User login
Delving Deeper
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 32-year-old, previously healthy woman presented to the emergency department (ED) with 3 days of nasal pain, congestion, and cough. A day prior, she had consulted with her primary care provider by phone and had been prescribed amoxicillin-clavulanate for presumed bacterial sinusitis. She subsequently developed fever (39 oC) and pleuritic, left-upper-quadrant abdominal pain. In the ED, chest radiograph demonstrated right hilar opacification. Laboratory studies and computed tomography (CT) of the abdomen and pelvis did not identify a cause for her pain. Given the pleuritic nature of her left-upper-quadrant pain, CT pulmonary angiography was ordered. The CT revealed “mass-like” right hilar opacification and lymphadenopathy. No pulmonary emboli were identified. Levofloxacin was prescribed for presumed pneumonia, and the patient was discharged home. The following week, mediastinal biopsy was arranged for evaluation of the right hilar abnormality.
This is a young woman presenting with upper respiratory symptoms, abdominal pain, fever, and hilar lymphadenopathy. Upper respiratory symptoms are common and usually indicate an inflammatory response to allergens or infection, though autoimmune disorders may affect the upper airways. Fever and hilar lymphadenopathy likely also signify an inflammatory response. Taken together, these findings can be associated with mycobacterial or fungal infection, malignancy, and, particularly in a young woman, sarcoidosis, which could explain her abdominal pain if her presentation included splenomegaly. At this point she likely has a systemic illness involving at least the upper, and possibly the lower, respiratory tract.
Within days, her symptoms resolved. Mediastinal biopsy of the hilar node revealed scant pus. Pathology demonstrated suppurative granulomata. Gram stain; bacterial, mycobacterial, and fungal cultures; and 16S ribosomal analyses for bacteria and fungi from the biopsy were unrevealing. For unclear reasons, prior to the biopsy, she was given intramuscular Haemophilus influenzae type B and tetanus, diphtheria, and pertussis vaccines. Two weeks later, she presented again with fever and left-upper-quadrant pain as well as painful skin nodules at her biopsy and vaccination sites. She was admitted for further evaluation. Chest CT showed expansion of the mediastinal lesion and splenic enlargement. Biopsy of a skin lesion revealed suppurative granulomatous dermatitis and panniculitis. Repeat blood cultures were negative, though serum β-D-glucan was weakly positive at 173 pg/mL (reference range, <60 pg/mL). Tissue cultures and Gram, acid-fast, Fite, and Warthin-Starry stains from the skin biopsy were negative. She was discharged on fluconazole and then readmitted 2 days later with dyspnea, fever, and leukocytosis.
The young woman’s symptoms resolved, only to recur days later; her granulomatous hilar lesions grew larger, and new cutaneous and splenic findings appeared. The granulomatous lesions prompt consideration of infectious, malignant, and immune-mediated processes. The negative cultures make infection less likely, although the elevated β-D-glucan may suggest fungal infection. By description, the skin lesions are consistent with pathergy, a phenomenon characterized by trauma-provoked cutaneous lesions or ulcers, which is associated with numerous syndromes, including Behçet syndrome, inflammatory bowel disease, and neutrophilic dermatoses such as pyoderma gangrenosum (PG) and Sweet syndrome. In addition to details about her medical history, it is important to seek evidence of oral ulcers or vasculitis, as Behçet syndrome may be associated with cutaneous, visceral, and ophthalmologic vasculitis.
Her medical history included hypertension and active, 10-pack-year cigarette use. During childhood, she had occasional ingrown hairs and folliculitis. She did not take medications prior to this acute illness. Family history was notable for cardiovascular disease. She rarely consumed alcohol and did not use illicit drugs. She lived in a rural town in the mid–Willamette Valley of Oregon and worked as an administrative assistant. She spent time outdoors, including trail running and golfing. A case of tularemia was recently reported in an area near her home. Her only travel outside of Oregon was to Puerto Vallarta, Mexico, 16 years previously. She grew up on a farm and had no known tuberculosis exposure.
Tularemia is an interesting diagnostic consideration and could explain her fever, cutaneous lesions, and hilar adenopathy. It is plausible that she had clinically mild pneumonic tularemia at the outset and that her cutaneous lesions are variants of ulceroglandular tularemia. Positive antibodies for Francisella tularensis would be expected if this were the cause of her illness. The ingrown hairs raise the possibility of a primary immune deficiency syndrome predisposing her to abscesses. However, they seem to have been of trivial significance to her, making an immune deficiency syndrome unlikely.
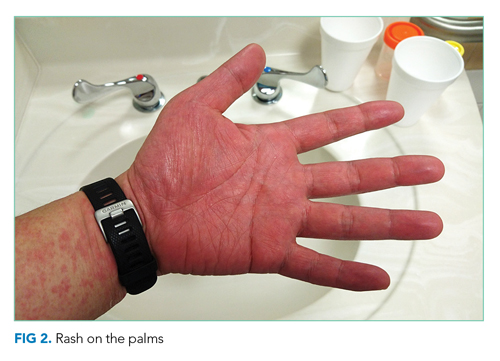
On readmission, she was afebrile, normotensive, and tachycardic (114 beats/min), with a normal respiratory rate and oxygen saturation. She was not ill appearing. She had noninjected conjunctiva and no oral lesions. Apart from tachycardia, cardiovascular examination was unremarkable. Abdominal examination was notable for mild distension and a palpable, tender spleen. Musculoskeletal and neurologic examinations were normal. Her skin was notable for various sized (8 cm × 4 cm to 10 cm × 15 cm) painful ulcers with violaceous, friable borders—some with fluctuance and purulent drainage—on her right hand, bilateral arms, right axilla, sternum, and legs (Figure 1).
Laboratory studies were notable for normocytic anemia (hemoglobin, 8.9 g/dL; range, 12.0-16.0 g/dL), leukocytosis (white blood cells, 24,900/µL; range, 4500-11,000/µL), thrombocytosis (platelet count, 690,000/µL; range, 150,000-400,000/µL), and elevated inflammatory markers (C-reactive protein, 33 mg/dL; range, <0.5 mg/dL; erythrocyte sedimentation rate, 78 mm/h; range, <20 mm/h). A complete metabolic panel was within normal limits. Repeat blood cultures and β -D-glucan and 16S ribosomal assays were negative. Polymerase chain reaction testing for Bartonella henselae was negative. Urine probes for Neisseria gonorrhoeae and Chlamydia trachomatis were negative. Rapid plasma regain (RPR) was negative. Antibodies to toxoplasmosis, histoplasmosis, blastomycosis, and aspergillosis were unrevealing. A Coccidioides test by immunodiffusion was negative. Serum antigen tests for Cryptococcus and Epstein-Barr virus (EBV) were negative. EBV, HIV, and hepatitis antibody tests were negative. Rheumatologic studies, including antinuclear, anti-double-stranded DNA, anti-Smith, anti–Sjögren syndrome antigens A and B, anticentromere, anti-topoisomerase (anti-Scl-70), anti-histidyl-transfer-RNA-synthetase (anti-Jo-1), and anti-nucleosome (anti-chromatic) antibodies, were unrevealing. Levels of angiotensin-converting enzyme, rheumatoid factor, complement, cytoplasmic, and perinuclear antineutrophil cytoplasmic antibodies were also normal. A neutrophil oxidative burst test was negative. In addition, peripheral flow cytology and serum and urine protein electrophoresis were negative. Chest CT revealed bilateral lower lobe consolidations concerning for necrotizing pneumonia, splenic enlargement, numerous hypodense splenic lesions, and a 1.3-cm right hilar node, which had decreased in size compared with 1 month prior.
In summary, the patient presented with recurrent upper respiratory symptoms, fever, and abdominal pain; expanding granulomatous hilar lesions, splenomegaly, and cutaneous lesions consistent with pathergy; elevated inflammatory markers and leukocytosis; and a possible exposure to F tularensis. She has had extensive negative infectious workups, except for a weakly positive β-D-glucan, and completed several courses of apparently unhelpful antimicrobials. At this point, the most notable findings are her splenomegaly and inflammatory masses suggesting an inflammatory process, which may be autoimmune in nature. Both vasculitis and sarcoidosis remain possibilities, and malignancy is possible. Given her possible exposure to F tularensis, obtaining serum antibodies to F tularensis, in addition to biopsies of the skin lesions, is advisable.
Laboratory studies revealed a positive F tularensis antibody with a titer of 1:320 and an IgM of 7 U/mL and IgG of 30 U/mL. This was repeated, revealing a titer of 1:540 and an IgM and IgG of 5 U/mL and 20 U/mL, respectively. Given the potential exposure history, the clinical syndrome compatible with tularemia, and an otherwise extensive yet unrevealing evaluation, she was treated with a 10-day course of streptomycin. Her fever persisted, and the splenic lesions increased in size and number, prompting addition of moxifloxacin without apparent benefit. Skin biopsies taken from the patient’s arm were notable for nodular, suppurative, neutrophilic infiltrates and histiocytes in the medium and deep dermis without multinucleated histiocytes or evidence of vasculitis. Fungal, mycobacterial, and bacterial stains from the biopsy were negative. The findings were consistent with but not diagnostic of an acute neutrophilic dermatosis.
At this point, the patient has a confirmed exposure to F tularensis; she also has persistent fever, progressive splenomegaly, and new skin biopsies consistent with neutrophilic dermatosis. Despite the F tularensis antibody positivity, her negative cultures and lack of improvement with multiple courses of antimicrobials argue against an infectious etiology. Accordingly, malignancy should be considered but seems less likely given that no laboratory, imaging, or tissue samples support it. This leaves immune-mediated etiologies, especially autoimmune conditions associated with neutrophilic dermatoses, as the most likely explanation of her inflammatory syndrome. Neutrophilic dermatoses include some vasculitides, Sweet syndrome, PG, Behçet syndrome, and other inflammatory entities. She has no evidence of vasculitis on biopsy. Given the evidence of inflammation and the history of pathergy, Behçet syndrome and PG should be seriously considered.
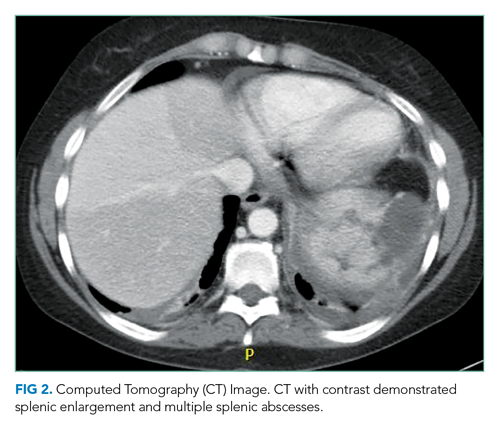
She underwent incision and drainage of the left leg and mediastinal lesions. A follow-up chest CT revealed stable cutaneous and deep tissue lesions and continued splenic enlargement. She was started on prednisone and dapsone for presumed cutaneous and visceral PG. The lesions improved dramatically and, following a month-long hospitalization, she was discharged on dapsone and a slow prednisone taper. Three weeks after discharge, while on dapsone and prednisone, she developed a new skin lesion. Cyclosporine was added, with improvement. Eight weeks after discharge, she developed fever, acute left-upper-quadrant pain, and marked splenomegaly with abscesses seen on CT imaging (Figure 2).
This continues to be a very puzzling case, and it is worth revisiting her clinical course once again. This is a previously healthy 32-year-old woman with multiple hospital presentations for upper-respiratory symptoms, persistent fever, abdominal pain, and painful cutaneous lesions consistent with pathergy; she was found to have granulomatous hilar lesions, progressive splenomegaly, and skin biopsies consistent with neutrophilic dermatosis. Exhaustive infectious and rheumatologic workup was negative, and no evident malignancy was found. Finally, despite multiple courses of antimicrobials, including standard treatments for tularemia (for which she had positive antibodies), her clinical course failed to improve until the addition of systemic anti-inflammatory agents, which resulted in rapid improvement. She then presented 8 weeks later with recurrent fever and splenomegaly. Given the recurrence and the severity of the splenic pathology, a diagnostic splenectomy is advisable for what appears to be visceral PG. In addition, attempting to identify a trigger of her syndrome is important. PG can be associated with inflammatory bowel disease, hematologic disorders (eg, leukemia, myeloma, myelodysplastic syndrome, and myelofibrosis), and autoimmune diseases, especially inflammatory arthritis.1 Therefore, a diagnostic colonoscopy and bone marrow biopsy should be considered. With no history or examination supporting inflammatory arthritis and a broad, unrevealing workup, her rheumatologic evaluation is sufficient.
The patient underwent splenectomy. Gross description of the spleen was notable for multiple abscesses, consisting on microscopy of large areas of necrosis with islands of dense neutrophil collections (Figure 3). Microscopic examination failed to demonstrate microorganisms on multiple stains, and there was no microscopic or flow cytometric evidence of lymphoma. The final pathologic diagnosis was multiple sterile splenic abscesses with siderosis, which, in the context of her overall syndrome, was consistent with an entity termed aseptic abscess syndrome (AAS). After discharge, she underwent a slow steroid taper and was ultimately maintained on daily low-dose prednisone. Cyclosporine and dapsone were discontinued in favor of infliximab infusions. She underwent additional diagnostic workup, including an unremarkable colonoscopy and a bone marrow biopsy, which showed monoclonal gammopathy of undetermined significance (MGUS) with an insignificant IgA monoclonal gammopathy. All cutaneous lesions healed. Three years after the splenectomy, while still on infliximab and prednisone, she developed a new aseptic lung abscess, which resolved after increasing her prednisone dose. Six years after splenectomy, she developed an aseptic liver abscess, which resolved after again increasing the frequency of her infliximab infusions.
DISCUSSION
Diagnostic uncertainty is an intrinsic feature of medical practice—in part because patients often present with undifferentiated and evolving symptoms.2 When faced with uncertainty, clinicians are well served by prioritizing a thoughtful differential diagnosis, adopting a stepwise management strategy, and engaging in iterative reassessments of the patient. In this case, a 32-year-old, previously healthy woman presented with an array of symptoms, including abdominal pain, fever, leukocytosis, necrotic skin lesions, necrotizing mediastinal lymphadenitis, pathergy, and splenomegaly. Elements of the history, examination, and diagnostic studies supported a differential diagnosis of tularemia, PG, and AAS. Through stepwise management and ongoing reassessment, she was ultimately diagnosed with AAS.
Tularemia was initially an important diagnostic consideration in this patient, given her potential exposure and positive F tularensis serum antibodies. Francisella tularensis is a Gram-negative coccobacillus found in more than 250 species of fish, ticks, birds, and mammals. In humans, an incubation period of 3 to 5 days is typical. Although clinical manifestations vary, they often include fever, headache, and malaise.3 Other findings may include lymphadenopathy with or without ulcerative cutaneous lesions (glandular or ulceroglandular tularemia) and cough, dyspnea, pleuritic chest pain, and hilar adenopathy (pneumonic tularemia). As noted by the discussant, a pneumonic tularemia syndrome could have explained this patient’s fever, respiratory symptoms, and hilar adenopathy; ulceroglandular tularemia might have explained her cutaneous lesions. Since splenomegaly may be seen in tularemia, this finding was also consistent with the diagnosis. Serum antibody testing is supportive of the diagnosis, while culture confirms it. Standard treatment consists of a 10- to 14-day course of streptomycin, and combination therapy with a fluoroquinolone is recommended in severe cases.4 In this patient, however, F tularensis was not demonstrated on culture. Furthermore, she did not experience the expected clinical improvement with treatment. Finally, because both IgG and IgM tularemia antibodies may co-occur up to 10 years following infection, her positive F tularensis serum antibodies did not provide evidence of acute infection.5
Recognizing inconsistencies in the diagnosis of tularemia, the focus shifted to PG owing to the patient’s neutrophilic cutaneous lesions, negative infectious workup, and pathergy. Pyoderma gangrenosum is a neutrophilic dermatosis—one of a heterogeneous group of skin conditions characterized by perivascular and diffuse neutrophilic infiltrates without an identifiable infectious agent.6 It is a chronic, recurrent cutaneous disease with several variants.7 The classic presentation includes painful lower-extremity ulcers with violaceous undermined borders and may be associated with pathergy. Guiding principles for the management of PG include controlling inflammation, optimizing wound healing, and minimizing exacerbating factors.1 As such, treatment mainstays include local and systemic anti-inflammatory agents and wound care. As the discussant highlighted, in this case the inflammatory skin lesions were suggestive of PG. However, other features of the case, notably, splenomegaly, splenic abscesses, and necrotizing mediastinal lymphadenitis, were more consistent with another diagnosis: AAS. Aseptic abscess syndrome is an autoinflammatory disorder defined by deep, noninfectious abscesses that preferentially affect the spleen.8 Additional clinical manifestations include weight loss, fever, abdominal pain, and leukocytosis. Lesions may also affect bone, kidney, liver, lung, lymph node, and skin. In one case series, neutrophilic dermatoses were seen in 20% of AAS cases.8 In all cases of AAS, extensive infectious workup is unrevealing, and antibiotics are ineffective. The pathophysiology of AAS is unknown.
Similar to PG, the majority of AAS cases are associated with inflammatory bowel disease, especially Crohn disease.9 However, AAS also has associations with conditions such as MGUS, rheumatoid arthritis, spondyloarthritis, and relapsing polychondritis. Histologically, early lesions demonstrate a necrotic core of neutrophils, with or without surrounding palisading histiocytes, and giant cells. In older lesions, neutrophils may be absent; fibrous tissue may be present.8 Treatment regimens include splenectomy, corticosteroids, colchicine, thalidomide, tumor necrosis factor (TNF) antagonists, and cyclophosphamide. The discussant astutely recommended a splenectomy for this patient, which was both diagnostic and therapeutic. As in this case, relapse is common. Optimal maintenance therapy is yet to be determined.9
Given the overlapping clinical manifestations, shared disease associations, and similar responsiveness to immunosuppression, it is unclear whether AAS represents a new disease entity or a variant of known autoinflammatory disorders. Aseptic abscess syndrome is likely part of a spectrum of autoinflammatory disorders with inflammatory bowel diseases, neutrophilic dermatoses, and other similar diseases.8 While infectious visceral abscesses remain more common, this case highlights the clinical manifestation of an emerging and likely underrecognized entity.
TEACHING POINTS
- Aseptic abscess syndrome should be considered in patients who present with visceral (particularly splenic) abscesses and negative infectious workup.
- Aseptic abscess syndrome is commonly associated with other autoinflammatory disorders; the majority of reported cases are associated with inflammatory bowel disease, especially Crohn disease.
- Up to 20% of AAS cases are associated with neutrophilic dermatoses such as PG.
- The initial treatment for this syndrome is high-dose intravenous glucocorticoids; maintenance treatment regimens include corticosteroids, colchicine, thalidomide, TNF antagonists, and cyclophosphamide.
Acknowledgments
The authors would thank Dr Bob Pelz and Dr John Townes for their contributions to the case.
1. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191-211. https://doi.org/10.2165/11595240-000000000-00000
2. Bhise V, Rajan SS, Sittig DF, Morgan RO, Chaudhary P, Singh H. Defining and measuring diagnostic uncertainty in medicine: a systematic review. J Gen Intern Med. 2018;33(1):103-115. https://doi.org/10.1007/s11606-017-4164-1
3. Penn RL. Francisella tualerensis (Tularemia). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Elsevier Saunders; 2015:2590-2602.
4. Eliasson H, Broman T, Forsman M, Bäck E. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am. 2006;20(2):289-311. https://doi.org/10.1016/j.idc.2006.03.002
5. Bevanger L, Maeland JA, Kvan AI. Comparative analysis of antibodies to Francisella tularensis antigens during the acute phase of tularemia and eight years later. Clin Diagn Lab Immunol. 1994;1(2):238-240.
6. Moschella SL, Davis MDP. Neutrophilic dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Saunders; 2012:424-438.
7. Dabade TS, Davis MDP. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet’s syndrome). Dermatol Ther. 2011;24(2):273-284. https://doi/org/10.1111/j.1529-8019.2011.01403.x
8. André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86(3):145-161. https://doi/org/10.1097/md.0b013e18064f9f3
9. Fillman H, Riquelme P, Sullivan PD, Mansoor AM. Aseptic abscess syndrome. BMJ Case Rep. 2020;13(10):e236437. https://doi.org/10.1136/bcr-2020-236437
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 32-year-old, previously healthy woman presented to the emergency department (ED) with 3 days of nasal pain, congestion, and cough. A day prior, she had consulted with her primary care provider by phone and had been prescribed amoxicillin-clavulanate for presumed bacterial sinusitis. She subsequently developed fever (39 oC) and pleuritic, left-upper-quadrant abdominal pain. In the ED, chest radiograph demonstrated right hilar opacification. Laboratory studies and computed tomography (CT) of the abdomen and pelvis did not identify a cause for her pain. Given the pleuritic nature of her left-upper-quadrant pain, CT pulmonary angiography was ordered. The CT revealed “mass-like” right hilar opacification and lymphadenopathy. No pulmonary emboli were identified. Levofloxacin was prescribed for presumed pneumonia, and the patient was discharged home. The following week, mediastinal biopsy was arranged for evaluation of the right hilar abnormality.
This is a young woman presenting with upper respiratory symptoms, abdominal pain, fever, and hilar lymphadenopathy. Upper respiratory symptoms are common and usually indicate an inflammatory response to allergens or infection, though autoimmune disorders may affect the upper airways. Fever and hilar lymphadenopathy likely also signify an inflammatory response. Taken together, these findings can be associated with mycobacterial or fungal infection, malignancy, and, particularly in a young woman, sarcoidosis, which could explain her abdominal pain if her presentation included splenomegaly. At this point she likely has a systemic illness involving at least the upper, and possibly the lower, respiratory tract.
Within days, her symptoms resolved. Mediastinal biopsy of the hilar node revealed scant pus. Pathology demonstrated suppurative granulomata. Gram stain; bacterial, mycobacterial, and fungal cultures; and 16S ribosomal analyses for bacteria and fungi from the biopsy were unrevealing. For unclear reasons, prior to the biopsy, she was given intramuscular Haemophilus influenzae type B and tetanus, diphtheria, and pertussis vaccines. Two weeks later, she presented again with fever and left-upper-quadrant pain as well as painful skin nodules at her biopsy and vaccination sites. She was admitted for further evaluation. Chest CT showed expansion of the mediastinal lesion and splenic enlargement. Biopsy of a skin lesion revealed suppurative granulomatous dermatitis and panniculitis. Repeat blood cultures were negative, though serum β-D-glucan was weakly positive at 173 pg/mL (reference range, <60 pg/mL). Tissue cultures and Gram, acid-fast, Fite, and Warthin-Starry stains from the skin biopsy were negative. She was discharged on fluconazole and then readmitted 2 days later with dyspnea, fever, and leukocytosis.
The young woman’s symptoms resolved, only to recur days later; her granulomatous hilar lesions grew larger, and new cutaneous and splenic findings appeared. The granulomatous lesions prompt consideration of infectious, malignant, and immune-mediated processes. The negative cultures make infection less likely, although the elevated β-D-glucan may suggest fungal infection. By description, the skin lesions are consistent with pathergy, a phenomenon characterized by trauma-provoked cutaneous lesions or ulcers, which is associated with numerous syndromes, including Behçet syndrome, inflammatory bowel disease, and neutrophilic dermatoses such as pyoderma gangrenosum (PG) and Sweet syndrome. In addition to details about her medical history, it is important to seek evidence of oral ulcers or vasculitis, as Behçet syndrome may be associated with cutaneous, visceral, and ophthalmologic vasculitis.
Her medical history included hypertension and active, 10-pack-year cigarette use. During childhood, she had occasional ingrown hairs and folliculitis. She did not take medications prior to this acute illness. Family history was notable for cardiovascular disease. She rarely consumed alcohol and did not use illicit drugs. She lived in a rural town in the mid–Willamette Valley of Oregon and worked as an administrative assistant. She spent time outdoors, including trail running and golfing. A case of tularemia was recently reported in an area near her home. Her only travel outside of Oregon was to Puerto Vallarta, Mexico, 16 years previously. She grew up on a farm and had no known tuberculosis exposure.
Tularemia is an interesting diagnostic consideration and could explain her fever, cutaneous lesions, and hilar adenopathy. It is plausible that she had clinically mild pneumonic tularemia at the outset and that her cutaneous lesions are variants of ulceroglandular tularemia. Positive antibodies for Francisella tularensis would be expected if this were the cause of her illness. The ingrown hairs raise the possibility of a primary immune deficiency syndrome predisposing her to abscesses. However, they seem to have been of trivial significance to her, making an immune deficiency syndrome unlikely.
On readmission, she was afebrile, normotensive, and tachycardic (114 beats/min), with a normal respiratory rate and oxygen saturation. She was not ill appearing. She had noninjected conjunctiva and no oral lesions. Apart from tachycardia, cardiovascular examination was unremarkable. Abdominal examination was notable for mild distension and a palpable, tender spleen. Musculoskeletal and neurologic examinations were normal. Her skin was notable for various sized (8 cm × 4 cm to 10 cm × 15 cm) painful ulcers with violaceous, friable borders—some with fluctuance and purulent drainage—on her right hand, bilateral arms, right axilla, sternum, and legs (Figure 1).
Laboratory studies were notable for normocytic anemia (hemoglobin, 8.9 g/dL; range, 12.0-16.0 g/dL), leukocytosis (white blood cells, 24,900/µL; range, 4500-11,000/µL), thrombocytosis (platelet count, 690,000/µL; range, 150,000-400,000/µL), and elevated inflammatory markers (C-reactive protein, 33 mg/dL; range, <0.5 mg/dL; erythrocyte sedimentation rate, 78 mm/h; range, <20 mm/h). A complete metabolic panel was within normal limits. Repeat blood cultures and β -D-glucan and 16S ribosomal assays were negative. Polymerase chain reaction testing for Bartonella henselae was negative. Urine probes for Neisseria gonorrhoeae and Chlamydia trachomatis were negative. Rapid plasma regain (RPR) was negative. Antibodies to toxoplasmosis, histoplasmosis, blastomycosis, and aspergillosis were unrevealing. A Coccidioides test by immunodiffusion was negative. Serum antigen tests for Cryptococcus and Epstein-Barr virus (EBV) were negative. EBV, HIV, and hepatitis antibody tests were negative. Rheumatologic studies, including antinuclear, anti-double-stranded DNA, anti-Smith, anti–Sjögren syndrome antigens A and B, anticentromere, anti-topoisomerase (anti-Scl-70), anti-histidyl-transfer-RNA-synthetase (anti-Jo-1), and anti-nucleosome (anti-chromatic) antibodies, were unrevealing. Levels of angiotensin-converting enzyme, rheumatoid factor, complement, cytoplasmic, and perinuclear antineutrophil cytoplasmic antibodies were also normal. A neutrophil oxidative burst test was negative. In addition, peripheral flow cytology and serum and urine protein electrophoresis were negative. Chest CT revealed bilateral lower lobe consolidations concerning for necrotizing pneumonia, splenic enlargement, numerous hypodense splenic lesions, and a 1.3-cm right hilar node, which had decreased in size compared with 1 month prior.
In summary, the patient presented with recurrent upper respiratory symptoms, fever, and abdominal pain; expanding granulomatous hilar lesions, splenomegaly, and cutaneous lesions consistent with pathergy; elevated inflammatory markers and leukocytosis; and a possible exposure to F tularensis. She has had extensive negative infectious workups, except for a weakly positive β-D-glucan, and completed several courses of apparently unhelpful antimicrobials. At this point, the most notable findings are her splenomegaly and inflammatory masses suggesting an inflammatory process, which may be autoimmune in nature. Both vasculitis and sarcoidosis remain possibilities, and malignancy is possible. Given her possible exposure to F tularensis, obtaining serum antibodies to F tularensis, in addition to biopsies of the skin lesions, is advisable.
Laboratory studies revealed a positive F tularensis antibody with a titer of 1:320 and an IgM of 7 U/mL and IgG of 30 U/mL. This was repeated, revealing a titer of 1:540 and an IgM and IgG of 5 U/mL and 20 U/mL, respectively. Given the potential exposure history, the clinical syndrome compatible with tularemia, and an otherwise extensive yet unrevealing evaluation, she was treated with a 10-day course of streptomycin. Her fever persisted, and the splenic lesions increased in size and number, prompting addition of moxifloxacin without apparent benefit. Skin biopsies taken from the patient’s arm were notable for nodular, suppurative, neutrophilic infiltrates and histiocytes in the medium and deep dermis without multinucleated histiocytes or evidence of vasculitis. Fungal, mycobacterial, and bacterial stains from the biopsy were negative. The findings were consistent with but not diagnostic of an acute neutrophilic dermatosis.
At this point, the patient has a confirmed exposure to F tularensis; she also has persistent fever, progressive splenomegaly, and new skin biopsies consistent with neutrophilic dermatosis. Despite the F tularensis antibody positivity, her negative cultures and lack of improvement with multiple courses of antimicrobials argue against an infectious etiology. Accordingly, malignancy should be considered but seems less likely given that no laboratory, imaging, or tissue samples support it. This leaves immune-mediated etiologies, especially autoimmune conditions associated with neutrophilic dermatoses, as the most likely explanation of her inflammatory syndrome. Neutrophilic dermatoses include some vasculitides, Sweet syndrome, PG, Behçet syndrome, and other inflammatory entities. She has no evidence of vasculitis on biopsy. Given the evidence of inflammation and the history of pathergy, Behçet syndrome and PG should be seriously considered.
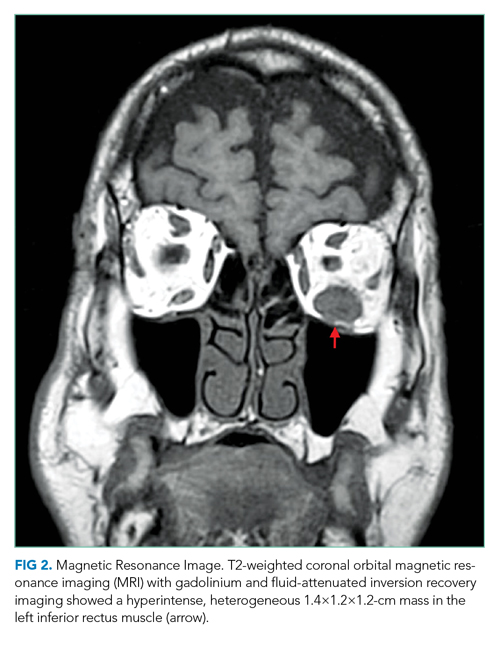
She underwent incision and drainage of the left leg and mediastinal lesions. A follow-up chest CT revealed stable cutaneous and deep tissue lesions and continued splenic enlargement. She was started on prednisone and dapsone for presumed cutaneous and visceral PG. The lesions improved dramatically and, following a month-long hospitalization, she was discharged on dapsone and a slow prednisone taper. Three weeks after discharge, while on dapsone and prednisone, she developed a new skin lesion. Cyclosporine was added, with improvement. Eight weeks after discharge, she developed fever, acute left-upper-quadrant pain, and marked splenomegaly with abscesses seen on CT imaging (Figure 2).
This continues to be a very puzzling case, and it is worth revisiting her clinical course once again. This is a previously healthy 32-year-old woman with multiple hospital presentations for upper-respiratory symptoms, persistent fever, abdominal pain, and painful cutaneous lesions consistent with pathergy; she was found to have granulomatous hilar lesions, progressive splenomegaly, and skin biopsies consistent with neutrophilic dermatosis. Exhaustive infectious and rheumatologic workup was negative, and no evident malignancy was found. Finally, despite multiple courses of antimicrobials, including standard treatments for tularemia (for which she had positive antibodies), her clinical course failed to improve until the addition of systemic anti-inflammatory agents, which resulted in rapid improvement. She then presented 8 weeks later with recurrent fever and splenomegaly. Given the recurrence and the severity of the splenic pathology, a diagnostic splenectomy is advisable for what appears to be visceral PG. In addition, attempting to identify a trigger of her syndrome is important. PG can be associated with inflammatory bowel disease, hematologic disorders (eg, leukemia, myeloma, myelodysplastic syndrome, and myelofibrosis), and autoimmune diseases, especially inflammatory arthritis.1 Therefore, a diagnostic colonoscopy and bone marrow biopsy should be considered. With no history or examination supporting inflammatory arthritis and a broad, unrevealing workup, her rheumatologic evaluation is sufficient.
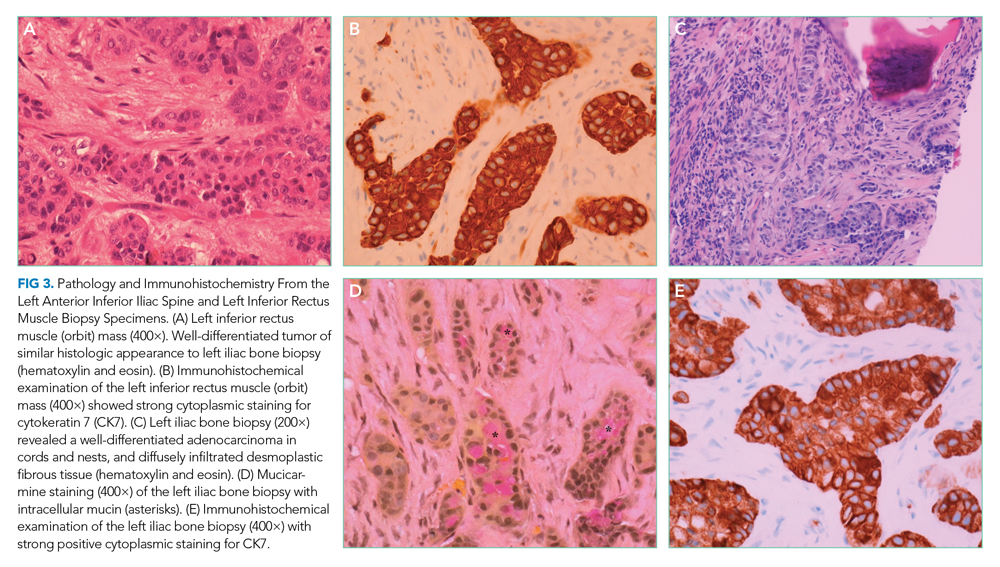
The patient underwent splenectomy. Gross description of the spleen was notable for multiple abscesses, consisting on microscopy of large areas of necrosis with islands of dense neutrophil collections (Figure 3). Microscopic examination failed to demonstrate microorganisms on multiple stains, and there was no microscopic or flow cytometric evidence of lymphoma. The final pathologic diagnosis was multiple sterile splenic abscesses with siderosis, which, in the context of her overall syndrome, was consistent with an entity termed aseptic abscess syndrome (AAS). After discharge, she underwent a slow steroid taper and was ultimately maintained on daily low-dose prednisone. Cyclosporine and dapsone were discontinued in favor of infliximab infusions. She underwent additional diagnostic workup, including an unremarkable colonoscopy and a bone marrow biopsy, which showed monoclonal gammopathy of undetermined significance (MGUS) with an insignificant IgA monoclonal gammopathy. All cutaneous lesions healed. Three years after the splenectomy, while still on infliximab and prednisone, she developed a new aseptic lung abscess, which resolved after increasing her prednisone dose. Six years after splenectomy, she developed an aseptic liver abscess, which resolved after again increasing the frequency of her infliximab infusions.
DISCUSSION
Diagnostic uncertainty is an intrinsic feature of medical practice—in part because patients often present with undifferentiated and evolving symptoms.2 When faced with uncertainty, clinicians are well served by prioritizing a thoughtful differential diagnosis, adopting a stepwise management strategy, and engaging in iterative reassessments of the patient. In this case, a 32-year-old, previously healthy woman presented with an array of symptoms, including abdominal pain, fever, leukocytosis, necrotic skin lesions, necrotizing mediastinal lymphadenitis, pathergy, and splenomegaly. Elements of the history, examination, and diagnostic studies supported a differential diagnosis of tularemia, PG, and AAS. Through stepwise management and ongoing reassessment, she was ultimately diagnosed with AAS.
Tularemia was initially an important diagnostic consideration in this patient, given her potential exposure and positive F tularensis serum antibodies. Francisella tularensis is a Gram-negative coccobacillus found in more than 250 species of fish, ticks, birds, and mammals. In humans, an incubation period of 3 to 5 days is typical. Although clinical manifestations vary, they often include fever, headache, and malaise.3 Other findings may include lymphadenopathy with or without ulcerative cutaneous lesions (glandular or ulceroglandular tularemia) and cough, dyspnea, pleuritic chest pain, and hilar adenopathy (pneumonic tularemia). As noted by the discussant, a pneumonic tularemia syndrome could have explained this patient’s fever, respiratory symptoms, and hilar adenopathy; ulceroglandular tularemia might have explained her cutaneous lesions. Since splenomegaly may be seen in tularemia, this finding was also consistent with the diagnosis. Serum antibody testing is supportive of the diagnosis, while culture confirms it. Standard treatment consists of a 10- to 14-day course of streptomycin, and combination therapy with a fluoroquinolone is recommended in severe cases.4 In this patient, however, F tularensis was not demonstrated on culture. Furthermore, she did not experience the expected clinical improvement with treatment. Finally, because both IgG and IgM tularemia antibodies may co-occur up to 10 years following infection, her positive F tularensis serum antibodies did not provide evidence of acute infection.5
Recognizing inconsistencies in the diagnosis of tularemia, the focus shifted to PG owing to the patient’s neutrophilic cutaneous lesions, negative infectious workup, and pathergy. Pyoderma gangrenosum is a neutrophilic dermatosis—one of a heterogeneous group of skin conditions characterized by perivascular and diffuse neutrophilic infiltrates without an identifiable infectious agent.6 It is a chronic, recurrent cutaneous disease with several variants.7 The classic presentation includes painful lower-extremity ulcers with violaceous undermined borders and may be associated with pathergy. Guiding principles for the management of PG include controlling inflammation, optimizing wound healing, and minimizing exacerbating factors.1 As such, treatment mainstays include local and systemic anti-inflammatory agents and wound care. As the discussant highlighted, in this case the inflammatory skin lesions were suggestive of PG. However, other features of the case, notably, splenomegaly, splenic abscesses, and necrotizing mediastinal lymphadenitis, were more consistent with another diagnosis: AAS. Aseptic abscess syndrome is an autoinflammatory disorder defined by deep, noninfectious abscesses that preferentially affect the spleen.8 Additional clinical manifestations include weight loss, fever, abdominal pain, and leukocytosis. Lesions may also affect bone, kidney, liver, lung, lymph node, and skin. In one case series, neutrophilic dermatoses were seen in 20% of AAS cases.8 In all cases of AAS, extensive infectious workup is unrevealing, and antibiotics are ineffective. The pathophysiology of AAS is unknown.
