User login
A Short-Lived Crisis
A 79-year-old woman presented to the emergency department with 1 day of nausea and vomiting. On the morning of presentation, she felt mild cramping in her legs and vomited twice. She denied chest or back pain, dyspnea, diaphoresis, cough, fever, dysuria, headache, and abdominal pain. Her medical history included hypertension, osteoporosis, and a right-sided acoustic neuroma treated with radiation 12 years prior. One month before this presentation, type 2 diabetes mellitus was diagnosed (hemoglobin A1c level, 7.3%) on routine testing by her primary care physician. Her medications were losartan and alendronate. She was born in China and immigrated to the United States 50 years prior. Her husband was chronically ill with several recent hospitalizations.
Nausea and vomiting are nonspecific symptoms that can arise from systemic illness, including hyperglycemia, a drug/toxin effect, or injury/inflammation of the gastrointestinal, central nervous system, or cardiovascular systems. An acoustic neuroma recurrence or malignancy in the radiation field could trigger nausea. Muscle cramping could arise from myositis or from hypokalemia secondary to vomiting. Her husband’s recent hospitalizations add an important psychosocial dimension to her care and should prompt consideration of a shared illness depending on the nature of his illness.
The patient’s temperature was 36.7 °C; heart rate, 99 beats per minute; blood pressure, 94/58 mm Hg;respiratory rate, 16 breaths per minute; and oxygen saturation, 98% while breathing room air. Her body mass index (BMI) was 18.7 kg/m2. She appeared comfortable. The heart, lung, jugular venous, and abdominal examinations were normal. She had no lower extremity edema or muscle tenderness.
The white blood cell (WBC) count was 14,500/µL (81% neutrophils, 9% lymphocytes, 8% monocytes), hemoglobin level was 17.5 g/dL (elevated from 14.2 g/dL 8 weeks prior), and platelet count was 238,000/µL. The metabolic panel revealed the following values: sodium, 139 mmol/L; potassium, 5.1 mmol/L; chloride, 96 mmol/L; bicarbonate, 17 mmol/L; blood urea nitrogen, 40 mg/dL; creatinine, 2.2 mg/dL (elevated from 0.7 mg/dL 8 weeks prior); glucose, 564 mg/dL; aspartate transaminase, 108 U/L; alanine transaminase, 130 U/L; total bilirubin, 0.6 mg/dL; and alkaline phosphatase, 105 U/L. Creatine kinase, amylase, and lipase levels were not measured. The urinalysis showed trace ketones, protein 100 mg/dL, glucose >500 mg/dL, and <5 WBCs per high-power field. The venous blood gas demonstrated a pH of 7.20 and lactate level of 13.2 mmol/L. Serum beta-hydroxybutyrate level was 0.27 mmol/L (reference range, 0.02-0.27), serum troponin I level was 8.5 µg/L (reference range, <0.05), and
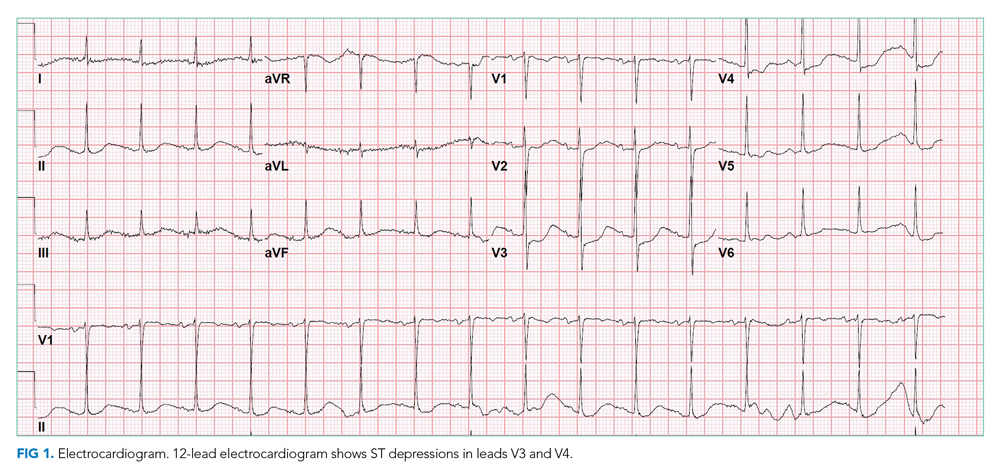
Chest x-ray showed bilateral perihilar opacities with normal heart size. Electrocardiogram (ECG) revealed new ST-segment depressions in the anterior precordial leads (Figure 1).
Her hypotension may signal septic, cardiogenic, or hypovolemic shock. The leukocytosis, anion gap acidosis, acute kidney injury, and elevated lactate are compatible with sepsis, although there is no identified source of infection. Although diabetic ketoacidosis (DKA) can explain many of these findings, the serum beta-hydroxybutyrate and urine ketones are lower than expected for that condition. Her low-normal BMI makes significant insulin resistance less likely and raises concern about pancreatic adenocarcinoma as a secondary cause of diabetes.
The nausea, ST depressions, elevated troponin and B-type natriuretic peptide levels, and bilateral infiltrates suggest acute coronary syndrome (ACS), complicated by acute heart failure leading to systemic hypoperfusion and associated lactic acidosis and kidney injury. Nonischemic causes of myocardial injury, such as sepsis, myocarditis, and stress cardiomyopathy, should also be considered. Alternatively, she could be experiencing multiorgan injury from widespread embolism (eg, endocarditis), thrombosis (eg, antiphospholipid syndrome), or inflammation (eg, vasculitis). Acute pancreatitis can cause acute hyperglycemia and multisystem disease, but she did not have abdominal pain or tenderness (and her lipase level was not measured). Treatment should include intravenous insulin, intravenous fluids (trying to balance possible sepsis or DKA with heart failure), medical management for non-ST elevation myocardial infarction (NSTEMI), and empiric antibiotics.
ACS was diagnosed, and aspirin, atorvastatin, clopidogrel, and heparin were prescribed. Insulin infusion and intravenous fluids (approximately 3 L overnight) were administered for hyperglycemia (and possible early DKA). On the night of admission, the patient became profoundly diaphoretic without fevers; the WBC count rose to 24,200/µL. Vancomycin and ertapenem were initiated for possible sepsis. Serum troponin I level increased to 11.9 µg/L; the patient did not have chest pain, and the ECG was unchanged.
The next morning, the patient reported new mild diffuse abdominal pain and had mild epigastric tenderness. The WBC count was 28,900/µL; hemoglobin, 13.2 g/dL; venous pH, 7.39; lactate, 2.9 mmol/L; lipase, 48 U/L; aspartate transaminase, 84 U/L; alanine transaminase, 72 U/L; total bilirubin, 0.7 mg/dL; alkaline phosphatase, 64 U/L; and creatinine, 1.2 mg/dL.
Her rising troponin without dynamic ECG changes makes the diagnosis of ACS less likely, although myocardial ischemia can present as abdominal pain. Other causes of myocardial injury to consider (in addition to the previously mentioned sepsis, myocarditis, and stress cardiomyopathy) are pulmonary embolism and proximal aortic dissection. The latter can lead to ischemia in multiple systems (cardiac, mesenteric, renal, and lower extremity, recalling her leg cramps on admission).
The leukocytosis and lactic acidosis in the setting of new abdominal pain raises the question of mesenteric ischemia or intra-abdominal sepsis. Her hemoglobin has decreased by 4 g, and while some of the change may be dilutional, it will be important to consider hemolysis (less likely with a normal bilirubin) or gastrointestinal bleeding (given current anticoagulant and antiplatelet therapy). An echocardiogram and computed tomography (CT) angiogram of the chest, abdomen, and pelvis are indicated to evaluate the vasculature and assess for intra-abdominal pathology.
