User login
High Rate of Inappropriate Fecal Immunochemical Testing at a Large Veterans Affairs Health Care System
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
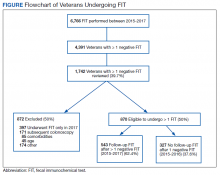
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
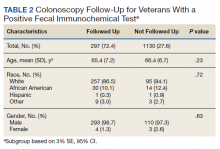
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
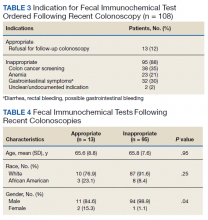
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
Performance of the Veterans Choice Program for Improving Access to Colonoscopy at a Tertiary VA Facility
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
Of Mice and Men
Fever in an elderly man is a nonspecific finding, occurring most commonly with infections but also with certain malignancies, rheumatologic disorders, and drug exposures. The complaint of rigors with diaphoresis makes an infection most likely. The acuity of his illness makes infections with more chronic presentations such as tuberculosis or actinomycosis less likely. The presence of frontal headache might suggest a sinus or brain source, but headache also occurs in generalized infections such as pneumonia, bacteremia from any cause, malaria, rickettsial infections, viral illnesses, and others. Additional history should include detailed inquiry into travel, vocational, and avocational exposures.
The patient's difficulty standing implies the development of lower extremity weakness and infections associated with neurological syndromes. His leg weakness may be related to early Guillain‐Barre syndrome, which is associated most commonly with Campylobacter jejuni, but also other bacteria and viruses such as Haemophilus influenza, Mycoplasma pneumonia, Influenza virus, Cytomegalovirus and hepatitis E. Other viral infections associated with pure motor deficits include echovirus, coxsackie virus, enterovirus, and West Nile virus (WNV). The paralytic syndrome associated with enteroviruses is more common in children, whereas the neuroinvasive variant of WNV more often affects the elderly and can be associated with encephalitis as well as a flaccid paralysis. Although acute paralytic shellfish poisoning could account for both his weakness and his acute gastrointestinal syndrome, this diagnosis is unlikely because the symptoms often have a prominent sensory component, and there is usually the history of recent ingestion of the suspect bivalves. Like all adults presenting for medical care, he should be screened for human immunodeficiency virus (HIV) infection; if testing is positive, the differential diagnosis for his current illness broadens significantly. Finally, he may have a spinal cord disorder or infection such as an epidural abscess, or transverse myelitis, which would present with lower extremity weakness and fever. It would be helpful to know the time of year of his illness, exposure to mosquito bites, his neurological exam findings, and results of blood and stool cultures. If the patient had signs of meningitis or encephalitis, cerebral spinal fluid analysis would be helpful. If his neurological exam was suggestive of cord involvement, it would be helpful to know the results of magnetic resonance imaging of the spinal cord.
The patient's past medical history includes relatively common problems for a 73‐year‐old man and does not substantially influence the differential diagnosis of his current illness. His travel history to Uganda a year previously may be relevant, because malaria (Plasmodium vivax) could present with fever and weakness. Less commonly, African trypanosomiasis (Trypanosoma brucei gambiense) can, in the late phase, present with fever and malaise, but also typically includes symptoms of encephalitis, including depressed mental status, confusion, ataxia, and possibly personality changes. His travel to Zurich should not impose any particular infection risk, unless he was hiking in the mountains around Zurich, where he could have contracted tick‐borne encephalitis; however, his travel more than 6 months prior to presentation makes this unlikely. Lyme disease due to Borrelia burgdorferi is also a potential exposure in the Swiss mountains, and can present with fever in the acute phase, as well as arthritis with chronic disease, but should not cause fever, rigor, diaphoresis, and headache many months later. Summering in Cape Cod puts him at risk for babesiosis, but an incubation period of 5 months is too long. Keeping chickens places him at risk for Salmonella exposure and typhoid fever. Ingesting raw oysters carries a risk for shellfish poisoning and Vibrio infections, but the incubation period (1 month) again seems too long to cause his current symptoms.
Notable physical findings are an ill‐appearing man with injected sclera and a high fever but normal blood pressure and heart rate. He also demonstrates proximal lower extremity weakness manifested by difficulty rising from a chair and a slow gait with short strides and deliberate (possibly on‐block) turning. His neurological exam is most consistent with Parkinsonian symptoms that have been described in patients with severe influenza A, which would explain all of his other symptoms as well. Pulse‐temperature dissociation is classically described with typhoid fever but usually occurs later in the disease course, and could be masked by the patient's metoprolol. Typhoid fever can also be associated with neurological symptoms including meningitis and movement disorders.
