/j.pec.2020.04.021.
7. Simpson N et al. Don’t forget shared decision-making in the COVID-19 crisis. Intern Med J. 2020 Jun;50(6):761-3. doi: 10.1111/imj.14862.
Apixaban a reasonable alternative to warfarin in patients with severe renal impairment
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
The top pediatric hospital medicine articles of 2020
The year 2020 was unlike any in recent history, particularly for those working in health care. With the onset of the SARs-CoV-2 pandemic, many physicians were met with increasing clinical demands, and hospitalists served an instrumental role in providing medical care as the world faced an unprecedented need for health care resources.
In addition, 2020 was a year in which many of us reflected on inequities both inside and outside of medicine. Many in health care witnessed the disproportionate burden that the SARs-CoV-2 pandemic placed on communities of color and inequities pertaining to vaccine distribution.
In spite of the challenges of 2020, the field of pediatric hospital medicine (PHM) has continued to grow and evolve, with an incredible amount of new literature published in 2020.
In this article, we identify the top 10 articles published in 2020, 5 of which are summarized below. These articles were presented at the Pediatric Update at SHM Converge 2021.
The top 5 articles
Association between parent comfort with English and adverse events among hospitalized children
Khan A et al. JAMA Pediatrics. December 2020.1
Background: Hospitalized children experience similar rates of medical errors compared to adult patients, but higher rates in areas that could cause harm.1 A major contributor to medical errors is communication failure, which language barriers frequently contribute to. Single-center data suggest that pediatric patients of families with limited comfort with English experience increased adverse events,2 but multicenter data are lacking.
Findings: This prospective cohort study observed adverse event rates among 2,148 patients from seven teaching hospitals from December 2014 to January 2017. Survey data revealed 147 of 1,666 (9%) parents of patient families expressed limited comfort in English, and Spanish was the predominant language in this group (71%). There were 217 adverse events reported, 142 (65%) of which were deemed preventable by study personnel. Nearly twice as many children of parents with limited comfort with English experienced an adverse event when compared to their English-speaking counterparts (26 of 147 [17.7%] vs. 146 of 1,519 [9.6%]; adjusted odds ratio, 2.1; 95% confidence interval, 1.2-3.7). Interpreter use was not measured.
Impact to practice: Children of parents with limited comfort with English are nearly twice as likely to experience adverse events when hospitalized. Hospitals should reflect on current practice and make efforts to improve their ability to identify and communicate with this vulnerable cohort.
Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children
Yeung F et al. Hospital Pediatrics. December 2020.3
Background: Peripheral intravenous catheter (PIV) insertion is performed on most hospitalized children. Unfortunately, PIVs frequently fail and need to be replaced. There is a widespread perception that infusing a crystalloid solution at a low rate through a PIV, a strategy known as “to keep vein open” (TKO) prolongs the patency of PIVs, however there is a lack of evidence to support this practice.4Findings: In this prospective, time-allocated study, 172 children were allocated to either a TKO strategy or a saline-lock strategy with a primary outcome of duration of PIV patency.3 Secondary outcomes included PIV–related complication rates and patient and caregiver satisfaction. The mean duration of PIV patency was 41.68 hours in the TKO group and 44.05 hours in the saline-lock group, which did not meet the prespecified definition of a clinically significant difference. There was no significant difference in prevalence of PIV-associated complications and patient satisfaction was similar between the two groups.
Impact to practice: Running fluid “to keep vein open” does not increase the duration of PIV patency compared to intermittent saline locks. Given that a TKO strategy limits a patient’s mobility, this low-value practice can be discontinued without increasing the risk of PIV failure.
Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol
Coon ER et al. Journal of Hospital Medicine. June 2020.5
Background: High Flow Nasal Cannula (HFNC) has been widely adopted for escalation of respiratory support in patients with bronchiolitis; however, its use is dictated by highly variant local protocols.6 Small-scale randomized control trials and systematic reviews show that early HFNC initiation in mild to moderate disease does not change patient outcomes.7Findings: In this retrospective cohort study of ward-based HFNC, the authors used the Pediatric Health Information System database to identify 12 hospitals that had adopted ward-based HFNC protocols. The study used an interrupted time series analysis to compare outcomes for patients ages 3-24 months hospitalized with bronchiolitis (n = 32,809) in the three seasons before and after protocol adoption. Ward-based HFNC adoption paradoxically increased ICU admission (absolute increase 3.1%, 95% confidence interval, 2.8-3.4%) and ICU length of stay (absolute difference 9.1 days/100 patients, 95% CI, 5.1-13.2). Total length of stay and rates of mechanical ventilation were similar between groups.5Impact to practice: Ward-based HFNC protocols are associated with increased ICU utilization. As bronchiolitis is the leading diagnosis in pediatrics, pediatric hospitals can lead ward-based quality efforts to decrease HFNC overutilization focused on decreased initiation or deimplementation.