Coronary angiography revealed a 40% stenosis in the proximal right coronary artery and no other angiographically significant disease; the left ventricular end-diastolic pressure (LVEDP) was 30 mm Hg. Transthoracic echocardiography demonstrated normal left ventricular size, left ventricular ejection fraction of 65% to 70%, impaired left ventricular relaxation, and an inferior vena cava <2 cm in diameter that collapsed with inspiration.
The angiogram shows modest coronary artery disease and points away from plaque rupture as the cause of myocardial injury. Another important consideration given her husband’s recurrent illness is stress cardiomyopathy, but she does not have the typical apical ballooning or left ventricular dysfunction. The increased LVEDP with normal left ventricular size and function with elevated filling pressures is consistent with left-sided heart failure with preserved ejection fraction. Cardiac magnetic resonance imaging could exclude an infiltrative disorder leading to diastolic dysfunction or a myocarditis that explains the troponin elevation, but both diagnoses seem unlikely.
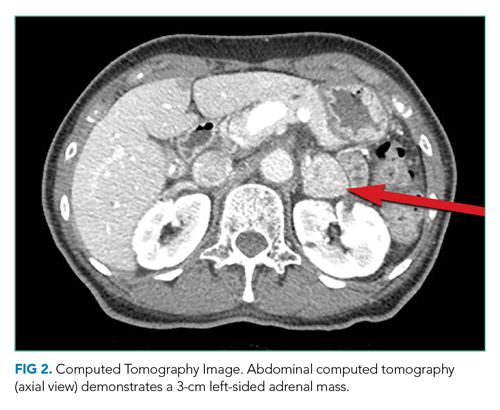
CT of the abdomen and pelvis demonstrated a heterogeneous 3-cm mass in the left adrenal gland (Figure 2).
An adrenal mass could be a functional or nonfunctional adenoma, primary adrenal carcinoma, a metastatic malignancy, or granulomatous infection such as tuberculosis. Secretion of excess glucocorticoid, mineralocorticoid, or catecholamine should be evaluated.
Cushing syndrome could explain her hyperglycemia, leukocytosis, and heart failure (mediated by the increased risk of atherosclerosis and hypertension with hypercortisolism), although her low BMI is atypical. Primary hyperaldosteronism causes hypertension but does not cause an acute multisystem disease. Pheochromocytoma could account for the diaphoresis, hypertension, hyperglycemia, leukocytosis, and cardiac injury. A more severe form—pheochromocytoma crisis—is characterized by widespread end-organ damage, including cardiomyopathy, bowel ischemia, hepatitis, hyperglycemia with ketoacidosis, and lactic acidosis. Measurement of serum cortisol and plasma and urine fractionated metanephrines, and a dexamethasone suppression test can determine whether the adrenal mass is functional.
The intravenous insulin infusion was changed to subcutaneous dosing on hospital day 2. She had no further nausea, diaphoresis, or abdominal pain, was walking around the hospital unit unassisted, and was consuming a regular diet. By hospital day 3, insulin was discontinued. The patient remained euglycemic for the remainder of her hospitalization; hemoglobin A1c value was 7.0%. Blood cultures were sterile, and the WBC count was 12,000/µL. Thyroid-stimulating hormone level was 0.31 mIU/L (reference range, 0.45-4.12), and the free thyroxine level was 12 pmol/L (reference range, 10-18). Antibiotics were discontinued. She remained euvolemic and never required diuretic therapy. The acute myocardial injury and diastolic dysfunction were attributed to an acute stress cardiomyopathy arising from the strain of her husband’s declining health. She was discharged on hospital day 5 with aspirin, atorvastatin, metoprolol, lisinopril, and outpatient follow-up.
The rapid resolution of her multisystem process suggests a self-limited process or successful treatment of the underlying cause. Although she received antibiotics, a bacterial infection never manifested. Cardiomyopathy with a high troponin level, ECG changes, and early heart failure often requires aggressive supportive measures, which were not required here. The rapid cessation of hyperglycemia and an insulin requirement within 1 day is atypical for DKA.
Pheochromocytoma is a rare secondary cause of diabetes in which excess catecholamines cause insulin resistance and suppress insulin release. It can explain both the adrenal mass and, in the form of pheochromocytoma crisis, the severe multisystem injury. However, the patient’s hypotension (which could be explained by concomitant cardiomyopathy) and older age are not typical for pheochromocytoma.
Results of testing for adrenal biomarkers, which were sent during her hospitalization, returned several days after hospital discharge. The plasma free metanephrine level was 687 pg/mL (reference range, <57) and the plasma free normetanephrine level was 508 pg/mL (reference range, <148). Metoprolol was discontinued by her primary care physician.
Elevated plasma free metanephrine and normetanephrine levels were confirmed in the endocrinology clinic 3 weeks later. The 24-hour urine metanephrine level was 1497 µg/24 hours (reference range, 90-315), and the 24-hour urine normetanephrine level was 379 µg/24 hours (reference range, 122-676). Serum aldosterone level was 8 ng/dL (reference range, 3-16), and morning cortisol level was 8 µg/dL (reference range, 4-19). Lisinopril was discontinued, and phenoxybenzamine was prescribed.
Adrenal-protocol CT of the abdomen demonstrated that the left adrenal mass was enhanced by contrast without definite washout, which could be consistent with a pheochromocytoma.
The diagnosis of pheochromocytoma has been confirmed by biochemistry and imaging. It was appropriate to stop metoprolol, as β-blockade can lead to unopposed α-receptor agonism and hypertension. Implementation of α-blockade with phenoxybenzamine and endocrine surgery referral are indicated.
On the day she intended to fill a phenoxybenzamine prescription, the patient experienced acute generalized weakness and presented to the emergency department with hyperglycemia (glucose, 661 mg/dL), acute kidney injury (creatinine, 1.6 mg/dL), troponin I elevation (0.14 µg/L), and lactic acidosis (4.7 mmol/L). She was admitted to the hospital and rapidly improved with intravenous fluids and insulin. Phenoxybenzamine 10 mg daily was administered, and she was discharged on hospital day 2. The dosage of phenoxybenzamine was gradually increased over 2 months.
Laparoscopic left adrenalectomy was performed, with removal of a 3-cm mass. The pathologic findings confirmed the diagnosis of pheochromocytoma. Two months later she felt well. Her hypertension was controlled with lisinopril 10 mg daily. Transthoracic echocardiography 3 months after adrenalectomy demonstrated a left ventricular ejection fraction of 60% to 65%. Six months later, her hemoglobin A1c was 6.6%.
DISCUSSION
Pheochromocytoma is an abnormal growth of cells of chromaffin origin that arises in the adrenal medulla.1,2 The incidence of these often benign tumors is estimated to be 2 to 8 cases per million in the general population, and 2 to 6 per 1000 in adult patients with hypertension.1,3,4 Although clinicians commonly associate these catecholamine-secreting tumors with intermittent hypertension or diaphoresis, they have a wide spectrum of manifestations, which range from asymptomatic adrenal mass to acute multiorgan illness that mimics other life-threatening conditions. Common signs and symptoms of pheochromocytoma include hypertension (60%-70% incidence), headache (50%), diaphoresis (50%), and palpitations (50%-60%).4 The textbook triad of headache, sweating, and palpitations is seen in fewer than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is often explained by a more common condition.1,4 Approximately 5% of adrenal “incidentalomas” are pheochromocytomas that are minimally symptomatic or asymptomatic.1,3 In a study of 102 patients who underwent pheochromocytoma resection, 33% were diagnosed during evaluation of an adrenal incidentaloma.5 At the other end of the spectrum is a pheochromocytoma crisis with its mimicry of ACS and sepsis, and manifestations including severe hyperglycemia, abdominal pain, acute heart failure, and syncope.2,5-9 Aside from chronic mild hypertension and a single episode of diaphoresis during admission, our patient had none of the classic signs or symptoms of pheochromocytoma. Rather, she presented with the abrupt onset of multiorgan injury.
