User login
Special Report II: Tackling Burnout
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Revolutionizing Headache Medicine: The Role of Artificial Intelligence
As we move further into the 21st century, technology continues to revolutionize various facets of our lives. Healthcare is a prime example. Advances in technology have dramatically reshaped the way we develop medications, diagnose diseases, and enhance patient care. The rise of artificial intelligence (AI) and the widespread adoption of digital health technologies have marked a significant milestone in improving the quality of care. AI, with its ability to leverage algorithms, deep learning, and machine learning to process data, make decisions, and perform tasks autonomously, is becoming an integral part of modern society. It is embedded in various technologies that we rely on daily, from smartphones and smart home devices to content recommendations on streaming services and social media platforms.
In healthcare, AI has applications in numerous fields, such as radiology. AI streamlines processes such as organizing patient appointments, optimizing radiation protocols for safety and efficiency, and enhancing the documentation process through advanced image analysis. AI technology plays an integral role in imaging tasks like image enhancement, lesion detection, and precise measurement. In difficult-to-interpret radiologic studies, such as some mammography images, it can be a crucial aid to the radiologist. Additionally, the use of AI has significantly improved remote patient monitoring that enables healthcare professionals to monitor and assess patient conditions without needing in-person visits. Remote patient monitoring gained prominence during the COVID-19 pandemic and continues to be a valuable tool in post pandemic care. Study results have highlighted that AI-driven ambient dictation tools have increased provider engagement with patients during consultations while reducing the time spent documenting in electronic health records.
Like many other medical specialties, headache medicine also uses AI. Most prominently, AI has been used in models and engines in assisting with headache diagnoses. A noteworthy example of AI in headache medicine is the development of an online, computer-based diagnostic engine (CDE) by Rapoport et al, called BonTriage. This tool is designed to diagnose headaches by employing a rule set based on the International Classification of Headache Disorders-3 (ICHD-3) criteria for primary headache disorders while also evaluating secondary headaches and medication overuse headaches. By leveraging machine learning, the CDE has the potential to streamline the diagnostic process, reducing the number of questions needed to reach a diagnosis and making the experience more efficient. This information can then be printed as a PDF file and taken by the patient to a healthcare professional for further discussion, fostering a more accurate, fluid, and conversational consultation.
A study was conducted to evaluate the accuracy of the CDE. Participants were randomly assigned to 1 of 2 sequences: (1) using the CDE followed by a structured standard interview with a headache specialist using the same ICHD-3 criteria or (2) starting with the structured standard interview followed by the CDE. The results demonstrated nearly perfect agreement in diagnosing migraine and probable migraine between the CDE and structured standard interview (κ = 0.82, 95% CI: 0.74, 0.90). The CDE demonstrated a diagnostic accuracy of 91.6% (95% CI: 86.9%, 95.0%), a sensitivity rate of 89.0% (95% CI: 82.5%, 93.7%), and a specificity rate of 97.0% (95% CI: 89.5%, 99.6%).
A diagnostic engine such as this can save time that clinicians spend on documentation and allow more time for discussion with the patient. For instance, a patient can take the printout received from the CDE to an appointment; the printout gives a detailed history plus information about social and psychological issues, a list of medications taken, and results of previous testing. The CDE system was originally designed to help patients see a specialist in the environment of a nationwide lack of headache specialists. There are currently 45 million patients with headaches who are seeking treatment with only around 550 certified headache specialists in the United States. The CDE printed information can help a patient obtain a consultation from a clinician quickly and start evaluation and treatment earlier. This expert online consultation is currently free of charge.
Kwon et al developed a machine learning–based model designed to automatically classify headache disorders using data from a questionnaire. Their model was able to predict diagnoses for conditions such as migraine, tension-type headaches, trigeminal autonomic cephalalgia, epicranial headache, and thunderclap headaches. The model was trained on data from 2162 patients, all diagnosed by headache specialists, and achieved an overall accuracy of 81%, with a sensitivity of 88% and a specificity of 95% for diagnosing migraines. However, the model’s performance was less robust when applied to other headache disorders.
Katsuki et al developed an AI model to help non specialists accurately diagnose headaches. This model analyzed 17 variables and was trained on data from 2800 patients, with additional testing and refinement using data from another 200 patients. To evaluate its effectiveness, 2 groups of non-headache specialists each assessed 50 patients: 1 group relied solely on their expertise, while the other used the AI model. The group without AI assistance achieved an overall accuracy of 46% (κ = 0.21), while the group using the AI model significantly improved, reaching an overall accuracy of 83.2% (κ = 0.68).
Building on their work with AI for diagnosing headaches, Katsuki et al conducted a study using a smartphone application that tracked user-reported headache events alongside local weather data. The AI model revealed that lower barometric pressure, higher humidity, and increased rainfall were linked to the onset of headache attacks. The application also identified triggers for headaches in specific weather patterns, such as a drop in barometric pressure noted 6 hours before headache onset. The application of AI in monitoring weather changes could be crucial, especially given concerns that the rising frequency of severe weather events due to climate change may be exacerbating the severity and burden of migraine. Additionally, recent post hoc analyses of fremanezumab clinical trials have provided further evidence that weather changes can trigger headaches.
Rapoport and colleagues have also developed an application called Migraine Mentor, which accurately tracks headaches, triggers, health data, and response to medication on a smartphone. The patient spends 3 minutes a day answering a few questions about their day and whether they had a headache or took any medication. At 1 or 2 months, Migraine Mentor can generate a detailed report with data and current trends that is sent to the patient, which the patient can then share with the clinician. The application also reminds patients when to document data and take medication.
However, although the use of AI in headache medicine appears promising, caution must be exercised to ensure proper results and information are disseminated. One rapidly expanding application of AI is the widely popular ChatGPT. ChatGPT, which stands for generative pretraining transformer, is a type of large language model (LLM). An LLM is a deep learning algorithm designed to recognize, translate, predict, summarize, and generate text responses based on a given prompt. This model is trained on an extensive dataset that includes a diverse array of books, articles, and websites, exposing it to various language structures and styles. This training enables ChatGPT to generate responses that closely mimic human communication. LLMs are being used more and more in medicine to assist with generating patient documentation and educational materials.
However, Dr Fred Cohen published a perspective piece detailing how LLMs (such as ChatGPT) can produce misleading and inaccurate answers. In his example, he tasked ChatGPT to describe the epidemiology of migraines in penguins; the AI model generated a well-written and highly believable manuscript titled, “Migraine Under the Ice: Understanding Headaches in Antarctica's Feathered Friends.” The manuscript highlights that migraines are more prevalent in male penguins compared to females, with the peak age of onset occurring between 4 and 5 years. Additionally, emperor and king penguins are identified as being more susceptible to developing migraines compared to other penguin species. The paper was fictitious (as no studies on migraine in penguins have been written to date), exemplifying that these models can produce nonfactual materials.
For years, technological advancements have been reshaping many aspects of life, and medicine is no exception. AI has been successfully applied to streamline medical documentation, develop new drug targets, and deepen our understanding of various diseases. The field of headache medicine now also uses AI. Recent developments show significant promise, with AI aiding in the diagnosis of migraine and other headache disorders. AI models have even been used in the identification of potential drug targets for migraine treatment. Although there are still limitations to overcome, the future of AI in headache medicine appears bright.
If you would like to read more about Dr. Cohen’s work on AI and migraine, please visit fredcohenmd.com or TikTok @fredcohenmd.
As we move further into the 21st century, technology continues to revolutionize various facets of our lives. Healthcare is a prime example. Advances in technology have dramatically reshaped the way we develop medications, diagnose diseases, and enhance patient care. The rise of artificial intelligence (AI) and the widespread adoption of digital health technologies have marked a significant milestone in improving the quality of care. AI, with its ability to leverage algorithms, deep learning, and machine learning to process data, make decisions, and perform tasks autonomously, is becoming an integral part of modern society. It is embedded in various technologies that we rely on daily, from smartphones and smart home devices to content recommendations on streaming services and social media platforms.
In healthcare, AI has applications in numerous fields, such as radiology. AI streamlines processes such as organizing patient appointments, optimizing radiation protocols for safety and efficiency, and enhancing the documentation process through advanced image analysis. AI technology plays an integral role in imaging tasks like image enhancement, lesion detection, and precise measurement. In difficult-to-interpret radiologic studies, such as some mammography images, it can be a crucial aid to the radiologist. Additionally, the use of AI has significantly improved remote patient monitoring that enables healthcare professionals to monitor and assess patient conditions without needing in-person visits. Remote patient monitoring gained prominence during the COVID-19 pandemic and continues to be a valuable tool in post pandemic care. Study results have highlighted that AI-driven ambient dictation tools have increased provider engagement with patients during consultations while reducing the time spent documenting in electronic health records.
Like many other medical specialties, headache medicine also uses AI. Most prominently, AI has been used in models and engines in assisting with headache diagnoses. A noteworthy example of AI in headache medicine is the development of an online, computer-based diagnostic engine (CDE) by Rapoport et al, called BonTriage. This tool is designed to diagnose headaches by employing a rule set based on the International Classification of Headache Disorders-3 (ICHD-3) criteria for primary headache disorders while also evaluating secondary headaches and medication overuse headaches. By leveraging machine learning, the CDE has the potential to streamline the diagnostic process, reducing the number of questions needed to reach a diagnosis and making the experience more efficient. This information can then be printed as a PDF file and taken by the patient to a healthcare professional for further discussion, fostering a more accurate, fluid, and conversational consultation.
A study was conducted to evaluate the accuracy of the CDE. Participants were randomly assigned to 1 of 2 sequences: (1) using the CDE followed by a structured standard interview with a headache specialist using the same ICHD-3 criteria or (2) starting with the structured standard interview followed by the CDE. The results demonstrated nearly perfect agreement in diagnosing migraine and probable migraine between the CDE and structured standard interview (κ = 0.82, 95% CI: 0.74, 0.90). The CDE demonstrated a diagnostic accuracy of 91.6% (95% CI: 86.9%, 95.0%), a sensitivity rate of 89.0% (95% CI: 82.5%, 93.7%), and a specificity rate of 97.0% (95% CI: 89.5%, 99.6%).
A diagnostic engine such as this can save time that clinicians spend on documentation and allow more time for discussion with the patient. For instance, a patient can take the printout received from the CDE to an appointment; the printout gives a detailed history plus information about social and psychological issues, a list of medications taken, and results of previous testing. The CDE system was originally designed to help patients see a specialist in the environment of a nationwide lack of headache specialists. There are currently 45 million patients with headaches who are seeking treatment with only around 550 certified headache specialists in the United States. The CDE printed information can help a patient obtain a consultation from a clinician quickly and start evaluation and treatment earlier. This expert online consultation is currently free of charge.
Kwon et al developed a machine learning–based model designed to automatically classify headache disorders using data from a questionnaire. Their model was able to predict diagnoses for conditions such as migraine, tension-type headaches, trigeminal autonomic cephalalgia, epicranial headache, and thunderclap headaches. The model was trained on data from 2162 patients, all diagnosed by headache specialists, and achieved an overall accuracy of 81%, with a sensitivity of 88% and a specificity of 95% for diagnosing migraines. However, the model’s performance was less robust when applied to other headache disorders.
Katsuki et al developed an AI model to help non specialists accurately diagnose headaches. This model analyzed 17 variables and was trained on data from 2800 patients, with additional testing and refinement using data from another 200 patients. To evaluate its effectiveness, 2 groups of non-headache specialists each assessed 50 patients: 1 group relied solely on their expertise, while the other used the AI model. The group without AI assistance achieved an overall accuracy of 46% (κ = 0.21), while the group using the AI model significantly improved, reaching an overall accuracy of 83.2% (κ = 0.68).
Building on their work with AI for diagnosing headaches, Katsuki et al conducted a study using a smartphone application that tracked user-reported headache events alongside local weather data. The AI model revealed that lower barometric pressure, higher humidity, and increased rainfall were linked to the onset of headache attacks. The application also identified triggers for headaches in specific weather patterns, such as a drop in barometric pressure noted 6 hours before headache onset. The application of AI in monitoring weather changes could be crucial, especially given concerns that the rising frequency of severe weather events due to climate change may be exacerbating the severity and burden of migraine. Additionally, recent post hoc analyses of fremanezumab clinical trials have provided further evidence that weather changes can trigger headaches.
Rapoport and colleagues have also developed an application called Migraine Mentor, which accurately tracks headaches, triggers, health data, and response to medication on a smartphone. The patient spends 3 minutes a day answering a few questions about their day and whether they had a headache or took any medication. At 1 or 2 months, Migraine Mentor can generate a detailed report with data and current trends that is sent to the patient, which the patient can then share with the clinician. The application also reminds patients when to document data and take medication.
However, although the use of AI in headache medicine appears promising, caution must be exercised to ensure proper results and information are disseminated. One rapidly expanding application of AI is the widely popular ChatGPT. ChatGPT, which stands for generative pretraining transformer, is a type of large language model (LLM). An LLM is a deep learning algorithm designed to recognize, translate, predict, summarize, and generate text responses based on a given prompt. This model is trained on an extensive dataset that includes a diverse array of books, articles, and websites, exposing it to various language structures and styles. This training enables ChatGPT to generate responses that closely mimic human communication. LLMs are being used more and more in medicine to assist with generating patient documentation and educational materials.
However, Dr Fred Cohen published a perspective piece detailing how LLMs (such as ChatGPT) can produce misleading and inaccurate answers. In his example, he tasked ChatGPT to describe the epidemiology of migraines in penguins; the AI model generated a well-written and highly believable manuscript titled, “Migraine Under the Ice: Understanding Headaches in Antarctica's Feathered Friends.” The manuscript highlights that migraines are more prevalent in male penguins compared to females, with the peak age of onset occurring between 4 and 5 years. Additionally, emperor and king penguins are identified as being more susceptible to developing migraines compared to other penguin species. The paper was fictitious (as no studies on migraine in penguins have been written to date), exemplifying that these models can produce nonfactual materials.
For years, technological advancements have been reshaping many aspects of life, and medicine is no exception. AI has been successfully applied to streamline medical documentation, develop new drug targets, and deepen our understanding of various diseases. The field of headache medicine now also uses AI. Recent developments show significant promise, with AI aiding in the diagnosis of migraine and other headache disorders. AI models have even been used in the identification of potential drug targets for migraine treatment. Although there are still limitations to overcome, the future of AI in headache medicine appears bright.
If you would like to read more about Dr. Cohen’s work on AI and migraine, please visit fredcohenmd.com or TikTok @fredcohenmd.
As we move further into the 21st century, technology continues to revolutionize various facets of our lives. Healthcare is a prime example. Advances in technology have dramatically reshaped the way we develop medications, diagnose diseases, and enhance patient care. The rise of artificial intelligence (AI) and the widespread adoption of digital health technologies have marked a significant milestone in improving the quality of care. AI, with its ability to leverage algorithms, deep learning, and machine learning to process data, make decisions, and perform tasks autonomously, is becoming an integral part of modern society. It is embedded in various technologies that we rely on daily, from smartphones and smart home devices to content recommendations on streaming services and social media platforms.
In healthcare, AI has applications in numerous fields, such as radiology. AI streamlines processes such as organizing patient appointments, optimizing radiation protocols for safety and efficiency, and enhancing the documentation process through advanced image analysis. AI technology plays an integral role in imaging tasks like image enhancement, lesion detection, and precise measurement. In difficult-to-interpret radiologic studies, such as some mammography images, it can be a crucial aid to the radiologist. Additionally, the use of AI has significantly improved remote patient monitoring that enables healthcare professionals to monitor and assess patient conditions without needing in-person visits. Remote patient monitoring gained prominence during the COVID-19 pandemic and continues to be a valuable tool in post pandemic care. Study results have highlighted that AI-driven ambient dictation tools have increased provider engagement with patients during consultations while reducing the time spent documenting in electronic health records.
Like many other medical specialties, headache medicine also uses AI. Most prominently, AI has been used in models and engines in assisting with headache diagnoses. A noteworthy example of AI in headache medicine is the development of an online, computer-based diagnostic engine (CDE) by Rapoport et al, called BonTriage. This tool is designed to diagnose headaches by employing a rule set based on the International Classification of Headache Disorders-3 (ICHD-3) criteria for primary headache disorders while also evaluating secondary headaches and medication overuse headaches. By leveraging machine learning, the CDE has the potential to streamline the diagnostic process, reducing the number of questions needed to reach a diagnosis and making the experience more efficient. This information can then be printed as a PDF file and taken by the patient to a healthcare professional for further discussion, fostering a more accurate, fluid, and conversational consultation.
