User login
A Painful Coincidence?
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
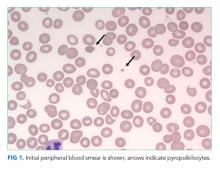
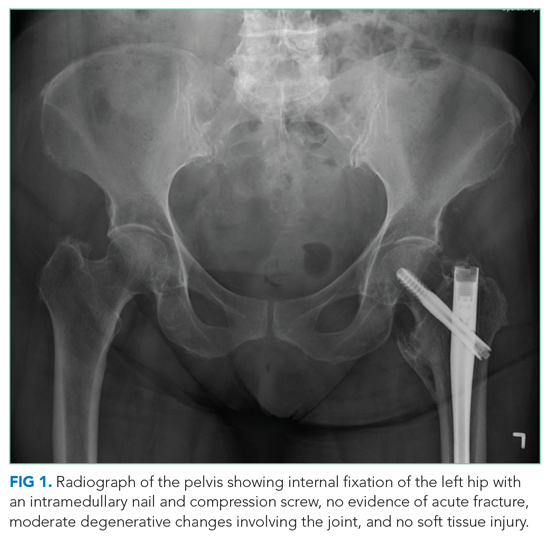
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.
The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
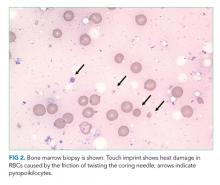
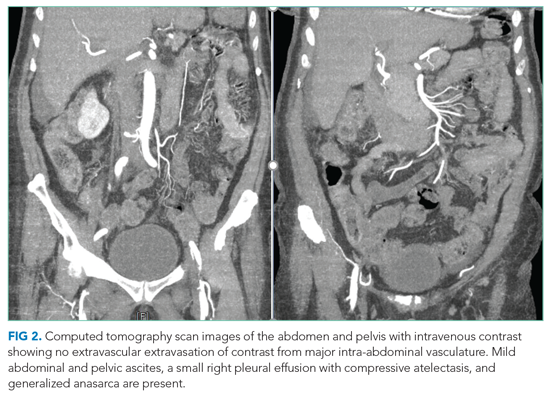
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170
The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
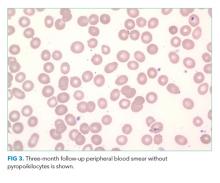
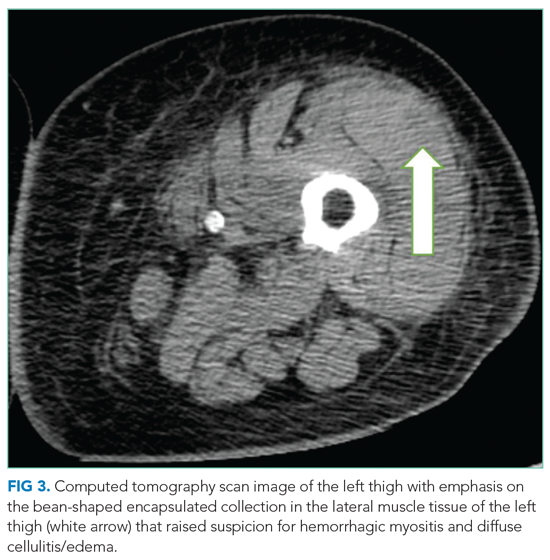
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.
Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
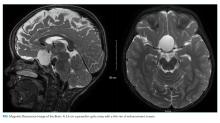
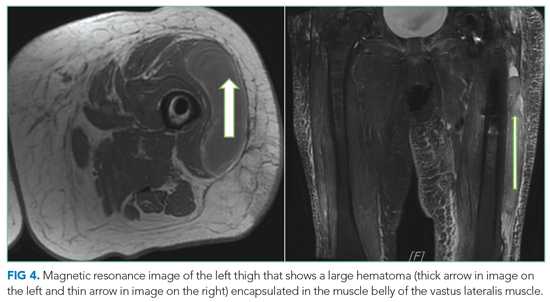
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.
DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.

The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170

The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.

Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.

DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.

The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170

The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.

Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.

DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
© 2021 Society of Hospital Medicine
Bronchiolitis: Less Is More, but Different Is Better
Bronchiolitis, the most common cause of hospital admission for infants, is responsible for more than $500 million in direct medical costs in the United States yearly. Recent efforts have focused on what can be safely avoided when caring for patients with bronchiolitis (eg, continuous pulse oximetry, bronchodilator administration). While there remains substantial room for improvement in avoiding such low-value (or no-value) practices, the incremental improvements from these de-escalations will reach an asymptote over time. Further improvements in care and value must occur by doing things differently—not just simply doing less.
In this month’s Journal of Hospital Medicine, Ohlsen et al1 describe an intervention to decrease length of stay (LOS) for patients with bronchiolitis They employed an interrupted time series analysis to evaluate implementation of an observation unit and home oxygen therapy (OU-HOT) model of care and found that LOS dramatically decreased immediately following implementation. This reduction was maintained over 9 years. Use of home oxygen decreased over the study period, while LOS remained low, suggesting that the most important intervention was a structural one—the admission of patients to a unit dedicated to efficient discharge.
Observation units, staffed 24/7 with attending physicians, are well adapted to care for patients with illnesses like bronchiolitis, where hospitalization, though often needed, may be brief.2 These units are designed more like an emergency department than an inpatient unit, with protocolized care and the expectation of rapid turnover.
Multiple studies have shown that physician-related delays are a primary driver of delayed discharge from inpatient units. Such delays include delayed or variable clinical decision-making, inadequate communication of discharge criteria, and waiting to staff patients with an attending physician.3-5 Addressing these issues could allow inpatient units to function more like observation units for specific diagnoses. Standardization of care around specific diagnoses can make decision-making and discharge more efficient. In 2014, White et al4 showed that standardizing discharge criteria for specific diagnoses (including bronchiolitis) and embedding these criteria in admission order sets resulted in a significant decrease in LOS without affecting readmission rates or patient satisfaction.
To address the issues of attending availability, we may need to rethink rounding. The daily structure of inpatient rounding has not meaningfully changed since the 1950s. While there has been a push for increased morning discharges, this approach misses many patients whose illness course is evolving and who may be ready for discharge in the afternoon or evening.6 The current structure of morning rounds on medical teams is based on the need for resident education, supervision, and time available for attendings to complete administrative tasks and teaching in the afternoons. Structural change in patient care requires academic institutions to rethink what “being on service” actually means. Since LOS in these cases is brief, multiple days of clinical continuity may not be as beneficial as with other diagnoses. Further, there is no reason that daytime rounding teams are the only teams that can discharge patients. Telemedicine could also offer an opportunity for attending physicians to remotely determine whether a patient is discharge appropriate. Standardization of discharge criteria at admission could allow for trainees to discharge patients when they meet those criteria.
Perhaps we should begin to adapt our work structure to our patients’ needs, rather than the other way around. In pediatrics, we have already made traditional rounding more patient-focused through the practice of family-centered rounding. We should identify, as the authors have, ways to do things differently to make further improvements in care.
Ultimately, the success of this OU-HOT protocol demonstrates the power of structural interventions aimed at changing how we do things rather than just doing more (or less) of the same.
1. Ohlsen T, Knudson A, Korgenski EK, et al. Nine seasons of a bronchiolitis observation unit and home oxygen therapy protocol. J Hosp Med. 2021;16(5):261-267.
2. Plamann JM, Zedreck-Gonzalez J, Fennimore L. Creation of an adult observation unit: improving outcomes. J Nurs Care Qual. 2018;33(1):72-78. https://doi.org/10.1097/NCQ.0000000000000267
3. Zoucha J, Hull M, Keniston A, et al. Barriers to early hospital discharge: a cross-sectional study at five academic hospitals. J Hosp Med. 2018;13(12):816-822. https://doi.org/10.12788/jhm.3074
4. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
5. Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481-485. https://doi.org/10.1002/jhm.490
6. Gordon SA, Garber D, Taufique Z, et al. Improving on-time discharge in otolaryngology admissions. Otolaryngol Head Neck Surg. 2020;163(2):188-193. https://doi.org/10.1177/0194599819898910
Bronchiolitis, the most common cause of hospital admission for infants, is responsible for more than $500 million in direct medical costs in the United States yearly. Recent efforts have focused on what can be safely avoided when caring for patients with bronchiolitis (eg, continuous pulse oximetry, bronchodilator administration). While there remains substantial room for improvement in avoiding such low-value (or no-value) practices, the incremental improvements from these de-escalations will reach an asymptote over time. Further improvements in care and value must occur by doing things differently—not just simply doing less.
In this month’s Journal of Hospital Medicine, Ohlsen et al1 describe an intervention to decrease length of stay (LOS) for patients with bronchiolitis They employed an interrupted time series analysis to evaluate implementation of an observation unit and home oxygen therapy (OU-HOT) model of care and found that LOS dramatically decreased immediately following implementation. This reduction was maintained over 9 years. Use of home oxygen decreased over the study period, while LOS remained low, suggesting that the most important intervention was a structural one—the admission of patients to a unit dedicated to efficient discharge.
Observation units, staffed 24/7 with attending physicians, are well adapted to care for patients with illnesses like bronchiolitis, where hospitalization, though often needed, may be brief.2 These units are designed more like an emergency department than an inpatient unit, with protocolized care and the expectation of rapid turnover.
Multiple studies have shown that physician-related delays are a primary driver of delayed discharge from inpatient units. Such delays include delayed or variable clinical decision-making, inadequate communication of discharge criteria, and waiting to staff patients with an attending physician.3-5 Addressing these issues could allow inpatient units to function more like observation units for specific diagnoses. Standardization of care around specific diagnoses can make decision-making and discharge more efficient. In 2014, White et al4 showed that standardizing discharge criteria for specific diagnoses (including bronchiolitis) and embedding these criteria in admission order sets resulted in a significant decrease in LOS without affecting readmission rates or patient satisfaction.
To address the issues of attending availability, we may need to rethink rounding. The daily structure of inpatient rounding has not meaningfully changed since the 1950s. While there has been a push for increased morning discharges, this approach misses many patients whose illness course is evolving and who may be ready for discharge in the afternoon or evening.6 The current structure of morning rounds on medical teams is based on the need for resident education, supervision, and time available for attendings to complete administrative tasks and teaching in the afternoons. Structural change in patient care requires academic institutions to rethink what “being on service” actually means. Since LOS in these cases is brief, multiple days of clinical continuity may not be as beneficial as with other diagnoses. Further, there is no reason that daytime rounding teams are the only teams that can discharge patients. Telemedicine could also offer an opportunity for attending physicians to remotely determine whether a patient is discharge appropriate. Standardization of discharge criteria at admission could allow for trainees to discharge patients when they meet those criteria.
Perhaps we should begin to adapt our work structure to our patients’ needs, rather than the other way around. In pediatrics, we have already made traditional rounding more patient-focused through the practice of family-centered rounding. We should identify, as the authors have, ways to do things differently to make further improvements in care.
Ultimately, the success of this OU-HOT protocol demonstrates the power of structural interventions aimed at changing how we do things rather than just doing more (or less) of the same.
Bronchiolitis, the most common cause of hospital admission for infants, is responsible for more than $500 million in direct medical costs in the United States yearly. Recent efforts have focused on what can be safely avoided when caring for patients with bronchiolitis (eg, continuous pulse oximetry, bronchodilator administration). While there remains substantial room for improvement in avoiding such low-value (or no-value) practices, the incremental improvements from these de-escalations will reach an asymptote over time. Further improvements in care and value must occur by doing things differently—not just simply doing less.
In this month’s Journal of Hospital Medicine, Ohlsen et al1 describe an intervention to decrease length of stay (LOS) for patients with bronchiolitis They employed an interrupted time series analysis to evaluate implementation of an observation unit and home oxygen therapy (OU-HOT) model of care and found that LOS dramatically decreased immediately following implementation. This reduction was maintained over 9 years. Use of home oxygen decreased over the study period, while LOS remained low, suggesting that the most important intervention was a structural one—the admission of patients to a unit dedicated to efficient discharge.
Observation units, staffed 24/7 with attending physicians, are well adapted to care for patients with illnesses like bronchiolitis, where hospitalization, though often needed, may be brief.2 These units are designed more like an emergency department than an inpatient unit, with protocolized care and the expectation of rapid turnover.
Multiple studies have shown that physician-related delays are a primary driver of delayed discharge from inpatient units. Such delays include delayed or variable clinical decision-making, inadequate communication of discharge criteria, and waiting to staff patients with an attending physician.3-5 Addressing these issues could allow inpatient units to function more like observation units for specific diagnoses. Standardization of care around specific diagnoses can make decision-making and discharge more efficient. In 2014, White et al4 showed that standardizing discharge criteria for specific diagnoses (including bronchiolitis) and embedding these criteria in admission order sets resulted in a significant decrease in LOS without affecting readmission rates or patient satisfaction.
To address the issues of attending availability, we may need to rethink rounding. The daily structure of inpatient rounding has not meaningfully changed since the 1950s. While there has been a push for increased morning discharges, this approach misses many patients whose illness course is evolving and who may be ready for discharge in the afternoon or evening.6 The current structure of morning rounds on medical teams is based on the need for resident education, supervision, and time available for attendings to complete administrative tasks and teaching in the afternoons. Structural change in patient care requires academic institutions to rethink what “being on service” actually means. Since LOS in these cases is brief, multiple days of clinical continuity may not be as beneficial as with other diagnoses. Further, there is no reason that daytime rounding teams are the only teams that can discharge patients. Telemedicine could also offer an opportunity for attending physicians to remotely determine whether a patient is discharge appropriate. Standardization of discharge criteria at admission could allow for trainees to discharge patients when they meet those criteria.
Perhaps we should begin to adapt our work structure to our patients’ needs, rather than the other way around. In pediatrics, we have already made traditional rounding more patient-focused through the practice of family-centered rounding. We should identify, as the authors have, ways to do things differently to make further improvements in care.
Ultimately, the success of this OU-HOT protocol demonstrates the power of structural interventions aimed at changing how we do things rather than just doing more (or less) of the same.
1. Ohlsen T, Knudson A, Korgenski EK, et al. Nine seasons of a bronchiolitis observation unit and home oxygen therapy protocol. J Hosp Med. 2021;16(5):261-267.
2. Plamann JM, Zedreck-Gonzalez J, Fennimore L. Creation of an adult observation unit: improving outcomes. J Nurs Care Qual. 2018;33(1):72-78. https://doi.org/10.1097/NCQ.0000000000000267
3. Zoucha J, Hull M, Keniston A, et al. Barriers to early hospital discharge: a cross-sectional study at five academic hospitals. J Hosp Med. 2018;13(12):816-822. https://doi.org/10.12788/jhm.3074
4. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
5. Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481-485. https://doi.org/10.1002/jhm.490
6. Gordon SA, Garber D, Taufique Z, et al. Improving on-time discharge in otolaryngology admissions. Otolaryngol Head Neck Surg. 2020;163(2):188-193. https://doi.org/10.1177/0194599819898910
1. Ohlsen T, Knudson A, Korgenski EK, et al. Nine seasons of a bronchiolitis observation unit and home oxygen therapy protocol. J Hosp Med. 2021;16(5):261-267.
2. Plamann JM, Zedreck-Gonzalez J, Fennimore L. Creation of an adult observation unit: improving outcomes. J Nurs Care Qual. 2018;33(1):72-78. https://doi.org/10.1097/NCQ.0000000000000267
3. Zoucha J, Hull M, Keniston A, et al. Barriers to early hospital discharge: a cross-sectional study at five academic hospitals. J Hosp Med. 2018;13(12):816-822. https://doi.org/10.12788/jhm.3074
4. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
5. Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481-485. https://doi.org/10.1002/jhm.490
6. Gordon SA, Garber D, Taufique Z, et al. Improving on-time discharge in otolaryngology admissions. Otolaryngol Head Neck Surg. 2020;163(2):188-193. https://doi.org/10.1177/0194599819898910
© 2021 Society of Hospital Medicine
Care Transitions: A Complex Problem That Requires a Complexity Mindset
In recent years, there has been increased scrutiny of transitions of care in medicine, particularly at hospital discharge. Much focus has been on preventing readmissions, motivated at least in part by the Affordable Care Act’s Hospital Readmissions Reduction Program, which financially penalizes hospitals for higher-than-expected readmission rates.1 However, the problem of transition from hospital to home is not just a readmissions issue—it is a quality and patient safety issue.2 Therefore, measuring readmissions alone is inadequate. More effective systems for transition from hospital to home are needed in order to deliver high-quality care that actually restores patient well-being after hospitalization.
In this month’s issue of Journal of Hospital Medicine, Schnipper and Samal, et al report the results of a stepped-wedge randomized trial examining the effect of a multifaceted intervention on postdischarge patient-centered outcomes when compared with usual care.3 At 30 days after discharge, adverse events were reduced from 23 per 100 patients in the usual care group to 18 per 100 patients in the intervention group, with an incidence rate ratio of 0.55 (95% CI, 0.35-0.84) after adjustment for study month and baseline characteristics. Interestingly, there was no statistically significant difference in nonelective readmissions, and penetrance was notably poor: The majority of components of the intervention were received by fewer than half of intended patients, and 13% failed to receive any component at all.
With such incomplete implementation, what explains the reduction in adverse events? To best answer this, it is helpful to recognize the transition from hospital to home as a complex problem rather than a complicated one.4 The difference is key. Complicated problems follow a predictable set of rules that can be thought of and planned for, and when the plan is methodically followed, complicated problems can be solved. Complex problems, on the other hand, have a more unpredictable interplay between multiple nonindependent and sometimes unknown factors. Complex problems cannot be solved by merely following a well-designed plan; rather, they require tremendous preparation, adaptability, and active management as the problem plays itself out.
Fortunately, Schnipper and Samal, et al properly identified the problem of transition from hospital to home as complex and approached it from a complexity mindset. In their design of a multifaceted intervention, they aimed high and cast a wide net. Understanding that different practices have different cultures and resources, they standardized the function of the intervention components rather than the exact form. As the trial progressed, they allowed for modification of the intervention, incorporating input from multiple stakeholders and feedback from early failures. Thus, by recognizing and embracing the complexity of the problem, the authors set themselves and their patients up for success. The most likely explanation for the observed effect of the intervention on this complex problem is therefore quite simple: The study design allowed for the components most likely to work to be most readily implemented on a patient-by-patient and practice-by-practice basis.
While the trial aims to imitate the “real world,” it does not leave clear-cut answers for real healthcare professionals. Without knowing if any individual component of the intervention was effective on its own, it may be difficult for institutions to justify the cost of implementation. And while there should be adequate incentive to action for any intervention that improves how patients function or feel, without a reduction in readmissions, the financial downside may in some instances be prohibitive.
Despite these limitations, the path forward is clear. Institutions looking to implement a similar program now should approach the problem with a complexity mindset, even if their downstream interventions may differ. Researchers looking to design similar trials should focus on narrowing the scope of the intervention while maintaining a complexity mindset, which might help lead to more widespread implementation of evidence-based interventions in the future. In teaching us more about the approach to finding a solution than the solution itself, the present study marks an important next step in hospital to home transitions of care and transitions-of-care research.
1. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/circulationaha.114.010270
2. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345-349.
3. Schnipper JL, Samal L, Nolido N, et al. The effects of a multifaceted intervention to improve care transitions within an accountable care organization: results of a stepped-wedge cluster-randomized trial. J Hosp Med. 2020:16:15-22. https://doi.org/10.12788/jhm.3513
4. Kinni T. “The critical difference between complex and complicated: featured excerpt from It’s Not Complicated: The Art and Science of Complexity for Business.” MIT Sloan Management Review. June 21, 2017. Accessed August 12, 2020. https://sloanreview.mit.edu/article/the-critical-difference-between-comp...
In recent years, there has been increased scrutiny of transitions of care in medicine, particularly at hospital discharge. Much focus has been on preventing readmissions, motivated at least in part by the Affordable Care Act’s Hospital Readmissions Reduction Program, which financially penalizes hospitals for higher-than-expected readmission rates.1 However, the problem of transition from hospital to home is not just a readmissions issue—it is a quality and patient safety issue.2 Therefore, measuring readmissions alone is inadequate. More effective systems for transition from hospital to home are needed in order to deliver high-quality care that actually restores patient well-being after hospitalization.
In this month’s issue of Journal of Hospital Medicine, Schnipper and Samal, et al report the results of a stepped-wedge randomized trial examining the effect of a multifaceted intervention on postdischarge patient-centered outcomes when compared with usual care.3 At 30 days after discharge, adverse events were reduced from 23 per 100 patients in the usual care group to 18 per 100 patients in the intervention group, with an incidence rate ratio of 0.55 (95% CI, 0.35-0.84) after adjustment for study month and baseline characteristics. Interestingly, there was no statistically significant difference in nonelective readmissions, and penetrance was notably poor: The majority of components of the intervention were received by fewer than half of intended patients, and 13% failed to receive any component at all.
With such incomplete implementation, what explains the reduction in adverse events? To best answer this, it is helpful to recognize the transition from hospital to home as a complex problem rather than a complicated one.4 The difference is key. Complicated problems follow a predictable set of rules that can be thought of and planned for, and when the plan is methodically followed, complicated problems can be solved. Complex problems, on the other hand, have a more unpredictable interplay between multiple nonindependent and sometimes unknown factors. Complex problems cannot be solved by merely following a well-designed plan; rather, they require tremendous preparation, adaptability, and active management as the problem plays itself out.
Fortunately, Schnipper and Samal, et al properly identified the problem of transition from hospital to home as complex and approached it from a complexity mindset. In their design of a multifaceted intervention, they aimed high and cast a wide net. Understanding that different practices have different cultures and resources, they standardized the function of the intervention components rather than the exact form. As the trial progressed, they allowed for modification of the intervention, incorporating input from multiple stakeholders and feedback from early failures. Thus, by recognizing and embracing the complexity of the problem, the authors set themselves and their patients up for success. The most likely explanation for the observed effect of the intervention on this complex problem is therefore quite simple: The study design allowed for the components most likely to work to be most readily implemented on a patient-by-patient and practice-by-practice basis.
While the trial aims to imitate the “real world,” it does not leave clear-cut answers for real healthcare professionals. Without knowing if any individual component of the intervention was effective on its own, it may be difficult for institutions to justify the cost of implementation. And while there should be adequate incentive to action for any intervention that improves how patients function or feel, without a reduction in readmissions, the financial downside may in some instances be prohibitive.
Despite these limitations, the path forward is clear. Institutions looking to implement a similar program now should approach the problem with a complexity mindset, even if their downstream interventions may differ. Researchers looking to design similar trials should focus on narrowing the scope of the intervention while maintaining a complexity mindset, which might help lead to more widespread implementation of evidence-based interventions in the future. In teaching us more about the approach to finding a solution than the solution itself, the present study marks an important next step in hospital to home transitions of care and transitions-of-care research.
In recent years, there has been increased scrutiny of transitions of care in medicine, particularly at hospital discharge. Much focus has been on preventing readmissions, motivated at least in part by the Affordable Care Act’s Hospital Readmissions Reduction Program, which financially penalizes hospitals for higher-than-expected readmission rates.1 However, the problem of transition from hospital to home is not just a readmissions issue—it is a quality and patient safety issue.2 Therefore, measuring readmissions alone is inadequate. More effective systems for transition from hospital to home are needed in order to deliver high-quality care that actually restores patient well-being after hospitalization.
In this month’s issue of Journal of Hospital Medicine, Schnipper and Samal, et al report the results of a stepped-wedge randomized trial examining the effect of a multifaceted intervention on postdischarge patient-centered outcomes when compared with usual care.3 At 30 days after discharge, adverse events were reduced from 23 per 100 patients in the usual care group to 18 per 100 patients in the intervention group, with an incidence rate ratio of 0.55 (95% CI, 0.35-0.84) after adjustment for study month and baseline characteristics. Interestingly, there was no statistically significant difference in nonelective readmissions, and penetrance was notably poor: The majority of components of the intervention were received by fewer than half of intended patients, and 13% failed to receive any component at all.
With such incomplete implementation, what explains the reduction in adverse events? To best answer this, it is helpful to recognize the transition from hospital to home as a complex problem rather than a complicated one.4 The difference is key. Complicated problems follow a predictable set of rules that can be thought of and planned for, and when the plan is methodically followed, complicated problems can be solved. Complex problems, on the other hand, have a more unpredictable interplay between multiple nonindependent and sometimes unknown factors. Complex problems cannot be solved by merely following a well-designed plan; rather, they require tremendous preparation, adaptability, and active management as the problem plays itself out.
Fortunately, Schnipper and Samal, et al properly identified the problem of transition from hospital to home as complex and approached it from a complexity mindset. In their design of a multifaceted intervention, they aimed high and cast a wide net. Understanding that different practices have different cultures and resources, they standardized the function of the intervention components rather than the exact form. As the trial progressed, they allowed for modification of the intervention, incorporating input from multiple stakeholders and feedback from early failures. Thus, by recognizing and embracing the complexity of the problem, the authors set themselves and their patients up for success. The most likely explanation for the observed effect of the intervention on this complex problem is therefore quite simple: The study design allowed for the components most likely to work to be most readily implemented on a patient-by-patient and practice-by-practice basis.
While the trial aims to imitate the “real world,” it does not leave clear-cut answers for real healthcare professionals. Without knowing if any individual component of the intervention was effective on its own, it may be difficult for institutions to justify the cost of implementation. And while there should be adequate incentive to action for any intervention that improves how patients function or feel, without a reduction in readmissions, the financial downside may in some instances be prohibitive.
Despite these limitations, the path forward is clear. Institutions looking to implement a similar program now should approach the problem with a complexity mindset, even if their downstream interventions may differ. Researchers looking to design similar trials should focus on narrowing the scope of the intervention while maintaining a complexity mindset, which might help lead to more widespread implementation of evidence-based interventions in the future. In teaching us more about the approach to finding a solution than the solution itself, the present study marks an important next step in hospital to home transitions of care and transitions-of-care research.
1. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/circulationaha.114.010270
2. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345-349.
3. Schnipper JL, Samal L, Nolido N, et al. The effects of a multifaceted intervention to improve care transitions within an accountable care organization: results of a stepped-wedge cluster-randomized trial. J Hosp Med. 2020:16:15-22. https://doi.org/10.12788/jhm.3513
4. Kinni T. “The critical difference between complex and complicated: featured excerpt from It’s Not Complicated: The Art and Science of Complexity for Business.” MIT Sloan Management Review. June 21, 2017. Accessed August 12, 2020. https://sloanreview.mit.edu/article/the-critical-difference-between-comp...
1. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/circulationaha.114.010270
2. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345-349.
3. Schnipper JL, Samal L, Nolido N, et al. The effects of a multifaceted intervention to improve care transitions within an accountable care organization: results of a stepped-wedge cluster-randomized trial. J Hosp Med. 2020:16:15-22. https://doi.org/10.12788/jhm.3513
4. Kinni T. “The critical difference between complex and complicated: featured excerpt from It’s Not Complicated: The Art and Science of Complexity for Business.” MIT Sloan Management Review. June 21, 2017. Accessed August 12, 2020. https://sloanreview.mit.edu/article/the-critical-difference-between-comp...
© 2021 Society of Hospital Medicine
Email: Brf9036@med.cornell.edu; Telephone: 212-746-4071.
Left Out in the Cold
A previously healthy 4-year-old boy presented to his pediatrician for nasal congestion, left ear pain, and intermittent fevers, which he’d been experiencing for 2 days. His exam was consistent with acute otitis media. Cefdinir was prescribed given a rash allergy to amoxicillin. His fever, congestion, and otalgia improved the next day.
Three days later he developed abdominal pain, fever, and labored breathing; his mother brought him to the emergency department (ED). His temperature was 38.0 °C, heart rate 141 beats per minute, blood pressure 117/71 mm Hg, respiratory rate 22 breaths per minute; he had oxygen saturation of 96% on ambient air. Despite mild accessory muscle use, he appeared comfortable and interactive. His left tympanic membrane was bulging without erythema. His neck was supple and mucous membranes moist. He had neither cervical lymphadenopathy nor conjunctival pallor. The cardiopulmonary exam was normal except for tachycardia. His abdomen was soft and not distended without organomegaly or tenderness.
Upper respiratory tract symptoms are commonly encountered in pediatrics and most often result from self-limited viral processes. Evaluation of a child with upper respiratory tract symptoms aims to identify serious causes like meningitis, as well as assessing the need for antimicrobial therapy. Supportive management is often appropriate in otitis media. His new, more concerning symptoms portend either a progression of the original process causing his upper respiratory tract symptoms or a separate etiology. It is key to determine which signs and symptoms are associated with the primary process and which are compensatory or secondary. If he were to be more ill appearing, for example, it is possible that his respiratory distress may be related to an underlying systemic illness rather than a primary lung process. Respiratory distress, abdominal pain, and fever could be a result of sepsis from an intrabdominal process such as ruptured appendicitis, intussusception, or malrotation with volvulus. Other causes of sepsis, such as meningitis or severe mastoiditis, both rare complications of otitis media, should be considered, although he does not appear severely ill. Acute myelogenous leukemia or other malignancies and illnesses associated with immunodeficiency can present with sepsis and chloromas in the middle ear that can be misconstrued as otitis media.
A chest radiograph demonstrated left lower lobe patchy opacities concerning for pneumonia. Rapid respiratory syncytial virus and influenza antigen test results were negative. Laboratory testing for general bloodwork was not obtained. He was administered a single dose of intramuscular ceftriaxone, prescribed a 5-day course of azithromycin, and discharged home. The child’s breathing gradually improved, but he continued to have subjective fevers. Two days later, he developed dark red urine. His mother brought him back to the outpatient clinic.
At the time of the ED visit, a diagnosis of community-acquired pneumonia was plausible given fever, mildly increased work of breathing, and an opacification on chest radiography. Most community-acquired pneumonia is caused by viruses; common bacterial causes for his age include Streptococcus pneumoniae and Moraxella catarrhalis. The first-line treatment for uncomplicated community-acquired pneumonia in children is amoxicillin, but this was appropriately avoided given his allergy.
The persistent fevers are surprising. The improvement in breathing corresponds to the treatment (and resolution) of community-acquired pneumonia. However, the development of dark urine does not. Red urine—in the absence of ingested pigments (such as those found in beets)—usually results from hematuria, hemoglobinuria, or myoglobinuria. Gross hematuria can originate from the kidneys to the urethral meatus. Abdominal masses, kidney trauma, or underlying kidney disease may all present with gross hematuria (or microscopic hematuria, seen only on urinalysis). The urine should be examined for the presence of heme, protein, and for evidence of infection; microscopy should be performed to examine for cellular casts and dysmorphic red cells. Tests of renal function, a comprehensive metabolic panel, evaluation of hematologic indexes, and assessments of inflammatory markers should be performed.
The child lived with his parents and had no siblings. He experienced no physical trauma, and there was no family history of kidney disease or hematuria. His father had a persistent cough and fever for 1 month, but recovered around the time the patient began to experience his initial symptoms. This was the patient’s third diagnosis of pneumonia. He had not traveled and was up to date with immunizations. He attended day care.
