User login
Improving Healthcare Value: Reducing Overuse in Hospital Pediatrics
Most hospital pediatricians can recall cases where an abnormal result in one unnecessary test led to a cascade of multiple further unnecessary treatments, procedures, and tests. These cases are well described in the literature and written off as a side effect of delivering high-quality, comprehensive pediatric care.1 Unfortunately, however, these frequent events are not without consequence and can cause significant harm to patients, as well as stress and fear for parents and families, and indirectly waste valuable resources.
As we look forward to recovering from the COVID-19 pandemic, there are calls to prioritize high-value and more equitable care in the postpandemic world.2 Choosing Wisely is a global movement comprised of clinician-led campaigns that partner with national specialty societies to develop lists of evidence-based recommendations of tests, treatments, and procedures that offer no added clinical value and may cause harm.3
In pediatrics, there is a growing recognition and published literature on the harms of overdiagnosis and unnecessary care in children.4-6 Choosing Wisely recommendations are being used as a resource to drive healthcare prioritization and ensure low-value care is avoided so that greater focus can be placed on areas of need exacerbated by the pandemic. Using a Choosing Wisely perspective can drive quality and help inform a shift in practice, creating a roadmap for reducing testing or treatment cascades that harm patients and waste resources as we move toward the goal of high-value pediatric care. However, adoption of Choosing Wisely recommendations in pediatrics has been slow. For example, the pediatric working group of the Society of Hospital Medicine released a Choosing Wisely® recommendation in 2013 against the use of continuous pulse oximetry monitoring in children with acute respiratory illness who are not on supplementary oxygen.7 Data from a cross-sectional study across 56 hospitals 6 years later found significant variation in this practice for infants hospitalized with bronchiolitis and not receiving supplemental oxygen; 46% were continuously monitored with pulse oximetry (range, 2%-92%).8
WHY HAS CHOOSING WISELY LAGGED IN PEDIATRICS?
Traditionally, attention in children’s healthcare has focused on underuse (eg, immunizations or mental health) rather than overuse. Further, the weakness of the evidence base, with very few randomized controlled trials in children, limits our ability to provide sufficient confidence in the evidence supporting some of our recommendations.9
Second, there is also tremendous anxiety for both parents and frontline clinicians around diagnostic uncertainty of any kind when it comes to children. We endeavour to reassure ourselves and patients’ families by leaving no stone unturned. This approach can lead to unnecessary care, including false-positive test results, “incidentalomas,” and adverse effects from unnecessary medications. Despite the best intentions of assuaging caregivers’ anxiety, overuse of invasive and uncomfortable tests can have the opposite effect of increasing stress and trauma for both children and parents.
Third, there is compelling evidence that practice habits, once established, are difficult to break.10 Particularly in the high-stakes practice of hospital pediatric medicine, where we are conditioned to expect the worst and anticipate the unexpected. This “do everything to everyone” approach, however, can lead to significant harms for pediatric patients. For example, the exposure to ionizing radiation through unnecessary computed tomography (CT) scans can increase a child’s lifetime cancer risk.11
The perpetuation of unnecessary care needs to change in pediatrics, especially for the most vulnerable young patients seeking hospital care. Implementation is a necessary next step to introduce recommendations into practice, and the Choosing Wisely efforts of the Hospital for Sick Children in Toronto, Canada, can offer insights into opportunities to embed this approach across similar quaternary care teaching hospitals, as well as general hospitals and the systems they support.
STEPS TO IMPLEMENTING CHOOSING WISELY HOSPITAL-WIDE
Creating Lists of Recommendations Aligned With Quality Metrics
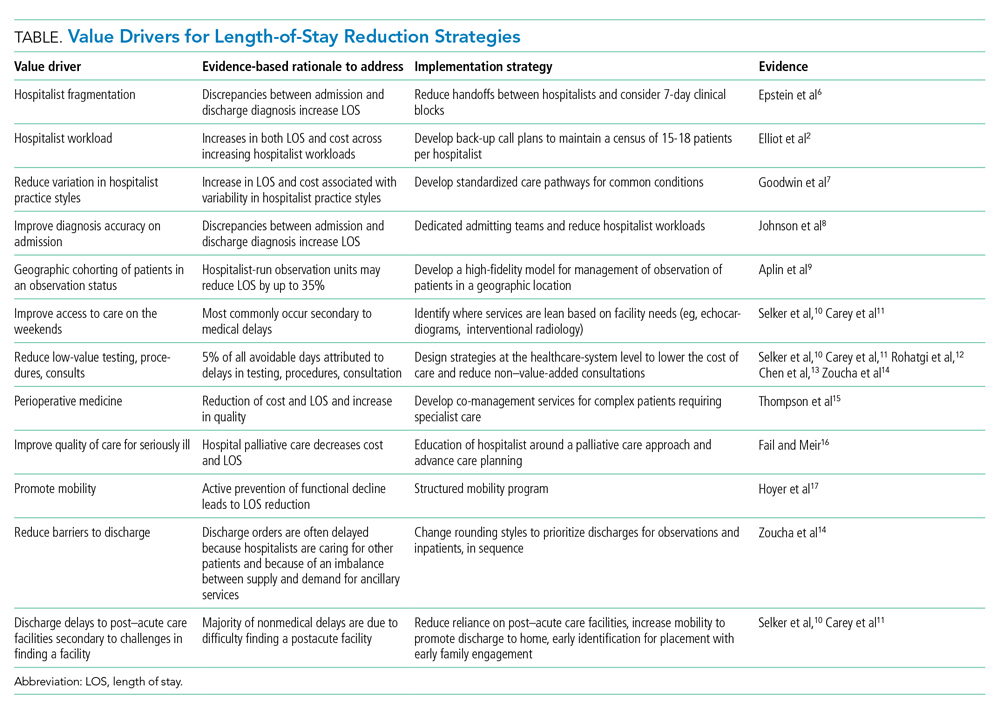
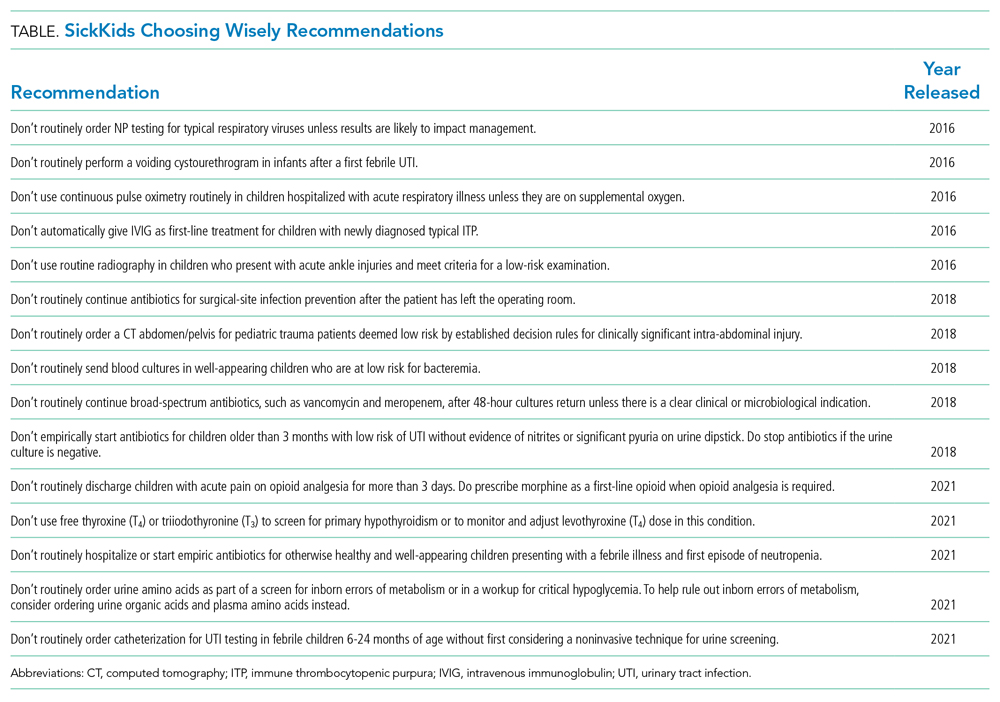
The Hospital for Sick Children developed a hospital-specific Choosing Wisely list in 2016 to address a gap in existing Choosing Wisely Canada campaign recommendations related to pediatric hospitals.12 Choosing Wisely Canada was initially focused on adult medicine, and a list of recommendations developed by the Canadian Paediatric Society relates mostly to overuse in pediatric outpatient settings and is not applicable to hospital-based practice.13 The Society of Hospital Medicine-Pediatric Hospital Medicine Choosing Wisely® list predominantly pertained to unnecessary care of infants with bronchiolitis (eg, not to order chest radiographs in uncomplicated asthma and bronchiolitis). We had measured our compliance with this recommendation and found it was already well below the achievable benchmark of care in the United States,14 so we preferred to create a list that would resonate with our clinicians. Since the original list was created at the Hospital for Sick Children,12 we have developed two subsequent lists of recommendations, which were released in 2018 and 2021 (Table).
The approach to list development used by staff pediatricians and trainees, with input from hospital staff and family advisors, has been described elsewhere.12 The goal was to self-identify five local practices that we felt would help us reduce unnecessary care. This list served as the foundation of an organization-wide quality initiative driven by a steering committee that consisted of the clinician champions as well as representation from various groups at the hospital, including decision support, information services, the family advisory committee, and public affairs.
Each recommendation needed to be evidence-based and measurable, have a clinician champion to implement the recommendation, and have the potential to improve the quality and safety of the care we provided. “Balancing” measures needed to be carefully monitored to ensure that no diagnoses were being missed or negative effects resulted from decreasing these interventions. In order for a recommendation to be considered, a subgroup of the pediatric department’s clinical advisory committee reviewed the references provided to ensure that what was being suggested was based on published evidence and part of current national guidelines. The clinician champion needed to agree to lead the implementation project, and specific outcomes, including appropriate balancing measures, needed to be identified a priori, in addition to an appropriate mechanism to collect the data. Hospital executive leaders were supportive of the initiative and facilitated access to “in-kind” hospital resources as required, although no financial budget was provided. After some early success, the Department of Paediatrics provided part-time project management support to help coordinate the growth and administration of the initiative.
Measuring and Supporting Practice Change
The main implementation principles included targeted education/awareness, transparent measurement with audit/feedback, and, most importantly, embedding changes in the ordering process, essentially making it easy for frontline clinicians to do the right thing (and trickier to do the “wrong” thing). Audit and feedback have been used at both the individual provider level (eg, respiratory viral testing–ordering practices) and the divisional level (eg, ordering of postoperative antibiotics). These quality improvement initiatives have had a compelling impact. Scorecards have been developed and results shared internally using local divisional as well as hospital-wide tools, varying from staff meetings to screensavers across hospital computers and television screens and the hospital intranet. Evaluation is ongoing, but many of the initial results have been encouraging.15-17
For example, the 2016 list includes recommendations related to emergency department (ED) test ordering. Implementation efforts to address unnecessary nasopharyngeal swabs for viral testing in bronchiolitis reduced this practice by 80%,15 and there has been a 50% reduction in ankle X-rays in children with acute ankle injuries who meet criteria for a low-risk examination.16 The 2016 list also included a recommendation related to inappropriate intravenous immunoglobulin (IVIG) use in children with typical acute immune thrombocytopenic purpura (ITP), and a targeted quality improvement initiative reduced inappropriate IVIG use by 50%, with no detectable increase in bleeding complications or readmission to hospital.17 These results have been sustained over a period of 3 or more years. Examples from the 2018 list include a 40% decrease in inappropriate urinary tract infection diagnosis and treatment in the ED and a four-fold decrease in the CT abdomen/pelvis imaging rate for low-risk trauma.18
The steering committee meets every 2 months and includes all of the clinician champions as well as representatives from strategic hospital resources and two family advisors (NGS). These meetings are chaired by the Associate Pediatrician-in-Chief (JNF) and the project manager. The progress of the active projects is discussed, and the experience of the group is used to problem-solve, plan ahead, and encourage academic presentation and publication of the various projects. Patient partnership and participation in committees has ensured that improvements to patient experience, satisfaction, and education are considered in the outcomes of implementation. Moreover, it has safeguarded that this effort is not misperceived as limiting care and remains focused on advancing quality, safety, and the patient experience.
SOME LESSONS LEARNED
While most projects have surpassed expectations, not all have proceeded as anticipated. The biggest challenge is finding a reliable and practical source for data collection. For example, at the time of initiation of the voiding cystourethrography (VCUG) recommendation, practice had presumably changed over the recent years, and compliance already exceeded the goal, illustrating the importance of current accurate data. The oxygen saturation–monitoring recommendation highlighted the challenge presented by data collection that requires manual audits; the inability to find staff to do this regularly significantly hampered this project. The critical role of the clinician champion was highlighted in a few projects when a lead was absent for a prolonged period of time (eg, due to a parental leave or change in job), with no willing replacement. There does seem to be a strong correlation between the commitment and passion of the clinician lead and the success of the project. We have incorporated the lessons learned into the development and rollout of the 2018 and 2021 lists.
SPREAD AND SCALE
The challenge is to scale up these successes to impact and change practice across the hospital pediatrics community. After 5 years, awareness of and engagement with this process are still not uniform across our hospital campus. Nevertheless, anecdotally, at the Hospital for Sick Children, there is a shift in culture where clinicians have processed the imperative to reduce overuse and unnecessary tests and treatments, with phrases such as “this is not very Choosing Wisely” entering the vernacular. It is becoming part of the culture. Second, the new generation of medical school trainees and residents has displayed a tremendous appetite and passion for stewardship and a sense that practice can change from the ground up. The SickKids Choosing Wisely efforts have been a hub for resident-led quality improvement projects and leadership for implementation of recommendations.19 As we continue to engage all providers at our hospital, we are also reaching out to the other community hospitals in our region, and all children’s hospitals in Canada, to share the principles and lessons learned from our program through a national community of practice.
CONCLUSION
Practicing pediatric medicine in a well-resourced hospital setting should not drive us to overuse in practice “just because we can.” The harms of this approach to our patients and health systems, coupled with the pressures of the pandemic, are compelling reasons to be responsible stewards. There are opportunities to reshape and rethink practice patterns and habits.20 Overuse and overdiagnosis harm our patients and families physically and emotionally and indirectly waste resources urgently needed for investment upstream. Providing safe, quality, high-value care to our young patients requires constant critical thinking. The time is here to advance Choosing Wisely into pediatric hospital practice.