A study was conducted to evaluate the accuracy of the CDE. Participants were randomly assigned to 1 of 2 sequences: (1) using the CDE followed by a structured standard interview with a headache specialist using the same ICHD-3 criteria or (2) starting with the structured standard interview followed by the CDE. The results demonstrated nearly perfect agreement in diagnosing migraine and probable migraine between the CDE and structured standard interview (κ = 0.82, 95% CI: 0.74, 0.90). The CDE demonstrated a diagnostic accuracy of 91.6% (95% CI: 86.9%, 95.0%), a sensitivity rate of 89.0% (95% CI: 82.5%, 93.7%), and a specificity rate of 97.0% (95% CI: 89.5%, 99.6%).
A diagnostic engine such as this can save time that clinicians spend on documentation and allow more time for discussion with the patient. For instance, a patient can take the printout received from the CDE to an appointment; the printout gives a detailed history plus information about social and psychological issues, a list of medications taken, and results of previous testing. The CDE system was originally designed to help patients see a specialist in the environment of a nationwide lack of headache specialists. There are currently 45 million patients with headaches who are seeking treatment with only around 550 certified headache specialists in the United States. The CDE printed information can help a patient obtain a consultation from a clinician quickly and start evaluation and treatment earlier. This expert online consultation is currently free of charge.
Kwon et al developed a machine learning–based model designed to automatically classify headache disorders using data from a questionnaire. Their model was able to predict diagnoses for conditions such as migraine, tension-type headaches, trigeminal autonomic cephalalgia, epicranial headache, and thunderclap headaches. The model was trained on data from 2162 patients, all diagnosed by headache specialists, and achieved an overall accuracy of 81%, with a sensitivity of 88% and a specificity of 95% for diagnosing migraines. However, the model’s performance was less robust when applied to other headache disorders.
Katsuki et al developed an AI model to help non specialists accurately diagnose headaches. This model analyzed 17 variables and was trained on data from 2800 patients, with additional testing and refinement using data from another 200 patients. To evaluate its effectiveness, 2 groups of non-headache specialists each assessed 50 patients: 1 group relied solely on their expertise, while the other used the AI model. The group without AI assistance achieved an overall accuracy of 46% (κ = 0.21), while the group using the AI model significantly improved, reaching an overall accuracy of 83.2% (κ = 0.68).
Building on their work with AI for diagnosing headaches, Katsuki et al conducted a study using a smartphone application that tracked user-reported headache events alongside local weather data. The AI model revealed that lower barometric pressure, higher humidity, and increased rainfall were linked to the onset of headache attacks. The application also identified triggers for headaches in specific weather patterns, such as a drop in barometric pressure noted 6 hours before headache onset. The application of AI in monitoring weather changes could be crucial, especially given concerns that the rising frequency of severe weather events due to climate change may be exacerbating the severity and burden of migraine. Additionally, recent post hoc analyses of fremanezumab clinical trials have provided further evidence that weather changes can trigger headaches.
Rapoport and colleagues have also developed an application called Migraine Mentor, which accurately tracks headaches, triggers, health data, and response to medication on a smartphone. The patient spends 3 minutes a day answering a few questions about their day and whether they had a headache or took any medication. At 1 or 2 months, Migraine Mentor can generate a detailed report with data and current trends that is sent to the patient, which the patient can then share with the clinician. The application also reminds patients when to document data and take medication.
However, although the use of AI in headache medicine appears promising, caution must be exercised to ensure proper results and information are disseminated. One rapidly expanding application of AI is the widely popular ChatGPT. ChatGPT, which stands for generative pretraining transformer, is a type of large language model (LLM). An LLM is a deep learning algorithm designed to recognize, translate, predict, summarize, and generate text responses based on a given prompt. This model is trained on an extensive dataset that includes a diverse array of books, articles, and websites, exposing it to various language structures and styles. This training enables ChatGPT to generate responses that closely mimic human communication. LLMs are being used more and more in medicine to assist with generating patient documentation and educational materials.
However, Dr Fred Cohen published a perspective piece detailing how LLMs (such as ChatGPT) can produce misleading and inaccurate answers. In his example, he tasked ChatGPT to describe the epidemiology of migraines in penguins; the AI model generated a well-written and highly believable manuscript titled, “Migraine Under the Ice: Understanding Headaches in Antarctica's Feathered Friends.” The manuscript highlights that migraines are more prevalent in male penguins compared to females, with the peak age of onset occurring between 4 and 5 years. Additionally, emperor and king penguins are identified as being more susceptible to developing migraines compared to other penguin species. The paper was fictitious (as no studies on migraine in penguins have been written to date), exemplifying that these models can produce nonfactual materials.
For years, technological advancements have been reshaping many aspects of life, and medicine is no exception. AI has been successfully applied to streamline medical documentation, develop new drug targets, and deepen our understanding of various diseases. The field of headache medicine now also uses AI. Recent developments show significant promise, with AI aiding in the diagnosis of migraine and other headache disorders. AI models have even been used in the identification of potential drug targets for migraine treatment. Although there are still limitations to overcome, the future of AI in headache medicine appears bright.
If you would like to read more about Dr. Cohen’s work on AI and migraine, please visit fredcohenmd.com or TikTok @fredcohenmd.
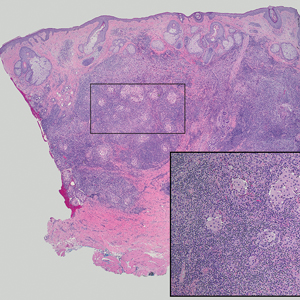
A Clonal Complete Remission Induced by IDH1 Inhibitor Ivosidenib in a Myelodysplastic Syndrome (MDS) With Co-Mutations of IDH1 and the ZRSR2 RNA Splicing Gene
Background
IDH1 mutations are detected in 3-4% of MDS, nearly always with one or more co-mutations. Treatment with IDH1 inhibitor ivosidenib typically resulted in regression of the abnormal clone in 15 reported responders. However, in a few cases differentiation was restored from the abnormal clone. Here we report a durable MDS remission despite sustained proliferation of a clone with IDH1 and ZRSR2 mutations.
Case Presentation
A 49-year-old man developed severe neutropenia and macrocytic anemia in January 2019. Mild marrow dysplasia developed by March 2020 with IDH1 (31.1%) and splicing gene ZRSR2 (55.7%) mutations. In October 2022 biopsy showed MDS with 4% blasts, megakaryocytic/granulocytic hypoplasia, normal cytogenetics and 43% IDH1/89% ZRSR2. After azacytidine failure, ivosidenib was started in November 2023 following FDA approval. Within weeks ANCs increased from 170 to 1580 and hemoglobin from 7.9 to 11.6 with MCV 115, reticulocytes 1.72%. At 3 months a CBC was normal except for MCV 111. IDH1 and ZRSR2 were 36.4% and 71%. After 6 months, ANC was 2380, hemoglobin 14.7, MCV 108.6, reticulo-cytes 1.77%. IDH1 PCR showed a 33.1% allele frequency consistent with a clonal remission.
Discussion
IDH1 mutations in MDS/AML frequently co-occur with mutations in RNA splicing genes SRSF2 or ZRSR2. For ZRSR2, we previously reported that isolated mutations of this gene cause refractory macrocytic anemias without dysplasia, thus presenting as clonal cytopenias of undetermined significance (Fleischman et al., Leuk Res, 2017). In this MDS case, after ivosidenib treatment the ZRSR2 splicing defect sustained clonal dominance over polyclonal hematopoiesis while accounting for macrocytosis. Longitudinal data for two ivosidenib-treated IDH1/SRSF2 MDS cases are incomplete, but one case of IDH2/SRSF2 MDS treated with the inhibitor enasidenib similarly achieved complete remission without regression of the mutated clone for 12 months.
Conclusions
Following the FDA approval of ivosidenib, all cases of MDS should have DNA sequencing performed at diagnosis to identify IDH1 mutations. Treatment induces high rates of remission even when polyclonal hematopoiesis does not recover. Moreover, the restoration of hematopoietic differentiation by the abnormal clone provides unique insights into the clinical phenotype and fitness advantage conferred by the co-existing driver mutations.
Background
IDH1 mutations are detected in 3-4% of MDS, nearly always with one or more co-mutations. Treatment with IDH1 inhibitor ivosidenib typically resulted in regression of the abnormal clone in 15 reported responders. However, in a few cases differentiation was restored from the abnormal clone. Here we report a durable MDS remission despite sustained proliferation of a clone with IDH1 and ZRSR2 mutations.
Case Presentation
A 49-year-old man developed severe neutropenia and macrocytic anemia in January 2019. Mild marrow dysplasia developed by March 2020 with IDH1 (31.1%) and splicing gene ZRSR2 (55.7%) mutations. In October 2022 biopsy showed MDS with 4% blasts, megakaryocytic/granulocytic hypoplasia, normal cytogenetics and 43% IDH1/89% ZRSR2. After azacytidine failure, ivosidenib was started in November 2023 following FDA approval. Within weeks ANCs increased from 170 to 1580 and hemoglobin from 7.9 to 11.6 with MCV 115, reticulocytes 1.72%. At 3 months a CBC was normal except for MCV 111. IDH1 and ZRSR2 were 36.4% and 71%. After 6 months, ANC was 2380, hemoglobin 14.7, MCV 108.6, reticulo-cytes 1.77%. IDH1 PCR showed a 33.1% allele frequency consistent with a clonal remission.
Discussion
IDH1 mutations in MDS/AML frequently co-occur with mutations in RNA splicing genes SRSF2 or ZRSR2. For ZRSR2, we previously reported that isolated mutations of this gene cause refractory macrocytic anemias without dysplasia, thus presenting as clonal cytopenias of undetermined significance (Fleischman et al., Leuk Res, 2017). In this MDS case, after ivosidenib treatment the ZRSR2 splicing defect sustained clonal dominance over polyclonal hematopoiesis while accounting for macrocytosis. Longitudinal data for two ivosidenib-treated IDH1/SRSF2 MDS cases are incomplete, but one case of IDH2/SRSF2 MDS treated with the inhibitor enasidenib similarly achieved complete remission without regression of the mutated clone for 12 months.
Conclusions
Following the FDA approval of ivosidenib, all cases of MDS should have DNA sequencing performed at diagnosis to identify IDH1 mutations. Treatment induces high rates of remission even when polyclonal hematopoiesis does not recover. Moreover, the restoration of hematopoietic differentiation by the abnormal clone provides unique insights into the clinical phenotype and fitness advantage conferred by the co-existing driver mutations.
Background
IDH1 mutations are detected in 3-4% of MDS, nearly always with one or more co-mutations. Treatment with IDH1 inhibitor ivosidenib typically resulted in regression of the abnormal clone in 15 reported responders. However, in a few cases differentiation was restored from the abnormal clone. Here we report a durable MDS remission despite sustained proliferation of a clone with IDH1 and ZRSR2 mutations.
Case Presentation
A 49-year-old man developed severe neutropenia and macrocytic anemia in January 2019. Mild marrow dysplasia developed by March 2020 with IDH1 (31.1%) and splicing gene ZRSR2 (55.7%) mutations. In October 2022 biopsy showed MDS with 4% blasts, megakaryocytic/granulocytic hypoplasia, normal cytogenetics and 43% IDH1/89% ZRSR2. After azacytidine failure, ivosidenib was started in November 2023 following FDA approval. Within weeks ANCs increased from 170 to 1580 and hemoglobin from 7.9 to 11.6 with MCV 115, reticulocytes 1.72%. At 3 months a CBC was normal except for MCV 111. IDH1 and ZRSR2 were 36.4% and 71%. After 6 months, ANC was 2380, hemoglobin 14.7, MCV 108.6, reticulo-cytes 1.77%. IDH1 PCR showed a 33.1% allele frequency consistent with a clonal remission.
Discussion
IDH1 mutations in MDS/AML frequently co-occur with mutations in RNA splicing genes SRSF2 or ZRSR2. For ZRSR2, we previously reported that isolated mutations of this gene cause refractory macrocytic anemias without dysplasia, thus presenting as clonal cytopenias of undetermined significance (Fleischman et al., Leuk Res, 2017). In this MDS case, after ivosidenib treatment the ZRSR2 splicing defect sustained clonal dominance over polyclonal hematopoiesis while accounting for macrocytosis. Longitudinal data for two ivosidenib-treated IDH1/SRSF2 MDS cases are incomplete, but one case of IDH2/SRSF2 MDS treated with the inhibitor enasidenib similarly achieved complete remission without regression of the mutated clone for 12 months.
Conclusions
Following the FDA approval of ivosidenib, all cases of MDS should have DNA sequencing performed at diagnosis to identify IDH1 mutations. Treatment induces high rates of remission even when polyclonal hematopoiesis does not recover. Moreover, the restoration of hematopoietic differentiation by the abnormal clone provides unique insights into the clinical phenotype and fitness advantage conferred by the co-existing driver mutations.
Creating a Urology Prostate Cancer Note, a National Oncology and Surgery Office Collaboration for Prostate Cancer Clinical Pathway Utilization
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Discussion
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. For example, the Very Low Risk flow map has seven unique Veterans entered, whereas the Molecular Testing flow map has over 3,900 unique Veterans entered. One clear reason for this disparity in pathway documentation use is that local prostate cancer is managed by urology and their documentation of the PCCP is not as widespread as the medical oncologists. The National Oncology Program developed clinical note templates to document PCCP that medical oncologist use which has increased utilization. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator. The UPCN will function as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. The UPCN is in the testing phase at three pilot test sites and is scheduled to be deployed summer 2024. The collaborative effort is aligned with the VHA directives outlined in the Cleland Dole Act.
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Discussion
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. For example, the Very Low Risk flow map has seven unique Veterans entered, whereas the Molecular Testing flow map has over 3,900 unique Veterans entered. One clear reason for this disparity in pathway documentation use is that local prostate cancer is managed by urology and their documentation of the PCCP is not as widespread as the medical oncologists. The National Oncology Program developed clinical note templates to document PCCP that medical oncologist use which has increased utilization. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator. The UPCN will function as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. The UPCN is in the testing phase at three pilot test sites and is scheduled to be deployed summer 2024. The collaborative effort is aligned with the VHA directives outlined in the Cleland Dole Act.
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Discussion
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. For example, the Very Low Risk flow map has seven unique Veterans entered, whereas the Molecular Testing flow map has over 3,900 unique Veterans entered. One clear reason for this disparity in pathway documentation use is that local prostate cancer is managed by urology and their documentation of the PCCP is not as widespread as the medical oncologists. The National Oncology Program developed clinical note templates to document PCCP that medical oncologist use which has increased utilization. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator. The UPCN will function as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. The UPCN is in the testing phase at three pilot test sites and is scheduled to be deployed summer 2024. The collaborative effort is aligned with the VHA directives outlined in the Cleland Dole Act.
Migraine Treatment Outcomes
Outcomes of Acute and Preventive Migraine Therapy Based on Patient Sex
I previously have addressed myths about migraine as they pertain to men and women. When I found an interesting study recently published in Cephalalgia investigating the effectiveness of calcitonin gene-related peptide receptor (CGRP-R) antagonists (gepants) for acute care and prevention of episodic migraine and CGRP monoclonal antibodies for preventive treatment of episodic and chronic migraine in men and women, I thought I would discuss it here.
The study’s aim was to discern if patient sex contributed to outcomes in each specific treatment arm. Female sex hormones have been recognized as factors in promoting migraine, and women show increased severity, persistence, and comorbidity in migraine profiles, and increased prevalence of migraine relative to men.
Gepants used for acute therapy (ubrogepant, rimegepant, zavegepant) and preventive therapy (atogepant, rimegepant) were studied in this trial. Erenumab, fremanezumab, galcanezumab, and eptinezumab are monoclonal antibodies that either sit on the CGRP receptor (erenumab) or inactivate the CGRP ligand (fremanezumab, galcanezumab, and eptinezumab) and are used for migraine prevention. CGRP-based therapies are not effective in all patients and understanding which patient groups respond preferentially could reduce trial and error in treatment selection. The effectiveness of treatments targeting CGRP or the CGRP receptor may not be uniform in men and women, highlighting the need for further research and understanding of CGRP neurobiology in both sexes.
Key findings:
- In the trial by Porreca et al: In women, the 3 gepants approved by the FDA for the acute care of migraine (ubrogepant, rimegepant, zavegepant) produced a statistically significant drug effect for the 2-hour pain freedom (2h-PF) endpoint, with an average drug effect of 9.5% (CI: 7.4 to 11.6) and an average number needed to treat (NNT) of 11.
- Men did not show statistically significant effects with the acute use of gepants. The average drug effect was 2.8%, and the average NNT was 36.
