User login
The First Patient in the Veteran Affairs System to Receive Chimeric Antigen Receptors T-cell Therapy for Refractory Multiple Myeloma and the Role of Intravenous Immunoglobulin in the Prevention of Therapy-associated Infections
Background
In 3/2021, chimeric antigen receptor (CAR) T-cell therapy was approved for the treatment of multiple myeloma in adult patients with refractory disease. Currently, only the Veterans Affair (VA) center at the Tennessee Valley Healthcare System (TVHS) offers this treatment. Herein, we report a significant healthcare milestone in 2024 when the first patient received CAR T-cell therapy for multiple myeloma in the VA system. Additionally, the rate of hypogammaglobulinemia is the highest for CAR T-cell therapy using idecabtagene vicleucel compared to therapies using other antineoplastic agents (Wat et al, 2021). The complications of hypogammaglobulinemia can be mitigated by intravenous immunoglobulin (IVIG) treatment.
Case Presentation
A 75-year-old male veteran was diagnosed with IgA Kappa multiple myeloma and received induction therapy with bortezomib, lenalidomide, and dexamethasone in 2014. The patient underwent autologous stem cell transplant (SCT) in the same year. His disease recurred in 3/2019, and the patient was started on daratumumab and pomalidomide. He received another autologous SCT in 2/2021, to which he was refractory. The veteran then received treatment with daratumumab and ixazomib, followed by carfilzomib and cyclophosphamide. Starting in 9/2022, the patient also required regular IVIG treatment for hypogammaglobulinemia. He eventually received CAR T-cell therapy with idecabtagene vicleucel at THVS on 4/18/2024. The patient tolerated the treatment well and is undergoing routine disease monitoring. Following CAR T-cell therapy, his hypogammaglobulinemia persists with immunoglobulins level less than 500 mg/dL, and the veteran is still receiving supportive care IVIG.
Discussion
A population estimate of 1.3 million veterans are uninsured and can only access healthcare through the VA (Nelson et al, 2007). This case highlights the first patient to receive CAR T-cell therapy for multiple myeloma in the VA system, indicating that veterans now have access to this life-saving treatment. The rate of hypogammaglobulinemia following CAR T-cell therapy for multiple myeloma is as high as 41%, with an associated infection risk of 70%. Following CAR T-cell therapy with idecabtagene vicleucel, around 61% of patients will require IVIG treatment (Wat el al, 2021). Our case adds to this growing literature on the prevalence of IVIG treatment following CAR T-cell therapy in this patient population.
Background
In 3/2021, chimeric antigen receptor (CAR) T-cell therapy was approved for the treatment of multiple myeloma in adult patients with refractory disease. Currently, only the Veterans Affair (VA) center at the Tennessee Valley Healthcare System (TVHS) offers this treatment. Herein, we report a significant healthcare milestone in 2024 when the first patient received CAR T-cell therapy for multiple myeloma in the VA system. Additionally, the rate of hypogammaglobulinemia is the highest for CAR T-cell therapy using idecabtagene vicleucel compared to therapies using other antineoplastic agents (Wat et al, 2021). The complications of hypogammaglobulinemia can be mitigated by intravenous immunoglobulin (IVIG) treatment.
Case Presentation
A 75-year-old male veteran was diagnosed with IgA Kappa multiple myeloma and received induction therapy with bortezomib, lenalidomide, and dexamethasone in 2014. The patient underwent autologous stem cell transplant (SCT) in the same year. His disease recurred in 3/2019, and the patient was started on daratumumab and pomalidomide. He received another autologous SCT in 2/2021, to which he was refractory. The veteran then received treatment with daratumumab and ixazomib, followed by carfilzomib and cyclophosphamide. Starting in 9/2022, the patient also required regular IVIG treatment for hypogammaglobulinemia. He eventually received CAR T-cell therapy with idecabtagene vicleucel at THVS on 4/18/2024. The patient tolerated the treatment well and is undergoing routine disease monitoring. Following CAR T-cell therapy, his hypogammaglobulinemia persists with immunoglobulins level less than 500 mg/dL, and the veteran is still receiving supportive care IVIG.
Discussion
A population estimate of 1.3 million veterans are uninsured and can only access healthcare through the VA (Nelson et al, 2007). This case highlights the first patient to receive CAR T-cell therapy for multiple myeloma in the VA system, indicating that veterans now have access to this life-saving treatment. The rate of hypogammaglobulinemia following CAR T-cell therapy for multiple myeloma is as high as 41%, with an associated infection risk of 70%. Following CAR T-cell therapy with idecabtagene vicleucel, around 61% of patients will require IVIG treatment (Wat el al, 2021). Our case adds to this growing literature on the prevalence of IVIG treatment following CAR T-cell therapy in this patient population.
Background
In 3/2021, chimeric antigen receptor (CAR) T-cell therapy was approved for the treatment of multiple myeloma in adult patients with refractory disease. Currently, only the Veterans Affair (VA) center at the Tennessee Valley Healthcare System (TVHS) offers this treatment. Herein, we report a significant healthcare milestone in 2024 when the first patient received CAR T-cell therapy for multiple myeloma in the VA system. Additionally, the rate of hypogammaglobulinemia is the highest for CAR T-cell therapy using idecabtagene vicleucel compared to therapies using other antineoplastic agents (Wat et al, 2021). The complications of hypogammaglobulinemia can be mitigated by intravenous immunoglobulin (IVIG) treatment.
Case Presentation
A 75-year-old male veteran was diagnosed with IgA Kappa multiple myeloma and received induction therapy with bortezomib, lenalidomide, and dexamethasone in 2014. The patient underwent autologous stem cell transplant (SCT) in the same year. His disease recurred in 3/2019, and the patient was started on daratumumab and pomalidomide. He received another autologous SCT in 2/2021, to which he was refractory. The veteran then received treatment with daratumumab and ixazomib, followed by carfilzomib and cyclophosphamide. Starting in 9/2022, the patient also required regular IVIG treatment for hypogammaglobulinemia. He eventually received CAR T-cell therapy with idecabtagene vicleucel at THVS on 4/18/2024. The patient tolerated the treatment well and is undergoing routine disease monitoring. Following CAR T-cell therapy, his hypogammaglobulinemia persists with immunoglobulins level less than 500 mg/dL, and the veteran is still receiving supportive care IVIG.
Discussion
A population estimate of 1.3 million veterans are uninsured and can only access healthcare through the VA (Nelson et al, 2007). This case highlights the first patient to receive CAR T-cell therapy for multiple myeloma in the VA system, indicating that veterans now have access to this life-saving treatment. The rate of hypogammaglobulinemia following CAR T-cell therapy for multiple myeloma is as high as 41%, with an associated infection risk of 70%. Following CAR T-cell therapy with idecabtagene vicleucel, around 61% of patients will require IVIG treatment (Wat el al, 2021). Our case adds to this growing literature on the prevalence of IVIG treatment following CAR T-cell therapy in this patient population.
Creating a Urology Prostate Cancer Note, a National Oncology and Surgery Office Collaboration for Prostate Cancer Clinical Pathway Utilization
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Discussion
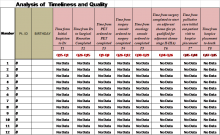
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. For example, the Very Low Risk flow map has seven unique Veterans entered, whereas the Molecular Testing flow map has over 3,900 unique Veterans entered. One clear reason for this disparity in pathway documentation use is that local prostate cancer is managed by urology and their documentation of the PCCP is not as widespread as the medical oncologists. The National Oncology Program developed clinical note templates to document PCCP that medical oncologist use which has increased utilization. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator. The UPCN will function as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. The UPCN is in the testing phase at three pilot test sites and is scheduled to be deployed summer 2024. The collaborative effort is aligned with the VHA directives outlined in the Cleland Dole Act.
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Discussion
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. For example, the Very Low Risk flow map has seven unique Veterans entered, whereas the Molecular Testing flow map has over 3,900 unique Veterans entered. One clear reason for this disparity in pathway documentation use is that local prostate cancer is managed by urology and their documentation of the PCCP is not as widespread as the medical oncologists. The National Oncology Program developed clinical note templates to document PCCP that medical oncologist use which has increased utilization. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator. The UPCN will function as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. The UPCN is in the testing phase at three pilot test sites and is scheduled to be deployed summer 2024. The collaborative effort is aligned with the VHA directives outlined in the Cleland Dole Act.
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Discussion
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. For example, the Very Low Risk flow map has seven unique Veterans entered, whereas the Molecular Testing flow map has over 3,900 unique Veterans entered. One clear reason for this disparity in pathway documentation use is that local prostate cancer is managed by urology and their documentation of the PCCP is not as widespread as the medical oncologists. The National Oncology Program developed clinical note templates to document PCCP that medical oncologist use which has increased utilization. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator. The UPCN will function as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. The UPCN is in the testing phase at three pilot test sites and is scheduled to be deployed summer 2024. The collaborative effort is aligned with the VHA directives outlined in the Cleland Dole Act.
A Learning Health System Approach to Long COVID Care
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
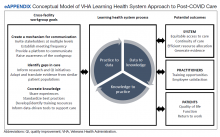
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
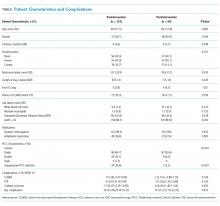
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
NY radiation oncologist loses license, poses ‘potential danger’
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
Less Lumens-Less Risk: A Pilot Intervention to Increase the Use of Single-Lumen Peripherally Inserted Central Catheters
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
© 2019 Society of Hospital Medicine
Treatment and Management of Patients With Non-Small Cell Lung Cancer (FULL)
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Cancer Care Collaborative Approach to Optimize Clinical Care (FULL)
A collaboration between clinicians and industrial engineers resulted in significant improvements in cancer screening, the development of toolkits, and more efficient care for hepatocellular carcinoma and breast, colorectal, lung, head and neck, and prostate cancers.
Cancer is one of the most common causes of premature death and disability that requires long-term follow-up surveillance and oftentimes ongoing treatment for survivors that can lead to important health, psychosocial
Like other cancer treatment systems, the VA faces some challenges in timeliness, surveillance, and quality of the cancer care process.12-18 Although implementation of cancer patientcentered home care and other efforts were developed to improve delivery and efficiency of cancer care in VA and non-VA facilities, the patient continuum of care remains convoluted.2,19-23
In 2004, the Clinical Cancer Care Collaborative (C4), a national VA program, was launched to improve timeliness, quality, access improvement, efficiency, and the “sustainability and spread” of successful programs at the VA. This program included representatives throughout the VA and encompassed cancer care coordinators (clinical nurse navigators), advisory panels, and a multidisciplinary team of clinicians.
In 2009, the VA promoted the Cancer Care Collaborative (CCC) to focus on optimizing the timeliness and quality of colorectal, breast, lung, prostate, and hematologic cancer care throughout the VA health care system. The VA Office of Systems Redesign (SR) partnered with the VA-Center for Applied Systems Engineering (VA-CASE) Veteran Engineering Resource Center (VERC), including industrial engineers (IEs) to provide their expertise and support. The CCC provided a forum to develop teams; set aims; and map, measure, analyze, and implement changes to assure timely diagnosis and initiation of evidence-based treatment and subsequently sustain the practices that led to improvements in these areas.
The CCC structure was separated into 6 distinct support areas: (1) industrial/systems engineering support; (2) informatics and clinical application support; (3) development and dissemination of improvement resource guides; (4) real-time and rapid-cycle evaluation tools and approaches; (5) application of advanced operational systems engineering techniques, such as simulation and modeling to inform further system optimization; and (6) advisory panels focused on quality topics that were identified, developed, implemented, and evaluated by the participants with support from the CCC faculty.
Here the authors describe the framework of the CCC model developed by VA-CASE, demonstrate the performance improvement results of teams focusing on several types of cancer, and highlight the key indicators to best practices.
Methods
Figure 1 outlines the CCC 3-Phase Conceptual Model. Phase 1 included diagnosis (screening and symptoms); phase 2 included treatment (from diagnosis to beyond treatment); and phase 3 was designed for hub and spoke facilities where screening/diagnosis occurs in a smaller (spoke) facility and treatment occurs in the larger (hub) facility.
In the first phase, 18 facility-based teams were selected through an application and interview process and immediately applied SR to their team’s specific improvement projects, which included the following cancers: breast, colorectal, lung, and prostate.
In addition to the cancer types covered in the initial phase, phase 2 also included hepatocellular carcinoma (HCC) and head and neck cancers. National VHA Toolkits were products that developed from and for use in lung and colorectal cancers (CRCs) (phases 1 and 2). These were organized and disseminated throughout the entire VA, offering specific knowledge and tools that could be applied to improving cancer care. The toolkit included guidance documents, specific process examples, and items that could be downloaded into Microsoft SharePoint (Redmond, WA) for adaptation and use by VA facilities. The toolkit contents were primarily developed and/or identified by CCC participants and funded by the VA Office of Quality and Performance (OQP) and SR. The toolkits included links to the following resources for each cancer type in phase 2: quality indicators, tool tables, timeliness measures, understanding the continuum of care, and a resource entitled, “How Can the Quality Metrics Help Me?” (eAppendix 1, available at fedprac.com/AVAHO).
The phase 3 collaborative was designed for hub and spoke facilities by focusing on current state vs ideal state processes, communication patterns, and care coordination between the hub and spoke facilities. There were 10 facilities in which all teams focused on lung cancer. Each facility was made up of 1 hub and had the ability to send up to 8 participants (from either the hub or the spoke facility) to the CCC workgroup meetings. Participants were specialists, radiologists, primary care providers, pathologists, nurses, nurse practitioners, or physician assistants.
Conceptual Model Deployment
The deployment of the CCC 3-phase conceptual model was based on the Institute for Healthcare Improvement (IHI) Breakthrough Series Collaborative Model.24,25 Implementation was carried out over 3 phases (2005-2011) after proper teaching, coaching, and learning sessions (LS)
Each LS incorporated instruction in basic systems engineering and Lean Six Sigma principles (an approach to quality improvement that focuses on reducing waste and variability) with practical, health care-based examples, case studies, and immediate application of the VA-TAMMCS (vision/analyze, team/aim, map, measure, change, sustain) SR organizational framework (Figure 2), tools, and methodologies to the process under investigation.26 The VA-TAMMCS (eAppendix 2, available at fedprac.com/AVAHO)
The CCC encouraged joint facilitation. A SR clinical coach, a VERC IE, and participating facilities were required to work together intensively (mentor and support) for 10 to 12 months. The mix of clinicians and engineers helped the facilities by bringing in diverse perspectives, which led to better decisions in the improvement of cancer care.28 During the CCC, the IEs partnered with and supported clinicians, using Lean Six Sigma and SR tools and approaches to health care quality improvement to quickly make improvements in efficiency and quality (eAppendix 3a, 3b, and 3c, available at fedprac.com/AVAHO).26,29
The IEs provided on-site support at all participating VAMCs during all 3 phases by providing the clinical teams with a variety of VA-TAMMCS process improvement tools to support the analysis and improvement of their organizations.
Data Collection
As part of the overall improvement process, the facilities worked on several aim statements in order to improve a primary constraint; such as timeliness and quality of care. An aim statement communicates what you want to do (eg, reduce, improve, or eliminate), by how much, and when. In order to improve timeliness, the CCC focused on measures from first evidence to tissue diagnosis, from diagnosis to treatment, and also intermediate measures, such as time from positron emission tomography scan ordered to completion. While working on overall quality of care unique to cancer, the CCC focused on measures related to documentation compliance and consistency of care provided to patients.
Phase 1
Facilities were to optimize their process (time from initial suspicion to diagnosis). Hence, participating facilities were allowed to simply identify their aim statement and pick and choose the area of focus.
Phases 2 and 3
Timeliness Aims. These aims were addressed through improvements in information technology in the Computerized Patient Record System (CPRS) electronic medical record by creating electronic order sets containing codes that alert providers daily to retrieve and follow up on abnormal test results. Primary care physicians and front desk staff also were educated on the use of these order sets and to schedule a follow-up test or specialist consult within 3 to 7 days.
Aim 1: Reduce to 15 days the time from initial suspicion to diagnosis within 1 year.
Aim 2: Reduce to 30 days the time from diagnosis to start of treatment within 1 year.
Quality Aim. Improve the compliance rate of identified quality indicators to 100% within 1 year.
Measurement Tools
The Cancer Care Measurement Tools were designed to support the OQP performance metric (Figure 3). The OQP creates and collects data on evidence-based national benchmarks to measure the quality of preventive and therapeutic health care services at the facility, VISN, and national levels. These metrics may be performance measures, performance monitors, quality indicators, and special studies, among other measures, to support clinicians, managers, and employees in improving care to veterans.
For each of the 3 CCC phases, VA-CASE IEs facilitated the development of standardized measurement and tracking tools for each cancer type. The tools identified key timeliness and quality measures as a function of entered patient data (eAppendixes 4-7, available at fedprac.com/AVAHO).
Quality Improvement Toolkit Series
The Quality Improvement Toolkit Series (QITS) was created for VA clinical managers and policy makers to improve diagnosis, treatment, and patient outcomes for high-priority conditions. The goal of the QITS is to serve as the cancer care improvement resource guide to produce and disseminate the National Quality Improvement Toolkit resource.
Each tool included in the QITS is matched to 1 or more metrics of the OQP (such as a performance measure or quality indicator). For example, the types of tools include CPRS order sets and templates, enhanced registries and patient databases, service agreements, and care process flow maps. Each toolkit served as a resource for improving facility performance on a specific set of established performance measures and/or quality indicators. Toolkits that helped VA facilities improve performance on OQP quality indicators and performance measures were based on the VA-TAMMCS model and continuous improvement that was tailored to the structure and needs of the VA system. The VA-CASE staff provided guidance on the criteria for inclusion in the toolkits to promote best practice and quality in clinical practice. The criteria used by a condition-specific expert panel were based on whether or not it was (1) not already part of VA routine care nationwide; (2) can be matched to 1 or more VA quality metrics/indicators; and (3) currently in use at a health care facility (innovative VA colleagues nationwide and by non-VA health care organizations).
Evaluation
After each LS, VA CCC evaluation data were collected using standardized 5-point Likert scale questions.
Results
Industrial engineers provided > 1,200 days of on-site support across the 60 teams and built 63 flow maps and 47 customized tools based on the team’s requests throughout the implementation period. Throughout the 3-phase CCC, the IEs developed standardized measurement and tracking tools for each cancer type (lung, colorectal, prostate, head and neck, and HCC). Outcomes included the sharing of best practices that spread across programs (uploaded to the national QITS site, available only to VA employees); as well as enterprisewide development of the special interest group (eg, VHA survivorship), which led to a national survivorship toolkit.
The table illustrates the overall collaborative impact across the CCC. In phase 1, 78% of the 64 aims (breast, CRC, lung, prostate) were met at 18 facilities. In phase 2, 72% of the 94 aims (CRC; HCC; and head and neck, lung, and prostate cancers) were met at 21 facilities. In phase 3, 47% of the 64 aims for head and neck and lung cancer were met at 11 facilities. The difference in the percentage of aims met during each phase was due to the variations in complexity of cancer types as well as additional logistic barriers at each institution.
Discussion
Overall, the CCC had a positive impact that improved timeliness, accessibility, and quality of the cancer care process in participating VAMCs. The majority of VAMCs focused on optimizing the lung cancer care process in all the phases of the collaborative, given that lung cancer suspicion-to-treatment process is highly complex, requiring multiple departments to coordinate workup and care, leading to the greatest room for improvement.
Industrial engineers introduced a variety of approaches to improvement to the collaborative teams, and they were integral to the development of standardized measurement and tracking tools for each type of cancer, introducing advanced SR methods for specific aims and performing appropriate data analysis. The ability of the VA system to recognize where improvements were needed was complemented by the efforts of VA clinicians and administration with direction from VERC IEs and their toolkits. Improvements were made, sometimes decreasing time from diagnosis to treatment by 50%. The VA facilities were encouraged to sustain this improvement using the toolkits with continued data gathering and implementation. In phase 1, lung cancer improvements included (1) establishing the multidisciplinary clinic, multidisciplinary rounds, and improved communication among key service lines; (2) developing a database (measurement tool) to prospectively track all cancer patients; (3) scheduling weekly multidisciplinary meetings to provide a mechanism to rapidly review patients and triage to appropriate pathways in the treatment algorithm; and (4) increasing physician participation, including oncologists, surgeons, radiologists, and radiation oncologists, to identify methods and process
changes that could eliminate wasteful steps and improve access for expediting diagnosis and treatment of patients with lung cancer who require surgery, chemotherapy, and/or radiation. The overall impact on time from abnormal CT to lung cancer surgery was reduced by > 5 months from 180 to 20 days. Substantial improvements were made in timeliness and reliability in caring for veterans with lung cancer.12
Groundbreaking work and exceptional results continued in the second phase for lung cancer care. In addition, the creation of a prostate cancer care web-based clinical measurement tool helped to improve the ability to proactively manage patients. The tool included same-day scheduling of biopsy and urology appointments for veterans with possible prostate cancer and the development of a protocol for expedited high-risk patients with metastatic disease. Ultimately, the wait time from urology consult to diagnosis was cut from 96 to 46 days for veterans with prostate cancer (Figure 4).
Once the face-to-face CCC process was established, tested, refined, and replicated successfully, the virtual team proved to be a cost-effective model. The virtual team did not travel to LSs, a major source of expense, so a process was set in place for their participation in all other facets of the collaborative. This led to the pilot testing of national virtual collaboratives (eg, specialty and surgical care collaboratives).
The toolkits for lung and CRC (phases 1 and 2) were organized, standardized, and disseminated throughout the VA to provide specific knowledge and tools to improve cancer care. The content of toolkits was primarily developed and/or identified by CCC participants. Funding for the toolkits was secured by OQP and SR, which led to the creation of the integration and crosswalk documents (eAppendix 7, available at fedprac.com/AVAHO).
In phase 3, lung cancer care teams showed the most improvement among all 3 phases of the collaborative. Aims statements in lung cancer process showed an increased percentage of improvement in all phases. Weekly multidisciplinary meetings provided a mechanism to rapidly review patients and triage appropriate pathways in the treatment algorithm. Open communication among sites and disciplines was vital and increased participation by physicians to identify ways to expedite diagnosis and treatment of lung cancer. In addition to access and timeliness of care (accommodating patients’ preference for scheduling), the teams identified areas they deemed important for successful programs and developed advisory panels that focused on quality, such as tumor boards, clinical trials, patient education, cancer care coordinator/navigator, survivorship, standard order sets and progress notes, reliable handoff, chemotherapy and radiation make/buy tools, head and neck toolkit, clinical documentation, chemotherapy efficiency, and Veterans Equitable Resource Allocation recovery for metastatic cancer.
Based on the evaluation results, participants gave their highest average ratings to items that asked about the general potential of SR to improve patient care and patient satisfaction, team dynamics, site leadership support; confidence in self, team, and coach; and the general potential of SR to improve staff satisfaction. Participants gave their lowest ratings to questions that asked about having the necessary time and resources to implement SR initiatives at their site as well as the level of active engagement by site leadership in SR work.
Click here to read the digital edition.
1. Klemp JR. Breast cancer prevention across the cancer care continuum. Semin Oncol Nurs. 2015;31(2):89-99.
2. Tralongo P, Ferraù F, Borsellino N, et al. Cancer patientcentered home care: a new model for health care in oncology. Ther Clin Risk Manag. 2011;7:387-392.
3. Institute of Medicine of the National Academy of Sciences. Delivering high-quality cancer care: charting a new course for a system in crisis. http://nationalacademies.org/hmd/~/media/Files/Report%20Files/2013/Quality-Cancer-Care/qualitycancercare_rb.pdf. Published September 2013. Accessed April 6, 2017.
4. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
5. Jabaaij L, van den Akker M, Schellevis FG. Excess of health care use in general practice and of comorbid chronic conditions in cancer patients compared to controls. BMC Fam Pract. 2012;13:60
6. Brazil K, Whelan T, O’Brien MA, Sussman J, Pyette N, Bainbridge D. Towards improving the co-ordination of supportive cancer care services in the community. Health Policy. 2004;70(1):125-131.
7. Husain A, Barbera L, Howell D, Moineddin R, Bezjak A, Sussman J. Advanced lung cancer patients’ experience with continuity of care and supportive care needs. Support Care Cancer. 2013;21(5):1351-1358.
8. Sayed S, Moloo Z, Bird P, et al. Breast cancer diagnosis in a resource poor environment through a collaborative multidisciplinary approach: the Kenyan experience. J Clin Pathol. 2013;66(4):307-311.
9. Morgan PA, Murray S, Moffatt CJ, Honnor A. The challenges of managing complex lymphoedema/chronic oedema in the UK and Canada. Int Wound J. 2011;9(1):54-69.
10. Renshaw M. Lymphorrhoea: ‘leaky legs’ are not just the nurse’s problem. Br J Community Nurs. 2007;12(4):S18-S21.
11. Morgan PA. Health professionals’ ideal roles in lympoedema management. Br J Community Nurs. 2006;11(suppl):5-8.
12. Hunnibell LS, Rose MG, Connery DM, et al. Using nurse navigation to improve timeliness of lung cancer care at a veterans hospital. Clin J Oncol Nurs. 2012;16(1):29-36.
13. Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179(7):
595-600.
14. Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133(5):1167-1173.
15. Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181.
16. Walling AM, Tisnado D, Asch SM, et al. The quality of supportive cancer care in the Veterans Affairs system and targets for improvement. JAMA Intern Med. 2013;173(22):2071-2079.
17. Keating NL, Landrum MB, Lamont EB, et al. Quality of care for older patients with cancer in the Veterans Health Administration versus the private sector: a cohort study. Ann Intern Med. 2011;154(11):727-736.
18. Kaiser AM, Nunoo-Mensah JW, Wasserberg N. Surgical volume and long-term survival following surgery for colorectal cancer in the Veterans Affairs Health-Care System. Am J Gastroenterol. 2005;100(1):250.
19. Abrahams E, Foti M, Kean MA. Accelerating the delivery of patient-centered, high-quality cancer care. Clin Cancer Res. 2015;21(10):2263-2267.
20. Taplin SH, Weaver S, Salas E, et al. Reviewing cancer care team effectiveness. J Oncol Pract. 2015;11(3):239-246.
21. Kosty MP, Bruinooge SS, Cox JV. Intentional approach to team-based oncology care: evidence-based teamwork to improve collaboration and patient engagement. J Oncol Pract. 2015;11(3):247-248.
22. Ko NY, Darnell JS, Calhoun E, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. J Clin Oncol. 2014;32(25):2758-2764.
23. Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12(1):4-13.
24. Institute for Healthcare Improvement. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston, MA: Institute for Healthcare Improvement; 2003.
25. Boushon B, Provost L, Gagnon J, Carver P. Using a virtual breakthrough series collaborative to improve access in primary care. Jt Comm J Qual Patient Saf. 2006;32(10):573-584.