Lower versus traditional treatment threshold for neonatal hypoglycemia
Van Kempen AAMW et al. New England Journal of Medicine. February 2020.8
Background: Hypoglycemia is the most common metabolic abnormality in newborns, and up to 30% of newborns are routinely monitored for hypoglycemia. There is no consensus regarding the appropriate threshold at which hypoglycemia should be treated in order to prevent neurologic injury. Prior studies of neonatal hypoglycemia have largely been observation and have yielded conflicting results.8Findings: In this multicenter, randomized, noninferiority trial, 689 infants born at 35 weeks gestational age or later with risk factors for hypoglycemia and a measured blood glucose of 36-46 mg/dL were randomized to either a lower glucose treatment threshold (36 mg/dL) or traditional glucose treatment threshold (47 mg/dL). The primary outcome was psychomotor development at 18 months, assessed via the Bayley Scale of Infant and Toddler Development, third edition. There was no significant difference in cognitive or motor scores at 18 months. The lower treatment threshold group had a higher frequency of severe hypoglycemia (< 36 mg/dL) and were more likely to have four or more episodes of hypoglycemia. The traditional treatment threshold group had more supplemental feeding and more IV glucose administration. Length of stay for the mother and baby did not differ between groups.8
Impact to practice: This prospective, randomized study suggests that reducing the treatment threshold for neonatal hypoglycemia did not affect neurodevelopmental at 18 months of age. In contrast, a recent meta-analysis by Shah et al. suggested that neonatal hypoglycemia was not associated with adverse neurodevelopmental outcomes in early childhood; however, differences in rates of neurodevelopmental impairment, low literacy, and low numeracy were detectable by age five.9
Factors associated with family experience in pediatric inpatient care
Feng JY et al. Pediatrics. March 2020 Mar.10
Background: Positive patient experience is associated with better health care outcomes and reduced health care use.11 Consequently, patient experience surveys have played a larger role in public reporting, financial risk sharing arrangements, and pay for performance programs. While adult studies have examined the importance of specific care dimensions for patient experience, data are lacking for inpatient pediatric populations.
Findings: A retrospective study collected Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys from 17,727 patients in 69 hospitals within the United States over a 14-month period.10 Of the 10 care dimensions analyzed, child comfort (aOR 1.50; 95% CI, 1.41-1.60) and nurse-parent communication (aOR 1.50; 95% CI, 1.42-1.58) were most strongly associated with a family’s willingness to recommend a hospital. Additional associated indices included preparing to leave the hospital (aOR 1.34; 95% CI, 1.27-1.41), doctor-parent communication (aOR 1.28; 95% CI, 1.21–1.35), and keeping parents informed (aOR 1.25; 95% CI, 1.18-1.33). Privacy and quietness, which are associated with positive patient experience in adult studies, were not significantly associated with willingness to recommend in this cohort.
Impact to practice: Hospitals seeking to improve patient experience will benefit most by focusing on improving patient comfort and nurse-parent communication. Factors that increase adult patient satisfaction may not be as important to the pediatric population and their families.
The other five articles that comprised the top 10 are listed below:
Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis
Coon ER et al. JAMA Pediatrics. September 2020.12
Clinical prediction rule for distinguishing bacterial from aseptic meningitis
Mintegi S et al. Pediatrics. September 2020.13
The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC Ullman AJ et al. Pediatrics. June 2020.14
A structured neonatal parenting elective: An approach for parenting leave during residency
Cree-Green M et al. Academic Pediatrics. Aug 2020.15
The KidzMed project: Teaching children to swallow tablet medication
Tse Y et al. Archives of Disease in Childhood. November 2020.16
Dr. Steed is an internal medicine and pediatrics hospitalist at Northwestern Memorial Hospital and Ann and Robert H. Lurie’s Children’s Hospital of Chicago. Dr. Fisher is a current fellow in hospice and palliative medicine and a clinical assistant professor at Michigan State University. Dr. Money is an assistant professor of pediatrics at the University of Utah and a fellowship-trained pediatric hospitalist at Utah Valley Hospital and Primary Children’s Hospital.
References
1. Khan A et al. Association between parent comfort with english and adverse events among hospitalized children. JAMA Pediatr. 2020 Dec 1;174(12):e203215. doi: 10.1001/jamapediatrics.2020.3215.
2. Wasserman M et al. Identifying and preventing medical errors in patients with limited English proficiency: Key findings and tools for the field. J Healthc Qual. May-Jun 2014;36(3):5-16. doi: 10.1111/jhq.12065.
3. Yeung F et al. Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children. Hosp Pediatr. 2020 Dec;10(12):1038-43. doi: 10.1542/hpeds.2020-0137.
4. Mok E et al. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract. 2007 Feb;13(1):33-45. doi: 10.1111/j.1440-172X.2006.00607.x.
5. Coon ER et al. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020 Jun;15(6):325-30. doi: 10.12788/jhm.3417.
6. Kalburgi S and Halley T. High-flow nasal cannula use outside of the ICU setting. Pediatrics. 2020;146(5):e20194083. doi: 10.1542/peds.2019-4083.
7. Leyenaar JK and Ralston SL. Widespread adoption of low-value therapy: The case of bronchiolitis and high-flow oxygen. Pediatrics. 2020 Nov;146(5):e2020021188. doi: 10.1542/peds.2020-021188.