- For both men and women, CGRP-targeting therapies for prevention of migraine (the 4 monoclonal antibodies) were equally effective; however, possible sex differences remain uncertain and need further study.
- In patients with chronic migraine, CGRP/CGRP-R antibodies were similarly effective in both men and women.
- For the 2-hour freedom from most bothersome symptom (2h-MBS) endpoint when gepants were given acutely, the effects were much better in women than men, with an average drug effect of 10.2% and an average NNT of 10.
- In men, these medications produced observed treatment effects on 2h-MBS with an average drug effect of 3.2% and an average NNT of 32.
- In men, 5 out of 12 estimates favored placebo over the active treatment, suggesting a treatment with little to no effect.
- The pooled treatment effects for women were 3 times as large, at 9.2% and 10.2%, respectively.
- The placebo response rates for 2 of the 3 ubrogepant studies and one of 2 zavegepant studies were higher in men than in women.
The study concludes that, while small molecule CGRP-R antagonists are dramatically effective for acute therapy of migraine in women, available data do not demonstrate effectiveness in men. The treatment effect was found to always favor active treatment numerically for both men and women for prevention of episodic and chronic migraine. The data highlight possible differential effects of CGRP-targeted therapies in different patient populations and the need for increased understanding of CGRP neurobiology in men and women. The study also emphasizes the need to understand which patient groups preferentially respond to CGRP-based therapies to reduce trial and error in treatment. Note that rimegepant data on prevention were not available for analysis at the time of the writing.
It would be interesting to perform a meta-analysis of multiple well-done, large, real-world studies to see if the same differences and similarities are found in men versus women for acute care of migraine and prevention of episodic and chronic migraine. I suspect that we would find that acute care results favor women but that some men do well.
The Effectiveness of Prednisolone for Treating Medication Overuse Headache
I often discuss medication overuse headache (MOH), as it is difficult to diagnose and treat, so I wanted to comment on another pertinent study. It is a post hoc analysis of the Registry for Load and Management of Medication Overuse Headache (RELEASE). The RELEASE trial is an ongoing, multicenter, observational, cohort study of MOH that has been conducted in Korea since April 2020. Findings were recently published in Headache by Lee et al.
MOH is a secondary headache disorder that develops in patients with a preexisting primary headache when they overuse acute care headache medications of any type except gepants. This includes prescription medications such as triptans, ergots, butalbital-containing medications; opioids; aspirin; acetaminophen; any type of combination medication often containing caffeine; or a combination of medications. This condition significantly impacts patients’ quality of life and productivity, usually increasing the frequency of headaches per month and leading to higher healthcare-related costs.
Treating MOH is challenging due to the lack of high-quality drug trials specifically designed for MOH and doctor inexperience. Current evidence is based largely on subgroup analyses of drug trials for the treatment of chronic migraine that contain these patient types.
Withdrawal of acute care headache medications that are being overused has traditionally been considered an important aspect of MOH treatment, although this may be changing. Withdrawal symptoms, such as increased intensity of headache pain, frequency of headaches, and other symptoms like agitation and sleep disturbance, can prevent patients from discontinuing overused medications. Systemic corticosteroids are widely used to reduce these withdrawal headaches, but clinical trials are sparse and have failed to meet proper endpoints. Despite this, corticosteroids have shown potential benefits, such as decreasing withdrawal headaches, reducing the use of rescue medications, and lowering headache intensity at certain time points after treatment.
Given these findings, this published study hypothesized that prednisolone may play a role in converting MOH to non-MOH at 3 months after treatment. The objective was to evaluate the outcome of prednisolone therapy in reversing medication overuse at 3 months posttreatment in patients with MOH using prospective multicenter registry data. Prednisolone was prescribed to 59 out of 309 patients (19.1%) enrolled during this observational study period, with doses ranging from 10 to 40 mg/day for 5-14 days. Of these patients, 228 (73.8%) completed the 3-month follow-up period.
Key findings:
- The MOH reversal rates at 3 months postbaseline were 76% (31/41) in the prednisolone group and 57.8% (108/187) in the no prednisolone group (p = 0.034).
- The steroid effect remained significant (adjusted odds ratio, 2.78; 95% confidence interval 1.27-6.1, p = 0.010) after adjusting for the number of monthly headache days at baseline, mode of discontinuation of overused medication, use of early preventive medications, and the number of combined preventive medications.
The study had several strengths, including the multicenter collection of data, prospective follow-ups, and comprehensiveness of data acquisition. However, it also had significant limitations, such as the noninterventional, observational nature of the study, potential bias in steroid prescription (every doctor prescribed what they wanted), and heterogeneity in the patient population. Also, there were a variety of treatments, and they were not standardized. Further external validation may be necessary before generalizing the study results.
Despite these limitations, the results do suggest that prednisolone may be one part of a valid treatment option for patients with MOH. I suspect, if the proper studies are done, we will see that using a good preventive medication, with few adverse events, and with careful education of the patient, formal detoxification will not be necessary when treating many patients with MOH. This has been my experience with MOH treatment utilizing the newer anti-CGRP preventive medications, including the older monoclonal antibodies and the newer gepants.
Outcomes of Acute and Preventive Migraine Therapy Based on Patient Sex
I previously have addressed myths about migraine as they pertain to men and women. When I found an interesting study recently published in Cephalalgia investigating the effectiveness of calcitonin gene-related peptide receptor (CGRP-R) antagonists (gepants) for acute care and prevention of episodic migraine and CGRP monoclonal antibodies for preventive treatment of episodic and chronic migraine in men and women, I thought I would discuss it here.
The study’s aim was to discern if patient sex contributed to outcomes in each specific treatment arm. Female sex hormones have been recognized as factors in promoting migraine, and women show increased severity, persistence, and comorbidity in migraine profiles, and increased prevalence of migraine relative to men.
Gepants used for acute therapy (ubrogepant, rimegepant, zavegepant) and preventive therapy (atogepant, rimegepant) were studied in this trial. Erenumab, fremanezumab, galcanezumab, and eptinezumab are monoclonal antibodies that either sit on the CGRP receptor (erenumab) or inactivate the CGRP ligand (fremanezumab, galcanezumab, and eptinezumab) and are used for migraine prevention. CGRP-based therapies are not effective in all patients and understanding which patient groups respond preferentially could reduce trial and error in treatment selection. The effectiveness of treatments targeting CGRP or the CGRP receptor may not be uniform in men and women, highlighting the need for further research and understanding of CGRP neurobiology in both sexes.
Key findings:
- In the trial by Porreca et al: In women, the 3 gepants approved by the FDA for the acute care of migraine (ubrogepant, rimegepant, zavegepant) produced a statistically significant drug effect for the 2-hour pain freedom (2h-PF) endpoint, with an average drug effect of 9.5% (CI: 7.4 to 11.6) and an average number needed to treat (NNT) of 11.
- Men did not show statistically significant effects with the acute use of gepants. The average drug effect was 2.8%, and the average NNT was 36.
- For both men and women, CGRP-targeting therapies for prevention of migraine (the 4 monoclonal antibodies) were equally effective; however, possible sex differences remain uncertain and need further study.
- In patients with chronic migraine, CGRP/CGRP-R antibodies were similarly effective in both men and women.
- For the 2-hour freedom from most bothersome symptom (2h-MBS) endpoint when gepants were given acutely, the effects were much better in women than men, with an average drug effect of 10.2% and an average NNT of 10.
- In men, these medications produced observed treatment effects on 2h-MBS with an average drug effect of 3.2% and an average NNT of 32.
- In men, 5 out of 12 estimates favored placebo over the active treatment, suggesting a treatment with little to no effect.
- The pooled treatment effects for women were 3 times as large, at 9.2% and 10.2%, respectively.
- The placebo response rates for 2 of the 3 ubrogepant studies and one of 2 zavegepant studies were higher in men than in women.
The study concludes that, while small molecule CGRP-R antagonists are dramatically effective for acute therapy of migraine in women, available data do not demonstrate effectiveness in men. The treatment effect was found to always favor active treatment numerically for both men and women for prevention of episodic and chronic migraine. The data highlight possible differential effects of CGRP-targeted therapies in different patient populations and the need for increased understanding of CGRP neurobiology in men and women. The study also emphasizes the need to understand which patient groups preferentially respond to CGRP-based therapies to reduce trial and error in treatment. Note that rimegepant data on prevention were not available for analysis at the time of the writing.
It would be interesting to perform a meta-analysis of multiple well-done, large, real-world studies to see if the same differences and similarities are found in men versus women for acute care of migraine and prevention of episodic and chronic migraine. I suspect that we would find that acute care results favor women but that some men do well.
The Effectiveness of Prednisolone for Treating Medication Overuse Headache
I often discuss medication overuse headache (MOH), as it is difficult to diagnose and treat, so I wanted to comment on another pertinent study. It is a post hoc analysis of the Registry for Load and Management of Medication Overuse Headache (RELEASE). The RELEASE trial is an ongoing, multicenter, observational, cohort study of MOH that has been conducted in Korea since April 2020. Findings were recently published in Headache by Lee et al.
MOH is a secondary headache disorder that develops in patients with a preexisting primary headache when they overuse acute care headache medications of any type except gepants. This includes prescription medications such as triptans, ergots, butalbital-containing medications; opioids; aspirin; acetaminophen; any type of combination medication often containing caffeine; or a combination of medications. This condition significantly impacts patients’ quality of life and productivity, usually increasing the frequency of headaches per month and leading to higher healthcare-related costs.
Treating MOH is challenging due to the lack of high-quality drug trials specifically designed for MOH and doctor inexperience. Current evidence is based largely on subgroup analyses of drug trials for the treatment of chronic migraine that contain these patient types.
Withdrawal of acute care headache medications that are being overused has traditionally been considered an important aspect of MOH treatment, although this may be changing. Withdrawal symptoms, such as increased intensity of headache pain, frequency of headaches, and other symptoms like agitation and sleep disturbance, can prevent patients from discontinuing overused medications. Systemic corticosteroids are widely used to reduce these withdrawal headaches, but clinical trials are sparse and have failed to meet proper endpoints. Despite this, corticosteroids have shown potential benefits, such as decreasing withdrawal headaches, reducing the use of rescue medications, and lowering headache intensity at certain time points after treatment.
Given these findings, this published study hypothesized that prednisolone may play a role in converting MOH to non-MOH at 3 months after treatment. The objective was to evaluate the outcome of prednisolone therapy in reversing medication overuse at 3 months posttreatment in patients with MOH using prospective multicenter registry data. Prednisolone was prescribed to 59 out of 309 patients (19.1%) enrolled during this observational study period, with doses ranging from 10 to 40 mg/day for 5-14 days. Of these patients, 228 (73.8%) completed the 3-month follow-up period.
Key findings:
- The MOH reversal rates at 3 months postbaseline were 76% (31/41) in the prednisolone group and 57.8% (108/187) in the no prednisolone group (p = 0.034).
- The steroid effect remained significant (adjusted odds ratio, 2.78; 95% confidence interval 1.27-6.1, p = 0.010) after adjusting for the number of monthly headache days at baseline, mode of discontinuation of overused medication, use of early preventive medications, and the number of combined preventive medications.
The study had several strengths, including the multicenter collection of data, prospective follow-ups, and comprehensiveness of data acquisition. However, it also had significant limitations, such as the noninterventional, observational nature of the study, potential bias in steroid prescription (every doctor prescribed what they wanted), and heterogeneity in the patient population. Also, there were a variety of treatments, and they were not standardized. Further external validation may be necessary before generalizing the study results.
Despite these limitations, the results do suggest that prednisolone may be one part of a valid treatment option for patients with MOH. I suspect, if the proper studies are done, we will see that using a good preventive medication, with few adverse events, and with careful education of the patient, formal detoxification will not be necessary when treating many patients with MOH. This has been my experience with MOH treatment utilizing the newer anti-CGRP preventive medications, including the older monoclonal antibodies and the newer gepants.
Outcomes of Acute and Preventive Migraine Therapy Based on Patient Sex
I previously have addressed myths about migraine as they pertain to men and women. When I found an interesting study recently published in Cephalalgia investigating the effectiveness of calcitonin gene-related peptide receptor (CGRP-R) antagonists (gepants) for acute care and prevention of episodic migraine and CGRP monoclonal antibodies for preventive treatment of episodic and chronic migraine in men and women, I thought I would discuss it here.
The study’s aim was to discern if patient sex contributed to outcomes in each specific treatment arm. Female sex hormones have been recognized as factors in promoting migraine, and women show increased severity, persistence, and comorbidity in migraine profiles, and increased prevalence of migraine relative to men.
Gepants used for acute therapy (ubrogepant, rimegepant, zavegepant) and preventive therapy (atogepant, rimegepant) were studied in this trial. Erenumab, fremanezumab, galcanezumab, and eptinezumab are monoclonal antibodies that either sit on the CGRP receptor (erenumab) or inactivate the CGRP ligand (fremanezumab, galcanezumab, and eptinezumab) and are used for migraine prevention. CGRP-based therapies are not effective in all patients and understanding which patient groups respond preferentially could reduce trial and error in treatment selection. The effectiveness of treatments targeting CGRP or the CGRP receptor may not be uniform in men and women, highlighting the need for further research and understanding of CGRP neurobiology in both sexes.
Key findings:
- In the trial by Porreca et al: In women, the 3 gepants approved by the FDA for the acute care of migraine (ubrogepant, rimegepant, zavegepant) produced a statistically significant drug effect for the 2-hour pain freedom (2h-PF) endpoint, with an average drug effect of 9.5% (CI: 7.4 to 11.6) and an average number needed to treat (NNT) of 11.
- Men did not show statistically significant effects with the acute use of gepants. The average drug effect was 2.8%, and the average NNT was 36.
- For both men and women, CGRP-targeting therapies for prevention of migraine (the 4 monoclonal antibodies) were equally effective; however, possible sex differences remain uncertain and need further study.
- In patients with chronic migraine, CGRP/CGRP-R antibodies were similarly effective in both men and women.
- For the 2-hour freedom from most bothersome symptom (2h-MBS) endpoint when gepants were given acutely, the effects were much better in women than men, with an average drug effect of 10.2% and an average NNT of 10.
- In men, these medications produced observed treatment effects on 2h-MBS with an average drug effect of 3.2% and an average NNT of 32.
- In men, 5 out of 12 estimates favored placebo over the active treatment, suggesting a treatment with little to no effect.
- The pooled treatment effects for women were 3 times as large, at 9.2% and 10.2%, respectively.
- The placebo response rates for 2 of the 3 ubrogepant studies and one of 2 zavegepant studies were higher in men than in women.
The study concludes that, while small molecule CGRP-R antagonists are dramatically effective for acute therapy of migraine in women, available data do not demonstrate effectiveness in men. The treatment effect was found to always favor active treatment numerically for both men and women for prevention of episodic and chronic migraine. The data highlight possible differential effects of CGRP-targeted therapies in different patient populations and the need for increased understanding of CGRP neurobiology in men and women. The study also emphasizes the need to understand which patient groups preferentially respond to CGRP-based therapies to reduce trial and error in treatment. Note that rimegepant data on prevention were not available for analysis at the time of the writing.
It would be interesting to perform a meta-analysis of multiple well-done, large, real-world studies to see if the same differences and similarities are found in men versus women for acute care of migraine and prevention of episodic and chronic migraine. I suspect that we would find that acute care results favor women but that some men do well.
The Effectiveness of Prednisolone for Treating Medication Overuse Headache
I often discuss medication overuse headache (MOH), as it is difficult to diagnose and treat, so I wanted to comment on another pertinent study. It is a post hoc analysis of the Registry for Load and Management of Medication Overuse Headache (RELEASE). The RELEASE trial is an ongoing, multicenter, observational, cohort study of MOH that has been conducted in Korea since April 2020. Findings were recently published in Headache by Lee et al.
MOH is a secondary headache disorder that develops in patients with a preexisting primary headache when they overuse acute care headache medications of any type except gepants. This includes prescription medications such as triptans, ergots, butalbital-containing medications; opioids; aspirin; acetaminophen; any type of combination medication often containing caffeine; or a combination of medications. This condition significantly impacts patients’ quality of life and productivity, usually increasing the frequency of headaches per month and leading to higher healthcare-related costs.
Treating MOH is challenging due to the lack of high-quality drug trials specifically designed for MOH and doctor inexperience. Current evidence is based largely on subgroup analyses of drug trials for the treatment of chronic migraine that contain these patient types.
