User login
Quality Improvement in Health Care: From Conceptual Frameworks and Definitions to Implementation
As the movement to improve quality in health care has evolved over the past several decades, organizations whose missions focus on supporting and promoting quality in health care have defined essential concepts, standards, and measures that comprise quality and that can be used to guide quality improvement (QI) work. The World Health Organization (WHO) defines quality in clinical care as safe, effective, and people-centered service.1 These 3 pillars of quality form the foundation of a quality system aiming to deliver health care in a timely, equitable, efficient, and integrated manner. The WHO estimates that 5.7 to 8.4 million deaths occur yearly in low- and middle-income countries due to poor quality care. Regarding safety, patient harm from unsafe care is estimated to be among the top 10 causes of death and disability worldwide.2 A health care QI plan involves identifying areas for improvement, setting measurable goals, implementing evidence-based strategies and interventions, monitoring progress toward achieving those goals, and continuously evaluating and adjusting the plan as needed to ensure sustained improvement over time. Such a plan can be implemented at various levels of health care organizations, from individual clinical units to entire hospitals or even regional health care systems.
The Institute of Medicine (IOM) identifies 5 domains of quality in health care: effectiveness, efficiency, equity, patient-centeredness, and safety.3 Effectiveness relies on providing care processes supported by scientific evidence and achieving desired outcomes in the IOM recommendations. The primary efficiency aim maximizes the quality of health care delivered or the benefits achieved for a given resource unit. Equity relates to providing health care of equal quality to all individuals, regardless of personal characteristics. Moreover, patient-centeredness relates to meeting patients’ needs and preferences and providing education and support. Safety relates to avoiding actual or potential harm. Timeliness relates to obtaining needed care while minimizing delays. Finally, the IOM defines health care quality as the systematic evaluation and provision of evidence-based and safe care characterized by a culture of continuous improvement, resulting in optimal health outcomes. Taking all these concepts into consideration, 4 key attributes have been identified as essential to the global definition of health care quality: effectiveness, safety, culture of continuous improvement, and desired outcomes. This conceptualization of health care quality encompasses the fundamental components and has the potential to enhance the delivery of care. This definition’s theoretical and practical implications provide a comprehensive and consistent understanding of the elements required to improve health care and maintain public trust.
Health care quality is a dynamic, ever-evolving construct that requires continuous assessment and evaluation to ensure the delivery of care meets the changing needs of society. The National Quality Forum’s National Voluntary Consensus Standards for health care provide measures, guidance, and recommendations on achieving effective outcomes through evidence-based practices.4 These standards establish criteria by which health care systems and providers can assess and improve their quality performance.
In the United States, in order to implement and disseminate best practices, the Centers for Medicare & Medicaid Services (CMS) developed Quality Payment Programs that offer incentives to health care providers to improve the quality of care delivery. This CMS program evaluates providers based on their performance in the Merit-Based Incentive Payment System performance categories.5 These include measures related to patient experience, cost, clinical quality, improvement activities, and the use of certified electronic health record technology. The scores that providers receive are used to determine their performance-based reimbursements under Medicare’s fee-for-service program.
The concept of health care quality is also applicable in other countries. In the United Kingdom, QI initiatives are led by the Department of Health and Social Care. The National Institute for Health and Care Excellence (NICE) produces guidelines on best practices to ensure that care delivery meets established safety and quality standards, reaching cost-effectiveness excellence.6 In Australia, the Australian Commission on Quality and Safety in Health Care is responsible for setting benchmarks for performance in health care systems through a clear, structured agenda.7 Ultimately, health care quality is a complex and multifaceted issue that requires a comprehensive approach to ensure the best outcomes for patients. With the implementation of measures such as the CMS Quality Payment Programs and NICE guidelines, health care organizations can take steps to ensure their systems of care delivery reflect evidence-based practices and demonstrate a commitment to providing high-quality care.
Implementing a health care QI plan that encompasses the 4 key attributes of health care quality—effectiveness, safety, culture of continuous improvement, and desired outcomes—requires collaboration among different departments and stakeholders and a data-driven approach to decision-making. Effective communication with patients and their families is critical to ensuring that their needs are being met and that they are active partners in their health care journey. While a health care QI plan is essential for delivering high-quality, safe patient care, it also helps health care organizations comply with regulatory requirements, meet accreditation standards, and stay competitive in the ever-evolving health care landscape.
Corresponding author: Ebrahim Barkoudah, MD, MPH; Ebrahim.Barkoudah@baystatehealth.org
1. World Health Organization. Quality of care. Accessed on May 17, 2023. www.who.int/health-topics/quality-of-care#tab=tab_1
2. World Health Organization. Patient safety. Accessed on May 17, 2023 www.who.int/news-room/fact-sheets/detail/patient-safety
3. Agency for Healthcare Research and Quality. Understanding quality measurement. Accessed on May 17, 2023. www.ahrq.gov/patient-safety/quality-resources/tools/chtoolbx/understand/index.html
4. Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33(6):737-744. doi:10.1016/j.jpainsymman.2007.02.024
5. U.S Centers for Medicare & Medicaid Services. Quality payment program. Accessed on March 14, 2023 qpp.cms.gov/mips/overview
6. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1-503, v-vi. doi: 10.3310/hta19140
7. Braithwaite J, Healy J, Dwan K. The Governance of Health Safety and Quality, Commonwealth of Australia. Accessed May 17, 2023. https://regnet.anu.edu.au/research/publications/3626/governance-health-safety-and-quality 2005
As the movement to improve quality in health care has evolved over the past several decades, organizations whose missions focus on supporting and promoting quality in health care have defined essential concepts, standards, and measures that comprise quality and that can be used to guide quality improvement (QI) work. The World Health Organization (WHO) defines quality in clinical care as safe, effective, and people-centered service.1 These 3 pillars of quality form the foundation of a quality system aiming to deliver health care in a timely, equitable, efficient, and integrated manner. The WHO estimates that 5.7 to 8.4 million deaths occur yearly in low- and middle-income countries due to poor quality care. Regarding safety, patient harm from unsafe care is estimated to be among the top 10 causes of death and disability worldwide.2 A health care QI plan involves identifying areas for improvement, setting measurable goals, implementing evidence-based strategies and interventions, monitoring progress toward achieving those goals, and continuously evaluating and adjusting the plan as needed to ensure sustained improvement over time. Such a plan can be implemented at various levels of health care organizations, from individual clinical units to entire hospitals or even regional health care systems.
The Institute of Medicine (IOM) identifies 5 domains of quality in health care: effectiveness, efficiency, equity, patient-centeredness, and safety.3 Effectiveness relies on providing care processes supported by scientific evidence and achieving desired outcomes in the IOM recommendations. The primary efficiency aim maximizes the quality of health care delivered or the benefits achieved for a given resource unit. Equity relates to providing health care of equal quality to all individuals, regardless of personal characteristics. Moreover, patient-centeredness relates to meeting patients’ needs and preferences and providing education and support. Safety relates to avoiding actual or potential harm. Timeliness relates to obtaining needed care while minimizing delays. Finally, the IOM defines health care quality as the systematic evaluation and provision of evidence-based and safe care characterized by a culture of continuous improvement, resulting in optimal health outcomes. Taking all these concepts into consideration, 4 key attributes have been identified as essential to the global definition of health care quality: effectiveness, safety, culture of continuous improvement, and desired outcomes. This conceptualization of health care quality encompasses the fundamental components and has the potential to enhance the delivery of care. This definition’s theoretical and practical implications provide a comprehensive and consistent understanding of the elements required to improve health care and maintain public trust.
Health care quality is a dynamic, ever-evolving construct that requires continuous assessment and evaluation to ensure the delivery of care meets the changing needs of society. The National Quality Forum’s National Voluntary Consensus Standards for health care provide measures, guidance, and recommendations on achieving effective outcomes through evidence-based practices.4 These standards establish criteria by which health care systems and providers can assess and improve their quality performance.
In the United States, in order to implement and disseminate best practices, the Centers for Medicare & Medicaid Services (CMS) developed Quality Payment Programs that offer incentives to health care providers to improve the quality of care delivery. This CMS program evaluates providers based on their performance in the Merit-Based Incentive Payment System performance categories.5 These include measures related to patient experience, cost, clinical quality, improvement activities, and the use of certified electronic health record technology. The scores that providers receive are used to determine their performance-based reimbursements under Medicare’s fee-for-service program.
The concept of health care quality is also applicable in other countries. In the United Kingdom, QI initiatives are led by the Department of Health and Social Care. The National Institute for Health and Care Excellence (NICE) produces guidelines on best practices to ensure that care delivery meets established safety and quality standards, reaching cost-effectiveness excellence.6 In Australia, the Australian Commission on Quality and Safety in Health Care is responsible for setting benchmarks for performance in health care systems through a clear, structured agenda.7 Ultimately, health care quality is a complex and multifaceted issue that requires a comprehensive approach to ensure the best outcomes for patients. With the implementation of measures such as the CMS Quality Payment Programs and NICE guidelines, health care organizations can take steps to ensure their systems of care delivery reflect evidence-based practices and demonstrate a commitment to providing high-quality care.
Implementing a health care QI plan that encompasses the 4 key attributes of health care quality—effectiveness, safety, culture of continuous improvement, and desired outcomes—requires collaboration among different departments and stakeholders and a data-driven approach to decision-making. Effective communication with patients and their families is critical to ensuring that their needs are being met and that they are active partners in their health care journey. While a health care QI plan is essential for delivering high-quality, safe patient care, it also helps health care organizations comply with regulatory requirements, meet accreditation standards, and stay competitive in the ever-evolving health care landscape.
Corresponding author: Ebrahim Barkoudah, MD, MPH; Ebrahim.Barkoudah@baystatehealth.org
As the movement to improve quality in health care has evolved over the past several decades, organizations whose missions focus on supporting and promoting quality in health care have defined essential concepts, standards, and measures that comprise quality and that can be used to guide quality improvement (QI) work. The World Health Organization (WHO) defines quality in clinical care as safe, effective, and people-centered service.1 These 3 pillars of quality form the foundation of a quality system aiming to deliver health care in a timely, equitable, efficient, and integrated manner. The WHO estimates that 5.7 to 8.4 million deaths occur yearly in low- and middle-income countries due to poor quality care. Regarding safety, patient harm from unsafe care is estimated to be among the top 10 causes of death and disability worldwide.2 A health care QI plan involves identifying areas for improvement, setting measurable goals, implementing evidence-based strategies and interventions, monitoring progress toward achieving those goals, and continuously evaluating and adjusting the plan as needed to ensure sustained improvement over time. Such a plan can be implemented at various levels of health care organizations, from individual clinical units to entire hospitals or even regional health care systems.
The Institute of Medicine (IOM) identifies 5 domains of quality in health care: effectiveness, efficiency, equity, patient-centeredness, and safety.3 Effectiveness relies on providing care processes supported by scientific evidence and achieving desired outcomes in the IOM recommendations. The primary efficiency aim maximizes the quality of health care delivered or the benefits achieved for a given resource unit. Equity relates to providing health care of equal quality to all individuals, regardless of personal characteristics. Moreover, patient-centeredness relates to meeting patients’ needs and preferences and providing education and support. Safety relates to avoiding actual or potential harm. Timeliness relates to obtaining needed care while minimizing delays. Finally, the IOM defines health care quality as the systematic evaluation and provision of evidence-based and safe care characterized by a culture of continuous improvement, resulting in optimal health outcomes. Taking all these concepts into consideration, 4 key attributes have been identified as essential to the global definition of health care quality: effectiveness, safety, culture of continuous improvement, and desired outcomes. This conceptualization of health care quality encompasses the fundamental components and has the potential to enhance the delivery of care. This definition’s theoretical and practical implications provide a comprehensive and consistent understanding of the elements required to improve health care and maintain public trust.
Health care quality is a dynamic, ever-evolving construct that requires continuous assessment and evaluation to ensure the delivery of care meets the changing needs of society. The National Quality Forum’s National Voluntary Consensus Standards for health care provide measures, guidance, and recommendations on achieving effective outcomes through evidence-based practices.4 These standards establish criteria by which health care systems and providers can assess and improve their quality performance.
In the United States, in order to implement and disseminate best practices, the Centers for Medicare & Medicaid Services (CMS) developed Quality Payment Programs that offer incentives to health care providers to improve the quality of care delivery. This CMS program evaluates providers based on their performance in the Merit-Based Incentive Payment System performance categories.5 These include measures related to patient experience, cost, clinical quality, improvement activities, and the use of certified electronic health record technology. The scores that providers receive are used to determine their performance-based reimbursements under Medicare’s fee-for-service program.
