User login
Patients Want the Facts Delivered in a Personal Story
Poor communication between physician and patient can cause a lot of harm, according to Joseph N. Cappella, PhD, Gerald R. Miller Professor Emeritus of Communication at the University of Pennsylvania in Philadelphia, and Richard N. Street Jr, PhD, professor of communication and media science at Texas A&M University in Houston, Texas. When a physician and patient talk past each other, it may impair the patient’s compliance with preventive measures, screening, and treatment; undermine the physician-patient relationship; exacerbate fears and concerns; and possibly lead patients to rely on misleading, incomplete, or simply incorrect information, turning away from evidence-based medicine.
Drs. Cappella and Street made these points in an essay recently published in JAMA. The essay marks the beginning of the JAMA series Communicating Medicine.
“Helping clinicians deliver accurate information more effectively can lead to better-informed patients,” wrote Anne R. Cappola, MD, professor of endocrinology, diabetes, and metabolism at the University of Pennsylvania, and Kirsten Bibbins-Domingo, MD, PhD, professor of medicine at the University of California, San Francisco, in an accompanying editorial. Drs. Cappola and Bibbins-Domingo also are editors of JAMA.
To establish a common understanding between physician and patient, Drs. Cappella and Street identified the following four responsibilities of the physician:
- Discover what the patient understands and why
- Provide accurate information in an understandable manner
- Promote the credibility of the information
- Verify whether the patient has understood.
“Research has shown that although medical facts need to be the basis for the clinician’s core message, those facts are more effectively communicated in a patient-clinician relationship characterized by trust and cooperation and when the information is presented in a manner that fosters patient understanding,” wrote Drs. Cappella and Street. This approach includes using interpreters for patients who do not fluently speak the physician’s language and supplementing explanations with simple written information, images, and videos.
Patients generally believe their physician’s information, and most patients view their physicians as a trustworthy source. Trust is based on the belief that the physician has the patient’s best interests at heart.
However, patients may be distrustful of their physician’s information if it contradicts their own belief system or personal experiences or because they inherently distrust the medical profession.
In addition, patients are less willing to accept explanations and recommendations if they feel misunderstood, judged, discriminated against, or rushed by the physician. The basis for effective communication is a relationship with patients that is built on trust and respect. Empirically supported strategies for expressing respect and building trust include the following:
- Affirming the patient’s values
- Anticipating and addressing false or misleading information
- Using simple, jargon-free language
- Embedding facts into a story, rather than presenting the scientific evidence dryly.
“Conveying factual material using these techniques makes facts more engaging and memorable,” wrote Drs. Cappella and Street. It is crucial to inquire about and consider the patient’s perspective, health beliefs, assumptions, concerns, needs, and stories in the conversation.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Poor communication between physician and patient can cause a lot of harm, according to Joseph N. Cappella, PhD, Gerald R. Miller Professor Emeritus of Communication at the University of Pennsylvania in Philadelphia, and Richard N. Street Jr, PhD, professor of communication and media science at Texas A&M University in Houston, Texas. When a physician and patient talk past each other, it may impair the patient’s compliance with preventive measures, screening, and treatment; undermine the physician-patient relationship; exacerbate fears and concerns; and possibly lead patients to rely on misleading, incomplete, or simply incorrect information, turning away from evidence-based medicine.
Drs. Cappella and Street made these points in an essay recently published in JAMA. The essay marks the beginning of the JAMA series Communicating Medicine.
“Helping clinicians deliver accurate information more effectively can lead to better-informed patients,” wrote Anne R. Cappola, MD, professor of endocrinology, diabetes, and metabolism at the University of Pennsylvania, and Kirsten Bibbins-Domingo, MD, PhD, professor of medicine at the University of California, San Francisco, in an accompanying editorial. Drs. Cappola and Bibbins-Domingo also are editors of JAMA.
To establish a common understanding between physician and patient, Drs. Cappella and Street identified the following four responsibilities of the physician:
- Discover what the patient understands and why
- Provide accurate information in an understandable manner
- Promote the credibility of the information
- Verify whether the patient has understood.
“Research has shown that although medical facts need to be the basis for the clinician’s core message, those facts are more effectively communicated in a patient-clinician relationship characterized by trust and cooperation and when the information is presented in a manner that fosters patient understanding,” wrote Drs. Cappella and Street. This approach includes using interpreters for patients who do not fluently speak the physician’s language and supplementing explanations with simple written information, images, and videos.
Patients generally believe their physician’s information, and most patients view their physicians as a trustworthy source. Trust is based on the belief that the physician has the patient’s best interests at heart.
However, patients may be distrustful of their physician’s information if it contradicts their own belief system or personal experiences or because they inherently distrust the medical profession.
In addition, patients are less willing to accept explanations and recommendations if they feel misunderstood, judged, discriminated against, or rushed by the physician. The basis for effective communication is a relationship with patients that is built on trust and respect. Empirically supported strategies for expressing respect and building trust include the following:
- Affirming the patient’s values
- Anticipating and addressing false or misleading information
- Using simple, jargon-free language
- Embedding facts into a story, rather than presenting the scientific evidence dryly.
“Conveying factual material using these techniques makes facts more engaging and memorable,” wrote Drs. Cappella and Street. It is crucial to inquire about and consider the patient’s perspective, health beliefs, assumptions, concerns, needs, and stories in the conversation.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Poor communication between physician and patient can cause a lot of harm, according to Joseph N. Cappella, PhD, Gerald R. Miller Professor Emeritus of Communication at the University of Pennsylvania in Philadelphia, and Richard N. Street Jr, PhD, professor of communication and media science at Texas A&M University in Houston, Texas. When a physician and patient talk past each other, it may impair the patient’s compliance with preventive measures, screening, and treatment; undermine the physician-patient relationship; exacerbate fears and concerns; and possibly lead patients to rely on misleading, incomplete, or simply incorrect information, turning away from evidence-based medicine.
Drs. Cappella and Street made these points in an essay recently published in JAMA. The essay marks the beginning of the JAMA series Communicating Medicine.
“Helping clinicians deliver accurate information more effectively can lead to better-informed patients,” wrote Anne R. Cappola, MD, professor of endocrinology, diabetes, and metabolism at the University of Pennsylvania, and Kirsten Bibbins-Domingo, MD, PhD, professor of medicine at the University of California, San Francisco, in an accompanying editorial. Drs. Cappola and Bibbins-Domingo also are editors of JAMA.
To establish a common understanding between physician and patient, Drs. Cappella and Street identified the following four responsibilities of the physician:
- Discover what the patient understands and why
- Provide accurate information in an understandable manner
- Promote the credibility of the information
- Verify whether the patient has understood.
“Research has shown that although medical facts need to be the basis for the clinician’s core message, those facts are more effectively communicated in a patient-clinician relationship characterized by trust and cooperation and when the information is presented in a manner that fosters patient understanding,” wrote Drs. Cappella and Street. This approach includes using interpreters for patients who do not fluently speak the physician’s language and supplementing explanations with simple written information, images, and videos.
Patients generally believe their physician’s information, and most patients view their physicians as a trustworthy source. Trust is based on the belief that the physician has the patient’s best interests at heart.
However, patients may be distrustful of their physician’s information if it contradicts their own belief system or personal experiences or because they inherently distrust the medical profession.
In addition, patients are less willing to accept explanations and recommendations if they feel misunderstood, judged, discriminated against, or rushed by the physician. The basis for effective communication is a relationship with patients that is built on trust and respect. Empirically supported strategies for expressing respect and building trust include the following:
- Affirming the patient’s values
- Anticipating and addressing false or misleading information
- Using simple, jargon-free language
- Embedding facts into a story, rather than presenting the scientific evidence dryly.
“Conveying factual material using these techniques makes facts more engaging and memorable,” wrote Drs. Cappella and Street. It is crucial to inquire about and consider the patient’s perspective, health beliefs, assumptions, concerns, needs, and stories in the conversation.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Preventing ASCVD Events: Using Coronary Artery Calcification Scores to Personalize Risk and Guide Statin Therapy
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
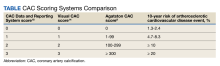
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
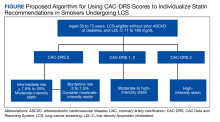
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
New guideline for managing toothache in children
Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or both medications together can effectively manage a child’s toothache as a stopgap until definitive treatment is available, according to a new guideline.
The guideline, published in the September issue of the Journal of the American Dental Association, does not recommend opioids for a toothache or after tooth extraction in this population.
Opioid prescriptions for children entail risk for hospitalization and death. Yet, some dentists continued to prescribe contraindicated opioids to young children after a Food and Drug Administration warning in 2017 about the use of tramadol and codeine in this population, the guideline notes.
Opioid prescribing to children also continued after the American Academy of Pediatric Dentistry in 2018 recommended acetaminophen and NSAIDs as first-line medications for pain management and said that the use of opioids should be “rare.”
Although the new guidance, which also covers pain management after tooth extraction, is geared toward general dentists, it could help emergency clinicians and primary care providers manage children’s pain when definitive treatment is not immediately available, the authors noted.
Definitive treatment could include pulpectomy, nonsurgical root canal, incision for drainage of an abscess, or tooth extraction.
If definitive care in 2-3 days is not possible, parents should let the health care team know, the guideline says.
“These pharmacologic strategies will alleviate dental pain temporarily until a referral for definitive dental treatment is in place,” the authors wrote.
The American Dental Association (ADA) endorsed the new guideline, which was developed by researchers with the ADA Science & Research Institute, the University of Pittsburgh School of Dental Medicine, and the Center for Integrative Global Oral Health at the University of Pennsylvania School of Dental Medicine in Philadelphia.
The guideline recommends ibuprofen and, for children older than 2 years, naproxen as NSAID options. The use of naproxen in children younger than 12 years for this purpose is off label, they noted.
The guideline suggests doses of acetaminophen and NSAIDs on the basis of age and weight that may differ from those on medication packaging.
“When acetaminophen or NSAIDs are administered as directed, the risk of harm to children from either medication is low,” the guideline states.
“While prescribing opioids to children has become less frequent overall, this guideline ensures that both dentists and parents have evidence-based recommendations to determine the most appropriate treatment for dental pain,” senior guideline author Paul Moore, DMD, PhD, MPH, professor emeritus at the University of Pittsburgh’s School of Dental Medicine, said in a news release from the ADA. “Parents and caregivers can take comfort that widely available medications that have no abuse potential, such as acetaminophen or ibuprofen, are safe and effective for helping their children find relief from short-term dental pain.”
A 2018 review by Dr. Moore and coauthors found that NSAIDs, with or without acetaminophen, were effective and minimized adverse events, relative to opioids, for acute dental pain across ages.
The new recommendations for children will “allow for better treatment of this kind of pain” and “will help prevent unnecessary prescribing of medications with abuse potential, including opioids,” Patrizia Cavazzoni, MD, director of the FDA Center for Drug Evaluation and Research, said in the news release.
The report stems from a 3-year, $1.5 million grant awarded by the FDA in 2020 to the University of Pittsburgh and the ADA Science & Research Institute to develop a clinical practice guideline for the management of acute pain in dentistry in children, adolescents, and adults. The recommendations for adolescents and adults are still in development.
The report was supported by an FDA grant, and the guideline authors received technical and methodologic support from the agency. Some authors disclosed ties to pharmaceutical companies.
A version of this article appeared on Medscape.com.
Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or both medications together can effectively manage a child’s toothache as a stopgap until definitive treatment is available, according to a new guideline.
The guideline, published in the September issue of the Journal of the American Dental Association, does not recommend opioids for a toothache or after tooth extraction in this population.
Opioid prescriptions for children entail risk for hospitalization and death. Yet, some dentists continued to prescribe contraindicated opioids to young children after a Food and Drug Administration warning in 2017 about the use of tramadol and codeine in this population, the guideline notes.
Opioid prescribing to children also continued after the American Academy of Pediatric Dentistry in 2018 recommended acetaminophen and NSAIDs as first-line medications for pain management and said that the use of opioids should be “rare.”
Although the new guidance, which also covers pain management after tooth extraction, is geared toward general dentists, it could help emergency clinicians and primary care providers manage children’s pain when definitive treatment is not immediately available, the authors noted.
Definitive treatment could include pulpectomy, nonsurgical root canal, incision for drainage of an abscess, or tooth extraction.
If definitive care in 2-3 days is not possible, parents should let the health care team know, the guideline says.
“These pharmacologic strategies will alleviate dental pain temporarily until a referral for definitive dental treatment is in place,” the authors wrote.
The American Dental Association (ADA) endorsed the new guideline, which was developed by researchers with the ADA Science & Research Institute, the University of Pittsburgh School of Dental Medicine, and the Center for Integrative Global Oral Health at the University of Pennsylvania School of Dental Medicine in Philadelphia.
The guideline recommends ibuprofen and, for children older than 2 years, naproxen as NSAID options. The use of naproxen in children younger than 12 years for this purpose is off label, they noted.
The guideline suggests doses of acetaminophen and NSAIDs on the basis of age and weight that may differ from those on medication packaging.
“When acetaminophen or NSAIDs are administered as directed, the risk of harm to children from either medication is low,” the guideline states.