Diagnostic evaluation for pheochromocytoma typically includes demonstration of elevated catecholamine byproducts (metanephrines) in plasma or urine and an adrenal mass on imaging.2,10 Biopsy is contraindicated because this can lead to release of catecholamines, which can trigger a pheochromocytoma crisis.5 The Endocrine Society guidelines recommend evaluating patients for pheochromocytoma who have: (1) a known or suspected genetic syndrome linked to pheochromocytoma (eg, multiple endocrine neoplasia type 2 or Von Hippel-Lindau syndrome), (2) an adrenal mass incidentally found on imaging, regardless of a history of hypertension, or (3) signs and symptoms of pheochromocytoma.3
Patients in pheochromocytoma crisis are typically very ill, requiring intensive care unit admission for hemodynamic stabilization.1,11 Initial management is typically directed at assessing and treating for common causes of systemic illness and hemodynamic instability, such as ACS and sepsis. Although some patients with pheochromocytoma crisis may have hemodynamic collapse requiring invasive circulatory support, others improve while receiving empiric treatment for mimicking conditions. Our patient had multiorgan injury and hemodynamic instability but returned to her preadmission state within 48 to 72 hours and remained stable after the withdrawal of all therapies, including insulin and antibiotics. This rapid improvement suggested a paroxysmal condition with an “on/off” capacity mediated by endogenous mediators. Once pheochromocytoma crisis is diagnosed, hemodynamic stabilization with α-adrenergic receptor blockade and intravascular volume repletion is essential. Confirmation of the diagnosis with repeat testing after hospital discharge is important because biochemical test results are less specific in the setting of acute illness. Surgery on an elective basis is the definitive treatment. Ongoing α-adrenergic receptor blockade is essential to minimize the risk of an intraoperative pheochromocytoma crisis (because of anesthesia or tumor manipulation) and prevent cardiovascular collapse after resection of tumor.11
Although the biochemical profile of a pheochromocytoma (eg, epinephrine predominant) is not tightly linked to the phenotype, the pattern of organ injury can reflect the pleotropic effects of specific catecholamines.12 While both norepinephrine and epinephrine bind the β1-adrenergic receptor with equal affinity, epinephrine has a higher affinity for the β2-adrenergic receptor. Our patient’s initial relative hypotension was likely caused by hypovolemia from decreased oral intake, vomiting, and hyperglycemia-mediated polyuria. However, β2-adrenergic receptor agonism could have caused vasodilation, and nocardiogenic hypotension has been observed with epinephrine-predominant pheochromocytomas.13 Several of the other clinical findings in this case can be explained by widespread β-adrenergic receptor agonism. Epinephrine (whether endogenously produced or exogenously administered) can lead to cardiac injury with elevated cardiac biomarkers.1,6,14 Epinephrine administration can cause leukocytosis, which is attributed to demargination of leukocyte subsets that express β2-adrenergic receptors.15,16 Lactic acidosis in the absence of tissue hypoxia (type B lactic acidosis) occurs during epinephrine infusions in healthy volunteers.17,18 Hyperglycemia from epinephrine infusions is attributed to β-adrenergic receptor stimulation causing increased gluconeogenesis and glycogenolysis and decreased insulin secretion and tissue glucose uptake.8 Resolution of hyperglycemia and diabetes is observed in the majority of patients after resection of pheochromocytoma, and hypoglycemia immediately after surgery is common, occasionally requiring glucose infusion.19,20
Pheochromocytomas are rare tumors with a wide range of manifestations that extend well beyond the classic triad. Pheochromocytomas can present as an asymptomatic adrenal mass with normal blood pressure, as new onset diabetes, or as multiorgan injury with cardiovascular collapse. Our patient suffered from two episodes of catecholamine excess that required hospitalization, but fortunately each proved to be a short-lived crisis.
TEACHING POINTS
- The classic triad of headache, sweating, and palpitations occurs in less than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is usually explained by a common medical condition.
- The presentation of pheochromocytoma varies widely, from asymptomatic adrenal incidentaloma to pheochromocytoma crisis causing multiorgan dysfunction with hemodynamic instability and mimicry of common critical illnesses like ACS, DKA, and sepsis.
- Biochemical screening for pheochromocytoma is recommended when a patient has a known or suspected genetic syndrome linked to pheochromocytoma, an adrenal mass incidentally found on imaging regardless of blood pressure, or signs and symptoms of a pheochromocytoma.
1. Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757-764. https://doi.org/10.1530/eje-15-0483
2. Whitelaw BC, Prague JK, Mustafa OG, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf). 2014;80(1):13-22. https://doi.org/10.1111/cen.12324
3. Lenders JW, Duh QY, Eisenhofer G, et al; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498
4. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24(12):2331-2339. https://doi.org/10.1097/01.hjh.0000251887.01885.54
5. Shen WT, Grogan R, Vriens M, Clark OH, Duh QY. One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg. 2010;145(9):893-897. https://doi.org/10.1001/archsurg.2010.159
6. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99(14):1438-1444. https://doi.org/10.1136/heartjnl-2013-304073
7. Lee TW, Lin KH, Chang CJ, Lew WH, Lee TI. Pheochromocytoma mimicking both acute coronary syndrome and sepsis: a case report. Med Princ Pract. 2013;22(4):405-407. https://doi.org/10.1159/000343578
8. Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017;23(8):999-1005. https://doi.org/10.4158/ep171914.ra
9. Ueda T, Oka N, Matsumoto A, et al. Pheochromocytoma presenting as recurrent hypotension and syncope. Intern Med. 2005;44(3):222-227. https://doi.org/10.2169/internalmedicine.44.222
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. https://doi.org/10.1056/nejmra1806651
11. Scholten A, Cisco RM, Vriens MR, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. 2013;98(2):581-591. https://doi.org/10.1210/jc.2012-3020
12. Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65-90.
13. Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf). 1992;37(3):304-306. https://doi.org/10.1111/j.1365-2265.1992.tb02326.x
14. Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3(1):76-80. https://doi.org/10.1016/j.jaip.2014.06.007
15. Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77-91. https://doi.org/10.1006/brbi.1996.0009
16. Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503-511. https://doi.org/10.4049/jimmunol.0902189
17. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-1140. https://doi.org/10.1016/j.mayocp.2013.06.012
18. Levy B. Bench-to-bedside review: is there a place for epinephrine in septic shock? Crit Care. 2005;9(6):561-565. https://doi.org/10.1186/cc3901
19. Chen Y, Hodin RA, Pandolfi C, Ruan DT, McKenzie TJ. Hypoglycemia after resection of pheochromocytoma. Surgery. 2014;156:1404-1408; discussion 1408-1409. https://doi.org/10.1016/j.surg.2014.08.020
20. Pogorzelski R, Toutounchi S, Krajewska E, et al. The effect of surgical treatment of phaeochromocytoma on concomitant arterial hypertension and diabetes mellitus in a single-centre retrospective study. Cent European J Urol. 2014;67(4):361-365. https://doi.org/10.5173/ceju.2014.04.art9
A 79-year-old woman presented to the emergency department with 1 day of nausea and vomiting. On the morning of presentation, she felt mild cramping in her legs and vomited twice. She denied chest or back pain, dyspnea, diaphoresis, cough, fever, dysuria, headache, and abdominal pain. Her medical history included hypertension, osteoporosis, and a right-sided acoustic neuroma treated with radiation 12 years prior. One month before this presentation, type 2 diabetes mellitus was diagnosed (hemoglobin A1c level, 7.3%) on routine testing by her primary care physician. Her medications were losartan and alendronate. She was born in China and immigrated to the United States 50 years prior. Her husband was chronically ill with several recent hospitalizations.
Nausea and vomiting are nonspecific symptoms that can arise from systemic illness, including hyperglycemia, a drug/toxin effect, or injury/inflammation of the gastrointestinal, central nervous system, or cardiovascular systems. An acoustic neuroma recurrence or malignancy in the radiation field could trigger nausea. Muscle cramping could arise from myositis or from hypokalemia secondary to vomiting. Her husband’s recent hospitalizations add an important psychosocial dimension to her care and should prompt consideration of a shared illness depending on the nature of his illness.