The concept of health care quality is also applicable in other countries. In the United Kingdom, QI initiatives are led by the Department of Health and Social Care. The National Institute for Health and Care Excellence (NICE) produces guidelines on best practices to ensure that care delivery meets established safety and quality standards, reaching cost-effectiveness excellence.6 In Australia, the Australian Commission on Quality and Safety in Health Care is responsible for setting benchmarks for performance in health care systems through a clear, structured agenda.7 Ultimately, health care quality is a complex and multifaceted issue that requires a comprehensive approach to ensure the best outcomes for patients. With the implementation of measures such as the CMS Quality Payment Programs and NICE guidelines, health care organizations can take steps to ensure their systems of care delivery reflect evidence-based practices and demonstrate a commitment to providing high-quality care.
Implementing a health care QI plan that encompasses the 4 key attributes of health care quality—effectiveness, safety, culture of continuous improvement, and desired outcomes—requires collaboration among different departments and stakeholders and a data-driven approach to decision-making. Effective communication with patients and their families is critical to ensuring that their needs are being met and that they are active partners in their health care journey. While a health care QI plan is essential for delivering high-quality, safe patient care, it also helps health care organizations comply with regulatory requirements, meet accreditation standards, and stay competitive in the ever-evolving health care landscape.
Corresponding author: Ebrahim Barkoudah, MD, MPH; Ebrahim.Barkoudah@baystatehealth.org
1. World Health Organization. Quality of care. Accessed on May 17, 2023. www.who.int/health-topics/quality-of-care#tab=tab_1
2. World Health Organization. Patient safety. Accessed on May 17, 2023 www.who.int/news-room/fact-sheets/detail/patient-safety
3. Agency for Healthcare Research and Quality. Understanding quality measurement. Accessed on May 17, 2023. www.ahrq.gov/patient-safety/quality-resources/tools/chtoolbx/understand/index.html
4. Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33(6):737-744. doi:10.1016/j.jpainsymman.2007.02.024
5. U.S Centers for Medicare & Medicaid Services. Quality payment program. Accessed on March 14, 2023 qpp.cms.gov/mips/overview
6. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1-503, v-vi. doi: 10.3310/hta19140
7. Braithwaite J, Healy J, Dwan K. The Governance of Health Safety and Quality, Commonwealth of Australia. Accessed May 17, 2023. https://regnet.anu.edu.au/research/publications/3626/governance-health-safety-and-quality 2005
1. World Health Organization. Quality of care. Accessed on May 17, 2023. www.who.int/health-topics/quality-of-care#tab=tab_1
2. World Health Organization. Patient safety. Accessed on May 17, 2023 www.who.int/news-room/fact-sheets/detail/patient-safety
3. Agency for Healthcare Research and Quality. Understanding quality measurement. Accessed on May 17, 2023. www.ahrq.gov/patient-safety/quality-resources/tools/chtoolbx/understand/index.html
4. Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33(6):737-744. doi:10.1016/j.jpainsymman.2007.02.024
5. U.S Centers for Medicare & Medicaid Services. Quality payment program. Accessed on March 14, 2023 qpp.cms.gov/mips/overview
6. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1-503, v-vi. doi: 10.3310/hta19140
7. Braithwaite J, Healy J, Dwan K. The Governance of Health Safety and Quality, Commonwealth of Australia. Accessed May 17, 2023. https://regnet.anu.edu.au/research/publications/3626/governance-health-safety-and-quality 2005
The Mission of Continuous Improvement in Health Care: A New Era for Clinical Outcomes Management
This issue of the Journal of Clinical Outcomes (JCOM) debuts a new cover design that brings forward the articles and features in each issue. Although the Journal’s cover has a new look, JCOM’s goals remain the same—improving care by disseminating evidence of quality improvement in health care and sharing access to the medical literature with our readers. We continue our mission to promote the best medical practice by providing clinicians with updates and communicating advances that lead to measurable improvement in health care delivery, quality, and outcomes.
As we continue the work of improving health care quality, knowledge gaps and unmet needs in the literature remain. These unmet needs are evident throughout all phases of health care delivery. Moreover, the Institutes of Medicine report that centered on efforts to build a safer health care environment by redesigning health care processes remains salient.1 The journey to continuous improvement in health care, where we achieve threshold change in the quality of each process and across the entire health care system, requires collective effort. Such efforts include establishing clear metrics and measurements for improvement goals throughout the patient’s journey through diagnosis, treatment, transitions of care, and disease management.2,3 To address evidence and knowledge gaps in the literature, JCOM publishes reports of original studies and quality improvement projects as well as reviews, providing its 30,000 readers with new evidence to implement in daily practice. We welcome submissions of original research reports, reports of quality improvement projects that follow the SQUIRE 2.0 standards,4 and perspectives on developments and innovations in health care delivery.
The next chapter in health care delivery improvement will encompass value-based care.5 This new era of clinical outcomes management will dictate the metrics and outcomes reporting6 and how to plan future investments. The value-based phase will increase innovation and shape policies that advance population health, transforming every step in the care delivery journey.7 The next phase in health care delivery will also create a viable financial structure while implementing effective performance measures for optimal outcomes through patient-centered care and optimization of cost and care strategies. In light of health care’s evolution toward a value-based model, JCOM welcomes submissions of manuscripts that explore themes central to this model, including patient-centered care, implementation of best practices, system design, safety, cost-effectiveness, and the balance between cost optimization and quality. For JCOM’s authors and readers, our editorial team remains commited to the highest standards in timely publishing to support our community through our collective expertise and dedication to quality improvement.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Washington (DC): National Academies Press (US); 2000.
2. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-10. doi:10.1136/bmjqs-2014-003675
3. Bates DW. Preventing medication errors: a summary. Am J Health Syst Pharm. 2007;64(14 Suppl 9):S3-9. doi:10.2146/ajhp070190
4. Revised Standards for Quality Improvement Reporting Excellence. SQUIRE 2.0. Accessed July 25, 2022. http://squire-statement.org
5. Gray M. Value based healthcare. BMJ. 2017;356:j437. doi:10.1136/bmj.j437
6. What is value-based healthcare? NEJM Catalyst. January 1, 2017. Accessed July 25, 2022. catalyst.nejm.org/doi/full/10.1056/CAT.17.0558
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
This issue of the Journal of Clinical Outcomes (JCOM) debuts a new cover design that brings forward the articles and features in each issue. Although the Journal’s cover has a new look, JCOM’s goals remain the same—improving care by disseminating evidence of quality improvement in health care and sharing access to the medical literature with our readers. We continue our mission to promote the best medical practice by providing clinicians with updates and communicating advances that lead to measurable improvement in health care delivery, quality, and outcomes.
As we continue the work of improving health care quality, knowledge gaps and unmet needs in the literature remain. These unmet needs are evident throughout all phases of health care delivery. Moreover, the Institutes of Medicine report that centered on efforts to build a safer health care environment by redesigning health care processes remains salient.1 The journey to continuous improvement in health care, where we achieve threshold change in the quality of each process and across the entire health care system, requires collective effort. Such efforts include establishing clear metrics and measurements for improvement goals throughout the patient’s journey through diagnosis, treatment, transitions of care, and disease management.2,3 To address evidence and knowledge gaps in the literature, JCOM publishes reports of original studies and quality improvement projects as well as reviews, providing its 30,000 readers with new evidence to implement in daily practice. We welcome submissions of original research reports, reports of quality improvement projects that follow the SQUIRE 2.0 standards,4 and perspectives on developments and innovations in health care delivery.
The next chapter in health care delivery improvement will encompass value-based care.5 This new era of clinical outcomes management will dictate the metrics and outcomes reporting6 and how to plan future investments. The value-based phase will increase innovation and shape policies that advance population health, transforming every step in the care delivery journey.7 The next phase in health care delivery will also create a viable financial structure while implementing effective performance measures for optimal outcomes through patient-centered care and optimization of cost and care strategies. In light of health care’s evolution toward a value-based model, JCOM welcomes submissions of manuscripts that explore themes central to this model, including patient-centered care, implementation of best practices, system design, safety, cost-effectiveness, and the balance between cost optimization and quality. For JCOM’s authors and readers, our editorial team remains commited to the highest standards in timely publishing to support our community through our collective expertise and dedication to quality improvement.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
This issue of the Journal of Clinical Outcomes (JCOM) debuts a new cover design that brings forward the articles and features in each issue. Although the Journal’s cover has a new look, JCOM’s goals remain the same—improving care by disseminating evidence of quality improvement in health care and sharing access to the medical literature with our readers. We continue our mission to promote the best medical practice by providing clinicians with updates and communicating advances that lead to measurable improvement in health care delivery, quality, and outcomes.
As we continue the work of improving health care quality, knowledge gaps and unmet needs in the literature remain. These unmet needs are evident throughout all phases of health care delivery. Moreover, the Institutes of Medicine report that centered on efforts to build a safer health care environment by redesigning health care processes remains salient.1 The journey to continuous improvement in health care, where we achieve threshold change in the quality of each process and across the entire health care system, requires collective effort. Such efforts include establishing clear metrics and measurements for improvement goals throughout the patient’s journey through diagnosis, treatment, transitions of care, and disease management.2,3 To address evidence and knowledge gaps in the literature, JCOM publishes reports of original studies and quality improvement projects as well as reviews, providing its 30,000 readers with new evidence to implement in daily practice. We welcome submissions of original research reports, reports of quality improvement projects that follow the SQUIRE 2.0 standards,4 and perspectives on developments and innovations in health care delivery.
The next chapter in health care delivery improvement will encompass value-based care.5 This new era of clinical outcomes management will dictate the metrics and outcomes reporting6 and how to plan future investments. The value-based phase will increase innovation and shape policies that advance population health, transforming every step in the care delivery journey.7 The next phase in health care delivery will also create a viable financial structure while implementing effective performance measures for optimal outcomes through patient-centered care and optimization of cost and care strategies. In light of health care’s evolution toward a value-based model, JCOM welcomes submissions of manuscripts that explore themes central to this model, including patient-centered care, implementation of best practices, system design, safety, cost-effectiveness, and the balance between cost optimization and quality. For JCOM’s authors and readers, our editorial team remains commited to the highest standards in timely publishing to support our community through our collective expertise and dedication to quality improvement.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Washington (DC): National Academies Press (US); 2000.
2. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-10. doi:10.1136/bmjqs-2014-003675
3. Bates DW. Preventing medication errors: a summary. Am J Health Syst Pharm. 2007;64(14 Suppl 9):S3-9. doi:10.2146/ajhp070190
4. Revised Standards for Quality Improvement Reporting Excellence. SQUIRE 2.0. Accessed July 25, 2022. http://squire-statement.org
5. Gray M. Value based healthcare. BMJ. 2017;356:j437. doi:10.1136/bmj.j437
6. What is value-based healthcare? NEJM Catalyst. January 1, 2017. Accessed July 25, 2022. catalyst.nejm.org/doi/full/10.1056/CAT.17.0558
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Washington (DC): National Academies Press (US); 2000.
2. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-10. doi:10.1136/bmjqs-2014-003675
3. Bates DW. Preventing medication errors: a summary. Am J Health Syst Pharm. 2007;64(14 Suppl 9):S3-9. doi:10.2146/ajhp070190
4. Revised Standards for Quality Improvement Reporting Excellence. SQUIRE 2.0. Accessed July 25, 2022. http://squire-statement.org
5. Gray M. Value based healthcare. BMJ. 2017;356:j437. doi:10.1136/bmj.j437
6. What is value-based healthcare? NEJM Catalyst. January 1, 2017. Accessed July 25, 2022. catalyst.nejm.org/doi/full/10.1056/CAT.17.0558
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
The Intersection of Clinical Quality Improvement Research and Implementation Science
The Institute of Medicine brought much-needed attention to the need for process improvement in medicine with its seminal report To Err Is Human: Building a Safer Health System, which was issued in 1999, leading to the quality movement’s call to close health care performance gaps in Crossing the Quality Chasm: A New Health System for the 21st Century.1,2 Quality improvement science in medicine has evolved over the past 2 decades to include a broad spectrum of approaches, from agile improvement to continuous learning and improvement. Current efforts focus on Lean-based process improvement along with a reduction in variation in clinical practice to align practice with the principles of evidence-based medicine in a patient-centered approach.3 Further, the definition of quality improvement under the Affordable Care Act was framed as an equitable, timely, value-based, patient-centered approach to achieving population-level health goals.4 Thus, the science of quality improvement drives the core principles of care delivery improvement, and the rigorous evidence needed to expand innovation is embedded within the same framework.5,6 In clinical practice, quality improvement projects aim to define gaps and then specific steps are undertaken to improve the evidence-based practice of a specific process. The overarching goal is to enhance the efficacy of the practice by reducing waste within a particular domain. Thus, quality improvement and implementation research eventually unify how clinical practice is advanced concurrently to bridge identified gaps.7
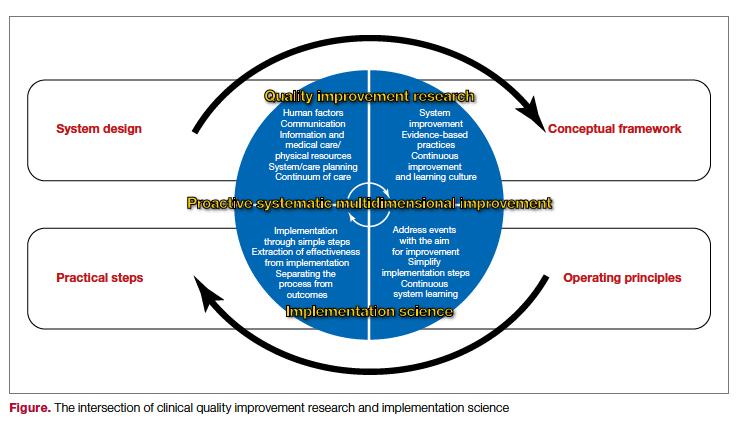
System redesign through a patient-centered framework forms the core of an overarching strategy to support system-level processes. Both require a deep understanding of the fields of quality improvement science and implementation science.8 Furthermore, aligning clinical research needs, system aims, patients’ values, and clinical care give the new design a clear path forward. Patient-centered improvement includes the essential elements of system redesign around human factors, including communication, physical resources, and updated information during episodes of care. The patient-centered improvement design is juxtaposed with care planning and establishing continuum of care processes.9 It is essential to note that safety is rooted within the quality domain as a top priority in medicine.10 The best implementation methods and approaches are discussed and debated, and the improvement progress continues on multiple fronts.11 Patient safety systems are implemented simultaneously during the redesign phase. Moreover, identifying and testing the health care delivery methods in the era of competing strategic priorities to achieve the desirable clinical outcomes highlights the importance of implementation, while contemplating the methods of dissemination, scalability, and sustainability of the best evidence-based clinical practice.
The cycle of quality improvement research completes the system implementation efforts. The conceptual framework of quality improvement includes multiple areas of care and transition, along with applying the best clinical practices in a culture that emphasizes continuous improvement and learning. At the same time, the operating principles should include continuous improvement in a simple and continuous system of learning as a core concept. Our proposed implementation approach involves taking simple and practical steps while separating the process from the outcomes measures, extracting effectiveness throughout the process. It is essential to keep in mind that building a proactive and systematic improvement environment requires a framework for safety, reliability, and effective care, as well as the alignment of the physical system, communication, and professional environment and culture (Figure).
In summary, system design for quality improvement research should incorporate the principles and conceptual framework that embody effective implementation strategies, with a focus on operational and practical steps. Continuous improvement will be reached through the multidimensional development of current health care system metrics and the incorporation of implementation science methods.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington (DC): National Academies Press (US); 2000.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001.
3. Berwick DM. The science of improvement. JAMA. 2008;299(10):1182-1184. doi:10.1001/jama.299.10.1182
4. Mazurenko O, Balio CP, Agarwal R, Carroll AE, Menachemi N. The effects of Medicaid expansion under the ACA: a systematic review. Health Affairs. 2018;37(6):944-950. doi: 10.1377/hlthaff.2017.1491
5. Fan E, Needham DM. The science of quality improvement. JAMA. 2008;300(4):390-391. doi:10.1001/jama.300.4.390-b
6. Alexander JA, Hearld LR. The science of quality improvement implementation: developing capacity to make a difference. Med Care. 2011:S6-20. doi:10.1097/MLR.0b013e3181e1709c
7. Rohweder C, Wangen M, Black M, et al. Understanding quality improvement collaboratives through an implementation science lens. Prev Med. 2019;129:105859. doi: 10.1016/j.ypmed.2019.105859
8. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296(23):2848-2851. doi:10.1001/jama.296.23.2848
9. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1(Suppl 1):i85-90. doi:10.1136/qhc.13.suppl_1.i85
10. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507. doi:10.1001/jama.288.4.501
11. Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608-613. doi:10.1056/NEJMsb070738
The Institute of Medicine brought much-needed attention to the need for process improvement in medicine with its seminal report To Err Is Human: Building a Safer Health System, which was issued in 1999, leading to the quality movement’s call to close health care performance gaps in Crossing the Quality Chasm: A New Health System for the 21st Century.1,2 Quality improvement science in medicine has evolved over the past 2 decades to include a broad spectrum of approaches, from agile improvement to continuous learning and improvement. Current efforts focus on Lean-based process improvement along with a reduction in variation in clinical practice to align practice with the principles of evidence-based medicine in a patient-centered approach.3 Further, the definition of quality improvement under the Affordable Care Act was framed as an equitable, timely, value-based, patient-centered approach to achieving population-level health goals.4 Thus, the science of quality improvement drives the core principles of care delivery improvement, and the rigorous evidence needed to expand innovation is embedded within the same framework.5,6 In clinical practice, quality improvement projects aim to define gaps and then specific steps are undertaken to improve the evidence-based practice of a specific process. The overarching goal is to enhance the efficacy of the practice by reducing waste within a particular domain. Thus, quality improvement and implementation research eventually unify how clinical practice is advanced concurrently to bridge identified gaps.7
System redesign through a patient-centered framework forms the core of an overarching strategy to support system-level processes. Both require a deep understanding of the fields of quality improvement science and implementation science.8 Furthermore, aligning clinical research needs, system aims, patients’ values, and clinical care give the new design a clear path forward. Patient-centered improvement includes the essential elements of system redesign around human factors, including communication, physical resources, and updated information during episodes of care. The patient-centered improvement design is juxtaposed with care planning and establishing continuum of care processes.9 It is essential to note that safety is rooted within the quality domain as a top priority in medicine.10 The best implementation methods and approaches are discussed and debated, and the improvement progress continues on multiple fronts.11 Patient safety systems are implemented simultaneously during the redesign phase. Moreover, identifying and testing the health care delivery methods in the era of competing strategic priorities to achieve the desirable clinical outcomes highlights the importance of implementation, while contemplating the methods of dissemination, scalability, and sustainability of the best evidence-based clinical practice.
The cycle of quality improvement research completes the system implementation efforts. The conceptual framework of quality improvement includes multiple areas of care and transition, along with applying the best clinical practices in a culture that emphasizes continuous improvement and learning. At the same time, the operating principles should include continuous improvement in a simple and continuous system of learning as a core concept. Our proposed implementation approach involves taking simple and practical steps while separating the process from the outcomes measures, extracting effectiveness throughout the process. It is essential to keep in mind that building a proactive and systematic improvement environment requires a framework for safety, reliability, and effective care, as well as the alignment of the physical system, communication, and professional environment and culture (Figure).
In summary, system design for quality improvement research should incorporate the principles and conceptual framework that embody effective implementation strategies, with a focus on operational and practical steps. Continuous improvement will be reached through the multidimensional development of current health care system metrics and the incorporation of implementation science methods.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
The Institute of Medicine brought much-needed attention to the need for process improvement in medicine with its seminal report To Err Is Human: Building a Safer Health System, which was issued in 1999, leading to the quality movement’s call to close health care performance gaps in Crossing the Quality Chasm: A New Health System for the 21st Century.1,2 Quality improvement science in medicine has evolved over the past 2 decades to include a broad spectrum of approaches, from agile improvement to continuous learning and improvement. Current efforts focus on Lean-based process improvement along with a reduction in variation in clinical practice to align practice with the principles of evidence-based medicine in a patient-centered approach.3 Further, the definition of quality improvement under the Affordable Care Act was framed as an equitable, timely, value-based, patient-centered approach to achieving population-level health goals.4 Thus, the science of quality improvement drives the core principles of care delivery improvement, and the rigorous evidence needed to expand innovation is embedded within the same framework.5,6 In clinical practice, quality improvement projects aim to define gaps and then specific steps are undertaken to improve the evidence-based practice of a specific process. The overarching goal is to enhance the efficacy of the practice by reducing waste within a particular domain. Thus, quality improvement and implementation research eventually unify how clinical practice is advanced concurrently to bridge identified gaps.7
System redesign through a patient-centered framework forms the core of an overarching strategy to support system-level processes. Both require a deep understanding of the fields of quality improvement science and implementation science.8 Furthermore, aligning clinical research needs, system aims, patients’ values, and clinical care give the new design a clear path forward. Patient-centered improvement includes the essential elements of system redesign around human factors, including communication, physical resources, and updated information during episodes of care. The patient-centered improvement design is juxtaposed with care planning and establishing continuum of care processes.9 It is essential to note that safety is rooted within the quality domain as a top priority in medicine.10 The best implementation methods and approaches are discussed and debated, and the improvement progress continues on multiple fronts.11 Patient safety systems are implemented simultaneously during the redesign phase. Moreover, identifying and testing the health care delivery methods in the era of competing strategic priorities to achieve the desirable clinical outcomes highlights the importance of implementation, while contemplating the methods of dissemination, scalability, and sustainability of the best evidence-based clinical practice.
The cycle of quality improvement research completes the system implementation efforts. The conceptual framework of quality improvement includes multiple areas of care and transition, along with applying the best clinical practices in a culture that emphasizes continuous improvement and learning. At the same time, the operating principles should include continuous improvement in a simple and continuous system of learning as a core concept. Our proposed implementation approach involves taking simple and practical steps while separating the process from the outcomes measures, extracting effectiveness throughout the process. It is essential to keep in mind that building a proactive and systematic improvement environment requires a framework for safety, reliability, and effective care, as well as the alignment of the physical system, communication, and professional environment and culture (Figure).
In summary, system design for quality improvement research should incorporate the principles and conceptual framework that embody effective implementation strategies, with a focus on operational and practical steps. Continuous improvement will be reached through the multidimensional development of current health care system metrics and the incorporation of implementation science methods.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington (DC): National Academies Press (US); 2000.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001.
3. Berwick DM. The science of improvement. JAMA. 2008;299(10):1182-1184. doi:10.1001/jama.299.10.1182
4. Mazurenko O, Balio CP, Agarwal R, Carroll AE, Menachemi N. The effects of Medicaid expansion under the ACA: a systematic review. Health Affairs. 2018;37(6):944-950. doi: 10.1377/hlthaff.2017.1491
5. Fan E, Needham DM. The science of quality improvement. JAMA. 2008;300(4):390-391. doi:10.1001/jama.300.4.390-b
6. Alexander JA, Hearld LR. The science of quality improvement implementation: developing capacity to make a difference. Med Care. 2011:S6-20. doi:10.1097/MLR.0b013e3181e1709c
7. Rohweder C, Wangen M, Black M, et al. Understanding quality improvement collaboratives through an implementation science lens. Prev Med. 2019;129:105859. doi: 10.1016/j.ypmed.2019.105859
8. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296(23):2848-2851. doi:10.1001/jama.296.23.2848
9. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1(Suppl 1):i85-90. doi:10.1136/qhc.13.suppl_1.i85
10. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507. doi:10.1001/jama.288.4.501
11. Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608-613. doi:10.1056/NEJMsb070738
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington (DC): National Academies Press (US); 2000.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001.
3. Berwick DM. The science of improvement. JAMA. 2008;299(10):1182-1184. doi:10.1001/jama.299.10.1182
4. Mazurenko O, Balio CP, Agarwal R, Carroll AE, Menachemi N. The effects of Medicaid expansion under the ACA: a systematic review. Health Affairs. 2018;37(6):944-950. doi: 10.1377/hlthaff.2017.1491
5. Fan E, Needham DM. The science of quality improvement. JAMA. 2008;300(4):390-391. doi:10.1001/jama.300.4.390-b
6. Alexander JA, Hearld LR. The science of quality improvement implementation: developing capacity to make a difference. Med Care. 2011:S6-20. doi:10.1097/MLR.0b013e3181e1709c
7. Rohweder C, Wangen M, Black M, et al. Understanding quality improvement collaboratives through an implementation science lens. Prev Med. 2019;129:105859. doi: 10.1016/j.ypmed.2019.105859
8. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296(23):2848-2851. doi:10.1001/jama.296.23.2848
9. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1(Suppl 1):i85-90. doi:10.1136/qhc.13.suppl_1.i85
10. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507. doi:10.1001/jama.288.4.501
11. Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608-613. doi:10.1056/NEJMsb070738
Aiming for System Improvement While Transitioning to the New Normal
As we transition out of the Omicron surge, the lessons we’ve learned from the prior surges carry forward and add to our knowledge foundation. Medical journals have published numerous research and perspectives manuscripts on all aspects of COVID-19 over the past 2 years, adding much-needed knowledge to our clinical practice during the pandemic. However, the story does not stop there, as the pandemic has impacted the usual, non-COVID-19 clinical care we provide. The value-based health care delivery model accounts for both COVID-19 clinical care and the usual care we provide our patients every day. Clinicians, administrators, and health care workers will need to know how to balance both worlds in the years to come.