The fact that this is not the first episode of “pneumonia” raises important possibilities. The most likely one is that the child has had multiple viral infections; however, he could have an underlying primary immunodeficiency (PI) that predisposes him to recurrent infections. More severe PIs often present with recurrent sepsis, bacteremia, and failure to thrive, none of which were present in this case. Less severe PIs (such as selective IgA deficiency) could be possible. Another possibility is that these recurrent episodes of pneumonia are a relapsing and remitting noninfectious process, such as an antineutrophil cytoplasmic antibodies–associated vasculitis or anti–glomerular basement membrane disease. The patient’s father’s recent prolonged respiratory symptoms may be suggestive of pertussis or a “walking pneumonia” potentially caused by Mycoplasma or another atypical bacterium.
His temperature was 36.9 °C, heart rate 107 beats per minute, blood pressure was 106/67 mm Hg, and respiratory rate was 24 breaths per minute with oxygen saturation of 100% on ambient air. He was well appearing. His mucous membranes were moist, and oropharynx was clear. He had scleral icterus. The cardiopulmonary exam was normal. He had no significant lymphadenopathy, hepatosplenomegaly, or rashes.
The finding of jaundice is an important diagnostic pivot point, especially when combined with hematuria. The next step is determining if the jaundice is resulting from unconjugated or conjugated hyperbilirubinemia; the former most often stems from hemolysis or impairment in conjugation, while the latter results from intrahepatic or extrahepatic biliary defects. Tests for hepatobiliary injury including evaluations of alanine and aspartate aminotransferases and alkaline phosphatase, as well as for hepatic function such as tests of coagulation, should be performed.
The patient was referred to the ED and admitted for further evaluation. A complete blood count revealed a white blood cell (WBC) count of 10,700/µL (61% polymorphonuclear neutrophils, 30% lymphocytes, 5% monocytes, 3% eosinophils, 1% basophils), hemoglobin count was 10.3 g/dL (reticulocyte 2% with absolute reticulocyte count 58,400/μL), and platelet count was 265,000/µL. Components of the basic metabolic panel were within reference ranges except for a mildly elevated blood urea nitrogen level of 14 mg/dL with normal creatinine level of 0.3 mg/dL. Total protein was 6.7 g/dL (reference range, 6.4-8.3) and albumin 3.9 g/dL (reference range, 3.4-4.8). Alkaline phosphatase level was 188 U/L (reference range, 44-147), aspartate aminotransferase level 76 U/L (reference range, 0-40), and alanine aminotransferase level 12 U/L (reference range, 7-40). Total bilirubin level was 2.4 mg/dL (reference range, less than 1.5) with direct bilirubin level of 0.4 mg/dL. His C-reactive protein level was 1.5 mg/mL (reference range, 0-0.75). Creatinine kinase (CK) level was 2,550 U/L (reference range, 2-198). International Normalized Ratio (INR) was 1.0. Urinalysis was notable for 2+ proteinuria, large hemoglobin pigment, and 6 red blood cells per high power field (reference range, 0-4).
His blood urea nitrogen is elevated, reflecting either prerenal azotemia or increased absorption of nitrogenous products. Unconjugated hyperbilirubinemia may result from impaired hepatic bilirubin uptake (such as in heart failure or portosystemic shunts), impaired bilirubin conjugation (resulting from genetic conditions or drugs), or excess bilirubin production (such as in hemolysis); his anemia and lack of other evidence of hepatic dysfunction point to hemolysis as the etiology. The reticulocyte production index is approximately 1%, which suggests that an increase in erythrocyte generation is present but inadequate. This, however, does not mean that an erythrocyte production abnormality is present since reticulocytosis can be delayed in many cases of acute hemolytic anemia. It is also possible that the same hemolytic process is affecting mature and immature erythrocytes. A peripheral blood smear should be reviewed for evidence of intravascular hemolysis and testing for autoimmune hemolysis should be performed. Notably, his white blood cell and platelet counts are preserved, which makes a bone marrow–involved malignancy or infiltrative process less likely. The alkaline phosphatase elevation may result from either intrahepatic or extrahepatic biliopathy; bone damage is also possible. The elevation of aspartate aminotransferase, CK, and potassium, along with marked urinary heme pigment, may indicate muscle damage; the most common myositis in children is benign acute childhood myositis resulting from viral infection. However, the moderate level of CK elevation seen in this case is nonspecific and can result from many different etiologies. A metabolic myopathy, such as carnitine palmitoyltransferase II deficiency, can be made worse by metabolic stress and result in rhabdomyolysis; the presentations of inborn errors of metabolism are varied and a planned-out, stepwise approach in evaluation is fundamental.
Lactic acid dehydrogenase (LDH) level was 1,457 U/L (reference range, 140-280), and haptoglobin level was less than 6 mg/dL (reference range, 30-200). Peripheral blood smear demonstrated occasional atypical, reactive-appearing lymphocytes with red cell clumping and agglutination, as well as rare target, burr, and fragmented red cells. Test results for urine myoglobin were negative. Results of urine culture were negative. No blood culture was collected.
The elevated LDH, decreased haptoglobin, and findings on the peripheral blood smear confirm hemolysis. The clumping of erythrocytes can be artifactual in the preparation of peripheral smears, but when considered in the context of hemolysis, may be clinically important. Clumping of erythrocytes on the peripheral smear indicates the binding of a protein to antigens on the erythrocyte membrane; when this occurs below body temperature, this is consistent with the presence of a “cold agglutinin,” usually an IgM antibody directed at erythrocyte surface antigens that causes agglutination and destruction, especially in cooler areas of the body. This is a well-known complication of Mycoplasma pneumoniae infections as well as Epstein-Barr virus (EBV) infections; it may also occur with lymphoid malignancies or autoimmune disease.
Direct Coombs IgG test findings were negative, direct Coombs C3 test was positive, and direct Coombs polyspecific test was positive. M pneumoniae IgG antibody level was 1.4 mg/dL (reference ranges: <0.9, negative; 0.91-1.09, equivocal; >1.1, positive); M pneumoniae IgM level was 529 U/mL (reference range: <770, negative). EBV capsid IgM and IgG levels were undetectable. EBV nuclear antigen IgG level was also undetectable. EBV viral load was fewer than 10 copies/mL. Antinuclear antibodies (ANA) level was negative. General IgE and IgM levels were normal, at 11 and 81 mg/dL, respectively. Repeat complete blood count showed WBC of 7,800/µL, hemoglobin of 8.7 g/dL, and platelet count of 341,000/µL. The patient’s hemoglobin remained stable during hospitalization.
This directed testing is helpful in further classifying the patient’s hemolytic anemia. Autoimmune hemolytic anemias are classified into warm antibody–mediated, cold antibody–mediated, and mixed-type forms; drug-induced and alloimmune hemolytic anemias also occur. In addition, both systemic lupus erythematosus and antiphospholipid antibody syndrome can have hemolytic anemia with variable Coombs testing results; neither fit well in this case. The absence of red blood cell–directed IgG antibodies substantially decreases the likelihood of warm antibody–mediated hemolytic anemia. In cold antibody–mediated hemolytic anemia, antibodies bind to the erythrocyte membrane and then adhere to complement C3, which leads to both intravascular and extravascular hemolysis. Important types of cold antibody–mediated hemolytic anemia in children are primary and secondary cold agglutinin disease, along with paroxysmal cold hemoglobinuria. The Donath-Landsteiner test can be helpful in differentiating these conditions. Antibodies to Mycoplasma may be delayed in response to acute infection, and a child who is reinfected may only produce IgG antibodies. Given the patient’s clinical stability and previous health, the most likely diagnosis is Mycoplasma-induced cold antibody–mediated hemolytic anemia. It may be helpful to check convalescent titers to Mycoplasma in 2 to 4 weeks.
Donath-Landsteiner (D-L) antibody test results were positive. Medication-derived hemolytic anemia testing was conducted, but the presence of positive D-L antibody makes the test results inconclusive. This ultimately led to a diagnosis of paroxysmal cold hemoglobinuria (PCH), presumably triggered by a viral syndrome. Convalescent titers to Mycoplasma were not checked given clinical improvement. Because the patient’s hemoglobin was stable during hospitalization, he was not treated with steroids. His parents were counseled on avoiding cold temperatures for several days. Within 1 month, his hemoglobin had recovered without further evidence of hemolysis.
DISCUSSION
Hemolytic anemia refers to the accelerated destruction of red blood cells and can be further classified as acquired or hereditary.1 Hereditary conditions causing hemolytic anemia include enzymopathies (eg, glucose-6-phosphate dehydrogenase deficiency), hemoglobinopathies (eg, sickle cell disease), and membrane abnormalities (eg, hereditary spherocytosis). Acquired pathologies include microangiopathic hemolytic anemia (MAHA), anemias directly caused by certain infections such as malaria, and immune-mediated (Coombs-positive) hemolytic anemias.
MAHA can sometimes be life-threatening and is therefore important to identify quickly. In the right clinical context, such processes may be rapidly recognized by the presence of schistocytes on blood smear in addition to an elevated serum LDH level. Schistocytes suggest mechanical destruction of erythrocytes in the vasculature, the hallmark of MAHA. Important MAHAs include thrombocytopenic purpura, hemolytic-uremic syndrome, and disseminated intravascular coagulation. Though this patient did have a mildly elevated LDH, MAHA was less likely because there were no schistocytes on the blood smear.
Autoimmune hemolytic anemias (AIHAs) are another important subset of acquired hemolytic anemias. AIHAs occur when there is antibody-mediated destruction of erythrocytes. The direct Coombs test evaluates for antibody- or complement-coated erythrocytes. After administration of anti-IgG and anti-C3 serum, the test evaluates for agglutination of the red cells caused by attached antibodies or complement. Coombs-positive AIHA can also be categorized by the temperature of agglutination. “Warm” hemolysis often involves IgG autoantibodies (ie, warm agglutinins), while “cold” antibodies, usually IgM autoantibodies, bind at colder temperatures (0-4 °C) and activate complements, including C3. In this patient, the Coombs C3 was positive while the Coombs IgG was negative, which is more suggestive of a cold complement–mediated pathway.
Cold AIHA can be further categorized into primary cold agglutinin disease, secondary cold agglutinin disease, and PCH. Primary cold agglutinin disease is an autoimmune disorder that mostly occurs in adults. Secondary cold AIHA can often be triggered by bacterial infection (commonly M pneumoniae) or viruses including EBV, measles, and mumps.2 Medications, including penicillin and cephalosporins, can also be implicated. Secondary cold AIHA is also linked with autoimmune diseases, such as systemic lupus erythematosus and lymphoproliferative disorders. PCH can be identified with the unique presence of a specific autoantibody (ie, D-L autoantibody) that agglutinates at cold temperatures but dissociates on subsequent rewarming.3 Complement remains affixed and activates hemolysis.
The D-L antibody responsible for PCH is an IgG antibody to the P-antigen present on the erythrocyte surface. Since the Coombs test is conducted at normal temperature, it will be positive for the affixed complement but not for IgG. The underlying mechanism for PCH was proposed by Julius Donath, MD, and Karl Landsteiner, MD, in 1904 and is considered to be the first description of autoimmune disease being precipitated by antibodies.4 The D-L antibody test itself is uncommonly performed and somewhat difficult to interpret, particularly in adults, and may lead to false-negative results.5
PCH is an acquired, cold AIHA more common to children6,7 and may account for up to 33% of pediatric AIHA cases.8 Typical presentation is after an upper respiratory tract illness; however, the trigger is often not identified. Implicated triggers include a number of viruses.9 Clinical presentation includes findings of intravascular hemolysis similar to those in our patient. The pathogenic IgG autoantibody is polyclonal and is likely formed because of immune stimulation, which is consistent with the predominance of nonmalignant triggers of this disease process.10 Hemolysis and associated symptoms are often exacerbated with cold exposure; both typically resolve within 2 weeks. In recurrent cases, which are a minority, immunosuppression may be considered.10
PCH remains an often-understated cause of hemolytic anemia particularly in children. Lacking obvious pathognomonic clinical symptoms, it may be overlooked for other forms of AIHA or MAHA. However, with a structured approach to evaluation, as with this patient who had hematuria and jaundice, early diagnosis can prevent an unnecessarily extensive workup and can provide reassurance to patient and parents. By understanding the basic categories of hemolytic anemia, the relevant blood testing available, and interpretation of Coombs test results, clinicians can ensure that PCH is a diagnosis that is not left out in the cold.
KEY TEACHING POINTS
- Examination for schistocytes on a blood smear can help identify life-threatening causes of hemolytic anemia.
- Characterization of cold AIHA includes defining the underlying etiology as primary cold agglutinin disease, secondary cold agglutinin disease, or PCH.
- PCH is a cold AIHA that is an underrecognized cause of hemolytic anemia in children. The diagnosis of PCH is made by testing for the presence of the D-L antibody.
1. Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599-2606.
2. Djaldetti M. Paroxysmal cold hemoglobinuria. CRC Crit Rev Clin Lab Sci. 1978;9(1):49-83. https://doi.org/10.3109/10408367809150915
3. Levine P, Celano MJ, Falkowski F. The specificity of the antibody in paroxysmal cold hemoglobinuria (P.C.H.). Transfusion. 1963;3(4):278-280. https://doi.org/10.1111/j.1537-2995.1963.tb04643.x
4. Donath J, Landsteiner K. Uber Paroxysmale Hamoglobinurie. Munch Med Wochenschr. 1904;51:1590-1593
5. Zeller MP, Arnold DM, Al Habsi K, et al. Paroxysmal cold hemoglobinuria: a difficult diagnosis in adult patients. Transfusion. 2017;57(1):137-143. https://doi.org/10.1111/trf.13888
6. Göttsche B, Salama A, Mueller-Eckhardt C. Donath-Landsteiner autoimmune hemolytic anemia in children. a study of 22 cases. Vox Sang. 1990;58(4):281-286. https://doi.org/10.1111/j.1423-0410.1990.tb05000.x
7. Sokol RJ, Booker DJ, Stamps R. Erythropoiesis: paroxysmal cold haemoglobinuria: a clinico-pathological study of patients with a positive Donath-Landsteiner test. Hematology. 1999;4(2):137-164. https://doi.org/10.1080/10245332.1999.11746439
8. Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22(1):1-15. https://doi.org/10.1016/j.blre.2007.08.002
9. Leibrandt R, Angelino K, Vizel-Schwartz M, Shapira I. Paroxysmal cold hemoglobinuria in an adult with respiratory syncytial virus. Case Rep Hematol. 2018;2018:1-3. https://doi.org/10.1155/2018/7586719
10. Gertz MA. Management of cold haemolytic syndrome. Br J Haematol. 2007;138(4):422-429. https://doi.org/10.1111/j.1365-2141.2007.06664.x
A previously healthy 4-year-old boy presented to his pediatrician for nasal congestion, left ear pain, and intermittent fevers, which he’d been experiencing for 2 days. His exam was consistent with acute otitis media. Cefdinir was prescribed given a rash allergy to amoxicillin. His fever, congestion, and otalgia improved the next day.
Three days later he developed abdominal pain, fever, and labored breathing; his mother brought him to the emergency department (ED). His temperature was 38.0 °C, heart rate 141 beats per minute, blood pressure 117/71 mm Hg, respiratory rate 22 breaths per minute; he had oxygen saturation of 96% on ambient air. Despite mild accessory muscle use, he appeared comfortable and interactive. His left tympanic membrane was bulging without erythema. His neck was supple and mucous membranes moist. He had neither cervical lymphadenopathy nor conjunctival pallor. The cardiopulmonary exam was normal except for tachycardia. His abdomen was soft and not distended without organomegaly or tenderness.
Upper respiratory tract symptoms are commonly encountered in pediatrics and most often result from self-limited viral processes. Evaluation of a child with upper respiratory tract symptoms aims to identify serious causes like meningitis, as well as assessing the need for antimicrobial therapy. Supportive management is often appropriate in otitis media. His new, more concerning symptoms portend either a progression of the original process causing his upper respiratory tract symptoms or a separate etiology. It is key to determine which signs and symptoms are associated with the primary process and which are compensatory or secondary. If he were to be more ill appearing, for example, it is possible that his respiratory distress may be related to an underlying systemic illness rather than a primary lung process. Respiratory distress, abdominal pain, and fever could be a result of sepsis from an intrabdominal process such as ruptured appendicitis, intussusception, or malrotation with volvulus. Other causes of sepsis, such as meningitis or severe mastoiditis, both rare complications of otitis media, should be considered, although he does not appear severely ill. Acute myelogenous leukemia or other malignancies and illnesses associated with immunodeficiency can present with sepsis and chloromas in the middle ear that can be misconstrued as otitis media.
A chest radiograph demonstrated left lower lobe patchy opacities concerning for pneumonia. Rapid respiratory syncytial virus and influenza antigen test results were negative. Laboratory testing for general bloodwork was not obtained. He was administered a single dose of intramuscular ceftriaxone, prescribed a 5-day course of azithromycin, and discharged home. The child’s breathing gradually improved, but he continued to have subjective fevers. Two days later, he developed dark red urine. His mother brought him back to the outpatient clinic.
At the time of the ED visit, a diagnosis of community-acquired pneumonia was plausible given fever, mildly increased work of breathing, and an opacification on chest radiography. Most community-acquired pneumonia is caused by viruses; common bacterial causes for his age include Streptococcus pneumoniae and Moraxella catarrhalis. The first-line treatment for uncomplicated community-acquired pneumonia in children is amoxicillin, but this was appropriately avoided given his allergy.
The persistent fevers are surprising. The improvement in breathing corresponds to the treatment (and resolution) of community-acquired pneumonia. However, the development of dark urine does not. Red urine—in the absence of ingested pigments (such as those found in beets)—usually results from hematuria, hemoglobinuria, or myoglobinuria. Gross hematuria can originate from the kidneys to the urethral meatus. Abdominal masses, kidney trauma, or underlying kidney disease may all present with gross hematuria (or microscopic hematuria, seen only on urinalysis). The urine should be examined for the presence of heme, protein, and for evidence of infection; microscopy should be performed to examine for cellular casts and dysmorphic red cells. Tests of renal function, a comprehensive metabolic panel, evaluation of hematologic indexes, and assessments of inflammatory markers should be performed.
The child lived with his parents and had no siblings. He experienced no physical trauma, and there was no family history of kidney disease or hematuria. His father had a persistent cough and fever for 1 month, but recovered around the time the patient began to experience his initial symptoms. This was the patient’s third diagnosis of pneumonia. He had not traveled and was up to date with immunizations. He attended day care.
The fact that this is not the first episode of “pneumonia” raises important possibilities. The most likely one is that the child has had multiple viral infections; however, he could have an underlying primary immunodeficiency (PI) that predisposes him to recurrent infections. More severe PIs often present with recurrent sepsis, bacteremia, and failure to thrive, none of which were present in this case. Less severe PIs (such as selective IgA deficiency) could be possible. Another possibility is that these recurrent episodes of pneumonia are a relapsing and remitting noninfectious process, such as an antineutrophil cytoplasmic antibodies–associated vasculitis or anti–glomerular basement membrane disease. The patient’s father’s recent prolonged respiratory symptoms may be suggestive of pertussis or a “walking pneumonia” potentially caused by Mycoplasma or another atypical bacterium.
His temperature was 36.9 °C, heart rate 107 beats per minute, blood pressure was 106/67 mm Hg, and respiratory rate was 24 breaths per minute with oxygen saturation of 100% on ambient air. He was well appearing. His mucous membranes were moist, and oropharynx was clear. He had scleral icterus. The cardiopulmonary exam was normal. He had no significant lymphadenopathy, hepatosplenomegaly, or rashes.
The finding of jaundice is an important diagnostic pivot point, especially when combined with hematuria. The next step is determining if the jaundice is resulting from unconjugated or conjugated hyperbilirubinemia; the former most often stems from hemolysis or impairment in conjugation, while the latter results from intrahepatic or extrahepatic biliary defects. Tests for hepatobiliary injury including evaluations of alanine and aspartate aminotransferases and alkaline phosphatase, as well as for hepatic function such as tests of coagulation, should be performed.
The patient was referred to the ED and admitted for further evaluation. A complete blood count revealed a white blood cell (WBC) count of 10,700/µL (61% polymorphonuclear neutrophils, 30% lymphocytes, 5% monocytes, 3% eosinophils, 1% basophils), hemoglobin count was 10.3 g/dL (reticulocyte 2% with absolute reticulocyte count 58,400/μL), and platelet count was 265,000/µL. Components of the basic metabolic panel were within reference ranges except for a mildly elevated blood urea nitrogen level of 14 mg/dL with normal creatinine level of 0.3 mg/dL. Total protein was 6.7 g/dL (reference range, 6.4-8.3) and albumin 3.9 g/dL (reference range, 3.4-4.8). Alkaline phosphatase level was 188 U/L (reference range, 44-147), aspartate aminotransferase level 76 U/L (reference range, 0-40), and alanine aminotransferase level 12 U/L (reference range, 7-40). Total bilirubin level was 2.4 mg/dL (reference range, less than 1.5) with direct bilirubin level of 0.4 mg/dL. His C-reactive protein level was 1.5 mg/mL (reference range, 0-0.75). Creatinine kinase (CK) level was 2,550 U/L (reference range, 2-198). International Normalized Ratio (INR) was 1.0. Urinalysis was notable for 2+ proteinuria, large hemoglobin pigment, and 6 red blood cells per high power field (reference range, 0-4).
His blood urea nitrogen is elevated, reflecting either prerenal azotemia or increased absorption of nitrogenous products. Unconjugated hyperbilirubinemia may result from impaired hepatic bilirubin uptake (such as in heart failure or portosystemic shunts), impaired bilirubin conjugation (resulting from genetic conditions or drugs), or excess bilirubin production (such as in hemolysis); his anemia and lack of other evidence of hepatic dysfunction point to hemolysis as the etiology. The reticulocyte production index is approximately 1%, which suggests that an increase in erythrocyte generation is present but inadequate. This, however, does not mean that an erythrocyte production abnormality is present since reticulocytosis can be delayed in many cases of acute hemolytic anemia. It is also possible that the same hemolytic process is affecting mature and immature erythrocytes. A peripheral blood smear should be reviewed for evidence of intravascular hemolysis and testing for autoimmune hemolysis should be performed. Notably, his white blood cell and platelet counts are preserved, which makes a bone marrow–involved malignancy or infiltrative process less likely. The alkaline phosphatase elevation may result from either intrahepatic or extrahepatic biliopathy; bone damage is also possible. The elevation of aspartate aminotransferase, CK, and potassium, along with marked urinary heme pigment, may indicate muscle damage; the most common myositis in children is benign acute childhood myositis resulting from viral infection. However, the moderate level of CK elevation seen in this case is nonspecific and can result from many different etiologies. A metabolic myopathy, such as carnitine palmitoyltransferase II deficiency, can be made worse by metabolic stress and result in rhabdomyolysis; the presentations of inborn errors of metabolism are varied and a planned-out, stepwise approach in evaluation is fundamental.
Lactic acid dehydrogenase (LDH) level was 1,457 U/L (reference range, 140-280), and haptoglobin level was less than 6 mg/dL (reference range, 30-200). Peripheral blood smear demonstrated occasional atypical, reactive-appearing lymphocytes with red cell clumping and agglutination, as well as rare target, burr, and fragmented red cells. Test results for urine myoglobin were negative. Results of urine culture were negative. No blood culture was collected.
The elevated LDH, decreased haptoglobin, and findings on the peripheral blood smear confirm hemolysis. The clumping of erythrocytes can be artifactual in the preparation of peripheral smears, but when considered in the context of hemolysis, may be clinically important. Clumping of erythrocytes on the peripheral smear indicates the binding of a protein to antigens on the erythrocyte membrane; when this occurs below body temperature, this is consistent with the presence of a “cold agglutinin,” usually an IgM antibody directed at erythrocyte surface antigens that causes agglutination and destruction, especially in cooler areas of the body. This is a well-known complication of Mycoplasma pneumoniae infections as well as Epstein-Barr virus (EBV) infections; it may also occur with lymphoid malignancies or autoimmune disease.
Direct Coombs IgG test findings were negative, direct Coombs C3 test was positive, and direct Coombs polyspecific test was positive. M pneumoniae IgG antibody level was 1.4 mg/dL (reference ranges: <0.9, negative; 0.91-1.09, equivocal; >1.1, positive); M pneumoniae IgM level was 529 U/mL (reference range: <770, negative). EBV capsid IgM and IgG levels were undetectable. EBV nuclear antigen IgG level was also undetectable. EBV viral load was fewer than 10 copies/mL. Antinuclear antibodies (ANA) level was negative. General IgE and IgM levels were normal, at 11 and 81 mg/dL, respectively. Repeat complete blood count showed WBC of 7,800/µL, hemoglobin of 8.7 g/dL, and platelet count of 341,000/µL. The patient’s hemoglobin remained stable during hospitalization.
This directed testing is helpful in further classifying the patient’s hemolytic anemia. Autoimmune hemolytic anemias are classified into warm antibody–mediated, cold antibody–mediated, and mixed-type forms; drug-induced and alloimmune hemolytic anemias also occur. In addition, both systemic lupus erythematosus and antiphospholipid antibody syndrome can have hemolytic anemia with variable Coombs testing results; neither fit well in this case. The absence of red blood cell–directed IgG antibodies substantially decreases the likelihood of warm antibody–mediated hemolytic anemia. In cold antibody–mediated hemolytic anemia, antibodies bind to the erythrocyte membrane and then adhere to complement C3, which leads to both intravascular and extravascular hemolysis. Important types of cold antibody–mediated hemolytic anemia in children are primary and secondary cold agglutinin disease, along with paroxysmal cold hemoglobinuria. The Donath-Landsteiner test can be helpful in differentiating these conditions. Antibodies to Mycoplasma may be delayed in response to acute infection, and a child who is reinfected may only produce IgG antibodies. Given the patient’s clinical stability and previous health, the most likely diagnosis is Mycoplasma-induced cold antibody–mediated hemolytic anemia. It may be helpful to check convalescent titers to Mycoplasma in 2 to 4 weeks.
Donath-Landsteiner (D-L) antibody test results were positive. Medication-derived hemolytic anemia testing was conducted, but the presence of positive D-L antibody makes the test results inconclusive. This ultimately led to a diagnosis of paroxysmal cold hemoglobinuria (PCH), presumably triggered by a viral syndrome. Convalescent titers to Mycoplasma were not checked given clinical improvement. Because the patient’s hemoglobin was stable during hospitalization, he was not treated with steroids. His parents were counseled on avoiding cold temperatures for several days. Within 1 month, his hemoglobin had recovered without further evidence of hemolysis.
DISCUSSION
Hemolytic anemia refers to the accelerated destruction of red blood cells and can be further classified as acquired or hereditary.1 Hereditary conditions causing hemolytic anemia include enzymopathies (eg, glucose-6-phosphate dehydrogenase deficiency), hemoglobinopathies (eg, sickle cell disease), and membrane abnormalities (eg, hereditary spherocytosis). Acquired pathologies include microangiopathic hemolytic anemia (MAHA), anemias directly caused by certain infections such as malaria, and immune-mediated (Coombs-positive) hemolytic anemias.
MAHA can sometimes be life-threatening and is therefore important to identify quickly. In the right clinical context, such processes may be rapidly recognized by the presence of schistocytes on blood smear in addition to an elevated serum LDH level. Schistocytes suggest mechanical destruction of erythrocytes in the vasculature, the hallmark of MAHA. Important MAHAs include thrombocytopenic purpura, hemolytic-uremic syndrome, and disseminated intravascular coagulation. Though this patient did have a mildly elevated LDH, MAHA was less likely because there were no schistocytes on the blood smear.
Autoimmune hemolytic anemias (AIHAs) are another important subset of acquired hemolytic anemias. AIHAs occur when there is antibody-mediated destruction of erythrocytes. The direct Coombs test evaluates for antibody- or complement-coated erythrocytes. After administration of anti-IgG and anti-C3 serum, the test evaluates for agglutination of the red cells caused by attached antibodies or complement. Coombs-positive AIHA can also be categorized by the temperature of agglutination. “Warm” hemolysis often involves IgG autoantibodies (ie, warm agglutinins), while “cold” antibodies, usually IgM autoantibodies, bind at colder temperatures (0-4 °C) and activate complements, including C3. In this patient, the Coombs C3 was positive while the Coombs IgG was negative, which is more suggestive of a cold complement–mediated pathway.
Cold AIHA can be further categorized into primary cold agglutinin disease, secondary cold agglutinin disease, and PCH. Primary cold agglutinin disease is an autoimmune disorder that mostly occurs in adults. Secondary cold AIHA can often be triggered by bacterial infection (commonly M pneumoniae) or viruses including EBV, measles, and mumps.2 Medications, including penicillin and cephalosporins, can also be implicated. Secondary cold AIHA is also linked with autoimmune diseases, such as systemic lupus erythematosus and lymphoproliferative disorders. PCH can be identified with the unique presence of a specific autoantibody (ie, D-L autoantibody) that agglutinates at cold temperatures but dissociates on subsequent rewarming.3 Complement remains affixed and activates hemolysis.
The D-L antibody responsible for PCH is an IgG antibody to the P-antigen present on the erythrocyte surface. Since the Coombs test is conducted at normal temperature, it will be positive for the affixed complement but not for IgG. The underlying mechanism for PCH was proposed by Julius Donath, MD, and Karl Landsteiner, MD, in 1904 and is considered to be the first description of autoimmune disease being precipitated by antibodies.4 The D-L antibody test itself is uncommonly performed and somewhat difficult to interpret, particularly in adults, and may lead to false-negative results.5
PCH is an acquired, cold AIHA more common to children6,7 and may account for up to 33% of pediatric AIHA cases.8 Typical presentation is after an upper respiratory tract illness; however, the trigger is often not identified. Implicated triggers include a number of viruses.9 Clinical presentation includes findings of intravascular hemolysis similar to those in our patient. The pathogenic IgG autoantibody is polyclonal and is likely formed because of immune stimulation, which is consistent with the predominance of nonmalignant triggers of this disease process.10 Hemolysis and associated symptoms are often exacerbated with cold exposure; both typically resolve within 2 weeks. In recurrent cases, which are a minority, immunosuppression may be considered.10
PCH remains an often-understated cause of hemolytic anemia particularly in children. Lacking obvious pathognomonic clinical symptoms, it may be overlooked for other forms of AIHA or MAHA. However, with a structured approach to evaluation, as with this patient who had hematuria and jaundice, early diagnosis can prevent an unnecessarily extensive workup and can provide reassurance to patient and parents. By understanding the basic categories of hemolytic anemia, the relevant blood testing available, and interpretation of Coombs test results, clinicians can ensure that PCH is a diagnosis that is not left out in the cold.
KEY TEACHING POINTS
- Examination for schistocytes on a blood smear can help identify life-threatening causes of hemolytic anemia.