The patient has a remarkable bandemia, suggesting a bacterial infection, as well as a slight reduction in hematocrit and platelet count. Additionally, his labs revealed a mild transaminitis, but with significantly elevated alkaline phosphatase and GGT, and microscopic hematuria. His ferritin is significantly elevated, which may simply represent an acute phase reactant. Infections associated with hepatitis, cytopenias, and hematuria include sepsis with disseminated intravascular coagulation, previously mentioned malaria, leptospirosis, dengue, ehrlichiosis, and rickettsial diseases, but he has no special risks for these infections, and other aspects of his illness (Parkinsonian features, bandemia) do not fit. His lung findings with hematuria might suggest a pulmonary/renal syndrome, but, once again, other features of his illness are not typical of these syndromes. Salmonella (typhoid fever) or influenza, now complicated by an early bacterial pneumonia, are viable possibilities.
The patient's ongoing clinical course is notable for a nontoxic (non‐SIRS) appearance but continued high‐grade fever with blood and urine cultures that are sterile. This argues against a common bacteremia with sepsis, and for either relapsing malaria (P vivax), influenza with a Parkinsonian‐like illness, typhoid fever, leptospirosis, dengue, or a rickettsial infection. Mycoplasma pneumonia is also possible given the atypical chest x‐ray appearance, slightly low hematocrit with elevated bilirubin, and neurological symptoms that may represent ataxia.
The subsequent negative laboratory tests listed are helpful in likely excluding many of the diagnoses suggested such as malaria, Babesia, common bacteremias, viral hepatitis, HIV, and WNV. Furthermore, the new history of mouse exposure brings to the forefront rodent‐associated infections, specifically exposure to mouse urine, a vehicle for leptospirosis. The patient's hepatitis, anemia, thrombocytopenia, scleral injection, along with the rest of his symptoms in the context of exposure to mouse urine makes leptospirosis the likely diagnosis. A negative Leptospira antibody early in his illness does not rule out the disease, and a convalescent titer should be obtained to confirm the diagnosis.
COMMENTARY
This case describes an elderly man who presented with a fever of unknown origin (FUO), and was eventually diagnosed with leptospirosis. FUO presents slightly differently in elderly patients, as elderly patients are less likely to mount a high fever, and when they do, the etiology is more likely to indicate a serious bacterial or viral infection. Additionally, an etiology for FUO in the elderly is found in over 70% of presenting cases, compared to 51% in patients under the age of 65 years.[1] A detailed, comprehensive social, travel, and exposure history and physical examination remains the cornerstone of elucidating the diagnosis for FUO. The exposure to mouse urine in this case was an unusual and a helpful piece of the history to further focus the differential diagnosis.
Leptospirosis is an emerging bacterial zoonosis, and causes both endemic and epidemic severe multisystem disease. The Leptospira spirochete is maintained in nature through a chronic renal infection in mammalian reservoir hosts, such as mice,[2, 4] and is transmitted through direct or aerosolized contact with infected urine or tissue. After a mean incubation period of 10 days, a variety of clinical manifestations may be seen. In this case, the patient's clinical presentation revealed many classic symptoms of leptospirosis, including fevers, rigors, headache, lower extremity myalgias, nausea, vomiting, and diarrhea; however, these symptoms are nonspecific. The presence of a conjunctival suffusion in leptospirosis infection had a specificity of 98% in a high‐incidence cohort of febrile patients in Sri Lanka,[3] and was an important diagnostic clue in this case. Leptospirosis is a self‐limited illness in most patients, with an initial septicemic, febrile phase followed by an immune phase. A more severe presentation may be seen in the immune phase of the illness, which includes renal and hepatic dysfunction (known as Weil's disease), as well as cardiac, pulmonary, and central nervous system abnormalities. With a 14% case fatality rate, the risk of death has been shown to be higher in patients over 40 years old, with altered mental status and multiorgan failure.[4]