“While prescribing opioids to children has become less frequent overall, this guideline ensures that both dentists and parents have evidence-based recommendations to determine the most appropriate treatment for dental pain,” senior guideline author Paul Moore, DMD, PhD, MPH, professor emeritus at the University of Pittsburgh’s School of Dental Medicine, said in a news release from the ADA. “Parents and caregivers can take comfort that widely available medications that have no abuse potential, such as acetaminophen or ibuprofen, are safe and effective for helping their children find relief from short-term dental pain.”
A 2018 review by Dr. Moore and coauthors found that NSAIDs, with or without acetaminophen, were effective and minimized adverse events, relative to opioids, for acute dental pain across ages.
The new recommendations for children will “allow for better treatment of this kind of pain” and “will help prevent unnecessary prescribing of medications with abuse potential, including opioids,” Patrizia Cavazzoni, MD, director of the FDA Center for Drug Evaluation and Research, said in the news release.
The report stems from a 3-year, $1.5 million grant awarded by the FDA in 2020 to the University of Pittsburgh and the ADA Science & Research Institute to develop a clinical practice guideline for the management of acute pain in dentistry in children, adolescents, and adults. The recommendations for adolescents and adults are still in development.
The report was supported by an FDA grant, and the guideline authors received technical and methodologic support from the agency. Some authors disclosed ties to pharmaceutical companies.
A version of this article appeared on Medscape.com.
Nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or both medications together can effectively manage a child’s toothache as a stopgap until definitive treatment is available, according to a new guideline.
The guideline, published in the September issue of the Journal of the American Dental Association, does not recommend opioids for a toothache or after tooth extraction in this population.
Opioid prescriptions for children entail risk for hospitalization and death. Yet, some dentists continued to prescribe contraindicated opioids to young children after a Food and Drug Administration warning in 2017 about the use of tramadol and codeine in this population, the guideline notes.
Opioid prescribing to children also continued after the American Academy of Pediatric Dentistry in 2018 recommended acetaminophen and NSAIDs as first-line medications for pain management and said that the use of opioids should be “rare.”
Although the new guidance, which also covers pain management after tooth extraction, is geared toward general dentists, it could help emergency clinicians and primary care providers manage children’s pain when definitive treatment is not immediately available, the authors noted.
Definitive treatment could include pulpectomy, nonsurgical root canal, incision for drainage of an abscess, or tooth extraction.
If definitive care in 2-3 days is not possible, parents should let the health care team know, the guideline says.
“These pharmacologic strategies will alleviate dental pain temporarily until a referral for definitive dental treatment is in place,” the authors wrote.
The American Dental Association (ADA) endorsed the new guideline, which was developed by researchers with the ADA Science & Research Institute, the University of Pittsburgh School of Dental Medicine, and the Center for Integrative Global Oral Health at the University of Pennsylvania School of Dental Medicine in Philadelphia.
The guideline recommends ibuprofen and, for children older than 2 years, naproxen as NSAID options. The use of naproxen in children younger than 12 years for this purpose is off label, they noted.
The guideline suggests doses of acetaminophen and NSAIDs on the basis of age and weight that may differ from those on medication packaging.
“When acetaminophen or NSAIDs are administered as directed, the risk of harm to children from either medication is low,” the guideline states.
“While prescribing opioids to children has become less frequent overall, this guideline ensures that both dentists and parents have evidence-based recommendations to determine the most appropriate treatment for dental pain,” senior guideline author Paul Moore, DMD, PhD, MPH, professor emeritus at the University of Pittsburgh’s School of Dental Medicine, said in a news release from the ADA. “Parents and caregivers can take comfort that widely available medications that have no abuse potential, such as acetaminophen or ibuprofen, are safe and effective for helping their children find relief from short-term dental pain.”
A 2018 review by Dr. Moore and coauthors found that NSAIDs, with or without acetaminophen, were effective and minimized adverse events, relative to opioids, for acute dental pain across ages.
The new recommendations for children will “allow for better treatment of this kind of pain” and “will help prevent unnecessary prescribing of medications with abuse potential, including opioids,” Patrizia Cavazzoni, MD, director of the FDA Center for Drug Evaluation and Research, said in the news release.
The report stems from a 3-year, $1.5 million grant awarded by the FDA in 2020 to the University of Pittsburgh and the ADA Science & Research Institute to develop a clinical practice guideline for the management of acute pain in dentistry in children, adolescents, and adults. The recommendations for adolescents and adults are still in development.
The report was supported by an FDA grant, and the guideline authors received technical and methodologic support from the agency. Some authors disclosed ties to pharmaceutical companies.
A version of this article appeared on Medscape.com.
Migraine in children and teens: managing the pain
By the time Mira Halker started high school, hardly a day passed that she wasn’t either getting a migraine attack or recovering from one. She missed volleyball team practice. She missed classes. She missed social events. And few people understood. After all, she looked healthy.
“A lot of times, people think I’m faking it,” said Mira, now 16, who lives in Phoenix. Friends called her flaky; her volleyball coaches questioned her dedication to the team. “I’m like, ‘I’m not trying to get out of this. This is not what this is about,’ ” she said.
Her mother, Rashmi B. Halker Singh, MD, is a neurologist at Mayo Clinic who happens to specialize in migraine. Even so, finding a solution was not easy. Neither ibuprofen nor triptans, nor various preventive measures such as a daily prescription for topiramate controlled the pain and associated symptoms. Mira was barely making it through her school day and had to quit volleyball. Then, in the spring of 10th grade, Mira told her mother that she couldn’t go to prom because the loud noises and lights could give her a migraine attack.
Mother and daughter decided it was time to get even more aggressive. “There are these key moments in life that you can’t get back,” Dr. Singh said. “Migraine steals so much from you.”
Diagnosis
One of the challenges Mira’s physicians faced was deciding which medications and other therapies to prescribe to a teenager. Drug companies have been releasing a steady stream of new treatments for migraine headaches, and researchers promise more are on the way soon. Here’s what works for children, what hasn’t yet been approved for use with minors, and how to diagnose migraines in the first place, from experts at some of the nation’s leading pediatric headache centers.
Migraine affects about 10% of children, according to the American Migraine Foundation. The headaches can strike children as early as age 3 or 4 years, said Robert Little, MD, a pediatric neurologist at Phoenix Children’s Hospital.
Before puberty, boys report more migraine attacks than girls, according to the American Academy of Pediatrics. But that reverses in adolescence: By age 17, as many as 8% of boys and 23% of girls have had migraine. To diagnose migraine, Juliana H. VanderPluym, MD, associate professor of neurology at Mayo Clinic in Phoenix, said she uses the criteria published in the latest edition of the International Classification of Headache Disorders (ICHD): A patient must have had at least five attacks in their life; and in children and adolescents, the attacks must last no less than 2 hours.
In addition, the headaches should exhibit at least two out of four features:
1. Occur more on one side of the head than the other (although Dr. VanderPluym said in children and adolescents headaches often are bilateral).
2. Be of moderate to severe intensity.
3. Have a pounding or throbbing quality.
4. Grow worse with activity or cause an avoidance of activity.
If the attacks meet those criteria, clinicians should check to see if they meet at least one out of the two following:
1. Are sensitive to light and sounds.
2. Are associated with nausea and/or vomiting.
A clinician should consider whether the headaches are not better accounted for by another diagnosis, according to the ICHD criteria. But, Dr. VanderPluym warned that does not necessarily mean running a slew of tests.
“In the absence of red flag features, it is more than likely going to be migraine headache,” she said. That’s especially true if a child has a family history of migraine, as the condition is often passed down from parent to child.
Ultimately, the diagnosis is fairly simple and can be made in a minute or less, said Jack Gladstein, MD, a pediatrician at the University of Maryland whose research focuses on the clinical care of children and adolescents with headache.
“Migraine is acute,” Dr. Gladstein said. “It’s really bad. And it’s recurrent.”
First line of treatment
Whatever a patient takes to treat a migraine, they should hit it early and hard, Dr. Gladstein said.
“The first thing you say, as a primary care physician, is treat your migraine at first twinge, whatever you use. Don’t wait, don’t wish it away,” he said. “The longer you wait, the less chance anything will work.”
The second piece of advice, Dr. Gladstein said, is that whatever drug a patient is taking, they should be on the highest feasible dose. “Work as fast as you can to treat them. You want the brain to reset as quickly as you can,” he said.
Patients should begin with over-the-counter pain relievers, Dr. Little said. If those prove insufficient, they can try a triptan. Rizatriptan is the only such agent that the Food and Drug Administration has approved for children aged 6-17 years. Other drugs in the class – sumatriptan/naproxen, almotriptan, and zolmitriptan – are approved for children 12 and older.
Another migraine therapy recently approved for children aged 12 and older is the use of neurostimulators. “It’s helpful to be aware of them,” Dr. VanderPluym said.
However, if neurostimulators and acute medications prove insufficient, clinicians should warn patients not to up their doses of triptans. Rebound headaches can occur if patients take triptans more than twice a week, or a maximum 10 days per month.
Another possibility is to add a preventive therapy. One mild, first option is nutraceuticals, like riboflavin (vitamin B2) or magnesium, said Anisa F. Kelley, MD, a neurologist and associate director of the headache program at the Ann and Robert H. Lurie Children’s Hospital of Chicago.
“We don’t have definitive evidence, but they’re probably doing more benefit than they are harm,” Dr. Kelley said of these therapies. “In patients who have anywhere from 4 to 8 migraine days a month, where you’re in that in-between period where you don’t necessarily need a [prescription] prophylactic, I will often start with a nutraceutical,” Dr. Kelley said.
For those patients who don’t respond to nutraceuticals, or who need more support, clinicians can prescribe amitriptyline or topiramate. Dr. VanderPluym said.
A 2017 study found such prophylactics to be no more effective than placebo in pediatric migraine patients, but experts caution the results should not be considered definitive.
For one thing, the study enrolled a highly selective group of participants, with milder forms of migraine who may have improved anyway, Dr. VanderPluym said. All participants also received lifestyle counseling.
Every time participants came in for a follow-up, they were asked questions such as how much water were they drinking and how much sleep were they getting, Dr. Kelley noted. The takeaway, she said: “Pediatric and adolescent migraine [management] is very, very much reliant on lifestyle factors.”
Lifestyle triggers
Clinicians should counsel their migraine patients about lifestyle changes, experts said. Getting adequate sleep, staying hydrated, and managing stress can help reduce the intensity and frequency of attacks.
Migraine patients should also be mindful of their screen time, Dr. Kelley added.
“I’ve had lots and lots of patients who find excessive screen time will trigger or worsen migraine,” she said.
As for other potential triggers of attacks, the evidence is mixed.
“There’s clearly an association with disrupted sleep and migraine, and that has been very well established,” Dr. Little said. “And there is some modest amount of evidence that regular exercise can be helpful.” But for reported food triggers, he said, there have been very inconclusive results.
Commonly reported triggers include MSG, red wine, chocolate, and aged cheese. When Dr. Little’s patients keep headache diaries, tracking their meals alongside when they got migraine attacks, they often discover individualized triggers – strawberries, for instance, in one case, he said.
Scientists believe migraines result from the inappropriate activation of the trigeminal ganglion. “The question is, what causes it to get triggered? And how does it get triggered?” Dr. Gladstein said. “And that’s where there’s a lot of difference of opinion and no conclusive evidence.” Clinicians also should make sure that something else – usually depression, anxiety, insomnia, and dizziness – is not hindering effective migraine management. “If someone has terrible insomnia, until you treat the insomnia, the headaches aren’t going to get better,” he said.
As for Mira, her migraine attacks did not significantly improve, despite trying triptans, prophylactics, lifestyle changes, and shots to block nerve pain. When the headaches threatened Mira’s chance to go to her prom, her neurologist suggested trying something different. The physician persuaded the family’s insurance to cover a calcitonin gene-related peptide antagonist, an injectable monoclonal antibody treatment for migraine that the FDA has currently approved only for use in adults.
The difference for Mira has been extraordinary.
“I can do so much more than I was able to do,” said Mira, who attended the dance migraine free. “I feel liberated.”
It’s only migraine
One of the greatest challenges in diagnosing migraine can be reassuring the patient, the parents, even clinicians themselves that migraine really is the cause of all this pain and discomfort, experts said.
“A lot of migraine treatment actually comes down to migraine education,” Dr. VanderPluym said.
Patients and their parents often wonder how they can be sure that this pain is not resulting from something more dangerous than migraine, Dr. Little said. In these cases, he cites practice guidelines published by the American Academy of Neurology.
“The gist of those guidelines is that most pediatric patients do not need further workup,” he said. “But I think that there’s always a fear that you’re missing something because we don’t have a test that we can do” for migraine.
Some warning signs that further tests might be warranted, Dr. Kelley said, include:
- Headaches that wake a patient up in the middle of the night.
- Headaches that start first thing in the morning, especially those that include vomiting.
- A headache pattern that suddenly gets much worse.
- Certain symptoms that accompany the headache, such as tingling, numbness or double vision.
Although all of these signs can still stem from migraines – tingling or numbness, for instance, can be signs of migraine aura – running additional tests can rule out more serious concerns, she said.
By the time Mira Halker started high school, hardly a day passed that she wasn’t either getting a migraine attack or recovering from one. She missed volleyball team practice. She missed classes. She missed social events. And few people understood. After all, she looked healthy.