- Characterization of cold AIHA includes defining the underlying etiology as primary cold agglutinin disease, secondary cold agglutinin disease, or PCH.
- PCH is a cold AIHA that is an underrecognized cause of hemolytic anemia in children. The diagnosis of PCH is made by testing for the presence of the D-L antibody.
A previously healthy 4-year-old boy presented to his pediatrician for nasal congestion, left ear pain, and intermittent fevers, which he’d been experiencing for 2 days. His exam was consistent with acute otitis media. Cefdinir was prescribed given a rash allergy to amoxicillin. His fever, congestion, and otalgia improved the next day.
Three days later he developed abdominal pain, fever, and labored breathing; his mother brought him to the emergency department (ED). His temperature was 38.0 °C, heart rate 141 beats per minute, blood pressure 117/71 mm Hg, respiratory rate 22 breaths per minute; he had oxygen saturation of 96% on ambient air. Despite mild accessory muscle use, he appeared comfortable and interactive. His left tympanic membrane was bulging without erythema. His neck was supple and mucous membranes moist. He had neither cervical lymphadenopathy nor conjunctival pallor. The cardiopulmonary exam was normal except for tachycardia. His abdomen was soft and not distended without organomegaly or tenderness.
Upper respiratory tract symptoms are commonly encountered in pediatrics and most often result from self-limited viral processes. Evaluation of a child with upper respiratory tract symptoms aims to identify serious causes like meningitis, as well as assessing the need for antimicrobial therapy. Supportive management is often appropriate in otitis media. His new, more concerning symptoms portend either a progression of the original process causing his upper respiratory tract symptoms or a separate etiology. It is key to determine which signs and symptoms are associated with the primary process and which are compensatory or secondary. If he were to be more ill appearing, for example, it is possible that his respiratory distress may be related to an underlying systemic illness rather than a primary lung process. Respiratory distress, abdominal pain, and fever could be a result of sepsis from an intrabdominal process such as ruptured appendicitis, intussusception, or malrotation with volvulus. Other causes of sepsis, such as meningitis or severe mastoiditis, both rare complications of otitis media, should be considered, although he does not appear severely ill. Acute myelogenous leukemia or other malignancies and illnesses associated with immunodeficiency can present with sepsis and chloromas in the middle ear that can be misconstrued as otitis media.
A chest radiograph demonstrated left lower lobe patchy opacities concerning for pneumonia. Rapid respiratory syncytial virus and influenza antigen test results were negative. Laboratory testing for general bloodwork was not obtained. He was administered a single dose of intramuscular ceftriaxone, prescribed a 5-day course of azithromycin, and discharged home. The child’s breathing gradually improved, but he continued to have subjective fevers. Two days later, he developed dark red urine. His mother brought him back to the outpatient clinic.
At the time of the ED visit, a diagnosis of community-acquired pneumonia was plausible given fever, mildly increased work of breathing, and an opacification on chest radiography. Most community-acquired pneumonia is caused by viruses; common bacterial causes for his age include Streptococcus pneumoniae and Moraxella catarrhalis. The first-line treatment for uncomplicated community-acquired pneumonia in children is amoxicillin, but this was appropriately avoided given his allergy.
The persistent fevers are surprising. The improvement in breathing corresponds to the treatment (and resolution) of community-acquired pneumonia. However, the development of dark urine does not. Red urine—in the absence of ingested pigments (such as those found in beets)—usually results from hematuria, hemoglobinuria, or myoglobinuria. Gross hematuria can originate from the kidneys to the urethral meatus. Abdominal masses, kidney trauma, or underlying kidney disease may all present with gross hematuria (or microscopic hematuria, seen only on urinalysis). The urine should be examined for the presence of heme, protein, and for evidence of infection; microscopy should be performed to examine for cellular casts and dysmorphic red cells. Tests of renal function, a comprehensive metabolic panel, evaluation of hematologic indexes, and assessments of inflammatory markers should be performed.
The child lived with his parents and had no siblings. He experienced no physical trauma, and there was no family history of kidney disease or hematuria. His father had a persistent cough and fever for 1 month, but recovered around the time the patient began to experience his initial symptoms. This was the patient’s third diagnosis of pneumonia. He had not traveled and was up to date with immunizations. He attended day care.
The fact that this is not the first episode of “pneumonia” raises important possibilities. The most likely one is that the child has had multiple viral infections; however, he could have an underlying primary immunodeficiency (PI) that predisposes him to recurrent infections. More severe PIs often present with recurrent sepsis, bacteremia, and failure to thrive, none of which were present in this case. Less severe PIs (such as selective IgA deficiency) could be possible. Another possibility is that these recurrent episodes of pneumonia are a relapsing and remitting noninfectious process, such as an antineutrophil cytoplasmic antibodies–associated vasculitis or anti–glomerular basement membrane disease. The patient’s father’s recent prolonged respiratory symptoms may be suggestive of pertussis or a “walking pneumonia” potentially caused by Mycoplasma or another atypical bacterium.
His temperature was 36.9 °C, heart rate 107 beats per minute, blood pressure was 106/67 mm Hg, and respiratory rate was 24 breaths per minute with oxygen saturation of 100% on ambient air. He was well appearing. His mucous membranes were moist, and oropharynx was clear. He had scleral icterus. The cardiopulmonary exam was normal. He had no significant lymphadenopathy, hepatosplenomegaly, or rashes.
The finding of jaundice is an important diagnostic pivot point, especially when combined with hematuria. The next step is determining if the jaundice is resulting from unconjugated or conjugated hyperbilirubinemia; the former most often stems from hemolysis or impairment in conjugation, while the latter results from intrahepatic or extrahepatic biliary defects. Tests for hepatobiliary injury including evaluations of alanine and aspartate aminotransferases and alkaline phosphatase, as well as for hepatic function such as tests of coagulation, should be performed.
The patient was referred to the ED and admitted for further evaluation. A complete blood count revealed a white blood cell (WBC) count of 10,700/µL (61% polymorphonuclear neutrophils, 30% lymphocytes, 5% monocytes, 3% eosinophils, 1% basophils), hemoglobin count was 10.3 g/dL (reticulocyte 2% with absolute reticulocyte count 58,400/μL), and platelet count was 265,000/µL. Components of the basic metabolic panel were within reference ranges except for a mildly elevated blood urea nitrogen level of 14 mg/dL with normal creatinine level of 0.3 mg/dL. Total protein was 6.7 g/dL (reference range, 6.4-8.3) and albumin 3.9 g/dL (reference range, 3.4-4.8). Alkaline phosphatase level was 188 U/L (reference range, 44-147), aspartate aminotransferase level 76 U/L (reference range, 0-40), and alanine aminotransferase level 12 U/L (reference range, 7-40). Total bilirubin level was 2.4 mg/dL (reference range, less than 1.5) with direct bilirubin level of 0.4 mg/dL. His C-reactive protein level was 1.5 mg/mL (reference range, 0-0.75). Creatinine kinase (CK) level was 2,550 U/L (reference range, 2-198). International Normalized Ratio (INR) was 1.0. Urinalysis was notable for 2+ proteinuria, large hemoglobin pigment, and 6 red blood cells per high power field (reference range, 0-4).
His blood urea nitrogen is elevated, reflecting either prerenal azotemia or increased absorption of nitrogenous products. Unconjugated hyperbilirubinemia may result from impaired hepatic bilirubin uptake (such as in heart failure or portosystemic shunts), impaired bilirubin conjugation (resulting from genetic conditions or drugs), or excess bilirubin production (such as in hemolysis); his anemia and lack of other evidence of hepatic dysfunction point to hemolysis as the etiology. The reticulocyte production index is approximately 1%, which suggests that an increase in erythrocyte generation is present but inadequate. This, however, does not mean that an erythrocyte production abnormality is present since reticulocytosis can be delayed in many cases of acute hemolytic anemia. It is also possible that the same hemolytic process is affecting mature and immature erythrocytes. A peripheral blood smear should be reviewed for evidence of intravascular hemolysis and testing for autoimmune hemolysis should be performed. Notably, his white blood cell and platelet counts are preserved, which makes a bone marrow–involved malignancy or infiltrative process less likely. The alkaline phosphatase elevation may result from either intrahepatic or extrahepatic biliopathy; bone damage is also possible. The elevation of aspartate aminotransferase, CK, and potassium, along with marked urinary heme pigment, may indicate muscle damage; the most common myositis in children is benign acute childhood myositis resulting from viral infection. However, the moderate level of CK elevation seen in this case is nonspecific and can result from many different etiologies. A metabolic myopathy, such as carnitine palmitoyltransferase II deficiency, can be made worse by metabolic stress and result in rhabdomyolysis; the presentations of inborn errors of metabolism are varied and a planned-out, stepwise approach in evaluation is fundamental.
Lactic acid dehydrogenase (LDH) level was 1,457 U/L (reference range, 140-280), and haptoglobin level was less than 6 mg/dL (reference range, 30-200). Peripheral blood smear demonstrated occasional atypical, reactive-appearing lymphocytes with red cell clumping and agglutination, as well as rare target, burr, and fragmented red cells. Test results for urine myoglobin were negative. Results of urine culture were negative. No blood culture was collected.
The elevated LDH, decreased haptoglobin, and findings on the peripheral blood smear confirm hemolysis. The clumping of erythrocytes can be artifactual in the preparation of peripheral smears, but when considered in the context of hemolysis, may be clinically important. Clumping of erythrocytes on the peripheral smear indicates the binding of a protein to antigens on the erythrocyte membrane; when this occurs below body temperature, this is consistent with the presence of a “cold agglutinin,” usually an IgM antibody directed at erythrocyte surface antigens that causes agglutination and destruction, especially in cooler areas of the body. This is a well-known complication of Mycoplasma pneumoniae infections as well as Epstein-Barr virus (EBV) infections; it may also occur with lymphoid malignancies or autoimmune disease.
Direct Coombs IgG test findings were negative, direct Coombs C3 test was positive, and direct Coombs polyspecific test was positive. M pneumoniae IgG antibody level was 1.4 mg/dL (reference ranges: <0.9, negative; 0.91-1.09, equivocal; >1.1, positive); M pneumoniae IgM level was 529 U/mL (reference range: <770, negative). EBV capsid IgM and IgG levels were undetectable. EBV nuclear antigen IgG level was also undetectable. EBV viral load was fewer than 10 copies/mL. Antinuclear antibodies (ANA) level was negative. General IgE and IgM levels were normal, at 11 and 81 mg/dL, respectively. Repeat complete blood count showed WBC of 7,800/µL, hemoglobin of 8.7 g/dL, and platelet count of 341,000/µL. The patient’s hemoglobin remained stable during hospitalization.
This directed testing is helpful in further classifying the patient’s hemolytic anemia. Autoimmune hemolytic anemias are classified into warm antibody–mediated, cold antibody–mediated, and mixed-type forms; drug-induced and alloimmune hemolytic anemias also occur. In addition, both systemic lupus erythematosus and antiphospholipid antibody syndrome can have hemolytic anemia with variable Coombs testing results; neither fit well in this case. The absence of red blood cell–directed IgG antibodies substantially decreases the likelihood of warm antibody–mediated hemolytic anemia. In cold antibody–mediated hemolytic anemia, antibodies bind to the erythrocyte membrane and then adhere to complement C3, which leads to both intravascular and extravascular hemolysis. Important types of cold antibody–mediated hemolytic anemia in children are primary and secondary cold agglutinin disease, along with paroxysmal cold hemoglobinuria. The Donath-Landsteiner test can be helpful in differentiating these conditions. Antibodies to Mycoplasma may be delayed in response to acute infection, and a child who is reinfected may only produce IgG antibodies. Given the patient’s clinical stability and previous health, the most likely diagnosis is Mycoplasma-induced cold antibody–mediated hemolytic anemia. It may be helpful to check convalescent titers to Mycoplasma in 2 to 4 weeks.
Donath-Landsteiner (D-L) antibody test results were positive. Medication-derived hemolytic anemia testing was conducted, but the presence of positive D-L antibody makes the test results inconclusive. This ultimately led to a diagnosis of paroxysmal cold hemoglobinuria (PCH), presumably triggered by a viral syndrome. Convalescent titers to Mycoplasma were not checked given clinical improvement. Because the patient’s hemoglobin was stable during hospitalization, he was not treated with steroids. His parents were counseled on avoiding cold temperatures for several days. Within 1 month, his hemoglobin had recovered without further evidence of hemolysis.
DISCUSSION
Hemolytic anemia refers to the accelerated destruction of red blood cells and can be further classified as acquired or hereditary.1 Hereditary conditions causing hemolytic anemia include enzymopathies (eg, glucose-6-phosphate dehydrogenase deficiency), hemoglobinopathies (eg, sickle cell disease), and membrane abnormalities (eg, hereditary spherocytosis). Acquired pathologies include microangiopathic hemolytic anemia (MAHA), anemias directly caused by certain infections such as malaria, and immune-mediated (Coombs-positive) hemolytic anemias.
MAHA can sometimes be life-threatening and is therefore important to identify quickly. In the right clinical context, such processes may be rapidly recognized by the presence of schistocytes on blood smear in addition to an elevated serum LDH level. Schistocytes suggest mechanical destruction of erythrocytes in the vasculature, the hallmark of MAHA. Important MAHAs include thrombocytopenic purpura, hemolytic-uremic syndrome, and disseminated intravascular coagulation. Though this patient did have a mildly elevated LDH, MAHA was less likely because there were no schistocytes on the blood smear.
Autoimmune hemolytic anemias (AIHAs) are another important subset of acquired hemolytic anemias. AIHAs occur when there is antibody-mediated destruction of erythrocytes. The direct Coombs test evaluates for antibody- or complement-coated erythrocytes. After administration of anti-IgG and anti-C3 serum, the test evaluates for agglutination of the red cells caused by attached antibodies or complement. Coombs-positive AIHA can also be categorized by the temperature of agglutination. “Warm” hemolysis often involves IgG autoantibodies (ie, warm agglutinins), while “cold” antibodies, usually IgM autoantibodies, bind at colder temperatures (0-4 °C) and activate complements, including C3. In this patient, the Coombs C3 was positive while the Coombs IgG was negative, which is more suggestive of a cold complement–mediated pathway.
Cold AIHA can be further categorized into primary cold agglutinin disease, secondary cold agglutinin disease, and PCH. Primary cold agglutinin disease is an autoimmune disorder that mostly occurs in adults. Secondary cold AIHA can often be triggered by bacterial infection (commonly M pneumoniae) or viruses including EBV, measles, and mumps.2 Medications, including penicillin and cephalosporins, can also be implicated. Secondary cold AIHA is also linked with autoimmune diseases, such as systemic lupus erythematosus and lymphoproliferative disorders. PCH can be identified with the unique presence of a specific autoantibody (ie, D-L autoantibody) that agglutinates at cold temperatures but dissociates on subsequent rewarming.3 Complement remains affixed and activates hemolysis.
The D-L antibody responsible for PCH is an IgG antibody to the P-antigen present on the erythrocyte surface. Since the Coombs test is conducted at normal temperature, it will be positive for the affixed complement but not for IgG. The underlying mechanism for PCH was proposed by Julius Donath, MD, and Karl Landsteiner, MD, in 1904 and is considered to be the first description of autoimmune disease being precipitated by antibodies.4 The D-L antibody test itself is uncommonly performed and somewhat difficult to interpret, particularly in adults, and may lead to false-negative results.5
PCH is an acquired, cold AIHA more common to children6,7 and may account for up to 33% of pediatric AIHA cases.8 Typical presentation is after an upper respiratory tract illness; however, the trigger is often not identified. Implicated triggers include a number of viruses.9 Clinical presentation includes findings of intravascular hemolysis similar to those in our patient. The pathogenic IgG autoantibody is polyclonal and is likely formed because of immune stimulation, which is consistent with the predominance of nonmalignant triggers of this disease process.10 Hemolysis and associated symptoms are often exacerbated with cold exposure; both typically resolve within 2 weeks. In recurrent cases, which are a minority, immunosuppression may be considered.10
PCH remains an often-understated cause of hemolytic anemia particularly in children. Lacking obvious pathognomonic clinical symptoms, it may be overlooked for other forms of AIHA or MAHA. However, with a structured approach to evaluation, as with this patient who had hematuria and jaundice, early diagnosis can prevent an unnecessarily extensive workup and can provide reassurance to patient and parents. By understanding the basic categories of hemolytic anemia, the relevant blood testing available, and interpretation of Coombs test results, clinicians can ensure that PCH is a diagnosis that is not left out in the cold.
KEY TEACHING POINTS
- Examination for schistocytes on a blood smear can help identify life-threatening causes of hemolytic anemia.
- Characterization of cold AIHA includes defining the underlying etiology as primary cold agglutinin disease, secondary cold agglutinin disease, or PCH.
- PCH is a cold AIHA that is an underrecognized cause of hemolytic anemia in children. The diagnosis of PCH is made by testing for the presence of the D-L antibody.
1. Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599-2606.
2. Djaldetti M. Paroxysmal cold hemoglobinuria. CRC Crit Rev Clin Lab Sci. 1978;9(1):49-83. https://doi.org/10.3109/10408367809150915
3. Levine P, Celano MJ, Falkowski F. The specificity of the antibody in paroxysmal cold hemoglobinuria (P.C.H.). Transfusion. 1963;3(4):278-280. https://doi.org/10.1111/j.1537-2995.1963.tb04643.x
4. Donath J, Landsteiner K. Uber Paroxysmale Hamoglobinurie. Munch Med Wochenschr. 1904;51:1590-1593
5. Zeller MP, Arnold DM, Al Habsi K, et al. Paroxysmal cold hemoglobinuria: a difficult diagnosis in adult patients. Transfusion. 2017;57(1):137-143. https://doi.org/10.1111/trf.13888
6. Göttsche B, Salama A, Mueller-Eckhardt C. Donath-Landsteiner autoimmune hemolytic anemia in children. a study of 22 cases. Vox Sang. 1990;58(4):281-286. https://doi.org/10.1111/j.1423-0410.1990.tb05000.x
7. Sokol RJ, Booker DJ, Stamps R. Erythropoiesis: paroxysmal cold haemoglobinuria: a clinico-pathological study of patients with a positive Donath-Landsteiner test. Hematology. 1999;4(2):137-164. https://doi.org/10.1080/10245332.1999.11746439
8. Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22(1):1-15. https://doi.org/10.1016/j.blre.2007.08.002
9. Leibrandt R, Angelino K, Vizel-Schwartz M, Shapira I. Paroxysmal cold hemoglobinuria in an adult with respiratory syncytial virus. Case Rep Hematol. 2018;2018:1-3. https://doi.org/10.1155/2018/7586719
10. Gertz MA. Management of cold haemolytic syndrome. Br J Haematol. 2007;138(4):422-429. https://doi.org/10.1111/j.1365-2141.2007.06664.x
1. Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599-2606.
2. Djaldetti M. Paroxysmal cold hemoglobinuria. CRC Crit Rev Clin Lab Sci. 1978;9(1):49-83. https://doi.org/10.3109/10408367809150915
3. Levine P, Celano MJ, Falkowski F. The specificity of the antibody in paroxysmal cold hemoglobinuria (P.C.H.). Transfusion. 1963;3(4):278-280. https://doi.org/10.1111/j.1537-2995.1963.tb04643.x
4. Donath J, Landsteiner K. Uber Paroxysmale Hamoglobinurie. Munch Med Wochenschr. 1904;51:1590-1593
5. Zeller MP, Arnold DM, Al Habsi K, et al. Paroxysmal cold hemoglobinuria: a difficult diagnosis in adult patients. Transfusion. 2017;57(1):137-143. https://doi.org/10.1111/trf.13888
6. Göttsche B, Salama A, Mueller-Eckhardt C. Donath-Landsteiner autoimmune hemolytic anemia in children. a study of 22 cases. Vox Sang. 1990;58(4):281-286. https://doi.org/10.1111/j.1423-0410.1990.tb05000.x
7. Sokol RJ, Booker DJ, Stamps R. Erythropoiesis: paroxysmal cold haemoglobinuria: a clinico-pathological study of patients with a positive Donath-Landsteiner test. Hematology. 1999;4(2):137-164. https://doi.org/10.1080/10245332.1999.11746439
8. Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22(1):1-15. https://doi.org/10.1016/j.blre.2007.08.002
9. Leibrandt R, Angelino K, Vizel-Schwartz M, Shapira I. Paroxysmal cold hemoglobinuria in an adult with respiratory syncytial virus. Case Rep Hematol. 2018;2018:1-3. https://doi.org/10.1155/2018/7586719
10. Gertz MA. Management of cold haemolytic syndrome. Br J Haematol. 2007;138(4):422-429. https://doi.org/10.1111/j.1365-2141.2007.06664.x
© 2020 Society of Hospital Medicine
A Fiery Pivot
A 62-year-old man with metastatic non–small cell lung cancer (NSCLC) presented to the Emergency Department with 3 days of progressive generalized weakness, anorexia, and nonbloody diarrhea. He denied fever, chills, nausea, vomiting, cough, shortness of breath, or abdominal pain. He had no sick contacts.
One diagnostic approach for patients with cancer who present with new symptoms is to consider diagnoses both related and unrelated to the cancer. Cancer-related diagnoses can include the broad categories of complications related to the tumor itself (such as mass effect), paraneoplastic phenomena, or treatment-related complications (such as infection from immunosuppression or chemotherapy toxicity).
For this patient with metastatic NSCLC, weakness, anorexia, and diarrhea are unlikely to be related to mass effect unless the patient has peritoneal metastases (an uncommon complication of NSCLC) with carcinomatosis-associated diarrhea.
Paraneoplastic phenomena, such as hypercalcemia or hyponatremia from the syndrome of inappropriate antidiuretic hormone (SIADH), are common with NSCLC and could both lead to weakness and anorexia. Hematologic consequences of NSCLC (or its treatment) include anemia, thrombosis, and thrombotic microangiopathy (TMA), though diarrhea, in the absence of abdominal pain or hematochezia, would be unexpected.
Weakness, anorexia, and diarrhea may also be symptoms of chemotherapy toxicity or an infection resulting from immunosuppression. It would be important to know what specific treatment the patient has received. Chemotherapy commonly causes neutropenia and predisposes to rapidly progressive infections, while immunotherapies have other toxicities. Diarrhea is a common toxicity of the checkpoint inhibitors and anaplastic lymphoma kinase (ALK) inhibitors that are frequently used to treat metastatic NSCLC. Checkpoint inhibitors also are known to cause a wide range of autoimmune phenomena including colitis.
Finally, the patient’s symptoms may be unrelated to the cancer. Weakness, anorexia, and nonbloody diarrhea could be signs of viral or bacterial gastroenteritis or Clostridioides difficile colitis particularly with frequent healthcare contact or antimicrobial use.
Two days prior, he had been diagnosed with nonsevere Clostridioides difficile colitis in an acute care clinic. He was started on oral metronidazole, but his diarrhea worsened over the next day and was accompanied by weakness and anorexia. Additional past medical history included untreated hepatitis C infection, chronic kidney disease stage 3, seizure disorder, and left lung NSCLC (adenocarcinoma). The lung cancer was diagnosed 8 months prior when he had presented with hemoptysis and 3 months of progressive constitutional symptoms. Imaging at that time revealed metastases to the contralateral lung and regional lymph nodes, as well as vertebrae, ribs, and pelvis. He had no abdominal metastases. He was initially treated with carboplatin and paclitaxel. After a partial response to initial chemotherapy, he developed peripheral neuropathy and was switched to gemcitabine 12 weeks ago. He received five cycles of gemcitabine over 10 weeks. He was last administered gemcitabine 2 weeks prior. He had not received any additional chemotherapy or immunotherapy. He had a 40 pack-year history of smoking, but quit when diagnosed with cancer. He did not drink alcohol. He had no recent travel or sick contacts. He was not on any medications. He was homeless but staying with family members in the area. Additional review of systems was negative for recent bleeding, bruising, hemoptysis, melena, hematochezia, or hematuria.
Recent treatment with gemcitabine could contribute to the presentation in a number of ways. First, gemcitabine is associated with myelosuppression and neutropenia that could predispose him to infectious colitis. Second, gemcitabine is known to cause anemia, anorexia, diarrhea, and fatigue. Third, gemcitabine may also cause renal injury that can contribute to worsening anemia. He may be at greater risk of anemia and renal toxicity because of preexisting chronic kidney disease. Finally, gemcitabine can rarely cause TMA with characteristics that mimic the hemolytic-uremic syndrome with microangiopathic hemolytic anemia, mild thrombocytopenia, and severe acute kidney injury (AKI).
In addition, worsening infectious colitis could certainly explain his presenting symptoms. At this point, local mass effect seems unlikely despite his metastatic disease. Lastly, it should be noted that, in an immunosuppressed cancer patient, multiple problems could be present at the same time. Laboratory testing should evaluate for hypercalcemia, SIADH, hematologic indexes, and renal function. If initial laboratory evaluation is unrevealing, abdominal imaging may be needed to assess for carcinomatosis, complications from colitis, typhlitis, abscess, or perforation.
On physical examination, the patient appeared fatigued. His temperature was 36.8°C, blood pressure 158/72 mm Hg, pulse 88 beats per minute, respiratory rate 16 breaths per minute, and oxygen saturation was 96% while breathing ambient air. There was neither scleral icterus nor conjunctival injection but he had mild conjunctival pallor. Cardiovascular and lung examinations were normal. Abdominal exam revealed normal bowel sounds without tenderness or organomegaly. He had no supraclavicular, axillary, or inguinal lymphadenopathy. He was alert and oriented. Cranial nerves II through XII were intact. He had decreased muscle bulk in his extremities without focal weakness. Gait and reflexes were not tested.
Initial laboratory testing revealed a white blood cell count of 5.5 K/mm3, hemoglobin of 5 g/dL (hemoglobin 1 month prior was 10.1 g/dL), and platelet count of 20 K/mm3 (platelet count 1 month prior was 246 K/mm3). Creatinine was 3.9 mg/dL (compared with a baseline of 1.8 mg/dL), and blood urea nitrogen was 39 mg/dL. His sodium was 137 mEq/L, potassium 4.2 mEq/L, chloride 105 mEq/L, bicarbonate 22 mEq/L, and thyroid stimulating hormone 0.9 mU/L. His total protein was 4.9 g/dL, albumin 2.1 g/dL, alkaline phosphatase 60 IU/L, alanine aminotransferase 17 IU/L, aspartate aminotransferase 60 IU/L, direct bilirubin 0.2 mg/dL, and total bilirubin 0.5 mg/dL. A chest x-ray showed no infiltrates.
The patient’s laboratory tests reveal several important new findings, including severe acute on chronic anemia, acute thrombocytopenia, and AKI, without clinical evidence of acute blood loss. These changes could be parts of a syndrome or multiple independent disorders. The most urgent priority is to evaluate for TMAs, many of which are fatal if not diagnosed and treated expeditiously. This includes thrombotic thrombocytopenic purpura (TTP), disseminated intravascular hemolysis (DIC), and atypical hemolytic uremic syndrome (aHUS). A manual review of a peripheral blood smear is required to evaluate for fragmented red blood cells (schistocytes). Thereafter, ancillary testing to confirm intravascular hemolysis would include measuring free plasma hemoglobin and lactate dehydrogenase (LDH). Additionally, in intravascular hemolysis, haptoglobin should be depleted and urinalysis should show heme-positive urine without RBCs. In this case the patient’s normal bilirubin studies argue against hemolysis; however, elevated bilirubin is variably present in hemolytic anemias depending on the liver’s ability to conjugate and excrete bilirubin, the relative degree of RBC turnover, and type of hemolysis. Patients with intravascular hemolysis lose hemoglobin directly into the urine leaving relatively little hemoglobin to be incorporated into bile once it has reached the reticuloendothelial system. This results in relatively normal bilirubin levels. More specific indicators of intravascular hemolysis include pink colored plasma on visual inspection (commonly done in the blood bank as part of assessing for hemolytic transfusion reactions), measuring plasma free hemoglobin, or by detecting hemoglobin in the urine.
If microangiopathic hemolytic anemia (MAHA) is excluded, then other causes of these laboratory abnormalities should be considered. Bleeding is the most common cause for anemia, and thrombocytopenia predisposes patients to bleeding. However, there is no evidence of bleeding in this patient, and such a rapid acute anemia is unlikely to be caused by occult blood loss alone. Concurrent anemia and thrombocytopenia could be evidence of bone marrow toxicity from chemotherapy or neoplastic infiltration. With marrow infiltration, there are typically signs on the peripheral smear of leukoerythroblastosis, with circulating nucleated red blood cells and early myeloid forms. Concurrent immune thrombocytopenia (ITP) and autoimmune hemolytic anemia (AIHA), or Evans’ Syndrome, should also be considered. AIHA would be suggested by spherocytes on the peripheral smear, elevated LDH and a positive direct antibody test (DAT).
Regarding the AKI, the patient has diarrhea, which could lead to prerenal azotemia and acute tubular necrosis. A formal urinalysis would evaluate for prerenal and intrinsic kidney disease. TMA can cause intrinsic kidney injury with a benign urinary sediment. The blood urea nitrogen-to-creatinine ratio is not elevated, but in a patient with malnutrition this may not indicate prerenal azotemia. In summary, to differentiate potential TMAs from other causes, the patient needs a blood smear, coagulation studies, and an evaluation for hemolysis, including a urinalysis for free heme and any evidence of intrinsic kidney disease.
Urinalysis showed amber-colored, dilute urine with no white blood cells, red blood cells, protein, or casts. It was positive for blood and negative for bilirubin and hemosiderin. LDH was 1,382 IU/L (reference range 135-225 IU/L), and haptoglobin was unmeasurably low. His ferritin was 2,267 ng/mL, serum iron was 57 mcg/dL, total iron-binding capacity was 241 mcg/dL, and transferrin was 162 mcg/dL. Reticulocyte count was 6% (reticulocyte index of 0.86). Vitamin B12 level was normal. DAT was negative; INR and aPTT were normal. Fibrinogen was 287 mg/dL (reference range 200-400 mg/dL), and D-dimer was 5,095 ng/mL (reference range 0-229 ng/mL).
The urinalysis shows no active sediment to suggest vasculitis or glomerulonephritis. The kidney injury could be the result of renal toxicity from free hemoglobin or as part of TMA caused by microvascular thrombosis. The dilute urine makes prerenal azotemia less likely.
There is clearly acute intravascular hemolysis occurring as evidenced by hemoglobinuria, very high LDH, and undetectable serum haptoglobin. The hemolysis is acute because chronic intravascular hemolysis would lead to positive urine hemosiderin via deposition in the renal tubules. Autoimmune hemolytic anemia is much less likely, but not ruled out, by a negative DAT.