8. Van Kempen AAMW et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020 Feb 6;382(6):534-44. doi: 10.1056/NEJMoa1905593.
9. Shah R et al. Neonatal glycaemia and neurodevelopmental outcomes: A systematic review and meta-analysis. Neonatology. 2019;115(2):116-26. doi: 10.1159/000492859.
10. Feng JY et al. Factors associated with family experience in pediatric inpatient care. Pediatrics. 2020 Mar;145(3):e20191264. doi: 10.1542/peds.2019-1264.
11. Anhang Price R et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014 Oct;71(5):522-54. doi: 10.1177/1077558714541480.
12. Coon ER et al. Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis: The Bronchiolitis Follow-up Intervention Trial (BeneFIT) randomized clinical trial. JAMA Pediatr. 2020 Sep 1;174(9):e201937. doi: 10.1001/jamapediatrics.2020.1937.
13. Mintegi S et al. Clinical prediction rule for distinguishing bacterial from aseptic meningitis. Pediatrics. 2020 Sept;146(3): e20201126. doi: 10.1542/peds.2020-1126.
14. Ullman AJ et al. The Michigan Appropriateness Guide for Intravenous Catheters in pediatrics: miniMAGIC. Pediatrics. 2020 Jun;145(Suppl 3):S269-S284. doi: 10.1542/peds.2019-3474I.
15. Cree-Green M et al. A structured neonatal parenting elective: an approach for parenting leave during residency. Acad Pediatr. 2021 Jan-Feb;21(1):16-18. doi: 10.1016/j.acap.2020.02.008.
16. Tse Y et al. The KidzMed project: Teaching children to swallow tablet medication. Arch Dis Child. 2020 Nov;105(11):1105-7. doi: 10.1136/archdischild-2019-317512.
The year 2020 was unlike any in recent history, particularly for those working in health care. With the onset of the SARs-CoV-2 pandemic, many physicians were met with increasing clinical demands, and hospitalists served an instrumental role in providing medical care as the world faced an unprecedented need for health care resources.
In addition, 2020 was a year in which many of us reflected on inequities both inside and outside of medicine. Many in health care witnessed the disproportionate burden that the SARs-CoV-2 pandemic placed on communities of color and inequities pertaining to vaccine distribution.
In spite of the challenges of 2020, the field of pediatric hospital medicine (PHM) has continued to grow and evolve, with an incredible amount of new literature published in 2020.
In this article, we identify the top 10 articles published in 2020, 5 of which are summarized below. These articles were presented at the Pediatric Update at SHM Converge 2021.
The top 5 articles
Association between parent comfort with English and adverse events among hospitalized children
Khan A et al. JAMA Pediatrics. December 2020.1
Background: Hospitalized children experience similar rates of medical errors compared to adult patients, but higher rates in areas that could cause harm.1 A major contributor to medical errors is communication failure, which language barriers frequently contribute to. Single-center data suggest that pediatric patients of families with limited comfort with English experience increased adverse events,2 but multicenter data are lacking.
Findings: This prospective cohort study observed adverse event rates among 2,148 patients from seven teaching hospitals from December 2014 to January 2017. Survey data revealed 147 of 1,666 (9%) parents of patient families expressed limited comfort in English, and Spanish was the predominant language in this group (71%). There were 217 adverse events reported, 142 (65%) of which were deemed preventable by study personnel. Nearly twice as many children of parents with limited comfort with English experienced an adverse event when compared to their English-speaking counterparts (26 of 147 [17.7%] vs. 146 of 1,519 [9.6%]; adjusted odds ratio, 2.1; 95% confidence interval, 1.2-3.7). Interpreter use was not measured.
Impact to practice: Children of parents with limited comfort with English are nearly twice as likely to experience adverse events when hospitalized. Hospitals should reflect on current practice and make efforts to improve their ability to identify and communicate with this vulnerable cohort.
Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children
Yeung F et al. Hospital Pediatrics. December 2020.3
Background: Peripheral intravenous catheter (PIV) insertion is performed on most hospitalized children. Unfortunately, PIVs frequently fail and need to be replaced. There is a widespread perception that infusing a crystalloid solution at a low rate through a PIV, a strategy known as “to keep vein open” (TKO) prolongs the patency of PIVs, however there is a lack of evidence to support this practice.4Findings: In this prospective, time-allocated study, 172 children were allocated to either a TKO strategy or a saline-lock strategy with a primary outcome of duration of PIV patency.3 Secondary outcomes included PIV–related complication rates and patient and caregiver satisfaction. The mean duration of PIV patency was 41.68 hours in the TKO group and 44.05 hours in the saline-lock group, which did not meet the prespecified definition of a clinically significant difference. There was no significant difference in prevalence of PIV-associated complications and patient satisfaction was similar between the two groups.
Impact to practice: Running fluid “to keep vein open” does not increase the duration of PIV patency compared to intermittent saline locks. Given that a TKO strategy limits a patient’s mobility, this low-value practice can be discontinued without increasing the risk of PIV failure.
Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol
Coon ER et al. Journal of Hospital Medicine. June 2020.5
Background: High Flow Nasal Cannula (HFNC) has been widely adopted for escalation of respiratory support in patients with bronchiolitis; however, its use is dictated by highly variant local protocols.6 Small-scale randomized control trials and systematic reviews show that early HFNC initiation in mild to moderate disease does not change patient outcomes.7Findings: In this retrospective cohort study of ward-based HFNC, the authors used the Pediatric Health Information System database to identify 12 hospitals that had adopted ward-based HFNC protocols. The study used an interrupted time series analysis to compare outcomes for patients ages 3-24 months hospitalized with bronchiolitis (n = 32,809) in the three seasons before and after protocol adoption. Ward-based HFNC adoption paradoxically increased ICU admission (absolute increase 3.1%, 95% confidence interval, 2.8-3.4%) and ICU length of stay (absolute difference 9.1 days/100 patients, 95% CI, 5.1-13.2). Total length of stay and rates of mechanical ventilation were similar between groups.5Impact to practice: Ward-based HFNC protocols are associated with increased ICU utilization. As bronchiolitis is the leading diagnosis in pediatrics, pediatric hospitals can lead ward-based quality efforts to decrease HFNC overutilization focused on decreased initiation or deimplementation.
Lower versus traditional treatment threshold for neonatal hypoglycemia
Van Kempen AAMW et al. New England Journal of Medicine. February 2020.8
Background: Hypoglycemia is the most common metabolic abnormality in newborns, and up to 30% of newborns are routinely monitored for hypoglycemia. There is no consensus regarding the appropriate threshold at which hypoglycemia should be treated in order to prevent neurologic injury. Prior studies of neonatal hypoglycemia have largely been observation and have yielded conflicting results.8Findings: In this multicenter, randomized, noninferiority trial, 689 infants born at 35 weeks gestational age or later with risk factors for hypoglycemia and a measured blood glucose of 36-46 mg/dL were randomized to either a lower glucose treatment threshold (36 mg/dL) or traditional glucose treatment threshold (47 mg/dL). The primary outcome was psychomotor development at 18 months, assessed via the Bayley Scale of Infant and Toddler Development, third edition. There was no significant difference in cognitive or motor scores at 18 months. The lower treatment threshold group had a higher frequency of severe hypoglycemia (< 36 mg/dL) and were more likely to have four or more episodes of hypoglycemia. The traditional treatment threshold group had more supplemental feeding and more IV glucose administration. Length of stay for the mother and baby did not differ between groups.8
Impact to practice: This prospective, randomized study suggests that reducing the treatment threshold for neonatal hypoglycemia did not affect neurodevelopmental at 18 months of age. In contrast, a recent meta-analysis by Shah et al. suggested that neonatal hypoglycemia was not associated with adverse neurodevelopmental outcomes in early childhood; however, differences in rates of neurodevelopmental impairment, low literacy, and low numeracy were detectable by age five.9
Factors associated with family experience in pediatric inpatient care
Feng JY et al. Pediatrics. March 2020 Mar.10
Background: Positive patient experience is associated with better health care outcomes and reduced health care use.11 Consequently, patient experience surveys have played a larger role in public reporting, financial risk sharing arrangements, and pay for performance programs. While adult studies have examined the importance of specific care dimensions for patient experience, data are lacking for inpatient pediatric populations.
Findings: A retrospective study collected Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys from 17,727 patients in 69 hospitals within the United States over a 14-month period.10 Of the 10 care dimensions analyzed, child comfort (aOR 1.50; 95% CI, 1.41-1.60) and nurse-parent communication (aOR 1.50; 95% CI, 1.42-1.58) were most strongly associated with a family’s willingness to recommend a hospital. Additional associated indices included preparing to leave the hospital (aOR 1.34; 95% CI, 1.27-1.41), doctor-parent communication (aOR 1.28; 95% CI, 1.21–1.35), and keeping parents informed (aOR 1.25; 95% CI, 1.18-1.33). Privacy and quietness, which are associated with positive patient experience in adult studies, were not significantly associated with willingness to recommend in this cohort.
Impact to practice: Hospitals seeking to improve patient experience will benefit most by focusing on improving patient comfort and nurse-parent communication. Factors that increase adult patient satisfaction may not be as important to the pediatric population and their families.
The other five articles that comprised the top 10 are listed below:
Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis
Coon ER et al. JAMA Pediatrics. September 2020.12
Clinical prediction rule for distinguishing bacterial from aseptic meningitis
Mintegi S et al. Pediatrics. September 2020.13
The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC Ullman AJ et al. Pediatrics. June 2020.14
A structured neonatal parenting elective: An approach for parenting leave during residency
Cree-Green M et al. Academic Pediatrics. Aug 2020.15
The KidzMed project: Teaching children to swallow tablet medication
Tse Y et al. Archives of Disease in Childhood. November 2020.16
Dr. Steed is an internal medicine and pediatrics hospitalist at Northwestern Memorial Hospital and Ann and Robert H. Lurie’s Children’s Hospital of Chicago. Dr. Fisher is a current fellow in hospice and palliative medicine and a clinical assistant professor at Michigan State University. Dr. Money is an assistant professor of pediatrics at the University of Utah and a fellowship-trained pediatric hospitalist at Utah Valley Hospital and Primary Children’s Hospital.