Similar to PG, the majority of AAS cases are associated with inflammatory bowel disease, especially Crohn disease.9 However, AAS also has associations with conditions such as MGUS, rheumatoid arthritis, spondyloarthritis, and relapsing polychondritis. Histologically, early lesions demonstrate a necrotic core of neutrophils, with or without surrounding palisading histiocytes, and giant cells. In older lesions, neutrophils may be absent; fibrous tissue may be present.8 Treatment regimens include splenectomy, corticosteroids, colchicine, thalidomide, tumor necrosis factor (TNF) antagonists, and cyclophosphamide. The discussant astutely recommended a splenectomy for this patient, which was both diagnostic and therapeutic. As in this case, relapse is common. Optimal maintenance therapy is yet to be determined.9
Given the overlapping clinical manifestations, shared disease associations, and similar responsiveness to immunosuppression, it is unclear whether AAS represents a new disease entity or a variant of known autoinflammatory disorders. Aseptic abscess syndrome is likely part of a spectrum of autoinflammatory disorders with inflammatory bowel diseases, neutrophilic dermatoses, and other similar diseases.8 While infectious visceral abscesses remain more common, this case highlights the clinical manifestation of an emerging and likely underrecognized entity.
TEACHING POINTS
- Aseptic abscess syndrome should be considered in patients who present with visceral (particularly splenic) abscesses and negative infectious workup.
- Aseptic abscess syndrome is commonly associated with other autoinflammatory disorders; the majority of reported cases are associated with inflammatory bowel disease, especially Crohn disease.
- Up to 20% of AAS cases are associated with neutrophilic dermatoses such as PG.
- The initial treatment for this syndrome is high-dose intravenous glucocorticoids; maintenance treatment regimens include corticosteroids, colchicine, thalidomide, TNF antagonists, and cyclophosphamide.
Acknowledgments
The authors would thank Dr Bob Pelz and Dr John Townes for their contributions to the case.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 32-year-old, previously healthy woman presented to the emergency department (ED) with 3 days of nasal pain, congestion, and cough. A day prior, she had consulted with her primary care provider by phone and had been prescribed amoxicillin-clavulanate for presumed bacterial sinusitis. She subsequently developed fever (39 oC) and pleuritic, left-upper-quadrant abdominal pain. In the ED, chest radiograph demonstrated right hilar opacification. Laboratory studies and computed tomography (CT) of the abdomen and pelvis did not identify a cause for her pain. Given the pleuritic nature of her left-upper-quadrant pain, CT pulmonary angiography was ordered. The CT revealed “mass-like” right hilar opacification and lymphadenopathy. No pulmonary emboli were identified. Levofloxacin was prescribed for presumed pneumonia, and the patient was discharged home. The following week, mediastinal biopsy was arranged for evaluation of the right hilar abnormality.
This is a young woman presenting with upper respiratory symptoms, abdominal pain, fever, and hilar lymphadenopathy. Upper respiratory symptoms are common and usually indicate an inflammatory response to allergens or infection, though autoimmune disorders may affect the upper airways. Fever and hilar lymphadenopathy likely also signify an inflammatory response. Taken together, these findings can be associated with mycobacterial or fungal infection, malignancy, and, particularly in a young woman, sarcoidosis, which could explain her abdominal pain if her presentation included splenomegaly. At this point she likely has a systemic illness involving at least the upper, and possibly the lower, respiratory tract.
Within days, her symptoms resolved. Mediastinal biopsy of the hilar node revealed scant pus. Pathology demonstrated suppurative granulomata. Gram stain; bacterial, mycobacterial, and fungal cultures; and 16S ribosomal analyses for bacteria and fungi from the biopsy were unrevealing. For unclear reasons, prior to the biopsy, she was given intramuscular Haemophilus influenzae type B and tetanus, diphtheria, and pertussis vaccines. Two weeks later, she presented again with fever and left-upper-quadrant pain as well as painful skin nodules at her biopsy and vaccination sites. She was admitted for further evaluation. Chest CT showed expansion of the mediastinal lesion and splenic enlargement. Biopsy of a skin lesion revealed suppurative granulomatous dermatitis and panniculitis. Repeat blood cultures were negative, though serum β-D-glucan was weakly positive at 173 pg/mL (reference range, <60 pg/mL). Tissue cultures and Gram, acid-fast, Fite, and Warthin-Starry stains from the skin biopsy were negative. She was discharged on fluconazole and then readmitted 2 days later with dyspnea, fever, and leukocytosis.
The young woman’s symptoms resolved, only to recur days later; her granulomatous hilar lesions grew larger, and new cutaneous and splenic findings appeared. The granulomatous lesions prompt consideration of infectious, malignant, and immune-mediated processes. The negative cultures make infection less likely, although the elevated β-D-glucan may suggest fungal infection. By description, the skin lesions are consistent with pathergy, a phenomenon characterized by trauma-provoked cutaneous lesions or ulcers, which is associated with numerous syndromes, including Behçet syndrome, inflammatory bowel disease, and neutrophilic dermatoses such as pyoderma gangrenosum (PG) and Sweet syndrome. In addition to details about her medical history, it is important to seek evidence of oral ulcers or vasculitis, as Behçet syndrome may be associated with cutaneous, visceral, and ophthalmologic vasculitis.
Her medical history included hypertension and active, 10-pack-year cigarette use. During childhood, she had occasional ingrown hairs and folliculitis. She did not take medications prior to this acute illness. Family history was notable for cardiovascular disease. She rarely consumed alcohol and did not use illicit drugs. She lived in a rural town in the mid–Willamette Valley of Oregon and worked as an administrative assistant. She spent time outdoors, including trail running and golfing. A case of tularemia was recently reported in an area near her home. Her only travel outside of Oregon was to Puerto Vallarta, Mexico, 16 years previously. She grew up on a farm and had no known tuberculosis exposure.
Tularemia is an interesting diagnostic consideration and could explain her fever, cutaneous lesions, and hilar adenopathy. It is plausible that she had clinically mild pneumonic tularemia at the outset and that her cutaneous lesions are variants of ulceroglandular tularemia. Positive antibodies for Francisella tularensis would be expected if this were the cause of her illness. The ingrown hairs raise the possibility of a primary immune deficiency syndrome predisposing her to abscesses. However, they seem to have been of trivial significance to her, making an immune deficiency syndrome unlikely.
On readmission, she was afebrile, normotensive, and tachycardic (114 beats/min), with a normal respiratory rate and oxygen saturation. She was not ill appearing. She had noninjected conjunctiva and no oral lesions. Apart from tachycardia, cardiovascular examination was unremarkable. Abdominal examination was notable for mild distension and a palpable, tender spleen. Musculoskeletal and neurologic examinations were normal. Her skin was notable for various sized (8 cm × 4 cm to 10 cm × 15 cm) painful ulcers with violaceous, friable borders—some with fluctuance and purulent drainage—on her right hand, bilateral arms, right axilla, sternum, and legs (Figure 1).
Laboratory studies were notable for normocytic anemia (hemoglobin, 8.9 g/dL; range, 12.0-16.0 g/dL), leukocytosis (white blood cells, 24,900/µL; range, 4500-11,000/µL), thrombocytosis (platelet count, 690,000/µL; range, 150,000-400,000/µL), and elevated inflammatory markers (C-reactive protein, 33 mg/dL; range, <0.5 mg/dL; erythrocyte sedimentation rate, 78 mm/h; range, <20 mm/h). A complete metabolic panel was within normal limits. Repeat blood cultures and β -D-glucan and 16S ribosomal assays were negative. Polymerase chain reaction testing for Bartonella henselae was negative. Urine probes for Neisseria gonorrhoeae and Chlamydia trachomatis were negative. Rapid plasma regain (RPR) was negative. Antibodies to toxoplasmosis, histoplasmosis, blastomycosis, and aspergillosis were unrevealing. A Coccidioides test by immunodiffusion was negative. Serum antigen tests for Cryptococcus and Epstein-Barr virus (EBV) were negative. EBV, HIV, and hepatitis antibody tests were negative. Rheumatologic studies, including antinuclear, anti-double-stranded DNA, anti-Smith, anti–Sjögren syndrome antigens A and B, anticentromere, anti-topoisomerase (anti-Scl-70), anti-histidyl-transfer-RNA-synthetase (anti-Jo-1), and anti-nucleosome (anti-chromatic) antibodies, were unrevealing. Levels of angiotensin-converting enzyme, rheumatoid factor, complement, cytoplasmic, and perinuclear antineutrophil cytoplasmic antibodies were also normal. A neutrophil oxidative burst test was negative. In addition, peripheral flow cytology and serum and urine protein electrophoresis were negative. Chest CT revealed bilateral lower lobe consolidations concerning for necrotizing pneumonia, splenic enlargement, numerous hypodense splenic lesions, and a 1.3-cm right hilar node, which had decreased in size compared with 1 month prior.
In summary, the patient presented with recurrent upper respiratory symptoms, fever, and abdominal pain; expanding granulomatous hilar lesions, splenomegaly, and cutaneous lesions consistent with pathergy; elevated inflammatory markers and leukocytosis; and a possible exposure to F tularensis. She has had extensive negative infectious workups, except for a weakly positive β-D-glucan, and completed several courses of apparently unhelpful antimicrobials. At this point, the most notable findings are her splenomegaly and inflammatory masses suggesting an inflammatory process, which may be autoimmune in nature. Both vasculitis and sarcoidosis remain possibilities, and malignancy is possible. Given her possible exposure to F tularensis, obtaining serum antibodies to F tularensis, in addition to biopsies of the skin lesions, is advisable.
Laboratory studies revealed a positive F tularensis antibody with a titer of 1:320 and an IgM of 7 U/mL and IgG of 30 U/mL. This was repeated, revealing a titer of 1:540 and an IgM and IgG of 5 U/mL and 20 U/mL, respectively. Given the potential exposure history, the clinical syndrome compatible with tularemia, and an otherwise extensive yet unrevealing evaluation, she was treated with a 10-day course of streptomycin. Her fever persisted, and the splenic lesions increased in size and number, prompting addition of moxifloxacin without apparent benefit. Skin biopsies taken from the patient’s arm were notable for nodular, suppurative, neutrophilic infiltrates and histiocytes in the medium and deep dermis without multinucleated histiocytes or evidence of vasculitis. Fungal, mycobacterial, and bacterial stains from the biopsy were negative. The findings were consistent with but not diagnostic of an acute neutrophilic dermatosis.
At this point, the patient has a confirmed exposure to F tularensis; she also has persistent fever, progressive splenomegaly, and new skin biopsies consistent with neutrophilic dermatosis. Despite the F tularensis antibody positivity, her negative cultures and lack of improvement with multiple courses of antimicrobials argue against an infectious etiology. Accordingly, malignancy should be considered but seems less likely given that no laboratory, imaging, or tissue samples support it. This leaves immune-mediated etiologies, especially autoimmune conditions associated with neutrophilic dermatoses, as the most likely explanation of her inflammatory syndrome. Neutrophilic dermatoses include some vasculitides, Sweet syndrome, PG, Behçet syndrome, and other inflammatory entities. She has no evidence of vasculitis on biopsy. Given the evidence of inflammation and the history of pathergy, Behçet syndrome and PG should be seriously considered.
She underwent incision and drainage of the left leg and mediastinal lesions. A follow-up chest CT revealed stable cutaneous and deep tissue lesions and continued splenic enlargement. She was started on prednisone and dapsone for presumed cutaneous and visceral PG. The lesions improved dramatically and, following a month-long hospitalization, she was discharged on dapsone and a slow prednisone taper. Three weeks after discharge, while on dapsone and prednisone, she developed a new skin lesion. Cyclosporine was added, with improvement. Eight weeks after discharge, she developed fever, acute left-upper-quadrant pain, and marked splenomegaly with abscesses seen on CT imaging (Figure 2).
This continues to be a very puzzling case, and it is worth revisiting her clinical course once again. This is a previously healthy 32-year-old woman with multiple hospital presentations for upper-respiratory symptoms, persistent fever, abdominal pain, and painful cutaneous lesions consistent with pathergy; she was found to have granulomatous hilar lesions, progressive splenomegaly, and skin biopsies consistent with neutrophilic dermatosis. Exhaustive infectious and rheumatologic workup was negative, and no evident malignancy was found. Finally, despite multiple courses of antimicrobials, including standard treatments for tularemia (for which she had positive antibodies), her clinical course failed to improve until the addition of systemic anti-inflammatory agents, which resulted in rapid improvement. She then presented 8 weeks later with recurrent fever and splenomegaly. Given the recurrence and the severity of the splenic pathology, a diagnostic splenectomy is advisable for what appears to be visceral PG. In addition, attempting to identify a trigger of her syndrome is important. PG can be associated with inflammatory bowel disease, hematologic disorders (eg, leukemia, myeloma, myelodysplastic syndrome, and myelofibrosis), and autoimmune diseases, especially inflammatory arthritis.1 Therefore, a diagnostic colonoscopy and bone marrow biopsy should be considered. With no history or examination supporting inflammatory arthritis and a broad, unrevealing workup, her rheumatologic evaluation is sufficient.
The patient underwent splenectomy. Gross description of the spleen was notable for multiple abscesses, consisting on microscopy of large areas of necrosis with islands of dense neutrophil collections (Figure 3). Microscopic examination failed to demonstrate microorganisms on multiple stains, and there was no microscopic or flow cytometric evidence of lymphoma. The final pathologic diagnosis was multiple sterile splenic abscesses with siderosis, which, in the context of her overall syndrome, was consistent with an entity termed aseptic abscess syndrome (AAS). After discharge, she underwent a slow steroid taper and was ultimately maintained on daily low-dose prednisone. Cyclosporine and dapsone were discontinued in favor of infliximab infusions. She underwent additional diagnostic workup, including an unremarkable colonoscopy and a bone marrow biopsy, which showed monoclonal gammopathy of undetermined significance (MGUS) with an insignificant IgA monoclonal gammopathy. All cutaneous lesions healed. Three years after the splenectomy, while still on infliximab and prednisone, she developed a new aseptic lung abscess, which resolved after increasing her prednisone dose. Six years after splenectomy, she developed an aseptic liver abscess, which resolved after again increasing the frequency of her infliximab infusions.
DISCUSSION
Diagnostic uncertainty is an intrinsic feature of medical practice—in part because patients often present with undifferentiated and evolving symptoms.2 When faced with uncertainty, clinicians are well served by prioritizing a thoughtful differential diagnosis, adopting a stepwise management strategy, and engaging in iterative reassessments of the patient. In this case, a 32-year-old, previously healthy woman presented with an array of symptoms, including abdominal pain, fever, leukocytosis, necrotic skin lesions, necrotizing mediastinal lymphadenitis, pathergy, and splenomegaly. Elements of the history, examination, and diagnostic studies supported a differential diagnosis of tularemia, PG, and AAS. Through stepwise management and ongoing reassessment, she was ultimately diagnosed with AAS.
Tularemia was initially an important diagnostic consideration in this patient, given her potential exposure and positive F tularensis serum antibodies. Francisella tularensis is a Gram-negative coccobacillus found in more than 250 species of fish, ticks, birds, and mammals. In humans, an incubation period of 3 to 5 days is typical. Although clinical manifestations vary, they often include fever, headache, and malaise.3 Other findings may include lymphadenopathy with or without ulcerative cutaneous lesions (glandular or ulceroglandular tularemia) and cough, dyspnea, pleuritic chest pain, and hilar adenopathy (pneumonic tularemia). As noted by the discussant, a pneumonic tularemia syndrome could have explained this patient’s fever, respiratory symptoms, and hilar adenopathy; ulceroglandular tularemia might have explained her cutaneous lesions. Since splenomegaly may be seen in tularemia, this finding was also consistent with the diagnosis. Serum antibody testing is supportive of the diagnosis, while culture confirms it. Standard treatment consists of a 10- to 14-day course of streptomycin, and combination therapy with a fluoroquinolone is recommended in severe cases.4 In this patient, however, F tularensis was not demonstrated on culture. Furthermore, she did not experience the expected clinical improvement with treatment. Finally, because both IgG and IgM tularemia antibodies may co-occur up to 10 years following infection, her positive F tularensis serum antibodies did not provide evidence of acute infection.5
Recognizing inconsistencies in the diagnosis of tularemia, the focus shifted to PG owing to the patient’s neutrophilic cutaneous lesions, negative infectious workup, and pathergy. Pyoderma gangrenosum is a neutrophilic dermatosis—one of a heterogeneous group of skin conditions characterized by perivascular and diffuse neutrophilic infiltrates without an identifiable infectious agent.6 It is a chronic, recurrent cutaneous disease with several variants.7 The classic presentation includes painful lower-extremity ulcers with violaceous undermined borders and may be associated with pathergy. Guiding principles for the management of PG include controlling inflammation, optimizing wound healing, and minimizing exacerbating factors.1 As such, treatment mainstays include local and systemic anti-inflammatory agents and wound care. As the discussant highlighted, in this case the inflammatory skin lesions were suggestive of PG. However, other features of the case, notably, splenomegaly, splenic abscesses, and necrotizing mediastinal lymphadenitis, were more consistent with another diagnosis: AAS. Aseptic abscess syndrome is an autoinflammatory disorder defined by deep, noninfectious abscesses that preferentially affect the spleen.8 Additional clinical manifestations include weight loss, fever, abdominal pain, and leukocytosis. Lesions may also affect bone, kidney, liver, lung, lymph node, and skin. In one case series, neutrophilic dermatoses were seen in 20% of AAS cases.8 In all cases of AAS, extensive infectious workup is unrevealing, and antibiotics are ineffective. The pathophysiology of AAS is unknown.
Similar to PG, the majority of AAS cases are associated with inflammatory bowel disease, especially Crohn disease.9 However, AAS also has associations with conditions such as MGUS, rheumatoid arthritis, spondyloarthritis, and relapsing polychondritis. Histologically, early lesions demonstrate a necrotic core of neutrophils, with or without surrounding palisading histiocytes, and giant cells. In older lesions, neutrophils may be absent; fibrous tissue may be present.8 Treatment regimens include splenectomy, corticosteroids, colchicine, thalidomide, tumor necrosis factor (TNF) antagonists, and cyclophosphamide. The discussant astutely recommended a splenectomy for this patient, which was both diagnostic and therapeutic. As in this case, relapse is common. Optimal maintenance therapy is yet to be determined.9
Given the overlapping clinical manifestations, shared disease associations, and similar responsiveness to immunosuppression, it is unclear whether AAS represents a new disease entity or a variant of known autoinflammatory disorders. Aseptic abscess syndrome is likely part of a spectrum of autoinflammatory disorders with inflammatory bowel diseases, neutrophilic dermatoses, and other similar diseases.8 While infectious visceral abscesses remain more common, this case highlights the clinical manifestation of an emerging and likely underrecognized entity.
TEACHING POINTS
- Aseptic abscess syndrome should be considered in patients who present with visceral (particularly splenic) abscesses and negative infectious workup.
- Aseptic abscess syndrome is commonly associated with other autoinflammatory disorders; the majority of reported cases are associated with inflammatory bowel disease, especially Crohn disease.
- Up to 20% of AAS cases are associated with neutrophilic dermatoses such as PG.
- The initial treatment for this syndrome is high-dose intravenous glucocorticoids; maintenance treatment regimens include corticosteroids, colchicine, thalidomide, TNF antagonists, and cyclophosphamide.
Acknowledgments
The authors would thank Dr Bob Pelz and Dr John Townes for their contributions to the case.
1. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191-211. https://doi.org/10.2165/11595240-000000000-00000
2. Bhise V, Rajan SS, Sittig DF, Morgan RO, Chaudhary P, Singh H. Defining and measuring diagnostic uncertainty in medicine: a systematic review. J Gen Intern Med. 2018;33(1):103-115. https://doi.org/10.1007/s11606-017-4164-1
3. Penn RL. Francisella tualerensis (Tularemia). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Elsevier Saunders; 2015:2590-2602.
4. Eliasson H, Broman T, Forsman M, Bäck E. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am. 2006;20(2):289-311. https://doi.org/10.1016/j.idc.2006.03.002
5. Bevanger L, Maeland JA, Kvan AI. Comparative analysis of antibodies to Francisella tularensis antigens during the acute phase of tularemia and eight years later. Clin Diagn Lab Immunol. 1994;1(2):238-240.
6. Moschella SL, Davis MDP. Neutrophilic dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Saunders; 2012:424-438.
7. Dabade TS, Davis MDP. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet’s syndrome). Dermatol Ther. 2011;24(2):273-284. https://doi/org/10.1111/j.1529-8019.2011.01403.x
8. André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86(3):145-161. https://doi/org/10.1097/md.0b013e18064f9f3
9. Fillman H, Riquelme P, Sullivan PD, Mansoor AM. Aseptic abscess syndrome. BMJ Case Rep. 2020;13(10):e236437. https://doi.org/10.1136/bcr-2020-236437
1. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191-211. https://doi.org/10.2165/11595240-000000000-00000
2. Bhise V, Rajan SS, Sittig DF, Morgan RO, Chaudhary P, Singh H. Defining and measuring diagnostic uncertainty in medicine: a systematic review. J Gen Intern Med. 2018;33(1):103-115. https://doi.org/10.1007/s11606-017-4164-1
3. Penn RL. Francisella tualerensis (Tularemia). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Elsevier Saunders; 2015:2590-2602.
4. Eliasson H, Broman T, Forsman M, Bäck E. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am. 2006;20(2):289-311. https://doi.org/10.1016/j.idc.2006.03.002
5. Bevanger L, Maeland JA, Kvan AI. Comparative analysis of antibodies to Francisella tularensis antigens during the acute phase of tularemia and eight years later. Clin Diagn Lab Immunol. 1994;1(2):238-240.
6. Moschella SL, Davis MDP. Neutrophilic dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Saunders; 2012:424-438.
7. Dabade TS, Davis MDP. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet’s syndrome). Dermatol Ther. 2011;24(2):273-284. https://doi/org/10.1111/j.1529-8019.2011.01403.x
8. André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86(3):145-161. https://doi/org/10.1097/md.0b013e18064f9f3
9. Fillman H, Riquelme P, Sullivan PD, Mansoor AM. Aseptic abscess syndrome. BMJ Case Rep. 2020;13(10):e236437. https://doi.org/10.1136/bcr-2020-236437
© 2021 Society of Hospital Medicine
Falling Through the Cracks
A 61-year-old man presented to the emergency department (ED) for persistent headache that began after he fell in his bathroom 4 days earlier. He described the headache as generalized and constant, rating the severity as a 5 on a scale of 0 to 10. The patient denied any associated neck pain or changes in headache quality with position change. He reported a 3-day history of nausea and four episodes of vomiting.
Headache after a fall raises concern for intracranial hemorrhage, particularly if this patient is on anticoagulant or antiplatelet medications. Subdural hematoma (SDH) would be more likely than epidural or subarachnoid hematoma (SAH) given the duration of days without progression. While nausea and vomiting are nonspecific, persistent vomiting may indicate increased intracranial pressure (eg, from an intracranial mass or SDH), particularly if provoked by positional changes. Without a history of fever or neck stiffness, meningitis is less likely unless the patient has a history of immunosuppression. Secondary causes of headache include vascular etiologies (eg, hemorrhagic cerebrovascular accident [CVA], arterial dissection, aneurysm, vasculitis), systemic causes (eg, chronic hypoxia/hypercapnia, hypertension), or medication overuse or withdrawal. In this patient, traumatic head injury with resultant postconcussive symptoms, though a diagnosis of exclusion, should also be considered. If the patient has a history of migraines, it is essential to obtain a history of typical migraine symptoms. More information regarding the mechanism of the fall is also essential to help elucidate a potential cause.
The patient had a 1-year history of recurrent loss of consciousness resulting in falls. After each fall, he quickly regained consciousness and exhibited no residual deficits or confusion. These episodes occurred suddenly when the patient was performing normal daily activities such as walking, driving, doing light chores, and standing up from a seated position. Immediately before this most recent fall, the patient stood up from a chair, walked toward the bathroom and, without any warning signs, lost consciousness. He denied dizziness, lightheadedness, nausea, or diaphoresis immediately before or after the fall. He also reported experiencing intermittent palpitations, but these did not appear to be related to the syncopal episodes. He denied experiencing chest pain, shortness of breath, or seizures.
The differential diagnosis for syncope is broad; therefore, it is important to identify features that suggest an etiology requiring urgent management. In this patient, cardiac etiologies such as arrhythmia (eg, atrial fibrillation [AF], ventricular tachycardia, heart block), ischemia, heart failure, and structural heart disease (eg, valvular abnormalities, cardiomyopathies) must be considered. His complaints of intermittent palpitations could suggest arrhythmia; however, the absence of a correlation to the syncopal episodes and other associated cardiac symptoms makes arrhythmias such as AF less likely. Medication side effects provoking cardiac conduction disturbances, heart block, or hypotension should be considered. Ischemic heart disease and heart failure are possible causes despite the absence of chest pain and dyspnea. While the exertional nature of the patient’s symptoms could support cardiac etiologies, it could also be indicative of recurrent pulmonary embolism or right ventricular dysfunction/strain, such as chronic thromboembolic pulmonary hypertension (CTEPH).
Neurologic causes of syncope should also be included in the differential diagnosis. Seizure is less likely the underlying cause in this case since the patient regained consciousness quickly after each episode and reported no residual deficits, confusion, incontinence, or oral trauma. While less likely, other neurovascular causes can be considered, including transient ischemic attack (TIA), CVA, SAH, or vertebrobasilar insufficiency.
Neurocardiogenic syncope is less likely due to lack of a clear trigger or classical prodromal symptoms. Without a history of volume loss, orthostatic syncope is also unlikely. Other possibilities include adrenal insufficiency or an autonomic dysfunction resulting from diabetic neuropathy, chronic kidney disease, amyloidosis, spinal cord injury, or neurologic diseases (eg, Parkinson disease, Lewy body dementia). Thus far, the provided history is not suggestive of these etiologies. Other causes for loss of consciousness include hypoglycemia, sleep disorders (eg, narcolepsy), or psychiatric causes.
About 10 months prior to this presentation, the patient had presented to the hospital for evaluation of headache and was found to have bilateral SDH requiring burr hole evacuation. At that time, he was on anticoagulation therapy for a history of left superficial femoral vein thrombosis with negative workup for hypercoagulability. Warfarin was discontinued after the SDH was diagnosed. Regarding the patient’s social history, although he reported drinking two glasses of wine with dinner each night and smoking marijuana afterward, all syncopal events occurred during the daytime.
The history of prior SDH should raise suspicion for recurrent SDH, particularly considering the patient’s ongoing alcohol use. History of deep vein thrombosis (DVT) and possible exertional syncope might suggest recurrent pulmonary embolism or CTEPH as an etiology. DVT and TIA/CVA secondary to paradoxical embolism are also possible. Depending on extent of alcohol use, intoxication and cardiomyopathy with secondary arrhythmias are possibilities.
The basic workup should focus on identifying any acute intracranial processes that may explain the patient’s presentation and evaluating for syncope. This includes a complete blood count with differential, electrolytes, hepatic panel (based on patient’s history of alcohol use), and coagulation studies. Troponins and B-type natriuretic peptide would help assess for cardiac disease, and a urine/serum drug test would be beneficial to screen for substance use. Considering the patient’s prior history of SDH, head imaging should be obtained. If the patient were to exhibit focal neurologic deficits or persistent alterations in consciousness (thereby raising the index of suspicion for TIA or CVA), perfusion/diffusion-weighted magnetic resonance imaging (MRI) studies should be obtained. If obtaining a brain MRI is not practical, then a computed tomography angiogram (CTA) of the head and neck should be obtained. A noncontrast head CT would be sufficient to reveal the presence of SDH. An electroencephalogram (EEG) to assess for seizure should be performed if the patient is noted to have any focal neurologic findings or complaints consistent with seizure. With possible exertional syncope, an electrocardiogram (ECG) and transthoracic echocardiogram (with bubble study to assess for patent foramen ovale) should be obtained urgently.
The patient had a history of hypertension and irritable bowel syndrome, for which he took metoprolol and duloxetine, respectively. Eight months prior to the current ED presentation, he was admitted to the hospital for a syncope workup after falling and sustaining a fractured jaw and torn rotator cuff. ECG and continuous telemetry monitoring showed normal sinus rhythm, normal intervals, and rare episodes of sinus tachycardia, but no evidence of arrhythmia. An echocardiogram demonstrated normal ejection fraction and chamber sizes; CT and MRI of the brain showed no residual SDH; and EEG monitoring showed no seizure activity. It was determined that the patient’s syncopal episodes were multifactorial; possible etiologies included episodic hypotension from irritable bowel syndrome—related diarrhea, paroxysmal arrhythmias, and ongoing substance use.
The patient was discharged home with a 14-day Holter monitor. Rare episodes of AF (total burden 0.4%) were detected, and dronedarone was prescribed for rhythm control; he remained off anticoagulation therapy due to the history of SDH. Over the next few months, cardiology, electrophysiology, and neurology consultants concluded that paroxysmal AF was the likely etiology of the patient’s syncopal episodes. The patient was considered high risk for CVA, but the risk of bleeding from syncope-related falls was too high to resume anticoagulation therapy.
One month prior to the current ED presentation, the patient underwent a left atrial appendage closure with a WATCHMAN implant to avoid long-term anticoagulation. After the procedure, he was started on warfarin with plans to permanently discontinue anticoagulation after 6 to 8 weeks of completed therapy. He had been on warfarin for 3 weeks prior the most recent fall and current ED visit.At the time of this presentation, the patient was on dronedarone, duloxetine, metoprolol, and warfarin. On exam, he was alert and in no distress. His temperature was 36.8 °C, heart rate 98 beats per minute , blood pressure (BP) 110/75 mm Hg (with no orthostatic changes), respiratory rate 18 breaths per minute, and oxygen saturation 95% on room air. He had a regular heart rate and rhythm, clear lung fields, and a benign abdominal exam. He was oriented to time, place, and person. His pupils were equal in size and reactive to light, and sensation and strength were equal bilaterally with no focal neurologic deficits. His neck was supple, and head movements did not cause any symptoms. His musculoskeletal exam was notable for right supraspinatus weakness upon abduction of arm to 90° and a positive impingement sign. ECG showed normal sinus rhythm with normal intervals. Laboratory findings were notable only for an international normalized ratio of 4.9. CT of the head did not show any pathology. The patient was admitted to the medicine floor for further evaluation.
At this point in his clinical course, the patient has had a thorough workup—one that has largely been unrevealing aside from paroxysmal AF. With his current presentation, acute intracranial causes remain on the differential, but the normal CT scan essentially excludes hemorrhage or mass. Although previous MRI studies have been negative and no focal neurologic findings have been described throughout his course, given the patient’s repeated presentations for syncope, intracranial vessel imaging should be obtained to exclude anatomical abnormalities or focal stenosis that could cause recurrent TIAs.
Seizure is also a consideration, but prior EEG and normal neurologic exam makes this less likely. While cardiac workup for syncope has been reassuring, the patient’s history of AF should continue to remain a consideration even though this is less likely the underlying cause since he is now taking dronedarone. He should be placed on telemetry upon admission. While negative orthostatic vital signs make orthostatic syncope less likely, this could be confounded by use of beta-blockers. Overall, the patient’s case remains a challenging one, with the etiology of his syncope remaining unclear at this time.
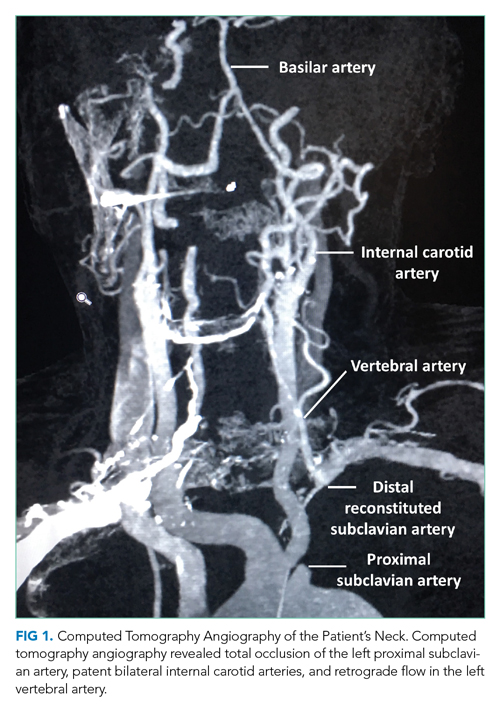
During this hospitalization, possible etiologies for recurrent syncope and falls were reviewed. The burden of verifiable AF was too low to explain the patient’s recurrent syncopal episodes. Further review of his medical record revealed that a carotid ultrasound was obtained a year earlier in the course of a previous hospitalization. The ultrasound report described patent carotid arteries and retrograde flow in the left vertebral artery consistent with ipsilateral subclavian stenosis. At the time, the ultrasound was interpreted as reassuring based on the lack of significant carotid stenosis; the findings were thought to be unrelated to the patient’s syncopal episodes. On further questioning, the patient noted that minimal exertion such as unloading a few items from the dishwasher caused left arm pain and paresthesia, accompanied by headache and lightheadedness. He also reported using his left arm more frequently following a right shoulder injury. Repeat physical exam found an inter-arm systolic BP difference (IASBPD) >40 mm Hg and left-arm claudication. CT-angiogram of the neck was obtained and showed total occlusion of the left proximal subclavian artery, patent bilateral internal carotid arteries, and retrograde flow in the left vertebral artery (Figure 1).
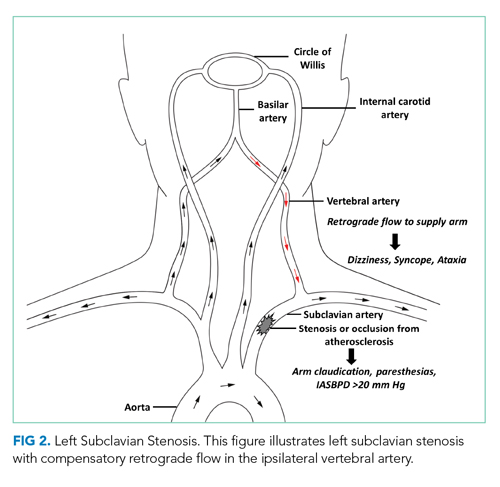
Subclavian steal syndrome (SSS) results from compromised flow to the distal arm or brainstem circulation due to a proximal subclavian artery occlusion or stenosis (prior to the origin of the vertebral artery).1,2 Subclavian stenosis may cause lowered pressure in the distal subclavian artery, creating a gradient for blood flow from the contralateral vertebral artery through the basilar artery to the ipsilateral vertebral artery, ultimately supplying blood flow to the affected subclavian artery distal to the occlusion (subclavian steal phenomenon). Flow reversal in the vertebrobasilar system can result in hypoperfusion of the brainstem (ie, vertebrobasilar insufficiency), which can cause a variety of neurologic symptoms, including SSS. While atherosclerosis is the most common cause of subclavian steal, it may be due to other conditions (eg, Takayasu arteritis, thoracic outlet syndrome, congenital heart disease).
Clinically, although many patients with proximal subclavian stenosis are asymptomatic (even in cases wherein angiographic flow reversal is detected), it is critical that clinicians be familiar with common symptoms associated with the diagnosis. Symptoms may include arm claudication related to hypoperfusion of the extremity, particularly when performing activities, as was observed in this patient. Neurologic symptoms are less common but include symptoms consistent with compromised posterior circulation such as dizziness, vertigo, ataxia, diplopia, nystagmus, impaired vision (blurring of vision, hemianopia), otologic symptoms (tinnitus, hearing loss), and/or syncope (ie, “drop attacks”). The patient’s initial complaints of sudden syncope are consistent with this presentation, as are his history of headache and lightheadedness upon use of his left arm.
Diagnostically, a gradient in upper extremity BP >15 mm Hg (as seen in this patient) or findings of arterial insufficiency would suggest subclavian stenosis. Duplex ultrasound is a reliable imaging modality and can demonstrate proximal subclavian stenosis (sensitivity of 90.9% and specificity of 82.5% for predicting >70% of stenosis cases) and ipsilateral vertebral artery flow reversal.1 Transcranial Doppler studies can be obtained to assess for basilar artery flow reversal as well. CTA/MRA can help delineate location, severity, and cause of stenosis. However, detection of vertebral or basilar artery flow reversal does not always correlate with the development of neurologic symptoms.
For patients with asymptomatic subclavian stenosis, medical management with aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, and statins should be considered given the high likelihood for other atherosclerotic disease. Management of SSS may include percutaneous/surgical intervention in combination with medical therapy, particularly for patients with severe symptomatic disease (arm claudication, posterior circulation deficits, or coronary ischemia in patients with history of coronary bypass utilizing the left internal mammary artery).
The patient was diagnosed with SSS. Cardiovascular medicine and vascular surgery services were asked to evaluate the patient for a revascularization procedure. Because the patient’s anterior circulation was intact, several specialists remained skeptical of SSS as the cause of his syncope. As such, further evaluation for arrhythmia was recommended. The patient’s arm claudication was thought to be due to SSS; however, the well-established retrograde flow via the vertebral artery made a revascularization procedure nonurgent. Moreover, continuation of warfarin was necessary in the setting of his recent left atrial appendage closure and prior history of DVT. It was determined that the risks of discontinuing anticoagulation in order to surgically treat his subclavian stenosis outweighed the benefits. In the meantime, brachial-radial index measurement and a 30-day event monitor were ordered to further assess for arrhythmias. The patient reported being overwhelmed by diagnostic testing without resolution of his syncopal episodes and missed some of his scheduled appointments. One month later, he fell again and sustained vertebral fractures at C1, C4, and L1, and a subsequent SDH requiring craniotomy with a bone flap followed by clot evacuation. The 30-day event monitor report did not reveal any arrhythmias before, during, or after multiple syncopal events that occurred in the period leading up to this fall. The patient later died in a neurology intensive care unit.