In this issue of JCOM, the work of balancing the demands of COVID-19 care with those of system improvement continues. Two original research articles address the former, with Liesching et al1 reporting data on improving clinical outcomes of patients with COVID-19 through acute care oxygen therapies, and Ali et al2 explaining the impact of COVID-19 on STEMI care delivery models. Liesching et al’s study showed that patients admitted for COVID-19 after the first surge were more likely to receive high-flow nasal cannula and had better outcomes, while Ali et al showed that patients with STEMI yet again experienced worse outcomes during the first wave.
On the system improvement front, Cusick et al3 report on a quality improvement (QI) project that addressed acute disease management of heparin-induced thrombocytopenia (HIT) during hospitalization, Sosa et al4 discuss efforts to improve comorbidity capture at their institution, and Uche et al5 present the results of a nonpharmacologic initiative to improve management of chronic pain among veterans. Cusick et al’s QI project showed that a HIT testing strategy could be safely implemented through an evidence-based process to nudge resource utilization using specific management pathways. While capturing and measuring the complexity of diseases and comorbidities can be challenging, accurate capture is essential, as patient acuity has implications for reimbursement and quality comparisons for hospitals and physicians; Sosa et al describe a series of initiatives implemented at their institution that improved comorbidity capture. Furthermore, Uche et al report on a 10-week complementary and integrative health program for veterans with noncancer chronic pain that reduced pain intensity and improved quality of life for its participants. These QI reports show that, though the health care landscape has changed over the past 2 years, the aim remains the same: to provide the best care for patients regardless of the diagnosis, location, or time.
Conducting QI projects during the COVID-19 pandemic has been difficult, especially in terms of implementing consistent processes and management pathways while contending with staff and supply shortages. The pandemic, however, has highlighted the importance of continuing QI efforts, specifically around infectious disease prevention and good clinical practices. Moreover, the recent continuous learning and implementation around COVID-19 patient care has been a significant achievement, as clinicians and administrators worked continuously to understand and improve processes, create a supporting culture, and redesign care delivery on the fly. The management of both COVID-19 care and our usual care QI efforts should incorporate the lessons learned from the pandemic and leverage system redesign for future steps. As we’ve seen, survival in COVID-19 improved dramatically since the beginning of the pandemic, as clinical trials became more adaptive and efficient and system upgrades like telemedicine and digital technologies in the public health response led to major advancements. The work to improve the care provided in the clinic and at the bedside will continue through one collective approach in the new normal.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
1. Liesching TN, Lei Y. Oxygen therapies and clinical outcomes for patients hospitalized with covid-19: first surge vs second surge. J Clin Outcomes Manag. 2022;29(2):58-64. doi:10.12788/jcom.0086
2. Ali SH, Hyer S, Davis K, Murrow JR. Acute STEMI during the COVID-19 pandemic at Piedmont Athens Regional: incidence, clinical characteristics, and outcomes. J Clin Outcomes Manag. 2022;29(2):65-71. doi:10.12788/jcom.0085
3. Cusick A, Hanigan S, Bashaw L, et al. A practical and cost-effective approach to the diagnosis of heparin-induced thrombocytopenia: a single-center quality improvement study. J Clin Outcomes Manag. 2022;29(2):72-77.
4. Sosa MA, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manag. 2022;29(2):80-87. doi:10.12788/jcom.00885. Uche JU, Jamison M, Waugh S. Evaluation of the Empower Veterans Program for military veterans with chronic pain. J Clin Outcomes Manag. 2022;29(2):88-95. doi:10.12788/jcom.0089
As we transition out of the Omicron surge, the lessons we’ve learned from the prior surges carry forward and add to our knowledge foundation. Medical journals have published numerous research and perspectives manuscripts on all aspects of COVID-19 over the past 2 years, adding much-needed knowledge to our clinical practice during the pandemic. However, the story does not stop there, as the pandemic has impacted the usual, non-COVID-19 clinical care we provide. The value-based health care delivery model accounts for both COVID-19 clinical care and the usual care we provide our patients every day. Clinicians, administrators, and health care workers will need to know how to balance both worlds in the years to come.
In this issue of JCOM, the work of balancing the demands of COVID-19 care with those of system improvement continues. Two original research articles address the former, with Liesching et al1 reporting data on improving clinical outcomes of patients with COVID-19 through acute care oxygen therapies, and Ali et al2 explaining the impact of COVID-19 on STEMI care delivery models. Liesching et al’s study showed that patients admitted for COVID-19 after the first surge were more likely to receive high-flow nasal cannula and had better outcomes, while Ali et al showed that patients with STEMI yet again experienced worse outcomes during the first wave.
On the system improvement front, Cusick et al3 report on a quality improvement (QI) project that addressed acute disease management of heparin-induced thrombocytopenia (HIT) during hospitalization, Sosa et al4 discuss efforts to improve comorbidity capture at their institution, and Uche et al5 present the results of a nonpharmacologic initiative to improve management of chronic pain among veterans. Cusick et al’s QI project showed that a HIT testing strategy could be safely implemented through an evidence-based process to nudge resource utilization using specific management pathways. While capturing and measuring the complexity of diseases and comorbidities can be challenging, accurate capture is essential, as patient acuity has implications for reimbursement and quality comparisons for hospitals and physicians; Sosa et al describe a series of initiatives implemented at their institution that improved comorbidity capture. Furthermore, Uche et al report on a 10-week complementary and integrative health program for veterans with noncancer chronic pain that reduced pain intensity and improved quality of life for its participants. These QI reports show that, though the health care landscape has changed over the past 2 years, the aim remains the same: to provide the best care for patients regardless of the diagnosis, location, or time.
Conducting QI projects during the COVID-19 pandemic has been difficult, especially in terms of implementing consistent processes and management pathways while contending with staff and supply shortages. The pandemic, however, has highlighted the importance of continuing QI efforts, specifically around infectious disease prevention and good clinical practices. Moreover, the recent continuous learning and implementation around COVID-19 patient care has been a significant achievement, as clinicians and administrators worked continuously to understand and improve processes, create a supporting culture, and redesign care delivery on the fly. The management of both COVID-19 care and our usual care QI efforts should incorporate the lessons learned from the pandemic and leverage system redesign for future steps. As we’ve seen, survival in COVID-19 improved dramatically since the beginning of the pandemic, as clinical trials became more adaptive and efficient and system upgrades like telemedicine and digital technologies in the public health response led to major advancements. The work to improve the care provided in the clinic and at the bedside will continue through one collective approach in the new normal.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
As we transition out of the Omicron surge, the lessons we’ve learned from the prior surges carry forward and add to our knowledge foundation. Medical journals have published numerous research and perspectives manuscripts on all aspects of COVID-19 over the past 2 years, adding much-needed knowledge to our clinical practice during the pandemic. However, the story does not stop there, as the pandemic has impacted the usual, non-COVID-19 clinical care we provide. The value-based health care delivery model accounts for both COVID-19 clinical care and the usual care we provide our patients every day. Clinicians, administrators, and health care workers will need to know how to balance both worlds in the years to come.
In this issue of JCOM, the work of balancing the demands of COVID-19 care with those of system improvement continues. Two original research articles address the former, with Liesching et al1 reporting data on improving clinical outcomes of patients with COVID-19 through acute care oxygen therapies, and Ali et al2 explaining the impact of COVID-19 on STEMI care delivery models. Liesching et al’s study showed that patients admitted for COVID-19 after the first surge were more likely to receive high-flow nasal cannula and had better outcomes, while Ali et al showed that patients with STEMI yet again experienced worse outcomes during the first wave.
On the system improvement front, Cusick et al3 report on a quality improvement (QI) project that addressed acute disease management of heparin-induced thrombocytopenia (HIT) during hospitalization, Sosa et al4 discuss efforts to improve comorbidity capture at their institution, and Uche et al5 present the results of a nonpharmacologic initiative to improve management of chronic pain among veterans. Cusick et al’s QI project showed that a HIT testing strategy could be safely implemented through an evidence-based process to nudge resource utilization using specific management pathways. While capturing and measuring the complexity of diseases and comorbidities can be challenging, accurate capture is essential, as patient acuity has implications for reimbursement and quality comparisons for hospitals and physicians; Sosa et al describe a series of initiatives implemented at their institution that improved comorbidity capture. Furthermore, Uche et al report on a 10-week complementary and integrative health program for veterans with noncancer chronic pain that reduced pain intensity and improved quality of life for its participants. These QI reports show that, though the health care landscape has changed over the past 2 years, the aim remains the same: to provide the best care for patients regardless of the diagnosis, location, or time.
Conducting QI projects during the COVID-19 pandemic has been difficult, especially in terms of implementing consistent processes and management pathways while contending with staff and supply shortages. The pandemic, however, has highlighted the importance of continuing QI efforts, specifically around infectious disease prevention and good clinical practices. Moreover, the recent continuous learning and implementation around COVID-19 patient care has been a significant achievement, as clinicians and administrators worked continuously to understand and improve processes, create a supporting culture, and redesign care delivery on the fly. The management of both COVID-19 care and our usual care QI efforts should incorporate the lessons learned from the pandemic and leverage system redesign for future steps. As we’ve seen, survival in COVID-19 improved dramatically since the beginning of the pandemic, as clinical trials became more adaptive and efficient and system upgrades like telemedicine and digital technologies in the public health response led to major advancements. The work to improve the care provided in the clinic and at the bedside will continue through one collective approach in the new normal.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
1. Liesching TN, Lei Y. Oxygen therapies and clinical outcomes for patients hospitalized with covid-19: first surge vs second surge. J Clin Outcomes Manag. 2022;29(2):58-64. doi:10.12788/jcom.0086
2. Ali SH, Hyer S, Davis K, Murrow JR. Acute STEMI during the COVID-19 pandemic at Piedmont Athens Regional: incidence, clinical characteristics, and outcomes. J Clin Outcomes Manag. 2022;29(2):65-71. doi:10.12788/jcom.0085
3. Cusick A, Hanigan S, Bashaw L, et al. A practical and cost-effective approach to the diagnosis of heparin-induced thrombocytopenia: a single-center quality improvement study. J Clin Outcomes Manag. 2022;29(2):72-77.
4. Sosa MA, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manag. 2022;29(2):80-87. doi:10.12788/jcom.00885. Uche JU, Jamison M, Waugh S. Evaluation of the Empower Veterans Program for military veterans with chronic pain. J Clin Outcomes Manag. 2022;29(2):88-95. doi:10.12788/jcom.0089
1. Liesching TN, Lei Y. Oxygen therapies and clinical outcomes for patients hospitalized with covid-19: first surge vs second surge. J Clin Outcomes Manag. 2022;29(2):58-64. doi:10.12788/jcom.0086
2. Ali SH, Hyer S, Davis K, Murrow JR. Acute STEMI during the COVID-19 pandemic at Piedmont Athens Regional: incidence, clinical characteristics, and outcomes. J Clin Outcomes Manag. 2022;29(2):65-71. doi:10.12788/jcom.0085
3. Cusick A, Hanigan S, Bashaw L, et al. A practical and cost-effective approach to the diagnosis of heparin-induced thrombocytopenia: a single-center quality improvement study. J Clin Outcomes Manag. 2022;29(2):72-77.
4. Sosa MA, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manag. 2022;29(2):80-87. doi:10.12788/jcom.00885. Uche JU, Jamison M, Waugh S. Evaluation of the Empower Veterans Program for military veterans with chronic pain. J Clin Outcomes Manag. 2022;29(2):88-95. doi:10.12788/jcom.0089
Simulation-Based Training in Medical Education: Immediate Growth or Cautious Optimism?
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
Centers for Medicare & Medicaid Services Price Publication Requirement: If You Post It, Will They Come?
Patients in the United States continue to experience rising out-of-pocket medical costs, with little access to the price information they desire when making decisions regarding medical care.1 The Centers for Medicare & Medicaid Services (CMS) has taken steps toward transparency by requiring hospitals to publish price information.2 In this issue of the Journal of Hospital Medicine, White and Liao3 break down the new rule, and we further discuss how this policy affects patients, hospitals, and hospitalists.
