User login
Goal-Concordant Care After Hospitalization for Serious Acute Illness: A Key Opportunity for Hospitalists in Patient-Centered Outcomes
Care concordant with patient goals of care (GOC) is a central component of quality. Communication about GOC is associated with improved quality of life, reduced resource utilization, and optimized end-of-life (EOL) care. Prior literature has focused on outpatient populations, with little knowledge based on preferences elicited from patients hospitalized for serious acute illness.1 The consequent knowledge gap relates to a dimension of practice through which hospitalists can improve patient-centered care by clarifying patient preferences for goal-directed treatments both during and following hospitalization.2 Implementing interventions that optimize shared decision-making through a personalized serious- illness care plan is a high-priority research area.2
In this issue, to estimate how frequently GOC are assessed during hospitalization for serious illness and the concordance between identified goals and postdischarge care, Taylor et al3 retrospectively evaluated a cohort of sepsis survivors through electronic health record (EHR) review. A standardized EHR care alignment tool and a comprehensive EHR assessment demonstrated that only 19% and 40% of patients, respectively, had identifiable GOC documented. Goal-concordant care was subsequently observed among 68% of patients with identified goals, consistent with prior work demonstrating goal-concordance in this range.1 Data on EOL care provided to decedents in an integrated health system notably showed that 89% received goal-concordant treatments.4 This difference may stem from clinicians’ emphasis on goal ascertainment at the EOL, a propensity reflected in the comparative characteristics of patients with goals documented in the current study’s Table.3 Investigators took advantage of unique inpatient and postdischarge clinical information from a sepsis patient sample to provide novel insights into the inadequacy of patient preference assessment and the substantial frequency of goal-discordant care resulting from insufficient attention to GOC.
This study suggests a critical need to improve practices related to identification of GOC in patients hospitalized with serious illness. After adjusting for relevant confounding characteristics, completion of a standardized EHR care alignment tool was strongly associated with receipt of goal-concordant care following discharge.3 Although this tool was only completed in 19% of patients, this finding suggests that elicitation of patient preferences is an under-addressed step in facilitating patient-centered transitions of care. In particular, the low 39% rate of goal-concordant care among patients prioritizing comfort over longevity is noteworthy, but consistent with prior literature.1 This degree of discordance highlights provision of goal-concordant care following hospitalization as a key, yet unfulfilled, patient-centered-care quality metric.
The identified shortcomings in communication and care represent an important opportunity for hospitalists to enhance the extent to which survivors of critical illness receive care respectful of their preferences and values. Given the importance of effective discharge handoff practices in hospital medicine,2 future work should address assertively incorporating GOC into transitions after serious acute illness. Enhancing communication of these goals at discharge may benefit patients at high risk of readmission and other postdischarge adverse events, particularly for patients with comfort-focused GOC.
The study is limited in its derivation from trial participants with a specific clinical syndrome in a single health system. Also, investigators’ classification of a single patient goal does not reflect the multifactorial objectives of health interventions. In addition, since patient-reported GOC discussions correlate more highly with goal-concordant care than those identified through EHRs,5 future work should ascertain the generalizability of the identified gaps in practice.
The findings of this study underscore the need for clinicians to promote GOC assessment and documentation during hospitalization for high-risk conditions, such as sepsis. Tracking rates of GOC elicitation and goal-concordant care following discharge should be incorporated into quality measurement systems as important patient-centered dimensions of care. Hospitalists can fill a critical void by helping to correct the deficiencies that exist in respecting the preferences of survivors of serious acute illness.
1. Modes ME, Heckbert SR, Engelberg RA, Nielsen EL, Curtis JR, Kross EK. Patient-reported receipt of goal-concordant care among seriously ill outpatients-prevalence and associated factors. J Pain Symptom Manage. 2020;60(4):765-773. https://doi.org/10.1016/j.jpainsymman.2020.04.026
2. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
3. Taylor SP, Kowalkowski MA, Courtright KR, et al. Deficits in identification of goals and goal-concordant care after sepsis hospitalization. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3714
4. Glass DP, Wang SE, Minardi PM, Kanter MH. Concordance of end-of-life care with end-of-life wishes in an integrated health care system. JAMA Netw Open. 2021;4(4):e213053. https://doi.org/10.1001/jamanetworkopen.2021.3053
5. Modes ME, Engelberg RA, Downey L, Nielsen EL, Curtis JR, Kross EK. Did a goals-of-care discussion happen? Differences in the occurrence of goals-of-care discussions as reported by patients, clinicians, and in the electronic health record. J Pain Symptom Manage. 2019;57(2):251-259. https://doi.org/10.1016/j.jpainsymman.2018.10.507
Care concordant with patient goals of care (GOC) is a central component of quality. Communication about GOC is associated with improved quality of life, reduced resource utilization, and optimized end-of-life (EOL) care. Prior literature has focused on outpatient populations, with little knowledge based on preferences elicited from patients hospitalized for serious acute illness.1 The consequent knowledge gap relates to a dimension of practice through which hospitalists can improve patient-centered care by clarifying patient preferences for goal-directed treatments both during and following hospitalization.2 Implementing interventions that optimize shared decision-making through a personalized serious- illness care plan is a high-priority research area.2
In this issue, to estimate how frequently GOC are assessed during hospitalization for serious illness and the concordance between identified goals and postdischarge care, Taylor et al3 retrospectively evaluated a cohort of sepsis survivors through electronic health record (EHR) review. A standardized EHR care alignment tool and a comprehensive EHR assessment demonstrated that only 19% and 40% of patients, respectively, had identifiable GOC documented. Goal-concordant care was subsequently observed among 68% of patients with identified goals, consistent with prior work demonstrating goal-concordance in this range.1 Data on EOL care provided to decedents in an integrated health system notably showed that 89% received goal-concordant treatments.4 This difference may stem from clinicians’ emphasis on goal ascertainment at the EOL, a propensity reflected in the comparative characteristics of patients with goals documented in the current study’s Table.3 Investigators took advantage of unique inpatient and postdischarge clinical information from a sepsis patient sample to provide novel insights into the inadequacy of patient preference assessment and the substantial frequency of goal-discordant care resulting from insufficient attention to GOC.
This study suggests a critical need to improve practices related to identification of GOC in patients hospitalized with serious illness. After adjusting for relevant confounding characteristics, completion of a standardized EHR care alignment tool was strongly associated with receipt of goal-concordant care following discharge.3 Although this tool was only completed in 19% of patients, this finding suggests that elicitation of patient preferences is an under-addressed step in facilitating patient-centered transitions of care. In particular, the low 39% rate of goal-concordant care among patients prioritizing comfort over longevity is noteworthy, but consistent with prior literature.1 This degree of discordance highlights provision of goal-concordant care following hospitalization as a key, yet unfulfilled, patient-centered-care quality metric.
The identified shortcomings in communication and care represent an important opportunity for hospitalists to enhance the extent to which survivors of critical illness receive care respectful of their preferences and values. Given the importance of effective discharge handoff practices in hospital medicine,2 future work should address assertively incorporating GOC into transitions after serious acute illness. Enhancing communication of these goals at discharge may benefit patients at high risk of readmission and other postdischarge adverse events, particularly for patients with comfort-focused GOC.
The study is limited in its derivation from trial participants with a specific clinical syndrome in a single health system. Also, investigators’ classification of a single patient goal does not reflect the multifactorial objectives of health interventions. In addition, since patient-reported GOC discussions correlate more highly with goal-concordant care than those identified through EHRs,5 future work should ascertain the generalizability of the identified gaps in practice.
The findings of this study underscore the need for clinicians to promote GOC assessment and documentation during hospitalization for high-risk conditions, such as sepsis. Tracking rates of GOC elicitation and goal-concordant care following discharge should be incorporated into quality measurement systems as important patient-centered dimensions of care. Hospitalists can fill a critical void by helping to correct the deficiencies that exist in respecting the preferences of survivors of serious acute illness.
Care concordant with patient goals of care (GOC) is a central component of quality. Communication about GOC is associated with improved quality of life, reduced resource utilization, and optimized end-of-life (EOL) care. Prior literature has focused on outpatient populations, with little knowledge based on preferences elicited from patients hospitalized for serious acute illness.1 The consequent knowledge gap relates to a dimension of practice through which hospitalists can improve patient-centered care by clarifying patient preferences for goal-directed treatments both during and following hospitalization.2 Implementing interventions that optimize shared decision-making through a personalized serious- illness care plan is a high-priority research area.2
In this issue, to estimate how frequently GOC are assessed during hospitalization for serious illness and the concordance between identified goals and postdischarge care, Taylor et al3 retrospectively evaluated a cohort of sepsis survivors through electronic health record (EHR) review. A standardized EHR care alignment tool and a comprehensive EHR assessment demonstrated that only 19% and 40% of patients, respectively, had identifiable GOC documented. Goal-concordant care was subsequently observed among 68% of patients with identified goals, consistent with prior work demonstrating goal-concordance in this range.1 Data on EOL care provided to decedents in an integrated health system notably showed that 89% received goal-concordant treatments.4 This difference may stem from clinicians’ emphasis on goal ascertainment at the EOL, a propensity reflected in the comparative characteristics of patients with goals documented in the current study’s Table.3 Investigators took advantage of unique inpatient and postdischarge clinical information from a sepsis patient sample to provide novel insights into the inadequacy of patient preference assessment and the substantial frequency of goal-discordant care resulting from insufficient attention to GOC.
This study suggests a critical need to improve practices related to identification of GOC in patients hospitalized with serious illness. After adjusting for relevant confounding characteristics, completion of a standardized EHR care alignment tool was strongly associated with receipt of goal-concordant care following discharge.3 Although this tool was only completed in 19% of patients, this finding suggests that elicitation of patient preferences is an under-addressed step in facilitating patient-centered transitions of care. In particular, the low 39% rate of goal-concordant care among patients prioritizing comfort over longevity is noteworthy, but consistent with prior literature.1 This degree of discordance highlights provision of goal-concordant care following hospitalization as a key, yet unfulfilled, patient-centered-care quality metric.
The identified shortcomings in communication and care represent an important opportunity for hospitalists to enhance the extent to which survivors of critical illness receive care respectful of their preferences and values. Given the importance of effective discharge handoff practices in hospital medicine,2 future work should address assertively incorporating GOC into transitions after serious acute illness. Enhancing communication of these goals at discharge may benefit patients at high risk of readmission and other postdischarge adverse events, particularly for patients with comfort-focused GOC.
The study is limited in its derivation from trial participants with a specific clinical syndrome in a single health system. Also, investigators’ classification of a single patient goal does not reflect the multifactorial objectives of health interventions. In addition, since patient-reported GOC discussions correlate more highly with goal-concordant care than those identified through EHRs,5 future work should ascertain the generalizability of the identified gaps in practice.
The findings of this study underscore the need for clinicians to promote GOC assessment and documentation during hospitalization for high-risk conditions, such as sepsis. Tracking rates of GOC elicitation and goal-concordant care following discharge should be incorporated into quality measurement systems as important patient-centered dimensions of care. Hospitalists can fill a critical void by helping to correct the deficiencies that exist in respecting the preferences of survivors of serious acute illness.
1. Modes ME, Heckbert SR, Engelberg RA, Nielsen EL, Curtis JR, Kross EK. Patient-reported receipt of goal-concordant care among seriously ill outpatients-prevalence and associated factors. J Pain Symptom Manage. 2020;60(4):765-773. https://doi.org/10.1016/j.jpainsymman.2020.04.026
2. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
3. Taylor SP, Kowalkowski MA, Courtright KR, et al. Deficits in identification of goals and goal-concordant care after sepsis hospitalization. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3714
4. Glass DP, Wang SE, Minardi PM, Kanter MH. Concordance of end-of-life care with end-of-life wishes in an integrated health care system. JAMA Netw Open. 2021;4(4):e213053. https://doi.org/10.1001/jamanetworkopen.2021.3053
5. Modes ME, Engelberg RA, Downey L, Nielsen EL, Curtis JR, Kross EK. Did a goals-of-care discussion happen? Differences in the occurrence of goals-of-care discussions as reported by patients, clinicians, and in the electronic health record. J Pain Symptom Manage. 2019;57(2):251-259. https://doi.org/10.1016/j.jpainsymman.2018.10.507
1. Modes ME, Heckbert SR, Engelberg RA, Nielsen EL, Curtis JR, Kross EK. Patient-reported receipt of goal-concordant care among seriously ill outpatients-prevalence and associated factors. J Pain Symptom Manage. 2020;60(4):765-773. https://doi.org/10.1016/j.jpainsymman.2020.04.026
2. Harrison JD, Archuleta M, Avitia E, et al. Developing a patient- and family-centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337. https://doi.org/10.12788/jhm.3386
3. Taylor SP, Kowalkowski MA, Courtright KR, et al. Deficits in identification of goals and goal-concordant care after sepsis hospitalization. J Hosp Med. 2021;16(11):645-651. https://doi.org/10.12788/jhm.3714
4. Glass DP, Wang SE, Minardi PM, Kanter MH. Concordance of end-of-life care with end-of-life wishes in an integrated health care system. JAMA Netw Open. 2021;4(4):e213053. https://doi.org/10.1001/jamanetworkopen.2021.3053
5. Modes ME, Engelberg RA, Downey L, Nielsen EL, Curtis JR, Kross EK. Did a goals-of-care discussion happen? Differences in the occurrence of goals-of-care discussions as reported by patients, clinicians, and in the electronic health record. J Pain Symptom Manage. 2019;57(2):251-259. https://doi.org/10.1016/j.jpainsymman.2018.10.507
© 2021 Society of Hospital Medicine
Predictors of COVID-19 Seropositivity Among Healthcare Workers: An Important Piece of an Incomplete Puzzle
SARS-CoV-2 seroprevalence studies of healthcare workers (HCWs) provide valuable insights into the excess risk of infection in this population and indirect evidence supporting the value of personal protective equipment (PPE) use. Seroprevalence estimates are composite measures of exposure risk and transmission mitigation both in the healthcare and community environments. The challenge of interpreting these studies arises from the diversity of HCW vocational roles and work settings in juxtaposition to heterogeneous community exposure risks. In this issue, two studies untangle some of these competing factors.
Investigators from Kashmir, India, assessed the relationship between seropositivity and specific HCW roles and work sites.1 They found a lower seroprevalence among HCWs at hospitals dedicated to COVID patients, relative to non-COVID hospitals. This seemingly paradoxical finding likely results from a combination of vigilant PPE adherence enforced through a buddy system, restrictive visitation policies, HCW residential dormitories reducing community exposure, and a spillover effect of careful in-hospital exposure avoidance practices on out-of-hospital behavior. A similar spillover effect has been hypothesized for low HCW seroprevalence relative to the surrounding community in California.2
In complement, researchers at a large New York City (NYC) hospital found higher overall HCW seropositivity rates compared with the community, though estimates were strikingly variable after detailed stratification by job function and location.3 The gradient of seroprevalence showed the highest risk among nurses and those in nonclinical, low-wage jobs (eg, patient transport, housekeeping), a finding also seen in another US study prior to adjustment for demographic and community factors.4 This finding highlights the association between socioeconomic status, structural community exposure risk factors such as multiple essential workers living within multigenerational households, and the challenges of sickness absenteeism. High seroprevalence among nurses and emergency department HCWs (who expeditiously evaluate many undifferentiated patients) may reflect both greater aggregate duration of exposure to infected patients and increased frequency of PPE donning and doffing, resulting in fatigue and diminished vigilance.5
A NYC-based study similarly showed high HCW seroprevalence, although no consistent associations with job function (albeit measured with less granularity) or community-based exposures were identified.6 Several studies comparing HCW to local community seropositivity rates have reached disparate conclusions.2,7 These contrasting data may result from variability in vigilance of PPE use, mask use in work rooms or during meals/breaktimes, sick leave policies driven by staffing demands, and neighborhood factors. In addition, selection biases and timing of blood sampling relative to viral transmission peaks (with differing degrees of temporal antibody waning) may contribute to the apparent discordance. In particular, comparative community-based samples vary greatly in their inclusion of asymptomatic patients, which can substantially affect such estimates by changing the denominator population.
We draw three conclusions: (1) Evidence for HCW exposure often tracks with community infection rates, suggesting that nonworkplace exposures are a dominant source of HCW seropositivity; (2) vigilant PPE use and assertively implemented protective measures unrelated to patient encounters can dramatically reduce infection risk, even among those with frequent exposures; and (3) HCW infection risk during future peaks can be effectively restrained with adequate resources and support, even in the presence of variants for which no effective vaccination or preventive pharmacotherapy exists. Given the divergent seroprevalence rates found in these studies after detailed stratification by job function and location, it is important for future studies to evaluate their relationship with infectious risk. Accurately quantifying the excess risks borne by HCWs may remain an elusive objective, but experiential knowledge offers numerous strategies worthy of proactive implementation to preserve HCW safety and well-being.
1. Khan M, Haq I, Qurieshi MA, et al. SARS-CoV-2 seroprevalence among healthcare workers by workplace exposure risk in Kashmir, India. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3609
2. Brant-Zawadzki M, Fridman D, Robinson PA, et al. Prevalence and longevity of SARS-CoV-2 antibodies among health care workers. Open Forum Infect Dis. 2021;8(2):ofab015. https://doi.org/10.1093/ofid/ofab015
3. Purswani MU, Bucciarelli J, Tiburcio J. SARS-CoV-2 seroprevalence among healthcare workers by job function and work location in a New York inner-city hospital. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3627
4. Jacob JT, Baker JM, Fridkin SK, et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open. 2021;4(3):e211283. https://doi.org/10.1001/jamanetworkopen.2021.1283
5. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
6. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: A cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2021(1);102:63-69. https://doi.org/10.1016/j.ijid.2020.10.0367. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120-134. https://doi.org/10.1016/j.jhin.2020.11.008
SARS-CoV-2 seroprevalence studies of healthcare workers (HCWs) provide valuable insights into the excess risk of infection in this population and indirect evidence supporting the value of personal protective equipment (PPE) use. Seroprevalence estimates are composite measures of exposure risk and transmission mitigation both in the healthcare and community environments. The challenge of interpreting these studies arises from the diversity of HCW vocational roles and work settings in juxtaposition to heterogeneous community exposure risks. In this issue, two studies untangle some of these competing factors.
Investigators from Kashmir, India, assessed the relationship between seropositivity and specific HCW roles and work sites.1 They found a lower seroprevalence among HCWs at hospitals dedicated to COVID patients, relative to non-COVID hospitals. This seemingly paradoxical finding likely results from a combination of vigilant PPE adherence enforced through a buddy system, restrictive visitation policies, HCW residential dormitories reducing community exposure, and a spillover effect of careful in-hospital exposure avoidance practices on out-of-hospital behavior. A similar spillover effect has been hypothesized for low HCW seroprevalence relative to the surrounding community in California.2
In complement, researchers at a large New York City (NYC) hospital found higher overall HCW seropositivity rates compared with the community, though estimates were strikingly variable after detailed stratification by job function and location.3 The gradient of seroprevalence showed the highest risk among nurses and those in nonclinical, low-wage jobs (eg, patient transport, housekeeping), a finding also seen in another US study prior to adjustment for demographic and community factors.4 This finding highlights the association between socioeconomic status, structural community exposure risk factors such as multiple essential workers living within multigenerational households, and the challenges of sickness absenteeism. High seroprevalence among nurses and emergency department HCWs (who expeditiously evaluate many undifferentiated patients) may reflect both greater aggregate duration of exposure to infected patients and increased frequency of PPE donning and doffing, resulting in fatigue and diminished vigilance.5
A NYC-based study similarly showed high HCW seroprevalence, although no consistent associations with job function (albeit measured with less granularity) or community-based exposures were identified.6 Several studies comparing HCW to local community seropositivity rates have reached disparate conclusions.2,7 These contrasting data may result from variability in vigilance of PPE use, mask use in work rooms or during meals/breaktimes, sick leave policies driven by staffing demands, and neighborhood factors. In addition, selection biases and timing of blood sampling relative to viral transmission peaks (with differing degrees of temporal antibody waning) may contribute to the apparent discordance. In particular, comparative community-based samples vary greatly in their inclusion of asymptomatic patients, which can substantially affect such estimates by changing the denominator population.