DISCUSSION
SSS often stems from atherosclerotic arterial disease that leads to stenosis or occlusion of the proximal subclavian artery, causing decreased pressure distal to the lesion. The left subclavian artery is affected more often than the right because of its acute angle of origin, which presumably causes turbulence and predisposes to atherosclerosis.3 Compromised blood flow to the arm causes exertional arm claudication and paresthesia. The compensatory retrograde flow in the ipsilateral vertebral artery causes symptoms of vertebrobasilar insufficiency such as dizziness, vertigo, and syncope (Figure 2). This conglomerate of symptoms from subclavian steal, by definition, comprises SSS. The most remarkable signs of SSS are IASBPD >20 mm Hg and, less commonly, reproducible arm claudication.
Diagnosis of SSS requires a careful correlation of clinical history, physical examination, and radiologic findings. Over 80% of patients with subclavian disease have concomitant lesions (eg, in carotid arteries) that can affect collateral circulation.4 While symptoms of SSS may vary depending on the adequacy of collaterals, patent anterior circulation does not, by default, prevent SSS in patients with subclavian stenosis.3 In one study, neurologic symptoms were found in 36% of individuals with subclavian stenosis and concomitant carotid atherosclerotic lesions, and in only 5% in patients without carotid lesions.5
A key step in diagnosis is measurement of bilateral arm BP as elevated IASBPDs are highly sensitive for subclavian steal. More than 80% of patients with IASBPD >20 mm Hg have evidence of this condition on Doppler ultrasound.3 Higher differentials in BP correlate with occurrence of symptoms (~30% of patients with IASBPD 40-50 mm Hg, and ~40% of those with IASBPD >50 mm Hg).6
The severity of subclavian stenosis is traditionally classified by imaging into three separate grades or stages based on the direction of blood flow in vertebral arteries. Grade I involves no retrograde flow; grade II involves cardiac cycle dependent alternating antegrade and retrograde flow; and grade III involves permanent retrograde flow (complete steal).7 Our patient’s care was impacted by an unsupported conventional belief that grade II SSS may involve more hemodynamic instability and produce more severe symptoms compared to permanent retrograde flow (grade III), which would result in more stability with a reset of hemodynamics in posterior circulation.7 This hypothesis has been disproven in the past, and our patient’s tragic outcome also demonstrates that complete steal is not harmless.8 Our patient had permanent retrograde flow in the left vertebral artery, and he suffered classic symptoms of SSS, with devastating consequences. Moreover, increased demand or exertion can enhance the retrograde flow even in grade III stenosis and can precipitate neurologic symptoms of SSS, including syncope. This case provides an important lesson: Management of patients with SSS should depend on the severity of symptoms, not solely on radiologic grading.
Management of SSS is often medical for atherosclerosis and hypertension, especially if symptoms are mild and infrequent. Less than 10% patients with radiologic evidence of subclavian stenosis are symptomatic, and <20% patients with symptomatic SSS require revascularization.3 Percutaneous transluminal angioplasty (PTA) and stenting have become the most favored surgical approach rather than extra-anatomic revascularization techniques.7 Both endovascular interventions and open revascularization carry an excellent success rate with low morbidity. Patients undergoing PTA have a combined rate of 3.6% for CVA and death9 and a 5-year primary patency rate10 of 82%. Bypass surgery appears similarly well tolerated, with low perioperative CVA/mortality, and a 10-year primary patency rate of 92%.11 For patients with SSS and coexisting disease in the anterior circulation, carotid endarterectomy is prioritized over subclavian revascularization as repair of the anterior circulation often resolves symptoms of SSS.12
In our patient, SSS presented with classic vertebrobasilar and brachial symptoms, but several features of his presentation made the diagnosis a challenge. First, his history suggested several potential causes of syncope, including arrhythmia, orthostatic hypotension, and substance use. Second, he reported arm paresthesia and claudication only when specifically prompted and after a targeted history was obtained. Third, there were no consistent triggers for his syncopal episodes. The patient noted that he lost consciousness when walking, driving, doing light chores, and arising from a seated position. These atypical triggers of syncope were not consistent with any of the illnesses considered during the initial workup, and therefore resulted in a broad differential, delaying the targeted workup for SSS. The wisdom of parsimony may also have played an unintended role: In clinical practice, common things are common, and explanation of most or all symptoms with a known diagnosis is often correct rather than addition of uncommon disorders.
Unfortunately, this patient kept falling through the cracks. Several providers believed that AF and alcohol use were the likely causes of his syncope. This assumption enabled a less than rigorous appraisal of the critical ultrasound report. If SSS had been on the differential, assessing the patient for the associated signs and symptoms might have led to an earlier diagnosis.
KEY TEACHING POINTS
- SSS should be included in the differential diagnosis of patients with syncope, especially when common diagnoses have been ruled out.
- Incidentally detected retrograde vertebral flow on ultrasound should never be dismissed, and the patients should be assessed for signs and symptoms of subclavian steal.
- A difference in inter-arm systolic blood pressure >20 mm Hg is highly suggestive of subclavian stenosis.
- SSS has excellent prognosis with appropriate medical treatment or revascularization.
1. Mousa AY, Morkous R, Broce M, et al. Validation of subclavian duplex velocity criteria to grade severity of subclavian artery stenosis. J Vasc Surg. 2017;65(6):1779-1785. https://doi.org/10.1016/j.jvs.2016.12.098
2. Potter BJ, Pinto DS. Subclavian steal syndrome. Circulation. 2014;129(22):2320-2323. https://doi.org/10.1161/circulationaha.113.006653
3. Labropoulos N, Nandivada P, Bekelis K. Prevalence and impact of the subclavian steal syndrome. Ann Surg. 2010;252(1):166-170. https://doi.org/10.1097/sla.0b013e3181e3375a
4. Fields WS, Lemak NA. Joint study of extracranial arterial occlusion. VII. Subclavian steal--a review of 168 cases. JAMA. 1972;222(9):1139-1143. https://doi.org/10.1001/jama.1972.03210090019004
5. Hennerici M, Klemm C, Rautenberg W. The subclavian steal phenomenon: a common vascular disorder with rare neurologic deficits. Neurology. 1988;38(5): 669-673. https://doi.org/10.1212/wnl.38.5.669
6. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379(9819):905-914. https://doi.org/10.1016/s0140-6736(11)61710-8
7. Osiro S, Zurada A, Gielecki J, Shoja MM, Tubbs RS, Loukas M. A review of subclavian steal syndrome with clinical correlation. Med Sci Monit. 2012;18(5):RA57-RA63. https://doi.org/10.12659/msm.882721
8. Thomassen L, Aarli JA. Subclavian steal phenomenon. Clinical and hemodynamic aspects. Acta Neurol Scand. 1994;90(4):241-244. https://doi.org/10.1111/j.1600-0404.1994.tb02714.x
9. De Vries JP, Jager LC, Van den Berg JC, et al. Durability of percutaneous transluminal angioplasty for obstructive lesions of proximal subclavian artery: long-term results. J Vasc Surg. 2005;41(1):19-23. https://doi.org/10.1016/j.jvs.2004.09.030
10. Wang KQ, Wang ZG, Yang BZ, et al. Long-term results of endovascular therapy for proximal subclavian arterial obstructive lesions. Chin Med J (Engl). 2010;123(1):45-50. https://doi.org/10.3760/cma.j.issn.0366-6999.2010.01.008
11. AbuRahma AF, Robinson PA, Jennings TG. Carotid-subclavian bypass grafting with polytetrafluoroethylene grafts for symptomatic subclavian artery stenosis or occlusion: a 20-year experience. J Vasc Surg. 2000;32(3):411-418; discussion 418-419. https://doi.org/10.1067/mva.2000.108644
12. Smith JM, Koury HI, Hafner CD, Welling RE. Subclavian steal syndrome. A review of 59 consecutive cases. J Cardiovasc Surg (Torino). 1994;35(1):11-14.
A 61-year-old man presented to the emergency department (ED) for persistent headache that began after he fell in his bathroom 4 days earlier. He described the headache as generalized and constant, rating the severity as a 5 on a scale of 0 to 10. The patient denied any associated neck pain or changes in headache quality with position change. He reported a 3-day history of nausea and four episodes of vomiting.
Headache after a fall raises concern for intracranial hemorrhage, particularly if this patient is on anticoagulant or antiplatelet medications. Subdural hematoma (SDH) would be more likely than epidural or subarachnoid hematoma (SAH) given the duration of days without progression. While nausea and vomiting are nonspecific, persistent vomiting may indicate increased intracranial pressure (eg, from an intracranial mass or SDH), particularly if provoked by positional changes. Without a history of fever or neck stiffness, meningitis is less likely unless the patient has a history of immunosuppression. Secondary causes of headache include vascular etiologies (eg, hemorrhagic cerebrovascular accident [CVA], arterial dissection, aneurysm, vasculitis), systemic causes (eg, chronic hypoxia/hypercapnia, hypertension), or medication overuse or withdrawal. In this patient, traumatic head injury with resultant postconcussive symptoms, though a diagnosis of exclusion, should also be considered. If the patient has a history of migraines, it is essential to obtain a history of typical migraine symptoms. More information regarding the mechanism of the fall is also essential to help elucidate a potential cause.
The patient had a 1-year history of recurrent loss of consciousness resulting in falls. After each fall, he quickly regained consciousness and exhibited no residual deficits or confusion. These episodes occurred suddenly when the patient was performing normal daily activities such as walking, driving, doing light chores, and standing up from a seated position. Immediately before this most recent fall, the patient stood up from a chair, walked toward the bathroom and, without any warning signs, lost consciousness. He denied dizziness, lightheadedness, nausea, or diaphoresis immediately before or after the fall. He also reported experiencing intermittent palpitations, but these did not appear to be related to the syncopal episodes. He denied experiencing chest pain, shortness of breath, or seizures.
The differential diagnosis for syncope is broad; therefore, it is important to identify features that suggest an etiology requiring urgent management. In this patient, cardiac etiologies such as arrhythmia (eg, atrial fibrillation [AF], ventricular tachycardia, heart block), ischemia, heart failure, and structural heart disease (eg, valvular abnormalities, cardiomyopathies) must be considered. His complaints of intermittent palpitations could suggest arrhythmia; however, the absence of a correlation to the syncopal episodes and other associated cardiac symptoms makes arrhythmias such as AF less likely. Medication side effects provoking cardiac conduction disturbances, heart block, or hypotension should be considered. Ischemic heart disease and heart failure are possible causes despite the absence of chest pain and dyspnea. While the exertional nature of the patient’s symptoms could support cardiac etiologies, it could also be indicative of recurrent pulmonary embolism or right ventricular dysfunction/strain, such as chronic thromboembolic pulmonary hypertension (CTEPH).
Neurologic causes of syncope should also be included in the differential diagnosis. Seizure is less likely the underlying cause in this case since the patient regained consciousness quickly after each episode and reported no residual deficits, confusion, incontinence, or oral trauma. While less likely, other neurovascular causes can be considered, including transient ischemic attack (TIA), CVA, SAH, or vertebrobasilar insufficiency.
Neurocardiogenic syncope is less likely due to lack of a clear trigger or classical prodromal symptoms. Without a history of volume loss, orthostatic syncope is also unlikely. Other possibilities include adrenal insufficiency or an autonomic dysfunction resulting from diabetic neuropathy, chronic kidney disease, amyloidosis, spinal cord injury, or neurologic diseases (eg, Parkinson disease, Lewy body dementia). Thus far, the provided history is not suggestive of these etiologies. Other causes for loss of consciousness include hypoglycemia, sleep disorders (eg, narcolepsy), or psychiatric causes.
About 10 months prior to this presentation, the patient had presented to the hospital for evaluation of headache and was found to have bilateral SDH requiring burr hole evacuation. At that time, he was on anticoagulation therapy for a history of left superficial femoral vein thrombosis with negative workup for hypercoagulability. Warfarin was discontinued after the SDH was diagnosed. Regarding the patient’s social history, although he reported drinking two glasses of wine with dinner each night and smoking marijuana afterward, all syncopal events occurred during the daytime.
The history of prior SDH should raise suspicion for recurrent SDH, particularly considering the patient’s ongoing alcohol use. History of deep vein thrombosis (DVT) and possible exertional syncope might suggest recurrent pulmonary embolism or CTEPH as an etiology. DVT and TIA/CVA secondary to paradoxical embolism are also possible. Depending on extent of alcohol use, intoxication and cardiomyopathy with secondary arrhythmias are possibilities.
The basic workup should focus on identifying any acute intracranial processes that may explain the patient’s presentation and evaluating for syncope. This includes a complete blood count with differential, electrolytes, hepatic panel (based on patient’s history of alcohol use), and coagulation studies. Troponins and B-type natriuretic peptide would help assess for cardiac disease, and a urine/serum drug test would be beneficial to screen for substance use. Considering the patient’s prior history of SDH, head imaging should be obtained. If the patient were to exhibit focal neurologic deficits or persistent alterations in consciousness (thereby raising the index of suspicion for TIA or CVA), perfusion/diffusion-weighted magnetic resonance imaging (MRI) studies should be obtained. If obtaining a brain MRI is not practical, then a computed tomography angiogram (CTA) of the head and neck should be obtained. A noncontrast head CT would be sufficient to reveal the presence of SDH. An electroencephalogram (EEG) to assess for seizure should be performed if the patient is noted to have any focal neurologic findings or complaints consistent with seizure. With possible exertional syncope, an electrocardiogram (ECG) and transthoracic echocardiogram (with bubble study to assess for patent foramen ovale) should be obtained urgently.
The patient had a history of hypertension and irritable bowel syndrome, for which he took metoprolol and duloxetine, respectively. Eight months prior to the current ED presentation, he was admitted to the hospital for a syncope workup after falling and sustaining a fractured jaw and torn rotator cuff. ECG and continuous telemetry monitoring showed normal sinus rhythm, normal intervals, and rare episodes of sinus tachycardia, but no evidence of arrhythmia. An echocardiogram demonstrated normal ejection fraction and chamber sizes; CT and MRI of the brain showed no residual SDH; and EEG monitoring showed no seizure activity. It was determined that the patient’s syncopal episodes were multifactorial; possible etiologies included episodic hypotension from irritable bowel syndrome—related diarrhea, paroxysmal arrhythmias, and ongoing substance use.
The patient was discharged home with a 14-day Holter monitor. Rare episodes of AF (total burden 0.4%) were detected, and dronedarone was prescribed for rhythm control; he remained off anticoagulation therapy due to the history of SDH. Over the next few months, cardiology, electrophysiology, and neurology consultants concluded that paroxysmal AF was the likely etiology of the patient’s syncopal episodes. The patient was considered high risk for CVA, but the risk of bleeding from syncope-related falls was too high to resume anticoagulation therapy.
One month prior to the current ED presentation, the patient underwent a left atrial appendage closure with a WATCHMAN implant to avoid long-term anticoagulation. After the procedure, he was started on warfarin with plans to permanently discontinue anticoagulation after 6 to 8 weeks of completed therapy. He had been on warfarin for 3 weeks prior the most recent fall and current ED visit.At the time of this presentation, the patient was on dronedarone, duloxetine, metoprolol, and warfarin. On exam, he was alert and in no distress. His temperature was 36.8 °C, heart rate 98 beats per minute , blood pressure (BP) 110/75 mm Hg (with no orthostatic changes), respiratory rate 18 breaths per minute, and oxygen saturation 95% on room air. He had a regular heart rate and rhythm, clear lung fields, and a benign abdominal exam. He was oriented to time, place, and person. His pupils were equal in size and reactive to light, and sensation and strength were equal bilaterally with no focal neurologic deficits. His neck was supple, and head movements did not cause any symptoms. His musculoskeletal exam was notable for right supraspinatus weakness upon abduction of arm to 90° and a positive impingement sign. ECG showed normal sinus rhythm with normal intervals. Laboratory findings were notable only for an international normalized ratio of 4.9. CT of the head did not show any pathology. The patient was admitted to the medicine floor for further evaluation.
At this point in his clinical course, the patient has had a thorough workup—one that has largely been unrevealing aside from paroxysmal AF. With his current presentation, acute intracranial causes remain on the differential, but the normal CT scan essentially excludes hemorrhage or mass. Although previous MRI studies have been negative and no focal neurologic findings have been described throughout his course, given the patient’s repeated presentations for syncope, intracranial vessel imaging should be obtained to exclude anatomical abnormalities or focal stenosis that could cause recurrent TIAs.
Seizure is also a consideration, but prior EEG and normal neurologic exam makes this less likely. While cardiac workup for syncope has been reassuring, the patient’s history of AF should continue to remain a consideration even though this is less likely the underlying cause since he is now taking dronedarone. He should be placed on telemetry upon admission. While negative orthostatic vital signs make orthostatic syncope less likely, this could be confounded by use of beta-blockers. Overall, the patient’s case remains a challenging one, with the etiology of his syncope remaining unclear at this time.
During this hospitalization, possible etiologies for recurrent syncope and falls were reviewed. The burden of verifiable AF was too low to explain the patient’s recurrent syncopal episodes. Further review of his medical record revealed that a carotid ultrasound was obtained a year earlier in the course of a previous hospitalization. The ultrasound report described patent carotid arteries and retrograde flow in the left vertebral artery consistent with ipsilateral subclavian stenosis. At the time, the ultrasound was interpreted as reassuring based on the lack of significant carotid stenosis; the findings were thought to be unrelated to the patient’s syncopal episodes. On further questioning, the patient noted that minimal exertion such as unloading a few items from the dishwasher caused left arm pain and paresthesia, accompanied by headache and lightheadedness. He also reported using his left arm more frequently following a right shoulder injury. Repeat physical exam found an inter-arm systolic BP difference (IASBPD) >40 mm Hg and left-arm claudication. CT-angiogram of the neck was obtained and showed total occlusion of the left proximal subclavian artery, patent bilateral internal carotid arteries, and retrograde flow in the left vertebral artery (Figure 1).
Subclavian steal syndrome (SSS) results from compromised flow to the distal arm or brainstem circulation due to a proximal subclavian artery occlusion or stenosis (prior to the origin of the vertebral artery).1,2 Subclavian stenosis may cause lowered pressure in the distal subclavian artery, creating a gradient for blood flow from the contralateral vertebral artery through the basilar artery to the ipsilateral vertebral artery, ultimately supplying blood flow to the affected subclavian artery distal to the occlusion (subclavian steal phenomenon). Flow reversal in the vertebrobasilar system can result in hypoperfusion of the brainstem (ie, vertebrobasilar insufficiency), which can cause a variety of neurologic symptoms, including SSS. While atherosclerosis is the most common cause of subclavian steal, it may be due to other conditions (eg, Takayasu arteritis, thoracic outlet syndrome, congenital heart disease).
Clinically, although many patients with proximal subclavian stenosis are asymptomatic (even in cases wherein angiographic flow reversal is detected), it is critical that clinicians be familiar with common symptoms associated with the diagnosis. Symptoms may include arm claudication related to hypoperfusion of the extremity, particularly when performing activities, as was observed in this patient. Neurologic symptoms are less common but include symptoms consistent with compromised posterior circulation such as dizziness, vertigo, ataxia, diplopia, nystagmus, impaired vision (blurring of vision, hemianopia), otologic symptoms (tinnitus, hearing loss), and/or syncope (ie, “drop attacks”). The patient’s initial complaints of sudden syncope are consistent with this presentation, as are his history of headache and lightheadedness upon use of his left arm.
Diagnostically, a gradient in upper extremity BP >15 mm Hg (as seen in this patient) or findings of arterial insufficiency would suggest subclavian stenosis. Duplex ultrasound is a reliable imaging modality and can demonstrate proximal subclavian stenosis (sensitivity of 90.9% and specificity of 82.5% for predicting >70% of stenosis cases) and ipsilateral vertebral artery flow reversal.1 Transcranial Doppler studies can be obtained to assess for basilar artery flow reversal as well. CTA/MRA can help delineate location, severity, and cause of stenosis. However, detection of vertebral or basilar artery flow reversal does not always correlate with the development of neurologic symptoms.
For patients with asymptomatic subclavian stenosis, medical management with aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, and statins should be considered given the high likelihood for other atherosclerotic disease. Management of SSS may include percutaneous/surgical intervention in combination with medical therapy, particularly for patients with severe symptomatic disease (arm claudication, posterior circulation deficits, or coronary ischemia in patients with history of coronary bypass utilizing the left internal mammary artery).
The patient was diagnosed with SSS. Cardiovascular medicine and vascular surgery services were asked to evaluate the patient for a revascularization procedure. Because the patient’s anterior circulation was intact, several specialists remained skeptical of SSS as the cause of his syncope. As such, further evaluation for arrhythmia was recommended. The patient’s arm claudication was thought to be due to SSS; however, the well-established retrograde flow via the vertebral artery made a revascularization procedure nonurgent. Moreover, continuation of warfarin was necessary in the setting of his recent left atrial appendage closure and prior history of DVT. It was determined that the risks of discontinuing anticoagulation in order to surgically treat his subclavian stenosis outweighed the benefits. In the meantime, brachial-radial index measurement and a 30-day event monitor were ordered to further assess for arrhythmias. The patient reported being overwhelmed by diagnostic testing without resolution of his syncopal episodes and missed some of his scheduled appointments. One month later, he fell again and sustained vertebral fractures at C1, C4, and L1, and a subsequent SDH requiring craniotomy with a bone flap followed by clot evacuation. The 30-day event monitor report did not reveal any arrhythmias before, during, or after multiple syncopal events that occurred in the period leading up to this fall. The patient later died in a neurology intensive care unit.
DISCUSSION
SSS often stems from atherosclerotic arterial disease that leads to stenosis or occlusion of the proximal subclavian artery, causing decreased pressure distal to the lesion. The left subclavian artery is affected more often than the right because of its acute angle of origin, which presumably causes turbulence and predisposes to atherosclerosis.3 Compromised blood flow to the arm causes exertional arm claudication and paresthesia. The compensatory retrograde flow in the ipsilateral vertebral artery causes symptoms of vertebrobasilar insufficiency such as dizziness, vertigo, and syncope (Figure 2). This conglomerate of symptoms from subclavian steal, by definition, comprises SSS. The most remarkable signs of SSS are IASBPD >20 mm Hg and, less commonly, reproducible arm claudication.
Diagnosis of SSS requires a careful correlation of clinical history, physical examination, and radiologic findings. Over 80% of patients with subclavian disease have concomitant lesions (eg, in carotid arteries) that can affect collateral circulation.4 While symptoms of SSS may vary depending on the adequacy of collaterals, patent anterior circulation does not, by default, prevent SSS in patients with subclavian stenosis.3 In one study, neurologic symptoms were found in 36% of individuals with subclavian stenosis and concomitant carotid atherosclerotic lesions, and in only 5% in patients without carotid lesions.5
A key step in diagnosis is measurement of bilateral arm BP as elevated IASBPDs are highly sensitive for subclavian steal. More than 80% of patients with IASBPD >20 mm Hg have evidence of this condition on Doppler ultrasound.3 Higher differentials in BP correlate with occurrence of symptoms (~30% of patients with IASBPD 40-50 mm Hg, and ~40% of those with IASBPD >50 mm Hg).6
The severity of subclavian stenosis is traditionally classified by imaging into three separate grades or stages based on the direction of blood flow in vertebral arteries. Grade I involves no retrograde flow; grade II involves cardiac cycle dependent alternating antegrade and retrograde flow; and grade III involves permanent retrograde flow (complete steal).7 Our patient’s care was impacted by an unsupported conventional belief that grade II SSS may involve more hemodynamic instability and produce more severe symptoms compared to permanent retrograde flow (grade III), which would result in more stability with a reset of hemodynamics in posterior circulation.7 This hypothesis has been disproven in the past, and our patient’s tragic outcome also demonstrates that complete steal is not harmless.8 Our patient had permanent retrograde flow in the left vertebral artery, and he suffered classic symptoms of SSS, with devastating consequences. Moreover, increased demand or exertion can enhance the retrograde flow even in grade III stenosis and can precipitate neurologic symptoms of SSS, including syncope. This case provides an important lesson: Management of patients with SSS should depend on the severity of symptoms, not solely on radiologic grading.
Management of SSS is often medical for atherosclerosis and hypertension, especially if symptoms are mild and infrequent. Less than 10% patients with radiologic evidence of subclavian stenosis are symptomatic, and <20% patients with symptomatic SSS require revascularization.3 Percutaneous transluminal angioplasty (PTA) and stenting have become the most favored surgical approach rather than extra-anatomic revascularization techniques.7 Both endovascular interventions and open revascularization carry an excellent success rate with low morbidity. Patients undergoing PTA have a combined rate of 3.6% for CVA and death9 and a 5-year primary patency rate10 of 82%. Bypass surgery appears similarly well tolerated, with low perioperative CVA/mortality, and a 10-year primary patency rate of 92%.11 For patients with SSS and coexisting disease in the anterior circulation, carotid endarterectomy is prioritized over subclavian revascularization as repair of the anterior circulation often resolves symptoms of SSS.12
In our patient, SSS presented with classic vertebrobasilar and brachial symptoms, but several features of his presentation made the diagnosis a challenge. First, his history suggested several potential causes of syncope, including arrhythmia, orthostatic hypotension, and substance use. Second, he reported arm paresthesia and claudication only when specifically prompted and after a targeted history was obtained. Third, there were no consistent triggers for his syncopal episodes. The patient noted that he lost consciousness when walking, driving, doing light chores, and arising from a seated position. These atypical triggers of syncope were not consistent with any of the illnesses considered during the initial workup, and therefore resulted in a broad differential, delaying the targeted workup for SSS. The wisdom of parsimony may also have played an unintended role: In clinical practice, common things are common, and explanation of most or all symptoms with a known diagnosis is often correct rather than addition of uncommon disorders.
Unfortunately, this patient kept falling through the cracks. Several providers believed that AF and alcohol use were the likely causes of his syncope. This assumption enabled a less than rigorous appraisal of the critical ultrasound report. If SSS had been on the differential, assessing the patient for the associated signs and symptoms might have led to an earlier diagnosis.
KEY TEACHING POINTS
- SSS should be included in the differential diagnosis of patients with syncope, especially when common diagnoses have been ruled out.
- Incidentally detected retrograde vertebral flow on ultrasound should never be dismissed, and the patients should be assessed for signs and symptoms of subclavian steal.
- A difference in inter-arm systolic blood pressure >20 mm Hg is highly suggestive of subclavian stenosis.
- SSS has excellent prognosis with appropriate medical treatment or revascularization.
A 61-year-old man presented to the emergency department (ED) for persistent headache that began after he fell in his bathroom 4 days earlier. He described the headache as generalized and constant, rating the severity as a 5 on a scale of 0 to 10. The patient denied any associated neck pain or changes in headache quality with position change. He reported a 3-day history of nausea and four episodes of vomiting.
Headache after a fall raises concern for intracranial hemorrhage, particularly if this patient is on anticoagulant or antiplatelet medications. Subdural hematoma (SDH) would be more likely than epidural or subarachnoid hematoma (SAH) given the duration of days without progression. While nausea and vomiting are nonspecific, persistent vomiting may indicate increased intracranial pressure (eg, from an intracranial mass or SDH), particularly if provoked by positional changes. Without a history of fever or neck stiffness, meningitis is less likely unless the patient has a history of immunosuppression. Secondary causes of headache include vascular etiologies (eg, hemorrhagic cerebrovascular accident [CVA], arterial dissection, aneurysm, vasculitis), systemic causes (eg, chronic hypoxia/hypercapnia, hypertension), or medication overuse or withdrawal. In this patient, traumatic head injury with resultant postconcussive symptoms, though a diagnosis of exclusion, should also be considered. If the patient has a history of migraines, it is essential to obtain a history of typical migraine symptoms. More information regarding the mechanism of the fall is also essential to help elucidate a potential cause.
The patient had a 1-year history of recurrent loss of consciousness resulting in falls. After each fall, he quickly regained consciousness and exhibited no residual deficits or confusion. These episodes occurred suddenly when the patient was performing normal daily activities such as walking, driving, doing light chores, and standing up from a seated position. Immediately before this most recent fall, the patient stood up from a chair, walked toward the bathroom and, without any warning signs, lost consciousness. He denied dizziness, lightheadedness, nausea, or diaphoresis immediately before or after the fall. He also reported experiencing intermittent palpitations, but these did not appear to be related to the syncopal episodes. He denied experiencing chest pain, shortness of breath, or seizures.
The differential diagnosis for syncope is broad; therefore, it is important to identify features that suggest an etiology requiring urgent management. In this patient, cardiac etiologies such as arrhythmia (eg, atrial fibrillation [AF], ventricular tachycardia, heart block), ischemia, heart failure, and structural heart disease (eg, valvular abnormalities, cardiomyopathies) must be considered. His complaints of intermittent palpitations could suggest arrhythmia; however, the absence of a correlation to the syncopal episodes and other associated cardiac symptoms makes arrhythmias such as AF less likely. Medication side effects provoking cardiac conduction disturbances, heart block, or hypotension should be considered. Ischemic heart disease and heart failure are possible causes despite the absence of chest pain and dyspnea. While the exertional nature of the patient’s symptoms could support cardiac etiologies, it could also be indicative of recurrent pulmonary embolism or right ventricular dysfunction/strain, such as chronic thromboembolic pulmonary hypertension (CTEPH).
Neurologic causes of syncope should also be included in the differential diagnosis. Seizure is less likely the underlying cause in this case since the patient regained consciousness quickly after each episode and reported no residual deficits, confusion, incontinence, or oral trauma. While less likely, other neurovascular causes can be considered, including transient ischemic attack (TIA), CVA, SAH, or vertebrobasilar insufficiency.
Neurocardiogenic syncope is less likely due to lack of a clear trigger or classical prodromal symptoms. Without a history of volume loss, orthostatic syncope is also unlikely. Other possibilities include adrenal insufficiency or an autonomic dysfunction resulting from diabetic neuropathy, chronic kidney disease, amyloidosis, spinal cord injury, or neurologic diseases (eg, Parkinson disease, Lewy body dementia). Thus far, the provided history is not suggestive of these etiologies. Other causes for loss of consciousness include hypoglycemia, sleep disorders (eg, narcolepsy), or psychiatric causes.
About 10 months prior to this presentation, the patient had presented to the hospital for evaluation of headache and was found to have bilateral SDH requiring burr hole evacuation. At that time, he was on anticoagulation therapy for a history of left superficial femoral vein thrombosis with negative workup for hypercoagulability. Warfarin was discontinued after the SDH was diagnosed. Regarding the patient’s social history, although he reported drinking two glasses of wine with dinner each night and smoking marijuana afterward, all syncopal events occurred during the daytime.
The history of prior SDH should raise suspicion for recurrent SDH, particularly considering the patient’s ongoing alcohol use. History of deep vein thrombosis (DVT) and possible exertional syncope might suggest recurrent pulmonary embolism or CTEPH as an etiology. DVT and TIA/CVA secondary to paradoxical embolism are also possible. Depending on extent of alcohol use, intoxication and cardiomyopathy with secondary arrhythmias are possibilities.
The basic workup should focus on identifying any acute intracranial processes that may explain the patient’s presentation and evaluating for syncope. This includes a complete blood count with differential, electrolytes, hepatic panel (based on patient’s history of alcohol use), and coagulation studies. Troponins and B-type natriuretic peptide would help assess for cardiac disease, and a urine/serum drug test would be beneficial to screen for substance use. Considering the patient’s prior history of SDH, head imaging should be obtained. If the patient were to exhibit focal neurologic deficits or persistent alterations in consciousness (thereby raising the index of suspicion for TIA or CVA), perfusion/diffusion-weighted magnetic resonance imaging (MRI) studies should be obtained. If obtaining a brain MRI is not practical, then a computed tomography angiogram (CTA) of the head and neck should be obtained. A noncontrast head CT would be sufficient to reveal the presence of SDH. An electroencephalogram (EEG) to assess for seizure should be performed if the patient is noted to have any focal neurologic findings or complaints consistent with seizure. With possible exertional syncope, an electrocardiogram (ECG) and transthoracic echocardiogram (with bubble study to assess for patent foramen ovale) should be obtained urgently.
The patient had a history of hypertension and irritable bowel syndrome, for which he took metoprolol and duloxetine, respectively. Eight months prior to the current ED presentation, he was admitted to the hospital for a syncope workup after falling and sustaining a fractured jaw and torn rotator cuff. ECG and continuous telemetry monitoring showed normal sinus rhythm, normal intervals, and rare episodes of sinus tachycardia, but no evidence of arrhythmia. An echocardiogram demonstrated normal ejection fraction and chamber sizes; CT and MRI of the brain showed no residual SDH; and EEG monitoring showed no seizure activity. It was determined that the patient’s syncopal episodes were multifactorial; possible etiologies included episodic hypotension from irritable bowel syndrome—related diarrhea, paroxysmal arrhythmias, and ongoing substance use.
The patient was discharged home with a 14-day Holter monitor. Rare episodes of AF (total burden 0.4%) were detected, and dronedarone was prescribed for rhythm control; he remained off anticoagulation therapy due to the history of SDH. Over the next few months, cardiology, electrophysiology, and neurology consultants concluded that paroxysmal AF was the likely etiology of the patient’s syncopal episodes. The patient was considered high risk for CVA, but the risk of bleeding from syncope-related falls was too high to resume anticoagulation therapy.
One month prior to the current ED presentation, the patient underwent a left atrial appendage closure with a WATCHMAN implant to avoid long-term anticoagulation. After the procedure, he was started on warfarin with plans to permanently discontinue anticoagulation after 6 to 8 weeks of completed therapy. He had been on warfarin for 3 weeks prior the most recent fall and current ED visit.At the time of this presentation, the patient was on dronedarone, duloxetine, metoprolol, and warfarin. On exam, he was alert and in no distress. His temperature was 36.8 °C, heart rate 98 beats per minute , blood pressure (BP) 110/75 mm Hg (with no orthostatic changes), respiratory rate 18 breaths per minute, and oxygen saturation 95% on room air. He had a regular heart rate and rhythm, clear lung fields, and a benign abdominal exam. He was oriented to time, place, and person. His pupils were equal in size and reactive to light, and sensation and strength were equal bilaterally with no focal neurologic deficits. His neck was supple, and head movements did not cause any symptoms. His musculoskeletal exam was notable for right supraspinatus weakness upon abduction of arm to 90° and a positive impingement sign. ECG showed normal sinus rhythm with normal intervals. Laboratory findings were notable only for an international normalized ratio of 4.9. CT of the head did not show any pathology. The patient was admitted to the medicine floor for further evaluation.
At this point in his clinical course, the patient has had a thorough workup—one that has largely been unrevealing aside from paroxysmal AF. With his current presentation, acute intracranial causes remain on the differential, but the normal CT scan essentially excludes hemorrhage or mass. Although previous MRI studies have been negative and no focal neurologic findings have been described throughout his course, given the patient’s repeated presentations for syncope, intracranial vessel imaging should be obtained to exclude anatomical abnormalities or focal stenosis that could cause recurrent TIAs.
Seizure is also a consideration, but prior EEG and normal neurologic exam makes this less likely. While cardiac workup for syncope has been reassuring, the patient’s history of AF should continue to remain a consideration even though this is less likely the underlying cause since he is now taking dronedarone. He should be placed on telemetry upon admission. While negative orthostatic vital signs make orthostatic syncope less likely, this could be confounded by use of beta-blockers. Overall, the patient’s case remains a challenging one, with the etiology of his syncope remaining unclear at this time.
During this hospitalization, possible etiologies for recurrent syncope and falls were reviewed. The burden of verifiable AF was too low to explain the patient’s recurrent syncopal episodes. Further review of his medical record revealed that a carotid ultrasound was obtained a year earlier in the course of a previous hospitalization. The ultrasound report described patent carotid arteries and retrograde flow in the left vertebral artery consistent with ipsilateral subclavian stenosis. At the time, the ultrasound was interpreted as reassuring based on the lack of significant carotid stenosis; the findings were thought to be unrelated to the patient’s syncopal episodes. On further questioning, the patient noted that minimal exertion such as unloading a few items from the dishwasher caused left arm pain and paresthesia, accompanied by headache and lightheadedness. He also reported using his left arm more frequently following a right shoulder injury. Repeat physical exam found an inter-arm systolic BP difference (IASBPD) >40 mm Hg and left-arm claudication. CT-angiogram of the neck was obtained and showed total occlusion of the left proximal subclavian artery, patent bilateral internal carotid arteries, and retrograde flow in the left vertebral artery (Figure 1).
Subclavian steal syndrome (SSS) results from compromised flow to the distal arm or brainstem circulation due to a proximal subclavian artery occlusion or stenosis (prior to the origin of the vertebral artery).1,2 Subclavian stenosis may cause lowered pressure in the distal subclavian artery, creating a gradient for blood flow from the contralateral vertebral artery through the basilar artery to the ipsilateral vertebral artery, ultimately supplying blood flow to the affected subclavian artery distal to the occlusion (subclavian steal phenomenon). Flow reversal in the vertebrobasilar system can result in hypoperfusion of the brainstem (ie, vertebrobasilar insufficiency), which can cause a variety of neurologic symptoms, including SSS. While atherosclerosis is the most common cause of subclavian steal, it may be due to other conditions (eg, Takayasu arteritis, thoracic outlet syndrome, congenital heart disease).
Clinically, although many patients with proximal subclavian stenosis are asymptomatic (even in cases wherein angiographic flow reversal is detected), it is critical that clinicians be familiar with common symptoms associated with the diagnosis. Symptoms may include arm claudication related to hypoperfusion of the extremity, particularly when performing activities, as was observed in this patient. Neurologic symptoms are less common but include symptoms consistent with compromised posterior circulation such as dizziness, vertigo, ataxia, diplopia, nystagmus, impaired vision (blurring of vision, hemianopia), otologic symptoms (tinnitus, hearing loss), and/or syncope (ie, “drop attacks”). The patient’s initial complaints of sudden syncope are consistent with this presentation, as are his history of headache and lightheadedness upon use of his left arm.
Diagnostically, a gradient in upper extremity BP >15 mm Hg (as seen in this patient) or findings of arterial insufficiency would suggest subclavian stenosis. Duplex ultrasound is a reliable imaging modality and can demonstrate proximal subclavian stenosis (sensitivity of 90.9% and specificity of 82.5% for predicting >70% of stenosis cases) and ipsilateral vertebral artery flow reversal.1 Transcranial Doppler studies can be obtained to assess for basilar artery flow reversal as well. CTA/MRA can help delineate location, severity, and cause of stenosis. However, detection of vertebral or basilar artery flow reversal does not always correlate with the development of neurologic symptoms.