The new CMS rule requires hospitals to publish the prices of 300 “shoppable” services, including those negotiated with different payors. The rule standardizes how this information is displayed and accessed, with a daily penalty for facilities that fail to comply. Clinics and ambulatory surgical centers are currently excluded, as are facility and ancillary fees, such as those billed by pathology or anesthesiology. As White and Liao point out, a limitation for hospitalists is that this rule will only affect orders for the outpatient setting at discharge. In addition, this rule separates cost from quality. Although quality data are publicly available via CMS, price data are posted directly by hospitals, making a true value assessment difficult. To strengthen the rule, White and Liao recommend the following: increasing the financial penalty for noncompliance; aggregating data centrally to allow for comparisons; adding quality data to cost; expanding included sites and types of services; and adding common additional fees to the service price.
The larger question is whether patients will use these data in the manner intended. Previous studies have found a paradoxical relationship between patients’ expressed desire to compare prices for medical services vs documented low levels of price-shopping behavior. Mehrotra et al1 found that lack of access to data as well as loyalty to providers were significant barriers to using price data effectively. The CMS rule increases access to the price information patients desire but cannot find. However, it is unclear whether available prices will be sufficient to change behaviors given that, aside from those with no insurance and those with high-deductible plans, most patients are fairly removed from the actual cost of service.
This rule may have a larger, unexpected impact on hospitals and access to care. Sharing price data could increase pressure on facilities to merge with larger systems in order to obtain more favorable rates via increased negotiating power. Hospitals that serve poorer communities may not be attractive merger candidates for large systems and could be left out of the push toward consolidation. Charging higher prices for the same services could lead to hospital closures or cuts in resources, potentially exacerbating health inequities for underserved populations.
On the provider end, it is unlikely that price transparency will influence resource utilization. Mummadi et al4 found that displaying price information in the electronic health record did not significantly influence physician ordering behavior. For hospitalists today, the emphasis on “high-value care” is already an important consideration when utilizing healthcare resources, considering the Accreditation Council for Graduate Medical Education (ACGME) requirements for residency, restrictive insurance protocols, and guidelines such as the ACR Appropriateness Criteria and the American Board of Internal Medicine’s Choosing Wisely® campaign. Outside of extremes, separate cost data likely will not make a difference in provider ordering practices.
Although the information from this rule may not cause dramatic practice change, it will allow us to help our patients by providing those interested in price-shopping with data. This policy represents a large step toward a more transparent healthcare system, though it may have limited impact on overall healthcare costs.
1. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
2. Price Transparency Requirements for Hospitals to Make Standard Charges Public. 45 CFR § 180.20 (2019).
3. White AA, Liao JM. Policy in clinical practice: hospital price transparency. J Hosp Med. 2021;16(11):688-690. https://doi.org/10.12788/jhm.3698
4. Mummadi SR, Mishra R. Effectiveness of provider price display in computerized physician order entry (CPOE) on healthcare quality: a systematic review. J Am Med Inform Assoc. 2018;25(9):1228-1239. https://doi.org/10.1093/jamia/ocy076
Patients in the United States continue to experience rising out-of-pocket medical costs, with little access to the price information they desire when making decisions regarding medical care.1 The Centers for Medicare & Medicaid Services (CMS) has taken steps toward transparency by requiring hospitals to publish price information.2 In this issue of the Journal of Hospital Medicine, White and Liao3 break down the new rule, and we further discuss how this policy affects patients, hospitals, and hospitalists.
The new CMS rule requires hospitals to publish the prices of 300 “shoppable” services, including those negotiated with different payors. The rule standardizes how this information is displayed and accessed, with a daily penalty for facilities that fail to comply. Clinics and ambulatory surgical centers are currently excluded, as are facility and ancillary fees, such as those billed by pathology or anesthesiology. As White and Liao point out, a limitation for hospitalists is that this rule will only affect orders for the outpatient setting at discharge. In addition, this rule separates cost from quality. Although quality data are publicly available via CMS, price data are posted directly by hospitals, making a true value assessment difficult. To strengthen the rule, White and Liao recommend the following: increasing the financial penalty for noncompliance; aggregating data centrally to allow for comparisons; adding quality data to cost; expanding included sites and types of services; and adding common additional fees to the service price.
The larger question is whether patients will use these data in the manner intended. Previous studies have found a paradoxical relationship between patients’ expressed desire to compare prices for medical services vs documented low levels of price-shopping behavior. Mehrotra et al1 found that lack of access to data as well as loyalty to providers were significant barriers to using price data effectively. The CMS rule increases access to the price information patients desire but cannot find. However, it is unclear whether available prices will be sufficient to change behaviors given that, aside from those with no insurance and those with high-deductible plans, most patients are fairly removed from the actual cost of service.
This rule may have a larger, unexpected impact on hospitals and access to care. Sharing price data could increase pressure on facilities to merge with larger systems in order to obtain more favorable rates via increased negotiating power. Hospitals that serve poorer communities may not be attractive merger candidates for large systems and could be left out of the push toward consolidation. Charging higher prices for the same services could lead to hospital closures or cuts in resources, potentially exacerbating health inequities for underserved populations.
On the provider end, it is unlikely that price transparency will influence resource utilization. Mummadi et al4 found that displaying price information in the electronic health record did not significantly influence physician ordering behavior. For hospitalists today, the emphasis on “high-value care” is already an important consideration when utilizing healthcare resources, considering the Accreditation Council for Graduate Medical Education (ACGME) requirements for residency, restrictive insurance protocols, and guidelines such as the ACR Appropriateness Criteria and the American Board of Internal Medicine’s Choosing Wisely® campaign. Outside of extremes, separate cost data likely will not make a difference in provider ordering practices.
Although the information from this rule may not cause dramatic practice change, it will allow us to help our patients by providing those interested in price-shopping with data. This policy represents a large step toward a more transparent healthcare system, though it may have limited impact on overall healthcare costs.
Patients in the United States continue to experience rising out-of-pocket medical costs, with little access to the price information they desire when making decisions regarding medical care.1 The Centers for Medicare & Medicaid Services (CMS) has taken steps toward transparency by requiring hospitals to publish price information.2 In this issue of the Journal of Hospital Medicine, White and Liao3 break down the new rule, and we further discuss how this policy affects patients, hospitals, and hospitalists.
The new CMS rule requires hospitals to publish the prices of 300 “shoppable” services, including those negotiated with different payors. The rule standardizes how this information is displayed and accessed, with a daily penalty for facilities that fail to comply. Clinics and ambulatory surgical centers are currently excluded, as are facility and ancillary fees, such as those billed by pathology or anesthesiology. As White and Liao point out, a limitation for hospitalists is that this rule will only affect orders for the outpatient setting at discharge. In addition, this rule separates cost from quality. Although quality data are publicly available via CMS, price data are posted directly by hospitals, making a true value assessment difficult. To strengthen the rule, White and Liao recommend the following: increasing the financial penalty for noncompliance; aggregating data centrally to allow for comparisons; adding quality data to cost; expanding included sites and types of services; and adding common additional fees to the service price.
The larger question is whether patients will use these data in the manner intended. Previous studies have found a paradoxical relationship between patients’ expressed desire to compare prices for medical services vs documented low levels of price-shopping behavior. Mehrotra et al1 found that lack of access to data as well as loyalty to providers were significant barriers to using price data effectively. The CMS rule increases access to the price information patients desire but cannot find. However, it is unclear whether available prices will be sufficient to change behaviors given that, aside from those with no insurance and those with high-deductible plans, most patients are fairly removed from the actual cost of service.
This rule may have a larger, unexpected impact on hospitals and access to care. Sharing price data could increase pressure on facilities to merge with larger systems in order to obtain more favorable rates via increased negotiating power. Hospitals that serve poorer communities may not be attractive merger candidates for large systems and could be left out of the push toward consolidation. Charging higher prices for the same services could lead to hospital closures or cuts in resources, potentially exacerbating health inequities for underserved populations.
On the provider end, it is unlikely that price transparency will influence resource utilization. Mummadi et al4 found that displaying price information in the electronic health record did not significantly influence physician ordering behavior. For hospitalists today, the emphasis on “high-value care” is already an important consideration when utilizing healthcare resources, considering the Accreditation Council for Graduate Medical Education (ACGME) requirements for residency, restrictive insurance protocols, and guidelines such as the ACR Appropriateness Criteria and the American Board of Internal Medicine’s Choosing Wisely® campaign. Outside of extremes, separate cost data likely will not make a difference in provider ordering practices.
Although the information from this rule may not cause dramatic practice change, it will allow us to help our patients by providing those interested in price-shopping with data. This policy represents a large step toward a more transparent healthcare system, though it may have limited impact on overall healthcare costs.
1. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
2. Price Transparency Requirements for Hospitals to Make Standard Charges Public. 45 CFR § 180.20 (2019).
3. White AA, Liao JM. Policy in clinical practice: hospital price transparency. J Hosp Med. 2021;16(11):688-690. https://doi.org/10.12788/jhm.3698
4. Mummadi SR, Mishra R. Effectiveness of provider price display in computerized physician order entry (CPOE) on healthcare quality: a systematic review. J Am Med Inform Assoc. 2018;25(9):1228-1239. https://doi.org/10.1093/jamia/ocy076
1. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
2. Price Transparency Requirements for Hospitals to Make Standard Charges Public. 45 CFR § 180.20 (2019).
3. White AA, Liao JM. Policy in clinical practice: hospital price transparency. J Hosp Med. 2021;16(11):688-690. https://doi.org/10.12788/jhm.3698
4. Mummadi SR, Mishra R. Effectiveness of provider price display in computerized physician order entry (CPOE) on healthcare quality: a systematic review. J Am Med Inform Assoc. 2018;25(9):1228-1239. https://doi.org/10.1093/jamia/ocy076
© 2021 Society of Hospital Medicine
Goal-Concordant Care After Hospitalization for Serious Acute Illness: A Key Opportunity for Hospitalists in Patient-Centered Outcomes
Care concordant with patient goals of care (GOC) is a central component of quality. Communication about GOC is associated with improved quality of life, reduced resource utilization, and optimized end-of-life (EOL) care. Prior literature has focused on outpatient populations, with little knowledge based on preferences elicited from patients hospitalized for serious acute illness.1 The consequent knowledge gap relates to a dimension of practice through which hospitalists can improve patient-centered care by clarifying patient preferences for goal-directed treatments both during and following hospitalization.2 Implementing interventions that optimize shared decision-making through a personalized serious- illness care plan is a high-priority research area.2
In this issue, to estimate how frequently GOC are assessed during hospitalization for serious illness and the concordance between identified goals and postdischarge care, Taylor et al3 retrospectively evaluated a cohort of sepsis survivors through electronic health record (EHR) review. A standardized EHR care alignment tool and a comprehensive EHR assessment demonstrated that only 19% and 40% of patients, respectively, had identifiable GOC documented. Goal-concordant care was subsequently observed among 68% of patients with identified goals, consistent with prior work demonstrating goal-concordance in this range.1 Data on EOL care provided to decedents in an integrated health system notably showed that 89% received goal-concordant treatments.4 This difference may stem from clinicians’ emphasis on goal ascertainment at the EOL, a propensity reflected in the comparative characteristics of patients with goals documented in the current study’s Table.3 Investigators took advantage of unique inpatient and postdischarge clinical information from a sepsis patient sample to provide novel insights into the inadequacy of patient preference assessment and the substantial frequency of goal-discordant care resulting from insufficient attention to GOC.
This study suggests a critical need to improve practices related to identification of GOC in patients hospitalized with serious illness. After adjusting for relevant confounding characteristics, completion of a standardized EHR care alignment tool was strongly associated with receipt of goal-concordant care following discharge.3 Although this tool was only completed in 19% of patients, this finding suggests that elicitation of patient preferences is an under-addressed step in facilitating patient-centered transitions of care. In particular, the low 39% rate of goal-concordant care among patients prioritizing comfort over longevity is noteworthy, but consistent with prior literature.1 This degree of discordance highlights provision of goal-concordant care following hospitalization as a key, yet unfulfilled, patient-centered-care quality metric.
The identified shortcomings in communication and care represent an important opportunity for hospitalists to enhance the extent to which survivors of critical illness receive care respectful of their preferences and values. Given the importance of effective discharge handoff practices in hospital medicine,2 future work should address assertively incorporating GOC into transitions after serious acute illness. Enhancing communication of these goals at discharge may benefit patients at high risk of readmission and other postdischarge adverse events, particularly for patients with comfort-focused GOC.