The patient’s temperature was 36.7 °C; heart rate, 99 beats per minute; blood pressure, 94/58 mm Hg;respiratory rate, 16 breaths per minute; and oxygen saturation, 98% while breathing room air. Her body mass index (BMI) was 18.7 kg/m2. She appeared comfortable. The heart, lung, jugular venous, and abdominal examinations were normal. She had no lower extremity edema or muscle tenderness.
The white blood cell (WBC) count was 14,500/µL (81% neutrophils, 9% lymphocytes, 8% monocytes), hemoglobin level was 17.5 g/dL (elevated from 14.2 g/dL 8 weeks prior), and platelet count was 238,000/µL. The metabolic panel revealed the following values: sodium, 139 mmol/L; potassium, 5.1 mmol/L; chloride, 96 mmol/L; bicarbonate, 17 mmol/L; blood urea nitrogen, 40 mg/dL; creatinine, 2.2 mg/dL (elevated from 0.7 mg/dL 8 weeks prior); glucose, 564 mg/dL; aspartate transaminase, 108 U/L; alanine transaminase, 130 U/L; total bilirubin, 0.6 mg/dL; and alkaline phosphatase, 105 U/L. Creatine kinase, amylase, and lipase levels were not measured. The urinalysis showed trace ketones, protein 100 mg/dL, glucose >500 mg/dL, and <5 WBCs per high-power field. The venous blood gas demonstrated a pH of 7.20 and lactate level of 13.2 mmol/L. Serum beta-hydroxybutyrate level was 0.27 mmol/L (reference range, 0.02-0.27), serum troponin I level was 8.5 µg/L (reference range, <0.05), and
Chest x-ray showed bilateral perihilar opacities with normal heart size. Electrocardiogram (ECG) revealed new ST-segment depressions in the anterior precordial leads (Figure 1).
Her hypotension may signal septic, cardiogenic, or hypovolemic shock. The leukocytosis, anion gap acidosis, acute kidney injury, and elevated lactate are compatible with sepsis, although there is no identified source of infection. Although diabetic ketoacidosis (DKA) can explain many of these findings, the serum beta-hydroxybutyrate and urine ketones are lower than expected for that condition. Her low-normal BMI makes significant insulin resistance less likely and raises concern about pancreatic adenocarcinoma as a secondary cause of diabetes.
The nausea, ST depressions, elevated troponin and B-type natriuretic peptide levels, and bilateral infiltrates suggest acute coronary syndrome (ACS), complicated by acute heart failure leading to systemic hypoperfusion and associated lactic acidosis and kidney injury. Nonischemic causes of myocardial injury, such as sepsis, myocarditis, and stress cardiomyopathy, should also be considered. Alternatively, she could be experiencing multiorgan injury from widespread embolism (eg, endocarditis), thrombosis (eg, antiphospholipid syndrome), or inflammation (eg, vasculitis). Acute pancreatitis can cause acute hyperglycemia and multisystem disease, but she did not have abdominal pain or tenderness (and her lipase level was not measured). Treatment should include intravenous insulin, intravenous fluids (trying to balance possible sepsis or DKA with heart failure), medical management for non-ST elevation myocardial infarction (NSTEMI), and empiric antibiotics.
ACS was diagnosed, and aspirin, atorvastatin, clopidogrel, and heparin were prescribed. Insulin infusion and intravenous fluids (approximately 3 L overnight) were administered for hyperglycemia (and possible early DKA). On the night of admission, the patient became profoundly diaphoretic without fevers; the WBC count rose to 24,200/µL. Vancomycin and ertapenem were initiated for possible sepsis. Serum troponin I level increased to 11.9 µg/L; the patient did not have chest pain, and the ECG was unchanged.
The next morning, the patient reported new mild diffuse abdominal pain and had mild epigastric tenderness. The WBC count was 28,900/µL; hemoglobin, 13.2 g/dL; venous pH, 7.39; lactate, 2.9 mmol/L; lipase, 48 U/L; aspartate transaminase, 84 U/L; alanine transaminase, 72 U/L; total bilirubin, 0.7 mg/dL; alkaline phosphatase, 64 U/L; and creatinine, 1.2 mg/dL.
Her rising troponin without dynamic ECG changes makes the diagnosis of ACS less likely, although myocardial ischemia can present as abdominal pain. Other causes of myocardial injury to consider (in addition to the previously mentioned sepsis, myocarditis, and stress cardiomyopathy) are pulmonary embolism and proximal aortic dissection. The latter can lead to ischemia in multiple systems (cardiac, mesenteric, renal, and lower extremity, recalling her leg cramps on admission).
The leukocytosis and lactic acidosis in the setting of new abdominal pain raises the question of mesenteric ischemia or intra-abdominal sepsis. Her hemoglobin has decreased by 4 g, and while some of the change may be dilutional, it will be important to consider hemolysis (less likely with a normal bilirubin) or gastrointestinal bleeding (given current anticoagulant and antiplatelet therapy). An echocardiogram and computed tomography (CT) angiogram of the chest, abdomen, and pelvis are indicated to evaluate the vasculature and assess for intra-abdominal pathology.
Coronary angiography revealed a 40% stenosis in the proximal right coronary artery and no other angiographically significant disease; the left ventricular end-diastolic pressure (LVEDP) was 30 mm Hg. Transthoracic echocardiography demonstrated normal left ventricular size, left ventricular ejection fraction of 65% to 70%, impaired left ventricular relaxation, and an inferior vena cava <2 cm in diameter that collapsed with inspiration.
The angiogram shows modest coronary artery disease and points away from plaque rupture as the cause of myocardial injury. Another important consideration given her husband’s recurrent illness is stress cardiomyopathy, but she does not have the typical apical ballooning or left ventricular dysfunction. The increased LVEDP with normal left ventricular size and function with elevated filling pressures is consistent with left-sided heart failure with preserved ejection fraction. Cardiac magnetic resonance imaging could exclude an infiltrative disorder leading to diastolic dysfunction or a myocarditis that explains the troponin elevation, but both diagnoses seem unlikely.
CT of the abdomen and pelvis demonstrated a heterogeneous 3-cm mass in the left adrenal gland (Figure 2).
An adrenal mass could be a functional or nonfunctional adenoma, primary adrenal carcinoma, a metastatic malignancy, or granulomatous infection such as tuberculosis. Secretion of excess glucocorticoid, mineralocorticoid, or catecholamine should be evaluated.
Cushing syndrome could explain her hyperglycemia, leukocytosis, and heart failure (mediated by the increased risk of atherosclerosis and hypertension with hypercortisolism), although her low BMI is atypical. Primary hyperaldosteronism causes hypertension but does not cause an acute multisystem disease. Pheochromocytoma could account for the diaphoresis, hypertension, hyperglycemia, leukocytosis, and cardiac injury. A more severe form—pheochromocytoma crisis—is characterized by widespread end-organ damage, including cardiomyopathy, bowel ischemia, hepatitis, hyperglycemia with ketoacidosis, and lactic acidosis. Measurement of serum cortisol and plasma and urine fractionated metanephrines, and a dexamethasone suppression test can determine whether the adrenal mass is functional.
The intravenous insulin infusion was changed to subcutaneous dosing on hospital day 2. She had no further nausea, diaphoresis, or abdominal pain, was walking around the hospital unit unassisted, and was consuming a regular diet. By hospital day 3, insulin was discontinued. The patient remained euglycemic for the remainder of her hospitalization; hemoglobin A1c value was 7.0%. Blood cultures were sterile, and the WBC count was 12,000/µL. Thyroid-stimulating hormone level was 0.31 mIU/L (reference range, 0.45-4.12), and the free thyroxine level was 12 pmol/L (reference range, 10-18). Antibiotics were discontinued. She remained euvolemic and never required diuretic therapy. The acute myocardial injury and diastolic dysfunction were attributed to an acute stress cardiomyopathy arising from the strain of her husband’s declining health. She was discharged on hospital day 5 with aspirin, atorvastatin, metoprolol, lisinopril, and outpatient follow-up.