For patients with asymptomatic subclavian stenosis, medical management with aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, and statins should be considered given the high likelihood for other atherosclerotic disease. Management of SSS may include percutaneous/surgical intervention in combination with medical therapy, particularly for patients with severe symptomatic disease (arm claudication, posterior circulation deficits, or coronary ischemia in patients with history of coronary bypass utilizing the left internal mammary artery).
The patient was diagnosed with SSS. Cardiovascular medicine and vascular surgery services were asked to evaluate the patient for a revascularization procedure. Because the patient’s anterior circulation was intact, several specialists remained skeptical of SSS as the cause of his syncope. As such, further evaluation for arrhythmia was recommended. The patient’s arm claudication was thought to be due to SSS; however, the well-established retrograde flow via the vertebral artery made a revascularization procedure nonurgent. Moreover, continuation of warfarin was necessary in the setting of his recent left atrial appendage closure and prior history of DVT. It was determined that the risks of discontinuing anticoagulation in order to surgically treat his subclavian stenosis outweighed the benefits. In the meantime, brachial-radial index measurement and a 30-day event monitor were ordered to further assess for arrhythmias. The patient reported being overwhelmed by diagnostic testing without resolution of his syncopal episodes and missed some of his scheduled appointments. One month later, he fell again and sustained vertebral fractures at C1, C4, and L1, and a subsequent SDH requiring craniotomy with a bone flap followed by clot evacuation. The 30-day event monitor report did not reveal any arrhythmias before, during, or after multiple syncopal events that occurred in the period leading up to this fall. The patient later died in a neurology intensive care unit.
DISCUSSION
SSS often stems from atherosclerotic arterial disease that leads to stenosis or occlusion of the proximal subclavian artery, causing decreased pressure distal to the lesion. The left subclavian artery is affected more often than the right because of its acute angle of origin, which presumably causes turbulence and predisposes to atherosclerosis.3 Compromised blood flow to the arm causes exertional arm claudication and paresthesia. The compensatory retrograde flow in the ipsilateral vertebral artery causes symptoms of vertebrobasilar insufficiency such as dizziness, vertigo, and syncope (Figure 2). This conglomerate of symptoms from subclavian steal, by definition, comprises SSS. The most remarkable signs of SSS are IASBPD >20 mm Hg and, less commonly, reproducible arm claudication.
Diagnosis of SSS requires a careful correlation of clinical history, physical examination, and radiologic findings. Over 80% of patients with subclavian disease have concomitant lesions (eg, in carotid arteries) that can affect collateral circulation.4 While symptoms of SSS may vary depending on the adequacy of collaterals, patent anterior circulation does not, by default, prevent SSS in patients with subclavian stenosis.3 In one study, neurologic symptoms were found in 36% of individuals with subclavian stenosis and concomitant carotid atherosclerotic lesions, and in only 5% in patients without carotid lesions.5
A key step in diagnosis is measurement of bilateral arm BP as elevated IASBPDs are highly sensitive for subclavian steal. More than 80% of patients with IASBPD >20 mm Hg have evidence of this condition on Doppler ultrasound.3 Higher differentials in BP correlate with occurrence of symptoms (~30% of patients with IASBPD 40-50 mm Hg, and ~40% of those with IASBPD >50 mm Hg).6
The severity of subclavian stenosis is traditionally classified by imaging into three separate grades or stages based on the direction of blood flow in vertebral arteries. Grade I involves no retrograde flow; grade II involves cardiac cycle dependent alternating antegrade and retrograde flow; and grade III involves permanent retrograde flow (complete steal).7 Our patient’s care was impacted by an unsupported conventional belief that grade II SSS may involve more hemodynamic instability and produce more severe symptoms compared to permanent retrograde flow (grade III), which would result in more stability with a reset of hemodynamics in posterior circulation.7 This hypothesis has been disproven in the past, and our patient’s tragic outcome also demonstrates that complete steal is not harmless.8 Our patient had permanent retrograde flow in the left vertebral artery, and he suffered classic symptoms of SSS, with devastating consequences. Moreover, increased demand or exertion can enhance the retrograde flow even in grade III stenosis and can precipitate neurologic symptoms of SSS, including syncope. This case provides an important lesson: Management of patients with SSS should depend on the severity of symptoms, not solely on radiologic grading.
Management of SSS is often medical for atherosclerosis and hypertension, especially if symptoms are mild and infrequent. Less than 10% patients with radiologic evidence of subclavian stenosis are symptomatic, and <20% patients with symptomatic SSS require revascularization.3 Percutaneous transluminal angioplasty (PTA) and stenting have become the most favored surgical approach rather than extra-anatomic revascularization techniques.7 Both endovascular interventions and open revascularization carry an excellent success rate with low morbidity. Patients undergoing PTA have a combined rate of 3.6% for CVA and death9 and a 5-year primary patency rate10 of 82%. Bypass surgery appears similarly well tolerated, with low perioperative CVA/mortality, and a 10-year primary patency rate of 92%.11 For patients with SSS and coexisting disease in the anterior circulation, carotid endarterectomy is prioritized over subclavian revascularization as repair of the anterior circulation often resolves symptoms of SSS.12
In our patient, SSS presented with classic vertebrobasilar and brachial symptoms, but several features of his presentation made the diagnosis a challenge. First, his history suggested several potential causes of syncope, including arrhythmia, orthostatic hypotension, and substance use. Second, he reported arm paresthesia and claudication only when specifically prompted and after a targeted history was obtained. Third, there were no consistent triggers for his syncopal episodes. The patient noted that he lost consciousness when walking, driving, doing light chores, and arising from a seated position. These atypical triggers of syncope were not consistent with any of the illnesses considered during the initial workup, and therefore resulted in a broad differential, delaying the targeted workup for SSS. The wisdom of parsimony may also have played an unintended role: In clinical practice, common things are common, and explanation of most or all symptoms with a known diagnosis is often correct rather than addition of uncommon disorders.
Unfortunately, this patient kept falling through the cracks. Several providers believed that AF and alcohol use were the likely causes of his syncope. This assumption enabled a less than rigorous appraisal of the critical ultrasound report. If SSS had been on the differential, assessing the patient for the associated signs and symptoms might have led to an earlier diagnosis.
KEY TEACHING POINTS
- SSS should be included in the differential diagnosis of patients with syncope, especially when common diagnoses have been ruled out.
- Incidentally detected retrograde vertebral flow on ultrasound should never be dismissed, and the patients should be assessed for signs and symptoms of subclavian steal.
- A difference in inter-arm systolic blood pressure >20 mm Hg is highly suggestive of subclavian stenosis.
- SSS has excellent prognosis with appropriate medical treatment or revascularization.
1. Mousa AY, Morkous R, Broce M, et al. Validation of subclavian duplex velocity criteria to grade severity of subclavian artery stenosis. J Vasc Surg. 2017;65(6):1779-1785. https://doi.org/10.1016/j.jvs.2016.12.098
2. Potter BJ, Pinto DS. Subclavian steal syndrome. Circulation. 2014;129(22):2320-2323. https://doi.org/10.1161/circulationaha.113.006653
3. Labropoulos N, Nandivada P, Bekelis K. Prevalence and impact of the subclavian steal syndrome. Ann Surg. 2010;252(1):166-170. https://doi.org/10.1097/sla.0b013e3181e3375a
4. Fields WS, Lemak NA. Joint study of extracranial arterial occlusion. VII. Subclavian steal--a review of 168 cases. JAMA. 1972;222(9):1139-1143. https://doi.org/10.1001/jama.1972.03210090019004
5. Hennerici M, Klemm C, Rautenberg W. The subclavian steal phenomenon: a common vascular disorder with rare neurologic deficits. Neurology. 1988;38(5): 669-673. https://doi.org/10.1212/wnl.38.5.669
6. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379(9819):905-914. https://doi.org/10.1016/s0140-6736(11)61710-8
7. Osiro S, Zurada A, Gielecki J, Shoja MM, Tubbs RS, Loukas M. A review of subclavian steal syndrome with clinical correlation. Med Sci Monit. 2012;18(5):RA57-RA63. https://doi.org/10.12659/msm.882721
8. Thomassen L, Aarli JA. Subclavian steal phenomenon. Clinical and hemodynamic aspects. Acta Neurol Scand. 1994;90(4):241-244. https://doi.org/10.1111/j.1600-0404.1994.tb02714.x
9. De Vries JP, Jager LC, Van den Berg JC, et al. Durability of percutaneous transluminal angioplasty for obstructive lesions of proximal subclavian artery: long-term results. J Vasc Surg. 2005;41(1):19-23. https://doi.org/10.1016/j.jvs.2004.09.030
10. Wang KQ, Wang ZG, Yang BZ, et al. Long-term results of endovascular therapy for proximal subclavian arterial obstructive lesions. Chin Med J (Engl). 2010;123(1):45-50. https://doi.org/10.3760/cma.j.issn.0366-6999.2010.01.008
11. AbuRahma AF, Robinson PA, Jennings TG. Carotid-subclavian bypass grafting with polytetrafluoroethylene grafts for symptomatic subclavian artery stenosis or occlusion: a 20-year experience. J Vasc Surg. 2000;32(3):411-418; discussion 418-419. https://doi.org/10.1067/mva.2000.108644
12. Smith JM, Koury HI, Hafner CD, Welling RE. Subclavian steal syndrome. A review of 59 consecutive cases. J Cardiovasc Surg (Torino). 1994;35(1):11-14.
1. Mousa AY, Morkous R, Broce M, et al. Validation of subclavian duplex velocity criteria to grade severity of subclavian artery stenosis. J Vasc Surg. 2017;65(6):1779-1785. https://doi.org/10.1016/j.jvs.2016.12.098
2. Potter BJ, Pinto DS. Subclavian steal syndrome. Circulation. 2014;129(22):2320-2323. https://doi.org/10.1161/circulationaha.113.006653
3. Labropoulos N, Nandivada P, Bekelis K. Prevalence and impact of the subclavian steal syndrome. Ann Surg. 2010;252(1):166-170. https://doi.org/10.1097/sla.0b013e3181e3375a
4. Fields WS, Lemak NA. Joint study of extracranial arterial occlusion. VII. Subclavian steal--a review of 168 cases. JAMA. 1972;222(9):1139-1143. https://doi.org/10.1001/jama.1972.03210090019004
5. Hennerici M, Klemm C, Rautenberg W. The subclavian steal phenomenon: a common vascular disorder with rare neurologic deficits. Neurology. 1988;38(5): 669-673. https://doi.org/10.1212/wnl.38.5.669
6. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379(9819):905-914. https://doi.org/10.1016/s0140-6736(11)61710-8
7. Osiro S, Zurada A, Gielecki J, Shoja MM, Tubbs RS, Loukas M. A review of subclavian steal syndrome with clinical correlation. Med Sci Monit. 2012;18(5):RA57-RA63. https://doi.org/10.12659/msm.882721
8. Thomassen L, Aarli JA. Subclavian steal phenomenon. Clinical and hemodynamic aspects. Acta Neurol Scand. 1994;90(4):241-244. https://doi.org/10.1111/j.1600-0404.1994.tb02714.x
9. De Vries JP, Jager LC, Van den Berg JC, et al. Durability of percutaneous transluminal angioplasty for obstructive lesions of proximal subclavian artery: long-term results. J Vasc Surg. 2005;41(1):19-23. https://doi.org/10.1016/j.jvs.2004.09.030
10. Wang KQ, Wang ZG, Yang BZ, et al. Long-term results of endovascular therapy for proximal subclavian arterial obstructive lesions. Chin Med J (Engl). 2010;123(1):45-50. https://doi.org/10.3760/cma.j.issn.0366-6999.2010.01.008
11. AbuRahma AF, Robinson PA, Jennings TG. Carotid-subclavian bypass grafting with polytetrafluoroethylene grafts for symptomatic subclavian artery stenosis or occlusion: a 20-year experience. J Vasc Surg. 2000;32(3):411-418; discussion 418-419. https://doi.org/10.1067/mva.2000.108644
12. Smith JM, Koury HI, Hafner CD, Welling RE. Subclavian steal syndrome. A review of 59 consecutive cases. J Cardiovasc Surg (Torino). 1994;35(1):11-14.
© 2021 Society of Hospital Medicine
Buried Deep
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
© 2021 Society of Hospital Medicine
A Short-Lived Crisis
A 79-year-old woman presented to the emergency department with 1 day of nausea and vomiting. On the morning of presentation, she felt mild cramping in her legs and vomited twice. She denied chest or back pain, dyspnea, diaphoresis, cough, fever, dysuria, headache, and abdominal pain. Her medical history included hypertension, osteoporosis, and a right-sided acoustic neuroma treated with radiation 12 years prior. One month before this presentation, type 2 diabetes mellitus was diagnosed (hemoglobin A1c level, 7.3%) on routine testing by her primary care physician. Her medications were losartan and alendronate. She was born in China and immigrated to the United States 50 years prior. Her husband was chronically ill with several recent hospitalizations.
Nausea and vomiting are nonspecific symptoms that can arise from systemic illness, including hyperglycemia, a drug/toxin effect, or injury/inflammation of the gastrointestinal, central nervous system, or cardiovascular systems. An acoustic neuroma recurrence or malignancy in the radiation field could trigger nausea. Muscle cramping could arise from myositis or from hypokalemia secondary to vomiting. Her husband’s recent hospitalizations add an important psychosocial dimension to her care and should prompt consideration of a shared illness depending on the nature of his illness.
The patient’s temperature was 36.7 °C; heart rate, 99 beats per minute; blood pressure, 94/58 mm Hg;respiratory rate, 16 breaths per minute; and oxygen saturation, 98% while breathing room air. Her body mass index (BMI) was 18.7 kg/m2. She appeared comfortable. The heart, lung, jugular venous, and abdominal examinations were normal. She had no lower extremity edema or muscle tenderness.
The white blood cell (WBC) count was 14,500/µL (81% neutrophils, 9% lymphocytes, 8% monocytes), hemoglobin level was 17.5 g/dL (elevated from 14.2 g/dL 8 weeks prior), and platelet count was 238,000/µL. The metabolic panel revealed the following values: sodium, 139 mmol/L; potassium, 5.1 mmol/L; chloride, 96 mmol/L; bicarbonate, 17 mmol/L; blood urea nitrogen, 40 mg/dL; creatinine, 2.2 mg/dL (elevated from 0.7 mg/dL 8 weeks prior); glucose, 564 mg/dL; aspartate transaminase, 108 U/L; alanine transaminase, 130 U/L; total bilirubin, 0.6 mg/dL; and alkaline phosphatase, 105 U/L. Creatine kinase, amylase, and lipase levels were not measured. The urinalysis showed trace ketones, protein 100 mg/dL, glucose >500 mg/dL, and <5 WBCs per high-power field. The venous blood gas demonstrated a pH of 7.20 and lactate level of 13.2 mmol/L. Serum beta-hydroxybutyrate level was 0.27 mmol/L (reference range, 0.02-0.27), serum troponin I level was 8.5 µg/L (reference range, <0.05), and
Chest x-ray showed bilateral perihilar opacities with normal heart size. Electrocardiogram (ECG) revealed new ST-segment depressions in the anterior precordial leads (Figure 1).
Her hypotension may signal septic, cardiogenic, or hypovolemic shock. The leukocytosis, anion gap acidosis, acute kidney injury, and elevated lactate are compatible with sepsis, although there is no identified source of infection. Although diabetic ketoacidosis (DKA) can explain many of these findings, the serum beta-hydroxybutyrate and urine ketones are lower than expected for that condition. Her low-normal BMI makes significant insulin resistance less likely and raises concern about pancreatic adenocarcinoma as a secondary cause of diabetes.
The nausea, ST depressions, elevated troponin and B-type natriuretic peptide levels, and bilateral infiltrates suggest acute coronary syndrome (ACS), complicated by acute heart failure leading to systemic hypoperfusion and associated lactic acidosis and kidney injury. Nonischemic causes of myocardial injury, such as sepsis, myocarditis, and stress cardiomyopathy, should also be considered. Alternatively, she could be experiencing multiorgan injury from widespread embolism (eg, endocarditis), thrombosis (eg, antiphospholipid syndrome), or inflammation (eg, vasculitis). Acute pancreatitis can cause acute hyperglycemia and multisystem disease, but she did not have abdominal pain or tenderness (and her lipase level was not measured). Treatment should include intravenous insulin, intravenous fluids (trying to balance possible sepsis or DKA with heart failure), medical management for non-ST elevation myocardial infarction (NSTEMI), and empiric antibiotics.
ACS was diagnosed, and aspirin, atorvastatin, clopidogrel, and heparin were prescribed. Insulin infusion and intravenous fluids (approximately 3 L overnight) were administered for hyperglycemia (and possible early DKA). On the night of admission, the patient became profoundly diaphoretic without fevers; the WBC count rose to 24,200/µL. Vancomycin and ertapenem were initiated for possible sepsis. Serum troponin I level increased to 11.9 µg/L; the patient did not have chest pain, and the ECG was unchanged.
The next morning, the patient reported new mild diffuse abdominal pain and had mild epigastric tenderness. The WBC count was 28,900/µL; hemoglobin, 13.2 g/dL; venous pH, 7.39; lactate, 2.9 mmol/L; lipase, 48 U/L; aspartate transaminase, 84 U/L; alanine transaminase, 72 U/L; total bilirubin, 0.7 mg/dL; alkaline phosphatase, 64 U/L; and creatinine, 1.2 mg/dL.
Her rising troponin without dynamic ECG changes makes the diagnosis of ACS less likely, although myocardial ischemia can present as abdominal pain. Other causes of myocardial injury to consider (in addition to the previously mentioned sepsis, myocarditis, and stress cardiomyopathy) are pulmonary embolism and proximal aortic dissection. The latter can lead to ischemia in multiple systems (cardiac, mesenteric, renal, and lower extremity, recalling her leg cramps on admission).
The leukocytosis and lactic acidosis in the setting of new abdominal pain raises the question of mesenteric ischemia or intra-abdominal sepsis. Her hemoglobin has decreased by 4 g, and while some of the change may be dilutional, it will be important to consider hemolysis (less likely with a normal bilirubin) or gastrointestinal bleeding (given current anticoagulant and antiplatelet therapy). An echocardiogram and computed tomography (CT) angiogram of the chest, abdomen, and pelvis are indicated to evaluate the vasculature and assess for intra-abdominal pathology.
Coronary angiography revealed a 40% stenosis in the proximal right coronary artery and no other angiographically significant disease; the left ventricular end-diastolic pressure (LVEDP) was 30 mm Hg. Transthoracic echocardiography demonstrated normal left ventricular size, left ventricular ejection fraction of 65% to 70%, impaired left ventricular relaxation, and an inferior vena cava <2 cm in diameter that collapsed with inspiration.
The angiogram shows modest coronary artery disease and points away from plaque rupture as the cause of myocardial injury. Another important consideration given her husband’s recurrent illness is stress cardiomyopathy, but she does not have the typical apical ballooning or left ventricular dysfunction. The increased LVEDP with normal left ventricular size and function with elevated filling pressures is consistent with left-sided heart failure with preserved ejection fraction. Cardiac magnetic resonance imaging could exclude an infiltrative disorder leading to diastolic dysfunction or a myocarditis that explains the troponin elevation, but both diagnoses seem unlikely.
CT of the abdomen and pelvis demonstrated a heterogeneous 3-cm mass in the left adrenal gland (Figure 2).
An adrenal mass could be a functional or nonfunctional adenoma, primary adrenal carcinoma, a metastatic malignancy, or granulomatous infection such as tuberculosis. Secretion of excess glucocorticoid, mineralocorticoid, or catecholamine should be evaluated.
Cushing syndrome could explain her hyperglycemia, leukocytosis, and heart failure (mediated by the increased risk of atherosclerosis and hypertension with hypercortisolism), although her low BMI is atypical. Primary hyperaldosteronism causes hypertension but does not cause an acute multisystem disease. Pheochromocytoma could account for the diaphoresis, hypertension, hyperglycemia, leukocytosis, and cardiac injury. A more severe form—pheochromocytoma crisis—is characterized by widespread end-organ damage, including cardiomyopathy, bowel ischemia, hepatitis, hyperglycemia with ketoacidosis, and lactic acidosis. Measurement of serum cortisol and plasma and urine fractionated metanephrines, and a dexamethasone suppression test can determine whether the adrenal mass is functional.
The intravenous insulin infusion was changed to subcutaneous dosing on hospital day 2. She had no further nausea, diaphoresis, or abdominal pain, was walking around the hospital unit unassisted, and was consuming a regular diet. By hospital day 3, insulin was discontinued. The patient remained euglycemic for the remainder of her hospitalization; hemoglobin A1c value was 7.0%. Blood cultures were sterile, and the WBC count was 12,000/µL. Thyroid-stimulating hormone level was 0.31 mIU/L (reference range, 0.45-4.12), and the free thyroxine level was 12 pmol/L (reference range, 10-18). Antibiotics were discontinued. She remained euvolemic and never required diuretic therapy. The acute myocardial injury and diastolic dysfunction were attributed to an acute stress cardiomyopathy arising from the strain of her husband’s declining health. She was discharged on hospital day 5 with aspirin, atorvastatin, metoprolol, lisinopril, and outpatient follow-up.
The rapid resolution of her multisystem process suggests a self-limited process or successful treatment of the underlying cause. Although she received antibiotics, a bacterial infection never manifested. Cardiomyopathy with a high troponin level, ECG changes, and early heart failure often requires aggressive supportive measures, which were not required here. The rapid cessation of hyperglycemia and an insulin requirement within 1 day is atypical for DKA.
Pheochromocytoma is a rare secondary cause of diabetes in which excess catecholamines cause insulin resistance and suppress insulin release. It can explain both the adrenal mass and, in the form of pheochromocytoma crisis, the severe multisystem injury. However, the patient’s hypotension (which could be explained by concomitant cardiomyopathy) and older age are not typical for pheochromocytoma.
Results of testing for adrenal biomarkers, which were sent during her hospitalization, returned several days after hospital discharge. The plasma free metanephrine level was 687 pg/mL (reference range, <57) and the plasma free normetanephrine level was 508 pg/mL (reference range, <148). Metoprolol was discontinued by her primary care physician.
Elevated plasma free metanephrine and normetanephrine levels were confirmed in the endocrinology clinic 3 weeks later. The 24-hour urine metanephrine level was 1497 µg/24 hours (reference range, 90-315), and the 24-hour urine normetanephrine level was 379 µg/24 hours (reference range, 122-676). Serum aldosterone level was 8 ng/dL (reference range, 3-16), and morning cortisol level was 8 µg/dL (reference range, 4-19). Lisinopril was discontinued, and phenoxybenzamine was prescribed.
Adrenal-protocol CT of the abdomen demonstrated that the left adrenal mass was enhanced by contrast without definite washout, which could be consistent with a pheochromocytoma.
The diagnosis of pheochromocytoma has been confirmed by biochemistry and imaging. It was appropriate to stop metoprolol, as β-blockade can lead to unopposed α-receptor agonism and hypertension. Implementation of α-blockade with phenoxybenzamine and endocrine surgery referral are indicated.
On the day she intended to fill a phenoxybenzamine prescription, the patient experienced acute generalized weakness and presented to the emergency department with hyperglycemia (glucose, 661 mg/dL), acute kidney injury (creatinine, 1.6 mg/dL), troponin I elevation (0.14 µg/L), and lactic acidosis (4.7 mmol/L). She was admitted to the hospital and rapidly improved with intravenous fluids and insulin. Phenoxybenzamine 10 mg daily was administered, and she was discharged on hospital day 2. The dosage of phenoxybenzamine was gradually increased over 2 months.
Laparoscopic left adrenalectomy was performed, with removal of a 3-cm mass. The pathologic findings confirmed the diagnosis of pheochromocytoma. Two months later she felt well. Her hypertension was controlled with lisinopril 10 mg daily. Transthoracic echocardiography 3 months after adrenalectomy demonstrated a left ventricular ejection fraction of 60% to 65%. Six months later, her hemoglobin A1c was 6.6%.
DISCUSSION
Pheochromocytoma is an abnormal growth of cells of chromaffin origin that arises in the adrenal medulla.1,2 The incidence of these often benign tumors is estimated to be 2 to 8 cases per million in the general population, and 2 to 6 per 1000 in adult patients with hypertension.1,3,4 Although clinicians commonly associate these catecholamine-secreting tumors with intermittent hypertension or diaphoresis, they have a wide spectrum of manifestations, which range from asymptomatic adrenal mass to acute multiorgan illness that mimics other life-threatening conditions. Common signs and symptoms of pheochromocytoma include hypertension (60%-70% incidence), headache (50%), diaphoresis (50%), and palpitations (50%-60%).4 The textbook triad of headache, sweating, and palpitations is seen in fewer than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is often explained by a more common condition.1,4 Approximately 5% of adrenal “incidentalomas” are pheochromocytomas that are minimally symptomatic or asymptomatic.1,3 In a study of 102 patients who underwent pheochromocytoma resection, 33% were diagnosed during evaluation of an adrenal incidentaloma.5 At the other end of the spectrum is a pheochromocytoma crisis with its mimicry of ACS and sepsis, and manifestations including severe hyperglycemia, abdominal pain, acute heart failure, and syncope.2,5-9 Aside from chronic mild hypertension and a single episode of diaphoresis during admission, our patient had none of the classic signs or symptoms of pheochromocytoma. Rather, she presented with the abrupt onset of multiorgan injury.
Diagnostic evaluation for pheochromocytoma typically includes demonstration of elevated catecholamine byproducts (metanephrines) in plasma or urine and an adrenal mass on imaging.2,10 Biopsy is contraindicated because this can lead to release of catecholamines, which can trigger a pheochromocytoma crisis.5 The Endocrine Society guidelines recommend evaluating patients for pheochromocytoma who have: (1) a known or suspected genetic syndrome linked to pheochromocytoma (eg, multiple endocrine neoplasia type 2 or Von Hippel-Lindau syndrome), (2) an adrenal mass incidentally found on imaging, regardless of a history of hypertension, or (3) signs and symptoms of pheochromocytoma.3
Patients in pheochromocytoma crisis are typically very ill, requiring intensive care unit admission for hemodynamic stabilization.1,11 Initial management is typically directed at assessing and treating for common causes of systemic illness and hemodynamic instability, such as ACS and sepsis. Although some patients with pheochromocytoma crisis may have hemodynamic collapse requiring invasive circulatory support, others improve while receiving empiric treatment for mimicking conditions. Our patient had multiorgan injury and hemodynamic instability but returned to her preadmission state within 48 to 72 hours and remained stable after the withdrawal of all therapies, including insulin and antibiotics. This rapid improvement suggested a paroxysmal condition with an “on/off” capacity mediated by endogenous mediators. Once pheochromocytoma crisis is diagnosed, hemodynamic stabilization with α-adrenergic receptor blockade and intravascular volume repletion is essential. Confirmation of the diagnosis with repeat testing after hospital discharge is important because biochemical test results are less specific in the setting of acute illness. Surgery on an elective basis is the definitive treatment. Ongoing α-adrenergic receptor blockade is essential to minimize the risk of an intraoperative pheochromocytoma crisis (because of anesthesia or tumor manipulation) and prevent cardiovascular collapse after resection of tumor.11
Although the biochemical profile of a pheochromocytoma (eg, epinephrine predominant) is not tightly linked to the phenotype, the pattern of organ injury can reflect the pleotropic effects of specific catecholamines.12 While both norepinephrine and epinephrine bind the β1-adrenergic receptor with equal affinity, epinephrine has a higher affinity for the β2-adrenergic receptor. Our patient’s initial relative hypotension was likely caused by hypovolemia from decreased oral intake, vomiting, and hyperglycemia-mediated polyuria. However, β2-adrenergic receptor agonism could have caused vasodilation, and nocardiogenic hypotension has been observed with epinephrine-predominant pheochromocytomas.13 Several of the other clinical findings in this case can be explained by widespread β-adrenergic receptor agonism. Epinephrine (whether endogenously produced or exogenously administered) can lead to cardiac injury with elevated cardiac biomarkers.1,6,14 Epinephrine administration can cause leukocytosis, which is attributed to demargination of leukocyte subsets that express β2-adrenergic receptors.15,16 Lactic acidosis in the absence of tissue hypoxia (type B lactic acidosis) occurs during epinephrine infusions in healthy volunteers.17,18 Hyperglycemia from epinephrine infusions is attributed to β-adrenergic receptor stimulation causing increased gluconeogenesis and glycogenolysis and decreased insulin secretion and tissue glucose uptake.8 Resolution of hyperglycemia and diabetes is observed in the majority of patients after resection of pheochromocytoma, and hypoglycemia immediately after surgery is common, occasionally requiring glucose infusion.19,20
Pheochromocytomas are rare tumors with a wide range of manifestations that extend well beyond the classic triad. Pheochromocytomas can present as an asymptomatic adrenal mass with normal blood pressure, as new onset diabetes, or as multiorgan injury with cardiovascular collapse. Our patient suffered from two episodes of catecholamine excess that required hospitalization, but fortunately each proved to be a short-lived crisis.
TEACHING POINTS
- The classic triad of headache, sweating, and palpitations occurs in less than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is usually explained by a common medical condition.
- The presentation of pheochromocytoma varies widely, from asymptomatic adrenal incidentaloma to pheochromocytoma crisis causing multiorgan dysfunction with hemodynamic instability and mimicry of common critical illnesses like ACS, DKA, and sepsis.
- Biochemical screening for pheochromocytoma is recommended when a patient has a known or suspected genetic syndrome linked to pheochromocytoma, an adrenal mass incidentally found on imaging regardless of blood pressure, or signs and symptoms of a pheochromocytoma.
1. Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757-764. https://doi.org/10.1530/eje-15-0483
2. Whitelaw BC, Prague JK, Mustafa OG, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf). 2014;80(1):13-22. https://doi.org/10.1111/cen.12324
3. Lenders JW, Duh QY, Eisenhofer G, et al; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498
4. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24(12):2331-2339. https://doi.org/10.1097/01.hjh.0000251887.01885.54
5. Shen WT, Grogan R, Vriens M, Clark OH, Duh QY. One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg. 2010;145(9):893-897. https://doi.org/10.1001/archsurg.2010.159
6. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99(14):1438-1444. https://doi.org/10.1136/heartjnl-2013-304073
7. Lee TW, Lin KH, Chang CJ, Lew WH, Lee TI. Pheochromocytoma mimicking both acute coronary syndrome and sepsis: a case report. Med Princ Pract. 2013;22(4):405-407. https://doi.org/10.1159/000343578
8. Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017;23(8):999-1005. https://doi.org/10.4158/ep171914.ra
9. Ueda T, Oka N, Matsumoto A, et al. Pheochromocytoma presenting as recurrent hypotension and syncope. Intern Med. 2005;44(3):222-227. https://doi.org/10.2169/internalmedicine.44.222
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. https://doi.org/10.1056/nejmra1806651
11. Scholten A, Cisco RM, Vriens MR, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. 2013;98(2):581-591. https://doi.org/10.1210/jc.2012-3020
12. Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65-90.
13. Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf). 1992;37(3):304-306. https://doi.org/10.1111/j.1365-2265.1992.tb02326.x
14. Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3(1):76-80. https://doi.org/10.1016/j.jaip.2014.06.007
15. Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77-91. https://doi.org/10.1006/brbi.1996.0009
16. Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503-511. https://doi.org/10.4049/jimmunol.0902189
17. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-1140. https://doi.org/10.1016/j.mayocp.2013.06.012
18. Levy B. Bench-to-bedside review: is there a place for epinephrine in septic shock? Crit Care. 2005;9(6):561-565. https://doi.org/10.1186/cc3901
19. Chen Y, Hodin RA, Pandolfi C, Ruan DT, McKenzie TJ. Hypoglycemia after resection of pheochromocytoma. Surgery. 2014;156:1404-1408; discussion 1408-1409. https://doi.org/10.1016/j.surg.2014.08.020
20. Pogorzelski R, Toutounchi S, Krajewska E, et al. The effect of surgical treatment of phaeochromocytoma on concomitant arterial hypertension and diabetes mellitus in a single-centre retrospective study. Cent European J Urol. 2014;67(4):361-365. https://doi.org/10.5173/ceju.2014.04.art9
A 79-year-old woman presented to the emergency department with 1 day of nausea and vomiting. On the morning of presentation, she felt mild cramping in her legs and vomited twice. She denied chest or back pain, dyspnea, diaphoresis, cough, fever, dysuria, headache, and abdominal pain. Her medical history included hypertension, osteoporosis, and a right-sided acoustic neuroma treated with radiation 12 years prior. One month before this presentation, type 2 diabetes mellitus was diagnosed (hemoglobin A1c level, 7.3%) on routine testing by her primary care physician. Her medications were losartan and alendronate. She was born in China and immigrated to the United States 50 years prior. Her husband was chronically ill with several recent hospitalizations.
Nausea and vomiting are nonspecific symptoms that can arise from systemic illness, including hyperglycemia, a drug/toxin effect, or injury/inflammation of the gastrointestinal, central nervous system, or cardiovascular systems. An acoustic neuroma recurrence or malignancy in the radiation field could trigger nausea. Muscle cramping could arise from myositis or from hypokalemia secondary to vomiting. Her husband’s recent hospitalizations add an important psychosocial dimension to her care and should prompt consideration of a shared illness depending on the nature of his illness.
The patient’s temperature was 36.7 °C; heart rate, 99 beats per minute; blood pressure, 94/58 mm Hg;respiratory rate, 16 breaths per minute; and oxygen saturation, 98% while breathing room air. Her body mass index (BMI) was 18.7 kg/m2. She appeared comfortable. The heart, lung, jugular venous, and abdominal examinations were normal. She had no lower extremity edema or muscle tenderness.
The white blood cell (WBC) count was 14,500/µL (81% neutrophils, 9% lymphocytes, 8% monocytes), hemoglobin level was 17.5 g/dL (elevated from 14.2 g/dL 8 weeks prior), and platelet count was 238,000/µL. The metabolic panel revealed the following values: sodium, 139 mmol/L; potassium, 5.1 mmol/L; chloride, 96 mmol/L; bicarbonate, 17 mmol/L; blood urea nitrogen, 40 mg/dL; creatinine, 2.2 mg/dL (elevated from 0.7 mg/dL 8 weeks prior); glucose, 564 mg/dL; aspartate transaminase, 108 U/L; alanine transaminase, 130 U/L; total bilirubin, 0.6 mg/dL; and alkaline phosphatase, 105 U/L. Creatine kinase, amylase, and lipase levels were not measured. The urinalysis showed trace ketones, protein 100 mg/dL, glucose >500 mg/dL, and <5 WBCs per high-power field. The venous blood gas demonstrated a pH of 7.20 and lactate level of 13.2 mmol/L. Serum beta-hydroxybutyrate level was 0.27 mmol/L (reference range, 0.02-0.27), serum troponin I level was 8.5 µg/L (reference range, <0.05), and
Chest x-ray showed bilateral perihilar opacities with normal heart size. Electrocardiogram (ECG) revealed new ST-segment depressions in the anterior precordial leads (Figure 1).
Her hypotension may signal septic, cardiogenic, or hypovolemic shock. The leukocytosis, anion gap acidosis, acute kidney injury, and elevated lactate are compatible with sepsis, although there is no identified source of infection. Although diabetic ketoacidosis (DKA) can explain many of these findings, the serum beta-hydroxybutyrate and urine ketones are lower than expected for that condition. Her low-normal BMI makes significant insulin resistance less likely and raises concern about pancreatic adenocarcinoma as a secondary cause of diabetes.
The nausea, ST depressions, elevated troponin and B-type natriuretic peptide levels, and bilateral infiltrates suggest acute coronary syndrome (ACS), complicated by acute heart failure leading to systemic hypoperfusion and associated lactic acidosis and kidney injury. Nonischemic causes of myocardial injury, such as sepsis, myocarditis, and stress cardiomyopathy, should also be considered. Alternatively, she could be experiencing multiorgan injury from widespread embolism (eg, endocarditis), thrombosis (eg, antiphospholipid syndrome), or inflammation (eg, vasculitis). Acute pancreatitis can cause acute hyperglycemia and multisystem disease, but she did not have abdominal pain or tenderness (and her lipase level was not measured). Treatment should include intravenous insulin, intravenous fluids (trying to balance possible sepsis or DKA with heart failure), medical management for non-ST elevation myocardial infarction (NSTEMI), and empiric antibiotics.
ACS was diagnosed, and aspirin, atorvastatin, clopidogrel, and heparin were prescribed. Insulin infusion and intravenous fluids (approximately 3 L overnight) were administered for hyperglycemia (and possible early DKA). On the night of admission, the patient became profoundly diaphoretic without fevers; the WBC count rose to 24,200/µL. Vancomycin and ertapenem were initiated for possible sepsis. Serum troponin I level increased to 11.9 µg/L; the patient did not have chest pain, and the ECG was unchanged.
The next morning, the patient reported new mild diffuse abdominal pain and had mild epigastric tenderness. The WBC count was 28,900/µL; hemoglobin, 13.2 g/dL; venous pH, 7.39; lactate, 2.9 mmol/L; lipase, 48 U/L; aspartate transaminase, 84 U/L; alanine transaminase, 72 U/L; total bilirubin, 0.7 mg/dL; alkaline phosphatase, 64 U/L; and creatinine, 1.2 mg/dL.
Her rising troponin without dynamic ECG changes makes the diagnosis of ACS less likely, although myocardial ischemia can present as abdominal pain. Other causes of myocardial injury to consider (in addition to the previously mentioned sepsis, myocarditis, and stress cardiomyopathy) are pulmonary embolism and proximal aortic dissection. The latter can lead to ischemia in multiple systems (cardiac, mesenteric, renal, and lower extremity, recalling her leg cramps on admission).
The leukocytosis and lactic acidosis in the setting of new abdominal pain raises the question of mesenteric ischemia or intra-abdominal sepsis. Her hemoglobin has decreased by 4 g, and while some of the change may be dilutional, it will be important to consider hemolysis (less likely with a normal bilirubin) or gastrointestinal bleeding (given current anticoagulant and antiplatelet therapy). An echocardiogram and computed tomography (CT) angiogram of the chest, abdomen, and pelvis are indicated to evaluate the vasculature and assess for intra-abdominal pathology.