This syndrome can be further refined from acute anemia to acute anemia with likely nonimmune intravascular hemolysis, acute thrombocytopenia, and AKI with hemoglobinuria and a bland urinary sediment. At this point, intravascular hemolysis and kidney injury could be part of a unifying diagnosis. However, this does not account for the patient’s thrombocytopenia, and TMA remains the best explanation for the constellation of findings. Review of the peripheral blood smear is urgent because evidence of MAHA would prompt urgent plasma exchange based on presumptive diagnosis of acquired TTP to later be confirmed with ADAMTS13 activity testing. Most TMAs are treated with supportive care only; TTP and aHUS have specific interventions that change the natural history of the disease (plasma exchange and anticomplement therapy, respectively). Given both the deadly natural history and opportunity to intervene with plasma exchange, patients with TMA should be treated with urgent plasma exchange until ADAMTS13 deficiency is confirmed or refuted. One TMA that can be excluded at this point is DIC. DIC in its acute and chronic forms nearly universally causes MAHA, thrombocytopenia, and consumptive coagulopathy including hypofibrinogenemia.
If MAHA is excluded, then other causes of intravascular hemolysis should be considered, along with causes of thrombocytopenia that might be occurring concurrently. Intravascular hemolysis can be further differentiated by etiologies primarily related to the RBC or whether the RBC is the innocent bystander amidst a systemic illness. RBC disorders include syndromes affecting RBC fragility like hereditary spherocytosis or RBC enzymopathies (G6PD deficiency), but these do not cause thrombocytopenia. One exception is an acquired membrane defect, paroxysmal nocturnal hemoglobinuria (PNH), in which RBCs and other blood cells become susceptible to complement-mediated lysis. Testing for PNH by peripheral blood flow cytometry should be considered if the blood film lacks schistocytes. Systemic disorders that cause intravascular hemolysis include severe burns (heat damage to RBCs), RBC trauma from “march hemoglobinuria” or mechanical heart valves, immune (antibody-mediated) hemolysis from Rh immune globulin administration, cold agglutinin disease or ABO mismatched transfusion, and infections including the intraerythrocyte parasites malaria, Bartonellosis, and Babesiosis, as well as organisms that induce RBC fragility such as Leishmaniasis, Clostridium perfringens, and Haemophilus influenzae B.
On review of additional history, the patient had not recently received blood products. He had received heparin during prior hospitalizations, but had no prior history of thrombosis. He had no history of tick exposure. Peripheral blood smear was obtained and reviewed by a
The blood smear helps narrow the differential further. The lack of schistocytes makes TMA far less likely and so plasma exchange is not urgently indicated. The differential still includes drug-induced TMA (gemcitabine being a well-known cause for TMA) and cancer-associated TMA could still cause these findings, but plasma exchange does not improve outcomes. Acquired (immune) TTP is very unlikely unless the patient did not improve with supportive care or developed neurologic symptoms. Similarly, atypical (complement-driven) HUS would only be considered if renal failure did not improve with supportive care.
The blood smear does show a surprising finding of pyropoikilocytosis. Pyropoikilocytosis refers to changes in RBC shape (poikilocytosis) typically seen with thermal injury or rare RBC membrane structural defects. Hereditary pyropoikilocytosis, a very rare disease, is characterized by chronic hyperproliferative, compensated anemia, and occasional hemolytic crises. These crises are associated with splenomegaly, reticulocytosis, and elevated bilirubin with jaundice. As the patient has no history of similar episodes, the blood smear changes are not due to a hereditary cause and obviously not due to thermal injury (ie, severe burns). Pyropoikilocytosis has been rarely reported in drug-induced TMA and in severe bacterial bloodstream infections (most commonly Gram-negative bacilli). This patient has received gemcitabine (a known cause of drug-induced TMA) and has a recently diagnosed infection (C difficile colitis), either of which could be linked to this rare blood smear finding. Both of these syndromes would be treated with supportive care plus avoidance of future gemcitabine.
Transfusion of packed RBCs is indicated given his profound anemia and symptoms of fatigue. One should obtain further testing for cold agglutinins, PNH, and echocardiography to exclude endocarditis. If he were to become critically ill, anuric, or encephalopathic, then one could consider plasma exchange for treatment of TMA and hemoglobin-mediated AKI. Pyropoikilocytosis should be considered the result of drug-induced TMA, severe C difficile colitis, or an occult infection.
The patient was transfused packed RBCs. Because of a concern for an acute TMA such as TTP, both a hematopathologist and the consulting hematology/oncology team reviewed the peripheral blood morphology emergently. He was given aggressive fluid resuscitation and received 3 L of IV lactated ringers’ solution. An echocardiogram did not show valvular abnormalities. A renal biopsy was contraindicated because of the severe thrombocytopenia.
Given the recently confirmed C difficile colitis along with the findings of pyropoikilocytosis on the peripheral smear, toxin-mediated intravascular hemolysis from systemic C difficile infection became the leading diagnosis. Positing that the C difficile colitis was inadequately treated with oral metronidazole, aggressive treatment for C difficile was initiated with oral vancomycin in addition to intravenous metronidazole. Intravenous metronidazole was included given his elevated creatinine, presence of severe colitis on imaging, and concern he may be at risk for translocation of colonic C difficile or exotoxin into the bloodstream.
Over the course of the next 3 days, the patient’s platelet count normalized and his hemoglobin, creatinine, and symptoms of fatigue improved. Blood cultures remained negative. The patient’s rapid improvement with antibiotics supported our final diagnosis of toxin-mediated hemolysis caused by a systemic C difficile infection. On follow-up testing after hospital discharge, hemoglobin had returned to prior baseline and there was no recurrent hemolysis. Gemcitabine was considered to be a possible cause of his hemolytic anemia and was not continued in further treatment for his NSCLC.
COMMENTARY
When evaluating patients with cancer who present with fatigue, hospitalists should consider a broad list of potential causes. The differential should include etiologies directly related to the malignancy, paraneoplastic phenomena, treatment-related complications, and diseases unrelated to cancer. In addition, as the number of medications used for cancer proliferates, hospitalists must take a detailed history of the agents used and be aware of major side effects. Using this information, hospitalists may undertake a targeted approach to diagnostics while searching for a cause of fatigue.
When lab testing reveals profound anemia, hospitalists must consider syndromes that may require emergent management. Anemia can be caused by decreased RBC production, and acute anemia in the absence of clear blood loss suggests hemolysis. Moreover, the combination of elevated LDH and low haptoglobin is quite specific of hemolytic anemia.1,2 Once hemolytic anemia is identified, DIC and TMA syndromes (such as TTP) need to be considered. The combination of hemolytic anemia and AKI may indicate a medical emergency and should prompt hospitalists to obtain an urgent peripheral blood smear to help narrow the differential.3
The absence of schistocytes on a blood smear does not rule out TTP or HUS, but does argue strongly against these diagnoses.4,5 Of note, consultation with a hematopathologist and hematology subspecialist should be done to ensure appropriate and timely review of the peripheral blood smear.
In this case, the blood smear led to a very rare finding of pyropoikilocytosis. The unexpected result should prompt a broader review of the medical history particularly as it relates to the patient’s broader symptoms and laboratory abnormalities. Acquired pyropoikilocytosis is a very specific finding known to be associated only with hyperthermal injury (seen in burn patients), drug-induced TMA, and bacterial bloodstream infections, mainly Gram-negative toxins and Clostridioidal infections.6-8 In this case, both drug-induced TMA and C difficile infection were considered.
Gemcitabine-induced TMA can occur with either short or long term use of the medication and can be difficult to distinguish from TTP. While both TTP and gemcitabine-induced TMA can cause thrombocytopenia, hemolytic anemia, and schistocytes on a blood smear, the latter causes acute kidney injury more frequently than TTP. In addition, gemcitabine-induced TMA may not lead to severe decrease in ADAMTS13 activity. A kidney biopsy could confirm drug-induced TMA but was contraindicated in this case because of the thrombocytopenia. Gemcitabine should not be restarted if this side effect is suspected.
Given the continued rise in C difficile incidence, hospitalists should be aware that C difficile infection can cause extraintestinal illness.9,10 Although uncommon, these extraintestinal complications are associated with high risk of mortality and frequently occur in those with a history of intestinal injury or inflammation and a concomitant bloodstream infection.10 Regarding the possibility of C difficile contributing to hemolysis in this case, the patient’s low blood counts and hemolysis improved concomitantly with more aggressive treatment of C difficile infection. Although his blood cultures were sterile, C difficile is notoriously difficult to culture. Prior case reports have associated C difficile with intravascular hemolysis, which leads to the possibility that the patient did have a very rare manifestation of this unfortunately common infection.11
This case provides an excellent example of a diagnostic pivot point
KEY TEACHING POINTS
- In evaluating symptomatic cancer patients, providers must consider sequelae of the tumor, paraneoplastic phenomena, and treatment-related complications.
- Hemolytic anemia may represent a life-threatening emergency particularly when accompanied by AKI and requires urgent peripheral blood smear evaluation.
- Acquired pyropoikilocytosis is a specific finding known to be associated only with thermal injury, drug-induced TMA, and bacterial toxin–mediated hemolysis.
Disclosures
The authors have nothing to disclose.
1. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013:139(1):9-29. https://doi.org/10.1309/AJCP50AEEYGREWUZ.
2. Marchand A, Galen RS, Van Lente F. The predictive value of serum haptoglobin in hemolytic disease. JAMA.1980;243(19):1909-1911. https://doi:10.1001/jama.1980.03300450023014.
3. Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599-2606.
4. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. https://doi.org/10.1182/blood-2016-10-709857.
5. Jokiranta TS. HUS and atypical HUS. Blood. 2017;129(21):2847-2856. https://doi.org/10.1182/blood-2016-11-709865.
6. Baar S, Arrowsmith DJ. Thermal damage to red cells. J Clin Path. 1970;23(7):572-576. https://doi.org/10.1136/jcp.23.7.572.
7. Meinders AJ, Dijkstra I. Massive hemolysis and erythrophagocytosis in severe sepsis. Blood. 2014;124(6):841. https://doi.org/10.1182/blood-2014-04-565663.
8. McIlwaine K, Leach MT. Clostridium perfringens septicaemia. Br J Haematol. 2013;163(5):549. https://doi.org/10.1111/bjh.12551.
9. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60 (Supp 2):S66-71. https://doi.org/10.1093/cid/civ140.
10. Gupta A, Patel R, Baddour LM, Pardi DS, Khanna S. Extraintestinal Clostridium difficile infections: a single-center experience. Mayo Clin Proc. 2014;89(11):1525-36. https://doi.org/10.1016/j.mayocp.2014.07.012.
11. Alvarado AS, Brodsky SV, Nadasdy T, Singh N. Hemolytic uremic syndrome associated with Clostridium difficile infection. Clin Nephrol. 2014;81(4):302-6. https://doi.org/10.5414/CN107691.
A 62-year-old man with metastatic non–small cell lung cancer (NSCLC) presented to the Emergency Department with 3 days of progressive generalized weakness, anorexia, and nonbloody diarrhea. He denied fever, chills, nausea, vomiting, cough, shortness of breath, or abdominal pain. He had no sick contacts.
One diagnostic approach for patients with cancer who present with new symptoms is to consider diagnoses both related and unrelated to the cancer. Cancer-related diagnoses can include the broad categories of complications related to the tumor itself (such as mass effect), paraneoplastic phenomena, or treatment-related complications (such as infection from immunosuppression or chemotherapy toxicity).
For this patient with metastatic NSCLC, weakness, anorexia, and diarrhea are unlikely to be related to mass effect unless the patient has peritoneal metastases (an uncommon complication of NSCLC) with carcinomatosis-associated diarrhea.
Paraneoplastic phenomena, such as hypercalcemia or hyponatremia from the syndrome of inappropriate antidiuretic hormone (SIADH), are common with NSCLC and could both lead to weakness and anorexia. Hematologic consequences of NSCLC (or its treatment) include anemia, thrombosis, and thrombotic microangiopathy (TMA), though diarrhea, in the absence of abdominal pain or hematochezia, would be unexpected.
Weakness, anorexia, and diarrhea may also be symptoms of chemotherapy toxicity or an infection resulting from immunosuppression. It would be important to know what specific treatment the patient has received. Chemotherapy commonly causes neutropenia and predisposes to rapidly progressive infections, while immunotherapies have other toxicities. Diarrhea is a common toxicity of the checkpoint inhibitors and anaplastic lymphoma kinase (ALK) inhibitors that are frequently used to treat metastatic NSCLC. Checkpoint inhibitors also are known to cause a wide range of autoimmune phenomena including colitis.
Finally, the patient’s symptoms may be unrelated to the cancer. Weakness, anorexia, and nonbloody diarrhea could be signs of viral or bacterial gastroenteritis or Clostridioides difficile colitis particularly with frequent healthcare contact or antimicrobial use.
Two days prior, he had been diagnosed with nonsevere Clostridioides difficile colitis in an acute care clinic. He was started on oral metronidazole, but his diarrhea worsened over the next day and was accompanied by weakness and anorexia. Additional past medical history included untreated hepatitis C infection, chronic kidney disease stage 3, seizure disorder, and left lung NSCLC (adenocarcinoma). The lung cancer was diagnosed 8 months prior when he had presented with hemoptysis and 3 months of progressive constitutional symptoms. Imaging at that time revealed metastases to the contralateral lung and regional lymph nodes, as well as vertebrae, ribs, and pelvis. He had no abdominal metastases. He was initially treated with carboplatin and paclitaxel. After a partial response to initial chemotherapy, he developed peripheral neuropathy and was switched to gemcitabine 12 weeks ago. He received five cycles of gemcitabine over 10 weeks. He was last administered gemcitabine 2 weeks prior. He had not received any additional chemotherapy or immunotherapy. He had a 40 pack-year history of smoking, but quit when diagnosed with cancer. He did not drink alcohol. He had no recent travel or sick contacts. He was not on any medications. He was homeless but staying with family members in the area. Additional review of systems was negative for recent bleeding, bruising, hemoptysis, melena, hematochezia, or hematuria.
Recent treatment with gemcitabine could contribute to the presentation in a number of ways. First, gemcitabine is associated with myelosuppression and neutropenia that could predispose him to infectious colitis. Second, gemcitabine is known to cause anemia, anorexia, diarrhea, and fatigue. Third, gemcitabine may also cause renal injury that can contribute to worsening anemia. He may be at greater risk of anemia and renal toxicity because of preexisting chronic kidney disease. Finally, gemcitabine can rarely cause TMA with characteristics that mimic the hemolytic-uremic syndrome with microangiopathic hemolytic anemia, mild thrombocytopenia, and severe acute kidney injury (AKI).
In addition, worsening infectious colitis could certainly explain his presenting symptoms. At this point, local mass effect seems unlikely despite his metastatic disease. Lastly, it should be noted that, in an immunosuppressed cancer patient, multiple problems could be present at the same time. Laboratory testing should evaluate for hypercalcemia, SIADH, hematologic indexes, and renal function. If initial laboratory evaluation is unrevealing, abdominal imaging may be needed to assess for carcinomatosis, complications from colitis, typhlitis, abscess, or perforation.
On physical examination, the patient appeared fatigued. His temperature was 36.8°C, blood pressure 158/72 mm Hg, pulse 88 beats per minute, respiratory rate 16 breaths per minute, and oxygen saturation was 96% while breathing ambient air. There was neither scleral icterus nor conjunctival injection but he had mild conjunctival pallor. Cardiovascular and lung examinations were normal. Abdominal exam revealed normal bowel sounds without tenderness or organomegaly. He had no supraclavicular, axillary, or inguinal lymphadenopathy. He was alert and oriented. Cranial nerves II through XII were intact. He had decreased muscle bulk in his extremities without focal weakness. Gait and reflexes were not tested.
Initial laboratory testing revealed a white blood cell count of 5.5 K/mm3, hemoglobin of 5 g/dL (hemoglobin 1 month prior was 10.1 g/dL), and platelet count of 20 K/mm3 (platelet count 1 month prior was 246 K/mm3). Creatinine was 3.9 mg/dL (compared with a baseline of 1.8 mg/dL), and blood urea nitrogen was 39 mg/dL. His sodium was 137 mEq/L, potassium 4.2 mEq/L, chloride 105 mEq/L, bicarbonate 22 mEq/L, and thyroid stimulating hormone 0.9 mU/L. His total protein was 4.9 g/dL, albumin 2.1 g/dL, alkaline phosphatase 60 IU/L, alanine aminotransferase 17 IU/L, aspartate aminotransferase 60 IU/L, direct bilirubin 0.2 mg/dL, and total bilirubin 0.5 mg/dL. A chest x-ray showed no infiltrates.
The patient’s laboratory tests reveal several important new findings, including severe acute on chronic anemia, acute thrombocytopenia, and AKI, without clinical evidence of acute blood loss. These changes could be parts of a syndrome or multiple independent disorders. The most urgent priority is to evaluate for TMAs, many of which are fatal if not diagnosed and treated expeditiously. This includes thrombotic thrombocytopenic purpura (TTP), disseminated intravascular hemolysis (DIC), and atypical hemolytic uremic syndrome (aHUS). A manual review of a peripheral blood smear is required to evaluate for fragmented red blood cells (schistocytes). Thereafter, ancillary testing to confirm intravascular hemolysis would include measuring free plasma hemoglobin and lactate dehydrogenase (LDH). Additionally, in intravascular hemolysis, haptoglobin should be depleted and urinalysis should show heme-positive urine without RBCs. In this case the patient’s normal bilirubin studies argue against hemolysis; however, elevated bilirubin is variably present in hemolytic anemias depending on the liver’s ability to conjugate and excrete bilirubin, the relative degree of RBC turnover, and type of hemolysis. Patients with intravascular hemolysis lose hemoglobin directly into the urine leaving relatively little hemoglobin to be incorporated into bile once it has reached the reticuloendothelial system. This results in relatively normal bilirubin levels. More specific indicators of intravascular hemolysis include pink colored plasma on visual inspection (commonly done in the blood bank as part of assessing for hemolytic transfusion reactions), measuring plasma free hemoglobin, or by detecting hemoglobin in the urine.
If microangiopathic hemolytic anemia (MAHA) is excluded, then other causes of these laboratory abnormalities should be considered. Bleeding is the most common cause for anemia, and thrombocytopenia predisposes patients to bleeding. However, there is no evidence of bleeding in this patient, and such a rapid acute anemia is unlikely to be caused by occult blood loss alone. Concurrent anemia and thrombocytopenia could be evidence of bone marrow toxicity from chemotherapy or neoplastic infiltration. With marrow infiltration, there are typically signs on the peripheral smear of leukoerythroblastosis, with circulating nucleated red blood cells and early myeloid forms. Concurrent immune thrombocytopenia (ITP) and autoimmune hemolytic anemia (AIHA), or Evans’ Syndrome, should also be considered. AIHA would be suggested by spherocytes on the peripheral smear, elevated LDH and a positive direct antibody test (DAT).
Regarding the AKI, the patient has diarrhea, which could lead to prerenal azotemia and acute tubular necrosis. A formal urinalysis would evaluate for prerenal and intrinsic kidney disease. TMA can cause intrinsic kidney injury with a benign urinary sediment. The blood urea nitrogen-to-creatinine ratio is not elevated, but in a patient with malnutrition this may not indicate prerenal azotemia. In summary, to differentiate potential TMAs from other causes, the patient needs a blood smear, coagulation studies, and an evaluation for hemolysis, including a urinalysis for free heme and any evidence of intrinsic kidney disease.
Urinalysis showed amber-colored, dilute urine with no white blood cells, red blood cells, protein, or casts. It was positive for blood and negative for bilirubin and hemosiderin. LDH was 1,382 IU/L (reference range 135-225 IU/L), and haptoglobin was unmeasurably low. His ferritin was 2,267 ng/mL, serum iron was 57 mcg/dL, total iron-binding capacity was 241 mcg/dL, and transferrin was 162 mcg/dL. Reticulocyte count was 6% (reticulocyte index of 0.86). Vitamin B12 level was normal. DAT was negative; INR and aPTT were normal. Fibrinogen was 287 mg/dL (reference range 200-400 mg/dL), and D-dimer was 5,095 ng/mL (reference range 0-229 ng/mL).
The urinalysis shows no active sediment to suggest vasculitis or glomerulonephritis. The kidney injury could be the result of renal toxicity from free hemoglobin or as part of TMA caused by microvascular thrombosis. The dilute urine makes prerenal azotemia less likely.
There is clearly acute intravascular hemolysis occurring as evidenced by hemoglobinuria, very high LDH, and undetectable serum haptoglobin. The hemolysis is acute because chronic intravascular hemolysis would lead to positive urine hemosiderin via deposition in the renal tubules. Autoimmune hemolytic anemia is much less likely, but not ruled out, by a negative DAT.
This syndrome can be further refined from acute anemia to acute anemia with likely nonimmune intravascular hemolysis, acute thrombocytopenia, and AKI with hemoglobinuria and a bland urinary sediment. At this point, intravascular hemolysis and kidney injury could be part of a unifying diagnosis. However, this does not account for the patient’s thrombocytopenia, and TMA remains the best explanation for the constellation of findings. Review of the peripheral blood smear is urgent because evidence of MAHA would prompt urgent plasma exchange based on presumptive diagnosis of acquired TTP to later be confirmed with ADAMTS13 activity testing. Most TMAs are treated with supportive care only; TTP and aHUS have specific interventions that change the natural history of the disease (plasma exchange and anticomplement therapy, respectively). Given both the deadly natural history and opportunity to intervene with plasma exchange, patients with TMA should be treated with urgent plasma exchange until ADAMTS13 deficiency is confirmed or refuted. One TMA that can be excluded at this point is DIC. DIC in its acute and chronic forms nearly universally causes MAHA, thrombocytopenia, and consumptive coagulopathy including hypofibrinogenemia.
If MAHA is excluded, then other causes of intravascular hemolysis should be considered, along with causes of thrombocytopenia that might be occurring concurrently. Intravascular hemolysis can be further differentiated by etiologies primarily related to the RBC or whether the RBC is the innocent bystander amidst a systemic illness. RBC disorders include syndromes affecting RBC fragility like hereditary spherocytosis or RBC enzymopathies (G6PD deficiency), but these do not cause thrombocytopenia. One exception is an acquired membrane defect, paroxysmal nocturnal hemoglobinuria (PNH), in which RBCs and other blood cells become susceptible to complement-mediated lysis. Testing for PNH by peripheral blood flow cytometry should be considered if the blood film lacks schistocytes. Systemic disorders that cause intravascular hemolysis include severe burns (heat damage to RBCs), RBC trauma from “march hemoglobinuria” or mechanical heart valves, immune (antibody-mediated) hemolysis from Rh immune globulin administration, cold agglutinin disease or ABO mismatched transfusion, and infections including the intraerythrocyte parasites malaria, Bartonellosis, and Babesiosis, as well as organisms that induce RBC fragility such as Leishmaniasis, Clostridium perfringens, and Haemophilus influenzae B.
On review of additional history, the patient had not recently received blood products. He had received heparin during prior hospitalizations, but had no prior history of thrombosis. He had no history of tick exposure. Peripheral blood smear was obtained and reviewed by a
The blood smear helps narrow the differential further. The lack of schistocytes makes TMA far less likely and so plasma exchange is not urgently indicated. The differential still includes drug-induced TMA (gemcitabine being a well-known cause for TMA) and cancer-associated TMA could still cause these findings, but plasma exchange does not improve outcomes. Acquired (immune) TTP is very unlikely unless the patient did not improve with supportive care or developed neurologic symptoms. Similarly, atypical (complement-driven) HUS would only be considered if renal failure did not improve with supportive care.
The blood smear does show a surprising finding of pyropoikilocytosis. Pyropoikilocytosis refers to changes in RBC shape (poikilocytosis) typically seen with thermal injury or rare RBC membrane structural defects. Hereditary pyropoikilocytosis, a very rare disease, is characterized by chronic hyperproliferative, compensated anemia, and occasional hemolytic crises. These crises are associated with splenomegaly, reticulocytosis, and elevated bilirubin with jaundice. As the patient has no history of similar episodes, the blood smear changes are not due to a hereditary cause and obviously not due to thermal injury (ie, severe burns). Pyropoikilocytosis has been rarely reported in drug-induced TMA and in severe bacterial bloodstream infections (most commonly Gram-negative bacilli). This patient has received gemcitabine (a known cause of drug-induced TMA) and has a recently diagnosed infection (C difficile colitis), either of which could be linked to this rare blood smear finding. Both of these syndromes would be treated with supportive care plus avoidance of future gemcitabine.
Transfusion of packed RBCs is indicated given his profound anemia and symptoms of fatigue. One should obtain further testing for cold agglutinins, PNH, and echocardiography to exclude endocarditis. If he were to become critically ill, anuric, or encephalopathic, then one could consider plasma exchange for treatment of TMA and hemoglobin-mediated AKI. Pyropoikilocytosis should be considered the result of drug-induced TMA, severe C difficile colitis, or an occult infection.
The patient was transfused packed RBCs. Because of a concern for an acute TMA such as TTP, both a hematopathologist and the consulting hematology/oncology team reviewed the peripheral blood morphology emergently. He was given aggressive fluid resuscitation and received 3 L of IV lactated ringers’ solution. An echocardiogram did not show valvular abnormalities. A renal biopsy was contraindicated because of the severe thrombocytopenia.
Given the recently confirmed C difficile colitis along with the findings of pyropoikilocytosis on the peripheral smear, toxin-mediated intravascular hemolysis from systemic C difficile infection became the leading diagnosis. Positing that the C difficile colitis was inadequately treated with oral metronidazole, aggressive treatment for C difficile was initiated with oral vancomycin in addition to intravenous metronidazole. Intravenous metronidazole was included given his elevated creatinine, presence of severe colitis on imaging, and concern he may be at risk for translocation of colonic C difficile or exotoxin into the bloodstream.
Over the course of the next 3 days, the patient’s platelet count normalized and his hemoglobin, creatinine, and symptoms of fatigue improved. Blood cultures remained negative. The patient’s rapid improvement with antibiotics supported our final diagnosis of toxin-mediated hemolysis caused by a systemic C difficile infection. On follow-up testing after hospital discharge, hemoglobin had returned to prior baseline and there was no recurrent hemolysis. Gemcitabine was considered to be a possible cause of his hemolytic anemia and was not continued in further treatment for his NSCLC.
COMMENTARY
When evaluating patients with cancer who present with fatigue, hospitalists should consider a broad list of potential causes. The differential should include etiologies directly related to the malignancy, paraneoplastic phenomena, treatment-related complications, and diseases unrelated to cancer. In addition, as the number of medications used for cancer proliferates, hospitalists must take a detailed history of the agents used and be aware of major side effects. Using this information, hospitalists may undertake a targeted approach to diagnostics while searching for a cause of fatigue.
When lab testing reveals profound anemia, hospitalists must consider syndromes that may require emergent management. Anemia can be caused by decreased RBC production, and acute anemia in the absence of clear blood loss suggests hemolysis. Moreover, the combination of elevated LDH and low haptoglobin is quite specific of hemolytic anemia.1,2 Once hemolytic anemia is identified, DIC and TMA syndromes (such as TTP) need to be considered. The combination of hemolytic anemia and AKI may indicate a medical emergency and should prompt hospitalists to obtain an urgent peripheral blood smear to help narrow the differential.3
The absence of schistocytes on a blood smear does not rule out TTP or HUS, but does argue strongly against these diagnoses.4,5 Of note, consultation with a hematopathologist and hematology subspecialist should be done to ensure appropriate and timely review of the peripheral blood smear.
In this case, the blood smear led to a very rare finding of pyropoikilocytosis. The unexpected result should prompt a broader review of the medical history particularly as it relates to the patient’s broader symptoms and laboratory abnormalities. Acquired pyropoikilocytosis is a very specific finding known to be associated only with hyperthermal injury (seen in burn patients), drug-induced TMA, and bacterial bloodstream infections, mainly Gram-negative toxins and Clostridioidal infections.6-8 In this case, both drug-induced TMA and C difficile infection were considered.
Gemcitabine-induced TMA can occur with either short or long term use of the medication and can be difficult to distinguish from TTP. While both TTP and gemcitabine-induced TMA can cause thrombocytopenia, hemolytic anemia, and schistocytes on a blood smear, the latter causes acute kidney injury more frequently than TTP. In addition, gemcitabine-induced TMA may not lead to severe decrease in ADAMTS13 activity. A kidney biopsy could confirm drug-induced TMA but was contraindicated in this case because of the thrombocytopenia. Gemcitabine should not be restarted if this side effect is suspected.
Given the continued rise in C difficile incidence, hospitalists should be aware that C difficile infection can cause extraintestinal illness.9,10 Although uncommon, these extraintestinal complications are associated with high risk of mortality and frequently occur in those with a history of intestinal injury or inflammation and a concomitant bloodstream infection.10 Regarding the possibility of C difficile contributing to hemolysis in this case, the patient’s low blood counts and hemolysis improved concomitantly with more aggressive treatment of C difficile infection. Although his blood cultures were sterile, C difficile is notoriously difficult to culture. Prior case reports have associated C difficile with intravascular hemolysis, which leads to the possibility that the patient did have a very rare manifestation of this unfortunately common infection.11
This case provides an excellent example of a diagnostic pivot point
KEY TEACHING POINTS
- In evaluating symptomatic cancer patients, providers must consider sequelae of the tumor, paraneoplastic phenomena, and treatment-related complications.
- Hemolytic anemia may represent a life-threatening emergency particularly when accompanied by AKI and requires urgent peripheral blood smear evaluation.
- Acquired pyropoikilocytosis is a specific finding known to be associated only with thermal injury, drug-induced TMA, and bacterial toxin–mediated hemolysis.
Disclosures
The authors have nothing to disclose.
A 62-year-old man with metastatic non–small cell lung cancer (NSCLC) presented to the Emergency Department with 3 days of progressive generalized weakness, anorexia, and nonbloody diarrhea. He denied fever, chills, nausea, vomiting, cough, shortness of breath, or abdominal pain. He had no sick contacts.
One diagnostic approach for patients with cancer who present with new symptoms is to consider diagnoses both related and unrelated to the cancer. Cancer-related diagnoses can include the broad categories of complications related to the tumor itself (such as mass effect), paraneoplastic phenomena, or treatment-related complications (such as infection from immunosuppression or chemotherapy toxicity).
For this patient with metastatic NSCLC, weakness, anorexia, and diarrhea are unlikely to be related to mass effect unless the patient has peritoneal metastases (an uncommon complication of NSCLC) with carcinomatosis-associated diarrhea.
Paraneoplastic phenomena, such as hypercalcemia or hyponatremia from the syndrome of inappropriate antidiuretic hormone (SIADH), are common with NSCLC and could both lead to weakness and anorexia. Hematologic consequences of NSCLC (or its treatment) include anemia, thrombosis, and thrombotic microangiopathy (TMA), though diarrhea, in the absence of abdominal pain or hematochezia, would be unexpected.
Weakness, anorexia, and diarrhea may also be symptoms of chemotherapy toxicity or an infection resulting from immunosuppression. It would be important to know what specific treatment the patient has received. Chemotherapy commonly causes neutropenia and predisposes to rapidly progressive infections, while immunotherapies have other toxicities. Diarrhea is a common toxicity of the checkpoint inhibitors and anaplastic lymphoma kinase (ALK) inhibitors that are frequently used to treat metastatic NSCLC. Checkpoint inhibitors also are known to cause a wide range of autoimmune phenomena including colitis.