1. Elliott DK, Rose SR, Ronan JC. Changing the culture around cultures. Hosp Pediatr. 2014;4(6):405-407. https://doi.org/10.1542/hpeds.2014-0064
2. Gupta R, Simpson LA, Morgan DJ. Prioritizing high-value, equitable care after the COVID-19 shutdown: an opportunity for a healthcare renaissance. J Hosp Med. 2021;16(2):114-116. https://doi.org/10.12788/jhm.3526
3. Born K, Kool T, Levinson W. Reducing overuse in healthcare: advancing Choosing Wisely. BMJ. 2019;367:l6317. https://doi.org/10.1136/bmj.l6317
4. Coon ER, Young PC, Quinonez RA, Morgan DJ, Dhruva SS, Schroeder AR. Update on pediatric overuse. Pediatrics. 2017;139(2):e20162797. https://doi.org/10.1542/peds.2016-2797
5. Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. https://doi.org/10.1542/peds.2014-1778
6. Wolf ER, Krist AH, Schroeder AR. Deimplementation in pediatrics: past, present, and future. JAMA Pediatr. 2021;175(3):230-232. https://doi.org/10.1001/jamapediatrics.2020.4681
7. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing Wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
8. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
9. Ralston SL, Schroeder AR. Why is it so hard to talk about overuse in pediatrics and why it matters. JAMA Pediatr. 2017;17(10):931-932. https://doi.org/10.1001/jamapediatrics.2017.2239
10. Stammen LA, Stalmeijer RE, Paternotte E, et al. Training physicians to provide high-value, cost-conscious care: a systematic review. JAMA. 2015;314(22):2384-2400. https://doi.org/10.1001/jama.2015.16353
11. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. https://doi.org/10.1136/bmj.f2360
12. Friedman JN. Saying yes to the less: making it easier to choose wisely [editorial]. J Pediatr. 2017;145:4-5. https://doi.org/10.1016/j.jpeds.2017.01.062
13. Canadian Paediatric Society. Five things physicians and patients should question. Choosing Wisely Canada. Updated July 2019. Accessed June 17, 2021. https://choosingwiselycanada.org/wp-content/uploads/2020/07/Paediatrics_EN.pdf
14. Parikh K, Hall M, Montalbano A, et al. Establishing benchmarks for the hospitalized care of children with asthma, bronciolitis, and pneumonia. Pediatrics. 2014;134(3):555-562. https://doi.org/10.1542/peds.2014-1052
15. Ostrow O, Richardson S, Savlov D, Friedman JN. Reducing unnecessary respiratory viral testing to promote high value care. Pediatrics. In press.
16. Al-Sani F, Ben-Yakov M, Harvey G, et al. P016: Low risk ankle rule, high reward—a quality improvement initiative to reduce ankle x-rays in the pediatric emergency department [poster]. CJEM. 2017;19(S1):S83. https://doi.org/10.1017/cem.2017.218
17. Beck CE, Carcao M, Cada M, Porter S, Blanchette VS, Parkin PC. A quality improvement bundle to improve informed choice for children with typical, newly diagnosed immune thrombocytopenia. J Pediatr Hematol Oncol. 2018;40(8):e537-e543. https://doi.org/10.1097/MPH.0000000000001247
18. Beno S, Lenton-Brym T, Rosenfield D, McDowall D, Wales P, Principi T. Safe reduction of abdominal CT imaging in pediatric trauma patients: a quality-improvement initiative [abstract]. Can J Surg. 2019;62(3 Suppl 2):S29-S30.
19. Bal C, Tesch M, Blair G, Ostrow O, Premji L. Engaging medical trainees in resource stewardship through resident-led teaching sessions: a choosing wisely educational initiative. Can Med Educ J. 2021;12(1):e98-e100. https://doi.org/10.36834/cmej.70563
20. Berwick DM. Choices for the “new normal.” JAMA. 2020;323(21):2125-2126. https://doi.org/10.1001/jama.2020.6949
Most hospital pediatricians can recall cases where an abnormal result in one unnecessary test led to a cascade of multiple further unnecessary treatments, procedures, and tests. These cases are well described in the literature and written off as a side effect of delivering high-quality, comprehensive pediatric care.1 Unfortunately, however, these frequent events are not without consequence and can cause significant harm to patients, as well as stress and fear for parents and families, and indirectly waste valuable resources.
As we look forward to recovering from the COVID-19 pandemic, there are calls to prioritize high-value and more equitable care in the postpandemic world.2 Choosing Wisely is a global movement comprised of clinician-led campaigns that partner with national specialty societies to develop lists of evidence-based recommendations of tests, treatments, and procedures that offer no added clinical value and may cause harm.3
In pediatrics, there is a growing recognition and published literature on the harms of overdiagnosis and unnecessary care in children.4-6 Choosing Wisely recommendations are being used as a resource to drive healthcare prioritization and ensure low-value care is avoided so that greater focus can be placed on areas of need exacerbated by the pandemic. Using a Choosing Wisely perspective can drive quality and help inform a shift in practice, creating a roadmap for reducing testing or treatment cascades that harm patients and waste resources as we move toward the goal of high-value pediatric care. However, adoption of Choosing Wisely recommendations in pediatrics has been slow. For example, the pediatric working group of the Society of Hospital Medicine released a Choosing Wisely® recommendation in 2013 against the use of continuous pulse oximetry monitoring in children with acute respiratory illness who are not on supplementary oxygen.7 Data from a cross-sectional study across 56 hospitals 6 years later found significant variation in this practice for infants hospitalized with bronchiolitis and not receiving supplemental oxygen; 46% were continuously monitored with pulse oximetry (range, 2%-92%).8
WHY HAS CHOOSING WISELY LAGGED IN PEDIATRICS?
Traditionally, attention in children’s healthcare has focused on underuse (eg, immunizations or mental health) rather than overuse. Further, the weakness of the evidence base, with very few randomized controlled trials in children, limits our ability to provide sufficient confidence in the evidence supporting some of our recommendations.9
Second, there is also tremendous anxiety for both parents and frontline clinicians around diagnostic uncertainty of any kind when it comes to children. We endeavour to reassure ourselves and patients’ families by leaving no stone unturned. This approach can lead to unnecessary care, including false-positive test results, “incidentalomas,” and adverse effects from unnecessary medications. Despite the best intentions of assuaging caregivers’ anxiety, overuse of invasive and uncomfortable tests can have the opposite effect of increasing stress and trauma for both children and parents.
Third, there is compelling evidence that practice habits, once established, are difficult to break.10 Particularly in the high-stakes practice of hospital pediatric medicine, where we are conditioned to expect the worst and anticipate the unexpected. This “do everything to everyone” approach, however, can lead to significant harms for pediatric patients. For example, the exposure to ionizing radiation through unnecessary computed tomography (CT) scans can increase a child’s lifetime cancer risk.11
The perpetuation of unnecessary care needs to change in pediatrics, especially for the most vulnerable young patients seeking hospital care. Implementation is a necessary next step to introduce recommendations into practice, and the Choosing Wisely efforts of the Hospital for Sick Children in Toronto, Canada, can offer insights into opportunities to embed this approach across similar quaternary care teaching hospitals, as well as general hospitals and the systems they support.
STEPS TO IMPLEMENTING CHOOSING WISELY HOSPITAL-WIDE
Creating Lists of Recommendations Aligned With Quality Metrics
The Hospital for Sick Children developed a hospital-specific Choosing Wisely list in 2016 to address a gap in existing Choosing Wisely Canada campaign recommendations related to pediatric hospitals.12 Choosing Wisely Canada was initially focused on adult medicine, and a list of recommendations developed by the Canadian Paediatric Society relates mostly to overuse in pediatric outpatient settings and is not applicable to hospital-based practice.13 The Society of Hospital Medicine-Pediatric Hospital Medicine Choosing Wisely® list predominantly pertained to unnecessary care of infants with bronchiolitis (eg, not to order chest radiographs in uncomplicated asthma and bronchiolitis). We had measured our compliance with this recommendation and found it was already well below the achievable benchmark of care in the United States,14 so we preferred to create a list that would resonate with our clinicians. Since the original list was created at the Hospital for Sick Children,12 we have developed two subsequent lists of recommendations, which were released in 2018 and 2021 (Table).

The approach to list development used by staff pediatricians and trainees, with input from hospital staff and family advisors, has been described elsewhere.12 The goal was to self-identify five local practices that we felt would help us reduce unnecessary care. This list served as the foundation of an organization-wide quality initiative driven by a steering committee that consisted of the clinician champions as well as representation from various groups at the hospital, including decision support, information services, the family advisory committee, and public affairs.
Each recommendation needed to be evidence-based and measurable, have a clinician champion to implement the recommendation, and have the potential to improve the quality and safety of the care we provided. “Balancing” measures needed to be carefully monitored to ensure that no diagnoses were being missed or negative effects resulted from decreasing these interventions. In order for a recommendation to be considered, a subgroup of the pediatric department’s clinical advisory committee reviewed the references provided to ensure that what was being suggested was based on published evidence and part of current national guidelines. The clinician champion needed to agree to lead the implementation project, and specific outcomes, including appropriate balancing measures, needed to be identified a priori, in addition to an appropriate mechanism to collect the data. Hospital executive leaders were supportive of the initiative and facilitated access to “in-kind” hospital resources as required, although no financial budget was provided. After some early success, the Department of Paediatrics provided part-time project management support to help coordinate the growth and administration of the initiative.
Measuring and Supporting Practice Change
The main implementation principles included targeted education/awareness, transparent measurement with audit/feedback, and, most importantly, embedding changes in the ordering process, essentially making it easy for frontline clinicians to do the right thing (and trickier to do the “wrong” thing). Audit and feedback have been used at both the individual provider level (eg, respiratory viral testing–ordering practices) and the divisional level (eg, ordering of postoperative antibiotics). These quality improvement initiatives have had a compelling impact. Scorecards have been developed and results shared internally using local divisional as well as hospital-wide tools, varying from staff meetings to screensavers across hospital computers and television screens and the hospital intranet. Evaluation is ongoing, but many of the initial results have been encouraging.15-17
For example, the 2016 list includes recommendations related to emergency department (ED) test ordering. Implementation efforts to address unnecessary nasopharyngeal swabs for viral testing in bronchiolitis reduced this practice by 80%,15 and there has been a 50% reduction in ankle X-rays in children with acute ankle injuries who meet criteria for a low-risk examination.16 The 2016 list also included a recommendation related to inappropriate intravenous immunoglobulin (IVIG) use in children with typical acute immune thrombocytopenic purpura (ITP), and a targeted quality improvement initiative reduced inappropriate IVIG use by 50%, with no detectable increase in bleeding complications or readmission to hospital.17 These results have been sustained over a period of 3 or more years. Examples from the 2018 list include a 40% decrease in inappropriate urinary tract infection diagnosis and treatment in the ED and a four-fold decrease in the CT abdomen/pelvis imaging rate for low-risk trauma.18
The steering committee meets every 2 months and includes all of the clinician champions as well as representatives from strategic hospital resources and two family advisors (NGS). These meetings are chaired by the Associate Pediatrician-in-Chief (JNF) and the project manager. The progress of the active projects is discussed, and the experience of the group is used to problem-solve, plan ahead, and encourage academic presentation and publication of the various projects. Patient partnership and participation in committees has ensured that improvements to patient experience, satisfaction, and education are considered in the outcomes of implementation. Moreover, it has safeguarded that this effort is not misperceived as limiting care and remains focused on advancing quality, safety, and the patient experience.
SOME LESSONS LEARNED
While most projects have surpassed expectations, not all have proceeded as anticipated. The biggest challenge is finding a reliable and practical source for data collection. For example, at the time of initiation of the voiding cystourethrography (VCUG) recommendation, practice had presumably changed over the recent years, and compliance already exceeded the goal, illustrating the importance of current accurate data. The oxygen saturation–monitoring recommendation highlighted the challenge presented by data collection that requires manual audits; the inability to find staff to do this regularly significantly hampered this project. The critical role of the clinician champion was highlighted in a few projects when a lead was absent for a prolonged period of time (eg, due to a parental leave or change in job), with no willing replacement. There does seem to be a strong correlation between the commitment and passion of the clinician lead and the success of the project. We have incorporated the lessons learned into the development and rollout of the 2018 and 2021 lists.
SPREAD AND SCALE
The challenge is to scale up these successes to impact and change practice across the hospital pediatrics community. After 5 years, awareness of and engagement with this process are still not uniform across our hospital campus. Nevertheless, anecdotally, at the Hospital for Sick Children, there is a shift in culture where clinicians have processed the imperative to reduce overuse and unnecessary tests and treatments, with phrases such as “this is not very Choosing Wisely” entering the vernacular. It is becoming part of the culture. Second, the new generation of medical school trainees and residents has displayed a tremendous appetite and passion for stewardship and a sense that practice can change from the ground up. The SickKids Choosing Wisely efforts have been a hub for resident-led quality improvement projects and leadership for implementation of recommendations.19 As we continue to engage all providers at our hospital, we are also reaching out to the other community hospitals in our region, and all children’s hospitals in Canada, to share the principles and lessons learned from our program through a national community of practice.
CONCLUSION
Practicing pediatric medicine in a well-resourced hospital setting should not drive us to overuse in practice “just because we can.” The harms of this approach to our patients and health systems, coupled with the pressures of the pandemic, are compelling reasons to be responsible stewards. There are opportunities to reshape and rethink practice patterns and habits.20 Overuse and overdiagnosis harm our patients and families physically and emotionally and indirectly waste resources urgently needed for investment upstream. Providing safe, quality, high-value care to our young patients requires constant critical thinking. The time is here to advance Choosing Wisely into pediatric hospital practice.
Most hospital pediatricians can recall cases where an abnormal result in one unnecessary test led to a cascade of multiple further unnecessary treatments, procedures, and tests. These cases are well described in the literature and written off as a side effect of delivering high-quality, comprehensive pediatric care.1 Unfortunately, however, these frequent events are not without consequence and can cause significant harm to patients, as well as stress and fear for parents and families, and indirectly waste valuable resources.