“A lot of times, people think I’m faking it,” said Mira, now 16, who lives in Phoenix. Friends called her flaky; her volleyball coaches questioned her dedication to the team. “I’m like, ‘I’m not trying to get out of this. This is not what this is about,’ ” she said.
Her mother, Rashmi B. Halker Singh, MD, is a neurologist at Mayo Clinic who happens to specialize in migraine. Even so, finding a solution was not easy. Neither ibuprofen nor triptans, nor various preventive measures such as a daily prescription for topiramate controlled the pain and associated symptoms. Mira was barely making it through her school day and had to quit volleyball. Then, in the spring of 10th grade, Mira told her mother that she couldn’t go to prom because the loud noises and lights could give her a migraine attack.
Mother and daughter decided it was time to get even more aggressive. “There are these key moments in life that you can’t get back,” Dr. Singh said. “Migraine steals so much from you.”
Diagnosis
One of the challenges Mira’s physicians faced was deciding which medications and other therapies to prescribe to a teenager. Drug companies have been releasing a steady stream of new treatments for migraine headaches, and researchers promise more are on the way soon. Here’s what works for children, what hasn’t yet been approved for use with minors, and how to diagnose migraines in the first place, from experts at some of the nation’s leading pediatric headache centers.
Migraine affects about 10% of children, according to the American Migraine Foundation. The headaches can strike children as early as age 3 or 4 years, said Robert Little, MD, a pediatric neurologist at Phoenix Children’s Hospital.
Before puberty, boys report more migraine attacks than girls, according to the American Academy of Pediatrics. But that reverses in adolescence: By age 17, as many as 8% of boys and 23% of girls have had migraine. To diagnose migraine, Juliana H. VanderPluym, MD, associate professor of neurology at Mayo Clinic in Phoenix, said she uses the criteria published in the latest edition of the International Classification of Headache Disorders (ICHD): A patient must have had at least five attacks in their life; and in children and adolescents, the attacks must last no less than 2 hours.
In addition, the headaches should exhibit at least two out of four features:
1. Occur more on one side of the head than the other (although Dr. VanderPluym said in children and adolescents headaches often are bilateral).
2. Be of moderate to severe intensity.
3. Have a pounding or throbbing quality.
4. Grow worse with activity or cause an avoidance of activity.
If the attacks meet those criteria, clinicians should check to see if they meet at least one out of the two following:
1. Are sensitive to light and sounds.
2. Are associated with nausea and/or vomiting.
A clinician should consider whether the headaches are not better accounted for by another diagnosis, according to the ICHD criteria. But, Dr. VanderPluym warned that does not necessarily mean running a slew of tests.
“In the absence of red flag features, it is more than likely going to be migraine headache,” she said. That’s especially true if a child has a family history of migraine, as the condition is often passed down from parent to child.
Ultimately, the diagnosis is fairly simple and can be made in a minute or less, said Jack Gladstein, MD, a pediatrician at the University of Maryland whose research focuses on the clinical care of children and adolescents with headache.
“Migraine is acute,” Dr. Gladstein said. “It’s really bad. And it’s recurrent.”
First line of treatment
Whatever a patient takes to treat a migraine, they should hit it early and hard, Dr. Gladstein said.
“The first thing you say, as a primary care physician, is treat your migraine at first twinge, whatever you use. Don’t wait, don’t wish it away,” he said. “The longer you wait, the less chance anything will work.”
The second piece of advice, Dr. Gladstein said, is that whatever drug a patient is taking, they should be on the highest feasible dose. “Work as fast as you can to treat them. You want the brain to reset as quickly as you can,” he said.
Patients should begin with over-the-counter pain relievers, Dr. Little said. If those prove insufficient, they can try a triptan. Rizatriptan is the only such agent that the Food and Drug Administration has approved for children aged 6-17 years. Other drugs in the class – sumatriptan/naproxen, almotriptan, and zolmitriptan – are approved for children 12 and older.
Another migraine therapy recently approved for children aged 12 and older is the use of neurostimulators. “It’s helpful to be aware of them,” Dr. VanderPluym said.
However, if neurostimulators and acute medications prove insufficient, clinicians should warn patients not to up their doses of triptans. Rebound headaches can occur if patients take triptans more than twice a week, or a maximum 10 days per month.
Another possibility is to add a preventive therapy. One mild, first option is nutraceuticals, like riboflavin (vitamin B2) or magnesium, said Anisa F. Kelley, MD, a neurologist and associate director of the headache program at the Ann and Robert H. Lurie Children’s Hospital of Chicago.
“We don’t have definitive evidence, but they’re probably doing more benefit than they are harm,” Dr. Kelley said of these therapies. “In patients who have anywhere from 4 to 8 migraine days a month, where you’re in that in-between period where you don’t necessarily need a [prescription] prophylactic, I will often start with a nutraceutical,” Dr. Kelley said.
For those patients who don’t respond to nutraceuticals, or who need more support, clinicians can prescribe amitriptyline or topiramate. Dr. VanderPluym said.
A 2017 study found such prophylactics to be no more effective than placebo in pediatric migraine patients, but experts caution the results should not be considered definitive.
For one thing, the study enrolled a highly selective group of participants, with milder forms of migraine who may have improved anyway, Dr. VanderPluym said. All participants also received lifestyle counseling.
Every time participants came in for a follow-up, they were asked questions such as how much water were they drinking and how much sleep were they getting, Dr. Kelley noted. The takeaway, she said: “Pediatric and adolescent migraine [management] is very, very much reliant on lifestyle factors.”
Lifestyle triggers
Clinicians should counsel their migraine patients about lifestyle changes, experts said. Getting adequate sleep, staying hydrated, and managing stress can help reduce the intensity and frequency of attacks.
Migraine patients should also be mindful of their screen time, Dr. Kelley added.
“I’ve had lots and lots of patients who find excessive screen time will trigger or worsen migraine,” she said.
As for other potential triggers of attacks, the evidence is mixed.
“There’s clearly an association with disrupted sleep and migraine, and that has been very well established,” Dr. Little said. “And there is some modest amount of evidence that regular exercise can be helpful.” But for reported food triggers, he said, there have been very inconclusive results.
Commonly reported triggers include MSG, red wine, chocolate, and aged cheese. When Dr. Little’s patients keep headache diaries, tracking their meals alongside when they got migraine attacks, they often discover individualized triggers – strawberries, for instance, in one case, he said.
Scientists believe migraines result from the inappropriate activation of the trigeminal ganglion. “The question is, what causes it to get triggered? And how does it get triggered?” Dr. Gladstein said. “And that’s where there’s a lot of difference of opinion and no conclusive evidence.” Clinicians also should make sure that something else – usually depression, anxiety, insomnia, and dizziness – is not hindering effective migraine management. “If someone has terrible insomnia, until you treat the insomnia, the headaches aren’t going to get better,” he said.
As for Mira, her migraine attacks did not significantly improve, despite trying triptans, prophylactics, lifestyle changes, and shots to block nerve pain. When the headaches threatened Mira’s chance to go to her prom, her neurologist suggested trying something different. The physician persuaded the family’s insurance to cover a calcitonin gene-related peptide antagonist, an injectable monoclonal antibody treatment for migraine that the FDA has currently approved only for use in adults.
The difference for Mira has been extraordinary.
“I can do so much more than I was able to do,” said Mira, who attended the dance migraine free. “I feel liberated.”
It’s only migraine
One of the greatest challenges in diagnosing migraine can be reassuring the patient, the parents, even clinicians themselves that migraine really is the cause of all this pain and discomfort, experts said.
“A lot of migraine treatment actually comes down to migraine education,” Dr. VanderPluym said.
Patients and their parents often wonder how they can be sure that this pain is not resulting from something more dangerous than migraine, Dr. Little said. In these cases, he cites practice guidelines published by the American Academy of Neurology.
“The gist of those guidelines is that most pediatric patients do not need further workup,” he said. “But I think that there’s always a fear that you’re missing something because we don’t have a test that we can do” for migraine.
Some warning signs that further tests might be warranted, Dr. Kelley said, include:
- Headaches that wake a patient up in the middle of the night.
- Headaches that start first thing in the morning, especially those that include vomiting.
- A headache pattern that suddenly gets much worse.
- Certain symptoms that accompany the headache, such as tingling, numbness or double vision.
Although all of these signs can still stem from migraines – tingling or numbness, for instance, can be signs of migraine aura – running additional tests can rule out more serious concerns, she said.
By the time Mira Halker started high school, hardly a day passed that she wasn’t either getting a migraine attack or recovering from one. She missed volleyball team practice. She missed classes. She missed social events. And few people understood. After all, she looked healthy.
“A lot of times, people think I’m faking it,” said Mira, now 16, who lives in Phoenix. Friends called her flaky; her volleyball coaches questioned her dedication to the team. “I’m like, ‘I’m not trying to get out of this. This is not what this is about,’ ” she said.
Her mother, Rashmi B. Halker Singh, MD, is a neurologist at Mayo Clinic who happens to specialize in migraine. Even so, finding a solution was not easy. Neither ibuprofen nor triptans, nor various preventive measures such as a daily prescription for topiramate controlled the pain and associated symptoms. Mira was barely making it through her school day and had to quit volleyball. Then, in the spring of 10th grade, Mira told her mother that she couldn’t go to prom because the loud noises and lights could give her a migraine attack.
Mother and daughter decided it was time to get even more aggressive. “There are these key moments in life that you can’t get back,” Dr. Singh said. “Migraine steals so much from you.”
Diagnosis
One of the challenges Mira’s physicians faced was deciding which medications and other therapies to prescribe to a teenager. Drug companies have been releasing a steady stream of new treatments for migraine headaches, and researchers promise more are on the way soon. Here’s what works for children, what hasn’t yet been approved for use with minors, and how to diagnose migraines in the first place, from experts at some of the nation’s leading pediatric headache centers.
Migraine affects about 10% of children, according to the American Migraine Foundation. The headaches can strike children as early as age 3 or 4 years, said Robert Little, MD, a pediatric neurologist at Phoenix Children’s Hospital.
Before puberty, boys report more migraine attacks than girls, according to the American Academy of Pediatrics. But that reverses in adolescence: By age 17, as many as 8% of boys and 23% of girls have had migraine. To diagnose migraine, Juliana H. VanderPluym, MD, associate professor of neurology at Mayo Clinic in Phoenix, said she uses the criteria published in the latest edition of the International Classification of Headache Disorders (ICHD): A patient must have had at least five attacks in their life; and in children and adolescents, the attacks must last no less than 2 hours.
In addition, the headaches should exhibit at least two out of four features:
1. Occur more on one side of the head than the other (although Dr. VanderPluym said in children and adolescents headaches often are bilateral).
2. Be of moderate to severe intensity.
3. Have a pounding or throbbing quality.
4. Grow worse with activity or cause an avoidance of activity.
If the attacks meet those criteria, clinicians should check to see if they meet at least one out of the two following:
1. Are sensitive to light and sounds.
2. Are associated with nausea and/or vomiting.
A clinician should consider whether the headaches are not better accounted for by another diagnosis, according to the ICHD criteria. But, Dr. VanderPluym warned that does not necessarily mean running a slew of tests.
“In the absence of red flag features, it is more than likely going to be migraine headache,” she said. That’s especially true if a child has a family history of migraine, as the condition is often passed down from parent to child.
Ultimately, the diagnosis is fairly simple and can be made in a minute or less, said Jack Gladstein, MD, a pediatrician at the University of Maryland whose research focuses on the clinical care of children and adolescents with headache.
“Migraine is acute,” Dr. Gladstein said. “It’s really bad. And it’s recurrent.”
First line of treatment
Whatever a patient takes to treat a migraine, they should hit it early and hard, Dr. Gladstein said.
“The first thing you say, as a primary care physician, is treat your migraine at first twinge, whatever you use. Don’t wait, don’t wish it away,” he said. “The longer you wait, the less chance anything will work.”
The second piece of advice, Dr. Gladstein said, is that whatever drug a patient is taking, they should be on the highest feasible dose. “Work as fast as you can to treat them. You want the brain to reset as quickly as you can,” he said.
Patients should begin with over-the-counter pain relievers, Dr. Little said. If those prove insufficient, they can try a triptan. Rizatriptan is the only such agent that the Food and Drug Administration has approved for children aged 6-17 years. Other drugs in the class – sumatriptan/naproxen, almotriptan, and zolmitriptan – are approved for children 12 and older.
Another migraine therapy recently approved for children aged 12 and older is the use of neurostimulators. “It’s helpful to be aware of them,” Dr. VanderPluym said.
However, if neurostimulators and acute medications prove insufficient, clinicians should warn patients not to up their doses of triptans. Rebound headaches can occur if patients take triptans more than twice a week, or a maximum 10 days per month.
Another possibility is to add a preventive therapy. One mild, first option is nutraceuticals, like riboflavin (vitamin B2) or magnesium, said Anisa F. Kelley, MD, a neurologist and associate director of the headache program at the Ann and Robert H. Lurie Children’s Hospital of Chicago.
“We don’t have definitive evidence, but they’re probably doing more benefit than they are harm,” Dr. Kelley said of these therapies. “In patients who have anywhere from 4 to 8 migraine days a month, where you’re in that in-between period where you don’t necessarily need a [prescription] prophylactic, I will often start with a nutraceutical,” Dr. Kelley said.