References
1. Khan A et al. Association between parent comfort with english and adverse events among hospitalized children. JAMA Pediatr. 2020 Dec 1;174(12):e203215. doi: 10.1001/jamapediatrics.2020.3215.
2. Wasserman M et al. Identifying and preventing medical errors in patients with limited English proficiency: Key findings and tools for the field. J Healthc Qual. May-Jun 2014;36(3):5-16. doi: 10.1111/jhq.12065.
3. Yeung F et al. Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children. Hosp Pediatr. 2020 Dec;10(12):1038-43. doi: 10.1542/hpeds.2020-0137.
4. Mok E et al. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract. 2007 Feb;13(1):33-45. doi: 10.1111/j.1440-172X.2006.00607.x.
5. Coon ER et al. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020 Jun;15(6):325-30. doi: 10.12788/jhm.3417.
6. Kalburgi S and Halley T. High-flow nasal cannula use outside of the ICU setting. Pediatrics. 2020;146(5):e20194083. doi: 10.1542/peds.2019-4083.
7. Leyenaar JK and Ralston SL. Widespread adoption of low-value therapy: The case of bronchiolitis and high-flow oxygen. Pediatrics. 2020 Nov;146(5):e2020021188. doi: 10.1542/peds.2020-021188.
8. Van Kempen AAMW et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020 Feb 6;382(6):534-44. doi: 10.1056/NEJMoa1905593.
9. Shah R et al. Neonatal glycaemia and neurodevelopmental outcomes: A systematic review and meta-analysis. Neonatology. 2019;115(2):116-26. doi: 10.1159/000492859.
10. Feng JY et al. Factors associated with family experience in pediatric inpatient care. Pediatrics. 2020 Mar;145(3):e20191264. doi: 10.1542/peds.2019-1264.
11. Anhang Price R et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014 Oct;71(5):522-54. doi: 10.1177/1077558714541480.
12. Coon ER et al. Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis: The Bronchiolitis Follow-up Intervention Trial (BeneFIT) randomized clinical trial. JAMA Pediatr. 2020 Sep 1;174(9):e201937. doi: 10.1001/jamapediatrics.2020.1937.
13. Mintegi S et al. Clinical prediction rule for distinguishing bacterial from aseptic meningitis. Pediatrics. 2020 Sept;146(3): e20201126. doi: 10.1542/peds.2020-1126.
14. Ullman AJ et al. The Michigan Appropriateness Guide for Intravenous Catheters in pediatrics: miniMAGIC. Pediatrics. 2020 Jun;145(Suppl 3):S269-S284. doi: 10.1542/peds.2019-3474I.
15. Cree-Green M et al. A structured neonatal parenting elective: an approach for parenting leave during residency. Acad Pediatr. 2021 Jan-Feb;21(1):16-18. doi: 10.1016/j.acap.2020.02.008.
16. Tse Y et al. The KidzMed project: Teaching children to swallow tablet medication. Arch Dis Child. 2020 Nov;105(11):1105-7. doi: 10.1136/archdischild-2019-317512.
The year 2020 was unlike any in recent history, particularly for those working in health care. With the onset of the SARs-CoV-2 pandemic, many physicians were met with increasing clinical demands, and hospitalists served an instrumental role in providing medical care as the world faced an unprecedented need for health care resources.
In addition, 2020 was a year in which many of us reflected on inequities both inside and outside of medicine. Many in health care witnessed the disproportionate burden that the SARs-CoV-2 pandemic placed on communities of color and inequities pertaining to vaccine distribution.
In spite of the challenges of 2020, the field of pediatric hospital medicine (PHM) has continued to grow and evolve, with an incredible amount of new literature published in 2020.
In this article, we identify the top 10 articles published in 2020, 5 of which are summarized below. These articles were presented at the Pediatric Update at SHM Converge 2021.
The top 5 articles
Association between parent comfort with English and adverse events among hospitalized children
Khan A et al. JAMA Pediatrics. December 2020.1
Background: Hospitalized children experience similar rates of medical errors compared to adult patients, but higher rates in areas that could cause harm.1 A major contributor to medical errors is communication failure, which language barriers frequently contribute to. Single-center data suggest that pediatric patients of families with limited comfort with English experience increased adverse events,2 but multicenter data are lacking.
Findings: This prospective cohort study observed adverse event rates among 2,148 patients from seven teaching hospitals from December 2014 to January 2017. Survey data revealed 147 of 1,666 (9%) parents of patient families expressed limited comfort in English, and Spanish was the predominant language in this group (71%). There were 217 adverse events reported, 142 (65%) of which were deemed preventable by study personnel. Nearly twice as many children of parents with limited comfort with English experienced an adverse event when compared to their English-speaking counterparts (26 of 147 [17.7%] vs. 146 of 1,519 [9.6%]; adjusted odds ratio, 2.1; 95% confidence interval, 1.2-3.7). Interpreter use was not measured.
Impact to practice: Children of parents with limited comfort with English are nearly twice as likely to experience adverse events when hospitalized. Hospitals should reflect on current practice and make efforts to improve their ability to identify and communicate with this vulnerable cohort.
Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children
Yeung F et al. Hospital Pediatrics. December 2020.3
Background: Peripheral intravenous catheter (PIV) insertion is performed on most hospitalized children. Unfortunately, PIVs frequently fail and need to be replaced. There is a widespread perception that infusing a crystalloid solution at a low rate through a PIV, a strategy known as “to keep vein open” (TKO) prolongs the patency of PIVs, however there is a lack of evidence to support this practice.4Findings: In this prospective, time-allocated study, 172 children were allocated to either a TKO strategy or a saline-lock strategy with a primary outcome of duration of PIV patency.3 Secondary outcomes included PIV–related complication rates and patient and caregiver satisfaction. The mean duration of PIV patency was 41.68 hours in the TKO group and 44.05 hours in the saline-lock group, which did not meet the prespecified definition of a clinically significant difference. There was no significant difference in prevalence of PIV-associated complications and patient satisfaction was similar between the two groups.
Impact to practice: Running fluid “to keep vein open” does not increase the duration of PIV patency compared to intermittent saline locks. Given that a TKO strategy limits a patient’s mobility, this low-value practice can be discontinued without increasing the risk of PIV failure.
Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol
Coon ER et al. Journal of Hospital Medicine. June 2020.5
Background: High Flow Nasal Cannula (HFNC) has been widely adopted for escalation of respiratory support in patients with bronchiolitis; however, its use is dictated by highly variant local protocols.6 Small-scale randomized control trials and systematic reviews show that early HFNC initiation in mild to moderate disease does not change patient outcomes.7Findings: In this retrospective cohort study of ward-based HFNC, the authors used the Pediatric Health Information System database to identify 12 hospitals that had adopted ward-based HFNC protocols. The study used an interrupted time series analysis to compare outcomes for patients ages 3-24 months hospitalized with bronchiolitis (n = 32,809) in the three seasons before and after protocol adoption. Ward-based HFNC adoption paradoxically increased ICU admission (absolute increase 3.1%, 95% confidence interval, 2.8-3.4%) and ICU length of stay (absolute difference 9.1 days/100 patients, 95% CI, 5.1-13.2). Total length of stay and rates of mechanical ventilation were similar between groups.5Impact to practice: Ward-based HFNC protocols are associated with increased ICU utilization. As bronchiolitis is the leading diagnosis in pediatrics, pediatric hospitals can lead ward-based quality efforts to decrease HFNC overutilization focused on decreased initiation or deimplementation.
Lower versus traditional treatment threshold for neonatal hypoglycemia
Van Kempen AAMW et al. New England Journal of Medicine. February 2020.8
Background: Hypoglycemia is the most common metabolic abnormality in newborns, and up to 30% of newborns are routinely monitored for hypoglycemia. There is no consensus regarding the appropriate threshold at which hypoglycemia should be treated in order to prevent neurologic injury. Prior studies of neonatal hypoglycemia have largely been observation and have yielded conflicting results.8Findings: In this multicenter, randomized, noninferiority trial, 689 infants born at 35 weeks gestational age or later with risk factors for hypoglycemia and a measured blood glucose of 36-46 mg/dL were randomized to either a lower glucose treatment threshold (36 mg/dL) or traditional glucose treatment threshold (47 mg/dL). The primary outcome was psychomotor development at 18 months, assessed via the Bayley Scale of Infant and Toddler Development, third edition. There was no significant difference in cognitive or motor scores at 18 months. The lower treatment threshold group had a higher frequency of severe hypoglycemia (< 36 mg/dL) and were more likely to have four or more episodes of hypoglycemia. The traditional treatment threshold group had more supplemental feeding and more IV glucose administration. Length of stay for the mother and baby did not differ between groups.8
Impact to practice: This prospective, randomized study suggests that reducing the treatment threshold for neonatal hypoglycemia did not affect neurodevelopmental at 18 months of age. In contrast, a recent meta-analysis by Shah et al. suggested that neonatal hypoglycemia was not associated with adverse neurodevelopmental outcomes in early childhood; however, differences in rates of neurodevelopmental impairment, low literacy, and low numeracy were detectable by age five.9
Factors associated with family experience in pediatric inpatient care
Feng JY et al. Pediatrics. March 2020 Mar.10
Background: Positive patient experience is associated with better health care outcomes and reduced health care use.11 Consequently, patient experience surveys have played a larger role in public reporting, financial risk sharing arrangements, and pay for performance programs. While adult studies have examined the importance of specific care dimensions for patient experience, data are lacking for inpatient pediatric populations.
Findings: A retrospective study collected Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys from 17,727 patients in 69 hospitals within the United States over a 14-month period.10 Of the 10 care dimensions analyzed, child comfort (aOR 1.50; 95% CI, 1.41-1.60) and nurse-parent communication (aOR 1.50; 95% CI, 1.42-1.58) were most strongly associated with a family’s willingness to recommend a hospital. Additional associated indices included preparing to leave the hospital (aOR 1.34; 95% CI, 1.27-1.41), doctor-parent communication (aOR 1.28; 95% CI, 1.21–1.35), and keeping parents informed (aOR 1.25; 95% CI, 1.18-1.33). Privacy and quietness, which are associated with positive patient experience in adult studies, were not significantly associated with willingness to recommend in this cohort.