The early diagnosis of leptospirosis relies heavily on physical exam findings and epidemiologic history. In this case, the patient's laboratory abnormalities, including immature granulocytes, thrombocytopenia, hyponatremia, hypokalemia, mild hepatitis, and pyuria with granular casts are all reported with leptospirosis infection2; however, independently, these laboratory findings are nonspecific. Patients may not have a detectable antibody levels in the acute phase of the disease. In this case, given the strong clinical suspicion based on the findings of conjunctival suffusion and exposure to mouse urine history, the lack of a more plausible alternate diagnosis, and known delay in antibody positivity, the patient was treated empirically with doxycycline for presumed leptospirosis.[5] Forthcoming novel diagnostic strategies such as next‐generation DNA sequencing techniques may provide real‐time diagnosis of this zoonotic infection, thus decreasing the window period between empirical antimicrobial coverage and diagnostic confirmation.[6]
Leptospirosis is prevalent in tropical climates and has been associated with impoverished communities.[7] Urban slums, with poor sanitation and high rodent density, are an ideal environment for leptospirosis. The reported risk of infection in a Brazilian slum was as high as 3% per year.[8] Additionally, rodent sightings, as well as the presence of chickens, were risk factors for leptospirosis transmission in urban slums.[9] Correspondingly in this case, we hypothesize that the patient's interest in urban farming, specifically the chickens he kept, likely attracted the mice infected with leptospirosis. Urban chicken farming is becoming increasingly popular in the United States,[10] and may be a developing risk factor for human leptospirosis infection. Leptospirosis is one of many emerging zoonoses, such as avian influenza, tick‐borne illness, and ebola, resulting from changing human ecology. Thus, when considering infectious etiologies, clinicians should ask patients about vocational and avocational exposures, including new trends such as urban farming, which may expose them to previously underappreciated illnesses.
TEACHING POINTS
- Elderly patients with a FUO are more likely to be diagnosed with an underlying serious bacterial or viral infection when compared to a younger cohort of FUO patients.
- The diagnosis of leptospirosis may initially be based on clinical suspicion in patients with classic features and exposures, noting the high specificity of conjunctival suffusion, and initial titers may be nondiagnostic; therefore, empiric treatment should be considered when clinical suspicion is high.
- Increased interest in urban chicken farming in the United States, with associated higher rodent density, may represent a newly recognized risk factor for human leptospirosis infection.
Disclosures
The authors report no conflicts of interest.
- , , . Fever of unknown origin in older persons. Infect Dis Clin North Am. 2007;21(4):937–945.
- . Leptospirosis. Clin Microbiol Rev. 2011;14(2):296–326.
- , , , et al. Leptospirosis as frequent cause of acute febrile illness in southern Sri Lanka. Emerg Infect Dis. 2011;17(9):1678–1684.
- , , , et al. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Philadelphia, PA: Elsevier; 2010.
- , . Antibiotics for leptospirosis. The Cochrane Database Syst Rev. 2012;2:CD008264.
- , , , et al. Actionable diagnosis of neuroleptospirosis by next‐generation sequencing. N Engl J Med. 2014;370(25):2408–2417.
- , , , . Cases distribution of leptospirosis in City of Manaus, State of Amazonas, Brazil, 2000–2010. Rev Soc Bras Med Trop. 2012;45(6):713–716.
- , , , et al. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the leptospira agent. PLoS Negl Trop Dis. 2014;8(5):e2927.
- , , , et al. Impact of environment and social gradient on leptospira infection in urban slums. PLoS Negl Trop Dis. 2008;2(4):e228.
- Urban chicken ownership in four U.S. cities. United States Department of Agriculture website. Available at: http://www.aphis.usda.gov/animal_health/nahms/poultry/downloads/poultry10/Poultry10_dr_Urban_Chicken_Four.pdf. Published April 2013. Accessed June 9, 2015.
Fever in an elderly man is a nonspecific finding, occurring most commonly with infections but also with certain malignancies, rheumatologic disorders, and drug exposures. The complaint of rigors with diaphoresis makes an infection most likely. The acuity of his illness makes infections with more chronic presentations such as tuberculosis or actinomycosis less likely. The presence of frontal headache might suggest a sinus or brain source, but headache also occurs in generalized infections such as pneumonia, bacteremia from any cause, malaria, rickettsial infections, viral illnesses, and others. Additional history should include detailed inquiry into travel, vocational, and avocational exposures.