We draw three conclusions: (1) Evidence for HCW exposure often tracks with community infection rates, suggesting that nonworkplace exposures are a dominant source of HCW seropositivity; (2) vigilant PPE use and assertively implemented protective measures unrelated to patient encounters can dramatically reduce infection risk, even among those with frequent exposures; and (3) HCW infection risk during future peaks can be effectively restrained with adequate resources and support, even in the presence of variants for which no effective vaccination or preventive pharmacotherapy exists. Given the divergent seroprevalence rates found in these studies after detailed stratification by job function and location, it is important for future studies to evaluate their relationship with infectious risk. Accurately quantifying the excess risks borne by HCWs may remain an elusive objective, but experiential knowledge offers numerous strategies worthy of proactive implementation to preserve HCW safety and well-being.
SARS-CoV-2 seroprevalence studies of healthcare workers (HCWs) provide valuable insights into the excess risk of infection in this population and indirect evidence supporting the value of personal protective equipment (PPE) use. Seroprevalence estimates are composite measures of exposure risk and transmission mitigation both in the healthcare and community environments. The challenge of interpreting these studies arises from the diversity of HCW vocational roles and work settings in juxtaposition to heterogeneous community exposure risks. In this issue, two studies untangle some of these competing factors.
Investigators from Kashmir, India, assessed the relationship between seropositivity and specific HCW roles and work sites.1 They found a lower seroprevalence among HCWs at hospitals dedicated to COVID patients, relative to non-COVID hospitals. This seemingly paradoxical finding likely results from a combination of vigilant PPE adherence enforced through a buddy system, restrictive visitation policies, HCW residential dormitories reducing community exposure, and a spillover effect of careful in-hospital exposure avoidance practices on out-of-hospital behavior. A similar spillover effect has been hypothesized for low HCW seroprevalence relative to the surrounding community in California.2
In complement, researchers at a large New York City (NYC) hospital found higher overall HCW seropositivity rates compared with the community, though estimates were strikingly variable after detailed stratification by job function and location.3 The gradient of seroprevalence showed the highest risk among nurses and those in nonclinical, low-wage jobs (eg, patient transport, housekeeping), a finding also seen in another US study prior to adjustment for demographic and community factors.4 This finding highlights the association between socioeconomic status, structural community exposure risk factors such as multiple essential workers living within multigenerational households, and the challenges of sickness absenteeism. High seroprevalence among nurses and emergency department HCWs (who expeditiously evaluate many undifferentiated patients) may reflect both greater aggregate duration of exposure to infected patients and increased frequency of PPE donning and doffing, resulting in fatigue and diminished vigilance.5
A NYC-based study similarly showed high HCW seroprevalence, although no consistent associations with job function (albeit measured with less granularity) or community-based exposures were identified.6 Several studies comparing HCW to local community seropositivity rates have reached disparate conclusions.2,7 These contrasting data may result from variability in vigilance of PPE use, mask use in work rooms or during meals/breaktimes, sick leave policies driven by staffing demands, and neighborhood factors. In addition, selection biases and timing of blood sampling relative to viral transmission peaks (with differing degrees of temporal antibody waning) may contribute to the apparent discordance. In particular, comparative community-based samples vary greatly in their inclusion of asymptomatic patients, which can substantially affect such estimates by changing the denominator population.
We draw three conclusions: (1) Evidence for HCW exposure often tracks with community infection rates, suggesting that nonworkplace exposures are a dominant source of HCW seropositivity; (2) vigilant PPE use and assertively implemented protective measures unrelated to patient encounters can dramatically reduce infection risk, even among those with frequent exposures; and (3) HCW infection risk during future peaks can be effectively restrained with adequate resources and support, even in the presence of variants for which no effective vaccination or preventive pharmacotherapy exists. Given the divergent seroprevalence rates found in these studies after detailed stratification by job function and location, it is important for future studies to evaluate their relationship with infectious risk. Accurately quantifying the excess risks borne by HCWs may remain an elusive objective, but experiential knowledge offers numerous strategies worthy of proactive implementation to preserve HCW safety and well-being.
1. Khan M, Haq I, Qurieshi MA, et al. SARS-CoV-2 seroprevalence among healthcare workers by workplace exposure risk in Kashmir, India. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3609
2. Brant-Zawadzki M, Fridman D, Robinson PA, et al. Prevalence and longevity of SARS-CoV-2 antibodies among health care workers. Open Forum Infect Dis. 2021;8(2):ofab015. https://doi.org/10.1093/ofid/ofab015
3. Purswani MU, Bucciarelli J, Tiburcio J. SARS-CoV-2 seroprevalence among healthcare workers by job function and work location in a New York inner-city hospital. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3627
4. Jacob JT, Baker JM, Fridkin SK, et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open. 2021;4(3):e211283. https://doi.org/10.1001/jamanetworkopen.2021.1283
5. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
6. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: A cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2021(1);102:63-69. https://doi.org/10.1016/j.ijid.2020.10.0367. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120-134. https://doi.org/10.1016/j.jhin.2020.11.008
1. Khan M, Haq I, Qurieshi MA, et al. SARS-CoV-2 seroprevalence among healthcare workers by workplace exposure risk in Kashmir, India. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3609
2. Brant-Zawadzki M, Fridman D, Robinson PA, et al. Prevalence and longevity of SARS-CoV-2 antibodies among health care workers. Open Forum Infect Dis. 2021;8(2):ofab015. https://doi.org/10.1093/ofid/ofab015
3. Purswani MU, Bucciarelli J, Tiburcio J. SARS-CoV-2 seroprevalence among healthcare workers by job function and work location in a New York inner-city hospital. J Hosp Med. 2021;16(5):274-281. https://doi.org/10.12788/jhm.3627
4. Jacob JT, Baker JM, Fridkin SK, et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open. 2021;4(3):e211283. https://doi.org/10.1001/jamanetworkopen.2021.1283
5. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
6. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: A cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2021(1);102:63-69. https://doi.org/10.1016/j.ijid.2020.10.0367. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120-134. https://doi.org/10.1016/j.jhin.2020.11.008
© 2021 Society of Hospital Medicine
Limiting Patient Autonomy: Mandatory COVID-19 Diagnostic Testing
Despite the important clinical and public health implications of a COVID-19 diagnosis, respect for autonomy allows patients to decline testing without explanation and with impunity. Whether physicians believe a test is indicated for clinical care of an individual patient, prevention of nosocomial transmission, or the greater public health, patients may refuse. Such refusals may be increasing due to quarantine requirements, concerns regarding contact tracing, and the persistent absence of a curative treatment.1,2 Mass screening of all healthcare workers (HCWs) is being considered to prevent hospital transmission,3 and universal screening in nursing homes has thwarted outbreaks while providing data to facilitate resource allocation.4 Given these circumstances, patients’ absolute right to refuse a noninvasive test with the potential for multifaceted downstream benefit is worthy of reconsideration, in favor of mandatory testing. Mandatory testing confers numerous benefits, including mitigating risk to other patients and HCWs, who play a central role in pandemic response. Because infected HCWs may transmit the virus to patients, they also should undergo mandatory testing,3 particularly in the presence of symptoms, since nasal secretions increase the diagnostic yield of testing.5 Although pretest probability (as an estimate of disease prevalence) typically determines the testing strategy for admitted patients, model-based analyses suggest that testing every 3 days for HCWs or continuously hospitalized patients would nearly eliminate infectivity.6
Tools for assisting frustrated HCWs navigating patients’ right to refuse testing have been developed that incorporate education, clear communication, and conflict resolution.7 Such approaches are, however, only moderately successful, making the use of personal protective equipment (PPE) based on a default assumption of COVID-19 positivity common.8 The burden and disheartening waste created by low-yield PPE use among patients unwilling to be tested becomes particularly evident in the context of shortages. Such vexing, stressful shortages, as well as the dual responsibilities of hospitals as stewards of both individual patient and population health, serve as reminders that efficient allocation of resources must be valued alongside the autonomous rights of patients.9 Moreover, recent reports suggest that test avoidance is a growing problem.1,2 Refusal to accept testing may be rooted in anxiety, concerns about the consequences of a positive result (eg, inability to attend school or work), or a desire for self-determination.1,2 The hesitancy that leads to refusal may also arise from misinformation, poor public health messaging, distrust in the establishment, and unproductive considerations related to conscientious objection without foundation.2 Concepts of individual liberty that often underlie steadfast adherence to the principles of self-determination created opposition to masks that antagonized public health efforts to limit the spread of COVID-19. Although influencing inpatients’ behavior to benefit both the public and HCWs may be distinct from community settings, the attitudes that lead to test refusal and defiance of mask-related ordinances likely have substantial commonalities.
THE PATIENT ROLE IN HEALTHCARE DECISIONS
As a pillar of ethical
ANALOGOUS EXPERIENCES: ETHICAL LESSONS AND PRACTICAL IMPLICATIONS
In non-healthcare settings, the controversies surrounding vaccination and access to schools for unvaccinated children are perhaps the public and professional debate most analogous to COVID-19 testing refusal.12 Although policymakers may distinguish between testing and vaccination, these interventions similarly hold the potential to limit disease incidence and mitigate health impact. To preserve public health, most states prevent (with varied exemptions) unvaccinated children from attending schools. COVID-19 testing may in the future become a requirement for participation in group social activities, athletic competitions, or physical presence in the workplace to facilitate quarantining and/or targeted use of PPE for transmission risk reduction. Given the dramatic mitigation benefits accruable on college campuses,13 required testing for in-person learning has become common.
There are also parallels, and therefore lessons, to be drawn from experience in testing for HIV, although HIV-related stigma and devalued status of the marginalized populations initially infected impacted the broader societal view of HIV compared with COVID-19. For example, antenatal HIV screening of pregnant women is strongly recommended to facilitate interventions that reduce the chance of vertical transmission.14 The limitations of purely elective testing are one justification for the current standard of opt-out screening. However, in this case, the health complications of refusal are largely the burden of the fetus, over whose future the mother holds a great deal of choice and responsibility, irrespective of HIV status. The public health implications of HIV test refusal are far less immediate than for COVID-19 infection because there is no effective curative therapy for COVID-19 and spread occurs through nonintimate, unintentional, and unpredictable exposure.
Translating societal attitudes and practices into the healthcare setting to consider mandated COVID-19 testing requires additional considerations related to both patients and providers: (1) HCWs have committed to a set of values and professional obligations that include tasks requiring risks15; (2) the public expects HCWs to perform their duties according to a social contract that has few restrictions16; (3) limiting patient access to hospital care due to COVID-19 testing refusal would contradict and create conflicts related to professional conceptions of hospitals and physicians as patient agents15; and (4) patients who conscientiously object to testing may seek healthcare less diligently, which may lead to health decrements. The associated postponement of essential care may unduly burden the healthcare system, particularly in situations such as ambulatory care–sensitive conditions.
HEALTHCARE WORKER PROTECTION, PATIENT ACCESS, AND THE VALUE OF PARSIMONY
The extent to which the public health justification for mandatory testing extends to hospitalized patients to protect HCWs is ambiguous. HCWs are of enormous instrumental value and are therefore essential for the pandemic response and health of the broader population. Their protection may therefore justify curtailing informed consent for diagnostic testing. Downstream effects on the supply of frontline HCWs may be realized. Poor control over working conditions may negatively impact motivation among HCWs. In addition, they may feel disenfranchised while obligatorily taking personal risks in caring for patients unwilling to commit to the common good through diagnostic test consent. Hospitalized patients who refuse testing may remain patients under investigation (PUIs), thus requiring special respiratory precautions (SRP) throughout their hospitalization, thereby placing a persistent burden on those with responsibilities requiring patient contact.17 Repeatedly donning and doffing PPE may remind at-risk HCWs that a myriad of benefits may accrue from frequent, ubiquitous testing. Their motivation may be tempered by the demoralizing requirement to care for patients who will not consent to a simple test, knowing that an opportunity to diminish the burdens of this communicable disease that has taken the lives of many HCWs is being relinquished.
Although HCWs could use SRP universally, their selective application in rooms of known COVID-19–positive patients and those with temporary PUI status has several advantages.17 First, we learned that HIV testing on patients was helpful in enabling surgeons to selectively implement special precautions among infected patients rather than universally applied intensive precautions. Even in the setting of high rates of HIV infection and educational interventions, HCWs do not reliably apply protective measures included in universal precautions.18 In keeping with these experiences, limiting the number of patients on SRP will minimize the “precautions fatigue” that drives nonadherent behavior among HCWs.17 As a result, minimizing the proportion of patients on SRP through testing (and liberation from unnecessary precautions in most cases) will improve uptake of crucial hand hygiene practices and adoption of vigilant PPE use. Second, definitive knowledge of COVID-19 status will increase patient access to care because, whether by personal choice or policy, many HCWs limit in-person contact with patients who are or may be COVID-19 positive. For example, many inpatient dialysis units do not accept patients without a negative COVID-19 nasal swab. Physical therapists may delay or avoid seeing a PUI, which will pose challenges for efficient determination of discharge disposition. Third, selective use of SRP will limit the environmental impact of disposed PPE, which is neither recyclable nor biodegradable. Infectious or regulated biomedical waste products are a significant source of environmental pollution, and the World Health Organization has recommended parsimonious, selective use of PPE to minimize the adverse environmental consequences of biomedical waste products.
CONCLUSION
In summary, there are substantial justifications for mandatory testing for COVID-19 in the hospital for HCWs and patients, as has been successfully piloted in selected long-term care facilities. Patients who refuse to allow testing may have to accept that their care may be compromised. For preservation of HCW supply and maintenance of HCW morale, hospital policies should make explicit, without punishment or coercion, that HCWs may modify the care they provide to patients who refuse to consent to COVID-19 testing.
1. Morris NP. Refusing testing during a pandemic. Am J Public Health. 2020;110(9):1354-1355. https://doi.org/10.2105/AJPH.2020.305810
2. Rubin R. First it was masks; now some refuse testing for SARS-CoV-2. JAMA. 2020;324(20):2015-2016. https://doi.org/10.1001/jama.2020.22003
3. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/S0140-6736(20)30917-X
4. McBee SM, Thomasson ED, Scott MA, et al. Notes from the field: universal statewide laboratory testing for SARS-CoV-2 in nursing homes—West Virginia, April 21–May 8, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(34):1177-1179. http://dx.doi.org/10.15585/mmwr.mm6934a4
5. Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis. 2021;72(2):323-326. https://doi.org/10.1093/cid/ciaa722
6. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. https://advances.sciencemag.org/content/7/1/eabd5393
7. Lu AC, Burgart AM. Elective surgery and COVID-19: a framework for the untested patient. Ann Surg. 2020;272(6):e291-e295. https://doi.org/10.1097/SLA.0000000000004474
8. Podboy A, Cholankeril G, Cianfichi L, Guzman E Jr, Ahmed A, Banerjee S. Implementation and impact of universal preprocedure testing of patients for COVID-19 before endoscopy. Gastroenterology. 2020;159(4):1586-1588. https://doi.org/10.1053/j.gastro.2020.06.022
9. O’Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4-7. https://doi.org/10.1136/jme.29.1.4
10. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part II: the autonomy model. Chest. 2011;139(6):1491-1497. https://doi.org/10.1378/chest.11-0516
11. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part I: the beneficence model. Chest. 2011;139(3):669-673. https://doi.org/10.1378/chest.10-2532
12. Hendrix KS, Sturm LA, Zimet GD, Meslin EM. Ethics and childhood vaccination policy in the United States. Am J Public Health. 2016;106(2):273-278. https://doi.org/10.2105/AJPH.2015.302952
13. Losina E, Leifer V, Millham L, et al. College campuses and COVID-19 mitigation: clinical and economic value. Ann Intern Med. Published online December 21, 2020. https://doi.org/10.7326/M20-6558
14. Selph SS, Bougatsos C, Dana T, Grusing S, Chou R. Screening for HIV infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(23):2349-2360. https://doi.org/10.1001/jama.2019.2593
15. Dranove D, White WD. Agency and the organization of health care delivery. Inquiry. 1987;24(4):405-415.
16. Huber SJ, Wynia MK. When pestilence prevails...physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-W11. https://www.tandfonline.com/doi/abs/10.1162/152651604773067497
17. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
18. Freeman SW, Chambers CV. Compliance with universal precautions in a medical practice with a high rate of HIV infection. J Am Board Fam Pract. 1992;5(3):313-318.
Despite the important clinical and public health implications of a COVID-19 diagnosis, respect for autonomy allows patients to decline testing without explanation and with impunity. Whether physicians believe a test is indicated for clinical care of an individual patient, prevention of nosocomial transmission, or the greater public health, patients may refuse. Such refusals may be increasing due to quarantine requirements, concerns regarding contact tracing, and the persistent absence of a curative treatment.1,2 Mass screening of all healthcare workers (HCWs) is being considered to prevent hospital transmission,3 and universal screening in nursing homes has thwarted outbreaks while providing data to facilitate resource allocation.4 Given these circumstances, patients’ absolute right to refuse a noninvasive test with the potential for multifaceted downstream benefit is worthy of reconsideration, in favor of mandatory testing. Mandatory testing confers numerous benefits, including mitigating risk to other patients and HCWs, who play a central role in pandemic response. Because infected HCWs may transmit the virus to patients, they also should undergo mandatory testing,3 particularly in the presence of symptoms, since nasal secretions increase the diagnostic yield of testing.5 Although pretest probability (as an estimate of disease prevalence) typically determines the testing strategy for admitted patients, model-based analyses suggest that testing every 3 days for HCWs or continuously hospitalized patients would nearly eliminate infectivity.6
Tools for assisting frustrated HCWs navigating patients’ right to refuse testing have been developed that incorporate education, clear communication, and conflict resolution.7 Such approaches are, however, only moderately successful, making the use of personal protective equipment (PPE) based on a default assumption of COVID-19 positivity common.8 The burden and disheartening waste created by low-yield PPE use among patients unwilling to be tested becomes particularly evident in the context of shortages. Such vexing, stressful shortages, as well as the dual responsibilities of hospitals as stewards of both individual patient and population health, serve as reminders that efficient allocation of resources must be valued alongside the autonomous rights of patients.9 Moreover, recent reports suggest that test avoidance is a growing problem.1,2 Refusal to accept testing may be rooted in anxiety, concerns about the consequences of a positive result (eg, inability to attend school or work), or a desire for self-determination.1,2 The hesitancy that leads to refusal may also arise from misinformation, poor public health messaging, distrust in the establishment, and unproductive considerations related to conscientious objection without foundation.2 Concepts of individual liberty that often underlie steadfast adherence to the principles of self-determination created opposition to masks that antagonized public health efforts to limit the spread of COVID-19. Although influencing inpatients’ behavior to benefit both the public and HCWs may be distinct from community settings, the attitudes that lead to test refusal and defiance of mask-related ordinances likely have substantial commonalities.