Coronary angiography revealed a 40% stenosis in the proximal right coronary artery and no other angiographically significant disease; the left ventricular end-diastolic pressure (LVEDP) was 30 mm Hg. Transthoracic echocardiography demonstrated normal left ventricular size, left ventricular ejection fraction of 65% to 70%, impaired left ventricular relaxation, and an inferior vena cava <2 cm in diameter that collapsed with inspiration.
The angiogram shows modest coronary artery disease and points away from plaque rupture as the cause of myocardial injury. Another important consideration given her husband’s recurrent illness is stress cardiomyopathy, but she does not have the typical apical ballooning or left ventricular dysfunction. The increased LVEDP with normal left ventricular size and function with elevated filling pressures is consistent with left-sided heart failure with preserved ejection fraction. Cardiac magnetic resonance imaging could exclude an infiltrative disorder leading to diastolic dysfunction or a myocarditis that explains the troponin elevation, but both diagnoses seem unlikely.
CT of the abdomen and pelvis demonstrated a heterogeneous 3-cm mass in the left adrenal gland (Figure 2).
An adrenal mass could be a functional or nonfunctional adenoma, primary adrenal carcinoma, a metastatic malignancy, or granulomatous infection such as tuberculosis. Secretion of excess glucocorticoid, mineralocorticoid, or catecholamine should be evaluated.
Cushing syndrome could explain her hyperglycemia, leukocytosis, and heart failure (mediated by the increased risk of atherosclerosis and hypertension with hypercortisolism), although her low BMI is atypical. Primary hyperaldosteronism causes hypertension but does not cause an acute multisystem disease. Pheochromocytoma could account for the diaphoresis, hypertension, hyperglycemia, leukocytosis, and cardiac injury. A more severe form—pheochromocytoma crisis—is characterized by widespread end-organ damage, including cardiomyopathy, bowel ischemia, hepatitis, hyperglycemia with ketoacidosis, and lactic acidosis. Measurement of serum cortisol and plasma and urine fractionated metanephrines, and a dexamethasone suppression test can determine whether the adrenal mass is functional.
The intravenous insulin infusion was changed to subcutaneous dosing on hospital day 2. She had no further nausea, diaphoresis, or abdominal pain, was walking around the hospital unit unassisted, and was consuming a regular diet. By hospital day 3, insulin was discontinued. The patient remained euglycemic for the remainder of her hospitalization; hemoglobin A1c value was 7.0%. Blood cultures were sterile, and the WBC count was 12,000/µL. Thyroid-stimulating hormone level was 0.31 mIU/L (reference range, 0.45-4.12), and the free thyroxine level was 12 pmol/L (reference range, 10-18). Antibiotics were discontinued. She remained euvolemic and never required diuretic therapy. The acute myocardial injury and diastolic dysfunction were attributed to an acute stress cardiomyopathy arising from the strain of her husband’s declining health. She was discharged on hospital day 5 with aspirin, atorvastatin, metoprolol, lisinopril, and outpatient follow-up.
The rapid resolution of her multisystem process suggests a self-limited process or successful treatment of the underlying cause. Although she received antibiotics, a bacterial infection never manifested. Cardiomyopathy with a high troponin level, ECG changes, and early heart failure often requires aggressive supportive measures, which were not required here. The rapid cessation of hyperglycemia and an insulin requirement within 1 day is atypical for DKA.
Pheochromocytoma is a rare secondary cause of diabetes in which excess catecholamines cause insulin resistance and suppress insulin release. It can explain both the adrenal mass and, in the form of pheochromocytoma crisis, the severe multisystem injury. However, the patient’s hypotension (which could be explained by concomitant cardiomyopathy) and older age are not typical for pheochromocytoma.
Results of testing for adrenal biomarkers, which were sent during her hospitalization, returned several days after hospital discharge. The plasma free metanephrine level was 687 pg/mL (reference range, <57) and the plasma free normetanephrine level was 508 pg/mL (reference range, <148). Metoprolol was discontinued by her primary care physician.
Elevated plasma free metanephrine and normetanephrine levels were confirmed in the endocrinology clinic 3 weeks later. The 24-hour urine metanephrine level was 1497 µg/24 hours (reference range, 90-315), and the 24-hour urine normetanephrine level was 379 µg/24 hours (reference range, 122-676). Serum aldosterone level was 8 ng/dL (reference range, 3-16), and morning cortisol level was 8 µg/dL (reference range, 4-19). Lisinopril was discontinued, and phenoxybenzamine was prescribed.
Adrenal-protocol CT of the abdomen demonstrated that the left adrenal mass was enhanced by contrast without definite washout, which could be consistent with a pheochromocytoma.
The diagnosis of pheochromocytoma has been confirmed by biochemistry and imaging. It was appropriate to stop metoprolol, as β-blockade can lead to unopposed α-receptor agonism and hypertension. Implementation of α-blockade with phenoxybenzamine and endocrine surgery referral are indicated.
On the day she intended to fill a phenoxybenzamine prescription, the patient experienced acute generalized weakness and presented to the emergency department with hyperglycemia (glucose, 661 mg/dL), acute kidney injury (creatinine, 1.6 mg/dL), troponin I elevation (0.14 µg/L), and lactic acidosis (4.7 mmol/L). She was admitted to the hospital and rapidly improved with intravenous fluids and insulin. Phenoxybenzamine 10 mg daily was administered, and she was discharged on hospital day 2. The dosage of phenoxybenzamine was gradually increased over 2 months.
Laparoscopic left adrenalectomy was performed, with removal of a 3-cm mass. The pathologic findings confirmed the diagnosis of pheochromocytoma. Two months later she felt well. Her hypertension was controlled with lisinopril 10 mg daily. Transthoracic echocardiography 3 months after adrenalectomy demonstrated a left ventricular ejection fraction of 60% to 65%. Six months later, her hemoglobin A1c was 6.6%.
DISCUSSION
Pheochromocytoma is an abnormal growth of cells of chromaffin origin that arises in the adrenal medulla.1,2 The incidence of these often benign tumors is estimated to be 2 to 8 cases per million in the general population, and 2 to 6 per 1000 in adult patients with hypertension.1,3,4 Although clinicians commonly associate these catecholamine-secreting tumors with intermittent hypertension or diaphoresis, they have a wide spectrum of manifestations, which range from asymptomatic adrenal mass to acute multiorgan illness that mimics other life-threatening conditions. Common signs and symptoms of pheochromocytoma include hypertension (60%-70% incidence), headache (50%), diaphoresis (50%), and palpitations (50%-60%).4 The textbook triad of headache, sweating, and palpitations is seen in fewer than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is often explained by a more common condition.1,4 Approximately 5% of adrenal “incidentalomas” are pheochromocytomas that are minimally symptomatic or asymptomatic.1,3 In a study of 102 patients who underwent pheochromocytoma resection, 33% were diagnosed during evaluation of an adrenal incidentaloma.5 At the other end of the spectrum is a pheochromocytoma crisis with its mimicry of ACS and sepsis, and manifestations including severe hyperglycemia, abdominal pain, acute heart failure, and syncope.2,5-9 Aside from chronic mild hypertension and a single episode of diaphoresis during admission, our patient had none of the classic signs or symptoms of pheochromocytoma. Rather, she presented with the abrupt onset of multiorgan injury.
Diagnostic evaluation for pheochromocytoma typically includes demonstration of elevated catecholamine byproducts (metanephrines) in plasma or urine and an adrenal mass on imaging.2,10 Biopsy is contraindicated because this can lead to release of catecholamines, which can trigger a pheochromocytoma crisis.5 The Endocrine Society guidelines recommend evaluating patients for pheochromocytoma who have: (1) a known or suspected genetic syndrome linked to pheochromocytoma (eg, multiple endocrine neoplasia type 2 or Von Hippel-Lindau syndrome), (2) an adrenal mass incidentally found on imaging, regardless of a history of hypertension, or (3) signs and symptoms of pheochromocytoma.3
Patients in pheochromocytoma crisis are typically very ill, requiring intensive care unit admission for hemodynamic stabilization.1,11 Initial management is typically directed at assessing and treating for common causes of systemic illness and hemodynamic instability, such as ACS and sepsis. Although some patients with pheochromocytoma crisis may have hemodynamic collapse requiring invasive circulatory support, others improve while receiving empiric treatment for mimicking conditions. Our patient had multiorgan injury and hemodynamic instability but returned to her preadmission state within 48 to 72 hours and remained stable after the withdrawal of all therapies, including insulin and antibiotics. This rapid improvement suggested a paroxysmal condition with an “on/off” capacity mediated by endogenous mediators. Once pheochromocytoma crisis is diagnosed, hemodynamic stabilization with α-adrenergic receptor blockade and intravascular volume repletion is essential. Confirmation of the diagnosis with repeat testing after hospital discharge is important because biochemical test results are less specific in the setting of acute illness. Surgery on an elective basis is the definitive treatment. Ongoing α-adrenergic receptor blockade is essential to minimize the risk of an intraoperative pheochromocytoma crisis (because of anesthesia or tumor manipulation) and prevent cardiovascular collapse after resection of tumor.11
Although the biochemical profile of a pheochromocytoma (eg, epinephrine predominant) is not tightly linked to the phenotype, the pattern of organ injury can reflect the pleotropic effects of specific catecholamines.12 While both norepinephrine and epinephrine bind the β1-adrenergic receptor with equal affinity, epinephrine has a higher affinity for the β2-adrenergic receptor. Our patient’s initial relative hypotension was likely caused by hypovolemia from decreased oral intake, vomiting, and hyperglycemia-mediated polyuria. However, β2-adrenergic receptor agonism could have caused vasodilation, and nocardiogenic hypotension has been observed with epinephrine-predominant pheochromocytomas.13 Several of the other clinical findings in this case can be explained by widespread β-adrenergic receptor agonism. Epinephrine (whether endogenously produced or exogenously administered) can lead to cardiac injury with elevated cardiac biomarkers.1,6,14 Epinephrine administration can cause leukocytosis, which is attributed to demargination of leukocyte subsets that express β2-adrenergic receptors.15,16 Lactic acidosis in the absence of tissue hypoxia (type B lactic acidosis) occurs during epinephrine infusions in healthy volunteers.17,18 Hyperglycemia from epinephrine infusions is attributed to β-adrenergic receptor stimulation causing increased gluconeogenesis and glycogenolysis and decreased insulin secretion and tissue glucose uptake.8 Resolution of hyperglycemia and diabetes is observed in the majority of patients after resection of pheochromocytoma, and hypoglycemia immediately after surgery is common, occasionally requiring glucose infusion.19,20
Pheochromocytomas are rare tumors with a wide range of manifestations that extend well beyond the classic triad. Pheochromocytomas can present as an asymptomatic adrenal mass with normal blood pressure, as new onset diabetes, or as multiorgan injury with cardiovascular collapse. Our patient suffered from two episodes of catecholamine excess that required hospitalization, but fortunately each proved to be a short-lived crisis.
TEACHING POINTS
- The classic triad of headache, sweating, and palpitations occurs in less than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is usually explained by a common medical condition.
- The presentation of pheochromocytoma varies widely, from asymptomatic adrenal incidentaloma to pheochromocytoma crisis causing multiorgan dysfunction with hemodynamic instability and mimicry of common critical illnesses like ACS, DKA, and sepsis.
- Biochemical screening for pheochromocytoma is recommended when a patient has a known or suspected genetic syndrome linked to pheochromocytoma, an adrenal mass incidentally found on imaging regardless of blood pressure, or signs and symptoms of a pheochromocytoma.
A 79-year-old woman presented to the emergency department with 1 day of nausea and vomiting. On the morning of presentation, she felt mild cramping in her legs and vomited twice. She denied chest or back pain, dyspnea, diaphoresis, cough, fever, dysuria, headache, and abdominal pain. Her medical history included hypertension, osteoporosis, and a right-sided acoustic neuroma treated with radiation 12 years prior. One month before this presentation, type 2 diabetes mellitus was diagnosed (hemoglobin A1c level, 7.3%) on routine testing by her primary care physician. Her medications were losartan and alendronate. She was born in China and immigrated to the United States 50 years prior. Her husband was chronically ill with several recent hospitalizations.
Nausea and vomiting are nonspecific symptoms that can arise from systemic illness, including hyperglycemia, a drug/toxin effect, or injury/inflammation of the gastrointestinal, central nervous system, or cardiovascular systems. An acoustic neuroma recurrence or malignancy in the radiation field could trigger nausea. Muscle cramping could arise from myositis or from hypokalemia secondary to vomiting. Her husband’s recent hospitalizations add an important psychosocial dimension to her care and should prompt consideration of a shared illness depending on the nature of his illness.
The patient’s temperature was 36.7 °C; heart rate, 99 beats per minute; blood pressure, 94/58 mm Hg;respiratory rate, 16 breaths per minute; and oxygen saturation, 98% while breathing room air. Her body mass index (BMI) was 18.7 kg/m2. She appeared comfortable. The heart, lung, jugular venous, and abdominal examinations were normal. She had no lower extremity edema or muscle tenderness.
The white blood cell (WBC) count was 14,500/µL (81% neutrophils, 9% lymphocytes, 8% monocytes), hemoglobin level was 17.5 g/dL (elevated from 14.2 g/dL 8 weeks prior), and platelet count was 238,000/µL. The metabolic panel revealed the following values: sodium, 139 mmol/L; potassium, 5.1 mmol/L; chloride, 96 mmol/L; bicarbonate, 17 mmol/L; blood urea nitrogen, 40 mg/dL; creatinine, 2.2 mg/dL (elevated from 0.7 mg/dL 8 weeks prior); glucose, 564 mg/dL; aspartate transaminase, 108 U/L; alanine transaminase, 130 U/L; total bilirubin, 0.6 mg/dL; and alkaline phosphatase, 105 U/L. Creatine kinase, amylase, and lipase levels were not measured. The urinalysis showed trace ketones, protein 100 mg/dL, glucose >500 mg/dL, and <5 WBCs per high-power field. The venous blood gas demonstrated a pH of 7.20 and lactate level of 13.2 mmol/L. Serum beta-hydroxybutyrate level was 0.27 mmol/L (reference range, 0.02-0.27), serum troponin I level was 8.5 µg/L (reference range, <0.05), and
Chest x-ray showed bilateral perihilar opacities with normal heart size. Electrocardiogram (ECG) revealed new ST-segment depressions in the anterior precordial leads (Figure 1).
Her hypotension may signal septic, cardiogenic, or hypovolemic shock. The leukocytosis, anion gap acidosis, acute kidney injury, and elevated lactate are compatible with sepsis, although there is no identified source of infection. Although diabetic ketoacidosis (DKA) can explain many of these findings, the serum beta-hydroxybutyrate and urine ketones are lower than expected for that condition. Her low-normal BMI makes significant insulin resistance less likely and raises concern about pancreatic adenocarcinoma as a secondary cause of diabetes.
The nausea, ST depressions, elevated troponin and B-type natriuretic peptide levels, and bilateral infiltrates suggest acute coronary syndrome (ACS), complicated by acute heart failure leading to systemic hypoperfusion and associated lactic acidosis and kidney injury. Nonischemic causes of myocardial injury, such as sepsis, myocarditis, and stress cardiomyopathy, should also be considered. Alternatively, she could be experiencing multiorgan injury from widespread embolism (eg, endocarditis), thrombosis (eg, antiphospholipid syndrome), or inflammation (eg, vasculitis). Acute pancreatitis can cause acute hyperglycemia and multisystem disease, but she did not have abdominal pain or tenderness (and her lipase level was not measured). Treatment should include intravenous insulin, intravenous fluids (trying to balance possible sepsis or DKA with heart failure), medical management for non-ST elevation myocardial infarction (NSTEMI), and empiric antibiotics.
ACS was diagnosed, and aspirin, atorvastatin, clopidogrel, and heparin were prescribed. Insulin infusion and intravenous fluids (approximately 3 L overnight) were administered for hyperglycemia (and possible early DKA). On the night of admission, the patient became profoundly diaphoretic without fevers; the WBC count rose to 24,200/µL. Vancomycin and ertapenem were initiated for possible sepsis. Serum troponin I level increased to 11.9 µg/L; the patient did not have chest pain, and the ECG was unchanged.
The next morning, the patient reported new mild diffuse abdominal pain and had mild epigastric tenderness. The WBC count was 28,900/µL; hemoglobin, 13.2 g/dL; venous pH, 7.39; lactate, 2.9 mmol/L; lipase, 48 U/L; aspartate transaminase, 84 U/L; alanine transaminase, 72 U/L; total bilirubin, 0.7 mg/dL; alkaline phosphatase, 64 U/L; and creatinine, 1.2 mg/dL.
Her rising troponin without dynamic ECG changes makes the diagnosis of ACS less likely, although myocardial ischemia can present as abdominal pain. Other causes of myocardial injury to consider (in addition to the previously mentioned sepsis, myocarditis, and stress cardiomyopathy) are pulmonary embolism and proximal aortic dissection. The latter can lead to ischemia in multiple systems (cardiac, mesenteric, renal, and lower extremity, recalling her leg cramps on admission).
The leukocytosis and lactic acidosis in the setting of new abdominal pain raises the question of mesenteric ischemia or intra-abdominal sepsis. Her hemoglobin has decreased by 4 g, and while some of the change may be dilutional, it will be important to consider hemolysis (less likely with a normal bilirubin) or gastrointestinal bleeding (given current anticoagulant and antiplatelet therapy). An echocardiogram and computed tomography (CT) angiogram of the chest, abdomen, and pelvis are indicated to evaluate the vasculature and assess for intra-abdominal pathology.
Coronary angiography revealed a 40% stenosis in the proximal right coronary artery and no other angiographically significant disease; the left ventricular end-diastolic pressure (LVEDP) was 30 mm Hg. Transthoracic echocardiography demonstrated normal left ventricular size, left ventricular ejection fraction of 65% to 70%, impaired left ventricular relaxation, and an inferior vena cava <2 cm in diameter that collapsed with inspiration.
The angiogram shows modest coronary artery disease and points away from plaque rupture as the cause of myocardial injury. Another important consideration given her husband’s recurrent illness is stress cardiomyopathy, but she does not have the typical apical ballooning or left ventricular dysfunction. The increased LVEDP with normal left ventricular size and function with elevated filling pressures is consistent with left-sided heart failure with preserved ejection fraction. Cardiac magnetic resonance imaging could exclude an infiltrative disorder leading to diastolic dysfunction or a myocarditis that explains the troponin elevation, but both diagnoses seem unlikely.
CT of the abdomen and pelvis demonstrated a heterogeneous 3-cm mass in the left adrenal gland (Figure 2).
An adrenal mass could be a functional or nonfunctional adenoma, primary adrenal carcinoma, a metastatic malignancy, or granulomatous infection such as tuberculosis. Secretion of excess glucocorticoid, mineralocorticoid, or catecholamine should be evaluated.
Cushing syndrome could explain her hyperglycemia, leukocytosis, and heart failure (mediated by the increased risk of atherosclerosis and hypertension with hypercortisolism), although her low BMI is atypical. Primary hyperaldosteronism causes hypertension but does not cause an acute multisystem disease. Pheochromocytoma could account for the diaphoresis, hypertension, hyperglycemia, leukocytosis, and cardiac injury. A more severe form—pheochromocytoma crisis—is characterized by widespread end-organ damage, including cardiomyopathy, bowel ischemia, hepatitis, hyperglycemia with ketoacidosis, and lactic acidosis. Measurement of serum cortisol and plasma and urine fractionated metanephrines, and a dexamethasone suppression test can determine whether the adrenal mass is functional.
The intravenous insulin infusion was changed to subcutaneous dosing on hospital day 2. She had no further nausea, diaphoresis, or abdominal pain, was walking around the hospital unit unassisted, and was consuming a regular diet. By hospital day 3, insulin was discontinued. The patient remained euglycemic for the remainder of her hospitalization; hemoglobin A1c value was 7.0%. Blood cultures were sterile, and the WBC count was 12,000/µL. Thyroid-stimulating hormone level was 0.31 mIU/L (reference range, 0.45-4.12), and the free thyroxine level was 12 pmol/L (reference range, 10-18). Antibiotics were discontinued. She remained euvolemic and never required diuretic therapy. The acute myocardial injury and diastolic dysfunction were attributed to an acute stress cardiomyopathy arising from the strain of her husband’s declining health. She was discharged on hospital day 5 with aspirin, atorvastatin, metoprolol, lisinopril, and outpatient follow-up.
The rapid resolution of her multisystem process suggests a self-limited process or successful treatment of the underlying cause. Although she received antibiotics, a bacterial infection never manifested. Cardiomyopathy with a high troponin level, ECG changes, and early heart failure often requires aggressive supportive measures, which were not required here. The rapid cessation of hyperglycemia and an insulin requirement within 1 day is atypical for DKA.
Pheochromocytoma is a rare secondary cause of diabetes in which excess catecholamines cause insulin resistance and suppress insulin release. It can explain both the adrenal mass and, in the form of pheochromocytoma crisis, the severe multisystem injury. However, the patient’s hypotension (which could be explained by concomitant cardiomyopathy) and older age are not typical for pheochromocytoma.
Results of testing for adrenal biomarkers, which were sent during her hospitalization, returned several days after hospital discharge. The plasma free metanephrine level was 687 pg/mL (reference range, <57) and the plasma free normetanephrine level was 508 pg/mL (reference range, <148). Metoprolol was discontinued by her primary care physician.
Elevated plasma free metanephrine and normetanephrine levels were confirmed in the endocrinology clinic 3 weeks later. The 24-hour urine metanephrine level was 1497 µg/24 hours (reference range, 90-315), and the 24-hour urine normetanephrine level was 379 µg/24 hours (reference range, 122-676). Serum aldosterone level was 8 ng/dL (reference range, 3-16), and morning cortisol level was 8 µg/dL (reference range, 4-19). Lisinopril was discontinued, and phenoxybenzamine was prescribed.
Adrenal-protocol CT of the abdomen demonstrated that the left adrenal mass was enhanced by contrast without definite washout, which could be consistent with a pheochromocytoma.
The diagnosis of pheochromocytoma has been confirmed by biochemistry and imaging. It was appropriate to stop metoprolol, as β-blockade can lead to unopposed α-receptor agonism and hypertension. Implementation of α-blockade with phenoxybenzamine and endocrine surgery referral are indicated.
On the day she intended to fill a phenoxybenzamine prescription, the patient experienced acute generalized weakness and presented to the emergency department with hyperglycemia (glucose, 661 mg/dL), acute kidney injury (creatinine, 1.6 mg/dL), troponin I elevation (0.14 µg/L), and lactic acidosis (4.7 mmol/L). She was admitted to the hospital and rapidly improved with intravenous fluids and insulin. Phenoxybenzamine 10 mg daily was administered, and she was discharged on hospital day 2. The dosage of phenoxybenzamine was gradually increased over 2 months.
Laparoscopic left adrenalectomy was performed, with removal of a 3-cm mass. The pathologic findings confirmed the diagnosis of pheochromocytoma. Two months later she felt well. Her hypertension was controlled with lisinopril 10 mg daily. Transthoracic echocardiography 3 months after adrenalectomy demonstrated a left ventricular ejection fraction of 60% to 65%. Six months later, her hemoglobin A1c was 6.6%.
DISCUSSION
Pheochromocytoma is an abnormal growth of cells of chromaffin origin that arises in the adrenal medulla.1,2 The incidence of these often benign tumors is estimated to be 2 to 8 cases per million in the general population, and 2 to 6 per 1000 in adult patients with hypertension.1,3,4 Although clinicians commonly associate these catecholamine-secreting tumors with intermittent hypertension or diaphoresis, they have a wide spectrum of manifestations, which range from asymptomatic adrenal mass to acute multiorgan illness that mimics other life-threatening conditions. Common signs and symptoms of pheochromocytoma include hypertension (60%-70% incidence), headache (50%), diaphoresis (50%), and palpitations (50%-60%).4 The textbook triad of headache, sweating, and palpitations is seen in fewer than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is often explained by a more common condition.1,4 Approximately 5% of adrenal “incidentalomas” are pheochromocytomas that are minimally symptomatic or asymptomatic.1,3 In a study of 102 patients who underwent pheochromocytoma resection, 33% were diagnosed during evaluation of an adrenal incidentaloma.5 At the other end of the spectrum is a pheochromocytoma crisis with its mimicry of ACS and sepsis, and manifestations including severe hyperglycemia, abdominal pain, acute heart failure, and syncope.2,5-9 Aside from chronic mild hypertension and a single episode of diaphoresis during admission, our patient had none of the classic signs or symptoms of pheochromocytoma. Rather, she presented with the abrupt onset of multiorgan injury.
Diagnostic evaluation for pheochromocytoma typically includes demonstration of elevated catecholamine byproducts (metanephrines) in plasma or urine and an adrenal mass on imaging.2,10 Biopsy is contraindicated because this can lead to release of catecholamines, which can trigger a pheochromocytoma crisis.5 The Endocrine Society guidelines recommend evaluating patients for pheochromocytoma who have: (1) a known or suspected genetic syndrome linked to pheochromocytoma (eg, multiple endocrine neoplasia type 2 or Von Hippel-Lindau syndrome), (2) an adrenal mass incidentally found on imaging, regardless of a history of hypertension, or (3) signs and symptoms of pheochromocytoma.3
Patients in pheochromocytoma crisis are typically very ill, requiring intensive care unit admission for hemodynamic stabilization.1,11 Initial management is typically directed at assessing and treating for common causes of systemic illness and hemodynamic instability, such as ACS and sepsis. Although some patients with pheochromocytoma crisis may have hemodynamic collapse requiring invasive circulatory support, others improve while receiving empiric treatment for mimicking conditions. Our patient had multiorgan injury and hemodynamic instability but returned to her preadmission state within 48 to 72 hours and remained stable after the withdrawal of all therapies, including insulin and antibiotics. This rapid improvement suggested a paroxysmal condition with an “on/off” capacity mediated by endogenous mediators. Once pheochromocytoma crisis is diagnosed, hemodynamic stabilization with α-adrenergic receptor blockade and intravascular volume repletion is essential. Confirmation of the diagnosis with repeat testing after hospital discharge is important because biochemical test results are less specific in the setting of acute illness. Surgery on an elective basis is the definitive treatment. Ongoing α-adrenergic receptor blockade is essential to minimize the risk of an intraoperative pheochromocytoma crisis (because of anesthesia or tumor manipulation) and prevent cardiovascular collapse after resection of tumor.11
Although the biochemical profile of a pheochromocytoma (eg, epinephrine predominant) is not tightly linked to the phenotype, the pattern of organ injury can reflect the pleotropic effects of specific catecholamines.12 While both norepinephrine and epinephrine bind the β1-adrenergic receptor with equal affinity, epinephrine has a higher affinity for the β2-adrenergic receptor. Our patient’s initial relative hypotension was likely caused by hypovolemia from decreased oral intake, vomiting, and hyperglycemia-mediated polyuria. However, β2-adrenergic receptor agonism could have caused vasodilation, and nocardiogenic hypotension has been observed with epinephrine-predominant pheochromocytomas.13 Several of the other clinical findings in this case can be explained by widespread β-adrenergic receptor agonism. Epinephrine (whether endogenously produced or exogenously administered) can lead to cardiac injury with elevated cardiac biomarkers.1,6,14 Epinephrine administration can cause leukocytosis, which is attributed to demargination of leukocyte subsets that express β2-adrenergic receptors.15,16 Lactic acidosis in the absence of tissue hypoxia (type B lactic acidosis) occurs during epinephrine infusions in healthy volunteers.17,18 Hyperglycemia from epinephrine infusions is attributed to β-adrenergic receptor stimulation causing increased gluconeogenesis and glycogenolysis and decreased insulin secretion and tissue glucose uptake.8 Resolution of hyperglycemia and diabetes is observed in the majority of patients after resection of pheochromocytoma, and hypoglycemia immediately after surgery is common, occasionally requiring glucose infusion.19,20
Pheochromocytomas are rare tumors with a wide range of manifestations that extend well beyond the classic triad. Pheochromocytomas can present as an asymptomatic adrenal mass with normal blood pressure, as new onset diabetes, or as multiorgan injury with cardiovascular collapse. Our patient suffered from two episodes of catecholamine excess that required hospitalization, but fortunately each proved to be a short-lived crisis.
TEACHING POINTS
- The classic triad of headache, sweating, and palpitations occurs in less than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is usually explained by a common medical condition.
- The presentation of pheochromocytoma varies widely, from asymptomatic adrenal incidentaloma to pheochromocytoma crisis causing multiorgan dysfunction with hemodynamic instability and mimicry of common critical illnesses like ACS, DKA, and sepsis.
- Biochemical screening for pheochromocytoma is recommended when a patient has a known or suspected genetic syndrome linked to pheochromocytoma, an adrenal mass incidentally found on imaging regardless of blood pressure, or signs and symptoms of a pheochromocytoma.
1. Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757-764. https://doi.org/10.1530/eje-15-0483
2. Whitelaw BC, Prague JK, Mustafa OG, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf). 2014;80(1):13-22. https://doi.org/10.1111/cen.12324
3. Lenders JW, Duh QY, Eisenhofer G, et al; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498
4. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24(12):2331-2339. https://doi.org/10.1097/01.hjh.0000251887.01885.54
5. Shen WT, Grogan R, Vriens M, Clark OH, Duh QY. One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg. 2010;145(9):893-897. https://doi.org/10.1001/archsurg.2010.159
6. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99(14):1438-1444. https://doi.org/10.1136/heartjnl-2013-304073
7. Lee TW, Lin KH, Chang CJ, Lew WH, Lee TI. Pheochromocytoma mimicking both acute coronary syndrome and sepsis: a case report. Med Princ Pract. 2013;22(4):405-407. https://doi.org/10.1159/000343578
8. Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017;23(8):999-1005. https://doi.org/10.4158/ep171914.ra
9. Ueda T, Oka N, Matsumoto A, et al. Pheochromocytoma presenting as recurrent hypotension and syncope. Intern Med. 2005;44(3):222-227. https://doi.org/10.2169/internalmedicine.44.222
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. https://doi.org/10.1056/nejmra1806651
11. Scholten A, Cisco RM, Vriens MR, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. 2013;98(2):581-591. https://doi.org/10.1210/jc.2012-3020
12. Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65-90.
13. Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf). 1992;37(3):304-306. https://doi.org/10.1111/j.1365-2265.1992.tb02326.x
14. Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3(1):76-80. https://doi.org/10.1016/j.jaip.2014.06.007
15. Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77-91. https://doi.org/10.1006/brbi.1996.0009
16. Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503-511. https://doi.org/10.4049/jimmunol.0902189
17. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-1140. https://doi.org/10.1016/j.mayocp.2013.06.012
18. Levy B. Bench-to-bedside review: is there a place for epinephrine in septic shock? Crit Care. 2005;9(6):561-565. https://doi.org/10.1186/cc3901
19. Chen Y, Hodin RA, Pandolfi C, Ruan DT, McKenzie TJ. Hypoglycemia after resection of pheochromocytoma. Surgery. 2014;156:1404-1408; discussion 1408-1409. https://doi.org/10.1016/j.surg.2014.08.020
20. Pogorzelski R, Toutounchi S, Krajewska E, et al. The effect of surgical treatment of phaeochromocytoma on concomitant arterial hypertension and diabetes mellitus in a single-centre retrospective study. Cent European J Urol. 2014;67(4):361-365. https://doi.org/10.5173/ceju.2014.04.art9
1. Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757-764. https://doi.org/10.1530/eje-15-0483
2. Whitelaw BC, Prague JK, Mustafa OG, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf). 2014;80(1):13-22. https://doi.org/10.1111/cen.12324
3. Lenders JW, Duh QY, Eisenhofer G, et al; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498
4. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24(12):2331-2339. https://doi.org/10.1097/01.hjh.0000251887.01885.54
5. Shen WT, Grogan R, Vriens M, Clark OH, Duh QY. One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg. 2010;145(9):893-897. https://doi.org/10.1001/archsurg.2010.159
6. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99(14):1438-1444. https://doi.org/10.1136/heartjnl-2013-304073
7. Lee TW, Lin KH, Chang CJ, Lew WH, Lee TI. Pheochromocytoma mimicking both acute coronary syndrome and sepsis: a case report. Med Princ Pract. 2013;22(4):405-407. https://doi.org/10.1159/000343578
8. Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017;23(8):999-1005. https://doi.org/10.4158/ep171914.ra
9. Ueda T, Oka N, Matsumoto A, et al. Pheochromocytoma presenting as recurrent hypotension and syncope. Intern Med. 2005;44(3):222-227. https://doi.org/10.2169/internalmedicine.44.222
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. https://doi.org/10.1056/nejmra1806651
11. Scholten A, Cisco RM, Vriens MR, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. 2013;98(2):581-591. https://doi.org/10.1210/jc.2012-3020
12. Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65-90.
13. Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf). 1992;37(3):304-306. https://doi.org/10.1111/j.1365-2265.1992.tb02326.x
14. Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3(1):76-80. https://doi.org/10.1016/j.jaip.2014.06.007
15. Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77-91. https://doi.org/10.1006/brbi.1996.0009
16. Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503-511. https://doi.org/10.4049/jimmunol.0902189
17. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-1140. https://doi.org/10.1016/j.mayocp.2013.06.012
18. Levy B. Bench-to-bedside review: is there a place for epinephrine in septic shock? Crit Care. 2005;9(6):561-565. https://doi.org/10.1186/cc3901
19. Chen Y, Hodin RA, Pandolfi C, Ruan DT, McKenzie TJ. Hypoglycemia after resection of pheochromocytoma. Surgery. 2014;156:1404-1408; discussion 1408-1409. https://doi.org/10.1016/j.surg.2014.08.020
20. Pogorzelski R, Toutounchi S, Krajewska E, et al. The effect of surgical treatment of phaeochromocytoma on concomitant arterial hypertension and diabetes mellitus in a single-centre retrospective study. Cent European J Urol. 2014;67(4):361-365. https://doi.org/10.5173/ceju.2014.04.art9
© 2021 Society of Hospital Medicine
An A-Peeling Diagnosis
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 39-year-old previously healthy man presented to the emergency department (ED) with abrupt-onset fever, headache, back pain, myalgias, chills, and photophobia. His past medical history included seasonal allergies and an episode of aseptic meningitis 8 years prior. He denied cough, dysuria, weakness, numbness, or visual changes. He denied using tobacco or injection drugs and rarely drank alcohol. His only medication was acetaminophen for fever.
The patient’s sudden fever indicates the rapid onset of an inflammatory state. While the headache and photophobia might be a result of an underlying systemic infection or an irritant like blood in the cerebral spinal fluid (CSF), one must consider meningitis. Potential sources for sudden meningitis include infectious, autoimmune (rheumatoid arthritis, systemic lupus erythematosus [SLE]), or drug-induced aseptic meningitis, and structural etiologies (ruptured cyst). Recrudescence of prior disease may also present acutely (Mollaret meningitis). Malignant etiologies, being more indolent, seem less likely. Back pain may indicate an epidural inflammatory process like epidural abscess; however, the patient denies risk factors such as injection drug use or recent procedures.
The patient’s temperature was 101.2 °F; blood pressure, 120/72 mm Hg; and heart rate, 112 bpm. He appeared comfortable, without meningismus or spinal tenderness. Pupils were reactive; eyes were without icterus, injection, or suffusion. Cardiac exam was normal. Lungs were clear to auscultation. He had no abdominal tenderness, hepatosplenomegaly, or lymphadenopathy. Cranial nerves II through XII, balance, coordination, strength, and sensation were intact. No rash was noted. Complete blood count (CBC), basic and hepatic chemistry panels, urinalysis, and serum lactate tests were within normal limits. Erythrocyte sedimentation rate (ESR) was elevated to 15 mm/h (normal range, 3-10 mm/h), C-reactive protein (CRP) to 2.4 mg/dL (normal range, <0.5 mg/dL), and procalcitonin to 0.07 ng/mL (normal range, <0.05 ng/mL). The patient was treated with intravenous (IV) fluids, ketorolac, dexamethasone, and acetaminophen, with resolution of symptoms. Given his rapid improvement, absence of meningismus, and lack of immunocompromise, lumbar puncture was deferred. A diagnosis of nonspecific viral syndrome was made. He was discharged home.
Certainly, a systemic infection (eg, influenza, adenovirus, arbovirus-related infection, HIV) could be a cause of this patient’s presentation. Notably, less than two-thirds of patients with meningitis present with the classic triad of fever, neck stiffness, and altered mental status. In this patient with fever, headache, and photophobia, aseptic meningitis should still be considered. While the negative procalcitonin and rapid clinical improvement without antibiotics make acute bacterial meningitis unlikely, nonbacterial causes of meningeal irritation can be severe and life-threatening. An assessment for jolt accentuation of the headache might have been helpful. Information about time of year, geographic exposures (vector-borne infections), and sick contacts (viral illness) can inform the clinical decision to pursue lumbar puncture. Additional history regarding his previous aseptic meningitis would be helpful, as it could suggest a recurrent inflammatory process. Causes of recurrent aseptic meningitis include infectious (herpes simplex virus [HSV], Epstein-Barr virus [EBV], syphilis), drug-related (nonsteroidal anti-inflammatory drugs [NSAIDs]), structural (epidermoid cyst with rupture), and autoimmune (lupus, Sjögren syndrome, Behçet disease) etiologies.
The mildly elevated inflammatory markers are nonspecific and reflect the patient’s known inflammatory state. The dexamethasone given for symptomatic management may have had some therapeutic effect in the setting of an autoimmune process, with additional contribution from ketorolac and acetaminophen.
He returned to the ED 3 days later with a pruritic, disseminated rash involving his palms and soles, accompanied by hand swelling and tingling. Although his headache and photophobia resolved, he reported a productive cough, nasal congestion, and sore throat. He also reported orange-pink urine without dysuria or urinary frequency. Additional questioning revealed a recent motorcycle trip to the Great Lakes region. During this trip, he did not camp, interact with animals or ticks, or swim in streams or lakes. He did not eat any raw, undercooked, or locally hunted meats. He denied new medications, soaps or detergents, or sexual contacts. He had started taking acetaminophen and ibuprofen around the clock since prior discharge.