Impact to practice: Hospitals seeking to improve patient experience will benefit most by focusing on improving patient comfort and nurse-parent communication. Factors that increase adult patient satisfaction may not be as important to the pediatric population and their families.
The other five articles that comprised the top 10 are listed below:
Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis
Coon ER et al. JAMA Pediatrics. September 2020.12
Clinical prediction rule for distinguishing bacterial from aseptic meningitis
Mintegi S et al. Pediatrics. September 2020.13
The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC Ullman AJ et al. Pediatrics. June 2020.14
A structured neonatal parenting elective: An approach for parenting leave during residency
Cree-Green M et al. Academic Pediatrics. Aug 2020.15
The KidzMed project: Teaching children to swallow tablet medication
Tse Y et al. Archives of Disease in Childhood. November 2020.16
Dr. Steed is an internal medicine and pediatrics hospitalist at Northwestern Memorial Hospital and Ann and Robert H. Lurie’s Children’s Hospital of Chicago. Dr. Fisher is a current fellow in hospice and palliative medicine and a clinical assistant professor at Michigan State University. Dr. Money is an assistant professor of pediatrics at the University of Utah and a fellowship-trained pediatric hospitalist at Utah Valley Hospital and Primary Children’s Hospital.
References
1. Khan A et al. Association between parent comfort with english and adverse events among hospitalized children. JAMA Pediatr. 2020 Dec 1;174(12):e203215. doi: 10.1001/jamapediatrics.2020.3215.
2. Wasserman M et al. Identifying and preventing medical errors in patients with limited English proficiency: Key findings and tools for the field. J Healthc Qual. May-Jun 2014;36(3):5-16. doi: 10.1111/jhq.12065.
3. Yeung F et al. Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children. Hosp Pediatr. 2020 Dec;10(12):1038-43. doi: 10.1542/hpeds.2020-0137.
4. Mok E et al. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract. 2007 Feb;13(1):33-45. doi: 10.1111/j.1440-172X.2006.00607.x.
5. Coon ER et al. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020 Jun;15(6):325-30. doi: 10.12788/jhm.3417.
6. Kalburgi S and Halley T. High-flow nasal cannula use outside of the ICU setting. Pediatrics. 2020;146(5):e20194083. doi: 10.1542/peds.2019-4083.
7. Leyenaar JK and Ralston SL. Widespread adoption of low-value therapy: The case of bronchiolitis and high-flow oxygen. Pediatrics. 2020 Nov;146(5):e2020021188. doi: 10.1542/peds.2020-021188.
8. Van Kempen AAMW et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020 Feb 6;382(6):534-44. doi: 10.1056/NEJMoa1905593.
9. Shah R et al. Neonatal glycaemia and neurodevelopmental outcomes: A systematic review and meta-analysis. Neonatology. 2019;115(2):116-26. doi: 10.1159/000492859.
10. Feng JY et al. Factors associated with family experience in pediatric inpatient care. Pediatrics. 2020 Mar;145(3):e20191264. doi: 10.1542/peds.2019-1264.
11. Anhang Price R et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014 Oct;71(5):522-54. doi: 10.1177/1077558714541480.
12. Coon ER et al. Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis: The Bronchiolitis Follow-up Intervention Trial (BeneFIT) randomized clinical trial. JAMA Pediatr. 2020 Sep 1;174(9):e201937. doi: 10.1001/jamapediatrics.2020.1937.
13. Mintegi S et al. Clinical prediction rule for distinguishing bacterial from aseptic meningitis. Pediatrics. 2020 Sept;146(3): e20201126. doi: 10.1542/peds.2020-1126.
14. Ullman AJ et al. The Michigan Appropriateness Guide for Intravenous Catheters in pediatrics: miniMAGIC. Pediatrics. 2020 Jun;145(Suppl 3):S269-S284. doi: 10.1542/peds.2019-3474I.
15. Cree-Green M et al. A structured neonatal parenting elective: an approach for parenting leave during residency. Acad Pediatr. 2021 Jan-Feb;21(1):16-18. doi: 10.1016/j.acap.2020.02.008.
16. Tse Y et al. The KidzMed project: Teaching children to swallow tablet medication. Arch Dis Child. 2020 Nov;105(11):1105-7. doi: 10.1136/archdischild-2019-317512.
Timing of initiation of renal-replacement therapy in acute kidney injury
Background: Acute kidney injury (AKI) is a common complication that occurs in seriously ill patients admitted to the ICU, and many of these patients eventually require RRT. When complicated by major metabolic disorders, it is usually clear when therapy should be initiated. However, when these complications are absent, the most appropriate time to initiate RRT is unclear. There are potential advantages to performing early RRT in patients with severe AKI, such as restoring acid-base balance, preventing fluid accumulation, and preventing major electrolyte disturbances.
Study design: Multinational, randomized, controlled trial.
Setting: 168 hospitals in 15 countries.