THE PATIENT ROLE IN HEALTHCARE DECISIONS
As a pillar of ethical
ANALOGOUS EXPERIENCES: ETHICAL LESSONS AND PRACTICAL IMPLICATIONS
In non-healthcare settings, the controversies surrounding vaccination and access to schools for unvaccinated children are perhaps the public and professional debate most analogous to COVID-19 testing refusal.12 Although policymakers may distinguish between testing and vaccination, these interventions similarly hold the potential to limit disease incidence and mitigate health impact. To preserve public health, most states prevent (with varied exemptions) unvaccinated children from attending schools. COVID-19 testing may in the future become a requirement for participation in group social activities, athletic competitions, or physical presence in the workplace to facilitate quarantining and/or targeted use of PPE for transmission risk reduction. Given the dramatic mitigation benefits accruable on college campuses,13 required testing for in-person learning has become common.
There are also parallels, and therefore lessons, to be drawn from experience in testing for HIV, although HIV-related stigma and devalued status of the marginalized populations initially infected impacted the broader societal view of HIV compared with COVID-19. For example, antenatal HIV screening of pregnant women is strongly recommended to facilitate interventions that reduce the chance of vertical transmission.14 The limitations of purely elective testing are one justification for the current standard of opt-out screening. However, in this case, the health complications of refusal are largely the burden of the fetus, over whose future the mother holds a great deal of choice and responsibility, irrespective of HIV status. The public health implications of HIV test refusal are far less immediate than for COVID-19 infection because there is no effective curative therapy for COVID-19 and spread occurs through nonintimate, unintentional, and unpredictable exposure.
Translating societal attitudes and practices into the healthcare setting to consider mandated COVID-19 testing requires additional considerations related to both patients and providers: (1) HCWs have committed to a set of values and professional obligations that include tasks requiring risks15; (2) the public expects HCWs to perform their duties according to a social contract that has few restrictions16; (3) limiting patient access to hospital care due to COVID-19 testing refusal would contradict and create conflicts related to professional conceptions of hospitals and physicians as patient agents15; and (4) patients who conscientiously object to testing may seek healthcare less diligently, which may lead to health decrements. The associated postponement of essential care may unduly burden the healthcare system, particularly in situations such as ambulatory care–sensitive conditions.
HEALTHCARE WORKER PROTECTION, PATIENT ACCESS, AND THE VALUE OF PARSIMONY
The extent to which the public health justification for mandatory testing extends to hospitalized patients to protect HCWs is ambiguous. HCWs are of enormous instrumental value and are therefore essential for the pandemic response and health of the broader population. Their protection may therefore justify curtailing informed consent for diagnostic testing. Downstream effects on the supply of frontline HCWs may be realized. Poor control over working conditions may negatively impact motivation among HCWs. In addition, they may feel disenfranchised while obligatorily taking personal risks in caring for patients unwilling to commit to the common good through diagnostic test consent. Hospitalized patients who refuse testing may remain patients under investigation (PUIs), thus requiring special respiratory precautions (SRP) throughout their hospitalization, thereby placing a persistent burden on those with responsibilities requiring patient contact.17 Repeatedly donning and doffing PPE may remind at-risk HCWs that a myriad of benefits may accrue from frequent, ubiquitous testing. Their motivation may be tempered by the demoralizing requirement to care for patients who will not consent to a simple test, knowing that an opportunity to diminish the burdens of this communicable disease that has taken the lives of many HCWs is being relinquished.
Although HCWs could use SRP universally, their selective application in rooms of known COVID-19–positive patients and those with temporary PUI status has several advantages.17 First, we learned that HIV testing on patients was helpful in enabling surgeons to selectively implement special precautions among infected patients rather than universally applied intensive precautions. Even in the setting of high rates of HIV infection and educational interventions, HCWs do not reliably apply protective measures included in universal precautions.18 In keeping with these experiences, limiting the number of patients on SRP will minimize the “precautions fatigue” that drives nonadherent behavior among HCWs.17 As a result, minimizing the proportion of patients on SRP through testing (and liberation from unnecessary precautions in most cases) will improve uptake of crucial hand hygiene practices and adoption of vigilant PPE use. Second, definitive knowledge of COVID-19 status will increase patient access to care because, whether by personal choice or policy, many HCWs limit in-person contact with patients who are or may be COVID-19 positive. For example, many inpatient dialysis units do not accept patients without a negative COVID-19 nasal swab. Physical therapists may delay or avoid seeing a PUI, which will pose challenges for efficient determination of discharge disposition. Third, selective use of SRP will limit the environmental impact of disposed PPE, which is neither recyclable nor biodegradable. Infectious or regulated biomedical waste products are a significant source of environmental pollution, and the World Health Organization has recommended parsimonious, selective use of PPE to minimize the adverse environmental consequences of biomedical waste products.
CONCLUSION
In summary, there are substantial justifications for mandatory testing for COVID-19 in the hospital for HCWs and patients, as has been successfully piloted in selected long-term care facilities. Patients who refuse to allow testing may have to accept that their care may be compromised. For preservation of HCW supply and maintenance of HCW morale, hospital policies should make explicit, without punishment or coercion, that HCWs may modify the care they provide to patients who refuse to consent to COVID-19 testing.
Despite the important clinical and public health implications of a COVID-19 diagnosis, respect for autonomy allows patients to decline testing without explanation and with impunity. Whether physicians believe a test is indicated for clinical care of an individual patient, prevention of nosocomial transmission, or the greater public health, patients may refuse. Such refusals may be increasing due to quarantine requirements, concerns regarding contact tracing, and the persistent absence of a curative treatment.1,2 Mass screening of all healthcare workers (HCWs) is being considered to prevent hospital transmission,3 and universal screening in nursing homes has thwarted outbreaks while providing data to facilitate resource allocation.4 Given these circumstances, patients’ absolute right to refuse a noninvasive test with the potential for multifaceted downstream benefit is worthy of reconsideration, in favor of mandatory testing. Mandatory testing confers numerous benefits, including mitigating risk to other patients and HCWs, who play a central role in pandemic response. Because infected HCWs may transmit the virus to patients, they also should undergo mandatory testing,3 particularly in the presence of symptoms, since nasal secretions increase the diagnostic yield of testing.5 Although pretest probability (as an estimate of disease prevalence) typically determines the testing strategy for admitted patients, model-based analyses suggest that testing every 3 days for HCWs or continuously hospitalized patients would nearly eliminate infectivity.6
Tools for assisting frustrated HCWs navigating patients’ right to refuse testing have been developed that incorporate education, clear communication, and conflict resolution.7 Such approaches are, however, only moderately successful, making the use of personal protective equipment (PPE) based on a default assumption of COVID-19 positivity common.8 The burden and disheartening waste created by low-yield PPE use among patients unwilling to be tested becomes particularly evident in the context of shortages. Such vexing, stressful shortages, as well as the dual responsibilities of hospitals as stewards of both individual patient and population health, serve as reminders that efficient allocation of resources must be valued alongside the autonomous rights of patients.9 Moreover, recent reports suggest that test avoidance is a growing problem.1,2 Refusal to accept testing may be rooted in anxiety, concerns about the consequences of a positive result (eg, inability to attend school or work), or a desire for self-determination.1,2 The hesitancy that leads to refusal may also arise from misinformation, poor public health messaging, distrust in the establishment, and unproductive considerations related to conscientious objection without foundation.2 Concepts of individual liberty that often underlie steadfast adherence to the principles of self-determination created opposition to masks that antagonized public health efforts to limit the spread of COVID-19. Although influencing inpatients’ behavior to benefit both the public and HCWs may be distinct from community settings, the attitudes that lead to test refusal and defiance of mask-related ordinances likely have substantial commonalities.
THE PATIENT ROLE IN HEALTHCARE DECISIONS
As a pillar of ethical
ANALOGOUS EXPERIENCES: ETHICAL LESSONS AND PRACTICAL IMPLICATIONS
In non-healthcare settings, the controversies surrounding vaccination and access to schools for unvaccinated children are perhaps the public and professional debate most analogous to COVID-19 testing refusal.12 Although policymakers may distinguish between testing and vaccination, these interventions similarly hold the potential to limit disease incidence and mitigate health impact. To preserve public health, most states prevent (with varied exemptions) unvaccinated children from attending schools. COVID-19 testing may in the future become a requirement for participation in group social activities, athletic competitions, or physical presence in the workplace to facilitate quarantining and/or targeted use of PPE for transmission risk reduction. Given the dramatic mitigation benefits accruable on college campuses,13 required testing for in-person learning has become common.
There are also parallels, and therefore lessons, to be drawn from experience in testing for HIV, although HIV-related stigma and devalued status of the marginalized populations initially infected impacted the broader societal view of HIV compared with COVID-19. For example, antenatal HIV screening of pregnant women is strongly recommended to facilitate interventions that reduce the chance of vertical transmission.14 The limitations of purely elective testing are one justification for the current standard of opt-out screening. However, in this case, the health complications of refusal are largely the burden of the fetus, over whose future the mother holds a great deal of choice and responsibility, irrespective of HIV status. The public health implications of HIV test refusal are far less immediate than for COVID-19 infection because there is no effective curative therapy for COVID-19 and spread occurs through nonintimate, unintentional, and unpredictable exposure.
Translating societal attitudes and practices into the healthcare setting to consider mandated COVID-19 testing requires additional considerations related to both patients and providers: (1) HCWs have committed to a set of values and professional obligations that include tasks requiring risks15; (2) the public expects HCWs to perform their duties according to a social contract that has few restrictions16; (3) limiting patient access to hospital care due to COVID-19 testing refusal would contradict and create conflicts related to professional conceptions of hospitals and physicians as patient agents15; and (4) patients who conscientiously object to testing may seek healthcare less diligently, which may lead to health decrements. The associated postponement of essential care may unduly burden the healthcare system, particularly in situations such as ambulatory care–sensitive conditions.
HEALTHCARE WORKER PROTECTION, PATIENT ACCESS, AND THE VALUE OF PARSIMONY
The extent to which the public health justification for mandatory testing extends to hospitalized patients to protect HCWs is ambiguous. HCWs are of enormous instrumental value and are therefore essential for the pandemic response and health of the broader population. Their protection may therefore justify curtailing informed consent for diagnostic testing. Downstream effects on the supply of frontline HCWs may be realized. Poor control over working conditions may negatively impact motivation among HCWs. In addition, they may feel disenfranchised while obligatorily taking personal risks in caring for patients unwilling to commit to the common good through diagnostic test consent. Hospitalized patients who refuse testing may remain patients under investigation (PUIs), thus requiring special respiratory precautions (SRP) throughout their hospitalization, thereby placing a persistent burden on those with responsibilities requiring patient contact.17 Repeatedly donning and doffing PPE may remind at-risk HCWs that a myriad of benefits may accrue from frequent, ubiquitous testing. Their motivation may be tempered by the demoralizing requirement to care for patients who will not consent to a simple test, knowing that an opportunity to diminish the burdens of this communicable disease that has taken the lives of many HCWs is being relinquished.
Although HCWs could use SRP universally, their selective application in rooms of known COVID-19–positive patients and those with temporary PUI status has several advantages.17 First, we learned that HIV testing on patients was helpful in enabling surgeons to selectively implement special precautions among infected patients rather than universally applied intensive precautions. Even in the setting of high rates of HIV infection and educational interventions, HCWs do not reliably apply protective measures included in universal precautions.18 In keeping with these experiences, limiting the number of patients on SRP will minimize the “precautions fatigue” that drives nonadherent behavior among HCWs.17 As a result, minimizing the proportion of patients on SRP through testing (and liberation from unnecessary precautions in most cases) will improve uptake of crucial hand hygiene practices and adoption of vigilant PPE use. Second, definitive knowledge of COVID-19 status will increase patient access to care because, whether by personal choice or policy, many HCWs limit in-person contact with patients who are or may be COVID-19 positive. For example, many inpatient dialysis units do not accept patients without a negative COVID-19 nasal swab. Physical therapists may delay or avoid seeing a PUI, which will pose challenges for efficient determination of discharge disposition. Third, selective use of SRP will limit the environmental impact of disposed PPE, which is neither recyclable nor biodegradable. Infectious or regulated biomedical waste products are a significant source of environmental pollution, and the World Health Organization has recommended parsimonious, selective use of PPE to minimize the adverse environmental consequences of biomedical waste products.
CONCLUSION
In summary, there are substantial justifications for mandatory testing for COVID-19 in the hospital for HCWs and patients, as has been successfully piloted in selected long-term care facilities. Patients who refuse to allow testing may have to accept that their care may be compromised. For preservation of HCW supply and maintenance of HCW morale, hospital policies should make explicit, without punishment or coercion, that HCWs may modify the care they provide to patients who refuse to consent to COVID-19 testing.
1. Morris NP. Refusing testing during a pandemic. Am J Public Health. 2020;110(9):1354-1355. https://doi.org/10.2105/AJPH.2020.305810
2. Rubin R. First it was masks; now some refuse testing for SARS-CoV-2. JAMA. 2020;324(20):2015-2016. https://doi.org/10.1001/jama.2020.22003
3. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/S0140-6736(20)30917-X
4. McBee SM, Thomasson ED, Scott MA, et al. Notes from the field: universal statewide laboratory testing for SARS-CoV-2 in nursing homes—West Virginia, April 21–May 8, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(34):1177-1179. http://dx.doi.org/10.15585/mmwr.mm6934a4
5. Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis. 2021;72(2):323-326. https://doi.org/10.1093/cid/ciaa722
6. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. https://advances.sciencemag.org/content/7/1/eabd5393
7. Lu AC, Burgart AM. Elective surgery and COVID-19: a framework for the untested patient. Ann Surg. 2020;272(6):e291-e295. https://doi.org/10.1097/SLA.0000000000004474
8. Podboy A, Cholankeril G, Cianfichi L, Guzman E Jr, Ahmed A, Banerjee S. Implementation and impact of universal preprocedure testing of patients for COVID-19 before endoscopy. Gastroenterology. 2020;159(4):1586-1588. https://doi.org/10.1053/j.gastro.2020.06.022
9. O’Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4-7. https://doi.org/10.1136/jme.29.1.4
10. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part II: the autonomy model. Chest. 2011;139(6):1491-1497. https://doi.org/10.1378/chest.11-0516
11. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part I: the beneficence model. Chest. 2011;139(3):669-673. https://doi.org/10.1378/chest.10-2532
12. Hendrix KS, Sturm LA, Zimet GD, Meslin EM. Ethics and childhood vaccination policy in the United States. Am J Public Health. 2016;106(2):273-278. https://doi.org/10.2105/AJPH.2015.302952
13. Losina E, Leifer V, Millham L, et al. College campuses and COVID-19 mitigation: clinical and economic value. Ann Intern Med. Published online December 21, 2020. https://doi.org/10.7326/M20-6558
14. Selph SS, Bougatsos C, Dana T, Grusing S, Chou R. Screening for HIV infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(23):2349-2360. https://doi.org/10.1001/jama.2019.2593
15. Dranove D, White WD. Agency and the organization of health care delivery. Inquiry. 1987;24(4):405-415.
16. Huber SJ, Wynia MK. When pestilence prevails...physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-W11. https://www.tandfonline.com/doi/abs/10.1162/152651604773067497
17. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
18. Freeman SW, Chambers CV. Compliance with universal precautions in a medical practice with a high rate of HIV infection. J Am Board Fam Pract. 1992;5(3):313-318.
1. Morris NP. Refusing testing during a pandemic. Am J Public Health. 2020;110(9):1354-1355. https://doi.org/10.2105/AJPH.2020.305810
2. Rubin R. First it was masks; now some refuse testing for SARS-CoV-2. JAMA. 2020;324(20):2015-2016. https://doi.org/10.1001/jama.2020.22003
3. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/S0140-6736(20)30917-X
4. McBee SM, Thomasson ED, Scott MA, et al. Notes from the field: universal statewide laboratory testing for SARS-CoV-2 in nursing homes—West Virginia, April 21–May 8, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(34):1177-1179. http://dx.doi.org/10.15585/mmwr.mm6934a4
5. Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis. 2021;72(2):323-326. https://doi.org/10.1093/cid/ciaa722
6. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. https://advances.sciencemag.org/content/7/1/eabd5393
7. Lu AC, Burgart AM. Elective surgery and COVID-19: a framework for the untested patient. Ann Surg. 2020;272(6):e291-e295. https://doi.org/10.1097/SLA.0000000000004474
8. Podboy A, Cholankeril G, Cianfichi L, Guzman E Jr, Ahmed A, Banerjee S. Implementation and impact of universal preprocedure testing of patients for COVID-19 before endoscopy. Gastroenterology. 2020;159(4):1586-1588. https://doi.org/10.1053/j.gastro.2020.06.022
9. O’Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4-7. https://doi.org/10.1136/jme.29.1.4
10. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part II: the autonomy model. Chest. 2011;139(6):1491-1497. https://doi.org/10.1378/chest.11-0516
11. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part I: the beneficence model. Chest. 2011;139(3):669-673. https://doi.org/10.1378/chest.10-2532
12. Hendrix KS, Sturm LA, Zimet GD, Meslin EM. Ethics and childhood vaccination policy in the United States. Am J Public Health. 2016;106(2):273-278. https://doi.org/10.2105/AJPH.2015.302952
13. Losina E, Leifer V, Millham L, et al. College campuses and COVID-19 mitigation: clinical and economic value. Ann Intern Med. Published online December 21, 2020. https://doi.org/10.7326/M20-6558
14. Selph SS, Bougatsos C, Dana T, Grusing S, Chou R. Screening for HIV infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(23):2349-2360. https://doi.org/10.1001/jama.2019.2593
15. Dranove D, White WD. Agency and the organization of health care delivery. Inquiry. 1987;24(4):405-415.
16. Huber SJ, Wynia MK. When pestilence prevails...physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-W11. https://www.tandfonline.com/doi/abs/10.1162/152651604773067497
17. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
18. Freeman SW, Chambers CV. Compliance with universal precautions in a medical practice with a high rate of HIV infection. J Am Board Fam Pract. 1992;5(3):313-318.
© 2021 Society of Hospital Medicine
Physician-Driven Discretionary Utilization: Measuring Overuse and Choosing Wisely
Overutilization and low-value care are important clinical and policy problems. Their measurement is challenging because it requires detailed clinical information. Additionally, there are inherent difficulties in identifying discretionary services likely to be inappropriate or low-value and demonstrating that certain services produce little/no health benefit. Quantifying “ideal” expected testing rates—ones that would reflect minimization of inappropriate/low-value care without excluding essential, high-yield diagnostic services—presents additional challenges. Consequently, of 521 unique measures specified by national measurement programs and professional guidelines, 91.6% targeted underuse, while only 6.5% targeted overuse.1
The potential for unintended consequences of implementing measures to eliminate overuse are a barrier to incorporating such measures into practice.2 For example, measuring, reporting, and penalizing overuse of inappropriate bone scanning may lead to underuse in patients for whom scanning is crucial.2 Most overuse measures based on inappropriate or low-value indications relate to imaging and medications.1 However, there is increasing interest in overutilization measures based on a broad set of health services. Identifying low-value testing or treatments often requires a substantial degree of clinical detail to avoid the damaging inclusion of beneficial services, which may lead to unintended negative outcomes, creating skepticism among clinicians. Ultimately, getting measurement of low-value care wrong would undermine adoption of interventions to reduce overuse.