The rapid resolution of her multisystem process suggests a self-limited process or successful treatment of the underlying cause. Although she received antibiotics, a bacterial infection never manifested. Cardiomyopathy with a high troponin level, ECG changes, and early heart failure often requires aggressive supportive measures, which were not required here. The rapid cessation of hyperglycemia and an insulin requirement within 1 day is atypical for DKA.
Pheochromocytoma is a rare secondary cause of diabetes in which excess catecholamines cause insulin resistance and suppress insulin release. It can explain both the adrenal mass and, in the form of pheochromocytoma crisis, the severe multisystem injury. However, the patient’s hypotension (which could be explained by concomitant cardiomyopathy) and older age are not typical for pheochromocytoma.
Results of testing for adrenal biomarkers, which were sent during her hospitalization, returned several days after hospital discharge. The plasma free metanephrine level was 687 pg/mL (reference range, <57) and the plasma free normetanephrine level was 508 pg/mL (reference range, <148). Metoprolol was discontinued by her primary care physician.
Elevated plasma free metanephrine and normetanephrine levels were confirmed in the endocrinology clinic 3 weeks later. The 24-hour urine metanephrine level was 1497 µg/24 hours (reference range, 90-315), and the 24-hour urine normetanephrine level was 379 µg/24 hours (reference range, 122-676). Serum aldosterone level was 8 ng/dL (reference range, 3-16), and morning cortisol level was 8 µg/dL (reference range, 4-19). Lisinopril was discontinued, and phenoxybenzamine was prescribed.
Adrenal-protocol CT of the abdomen demonstrated that the left adrenal mass was enhanced by contrast without definite washout, which could be consistent with a pheochromocytoma.
The diagnosis of pheochromocytoma has been confirmed by biochemistry and imaging. It was appropriate to stop metoprolol, as β-blockade can lead to unopposed α-receptor agonism and hypertension. Implementation of α-blockade with phenoxybenzamine and endocrine surgery referral are indicated.
On the day she intended to fill a phenoxybenzamine prescription, the patient experienced acute generalized weakness and presented to the emergency department with hyperglycemia (glucose, 661 mg/dL), acute kidney injury (creatinine, 1.6 mg/dL), troponin I elevation (0.14 µg/L), and lactic acidosis (4.7 mmol/L). She was admitted to the hospital and rapidly improved with intravenous fluids and insulin. Phenoxybenzamine 10 mg daily was administered, and she was discharged on hospital day 2. The dosage of phenoxybenzamine was gradually increased over 2 months.
Laparoscopic left adrenalectomy was performed, with removal of a 3-cm mass. The pathologic findings confirmed the diagnosis of pheochromocytoma. Two months later she felt well. Her hypertension was controlled with lisinopril 10 mg daily. Transthoracic echocardiography 3 months after adrenalectomy demonstrated a left ventricular ejection fraction of 60% to 65%. Six months later, her hemoglobin A1c was 6.6%.
DISCUSSION
Pheochromocytoma is an abnormal growth of cells of chromaffin origin that arises in the adrenal medulla.1,2 The incidence of these often benign tumors is estimated to be 2 to 8 cases per million in the general population, and 2 to 6 per 1000 in adult patients with hypertension.1,3,4 Although clinicians commonly associate these catecholamine-secreting tumors with intermittent hypertension or diaphoresis, they have a wide spectrum of manifestations, which range from asymptomatic adrenal mass to acute multiorgan illness that mimics other life-threatening conditions. Common signs and symptoms of pheochromocytoma include hypertension (60%-70% incidence), headache (50%), diaphoresis (50%), and palpitations (50%-60%).4 The textbook triad of headache, sweating, and palpitations is seen in fewer than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is often explained by a more common condition.1,4 Approximately 5% of adrenal “incidentalomas” are pheochromocytomas that are minimally symptomatic or asymptomatic.1,3 In a study of 102 patients who underwent pheochromocytoma resection, 33% were diagnosed during evaluation of an adrenal incidentaloma.5 At the other end of the spectrum is a pheochromocytoma crisis with its mimicry of ACS and sepsis, and manifestations including severe hyperglycemia, abdominal pain, acute heart failure, and syncope.2,5-9 Aside from chronic mild hypertension and a single episode of diaphoresis during admission, our patient had none of the classic signs or symptoms of pheochromocytoma. Rather, she presented with the abrupt onset of multiorgan injury.
Diagnostic evaluation for pheochromocytoma typically includes demonstration of elevated catecholamine byproducts (metanephrines) in plasma or urine and an adrenal mass on imaging.2,10 Biopsy is contraindicated because this can lead to release of catecholamines, which can trigger a pheochromocytoma crisis.5 The Endocrine Society guidelines recommend evaluating patients for pheochromocytoma who have: (1) a known or suspected genetic syndrome linked to pheochromocytoma (eg, multiple endocrine neoplasia type 2 or Von Hippel-Lindau syndrome), (2) an adrenal mass incidentally found on imaging, regardless of a history of hypertension, or (3) signs and symptoms of pheochromocytoma.3
Patients in pheochromocytoma crisis are typically very ill, requiring intensive care unit admission for hemodynamic stabilization.1,11 Initial management is typically directed at assessing and treating for common causes of systemic illness and hemodynamic instability, such as ACS and sepsis. Although some patients with pheochromocytoma crisis may have hemodynamic collapse requiring invasive circulatory support, others improve while receiving empiric treatment for mimicking conditions. Our patient had multiorgan injury and hemodynamic instability but returned to her preadmission state within 48 to 72 hours and remained stable after the withdrawal of all therapies, including insulin and antibiotics. This rapid improvement suggested a paroxysmal condition with an “on/off” capacity mediated by endogenous mediators. Once pheochromocytoma crisis is diagnosed, hemodynamic stabilization with α-adrenergic receptor blockade and intravascular volume repletion is essential. Confirmation of the diagnosis with repeat testing after hospital discharge is important because biochemical test results are less specific in the setting of acute illness. Surgery on an elective basis is the definitive treatment. Ongoing α-adrenergic receptor blockade is essential to minimize the risk of an intraoperative pheochromocytoma crisis (because of anesthesia or tumor manipulation) and prevent cardiovascular collapse after resection of tumor.11
Although the biochemical profile of a pheochromocytoma (eg, epinephrine predominant) is not tightly linked to the phenotype, the pattern of organ injury can reflect the pleotropic effects of specific catecholamines.12 While both norepinephrine and epinephrine bind the β1-adrenergic receptor with equal affinity, epinephrine has a higher affinity for the β2-adrenergic receptor. Our patient’s initial relative hypotension was likely caused by hypovolemia from decreased oral intake, vomiting, and hyperglycemia-mediated polyuria. However, β2-adrenergic receptor agonism could have caused vasodilation, and nocardiogenic hypotension has been observed with epinephrine-predominant pheochromocytomas.13 Several of the other clinical findings in this case can be explained by widespread β-adrenergic receptor agonism. Epinephrine (whether endogenously produced or exogenously administered) can lead to cardiac injury with elevated cardiac biomarkers.1,6,14 Epinephrine administration can cause leukocytosis, which is attributed to demargination of leukocyte subsets that express β2-adrenergic receptors.15,16 Lactic acidosis in the absence of tissue hypoxia (type B lactic acidosis) occurs during epinephrine infusions in healthy volunteers.17,18 Hyperglycemia from epinephrine infusions is attributed to β-adrenergic receptor stimulation causing increased gluconeogenesis and glycogenolysis and decreased insulin secretion and tissue glucose uptake.8 Resolution of hyperglycemia and diabetes is observed in the majority of patients after resection of pheochromocytoma, and hypoglycemia immediately after surgery is common, occasionally requiring glucose infusion.19,20
Pheochromocytomas are rare tumors with a wide range of manifestations that extend well beyond the classic triad. Pheochromocytomas can present as an asymptomatic adrenal mass with normal blood pressure, as new onset diabetes, or as multiorgan injury with cardiovascular collapse. Our patient suffered from two episodes of catecholamine excess that required hospitalization, but fortunately each proved to be a short-lived crisis.