As we look forward to recovering from the COVID-19 pandemic, there are calls to prioritize high-value and more equitable care in the postpandemic world.2 Choosing Wisely is a global movement comprised of clinician-led campaigns that partner with national specialty societies to develop lists of evidence-based recommendations of tests, treatments, and procedures that offer no added clinical value and may cause harm.3
In pediatrics, there is a growing recognition and published literature on the harms of overdiagnosis and unnecessary care in children.4-6 Choosing Wisely recommendations are being used as a resource to drive healthcare prioritization and ensure low-value care is avoided so that greater focus can be placed on areas of need exacerbated by the pandemic. Using a Choosing Wisely perspective can drive quality and help inform a shift in practice, creating a roadmap for reducing testing or treatment cascades that harm patients and waste resources as we move toward the goal of high-value pediatric care. However, adoption of Choosing Wisely recommendations in pediatrics has been slow. For example, the pediatric working group of the Society of Hospital Medicine released a Choosing Wisely® recommendation in 2013 against the use of continuous pulse oximetry monitoring in children with acute respiratory illness who are not on supplementary oxygen.7 Data from a cross-sectional study across 56 hospitals 6 years later found significant variation in this practice for infants hospitalized with bronchiolitis and not receiving supplemental oxygen; 46% were continuously monitored with pulse oximetry (range, 2%-92%).8
WHY HAS CHOOSING WISELY LAGGED IN PEDIATRICS?
Traditionally, attention in children’s healthcare has focused on underuse (eg, immunizations or mental health) rather than overuse. Further, the weakness of the evidence base, with very few randomized controlled trials in children, limits our ability to provide sufficient confidence in the evidence supporting some of our recommendations.9
Second, there is also tremendous anxiety for both parents and frontline clinicians around diagnostic uncertainty of any kind when it comes to children. We endeavour to reassure ourselves and patients’ families by leaving no stone unturned. This approach can lead to unnecessary care, including false-positive test results, “incidentalomas,” and adverse effects from unnecessary medications. Despite the best intentions of assuaging caregivers’ anxiety, overuse of invasive and uncomfortable tests can have the opposite effect of increasing stress and trauma for both children and parents.
Third, there is compelling evidence that practice habits, once established, are difficult to break.10 Particularly in the high-stakes practice of hospital pediatric medicine, where we are conditioned to expect the worst and anticipate the unexpected. This “do everything to everyone” approach, however, can lead to significant harms for pediatric patients. For example, the exposure to ionizing radiation through unnecessary computed tomography (CT) scans can increase a child’s lifetime cancer risk.11
The perpetuation of unnecessary care needs to change in pediatrics, especially for the most vulnerable young patients seeking hospital care. Implementation is a necessary next step to introduce recommendations into practice, and the Choosing Wisely efforts of the Hospital for Sick Children in Toronto, Canada, can offer insights into opportunities to embed this approach across similar quaternary care teaching hospitals, as well as general hospitals and the systems they support.
STEPS TO IMPLEMENTING CHOOSING WISELY HOSPITAL-WIDE
Creating Lists of Recommendations Aligned With Quality Metrics
The Hospital for Sick Children developed a hospital-specific Choosing Wisely list in 2016 to address a gap in existing Choosing Wisely Canada campaign recommendations related to pediatric hospitals.12 Choosing Wisely Canada was initially focused on adult medicine, and a list of recommendations developed by the Canadian Paediatric Society relates mostly to overuse in pediatric outpatient settings and is not applicable to hospital-based practice.13 The Society of Hospital Medicine-Pediatric Hospital Medicine Choosing Wisely® list predominantly pertained to unnecessary care of infants with bronchiolitis (eg, not to order chest radiographs in uncomplicated asthma and bronchiolitis). We had measured our compliance with this recommendation and found it was already well below the achievable benchmark of care in the United States,14 so we preferred to create a list that would resonate with our clinicians. Since the original list was created at the Hospital for Sick Children,12 we have developed two subsequent lists of recommendations, which were released in 2018 and 2021 (Table).

The approach to list development used by staff pediatricians and trainees, with input from hospital staff and family advisors, has been described elsewhere.12 The goal was to self-identify five local practices that we felt would help us reduce unnecessary care. This list served as the foundation of an organization-wide quality initiative driven by a steering committee that consisted of the clinician champions as well as representation from various groups at the hospital, including decision support, information services, the family advisory committee, and public affairs.
Each recommendation needed to be evidence-based and measurable, have a clinician champion to implement the recommendation, and have the potential to improve the quality and safety of the care we provided. “Balancing” measures needed to be carefully monitored to ensure that no diagnoses were being missed or negative effects resulted from decreasing these interventions. In order for a recommendation to be considered, a subgroup of the pediatric department’s clinical advisory committee reviewed the references provided to ensure that what was being suggested was based on published evidence and part of current national guidelines. The clinician champion needed to agree to lead the implementation project, and specific outcomes, including appropriate balancing measures, needed to be identified a priori, in addition to an appropriate mechanism to collect the data. Hospital executive leaders were supportive of the initiative and facilitated access to “in-kind” hospital resources as required, although no financial budget was provided. After some early success, the Department of Paediatrics provided part-time project management support to help coordinate the growth and administration of the initiative.
Measuring and Supporting Practice Change
The main implementation principles included targeted education/awareness, transparent measurement with audit/feedback, and, most importantly, embedding changes in the ordering process, essentially making it easy for frontline clinicians to do the right thing (and trickier to do the “wrong” thing). Audit and feedback have been used at both the individual provider level (eg, respiratory viral testing–ordering practices) and the divisional level (eg, ordering of postoperative antibiotics). These quality improvement initiatives have had a compelling impact. Scorecards have been developed and results shared internally using local divisional as well as hospital-wide tools, varying from staff meetings to screensavers across hospital computers and television screens and the hospital intranet. Evaluation is ongoing, but many of the initial results have been encouraging.15-17
For example, the 2016 list includes recommendations related to emergency department (ED) test ordering. Implementation efforts to address unnecessary nasopharyngeal swabs for viral testing in bronchiolitis reduced this practice by 80%,15 and there has been a 50% reduction in ankle X-rays in children with acute ankle injuries who meet criteria for a low-risk examination.16 The 2016 list also included a recommendation related to inappropriate intravenous immunoglobulin (IVIG) use in children with typical acute immune thrombocytopenic purpura (ITP), and a targeted quality improvement initiative reduced inappropriate IVIG use by 50%, with no detectable increase in bleeding complications or readmission to hospital.17 These results have been sustained over a period of 3 or more years. Examples from the 2018 list include a 40% decrease in inappropriate urinary tract infection diagnosis and treatment in the ED and a four-fold decrease in the CT abdomen/pelvis imaging rate for low-risk trauma.18
The steering committee meets every 2 months and includes all of the clinician champions as well as representatives from strategic hospital resources and two family advisors (NGS). These meetings are chaired by the Associate Pediatrician-in-Chief (JNF) and the project manager. The progress of the active projects is discussed, and the experience of the group is used to problem-solve, plan ahead, and encourage academic presentation and publication of the various projects. Patient partnership and participation in committees has ensured that improvements to patient experience, satisfaction, and education are considered in the outcomes of implementation. Moreover, it has safeguarded that this effort is not misperceived as limiting care and remains focused on advancing quality, safety, and the patient experience.
SOME LESSONS LEARNED
While most projects have surpassed expectations, not all have proceeded as anticipated. The biggest challenge is finding a reliable and practical source for data collection. For example, at the time of initiation of the voiding cystourethrography (VCUG) recommendation, practice had presumably changed over the recent years, and compliance already exceeded the goal, illustrating the importance of current accurate data. The oxygen saturation–monitoring recommendation highlighted the challenge presented by data collection that requires manual audits; the inability to find staff to do this regularly significantly hampered this project. The critical role of the clinician champion was highlighted in a few projects when a lead was absent for a prolonged period of time (eg, due to a parental leave or change in job), with no willing replacement. There does seem to be a strong correlation between the commitment and passion of the clinician lead and the success of the project. We have incorporated the lessons learned into the development and rollout of the 2018 and 2021 lists.
SPREAD AND SCALE
The challenge is to scale up these successes to impact and change practice across the hospital pediatrics community. After 5 years, awareness of and engagement with this process are still not uniform across our hospital campus. Nevertheless, anecdotally, at the Hospital for Sick Children, there is a shift in culture where clinicians have processed the imperative to reduce overuse and unnecessary tests and treatments, with phrases such as “this is not very Choosing Wisely” entering the vernacular. It is becoming part of the culture. Second, the new generation of medical school trainees and residents has displayed a tremendous appetite and passion for stewardship and a sense that practice can change from the ground up. The SickKids Choosing Wisely efforts have been a hub for resident-led quality improvement projects and leadership for implementation of recommendations.19 As we continue to engage all providers at our hospital, we are also reaching out to the other community hospitals in our region, and all children’s hospitals in Canada, to share the principles and lessons learned from our program through a national community of practice.
CONCLUSION
Practicing pediatric medicine in a well-resourced hospital setting should not drive us to overuse in practice “just because we can.” The harms of this approach to our patients and health systems, coupled with the pressures of the pandemic, are compelling reasons to be responsible stewards. There are opportunities to reshape and rethink practice patterns and habits.20 Overuse and overdiagnosis harm our patients and families physically and emotionally and indirectly waste resources urgently needed for investment upstream. Providing safe, quality, high-value care to our young patients requires constant critical thinking. The time is here to advance Choosing Wisely into pediatric hospital practice.
1. Elliott DK, Rose SR, Ronan JC. Changing the culture around cultures. Hosp Pediatr. 2014;4(6):405-407. https://doi.org/10.1542/hpeds.2014-0064
2. Gupta R, Simpson LA, Morgan DJ. Prioritizing high-value, equitable care after the COVID-19 shutdown: an opportunity for a healthcare renaissance. J Hosp Med. 2021;16(2):114-116. https://doi.org/10.12788/jhm.3526
3. Born K, Kool T, Levinson W. Reducing overuse in healthcare: advancing Choosing Wisely. BMJ. 2019;367:l6317. https://doi.org/10.1136/bmj.l6317
4. Coon ER, Young PC, Quinonez RA, Morgan DJ, Dhruva SS, Schroeder AR. Update on pediatric overuse. Pediatrics. 2017;139(2):e20162797. https://doi.org/10.1542/peds.2016-2797
5. Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. https://doi.org/10.1542/peds.2014-1778
6. Wolf ER, Krist AH, Schroeder AR. Deimplementation in pediatrics: past, present, and future. JAMA Pediatr. 2021;175(3):230-232. https://doi.org/10.1001/jamapediatrics.2020.4681
7. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing Wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
8. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
9. Ralston SL, Schroeder AR. Why is it so hard to talk about overuse in pediatrics and why it matters. JAMA Pediatr. 2017;17(10):931-932. https://doi.org/10.1001/jamapediatrics.2017.2239
10. Stammen LA, Stalmeijer RE, Paternotte E, et al. Training physicians to provide high-value, cost-conscious care: a systematic review. JAMA. 2015;314(22):2384-2400. https://doi.org/10.1001/jama.2015.16353
11. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. https://doi.org/10.1136/bmj.f2360
12. Friedman JN. Saying yes to the less: making it easier to choose wisely [editorial]. J Pediatr. 2017;145:4-5. https://doi.org/10.1016/j.jpeds.2017.01.062
13. Canadian Paediatric Society. Five things physicians and patients should question. Choosing Wisely Canada. Updated July 2019. Accessed June 17, 2021. https://choosingwiselycanada.org/wp-content/uploads/2020/07/Paediatrics_EN.pdf
14. Parikh K, Hall M, Montalbano A, et al. Establishing benchmarks for the hospitalized care of children with asthma, bronciolitis, and pneumonia. Pediatrics. 2014;134(3):555-562. https://doi.org/10.1542/peds.2014-1052
15. Ostrow O, Richardson S, Savlov D, Friedman JN. Reducing unnecessary respiratory viral testing to promote high value care. Pediatrics. In press.
16. Al-Sani F, Ben-Yakov M, Harvey G, et al. P016: Low risk ankle rule, high reward—a quality improvement initiative to reduce ankle x-rays in the pediatric emergency department [poster]. CJEM. 2017;19(S1):S83. https://doi.org/10.1017/cem.2017.218
17. Beck CE, Carcao M, Cada M, Porter S, Blanchette VS, Parkin PC. A quality improvement bundle to improve informed choice for children with typical, newly diagnosed immune thrombocytopenia. J Pediatr Hematol Oncol. 2018;40(8):e537-e543. https://doi.org/10.1097/MPH.0000000000001247
18. Beno S, Lenton-Brym T, Rosenfield D, McDowall D, Wales P, Principi T. Safe reduction of abdominal CT imaging in pediatric trauma patients: a quality-improvement initiative [abstract]. Can J Surg. 2019;62(3 Suppl 2):S29-S30.
19. Bal C, Tesch M, Blair G, Ostrow O, Premji L. Engaging medical trainees in resource stewardship through resident-led teaching sessions: a choosing wisely educational initiative. Can Med Educ J. 2021;12(1):e98-e100. https://doi.org/10.36834/cmej.70563
20. Berwick DM. Choices for the “new normal.” JAMA. 2020;323(21):2125-2126. https://doi.org/10.1001/jama.2020.6949
1. Elliott DK, Rose SR, Ronan JC. Changing the culture around cultures. Hosp Pediatr. 2014;4(6):405-407. https://doi.org/10.1542/hpeds.2014-0064
2. Gupta R, Simpson LA, Morgan DJ. Prioritizing high-value, equitable care after the COVID-19 shutdown: an opportunity for a healthcare renaissance. J Hosp Med. 2021;16(2):114-116. https://doi.org/10.12788/jhm.3526
3. Born K, Kool T, Levinson W. Reducing overuse in healthcare: advancing Choosing Wisely. BMJ. 2019;367:l6317. https://doi.org/10.1136/bmj.l6317
4. Coon ER, Young PC, Quinonez RA, Morgan DJ, Dhruva SS, Schroeder AR. Update on pediatric overuse. Pediatrics. 2017;139(2):e20162797. https://doi.org/10.1542/peds.2016-2797
5. Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. https://doi.org/10.1542/peds.2014-1778
6. Wolf ER, Krist AH, Schroeder AR. Deimplementation in pediatrics: past, present, and future. JAMA Pediatr. 2021;175(3):230-232. https://doi.org/10.1001/jamapediatrics.2020.4681
7. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing Wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
8. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
9. Ralston SL, Schroeder AR. Why is it so hard to talk about overuse in pediatrics and why it matters. JAMA Pediatr. 2017;17(10):931-932. https://doi.org/10.1001/jamapediatrics.2017.2239
10. Stammen LA, Stalmeijer RE, Paternotte E, et al. Training physicians to provide high-value, cost-conscious care: a systematic review. JAMA. 2015;314(22):2384-2400. https://doi.org/10.1001/jama.2015.16353
11. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. https://doi.org/10.1136/bmj.f2360
12. Friedman JN. Saying yes to the less: making it easier to choose wisely [editorial]. J Pediatr. 2017;145:4-5. https://doi.org/10.1016/j.jpeds.2017.01.062
13. Canadian Paediatric Society. Five things physicians and patients should question. Choosing Wisely Canada. Updated July 2019. Accessed June 17, 2021. https://choosingwiselycanada.org/wp-content/uploads/2020/07/Paediatrics_EN.pdf
14. Parikh K, Hall M, Montalbano A, et al. Establishing benchmarks for the hospitalized care of children with asthma, bronciolitis, and pneumonia. Pediatrics. 2014;134(3):555-562. https://doi.org/10.1542/peds.2014-1052
15. Ostrow O, Richardson S, Savlov D, Friedman JN. Reducing unnecessary respiratory viral testing to promote high value care. Pediatrics. In press.
