User login
Policy in Clinical Practice: Hospital Price Transparency
CLINICAL SCENARIO
A 59-year-old man is observed in the hospital for substernal chest pain initially concerning for angina. Serial troponin testing is negative, and based on additional history of intermittent dysphagia, an elective upper endoscopy is recommended after discharge. The patient does not have health insurance and expresses anxiety about the cost of endoscopy. He asks how he could compare the costs at different hospitals. How do federal price transparency rules assist the hospitalist in addressing this patient’s question?
BACKGROUND AND HISTORY
Healthcare costs continue to rise in the United States despite mounting concerns about wasteful spending and unaffordability.1 One contributor is a lack of price transparency.2 In theory, price transparency allows individuals to shop for services, spurring competition and lower prices. However, healthcare prices have historically been opaque to both physicians and patients; unlike other licensed professionals who provide clients estimates for their work (eg, lawyers, electricians), physicians are rarely able to offer patients real-time insight or guidance about costs, which most patients discover only when the bill arrives. The situation is particularly problematic for patients who bear higher out-of-pocket costs, such as the uninsured or those with high-deductible health plans.3
Decades of work to improve healthcare price transparency have unfortunately borne little fruit. Multiple states and organizations have attempted to disseminate price information on comparison websites.4 These efforts only modestly reduced some prices, with benefits confined to elective, single-episode, commodifiable services such as magnetic resonance imaging scans.5 The Affordable Care Act required hospitals to publish standard charges, also called a chargemaster (Table).6 However, chargemaster fees are notoriously inflated and inaccessible at the point of service, undercutting transparency.

POLICY IN CLINICAL PRACTICE
Beginning January 2021, the Centers for Medicare & Medicaid Services (CMS) required all hospitals to publish negotiated prices—including payor-specific negotiated charges—for 300 “shoppable services” (Table).6 The list must include 70 common CMS-specified services, such as a basic metabolic panel, upper endoscopy, and prostate biopsy, as well as another 230 services that each hospital determines relevant to its patient population.
In circumstances where hospitals have negotiated different prices for a service, they must list each third-party payor and their payor-specific charge. The information must be prominently displayed, accessible without requiring the patient to enter personal information, and provided in a machine-readable file. CMS may impose a $300 daily penalty on hospitals failing to comply with the policy. Of note, the policy does not apply to clinics or ambulatory surgery centers.
As more hospitals share data, this policy will directly benefit both patients and physicians. It can benefit patients with the time, foresight, and ability to search for the lowest price for shoppable services. Other patients may also benefit indirectly, to the extent that insurers and other purchasers apply this information to negotiate lower and more uniform prices. Decreased price variation may also encourage hospitals to compete on quality to distinguish the value of their services. Hospitalists could benefit through the ability to directly help patients locate price information.
Despite these potential benefits, the policy has limitations. Price information about shoppable services is most useful for discharge planning, and other solutions are needed to address transparency before and during unplanned admissions. Patients who prioritize continuity with a hospital or physician may be less price sensitive, particularly for more complex services. Patients with commercial insurance may be shielded from cost considerations and personal incentives to comparison shop. Interpreting hospitals’ estimates remains difficult, as it can be unclear if professional fees are included or if certain prices are offered to outpatients.7 Price information is not accompanied by corresponding quality data. Additionally, price transparency may also fail to lower prices in heavily concentrated payor or provider markets, and it remains unknown whether some providers may actually raise prices after learning about higher rates negotiated by competitors.8,9
Another issue is hospital participation. Early evidence suggests that most hospitals have not complied with the letter or spirit of the regulation.
Despite its limitations, this policy represents a meaningful advance for healthcare competition and patient empowerment. Additionally, it signals federal willingness to address the lack of price transparency as a source of widespread patient and clinician frustration—a commitment that will be needed to sustain this policy and implement additional measures in the future.
COMMENTARY AND RECOMMENDATIONS
CMS could consider five steps to augment the policy and maximize transparency and value for patients.
First, CMS could consider increasing daily nonparticipation penalties. Hospitals, particularly those in areas with less competition, have less incentive to participate given meager current penalties. Because the magnitude needed to compel action remains unknown, CMS could gradually escalate penalties over time until there is broader participation across hospitals.
Second, policymakers could aggregate price information centrally, organize the data around patients’ clinical scenarios, and advertise its availability. Currently, this information is scattered and time-consuming for hospitalists and patients to gather for decision-making. Additionally, CMS could encourage the development of third-party tools that aggregate and analyze machine-readable price data or require that prices be posted at the point of service.
Third, CMS could revise the policy to include quality as well as price information. Price alone does not offer a full enough picture of what consumers can expect from hospitals for shoppable services. Pairing price and quality information is better aligned to addressing costs in the context of value, rather than cost-cutting for its own purposes.
Fourth, over time, CMS could expand the list of services and sites required to report (eg, clinics and ambulatory surgical centers as well as hospitals).
Fifth, CMS rule-makers could set reporting standards and contextualize price information in common clinical scenarios. Patients may have difficulty shopping for complex healthcare services without understanding how they apply in different clinical situations. Decision-making would also be aided by reporting standards—for instance, for how prices are displayed and whether they include certain fees (eg, professional fees, pathology studies).
WHAT SHOULD I TELL MY PATIENT?
Hospitalists planning follow-up care should inform patients that price information is increasingly available and encourage them to search on the internet or contact hospital billing offices to request information (eg, discounted cash prices and minimum negotiated charges) before obtaining elective services after discharge. Hospitalists can also encourage patients to discuss shoppable services with their primary care physicians to understand the clinical context and make high-value decisions. Hospitalists who wish to build communication skills discussing costs with patients can increasingly find resources for these conversations and request that prices be displayed in the electronic health record for this purpose.13,14 As conversations occur, hospitalists should seek to understand other factors, such as convenience and continuity relationships, that might influence choices.
CONCLUSIONS
Starting in 2021, CMS policy requires that hospitals report prices for services such as the endoscopy recommended for the patient in the scenario. Though the policy gives patients new hope for greater transparency and better prices, additional steps are needed to help patients and hospitalists achieve these benefits.
1. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
2. Wetzell S. Transparency: a needed step towards health care affordability. American Health Policy Institute. March 2014. Accessed August 26, 2021. https://www.americanhealthpolicy.org/Content/documents/resources/Transparency%20Study%201%20-%20The%20Need%20for%20Health%20Care%20Transparency.pdf
3. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
4. Kullgren JT, Duey KA, Werner RM. A census of state health care price transparency websites. JAMA. 2013;309(23):2437-2438. https://doi.org/10.1001/jama.2013.6557
5. Brown ZY. Equilibrium effects of health care price information. Rev Econ Stat. 2019;101(4):699-712. https://doi.org/10.1162/rest_a_00765
6. Medicare and Medicaid Programs: CY 2020 hospital outpatient PPS policy changes and payment rates and ambulatory surgical center payment system policy changes and payment rates. Price transparency requirements for hospitals to make standard charges public. 45 CFR §180.20 (2019).
7. Kurani N, Ramirez G, Hudman J, Cox C, Kamal R. Early results from federal price transparency rule show difficulty in estimating the cost of care. Peterson-Kaiser Family Foundation. April 9, 2021. Accessed August 26, 2021. https://www.healthsystemtracker.org/brief/early-results-from-federal-price-transparency-rule-show-difficultly-in-estimating-the-cost-of-care/
8. Miller BJ, Mandelberg MC, Griffith NC, Ehrenfeld JM. Price transparency: empowering patient choice and promoting provider competition. J Med Syst. 2020;44(4):80. https://doi.org/10.1007/s10916-020-01553-2
9. Glied S. Price transparency–promise and peril. JAMA. 2021;325(15):1496-1497. https://doi.org/10.1001/jama.2021.4640
10. Haque W, Ahmadzada M, Allahrakha H, Haque E, Hsiehchen D. Transparency, accessibility, and variability of US hospital price data. JAMA Netw Open. 2021;4(5):e2110109. https://doi.org/10.1001/jamanetworkopen.2021.10109
11. Henderson M, Mouslim MC. Low compliance from big hospitals on CMS’s hospital price transparency rule. Health Affairs Blog. March 16, 2021. Accessed August 26, 2021. https://doi.org/10.1377/hblog20210311.899634
12. McGinty T, Wilde Mathews A, Evans M. Hospitals hide pricing data from search results. The Wall Street Journal. March 22, 2021. Accessed August 26, 2021. https://www.wsj.com/articles/hospitals-hide-pricing-data-from-search-results-11616405402
13. Dine CJ, Masi D, Smith CD. Tools to help overcome barriers to cost-of-care conversations. Ann Intern Med. 2019;170(9 suppl):S36-S38. https://doi.org/10.7326/M19-0778
14. Miller BJ, Slota JM, Ehrenfeld JM. Redefining the physician’s role in cost-conscious care: the potential role of the electronic health record. JAMA. 2019;322(8):721-722. https://doi.org/10.1001/jama.2019.9114
CLINICAL SCENARIO
A 59-year-old man is observed in the hospital for substernal chest pain initially concerning for angina. Serial troponin testing is negative, and based on additional history of intermittent dysphagia, an elective upper endoscopy is recommended after discharge. The patient does not have health insurance and expresses anxiety about the cost of endoscopy. He asks how he could compare the costs at different hospitals. How do federal price transparency rules assist the hospitalist in addressing this patient’s question?
BACKGROUND AND HISTORY
Healthcare costs continue to rise in the United States despite mounting concerns about wasteful spending and unaffordability.1 One contributor is a lack of price transparency.2 In theory, price transparency allows individuals to shop for services, spurring competition and lower prices. However, healthcare prices have historically been opaque to both physicians and patients; unlike other licensed professionals who provide clients estimates for their work (eg, lawyers, electricians), physicians are rarely able to offer patients real-time insight or guidance about costs, which most patients discover only when the bill arrives. The situation is particularly problematic for patients who bear higher out-of-pocket costs, such as the uninsured or those with high-deductible health plans.3
Decades of work to improve healthcare price transparency have unfortunately borne little fruit. Multiple states and organizations have attempted to disseminate price information on comparison websites.4 These efforts only modestly reduced some prices, with benefits confined to elective, single-episode, commodifiable services such as magnetic resonance imaging scans.5 The Affordable Care Act required hospitals to publish standard charges, also called a chargemaster (Table).6 However, chargemaster fees are notoriously inflated and inaccessible at the point of service, undercutting transparency.

POLICY IN CLINICAL PRACTICE
Beginning January 2021, the Centers for Medicare & Medicaid Services (CMS) required all hospitals to publish negotiated prices—including payor-specific negotiated charges—for 300 “shoppable services” (Table).6 The list must include 70 common CMS-specified services, such as a basic metabolic panel, upper endoscopy, and prostate biopsy, as well as another 230 services that each hospital determines relevant to its patient population.
In circumstances where hospitals have negotiated different prices for a service, they must list each third-party payor and their payor-specific charge. The information must be prominently displayed, accessible without requiring the patient to enter personal information, and provided in a machine-readable file. CMS may impose a $300 daily penalty on hospitals failing to comply with the policy. Of note, the policy does not apply to clinics or ambulatory surgery centers.
As more hospitals share data, this policy will directly benefit both patients and physicians. It can benefit patients with the time, foresight, and ability to search for the lowest price for shoppable services. Other patients may also benefit indirectly, to the extent that insurers and other purchasers apply this information to negotiate lower and more uniform prices. Decreased price variation may also encourage hospitals to compete on quality to distinguish the value of their services. Hospitalists could benefit through the ability to directly help patients locate price information.
Despite these potential benefits, the policy has limitations. Price information about shoppable services is most useful for discharge planning, and other solutions are needed to address transparency before and during unplanned admissions. Patients who prioritize continuity with a hospital or physician may be less price sensitive, particularly for more complex services. Patients with commercial insurance may be shielded from cost considerations and personal incentives to comparison shop. Interpreting hospitals’ estimates remains difficult, as it can be unclear if professional fees are included or if certain prices are offered to outpatients.7 Price information is not accompanied by corresponding quality data. Additionally, price transparency may also fail to lower prices in heavily concentrated payor or provider markets, and it remains unknown whether some providers may actually raise prices after learning about higher rates negotiated by competitors.8,9
Another issue is hospital participation. Early evidence suggests that most hospitals have not complied with the letter or spirit of the regulation.
Despite its limitations, this policy represents a meaningful advance for healthcare competition and patient empowerment. Additionally, it signals federal willingness to address the lack of price transparency as a source of widespread patient and clinician frustration—a commitment that will be needed to sustain this policy and implement additional measures in the future.
COMMENTARY AND RECOMMENDATIONS
CMS could consider five steps to augment the policy and maximize transparency and value for patients.
First, CMS could consider increasing daily nonparticipation penalties. Hospitals, particularly those in areas with less competition, have less incentive to participate given meager current penalties. Because the magnitude needed to compel action remains unknown, CMS could gradually escalate penalties over time until there is broader participation across hospitals.
Second, policymakers could aggregate price information centrally, organize the data around patients’ clinical scenarios, and advertise its availability. Currently, this information is scattered and time-consuming for hospitalists and patients to gather for decision-making. Additionally, CMS could encourage the development of third-party tools that aggregate and analyze machine-readable price data or require that prices be posted at the point of service.
Third, CMS could revise the policy to include quality as well as price information. Price alone does not offer a full enough picture of what consumers can expect from hospitals for shoppable services. Pairing price and quality information is better aligned to addressing costs in the context of value, rather than cost-cutting for its own purposes.
Fourth, over time, CMS could expand the list of services and sites required to report (eg, clinics and ambulatory surgical centers as well as hospitals).
Fifth, CMS rule-makers could set reporting standards and contextualize price information in common clinical scenarios. Patients may have difficulty shopping for complex healthcare services without understanding how they apply in different clinical situations. Decision-making would also be aided by reporting standards—for instance, for how prices are displayed and whether they include certain fees (eg, professional fees, pathology studies).
WHAT SHOULD I TELL MY PATIENT?
Hospitalists planning follow-up care should inform patients that price information is increasingly available and encourage them to search on the internet or contact hospital billing offices to request information (eg, discounted cash prices and minimum negotiated charges) before obtaining elective services after discharge. Hospitalists can also encourage patients to discuss shoppable services with their primary care physicians to understand the clinical context and make high-value decisions. Hospitalists who wish to build communication skills discussing costs with patients can increasingly find resources for these conversations and request that prices be displayed in the electronic health record for this purpose.13,14 As conversations occur, hospitalists should seek to understand other factors, such as convenience and continuity relationships, that might influence choices.
CONCLUSIONS
Starting in 2021, CMS policy requires that hospitals report prices for services such as the endoscopy recommended for the patient in the scenario. Though the policy gives patients new hope for greater transparency and better prices, additional steps are needed to help patients and hospitalists achieve these benefits.
CLINICAL SCENARIO
A 59-year-old man is observed in the hospital for substernal chest pain initially concerning for angina. Serial troponin testing is negative, and based on additional history of intermittent dysphagia, an elective upper endoscopy is recommended after discharge. The patient does not have health insurance and expresses anxiety about the cost of endoscopy. He asks how he could compare the costs at different hospitals. How do federal price transparency rules assist the hospitalist in addressing this patient’s question?
BACKGROUND AND HISTORY
Healthcare costs continue to rise in the United States despite mounting concerns about wasteful spending and unaffordability.1 One contributor is a lack of price transparency.2 In theory, price transparency allows individuals to shop for services, spurring competition and lower prices. However, healthcare prices have historically been opaque to both physicians and patients; unlike other licensed professionals who provide clients estimates for their work (eg, lawyers, electricians), physicians are rarely able to offer patients real-time insight or guidance about costs, which most patients discover only when the bill arrives. The situation is particularly problematic for patients who bear higher out-of-pocket costs, such as the uninsured or those with high-deductible health plans.3
Decades of work to improve healthcare price transparency have unfortunately borne little fruit. Multiple states and organizations have attempted to disseminate price information on comparison websites.4 These efforts only modestly reduced some prices, with benefits confined to elective, single-episode, commodifiable services such as magnetic resonance imaging scans.5 The Affordable Care Act required hospitals to publish standard charges, also called a chargemaster (Table).6 However, chargemaster fees are notoriously inflated and inaccessible at the point of service, undercutting transparency.

POLICY IN CLINICAL PRACTICE
Beginning January 2021, the Centers for Medicare & Medicaid Services (CMS) required all hospitals to publish negotiated prices—including payor-specific negotiated charges—for 300 “shoppable services” (Table).6 The list must include 70 common CMS-specified services, such as a basic metabolic panel, upper endoscopy, and prostate biopsy, as well as another 230 services that each hospital determines relevant to its patient population.
In circumstances where hospitals have negotiated different prices for a service, they must list each third-party payor and their payor-specific charge. The information must be prominently displayed, accessible without requiring the patient to enter personal information, and provided in a machine-readable file. CMS may impose a $300 daily penalty on hospitals failing to comply with the policy. Of note, the policy does not apply to clinics or ambulatory surgery centers.
As more hospitals share data, this policy will directly benefit both patients and physicians. It can benefit patients with the time, foresight, and ability to search for the lowest price for shoppable services. Other patients may also benefit indirectly, to the extent that insurers and other purchasers apply this information to negotiate lower and more uniform prices. Decreased price variation may also encourage hospitals to compete on quality to distinguish the value of their services. Hospitalists could benefit through the ability to directly help patients locate price information.
Despite these potential benefits, the policy has limitations. Price information about shoppable services is most useful for discharge planning, and other solutions are needed to address transparency before and during unplanned admissions. Patients who prioritize continuity with a hospital or physician may be less price sensitive, particularly for more complex services. Patients with commercial insurance may be shielded from cost considerations and personal incentives to comparison shop. Interpreting hospitals’ estimates remains difficult, as it can be unclear if professional fees are included or if certain prices are offered to outpatients.7 Price information is not accompanied by corresponding quality data. Additionally, price transparency may also fail to lower prices in heavily concentrated payor or provider markets, and it remains unknown whether some providers may actually raise prices after learning about higher rates negotiated by competitors.8,9
Another issue is hospital participation. Early evidence suggests that most hospitals have not complied with the letter or spirit of the regulation.
Despite its limitations, this policy represents a meaningful advance for healthcare competition and patient empowerment. Additionally, it signals federal willingness to address the lack of price transparency as a source of widespread patient and clinician frustration—a commitment that will be needed to sustain this policy and implement additional measures in the future.
COMMENTARY AND RECOMMENDATIONS
CMS could consider five steps to augment the policy and maximize transparency and value for patients.
First, CMS could consider increasing daily nonparticipation penalties. Hospitals, particularly those in areas with less competition, have less incentive to participate given meager current penalties. Because the magnitude needed to compel action remains unknown, CMS could gradually escalate penalties over time until there is broader participation across hospitals.
Second, policymakers could aggregate price information centrally, organize the data around patients’ clinical scenarios, and advertise its availability. Currently, this information is scattered and time-consuming for hospitalists and patients to gather for decision-making. Additionally, CMS could encourage the development of third-party tools that aggregate and analyze machine-readable price data or require that prices be posted at the point of service.
Third, CMS could revise the policy to include quality as well as price information. Price alone does not offer a full enough picture of what consumers can expect from hospitals for shoppable services. Pairing price and quality information is better aligned to addressing costs in the context of value, rather than cost-cutting for its own purposes.
Fourth, over time, CMS could expand the list of services and sites required to report (eg, clinics and ambulatory surgical centers as well as hospitals).
Fifth, CMS rule-makers could set reporting standards and contextualize price information in common clinical scenarios. Patients may have difficulty shopping for complex healthcare services without understanding how they apply in different clinical situations. Decision-making would also be aided by reporting standards—for instance, for how prices are displayed and whether they include certain fees (eg, professional fees, pathology studies).
WHAT SHOULD I TELL MY PATIENT?
Hospitalists planning follow-up care should inform patients that price information is increasingly available and encourage them to search on the internet or contact hospital billing offices to request information (eg, discounted cash prices and minimum negotiated charges) before obtaining elective services after discharge. Hospitalists can also encourage patients to discuss shoppable services with their primary care physicians to understand the clinical context and make high-value decisions. Hospitalists who wish to build communication skills discussing costs with patients can increasingly find resources for these conversations and request that prices be displayed in the electronic health record for this purpose.13,14 As conversations occur, hospitalists should seek to understand other factors, such as convenience and continuity relationships, that might influence choices.
CONCLUSIONS
Starting in 2021, CMS policy requires that hospitals report prices for services such as the endoscopy recommended for the patient in the scenario. Though the policy gives patients new hope for greater transparency and better prices, additional steps are needed to help patients and hospitalists achieve these benefits.
1. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
2. Wetzell S. Transparency: a needed step towards health care affordability. American Health Policy Institute. March 2014. Accessed August 26, 2021. https://www.americanhealthpolicy.org/Content/documents/resources/Transparency%20Study%201%20-%20The%20Need%20for%20Health%20Care%20Transparency.pdf
3. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
4. Kullgren JT, Duey KA, Werner RM. A census of state health care price transparency websites. JAMA. 2013;309(23):2437-2438. https://doi.org/10.1001/jama.2013.6557
5. Brown ZY. Equilibrium effects of health care price information. Rev Econ Stat. 2019;101(4):699-712. https://doi.org/10.1162/rest_a_00765
6. Medicare and Medicaid Programs: CY 2020 hospital outpatient PPS policy changes and payment rates and ambulatory surgical center payment system policy changes and payment rates. Price transparency requirements for hospitals to make standard charges public. 45 CFR §180.20 (2019).
7. Kurani N, Ramirez G, Hudman J, Cox C, Kamal R. Early results from federal price transparency rule show difficulty in estimating the cost of care. Peterson-Kaiser Family Foundation. April 9, 2021. Accessed August 26, 2021. https://www.healthsystemtracker.org/brief/early-results-from-federal-price-transparency-rule-show-difficultly-in-estimating-the-cost-of-care/
8. Miller BJ, Mandelberg MC, Griffith NC, Ehrenfeld JM. Price transparency: empowering patient choice and promoting provider competition. J Med Syst. 2020;44(4):80. https://doi.org/10.1007/s10916-020-01553-2
9. Glied S. Price transparency–promise and peril. JAMA. 2021;325(15):1496-1497. https://doi.org/10.1001/jama.2021.4640
10. Haque W, Ahmadzada M, Allahrakha H, Haque E, Hsiehchen D. Transparency, accessibility, and variability of US hospital price data. JAMA Netw Open. 2021;4(5):e2110109. https://doi.org/10.1001/jamanetworkopen.2021.10109
11. Henderson M, Mouslim MC. Low compliance from big hospitals on CMS’s hospital price transparency rule. Health Affairs Blog. March 16, 2021. Accessed August 26, 2021. https://doi.org/10.1377/hblog20210311.899634
12. McGinty T, Wilde Mathews A, Evans M. Hospitals hide pricing data from search results. The Wall Street Journal. March 22, 2021. Accessed August 26, 2021. https://www.wsj.com/articles/hospitals-hide-pricing-data-from-search-results-11616405402
13. Dine CJ, Masi D, Smith CD. Tools to help overcome barriers to cost-of-care conversations. Ann Intern Med. 2019;170(9 suppl):S36-S38. https://doi.org/10.7326/M19-0778
14. Miller BJ, Slota JM, Ehrenfeld JM. Redefining the physician’s role in cost-conscious care: the potential role of the electronic health record. JAMA. 2019;322(8):721-722. https://doi.org/10.1001/jama.2019.9114
1. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
2. Wetzell S. Transparency: a needed step towards health care affordability. American Health Policy Institute. March 2014. Accessed August 26, 2021. https://www.americanhealthpolicy.org/Content/documents/resources/Transparency%20Study%201%20-%20The%20Need%20for%20Health%20Care%20Transparency.pdf
3. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
4. Kullgren JT, Duey KA, Werner RM. A census of state health care price transparency websites. JAMA. 2013;309(23):2437-2438. https://doi.org/10.1001/jama.2013.6557
5. Brown ZY. Equilibrium effects of health care price information. Rev Econ Stat. 2019;101(4):699-712. https://doi.org/10.1162/rest_a_00765
6. Medicare and Medicaid Programs: CY 2020 hospital outpatient PPS policy changes and payment rates and ambulatory surgical center payment system policy changes and payment rates. Price transparency requirements for hospitals to make standard charges public. 45 CFR §180.20 (2019).
7. Kurani N, Ramirez G, Hudman J, Cox C, Kamal R. Early results from federal price transparency rule show difficulty in estimating the cost of care. Peterson-Kaiser Family Foundation. April 9, 2021. Accessed August 26, 2021. https://www.healthsystemtracker.org/brief/early-results-from-federal-price-transparency-rule-show-difficultly-in-estimating-the-cost-of-care/
8. Miller BJ, Mandelberg MC, Griffith NC, Ehrenfeld JM. Price transparency: empowering patient choice and promoting provider competition. J Med Syst. 2020;44(4):80. https://doi.org/10.1007/s10916-020-01553-2
9. Glied S. Price transparency–promise and peril. JAMA. 2021;325(15):1496-1497. https://doi.org/10.1001/jama.2021.4640
10. Haque W, Ahmadzada M, Allahrakha H, Haque E, Hsiehchen D. Transparency, accessibility, and variability of US hospital price data. JAMA Netw Open. 2021;4(5):e2110109. https://doi.org/10.1001/jamanetworkopen.2021.10109
11. Henderson M, Mouslim MC. Low compliance from big hospitals on CMS’s hospital price transparency rule. Health Affairs Blog. March 16, 2021. Accessed August 26, 2021. https://doi.org/10.1377/hblog20210311.899634
12. McGinty T, Wilde Mathews A, Evans M. Hospitals hide pricing data from search results. The Wall Street Journal. March 22, 2021. Accessed August 26, 2021. https://www.wsj.com/articles/hospitals-hide-pricing-data-from-search-results-11616405402
13. Dine CJ, Masi D, Smith CD. Tools to help overcome barriers to cost-of-care conversations. Ann Intern Med. 2019;170(9 suppl):S36-S38. https://doi.org/10.7326/M19-0778
14. Miller BJ, Slota JM, Ehrenfeld JM. Redefining the physician’s role in cost-conscious care: the potential role of the electronic health record. JAMA. 2019;322(8):721-722. https://doi.org/10.1001/jama.2019.9114
© 2021 Society of Hospital Medicine
An A-Peeling Diagnosis
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 39-year-old previously healthy man presented to the emergency department (ED) with abrupt-onset fever, headache, back pain, myalgias, chills, and photophobia. His past medical history included seasonal allergies and an episode of aseptic meningitis 8 years prior. He denied cough, dysuria, weakness, numbness, or visual changes. He denied using tobacco or injection drugs and rarely drank alcohol. His only medication was acetaminophen for fever.
The patient’s sudden fever indicates the rapid onset of an inflammatory state. While the headache and photophobia might be a result of an underlying systemic infection or an irritant like blood in the cerebral spinal fluid (CSF), one must consider meningitis. Potential sources for sudden meningitis include infectious, autoimmune (rheumatoid arthritis, systemic lupus erythematosus [SLE]), or drug-induced aseptic meningitis, and structural etiologies (ruptured cyst). Recrudescence of prior disease may also present acutely (Mollaret meningitis). Malignant etiologies, being more indolent, seem less likely. Back pain may indicate an epidural inflammatory process like epidural abscess; however, the patient denies risk factors such as injection drug use or recent procedures.
The patient’s temperature was 101.2 °F; blood pressure, 120/72 mm Hg; and heart rate, 112 bpm. He appeared comfortable, without meningismus or spinal tenderness. Pupils were reactive; eyes were without icterus, injection, or suffusion. Cardiac exam was normal. Lungs were clear to auscultation. He had no abdominal tenderness, hepatosplenomegaly, or lymphadenopathy. Cranial nerves II through XII, balance, coordination, strength, and sensation were intact. No rash was noted. Complete blood count (CBC), basic and hepatic chemistry panels, urinalysis, and serum lactate tests were within normal limits. Erythrocyte sedimentation rate (ESR) was elevated to 15 mm/h (normal range, 3-10 mm/h), C-reactive protein (CRP) to 2.4 mg/dL (normal range, <0.5 mg/dL), and procalcitonin to 0.07 ng/mL (normal range, <0.05 ng/mL). The patient was treated with intravenous (IV) fluids, ketorolac, dexamethasone, and acetaminophen, with resolution of symptoms. Given his rapid improvement, absence of meningismus, and lack of immunocompromise, lumbar puncture was deferred. A diagnosis of nonspecific viral syndrome was made. He was discharged home.
Certainly, a systemic infection (eg, influenza, adenovirus, arbovirus-related infection, HIV) could be a cause of this patient’s presentation. Notably, less than two-thirds of patients with meningitis present with the classic triad of fever, neck stiffness, and altered mental status. In this patient with fever, headache, and photophobia, aseptic meningitis should still be considered. While the negative procalcitonin and rapid clinical improvement without antibiotics make acute bacterial meningitis unlikely, nonbacterial causes of meningeal irritation can be severe and life-threatening. An assessment for jolt accentuation of the headache might have been helpful. Information about time of year, geographic exposures (vector-borne infections), and sick contacts (viral illness) can inform the clinical decision to pursue lumbar puncture. Additional history regarding his previous aseptic meningitis would be helpful, as it could suggest a recurrent inflammatory process. Causes of recurrent aseptic meningitis include infectious (herpes simplex virus [HSV], Epstein-Barr virus [EBV], syphilis), drug-related (nonsteroidal anti-inflammatory drugs [NSAIDs]), structural (epidermoid cyst with rupture), and autoimmune (lupus, Sjögren syndrome, Behçet disease) etiologies.
The mildly elevated inflammatory markers are nonspecific and reflect the patient’s known inflammatory state. The dexamethasone given for symptomatic management may have had some therapeutic effect in the setting of an autoimmune process, with additional contribution from ketorolac and acetaminophen.
He returned to the ED 3 days later with a pruritic, disseminated rash involving his palms and soles, accompanied by hand swelling and tingling. Although his headache and photophobia resolved, he reported a productive cough, nasal congestion, and sore throat. He also reported orange-pink urine without dysuria or urinary frequency. Additional questioning revealed a recent motorcycle trip to the Great Lakes region. During this trip, he did not camp, interact with animals or ticks, or swim in streams or lakes. He did not eat any raw, undercooked, or locally hunted meats. He denied new medications, soaps or detergents, or sexual contacts. He had started taking acetaminophen and ibuprofen around the clock since prior discharge.
The orange-pink urine and acute-onset palmoplantar rash with recent fever help narrow the differential. Orange-pink urine might suggest bilirubinuria from liver injury, hemolysis with hemoglobinuria, or myoglobinuria. Most concerning would be hematuria associated with glomerular injury and a systemic vasculopathy.
The rash on the palms and soles should be further characterized as blanching or nonblanching. Blanching, indicating vasodilation of intact blood vessels, is seen with many drug eruptions and viral exanthems. Nonblanching, suggesting broken capillaries (petechiae or purpura), would suggest vasculitis or vasculopathy from emboli, infection, or inflammation. A palmoplantar rash in febrile illness should first prompt evaluation for life-threatening conditions, followed by consideration of both infectious and noninfectious etiologies. Acutely fatal infections include Rocky Mountain spotted fever (RMSF), meningococcemia, toxic shock syndrome, infective endocarditis, and rat-bite fever. The rash, fever, headache, and outdoor exposure raise the possibility of a rickettsial infection, including RMSF, which can be contracted rarely around the Great Lakes. Other life-threatening infections seem unlikely, as the patient would have significantly deteriorated without proper medical care by now. Palmoplantar rash with fever can also be seen in other bacterial infections (eg, secondary syphilis, arbovirus infections, typhus) and in viral infections (eg, cytomegalovirus [CMV], EBV, human herpesvirus-6 [HHV-6], HIV, coxsackievirus, and papular-purpuric gloves and socks syndrome caused by parvovirus B19). Noninfectious considerations include drug hypersensitivity rashes, neoplasm (eg, cutaneous T-cell lymphoma), or inflammatory conditions (eg, SLE, vasculitis). Drug reaction with eosinophilia and systemic symptoms (may also present with severe illness.
The acetaminophen and ibuprofen may be masking ongoing fevers. The cough, nasal congestion, and sore throat might be part of a viral prodrome or, in tandem with fever, associated with a vasculitis such as granulomatosis with polyangiitis.
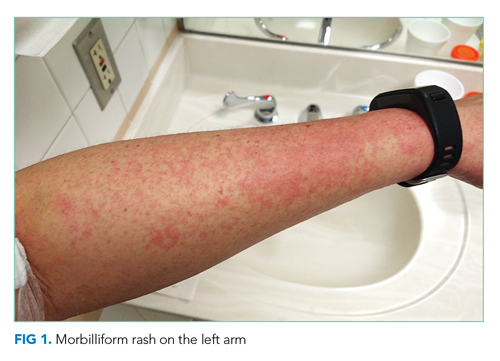
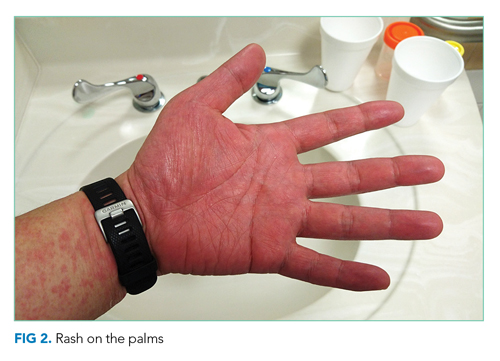
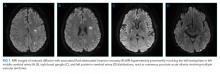
Vital signs were normal, and the patient appeared nontoxic. Physical examination demonstrated mildly cracked lips, oropharyngeal erythema with small petechiae on the soft palate, a morbilliform rash throughout his extremities and trunk (Figure 1), and confluent, brightly erythematous patches on his palms and soles with associated edema (Figure 2 and Appendix Figure). No lymphadenopathy, hepatosplenomegaly, or joint swelling was noted. CBC and basic chemistry panel remained normal; however, hepatic chemistries were notable for alanine aminotransferase (ALT) of 128 U/L, aspartate aminotransferase (AST) of 49 U/L, total bilirubin of 3.7 mg/dL, direct bilirubin of 2.4 mg/dL, total protein of 7.1 g/dL, albumin of 4.1 g/dL, and alkaline phosphatase of 197 U/L. Urinalysis detected bilirubin without blood, protein, bacteria, cells, or casts. The patient was admitted to the hospital.