Finally, the patient’s symptoms may be unrelated to the cancer. Weakness, anorexia, and nonbloody diarrhea could be signs of viral or bacterial gastroenteritis or Clostridioides difficile colitis particularly with frequent healthcare contact or antimicrobial use.
Two days prior, he had been diagnosed with nonsevere Clostridioides difficile colitis in an acute care clinic. He was started on oral metronidazole, but his diarrhea worsened over the next day and was accompanied by weakness and anorexia. Additional past medical history included untreated hepatitis C infection, chronic kidney disease stage 3, seizure disorder, and left lung NSCLC (adenocarcinoma). The lung cancer was diagnosed 8 months prior when he had presented with hemoptysis and 3 months of progressive constitutional symptoms. Imaging at that time revealed metastases to the contralateral lung and regional lymph nodes, as well as vertebrae, ribs, and pelvis. He had no abdominal metastases. He was initially treated with carboplatin and paclitaxel. After a partial response to initial chemotherapy, he developed peripheral neuropathy and was switched to gemcitabine 12 weeks ago. He received five cycles of gemcitabine over 10 weeks. He was last administered gemcitabine 2 weeks prior. He had not received any additional chemotherapy or immunotherapy. He had a 40 pack-year history of smoking, but quit when diagnosed with cancer. He did not drink alcohol. He had no recent travel or sick contacts. He was not on any medications. He was homeless but staying with family members in the area. Additional review of systems was negative for recent bleeding, bruising, hemoptysis, melena, hematochezia, or hematuria.
Recent treatment with gemcitabine could contribute to the presentation in a number of ways. First, gemcitabine is associated with myelosuppression and neutropenia that could predispose him to infectious colitis. Second, gemcitabine is known to cause anemia, anorexia, diarrhea, and fatigue. Third, gemcitabine may also cause renal injury that can contribute to worsening anemia. He may be at greater risk of anemia and renal toxicity because of preexisting chronic kidney disease. Finally, gemcitabine can rarely cause TMA with characteristics that mimic the hemolytic-uremic syndrome with microangiopathic hemolytic anemia, mild thrombocytopenia, and severe acute kidney injury (AKI).
In addition, worsening infectious colitis could certainly explain his presenting symptoms. At this point, local mass effect seems unlikely despite his metastatic disease. Lastly, it should be noted that, in an immunosuppressed cancer patient, multiple problems could be present at the same time. Laboratory testing should evaluate for hypercalcemia, SIADH, hematologic indexes, and renal function. If initial laboratory evaluation is unrevealing, abdominal imaging may be needed to assess for carcinomatosis, complications from colitis, typhlitis, abscess, or perforation.
On physical examination, the patient appeared fatigued. His temperature was 36.8°C, blood pressure 158/72 mm Hg, pulse 88 beats per minute, respiratory rate 16 breaths per minute, and oxygen saturation was 96% while breathing ambient air. There was neither scleral icterus nor conjunctival injection but he had mild conjunctival pallor. Cardiovascular and lung examinations were normal. Abdominal exam revealed normal bowel sounds without tenderness or organomegaly. He had no supraclavicular, axillary, or inguinal lymphadenopathy. He was alert and oriented. Cranial nerves II through XII were intact. He had decreased muscle bulk in his extremities without focal weakness. Gait and reflexes were not tested.
Initial laboratory testing revealed a white blood cell count of 5.5 K/mm3, hemoglobin of 5 g/dL (hemoglobin 1 month prior was 10.1 g/dL), and platelet count of 20 K/mm3 (platelet count 1 month prior was 246 K/mm3). Creatinine was 3.9 mg/dL (compared with a baseline of 1.8 mg/dL), and blood urea nitrogen was 39 mg/dL. His sodium was 137 mEq/L, potassium 4.2 mEq/L, chloride 105 mEq/L, bicarbonate 22 mEq/L, and thyroid stimulating hormone 0.9 mU/L. His total protein was 4.9 g/dL, albumin 2.1 g/dL, alkaline phosphatase 60 IU/L, alanine aminotransferase 17 IU/L, aspartate aminotransferase 60 IU/L, direct bilirubin 0.2 mg/dL, and total bilirubin 0.5 mg/dL. A chest x-ray showed no infiltrates.
The patient’s laboratory tests reveal several important new findings, including severe acute on chronic anemia, acute thrombocytopenia, and AKI, without clinical evidence of acute blood loss. These changes could be parts of a syndrome or multiple independent disorders. The most urgent priority is to evaluate for TMAs, many of which are fatal if not diagnosed and treated expeditiously. This includes thrombotic thrombocytopenic purpura (TTP), disseminated intravascular hemolysis (DIC), and atypical hemolytic uremic syndrome (aHUS). A manual review of a peripheral blood smear is required to evaluate for fragmented red blood cells (schistocytes). Thereafter, ancillary testing to confirm intravascular hemolysis would include measuring free plasma hemoglobin and lactate dehydrogenase (LDH). Additionally, in intravascular hemolysis, haptoglobin should be depleted and urinalysis should show heme-positive urine without RBCs. In this case the patient’s normal bilirubin studies argue against hemolysis; however, elevated bilirubin is variably present in hemolytic anemias depending on the liver’s ability to conjugate and excrete bilirubin, the relative degree of RBC turnover, and type of hemolysis. Patients with intravascular hemolysis lose hemoglobin directly into the urine leaving relatively little hemoglobin to be incorporated into bile once it has reached the reticuloendothelial system. This results in relatively normal bilirubin levels. More specific indicators of intravascular hemolysis include pink colored plasma on visual inspection (commonly done in the blood bank as part of assessing for hemolytic transfusion reactions), measuring plasma free hemoglobin, or by detecting hemoglobin in the urine.
If microangiopathic hemolytic anemia (MAHA) is excluded, then other causes of these laboratory abnormalities should be considered. Bleeding is the most common cause for anemia, and thrombocytopenia predisposes patients to bleeding. However, there is no evidence of bleeding in this patient, and such a rapid acute anemia is unlikely to be caused by occult blood loss alone. Concurrent anemia and thrombocytopenia could be evidence of bone marrow toxicity from chemotherapy or neoplastic infiltration. With marrow infiltration, there are typically signs on the peripheral smear of leukoerythroblastosis, with circulating nucleated red blood cells and early myeloid forms. Concurrent immune thrombocytopenia (ITP) and autoimmune hemolytic anemia (AIHA), or Evans’ Syndrome, should also be considered. AIHA would be suggested by spherocytes on the peripheral smear, elevated LDH and a positive direct antibody test (DAT).
Regarding the AKI, the patient has diarrhea, which could lead to prerenal azotemia and acute tubular necrosis. A formal urinalysis would evaluate for prerenal and intrinsic kidney disease. TMA can cause intrinsic kidney injury with a benign urinary sediment. The blood urea nitrogen-to-creatinine ratio is not elevated, but in a patient with malnutrition this may not indicate prerenal azotemia. In summary, to differentiate potential TMAs from other causes, the patient needs a blood smear, coagulation studies, and an evaluation for hemolysis, including a urinalysis for free heme and any evidence of intrinsic kidney disease.
Urinalysis showed amber-colored, dilute urine with no white blood cells, red blood cells, protein, or casts. It was positive for blood and negative for bilirubin and hemosiderin. LDH was 1,382 IU/L (reference range 135-225 IU/L), and haptoglobin was unmeasurably low. His ferritin was 2,267 ng/mL, serum iron was 57 mcg/dL, total iron-binding capacity was 241 mcg/dL, and transferrin was 162 mcg/dL. Reticulocyte count was 6% (reticulocyte index of 0.86). Vitamin B12 level was normal. DAT was negative; INR and aPTT were normal. Fibrinogen was 287 mg/dL (reference range 200-400 mg/dL), and D-dimer was 5,095 ng/mL (reference range 0-229 ng/mL).
The urinalysis shows no active sediment to suggest vasculitis or glomerulonephritis. The kidney injury could be the result of renal toxicity from free hemoglobin or as part of TMA caused by microvascular thrombosis. The dilute urine makes prerenal azotemia less likely.
There is clearly acute intravascular hemolysis occurring as evidenced by hemoglobinuria, very high LDH, and undetectable serum haptoglobin. The hemolysis is acute because chronic intravascular hemolysis would lead to positive urine hemosiderin via deposition in the renal tubules. Autoimmune hemolytic anemia is much less likely, but not ruled out, by a negative DAT.
This syndrome can be further refined from acute anemia to acute anemia with likely nonimmune intravascular hemolysis, acute thrombocytopenia, and AKI with hemoglobinuria and a bland urinary sediment. At this point, intravascular hemolysis and kidney injury could be part of a unifying diagnosis. However, this does not account for the patient’s thrombocytopenia, and TMA remains the best explanation for the constellation of findings. Review of the peripheral blood smear is urgent because evidence of MAHA would prompt urgent plasma exchange based on presumptive diagnosis of acquired TTP to later be confirmed with ADAMTS13 activity testing. Most TMAs are treated with supportive care only; TTP and aHUS have specific interventions that change the natural history of the disease (plasma exchange and anticomplement therapy, respectively). Given both the deadly natural history and opportunity to intervene with plasma exchange, patients with TMA should be treated with urgent plasma exchange until ADAMTS13 deficiency is confirmed or refuted. One TMA that can be excluded at this point is DIC. DIC in its acute and chronic forms nearly universally causes MAHA, thrombocytopenia, and consumptive coagulopathy including hypofibrinogenemia.
If MAHA is excluded, then other causes of intravascular hemolysis should be considered, along with causes of thrombocytopenia that might be occurring concurrently. Intravascular hemolysis can be further differentiated by etiologies primarily related to the RBC or whether the RBC is the innocent bystander amidst a systemic illness. RBC disorders include syndromes affecting RBC fragility like hereditary spherocytosis or RBC enzymopathies (G6PD deficiency), but these do not cause thrombocytopenia. One exception is an acquired membrane defect, paroxysmal nocturnal hemoglobinuria (PNH), in which RBCs and other blood cells become susceptible to complement-mediated lysis. Testing for PNH by peripheral blood flow cytometry should be considered if the blood film lacks schistocytes. Systemic disorders that cause intravascular hemolysis include severe burns (heat damage to RBCs), RBC trauma from “march hemoglobinuria” or mechanical heart valves, immune (antibody-mediated) hemolysis from Rh immune globulin administration, cold agglutinin disease or ABO mismatched transfusion, and infections including the intraerythrocyte parasites malaria, Bartonellosis, and Babesiosis, as well as organisms that induce RBC fragility such as Leishmaniasis, Clostridium perfringens, and Haemophilus influenzae B.
On review of additional history, the patient had not recently received blood products. He had received heparin during prior hospitalizations, but had no prior history of thrombosis. He had no history of tick exposure. Peripheral blood smear was obtained and reviewed by a
The blood smear helps narrow the differential further. The lack of schistocytes makes TMA far less likely and so plasma exchange is not urgently indicated. The differential still includes drug-induced TMA (gemcitabine being a well-known cause for TMA) and cancer-associated TMA could still cause these findings, but plasma exchange does not improve outcomes. Acquired (immune) TTP is very unlikely unless the patient did not improve with supportive care or developed neurologic symptoms. Similarly, atypical (complement-driven) HUS would only be considered if renal failure did not improve with supportive care.
The blood smear does show a surprising finding of pyropoikilocytosis. Pyropoikilocytosis refers to changes in RBC shape (poikilocytosis) typically seen with thermal injury or rare RBC membrane structural defects. Hereditary pyropoikilocytosis, a very rare disease, is characterized by chronic hyperproliferative, compensated anemia, and occasional hemolytic crises. These crises are associated with splenomegaly, reticulocytosis, and elevated bilirubin with jaundice. As the patient has no history of similar episodes, the blood smear changes are not due to a hereditary cause and obviously not due to thermal injury (ie, severe burns). Pyropoikilocytosis has been rarely reported in drug-induced TMA and in severe bacterial bloodstream infections (most commonly Gram-negative bacilli). This patient has received gemcitabine (a known cause of drug-induced TMA) and has a recently diagnosed infection (C difficile colitis), either of which could be linked to this rare blood smear finding. Both of these syndromes would be treated with supportive care plus avoidance of future gemcitabine.
Transfusion of packed RBCs is indicated given his profound anemia and symptoms of fatigue. One should obtain further testing for cold agglutinins, PNH, and echocardiography to exclude endocarditis. If he were to become critically ill, anuric, or encephalopathic, then one could consider plasma exchange for treatment of TMA and hemoglobin-mediated AKI. Pyropoikilocytosis should be considered the result of drug-induced TMA, severe C difficile colitis, or an occult infection.
The patient was transfused packed RBCs. Because of a concern for an acute TMA such as TTP, both a hematopathologist and the consulting hematology/oncology team reviewed the peripheral blood morphology emergently. He was given aggressive fluid resuscitation and received 3 L of IV lactated ringers’ solution. An echocardiogram did not show valvular abnormalities. A renal biopsy was contraindicated because of the severe thrombocytopenia.
Given the recently confirmed C difficile colitis along with the findings of pyropoikilocytosis on the peripheral smear, toxin-mediated intravascular hemolysis from systemic C difficile infection became the leading diagnosis. Positing that the C difficile colitis was inadequately treated with oral metronidazole, aggressive treatment for C difficile was initiated with oral vancomycin in addition to intravenous metronidazole. Intravenous metronidazole was included given his elevated creatinine, presence of severe colitis on imaging, and concern he may be at risk for translocation of colonic C difficile or exotoxin into the bloodstream.
Over the course of the next 3 days, the patient’s platelet count normalized and his hemoglobin, creatinine, and symptoms of fatigue improved. Blood cultures remained negative. The patient’s rapid improvement with antibiotics supported our final diagnosis of toxin-mediated hemolysis caused by a systemic C difficile infection. On follow-up testing after hospital discharge, hemoglobin had returned to prior baseline and there was no recurrent hemolysis. Gemcitabine was considered to be a possible cause of his hemolytic anemia and was not continued in further treatment for his NSCLC.
COMMENTARY
When evaluating patients with cancer who present with fatigue, hospitalists should consider a broad list of potential causes. The differential should include etiologies directly related to the malignancy, paraneoplastic phenomena, treatment-related complications, and diseases unrelated to cancer. In addition, as the number of medications used for cancer proliferates, hospitalists must take a detailed history of the agents used and be aware of major side effects. Using this information, hospitalists may undertake a targeted approach to diagnostics while searching for a cause of fatigue.
When lab testing reveals profound anemia, hospitalists must consider syndromes that may require emergent management. Anemia can be caused by decreased RBC production, and acute anemia in the absence of clear blood loss suggests hemolysis. Moreover, the combination of elevated LDH and low haptoglobin is quite specific of hemolytic anemia.1,2 Once hemolytic anemia is identified, DIC and TMA syndromes (such as TTP) need to be considered. The combination of hemolytic anemia and AKI may indicate a medical emergency and should prompt hospitalists to obtain an urgent peripheral blood smear to help narrow the differential.3
The absence of schistocytes on a blood smear does not rule out TTP or HUS, but does argue strongly against these diagnoses.4,5 Of note, consultation with a hematopathologist and hematology subspecialist should be done to ensure appropriate and timely review of the peripheral blood smear.
In this case, the blood smear led to a very rare finding of pyropoikilocytosis. The unexpected result should prompt a broader review of the medical history particularly as it relates to the patient’s broader symptoms and laboratory abnormalities. Acquired pyropoikilocytosis is a very specific finding known to be associated only with hyperthermal injury (seen in burn patients), drug-induced TMA, and bacterial bloodstream infections, mainly Gram-negative toxins and Clostridioidal infections.6-8 In this case, both drug-induced TMA and C difficile infection were considered.
Gemcitabine-induced TMA can occur with either short or long term use of the medication and can be difficult to distinguish from TTP. While both TTP and gemcitabine-induced TMA can cause thrombocytopenia, hemolytic anemia, and schistocytes on a blood smear, the latter causes acute kidney injury more frequently than TTP. In addition, gemcitabine-induced TMA may not lead to severe decrease in ADAMTS13 activity. A kidney biopsy could confirm drug-induced TMA but was contraindicated in this case because of the thrombocytopenia. Gemcitabine should not be restarted if this side effect is suspected.
Given the continued rise in C difficile incidence, hospitalists should be aware that C difficile infection can cause extraintestinal illness.9,10 Although uncommon, these extraintestinal complications are associated with high risk of mortality and frequently occur in those with a history of intestinal injury or inflammation and a concomitant bloodstream infection.10 Regarding the possibility of C difficile contributing to hemolysis in this case, the patient’s low blood counts and hemolysis improved concomitantly with more aggressive treatment of C difficile infection. Although his blood cultures were sterile, C difficile is notoriously difficult to culture. Prior case reports have associated C difficile with intravascular hemolysis, which leads to the possibility that the patient did have a very rare manifestation of this unfortunately common infection.11
This case provides an excellent example of a diagnostic pivot point
KEY TEACHING POINTS
- In evaluating symptomatic cancer patients, providers must consider sequelae of the tumor, paraneoplastic phenomena, and treatment-related complications.
- Hemolytic anemia may represent a life-threatening emergency particularly when accompanied by AKI and requires urgent peripheral blood smear evaluation.
- Acquired pyropoikilocytosis is a specific finding known to be associated only with thermal injury, drug-induced TMA, and bacterial toxin–mediated hemolysis.
Disclosures
The authors have nothing to disclose.
1. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013:139(1):9-29. https://doi.org/10.1309/AJCP50AEEYGREWUZ.
2. Marchand A, Galen RS, Van Lente F. The predictive value of serum haptoglobin in hemolytic disease. JAMA.1980;243(19):1909-1911. https://doi:10.1001/jama.1980.03300450023014.
3. Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599-2606.
4. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. https://doi.org/10.1182/blood-2016-10-709857.
5. Jokiranta TS. HUS and atypical HUS. Blood. 2017;129(21):2847-2856. https://doi.org/10.1182/blood-2016-11-709865.
6. Baar S, Arrowsmith DJ. Thermal damage to red cells. J Clin Path. 1970;23(7):572-576. https://doi.org/10.1136/jcp.23.7.572.
7. Meinders AJ, Dijkstra I. Massive hemolysis and erythrophagocytosis in severe sepsis. Blood. 2014;124(6):841. https://doi.org/10.1182/blood-2014-04-565663.
8. McIlwaine K, Leach MT. Clostridium perfringens septicaemia. Br J Haematol. 2013;163(5):549. https://doi.org/10.1111/bjh.12551.
9. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60 (Supp 2):S66-71. https://doi.org/10.1093/cid/civ140.
10. Gupta A, Patel R, Baddour LM, Pardi DS, Khanna S. Extraintestinal Clostridium difficile infections: a single-center experience. Mayo Clin Proc. 2014;89(11):1525-36. https://doi.org/10.1016/j.mayocp.2014.07.012.
11. Alvarado AS, Brodsky SV, Nadasdy T, Singh N. Hemolytic uremic syndrome associated with Clostridium difficile infection. Clin Nephrol. 2014;81(4):302-6. https://doi.org/10.5414/CN107691.
1. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013:139(1):9-29. https://doi.org/10.1309/AJCP50AEEYGREWUZ.
2. Marchand A, Galen RS, Van Lente F. The predictive value of serum haptoglobin in hemolytic disease. JAMA.1980;243(19):1909-1911. https://doi:10.1001/jama.1980.03300450023014.
3. Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599-2606.
4. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. https://doi.org/10.1182/blood-2016-10-709857.
5. Jokiranta TS. HUS and atypical HUS. Blood. 2017;129(21):2847-2856. https://doi.org/10.1182/blood-2016-11-709865.
6. Baar S, Arrowsmith DJ. Thermal damage to red cells. J Clin Path. 1970;23(7):572-576. https://doi.org/10.1136/jcp.23.7.572.
7. Meinders AJ, Dijkstra I. Massive hemolysis and erythrophagocytosis in severe sepsis. Blood. 2014;124(6):841. https://doi.org/10.1182/blood-2014-04-565663.
8. McIlwaine K, Leach MT. Clostridium perfringens septicaemia. Br J Haematol. 2013;163(5):549. https://doi.org/10.1111/bjh.12551.
9. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60 (Supp 2):S66-71. https://doi.org/10.1093/cid/civ140.
10. Gupta A, Patel R, Baddour LM, Pardi DS, Khanna S. Extraintestinal Clostridium difficile infections: a single-center experience. Mayo Clin Proc. 2014;89(11):1525-36. https://doi.org/10.1016/j.mayocp.2014.07.012.
11. Alvarado AS, Brodsky SV, Nadasdy T, Singh N. Hemolytic uremic syndrome associated with Clostridium difficile infection. Clin Nephrol. 2014;81(4):302-6. https://doi.org/10.5414/CN107691.
© 2020 Society of Hospital Medicine
Developing Trust With Early Medical School Graduates During the COVID-19 Pandemic
The coronavirus disease of 2019 (COVID-19) pandemic has strained the healthcare system by rapidly depleting multiple resources including hospital space, medications, ventilators, personal protective equipment (PPE), clinical revenue, and morale. One of the most essential at-risk resources is healthcare providers. Healthcare providers have been overwhelmed as hospital systems have experienced local surges in COVID-19 patients. Compounding this is the fact that providers are more likely to contract COVID-19, which could sideline portions of an already taxed workforce.
Multiple “surge” interventions have been planned or implemented to mitigate a current or anticipated dearth of physicians. Some institutions are reallocating subspecialists and surgeons to general ward and intensive care unit (ICU) roles, often with support from hospitalists and ICU physicians.1 Others have used telemedicine to reduce personnel exposure and conserve PPE.2 A novel and perhaps paradigm-shifting solution arose in March 2020 when several medical schools around the world announced they would graduate final year students early to allow them to join the workforce during the COVID-19 surge.3-7 In the United States, fourth-year medical students at multiple institutions in cities such as New York, Boston, Phoenix, Tucson, Newark, Portland, and Bethesda were offered the opportunity to graduate in April rather than in May or June. The Liaison Committee on Medical Education stated that for students to graduate early, they must have already met all curricular requirements and be deemed ready by an evaluations and promotions committee.8 What these early graduates do with their “gap time” before residency is neither standardized nor prescribed. The Accreditation Council for Graduate Medical Education has discouraged individuals from joining their newly matched residency programs early.9 Some early graduates who wish to bolster the workforce have signed temporary training agreements with local healthcare systems to work for a 1- to 2-month period before moving on to their matched residency program. Some institutions have already been working with local and state officials to rapidly grant provisional temporary licenses for this purpose.10
Early medical school graduation in times of international crisis is not without precedent. When faced with physician shortages during World War II, the United States federal government urged medical colleges to graduate trainees in 3 years.11 The national medical education milieu was different then, with standardized medical school training still crystalizing merely 30 years following the Flexner report. However, there was pressure from the federal government during World War II, whereas decisions around early graduation today are driven by institutional and local officials. While a few accelerated programs persist today, there has not been an urgent, unplanned early release of graduates to meet a public health need on such a large scale in recent history. The seasonal timing of the pandemic surge in the United States may have been a key factor in deciding to graduate students early. With a late winter and early spring peak, final year students are graduating only 2 to 3 months early. But what if another peak occurs in late summer or early fall, and some students are graduated even earlier? With which aspects of patient care would hospitalists trust these graduates, and with what level of supervision? Whether now or with a future COVID-19 peak, we describe how trust develops with learners and provide hospitalists with a framework for deliberate entrustment if and when they are asked to integrate early medical school graduates into their workforce.
PROGRESSION OF TRUST WITH LEARNERS
The degree of supervision that is provided to a learner is linked to how much a supervisor trusts the learner, as well as the specific context. Trust has many forms, often depending on what type of information informs it. Presumptive trust is trust based on credentials, without any actual interaction with the learner.12 Healthcare systems typically assume that medical school graduates are ready to perform intern-level tasks based on their medical degree. This presumptive trust may be bolstered by the assumption that a residency program director has vetted a learner’s credentials during the match process. On meeting a learner, we develop initial trust, which is based on first impressions and snap-judgment. Over time, presumptive and initial trust can be replaced by grounded trust, or trust based on demonstrated performance after prolonged experience with a learner. Under normal circumstances, supervisors use observations of learner performance in the clinical environment to develop grounded trust. With early graduates, especially those who sign temporary work agreements, the usual progression of trust may be compressed. Hospitalists may have less presumptive trust because these students graduated early and little time to develop grounded trust before integrating new graduates into patient care. How should hospitalists navigate supervision in this setting?
PRESUMPTIVE TRUST FOR CURRENT EARLY GRADUATES
Missing a few months at the end of medical school likely does not significantly affect competence and, therefore, should not affect presumptive trust. The value of the fourth year of medical school has been questioned because, after fulfilling graduation requirements, students often spend significant amounts of time interviewing, traveling, taking electives with lighter workloads, or exploring nonclinical interests late in the year.13 More intense “subintern” rotations, which are important for the residency application process, occur earlier in the academic year. It is therefore reasonable to presume that most students graduating in April are not less prepared than those graduating in June.
Additionally, there is significant interlearner variability in rates of competence attainment.14 This means that there is no magic point in time at which students are fully ready for resident-level responsibilities. Some students are likely competent to be interns without a fourth year at all, while others are still facing challenges in their development at the end of medical school. As Englander and Carraccio wrote, “The notion that every medical student across the nation has somehow achieved all the competencies necessary to start residency training on July 1 of their graduation year is magical thinking.”15 Since there is no universal, time-based finish line for competence, we should not be thrown by a slight change in the arbitrary line currently drawn in June. Whether students graduate in April or June, it remains true that some will be more ready than others.
INITIAL TRUST—HIGH RISK FOR BIAS
With compressed timelines, hospitalists may default to initial trust, relying heavily on first impressions to determine how much supervision an early graduate requires. For example, a graduate who is extroverted, assertive, and articulate may give off an air of confidence, which could entice a supervising hospitalist to give a “longer leash” with higher-risk patient care tasks. It is easy to fall prey to the “confidence equals competence” heuristic, but this has been shown to be unreliable.16 Initial trust is influenced by both social biases (eg, gender, race, age) and cognitive biases (eg, halo effect) that have little or nothing to do with the actual abilities of learners. While initial trust and accompanying biases often develop unconsciously, it is important to reflect on how unfounded first impressions can influence trust and supervision decisions.
GROUNDED TRUST BUILT THROUGH DIRECT OBSERVATION
Hospitalists must be deliberate with entrustment decisions, especially in a pandemic environment. There are useful guides for making these decisions that can be used in a point-of-care manner.17 First, it is important to acknowledge that entrustment is based in part on the perceived trustworthiness of a person. Kennedy and colleagues have described four components of trustworthiness, all of which can be assessed by hospitalists in the moment of care delivery: (1) knowledge and skill (Does the trainee possess the requisite knowledge and skill to perform the task?), (2) conscientiousness (Does the trainee follow through on tasks? Are they thorough and dependable?), (3) discernment (Does the trainee recognize personal limitations and seek help when needed?), and (4) truthfulness (Does the trainee tell the truth?).17
Entrustment decisions also depend on the specific task being observed (eg, high risk vs low risk) and context (eg, severity of illness of the patient, acuity of the setting).18 Trust is linked with perceived risk and benefits.19 More entrustment (less supervision) may be given when perceived risk is low, such as prescribing acetaminophen on a stable patient or taking an initial history. Less entrustment (more supervision) may be given when perceived risk is high, such as with managing septic shock or inserting a central venous catheter. However, the duress of the COVID-19 pandemic may tilt the risk/benefit balance toward less-than-usual supervision if an early graduate is the only provider available for some higher-risk tasks. This underscores the importance of direct observation leading to grounded trust with progressively higher-risk tasks as dictated by the local pandemic environment.
As much as possible, trust should be determined based on direct observation, not fallible first impressions or inference. Supervisors often use inference when assuming that performance on one task reflects performance on others. For example, if learners are observed to be competent when interpreting electrocardiograms, one might infer they also know how to manage tachyarrhythmias. If they can manage tachyarrhythmias, one might infer they also know how to manage acute coronary syndrome. These inferences are not the way to build grounded trust because competence is task and context dependent.
Direct observation can include watching patient interactions, being present for procedures, think-alouds during didactics, cognitive autopsies, reviewing notes, and informal conversations. Being deliberate with direct observation and entrustment decision-making can be challenging because of the high cognitive load of caring for sick and complex patients, maintaining proper PPE practices, and simultaneously assessing an early graduate’s performance. However, maintaining a level of supervision that is appropriate for trainee competence is paramount for patient safety. It may be valuable to identify tasks needing to be performed by early graduates and using focused simulation to generate a significant number of observations over a short period of time. Trust should be gained once competence is observed, not inferred or assumed. Instead of “trust, but verify,” we should “observe, then trust.”
CONCLUSION
There is a moral obligation to patients to avoid placing trainees in situations for which they are ill prepared based on their current abilities. We must balance the risk that exists both in leaving early graduates on the sidelines (overprotecting them as learners) and in asking them to perform tasks for which they are not prepared (overextending them as a workforce). Focusing on grounded trust derived from direct observation of performance while also balancing the risks and benefits inherent in the local pandemic context can help hospitalists calibrate supervision to a level that helps extend the workforce in a time of crisis while maintaining patient safety.
1. Cram P, Anderson ML, Shaughnessy EE. All hands on deck: learning to “unspecialize” in the COVID-19 pandemic. J Hosp Med. 2020;15(5):314‐315. https://doi.org/10.12788/jhm.3426.
2. Doshi A, Platt Y, Dressen JR, Mathews BK, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(5):302‐304 https://doi.org/10.12788/jhm.3419.
3. Cole B. 10,000 med school graduates in Italy skip final exam, get sent directly into health service to help fight COVID-19. Newsweek. March 18, 2020. https://www.newsweek.com/italy-coronavirus-covid-19-medical-students-1492996. Accessed April 18, 2020.
4. Goldberg E. Early graduation could send medical students to virus front lines. New York Times. March 26, 2020. https://www.nytimes.com/2020/03/26/health/coronavirus-medical-students-graduation.html. Accessed April 18, 2020.
5. OHSU students enter medical residency early to aid in battle against COVID-19. MSN News. March 28, 2020. https://www.msn.com/en-us/news/us/ohsu-students-enter-medical-residency-early-to-aid-in-battle-against-covid-19/ar-BB11QlM4. Accessed April 18, 2020.
6. Siddique H. Final-year medical students graduate early to fight Covid-19. The Guardian. March 20, 2020. https://www.theguardian.com/world/2020/mar/20/final-year-medical-students-graduate-early-fight-coronavirus-covid-19. Accessed April 18, 2020.
7. Kime P. Military medical school to graduate students early, rush to COVID-19 response. Military.com. March 27, 2020. https://www.military.com/daily-news/2020/03/27/military-medical-school-graduate-students-early-rush-covid-19-response.html. Accessed April 18, 2020.