The orange-pink urine and acute-onset palmoplantar rash with recent fever help narrow the differential. Orange-pink urine might suggest bilirubinuria from liver injury, hemolysis with hemoglobinuria, or myoglobinuria. Most concerning would be hematuria associated with glomerular injury and a systemic vasculopathy.
The rash on the palms and soles should be further characterized as blanching or nonblanching. Blanching, indicating vasodilation of intact blood vessels, is seen with many drug eruptions and viral exanthems. Nonblanching, suggesting broken capillaries (petechiae or purpura), would suggest vasculitis or vasculopathy from emboli, infection, or inflammation. A palmoplantar rash in febrile illness should first prompt evaluation for life-threatening conditions, followed by consideration of both infectious and noninfectious etiologies. Acutely fatal infections include Rocky Mountain spotted fever (RMSF), meningococcemia, toxic shock syndrome, infective endocarditis, and rat-bite fever. The rash, fever, headache, and outdoor exposure raise the possibility of a rickettsial infection, including RMSF, which can be contracted rarely around the Great Lakes. Other life-threatening infections seem unlikely, as the patient would have significantly deteriorated without proper medical care by now. Palmoplantar rash with fever can also be seen in other bacterial infections (eg, secondary syphilis, arbovirus infections, typhus) and in viral infections (eg, cytomegalovirus [CMV], EBV, human herpesvirus-6 [HHV-6], HIV, coxsackievirus, and papular-purpuric gloves and socks syndrome caused by parvovirus B19). Noninfectious considerations include drug hypersensitivity rashes, neoplasm (eg, cutaneous T-cell lymphoma), or inflammatory conditions (eg, SLE, vasculitis). Drug reaction with eosinophilia and systemic symptoms (may also present with severe illness.
The acetaminophen and ibuprofen may be masking ongoing fevers. The cough, nasal congestion, and sore throat might be part of a viral prodrome or, in tandem with fever, associated with a vasculitis such as granulomatosis with polyangiitis.
Vital signs were normal, and the patient appeared nontoxic. Physical examination demonstrated mildly cracked lips, oropharyngeal erythema with small petechiae on the soft palate, a morbilliform rash throughout his extremities and trunk (Figure 1), and confluent, brightly erythematous patches on his palms and soles with associated edema (Figure 2 and Appendix Figure). No lymphadenopathy, hepatosplenomegaly, or joint swelling was noted. CBC and basic chemistry panel remained normal; however, hepatic chemistries were notable for alanine aminotransferase (ALT) of 128 U/L, aspartate aminotransferase (AST) of 49 U/L, total bilirubin of 3.7 mg/dL, direct bilirubin of 2.4 mg/dL, total protein of 7.1 g/dL, albumin of 4.1 g/dL, and alkaline phosphatase of 197 U/L. Urinalysis detected bilirubin without blood, protein, bacteria, cells, or casts. The patient was admitted to the hospital.
The patient now has acute-onset upper respiratory symptoms with oral mucosal erythema, edema and erythema of the hands and feet with morbilliform rash of the extremities, and liver injury causing bilirubinuria. The patient’s initial symptoms may have had some response to therapy, but the current presentation suggests ongoing evolution of disease. Reactive infectious mucocutaneous eruptions include chlamydia, influenza, parainfluenza, and enteroviruses. Measles is possible given its recent resurgence; however, absence of coryza or Koplik spots and the peripheral distribution of the rash without initial truncal involvement make this less likely. Mycoplasma pneumonia–induced rash and mucositis might present with respiratory symptoms and this rash distribution, but typically involves two or more mucosal sites.
Iatrogenic causes are important to consider given the recent exposure to NSAIDs, specifically Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). In this patient, however, SJS/TEN is unlikely as it typically presents 1 to 3 weeks after exposure, with a truncal-predominant rash rarely involving the palms and soles.
Despite the absence of conjunctivitis and cervical lymphadenopathy, one additional consideration is Kawasaki disease (KD). Though more common in children, it may rarely present in adulthood. The time course of manifesting symptoms with potential steroid responsiveness raises suspicion for this diagnosis.
During a 4-day hospitalization, he developed mild bilateral conjunctivitis, peeling lips, and scleral icterus. CBC remained within normal limits. A peripheral smear demonstrated toxic neutrophilic granulation with normal erythrocytes and platelets. HIV and hepatitis A, B, and C serologies were negative. Blood cultures were negative. CRP and ESR increased to 4.3 mg/dL and 56 mm/h, respectively. Hepatic chemistries increased to ALT 155 U/L, AST 101 U/L, total bilirubin 5.1 mg/dL, direct bilirubin 3.3 mg/dL, and alkaline phosphatase 211 U/L. Right upper-quadrant ultrasound demonstrated gallbladder distention (11.3 cm × 5.0 cm; normal, 10.0 cm × 4.0 cm) without stones, wall thickening, or pericholecystic fluid; sonographic Murphy sign was negative. The liver was unremarkable with normal flow in the portal vein.
The patient’s persistent reactive neutrophilic granulation and rising CRP and ESR indicate ongoing inflammation. The largely direct hyperbilirubinemia with hepatitis, minimal findings on ultrasound imaging, and lack of Murphy sign suggest either direct infection of the liver or cholestasis. Viral serologies for EBV, HSV, and CMV should be sent, although these viruses are less commonly associated with oral rash and conjunctivitis. The marked degree of cholestasis makes adenovirus and mycoplasma less likely. Leptospirosis should be considered given the degree of liver injury with potential conjunctival suffusion. However, oral involvement would be atypical; renal injury is absent; and the patient denied pertinent exposures, vomiting, diarrhea, or persistent myalgias.
It is important to know whether the patient continued to receive
Low-grade fevers resolved without intervention. Tests were sent for tick-borne (ehrlichiosis, babesiosis, RMSF, anaplasmosis), viral (EBV, West Nile virus, parvovirus, CMV, coxsackievirus, adenovirus), other bacterial and protozoal (syphilis, Coxiella, leptospirosis, Lyme, Giardia), and autoimmune (antinuclear antibody, perinuclear antineutrophil cytoplasmic antibody, double-stranded DNA) diseases. Topical steroids and antihistamines were prescribed for a suspected viral exanthem. Empiric doxycycline was prescribed to treat possible tick-borne disease, and the patient was discharged home. At home, progressive darkening of the urine was noted. Outpatient testing demonstrated rising ALT to 377 U/L, AST to 183 U/L, total bilirubin to 5.9 mg/dL, direct bilirubin to 3.5 mg/dL, and alkaline phosphatase to 301 U/L. The patient was readmitted for further evaluation.
Despite concerns of the treating physicians, features of this case make tick-borne infections less likely. Lyme disease does not typically cause significant laboratory abnormalities and is classically associated with erythema migrans rather than a mucocutaneous rash. Relapsing fever, ehrlichioses, and rickettsial infections are associated with leukopenia and thrombocytopenia in addition to hepatocellular, rather than cholestatic, liver injury. The lack of response to doxycycline is helpful diagnostically: most tick-borne infections, in addition to leptospirosis, respond well to treatment. While babesiosis, tularemia, and Powassan or Heartland viruses transmitted by ticks are not treated with doxycycline, babesiosis often involves a hemolytic anemia (not seen in this case), and this patient’s laboratory abnormalities and rash are not characteristic of tularemia or viral tick-borne infections.
Either a new or reactivated viral infection with liver inflammation or an autoimmune etiology, specifically KD, remain the most likely etiology of the patient’s symptoms.
He remained asymptomatic during a 6-day hospitalization. His oral lesions resolved. The morbilliform rash coalesced into confluent macules with fine desquamation on the extremities and trunk. There was prominent periungual and palmar/plantar desquamation (Figure 3 and Figure 4). CBC demonstrated hemoglobin of 12.6 g/dL and platelets of 399,000/μL. CRP was undetectable at <0.5 mg/dL; however, ESR increased to 110 mm/h. Transaminases increased to ALT 551 U/L and AST 219 U/L. Serum alkaline phosphatase and bilirubin decreased without intervention. Albumin and total protein remained unchanged. All infectious and autoimmune testing sent from the prior admission returned negative.
An acute-onset viral-like prodrome with fevers potentially responsive to steroids, followed by conjunctivitis, oral erythema and cracked lips, morbilliform rash with hand and foot erythema and edema, cholestatic hepatitis, and subsequent periungual desquamation is highly suggestive of KD. It would be interesting to revisit the patient’s prior episode of aseptic meningitis to see whether any other symptoms were suggestive of KD. While intravenous immunoglobulin (IVIg) and aspirin are standard therapies for the acute febrile phase of KD, the patient is now nearly 2 weeks into his clinical course, rendering their utility uncertain. Nonetheless, screening for coronary aneurysms should be pursued, which may help confirm the diagnosis.
Upon reviewing the evolution of the findings, a diagnosis of adult-onset KD was made. IVIg 2g/kg and aspirin 325 mg were administered. Echocardiogram did not show any evidence of coronary artery aneurysm, myocarditis, pericarditis, wall motion abnormalities, or pericardial effusion. Computed tomography (CT) coronary angiogram confirmed normal coronary arteries without aneurysm. The patient was discharged home without fever on daily aspirin, and all hepatic chemistries and inflammatory markers normalized. Follow-up cardiac magnetic resonance imaging at 3 months and CT angiogram at 6 months remained normal. The patient remains well now 2 years after the original diagnosis and treatment.
DISCUSSION
KD, also known as mucocutaneous lymph node syndrome, is a vasculitis that typically affects children younger than 5 years.1 Having a sibling with KD confers a 10- to 15-fold higher risk, suggesting a genetic component to the disease.2 The highest incidence of KD is in persons of East Asian descent, but KD can affect patients of all races and ethnicities. In the United States, the majority of patients with KD are non-Hispanic White, followed by Black, Hispanic, and Asian.3 The etiology is still unknown, but it is posited that an unidentified, ubiquitous infectious agent may trigger KD in genetically susceptible individuals.4
KD can cause aneurysms and thromboses in medium-sized blood vessels throughout the body.5,6 The classic presentation involves 5 days of high fever plus four or more of the symptoms in the mnemonic CRASH: conjunctival injection, rash (polymorphous), adenopathy (cervical), strawberry tongue (or red, cracked lips and oropharyngeal edema), hand (erythema and induration of hands or feet, followed by periungual desquamation).7 Multiple organ systems may be affected, manifesting as abdominal pain, arthritis, pneumonitis, aseptic meningitis, and acalculous distention of the gallbladder (hydrops).7 The most feared consequence is coronary artery involvement, which leads to aneurysm, thrombosis, and sudden death.
Though no definitive diagnostic test exists, certain laboratory findings support the diagnosis, such as sterile pyuria, thrombocytosis, elevated CRP and ESR, transaminitis, and hypoalbuminemia.7 Diagnosis requires exclusion of illnesses with similar presentations, such as bacterial, viral, and tick-borne infections; drug hypersensitivity reactions; toxic shock syndrome; scarlet fever; juvenile rheumatoid arthritis; and other rheumatologic conditions. Some cases of KD present with fewer than four of the principal (CRASH) symptoms—these are termed “incomplete” KD. The combination of supportive laboratory findings and echocardiogram can facilitate diagnosis of incomplete KD, which carries a similar risk of coronary artery aneurysm.7
Though primarily a disease of childhood, KD can present in adults.8 Adults, compared with children, are less likely to have thrombocytosis and more likely to have cervical adenopathy, arthralgias, and hepatic test abnormalities.8 Although coronary artery aneurysms occur less frequently in adults compared with children, timely diagnosis and treatment is key to preventing this life-threatening complication.8
In children, treatment is IVIg 2 g/kg and aspirin 80 to 100 mg/kg daily until afebrile for several days.9 Some require a second dose of IVIg.9 Children are then maintained on 3 to 5 mg/kg of aspirin daily for 6 to 8 weeks.9 IVIg, given within 10 days of the onset of fever, is highly effective at preventing coronary artery aneurysms.10,11 When coronary aneurysms do occur, treatment is with aspirin or clopidogrel. Very large aneurysms require systemic anticoagulation. After the acute illness, children are monitored with serial cardiac imaging at 2 weeks and 6 to 8 weeks after diagnosis.7 In adults, the optimal imaging timing is unknown. Echocardiography often cannot visualize the coronary arteries, necessitating coronary CT angiography or cardiac MRI.
Despite the presence of classic features, this patient’s diagnosis was delayed because of the rarity of KD in adults and the need to exclude more common diseases. Furthermore, the administration of dexamethasone likely shortened his febrile period and ameliorated some symptoms,12 affecting the natural history of his illness. The diagnosis relied on three components: ruling out common diagnoses, noting two unusual findings (gallbladder hydrops, desquamating periungual rash), and broadening the differential to include adult presentations of childhood disease. Review of the literature suggests very few causes for gallbladder hydrops: impacted stones, cystic fibrosis, cystic duct narrowing due to tumor or lymph nodes, KD, and bacterial and parasitic disease (eg, salmonella, ascariasis). Gallbladder hydrops and periungual desquamation are seen together only in KD.13 Given the complexity of diagnosis in adults, the time to diagnosis is often delayed compared with that for children. While IVIg treatment is preferred within 10 days of the onset of fever, this patient received IVIg on day 14, given the relatively benign nature of IVIg and the considerable morbidity associated with coronary artery aneurysms. Dosing for aspirin is unclear in adults.8 This patient was started on 325 mg aspirin daily. He recovered fully and remains free of coronary changes at two years after initial diagnosis. This case is an excellent reminder that, after exclusion of common diagnoses, reflection on the most unusual aspects of the case and consideration of childhood diseases is particularly important in our younger patients.
TEACHING POINTS
- Extended fever should broaden the differential to include rheumatologic diagnoses.
- KD is rare in adults but can present with classic findings from childhood.
- Early treatment with IVIg and aspirin can be lifesaving in patients with KD, including adults.
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Article in Japanese. Arerugi. 1967;16(3):178-222.
2. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9(4):185-194. https://doi.org/10.1016/j.ijid.2005.03.002
3. Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-488. https://doi.org/10.1097/INF.0b013e3181cf8705
4. Rowley A, Baker S, Arollo D, et al. A hepacivirus-like protein is targeted by the antibody response to Kawasaki disease (KD) [abstract]. Open Forum Infect Dis. 2019;6(suppl 2):S48.
5. Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5(9):e003289. https://doi.org/10.1161/JAHA.116.003289
6. Zhao QM, Chu C, Wu L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. 2019;144(6):e20192254. https://doi.org/10.1542/peds.2019-2254
7. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. https://doi.org/10.1542/peds.2004-2182
8. Sève P, Stankovic K, Smail A, Durand DV, Marchand G, Broussolle C. Adult Kawasaki disease: report of two cases and literature review. Semin Arthritis Rheum. 2005;34(6):785-792. https://doi.org/10.1016/j.semarthrit.2005.01.012
9. Shulman ST. Intravenous immunoglobulin for the treatment of Kawasaki disease. Pediatr Ann. 2017;46(1):e25-e28. https://doi.org/10.3928/19382359-20161212-01
10. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341-347. https://doi.org/10.1056/NEJM198608073150601
11. Rowley AH, Duffy CE, Shulman ST. Prevention of giant coronary artery aneurysms in Kawasaki disease by intravenous gamma globulin therapy. J Pediatr. 1988;113(2):290-294. https://doi/org/10.1016/s0022-3476(88)80267-1
12. Lim YJ, Jung JW. Clinical outcomes of initial dexamethasone treatment combined with a single high dose of intravenous immunoglobulin for primary treatment of Kawasaki disease. Yonsei Med J. 2014;55(5):1260-1266. https://doi.org/10.3349/ymj.2014.55.5.1260
13. Sun Q, Zhang J, Yang Y. Gallbladder hydrops associated with Kawasaki disease: a case report and literature review. Clin Pediatr (Phila). 2018;57(3):341-343. https://doi.org/10.1177/0009922817696468
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 39-year-old previously healthy man presented to the emergency department (ED) with abrupt-onset fever, headache, back pain, myalgias, chills, and photophobia. His past medical history included seasonal allergies and an episode of aseptic meningitis 8 years prior. He denied cough, dysuria, weakness, numbness, or visual changes. He denied using tobacco or injection drugs and rarely drank alcohol. His only medication was acetaminophen for fever.
The patient’s sudden fever indicates the rapid onset of an inflammatory state. While the headache and photophobia might be a result of an underlying systemic infection or an irritant like blood in the cerebral spinal fluid (CSF), one must consider meningitis. Potential sources for sudden meningitis include infectious, autoimmune (rheumatoid arthritis, systemic lupus erythematosus [SLE]), or drug-induced aseptic meningitis, and structural etiologies (ruptured cyst). Recrudescence of prior disease may also present acutely (Mollaret meningitis). Malignant etiologies, being more indolent, seem less likely. Back pain may indicate an epidural inflammatory process like epidural abscess; however, the patient denies risk factors such as injection drug use or recent procedures.
The patient’s temperature was 101.2 °F; blood pressure, 120/72 mm Hg; and heart rate, 112 bpm. He appeared comfortable, without meningismus or spinal tenderness. Pupils were reactive; eyes were without icterus, injection, or suffusion. Cardiac exam was normal. Lungs were clear to auscultation. He had no abdominal tenderness, hepatosplenomegaly, or lymphadenopathy. Cranial nerves II through XII, balance, coordination, strength, and sensation were intact. No rash was noted. Complete blood count (CBC), basic and hepatic chemistry panels, urinalysis, and serum lactate tests were within normal limits. Erythrocyte sedimentation rate (ESR) was elevated to 15 mm/h (normal range, 3-10 mm/h), C-reactive protein (CRP) to 2.4 mg/dL (normal range, <0.5 mg/dL), and procalcitonin to 0.07 ng/mL (normal range, <0.05 ng/mL). The patient was treated with intravenous (IV) fluids, ketorolac, dexamethasone, and acetaminophen, with resolution of symptoms. Given his rapid improvement, absence of meningismus, and lack of immunocompromise, lumbar puncture was deferred. A diagnosis of nonspecific viral syndrome was made. He was discharged home.
Certainly, a systemic infection (eg, influenza, adenovirus, arbovirus-related infection, HIV) could be a cause of this patient’s presentation. Notably, less than two-thirds of patients with meningitis present with the classic triad of fever, neck stiffness, and altered mental status. In this patient with fever, headache, and photophobia, aseptic meningitis should still be considered. While the negative procalcitonin and rapid clinical improvement without antibiotics make acute bacterial meningitis unlikely, nonbacterial causes of meningeal irritation can be severe and life-threatening. An assessment for jolt accentuation of the headache might have been helpful. Information about time of year, geographic exposures (vector-borne infections), and sick contacts (viral illness) can inform the clinical decision to pursue lumbar puncture. Additional history regarding his previous aseptic meningitis would be helpful, as it could suggest a recurrent inflammatory process. Causes of recurrent aseptic meningitis include infectious (herpes simplex virus [HSV], Epstein-Barr virus [EBV], syphilis), drug-related (nonsteroidal anti-inflammatory drugs [NSAIDs]), structural (epidermoid cyst with rupture), and autoimmune (lupus, Sjögren syndrome, Behçet disease) etiologies.
The mildly elevated inflammatory markers are nonspecific and reflect the patient’s known inflammatory state. The dexamethasone given for symptomatic management may have had some therapeutic effect in the setting of an autoimmune process, with additional contribution from ketorolac and acetaminophen.
He returned to the ED 3 days later with a pruritic, disseminated rash involving his palms and soles, accompanied by hand swelling and tingling. Although his headache and photophobia resolved, he reported a productive cough, nasal congestion, and sore throat. He also reported orange-pink urine without dysuria or urinary frequency. Additional questioning revealed a recent motorcycle trip to the Great Lakes region. During this trip, he did not camp, interact with animals or ticks, or swim in streams or lakes. He did not eat any raw, undercooked, or locally hunted meats. He denied new medications, soaps or detergents, or sexual contacts. He had started taking acetaminophen and ibuprofen around the clock since prior discharge.
The orange-pink urine and acute-onset palmoplantar rash with recent fever help narrow the differential. Orange-pink urine might suggest bilirubinuria from liver injury, hemolysis with hemoglobinuria, or myoglobinuria. Most concerning would be hematuria associated with glomerular injury and a systemic vasculopathy.
The rash on the palms and soles should be further characterized as blanching or nonblanching. Blanching, indicating vasodilation of intact blood vessels, is seen with many drug eruptions and viral exanthems. Nonblanching, suggesting broken capillaries (petechiae or purpura), would suggest vasculitis or vasculopathy from emboli, infection, or inflammation. A palmoplantar rash in febrile illness should first prompt evaluation for life-threatening conditions, followed by consideration of both infectious and noninfectious etiologies. Acutely fatal infections include Rocky Mountain spotted fever (RMSF), meningococcemia, toxic shock syndrome, infective endocarditis, and rat-bite fever. The rash, fever, headache, and outdoor exposure raise the possibility of a rickettsial infection, including RMSF, which can be contracted rarely around the Great Lakes. Other life-threatening infections seem unlikely, as the patient would have significantly deteriorated without proper medical care by now. Palmoplantar rash with fever can also be seen in other bacterial infections (eg, secondary syphilis, arbovirus infections, typhus) and in viral infections (eg, cytomegalovirus [CMV], EBV, human herpesvirus-6 [HHV-6], HIV, coxsackievirus, and papular-purpuric gloves and socks syndrome caused by parvovirus B19). Noninfectious considerations include drug hypersensitivity rashes, neoplasm (eg, cutaneous T-cell lymphoma), or inflammatory conditions (eg, SLE, vasculitis). Drug reaction with eosinophilia and systemic symptoms (may also present with severe illness.
The acetaminophen and ibuprofen may be masking ongoing fevers. The cough, nasal congestion, and sore throat might be part of a viral prodrome or, in tandem with fever, associated with a vasculitis such as granulomatosis with polyangiitis.
Vital signs were normal, and the patient appeared nontoxic. Physical examination demonstrated mildly cracked lips, oropharyngeal erythema with small petechiae on the soft palate, a morbilliform rash throughout his extremities and trunk (Figure 1), and confluent, brightly erythematous patches on his palms and soles with associated edema (Figure 2 and Appendix Figure). No lymphadenopathy, hepatosplenomegaly, or joint swelling was noted. CBC and basic chemistry panel remained normal; however, hepatic chemistries were notable for alanine aminotransferase (ALT) of 128 U/L, aspartate aminotransferase (AST) of 49 U/L, total bilirubin of 3.7 mg/dL, direct bilirubin of 2.4 mg/dL, total protein of 7.1 g/dL, albumin of 4.1 g/dL, and alkaline phosphatase of 197 U/L. Urinalysis detected bilirubin without blood, protein, bacteria, cells, or casts. The patient was admitted to the hospital.
The patient now has acute-onset upper respiratory symptoms with oral mucosal erythema, edema and erythema of the hands and feet with morbilliform rash of the extremities, and liver injury causing bilirubinuria. The patient’s initial symptoms may have had some response to therapy, but the current presentation suggests ongoing evolution of disease. Reactive infectious mucocutaneous eruptions include chlamydia, influenza, parainfluenza, and enteroviruses. Measles is possible given its recent resurgence; however, absence of coryza or Koplik spots and the peripheral distribution of the rash without initial truncal involvement make this less likely. Mycoplasma pneumonia–induced rash and mucositis might present with respiratory symptoms and this rash distribution, but typically involves two or more mucosal sites.
Iatrogenic causes are important to consider given the recent exposure to NSAIDs, specifically Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). In this patient, however, SJS/TEN is unlikely as it typically presents 1 to 3 weeks after exposure, with a truncal-predominant rash rarely involving the palms and soles.
Despite the absence of conjunctivitis and cervical lymphadenopathy, one additional consideration is Kawasaki disease (KD). Though more common in children, it may rarely present in adulthood. The time course of manifesting symptoms with potential steroid responsiveness raises suspicion for this diagnosis.
During a 4-day hospitalization, he developed mild bilateral conjunctivitis, peeling lips, and scleral icterus. CBC remained within normal limits. A peripheral smear demonstrated toxic neutrophilic granulation with normal erythrocytes and platelets. HIV and hepatitis A, B, and C serologies were negative. Blood cultures were negative. CRP and ESR increased to 4.3 mg/dL and 56 mm/h, respectively. Hepatic chemistries increased to ALT 155 U/L, AST 101 U/L, total bilirubin 5.1 mg/dL, direct bilirubin 3.3 mg/dL, and alkaline phosphatase 211 U/L. Right upper-quadrant ultrasound demonstrated gallbladder distention (11.3 cm × 5.0 cm; normal, 10.0 cm × 4.0 cm) without stones, wall thickening, or pericholecystic fluid; sonographic Murphy sign was negative. The liver was unremarkable with normal flow in the portal vein.
The patient’s persistent reactive neutrophilic granulation and rising CRP and ESR indicate ongoing inflammation. The largely direct hyperbilirubinemia with hepatitis, minimal findings on ultrasound imaging, and lack of Murphy sign suggest either direct infection of the liver or cholestasis. Viral serologies for EBV, HSV, and CMV should be sent, although these viruses are less commonly associated with oral rash and conjunctivitis. The marked degree of cholestasis makes adenovirus and mycoplasma less likely. Leptospirosis should be considered given the degree of liver injury with potential conjunctival suffusion. However, oral involvement would be atypical; renal injury is absent; and the patient denied pertinent exposures, vomiting, diarrhea, or persistent myalgias.
It is important to know whether the patient continued to receive
Low-grade fevers resolved without intervention. Tests were sent for tick-borne (ehrlichiosis, babesiosis, RMSF, anaplasmosis), viral (EBV, West Nile virus, parvovirus, CMV, coxsackievirus, adenovirus), other bacterial and protozoal (syphilis, Coxiella, leptospirosis, Lyme, Giardia), and autoimmune (antinuclear antibody, perinuclear antineutrophil cytoplasmic antibody, double-stranded DNA) diseases. Topical steroids and antihistamines were prescribed for a suspected viral exanthem. Empiric doxycycline was prescribed to treat possible tick-borne disease, and the patient was discharged home. At home, progressive darkening of the urine was noted. Outpatient testing demonstrated rising ALT to 377 U/L, AST to 183 U/L, total bilirubin to 5.9 mg/dL, direct bilirubin to 3.5 mg/dL, and alkaline phosphatase to 301 U/L. The patient was readmitted for further evaluation.
Despite concerns of the treating physicians, features of this case make tick-borne infections less likely. Lyme disease does not typically cause significant laboratory abnormalities and is classically associated with erythema migrans rather than a mucocutaneous rash. Relapsing fever, ehrlichioses, and rickettsial infections are associated with leukopenia and thrombocytopenia in addition to hepatocellular, rather than cholestatic, liver injury. The lack of response to doxycycline is helpful diagnostically: most tick-borne infections, in addition to leptospirosis, respond well to treatment. While babesiosis, tularemia, and Powassan or Heartland viruses transmitted by ticks are not treated with doxycycline, babesiosis often involves a hemolytic anemia (not seen in this case), and this patient’s laboratory abnormalities and rash are not characteristic of tularemia or viral tick-borne infections.
Either a new or reactivated viral infection with liver inflammation or an autoimmune etiology, specifically KD, remain the most likely etiology of the patient’s symptoms.
He remained asymptomatic during a 6-day hospitalization. His oral lesions resolved. The morbilliform rash coalesced into confluent macules with fine desquamation on the extremities and trunk. There was prominent periungual and palmar/plantar desquamation (Figure 3 and Figure 4). CBC demonstrated hemoglobin of 12.6 g/dL and platelets of 399,000/μL. CRP was undetectable at <0.5 mg/dL; however, ESR increased to 110 mm/h. Transaminases increased to ALT 551 U/L and AST 219 U/L. Serum alkaline phosphatase and bilirubin decreased without intervention. Albumin and total protein remained unchanged. All infectious and autoimmune testing sent from the prior admission returned negative.
An acute-onset viral-like prodrome with fevers potentially responsive to steroids, followed by conjunctivitis, oral erythema and cracked lips, morbilliform rash with hand and foot erythema and edema, cholestatic hepatitis, and subsequent periungual desquamation is highly suggestive of KD. It would be interesting to revisit the patient’s prior episode of aseptic meningitis to see whether any other symptoms were suggestive of KD. While intravenous immunoglobulin (IVIg) and aspirin are standard therapies for the acute febrile phase of KD, the patient is now nearly 2 weeks into his clinical course, rendering their utility uncertain. Nonetheless, screening for coronary aneurysms should be pursued, which may help confirm the diagnosis.
Upon reviewing the evolution of the findings, a diagnosis of adult-onset KD was made. IVIg 2g/kg and aspirin 325 mg were administered. Echocardiogram did not show any evidence of coronary artery aneurysm, myocarditis, pericarditis, wall motion abnormalities, or pericardial effusion. Computed tomography (CT) coronary angiogram confirmed normal coronary arteries without aneurysm. The patient was discharged home without fever on daily aspirin, and all hepatic chemistries and inflammatory markers normalized. Follow-up cardiac magnetic resonance imaging at 3 months and CT angiogram at 6 months remained normal. The patient remains well now 2 years after the original diagnosis and treatment.
DISCUSSION
KD, also known as mucocutaneous lymph node syndrome, is a vasculitis that typically affects children younger than 5 years.1 Having a sibling with KD confers a 10- to 15-fold higher risk, suggesting a genetic component to the disease.2 The highest incidence of KD is in persons of East Asian descent, but KD can affect patients of all races and ethnicities. In the United States, the majority of patients with KD are non-Hispanic White, followed by Black, Hispanic, and Asian.3 The etiology is still unknown, but it is posited that an unidentified, ubiquitous infectious agent may trigger KD in genetically susceptible individuals.4
KD can cause aneurysms and thromboses in medium-sized blood vessels throughout the body.5,6 The classic presentation involves 5 days of high fever plus four or more of the symptoms in the mnemonic CRASH: conjunctival injection, rash (polymorphous), adenopathy (cervical), strawberry tongue (or red, cracked lips and oropharyngeal edema), hand (erythema and induration of hands or feet, followed by periungual desquamation).7 Multiple organ systems may be affected, manifesting as abdominal pain, arthritis, pneumonitis, aseptic meningitis, and acalculous distention of the gallbladder (hydrops).7 The most feared consequence is coronary artery involvement, which leads to aneurysm, thrombosis, and sudden death.
Though no definitive diagnostic test exists, certain laboratory findings support the diagnosis, such as sterile pyuria, thrombocytosis, elevated CRP and ESR, transaminitis, and hypoalbuminemia.7 Diagnosis requires exclusion of illnesses with similar presentations, such as bacterial, viral, and tick-borne infections; drug hypersensitivity reactions; toxic shock syndrome; scarlet fever; juvenile rheumatoid arthritis; and other rheumatologic conditions. Some cases of KD present with fewer than four of the principal (CRASH) symptoms—these are termed “incomplete” KD. The combination of supportive laboratory findings and echocardiogram can facilitate diagnosis of incomplete KD, which carries a similar risk of coronary artery aneurysm.7
Though primarily a disease of childhood, KD can present in adults.8 Adults, compared with children, are less likely to have thrombocytosis and more likely to have cervical adenopathy, arthralgias, and hepatic test abnormalities.8 Although coronary artery aneurysms occur less frequently in adults compared with children, timely diagnosis and treatment is key to preventing this life-threatening complication.8
In children, treatment is IVIg 2 g/kg and aspirin 80 to 100 mg/kg daily until afebrile for several days.9 Some require a second dose of IVIg.9 Children are then maintained on 3 to 5 mg/kg of aspirin daily for 6 to 8 weeks.9 IVIg, given within 10 days of the onset of fever, is highly effective at preventing coronary artery aneurysms.10,11 When coronary aneurysms do occur, treatment is with aspirin or clopidogrel. Very large aneurysms require systemic anticoagulation. After the acute illness, children are monitored with serial cardiac imaging at 2 weeks and 6 to 8 weeks after diagnosis.7 In adults, the optimal imaging timing is unknown. Echocardiography often cannot visualize the coronary arteries, necessitating coronary CT angiography or cardiac MRI.
Despite the presence of classic features, this patient’s diagnosis was delayed because of the rarity of KD in adults and the need to exclude more common diseases. Furthermore, the administration of dexamethasone likely shortened his febrile period and ameliorated some symptoms,12 affecting the natural history of his illness. The diagnosis relied on three components: ruling out common diagnoses, noting two unusual findings (gallbladder hydrops, desquamating periungual rash), and broadening the differential to include adult presentations of childhood disease. Review of the literature suggests very few causes for gallbladder hydrops: impacted stones, cystic fibrosis, cystic duct narrowing due to tumor or lymph nodes, KD, and bacterial and parasitic disease (eg, salmonella, ascariasis). Gallbladder hydrops and periungual desquamation are seen together only in KD.13 Given the complexity of diagnosis in adults, the time to diagnosis is often delayed compared with that for children. While IVIg treatment is preferred within 10 days of the onset of fever, this patient received IVIg on day 14, given the relatively benign nature of IVIg and the considerable morbidity associated with coronary artery aneurysms. Dosing for aspirin is unclear in adults.8 This patient was started on 325 mg aspirin daily. He recovered fully and remains free of coronary changes at two years after initial diagnosis. This case is an excellent reminder that, after exclusion of common diagnoses, reflection on the most unusual aspects of the case and consideration of childhood diseases is particularly important in our younger patients.
TEACHING POINTS
- Extended fever should broaden the differential to include rheumatologic diagnoses.
- KD is rare in adults but can present with classic findings from childhood.
- Early treatment with IVIg and aspirin can be lifesaving in patients with KD, including adults.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 39-year-old previously healthy man presented to the emergency department (ED) with abrupt-onset fever, headache, back pain, myalgias, chills, and photophobia. His past medical history included seasonal allergies and an episode of aseptic meningitis 8 years prior. He denied cough, dysuria, weakness, numbness, or visual changes. He denied using tobacco or injection drugs and rarely drank alcohol. His only medication was acetaminophen for fever.
The patient’s sudden fever indicates the rapid onset of an inflammatory state. While the headache and photophobia might be a result of an underlying systemic infection or an irritant like blood in the cerebral spinal fluid (CSF), one must consider meningitis. Potential sources for sudden meningitis include infectious, autoimmune (rheumatoid arthritis, systemic lupus erythematosus [SLE]), or drug-induced aseptic meningitis, and structural etiologies (ruptured cyst). Recrudescence of prior disease may also present acutely (Mollaret meningitis). Malignant etiologies, being more indolent, seem less likely. Back pain may indicate an epidural inflammatory process like epidural abscess; however, the patient denies risk factors such as injection drug use or recent procedures.
The patient’s temperature was 101.2 °F; blood pressure, 120/72 mm Hg; and heart rate, 112 bpm. He appeared comfortable, without meningismus or spinal tenderness. Pupils were reactive; eyes were without icterus, injection, or suffusion. Cardiac exam was normal. Lungs were clear to auscultation. He had no abdominal tenderness, hepatosplenomegaly, or lymphadenopathy. Cranial nerves II through XII, balance, coordination, strength, and sensation were intact. No rash was noted. Complete blood count (CBC), basic and hepatic chemistry panels, urinalysis, and serum lactate tests were within normal limits. Erythrocyte sedimentation rate (ESR) was elevated to 15 mm/h (normal range, 3-10 mm/h), C-reactive protein (CRP) to 2.4 mg/dL (normal range, <0.5 mg/dL), and procalcitonin to 0.07 ng/mL (normal range, <0.05 ng/mL). The patient was treated with intravenous (IV) fluids, ketorolac, dexamethasone, and acetaminophen, with resolution of symptoms. Given his rapid improvement, absence of meningismus, and lack of immunocompromise, lumbar puncture was deferred. A diagnosis of nonspecific viral syndrome was made. He was discharged home.
Certainly, a systemic infection (eg, influenza, adenovirus, arbovirus-related infection, HIV) could be a cause of this patient’s presentation. Notably, less than two-thirds of patients with meningitis present with the classic triad of fever, neck stiffness, and altered mental status. In this patient with fever, headache, and photophobia, aseptic meningitis should still be considered. While the negative procalcitonin and rapid clinical improvement without antibiotics make acute bacterial meningitis unlikely, nonbacterial causes of meningeal irritation can be severe and life-threatening. An assessment for jolt accentuation of the headache might have been helpful. Information about time of year, geographic exposures (vector-borne infections), and sick contacts (viral illness) can inform the clinical decision to pursue lumbar puncture. Additional history regarding his previous aseptic meningitis would be helpful, as it could suggest a recurrent inflammatory process. Causes of recurrent aseptic meningitis include infectious (herpes simplex virus [HSV], Epstein-Barr virus [EBV], syphilis), drug-related (nonsteroidal anti-inflammatory drugs [NSAIDs]), structural (epidermoid cyst with rupture), and autoimmune (lupus, Sjögren syndrome, Behçet disease) etiologies.
The mildly elevated inflammatory markers are nonspecific and reflect the patient’s known inflammatory state. The dexamethasone given for symptomatic management may have had some therapeutic effect in the setting of an autoimmune process, with additional contribution from ketorolac and acetaminophen.
He returned to the ED 3 days later with a pruritic, disseminated rash involving his palms and soles, accompanied by hand swelling and tingling. Although his headache and photophobia resolved, he reported a productive cough, nasal congestion, and sore throat. He also reported orange-pink urine without dysuria or urinary frequency. Additional questioning revealed a recent motorcycle trip to the Great Lakes region. During this trip, he did not camp, interact with animals or ticks, or swim in streams or lakes. He did not eat any raw, undercooked, or locally hunted meats. He denied new medications, soaps or detergents, or sexual contacts. He had started taking acetaminophen and ibuprofen around the clock since prior discharge.
The orange-pink urine and acute-onset palmoplantar rash with recent fever help narrow the differential. Orange-pink urine might suggest bilirubinuria from liver injury, hemolysis with hemoglobinuria, or myoglobinuria. Most concerning would be hematuria associated with glomerular injury and a systemic vasculopathy.