The patient now has acute-onset upper respiratory symptoms with oral mucosal erythema, edema and erythema of the hands and feet with morbilliform rash of the extremities, and liver injury causing bilirubinuria. The patient’s initial symptoms may have had some response to therapy, but the current presentation suggests ongoing evolution of disease. Reactive infectious mucocutaneous eruptions include chlamydia, influenza, parainfluenza, and enteroviruses. Measles is possible given its recent resurgence; however, absence of coryza or Koplik spots and the peripheral distribution of the rash without initial truncal involvement make this less likely. Mycoplasma pneumonia–induced rash and mucositis might present with respiratory symptoms and this rash distribution, but typically involves two or more mucosal sites.
Iatrogenic causes are important to consider given the recent exposure to NSAIDs, specifically Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). In this patient, however, SJS/TEN is unlikely as it typically presents 1 to 3 weeks after exposure, with a truncal-predominant rash rarely involving the palms and soles.
Despite the absence of conjunctivitis and cervical lymphadenopathy, one additional consideration is Kawasaki disease (KD). Though more common in children, it may rarely present in adulthood. The time course of manifesting symptoms with potential steroid responsiveness raises suspicion for this diagnosis.
During a 4-day hospitalization, he developed mild bilateral conjunctivitis, peeling lips, and scleral icterus. CBC remained within normal limits. A peripheral smear demonstrated toxic neutrophilic granulation with normal erythrocytes and platelets. HIV and hepatitis A, B, and C serologies were negative. Blood cultures were negative. CRP and ESR increased to 4.3 mg/dL and 56 mm/h, respectively. Hepatic chemistries increased to ALT 155 U/L, AST 101 U/L, total bilirubin 5.1 mg/dL, direct bilirubin 3.3 mg/dL, and alkaline phosphatase 211 U/L. Right upper-quadrant ultrasound demonstrated gallbladder distention (11.3 cm × 5.0 cm; normal, 10.0 cm × 4.0 cm) without stones, wall thickening, or pericholecystic fluid; sonographic Murphy sign was negative. The liver was unremarkable with normal flow in the portal vein.
The patient’s persistent reactive neutrophilic granulation and rising CRP and ESR indicate ongoing inflammation. The largely direct hyperbilirubinemia with hepatitis, minimal findings on ultrasound imaging, and lack of Murphy sign suggest either direct infection of the liver or cholestasis. Viral serologies for EBV, HSV, and CMV should be sent, although these viruses are less commonly associated with oral rash and conjunctivitis. The marked degree of cholestasis makes adenovirus and mycoplasma less likely. Leptospirosis should be considered given the degree of liver injury with potential conjunctival suffusion. However, oral involvement would be atypical; renal injury is absent; and the patient denied pertinent exposures, vomiting, diarrhea, or persistent myalgias.
It is important to know whether the patient continued to receive
Low-grade fevers resolved without intervention. Tests were sent for tick-borne (ehrlichiosis, babesiosis, RMSF, anaplasmosis), viral (EBV, West Nile virus, parvovirus, CMV, coxsackievirus, adenovirus), other bacterial and protozoal (syphilis, Coxiella, leptospirosis, Lyme, Giardia), and autoimmune (antinuclear antibody, perinuclear antineutrophil cytoplasmic antibody, double-stranded DNA) diseases. Topical steroids and antihistamines were prescribed for a suspected viral exanthem. Empiric doxycycline was prescribed to treat possible tick-borne disease, and the patient was discharged home. At home, progressive darkening of the urine was noted. Outpatient testing demonstrated rising ALT to 377 U/L, AST to 183 U/L, total bilirubin to 5.9 mg/dL, direct bilirubin to 3.5 mg/dL, and alkaline phosphatase to 301 U/L. The patient was readmitted for further evaluation.
Despite concerns of the treating physicians, features of this case make tick-borne infections less likely. Lyme disease does not typically cause significant laboratory abnormalities and is classically associated with erythema migrans rather than a mucocutaneous rash. Relapsing fever, ehrlichioses, and rickettsial infections are associated with leukopenia and thrombocytopenia in addition to hepatocellular, rather than cholestatic, liver injury. The lack of response to doxycycline is helpful diagnostically: most tick-borne infections, in addition to leptospirosis, respond well to treatment. While babesiosis, tularemia, and Powassan or Heartland viruses transmitted by ticks are not treated with doxycycline, babesiosis often involves a hemolytic anemia (not seen in this case), and this patient’s laboratory abnormalities and rash are not characteristic of tularemia or viral tick-borne infections.
Either a new or reactivated viral infection with liver inflammation or an autoimmune etiology, specifically KD, remain the most likely etiology of the patient’s symptoms.
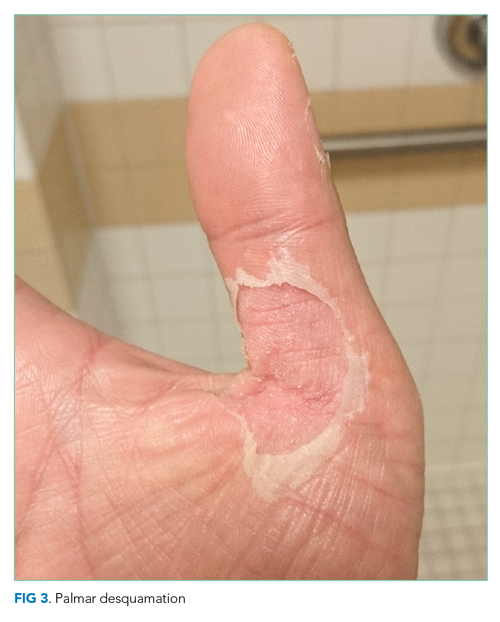
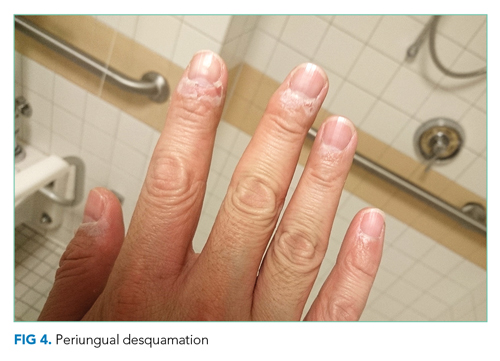
He remained asymptomatic during a 6-day hospitalization. His oral lesions resolved. The morbilliform rash coalesced into confluent macules with fine desquamation on the extremities and trunk. There was prominent periungual and palmar/plantar desquamation (Figure 3 and Figure 4). CBC demonstrated hemoglobin of 12.6 g/dL and platelets of 399,000/μL. CRP was undetectable at <0.5 mg/dL; however, ESR increased to 110 mm/h. Transaminases increased to ALT 551 U/L and AST 219 U/L. Serum alkaline phosphatase and bilirubin decreased without intervention. Albumin and total protein remained unchanged. All infectious and autoimmune testing sent from the prior admission returned negative.
An acute-onset viral-like prodrome with fevers potentially responsive to steroids, followed by conjunctivitis, oral erythema and cracked lips, morbilliform rash with hand and foot erythema and edema, cholestatic hepatitis, and subsequent periungual desquamation is highly suggestive of KD. It would be interesting to revisit the patient’s prior episode of aseptic meningitis to see whether any other symptoms were suggestive of KD. While intravenous immunoglobulin (IVIg) and aspirin are standard therapies for the acute febrile phase of KD, the patient is now nearly 2 weeks into his clinical course, rendering their utility uncertain. Nonetheless, screening for coronary aneurysms should be pursued, which may help confirm the diagnosis.
Upon reviewing the evolution of the findings, a diagnosis of adult-onset KD was made. IVIg 2g/kg and aspirin 325 mg were administered. Echocardiogram did not show any evidence of coronary artery aneurysm, myocarditis, pericarditis, wall motion abnormalities, or pericardial effusion. Computed tomography (CT) coronary angiogram confirmed normal coronary arteries without aneurysm. The patient was discharged home without fever on daily aspirin, and all hepatic chemistries and inflammatory markers normalized. Follow-up cardiac magnetic resonance imaging at 3 months and CT angiogram at 6 months remained normal. The patient remains well now 2 years after the original diagnosis and treatment.
DISCUSSION
KD, also known as mucocutaneous lymph node syndrome, is a vasculitis that typically affects children younger than 5 years.1 Having a sibling with KD confers a 10- to 15-fold higher risk, suggesting a genetic component to the disease.2 The highest incidence of KD is in persons of East Asian descent, but KD can affect patients of all races and ethnicities. In the United States, the majority of patients with KD are non-Hispanic White, followed by Black, Hispanic, and Asian.3 The etiology is still unknown, but it is posited that an unidentified, ubiquitous infectious agent may trigger KD in genetically susceptible individuals.4
KD can cause aneurysms and thromboses in medium-sized blood vessels throughout the body.5,6 The classic presentation involves 5 days of high fever plus four or more of the symptoms in the mnemonic CRASH: conjunctival injection, rash (polymorphous), adenopathy (cervical), strawberry tongue (or red, cracked lips and oropharyngeal edema), hand (erythema and induration of hands or feet, followed by periungual desquamation).7 Multiple organ systems may be affected, manifesting as abdominal pain, arthritis, pneumonitis, aseptic meningitis, and acalculous distention of the gallbladder (hydrops).7 The most feared consequence is coronary artery involvement, which leads to aneurysm, thrombosis, and sudden death.
Though no definitive diagnostic test exists, certain laboratory findings support the diagnosis, such as sterile pyuria, thrombocytosis, elevated CRP and ESR, transaminitis, and hypoalbuminemia.7 Diagnosis requires exclusion of illnesses with similar presentations, such as bacterial, viral, and tick-borne infections; drug hypersensitivity reactions; toxic shock syndrome; scarlet fever; juvenile rheumatoid arthritis; and other rheumatologic conditions. Some cases of KD present with fewer than four of the principal (CRASH) symptoms—these are termed “incomplete” KD. The combination of supportive laboratory findings and echocardiogram can facilitate diagnosis of incomplete KD, which carries a similar risk of coronary artery aneurysm.7
Though primarily a disease of childhood, KD can present in adults.8 Adults, compared with children, are less likely to have thrombocytosis and more likely to have cervical adenopathy, arthralgias, and hepatic test abnormalities.8 Although coronary artery aneurysms occur less frequently in adults compared with children, timely diagnosis and treatment is key to preventing this life-threatening complication.8
In children, treatment is IVIg 2 g/kg and aspirin 80 to 100 mg/kg daily until afebrile for several days.9 Some require a second dose of IVIg.9 Children are then maintained on 3 to 5 mg/kg of aspirin daily for 6 to 8 weeks.9 IVIg, given within 10 days of the onset of fever, is highly effective at preventing coronary artery aneurysms.10,11 When coronary aneurysms do occur, treatment is with aspirin or clopidogrel. Very large aneurysms require systemic anticoagulation. After the acute illness, children are monitored with serial cardiac imaging at 2 weeks and 6 to 8 weeks after diagnosis.7 In adults, the optimal imaging timing is unknown. Echocardiography often cannot visualize the coronary arteries, necessitating coronary CT angiography or cardiac MRI.
Despite the presence of classic features, this patient’s diagnosis was delayed because of the rarity of KD in adults and the need to exclude more common diseases. Furthermore, the administration of dexamethasone likely shortened his febrile period and ameliorated some symptoms,12 affecting the natural history of his illness. The diagnosis relied on three components: ruling out common diagnoses, noting two unusual findings (gallbladder hydrops, desquamating periungual rash), and broadening the differential to include adult presentations of childhood disease. Review of the literature suggests very few causes for gallbladder hydrops: impacted stones, cystic fibrosis, cystic duct narrowing due to tumor or lymph nodes, KD, and bacterial and parasitic disease (eg, salmonella, ascariasis). Gallbladder hydrops and periungual desquamation are seen together only in KD.13 Given the complexity of diagnosis in adults, the time to diagnosis is often delayed compared with that for children. While IVIg treatment is preferred within 10 days of the onset of fever, this patient received IVIg on day 14, given the relatively benign nature of IVIg and the considerable morbidity associated with coronary artery aneurysms. Dosing for aspirin is unclear in adults.8 This patient was started on 325 mg aspirin daily. He recovered fully and remains free of coronary changes at two years after initial diagnosis. This case is an excellent reminder that, after exclusion of common diagnoses, reflection on the most unusual aspects of the case and consideration of childhood diseases is particularly important in our younger patients.
TEACHING POINTS
- Extended fever should broaden the differential to include rheumatologic diagnoses.
- KD is rare in adults but can present with classic findings from childhood.
- Early treatment with IVIg and aspirin can be lifesaving in patients with KD, including adults.
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Article in Japanese. Arerugi. 1967;16(3):178-222.
2. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9(4):185-194. https://doi.org/10.1016/j.ijid.2005.03.002
3. Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-488. https://doi.org/10.1097/INF.0b013e3181cf8705
4. Rowley A, Baker S, Arollo D, et al. A hepacivirus-like protein is targeted by the antibody response to Kawasaki disease (KD) [abstract]. Open Forum Infect Dis. 2019;6(suppl 2):S48.
5. Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5(9):e003289. https://doi.org/10.1161/JAHA.116.003289
6. Zhao QM, Chu C, Wu L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. 2019;144(6):e20192254. https://doi.org/10.1542/peds.2019-2254
7. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. https://doi.org/10.1542/peds.2004-2182
8. Sève P, Stankovic K, Smail A, Durand DV, Marchand G, Broussolle C. Adult Kawasaki disease: report of two cases and literature review. Semin Arthritis Rheum. 2005;34(6):785-792. https://doi.org/10.1016/j.semarthrit.2005.01.012
9. Shulman ST. Intravenous immunoglobulin for the treatment of Kawasaki disease. Pediatr Ann. 2017;46(1):e25-e28. https://doi.org/10.3928/19382359-20161212-01
10. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341-347. https://doi.org/10.1056/NEJM198608073150601
11. Rowley AH, Duffy CE, Shulman ST. Prevention of giant coronary artery aneurysms in Kawasaki disease by intravenous gamma globulin therapy. J Pediatr. 1988;113(2):290-294. https://doi/org/10.1016/s0022-3476(88)80267-1
12. Lim YJ, Jung JW. Clinical outcomes of initial dexamethasone treatment combined with a single high dose of intravenous immunoglobulin for primary treatment of Kawasaki disease. Yonsei Med J. 2014;55(5):1260-1266. https://doi.org/10.3349/ymj.2014.55.5.1260
13. Sun Q, Zhang J, Yang Y. Gallbladder hydrops associated with Kawasaki disease: a case report and literature review. Clin Pediatr (Phila). 2018;57(3):341-343. https://doi.org/10.1177/0009922817696468
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 39-year-old previously healthy man presented to the emergency department (ED) with abrupt-onset fever, headache, back pain, myalgias, chills, and photophobia. His past medical history included seasonal allergies and an episode of aseptic meningitis 8 years prior. He denied cough, dysuria, weakness, numbness, or visual changes. He denied using tobacco or injection drugs and rarely drank alcohol. His only medication was acetaminophen for fever.
The patient’s sudden fever indicates the rapid onset of an inflammatory state. While the headache and photophobia might be a result of an underlying systemic infection or an irritant like blood in the cerebral spinal fluid (CSF), one must consider meningitis. Potential sources for sudden meningitis include infectious, autoimmune (rheumatoid arthritis, systemic lupus erythematosus [SLE]), or drug-induced aseptic meningitis, and structural etiologies (ruptured cyst). Recrudescence of prior disease may also present acutely (Mollaret meningitis). Malignant etiologies, being more indolent, seem less likely. Back pain may indicate an epidural inflammatory process like epidural abscess; however, the patient denies risk factors such as injection drug use or recent procedures.
The patient’s temperature was 101.2 °F; blood pressure, 120/72 mm Hg; and heart rate, 112 bpm. He appeared comfortable, without meningismus or spinal tenderness. Pupils were reactive; eyes were without icterus, injection, or suffusion. Cardiac exam was normal. Lungs were clear to auscultation. He had no abdominal tenderness, hepatosplenomegaly, or lymphadenopathy. Cranial nerves II through XII, balance, coordination, strength, and sensation were intact. No rash was noted. Complete blood count (CBC), basic and hepatic chemistry panels, urinalysis, and serum lactate tests were within normal limits. Erythrocyte sedimentation rate (ESR) was elevated to 15 mm/h (normal range, 3-10 mm/h), C-reactive protein (CRP) to 2.4 mg/dL (normal range, <0.5 mg/dL), and procalcitonin to 0.07 ng/mL (normal range, <0.05 ng/mL). The patient was treated with intravenous (IV) fluids, ketorolac, dexamethasone, and acetaminophen, with resolution of symptoms. Given his rapid improvement, absence of meningismus, and lack of immunocompromise, lumbar puncture was deferred. A diagnosis of nonspecific viral syndrome was made. He was discharged home.
Certainly, a systemic infection (eg, influenza, adenovirus, arbovirus-related infection, HIV) could be a cause of this patient’s presentation. Notably, less than two-thirds of patients with meningitis present with the classic triad of fever, neck stiffness, and altered mental status. In this patient with fever, headache, and photophobia, aseptic meningitis should still be considered. While the negative procalcitonin and rapid clinical improvement without antibiotics make acute bacterial meningitis unlikely, nonbacterial causes of meningeal irritation can be severe and life-threatening. An assessment for jolt accentuation of the headache might have been helpful. Information about time of year, geographic exposures (vector-borne infections), and sick contacts (viral illness) can inform the clinical decision to pursue lumbar puncture. Additional history regarding his previous aseptic meningitis would be helpful, as it could suggest a recurrent inflammatory process. Causes of recurrent aseptic meningitis include infectious (herpes simplex virus [HSV], Epstein-Barr virus [EBV], syphilis), drug-related (nonsteroidal anti-inflammatory drugs [NSAIDs]), structural (epidermoid cyst with rupture), and autoimmune (lupus, Sjögren syndrome, Behçet disease) etiologies.
The mildly elevated inflammatory markers are nonspecific and reflect the patient’s known inflammatory state. The dexamethasone given for symptomatic management may have had some therapeutic effect in the setting of an autoimmune process, with additional contribution from ketorolac and acetaminophen.
He returned to the ED 3 days later with a pruritic, disseminated rash involving his palms and soles, accompanied by hand swelling and tingling. Although his headache and photophobia resolved, he reported a productive cough, nasal congestion, and sore throat. He also reported orange-pink urine without dysuria or urinary frequency. Additional questioning revealed a recent motorcycle trip to the Great Lakes region. During this trip, he did not camp, interact with animals or ticks, or swim in streams or lakes. He did not eat any raw, undercooked, or locally hunted meats. He denied new medications, soaps or detergents, or sexual contacts. He had started taking acetaminophen and ibuprofen around the clock since prior discharge.
The orange-pink urine and acute-onset palmoplantar rash with recent fever help narrow the differential. Orange-pink urine might suggest bilirubinuria from liver injury, hemolysis with hemoglobinuria, or myoglobinuria. Most concerning would be hematuria associated with glomerular injury and a systemic vasculopathy.
The rash on the palms and soles should be further characterized as blanching or nonblanching. Blanching, indicating vasodilation of intact blood vessels, is seen with many drug eruptions and viral exanthems. Nonblanching, suggesting broken capillaries (petechiae or purpura), would suggest vasculitis or vasculopathy from emboli, infection, or inflammation. A palmoplantar rash in febrile illness should first prompt evaluation for life-threatening conditions, followed by consideration of both infectious and noninfectious etiologies. Acutely fatal infections include Rocky Mountain spotted fever (RMSF), meningococcemia, toxic shock syndrome, infective endocarditis, and rat-bite fever. The rash, fever, headache, and outdoor exposure raise the possibility of a rickettsial infection, including RMSF, which can be contracted rarely around the Great Lakes. Other life-threatening infections seem unlikely, as the patient would have significantly deteriorated without proper medical care by now. Palmoplantar rash with fever can also be seen in other bacterial infections (eg, secondary syphilis, arbovirus infections, typhus) and in viral infections (eg, cytomegalovirus [CMV], EBV, human herpesvirus-6 [HHV-6], HIV, coxsackievirus, and papular-purpuric gloves and socks syndrome caused by parvovirus B19). Noninfectious considerations include drug hypersensitivity rashes, neoplasm (eg, cutaneous T-cell lymphoma), or inflammatory conditions (eg, SLE, vasculitis). Drug reaction with eosinophilia and systemic symptoms (may also present with severe illness.
The acetaminophen and ibuprofen may be masking ongoing fevers. The cough, nasal congestion, and sore throat might be part of a viral prodrome or, in tandem with fever, associated with a vasculitis such as granulomatosis with polyangiitis.
Vital signs were normal, and the patient appeared nontoxic. Physical examination demonstrated mildly cracked lips, oropharyngeal erythema with small petechiae on the soft palate, a morbilliform rash throughout his extremities and trunk (Figure 1), and confluent, brightly erythematous patches on his palms and soles with associated edema (Figure 2 and Appendix Figure). No lymphadenopathy, hepatosplenomegaly, or joint swelling was noted. CBC and basic chemistry panel remained normal; however, hepatic chemistries were notable for alanine aminotransferase (ALT) of 128 U/L, aspartate aminotransferase (AST) of 49 U/L, total bilirubin of 3.7 mg/dL, direct bilirubin of 2.4 mg/dL, total protein of 7.1 g/dL, albumin of 4.1 g/dL, and alkaline phosphatase of 197 U/L. Urinalysis detected bilirubin without blood, protein, bacteria, cells, or casts. The patient was admitted to the hospital.
The patient now has acute-onset upper respiratory symptoms with oral mucosal erythema, edema and erythema of the hands and feet with morbilliform rash of the extremities, and liver injury causing bilirubinuria. The patient’s initial symptoms may have had some response to therapy, but the current presentation suggests ongoing evolution of disease. Reactive infectious mucocutaneous eruptions include chlamydia, influenza, parainfluenza, and enteroviruses. Measles is possible given its recent resurgence; however, absence of coryza or Koplik spots and the peripheral distribution of the rash without initial truncal involvement make this less likely. Mycoplasma pneumonia–induced rash and mucositis might present with respiratory symptoms and this rash distribution, but typically involves two or more mucosal sites.
Iatrogenic causes are important to consider given the recent exposure to NSAIDs, specifically Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). In this patient, however, SJS/TEN is unlikely as it typically presents 1 to 3 weeks after exposure, with a truncal-predominant rash rarely involving the palms and soles.
Despite the absence of conjunctivitis and cervical lymphadenopathy, one additional consideration is Kawasaki disease (KD). Though more common in children, it may rarely present in adulthood. The time course of manifesting symptoms with potential steroid responsiveness raises suspicion for this diagnosis.
During a 4-day hospitalization, he developed mild bilateral conjunctivitis, peeling lips, and scleral icterus. CBC remained within normal limits. A peripheral smear demonstrated toxic neutrophilic granulation with normal erythrocytes and platelets. HIV and hepatitis A, B, and C serologies were negative. Blood cultures were negative. CRP and ESR increased to 4.3 mg/dL and 56 mm/h, respectively. Hepatic chemistries increased to ALT 155 U/L, AST 101 U/L, total bilirubin 5.1 mg/dL, direct bilirubin 3.3 mg/dL, and alkaline phosphatase 211 U/L. Right upper-quadrant ultrasound demonstrated gallbladder distention (11.3 cm × 5.0 cm; normal, 10.0 cm × 4.0 cm) without stones, wall thickening, or pericholecystic fluid; sonographic Murphy sign was negative. The liver was unremarkable with normal flow in the portal vein.
The patient’s persistent reactive neutrophilic granulation and rising CRP and ESR indicate ongoing inflammation. The largely direct hyperbilirubinemia with hepatitis, minimal findings on ultrasound imaging, and lack of Murphy sign suggest either direct infection of the liver or cholestasis. Viral serologies for EBV, HSV, and CMV should be sent, although these viruses are less commonly associated with oral rash and conjunctivitis. The marked degree of cholestasis makes adenovirus and mycoplasma less likely. Leptospirosis should be considered given the degree of liver injury with potential conjunctival suffusion. However, oral involvement would be atypical; renal injury is absent; and the patient denied pertinent exposures, vomiting, diarrhea, or persistent myalgias.
It is important to know whether the patient continued to receive
Low-grade fevers resolved without intervention. Tests were sent for tick-borne (ehrlichiosis, babesiosis, RMSF, anaplasmosis), viral (EBV, West Nile virus, parvovirus, CMV, coxsackievirus, adenovirus), other bacterial and protozoal (syphilis, Coxiella, leptospirosis, Lyme, Giardia), and autoimmune (antinuclear antibody, perinuclear antineutrophil cytoplasmic antibody, double-stranded DNA) diseases. Topical steroids and antihistamines were prescribed for a suspected viral exanthem. Empiric doxycycline was prescribed to treat possible tick-borne disease, and the patient was discharged home. At home, progressive darkening of the urine was noted. Outpatient testing demonstrated rising ALT to 377 U/L, AST to 183 U/L, total bilirubin to 5.9 mg/dL, direct bilirubin to 3.5 mg/dL, and alkaline phosphatase to 301 U/L. The patient was readmitted for further evaluation.
Despite concerns of the treating physicians, features of this case make tick-borne infections less likely. Lyme disease does not typically cause significant laboratory abnormalities and is classically associated with erythema migrans rather than a mucocutaneous rash. Relapsing fever, ehrlichioses, and rickettsial infections are associated with leukopenia and thrombocytopenia in addition to hepatocellular, rather than cholestatic, liver injury. The lack of response to doxycycline is helpful diagnostically: most tick-borne infections, in addition to leptospirosis, respond well to treatment. While babesiosis, tularemia, and Powassan or Heartland viruses transmitted by ticks are not treated with doxycycline, babesiosis often involves a hemolytic anemia (not seen in this case), and this patient’s laboratory abnormalities and rash are not characteristic of tularemia or viral tick-borne infections.
Either a new or reactivated viral infection with liver inflammation or an autoimmune etiology, specifically KD, remain the most likely etiology of the patient’s symptoms.
He remained asymptomatic during a 6-day hospitalization. His oral lesions resolved. The morbilliform rash coalesced into confluent macules with fine desquamation on the extremities and trunk. There was prominent periungual and palmar/plantar desquamation (Figure 3 and Figure 4). CBC demonstrated hemoglobin of 12.6 g/dL and platelets of 399,000/μL. CRP was undetectable at <0.5 mg/dL; however, ESR increased to 110 mm/h. Transaminases increased to ALT 551 U/L and AST 219 U/L. Serum alkaline phosphatase and bilirubin decreased without intervention. Albumin and total protein remained unchanged. All infectious and autoimmune testing sent from the prior admission returned negative.
An acute-onset viral-like prodrome with fevers potentially responsive to steroids, followed by conjunctivitis, oral erythema and cracked lips, morbilliform rash with hand and foot erythema and edema, cholestatic hepatitis, and subsequent periungual desquamation is highly suggestive of KD. It would be interesting to revisit the patient’s prior episode of aseptic meningitis to see whether any other symptoms were suggestive of KD. While intravenous immunoglobulin (IVIg) and aspirin are standard therapies for the acute febrile phase of KD, the patient is now nearly 2 weeks into his clinical course, rendering their utility uncertain. Nonetheless, screening for coronary aneurysms should be pursued, which may help confirm the diagnosis.
Upon reviewing the evolution of the findings, a diagnosis of adult-onset KD was made. IVIg 2g/kg and aspirin 325 mg were administered. Echocardiogram did not show any evidence of coronary artery aneurysm, myocarditis, pericarditis, wall motion abnormalities, or pericardial effusion. Computed tomography (CT) coronary angiogram confirmed normal coronary arteries without aneurysm. The patient was discharged home without fever on daily aspirin, and all hepatic chemistries and inflammatory markers normalized. Follow-up cardiac magnetic resonance imaging at 3 months and CT angiogram at 6 months remained normal. The patient remains well now 2 years after the original diagnosis and treatment.
DISCUSSION
KD, also known as mucocutaneous lymph node syndrome, is a vasculitis that typically affects children younger than 5 years.1 Having a sibling with KD confers a 10- to 15-fold higher risk, suggesting a genetic component to the disease.2 The highest incidence of KD is in persons of East Asian descent, but KD can affect patients of all races and ethnicities. In the United States, the majority of patients with KD are non-Hispanic White, followed by Black, Hispanic, and Asian.3 The etiology is still unknown, but it is posited that an unidentified, ubiquitous infectious agent may trigger KD in genetically susceptible individuals.4
KD can cause aneurysms and thromboses in medium-sized blood vessels throughout the body.5,6 The classic presentation involves 5 days of high fever plus four or more of the symptoms in the mnemonic CRASH: conjunctival injection, rash (polymorphous), adenopathy (cervical), strawberry tongue (or red, cracked lips and oropharyngeal edema), hand (erythema and induration of hands or feet, followed by periungual desquamation).7 Multiple organ systems may be affected, manifesting as abdominal pain, arthritis, pneumonitis, aseptic meningitis, and acalculous distention of the gallbladder (hydrops).7 The most feared consequence is coronary artery involvement, which leads to aneurysm, thrombosis, and sudden death.
Though no definitive diagnostic test exists, certain laboratory findings support the diagnosis, such as sterile pyuria, thrombocytosis, elevated CRP and ESR, transaminitis, and hypoalbuminemia.7 Diagnosis requires exclusion of illnesses with similar presentations, such as bacterial, viral, and tick-borne infections; drug hypersensitivity reactions; toxic shock syndrome; scarlet fever; juvenile rheumatoid arthritis; and other rheumatologic conditions. Some cases of KD present with fewer than four of the principal (CRASH) symptoms—these are termed “incomplete” KD. The combination of supportive laboratory findings and echocardiogram can facilitate diagnosis of incomplete KD, which carries a similar risk of coronary artery aneurysm.7
Though primarily a disease of childhood, KD can present in adults.8 Adults, compared with children, are less likely to have thrombocytosis and more likely to have cervical adenopathy, arthralgias, and hepatic test abnormalities.8 Although coronary artery aneurysms occur less frequently in adults compared with children, timely diagnosis and treatment is key to preventing this life-threatening complication.8
In children, treatment is IVIg 2 g/kg and aspirin 80 to 100 mg/kg daily until afebrile for several days.9 Some require a second dose of IVIg.9 Children are then maintained on 3 to 5 mg/kg of aspirin daily for 6 to 8 weeks.9 IVIg, given within 10 days of the onset of fever, is highly effective at preventing coronary artery aneurysms.10,11 When coronary aneurysms do occur, treatment is with aspirin or clopidogrel. Very large aneurysms require systemic anticoagulation. After the acute illness, children are monitored with serial cardiac imaging at 2 weeks and 6 to 8 weeks after diagnosis.7 In adults, the optimal imaging timing is unknown. Echocardiography often cannot visualize the coronary arteries, necessitating coronary CT angiography or cardiac MRI.
Despite the presence of classic features, this patient’s diagnosis was delayed because of the rarity of KD in adults and the need to exclude more common diseases. Furthermore, the administration of dexamethasone likely shortened his febrile period and ameliorated some symptoms,12 affecting the natural history of his illness. The diagnosis relied on three components: ruling out common diagnoses, noting two unusual findings (gallbladder hydrops, desquamating periungual rash), and broadening the differential to include adult presentations of childhood disease. Review of the literature suggests very few causes for gallbladder hydrops: impacted stones, cystic fibrosis, cystic duct narrowing due to tumor or lymph nodes, KD, and bacterial and parasitic disease (eg, salmonella, ascariasis). Gallbladder hydrops and periungual desquamation are seen together only in KD.13 Given the complexity of diagnosis in adults, the time to diagnosis is often delayed compared with that for children. While IVIg treatment is preferred within 10 days of the onset of fever, this patient received IVIg on day 14, given the relatively benign nature of IVIg and the considerable morbidity associated with coronary artery aneurysms. Dosing for aspirin is unclear in adults.8 This patient was started on 325 mg aspirin daily. He recovered fully and remains free of coronary changes at two years after initial diagnosis. This case is an excellent reminder that, after exclusion of common diagnoses, reflection on the most unusual aspects of the case and consideration of childhood diseases is particularly important in our younger patients.
TEACHING POINTS
- Extended fever should broaden the differential to include rheumatologic diagnoses.
- KD is rare in adults but can present with classic findings from childhood.
- Early treatment with IVIg and aspirin can be lifesaving in patients with KD, including adults.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 39-year-old previously healthy man presented to the emergency department (ED) with abrupt-onset fever, headache, back pain, myalgias, chills, and photophobia. His past medical history included seasonal allergies and an episode of aseptic meningitis 8 years prior. He denied cough, dysuria, weakness, numbness, or visual changes. He denied using tobacco or injection drugs and rarely drank alcohol. His only medication was acetaminophen for fever.
The patient’s sudden fever indicates the rapid onset of an inflammatory state. While the headache and photophobia might be a result of an underlying systemic infection or an irritant like blood in the cerebral spinal fluid (CSF), one must consider meningitis. Potential sources for sudden meningitis include infectious, autoimmune (rheumatoid arthritis, systemic lupus erythematosus [SLE]), or drug-induced aseptic meningitis, and structural etiologies (ruptured cyst). Recrudescence of prior disease may also present acutely (Mollaret meningitis). Malignant etiologies, being more indolent, seem less likely. Back pain may indicate an epidural inflammatory process like epidural abscess; however, the patient denies risk factors such as injection drug use or recent procedures.