TEACHING POINTS
- The classic triad of headache, sweating, and palpitations occurs in less than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is usually explained by a common medical condition.
- The presentation of pheochromocytoma varies widely, from asymptomatic adrenal incidentaloma to pheochromocytoma crisis causing multiorgan dysfunction with hemodynamic instability and mimicry of common critical illnesses like ACS, DKA, and sepsis.
- Biochemical screening for pheochromocytoma is recommended when a patient has a known or suspected genetic syndrome linked to pheochromocytoma, an adrenal mass incidentally found on imaging regardless of blood pressure, or signs and symptoms of a pheochromocytoma.
A 79-year-old woman presented to the emergency department with 1 day of nausea and vomiting. On the morning of presentation, she felt mild cramping in her legs and vomited twice. She denied chest or back pain, dyspnea, diaphoresis, cough, fever, dysuria, headache, and abdominal pain. Her medical history included hypertension, osteoporosis, and a right-sided acoustic neuroma treated with radiation 12 years prior. One month before this presentation, type 2 diabetes mellitus was diagnosed (hemoglobin A1c level, 7.3%) on routine testing by her primary care physician. Her medications were losartan and alendronate. She was born in China and immigrated to the United States 50 years prior. Her husband was chronically ill with several recent hospitalizations.
Nausea and vomiting are nonspecific symptoms that can arise from systemic illness, including hyperglycemia, a drug/toxin effect, or injury/inflammation of the gastrointestinal, central nervous system, or cardiovascular systems. An acoustic neuroma recurrence or malignancy in the radiation field could trigger nausea. Muscle cramping could arise from myositis or from hypokalemia secondary to vomiting. Her husband’s recent hospitalizations add an important psychosocial dimension to her care and should prompt consideration of a shared illness depending on the nature of his illness.
The patient’s temperature was 36.7 °C; heart rate, 99 beats per minute; blood pressure, 94/58 mm Hg;respiratory rate, 16 breaths per minute; and oxygen saturation, 98% while breathing room air. Her body mass index (BMI) was 18.7 kg/m2. She appeared comfortable. The heart, lung, jugular venous, and abdominal examinations were normal. She had no lower extremity edema or muscle tenderness.
The white blood cell (WBC) count was 14,500/µL (81% neutrophils, 9% lymphocytes, 8% monocytes), hemoglobin level was 17.5 g/dL (elevated from 14.2 g/dL 8 weeks prior), and platelet count was 238,000/µL. The metabolic panel revealed the following values: sodium, 139 mmol/L; potassium, 5.1 mmol/L; chloride, 96 mmol/L; bicarbonate, 17 mmol/L; blood urea nitrogen, 40 mg/dL; creatinine, 2.2 mg/dL (elevated from 0.7 mg/dL 8 weeks prior); glucose, 564 mg/dL; aspartate transaminase, 108 U/L; alanine transaminase, 130 U/L; total bilirubin, 0.6 mg/dL; and alkaline phosphatase, 105 U/L. Creatine kinase, amylase, and lipase levels were not measured. The urinalysis showed trace ketones, protein 100 mg/dL, glucose >500 mg/dL, and <5 WBCs per high-power field. The venous blood gas demonstrated a pH of 7.20 and lactate level of 13.2 mmol/L. Serum beta-hydroxybutyrate level was 0.27 mmol/L (reference range, 0.02-0.27), serum troponin I level was 8.5 µg/L (reference range, <0.05), and
Chest x-ray showed bilateral perihilar opacities with normal heart size. Electrocardiogram (ECG) revealed new ST-segment depressions in the anterior precordial leads (Figure 1).
Her hypotension may signal septic, cardiogenic, or hypovolemic shock. The leukocytosis, anion gap acidosis, acute kidney injury, and elevated lactate are compatible with sepsis, although there is no identified source of infection. Although diabetic ketoacidosis (DKA) can explain many of these findings, the serum beta-hydroxybutyrate and urine ketones are lower than expected for that condition. Her low-normal BMI makes significant insulin resistance less likely and raises concern about pancreatic adenocarcinoma as a secondary cause of diabetes.
The nausea, ST depressions, elevated troponin and B-type natriuretic peptide levels, and bilateral infiltrates suggest acute coronary syndrome (ACS), complicated by acute heart failure leading to systemic hypoperfusion and associated lactic acidosis and kidney injury. Nonischemic causes of myocardial injury, such as sepsis, myocarditis, and stress cardiomyopathy, should also be considered. Alternatively, she could be experiencing multiorgan injury from widespread embolism (eg, endocarditis), thrombosis (eg, antiphospholipid syndrome), or inflammation (eg, vasculitis). Acute pancreatitis can cause acute hyperglycemia and multisystem disease, but she did not have abdominal pain or tenderness (and her lipase level was not measured). Treatment should include intravenous insulin, intravenous fluids (trying to balance possible sepsis or DKA with heart failure), medical management for non-ST elevation myocardial infarction (NSTEMI), and empiric antibiotics.
ACS was diagnosed, and aspirin, atorvastatin, clopidogrel, and heparin were prescribed. Insulin infusion and intravenous fluids (approximately 3 L overnight) were administered for hyperglycemia (and possible early DKA). On the night of admission, the patient became profoundly diaphoretic without fevers; the WBC count rose to 24,200/µL. Vancomycin and ertapenem were initiated for possible sepsis. Serum troponin I level increased to 11.9 µg/L; the patient did not have chest pain, and the ECG was unchanged.
The next morning, the patient reported new mild diffuse abdominal pain and had mild epigastric tenderness. The WBC count was 28,900/µL; hemoglobin, 13.2 g/dL; venous pH, 7.39; lactate, 2.9 mmol/L; lipase, 48 U/L; aspartate transaminase, 84 U/L; alanine transaminase, 72 U/L; total bilirubin, 0.7 mg/dL; alkaline phosphatase, 64 U/L; and creatinine, 1.2 mg/dL.
Her rising troponin without dynamic ECG changes makes the diagnosis of ACS less likely, although myocardial ischemia can present as abdominal pain. Other causes of myocardial injury to consider (in addition to the previously mentioned sepsis, myocarditis, and stress cardiomyopathy) are pulmonary embolism and proximal aortic dissection. The latter can lead to ischemia in multiple systems (cardiac, mesenteric, renal, and lower extremity, recalling her leg cramps on admission).
The leukocytosis and lactic acidosis in the setting of new abdominal pain raises the question of mesenteric ischemia or intra-abdominal sepsis. Her hemoglobin has decreased by 4 g, and while some of the change may be dilutional, it will be important to consider hemolysis (less likely with a normal bilirubin) or gastrointestinal bleeding (given current anticoagulant and antiplatelet therapy). An echocardiogram and computed tomography (CT) angiogram of the chest, abdomen, and pelvis are indicated to evaluate the vasculature and assess for intra-abdominal pathology.
Coronary angiography revealed a 40% stenosis in the proximal right coronary artery and no other angiographically significant disease; the left ventricular end-diastolic pressure (LVEDP) was 30 mm Hg. Transthoracic echocardiography demonstrated normal left ventricular size, left ventricular ejection fraction of 65% to 70%, impaired left ventricular relaxation, and an inferior vena cava <2 cm in diameter that collapsed with inspiration.
The angiogram shows modest coronary artery disease and points away from plaque rupture as the cause of myocardial injury. Another important consideration given her husband’s recurrent illness is stress cardiomyopathy, but she does not have the typical apical ballooning or left ventricular dysfunction. The increased LVEDP with normal left ventricular size and function with elevated filling pressures is consistent with left-sided heart failure with preserved ejection fraction. Cardiac magnetic resonance imaging could exclude an infiltrative disorder leading to diastolic dysfunction or a myocarditis that explains the troponin elevation, but both diagnoses seem unlikely.