16. Al-Sani F, Ben-Yakov M, Harvey G, et al. P016: Low risk ankle rule, high reward—a quality improvement initiative to reduce ankle x-rays in the pediatric emergency department [poster]. CJEM. 2017;19(S1):S83. https://doi.org/10.1017/cem.2017.218
17. Beck CE, Carcao M, Cada M, Porter S, Blanchette VS, Parkin PC. A quality improvement bundle to improve informed choice for children with typical, newly diagnosed immune thrombocytopenia. J Pediatr Hematol Oncol. 2018;40(8):e537-e543. https://doi.org/10.1097/MPH.0000000000001247
18. Beno S, Lenton-Brym T, Rosenfield D, McDowall D, Wales P, Principi T. Safe reduction of abdominal CT imaging in pediatric trauma patients: a quality-improvement initiative [abstract]. Can J Surg. 2019;62(3 Suppl 2):S29-S30.
19. Bal C, Tesch M, Blair G, Ostrow O, Premji L. Engaging medical trainees in resource stewardship through resident-led teaching sessions: a choosing wisely educational initiative. Can Med Educ J. 2021;12(1):e98-e100. https://doi.org/10.36834/cmej.70563
20. Berwick DM. Choices for the “new normal.” JAMA. 2020;323(21):2125-2126. https://doi.org/10.1001/jama.2020.6949
© 2021 Society of Hospital Medicine
Policy in Clinical Practice: Hospital Price Transparency
CLINICAL SCENARIO
A 59-year-old man is observed in the hospital for substernal chest pain initially concerning for angina. Serial troponin testing is negative, and based on additional history of intermittent dysphagia, an elective upper endoscopy is recommended after discharge. The patient does not have health insurance and expresses anxiety about the cost of endoscopy. He asks how he could compare the costs at different hospitals. How do federal price transparency rules assist the hospitalist in addressing this patient’s question?
BACKGROUND AND HISTORY
Healthcare costs continue to rise in the United States despite mounting concerns about wasteful spending and unaffordability.1 One contributor is a lack of price transparency.2 In theory, price transparency allows individuals to shop for services, spurring competition and lower prices. However, healthcare prices have historically been opaque to both physicians and patients; unlike other licensed professionals who provide clients estimates for their work (eg, lawyers, electricians), physicians are rarely able to offer patients real-time insight or guidance about costs, which most patients discover only when the bill arrives. The situation is particularly problematic for patients who bear higher out-of-pocket costs, such as the uninsured or those with high-deductible health plans.3
Decades of work to improve healthcare price transparency have unfortunately borne little fruit. Multiple states and organizations have attempted to disseminate price information on comparison websites.4 These efforts only modestly reduced some prices, with benefits confined to elective, single-episode, commodifiable services such as magnetic resonance imaging scans.5 The Affordable Care Act required hospitals to publish standard charges, also called a chargemaster (Table).6 However, chargemaster fees are notoriously inflated and inaccessible at the point of service, undercutting transparency.

POLICY IN CLINICAL PRACTICE
Beginning January 2021, the Centers for Medicare & Medicaid Services (CMS) required all hospitals to publish negotiated prices—including payor-specific negotiated charges—for 300 “shoppable services” (Table).6 The list must include 70 common CMS-specified services, such as a basic metabolic panel, upper endoscopy, and prostate biopsy, as well as another 230 services that each hospital determines relevant to its patient population.
In circumstances where hospitals have negotiated different prices for a service, they must list each third-party payor and their payor-specific charge. The information must be prominently displayed, accessible without requiring the patient to enter personal information, and provided in a machine-readable file. CMS may impose a $300 daily penalty on hospitals failing to comply with the policy. Of note, the policy does not apply to clinics or ambulatory surgery centers.
As more hospitals share data, this policy will directly benefit both patients and physicians. It can benefit patients with the time, foresight, and ability to search for the lowest price for shoppable services. Other patients may also benefit indirectly, to the extent that insurers and other purchasers apply this information to negotiate lower and more uniform prices. Decreased price variation may also encourage hospitals to compete on quality to distinguish the value of their services. Hospitalists could benefit through the ability to directly help patients locate price information.
Despite these potential benefits, the policy has limitations. Price information about shoppable services is most useful for discharge planning, and other solutions are needed to address transparency before and during unplanned admissions. Patients who prioritize continuity with a hospital or physician may be less price sensitive, particularly for more complex services. Patients with commercial insurance may be shielded from cost considerations and personal incentives to comparison shop. Interpreting hospitals’ estimates remains difficult, as it can be unclear if professional fees are included or if certain prices are offered to outpatients.7 Price information is not accompanied by corresponding quality data. Additionally, price transparency may also fail to lower prices in heavily concentrated payor or provider markets, and it remains unknown whether some providers may actually raise prices after learning about higher rates negotiated by competitors.8,9
Another issue is hospital participation. Early evidence suggests that most hospitals have not complied with the letter or spirit of the regulation.
Despite its limitations, this policy represents a meaningful advance for healthcare competition and patient empowerment. Additionally, it signals federal willingness to address the lack of price transparency as a source of widespread patient and clinician frustration—a commitment that will be needed to sustain this policy and implement additional measures in the future.
COMMENTARY AND RECOMMENDATIONS
CMS could consider five steps to augment the policy and maximize transparency and value for patients.
First, CMS could consider increasing daily nonparticipation penalties. Hospitals, particularly those in areas with less competition, have less incentive to participate given meager current penalties. Because the magnitude needed to compel action remains unknown, CMS could gradually escalate penalties over time until there is broader participation across hospitals.
Second, policymakers could aggregate price information centrally, organize the data around patients’ clinical scenarios, and advertise its availability. Currently, this information is scattered and time-consuming for hospitalists and patients to gather for decision-making. Additionally, CMS could encourage the development of third-party tools that aggregate and analyze machine-readable price data or require that prices be posted at the point of service.
Third, CMS could revise the policy to include quality as well as price information. Price alone does not offer a full enough picture of what consumers can expect from hospitals for shoppable services. Pairing price and quality information is better aligned to addressing costs in the context of value, rather than cost-cutting for its own purposes.
Fourth, over time, CMS could expand the list of services and sites required to report (eg, clinics and ambulatory surgical centers as well as hospitals).
Fifth, CMS rule-makers could set reporting standards and contextualize price information in common clinical scenarios. Patients may have difficulty shopping for complex healthcare services without understanding how they apply in different clinical situations. Decision-making would also be aided by reporting standards—for instance, for how prices are displayed and whether they include certain fees (eg, professional fees, pathology studies).
WHAT SHOULD I TELL MY PATIENT?
Hospitalists planning follow-up care should inform patients that price information is increasingly available and encourage them to search on the internet or contact hospital billing offices to request information (eg, discounted cash prices and minimum negotiated charges) before obtaining elective services after discharge. Hospitalists can also encourage patients to discuss shoppable services with their primary care physicians to understand the clinical context and make high-value decisions. Hospitalists who wish to build communication skills discussing costs with patients can increasingly find resources for these conversations and request that prices be displayed in the electronic health record for this purpose.13,14 As conversations occur, hospitalists should seek to understand other factors, such as convenience and continuity relationships, that might influence choices.
CONCLUSIONS
Starting in 2021, CMS policy requires that hospitals report prices for services such as the endoscopy recommended for the patient in the scenario. Though the policy gives patients new hope for greater transparency and better prices, additional steps are needed to help patients and hospitalists achieve these benefits.
1. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
2. Wetzell S. Transparency: a needed step towards health care affordability. American Health Policy Institute. March 2014. Accessed August 26, 2021. https://www.americanhealthpolicy.org/Content/documents/resources/Transparency%20Study%201%20-%20The%20Need%20for%20Health%20Care%20Transparency.pdf
3. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
4. Kullgren JT, Duey KA, Werner RM. A census of state health care price transparency websites. JAMA. 2013;309(23):2437-2438. https://doi.org/10.1001/jama.2013.6557
5. Brown ZY. Equilibrium effects of health care price information. Rev Econ Stat. 2019;101(4):699-712. https://doi.org/10.1162/rest_a_00765
6. Medicare and Medicaid Programs: CY 2020 hospital outpatient PPS policy changes and payment rates and ambulatory surgical center payment system policy changes and payment rates. Price transparency requirements for hospitals to make standard charges public. 45 CFR §180.20 (2019).
7. Kurani N, Ramirez G, Hudman J, Cox C, Kamal R. Early results from federal price transparency rule show difficulty in estimating the cost of care. Peterson-Kaiser Family Foundation. April 9, 2021. Accessed August 26, 2021. https://www.healthsystemtracker.org/brief/early-results-from-federal-price-transparency-rule-show-difficultly-in-estimating-the-cost-of-care/
8. Miller BJ, Mandelberg MC, Griffith NC, Ehrenfeld JM. Price transparency: empowering patient choice and promoting provider competition. J Med Syst. 2020;44(4):80. https://doi.org/10.1007/s10916-020-01553-2
9. Glied S. Price transparency–promise and peril. JAMA. 2021;325(15):1496-1497. https://doi.org/10.1001/jama.2021.4640
10. Haque W, Ahmadzada M, Allahrakha H, Haque E, Hsiehchen D. Transparency, accessibility, and variability of US hospital price data. JAMA Netw Open. 2021;4(5):e2110109. https://doi.org/10.1001/jamanetworkopen.2021.10109
11. Henderson M, Mouslim MC. Low compliance from big hospitals on CMS’s hospital price transparency rule. Health Affairs Blog. March 16, 2021. Accessed August 26, 2021. https://doi.org/10.1377/hblog20210311.899634
12. McGinty T, Wilde Mathews A, Evans M. Hospitals hide pricing data from search results. The Wall Street Journal. March 22, 2021. Accessed August 26, 2021. https://www.wsj.com/articles/hospitals-hide-pricing-data-from-search-results-11616405402
13. Dine CJ, Masi D, Smith CD. Tools to help overcome barriers to cost-of-care conversations. Ann Intern Med. 2019;170(9 suppl):S36-S38. https://doi.org/10.7326/M19-0778
14. Miller BJ, Slota JM, Ehrenfeld JM. Redefining the physician’s role in cost-conscious care: the potential role of the electronic health record. JAMA. 2019;322(8):721-722. https://doi.org/10.1001/jama.2019.9114
CLINICAL SCENARIO
A 59-year-old man is observed in the hospital for substernal chest pain initially concerning for angina. Serial troponin testing is negative, and based on additional history of intermittent dysphagia, an elective upper endoscopy is recommended after discharge. The patient does not have health insurance and expresses anxiety about the cost of endoscopy. He asks how he could compare the costs at different hospitals. How do federal price transparency rules assist the hospitalist in addressing this patient’s question?
BACKGROUND AND HISTORY
Healthcare costs continue to rise in the United States despite mounting concerns about wasteful spending and unaffordability.1 One contributor is a lack of price transparency.2 In theory, price transparency allows individuals to shop for services, spurring competition and lower prices. However, healthcare prices have historically been opaque to both physicians and patients; unlike other licensed professionals who provide clients estimates for their work (eg, lawyers, electricians), physicians are rarely able to offer patients real-time insight or guidance about costs, which most patients discover only when the bill arrives. The situation is particularly problematic for patients who bear higher out-of-pocket costs, such as the uninsured or those with high-deductible health plans.3
Decades of work to improve healthcare price transparency have unfortunately borne little fruit. Multiple states and organizations have attempted to disseminate price information on comparison websites.4 These efforts only modestly reduced some prices, with benefits confined to elective, single-episode, commodifiable services such as magnetic resonance imaging scans.5 The Affordable Care Act required hospitals to publish standard charges, also called a chargemaster (Table).6 However, chargemaster fees are notoriously inflated and inaccessible at the point of service, undercutting transparency.

POLICY IN CLINICAL PRACTICE
Beginning January 2021, the Centers for Medicare & Medicaid Services (CMS) required all hospitals to publish negotiated prices—including payor-specific negotiated charges—for 300 “shoppable services” (Table).6 The list must include 70 common CMS-specified services, such as a basic metabolic panel, upper endoscopy, and prostate biopsy, as well as another 230 services that each hospital determines relevant to its patient population.
In circumstances where hospitals have negotiated different prices for a service, they must list each third-party payor and their payor-specific charge. The information must be prominently displayed, accessible without requiring the patient to enter personal information, and provided in a machine-readable file. CMS may impose a $300 daily penalty on hospitals failing to comply with the policy. Of note, the policy does not apply to clinics or ambulatory surgery centers.
As more hospitals share data, this policy will directly benefit both patients and physicians. It can benefit patients with the time, foresight, and ability to search for the lowest price for shoppable services. Other patients may also benefit indirectly, to the extent that insurers and other purchasers apply this information to negotiate lower and more uniform prices. Decreased price variation may also encourage hospitals to compete on quality to distinguish the value of their services. Hospitalists could benefit through the ability to directly help patients locate price information.
Despite these potential benefits, the policy has limitations. Price information about shoppable services is most useful for discharge planning, and other solutions are needed to address transparency before and during unplanned admissions. Patients who prioritize continuity with a hospital or physician may be less price sensitive, particularly for more complex services. Patients with commercial insurance may be shielded from cost considerations and personal incentives to comparison shop. Interpreting hospitals’ estimates remains difficult, as it can be unclear if professional fees are included or if certain prices are offered to outpatients.7 Price information is not accompanied by corresponding quality data. Additionally, price transparency may also fail to lower prices in heavily concentrated payor or provider markets, and it remains unknown whether some providers may actually raise prices after learning about higher rates negotiated by competitors.8,9
Another issue is hospital participation. Early evidence suggests that most hospitals have not complied with the letter or spirit of the regulation.
Despite its limitations, this policy represents a meaningful advance for healthcare competition and patient empowerment. Additionally, it signals federal willingness to address the lack of price transparency as a source of widespread patient and clinician frustration—a commitment that will be needed to sustain this policy and implement additional measures in the future.
COMMENTARY AND RECOMMENDATIONS
CMS could consider five steps to augment the policy and maximize transparency and value for patients.