The patient’s temperature was 101.2 °F; blood pressure, 120/72 mm Hg; and heart rate, 112 bpm. He appeared comfortable, without meningismus or spinal tenderness. Pupils were reactive; eyes were without icterus, injection, or suffusion. Cardiac exam was normal. Lungs were clear to auscultation. He had no abdominal tenderness, hepatosplenomegaly, or lymphadenopathy. Cranial nerves II through XII, balance, coordination, strength, and sensation were intact. No rash was noted. Complete blood count (CBC), basic and hepatic chemistry panels, urinalysis, and serum lactate tests were within normal limits. Erythrocyte sedimentation rate (ESR) was elevated to 15 mm/h (normal range, 3-10 mm/h), C-reactive protein (CRP) to 2.4 mg/dL (normal range, <0.5 mg/dL), and procalcitonin to 0.07 ng/mL (normal range, <0.05 ng/mL). The patient was treated with intravenous (IV) fluids, ketorolac, dexamethasone, and acetaminophen, with resolution of symptoms. Given his rapid improvement, absence of meningismus, and lack of immunocompromise, lumbar puncture was deferred. A diagnosis of nonspecific viral syndrome was made. He was discharged home.
Certainly, a systemic infection (eg, influenza, adenovirus, arbovirus-related infection, HIV) could be a cause of this patient’s presentation. Notably, less than two-thirds of patients with meningitis present with the classic triad of fever, neck stiffness, and altered mental status. In this patient with fever, headache, and photophobia, aseptic meningitis should still be considered. While the negative procalcitonin and rapid clinical improvement without antibiotics make acute bacterial meningitis unlikely, nonbacterial causes of meningeal irritation can be severe and life-threatening. An assessment for jolt accentuation of the headache might have been helpful. Information about time of year, geographic exposures (vector-borne infections), and sick contacts (viral illness) can inform the clinical decision to pursue lumbar puncture. Additional history regarding his previous aseptic meningitis would be helpful, as it could suggest a recurrent inflammatory process. Causes of recurrent aseptic meningitis include infectious (herpes simplex virus [HSV], Epstein-Barr virus [EBV], syphilis), drug-related (nonsteroidal anti-inflammatory drugs [NSAIDs]), structural (epidermoid cyst with rupture), and autoimmune (lupus, Sjögren syndrome, Behçet disease) etiologies.
The mildly elevated inflammatory markers are nonspecific and reflect the patient’s known inflammatory state. The dexamethasone given for symptomatic management may have had some therapeutic effect in the setting of an autoimmune process, with additional contribution from ketorolac and acetaminophen.
He returned to the ED 3 days later with a pruritic, disseminated rash involving his palms and soles, accompanied by hand swelling and tingling. Although his headache and photophobia resolved, he reported a productive cough, nasal congestion, and sore throat. He also reported orange-pink urine without dysuria or urinary frequency. Additional questioning revealed a recent motorcycle trip to the Great Lakes region. During this trip, he did not camp, interact with animals or ticks, or swim in streams or lakes. He did not eat any raw, undercooked, or locally hunted meats. He denied new medications, soaps or detergents, or sexual contacts. He had started taking acetaminophen and ibuprofen around the clock since prior discharge.
The orange-pink urine and acute-onset palmoplantar rash with recent fever help narrow the differential. Orange-pink urine might suggest bilirubinuria from liver injury, hemolysis with hemoglobinuria, or myoglobinuria. Most concerning would be hematuria associated with glomerular injury and a systemic vasculopathy.
The rash on the palms and soles should be further characterized as blanching or nonblanching. Blanching, indicating vasodilation of intact blood vessels, is seen with many drug eruptions and viral exanthems. Nonblanching, suggesting broken capillaries (petechiae or purpura), would suggest vasculitis or vasculopathy from emboli, infection, or inflammation. A palmoplantar rash in febrile illness should first prompt evaluation for life-threatening conditions, followed by consideration of both infectious and noninfectious etiologies. Acutely fatal infections include Rocky Mountain spotted fever (RMSF), meningococcemia, toxic shock syndrome, infective endocarditis, and rat-bite fever. The rash, fever, headache, and outdoor exposure raise the possibility of a rickettsial infection, including RMSF, which can be contracted rarely around the Great Lakes. Other life-threatening infections seem unlikely, as the patient would have significantly deteriorated without proper medical care by now. Palmoplantar rash with fever can also be seen in other bacterial infections (eg, secondary syphilis, arbovirus infections, typhus) and in viral infections (eg, cytomegalovirus [CMV], EBV, human herpesvirus-6 [HHV-6], HIV, coxsackievirus, and papular-purpuric gloves and socks syndrome caused by parvovirus B19). Noninfectious considerations include drug hypersensitivity rashes, neoplasm (eg, cutaneous T-cell lymphoma), or inflammatory conditions (eg, SLE, vasculitis). Drug reaction with eosinophilia and systemic symptoms (may also present with severe illness.
The acetaminophen and ibuprofen may be masking ongoing fevers. The cough, nasal congestion, and sore throat might be part of a viral prodrome or, in tandem with fever, associated with a vasculitis such as granulomatosis with polyangiitis.
Vital signs were normal, and the patient appeared nontoxic. Physical examination demonstrated mildly cracked lips, oropharyngeal erythema with small petechiae on the soft palate, a morbilliform rash throughout his extremities and trunk (Figure 1), and confluent, brightly erythematous patches on his palms and soles with associated edema (Figure 2 and Appendix Figure). No lymphadenopathy, hepatosplenomegaly, or joint swelling was noted. CBC and basic chemistry panel remained normal; however, hepatic chemistries were notable for alanine aminotransferase (ALT) of 128 U/L, aspartate aminotransferase (AST) of 49 U/L, total bilirubin of 3.7 mg/dL, direct bilirubin of 2.4 mg/dL, total protein of 7.1 g/dL, albumin of 4.1 g/dL, and alkaline phosphatase of 197 U/L. Urinalysis detected bilirubin without blood, protein, bacteria, cells, or casts. The patient was admitted to the hospital.
The patient now has acute-onset upper respiratory symptoms with oral mucosal erythema, edema and erythema of the hands and feet with morbilliform rash of the extremities, and liver injury causing bilirubinuria. The patient’s initial symptoms may have had some response to therapy, but the current presentation suggests ongoing evolution of disease. Reactive infectious mucocutaneous eruptions include chlamydia, influenza, parainfluenza, and enteroviruses. Measles is possible given its recent resurgence; however, absence of coryza or Koplik spots and the peripheral distribution of the rash without initial truncal involvement make this less likely. Mycoplasma pneumonia–induced rash and mucositis might present with respiratory symptoms and this rash distribution, but typically involves two or more mucosal sites.
Iatrogenic causes are important to consider given the recent exposure to NSAIDs, specifically Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). In this patient, however, SJS/TEN is unlikely as it typically presents 1 to 3 weeks after exposure, with a truncal-predominant rash rarely involving the palms and soles.
Despite the absence of conjunctivitis and cervical lymphadenopathy, one additional consideration is Kawasaki disease (KD). Though more common in children, it may rarely present in adulthood. The time course of manifesting symptoms with potential steroid responsiveness raises suspicion for this diagnosis.
During a 4-day hospitalization, he developed mild bilateral conjunctivitis, peeling lips, and scleral icterus. CBC remained within normal limits. A peripheral smear demonstrated toxic neutrophilic granulation with normal erythrocytes and platelets. HIV and hepatitis A, B, and C serologies were negative. Blood cultures were negative. CRP and ESR increased to 4.3 mg/dL and 56 mm/h, respectively. Hepatic chemistries increased to ALT 155 U/L, AST 101 U/L, total bilirubin 5.1 mg/dL, direct bilirubin 3.3 mg/dL, and alkaline phosphatase 211 U/L. Right upper-quadrant ultrasound demonstrated gallbladder distention (11.3 cm × 5.0 cm; normal, 10.0 cm × 4.0 cm) without stones, wall thickening, or pericholecystic fluid; sonographic Murphy sign was negative. The liver was unremarkable with normal flow in the portal vein.
The patient’s persistent reactive neutrophilic granulation and rising CRP and ESR indicate ongoing inflammation. The largely direct hyperbilirubinemia with hepatitis, minimal findings on ultrasound imaging, and lack of Murphy sign suggest either direct infection of the liver or cholestasis. Viral serologies for EBV, HSV, and CMV should be sent, although these viruses are less commonly associated with oral rash and conjunctivitis. The marked degree of cholestasis makes adenovirus and mycoplasma less likely. Leptospirosis should be considered given the degree of liver injury with potential conjunctival suffusion. However, oral involvement would be atypical; renal injury is absent; and the patient denied pertinent exposures, vomiting, diarrhea, or persistent myalgias.
It is important to know whether the patient continued to receive
Low-grade fevers resolved without intervention. Tests were sent for tick-borne (ehrlichiosis, babesiosis, RMSF, anaplasmosis), viral (EBV, West Nile virus, parvovirus, CMV, coxsackievirus, adenovirus), other bacterial and protozoal (syphilis, Coxiella, leptospirosis, Lyme, Giardia), and autoimmune (antinuclear antibody, perinuclear antineutrophil cytoplasmic antibody, double-stranded DNA) diseases. Topical steroids and antihistamines were prescribed for a suspected viral exanthem. Empiric doxycycline was prescribed to treat possible tick-borne disease, and the patient was discharged home. At home, progressive darkening of the urine was noted. Outpatient testing demonstrated rising ALT to 377 U/L, AST to 183 U/L, total bilirubin to 5.9 mg/dL, direct bilirubin to 3.5 mg/dL, and alkaline phosphatase to 301 U/L. The patient was readmitted for further evaluation.
Despite concerns of the treating physicians, features of this case make tick-borne infections less likely. Lyme disease does not typically cause significant laboratory abnormalities and is classically associated with erythema migrans rather than a mucocutaneous rash. Relapsing fever, ehrlichioses, and rickettsial infections are associated with leukopenia and thrombocytopenia in addition to hepatocellular, rather than cholestatic, liver injury. The lack of response to doxycycline is helpful diagnostically: most tick-borne infections, in addition to leptospirosis, respond well to treatment. While babesiosis, tularemia, and Powassan or Heartland viruses transmitted by ticks are not treated with doxycycline, babesiosis often involves a hemolytic anemia (not seen in this case), and this patient’s laboratory abnormalities and rash are not characteristic of tularemia or viral tick-borne infections.
Either a new or reactivated viral infection with liver inflammation or an autoimmune etiology, specifically KD, remain the most likely etiology of the patient’s symptoms.
He remained asymptomatic during a 6-day hospitalization. His oral lesions resolved. The morbilliform rash coalesced into confluent macules with fine desquamation on the extremities and trunk. There was prominent periungual and palmar/plantar desquamation (Figure 3 and Figure 4). CBC demonstrated hemoglobin of 12.6 g/dL and platelets of 399,000/μL. CRP was undetectable at <0.5 mg/dL; however, ESR increased to 110 mm/h. Transaminases increased to ALT 551 U/L and AST 219 U/L. Serum alkaline phosphatase and bilirubin decreased without intervention. Albumin and total protein remained unchanged. All infectious and autoimmune testing sent from the prior admission returned negative.
An acute-onset viral-like prodrome with fevers potentially responsive to steroids, followed by conjunctivitis, oral erythema and cracked lips, morbilliform rash with hand and foot erythema and edema, cholestatic hepatitis, and subsequent periungual desquamation is highly suggestive of KD. It would be interesting to revisit the patient’s prior episode of aseptic meningitis to see whether any other symptoms were suggestive of KD. While intravenous immunoglobulin (IVIg) and aspirin are standard therapies for the acute febrile phase of KD, the patient is now nearly 2 weeks into his clinical course, rendering their utility uncertain. Nonetheless, screening for coronary aneurysms should be pursued, which may help confirm the diagnosis.
Upon reviewing the evolution of the findings, a diagnosis of adult-onset KD was made. IVIg 2g/kg and aspirin 325 mg were administered. Echocardiogram did not show any evidence of coronary artery aneurysm, myocarditis, pericarditis, wall motion abnormalities, or pericardial effusion. Computed tomography (CT) coronary angiogram confirmed normal coronary arteries without aneurysm. The patient was discharged home without fever on daily aspirin, and all hepatic chemistries and inflammatory markers normalized. Follow-up cardiac magnetic resonance imaging at 3 months and CT angiogram at 6 months remained normal. The patient remains well now 2 years after the original diagnosis and treatment.
DISCUSSION
KD, also known as mucocutaneous lymph node syndrome, is a vasculitis that typically affects children younger than 5 years.1 Having a sibling with KD confers a 10- to 15-fold higher risk, suggesting a genetic component to the disease.2 The highest incidence of KD is in persons of East Asian descent, but KD can affect patients of all races and ethnicities. In the United States, the majority of patients with KD are non-Hispanic White, followed by Black, Hispanic, and Asian.3 The etiology is still unknown, but it is posited that an unidentified, ubiquitous infectious agent may trigger KD in genetically susceptible individuals.4
KD can cause aneurysms and thromboses in medium-sized blood vessels throughout the body.5,6 The classic presentation involves 5 days of high fever plus four or more of the symptoms in the mnemonic CRASH: conjunctival injection, rash (polymorphous), adenopathy (cervical), strawberry tongue (or red, cracked lips and oropharyngeal edema), hand (erythema and induration of hands or feet, followed by periungual desquamation).7 Multiple organ systems may be affected, manifesting as abdominal pain, arthritis, pneumonitis, aseptic meningitis, and acalculous distention of the gallbladder (hydrops).7 The most feared consequence is coronary artery involvement, which leads to aneurysm, thrombosis, and sudden death.
Though no definitive diagnostic test exists, certain laboratory findings support the diagnosis, such as sterile pyuria, thrombocytosis, elevated CRP and ESR, transaminitis, and hypoalbuminemia.7 Diagnosis requires exclusion of illnesses with similar presentations, such as bacterial, viral, and tick-borne infections; drug hypersensitivity reactions; toxic shock syndrome; scarlet fever; juvenile rheumatoid arthritis; and other rheumatologic conditions. Some cases of KD present with fewer than four of the principal (CRASH) symptoms—these are termed “incomplete” KD. The combination of supportive laboratory findings and echocardiogram can facilitate diagnosis of incomplete KD, which carries a similar risk of coronary artery aneurysm.7
Though primarily a disease of childhood, KD can present in adults.8 Adults, compared with children, are less likely to have thrombocytosis and more likely to have cervical adenopathy, arthralgias, and hepatic test abnormalities.8 Although coronary artery aneurysms occur less frequently in adults compared with children, timely diagnosis and treatment is key to preventing this life-threatening complication.8
In children, treatment is IVIg 2 g/kg and aspirin 80 to 100 mg/kg daily until afebrile for several days.9 Some require a second dose of IVIg.9 Children are then maintained on 3 to 5 mg/kg of aspirin daily for 6 to 8 weeks.9 IVIg, given within 10 days of the onset of fever, is highly effective at preventing coronary artery aneurysms.10,11 When coronary aneurysms do occur, treatment is with aspirin or clopidogrel. Very large aneurysms require systemic anticoagulation. After the acute illness, children are monitored with serial cardiac imaging at 2 weeks and 6 to 8 weeks after diagnosis.7 In adults, the optimal imaging timing is unknown. Echocardiography often cannot visualize the coronary arteries, necessitating coronary CT angiography or cardiac MRI.
Despite the presence of classic features, this patient’s diagnosis was delayed because of the rarity of KD in adults and the need to exclude more common diseases. Furthermore, the administration of dexamethasone likely shortened his febrile period and ameliorated some symptoms,12 affecting the natural history of his illness. The diagnosis relied on three components: ruling out common diagnoses, noting two unusual findings (gallbladder hydrops, desquamating periungual rash), and broadening the differential to include adult presentations of childhood disease. Review of the literature suggests very few causes for gallbladder hydrops: impacted stones, cystic fibrosis, cystic duct narrowing due to tumor or lymph nodes, KD, and bacterial and parasitic disease (eg, salmonella, ascariasis). Gallbladder hydrops and periungual desquamation are seen together only in KD.13 Given the complexity of diagnosis in adults, the time to diagnosis is often delayed compared with that for children. While IVIg treatment is preferred within 10 days of the onset of fever, this patient received IVIg on day 14, given the relatively benign nature of IVIg and the considerable morbidity associated with coronary artery aneurysms. Dosing for aspirin is unclear in adults.8 This patient was started on 325 mg aspirin daily. He recovered fully and remains free of coronary changes at two years after initial diagnosis. This case is an excellent reminder that, after exclusion of common diagnoses, reflection on the most unusual aspects of the case and consideration of childhood diseases is particularly important in our younger patients.
TEACHING POINTS
- Extended fever should broaden the differential to include rheumatologic diagnoses.
- KD is rare in adults but can present with classic findings from childhood.
- Early treatment with IVIg and aspirin can be lifesaving in patients with KD, including adults.
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Article in Japanese. Arerugi. 1967;16(3):178-222.
2. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9(4):185-194. https://doi.org/10.1016/j.ijid.2005.03.002
3. Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-488. https://doi.org/10.1097/INF.0b013e3181cf8705
4. Rowley A, Baker S, Arollo D, et al. A hepacivirus-like protein is targeted by the antibody response to Kawasaki disease (KD) [abstract]. Open Forum Infect Dis. 2019;6(suppl 2):S48.
5. Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5(9):e003289. https://doi.org/10.1161/JAHA.116.003289
6. Zhao QM, Chu C, Wu L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. 2019;144(6):e20192254. https://doi.org/10.1542/peds.2019-2254
7. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. https://doi.org/10.1542/peds.2004-2182
8. Sève P, Stankovic K, Smail A, Durand DV, Marchand G, Broussolle C. Adult Kawasaki disease: report of two cases and literature review. Semin Arthritis Rheum. 2005;34(6):785-792. https://doi.org/10.1016/j.semarthrit.2005.01.012
9. Shulman ST. Intravenous immunoglobulin for the treatment of Kawasaki disease. Pediatr Ann. 2017;46(1):e25-e28. https://doi.org/10.3928/19382359-20161212-01
10. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341-347. https://doi.org/10.1056/NEJM198608073150601
11. Rowley AH, Duffy CE, Shulman ST. Prevention of giant coronary artery aneurysms in Kawasaki disease by intravenous gamma globulin therapy. J Pediatr. 1988;113(2):290-294. https://doi/org/10.1016/s0022-3476(88)80267-1
12. Lim YJ, Jung JW. Clinical outcomes of initial dexamethasone treatment combined with a single high dose of intravenous immunoglobulin for primary treatment of Kawasaki disease. Yonsei Med J. 2014;55(5):1260-1266. https://doi.org/10.3349/ymj.2014.55.5.1260
13. Sun Q, Zhang J, Yang Y. Gallbladder hydrops associated with Kawasaki disease: a case report and literature review. Clin Pediatr (Phila). 2018;57(3):341-343. https://doi.org/10.1177/0009922817696468
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Article in Japanese. Arerugi. 1967;16(3):178-222.
2. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9(4):185-194. https://doi.org/10.1016/j.ijid.2005.03.002
3. Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-488. https://doi.org/10.1097/INF.0b013e3181cf8705
4. Rowley A, Baker S, Arollo D, et al. A hepacivirus-like protein is targeted by the antibody response to Kawasaki disease (KD) [abstract]. Open Forum Infect Dis. 2019;6(suppl 2):S48.
5. Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5(9):e003289. https://doi.org/10.1161/JAHA.116.003289
6. Zhao QM, Chu C, Wu L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. 2019;144(6):e20192254. https://doi.org/10.1542/peds.2019-2254
7. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. https://doi.org/10.1542/peds.2004-2182
8. Sève P, Stankovic K, Smail A, Durand DV, Marchand G, Broussolle C. Adult Kawasaki disease: report of two cases and literature review. Semin Arthritis Rheum. 2005;34(6):785-792. https://doi.org/10.1016/j.semarthrit.2005.01.012
9. Shulman ST. Intravenous immunoglobulin for the treatment of Kawasaki disease. Pediatr Ann. 2017;46(1):e25-e28. https://doi.org/10.3928/19382359-20161212-01
10. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341-347. https://doi.org/10.1056/NEJM198608073150601
11. Rowley AH, Duffy CE, Shulman ST. Prevention of giant coronary artery aneurysms in Kawasaki disease by intravenous gamma globulin therapy. J Pediatr. 1988;113(2):290-294. https://doi/org/10.1016/s0022-3476(88)80267-1
12. Lim YJ, Jung JW. Clinical outcomes of initial dexamethasone treatment combined with a single high dose of intravenous immunoglobulin for primary treatment of Kawasaki disease. Yonsei Med J. 2014;55(5):1260-1266. https://doi.org/10.3349/ymj.2014.55.5.1260
13. Sun Q, Zhang J, Yang Y. Gallbladder hydrops associated with Kawasaki disease: a case report and literature review. Clin Pediatr (Phila). 2018;57(3):341-343. https://doi.org/10.1177/0009922817696468
© 2021 Society of Hospital Medicine
Aspiring to Treat Wisely: Challenges in Diagnosing and Optimizing Antibiotic Therapy for Aspiration Pneumonia
In this issue of the Journal of Hospital Medicine, Dr. Thomson and colleagues present an analysis of 4,700 hospitalizations in the Pediatric Health Information System (PHIS) database to compare the effectiveness of different antibiotic regimens for children with neurological impairment and aspiration pneumonia.1 After adjusting for potential confounders, including illness severity markers and demographic factors, they observed that receiving anaerobic coverage was associated with improvements in rates of acute respiratory failure, intensive care unit (ICU) transfer frequency, and length of stay. Given that the authors used an administrative database, several considerations limit the generalizability of the current study. These limitations include that only patients hospitalized at freestanding children’s hospitals were included, the incomplete ability to assess illness severity, and the absence of validated clinical criteria for the diagnosis of aspiration pneumonia. Despite the limitations of a retrospective study using administrative data, the authors should be commended for their rigorous analyses and for their important contribution to the care of this understudied population.
Optimizing appropriate antibiotic therapy for children with suspected aspiration pneumonia is challenging for several reasons. First, previous epidemiological studies demonstrated that viruses cause most pediatric community-acquired pneumonia2; however, we lack tools to identify patients who do not require antibiotic therapy. Second, current clinical guidelines on community-acquired pneumonia do not address aspiration pneumonia diagnosis and management.3 Similar to community-acquired pneumonia, aspiration pneumonia is a clinical diagnosis supported by patient history and laboratory and radiographic data. Given the lack of a gold standard, diagnosis of aspiration pneumonia is difficult to confirm. Previous studies using the PHIS database have demonstrated that, compared with children with nonaspiration pneumonia, those with aspiration pneumonia International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes feature higher rates of mortality, ICU-level care, and 30-day readmission rates.4,5 However, in these studies, patients with an ICD-9-CM code for aspiration pneumonia were also more medically complex, with a higher number of complex chronic conditions and rates of technology use. Lastly, aspiration pneumonia is occasionally synonymous with pneumonia in medically complex patients, which leads to the increased exposure to broad-spectrum antibiotics. The exposure to broad-spectrum antibiotics causes complications, such as Clostridioides difficile infection and potential antibiotic resistance in a patient population that already experiences significant antibiotic exposure.
Growing concerns about antibiotic overuse and the declining prevalence of anaerobic isolates among adult pneumonia patients recently prompted the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) to discourage routine anaerobic coverage among adults with suspected aspiration pneumonia and no abscess or empyema.6 These guidelines overturn years of habit for most adult hospitalists, although the IDSA and ATS acknowledge the extremely low quality of evidence informing the recommendation. Thus, the dilemma is whether the IDSA/ATS guidelines should be reconciled with the conclusions of Thomson et al. The answer is “not necessarily.” Fundamentally, different causes of neurological impairment, such as dementia and stroke, afflict elderly adults with aspiration pneumonia along with important differences in physiological and microbiological exposures. Instead, adult and pediatric hospitalists can find common ground around the shared uncertainty and variability in diagnosing aspiration pneumonia and the need for more credible evidence. Unfortunately, wide variation in diagnosis and coding practices might complicate the efforts to reproduce Thomson’s rigorous retrospective cohort study in large adult databases7 given that Medicare-quality comparison programs may have inadvertently encouraged changes in coding behaviors during the last decade. Attributing pneumonia cases to aspiration removed high-risk patients from reporting cohorts, thus improving a hospital’s apparent mortality rate for community-acquired pneumonia. Although the United States Centers for Medicare & Medicaid Services amended rules in 2017 to address this concern, years of overdiagnosis of aspiration pneumonia possibly biased adult administrative data sets.
Although the association between the use of anaerobic antibiotic coverage and improved pediatric outcomes is promising, these results also point out the need for rigorous prospective studies to improve the evidence base for the diagnosis and treatment of suspected aspiration pneumonia in hospitalized patients of all ages. Given the heterogeneity in the use of aspiration pneumonia diagnoses, foundational work might include assessing the factors that influence clinicians in deciding on the diagnosis of aspiration pneumonia (versus community-acquired pneumonia). On the patient side, parallel trials may start with multicenter, prospective cohort studies to gain insights into the demographic, clinical, and laboratory factors that are associated with the diagnosis of aspiration pneumonia. This research direction may lead to the development and standardization of diagnostic criteria for aspiration pneumonia. Ultimately, prospective randomized controlled trials are needed to assess the comparative effectiveness of different antibiotic choices on clinical outcomes.
1. Thomson J, Hall M, Ambroggio L, et al. Antibiotics for aspiration pneumonia in neurologically impaired children. J Hosp Med. 2020;15(7):395-402. https://doi.org/10.12788/jhm.3338
2. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870
3. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-76. https://doi.org/10.1093/cid/cir531
4. Hirsch AW, Monuteaux MC, Fruchtman G, Bachur RG, Neuman MI. Characteristics of children hospitalized with aspiration pneumonia. Hosp Pediatr. 2016;6(11):659-666. https://doi.org/10.1542/hpeds.2016-0064
5. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):1-10. https://doi.org/10.1542/peds.2015-1612
6. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581ST
7. Lindenauer PK, Strait KM, Grady JN, et al. Variation in the diagnosis of aspiration pneumonia and association with hospital pneumonia outcomes. Ann Am Thorac Soc. 2018;15(5):562-569. https://doi.org/10.1513/AnnalsATS.201709-728OC
In this issue of the Journal of Hospital Medicine, Dr. Thomson and colleagues present an analysis of 4,700 hospitalizations in the Pediatric Health Information System (PHIS) database to compare the effectiveness of different antibiotic regimens for children with neurological impairment and aspiration pneumonia.1 After adjusting for potential confounders, including illness severity markers and demographic factors, they observed that receiving anaerobic coverage was associated with improvements in rates of acute respiratory failure, intensive care unit (ICU) transfer frequency, and length of stay. Given that the authors used an administrative database, several considerations limit the generalizability of the current study. These limitations include that only patients hospitalized at freestanding children’s hospitals were included, the incomplete ability to assess illness severity, and the absence of validated clinical criteria for the diagnosis of aspiration pneumonia. Despite the limitations of a retrospective study using administrative data, the authors should be commended for their rigorous analyses and for their important contribution to the care of this understudied population.
Optimizing appropriate antibiotic therapy for children with suspected aspiration pneumonia is challenging for several reasons. First, previous epidemiological studies demonstrated that viruses cause most pediatric community-acquired pneumonia2; however, we lack tools to identify patients who do not require antibiotic therapy. Second, current clinical guidelines on community-acquired pneumonia do not address aspiration pneumonia diagnosis and management.3 Similar to community-acquired pneumonia, aspiration pneumonia is a clinical diagnosis supported by patient history and laboratory and radiographic data. Given the lack of a gold standard, diagnosis of aspiration pneumonia is difficult to confirm. Previous studies using the PHIS database have demonstrated that, compared with children with nonaspiration pneumonia, those with aspiration pneumonia International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes feature higher rates of mortality, ICU-level care, and 30-day readmission rates.4,5 However, in these studies, patients with an ICD-9-CM code for aspiration pneumonia were also more medically complex, with a higher number of complex chronic conditions and rates of technology use. Lastly, aspiration pneumonia is occasionally synonymous with pneumonia in medically complex patients, which leads to the increased exposure to broad-spectrum antibiotics. The exposure to broad-spectrum antibiotics causes complications, such as Clostridioides difficile infection and potential antibiotic resistance in a patient population that already experiences significant antibiotic exposure.
Growing concerns about antibiotic overuse and the declining prevalence of anaerobic isolates among adult pneumonia patients recently prompted the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) to discourage routine anaerobic coverage among adults with suspected aspiration pneumonia and no abscess or empyema.6 These guidelines overturn years of habit for most adult hospitalists, although the IDSA and ATS acknowledge the extremely low quality of evidence informing the recommendation. Thus, the dilemma is whether the IDSA/ATS guidelines should be reconciled with the conclusions of Thomson et al. The answer is “not necessarily.” Fundamentally, different causes of neurological impairment, such as dementia and stroke, afflict elderly adults with aspiration pneumonia along with important differences in physiological and microbiological exposures. Instead, adult and pediatric hospitalists can find common ground around the shared uncertainty and variability in diagnosing aspiration pneumonia and the need for more credible evidence. Unfortunately, wide variation in diagnosis and coding practices might complicate the efforts to reproduce Thomson’s rigorous retrospective cohort study in large adult databases7 given that Medicare-quality comparison programs may have inadvertently encouraged changes in coding behaviors during the last decade. Attributing pneumonia cases to aspiration removed high-risk patients from reporting cohorts, thus improving a hospital’s apparent mortality rate for community-acquired pneumonia. Although the United States Centers for Medicare & Medicaid Services amended rules in 2017 to address this concern, years of overdiagnosis of aspiration pneumonia possibly biased adult administrative data sets.
Although the association between the use of anaerobic antibiotic coverage and improved pediatric outcomes is promising, these results also point out the need for rigorous prospective studies to improve the evidence base for the diagnosis and treatment of suspected aspiration pneumonia in hospitalized patients of all ages. Given the heterogeneity in the use of aspiration pneumonia diagnoses, foundational work might include assessing the factors that influence clinicians in deciding on the diagnosis of aspiration pneumonia (versus community-acquired pneumonia). On the patient side, parallel trials may start with multicenter, prospective cohort studies to gain insights into the demographic, clinical, and laboratory factors that are associated with the diagnosis of aspiration pneumonia. This research direction may lead to the development and standardization of diagnostic criteria for aspiration pneumonia. Ultimately, prospective randomized controlled trials are needed to assess the comparative effectiveness of different antibiotic choices on clinical outcomes.
In this issue of the Journal of Hospital Medicine, Dr. Thomson and colleagues present an analysis of 4,700 hospitalizations in the Pediatric Health Information System (PHIS) database to compare the effectiveness of different antibiotic regimens for children with neurological impairment and aspiration pneumonia.1 After adjusting for potential confounders, including illness severity markers and demographic factors, they observed that receiving anaerobic coverage was associated with improvements in rates of acute respiratory failure, intensive care unit (ICU) transfer frequency, and length of stay. Given that the authors used an administrative database, several considerations limit the generalizability of the current study. These limitations include that only patients hospitalized at freestanding children’s hospitals were included, the incomplete ability to assess illness severity, and the absence of validated clinical criteria for the diagnosis of aspiration pneumonia. Despite the limitations of a retrospective study using administrative data, the authors should be commended for their rigorous analyses and for their important contribution to the care of this understudied population.
Optimizing appropriate antibiotic therapy for children with suspected aspiration pneumonia is challenging for several reasons. First, previous epidemiological studies demonstrated that viruses cause most pediatric community-acquired pneumonia2; however, we lack tools to identify patients who do not require antibiotic therapy. Second, current clinical guidelines on community-acquired pneumonia do not address aspiration pneumonia diagnosis and management.3 Similar to community-acquired pneumonia, aspiration pneumonia is a clinical diagnosis supported by patient history and laboratory and radiographic data. Given the lack of a gold standard, diagnosis of aspiration pneumonia is difficult to confirm. Previous studies using the PHIS database have demonstrated that, compared with children with nonaspiration pneumonia, those with aspiration pneumonia International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes feature higher rates of mortality, ICU-level care, and 30-day readmission rates.4,5 However, in these studies, patients with an ICD-9-CM code for aspiration pneumonia were also more medically complex, with a higher number of complex chronic conditions and rates of technology use. Lastly, aspiration pneumonia is occasionally synonymous with pneumonia in medically complex patients, which leads to the increased exposure to broad-spectrum antibiotics. The exposure to broad-spectrum antibiotics causes complications, such as Clostridioides difficile infection and potential antibiotic resistance in a patient population that already experiences significant antibiotic exposure.
Growing concerns about antibiotic overuse and the declining prevalence of anaerobic isolates among adult pneumonia patients recently prompted the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) to discourage routine anaerobic coverage among adults with suspected aspiration pneumonia and no abscess or empyema.6 These guidelines overturn years of habit for most adult hospitalists, although the IDSA and ATS acknowledge the extremely low quality of evidence informing the recommendation. Thus, the dilemma is whether the IDSA/ATS guidelines should be reconciled with the conclusions of Thomson et al. The answer is “not necessarily.” Fundamentally, different causes of neurological impairment, such as dementia and stroke, afflict elderly adults with aspiration pneumonia along with important differences in physiological and microbiological exposures. Instead, adult and pediatric hospitalists can find common ground around the shared uncertainty and variability in diagnosing aspiration pneumonia and the need for more credible evidence. Unfortunately, wide variation in diagnosis and coding practices might complicate the efforts to reproduce Thomson’s rigorous retrospective cohort study in large adult databases7 given that Medicare-quality comparison programs may have inadvertently encouraged changes in coding behaviors during the last decade. Attributing pneumonia cases to aspiration removed high-risk patients from reporting cohorts, thus improving a hospital’s apparent mortality rate for community-acquired pneumonia. Although the United States Centers for Medicare & Medicaid Services amended rules in 2017 to address this concern, years of overdiagnosis of aspiration pneumonia possibly biased adult administrative data sets.