26. Womack JP, Jones DT. Lean Thinking: Banish Waste and Create Wealth in Your Corporation. New York, NY: Simon & Schuster; 1996.
27. Bidassie B, Davies ML, Stark R, Boushon B. VA experience in implementing patient-centered medical home using a breakthrough series collaborative. J Gen Intern Med. 2014;29(suppl 2):S563-S5671.
28. Bidassie B, Williams LS, Woodward-Hagg H, Matthias MS, Damush TM. Key components of external facilitation in an acute stroke quality improvement collaborative in the Veterans Health Administration. Implement Sci. 2015;10(1):69.
29. Woodward-Hagg H, Workman-Germann J, Flanagan M, et al. Implementation of systems redesign: approaches to spread and sustain adoption. In: Henriksen K, Battles J, Keyes M, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches: Vol. 2: Culture and Redesign. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
30. American Cancer Society. National roundtable recognizes leaders in colorectal cancer prevention effort with 80% by 2018 National Achievement Award. [press release]. http://pressroom.cancer.org/2017-02-01-National-Colorectal-Cancer-Roundtable-Recognizes-Leaders-in-Colorectal-Cancer-Prevention-Effort-with-80-by-2018-National-Achievement-Award. Published February 1, 2017. Accessed March 29, 2017.
31. Margolis PA, Lannon CM, Stuart JM, Fried BJ, Keyes-Elstein L, Moore DE Jr. Practice based education to improve delivery systems for prevention in primary care: randomized trial. BMJ. 2004;328(7436):388.
A collaboration between clinicians and industrial engineers resulted in significant improvements in cancer screening, the development of toolkits, and more efficient care for hepatocellular carcinoma and breast, colorectal, lung, head and neck, and prostate cancers.
Cancer is one of the most common causes of premature death and disability that requires long-term follow-up surveillance and oftentimes ongoing treatment for survivors that can lead to important health, psychosocial
Like other cancer treatment systems, the VA faces some challenges in timeliness, surveillance, and quality of the cancer care process.12-18 Although implementation of cancer patientcentered home care and other efforts were developed to improve delivery and efficiency of cancer care in VA and non-VA facilities, the patient continuum of care remains convoluted.2,19-23
In 2004, the Clinical Cancer Care Collaborative (C4), a national VA program, was launched to improve timeliness, quality, access improvement, efficiency, and the “sustainability and spread” of successful programs at the VA. This program included representatives throughout the VA and encompassed cancer care coordinators (clinical nurse navigators), advisory panels, and a multidisciplinary team of clinicians.
In 2009, the VA promoted the Cancer Care Collaborative (CCC) to focus on optimizing the timeliness and quality of colorectal, breast, lung, prostate, and hematologic cancer care throughout the VA health care system. The VA Office of Systems Redesign (SR) partnered with the VA-Center for Applied Systems Engineering (VA-CASE) Veteran Engineering Resource Center (VERC), including industrial engineers (IEs) to provide their expertise and support. The CCC provided a forum to develop teams; set aims; and map, measure, analyze, and implement changes to assure timely diagnosis and initiation of evidence-based treatment and subsequently sustain the practices that led to improvements in these areas.
The CCC structure was separated into 6 distinct support areas: (1) industrial/systems engineering support; (2) informatics and clinical application support; (3) development and dissemination of improvement resource guides; (4) real-time and rapid-cycle evaluation tools and approaches; (5) application of advanced operational systems engineering techniques, such as simulation and modeling to inform further system optimization; and (6) advisory panels focused on quality topics that were identified, developed, implemented, and evaluated by the participants with support from the CCC faculty.
Here the authors describe the framework of the CCC model developed by VA-CASE, demonstrate the performance improvement results of teams focusing on several types of cancer, and highlight the key indicators to best practices.
Methods
Figure 1 outlines the CCC 3-Phase Conceptual Model. Phase 1 included diagnosis (screening and symptoms); phase 2 included treatment (from diagnosis to beyond treatment); and phase 3 was designed for hub and spoke facilities where screening/diagnosis occurs in a smaller (spoke) facility and treatment occurs in the larger (hub) facility.
In the first phase, 18 facility-based teams were selected through an application and interview process and immediately applied SR to their team’s specific improvement projects, which included the following cancers: breast, colorectal, lung, and prostate.
In addition to the cancer types covered in the initial phase, phase 2 also included hepatocellular carcinoma (HCC) and head and neck cancers. National VHA Toolkits were products that developed from and for use in lung and colorectal cancers (CRCs) (phases 1 and 2). These were organized and disseminated throughout the entire VA, offering specific knowledge and tools that could be applied to improving cancer care. The toolkit included guidance documents, specific process examples, and items that could be downloaded into Microsoft SharePoint (Redmond, WA) for adaptation and use by VA facilities. The toolkit contents were primarily developed and/or identified by CCC participants and funded by the VA Office of Quality and Performance (OQP) and SR. The toolkits included links to the following resources for each cancer type in phase 2: quality indicators, tool tables, timeliness measures, understanding the continuum of care, and a resource entitled, “How Can the Quality Metrics Help Me?” (eAppendix 1, available at fedprac.com/AVAHO).
The phase 3 collaborative was designed for hub and spoke facilities by focusing on current state vs ideal state processes, communication patterns, and care coordination between the hub and spoke facilities. There were 10 facilities in which all teams focused on lung cancer. Each facility was made up of 1 hub and had the ability to send up to 8 participants (from either the hub or the spoke facility) to the CCC workgroup meetings. Participants were specialists, radiologists, primary care providers, pathologists, nurses, nurse practitioners, or physician assistants.
Conceptual Model Deployment
The deployment of the CCC 3-phase conceptual model was based on the Institute for Healthcare Improvement (IHI) Breakthrough Series Collaborative Model.24,25 Implementation was carried out over 3 phases (2005-2011) after proper teaching, coaching, and learning sessions (LS)
Each LS incorporated instruction in basic systems engineering and Lean Six Sigma principles (an approach to quality improvement that focuses on reducing waste and variability) with practical, health care-based examples, case studies, and immediate application of the VA-TAMMCS (vision/analyze, team/aim, map, measure, change, sustain) SR organizational framework (Figure 2), tools, and methodologies to the process under investigation.26 The VA-TAMMCS (eAppendix 2, available at fedprac.com/AVAHO)
The CCC encouraged joint facilitation. A SR clinical coach, a VERC IE, and participating facilities were required to work together intensively (mentor and support) for 10 to 12 months. The mix of clinicians and engineers helped the facilities by bringing in diverse perspectives, which led to better decisions in the improvement of cancer care.28 During the CCC, the IEs partnered with and supported clinicians, using Lean Six Sigma and SR tools and approaches to health care quality improvement to quickly make improvements in efficiency and quality (eAppendix 3a, 3b, and 3c, available at fedprac.com/AVAHO).26,29
The IEs provided on-site support at all participating VAMCs during all 3 phases by providing the clinical teams with a variety of VA-TAMMCS process improvement tools to support the analysis and improvement of their organizations.
Data Collection
As part of the overall improvement process, the facilities worked on several aim statements in order to improve a primary constraint; such as timeliness and quality of care. An aim statement communicates what you want to do (eg, reduce, improve, or eliminate), by how much, and when. In order to improve timeliness, the CCC focused on measures from first evidence to tissue diagnosis, from diagnosis to treatment, and also intermediate measures, such as time from positron emission tomography scan ordered to completion. While working on overall quality of care unique to cancer, the CCC focused on measures related to documentation compliance and consistency of care provided to patients.
Phase 1
Facilities were to optimize their process (time from initial suspicion to diagnosis). Hence, participating facilities were allowed to simply identify their aim statement and pick and choose the area of focus.
Phases 2 and 3
Timeliness Aims. These aims were addressed through improvements in information technology in the Computerized Patient Record System (CPRS) electronic medical record by creating electronic order sets containing codes that alert providers daily to retrieve and follow up on abnormal test results. Primary care physicians and front desk staff also were educated on the use of these order sets and to schedule a follow-up test or specialist consult within 3 to 7 days.
Aim 1: Reduce to 15 days the time from initial suspicion to diagnosis within 1 year.
Aim 2: Reduce to 30 days the time from diagnosis to start of treatment within 1 year.
Quality Aim. Improve the compliance rate of identified quality indicators to 100% within 1 year.
Measurement Tools
The Cancer Care Measurement Tools were designed to support the OQP performance metric (Figure 3). The OQP creates and collects data on evidence-based national benchmarks to measure the quality of preventive and therapeutic health care services at the facility, VISN, and national levels. These metrics may be performance measures, performance monitors, quality indicators, and special studies, among other measures, to support clinicians, managers, and employees in improving care to veterans.
For each of the 3 CCC phases, VA-CASE IEs facilitated the development of standardized measurement and tracking tools for each cancer type. The tools identified key timeliness and quality measures as a function of entered patient data (eAppendixes 4-7, available at fedprac.com/AVAHO).
Quality Improvement Toolkit Series
The Quality Improvement Toolkit Series (QITS) was created for VA clinical managers and policy makers to improve diagnosis, treatment, and patient outcomes for high-priority conditions. The goal of the QITS is to serve as the cancer care improvement resource guide to produce and disseminate the National Quality Improvement Toolkit resource.
Each tool included in the QITS is matched to 1 or more metrics of the OQP (such as a performance measure or quality indicator). For example, the types of tools include CPRS order sets and templates, enhanced registries and patient databases, service agreements, and care process flow maps. Each toolkit served as a resource for improving facility performance on a specific set of established performance measures and/or quality indicators. Toolkits that helped VA facilities improve performance on OQP quality indicators and performance measures were based on the VA-TAMMCS model and continuous improvement that was tailored to the structure and needs of the VA system. The VA-CASE staff provided guidance on the criteria for inclusion in the toolkits to promote best practice and quality in clinical practice. The criteria used by a condition-specific expert panel were based on whether or not it was (1) not already part of VA routine care nationwide; (2) can be matched to 1 or more VA quality metrics/indicators; and (3) currently in use at a health care facility (innovative VA colleagues nationwide and by non-VA health care organizations).
Evaluation
After each LS, VA CCC evaluation data were collected using standardized 5-point Likert scale questions.
Results
Industrial engineers provided > 1,200 days of on-site support across the 60 teams and built 63 flow maps and 47 customized tools based on the team’s requests throughout the implementation period. Throughout the 3-phase CCC, the IEs developed standardized measurement and tracking tools for each cancer type (lung, colorectal, prostate, head and neck, and HCC). Outcomes included the sharing of best practices that spread across programs (uploaded to the national QITS site, available only to VA employees); as well as enterprisewide development of the special interest group (eg, VHA survivorship), which led to a national survivorship toolkit.
The table illustrates the overall collaborative impact across the CCC. In phase 1, 78% of the 64 aims (breast, CRC, lung, prostate) were met at 18 facilities. In phase 2, 72% of the 94 aims (CRC; HCC; and head and neck, lung, and prostate cancers) were met at 21 facilities. In phase 3, 47% of the 64 aims for head and neck and lung cancer were met at 11 facilities. The difference in the percentage of aims met during each phase was due to the variations in complexity of cancer types as well as additional logistic barriers at each institution.
Discussion
Overall, the CCC had a positive impact that improved timeliness, accessibility, and quality of the cancer care process in participating VAMCs. The majority of VAMCs focused on optimizing the lung cancer care process in all the phases of the collaborative, given that lung cancer suspicion-to-treatment process is highly complex, requiring multiple departments to coordinate workup and care, leading to the greatest room for improvement.
Industrial engineers introduced a variety of approaches to improvement to the collaborative teams, and they were integral to the development of standardized measurement and tracking tools for each type of cancer, introducing advanced SR methods for specific aims and performing appropriate data analysis. The ability of the VA system to recognize where improvements were needed was complemented by the efforts of VA clinicians and administration with direction from VERC IEs and their toolkits. Improvements were made, sometimes decreasing time from diagnosis to treatment by 50%. The VA facilities were encouraged to sustain this improvement using the toolkits with continued data gathering and implementation. In phase 1, lung cancer improvements included (1) establishing the multidisciplinary clinic, multidisciplinary rounds, and improved communication among key service lines; (2) developing a database (measurement tool) to prospectively track all cancer patients; (3) scheduling weekly multidisciplinary meetings to provide a mechanism to rapidly review patients and triage to appropriate pathways in the treatment algorithm; and (4) increasing physician participation, including oncologists, surgeons, radiologists, and radiation oncologists, to identify methods and process
changes that could eliminate wasteful steps and improve access for expediting diagnosis and treatment of patients with lung cancer who require surgery, chemotherapy, and/or radiation. The overall impact on time from abnormal CT to lung cancer surgery was reduced by > 5 months from 180 to 20 days. Substantial improvements were made in timeliness and reliability in caring for veterans with lung cancer.12
Groundbreaking work and exceptional results continued in the second phase for lung cancer care. In addition, the creation of a prostate cancer care web-based clinical measurement tool helped to improve the ability to proactively manage patients. The tool included same-day scheduling of biopsy and urology appointments for veterans with possible prostate cancer and the development of a protocol for expedited high-risk patients with metastatic disease. Ultimately, the wait time from urology consult to diagnosis was cut from 96 to 46 days for veterans with prostate cancer (Figure 4).
Once the face-to-face CCC process was established, tested, refined, and replicated successfully, the virtual team proved to be a cost-effective model. The virtual team did not travel to LSs, a major source of expense, so a process was set in place for their participation in all other facets of the collaborative. This led to the pilot testing of national virtual collaboratives (eg, specialty and surgical care collaboratives).
The toolkits for lung and CRC (phases 1 and 2) were organized, standardized, and disseminated throughout the VA to provide specific knowledge and tools to improve cancer care. The content of toolkits was primarily developed and/or identified by CCC participants. Funding for the toolkits was secured by OQP and SR, which led to the creation of the integration and crosswalk documents (eAppendix 7, available at fedprac.com/AVAHO).
In phase 3, lung cancer care teams showed the most improvement among all 3 phases of the collaborative. Aims statements in lung cancer process showed an increased percentage of improvement in all phases. Weekly multidisciplinary meetings provided a mechanism to rapidly review patients and triage appropriate pathways in the treatment algorithm. Open communication among sites and disciplines was vital and increased participation by physicians to identify ways to expedite diagnosis and treatment of lung cancer. In addition to access and timeliness of care (accommodating patients’ preference for scheduling), the teams identified areas they deemed important for successful programs and developed advisory panels that focused on quality, such as tumor boards, clinical trials, patient education, cancer care coordinator/navigator, survivorship, standard order sets and progress notes, reliable handoff, chemotherapy and radiation make/buy tools, head and neck toolkit, clinical documentation, chemotherapy efficiency, and Veterans Equitable Resource Allocation recovery for metastatic cancer.
Based on the evaluation results, participants gave their highest average ratings to items that asked about the general potential of SR to improve patient care and patient satisfaction, team dynamics, site leadership support; confidence in self, team, and coach; and the general potential of SR to improve staff satisfaction. Participants gave their lowest ratings to questions that asked about having the necessary time and resources to implement SR initiatives at their site as well as the level of active engagement by site leadership in SR work.
Click here to read the digital edition.
A collaboration between clinicians and industrial engineers resulted in significant improvements in cancer screening, the development of toolkits, and more efficient care for hepatocellular carcinoma and breast, colorectal, lung, head and neck, and prostate cancers.
Cancer is one of the most common causes of premature death and disability that requires long-term follow-up surveillance and oftentimes ongoing treatment for survivors that can lead to important health, psychosocial
Like other cancer treatment systems, the VA faces some challenges in timeliness, surveillance, and quality of the cancer care process.12-18 Although implementation of cancer patientcentered home care and other efforts were developed to improve delivery and efficiency of cancer care in VA and non-VA facilities, the patient continuum of care remains convoluted.2,19-23
In 2004, the Clinical Cancer Care Collaborative (C4), a national VA program, was launched to improve timeliness, quality, access improvement, efficiency, and the “sustainability and spread” of successful programs at the VA. This program included representatives throughout the VA and encompassed cancer care coordinators (clinical nurse navigators), advisory panels, and a multidisciplinary team of clinicians.
In 2009, the VA promoted the Cancer Care Collaborative (CCC) to focus on optimizing the timeliness and quality of colorectal, breast, lung, prostate, and hematologic cancer care throughout the VA health care system. The VA Office of Systems Redesign (SR) partnered with the VA-Center for Applied Systems Engineering (VA-CASE) Veteran Engineering Resource Center (VERC), including industrial engineers (IEs) to provide their expertise and support. The CCC provided a forum to develop teams; set aims; and map, measure, analyze, and implement changes to assure timely diagnosis and initiation of evidence-based treatment and subsequently sustain the practices that led to improvements in these areas.
The CCC structure was separated into 6 distinct support areas: (1) industrial/systems engineering support; (2) informatics and clinical application support; (3) development and dissemination of improvement resource guides; (4) real-time and rapid-cycle evaluation tools and approaches; (5) application of advanced operational systems engineering techniques, such as simulation and modeling to inform further system optimization; and (6) advisory panels focused on quality topics that were identified, developed, implemented, and evaluated by the participants with support from the CCC faculty.
Here the authors describe the framework of the CCC model developed by VA-CASE, demonstrate the performance improvement results of teams focusing on several types of cancer, and highlight the key indicators to best practices.
Methods
Figure 1 outlines the CCC 3-Phase Conceptual Model. Phase 1 included diagnosis (screening and symptoms); phase 2 included treatment (from diagnosis to beyond treatment); and phase 3 was designed for hub and spoke facilities where screening/diagnosis occurs in a smaller (spoke) facility and treatment occurs in the larger (hub) facility.
In the first phase, 18 facility-based teams were selected through an application and interview process and immediately applied SR to their team’s specific improvement projects, which included the following cancers: breast, colorectal, lung, and prostate.
In addition to the cancer types covered in the initial phase, phase 2 also included hepatocellular carcinoma (HCC) and head and neck cancers. National VHA Toolkits were products that developed from and for use in lung and colorectal cancers (CRCs) (phases 1 and 2). These were organized and disseminated throughout the entire VA, offering specific knowledge and tools that could be applied to improving cancer care. The toolkit included guidance documents, specific process examples, and items that could be downloaded into Microsoft SharePoint (Redmond, WA) for adaptation and use by VA facilities. The toolkit contents were primarily developed and/or identified by CCC participants and funded by the VA Office of Quality and Performance (OQP) and SR. The toolkits included links to the following resources for each cancer type in phase 2: quality indicators, tool tables, timeliness measures, understanding the continuum of care, and a resource entitled, “How Can the Quality Metrics Help Me?” (eAppendix 1, available at fedprac.com/AVAHO).
The phase 3 collaborative was designed for hub and spoke facilities by focusing on current state vs ideal state processes, communication patterns, and care coordination between the hub and spoke facilities. There were 10 facilities in which all teams focused on lung cancer. Each facility was made up of 1 hub and had the ability to send up to 8 participants (from either the hub or the spoke facility) to the CCC workgroup meetings. Participants were specialists, radiologists, primary care providers, pathologists, nurses, nurse practitioners, or physician assistants.
Conceptual Model Deployment
The deployment of the CCC 3-phase conceptual model was based on the Institute for Healthcare Improvement (IHI) Breakthrough Series Collaborative Model.24,25 Implementation was carried out over 3 phases (2005-2011) after proper teaching, coaching, and learning sessions (LS)
Each LS incorporated instruction in basic systems engineering and Lean Six Sigma principles (an approach to quality improvement that focuses on reducing waste and variability) with practical, health care-based examples, case studies, and immediate application of the VA-TAMMCS (vision/analyze, team/aim, map, measure, change, sustain) SR organizational framework (Figure 2), tools, and methodologies to the process under investigation.26 The VA-TAMMCS (eAppendix 2, available at fedprac.com/AVAHO)
The CCC encouraged joint facilitation. A SR clinical coach, a VERC IE, and participating facilities were required to work together intensively (mentor and support) for 10 to 12 months. The mix of clinicians and engineers helped the facilities by bringing in diverse perspectives, which led to better decisions in the improvement of cancer care.28 During the CCC, the IEs partnered with and supported clinicians, using Lean Six Sigma and SR tools and approaches to health care quality improvement to quickly make improvements in efficiency and quality (eAppendix 3a, 3b, and 3c, available at fedprac.com/AVAHO).26,29
The IEs provided on-site support at all participating VAMCs during all 3 phases by providing the clinical teams with a variety of VA-TAMMCS process improvement tools to support the analysis and improvement of their organizations.
Data Collection
As part of the overall improvement process, the facilities worked on several aim statements in order to improve a primary constraint; such as timeliness and quality of care. An aim statement communicates what you want to do (eg, reduce, improve, or eliminate), by how much, and when. In order to improve timeliness, the CCC focused on measures from first evidence to tissue diagnosis, from diagnosis to treatment, and also intermediate measures, such as time from positron emission tomography scan ordered to completion. While working on overall quality of care unique to cancer, the CCC focused on measures related to documentation compliance and consistency of care provided to patients.
Phase 1
Facilities were to optimize their process (time from initial suspicion to diagnosis). Hence, participating facilities were allowed to simply identify their aim statement and pick and choose the area of focus.
Phases 2 and 3
Timeliness Aims. These aims were addressed through improvements in information technology in the Computerized Patient Record System (CPRS) electronic medical record by creating electronic order sets containing codes that alert providers daily to retrieve and follow up on abnormal test results. Primary care physicians and front desk staff also were educated on the use of these order sets and to schedule a follow-up test or specialist consult within 3 to 7 days.
Aim 1: Reduce to 15 days the time from initial suspicion to diagnosis within 1 year.
Aim 2: Reduce to 30 days the time from diagnosis to start of treatment within 1 year.
Quality Aim. Improve the compliance rate of identified quality indicators to 100% within 1 year.
Measurement Tools
The Cancer Care Measurement Tools were designed to support the OQP performance metric (Figure 3). The OQP creates and collects data on evidence-based national benchmarks to measure the quality of preventive and therapeutic health care services at the facility, VISN, and national levels. These metrics may be performance measures, performance monitors, quality indicators, and special studies, among other measures, to support clinicians, managers, and employees in improving care to veterans.
For each of the 3 CCC phases, VA-CASE IEs facilitated the development of standardized measurement and tracking tools for each cancer type. The tools identified key timeliness and quality measures as a function of entered patient data (eAppendixes 4-7, available at fedprac.com/AVAHO).
Quality Improvement Toolkit Series
The Quality Improvement Toolkit Series (QITS) was created for VA clinical managers and policy makers to improve diagnosis, treatment, and patient outcomes for high-priority conditions. The goal of the QITS is to serve as the cancer care improvement resource guide to produce and disseminate the National Quality Improvement Toolkit resource.
Each tool included in the QITS is matched to 1 or more metrics of the OQP (such as a performance measure or quality indicator). For example, the types of tools include CPRS order sets and templates, enhanced registries and patient databases, service agreements, and care process flow maps. Each toolkit served as a resource for improving facility performance on a specific set of established performance measures and/or quality indicators. Toolkits that helped VA facilities improve performance on OQP quality indicators and performance measures were based on the VA-TAMMCS model and continuous improvement that was tailored to the structure and needs of the VA system. The VA-CASE staff provided guidance on the criteria for inclusion in the toolkits to promote best practice and quality in clinical practice. The criteria used by a condition-specific expert panel were based on whether or not it was (1) not already part of VA routine care nationwide; (2) can be matched to 1 or more VA quality metrics/indicators; and (3) currently in use at a health care facility (innovative VA colleagues nationwide and by non-VA health care organizations).
Evaluation
After each LS, VA CCC evaluation data were collected using standardized 5-point Likert scale questions.
Results
Industrial engineers provided > 1,200 days of on-site support across the 60 teams and built 63 flow maps and 47 customized tools based on the team’s requests throughout the implementation period. Throughout the 3-phase CCC, the IEs developed standardized measurement and tracking tools for each cancer type (lung, colorectal, prostate, head and neck, and HCC). Outcomes included the sharing of best practices that spread across programs (uploaded to the national QITS site, available only to VA employees); as well as enterprisewide development of the special interest group (eg, VHA survivorship), which led to a national survivorship toolkit.
The table illustrates the overall collaborative impact across the CCC. In phase 1, 78% of the 64 aims (breast, CRC, lung, prostate) were met at 18 facilities. In phase 2, 72% of the 94 aims (CRC; HCC; and head and neck, lung, and prostate cancers) were met at 21 facilities. In phase 3, 47% of the 64 aims for head and neck and lung cancer were met at 11 facilities. The difference in the percentage of aims met during each phase was due to the variations in complexity of cancer types as well as additional logistic barriers at each institution.
Discussion
Overall, the CCC had a positive impact that improved timeliness, accessibility, and quality of the cancer care process in participating VAMCs. The majority of VAMCs focused on optimizing the lung cancer care process in all the phases of the collaborative, given that lung cancer suspicion-to-treatment process is highly complex, requiring multiple departments to coordinate workup and care, leading to the greatest room for improvement.
Industrial engineers introduced a variety of approaches to improvement to the collaborative teams, and they were integral to the development of standardized measurement and tracking tools for each type of cancer, introducing advanced SR methods for specific aims and performing appropriate data analysis. The ability of the VA system to recognize where improvements were needed was complemented by the efforts of VA clinicians and administration with direction from VERC IEs and their toolkits. Improvements were made, sometimes decreasing time from diagnosis to treatment by 50%. The VA facilities were encouraged to sustain this improvement using the toolkits with continued data gathering and implementation. In phase 1, lung cancer improvements included (1) establishing the multidisciplinary clinic, multidisciplinary rounds, and improved communication among key service lines; (2) developing a database (measurement tool) to prospectively track all cancer patients; (3) scheduling weekly multidisciplinary meetings to provide a mechanism to rapidly review patients and triage to appropriate pathways in the treatment algorithm; and (4) increasing physician participation, including oncologists, surgeons, radiologists, and radiation oncologists, to identify methods and process
changes that could eliminate wasteful steps and improve access for expediting diagnosis and treatment of patients with lung cancer who require surgery, chemotherapy, and/or radiation. The overall impact on time from abnormal CT to lung cancer surgery was reduced by > 5 months from 180 to 20 days. Substantial improvements were made in timeliness and reliability in caring for veterans with lung cancer.12
Groundbreaking work and exceptional results continued in the second phase for lung cancer care. In addition, the creation of a prostate cancer care web-based clinical measurement tool helped to improve the ability to proactively manage patients. The tool included same-day scheduling of biopsy and urology appointments for veterans with possible prostate cancer and the development of a protocol for expedited high-risk patients with metastatic disease. Ultimately, the wait time from urology consult to diagnosis was cut from 96 to 46 days for veterans with prostate cancer (Figure 4).
Once the face-to-face CCC process was established, tested, refined, and replicated successfully, the virtual team proved to be a cost-effective model. The virtual team did not travel to LSs, a major source of expense, so a process was set in place for their participation in all other facets of the collaborative. This led to the pilot testing of national virtual collaboratives (eg, specialty and surgical care collaboratives).