The patient's difficulty standing implies the development of lower extremity weakness and infections associated with neurological syndromes. His leg weakness may be related to early Guillain‐Barre syndrome, which is associated most commonly with Campylobacter jejuni, but also other bacteria and viruses such as Haemophilus influenza, Mycoplasma pneumonia, Influenza virus, Cytomegalovirus and hepatitis E. Other viral infections associated with pure motor deficits include echovirus, coxsackie virus, enterovirus, and West Nile virus (WNV). The paralytic syndrome associated with enteroviruses is more common in children, whereas the neuroinvasive variant of WNV more often affects the elderly and can be associated with encephalitis as well as a flaccid paralysis. Although acute paralytic shellfish poisoning could account for both his weakness and his acute gastrointestinal syndrome, this diagnosis is unlikely because the symptoms often have a prominent sensory component, and there is usually the history of recent ingestion of the suspect bivalves. Like all adults presenting for medical care, he should be screened for human immunodeficiency virus (HIV) infection; if testing is positive, the differential diagnosis for his current illness broadens significantly. Finally, he may have a spinal cord disorder or infection such as an epidural abscess, or transverse myelitis, which would present with lower extremity weakness and fever. It would be helpful to know the time of year of his illness, exposure to mosquito bites, his neurological exam findings, and results of blood and stool cultures. If the patient had signs of meningitis or encephalitis, cerebral spinal fluid analysis would be helpful. If his neurological exam was suggestive of cord involvement, it would be helpful to know the results of magnetic resonance imaging of the spinal cord.
The patient's past medical history includes relatively common problems for a 73‐year‐old man and does not substantially influence the differential diagnosis of his current illness. His travel history to Uganda a year previously may be relevant, because malaria (Plasmodium vivax) could present with fever and weakness. Less commonly, African trypanosomiasis (Trypanosoma brucei gambiense) can, in the late phase, present with fever and malaise, but also typically includes symptoms of encephalitis, including depressed mental status, confusion, ataxia, and possibly personality changes. His travel to Zurich should not impose any particular infection risk, unless he was hiking in the mountains around Zurich, where he could have contracted tick‐borne encephalitis; however, his travel more than 6 months prior to presentation makes this unlikely. Lyme disease due to Borrelia burgdorferi is also a potential exposure in the Swiss mountains, and can present with fever in the acute phase, as well as arthritis with chronic disease, but should not cause fever, rigor, diaphoresis, and headache many months later. Summering in Cape Cod puts him at risk for babesiosis, but an incubation period of 5 months is too long. Keeping chickens places him at risk for Salmonella exposure and typhoid fever. Ingesting raw oysters carries a risk for shellfish poisoning and Vibrio infections, but the incubation period (1 month) again seems too long to cause his current symptoms.
Notable physical findings are an ill‐appearing man with injected sclera and a high fever but normal blood pressure and heart rate. He also demonstrates proximal lower extremity weakness manifested by difficulty rising from a chair and a slow gait with short strides and deliberate (possibly on‐block) turning. His neurological exam is most consistent with Parkinsonian symptoms that have been described in patients with severe influenza A, which would explain all of his other symptoms as well. Pulse‐temperature dissociation is classically described with typhoid fever but usually occurs later in the disease course, and could be masked by the patient's metoprolol. Typhoid fever can also be associated with neurological symptoms including meningitis and movement disorders.
The patient has a remarkable bandemia, suggesting a bacterial infection, as well as a slight reduction in hematocrit and platelet count. Additionally, his labs revealed a mild transaminitis, but with significantly elevated alkaline phosphatase and GGT, and microscopic hematuria. His ferritin is significantly elevated, which may simply represent an acute phase reactant. Infections associated with hepatitis, cytopenias, and hematuria include sepsis with disseminated intravascular coagulation, previously mentioned malaria, leptospirosis, dengue, ehrlichiosis, and rickettsial diseases, but he has no special risks for these infections, and other aspects of his illness (Parkinsonian features, bandemia) do not fit. His lung findings with hematuria might suggest a pulmonary/renal syndrome, but, once again, other features of his illness are not typical of these syndromes. Salmonella (typhoid fever) or influenza, now complicated by an early bacterial pneumonia, are viable possibilities.
The patient's ongoing clinical course is notable for a nontoxic (non‐SIRS) appearance but continued high‐grade fever with blood and urine cultures that are sterile. This argues against a common bacteremia with sepsis, and for either relapsing malaria (P vivax), influenza with a Parkinsonian‐like illness, typhoid fever, leptospirosis, dengue, or a rickettsial infection. Mycoplasma pneumonia is also possible given the atypical chest x‐ray appearance, slightly low hematocrit with elevated bilirubin, and neurological symptoms that may represent ataxia.