Although the association between the use of anaerobic antibiotic coverage and improved pediatric outcomes is promising, these results also point out the need for rigorous prospective studies to improve the evidence base for the diagnosis and treatment of suspected aspiration pneumonia in hospitalized patients of all ages. Given the heterogeneity in the use of aspiration pneumonia diagnoses, foundational work might include assessing the factors that influence clinicians in deciding on the diagnosis of aspiration pneumonia (versus community-acquired pneumonia). On the patient side, parallel trials may start with multicenter, prospective cohort studies to gain insights into the demographic, clinical, and laboratory factors that are associated with the diagnosis of aspiration pneumonia. This research direction may lead to the development and standardization of diagnostic criteria for aspiration pneumonia. Ultimately, prospective randomized controlled trials are needed to assess the comparative effectiveness of different antibiotic choices on clinical outcomes.
1. Thomson J, Hall M, Ambroggio L, et al. Antibiotics for aspiration pneumonia in neurologically impaired children. J Hosp Med. 2020;15(7):395-402. https://doi.org/10.12788/jhm.3338
2. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870
3. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-76. https://doi.org/10.1093/cid/cir531
4. Hirsch AW, Monuteaux MC, Fruchtman G, Bachur RG, Neuman MI. Characteristics of children hospitalized with aspiration pneumonia. Hosp Pediatr. 2016;6(11):659-666. https://doi.org/10.1542/hpeds.2016-0064
5. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):1-10. https://doi.org/10.1542/peds.2015-1612
6. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581ST
7. Lindenauer PK, Strait KM, Grady JN, et al. Variation in the diagnosis of aspiration pneumonia and association with hospital pneumonia outcomes. Ann Am Thorac Soc. 2018;15(5):562-569. https://doi.org/10.1513/AnnalsATS.201709-728OC
1. Thomson J, Hall M, Ambroggio L, et al. Antibiotics for aspiration pneumonia in neurologically impaired children. J Hosp Med. 2020;15(7):395-402. https://doi.org/10.12788/jhm.3338
2. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870
3. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-76. https://doi.org/10.1093/cid/cir531
4. Hirsch AW, Monuteaux MC, Fruchtman G, Bachur RG, Neuman MI. Characteristics of children hospitalized with aspiration pneumonia. Hosp Pediatr. 2016;6(11):659-666. https://doi.org/10.1542/hpeds.2016-0064
5. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):1-10. https://doi.org/10.1542/peds.2015-1612
6. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581ST
7. Lindenauer PK, Strait KM, Grady JN, et al. Variation in the diagnosis of aspiration pneumonia and association with hospital pneumonia outcomes. Ann Am Thorac Soc. 2018;15(5):562-569. https://doi.org/10.1513/AnnalsATS.201709-728OC
© 2020 Society of Hospital Medicine
Hospitalists as Triagists: Description of the Triagist Role across Academic Medical Centers
Hospital medicine has grown dramatically over the past 20 years.1,2 A recent survey regarding hospitalists’ clinical roles showed an expansion to triaging emergency department (ED) medical admissions and transfers from outside hospitals.3 From the hospitalist perspective, triaging involves the evaluation of patients for potential admission.4 With scrutiny on ED metrics, such as wait times (https://www.medicare.gov/hospitalcompare/search.html), health system administrators have heightened expectations for efficient patient flow, which increasingly falls to hospitalists.5-7
Despite the growth in hospitalists’ triagist activities, there has been little formal assessment of their role. We hypothesized that this role differs from inpatient care in significant ways.6-8 We sought to describe the triagist role in adult academic inpatient medicine settings to understand the responsibilities and skill set required.
METHODS
Ten academic medical center (AMC) sites were recruited from Research Committee session attendees at the 2014 Society of Hospital Medicine national meeting and the 2014 Society of General Internal Medicine southern regional meeting. The AMCs were geographically diverse: three Western, two Midwestern, two Southern, one Northeastern, and two Southeastern. Site representatives were identified and completed a web-based questionnaire about their AMC (see Appendix 1 for the information collected). Clarifications regarding survey responses were performed via conference calls between the authors (STV, ESW) and site representatives.
Hospitalist Survey
In January 2018, surveys were sent to 583 physicians who worked as triagists. Participants received an anonymous 28-item RedCap survey by e-mail and were sent up to five reminder e-mails over six weeks (see Appendix 2 for the questions analyzed in this paper). Respondents were given the option to be entered in a gift card drawing.
Demographic information and individual workflow/practices were obtained. A 5-point Likert scale (strongly disagree – strongly agree) was used to assess hospitalists’ concurrence with current providers (eg, ED, clinic providers) regarding the management and whether patients must meet the utilization management (UM) criteria for admission. Time estimates used 5% increments and were categorized into four frequency categories based on the local modes provided in responses: Seldom (0%-10%), Occasional (15%-35%), Half-the-Time (40%-60%), and Frequently (65%-100%). Free text responses on effective/ineffective triagist qualities were elicited. Responses were included for analysis if at least 70% of questions were completed.
Data Analysis
Quantitative
Descriptive statistics were calculated for each variable. The Kruskal-Wallis test was used to evaluate differences across AMCs in the time spent on in-person evaluation and communication. Weighting, based on the ratio of hospitalists to survey respondents at each AMC, was used to calculate the average institutional percentages across the study sample.
Qualitative
Responses to open-ended questions were analyzed using thematic analysis.9 Three independent reviewers (STV, JC, ESW) read, analyzed, and grouped the responses by codes. Codes were then assessed for overlap and grouped into themes by one reviewer (STV). A table of themes with supporting quotes and the number of mentions was subsequently developed by all three reviewers. Similar themes were combined to create domains. The domains were reviewed by the steering committee members to create a consensus description (Appendix 3).
The University of Texas Health San Antonio’s Institutional Review Board and participating institutions approved the study as exempt.
RESULTS
Site Characteristics
Representatives from 10 AMCs reported data on a range of one to four hospitals for a total of 22 hospitals. The median reported that the number of medical patients admitted in a 24-hour period was 31-40 (range, 11-20 to >50). The median group size of hospitalists was 41-50 (range, 0-10 to >70).
The survey response rate was 40% (n = 235), ranging from 9%-70% between institutions. Self-identified female hospitalists accounted for 52% of respondents. Four percent were 25-29 years old, 66% were 30-39 years old, 24% were 40-49 years old, and 6% were ≥50 years old. The average clinical time spent as a triagist was 16%.
Description of Triagist Activities
The activities identified by the majority of respondents across all sites included transferring patients within the hospital (73%), and assessing/approving patient transfers from outside hospitals and clinics (82%). Internal transfer activities reported by >50% of respondents included allocating patients within the hospital or bed capacity coordination, assessing intensive care unit transfers, assigning ED admissions, and consulting other services. The ED accounted for an average of 55% of calls received. Respondents also reported being involved with the documentation related to these activities.
Similarities and Differences across AMCs
Two AMCs did not have a dedicated triagist; instead, physicians supervised residents and advanced practice providers. Among the eight sites with triagists, triaging was predominantly done by faculty physicians contacted via pagers. At seven of these sites, 100% of hospitalists worked as triagists. The triage service was covered by faculty physicians from 8-24 hours per day.
Bed boards and transfer centers staffed by registered nurses, nurse coordinators, house supervisors, or physicians were common support systems, though this infrastructure was organized differently across institutions. A UM review before admission was performed at three institutions 24 hours/day. The remaining institutions reviewed patients retrospectively.
Twenty-eight percent of hospitalists across all sites “Disagreed” or “Strongly disagreed” that a patient must meet UM criteria for admission. Forty-two percent had “Frequent” different opinions regarding patient management than the consulting provider.
Triagist and current provider communication practices varied widely across AMCs (Figure). There was significant variability in verbal communication (P = .02), with >70% of respondents at two AMCs reporting verbal communication at least half the time, but <30% reporting this frequency at two other AMCs. Respondents reported variable use of electronic communication (ie, notes/orders in the electronic health record) across AMCs (
The practice of evaluating patients in person also varied significantly across AMCs (P < .0001, Figure). Across hospitalists, only 28% see patients in person about “Half-the-Time” or more.
Differences within AMCs
Variability within AMCs was greatest for the rate of verbal communication practices, with a typical interquartile range (IQR) of 20% to 90% among the hospitalists within a given AMC and for the rate of electronic communication with a typical IQR of 0% to 50%. For other survey questions, the IQR was typically 15 to 20 percentage points.
Thematic Analysis
We received 207 and 203 responses (88% and 86%, respectively) to the open-ended questions “What qualities does an effective triagist have?’ and ‘What qualities make a triagist ineffective?” We identified 22 themes for effective and ineffective qualities, which were grouped into seven domains (Table). All themes had at least three mentions by respondents. The three most frequently mentioned themes, communication skills, efficiency, and systems knowledge, had greater than 60 mentions.
DISCUSSION
Our study of the triagist role at 10 AMCs describes critical triagist functions and identifies key findings across and within AMCs. Twenty-eight percent of hospitalists reported admitting patients even when the patient did not meet the admission criteria, consistent with previous research demonstrating the influence of factors other than clinical disease severity on triage decisions.10 However, preventable admissions remain a hospital-level quality metric.11,12 Triagists must often balance each patient’s circumstances with the complexities of the system. Juggling the competing demands of the system while providing patient-centered care can be challenging and may explain why attending physicians are more frequently filling this role.13
Local context/culture is likely to play a role in the variation across sites; however, compensation for the time spent may also be a factor. If triage activities are not reimbursable, this could lead to less documentation and a lower likelihood that patients are evaluated in person.14 This reason may also explain why all hospitalists were required to serve as a triagist at most sites.
Currently, no consensus definition of the triagist role has been developed. Our results demonstrate that this role is heterogeneous and grounded in the local healthcare system practices. We propose the following working definition of the triagist: a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting. A triagist should be equipped with a skill set that includes not only clinical knowledge but also emphasizes systems knowledge, awareness of others’ goals, efficiency, an ability to communicate effectively, and the knowledge of UM. We recommend that medical directors of hospitalist programs focus their attention on locally specific, systems-based skills development when orienting new hospitalists. The financial aspects of cost should be considered and delineated as well.
Our analysis is limited in several respects. Participant AMCs were not randomly chosen, but do represent a broad array of facility types, group size, and geographic regions. The low response rates at some AMCs may result in an inaccurate representation of those sites. Data was not obtained on hospitalists that did not respond to the survey; therefore, nonresponse bias may affect outcomes. This research used self-report rather than direct observation, which could be subject to recall and social desirability bias. Finally, our results may not be generalizable to nonacademic institutions.
CONCLUSION
The hospitalist role as triagist at AMCs emphasizes communication, organizational skills, efficiency, systems-based practice, and UM knowledge. Although we found significant variation across and within AMCs, internal transfer activities were common across programs. Hospitalist programs should focus on systems-based skills development to prepare hospitalists for the role. The skill set necessary for triagist responsibilities also has implications for internal medicine resident education.4 With increasing emphasis on value and system effectiveness in care delivery, further studies of the triagist role should be undertaken.
Acknowledgments
The TRIAGIST Collaborative Group consists of: Maralyssa Bann, MD, Andrew White, MD (University of Washington); Jagriti Chadha, MD (University of Kentucky); Joel Boggan, MD (Duke University); Sherwin Hsu, MD (UCLA); Jeff Liao, MD (Harvard Medical School); Tabatha Matthias, DO (University of Nebraska Medical Center); Tresa McNeal, MD (Scott and White Texas A&M); Roxana Naderi, MD, Khooshbu Shah, MD (University of Colorado); David Schmit, MD (University of Texas Health San Antonio); Manivannan Veerasamy, MD (Michigan State University).
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the po
1. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015; 42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003.
2. Wachter, RM, Goldman, L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11): 1009-1011. https://doi.org/10.1056/NEJMp1607958.
3. Vasilevskis EE, Knebel RJ, Wachter RM, Auerbach AD. California hospital leaders’ views of hospitalists: meeting needs of the present and future. J Hosp Med. 2009;4:528-534. https://doi.org/10.1002/jhm.529.
4. Wang ES, Velásquez ST, Smith CJ, et al. Triaging inpatient admissions: an opportunity for resident education. J Gen Intern Med. 2019; 34(5):754-757. https://doi.org/10.1007/s11606-019-04882-2.
5. Briones A, Markoff B, Kathuria N, et al. A model of a hospitalist role in the care of admitted patients in the emergency department. J Hosp Med. 2010;5(6):360-364. https://doi.org/10.1002/jhm.636.
6. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. https://doi.org/10.1111/j.1525-1497.2004.30431.x.
7. Howell E, Bessman E, Marshall R, Wright S. Hospitalist bed management effecting throughput from the emergency department to the intensive care unit. J Crit Care. 2010;25:184-189. https://doi.org/10.1016/j.jcrc.2009.08.004.
8. Chadaga SR, Shockley L, Keniston A, et al. Hospitalist-led medicine emergency department team: associations with throughput, timeliness of patient care, and satisfaction. J Hosp Med. 2012;7:562-566. https://doi.org/10.1002/jhm.1957.
9. Braun, V. Clarke, V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;77-101. https://doi.org/10.1191/1478088706qp063oa.
10. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of non-critically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
11. Patel KK, Vakharia N, Pile J, Howell EH, Rothberg MB. Preventable admissions on a general medicine service: prevalence, causes and comparison with AHRQ prevention quality indicators-a cross-sectional analysis. J Gen Intern Med. 2016;31(6):597-601. https://doi.org/10.1007/s11606-016-3615-4.
12. Daniels LM1, Sorita A2, Kashiwagi DT, et al. Characterizing potentially preventable admissions: a mixed methods study of rates, associated factors, outcomes, and physician decision-making. J Gen Intern Med. 2018;33(5):737-744. https://doi.org/10.1007/s11606-017-4285-6.
13. Howard-Anderson J, Lonowski S, Vangala S, Tseng CH, Busuttil A, Afsar-Manesh N. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870-1872. https://doi.org/10.1001/jamainternmed.2014.4782.
14. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB, Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
Hospital medicine has grown dramatically over the past 20 years.1,2 A recent survey regarding hospitalists’ clinical roles showed an expansion to triaging emergency department (ED) medical admissions and transfers from outside hospitals.3 From the hospitalist perspective, triaging involves the evaluation of patients for potential admission.4 With scrutiny on ED metrics, such as wait times (https://www.medicare.gov/hospitalcompare/search.html), health system administrators have heightened expectations for efficient patient flow, which increasingly falls to hospitalists.5-7
Despite the growth in hospitalists’ triagist activities, there has been little formal assessment of their role. We hypothesized that this role differs from inpatient care in significant ways.6-8 We sought to describe the triagist role in adult academic inpatient medicine settings to understand the responsibilities and skill set required.
METHODS
Ten academic medical center (AMC) sites were recruited from Research Committee session attendees at the 2014 Society of Hospital Medicine national meeting and the 2014 Society of General Internal Medicine southern regional meeting. The AMCs were geographically diverse: three Western, two Midwestern, two Southern, one Northeastern, and two Southeastern. Site representatives were identified and completed a web-based questionnaire about their AMC (see Appendix 1 for the information collected). Clarifications regarding survey responses were performed via conference calls between the authors (STV, ESW) and site representatives.
Hospitalist Survey
In January 2018, surveys were sent to 583 physicians who worked as triagists. Participants received an anonymous 28-item RedCap survey by e-mail and were sent up to five reminder e-mails over six weeks (see Appendix 2 for the questions analyzed in this paper). Respondents were given the option to be entered in a gift card drawing.
Demographic information and individual workflow/practices were obtained. A 5-point Likert scale (strongly disagree – strongly agree) was used to assess hospitalists’ concurrence with current providers (eg, ED, clinic providers) regarding the management and whether patients must meet the utilization management (UM) criteria for admission. Time estimates used 5% increments and were categorized into four frequency categories based on the local modes provided in responses: Seldom (0%-10%), Occasional (15%-35%), Half-the-Time (40%-60%), and Frequently (65%-100%). Free text responses on effective/ineffective triagist qualities were elicited. Responses were included for analysis if at least 70% of questions were completed.
Data Analysis
Quantitative
Descriptive statistics were calculated for each variable. The Kruskal-Wallis test was used to evaluate differences across AMCs in the time spent on in-person evaluation and communication. Weighting, based on the ratio of hospitalists to survey respondents at each AMC, was used to calculate the average institutional percentages across the study sample.
Qualitative
Responses to open-ended questions were analyzed using thematic analysis.9 Three independent reviewers (STV, JC, ESW) read, analyzed, and grouped the responses by codes. Codes were then assessed for overlap and grouped into themes by one reviewer (STV). A table of themes with supporting quotes and the number of mentions was subsequently developed by all three reviewers. Similar themes were combined to create domains. The domains were reviewed by the steering committee members to create a consensus description (Appendix 3).
The University of Texas Health San Antonio’s Institutional Review Board and participating institutions approved the study as exempt.
RESULTS
Site Characteristics
Representatives from 10 AMCs reported data on a range of one to four hospitals for a total of 22 hospitals. The median reported that the number of medical patients admitted in a 24-hour period was 31-40 (range, 11-20 to >50). The median group size of hospitalists was 41-50 (range, 0-10 to >70).
The survey response rate was 40% (n = 235), ranging from 9%-70% between institutions. Self-identified female hospitalists accounted for 52% of respondents. Four percent were 25-29 years old, 66% were 30-39 years old, 24% were 40-49 years old, and 6% were ≥50 years old. The average clinical time spent as a triagist was 16%.
Description of Triagist Activities
The activities identified by the majority of respondents across all sites included transferring patients within the hospital (73%), and assessing/approving patient transfers from outside hospitals and clinics (82%). Internal transfer activities reported by >50% of respondents included allocating patients within the hospital or bed capacity coordination, assessing intensive care unit transfers, assigning ED admissions, and consulting other services. The ED accounted for an average of 55% of calls received. Respondents also reported being involved with the documentation related to these activities.
Similarities and Differences across AMCs
Two AMCs did not have a dedicated triagist; instead, physicians supervised residents and advanced practice providers. Among the eight sites with triagists, triaging was predominantly done by faculty physicians contacted via pagers. At seven of these sites, 100% of hospitalists worked as triagists. The triage service was covered by faculty physicians from 8-24 hours per day.
Bed boards and transfer centers staffed by registered nurses, nurse coordinators, house supervisors, or physicians were common support systems, though this infrastructure was organized differently across institutions. A UM review before admission was performed at three institutions 24 hours/day. The remaining institutions reviewed patients retrospectively.
Twenty-eight percent of hospitalists across all sites “Disagreed” or “Strongly disagreed” that a patient must meet UM criteria for admission. Forty-two percent had “Frequent” different opinions regarding patient management than the consulting provider.
Triagist and current provider communication practices varied widely across AMCs (Figure). There was significant variability in verbal communication (P = .02), with >70% of respondents at two AMCs reporting verbal communication at least half the time, but <30% reporting this frequency at two other AMCs. Respondents reported variable use of electronic communication (ie, notes/orders in the electronic health record) across AMCs (
The practice of evaluating patients in person also varied significantly across AMCs (P < .0001, Figure). Across hospitalists, only 28% see patients in person about “Half-the-Time” or more.
Differences within AMCs
Variability within AMCs was greatest for the rate of verbal communication practices, with a typical interquartile range (IQR) of 20% to 90% among the hospitalists within a given AMC and for the rate of electronic communication with a typical IQR of 0% to 50%. For other survey questions, the IQR was typically 15 to 20 percentage points.
Thematic Analysis
We received 207 and 203 responses (88% and 86%, respectively) to the open-ended questions “What qualities does an effective triagist have?’ and ‘What qualities make a triagist ineffective?” We identified 22 themes for effective and ineffective qualities, which were grouped into seven domains (Table). All themes had at least three mentions by respondents. The three most frequently mentioned themes, communication skills, efficiency, and systems knowledge, had greater than 60 mentions.
DISCUSSION
Our study of the triagist role at 10 AMCs describes critical triagist functions and identifies key findings across and within AMCs. Twenty-eight percent of hospitalists reported admitting patients even when the patient did not meet the admission criteria, consistent with previous research demonstrating the influence of factors other than clinical disease severity on triage decisions.10 However, preventable admissions remain a hospital-level quality metric.11,12 Triagists must often balance each patient’s circumstances with the complexities of the system. Juggling the competing demands of the system while providing patient-centered care can be challenging and may explain why attending physicians are more frequently filling this role.13
Local context/culture is likely to play a role in the variation across sites; however, compensation for the time spent may also be a factor. If triage activities are not reimbursable, this could lead to less documentation and a lower likelihood that patients are evaluated in person.14 This reason may also explain why all hospitalists were required to serve as a triagist at most sites.
Currently, no consensus definition of the triagist role has been developed. Our results demonstrate that this role is heterogeneous and grounded in the local healthcare system practices. We propose the following working definition of the triagist: a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting. A triagist should be equipped with a skill set that includes not only clinical knowledge but also emphasizes systems knowledge, awareness of others’ goals, efficiency, an ability to communicate effectively, and the knowledge of UM. We recommend that medical directors of hospitalist programs focus their attention on locally specific, systems-based skills development when orienting new hospitalists. The financial aspects of cost should be considered and delineated as well.
Our analysis is limited in several respects. Participant AMCs were not randomly chosen, but do represent a broad array of facility types, group size, and geographic regions. The low response rates at some AMCs may result in an inaccurate representation of those sites. Data was not obtained on hospitalists that did not respond to the survey; therefore, nonresponse bias may affect outcomes. This research used self-report rather than direct observation, which could be subject to recall and social desirability bias. Finally, our results may not be generalizable to nonacademic institutions.
CONCLUSION
The hospitalist role as triagist at AMCs emphasizes communication, organizational skills, efficiency, systems-based practice, and UM knowledge. Although we found significant variation across and within AMCs, internal transfer activities were common across programs. Hospitalist programs should focus on systems-based skills development to prepare hospitalists for the role. The skill set necessary for triagist responsibilities also has implications for internal medicine resident education.4 With increasing emphasis on value and system effectiveness in care delivery, further studies of the triagist role should be undertaken.
Acknowledgments
The TRIAGIST Collaborative Group consists of: Maralyssa Bann, MD, Andrew White, MD (University of Washington); Jagriti Chadha, MD (University of Kentucky); Joel Boggan, MD (Duke University); Sherwin Hsu, MD (UCLA); Jeff Liao, MD (Harvard Medical School); Tabatha Matthias, DO (University of Nebraska Medical Center); Tresa McNeal, MD (Scott and White Texas A&M); Roxana Naderi, MD, Khooshbu Shah, MD (University of Colorado); David Schmit, MD (University of Texas Health San Antonio); Manivannan Veerasamy, MD (Michigan State University).
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the po
Hospital medicine has grown dramatically over the past 20 years.1,2 A recent survey regarding hospitalists’ clinical roles showed an expansion to triaging emergency department (ED) medical admissions and transfers from outside hospitals.3 From the hospitalist perspective, triaging involves the evaluation of patients for potential admission.4 With scrutiny on ED metrics, such as wait times (https://www.medicare.gov/hospitalcompare/search.html), health system administrators have heightened expectations for efficient patient flow, which increasingly falls to hospitalists.5-7
Despite the growth in hospitalists’ triagist activities, there has been little formal assessment of their role. We hypothesized that this role differs from inpatient care in significant ways.6-8 We sought to describe the triagist role in adult academic inpatient medicine settings to understand the responsibilities and skill set required.
METHODS
Ten academic medical center (AMC) sites were recruited from Research Committee session attendees at the 2014 Society of Hospital Medicine national meeting and the 2014 Society of General Internal Medicine southern regional meeting. The AMCs were geographically diverse: three Western, two Midwestern, two Southern, one Northeastern, and two Southeastern. Site representatives were identified and completed a web-based questionnaire about their AMC (see Appendix 1 for the information collected). Clarifications regarding survey responses were performed via conference calls between the authors (STV, ESW) and site representatives.
Hospitalist Survey
In January 2018, surveys were sent to 583 physicians who worked as triagists. Participants received an anonymous 28-item RedCap survey by e-mail and were sent up to five reminder e-mails over six weeks (see Appendix 2 for the questions analyzed in this paper). Respondents were given the option to be entered in a gift card drawing.
Demographic information and individual workflow/practices were obtained. A 5-point Likert scale (strongly disagree – strongly agree) was used to assess hospitalists’ concurrence with current providers (eg, ED, clinic providers) regarding the management and whether patients must meet the utilization management (UM) criteria for admission. Time estimates used 5% increments and were categorized into four frequency categories based on the local modes provided in responses: Seldom (0%-10%), Occasional (15%-35%), Half-the-Time (40%-60%), and Frequently (65%-100%). Free text responses on effective/ineffective triagist qualities were elicited. Responses were included for analysis if at least 70% of questions were completed.
Data Analysis
Quantitative
Descriptive statistics were calculated for each variable. The Kruskal-Wallis test was used to evaluate differences across AMCs in the time spent on in-person evaluation and communication. Weighting, based on the ratio of hospitalists to survey respondents at each AMC, was used to calculate the average institutional percentages across the study sample.
Qualitative
Responses to open-ended questions were analyzed using thematic analysis.9 Three independent reviewers (STV, JC, ESW) read, analyzed, and grouped the responses by codes. Codes were then assessed for overlap and grouped into themes by one reviewer (STV). A table of themes with supporting quotes and the number of mentions was subsequently developed by all three reviewers. Similar themes were combined to create domains. The domains were reviewed by the steering committee members to create a consensus description (Appendix 3).
The University of Texas Health San Antonio’s Institutional Review Board and participating institutions approved the study as exempt.
RESULTS
Site Characteristics
Representatives from 10 AMCs reported data on a range of one to four hospitals for a total of 22 hospitals. The median reported that the number of medical patients admitted in a 24-hour period was 31-40 (range, 11-20 to >50). The median group size of hospitalists was 41-50 (range, 0-10 to >70).
The survey response rate was 40% (n = 235), ranging from 9%-70% between institutions. Self-identified female hospitalists accounted for 52% of respondents. Four percent were 25-29 years old, 66% were 30-39 years old, 24% were 40-49 years old, and 6% were ≥50 years old. The average clinical time spent as a triagist was 16%.
Description of Triagist Activities
The activities identified by the majority of respondents across all sites included transferring patients within the hospital (73%), and assessing/approving patient transfers from outside hospitals and clinics (82%). Internal transfer activities reported by >50% of respondents included allocating patients within the hospital or bed capacity coordination, assessing intensive care unit transfers, assigning ED admissions, and consulting other services. The ED accounted for an average of 55% of calls received. Respondents also reported being involved with the documentation related to these activities.
Similarities and Differences across AMCs
Two AMCs did not have a dedicated triagist; instead, physicians supervised residents and advanced practice providers. Among the eight sites with triagists, triaging was predominantly done by faculty physicians contacted via pagers. At seven of these sites, 100% of hospitalists worked as triagists. The triage service was covered by faculty physicians from 8-24 hours per day.
Bed boards and transfer centers staffed by registered nurses, nurse coordinators, house supervisors, or physicians were common support systems, though this infrastructure was organized differently across institutions. A UM review before admission was performed at three institutions 24 hours/day. The remaining institutions reviewed patients retrospectively.
Twenty-eight percent of hospitalists across all sites “Disagreed” or “Strongly disagreed” that a patient must meet UM criteria for admission. Forty-two percent had “Frequent” different opinions regarding patient management than the consulting provider.
Triagist and current provider communication practices varied widely across AMCs (Figure). There was significant variability in verbal communication (P = .02), with >70% of respondents at two AMCs reporting verbal communication at least half the time, but <30% reporting this frequency at two other AMCs. Respondents reported variable use of electronic communication (ie, notes/orders in the electronic health record) across AMCs (
The practice of evaluating patients in person also varied significantly across AMCs (P < .0001, Figure). Across hospitalists, only 28% see patients in person about “Half-the-Time” or more.
Differences within AMCs
Variability within AMCs was greatest for the rate of verbal communication practices, with a typical interquartile range (IQR) of 20% to 90% among the hospitalists within a given AMC and for the rate of electronic communication with a typical IQR of 0% to 50%. For other survey questions, the IQR was typically 15 to 20 percentage points.
Thematic Analysis
We received 207 and 203 responses (88% and 86%, respectively) to the open-ended questions “What qualities does an effective triagist have?’ and ‘What qualities make a triagist ineffective?” We identified 22 themes for effective and ineffective qualities, which were grouped into seven domains (Table). All themes had at least three mentions by respondents. The three most frequently mentioned themes, communication skills, efficiency, and systems knowledge, had greater than 60 mentions.
DISCUSSION
Our study of the triagist role at 10 AMCs describes critical triagist functions and identifies key findings across and within AMCs. Twenty-eight percent of hospitalists reported admitting patients even when the patient did not meet the admission criteria, consistent with previous research demonstrating the influence of factors other than clinical disease severity on triage decisions.10 However, preventable admissions remain a hospital-level quality metric.11,12 Triagists must often balance each patient’s circumstances with the complexities of the system. Juggling the competing demands of the system while providing patient-centered care can be challenging and may explain why attending physicians are more frequently filling this role.13
Local context/culture is likely to play a role in the variation across sites; however, compensation for the time spent may also be a factor. If triage activities are not reimbursable, this could lead to less documentation and a lower likelihood that patients are evaluated in person.14 This reason may also explain why all hospitalists were required to serve as a triagist at most sites.
Currently, no consensus definition of the triagist role has been developed. Our results demonstrate that this role is heterogeneous and grounded in the local healthcare system practices. We propose the following working definition of the triagist: a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting. A triagist should be equipped with a skill set that includes not only clinical knowledge but also emphasizes systems knowledge, awareness of others’ goals, efficiency, an ability to communicate effectively, and the knowledge of UM. We recommend that medical directors of hospitalist programs focus their attention on locally specific, systems-based skills development when orienting new hospitalists. The financial aspects of cost should be considered and delineated as well.
Our analysis is limited in several respects. Participant AMCs were not randomly chosen, but do represent a broad array of facility types, group size, and geographic regions. The low response rates at some AMCs may result in an inaccurate representation of those sites. Data was not obtained on hospitalists that did not respond to the survey; therefore, nonresponse bias may affect outcomes. This research used self-report rather than direct observation, which could be subject to recall and social desirability bias. Finally, our results may not be generalizable to nonacademic institutions.
CONCLUSION
The hospitalist role as triagist at AMCs emphasizes communication, organizational skills, efficiency, systems-based practice, and UM knowledge. Although we found significant variation across and within AMCs, internal transfer activities were common across programs. Hospitalist programs should focus on systems-based skills development to prepare hospitalists for the role. The skill set necessary for triagist responsibilities also has implications for internal medicine resident education.4 With increasing emphasis on value and system effectiveness in care delivery, further studies of the triagist role should be undertaken.
Acknowledgments
The TRIAGIST Collaborative Group consists of: Maralyssa Bann, MD, Andrew White, MD (University of Washington); Jagriti Chadha, MD (University of Kentucky); Joel Boggan, MD (Duke University); Sherwin Hsu, MD (UCLA); Jeff Liao, MD (Harvard Medical School); Tabatha Matthias, DO (University of Nebraska Medical Center); Tresa McNeal, MD (Scott and White Texas A&M); Roxana Naderi, MD, Khooshbu Shah, MD (University of Colorado); David Schmit, MD (University of Texas Health San Antonio); Manivannan Veerasamy, MD (Michigan State University).
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the po
1. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015; 42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003.
2. Wachter, RM, Goldman, L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11): 1009-1011. https://doi.org/10.1056/NEJMp1607958.
3. Vasilevskis EE, Knebel RJ, Wachter RM, Auerbach AD. California hospital leaders’ views of hospitalists: meeting needs of the present and future. J Hosp Med. 2009;4:528-534. https://doi.org/10.1002/jhm.529.
4. Wang ES, Velásquez ST, Smith CJ, et al. Triaging inpatient admissions: an opportunity for resident education. J Gen Intern Med. 2019; 34(5):754-757. https://doi.org/10.1007/s11606-019-04882-2.
5. Briones A, Markoff B, Kathuria N, et al. A model of a hospitalist role in the care of admitted patients in the emergency department. J Hosp Med. 2010;5(6):360-364. https://doi.org/10.1002/jhm.636.
6. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. https://doi.org/10.1111/j.1525-1497.2004.30431.x.
7. Howell E, Bessman E, Marshall R, Wright S. Hospitalist bed management effecting throughput from the emergency department to the intensive care unit. J Crit Care. 2010;25:184-189. https://doi.org/10.1016/j.jcrc.2009.08.004.
8. Chadaga SR, Shockley L, Keniston A, et al. Hospitalist-led medicine emergency department team: associations with throughput, timeliness of patient care, and satisfaction. J Hosp Med. 2012;7:562-566. https://doi.org/10.1002/jhm.1957.
9. Braun, V. Clarke, V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;77-101. https://doi.org/10.1191/1478088706qp063oa.
10. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of non-critically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
11. Patel KK, Vakharia N, Pile J, Howell EH, Rothberg MB. Preventable admissions on a general medicine service: prevalence, causes and comparison with AHRQ prevention quality indicators-a cross-sectional analysis. J Gen Intern Med. 2016;31(6):597-601. https://doi.org/10.1007/s11606-016-3615-4.