First, CMS could consider increasing daily nonparticipation penalties. Hospitals, particularly those in areas with less competition, have less incentive to participate given meager current penalties. Because the magnitude needed to compel action remains unknown, CMS could gradually escalate penalties over time until there is broader participation across hospitals.
Second, policymakers could aggregate price information centrally, organize the data around patients’ clinical scenarios, and advertise its availability. Currently, this information is scattered and time-consuming for hospitalists and patients to gather for decision-making. Additionally, CMS could encourage the development of third-party tools that aggregate and analyze machine-readable price data or require that prices be posted at the point of service.
Third, CMS could revise the policy to include quality as well as price information. Price alone does not offer a full enough picture of what consumers can expect from hospitals for shoppable services. Pairing price and quality information is better aligned to addressing costs in the context of value, rather than cost-cutting for its own purposes.
Fourth, over time, CMS could expand the list of services and sites required to report (eg, clinics and ambulatory surgical centers as well as hospitals).
Fifth, CMS rule-makers could set reporting standards and contextualize price information in common clinical scenarios. Patients may have difficulty shopping for complex healthcare services without understanding how they apply in different clinical situations. Decision-making would also be aided by reporting standards—for instance, for how prices are displayed and whether they include certain fees (eg, professional fees, pathology studies).
WHAT SHOULD I TELL MY PATIENT?
Hospitalists planning follow-up care should inform patients that price information is increasingly available and encourage them to search on the internet or contact hospital billing offices to request information (eg, discounted cash prices and minimum negotiated charges) before obtaining elective services after discharge. Hospitalists can also encourage patients to discuss shoppable services with their primary care physicians to understand the clinical context and make high-value decisions. Hospitalists who wish to build communication skills discussing costs with patients can increasingly find resources for these conversations and request that prices be displayed in the electronic health record for this purpose.13,14 As conversations occur, hospitalists should seek to understand other factors, such as convenience and continuity relationships, that might influence choices.
CONCLUSIONS
Starting in 2021, CMS policy requires that hospitals report prices for services such as the endoscopy recommended for the patient in the scenario. Though the policy gives patients new hope for greater transparency and better prices, additional steps are needed to help patients and hospitalists achieve these benefits.
CLINICAL SCENARIO
A 59-year-old man is observed in the hospital for substernal chest pain initially concerning for angina. Serial troponin testing is negative, and based on additional history of intermittent dysphagia, an elective upper endoscopy is recommended after discharge. The patient does not have health insurance and expresses anxiety about the cost of endoscopy. He asks how he could compare the costs at different hospitals. How do federal price transparency rules assist the hospitalist in addressing this patient’s question?
BACKGROUND AND HISTORY
Healthcare costs continue to rise in the United States despite mounting concerns about wasteful spending and unaffordability.1 One contributor is a lack of price transparency.2 In theory, price transparency allows individuals to shop for services, spurring competition and lower prices. However, healthcare prices have historically been opaque to both physicians and patients; unlike other licensed professionals who provide clients estimates for their work (eg, lawyers, electricians), physicians are rarely able to offer patients real-time insight or guidance about costs, which most patients discover only when the bill arrives. The situation is particularly problematic for patients who bear higher out-of-pocket costs, such as the uninsured or those with high-deductible health plans.3
Decades of work to improve healthcare price transparency have unfortunately borne little fruit. Multiple states and organizations have attempted to disseminate price information on comparison websites.4 These efforts only modestly reduced some prices, with benefits confined to elective, single-episode, commodifiable services such as magnetic resonance imaging scans.5 The Affordable Care Act required hospitals to publish standard charges, also called a chargemaster (Table).6 However, chargemaster fees are notoriously inflated and inaccessible at the point of service, undercutting transparency.

POLICY IN CLINICAL PRACTICE
Beginning January 2021, the Centers for Medicare & Medicaid Services (CMS) required all hospitals to publish negotiated prices—including payor-specific negotiated charges—for 300 “shoppable services” (Table).6 The list must include 70 common CMS-specified services, such as a basic metabolic panel, upper endoscopy, and prostate biopsy, as well as another 230 services that each hospital determines relevant to its patient population.
In circumstances where hospitals have negotiated different prices for a service, they must list each third-party payor and their payor-specific charge. The information must be prominently displayed, accessible without requiring the patient to enter personal information, and provided in a machine-readable file. CMS may impose a $300 daily penalty on hospitals failing to comply with the policy. Of note, the policy does not apply to clinics or ambulatory surgery centers.
As more hospitals share data, this policy will directly benefit both patients and physicians. It can benefit patients with the time, foresight, and ability to search for the lowest price for shoppable services. Other patients may also benefit indirectly, to the extent that insurers and other purchasers apply this information to negotiate lower and more uniform prices. Decreased price variation may also encourage hospitals to compete on quality to distinguish the value of their services. Hospitalists could benefit through the ability to directly help patients locate price information.
Despite these potential benefits, the policy has limitations. Price information about shoppable services is most useful for discharge planning, and other solutions are needed to address transparency before and during unplanned admissions. Patients who prioritize continuity with a hospital or physician may be less price sensitive, particularly for more complex services. Patients with commercial insurance may be shielded from cost considerations and personal incentives to comparison shop. Interpreting hospitals’ estimates remains difficult, as it can be unclear if professional fees are included or if certain prices are offered to outpatients.7 Price information is not accompanied by corresponding quality data. Additionally, price transparency may also fail to lower prices in heavily concentrated payor or provider markets, and it remains unknown whether some providers may actually raise prices after learning about higher rates negotiated by competitors.8,9
Another issue is hospital participation. Early evidence suggests that most hospitals have not complied with the letter or spirit of the regulation.
Despite its limitations, this policy represents a meaningful advance for healthcare competition and patient empowerment. Additionally, it signals federal willingness to address the lack of price transparency as a source of widespread patient and clinician frustration—a commitment that will be needed to sustain this policy and implement additional measures in the future.
COMMENTARY AND RECOMMENDATIONS
CMS could consider five steps to augment the policy and maximize transparency and value for patients.
First, CMS could consider increasing daily nonparticipation penalties. Hospitals, particularly those in areas with less competition, have less incentive to participate given meager current penalties. Because the magnitude needed to compel action remains unknown, CMS could gradually escalate penalties over time until there is broader participation across hospitals.
Second, policymakers could aggregate price information centrally, organize the data around patients’ clinical scenarios, and advertise its availability. Currently, this information is scattered and time-consuming for hospitalists and patients to gather for decision-making. Additionally, CMS could encourage the development of third-party tools that aggregate and analyze machine-readable price data or require that prices be posted at the point of service.
Third, CMS could revise the policy to include quality as well as price information. Price alone does not offer a full enough picture of what consumers can expect from hospitals for shoppable services. Pairing price and quality information is better aligned to addressing costs in the context of value, rather than cost-cutting for its own purposes.
Fourth, over time, CMS could expand the list of services and sites required to report (eg, clinics and ambulatory surgical centers as well as hospitals).
Fifth, CMS rule-makers could set reporting standards and contextualize price information in common clinical scenarios. Patients may have difficulty shopping for complex healthcare services without understanding how they apply in different clinical situations. Decision-making would also be aided by reporting standards—for instance, for how prices are displayed and whether they include certain fees (eg, professional fees, pathology studies).
WHAT SHOULD I TELL MY PATIENT?
Hospitalists planning follow-up care should inform patients that price information is increasingly available and encourage them to search on the internet or contact hospital billing offices to request information (eg, discounted cash prices and minimum negotiated charges) before obtaining elective services after discharge. Hospitalists can also encourage patients to discuss shoppable services with their primary care physicians to understand the clinical context and make high-value decisions. Hospitalists who wish to build communication skills discussing costs with patients can increasingly find resources for these conversations and request that prices be displayed in the electronic health record for this purpose.13,14 As conversations occur, hospitalists should seek to understand other factors, such as convenience and continuity relationships, that might influence choices.
CONCLUSIONS
Starting in 2021, CMS policy requires that hospitals report prices for services such as the endoscopy recommended for the patient in the scenario. Though the policy gives patients new hope for greater transparency and better prices, additional steps are needed to help patients and hospitalists achieve these benefits.
1. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
2. Wetzell S. Transparency: a needed step towards health care affordability. American Health Policy Institute. March 2014. Accessed August 26, 2021. https://www.americanhealthpolicy.org/Content/documents/resources/Transparency%20Study%201%20-%20The%20Need%20for%20Health%20Care%20Transparency.pdf
3. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
4. Kullgren JT, Duey KA, Werner RM. A census of state health care price transparency websites. JAMA. 2013;309(23):2437-2438. https://doi.org/10.1001/jama.2013.6557
5. Brown ZY. Equilibrium effects of health care price information. Rev Econ Stat. 2019;101(4):699-712. https://doi.org/10.1162/rest_a_00765
6. Medicare and Medicaid Programs: CY 2020 hospital outpatient PPS policy changes and payment rates and ambulatory surgical center payment system policy changes and payment rates. Price transparency requirements for hospitals to make standard charges public. 45 CFR §180.20 (2019).
7. Kurani N, Ramirez G, Hudman J, Cox C, Kamal R. Early results from federal price transparency rule show difficulty in estimating the cost of care. Peterson-Kaiser Family Foundation. April 9, 2021. Accessed August 26, 2021. https://www.healthsystemtracker.org/brief/early-results-from-federal-price-transparency-rule-show-difficultly-in-estimating-the-cost-of-care/
8. Miller BJ, Mandelberg MC, Griffith NC, Ehrenfeld JM. Price transparency: empowering patient choice and promoting provider competition. J Med Syst. 2020;44(4):80. https://doi.org/10.1007/s10916-020-01553-2
9. Glied S. Price transparency–promise and peril. JAMA. 2021;325(15):1496-1497. https://doi.org/10.1001/jama.2021.4640
10. Haque W, Ahmadzada M, Allahrakha H, Haque E, Hsiehchen D. Transparency, accessibility, and variability of US hospital price data. JAMA Netw Open. 2021;4(5):e2110109. https://doi.org/10.1001/jamanetworkopen.2021.10109
11. Henderson M, Mouslim MC. Low compliance from big hospitals on CMS’s hospital price transparency rule. Health Affairs Blog. March 16, 2021. Accessed August 26, 2021. https://doi.org/10.1377/hblog20210311.899634
12. McGinty T, Wilde Mathews A, Evans M. Hospitals hide pricing data from search results. The Wall Street Journal. March 22, 2021. Accessed August 26, 2021. https://www.wsj.com/articles/hospitals-hide-pricing-data-from-search-results-11616405402
13. Dine CJ, Masi D, Smith CD. Tools to help overcome barriers to cost-of-care conversations. Ann Intern Med. 2019;170(9 suppl):S36-S38. https://doi.org/10.7326/M19-0778
14. Miller BJ, Slota JM, Ehrenfeld JM. Redefining the physician’s role in cost-conscious care: the potential role of the electronic health record. JAMA. 2019;322(8):721-722. https://doi.org/10.1001/jama.2019.9114
1. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
2. Wetzell S. Transparency: a needed step towards health care affordability. American Health Policy Institute. March 2014. Accessed August 26, 2021. https://www.americanhealthpolicy.org/Content/documents/resources/Transparency%20Study%201%20-%20The%20Need%20for%20Health%20Care%20Transparency.pdf
3. Mehrotra A, Dean KM, Sinaiko AD, Sood N. Americans support price shopping for health care, but few actually seek out price information. Health Aff (Millwood). 2017;36(8):1392-1400. https://doi.org/10.1377/hlthaff.2016.1471
4. Kullgren JT, Duey KA, Werner RM. A census of state health care price transparency websites. JAMA. 2013;309(23):2437-2438. https://doi.org/10.1001/jama.2013.6557
5. Brown ZY. Equilibrium effects of health care price information. Rev Econ Stat. 2019;101(4):699-712. https://doi.org/10.1162/rest_a_00765
6. Medicare and Medicaid Programs: CY 2020 hospital outpatient PPS policy changes and payment rates and ambulatory surgical center payment system policy changes and payment rates. Price transparency requirements for hospitals to make standard charges public. 45 CFR §180.20 (2019).
7. Kurani N, Ramirez G, Hudman J, Cox C, Kamal R. Early results from federal price transparency rule show difficulty in estimating the cost of care. Peterson-Kaiser Family Foundation. April 9, 2021. Accessed August 26, 2021. https://www.healthsystemtracker.org/brief/early-results-from-federal-price-transparency-rule-show-difficultly-in-estimating-the-cost-of-care/
8. Miller BJ, Mandelberg MC, Griffith NC, Ehrenfeld JM. Price transparency: empowering patient choice and promoting provider competition. J Med Syst. 2020;44(4):80. https://doi.org/10.1007/s10916-020-01553-2
9. Glied S. Price transparency–promise and peril. JAMA. 2021;325(15):1496-1497. https://doi.org/10.1001/jama.2021.4640
10. Haque W, Ahmadzada M, Allahrakha H, Haque E, Hsiehchen D. Transparency, accessibility, and variability of US hospital price data. JAMA Netw Open. 2021;4(5):e2110109. https://doi.org/10.1001/jamanetworkopen.2021.10109
11. Henderson M, Mouslim MC. Low compliance from big hospitals on CMS’s hospital price transparency rule. Health Affairs Blog. March 16, 2021. Accessed August 26, 2021. https://doi.org/10.1377/hblog20210311.899634
12. McGinty T, Wilde Mathews A, Evans M. Hospitals hide pricing data from search results. The Wall Street Journal. March 22, 2021. Accessed August 26, 2021. https://www.wsj.com/articles/hospitals-hide-pricing-data-from-search-results-11616405402
13. Dine CJ, Masi D, Smith CD. Tools to help overcome barriers to cost-of-care conversations. Ann Intern Med. 2019;170(9 suppl):S36-S38. https://doi.org/10.7326/M19-0778
14. Miller BJ, Slota JM, Ehrenfeld JM. Redefining the physician’s role in cost-conscious care: the potential role of the electronic health record. JAMA. 2019;322(8):721-722. https://doi.org/10.1001/jama.2019.9114
© 2021 Society of Hospital Medicine
Policy in Clinical Practice: Emergency Medicaid and Access to Allogeneic Stem Cell Transplant for Undocumented Immigrants
Clinical Scenario
Juan, a 50-year-old man with acute myeloid leukemia (AML), sat on the edge of his bed, dejected. Juan’s leukemia had relapsed for a third time, and he was low on options and optimism. Originally from Mexico, he had made the journey to Colorado to work as a mechanic and care for his disabled son. Like millions of other individuals in the United States, he did not obtain a visa and had no affordable options for health insurance. For nearly a decade, that had seemed not to matter, until he became ill. Initially presenting to the emergency department with fatigue and night sweats, Juan was diagnosed with poor-risk AML and underwent emergent induction chemotherapy reimbursed under Emergency Medicaid (Table). Just when his bone marrow biopsy showed remission, however, Juan was told there was no chance to cure him, as his documentation status precluded him from receiving the next recommended therapy: stem cell transplant (SCT). Without transplant, Juan’s leukemia relapsed within a few months. He decided to undergo all the salvage chemotherapy that was offered, worrying about how his son would survive without his father.