Withdrawal of acute care headache medications that are being overused has traditionally been considered an important aspect of MOH treatment, although this may be changing. Withdrawal symptoms, such as increased intensity of headache pain, frequency of headaches, and other symptoms like agitation and sleep disturbance, can prevent patients from discontinuing overused medications. Systemic corticosteroids are widely used to reduce these withdrawal headaches, but clinical trials are sparse and have failed to meet proper endpoints. Despite this, corticosteroids have shown potential benefits, such as decreasing withdrawal headaches, reducing the use of rescue medications, and lowering headache intensity at certain time points after treatment.
Given these findings, this published study hypothesized that prednisolone may play a role in converting MOH to non-MOH at 3 months after treatment. The objective was to evaluate the outcome of prednisolone therapy in reversing medication overuse at 3 months posttreatment in patients with MOH using prospective multicenter registry data. Prednisolone was prescribed to 59 out of 309 patients (19.1%) enrolled during this observational study period, with doses ranging from 10 to 40 mg/day for 5-14 days. Of these patients, 228 (73.8%) completed the 3-month follow-up period.
Key findings:
- The MOH reversal rates at 3 months postbaseline were 76% (31/41) in the prednisolone group and 57.8% (108/187) in the no prednisolone group (p = 0.034).
- The steroid effect remained significant (adjusted odds ratio, 2.78; 95% confidence interval 1.27-6.1, p = 0.010) after adjusting for the number of monthly headache days at baseline, mode of discontinuation of overused medication, use of early preventive medications, and the number of combined preventive medications.
The study had several strengths, including the multicenter collection of data, prospective follow-ups, and comprehensiveness of data acquisition. However, it also had significant limitations, such as the noninterventional, observational nature of the study, potential bias in steroid prescription (every doctor prescribed what they wanted), and heterogeneity in the patient population. Also, there were a variety of treatments, and they were not standardized. Further external validation may be necessary before generalizing the study results.
Despite these limitations, the results do suggest that prednisolone may be one part of a valid treatment option for patients with MOH. I suspect, if the proper studies are done, we will see that using a good preventive medication, with few adverse events, and with careful education of the patient, formal detoxification will not be necessary when treating many patients with MOH. This has been my experience with MOH treatment utilizing the newer anti-CGRP preventive medications, including the older monoclonal antibodies and the newer gepants.
Tender Dermal Nodule on the Temple
The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2
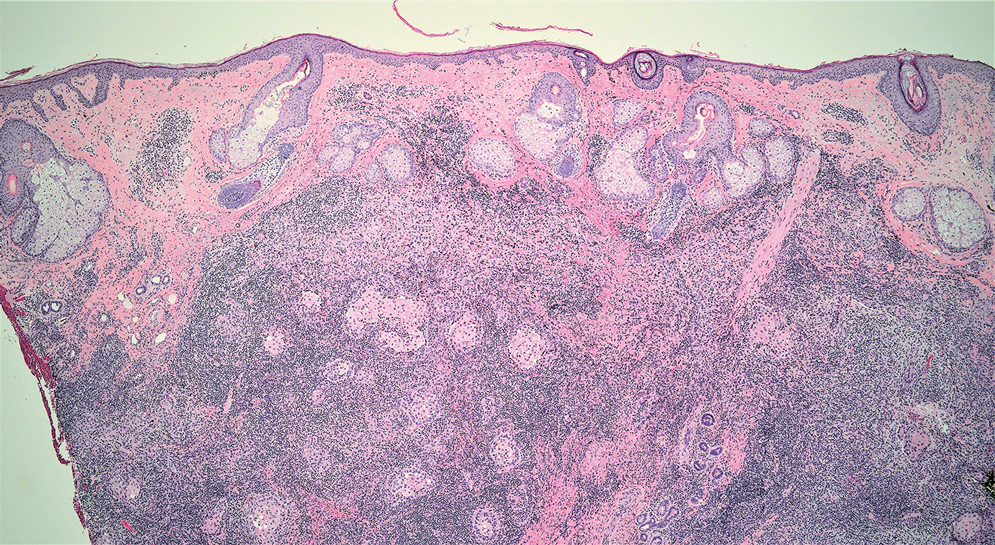
The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4
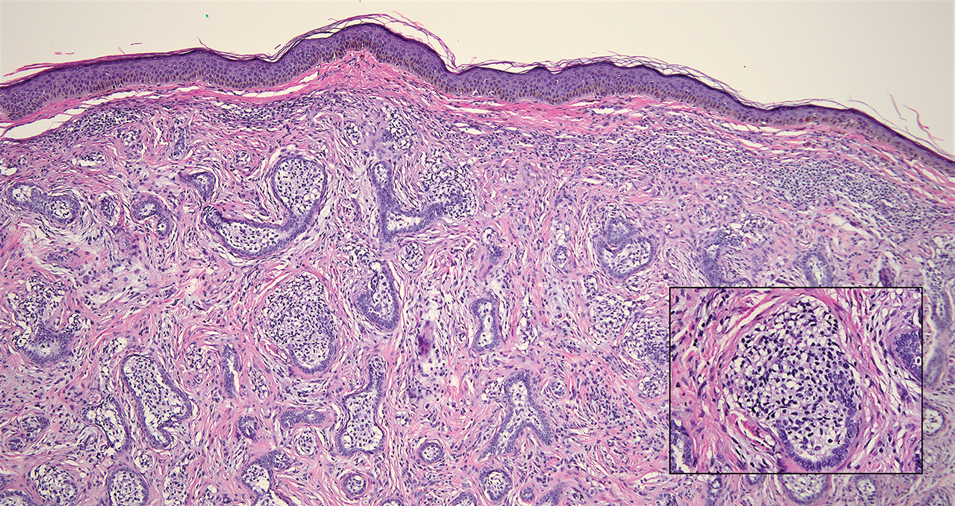
Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8
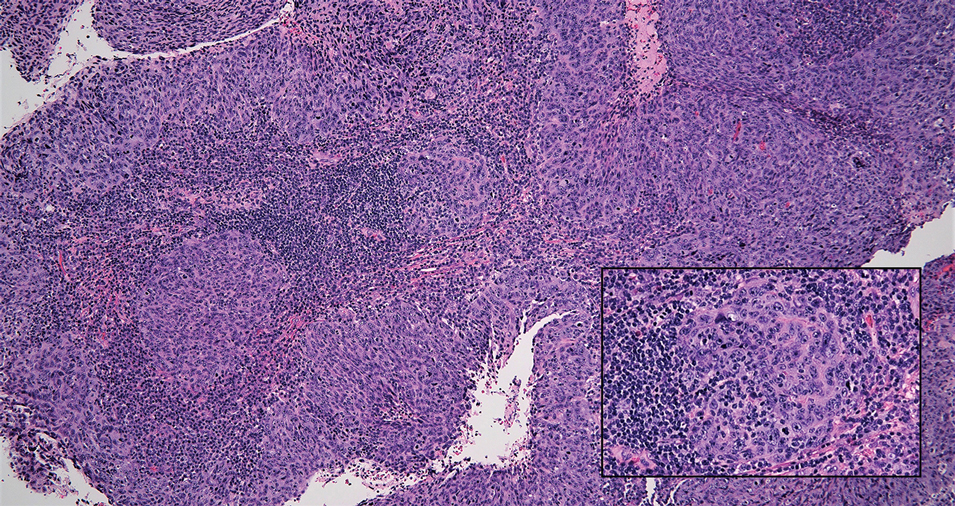
Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3
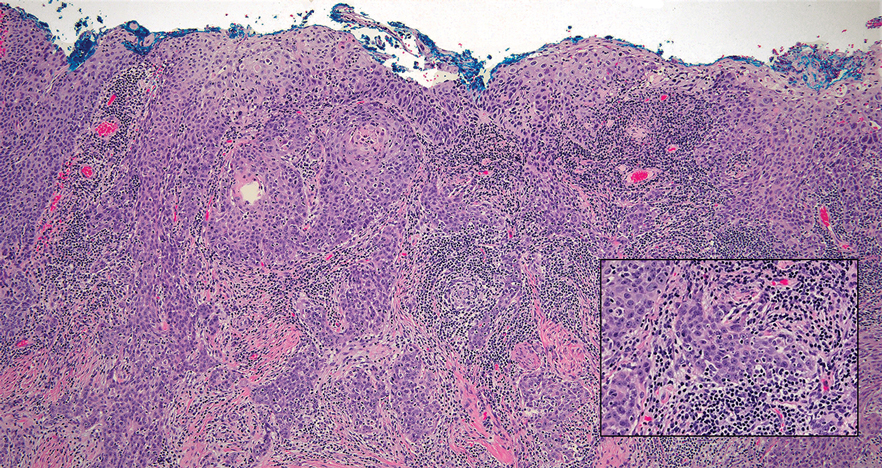
Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12
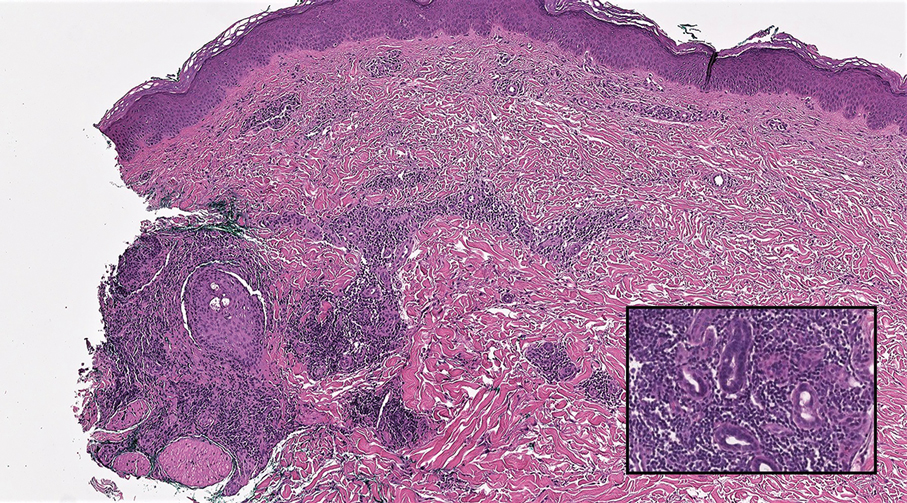
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2

The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4

Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8

Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3

Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12

The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2

The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4

Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8

Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3

Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12

- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
A 77-year-old man presented with a 1.2-cm dermal nodule on the left temple of 1 year’s duration. The lesion had become tender and darker in color. An excision was performed and submitted for histologic examination. Additional immunohistochemistry staining for Epstein-Barr virus was negative.
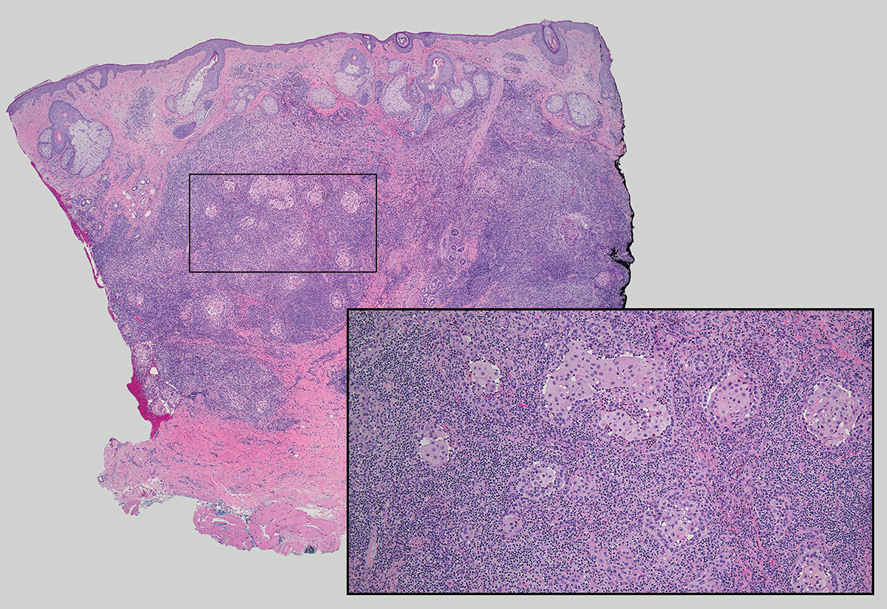
E-Consults in Dermatology: A Retrospective Analysis
Dermatologic conditions affect approximately one-third of individuals in the United States.1,2 Nearly 1 in 4 physician office visits in the United States are for skin conditions, and less than one-third of these visits are with dermatologists. Although many of these patients may prefer to see a dermatologist for their concerns, they may not be able to access specialist care.3 The limited supply and urban-focused distribution of dermatologists along with reduced acceptance of state-funded insurance plans and long appointment wait times all pose considerable challenges to individuals seeking dermatologic care.2 Electronic consultations (e-consults) have emerged as a promising solution to overcoming these barriers while providing high-quality dermatologic care to a large diverse patient population.2,4 Although e-consults can be of service to all dermatology patients, this modality may be especially beneficial to underserved populations, such as the uninsured and Medicaid patients—groups that historically have experienced limited access to dermatology care due to the low reimbursement rates and high administrative burdens accompanying care delivery.4 This limited access leads to inequity in care, as timely access to dermatology is associated with improved diagnostic accuracy and disease outcomes.3 E-consult implementation can facilitate timely access for these underserved populations and bypass additional barriers to care such as lack of transportation or time off work. Prior e-consult studies have demonstrated relatively high numbers of Medicaid patients utilizing e-consult services.3,5
Although in-person visits remain the gold standard for diagnosis and treatment of dermatologic conditions, e-consults placed by primary care providers (PCPs) can improve access and help triage patients who require in-person dermatology visits.6 In this study, we conducted a retrospective chart review to characterize the e-consults requested of the dermatology department at a large tertiary care medical center in Winston-Salem, North Carolina.
Methods
The electronic health record (EHR) of Atrium Health Wake Forest Baptist (Winston-Salem, North Carolina) was screened for eligible patients from January 1, 2020, to May 31, 2021. Patients—both adult (aged ≥18 years) and pediatric (aged <18 years)—were included if they underwent a dermatology e-consult within this time frame. Provider notes in the medical records were reviewed to determine the nature of the lesion, how long the dermatologist took to complete the e-consult, whether an in-person appointment was recommended, and whether the patient was seen by dermatology within 90 days of the e-consult. Institutional review board approval was obtained.
For each e-consult, the PCP obtained clinical photographs of the lesion in question either through the EHR mobile application or by having patients upload their own photographs directly to their medical records. The referring PCP then completed a brief template regarding the patient’s clinical question and medical history and then sent the completed information to the consulting dermatologist’s EHR inbox. From there, the dermatologist could view the clinical question, documented photographs, and patient medical record to create a brief consult note with recommendations. The note was then sent back via EHR to the PCP to follow up with the patient. Patients were not charged for the e-consult.