12. Daniels LM1, Sorita A2, Kashiwagi DT, et al. Characterizing potentially preventable admissions: a mixed methods study of rates, associated factors, outcomes, and physician decision-making. J Gen Intern Med. 2018;33(5):737-744. https://doi.org/10.1007/s11606-017-4285-6.
13. Howard-Anderson J, Lonowski S, Vangala S, Tseng CH, Busuttil A, Afsar-Manesh N. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870-1872. https://doi.org/10.1001/jamainternmed.2014.4782.
14. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB, Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
1. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015; 42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003.
2. Wachter, RM, Goldman, L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11): 1009-1011. https://doi.org/10.1056/NEJMp1607958.
3. Vasilevskis EE, Knebel RJ, Wachter RM, Auerbach AD. California hospital leaders’ views of hospitalists: meeting needs of the present and future. J Hosp Med. 2009;4:528-534. https://doi.org/10.1002/jhm.529.
4. Wang ES, Velásquez ST, Smith CJ, et al. Triaging inpatient admissions: an opportunity for resident education. J Gen Intern Med. 2019; 34(5):754-757. https://doi.org/10.1007/s11606-019-04882-2.
5. Briones A, Markoff B, Kathuria N, et al. A model of a hospitalist role in the care of admitted patients in the emergency department. J Hosp Med. 2010;5(6):360-364. https://doi.org/10.1002/jhm.636.
6. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. https://doi.org/10.1111/j.1525-1497.2004.30431.x.
7. Howell E, Bessman E, Marshall R, Wright S. Hospitalist bed management effecting throughput from the emergency department to the intensive care unit. J Crit Care. 2010;25:184-189. https://doi.org/10.1016/j.jcrc.2009.08.004.
8. Chadaga SR, Shockley L, Keniston A, et al. Hospitalist-led medicine emergency department team: associations with throughput, timeliness of patient care, and satisfaction. J Hosp Med. 2012;7:562-566. https://doi.org/10.1002/jhm.1957.
9. Braun, V. Clarke, V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;77-101. https://doi.org/10.1191/1478088706qp063oa.
10. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of non-critically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
11. Patel KK, Vakharia N, Pile J, Howell EH, Rothberg MB. Preventable admissions on a general medicine service: prevalence, causes and comparison with AHRQ prevention quality indicators-a cross-sectional analysis. J Gen Intern Med. 2016;31(6):597-601. https://doi.org/10.1007/s11606-016-3615-4.
12. Daniels LM1, Sorita A2, Kashiwagi DT, et al. Characterizing potentially preventable admissions: a mixed methods study of rates, associated factors, outcomes, and physician decision-making. J Gen Intern Med. 2018;33(5):737-744. https://doi.org/10.1007/s11606-017-4285-6.
13. Howard-Anderson J, Lonowski S, Vangala S, Tseng CH, Busuttil A, Afsar-Manesh N. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870-1872. https://doi.org/10.1001/jamainternmed.2014.4782.
14. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB, Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
© 2019 Society of Hospital Medicine
Nurturing Sustainability in a Growing Community Pediatric Hospital Medicine Workforce
Systematic efforts to measure and compare work hours emerged in the 19th century as laborers shifted from artisanal shops to factories, sparking debate over the appropriate length and intensity of work.1 Two centuries of unionization and regulation defined work hours for many United States employees, including graduate medical trainees, but left attending physicians largely untouched. Instead, the medical workforce has long relied on survey data to shape jobs that balance professional norms with local market demands. Leaders in young, dynamic specialties, such as pediatric hospital medicine (PHM), particularly require such data to recruit and retain talent.
PHM progressed swiftly from acknowledgment as a “distinct area of practice” in 1999 to a subspecialty recognition.2 Currently, at least 3,000 pediatric hospitalists3 practice in more than 800 US sites (Snow C, Personal communication regarding community PHM workforce survey). Approximately half of them work at community hospitals, where PHM groups often comprise fewer than five full-time equivalents (FTEs) and face unique challenges. Community PHM practices may assume broader responsibilities than university/children’s hospital colleagues, including advocacy for the needs of children in predominantly adult-oriented hospitals.4 Although data regarding academic PHM work demands are available,5 there is little information pertaining to community hospitalists regarding typical workloads or other characteristics of thriving practices.
In this issue of the Journal of Hospital Medicine, Alvarez et al. present the findings of structured interviews with 70 community PHM group leaders.6 Each participant answered 12 questions about their group, addressing the definition of a full-time workload and hours, the design of backup systems, and the respondent’s perception of the program’s sustainability. The sample is robust, with the caveats that it disproportionately represents the Midwest and West (34.3% each) and more than half of the groups were employed by an academic institution. The authors found a median work expectation per FTE of 1,882 hours/year and 21 weekends per year, although they noted significant variability in employers’ demands and services provided. The majority of hospitalist groups lacked census caps, formal backup systems, or processes to expand coverage during busy seasons. Among the site leaders, 63% perceived their program as sustainable, but no program design or employer characteristic was clearly associated with this perception.
The importance of this study derives from aggregating data about the largest cross section of community PHM groups yet reported. For many PHM group leaders, this will offer a new point of reference for key practice characteristics. Furthermore, the authors should be commended for attempting to distinguish how program sustainability manifests in community PHM, where hospitalists shoulder longer patient care hours and many of them sustain academic endeavors. It is concerning that more than a third of leaders do not perceive their program as sustainable, but the implications for the field are unclear. Perhaps part of this uncertainty arises from the terminology, as sustainability lacks a technical or a consensus definition and the authors purposefully did not define the term for the respondents. While many respondents probably worried about physician burnout, others might have channeled fears about group finances or competition with adult service lines for beds. In addition, leaders’ fears about sustainability may not exactly represent the concerns of front-line employees.
Sustainable work environments are complex constructs with several inputs. For example, supportive leaders, efficient delivery systems, optimized EHRs, competitive pay, and confidence about service line stability might all mitigate higher workloads. Ultimately, this complexity underscores an important caution about all workplace surveys in medicine; ie, average values can inform practice design, but hospitalists and administrators should always consider the local context. Blindly applying medians as benchmarks and ignoring the myriad other contributors to sustainable practice risk disrupting successful PHM programs. In other words, surveys describe how the world is, not how it should be. The spectrum of academic work and norms permeating community PHM groups instead call for a nuanced approach.
How does the field build upon this useful paper? First, the Society of Hospital Medicine (SHM) should engage PHM leaders to increase participation in regular remeasurement, a critical endeavor for this dynamic field. SHM’s State of Hospital Medicine Report queries about a wider variety of practice characteristics, but it has a smaller sample size that must grow to fill this void.7 As the work of repeated surveys transitions from academic inquiry to professional society service, SHM’s Practice Analysis Committee can meet the needs of PHM through relevant questions and efforts to foster adequate participation. Second, all practice leaders should follow the ballooning bodies of literature about burnout and healthcare value. Just as labor leaders had discovered in the industrial revolution, sustainable careers require not only measuring work hours but also advocating for safe, meaningful, and engaging work conditions. By continuously creating value for patients, families, and hospitals, we can strengthen our claim to the resources needed to optimize the work environment.
Disclosure
Andrew White is Chair of the Society of Hospital Medicine’s Practice Analysis Committee, an unpaid position. Dr. Marek serves on the American Academy of Pediatrics Section on Hospital Medicine Executive Committee which is a voluntary, unpaid, elected position.
1. Whaples R. Hours of Work in U.S. History. EH Net Encyclopedia. 2001. http://eh.net/encyclopedia/hours-of-work-in-u-s-history/. Accessed June 25, 2019.
2. Pediatric Hospital Medicine Certification. The American Board of Pediatrics.
https://www.abp.org/content/pediatric-hospital-medicine-certification.
Accessed 28 February, 2018.
3. Harbuck SM, Follmer AD, Dill MJ, Erikson C. Estimating the number and characteristics
of hospitalist physicians in the United States and their possible workforce
implications. Association of Medical Colleges. 2012. www.aamc.org/download/
300620/data/aibvol12_no3-hospitalist.pdf. Accessed June 25, 2019.
4. Roberts KB, Brown J, Quinonez RA, Percelay JM. Institutions and individuals:
what makes a hospitalist “academic”? Hosp Pediatr. 2014;4(5);326-327.
https://doi.org/10.1542/hpeds.2014-00.
5. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist
workload and sustainability in university-based programs: results from a
national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.
org/10.12788/jhm.2977.
6. Alvarez, F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist
workload: results from a national survey. J Hosp Med. 2019; 14(11):682-685. https://
doi.org/10.12788/jhm.3263.
7. 2018 State of Hospital Medicine Report. Society of Hospital Medicine: Philadelphia,
Pennsylvania; 2019. https://www.hospitalmedicine.org/practice-management/
shms-state-of-hospital-medicine/. Accessed July 27, 2019.
Systematic efforts to measure and compare work hours emerged in the 19th century as laborers shifted from artisanal shops to factories, sparking debate over the appropriate length and intensity of work.1 Two centuries of unionization and regulation defined work hours for many United States employees, including graduate medical trainees, but left attending physicians largely untouched. Instead, the medical workforce has long relied on survey data to shape jobs that balance professional norms with local market demands. Leaders in young, dynamic specialties, such as pediatric hospital medicine (PHM), particularly require such data to recruit and retain talent.
PHM progressed swiftly from acknowledgment as a “distinct area of practice” in 1999 to a subspecialty recognition.2 Currently, at least 3,000 pediatric hospitalists3 practice in more than 800 US sites (Snow C, Personal communication regarding community PHM workforce survey). Approximately half of them work at community hospitals, where PHM groups often comprise fewer than five full-time equivalents (FTEs) and face unique challenges. Community PHM practices may assume broader responsibilities than university/children’s hospital colleagues, including advocacy for the needs of children in predominantly adult-oriented hospitals.4 Although data regarding academic PHM work demands are available,5 there is little information pertaining to community hospitalists regarding typical workloads or other characteristics of thriving practices.
In this issue of the Journal of Hospital Medicine, Alvarez et al. present the findings of structured interviews with 70 community PHM group leaders.6 Each participant answered 12 questions about their group, addressing the definition of a full-time workload and hours, the design of backup systems, and the respondent’s perception of the program’s sustainability. The sample is robust, with the caveats that it disproportionately represents the Midwest and West (34.3% each) and more than half of the groups were employed by an academic institution. The authors found a median work expectation per FTE of 1,882 hours/year and 21 weekends per year, although they noted significant variability in employers’ demands and services provided. The majority of hospitalist groups lacked census caps, formal backup systems, or processes to expand coverage during busy seasons. Among the site leaders, 63% perceived their program as sustainable, but no program design or employer characteristic was clearly associated with this perception.
The importance of this study derives from aggregating data about the largest cross section of community PHM groups yet reported. For many PHM group leaders, this will offer a new point of reference for key practice characteristics. Furthermore, the authors should be commended for attempting to distinguish how program sustainability manifests in community PHM, where hospitalists shoulder longer patient care hours and many of them sustain academic endeavors. It is concerning that more than a third of leaders do not perceive their program as sustainable, but the implications for the field are unclear. Perhaps part of this uncertainty arises from the terminology, as sustainability lacks a technical or a consensus definition and the authors purposefully did not define the term for the respondents. While many respondents probably worried about physician burnout, others might have channeled fears about group finances or competition with adult service lines for beds. In addition, leaders’ fears about sustainability may not exactly represent the concerns of front-line employees.
Sustainable work environments are complex constructs with several inputs. For example, supportive leaders, efficient delivery systems, optimized EHRs, competitive pay, and confidence about service line stability might all mitigate higher workloads. Ultimately, this complexity underscores an important caution about all workplace surveys in medicine; ie, average values can inform practice design, but hospitalists and administrators should always consider the local context. Blindly applying medians as benchmarks and ignoring the myriad other contributors to sustainable practice risk disrupting successful PHM programs. In other words, surveys describe how the world is, not how it should be. The spectrum of academic work and norms permeating community PHM groups instead call for a nuanced approach.
How does the field build upon this useful paper? First, the Society of Hospital Medicine (SHM) should engage PHM leaders to increase participation in regular remeasurement, a critical endeavor for this dynamic field. SHM’s State of Hospital Medicine Report queries about a wider variety of practice characteristics, but it has a smaller sample size that must grow to fill this void.7 As the work of repeated surveys transitions from academic inquiry to professional society service, SHM’s Practice Analysis Committee can meet the needs of PHM through relevant questions and efforts to foster adequate participation. Second, all practice leaders should follow the ballooning bodies of literature about burnout and healthcare value. Just as labor leaders had discovered in the industrial revolution, sustainable careers require not only measuring work hours but also advocating for safe, meaningful, and engaging work conditions. By continuously creating value for patients, families, and hospitals, we can strengthen our claim to the resources needed to optimize the work environment.
Disclosure
Andrew White is Chair of the Society of Hospital Medicine’s Practice Analysis Committee, an unpaid position. Dr. Marek serves on the American Academy of Pediatrics Section on Hospital Medicine Executive Committee which is a voluntary, unpaid, elected position.
Systematic efforts to measure and compare work hours emerged in the 19th century as laborers shifted from artisanal shops to factories, sparking debate over the appropriate length and intensity of work.1 Two centuries of unionization and regulation defined work hours for many United States employees, including graduate medical trainees, but left attending physicians largely untouched. Instead, the medical workforce has long relied on survey data to shape jobs that balance professional norms with local market demands. Leaders in young, dynamic specialties, such as pediatric hospital medicine (PHM), particularly require such data to recruit and retain talent.
PHM progressed swiftly from acknowledgment as a “distinct area of practice” in 1999 to a subspecialty recognition.2 Currently, at least 3,000 pediatric hospitalists3 practice in more than 800 US sites (Snow C, Personal communication regarding community PHM workforce survey). Approximately half of them work at community hospitals, where PHM groups often comprise fewer than five full-time equivalents (FTEs) and face unique challenges. Community PHM practices may assume broader responsibilities than university/children’s hospital colleagues, including advocacy for the needs of children in predominantly adult-oriented hospitals.4 Although data regarding academic PHM work demands are available,5 there is little information pertaining to community hospitalists regarding typical workloads or other characteristics of thriving practices.
In this issue of the Journal of Hospital Medicine, Alvarez et al. present the findings of structured interviews with 70 community PHM group leaders.6 Each participant answered 12 questions about their group, addressing the definition of a full-time workload and hours, the design of backup systems, and the respondent’s perception of the program’s sustainability. The sample is robust, with the caveats that it disproportionately represents the Midwest and West (34.3% each) and more than half of the groups were employed by an academic institution. The authors found a median work expectation per FTE of 1,882 hours/year and 21 weekends per year, although they noted significant variability in employers’ demands and services provided. The majority of hospitalist groups lacked census caps, formal backup systems, or processes to expand coverage during busy seasons. Among the site leaders, 63% perceived their program as sustainable, but no program design or employer characteristic was clearly associated with this perception.
The importance of this study derives from aggregating data about the largest cross section of community PHM groups yet reported. For many PHM group leaders, this will offer a new point of reference for key practice characteristics. Furthermore, the authors should be commended for attempting to distinguish how program sustainability manifests in community PHM, where hospitalists shoulder longer patient care hours and many of them sustain academic endeavors. It is concerning that more than a third of leaders do not perceive their program as sustainable, but the implications for the field are unclear. Perhaps part of this uncertainty arises from the terminology, as sustainability lacks a technical or a consensus definition and the authors purposefully did not define the term for the respondents. While many respondents probably worried about physician burnout, others might have channeled fears about group finances or competition with adult service lines for beds. In addition, leaders’ fears about sustainability may not exactly represent the concerns of front-line employees.
Sustainable work environments are complex constructs with several inputs. For example, supportive leaders, efficient delivery systems, optimized EHRs, competitive pay, and confidence about service line stability might all mitigate higher workloads. Ultimately, this complexity underscores an important caution about all workplace surveys in medicine; ie, average values can inform practice design, but hospitalists and administrators should always consider the local context. Blindly applying medians as benchmarks and ignoring the myriad other contributors to sustainable practice risk disrupting successful PHM programs. In other words, surveys describe how the world is, not how it should be. The spectrum of academic work and norms permeating community PHM groups instead call for a nuanced approach.
How does the field build upon this useful paper? First, the Society of Hospital Medicine (SHM) should engage PHM leaders to increase participation in regular remeasurement, a critical endeavor for this dynamic field. SHM’s State of Hospital Medicine Report queries about a wider variety of practice characteristics, but it has a smaller sample size that must grow to fill this void.7 As the work of repeated surveys transitions from academic inquiry to professional society service, SHM’s Practice Analysis Committee can meet the needs of PHM through relevant questions and efforts to foster adequate participation. Second, all practice leaders should follow the ballooning bodies of literature about burnout and healthcare value. Just as labor leaders had discovered in the industrial revolution, sustainable careers require not only measuring work hours but also advocating for safe, meaningful, and engaging work conditions. By continuously creating value for patients, families, and hospitals, we can strengthen our claim to the resources needed to optimize the work environment.
Disclosure
Andrew White is Chair of the Society of Hospital Medicine’s Practice Analysis Committee, an unpaid position. Dr. Marek serves on the American Academy of Pediatrics Section on Hospital Medicine Executive Committee which is a voluntary, unpaid, elected position.
1. Whaples R. Hours of Work in U.S. History. EH Net Encyclopedia. 2001. http://eh.net/encyclopedia/hours-of-work-in-u-s-history/. Accessed June 25, 2019.
2. Pediatric Hospital Medicine Certification. The American Board of Pediatrics.
https://www.abp.org/content/pediatric-hospital-medicine-certification.
Accessed 28 February, 2018.
3. Harbuck SM, Follmer AD, Dill MJ, Erikson C. Estimating the number and characteristics
of hospitalist physicians in the United States and their possible workforce
implications. Association of Medical Colleges. 2012. www.aamc.org/download/
300620/data/aibvol12_no3-hospitalist.pdf. Accessed June 25, 2019.
4. Roberts KB, Brown J, Quinonez RA, Percelay JM. Institutions and individuals:
what makes a hospitalist “academic”? Hosp Pediatr. 2014;4(5);326-327.
https://doi.org/10.1542/hpeds.2014-00.
5. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist
workload and sustainability in university-based programs: results from a
national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.
org/10.12788/jhm.2977.
6. Alvarez, F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist
workload: results from a national survey. J Hosp Med. 2019; 14(11):682-685. https://
doi.org/10.12788/jhm.3263.
7. 2018 State of Hospital Medicine Report. Society of Hospital Medicine: Philadelphia,
Pennsylvania; 2019. https://www.hospitalmedicine.org/practice-management/
shms-state-of-hospital-medicine/. Accessed July 27, 2019.
1. Whaples R. Hours of Work in U.S. History. EH Net Encyclopedia. 2001. http://eh.net/encyclopedia/hours-of-work-in-u-s-history/. Accessed June 25, 2019.
2. Pediatric Hospital Medicine Certification. The American Board of Pediatrics.
https://www.abp.org/content/pediatric-hospital-medicine-certification.
Accessed 28 February, 2018.
3. Harbuck SM, Follmer AD, Dill MJ, Erikson C. Estimating the number and characteristics
of hospitalist physicians in the United States and their possible workforce
implications. Association of Medical Colleges. 2012. www.aamc.org/download/
300620/data/aibvol12_no3-hospitalist.pdf. Accessed June 25, 2019.
4. Roberts KB, Brown J, Quinonez RA, Percelay JM. Institutions and individuals:
what makes a hospitalist “academic”? Hosp Pediatr. 2014;4(5);326-327.
https://doi.org/10.1542/hpeds.2014-00.
5. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist
workload and sustainability in university-based programs: results from a
national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.
org/10.12788/jhm.2977.
6. Alvarez, F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist
workload: results from a national survey. J Hosp Med. 2019; 14(11):682-685. https://
doi.org/10.12788/jhm.3263.
7. 2018 State of Hospital Medicine Report. Society of Hospital Medicine: Philadelphia,
Pennsylvania; 2019. https://www.hospitalmedicine.org/practice-management/
shms-state-of-hospital-medicine/. Accessed July 27, 2019.
© 2019 Society of Hospital Medicine
Collaboration, Not Calculation: A Qualitative Study of How Hospital Executives Value Hospital Medicine Groups
The field of hospital medicine has expanded rapidly since its inception in the late 1990s, and currently, most hospitals in the United States employ or contract with hospital medicine groups (HMGs).1-4 This dramatic growth began in response to several factors: primary care physicians (PCPs) opting out of inpatient care, the increasing acuity and complexity of inpatient care, and cost pressures on hospitals.5,6 Recent studies associate greater use of hospitalists with increased hospital revenues and modest improvements in hospital financial performance.7 However, funding the hospitalist delivery model required hospitals to share the savings hospitalists generate through facility billing and quality incentives.
Hospitalists’ professional fee revenues alone generally do not fund their salaries. An average HMG serving adult patients requires $176,658 from the hospital to support a full-time physician.8 Determining the appropriate level of HMG support typically occurs through negotiation with hospital executives. During the last 10 years, HMG size and hospitalist compensation have risen steadily, combining to increase the hospitalist labor costs borne by hospitals.4,8 Accordingly, hospital executives in challenging economic environments may pressure HMG leaders to accept diminished support or to demonstrate a better return on the hospital’s investment.
These negotiations are influenced by the beliefs of hospital executives about the value of the hospitalist labor model. Little is known about how hospital and health system executive leadership assess the value of hospitalists. A deeper understanding of executive attitudes and beliefs could inform HMG leaders seeking integrative (“win-win”) outcomes in contract and compensation negotiations. Members of the Society of Hospital Medicine (SHM) Practice Management Committee surveyed hospital executives to guide SHM program development. We sought to analyze transcripts from these interviews to describe how executives assess HMGs and to test the hypothesis that hospital executives apply specific financial models when determining the return on investment (ROI) from subsidizing an HMG.
METHODS
Study Design, Setting, and Participants
Members of the SHM Practice Management Committee conducted interviews with a convenience sample of 24 key informants representing the following stakeholders at hospitals employing hospitalists: Chief Executive Officers (CEOs), Presidents, Vice Presidents, Chief Medical Officers (CMOs), and Chief Financial Officers (CFOs). Participants were recruited from 17 fee-for-service healthcare organizations, including rural, suburban, urban, community, and academic medical centers. The semi-structured interviews occurred in person between January and March 2018; each one lasted an average of 45 minutes and were designed to guide SHM program and product development. Twenty-eight executives were recruited by e-mail, and four did not complete the interview due to scheduling difficulty. All the participants provided informed consent. The University of Washington Institutional Review Board approved the secondary analysis of deidentified transcripts.
Interview Guide and Data Collection
All interviews followed a guide with eight demographic questions and 10 open-ended questions (Appendix). Cognitive interviews were performed with two hospital executives outside the study cohort, resulting in the addition of one question and rewording one question for clarity. One-on-one interviews were performed by 10 committee members (range, 1-3 interviews). All interview audios were recorded, and no field notes were kept. The goal of the interviews was to obtain an understanding of how hospital executives value the contributions and costs of hospitalist groups.
The interviews began with questions about the informant’s current interactions with hospitalists and the origin of the hospitalist group at their facility. Informants then described the value they feel hospitalists bring to their hospital and occasions they were surprised or dissatisfied with the clinical or financial value delivered by the hospitalists. Participants described how they calculate a return on investment (ROI) for their hospitalist group, nonfinancial benefits and disadvantages to hospitalists, and how they believe hospitalists should participate in risk-sharing contracts.
Data Analysis
The interview audiotapes were transcribed and deidentified. A sample of eight transcripts was verified by participants to ensure accuracy. Three investigators (AAW, RC, CC) reviewed a random sample of five transcripts to identify and codify preliminary themes. We applied a general inductive framework with a content analysis approach. Two investigators (TM and MC) read all transcripts independently, coding the presence of each theme and quotations exemplifying these themes using qualitative analysis software (Dedoose Version 7.0.23, SocioCultural Research Consultants). A third investigator (AAW) read all the transcripts and resolved differences of opinion. Themes and code application were discussed among the study team after the second and fifth transcripts to add or clarify codes. No new codes were identified after the first review of the preliminary codebook, although investigators intermittently used an “unknown” code through the 20th transcript. After discussion to reach consensus, excerpts initially coded “unknown” were assigned existing codes; the 20th transcript represents the approximate point of reaching thematic saturation.
RESULTS
Of the 24 participants, 18 (75%) were male, representing a variety of roles: 7 (29.2%) CMOs, 5 (20.8%) Presidents, 5 (20.8%) CFOs, 4 (16.7%) CEOs, and 3 (12.5%) Vice Presidents. The participants represented all regions (Midwest 12 [50%], South 6 [25%], West 4 [16.7%], and East 2 [8.3%], community size (Urban 11 [45.8%], Suburban 8 [33.3%], and Rural 5 [20.8%]), and Hospital Types (Community 11 [45.8%], Multihospital System 5 [20.8%], Academic 5 [20.8%], Safety Net 2 [8.3%], and Critical Access 1 [4.2%]). We present specific themes below and supporting quotations in Tables 1 and 2.
Current Value of the HMG at the Respondent’s Hospital
Most executives reported their hospital’s HMG had operated for over a decade and had developed an earlier, outdated value framework. Interviewees described an initial mix of financial pressures, shifts in physician work preferences, increasing patient acuity, resident labor shortages, and unsolved hospital throughput needs that triggered a reactive conversion from community PCP staffing to hospitalist care teams, followed by refinements to realize value.
“I think initially here it was to deal with the resident caps, right? So, at that moment, the solution that was put in place probably made a lot of sense. If that’s all someone came in with, now I’d be scratching my head and said, what are you thinking?” (President, #2)
Respondents perceived that HMGs provide value in many domains, including financial contributions, high-quality care, organizational efficiency, academics, leadership of interprofessional teams, effective communication, system improvement, and beneficial influence on the care environment and other employees. Regarding the measurable generation of financial benefit, documentation for improved billing accuracy, increased hospital efficiency (eg, lower length of stay, early discharges), and comanagement arrangements were commonly identified.
“I don’t want a urologist with a stethoscope, so I’m happy to have the hospitalists say, ‘Look, I’ll take care of the patient. You do the procedure.’ Well, that’s inherently valuable, whether we measure it or whether we don’t.” (CMO, #21)
Executives generally expressed satisfaction with their HMG’s quality of care and the related pay-for-performance financial benefits from payers, attributing success to hospitalists’ familiarity with inpatient systems and willingness to standardize.
“I just think it’s having one structure, one group to go to, a standard rather than trying to push it through the medical staff.” (VP, #18)
Executives reported that HMGs generate substantial value that is difficult to measure financially. For example, a large bundle of excerpts organized around communication with patients, nurses, and other providers.
“If we have the right hospitalist staff, to engage them with the nursing staff would help to reduce my turnover rate…and create a very positive morale within the nursing units. That’s huge. That’s nonfinancial” (President, #15)
Executives particularly appreciated hospitalists’ work to aggregate input from multiple specialists and present a cohesive explanation to patients. Executives also felt that HMGs create significant unmeasured value by improving processes and outcomes on service lines beyond hospital medicine, achieving this through culture change, involvement in leadership, hospital-wide process redesign, and running rapid response teams. Some executives expressed a desire for hospitalists to assume this global quality responsibility more explicitly as a job expectation.
Executives described how they would evaluate a de novo proposal for hospitalist services, usually enumerating key general domains without explaining specifically how they would measure each element. The following priorities emerged: clinical excellence, capacity to collaborate with hospital leadership, the scope of services provided, cultural fit/alignment, financial performance, contract cost, pay-for-performance measures, and turnover. Regarding financial performance, respondents expected to know the cost of the proposal but lacked a specific price threshold. Instead, they sought to understand the total value of the proposal through its effect on metrics such as facility fees or resource use. Nonetheless, cultural fit was a critical, overriding driver of the hypothetical decision, despite difficulty defining beyond estimates of teamwork, alignment with hospital priorities, and qualities of the group leader.
“For us, it usually ends being how do we mix personally, do we like them?” (CMO, #5)
Alignment and Collaboration
The related concepts of “collaboration” and “alignment” emerged as prominent themes during all interviews. Executives highly valued hospitalist groups that could demonstrate alignment with hospital priorities and often used this concept to summarize the HMG’s success or failure across a group of value domains.
“If you’re just coming in to fill a shift and see 10 patients, you have much less value than somebody who’s going to play that active partnership role… hospitalist services need to partner with hospitals and be intimately involved with the success of the hospital.” (CMO, #20)
Alignment sometimes manifested in a quantified, explicit way, through incentive plans or shared savings plans. However, it most often manifested as a broader sense that the hospitalists’ work targeted the same priorities as the executive leaders and that hospitalists genuinely cared about those priorities. A “shift-work mentality” was expressed by some as the antithesis of alignment. Incorporating hospitalist leaders in hospital leadership and frequent communication arose as mechanisms to increase alignment.
Ways HMGs Fail to Meet Expectations
Respondents described unresolved disadvantages to the hospitalist care model.
“I mean, OPPE, how do you do that for a hospitalist? How can you do it? It’s hard to attribute a patient to someone….it is a weakness and I think we all know it.” (CMO, #21)
Executives also worried about inconsistent handoffs with primary care providers and the field’s demographics, finding it disproportionately comprised of junior or transient physicians. They also hoped that hospitalist innovators would solve clinician burnout and the high cost of inpatient care. Disappointments specific to the local HMG revolved around difficulty developing shared models of value and mechanisms to achieve them.
“I would like to have more dialog between the hospital leadership team and the hospitalist group…I would like to see a little bit more collaboration.” (President, #13)
These challenges emerged not as a deficiency with hospital medicine as a specialty, but a failure at their specific facility to achieve the goal of alignment through joint strategic planning.
Calculating Value
When asked if their hospital had a formal process to evaluate ROI for their HMG, two dominant answers emerged: (1) the executive lacked a formal process for determining ROI and was unaware of one used at their facility or (2) the executive evaluated HMG performance based on multiple measures, including cost, but did not attempt to calculate ROI or a summary value. Several described the financial evaluation process as too difficult or unnecessary.
“No. It’s too difficult to extract that data. I would say the best proxy that we could do it is our case mix index on our medicine service line.” (CMO, #20)
“No, not a formal process, no… I question the value of some of the other things we do with the medical group…but not the value of the hospitalists… I don’t think we’ve done a formal assessment. I appreciate the flexibility, especially in a small hospital.” (President, #10)
Rarely, executives described specific financial calculations that served as a proxy for ROI. These included calculating a contribution margin to compare against the cost of salary support or the application of external survey benchmarking comparisons for productivity and salary to evaluate the appropriateness of a limited set of financial indicators. Twice respondents alluded to more sophisticated measurements conducted by the finance department but lacked familiarity with the process. Several executives described ROI calculations for specific projects and discrete business decisions involving hospitalists, particularly considering hiring an additional hospitalist.
Executives generally struggled to recall specific ways that the nonfinancial contributions of hospitalists were incorporated into executive decisions regarding the hospitalist group. Two related themes emerged: first, the belief that hospitals could not function effectively without hospitalists, making their presence an expected cost of doing business. Second, absent measures of HMG ROI, executives appeared to determine an approximate overall value of hospitalists, rather than parsing the various contributions. A few respondents expressed alarm at the rise in hospitalist salaries, whereas others acknowledged market forces beyond their control.
“… there is going to be more of a demand for hospitalists, which is definitely going to drive up the compensation. So, I don’t worry that the compensation will be driven up so high that there won’t be a return [on investment].” (CFO, #16)
Some urged individual hospitalists to develop a deeper understanding of what supports their salary to avoid strained relationships with executives.
Evolution and Risk-Sharing Contracts
Respondents described an evolving conceptualization of the hospitalist’s value, occurring at both a broad, long-term scale and at an incremental, annual scale through minor modifications to incentive pay schemes. For most executives, hiring hospitalists as replacements for PCPs had become necessary and not a source of novel value; many executives described it as “the cost of doing business.” Some described gradually deemphasizing relative value unit (RVU) production to recognize other contributions. Several reported their general appreciation of hospitalists evolved as specific hospitalists matured and demonstrated new contributions to hospital function. Some leaders tried to speculate about future phases of this evolution, although details were sparse.
Among respondents with greater implementation of risk-sharing contracts or ACOs, executives did not describe significantly different goals for hospitalists; executives emphasized that hospitalists should accelerate existing efforts to reduce inpatient costs, length of stay, healthcare-acquired conditions, unnecessary testing, and readmissions. A theme emerged around hospitalists supporting the continuum of care, through improved communication and increased alignment with health systems.
“Where I see the real benefit…is to figure out a way to use hospitalists and match them up with the primary care physicians on the outpatient side to truly develop an integrated population-based medicine practice for all our patients.” (President, #15)
Executives believed that communication and collaboration with PCPs and postacute care providers would attract more measurement.
DISCUSSION
Our findings provide hospitalists with insight into the approach hospital executives may follow when determining the rationale for and extent of financial support for HMGs. The results did not support our hypothesis that executives commonly determine the appropriate support by summing detailed quantitative models for various HMG contributions. Instead, most hospital executives appear to make decisions about the appropriateness of financial support based on a small number of basic financial or care quality metrics combined with a subjective assessment of the HMG’s broader alignment with hospital priorities. However, we did find substantial evidence that hospital executives’ expectations of hospitalists have evolved in the last decade, creating the potential for dissociation from how hospitalists prioritize and value their own efforts. Together, our findings suggest that enhanced communication, relationship building, and collaboration with hospital leaders may help HMGs to maintain a shared model of value with hospital executives.