For those patients who don’t respond to nutraceuticals, or who need more support, clinicians can prescribe amitriptyline or topiramate. Dr. VanderPluym said.
A 2017 study found such prophylactics to be no more effective than placebo in pediatric migraine patients, but experts caution the results should not be considered definitive.
For one thing, the study enrolled a highly selective group of participants, with milder forms of migraine who may have improved anyway, Dr. VanderPluym said. All participants also received lifestyle counseling.
Every time participants came in for a follow-up, they were asked questions such as how much water were they drinking and how much sleep were they getting, Dr. Kelley noted. The takeaway, she said: “Pediatric and adolescent migraine [management] is very, very much reliant on lifestyle factors.”
Lifestyle triggers
Clinicians should counsel their migraine patients about lifestyle changes, experts said. Getting adequate sleep, staying hydrated, and managing stress can help reduce the intensity and frequency of attacks.
Migraine patients should also be mindful of their screen time, Dr. Kelley added.
“I’ve had lots and lots of patients who find excessive screen time will trigger or worsen migraine,” she said.
As for other potential triggers of attacks, the evidence is mixed.
“There’s clearly an association with disrupted sleep and migraine, and that has been very well established,” Dr. Little said. “And there is some modest amount of evidence that regular exercise can be helpful.” But for reported food triggers, he said, there have been very inconclusive results.
Commonly reported triggers include MSG, red wine, chocolate, and aged cheese. When Dr. Little’s patients keep headache diaries, tracking their meals alongside when they got migraine attacks, they often discover individualized triggers – strawberries, for instance, in one case, he said.
Scientists believe migraines result from the inappropriate activation of the trigeminal ganglion. “The question is, what causes it to get triggered? And how does it get triggered?” Dr. Gladstein said. “And that’s where there’s a lot of difference of opinion and no conclusive evidence.” Clinicians also should make sure that something else – usually depression, anxiety, insomnia, and dizziness – is not hindering effective migraine management. “If someone has terrible insomnia, until you treat the insomnia, the headaches aren’t going to get better,” he said.
As for Mira, her migraine attacks did not significantly improve, despite trying triptans, prophylactics, lifestyle changes, and shots to block nerve pain. When the headaches threatened Mira’s chance to go to her prom, her neurologist suggested trying something different. The physician persuaded the family’s insurance to cover a calcitonin gene-related peptide antagonist, an injectable monoclonal antibody treatment for migraine that the FDA has currently approved only for use in adults.
The difference for Mira has been extraordinary.
“I can do so much more than I was able to do,” said Mira, who attended the dance migraine free. “I feel liberated.”
It’s only migraine
One of the greatest challenges in diagnosing migraine can be reassuring the patient, the parents, even clinicians themselves that migraine really is the cause of all this pain and discomfort, experts said.
“A lot of migraine treatment actually comes down to migraine education,” Dr. VanderPluym said.
Patients and their parents often wonder how they can be sure that this pain is not resulting from something more dangerous than migraine, Dr. Little said. In these cases, he cites practice guidelines published by the American Academy of Neurology.
“The gist of those guidelines is that most pediatric patients do not need further workup,” he said. “But I think that there’s always a fear that you’re missing something because we don’t have a test that we can do” for migraine.
Some warning signs that further tests might be warranted, Dr. Kelley said, include:
- Headaches that wake a patient up in the middle of the night.
- Headaches that start first thing in the morning, especially those that include vomiting.
- A headache pattern that suddenly gets much worse.
- Certain symptoms that accompany the headache, such as tingling, numbness or double vision.
Although all of these signs can still stem from migraines – tingling or numbness, for instance, can be signs of migraine aura – running additional tests can rule out more serious concerns, she said.
Best practices for an LGBTQ+ friendly medical space
While rainbow-colored flags may wave proudly from hotel balconies and sports arenas, LGBTQ+ patients might still feel some discrimination in the medical space, according to a Center for American Progress survey.
“Despite health care being considered a basic human right by the World Health Organization, it’s common for LGBTQ+ folks to face difficulties not only when trying to access care but also within the walls of the doctor’s office or hospital,” says Samantha Estevez, MD, a reproductive endocrinology and infertility fellow in New York.
In Medscape’s Physicians’ Views on LGBTQ+ Rights Issues Report 2022: Strong Emotions, Contrary Opinions, physicians were asked whether they see disparities in the care LGBTQ+ patients receive in comparison with the care that non-LGBTQ+ patients receive. About 35% of physicians said LGBTQ+ patients receive a different level of care; 52% of respondents younger than 45 said so.
It’s an issue unlikely to be resolved without the medical community’s awareness. With insights from four LGBTQ+ clinicians, here are several steps physicians can take to close the disparity gap.
Update intake forms
Many patient medical forms are populated with checkboxes. These forms may make it easier for patients to share their medical information and for practices to collect data. But unfortunately, they don’t allow for patients to fill in contextual information.
“It’s extremely important for health care professionals to understand the people they are serving,” says Nicholas Grant, PhD, ABPP, president of GLMA: Health Professionals Advancing LGBTQ+ Equality. Dr. Grant is a board-certified clinical psychologist in Hawaii. “The more accurate we are with our information gathering and paperwork, the more accurate we will be at serving our LGBTQ+ communities.”
Dr. Grant recommends asking open-ended questions, such as the following:
- What is your gender identity?
- What was your assigned sex at birth?
- What pronouns do you prefer?
- What gender(s) are your sexual partners?
However, Frances Grimstad, MD, a Boston-based ob/gyn and GLMA board member, adds this advice: Before revising intake forms, consider their purpose.
“As an ob/gyn, information about a patient’s sexual orientation and their sexual activity is beneficial for my care,” says Dr. Grimstad. “But that information may not be relevant for a physical therapy clinic where most patients are coming in with knee injuries. So, you shouldn’t just place items on your intake forms by default. Instead, clinicians should consider what is relevant to the encounter you’re having and how you are going to use the information.”
Change signage
Take stock of posters and brochures in the office and signs outside restrooms. If they communicate traditional gender roles, then it may be time for a change.
“It’s important to ensure representation of all types of people and families in your office,” says Chase Anderson, MD, an assistant professor of child and adolescent psychiatry in San Francisco.
Hang posters with images of diverse families. Display brochures that address LGBTQ+ health concerns when warranted. And for restrooms, replace traditional binary images with gender-neutral ones. You can also add signage about each bathroom’s purpose, suggests Dr. Grimstad.
“Let’s not just de-gender bathrooms,” she says. “Let’s hang signs that tell if the bathroom has multiple stalls, urinals, or handicap access. Let signage focus on the functions of each bathroom, not gender.”
Ask for feedback
Feedback forms give LBGTQ+ patients a platform to share concerns. For example, consider an email with a linked document that all patients can fill out anonymously. Ask questions such as the following:
- Did you feel affirmed during your appointment? If so, how? If not, how can we improve?
- Did we use the proper pronouns?
- Did signage make you feel like you were in a safe space? What didn’t make you feel safe?
Set up a system with team members to process feedback and implement changes.
Also, if you have a large-scale practice, consider forming an LGBTQ+ community advisory board. “They can offer feedback about your practice’s clinical structure,” Dr. Grimstad tells Medscape.
Hire diverse employees
Building a diverse and inclusive workforce is critical to serving the LBGTQ+ community. Team members should reflect your patient population.
“Diversity isn’t a monolith,” says Dr. Grimstad. “It isn’t just racial diversity, or sexual or gender diversity. Even in a town which appears homogeneous in one area of diversity, such as a majority White town, it’s important to remember all the other facets of diversity that exist, such as gender, sexual orientation, cultural diversity.”
A diverse team may offer a surprising boost to your practice. According to a study published in the Journal of the National Medical Association, patient outcomes improve when a more diverse team provides care. In fact, diverse teams fare better in innovation, communication, risk assessment, and financial performance.
Dr. Anderson also recommends allowing team members “to be themselves.” For example, let employees wear their hair in whatever way they prefer or display their tattoos.
“This signals to patients that if staff members can be themselves here, patients can be themselves here, too,” says Dr. Anderson.
Provide training
Medical staff may sometimes feel uncomfortable serving LBGTQ+ patients because of their own biases, attitudes, or lack of knowledge about the community. Regular training can ease their discomfort.
“Make sure all health professionals are trained and educated on the needs of LGBTQ+ patients,” says Dr. Grant. “Understanding their health needs is the provider’s responsibility.”
For basic information, Dr. Anderson recommends visiting The Trevor Project, an organization that serves LGBTQ+ youth. “They’re really good at keeping up with changing verbiage and trends,” says Dr. Anderson.
To strengthen community connections, Dr. Grimstad recommends using trainers from your local area if possible. Do a Google search to find an LGBTQ+ center nearby or in the closest major city. Invite them to staff meetings or ask them to organize a workshop.
By implementing these strategies, you can start building a bridge between your practice and the LGBTQ+ community and provide better care for them as patients.
“Whether it’s knowing about PrEP ... or ensuring staff members are trained in caring for patients with any general or sexual identity, we as doctors and medical professionals must continue to move forward and serve our LGBTQ+ patients in big and small ways,” says Dr. Estevez.
For in-depth training, check the following organizations:
National LGBTQIA+ Health Education Center at the Fenway Institute provides educational programs and resources to health care organizations.
GLMA has a top 10 health issues webpage that doctors can use to educate themselves and staff members on the LGBTQ+ community’s most urgent health needs.
Alliance for Full Acceptance offers LGBTQ cultural competency training, including a 1-hour awareness class and a 3-hour inclusivity workshop for clinicians.
The Substance Abuse and Mental Health Services Administration has compiled a list of training curricula for behavioral health counselors and primary care providers.
UCSF’s Lesbian, Gay, Bisexual, and Transgender Resource Center has a list of training and educational materials for medical professionals.
Equality California Institute offers both in-person and virtual training covering basic terminology, data on LGBTQ+ health issues, and how to create an inclusive environment.
A version of this article first appeared on Medscape.com.
While rainbow-colored flags may wave proudly from hotel balconies and sports arenas, LGBTQ+ patients might still feel some discrimination in the medical space, according to a Center for American Progress survey.
“Despite health care being considered a basic human right by the World Health Organization, it’s common for LGBTQ+ folks to face difficulties not only when trying to access care but also within the walls of the doctor’s office or hospital,” says Samantha Estevez, MD, a reproductive endocrinology and infertility fellow in New York.
In Medscape’s Physicians’ Views on LGBTQ+ Rights Issues Report 2022: Strong Emotions, Contrary Opinions, physicians were asked whether they see disparities in the care LGBTQ+ patients receive in comparison with the care that non-LGBTQ+ patients receive. About 35% of physicians said LGBTQ+ patients receive a different level of care; 52% of respondents younger than 45 said so.
It’s an issue unlikely to be resolved without the medical community’s awareness. With insights from four LGBTQ+ clinicians, here are several steps physicians can take to close the disparity gap.
Update intake forms
Many patient medical forms are populated with checkboxes. These forms may make it easier for patients to share their medical information and for practices to collect data. But unfortunately, they don’t allow for patients to fill in contextual information.
“It’s extremely important for health care professionals to understand the people they are serving,” says Nicholas Grant, PhD, ABPP, president of GLMA: Health Professionals Advancing LGBTQ+ Equality. Dr. Grant is a board-certified clinical psychologist in Hawaii. “The more accurate we are with our information gathering and paperwork, the more accurate we will be at serving our LGBTQ+ communities.”
Dr. Grant recommends asking open-ended questions, such as the following:
- What is your gender identity?
- What was your assigned sex at birth?
- What pronouns do you prefer?
- What gender(s) are your sexual partners?
However, Frances Grimstad, MD, a Boston-based ob/gyn and GLMA board member, adds this advice: Before revising intake forms, consider their purpose.
“As an ob/gyn, information about a patient’s sexual orientation and their sexual activity is beneficial for my care,” says Dr. Grimstad. “But that information may not be relevant for a physical therapy clinic where most patients are coming in with knee injuries. So, you shouldn’t just place items on your intake forms by default. Instead, clinicians should consider what is relevant to the encounter you’re having and how you are going to use the information.”
Change signage
Take stock of posters and brochures in the office and signs outside restrooms. If they communicate traditional gender roles, then it may be time for a change.
“It’s important to ensure representation of all types of people and families in your office,” says Chase Anderson, MD, an assistant professor of child and adolescent psychiatry in San Francisco.
Hang posters with images of diverse families. Display brochures that address LGBTQ+ health concerns when warranted. And for restrooms, replace traditional binary images with gender-neutral ones. You can also add signage about each bathroom’s purpose, suggests Dr. Grimstad.
“Let’s not just de-gender bathrooms,” she says. “Let’s hang signs that tell if the bathroom has multiple stalls, urinals, or handicap access. Let signage focus on the functions of each bathroom, not gender.”
Ask for feedback
Feedback forms give LBGTQ+ patients a platform to share concerns. For example, consider an email with a linked document that all patients can fill out anonymously. Ask questions such as the following:
- Did you feel affirmed during your appointment? If so, how? If not, how can we improve?
- Did we use the proper pronouns?
- Did signage make you feel like you were in a safe space? What didn’t make you feel safe?