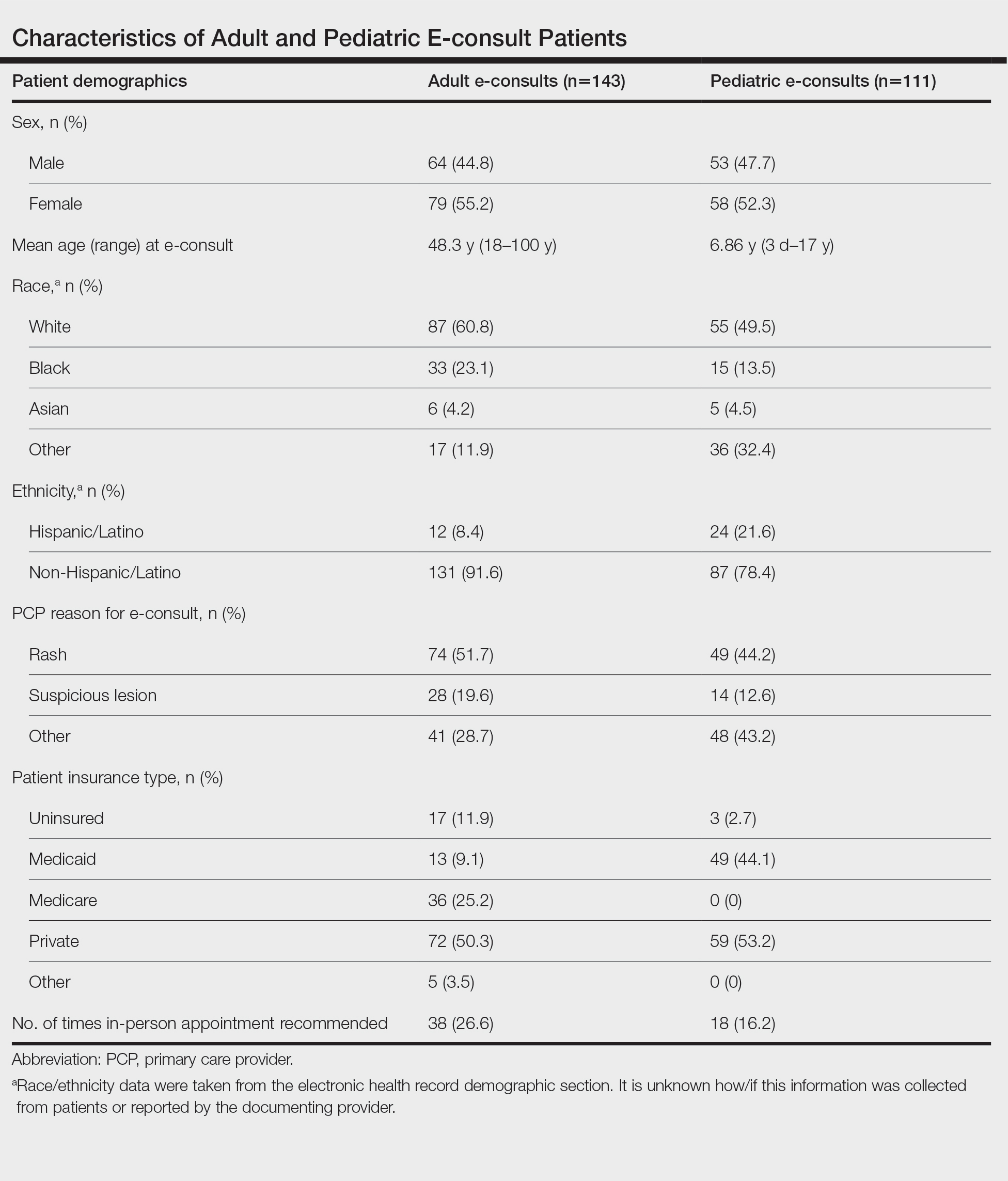
Results
Two hundred fifty-four dermatology e-consults were requested by providers at the study center (eTable), which included 252 unique patients (2 patients had 2 separate e-consults regarding different clinical questions). The median time for completion of the e-consult—from submission of the PCP’s e-consult request to dermatologist completion—was 0.37 days. Fifty-six patients (22.0%) were recommended for an in-person appointment (Figure), 33 (58.9%) of whom ultimately scheduled the in-person appointment, and the median length of time between the completion of the e-consult and the in-person appointment was 16.5 days. The remaining 198 patients (78.0%) were not triaged to receive an in-person appointment following the e-consult,but 2 patients (8.7%) were ultimately seen in-person anyway via other referral pathways, with a median length of 33 days between e-consult completion and the in-person appointment. One hundred seventy-six patients (69.8%) avoided an in-person dermatology visit, although 38 (21.6%) of those patients were fewer than 90 days out from their e-consults at the time of data collection. The 254 e-consults included patients from 50 different zip codes, 49 (98.0%) of which were in North Carolina.
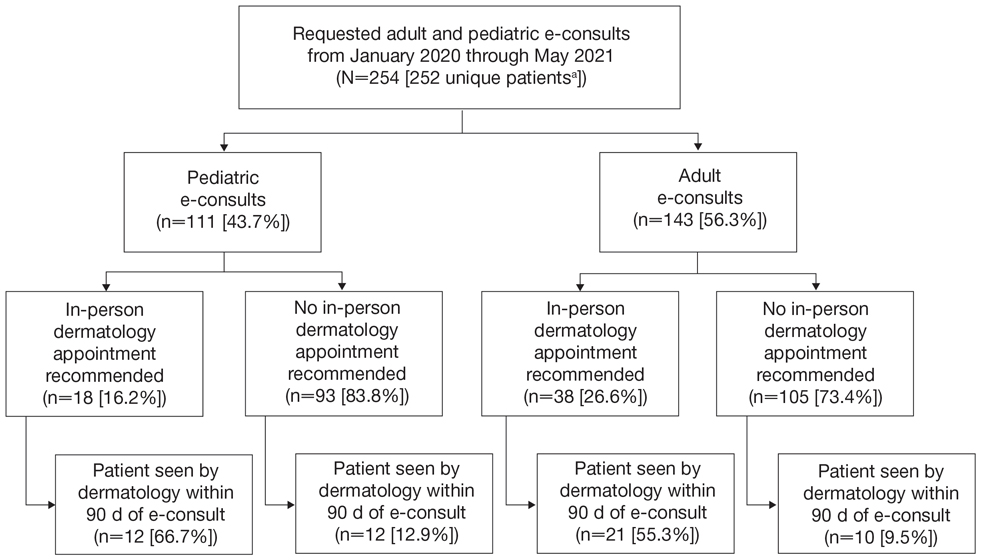
Comment
An e-consult is an asynchronous telehealth modality through which PCPs can request specialty evaluation to provide diagnostic and therapeutic guidance, facilitate PCP-specialist coordination of care, and increase access to specialty care with reduced wait times.7,8 Increased care access is especially important, as specialty referral can decrease overall health care expenditure; however, the demand for specialists often exceeds the availability.8 Our e-consult program drastically reduced the time from patients’ initial presentation at their PCP’s office to dermatologist recommendations for treatment or need for in-person dermatology follow-up.
In our analysis, patients were of different racial, ethnic, and socioeconomic backgrounds and lived across a variety of zip codes, predominantly in central and western North Carolina. Almost three-quarters of the patients resided in zip codes where the average income was less than the North Carolina median household income ($66,196).9 Additionally, 82 patients (32.3%) were uninsured or on Medicaid (eTable). These economically disadvantaged patient populations historically have had limited access to dermatologic care.4 One study showed that privately insured individuals were accepted as new patients by dermatologists 91% of the time compared to a 29.8% acceptance rate for publicly insured individuals.10 Uninsured and Medicaid patients also have to wait 34% longer for an appointment compared to individuals with Medicare or private insurance.2 Considering these patients may already be at an economic disadvantage when it comes to seeing and paying for dermatologic services, e-consults may reduce patient travel and appointment expenses while increasing access to specialty care. Based on a 2020 study, each e-consult generates an estimated savings of $80 out-of-pocket per patient per avoided in-person visit.11
In our study, the most common condition for an e-consult in both adult and pediatric patients was rash, which is consistent with prior e-consult studies.5,11 We found that most e-consult patients were not recommended for an in-person dermatology visit, and for those who were recommended to have an in-person visit, the wait time was reduced (Figure). These results corroborate that e-consults may be used as an important triage tool for determining whether a specialist appointment is indicated as well as a public health tool, as timely evaluation is associated with better dermatologic health care outcomes.3 However, the number of patients who did not present for an in-person appointment in our study may be overestimated, as 38 patients’ (21.6%) e-consults were conducted fewer than 90 days before our data collection. Although none of these patients had been seen in person, it is possible they requested an in-person visit after their medical records were reviewed for this study. Additionally, it is possible patients sought care from outside providers not documented in the EHR.
With regard to the payment model for the e-consult program, Atrium Health Wake Forest Baptist initially piloted the e-consult system through a partnership with the American Academy of Medical Colleges’ Project CORE: Coordinating Optimal Referral Experiences (https://www.aamc.org/what-we-do/mission-areas/health-care/project-core). Grant funding through Project CORE allowed both the referring PCP and the specialist completing the e-consult to each receive approximately 0.5 relative value units in payment for each consult completed. Based on early adoption successes, the institution has created additional internal funding to support the continued expansion of the e-consult system and is incentivized to continue funding, as proper utilization of e-consults improves patient access to timely specialist care, avoids no-shows or last-minute cancellations for specialist appointments, and decreases back-door access to specialist care through the emergency department and urgent care facilities.5 Although 0.5 relative value units is not equivalent compensation to an in-person office visit, our study showed that e-consults can be completed much more quickly and efficiently and do not utilize nursing staff or other office resources.
Conclusion
E-consults are an effective telehealth modality that can increase patients’ access to dermatologic specialty care.
Acknowledgments—The authors thank the Wake Forest University School of Medicine Department of Medical Education and Department of Dermatology (Winston-Salem, North Carolina) for their contributions to this research study as well as the Wake Forest Clinical and Translational Science Institute (Winston-Salem, North Carolina) for their help extracting EHR data.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Naka F, Lu J, Porto A, et al. Impact of dermatology econsults on access to care and skin cancer screening in underserved populations: a model for teledermatology services in community health centers. J Am Acad Dermatol. 2018;78:293-302.
- Mulcahy A, Mehrotra A, Edison K, et al. Variation in dermatologist visits by sociodemographic characteristics. J Am Acad Dermatol. 2017;76:918-924.
- Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81:758-764.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an econsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2020;83:1633-1638.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Parikh PJ, Mowrey C, Gallimore J, et al. Evaluating e-consultation implementations based on use and time-line across various specialties. Int J Med Inform. 2017;108:42-48.
- Wasfy JH, Rao SK, Kalwani N, et al. Longer-term impact of cardiology e-consults. Am Heart J. 2016;173:86-93.
- United States Census Bureau. QuickFacts: North Carolina; United States. Accessed February 26, 2024. https://www.census.gov/quickfacts/fact/table/NC,US/PST045222
- Alghothani L, Jacks SK, Vander Horst A, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
- Seiger K, Hawryluk EB, Kroshinsky D, et al. Pediatric dermatology econsults: reduced wait times and dermatology office visits. Pediatr Dermatol. 2020;37:804-810.
Dermatologic conditions affect approximately one-third of individuals in the United States.1,2 Nearly 1 in 4 physician office visits in the United States are for skin conditions, and less than one-third of these visits are with dermatologists. Although many of these patients may prefer to see a dermatologist for their concerns, they may not be able to access specialist care.3 The limited supply and urban-focused distribution of dermatologists along with reduced acceptance of state-funded insurance plans and long appointment wait times all pose considerable challenges to individuals seeking dermatologic care.2 Electronic consultations (e-consults) have emerged as a promising solution to overcoming these barriers while providing high-quality dermatologic care to a large diverse patient population.2,4 Although e-consults can be of service to all dermatology patients, this modality may be especially beneficial to underserved populations, such as the uninsured and Medicaid patients—groups that historically have experienced limited access to dermatology care due to the low reimbursement rates and high administrative burdens accompanying care delivery.4 This limited access leads to inequity in care, as timely access to dermatology is associated with improved diagnostic accuracy and disease outcomes.3 E-consult implementation can facilitate timely access for these underserved populations and bypass additional barriers to care such as lack of transportation or time off work. Prior e-consult studies have demonstrated relatively high numbers of Medicaid patients utilizing e-consult services.3,5
Although in-person visits remain the gold standard for diagnosis and treatment of dermatologic conditions, e-consults placed by primary care providers (PCPs) can improve access and help triage patients who require in-person dermatology visits.6 In this study, we conducted a retrospective chart review to characterize the e-consults requested of the dermatology department at a large tertiary care medical center in Winston-Salem, North Carolina.
Methods
The electronic health record (EHR) of Atrium Health Wake Forest Baptist (Winston-Salem, North Carolina) was screened for eligible patients from January 1, 2020, to May 31, 2021. Patients—both adult (aged ≥18 years) and pediatric (aged <18 years)—were included if they underwent a dermatology e-consult within this time frame. Provider notes in the medical records were reviewed to determine the nature of the lesion, how long the dermatologist took to complete the e-consult, whether an in-person appointment was recommended, and whether the patient was seen by dermatology within 90 days of the e-consult. Institutional review board approval was obtained.
For each e-consult, the PCP obtained clinical photographs of the lesion in question either through the EHR mobile application or by having patients upload their own photographs directly to their medical records. The referring PCP then completed a brief template regarding the patient’s clinical question and medical history and then sent the completed information to the consulting dermatologist’s EHR inbox. From there, the dermatologist could view the clinical question, documented photographs, and patient medical record to create a brief consult note with recommendations. The note was then sent back via EHR to the PCP to follow up with the patient. Patients were not charged for the e-consult.

Results
Two hundred fifty-four dermatology e-consults were requested by providers at the study center (eTable), which included 252 unique patients (2 patients had 2 separate e-consults regarding different clinical questions). The median time for completion of the e-consult—from submission of the PCP’s e-consult request to dermatologist completion—was 0.37 days. Fifty-six patients (22.0%) were recommended for an in-person appointment (Figure), 33 (58.9%) of whom ultimately scheduled the in-person appointment, and the median length of time between the completion of the e-consult and the in-person appointment was 16.5 days. The remaining 198 patients (78.0%) were not triaged to receive an in-person appointment following the e-consult,but 2 patients (8.7%) were ultimately seen in-person anyway via other referral pathways, with a median length of 33 days between e-consult completion and the in-person appointment. One hundred seventy-six patients (69.8%) avoided an in-person dermatology visit, although 38 (21.6%) of those patients were fewer than 90 days out from their e-consults at the time of data collection. The 254 e-consults included patients from 50 different zip codes, 49 (98.0%) of which were in North Carolina.

Comment
An e-consult is an asynchronous telehealth modality through which PCPs can request specialty evaluation to provide diagnostic and therapeutic guidance, facilitate PCP-specialist coordination of care, and increase access to specialty care with reduced wait times.7,8 Increased care access is especially important, as specialty referral can decrease overall health care expenditure; however, the demand for specialists often exceeds the availability.8 Our e-consult program drastically reduced the time from patients’ initial presentation at their PCP’s office to dermatologist recommendations for treatment or need for in-person dermatology follow-up.
In our analysis, patients were of different racial, ethnic, and socioeconomic backgrounds and lived across a variety of zip codes, predominantly in central and western North Carolina. Almost three-quarters of the patients resided in zip codes where the average income was less than the North Carolina median household income ($66,196).9 Additionally, 82 patients (32.3%) were uninsured or on Medicaid (eTable). These economically disadvantaged patient populations historically have had limited access to dermatologic care.4 One study showed that privately insured individuals were accepted as new patients by dermatologists 91% of the time compared to a 29.8% acceptance rate for publicly insured individuals.10 Uninsured and Medicaid patients also have to wait 34% longer for an appointment compared to individuals with Medicare or private insurance.2 Considering these patients may already be at an economic disadvantage when it comes to seeing and paying for dermatologic services, e-consults may reduce patient travel and appointment expenses while increasing access to specialty care. Based on a 2020 study, each e-consult generates an estimated savings of $80 out-of-pocket per patient per avoided in-person visit.11
In our study, the most common condition for an e-consult in both adult and pediatric patients was rash, which is consistent with prior e-consult studies.5,11 We found that most e-consult patients were not recommended for an in-person dermatology visit, and for those who were recommended to have an in-person visit, the wait time was reduced (Figure). These results corroborate that e-consults may be used as an important triage tool for determining whether a specialist appointment is indicated as well as a public health tool, as timely evaluation is associated with better dermatologic health care outcomes.3 However, the number of patients who did not present for an in-person appointment in our study may be overestimated, as 38 patients’ (21.6%) e-consults were conducted fewer than 90 days before our data collection. Although none of these patients had been seen in person, it is possible they requested an in-person visit after their medical records were reviewed for this study. Additionally, it is possible patients sought care from outside providers not documented in the EHR.
With regard to the payment model for the e-consult program, Atrium Health Wake Forest Baptist initially piloted the e-consult system through a partnership with the American Academy of Medical Colleges’ Project CORE: Coordinating Optimal Referral Experiences (https://www.aamc.org/what-we-do/mission-areas/health-care/project-core). Grant funding through Project CORE allowed both the referring PCP and the specialist completing the e-consult to each receive approximately 0.5 relative value units in payment for each consult completed. Based on early adoption successes, the institution has created additional internal funding to support the continued expansion of the e-consult system and is incentivized to continue funding, as proper utilization of e-consults improves patient access to timely specialist care, avoids no-shows or last-minute cancellations for specialist appointments, and decreases back-door access to specialist care through the emergency department and urgent care facilities.5 Although 0.5 relative value units is not equivalent compensation to an in-person office visit, our study showed that e-consults can be completed much more quickly and efficiently and do not utilize nursing staff or other office resources.
Conclusion
E-consults are an effective telehealth modality that can increase patients’ access to dermatologic specialty care.
Acknowledgments—The authors thank the Wake Forest University School of Medicine Department of Medical Education and Department of Dermatology (Winston-Salem, North Carolina) for their contributions to this research study as well as the Wake Forest Clinical and Translational Science Institute (Winston-Salem, North Carolina) for their help extracting EHR data.