The toolkits for lung and CRC (phases 1 and 2) were organized, standardized, and disseminated throughout the VA to provide specific knowledge and tools to improve cancer care. The content of toolkits was primarily developed and/or identified by CCC participants. Funding for the toolkits was secured by OQP and SR, which led to the creation of the integration and crosswalk documents (eAppendix 7, available at fedprac.com/AVAHO).
In phase 3, lung cancer care teams showed the most improvement among all 3 phases of the collaborative. Aims statements in lung cancer process showed an increased percentage of improvement in all phases. Weekly multidisciplinary meetings provided a mechanism to rapidly review patients and triage appropriate pathways in the treatment algorithm. Open communication among sites and disciplines was vital and increased participation by physicians to identify ways to expedite diagnosis and treatment of lung cancer. In addition to access and timeliness of care (accommodating patients’ preference for scheduling), the teams identified areas they deemed important for successful programs and developed advisory panels that focused on quality, such as tumor boards, clinical trials, patient education, cancer care coordinator/navigator, survivorship, standard order sets and progress notes, reliable handoff, chemotherapy and radiation make/buy tools, head and neck toolkit, clinical documentation, chemotherapy efficiency, and Veterans Equitable Resource Allocation recovery for metastatic cancer.
Based on the evaluation results, participants gave their highest average ratings to items that asked about the general potential of SR to improve patient care and patient satisfaction, team dynamics, site leadership support; confidence in self, team, and coach; and the general potential of SR to improve staff satisfaction. Participants gave their lowest ratings to questions that asked about having the necessary time and resources to implement SR initiatives at their site as well as the level of active engagement by site leadership in SR work.
Click here to read the digital edition.
1. Klemp JR. Breast cancer prevention across the cancer care continuum. Semin Oncol Nurs. 2015;31(2):89-99.
2. Tralongo P, Ferraù F, Borsellino N, et al. Cancer patientcentered home care: a new model for health care in oncology. Ther Clin Risk Manag. 2011;7:387-392.
3. Institute of Medicine of the National Academy of Sciences. Delivering high-quality cancer care: charting a new course for a system in crisis. http://nationalacademies.org/hmd/~/media/Files/Report%20Files/2013/Quality-Cancer-Care/qualitycancercare_rb.pdf. Published September 2013. Accessed April 6, 2017.
4. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
5. Jabaaij L, van den Akker M, Schellevis FG. Excess of health care use in general practice and of comorbid chronic conditions in cancer patients compared to controls. BMC Fam Pract. 2012;13:60
6. Brazil K, Whelan T, O’Brien MA, Sussman J, Pyette N, Bainbridge D. Towards improving the co-ordination of supportive cancer care services in the community. Health Policy. 2004;70(1):125-131.
7. Husain A, Barbera L, Howell D, Moineddin R, Bezjak A, Sussman J. Advanced lung cancer patients’ experience with continuity of care and supportive care needs. Support Care Cancer. 2013;21(5):1351-1358.
8. Sayed S, Moloo Z, Bird P, et al. Breast cancer diagnosis in a resource poor environment through a collaborative multidisciplinary approach: the Kenyan experience. J Clin Pathol. 2013;66(4):307-311.
9. Morgan PA, Murray S, Moffatt CJ, Honnor A. The challenges of managing complex lymphoedema/chronic oedema in the UK and Canada. Int Wound J. 2011;9(1):54-69.
10. Renshaw M. Lymphorrhoea: ‘leaky legs’ are not just the nurse’s problem. Br J Community Nurs. 2007;12(4):S18-S21.
11. Morgan PA. Health professionals’ ideal roles in lympoedema management. Br J Community Nurs. 2006;11(suppl):5-8.
12. Hunnibell LS, Rose MG, Connery DM, et al. Using nurse navigation to improve timeliness of lung cancer care at a veterans hospital. Clin J Oncol Nurs. 2012;16(1):29-36.
13. Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179(7):
595-600.
14. Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133(5):1167-1173.
15. Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181.
16. Walling AM, Tisnado D, Asch SM, et al. The quality of supportive cancer care in the Veterans Affairs system and targets for improvement. JAMA Intern Med. 2013;173(22):2071-2079.
17. Keating NL, Landrum MB, Lamont EB, et al. Quality of care for older patients with cancer in the Veterans Health Administration versus the private sector: a cohort study. Ann Intern Med. 2011;154(11):727-736.
18. Kaiser AM, Nunoo-Mensah JW, Wasserberg N. Surgical volume and long-term survival following surgery for colorectal cancer in the Veterans Affairs Health-Care System. Am J Gastroenterol. 2005;100(1):250.
19. Abrahams E, Foti M, Kean MA. Accelerating the delivery of patient-centered, high-quality cancer care. Clin Cancer Res. 2015;21(10):2263-2267.
20. Taplin SH, Weaver S, Salas E, et al. Reviewing cancer care team effectiveness. J Oncol Pract. 2015;11(3):239-246.
21. Kosty MP, Bruinooge SS, Cox JV. Intentional approach to team-based oncology care: evidence-based teamwork to improve collaboration and patient engagement. J Oncol Pract. 2015;11(3):247-248.
22. Ko NY, Darnell JS, Calhoun E, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. J Clin Oncol. 2014;32(25):2758-2764.
23. Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12(1):4-13.
24. Institute for Healthcare Improvement. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston, MA: Institute for Healthcare Improvement; 2003.
25. Boushon B, Provost L, Gagnon J, Carver P. Using a virtual breakthrough series collaborative to improve access in primary care. Jt Comm J Qual Patient Saf. 2006;32(10):573-584.
26. Womack JP, Jones DT. Lean Thinking: Banish Waste and Create Wealth in Your Corporation. New York, NY: Simon & Schuster; 1996.
27. Bidassie B, Davies ML, Stark R, Boushon B. VA experience in implementing patient-centered medical home using a breakthrough series collaborative. J Gen Intern Med. 2014;29(suppl 2):S563-S5671.
28. Bidassie B, Williams LS, Woodward-Hagg H, Matthias MS, Damush TM. Key components of external facilitation in an acute stroke quality improvement collaborative in the Veterans Health Administration. Implement Sci. 2015;10(1):69.
29. Woodward-Hagg H, Workman-Germann J, Flanagan M, et al. Implementation of systems redesign: approaches to spread and sustain adoption. In: Henriksen K, Battles J, Keyes M, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches: Vol. 2: Culture and Redesign. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
30. American Cancer Society. National roundtable recognizes leaders in colorectal cancer prevention effort with 80% by 2018 National Achievement Award. [press release]. http://pressroom.cancer.org/2017-02-01-National-Colorectal-Cancer-Roundtable-Recognizes-Leaders-in-Colorectal-Cancer-Prevention-Effort-with-80-by-2018-National-Achievement-Award. Published February 1, 2017. Accessed March 29, 2017.
31. Margolis PA, Lannon CM, Stuart JM, Fried BJ, Keyes-Elstein L, Moore DE Jr. Practice based education to improve delivery systems for prevention in primary care: randomized trial. BMJ. 2004;328(7436):388.
1. Klemp JR. Breast cancer prevention across the cancer care continuum. Semin Oncol Nurs. 2015;31(2):89-99.
2. Tralongo P, Ferraù F, Borsellino N, et al. Cancer patientcentered home care: a new model for health care in oncology. Ther Clin Risk Manag. 2011;7:387-392.
3. Institute of Medicine of the National Academy of Sciences. Delivering high-quality cancer care: charting a new course for a system in crisis. http://nationalacademies.org/hmd/~/media/Files/Report%20Files/2013/Quality-Cancer-Care/qualitycancercare_rb.pdf. Published September 2013. Accessed April 6, 2017.
4. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
5. Jabaaij L, van den Akker M, Schellevis FG. Excess of health care use in general practice and of comorbid chronic conditions in cancer patients compared to controls. BMC Fam Pract. 2012;13:60
6. Brazil K, Whelan T, O’Brien MA, Sussman J, Pyette N, Bainbridge D. Towards improving the co-ordination of supportive cancer care services in the community. Health Policy. 2004;70(1):125-131.
7. Husain A, Barbera L, Howell D, Moineddin R, Bezjak A, Sussman J. Advanced lung cancer patients’ experience with continuity of care and supportive care needs. Support Care Cancer. 2013;21(5):1351-1358.
8. Sayed S, Moloo Z, Bird P, et al. Breast cancer diagnosis in a resource poor environment through a collaborative multidisciplinary approach: the Kenyan experience. J Clin Pathol. 2013;66(4):307-311.
9. Morgan PA, Murray S, Moffatt CJ, Honnor A. The challenges of managing complex lymphoedema/chronic oedema in the UK and Canada. Int Wound J. 2011;9(1):54-69.
10. Renshaw M. Lymphorrhoea: ‘leaky legs’ are not just the nurse’s problem. Br J Community Nurs. 2007;12(4):S18-S21.
11. Morgan PA. Health professionals’ ideal roles in lympoedema management. Br J Community Nurs. 2006;11(suppl):5-8.
12. Hunnibell LS, Rose MG, Connery DM, et al. Using nurse navigation to improve timeliness of lung cancer care at a veterans hospital. Clin J Oncol Nurs. 2012;16(1):29-36.
13. Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179(7):
595-600.
14. Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133(5):1167-1173.
15. Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176-3181.
16. Walling AM, Tisnado D, Asch SM, et al. The quality of supportive cancer care in the Veterans Affairs system and targets for improvement. JAMA Intern Med. 2013;173(22):2071-2079.
17. Keating NL, Landrum MB, Lamont EB, et al. Quality of care for older patients with cancer in the Veterans Health Administration versus the private sector: a cohort study. Ann Intern Med. 2011;154(11):727-736.
18. Kaiser AM, Nunoo-Mensah JW, Wasserberg N. Surgical volume and long-term survival following surgery for colorectal cancer in the Veterans Affairs Health-Care System. Am J Gastroenterol. 2005;100(1):250.
19. Abrahams E, Foti M, Kean MA. Accelerating the delivery of patient-centered, high-quality cancer care. Clin Cancer Res. 2015;21(10):2263-2267.
20. Taplin SH, Weaver S, Salas E, et al. Reviewing cancer care team effectiveness. J Oncol Pract. 2015;11(3):239-246.
21. Kosty MP, Bruinooge SS, Cox JV. Intentional approach to team-based oncology care: evidence-based teamwork to improve collaboration and patient engagement. J Oncol Pract. 2015;11(3):247-248.
22. Ko NY, Darnell JS, Calhoun E, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. J Clin Oncol. 2014;32(25):2758-2764.
23. Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12(1):4-13.
24. Institute for Healthcare Improvement. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston, MA: Institute for Healthcare Improvement; 2003.
25. Boushon B, Provost L, Gagnon J, Carver P. Using a virtual breakthrough series collaborative to improve access in primary care. Jt Comm J Qual Patient Saf. 2006;32(10):573-584.
26. Womack JP, Jones DT. Lean Thinking: Banish Waste and Create Wealth in Your Corporation. New York, NY: Simon & Schuster; 1996.
27. Bidassie B, Davies ML, Stark R, Boushon B. VA experience in implementing patient-centered medical home using a breakthrough series collaborative. J Gen Intern Med. 2014;29(suppl 2):S563-S5671.
28. Bidassie B, Williams LS, Woodward-Hagg H, Matthias MS, Damush TM. Key components of external facilitation in an acute stroke quality improvement collaborative in the Veterans Health Administration. Implement Sci. 2015;10(1):69.
29. Woodward-Hagg H, Workman-Germann J, Flanagan M, et al. Implementation of systems redesign: approaches to spread and sustain adoption. In: Henriksen K, Battles J, Keyes M, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches: Vol. 2: Culture and Redesign. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
30. American Cancer Society. National roundtable recognizes leaders in colorectal cancer prevention effort with 80% by 2018 National Achievement Award. [press release]. http://pressroom.cancer.org/2017-02-01-National-Colorectal-Cancer-Roundtable-Recognizes-Leaders-in-Colorectal-Cancer-Prevention-Effort-with-80-by-2018-National-Achievement-Award. Published February 1, 2017. Accessed March 29, 2017.
31. Margolis PA, Lannon CM, Stuart JM, Fried BJ, Keyes-Elstein L, Moore DE Jr. Practice based education to improve delivery systems for prevention in primary care: randomized trial. BMJ. 2004;328(7436):388.
Interdisciplinary Geriatric Difficult Case Conference: Innovative Education Across the Continuum
From Wheaton Franciscan Healthcare (Ms. Fedel), Aspirus (Ms. Hackbarth), and Aurora Health Care (Mr. Malsch and Ms. Pagel).
Abstract
- Background: There is a nationwide shortage of geriatric prepared providers. Caring for complex older adults is challenging.
- Objective: To develop an efficient and affordable way to educate members of the interdisciplinary team involved in the care of geriatric patients.
- Methods: A team from 3 area health systems developed a plan to present monthly case studies via teleconference. Cases are presented by a direct caregiver using the Wisconsin Star Method to facilitate analysis of the case. A geriatric expert and another member of the team presents teaching points, and questions are elicited and discussed.
- Results: The team has completed 18 consecutive monthly teleconferences. Participant satisfaction has been favorable. Participation on the call has increased approximately 300% since the initiation of the program.
- Conclusion: The case teleconference provides an accessible and affordable educational forum that provides learners an opportunity to improve their knowledge in care of older adults.
The number of older adults in the United States will nearly double between 2005 and 2030 [1] as the baby boom generation begins turning 65 and as life expectancy for older Americans increases. The Institute of Medicine’s (IOM) landmark report Retooling for an Aging America: Building the Health Care Workforce states that “unless action is taken immediately, the health care workforce will lack the capacity (in both size and ability) to meet the needs of older patients in the future [1].” One of their recommendations is to explore ways to widen the duties and responsibilities of workers at various levels of training. More health care providers need to be trained in the basics of geriatric care and should be capable of caring for older patients.
Team-based care is becoming more prevalent. Care delivered by interdisciplinary teams have been shown to improve patient outcomes [2]. A team led by one of the authors (PF) developed an intervention to increase the geriatric and teamwork competencies of interdisciplinary teams who serve patients throughout Wisconsin. The Interdisciplinary Geriatric Difficult Case Conference Call (IGDCC) is sponsored monthly by 3 Wisconsin health systems. The purpose is to provide opportunities to discuss clinical cases, to learn from one another and from experts, and to elevate the level of geriatric care in the states of Wisconsin, Michigan, and beyond. Each month a difficult case is presented by a clinician involved in that patient’s care. Time is allotted for participants to ask questions, and teaching points are shared by a clinical expert to highlight concepts and provide additional context. The IGDCC is meant to be a joint learning exercise to explore a specific difficult patient situation and learn skills and knowledge to improve care and transitions for older adults. The conference call is not a critique of the care, but rather an opportunity to jointly learn from the challenging situations all experience.
Background
The IGDCC was created by four members of 3 health systems in Wisconsin: Wheaton Franciscan Healthcare, Aspirus, and Aurora Health Care. The health systems serve and partially overlap on a broad geographic and demographic area of Wisconsin. The 4 members collaborated on numerous projects in the past, including Nurses Improving Case for Health System Elders (NICHE) implementation [3]. A common concern among the team is the management of challenging geriatric clinical patients and having a prepared workforce to meet those challenges.
Problem/Issue
As mentioned above, the older adult population is increasing, and these statistics are reflected in our service area [4]. Exacerbating these demographic changes is a shortage of health care workers in all disciplines, inadequate geriatric training, and the increased prevalence of multiple chronic conditions. Older adults also have higher rates of 30-day readmissions as well as higher rates of functional decline and medical errors during hospital stays [5,6]. Effective interprofessional teamwork is essential for the delivery of high-quality patient care in an increasingly complex health environment [7]. The IOM’s Future of Nursing report recommends that nurses, who represent the largest segment of the US health workforce, should achieve higher levels of training and be full partners in redesigning health care [8]. Unfortunately, effective care is hampered by poor coordination, limited communication, boundary infringement, and lack of understanding of roles [9]. Meta-analyses have demonstrated that there is a positive relationship between team training interventions and outcomes [10,11].
Objectives
The objective of the IGDCC is to elevate the level of geriatric care in the region by providing an accessible and affordable forum for the education of health care workers involved in the care of our most vulnerable population. To meet this challenge, the 4 founding members of IGDCC utilized the Aurora Health Care Geriatric Fellow’s Most Difficult Case (GFMCC) conference format as a model [12,13]. All disciplines are encouraged to participate, with announcements sent out via the leadership at the participating hospital systems. Participants have the option to call into the conference and teleconference via their own personal telephone and computer; in addition, each participating hospital system frequently hosts an open forum teleconference room where participants also may join a group.
Conference Components
The team uses the Wisconsin Star Method framework for presentation and discussion of the case. The Star Method, developed by Timothy Howell, enables clinical data about a person to be mapped out onto a single field with 5 domains: medications, medical, behavioral, personal, and social [14], creating a visual representation of the complicated and interacting physical, emotional, and social issues of older adults (Figure). By becoming comfortable using this method, the learner can use a similar approach in their clinical practice to address the needs of the patient in a holistic manner.
The case call concludes with expert teaching points from both a geriatric expert and a member of the interdisciplinary team. The interdisciplinary team member is chosen based on the key issues raised by the case. For example, cases that are made complex due to polypharmacy and adverse drug reactions might have a pharmacist presenting pertinent take-home message for the learner. In addition, geriatric teaching experts (ie, a geriatrician or advanced practice geriatric nursing specialist) provide the learner with insights that they can apply to their future practice. Often times the teaching points consist of an analysis of the various geriatric syndromes and how they can be managed in the complex older adult.
Implementation
Implementation of the IGDCC is coordinated by an oversight team with representation from each of the 3 sponsoring health systems. The oversight team currently includes 4 members: 3 geriatric clinical nurse specialists and a geriatric service line administrator. The team is responsible for:
- Planning the conference call schedule
- Making arrangements for case presenters and experts to contribute teaching points
- Registering participants and sharing written materials with participants
- Publicizing and encouraging attendance
- Soliciting feedback for continual improvement
- Exploring and implementing new ways to maximize learning.
Team members share duties and rotate case presentations. The Aurora and Wheaton Franciscan systems provide the geriatric specialists who provide the expert teaching points. The Aspirus system provides the conference line and webinar application and supports publicity and evaluations. All 3 systems are supported by a geriatric clinical nurse specialist who identifies and helps prepare presenters, case presentations, and call participants. Over time, the conference call format has evolved into a webinar format, allowing participants to either phone into the call for audio only or participate via both audio and visual. The visual allows participants to watch on their computer screens while the case is presented using the Star Method. During the call, a member of the oversight team adds clinical details by typing into a Word template of a blank star, adding information for each of the 5 domains in real-time as the case is discussed. Another member of the team facilitates the call, introducing presenters and experts, describing the Star Method, and offering “housekeeping” announcements. The facilitator also watches the timing to make certain the agenda is followed and the call begins and ends on time. During the call, another member of the team updates the attendance spreadsheet and makes a recording of each session.
Some participating facilities reserve a meeting room and project the webinar onto a screen for shared viewing. One of the participating sites has done this quite successfully with a growing group of participants coming together to watch the case during their lunch hour. This allows an opportunity for group discussion—when the conference call is on “mute” so as not to disrupt learners at other locations.
Measurement/Analysis
Attendance has steadily increased. In CY2015 from January to September, the mean attendance per month was 29.1 (mode, 17). The maximum per month was 62 (September 2015). The program enjoyed a boost in attendance beginning in July 2015 when Nurses Improving Care of Healthsystem Elders (NICHE) [3] began promoting the call-in opportunity to its NICHE Coordinators at member health systems. In June 2015, the technology was improved to allow for recorded sessions, and the recordings are growing in popularity from 2 listeners per month in July 2014 to 23 listeners per month in September 2015.
Lessons Learned
In comparing the IGDCC with similar conference call educational offerings, the team found that the program was unique in 2 areas. First, in addition to having a rich discussion in the care of frail older adults with experts in the field, the team also sought to help our staff learn how to present a difficult case to their peers. Three of our 4 committee members are geriatric clinical nurse specialists (a fourth is a clinical nurse specialist from Aspirus who assists periodically) who have been able to mentor, guide, and encourage interdisciplinary team members to present a challenging case. Many presenters had never presented a difficult case in this format. Presenters found the process fun and rewarding and have offered to present cases again in the future.
A second unique feature was utilizing the Wisconsin Star Method rather than focusing on a typical medical model framework for discussing a challenging case. The Star Method allows participants to increase their proficiency in providing comprehensive care while being more confident and mindful in addressing the complicated interacting physical, emotional and social issues of older adults [13].
A monthly post-call debriefing with committee members to review the strengths and weakness of the call was key to growing the program. The committee was able to critically review the process of the call, review participant surveys and discuss next steps. Adding a webinar approach, automatic email notification of calls, participant electronic survey, recording the call, and the addition of offering contact hours were some of the action items that were a result of monthly debriefing calls.
The team also found the 3-system collaboration to be beneficial. Aspirus has a large rural population, and Wheaton and Aurora have a diverse population, and each adds to the participant’s experience. Each IGDCC was rotated between the systems, which did not put the burden on any one health system. An annual call assignment listing was maintained for noting which system was responsible for the case each month and whether the geriatric expert was assigned/confirmed. Identifying the committee’s individual and collective group expertise was helpful in the overall project planning. The committee also developed a standard presenter guide and template and an expert teaching guide so the monthly IGDCC were consistent.
Challenges
The committee did not have a budget. Participation on the committee was in-kind funding from each system. Aspirus used its electronic system in place at the time to support the project. Interactive conference call education platform can be challenging with multiple participants on an open line who may not mute their phone. Often times, when a group of participants are calling in from one phone line it is difficult to know how many people are attending the IGDCC. It can be challenging at times to facilitate the call during the discussion component as participants occasionally talk over each other.
Current Status/Future Directions
The team has completed 18 consecutive monthly IGDCCs. Our participation rate has tripled. Participant satisfaction remains favorable. The team is now offering 1 contact hour to participants, and our invitations to participate have been extended to national health care groups. Challenging cases will be presented from community sources outside the hospital. Focusing attention on elevating the level of geriatric care in our region using a community educational approach will give us new opportunities for collaborating on best practice in multiple settings across the care continuum.
Acknowledgment: The planning team acknowledges Evalyn Michira, MSN, RN, PHN, AGCNS-BC, for her assistance in call presentations.
Corresponding author: Margie Hackbarth, MBA, margie.hackbarth@aspirus.org.
Financial disclosures: none.
1. Institute of Medicine. Retooling for an aging America: Building the health care workforce. Washington, DC: National Academies Press; 2008.
2. Mitchell P, Wynia M, Golden R, et al. Core principles and values of effective team-based health care. Discussion paper. Washington, DC; Institute of Medicine; 2012.
3. Nurses Improving Care for Healthsystem Elders. Accessed 1 Dec 2015 at www.nicheprogram.org/.
4. Wisconsin Department of Health Services. Southeastern region population report: 1 Jul 2013. Accessed 16 Feb 2015 at www.dhs.wisconsin.gov/sites/default/files/legacy/population/13data/southeastern.pdf.
5. From the Centers for Disease Control and Prevention. Public health and aging: trends in aging--United States and worldwide. JAMA 2003;289:1371–3.
6. Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010;(29):1–20, 24.
7. Nembhard IM, Edmondson AC. Making it safe: The effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav 2006; 27:941–66.
8. Institute of Medicine. The future of nursing: leading change, advancing health. National Academies Press; 2011.
9. Reeves S, Zwarenstein M, Goldman et al. Interprofessional education: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2013;3:CD002213.
10. Salas E, Diaz Granados D, Klein C, et al. Does team training improve team performance? A meta-analysis. Hum Factors 2008;50:903–33.
11. Strasser DD, Burridge AB, Falconer JA, et al. Toward spanning the quality chasm: an examination of team functioning measures. Arch Phys Med Rehabil 2014;95:2220–3.
12. Roche VM, Torregosa H, Howell T, Malone ML. Establishing a treatment plan for an elder with a complex and incomplete medical history and multiple medical providers, diagnoses, and medications. Ann Long-Term Care 2012;20(9).
13. Roche VM, Arnouville J, Danto-Nocton ES, et al. Optimal management of an older patient with multiple comorbidities and a complex psychosocial history. Ann Long-Term Care 2011;19(9).
14. Wisconsin Geriatric Psychiatry Initiative. The Wisconsin Star Method. Accessed 19 Jan 2015 at wgpi.wisc.edu/wisconsin-star-method/.
From Wheaton Franciscan Healthcare (Ms. Fedel), Aspirus (Ms. Hackbarth), and Aurora Health Care (Mr. Malsch and Ms. Pagel).
Abstract
- Background: There is a nationwide shortage of geriatric prepared providers. Caring for complex older adults is challenging.
- Objective: To develop an efficient and affordable way to educate members of the interdisciplinary team involved in the care of geriatric patients.
- Methods: A team from 3 area health systems developed a plan to present monthly case studies via teleconference. Cases are presented by a direct caregiver using the Wisconsin Star Method to facilitate analysis of the case. A geriatric expert and another member of the team presents teaching points, and questions are elicited and discussed.
- Results: The team has completed 18 consecutive monthly teleconferences. Participant satisfaction has been favorable. Participation on the call has increased approximately 300% since the initiation of the program.
- Conclusion: The case teleconference provides an accessible and affordable educational forum that provides learners an opportunity to improve their knowledge in care of older adults.
The number of older adults in the United States will nearly double between 2005 and 2030 [1] as the baby boom generation begins turning 65 and as life expectancy for older Americans increases. The Institute of Medicine’s (IOM) landmark report Retooling for an Aging America: Building the Health Care Workforce states that “unless action is taken immediately, the health care workforce will lack the capacity (in both size and ability) to meet the needs of older patients in the future [1].” One of their recommendations is to explore ways to widen the duties and responsibilities of workers at various levels of training. More health care providers need to be trained in the basics of geriatric care and should be capable of caring for older patients.
Team-based care is becoming more prevalent. Care delivered by interdisciplinary teams have been shown to improve patient outcomes [2]. A team led by one of the authors (PF) developed an intervention to increase the geriatric and teamwork competencies of interdisciplinary teams who serve patients throughout Wisconsin. The Interdisciplinary Geriatric Difficult Case Conference Call (IGDCC) is sponsored monthly by 3 Wisconsin health systems. The purpose is to provide opportunities to discuss clinical cases, to learn from one another and from experts, and to elevate the level of geriatric care in the states of Wisconsin, Michigan, and beyond. Each month a difficult case is presented by a clinician involved in that patient’s care. Time is allotted for participants to ask questions, and teaching points are shared by a clinical expert to highlight concepts and provide additional context. The IGDCC is meant to be a joint learning exercise to explore a specific difficult patient situation and learn skills and knowledge to improve care and transitions for older adults. The conference call is not a critique of the care, but rather an opportunity to jointly learn from the challenging situations all experience.