To reduce low-value care through expansive measures of provider ordering behavior,3 Ellenbogen et al4 derived a novel index to identify hospitals with high rates of low-yield diagnostic testing. This index is based on the concept that, in the presence of nonspecific, symptom-based principal diagnoses, a substantial proportion of (apparently) non-diagnostic related studies were probably ordered despite a low pretest probability of serious disease. Since such symptom-based diagnoses reflect the absence of a more specific diagnosis, the examinations observed are markers of physician-driven decisions leading to discretionary utilization likely to be of low-value to patients. This study fills a critical gap in dual measures of appropriateness and yield, rather than simply utilization, to advance the Choosing Wisely campaign.3
Advantages of this overuse index include its derivation from administrative data, obviating the need for electronic health records, and incorporation of diagnostic yield at the inpatient-encounter level. One study selected procedures identifiable solely with claims from a set deemed overused by professional/consumer groups.5 However, the yield of physician decisions in specific cases was not measured. In contrast, this novel index is derived from an assessment of diagnostic yield.4 Although test results are not known with certainty, the absence of a specific discharge diagnosis serves as a test result proxy. Measurement of diagnostic examination yield at the patient-level (aggregated to the hospital-level) may be applicable across hospitals with varied patient populations, which include large differences in patient and/or family preferences to seek medical attention and engage in shared decision-making. The role that patient preferences play in decisions creates a limitation in this index—while decisions for the candidate diagnostic tests are physician driven, patient demand may be a confounding factor. This index cannot therefore be considered purely a measure of physician-induced intensity of diagnostic services. Patient-reported data would enhance future analyses by more fully capturing all dimensions of care necessary to identify low-value services. Subjective outcomes are critical in completely measuring the aggregate benefits of tests and interventions judged low-value based on objective metrics. Such data would also aid in quantifying the relative contributions of patient and physician preferences in driving discretionary utilization.
Finally, the derived index is restricted to diagnostic decision-making and may not be applicable to treatment-related practice patterns. However, the literature suggests strong correlations between diagnostic and therapeutic intensity. Application of this novel index will play an important role in reducing low-value discretionary utilization.
1. Newton EH, Zazzera EA, Van Moorsel G, Sirovich BE. Undermeasuring overuse--an examination of national clinical performance measures. JAMA Intern Med. 2015;175(10):1709-1711. https://doi.org/10.1001/jamainternmed.2015.4025
2. Mathias JS, Baker DW. Developing quality measures to address overuse. JAMA. 2013;309(18):1897-1898. https://doi.org/10.1001/jama.2013.3588
3. Bhatia RS, Levinson W, Shortt S, et al. Measuring the effect of Choosing Wisely: an integrated framework to assess campaign impact on low-value care. BMJ Qual Saf. 2015;24(8):523-531. https://doi.org/10.1136/bmjqs-2015-004070
4. Ellenbogen MI, Prichett L, Johnson PT, Brotman DJ. Development of a simple index to measure overuse of diagnostic testing at the hospital level using administrative data. J Hosp Med. 2021;16:xxx-xxx. https://doi.org/10.12788/jhm.3547
5. Segal JB, Bridges JF, Chang HY, et al. Identifying possible indicators of systematic overuse of health care procedures with claims data. Med Care. 2014;52(2):157-163. https://doi.org/10.1097/MLR.0000000000000052
Overutilization and low-value care are important clinical and policy problems. Their measurement is challenging because it requires detailed clinical information. Additionally, there are inherent difficulties in identifying discretionary services likely to be inappropriate or low-value and demonstrating that certain services produce little/no health benefit. Quantifying “ideal” expected testing rates—ones that would reflect minimization of inappropriate/low-value care without excluding essential, high-yield diagnostic services—presents additional challenges. Consequently, of 521 unique measures specified by national measurement programs and professional guidelines, 91.6% targeted underuse, while only 6.5% targeted overuse.1
The potential for unintended consequences of implementing measures to eliminate overuse are a barrier to incorporating such measures into practice.2 For example, measuring, reporting, and penalizing overuse of inappropriate bone scanning may lead to underuse in patients for whom scanning is crucial.2 Most overuse measures based on inappropriate or low-value indications relate to imaging and medications.1 However, there is increasing interest in overutilization measures based on a broad set of health services. Identifying low-value testing or treatments often requires a substantial degree of clinical detail to avoid the damaging inclusion of beneficial services, which may lead to unintended negative outcomes, creating skepticism among clinicians. Ultimately, getting measurement of low-value care wrong would undermine adoption of interventions to reduce overuse.
To reduce low-value care through expansive measures of provider ordering behavior,3 Ellenbogen et al4 derived a novel index to identify hospitals with high rates of low-yield diagnostic testing. This index is based on the concept that, in the presence of nonspecific, symptom-based principal diagnoses, a substantial proportion of (apparently) non-diagnostic related studies were probably ordered despite a low pretest probability of serious disease. Since such symptom-based diagnoses reflect the absence of a more specific diagnosis, the examinations observed are markers of physician-driven decisions leading to discretionary utilization likely to be of low-value to patients. This study fills a critical gap in dual measures of appropriateness and yield, rather than simply utilization, to advance the Choosing Wisely campaign.3
Advantages of this overuse index include its derivation from administrative data, obviating the need for electronic health records, and incorporation of diagnostic yield at the inpatient-encounter level. One study selected procedures identifiable solely with claims from a set deemed overused by professional/consumer groups.5 However, the yield of physician decisions in specific cases was not measured. In contrast, this novel index is derived from an assessment of diagnostic yield.4 Although test results are not known with certainty, the absence of a specific discharge diagnosis serves as a test result proxy. Measurement of diagnostic examination yield at the patient-level (aggregated to the hospital-level) may be applicable across hospitals with varied patient populations, which include large differences in patient and/or family preferences to seek medical attention and engage in shared decision-making. The role that patient preferences play in decisions creates a limitation in this index—while decisions for the candidate diagnostic tests are physician driven, patient demand may be a confounding factor. This index cannot therefore be considered purely a measure of physician-induced intensity of diagnostic services. Patient-reported data would enhance future analyses by more fully capturing all dimensions of care necessary to identify low-value services. Subjective outcomes are critical in completely measuring the aggregate benefits of tests and interventions judged low-value based on objective metrics. Such data would also aid in quantifying the relative contributions of patient and physician preferences in driving discretionary utilization.
Finally, the derived index is restricted to diagnostic decision-making and may not be applicable to treatment-related practice patterns. However, the literature suggests strong correlations between diagnostic and therapeutic intensity. Application of this novel index will play an important role in reducing low-value discretionary utilization.
Overutilization and low-value care are important clinical and policy problems. Their measurement is challenging because it requires detailed clinical information. Additionally, there are inherent difficulties in identifying discretionary services likely to be inappropriate or low-value and demonstrating that certain services produce little/no health benefit. Quantifying “ideal” expected testing rates—ones that would reflect minimization of inappropriate/low-value care without excluding essential, high-yield diagnostic services—presents additional challenges. Consequently, of 521 unique measures specified by national measurement programs and professional guidelines, 91.6% targeted underuse, while only 6.5% targeted overuse.1
The potential for unintended consequences of implementing measures to eliminate overuse are a barrier to incorporating such measures into practice.2 For example, measuring, reporting, and penalizing overuse of inappropriate bone scanning may lead to underuse in patients for whom scanning is crucial.2 Most overuse measures based on inappropriate or low-value indications relate to imaging and medications.1 However, there is increasing interest in overutilization measures based on a broad set of health services. Identifying low-value testing or treatments often requires a substantial degree of clinical detail to avoid the damaging inclusion of beneficial services, which may lead to unintended negative outcomes, creating skepticism among clinicians. Ultimately, getting measurement of low-value care wrong would undermine adoption of interventions to reduce overuse.
To reduce low-value care through expansive measures of provider ordering behavior,3 Ellenbogen et al4 derived a novel index to identify hospitals with high rates of low-yield diagnostic testing. This index is based on the concept that, in the presence of nonspecific, symptom-based principal diagnoses, a substantial proportion of (apparently) non-diagnostic related studies were probably ordered despite a low pretest probability of serious disease. Since such symptom-based diagnoses reflect the absence of a more specific diagnosis, the examinations observed are markers of physician-driven decisions leading to discretionary utilization likely to be of low-value to patients. This study fills a critical gap in dual measures of appropriateness and yield, rather than simply utilization, to advance the Choosing Wisely campaign.3
Advantages of this overuse index include its derivation from administrative data, obviating the need for electronic health records, and incorporation of diagnostic yield at the inpatient-encounter level. One study selected procedures identifiable solely with claims from a set deemed overused by professional/consumer groups.5 However, the yield of physician decisions in specific cases was not measured. In contrast, this novel index is derived from an assessment of diagnostic yield.4 Although test results are not known with certainty, the absence of a specific discharge diagnosis serves as a test result proxy. Measurement of diagnostic examination yield at the patient-level (aggregated to the hospital-level) may be applicable across hospitals with varied patient populations, which include large differences in patient and/or family preferences to seek medical attention and engage in shared decision-making. The role that patient preferences play in decisions creates a limitation in this index—while decisions for the candidate diagnostic tests are physician driven, patient demand may be a confounding factor. This index cannot therefore be considered purely a measure of physician-induced intensity of diagnostic services. Patient-reported data would enhance future analyses by more fully capturing all dimensions of care necessary to identify low-value services. Subjective outcomes are critical in completely measuring the aggregate benefits of tests and interventions judged low-value based on objective metrics. Such data would also aid in quantifying the relative contributions of patient and physician preferences in driving discretionary utilization.
Finally, the derived index is restricted to diagnostic decision-making and may not be applicable to treatment-related practice patterns. However, the literature suggests strong correlations between diagnostic and therapeutic intensity. Application of this novel index will play an important role in reducing low-value discretionary utilization.
1. Newton EH, Zazzera EA, Van Moorsel G, Sirovich BE. Undermeasuring overuse--an examination of national clinical performance measures. JAMA Intern Med. 2015;175(10):1709-1711. https://doi.org/10.1001/jamainternmed.2015.4025
2. Mathias JS, Baker DW. Developing quality measures to address overuse. JAMA. 2013;309(18):1897-1898. https://doi.org/10.1001/jama.2013.3588
3. Bhatia RS, Levinson W, Shortt S, et al. Measuring the effect of Choosing Wisely: an integrated framework to assess campaign impact on low-value care. BMJ Qual Saf. 2015;24(8):523-531. https://doi.org/10.1136/bmjqs-2015-004070
4. Ellenbogen MI, Prichett L, Johnson PT, Brotman DJ. Development of a simple index to measure overuse of diagnostic testing at the hospital level using administrative data. J Hosp Med. 2021;16:xxx-xxx. https://doi.org/10.12788/jhm.3547
5. Segal JB, Bridges JF, Chang HY, et al. Identifying possible indicators of systematic overuse of health care procedures with claims data. Med Care. 2014;52(2):157-163. https://doi.org/10.1097/MLR.0000000000000052
1. Newton EH, Zazzera EA, Van Moorsel G, Sirovich BE. Undermeasuring overuse--an examination of national clinical performance measures. JAMA Intern Med. 2015;175(10):1709-1711. https://doi.org/10.1001/jamainternmed.2015.4025
2. Mathias JS, Baker DW. Developing quality measures to address overuse. JAMA. 2013;309(18):1897-1898. https://doi.org/10.1001/jama.2013.3588
3. Bhatia RS, Levinson W, Shortt S, et al. Measuring the effect of Choosing Wisely: an integrated framework to assess campaign impact on low-value care. BMJ Qual Saf. 2015;24(8):523-531. https://doi.org/10.1136/bmjqs-2015-004070
4. Ellenbogen MI, Prichett L, Johnson PT, Brotman DJ. Development of a simple index to measure overuse of diagnostic testing at the hospital level using administrative data. J Hosp Med. 2021;16:xxx-xxx. https://doi.org/10.12788/jhm.3547
5. Segal JB, Bridges JF, Chang HY, et al. Identifying possible indicators of systematic overuse of health care procedures with claims data. Med Care. 2014;52(2):157-163. https://doi.org/10.1097/MLR.0000000000000052
© 2021Society of Hospital Medicine
Hospital Ward Adaptation During the COVID-19 Pandemic: A National Survey of Academic Medical Centers
The coronavirus disease of 2019 (COVID-19) pandemic has resulted in a surge in hospitalizations of patients with a novel, serious, and highly contagious infectious disease for which there is yet no proven treatment. Currently, much of the focus has been on intensive care unit (ICU) and ventilator capacity for the sickest of these patients who develop respiratory failure. However, most hospitalized patients are being cared for in general medical units.1 Some evidence exists to describe adaptations to capacity needs outside of medical wards,2-4 but few studies have specifically addressed the ward setting. Therefore, there is a pressing need for evidence to describe how to expand capacity and deliver medical ward–based care.
To better understand how inpatient care in the United States is adapting to the COVID-19 pandemic, we surveyed 72 sites participating in the Hospital Medicine Reengineering Network (HOMERuN), a national consortium of hospital medicine groups.5 We report results of this survey, carried out between April 3 and April 5, 2020.
METHODS
Sites and Subjects
HOMERuN is a collaborative network of hospitalists from across the United States whose primary goal is to catalyze research and share best practices across hospital medicine groups. Using surveys of Hospital Medicine leaders, targeted medical record review, and other methods, HOMERuN’s funded research interests to date have included care transitions, workforce issues, patient and family engagement, and diagnostic errors. Sites participating in HOMERuN sites are relatively large urban academic medical centers (Appendix).
Survey Development and Deployment
We designed a focused survey that aimed to provide a snapshot of evolving operational and clinical aspects of COVID-19 care (Appendix). Domains included COVID-19 testing turnaround times, personal protective equipment (PPE) stewardship,6 features of respiratory isolation units (RIUs; ie, dedicated units for patients with known or suspected COVID-19), and observed effects on clinical care. We tested the instrument to ensure feasibility and clarity internally, performed brief cognitive testing with several hospital medicine leaders in HOMERuN, then disseminated the survey by email on April 3, with two follow-up emails on 2 subsequent days. Our study was deemed non–human subjects research by the University of California, San Francisco, Committee on Human Research. Descriptive statistics were used to characterize survey responses.
RESULTS
Of 72 hospitals surveyed, 51 (71%) responded. Mean hospital bed count was 940, three were safety-net hospitals, and one was a community-based teaching center; responding and nonresponding hospitals did not differ significantly in terms of bed count (Appendix).
Health System Adaptations, Testing, and PPE Status
Nearly all responding hospitals (46 of 51; 90%) had RIUs for patients with known or suspected COVID-19 (Table 1). Nearly all hospitals took steps to keep potentially sick healthcare providers from infecting others (eg, staying home if sick or exposed). Among respondents, 32% had rapid response teams, 24% had respiratory therapy teams, and 29% had case management teams that were dedicated to COVID-19 care. Thirty-two (63%) had developed models, such as ethics or palliative care consult services, to assist with difficult resource-allocation decisions (eg, how to prioritize ventilator use if demand exceeded supply). Twenty-three (45%) had developed post-acute care monitoring programs dedicated to COVID-19 patients.
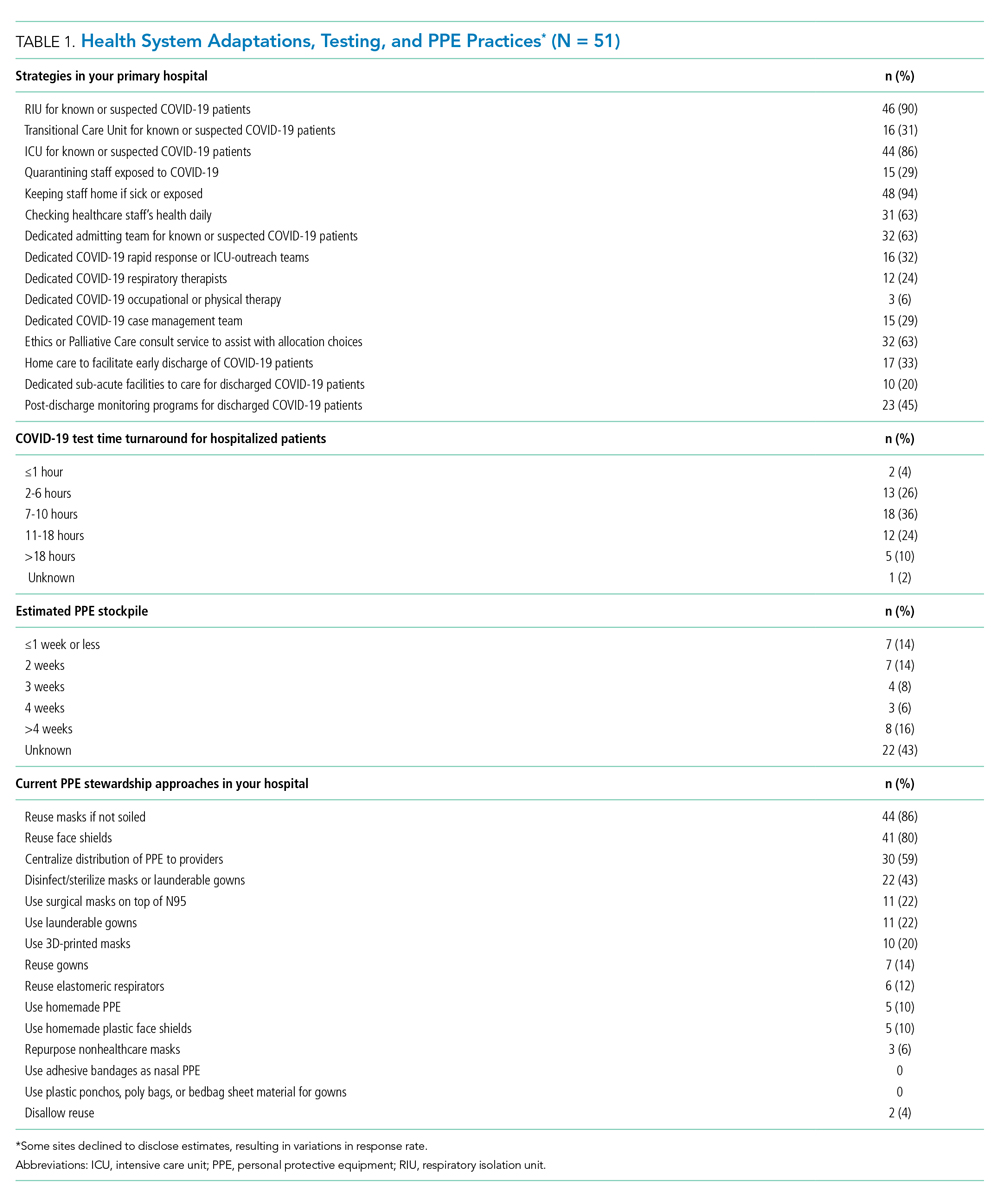
At the time of our survey, only 2 sites (4%) reported COVID-19 test time turnaround under 1 hour, and 15 (30%) reported turnaround in less than 6 hours. Of the 29 sites able to provide estimates of PPE stockpile, 14 (48%) reported a supply of 2 weeks or less. The most common approaches to PPE stewardship focused on reuse of masks and face shields if not obviously soiled, centralizing PPE distribution, and disinfecting or sterilizing masks. Ten sites (20%) were utilizing 3-D printed masks, while 10% used homemade face shields or masks.