Dermatologic conditions affect approximately one-third of individuals in the United States.1,2 Nearly 1 in 4 physician office visits in the United States are for skin conditions, and less than one-third of these visits are with dermatologists. Although many of these patients may prefer to see a dermatologist for their concerns, they may not be able to access specialist care.3 The limited supply and urban-focused distribution of dermatologists along with reduced acceptance of state-funded insurance plans and long appointment wait times all pose considerable challenges to individuals seeking dermatologic care.2 Electronic consultations (e-consults) have emerged as a promising solution to overcoming these barriers while providing high-quality dermatologic care to a large diverse patient population.2,4 Although e-consults can be of service to all dermatology patients, this modality may be especially beneficial to underserved populations, such as the uninsured and Medicaid patients—groups that historically have experienced limited access to dermatology care due to the low reimbursement rates and high administrative burdens accompanying care delivery.4 This limited access leads to inequity in care, as timely access to dermatology is associated with improved diagnostic accuracy and disease outcomes.3 E-consult implementation can facilitate timely access for these underserved populations and bypass additional barriers to care such as lack of transportation or time off work. Prior e-consult studies have demonstrated relatively high numbers of Medicaid patients utilizing e-consult services.3,5
Although in-person visits remain the gold standard for diagnosis and treatment of dermatologic conditions, e-consults placed by primary care providers (PCPs) can improve access and help triage patients who require in-person dermatology visits.6 In this study, we conducted a retrospective chart review to characterize the e-consults requested of the dermatology department at a large tertiary care medical center in Winston-Salem, North Carolina.
Methods
The electronic health record (EHR) of Atrium Health Wake Forest Baptist (Winston-Salem, North Carolina) was screened for eligible patients from January 1, 2020, to May 31, 2021. Patients—both adult (aged ≥18 years) and pediatric (aged <18 years)—were included if they underwent a dermatology e-consult within this time frame. Provider notes in the medical records were reviewed to determine the nature of the lesion, how long the dermatologist took to complete the e-consult, whether an in-person appointment was recommended, and whether the patient was seen by dermatology within 90 days of the e-consult. Institutional review board approval was obtained.
For each e-consult, the PCP obtained clinical photographs of the lesion in question either through the EHR mobile application or by having patients upload their own photographs directly to their medical records. The referring PCP then completed a brief template regarding the patient’s clinical question and medical history and then sent the completed information to the consulting dermatologist’s EHR inbox. From there, the dermatologist could view the clinical question, documented photographs, and patient medical record to create a brief consult note with recommendations. The note was then sent back via EHR to the PCP to follow up with the patient. Patients were not charged for the e-consult.

Results
Two hundred fifty-four dermatology e-consults were requested by providers at the study center (eTable), which included 252 unique patients (2 patients had 2 separate e-consults regarding different clinical questions). The median time for completion of the e-consult—from submission of the PCP’s e-consult request to dermatologist completion—was 0.37 days. Fifty-six patients (22.0%) were recommended for an in-person appointment (Figure), 33 (58.9%) of whom ultimately scheduled the in-person appointment, and the median length of time between the completion of the e-consult and the in-person appointment was 16.5 days. The remaining 198 patients (78.0%) were not triaged to receive an in-person appointment following the e-consult,but 2 patients (8.7%) were ultimately seen in-person anyway via other referral pathways, with a median length of 33 days between e-consult completion and the in-person appointment. One hundred seventy-six patients (69.8%) avoided an in-person dermatology visit, although 38 (21.6%) of those patients were fewer than 90 days out from their e-consults at the time of data collection. The 254 e-consults included patients from 50 different zip codes, 49 (98.0%) of which were in North Carolina.

Comment
An e-consult is an asynchronous telehealth modality through which PCPs can request specialty evaluation to provide diagnostic and therapeutic guidance, facilitate PCP-specialist coordination of care, and increase access to specialty care with reduced wait times.7,8 Increased care access is especially important, as specialty referral can decrease overall health care expenditure; however, the demand for specialists often exceeds the availability.8 Our e-consult program drastically reduced the time from patients’ initial presentation at their PCP’s office to dermatologist recommendations for treatment or need for in-person dermatology follow-up.
In our analysis, patients were of different racial, ethnic, and socioeconomic backgrounds and lived across a variety of zip codes, predominantly in central and western North Carolina. Almost three-quarters of the patients resided in zip codes where the average income was less than the North Carolina median household income ($66,196).9 Additionally, 82 patients (32.3%) were uninsured or on Medicaid (eTable). These economically disadvantaged patient populations historically have had limited access to dermatologic care.4 One study showed that privately insured individuals were accepted as new patients by dermatologists 91% of the time compared to a 29.8% acceptance rate for publicly insured individuals.10 Uninsured and Medicaid patients also have to wait 34% longer for an appointment compared to individuals with Medicare or private insurance.2 Considering these patients may already be at an economic disadvantage when it comes to seeing and paying for dermatologic services, e-consults may reduce patient travel and appointment expenses while increasing access to specialty care. Based on a 2020 study, each e-consult generates an estimated savings of $80 out-of-pocket per patient per avoided in-person visit.11
In our study, the most common condition for an e-consult in both adult and pediatric patients was rash, which is consistent with prior e-consult studies.5,11 We found that most e-consult patients were not recommended for an in-person dermatology visit, and for those who were recommended to have an in-person visit, the wait time was reduced (Figure). These results corroborate that e-consults may be used as an important triage tool for determining whether a specialist appointment is indicated as well as a public health tool, as timely evaluation is associated with better dermatologic health care outcomes.3 However, the number of patients who did not present for an in-person appointment in our study may be overestimated, as 38 patients’ (21.6%) e-consults were conducted fewer than 90 days before our data collection. Although none of these patients had been seen in person, it is possible they requested an in-person visit after their medical records were reviewed for this study. Additionally, it is possible patients sought care from outside providers not documented in the EHR.
With regard to the payment model for the e-consult program, Atrium Health Wake Forest Baptist initially piloted the e-consult system through a partnership with the American Academy of Medical Colleges’ Project CORE: Coordinating Optimal Referral Experiences (https://www.aamc.org/what-we-do/mission-areas/health-care/project-core). Grant funding through Project CORE allowed both the referring PCP and the specialist completing the e-consult to each receive approximately 0.5 relative value units in payment for each consult completed. Based on early adoption successes, the institution has created additional internal funding to support the continued expansion of the e-consult system and is incentivized to continue funding, as proper utilization of e-consults improves patient access to timely specialist care, avoids no-shows or last-minute cancellations for specialist appointments, and decreases back-door access to specialist care through the emergency department and urgent care facilities.5 Although 0.5 relative value units is not equivalent compensation to an in-person office visit, our study showed that e-consults can be completed much more quickly and efficiently and do not utilize nursing staff or other office resources.
Conclusion
E-consults are an effective telehealth modality that can increase patients’ access to dermatologic specialty care.
Acknowledgments—The authors thank the Wake Forest University School of Medicine Department of Medical Education and Department of Dermatology (Winston-Salem, North Carolina) for their contributions to this research study as well as the Wake Forest Clinical and Translational Science Institute (Winston-Salem, North Carolina) for their help extracting EHR data.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Naka F, Lu J, Porto A, et al. Impact of dermatology econsults on access to care and skin cancer screening in underserved populations: a model for teledermatology services in community health centers. J Am Acad Dermatol. 2018;78:293-302.
- Mulcahy A, Mehrotra A, Edison K, et al. Variation in dermatologist visits by sociodemographic characteristics. J Am Acad Dermatol. 2017;76:918-924.
- Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81:758-764.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an econsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2020;83:1633-1638.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Parikh PJ, Mowrey C, Gallimore J, et al. Evaluating e-consultation implementations based on use and time-line across various specialties. Int J Med Inform. 2017;108:42-48.
- Wasfy JH, Rao SK, Kalwani N, et al. Longer-term impact of cardiology e-consults. Am Heart J. 2016;173:86-93.
- United States Census Bureau. QuickFacts: North Carolina; United States. Accessed February 26, 2024. https://www.census.gov/quickfacts/fact/table/NC,US/PST045222
- Alghothani L, Jacks SK, Vander Horst A, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
- Seiger K, Hawryluk EB, Kroshinsky D, et al. Pediatric dermatology econsults: reduced wait times and dermatology office visits. Pediatr Dermatol. 2020;37:804-810.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Naka F, Lu J, Porto A, et al. Impact of dermatology econsults on access to care and skin cancer screening in underserved populations: a model for teledermatology services in community health centers. J Am Acad Dermatol. 2018;78:293-302.
- Mulcahy A, Mehrotra A, Edison K, et al. Variation in dermatologist visits by sociodemographic characteristics. J Am Acad Dermatol. 2017;76:918-924.
- Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81:758-764.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an econsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2020;83:1633-1638.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Parikh PJ, Mowrey C, Gallimore J, et al. Evaluating e-consultation implementations based on use and time-line across various specialties. Int J Med Inform. 2017;108:42-48.
- Wasfy JH, Rao SK, Kalwani N, et al. Longer-term impact of cardiology e-consults. Am Heart J. 2016;173:86-93.
- United States Census Bureau. QuickFacts: North Carolina; United States. Accessed February 26, 2024. https://www.census.gov/quickfacts/fact/table/NC,US/PST045222
- Alghothani L, Jacks SK, Vander Horst A, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
- Seiger K, Hawryluk EB, Kroshinsky D, et al. Pediatric dermatology econsults: reduced wait times and dermatology office visits. Pediatr Dermatol. 2020;37:804-810.
Practice Points
- Most electronic consult patients may be able to avoid in-person dermatology appointments.
- E-consults can increase patient access to dermatologic specialty care.
Commentary: PPI Dosing, Biomarkers, and Eating Behaviors in Patients With EoE, March 2024
This study provides compelling evidence that a twice-daily dosing regimen of moderate-dose proton pump inhibitors (PPIs) is superior to a once-daily regimen for inducing histologic remission in eosinophilic esophagitis (EoE). This finding suggests a significant paradigm shift in EoE management, challenging the current standard treatment guideline that recommends a PPI trial of 20-40 mg twice daily. The limited data on various dosing regimens for EoE treatment underscores the importance of this research. Dr Muftah and colleagues from Brigham and Women's Hospital have conducted a novel retrospective cohort study to address the question: Does a twice-daily PPI dose induce a higher remission rate in EoE than a once-daily regimen does regardless of the total daily dose?
The study enrolled adult patients with newly-diagnosed treatment-naive EoE at a tertiary care center, dividing participants into four groups on the basis of their treatment regimen: once-daily standard dose (20 mg omeprazole), once-daily moderate dose (40 mg), twice-daily moderate dose (20 mg), and twice-daily high dose (40 mg). Patients underwent endoscopy 8-12 weeks after initiating PPI treatment, with the primary outcome being the histologic response to PPI, defined as fewer than 15 eosinophils/high power field in repeat esophageal biopsies.
Out of 305 patients (54.6% men, mean age 44.7 ± 16.7 years), 42.3% achieved a histologic response to PPI treatment. Patients receiving the standard PPI dose (20 mg omeprazole once daily) vs those on twice-daily moderate and high doses showed significantly higher histologic response rates (52.8% vs 11.8%, P < .0001; and 54.3% vs 11.8%, P < .0001; respectively). Multivariable analysis revealed that twice-daily moderate and high doses were significantly more effective (adjusted odds ration [aOR] 6.75; CI 2.53-18.0, P = .0008; and aOR 12.8, CI 4.69-34.8, P < .001; respectively).
However, the study's retrospective design limits its ability to establish causality and may introduce selection bias. In addition, the lack of specified adjustments for PPI dosing based on diet and lifestyle factors across the cohort could influence treatment response and outcomes. Last, as a single-center study, the results may not generalize across diverse patient populations, particularly those with different demographics or disease severities.
This research heralds a shift toward a more effective treatment strategy in EoE management, suggesting that a twice-daily PPI regimen may be more beneficial than once-daily dosing is for inducing histologic remission, especially in patients inadequately responding to once-daily PPI treatment. It advocates for a personalized treatment approach, considering factors such as symptom severity, previous PPI response, and potential for adherence to a twice-daily regimen.
Distinguishing between inflammatory bowel disease (IBD)–induced eosinophilia and EoE poses a significant challenge for clinicians. Given that the incidence of EoE is 3-5 times higher in patients with IBD compared with the general population, there is a pressing need for new biomarkers to differentiate between these two conditions. In response to this need, Dr Butzke and colleagues at Nemours Children's Health in Wilmington, Delaware, conducted a retrospective study to evaluate the roles of Major Basic Protein (MBP) and interleukin (IL)-13 in distinguishing these diseases. The study included participants who underwent esophagogastroduodenoscopy with esophageal biopsies for IBD workup or suspicion of EoE. It comprised 27 patients with EoE-IBD, 39 with EoE, 29 with IBD eosinophilia, 30 with IBD only, and 30 control patients. The biopsies were stained with MBP and IL-13 antibodies, and the results (percent staining/total tissue area), demographic, and clinical findings were compared among the groups.
The study revealed that MBP staining levels among patients with EoE-IBD were 3.8 units, which is significantly lower than those in the EoE group at 52.8 units and higher than those with IBD eosinophilia at 0.2 units (P < .001). IL-13 expression was significantly higher only compared with the IBD and control groups and not with EoE-IBD or IBD eosinophilia. MBP predicted EoE with 100% sensitivity and 99% specificity, whereas IL-13 demonstrated 83% sensitivity and 90% specificity using a cutoff point from the cohort of patients without EoE-IBD. Based on the MBP cutoff point of 3.49 units that distinguished between EoE and non-EoE cases, 100% of patients with EoE were MBP-positive compared with 3% of patients with IBD-associated eosinophilia (P < .05).
To implement this new biomarker into clinical practice, guidelines for interpreting MBP staining results should be developed and established, including defining cutoff points for positive and negative results. However, this study faces several limitations, such as not evaluating the differences in MBP results based on EoE-IBD type and disease activity. The retrospective nature of the study and its small sample size limit its power. In addition, the study did not assess how different treatments and disease activity affect MBP levels nor did it address the lack of longitudinal evaluation in assessing MBP levels.
Despite these limitations, the study presents a compelling case for the use of MBP as a biomarker to distinguish true EoE from EoE-IBD. This differentiation is crucial because it can guide therapeutic approaches, influencing medication choices and dietary interventions. MBP shows promise as an excellent biomarker for distinguishing true EoE from eosinophilia caused by IBD. When combined with endoscopic and histologic changes, MBP can assist with the diagnosis of EoE in IBD patients, thereby reducing the possibility of misdiagnosis.
Being diagnosed with EoE poses a challenging and life-altering experience for patients and their families. They face numerous challenges, from undergoing diagnostic procedures and treatments to adapting daily diets. Limited information is available on the eating habits of patients diagnosed with EoE. In this study, Dr Kennedy and colleagues explored how a diagnosis of EoE affects eating behaviors among pediatric patients.
The researchers conducted a prospective study involving 27 patients diagnosed with EoE and compared their eating behaviors to those of 25 healthy control participants. The participants were evaluated on the basis of their responses to four food textures (puree, soft solid, chewable, and hard solid), focusing on the number of chews per bite, sips of fluid per food, and consumption time.
The study found that, on average, patients with EoE (63.5% boys, mean age 11 years) required more chews per bite across several food textures (soft solid P = .031; chewable P = .047; and hard solid P = .037) and demonstrated increased consumption time for soft solid (P = .002), chewable (P = .005), and hard solid foods (P = .034) compared to healthy controls. In addition, endoscopic reference scores positively correlated with consumption time (r = 0.53; P = .008) and the number of chews (r = 0.45; P = .027) for chewable foods as well as with the number of chews (r = 0.44; P = .043) for hard solid foods. Increased consumption time also correlated with increased eosinophil counts (r = 0.42; P = .050) and decreased esophageal distensibility (r = -0.82; P < .0001).
Though these findings open promising avenues for the noninvasive assessment and personalized management of EoE, further research with larger, longitudinal studies is essential to validate these behaviors as reliable clinical biomarkers. Increasing the sample size would enhance the study's power and broaden the generalizability of its findings to a wider pediatric EoE population. The study's cross-sectional nature limits the ability to assess how eating behaviors change over time with treatment or disease progression.
This study underscores the potential of eating behaviors as clinical markers for pediatric patients with EoE, enabling early identification through increased chewing and consumption times, especially with harder textures. Such markers could prompt diagnostic evaluations in settings where endoscopy and biopsy are gold standards for diagnosing EoE. Moreover, eating patterns could assist in monitoring disease activity and progression, offering a noninvasive means of assessing disease status and response to therapy, thus allowing for more frequent assessments of disease status without the need for invasive procedures. Understanding these behaviors allows healthcare providers to tailor dietary advice and interventions, potentially enhancing treatment compliance and improving the quality of life for pediatric patients with EoE.