Background
The IGDCC was created by four members of 3 health systems in Wisconsin: Wheaton Franciscan Healthcare, Aspirus, and Aurora Health Care. The health systems serve and partially overlap on a broad geographic and demographic area of Wisconsin. The 4 members collaborated on numerous projects in the past, including Nurses Improving Case for Health System Elders (NICHE) implementation [3]. A common concern among the team is the management of challenging geriatric clinical patients and having a prepared workforce to meet those challenges.
Problem/Issue
As mentioned above, the older adult population is increasing, and these statistics are reflected in our service area [4]. Exacerbating these demographic changes is a shortage of health care workers in all disciplines, inadequate geriatric training, and the increased prevalence of multiple chronic conditions. Older adults also have higher rates of 30-day readmissions as well as higher rates of functional decline and medical errors during hospital stays [5,6]. Effective interprofessional teamwork is essential for the delivery of high-quality patient care in an increasingly complex health environment [7]. The IOM’s Future of Nursing report recommends that nurses, who represent the largest segment of the US health workforce, should achieve higher levels of training and be full partners in redesigning health care [8]. Unfortunately, effective care is hampered by poor coordination, limited communication, boundary infringement, and lack of understanding of roles [9]. Meta-analyses have demonstrated that there is a positive relationship between team training interventions and outcomes [10,11].
Objectives
The objective of the IGDCC is to elevate the level of geriatric care in the region by providing an accessible and affordable forum for the education of health care workers involved in the care of our most vulnerable population. To meet this challenge, the 4 founding members of IGDCC utilized the Aurora Health Care Geriatric Fellow’s Most Difficult Case (GFMCC) conference format as a model [12,13]. All disciplines are encouraged to participate, with announcements sent out via the leadership at the participating hospital systems. Participants have the option to call into the conference and teleconference via their own personal telephone and computer; in addition, each participating hospital system frequently hosts an open forum teleconference room where participants also may join a group.
Conference Components
The team uses the Wisconsin Star Method framework for presentation and discussion of the case. The Star Method, developed by Timothy Howell, enables clinical data about a person to be mapped out onto a single field with 5 domains: medications, medical, behavioral, personal, and social [14], creating a visual representation of the complicated and interacting physical, emotional, and social issues of older adults (Figure). By becoming comfortable using this method, the learner can use a similar approach in their clinical practice to address the needs of the patient in a holistic manner.
The case call concludes with expert teaching points from both a geriatric expert and a member of the interdisciplinary team. The interdisciplinary team member is chosen based on the key issues raised by the case. For example, cases that are made complex due to polypharmacy and adverse drug reactions might have a pharmacist presenting pertinent take-home message for the learner. In addition, geriatric teaching experts (ie, a geriatrician or advanced practice geriatric nursing specialist) provide the learner with insights that they can apply to their future practice. Often times the teaching points consist of an analysis of the various geriatric syndromes and how they can be managed in the complex older adult.
Implementation
Implementation of the IGDCC is coordinated by an oversight team with representation from each of the 3 sponsoring health systems. The oversight team currently includes 4 members: 3 geriatric clinical nurse specialists and a geriatric service line administrator. The team is responsible for:
- Planning the conference call schedule
- Making arrangements for case presenters and experts to contribute teaching points
- Registering participants and sharing written materials with participants
- Publicizing and encouraging attendance
- Soliciting feedback for continual improvement
- Exploring and implementing new ways to maximize learning.
Team members share duties and rotate case presentations. The Aurora and Wheaton Franciscan systems provide the geriatric specialists who provide the expert teaching points. The Aspirus system provides the conference line and webinar application and supports publicity and evaluations. All 3 systems are supported by a geriatric clinical nurse specialist who identifies and helps prepare presenters, case presentations, and call participants. Over time, the conference call format has evolved into a webinar format, allowing participants to either phone into the call for audio only or participate via both audio and visual. The visual allows participants to watch on their computer screens while the case is presented using the Star Method. During the call, a member of the oversight team adds clinical details by typing into a Word template of a blank star, adding information for each of the 5 domains in real-time as the case is discussed. Another member of the team facilitates the call, introducing presenters and experts, describing the Star Method, and offering “housekeeping” announcements. The facilitator also watches the timing to make certain the agenda is followed and the call begins and ends on time. During the call, another member of the team updates the attendance spreadsheet and makes a recording of each session.
Some participating facilities reserve a meeting room and project the webinar onto a screen for shared viewing. One of the participating sites has done this quite successfully with a growing group of participants coming together to watch the case during their lunch hour. This allows an opportunity for group discussion—when the conference call is on “mute” so as not to disrupt learners at other locations.
Measurement/Analysis
Attendance has steadily increased. In CY2015 from January to September, the mean attendance per month was 29.1 (mode, 17). The maximum per month was 62 (September 2015). The program enjoyed a boost in attendance beginning in July 2015 when Nurses Improving Care of Healthsystem Elders (NICHE) [3] began promoting the call-in opportunity to its NICHE Coordinators at member health systems. In June 2015, the technology was improved to allow for recorded sessions, and the recordings are growing in popularity from 2 listeners per month in July 2014 to 23 listeners per month in September 2015.
Lessons Learned
In comparing the IGDCC with similar conference call educational offerings, the team found that the program was unique in 2 areas. First, in addition to having a rich discussion in the care of frail older adults with experts in the field, the team also sought to help our staff learn how to present a difficult case to their peers. Three of our 4 committee members are geriatric clinical nurse specialists (a fourth is a clinical nurse specialist from Aspirus who assists periodically) who have been able to mentor, guide, and encourage interdisciplinary team members to present a challenging case. Many presenters had never presented a difficult case in this format. Presenters found the process fun and rewarding and have offered to present cases again in the future.
A second unique feature was utilizing the Wisconsin Star Method rather than focusing on a typical medical model framework for discussing a challenging case. The Star Method allows participants to increase their proficiency in providing comprehensive care while being more confident and mindful in addressing the complicated interacting physical, emotional and social issues of older adults [13].
A monthly post-call debriefing with committee members to review the strengths and weakness of the call was key to growing the program. The committee was able to critically review the process of the call, review participant surveys and discuss next steps. Adding a webinar approach, automatic email notification of calls, participant electronic survey, recording the call, and the addition of offering contact hours were some of the action items that were a result of monthly debriefing calls.
The team also found the 3-system collaboration to be beneficial. Aspirus has a large rural population, and Wheaton and Aurora have a diverse population, and each adds to the participant’s experience. Each IGDCC was rotated between the systems, which did not put the burden on any one health system. An annual call assignment listing was maintained for noting which system was responsible for the case each month and whether the geriatric expert was assigned/confirmed. Identifying the committee’s individual and collective group expertise was helpful in the overall project planning. The committee also developed a standard presenter guide and template and an expert teaching guide so the monthly IGDCC were consistent.
Challenges
The committee did not have a budget. Participation on the committee was in-kind funding from each system. Aspirus used its electronic system in place at the time to support the project. Interactive conference call education platform can be challenging with multiple participants on an open line who may not mute their phone. Often times, when a group of participants are calling in from one phone line it is difficult to know how many people are attending the IGDCC. It can be challenging at times to facilitate the call during the discussion component as participants occasionally talk over each other.
Current Status/Future Directions
The team has completed 18 consecutive monthly IGDCCs. Our participation rate has tripled. Participant satisfaction remains favorable. The team is now offering 1 contact hour to participants, and our invitations to participate have been extended to national health care groups. Challenging cases will be presented from community sources outside the hospital. Focusing attention on elevating the level of geriatric care in our region using a community educational approach will give us new opportunities for collaborating on best practice in multiple settings across the care continuum.
Acknowledgment: The planning team acknowledges Evalyn Michira, MSN, RN, PHN, AGCNS-BC, for her assistance in call presentations.
Corresponding author: Margie Hackbarth, MBA, margie.hackbarth@aspirus.org.
Financial disclosures: none.
From Wheaton Franciscan Healthcare (Ms. Fedel), Aspirus (Ms. Hackbarth), and Aurora Health Care (Mr. Malsch and Ms. Pagel).
Abstract
- Background: There is a nationwide shortage of geriatric prepared providers. Caring for complex older adults is challenging.
- Objective: To develop an efficient and affordable way to educate members of the interdisciplinary team involved in the care of geriatric patients.
- Methods: A team from 3 area health systems developed a plan to present monthly case studies via teleconference. Cases are presented by a direct caregiver using the Wisconsin Star Method to facilitate analysis of the case. A geriatric expert and another member of the team presents teaching points, and questions are elicited and discussed.
- Results: The team has completed 18 consecutive monthly teleconferences. Participant satisfaction has been favorable. Participation on the call has increased approximately 300% since the initiation of the program.
- Conclusion: The case teleconference provides an accessible and affordable educational forum that provides learners an opportunity to improve their knowledge in care of older adults.
The number of older adults in the United States will nearly double between 2005 and 2030 [1] as the baby boom generation begins turning 65 and as life expectancy for older Americans increases. The Institute of Medicine’s (IOM) landmark report Retooling for an Aging America: Building the Health Care Workforce states that “unless action is taken immediately, the health care workforce will lack the capacity (in both size and ability) to meet the needs of older patients in the future [1].” One of their recommendations is to explore ways to widen the duties and responsibilities of workers at various levels of training. More health care providers need to be trained in the basics of geriatric care and should be capable of caring for older patients.
Team-based care is becoming more prevalent. Care delivered by interdisciplinary teams have been shown to improve patient outcomes [2]. A team led by one of the authors (PF) developed an intervention to increase the geriatric and teamwork competencies of interdisciplinary teams who serve patients throughout Wisconsin. The Interdisciplinary Geriatric Difficult Case Conference Call (IGDCC) is sponsored monthly by 3 Wisconsin health systems. The purpose is to provide opportunities to discuss clinical cases, to learn from one another and from experts, and to elevate the level of geriatric care in the states of Wisconsin, Michigan, and beyond. Each month a difficult case is presented by a clinician involved in that patient’s care. Time is allotted for participants to ask questions, and teaching points are shared by a clinical expert to highlight concepts and provide additional context. The IGDCC is meant to be a joint learning exercise to explore a specific difficult patient situation and learn skills and knowledge to improve care and transitions for older adults. The conference call is not a critique of the care, but rather an opportunity to jointly learn from the challenging situations all experience.
Background
The IGDCC was created by four members of 3 health systems in Wisconsin: Wheaton Franciscan Healthcare, Aspirus, and Aurora Health Care. The health systems serve and partially overlap on a broad geographic and demographic area of Wisconsin. The 4 members collaborated on numerous projects in the past, including Nurses Improving Case for Health System Elders (NICHE) implementation [3]. A common concern among the team is the management of challenging geriatric clinical patients and having a prepared workforce to meet those challenges.
Problem/Issue
As mentioned above, the older adult population is increasing, and these statistics are reflected in our service area [4]. Exacerbating these demographic changes is a shortage of health care workers in all disciplines, inadequate geriatric training, and the increased prevalence of multiple chronic conditions. Older adults also have higher rates of 30-day readmissions as well as higher rates of functional decline and medical errors during hospital stays [5,6]. Effective interprofessional teamwork is essential for the delivery of high-quality patient care in an increasingly complex health environment [7]. The IOM’s Future of Nursing report recommends that nurses, who represent the largest segment of the US health workforce, should achieve higher levels of training and be full partners in redesigning health care [8]. Unfortunately, effective care is hampered by poor coordination, limited communication, boundary infringement, and lack of understanding of roles [9]. Meta-analyses have demonstrated that there is a positive relationship between team training interventions and outcomes [10,11].
Objectives
The objective of the IGDCC is to elevate the level of geriatric care in the region by providing an accessible and affordable forum for the education of health care workers involved in the care of our most vulnerable population. To meet this challenge, the 4 founding members of IGDCC utilized the Aurora Health Care Geriatric Fellow’s Most Difficult Case (GFMCC) conference format as a model [12,13]. All disciplines are encouraged to participate, with announcements sent out via the leadership at the participating hospital systems. Participants have the option to call into the conference and teleconference via their own personal telephone and computer; in addition, each participating hospital system frequently hosts an open forum teleconference room where participants also may join a group.
Conference Components
The team uses the Wisconsin Star Method framework for presentation and discussion of the case. The Star Method, developed by Timothy Howell, enables clinical data about a person to be mapped out onto a single field with 5 domains: medications, medical, behavioral, personal, and social [14], creating a visual representation of the complicated and interacting physical, emotional, and social issues of older adults (Figure). By becoming comfortable using this method, the learner can use a similar approach in their clinical practice to address the needs of the patient in a holistic manner.
The case call concludes with expert teaching points from both a geriatric expert and a member of the interdisciplinary team. The interdisciplinary team member is chosen based on the key issues raised by the case. For example, cases that are made complex due to polypharmacy and adverse drug reactions might have a pharmacist presenting pertinent take-home message for the learner. In addition, geriatric teaching experts (ie, a geriatrician or advanced practice geriatric nursing specialist) provide the learner with insights that they can apply to their future practice. Often times the teaching points consist of an analysis of the various geriatric syndromes and how they can be managed in the complex older adult.
Implementation
Implementation of the IGDCC is coordinated by an oversight team with representation from each of the 3 sponsoring health systems. The oversight team currently includes 4 members: 3 geriatric clinical nurse specialists and a geriatric service line administrator. The team is responsible for:
- Planning the conference call schedule
- Making arrangements for case presenters and experts to contribute teaching points
- Registering participants and sharing written materials with participants
- Publicizing and encouraging attendance
- Soliciting feedback for continual improvement
- Exploring and implementing new ways to maximize learning.
Team members share duties and rotate case presentations. The Aurora and Wheaton Franciscan systems provide the geriatric specialists who provide the expert teaching points. The Aspirus system provides the conference line and webinar application and supports publicity and evaluations. All 3 systems are supported by a geriatric clinical nurse specialist who identifies and helps prepare presenters, case presentations, and call participants. Over time, the conference call format has evolved into a webinar format, allowing participants to either phone into the call for audio only or participate via both audio and visual. The visual allows participants to watch on their computer screens while the case is presented using the Star Method. During the call, a member of the oversight team adds clinical details by typing into a Word template of a blank star, adding information for each of the 5 domains in real-time as the case is discussed. Another member of the team facilitates the call, introducing presenters and experts, describing the Star Method, and offering “housekeeping” announcements. The facilitator also watches the timing to make certain the agenda is followed and the call begins and ends on time. During the call, another member of the team updates the attendance spreadsheet and makes a recording of each session.
Some participating facilities reserve a meeting room and project the webinar onto a screen for shared viewing. One of the participating sites has done this quite successfully with a growing group of participants coming together to watch the case during their lunch hour. This allows an opportunity for group discussion—when the conference call is on “mute” so as not to disrupt learners at other locations.
Measurement/Analysis
Attendance has steadily increased. In CY2015 from January to September, the mean attendance per month was 29.1 (mode, 17). The maximum per month was 62 (September 2015). The program enjoyed a boost in attendance beginning in July 2015 when Nurses Improving Care of Healthsystem Elders (NICHE) [3] began promoting the call-in opportunity to its NICHE Coordinators at member health systems. In June 2015, the technology was improved to allow for recorded sessions, and the recordings are growing in popularity from 2 listeners per month in July 2014 to 23 listeners per month in September 2015.
Lessons Learned
In comparing the IGDCC with similar conference call educational offerings, the team found that the program was unique in 2 areas. First, in addition to having a rich discussion in the care of frail older adults with experts in the field, the team also sought to help our staff learn how to present a difficult case to their peers. Three of our 4 committee members are geriatric clinical nurse specialists (a fourth is a clinical nurse specialist from Aspirus who assists periodically) who have been able to mentor, guide, and encourage interdisciplinary team members to present a challenging case. Many presenters had never presented a difficult case in this format. Presenters found the process fun and rewarding and have offered to present cases again in the future.
A second unique feature was utilizing the Wisconsin Star Method rather than focusing on a typical medical model framework for discussing a challenging case. The Star Method allows participants to increase their proficiency in providing comprehensive care while being more confident and mindful in addressing the complicated interacting physical, emotional and social issues of older adults [13].
A monthly post-call debriefing with committee members to review the strengths and weakness of the call was key to growing the program. The committee was able to critically review the process of the call, review participant surveys and discuss next steps. Adding a webinar approach, automatic email notification of calls, participant electronic survey, recording the call, and the addition of offering contact hours were some of the action items that were a result of monthly debriefing calls.
The team also found the 3-system collaboration to be beneficial. Aspirus has a large rural population, and Wheaton and Aurora have a diverse population, and each adds to the participant’s experience. Each IGDCC was rotated between the systems, which did not put the burden on any one health system. An annual call assignment listing was maintained for noting which system was responsible for the case each month and whether the geriatric expert was assigned/confirmed. Identifying the committee’s individual and collective group expertise was helpful in the overall project planning. The committee also developed a standard presenter guide and template and an expert teaching guide so the monthly IGDCC were consistent.
Challenges
The committee did not have a budget. Participation on the committee was in-kind funding from each system. Aspirus used its electronic system in place at the time to support the project. Interactive conference call education platform can be challenging with multiple participants on an open line who may not mute their phone. Often times, when a group of participants are calling in from one phone line it is difficult to know how many people are attending the IGDCC. It can be challenging at times to facilitate the call during the discussion component as participants occasionally talk over each other.
Current Status/Future Directions
The team has completed 18 consecutive monthly IGDCCs. Our participation rate has tripled. Participant satisfaction remains favorable. The team is now offering 1 contact hour to participants, and our invitations to participate have been extended to national health care groups. Challenging cases will be presented from community sources outside the hospital. Focusing attention on elevating the level of geriatric care in our region using a community educational approach will give us new opportunities for collaborating on best practice in multiple settings across the care continuum.
Acknowledgment: The planning team acknowledges Evalyn Michira, MSN, RN, PHN, AGCNS-BC, for her assistance in call presentations.
Corresponding author: Margie Hackbarth, MBA, margie.hackbarth@aspirus.org.
Financial disclosures: none.
1. Institute of Medicine. Retooling for an aging America: Building the health care workforce. Washington, DC: National Academies Press; 2008.
2. Mitchell P, Wynia M, Golden R, et al. Core principles and values of effective team-based health care. Discussion paper. Washington, DC; Institute of Medicine; 2012.
3. Nurses Improving Care for Healthsystem Elders. Accessed 1 Dec 2015 at www.nicheprogram.org/.
4. Wisconsin Department of Health Services. Southeastern region population report: 1 Jul 2013. Accessed 16 Feb 2015 at www.dhs.wisconsin.gov/sites/default/files/legacy/population/13data/southeastern.pdf.
5. From the Centers for Disease Control and Prevention. Public health and aging: trends in aging--United States and worldwide. JAMA 2003;289:1371–3.
6. Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010;(29):1–20, 24.
7. Nembhard IM, Edmondson AC. Making it safe: The effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav 2006; 27:941–66.
8. Institute of Medicine. The future of nursing: leading change, advancing health. National Academies Press; 2011.
9. Reeves S, Zwarenstein M, Goldman et al. Interprofessional education: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2013;3:CD002213.
10. Salas E, Diaz Granados D, Klein C, et al. Does team training improve team performance? A meta-analysis. Hum Factors 2008;50:903–33.
11. Strasser DD, Burridge AB, Falconer JA, et al. Toward spanning the quality chasm: an examination of team functioning measures. Arch Phys Med Rehabil 2014;95:2220–3.
12. Roche VM, Torregosa H, Howell T, Malone ML. Establishing a treatment plan for an elder with a complex and incomplete medical history and multiple medical providers, diagnoses, and medications. Ann Long-Term Care 2012;20(9).
13. Roche VM, Arnouville J, Danto-Nocton ES, et al. Optimal management of an older patient with multiple comorbidities and a complex psychosocial history. Ann Long-Term Care 2011;19(9).
14. Wisconsin Geriatric Psychiatry Initiative. The Wisconsin Star Method. Accessed 19 Jan 2015 at wgpi.wisc.edu/wisconsin-star-method/.
1. Institute of Medicine. Retooling for an aging America: Building the health care workforce. Washington, DC: National Academies Press; 2008.
2. Mitchell P, Wynia M, Golden R, et al. Core principles and values of effective team-based health care. Discussion paper. Washington, DC; Institute of Medicine; 2012.
3. Nurses Improving Care for Healthsystem Elders. Accessed 1 Dec 2015 at www.nicheprogram.org/.
4. Wisconsin Department of Health Services. Southeastern region population report: 1 Jul 2013. Accessed 16 Feb 2015 at www.dhs.wisconsin.gov/sites/default/files/legacy/population/13data/southeastern.pdf.
5. From the Centers for Disease Control and Prevention. Public health and aging: trends in aging--United States and worldwide. JAMA 2003;289:1371–3.
6. Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010;(29):1–20, 24.
7. Nembhard IM, Edmondson AC. Making it safe: The effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav 2006; 27:941–66.
8. Institute of Medicine. The future of nursing: leading change, advancing health. National Academies Press; 2011.
9. Reeves S, Zwarenstein M, Goldman et al. Interprofessional education: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2013;3:CD002213.
10. Salas E, Diaz Granados D, Klein C, et al. Does team training improve team performance? A meta-analysis. Hum Factors 2008;50:903–33.
11. Strasser DD, Burridge AB, Falconer JA, et al. Toward spanning the quality chasm: an examination of team functioning measures. Arch Phys Med Rehabil 2014;95:2220–3.
12. Roche VM, Torregosa H, Howell T, Malone ML. Establishing a treatment plan for an elder with a complex and incomplete medical history and multiple medical providers, diagnoses, and medications. Ann Long-Term Care 2012;20(9).
13. Roche VM, Arnouville J, Danto-Nocton ES, et al. Optimal management of an older patient with multiple comorbidities and a complex psychosocial history. Ann Long-Term Care 2011;19(9).
14. Wisconsin Geriatric Psychiatry Initiative. The Wisconsin Star Method. Accessed 19 Jan 2015 at wgpi.wisc.edu/wisconsin-star-method/.
Unplanned Exubations in the ICU: Risk Factors and Strategies for Reducing Adverse Events
From the MetroHealth System, Cleveland, OH.
Abstract
- Objective: To describe risk factors for unplanned extubation (UE) among critically ill adults requiring mechanical ventilation and to identify strategies to reduce the occurrence of this adverse event.
- Methods: Review of the literature.
- Results: Inadvertent removal of an endotracheal tube, or a UE, occurs in 7% to 22.5% of mechanically ventilated adult patients and is often due to deliberate patient removal. Despite the multitude of research examining risk factors and predictors of UE, rates have remained unchanged for the past 2 decades. Risk factors can be classified by intensive care unit (ICU) type, including medical ICUs, surgical ICUs, and mixed medical-surgical ICUs. The majority of risk factors for UEs across ICUs may be amenable to changes in unit processes, such as programs for agitation management, use of weaning protocols, increased surveillance of patients, and ongoing education for patients and health care staff.
- Conclusion: Prevention of UE remains an elusive target. Changes in unit processes that target identified risk factors may be an effective method to decrease prevalence of UE.
Unplanned extubation (UE) is the inadvertent removal of an endotracheal tube, either by a patient (deliberate self-extubation), or by a member of the health care team providing routine care such as repositioning, suctioning, or procedures (accidental extubation). Approximately 7% to 22.5% of mechanically ventilated patients in the intensive care unit (ICU) experience UE [1–7]. Estimates are likely higher, as current regulatory and accreditation standards do not include mandatory reporting of this event. Despite numerous studies investigating risk factors associated with UE, it remains a prevalent problem with adverse outcomes for patients and hospitals. The purpose of this review is to provide a summary of the literature on risk factors for UE, review effects on patient and organizational outcomes, and identify evidence-based strategies for reducing occurrence of UE among mechanically ventilated patients.
Prevalence of Unplanned Exubation
There is substantial heterogeneity in how UE is calculated and reported in the research literature. UE is calculated as the number of UE events per 100 or 1000 patient days, or the number of UE per total ventilator days. Rates of UE are also reported as the proportion of patients who experience UE out of all intubated patients over a set time period [8]. Despite efforts aimed at mitigating risk factors for UE, rates have remained static over the past 2 decades. Reported UE rates from 1994–2002 were 2.6% to 14% [3,6,9–11], while rates from 2004–2014 ranged from 1% to 22% [3–5,8,12–15]. Interventions utilizing a multidisciplinary approach have been implemented with the aim of decreasing UE, yet few have proven successful on improving rates nationally.
Unplanned self-extubation by the patient (deliberate self-extubation) is the most common type of UE [3,10,12,16–18]. A multicenter trial of 426 patients from 11 medical centers indicates that 46 patients experienced UE, with 36 of these (78.2%) caused by patient self-extubation [6]. Prospective single-site studies report similar or higher estimates of patient self-extubation, ranging from 75.8% to 91.7% [3,5], while a multisite study of 10,112 patients revealed 32 of 35 UE (91.4%) were due to patient self-extubation [12]. Similarly, a 4-year analysis of 85 UEs reported 82 incidences (96.5%) were a result of deliberate patient removal [13]. Patients either physically pull out the endotracheal tube or use their tongue or coughing/gagging maneuvers to displace or intentionally remove the endotracheal tube [5]. Only 3% to 8% of UEs are caused by inadvertent removal by health care staff [3,5,12,13].
Effects on Patient and Organizational Outcomes
Regardless of the cause of the UE, there are adverse consequences for both patients and hospitals. Some patients who experience UE have higher rates of in-hospital mortality; however, this is often due to contributing factors associated with severity of injury, the need for reintubation, and underlying chronic diseases [13]. Patients who experience accidental UE have higher incidence of nosocomial pneumonia (27.6% vs. 138%, P = 0.002) [11], longer duration of mechanical ventilation, and increased length of stay (LOS) [7,13]. While some studies report UE can result in serious consequences such as respiratory distress, hypoxia [13], and even death [6,12], others report lower mortality and length of stay when UE occurs, likely due to the fact that many patients are ready for liberation from mechanical ventilation at the time of UE [5,15].
Despite the emergent nature of UE, not all patients experience immediate reintubation. Many instances of UE occur during patient weaning trials or in preparation for planned extubations [5,11], which explains why only 10% to 60% of patients require reintubation [3,5,10,11,15,19,20]. When reintubation is necessary, it results in increased number of ventilator days [10,11], and increased ICU and hospital LOS [1,11]. There is little evidence directly linking reintubation with in-hospital mortality; however, it can cause serious complications such as hypotension, hypertension, arrhythmias, and airway trauma [21]. For hospitals and health care organizations, the need for reintubation results in increased hospital costs, estimated to be $1000 per reintubation event [17,22]. This estimate does not take into account additional costs incurred with increased ICU care, longer periods of mechanical ventilation, and increased LOS. Estimates of these additional costs in pediatric patients are approximately $36,000 [23]. Costs are likely higher in adult patients, due to multiple comorbidities that often accompany the need for mechanical ventilation, as well as increased pharmacy, lab, and diagnostic charges [1].