Background and History
For the patient with a new cancer diagnosis, a difference in immigration status may be the difference between life and death. Undocumented immigrants are excluded from federally funded benefits, including those offered under Medicare, most Medicaid programs, and the Patient Protection and Affordable Care Act (Table).1 The nearly 11 million undocumented immigrants residing in the United States are integral to the workforce and economy. Although they pay taxes that fund Medicaid, contributing approximately $11.7 billion nationally in 2017, undocumented immigrants are ineligible to benefit from such programs.2 The inequity of this policy is highlighted by Juan, an undocumented immigrant presenting with a new diagnosis of AML.
The Emergency Medical Treatment and Active Labor Act (EMTALA) is a 1986 federal law which mandates that patients who present to the hospital with an emergency medical condition receive appropriate evaluation and stabilizing treatment. An emergency condition is defined as “manifesting itself by acute symptoms of sufficient severity … such that the absence of immediate medical attention could reasonably be expected to result in (A) placing the patient’s health in serious jeopardy; (B) serious impairment to bodily functions; or (C) serious dysfunction of any bodily organ or part” (Table).3,4 The Centers for Medicare & Medicaid manual restates the EMTALA definition and notes that services for an emergency medical condition cannot include care related to organ transplantation. Most state Emergency Medicaid programs have adopted the federal definition of what constitutes a medical emergency.5 As a result, undocumented individuals who qualify for Medicaid benefits but who do not meet citizenship requirements are eligible to “receive Medical Assistance benefits for emergency medical care only.”3
Similar to our patient Juan, individuals who initially present with an acute leukemia would be eligible for induction chemotherapy, as blast crisis is imminently fatal. Once in remission, however, standard-of-care therapy for patients without disqualifying comorbidities, depending on cytogenetic disease phenotypes, recommends the only current potential cure: allogeneic SCT, a treatment that was far from routine practice at the time EMTALA was enacted.6 When preparing for transplant, a patient is stable and no longer fits EMTALA’s “emergency” criteria, even though their health is still in “serious jeopardy,” as their cancer has been incompletely treated. Because most state Emergency Medicaid programs adopt the federal definition of an emergency medical condition, the cure is out of reach.
Policy in Clinical Practice
This policy requires clinicians to deviate from the usual standard of care and results in inferior outcomes. For AML patients in the poor-risk category, allogeneic SCT is recommended following induction chemotherapy.7 The risk of relapse is 30% to 40% if consolidation therapy includes SCT, vs 70% to 80% if treated with chemotherapeutic consolidation alone.6 AML patients in the intermediate-, and sometimes even favorable- risk categories, have been shown to benefit from allogeneic SCT as well, with risk of relapse half that of a patient who undergoes consolidation without transplant. Undocumented individuals with AML are therefore resigned to inadequate cancer treatment, including lifelong salvage chemotherapy, and have a substantially decreased chance of achieving sustained remission.6 Furthermore, providing inequitable care for undocumented patients with other medical conditions, such as end-stage kidney disease (ESKD), has been associated with inferior patient-reported outcomes, higher mortality and hospital costs, and clinician burnout. In many states, undocumented immigrants with ESKD rely on emergency dialysis (dialysis made available only after presenting critically ill to an emergency department). In 2019, Colorado’s Medicaid agency opted to include ESKD as a qualifying condition for Emergency Medicaid, thereby expanding access to scheduled dialysis. This led to improved patient quality of life, a decreased emotional toll on patients and clinicians, and reduced costs.8,9
Economic Considerations
Policy discussions must consider cost. The average cost of allogeneic SCT in the United States was approximately $226,000 in 2018, which is often compared to the cost of managing a patient with refractory disease who does not receive transplant.10 This study reported a cost of active disease without transplant, including chemotherapy and hospitalizations, of approximately $69,000, plus terminal care costs of nearly $89,000; at a total of $158,000, this comes out to $68,000 less than SCT.10 This cost savings, however, results in a patient’s death rather than an up to 85% chance of long-term, relapse-free survival.6
To more completely capture the relationship between the healthcare value and cost-effectiveness of SCT, a second study calculated the incremental cost-effectiveness ratio (ICER) of transplantation in acute leukemias in the first 100 days post transplant, including management of complications, such as hospitalization, acute graft-versus-host disease (GVHD), infection, and blood product transfusions. ICER represents the economic value of an intervention compared to an alternative, calculated as cost per quality-adjusted life years. The ICER of SCT compared to no transplant is $16,346 to $34,360, depending on type of transplant and conditioning regimen.11 An ICER of less than $50,000 is considered an acceptable expense for the value achieved—in this case, a significant opportunity for cure. This finding supports SCT, including management of complications, as an economically valuable intervention. Furthermore, if a sustained remission is achieved with SCT, this difference in expense buys the individual patient potentially decades of productivity to contribute back into society and the economy. According to the National Bureau of Economic Research, undocumented workers as a whole contribute $5 trillion to the US Gross Domestic Product over a 10-year period, or about $45,000 per worker per year.12 According to the costs cited, curing a single undocumented worker with acute leukemia via SCT and allowing them to return to work would lead to a return on investment in less than 2 years. If the goal is high-quality, high-value, equitable care, it is logical to spend the money upfront and allow all patients the best chance for recovery.
One might suggest that patients instead receive treatment in their country of origin. This proposition, however, is often unrealistic. Latin American countries, for example, lack access to many standard-of-care cancer treatments available domestically. In Mexico, SCT is only available at a single facility in Mexico City, which is unable to track outcomes.13 The mortality-to-incidence ratio for cancer, a marker of availability of effective treatment, for Latin America is 0.48, substantially inferior to that of the United States (0.29).14 Importantly, almost two thirds of undocumented immigrants in the United States have lived in the country for 10 or more years, and 43% are parents of minor children, an increasing proportion of whom are American citizens.15 This highlights the impracticality of these individuals returning to their country of origin for treatment.
Commentary and Recommendations
Medicaid laws in several states have made it possible for undocumented immigrants to receive access to standard-of-care therapies. Washington and California have included provisions that enable undocumented immigrants to receive allogeneic SCT if they are otherwise medically eligible. In the course of this policy change, legal arguments from the California Court of Appeals expressed that the language of the law was not intended to deny lifesaving treatment to an individual.16 California’s Emergency Medicaid policy is comparable to that of other states, but because the courts considered SCT a “continuation of medically necessary inpatient hospital services … directly related to the emergency” for which the patient initially presented, they concluded that it could be covered under California Medicaid. Despite covering SCT for undocumented immigrants, California maintains lower costs for those patients compared to US citizens on Medicaid while providing evidence-based cancer care.17 This exemplifies sustainable and equitable healthcare policy for the rest of the nation.
A proposed change in policy could occur at either the federal or state level. One option would be to follow the example set by the State of Washington. Under Emergency Medicaid, Washington modified qualifying conditions to include “emergency room care, inpatient admission, or outpatient surgery; a cancer treatment plan; dialysis treatment; anti-rejection medication for an organ transplant” and long-term care services.18 Federal policy reform for undocumented immigrants would also improve access to care. The US Citizenship Act of 2021, introduced to the House of Representatives in February 2021, offers a path to citizenship for undocumented immigrants, ultimately allowing for undocumented individuals to be eligible for the same programs as citizens, though after a period of up to 8 years.19 More immediate revisions of qualifying conditions under state Emergency Medicaid programs, coupled with a path to citizenship, would make significant progress towards reducing structural health inequities. Such policy change would also have broader implications. Three quarters of undocumented immigrants in the United States originate from Mexico, Central America, and South America, and the incidence rate of AML for Latinx individuals is 3.6 per 100,000, a figure which can be extrapolated to an estimated 380 cases per year in the US undocumented population.20-22 In addition to benefiting patients with acute leukemias, the proposed policy change would also benefit numerous others who are frequently hospitalized for acute decompensations of chronic conditions, including congestive heart failure, liver disease, ESKD, and chronic lung conditions. Enabling follow-up care for these diseases under Emergency Medicaid would likewise be expected to reduce costs and improve both quality of care and patient-centered and clinical outcomes.
What Should I Tell My Patient?
Hospitalists frequently care for undocumented immigrants with acute leukemias because the hospital can only be reimbursed by Emergency Medicaid when a patient is admitted to the hospital. Patients may ask about what they can expect in the course of their illness and, while details may be left to the oncologist, hospitalists will be faced with responding to many of these questions. Clinicians at our institution hold honest conversations with patients like Juan. We are compelled to provide the care that hospital and state policies allow, and can only offer the best care available to them because of the restrictions of an insurance system to which they contribute financially, yet cannot benefit from, in their time of need. We can tell our undocumented immigrant patients that we find this unacceptable and are actively advocating to change this policy.
Conclusion
The State of Colorado and the nation must amend its healthcare policy to include comprehensive cancer care for everyone. Offering standard-of-care therapy to all patients is not only ethical, but also an economically sound policy benefiting patients, clinicians, and the workforce.
1. Skopec L, Holahan J, Elmendorf C. Changes in Health Insurance Coverage in 2013-2016: Medicaid Expansion States Lead the Way. Urban Institute. September 11, 2018. Accessed July 12, 2021. https://www.urban.org/research/publication/changes-health-insurance-coverage-2013-2016-medicaid-expansion-states-lead-way
2. Christensen Gee L, Gardner M, Hill ME, Wiehe M. Undocumented Immigrants’ State & Local Tax Contributions. Institute on Taxation & Economic Policy. Updated March 2017. Accessed July 12, 2021. https://www.immigrationresearch.org/system/files/immigration_taxes_2017.pdf
3. Emergency Medical Treatment and Labor Act (EMTALA), Public Law 42 U.S.C. 1395dd. 2010.
4. Social Security Act. Sec. 1903 [42 U.S.C. 1396b]. Accessed July 12, 2021. https://www.ssa.gov/OP_Home/ssact/title19/1903.htm.
5. Cervantes L, Mundo W, Powe NR. The status of provision of standard outpatient dialysis for US undocumented immigrants with ESKD. Clin J Am Soc Nephrol. 2019;14(8):1258-1260. https://doi.org/10.2215/CJN.03460319
6. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. https://doi.org/10.1182/blood-2015-07-604546
7. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. 2021.
8. Cervantes L, Richardson S, Raghavan R, et al. Clinicians’ perspectives on providing emergency-only hemodialysis to undocumented immigrants: a qualitative study. Ann Intern Med. 2018;169(2):78-86. https://doi.org/10.7326/M18-0400
9. Cervantes L, Tong A, Camacho C, Collings A, Powe NR. Patient-reported outcomes and experiences in the transition of undocumented patients from emergency to scheduled hemodialysis. Kidney Int. 2021;99(1):198-207. https://doi.org/10.1016/j.kint.2020.07.024
10. Stein E, Xie J, Duchesneau E, et al. Cost effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in the United States. Pharmacoeconomics. 2019;37(2):239-253. https://doi.org/10.1007/s40273-018-0732-4
11. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620-1628. https://doi.org/10.1016/j.bbmt.2012.04.001
12. Edwards R, Ortega F. The Economic Contribution of Unauthorized Workers: An Industry Analysis. National Bureau of Economic Research. November 2016. Accessed July 12, 2021. https://www.nber.org/system/files/working_papers/w22834/w22834.pdf
13. Nunnery SE, Fintel AE, Jackson WC, Chandler JC, Ugwueke MO, Martin MG. Treatment disparities faced by undocumented workers from low- and middle-income countries in the United States with hematologic malignancies. J Natl Compr Canc Netw. 2016;14(4):483-486. https://doi.org/10.6004/jnccn.2016.0053
14. World Cancer Initiative. Cancer Preparedness in Latin America: The Need to Build on Recent Progress. 2019. Accessed July 7, 2021. https://worldcancerinitiative.economist.com/cancer-preparedness-latin-america
15. Taylor P, Lopez MH, Passel JS, Motel S; Pew Research Center. Unauthorized Immigrants: Length of Residency, Patterns of Parenthood. December 1, 2011. Accessed July 12, 2021. https://www.pewresearch.org/hispanic/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
16. California Supreme Court, Records and Briefs: S019427, Dominguez vs. Superior Court of Alameda County. 1990.
17. Wallace SP, Torres J, Sadegh-Nobari T, Pourat N, Brown ER. Undocumented Immigrants and Health Care Reform. UCLA Center for Health Policy Research. August 31, 2012. Accessed July 7, 2021. https://healthpolicy.ucla.edu/publications/Documents/PDF/undocumentedreport-aug2013.pdf
18. Washington State Health Care Authority. Health care services and supports. Noncitizens. Accessed July 12, 2021. https://www.hca.wa.gov/health-care-services-supports/apple-health-medicaid-coverage/non-citizens
19. 117th Congress of the United States. H.R.1177, U.S. Citizenship Act of 2021.
20. National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program. Accessed July 7, 2021. https://seer.cancer.gov/
21. Migration Policy Institute. Profile of the unauthorized population: United States. Accessed July 12, 2021. https://www.migrationpolicy.org/data/unauthorized-immigrant-population/state/US. 2021.
22. Torres L. Latinx? Lat Stud. 2018;16:283-285. https://doi.org/10.1057/s41276-018-0142-y
Clinical Scenario
Juan, a 50-year-old man with acute myeloid leukemia (AML), sat on the edge of his bed, dejected. Juan’s leukemia had relapsed for a third time, and he was low on options and optimism. Originally from Mexico, he had made the journey to Colorado to work as a mechanic and care for his disabled son. Like millions of other individuals in the United States, he did not obtain a visa and had no affordable options for health insurance. For nearly a decade, that had seemed not to matter, until he became ill. Initially presenting to the emergency department with fatigue and night sweats, Juan was diagnosed with poor-risk AML and underwent emergent induction chemotherapy reimbursed under Emergency Medicaid (Table). Just when his bone marrow biopsy showed remission, however, Juan was told there was no chance to cure him, as his documentation status precluded him from receiving the next recommended therapy: stem cell transplant (SCT). Without transplant, Juan’s leukemia relapsed within a few months. He decided to undergo all the salvage chemotherapy that was offered, worrying about how his son would survive without his father.