This study provides compelling evidence that a twice-daily dosing regimen of moderate-dose proton pump inhibitors (PPIs) is superior to a once-daily regimen for inducing histologic remission in eosinophilic esophagitis (EoE). This finding suggests a significant paradigm shift in EoE management, challenging the current standard treatment guideline that recommends a PPI trial of 20-40 mg twice daily. The limited data on various dosing regimens for EoE treatment underscores the importance of this research. Dr Muftah and colleagues from Brigham and Women's Hospital have conducted a novel retrospective cohort study to address the question: Does a twice-daily PPI dose induce a higher remission rate in EoE than a once-daily regimen does regardless of the total daily dose?
The study enrolled adult patients with newly-diagnosed treatment-naive EoE at a tertiary care center, dividing participants into four groups on the basis of their treatment regimen: once-daily standard dose (20 mg omeprazole), once-daily moderate dose (40 mg), twice-daily moderate dose (20 mg), and twice-daily high dose (40 mg). Patients underwent endoscopy 8-12 weeks after initiating PPI treatment, with the primary outcome being the histologic response to PPI, defined as fewer than 15 eosinophils/high power field in repeat esophageal biopsies.
Out of 305 patients (54.6% men, mean age 44.7 ± 16.7 years), 42.3% achieved a histologic response to PPI treatment. Patients receiving the standard PPI dose (20 mg omeprazole once daily) vs those on twice-daily moderate and high doses showed significantly higher histologic response rates (52.8% vs 11.8%, P < .0001; and 54.3% vs 11.8%, P < .0001; respectively). Multivariable analysis revealed that twice-daily moderate and high doses were significantly more effective (adjusted odds ration [aOR] 6.75; CI 2.53-18.0, P = .0008; and aOR 12.8, CI 4.69-34.8, P < .001; respectively).
However, the study's retrospective design limits its ability to establish causality and may introduce selection bias. In addition, the lack of specified adjustments for PPI dosing based on diet and lifestyle factors across the cohort could influence treatment response and outcomes. Last, as a single-center study, the results may not generalize across diverse patient populations, particularly those with different demographics or disease severities.
This research heralds a shift toward a more effective treatment strategy in EoE management, suggesting that a twice-daily PPI regimen may be more beneficial than once-daily dosing is for inducing histologic remission, especially in patients inadequately responding to once-daily PPI treatment. It advocates for a personalized treatment approach, considering factors such as symptom severity, previous PPI response, and potential for adherence to a twice-daily regimen.
Distinguishing between inflammatory bowel disease (IBD)–induced eosinophilia and EoE poses a significant challenge for clinicians. Given that the incidence of EoE is 3-5 times higher in patients with IBD compared with the general population, there is a pressing need for new biomarkers to differentiate between these two conditions. In response to this need, Dr Butzke and colleagues at Nemours Children's Health in Wilmington, Delaware, conducted a retrospective study to evaluate the roles of Major Basic Protein (MBP) and interleukin (IL)-13 in distinguishing these diseases. The study included participants who underwent esophagogastroduodenoscopy with esophageal biopsies for IBD workup or suspicion of EoE. It comprised 27 patients with EoE-IBD, 39 with EoE, 29 with IBD eosinophilia, 30 with IBD only, and 30 control patients. The biopsies were stained with MBP and IL-13 antibodies, and the results (percent staining/total tissue area), demographic, and clinical findings were compared among the groups.
The study revealed that MBP staining levels among patients with EoE-IBD were 3.8 units, which is significantly lower than those in the EoE group at 52.8 units and higher than those with IBD eosinophilia at 0.2 units (P < .001). IL-13 expression was significantly higher only compared with the IBD and control groups and not with EoE-IBD or IBD eosinophilia. MBP predicted EoE with 100% sensitivity and 99% specificity, whereas IL-13 demonstrated 83% sensitivity and 90% specificity using a cutoff point from the cohort of patients without EoE-IBD. Based on the MBP cutoff point of 3.49 units that distinguished between EoE and non-EoE cases, 100% of patients with EoE were MBP-positive compared with 3% of patients with IBD-associated eosinophilia (P < .05).
To implement this new biomarker into clinical practice, guidelines for interpreting MBP staining results should be developed and established, including defining cutoff points for positive and negative results. However, this study faces several limitations, such as not evaluating the differences in MBP results based on EoE-IBD type and disease activity. The retrospective nature of the study and its small sample size limit its power. In addition, the study did not assess how different treatments and disease activity affect MBP levels nor did it address the lack of longitudinal evaluation in assessing MBP levels.
Despite these limitations, the study presents a compelling case for the use of MBP as a biomarker to distinguish true EoE from EoE-IBD. This differentiation is crucial because it can guide therapeutic approaches, influencing medication choices and dietary interventions. MBP shows promise as an excellent biomarker for distinguishing true EoE from eosinophilia caused by IBD. When combined with endoscopic and histologic changes, MBP can assist with the diagnosis of EoE in IBD patients, thereby reducing the possibility of misdiagnosis.
Being diagnosed with EoE poses a challenging and life-altering experience for patients and their families. They face numerous challenges, from undergoing diagnostic procedures and treatments to adapting daily diets. Limited information is available on the eating habits of patients diagnosed with EoE. In this study, Dr Kennedy and colleagues explored how a diagnosis of EoE affects eating behaviors among pediatric patients.
The researchers conducted a prospective study involving 27 patients diagnosed with EoE and compared their eating behaviors to those of 25 healthy control participants. The participants were evaluated on the basis of their responses to four food textures (puree, soft solid, chewable, and hard solid), focusing on the number of chews per bite, sips of fluid per food, and consumption time.
The study found that, on average, patients with EoE (63.5% boys, mean age 11 years) required more chews per bite across several food textures (soft solid P = .031; chewable P = .047; and hard solid P = .037) and demonstrated increased consumption time for soft solid (P = .002), chewable (P = .005), and hard solid foods (P = .034) compared to healthy controls. In addition, endoscopic reference scores positively correlated with consumption time (r = 0.53; P = .008) and the number of chews (r = 0.45; P = .027) for chewable foods as well as with the number of chews (r = 0.44; P = .043) for hard solid foods. Increased consumption time also correlated with increased eosinophil counts (r = 0.42; P = .050) and decreased esophageal distensibility (r = -0.82; P < .0001).
Though these findings open promising avenues for the noninvasive assessment and personalized management of EoE, further research with larger, longitudinal studies is essential to validate these behaviors as reliable clinical biomarkers. Increasing the sample size would enhance the study's power and broaden the generalizability of its findings to a wider pediatric EoE population. The study's cross-sectional nature limits the ability to assess how eating behaviors change over time with treatment or disease progression.
This study underscores the potential of eating behaviors as clinical markers for pediatric patients with EoE, enabling early identification through increased chewing and consumption times, especially with harder textures. Such markers could prompt diagnostic evaluations in settings where endoscopy and biopsy are gold standards for diagnosing EoE. Moreover, eating patterns could assist in monitoring disease activity and progression, offering a noninvasive means of assessing disease status and response to therapy, thus allowing for more frequent assessments of disease status without the need for invasive procedures. Understanding these behaviors allows healthcare providers to tailor dietary advice and interventions, potentially enhancing treatment compliance and improving the quality of life for pediatric patients with EoE.
This study provides compelling evidence that a twice-daily dosing regimen of moderate-dose proton pump inhibitors (PPIs) is superior to a once-daily regimen for inducing histologic remission in eosinophilic esophagitis (EoE). This finding suggests a significant paradigm shift in EoE management, challenging the current standard treatment guideline that recommends a PPI trial of 20-40 mg twice daily. The limited data on various dosing regimens for EoE treatment underscores the importance of this research. Dr Muftah and colleagues from Brigham and Women's Hospital have conducted a novel retrospective cohort study to address the question: Does a twice-daily PPI dose induce a higher remission rate in EoE than a once-daily regimen does regardless of the total daily dose?
The study enrolled adult patients with newly-diagnosed treatment-naive EoE at a tertiary care center, dividing participants into four groups on the basis of their treatment regimen: once-daily standard dose (20 mg omeprazole), once-daily moderate dose (40 mg), twice-daily moderate dose (20 mg), and twice-daily high dose (40 mg). Patients underwent endoscopy 8-12 weeks after initiating PPI treatment, with the primary outcome being the histologic response to PPI, defined as fewer than 15 eosinophils/high power field in repeat esophageal biopsies.
Out of 305 patients (54.6% men, mean age 44.7 ± 16.7 years), 42.3% achieved a histologic response to PPI treatment. Patients receiving the standard PPI dose (20 mg omeprazole once daily) vs those on twice-daily moderate and high doses showed significantly higher histologic response rates (52.8% vs 11.8%, P < .0001; and 54.3% vs 11.8%, P < .0001; respectively). Multivariable analysis revealed that twice-daily moderate and high doses were significantly more effective (adjusted odds ration [aOR] 6.75; CI 2.53-18.0, P = .0008; and aOR 12.8, CI 4.69-34.8, P < .001; respectively).
However, the study's retrospective design limits its ability to establish causality and may introduce selection bias. In addition, the lack of specified adjustments for PPI dosing based on diet and lifestyle factors across the cohort could influence treatment response and outcomes. Last, as a single-center study, the results may not generalize across diverse patient populations, particularly those with different demographics or disease severities.
This research heralds a shift toward a more effective treatment strategy in EoE management, suggesting that a twice-daily PPI regimen may be more beneficial than once-daily dosing is for inducing histologic remission, especially in patients inadequately responding to once-daily PPI treatment. It advocates for a personalized treatment approach, considering factors such as symptom severity, previous PPI response, and potential for adherence to a twice-daily regimen.
Distinguishing between inflammatory bowel disease (IBD)–induced eosinophilia and EoE poses a significant challenge for clinicians. Given that the incidence of EoE is 3-5 times higher in patients with IBD compared with the general population, there is a pressing need for new biomarkers to differentiate between these two conditions. In response to this need, Dr Butzke and colleagues at Nemours Children's Health in Wilmington, Delaware, conducted a retrospective study to evaluate the roles of Major Basic Protein (MBP) and interleukin (IL)-13 in distinguishing these diseases. The study included participants who underwent esophagogastroduodenoscopy with esophageal biopsies for IBD workup or suspicion of EoE. It comprised 27 patients with EoE-IBD, 39 with EoE, 29 with IBD eosinophilia, 30 with IBD only, and 30 control patients. The biopsies were stained with MBP and IL-13 antibodies, and the results (percent staining/total tissue area), demographic, and clinical findings were compared among the groups.
The study revealed that MBP staining levels among patients with EoE-IBD were 3.8 units, which is significantly lower than those in the EoE group at 52.8 units and higher than those with IBD eosinophilia at 0.2 units (P < .001). IL-13 expression was significantly higher only compared with the IBD and control groups and not with EoE-IBD or IBD eosinophilia. MBP predicted EoE with 100% sensitivity and 99% specificity, whereas IL-13 demonstrated 83% sensitivity and 90% specificity using a cutoff point from the cohort of patients without EoE-IBD. Based on the MBP cutoff point of 3.49 units that distinguished between EoE and non-EoE cases, 100% of patients with EoE were MBP-positive compared with 3% of patients with IBD-associated eosinophilia (P < .05).
To implement this new biomarker into clinical practice, guidelines for interpreting MBP staining results should be developed and established, including defining cutoff points for positive and negative results. However, this study faces several limitations, such as not evaluating the differences in MBP results based on EoE-IBD type and disease activity. The retrospective nature of the study and its small sample size limit its power. In addition, the study did not assess how different treatments and disease activity affect MBP levels nor did it address the lack of longitudinal evaluation in assessing MBP levels.
Despite these limitations, the study presents a compelling case for the use of MBP as a biomarker to distinguish true EoE from EoE-IBD. This differentiation is crucial because it can guide therapeutic approaches, influencing medication choices and dietary interventions. MBP shows promise as an excellent biomarker for distinguishing true EoE from eosinophilia caused by IBD. When combined with endoscopic and histologic changes, MBP can assist with the diagnosis of EoE in IBD patients, thereby reducing the possibility of misdiagnosis.
Being diagnosed with EoE poses a challenging and life-altering experience for patients and their families. They face numerous challenges, from undergoing diagnostic procedures and treatments to adapting daily diets. Limited information is available on the eating habits of patients diagnosed with EoE. In this study, Dr Kennedy and colleagues explored how a diagnosis of EoE affects eating behaviors among pediatric patients.
The researchers conducted a prospective study involving 27 patients diagnosed with EoE and compared their eating behaviors to those of 25 healthy control participants. The participants were evaluated on the basis of their responses to four food textures (puree, soft solid, chewable, and hard solid), focusing on the number of chews per bite, sips of fluid per food, and consumption time.
The study found that, on average, patients with EoE (63.5% boys, mean age 11 years) required more chews per bite across several food textures (soft solid P = .031; chewable P = .047; and hard solid P = .037) and demonstrated increased consumption time for soft solid (P = .002), chewable (P = .005), and hard solid foods (P = .034) compared to healthy controls. In addition, endoscopic reference scores positively correlated with consumption time (r = 0.53; P = .008) and the number of chews (r = 0.45; P = .027) for chewable foods as well as with the number of chews (r = 0.44; P = .043) for hard solid foods. Increased consumption time also correlated with increased eosinophil counts (r = 0.42; P = .050) and decreased esophageal distensibility (r = -0.82; P < .0001).
Though these findings open promising avenues for the noninvasive assessment and personalized management of EoE, further research with larger, longitudinal studies is essential to validate these behaviors as reliable clinical biomarkers. Increasing the sample size would enhance the study's power and broaden the generalizability of its findings to a wider pediatric EoE population. The study's cross-sectional nature limits the ability to assess how eating behaviors change over time with treatment or disease progression.
This study underscores the potential of eating behaviors as clinical markers for pediatric patients with EoE, enabling early identification through increased chewing and consumption times, especially with harder textures. Such markers could prompt diagnostic evaluations in settings where endoscopy and biopsy are gold standards for diagnosing EoE. Moreover, eating patterns could assist in monitoring disease activity and progression, offering a noninvasive means of assessing disease status and response to therapy, thus allowing for more frequent assessments of disease status without the need for invasive procedures. Understanding these behaviors allows healthcare providers to tailor dietary advice and interventions, potentially enhancing treatment compliance and improving the quality of life for pediatric patients with EoE.
Commentary: New Research on BC Chemotherapies, March 2024
The phase 3 KEYNOTE-355 trial established the role of chemotherapy in combination with pembrolizumab in the first-line setting for programmed death-ligand 1 (PD-L1)–positive advanced triple-negative breast cancer (TNBC). Patients unselected for PD-L1 status in this trial who received platinum- or taxane-based chemotherapy with placebo had a median progression-free survival of 5.6 months.[3] Strategies to improve upon efficacy and tolerability are desired in this space, and various trials have evaluated "switch maintenance" that involves receipt of an intensive induction regimen followed by a switch to an alternative/more tolerable regimen after response is achieved.[4] The phase II DORA trial randomized 45 patients with advanced TNBC and ongoing stable disease or complete or partial response from first- or second-line platinum-based chemotherapy to a maintenance regimen of olaparib (300 mg orally twice daily) with or without durvalumab (1500 mg on day 1 and every 4 weeks) (Tan et al). At a median follow-up of 9.8 months, median progression-free survival was 4.0 months (95% CI 2.6-6.1) with olaparib and 6.1 months (95% CI 3.7-10.1) with the combination; both were significantly longer than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). Durable disease control appeared more pronounced in patients with complete or partial response to prior platinum therapy, and no new safety signals were observed. Future efforts to study this approach include the phase 2/3 KEYLYNK-009 trial, which is evaluating olaparib plus pembrolizumab maintenance therapy after first-line chemotherapy plus pembrolizumab for TNBC.[5]
TNBC is a heterogenous subtype, characterized by aggressive biology, and it benefits from chemotherapy and immunotherapy treatment approaches. Presently, the management of early-stage TNBC often involves neoadjuvant systemic therapy; however, a proportion of patients receive treatment in the postoperative setting, highlighting the relevance of time to initiation of adjuvant therapy as well.[6] Various prior studies have showed that delayed administration of adjuvant chemotherapy for EBC can lead to adverse survival outcomes. Furthermore, this effect is subtype-dependent, with more aggressive tumors (luminal B, triple-negative, human epidermal growth factor receptor 2 [HER2]-positive) exhibiting inferior outcomes with delayed chemotherapy.[7] A retrospective cohort study that included 245 patients with early TNBC who received adjuvant chemotherapy after surgery evaluated the impact of time to initiation of adjuvant therapy in this population (Hatzipanagiotou et al). Superior survival outcomes were observed for the group receiving systemic therapy 22-28 days after surgery (median overall survival 10.2 years) compared with those receiving adjuvant chemotherapy at later time points (29-35 days, 36-42 days, and >6 weeks after surgery; median overall survival 8.3 years, 7.8 years, and 6.9 years, respectively). Patients receiving chemotherapy 22-28 days after surgery had significantly better survival than those receiving chemotherapy 29-35 days (P = .043) and >6 weeks (P = 0.033) postoperatively. This study emphasizes the importance of timely administration of adjuvant chemotherapy for early TNBC, and efforts aimed to identify potential challenges and propose solutions to optimize outcomes in this space are valuable.