8. Barzansky B, Catanese VM. LCME update of medical students, patients, and COVID-19: guiding principles for early graduation of final-year medical students. March 25, 2020. https://lcme.org/wp-content/uploads/filebase/March-25-2020-LCME-Guidance-for-Medical-Schools-Considering-Early-Graduation-Option.pdf. Accessed April 18, 2020.
9. ACGME statement on early graduation from US medical schools and early appointment to the clinical learning environment. ACGME News. April 3, 2020. https://acgme.org/Newsroom/Newsroom-Details/ArticleID/10184/ACGME-Statement-on-Early-Graduation-from-US-Medical-Schools-and-Early-Appointment-to-ACGME-Accredited-Programs. Accessed April 18, 2020.
10. Mitchell J. Baker requests federal disaster assistance, asks med schools to graduate students early. WBUR News. March 26, 2020. https://www.wbur.org/news/2020/03/26/baker-massachusetts-coronavirus. Accessed April 18, 2020.
11. Schwartz CC, Ajjarapu AS, Stamy CD, Schwinn DA. Comprehensive history of 3-year and accelerated US medical school programs: a century in review. Med Educ Online. 2018;23(1):1530557. https://doi.org/10.1080/10872981.2018.1530557.
12. Ten Cate O, Hart D, Ankel F, et al. Entrustment decision making in clinical training. Acad Med. 2016;91(2):191-198. https://doi.org/10.1097/acm.0000000000001044.
13. Walling A, Merando A. The fourth year of medical education: a literature review. Acad Med. 2010;85(11):1698-1704. https://doi.org/10.1097/acm.0b013e3181f52dc6.
14. Pusic MV, Boutis K, Hatala R, Cook DA. Learning curves in health professions education. Acad Med. 2015;90(8):1034-1042. https://doi.org/10.1097/acm.0000000000000681.
15. Englander R, Carraccio C. A lack of continuity in education, training, and practice violates the “do no harm” principle. Acad Med. 2018;93(3S):S12-S16. https://doi.org/10.1097/acm.0000000000002071.
16. Dunning D, Heath C, Suls JM. Flawed self-assessment: implications for health, education, and the workplace. Psychol Sci Public Interest. 2004;5(3):69-106. https://doi.org/10.1111/j.1529-1006.2004.00018.x.
17. Kennedy TJ, Regehr G, Baker GR, Lingard L. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008;83(10 Suppl):S89-S92. https://doi.org/10.1097/acm.0b013e318183c8b7.
18. Hauer KE, Ten Cate O, Boscardin C, Irby DM, Iobst W, O’Sullivan PS. Understanding trust as an essential element of trainee supervision and learning in the workplace. Adv Health Sci Educ Theory Pract. 2014;19(3):435-456. https://doi.org/10.1007/s10459-013-9474-4.
19. Ten Cate O. Managing risks and benefits: key issues in entrustment decisions. Med Educ. 2017;51(9):879-881. https://doi.org/10.1111/medu.13362.
The coronavirus disease of 2019 (COVID-19) pandemic has strained the healthcare system by rapidly depleting multiple resources including hospital space, medications, ventilators, personal protective equipment (PPE), clinical revenue, and morale. One of the most essential at-risk resources is healthcare providers. Healthcare providers have been overwhelmed as hospital systems have experienced local surges in COVID-19 patients. Compounding this is the fact that providers are more likely to contract COVID-19, which could sideline portions of an already taxed workforce.
Multiple “surge” interventions have been planned or implemented to mitigate a current or anticipated dearth of physicians. Some institutions are reallocating subspecialists and surgeons to general ward and intensive care unit (ICU) roles, often with support from hospitalists and ICU physicians.1 Others have used telemedicine to reduce personnel exposure and conserve PPE.2 A novel and perhaps paradigm-shifting solution arose in March 2020 when several medical schools around the world announced they would graduate final year students early to allow them to join the workforce during the COVID-19 surge.3-7 In the United States, fourth-year medical students at multiple institutions in cities such as New York, Boston, Phoenix, Tucson, Newark, Portland, and Bethesda were offered the opportunity to graduate in April rather than in May or June. The Liaison Committee on Medical Education stated that for students to graduate early, they must have already met all curricular requirements and be deemed ready by an evaluations and promotions committee.8 What these early graduates do with their “gap time” before residency is neither standardized nor prescribed. The Accreditation Council for Graduate Medical Education has discouraged individuals from joining their newly matched residency programs early.9 Some early graduates who wish to bolster the workforce have signed temporary training agreements with local healthcare systems to work for a 1- to 2-month period before moving on to their matched residency program. Some institutions have already been working with local and state officials to rapidly grant provisional temporary licenses for this purpose.10
Early medical school graduation in times of international crisis is not without precedent. When faced with physician shortages during World War II, the United States federal government urged medical colleges to graduate trainees in 3 years.11 The national medical education milieu was different then, with standardized medical school training still crystalizing merely 30 years following the Flexner report. However, there was pressure from the federal government during World War II, whereas decisions around early graduation today are driven by institutional and local officials. While a few accelerated programs persist today, there has not been an urgent, unplanned early release of graduates to meet a public health need on such a large scale in recent history. The seasonal timing of the pandemic surge in the United States may have been a key factor in deciding to graduate students early. With a late winter and early spring peak, final year students are graduating only 2 to 3 months early. But what if another peak occurs in late summer or early fall, and some students are graduated even earlier? With which aspects of patient care would hospitalists trust these graduates, and with what level of supervision? Whether now or with a future COVID-19 peak, we describe how trust develops with learners and provide hospitalists with a framework for deliberate entrustment if and when they are asked to integrate early medical school graduates into their workforce.
PROGRESSION OF TRUST WITH LEARNERS
The degree of supervision that is provided to a learner is linked to how much a supervisor trusts the learner, as well as the specific context. Trust has many forms, often depending on what type of information informs it. Presumptive trust is trust based on credentials, without any actual interaction with the learner.12 Healthcare systems typically assume that medical school graduates are ready to perform intern-level tasks based on their medical degree. This presumptive trust may be bolstered by the assumption that a residency program director has vetted a learner’s credentials during the match process. On meeting a learner, we develop initial trust, which is based on first impressions and snap-judgment. Over time, presumptive and initial trust can be replaced by grounded trust, or trust based on demonstrated performance after prolonged experience with a learner. Under normal circumstances, supervisors use observations of learner performance in the clinical environment to develop grounded trust. With early graduates, especially those who sign temporary work agreements, the usual progression of trust may be compressed. Hospitalists may have less presumptive trust because these students graduated early and little time to develop grounded trust before integrating new graduates into patient care. How should hospitalists navigate supervision in this setting?
PRESUMPTIVE TRUST FOR CURRENT EARLY GRADUATES
Missing a few months at the end of medical school likely does not significantly affect competence and, therefore, should not affect presumptive trust. The value of the fourth year of medical school has been questioned because, after fulfilling graduation requirements, students often spend significant amounts of time interviewing, traveling, taking electives with lighter workloads, or exploring nonclinical interests late in the year.13 More intense “subintern” rotations, which are important for the residency application process, occur earlier in the academic year. It is therefore reasonable to presume that most students graduating in April are not less prepared than those graduating in June.
Additionally, there is significant interlearner variability in rates of competence attainment.14 This means that there is no magic point in time at which students are fully ready for resident-level responsibilities. Some students are likely competent to be interns without a fourth year at all, while others are still facing challenges in their development at the end of medical school. As Englander and Carraccio wrote, “The notion that every medical student across the nation has somehow achieved all the competencies necessary to start residency training on July 1 of their graduation year is magical thinking.”15 Since there is no universal, time-based finish line for competence, we should not be thrown by a slight change in the arbitrary line currently drawn in June. Whether students graduate in April or June, it remains true that some will be more ready than others.
INITIAL TRUST—HIGH RISK FOR BIAS
With compressed timelines, hospitalists may default to initial trust, relying heavily on first impressions to determine how much supervision an early graduate requires. For example, a graduate who is extroverted, assertive, and articulate may give off an air of confidence, which could entice a supervising hospitalist to give a “longer leash” with higher-risk patient care tasks. It is easy to fall prey to the “confidence equals competence” heuristic, but this has been shown to be unreliable.16 Initial trust is influenced by both social biases (eg, gender, race, age) and cognitive biases (eg, halo effect) that have little or nothing to do with the actual abilities of learners. While initial trust and accompanying biases often develop unconsciously, it is important to reflect on how unfounded first impressions can influence trust and supervision decisions.
GROUNDED TRUST BUILT THROUGH DIRECT OBSERVATION
Hospitalists must be deliberate with entrustment decisions, especially in a pandemic environment. There are useful guides for making these decisions that can be used in a point-of-care manner.17 First, it is important to acknowledge that entrustment is based in part on the perceived trustworthiness of a person. Kennedy and colleagues have described four components of trustworthiness, all of which can be assessed by hospitalists in the moment of care delivery: (1) knowledge and skill (Does the trainee possess the requisite knowledge and skill to perform the task?), (2) conscientiousness (Does the trainee follow through on tasks? Are they thorough and dependable?), (3) discernment (Does the trainee recognize personal limitations and seek help when needed?), and (4) truthfulness (Does the trainee tell the truth?).17
Entrustment decisions also depend on the specific task being observed (eg, high risk vs low risk) and context (eg, severity of illness of the patient, acuity of the setting).18 Trust is linked with perceived risk and benefits.19 More entrustment (less supervision) may be given when perceived risk is low, such as prescribing acetaminophen on a stable patient or taking an initial history. Less entrustment (more supervision) may be given when perceived risk is high, such as with managing septic shock or inserting a central venous catheter. However, the duress of the COVID-19 pandemic may tilt the risk/benefit balance toward less-than-usual supervision if an early graduate is the only provider available for some higher-risk tasks. This underscores the importance of direct observation leading to grounded trust with progressively higher-risk tasks as dictated by the local pandemic environment.
As much as possible, trust should be determined based on direct observation, not fallible first impressions or inference. Supervisors often use inference when assuming that performance on one task reflects performance on others. For example, if learners are observed to be competent when interpreting electrocardiograms, one might infer they also know how to manage tachyarrhythmias. If they can manage tachyarrhythmias, one might infer they also know how to manage acute coronary syndrome. These inferences are not the way to build grounded trust because competence is task and context dependent.
Direct observation can include watching patient interactions, being present for procedures, think-alouds during didactics, cognitive autopsies, reviewing notes, and informal conversations. Being deliberate with direct observation and entrustment decision-making can be challenging because of the high cognitive load of caring for sick and complex patients, maintaining proper PPE practices, and simultaneously assessing an early graduate’s performance. However, maintaining a level of supervision that is appropriate for trainee competence is paramount for patient safety. It may be valuable to identify tasks needing to be performed by early graduates and using focused simulation to generate a significant number of observations over a short period of time. Trust should be gained once competence is observed, not inferred or assumed. Instead of “trust, but verify,” we should “observe, then trust.”
CONCLUSION
There is a moral obligation to patients to avoid placing trainees in situations for which they are ill prepared based on their current abilities. We must balance the risk that exists both in leaving early graduates on the sidelines (overprotecting them as learners) and in asking them to perform tasks for which they are not prepared (overextending them as a workforce). Focusing on grounded trust derived from direct observation of performance while also balancing the risks and benefits inherent in the local pandemic context can help hospitalists calibrate supervision to a level that helps extend the workforce in a time of crisis while maintaining patient safety.
The coronavirus disease of 2019 (COVID-19) pandemic has strained the healthcare system by rapidly depleting multiple resources including hospital space, medications, ventilators, personal protective equipment (PPE), clinical revenue, and morale. One of the most essential at-risk resources is healthcare providers. Healthcare providers have been overwhelmed as hospital systems have experienced local surges in COVID-19 patients. Compounding this is the fact that providers are more likely to contract COVID-19, which could sideline portions of an already taxed workforce.
Multiple “surge” interventions have been planned or implemented to mitigate a current or anticipated dearth of physicians. Some institutions are reallocating subspecialists and surgeons to general ward and intensive care unit (ICU) roles, often with support from hospitalists and ICU physicians.1 Others have used telemedicine to reduce personnel exposure and conserve PPE.2 A novel and perhaps paradigm-shifting solution arose in March 2020 when several medical schools around the world announced they would graduate final year students early to allow them to join the workforce during the COVID-19 surge.3-7 In the United States, fourth-year medical students at multiple institutions in cities such as New York, Boston, Phoenix, Tucson, Newark, Portland, and Bethesda were offered the opportunity to graduate in April rather than in May or June. The Liaison Committee on Medical Education stated that for students to graduate early, they must have already met all curricular requirements and be deemed ready by an evaluations and promotions committee.8 What these early graduates do with their “gap time” before residency is neither standardized nor prescribed. The Accreditation Council for Graduate Medical Education has discouraged individuals from joining their newly matched residency programs early.9 Some early graduates who wish to bolster the workforce have signed temporary training agreements with local healthcare systems to work for a 1- to 2-month period before moving on to their matched residency program. Some institutions have already been working with local and state officials to rapidly grant provisional temporary licenses for this purpose.10
Early medical school graduation in times of international crisis is not without precedent. When faced with physician shortages during World War II, the United States federal government urged medical colleges to graduate trainees in 3 years.11 The national medical education milieu was different then, with standardized medical school training still crystalizing merely 30 years following the Flexner report. However, there was pressure from the federal government during World War II, whereas decisions around early graduation today are driven by institutional and local officials. While a few accelerated programs persist today, there has not been an urgent, unplanned early release of graduates to meet a public health need on such a large scale in recent history. The seasonal timing of the pandemic surge in the United States may have been a key factor in deciding to graduate students early. With a late winter and early spring peak, final year students are graduating only 2 to 3 months early. But what if another peak occurs in late summer or early fall, and some students are graduated even earlier? With which aspects of patient care would hospitalists trust these graduates, and with what level of supervision? Whether now or with a future COVID-19 peak, we describe how trust develops with learners and provide hospitalists with a framework for deliberate entrustment if and when they are asked to integrate early medical school graduates into their workforce.
PROGRESSION OF TRUST WITH LEARNERS
The degree of supervision that is provided to a learner is linked to how much a supervisor trusts the learner, as well as the specific context. Trust has many forms, often depending on what type of information informs it. Presumptive trust is trust based on credentials, without any actual interaction with the learner.12 Healthcare systems typically assume that medical school graduates are ready to perform intern-level tasks based on their medical degree. This presumptive trust may be bolstered by the assumption that a residency program director has vetted a learner’s credentials during the match process. On meeting a learner, we develop initial trust, which is based on first impressions and snap-judgment. Over time, presumptive and initial trust can be replaced by grounded trust, or trust based on demonstrated performance after prolonged experience with a learner. Under normal circumstances, supervisors use observations of learner performance in the clinical environment to develop grounded trust. With early graduates, especially those who sign temporary work agreements, the usual progression of trust may be compressed. Hospitalists may have less presumptive trust because these students graduated early and little time to develop grounded trust before integrating new graduates into patient care. How should hospitalists navigate supervision in this setting?
PRESUMPTIVE TRUST FOR CURRENT EARLY GRADUATES
Missing a few months at the end of medical school likely does not significantly affect competence and, therefore, should not affect presumptive trust. The value of the fourth year of medical school has been questioned because, after fulfilling graduation requirements, students often spend significant amounts of time interviewing, traveling, taking electives with lighter workloads, or exploring nonclinical interests late in the year.13 More intense “subintern” rotations, which are important for the residency application process, occur earlier in the academic year. It is therefore reasonable to presume that most students graduating in April are not less prepared than those graduating in June.
Additionally, there is significant interlearner variability in rates of competence attainment.14 This means that there is no magic point in time at which students are fully ready for resident-level responsibilities. Some students are likely competent to be interns without a fourth year at all, while others are still facing challenges in their development at the end of medical school. As Englander and Carraccio wrote, “The notion that every medical student across the nation has somehow achieved all the competencies necessary to start residency training on July 1 of their graduation year is magical thinking.”15 Since there is no universal, time-based finish line for competence, we should not be thrown by a slight change in the arbitrary line currently drawn in June. Whether students graduate in April or June, it remains true that some will be more ready than others.
INITIAL TRUST—HIGH RISK FOR BIAS
With compressed timelines, hospitalists may default to initial trust, relying heavily on first impressions to determine how much supervision an early graduate requires. For example, a graduate who is extroverted, assertive, and articulate may give off an air of confidence, which could entice a supervising hospitalist to give a “longer leash” with higher-risk patient care tasks. It is easy to fall prey to the “confidence equals competence” heuristic, but this has been shown to be unreliable.16 Initial trust is influenced by both social biases (eg, gender, race, age) and cognitive biases (eg, halo effect) that have little or nothing to do with the actual abilities of learners. While initial trust and accompanying biases often develop unconsciously, it is important to reflect on how unfounded first impressions can influence trust and supervision decisions.
GROUNDED TRUST BUILT THROUGH DIRECT OBSERVATION
Hospitalists must be deliberate with entrustment decisions, especially in a pandemic environment. There are useful guides for making these decisions that can be used in a point-of-care manner.17 First, it is important to acknowledge that entrustment is based in part on the perceived trustworthiness of a person. Kennedy and colleagues have described four components of trustworthiness, all of which can be assessed by hospitalists in the moment of care delivery: (1) knowledge and skill (Does the trainee possess the requisite knowledge and skill to perform the task?), (2) conscientiousness (Does the trainee follow through on tasks? Are they thorough and dependable?), (3) discernment (Does the trainee recognize personal limitations and seek help when needed?), and (4) truthfulness (Does the trainee tell the truth?).17
Entrustment decisions also depend on the specific task being observed (eg, high risk vs low risk) and context (eg, severity of illness of the patient, acuity of the setting).18 Trust is linked with perceived risk and benefits.19 More entrustment (less supervision) may be given when perceived risk is low, such as prescribing acetaminophen on a stable patient or taking an initial history. Less entrustment (more supervision) may be given when perceived risk is high, such as with managing septic shock or inserting a central venous catheter. However, the duress of the COVID-19 pandemic may tilt the risk/benefit balance toward less-than-usual supervision if an early graduate is the only provider available for some higher-risk tasks. This underscores the importance of direct observation leading to grounded trust with progressively higher-risk tasks as dictated by the local pandemic environment.
As much as possible, trust should be determined based on direct observation, not fallible first impressions or inference. Supervisors often use inference when assuming that performance on one task reflects performance on others. For example, if learners are observed to be competent when interpreting electrocardiograms, one might infer they also know how to manage tachyarrhythmias. If they can manage tachyarrhythmias, one might infer they also know how to manage acute coronary syndrome. These inferences are not the way to build grounded trust because competence is task and context dependent.
Direct observation can include watching patient interactions, being present for procedures, think-alouds during didactics, cognitive autopsies, reviewing notes, and informal conversations. Being deliberate with direct observation and entrustment decision-making can be challenging because of the high cognitive load of caring for sick and complex patients, maintaining proper PPE practices, and simultaneously assessing an early graduate’s performance. However, maintaining a level of supervision that is appropriate for trainee competence is paramount for patient safety. It may be valuable to identify tasks needing to be performed by early graduates and using focused simulation to generate a significant number of observations over a short period of time. Trust should be gained once competence is observed, not inferred or assumed. Instead of “trust, but verify,” we should “observe, then trust.”
CONCLUSION
There is a moral obligation to patients to avoid placing trainees in situations for which they are ill prepared based on their current abilities. We must balance the risk that exists both in leaving early graduates on the sidelines (overprotecting them as learners) and in asking them to perform tasks for which they are not prepared (overextending them as a workforce). Focusing on grounded trust derived from direct observation of performance while also balancing the risks and benefits inherent in the local pandemic context can help hospitalists calibrate supervision to a level that helps extend the workforce in a time of crisis while maintaining patient safety.
1. Cram P, Anderson ML, Shaughnessy EE. All hands on deck: learning to “unspecialize” in the COVID-19 pandemic. J Hosp Med. 2020;15(5):314‐315. https://doi.org/10.12788/jhm.3426.
2. Doshi A, Platt Y, Dressen JR, Mathews BK, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(5):302‐304 https://doi.org/10.12788/jhm.3419.
3. Cole B. 10,000 med school graduates in Italy skip final exam, get sent directly into health service to help fight COVID-19. Newsweek. March 18, 2020. https://www.newsweek.com/italy-coronavirus-covid-19-medical-students-1492996. Accessed April 18, 2020.
4. Goldberg E. Early graduation could send medical students to virus front lines. New York Times. March 26, 2020. https://www.nytimes.com/2020/03/26/health/coronavirus-medical-students-graduation.html. Accessed April 18, 2020.
5. OHSU students enter medical residency early to aid in battle against COVID-19. MSN News. March 28, 2020. https://www.msn.com/en-us/news/us/ohsu-students-enter-medical-residency-early-to-aid-in-battle-against-covid-19/ar-BB11QlM4. Accessed April 18, 2020.
6. Siddique H. Final-year medical students graduate early to fight Covid-19. The Guardian. March 20, 2020. https://www.theguardian.com/world/2020/mar/20/final-year-medical-students-graduate-early-fight-coronavirus-covid-19. Accessed April 18, 2020.
7. Kime P. Military medical school to graduate students early, rush to COVID-19 response. Military.com. March 27, 2020. https://www.military.com/daily-news/2020/03/27/military-medical-school-graduate-students-early-rush-covid-19-response.html. Accessed April 18, 2020.
8. Barzansky B, Catanese VM. LCME update of medical students, patients, and COVID-19: guiding principles for early graduation of final-year medical students. March 25, 2020. https://lcme.org/wp-content/uploads/filebase/March-25-2020-LCME-Guidance-for-Medical-Schools-Considering-Early-Graduation-Option.pdf. Accessed April 18, 2020.
9. ACGME statement on early graduation from US medical schools and early appointment to the clinical learning environment. ACGME News. April 3, 2020. https://acgme.org/Newsroom/Newsroom-Details/ArticleID/10184/ACGME-Statement-on-Early-Graduation-from-US-Medical-Schools-and-Early-Appointment-to-ACGME-Accredited-Programs. Accessed April 18, 2020.
10. Mitchell J. Baker requests federal disaster assistance, asks med schools to graduate students early. WBUR News. March 26, 2020. https://www.wbur.org/news/2020/03/26/baker-massachusetts-coronavirus. Accessed April 18, 2020.
11. Schwartz CC, Ajjarapu AS, Stamy CD, Schwinn DA. Comprehensive history of 3-year and accelerated US medical school programs: a century in review. Med Educ Online. 2018;23(1):1530557. https://doi.org/10.1080/10872981.2018.1530557.
12. Ten Cate O, Hart D, Ankel F, et al. Entrustment decision making in clinical training. Acad Med. 2016;91(2):191-198. https://doi.org/10.1097/acm.0000000000001044.
13. Walling A, Merando A. The fourth year of medical education: a literature review. Acad Med. 2010;85(11):1698-1704. https://doi.org/10.1097/acm.0b013e3181f52dc6.
14. Pusic MV, Boutis K, Hatala R, Cook DA. Learning curves in health professions education. Acad Med. 2015;90(8):1034-1042. https://doi.org/10.1097/acm.0000000000000681.
15. Englander R, Carraccio C. A lack of continuity in education, training, and practice violates the “do no harm” principle. Acad Med. 2018;93(3S):S12-S16. https://doi.org/10.1097/acm.0000000000002071.
16. Dunning D, Heath C, Suls JM. Flawed self-assessment: implications for health, education, and the workplace. Psychol Sci Public Interest. 2004;5(3):69-106. https://doi.org/10.1111/j.1529-1006.2004.00018.x.
17. Kennedy TJ, Regehr G, Baker GR, Lingard L. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008;83(10 Suppl):S89-S92. https://doi.org/10.1097/acm.0b013e318183c8b7.
18. Hauer KE, Ten Cate O, Boscardin C, Irby DM, Iobst W, O’Sullivan PS. Understanding trust as an essential element of trainee supervision and learning in the workplace. Adv Health Sci Educ Theory Pract. 2014;19(3):435-456. https://doi.org/10.1007/s10459-013-9474-4.
19. Ten Cate O. Managing risks and benefits: key issues in entrustment decisions. Med Educ. 2017;51(9):879-881. https://doi.org/10.1111/medu.13362.
1. Cram P, Anderson ML, Shaughnessy EE. All hands on deck: learning to “unspecialize” in the COVID-19 pandemic. J Hosp Med. 2020;15(5):314‐315. https://doi.org/10.12788/jhm.3426.
2. Doshi A, Platt Y, Dressen JR, Mathews BK, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(5):302‐304 https://doi.org/10.12788/jhm.3419.
3. Cole B. 10,000 med school graduates in Italy skip final exam, get sent directly into health service to help fight COVID-19. Newsweek. March 18, 2020. https://www.newsweek.com/italy-coronavirus-covid-19-medical-students-1492996. Accessed April 18, 2020.
4. Goldberg E. Early graduation could send medical students to virus front lines. New York Times. March 26, 2020. https://www.nytimes.com/2020/03/26/health/coronavirus-medical-students-graduation.html. Accessed April 18, 2020.
5. OHSU students enter medical residency early to aid in battle against COVID-19. MSN News. March 28, 2020. https://www.msn.com/en-us/news/us/ohsu-students-enter-medical-residency-early-to-aid-in-battle-against-covid-19/ar-BB11QlM4. Accessed April 18, 2020.
6. Siddique H. Final-year medical students graduate early to fight Covid-19. The Guardian. March 20, 2020. https://www.theguardian.com/world/2020/mar/20/final-year-medical-students-graduate-early-fight-coronavirus-covid-19. Accessed April 18, 2020.
7. Kime P. Military medical school to graduate students early, rush to COVID-19 response. Military.com. March 27, 2020. https://www.military.com/daily-news/2020/03/27/military-medical-school-graduate-students-early-rush-covid-19-response.html. Accessed April 18, 2020.
8. Barzansky B, Catanese VM. LCME update of medical students, patients, and COVID-19: guiding principles for early graduation of final-year medical students. March 25, 2020. https://lcme.org/wp-content/uploads/filebase/March-25-2020-LCME-Guidance-for-Medical-Schools-Considering-Early-Graduation-Option.pdf. Accessed April 18, 2020.
9. ACGME statement on early graduation from US medical schools and early appointment to the clinical learning environment. ACGME News. April 3, 2020. https://acgme.org/Newsroom/Newsroom-Details/ArticleID/10184/ACGME-Statement-on-Early-Graduation-from-US-Medical-Schools-and-Early-Appointment-to-ACGME-Accredited-Programs. Accessed April 18, 2020.
10. Mitchell J. Baker requests federal disaster assistance, asks med schools to graduate students early. WBUR News. March 26, 2020. https://www.wbur.org/news/2020/03/26/baker-massachusetts-coronavirus. Accessed April 18, 2020.
11. Schwartz CC, Ajjarapu AS, Stamy CD, Schwinn DA. Comprehensive history of 3-year and accelerated US medical school programs: a century in review. Med Educ Online. 2018;23(1):1530557. https://doi.org/10.1080/10872981.2018.1530557.
12. Ten Cate O, Hart D, Ankel F, et al. Entrustment decision making in clinical training. Acad Med. 2016;91(2):191-198. https://doi.org/10.1097/acm.0000000000001044.
13. Walling A, Merando A. The fourth year of medical education: a literature review. Acad Med. 2010;85(11):1698-1704. https://doi.org/10.1097/acm.0b013e3181f52dc6.
14. Pusic MV, Boutis K, Hatala R, Cook DA. Learning curves in health professions education. Acad Med. 2015;90(8):1034-1042. https://doi.org/10.1097/acm.0000000000000681.
15. Englander R, Carraccio C. A lack of continuity in education, training, and practice violates the “do no harm” principle. Acad Med. 2018;93(3S):S12-S16. https://doi.org/10.1097/acm.0000000000002071.
16. Dunning D, Heath C, Suls JM. Flawed self-assessment: implications for health, education, and the workplace. Psychol Sci Public Interest. 2004;5(3):69-106. https://doi.org/10.1111/j.1529-1006.2004.00018.x.
17. Kennedy TJ, Regehr G, Baker GR, Lingard L. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008;83(10 Suppl):S89-S92. https://doi.org/10.1097/acm.0b013e318183c8b7.
18. Hauer KE, Ten Cate O, Boscardin C, Irby DM, Iobst W, O’Sullivan PS. Understanding trust as an essential element of trainee supervision and learning in the workplace. Adv Health Sci Educ Theory Pract. 2014;19(3):435-456. https://doi.org/10.1007/s10459-013-9474-4.
19. Ten Cate O. Managing risks and benefits: key issues in entrustment decisions. Med Educ. 2017;51(9):879-881. https://doi.org/10.1111/medu.13362.
© 2020 Society of Hospital Medicine
When Horses and Zebras Coexist: Achieving Diagnostic Excellence in the Age of High-Value Care
Safe, timely, and efficient diagnosis is fundamental for high-quality, effective healthcare. Why is diagnosis so important? First, it informs the two other main areas of medical decision-making: treatment and prognosis. These are the means by which physicians can actually change health outcomes for patients, as well as ensure that patients and their families have a realistic and accurate understanding of what the future holds with respect to their health. Second, patients and families tend to feel a sense of closure from having a name and an explanation for symptoms, even in the absence of specific treatment. Proper labeling allows patients and families to connect with others with the same diagnosis, who are best positioned to offer empathy by virtue of their similar experiences.
Despite the fundamental role of diagnosis, diagnostic error is pervasive in medicine, with unacceptable levels of resultant harm.1 In 2015, the Institute of Medicine published a landmark report, “Improving Diagnosis in Health Care,” bringing the problem to the forefront of the minds of healthcare professionals and the general public alike. According to the report, “improving the diagnostic process…represents a moral, professional, and public health imperative.”1 We must do more than avoid diagnostic error, however—we must aim to achieve diagnostic excellence. Not getting it wrong is not enough.
There are real challenges to achieving diagnostic safety, let alone excellence. The “churn” of modern hospital medicine does not reward deep diagnostic thought, nor does it often encourage reflection or collaboration, important components of being able to achieve diagnostic excellence.2 Furthermore, despite their years of training, physicians often have difficulty applying probabilistic reasoning and appropriately incorporating diagnostic information in the best evidence-based manner.3,4 In addition, there are no validated measures of diagnostic performance in practice. It is telling that many hospitalists, despite a professed interest in complex diagnosis, would rather be assigned to care for a patient with cellulitis than a patient with a complicated differential diagnosis.
Given these challenges, how can the modern healthcare ecosystem be changed to achieve diagnostic excellence? In this month’s issue of Journal of Hospital Medicine, Singer and colleagues describe a pilot project of a proposed solution to the problem.5 Aptly named, the Socrates Project is an intervention that makes available a team of “diagnosticians” that can be consulted for assistance with challenging diagnostic cases. The physicians on the team volunteer their time, allowing for deep diagnostic evaluation that is not limited by one’s daily workload, thus overcoming one of the major hurdles to achieving diagnostic excellence. The described program also focuses on harnessing the power of teamwork, which is especially relevant given recent descriptions of the effectiveness of collective intelligence in improving diagnostic performance.6 Importantly, the authors recognize that their intervention will not achieve a diagnosis in every case for which they are consulted; rather, they hope that their thorough evaluation will uncover additional potential diagnostic avenues for the referring team to pursue, with a goal to “improve patient care by providing…ideas to reduce—or at least manage—diagnostic uncertainty.”