The general absence of summary value calculations suggests specific opportunities, benefits, and risks for HMG group leaders (Table 3). An important opportunity relates to the communication agenda about unmeasured or nonfinancial contributions. Although executives recognized many of these, our data suggest a need for HMG leaders to educate hospital leaders about their unmeasured contributions proactively. Although some might recommend doing so by quantifying and financially rewarding key intangible contributions (eg, measuring leadership in culture change9), this entails important risks.10 Some experts propose that the proliferation of physician pay-for-performance schemes threatens medical professionalism, fails patients, and misunderstands what motivates physicians.11 HMG groups that feel undervalued should hesitate before monetizing all aspects of their work, and consider emphasizing relationship-building as a platform for communication about their performance. Achieving better alignment with executives is not just an opportunity for HMG leaders, but for each hospitalist within the group. Although executives wanted to have deeper relationships with group members, this may not be feasible in large organizations. Instead, it is incumbent for HMG leaders to translate executives’ expectations and forge better alignment.
Residency may not adequately prepare hospitalists to meet key expectations hospital executives hold related to system leadership and interprofessional team leadership. For example, hospital leaders particularly valued HMGs’ perceived ability to improve nurse retention and morale. Unfortunately, residency curricula generally lack concerted instruction on the skills required to produce such interprofessional inpatient teams reliably. Similarly, executives strongly wanted HMGs to acknowledge a role as partners in running the quality, stewardship, and safety missions of the entire hospital. While residency training builds clinical competence through the care of individual patients, many residents do not receive experiential education in system design and leadership. This suggests a need for HMGs to provide early career training or mentorship in quality improvement and interprofessional teamwork. Executives and HMG leaders seeking a stable, mature workforce, should allocate resources to retaining mid and late career hospitalists through leadership roles or financial incentives for longevity.
As with many qualitative studies, the generalizability of our findings may be limited, particularly outside the US healthcare system. We invited executives from diverse practice settings but may not have captured all the relevant viewpoints. This study did not include Veterans Affairs hospitals, safety net hospitals were underrepresented, Midwestern hospitals were overrepresented and the participants were predominantly male. We were unable to determine the influence of employment model on participant beliefs about HMGs, nor did we elicit comparisons to other physician specialties that would highlight a distinct approach to negotiating with HMGs. Because we used hospitalists as interviewers, including some from the same institution as the interviewee, respondents may have dampened critiques or descriptions of unmet expectations. Our data do not provide quantitative support for any approach to determining or negotiating appropriate financial support for an HMG.
CONCLUSIONS
This work contributes new understanding of the expectations executives have for HMGs and individual hospitalists. This highlights opportunities for group leaders, hospitalists, medical educators, and quality improvement experts to produce a hospitalist labor force that can engage in productive and mutually satisfying relationships with hospital leaders. Hospitalists should strive to improve alignment and communication with executive groups.
Disclosures
The authors report no potential conflict of interest.
1. Lapps J, Flansbaum B, Leykum L, et al. Updating threshold-based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45-47. https://doi.org/10.1002/jhm.2480.
2. Wachter RM, Goldman L. Zero to 50,000–the 20th Anniversary of the hospitalist. NEJM. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958.
3. Stevens JP, Nyweide DJ, Maresh S, et al. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781-1787. https://doi.org/10.1001/jamainternmed.2017.5824.
4. American Hospital Association (AHA) (2017), Hospital Statistics, AHA, Chicago, IL.
5. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. NEJM. 1996;335(7):514-517. https://doi.org/10.1093/ajhp/53.20.2389a.
6. Pham HH, Devers KJ, Kuo S, et al. Health care market trends and the evolution of hospitalist use and roles. J Gen Intern Med. 2005;20(2):101-107. https://doi.org/10.1111/j.1525-1497.2005.40184.x.
7. Epané JP, Weech-Maldonado R, Hearld L, et al. Hospitals’ use of hospitalistas: implications for financial performance. Health Care Manage Rev. 2019;44(1):10-18. https://doi.org/10.1097/hmr.0000000000000170.
8. State of Hospital Medicine: 2018 Report Based on 2017 Data. Society of Hospital Medicine. https://sohm.hospitalmedicine.org/ Accessed December 9, 2018.
9. Carmeli A, Tishler A. The relationships between intangible organizational elements and organizational performance. Strategic Manag J. 2004;25(13):1257-1278. https://doi.org/10.1002/smj.428.
10. Bernard M. Strategic performance management: leveraging and measuring your intangible value drivers. Amsterdam: Butterworth-Heinemann, 2006.
11. Khullar D, Wolfson D, Casalino LP. Professionalism, performance, and the future of physician incentives. JAMA. 2018;320(23):2419-2420. https://doi.org/10.1001/jama.2018.17719.
The field of hospital medicine has expanded rapidly since its inception in the late 1990s, and currently, most hospitals in the United States employ or contract with hospital medicine groups (HMGs).1-4 This dramatic growth began in response to several factors: primary care physicians (PCPs) opting out of inpatient care, the increasing acuity and complexity of inpatient care, and cost pressures on hospitals.5,6 Recent studies associate greater use of hospitalists with increased hospital revenues and modest improvements in hospital financial performance.7 However, funding the hospitalist delivery model required hospitals to share the savings hospitalists generate through facility billing and quality incentives.
Hospitalists’ professional fee revenues alone generally do not fund their salaries. An average HMG serving adult patients requires $176,658 from the hospital to support a full-time physician.8 Determining the appropriate level of HMG support typically occurs through negotiation with hospital executives. During the last 10 years, HMG size and hospitalist compensation have risen steadily, combining to increase the hospitalist labor costs borne by hospitals.4,8 Accordingly, hospital executives in challenging economic environments may pressure HMG leaders to accept diminished support or to demonstrate a better return on the hospital’s investment.
These negotiations are influenced by the beliefs of hospital executives about the value of the hospitalist labor model. Little is known about how hospital and health system executive leadership assess the value of hospitalists. A deeper understanding of executive attitudes and beliefs could inform HMG leaders seeking integrative (“win-win”) outcomes in contract and compensation negotiations. Members of the Society of Hospital Medicine (SHM) Practice Management Committee surveyed hospital executives to guide SHM program development. We sought to analyze transcripts from these interviews to describe how executives assess HMGs and to test the hypothesis that hospital executives apply specific financial models when determining the return on investment (ROI) from subsidizing an HMG.
METHODS
Study Design, Setting, and Participants
Members of the SHM Practice Management Committee conducted interviews with a convenience sample of 24 key informants representing the following stakeholders at hospitals employing hospitalists: Chief Executive Officers (CEOs), Presidents, Vice Presidents, Chief Medical Officers (CMOs), and Chief Financial Officers (CFOs). Participants were recruited from 17 fee-for-service healthcare organizations, including rural, suburban, urban, community, and academic medical centers. The semi-structured interviews occurred in person between January and March 2018; each one lasted an average of 45 minutes and were designed to guide SHM program and product development. Twenty-eight executives were recruited by e-mail, and four did not complete the interview due to scheduling difficulty. All the participants provided informed consent. The University of Washington Institutional Review Board approved the secondary analysis of deidentified transcripts.
Interview Guide and Data Collection
All interviews followed a guide with eight demographic questions and 10 open-ended questions (Appendix). Cognitive interviews were performed with two hospital executives outside the study cohort, resulting in the addition of one question and rewording one question for clarity. One-on-one interviews were performed by 10 committee members (range, 1-3 interviews). All interview audios were recorded, and no field notes were kept. The goal of the interviews was to obtain an understanding of how hospital executives value the contributions and costs of hospitalist groups.
The interviews began with questions about the informant’s current interactions with hospitalists and the origin of the hospitalist group at their facility. Informants then described the value they feel hospitalists bring to their hospital and occasions they were surprised or dissatisfied with the clinical or financial value delivered by the hospitalists. Participants described how they calculate a return on investment (ROI) for their hospitalist group, nonfinancial benefits and disadvantages to hospitalists, and how they believe hospitalists should participate in risk-sharing contracts.
Data Analysis
The interview audiotapes were transcribed and deidentified. A sample of eight transcripts was verified by participants to ensure accuracy. Three investigators (AAW, RC, CC) reviewed a random sample of five transcripts to identify and codify preliminary themes. We applied a general inductive framework with a content analysis approach. Two investigators (TM and MC) read all transcripts independently, coding the presence of each theme and quotations exemplifying these themes using qualitative analysis software (Dedoose Version 7.0.23, SocioCultural Research Consultants). A third investigator (AAW) read all the transcripts and resolved differences of opinion. Themes and code application were discussed among the study team after the second and fifth transcripts to add or clarify codes. No new codes were identified after the first review of the preliminary codebook, although investigators intermittently used an “unknown” code through the 20th transcript. After discussion to reach consensus, excerpts initially coded “unknown” were assigned existing codes; the 20th transcript represents the approximate point of reaching thematic saturation.
RESULTS
Of the 24 participants, 18 (75%) were male, representing a variety of roles: 7 (29.2%) CMOs, 5 (20.8%) Presidents, 5 (20.8%) CFOs, 4 (16.7%) CEOs, and 3 (12.5%) Vice Presidents. The participants represented all regions (Midwest 12 [50%], South 6 [25%], West 4 [16.7%], and East 2 [8.3%], community size (Urban 11 [45.8%], Suburban 8 [33.3%], and Rural 5 [20.8%]), and Hospital Types (Community 11 [45.8%], Multihospital System 5 [20.8%], Academic 5 [20.8%], Safety Net 2 [8.3%], and Critical Access 1 [4.2%]). We present specific themes below and supporting quotations in Tables 1 and 2.
Current Value of the HMG at the Respondent’s Hospital
Most executives reported their hospital’s HMG had operated for over a decade and had developed an earlier, outdated value framework. Interviewees described an initial mix of financial pressures, shifts in physician work preferences, increasing patient acuity, resident labor shortages, and unsolved hospital throughput needs that triggered a reactive conversion from community PCP staffing to hospitalist care teams, followed by refinements to realize value.
“I think initially here it was to deal with the resident caps, right? So, at that moment, the solution that was put in place probably made a lot of sense. If that’s all someone came in with, now I’d be scratching my head and said, what are you thinking?” (President, #2)
Respondents perceived that HMGs provide value in many domains, including financial contributions, high-quality care, organizational efficiency, academics, leadership of interprofessional teams, effective communication, system improvement, and beneficial influence on the care environment and other employees. Regarding the measurable generation of financial benefit, documentation for improved billing accuracy, increased hospital efficiency (eg, lower length of stay, early discharges), and comanagement arrangements were commonly identified.
“I don’t want a urologist with a stethoscope, so I’m happy to have the hospitalists say, ‘Look, I’ll take care of the patient. You do the procedure.’ Well, that’s inherently valuable, whether we measure it or whether we don’t.” (CMO, #21)
Executives generally expressed satisfaction with their HMG’s quality of care and the related pay-for-performance financial benefits from payers, attributing success to hospitalists’ familiarity with inpatient systems and willingness to standardize.
“I just think it’s having one structure, one group to go to, a standard rather than trying to push it through the medical staff.” (VP, #18)
Executives reported that HMGs generate substantial value that is difficult to measure financially. For example, a large bundle of excerpts organized around communication with patients, nurses, and other providers.
“If we have the right hospitalist staff, to engage them with the nursing staff would help to reduce my turnover rate…and create a very positive morale within the nursing units. That’s huge. That’s nonfinancial” (President, #15)
Executives particularly appreciated hospitalists’ work to aggregate input from multiple specialists and present a cohesive explanation to patients. Executives also felt that HMGs create significant unmeasured value by improving processes and outcomes on service lines beyond hospital medicine, achieving this through culture change, involvement in leadership, hospital-wide process redesign, and running rapid response teams. Some executives expressed a desire for hospitalists to assume this global quality responsibility more explicitly as a job expectation.
Executives described how they would evaluate a de novo proposal for hospitalist services, usually enumerating key general domains without explaining specifically how they would measure each element. The following priorities emerged: clinical excellence, capacity to collaborate with hospital leadership, the scope of services provided, cultural fit/alignment, financial performance, contract cost, pay-for-performance measures, and turnover. Regarding financial performance, respondents expected to know the cost of the proposal but lacked a specific price threshold. Instead, they sought to understand the total value of the proposal through its effect on metrics such as facility fees or resource use. Nonetheless, cultural fit was a critical, overriding driver of the hypothetical decision, despite difficulty defining beyond estimates of teamwork, alignment with hospital priorities, and qualities of the group leader.
“For us, it usually ends being how do we mix personally, do we like them?” (CMO, #5)
Alignment and Collaboration
The related concepts of “collaboration” and “alignment” emerged as prominent themes during all interviews. Executives highly valued hospitalist groups that could demonstrate alignment with hospital priorities and often used this concept to summarize the HMG’s success or failure across a group of value domains.
“If you’re just coming in to fill a shift and see 10 patients, you have much less value than somebody who’s going to play that active partnership role… hospitalist services need to partner with hospitals and be intimately involved with the success of the hospital.” (CMO, #20)
Alignment sometimes manifested in a quantified, explicit way, through incentive plans or shared savings plans. However, it most often manifested as a broader sense that the hospitalists’ work targeted the same priorities as the executive leaders and that hospitalists genuinely cared about those priorities. A “shift-work mentality” was expressed by some as the antithesis of alignment. Incorporating hospitalist leaders in hospital leadership and frequent communication arose as mechanisms to increase alignment.
Ways HMGs Fail to Meet Expectations
Respondents described unresolved disadvantages to the hospitalist care model.
“I mean, OPPE, how do you do that for a hospitalist? How can you do it? It’s hard to attribute a patient to someone….it is a weakness and I think we all know it.” (CMO, #21)
Executives also worried about inconsistent handoffs with primary care providers and the field’s demographics, finding it disproportionately comprised of junior or transient physicians. They also hoped that hospitalist innovators would solve clinician burnout and the high cost of inpatient care. Disappointments specific to the local HMG revolved around difficulty developing shared models of value and mechanisms to achieve them.
“I would like to have more dialog between the hospital leadership team and the hospitalist group…I would like to see a little bit more collaboration.” (President, #13)
These challenges emerged not as a deficiency with hospital medicine as a specialty, but a failure at their specific facility to achieve the goal of alignment through joint strategic planning.
Calculating Value
When asked if their hospital had a formal process to evaluate ROI for their HMG, two dominant answers emerged: (1) the executive lacked a formal process for determining ROI and was unaware of one used at their facility or (2) the executive evaluated HMG performance based on multiple measures, including cost, but did not attempt to calculate ROI or a summary value. Several described the financial evaluation process as too difficult or unnecessary.
“No. It’s too difficult to extract that data. I would say the best proxy that we could do it is our case mix index on our medicine service line.” (CMO, #20)
“No, not a formal process, no… I question the value of some of the other things we do with the medical group…but not the value of the hospitalists… I don’t think we’ve done a formal assessment. I appreciate the flexibility, especially in a small hospital.” (President, #10)
Rarely, executives described specific financial calculations that served as a proxy for ROI. These included calculating a contribution margin to compare against the cost of salary support or the application of external survey benchmarking comparisons for productivity and salary to evaluate the appropriateness of a limited set of financial indicators. Twice respondents alluded to more sophisticated measurements conducted by the finance department but lacked familiarity with the process. Several executives described ROI calculations for specific projects and discrete business decisions involving hospitalists, particularly considering hiring an additional hospitalist.
Executives generally struggled to recall specific ways that the nonfinancial contributions of hospitalists were incorporated into executive decisions regarding the hospitalist group. Two related themes emerged: first, the belief that hospitals could not function effectively without hospitalists, making their presence an expected cost of doing business. Second, absent measures of HMG ROI, executives appeared to determine an approximate overall value of hospitalists, rather than parsing the various contributions. A few respondents expressed alarm at the rise in hospitalist salaries, whereas others acknowledged market forces beyond their control.
“… there is going to be more of a demand for hospitalists, which is definitely going to drive up the compensation. So, I don’t worry that the compensation will be driven up so high that there won’t be a return [on investment].” (CFO, #16)
Some urged individual hospitalists to develop a deeper understanding of what supports their salary to avoid strained relationships with executives.
Evolution and Risk-Sharing Contracts
Respondents described an evolving conceptualization of the hospitalist’s value, occurring at both a broad, long-term scale and at an incremental, annual scale through minor modifications to incentive pay schemes. For most executives, hiring hospitalists as replacements for PCPs had become necessary and not a source of novel value; many executives described it as “the cost of doing business.” Some described gradually deemphasizing relative value unit (RVU) production to recognize other contributions. Several reported their general appreciation of hospitalists evolved as specific hospitalists matured and demonstrated new contributions to hospital function. Some leaders tried to speculate about future phases of this evolution, although details were sparse.
Among respondents with greater implementation of risk-sharing contracts or ACOs, executives did not describe significantly different goals for hospitalists; executives emphasized that hospitalists should accelerate existing efforts to reduce inpatient costs, length of stay, healthcare-acquired conditions, unnecessary testing, and readmissions. A theme emerged around hospitalists supporting the continuum of care, through improved communication and increased alignment with health systems.
“Where I see the real benefit…is to figure out a way to use hospitalists and match them up with the primary care physicians on the outpatient side to truly develop an integrated population-based medicine practice for all our patients.” (President, #15)
Executives believed that communication and collaboration with PCPs and postacute care providers would attract more measurement.
DISCUSSION
Our findings provide hospitalists with insight into the approach hospital executives may follow when determining the rationale for and extent of financial support for HMGs. The results did not support our hypothesis that executives commonly determine the appropriate support by summing detailed quantitative models for various HMG contributions. Instead, most hospital executives appear to make decisions about the appropriateness of financial support based on a small number of basic financial or care quality metrics combined with a subjective assessment of the HMG’s broader alignment with hospital priorities. However, we did find substantial evidence that hospital executives’ expectations of hospitalists have evolved in the last decade, creating the potential for dissociation from how hospitalists prioritize and value their own efforts. Together, our findings suggest that enhanced communication, relationship building, and collaboration with hospital leaders may help HMGs to maintain a shared model of value with hospital executives.
The general absence of summary value calculations suggests specific opportunities, benefits, and risks for HMG group leaders (Table 3). An important opportunity relates to the communication agenda about unmeasured or nonfinancial contributions. Although executives recognized many of these, our data suggest a need for HMG leaders to educate hospital leaders about their unmeasured contributions proactively. Although some might recommend doing so by quantifying and financially rewarding key intangible contributions (eg, measuring leadership in culture change9), this entails important risks.10 Some experts propose that the proliferation of physician pay-for-performance schemes threatens medical professionalism, fails patients, and misunderstands what motivates physicians.11 HMG groups that feel undervalued should hesitate before monetizing all aspects of their work, and consider emphasizing relationship-building as a platform for communication about their performance. Achieving better alignment with executives is not just an opportunity for HMG leaders, but for each hospitalist within the group. Although executives wanted to have deeper relationships with group members, this may not be feasible in large organizations. Instead, it is incumbent for HMG leaders to translate executives’ expectations and forge better alignment.
Residency may not adequately prepare hospitalists to meet key expectations hospital executives hold related to system leadership and interprofessional team leadership. For example, hospital leaders particularly valued HMGs’ perceived ability to improve nurse retention and morale. Unfortunately, residency curricula generally lack concerted instruction on the skills required to produce such interprofessional inpatient teams reliably. Similarly, executives strongly wanted HMGs to acknowledge a role as partners in running the quality, stewardship, and safety missions of the entire hospital. While residency training builds clinical competence through the care of individual patients, many residents do not receive experiential education in system design and leadership. This suggests a need for HMGs to provide early career training or mentorship in quality improvement and interprofessional teamwork. Executives and HMG leaders seeking a stable, mature workforce, should allocate resources to retaining mid and late career hospitalists through leadership roles or financial incentives for longevity.
As with many qualitative studies, the generalizability of our findings may be limited, particularly outside the US healthcare system. We invited executives from diverse practice settings but may not have captured all the relevant viewpoints. This study did not include Veterans Affairs hospitals, safety net hospitals were underrepresented, Midwestern hospitals were overrepresented and the participants were predominantly male. We were unable to determine the influence of employment model on participant beliefs about HMGs, nor did we elicit comparisons to other physician specialties that would highlight a distinct approach to negotiating with HMGs. Because we used hospitalists as interviewers, including some from the same institution as the interviewee, respondents may have dampened critiques or descriptions of unmet expectations. Our data do not provide quantitative support for any approach to determining or negotiating appropriate financial support for an HMG.
CONCLUSIONS
This work contributes new understanding of the expectations executives have for HMGs and individual hospitalists. This highlights opportunities for group leaders, hospitalists, medical educators, and quality improvement experts to produce a hospitalist labor force that can engage in productive and mutually satisfying relationships with hospital leaders. Hospitalists should strive to improve alignment and communication with executive groups.
Disclosures
The authors report no potential conflict of interest.
The field of hospital medicine has expanded rapidly since its inception in the late 1990s, and currently, most hospitals in the United States employ or contract with hospital medicine groups (HMGs).1-4 This dramatic growth began in response to several factors: primary care physicians (PCPs) opting out of inpatient care, the increasing acuity and complexity of inpatient care, and cost pressures on hospitals.5,6 Recent studies associate greater use of hospitalists with increased hospital revenues and modest improvements in hospital financial performance.7 However, funding the hospitalist delivery model required hospitals to share the savings hospitalists generate through facility billing and quality incentives.
Hospitalists’ professional fee revenues alone generally do not fund their salaries. An average HMG serving adult patients requires $176,658 from the hospital to support a full-time physician.8 Determining the appropriate level of HMG support typically occurs through negotiation with hospital executives. During the last 10 years, HMG size and hospitalist compensation have risen steadily, combining to increase the hospitalist labor costs borne by hospitals.4,8 Accordingly, hospital executives in challenging economic environments may pressure HMG leaders to accept diminished support or to demonstrate a better return on the hospital’s investment.
These negotiations are influenced by the beliefs of hospital executives about the value of the hospitalist labor model. Little is known about how hospital and health system executive leadership assess the value of hospitalists. A deeper understanding of executive attitudes and beliefs could inform HMG leaders seeking integrative (“win-win”) outcomes in contract and compensation negotiations. Members of the Society of Hospital Medicine (SHM) Practice Management Committee surveyed hospital executives to guide SHM program development. We sought to analyze transcripts from these interviews to describe how executives assess HMGs and to test the hypothesis that hospital executives apply specific financial models when determining the return on investment (ROI) from subsidizing an HMG.
METHODS
Study Design, Setting, and Participants
Members of the SHM Practice Management Committee conducted interviews with a convenience sample of 24 key informants representing the following stakeholders at hospitals employing hospitalists: Chief Executive Officers (CEOs), Presidents, Vice Presidents, Chief Medical Officers (CMOs), and Chief Financial Officers (CFOs). Participants were recruited from 17 fee-for-service healthcare organizations, including rural, suburban, urban, community, and academic medical centers. The semi-structured interviews occurred in person between January and March 2018; each one lasted an average of 45 minutes and were designed to guide SHM program and product development. Twenty-eight executives were recruited by e-mail, and four did not complete the interview due to scheduling difficulty. All the participants provided informed consent. The University of Washington Institutional Review Board approved the secondary analysis of deidentified transcripts.
Interview Guide and Data Collection
All interviews followed a guide with eight demographic questions and 10 open-ended questions (Appendix). Cognitive interviews were performed with two hospital executives outside the study cohort, resulting in the addition of one question and rewording one question for clarity. One-on-one interviews were performed by 10 committee members (range, 1-3 interviews). All interview audios were recorded, and no field notes were kept. The goal of the interviews was to obtain an understanding of how hospital executives value the contributions and costs of hospitalist groups.
The interviews began with questions about the informant’s current interactions with hospitalists and the origin of the hospitalist group at their facility. Informants then described the value they feel hospitalists bring to their hospital and occasions they were surprised or dissatisfied with the clinical or financial value delivered by the hospitalists. Participants described how they calculate a return on investment (ROI) for their hospitalist group, nonfinancial benefits and disadvantages to hospitalists, and how they believe hospitalists should participate in risk-sharing contracts.
Data Analysis
The interview audiotapes were transcribed and deidentified. A sample of eight transcripts was verified by participants to ensure accuracy. Three investigators (AAW, RC, CC) reviewed a random sample of five transcripts to identify and codify preliminary themes. We applied a general inductive framework with a content analysis approach. Two investigators (TM and MC) read all transcripts independently, coding the presence of each theme and quotations exemplifying these themes using qualitative analysis software (Dedoose Version 7.0.23, SocioCultural Research Consultants). A third investigator (AAW) read all the transcripts and resolved differences of opinion. Themes and code application were discussed among the study team after the second and fifth transcripts to add or clarify codes. No new codes were identified after the first review of the preliminary codebook, although investigators intermittently used an “unknown” code through the 20th transcript. After discussion to reach consensus, excerpts initially coded “unknown” were assigned existing codes; the 20th transcript represents the approximate point of reaching thematic saturation.
RESULTS
Of the 24 participants, 18 (75%) were male, representing a variety of roles: 7 (29.2%) CMOs, 5 (20.8%) Presidents, 5 (20.8%) CFOs, 4 (16.7%) CEOs, and 3 (12.5%) Vice Presidents. The participants represented all regions (Midwest 12 [50%], South 6 [25%], West 4 [16.7%], and East 2 [8.3%], community size (Urban 11 [45.8%], Suburban 8 [33.3%], and Rural 5 [20.8%]), and Hospital Types (Community 11 [45.8%], Multihospital System 5 [20.8%], Academic 5 [20.8%], Safety Net 2 [8.3%], and Critical Access 1 [4.2%]). We present specific themes below and supporting quotations in Tables 1 and 2.
Current Value of the HMG at the Respondent’s Hospital
Most executives reported their hospital’s HMG had operated for over a decade and had developed an earlier, outdated value framework. Interviewees described an initial mix of financial pressures, shifts in physician work preferences, increasing patient acuity, resident labor shortages, and unsolved hospital throughput needs that triggered a reactive conversion from community PCP staffing to hospitalist care teams, followed by refinements to realize value.
“I think initially here it was to deal with the resident caps, right? So, at that moment, the solution that was put in place probably made a lot of sense. If that’s all someone came in with, now I’d be scratching my head and said, what are you thinking?” (President, #2)
Respondents perceived that HMGs provide value in many domains, including financial contributions, high-quality care, organizational efficiency, academics, leadership of interprofessional teams, effective communication, system improvement, and beneficial influence on the care environment and other employees. Regarding the measurable generation of financial benefit, documentation for improved billing accuracy, increased hospital efficiency (eg, lower length of stay, early discharges), and comanagement arrangements were commonly identified.
“I don’t want a urologist with a stethoscope, so I’m happy to have the hospitalists say, ‘Look, I’ll take care of the patient. You do the procedure.’ Well, that’s inherently valuable, whether we measure it or whether we don’t.” (CMO, #21)
Executives generally expressed satisfaction with their HMG’s quality of care and the related pay-for-performance financial benefits from payers, attributing success to hospitalists’ familiarity with inpatient systems and willingness to standardize.
“I just think it’s having one structure, one group to go to, a standard rather than trying to push it through the medical staff.” (VP, #18)
Executives reported that HMGs generate substantial value that is difficult to measure financially. For example, a large bundle of excerpts organized around communication with patients, nurses, and other providers.
“If we have the right hospitalist staff, to engage them with the nursing staff would help to reduce my turnover rate…and create a very positive morale within the nursing units. That’s huge. That’s nonfinancial” (President, #15)
Executives particularly appreciated hospitalists’ work to aggregate input from multiple specialists and present a cohesive explanation to patients. Executives also felt that HMGs create significant unmeasured value by improving processes and outcomes on service lines beyond hospital medicine, achieving this through culture change, involvement in leadership, hospital-wide process redesign, and running rapid response teams. Some executives expressed a desire for hospitalists to assume this global quality responsibility more explicitly as a job expectation.
Executives described how they would evaluate a de novo proposal for hospitalist services, usually enumerating key general domains without explaining specifically how they would measure each element. The following priorities emerged: clinical excellence, capacity to collaborate with hospital leadership, the scope of services provided, cultural fit/alignment, financial performance, contract cost, pay-for-performance measures, and turnover. Regarding financial performance, respondents expected to know the cost of the proposal but lacked a specific price threshold. Instead, they sought to understand the total value of the proposal through its effect on metrics such as facility fees or resource use. Nonetheless, cultural fit was a critical, overriding driver of the hypothetical decision, despite difficulty defining beyond estimates of teamwork, alignment with hospital priorities, and qualities of the group leader.
“For us, it usually ends being how do we mix personally, do we like them?” (CMO, #5)
Alignment and Collaboration
The related concepts of “collaboration” and “alignment” emerged as prominent themes during all interviews. Executives highly valued hospitalist groups that could demonstrate alignment with hospital priorities and often used this concept to summarize the HMG’s success or failure across a group of value domains.
“If you’re just coming in to fill a shift and see 10 patients, you have much less value than somebody who’s going to play that active partnership role… hospitalist services need to partner with hospitals and be intimately involved with the success of the hospital.” (CMO, #20)
Alignment sometimes manifested in a quantified, explicit way, through incentive plans or shared savings plans. However, it most often manifested as a broader sense that the hospitalists’ work targeted the same priorities as the executive leaders and that hospitalists genuinely cared about those priorities. A “shift-work mentality” was expressed by some as the antithesis of alignment. Incorporating hospitalist leaders in hospital leadership and frequent communication arose as mechanisms to increase alignment.
Ways HMGs Fail to Meet Expectations
Respondents described unresolved disadvantages to the hospitalist care model.
“I mean, OPPE, how do you do that for a hospitalist? How can you do it? It’s hard to attribute a patient to someone….it is a weakness and I think we all know it.” (CMO, #21)
Executives also worried about inconsistent handoffs with primary care providers and the field’s demographics, finding it disproportionately comprised of junior or transient physicians. They also hoped that hospitalist innovators would solve clinician burnout and the high cost of inpatient care. Disappointments specific to the local HMG revolved around difficulty developing shared models of value and mechanisms to achieve them.
“I would like to have more dialog between the hospital leadership team and the hospitalist group…I would like to see a little bit more collaboration.” (President, #13)
These challenges emerged not as a deficiency with hospital medicine as a specialty, but a failure at their specific facility to achieve the goal of alignment through joint strategic planning.
Calculating Value
When asked if their hospital had a formal process to evaluate ROI for their HMG, two dominant answers emerged: (1) the executive lacked a formal process for determining ROI and was unaware of one used at their facility or (2) the executive evaluated HMG performance based on multiple measures, including cost, but did not attempt to calculate ROI or a summary value. Several described the financial evaluation process as too difficult or unnecessary.
“No. It’s too difficult to extract that data. I would say the best proxy that we could do it is our case mix index on our medicine service line.” (CMO, #20)
“No, not a formal process, no… I question the value of some of the other things we do with the medical group…but not the value of the hospitalists… I don’t think we’ve done a formal assessment. I appreciate the flexibility, especially in a small hospital.” (President, #10)
Rarely, executives described specific financial calculations that served as a proxy for ROI. These included calculating a contribution margin to compare against the cost of salary support or the application of external survey benchmarking comparisons for productivity and salary to evaluate the appropriateness of a limited set of financial indicators. Twice respondents alluded to more sophisticated measurements conducted by the finance department but lacked familiarity with the process. Several executives described ROI calculations for specific projects and discrete business decisions involving hospitalists, particularly considering hiring an additional hospitalist.
Executives generally struggled to recall specific ways that the nonfinancial contributions of hospitalists were incorporated into executive decisions regarding the hospitalist group. Two related themes emerged: first, the belief that hospitals could not function effectively without hospitalists, making their presence an expected cost of doing business. Second, absent measures of HMG ROI, executives appeared to determine an approximate overall value of hospitalists, rather than parsing the various contributions. A few respondents expressed alarm at the rise in hospitalist salaries, whereas others acknowledged market forces beyond their control.
“… there is going to be more of a demand for hospitalists, which is definitely going to drive up the compensation. So, I don’t worry that the compensation will be driven up so high that there won’t be a return [on investment].” (CFO, #16)
Some urged individual hospitalists to develop a deeper understanding of what supports their salary to avoid strained relationships with executives.
Evolution and Risk-Sharing Contracts
Respondents described an evolving conceptualization of the hospitalist’s value, occurring at both a broad, long-term scale and at an incremental, annual scale through minor modifications to incentive pay schemes. For most executives, hiring hospitalists as replacements for PCPs had become necessary and not a source of novel value; many executives described it as “the cost of doing business.” Some described gradually deemphasizing relative value unit (RVU) production to recognize other contributions. Several reported their general appreciation of hospitalists evolved as specific hospitalists matured and demonstrated new contributions to hospital function. Some leaders tried to speculate about future phases of this evolution, although details were sparse.
Among respondents with greater implementation of risk-sharing contracts or ACOs, executives did not describe significantly different goals for hospitalists; executives emphasized that hospitalists should accelerate existing efforts to reduce inpatient costs, length of stay, healthcare-acquired conditions, unnecessary testing, and readmissions. A theme emerged around hospitalists supporting the continuum of care, through improved communication and increased alignment with health systems.
“Where I see the real benefit…is to figure out a way to use hospitalists and match them up with the primary care physicians on the outpatient side to truly develop an integrated population-based medicine practice for all our patients.” (President, #15)
Executives believed that communication and collaboration with PCPs and postacute care providers would attract more measurement.
DISCUSSION
Our findings provide hospitalists with insight into the approach hospital executives may follow when determining the rationale for and extent of financial support for HMGs. The results did not support our hypothesis that executives commonly determine the appropriate support by summing detailed quantitative models for various HMG contributions. Instead, most hospital executives appear to make decisions about the appropriateness of financial support based on a small number of basic financial or care quality metrics combined with a subjective assessment of the HMG’s broader alignment with hospital priorities. However, we did find substantial evidence that hospital executives’ expectations of hospitalists have evolved in the last decade, creating the potential for dissociation from how hospitalists prioritize and value their own efforts. Together, our findings suggest that enhanced communication, relationship building, and collaboration with hospital leaders may help HMGs to maintain a shared model of value with hospital executives.