CT of the abdomen and pelvis demonstrated a heterogeneous 3-cm mass in the left adrenal gland (Figure 2).
An adrenal mass could be a functional or nonfunctional adenoma, primary adrenal carcinoma, a metastatic malignancy, or granulomatous infection such as tuberculosis. Secretion of excess glucocorticoid, mineralocorticoid, or catecholamine should be evaluated.
Cushing syndrome could explain her hyperglycemia, leukocytosis, and heart failure (mediated by the increased risk of atherosclerosis and hypertension with hypercortisolism), although her low BMI is atypical. Primary hyperaldosteronism causes hypertension but does not cause an acute multisystem disease. Pheochromocytoma could account for the diaphoresis, hypertension, hyperglycemia, leukocytosis, and cardiac injury. A more severe form—pheochromocytoma crisis—is characterized by widespread end-organ damage, including cardiomyopathy, bowel ischemia, hepatitis, hyperglycemia with ketoacidosis, and lactic acidosis. Measurement of serum cortisol and plasma and urine fractionated metanephrines, and a dexamethasone suppression test can determine whether the adrenal mass is functional.
The intravenous insulin infusion was changed to subcutaneous dosing on hospital day 2. She had no further nausea, diaphoresis, or abdominal pain, was walking around the hospital unit unassisted, and was consuming a regular diet. By hospital day 3, insulin was discontinued. The patient remained euglycemic for the remainder of her hospitalization; hemoglobin A1c value was 7.0%. Blood cultures were sterile, and the WBC count was 12,000/µL. Thyroid-stimulating hormone level was 0.31 mIU/L (reference range, 0.45-4.12), and the free thyroxine level was 12 pmol/L (reference range, 10-18). Antibiotics were discontinued. She remained euvolemic and never required diuretic therapy. The acute myocardial injury and diastolic dysfunction were attributed to an acute stress cardiomyopathy arising from the strain of her husband’s declining health. She was discharged on hospital day 5 with aspirin, atorvastatin, metoprolol, lisinopril, and outpatient follow-up.
The rapid resolution of her multisystem process suggests a self-limited process or successful treatment of the underlying cause. Although she received antibiotics, a bacterial infection never manifested. Cardiomyopathy with a high troponin level, ECG changes, and early heart failure often requires aggressive supportive measures, which were not required here. The rapid cessation of hyperglycemia and an insulin requirement within 1 day is atypical for DKA.
Pheochromocytoma is a rare secondary cause of diabetes in which excess catecholamines cause insulin resistance and suppress insulin release. It can explain both the adrenal mass and, in the form of pheochromocytoma crisis, the severe multisystem injury. However, the patient’s hypotension (which could be explained by concomitant cardiomyopathy) and older age are not typical for pheochromocytoma.
Results of testing for adrenal biomarkers, which were sent during her hospitalization, returned several days after hospital discharge. The plasma free metanephrine level was 687 pg/mL (reference range, <57) and the plasma free normetanephrine level was 508 pg/mL (reference range, <148). Metoprolol was discontinued by her primary care physician.
Elevated plasma free metanephrine and normetanephrine levels were confirmed in the endocrinology clinic 3 weeks later. The 24-hour urine metanephrine level was 1497 µg/24 hours (reference range, 90-315), and the 24-hour urine normetanephrine level was 379 µg/24 hours (reference range, 122-676). Serum aldosterone level was 8 ng/dL (reference range, 3-16), and morning cortisol level was 8 µg/dL (reference range, 4-19). Lisinopril was discontinued, and phenoxybenzamine was prescribed.
Adrenal-protocol CT of the abdomen demonstrated that the left adrenal mass was enhanced by contrast without definite washout, which could be consistent with a pheochromocytoma.
The diagnosis of pheochromocytoma has been confirmed by biochemistry and imaging. It was appropriate to stop metoprolol, as β-blockade can lead to unopposed α-receptor agonism and hypertension. Implementation of α-blockade with phenoxybenzamine and endocrine surgery referral are indicated.
On the day she intended to fill a phenoxybenzamine prescription, the patient experienced acute generalized weakness and presented to the emergency department with hyperglycemia (glucose, 661 mg/dL), acute kidney injury (creatinine, 1.6 mg/dL), troponin I elevation (0.14 µg/L), and lactic acidosis (4.7 mmol/L). She was admitted to the hospital and rapidly improved with intravenous fluids and insulin. Phenoxybenzamine 10 mg daily was administered, and she was discharged on hospital day 2. The dosage of phenoxybenzamine was gradually increased over 2 months.
Laparoscopic left adrenalectomy was performed, with removal of a 3-cm mass. The pathologic findings confirmed the diagnosis of pheochromocytoma. Two months later she felt well. Her hypertension was controlled with lisinopril 10 mg daily. Transthoracic echocardiography 3 months after adrenalectomy demonstrated a left ventricular ejection fraction of 60% to 65%. Six months later, her hemoglobin A1c was 6.6%.
DISCUSSION
Pheochromocytoma is an abnormal growth of cells of chromaffin origin that arises in the adrenal medulla.1,2 The incidence of these often benign tumors is estimated to be 2 to 8 cases per million in the general population, and 2 to 6 per 1000 in adult patients with hypertension.1,3,4 Although clinicians commonly associate these catecholamine-secreting tumors with intermittent hypertension or diaphoresis, they have a wide spectrum of manifestations, which range from asymptomatic adrenal mass to acute multiorgan illness that mimics other life-threatening conditions. Common signs and symptoms of pheochromocytoma include hypertension (60%-70% incidence), headache (50%), diaphoresis (50%), and palpitations (50%-60%).4 The textbook triad of headache, sweating, and palpitations is seen in fewer than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is often explained by a more common condition.1,4 Approximately 5% of adrenal “incidentalomas” are pheochromocytomas that are minimally symptomatic or asymptomatic.1,3 In a study of 102 patients who underwent pheochromocytoma resection, 33% were diagnosed during evaluation of an adrenal incidentaloma.5 At the other end of the spectrum is a pheochromocytoma crisis with its mimicry of ACS and sepsis, and manifestations including severe hyperglycemia, abdominal pain, acute heart failure, and syncope.2,5-9 Aside from chronic mild hypertension and a single episode of diaphoresis during admission, our patient had none of the classic signs or symptoms of pheochromocytoma. Rather, she presented with the abrupt onset of multiorgan injury.