The study is limited in its derivation from trial participants with a specific clinical syndrome in a single health system. Also, investigators’ classification of a single patient goal does not reflect the multifactorial objectives of health interventions. In addition, since patient-reported GOC discussions correlate more highly with goal-concordant care than those identified through EHRs,5 future work should ascertain the generalizability of the identified gaps in practice.
The findings of this study underscore the need for clinicians to promote GOC assessment and documentation during hospitalization for high-risk conditions, such as sepsis. Tracking rates of GOC elicitation and goal-concordant care following discharge should be incorporated into quality measurement systems as important patient-centered dimensions of care. Hospitalists can fill a critical void by helping to correct the deficiencies that exist in respecting the preferences of survivors of serious acute illness.
1. Modes ME, Heckbert SR, Engelberg RA, Nielsen EL, Curtis JR, Kross EK. Patient-reported receipt of goal-concordant care among seriously ill outpatients-prevalence and associated factors. J Pain Symptom Manage. 2020;60(4):765-773. https://doi.org/10.1016/j.jpainsymman.2020.04.026
2. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
3. Taylor SP, Kowalkowski MA, Courtright KR, et al. Deficits in identification of goals and goal-concordant care after sepsis hospitalization. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3714
4. Glass DP, Wang SE, Minardi PM, Kanter MH. Concordance of end-of-life care with end-of-life wishes in an integrated health care system. JAMA Netw Open. 2021;4(4):e213053. https://doi.org/10.1001/jamanetworkopen.2021.3053
5. Modes ME, Engelberg RA, Downey L, Nielsen EL, Curtis JR, Kross EK. Did a goals-of-care discussion happen? Differences in the occurrence of goals-of-care discussions as reported by patients, clinicians, and in the electronic health record. J Pain Symptom Manage. 2019;57(2):251-259. https://doi.org/10.1016/j.jpainsymman.2018.10.507
Care concordant with patient goals of care (GOC) is a central component of quality. Communication about GOC is associated with improved quality of life, reduced resource utilization, and optimized end-of-life (EOL) care. Prior literature has focused on outpatient populations, with little knowledge based on preferences elicited from patients hospitalized for serious acute illness.1 The consequent knowledge gap relates to a dimension of practice through which hospitalists can improve patient-centered care by clarifying patient preferences for goal-directed treatments both during and following hospitalization.2 Implementing interventions that optimize shared decision-making through a personalized serious- illness care plan is a high-priority research area.2
In this issue, to estimate how frequently GOC are assessed during hospitalization for serious illness and the concordance between identified goals and postdischarge care, Taylor et al3 retrospectively evaluated a cohort of sepsis survivors through electronic health record (EHR) review. A standardized EHR care alignment tool and a comprehensive EHR assessment demonstrated that only 19% and 40% of patients, respectively, had identifiable GOC documented. Goal-concordant care was subsequently observed among 68% of patients with identified goals, consistent with prior work demonstrating goal-concordance in this range.1 Data on EOL care provided to decedents in an integrated health system notably showed that 89% received goal-concordant treatments.4 This difference may stem from clinicians’ emphasis on goal ascertainment at the EOL, a propensity reflected in the comparative characteristics of patients with goals documented in the current study’s Table.3 Investigators took advantage of unique inpatient and postdischarge clinical information from a sepsis patient sample to provide novel insights into the inadequacy of patient preference assessment and the substantial frequency of goal-discordant care resulting from insufficient attention to GOC.
This study suggests a critical need to improve practices related to identification of GOC in patients hospitalized with serious illness. After adjusting for relevant confounding characteristics, completion of a standardized EHR care alignment tool was strongly associated with receipt of goal-concordant care following discharge.3 Although this tool was only completed in 19% of patients, this finding suggests that elicitation of patient preferences is an under-addressed step in facilitating patient-centered transitions of care. In particular, the low 39% rate of goal-concordant care among patients prioritizing comfort over longevity is noteworthy, but consistent with prior literature.1 This degree of discordance highlights provision of goal-concordant care following hospitalization as a key, yet unfulfilled, patient-centered-care quality metric.
The identified shortcomings in communication and care represent an important opportunity for hospitalists to enhance the extent to which survivors of critical illness receive care respectful of their preferences and values. Given the importance of effective discharge handoff practices in hospital medicine,2 future work should address assertively incorporating GOC into transitions after serious acute illness. Enhancing communication of these goals at discharge may benefit patients at high risk of readmission and other postdischarge adverse events, particularly for patients with comfort-focused GOC.
The study is limited in its derivation from trial participants with a specific clinical syndrome in a single health system. Also, investigators’ classification of a single patient goal does not reflect the multifactorial objectives of health interventions. In addition, since patient-reported GOC discussions correlate more highly with goal-concordant care than those identified through EHRs,5 future work should ascertain the generalizability of the identified gaps in practice.
The findings of this study underscore the need for clinicians to promote GOC assessment and documentation during hospitalization for high-risk conditions, such as sepsis. Tracking rates of GOC elicitation and goal-concordant care following discharge should be incorporated into quality measurement systems as important patient-centered dimensions of care. Hospitalists can fill a critical void by helping to correct the deficiencies that exist in respecting the preferences of survivors of serious acute illness.
Care concordant with patient goals of care (GOC) is a central component of quality. Communication about GOC is associated with improved quality of life, reduced resource utilization, and optimized end-of-life (EOL) care. Prior literature has focused on outpatient populations, with little knowledge based on preferences elicited from patients hospitalized for serious acute illness.1 The consequent knowledge gap relates to a dimension of practice through which hospitalists can improve patient-centered care by clarifying patient preferences for goal-directed treatments both during and following hospitalization.2 Implementing interventions that optimize shared decision-making through a personalized serious- illness care plan is a high-priority research area.2
In this issue, to estimate how frequently GOC are assessed during hospitalization for serious illness and the concordance between identified goals and postdischarge care, Taylor et al3 retrospectively evaluated a cohort of sepsis survivors through electronic health record (EHR) review. A standardized EHR care alignment tool and a comprehensive EHR assessment demonstrated that only 19% and 40% of patients, respectively, had identifiable GOC documented. Goal-concordant care was subsequently observed among 68% of patients with identified goals, consistent with prior work demonstrating goal-concordance in this range.1 Data on EOL care provided to decedents in an integrated health system notably showed that 89% received goal-concordant treatments.4 This difference may stem from clinicians’ emphasis on goal ascertainment at the EOL, a propensity reflected in the comparative characteristics of patients with goals documented in the current study’s Table.3 Investigators took advantage of unique inpatient and postdischarge clinical information from a sepsis patient sample to provide novel insights into the inadequacy of patient preference assessment and the substantial frequency of goal-discordant care resulting from insufficient attention to GOC.
This study suggests a critical need to improve practices related to identification of GOC in patients hospitalized with serious illness. After adjusting for relevant confounding characteristics, completion of a standardized EHR care alignment tool was strongly associated with receipt of goal-concordant care following discharge.3 Although this tool was only completed in 19% of patients, this finding suggests that elicitation of patient preferences is an under-addressed step in facilitating patient-centered transitions of care. In particular, the low 39% rate of goal-concordant care among patients prioritizing comfort over longevity is noteworthy, but consistent with prior literature.1 This degree of discordance highlights provision of goal-concordant care following hospitalization as a key, yet unfulfilled, patient-centered-care quality metric.
The identified shortcomings in communication and care represent an important opportunity for hospitalists to enhance the extent to which survivors of critical illness receive care respectful of their preferences and values. Given the importance of effective discharge handoff practices in hospital medicine,2 future work should address assertively incorporating GOC into transitions after serious acute illness. Enhancing communication of these goals at discharge may benefit patients at high risk of readmission and other postdischarge adverse events, particularly for patients with comfort-focused GOC.
The study is limited in its derivation from trial participants with a specific clinical syndrome in a single health system. Also, investigators’ classification of a single patient goal does not reflect the multifactorial objectives of health interventions. In addition, since patient-reported GOC discussions correlate more highly with goal-concordant care than those identified through EHRs,5 future work should ascertain the generalizability of the identified gaps in practice.
The findings of this study underscore the need for clinicians to promote GOC assessment and documentation during hospitalization for high-risk conditions, such as sepsis. Tracking rates of GOC elicitation and goal-concordant care following discharge should be incorporated into quality measurement systems as important patient-centered dimensions of care. Hospitalists can fill a critical void by helping to correct the deficiencies that exist in respecting the preferences of survivors of serious acute illness.
1. Modes ME, Heckbert SR, Engelberg RA, Nielsen EL, Curtis JR, Kross EK. Patient-reported receipt of goal-concordant care among seriously ill outpatients-prevalence and associated factors. J Pain Symptom Manage. 2020;60(4):765-773. https://doi.org/10.1016/j.jpainsymman.2020.04.026
2. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
3. Taylor SP, Kowalkowski MA, Courtright KR, et al. Deficits in identification of goals and goal-concordant care after sepsis hospitalization. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3714
4. Glass DP, Wang SE, Minardi PM, Kanter MH. Concordance of end-of-life care with end-of-life wishes in an integrated health care system. JAMA Netw Open. 2021;4(4):e213053. https://doi.org/10.1001/jamanetworkopen.2021.3053
5. Modes ME, Engelberg RA, Downey L, Nielsen EL, Curtis JR, Kross EK. Did a goals-of-care discussion happen? Differences in the occurrence of goals-of-care discussions as reported by patients, clinicians, and in the electronic health record. J Pain Symptom Manage. 2019;57(2):251-259. https://doi.org/10.1016/j.jpainsymman.2018.10.507
1. Modes ME, Heckbert SR, Engelberg RA, Nielsen EL, Curtis JR, Kross EK. Patient-reported receipt of goal-concordant care among seriously ill outpatients-prevalence and associated factors. J Pain Symptom Manage. 2020;60(4):765-773. https://doi.org/10.1016/j.jpainsymman.2020.04.026
2. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
3. Taylor SP, Kowalkowski MA, Courtright KR, et al. Deficits in identification of goals and goal-concordant care after sepsis hospitalization. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3714
4. Glass DP, Wang SE, Minardi PM, Kanter MH. Concordance of end-of-life care with end-of-life wishes in an integrated health care system. JAMA Netw Open. 2021;4(4):e213053. https://doi.org/10.1001/jamanetworkopen.2021.3053
5. Modes ME, Engelberg RA, Downey L, Nielsen EL, Curtis JR, Kross EK. Did a goals-of-care discussion happen? Differences in the occurrence of goals-of-care discussions as reported by patients, clinicians, and in the electronic health record. J Pain Symptom Manage. 2019;57(2):251-259. https://doi.org/10.1016/j.jpainsymman.2018.10.507
© 2021 Society of Hospital Medicine
Where Have All the Medicare Inpatients Gone?
The advent of COVID-19 saw a precipitous decline in inpatient admissions. Even before the COVID-19 pandemic, hospitals were seeing a trend toward fewer inpatient admissions for Medicare beneficiaries, which has not been thoroughly examined or explained.1 In this issue, Keohane et al2 studied Medicare inpatient episode trends between 2009 and 2017 and found that, during this period, inpatient episodes per 1000 Medicare fee-for-service (FFS) beneficiaries declined by 18.2%, from 326 to 267 per 1000 beneficiaries.
This trend can be partly explained by changes in the way that care is delivered. First, observation stays have risen, and these are excluded in the authors’ analysis. From 2010 to 2017, observation visits per 1000 beneficiaries increased from 28 to 51.1 Second, due to improved outpatient management, margin constraints, and efficiency gains, hospitals are less likely to admit patients with less complex problems or keep patients overnight for uncomplicated procedural interventions. In cardiology, there has been an increase in the proportion of same-day percutaneous coronary interventions, from 4.5% in 2009 to 28.6% in 2017.3 The authors do not include a quantitative measure of complexity, but their data support this conclusion as they find larger declines in episodes that began with a planned admission and those that involved no use of post–acute care services, and thus were likely less complicated admissions. Finally, the increased use of alternative care sites such as home-based care settings and urgent care clinics, the proliferation of telemedicine, and the continual development of guideline-based therapy have resulted in better outpatient management of diseases.