Background and History
For the patient with a new cancer diagnosis, a difference in immigration status may be the difference between life and death. Undocumented immigrants are excluded from federally funded benefits, including those offered under Medicare, most Medicaid programs, and the Patient Protection and Affordable Care Act (Table).1 The nearly 11 million undocumented immigrants residing in the United States are integral to the workforce and economy. Although they pay taxes that fund Medicaid, contributing approximately $11.7 billion nationally in 2017, undocumented immigrants are ineligible to benefit from such programs.2 The inequity of this policy is highlighted by Juan, an undocumented immigrant presenting with a new diagnosis of AML.
The Emergency Medical Treatment and Active Labor Act (EMTALA) is a 1986 federal law which mandates that patients who present to the hospital with an emergency medical condition receive appropriate evaluation and stabilizing treatment. An emergency condition is defined as “manifesting itself by acute symptoms of sufficient severity … such that the absence of immediate medical attention could reasonably be expected to result in (A) placing the patient’s health in serious jeopardy; (B) serious impairment to bodily functions; or (C) serious dysfunction of any bodily organ or part” (Table).3,4 The Centers for Medicare & Medicaid manual restates the EMTALA definition and notes that services for an emergency medical condition cannot include care related to organ transplantation. Most state Emergency Medicaid programs have adopted the federal definition of what constitutes a medical emergency.5 As a result, undocumented individuals who qualify for Medicaid benefits but who do not meet citizenship requirements are eligible to “receive Medical Assistance benefits for emergency medical care only.”3
Similar to our patient Juan, individuals who initially present with an acute leukemia would be eligible for induction chemotherapy, as blast crisis is imminently fatal. Once in remission, however, standard-of-care therapy for patients without disqualifying comorbidities, depending on cytogenetic disease phenotypes, recommends the only current potential cure: allogeneic SCT, a treatment that was far from routine practice at the time EMTALA was enacted.6 When preparing for transplant, a patient is stable and no longer fits EMTALA’s “emergency” criteria, even though their health is still in “serious jeopardy,” as their cancer has been incompletely treated. Because most state Emergency Medicaid programs adopt the federal definition of an emergency medical condition, the cure is out of reach.
Policy in Clinical Practice
This policy requires clinicians to deviate from the usual standard of care and results in inferior outcomes. For AML patients in the poor-risk category, allogeneic SCT is recommended following induction chemotherapy.7 The risk of relapse is 30% to 40% if consolidation therapy includes SCT, vs 70% to 80% if treated with chemotherapeutic consolidation alone.6 AML patients in the intermediate-, and sometimes even favorable- risk categories, have been shown to benefit from allogeneic SCT as well, with risk of relapse half that of a patient who undergoes consolidation without transplant. Undocumented individuals with AML are therefore resigned to inadequate cancer treatment, including lifelong salvage chemotherapy, and have a substantially decreased chance of achieving sustained remission.6 Furthermore, providing inequitable care for undocumented patients with other medical conditions, such as end-stage kidney disease (ESKD), has been associated with inferior patient-reported outcomes, higher mortality and hospital costs, and clinician burnout. In many states, undocumented immigrants with ESKD rely on emergency dialysis (dialysis made available only after presenting critically ill to an emergency department). In 2019, Colorado’s Medicaid agency opted to include ESKD as a qualifying condition for Emergency Medicaid, thereby expanding access to scheduled dialysis. This led to improved patient quality of life, a decreased emotional toll on patients and clinicians, and reduced costs.8,9
Economic Considerations
Policy discussions must consider cost. The average cost of allogeneic SCT in the United States was approximately $226,000 in 2018, which is often compared to the cost of managing a patient with refractory disease who does not receive transplant.10 This study reported a cost of active disease without transplant, including chemotherapy and hospitalizations, of approximately $69,000, plus terminal care costs of nearly $89,000; at a total of $158,000, this comes out to $68,000 less than SCT.10 This cost savings, however, results in a patient’s death rather than an up to 85% chance of long-term, relapse-free survival.6
To more completely capture the relationship between the healthcare value and cost-effectiveness of SCT, a second study calculated the incremental cost-effectiveness ratio (ICER) of transplantation in acute leukemias in the first 100 days post transplant, including management of complications, such as hospitalization, acute graft-versus-host disease (GVHD), infection, and blood product transfusions. ICER represents the economic value of an intervention compared to an alternative, calculated as cost per quality-adjusted life years. The ICER of SCT compared to no transplant is $16,346 to $34,360, depending on type of transplant and conditioning regimen.11 An ICER of less than $50,000 is considered an acceptable expense for the value achieved—in this case, a significant opportunity for cure. This finding supports SCT, including management of complications, as an economically valuable intervention. Furthermore, if a sustained remission is achieved with SCT, this difference in expense buys the individual patient potentially decades of productivity to contribute back into society and the economy. According to the National Bureau of Economic Research, undocumented workers as a whole contribute $5 trillion to the US Gross Domestic Product over a 10-year period, or about $45,000 per worker per year.12 According to the costs cited, curing a single undocumented worker with acute leukemia via SCT and allowing them to return to work would lead to a return on investment in less than 2 years. If the goal is high-quality, high-value, equitable care, it is logical to spend the money upfront and allow all patients the best chance for recovery.
One might suggest that patients instead receive treatment in their country of origin. This proposition, however, is often unrealistic. Latin American countries, for example, lack access to many standard-of-care cancer treatments available domestically. In Mexico, SCT is only available at a single facility in Mexico City, which is unable to track outcomes.13 The mortality-to-incidence ratio for cancer, a marker of availability of effective treatment, for Latin America is 0.48, substantially inferior to that of the United States (0.29).14 Importantly, almost two thirds of undocumented immigrants in the United States have lived in the country for 10 or more years, and 43% are parents of minor children, an increasing proportion of whom are American citizens.15 This highlights the impracticality of these individuals returning to their country of origin for treatment.
Commentary and Recommendations
Medicaid laws in several states have made it possible for undocumented immigrants to receive access to standard-of-care therapies. Washington and California have included provisions that enable undocumented immigrants to receive allogeneic SCT if they are otherwise medically eligible. In the course of this policy change, legal arguments from the California Court of Appeals expressed that the language of the law was not intended to deny lifesaving treatment to an individual.16 California’s Emergency Medicaid policy is comparable to that of other states, but because the courts considered SCT a “continuation of medically necessary inpatient hospital services … directly related to the emergency” for which the patient initially presented, they concluded that it could be covered under California Medicaid. Despite covering SCT for undocumented immigrants, California maintains lower costs for those patients compared to US citizens on Medicaid while providing evidence-based cancer care.17 This exemplifies sustainable and equitable healthcare policy for the rest of the nation.
A proposed change in policy could occur at either the federal or state level. One option would be to follow the example set by the State of Washington. Under Emergency Medicaid, Washington modified qualifying conditions to include “emergency room care, inpatient admission, or outpatient surgery; a cancer treatment plan; dialysis treatment; anti-rejection medication for an organ transplant” and long-term care services.18 Federal policy reform for undocumented immigrants would also improve access to care. The US Citizenship Act of 2021, introduced to the House of Representatives in February 2021, offers a path to citizenship for undocumented immigrants, ultimately allowing for undocumented individuals to be eligible for the same programs as citizens, though after a period of up to 8 years.19 More immediate revisions of qualifying conditions under state Emergency Medicaid programs, coupled with a path to citizenship, would make significant progress towards reducing structural health inequities. Such policy change would also have broader implications. Three quarters of undocumented immigrants in the United States originate from Mexico, Central America, and South America, and the incidence rate of AML for Latinx individuals is 3.6 per 100,000, a figure which can be extrapolated to an estimated 380 cases per year in the US undocumented population.20-22 In addition to benefiting patients with acute leukemias, the proposed policy change would also benefit numerous others who are frequently hospitalized for acute decompensations of chronic conditions, including congestive heart failure, liver disease, ESKD, and chronic lung conditions. Enabling follow-up care for these diseases under Emergency Medicaid would likewise be expected to reduce costs and improve both quality of care and patient-centered and clinical outcomes.
What Should I Tell My Patient?
Hospitalists frequently care for undocumented immigrants with acute leukemias because the hospital can only be reimbursed by Emergency Medicaid when a patient is admitted to the hospital. Patients may ask about what they can expect in the course of their illness and, while details may be left to the oncologist, hospitalists will be faced with responding to many of these questions. Clinicians at our institution hold honest conversations with patients like Juan. We are compelled to provide the care that hospital and state policies allow, and can only offer the best care available to them because of the restrictions of an insurance system to which they contribute financially, yet cannot benefit from, in their time of need. We can tell our undocumented immigrant patients that we find this unacceptable and are actively advocating to change this policy.
Conclusion
The State of Colorado and the nation must amend its healthcare policy to include comprehensive cancer care for everyone. Offering standard-of-care therapy to all patients is not only ethical, but also an economically sound policy benefiting patients, clinicians, and the workforce.
Clinical Scenario
Juan, a 50-year-old man with acute myeloid leukemia (AML), sat on the edge of his bed, dejected. Juan’s leukemia had relapsed for a third time, and he was low on options and optimism. Originally from Mexico, he had made the journey to Colorado to work as a mechanic and care for his disabled son. Like millions of other individuals in the United States, he did not obtain a visa and had no affordable options for health insurance. For nearly a decade, that had seemed not to matter, until he became ill. Initially presenting to the emergency department with fatigue and night sweats, Juan was diagnosed with poor-risk AML and underwent emergent induction chemotherapy reimbursed under Emergency Medicaid (Table). Just when his bone marrow biopsy showed remission, however, Juan was told there was no chance to cure him, as his documentation status precluded him from receiving the next recommended therapy: stem cell transplant (SCT). Without transplant, Juan’s leukemia relapsed within a few months. He decided to undergo all the salvage chemotherapy that was offered, worrying about how his son would survive without his father.
Background and History
For the patient with a new cancer diagnosis, a difference in immigration status may be the difference between life and death. Undocumented immigrants are excluded from federally funded benefits, including those offered under Medicare, most Medicaid programs, and the Patient Protection and Affordable Care Act (Table).1 The nearly 11 million undocumented immigrants residing in the United States are integral to the workforce and economy. Although they pay taxes that fund Medicaid, contributing approximately $11.7 billion nationally in 2017, undocumented immigrants are ineligible to benefit from such programs.2 The inequity of this policy is highlighted by Juan, an undocumented immigrant presenting with a new diagnosis of AML.
The Emergency Medical Treatment and Active Labor Act (EMTALA) is a 1986 federal law which mandates that patients who present to the hospital with an emergency medical condition receive appropriate evaluation and stabilizing treatment. An emergency condition is defined as “manifesting itself by acute symptoms of sufficient severity … such that the absence of immediate medical attention could reasonably be expected to result in (A) placing the patient’s health in serious jeopardy; (B) serious impairment to bodily functions; or (C) serious dysfunction of any bodily organ or part” (Table).3,4 The Centers for Medicare & Medicaid manual restates the EMTALA definition and notes that services for an emergency medical condition cannot include care related to organ transplantation. Most state Emergency Medicaid programs have adopted the federal definition of what constitutes a medical emergency.5 As a result, undocumented individuals who qualify for Medicaid benefits but who do not meet citizenship requirements are eligible to “receive Medical Assistance benefits for emergency medical care only.”3
Similar to our patient Juan, individuals who initially present with an acute leukemia would be eligible for induction chemotherapy, as blast crisis is imminently fatal. Once in remission, however, standard-of-care therapy for patients without disqualifying comorbidities, depending on cytogenetic disease phenotypes, recommends the only current potential cure: allogeneic SCT, a treatment that was far from routine practice at the time EMTALA was enacted.6 When preparing for transplant, a patient is stable and no longer fits EMTALA’s “emergency” criteria, even though their health is still in “serious jeopardy,” as their cancer has been incompletely treated. Because most state Emergency Medicaid programs adopt the federal definition of an emergency medical condition, the cure is out of reach.
Policy in Clinical Practice
This policy requires clinicians to deviate from the usual standard of care and results in inferior outcomes. For AML patients in the poor-risk category, allogeneic SCT is recommended following induction chemotherapy.7 The risk of relapse is 30% to 40% if consolidation therapy includes SCT, vs 70% to 80% if treated with chemotherapeutic consolidation alone.6 AML patients in the intermediate-, and sometimes even favorable- risk categories, have been shown to benefit from allogeneic SCT as well, with risk of relapse half that of a patient who undergoes consolidation without transplant. Undocumented individuals with AML are therefore resigned to inadequate cancer treatment, including lifelong salvage chemotherapy, and have a substantially decreased chance of achieving sustained remission.6 Furthermore, providing inequitable care for undocumented patients with other medical conditions, such as end-stage kidney disease (ESKD), has been associated with inferior patient-reported outcomes, higher mortality and hospital costs, and clinician burnout. In many states, undocumented immigrants with ESKD rely on emergency dialysis (dialysis made available only after presenting critically ill to an emergency department). In 2019, Colorado’s Medicaid agency opted to include ESKD as a qualifying condition for Emergency Medicaid, thereby expanding access to scheduled dialysis. This led to improved patient quality of life, a decreased emotional toll on patients and clinicians, and reduced costs.8,9
Economic Considerations
Policy discussions must consider cost. The average cost of allogeneic SCT in the United States was approximately $226,000 in 2018, which is often compared to the cost of managing a patient with refractory disease who does not receive transplant.10 This study reported a cost of active disease without transplant, including chemotherapy and hospitalizations, of approximately $69,000, plus terminal care costs of nearly $89,000; at a total of $158,000, this comes out to $68,000 less than SCT.10 This cost savings, however, results in a patient’s death rather than an up to 85% chance of long-term, relapse-free survival.6
To more completely capture the relationship between the healthcare value and cost-effectiveness of SCT, a second study calculated the incremental cost-effectiveness ratio (ICER) of transplantation in acute leukemias in the first 100 days post transplant, including management of complications, such as hospitalization, acute graft-versus-host disease (GVHD), infection, and blood product transfusions. ICER represents the economic value of an intervention compared to an alternative, calculated as cost per quality-adjusted life years. The ICER of SCT compared to no transplant is $16,346 to $34,360, depending on type of transplant and conditioning regimen.11 An ICER of less than $50,000 is considered an acceptable expense for the value achieved—in this case, a significant opportunity for cure. This finding supports SCT, including management of complications, as an economically valuable intervention. Furthermore, if a sustained remission is achieved with SCT, this difference in expense buys the individual patient potentially decades of productivity to contribute back into society and the economy. According to the National Bureau of Economic Research, undocumented workers as a whole contribute $5 trillion to the US Gross Domestic Product over a 10-year period, or about $45,000 per worker per year.12 According to the costs cited, curing a single undocumented worker with acute leukemia via SCT and allowing them to return to work would lead to a return on investment in less than 2 years. If the goal is high-quality, high-value, equitable care, it is logical to spend the money upfront and allow all patients the best chance for recovery.