Risk Factors for Unplanned Extubation
Medical ICU Risk Factors
MICUs traditionally have the highest rates of UE [4,8]. Data from a national prevalence study indicated that there were 23.4 episodes of UE in MICUs per 1000 ventilator days [4]. Approximately 9.5% to 15% of all ventilated patients in the MICU experience UE [4,5,8]. Patients in the MICU who require mechanical ventilation often have complex chronic illness with underlying respiratory disease, which can result in prolonged periods of ventilation and increased risk of UE. Specific risk factors investigated in UE research include patient specific factors (age, gender, diagnosis, comorbidities, agitation, level of consciousness, laboratory values), ventilatory factors (ventilator type and setting, type of tracheal tube, method of tube fixation), as well as type of sedation and use of protocols [5,6,24]. Surprisingly, few variables emerge as significant risk factors for UE among MICU patients. Risk factors associated with UE have included male gender [24], presence of chronic obstructive pulmonary disease (COPD) [24], increased level of consciousness [25], and use of weaning protocols [5]. While gender, COPD, and level of consciousness increase risk of UE, the presence of weaning protocols is shown to decrease risk of UE [5]. Although UE are reported most often in MICUs, few risk factors consistently emerge for this specific cohort, making definitive recommendations for prevention of UE difficult.
Surgical ICU Risk Factors
The prevalence of UE for mechanically ventilated patients in the SICU tend to be lower than those for MICU cohorts. Prevalence of UE in the SICU is reported at 1.41 episodes per 100 ventilator days [13], or 6.8 episodes per 1000 ventilator days [4]. Percentages of UE in the SICU range from 2% to 6% [4,8,19]. Similar to MICU patients, critically ill patients in the SICU often have specific risk factors placing them at risk for UE. Causative factors examined in research studies with this population include gender, age, sedation scale scores, need for reintubation, time from intubation to extubation, use of sedatives/analgesics, restraints, ICU nurse experience, location of staff at time of UE, and criteria for extubation [17,19]. Similar to MICU cohorts, few variables are identified as predictors of UE. Significant predictors include use of restraints, decreased sedation [17], and meeting criteria for extubation [19]. Among patients who experienced an UE, 87% were restrained at the time of the UE [17], and most had low levels of sedation (mean Ramsay sedation scale score = 2.42 in the hour preceding the UE). Approximately 64% of patients who experienced UE met criteria for planned extubation and did not require re-intubation [19], suggesting many patients were essentially ready for planned extubation.
Mixed ICU Risk Factors
The majority of research investigating risk factors for UE is conducted within medical-surgical or mixed/general ICUs. The prevalence of UE within this type of unit is reported at 1.59 episodes per 100 patient days [6], or approximately 2% to 10% [4,6,7]. Among this population, potential risk factors are similar to those included in solely MICU or SICU studies. Because of the high number of studies investigating UE in a mixed ICU setting, there are significantly more variables included in as potential risk factors. Variables include patient age, gender, admission diagnosis, injury severity using Acute Physiological and Chronic Health Evaluation (APACHE II), ICU and hospital LOS, patient level of consciousness, agitation, days of mechanical ventilation, ventilator settings, nosocomial infection, sedation, physical restraints, vital signs [7,14,26], laboratory values, medication types, and body mass index [15,26]. One study also included time of UE and ICU nurse level of experience [3]. Among all factors, several were significant predictors of UE: male gender [15], decreased sedation and increased level of consciousness [8], agitation [3,19,26], use of restraints [3,7], sedation practices (particularly use of benzodiazapines) [3,7,15,26,27], lack of strong tube fixation, absence of IV sedation, and orotracheal intubation [6]. UE were more likely to occur on the night shift and among staff that included nurses with fewer years of experience [3]. Many episodes of UE occurred during weaning [10] or among patients who could communicate and were alert [3]. One study reports 57% of patients who intentionally self-extubated explained they simply removed the tube because it was uncomfortable [3].
Strategies for Reducing Adverse Events
Agitation Management
The majority of studies cited agitation, altered level of consciousness, or inadequate sedation as risk factors for UE [3,6–8,15,17,18,25,26,28,29]. These factors directly impact restraint use, another common risk factor for UE [3,7,17]. A key recommendation for agitation management is to identify the source of agitation, which is often caused by delirium onset in the ICU [30–32]. Prevalence of delirium in the ICU ranges from 20% to 80% [33–35]. ICU patients are at high risk for delirium due to sleep deprivation, older age, restraints, abnormal lab values, medications, infection, and respiratory complications [31]. Treatment for delirium centers on prevention, early recognition, interdisciplinary and pharmacologic protocols, increased nursing presence, and use of short-acting sedation when necessary [30–32,36]. While there is no research specifically linking delirium to UE, a quality analysis of risk factors present at the time of UE using bow-tie analysis methods identified delirium as a key factor present in the majority of UE cases [36]. It is possible that agitation reported in other studies investigating risk factors for UE may actually be reflective of underlying delirium. Routine screening using validated tools, such as the Confusion Assessment Method-ICU (CAM-ICU) [37] would aid in early detection and management of delirium, and would provide a standardized method for exploring the relationship of delirium and UE in future trials.
Integration of Weaning Protocols
Protocol-directed weaning is beneficial for decreasing ventilator days, time to wean from mechanical ventilation, and ICU LOS [38]. A systematic review including 7 trials (2434 patients) comparing protocol/non-protocol for weaning from mechanical ventilation reported a 26% decrease in the mean duration of mechanical ventilation for the protocol groups (95% CI 13%–37%, P < 0.001), a 70% reduction in time to wean, (95% CI 27%–88%, P = 0.009), and a decrease in ICU LOS by 11% (95% CI 3%–19%, P = 0.01). Weaning protocols are also an important risk factor for UE [5]. Findings from a prospective cohort study specifically identify the presence of weaning protocols as an important factor for reducing UE; patients who had weaning protocols ordered and followed were least likely to experience UE (P = 0.02) [5]. A separate quality improvement initiative demonstrated an overall decrease in the number of UEs (from 5.2% to 0.9%) after implementing weaning protocols as standard of care [39]. Considering many UEs occur during weaning [10], integration of weaning protocols aids in expediting the process and ensuring timely extubation.
Increased Surveillance
Increasing surveillance and monitoring of ventilated patients is a recommendation based on risk factors presented at the time of UE. Specifically, staffing levels and shifts and the use of physical restraints are variables associated with UE that are amendable to changes in unit processes based on increased surveillance. It is reported that 40% to 76% of UEs occurred during the night shift [14,17,24,40]; many more occur during change of shift or when there is not a nurse present at the bedside [3,17]. Recent trends towards mandatory bedside reporting is a specific intervention that may positively impact UE among patients in the ICU [41]. Meta-analyses of observational studies investigating the effect of nurse staffing on hospital outcomes indicate that increasing the number of RNs is associated with decreased risk of adverse patient outcomes, including UE [42,43]. The addition of 1 additional nurse per patient day can result in a 51% decrease in UE, while a decrease in nursing workload could result in a 45% decrease in UE [42]. Data from a national prevalence study reports ICUs with fewer available resources, including staff, experienced a higher number of UEs [4].
Increasing surveillance by nursing and health care staff may also impact prevalence of physical restraint use. A significant number of patients who experience UE are physically restrained at the time of the incident, ranging from 40% to 90% of intubated patients [5–7,14,17,40]. It is well documented that UE continue to occur despite the use of restraints [5,7,28,29,44] Patients who are physically restrained often experience higher rates of unplanned extubation (42.9% vs. 16.5% , P < 0.001 in Chang et al’s study [7]), and longer ICU LOS (20.3 days vs. 15.8 days, P = 0.009) [7]. Soft wrist restraints are commonly used to prevent pulling of the endotracheal tube; however, research evidence on UE demonstrates this is not always an effective intervention. Increasing surveillance of ventilated patients, treating their agitation and screening for underlying delirium, and integration of weaning protocols are all interventions that may decrease UE and the need for routine use of physical restraints.
Ongoing Education for Patients and Health Care Staff
Initial and ongoing education about UE, risk factors, and effective interventions is beneficial for patients and health care staff. Although there are no trials investigating effects of educational interventions for patients on UE outcomes, pre-education of surgical patients regarding what to expect while intubated may aid in decreasing delirium risk, agitation, physical restraint use, and possibly UE. Verbal and written educational information during pre-admission testing is a feasible method easily integrated into pre-operative programs.
Because UEs often occur more frequently among less experienced staff, initial education about risk factors for UE is crucial to include in ICU staff orientation programs [3,7]. Educational initiatives should incorporate training on routine delirium screening and avoidance of agitation, use of protocols, and increased surveillance of patients receiving mechanical ventilation [5,15,17,39,45]. Ongoing education of staff regarding ventilatory equipment and risk factors for UE can be particularly effective in decreasing UE [46]. Initial educational efforts should be followed by routine updates for all members of the healthcare team about ongoing quality improvement efforts to monitor UE. Associated factors for UE that may be unit- or process-specific, including methods for endotracheal tube securement and intra-hospital transport, should be communicated with all individuals involved in patient care. Integration of continuous quality improvement programs can decrease UE rates by 22% to 53% [16]. Quality efforts typically focus on standardization of reporting and tracking tools, protocol implementations, and ongoing monitoring, auditing, and recording of UE.
Current Trends and Future Directions
Recent trends in critical care recommendations may mitigate potential risk factors identified in UE research. Integration of lightened sedation and daily wake up periods for intubated patients may decrease prevalence of risk factors for UE, specifically agitation, physical restraint use, and altered level of consciousness [30], while routine weaning protocols may improve ventilatory outcomes, including UE [5,38,40]. Nursing bedside report and purposeful hourly rounding are quickly emerging as mainstays of professional nursing care [41]. Inherent in these 2 initiatives are increased surveillance and vigilance by health care staff, which can result in timely extubation of those who indicate readiness, as well as decreased incidence of adverse events. Delirium remains a key factor that may be a likely cause for UE; recent trends towards early detection and proper management of delirium among ICU staff may result in improved ventilatory outcomes, including weaning, planned extubation, and the prevalence of UE.
Another important trend in critical care is the emergence of a neurocritical care specialty and routine admission of neurocritically ill patients to neuroscience ICUs [47,48]. However, there are no studies investigating prevalence of UE among these patients, who often have higher rates of agitation or restlessness due to cognitive impairment. Among general ICUs, patients with a primary respiratory diagnosis accounted for 23% of all UE in one study, while those with a neurological diagnosis accounted for the second highest percentage (12%) among the study population [15]. A separate study concluded that presence of neurological injury with a concomitant nosocomial infection increased risk of UE among patients in a mixed ICU [7]. A recent systematic review of weaning protocols highlights positive effects on ventilatory outcomes but cites lack of evidence for effectiveness of protocols among those with neurological injury [38]. Areas for future UE research should include factors specific to this patient population, as they may be at higher risk for adverse ventilatory outcomes due to the nature of the neurological injury.
Conclusion
Prevention of UE remains an elusive target, evidenced by little change in reported rates over 2 decades. Research provides data on risk factors that may be patient, unit, or process related. Structuring prevention efforts around modifiable risk factors for UE is a feasible approach amenable to ongoing monitoring for effectiveness. Integration of current trends in health care safety and quality may produce an added benefit of reducing the occurrence of UE in critical care units. Future research evaluating these trends and the prevalence of UE in subspecialty populations is warranted.
Corresponding author: Molly McNett, PhD, RN, CNRN, Attn: NBO, MetroHealth Medical Center, 2500 MetroHealth Drive; Cleveland, OH 44109, mmcnett@metrohealth.org.
Financial disclosures: None.
1. Krinsley JS, Barone JE. The drive to survive: unplanned extubation in the ICU. Chest 2005;128:560–6.
2. Coppolo DP, May JJ. Self-extubations. A 12-month experience. Chest 1990;98:165–9.
3. Yeh SH, Lee LN, Ho TH, et al. Implications of nursing care in the occurrence and consequences of unplanned extubation in adult intensive care units. Int J Nurs Stud 2004;41:255–62.
4. Mion LC, Minnick AF, Leipzig R, et al. Patient-initiated device removal in intensive care units: a national prevalence study. Crit Care Med 2007;35:2714–20.
5. Jarachovic M, Mason M, Kerber K. The role of standardized protocols in unplanned extubations in a medical intensive care unit. Am J Crit Care 2011;20:304–11.
6. Boulain T. Unplanned extubations in the adult intensive care unit: a prospective multicenter study. Association des Reanimateurs du Centre-Ouest. Am J Resp Crit Care Med 1998;157(4 Pt 1):1131–7.
7. Chang LY, Wang KW, Chao YF. Influence of physical restraint on unplanned extubation of adult intensive care patients: a case-control study. Am J Crit Care 2008;17:408–15.
8. Moons P, Sels K, De Becker W, et al. Development of a risk assessment tool for deliberate self-extubation in intensive care patients. Intensive Care Med 2004;30:1348–55.
9. Chiang AA, Lee KC, Lee JC, Wei CH. Effectiveness of a continuous quality improvement program aiming to reduce unplanned extubation: a prospective study. Intensive Care Med 1996;22:1269–71.
10. Betbese AJ, Perez M, Bak E, et al. A prospective study of unplanned endotracheal extubation in intensive care unit patients. Crit Care Med 1998;26:1180–6.
11. de Lassence A, Alberti C, Azoulay E, et al. Impact of unplanned extubation and reintubation after weaning on nosocomial pneumonia risk in the intensive care unit: a prospective multicenter study. Anesthesiology 2002;97:148–56.
12. Kapadia FN, Tekawade PC, Nath SS, et al. A prolonged observational study of tracheal tube displacements: Benchmarking an incidence <0.5-1% in a medical-surgical adult intensive care unit. Ind J Crit Care Med 2014;18:273–7.
13. Lee JH, Lee HC, Jeon YT, et al. Clinical outcomes after unplanned extubation in a surgical intensive care population. World J Surg 2014;38:203–10.
14. Chang LC, Liu PF, Huang YL, et al. Risk factors associated with unplanned endotracheal self-extubation of hospitalized intubated patients: a 3-year retrospective case-control study. Appl Nurs Res 2011;24:188–92.
15. de Groot RI, Dekkers OM, Herold IH, et al. Risk factors and outcomes after unplanned extubations on the ICU: a case-control study. Crit Care 2011;15:R19.
16. da Silva PS, Fonseca MC. Unplanned endotracheal extubations in the intensive care unit: systematic review, critical appraisal, and evidence-based recommendations. Anesth Analg 2012;114:1003–14.
17. Curry K, Cobb S, Kutash M, Diggs C. Characteristics associated with unplanned extubations in a surgical intensive care unit. Am J Crit Care 2008;17:45–51.
18. Christie JM, Dethlefsen M, Cane RD. Unplanned endotracheal extubation in the intensive care unit. J Clin Anesth 1996;8:289–93.
19. Huang YT. Factors leading to self-extubation of endotracheal tubes in the intensive care unit. Nurs Crit Care 2009;14:68–74.
20. Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126–34.
21. Mort TC. Unplanned tracheal extubation outside the operating room: a quality improvement audit of hemodynamic and tracheal airway complications associated with emergency tracheal reintubation. Anesth Analg 1998;86:1171–6.
22. Jaber S, Chanques G, Altairac C, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest 2005;128:2749–57.
23. Roddy DJ, Spaeder MC, Pastor W, Stockwell DC, Klugman D. Unplanned extubations in children: impact on hospital cost and length of stay. Ped Crit Care Med 2015.
24. Bouza C, Garcia E, Diaz M, et al. Unplanned extubation in orally intubated medical patients in the intensive care unit: a prospective cohort study. Heart Lung 2007;36:270–6.
25. Vassal T, Anh NG, Gabillet JM, et al. Prospective evaluation of self-extubations in a medical intensive care unit. Intensive Care Med 1993;19:340-342.
26. Tung A, Tadimeti L, Caruana-Montaldo B, et al. The relationship of sedation to deliberate self-extubation. J Clin Anesth 2001;13:24–9.
27. Tanios M, Epstein S, Grzeskowiak M, et al. Influence of sedation strategies on unplanned extubation in a mixed intensive care unit. Am J Crit Care 2014;23:306–14.
28. Atkins PM, Mion LC, Mendelson W, et al. Characteristics and outcomes of patients who self-extubate from ventilatory support: a case-control study. Chest 1997;112:1317–23.
29. Chevron V, Menard JF, Richard JC, et al. Unplanned extubation: risk factors of development and predictive criteria for reintubation. Crit Care Med 1998;26:1049–53.
30. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
31. Morandi A, Jackson JC. Delirium in the intensive care unit: a review. Neurol Clin 2011;29:749–63.
32. Banerjee A, Vasilevskis, EE, Pandharipande, P. Strategies to improve delirium assessment practices in the intensive care unit. J Clin Outcomes Manag 2010;17:459–68.
33. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
34. Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med 2004;32:106–12.
35. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc 2003;51:591–8.
36. Kerckhoffs MC, van der Sluijs AF, Binnekade JM, Dongelmans DA. Improving patient safety in the ICU by prospective identification of missing safety barriers using the bow-tie prospective risk analysis model. J Patient Safe 2013;9:154–9.
37. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8.
38. Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 2014;11:CD006904.
39. Chia PL, Santos DR, Tan TC, et al. Clinical quality improvement: eliminating unplanned extubation in the CCU. Int J Health Care Qual Ass 2013;26:642–52.
40. Balon JA. Common factors of spontaneous self-extubation in a critical care setting. Int J Trauma Nurs 2001;7:93–9.
41. Gregory S, Tan D, Tilrico M, et al. Bedside shift reports: what does the evidence say? J Nurs Admin 2014;44:541–5.
42. Kane RL, Shamliyan TA, Mueller C, et al. The association of registered nurse staffing levels and patient outcomes: systematic review and meta-analysis. Med Care 2007;45:1195–204.
43. Penoyer DA. Nurse staffing and patient outcomes in critical care: a concise review. Crit Care Med 2010;38:1521–8; quiz 1529.
44. Tindol GA, Jr., DiBenedetto RJ, Kosciuk L. Unplanned extubations. Chest 1994;105:1804–7.
45. Chen CM CK, Fong Y, Hsing SC, et al. Age is an important predictor of failed unplanned extubation. Int J Gerontol 2010;4:120–9.
46. Richmond AL, Jarog DL, Hanson VM. Unplanned extubation in adult critical care. Quality improvement and education payoff. Crit Care Nurs 2004;24:32–7.
47. Kurtz P, Fitts V, Sumer Z, et al. How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU? Neurocrit Care 2011;15:477–80.
48. McNett MM, Horowitz DA. International multidisciplinary consensus conference on multimodality monitoring: ICU processes of care. Neurocrit Care 2014;21 Suppl 2:S215–28.
49. Gardner A, Hughes, D, Cook R, et al. Best practice in stabilisation of oral endotracheal tubes: a systematic review. Database of abstracts of reivews of effects (DARE): Quality-assessed reviews. 2005. York: Center for Reviews and Dissemination.
50. Hofso K, Coyer FM. Part 1: Chemical and physical restraints in the management of mechanically ventliated patients in the ICU: Contributing factors. Intensive Crit Care Nurs 2007; 23:249–55.
51. Kiekkas P, Diamanto A, Panteli E, et al. Unplanned extubation in critially ill adults: Clinical reviews. Nurs Crit Care 2012;18:123–34.
52. King JN, Elliiot VA. Self/unplanned extubation: Safety, surveillance, and monitoring of the mechanically ventilated patient. Crit Care Nurs Clin North Am 2012;24:469–79.
From the MetroHealth System, Cleveland, OH.
Abstract
- Objective: To describe risk factors for unplanned extubation (UE) among critically ill adults requiring mechanical ventilation and to identify strategies to reduce the occurrence of this adverse event.
- Methods: Review of the literature.
- Results: Inadvertent removal of an endotracheal tube, or a UE, occurs in 7% to 22.5% of mechanically ventilated adult patients and is often due to deliberate patient removal. Despite the multitude of research examining risk factors and predictors of UE, rates have remained unchanged for the past 2 decades. Risk factors can be classified by intensive care unit (ICU) type, including medical ICUs, surgical ICUs, and mixed medical-surgical ICUs. The majority of risk factors for UEs across ICUs may be amenable to changes in unit processes, such as programs for agitation management, use of weaning protocols, increased surveillance of patients, and ongoing education for patients and health care staff.
- Conclusion: Prevention of UE remains an elusive target. Changes in unit processes that target identified risk factors may be an effective method to decrease prevalence of UE.
Unplanned extubation (UE) is the inadvertent removal of an endotracheal tube, either by a patient (deliberate self-extubation), or by a member of the health care team providing routine care such as repositioning, suctioning, or procedures (accidental extubation). Approximately 7% to 22.5% of mechanically ventilated patients in the intensive care unit (ICU) experience UE [1–7]. Estimates are likely higher, as current regulatory and accreditation standards do not include mandatory reporting of this event. Despite numerous studies investigating risk factors associated with UE, it remains a prevalent problem with adverse outcomes for patients and hospitals. The purpose of this review is to provide a summary of the literature on risk factors for UE, review effects on patient and organizational outcomes, and identify evidence-based strategies for reducing occurrence of UE among mechanically ventilated patients.
Prevalence of Unplanned Exubation
There is substantial heterogeneity in how UE is calculated and reported in the research literature. UE is calculated as the number of UE events per 100 or 1000 patient days, or the number of UE per total ventilator days. Rates of UE are also reported as the proportion of patients who experience UE out of all intubated patients over a set time period [8]. Despite efforts aimed at mitigating risk factors for UE, rates have remained static over the past 2 decades. Reported UE rates from 1994–2002 were 2.6% to 14% [3,6,9–11], while rates from 2004–2014 ranged from 1% to 22% [3–5,8,12–15]. Interventions utilizing a multidisciplinary approach have been implemented with the aim of decreasing UE, yet few have proven successful on improving rates nationally.
Unplanned self-extubation by the patient (deliberate self-extubation) is the most common type of UE [3,10,12,16–18]. A multicenter trial of 426 patients from 11 medical centers indicates that 46 patients experienced UE, with 36 of these (78.2%) caused by patient self-extubation [6]. Prospective single-site studies report similar or higher estimates of patient self-extubation, ranging from 75.8% to 91.7% [3,5], while a multisite study of 10,112 patients revealed 32 of 35 UE (91.4%) were due to patient self-extubation [12]. Similarly, a 4-year analysis of 85 UEs reported 82 incidences (96.5%) were a result of deliberate patient removal [13]. Patients either physically pull out the endotracheal tube or use their tongue or coughing/gagging maneuvers to displace or intentionally remove the endotracheal tube [5]. Only 3% to 8% of UEs are caused by inadvertent removal by health care staff [3,5,12,13].
Effects on Patient and Organizational Outcomes
Regardless of the cause of the UE, there are adverse consequences for both patients and hospitals. Some patients who experience UE have higher rates of in-hospital mortality; however, this is often due to contributing factors associated with severity of injury, the need for reintubation, and underlying chronic diseases [13]. Patients who experience accidental UE have higher incidence of nosocomial pneumonia (27.6% vs. 138%, P = 0.002) [11], longer duration of mechanical ventilation, and increased length of stay (LOS) [7,13]. While some studies report UE can result in serious consequences such as respiratory distress, hypoxia [13], and even death [6,12], others report lower mortality and length of stay when UE occurs, likely due to the fact that many patients are ready for liberation from mechanical ventilation at the time of UE [5,15].
Despite the emergent nature of UE, not all patients experience immediate reintubation. Many instances of UE occur during patient weaning trials or in preparation for planned extubations [5,11], which explains why only 10% to 60% of patients require reintubation [3,5,10,11,15,19,20]. When reintubation is necessary, it results in increased number of ventilator days [10,11], and increased ICU and hospital LOS [1,11]. There is little evidence directly linking reintubation with in-hospital mortality; however, it can cause serious complications such as hypotension, hypertension, arrhythmias, and airway trauma [21]. For hospitals and health care organizations, the need for reintubation results in increased hospital costs, estimated to be $1000 per reintubation event [17,22]. This estimate does not take into account additional costs incurred with increased ICU care, longer periods of mechanical ventilation, and increased LOS. Estimates of these additional costs in pediatric patients are approximately $36,000 [23]. Costs are likely higher in adult patients, due to multiple comorbidities that often accompany the need for mechanical ventilation, as well as increased pharmacy, lab, and diagnostic charges [1].
Risk Factors for Unplanned Extubation
Medical ICU Risk Factors
MICUs traditionally have the highest rates of UE [4,8]. Data from a national prevalence study indicated that there were 23.4 episodes of UE in MICUs per 1000 ventilator days [4]. Approximately 9.5% to 15% of all ventilated patients in the MICU experience UE [4,5,8]. Patients in the MICU who require mechanical ventilation often have complex chronic illness with underlying respiratory disease, which can result in prolonged periods of ventilation and increased risk of UE. Specific risk factors investigated in UE research include patient specific factors (age, gender, diagnosis, comorbidities, agitation, level of consciousness, laboratory values), ventilatory factors (ventilator type and setting, type of tracheal tube, method of tube fixation), as well as type of sedation and use of protocols [5,6,24]. Surprisingly, few variables emerge as significant risk factors for UE among MICU patients. Risk factors associated with UE have included male gender [24], presence of chronic obstructive pulmonary disease (COPD) [24], increased level of consciousness [25], and use of weaning protocols [5]. While gender, COPD, and level of consciousness increase risk of UE, the presence of weaning protocols is shown to decrease risk of UE [5]. Although UE are reported most often in MICUs, few risk factors consistently emerge for this specific cohort, making definitive recommendations for prevention of UE difficult.
Surgical ICU Risk Factors
The prevalence of UE for mechanically ventilated patients in the SICU tend to be lower than those for MICU cohorts. Prevalence of UE in the SICU is reported at 1.41 episodes per 100 ventilator days [13], or 6.8 episodes per 1000 ventilator days [4]. Percentages of UE in the SICU range from 2% to 6% [4,8,19]. Similar to MICU patients, critically ill patients in the SICU often have specific risk factors placing them at risk for UE. Causative factors examined in research studies with this population include gender, age, sedation scale scores, need for reintubation, time from intubation to extubation, use of sedatives/analgesics, restraints, ICU nurse experience, location of staff at time of UE, and criteria for extubation [17,19]. Similar to MICU cohorts, few variables are identified as predictors of UE. Significant predictors include use of restraints, decreased sedation [17], and meeting criteria for extubation [19]. Among patients who experienced an UE, 87% were restrained at the time of the UE [17], and most had low levels of sedation (mean Ramsay sedation scale score = 2.42 in the hour preceding the UE). Approximately 64% of patients who experienced UE met criteria for planned extubation and did not require re-intubation [19], suggesting many patients were essentially ready for planned extubation.