The growth of value-based care has also contributed to the reduction in inpatient admission. The past decade has seen the growth of bundled-payment contracts, accountable care organizations (ACO), and advanced primary care models. In 2018, an estimated 20% of Medicare beneficiaries were part of an ACO.4 These changes have led healthcare systems to invest in care management and postdischarge interventions, such as postdischarge phone calls, transitional clinics, and transition guides to reduce admissions and readmissions. Johns Hopkins adopted all these strategies to drive performance on the Maryland Total Cost of Care Model, which like an ACO holds hospitals accountable for both inpatient and outpatient costs incurred by Medicare FFS beneficiaries. A consistent theme among successful ACOs has been a reduction in inpatient spending.5
The authors are likely undercounting the volume of admissions by Medicare beneficiaries. First, to define an episode, they leverage the Medicare definition of bundles and include traditional Medicare inpatient, outpatient, and Part D services 30 days prior to hospitalizations and up to 90 days after. Admissions for the same diagnosis related group that occur in the 90 days after the anchor hospitalization are included in the same episode. From a clinical perspective, it is not intuitively clear why an admission for heart failure or pneumonia that occurs 3 months after an anchor hospitalization would not be defined as a separate and distinct admission rather than a readmission. Second, their analysis focuses on Medicare FFS and does not include Medicare Advantage, which now accounts for 42% of total Medicare beneficiaries. In fact, Medicare Advantage experienced significant growth in enrollment during the study period, increasing from 10 million to 24 million beneficiaries.6
Despite the reduction in inpatient volumes, the authors find that inpatient spending has increased. Spending per episode increased by 11.4% over this period, when adjusted for Medicare payment increases. Actual spending per episode unadjusted for payment increases rose by 25%. Thus, they astutely point out that most of the increase has been driven by Medicare payment increases. It is likely that increases in the complexity of patients and more dedicated focus on appropriate coding have also contributed. The authors, however, do not provide information on changes to the total cost of care outside of their defined inpatient episodes, a relevant measure to those participating in value-based models.
It is likely that the trend toward fewer inpatient admissions and increased outpatient management of medical conditions will continue as value-based care models grow. Studies like these are important in documenting this trend, but it will be important in future studies to understand how these changes have impacted the quality of care delivered to patients. Prior studies have found that reductions in readmissions through the Hospital Readmission Reduction Program were associated with increases in mortality as a potential unintended consequence.7
1. The Medicare Payment Advisory Commission. A Data Book: Health Care Spending and the Medicare Program. June 2018. Accessed October 25, 2021. http://medpac.gov/docs/default-source/data-book/jun19_databook_entirereport_sec.pdf
2. Keohane LM, Kripalani S, Buntin MB, et al. Traditional Medicare spending on inpatient episodes as hospitalizations decline. J Hosp Med. 2021;16(11):652-658. https://doi.org/10.12788/jhm.3699
3. Bradley SM, Kaltenbach LA, Xiang K, et al. Trends in use and outcomes of same-day discharge following elective percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14(15):1655-1666. https://doi.org/10.1016/j.jcin.2021.05.043
4. National Association of ACOs. NAACOs overview of the 2018 Medicare ACO class. Accessed October 25, 2021. https://www.naacos.com/overview-of-the-2018-medicare-aco-class
5. McWilliams JM, Hatfield LA, Landon BE, Hamed P, Chernew ME. Medicare spending after 3 years of the Medicare shared savings program. N Engl J Med. 2018;379(12):1139-1149. https://doi.org/10.1056/NEJMsa1803388
6. Freed M, Fuglesten Biniek J, Damico A, Neuman T. Medicare Advantage in 2021: Enrollment update and key trends. KFF. June 21, 2021. Accessed October 25, 2021. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2021-enrollment-update-and-key-trends/
7. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265
The advent of COVID-19 saw a precipitous decline in inpatient admissions. Even before the COVID-19 pandemic, hospitals were seeing a trend toward fewer inpatient admissions for Medicare beneficiaries, which has not been thoroughly examined or explained.1 In this issue, Keohane et al2 studied Medicare inpatient episode trends between 2009 and 2017 and found that, during this period, inpatient episodes per 1000 Medicare fee-for-service (FFS) beneficiaries declined by 18.2%, from 326 to 267 per 1000 beneficiaries.
This trend can be partly explained by changes in the way that care is delivered. First, observation stays have risen, and these are excluded in the authors’ analysis. From 2010 to 2017, observation visits per 1000 beneficiaries increased from 28 to 51.1 Second, due to improved outpatient management, margin constraints, and efficiency gains, hospitals are less likely to admit patients with less complex problems or keep patients overnight for uncomplicated procedural interventions. In cardiology, there has been an increase in the proportion of same-day percutaneous coronary interventions, from 4.5% in 2009 to 28.6% in 2017.3 The authors do not include a quantitative measure of complexity, but their data support this conclusion as they find larger declines in episodes that began with a planned admission and those that involved no use of post–acute care services, and thus were likely less complicated admissions. Finally, the increased use of alternative care sites such as home-based care settings and urgent care clinics, the proliferation of telemedicine, and the continual development of guideline-based therapy have resulted in better outpatient management of diseases.
The growth of value-based care has also contributed to the reduction in inpatient admission. The past decade has seen the growth of bundled-payment contracts, accountable care organizations (ACO), and advanced primary care models. In 2018, an estimated 20% of Medicare beneficiaries were part of an ACO.4 These changes have led healthcare systems to invest in care management and postdischarge interventions, such as postdischarge phone calls, transitional clinics, and transition guides to reduce admissions and readmissions. Johns Hopkins adopted all these strategies to drive performance on the Maryland Total Cost of Care Model, which like an ACO holds hospitals accountable for both inpatient and outpatient costs incurred by Medicare FFS beneficiaries. A consistent theme among successful ACOs has been a reduction in inpatient spending.5
The authors are likely undercounting the volume of admissions by Medicare beneficiaries. First, to define an episode, they leverage the Medicare definition of bundles and include traditional Medicare inpatient, outpatient, and Part D services 30 days prior to hospitalizations and up to 90 days after. Admissions for the same diagnosis related group that occur in the 90 days after the anchor hospitalization are included in the same episode. From a clinical perspective, it is not intuitively clear why an admission for heart failure or pneumonia that occurs 3 months after an anchor hospitalization would not be defined as a separate and distinct admission rather than a readmission. Second, their analysis focuses on Medicare FFS and does not include Medicare Advantage, which now accounts for 42% of total Medicare beneficiaries. In fact, Medicare Advantage experienced significant growth in enrollment during the study period, increasing from 10 million to 24 million beneficiaries.6
Despite the reduction in inpatient volumes, the authors find that inpatient spending has increased. Spending per episode increased by 11.4% over this period, when adjusted for Medicare payment increases. Actual spending per episode unadjusted for payment increases rose by 25%. Thus, they astutely point out that most of the increase has been driven by Medicare payment increases. It is likely that increases in the complexity of patients and more dedicated focus on appropriate coding have also contributed. The authors, however, do not provide information on changes to the total cost of care outside of their defined inpatient episodes, a relevant measure to those participating in value-based models.
It is likely that the trend toward fewer inpatient admissions and increased outpatient management of medical conditions will continue as value-based care models grow. Studies like these are important in documenting this trend, but it will be important in future studies to understand how these changes have impacted the quality of care delivered to patients. Prior studies have found that reductions in readmissions through the Hospital Readmission Reduction Program were associated with increases in mortality as a potential unintended consequence.7
The advent of COVID-19 saw a precipitous decline in inpatient admissions. Even before the COVID-19 pandemic, hospitals were seeing a trend toward fewer inpatient admissions for Medicare beneficiaries, which has not been thoroughly examined or explained.1 In this issue, Keohane et al2 studied Medicare inpatient episode trends between 2009 and 2017 and found that, during this period, inpatient episodes per 1000 Medicare fee-for-service (FFS) beneficiaries declined by 18.2%, from 326 to 267 per 1000 beneficiaries.
This trend can be partly explained by changes in the way that care is delivered. First, observation stays have risen, and these are excluded in the authors’ analysis. From 2010 to 2017, observation visits per 1000 beneficiaries increased from 28 to 51.1 Second, due to improved outpatient management, margin constraints, and efficiency gains, hospitals are less likely to admit patients with less complex problems or keep patients overnight for uncomplicated procedural interventions. In cardiology, there has been an increase in the proportion of same-day percutaneous coronary interventions, from 4.5% in 2009 to 28.6% in 2017.3 The authors do not include a quantitative measure of complexity, but their data support this conclusion as they find larger declines in episodes that began with a planned admission and those that involved no use of post–acute care services, and thus were likely less complicated admissions. Finally, the increased use of alternative care sites such as home-based care settings and urgent care clinics, the proliferation of telemedicine, and the continual development of guideline-based therapy have resulted in better outpatient management of diseases.
The growth of value-based care has also contributed to the reduction in inpatient admission. The past decade has seen the growth of bundled-payment contracts, accountable care organizations (ACO), and advanced primary care models. In 2018, an estimated 20% of Medicare beneficiaries were part of an ACO.4 These changes have led healthcare systems to invest in care management and postdischarge interventions, such as postdischarge phone calls, transitional clinics, and transition guides to reduce admissions and readmissions. Johns Hopkins adopted all these strategies to drive performance on the Maryland Total Cost of Care Model, which like an ACO holds hospitals accountable for both inpatient and outpatient costs incurred by Medicare FFS beneficiaries. A consistent theme among successful ACOs has been a reduction in inpatient spending.5
The authors are likely undercounting the volume of admissions by Medicare beneficiaries. First, to define an episode, they leverage the Medicare definition of bundles and include traditional Medicare inpatient, outpatient, and Part D services 30 days prior to hospitalizations and up to 90 days after. Admissions for the same diagnosis related group that occur in the 90 days after the anchor hospitalization are included in the same episode. From a clinical perspective, it is not intuitively clear why an admission for heart failure or pneumonia that occurs 3 months after an anchor hospitalization would not be defined as a separate and distinct admission rather than a readmission. Second, their analysis focuses on Medicare FFS and does not include Medicare Advantage, which now accounts for 42% of total Medicare beneficiaries. In fact, Medicare Advantage experienced significant growth in enrollment during the study period, increasing from 10 million to 24 million beneficiaries.6
Despite the reduction in inpatient volumes, the authors find that inpatient spending has increased. Spending per episode increased by 11.4% over this period, when adjusted for Medicare payment increases. Actual spending per episode unadjusted for payment increases rose by 25%. Thus, they astutely point out that most of the increase has been driven by Medicare payment increases. It is likely that increases in the complexity of patients and more dedicated focus on appropriate coding have also contributed. The authors, however, do not provide information on changes to the total cost of care outside of their defined inpatient episodes, a relevant measure to those participating in value-based models.
It is likely that the trend toward fewer inpatient admissions and increased outpatient management of medical conditions will continue as value-based care models grow. Studies like these are important in documenting this trend, but it will be important in future studies to understand how these changes have impacted the quality of care delivered to patients. Prior studies have found that reductions in readmissions through the Hospital Readmission Reduction Program were associated with increases in mortality as a potential unintended consequence.7
1. The Medicare Payment Advisory Commission. A Data Book: Health Care Spending and the Medicare Program. June 2018. Accessed October 25, 2021. http://medpac.gov/docs/default-source/data-book/jun19_databook_entirereport_sec.pdf
2. Keohane LM, Kripalani S, Buntin MB, et al. Traditional Medicare spending on inpatient episodes as hospitalizations decline. J Hosp Med. 2021;16(11):652-658. https://doi.org/10.12788/jhm.3699
3. Bradley SM, Kaltenbach LA, Xiang K, et al. Trends in use and outcomes of same-day discharge following elective percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14(15):1655-1666. https://doi.org/10.1016/j.jcin.2021.05.043
4. National Association of ACOs. NAACOs overview of the 2018 Medicare ACO class. Accessed October 25, 2021. https://www.naacos.com/overview-of-the-2018-medicare-aco-class
5. McWilliams JM, Hatfield LA, Landon BE, Hamed P, Chernew ME. Medicare spending after 3 years of the Medicare shared savings program. N Engl J Med. 2018;379(12):1139-1149. https://doi.org/10.1056/NEJMsa1803388
6. Freed M, Fuglesten Biniek J, Damico A, Neuman T. Medicare Advantage in 2021: Enrollment update and key trends. KFF. June 21, 2021. Accessed October 25, 2021. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2021-enrollment-update-and-key-trends/
7. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265
1. The Medicare Payment Advisory Commission. A Data Book: Health Care Spending and the Medicare Program. June 2018. Accessed October 25, 2021. http://medpac.gov/docs/default-source/data-book/jun19_databook_entirereport_sec.pdf
2. Keohane LM, Kripalani S, Buntin MB, et al. Traditional Medicare spending on inpatient episodes as hospitalizations decline. J Hosp Med. 2021;16(11):652-658. https://doi.org/10.12788/jhm.3699
3. Bradley SM, Kaltenbach LA, Xiang K, et al. Trends in use and outcomes of same-day discharge following elective percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14(15):1655-1666. https://doi.org/10.1016/j.jcin.2021.05.043
4. National Association of ACOs. NAACOs overview of the 2018 Medicare ACO class. Accessed October 25, 2021. https://www.naacos.com/overview-of-the-2018-medicare-aco-class
5. McWilliams JM, Hatfield LA, Landon BE, Hamed P, Chernew ME. Medicare spending after 3 years of the Medicare shared savings program. N Engl J Med. 2018;379(12):1139-1149. https://doi.org/10.1056/NEJMsa1803388
6. Freed M, Fuglesten Biniek J, Damico A, Neuman T. Medicare Advantage in 2021: Enrollment update and key trends. KFF. June 21, 2021. Accessed October 25, 2021. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2021-enrollment-update-and-key-trends/
7. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265
© 2021 Society of Hospital Medicine
Problematic Trends in Observation Status for Children’s Hospitals
Two children who presented to emergency departments in different cities were diagnosed with diabetes mellitus and ketoacidosis. On presentation, both had significant anion gap metabolic acidosis. Because the patients were deemed unsafe for discharge, the admitting physician placed orders that dictated hospital care, including an order that designated the stay as observation (OBS) or inpatient (IP) status. During their stay, both patients received care including continuous infusion of insulin, intravenous fluids, and frequent lab monitoring. Each child recovered quickly and was discharged in less than 48 hours.