Additional References
- Gnant M, Frantal S, Pfeiler G, et al, for the Austrian Breast & Colorectal Cancer Study Group. Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid. 2022;1:EVIDoa2200162. doi: 10.1056/EVIDoa2200162 Source
- Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 2018;23:293-30 doi: 10.1007/s40519-018-0505-2 Source
- Cortes J, Cescon DW, Rugo HS, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. doi: 10.1016/S0140-6736(20)32531-9 Source
- Bachelot T, Filleron T, Bieche I, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27:250-255. doi: 10.1038/s41591-020-01189-2 Source
- Saji S, Cussac AL, Andre F, et al. 68TiP KEYLYNK-009: a phase II/III, open-label, randomized study of pembrolizumab (pembro) + olaparib (ola) vs pembro + chemotherapy after induction with first-line (1L) pembro + chemo in patients (pts) with locally recurrent inoperable or metastatic TNBC (abstract). Ann Oncol. 2020;31(Suppl 6):S1268. doi: 10.1016/j.annonc.2020.10.088 Source
- Ortmann O, Blohmer JU, Sibert NT, et al for 55 breast cancer centers certified by the German Cancer Society. Current clinical practice and outcome of neoadjuvant chemotherapy for early breast cancer: Analysis of individual data from 94,638 patients treated in 55 breast cancer centers. J Cancer Res Clin Oncol. 2023;149:1195-1209. doi: 10.1007/s00432-022-03938-x Source
- Yu KD, Fan L, Qiu LX, et al. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8:46549-46556. doi: 10.18632/oncotarget.10551 Source
The phase 3 KEYNOTE-355 trial established the role of chemotherapy in combination with pembrolizumab in the first-line setting for programmed death-ligand 1 (PD-L1)–positive advanced triple-negative breast cancer (TNBC). Patients unselected for PD-L1 status in this trial who received platinum- or taxane-based chemotherapy with placebo had a median progression-free survival of 5.6 months.[3] Strategies to improve upon efficacy and tolerability are desired in this space, and various trials have evaluated "switch maintenance" that involves receipt of an intensive induction regimen followed by a switch to an alternative/more tolerable regimen after response is achieved.[4] The phase II DORA trial randomized 45 patients with advanced TNBC and ongoing stable disease or complete or partial response from first- or second-line platinum-based chemotherapy to a maintenance regimen of olaparib (300 mg orally twice daily) with or without durvalumab (1500 mg on day 1 and every 4 weeks) (Tan et al). At a median follow-up of 9.8 months, median progression-free survival was 4.0 months (95% CI 2.6-6.1) with olaparib and 6.1 months (95% CI 3.7-10.1) with the combination; both were significantly longer than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). Durable disease control appeared more pronounced in patients with complete or partial response to prior platinum therapy, and no new safety signals were observed. Future efforts to study this approach include the phase 2/3 KEYLYNK-009 trial, which is evaluating olaparib plus pembrolizumab maintenance therapy after first-line chemotherapy plus pembrolizumab for TNBC.[5]
TNBC is a heterogenous subtype, characterized by aggressive biology, and it benefits from chemotherapy and immunotherapy treatment approaches. Presently, the management of early-stage TNBC often involves neoadjuvant systemic therapy; however, a proportion of patients receive treatment in the postoperative setting, highlighting the relevance of time to initiation of adjuvant therapy as well.[6] Various prior studies have showed that delayed administration of adjuvant chemotherapy for EBC can lead to adverse survival outcomes. Furthermore, this effect is subtype-dependent, with more aggressive tumors (luminal B, triple-negative, human epidermal growth factor receptor 2 [HER2]-positive) exhibiting inferior outcomes with delayed chemotherapy.[7] A retrospective cohort study that included 245 patients with early TNBC who received adjuvant chemotherapy after surgery evaluated the impact of time to initiation of adjuvant therapy in this population (Hatzipanagiotou et al). Superior survival outcomes were observed for the group receiving systemic therapy 22-28 days after surgery (median overall survival 10.2 years) compared with those receiving adjuvant chemotherapy at later time points (29-35 days, 36-42 days, and >6 weeks after surgery; median overall survival 8.3 years, 7.8 years, and 6.9 years, respectively). Patients receiving chemotherapy 22-28 days after surgery had significantly better survival than those receiving chemotherapy 29-35 days (P = .043) and >6 weeks (P = 0.033) postoperatively. This study emphasizes the importance of timely administration of adjuvant chemotherapy for early TNBC, and efforts aimed to identify potential challenges and propose solutions to optimize outcomes in this space are valuable.
Additional References
- Gnant M, Frantal S, Pfeiler G, et al, for the Austrian Breast & Colorectal Cancer Study Group. Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid. 2022;1:EVIDoa2200162. doi: 10.1056/EVIDoa2200162 Source
- Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 2018;23:293-30 doi: 10.1007/s40519-018-0505-2 Source
- Cortes J, Cescon DW, Rugo HS, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. doi: 10.1016/S0140-6736(20)32531-9 Source
- Bachelot T, Filleron T, Bieche I, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27:250-255. doi: 10.1038/s41591-020-01189-2 Source
- Saji S, Cussac AL, Andre F, et al. 68TiP KEYLYNK-009: a phase II/III, open-label, randomized study of pembrolizumab (pembro) + olaparib (ola) vs pembro + chemotherapy after induction with first-line (1L) pembro + chemo in patients (pts) with locally recurrent inoperable or metastatic TNBC (abstract). Ann Oncol. 2020;31(Suppl 6):S1268. doi: 10.1016/j.annonc.2020.10.088 Source
- Ortmann O, Blohmer JU, Sibert NT, et al for 55 breast cancer centers certified by the German Cancer Society. Current clinical practice and outcome of neoadjuvant chemotherapy for early breast cancer: Analysis of individual data from 94,638 patients treated in 55 breast cancer centers. J Cancer Res Clin Oncol. 2023;149:1195-1209. doi: 10.1007/s00432-022-03938-x Source
- Yu KD, Fan L, Qiu LX, et al. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8:46549-46556. doi: 10.18632/oncotarget.10551 Source
The phase 3 KEYNOTE-355 trial established the role of chemotherapy in combination with pembrolizumab in the first-line setting for programmed death-ligand 1 (PD-L1)–positive advanced triple-negative breast cancer (TNBC). Patients unselected for PD-L1 status in this trial who received platinum- or taxane-based chemotherapy with placebo had a median progression-free survival of 5.6 months.[3] Strategies to improve upon efficacy and tolerability are desired in this space, and various trials have evaluated "switch maintenance" that involves receipt of an intensive induction regimen followed by a switch to an alternative/more tolerable regimen after response is achieved.[4] The phase II DORA trial randomized 45 patients with advanced TNBC and ongoing stable disease or complete or partial response from first- or second-line platinum-based chemotherapy to a maintenance regimen of olaparib (300 mg orally twice daily) with or without durvalumab (1500 mg on day 1 and every 4 weeks) (Tan et al). At a median follow-up of 9.8 months, median progression-free survival was 4.0 months (95% CI 2.6-6.1) with olaparib and 6.1 months (95% CI 3.7-10.1) with the combination; both were significantly longer than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). Durable disease control appeared more pronounced in patients with complete or partial response to prior platinum therapy, and no new safety signals were observed. Future efforts to study this approach include the phase 2/3 KEYLYNK-009 trial, which is evaluating olaparib plus pembrolizumab maintenance therapy after first-line chemotherapy plus pembrolizumab for TNBC.[5]
TNBC is a heterogenous subtype, characterized by aggressive biology, and it benefits from chemotherapy and immunotherapy treatment approaches. Presently, the management of early-stage TNBC often involves neoadjuvant systemic therapy; however, a proportion of patients receive treatment in the postoperative setting, highlighting the relevance of time to initiation of adjuvant therapy as well.[6] Various prior studies have showed that delayed administration of adjuvant chemotherapy for EBC can lead to adverse survival outcomes. Furthermore, this effect is subtype-dependent, with more aggressive tumors (luminal B, triple-negative, human epidermal growth factor receptor 2 [HER2]-positive) exhibiting inferior outcomes with delayed chemotherapy.[7] A retrospective cohort study that included 245 patients with early TNBC who received adjuvant chemotherapy after surgery evaluated the impact of time to initiation of adjuvant therapy in this population (Hatzipanagiotou et al). Superior survival outcomes were observed for the group receiving systemic therapy 22-28 days after surgery (median overall survival 10.2 years) compared with those receiving adjuvant chemotherapy at later time points (29-35 days, 36-42 days, and >6 weeks after surgery; median overall survival 8.3 years, 7.8 years, and 6.9 years, respectively). Patients receiving chemotherapy 22-28 days after surgery had significantly better survival than those receiving chemotherapy 29-35 days (P = .043) and >6 weeks (P = 0.033) postoperatively. This study emphasizes the importance of timely administration of adjuvant chemotherapy for early TNBC, and efforts aimed to identify potential challenges and propose solutions to optimize outcomes in this space are valuable.
Additional References
- Gnant M, Frantal S, Pfeiler G, et al, for the Austrian Breast & Colorectal Cancer Study Group. Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid. 2022;1:EVIDoa2200162. doi: 10.1056/EVIDoa2200162 Source
- Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 2018;23:293-30 doi: 10.1007/s40519-018-0505-2 Source
- Cortes J, Cescon DW, Rugo HS, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. doi: 10.1016/S0140-6736(20)32531-9 Source
- Bachelot T, Filleron T, Bieche I, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27:250-255. doi: 10.1038/s41591-020-01189-2 Source
- Saji S, Cussac AL, Andre F, et al. 68TiP KEYLYNK-009: a phase II/III, open-label, randomized study of pembrolizumab (pembro) + olaparib (ola) vs pembro + chemotherapy after induction with first-line (1L) pembro + chemo in patients (pts) with locally recurrent inoperable or metastatic TNBC (abstract). Ann Oncol. 2020;31(Suppl 6):S1268. doi: 10.1016/j.annonc.2020.10.088 Source
- Ortmann O, Blohmer JU, Sibert NT, et al for 55 breast cancer centers certified by the German Cancer Society. Current clinical practice and outcome of neoadjuvant chemotherapy for early breast cancer: Analysis of individual data from 94,638 patients treated in 55 breast cancer centers. J Cancer Res Clin Oncol. 2023;149:1195-1209. doi: 10.1007/s00432-022-03938-x Source
- Yu KD, Fan L, Qiu LX, et al. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8:46549-46556. doi: 10.18632/oncotarget.10551 Source
Commentary: Medication Timing and Other Dupilumab Concerns, March 2024
When skin diseases affect the palm or sole, they can have a disproportionately large negative effect on patients' lives. Hand and foot dermatitis can be disabling. Simpson and colleagues find that dupilumab is an effective treatment for AD of the hands and feet. Having safe and effective treatment for hand and foot dermatitis will be life-changing for many of our patients.
Patients often do very well with biologic treatment. When they do, they often wonder, Do I need to continue taking the medication? Lasheras-Pérez and colleagues found that the great majority of patients doing well taking dupilumab for AD could stretch out their dosing interval. I suspect a lot of our patients are doing this already. I used to worry that stretching out the dosing interval might lead to antidrug antibodies and loss of activity. Such loss of activity doesn't appear common. Because we also have multiple alternative treatments for severe AD, I think it may be quite reasonable for patients to try spreading out their doses after their disease has been well controlled for a good long time.
Superficial skin infections aren't rare in children, particularly children with AD. Paller and colleagues' study is informative about the safety of dupilumab in children. The drug, which blocks a pathway of the immune system, was associated with fewer infections. This is good news. The reduction in infections could be through restoring "immune balance" (whatever that means) or by improving skin barrier function. Perhaps the low rate of infection explains why dupilumab is not considered immunosuppressive.
I love studies of drug survival because I think that knowing the percentage of patients who stay with drug treatment is a good measure of overall safety and efficacy. Pezzolo and colleagues found — perhaps not surprisingly given the extraordinary efficacy of upadacitinib for AD — that almost no one discontinued the drug over 1.5 years due to lack of efficacy. There were patients who discontinued due to adverse events (and additional patients lost to follow-up who perhaps also discontinued the drug), but 80% of patients were still in the study at the end of 1.5 years. Three patients who weren't vaccinated for shingles developed shingles; encouraging patients to get the shingles vaccine may be a prudent measure when starting patients taking upadacitinib.
When skin diseases affect the palm or sole, they can have a disproportionately large negative effect on patients' lives. Hand and foot dermatitis can be disabling. Simpson and colleagues find that dupilumab is an effective treatment for AD of the hands and feet. Having safe and effective treatment for hand and foot dermatitis will be life-changing for many of our patients.
Patients often do very well with biologic treatment. When they do, they often wonder, Do I need to continue taking the medication? Lasheras-Pérez and colleagues found that the great majority of patients doing well taking dupilumab for AD could stretch out their dosing interval. I suspect a lot of our patients are doing this already. I used to worry that stretching out the dosing interval might lead to antidrug antibodies and loss of activity. Such loss of activity doesn't appear common. Because we also have multiple alternative treatments for severe AD, I think it may be quite reasonable for patients to try spreading out their doses after their disease has been well controlled for a good long time.
Superficial skin infections aren't rare in children, particularly children with AD. Paller and colleagues' study is informative about the safety of dupilumab in children. The drug, which blocks a pathway of the immune system, was associated with fewer infections. This is good news. The reduction in infections could be through restoring "immune balance" (whatever that means) or by improving skin barrier function. Perhaps the low rate of infection explains why dupilumab is not considered immunosuppressive.
I love studies of drug survival because I think that knowing the percentage of patients who stay with drug treatment is a good measure of overall safety and efficacy. Pezzolo and colleagues found — perhaps not surprisingly given the extraordinary efficacy of upadacitinib for AD — that almost no one discontinued the drug over 1.5 years due to lack of efficacy. There were patients who discontinued due to adverse events (and additional patients lost to follow-up who perhaps also discontinued the drug), but 80% of patients were still in the study at the end of 1.5 years. Three patients who weren't vaccinated for shingles developed shingles; encouraging patients to get the shingles vaccine may be a prudent measure when starting patients taking upadacitinib.
When skin diseases affect the palm or sole, they can have a disproportionately large negative effect on patients' lives. Hand and foot dermatitis can be disabling. Simpson and colleagues find that dupilumab is an effective treatment for AD of the hands and feet. Having safe and effective treatment for hand and foot dermatitis will be life-changing for many of our patients.
Patients often do very well with biologic treatment. When they do, they often wonder, Do I need to continue taking the medication? Lasheras-Pérez and colleagues found that the great majority of patients doing well taking dupilumab for AD could stretch out their dosing interval. I suspect a lot of our patients are doing this already. I used to worry that stretching out the dosing interval might lead to antidrug antibodies and loss of activity. Such loss of activity doesn't appear common. Because we also have multiple alternative treatments for severe AD, I think it may be quite reasonable for patients to try spreading out their doses after their disease has been well controlled for a good long time.
Superficial skin infections aren't rare in children, particularly children with AD. Paller and colleagues' study is informative about the safety of dupilumab in children. The drug, which blocks a pathway of the immune system, was associated with fewer infections. This is good news. The reduction in infections could be through restoring "immune balance" (whatever that means) or by improving skin barrier function. Perhaps the low rate of infection explains why dupilumab is not considered immunosuppressive.
I love studies of drug survival because I think that knowing the percentage of patients who stay with drug treatment is a good measure of overall safety and efficacy. Pezzolo and colleagues found — perhaps not surprisingly given the extraordinary efficacy of upadacitinib for AD — that almost no one discontinued the drug over 1.5 years due to lack of efficacy. There were patients who discontinued due to adverse events (and additional patients lost to follow-up who perhaps also discontinued the drug), but 80% of patients were still in the study at the end of 1.5 years. Three patients who weren't vaccinated for shingles developed shingles; encouraging patients to get the shingles vaccine may be a prudent measure when starting patients taking upadacitinib.