Mixed ICU Risk Factors
The majority of research investigating risk factors for UE is conducted within medical-surgical or mixed/general ICUs. The prevalence of UE within this type of unit is reported at 1.59 episodes per 100 patient days [6], or approximately 2% to 10% [4,6,7]. Among this population, potential risk factors are similar to those included in solely MICU or SICU studies. Because of the high number of studies investigating UE in a mixed ICU setting, there are significantly more variables included in as potential risk factors. Variables include patient age, gender, admission diagnosis, injury severity using Acute Physiological and Chronic Health Evaluation (APACHE II), ICU and hospital LOS, patient level of consciousness, agitation, days of mechanical ventilation, ventilator settings, nosocomial infection, sedation, physical restraints, vital signs [7,14,26], laboratory values, medication types, and body mass index [15,26]. One study also included time of UE and ICU nurse level of experience [3]. Among all factors, several were significant predictors of UE: male gender [15], decreased sedation and increased level of consciousness [8], agitation [3,19,26], use of restraints [3,7], sedation practices (particularly use of benzodiazapines) [3,7,15,26,27], lack of strong tube fixation, absence of IV sedation, and orotracheal intubation [6]. UE were more likely to occur on the night shift and among staff that included nurses with fewer years of experience [3]. Many episodes of UE occurred during weaning [10] or among patients who could communicate and were alert [3]. One study reports 57% of patients who intentionally self-extubated explained they simply removed the tube because it was uncomfortable [3].
Strategies for Reducing Adverse Events
Agitation Management
The majority of studies cited agitation, altered level of consciousness, or inadequate sedation as risk factors for UE [3,6–8,15,17,18,25,26,28,29]. These factors directly impact restraint use, another common risk factor for UE [3,7,17]. A key recommendation for agitation management is to identify the source of agitation, which is often caused by delirium onset in the ICU [30–32]. Prevalence of delirium in the ICU ranges from 20% to 80% [33–35]. ICU patients are at high risk for delirium due to sleep deprivation, older age, restraints, abnormal lab values, medications, infection, and respiratory complications [31]. Treatment for delirium centers on prevention, early recognition, interdisciplinary and pharmacologic protocols, increased nursing presence, and use of short-acting sedation when necessary [30–32,36]. While there is no research specifically linking delirium to UE, a quality analysis of risk factors present at the time of UE using bow-tie analysis methods identified delirium as a key factor present in the majority of UE cases [36]. It is possible that agitation reported in other studies investigating risk factors for UE may actually be reflective of underlying delirium. Routine screening using validated tools, such as the Confusion Assessment Method-ICU (CAM-ICU) [37] would aid in early detection and management of delirium, and would provide a standardized method for exploring the relationship of delirium and UE in future trials.
Integration of Weaning Protocols
Protocol-directed weaning is beneficial for decreasing ventilator days, time to wean from mechanical ventilation, and ICU LOS [38]. A systematic review including 7 trials (2434 patients) comparing protocol/non-protocol for weaning from mechanical ventilation reported a 26% decrease in the mean duration of mechanical ventilation for the protocol groups (95% CI 13%–37%, P < 0.001), a 70% reduction in time to wean, (95% CI 27%–88%, P = 0.009), and a decrease in ICU LOS by 11% (95% CI 3%–19%, P = 0.01). Weaning protocols are also an important risk factor for UE [5]. Findings from a prospective cohort study specifically identify the presence of weaning protocols as an important factor for reducing UE; patients who had weaning protocols ordered and followed were least likely to experience UE (P = 0.02) [5]. A separate quality improvement initiative demonstrated an overall decrease in the number of UEs (from 5.2% to 0.9%) after implementing weaning protocols as standard of care [39]. Considering many UEs occur during weaning [10], integration of weaning protocols aids in expediting the process and ensuring timely extubation.
Increased Surveillance
Increasing surveillance and monitoring of ventilated patients is a recommendation based on risk factors presented at the time of UE. Specifically, staffing levels and shifts and the use of physical restraints are variables associated with UE that are amendable to changes in unit processes based on increased surveillance. It is reported that 40% to 76% of UEs occurred during the night shift [14,17,24,40]; many more occur during change of shift or when there is not a nurse present at the bedside [3,17]. Recent trends towards mandatory bedside reporting is a specific intervention that may positively impact UE among patients in the ICU [41]. Meta-analyses of observational studies investigating the effect of nurse staffing on hospital outcomes indicate that increasing the number of RNs is associated with decreased risk of adverse patient outcomes, including UE [42,43]. The addition of 1 additional nurse per patient day can result in a 51% decrease in UE, while a decrease in nursing workload could result in a 45% decrease in UE [42]. Data from a national prevalence study reports ICUs with fewer available resources, including staff, experienced a higher number of UEs [4].
Increasing surveillance by nursing and health care staff may also impact prevalence of physical restraint use. A significant number of patients who experience UE are physically restrained at the time of the incident, ranging from 40% to 90% of intubated patients [5–7,14,17,40]. It is well documented that UE continue to occur despite the use of restraints [5,7,28,29,44] Patients who are physically restrained often experience higher rates of unplanned extubation (42.9% vs. 16.5% , P < 0.001 in Chang et al’s study [7]), and longer ICU LOS (20.3 days vs. 15.8 days, P = 0.009) [7]. Soft wrist restraints are commonly used to prevent pulling of the endotracheal tube; however, research evidence on UE demonstrates this is not always an effective intervention. Increasing surveillance of ventilated patients, treating their agitation and screening for underlying delirium, and integration of weaning protocols are all interventions that may decrease UE and the need for routine use of physical restraints.
Ongoing Education for Patients and Health Care Staff
Initial and ongoing education about UE, risk factors, and effective interventions is beneficial for patients and health care staff. Although there are no trials investigating effects of educational interventions for patients on UE outcomes, pre-education of surgical patients regarding what to expect while intubated may aid in decreasing delirium risk, agitation, physical restraint use, and possibly UE. Verbal and written educational information during pre-admission testing is a feasible method easily integrated into pre-operative programs.
Because UEs often occur more frequently among less experienced staff, initial education about risk factors for UE is crucial to include in ICU staff orientation programs [3,7]. Educational initiatives should incorporate training on routine delirium screening and avoidance of agitation, use of protocols, and increased surveillance of patients receiving mechanical ventilation [5,15,17,39,45]. Ongoing education of staff regarding ventilatory equipment and risk factors for UE can be particularly effective in decreasing UE [46]. Initial educational efforts should be followed by routine updates for all members of the healthcare team about ongoing quality improvement efforts to monitor UE. Associated factors for UE that may be unit- or process-specific, including methods for endotracheal tube securement and intra-hospital transport, should be communicated with all individuals involved in patient care. Integration of continuous quality improvement programs can decrease UE rates by 22% to 53% [16]. Quality efforts typically focus on standardization of reporting and tracking tools, protocol implementations, and ongoing monitoring, auditing, and recording of UE.
Current Trends and Future Directions
Recent trends in critical care recommendations may mitigate potential risk factors identified in UE research. Integration of lightened sedation and daily wake up periods for intubated patients may decrease prevalence of risk factors for UE, specifically agitation, physical restraint use, and altered level of consciousness [30], while routine weaning protocols may improve ventilatory outcomes, including UE [5,38,40]. Nursing bedside report and purposeful hourly rounding are quickly emerging as mainstays of professional nursing care [41]. Inherent in these 2 initiatives are increased surveillance and vigilance by health care staff, which can result in timely extubation of those who indicate readiness, as well as decreased incidence of adverse events. Delirium remains a key factor that may be a likely cause for UE; recent trends towards early detection and proper management of delirium among ICU staff may result in improved ventilatory outcomes, including weaning, planned extubation, and the prevalence of UE.
Another important trend in critical care is the emergence of a neurocritical care specialty and routine admission of neurocritically ill patients to neuroscience ICUs [47,48]. However, there are no studies investigating prevalence of UE among these patients, who often have higher rates of agitation or restlessness due to cognitive impairment. Among general ICUs, patients with a primary respiratory diagnosis accounted for 23% of all UE in one study, while those with a neurological diagnosis accounted for the second highest percentage (12%) among the study population [15]. A separate study concluded that presence of neurological injury with a concomitant nosocomial infection increased risk of UE among patients in a mixed ICU [7]. A recent systematic review of weaning protocols highlights positive effects on ventilatory outcomes but cites lack of evidence for effectiveness of protocols among those with neurological injury [38]. Areas for future UE research should include factors specific to this patient population, as they may be at higher risk for adverse ventilatory outcomes due to the nature of the neurological injury.
Conclusion
Prevention of UE remains an elusive target, evidenced by little change in reported rates over 2 decades. Research provides data on risk factors that may be patient, unit, or process related. Structuring prevention efforts around modifiable risk factors for UE is a feasible approach amenable to ongoing monitoring for effectiveness. Integration of current trends in health care safety and quality may produce an added benefit of reducing the occurrence of UE in critical care units. Future research evaluating these trends and the prevalence of UE in subspecialty populations is warranted.
Corresponding author: Molly McNett, PhD, RN, CNRN, Attn: NBO, MetroHealth Medical Center, 2500 MetroHealth Drive; Cleveland, OH 44109, mmcnett@metrohealth.org.
Financial disclosures: None.
From the MetroHealth System, Cleveland, OH.
Abstract
- Objective: To describe risk factors for unplanned extubation (UE) among critically ill adults requiring mechanical ventilation and to identify strategies to reduce the occurrence of this adverse event.
- Methods: Review of the literature.
- Results: Inadvertent removal of an endotracheal tube, or a UE, occurs in 7% to 22.5% of mechanically ventilated adult patients and is often due to deliberate patient removal. Despite the multitude of research examining risk factors and predictors of UE, rates have remained unchanged for the past 2 decades. Risk factors can be classified by intensive care unit (ICU) type, including medical ICUs, surgical ICUs, and mixed medical-surgical ICUs. The majority of risk factors for UEs across ICUs may be amenable to changes in unit processes, such as programs for agitation management, use of weaning protocols, increased surveillance of patients, and ongoing education for patients and health care staff.
- Conclusion: Prevention of UE remains an elusive target. Changes in unit processes that target identified risk factors may be an effective method to decrease prevalence of UE.
Unplanned extubation (UE) is the inadvertent removal of an endotracheal tube, either by a patient (deliberate self-extubation), or by a member of the health care team providing routine care such as repositioning, suctioning, or procedures (accidental extubation). Approximately 7% to 22.5% of mechanically ventilated patients in the intensive care unit (ICU) experience UE [1–7]. Estimates are likely higher, as current regulatory and accreditation standards do not include mandatory reporting of this event. Despite numerous studies investigating risk factors associated with UE, it remains a prevalent problem with adverse outcomes for patients and hospitals. The purpose of this review is to provide a summary of the literature on risk factors for UE, review effects on patient and organizational outcomes, and identify evidence-based strategies for reducing occurrence of UE among mechanically ventilated patients.
Prevalence of Unplanned Exubation
There is substantial heterogeneity in how UE is calculated and reported in the research literature. UE is calculated as the number of UE events per 100 or 1000 patient days, or the number of UE per total ventilator days. Rates of UE are also reported as the proportion of patients who experience UE out of all intubated patients over a set time period [8]. Despite efforts aimed at mitigating risk factors for UE, rates have remained static over the past 2 decades. Reported UE rates from 1994–2002 were 2.6% to 14% [3,6,9–11], while rates from 2004–2014 ranged from 1% to 22% [3–5,8,12–15]. Interventions utilizing a multidisciplinary approach have been implemented with the aim of decreasing UE, yet few have proven successful on improving rates nationally.
Unplanned self-extubation by the patient (deliberate self-extubation) is the most common type of UE [3,10,12,16–18]. A multicenter trial of 426 patients from 11 medical centers indicates that 46 patients experienced UE, with 36 of these (78.2%) caused by patient self-extubation [6]. Prospective single-site studies report similar or higher estimates of patient self-extubation, ranging from 75.8% to 91.7% [3,5], while a multisite study of 10,112 patients revealed 32 of 35 UE (91.4%) were due to patient self-extubation [12]. Similarly, a 4-year analysis of 85 UEs reported 82 incidences (96.5%) were a result of deliberate patient removal [13]. Patients either physically pull out the endotracheal tube or use their tongue or coughing/gagging maneuvers to displace or intentionally remove the endotracheal tube [5]. Only 3% to 8% of UEs are caused by inadvertent removal by health care staff [3,5,12,13].
Effects on Patient and Organizational Outcomes
Regardless of the cause of the UE, there are adverse consequences for both patients and hospitals. Some patients who experience UE have higher rates of in-hospital mortality; however, this is often due to contributing factors associated with severity of injury, the need for reintubation, and underlying chronic diseases [13]. Patients who experience accidental UE have higher incidence of nosocomial pneumonia (27.6% vs. 138%, P = 0.002) [11], longer duration of mechanical ventilation, and increased length of stay (LOS) [7,13]. While some studies report UE can result in serious consequences such as respiratory distress, hypoxia [13], and even death [6,12], others report lower mortality and length of stay when UE occurs, likely due to the fact that many patients are ready for liberation from mechanical ventilation at the time of UE [5,15].
Despite the emergent nature of UE, not all patients experience immediate reintubation. Many instances of UE occur during patient weaning trials or in preparation for planned extubations [5,11], which explains why only 10% to 60% of patients require reintubation [3,5,10,11,15,19,20]. When reintubation is necessary, it results in increased number of ventilator days [10,11], and increased ICU and hospital LOS [1,11]. There is little evidence directly linking reintubation with in-hospital mortality; however, it can cause serious complications such as hypotension, hypertension, arrhythmias, and airway trauma [21]. For hospitals and health care organizations, the need for reintubation results in increased hospital costs, estimated to be $1000 per reintubation event [17,22]. This estimate does not take into account additional costs incurred with increased ICU care, longer periods of mechanical ventilation, and increased LOS. Estimates of these additional costs in pediatric patients are approximately $36,000 [23]. Costs are likely higher in adult patients, due to multiple comorbidities that often accompany the need for mechanical ventilation, as well as increased pharmacy, lab, and diagnostic charges [1].
Risk Factors for Unplanned Extubation
Medical ICU Risk Factors
MICUs traditionally have the highest rates of UE [4,8]. Data from a national prevalence study indicated that there were 23.4 episodes of UE in MICUs per 1000 ventilator days [4]. Approximately 9.5% to 15% of all ventilated patients in the MICU experience UE [4,5,8]. Patients in the MICU who require mechanical ventilation often have complex chronic illness with underlying respiratory disease, which can result in prolonged periods of ventilation and increased risk of UE. Specific risk factors investigated in UE research include patient specific factors (age, gender, diagnosis, comorbidities, agitation, level of consciousness, laboratory values), ventilatory factors (ventilator type and setting, type of tracheal tube, method of tube fixation), as well as type of sedation and use of protocols [5,6,24]. Surprisingly, few variables emerge as significant risk factors for UE among MICU patients. Risk factors associated with UE have included male gender [24], presence of chronic obstructive pulmonary disease (COPD) [24], increased level of consciousness [25], and use of weaning protocols [5]. While gender, COPD, and level of consciousness increase risk of UE, the presence of weaning protocols is shown to decrease risk of UE [5]. Although UE are reported most often in MICUs, few risk factors consistently emerge for this specific cohort, making definitive recommendations for prevention of UE difficult.
Surgical ICU Risk Factors
The prevalence of UE for mechanically ventilated patients in the SICU tend to be lower than those for MICU cohorts. Prevalence of UE in the SICU is reported at 1.41 episodes per 100 ventilator days [13], or 6.8 episodes per 1000 ventilator days [4]. Percentages of UE in the SICU range from 2% to 6% [4,8,19]. Similar to MICU patients, critically ill patients in the SICU often have specific risk factors placing them at risk for UE. Causative factors examined in research studies with this population include gender, age, sedation scale scores, need for reintubation, time from intubation to extubation, use of sedatives/analgesics, restraints, ICU nurse experience, location of staff at time of UE, and criteria for extubation [17,19]. Similar to MICU cohorts, few variables are identified as predictors of UE. Significant predictors include use of restraints, decreased sedation [17], and meeting criteria for extubation [19]. Among patients who experienced an UE, 87% were restrained at the time of the UE [17], and most had low levels of sedation (mean Ramsay sedation scale score = 2.42 in the hour preceding the UE). Approximately 64% of patients who experienced UE met criteria for planned extubation and did not require re-intubation [19], suggesting many patients were essentially ready for planned extubation.
Mixed ICU Risk Factors
The majority of research investigating risk factors for UE is conducted within medical-surgical or mixed/general ICUs. The prevalence of UE within this type of unit is reported at 1.59 episodes per 100 patient days [6], or approximately 2% to 10% [4,6,7]. Among this population, potential risk factors are similar to those included in solely MICU or SICU studies. Because of the high number of studies investigating UE in a mixed ICU setting, there are significantly more variables included in as potential risk factors. Variables include patient age, gender, admission diagnosis, injury severity using Acute Physiological and Chronic Health Evaluation (APACHE II), ICU and hospital LOS, patient level of consciousness, agitation, days of mechanical ventilation, ventilator settings, nosocomial infection, sedation, physical restraints, vital signs [7,14,26], laboratory values, medication types, and body mass index [15,26]. One study also included time of UE and ICU nurse level of experience [3]. Among all factors, several were significant predictors of UE: male gender [15], decreased sedation and increased level of consciousness [8], agitation [3,19,26], use of restraints [3,7], sedation practices (particularly use of benzodiazapines) [3,7,15,26,27], lack of strong tube fixation, absence of IV sedation, and orotracheal intubation [6]. UE were more likely to occur on the night shift and among staff that included nurses with fewer years of experience [3]. Many episodes of UE occurred during weaning [10] or among patients who could communicate and were alert [3]. One study reports 57% of patients who intentionally self-extubated explained they simply removed the tube because it was uncomfortable [3].
Strategies for Reducing Adverse Events
Agitation Management
The majority of studies cited agitation, altered level of consciousness, or inadequate sedation as risk factors for UE [3,6–8,15,17,18,25,26,28,29]. These factors directly impact restraint use, another common risk factor for UE [3,7,17]. A key recommendation for agitation management is to identify the source of agitation, which is often caused by delirium onset in the ICU [30–32]. Prevalence of delirium in the ICU ranges from 20% to 80% [33–35]. ICU patients are at high risk for delirium due to sleep deprivation, older age, restraints, abnormal lab values, medications, infection, and respiratory complications [31]. Treatment for delirium centers on prevention, early recognition, interdisciplinary and pharmacologic protocols, increased nursing presence, and use of short-acting sedation when necessary [30–32,36]. While there is no research specifically linking delirium to UE, a quality analysis of risk factors present at the time of UE using bow-tie analysis methods identified delirium as a key factor present in the majority of UE cases [36]. It is possible that agitation reported in other studies investigating risk factors for UE may actually be reflective of underlying delirium. Routine screening using validated tools, such as the Confusion Assessment Method-ICU (CAM-ICU) [37] would aid in early detection and management of delirium, and would provide a standardized method for exploring the relationship of delirium and UE in future trials.
Integration of Weaning Protocols
Protocol-directed weaning is beneficial for decreasing ventilator days, time to wean from mechanical ventilation, and ICU LOS [38]. A systematic review including 7 trials (2434 patients) comparing protocol/non-protocol for weaning from mechanical ventilation reported a 26% decrease in the mean duration of mechanical ventilation for the protocol groups (95% CI 13%–37%, P < 0.001), a 70% reduction in time to wean, (95% CI 27%–88%, P = 0.009), and a decrease in ICU LOS by 11% (95% CI 3%–19%, P = 0.01). Weaning protocols are also an important risk factor for UE [5]. Findings from a prospective cohort study specifically identify the presence of weaning protocols as an important factor for reducing UE; patients who had weaning protocols ordered and followed were least likely to experience UE (P = 0.02) [5]. A separate quality improvement initiative demonstrated an overall decrease in the number of UEs (from 5.2% to 0.9%) after implementing weaning protocols as standard of care [39]. Considering many UEs occur during weaning [10], integration of weaning protocols aids in expediting the process and ensuring timely extubation.
Increased Surveillance
Increasing surveillance and monitoring of ventilated patients is a recommendation based on risk factors presented at the time of UE. Specifically, staffing levels and shifts and the use of physical restraints are variables associated with UE that are amendable to changes in unit processes based on increased surveillance. It is reported that 40% to 76% of UEs occurred during the night shift [14,17,24,40]; many more occur during change of shift or when there is not a nurse present at the bedside [3,17]. Recent trends towards mandatory bedside reporting is a specific intervention that may positively impact UE among patients in the ICU [41]. Meta-analyses of observational studies investigating the effect of nurse staffing on hospital outcomes indicate that increasing the number of RNs is associated with decreased risk of adverse patient outcomes, including UE [42,43]. The addition of 1 additional nurse per patient day can result in a 51% decrease in UE, while a decrease in nursing workload could result in a 45% decrease in UE [42]. Data from a national prevalence study reports ICUs with fewer available resources, including staff, experienced a higher number of UEs [4].
Increasing surveillance by nursing and health care staff may also impact prevalence of physical restraint use. A significant number of patients who experience UE are physically restrained at the time of the incident, ranging from 40% to 90% of intubated patients [5–7,14,17,40]. It is well documented that UE continue to occur despite the use of restraints [5,7,28,29,44] Patients who are physically restrained often experience higher rates of unplanned extubation (42.9% vs. 16.5% , P < 0.001 in Chang et al’s study [7]), and longer ICU LOS (20.3 days vs. 15.8 days, P = 0.009) [7]. Soft wrist restraints are commonly used to prevent pulling of the endotracheal tube; however, research evidence on UE demonstrates this is not always an effective intervention. Increasing surveillance of ventilated patients, treating their agitation and screening for underlying delirium, and integration of weaning protocols are all interventions that may decrease UE and the need for routine use of physical restraints.
Ongoing Education for Patients and Health Care Staff
Initial and ongoing education about UE, risk factors, and effective interventions is beneficial for patients and health care staff. Although there are no trials investigating effects of educational interventions for patients on UE outcomes, pre-education of surgical patients regarding what to expect while intubated may aid in decreasing delirium risk, agitation, physical restraint use, and possibly UE. Verbal and written educational information during pre-admission testing is a feasible method easily integrated into pre-operative programs.
Because UEs often occur more frequently among less experienced staff, initial education about risk factors for UE is crucial to include in ICU staff orientation programs [3,7]. Educational initiatives should incorporate training on routine delirium screening and avoidance of agitation, use of protocols, and increased surveillance of patients receiving mechanical ventilation [5,15,17,39,45]. Ongoing education of staff regarding ventilatory equipment and risk factors for UE can be particularly effective in decreasing UE [46]. Initial educational efforts should be followed by routine updates for all members of the healthcare team about ongoing quality improvement efforts to monitor UE. Associated factors for UE that may be unit- or process-specific, including methods for endotracheal tube securement and intra-hospital transport, should be communicated with all individuals involved in patient care. Integration of continuous quality improvement programs can decrease UE rates by 22% to 53% [16]. Quality efforts typically focus on standardization of reporting and tracking tools, protocol implementations, and ongoing monitoring, auditing, and recording of UE.
Current Trends and Future Directions
Recent trends in critical care recommendations may mitigate potential risk factors identified in UE research. Integration of lightened sedation and daily wake up periods for intubated patients may decrease prevalence of risk factors for UE, specifically agitation, physical restraint use, and altered level of consciousness [30], while routine weaning protocols may improve ventilatory outcomes, including UE [5,38,40]. Nursing bedside report and purposeful hourly rounding are quickly emerging as mainstays of professional nursing care [41]. Inherent in these 2 initiatives are increased surveillance and vigilance by health care staff, which can result in timely extubation of those who indicate readiness, as well as decreased incidence of adverse events. Delirium remains a key factor that may be a likely cause for UE; recent trends towards early detection and proper management of delirium among ICU staff may result in improved ventilatory outcomes, including weaning, planned extubation, and the prevalence of UE.
Another important trend in critical care is the emergence of a neurocritical care specialty and routine admission of neurocritically ill patients to neuroscience ICUs [47,48]. However, there are no studies investigating prevalence of UE among these patients, who often have higher rates of agitation or restlessness due to cognitive impairment. Among general ICUs, patients with a primary respiratory diagnosis accounted for 23% of all UE in one study, while those with a neurological diagnosis accounted for the second highest percentage (12%) among the study population [15]. A separate study concluded that presence of neurological injury with a concomitant nosocomial infection increased risk of UE among patients in a mixed ICU [7]. A recent systematic review of weaning protocols highlights positive effects on ventilatory outcomes but cites lack of evidence for effectiveness of protocols among those with neurological injury [38]. Areas for future UE research should include factors specific to this patient population, as they may be at higher risk for adverse ventilatory outcomes due to the nature of the neurological injury.
Conclusion
Prevention of UE remains an elusive target, evidenced by little change in reported rates over 2 decades. Research provides data on risk factors that may be patient, unit, or process related. Structuring prevention efforts around modifiable risk factors for UE is a feasible approach amenable to ongoing monitoring for effectiveness. Integration of current trends in health care safety and quality may produce an added benefit of reducing the occurrence of UE in critical care units. Future research evaluating these trends and the prevalence of UE in subspecialty populations is warranted.
Corresponding author: Molly McNett, PhD, RN, CNRN, Attn: NBO, MetroHealth Medical Center, 2500 MetroHealth Drive; Cleveland, OH 44109, mmcnett@metrohealth.org.
Financial disclosures: None.
1. Krinsley JS, Barone JE. The drive to survive: unplanned extubation in the ICU. Chest 2005;128:560–6.
2. Coppolo DP, May JJ. Self-extubations. A 12-month experience. Chest 1990;98:165–9.
3. Yeh SH, Lee LN, Ho TH, et al. Implications of nursing care in the occurrence and consequences of unplanned extubation in adult intensive care units. Int J Nurs Stud 2004;41:255–62.
4. Mion LC, Minnick AF, Leipzig R, et al. Patient-initiated device removal in intensive care units: a national prevalence study. Crit Care Med 2007;35:2714–20.
5. Jarachovic M, Mason M, Kerber K. The role of standardized protocols in unplanned extubations in a medical intensive care unit. Am J Crit Care 2011;20:304–11.
6. Boulain T. Unplanned extubations in the adult intensive care unit: a prospective multicenter study. Association des Reanimateurs du Centre-Ouest. Am J Resp Crit Care Med 1998;157(4 Pt 1):1131–7.
7. Chang LY, Wang KW, Chao YF. Influence of physical restraint on unplanned extubation of adult intensive care patients: a case-control study. Am J Crit Care 2008;17:408–15.
8. Moons P, Sels K, De Becker W, et al. Development of a risk assessment tool for deliberate self-extubation in intensive care patients. Intensive Care Med 2004;30:1348–55.
9. Chiang AA, Lee KC, Lee JC, Wei CH. Effectiveness of a continuous quality improvement program aiming to reduce unplanned extubation: a prospective study. Intensive Care Med 1996;22:1269–71.
10. Betbese AJ, Perez M, Bak E, et al. A prospective study of unplanned endotracheal extubation in intensive care unit patients. Crit Care Med 1998;26:1180–6.
11. de Lassence A, Alberti C, Azoulay E, et al. Impact of unplanned extubation and reintubation after weaning on nosocomial pneumonia risk in the intensive care unit: a prospective multicenter study. Anesthesiology 2002;97:148–56.