Characteristics of COVID-19 RIUs
Forty-six hospitals (90% of all respondents) in our cohort had developed RIUs at the time of survey administration. The earliest RIU implementation date was February 10, 2020, and the most recent was launched on the day of our survey. Admission to RIUs was primarily based on clinical factors associated with known or suspected COVID-19 infection (Table 2). The number of non–critical care RIU beds among locations at that time ranged from 10 or less to more than 50. The mean number of hospitalist attendings caring for patients in the RIUs was 10.2, with a mean 4.1 advanced practice providers, 5.5 residents, and 0 medical students. The number of planned patients per attending was typically 5 to 15. Nurses and physicians typically rounded separately. Medical distancing (eg, reducing patient room entry) was accomplished most commonly by grouped timing of medication administration (76% of sites), video links to room outside of rounding times (54% of sites), the use of video or telemedicine during rounds (17%), and clustering of activities such as medication administration or phlebotomy. The most common criteria prompting discharge from the RIU were a negative COVID-19 test (59%) and hospital discharge (57%), though comments from many respondents suggested that discharge criteria were changing rapidly.

Effects of Isolation Measures on In-Room Encounters and Diagnostic Processes
More than 90% of sites reported decreases in in-room encounter frequency across all provider types whether as a result of policies in place or not. Reductions were reported among hospitalists, advanced practice providers, residents, consultants, and therapists (Table 3). Reduced room entry most often resulted from an established or developing policy, but many noted reduced room entry without formal policies in place. Nearly all sites reported moving specialty consultations to phone or video evaluations. Diagnostic error was commonly reported, with missed non–COVID-19 medical diagnoses among COVID-19 infected patients being reported by 22 sites (46%) and missed COVID-19 diagnoses in patients admitted for other reasons by 22 sites (45%).
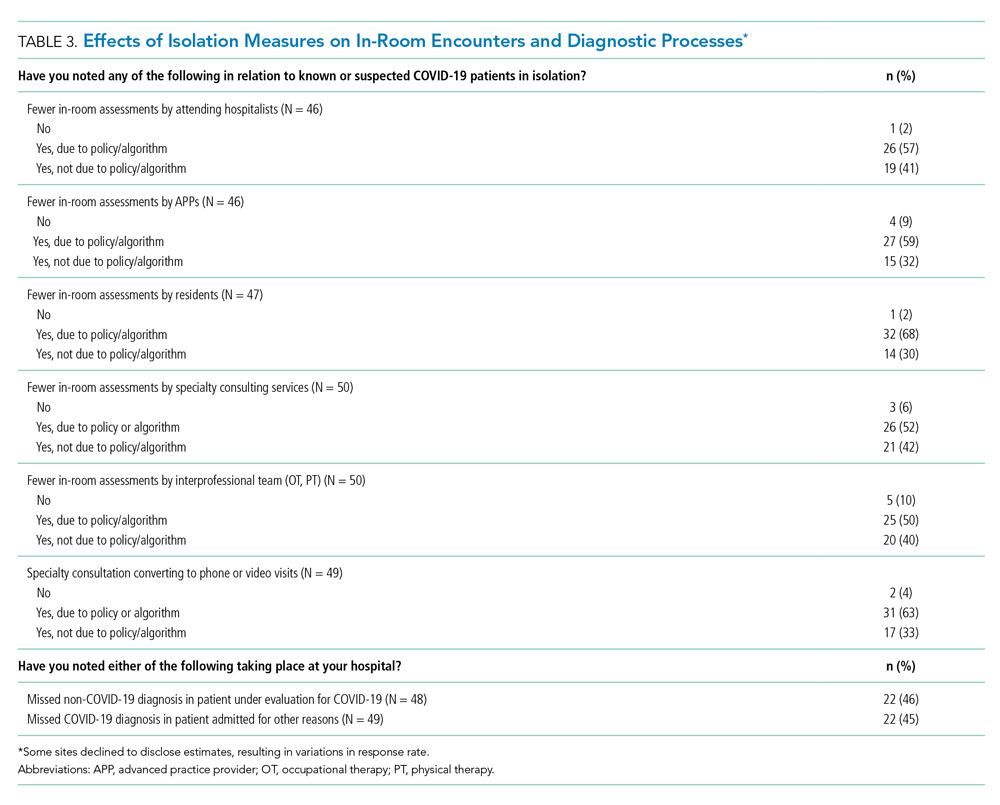
DISCUSSION
In this study of medical wards at academic medical centers, we found that, in response to the COVID-19 pandemic, hospitals made several changes in a short period of time to adapt to the crisis. These included implementation and rapid expansion of dedicated RIUs, greatly expanded use of inpatient telehealth for patient assessments and consultation, implementation of other approaches to minimize room entry (such as grouping in-room activities), and deployment of ethics consultation services to help manage issues around potential scarcity of life-saving measures such as ventilators. We also found that availability of PPE and timely testing was limited. Finally, a large proportion of sites reported potential diagnostic problems in the assessment of both patients suspected and those not suspected of having COVID-19.
RIUs are emerging as a primary modality for caring for non-ICU COVID-19 patients, though they never involved medical students; we hope the role of students in particular will increase as new models of training emerge in response to the pandemic.7 In contrast, telemedicine evolved rapidly to hold a substantial role in RIUs, with both ward and specialty teams using video visit technology to communicate with patients. COVID-19 has been viewed as a perfect use case for outpatient telemedicine,8 and a growing number of studies are examining its outpatient use9,10; however, to date, somewhat less attention has been paid to inpatient deployment. Although our data suggest telemedicine has found a prominent place in RIUs, it remains to be seen whether it is associated with differences in patient or provider outcomes. For example, deficiencies in the physical examination, limited face-to-face contact, and lack of physical presence could all affect the patient–provider relationship, patient engagement, and the accuracy of the diagnostic process.
Our data suggest the possibility of missing non–COVID-19 diagnoses in patients suspected of COVID-19 and missing COVID-19 in those admitted for nonrespiratory reasons. The latter may be addressed as routine COVID-19 screening of admitted patients becomes commonplace. For the former, however, it is possible that physicians are “anchoring” their thinking on COVID-19 to the exclusion of other diagnoses, that physicians are not fully aware of complications unique to COVID-19 infection (such as thromboembolism), and/or that the above-mentioned limitations of telemedicine have decreased diagnostic performance.
Although PPE stockpile data were not easily available for some sites, a distressingly large number reported stockpiles of 2 weeks or less, with reuse being the most common approach to extending PPE supply. We also found it concerning that 43% of hospital leaders did not know their stockpile data; we believe this is an important question that hospital leaders need to be asking. Most sites in our study reported test turnaround times of longer than 6 hours; lack of rapid COVID-19 testing further stresses PPE stockpile and may slow patients’ transition out of the RIU or discharge to home.
Our study has several limitations, including the evolving nature of the pandemic and rapid adaptations of care systems in the pandemic’s surge phase. However, we attempted to frame our questions in ways that provided a focused snapshot of care. Furthermore, respondents may not have had exhaustive knowledge of their institution’s COVID-19 response strategies, but most were the directors of their hospitalist services, and we encouraged the respondents to confer with others to gather high-fidelity data. Finally, as a survey of large academic medical centers, our results may not apply to nonacademic centers.
Approaches to caring for non-ICU patients during the COVID-19 pandemic are rapidly evolving. Expansion of RIUs and developing the workforce to support them has been a primary focus, with rapid innovation in use of technology emerging as a critical adaptation while PPE limitations persist and needs for “medical distancing” continue to grow. Although rates of missed COVID-19 diagnoses will likely be reduced with testing and systems improvements, physicians and systems will also need to consider how to utilize emerging technology in ways that can improve clinical care and provider safety while aiding diagnostic thinking. This survey illustrates the rapid adaptations made by our hospitals in response to the pandemic; ongoing adaptation will likely be needed to optimally care for hospitalized patients with COVID-19 while the pandemic continues to evolve.
Acknowledgment
Thanks to members of the HOMERuN COVID-19 Collaborative Group: Baylor Scott & White Medical Center – Temple, Texas - Tresa McNeal MD; Beth Israel Deaconess Medical Center - Shani Herzig MD MPH, Joseph Li MD, Julius Yang MD PhD; Brigham and Women’s Hospital - Christopher Roy MD, Jeffrey Schnipper MD MPH; Cedars-Sinai Medical Center - Ed Seferian MD, ; ChristianaCare - Surekha Bhamidipati MD; Cleveland Clinic - Matthew Pappas MD MPH; Dartmouth-Hitchcock Medical Center - Jonathan Lurie MD MS; Dell Medical School at The University of Texas at Austin - Chris Moriates MD, Luci Leykum MD MBA MSc; Denver Health and Hospitals Authority - Diana Mancini MD; Emory University Hospital - Dan Hunt MD; Johns Hopkins Hospital - Daniel J Brotman MD, Zishan K Siddiqui MD, Shaker Eid MD MBA; Maine Medical Center - Daniel A Meyer MD, Robert Trowbridge MD; Massachusetts General Hospital - Melissa Mattison MD; Mayo Clinic Rochester – Caroline Burton MD, Sagar Dugani MD PhD; Medical College of Wisconsin - Sanjay Bhandari MD; Miriam Hospital - Kwame Dapaah-Afriyie MD MBA; Mount Sinai Hospital - Andrew Dunn MD; NorthShore - David Lovinger MD; Northwestern Memorial Hospital - Kevin O’Leary MD MS; Ohio State University Wexner Medical Center - Eric Schumacher DO; Oregon Health & Science University - Angela Alday MD; Penn Medicine - Ryan Greysen MD MHS MA; Rutgers- Robert Wood Johnson University Hospital - Michael Steinberg MD MPH; Stanford University School of Medicine - Neera Ahuja MD; Tulane Hospital and University Medical Center - Geraldine Ménard MD; UC San Diego Health - Ian Jenkins MD; UC Los Angeles Health - Michael Lazarus MD, Magdalena E. Ptaszny, MD; UC San Francisco Health - Bradley A Sharpe, MD, Margaret Fang MD MPH; UK HealthCare - Mark Williams MD MHM, John Romond MD; University of Chicago – David Meltzer MD PhD, Gregory Ruhnke MD; University of Colorado - Marisha Burden MD; University of Florida - Nila Radhakrishnan MD; University of Iowa Hospitals and Clinics - Kevin Glenn MD MS; University of Miami - Efren Manjarrez MD; University of Michigan - Vineet Chopra MD MSc, Valerie Vaughn MD MSc; University of Missouri-Columbia Hospital - Hasan Naqvi MD; University of Nebraska Medical Center - Chad Vokoun MD; University of North Carolina at Chapel Hill - David Hemsey MD; University of Pittsburgh Medical Center - Gena Marie Walker MD; University of Vermont Medical Center - Steven Grant MD; University of Washington Medical Center - Christopher Kim MD MBA, Andrew White MD; University of Washington-Harborview Medical Center - Maralyssa Bann MD; University of Wisconsin Hospital and Clinics - David Sterken MD, Farah Kaiksow MD MPP, Ann Sheehy MD MS, Jordan Kenik MD MPH; UW Northwest Campus - Ben Wolpaw MD; Vanderbilt University Medical Center - Sunil Kripalani MD MSc, Eduard E Vasilevskis MD, Kathleene T Wooldridge MD MPH; Wake Forest Baptist Health - Erik Summers MD; Washington University St. Louis - Michael Lin MD; Weill Cornell - Justin Choi MD; Yale New Haven Hospital - William Cushing MA, Chris Sankey MD; Zuckerberg San Francisco General Hospital - Sumant Ranji MD.
1. Institute for Health Metrics and Evaluation. COVID-19 Projections: United States of America. 2020. Accessed May 5, 2020. https://covid19.healthdata.org/united-states-of-america
2. Iserson KV. Alternative care sites: an option in disasters. West J Emerg Med. 2020;21(3):484‐489. https://doi.org/10.5811/westjem.2020.4.47552
3. Paganini M, Conti A, Weinstein E, Della Corte F, Ragazzoni L. Translating COVID-19 pandemic surge theory to practice in the emergency department: how to expand structure [online first]. Disaster Med Public Health Prep. 2020:1-10. https://doi.org/10.1017/dmp.2020.57
4. Kumaraiah D, Yip N, Ivascu N, Hill L. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM: Catalyst. April 28, 2020. Accessed May 5, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0158
5. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
6. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.5317
7. Bauchner H, Sharfstein J. A bold response to the COVID-19 pandemic: medical students, national service, and public health [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6166
8. Hollander JE, Carr BG. Virtually perfect? telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679‐1681. https://doi.org/10.1056/nejmp2003539
9. Hau YS, Kim JK, Hur J, Chang MC. How about actively using telemedicine during the COVID-19 pandemic? J Med Syst. 2020;44(6):108. https://doi.org/10.1007/s10916-020-01580-z
10. Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic [online first]. J Am Coll Surg. 2020. https://doi.org/10.1016/j.jamcollsurg.2020.04.030
The coronavirus disease of 2019 (COVID-19) pandemic has resulted in a surge in hospitalizations of patients with a novel, serious, and highly contagious infectious disease for which there is yet no proven treatment. Currently, much of the focus has been on intensive care unit (ICU) and ventilator capacity for the sickest of these patients who develop respiratory failure. However, most hospitalized patients are being cared for in general medical units.1 Some evidence exists to describe adaptations to capacity needs outside of medical wards,2-4 but few studies have specifically addressed the ward setting. Therefore, there is a pressing need for evidence to describe how to expand capacity and deliver medical ward–based care.
To better understand how inpatient care in the United States is adapting to the COVID-19 pandemic, we surveyed 72 sites participating in the Hospital Medicine Reengineering Network (HOMERuN), a national consortium of hospital medicine groups.5 We report results of this survey, carried out between April 3 and April 5, 2020.
METHODS
Sites and Subjects
HOMERuN is a collaborative network of hospitalists from across the United States whose primary goal is to catalyze research and share best practices across hospital medicine groups. Using surveys of Hospital Medicine leaders, targeted medical record review, and other methods, HOMERuN’s funded research interests to date have included care transitions, workforce issues, patient and family engagement, and diagnostic errors. Sites participating in HOMERuN sites are relatively large urban academic medical centers (Appendix).
Survey Development and Deployment
We designed a focused survey that aimed to provide a snapshot of evolving operational and clinical aspects of COVID-19 care (Appendix). Domains included COVID-19 testing turnaround times, personal protective equipment (PPE) stewardship,6 features of respiratory isolation units (RIUs; ie, dedicated units for patients with known or suspected COVID-19), and observed effects on clinical care. We tested the instrument to ensure feasibility and clarity internally, performed brief cognitive testing with several hospital medicine leaders in HOMERuN, then disseminated the survey by email on April 3, with two follow-up emails on 2 subsequent days. Our study was deemed non–human subjects research by the University of California, San Francisco, Committee on Human Research. Descriptive statistics were used to characterize survey responses.
RESULTS
Of 72 hospitals surveyed, 51 (71%) responded. Mean hospital bed count was 940, three were safety-net hospitals, and one was a community-based teaching center; responding and nonresponding hospitals did not differ significantly in terms of bed count (Appendix).
Health System Adaptations, Testing, and PPE Status
Nearly all responding hospitals (46 of 51; 90%) had RIUs for patients with known or suspected COVID-19 (Table 1). Nearly all hospitals took steps to keep potentially sick healthcare providers from infecting others (eg, staying home if sick or exposed). Among respondents, 32% had rapid response teams, 24% had respiratory therapy teams, and 29% had case management teams that were dedicated to COVID-19 care. Thirty-two (63%) had developed models, such as ethics or palliative care consult services, to assist with difficult resource-allocation decisions (eg, how to prioritize ventilator use if demand exceeded supply). Twenty-three (45%) had developed post-acute care monitoring programs dedicated to COVID-19 patients.

At the time of our survey, only 2 sites (4%) reported COVID-19 test time turnaround under 1 hour, and 15 (30%) reported turnaround in less than 6 hours. Of the 29 sites able to provide estimates of PPE stockpile, 14 (48%) reported a supply of 2 weeks or less. The most common approaches to PPE stewardship focused on reuse of masks and face shields if not obviously soiled, centralizing PPE distribution, and disinfecting or sterilizing masks. Ten sites (20%) were utilizing 3-D printed masks, while 10% used homemade face shields or masks.
Characteristics of COVID-19 RIUs
Forty-six hospitals (90% of all respondents) in our cohort had developed RIUs at the time of survey administration. The earliest RIU implementation date was February 10, 2020, and the most recent was launched on the day of our survey. Admission to RIUs was primarily based on clinical factors associated with known or suspected COVID-19 infection (Table 2). The number of non–critical care RIU beds among locations at that time ranged from 10 or less to more than 50. The mean number of hospitalist attendings caring for patients in the RIUs was 10.2, with a mean 4.1 advanced practice providers, 5.5 residents, and 0 medical students. The number of planned patients per attending was typically 5 to 15. Nurses and physicians typically rounded separately. Medical distancing (eg, reducing patient room entry) was accomplished most commonly by grouped timing of medication administration (76% of sites), video links to room outside of rounding times (54% of sites), the use of video or telemedicine during rounds (17%), and clustering of activities such as medication administration or phlebotomy. The most common criteria prompting discharge from the RIU were a negative COVID-19 test (59%) and hospital discharge (57%), though comments from many respondents suggested that discharge criteria were changing rapidly.

Effects of Isolation Measures on In-Room Encounters and Diagnostic Processes
More than 90% of sites reported decreases in in-room encounter frequency across all provider types whether as a result of policies in place or not. Reductions were reported among hospitalists, advanced practice providers, residents, consultants, and therapists (Table 3). Reduced room entry most often resulted from an established or developing policy, but many noted reduced room entry without formal policies in place. Nearly all sites reported moving specialty consultations to phone or video evaluations. Diagnostic error was commonly reported, with missed non–COVID-19 medical diagnoses among COVID-19 infected patients being reported by 22 sites (46%) and missed COVID-19 diagnoses in patients admitted for other reasons by 22 sites (45%).

DISCUSSION
In this study of medical wards at academic medical centers, we found that, in response to the COVID-19 pandemic, hospitals made several changes in a short period of time to adapt to the crisis. These included implementation and rapid expansion of dedicated RIUs, greatly expanded use of inpatient telehealth for patient assessments and consultation, implementation of other approaches to minimize room entry (such as grouping in-room activities), and deployment of ethics consultation services to help manage issues around potential scarcity of life-saving measures such as ventilators. We also found that availability of PPE and timely testing was limited. Finally, a large proportion of sites reported potential diagnostic problems in the assessment of both patients suspected and those not suspected of having COVID-19.
RIUs are emerging as a primary modality for caring for non-ICU COVID-19 patients, though they never involved medical students; we hope the role of students in particular will increase as new models of training emerge in response to the pandemic.7 In contrast, telemedicine evolved rapidly to hold a substantial role in RIUs, with both ward and specialty teams using video visit technology to communicate with patients. COVID-19 has been viewed as a perfect use case for outpatient telemedicine,8 and a growing number of studies are examining its outpatient use9,10; however, to date, somewhat less attention has been paid to inpatient deployment. Although our data suggest telemedicine has found a prominent place in RIUs, it remains to be seen whether it is associated with differences in patient or provider outcomes. For example, deficiencies in the physical examination, limited face-to-face contact, and lack of physical presence could all affect the patient–provider relationship, patient engagement, and the accuracy of the diagnostic process.