The subsequent negative laboratory tests listed are helpful in likely excluding many of the diagnoses suggested such as malaria, Babesia, common bacteremias, viral hepatitis, HIV, and WNV. Furthermore, the new history of mouse exposure brings to the forefront rodent‐associated infections, specifically exposure to mouse urine, a vehicle for leptospirosis. The patient's hepatitis, anemia, thrombocytopenia, scleral injection, along with the rest of his symptoms in the context of exposure to mouse urine makes leptospirosis the likely diagnosis. A negative Leptospira antibody early in his illness does not rule out the disease, and a convalescent titer should be obtained to confirm the diagnosis.
COMMENTARY
This case describes an elderly man who presented with a fever of unknown origin (FUO), and was eventually diagnosed with leptospirosis. FUO presents slightly differently in elderly patients, as elderly patients are less likely to mount a high fever, and when they do, the etiology is more likely to indicate a serious bacterial or viral infection. Additionally, an etiology for FUO in the elderly is found in over 70% of presenting cases, compared to 51% in patients under the age of 65 years.[1] A detailed, comprehensive social, travel, and exposure history and physical examination remains the cornerstone of elucidating the diagnosis for FUO. The exposure to mouse urine in this case was an unusual and a helpful piece of the history to further focus the differential diagnosis.
Leptospirosis is an emerging bacterial zoonosis, and causes both endemic and epidemic severe multisystem disease. The Leptospira spirochete is maintained in nature through a chronic renal infection in mammalian reservoir hosts, such as mice,[2, 4] and is transmitted through direct or aerosolized contact with infected urine or tissue. After a mean incubation period of 10 days, a variety of clinical manifestations may be seen. In this case, the patient's clinical presentation revealed many classic symptoms of leptospirosis, including fevers, rigors, headache, lower extremity myalgias, nausea, vomiting, and diarrhea; however, these symptoms are nonspecific. The presence of a conjunctival suffusion in leptospirosis infection had a specificity of 98% in a high‐incidence cohort of febrile patients in Sri Lanka,[3] and was an important diagnostic clue in this case. Leptospirosis is a self‐limited illness in most patients, with an initial septicemic, febrile phase followed by an immune phase. A more severe presentation may be seen in the immune phase of the illness, which includes renal and hepatic dysfunction (known as Weil's disease), as well as cardiac, pulmonary, and central nervous system abnormalities. With a 14% case fatality rate, the risk of death has been shown to be higher in patients over 40 years old, with altered mental status and multiorgan failure.[4]
The early diagnosis of leptospirosis relies heavily on physical exam findings and epidemiologic history. In this case, the patient's laboratory abnormalities, including immature granulocytes, thrombocytopenia, hyponatremia, hypokalemia, mild hepatitis, and pyuria with granular casts are all reported with leptospirosis infection2; however, independently, these laboratory findings are nonspecific. Patients may not have a detectable antibody levels in the acute phase of the disease. In this case, given the strong clinical suspicion based on the findings of conjunctival suffusion and exposure to mouse urine history, the lack of a more plausible alternate diagnosis, and known delay in antibody positivity, the patient was treated empirically with doxycycline for presumed leptospirosis.[5] Forthcoming novel diagnostic strategies such as next‐generation DNA sequencing techniques may provide real‐time diagnosis of this zoonotic infection, thus decreasing the window period between empirical antimicrobial coverage and diagnostic confirmation.[6]
Leptospirosis is prevalent in tropical climates and has been associated with impoverished communities.[7] Urban slums, with poor sanitation and high rodent density, are an ideal environment for leptospirosis. The reported risk of infection in a Brazilian slum was as high as 3% per year.[8] Additionally, rodent sightings, as well as the presence of chickens, were risk factors for leptospirosis transmission in urban slums.[9] Correspondingly in this case, we hypothesize that the patient's interest in urban farming, specifically the chickens he kept, likely attracted the mice infected with leptospirosis. Urban chicken farming is becoming increasingly popular in the United States,[10] and may be a developing risk factor for human leptospirosis infection. Leptospirosis is one of many emerging zoonoses, such as avian influenza, tick‐borne illness, and ebola, resulting from changing human ecology. Thus, when considering infectious etiologies, clinicians should ask patients about vocational and avocational exposures, including new trends such as urban farming, which may expose them to previously underappreciated illnesses.
TEACHING POINTS
- Elderly patients with a FUO are more likely to be diagnosed with an underlying serious bacterial or viral infection when compared to a younger cohort of FUO patients.
- The diagnosis of leptospirosis may initially be based on clinical suspicion in patients with classic features and exposures, noting the high specificity of conjunctival suffusion, and initial titers may be nondiagnostic; therefore, empiric treatment should be considered when clinical suspicion is high.