Despite both patients receiving comparable care, recovering well, and being discharged home after a similar length of stay, their encounter designation may be different. Although the patient outcome is of utmost importance, the consequences of labeling an encounter as OBS or IP status are complex and may impact the financial standing of patients, hospitals, and payors. Determining the trajectory of OBS status and its utilization in the pediatric population is vital to understanding the consequences of this designation.
In this issue of the Journal of Hospital Medicine, Tian et al1 describe the increase in OBS status hospitalizations between 2010 and 2019, with OBS stays accounting for approximately one-third of pediatric hospitalizations within children’s hospitals in 2019. The increase in OBS status use was described in 19 of 20 of the most common All Patient Refined Diagnosis Related Groups, with the highest growth noted for surgical conditions and diabetes mellitus.1 These frequently seen, high-stakes conditions, when expertly managed, may result in a safe discharge within 48 hours of admission, but the labor-intensive technical skills to ensure patient safety and high-value care often differ greatly from the idea of simply “observing” a patient.
The scope creep of OBS status in pediatrics is evident. OBS status was initially designed to acknowledge a prolonged outpatient period of monitoring with a goal of determining whether inpatient hospitalization was warranted. However, in most circumstances, the care of children under OBS status differs little from those under IP status; OBS status patients are usually cared for in the same wards and by the same providers as IP status patients. The similarities in care lead to nearly equivalent hospital costs for IP and OBS stays.2 Comparable hospital costs would be less concerning if reimbursement were proportional, but OBS status hospitalizations are reimbursed at lower outpatient rates.3 The combination of similar costs and lower reimbursement results in a financial liability for children’s hospitals.
Tian et al add to the growing body of literature that underscores concerns with OBS stays.1 Its increasing use over the past decade represents a troubling continuation of increased OBS status use described by Macy et al3 nearly a decade ago, and the variability with which it is applied suggests that the designation has little connection to the clinical status of patients. Instead, its use is more likely influenced by local payor contracts, individual state laws, and provider culture. For individual institutions, this differential application affects more than just reimbursement. OBS stays are often excluded from nationally representative administrative databases, which makes hospital benchmarking, research on outcomes, and accurate comparison of patient populations impossible.4,5
The trends described by Tian et al1 raise concerns about the potential impact that OBS stays have on patients and hospital systems across the country. OBS status was created to serve a clinical need, but its inconsistent use places hospitals and the children they treat at risk. This erratic application of OBS status and the serious results of its assignment to pediatric hospitalizations provide evidence that criteria for OBS need to be standardized or otherwise abandoned outright.
1. Tian Y, Hall M, Ingram M-CE, Hu A, Raval MV. Trends and variation in the use of observation stays at children’s hospitals. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3622
2. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058. https://doi.org/10.1542/peds.2012-2494
3. Macy ML, Hall M, Shah SS, et al. Pediatric observation status: are we overlooking a growing population in children’s hospitals? J Hosp Med. 2012;7(7):530-536. https://doi.org/10.1002/jhm.1923
4. Synhorst DC, Hall M, Harris M, et al. Hospital observation status and readmission rates. Pediatrics. 2020;146(5):e2020003954. https://doi.org/10.1542/peds.2020-003954
5. Gay JC, Hall M, Morse R, Fieldston ES, Synhorst D, Macy ML. Observation encounters and length of stay benchmarking in children’s hospitals. Pediatrics. 2020;146(5):e20200120. https://doi.org/10.1542/peds.2020-0120
Two children who presented to emergency departments in different cities were diagnosed with diabetes mellitus and ketoacidosis. On presentation, both had significant anion gap metabolic acidosis. Because the patients were deemed unsafe for discharge, the admitting physician placed orders that dictated hospital care, including an order that designated the stay as observation (OBS) or inpatient (IP) status. During their stay, both patients received care including continuous infusion of insulin, intravenous fluids, and frequent lab monitoring. Each child recovered quickly and was discharged in less than 48 hours.
Despite both patients receiving comparable care, recovering well, and being discharged home after a similar length of stay, their encounter designation may be different. Although the patient outcome is of utmost importance, the consequences of labeling an encounter as OBS or IP status are complex and may impact the financial standing of patients, hospitals, and payors. Determining the trajectory of OBS status and its utilization in the pediatric population is vital to understanding the consequences of this designation.
In this issue of the Journal of Hospital Medicine, Tian et al1 describe the increase in OBS status hospitalizations between 2010 and 2019, with OBS stays accounting for approximately one-third of pediatric hospitalizations within children’s hospitals in 2019. The increase in OBS status use was described in 19 of 20 of the most common All Patient Refined Diagnosis Related Groups, with the highest growth noted for surgical conditions and diabetes mellitus.1 These frequently seen, high-stakes conditions, when expertly managed, may result in a safe discharge within 48 hours of admission, but the labor-intensive technical skills to ensure patient safety and high-value care often differ greatly from the idea of simply “observing” a patient.
The scope creep of OBS status in pediatrics is evident. OBS status was initially designed to acknowledge a prolonged outpatient period of monitoring with a goal of determining whether inpatient hospitalization was warranted. However, in most circumstances, the care of children under OBS status differs little from those under IP status; OBS status patients are usually cared for in the same wards and by the same providers as IP status patients. The similarities in care lead to nearly equivalent hospital costs for IP and OBS stays.2 Comparable hospital costs would be less concerning if reimbursement were proportional, but OBS status hospitalizations are reimbursed at lower outpatient rates.3 The combination of similar costs and lower reimbursement results in a financial liability for children’s hospitals.
Tian et al add to the growing body of literature that underscores concerns with OBS stays.1 Its increasing use over the past decade represents a troubling continuation of increased OBS status use described by Macy et al3 nearly a decade ago, and the variability with which it is applied suggests that the designation has little connection to the clinical status of patients. Instead, its use is more likely influenced by local payor contracts, individual state laws, and provider culture. For individual institutions, this differential application affects more than just reimbursement. OBS stays are often excluded from nationally representative administrative databases, which makes hospital benchmarking, research on outcomes, and accurate comparison of patient populations impossible.4,5
The trends described by Tian et al1 raise concerns about the potential impact that OBS stays have on patients and hospital systems across the country. OBS status was created to serve a clinical need, but its inconsistent use places hospitals and the children they treat at risk. This erratic application of OBS status and the serious results of its assignment to pediatric hospitalizations provide evidence that criteria for OBS need to be standardized or otherwise abandoned outright.
Two children who presented to emergency departments in different cities were diagnosed with diabetes mellitus and ketoacidosis. On presentation, both had significant anion gap metabolic acidosis. Because the patients were deemed unsafe for discharge, the admitting physician placed orders that dictated hospital care, including an order that designated the stay as observation (OBS) or inpatient (IP) status. During their stay, both patients received care including continuous infusion of insulin, intravenous fluids, and frequent lab monitoring. Each child recovered quickly and was discharged in less than 48 hours.
Despite both patients receiving comparable care, recovering well, and being discharged home after a similar length of stay, their encounter designation may be different. Although the patient outcome is of utmost importance, the consequences of labeling an encounter as OBS or IP status are complex and may impact the financial standing of patients, hospitals, and payors. Determining the trajectory of OBS status and its utilization in the pediatric population is vital to understanding the consequences of this designation.
In this issue of the Journal of Hospital Medicine, Tian et al1 describe the increase in OBS status hospitalizations between 2010 and 2019, with OBS stays accounting for approximately one-third of pediatric hospitalizations within children’s hospitals in 2019. The increase in OBS status use was described in 19 of 20 of the most common All Patient Refined Diagnosis Related Groups, with the highest growth noted for surgical conditions and diabetes mellitus.1 These frequently seen, high-stakes conditions, when expertly managed, may result in a safe discharge within 48 hours of admission, but the labor-intensive technical skills to ensure patient safety and high-value care often differ greatly from the idea of simply “observing” a patient.
The scope creep of OBS status in pediatrics is evident. OBS status was initially designed to acknowledge a prolonged outpatient period of monitoring with a goal of determining whether inpatient hospitalization was warranted. However, in most circumstances, the care of children under OBS status differs little from those under IP status; OBS status patients are usually cared for in the same wards and by the same providers as IP status patients. The similarities in care lead to nearly equivalent hospital costs for IP and OBS stays.2 Comparable hospital costs would be less concerning if reimbursement were proportional, but OBS status hospitalizations are reimbursed at lower outpatient rates.3 The combination of similar costs and lower reimbursement results in a financial liability for children’s hospitals.
Tian et al add to the growing body of literature that underscores concerns with OBS stays.1 Its increasing use over the past decade represents a troubling continuation of increased OBS status use described by Macy et al3 nearly a decade ago, and the variability with which it is applied suggests that the designation has little connection to the clinical status of patients. Instead, its use is more likely influenced by local payor contracts, individual state laws, and provider culture. For individual institutions, this differential application affects more than just reimbursement. OBS stays are often excluded from nationally representative administrative databases, which makes hospital benchmarking, research on outcomes, and accurate comparison of patient populations impossible.4,5
The trends described by Tian et al1 raise concerns about the potential impact that OBS stays have on patients and hospital systems across the country. OBS status was created to serve a clinical need, but its inconsistent use places hospitals and the children they treat at risk. This erratic application of OBS status and the serious results of its assignment to pediatric hospitalizations provide evidence that criteria for OBS need to be standardized or otherwise abandoned outright.
1. Tian Y, Hall M, Ingram M-CE, Hu A, Raval MV. Trends and variation in the use of observation stays at children’s hospitals. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3622
2. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058. https://doi.org/10.1542/peds.2012-2494
3. Macy ML, Hall M, Shah SS, et al. Pediatric observation status: are we overlooking a growing population in children’s hospitals? J Hosp Med. 2012;7(7):530-536. https://doi.org/10.1002/jhm.1923
4. Synhorst DC, Hall M, Harris M, et al. Hospital observation status and readmission rates. Pediatrics. 2020;146(5):e2020003954. https://doi.org/10.1542/peds.2020-003954
5. Gay JC, Hall M, Morse R, Fieldston ES, Synhorst D, Macy ML. Observation encounters and length of stay benchmarking in children’s hospitals. Pediatrics. 2020;146(5):e20200120. https://doi.org/10.1542/peds.2020-0120
1. Tian Y, Hall M, Ingram M-CE, Hu A, Raval MV. Trends and variation in the use of observation stays at children’s hospitals. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3622
2. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058. https://doi.org/10.1542/peds.2012-2494
3. Macy ML, Hall M, Shah SS, et al. Pediatric observation status: are we overlooking a growing population in children’s hospitals? J Hosp Med. 2012;7(7):530-536. https://doi.org/10.1002/jhm.1923
4. Synhorst DC, Hall M, Harris M, et al. Hospital observation status and readmission rates. Pediatrics. 2020;146(5):e2020003954. https://doi.org/10.1542/peds.2020-003954
5. Gay JC, Hall M, Morse R, Fieldston ES, Synhorst D, Macy ML. Observation encounters and length of stay benchmarking in children’s hospitals. Pediatrics. 2020;146(5):e20200120. https://doi.org/10.1542/peds.2020-0120
© 2021 Society of Hospital Medicine