Our data suggest the possibility of missing non–COVID-19 diagnoses in patients suspected of COVID-19 and missing COVID-19 in those admitted for nonrespiratory reasons. The latter may be addressed as routine COVID-19 screening of admitted patients becomes commonplace. For the former, however, it is possible that physicians are “anchoring” their thinking on COVID-19 to the exclusion of other diagnoses, that physicians are not fully aware of complications unique to COVID-19 infection (such as thromboembolism), and/or that the above-mentioned limitations of telemedicine have decreased diagnostic performance.
Although PPE stockpile data were not easily available for some sites, a distressingly large number reported stockpiles of 2 weeks or less, with reuse being the most common approach to extending PPE supply. We also found it concerning that 43% of hospital leaders did not know their stockpile data; we believe this is an important question that hospital leaders need to be asking. Most sites in our study reported test turnaround times of longer than 6 hours; lack of rapid COVID-19 testing further stresses PPE stockpile and may slow patients’ transition out of the RIU or discharge to home.
Our study has several limitations, including the evolving nature of the pandemic and rapid adaptations of care systems in the pandemic’s surge phase. However, we attempted to frame our questions in ways that provided a focused snapshot of care. Furthermore, respondents may not have had exhaustive knowledge of their institution’s COVID-19 response strategies, but most were the directors of their hospitalist services, and we encouraged the respondents to confer with others to gather high-fidelity data. Finally, as a survey of large academic medical centers, our results may not apply to nonacademic centers.
Approaches to caring for non-ICU patients during the COVID-19 pandemic are rapidly evolving. Expansion of RIUs and developing the workforce to support them has been a primary focus, with rapid innovation in use of technology emerging as a critical adaptation while PPE limitations persist and needs for “medical distancing” continue to grow. Although rates of missed COVID-19 diagnoses will likely be reduced with testing and systems improvements, physicians and systems will also need to consider how to utilize emerging technology in ways that can improve clinical care and provider safety while aiding diagnostic thinking. This survey illustrates the rapid adaptations made by our hospitals in response to the pandemic; ongoing adaptation will likely be needed to optimally care for hospitalized patients with COVID-19 while the pandemic continues to evolve.
Acknowledgment
Thanks to members of the HOMERuN COVID-19 Collaborative Group: Baylor Scott & White Medical Center – Temple, Texas - Tresa McNeal MD; Beth Israel Deaconess Medical Center - Shani Herzig MD MPH, Joseph Li MD, Julius Yang MD PhD; Brigham and Women’s Hospital - Christopher Roy MD, Jeffrey Schnipper MD MPH; Cedars-Sinai Medical Center - Ed Seferian MD, ; ChristianaCare - Surekha Bhamidipati MD; Cleveland Clinic - Matthew Pappas MD MPH; Dartmouth-Hitchcock Medical Center - Jonathan Lurie MD MS; Dell Medical School at The University of Texas at Austin - Chris Moriates MD, Luci Leykum MD MBA MSc; Denver Health and Hospitals Authority - Diana Mancini MD; Emory University Hospital - Dan Hunt MD; Johns Hopkins Hospital - Daniel J Brotman MD, Zishan K Siddiqui MD, Shaker Eid MD MBA; Maine Medical Center - Daniel A Meyer MD, Robert Trowbridge MD; Massachusetts General Hospital - Melissa Mattison MD; Mayo Clinic Rochester – Caroline Burton MD, Sagar Dugani MD PhD; Medical College of Wisconsin - Sanjay Bhandari MD; Miriam Hospital - Kwame Dapaah-Afriyie MD MBA; Mount Sinai Hospital - Andrew Dunn MD; NorthShore - David Lovinger MD; Northwestern Memorial Hospital - Kevin O’Leary MD MS; Ohio State University Wexner Medical Center - Eric Schumacher DO; Oregon Health & Science University - Angela Alday MD; Penn Medicine - Ryan Greysen MD MHS MA; Rutgers- Robert Wood Johnson University Hospital - Michael Steinberg MD MPH; Stanford University School of Medicine - Neera Ahuja MD; Tulane Hospital and University Medical Center - Geraldine Ménard MD; UC San Diego Health - Ian Jenkins MD; UC Los Angeles Health - Michael Lazarus MD, Magdalena E. Ptaszny, MD; UC San Francisco Health - Bradley A Sharpe, MD, Margaret Fang MD MPH; UK HealthCare - Mark Williams MD MHM, John Romond MD; University of Chicago – David Meltzer MD PhD, Gregory Ruhnke MD; University of Colorado - Marisha Burden MD; University of Florida - Nila Radhakrishnan MD; University of Iowa Hospitals and Clinics - Kevin Glenn MD MS; University of Miami - Efren Manjarrez MD; University of Michigan - Vineet Chopra MD MSc, Valerie Vaughn MD MSc; University of Missouri-Columbia Hospital - Hasan Naqvi MD; University of Nebraska Medical Center - Chad Vokoun MD; University of North Carolina at Chapel Hill - David Hemsey MD; University of Pittsburgh Medical Center - Gena Marie Walker MD; University of Vermont Medical Center - Steven Grant MD; University of Washington Medical Center - Christopher Kim MD MBA, Andrew White MD; University of Washington-Harborview Medical Center - Maralyssa Bann MD; University of Wisconsin Hospital and Clinics - David Sterken MD, Farah Kaiksow MD MPP, Ann Sheehy MD MS, Jordan Kenik MD MPH; UW Northwest Campus - Ben Wolpaw MD; Vanderbilt University Medical Center - Sunil Kripalani MD MSc, Eduard E Vasilevskis MD, Kathleene T Wooldridge MD MPH; Wake Forest Baptist Health - Erik Summers MD; Washington University St. Louis - Michael Lin MD; Weill Cornell - Justin Choi MD; Yale New Haven Hospital - William Cushing MA, Chris Sankey MD; Zuckerberg San Francisco General Hospital - Sumant Ranji MD.
The coronavirus disease of 2019 (COVID-19) pandemic has resulted in a surge in hospitalizations of patients with a novel, serious, and highly contagious infectious disease for which there is yet no proven treatment. Currently, much of the focus has been on intensive care unit (ICU) and ventilator capacity for the sickest of these patients who develop respiratory failure. However, most hospitalized patients are being cared for in general medical units.1 Some evidence exists to describe adaptations to capacity needs outside of medical wards,2-4 but few studies have specifically addressed the ward setting. Therefore, there is a pressing need for evidence to describe how to expand capacity and deliver medical ward–based care.
To better understand how inpatient care in the United States is adapting to the COVID-19 pandemic, we surveyed 72 sites participating in the Hospital Medicine Reengineering Network (HOMERuN), a national consortium of hospital medicine groups.5 We report results of this survey, carried out between April 3 and April 5, 2020.
METHODS
Sites and Subjects
HOMERuN is a collaborative network of hospitalists from across the United States whose primary goal is to catalyze research and share best practices across hospital medicine groups. Using surveys of Hospital Medicine leaders, targeted medical record review, and other methods, HOMERuN’s funded research interests to date have included care transitions, workforce issues, patient and family engagement, and diagnostic errors. Sites participating in HOMERuN sites are relatively large urban academic medical centers (Appendix).
Survey Development and Deployment
We designed a focused survey that aimed to provide a snapshot of evolving operational and clinical aspects of COVID-19 care (Appendix). Domains included COVID-19 testing turnaround times, personal protective equipment (PPE) stewardship,6 features of respiratory isolation units (RIUs; ie, dedicated units for patients with known or suspected COVID-19), and observed effects on clinical care. We tested the instrument to ensure feasibility and clarity internally, performed brief cognitive testing with several hospital medicine leaders in HOMERuN, then disseminated the survey by email on April 3, with two follow-up emails on 2 subsequent days. Our study was deemed non–human subjects research by the University of California, San Francisco, Committee on Human Research. Descriptive statistics were used to characterize survey responses.
RESULTS
Of 72 hospitals surveyed, 51 (71%) responded. Mean hospital bed count was 940, three were safety-net hospitals, and one was a community-based teaching center; responding and nonresponding hospitals did not differ significantly in terms of bed count (Appendix).
Health System Adaptations, Testing, and PPE Status
Nearly all responding hospitals (46 of 51; 90%) had RIUs for patients with known or suspected COVID-19 (Table 1). Nearly all hospitals took steps to keep potentially sick healthcare providers from infecting others (eg, staying home if sick or exposed). Among respondents, 32% had rapid response teams, 24% had respiratory therapy teams, and 29% had case management teams that were dedicated to COVID-19 care. Thirty-two (63%) had developed models, such as ethics or palliative care consult services, to assist with difficult resource-allocation decisions (eg, how to prioritize ventilator use if demand exceeded supply). Twenty-three (45%) had developed post-acute care monitoring programs dedicated to COVID-19 patients.

At the time of our survey, only 2 sites (4%) reported COVID-19 test time turnaround under 1 hour, and 15 (30%) reported turnaround in less than 6 hours. Of the 29 sites able to provide estimates of PPE stockpile, 14 (48%) reported a supply of 2 weeks or less. The most common approaches to PPE stewardship focused on reuse of masks and face shields if not obviously soiled, centralizing PPE distribution, and disinfecting or sterilizing masks. Ten sites (20%) were utilizing 3-D printed masks, while 10% used homemade face shields or masks.
Characteristics of COVID-19 RIUs
Forty-six hospitals (90% of all respondents) in our cohort had developed RIUs at the time of survey administration. The earliest RIU implementation date was February 10, 2020, and the most recent was launched on the day of our survey. Admission to RIUs was primarily based on clinical factors associated with known or suspected COVID-19 infection (Table 2). The number of non–critical care RIU beds among locations at that time ranged from 10 or less to more than 50. The mean number of hospitalist attendings caring for patients in the RIUs was 10.2, with a mean 4.1 advanced practice providers, 5.5 residents, and 0 medical students. The number of planned patients per attending was typically 5 to 15. Nurses and physicians typically rounded separately. Medical distancing (eg, reducing patient room entry) was accomplished most commonly by grouped timing of medication administration (76% of sites), video links to room outside of rounding times (54% of sites), the use of video or telemedicine during rounds (17%), and clustering of activities such as medication administration or phlebotomy. The most common criteria prompting discharge from the RIU were a negative COVID-19 test (59%) and hospital discharge (57%), though comments from many respondents suggested that discharge criteria were changing rapidly.

Effects of Isolation Measures on In-Room Encounters and Diagnostic Processes
More than 90% of sites reported decreases in in-room encounter frequency across all provider types whether as a result of policies in place or not. Reductions were reported among hospitalists, advanced practice providers, residents, consultants, and therapists (Table 3). Reduced room entry most often resulted from an established or developing policy, but many noted reduced room entry without formal policies in place. Nearly all sites reported moving specialty consultations to phone or video evaluations. Diagnostic error was commonly reported, with missed non–COVID-19 medical diagnoses among COVID-19 infected patients being reported by 22 sites (46%) and missed COVID-19 diagnoses in patients admitted for other reasons by 22 sites (45%).

DISCUSSION
In this study of medical wards at academic medical centers, we found that, in response to the COVID-19 pandemic, hospitals made several changes in a short period of time to adapt to the crisis. These included implementation and rapid expansion of dedicated RIUs, greatly expanded use of inpatient telehealth for patient assessments and consultation, implementation of other approaches to minimize room entry (such as grouping in-room activities), and deployment of ethics consultation services to help manage issues around potential scarcity of life-saving measures such as ventilators. We also found that availability of PPE and timely testing was limited. Finally, a large proportion of sites reported potential diagnostic problems in the assessment of both patients suspected and those not suspected of having COVID-19.
RIUs are emerging as a primary modality for caring for non-ICU COVID-19 patients, though they never involved medical students; we hope the role of students in particular will increase as new models of training emerge in response to the pandemic.7 In contrast, telemedicine evolved rapidly to hold a substantial role in RIUs, with both ward and specialty teams using video visit technology to communicate with patients. COVID-19 has been viewed as a perfect use case for outpatient telemedicine,8 and a growing number of studies are examining its outpatient use9,10; however, to date, somewhat less attention has been paid to inpatient deployment. Although our data suggest telemedicine has found a prominent place in RIUs, it remains to be seen whether it is associated with differences in patient or provider outcomes. For example, deficiencies in the physical examination, limited face-to-face contact, and lack of physical presence could all affect the patient–provider relationship, patient engagement, and the accuracy of the diagnostic process.
Our data suggest the possibility of missing non–COVID-19 diagnoses in patients suspected of COVID-19 and missing COVID-19 in those admitted for nonrespiratory reasons. The latter may be addressed as routine COVID-19 screening of admitted patients becomes commonplace. For the former, however, it is possible that physicians are “anchoring” their thinking on COVID-19 to the exclusion of other diagnoses, that physicians are not fully aware of complications unique to COVID-19 infection (such as thromboembolism), and/or that the above-mentioned limitations of telemedicine have decreased diagnostic performance.
Although PPE stockpile data were not easily available for some sites, a distressingly large number reported stockpiles of 2 weeks or less, with reuse being the most common approach to extending PPE supply. We also found it concerning that 43% of hospital leaders did not know their stockpile data; we believe this is an important question that hospital leaders need to be asking. Most sites in our study reported test turnaround times of longer than 6 hours; lack of rapid COVID-19 testing further stresses PPE stockpile and may slow patients’ transition out of the RIU or discharge to home.
Our study has several limitations, including the evolving nature of the pandemic and rapid adaptations of care systems in the pandemic’s surge phase. However, we attempted to frame our questions in ways that provided a focused snapshot of care. Furthermore, respondents may not have had exhaustive knowledge of their institution’s COVID-19 response strategies, but most were the directors of their hospitalist services, and we encouraged the respondents to confer with others to gather high-fidelity data. Finally, as a survey of large academic medical centers, our results may not apply to nonacademic centers.
Approaches to caring for non-ICU patients during the COVID-19 pandemic are rapidly evolving. Expansion of RIUs and developing the workforce to support them has been a primary focus, with rapid innovation in use of technology emerging as a critical adaptation while PPE limitations persist and needs for “medical distancing” continue to grow. Although rates of missed COVID-19 diagnoses will likely be reduced with testing and systems improvements, physicians and systems will also need to consider how to utilize emerging technology in ways that can improve clinical care and provider safety while aiding diagnostic thinking. This survey illustrates the rapid adaptations made by our hospitals in response to the pandemic; ongoing adaptation will likely be needed to optimally care for hospitalized patients with COVID-19 while the pandemic continues to evolve.
Acknowledgment
Thanks to members of the HOMERuN COVID-19 Collaborative Group: Baylor Scott & White Medical Center – Temple, Texas - Tresa McNeal MD; Beth Israel Deaconess Medical Center - Shani Herzig MD MPH, Joseph Li MD, Julius Yang MD PhD; Brigham and Women’s Hospital - Christopher Roy MD, Jeffrey Schnipper MD MPH; Cedars-Sinai Medical Center - Ed Seferian MD, ; ChristianaCare - Surekha Bhamidipati MD; Cleveland Clinic - Matthew Pappas MD MPH; Dartmouth-Hitchcock Medical Center - Jonathan Lurie MD MS; Dell Medical School at The University of Texas at Austin - Chris Moriates MD, Luci Leykum MD MBA MSc; Denver Health and Hospitals Authority - Diana Mancini MD; Emory University Hospital - Dan Hunt MD; Johns Hopkins Hospital - Daniel J Brotman MD, Zishan K Siddiqui MD, Shaker Eid MD MBA; Maine Medical Center - Daniel A Meyer MD, Robert Trowbridge MD; Massachusetts General Hospital - Melissa Mattison MD; Mayo Clinic Rochester – Caroline Burton MD, Sagar Dugani MD PhD; Medical College of Wisconsin - Sanjay Bhandari MD; Miriam Hospital - Kwame Dapaah-Afriyie MD MBA; Mount Sinai Hospital - Andrew Dunn MD; NorthShore - David Lovinger MD; Northwestern Memorial Hospital - Kevin O’Leary MD MS; Ohio State University Wexner Medical Center - Eric Schumacher DO; Oregon Health & Science University - Angela Alday MD; Penn Medicine - Ryan Greysen MD MHS MA; Rutgers- Robert Wood Johnson University Hospital - Michael Steinberg MD MPH; Stanford University School of Medicine - Neera Ahuja MD; Tulane Hospital and University Medical Center - Geraldine Ménard MD; UC San Diego Health - Ian Jenkins MD; UC Los Angeles Health - Michael Lazarus MD, Magdalena E. Ptaszny, MD; UC San Francisco Health - Bradley A Sharpe, MD, Margaret Fang MD MPH; UK HealthCare - Mark Williams MD MHM, John Romond MD; University of Chicago – David Meltzer MD PhD, Gregory Ruhnke MD; University of Colorado - Marisha Burden MD; University of Florida - Nila Radhakrishnan MD; University of Iowa Hospitals and Clinics - Kevin Glenn MD MS; University of Miami - Efren Manjarrez MD; University of Michigan - Vineet Chopra MD MSc, Valerie Vaughn MD MSc; University of Missouri-Columbia Hospital - Hasan Naqvi MD; University of Nebraska Medical Center - Chad Vokoun MD; University of North Carolina at Chapel Hill - David Hemsey MD; University of Pittsburgh Medical Center - Gena Marie Walker MD; University of Vermont Medical Center - Steven Grant MD; University of Washington Medical Center - Christopher Kim MD MBA, Andrew White MD; University of Washington-Harborview Medical Center - Maralyssa Bann MD; University of Wisconsin Hospital and Clinics - David Sterken MD, Farah Kaiksow MD MPP, Ann Sheehy MD MS, Jordan Kenik MD MPH; UW Northwest Campus - Ben Wolpaw MD; Vanderbilt University Medical Center - Sunil Kripalani MD MSc, Eduard E Vasilevskis MD, Kathleene T Wooldridge MD MPH; Wake Forest Baptist Health - Erik Summers MD; Washington University St. Louis - Michael Lin MD; Weill Cornell - Justin Choi MD; Yale New Haven Hospital - William Cushing MA, Chris Sankey MD; Zuckerberg San Francisco General Hospital - Sumant Ranji MD.