The rash on the palms and soles should be further characterized as blanching or nonblanching. Blanching, indicating vasodilation of intact blood vessels, is seen with many drug eruptions and viral exanthems. Nonblanching, suggesting broken capillaries (petechiae or purpura), would suggest vasculitis or vasculopathy from emboli, infection, or inflammation. A palmoplantar rash in febrile illness should first prompt evaluation for life-threatening conditions, followed by consideration of both infectious and noninfectious etiologies. Acutely fatal infections include Rocky Mountain spotted fever (RMSF), meningococcemia, toxic shock syndrome, infective endocarditis, and rat-bite fever. The rash, fever, headache, and outdoor exposure raise the possibility of a rickettsial infection, including RMSF, which can be contracted rarely around the Great Lakes. Other life-threatening infections seem unlikely, as the patient would have significantly deteriorated without proper medical care by now. Palmoplantar rash with fever can also be seen in other bacterial infections (eg, secondary syphilis, arbovirus infections, typhus) and in viral infections (eg, cytomegalovirus [CMV], EBV, human herpesvirus-6 [HHV-6], HIV, coxsackievirus, and papular-purpuric gloves and socks syndrome caused by parvovirus B19). Noninfectious considerations include drug hypersensitivity rashes, neoplasm (eg, cutaneous T-cell lymphoma), or inflammatory conditions (eg, SLE, vasculitis). Drug reaction with eosinophilia and systemic symptoms (may also present with severe illness.
The acetaminophen and ibuprofen may be masking ongoing fevers. The cough, nasal congestion, and sore throat might be part of a viral prodrome or, in tandem with fever, associated with a vasculitis such as granulomatosis with polyangiitis.
Vital signs were normal, and the patient appeared nontoxic. Physical examination demonstrated mildly cracked lips, oropharyngeal erythema with small petechiae on the soft palate, a morbilliform rash throughout his extremities and trunk (Figure 1), and confluent, brightly erythematous patches on his palms and soles with associated edema (Figure 2 and Appendix Figure). No lymphadenopathy, hepatosplenomegaly, or joint swelling was noted. CBC and basic chemistry panel remained normal; however, hepatic chemistries were notable for alanine aminotransferase (ALT) of 128 U/L, aspartate aminotransferase (AST) of 49 U/L, total bilirubin of 3.7 mg/dL, direct bilirubin of 2.4 mg/dL, total protein of 7.1 g/dL, albumin of 4.1 g/dL, and alkaline phosphatase of 197 U/L. Urinalysis detected bilirubin without blood, protein, bacteria, cells, or casts. The patient was admitted to the hospital.
The patient now has acute-onset upper respiratory symptoms with oral mucosal erythema, edema and erythema of the hands and feet with morbilliform rash of the extremities, and liver injury causing bilirubinuria. The patient’s initial symptoms may have had some response to therapy, but the current presentation suggests ongoing evolution of disease. Reactive infectious mucocutaneous eruptions include chlamydia, influenza, parainfluenza, and enteroviruses. Measles is possible given its recent resurgence; however, absence of coryza or Koplik spots and the peripheral distribution of the rash without initial truncal involvement make this less likely. Mycoplasma pneumonia–induced rash and mucositis might present with respiratory symptoms and this rash distribution, but typically involves two or more mucosal sites.
Iatrogenic causes are important to consider given the recent exposure to NSAIDs, specifically Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). In this patient, however, SJS/TEN is unlikely as it typically presents 1 to 3 weeks after exposure, with a truncal-predominant rash rarely involving the palms and soles.
Despite the absence of conjunctivitis and cervical lymphadenopathy, one additional consideration is Kawasaki disease (KD). Though more common in children, it may rarely present in adulthood. The time course of manifesting symptoms with potential steroid responsiveness raises suspicion for this diagnosis.
During a 4-day hospitalization, he developed mild bilateral conjunctivitis, peeling lips, and scleral icterus. CBC remained within normal limits. A peripheral smear demonstrated toxic neutrophilic granulation with normal erythrocytes and platelets. HIV and hepatitis A, B, and C serologies were negative. Blood cultures were negative. CRP and ESR increased to 4.3 mg/dL and 56 mm/h, respectively. Hepatic chemistries increased to ALT 155 U/L, AST 101 U/L, total bilirubin 5.1 mg/dL, direct bilirubin 3.3 mg/dL, and alkaline phosphatase 211 U/L. Right upper-quadrant ultrasound demonstrated gallbladder distention (11.3 cm × 5.0 cm; normal, 10.0 cm × 4.0 cm) without stones, wall thickening, or pericholecystic fluid; sonographic Murphy sign was negative. The liver was unremarkable with normal flow in the portal vein.
The patient’s persistent reactive neutrophilic granulation and rising CRP and ESR indicate ongoing inflammation. The largely direct hyperbilirubinemia with hepatitis, minimal findings on ultrasound imaging, and lack of Murphy sign suggest either direct infection of the liver or cholestasis. Viral serologies for EBV, HSV, and CMV should be sent, although these viruses are less commonly associated with oral rash and conjunctivitis. The marked degree of cholestasis makes adenovirus and mycoplasma less likely. Leptospirosis should be considered given the degree of liver injury with potential conjunctival suffusion. However, oral involvement would be atypical; renal injury is absent; and the patient denied pertinent exposures, vomiting, diarrhea, or persistent myalgias.
It is important to know whether the patient continued to receive
Low-grade fevers resolved without intervention. Tests were sent for tick-borne (ehrlichiosis, babesiosis, RMSF, anaplasmosis), viral (EBV, West Nile virus, parvovirus, CMV, coxsackievirus, adenovirus), other bacterial and protozoal (syphilis, Coxiella, leptospirosis, Lyme, Giardia), and autoimmune (antinuclear antibody, perinuclear antineutrophil cytoplasmic antibody, double-stranded DNA) diseases. Topical steroids and antihistamines were prescribed for a suspected viral exanthem. Empiric doxycycline was prescribed to treat possible tick-borne disease, and the patient was discharged home. At home, progressive darkening of the urine was noted. Outpatient testing demonstrated rising ALT to 377 U/L, AST to 183 U/L, total bilirubin to 5.9 mg/dL, direct bilirubin to 3.5 mg/dL, and alkaline phosphatase to 301 U/L. The patient was readmitted for further evaluation.
Despite concerns of the treating physicians, features of this case make tick-borne infections less likely. Lyme disease does not typically cause significant laboratory abnormalities and is classically associated with erythema migrans rather than a mucocutaneous rash. Relapsing fever, ehrlichioses, and rickettsial infections are associated with leukopenia and thrombocytopenia in addition to hepatocellular, rather than cholestatic, liver injury. The lack of response to doxycycline is helpful diagnostically: most tick-borne infections, in addition to leptospirosis, respond well to treatment. While babesiosis, tularemia, and Powassan or Heartland viruses transmitted by ticks are not treated with doxycycline, babesiosis often involves a hemolytic anemia (not seen in this case), and this patient’s laboratory abnormalities and rash are not characteristic of tularemia or viral tick-borne infections.
Either a new or reactivated viral infection with liver inflammation or an autoimmune etiology, specifically KD, remain the most likely etiology of the patient’s symptoms.
He remained asymptomatic during a 6-day hospitalization. His oral lesions resolved. The morbilliform rash coalesced into confluent macules with fine desquamation on the extremities and trunk. There was prominent periungual and palmar/plantar desquamation (Figure 3 and Figure 4). CBC demonstrated hemoglobin of 12.6 g/dL and platelets of 399,000/μL. CRP was undetectable at <0.5 mg/dL; however, ESR increased to 110 mm/h. Transaminases increased to ALT 551 U/L and AST 219 U/L. Serum alkaline phosphatase and bilirubin decreased without intervention. Albumin and total protein remained unchanged. All infectious and autoimmune testing sent from the prior admission returned negative.
An acute-onset viral-like prodrome with fevers potentially responsive to steroids, followed by conjunctivitis, oral erythema and cracked lips, morbilliform rash with hand and foot erythema and edema, cholestatic hepatitis, and subsequent periungual desquamation is highly suggestive of KD. It would be interesting to revisit the patient’s prior episode of aseptic meningitis to see whether any other symptoms were suggestive of KD. While intravenous immunoglobulin (IVIg) and aspirin are standard therapies for the acute febrile phase of KD, the patient is now nearly 2 weeks into his clinical course, rendering their utility uncertain. Nonetheless, screening for coronary aneurysms should be pursued, which may help confirm the diagnosis.
Upon reviewing the evolution of the findings, a diagnosis of adult-onset KD was made. IVIg 2g/kg and aspirin 325 mg were administered. Echocardiogram did not show any evidence of coronary artery aneurysm, myocarditis, pericarditis, wall motion abnormalities, or pericardial effusion. Computed tomography (CT) coronary angiogram confirmed normal coronary arteries without aneurysm. The patient was discharged home without fever on daily aspirin, and all hepatic chemistries and inflammatory markers normalized. Follow-up cardiac magnetic resonance imaging at 3 months and CT angiogram at 6 months remained normal. The patient remains well now 2 years after the original diagnosis and treatment.
DISCUSSION
KD, also known as mucocutaneous lymph node syndrome, is a vasculitis that typically affects children younger than 5 years.1 Having a sibling with KD confers a 10- to 15-fold higher risk, suggesting a genetic component to the disease.2 The highest incidence of KD is in persons of East Asian descent, but KD can affect patients of all races and ethnicities. In the United States, the majority of patients with KD are non-Hispanic White, followed by Black, Hispanic, and Asian.3 The etiology is still unknown, but it is posited that an unidentified, ubiquitous infectious agent may trigger KD in genetically susceptible individuals.4
KD can cause aneurysms and thromboses in medium-sized blood vessels throughout the body.5,6 The classic presentation involves 5 days of high fever plus four or more of the symptoms in the mnemonic CRASH: conjunctival injection, rash (polymorphous), adenopathy (cervical), strawberry tongue (or red, cracked lips and oropharyngeal edema), hand (erythema and induration of hands or feet, followed by periungual desquamation).7 Multiple organ systems may be affected, manifesting as abdominal pain, arthritis, pneumonitis, aseptic meningitis, and acalculous distention of the gallbladder (hydrops).7 The most feared consequence is coronary artery involvement, which leads to aneurysm, thrombosis, and sudden death.
Though no definitive diagnostic test exists, certain laboratory findings support the diagnosis, such as sterile pyuria, thrombocytosis, elevated CRP and ESR, transaminitis, and hypoalbuminemia.7 Diagnosis requires exclusion of illnesses with similar presentations, such as bacterial, viral, and tick-borne infections; drug hypersensitivity reactions; toxic shock syndrome; scarlet fever; juvenile rheumatoid arthritis; and other rheumatologic conditions. Some cases of KD present with fewer than four of the principal (CRASH) symptoms—these are termed “incomplete” KD. The combination of supportive laboratory findings and echocardiogram can facilitate diagnosis of incomplete KD, which carries a similar risk of coronary artery aneurysm.7
Though primarily a disease of childhood, KD can present in adults.8 Adults, compared with children, are less likely to have thrombocytosis and more likely to have cervical adenopathy, arthralgias, and hepatic test abnormalities.8 Although coronary artery aneurysms occur less frequently in adults compared with children, timely diagnosis and treatment is key to preventing this life-threatening complication.8
In children, treatment is IVIg 2 g/kg and aspirin 80 to 100 mg/kg daily until afebrile for several days.9 Some require a second dose of IVIg.9 Children are then maintained on 3 to 5 mg/kg of aspirin daily for 6 to 8 weeks.9 IVIg, given within 10 days of the onset of fever, is highly effective at preventing coronary artery aneurysms.10,11 When coronary aneurysms do occur, treatment is with aspirin or clopidogrel. Very large aneurysms require systemic anticoagulation. After the acute illness, children are monitored with serial cardiac imaging at 2 weeks and 6 to 8 weeks after diagnosis.7 In adults, the optimal imaging timing is unknown. Echocardiography often cannot visualize the coronary arteries, necessitating coronary CT angiography or cardiac MRI.
Despite the presence of classic features, this patient’s diagnosis was delayed because of the rarity of KD in adults and the need to exclude more common diseases. Furthermore, the administration of dexamethasone likely shortened his febrile period and ameliorated some symptoms,12 affecting the natural history of his illness. The diagnosis relied on three components: ruling out common diagnoses, noting two unusual findings (gallbladder hydrops, desquamating periungual rash), and broadening the differential to include adult presentations of childhood disease. Review of the literature suggests very few causes for gallbladder hydrops: impacted stones, cystic fibrosis, cystic duct narrowing due to tumor or lymph nodes, KD, and bacterial and parasitic disease (eg, salmonella, ascariasis). Gallbladder hydrops and periungual desquamation are seen together only in KD.13 Given the complexity of diagnosis in adults, the time to diagnosis is often delayed compared with that for children. While IVIg treatment is preferred within 10 days of the onset of fever, this patient received IVIg on day 14, given the relatively benign nature of IVIg and the considerable morbidity associated with coronary artery aneurysms. Dosing for aspirin is unclear in adults.8 This patient was started on 325 mg aspirin daily. He recovered fully and remains free of coronary changes at two years after initial diagnosis. This case is an excellent reminder that, after exclusion of common diagnoses, reflection on the most unusual aspects of the case and consideration of childhood diseases is particularly important in our younger patients.
TEACHING POINTS
- Extended fever should broaden the differential to include rheumatologic diagnoses.
- KD is rare in adults but can present with classic findings from childhood.
- Early treatment with IVIg and aspirin can be lifesaving in patients with KD, including adults.
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Article in Japanese. Arerugi. 1967;16(3):178-222.
2. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9(4):185-194. https://doi.org/10.1016/j.ijid.2005.03.002
3. Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-488. https://doi.org/10.1097/INF.0b013e3181cf8705
4. Rowley A, Baker S, Arollo D, et al. A hepacivirus-like protein is targeted by the antibody response to Kawasaki disease (KD) [abstract]. Open Forum Infect Dis. 2019;6(suppl 2):S48.
5. Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5(9):e003289. https://doi.org/10.1161/JAHA.116.003289
6. Zhao QM, Chu C, Wu L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. 2019;144(6):e20192254. https://doi.org/10.1542/peds.2019-2254
7. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. https://doi.org/10.1542/peds.2004-2182
8. Sève P, Stankovic K, Smail A, Durand DV, Marchand G, Broussolle C. Adult Kawasaki disease: report of two cases and literature review. Semin Arthritis Rheum. 2005;34(6):785-792. https://doi.org/10.1016/j.semarthrit.2005.01.012
9. Shulman ST. Intravenous immunoglobulin for the treatment of Kawasaki disease. Pediatr Ann. 2017;46(1):e25-e28. https://doi.org/10.3928/19382359-20161212-01
10. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341-347. https://doi.org/10.1056/NEJM198608073150601
11. Rowley AH, Duffy CE, Shulman ST. Prevention of giant coronary artery aneurysms in Kawasaki disease by intravenous gamma globulin therapy. J Pediatr. 1988;113(2):290-294. https://doi/org/10.1016/s0022-3476(88)80267-1
12. Lim YJ, Jung JW. Clinical outcomes of initial dexamethasone treatment combined with a single high dose of intravenous immunoglobulin for primary treatment of Kawasaki disease. Yonsei Med J. 2014;55(5):1260-1266. https://doi.org/10.3349/ymj.2014.55.5.1260
13. Sun Q, Zhang J, Yang Y. Gallbladder hydrops associated with Kawasaki disease: a case report and literature review. Clin Pediatr (Phila). 2018;57(3):341-343. https://doi.org/10.1177/0009922817696468
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Article in Japanese. Arerugi. 1967;16(3):178-222.
2. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9(4):185-194. https://doi.org/10.1016/j.ijid.2005.03.002
3. Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-488. https://doi.org/10.1097/INF.0b013e3181cf8705
4. Rowley A, Baker S, Arollo D, et al. A hepacivirus-like protein is targeted by the antibody response to Kawasaki disease (KD) [abstract]. Open Forum Infect Dis. 2019;6(suppl 2):S48.
5. Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5(9):e003289. https://doi.org/10.1161/JAHA.116.003289
6. Zhao QM, Chu C, Wu L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. 2019;144(6):e20192254. https://doi.org/10.1542/peds.2019-2254
7. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. https://doi.org/10.1542/peds.2004-2182
8. Sève P, Stankovic K, Smail A, Durand DV, Marchand G, Broussolle C. Adult Kawasaki disease: report of two cases and literature review. Semin Arthritis Rheum. 2005;34(6):785-792. https://doi.org/10.1016/j.semarthrit.2005.01.012
9. Shulman ST. Intravenous immunoglobulin for the treatment of Kawasaki disease. Pediatr Ann. 2017;46(1):e25-e28. https://doi.org/10.3928/19382359-20161212-01
10. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341-347. https://doi.org/10.1056/NEJM198608073150601
11. Rowley AH, Duffy CE, Shulman ST. Prevention of giant coronary artery aneurysms in Kawasaki disease by intravenous gamma globulin therapy. J Pediatr. 1988;113(2):290-294. https://doi/org/10.1016/s0022-3476(88)80267-1
12. Lim YJ, Jung JW. Clinical outcomes of initial dexamethasone treatment combined with a single high dose of intravenous immunoglobulin for primary treatment of Kawasaki disease. Yonsei Med J. 2014;55(5):1260-1266. https://doi.org/10.3349/ymj.2014.55.5.1260
13. Sun Q, Zhang J, Yang Y. Gallbladder hydrops associated with Kawasaki disease: a case report and literature review. Clin Pediatr (Phila). 2018;57(3):341-343. https://doi.org/10.1177/0009922817696468
© 2021 Society of Hospital Medicine
Out of Sight, Not Out of Mind
A 73-year-old man presented to clinic with 6 weeks of headache. He occasionally experienced generalized headaches throughout his life that resolved with naproxen. His new headache was characterized by a progressively worsening sensation of left-eye pressure with radiation to the left temple. Over the previous week, he had intermittent diplopia, left ptosis, and left lacrimation. He denied head trauma, fever, vision loss, photophobia, dysphagia, dysarthria, nausea, vomiting, or jaw claudication.
Primary headaches include tension type, migraine, and trigeminal autonomic cephalalgias (eg, cluster headache). A new headache in an older patient, particularly if protracted and progressive, prioritizes consideration of a secondary headache, which may reflect pathology within the brain parenchyma (eg, intracranial mass), blood vessels (eg, giant cell arteritis), meninges (eg, meningitis), or ventricles (eg, intraventricular cyst). Eye pain may arise from ocular and extraocular disease. Corneal abrasions, infectious keratitis, scleritis, uveitis, or acute angle-closure glaucoma are painful, although the latter is less likely given the prolonged duration of symptoms. Thyroid eye disease or other infiltrative disorders of the orbit can also cause eye discomfort.
Ptosis commonly results from degeneration of the levator aponeurosis. Other causes include third cranial nerve palsy and myasthenia gravis. Interruption of sympathetic innervation of the eyelid by lesions in the brain stem, spinal cord, lung (eg, Pancoast tumor), or cavernous sinus also can result in ptosis.
Whether the patient has monocular or binocular diplopia is uncertain. Monocular diplopia persists with only one eye open and can arise from uncorrected refractive error, corneal irregularities, lenticular opacities, or unilateral macular disease. Binocular diplopia develops from ocular misalignment due to neuromuscular weakness, extraocular muscle entrapment, or an orbital mass displacing the globe. An orbital mass would also explain the unilateral headache and unilateral ptosis.
His medical history included coronary artery disease, seronegative rheumatoid arthritis, osteoporosis, benign prostatic hypertrophy, and ureteral strictures from chronic nephrolithiasis. Following a cholecystectomy for gallstone pancreatitis 13 years earlier, he was hospitalized five more times for pancreatitis. The last episode was 6 years prior to this presentation. At that time, magnetic resonance cholangiopancreatography (MRCP) did not reveal pancreatic divisum, annular pancreas, biliary strictures, or a pancreatic mass. Esophagogastroduodenoscopy peformed during the same hospitalization showed mild gastritis. His recurrent pancreatitis was deemed idiopathic.
His medications were folic acid, cholecalciferol, lisinopril, metoprolol, omeprazole, simvastatin, aspirin, and weekly methotrexate. His sister had breast and ovarian cancer, and his brother had gastric cancer. He had two subcentimeter tubular adenomas removed during a screening colonoscopy 3 years prior. He had a 30 pack-year smoking history and quit 28 years earlier. He did not use alcohol or drugs. He was a retired chemical plant worker.
Choledocholithiasis (as discrete stones or biliary sludge) can trigger pancreatitis despite a cholecystectomy, but the recurrent episodes and negative MRCP should prompt consideration of other causes, such as alcohol. Hypercalcemia, hypertriglyceridemia, and medications are infrequent causes of pancreatic inflammation. IgG4-related disease (IgG4-RD) causes autoimmune pancreatitis and can infiltrate the eyelids, lacrimal glands, extraocular muscles, or orbital connective tissue. Malignancy of the pancreas or ampulla can trigger pancreatitis by causing pancreatic duct obstruction but would not go undetected for 13 years.
The patient was evaluated by an ophthalmologist and a neurologist. His heart rate was 52 beats per minute and blood pressure, 174/70 mm Hg; other vital signs were normal. He had conjunctival chemosis, ptosis, and nonpulsatile proptosis of the left eye with tenderness and increased resistance to retropulsion compared to the right eye (Figure 1). Visual acuity was 20/25 for the right eye and hand motions only in the left eye. The pupils were reactive and symmetric without afferent pupillary defect. There was no optic nerve swelling or pallor. Abduction, adduction, and elevation of the left eye were restricted and associated with diplopia. Movement of the right eye was unrestricted. There was no other facial asymmetry. Facial sensation was normal. Corneal reflexes were intact. Shoulder shrug strength was equal and symmetric. Tongue protrusion was midline. Olfaction and hearing were not assessed. Strength, sensation, and deep tendon reflexes were normal in all extremities. The plantar response was flexor bilaterally.
Unilateral ptosis, chemosis, proptosis, ophthalmoplegia, eye tenderness, and visual loss collectively point to a space-occupying orbital disease. Orbital masses are caused by cancers, infections such as mucormycosis (usually in an immunocompromised host), and inflammatory disorders such as thyroid orbitopathy, sarcoidosis, IgG4-related orbitopathy, granulomatosis with polyangiitis, and orbital pseudotumor (idiopathic inflammation of the orbit). Chemosis reflects edema of the conjunctiva, which can arise from direct conjunctival injury (eg, allergy, infection, or trauma), interruption of the venous drainage of the conjunctiva by vascular disorders (eg, cavernous sinus thrombosis or carotid-cavernous fistula), or space-occupying diseases of the orbit. Monocular visual loss arises from a prechiasmal lesion, and acute monocular visual loss is more commonly caused by posterior ocular pathology (eg, retina or optic nerve) than anterior disease (eg, keratitis). Visual loss in the presence of an orbital process suggests a compressive or infiltrative disease of the optic nerve.
Complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and thyroid function tests were normal. Interferon-gamma release assay, HIV antibody, rapid plasma reagin, Lyme antibody, antinuclear antibody, and antineutrophil cytoplasmic antibody (ANCA) tests were negative. A noncontrast computed tomography (CT) scan of the head revealed thickening of the left inferior rectus muscle. Orbital magnetic resonance imaging (MRI) with gadolinium and fluid-attenuated inversion recovery imaging demonstrated a T2 hyperintense, heterogeneous 1.4-cm mass in the left inferior rectus muscle (Figure 2). There was no carotid-cavernous fistula, brain mass, or meningeal enhancement.
An isolated mass in one ocular muscle raises the probability of a cancer. The most common malignant orbital tumor is B-cell lymphoma. Metastatic cancer to the eye is rare; breast, prostate, and lung cancer account for the majority of cases. The family history of breast and ovarian cancer raises the possibility of a BRCA mutation, which is also associated with gastric, pancreatic, and prostate malignancies. Granulomatosis with polyangiitis may be ANCA negative in localized sino-orbital disease. Biopsy of the orbital mass is the next step.
The patient underwent transconjunctival orbitotomy with excision of the left inferior rectus mass. Two days later, he presented to the emergency department with acute onset epigastric pain, nausea, and vomiting. A comprehensive review of systems, which had not been performed until this visit, revealed an unintentional 20-lb weight loss over the previous 3 months. He had a progressive ache in the left anterior groin that was dull, tender, nonradiating, and worse with weight bearing. He denied melena or hematochezia.
His temperature was 37 °C; heart rate, 98 beats per minute; and blood pressure, 128/63 mm Hg. He had midepigastric tenderness and point tenderness over the anterior iliac spine. White blood cell count was 12,600/μL; hemo globin, 14.5 g/dL; and platelet count, 158,000/μL. Serum lipase was 7,108 U/L. Serum creatinine, calcium, and triglyceride levels were normal. Alkaline phosphatase was 117 U/L (normal, 34-104 U/L); total bilirubin, 1.1 mg/dL; alanine aminotransferase (ALT), 119 U/L (normal, 7-52 U/L); and aspartate aminotransferase (AST), 236 U/L (normal, 13-39 U/L). Troponin I was undetectable, and an electrocardiogram demonstrated sinus tachycardia. Urinalysis was normal.
Concomitant pancreatitis and hepatitis with an elevated AST-to-ALT ratio should prompt evaluation of recurrent choledocholithiasis and a repeat inquiry about alcohol use. His medications should be reviewed for an association with pancreatitis. Anterior groin discomfort usually reflects osteoarthritis of the hip joint, inguinal hernia, or inguinal lymphadenopathy. Groin pain may be referred from spinal nerve root compression, aortoiliac occlusion, or nephrolithiasis. Weight loss in the presence of an inferior rectus mass suggests one of the aforementioned systemic diseases with orbital manifestations. Pancreatitis and groin discomfort may be important clues, but the chronicity of the recurrent pancreatitis and the high prevalence of hip osteoarthritis make it equally likely that they are unrelated to the eye disease.
CT scan of the abdomen and pelvis with contrast showed peripancreatic edema with fat stranding but no pancreatic or hepatobiliary mass. The common bile duct was normal. A 2.2×1.3-cm mass in the right posterior subphrenic space, a lytic lesion in the left anterior inferior iliac spine, and right nonobstructive nephrolithiasis were identified. CT scan of the chest with contrast showed multiple subpleural nodules and innumerable parenchymal nodules. Subcentimeter hilar, mediastinal, and prevascular lymphadenopathy were present, as well as multiple sclerotic lesions in the right fourth and sixth ribs. Prostate-specific antigen was 0.7 ng/mL (normal, ≤ 4.0 ng/mL). Cancer antigen 19-9 level was 5.5 U/mL (normal, < 37.0 U/mL), and carcinoembryonic antigen (CEA) was 100.1 ng/mL (normal, 0-3 U/mL).
Widespread pulmonary nodules, diffuse lymphadenopathy, and bony lesions raise concern for a metastatic malignancy. There is no evidence of a primary carcinoma. The lack of hepatic involvement reduces the likelihood of a gastrointestinal tumor, although a rectal cancer, which may drain directly into the inferior vena cava and bypass the portal circulation, could present as lung metastases on CT imaging. Lymphoma is plausible given the diffuse lymphadenopathy and orbital mass. Sarcoidosis and histiocytic disorders (eg, Langerhans cell histiocytosis) also cause orbital disease, pulmonary nodules, lymphadenopathy, and bone lesions, although a subphrenic mass would be atypical for both disorders; furthermore, the majority of patients with adult Langerhans cell histiocytosis smoke cigarettes. The elevated CEA makes a metastatic solid tumor more likely than lymphoma but does not specify the location of the primary tumor.
Pathology of the inferior rectus muscle mass showed well-differentiated adenocarcinoma (Figure 3A and 3B). A CT-guided biopsy of the left anterior inferior iliac spine revealed well-differentiated adenocarcinoma (Figure 3C). Adenocarcinoma of unknown primary wasdiagnosed.
Subsequent immunohistochemical (IHC) staining was positive for cytokeratin 7 (CK7) and mucicarmine (Figure 3D and 3E) and negative for cytokeratin 20 (CK20) and thyroid transcription factor 1 (TTF1). This IHC profile suggested pancreatic or upper gastrointestinal tract lineage. Positron emission tomography–CT (PET-CT) scan was aborted because of dyspnea and chest pressure following contrast administration. He declined further imaging or endoscopy. He received palliative radiation and three cycles of paclitaxel and gemcitabine for cancer of unknown primary (CUP). Two months later, he developed bilateral upper-arm weakness due to C7 and T2 cord compression from vertebral and epidural metastases; his symptoms progressed despite salvage chemotherapy. He was transitioned to comfort care and died at home 9 months after diagnosis.
DISCUSSION
This patient’s new headache and ocular abnormalities led to the discovery of an inferior rectus muscle mass. Initially unrecognized unintentional weight loss and hip pain recast a localized orbital syndrome as a systemic disease with pancreatic, ocular, pulmonary, lymph node, and skeletal pathology. Biopsies of the orbital rectus muscle and iliac bone demonstrated metastatic adenocarcinoma. Imaging studies did not identify a primary cancer, but IHC analysis suggested carcinoma of upper gastrointestinal or pancreatic origin.
Acute and chronic pancreatitis are both associated with pancreatic cancer.1 Chronic pancreatitis is associated with an increasing cumulative risk of pancreatic cancer; a potential mechanism is chronic inflammation with malignant transformation.2,3 There is also a 20-fold increased risk of pancreatic cancer in the first 2 years following an episode of acute pancreatitis,4 which may develop from malignant pancreatic duct obstruction. Although the post–acute pancreatitis risk of pancreatic cancer attenuates over time, a two-fold increased risk of pancreatic cancer remains after 10 years,4 which suggests that acute pancreatitis (particularly when idiopathic) either contributes to or shares pathogenesis with pancreatic adenocarcinoma. In elderly patients without gallstones or alcohol use, an abdominal CT scan or MRI shortly after resolution of the acute pancreatitis may be considered to assess for an underlying pancreatic tumor.5
CUP is a histologically defined malignancy without a known primary anatomic site despite an extensive evaluation. CUP accounts for up to 10% of all cancer diagnoses.6 CUP is ascribed to a primary cancer that remains too small to be detected or spontaneous regression of the primary cancer.7 Approximately 70% of autopsies of patients with CUP identify the primary tumor, which most commonly originates in the lung, gastrointestinal tract, breast, or pancreas.8
When a metastatic focus of cancer is found but the initial diagnostic evaluation (including CT scan of the chest, abdomen, and pelvis) fails to locate a primary cancer, the next step in searching for the tissue of origin is an IHC analysis of the tumor specimen. IHC analysis is a multistep staining process that can identify major categories of cancer, including carcinoma (adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma) and poorly or undifferentiated neoplasms (including carcinoma, lymphoma, sarcoma, or melanoma). Eighty-five percent of CUP cases are adenocarcinoma, 10% are squamous cell carcinoma, and the remaining 5% are undifferentiated neoplasms.9
There are no consensus guidelines for imaging in patients with CUP who have already undergone a CT scan of the chest, abdomen, and pelvis. Mammography is indicated in women with metastatic adenocarcinoma or axillary lymphadenopathy.7 MRI of the breast is obtained when mammography is nondiagnostic and the suspicion for breast cancer is high. Small clinical studies and meta-analyses support the use of PET-CT scans,7 although one study found that a PET-CT scan was not superior to CT imaging in identifying the primary tumor site in CUP.10 Endoscopy of the upper airway or gastrointestinal tract is rarely diagnostic in the absence of referable symptoms or a suggestive IHC profile (eg, CK7−, CK20+ suggestive of colon cancer).6
Molecular cancer classification has emerged as a useful diagnostic technique in CUP. Cancer cells retain gene expression patterns based on cellular origin, and a tumor’s profile can be compared with a reference database of known cancers, aiding in the identification of the primary tumor type. Molecular cancer classifier assays that use gene expression profiling can accurately determine a primary site11 and have been shown to be concordant with IHC testing.12 Molecular cancer classification is distinct from genetic assays that identify mutations for which there are approved therapies. Serum tumor markers are generally not useful in establishing the primary tumor and should be considered based on the clinical presentation (eg, prostate-specific antigen testing in a man with adenocarcinoma of unknown primary and osteoblastic metastases).
CUP is classified as favorable or unfavorable based on the IHC, pattern of spread, and serum markers in certain cases.6 Approximately 20% of CUP patients can be categorized into favorable subsets, such as adenocarcinoma in a single axillary lymph node in a female patient suggestive of a breast primary cancer, or squamous cell carcinoma in a cervical lymph node suggestive of a head or neck primary cancer.7 The remaining 80% of cases are categorized as unfavorable CUP and often have multiple metastases. Our patient’s pattern of spread and limited response to chemotherapy is characteristic of the unfavorable subset of CUP. The median survival of this group is 9 months, and only 25% of patients survive longer than 1 year.13
Biomarker-driven treatment of specific molecular targets independent of the tissue of origin (tissue-agnostic therapy) has shown promising results in the treatment of skin, lung, thyroid, colorectal, and gastric cancers.14 Pembrolizumab was the first drug approved by the US Food and Drug Administration based on a tumor’s biomarker without regard to its primary location. Data to support this approach for treating CUP are evolving and offer hope for patients with specific molecular targets.
Following the focused neuro-ophthalmologic evaluations, with focused examination and imaging, the hospitalist’s review of systems at the time of the final admission for pancreatitis set in motion an evaluation that led to a diagnosis of metastatic cancer. The risk factor of recurrent pancreatitis and IHC results suggested that pancreatic adenocarcinoma was the most likely primary tumor. As the focus of cancer treatment shifts away from the tissue of origin and toward molecular and genetic profiles, the search for the primary site may decrease in importance. In the future, even when we do not know the cancer’s origin, we may still know precisely what to do. But for now, as in this patient, our treatments continue to be based on a tumor that is out of sight, but not out of mind.
KEY TEACHING POINTS
- Acute and chronic pancreatitis are associated with an increased risk of pancreatic adenocarcinoma.
- CUP is a cancer in which diagnostic testing does not identify a primary tumor site. Immunohistochemistry and molecular analysis, imaging, and endoscopy are utilized selectively to identify a primary tumor type.
- Treatment of CUP currently depends on the suspected tissue of origin and pattern of spread.
- Tissue-agnostic therapy could allow for treatment for CUP patients independent of the tissue of origin.
Acknowledgments
We thank Andrew Mick, OD, for his review of an earlier version of this manuscript and Peter Phillips, MD, for his interpretation of the pathologic images.
1. Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(111):1711-1719. https://doi.org/10.1038/s41395-018-0255-9
2. Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23(11):2964-2970. https://doi.org/10.1093/annonc/mds140
3. Ekbom A, McLaughlin JK, Nyren O. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;329(20):1502-1503. https://doi.org/10.1056/NEJM199311113292016
4. Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(156):1729-1736. https://doi.org/10.1053/j.gastro.2018.02.011
5. Frampas E, Morla O, Regenet N, Eugene T, Dupas B, Meurette G. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94(7-8):741-755. https://doi.org/10.1016/j.diii.2013.03.013
6. Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765. https://doi.org/10.1056/NEJMra1303917
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017. 2018;35(3):199-206. https://doi.org//10.1053/j.semdp.2017.11.013
8. Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43(14):2026-2036. https://doi.org/10.1016/j.ejca.2007.06.023
9. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69(3):271-278. https://doi.org/10.1016/j.critrevonc.2008.09.005
10. Moller AK, Loft A, Berthelsen AK, et al. A prospective comparison of 18F-FDG PET/CT and CT as diagnostic tools to identify the primary tumor site in patients with extracervical carcinoma of unknown primary site. Oncologist. 2012;17(9):1146-1154. https://doi.org/10.1634/theoncologist.2011-0449
11. Economopoulou P, Mountzios G, Pavlidis N, Pentheroudakis G. Cancer of unknown primary origin in the genomic era: elucidating the dark box of cancer. Cancer Treat Rev. 2015;41(7):598-604. https://doi.org/10.1016/j.ctrv.2015.05.010
12. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642. https://doi.org/10.1007/s11864-013-0257-1
13. Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol. 2011;8(12):701-710. https://doi.org/10.1038/nrclinonc.2011.158
14. Luoh SW, Flaherty KT. When tissue is no longer the issue: tissue-agnostic cancer therapy comes of age. Ann Intern Med. 2018;169(4):233-239. https://doi.org/10.7326/M17-2832
A 73-year-old man presented to clinic with 6 weeks of headache. He occasionally experienced generalized headaches throughout his life that resolved with naproxen. His new headache was characterized by a progressively worsening sensation of left-eye pressure with radiation to the left temple. Over the previous week, he had intermittent diplopia, left ptosis, and left lacrimation. He denied head trauma, fever, vision loss, photophobia, dysphagia, dysarthria, nausea, vomiting, or jaw claudication.
Primary headaches include tension type, migraine, and trigeminal autonomic cephalalgias (eg, cluster headache). A new headache in an older patient, particularly if protracted and progressive, prioritizes consideration of a secondary headache, which may reflect pathology within the brain parenchyma (eg, intracranial mass), blood vessels (eg, giant cell arteritis), meninges (eg, meningitis), or ventricles (eg, intraventricular cyst). Eye pain may arise from ocular and extraocular disease. Corneal abrasions, infectious keratitis, scleritis, uveitis, or acute angle-closure glaucoma are painful, although the latter is less likely given the prolonged duration of symptoms. Thyroid eye disease or other infiltrative disorders of the orbit can also cause eye discomfort.
Ptosis commonly results from degeneration of the levator aponeurosis. Other causes include third cranial nerve palsy and myasthenia gravis. Interruption of sympathetic innervation of the eyelid by lesions in the brain stem, spinal cord, lung (eg, Pancoast tumor), or cavernous sinus also can result in ptosis.
Whether the patient has monocular or binocular diplopia is uncertain. Monocular diplopia persists with only one eye open and can arise from uncorrected refractive error, corneal irregularities, lenticular opacities, or unilateral macular disease. Binocular diplopia develops from ocular misalignment due to neuromuscular weakness, extraocular muscle entrapment, or an orbital mass displacing the globe. An orbital mass would also explain the unilateral headache and unilateral ptosis.
His medical history included coronary artery disease, seronegative rheumatoid arthritis, osteoporosis, benign prostatic hypertrophy, and ureteral strictures from chronic nephrolithiasis. Following a cholecystectomy for gallstone pancreatitis 13 years earlier, he was hospitalized five more times for pancreatitis. The last episode was 6 years prior to this presentation. At that time, magnetic resonance cholangiopancreatography (MRCP) did not reveal pancreatic divisum, annular pancreas, biliary strictures, or a pancreatic mass. Esophagogastroduodenoscopy peformed during the same hospitalization showed mild gastritis. His recurrent pancreatitis was deemed idiopathic.