Set up a system with team members to process feedback and implement changes.
Also, if you have a large-scale practice, consider forming an LGBTQ+ community advisory board. “They can offer feedback about your practice’s clinical structure,” Dr. Grimstad tells Medscape.
Hire diverse employees
Building a diverse and inclusive workforce is critical to serving the LBGTQ+ community. Team members should reflect your patient population.
“Diversity isn’t a monolith,” says Dr. Grimstad. “It isn’t just racial diversity, or sexual or gender diversity. Even in a town which appears homogeneous in one area of diversity, such as a majority White town, it’s important to remember all the other facets of diversity that exist, such as gender, sexual orientation, cultural diversity.”
A diverse team may offer a surprising boost to your practice. According to a study published in the Journal of the National Medical Association, patient outcomes improve when a more diverse team provides care. In fact, diverse teams fare better in innovation, communication, risk assessment, and financial performance.
Dr. Anderson also recommends allowing team members “to be themselves.” For example, let employees wear their hair in whatever way they prefer or display their tattoos.
“This signals to patients that if staff members can be themselves here, patients can be themselves here, too,” says Dr. Anderson.
Provide training
Medical staff may sometimes feel uncomfortable serving LBGTQ+ patients because of their own biases, attitudes, or lack of knowledge about the community. Regular training can ease their discomfort.
“Make sure all health professionals are trained and educated on the needs of LGBTQ+ patients,” says Dr. Grant. “Understanding their health needs is the provider’s responsibility.”
For basic information, Dr. Anderson recommends visiting The Trevor Project, an organization that serves LGBTQ+ youth. “They’re really good at keeping up with changing verbiage and trends,” says Dr. Anderson.
To strengthen community connections, Dr. Grimstad recommends using trainers from your local area if possible. Do a Google search to find an LGBTQ+ center nearby or in the closest major city. Invite them to staff meetings or ask them to organize a workshop.
By implementing these strategies, you can start building a bridge between your practice and the LGBTQ+ community and provide better care for them as patients.
“Whether it’s knowing about PrEP ... or ensuring staff members are trained in caring for patients with any general or sexual identity, we as doctors and medical professionals must continue to move forward and serve our LGBTQ+ patients in big and small ways,” says Dr. Estevez.
For in-depth training, check the following organizations:
National LGBTQIA+ Health Education Center at the Fenway Institute provides educational programs and resources to health care organizations.
GLMA has a top 10 health issues webpage that doctors can use to educate themselves and staff members on the LGBTQ+ community’s most urgent health needs.
Alliance for Full Acceptance offers LGBTQ cultural competency training, including a 1-hour awareness class and a 3-hour inclusivity workshop for clinicians.
The Substance Abuse and Mental Health Services Administration has compiled a list of training curricula for behavioral health counselors and primary care providers.
UCSF’s Lesbian, Gay, Bisexual, and Transgender Resource Center has a list of training and educational materials for medical professionals.
Equality California Institute offers both in-person and virtual training covering basic terminology, data on LGBTQ+ health issues, and how to create an inclusive environment.
A version of this article first appeared on Medscape.com.
While rainbow-colored flags may wave proudly from hotel balconies and sports arenas, LGBTQ+ patients might still feel some discrimination in the medical space, according to a Center for American Progress survey.
“Despite health care being considered a basic human right by the World Health Organization, it’s common for LGBTQ+ folks to face difficulties not only when trying to access care but also within the walls of the doctor’s office or hospital,” says Samantha Estevez, MD, a reproductive endocrinology and infertility fellow in New York.
In Medscape’s Physicians’ Views on LGBTQ+ Rights Issues Report 2022: Strong Emotions, Contrary Opinions, physicians were asked whether they see disparities in the care LGBTQ+ patients receive in comparison with the care that non-LGBTQ+ patients receive. About 35% of physicians said LGBTQ+ patients receive a different level of care; 52% of respondents younger than 45 said so.
It’s an issue unlikely to be resolved without the medical community’s awareness. With insights from four LGBTQ+ clinicians, here are several steps physicians can take to close the disparity gap.
Update intake forms
Many patient medical forms are populated with checkboxes. These forms may make it easier for patients to share their medical information and for practices to collect data. But unfortunately, they don’t allow for patients to fill in contextual information.
“It’s extremely important for health care professionals to understand the people they are serving,” says Nicholas Grant, PhD, ABPP, president of GLMA: Health Professionals Advancing LGBTQ+ Equality. Dr. Grant is a board-certified clinical psychologist in Hawaii. “The more accurate we are with our information gathering and paperwork, the more accurate we will be at serving our LGBTQ+ communities.”
Dr. Grant recommends asking open-ended questions, such as the following:
- What is your gender identity?
- What was your assigned sex at birth?
- What pronouns do you prefer?
- What gender(s) are your sexual partners?
However, Frances Grimstad, MD, a Boston-based ob/gyn and GLMA board member, adds this advice: Before revising intake forms, consider their purpose.
“As an ob/gyn, information about a patient’s sexual orientation and their sexual activity is beneficial for my care,” says Dr. Grimstad. “But that information may not be relevant for a physical therapy clinic where most patients are coming in with knee injuries. So, you shouldn’t just place items on your intake forms by default. Instead, clinicians should consider what is relevant to the encounter you’re having and how you are going to use the information.”
Change signage
Take stock of posters and brochures in the office and signs outside restrooms. If they communicate traditional gender roles, then it may be time for a change.
“It’s important to ensure representation of all types of people and families in your office,” says Chase Anderson, MD, an assistant professor of child and adolescent psychiatry in San Francisco.
Hang posters with images of diverse families. Display brochures that address LGBTQ+ health concerns when warranted. And for restrooms, replace traditional binary images with gender-neutral ones. You can also add signage about each bathroom’s purpose, suggests Dr. Grimstad.
“Let’s not just de-gender bathrooms,” she says. “Let’s hang signs that tell if the bathroom has multiple stalls, urinals, or handicap access. Let signage focus on the functions of each bathroom, not gender.”
Ask for feedback
Feedback forms give LBGTQ+ patients a platform to share concerns. For example, consider an email with a linked document that all patients can fill out anonymously. Ask questions such as the following:
- Did you feel affirmed during your appointment? If so, how? If not, how can we improve?
- Did we use the proper pronouns?
- Did signage make you feel like you were in a safe space? What didn’t make you feel safe?
Set up a system with team members to process feedback and implement changes.
Also, if you have a large-scale practice, consider forming an LGBTQ+ community advisory board. “They can offer feedback about your practice’s clinical structure,” Dr. Grimstad tells Medscape.
Hire diverse employees
Building a diverse and inclusive workforce is critical to serving the LBGTQ+ community. Team members should reflect your patient population.
“Diversity isn’t a monolith,” says Dr. Grimstad. “It isn’t just racial diversity, or sexual or gender diversity. Even in a town which appears homogeneous in one area of diversity, such as a majority White town, it’s important to remember all the other facets of diversity that exist, such as gender, sexual orientation, cultural diversity.”
A diverse team may offer a surprising boost to your practice. According to a study published in the Journal of the National Medical Association, patient outcomes improve when a more diverse team provides care. In fact, diverse teams fare better in innovation, communication, risk assessment, and financial performance.
Dr. Anderson also recommends allowing team members “to be themselves.” For example, let employees wear their hair in whatever way they prefer or display their tattoos.
“This signals to patients that if staff members can be themselves here, patients can be themselves here, too,” says Dr. Anderson.
Provide training
Medical staff may sometimes feel uncomfortable serving LBGTQ+ patients because of their own biases, attitudes, or lack of knowledge about the community. Regular training can ease their discomfort.
“Make sure all health professionals are trained and educated on the needs of LGBTQ+ patients,” says Dr. Grant. “Understanding their health needs is the provider’s responsibility.”
For basic information, Dr. Anderson recommends visiting The Trevor Project, an organization that serves LGBTQ+ youth. “They’re really good at keeping up with changing verbiage and trends,” says Dr. Anderson.
To strengthen community connections, Dr. Grimstad recommends using trainers from your local area if possible. Do a Google search to find an LGBTQ+ center nearby or in the closest major city. Invite them to staff meetings or ask them to organize a workshop.
By implementing these strategies, you can start building a bridge between your practice and the LGBTQ+ community and provide better care for them as patients.
“Whether it’s knowing about PrEP ... or ensuring staff members are trained in caring for patients with any general or sexual identity, we as doctors and medical professionals must continue to move forward and serve our LGBTQ+ patients in big and small ways,” says Dr. Estevez.
For in-depth training, check the following organizations:
National LGBTQIA+ Health Education Center at the Fenway Institute provides educational programs and resources to health care organizations.
GLMA has a top 10 health issues webpage that doctors can use to educate themselves and staff members on the LGBTQ+ community’s most urgent health needs.
Alliance for Full Acceptance offers LGBTQ cultural competency training, including a 1-hour awareness class and a 3-hour inclusivity workshop for clinicians.
The Substance Abuse and Mental Health Services Administration has compiled a list of training curricula for behavioral health counselors and primary care providers.
UCSF’s Lesbian, Gay, Bisexual, and Transgender Resource Center has a list of training and educational materials for medical professionals.
Equality California Institute offers both in-person and virtual training covering basic terminology, data on LGBTQ+ health issues, and how to create an inclusive environment.
A version of this article first appeared on Medscape.com.
Audit and Feedback: A Quality Improvement Study to Improve Antimicrobial Stewardship
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
High Rate of Inappropriate Fecal Immunochemical Testing at a Large Veterans Affairs Health Care System
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
Veteran and Provider Perspectives on Telehealth for Vocational Rehabilitation Services
Vocational rehabilitation (VR) interventions are offered through Compensated Work Therapy (CWT) as part of clinical care in the Veterans Health Administration (VHA) to improve employment and quality of life outcomes for veterans with life-altering disabilities.1–5 CWT vocational services range from assessment, vocational counseling, and treatment plan development to job placement, coaching, and follow-along support.1 However, many veterans receive care in community-based clinics that are not staffed with a VR specialist (VRS) to provide these services.6–8 Telehealth may increase patient access to VR, especially for rural veterans and those with travel barriers, but it is not known whether veterans and VRS would find this to be a satisfactory service delivery method.8,9 This paper examines veteran and VRS provider perspectives on VR provided by telehealth (VRtele) as part of a VHA clinical demonstration project. To our knowledge, this is the first report of using real-time, clinic-based VRtele.
Methods
The Rural Veterans Supported Employment Telerehabilitation Initiative (RVSETI) was conducted as a field-initiated demonstration project at 2 US Department of Veterans Affairs (VA) medical centers (VAMCs) in Florida between 2014 and 2016: James A. Haley Veterans’ Hospital & Clinics (Tampa) and Malcom Randall VAMC (Gainesville). This retrospective evaluation of its first year did not require institutional review board approval as it was determined to be a quality improvement project by the local research service.
The patient population for the project was veterans with disabilities who were referred by clinical consults to the CWT service, a recovery-oriented vocational program. During the project years, veterans were offered the option of receiving VR services, such as supported employment, community-based employment services, or vocational assistance, through VRtele rather than traditional face-to-face meetings. The specific interventions delivered included patient orientation, interview assessment, treatment plan development, referral activities, vocational counseling, assessment of workplace for accommodation needs, vocational case management, and other employment supports. VR staff participating in the project included 2 VR supervisors, 1 supported employment mentor trainer, and 5 VRSs.
Each clinic was set up for VRtele, and codes were added to the electronic health record (EHR) to ensure proper documentation. Participating VRSs completed teleconferencing training, including a skills assessment using the equipment for real-time, high-quality video streaming over an encrypted network to provide services in a patient’s home or other remote locations. VRS staff provided veterans with instructions on using a VA-provided tablet or their own device and assisted them with establishing connectivity with the network. Video equipment included speakers, camera, and headphones connected to the desktop computer or laptop of the VRS. A patient’s first VRtele
Demographic data, primary diagnosis, VR usage data, and zip codes of participating veterans were extracted from the EHR. Veterans completed a 2-part satisfaction survey administered 90 days after enrollment and at discharge. Part 1 was composed of 15 items, most with a 5-point Likert scale (higher ratings indicated greater satisfaction), on various aspects of the VRtele experience, such as audio and video quality and wait times.10 Part 2 addressed VR services and the VRS and consisted of 8 Likert scale items with the option to add a comment for each and 2 open-ended items that asked the participant to list what they liked best and least about VRtele.
Semistructured, in-person 30- to 60-minute interviews were conducted with VRSs at the initiation of VRtele
After ≥ 2 months of VRtele use
Analyses
Descriptive statistics were used for EHR data and satisfaction surveys. For qualitative analysis, each transcript was read in full by 2 researchers to get an overview of the data, and a rapid analysis approach was used to identify central themes focused on how technology was used and the experiences of the participants.11,12 Relevant text for each topic was tabulated, and a summary table was created that highlighted overlapping ideas discussed by the interviewees as well as differences.