The general absence of summary value calculations suggests specific opportunities, benefits, and risks for HMG group leaders (Table 3). An important opportunity relates to the communication agenda about unmeasured or nonfinancial contributions. Although executives recognized many of these, our data suggest a need for HMG leaders to educate hospital leaders about their unmeasured contributions proactively. Although some might recommend doing so by quantifying and financially rewarding key intangible contributions (eg, measuring leadership in culture change9), this entails important risks.10 Some experts propose that the proliferation of physician pay-for-performance schemes threatens medical professionalism, fails patients, and misunderstands what motivates physicians.11 HMG groups that feel undervalued should hesitate before monetizing all aspects of their work, and consider emphasizing relationship-building as a platform for communication about their performance. Achieving better alignment with executives is not just an opportunity for HMG leaders, but for each hospitalist within the group. Although executives wanted to have deeper relationships with group members, this may not be feasible in large organizations. Instead, it is incumbent for HMG leaders to translate executives’ expectations and forge better alignment.
Residency may not adequately prepare hospitalists to meet key expectations hospital executives hold related to system leadership and interprofessional team leadership. For example, hospital leaders particularly valued HMGs’ perceived ability to improve nurse retention and morale. Unfortunately, residency curricula generally lack concerted instruction on the skills required to produce such interprofessional inpatient teams reliably. Similarly, executives strongly wanted HMGs to acknowledge a role as partners in running the quality, stewardship, and safety missions of the entire hospital. While residency training builds clinical competence through the care of individual patients, many residents do not receive experiential education in system design and leadership. This suggests a need for HMGs to provide early career training or mentorship in quality improvement and interprofessional teamwork. Executives and HMG leaders seeking a stable, mature workforce, should allocate resources to retaining mid and late career hospitalists through leadership roles or financial incentives for longevity.
As with many qualitative studies, the generalizability of our findings may be limited, particularly outside the US healthcare system. We invited executives from diverse practice settings but may not have captured all the relevant viewpoints. This study did not include Veterans Affairs hospitals, safety net hospitals were underrepresented, Midwestern hospitals were overrepresented and the participants were predominantly male. We were unable to determine the influence of employment model on participant beliefs about HMGs, nor did we elicit comparisons to other physician specialties that would highlight a distinct approach to negotiating with HMGs. Because we used hospitalists as interviewers, including some from the same institution as the interviewee, respondents may have dampened critiques or descriptions of unmet expectations. Our data do not provide quantitative support for any approach to determining or negotiating appropriate financial support for an HMG.
CONCLUSIONS
This work contributes new understanding of the expectations executives have for HMGs and individual hospitalists. This highlights opportunities for group leaders, hospitalists, medical educators, and quality improvement experts to produce a hospitalist labor force that can engage in productive and mutually satisfying relationships with hospital leaders. Hospitalists should strive to improve alignment and communication with executive groups.
Disclosures
The authors report no potential conflict of interest.
1. Lapps J, Flansbaum B, Leykum L, et al. Updating threshold-based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45-47. https://doi.org/10.1002/jhm.2480.
2. Wachter RM, Goldman L. Zero to 50,000–the 20th Anniversary of the hospitalist. NEJM. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958.
3. Stevens JP, Nyweide DJ, Maresh S, et al. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781-1787. https://doi.org/10.1001/jamainternmed.2017.5824.
4. American Hospital Association (AHA) (2017), Hospital Statistics, AHA, Chicago, IL.
5. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. NEJM. 1996;335(7):514-517. https://doi.org/10.1093/ajhp/53.20.2389a.
6. Pham HH, Devers KJ, Kuo S, et al. Health care market trends and the evolution of hospitalist use and roles. J Gen Intern Med. 2005;20(2):101-107. https://doi.org/10.1111/j.1525-1497.2005.40184.x.
7. Epané JP, Weech-Maldonado R, Hearld L, et al. Hospitals’ use of hospitalistas: implications for financial performance. Health Care Manage Rev. 2019;44(1):10-18. https://doi.org/10.1097/hmr.0000000000000170.
8. State of Hospital Medicine: 2018 Report Based on 2017 Data. Society of Hospital Medicine. https://sohm.hospitalmedicine.org/ Accessed December 9, 2018.
9. Carmeli A, Tishler A. The relationships between intangible organizational elements and organizational performance. Strategic Manag J. 2004;25(13):1257-1278. https://doi.org/10.1002/smj.428.
10. Bernard M. Strategic performance management: leveraging and measuring your intangible value drivers. Amsterdam: Butterworth-Heinemann, 2006.
11. Khullar D, Wolfson D, Casalino LP. Professionalism, performance, and the future of physician incentives. JAMA. 2018;320(23):2419-2420. https://doi.org/10.1001/jama.2018.17719.
1. Lapps J, Flansbaum B, Leykum L, et al. Updating threshold-based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45-47. https://doi.org/10.1002/jhm.2480.
2. Wachter RM, Goldman L. Zero to 50,000–the 20th Anniversary of the hospitalist. NEJM. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958.
3. Stevens JP, Nyweide DJ, Maresh S, et al. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781-1787. https://doi.org/10.1001/jamainternmed.2017.5824.
4. American Hospital Association (AHA) (2017), Hospital Statistics, AHA, Chicago, IL.
5. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. NEJM. 1996;335(7):514-517. https://doi.org/10.1093/ajhp/53.20.2389a.
6. Pham HH, Devers KJ, Kuo S, et al. Health care market trends and the evolution of hospitalist use and roles. J Gen Intern Med. 2005;20(2):101-107. https://doi.org/10.1111/j.1525-1497.2005.40184.x.
7. Epané JP, Weech-Maldonado R, Hearld L, et al. Hospitals’ use of hospitalistas: implications for financial performance. Health Care Manage Rev. 2019;44(1):10-18. https://doi.org/10.1097/hmr.0000000000000170.
8. State of Hospital Medicine: 2018 Report Based on 2017 Data. Society of Hospital Medicine. https://sohm.hospitalmedicine.org/ Accessed December 9, 2018.
9. Carmeli A, Tishler A. The relationships between intangible organizational elements and organizational performance. Strategic Manag J. 2004;25(13):1257-1278. https://doi.org/10.1002/smj.428.
10. Bernard M. Strategic performance management: leveraging and measuring your intangible value drivers. Amsterdam: Butterworth-Heinemann, 2006.
11. Khullar D, Wolfson D, Casalino LP. Professionalism, performance, and the future of physician incentives. JAMA. 2018;320(23):2419-2420. https://doi.org/10.1001/jama.2018.17719.
© 2019 Society of Hospital Medicine
A Protean Protein
A 39-year-old man presented to a neurologist with three weeks of progressive leg weakness associated with numbness in his feet and fingertips. His medical history included hypertriglyceridemia, hypogonadism, and gout. He was taking fenofibrate and colchicine as needed. There was no family history of neurologic issues. He did not smoke or drink alcohol.
The patient appeared well with a heart rate of 76 beats per minute, blood pressure 133/72 mm Hg, temperature 36.6°C, respiratory rate 16 breaths per minute, and oxygen saturation 100% on room air. His cardiopulmonary and abdominal examinations were normal. His skin was warm and dry without rashes. On neurologic examination, upper extremity strength and sensation was normal. Bilateral hip flexion, knee flexion, and knee extension strength was 4/5; bilateral ankle dorsiflexion and plantar flexion strength was 3/5. Reflexes were trace in the arms and absent at the patellae and ankles. He had symmetric, length-dependent reduction in vibration, pinprick, and light touch sensation in his legs.
Peripheral neuropathy presenting with ascending symmetric motor and sensory deficits progressing over three weeks raises the suspicion of an acquired inflammatory demyelinating polyneuropathy (AIDP), a variant of Guillain-Barre Syndrome. Alternative causes of acute polyneuropathy include thiamine (B1) deficiency, vasculitis, sarcoidosis, or malignancy, particularly lymphoma and multiple myeloma. Further evaluation should include electromyography, nerve conduction studies, lumbar puncture with cerebrospinal fluid (CSF) protein, glucose, and cell count differential. Follow-up laboratory testing based on results of the above may include serum protein electrophoresis (SPEP), serum free light chains (sFLC), vitamin B12, human immunodeficiency virus (HIV), hepatitis B and C testing, antinuclear antibody, and erythrocyte sedimentation rate.
Electromyography and nerve conduction studies revealed a sensorimotor mixed axonal/demyelinating polyneuropathy in all extremities. CSF analysis found one white cell per mm3, glucose of 93 mg/dL, and protein of 313 mg/dL. Magnetic resonance imaging (MRI) of the spine without contrast showed normal cord parenchyma. The vitamin B12 level was 441 pg/mL (normal >200 pg/mL). Antibodies to HIV-1, HIV-2, hepatitis C virus, and Borrelia burgdorferi were negative. Serum protein electrophoresis (SPEP) and immunofixation were normal.
The patient received two courses of intravenous immunoglobulin (IVIG) for suspected AIDP. His weakness progressed over the next several weeks to the point that he required a wheelchair.
Progression of symptoms beyond three weeks and lack of response to IVIG are atypical for AIDP. Alternate diagnoses for a sensorimotor polyneuropathy should be considered. Causes of subacute or chronic demyelinating polyneuropathy include inflammatory conditions (chronic inflammatory demyelinating polyneuropathy [CIDP], connective-tissue disorders), paraprotein disorders (myeloma, amyloidosis, lymphoplasmacytic lymphoma), paraneoplastic syndromes, infectious diseases (HIV, Lyme disease), infiltrative disorders (sarcoidosis), medications or toxins, and hereditary disorders. Of these etiologies, the first three seem the most likely given the history and clinical course, the negative HIV and Lyme testing, and the absence of exposures and family history. Normal SPEP and immunofixation make paraprotein disorders less likely, but sFLC testing should be sent to evaluate for a light chain-only paraprotein. A paraneoplastic antibody panel and a CT of the chest, abdomen, and pelvis should be ordered to evaluate for sarcoidosis, lymphoma, or other malignancies. Although a peripheral nerve biopsy would further classify the polyneuropathy, it is of low diagnostic yield in patients with subacute and chronic distal symmetric polyneuropathies and is associated with significant morbidity. In the absence of history or physical exam findings to narrow the differential diagnosis for polyneuropathy, testing for paraneoplastic antibodies and imaging is appropriate.
The patient tested negative for antiganglioside GM1 and antimyelin-associated glycoprotein antibodies. Urine arsenic, lead, and mercury levels were normal. Tests for serum antinuclear antibody, rapid plasmin reagin, and a paraneoplastic neuropathy panel including amphiphysin antibody, CV2 antibody, and Hu auto-antibody were negative. Repeat electrodiagnostic testing was consistent with CIDP. The patient received prednisone 60 mg daily for six weeks and was then tapered to 30 mg daily over six weeks. Concurrently, he underwent twelve cycles of plasma exchange. His strength improved, and he could walk with a cane; however, weakness recurred when steroids were further tapered.
He was maintained on prednisone 50 mg daily. Over the next year, the patient’s lower extremities became flaccid and severely atrophied. He developed hyperpigmented patches on his trunk, severe gastroesophageal reflux disease (GERD), dysphonia, and gynecomastia. He had lost 60 pounds since symptom onset. He was prescribed levothyroxine for subclinical hypothyroidism (thyroid stimulating hormone 12.63 µIU/mL [normal 0.10-5.50 µIU/mL], free thyroxine 0.8 ng/dL [0.8-1.7 ng/dL]).
At this point, the diagnosis of CIDP should be questioned, and additional investigation is warranted. Although improvement was initially observed with plasma exchange and steroids, subsequent progression of symptoms despite prednisone suggests a nonimmune-mediated etiology, such as a neoplastic or infiltrative process. Conversely, negative serologic testing for paraneoplastic antibodies may be due to an antibody that has not been well characterized.
While prednisone could explain GERD and gynecomastia, the weight loss, dysphonia, and subclinical hypothyroidism may offer clues to the diagnosis underlying the neurological symptoms. Weight loss raises suspicion of a hypercatabolic process such as cancer, cachexia, systemic inflammation, heart failure, or chronic obstructive pulmonary disease. Causes of dysphonia relevant to this presentation include neurologic dysfunction related to malignant invasion of the vagus nerve or demyelinating disease. Subclinical hypothyroidism due to chronic autoimmune thyroiditis seems most likely in the absence of a medication effect or thyroid injury, yet infiltrative disorders of the thyroid (eg, amyloidosis, sarcoidosis, lymphoma) should also be considered. A diagnosis that unifies the neurologic and nonneurologic findings would be desirable; lymphoma with paraneoplastic peripheral neuropathy manifesting as CIDP seems most likely. As of yet, CT of the chest, abdomen, and pelvis or an 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) scan have not been obtained and would be helpful to evaluate for underlying malignancy. Further evaluation for a paraprotein disorder that includes sFLC is also still indicated to rule out a paraneoplastic disorder that may be associated with polyneuropathy.
Repeat SPEP and serum immunofixation were normal. sFLC assay showed elevated levels of both kappa and lambda light chains with a ratio of 0.61 (reference range: 0.26-1.25). Urine protein electrophoresis (UPEP) from a 24-hour specimen showed a homogenous band in the gamma region, but urine immunofixation demonstrated polyclonal light chains. The plasma vascular endothelial growth factor (VEGF) level was 612 pg/mL (reference range, 31-86 pg/mL).
CT imaging of the chest, abdomen, and pelvis with contrast demonstrated an enlarged liver and spleen and possible splenic infarcts. A skeletal survey and whole-body FGD-PET scan were normal. The patient declined bone marrow biopsy.
Polyneuropathy secondary to a monoclonal protein was previously considered, and an SPEP was normal. Full evaluation for a monoclonal protein additionally requires sFLC testing. If clinical suspicion remains high after a negative result, 24-hour UPEP and urine immunofixation should be obtained. Normal results in this case argue against the presence of a monoclonal protein.
The presence of a monoclonal protein and polyneuropathy are mandatory diagnostic criteria for POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes), a plasma cell proliferative disorder. Major diagnostic criteria include osteosclerotic bone lesions, Castleman’s disease, and markedly elevated VEGF levels. Castleman’s disease is a lymphoproliferative disorder characterized by angiofollicular lymphoid hyperplasia that results in lymphadenopathy in one or multiple lymph node regions. Imaging studies reveal organomegaly, one of many minor criteria, but not bone lesions or lymphadenopathy. A diagnosis of POEMS syndrome requires the presence of both mandatory, one major, and one minor criteria. Since only one of two of the mandatory criteria are met at this point, a diagnosis of POEMS syndrome cannot be made.
Eighteen months after symptom onset, the patient presented to the emergency department with dyspnea, orthopnea, and lower extremity edema. B-type natriuretic peptide was 1564 pg/mL. Transthoracic echocardiography showed a severely dilated and hypertrophied left ventricle. Left ventricular ejection fraction was 20%. A furosemide infusion was initiated. Angiography of the coronary vessels was not performed. Congo red stain of an abdominal adipose biopsy was negative for amyloid.
On hospital day five, he developed gangrenous changes in his right first toe. CT angiography of the abdomen and lower extremities demonstrated patent three vessel runoff to the foot with an infrarenal aortic thrombus. Heparin infusion was started. On hospital day 10, the patient developed expressive aphasia and somnolence, prompting intubation for airway protection. MRI and MR angiography (MRA) of the brain and cerebral vessels revealed multiple bilateral acute ischemic strokes (Figure 1) without flow limiting stenosis in cerebral vessels.
These clinical developments lead to an important opportunity to rethink this patient’s working diagnosis. The new diagnosis of heart failure in this young patient with polyneuropathy raises suspicion for an infiltrative cardiomyopathy such as amyloidosis, sarcoidosis, or Fabry disease. Of these, Fabry disease is the least likely because it is typically characterized by a painful burning sensation in response to specific triggers. Although polyneuropathy and heart failure may be concurrently observed with both sarcoidosis and amyloidosis, the absence of an apparent arrhythmia make amyloidosis the more likely of these two diagnoses. The development of an arterial thrombus and multiple strokes may represent emboli from a cardiac thrombus.
Cardiac imaging and tissue biopsy of the heart or other affected organs would distinguish between these diagnostic possibilities. An abdominal adipose biopsy negative for amyloid does not rule out amyloidosis, as the test is approximately 80% sensitive when cardiac amyloidosis is present and varies depending on the etiology of the amyloid protein (ie, light chain vs transthyretin). Evaluation of cardiac amyloid in the setting of peripheral neuropathy should include echocardiography (as was performed here) and repeat testing for a monoclonal protein.
If clinical suspicion of a paraprotein-associated disorder remains high and both SPEP and sFLC are normal, it is important to obtain a 24-hour UPEP and immunofixation. A monoclonal protein can be overlooked by SPEP and serum immunofixation if the monoclonal protein is composed only of a light chain or if the monoclonal protein is IgD or IgE. In these rare circumstances, sFLC analysis or 24-hour UPEP and immunofixation should mitigate the potential for a falsely negative SPEP/IFE. These studies are normal in this case, which argues against the presence of a monoclonal protein.
Transesophageal echocardiography showed grade IV atheromatous plaque within the descending thoracic aorta with mobile elements suggesting a superimposed thrombus; there was no intracardiac shunt or thrombus. MRA of the neck and great vessels was normal.
Testing for heparin-induced thrombocytopenia (HIT) was sent due to thrombocytopenia and the presence of thrombosis. An immunoassay for antiheparin-platelet factor 4 (anti-PF4) antibodies was substantially positive (optical density 2.178); however, functional testing with a washed platelet heparin-induced platelet activation assay was negative. Anticoagulation was changed to argatroban due to concern for HIT. Dry gangrenous changes developed in all distal toes on the right foot and three toes on the left foot. A right radial artery thrombus formed at the site of a prior arterial line.
Thrombocytopenia that develops between the fifth and tenth day following heparin exposure in a patient with new thromboses is consistent with HIT. However, the patient’s infrarenal aortic thrombus preceded the initiation of heparin, and negative functional testing undermines the diagnosis of HIT in this case. Therefore, the arterial thromboses may be related to an underlying unifying diagnosis.
A third SPEP showed a 0.1 g/dL M-spike in the gamma region, but standard immunofixation did not reveal a monoclonal protein (Figure 2). However, a specific request for immunofixation testing using IgD antisera detected an IgD heavy chain. A lambda chain comprising 3% of urine protein was detected on 24-hour urine immunofixation but was not detectable by serum immunofixation. Bone marrow biopsy demonstrated plasma cells comprising 5% of bone marrow cellularity (Figure 3); flow cytometry of the aspirate demonstrated an abnormal lambda-restricted plasma cell population.
When a monoclonal protein is identified but does not react with standard antisera to detect IgG, IgM, and IgA, immunofixation with IgD and IgE antisera are necessary to rule out a monoclonal IgD or IgE protein. The underlying IgD isotype coupled with its low abundance made detection of this monoclonal protein especially challenging. With the discovery of a monoclonal protein in the context of polyneuropathy, the mandatory criteria of POEMS syndrome are met. The elevated VEGF level and hypothyroidism meet major and minor criteria, respectively. Arterial thromboses and heart failure are other features that may be observed in cases of POEMS syndrome.
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) was diagnosed. Prednisone was continued, and weekly cyclophosphamide was initiated. After six weeks, the VEGF level remained elevated, and a neurologic examination showed minimal improvement. Due to poor respiratory muscle strength and difficulty managing secretions, he underwent percutaneous tracheostomy and gastrostomy tube placement. Unfortunately, his condition further deteriorated and he subsequently died of sepsis from pneumonia.
An autopsy revealed acute bronchopneumonia and multiple acute and subacute cerebral infarctions. There was extensive peripheral mixed axonal/demyelinating neuropathy, hepatosplenomegaly, atrophy of the thyroid and adrenal glands, hyperpigmented patches and thickened integument, and severe aortic and coronary atherosclerotic disease with a healed myocardial infarction.
DISCUSSION
POEMS syndrome1 is a rare constellation of clinical and laboratory findings resulting from an underlying plasma cell proliferative disorder. This paraneoplastic syndrome is characterized by the chronic overproduction of proinflammatory and proangiogenic cytokines, including VEGF, which are postulated to drive its manifestations,2 though the exact pathogenesis is not understood. Some of the disease’s most common features are summarized by its name: polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.3
The International Myeloma Working Group (IMWG) diagnostic criteria1 (Table) require the presence of both mandatory criteria (polyneuropathy and monoclonal plasma cell proliferation), plus at least one major and one minor criterion. Delayed diagnosis or misdiagnosis of this protean disorder is often driven by its rarity and clinical overlap with other paraprotein-associated polyneuropathies. These include amyloidosis, cryoglobulinemia, and monoclonal gammopathy of undetermined significance (MGUS), which can all produce antibodies directed against neural antigens. In addition, polyneuropathy is often the first and most striking manifestation of POEMS syndrome, fostering confusion with CIDP as both disorders are subacute, symmetric, motor-dominant, mixed axonal/demyelinating polyneuropathies.4
IgD and IgE monoclonal gammopathies are extremely rare. IgD myeloma, for instance, accounts for 2% of multiple myeloma cases, and IgE myeloma has been reported fewer than 50 times.5 IgD is secreted only in very small amounts, ordinarily representing 0.25% of the immunoglobulins in serum, while the majority is found in the plasma membranes of mature B-cells.6 These monoclonal gammopathies often escape detection for two reasons: (1) the very low paraprotein concentration produces undetectable or small M-protein levels on electrophoresis,5 and (2) immunofixation is routinely performed without antisera against IgD and IgE heavy chains.7
While this case depicts a rare manifestation of a rare disease, the principles underlying its elusive diagnosis are routinely encountered. Recognition of the specific limitations of the SPEP, UPEP, sFLC, and immunofixation tests, outlined below, can assist the hospitalist when suspicion for paraproteinemia is high.
First, low levels of monoclonal proteins may be associated with a normal SPEP. Accordingly, suspicion of a plasma cell dyscrasia should prompt serum immunofixation, even when the electrophoretic pattern appears normal.8
Second, laboratories routinely perform immunofixation with antisera against IgG, IgA, and IgM heavy chains and kappa and lambda light chains, whereas testing with IgD or IgE antisera must be specifically requested. Thus, clinicians should screen for the presence of IgD and IgE in patients with an apparently free monoclonal immunoglobulin light chain in the serum or with a monoclonal serum protein and negative immunofixation. In this case, the paraprotein was not detected on the first two serum electrophoreses, likely due to a low serum concentration, then missed on immunofixation due to a lack of IgD antiserum. On admission to the hospital, this patient had a very low paraprotein concentration (0.1 g/dL) on SPEP, and the lab initially reported negative immunofixation. When asked to test specifically for IgD and IgE, the lab ran a more comprehensive immunofixation revealing IgD heavy chain paraprotein.
Third, this case illustrates the limitations of the sFLC assay. IMWG guidelines specify that sFLC assay in combination with SPEP and serum immunofixation is sufficient to screen for monoclonal plasma cell proliferative disorders other than light chain amyloidosis (which requires all the serum tests as well as 24-hour urine immunofixation).9 Though the sFLC assay has been demonstrated to be more sensitive than urine analysis for detecting monoclonal free light chains,10 it is still subject to false negatives. Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal sFLC,11 the latter of which likely explains why the sFLC ratio was repeatedly normal in this case. In these circumstances, monoclonal free light chains can be identified by urine studies.11 In this case, 24-hour urine immunofixation detected the excess light chain that was not evident on the sFLC assay. Even with these pitfalls in mind, there is still no evident explanation as to why the 24-hour urine studies done prior to the patient’s hospital admission did not reveal a monoclonal light chain.
This case also highlights the thrombotic diathesis in POEMS syndrome. Although the patient was treated with argatroban for suspected HIT, it is likely that the HIT antibody result was a false positive, and his thrombi were better explained by POEMS syndrome in and of itself. Coronary, limb, and cerebral artery thromboses have been linked to POEMS syndrome,12,13 all of which were present in this case. Laboratory testing for HIT involves an immunoassay to detect circulating HIT antibody and a functional assay to measure platelet activity in the presence of patient serum and heparin. The immunoassay binds anti-PF4/heparin complex irrespective of its ability to activate platelets. The presence of nonspecific antibodies may lead to cross-reactions with the immunoassay test components, which has been demonstrated in cases of MGUS.14 In this case, elevated production of monoclonal antibodies by plasma cells may have led to false-positive results. With moderate to high clinical suspicion of HIT, the combination of a positive immunoassay and negative functional assay (as in this case) make the diagnosis of HIT indeterminate.15
TEACHING POINTS
- If a monoclonal protein is suggested by SPEP but cannot be identified by standard immunofixation, request immunofixation for IgD or IgE. Screen patients for IgD and IgE paraproteins before making a diagnosis of light chain multiple myeloma.
- Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal FLC and result in a normal sFLC ratio.
- Thrombosis is a less-recognized but documented feature of POEMS syndrome which may be mediated by the overproduction of proinflammatory and proangiogenic cytokines, though the precise pathogenesis is unknown.
Acknowledgment
The authors thank Dr. Theodore Kurtz and Dr. Anne Deucher from the department of laboratory medicine at the University of California, San Francisco for providing their respective expertise in clinical chemistry and hematopathology.
Disclosures
The authors have no conflicts of interests to disclose.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5. PubMed
2. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. doi: 10.1016/S0140-6736(96)91261-1. PubMed
3. Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119(24):5650-5658. doi: 10.1182/blood-2012-03-378992. PubMed
4. Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2012;83(5):476-479. doi: 10.1136/jnnp-2011-301706. PubMed
5. Pandey S, Kyle RA. Unusual myelomas: a review of IgD and IgE variants. Oncology. 2013;27(8):798-803. PubMed
6. Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7(2):131-140. doi: 10.1128/CDLI.7.2.131-140.2000. PubMed
7. Sinclair D, Cranfield T. IgD myeloma: A potential missed diagnosis. Ann Clin Biochem. 2001;38(5):564-565. doi: 10.1177/000456320103800517. PubMed
8. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705. doi: 10.1182/blood-2010-10-299529. PubMed
9. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215-224. doi: 10.1038/leu.2008.307. PubMed
10. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356-362. doi: 10.3324/haematol.2015.126797. PubMed
11. Levinson SS. Polyclonal free light chain of Ig may interfere with interpretation of monoclonal free light chain κ/λ ratio. Ann Clin Lab Sci. 2010;40(4):348-353. PubMed
12. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496-2506. doi: 10.1182/blood-2002-07-2299. PubMed
13. Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308-1312. doi: 10.1212/WNL.0b013e3181bd136b. PubMed
14. Markovic I, Debeljak Z, Bosnjak B, Marijanovic M. False positive immunoassay for heparin-induced thrombocytopenia in the presence of monoclonal gammopathy: a case report. Biochemia Medica. 2017;27(3):030801. doi: 10.11613/BM.2017.030801. PubMed
15. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
A 39-year-old man presented to a neurologist with three weeks of progressive leg weakness associated with numbness in his feet and fingertips. His medical history included hypertriglyceridemia, hypogonadism, and gout. He was taking fenofibrate and colchicine as needed. There was no family history of neurologic issues. He did not smoke or drink alcohol.
The patient appeared well with a heart rate of 76 beats per minute, blood pressure 133/72 mm Hg, temperature 36.6°C, respiratory rate 16 breaths per minute, and oxygen saturation 100% on room air. His cardiopulmonary and abdominal examinations were normal. His skin was warm and dry without rashes. On neurologic examination, upper extremity strength and sensation was normal. Bilateral hip flexion, knee flexion, and knee extension strength was 4/5; bilateral ankle dorsiflexion and plantar flexion strength was 3/5. Reflexes were trace in the arms and absent at the patellae and ankles. He had symmetric, length-dependent reduction in vibration, pinprick, and light touch sensation in his legs.
Peripheral neuropathy presenting with ascending symmetric motor and sensory deficits progressing over three weeks raises the suspicion of an acquired inflammatory demyelinating polyneuropathy (AIDP), a variant of Guillain-Barre Syndrome. Alternative causes of acute polyneuropathy include thiamine (B1) deficiency, vasculitis, sarcoidosis, or malignancy, particularly lymphoma and multiple myeloma. Further evaluation should include electromyography, nerve conduction studies, lumbar puncture with cerebrospinal fluid (CSF) protein, glucose, and cell count differential. Follow-up laboratory testing based on results of the above may include serum protein electrophoresis (SPEP), serum free light chains (sFLC), vitamin B12, human immunodeficiency virus (HIV), hepatitis B and C testing, antinuclear antibody, and erythrocyte sedimentation rate.
Electromyography and nerve conduction studies revealed a sensorimotor mixed axonal/demyelinating polyneuropathy in all extremities. CSF analysis found one white cell per mm3, glucose of 93 mg/dL, and protein of 313 mg/dL. Magnetic resonance imaging (MRI) of the spine without contrast showed normal cord parenchyma. The vitamin B12 level was 441 pg/mL (normal >200 pg/mL). Antibodies to HIV-1, HIV-2, hepatitis C virus, and Borrelia burgdorferi were negative. Serum protein electrophoresis (SPEP) and immunofixation were normal.
The patient received two courses of intravenous immunoglobulin (IVIG) for suspected AIDP. His weakness progressed over the next several weeks to the point that he required a wheelchair.
Progression of symptoms beyond three weeks and lack of response to IVIG are atypical for AIDP. Alternate diagnoses for a sensorimotor polyneuropathy should be considered. Causes of subacute or chronic demyelinating polyneuropathy include inflammatory conditions (chronic inflammatory demyelinating polyneuropathy [CIDP], connective-tissue disorders), paraprotein disorders (myeloma, amyloidosis, lymphoplasmacytic lymphoma), paraneoplastic syndromes, infectious diseases (HIV, Lyme disease), infiltrative disorders (sarcoidosis), medications or toxins, and hereditary disorders. Of these etiologies, the first three seem the most likely given the history and clinical course, the negative HIV and Lyme testing, and the absence of exposures and family history. Normal SPEP and immunofixation make paraprotein disorders less likely, but sFLC testing should be sent to evaluate for a light chain-only paraprotein. A paraneoplastic antibody panel and a CT of the chest, abdomen, and pelvis should be ordered to evaluate for sarcoidosis, lymphoma, or other malignancies. Although a peripheral nerve biopsy would further classify the polyneuropathy, it is of low diagnostic yield in patients with subacute and chronic distal symmetric polyneuropathies and is associated with significant morbidity. In the absence of history or physical exam findings to narrow the differential diagnosis for polyneuropathy, testing for paraneoplastic antibodies and imaging is appropriate.
The patient tested negative for antiganglioside GM1 and antimyelin-associated glycoprotein antibodies. Urine arsenic, lead, and mercury levels were normal. Tests for serum antinuclear antibody, rapid plasmin reagin, and a paraneoplastic neuropathy panel including amphiphysin antibody, CV2 antibody, and Hu auto-antibody were negative. Repeat electrodiagnostic testing was consistent with CIDP. The patient received prednisone 60 mg daily for six weeks and was then tapered to 30 mg daily over six weeks. Concurrently, he underwent twelve cycles of plasma exchange. His strength improved, and he could walk with a cane; however, weakness recurred when steroids were further tapered.
He was maintained on prednisone 50 mg daily. Over the next year, the patient’s lower extremities became flaccid and severely atrophied. He developed hyperpigmented patches on his trunk, severe gastroesophageal reflux disease (GERD), dysphonia, and gynecomastia. He had lost 60 pounds since symptom onset. He was prescribed levothyroxine for subclinical hypothyroidism (thyroid stimulating hormone 12.63 µIU/mL [normal 0.10-5.50 µIU/mL], free thyroxine 0.8 ng/dL [0.8-1.7 ng/dL]).
At this point, the diagnosis of CIDP should be questioned, and additional investigation is warranted. Although improvement was initially observed with plasma exchange and steroids, subsequent progression of symptoms despite prednisone suggests a nonimmune-mediated etiology, such as a neoplastic or infiltrative process. Conversely, negative serologic testing for paraneoplastic antibodies may be due to an antibody that has not been well characterized.
While prednisone could explain GERD and gynecomastia, the weight loss, dysphonia, and subclinical hypothyroidism may offer clues to the diagnosis underlying the neurological symptoms. Weight loss raises suspicion of a hypercatabolic process such as cancer, cachexia, systemic inflammation, heart failure, or chronic obstructive pulmonary disease. Causes of dysphonia relevant to this presentation include neurologic dysfunction related to malignant invasion of the vagus nerve or demyelinating disease. Subclinical hypothyroidism due to chronic autoimmune thyroiditis seems most likely in the absence of a medication effect or thyroid injury, yet infiltrative disorders of the thyroid (eg, amyloidosis, sarcoidosis, lymphoma) should also be considered. A diagnosis that unifies the neurologic and nonneurologic findings would be desirable; lymphoma with paraneoplastic peripheral neuropathy manifesting as CIDP seems most likely. As of yet, CT of the chest, abdomen, and pelvis or an 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) scan have not been obtained and would be helpful to evaluate for underlying malignancy. Further evaluation for a paraprotein disorder that includes sFLC is also still indicated to rule out a paraneoplastic disorder that may be associated with polyneuropathy.
Repeat SPEP and serum immunofixation were normal. sFLC assay showed elevated levels of both kappa and lambda light chains with a ratio of 0.61 (reference range: 0.26-1.25). Urine protein electrophoresis (UPEP) from a 24-hour specimen showed a homogenous band in the gamma region, but urine immunofixation demonstrated polyclonal light chains. The plasma vascular endothelial growth factor (VEGF) level was 612 pg/mL (reference range, 31-86 pg/mL).
CT imaging of the chest, abdomen, and pelvis with contrast demonstrated an enlarged liver and spleen and possible splenic infarcts. A skeletal survey and whole-body FGD-PET scan were normal. The patient declined bone marrow biopsy.