1. Institute for Health Metrics and Evaluation. COVID-19 Projections: United States of America. 2020. Accessed May 5, 2020. https://covid19.healthdata.org/united-states-of-america
2. Iserson KV. Alternative care sites: an option in disasters. West J Emerg Med. 2020;21(3):484‐489. https://doi.org/10.5811/westjem.2020.4.47552
3. Paganini M, Conti A, Weinstein E, Della Corte F, Ragazzoni L. Translating COVID-19 pandemic surge theory to practice in the emergency department: how to expand structure [online first]. Disaster Med Public Health Prep. 2020:1-10. https://doi.org/10.1017/dmp.2020.57
4. Kumaraiah D, Yip N, Ivascu N, Hill L. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM: Catalyst. April 28, 2020. Accessed May 5, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0158
5. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
6. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.5317
7. Bauchner H, Sharfstein J. A bold response to the COVID-19 pandemic: medical students, national service, and public health [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6166
8. Hollander JE, Carr BG. Virtually perfect? telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679‐1681. https://doi.org/10.1056/nejmp2003539
9. Hau YS, Kim JK, Hur J, Chang MC. How about actively using telemedicine during the COVID-19 pandemic? J Med Syst. 2020;44(6):108. https://doi.org/10.1007/s10916-020-01580-z
10. Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic [online first]. J Am Coll Surg. 2020. https://doi.org/10.1016/j.jamcollsurg.2020.04.030
1. Institute for Health Metrics and Evaluation. COVID-19 Projections: United States of America. 2020. Accessed May 5, 2020. https://covid19.healthdata.org/united-states-of-america
2. Iserson KV. Alternative care sites: an option in disasters. West J Emerg Med. 2020;21(3):484‐489. https://doi.org/10.5811/westjem.2020.4.47552
3. Paganini M, Conti A, Weinstein E, Della Corte F, Ragazzoni L. Translating COVID-19 pandemic surge theory to practice in the emergency department: how to expand structure [online first]. Disaster Med Public Health Prep. 2020:1-10. https://doi.org/10.1017/dmp.2020.57
4. Kumaraiah D, Yip N, Ivascu N, Hill L. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM: Catalyst. April 28, 2020. Accessed May 5, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0158
5. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
6. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.5317
7. Bauchner H, Sharfstein J. A bold response to the COVID-19 pandemic: medical students, national service, and public health [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6166
8. Hollander JE, Carr BG. Virtually perfect? telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679‐1681. https://doi.org/10.1056/nejmp2003539
9. Hau YS, Kim JK, Hur J, Chang MC. How about actively using telemedicine during the COVID-19 pandemic? J Med Syst. 2020;44(6):108. https://doi.org/10.1007/s10916-020-01580-z
10. Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic [online first]. J Am Coll Surg. 2020. https://doi.org/10.1016/j.jamcollsurg.2020.04.030
© 2020 Society of Hospital Medicine
Education in a Crisis: The Opportunity of Our Lives
In a few short months, the novel coronavirus SARS-CoV-2 has spread across the world, and illness caused by coronavirus 2019, or COVID-19, now affects every corner of the United States.1 As healthcare systems prepare to care for a wave of affected patients, those with a teaching mission face the added challenge of balancing the educational needs and safety of trainees with those of delivering patient care. In response to concerns for student welfare, medical and nursing schools have suspended classroom-based education and clinical rotations.2 The Accreditation Council for Graduate Medical Education (ACGME) and American Association of Colleges of Nursing (AACN) have emphasized the importance of adequate training in the use of personal protective equipment (PPE) for their trainees.3 The National League for Nursing has called on training programs to allow flexibility for graduating students who may have been removed from clinical rotations because of safety concerns.4
These decisions have precedent: During the SARS-CoV epidemic in 2003, medical and nursing student education was temporarily halted in affected areas.5-6 Healthcare trainees described concerns for their safety and reported adverse emotional impact.7-9 In the current pandemic, there is variation in how countries around the world are approaching the role of learners, with options ranging from removing learners from the clinical environment to encouraging early graduation for students in hopes of ameliorating the impending physician shortage.10-13 The need to balance educational goals with ethical concerns raised by this pandemic affects health professions trainees broadly.
Despite the challenges, there are unique educational opportunities at hand. In this Perspective, we draw on our collective experience, multiple informal interviews with educational leaders across the country, and educational literature to create a framework for health professions education during a crisis. From this framework, we propose a set of recommendations to assist educational policymakers and those working directly with learners to navigate these issues effectively.
KEY EDUCATIONAL ISSUES
Patient and Hospital Welfare
There are significant concerns about nosocomial spread of SARS-CoV-2. Having learners directly see COVID-19 patients can increase the risk of nosocomial spread. In one of the original case series, 29% of those infected were health care workers and 12.3% were patients hospitalized prior to infection.14 Additionally, preserving supplies of personal protective equipment (PPE) for healthcare workers has been a commonly cited reason for suspending student presence on clinical rotations. Insufficient supply of PPE has forced hospitals to relax PPE guidelines for those seeing patients under investigation and liberalize quarantine requirements for exposed health care workers, so many hospitals have reduced provider-patient interactions to only those considered essential.
Learner Welfare
As educators, we have a duty to keep our learners safe and psychologically well. The COVID-19 pandemic poses a risk of illness, permanent injury, or death among those infected. In some instances, the risks of exposure may be greater than the educational benefits of remaining in that clinical setting; however, health professions trainees at many institutions play such a central operational role that their absence could seriously impair overall care delivery. Furthermore, trainees are usually younger and healthier than supervising clinicians, which could leave them feeling an obligation to conduct a disproportionately large share of the direct patient contact. Despite these valid concerns, those being removed from the clinical environment for their safety could misinterpret it as a sign that their contributions or educational interests are not valued.
Educational Experience
Canceled clinical rotations will have significant negative educational effects on undergraduate learners. Depending on the extent of the pandemic’s effects, for example, third-year medical students may lack core rotations prior to applying for residency training. Other health professions face similar challenges—nursing students in their final year are likely missing their last opportunity for hands-on clinical training before graduation. Advanced practice nursing students may not be able to complete the required number of contact hours or clinical experiences mandated for accreditation. Graduate training programs must accommodate and adapt to these disparities when reviewing their applicant pools.
Absence from the clinical front lines, though, risks failing to capitalize on the unique educational opportunities presented by this pandemic. Students might miss the chance to learn about a new clinical entity and its increasingly varied clinical presentations, crisis medicine, infection control measures, emergency preparedness, ethics in the setting of scarce resources, public health and community response, communication in the setting of uncertainty and fear, and professionalism in the response to this singular situation. Trainees at all levels may miss the opportunity to stand alongside their teachers and peers to give care to those who need it most.
Heterogeneity of COVID-19 Responses Across the Country
The diversity of training sites in US health professions education has led to a wide range of responses to these challenges. In addition to regional variations, sites within individual academic programs are creating different educational and clinical polices, including the role of learners in the care of COVID-19 patients and even PPE requirements. Although educational accreditation bodies have offered guidance, implementation of creative responses has been left to individual schools, programs, and hospitals, creating important differences in learner training and experience.
A FRAMEWORK FOR PANDEMIC HEALTH PROFESSIONS EDUCATION
Given these challenges, we offer four broad principles to guide health professions education in response to this pandemic. Within this framework, we offer multiple suggestions to individual educators, health professions programs, healthcare systems, and educational policymakers.
1. Prioritize healthcare system welfare: Patients are the core of our professional responsibility, and their needs take precedence. First and foremost, plans for our learners must always promote and support the proper functioning of the health system and its individual healthcare workers. To support care delivery, healthcare systems should do the following:
- Ensure educational activities minimize the risk of nosocomial transmission and adverse effects on patient safety. For example, hospitals can modify bedside care to reduce exposure by using phone or video for patient-trainee contact, performing selective physical examination only, and, when needed, prioritizing a single skilled examiner.
- Ensure learner use of PPE does not negatively affect availability for others, both now and as the pandemic unfolds.
- Engage learners in authentic, value-added healthcare activities outside of direct patient contact: tele-medicine, meeting with families, or spending video time with inpatients not under their direct care.
2. Promote learner welfare: Educators have a duty to ensure the physical and psychological safety of learners across the health professions continuum. By virtue of power differentials in the hierarchy of the teaching environment, learners can be particularly vulnerable. To promote learner wellbeing, educators should do the following:
- Deploy technology to maximize opportunities for and quality of non–face-to-face clinical, didactic, and interprofessional learning.
- Ensure learners have access to and proper training in the use of PPE, independent of whether they may be using PPE as part of clinical responsibilities, while remaining aware of the potential supply constraints during a pandemic.
- Deliberately include stop points during teaching for dialogue around fears, stress, resilience, and coping.15 Deploy additional resources for support, including in-person or virtual psychological and psychiatric care and crisis intervention counseling.
- Maintain flexibility regarding trainee’s educational needs. For example, welcome trainees from other services joining inpatient medicine or ICU teams. Acknowledge the stress they may feel and support them as they learn and adapt. This can be a unique opportunity for lessons in professionalism, teamwork, and communication.
3. Maximize educational value: Efforts must be made to preserve educational quality and content, limit educational cost, and leverage unique opportunities that may only be available during this time. Educators and programs should do the following:
- Adapt teaching to reflect changes in the hospital environment. A student may have spent more time on the phone with a patient; the nurse may have examined the patient; a resident may have vital sign and lab data; the attending may have spoken to the family or know about local policy changes affecting care. The usual modes of rounding should adapt, focusing on sharing and synthesizing multisource data to generate rapid, intelligent plans while mitigating risk.
- Turn the potential challenge of diminished access to previously routine diagnostic testing into an opportunity for trainees to assertively develop clinical skills often underutilized in practice environments without resource limitation.
- Discuss learning opportunities for healthcare ethics. Multiple aspects of this pandemic raise ethical issues around allocation of scarce resources and principles such as contingency and crisis standards of care: the availability and application of testing, potential changes to patient triage standards in which patients sicker than ever may be sent home, and crisis allocation of life support resources.
- Highlight opportunities to support interprofessional education and collaborative practice. As traditional professional boundaries are temporarily blurred, we may find nurses asking gowned physicians to perform nursing tasks (eg, inflate blood pressure cuffs). Physicians may ask nurses for patient-related information (eg, physical examination findings), all to limit collective risk, maximize efficiency, and minimize the use of scarce PPE.
- Teach telemedicine. This is an opportunity to create a cadre of clinicians adept with this type of practice for the future—even outside pandemics. Now may be the time for virtual visits to be better integrated into clinical practice, which has been of interest to patients and providers for some time, and to address the constraints of reimbursement policies.
- Provide explicit role modeling to ensure learners recognize and learn from the key components of faculty activity—modeling communication skills, engaging in clinical reasoning, or navigating clinical and professional uncertainty.16 For example, faculty could share their clinical reasoning regarding diagnosis of respiratory complaints. While COVID-19 may be the most urgent diagnostic consideration, educators can emphasize the risk and implications of anchoring bias as an important cause of diagnostic errors.
- Identify opportunities for educational scholarship around these and other changes resulting from the pandemic. Seek to engage learners in this work.
4. Communicate transparently: Learners must be witness to decision-making processes; this will demonstrate that their safety and education are valued. Wherever possible, include learners in decision-making discussions and in the process of disseminating information.
- At the institutional level, generate, modify, and share communication regarding the ways that education is changing and the values and goals behind those changes.
- Invite trainees as active contributors to intellectual exchanges regarding changes in the learning environment.
- Limit the negative impact of the “rumor mill” by replacing it with frequent, targeted, and accurate messaging that relies on evidence to the greatest extent possible.
- Strive for consistency in communication content but diversity in distribution to reach the learners in the most effective ways. In times of uncertainty and stress, err on the side of overcommunication.
SUMMARY
Healthcare and medical education face a challenge unprecedented in our lifetimes. The COVID-19 pandemic will touch every aspect of how we care for patients, train the next generation of health professionals, and keep ourselves safe. By highlighting key issues facing health professions educators, offering a framework for education during pandemics, and providing specific suggestions for applying this framework, we hope to provide clarity on how we may advance our teaching mission and realize the educational opportunities as we face this crisis together.
1. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): Cases in the US. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 31, 2020.
2. Association of American Medical Colleges. Guidance on Medical Students’ Clinical Participation: Effective Immediately. https://www.aamc.org/system/files/2020-03/Guidance%20on%20Student%20Clinical%20Participation%203.17.20%20Final.pdf. Accessed March 30, 2020.
3. Updated: ACGME Guidance Statement on Coronavirus (COVID-19) and Resident/Fellow Education in the United States. https://acgme.org/COVID-19/Stage-2-Increased-Clinical-Demands-Guidance, Accessed April 6, 2020.
4. National League for Nursing. Coronavirus Resource Center. http://www.nln.org/coronavirus-resource-center. Accessed March 28, 2020.
5. Patil NG, Yan YC. SARS and its effect on medical education in Hong Kong. Med Educ. 2003;37(12):1127-1128. https://doi.org/10.1046/j.1365-2923.2003.01723.x.
6. Clark J. Fear of SARS thwarts medical education in Toronto. BMJ. 2003;326(7393):784. https://doi.org/10.1136/bmj.326.7393.784/c.
7. Sherbino J, Atzema C. SARS-Ed: severe acute respiratory syndrome and the impact on medical education. Ann Acad Emerg. 2004;44(3):229-231. https://doi.org/10.1016/j.annemergmed.2004.05.021.
8. Rambaldini G, Wilson K, Rath D, et al. The impact of severe acute respiratory syndrome on medical house staff: a qualitative study. J Gen Intern Med. 2005;20(5):381-385. https://doi.org/10.1111/j.1525-1497.2005.0099.x.
9. Lim EC, Oh VM, Koh DR, Seet RC. The challenges of “continuing medical education” in a pandemic era. Ann Acad Med Singapore. 2009;38(8):724-726.
10. Cole B. 10,000 Med school graduates in Italy skip final exam, get sent directly into health service to help fight COVID-19. Newsweek. March 18, 2020. https://www.newsweek.com/italy-coronavirus-covid-19-medical-students-1492996. Accessed March 27, 2020.
11. Siddique H. Final-year medical students graduate early to fight Covid-19. The Guardian. March 20, 2020. https://www.theguardian.com/world/2020/mar/20/final-year-medical-students-graduate-early-fight-coronavirus-covid-19. Accessed March 27, 2020.
12. Ahmed H, Allaf M, Elghazaly H. COVID-19 and medical education. Lancet Infect Dis. 2020. https://doi.org/10.1016/S1473-3099(20)30226-7.
13. Ducharme J. NYU med school will graduate students early to help New York fight coronavirus. Time. March 25, 2020. https://time.com/5809630/nyu-medical-school-early-graduation/. Accessed March 30, 2020.
14. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel-coronavirus infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. https://doi.org/10.1001/jama.2020.1585.
15. Markakis KM, Beckman HB, Suchman AL, Frankel RM. The path to professionalism: cultivating humanistic values and attitudes in residency training. Acad Med. 2000;75(2):141-150. https://doi.org/10.1097/00001888-200002000-00009.
16. Jochemsen-van der Leeuw HG, van Dijk N, van Etten-Jamaludin FS, Wieringa-de Waard M. The attributes of the clinical teacher as role model: a systematic review. Acad Med. 2013;88(1):26-34. https://doi.org/10.1097/ACM.0b013e318276d070.
In a few short months, the novel coronavirus SARS-CoV-2 has spread across the world, and illness caused by coronavirus 2019, or COVID-19, now affects every corner of the United States.1 As healthcare systems prepare to care for a wave of affected patients, those with a teaching mission face the added challenge of balancing the educational needs and safety of trainees with those of delivering patient care. In response to concerns for student welfare, medical and nursing schools have suspended classroom-based education and clinical rotations.2 The Accreditation Council for Graduate Medical Education (ACGME) and American Association of Colleges of Nursing (AACN) have emphasized the importance of adequate training in the use of personal protective equipment (PPE) for their trainees.3 The National League for Nursing has called on training programs to allow flexibility for graduating students who may have been removed from clinical rotations because of safety concerns.4
These decisions have precedent: During the SARS-CoV epidemic in 2003, medical and nursing student education was temporarily halted in affected areas.5-6 Healthcare trainees described concerns for their safety and reported adverse emotional impact.7-9 In the current pandemic, there is variation in how countries around the world are approaching the role of learners, with options ranging from removing learners from the clinical environment to encouraging early graduation for students in hopes of ameliorating the impending physician shortage.10-13 The need to balance educational goals with ethical concerns raised by this pandemic affects health professions trainees broadly.
Despite the challenges, there are unique educational opportunities at hand. In this Perspective, we draw on our collective experience, multiple informal interviews with educational leaders across the country, and educational literature to create a framework for health professions education during a crisis. From this framework, we propose a set of recommendations to assist educational policymakers and those working directly with learners to navigate these issues effectively.
KEY EDUCATIONAL ISSUES
Patient and Hospital Welfare
There are significant concerns about nosocomial spread of SARS-CoV-2. Having learners directly see COVID-19 patients can increase the risk of nosocomial spread. In one of the original case series, 29% of those infected were health care workers and 12.3% were patients hospitalized prior to infection.14 Additionally, preserving supplies of personal protective equipment (PPE) for healthcare workers has been a commonly cited reason for suspending student presence on clinical rotations. Insufficient supply of PPE has forced hospitals to relax PPE guidelines for those seeing patients under investigation and liberalize quarantine requirements for exposed health care workers, so many hospitals have reduced provider-patient interactions to only those considered essential.
Learner Welfare
As educators, we have a duty to keep our learners safe and psychologically well. The COVID-19 pandemic poses a risk of illness, permanent injury, or death among those infected. In some instances, the risks of exposure may be greater than the educational benefits of remaining in that clinical setting; however, health professions trainees at many institutions play such a central operational role that their absence could seriously impair overall care delivery. Furthermore, trainees are usually younger and healthier than supervising clinicians, which could leave them feeling an obligation to conduct a disproportionately large share of the direct patient contact. Despite these valid concerns, those being removed from the clinical environment for their safety could misinterpret it as a sign that their contributions or educational interests are not valued.
Educational Experience
Canceled clinical rotations will have significant negative educational effects on undergraduate learners. Depending on the extent of the pandemic’s effects, for example, third-year medical students may lack core rotations prior to applying for residency training. Other health professions face similar challenges—nursing students in their final year are likely missing their last opportunity for hands-on clinical training before graduation. Advanced practice nursing students may not be able to complete the required number of contact hours or clinical experiences mandated for accreditation. Graduate training programs must accommodate and adapt to these disparities when reviewing their applicant pools.
Absence from the clinical front lines, though, risks failing to capitalize on the unique educational opportunities presented by this pandemic. Students might miss the chance to learn about a new clinical entity and its increasingly varied clinical presentations, crisis medicine, infection control measures, emergency preparedness, ethics in the setting of scarce resources, public health and community response, communication in the setting of uncertainty and fear, and professionalism in the response to this singular situation. Trainees at all levels may miss the opportunity to stand alongside their teachers and peers to give care to those who need it most.
Heterogeneity of COVID-19 Responses Across the Country
The diversity of training sites in US health professions education has led to a wide range of responses to these challenges. In addition to regional variations, sites within individual academic programs are creating different educational and clinical polices, including the role of learners in the care of COVID-19 patients and even PPE requirements. Although educational accreditation bodies have offered guidance, implementation of creative responses has been left to individual schools, programs, and hospitals, creating important differences in learner training and experience.
A FRAMEWORK FOR PANDEMIC HEALTH PROFESSIONS EDUCATION
Given these challenges, we offer four broad principles to guide health professions education in response to this pandemic. Within this framework, we offer multiple suggestions to individual educators, health professions programs, healthcare systems, and educational policymakers.
1. Prioritize healthcare system welfare: Patients are the core of our professional responsibility, and their needs take precedence. First and foremost, plans for our learners must always promote and support the proper functioning of the health system and its individual healthcare workers. To support care delivery, healthcare systems should do the following:
- Ensure educational activities minimize the risk of nosocomial transmission and adverse effects on patient safety. For example, hospitals can modify bedside care to reduce exposure by using phone or video for patient-trainee contact, performing selective physical examination only, and, when needed, prioritizing a single skilled examiner.
- Ensure learner use of PPE does not negatively affect availability for others, both now and as the pandemic unfolds.
- Engage learners in authentic, value-added healthcare activities outside of direct patient contact: tele-medicine, meeting with families, or spending video time with inpatients not under their direct care.
2. Promote learner welfare: Educators have a duty to ensure the physical and psychological safety of learners across the health professions continuum. By virtue of power differentials in the hierarchy of the teaching environment, learners can be particularly vulnerable. To promote learner wellbeing, educators should do the following:
- Deploy technology to maximize opportunities for and quality of non–face-to-face clinical, didactic, and interprofessional learning.