Results
Of the 22 veterans who participated in the project, 11 completed satisfaction surveys and 4 participated in qualitative interviews. The rural and nonrural groups did not differ demographically or by diagnosis, which was predominantly mental health related. Only 1 veteran in each group owned a tablet; the majority of both groups required VA-issued devices: 80% (n = 8) rural and 91.7% (n = 11) nonrural. The number of VRtele sessions for the groups also was similar, 53 for rural and 60 for nonrural, as was the mean (SD) number of sessions per veteran: 5.3 (SD, 3.2) rural and 5.0 (SD, 2.5) urban. Overall, 63 miles per session were saved, mostly for rural veterans, and the number of mean (SD) miles saved per veteran was greater for rural than nonrural veterans: 379.2 (243.0) and 256.1 (275.9), respectively. One veteran who moved to a different state during the program continued VRtele at the new location. In a qualitative sampling of 5 VRtele sessions, all the VRSs used office desktop computers.
Level of satisfaction with aspects of VRtele related to the technology rated was consistently > 4 on the Likert scale. The lowest mean (SD) ratings were 4.2 (1.0) for audio quality and 4.4 (0.5) for video quality, and the highest rating was given for equipment operation explanation and privacy was respected, 4.9 (0.3) for both. All questions related to satisfaction with services were also rated high: The mean (SD) lowest ratings were 4.3 (1.0) given to both vocational needs 4.3 (1.0) and tasks effectively helped achieve goals 4.3 (0.7). The highest mean (SD) ratings were 4.6 (0.5) given to VR program service explained and 4.7 (0.5) for appointment timeliness.
Qualitative Results
At first, some VRSs thought the teleconferencing system might be difficult or awkward to use, but they found it easier to set up than expected and seamless to use. VRS staff reported being surprised at how well it worked despite some issues that occurred with loading the software. Once loaded, however, the connection worked well, one VRS noting that following step-by-step instructions solved the problem. Some VRSs indicated they did not invite all the veterans on their caseload to participate in VRtele due to concerns with the patient’s familiarity with technology, but one VRS stated, “I haven’t had anybody that failed to do a [session] that I couldn’t get them up and running within a few minutes.”
When working in the community, VRSs reported using laptops for VRtele but found that these devices were unreliable due to lack of internet access and were slow to start; several VRSs thought tablets would have been more helpful. Some veterans reported technical glitches, lack of comfort with technology, or a problem with sound due to a tablet’s protective case blocking the speakers. To solve the sound issue, a veteran used headphones. This veteran also explained that the log-on process required a new password every time, so he would keep a pen and paper ready to write it down. Because signing in and setting up takes a little time, this veteran and his VRS agreed to start connecting 5 minutes before their meeting time to allow for that set- up time.
Initially, some VRSs expressed concern that transitioning to VRtele would affect the quality of interactions with the veterans. However, VRSs also identified strengths of VRtele, including flexibility, saved time, and increased interaction. One VRS discussed a veteran’s adaptation by saying, “I think he feels even more involved in his plan [and] enjoys the increased interaction.” Veterans reported enjoying using tablets and identified the main strength of VRtele as being able to talk face-to-face with the VRS. Echoing the VRSs, veterans reported teleconferencing saved time by avoiding travel and enabled spontaneous meetings. One of the veterans summed up the benefits of using VRtele: “I’d rather just connect. It’s going to take us 40 to 50 minutes [to meet in person] when we can just connect right here and it takes 15 to 20. We don’t have to go through the driving.… So this right here, doing it ahead of time and having the appointment, it’s a lot easier.”
In their interviews, VRSs talked about enjoying VRtele. A VRS explained: “It makes it a lot easier. It makes me feel less guilty. This way [veterans] don’t have to use their gas money, use their time. I know [the veteran] had something else he needed to do today.” Thus, both veterans and VRSs were satisfied with their VRtele experiences.
Discussion
This first report on the perspective of providers and veterans using VRtele suggests that it is a viable option for service delivery and that is highly satisfactory for serving veterans with disabilities, many of whom live in rural areas or have travel barriers. These findings are consistent with data on telerehabilitation for veterans with cognitive, physical, and mental disabilities.13-22 Further, the data support the notion of using VRtele to facilitate long-term VR follow-up for persons with disabilities, as illustrated by successful continuation of vocational services after a veteran moved out of state.23
Similar to other reports, our experience highlighted 2 factors that affect successful VRtele: (1) Troubleshooting technology barriers for both VR providers and clients; and (2) supportive leadership to facilitate implementation
Changes to technology and increased usage of VA Video Connect may indicate that the barriers identified from the earlier process described here have been diminished or eliminated. More evaluation is needed to assess whether system upgrades have increased ease of use and access for veterans with disabilities.
Conclusions
Encouragingly, this clinical demonstration project showed that both providers and clients recognize the benefits of VRtele. Patient satisfaction and decreased travel costs were clear advantages to using VRtele for this small group of veterans who had barriers to care due to travel or disability barriers. As this program evaluation was limited by a small sample, absence of a comparison group, and lack of outcome data (eg, employment rates, hours, wages, retention), future research is needed on implementation and outcomes of VRtele
Acknowledgments
The authors thank Lynn Dirk, MAMC, for substantial editorial assistance. This material was based on work supported by Rural Veterans Supported Employment TeleRehabilitation Initiative (RVSETI), funded by the VA Office of Rural Health (Project # N08-FY14Q3-S2-P01222) and by support of the VA Health Services Research and Development Service. This work was presented in part at the 114th Annual Meeting of the American Anthropological Association at Denver, Colorado, November 21, 2015; a field-based Health Services Research and Development Service meeting, US Department of Veterans Affairs at Washington, DC, September 12, 2016; and the 2016 Annual Conference of the American Congress for Rehabilitation Medicine at Chicago, Illinois, October-November 2016.
1. Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA therapeutic and supported employment services programs. Psychiatr Serv. 2017;68(9):938-946. doi:10.1176/appi.ps.201600412
2. Davis LL, Kyriakides TC, Suris AM, et al. Effect of evidence-based supported employment vs transitional work on achieving steady work among veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2018;75(4):316. doi:10.1001/jamapsychiatry.2017.4472
3. Ottomanelli L, Goetz LL, Suris A, et al. Effectiveness of supported employment for veterans with spinal cord injuries: results from a randomized multisite study. Arch Phys Med Rehabil. 2012;93(5):740-747. doi:10.1016/j.apmr.2012.01.002
4. Ottomanelli L, Goetz LL, Barnett SD, et al. Individual placement and support in spinal cord injury: a longitudinal observational study of employment outcomes. Arch Phys Med Rehabil. 2017;98(8):1567-1575. doi:10.1016/j.apmr.2016.12.010
5. Cotner BA, Ottomanelli L, O’Connor DR, Njoh EN, Barnett SD, Miech EJ. Quality of life outcomes for veterans with spinal cord injury receiving individual placement and support (IPS). Top Spinal Cord Inj Rehabil. 2018;24(4):325-335. doi:10.1310/sci17-00046
6. Metzel DS, Giordano A. Locations of employment services and people with disabilities: a geographical analysis of accessibility. J Disabil Policy Stud. 2007;18(2):88-97. doi:10.1177/10442073070180020501
7. Landon T, Connor A, McKnight-Lizotte M, Peña J. Rehabilitation counseling in rural settings: a phenomenological study on barriers and supports. J Rehabil. 2019;85(2):47-57.
8. Riemer-Reiss M. Vocational rehabilitation counseling at a distance: Challenges, strategies and ethics to consider. J Rehabil. 2000;66(1):11-17.
9. Schmeler MR, Schein RM, McCue M, Betz K. Telerehabilitation clinical and vocational applications for assistive technology: research, opportunities, and challenges. Int J Telerehabilitation. 2009;1(1):59-72.
10. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370. doi:10.1682/JRRD.2014.10.0239
11. McMullen CK, Ash JS, Sittig DF, et al. Rapid assessment of clinical information systems in the healthcare setting: an efficient method for time-pressed evaluation. Methods Inf Med. 2011;50(4):299-307. doi:10.3414/ME10-01-0042
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
13. Egede LE, Acierno R, Knapp RG, et al. Psychotherapy for depression in older veterans via telemedicine: a randomised, open-label, non-inferiority trial. Lancet Psychiatry. 2015;2(8):693-701. doi:10.1016/S2215-0366(15)00122-4
14. Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22(8):1086-1093. doi:10.1007/s11606-007-0201-9
15. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58. doi:10.1001/jamapsychiatry.2014.1575
16. Grubbs KM, Fortney JC, Dean T, Williams JS, Godleski L. A comparison of mental health diagnoses treated via interactive video and face to face in the Veterans Healthcare Administration. Telemed E-Health. 2015;21(7):564-566. doi:10.1089/tmj.2014.0152
17. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213. doi:10.1177/1357633X15572201
18. Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed E-Health. 2010;16(4):417-423. doi:10.1089/tmj.2009.0118
19. Brennan DM, Georgeadis AC, Baron CR, Barker LM. The effect of videoconference-based telerehabilitation on story retelling performance by brain-injured subjects and its implications for remote speech-language therapy. Telemed J E Health. 2004;10(2):147-154. doi:10.1089/tmj.2004.10.147
20. Dallolio L, Menarini M, China S, et al. Functional and clinical outcomes of telemedicine in patients with spinal cord injury. Arch Phys Med Rehabil. 2008;89(12):2332-2341. doi:10.1016/j.apmr.2008.06.012
21. Houlihan BV, Jette A, Friedman RH, et al. A pilot study of a telehealth intervention for persons with spinal cord dysfunction. Spinal Cord. 2013;51(9):715-720.doi:10.1038/sc.2013.45
22. Smith MW, Hill ML, Hopkins KL, Kiratli BJ, Cronkite RC. A modeled analysis of telehealth methods for treating pressure ulcers after spinal cord injury. Int J Telemed Appl. 2012;2012:1-10. doi:10.1155/2012/729492
23. Balcazar FE, Keys CB, Davis M, Lardon C, Jones C. Strengths and challenges of intervention research in vocational rehabilitation: an illustration of agency-university collaboration. J Rehabil. 2005;71(2):40-48.
24. Martinez RN, Hogan TP, Balbale S, et al. Sociotechnical perspective on implementing clinical video telehealth for veterans with spinal cord injuries and disorders. Telemed J E Health. 2017;23(7):567-576. doi:10.1089/tmj.2016.0200
25. Martinez RN, Hogan TP, Lones K, et al. Evaluation and treatment of mild traumatic brain injury through the implementation of clinical video telehealth: provider perspectives from the Veterans Health Administration. PM R. 2017;9(3):231-240. doi:10.1016/j.pmrj.2016.07.002
26. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26(5):309-313. doi:10.1177/1357633X20916567
27. Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019; 36(3):122-128.
28. US Department of Veterans Affairs. Veterans VA Video Connect. Published May 22, 2020. Accessed May 29, 2020. https://mobile.va.gov/app/va-video-connect#AppDescription.
29. US Department of Veterans Affairs. VA telehealth at home. Accessed May 29, 2020. https://telehealth.va.gov/type/home
Vocational rehabilitation (VR) interventions are offered through Compensated Work Therapy (CWT) as part of clinical care in the Veterans Health Administration (VHA) to improve employment and quality of life outcomes for veterans with life-altering disabilities.1–5 CWT vocational services range from assessment, vocational counseling, and treatment plan development to job placement, coaching, and follow-along support.1 However, many veterans receive care in community-based clinics that are not staffed with a VR specialist (VRS) to provide these services.6–8 Telehealth may increase patient access to VR, especially for rural veterans and those with travel barriers, but it is not known whether veterans and VRS would find this to be a satisfactory service delivery method.8,9 This paper examines veteran and VRS provider perspectives on VR provided by telehealth (VRtele) as part of a VHA clinical demonstration project. To our knowledge, this is the first report of using real-time, clinic-based VRtele.
Methods
The Rural Veterans Supported Employment Telerehabilitation Initiative (RVSETI) was conducted as a field-initiated demonstration project at 2 US Department of Veterans Affairs (VA) medical centers (VAMCs) in Florida between 2014 and 2016: James A. Haley Veterans’ Hospital & Clinics (Tampa) and Malcom Randall VAMC (Gainesville). This retrospective evaluation of its first year did not require institutional review board approval as it was determined to be a quality improvement project by the local research service.
The patient population for the project was veterans with disabilities who were referred by clinical consults to the CWT service, a recovery-oriented vocational program. During the project years, veterans were offered the option of receiving VR services, such as supported employment, community-based employment services, or vocational assistance, through VRtele rather than traditional face-to-face meetings. The specific interventions delivered included patient orientation, interview assessment, treatment plan development, referral activities, vocational counseling, assessment of workplace for accommodation needs, vocational case management, and other employment supports. VR staff participating in the project included 2 VR supervisors, 1 supported employment mentor trainer, and 5 VRSs.
Each clinic was set up for VRtele, and codes were added to the electronic health record (EHR) to ensure proper documentation. Participating VRSs completed teleconferencing training, including a skills assessment using the equipment for real-time, high-quality video streaming over an encrypted network to provide services in a patient’s home or other remote locations. VRS staff provided veterans with instructions on using a VA-provided tablet or their own device and assisted them with establishing connectivity with the network. Video equipment included speakers, camera, and headphones connected to the desktop computer or laptop of the VRS. A patient’s first VRtele
Demographic data, primary diagnosis, VR usage data, and zip codes of participating veterans were extracted from the EHR. Veterans completed a 2-part satisfaction survey administered 90 days after enrollment and at discharge. Part 1 was composed of 15 items, most with a 5-point Likert scale (higher ratings indicated greater satisfaction), on various aspects of the VRtele experience, such as audio and video quality and wait times.10 Part 2 addressed VR services and the VRS and consisted of 8 Likert scale items with the option to add a comment for each and 2 open-ended items that asked the participant to list what they liked best and least about VRtele.