12. Kapadia FN, Tekawade PC, Nath SS, et al. A prolonged observational study of tracheal tube displacements: Benchmarking an incidence <0.5-1% in a medical-surgical adult intensive care unit. Ind J Crit Care Med 2014;18:273–7.
13. Lee JH, Lee HC, Jeon YT, et al. Clinical outcomes after unplanned extubation in a surgical intensive care population. World J Surg 2014;38:203–10.
14. Chang LC, Liu PF, Huang YL, et al. Risk factors associated with unplanned endotracheal self-extubation of hospitalized intubated patients: a 3-year retrospective case-control study. Appl Nurs Res 2011;24:188–92.
15. de Groot RI, Dekkers OM, Herold IH, et al. Risk factors and outcomes after unplanned extubations on the ICU: a case-control study. Crit Care 2011;15:R19.
16. da Silva PS, Fonseca MC. Unplanned endotracheal extubations in the intensive care unit: systematic review, critical appraisal, and evidence-based recommendations. Anesth Analg 2012;114:1003–14.
17. Curry K, Cobb S, Kutash M, Diggs C. Characteristics associated with unplanned extubations in a surgical intensive care unit. Am J Crit Care 2008;17:45–51.
18. Christie JM, Dethlefsen M, Cane RD. Unplanned endotracheal extubation in the intensive care unit. J Clin Anesth 1996;8:289–93.
19. Huang YT. Factors leading to self-extubation of endotracheal tubes in the intensive care unit. Nurs Crit Care 2009;14:68–74.
20. Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126–34.
21. Mort TC. Unplanned tracheal extubation outside the operating room: a quality improvement audit of hemodynamic and tracheal airway complications associated with emergency tracheal reintubation. Anesth Analg 1998;86:1171–6.
22. Jaber S, Chanques G, Altairac C, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest 2005;128:2749–57.
23. Roddy DJ, Spaeder MC, Pastor W, Stockwell DC, Klugman D. Unplanned extubations in children: impact on hospital cost and length of stay. Ped Crit Care Med 2015.
24. Bouza C, Garcia E, Diaz M, et al. Unplanned extubation in orally intubated medical patients in the intensive care unit: a prospective cohort study. Heart Lung 2007;36:270–6.
25. Vassal T, Anh NG, Gabillet JM, et al. Prospective evaluation of self-extubations in a medical intensive care unit. Intensive Care Med 1993;19:340-342.
26. Tung A, Tadimeti L, Caruana-Montaldo B, et al. The relationship of sedation to deliberate self-extubation. J Clin Anesth 2001;13:24–9.
27. Tanios M, Epstein S, Grzeskowiak M, et al. Influence of sedation strategies on unplanned extubation in a mixed intensive care unit. Am J Crit Care 2014;23:306–14.
28. Atkins PM, Mion LC, Mendelson W, et al. Characteristics and outcomes of patients who self-extubate from ventilatory support: a case-control study. Chest 1997;112:1317–23.
29. Chevron V, Menard JF, Richard JC, et al. Unplanned extubation: risk factors of development and predictive criteria for reintubation. Crit Care Med 1998;26:1049–53.
30. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
31. Morandi A, Jackson JC. Delirium in the intensive care unit: a review. Neurol Clin 2011;29:749–63.
32. Banerjee A, Vasilevskis, EE, Pandharipande, P. Strategies to improve delirium assessment practices in the intensive care unit. J Clin Outcomes Manag 2010;17:459–68.
33. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
34. Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med 2004;32:106–12.
35. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc 2003;51:591–8.
36. Kerckhoffs MC, van der Sluijs AF, Binnekade JM, Dongelmans DA. Improving patient safety in the ICU by prospective identification of missing safety barriers using the bow-tie prospective risk analysis model. J Patient Safe 2013;9:154–9.
37. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8.
38. Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 2014;11:CD006904.
39. Chia PL, Santos DR, Tan TC, et al. Clinical quality improvement: eliminating unplanned extubation in the CCU. Int J Health Care Qual Ass 2013;26:642–52.
40. Balon JA. Common factors of spontaneous self-extubation in a critical care setting. Int J Trauma Nurs 2001;7:93–9.
41. Gregory S, Tan D, Tilrico M, et al. Bedside shift reports: what does the evidence say? J Nurs Admin 2014;44:541–5.
42. Kane RL, Shamliyan TA, Mueller C, et al. The association of registered nurse staffing levels and patient outcomes: systematic review and meta-analysis. Med Care 2007;45:1195–204.
43. Penoyer DA. Nurse staffing and patient outcomes in critical care: a concise review. Crit Care Med 2010;38:1521–8; quiz 1529.
44. Tindol GA, Jr., DiBenedetto RJ, Kosciuk L. Unplanned extubations. Chest 1994;105:1804–7.
45. Chen CM CK, Fong Y, Hsing SC, et al. Age is an important predictor of failed unplanned extubation. Int J Gerontol 2010;4:120–9.
46. Richmond AL, Jarog DL, Hanson VM. Unplanned extubation in adult critical care. Quality improvement and education payoff. Crit Care Nurs 2004;24:32–7.
47. Kurtz P, Fitts V, Sumer Z, et al. How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU? Neurocrit Care 2011;15:477–80.
48. McNett MM, Horowitz DA. International multidisciplinary consensus conference on multimodality monitoring: ICU processes of care. Neurocrit Care 2014;21 Suppl 2:S215–28.
49. Gardner A, Hughes, D, Cook R, et al. Best practice in stabilisation of oral endotracheal tubes: a systematic review. Database of abstracts of reivews of effects (DARE): Quality-assessed reviews. 2005. York: Center for Reviews and Dissemination.
50. Hofso K, Coyer FM. Part 1: Chemical and physical restraints in the management of mechanically ventliated patients in the ICU: Contributing factors. Intensive Crit Care Nurs 2007; 23:249–55.
51. Kiekkas P, Diamanto A, Panteli E, et al. Unplanned extubation in critially ill adults: Clinical reviews. Nurs Crit Care 2012;18:123–34.
52. King JN, Elliiot VA. Self/unplanned extubation: Safety, surveillance, and monitoring of the mechanically ventilated patient. Crit Care Nurs Clin North Am 2012;24:469–79.
1. Krinsley JS, Barone JE. The drive to survive: unplanned extubation in the ICU. Chest 2005;128:560–6.
2. Coppolo DP, May JJ. Self-extubations. A 12-month experience. Chest 1990;98:165–9.
3. Yeh SH, Lee LN, Ho TH, et al. Implications of nursing care in the occurrence and consequences of unplanned extubation in adult intensive care units. Int J Nurs Stud 2004;41:255–62.
4. Mion LC, Minnick AF, Leipzig R, et al. Patient-initiated device removal in intensive care units: a national prevalence study. Crit Care Med 2007;35:2714–20.
5. Jarachovic M, Mason M, Kerber K. The role of standardized protocols in unplanned extubations in a medical intensive care unit. Am J Crit Care 2011;20:304–11.
6. Boulain T. Unplanned extubations in the adult intensive care unit: a prospective multicenter study. Association des Reanimateurs du Centre-Ouest. Am J Resp Crit Care Med 1998;157(4 Pt 1):1131–7.
7. Chang LY, Wang KW, Chao YF. Influence of physical restraint on unplanned extubation of adult intensive care patients: a case-control study. Am J Crit Care 2008;17:408–15.
8. Moons P, Sels K, De Becker W, et al. Development of a risk assessment tool for deliberate self-extubation in intensive care patients. Intensive Care Med 2004;30:1348–55.
9. Chiang AA, Lee KC, Lee JC, Wei CH. Effectiveness of a continuous quality improvement program aiming to reduce unplanned extubation: a prospective study. Intensive Care Med 1996;22:1269–71.
10. Betbese AJ, Perez M, Bak E, et al. A prospective study of unplanned endotracheal extubation in intensive care unit patients. Crit Care Med 1998;26:1180–6.
11. de Lassence A, Alberti C, Azoulay E, et al. Impact of unplanned extubation and reintubation after weaning on nosocomial pneumonia risk in the intensive care unit: a prospective multicenter study. Anesthesiology 2002;97:148–56.
12. Kapadia FN, Tekawade PC, Nath SS, et al. A prolonged observational study of tracheal tube displacements: Benchmarking an incidence <0.5-1% in a medical-surgical adult intensive care unit. Ind J Crit Care Med 2014;18:273–7.
13. Lee JH, Lee HC, Jeon YT, et al. Clinical outcomes after unplanned extubation in a surgical intensive care population. World J Surg 2014;38:203–10.
14. Chang LC, Liu PF, Huang YL, et al. Risk factors associated with unplanned endotracheal self-extubation of hospitalized intubated patients: a 3-year retrospective case-control study. Appl Nurs Res 2011;24:188–92.
15. de Groot RI, Dekkers OM, Herold IH, et al. Risk factors and outcomes after unplanned extubations on the ICU: a case-control study. Crit Care 2011;15:R19.
16. da Silva PS, Fonseca MC. Unplanned endotracheal extubations in the intensive care unit: systematic review, critical appraisal, and evidence-based recommendations. Anesth Analg 2012;114:1003–14.
17. Curry K, Cobb S, Kutash M, Diggs C. Characteristics associated with unplanned extubations in a surgical intensive care unit. Am J Crit Care 2008;17:45–51.
18. Christie JM, Dethlefsen M, Cane RD. Unplanned endotracheal extubation in the intensive care unit. J Clin Anesth 1996;8:289–93.
19. Huang YT. Factors leading to self-extubation of endotracheal tubes in the intensive care unit. Nurs Crit Care 2009;14:68–74.
20. Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126–34.
21. Mort TC. Unplanned tracheal extubation outside the operating room: a quality improvement audit of hemodynamic and tracheal airway complications associated with emergency tracheal reintubation. Anesth Analg 1998;86:1171–6.
22. Jaber S, Chanques G, Altairac C, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest 2005;128:2749–57.
23. Roddy DJ, Spaeder MC, Pastor W, Stockwell DC, Klugman D. Unplanned extubations in children: impact on hospital cost and length of stay. Ped Crit Care Med 2015.
24. Bouza C, Garcia E, Diaz M, et al. Unplanned extubation in orally intubated medical patients in the intensive care unit: a prospective cohort study. Heart Lung 2007;36:270–6.
25. Vassal T, Anh NG, Gabillet JM, et al. Prospective evaluation of self-extubations in a medical intensive care unit. Intensive Care Med 1993;19:340-342.
26. Tung A, Tadimeti L, Caruana-Montaldo B, et al. The relationship of sedation to deliberate self-extubation. J Clin Anesth 2001;13:24–9.
27. Tanios M, Epstein S, Grzeskowiak M, et al. Influence of sedation strategies on unplanned extubation in a mixed intensive care unit. Am J Crit Care 2014;23:306–14.
28. Atkins PM, Mion LC, Mendelson W, et al. Characteristics and outcomes of patients who self-extubate from ventilatory support: a case-control study. Chest 1997;112:1317–23.
29. Chevron V, Menard JF, Richard JC, et al. Unplanned extubation: risk factors of development and predictive criteria for reintubation. Crit Care Med 1998;26:1049–53.
30. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306.
31. Morandi A, Jackson JC. Delirium in the intensive care unit: a review. Neurol Clin 2011;29:749–63.
32. Banerjee A, Vasilevskis, EE, Pandharipande, P. Strategies to improve delirium assessment practices in the intensive care unit. J Clin Outcomes Manag 2010;17:459–68.
33. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10.
34. Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med 2004;32:106–12.
35. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc 2003;51:591–8.
36. Kerckhoffs MC, van der Sluijs AF, Binnekade JM, Dongelmans DA. Improving patient safety in the ICU by prospective identification of missing safety barriers using the bow-tie prospective risk analysis model. J Patient Safe 2013;9:154–9.
37. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8.
38. Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 2014;11:CD006904.
39. Chia PL, Santos DR, Tan TC, et al. Clinical quality improvement: eliminating unplanned extubation in the CCU. Int J Health Care Qual Ass 2013;26:642–52.
40. Balon JA. Common factors of spontaneous self-extubation in a critical care setting. Int J Trauma Nurs 2001;7:93–9.
41. Gregory S, Tan D, Tilrico M, et al. Bedside shift reports: what does the evidence say? J Nurs Admin 2014;44:541–5.
42. Kane RL, Shamliyan TA, Mueller C, et al. The association of registered nurse staffing levels and patient outcomes: systematic review and meta-analysis. Med Care 2007;45:1195–204.
43. Penoyer DA. Nurse staffing and patient outcomes in critical care: a concise review. Crit Care Med 2010;38:1521–8; quiz 1529.
44. Tindol GA, Jr., DiBenedetto RJ, Kosciuk L. Unplanned extubations. Chest 1994;105:1804–7.
45. Chen CM CK, Fong Y, Hsing SC, et al. Age is an important predictor of failed unplanned extubation. Int J Gerontol 2010;4:120–9.
46. Richmond AL, Jarog DL, Hanson VM. Unplanned extubation in adult critical care. Quality improvement and education payoff. Crit Care Nurs 2004;24:32–7.
47. Kurtz P, Fitts V, Sumer Z, et al. How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU? Neurocrit Care 2011;15:477–80.
48. McNett MM, Horowitz DA. International multidisciplinary consensus conference on multimodality monitoring: ICU processes of care. Neurocrit Care 2014;21 Suppl 2:S215–28.
49. Gardner A, Hughes, D, Cook R, et al. Best practice in stabilisation of oral endotracheal tubes: a systematic review. Database of abstracts of reivews of effects (DARE): Quality-assessed reviews. 2005. York: Center for Reviews and Dissemination.
50. Hofso K, Coyer FM. Part 1: Chemical and physical restraints in the management of mechanically ventliated patients in the ICU: Contributing factors. Intensive Crit Care Nurs 2007; 23:249–55.
51. Kiekkas P, Diamanto A, Panteli E, et al. Unplanned extubation in critially ill adults: Clinical reviews. Nurs Crit Care 2012;18:123–34.
52. King JN, Elliiot VA. Self/unplanned extubation: Safety, surveillance, and monitoring of the mechanically ventilated patient. Crit Care Nurs Clin North Am 2012;24:469–79.
How Effective Is Group Cognitive Behavioral Therapy to Treat PTSD?
Anxiety is a necessary and natural reaction to trauma, but, sometimes, anxiety symptoms become excessive and problematic, as experienced with posttraumatic stress disorder (PTSD). Some patients who struggle with PTSD endure a relentless apprehension so intense that it keeps them from participating in everyday activities, such as attending work and partaking in social activities. Associated anxiety symptoms severely impair everyday function and include increased heart rate, sweating, intrusive images, poor attention, fear, or insomnia. Posttraumatic stress disorder symptoms often lead to occupational dysfunction, relationship difficulty, and numerous other functional impairments.
Approximately 300,000 veterans meet the criteria for PTSD related to ongoing or recent wars.1 The veteran does not bear the personal and functional burden alone; however, the financial load is felt throughout society. One recent study suggests that for veterans diagnosed with PTSD, the first 2 years after deployment cost society an estimated $7,000 per individial.2 Current research suggests that this potentially debilitating disorder occurs in about 14% of Operation Iraqi Freedom/Operation Enduring Freedom combat troops, whereas the similar U.S. demographic population experiences PTSD at a rate of about 7%.1,3 The ongoing military trauma exposures are compelling the mental health community to establish efficient and effective treatment options.4,5
Several treatment strategies exist to reduce PTSD symptoms, but health care professionals must seek a balance between therapeutic benefit and cost. The treatment of PTSD is diverse and variable; however, in the most recent Clinical Practice Guideline (CPG) for PTSD, the VA and DoD specifically endorse some psychotherapeutic interventions while dissuading the use of others.6 Of note, the VA and DoD CPG strongly encourages Stress Inoculation Training (SIT) and similar cognitive therapies aimed at guiding patients through the process of consciously understanding the relationship between thoughts and feelings and then modifying thoughts to appropriately manage stressors.6 Meanwhile, group psychotherapy has been determined to be “somewhat helpful.”6 Even though cognitive- and group-based therapies have long been established as efficacious for numerous psychological disorders (depression, obsessive compulsive disorder, eating disorders, etc), neither the American Group Psychotherapy Association nor the VA and DoD CPG directly endorse the use of group cognitive behavioral therapy (GCBT) for the treatment of PTSD.6,7 However, both VA and DoD mental health providers commonly practice CBT and various group psychotherapies for the treatment of PTSD.
Despite the widespread use of CBT, there is a gap in the clinical understanding of the evidence supporting GCBT for PTSD. The goal of this synthesis was to understand the efficacy of treating PTSD symptoms with group psychotherapy. To begin this investigation, the following PICO (population, intervention, comparison, outcome) question was asked: In adults diagnosed with PTSD, how effective is group cognitive behavioral therapy in reducing PTSD-related symptoms?
Methods
Research articles addressing the use of GCBT in PTSD were obtained via database searches that took place during October 2012 (Table). Searched databases included the Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews Randomized Controlled Trials, Psychological Information (PsycINFO), and Public Medicine (PubMed).
The PubMed database was searched using the following MeSH (medical subject heading) terms: “psychotherapy, group” and “stress disorders, post-traumatic” and “cognitive therapy.” Limitations were set to include only patients aged ≥ 18 years, results in English, those involving human subjects, and articles published within the past 5 years. A manual search of references was also conducted, and relevant articles were retained.
Articles that addressed primary substance abuse, other DSM Axis I disorders, intimate partner violence, or family issues were excluded from the evidence sample due to concerns of an alternate treatment focus. Articles with a focus on telehealth or alternative medicine were considered confounding to the scope of this review were also excluded. It was also noted that the term CBT is used collectively for an umbrella of treatments; however, treatments that focused on elements other than the components of CBT being delivered in a group were not included. To prevent duplication of the results, research from an inclusive review was not considered individually.
SUMMARY OF EVIDENCE
Six works fulfilled the PICO criteria and were of sufficient quality to be synthesized. Of the 6 articles retained for synthesis, 2 were high-level reviews. Both reviews supported the use of GCBT for PTSD treatment. Barrera and colleagues reported an overall large effect size regardless of the presence of exposure in-group among the 12 treatment conditions and 651 study participants.8 These researchers also reported that in-group exposure did not further traumatize other group members.
Similarly, although a notably older and smaller review, Bisson and Andrew reported a significant standard mean deviation between 4 GCBT treatment and wait list controls. These reviewers did not find a significant difference between trauma- and nontrauma-focused treatment groups. The reviews also noted that individual psychotherapy and/or pharmacotherapy was most often continued throughout the reviewed studies.8,9
The 4 other studies contribute substantively to this synthesis but arguably represent lower evidence quality. A large longitudinal study of 496 Australian veterans reported a large effect size that was sustained 9 months after treatment began.10 These researchers used an intensive outpatient program that included medication and other treatment modalities as the basis for GCBT delivery. They reported that the majority of the patients revealed improvement in PTSD symptoms.
Another study sampled a similar group of 10 combat veterans but focused particular attention on sleep-related PTSD symptoms of insomnia, nightmares, and sleep quality.11 Although these researchers were unable to report a significant difference in overall PTSD symptoms for the 8 subjects who completed the protocol, they did find a large effect size on insomnia severity and a medium effect size on sleep quality. Regular treatment, including medication, continued throughout this study.
Other researchers reported a medium effect size on PTSD symptoms while using GCBT in a heterogeneous group with various anxiety disorders, including obsessive compulsive disorder, generalized anxiety disorder, social phobia, panic disorders, and PTSD.12 Although reporting similar results as all other included studies, this study has some significant limitations, including a 26% dropout rate among the 152 participants. The final study included for synthesis reported a remarkable 67% elimination of the PTSD diagnosis among 6 motor vehicle accident survivors in the small, uncontrolled study.13 Concomitant treatments, including medications, were not reported in detail for these 4 studies except as mentioned.
As a whole, the 6 studies revealed some appreciable commonalities. Time since diagnosis did not seem to influence the results. Attrition was consistently found to be similar to other PTSD treatments. The reported session topics were loosely based on common CBT tenets (ie, education, challenging cognitions, and relaxation techniques) and were typically similar among treatment groups, including the use of homework.
DISCUSSION
As the diagnosis of PTSD increases to unfamiliar levels, GCBT has the potential to be helpful to clinicians and patients seeking alternatives to their current treatments.1,4,14 The reported results imply that GCBT can be useful in PTSD symptom reduction. This could be particularly useful to VA and military providers or rural providers operating with limited resources.
Treatment protocols are not well established and should be approached with care prior to the establishment of CBT treatment groups for those diagnosed with PTSD. Session overviews and descriptions, such as those mentioned in Thompson and colleagues, could provide a reference point for future use.13
Also worth considering, CBT can be an ambiguous term requiring deliberate definition within treatment protocols. As noted in the VA and DoD CPG, exposure- and trauma-focused treatment designs can be efficacious, but these elements do not seem to be required within the GCBT treatment setting.
The current research also suggests GCBT efficacy regardless of the index trauma. This does not suggest that heterogeneous groups were frequently studied nor can conclusions be drawn regarding heterogeneous treatment groups. Elements such as group size and session length are inconsistently reported and require specific consideration as well. There is a distinct lack of research directly comparing individual CBT with GCBT directly, which prohibits meaningful conclusions regarding PTSD symptom reduction. This research gap may well have influenced the recommendations within the VA and DoD CPG. Although some higher quality studies exist, many of the published reports on GCBT have noteworthy design flaw, such as inadequate controls and statistical analysis.
LIMITATIONS
There are some limitations to this literature synthesis. Although the search was limited to the past 5 years, the inclusion of reviews accounts for older evidence. As alluded to earlier, the lack of a standardized GCBT treatment protocol challenges results comparisons as well. The consequent treatment variations make direct interstudy comparison and synthesis difficult. Similarly, outcome measures varied between studies. Also, group psychotherapy is well established and accepted. Therefore, much of the supporting research was accomplished outside the parameters of this literature search. This empirical view of group psychotherapy among mental health providers may also contribute to the lack of available research.
It is also worth noting that studies finding neutral or negative results are often unpublished. This publication bias could account for the lack of available evidence. The research reports do not consistently report therapist qualifications; however, board certificates in group psychotherapy and CBT are undeniably variables available for debate. The inclusion of uncontrolled trials limits these findings as well. Although the above limitations are not exhaustive, they do provide necessary caveats to future generalizations.
FUTURE IMPLICATIONS
Perhaps the most important information to gain from future research is that of treatment outcomes. Studies that include a detailed outcome evaluation could reveal patient satisfaction, efficacy, and financial considerations. In the presence of adequate supportive data, GCBT could contribute outcome data regarding trauma survivor symptom normalization, peer support formation, access to care, treatment efficiency, and health care resources utilization. As noted in Barrera and colleagues, future analysis will require a greater volume of trials with an overall increase in methodological rigor.8
Current research has demonstrated a solid base from which to spawn specific treatment protocols. The available research is investigational in terms of treatment procedures. Replication of these studies could dictate treatment protocol and contribute substantively to future VA and DoD CPG updates. Future researchers should consider the use of a standard PTSD symptom assessment tool to make interstudy comparisons more meaningful. The length of treatment and exposure elements should be targeted specifically in future research as these components currently vary the most.
The military represents an obvious avenue for future research due to increased PTSD diagnosis in recent years. Although the etiology of the increase in PTSD is unclear and most likely multifactorial (decreased resilience, increased awareness, increased pursuit of secondary gains, etc), the need for treatment options is apparent.1 Group cohesion has been shown to be a core component of successful group psychotherapy, so military members who are accustomed to unit cohesion might represent a uniquely suitable population for this modality.15 Interestingly and for reasons not currently understood, veterans do not see effects of therapy as large as their civilian counterparts.8 This underscores the need for further evaluation of military-specific outcomes.
CONCLUSIONS
Although the available evidence is not robust, results do support the careful use of GCBT as an effective treatment for PTSD symptom reduction.8 Group psychotherapy has been generally regarded as an efficacious and cost-effective method to achieve similar outcomes to individual therapy. Increasing PTSD prevalence compels mental health care providers to explore all available treatment options. The potential for GCBT as an option is exciting, especially for mental health providers and those with limited resources. Rising health care standards and the current national fiscal situation is dictating a reevaluation of treatment options; so perhaps all health care providers will soon consider the use of GCBT.
As with any group assignment, the clinician should carefully consider the individual’s suitability and desire for group participation.16 With GCBT, providers could facilitate the relief of relentless apprehension and functional impairment for several patients simultaneously. Although there are many details left to explore regarding the use of GCBT for PTSD, the therapy’s foundation for use as a PTSD treatment is apparent.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Litz B, Schlenger W. Posttraumatic stress disorder in service members and new veterans of the Iraq and Afghanistan wars: A bibliography and critique. PTSD Res Q. 2009;20(1):1-3.
2. Tanielian T. Assessing combat exposure and post-traumatic stress disorder in troops and estimating the costs to society: Implications from the RAND Invisible Wounds of War Study. http://www.rand.org/pubs/testimonies/CT321.html. Published 2009. Accessed September 29, 2014.
3. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Cukor J, Spitalnick J, Difede J, Rizzo A, Rothbaum BO. Emerging treatments for PTSD. Clinical Psychol Rev. 2009;29(8):715-726.
5. Hoge CW. Interventions for war-related posttraumatic stress disorder: Meeting veterans where they are. JAMA. 2011;306(5):549-551.
6. Veterans Health Administration, Department of Defense. VA/DoD Clinical Practice Guideline: Management of Post-Traumatic Stress, Version 2.0. Washington, DC: Veterans Health Administration and Department of Defense; 2010.
7. Burlingame GM, Fuhriman A, Mosier J. The differential effectiveness of group psychotherapy: A meta-analytic perspective. Group Dyn. 2003;7(1):3-12.
8. Barrera TL, Mott JM, Hofstein RF, Teng EJ. A meta-analytic review of exposure in group cognitive behavioral therapy for posttraumatic stress disorder. Clin Psychol Rev. 2013;33(1):24-32.
9. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007;(3):CD003388.
10. Khoo A, Dent MT, Oei TS. (2011). Group cognitive behaviour therapy for military service-related post-traumatic stress disorder: Effectiveness, sustainability and repeatability. Aust N Z J Psychiatry. 2011;45(8):663-672.
11. Swanson LM, Favorite TK, Horin E, Arnedt JT. A combined group treatment for nightmares and insomnia in combat veterans: A pilot study. J Trauma Stress. 2009;22(6):639-642.
12. Erickson DH, Janeck A, Tallman K. A cognitive-behavioral group for patients with various anxiety disorders. Psychiatr Serv. 2007;58(9):1205-1211.
13. Thompson AR, Wilde E, Boon K. The development of group CBT for the treatment of road-traffic-accident-related post-traumatic stress disorder: A pilot study. Cognitive Behav Therapist. 2010;2(1):32-42.
14. Slade T, Johnston A, Oakley-Browne MA, Andrews G, Whiteford H. 2007 National Survey of Mental Health and Wellbeing: Methods and key findings. Aust N Z J Psychiatry. 2009;43(7):594-605.