Programs of this nature are exciting for hospitalists. Hospital medicine is, perhaps, a place in modern medicine where diagnostic excellence has a natural home. Patients admitted to the hospital are acutely (and often severely) ill, and hospitalists are tasked with rapidly identifying the cause of their illness in order to initiate appropriate treatment and accurately inform prognosis. Hospitalists, as generalists, take a broad approach to challenging cases, and they tend to practice in well-resourced environments with nearly every diagnostic modality at their disposal. Many hospitalists would envy participating in a program such as the Socrates Project.
While Singer et al.’s innovation—and the institutional support thereof—should be lauded, some discussion must be had about how to assess the effectiveness of such a program. The authors acknowledge the need for evaluation of both the diagnostic process and the outcomes that process achieves. Measuring diagnostic performance is challenging, however, and while there is substantial progress being made in this area, recent efforts tend to focus on identifying diagnostic errors rather than measuring diagnostic excellence. Moreover, even if a program does improve diagnostic performance, how should we evaluate for unintended consequences of its implementation? In the age of high-value care, how can we ensure that efforts to do a better job of spotting proverbial zebras do not come at the cost of harming too many horses?7
Hospitalists are well primed to answer this question. The juxtaposition of Singer et al.’s article with the Journal of Hospital Medicine’s long-running series on Choosing Wisely®: Things We Do for No Reason™ provides a natural synergy to begin crafting a framework to evaluate unintended consequences of a program in diagnostic excellence. More diagnosis is not the goal; more appropriate diagnosis is what is needed. A clinical program aimed at achieving diagnostic excellence should not employ low-value, wasteful strategies that do not add substantively to the diagnostic process but should instead seek to improve the overall efficiency of even complicated diagnostic odysseys. Avoiding waste throughout will allow for allocation of diagnostic resources where they are needed. In turn, hospitalists can do a better job of correctly identifying both horses and zebras for what they are. While a given hospitalization for a diagnostically complex patient may be relatively expensive, better diagnosis during an index hospitalization is likely to lead to decreased downstream costs, such as those related to readmissions and further testing, as well as better health outcomes.
The Socrates Project, along with similar programs at other institutions, are exciting innovations. These programs are not only likely to be good for patients but are also good for hospitalists. The field of hospital medicine should leverage its collective expertise in clinical medicine, systems of care, and high-value care to become a home for diagnostic excellence.
1. National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015. https://doi.org/10.17226/21794
2. Olson A, Rencic J, Cosby K, et al. Competencies for improving diagnosis: an interprofessional framework for education and training in health care. Diagnosis. 2019;6(4):335-341. https://doi.org/10.1515/dx-2018-0107.
3. Baduashvili A, Guyatt G, Evans AT. ROC anatomy—getting the most out of your diagnostic test. J Gen Intern Med. 2019;34(9):1892-1898. https://doi.org/10.1007/s11606-019-05125-0.
4. Manrai AK, Bhatia G, Strymish J, Kohane IS, Jain SH. Medicine’s uncomfortable relationship with math: calculating positive predictive value. JAMA Intern Med. 2014;174(6):991-993. https://doi.org/10.1001/jamainternmed.2014.1059.
5. Singer BD, Goodwin AM, Patel AA, Vaughan DE. The Socrates Project for difficult diagnosis at Northwestern Medicine. J Hosp Med. 2020;15(2):116-118. https://doi.org/ 10.12788/jhm.3335.
6. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
7. Dhaliwal G. Bringing high-value care to the inpatient teaching service. JAMA Intern Med. 2014;174(7):1021-1022. https://doi.org/10.1001/jamainternmed.2014.2012.
Safe, timely, and efficient diagnosis is fundamental for high-quality, effective healthcare. Why is diagnosis so important? First, it informs the two other main areas of medical decision-making: treatment and prognosis. These are the means by which physicians can actually change health outcomes for patients, as well as ensure that patients and their families have a realistic and accurate understanding of what the future holds with respect to their health. Second, patients and families tend to feel a sense of closure from having a name and an explanation for symptoms, even in the absence of specific treatment. Proper labeling allows patients and families to connect with others with the same diagnosis, who are best positioned to offer empathy by virtue of their similar experiences.
Despite the fundamental role of diagnosis, diagnostic error is pervasive in medicine, with unacceptable levels of resultant harm.1 In 2015, the Institute of Medicine published a landmark report, “Improving Diagnosis in Health Care,” bringing the problem to the forefront of the minds of healthcare professionals and the general public alike. According to the report, “improving the diagnostic process…represents a moral, professional, and public health imperative.”1 We must do more than avoid diagnostic error, however—we must aim to achieve diagnostic excellence. Not getting it wrong is not enough.
There are real challenges to achieving diagnostic safety, let alone excellence. The “churn” of modern hospital medicine does not reward deep diagnostic thought, nor does it often encourage reflection or collaboration, important components of being able to achieve diagnostic excellence.2 Furthermore, despite their years of training, physicians often have difficulty applying probabilistic reasoning and appropriately incorporating diagnostic information in the best evidence-based manner.3,4 In addition, there are no validated measures of diagnostic performance in practice. It is telling that many hospitalists, despite a professed interest in complex diagnosis, would rather be assigned to care for a patient with cellulitis than a patient with a complicated differential diagnosis.
Given these challenges, how can the modern healthcare ecosystem be changed to achieve diagnostic excellence? In this month’s issue of Journal of Hospital Medicine, Singer and colleagues describe a pilot project of a proposed solution to the problem.5 Aptly named, the Socrates Project is an intervention that makes available a team of “diagnosticians” that can be consulted for assistance with challenging diagnostic cases. The physicians on the team volunteer their time, allowing for deep diagnostic evaluation that is not limited by one’s daily workload, thus overcoming one of the major hurdles to achieving diagnostic excellence. The described program also focuses on harnessing the power of teamwork, which is especially relevant given recent descriptions of the effectiveness of collective intelligence in improving diagnostic performance.6 Importantly, the authors recognize that their intervention will not achieve a diagnosis in every case for which they are consulted; rather, they hope that their thorough evaluation will uncover additional potential diagnostic avenues for the referring team to pursue, with a goal to “improve patient care by providing…ideas to reduce—or at least manage—diagnostic uncertainty.”
Programs of this nature are exciting for hospitalists. Hospital medicine is, perhaps, a place in modern medicine where diagnostic excellence has a natural home. Patients admitted to the hospital are acutely (and often severely) ill, and hospitalists are tasked with rapidly identifying the cause of their illness in order to initiate appropriate treatment and accurately inform prognosis. Hospitalists, as generalists, take a broad approach to challenging cases, and they tend to practice in well-resourced environments with nearly every diagnostic modality at their disposal. Many hospitalists would envy participating in a program such as the Socrates Project.
While Singer et al.’s innovation—and the institutional support thereof—should be lauded, some discussion must be had about how to assess the effectiveness of such a program. The authors acknowledge the need for evaluation of both the diagnostic process and the outcomes that process achieves. Measuring diagnostic performance is challenging, however, and while there is substantial progress being made in this area, recent efforts tend to focus on identifying diagnostic errors rather than measuring diagnostic excellence. Moreover, even if a program does improve diagnostic performance, how should we evaluate for unintended consequences of its implementation? In the age of high-value care, how can we ensure that efforts to do a better job of spotting proverbial zebras do not come at the cost of harming too many horses?7
Hospitalists are well primed to answer this question. The juxtaposition of Singer et al.’s article with the Journal of Hospital Medicine’s long-running series on Choosing Wisely®: Things We Do for No Reason™ provides a natural synergy to begin crafting a framework to evaluate unintended consequences of a program in diagnostic excellence. More diagnosis is not the goal; more appropriate diagnosis is what is needed. A clinical program aimed at achieving diagnostic excellence should not employ low-value, wasteful strategies that do not add substantively to the diagnostic process but should instead seek to improve the overall efficiency of even complicated diagnostic odysseys. Avoiding waste throughout will allow for allocation of diagnostic resources where they are needed. In turn, hospitalists can do a better job of correctly identifying both horses and zebras for what they are. While a given hospitalization for a diagnostically complex patient may be relatively expensive, better diagnosis during an index hospitalization is likely to lead to decreased downstream costs, such as those related to readmissions and further testing, as well as better health outcomes.
The Socrates Project, along with similar programs at other institutions, are exciting innovations. These programs are not only likely to be good for patients but are also good for hospitalists. The field of hospital medicine should leverage its collective expertise in clinical medicine, systems of care, and high-value care to become a home for diagnostic excellence.
Safe, timely, and efficient diagnosis is fundamental for high-quality, effective healthcare. Why is diagnosis so important? First, it informs the two other main areas of medical decision-making: treatment and prognosis. These are the means by which physicians can actually change health outcomes for patients, as well as ensure that patients and their families have a realistic and accurate understanding of what the future holds with respect to their health. Second, patients and families tend to feel a sense of closure from having a name and an explanation for symptoms, even in the absence of specific treatment. Proper labeling allows patients and families to connect with others with the same diagnosis, who are best positioned to offer empathy by virtue of their similar experiences.
Despite the fundamental role of diagnosis, diagnostic error is pervasive in medicine, with unacceptable levels of resultant harm.1 In 2015, the Institute of Medicine published a landmark report, “Improving Diagnosis in Health Care,” bringing the problem to the forefront of the minds of healthcare professionals and the general public alike. According to the report, “improving the diagnostic process…represents a moral, professional, and public health imperative.”1 We must do more than avoid diagnostic error, however—we must aim to achieve diagnostic excellence. Not getting it wrong is not enough.
There are real challenges to achieving diagnostic safety, let alone excellence. The “churn” of modern hospital medicine does not reward deep diagnostic thought, nor does it often encourage reflection or collaboration, important components of being able to achieve diagnostic excellence.2 Furthermore, despite their years of training, physicians often have difficulty applying probabilistic reasoning and appropriately incorporating diagnostic information in the best evidence-based manner.3,4 In addition, there are no validated measures of diagnostic performance in practice. It is telling that many hospitalists, despite a professed interest in complex diagnosis, would rather be assigned to care for a patient with cellulitis than a patient with a complicated differential diagnosis.
Given these challenges, how can the modern healthcare ecosystem be changed to achieve diagnostic excellence? In this month’s issue of Journal of Hospital Medicine, Singer and colleagues describe a pilot project of a proposed solution to the problem.5 Aptly named, the Socrates Project is an intervention that makes available a team of “diagnosticians” that can be consulted for assistance with challenging diagnostic cases. The physicians on the team volunteer their time, allowing for deep diagnostic evaluation that is not limited by one’s daily workload, thus overcoming one of the major hurdles to achieving diagnostic excellence. The described program also focuses on harnessing the power of teamwork, which is especially relevant given recent descriptions of the effectiveness of collective intelligence in improving diagnostic performance.6 Importantly, the authors recognize that their intervention will not achieve a diagnosis in every case for which they are consulted; rather, they hope that their thorough evaluation will uncover additional potential diagnostic avenues for the referring team to pursue, with a goal to “improve patient care by providing…ideas to reduce—or at least manage—diagnostic uncertainty.”
Programs of this nature are exciting for hospitalists. Hospital medicine is, perhaps, a place in modern medicine where diagnostic excellence has a natural home. Patients admitted to the hospital are acutely (and often severely) ill, and hospitalists are tasked with rapidly identifying the cause of their illness in order to initiate appropriate treatment and accurately inform prognosis. Hospitalists, as generalists, take a broad approach to challenging cases, and they tend to practice in well-resourced environments with nearly every diagnostic modality at their disposal. Many hospitalists would envy participating in a program such as the Socrates Project.
While Singer et al.’s innovation—and the institutional support thereof—should be lauded, some discussion must be had about how to assess the effectiveness of such a program. The authors acknowledge the need for evaluation of both the diagnostic process and the outcomes that process achieves. Measuring diagnostic performance is challenging, however, and while there is substantial progress being made in this area, recent efforts tend to focus on identifying diagnostic errors rather than measuring diagnostic excellence. Moreover, even if a program does improve diagnostic performance, how should we evaluate for unintended consequences of its implementation? In the age of high-value care, how can we ensure that efforts to do a better job of spotting proverbial zebras do not come at the cost of harming too many horses?7
Hospitalists are well primed to answer this question. The juxtaposition of Singer et al.’s article with the Journal of Hospital Medicine’s long-running series on Choosing Wisely®: Things We Do for No Reason™ provides a natural synergy to begin crafting a framework to evaluate unintended consequences of a program in diagnostic excellence. More diagnosis is not the goal; more appropriate diagnosis is what is needed. A clinical program aimed at achieving diagnostic excellence should not employ low-value, wasteful strategies that do not add substantively to the diagnostic process but should instead seek to improve the overall efficiency of even complicated diagnostic odysseys. Avoiding waste throughout will allow for allocation of diagnostic resources where they are needed. In turn, hospitalists can do a better job of correctly identifying both horses and zebras for what they are. While a given hospitalization for a diagnostically complex patient may be relatively expensive, better diagnosis during an index hospitalization is likely to lead to decreased downstream costs, such as those related to readmissions and further testing, as well as better health outcomes.
The Socrates Project, along with similar programs at other institutions, are exciting innovations. These programs are not only likely to be good for patients but are also good for hospitalists. The field of hospital medicine should leverage its collective expertise in clinical medicine, systems of care, and high-value care to become a home for diagnostic excellence.
1. National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015. https://doi.org/10.17226/21794
2. Olson A, Rencic J, Cosby K, et al. Competencies for improving diagnosis: an interprofessional framework for education and training in health care. Diagnosis. 2019;6(4):335-341. https://doi.org/10.1515/dx-2018-0107.
3. Baduashvili A, Guyatt G, Evans AT. ROC anatomy—getting the most out of your diagnostic test. J Gen Intern Med. 2019;34(9):1892-1898. https://doi.org/10.1007/s11606-019-05125-0.
4. Manrai AK, Bhatia G, Strymish J, Kohane IS, Jain SH. Medicine’s uncomfortable relationship with math: calculating positive predictive value. JAMA Intern Med. 2014;174(6):991-993. https://doi.org/10.1001/jamainternmed.2014.1059.
5. Singer BD, Goodwin AM, Patel AA, Vaughan DE. The Socrates Project for difficult diagnosis at Northwestern Medicine. J Hosp Med. 2020;15(2):116-118. https://doi.org/ 10.12788/jhm.3335.
6. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
7. Dhaliwal G. Bringing high-value care to the inpatient teaching service. JAMA Intern Med. 2014;174(7):1021-1022. https://doi.org/10.1001/jamainternmed.2014.2012.
1. National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015. https://doi.org/10.17226/21794
2. Olson A, Rencic J, Cosby K, et al. Competencies for improving diagnosis: an interprofessional framework for education and training in health care. Diagnosis. 2019;6(4):335-341. https://doi.org/10.1515/dx-2018-0107.
3. Baduashvili A, Guyatt G, Evans AT. ROC anatomy—getting the most out of your diagnostic test. J Gen Intern Med. 2019;34(9):1892-1898. https://doi.org/10.1007/s11606-019-05125-0.
4. Manrai AK, Bhatia G, Strymish J, Kohane IS, Jain SH. Medicine’s uncomfortable relationship with math: calculating positive predictive value. JAMA Intern Med. 2014;174(6):991-993. https://doi.org/10.1001/jamainternmed.2014.1059.
5. Singer BD, Goodwin AM, Patel AA, Vaughan DE. The Socrates Project for difficult diagnosis at Northwestern Medicine. J Hosp Med. 2020;15(2):116-118. https://doi.org/ 10.12788/jhm.3335.
6. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
7. Dhaliwal G. Bringing high-value care to the inpatient teaching service. JAMA Intern Med. 2014;174(7):1021-1022. https://doi.org/10.1001/jamainternmed.2014.2012.
© 2020 Society of Hospital Medicine
A Plea to Reconsider the Diagnosis
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
© 2019 Society of Hospital Medicine
Improving Resident Feedback on Diagnostic Reasoning after Handovers: The LOOP Project
One of the most promising methods for improving medical decision-making is learning from the outcomes of one’s decisions and either maintaining or modifying future decision-making based on those outcomes.1-3 This process of iterative improvement over time based on feedback is called calibration and is one of the most important drivers of lifelong learning and improvement.1
Despite the importance of knowing the outcomes of one’s decisions, this seldom occurs in modern medical education.4 Learners do not often obtain specific feedback about the decisions they make within a short enough time frame to intentionally reflect upon and modify that decision-making process.3,5 In addition, almost every patient admitted to a teaching hospital will be cared for by multiple physicians over the course of a hospitalization. These care transitions may be seen as barriers to high-quality care and education, but we suggest a different paradigm: transitions of care present opportunities for trainees to be teammates in each other’s calibration. Peers can provide specific feedback about the diagnostic process and inform one another about patient outcomes. Transitions of care allow for built-in “second opinions,” and trainees can intentionally learn by comparing the clinical reasoning involved at different points in a patient’s course. The diagnostic process is dynamic and complex; it is fundamental that trainees have the opportunity to reflect on the process to identify how and why the diagnostic process evolved throughout a patient’s hospitalization. Most inpatient diagnoses are “working diagnoses” that are likely to change. Thus, identifying the twists and turns in a patient’s diagnostic journey provides invaluable learning for future practice.
Herein, we describe the implementation and impact of a multisite initiative to engage residents in delivering feedback to their peers about medical decisions around transitions of care.
METHODS
The LOOP Project is a prospective clinical educational study that aimed to engage resident physicians to deliver feedback and updates about their colleagues’ diagnostic decision-making around care transitions. This study was deemed exempt from review by the University of Minnesota Institutional Review Board and either approved or deemed exempt by the corresponding Institutional Review Boards at all participating institutions. The study was conducted by seven programs at six institutions and included Internal Medicine, Pediatrics, and Internal Medicine–Pediatrics (PGY 1-4) residents from February 2017 to June 2017. Residents rotating through participating clinical services during the study period were invited to participate and given further information by site leads via informational presentations, written handouts, and/or emails.
The intervention entailed residents delivering structured feedback to their colleagues regarding their patients’ diagnoses after transitions of care. The predominant setting was the inpatient hospital medicine day-shift team providing feedback to the night-shift team regarding overnight admissions. Feedback about patients (usually chosen by the day-shift team) was delivered through completion of a standard templated form (Figure) usually sent within 24 hours after hospital admission through secure messaging (ie, EPIC In-Basket message utilizing a Smartphrase of the LOOP feedback form). A 24-hour time period was chosen to allow for rapid cycling of feedback focusing on initial diagnostic assessment. Site leads and resident champions promoted the project through presentations, informal discussions, and prizes for high completion rates of forms and surveys (ie, coffee cards and pizza).
Feedback forms were collected by site leads. A categorization rubric was developed during a pilot phase. Diagnoses before and after the transition of care were categorized as no change, diagnostic refinement (ie, the initial diagnosis was modified to be more specific), disease evolution (ie, the patient’s physiology or disease course changed), or major diagnostic change (ie, the initial and subsequent diagnoses differed substantially). Site leads acted as single-coders and conference calls were held to discuss coding and build consensus regarding the taxonomy. Diagnoses were not labeled as “right” or “wrong”; instead, categorization focused on differences between diagnoses before and after transitions of care.
Residents were invited to complete surveys before and after the rotation during which they had the opportunity to give or receive feedback. A unique identifier was entered by each participant to allow pairing of pre- and postsurveys. The survey (Appendix 1) was developed and refined during the initial pilot phase at the University of Minnesota. Surveys were collected using RedCap and analyzed using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina). Differences between pre- and postsurveys were calculated using paired t-tests for continuous variables, and descriptive statistics were used for demographic and other items. Only surveys completed by individuals who completed both pre- and postsurveys were included in the analysis.
RESULTS
Overall, there were 716 current residents in the training programs that participated in this study; one site planned on participating but did not complete any forms. A total of 405 residents were eligible to participate during the study period. Overall, 221 (54.5%)
Survey results (Table) indicated significantly improved self-efficacy in identifying cognitive errors in residents’ own practice, identifying why those errors occurred, and identifying strategies to decrease future diagnostic errors. Participants noted increased frequency of discussions within teams regarding differential diagnoses, diagnostic errors, and why diagnoses changed over time. The feedback process was viewed positively by participants, who were also generally satisfied with the overall quality, frequency, and value of the feedback received. After the intervention, participants reported an increase in the amount of feedback received for night admissions and an overall increase in the perception that nighttime admissions were as “educational” as daytime admissions.
Of 544 collected forms, 238 (43.7%) showed some diagnostic change. These changes were further categorized into disease evolution (60 forms, 11.0%), diagnostic refinement (109 forms, 20.0%), and major diagnostic change (69 forms, 12.7%).
CONCLUSION
This study suggests that an intervention to operationalize standardized, structured feedback about diagnostic decision-making around transitions of care is a promising approach to improve residents’ understanding of changes in, and evolution of, the diagnostic process, as well as improve the perceived educational value of overnight admissions. In our results, over 40% of the patients admitted by residents had some change in their diagnoses after a transition of care during their early hospitalization. This finding highlights the importance of ensuring that trainees have the opportunity to know the outcomes of their decisions. Indeed, residents should be encouraged to follow-up on their own patients without prompting; however, studies show that this practice is uncommon and interventions beyond admonition are necessary.4
The diagnostic change rate observed in this study confirms that diagnosis is an iterative process and that the concept of a working diagnosis is key—a diagnosis made at admission will very likely be modified by time, the natural history of the disease, and new clinical information. When diagnoses are viewed as working diagnoses, trainees may be empowered to better understand the diagnostic process. As learners and teachers adopt this perspective, training programs are more likely to be successful in helping learners calibrate toward expertise.
Previous studies have questioned whether resident physicians view overnight admissions as valuable.6 After our intervention, we found an increase in both the amount of feedback received and the proportion of participants who agreed that night and day admissions were equally educational, suggesting that targeted diagnostic reasoning feedback can bolster educational value of nighttime admissions.
This study presents a number of limitations. First, the survey response rate was low, which could potentially lead to biased results. We excluded those respondents who did not respond to both the pre- and postsurveys from the analysis. Second, we did not measure actual change in diagnostic performance. While learners did report learning and saw feedback as valuable, self-identified learning points may not always translate to improved patient care. Additionally, residents chose the patients for whom feedback was provided, and the diagnostic change rate described may be overestimated. We did not track the total number of admissions for which feedback could have been delivered during the study. We did not include a control group, and the intervention may not be responsible for changing learners’ perceptions. However, the included programs were not implementing other new protocols focused on diagnostic reasoning during the study period. In addition, we addressed diagnostic changes early in a hospital course; a comprehensive program should address more feedback loops (eg, discharging team to admitting team).
This work is a pilot study; for future interventions focused on improving calibration to be sustainable, they should be congruent with existing clinical workflows and avoid adding to the stress and/or cognitive load of an already-busy clinical experience. The most optimal strategies for delivering feedback about clinical reasoning remain unclear.
In summary, a program to deliver structured feedback among resident physicians about diagnostic reasoning across care transitions for selected hospitalized patients is viewed positively by trainees, is feasible, and leads to changes in resident perception and self-efficacy. Future studies and interventions should aim to provide feedback more systematically, rather than just for selected patients, and objectively track diagnostic changes over time in hospitalized patients. While truly objective diagnostic information is challenging to obtain, comparing admission and other inpatient diagnoses to discharge diagnoses or diagnoses from primary care follow-up visits may be helpful. In addition, studies should aim to track trainees’ clinical decision-making over time and determine the effectiveness of feedback at improving diagnostic performance through calibration.
Acknowledgments
The authors thank the trainees who participated in this study, as well as the residency leadership at participating institutions. The authors also thank Qi Wang, PhD, for providing statistical analysis.
Disclosures
The authors have nothing to disclose.
Funding
The study was funded by an AAIM Innovation Grant and local support at each participating institution.
1. Croskerry P. The feedback sanction. Acad Emerg Med. 2000;7(11):1232-1238. https://doi.org/10.1111/j.1553-2712.2000.tb00468.x.
2. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(Suppl 2):ii28-ii32. https://doi.org/10.1136/bmjqs-2012-001622.
3. Dhaliwal G. Clinical excellence: make it a habit. Acad Med. 2012;87(11):1473. https://doi.org/10.1097/ACM.0b013e31826d68d9.
4. Shenvi EC, Feupe SF, Yang H, El-Kareh R. Closing the loop: a mixed-methods study about resident learning from outcome feedback after patient handoffs. Diagnosis. 2018;5(4):235-242. https://doi.org/10.1515/dx-2018-0013.
5. Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892. https://doi.org/10.3109/0142159X.2011.558142.
6. Bump GM, Zimmer SM, McNeil MA, Elnicki DM. Hold-over admissions: are they educational for residents? J Gen Intern Med. 2014;29(3):463-467. https://doi.org/10.1007/s11606-013-2667-y.
One of the most promising methods for improving medical decision-making is learning from the outcomes of one’s decisions and either maintaining or modifying future decision-making based on those outcomes.1-3 This process of iterative improvement over time based on feedback is called calibration and is one of the most important drivers of lifelong learning and improvement.1
Despite the importance of knowing the outcomes of one’s decisions, this seldom occurs in modern medical education.4 Learners do not often obtain specific feedback about the decisions they make within a short enough time frame to intentionally reflect upon and modify that decision-making process.3,5 In addition, almost every patient admitted to a teaching hospital will be cared for by multiple physicians over the course of a hospitalization. These care transitions may be seen as barriers to high-quality care and education, but we suggest a different paradigm: transitions of care present opportunities for trainees to be teammates in each other’s calibration. Peers can provide specific feedback about the diagnostic process and inform one another about patient outcomes. Transitions of care allow for built-in “second opinions,” and trainees can intentionally learn by comparing the clinical reasoning involved at different points in a patient’s course. The diagnostic process is dynamic and complex; it is fundamental that trainees have the opportunity to reflect on the process to identify how and why the diagnostic process evolved throughout a patient’s hospitalization. Most inpatient diagnoses are “working diagnoses” that are likely to change. Thus, identifying the twists and turns in a patient’s diagnostic journey provides invaluable learning for future practice.
Herein, we describe the implementation and impact of a multisite initiative to engage residents in delivering feedback to their peers about medical decisions around transitions of care.
METHODS
The LOOP Project is a prospective clinical educational study that aimed to engage resident physicians to deliver feedback and updates about their colleagues’ diagnostic decision-making around care transitions. This study was deemed exempt from review by the University of Minnesota Institutional Review Board and either approved or deemed exempt by the corresponding Institutional Review Boards at all participating institutions. The study was conducted by seven programs at six institutions and included Internal Medicine, Pediatrics, and Internal Medicine–Pediatrics (PGY 1-4) residents from February 2017 to June 2017. Residents rotating through participating clinical services during the study period were invited to participate and given further information by site leads via informational presentations, written handouts, and/or emails.
The intervention entailed residents delivering structured feedback to their colleagues regarding their patients’ diagnoses after transitions of care. The predominant setting was the inpatient hospital medicine day-shift team providing feedback to the night-shift team regarding overnight admissions. Feedback about patients (usually chosen by the day-shift team) was delivered through completion of a standard templated form (Figure) usually sent within 24 hours after hospital admission through secure messaging (ie, EPIC In-Basket message utilizing a Smartphrase of the LOOP feedback form). A 24-hour time period was chosen to allow for rapid cycling of feedback focusing on initial diagnostic assessment. Site leads and resident champions promoted the project through presentations, informal discussions, and prizes for high completion rates of forms and surveys (ie, coffee cards and pizza).
Feedback forms were collected by site leads. A categorization rubric was developed during a pilot phase. Diagnoses before and after the transition of care were categorized as no change, diagnostic refinement (ie, the initial diagnosis was modified to be more specific), disease evolution (ie, the patient’s physiology or disease course changed), or major diagnostic change (ie, the initial and subsequent diagnoses differed substantially). Site leads acted as single-coders and conference calls were held to discuss coding and build consensus regarding the taxonomy. Diagnoses were not labeled as “right” or “wrong”; instead, categorization focused on differences between diagnoses before and after transitions of care.
Residents were invited to complete surveys before and after the rotation during which they had the opportunity to give or receive feedback. A unique identifier was entered by each participant to allow pairing of pre- and postsurveys. The survey (Appendix 1) was developed and refined during the initial pilot phase at the University of Minnesota. Surveys were collected using RedCap and analyzed using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina). Differences between pre- and postsurveys were calculated using paired t-tests for continuous variables, and descriptive statistics were used for demographic and other items. Only surveys completed by individuals who completed both pre- and postsurveys were included in the analysis.
RESULTS
Overall, there were 716 current residents in the training programs that participated in this study; one site planned on participating but did not complete any forms. A total of 405 residents were eligible to participate during the study period. Overall, 221 (54.5%)
Survey results (Table) indicated significantly improved self-efficacy in identifying cognitive errors in residents’ own practice, identifying why those errors occurred, and identifying strategies to decrease future diagnostic errors. Participants noted increased frequency of discussions within teams regarding differential diagnoses, diagnostic errors, and why diagnoses changed over time. The feedback process was viewed positively by participants, who were also generally satisfied with the overall quality, frequency, and value of the feedback received. After the intervention, participants reported an increase in the amount of feedback received for night admissions and an overall increase in the perception that nighttime admissions were as “educational” as daytime admissions.
Of 544 collected forms, 238 (43.7%) showed some diagnostic change. These changes were further categorized into disease evolution (60 forms, 11.0%), diagnostic refinement (109 forms, 20.0%), and major diagnostic change (69 forms, 12.7%).
CONCLUSION
This study suggests that an intervention to operationalize standardized, structured feedback about diagnostic decision-making around transitions of care is a promising approach to improve residents’ understanding of changes in, and evolution of, the diagnostic process, as well as improve the perceived educational value of overnight admissions. In our results, over 40% of the patients admitted by residents had some change in their diagnoses after a transition of care during their early hospitalization. This finding highlights the importance of ensuring that trainees have the opportunity to know the outcomes of their decisions. Indeed, residents should be encouraged to follow-up on their own patients without prompting; however, studies show that this practice is uncommon and interventions beyond admonition are necessary.4
The diagnostic change rate observed in this study confirms that diagnosis is an iterative process and that the concept of a working diagnosis is key—a diagnosis made at admission will very likely be modified by time, the natural history of the disease, and new clinical information. When diagnoses are viewed as working diagnoses, trainees may be empowered to better understand the diagnostic process. As learners and teachers adopt this perspective, training programs are more likely to be successful in helping learners calibrate toward expertise.
Previous studies have questioned whether resident physicians view overnight admissions as valuable.6 After our intervention, we found an increase in both the amount of feedback received and the proportion of participants who agreed that night and day admissions were equally educational, suggesting that targeted diagnostic reasoning feedback can bolster educational value of nighttime admissions.