- Increased interest in urban chicken farming in the United States, with associated higher rodent density, may represent a newly recognized risk factor for human leptospirosis infection.
Disclosures
The authors report no conflicts of interest.
Fever in an elderly man is a nonspecific finding, occurring most commonly with infections but also with certain malignancies, rheumatologic disorders, and drug exposures. The complaint of rigors with diaphoresis makes an infection most likely. The acuity of his illness makes infections with more chronic presentations such as tuberculosis or actinomycosis less likely. The presence of frontal headache might suggest a sinus or brain source, but headache also occurs in generalized infections such as pneumonia, bacteremia from any cause, malaria, rickettsial infections, viral illnesses, and others. Additional history should include detailed inquiry into travel, vocational, and avocational exposures.
The patient's difficulty standing implies the development of lower extremity weakness and infections associated with neurological syndromes. His leg weakness may be related to early Guillain‐Barre syndrome, which is associated most commonly with Campylobacter jejuni, but also other bacteria and viruses such as Haemophilus influenza, Mycoplasma pneumonia, Influenza virus, Cytomegalovirus and hepatitis E. Other viral infections associated with pure motor deficits include echovirus, coxsackie virus, enterovirus, and West Nile virus (WNV). The paralytic syndrome associated with enteroviruses is more common in children, whereas the neuroinvasive variant of WNV more often affects the elderly and can be associated with encephalitis as well as a flaccid paralysis. Although acute paralytic shellfish poisoning could account for both his weakness and his acute gastrointestinal syndrome, this diagnosis is unlikely because the symptoms often have a prominent sensory component, and there is usually the history of recent ingestion of the suspect bivalves. Like all adults presenting for medical care, he should be screened for human immunodeficiency virus (HIV) infection; if testing is positive, the differential diagnosis for his current illness broadens significantly. Finally, he may have a spinal cord disorder or infection such as an epidural abscess, or transverse myelitis, which would present with lower extremity weakness and fever. It would be helpful to know the time of year of his illness, exposure to mosquito bites, his neurological exam findings, and results of blood and stool cultures. If the patient had signs of meningitis or encephalitis, cerebral spinal fluid analysis would be helpful. If his neurological exam was suggestive of cord involvement, it would be helpful to know the results of magnetic resonance imaging of the spinal cord.
The patient's past medical history includes relatively common problems for a 73‐year‐old man and does not substantially influence the differential diagnosis of his current illness. His travel history to Uganda a year previously may be relevant, because malaria (Plasmodium vivax) could present with fever and weakness. Less commonly, African trypanosomiasis (Trypanosoma brucei gambiense) can, in the late phase, present with fever and malaise, but also typically includes symptoms of encephalitis, including depressed mental status, confusion, ataxia, and possibly personality changes. His travel to Zurich should not impose any particular infection risk, unless he was hiking in the mountains around Zurich, where he could have contracted tick‐borne encephalitis; however, his travel more than 6 months prior to presentation makes this unlikely. Lyme disease due to Borrelia burgdorferi is also a potential exposure in the Swiss mountains, and can present with fever in the acute phase, as well as arthritis with chronic disease, but should not cause fever, rigor, diaphoresis, and headache many months later. Summering in Cape Cod puts him at risk for babesiosis, but an incubation period of 5 months is too long. Keeping chickens places him at risk for Salmonella exposure and typhoid fever. Ingesting raw oysters carries a risk for shellfish poisoning and Vibrio infections, but the incubation period (1 month) again seems too long to cause his current symptoms.
Notable physical findings are an ill‐appearing man with injected sclera and a high fever but normal blood pressure and heart rate. He also demonstrates proximal lower extremity weakness manifested by difficulty rising from a chair and a slow gait with short strides and deliberate (possibly on‐block) turning. His neurological exam is most consistent with Parkinsonian symptoms that have been described in patients with severe influenza A, which would explain all of his other symptoms as well. Pulse‐temperature dissociation is classically described with typhoid fever but usually occurs later in the disease course, and could be masked by the patient's metoprolol. Typhoid fever can also be associated with neurological symptoms including meningitis and movement disorders.