His medications were folic acid, cholecalciferol, lisinopril, metoprolol, omeprazole, simvastatin, aspirin, and weekly methotrexate. His sister had breast and ovarian cancer, and his brother had gastric cancer. He had two subcentimeter tubular adenomas removed during a screening colonoscopy 3 years prior. He had a 30 pack-year smoking history and quit 28 years earlier. He did not use alcohol or drugs. He was a retired chemical plant worker.
Choledocholithiasis (as discrete stones or biliary sludge) can trigger pancreatitis despite a cholecystectomy, but the recurrent episodes and negative MRCP should prompt consideration of other causes, such as alcohol. Hypercalcemia, hypertriglyceridemia, and medications are infrequent causes of pancreatic inflammation. IgG4-related disease (IgG4-RD) causes autoimmune pancreatitis and can infiltrate the eyelids, lacrimal glands, extraocular muscles, or orbital connective tissue. Malignancy of the pancreas or ampulla can trigger pancreatitis by causing pancreatic duct obstruction but would not go undetected for 13 years.
The patient was evaluated by an ophthalmologist and a neurologist. His heart rate was 52 beats per minute and blood pressure, 174/70 mm Hg; other vital signs were normal. He had conjunctival chemosis, ptosis, and nonpulsatile proptosis of the left eye with tenderness and increased resistance to retropulsion compared to the right eye (Figure 1). Visual acuity was 20/25 for the right eye and hand motions only in the left eye. The pupils were reactive and symmetric without afferent pupillary defect. There was no optic nerve swelling or pallor. Abduction, adduction, and elevation of the left eye were restricted and associated with diplopia. Movement of the right eye was unrestricted. There was no other facial asymmetry. Facial sensation was normal. Corneal reflexes were intact. Shoulder shrug strength was equal and symmetric. Tongue protrusion was midline. Olfaction and hearing were not assessed. Strength, sensation, and deep tendon reflexes were normal in all extremities. The plantar response was flexor bilaterally.
Unilateral ptosis, chemosis, proptosis, ophthalmoplegia, eye tenderness, and visual loss collectively point to a space-occupying orbital disease. Orbital masses are caused by cancers, infections such as mucormycosis (usually in an immunocompromised host), and inflammatory disorders such as thyroid orbitopathy, sarcoidosis, IgG4-related orbitopathy, granulomatosis with polyangiitis, and orbital pseudotumor (idiopathic inflammation of the orbit). Chemosis reflects edema of the conjunctiva, which can arise from direct conjunctival injury (eg, allergy, infection, or trauma), interruption of the venous drainage of the conjunctiva by vascular disorders (eg, cavernous sinus thrombosis or carotid-cavernous fistula), or space-occupying diseases of the orbit. Monocular visual loss arises from a prechiasmal lesion, and acute monocular visual loss is more commonly caused by posterior ocular pathology (eg, retina or optic nerve) than anterior disease (eg, keratitis). Visual loss in the presence of an orbital process suggests a compressive or infiltrative disease of the optic nerve.
Complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and thyroid function tests were normal. Interferon-gamma release assay, HIV antibody, rapid plasma reagin, Lyme antibody, antinuclear antibody, and antineutrophil cytoplasmic antibody (ANCA) tests were negative. A noncontrast computed tomography (CT) scan of the head revealed thickening of the left inferior rectus muscle. Orbital magnetic resonance imaging (MRI) with gadolinium and fluid-attenuated inversion recovery imaging demonstrated a T2 hyperintense, heterogeneous 1.4-cm mass in the left inferior rectus muscle (Figure 2). There was no carotid-cavernous fistula, brain mass, or meningeal enhancement.
An isolated mass in one ocular muscle raises the probability of a cancer. The most common malignant orbital tumor is B-cell lymphoma. Metastatic cancer to the eye is rare; breast, prostate, and lung cancer account for the majority of cases. The family history of breast and ovarian cancer raises the possibility of a BRCA mutation, which is also associated with gastric, pancreatic, and prostate malignancies. Granulomatosis with polyangiitis may be ANCA negative in localized sino-orbital disease. Biopsy of the orbital mass is the next step.
The patient underwent transconjunctival orbitotomy with excision of the left inferior rectus mass. Two days later, he presented to the emergency department with acute onset epigastric pain, nausea, and vomiting. A comprehensive review of systems, which had not been performed until this visit, revealed an unintentional 20-lb weight loss over the previous 3 months. He had a progressive ache in the left anterior groin that was dull, tender, nonradiating, and worse with weight bearing. He denied melena or hematochezia.
His temperature was 37 °C; heart rate, 98 beats per minute; and blood pressure, 128/63 mm Hg. He had midepigastric tenderness and point tenderness over the anterior iliac spine. White blood cell count was 12,600/μL; hemo globin, 14.5 g/dL; and platelet count, 158,000/μL. Serum lipase was 7,108 U/L. Serum creatinine, calcium, and triglyceride levels were normal. Alkaline phosphatase was 117 U/L (normal, 34-104 U/L); total bilirubin, 1.1 mg/dL; alanine aminotransferase (ALT), 119 U/L (normal, 7-52 U/L); and aspartate aminotransferase (AST), 236 U/L (normal, 13-39 U/L). Troponin I was undetectable, and an electrocardiogram demonstrated sinus tachycardia. Urinalysis was normal.
Concomitant pancreatitis and hepatitis with an elevated AST-to-ALT ratio should prompt evaluation of recurrent choledocholithiasis and a repeat inquiry about alcohol use. His medications should be reviewed for an association with pancreatitis. Anterior groin discomfort usually reflects osteoarthritis of the hip joint, inguinal hernia, or inguinal lymphadenopathy. Groin pain may be referred from spinal nerve root compression, aortoiliac occlusion, or nephrolithiasis. Weight loss in the presence of an inferior rectus mass suggests one of the aforementioned systemic diseases with orbital manifestations. Pancreatitis and groin discomfort may be important clues, but the chronicity of the recurrent pancreatitis and the high prevalence of hip osteoarthritis make it equally likely that they are unrelated to the eye disease.
CT scan of the abdomen and pelvis with contrast showed peripancreatic edema with fat stranding but no pancreatic or hepatobiliary mass. The common bile duct was normal. A 2.2×1.3-cm mass in the right posterior subphrenic space, a lytic lesion in the left anterior inferior iliac spine, and right nonobstructive nephrolithiasis were identified. CT scan of the chest with contrast showed multiple subpleural nodules and innumerable parenchymal nodules. Subcentimeter hilar, mediastinal, and prevascular lymphadenopathy were present, as well as multiple sclerotic lesions in the right fourth and sixth ribs. Prostate-specific antigen was 0.7 ng/mL (normal, ≤ 4.0 ng/mL). Cancer antigen 19-9 level was 5.5 U/mL (normal, < 37.0 U/mL), and carcinoembryonic antigen (CEA) was 100.1 ng/mL (normal, 0-3 U/mL).
Widespread pulmonary nodules, diffuse lymphadenopathy, and bony lesions raise concern for a metastatic malignancy. There is no evidence of a primary carcinoma. The lack of hepatic involvement reduces the likelihood of a gastrointestinal tumor, although a rectal cancer, which may drain directly into the inferior vena cava and bypass the portal circulation, could present as lung metastases on CT imaging. Lymphoma is plausible given the diffuse lymphadenopathy and orbital mass. Sarcoidosis and histiocytic disorders (eg, Langerhans cell histiocytosis) also cause orbital disease, pulmonary nodules, lymphadenopathy, and bone lesions, although a subphrenic mass would be atypical for both disorders; furthermore, the majority of patients with adult Langerhans cell histiocytosis smoke cigarettes. The elevated CEA makes a metastatic solid tumor more likely than lymphoma but does not specify the location of the primary tumor.
Pathology of the inferior rectus muscle mass showed well-differentiated adenocarcinoma (Figure 3A and 3B). A CT-guided biopsy of the left anterior inferior iliac spine revealed well-differentiated adenocarcinoma (Figure 3C). Adenocarcinoma of unknown primary wasdiagnosed.
Subsequent immunohistochemical (IHC) staining was positive for cytokeratin 7 (CK7) and mucicarmine (Figure 3D and 3E) and negative for cytokeratin 20 (CK20) and thyroid transcription factor 1 (TTF1). This IHC profile suggested pancreatic or upper gastrointestinal tract lineage. Positron emission tomography–CT (PET-CT) scan was aborted because of dyspnea and chest pressure following contrast administration. He declined further imaging or endoscopy. He received palliative radiation and three cycles of paclitaxel and gemcitabine for cancer of unknown primary (CUP). Two months later, he developed bilateral upper-arm weakness due to C7 and T2 cord compression from vertebral and epidural metastases; his symptoms progressed despite salvage chemotherapy. He was transitioned to comfort care and died at home 9 months after diagnosis.
DISCUSSION
This patient’s new headache and ocular abnormalities led to the discovery of an inferior rectus muscle mass. Initially unrecognized unintentional weight loss and hip pain recast a localized orbital syndrome as a systemic disease with pancreatic, ocular, pulmonary, lymph node, and skeletal pathology. Biopsies of the orbital rectus muscle and iliac bone demonstrated metastatic adenocarcinoma. Imaging studies did not identify a primary cancer, but IHC analysis suggested carcinoma of upper gastrointestinal or pancreatic origin.
Acute and chronic pancreatitis are both associated with pancreatic cancer.1 Chronic pancreatitis is associated with an increasing cumulative risk of pancreatic cancer; a potential mechanism is chronic inflammation with malignant transformation.2,3 There is also a 20-fold increased risk of pancreatic cancer in the first 2 years following an episode of acute pancreatitis,4 which may develop from malignant pancreatic duct obstruction. Although the post–acute pancreatitis risk of pancreatic cancer attenuates over time, a two-fold increased risk of pancreatic cancer remains after 10 years,4 which suggests that acute pancreatitis (particularly when idiopathic) either contributes to or shares pathogenesis with pancreatic adenocarcinoma. In elderly patients without gallstones or alcohol use, an abdominal CT scan or MRI shortly after resolution of the acute pancreatitis may be considered to assess for an underlying pancreatic tumor.5
CUP is a histologically defined malignancy without a known primary anatomic site despite an extensive evaluation. CUP accounts for up to 10% of all cancer diagnoses.6 CUP is ascribed to a primary cancer that remains too small to be detected or spontaneous regression of the primary cancer.7 Approximately 70% of autopsies of patients with CUP identify the primary tumor, which most commonly originates in the lung, gastrointestinal tract, breast, or pancreas.8
When a metastatic focus of cancer is found but the initial diagnostic evaluation (including CT scan of the chest, abdomen, and pelvis) fails to locate a primary cancer, the next step in searching for the tissue of origin is an IHC analysis of the tumor specimen. IHC analysis is a multistep staining process that can identify major categories of cancer, including carcinoma (adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma) and poorly or undifferentiated neoplasms (including carcinoma, lymphoma, sarcoma, or melanoma). Eighty-five percent of CUP cases are adenocarcinoma, 10% are squamous cell carcinoma, and the remaining 5% are undifferentiated neoplasms.9
There are no consensus guidelines for imaging in patients with CUP who have already undergone a CT scan of the chest, abdomen, and pelvis. Mammography is indicated in women with metastatic adenocarcinoma or axillary lymphadenopathy.7 MRI of the breast is obtained when mammography is nondiagnostic and the suspicion for breast cancer is high. Small clinical studies and meta-analyses support the use of PET-CT scans,7 although one study found that a PET-CT scan was not superior to CT imaging in identifying the primary tumor site in CUP.10 Endoscopy of the upper airway or gastrointestinal tract is rarely diagnostic in the absence of referable symptoms or a suggestive IHC profile (eg, CK7−, CK20+ suggestive of colon cancer).6
Molecular cancer classification has emerged as a useful diagnostic technique in CUP. Cancer cells retain gene expression patterns based on cellular origin, and a tumor’s profile can be compared with a reference database of known cancers, aiding in the identification of the primary tumor type. Molecular cancer classifier assays that use gene expression profiling can accurately determine a primary site11 and have been shown to be concordant with IHC testing.12 Molecular cancer classification is distinct from genetic assays that identify mutations for which there are approved therapies. Serum tumor markers are generally not useful in establishing the primary tumor and should be considered based on the clinical presentation (eg, prostate-specific antigen testing in a man with adenocarcinoma of unknown primary and osteoblastic metastases).
CUP is classified as favorable or unfavorable based on the IHC, pattern of spread, and serum markers in certain cases.6 Approximately 20% of CUP patients can be categorized into favorable subsets, such as adenocarcinoma in a single axillary lymph node in a female patient suggestive of a breast primary cancer, or squamous cell carcinoma in a cervical lymph node suggestive of a head or neck primary cancer.7 The remaining 80% of cases are categorized as unfavorable CUP and often have multiple metastases. Our patient’s pattern of spread and limited response to chemotherapy is characteristic of the unfavorable subset of CUP. The median survival of this group is 9 months, and only 25% of patients survive longer than 1 year.13
Biomarker-driven treatment of specific molecular targets independent of the tissue of origin (tissue-agnostic therapy) has shown promising results in the treatment of skin, lung, thyroid, colorectal, and gastric cancers.14 Pembrolizumab was the first drug approved by the US Food and Drug Administration based on a tumor’s biomarker without regard to its primary location. Data to support this approach for treating CUP are evolving and offer hope for patients with specific molecular targets.
Following the focused neuro-ophthalmologic evaluations, with focused examination and imaging, the hospitalist’s review of systems at the time of the final admission for pancreatitis set in motion an evaluation that led to a diagnosis of metastatic cancer. The risk factor of recurrent pancreatitis and IHC results suggested that pancreatic adenocarcinoma was the most likely primary tumor. As the focus of cancer treatment shifts away from the tissue of origin and toward molecular and genetic profiles, the search for the primary site may decrease in importance. In the future, even when we do not know the cancer’s origin, we may still know precisely what to do. But for now, as in this patient, our treatments continue to be based on a tumor that is out of sight, but not out of mind.
KEY TEACHING POINTS
- Acute and chronic pancreatitis are associated with an increased risk of pancreatic adenocarcinoma.
- CUP is a cancer in which diagnostic testing does not identify a primary tumor site. Immunohistochemistry and molecular analysis, imaging, and endoscopy are utilized selectively to identify a primary tumor type.
- Treatment of CUP currently depends on the suspected tissue of origin and pattern of spread.
- Tissue-agnostic therapy could allow for treatment for CUP patients independent of the tissue of origin.
Acknowledgments
We thank Andrew Mick, OD, for his review of an earlier version of this manuscript and Peter Phillips, MD, for his interpretation of the pathologic images.
A 73-year-old man presented to clinic with 6 weeks of headache. He occasionally experienced generalized headaches throughout his life that resolved with naproxen. His new headache was characterized by a progressively worsening sensation of left-eye pressure with radiation to the left temple. Over the previous week, he had intermittent diplopia, left ptosis, and left lacrimation. He denied head trauma, fever, vision loss, photophobia, dysphagia, dysarthria, nausea, vomiting, or jaw claudication.
Primary headaches include tension type, migraine, and trigeminal autonomic cephalalgias (eg, cluster headache). A new headache in an older patient, particularly if protracted and progressive, prioritizes consideration of a secondary headache, which may reflect pathology within the brain parenchyma (eg, intracranial mass), blood vessels (eg, giant cell arteritis), meninges (eg, meningitis), or ventricles (eg, intraventricular cyst). Eye pain may arise from ocular and extraocular disease. Corneal abrasions, infectious keratitis, scleritis, uveitis, or acute angle-closure glaucoma are painful, although the latter is less likely given the prolonged duration of symptoms. Thyroid eye disease or other infiltrative disorders of the orbit can also cause eye discomfort.
Ptosis commonly results from degeneration of the levator aponeurosis. Other causes include third cranial nerve palsy and myasthenia gravis. Interruption of sympathetic innervation of the eyelid by lesions in the brain stem, spinal cord, lung (eg, Pancoast tumor), or cavernous sinus also can result in ptosis.
Whether the patient has monocular or binocular diplopia is uncertain. Monocular diplopia persists with only one eye open and can arise from uncorrected refractive error, corneal irregularities, lenticular opacities, or unilateral macular disease. Binocular diplopia develops from ocular misalignment due to neuromuscular weakness, extraocular muscle entrapment, or an orbital mass displacing the globe. An orbital mass would also explain the unilateral headache and unilateral ptosis.
His medical history included coronary artery disease, seronegative rheumatoid arthritis, osteoporosis, benign prostatic hypertrophy, and ureteral strictures from chronic nephrolithiasis. Following a cholecystectomy for gallstone pancreatitis 13 years earlier, he was hospitalized five more times for pancreatitis. The last episode was 6 years prior to this presentation. At that time, magnetic resonance cholangiopancreatography (MRCP) did not reveal pancreatic divisum, annular pancreas, biliary strictures, or a pancreatic mass. Esophagogastroduodenoscopy peformed during the same hospitalization showed mild gastritis. His recurrent pancreatitis was deemed idiopathic.
His medications were folic acid, cholecalciferol, lisinopril, metoprolol, omeprazole, simvastatin, aspirin, and weekly methotrexate. His sister had breast and ovarian cancer, and his brother had gastric cancer. He had two subcentimeter tubular adenomas removed during a screening colonoscopy 3 years prior. He had a 30 pack-year smoking history and quit 28 years earlier. He did not use alcohol or drugs. He was a retired chemical plant worker.
Choledocholithiasis (as discrete stones or biliary sludge) can trigger pancreatitis despite a cholecystectomy, but the recurrent episodes and negative MRCP should prompt consideration of other causes, such as alcohol. Hypercalcemia, hypertriglyceridemia, and medications are infrequent causes of pancreatic inflammation. IgG4-related disease (IgG4-RD) causes autoimmune pancreatitis and can infiltrate the eyelids, lacrimal glands, extraocular muscles, or orbital connective tissue. Malignancy of the pancreas or ampulla can trigger pancreatitis by causing pancreatic duct obstruction but would not go undetected for 13 years.
The patient was evaluated by an ophthalmologist and a neurologist. His heart rate was 52 beats per minute and blood pressure, 174/70 mm Hg; other vital signs were normal. He had conjunctival chemosis, ptosis, and nonpulsatile proptosis of the left eye with tenderness and increased resistance to retropulsion compared to the right eye (Figure 1). Visual acuity was 20/25 for the right eye and hand motions only in the left eye. The pupils were reactive and symmetric without afferent pupillary defect. There was no optic nerve swelling or pallor. Abduction, adduction, and elevation of the left eye were restricted and associated with diplopia. Movement of the right eye was unrestricted. There was no other facial asymmetry. Facial sensation was normal. Corneal reflexes were intact. Shoulder shrug strength was equal and symmetric. Tongue protrusion was midline. Olfaction and hearing were not assessed. Strength, sensation, and deep tendon reflexes were normal in all extremities. The plantar response was flexor bilaterally.
Unilateral ptosis, chemosis, proptosis, ophthalmoplegia, eye tenderness, and visual loss collectively point to a space-occupying orbital disease. Orbital masses are caused by cancers, infections such as mucormycosis (usually in an immunocompromised host), and inflammatory disorders such as thyroid orbitopathy, sarcoidosis, IgG4-related orbitopathy, granulomatosis with polyangiitis, and orbital pseudotumor (idiopathic inflammation of the orbit). Chemosis reflects edema of the conjunctiva, which can arise from direct conjunctival injury (eg, allergy, infection, or trauma), interruption of the venous drainage of the conjunctiva by vascular disorders (eg, cavernous sinus thrombosis or carotid-cavernous fistula), or space-occupying diseases of the orbit. Monocular visual loss arises from a prechiasmal lesion, and acute monocular visual loss is more commonly caused by posterior ocular pathology (eg, retina or optic nerve) than anterior disease (eg, keratitis). Visual loss in the presence of an orbital process suggests a compressive or infiltrative disease of the optic nerve.
Complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and thyroid function tests were normal. Interferon-gamma release assay, HIV antibody, rapid plasma reagin, Lyme antibody, antinuclear antibody, and antineutrophil cytoplasmic antibody (ANCA) tests were negative. A noncontrast computed tomography (CT) scan of the head revealed thickening of the left inferior rectus muscle. Orbital magnetic resonance imaging (MRI) with gadolinium and fluid-attenuated inversion recovery imaging demonstrated a T2 hyperintense, heterogeneous 1.4-cm mass in the left inferior rectus muscle (Figure 2). There was no carotid-cavernous fistula, brain mass, or meningeal enhancement.
An isolated mass in one ocular muscle raises the probability of a cancer. The most common malignant orbital tumor is B-cell lymphoma. Metastatic cancer to the eye is rare; breast, prostate, and lung cancer account for the majority of cases. The family history of breast and ovarian cancer raises the possibility of a BRCA mutation, which is also associated with gastric, pancreatic, and prostate malignancies. Granulomatosis with polyangiitis may be ANCA negative in localized sino-orbital disease. Biopsy of the orbital mass is the next step.
The patient underwent transconjunctival orbitotomy with excision of the left inferior rectus mass. Two days later, he presented to the emergency department with acute onset epigastric pain, nausea, and vomiting. A comprehensive review of systems, which had not been performed until this visit, revealed an unintentional 20-lb weight loss over the previous 3 months. He had a progressive ache in the left anterior groin that was dull, tender, nonradiating, and worse with weight bearing. He denied melena or hematochezia.
His temperature was 37 °C; heart rate, 98 beats per minute; and blood pressure, 128/63 mm Hg. He had midepigastric tenderness and point tenderness over the anterior iliac spine. White blood cell count was 12,600/μL; hemo globin, 14.5 g/dL; and platelet count, 158,000/μL. Serum lipase was 7,108 U/L. Serum creatinine, calcium, and triglyceride levels were normal. Alkaline phosphatase was 117 U/L (normal, 34-104 U/L); total bilirubin, 1.1 mg/dL; alanine aminotransferase (ALT), 119 U/L (normal, 7-52 U/L); and aspartate aminotransferase (AST), 236 U/L (normal, 13-39 U/L). Troponin I was undetectable, and an electrocardiogram demonstrated sinus tachycardia. Urinalysis was normal.
Concomitant pancreatitis and hepatitis with an elevated AST-to-ALT ratio should prompt evaluation of recurrent choledocholithiasis and a repeat inquiry about alcohol use. His medications should be reviewed for an association with pancreatitis. Anterior groin discomfort usually reflects osteoarthritis of the hip joint, inguinal hernia, or inguinal lymphadenopathy. Groin pain may be referred from spinal nerve root compression, aortoiliac occlusion, or nephrolithiasis. Weight loss in the presence of an inferior rectus mass suggests one of the aforementioned systemic diseases with orbital manifestations. Pancreatitis and groin discomfort may be important clues, but the chronicity of the recurrent pancreatitis and the high prevalence of hip osteoarthritis make it equally likely that they are unrelated to the eye disease.
CT scan of the abdomen and pelvis with contrast showed peripancreatic edema with fat stranding but no pancreatic or hepatobiliary mass. The common bile duct was normal. A 2.2×1.3-cm mass in the right posterior subphrenic space, a lytic lesion in the left anterior inferior iliac spine, and right nonobstructive nephrolithiasis were identified. CT scan of the chest with contrast showed multiple subpleural nodules and innumerable parenchymal nodules. Subcentimeter hilar, mediastinal, and prevascular lymphadenopathy were present, as well as multiple sclerotic lesions in the right fourth and sixth ribs. Prostate-specific antigen was 0.7 ng/mL (normal, ≤ 4.0 ng/mL). Cancer antigen 19-9 level was 5.5 U/mL (normal, < 37.0 U/mL), and carcinoembryonic antigen (CEA) was 100.1 ng/mL (normal, 0-3 U/mL).
Widespread pulmonary nodules, diffuse lymphadenopathy, and bony lesions raise concern for a metastatic malignancy. There is no evidence of a primary carcinoma. The lack of hepatic involvement reduces the likelihood of a gastrointestinal tumor, although a rectal cancer, which may drain directly into the inferior vena cava and bypass the portal circulation, could present as lung metastases on CT imaging. Lymphoma is plausible given the diffuse lymphadenopathy and orbital mass. Sarcoidosis and histiocytic disorders (eg, Langerhans cell histiocytosis) also cause orbital disease, pulmonary nodules, lymphadenopathy, and bone lesions, although a subphrenic mass would be atypical for both disorders; furthermore, the majority of patients with adult Langerhans cell histiocytosis smoke cigarettes. The elevated CEA makes a metastatic solid tumor more likely than lymphoma but does not specify the location of the primary tumor.
Pathology of the inferior rectus muscle mass showed well-differentiated adenocarcinoma (Figure 3A and 3B). A CT-guided biopsy of the left anterior inferior iliac spine revealed well-differentiated adenocarcinoma (Figure 3C). Adenocarcinoma of unknown primary wasdiagnosed.
Subsequent immunohistochemical (IHC) staining was positive for cytokeratin 7 (CK7) and mucicarmine (Figure 3D and 3E) and negative for cytokeratin 20 (CK20) and thyroid transcription factor 1 (TTF1). This IHC profile suggested pancreatic or upper gastrointestinal tract lineage. Positron emission tomography–CT (PET-CT) scan was aborted because of dyspnea and chest pressure following contrast administration. He declined further imaging or endoscopy. He received palliative radiation and three cycles of paclitaxel and gemcitabine for cancer of unknown primary (CUP). Two months later, he developed bilateral upper-arm weakness due to C7 and T2 cord compression from vertebral and epidural metastases; his symptoms progressed despite salvage chemotherapy. He was transitioned to comfort care and died at home 9 months after diagnosis.
DISCUSSION
This patient’s new headache and ocular abnormalities led to the discovery of an inferior rectus muscle mass. Initially unrecognized unintentional weight loss and hip pain recast a localized orbital syndrome as a systemic disease with pancreatic, ocular, pulmonary, lymph node, and skeletal pathology. Biopsies of the orbital rectus muscle and iliac bone demonstrated metastatic adenocarcinoma. Imaging studies did not identify a primary cancer, but IHC analysis suggested carcinoma of upper gastrointestinal or pancreatic origin.
Acute and chronic pancreatitis are both associated with pancreatic cancer.1 Chronic pancreatitis is associated with an increasing cumulative risk of pancreatic cancer; a potential mechanism is chronic inflammation with malignant transformation.2,3 There is also a 20-fold increased risk of pancreatic cancer in the first 2 years following an episode of acute pancreatitis,4 which may develop from malignant pancreatic duct obstruction. Although the post–acute pancreatitis risk of pancreatic cancer attenuates over time, a two-fold increased risk of pancreatic cancer remains after 10 years,4 which suggests that acute pancreatitis (particularly when idiopathic) either contributes to or shares pathogenesis with pancreatic adenocarcinoma. In elderly patients without gallstones or alcohol use, an abdominal CT scan or MRI shortly after resolution of the acute pancreatitis may be considered to assess for an underlying pancreatic tumor.5
CUP is a histologically defined malignancy without a known primary anatomic site despite an extensive evaluation. CUP accounts for up to 10% of all cancer diagnoses.6 CUP is ascribed to a primary cancer that remains too small to be detected or spontaneous regression of the primary cancer.7 Approximately 70% of autopsies of patients with CUP identify the primary tumor, which most commonly originates in the lung, gastrointestinal tract, breast, or pancreas.8
When a metastatic focus of cancer is found but the initial diagnostic evaluation (including CT scan of the chest, abdomen, and pelvis) fails to locate a primary cancer, the next step in searching for the tissue of origin is an IHC analysis of the tumor specimen. IHC analysis is a multistep staining process that can identify major categories of cancer, including carcinoma (adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma) and poorly or undifferentiated neoplasms (including carcinoma, lymphoma, sarcoma, or melanoma). Eighty-five percent of CUP cases are adenocarcinoma, 10% are squamous cell carcinoma, and the remaining 5% are undifferentiated neoplasms.9
There are no consensus guidelines for imaging in patients with CUP who have already undergone a CT scan of the chest, abdomen, and pelvis. Mammography is indicated in women with metastatic adenocarcinoma or axillary lymphadenopathy.7 MRI of the breast is obtained when mammography is nondiagnostic and the suspicion for breast cancer is high. Small clinical studies and meta-analyses support the use of PET-CT scans,7 although one study found that a PET-CT scan was not superior to CT imaging in identifying the primary tumor site in CUP.10 Endoscopy of the upper airway or gastrointestinal tract is rarely diagnostic in the absence of referable symptoms or a suggestive IHC profile (eg, CK7−, CK20+ suggestive of colon cancer).6
Molecular cancer classification has emerged as a useful diagnostic technique in CUP. Cancer cells retain gene expression patterns based on cellular origin, and a tumor’s profile can be compared with a reference database of known cancers, aiding in the identification of the primary tumor type. Molecular cancer classifier assays that use gene expression profiling can accurately determine a primary site11 and have been shown to be concordant with IHC testing.12 Molecular cancer classification is distinct from genetic assays that identify mutations for which there are approved therapies. Serum tumor markers are generally not useful in establishing the primary tumor and should be considered based on the clinical presentation (eg, prostate-specific antigen testing in a man with adenocarcinoma of unknown primary and osteoblastic metastases).
CUP is classified as favorable or unfavorable based on the IHC, pattern of spread, and serum markers in certain cases.6 Approximately 20% of CUP patients can be categorized into favorable subsets, such as adenocarcinoma in a single axillary lymph node in a female patient suggestive of a breast primary cancer, or squamous cell carcinoma in a cervical lymph node suggestive of a head or neck primary cancer.7 The remaining 80% of cases are categorized as unfavorable CUP and often have multiple metastases. Our patient’s pattern of spread and limited response to chemotherapy is characteristic of the unfavorable subset of CUP. The median survival of this group is 9 months, and only 25% of patients survive longer than 1 year.13
Biomarker-driven treatment of specific molecular targets independent of the tissue of origin (tissue-agnostic therapy) has shown promising results in the treatment of skin, lung, thyroid, colorectal, and gastric cancers.14 Pembrolizumab was the first drug approved by the US Food and Drug Administration based on a tumor’s biomarker without regard to its primary location. Data to support this approach for treating CUP are evolving and offer hope for patients with specific molecular targets.
Following the focused neuro-ophthalmologic evaluations, with focused examination and imaging, the hospitalist’s review of systems at the time of the final admission for pancreatitis set in motion an evaluation that led to a diagnosis of metastatic cancer. The risk factor of recurrent pancreatitis and IHC results suggested that pancreatic adenocarcinoma was the most likely primary tumor. As the focus of cancer treatment shifts away from the tissue of origin and toward molecular and genetic profiles, the search for the primary site may decrease in importance. In the future, even when we do not know the cancer’s origin, we may still know precisely what to do. But for now, as in this patient, our treatments continue to be based on a tumor that is out of sight, but not out of mind.
KEY TEACHING POINTS
- Acute and chronic pancreatitis are associated with an increased risk of pancreatic adenocarcinoma.
- CUP is a cancer in which diagnostic testing does not identify a primary tumor site. Immunohistochemistry and molecular analysis, imaging, and endoscopy are utilized selectively to identify a primary tumor type.
- Treatment of CUP currently depends on the suspected tissue of origin and pattern of spread.
- Tissue-agnostic therapy could allow for treatment for CUP patients independent of the tissue of origin.
Acknowledgments
We thank Andrew Mick, OD, for his review of an earlier version of this manuscript and Peter Phillips, MD, for his interpretation of the pathologic images.
1. Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(111):1711-1719. https://doi.org/10.1038/s41395-018-0255-9
2. Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23(11):2964-2970. https://doi.org/10.1093/annonc/mds140
3. Ekbom A, McLaughlin JK, Nyren O. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;329(20):1502-1503. https://doi.org/10.1056/NEJM199311113292016
4. Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(156):1729-1736. https://doi.org/10.1053/j.gastro.2018.02.011
5. Frampas E, Morla O, Regenet N, Eugene T, Dupas B, Meurette G. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94(7-8):741-755. https://doi.org/10.1016/j.diii.2013.03.013
6. Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765. https://doi.org/10.1056/NEJMra1303917
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017. 2018;35(3):199-206. https://doi.org//10.1053/j.semdp.2017.11.013
8. Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43(14):2026-2036. https://doi.org/10.1016/j.ejca.2007.06.023
9. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69(3):271-278. https://doi.org/10.1016/j.critrevonc.2008.09.005
10. Moller AK, Loft A, Berthelsen AK, et al. A prospective comparison of 18F-FDG PET/CT and CT as diagnostic tools to identify the primary tumor site in patients with extracervical carcinoma of unknown primary site. Oncologist. 2012;17(9):1146-1154. https://doi.org/10.1634/theoncologist.2011-0449
11. Economopoulou P, Mountzios G, Pavlidis N, Pentheroudakis G. Cancer of unknown primary origin in the genomic era: elucidating the dark box of cancer. Cancer Treat Rev. 2015;41(7):598-604. https://doi.org/10.1016/j.ctrv.2015.05.010
12. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642. https://doi.org/10.1007/s11864-013-0257-1
13. Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol. 2011;8(12):701-710. https://doi.org/10.1038/nrclinonc.2011.158
14. Luoh SW, Flaherty KT. When tissue is no longer the issue: tissue-agnostic cancer therapy comes of age. Ann Intern Med. 2018;169(4):233-239. https://doi.org/10.7326/M17-2832
1. Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(111):1711-1719. https://doi.org/10.1038/s41395-018-0255-9
2. Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23(11):2964-2970. https://doi.org/10.1093/annonc/mds140
3. Ekbom A, McLaughlin JK, Nyren O. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;329(20):1502-1503. https://doi.org/10.1056/NEJM199311113292016
4. Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(156):1729-1736. https://doi.org/10.1053/j.gastro.2018.02.011
5. Frampas E, Morla O, Regenet N, Eugene T, Dupas B, Meurette G. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94(7-8):741-755. https://doi.org/10.1016/j.diii.2013.03.013
6. Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765. https://doi.org/10.1056/NEJMra1303917
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017. 2018;35(3):199-206. https://doi.org//10.1053/j.semdp.2017.11.013
8. Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43(14):2026-2036. https://doi.org/10.1016/j.ejca.2007.06.023
9. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69(3):271-278. https://doi.org/10.1016/j.critrevonc.2008.09.005
10. Moller AK, Loft A, Berthelsen AK, et al. A prospective comparison of 18F-FDG PET/CT and CT as diagnostic tools to identify the primary tumor site in patients with extracervical carcinoma of unknown primary site. Oncologist. 2012;17(9):1146-1154. https://doi.org/10.1634/theoncologist.2011-0449
11. Economopoulou P, Mountzios G, Pavlidis N, Pentheroudakis G. Cancer of unknown primary origin in the genomic era: elucidating the dark box of cancer. Cancer Treat Rev. 2015;41(7):598-604. https://doi.org/10.1016/j.ctrv.2015.05.010
12. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642. https://doi.org/10.1007/s11864-013-0257-1
13. Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol. 2011;8(12):701-710. https://doi.org/10.1038/nrclinonc.2011.158
14. Luoh SW, Flaherty KT. When tissue is no longer the issue: tissue-agnostic cancer therapy comes of age. Ann Intern Med. 2018;169(4):233-239. https://doi.org/10.7326/M17-2832
© 2021 Society of Hospital Medicine
As the Story Unfolds
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).
Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1
After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.
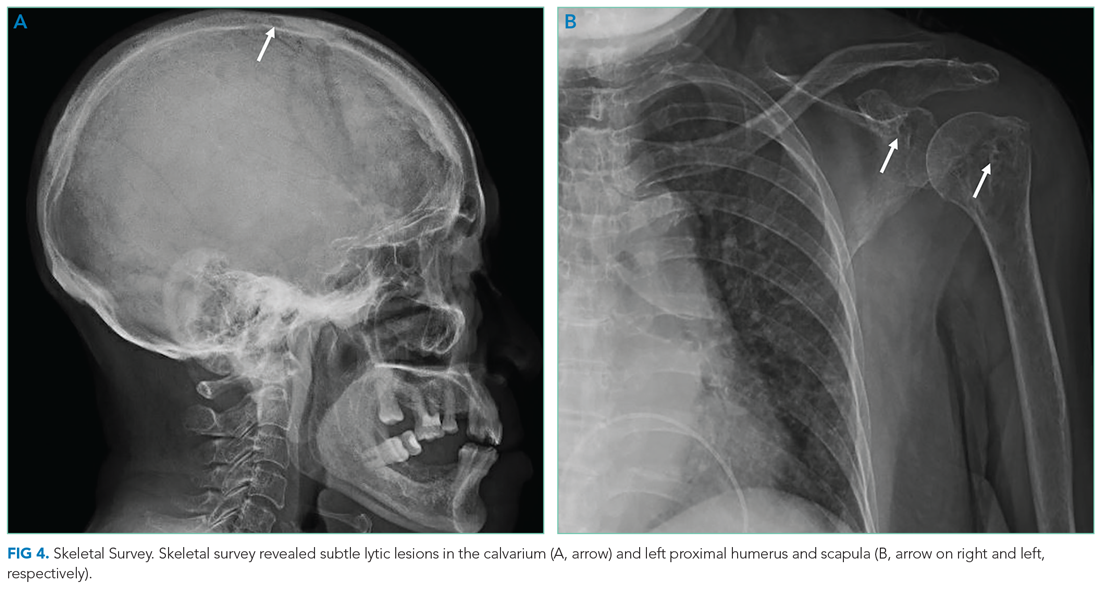
DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).

Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1

After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.

DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).

Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1

After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.

DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
© 2021 Society of Hospital Medicine
A Painful Coincidence?
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.
The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170
The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.
Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.
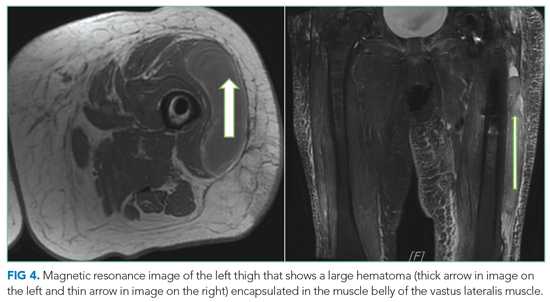
DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.

The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170

The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.

Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.

DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.

The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170

The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.

Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.

DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
© 2021 Society of Hospital Medicine