Synopsis: Eligible patients were adults admitted to an ICU with severe AKI. Patients were randomly assigned to an accelerated strategy of RRT (initiated within 12 hours, 1,465 patients) or a standard strategy of RRT (held until conventional indications developed or AKI lasted more than 72 hours, 1,462 patients). RRT was performed in 1,418 (96.8%) in the accelerated group and 903 (61.8%) in the standard group. At 90 days, 643 deaths (43.9%) occurred in the accelerated group and 639 deaths (43.7%) occurred in the standard group (RR, 1.00; 95% CI, 0.93-1.09; P = .92). Among survivors at 90 days, 85 out of 814 accelerated patients (10.4%) and 49 of 815 standard patients (6.0%) continued to require RRT (RR, 1.75; 95% CI, 1.24-2.43), suggesting the possibility of increased dependence on long-term RRT if introduced early. Limitations include use of clinical equipoise to confirm full eligibility, introducing possible patient heterogeneity into the trial. In addition, broad discretion was given to clinicians on when to start RRT in the standard group resulting in variable initiation times.
Bottom line: In critically ill patients with severe AKI, earlier RRT did not result in lower mortality at 90 days compared with standard therapy and increased the risk of requiring RRT at 90 days.
Citation: Bagshaw SM et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240-51. doi: 10.1056/NEJMoa2000741.
Dr. Kim is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Acute kidney injury (AKI) is a common complication that occurs in seriously ill patients admitted to the ICU, and many of these patients eventually require RRT. When complicated by major metabolic disorders, it is usually clear when therapy should be initiated. However, when these complications are absent, the most appropriate time to initiate RRT is unclear. There are potential advantages to performing early RRT in patients with severe AKI, such as restoring acid-base balance, preventing fluid accumulation, and preventing major electrolyte disturbances.
Study design: Multinational, randomized, controlled trial.
Setting: 168 hospitals in 15 countries.
Synopsis: Eligible patients were adults admitted to an ICU with severe AKI. Patients were randomly assigned to an accelerated strategy of RRT (initiated within 12 hours, 1,465 patients) or a standard strategy of RRT (held until conventional indications developed or AKI lasted more than 72 hours, 1,462 patients). RRT was performed in 1,418 (96.8%) in the accelerated group and 903 (61.8%) in the standard group. At 90 days, 643 deaths (43.9%) occurred in the accelerated group and 639 deaths (43.7%) occurred in the standard group (RR, 1.00; 95% CI, 0.93-1.09; P = .92). Among survivors at 90 days, 85 out of 814 accelerated patients (10.4%) and 49 of 815 standard patients (6.0%) continued to require RRT (RR, 1.75; 95% CI, 1.24-2.43), suggesting the possibility of increased dependence on long-term RRT if introduced early. Limitations include use of clinical equipoise to confirm full eligibility, introducing possible patient heterogeneity into the trial. In addition, broad discretion was given to clinicians on when to start RRT in the standard group resulting in variable initiation times.
Bottom line: In critically ill patients with severe AKI, earlier RRT did not result in lower mortality at 90 days compared with standard therapy and increased the risk of requiring RRT at 90 days.
Citation: Bagshaw SM et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240-51. doi: 10.1056/NEJMoa2000741.
Dr. Kim is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Acute kidney injury (AKI) is a common complication that occurs in seriously ill patients admitted to the ICU, and many of these patients eventually require RRT. When complicated by major metabolic disorders, it is usually clear when therapy should be initiated. However, when these complications are absent, the most appropriate time to initiate RRT is unclear. There are potential advantages to performing early RRT in patients with severe AKI, such as restoring acid-base balance, preventing fluid accumulation, and preventing major electrolyte disturbances.
Study design: Multinational, randomized, controlled trial.
Setting: 168 hospitals in 15 countries.
Synopsis: Eligible patients were adults admitted to an ICU with severe AKI. Patients were randomly assigned to an accelerated strategy of RRT (initiated within 12 hours, 1,465 patients) or a standard strategy of RRT (held until conventional indications developed or AKI lasted more than 72 hours, 1,462 patients). RRT was performed in 1,418 (96.8%) in the accelerated group and 903 (61.8%) in the standard group. At 90 days, 643 deaths (43.9%) occurred in the accelerated group and 639 deaths (43.7%) occurred in the standard group (RR, 1.00; 95% CI, 0.93-1.09; P = .92). Among survivors at 90 days, 85 out of 814 accelerated patients (10.4%) and 49 of 815 standard patients (6.0%) continued to require RRT (RR, 1.75; 95% CI, 1.24-2.43), suggesting the possibility of increased dependence on long-term RRT if introduced early. Limitations include use of clinical equipoise to confirm full eligibility, introducing possible patient heterogeneity into the trial. In addition, broad discretion was given to clinicians on when to start RRT in the standard group resulting in variable initiation times.
Bottom line: In critically ill patients with severe AKI, earlier RRT did not result in lower mortality at 90 days compared with standard therapy and increased the risk of requiring RRT at 90 days.
Citation: Bagshaw SM et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240-51. doi: 10.1056/NEJMoa2000741.
Dr. Kim is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.