The patient has a remarkable bandemia, suggesting a bacterial infection, as well as a slight reduction in hematocrit and platelet count. Additionally, his labs revealed a mild transaminitis, but with significantly elevated alkaline phosphatase and GGT, and microscopic hematuria. His ferritin is significantly elevated, which may simply represent an acute phase reactant. Infections associated with hepatitis, cytopenias, and hematuria include sepsis with disseminated intravascular coagulation, previously mentioned malaria, leptospirosis, dengue, ehrlichiosis, and rickettsial diseases, but he has no special risks for these infections, and other aspects of his illness (Parkinsonian features, bandemia) do not fit. His lung findings with hematuria might suggest a pulmonary/renal syndrome, but, once again, other features of his illness are not typical of these syndromes. Salmonella (typhoid fever) or influenza, now complicated by an early bacterial pneumonia, are viable possibilities.
The patient's ongoing clinical course is notable for a nontoxic (non‐SIRS) appearance but continued high‐grade fever with blood and urine cultures that are sterile. This argues against a common bacteremia with sepsis, and for either relapsing malaria (P vivax), influenza with a Parkinsonian‐like illness, typhoid fever, leptospirosis, dengue, or a rickettsial infection. Mycoplasma pneumonia is also possible given the atypical chest x‐ray appearance, slightly low hematocrit with elevated bilirubin, and neurological symptoms that may represent ataxia.
The subsequent negative laboratory tests listed are helpful in likely excluding many of the diagnoses suggested such as malaria, Babesia, common bacteremias, viral hepatitis, HIV, and WNV. Furthermore, the new history of mouse exposure brings to the forefront rodent‐associated infections, specifically exposure to mouse urine, a vehicle for leptospirosis. The patient's hepatitis, anemia, thrombocytopenia, scleral injection, along with the rest of his symptoms in the context of exposure to mouse urine makes leptospirosis the likely diagnosis. A negative Leptospira antibody early in his illness does not rule out the disease, and a convalescent titer should be obtained to confirm the diagnosis.
COMMENTARY
This case describes an elderly man who presented with a fever of unknown origin (FUO), and was eventually diagnosed with leptospirosis. FUO presents slightly differently in elderly patients, as elderly patients are less likely to mount a high fever, and when they do, the etiology is more likely to indicate a serious bacterial or viral infection. Additionally, an etiology for FUO in the elderly is found in over 70% of presenting cases, compared to 51% in patients under the age of 65 years.[1] A detailed, comprehensive social, travel, and exposure history and physical examination remains the cornerstone of elucidating the diagnosis for FUO. The exposure to mouse urine in this case was an unusual and a helpful piece of the history to further focus the differential diagnosis.
Leptospirosis is an emerging bacterial zoonosis, and causes both endemic and epidemic severe multisystem disease. The Leptospira spirochete is maintained in nature through a chronic renal infection in mammalian reservoir hosts, such as mice,[2, 4] and is transmitted through direct or aerosolized contact with infected urine or tissue. After a mean incubation period of 10 days, a variety of clinical manifestations may be seen. In this case, the patient's clinical presentation revealed many classic symptoms of leptospirosis, including fevers, rigors, headache, lower extremity myalgias, nausea, vomiting, and diarrhea; however, these symptoms are nonspecific. The presence of a conjunctival suffusion in leptospirosis infection had a specificity of 98% in a high‐incidence cohort of febrile patients in Sri Lanka,[3] and was an important diagnostic clue in this case. Leptospirosis is a self‐limited illness in most patients, with an initial septicemic, febrile phase followed by an immune phase. A more severe presentation may be seen in the immune phase of the illness, which includes renal and hepatic dysfunction (known as Weil's disease), as well as cardiac, pulmonary, and central nervous system abnormalities. With a 14% case fatality rate, the risk of death has been shown to be higher in patients over 40 years old, with altered mental status and multiorgan failure.[4]
The early diagnosis of leptospirosis relies heavily on physical exam findings and epidemiologic history. In this case, the patient's laboratory abnormalities, including immature granulocytes, thrombocytopenia, hyponatremia, hypokalemia, mild hepatitis, and pyuria with granular casts are all reported with leptospirosis infection2; however, independently, these laboratory findings are nonspecific. Patients may not have a detectable antibody levels in the acute phase of the disease. In this case, given the strong clinical suspicion based on the findings of conjunctival suffusion and exposure to mouse urine history, the lack of a more plausible alternate diagnosis, and known delay in antibody positivity, the patient was treated empirically with doxycycline for presumed leptospirosis.[5] Forthcoming novel diagnostic strategies such as next‐generation DNA sequencing techniques may provide real‐time diagnosis of this zoonotic infection, thus decreasing the window period between empirical antimicrobial coverage and diagnostic confirmation.[6]
Leptospirosis is prevalent in tropical climates and has been associated with impoverished communities.[7] Urban slums, with poor sanitation and high rodent density, are an ideal environment for leptospirosis. The reported risk of infection in a Brazilian slum was as high as 3% per year.[8] Additionally, rodent sightings, as well as the presence of chickens, were risk factors for leptospirosis transmission in urban slums.[9] Correspondingly in this case, we hypothesize that the patient's interest in urban farming, specifically the chickens he kept, likely attracted the mice infected with leptospirosis. Urban chicken farming is becoming increasingly popular in the United States,[10] and may be a developing risk factor for human leptospirosis infection. Leptospirosis is one of many emerging zoonoses, such as avian influenza, tick‐borne illness, and ebola, resulting from changing human ecology. Thus, when considering infectious etiologies, clinicians should ask patients about vocational and avocational exposures, including new trends such as urban farming, which may expose them to previously underappreciated illnesses.