- Ensure learners have access to and proper training in the use of PPE, independent of whether they may be using PPE as part of clinical responsibilities, while remaining aware of the potential supply constraints during a pandemic.
- Deliberately include stop points during teaching for dialogue around fears, stress, resilience, and coping.15 Deploy additional resources for support, including in-person or virtual psychological and psychiatric care and crisis intervention counseling.
- Maintain flexibility regarding trainee’s educational needs. For example, welcome trainees from other services joining inpatient medicine or ICU teams. Acknowledge the stress they may feel and support them as they learn and adapt. This can be a unique opportunity for lessons in professionalism, teamwork, and communication.
3. Maximize educational value: Efforts must be made to preserve educational quality and content, limit educational cost, and leverage unique opportunities that may only be available during this time. Educators and programs should do the following:
- Adapt teaching to reflect changes in the hospital environment. A student may have spent more time on the phone with a patient; the nurse may have examined the patient; a resident may have vital sign and lab data; the attending may have spoken to the family or know about local policy changes affecting care. The usual modes of rounding should adapt, focusing on sharing and synthesizing multisource data to generate rapid, intelligent plans while mitigating risk.
- Turn the potential challenge of diminished access to previously routine diagnostic testing into an opportunity for trainees to assertively develop clinical skills often underutilized in practice environments without resource limitation.
- Discuss learning opportunities for healthcare ethics. Multiple aspects of this pandemic raise ethical issues around allocation of scarce resources and principles such as contingency and crisis standards of care: the availability and application of testing, potential changes to patient triage standards in which patients sicker than ever may be sent home, and crisis allocation of life support resources.
- Highlight opportunities to support interprofessional education and collaborative practice. As traditional professional boundaries are temporarily blurred, we may find nurses asking gowned physicians to perform nursing tasks (eg, inflate blood pressure cuffs). Physicians may ask nurses for patient-related information (eg, physical examination findings), all to limit collective risk, maximize efficiency, and minimize the use of scarce PPE.
- Teach telemedicine. This is an opportunity to create a cadre of clinicians adept with this type of practice for the future—even outside pandemics. Now may be the time for virtual visits to be better integrated into clinical practice, which has been of interest to patients and providers for some time, and to address the constraints of reimbursement policies.
- Provide explicit role modeling to ensure learners recognize and learn from the key components of faculty activity—modeling communication skills, engaging in clinical reasoning, or navigating clinical and professional uncertainty.16 For example, faculty could share their clinical reasoning regarding diagnosis of respiratory complaints. While COVID-19 may be the most urgent diagnostic consideration, educators can emphasize the risk and implications of anchoring bias as an important cause of diagnostic errors.
- Identify opportunities for educational scholarship around these and other changes resulting from the pandemic. Seek to engage learners in this work.
4. Communicate transparently: Learners must be witness to decision-making processes; this will demonstrate that their safety and education are valued. Wherever possible, include learners in decision-making discussions and in the process of disseminating information.
- At the institutional level, generate, modify, and share communication regarding the ways that education is changing and the values and goals behind those changes.
- Invite trainees as active contributors to intellectual exchanges regarding changes in the learning environment.
- Limit the negative impact of the “rumor mill” by replacing it with frequent, targeted, and accurate messaging that relies on evidence to the greatest extent possible.
- Strive for consistency in communication content but diversity in distribution to reach the learners in the most effective ways. In times of uncertainty and stress, err on the side of overcommunication.
SUMMARY
Healthcare and medical education face a challenge unprecedented in our lifetimes. The COVID-19 pandemic will touch every aspect of how we care for patients, train the next generation of health professionals, and keep ourselves safe. By highlighting key issues facing health professions educators, offering a framework for education during pandemics, and providing specific suggestions for applying this framework, we hope to provide clarity on how we may advance our teaching mission and realize the educational opportunities as we face this crisis together.
In a few short months, the novel coronavirus SARS-CoV-2 has spread across the world, and illness caused by coronavirus 2019, or COVID-19, now affects every corner of the United States.1 As healthcare systems prepare to care for a wave of affected patients, those with a teaching mission face the added challenge of balancing the educational needs and safety of trainees with those of delivering patient care. In response to concerns for student welfare, medical and nursing schools have suspended classroom-based education and clinical rotations.2 The Accreditation Council for Graduate Medical Education (ACGME) and American Association of Colleges of Nursing (AACN) have emphasized the importance of adequate training in the use of personal protective equipment (PPE) for their trainees.3 The National League for Nursing has called on training programs to allow flexibility for graduating students who may have been removed from clinical rotations because of safety concerns.4
These decisions have precedent: During the SARS-CoV epidemic in 2003, medical and nursing student education was temporarily halted in affected areas.5-6 Healthcare trainees described concerns for their safety and reported adverse emotional impact.7-9 In the current pandemic, there is variation in how countries around the world are approaching the role of learners, with options ranging from removing learners from the clinical environment to encouraging early graduation for students in hopes of ameliorating the impending physician shortage.10-13 The need to balance educational goals with ethical concerns raised by this pandemic affects health professions trainees broadly.
Despite the challenges, there are unique educational opportunities at hand. In this Perspective, we draw on our collective experience, multiple informal interviews with educational leaders across the country, and educational literature to create a framework for health professions education during a crisis. From this framework, we propose a set of recommendations to assist educational policymakers and those working directly with learners to navigate these issues effectively.
KEY EDUCATIONAL ISSUES
Patient and Hospital Welfare
There are significant concerns about nosocomial spread of SARS-CoV-2. Having learners directly see COVID-19 patients can increase the risk of nosocomial spread. In one of the original case series, 29% of those infected were health care workers and 12.3% were patients hospitalized prior to infection.14 Additionally, preserving supplies of personal protective equipment (PPE) for healthcare workers has been a commonly cited reason for suspending student presence on clinical rotations. Insufficient supply of PPE has forced hospitals to relax PPE guidelines for those seeing patients under investigation and liberalize quarantine requirements for exposed health care workers, so many hospitals have reduced provider-patient interactions to only those considered essential.
Learner Welfare
As educators, we have a duty to keep our learners safe and psychologically well. The COVID-19 pandemic poses a risk of illness, permanent injury, or death among those infected. In some instances, the risks of exposure may be greater than the educational benefits of remaining in that clinical setting; however, health professions trainees at many institutions play such a central operational role that their absence could seriously impair overall care delivery. Furthermore, trainees are usually younger and healthier than supervising clinicians, which could leave them feeling an obligation to conduct a disproportionately large share of the direct patient contact. Despite these valid concerns, those being removed from the clinical environment for their safety could misinterpret it as a sign that their contributions or educational interests are not valued.
Educational Experience
Canceled clinical rotations will have significant negative educational effects on undergraduate learners. Depending on the extent of the pandemic’s effects, for example, third-year medical students may lack core rotations prior to applying for residency training. Other health professions face similar challenges—nursing students in their final year are likely missing their last opportunity for hands-on clinical training before graduation. Advanced practice nursing students may not be able to complete the required number of contact hours or clinical experiences mandated for accreditation. Graduate training programs must accommodate and adapt to these disparities when reviewing their applicant pools.
Absence from the clinical front lines, though, risks failing to capitalize on the unique educational opportunities presented by this pandemic. Students might miss the chance to learn about a new clinical entity and its increasingly varied clinical presentations, crisis medicine, infection control measures, emergency preparedness, ethics in the setting of scarce resources, public health and community response, communication in the setting of uncertainty and fear, and professionalism in the response to this singular situation. Trainees at all levels may miss the opportunity to stand alongside their teachers and peers to give care to those who need it most.
Heterogeneity of COVID-19 Responses Across the Country
The diversity of training sites in US health professions education has led to a wide range of responses to these challenges. In addition to regional variations, sites within individual academic programs are creating different educational and clinical polices, including the role of learners in the care of COVID-19 patients and even PPE requirements. Although educational accreditation bodies have offered guidance, implementation of creative responses has been left to individual schools, programs, and hospitals, creating important differences in learner training and experience.
A FRAMEWORK FOR PANDEMIC HEALTH PROFESSIONS EDUCATION
Given these challenges, we offer four broad principles to guide health professions education in response to this pandemic. Within this framework, we offer multiple suggestions to individual educators, health professions programs, healthcare systems, and educational policymakers.
1. Prioritize healthcare system welfare: Patients are the core of our professional responsibility, and their needs take precedence. First and foremost, plans for our learners must always promote and support the proper functioning of the health system and its individual healthcare workers. To support care delivery, healthcare systems should do the following:
- Ensure educational activities minimize the risk of nosocomial transmission and adverse effects on patient safety. For example, hospitals can modify bedside care to reduce exposure by using phone or video for patient-trainee contact, performing selective physical examination only, and, when needed, prioritizing a single skilled examiner.
- Ensure learner use of PPE does not negatively affect availability for others, both now and as the pandemic unfolds.
- Engage learners in authentic, value-added healthcare activities outside of direct patient contact: tele-medicine, meeting with families, or spending video time with inpatients not under their direct care.
2. Promote learner welfare: Educators have a duty to ensure the physical and psychological safety of learners across the health professions continuum. By virtue of power differentials in the hierarchy of the teaching environment, learners can be particularly vulnerable. To promote learner wellbeing, educators should do the following:
- Deploy technology to maximize opportunities for and quality of non–face-to-face clinical, didactic, and interprofessional learning.
- Ensure learners have access to and proper training in the use of PPE, independent of whether they may be using PPE as part of clinical responsibilities, while remaining aware of the potential supply constraints during a pandemic.
- Deliberately include stop points during teaching for dialogue around fears, stress, resilience, and coping.15 Deploy additional resources for support, including in-person or virtual psychological and psychiatric care and crisis intervention counseling.
- Maintain flexibility regarding trainee’s educational needs. For example, welcome trainees from other services joining inpatient medicine or ICU teams. Acknowledge the stress they may feel and support them as they learn and adapt. This can be a unique opportunity for lessons in professionalism, teamwork, and communication.
3. Maximize educational value: Efforts must be made to preserve educational quality and content, limit educational cost, and leverage unique opportunities that may only be available during this time. Educators and programs should do the following:
- Adapt teaching to reflect changes in the hospital environment. A student may have spent more time on the phone with a patient; the nurse may have examined the patient; a resident may have vital sign and lab data; the attending may have spoken to the family or know about local policy changes affecting care. The usual modes of rounding should adapt, focusing on sharing and synthesizing multisource data to generate rapid, intelligent plans while mitigating risk.
- Turn the potential challenge of diminished access to previously routine diagnostic testing into an opportunity for trainees to assertively develop clinical skills often underutilized in practice environments without resource limitation.
- Discuss learning opportunities for healthcare ethics. Multiple aspects of this pandemic raise ethical issues around allocation of scarce resources and principles such as contingency and crisis standards of care: the availability and application of testing, potential changes to patient triage standards in which patients sicker than ever may be sent home, and crisis allocation of life support resources.
- Highlight opportunities to support interprofessional education and collaborative practice. As traditional professional boundaries are temporarily blurred, we may find nurses asking gowned physicians to perform nursing tasks (eg, inflate blood pressure cuffs). Physicians may ask nurses for patient-related information (eg, physical examination findings), all to limit collective risk, maximize efficiency, and minimize the use of scarce PPE.
- Teach telemedicine. This is an opportunity to create a cadre of clinicians adept with this type of practice for the future—even outside pandemics. Now may be the time for virtual visits to be better integrated into clinical practice, which has been of interest to patients and providers for some time, and to address the constraints of reimbursement policies.
- Provide explicit role modeling to ensure learners recognize and learn from the key components of faculty activity—modeling communication skills, engaging in clinical reasoning, or navigating clinical and professional uncertainty.16 For example, faculty could share their clinical reasoning regarding diagnosis of respiratory complaints. While COVID-19 may be the most urgent diagnostic consideration, educators can emphasize the risk and implications of anchoring bias as an important cause of diagnostic errors.
- Identify opportunities for educational scholarship around these and other changes resulting from the pandemic. Seek to engage learners in this work.
4. Communicate transparently: Learners must be witness to decision-making processes; this will demonstrate that their safety and education are valued. Wherever possible, include learners in decision-making discussions and in the process of disseminating information.
- At the institutional level, generate, modify, and share communication regarding the ways that education is changing and the values and goals behind those changes.
- Invite trainees as active contributors to intellectual exchanges regarding changes in the learning environment.
- Limit the negative impact of the “rumor mill” by replacing it with frequent, targeted, and accurate messaging that relies on evidence to the greatest extent possible.
- Strive for consistency in communication content but diversity in distribution to reach the learners in the most effective ways. In times of uncertainty and stress, err on the side of overcommunication.
SUMMARY
Healthcare and medical education face a challenge unprecedented in our lifetimes. The COVID-19 pandemic will touch every aspect of how we care for patients, train the next generation of health professionals, and keep ourselves safe. By highlighting key issues facing health professions educators, offering a framework for education during pandemics, and providing specific suggestions for applying this framework, we hope to provide clarity on how we may advance our teaching mission and realize the educational opportunities as we face this crisis together.
1. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): Cases in the US. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 31, 2020.
2. Association of American Medical Colleges. Guidance on Medical Students’ Clinical Participation: Effective Immediately. https://www.aamc.org/system/files/2020-03/Guidance%20on%20Student%20Clinical%20Participation%203.17.20%20Final.pdf. Accessed March 30, 2020.
3. Updated: ACGME Guidance Statement on Coronavirus (COVID-19) and Resident/Fellow Education in the United States. https://acgme.org/COVID-19/Stage-2-Increased-Clinical-Demands-Guidance, Accessed April 6, 2020.
4. National League for Nursing. Coronavirus Resource Center. http://www.nln.org/coronavirus-resource-center. Accessed March 28, 2020.
5. Patil NG, Yan YC. SARS and its effect on medical education in Hong Kong. Med Educ. 2003;37(12):1127-1128. https://doi.org/10.1046/j.1365-2923.2003.01723.x.
6. Clark J. Fear of SARS thwarts medical education in Toronto. BMJ. 2003;326(7393):784. https://doi.org/10.1136/bmj.326.7393.784/c.
7. Sherbino J, Atzema C. SARS-Ed: severe acute respiratory syndrome and the impact on medical education. Ann Acad Emerg. 2004;44(3):229-231. https://doi.org/10.1016/j.annemergmed.2004.05.021.
8. Rambaldini G, Wilson K, Rath D, et al. The impact of severe acute respiratory syndrome on medical house staff: a qualitative study. J Gen Intern Med. 2005;20(5):381-385. https://doi.org/10.1111/j.1525-1497.2005.0099.x.
9. Lim EC, Oh VM, Koh DR, Seet RC. The challenges of “continuing medical education” in a pandemic era. Ann Acad Med Singapore. 2009;38(8):724-726.
10. Cole B. 10,000 Med school graduates in Italy skip final exam, get sent directly into health service to help fight COVID-19. Newsweek. March 18, 2020. https://www.newsweek.com/italy-coronavirus-covid-19-medical-students-1492996. Accessed March 27, 2020.
11. Siddique H. Final-year medical students graduate early to fight Covid-19. The Guardian. March 20, 2020. https://www.theguardian.com/world/2020/mar/20/final-year-medical-students-graduate-early-fight-coronavirus-covid-19. Accessed March 27, 2020.
12. Ahmed H, Allaf M, Elghazaly H. COVID-19 and medical education. Lancet Infect Dis. 2020. https://doi.org/10.1016/S1473-3099(20)30226-7.
13. Ducharme J. NYU med school will graduate students early to help New York fight coronavirus. Time. March 25, 2020. https://time.com/5809630/nyu-medical-school-early-graduation/. Accessed March 30, 2020.
14. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel-coronavirus infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. https://doi.org/10.1001/jama.2020.1585.
15. Markakis KM, Beckman HB, Suchman AL, Frankel RM. The path to professionalism: cultivating humanistic values and attitudes in residency training. Acad Med. 2000;75(2):141-150. https://doi.org/10.1097/00001888-200002000-00009.
16. Jochemsen-van der Leeuw HG, van Dijk N, van Etten-Jamaludin FS, Wieringa-de Waard M. The attributes of the clinical teacher as role model: a systematic review. Acad Med. 2013;88(1):26-34. https://doi.org/10.1097/ACM.0b013e318276d070.
1. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): Cases in the US. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 31, 2020.
2. Association of American Medical Colleges. Guidance on Medical Students’ Clinical Participation: Effective Immediately. https://www.aamc.org/system/files/2020-03/Guidance%20on%20Student%20Clinical%20Participation%203.17.20%20Final.pdf. Accessed March 30, 2020.
3. Updated: ACGME Guidance Statement on Coronavirus (COVID-19) and Resident/Fellow Education in the United States. https://acgme.org/COVID-19/Stage-2-Increased-Clinical-Demands-Guidance, Accessed April 6, 2020.
4. National League for Nursing. Coronavirus Resource Center. http://www.nln.org/coronavirus-resource-center. Accessed March 28, 2020.
5. Patil NG, Yan YC. SARS and its effect on medical education in Hong Kong. Med Educ. 2003;37(12):1127-1128. https://doi.org/10.1046/j.1365-2923.2003.01723.x.
6. Clark J. Fear of SARS thwarts medical education in Toronto. BMJ. 2003;326(7393):784. https://doi.org/10.1136/bmj.326.7393.784/c.
7. Sherbino J, Atzema C. SARS-Ed: severe acute respiratory syndrome and the impact on medical education. Ann Acad Emerg. 2004;44(3):229-231. https://doi.org/10.1016/j.annemergmed.2004.05.021.
8. Rambaldini G, Wilson K, Rath D, et al. The impact of severe acute respiratory syndrome on medical house staff: a qualitative study. J Gen Intern Med. 2005;20(5):381-385. https://doi.org/10.1111/j.1525-1497.2005.0099.x.
9. Lim EC, Oh VM, Koh DR, Seet RC. The challenges of “continuing medical education” in a pandemic era. Ann Acad Med Singapore. 2009;38(8):724-726.
10. Cole B. 10,000 Med school graduates in Italy skip final exam, get sent directly into health service to help fight COVID-19. Newsweek. March 18, 2020. https://www.newsweek.com/italy-coronavirus-covid-19-medical-students-1492996. Accessed March 27, 2020.
11. Siddique H. Final-year medical students graduate early to fight Covid-19. The Guardian. March 20, 2020. https://www.theguardian.com/world/2020/mar/20/final-year-medical-students-graduate-early-fight-coronavirus-covid-19. Accessed March 27, 2020.
12. Ahmed H, Allaf M, Elghazaly H. COVID-19 and medical education. Lancet Infect Dis. 2020. https://doi.org/10.1016/S1473-3099(20)30226-7.
13. Ducharme J. NYU med school will graduate students early to help New York fight coronavirus. Time. March 25, 2020. https://time.com/5809630/nyu-medical-school-early-graduation/. Accessed March 30, 2020.
14. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel-coronavirus infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. https://doi.org/10.1001/jama.2020.1585.
15. Markakis KM, Beckman HB, Suchman AL, Frankel RM. The path to professionalism: cultivating humanistic values and attitudes in residency training. Acad Med. 2000;75(2):141-150. https://doi.org/10.1097/00001888-200002000-00009.
16. Jochemsen-van der Leeuw HG, van Dijk N, van Etten-Jamaludin FS, Wieringa-de Waard M. The attributes of the clinical teacher as role model: a systematic review. Acad Med. 2013;88(1):26-34. https://doi.org/10.1097/ACM.0b013e318276d070.
© 2020 Society of Hospital Medicine