Polyneuropathy secondary to a monoclonal protein was previously considered, and an SPEP was normal. Full evaluation for a monoclonal protein additionally requires sFLC testing. If clinical suspicion remains high after a negative result, 24-hour UPEP and urine immunofixation should be obtained. Normal results in this case argue against the presence of a monoclonal protein.
The presence of a monoclonal protein and polyneuropathy are mandatory diagnostic criteria for POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes), a plasma cell proliferative disorder. Major diagnostic criteria include osteosclerotic bone lesions, Castleman’s disease, and markedly elevated VEGF levels. Castleman’s disease is a lymphoproliferative disorder characterized by angiofollicular lymphoid hyperplasia that results in lymphadenopathy in one or multiple lymph node regions. Imaging studies reveal organomegaly, one of many minor criteria, but not bone lesions or lymphadenopathy. A diagnosis of POEMS syndrome requires the presence of both mandatory, one major, and one minor criteria. Since only one of two of the mandatory criteria are met at this point, a diagnosis of POEMS syndrome cannot be made.
Eighteen months after symptom onset, the patient presented to the emergency department with dyspnea, orthopnea, and lower extremity edema. B-type natriuretic peptide was 1564 pg/mL. Transthoracic echocardiography showed a severely dilated and hypertrophied left ventricle. Left ventricular ejection fraction was 20%. A furosemide infusion was initiated. Angiography of the coronary vessels was not performed. Congo red stain of an abdominal adipose biopsy was negative for amyloid.
On hospital day five, he developed gangrenous changes in his right first toe. CT angiography of the abdomen and lower extremities demonstrated patent three vessel runoff to the foot with an infrarenal aortic thrombus. Heparin infusion was started. On hospital day 10, the patient developed expressive aphasia and somnolence, prompting intubation for airway protection. MRI and MR angiography (MRA) of the brain and cerebral vessels revealed multiple bilateral acute ischemic strokes (Figure 1) without flow limiting stenosis in cerebral vessels.
These clinical developments lead to an important opportunity to rethink this patient’s working diagnosis. The new diagnosis of heart failure in this young patient with polyneuropathy raises suspicion for an infiltrative cardiomyopathy such as amyloidosis, sarcoidosis, or Fabry disease. Of these, Fabry disease is the least likely because it is typically characterized by a painful burning sensation in response to specific triggers. Although polyneuropathy and heart failure may be concurrently observed with both sarcoidosis and amyloidosis, the absence of an apparent arrhythmia make amyloidosis the more likely of these two diagnoses. The development of an arterial thrombus and multiple strokes may represent emboli from a cardiac thrombus.
Cardiac imaging and tissue biopsy of the heart or other affected organs would distinguish between these diagnostic possibilities. An abdominal adipose biopsy negative for amyloid does not rule out amyloidosis, as the test is approximately 80% sensitive when cardiac amyloidosis is present and varies depending on the etiology of the amyloid protein (ie, light chain vs transthyretin). Evaluation of cardiac amyloid in the setting of peripheral neuropathy should include echocardiography (as was performed here) and repeat testing for a monoclonal protein.
If clinical suspicion of a paraprotein-associated disorder remains high and both SPEP and sFLC are normal, it is important to obtain a 24-hour UPEP and immunofixation. A monoclonal protein can be overlooked by SPEP and serum immunofixation if the monoclonal protein is composed only of a light chain or if the monoclonal protein is IgD or IgE. In these rare circumstances, sFLC analysis or 24-hour UPEP and immunofixation should mitigate the potential for a falsely negative SPEP/IFE. These studies are normal in this case, which argues against the presence of a monoclonal protein.
Transesophageal echocardiography showed grade IV atheromatous plaque within the descending thoracic aorta with mobile elements suggesting a superimposed thrombus; there was no intracardiac shunt or thrombus. MRA of the neck and great vessels was normal.
Testing for heparin-induced thrombocytopenia (HIT) was sent due to thrombocytopenia and the presence of thrombosis. An immunoassay for antiheparin-platelet factor 4 (anti-PF4) antibodies was substantially positive (optical density 2.178); however, functional testing with a washed platelet heparin-induced platelet activation assay was negative. Anticoagulation was changed to argatroban due to concern for HIT. Dry gangrenous changes developed in all distal toes on the right foot and three toes on the left foot. A right radial artery thrombus formed at the site of a prior arterial line.
Thrombocytopenia that develops between the fifth and tenth day following heparin exposure in a patient with new thromboses is consistent with HIT. However, the patient’s infrarenal aortic thrombus preceded the initiation of heparin, and negative functional testing undermines the diagnosis of HIT in this case. Therefore, the arterial thromboses may be related to an underlying unifying diagnosis.
A third SPEP showed a 0.1 g/dL M-spike in the gamma region, but standard immunofixation did not reveal a monoclonal protein (Figure 2). However, a specific request for immunofixation testing using IgD antisera detected an IgD heavy chain. A lambda chain comprising 3% of urine protein was detected on 24-hour urine immunofixation but was not detectable by serum immunofixation. Bone marrow biopsy demonstrated plasma cells comprising 5% of bone marrow cellularity (Figure 3); flow cytometry of the aspirate demonstrated an abnormal lambda-restricted plasma cell population.
When a monoclonal protein is identified but does not react with standard antisera to detect IgG, IgM, and IgA, immunofixation with IgD and IgE antisera are necessary to rule out a monoclonal IgD or IgE protein. The underlying IgD isotype coupled with its low abundance made detection of this monoclonal protein especially challenging. With the discovery of a monoclonal protein in the context of polyneuropathy, the mandatory criteria of POEMS syndrome are met. The elevated VEGF level and hypothyroidism meet major and minor criteria, respectively. Arterial thromboses and heart failure are other features that may be observed in cases of POEMS syndrome.
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) was diagnosed. Prednisone was continued, and weekly cyclophosphamide was initiated. After six weeks, the VEGF level remained elevated, and a neurologic examination showed minimal improvement. Due to poor respiratory muscle strength and difficulty managing secretions, he underwent percutaneous tracheostomy and gastrostomy tube placement. Unfortunately, his condition further deteriorated and he subsequently died of sepsis from pneumonia.
An autopsy revealed acute bronchopneumonia and multiple acute and subacute cerebral infarctions. There was extensive peripheral mixed axonal/demyelinating neuropathy, hepatosplenomegaly, atrophy of the thyroid and adrenal glands, hyperpigmented patches and thickened integument, and severe aortic and coronary atherosclerotic disease with a healed myocardial infarction.
DISCUSSION
POEMS syndrome1 is a rare constellation of clinical and laboratory findings resulting from an underlying plasma cell proliferative disorder. This paraneoplastic syndrome is characterized by the chronic overproduction of proinflammatory and proangiogenic cytokines, including VEGF, which are postulated to drive its manifestations,2 though the exact pathogenesis is not understood. Some of the disease’s most common features are summarized by its name: polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.3
The International Myeloma Working Group (IMWG) diagnostic criteria1 (Table) require the presence of both mandatory criteria (polyneuropathy and monoclonal plasma cell proliferation), plus at least one major and one minor criterion. Delayed diagnosis or misdiagnosis of this protean disorder is often driven by its rarity and clinical overlap with other paraprotein-associated polyneuropathies. These include amyloidosis, cryoglobulinemia, and monoclonal gammopathy of undetermined significance (MGUS), which can all produce antibodies directed against neural antigens. In addition, polyneuropathy is often the first and most striking manifestation of POEMS syndrome, fostering confusion with CIDP as both disorders are subacute, symmetric, motor-dominant, mixed axonal/demyelinating polyneuropathies.4
IgD and IgE monoclonal gammopathies are extremely rare. IgD myeloma, for instance, accounts for 2% of multiple myeloma cases, and IgE myeloma has been reported fewer than 50 times.5 IgD is secreted only in very small amounts, ordinarily representing 0.25% of the immunoglobulins in serum, while the majority is found in the plasma membranes of mature B-cells.6 These monoclonal gammopathies often escape detection for two reasons: (1) the very low paraprotein concentration produces undetectable or small M-protein levels on electrophoresis,5 and (2) immunofixation is routinely performed without antisera against IgD and IgE heavy chains.7
While this case depicts a rare manifestation of a rare disease, the principles underlying its elusive diagnosis are routinely encountered. Recognition of the specific limitations of the SPEP, UPEP, sFLC, and immunofixation tests, outlined below, can assist the hospitalist when suspicion for paraproteinemia is high.
First, low levels of monoclonal proteins may be associated with a normal SPEP. Accordingly, suspicion of a plasma cell dyscrasia should prompt serum immunofixation, even when the electrophoretic pattern appears normal.8
Second, laboratories routinely perform immunofixation with antisera against IgG, IgA, and IgM heavy chains and kappa and lambda light chains, whereas testing with IgD or IgE antisera must be specifically requested. Thus, clinicians should screen for the presence of IgD and IgE in patients with an apparently free monoclonal immunoglobulin light chain in the serum or with a monoclonal serum protein and negative immunofixation. In this case, the paraprotein was not detected on the first two serum electrophoreses, likely due to a low serum concentration, then missed on immunofixation due to a lack of IgD antiserum. On admission to the hospital, this patient had a very low paraprotein concentration (0.1 g/dL) on SPEP, and the lab initially reported negative immunofixation. When asked to test specifically for IgD and IgE, the lab ran a more comprehensive immunofixation revealing IgD heavy chain paraprotein.
Third, this case illustrates the limitations of the sFLC assay. IMWG guidelines specify that sFLC assay in combination with SPEP and serum immunofixation is sufficient to screen for monoclonal plasma cell proliferative disorders other than light chain amyloidosis (which requires all the serum tests as well as 24-hour urine immunofixation).9 Though the sFLC assay has been demonstrated to be more sensitive than urine analysis for detecting monoclonal free light chains,10 it is still subject to false negatives. Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal sFLC,11 the latter of which likely explains why the sFLC ratio was repeatedly normal in this case. In these circumstances, monoclonal free light chains can be identified by urine studies.11 In this case, 24-hour urine immunofixation detected the excess light chain that was not evident on the sFLC assay. Even with these pitfalls in mind, there is still no evident explanation as to why the 24-hour urine studies done prior to the patient’s hospital admission did not reveal a monoclonal light chain.
This case also highlights the thrombotic diathesis in POEMS syndrome. Although the patient was treated with argatroban for suspected HIT, it is likely that the HIT antibody result was a false positive, and his thrombi were better explained by POEMS syndrome in and of itself. Coronary, limb, and cerebral artery thromboses have been linked to POEMS syndrome,12,13 all of which were present in this case. Laboratory testing for HIT involves an immunoassay to detect circulating HIT antibody and a functional assay to measure platelet activity in the presence of patient serum and heparin. The immunoassay binds anti-PF4/heparin complex irrespective of its ability to activate platelets. The presence of nonspecific antibodies may lead to cross-reactions with the immunoassay test components, which has been demonstrated in cases of MGUS.14 In this case, elevated production of monoclonal antibodies by plasma cells may have led to false-positive results. With moderate to high clinical suspicion of HIT, the combination of a positive immunoassay and negative functional assay (as in this case) make the diagnosis of HIT indeterminate.15
TEACHING POINTS
- If a monoclonal protein is suggested by SPEP but cannot be identified by standard immunofixation, request immunofixation for IgD or IgE. Screen patients for IgD and IgE paraproteins before making a diagnosis of light chain multiple myeloma.
- Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal FLC and result in a normal sFLC ratio.
- Thrombosis is a less-recognized but documented feature of POEMS syndrome which may be mediated by the overproduction of proinflammatory and proangiogenic cytokines, though the precise pathogenesis is unknown.
Acknowledgment
The authors thank Dr. Theodore Kurtz and Dr. Anne Deucher from the department of laboratory medicine at the University of California, San Francisco for providing their respective expertise in clinical chemistry and hematopathology.
Disclosures
The authors have no conflicts of interests to disclose.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5.
A 39-year-old man presented to a neurologist with three weeks of progressive leg weakness associated with numbness in his feet and fingertips. His medical history included hypertriglyceridemia, hypogonadism, and gout. He was taking fenofibrate and colchicine as needed. There was no family history of neurologic issues. He did not smoke or drink alcohol.
The patient appeared well with a heart rate of 76 beats per minute, blood pressure 133/72 mm Hg, temperature 36.6°C, respiratory rate 16 breaths per minute, and oxygen saturation 100% on room air. His cardiopulmonary and abdominal examinations were normal. His skin was warm and dry without rashes. On neurologic examination, upper extremity strength and sensation was normal. Bilateral hip flexion, knee flexion, and knee extension strength was 4/5; bilateral ankle dorsiflexion and plantar flexion strength was 3/5. Reflexes were trace in the arms and absent at the patellae and ankles. He had symmetric, length-dependent reduction in vibration, pinprick, and light touch sensation in his legs.
Peripheral neuropathy presenting with ascending symmetric motor and sensory deficits progressing over three weeks raises the suspicion of an acquired inflammatory demyelinating polyneuropathy (AIDP), a variant of Guillain-Barre Syndrome. Alternative causes of acute polyneuropathy include thiamine (B1) deficiency, vasculitis, sarcoidosis, or malignancy, particularly lymphoma and multiple myeloma. Further evaluation should include electromyography, nerve conduction studies, lumbar puncture with cerebrospinal fluid (CSF) protein, glucose, and cell count differential. Follow-up laboratory testing based on results of the above may include serum protein electrophoresis (SPEP), serum free light chains (sFLC), vitamin B12, human immunodeficiency virus (HIV), hepatitis B and C testing, antinuclear antibody, and erythrocyte sedimentation rate.
Electromyography and nerve conduction studies revealed a sensorimotor mixed axonal/demyelinating polyneuropathy in all extremities. CSF analysis found one white cell per mm3, glucose of 93 mg/dL, and protein of 313 mg/dL. Magnetic resonance imaging (MRI) of the spine without contrast showed normal cord parenchyma. The vitamin B12 level was 441 pg/mL (normal >200 pg/mL). Antibodies to HIV-1, HIV-2, hepatitis C virus, and Borrelia burgdorferi were negative. Serum protein electrophoresis (SPEP) and immunofixation were normal.
The patient received two courses of intravenous immunoglobulin (IVIG) for suspected AIDP. His weakness progressed over the next several weeks to the point that he required a wheelchair.
Progression of symptoms beyond three weeks and lack of response to IVIG are atypical for AIDP. Alternate diagnoses for a sensorimotor polyneuropathy should be considered. Causes of subacute or chronic demyelinating polyneuropathy include inflammatory conditions (chronic inflammatory demyelinating polyneuropathy [CIDP], connective-tissue disorders), paraprotein disorders (myeloma, amyloidosis, lymphoplasmacytic lymphoma), paraneoplastic syndromes, infectious diseases (HIV, Lyme disease), infiltrative disorders (sarcoidosis), medications or toxins, and hereditary disorders. Of these etiologies, the first three seem the most likely given the history and clinical course, the negative HIV and Lyme testing, and the absence of exposures and family history. Normal SPEP and immunofixation make paraprotein disorders less likely, but sFLC testing should be sent to evaluate for a light chain-only paraprotein. A paraneoplastic antibody panel and a CT of the chest, abdomen, and pelvis should be ordered to evaluate for sarcoidosis, lymphoma, or other malignancies. Although a peripheral nerve biopsy would further classify the polyneuropathy, it is of low diagnostic yield in patients with subacute and chronic distal symmetric polyneuropathies and is associated with significant morbidity. In the absence of history or physical exam findings to narrow the differential diagnosis for polyneuropathy, testing for paraneoplastic antibodies and imaging is appropriate.
The patient tested negative for antiganglioside GM1 and antimyelin-associated glycoprotein antibodies. Urine arsenic, lead, and mercury levels were normal. Tests for serum antinuclear antibody, rapid plasmin reagin, and a paraneoplastic neuropathy panel including amphiphysin antibody, CV2 antibody, and Hu auto-antibody were negative. Repeat electrodiagnostic testing was consistent with CIDP. The patient received prednisone 60 mg daily for six weeks and was then tapered to 30 mg daily over six weeks. Concurrently, he underwent twelve cycles of plasma exchange. His strength improved, and he could walk with a cane; however, weakness recurred when steroids were further tapered.
He was maintained on prednisone 50 mg daily. Over the next year, the patient’s lower extremities became flaccid and severely atrophied. He developed hyperpigmented patches on his trunk, severe gastroesophageal reflux disease (GERD), dysphonia, and gynecomastia. He had lost 60 pounds since symptom onset. He was prescribed levothyroxine for subclinical hypothyroidism (thyroid stimulating hormone 12.63 µIU/mL [normal 0.10-5.50 µIU/mL], free thyroxine 0.8 ng/dL [0.8-1.7 ng/dL]).
At this point, the diagnosis of CIDP should be questioned, and additional investigation is warranted. Although improvement was initially observed with plasma exchange and steroids, subsequent progression of symptoms despite prednisone suggests a nonimmune-mediated etiology, such as a neoplastic or infiltrative process. Conversely, negative serologic testing for paraneoplastic antibodies may be due to an antibody that has not been well characterized.
While prednisone could explain GERD and gynecomastia, the weight loss, dysphonia, and subclinical hypothyroidism may offer clues to the diagnosis underlying the neurological symptoms. Weight loss raises suspicion of a hypercatabolic process such as cancer, cachexia, systemic inflammation, heart failure, or chronic obstructive pulmonary disease. Causes of dysphonia relevant to this presentation include neurologic dysfunction related to malignant invasion of the vagus nerve or demyelinating disease. Subclinical hypothyroidism due to chronic autoimmune thyroiditis seems most likely in the absence of a medication effect or thyroid injury, yet infiltrative disorders of the thyroid (eg, amyloidosis, sarcoidosis, lymphoma) should also be considered. A diagnosis that unifies the neurologic and nonneurologic findings would be desirable; lymphoma with paraneoplastic peripheral neuropathy manifesting as CIDP seems most likely. As of yet, CT of the chest, abdomen, and pelvis or an 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) scan have not been obtained and would be helpful to evaluate for underlying malignancy. Further evaluation for a paraprotein disorder that includes sFLC is also still indicated to rule out a paraneoplastic disorder that may be associated with polyneuropathy.
Repeat SPEP and serum immunofixation were normal. sFLC assay showed elevated levels of both kappa and lambda light chains with a ratio of 0.61 (reference range: 0.26-1.25). Urine protein electrophoresis (UPEP) from a 24-hour specimen showed a homogenous band in the gamma region, but urine immunofixation demonstrated polyclonal light chains. The plasma vascular endothelial growth factor (VEGF) level was 612 pg/mL (reference range, 31-86 pg/mL).
CT imaging of the chest, abdomen, and pelvis with contrast demonstrated an enlarged liver and spleen and possible splenic infarcts. A skeletal survey and whole-body FGD-PET scan were normal. The patient declined bone marrow biopsy.
Polyneuropathy secondary to a monoclonal protein was previously considered, and an SPEP was normal. Full evaluation for a monoclonal protein additionally requires sFLC testing. If clinical suspicion remains high after a negative result, 24-hour UPEP and urine immunofixation should be obtained. Normal results in this case argue against the presence of a monoclonal protein.
The presence of a monoclonal protein and polyneuropathy are mandatory diagnostic criteria for POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes), a plasma cell proliferative disorder. Major diagnostic criteria include osteosclerotic bone lesions, Castleman’s disease, and markedly elevated VEGF levels. Castleman’s disease is a lymphoproliferative disorder characterized by angiofollicular lymphoid hyperplasia that results in lymphadenopathy in one or multiple lymph node regions. Imaging studies reveal organomegaly, one of many minor criteria, but not bone lesions or lymphadenopathy. A diagnosis of POEMS syndrome requires the presence of both mandatory, one major, and one minor criteria. Since only one of two of the mandatory criteria are met at this point, a diagnosis of POEMS syndrome cannot be made.
Eighteen months after symptom onset, the patient presented to the emergency department with dyspnea, orthopnea, and lower extremity edema. B-type natriuretic peptide was 1564 pg/mL. Transthoracic echocardiography showed a severely dilated and hypertrophied left ventricle. Left ventricular ejection fraction was 20%. A furosemide infusion was initiated. Angiography of the coronary vessels was not performed. Congo red stain of an abdominal adipose biopsy was negative for amyloid.
On hospital day five, he developed gangrenous changes in his right first toe. CT angiography of the abdomen and lower extremities demonstrated patent three vessel runoff to the foot with an infrarenal aortic thrombus. Heparin infusion was started. On hospital day 10, the patient developed expressive aphasia and somnolence, prompting intubation for airway protection. MRI and MR angiography (MRA) of the brain and cerebral vessels revealed multiple bilateral acute ischemic strokes (Figure 1) without flow limiting stenosis in cerebral vessels.
These clinical developments lead to an important opportunity to rethink this patient’s working diagnosis. The new diagnosis of heart failure in this young patient with polyneuropathy raises suspicion for an infiltrative cardiomyopathy such as amyloidosis, sarcoidosis, or Fabry disease. Of these, Fabry disease is the least likely because it is typically characterized by a painful burning sensation in response to specific triggers. Although polyneuropathy and heart failure may be concurrently observed with both sarcoidosis and amyloidosis, the absence of an apparent arrhythmia make amyloidosis the more likely of these two diagnoses. The development of an arterial thrombus and multiple strokes may represent emboli from a cardiac thrombus.
Cardiac imaging and tissue biopsy of the heart or other affected organs would distinguish between these diagnostic possibilities. An abdominal adipose biopsy negative for amyloid does not rule out amyloidosis, as the test is approximately 80% sensitive when cardiac amyloidosis is present and varies depending on the etiology of the amyloid protein (ie, light chain vs transthyretin). Evaluation of cardiac amyloid in the setting of peripheral neuropathy should include echocardiography (as was performed here) and repeat testing for a monoclonal protein.
If clinical suspicion of a paraprotein-associated disorder remains high and both SPEP and sFLC are normal, it is important to obtain a 24-hour UPEP and immunofixation. A monoclonal protein can be overlooked by SPEP and serum immunofixation if the monoclonal protein is composed only of a light chain or if the monoclonal protein is IgD or IgE. In these rare circumstances, sFLC analysis or 24-hour UPEP and immunofixation should mitigate the potential for a falsely negative SPEP/IFE. These studies are normal in this case, which argues against the presence of a monoclonal protein.
Transesophageal echocardiography showed grade IV atheromatous plaque within the descending thoracic aorta with mobile elements suggesting a superimposed thrombus; there was no intracardiac shunt or thrombus. MRA of the neck and great vessels was normal.
Testing for heparin-induced thrombocytopenia (HIT) was sent due to thrombocytopenia and the presence of thrombosis. An immunoassay for antiheparin-platelet factor 4 (anti-PF4) antibodies was substantially positive (optical density 2.178); however, functional testing with a washed platelet heparin-induced platelet activation assay was negative. Anticoagulation was changed to argatroban due to concern for HIT. Dry gangrenous changes developed in all distal toes on the right foot and three toes on the left foot. A right radial artery thrombus formed at the site of a prior arterial line.
Thrombocytopenia that develops between the fifth and tenth day following heparin exposure in a patient with new thromboses is consistent with HIT. However, the patient’s infrarenal aortic thrombus preceded the initiation of heparin, and negative functional testing undermines the diagnosis of HIT in this case. Therefore, the arterial thromboses may be related to an underlying unifying diagnosis.
A third SPEP showed a 0.1 g/dL M-spike in the gamma region, but standard immunofixation did not reveal a monoclonal protein (Figure 2). However, a specific request for immunofixation testing using IgD antisera detected an IgD heavy chain. A lambda chain comprising 3% of urine protein was detected on 24-hour urine immunofixation but was not detectable by serum immunofixation. Bone marrow biopsy demonstrated plasma cells comprising 5% of bone marrow cellularity (Figure 3); flow cytometry of the aspirate demonstrated an abnormal lambda-restricted plasma cell population.
When a monoclonal protein is identified but does not react with standard antisera to detect IgG, IgM, and IgA, immunofixation with IgD and IgE antisera are necessary to rule out a monoclonal IgD or IgE protein. The underlying IgD isotype coupled with its low abundance made detection of this monoclonal protein especially challenging. With the discovery of a monoclonal protein in the context of polyneuropathy, the mandatory criteria of POEMS syndrome are met. The elevated VEGF level and hypothyroidism meet major and minor criteria, respectively. Arterial thromboses and heart failure are other features that may be observed in cases of POEMS syndrome.
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) was diagnosed. Prednisone was continued, and weekly cyclophosphamide was initiated. After six weeks, the VEGF level remained elevated, and a neurologic examination showed minimal improvement. Due to poor respiratory muscle strength and difficulty managing secretions, he underwent percutaneous tracheostomy and gastrostomy tube placement. Unfortunately, his condition further deteriorated and he subsequently died of sepsis from pneumonia.
An autopsy revealed acute bronchopneumonia and multiple acute and subacute cerebral infarctions. There was extensive peripheral mixed axonal/demyelinating neuropathy, hepatosplenomegaly, atrophy of the thyroid and adrenal glands, hyperpigmented patches and thickened integument, and severe aortic and coronary atherosclerotic disease with a healed myocardial infarction.
DISCUSSION
POEMS syndrome1 is a rare constellation of clinical and laboratory findings resulting from an underlying plasma cell proliferative disorder. This paraneoplastic syndrome is characterized by the chronic overproduction of proinflammatory and proangiogenic cytokines, including VEGF, which are postulated to drive its manifestations,2 though the exact pathogenesis is not understood. Some of the disease’s most common features are summarized by its name: polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.3
The International Myeloma Working Group (IMWG) diagnostic criteria1 (Table) require the presence of both mandatory criteria (polyneuropathy and monoclonal plasma cell proliferation), plus at least one major and one minor criterion. Delayed diagnosis or misdiagnosis of this protean disorder is often driven by its rarity and clinical overlap with other paraprotein-associated polyneuropathies. These include amyloidosis, cryoglobulinemia, and monoclonal gammopathy of undetermined significance (MGUS), which can all produce antibodies directed against neural antigens. In addition, polyneuropathy is often the first and most striking manifestation of POEMS syndrome, fostering confusion with CIDP as both disorders are subacute, symmetric, motor-dominant, mixed axonal/demyelinating polyneuropathies.4
IgD and IgE monoclonal gammopathies are extremely rare. IgD myeloma, for instance, accounts for 2% of multiple myeloma cases, and IgE myeloma has been reported fewer than 50 times.5 IgD is secreted only in very small amounts, ordinarily representing 0.25% of the immunoglobulins in serum, while the majority is found in the plasma membranes of mature B-cells.6 These monoclonal gammopathies often escape detection for two reasons: (1) the very low paraprotein concentration produces undetectable or small M-protein levels on electrophoresis,5 and (2) immunofixation is routinely performed without antisera against IgD and IgE heavy chains.7
While this case depicts a rare manifestation of a rare disease, the principles underlying its elusive diagnosis are routinely encountered. Recognition of the specific limitations of the SPEP, UPEP, sFLC, and immunofixation tests, outlined below, can assist the hospitalist when suspicion for paraproteinemia is high.
First, low levels of monoclonal proteins may be associated with a normal SPEP. Accordingly, suspicion of a plasma cell dyscrasia should prompt serum immunofixation, even when the electrophoretic pattern appears normal.8
Second, laboratories routinely perform immunofixation with antisera against IgG, IgA, and IgM heavy chains and kappa and lambda light chains, whereas testing with IgD or IgE antisera must be specifically requested. Thus, clinicians should screen for the presence of IgD and IgE in patients with an apparently free monoclonal immunoglobulin light chain in the serum or with a monoclonal serum protein and negative immunofixation. In this case, the paraprotein was not detected on the first two serum electrophoreses, likely due to a low serum concentration, then missed on immunofixation due to a lack of IgD antiserum. On admission to the hospital, this patient had a very low paraprotein concentration (0.1 g/dL) on SPEP, and the lab initially reported negative immunofixation. When asked to test specifically for IgD and IgE, the lab ran a more comprehensive immunofixation revealing IgD heavy chain paraprotein.
Third, this case illustrates the limitations of the sFLC assay. IMWG guidelines specify that sFLC assay in combination with SPEP and serum immunofixation is sufficient to screen for monoclonal plasma cell proliferative disorders other than light chain amyloidosis (which requires all the serum tests as well as 24-hour urine immunofixation).9 Though the sFLC assay has been demonstrated to be more sensitive than urine analysis for detecting monoclonal free light chains,10 it is still subject to false negatives. Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal sFLC,11 the latter of which likely explains why the sFLC ratio was repeatedly normal in this case. In these circumstances, monoclonal free light chains can be identified by urine studies.11 In this case, 24-hour urine immunofixation detected the excess light chain that was not evident on the sFLC assay. Even with these pitfalls in mind, there is still no evident explanation as to why the 24-hour urine studies done prior to the patient’s hospital admission did not reveal a monoclonal light chain.
This case also highlights the thrombotic diathesis in POEMS syndrome. Although the patient was treated with argatroban for suspected HIT, it is likely that the HIT antibody result was a false positive, and his thrombi were better explained by POEMS syndrome in and of itself. Coronary, limb, and cerebral artery thromboses have been linked to POEMS syndrome,12,13 all of which were present in this case. Laboratory testing for HIT involves an immunoassay to detect circulating HIT antibody and a functional assay to measure platelet activity in the presence of patient serum and heparin. The immunoassay binds anti-PF4/heparin complex irrespective of its ability to activate platelets. The presence of nonspecific antibodies may lead to cross-reactions with the immunoassay test components, which has been demonstrated in cases of MGUS.14 In this case, elevated production of monoclonal antibodies by plasma cells may have led to false-positive results. With moderate to high clinical suspicion of HIT, the combination of a positive immunoassay and negative functional assay (as in this case) make the diagnosis of HIT indeterminate.15
TEACHING POINTS
- If a monoclonal protein is suggested by SPEP but cannot be identified by standard immunofixation, request immunofixation for IgD or IgE. Screen patients for IgD and IgE paraproteins before making a diagnosis of light chain multiple myeloma.
- Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal FLC and result in a normal sFLC ratio.
- Thrombosis is a less-recognized but documented feature of POEMS syndrome which may be mediated by the overproduction of proinflammatory and proangiogenic cytokines, though the precise pathogenesis is unknown.
Acknowledgment
The authors thank Dr. Theodore Kurtz and Dr. Anne Deucher from the department of laboratory medicine at the University of California, San Francisco for providing their respective expertise in clinical chemistry and hematopathology.
Disclosures
The authors have no conflicts of interests to disclose.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5. PubMed
2. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. doi: 10.1016/S0140-6736(96)91261-1. PubMed
3. Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119(24):5650-5658. doi: 10.1182/blood-2012-03-378992. PubMed
4. Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2012;83(5):476-479. doi: 10.1136/jnnp-2011-301706. PubMed
5. Pandey S, Kyle RA. Unusual myelomas: a review of IgD and IgE variants. Oncology. 2013;27(8):798-803. PubMed
6. Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7(2):131-140. doi: 10.1128/CDLI.7.2.131-140.2000. PubMed
7. Sinclair D, Cranfield T. IgD myeloma: A potential missed diagnosis. Ann Clin Biochem. 2001;38(5):564-565. doi: 10.1177/000456320103800517. PubMed
8. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705. doi: 10.1182/blood-2010-10-299529. PubMed
9. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215-224. doi: 10.1038/leu.2008.307. PubMed
10. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356-362. doi: 10.3324/haematol.2015.126797. PubMed
11. Levinson SS. Polyclonal free light chain of Ig may interfere with interpretation of monoclonal free light chain κ/λ ratio. Ann Clin Lab Sci. 2010;40(4):348-353. PubMed
12. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496-2506. doi: 10.1182/blood-2002-07-2299. PubMed
13. Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308-1312. doi: 10.1212/WNL.0b013e3181bd136b. PubMed
14. Markovic I, Debeljak Z, Bosnjak B, Marijanovic M. False positive immunoassay for heparin-induced thrombocytopenia in the presence of monoclonal gammopathy: a case report. Biochemia Medica. 2017;27(3):030801. doi: 10.11613/BM.2017.030801. PubMed
15. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5. PubMed
2. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. doi: 10.1016/S0140-6736(96)91261-1. PubMed
3. Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119(24):5650-5658. doi: 10.1182/blood-2012-03-378992. PubMed
4. Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2012;83(5):476-479. doi: 10.1136/jnnp-2011-301706. PubMed
5. Pandey S, Kyle RA. Unusual myelomas: a review of IgD and IgE variants. Oncology. 2013;27(8):798-803. PubMed
6. Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7(2):131-140. doi: 10.1128/CDLI.7.2.131-140.2000. PubMed
7. Sinclair D, Cranfield T. IgD myeloma: A potential missed diagnosis. Ann Clin Biochem. 2001;38(5):564-565. doi: 10.1177/000456320103800517. PubMed
8. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705. doi: 10.1182/blood-2010-10-299529. PubMed
9. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215-224. doi: 10.1038/leu.2008.307. PubMed
10. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356-362. doi: 10.3324/haematol.2015.126797. PubMed
11. Levinson SS. Polyclonal free light chain of Ig may interfere with interpretation of monoclonal free light chain κ/λ ratio. Ann Clin Lab Sci. 2010;40(4):348-353. PubMed
12. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496-2506. doi: 10.1182/blood-2002-07-2299. PubMed
13. Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308-1312. doi: 10.1212/WNL.0b013e3181bd136b. PubMed
14. Markovic I, Debeljak Z, Bosnjak B, Marijanovic M. False positive immunoassay for heparin-induced thrombocytopenia in the presence of monoclonal gammopathy: a case report. Biochemia Medica. 2017;27(3):030801. doi: 10.11613/BM.2017.030801. PubMed
15. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
© 2019 Society of Hospital Medicine