Semistructured, in-person 30- to 60-minute interviews were conducted with VRSs at the initiation of VRtele
After ≥ 2 months of VRtele use
Analyses
Descriptive statistics were used for EHR data and satisfaction surveys. For qualitative analysis, each transcript was read in full by 2 researchers to get an overview of the data, and a rapid analysis approach was used to identify central themes focused on how technology was used and the experiences of the participants.11,12 Relevant text for each topic was tabulated, and a summary table was created that highlighted overlapping ideas discussed by the interviewees as well as differences.
Results
Of the 22 veterans who participated in the project, 11 completed satisfaction surveys and 4 participated in qualitative interviews. The rural and nonrural groups did not differ demographically or by diagnosis, which was predominantly mental health related. Only 1 veteran in each group owned a tablet; the majority of both groups required VA-issued devices: 80% (n = 8) rural and 91.7% (n = 11) nonrural. The number of VRtele sessions for the groups also was similar, 53 for rural and 60 for nonrural, as was the mean (SD) number of sessions per veteran: 5.3 (SD, 3.2) rural and 5.0 (SD, 2.5) urban. Overall, 63 miles per session were saved, mostly for rural veterans, and the number of mean (SD) miles saved per veteran was greater for rural than nonrural veterans: 379.2 (243.0) and 256.1 (275.9), respectively. One veteran who moved to a different state during the program continued VRtele at the new location. In a qualitative sampling of 5 VRtele sessions, all the VRSs used office desktop computers.
Level of satisfaction with aspects of VRtele related to the technology rated was consistently > 4 on the Likert scale. The lowest mean (SD) ratings were 4.2 (1.0) for audio quality and 4.4 (0.5) for video quality, and the highest rating was given for equipment operation explanation and privacy was respected, 4.9 (0.3) for both. All questions related to satisfaction with services were also rated high: The mean (SD) lowest ratings were 4.3 (1.0) given to both vocational needs 4.3 (1.0) and tasks effectively helped achieve goals 4.3 (0.7). The highest mean (SD) ratings were 4.6 (0.5) given to VR program service explained and 4.7 (0.5) for appointment timeliness.
Qualitative Results
At first, some VRSs thought the teleconferencing system might be difficult or awkward to use, but they found it easier to set up than expected and seamless to use. VRS staff reported being surprised at how well it worked despite some issues that occurred with loading the software. Once loaded, however, the connection worked well, one VRS noting that following step-by-step instructions solved the problem. Some VRSs indicated they did not invite all the veterans on their caseload to participate in VRtele due to concerns with the patient’s familiarity with technology, but one VRS stated, “I haven’t had anybody that failed to do a [session] that I couldn’t get them up and running within a few minutes.”
When working in the community, VRSs reported using laptops for VRtele but found that these devices were unreliable due to lack of internet access and were slow to start; several VRSs thought tablets would have been more helpful. Some veterans reported technical glitches, lack of comfort with technology, or a problem with sound due to a tablet’s protective case blocking the speakers. To solve the sound issue, a veteran used headphones. This veteran also explained that the log-on process required a new password every time, so he would keep a pen and paper ready to write it down. Because signing in and setting up takes a little time, this veteran and his VRS agreed to start connecting 5 minutes before their meeting time to allow for that set- up time.
Initially, some VRSs expressed concern that transitioning to VRtele would affect the quality of interactions with the veterans. However, VRSs also identified strengths of VRtele, including flexibility, saved time, and increased interaction. One VRS discussed a veteran’s adaptation by saying, “I think he feels even more involved in his plan [and] enjoys the increased interaction.” Veterans reported enjoying using tablets and identified the main strength of VRtele as being able to talk face-to-face with the VRS. Echoing the VRSs, veterans reported teleconferencing saved time by avoiding travel and enabled spontaneous meetings. One of the veterans summed up the benefits of using VRtele: “I’d rather just connect. It’s going to take us 40 to 50 minutes [to meet in person] when we can just connect right here and it takes 15 to 20. We don’t have to go through the driving.… So this right here, doing it ahead of time and having the appointment, it’s a lot easier.”
In their interviews, VRSs talked about enjoying VRtele. A VRS explained: “It makes it a lot easier. It makes me feel less guilty. This way [veterans] don’t have to use their gas money, use their time. I know [the veteran] had something else he needed to do today.” Thus, both veterans and VRSs were satisfied with their VRtele experiences.
Discussion
This first report on the perspective of providers and veterans using VRtele suggests that it is a viable option for service delivery and that is highly satisfactory for serving veterans with disabilities, many of whom live in rural areas or have travel barriers. These findings are consistent with data on telerehabilitation for veterans with cognitive, physical, and mental disabilities.13-22 Further, the data support the notion of using VRtele to facilitate long-term VR follow-up for persons with disabilities, as illustrated by successful continuation of vocational services after a veteran moved out of state.23
Similar to other reports, our experience highlighted 2 factors that affect successful VRtele: (1) Troubleshooting technology barriers for both VR providers and clients; and (2) supportive leadership to facilitate implementation
Changes to technology and increased usage of VA Video Connect may indicate that the barriers identified from the earlier process described here have been diminished or eliminated. More evaluation is needed to assess whether system upgrades have increased ease of use and access for veterans with disabilities.
Conclusions
Encouragingly, this clinical demonstration project showed that both providers and clients recognize the benefits of VRtele. Patient satisfaction and decreased travel costs were clear advantages to using VRtele for this small group of veterans who had barriers to care due to travel or disability barriers. As this program evaluation was limited by a small sample, absence of a comparison group, and lack of outcome data (eg, employment rates, hours, wages, retention), future research is needed on implementation and outcomes of VRtele
Acknowledgments
The authors thank Lynn Dirk, MAMC, for substantial editorial assistance. This material was based on work supported by Rural Veterans Supported Employment TeleRehabilitation Initiative (RVSETI), funded by the VA Office of Rural Health (Project # N08-FY14Q3-S2-P01222) and by support of the VA Health Services Research and Development Service. This work was presented in part at the 114th Annual Meeting of the American Anthropological Association at Denver, Colorado, November 21, 2015; a field-based Health Services Research and Development Service meeting, US Department of Veterans Affairs at Washington, DC, September 12, 2016; and the 2016 Annual Conference of the American Congress for Rehabilitation Medicine at Chicago, Illinois, October-November 2016.
Vocational rehabilitation (VR) interventions are offered through Compensated Work Therapy (CWT) as part of clinical care in the Veterans Health Administration (VHA) to improve employment and quality of life outcomes for veterans with life-altering disabilities.1–5 CWT vocational services range from assessment, vocational counseling, and treatment plan development to job placement, coaching, and follow-along support.1 However, many veterans receive care in community-based clinics that are not staffed with a VR specialist (VRS) to provide these services.6–8 Telehealth may increase patient access to VR, especially for rural veterans and those with travel barriers, but it is not known whether veterans and VRS would find this to be a satisfactory service delivery method.8,9 This paper examines veteran and VRS provider perspectives on VR provided by telehealth (VRtele) as part of a VHA clinical demonstration project. To our knowledge, this is the first report of using real-time, clinic-based VRtele.
Methods
The Rural Veterans Supported Employment Telerehabilitation Initiative (RVSETI) was conducted as a field-initiated demonstration project at 2 US Department of Veterans Affairs (VA) medical centers (VAMCs) in Florida between 2014 and 2016: James A. Haley Veterans’ Hospital & Clinics (Tampa) and Malcom Randall VAMC (Gainesville). This retrospective evaluation of its first year did not require institutional review board approval as it was determined to be a quality improvement project by the local research service.
The patient population for the project was veterans with disabilities who were referred by clinical consults to the CWT service, a recovery-oriented vocational program. During the project years, veterans were offered the option of receiving VR services, such as supported employment, community-based employment services, or vocational assistance, through VRtele rather than traditional face-to-face meetings. The specific interventions delivered included patient orientation, interview assessment, treatment plan development, referral activities, vocational counseling, assessment of workplace for accommodation needs, vocational case management, and other employment supports. VR staff participating in the project included 2 VR supervisors, 1 supported employment mentor trainer, and 5 VRSs.
Each clinic was set up for VRtele, and codes were added to the electronic health record (EHR) to ensure proper documentation. Participating VRSs completed teleconferencing training, including a skills assessment using the equipment for real-time, high-quality video streaming over an encrypted network to provide services in a patient’s home or other remote locations. VRS staff provided veterans with instructions on using a VA-provided tablet or their own device and assisted them with establishing connectivity with the network. Video equipment included speakers, camera, and headphones connected to the desktop computer or laptop of the VRS. A patient’s first VRtele
Demographic data, primary diagnosis, VR usage data, and zip codes of participating veterans were extracted from the EHR. Veterans completed a 2-part satisfaction survey administered 90 days after enrollment and at discharge. Part 1 was composed of 15 items, most with a 5-point Likert scale (higher ratings indicated greater satisfaction), on various aspects of the VRtele experience, such as audio and video quality and wait times.10 Part 2 addressed VR services and the VRS and consisted of 8 Likert scale items with the option to add a comment for each and 2 open-ended items that asked the participant to list what they liked best and least about VRtele.
Semistructured, in-person 30- to 60-minute interviews were conducted with VRSs at the initiation of VRtele
After ≥ 2 months of VRtele use
Analyses
Descriptive statistics were used for EHR data and satisfaction surveys. For qualitative analysis, each transcript was read in full by 2 researchers to get an overview of the data, and a rapid analysis approach was used to identify central themes focused on how technology was used and the experiences of the participants.11,12 Relevant text for each topic was tabulated, and a summary table was created that highlighted overlapping ideas discussed by the interviewees as well as differences.
Results
Of the 22 veterans who participated in the project, 11 completed satisfaction surveys and 4 participated in qualitative interviews. The rural and nonrural groups did not differ demographically or by diagnosis, which was predominantly mental health related. Only 1 veteran in each group owned a tablet; the majority of both groups required VA-issued devices: 80% (n = 8) rural and 91.7% (n = 11) nonrural. The number of VRtele sessions for the groups also was similar, 53 for rural and 60 for nonrural, as was the mean (SD) number of sessions per veteran: 5.3 (SD, 3.2) rural and 5.0 (SD, 2.5) urban. Overall, 63 miles per session were saved, mostly for rural veterans, and the number of mean (SD) miles saved per veteran was greater for rural than nonrural veterans: 379.2 (243.0) and 256.1 (275.9), respectively. One veteran who moved to a different state during the program continued VRtele at the new location. In a qualitative sampling of 5 VRtele sessions, all the VRSs used office desktop computers.
Level of satisfaction with aspects of VRtele related to the technology rated was consistently > 4 on the Likert scale. The lowest mean (SD) ratings were 4.2 (1.0) for audio quality and 4.4 (0.5) for video quality, and the highest rating was given for equipment operation explanation and privacy was respected, 4.9 (0.3) for both. All questions related to satisfaction with services were also rated high: The mean (SD) lowest ratings were 4.3 (1.0) given to both vocational needs 4.3 (1.0) and tasks effectively helped achieve goals 4.3 (0.7). The highest mean (SD) ratings were 4.6 (0.5) given to VR program service explained and 4.7 (0.5) for appointment timeliness.
Qualitative Results
At first, some VRSs thought the teleconferencing system might be difficult or awkward to use, but they found it easier to set up than expected and seamless to use. VRS staff reported being surprised at how well it worked despite some issues that occurred with loading the software. Once loaded, however, the connection worked well, one VRS noting that following step-by-step instructions solved the problem. Some VRSs indicated they did not invite all the veterans on their caseload to participate in VRtele due to concerns with the patient’s familiarity with technology, but one VRS stated, “I haven’t had anybody that failed to do a [session] that I couldn’t get them up and running within a few minutes.”
When working in the community, VRSs reported using laptops for VRtele but found that these devices were unreliable due to lack of internet access and were slow to start; several VRSs thought tablets would have been more helpful. Some veterans reported technical glitches, lack of comfort with technology, or a problem with sound due to a tablet’s protective case blocking the speakers. To solve the sound issue, a veteran used headphones. This veteran also explained that the log-on process required a new password every time, so he would keep a pen and paper ready to write it down. Because signing in and setting up takes a little time, this veteran and his VRS agreed to start connecting 5 minutes before their meeting time to allow for that set- up time.
Initially, some VRSs expressed concern that transitioning to VRtele would affect the quality of interactions with the veterans. However, VRSs also identified strengths of VRtele, including flexibility, saved time, and increased interaction. One VRS discussed a veteran’s adaptation by saying, “I think he feels even more involved in his plan [and] enjoys the increased interaction.” Veterans reported enjoying using tablets and identified the main strength of VRtele as being able to talk face-to-face with the VRS. Echoing the VRSs, veterans reported teleconferencing saved time by avoiding travel and enabled spontaneous meetings. One of the veterans summed up the benefits of using VRtele: “I’d rather just connect. It’s going to take us 40 to 50 minutes [to meet in person] when we can just connect right here and it takes 15 to 20. We don’t have to go through the driving.… So this right here, doing it ahead of time and having the appointment, it’s a lot easier.”