TEACHING POINTS
- Elderly patients with a FUO are more likely to be diagnosed with an underlying serious bacterial or viral infection when compared to a younger cohort of FUO patients.
- The diagnosis of leptospirosis may initially be based on clinical suspicion in patients with classic features and exposures, noting the high specificity of conjunctival suffusion, and initial titers may be nondiagnostic; therefore, empiric treatment should be considered when clinical suspicion is high.
- Increased interest in urban chicken farming in the United States, with associated higher rodent density, may represent a newly recognized risk factor for human leptospirosis infection.
Disclosures
The authors report no conflicts of interest.
- , , . Fever of unknown origin in older persons. Infect Dis Clin North Am. 2007;21(4):937–945.
- . Leptospirosis. Clin Microbiol Rev. 2011;14(2):296–326.
- , , , et al. Leptospirosis as frequent cause of acute febrile illness in southern Sri Lanka. Emerg Infect Dis. 2011;17(9):1678–1684.
- , , , et al. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Philadelphia, PA: Elsevier; 2010.
- , . Antibiotics for leptospirosis. The Cochrane Database Syst Rev. 2012;2:CD008264.
- , , , et al. Actionable diagnosis of neuroleptospirosis by next‐generation sequencing. N Engl J Med. 2014;370(25):2408–2417.
- , , , . Cases distribution of leptospirosis in City of Manaus, State of Amazonas, Brazil, 2000–2010. Rev Soc Bras Med Trop. 2012;45(6):713–716.
- , , , et al. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the leptospira agent. PLoS Negl Trop Dis. 2014;8(5):e2927.
- , , , et al. Impact of environment and social gradient on leptospira infection in urban slums. PLoS Negl Trop Dis. 2008;2(4):e228.
- Urban chicken ownership in four U.S. cities. United States Department of Agriculture website. Available at: http://www.aphis.usda.gov/animal_health/nahms/poultry/downloads/poultry10/Poultry10_dr_Urban_Chicken_Four.pdf. Published April 2013. Accessed June 9, 2015.
- , , . Fever of unknown origin in older persons. Infect Dis Clin North Am. 2007;21(4):937–945.
- . Leptospirosis. Clin Microbiol Rev. 2011;14(2):296–326.
- , , , et al. Leptospirosis as frequent cause of acute febrile illness in southern Sri Lanka. Emerg Infect Dis. 2011;17(9):1678–1684.
- , , , et al. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Philadelphia, PA: Elsevier; 2010.
- , . Antibiotics for leptospirosis. The Cochrane Database Syst Rev. 2012;2:CD008264.
- , , , et al. Actionable diagnosis of neuroleptospirosis by next‐generation sequencing. N Engl J Med. 2014;370(25):2408–2417.
- , , , . Cases distribution of leptospirosis in City of Manaus, State of Amazonas, Brazil, 2000–2010. Rev Soc Bras Med Trop. 2012;45(6):713–716.
- , , , et al. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the leptospira agent. PLoS Negl Trop Dis. 2014;8(5):e2927.
- , , , et al. Impact of environment and social gradient on leptospira infection in urban slums. PLoS Negl Trop Dis. 2008;2(4):e228.
- Urban chicken ownership in four U.S. cities. United States Department of Agriculture website. Available at: http://www.aphis.usda.gov/animal_health/nahms/poultry/downloads/poultry10/Poultry10_dr_Urban_Chicken_Four.pdf. Published April 2013. Accessed June 9, 2015.