This study presents a number of limitations. First, the survey response rate was low, which could potentially lead to biased results. We excluded those respondents who did not respond to both the pre- and postsurveys from the analysis. Second, we did not measure actual change in diagnostic performance. While learners did report learning and saw feedback as valuable, self-identified learning points may not always translate to improved patient care. Additionally, residents chose the patients for whom feedback was provided, and the diagnostic change rate described may be overestimated. We did not track the total number of admissions for which feedback could have been delivered during the study. We did not include a control group, and the intervention may not be responsible for changing learners’ perceptions. However, the included programs were not implementing other new protocols focused on diagnostic reasoning during the study period. In addition, we addressed diagnostic changes early in a hospital course; a comprehensive program should address more feedback loops (eg, discharging team to admitting team).
This work is a pilot study; for future interventions focused on improving calibration to be sustainable, they should be congruent with existing clinical workflows and avoid adding to the stress and/or cognitive load of an already-busy clinical experience. The most optimal strategies for delivering feedback about clinical reasoning remain unclear.
In summary, a program to deliver structured feedback among resident physicians about diagnostic reasoning across care transitions for selected hospitalized patients is viewed positively by trainees, is feasible, and leads to changes in resident perception and self-efficacy. Future studies and interventions should aim to provide feedback more systematically, rather than just for selected patients, and objectively track diagnostic changes over time in hospitalized patients. While truly objective diagnostic information is challenging to obtain, comparing admission and other inpatient diagnoses to discharge diagnoses or diagnoses from primary care follow-up visits may be helpful. In addition, studies should aim to track trainees’ clinical decision-making over time and determine the effectiveness of feedback at improving diagnostic performance through calibration.
Acknowledgments
The authors thank the trainees who participated in this study, as well as the residency leadership at participating institutions. The authors also thank Qi Wang, PhD, for providing statistical analysis.
Disclosures
The authors have nothing to disclose.
Funding
The study was funded by an AAIM Innovation Grant and local support at each participating institution.
One of the most promising methods for improving medical decision-making is learning from the outcomes of one’s decisions and either maintaining or modifying future decision-making based on those outcomes.1-3 This process of iterative improvement over time based on feedback is called calibration and is one of the most important drivers of lifelong learning and improvement.1
Despite the importance of knowing the outcomes of one’s decisions, this seldom occurs in modern medical education.4 Learners do not often obtain specific feedback about the decisions they make within a short enough time frame to intentionally reflect upon and modify that decision-making process.3,5 In addition, almost every patient admitted to a teaching hospital will be cared for by multiple physicians over the course of a hospitalization. These care transitions may be seen as barriers to high-quality care and education, but we suggest a different paradigm: transitions of care present opportunities for trainees to be teammates in each other’s calibration. Peers can provide specific feedback about the diagnostic process and inform one another about patient outcomes. Transitions of care allow for built-in “second opinions,” and trainees can intentionally learn by comparing the clinical reasoning involved at different points in a patient’s course. The diagnostic process is dynamic and complex; it is fundamental that trainees have the opportunity to reflect on the process to identify how and why the diagnostic process evolved throughout a patient’s hospitalization. Most inpatient diagnoses are “working diagnoses” that are likely to change. Thus, identifying the twists and turns in a patient’s diagnostic journey provides invaluable learning for future practice.
Herein, we describe the implementation and impact of a multisite initiative to engage residents in delivering feedback to their peers about medical decisions around transitions of care.
METHODS
The LOOP Project is a prospective clinical educational study that aimed to engage resident physicians to deliver feedback and updates about their colleagues’ diagnostic decision-making around care transitions. This study was deemed exempt from review by the University of Minnesota Institutional Review Board and either approved or deemed exempt by the corresponding Institutional Review Boards at all participating institutions. The study was conducted by seven programs at six institutions and included Internal Medicine, Pediatrics, and Internal Medicine–Pediatrics (PGY 1-4) residents from February 2017 to June 2017. Residents rotating through participating clinical services during the study period were invited to participate and given further information by site leads via informational presentations, written handouts, and/or emails.
The intervention entailed residents delivering structured feedback to their colleagues regarding their patients’ diagnoses after transitions of care. The predominant setting was the inpatient hospital medicine day-shift team providing feedback to the night-shift team regarding overnight admissions. Feedback about patients (usually chosen by the day-shift team) was delivered through completion of a standard templated form (Figure) usually sent within 24 hours after hospital admission through secure messaging (ie, EPIC In-Basket message utilizing a Smartphrase of the LOOP feedback form). A 24-hour time period was chosen to allow for rapid cycling of feedback focusing on initial diagnostic assessment. Site leads and resident champions promoted the project through presentations, informal discussions, and prizes for high completion rates of forms and surveys (ie, coffee cards and pizza).
Feedback forms were collected by site leads. A categorization rubric was developed during a pilot phase. Diagnoses before and after the transition of care were categorized as no change, diagnostic refinement (ie, the initial diagnosis was modified to be more specific), disease evolution (ie, the patient’s physiology or disease course changed), or major diagnostic change (ie, the initial and subsequent diagnoses differed substantially). Site leads acted as single-coders and conference calls were held to discuss coding and build consensus regarding the taxonomy. Diagnoses were not labeled as “right” or “wrong”; instead, categorization focused on differences between diagnoses before and after transitions of care.
Residents were invited to complete surveys before and after the rotation during which they had the opportunity to give or receive feedback. A unique identifier was entered by each participant to allow pairing of pre- and postsurveys. The survey (Appendix 1) was developed and refined during the initial pilot phase at the University of Minnesota. Surveys were collected using RedCap and analyzed using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina). Differences between pre- and postsurveys were calculated using paired t-tests for continuous variables, and descriptive statistics were used for demographic and other items. Only surveys completed by individuals who completed both pre- and postsurveys were included in the analysis.
RESULTS
Overall, there were 716 current residents in the training programs that participated in this study; one site planned on participating but did not complete any forms. A total of 405 residents were eligible to participate during the study period. Overall, 221 (54.5%)
Survey results (Table) indicated significantly improved self-efficacy in identifying cognitive errors in residents’ own practice, identifying why those errors occurred, and identifying strategies to decrease future diagnostic errors. Participants noted increased frequency of discussions within teams regarding differential diagnoses, diagnostic errors, and why diagnoses changed over time. The feedback process was viewed positively by participants, who were also generally satisfied with the overall quality, frequency, and value of the feedback received. After the intervention, participants reported an increase in the amount of feedback received for night admissions and an overall increase in the perception that nighttime admissions were as “educational” as daytime admissions.
Of 544 collected forms, 238 (43.7%) showed some diagnostic change. These changes were further categorized into disease evolution (60 forms, 11.0%), diagnostic refinement (109 forms, 20.0%), and major diagnostic change (69 forms, 12.7%).
CONCLUSION
This study suggests that an intervention to operationalize standardized, structured feedback about diagnostic decision-making around transitions of care is a promising approach to improve residents’ understanding of changes in, and evolution of, the diagnostic process, as well as improve the perceived educational value of overnight admissions. In our results, over 40% of the patients admitted by residents had some change in their diagnoses after a transition of care during their early hospitalization. This finding highlights the importance of ensuring that trainees have the opportunity to know the outcomes of their decisions. Indeed, residents should be encouraged to follow-up on their own patients without prompting; however, studies show that this practice is uncommon and interventions beyond admonition are necessary.4
The diagnostic change rate observed in this study confirms that diagnosis is an iterative process and that the concept of a working diagnosis is key—a diagnosis made at admission will very likely be modified by time, the natural history of the disease, and new clinical information. When diagnoses are viewed as working diagnoses, trainees may be empowered to better understand the diagnostic process. As learners and teachers adopt this perspective, training programs are more likely to be successful in helping learners calibrate toward expertise.
Previous studies have questioned whether resident physicians view overnight admissions as valuable.6 After our intervention, we found an increase in both the amount of feedback received and the proportion of participants who agreed that night and day admissions were equally educational, suggesting that targeted diagnostic reasoning feedback can bolster educational value of nighttime admissions.
This study presents a number of limitations. First, the survey response rate was low, which could potentially lead to biased results. We excluded those respondents who did not respond to both the pre- and postsurveys from the analysis. Second, we did not measure actual change in diagnostic performance. While learners did report learning and saw feedback as valuable, self-identified learning points may not always translate to improved patient care. Additionally, residents chose the patients for whom feedback was provided, and the diagnostic change rate described may be overestimated. We did not track the total number of admissions for which feedback could have been delivered during the study. We did not include a control group, and the intervention may not be responsible for changing learners’ perceptions. However, the included programs were not implementing other new protocols focused on diagnostic reasoning during the study period. In addition, we addressed diagnostic changes early in a hospital course; a comprehensive program should address more feedback loops (eg, discharging team to admitting team).
This work is a pilot study; for future interventions focused on improving calibration to be sustainable, they should be congruent with existing clinical workflows and avoid adding to the stress and/or cognitive load of an already-busy clinical experience. The most optimal strategies for delivering feedback about clinical reasoning remain unclear.
In summary, a program to deliver structured feedback among resident physicians about diagnostic reasoning across care transitions for selected hospitalized patients is viewed positively by trainees, is feasible, and leads to changes in resident perception and self-efficacy. Future studies and interventions should aim to provide feedback more systematically, rather than just for selected patients, and objectively track diagnostic changes over time in hospitalized patients. While truly objective diagnostic information is challenging to obtain, comparing admission and other inpatient diagnoses to discharge diagnoses or diagnoses from primary care follow-up visits may be helpful. In addition, studies should aim to track trainees’ clinical decision-making over time and determine the effectiveness of feedback at improving diagnostic performance through calibration.
Acknowledgments
The authors thank the trainees who participated in this study, as well as the residency leadership at participating institutions. The authors also thank Qi Wang, PhD, for providing statistical analysis.
Disclosures
The authors have nothing to disclose.
Funding
The study was funded by an AAIM Innovation Grant and local support at each participating institution.
1. Croskerry P. The feedback sanction. Acad Emerg Med. 2000;7(11):1232-1238. https://doi.org/10.1111/j.1553-2712.2000.tb00468.x.
2. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(Suppl 2):ii28-ii32. https://doi.org/10.1136/bmjqs-2012-001622.
3. Dhaliwal G. Clinical excellence: make it a habit. Acad Med. 2012;87(11):1473. https://doi.org/10.1097/ACM.0b013e31826d68d9.
4. Shenvi EC, Feupe SF, Yang H, El-Kareh R. Closing the loop: a mixed-methods study about resident learning from outcome feedback after patient handoffs. Diagnosis. 2018;5(4):235-242. https://doi.org/10.1515/dx-2018-0013.
5. Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892. https://doi.org/10.3109/0142159X.2011.558142.
6. Bump GM, Zimmer SM, McNeil MA, Elnicki DM. Hold-over admissions: are they educational for residents? J Gen Intern Med. 2014;29(3):463-467. https://doi.org/10.1007/s11606-013-2667-y.
1. Croskerry P. The feedback sanction. Acad Emerg Med. 2000;7(11):1232-1238. https://doi.org/10.1111/j.1553-2712.2000.tb00468.x.
2. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(Suppl 2):ii28-ii32. https://doi.org/10.1136/bmjqs-2012-001622.
3. Dhaliwal G. Clinical excellence: make it a habit. Acad Med. 2012;87(11):1473. https://doi.org/10.1097/ACM.0b013e31826d68d9.
4. Shenvi EC, Feupe SF, Yang H, El-Kareh R. Closing the loop: a mixed-methods study about resident learning from outcome feedback after patient handoffs. Diagnosis. 2018;5(4):235-242. https://doi.org/10.1515/dx-2018-0013.
5. Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892. https://doi.org/10.3109/0142159X.2011.558142.
6. Bump GM, Zimmer SM, McNeil MA, Elnicki DM. Hold-over admissions: are they educational for residents? J Gen Intern Med. 2014;29(3):463-467. https://doi.org/10.1007/s11606-013-2667-y.
© 2019 Society of Hospital Medicine
Quantity, Quality, or Neither–Measuring the Effectiveness of Rounds
Medicine has a rich history of attending-led rounds, with some iteration of this ritual occurring as far back as the 1600s.1 In the early 1900s, the concept of “bedside rounds” was popularized by William Osler, who widely espoused their importance as a clinical and educational tool. Despite our best intentions, however, rounds today may be little reminiscent of the rounds of Osler’s day. Recent investigations into the characteristics of rounds have specifically revealed a “shift in the format from the beside to conference rooms and hallways.”2 Most of our practices for rounding in the modern era are built on tradition and belief rather than evidence. The ecosystem of modern hospital care is dramatically different than that of Osler’s day, and fundamental questions about the format, content, stakeholders, and processes of rounds remain. Perhaps the greatest and most needed change in rounding in recent years is the shift of rounds from a physician-centric activity to an activity that values the modern interprofessional hospital team. Ultimately, the very definition of “rounds” and the purpose they are meant to serve in the context of a dynamic and complicated hospital ecosystem has become increasingly complex and thus, difficult to assess and improve.
In this month’s Journal of Hospital Medicine, Sang et al.3 address this complexity by returning to basics and utilizing a novel approach to precisely measure the frequency and duration of a necessary (albeit insufficient) condition for interdisciplinary bedside rounding to occur: colocation of physician, nurse, and patient. Ultimately, their results provide a springboard to ask more complex and meaningful questions. Why, despite a recent culture shift prioritizing a return to bedside, is substantive physician and nurse colocation so persistently difficult to attain? How can we study outcomes of interdisciplinary bedside rounds if we cannot reliably facilitate their occurrence? What does “effective” rounding even mean? That is, what variables would be both meaningful and sensitive to changes in rounds?
After centuries of rounding, the medical community would be presumed to have perfected this art; however, we are instead left with more questions than answers. Prior research efforts have demonstrated the shifting of rounds away from the bedside, with bedside rounds occurring only 10%-40% of the time based on bias-prone survey data.2,4 Interestingly, a study by Huang et al., designed specifically to increase implementation of interdisciplinary bedside rounds, showed a frequency of only 64%.5 These studies are focused primarily on parameters such as patient and nursing satisfaction and did not include other important outcomes such as length of stay, readmission rates, diagnostic quality, patient engagement, or mortality.2,4,6
In Sang et al.,3 the authors utilized a real-time locator system, namely, radiofrequency identification, to precisely track the physical workflow of both attending hospitalists and bedside nurses and then subsequently used the data obtained to measure the frequency and duration of colocation at the patient bedside. The authors defined a physician “rounding event” as the physician’s presence in a single bed patient room for at least 10 seconds. The study revealed that colocation of physician and nurse (for at least 10 seconds) occurred in only 30% of all physician rounding events recorded. The duration of a physician rounding event was 5.68 minutes without nurse colocation and 9.56 minutes if a nurse was present. No difference in the frequency of physician-nurse overlap was observed between weekdays and weekends. Interestingly and not surprisingly, patient rooms located farther from the nursing station had a decreased likelihood of physician-nurse overlap.
A greater understanding of the medical community’s inability to reliably implement interdisciplinary bedside rounding may be found by examining the ecosystem of inpatient medicine. Physicians and nurses function in an environment with increasingly complex patients, more stringent (and non- evidence-based) documentation requirements, the physical decoupling of patients and their clinical information, and, as Sang et al.3 illuminate, complex geographical ward structures. As the rapidity with which patients are diagnosed and treated continues to escalate, physicians and nurses are also asked to attempt to squeeze an Oslerian-type rounding system into an ecosystem that is in overdrive. That the puzzle pieces do not fit should not be a surprise.
There is a risk that systems may implement interventions to “check the box” for interdisciplinary bedside rounding instead of seeking to change outcomes. How much time is time enough together at the bedside? Sang et al., among others, ponder whether a rounding duration of just under 10 minutes is enough.3,6 However, Rothberg et al. demonstrated that increased duration of communication alone is not necessarily associated with increased patient satisfaction or nurse–physician agreement on plan of care,7 suggesting that colocation and communication are necessary but not sufficient for true interdisciplinary patient care. The discordance between communication and understanding can potentially be explained by the varying agendas of the members of the interdisciplinary team during the same interaction.8
Ultimately, the future of interdisciplinary bedside rounding, and rounding in general, remains uncertain. Potential areas for improvement and further study include patient regionalization,3,5 tools to align agendas among stakeholders,8 integrating recommendations for interdisciplinary communication,9 and utilizing a common definition and taxonomy for study design.10 These interventions may improve future study designs and outcomes. However, these interventions are small tweaks in a complex ecosystem, and the return on these interventions may eventually reach an asymptote. Perhaps the concept of rounding as we know it is broken beyond repair, and a more radical approach is needed: either the creation of a completely innovative shared mental model of acute care that acknowledges the complex environment of inpatient medicine, or a complete restructuring of the ecosystem itself. Nonetheless, the findings of Sang et al.3 with respect to the ongoing difficulty of implementing interdisciplinary bedside rounding elucidate the need for innovation in study design and rounding implementation strategies; they also prompt us to ask—and answer—the complicated questions related to this integral component of our practice.
Disclosures
The authors have nothing to disclose.
1. Linfors EW, Neelon FA. The case for bedside rounds. N Engl J Med. 1980;303(21):1230-1233. https://doi.org/10.1056/NEJM198011203032110.
2. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era. JAMA Intern Med. 2013;173(12):1084. https://doi.org/10.1001/jamainternmed.2013.6041.
3. Sang AX, Tisdale RL, Nielson D, et al. How much time are physicians and nurses spending together at the patient bedside? J Hosp Med. 2019;14(8):468-473. https://doi.org/10.12788/jhm.3204.
4. O’leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2015-005035.
5. Huang KTL, Minahan J, Brita-Rossi P, et al. All together now: impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds OR. J Hosp Med. 2017;12(3):150-156. https://doi.org/10.12788/jhm.2696.
6. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
7. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185-189. https://doi.org/10.1007/s11606-011-1857-8.
8. Holton R, Patel R, Eggebrecht M, et al. Rounding on rounds. Am J Med Qual. 2015;30(5):493-493. https://doi.org/10.1177/1062860615596388.
9. Kassutto S , Seam N, Carlos WG, et al. Twelve tips for conducting successful interprofessional teaching rounds [published online ahead of print February 1, 2019]. Med Teach. https://doi.org/10.1080/0142159X.2018.1545086.
10. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. https://doi.org/10.1002/jhm.2575.
Medicine has a rich history of attending-led rounds, with some iteration of this ritual occurring as far back as the 1600s.1 In the early 1900s, the concept of “bedside rounds” was popularized by William Osler, who widely espoused their importance as a clinical and educational tool. Despite our best intentions, however, rounds today may be little reminiscent of the rounds of Osler’s day. Recent investigations into the characteristics of rounds have specifically revealed a “shift in the format from the beside to conference rooms and hallways.”2 Most of our practices for rounding in the modern era are built on tradition and belief rather than evidence. The ecosystem of modern hospital care is dramatically different than that of Osler’s day, and fundamental questions about the format, content, stakeholders, and processes of rounds remain. Perhaps the greatest and most needed change in rounding in recent years is the shift of rounds from a physician-centric activity to an activity that values the modern interprofessional hospital team. Ultimately, the very definition of “rounds” and the purpose they are meant to serve in the context of a dynamic and complicated hospital ecosystem has become increasingly complex and thus, difficult to assess and improve.
In this month’s Journal of Hospital Medicine, Sang et al.3 address this complexity by returning to basics and utilizing a novel approach to precisely measure the frequency and duration of a necessary (albeit insufficient) condition for interdisciplinary bedside rounding to occur: colocation of physician, nurse, and patient. Ultimately, their results provide a springboard to ask more complex and meaningful questions. Why, despite a recent culture shift prioritizing a return to bedside, is substantive physician and nurse colocation so persistently difficult to attain? How can we study outcomes of interdisciplinary bedside rounds if we cannot reliably facilitate their occurrence? What does “effective” rounding even mean? That is, what variables would be both meaningful and sensitive to changes in rounds?
After centuries of rounding, the medical community would be presumed to have perfected this art; however, we are instead left with more questions than answers. Prior research efforts have demonstrated the shifting of rounds away from the bedside, with bedside rounds occurring only 10%-40% of the time based on bias-prone survey data.2,4 Interestingly, a study by Huang et al., designed specifically to increase implementation of interdisciplinary bedside rounds, showed a frequency of only 64%.5 These studies are focused primarily on parameters such as patient and nursing satisfaction and did not include other important outcomes such as length of stay, readmission rates, diagnostic quality, patient engagement, or mortality.2,4,6
In Sang et al.,3 the authors utilized a real-time locator system, namely, radiofrequency identification, to precisely track the physical workflow of both attending hospitalists and bedside nurses and then subsequently used the data obtained to measure the frequency and duration of colocation at the patient bedside. The authors defined a physician “rounding event” as the physician’s presence in a single bed patient room for at least 10 seconds. The study revealed that colocation of physician and nurse (for at least 10 seconds) occurred in only 30% of all physician rounding events recorded. The duration of a physician rounding event was 5.68 minutes without nurse colocation and 9.56 minutes if a nurse was present. No difference in the frequency of physician-nurse overlap was observed between weekdays and weekends. Interestingly and not surprisingly, patient rooms located farther from the nursing station had a decreased likelihood of physician-nurse overlap.
A greater understanding of the medical community’s inability to reliably implement interdisciplinary bedside rounding may be found by examining the ecosystem of inpatient medicine. Physicians and nurses function in an environment with increasingly complex patients, more stringent (and non- evidence-based) documentation requirements, the physical decoupling of patients and their clinical information, and, as Sang et al.3 illuminate, complex geographical ward structures. As the rapidity with which patients are diagnosed and treated continues to escalate, physicians and nurses are also asked to attempt to squeeze an Oslerian-type rounding system into an ecosystem that is in overdrive. That the puzzle pieces do not fit should not be a surprise.
There is a risk that systems may implement interventions to “check the box” for interdisciplinary bedside rounding instead of seeking to change outcomes. How much time is time enough together at the bedside? Sang et al., among others, ponder whether a rounding duration of just under 10 minutes is enough.3,6 However, Rothberg et al. demonstrated that increased duration of communication alone is not necessarily associated with increased patient satisfaction or nurse–physician agreement on plan of care,7 suggesting that colocation and communication are necessary but not sufficient for true interdisciplinary patient care. The discordance between communication and understanding can potentially be explained by the varying agendas of the members of the interdisciplinary team during the same interaction.8
Ultimately, the future of interdisciplinary bedside rounding, and rounding in general, remains uncertain. Potential areas for improvement and further study include patient regionalization,3,5 tools to align agendas among stakeholders,8 integrating recommendations for interdisciplinary communication,9 and utilizing a common definition and taxonomy for study design.10 These interventions may improve future study designs and outcomes. However, these interventions are small tweaks in a complex ecosystem, and the return on these interventions may eventually reach an asymptote. Perhaps the concept of rounding as we know it is broken beyond repair, and a more radical approach is needed: either the creation of a completely innovative shared mental model of acute care that acknowledges the complex environment of inpatient medicine, or a complete restructuring of the ecosystem itself. Nonetheless, the findings of Sang et al.3 with respect to the ongoing difficulty of implementing interdisciplinary bedside rounding elucidate the need for innovation in study design and rounding implementation strategies; they also prompt us to ask—and answer—the complicated questions related to this integral component of our practice.
Disclosures
The authors have nothing to disclose.
Medicine has a rich history of attending-led rounds, with some iteration of this ritual occurring as far back as the 1600s.1 In the early 1900s, the concept of “bedside rounds” was popularized by William Osler, who widely espoused their importance as a clinical and educational tool. Despite our best intentions, however, rounds today may be little reminiscent of the rounds of Osler’s day. Recent investigations into the characteristics of rounds have specifically revealed a “shift in the format from the beside to conference rooms and hallways.”2 Most of our practices for rounding in the modern era are built on tradition and belief rather than evidence. The ecosystem of modern hospital care is dramatically different than that of Osler’s day, and fundamental questions about the format, content, stakeholders, and processes of rounds remain. Perhaps the greatest and most needed change in rounding in recent years is the shift of rounds from a physician-centric activity to an activity that values the modern interprofessional hospital team. Ultimately, the very definition of “rounds” and the purpose they are meant to serve in the context of a dynamic and complicated hospital ecosystem has become increasingly complex and thus, difficult to assess and improve.
In this month’s Journal of Hospital Medicine, Sang et al.3 address this complexity by returning to basics and utilizing a novel approach to precisely measure the frequency and duration of a necessary (albeit insufficient) condition for interdisciplinary bedside rounding to occur: colocation of physician, nurse, and patient. Ultimately, their results provide a springboard to ask more complex and meaningful questions. Why, despite a recent culture shift prioritizing a return to bedside, is substantive physician and nurse colocation so persistently difficult to attain? How can we study outcomes of interdisciplinary bedside rounds if we cannot reliably facilitate their occurrence? What does “effective” rounding even mean? That is, what variables would be both meaningful and sensitive to changes in rounds?
After centuries of rounding, the medical community would be presumed to have perfected this art; however, we are instead left with more questions than answers. Prior research efforts have demonstrated the shifting of rounds away from the bedside, with bedside rounds occurring only 10%-40% of the time based on bias-prone survey data.2,4 Interestingly, a study by Huang et al., designed specifically to increase implementation of interdisciplinary bedside rounds, showed a frequency of only 64%.5 These studies are focused primarily on parameters such as patient and nursing satisfaction and did not include other important outcomes such as length of stay, readmission rates, diagnostic quality, patient engagement, or mortality.2,4,6
In Sang et al.,3 the authors utilized a real-time locator system, namely, radiofrequency identification, to precisely track the physical workflow of both attending hospitalists and bedside nurses and then subsequently used the data obtained to measure the frequency and duration of colocation at the patient bedside. The authors defined a physician “rounding event” as the physician’s presence in a single bed patient room for at least 10 seconds. The study revealed that colocation of physician and nurse (for at least 10 seconds) occurred in only 30% of all physician rounding events recorded. The duration of a physician rounding event was 5.68 minutes without nurse colocation and 9.56 minutes if a nurse was present. No difference in the frequency of physician-nurse overlap was observed between weekdays and weekends. Interestingly and not surprisingly, patient rooms located farther from the nursing station had a decreased likelihood of physician-nurse overlap.
A greater understanding of the medical community’s inability to reliably implement interdisciplinary bedside rounding may be found by examining the ecosystem of inpatient medicine. Physicians and nurses function in an environment with increasingly complex patients, more stringent (and non- evidence-based) documentation requirements, the physical decoupling of patients and their clinical information, and, as Sang et al.3 illuminate, complex geographical ward structures. As the rapidity with which patients are diagnosed and treated continues to escalate, physicians and nurses are also asked to attempt to squeeze an Oslerian-type rounding system into an ecosystem that is in overdrive. That the puzzle pieces do not fit should not be a surprise.
There is a risk that systems may implement interventions to “check the box” for interdisciplinary bedside rounding instead of seeking to change outcomes. How much time is time enough together at the bedside? Sang et al., among others, ponder whether a rounding duration of just under 10 minutes is enough.3,6 However, Rothberg et al. demonstrated that increased duration of communication alone is not necessarily associated with increased patient satisfaction or nurse–physician agreement on plan of care,7 suggesting that colocation and communication are necessary but not sufficient for true interdisciplinary patient care. The discordance between communication and understanding can potentially be explained by the varying agendas of the members of the interdisciplinary team during the same interaction.8
Ultimately, the future of interdisciplinary bedside rounding, and rounding in general, remains uncertain. Potential areas for improvement and further study include patient regionalization,3,5 tools to align agendas among stakeholders,8 integrating recommendations for interdisciplinary communication,9 and utilizing a common definition and taxonomy for study design.10 These interventions may improve future study designs and outcomes. However, these interventions are small tweaks in a complex ecosystem, and the return on these interventions may eventually reach an asymptote. Perhaps the concept of rounding as we know it is broken beyond repair, and a more radical approach is needed: either the creation of a completely innovative shared mental model of acute care that acknowledges the complex environment of inpatient medicine, or a complete restructuring of the ecosystem itself. Nonetheless, the findings of Sang et al.3 with respect to the ongoing difficulty of implementing interdisciplinary bedside rounding elucidate the need for innovation in study design and rounding implementation strategies; they also prompt us to ask—and answer—the complicated questions related to this integral component of our practice.
Disclosures
The authors have nothing to disclose.
1. Linfors EW, Neelon FA. The case for bedside rounds. N Engl J Med. 1980;303(21):1230-1233. https://doi.org/10.1056/NEJM198011203032110.
2. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era. JAMA Intern Med. 2013;173(12):1084. https://doi.org/10.1001/jamainternmed.2013.6041.
3. Sang AX, Tisdale RL, Nielson D, et al. How much time are physicians and nurses spending together at the patient bedside? J Hosp Med. 2019;14(8):468-473. https://doi.org/10.12788/jhm.3204.
4. O’leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2015-005035.
5. Huang KTL, Minahan J, Brita-Rossi P, et al. All together now: impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds OR. J Hosp Med. 2017;12(3):150-156. https://doi.org/10.12788/jhm.2696.
6. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
7. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185-189. https://doi.org/10.1007/s11606-011-1857-8.
8. Holton R, Patel R, Eggebrecht M, et al. Rounding on rounds. Am J Med Qual. 2015;30(5):493-493. https://doi.org/10.1177/1062860615596388.
9. Kassutto S , Seam N, Carlos WG, et al. Twelve tips for conducting successful interprofessional teaching rounds [published online ahead of print February 1, 2019]. Med Teach. https://doi.org/10.1080/0142159X.2018.1545086.
10. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. https://doi.org/10.1002/jhm.2575.
1. Linfors EW, Neelon FA. The case for bedside rounds. N Engl J Med. 1980;303(21):1230-1233. https://doi.org/10.1056/NEJM198011203032110.
2. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era. JAMA Intern Med. 2013;173(12):1084. https://doi.org/10.1001/jamainternmed.2013.6041.
3. Sang AX, Tisdale RL, Nielson D, et al. How much time are physicians and nurses spending together at the patient bedside? J Hosp Med. 2019;14(8):468-473. https://doi.org/10.12788/jhm.3204.
4. O’leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2015-005035.
5. Huang KTL, Minahan J, Brita-Rossi P, et al. All together now: impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds OR. J Hosp Med. 2017;12(3):150-156. https://doi.org/10.12788/jhm.2696.
6. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
7. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185-189. https://doi.org/10.1007/s11606-011-1857-8.
8. Holton R, Patel R, Eggebrecht M, et al. Rounding on rounds. Am J Med Qual. 2015;30(5):493-493. https://doi.org/10.1177/1062860615596388.
9. Kassutto S , Seam N, Carlos WG, et al. Twelve tips for conducting successful interprofessional teaching rounds [published online ahead of print February 1, 2019]. Med Teach. https://doi.org/10.1080/0142159X.2018.1545086.
10. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. https://doi.org/10.1002/jhm.2575.
© 2019 Society of Hospital Medicine