In their interviews, VRSs talked about enjoying VRtele. A VRS explained: “It makes it a lot easier. It makes me feel less guilty. This way [veterans] don’t have to use their gas money, use their time. I know [the veteran] had something else he needed to do today.” Thus, both veterans and VRSs were satisfied with their VRtele experiences.
Discussion
This first report on the perspective of providers and veterans using VRtele suggests that it is a viable option for service delivery and that is highly satisfactory for serving veterans with disabilities, many of whom live in rural areas or have travel barriers. These findings are consistent with data on telerehabilitation for veterans with cognitive, physical, and mental disabilities.13-22 Further, the data support the notion of using VRtele to facilitate long-term VR follow-up for persons with disabilities, as illustrated by successful continuation of vocational services after a veteran moved out of state.23
Similar to other reports, our experience highlighted 2 factors that affect successful VRtele: (1) Troubleshooting technology barriers for both VR providers and clients; and (2) supportive leadership to facilitate implementation
Changes to technology and increased usage of VA Video Connect may indicate that the barriers identified from the earlier process described here have been diminished or eliminated. More evaluation is needed to assess whether system upgrades have increased ease of use and access for veterans with disabilities.
Conclusions
Encouragingly, this clinical demonstration project showed that both providers and clients recognize the benefits of VRtele. Patient satisfaction and decreased travel costs were clear advantages to using VRtele for this small group of veterans who had barriers to care due to travel or disability barriers. As this program evaluation was limited by a small sample, absence of a comparison group, and lack of outcome data (eg, employment rates, hours, wages, retention), future research is needed on implementation and outcomes of VRtele
Acknowledgments
The authors thank Lynn Dirk, MAMC, for substantial editorial assistance. This material was based on work supported by Rural Veterans Supported Employment TeleRehabilitation Initiative (RVSETI), funded by the VA Office of Rural Health (Project # N08-FY14Q3-S2-P01222) and by support of the VA Health Services Research and Development Service. This work was presented in part at the 114th Annual Meeting of the American Anthropological Association at Denver, Colorado, November 21, 2015; a field-based Health Services Research and Development Service meeting, US Department of Veterans Affairs at Washington, DC, September 12, 2016; and the 2016 Annual Conference of the American Congress for Rehabilitation Medicine at Chicago, Illinois, October-November 2016.
1. Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA therapeutic and supported employment services programs. Psychiatr Serv. 2017;68(9):938-946. doi:10.1176/appi.ps.201600412
2. Davis LL, Kyriakides TC, Suris AM, et al. Effect of evidence-based supported employment vs transitional work on achieving steady work among veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2018;75(4):316. doi:10.1001/jamapsychiatry.2017.4472
3. Ottomanelli L, Goetz LL, Suris A, et al. Effectiveness of supported employment for veterans with spinal cord injuries: results from a randomized multisite study. Arch Phys Med Rehabil. 2012;93(5):740-747. doi:10.1016/j.apmr.2012.01.002
4. Ottomanelli L, Goetz LL, Barnett SD, et al. Individual placement and support in spinal cord injury: a longitudinal observational study of employment outcomes. Arch Phys Med Rehabil. 2017;98(8):1567-1575. doi:10.1016/j.apmr.2016.12.010
5. Cotner BA, Ottomanelli L, O’Connor DR, Njoh EN, Barnett SD, Miech EJ. Quality of life outcomes for veterans with spinal cord injury receiving individual placement and support (IPS). Top Spinal Cord Inj Rehabil. 2018;24(4):325-335. doi:10.1310/sci17-00046
6. Metzel DS, Giordano A. Locations of employment services and people with disabilities: a geographical analysis of accessibility. J Disabil Policy Stud. 2007;18(2):88-97. doi:10.1177/10442073070180020501
7. Landon T, Connor A, McKnight-Lizotte M, Peña J. Rehabilitation counseling in rural settings: a phenomenological study on barriers and supports. J Rehabil. 2019;85(2):47-57.
8. Riemer-Reiss M. Vocational rehabilitation counseling at a distance: Challenges, strategies and ethics to consider. J Rehabil. 2000;66(1):11-17.
9. Schmeler MR, Schein RM, McCue M, Betz K. Telerehabilitation clinical and vocational applications for assistive technology: research, opportunities, and challenges. Int J Telerehabilitation. 2009;1(1):59-72.
10. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370. doi:10.1682/JRRD.2014.10.0239
11. McMullen CK, Ash JS, Sittig DF, et al. Rapid assessment of clinical information systems in the healthcare setting: an efficient method for time-pressed evaluation. Methods Inf Med. 2011;50(4):299-307. doi:10.3414/ME10-01-0042
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
13. Egede LE, Acierno R, Knapp RG, et al. Psychotherapy for depression in older veterans via telemedicine: a randomised, open-label, non-inferiority trial. Lancet Psychiatry. 2015;2(8):693-701. doi:10.1016/S2215-0366(15)00122-4
14. Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22(8):1086-1093. doi:10.1007/s11606-007-0201-9
15. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58. doi:10.1001/jamapsychiatry.2014.1575
16. Grubbs KM, Fortney JC, Dean T, Williams JS, Godleski L. A comparison of mental health diagnoses treated via interactive video and face to face in the Veterans Healthcare Administration. Telemed E-Health. 2015;21(7):564-566. doi:10.1089/tmj.2014.0152
17. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213. doi:10.1177/1357633X15572201
18. Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed E-Health. 2010;16(4):417-423. doi:10.1089/tmj.2009.0118
19. Brennan DM, Georgeadis AC, Baron CR, Barker LM. The effect of videoconference-based telerehabilitation on story retelling performance by brain-injured subjects and its implications for remote speech-language therapy. Telemed J E Health. 2004;10(2):147-154. doi:10.1089/tmj.2004.10.147
20. Dallolio L, Menarini M, China S, et al. Functional and clinical outcomes of telemedicine in patients with spinal cord injury. Arch Phys Med Rehabil. 2008;89(12):2332-2341. doi:10.1016/j.apmr.2008.06.012
21. Houlihan BV, Jette A, Friedman RH, et al. A pilot study of a telehealth intervention for persons with spinal cord dysfunction. Spinal Cord. 2013;51(9):715-720.doi:10.1038/sc.2013.45
22. Smith MW, Hill ML, Hopkins KL, Kiratli BJ, Cronkite RC. A modeled analysis of telehealth methods for treating pressure ulcers after spinal cord injury. Int J Telemed Appl. 2012;2012:1-10. doi:10.1155/2012/729492
23. Balcazar FE, Keys CB, Davis M, Lardon C, Jones C. Strengths and challenges of intervention research in vocational rehabilitation: an illustration of agency-university collaboration. J Rehabil. 2005;71(2):40-48.
24. Martinez RN, Hogan TP, Balbale S, et al. Sociotechnical perspective on implementing clinical video telehealth for veterans with spinal cord injuries and disorders. Telemed J E Health. 2017;23(7):567-576. doi:10.1089/tmj.2016.0200
25. Martinez RN, Hogan TP, Lones K, et al. Evaluation and treatment of mild traumatic brain injury through the implementation of clinical video telehealth: provider perspectives from the Veterans Health Administration. PM R. 2017;9(3):231-240. doi:10.1016/j.pmrj.2016.07.002
26. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26(5):309-313. doi:10.1177/1357633X20916567
27. Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019; 36(3):122-128.
28. US Department of Veterans Affairs. Veterans VA Video Connect. Published May 22, 2020. Accessed May 29, 2020. https://mobile.va.gov/app/va-video-connect#AppDescription.
29. US Department of Veterans Affairs. VA telehealth at home. Accessed May 29, 2020. https://telehealth.va.gov/type/home
1. Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA therapeutic and supported employment services programs. Psychiatr Serv. 2017;68(9):938-946. doi:10.1176/appi.ps.201600412
2. Davis LL, Kyriakides TC, Suris AM, et al. Effect of evidence-based supported employment vs transitional work on achieving steady work among veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2018;75(4):316. doi:10.1001/jamapsychiatry.2017.4472
3. Ottomanelli L, Goetz LL, Suris A, et al. Effectiveness of supported employment for veterans with spinal cord injuries: results from a randomized multisite study. Arch Phys Med Rehabil. 2012;93(5):740-747. doi:10.1016/j.apmr.2012.01.002
4. Ottomanelli L, Goetz LL, Barnett SD, et al. Individual placement and support in spinal cord injury: a longitudinal observational study of employment outcomes. Arch Phys Med Rehabil. 2017;98(8):1567-1575. doi:10.1016/j.apmr.2016.12.010
5. Cotner BA, Ottomanelli L, O’Connor DR, Njoh EN, Barnett SD, Miech EJ. Quality of life outcomes for veterans with spinal cord injury receiving individual placement and support (IPS). Top Spinal Cord Inj Rehabil. 2018;24(4):325-335. doi:10.1310/sci17-00046
6. Metzel DS, Giordano A. Locations of employment services and people with disabilities: a geographical analysis of accessibility. J Disabil Policy Stud. 2007;18(2):88-97. doi:10.1177/10442073070180020501
7. Landon T, Connor A, McKnight-Lizotte M, Peña J. Rehabilitation counseling in rural settings: a phenomenological study on barriers and supports. J Rehabil. 2019;85(2):47-57.
8. Riemer-Reiss M. Vocational rehabilitation counseling at a distance: Challenges, strategies and ethics to consider. J Rehabil. 2000;66(1):11-17.
9. Schmeler MR, Schein RM, McCue M, Betz K. Telerehabilitation clinical and vocational applications for assistive technology: research, opportunities, and challenges. Int J Telerehabilitation. 2009;1(1):59-72.
10. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370. doi:10.1682/JRRD.2014.10.0239
11. McMullen CK, Ash JS, Sittig DF, et al. Rapid assessment of clinical information systems in the healthcare setting: an efficient method for time-pressed evaluation. Methods Inf Med. 2011;50(4):299-307. doi:10.3414/ME10-01-0042
12. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
13. Egede LE, Acierno R, Knapp RG, et al. Psychotherapy for depression in older veterans via telemedicine: a randomised, open-label, non-inferiority trial. Lancet Psychiatry. 2015;2(8):693-701. doi:10.1016/S2215-0366(15)00122-4
14. Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22(8):1086-1093. doi:10.1007/s11606-007-0201-9
15. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58. doi:10.1001/jamapsychiatry.2014.1575
16. Grubbs KM, Fortney JC, Dean T, Williams JS, Godleski L. A comparison of mental health diagnoses treated via interactive video and face to face in the Veterans Healthcare Administration. Telemed E-Health. 2015;21(7):564-566. doi:10.1089/tmj.2014.0152
17. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213. doi:10.1177/1357633X15572201
18. Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed E-Health. 2010;16(4):417-423. doi:10.1089/tmj.2009.0118
19. Brennan DM, Georgeadis AC, Baron CR, Barker LM. The effect of videoconference-based telerehabilitation on story retelling performance by brain-injured subjects and its implications for remote speech-language therapy. Telemed J E Health. 2004;10(2):147-154. doi:10.1089/tmj.2004.10.147
20. Dallolio L, Menarini M, China S, et al. Functional and clinical outcomes of telemedicine in patients with spinal cord injury. Arch Phys Med Rehabil. 2008;89(12):2332-2341. doi:10.1016/j.apmr.2008.06.012
21. Houlihan BV, Jette A, Friedman RH, et al. A pilot study of a telehealth intervention for persons with spinal cord dysfunction. Spinal Cord. 2013;51(9):715-720.doi:10.1038/sc.2013.45
22. Smith MW, Hill ML, Hopkins KL, Kiratli BJ, Cronkite RC. A modeled analysis of telehealth methods for treating pressure ulcers after spinal cord injury. Int J Telemed Appl. 2012;2012:1-10. doi:10.1155/2012/729492
23. Balcazar FE, Keys CB, Davis M, Lardon C, Jones C. Strengths and challenges of intervention research in vocational rehabilitation: an illustration of agency-university collaboration. J Rehabil. 2005;71(2):40-48.
24. Martinez RN, Hogan TP, Balbale S, et al. Sociotechnical perspective on implementing clinical video telehealth for veterans with spinal cord injuries and disorders. Telemed J E Health. 2017;23(7):567-576. doi:10.1089/tmj.2016.0200
25. Martinez RN, Hogan TP, Lones K, et al. Evaluation and treatment of mild traumatic brain injury through the implementation of clinical video telehealth: provider perspectives from the Veterans Health Administration. PM R. 2017;9(3):231-240. doi:10.1016/j.pmrj.2016.07.002
26. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26(5):309-313. doi:10.1177/1357633X20916567
27. Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019; 36(3):122-128.
28. US Department of Veterans Affairs. Veterans VA Video Connect. Published May 22, 2020. Accessed May 29, 2020. https://mobile.va.gov/app/va-video-connect#AppDescription.
29. US Department of Veterans Affairs. VA telehealth at home. Accessed May 29, 2020. https://telehealth.va.gov/type/home
COVID-19 Vaccine in Veterans with Multiple Sclerosis: Protect the Vulnerable
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
Behavioral Interventions in Multiple Sclerosis
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.