15. Crowe TP, Grenyer BF. Is therapist alliance or whole group cohesion more influential in group psychotherapy outcomes? Clin Psychol Psychother. 2008;15(4):239-246.
16. Leszcz M, Kobos JC. Evidence-based group psychotherapy: Using AGPA’s practice guidelines to enhance clinical effectiveness. J Clin Psychol. 2008;64(11):1238-1260.
Anxiety is a necessary and natural reaction to trauma, but, sometimes, anxiety symptoms become excessive and problematic, as experienced with posttraumatic stress disorder (PTSD). Some patients who struggle with PTSD endure a relentless apprehension so intense that it keeps them from participating in everyday activities, such as attending work and partaking in social activities. Associated anxiety symptoms severely impair everyday function and include increased heart rate, sweating, intrusive images, poor attention, fear, or insomnia. Posttraumatic stress disorder symptoms often lead to occupational dysfunction, relationship difficulty, and numerous other functional impairments.
Approximately 300,000 veterans meet the criteria for PTSD related to ongoing or recent wars.1 The veteran does not bear the personal and functional burden alone; however, the financial load is felt throughout society. One recent study suggests that for veterans diagnosed with PTSD, the first 2 years after deployment cost society an estimated $7,000 per individial.2 Current research suggests that this potentially debilitating disorder occurs in about 14% of Operation Iraqi Freedom/Operation Enduring Freedom combat troops, whereas the similar U.S. demographic population experiences PTSD at a rate of about 7%.1,3 The ongoing military trauma exposures are compelling the mental health community to establish efficient and effective treatment options.4,5
Several treatment strategies exist to reduce PTSD symptoms, but health care professionals must seek a balance between therapeutic benefit and cost. The treatment of PTSD is diverse and variable; however, in the most recent Clinical Practice Guideline (CPG) for PTSD, the VA and DoD specifically endorse some psychotherapeutic interventions while dissuading the use of others.6 Of note, the VA and DoD CPG strongly encourages Stress Inoculation Training (SIT) and similar cognitive therapies aimed at guiding patients through the process of consciously understanding the relationship between thoughts and feelings and then modifying thoughts to appropriately manage stressors.6 Meanwhile, group psychotherapy has been determined to be “somewhat helpful.”6 Even though cognitive- and group-based therapies have long been established as efficacious for numerous psychological disorders (depression, obsessive compulsive disorder, eating disorders, etc), neither the American Group Psychotherapy Association nor the VA and DoD CPG directly endorse the use of group cognitive behavioral therapy (GCBT) for the treatment of PTSD.6,7 However, both VA and DoD mental health providers commonly practice CBT and various group psychotherapies for the treatment of PTSD.
Despite the widespread use of CBT, there is a gap in the clinical understanding of the evidence supporting GCBT for PTSD. The goal of this synthesis was to understand the efficacy of treating PTSD symptoms with group psychotherapy. To begin this investigation, the following PICO (population, intervention, comparison, outcome) question was asked: In adults diagnosed with PTSD, how effective is group cognitive behavioral therapy in reducing PTSD-related symptoms?
Methods
Research articles addressing the use of GCBT in PTSD were obtained via database searches that took place during October 2012 (Table). Searched databases included the Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews Randomized Controlled Trials, Psychological Information (PsycINFO), and Public Medicine (PubMed).
The PubMed database was searched using the following MeSH (medical subject heading) terms: “psychotherapy, group” and “stress disorders, post-traumatic” and “cognitive therapy.” Limitations were set to include only patients aged ≥ 18 years, results in English, those involving human subjects, and articles published within the past 5 years. A manual search of references was also conducted, and relevant articles were retained.
Articles that addressed primary substance abuse, other DSM Axis I disorders, intimate partner violence, or family issues were excluded from the evidence sample due to concerns of an alternate treatment focus. Articles with a focus on telehealth or alternative medicine were considered confounding to the scope of this review were also excluded. It was also noted that the term CBT is used collectively for an umbrella of treatments; however, treatments that focused on elements other than the components of CBT being delivered in a group were not included. To prevent duplication of the results, research from an inclusive review was not considered individually.
SUMMARY OF EVIDENCE
Six works fulfilled the PICO criteria and were of sufficient quality to be synthesized. Of the 6 articles retained for synthesis, 2 were high-level reviews. Both reviews supported the use of GCBT for PTSD treatment. Barrera and colleagues reported an overall large effect size regardless of the presence of exposure in-group among the 12 treatment conditions and 651 study participants.8 These researchers also reported that in-group exposure did not further traumatize other group members.
Similarly, although a notably older and smaller review, Bisson and Andrew reported a significant standard mean deviation between 4 GCBT treatment and wait list controls. These reviewers did not find a significant difference between trauma- and nontrauma-focused treatment groups. The reviews also noted that individual psychotherapy and/or pharmacotherapy was most often continued throughout the reviewed studies.8,9
The 4 other studies contribute substantively to this synthesis but arguably represent lower evidence quality. A large longitudinal study of 496 Australian veterans reported a large effect size that was sustained 9 months after treatment began.10 These researchers used an intensive outpatient program that included medication and other treatment modalities as the basis for GCBT delivery. They reported that the majority of the patients revealed improvement in PTSD symptoms.
Another study sampled a similar group of 10 combat veterans but focused particular attention on sleep-related PTSD symptoms of insomnia, nightmares, and sleep quality.11 Although these researchers were unable to report a significant difference in overall PTSD symptoms for the 8 subjects who completed the protocol, they did find a large effect size on insomnia severity and a medium effect size on sleep quality. Regular treatment, including medication, continued throughout this study.
Other researchers reported a medium effect size on PTSD symptoms while using GCBT in a heterogeneous group with various anxiety disorders, including obsessive compulsive disorder, generalized anxiety disorder, social phobia, panic disorders, and PTSD.12 Although reporting similar results as all other included studies, this study has some significant limitations, including a 26% dropout rate among the 152 participants. The final study included for synthesis reported a remarkable 67% elimination of the PTSD diagnosis among 6 motor vehicle accident survivors in the small, uncontrolled study.13 Concomitant treatments, including medications, were not reported in detail for these 4 studies except as mentioned.
As a whole, the 6 studies revealed some appreciable commonalities. Time since diagnosis did not seem to influence the results. Attrition was consistently found to be similar to other PTSD treatments. The reported session topics were loosely based on common CBT tenets (ie, education, challenging cognitions, and relaxation techniques) and were typically similar among treatment groups, including the use of homework.
DISCUSSION
As the diagnosis of PTSD increases to unfamiliar levels, GCBT has the potential to be helpful to clinicians and patients seeking alternatives to their current treatments.1,4,14 The reported results imply that GCBT can be useful in PTSD symptom reduction. This could be particularly useful to VA and military providers or rural providers operating with limited resources.
Treatment protocols are not well established and should be approached with care prior to the establishment of CBT treatment groups for those diagnosed with PTSD. Session overviews and descriptions, such as those mentioned in Thompson and colleagues, could provide a reference point for future use.13
Also worth considering, CBT can be an ambiguous term requiring deliberate definition within treatment protocols. As noted in the VA and DoD CPG, exposure- and trauma-focused treatment designs can be efficacious, but these elements do not seem to be required within the GCBT treatment setting.
The current research also suggests GCBT efficacy regardless of the index trauma. This does not suggest that heterogeneous groups were frequently studied nor can conclusions be drawn regarding heterogeneous treatment groups. Elements such as group size and session length are inconsistently reported and require specific consideration as well. There is a distinct lack of research directly comparing individual CBT with GCBT directly, which prohibits meaningful conclusions regarding PTSD symptom reduction. This research gap may well have influenced the recommendations within the VA and DoD CPG. Although some higher quality studies exist, many of the published reports on GCBT have noteworthy design flaw, such as inadequate controls and statistical analysis.
LIMITATIONS
There are some limitations to this literature synthesis. Although the search was limited to the past 5 years, the inclusion of reviews accounts for older evidence. As alluded to earlier, the lack of a standardized GCBT treatment protocol challenges results comparisons as well. The consequent treatment variations make direct interstudy comparison and synthesis difficult. Similarly, outcome measures varied between studies. Also, group psychotherapy is well established and accepted. Therefore, much of the supporting research was accomplished outside the parameters of this literature search. This empirical view of group psychotherapy among mental health providers may also contribute to the lack of available research.
It is also worth noting that studies finding neutral or negative results are often unpublished. This publication bias could account for the lack of available evidence. The research reports do not consistently report therapist qualifications; however, board certificates in group psychotherapy and CBT are undeniably variables available for debate. The inclusion of uncontrolled trials limits these findings as well. Although the above limitations are not exhaustive, they do provide necessary caveats to future generalizations.
FUTURE IMPLICATIONS
Perhaps the most important information to gain from future research is that of treatment outcomes. Studies that include a detailed outcome evaluation could reveal patient satisfaction, efficacy, and financial considerations. In the presence of adequate supportive data, GCBT could contribute outcome data regarding trauma survivor symptom normalization, peer support formation, access to care, treatment efficiency, and health care resources utilization. As noted in Barrera and colleagues, future analysis will require a greater volume of trials with an overall increase in methodological rigor.8
Current research has demonstrated a solid base from which to spawn specific treatment protocols. The available research is investigational in terms of treatment procedures. Replication of these studies could dictate treatment protocol and contribute substantively to future VA and DoD CPG updates. Future researchers should consider the use of a standard PTSD symptom assessment tool to make interstudy comparisons more meaningful. The length of treatment and exposure elements should be targeted specifically in future research as these components currently vary the most.
The military represents an obvious avenue for future research due to increased PTSD diagnosis in recent years. Although the etiology of the increase in PTSD is unclear and most likely multifactorial (decreased resilience, increased awareness, increased pursuit of secondary gains, etc), the need for treatment options is apparent.1 Group cohesion has been shown to be a core component of successful group psychotherapy, so military members who are accustomed to unit cohesion might represent a uniquely suitable population for this modality.15 Interestingly and for reasons not currently understood, veterans do not see effects of therapy as large as their civilian counterparts.8 This underscores the need for further evaluation of military-specific outcomes.
CONCLUSIONS
Although the available evidence is not robust, results do support the careful use of GCBT as an effective treatment for PTSD symptom reduction.8 Group psychotherapy has been generally regarded as an efficacious and cost-effective method to achieve similar outcomes to individual therapy. Increasing PTSD prevalence compels mental health care providers to explore all available treatment options. The potential for GCBT as an option is exciting, especially for mental health providers and those with limited resources. Rising health care standards and the current national fiscal situation is dictating a reevaluation of treatment options; so perhaps all health care providers will soon consider the use of GCBT.
As with any group assignment, the clinician should carefully consider the individual’s suitability and desire for group participation.16 With GCBT, providers could facilitate the relief of relentless apprehension and functional impairment for several patients simultaneously. Although there are many details left to explore regarding the use of GCBT for PTSD, the therapy’s foundation for use as a PTSD treatment is apparent.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Anxiety is a necessary and natural reaction to trauma, but, sometimes, anxiety symptoms become excessive and problematic, as experienced with posttraumatic stress disorder (PTSD). Some patients who struggle with PTSD endure a relentless apprehension so intense that it keeps them from participating in everyday activities, such as attending work and partaking in social activities. Associated anxiety symptoms severely impair everyday function and include increased heart rate, sweating, intrusive images, poor attention, fear, or insomnia. Posttraumatic stress disorder symptoms often lead to occupational dysfunction, relationship difficulty, and numerous other functional impairments.
Approximately 300,000 veterans meet the criteria for PTSD related to ongoing or recent wars.1 The veteran does not bear the personal and functional burden alone; however, the financial load is felt throughout society. One recent study suggests that for veterans diagnosed with PTSD, the first 2 years after deployment cost society an estimated $7,000 per individial.2 Current research suggests that this potentially debilitating disorder occurs in about 14% of Operation Iraqi Freedom/Operation Enduring Freedom combat troops, whereas the similar U.S. demographic population experiences PTSD at a rate of about 7%.1,3 The ongoing military trauma exposures are compelling the mental health community to establish efficient and effective treatment options.4,5
Several treatment strategies exist to reduce PTSD symptoms, but health care professionals must seek a balance between therapeutic benefit and cost. The treatment of PTSD is diverse and variable; however, in the most recent Clinical Practice Guideline (CPG) for PTSD, the VA and DoD specifically endorse some psychotherapeutic interventions while dissuading the use of others.6 Of note, the VA and DoD CPG strongly encourages Stress Inoculation Training (SIT) and similar cognitive therapies aimed at guiding patients through the process of consciously understanding the relationship between thoughts and feelings and then modifying thoughts to appropriately manage stressors.6 Meanwhile, group psychotherapy has been determined to be “somewhat helpful.”6 Even though cognitive- and group-based therapies have long been established as efficacious for numerous psychological disorders (depression, obsessive compulsive disorder, eating disorders, etc), neither the American Group Psychotherapy Association nor the VA and DoD CPG directly endorse the use of group cognitive behavioral therapy (GCBT) for the treatment of PTSD.6,7 However, both VA and DoD mental health providers commonly practice CBT and various group psychotherapies for the treatment of PTSD.
Despite the widespread use of CBT, there is a gap in the clinical understanding of the evidence supporting GCBT for PTSD. The goal of this synthesis was to understand the efficacy of treating PTSD symptoms with group psychotherapy. To begin this investigation, the following PICO (population, intervention, comparison, outcome) question was asked: In adults diagnosed with PTSD, how effective is group cognitive behavioral therapy in reducing PTSD-related symptoms?
Methods
Research articles addressing the use of GCBT in PTSD were obtained via database searches that took place during October 2012 (Table). Searched databases included the Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews Randomized Controlled Trials, Psychological Information (PsycINFO), and Public Medicine (PubMed).
The PubMed database was searched using the following MeSH (medical subject heading) terms: “psychotherapy, group” and “stress disorders, post-traumatic” and “cognitive therapy.” Limitations were set to include only patients aged ≥ 18 years, results in English, those involving human subjects, and articles published within the past 5 years. A manual search of references was also conducted, and relevant articles were retained.
Articles that addressed primary substance abuse, other DSM Axis I disorders, intimate partner violence, or family issues were excluded from the evidence sample due to concerns of an alternate treatment focus. Articles with a focus on telehealth or alternative medicine were considered confounding to the scope of this review were also excluded. It was also noted that the term CBT is used collectively for an umbrella of treatments; however, treatments that focused on elements other than the components of CBT being delivered in a group were not included. To prevent duplication of the results, research from an inclusive review was not considered individually.
SUMMARY OF EVIDENCE
Six works fulfilled the PICO criteria and were of sufficient quality to be synthesized. Of the 6 articles retained for synthesis, 2 were high-level reviews. Both reviews supported the use of GCBT for PTSD treatment. Barrera and colleagues reported an overall large effect size regardless of the presence of exposure in-group among the 12 treatment conditions and 651 study participants.8 These researchers also reported that in-group exposure did not further traumatize other group members.
Similarly, although a notably older and smaller review, Bisson and Andrew reported a significant standard mean deviation between 4 GCBT treatment and wait list controls. These reviewers did not find a significant difference between trauma- and nontrauma-focused treatment groups. The reviews also noted that individual psychotherapy and/or pharmacotherapy was most often continued throughout the reviewed studies.8,9
The 4 other studies contribute substantively to this synthesis but arguably represent lower evidence quality. A large longitudinal study of 496 Australian veterans reported a large effect size that was sustained 9 months after treatment began.10 These researchers used an intensive outpatient program that included medication and other treatment modalities as the basis for GCBT delivery. They reported that the majority of the patients revealed improvement in PTSD symptoms.
Another study sampled a similar group of 10 combat veterans but focused particular attention on sleep-related PTSD symptoms of insomnia, nightmares, and sleep quality.11 Although these researchers were unable to report a significant difference in overall PTSD symptoms for the 8 subjects who completed the protocol, they did find a large effect size on insomnia severity and a medium effect size on sleep quality. Regular treatment, including medication, continued throughout this study.
Other researchers reported a medium effect size on PTSD symptoms while using GCBT in a heterogeneous group with various anxiety disorders, including obsessive compulsive disorder, generalized anxiety disorder, social phobia, panic disorders, and PTSD.12 Although reporting similar results as all other included studies, this study has some significant limitations, including a 26% dropout rate among the 152 participants. The final study included for synthesis reported a remarkable 67% elimination of the PTSD diagnosis among 6 motor vehicle accident survivors in the small, uncontrolled study.13 Concomitant treatments, including medications, were not reported in detail for these 4 studies except as mentioned.
As a whole, the 6 studies revealed some appreciable commonalities. Time since diagnosis did not seem to influence the results. Attrition was consistently found to be similar to other PTSD treatments. The reported session topics were loosely based on common CBT tenets (ie, education, challenging cognitions, and relaxation techniques) and were typically similar among treatment groups, including the use of homework.
DISCUSSION
As the diagnosis of PTSD increases to unfamiliar levels, GCBT has the potential to be helpful to clinicians and patients seeking alternatives to their current treatments.1,4,14 The reported results imply that GCBT can be useful in PTSD symptom reduction. This could be particularly useful to VA and military providers or rural providers operating with limited resources.
Treatment protocols are not well established and should be approached with care prior to the establishment of CBT treatment groups for those diagnosed with PTSD. Session overviews and descriptions, such as those mentioned in Thompson and colleagues, could provide a reference point for future use.13
Also worth considering, CBT can be an ambiguous term requiring deliberate definition within treatment protocols. As noted in the VA and DoD CPG, exposure- and trauma-focused treatment designs can be efficacious, but these elements do not seem to be required within the GCBT treatment setting.
The current research also suggests GCBT efficacy regardless of the index trauma. This does not suggest that heterogeneous groups were frequently studied nor can conclusions be drawn regarding heterogeneous treatment groups. Elements such as group size and session length are inconsistently reported and require specific consideration as well. There is a distinct lack of research directly comparing individual CBT with GCBT directly, which prohibits meaningful conclusions regarding PTSD symptom reduction. This research gap may well have influenced the recommendations within the VA and DoD CPG. Although some higher quality studies exist, many of the published reports on GCBT have noteworthy design flaw, such as inadequate controls and statistical analysis.
LIMITATIONS
There are some limitations to this literature synthesis. Although the search was limited to the past 5 years, the inclusion of reviews accounts for older evidence. As alluded to earlier, the lack of a standardized GCBT treatment protocol challenges results comparisons as well. The consequent treatment variations make direct interstudy comparison and synthesis difficult. Similarly, outcome measures varied between studies. Also, group psychotherapy is well established and accepted. Therefore, much of the supporting research was accomplished outside the parameters of this literature search. This empirical view of group psychotherapy among mental health providers may also contribute to the lack of available research.
It is also worth noting that studies finding neutral or negative results are often unpublished. This publication bias could account for the lack of available evidence. The research reports do not consistently report therapist qualifications; however, board certificates in group psychotherapy and CBT are undeniably variables available for debate. The inclusion of uncontrolled trials limits these findings as well. Although the above limitations are not exhaustive, they do provide necessary caveats to future generalizations.
FUTURE IMPLICATIONS
Perhaps the most important information to gain from future research is that of treatment outcomes. Studies that include a detailed outcome evaluation could reveal patient satisfaction, efficacy, and financial considerations. In the presence of adequate supportive data, GCBT could contribute outcome data regarding trauma survivor symptom normalization, peer support formation, access to care, treatment efficiency, and health care resources utilization. As noted in Barrera and colleagues, future analysis will require a greater volume of trials with an overall increase in methodological rigor.8
Current research has demonstrated a solid base from which to spawn specific treatment protocols. The available research is investigational in terms of treatment procedures. Replication of these studies could dictate treatment protocol and contribute substantively to future VA and DoD CPG updates. Future researchers should consider the use of a standard PTSD symptom assessment tool to make interstudy comparisons more meaningful. The length of treatment and exposure elements should be targeted specifically in future research as these components currently vary the most.
The military represents an obvious avenue for future research due to increased PTSD diagnosis in recent years. Although the etiology of the increase in PTSD is unclear and most likely multifactorial (decreased resilience, increased awareness, increased pursuit of secondary gains, etc), the need for treatment options is apparent.1 Group cohesion has been shown to be a core component of successful group psychotherapy, so military members who are accustomed to unit cohesion might represent a uniquely suitable population for this modality.15 Interestingly and for reasons not currently understood, veterans do not see effects of therapy as large as their civilian counterparts.8 This underscores the need for further evaluation of military-specific outcomes.
CONCLUSIONS
Although the available evidence is not robust, results do support the careful use of GCBT as an effective treatment for PTSD symptom reduction.8 Group psychotherapy has been generally regarded as an efficacious and cost-effective method to achieve similar outcomes to individual therapy. Increasing PTSD prevalence compels mental health care providers to explore all available treatment options. The potential for GCBT as an option is exciting, especially for mental health providers and those with limited resources. Rising health care standards and the current national fiscal situation is dictating a reevaluation of treatment options; so perhaps all health care providers will soon consider the use of GCBT.
As with any group assignment, the clinician should carefully consider the individual’s suitability and desire for group participation.16 With GCBT, providers could facilitate the relief of relentless apprehension and functional impairment for several patients simultaneously. Although there are many details left to explore regarding the use of GCBT for PTSD, the therapy’s foundation for use as a PTSD treatment is apparent.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Litz B, Schlenger W. Posttraumatic stress disorder in service members and new veterans of the Iraq and Afghanistan wars: A bibliography and critique. PTSD Res Q. 2009;20(1):1-3.
2. Tanielian T. Assessing combat exposure and post-traumatic stress disorder in troops and estimating the costs to society: Implications from the RAND Invisible Wounds of War Study. http://www.rand.org/pubs/testimonies/CT321.html. Published 2009. Accessed September 29, 2014.
3. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Cukor J, Spitalnick J, Difede J, Rizzo A, Rothbaum BO. Emerging treatments for PTSD. Clinical Psychol Rev. 2009;29(8):715-726.
5. Hoge CW. Interventions for war-related posttraumatic stress disorder: Meeting veterans where they are. JAMA. 2011;306(5):549-551.
6. Veterans Health Administration, Department of Defense. VA/DoD Clinical Practice Guideline: Management of Post-Traumatic Stress, Version 2.0. Washington, DC: Veterans Health Administration and Department of Defense; 2010.
7. Burlingame GM, Fuhriman A, Mosier J. The differential effectiveness of group psychotherapy: A meta-analytic perspective. Group Dyn. 2003;7(1):3-12.
8. Barrera TL, Mott JM, Hofstein RF, Teng EJ. A meta-analytic review of exposure in group cognitive behavioral therapy for posttraumatic stress disorder. Clin Psychol Rev. 2013;33(1):24-32.
9. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007;(3):CD003388.
10. Khoo A, Dent MT, Oei TS. (2011). Group cognitive behaviour therapy for military service-related post-traumatic stress disorder: Effectiveness, sustainability and repeatability. Aust N Z J Psychiatry. 2011;45(8):663-672.
11. Swanson LM, Favorite TK, Horin E, Arnedt JT. A combined group treatment for nightmares and insomnia in combat veterans: A pilot study. J Trauma Stress. 2009;22(6):639-642.
12. Erickson DH, Janeck A, Tallman K. A cognitive-behavioral group for patients with various anxiety disorders. Psychiatr Serv. 2007;58(9):1205-1211.
13. Thompson AR, Wilde E, Boon K. The development of group CBT for the treatment of road-traffic-accident-related post-traumatic stress disorder: A pilot study. Cognitive Behav Therapist. 2010;2(1):32-42.
14. Slade T, Johnston A, Oakley-Browne MA, Andrews G, Whiteford H. 2007 National Survey of Mental Health and Wellbeing: Methods and key findings. Aust N Z J Psychiatry. 2009;43(7):594-605.
15. Crowe TP, Grenyer BF. Is therapist alliance or whole group cohesion more influential in group psychotherapy outcomes? Clin Psychol Psychother. 2008;15(4):239-246.
16. Leszcz M, Kobos JC. Evidence-based group psychotherapy: Using AGPA’s practice guidelines to enhance clinical effectiveness. J Clin Psychol. 2008;64(11):1238-1260.
1. Litz B, Schlenger W. Posttraumatic stress disorder in service members and new veterans of the Iraq and Afghanistan wars: A bibliography and critique. PTSD Res Q. 2009;20(1):1-3.
2. Tanielian T. Assessing combat exposure and post-traumatic stress disorder in troops and estimating the costs to society: Implications from the RAND Invisible Wounds of War Study. http://www.rand.org/pubs/testimonies/CT321.html. Published 2009. Accessed September 29, 2014.
3. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Cukor J, Spitalnick J, Difede J, Rizzo A, Rothbaum BO. Emerging treatments for PTSD. Clinical Psychol Rev. 2009;29(8):715-726.
5. Hoge CW. Interventions for war-related posttraumatic stress disorder: Meeting veterans where they are. JAMA. 2011;306(5):549-551.
6. Veterans Health Administration, Department of Defense. VA/DoD Clinical Practice Guideline: Management of Post-Traumatic Stress, Version 2.0. Washington, DC: Veterans Health Administration and Department of Defense; 2010.
7. Burlingame GM, Fuhriman A, Mosier J. The differential effectiveness of group psychotherapy: A meta-analytic perspective. Group Dyn. 2003;7(1):3-12.
8. Barrera TL, Mott JM, Hofstein RF, Teng EJ. A meta-analytic review of exposure in group cognitive behavioral therapy for posttraumatic stress disorder. Clin Psychol Rev. 2013;33(1):24-32.
9. Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007;(3):CD003388.
10. Khoo A, Dent MT, Oei TS. (2011). Group cognitive behaviour therapy for military service-related post-traumatic stress disorder: Effectiveness, sustainability and repeatability. Aust N Z J Psychiatry. 2011;45(8):663-672.
11. Swanson LM, Favorite TK, Horin E, Arnedt JT. A combined group treatment for nightmares and insomnia in combat veterans: A pilot study. J Trauma Stress. 2009;22(6):639-642.
12. Erickson DH, Janeck A, Tallman K. A cognitive-behavioral group for patients with various anxiety disorders. Psychiatr Serv. 2007;58(9):1205-1211.
13. Thompson AR, Wilde E, Boon K. The development of group CBT for the treatment of road-traffic-accident-related post-traumatic stress disorder: A pilot study. Cognitive Behav Therapist. 2010;2(1):32-42.
14. Slade T, Johnston A, Oakley-Browne MA, Andrews G, Whiteford H. 2007 National Survey of Mental Health and Wellbeing: Methods and key findings. Aust N Z J Psychiatry. 2009;43(7):594-605.
15. Crowe TP, Grenyer BF. Is therapist alliance or whole group cohesion more influential in group psychotherapy outcomes? Clin Psychol Psychother. 2008;15(4):239-246.
16. Leszcz M, Kobos JC. Evidence-based group psychotherapy: Using AGPA’s practice guidelines to enhance clinical effectiveness. J Clin Psychol. 2008;64(11):1238-1260.