Diagnostic evaluation for pheochromocytoma typically includes demonstration of elevated catecholamine byproducts (metanephrines) in plasma or urine and an adrenal mass on imaging.2,10 Biopsy is contraindicated because this can lead to release of catecholamines, which can trigger a pheochromocytoma crisis.5 The Endocrine Society guidelines recommend evaluating patients for pheochromocytoma who have: (1) a known or suspected genetic syndrome linked to pheochromocytoma (eg, multiple endocrine neoplasia type 2 or Von Hippel-Lindau syndrome), (2) an adrenal mass incidentally found on imaging, regardless of a history of hypertension, or (3) signs and symptoms of pheochromocytoma.3
Patients in pheochromocytoma crisis are typically very ill, requiring intensive care unit admission for hemodynamic stabilization.1,11 Initial management is typically directed at assessing and treating for common causes of systemic illness and hemodynamic instability, such as ACS and sepsis. Although some patients with pheochromocytoma crisis may have hemodynamic collapse requiring invasive circulatory support, others improve while receiving empiric treatment for mimicking conditions. Our patient had multiorgan injury and hemodynamic instability but returned to her preadmission state within 48 to 72 hours and remained stable after the withdrawal of all therapies, including insulin and antibiotics. This rapid improvement suggested a paroxysmal condition with an “on/off” capacity mediated by endogenous mediators. Once pheochromocytoma crisis is diagnosed, hemodynamic stabilization with α-adrenergic receptor blockade and intravascular volume repletion is essential. Confirmation of the diagnosis with repeat testing after hospital discharge is important because biochemical test results are less specific in the setting of acute illness. Surgery on an elective basis is the definitive treatment. Ongoing α-adrenergic receptor blockade is essential to minimize the risk of an intraoperative pheochromocytoma crisis (because of anesthesia or tumor manipulation) and prevent cardiovascular collapse after resection of tumor.11
Although the biochemical profile of a pheochromocytoma (eg, epinephrine predominant) is not tightly linked to the phenotype, the pattern of organ injury can reflect the pleotropic effects of specific catecholamines.12 While both norepinephrine and epinephrine bind the β1-adrenergic receptor with equal affinity, epinephrine has a higher affinity for the β2-adrenergic receptor. Our patient’s initial relative hypotension was likely caused by hypovolemia from decreased oral intake, vomiting, and hyperglycemia-mediated polyuria. However, β2-adrenergic receptor agonism could have caused vasodilation, and nocardiogenic hypotension has been observed with epinephrine-predominant pheochromocytomas.13 Several of the other clinical findings in this case can be explained by widespread β-adrenergic receptor agonism. Epinephrine (whether endogenously produced or exogenously administered) can lead to cardiac injury with elevated cardiac biomarkers.1,6,14 Epinephrine administration can cause leukocytosis, which is attributed to demargination of leukocyte subsets that express β2-adrenergic receptors.15,16 Lactic acidosis in the absence of tissue hypoxia (type B lactic acidosis) occurs during epinephrine infusions in healthy volunteers.17,18 Hyperglycemia from epinephrine infusions is attributed to β-adrenergic receptor stimulation causing increased gluconeogenesis and glycogenolysis and decreased insulin secretion and tissue glucose uptake.8 Resolution of hyperglycemia and diabetes is observed in the majority of patients after resection of pheochromocytoma, and hypoglycemia immediately after surgery is common, occasionally requiring glucose infusion.19,20
Pheochromocytomas are rare tumors with a wide range of manifestations that extend well beyond the classic triad. Pheochromocytomas can present as an asymptomatic adrenal mass with normal blood pressure, as new onset diabetes, or as multiorgan injury with cardiovascular collapse. Our patient suffered from two episodes of catecholamine excess that required hospitalization, but fortunately each proved to be a short-lived crisis.
TEACHING POINTS
- The classic triad of headache, sweating, and palpitations occurs in less than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is usually explained by a common medical condition.
- The presentation of pheochromocytoma varies widely, from asymptomatic adrenal incidentaloma to pheochromocytoma crisis causing multiorgan dysfunction with hemodynamic instability and mimicry of common critical illnesses like ACS, DKA, and sepsis.
- Biochemical screening for pheochromocytoma is recommended when a patient has a known or suspected genetic syndrome linked to pheochromocytoma, an adrenal mass incidentally found on imaging regardless of blood pressure, or signs and symptoms of a pheochromocytoma.
1. Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757-764. https://doi.org/10.1530/eje-15-0483
2. Whitelaw BC, Prague JK, Mustafa OG, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf). 2014;80(1):13-22. https://doi.org/10.1111/cen.12324
3. Lenders JW, Duh QY, Eisenhofer G, et al; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498
4. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24(12):2331-2339. https://doi.org/10.1097/01.hjh.0000251887.01885.54
5. Shen WT, Grogan R, Vriens M, Clark OH, Duh QY. One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg. 2010;145(9):893-897. https://doi.org/10.1001/archsurg.2010.159
6. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99(14):1438-1444. https://doi.org/10.1136/heartjnl-2013-304073
7. Lee TW, Lin KH, Chang CJ, Lew WH, Lee TI. Pheochromocytoma mimicking both acute coronary syndrome and sepsis: a case report. Med Princ Pract. 2013;22(4):405-407. https://doi.org/10.1159/000343578
8. Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017;23(8):999-1005. https://doi.org/10.4158/ep171914.ra
9. Ueda T, Oka N, Matsumoto A, et al. Pheochromocytoma presenting as recurrent hypotension and syncope. Intern Med. 2005;44(3):222-227. https://doi.org/10.2169/internalmedicine.44.222
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. https://doi.org/10.1056/nejmra1806651
11. Scholten A, Cisco RM, Vriens MR, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. 2013;98(2):581-591. https://doi.org/10.1210/jc.2012-3020
12. Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65-90.
13. Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf). 1992;37(3):304-306. https://doi.org/10.1111/j.1365-2265.1992.tb02326.x
14. Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3(1):76-80. https://doi.org/10.1016/j.jaip.2014.06.007
15. Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77-91. https://doi.org/10.1006/brbi.1996.0009
16. Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503-511. https://doi.org/10.4049/jimmunol.0902189
17. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-1140. https://doi.org/10.1016/j.mayocp.2013.06.012
18. Levy B. Bench-to-bedside review: is there a place for epinephrine in septic shock? Crit Care. 2005;9(6):561-565. https://doi.org/10.1186/cc3901
19. Chen Y, Hodin RA, Pandolfi C, Ruan DT, McKenzie TJ. Hypoglycemia after resection of pheochromocytoma. Surgery. 2014;156:1404-1408; discussion 1408-1409. https://doi.org/10.1016/j.surg.2014.08.020
20. Pogorzelski R, Toutounchi S, Krajewska E, et al. The effect of surgical treatment of phaeochromocytoma on concomitant arterial hypertension and diabetes mellitus in a single-centre retrospective study. Cent European J Urol. 2014;67(4):361-365. https://doi.org/10.5173/ceju.2014.04.art9
1. Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757-764. https://doi.org/10.1530/eje-15-0483
2. Whitelaw BC, Prague JK, Mustafa OG, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf). 2014;80(1):13-22. https://doi.org/10.1111/cen.12324
3. Lenders JW, Duh QY, Eisenhofer G, et al; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498
4. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24(12):2331-2339. https://doi.org/10.1097/01.hjh.0000251887.01885.54
5. Shen WT, Grogan R, Vriens M, Clark OH, Duh QY. One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg. 2010;145(9):893-897. https://doi.org/10.1001/archsurg.2010.159
6. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99(14):1438-1444. https://doi.org/10.1136/heartjnl-2013-304073
7. Lee TW, Lin KH, Chang CJ, Lew WH, Lee TI. Pheochromocytoma mimicking both acute coronary syndrome and sepsis: a case report. Med Princ Pract. 2013;22(4):405-407. https://doi.org/10.1159/000343578
8. Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017;23(8):999-1005. https://doi.org/10.4158/ep171914.ra
9. Ueda T, Oka N, Matsumoto A, et al. Pheochromocytoma presenting as recurrent hypotension and syncope. Intern Med. 2005;44(3):222-227. https://doi.org/10.2169/internalmedicine.44.222
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. https://doi.org/10.1056/nejmra1806651
11. Scholten A, Cisco RM, Vriens MR, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. 2013;98(2):581-591. https://doi.org/10.1210/jc.2012-3020
12. Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65-90.
13. Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf). 1992;37(3):304-306. https://doi.org/10.1111/j.1365-2265.1992.tb02326.x
14. Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3(1):76-80. https://doi.org/10.1016/j.jaip.2014.06.007
15. Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77-91. https://doi.org/10.1006/brbi.1996.0009
16. Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503-511. https://doi.org/10.4049/jimmunol.0902189
17. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-1140. https://doi.org/10.1016/j.mayocp.2013.06.012
18. Levy B. Bench-to-bedside review: is there a place for epinephrine in septic shock? Crit Care. 2005;9(6):561-565. https://doi.org/10.1186/cc3901
19. Chen Y, Hodin RA, Pandolfi C, Ruan DT, McKenzie TJ. Hypoglycemia after resection of pheochromocytoma. Surgery. 2014;156:1404-1408; discussion 1408-1409. https://doi.org/10.1016/j.surg.2014.08.020
20. Pogorzelski R, Toutounchi S, Krajewska E, et al. The effect of surgical treatment of phaeochromocytoma on concomitant arterial hypertension and diabetes mellitus in a single-centre retrospective study. Cent European J Urol. 2014;67(4):361-365. https://doi.org/10.5173/ceju.2014.04.art9
© 2021 Society of Hospital Medicine