One might suggest that patients instead receive treatment in their country of origin. This proposition, however, is often unrealistic. Latin American countries, for example, lack access to many standard-of-care cancer treatments available domestically. In Mexico, SCT is only available at a single facility in Mexico City, which is unable to track outcomes.13 The mortality-to-incidence ratio for cancer, a marker of availability of effective treatment, for Latin America is 0.48, substantially inferior to that of the United States (0.29).14 Importantly, almost two thirds of undocumented immigrants in the United States have lived in the country for 10 or more years, and 43% are parents of minor children, an increasing proportion of whom are American citizens.15 This highlights the impracticality of these individuals returning to their country of origin for treatment.
Commentary and Recommendations
Medicaid laws in several states have made it possible for undocumented immigrants to receive access to standard-of-care therapies. Washington and California have included provisions that enable undocumented immigrants to receive allogeneic SCT if they are otherwise medically eligible. In the course of this policy change, legal arguments from the California Court of Appeals expressed that the language of the law was not intended to deny lifesaving treatment to an individual.16 California’s Emergency Medicaid policy is comparable to that of other states, but because the courts considered SCT a “continuation of medically necessary inpatient hospital services … directly related to the emergency” for which the patient initially presented, they concluded that it could be covered under California Medicaid. Despite covering SCT for undocumented immigrants, California maintains lower costs for those patients compared to US citizens on Medicaid while providing evidence-based cancer care.17 This exemplifies sustainable and equitable healthcare policy for the rest of the nation.
A proposed change in policy could occur at either the federal or state level. One option would be to follow the example set by the State of Washington. Under Emergency Medicaid, Washington modified qualifying conditions to include “emergency room care, inpatient admission, or outpatient surgery; a cancer treatment plan; dialysis treatment; anti-rejection medication for an organ transplant” and long-term care services.18 Federal policy reform for undocumented immigrants would also improve access to care. The US Citizenship Act of 2021, introduced to the House of Representatives in February 2021, offers a path to citizenship for undocumented immigrants, ultimately allowing for undocumented individuals to be eligible for the same programs as citizens, though after a period of up to 8 years.19 More immediate revisions of qualifying conditions under state Emergency Medicaid programs, coupled with a path to citizenship, would make significant progress towards reducing structural health inequities. Such policy change would also have broader implications. Three quarters of undocumented immigrants in the United States originate from Mexico, Central America, and South America, and the incidence rate of AML for Latinx individuals is 3.6 per 100,000, a figure which can be extrapolated to an estimated 380 cases per year in the US undocumented population.20-22 In addition to benefiting patients with acute leukemias, the proposed policy change would also benefit numerous others who are frequently hospitalized for acute decompensations of chronic conditions, including congestive heart failure, liver disease, ESKD, and chronic lung conditions. Enabling follow-up care for these diseases under Emergency Medicaid would likewise be expected to reduce costs and improve both quality of care and patient-centered and clinical outcomes.
What Should I Tell My Patient?
Hospitalists frequently care for undocumented immigrants with acute leukemias because the hospital can only be reimbursed by Emergency Medicaid when a patient is admitted to the hospital. Patients may ask about what they can expect in the course of their illness and, while details may be left to the oncologist, hospitalists will be faced with responding to many of these questions. Clinicians at our institution hold honest conversations with patients like Juan. We are compelled to provide the care that hospital and state policies allow, and can only offer the best care available to them because of the restrictions of an insurance system to which they contribute financially, yet cannot benefit from, in their time of need. We can tell our undocumented immigrant patients that we find this unacceptable and are actively advocating to change this policy.
Conclusion
The State of Colorado and the nation must amend its healthcare policy to include comprehensive cancer care for everyone. Offering standard-of-care therapy to all patients is not only ethical, but also an economically sound policy benefiting patients, clinicians, and the workforce.
1. Skopec L, Holahan J, Elmendorf C. Changes in Health Insurance Coverage in 2013-2016: Medicaid Expansion States Lead the Way. Urban Institute. September 11, 2018. Accessed July 12, 2021. https://www.urban.org/research/publication/changes-health-insurance-coverage-2013-2016-medicaid-expansion-states-lead-way
2. Christensen Gee L, Gardner M, Hill ME, Wiehe M. Undocumented Immigrants’ State & Local Tax Contributions. Institute on Taxation & Economic Policy. Updated March 2017. Accessed July 12, 2021. https://www.immigrationresearch.org/system/files/immigration_taxes_2017.pdf
3. Emergency Medical Treatment and Labor Act (EMTALA), Public Law 42 U.S.C. 1395dd. 2010.
4. Social Security Act. Sec. 1903 [42 U.S.C. 1396b]. Accessed July 12, 2021. https://www.ssa.gov/OP_Home/ssact/title19/1903.htm.
5. Cervantes L, Mundo W, Powe NR. The status of provision of standard outpatient dialysis for US undocumented immigrants with ESKD. Clin J Am Soc Nephrol. 2019;14(8):1258-1260. https://doi.org/10.2215/CJN.03460319
6. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. https://doi.org/10.1182/blood-2015-07-604546
7. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. 2021.
8. Cervantes L, Richardson S, Raghavan R, et al. Clinicians’ perspectives on providing emergency-only hemodialysis to undocumented immigrants: a qualitative study. Ann Intern Med. 2018;169(2):78-86. https://doi.org/10.7326/M18-0400
9. Cervantes L, Tong A, Camacho C, Collings A, Powe NR. Patient-reported outcomes and experiences in the transition of undocumented patients from emergency to scheduled hemodialysis. Kidney Int. 2021;99(1):198-207. https://doi.org/10.1016/j.kint.2020.07.024
10. Stein E, Xie J, Duchesneau E, et al. Cost effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in the United States. Pharmacoeconomics. 2019;37(2):239-253. https://doi.org/10.1007/s40273-018-0732-4
11. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620-1628. https://doi.org/10.1016/j.bbmt.2012.04.001
12. Edwards R, Ortega F. The Economic Contribution of Unauthorized Workers: An Industry Analysis. National Bureau of Economic Research. November 2016. Accessed July 12, 2021. https://www.nber.org/system/files/working_papers/w22834/w22834.pdf
13. Nunnery SE, Fintel AE, Jackson WC, Chandler JC, Ugwueke MO, Martin MG. Treatment disparities faced by undocumented workers from low- and middle-income countries in the United States with hematologic malignancies. J Natl Compr Canc Netw. 2016;14(4):483-486. https://doi.org/10.6004/jnccn.2016.0053
14. World Cancer Initiative. Cancer Preparedness in Latin America: The Need to Build on Recent Progress. 2019. Accessed July 7, 2021. https://worldcancerinitiative.economist.com/cancer-preparedness-latin-america
15. Taylor P, Lopez MH, Passel JS, Motel S; Pew Research Center. Unauthorized Immigrants: Length of Residency, Patterns of Parenthood. December 1, 2011. Accessed July 12, 2021. https://www.pewresearch.org/hispanic/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
16. California Supreme Court, Records and Briefs: S019427, Dominguez vs. Superior Court of Alameda County. 1990.
17. Wallace SP, Torres J, Sadegh-Nobari T, Pourat N, Brown ER. Undocumented Immigrants and Health Care Reform. UCLA Center for Health Policy Research. August 31, 2012. Accessed July 7, 2021. https://healthpolicy.ucla.edu/publications/Documents/PDF/undocumentedreport-aug2013.pdf
18. Washington State Health Care Authority. Health care services and supports. Noncitizens. Accessed July 12, 2021. https://www.hca.wa.gov/health-care-services-supports/apple-health-medicaid-coverage/non-citizens
19. 117th Congress of the United States. H.R.1177, U.S. Citizenship Act of 2021.
20. National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program. Accessed July 7, 2021. https://seer.cancer.gov/
21. Migration Policy Institute. Profile of the unauthorized population: United States. Accessed July 12, 2021. https://www.migrationpolicy.org/data/unauthorized-immigrant-population/state/US. 2021.
22. Torres L. Latinx? Lat Stud. 2018;16:283-285. https://doi.org/10.1057/s41276-018-0142-y
1. Skopec L, Holahan J, Elmendorf C. Changes in Health Insurance Coverage in 2013-2016: Medicaid Expansion States Lead the Way. Urban Institute. September 11, 2018. Accessed July 12, 2021. https://www.urban.org/research/publication/changes-health-insurance-coverage-2013-2016-medicaid-expansion-states-lead-way
2. Christensen Gee L, Gardner M, Hill ME, Wiehe M. Undocumented Immigrants’ State & Local Tax Contributions. Institute on Taxation & Economic Policy. Updated March 2017. Accessed July 12, 2021. https://www.immigrationresearch.org/system/files/immigration_taxes_2017.pdf
3. Emergency Medical Treatment and Labor Act (EMTALA), Public Law 42 U.S.C. 1395dd. 2010.
4. Social Security Act. Sec. 1903 [42 U.S.C. 1396b]. Accessed July 12, 2021. https://www.ssa.gov/OP_Home/ssact/title19/1903.htm.
5. Cervantes L, Mundo W, Powe NR. The status of provision of standard outpatient dialysis for US undocumented immigrants with ESKD. Clin J Am Soc Nephrol. 2019;14(8):1258-1260. https://doi.org/10.2215/CJN.03460319
6. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. https://doi.org/10.1182/blood-2015-07-604546
7. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. 2021.
8. Cervantes L, Richardson S, Raghavan R, et al. Clinicians’ perspectives on providing emergency-only hemodialysis to undocumented immigrants: a qualitative study. Ann Intern Med. 2018;169(2):78-86. https://doi.org/10.7326/M18-0400
9. Cervantes L, Tong A, Camacho C, Collings A, Powe NR. Patient-reported outcomes and experiences in the transition of undocumented patients from emergency to scheduled hemodialysis. Kidney Int. 2021;99(1):198-207. https://doi.org/10.1016/j.kint.2020.07.024
10. Stein E, Xie J, Duchesneau E, et al. Cost effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in the United States. Pharmacoeconomics. 2019;37(2):239-253. https://doi.org/10.1007/s40273-018-0732-4
11. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620-1628. https://doi.org/10.1016/j.bbmt.2012.04.001
12. Edwards R, Ortega F. The Economic Contribution of Unauthorized Workers: An Industry Analysis. National Bureau of Economic Research. November 2016. Accessed July 12, 2021. https://www.nber.org/system/files/working_papers/w22834/w22834.pdf
13. Nunnery SE, Fintel AE, Jackson WC, Chandler JC, Ugwueke MO, Martin MG. Treatment disparities faced by undocumented workers from low- and middle-income countries in the United States with hematologic malignancies. J Natl Compr Canc Netw. 2016;14(4):483-486. https://doi.org/10.6004/jnccn.2016.0053
14. World Cancer Initiative. Cancer Preparedness in Latin America: The Need to Build on Recent Progress. 2019. Accessed July 7, 2021. https://worldcancerinitiative.economist.com/cancer-preparedness-latin-america
15. Taylor P, Lopez MH, Passel JS, Motel S; Pew Research Center. Unauthorized Immigrants: Length of Residency, Patterns of Parenthood. December 1, 2011. Accessed July 12, 2021. https://www.pewresearch.org/hispanic/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
16. California Supreme Court, Records and Briefs: S019427, Dominguez vs. Superior Court of Alameda County. 1990.
17. Wallace SP, Torres J, Sadegh-Nobari T, Pourat N, Brown ER. Undocumented Immigrants and Health Care Reform. UCLA Center for Health Policy Research. August 31, 2012. Accessed July 7, 2021. https://healthpolicy.ucla.edu/publications/Documents/PDF/undocumentedreport-aug2013.pdf
18. Washington State Health Care Authority. Health care services and supports. Noncitizens. Accessed July 12, 2021. https://www.hca.wa.gov/health-care-services-supports/apple-health-medicaid-coverage/non-citizens
19. 117th Congress of the United States. H.R.1177, U.S. Citizenship Act of 2021.
20. National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program. Accessed July 7, 2021. https://seer.cancer.gov/
21. Migration Policy Institute. Profile of the unauthorized population: United States. Accessed July 12, 2021. https://www.migrationpolicy.org/data/unauthorized-immigrant-population/state/US. 2021.
22. Torres L. Latinx? Lat Stud. 2018;16:283-285. https://doi.org/10.1057/s41276-018-0142-y
© 2021 Society of Hospital Medicine
Improving Healthcare Value: Managing Length of Stay and Improving the Hospital Medicine Value Proposition
Healthcare payment model reform has increased pressure on healthcare systems and hospitalists to improve efficiency and reduce the cost of care. These pressures on the healthcare system have been exacerbated by a global pandemic and an aging patient population straining hospital capacity and resources. Hospital capacity constraints may contribute to hospital crowding and can compromise patient outcomes.1 Increasing hospital capacity also contributes to an increase in hospitalist census. This increase in census is accompanied by proportional increases in hospitalist burnout, cost of care, and prolonged length of stay (LOS).2 Managing LOS reduces “waste” (or non–value-added inpatient days) and can improve outcomes and efficiency within the hospital system.
The benefits for LOS reduction when patients are managed by hospitalists compared with primary care practitioners are well described and are associated with decreases in average LOS and cost.3-5 The shorter LOS with hospitalist care is most pronounced in older patients with more complex disease processes, which has temporal importance. The Department of Health and Human Services expects the number of American adults aged >65 years to approach 72 million (20% of the US population) by 2030. Hospitalists are positioned to drive evidence-based care pathways and improve the quality of patient care in this growing patient population. We examine the reasons for managing LOS, summarize factors that contribute to an increased LOS (“waste”), and propose a list of evidence-based value drivers for LOS reduction (Table).2,6-17 Our experience utilizing this approach within Cleveland Clinic Florida following implementation of many of these evidence-based strategies to reduce non–value-added hospital days is also described in the Appendix Figure.
WHY MANAGE LOS?
Barriers to sustainable LOS-reduction strategies have evolved, in part, since the introduction of the Medicare Prospective Payment System, which moved hospital Medicare payments to a predetermined fixed rate for each diagnosis-related group. This led to financial pressures on healthcare systems to identify methods to reduce cost and, in turn, contributed to an increase in postacute facility utilization, with alternative payment models developing in parallel.18,19 These changes along with disaggregated payments between hospitals and postacute facilities have created a formidable challenge to LOS and cost-reduction plans.19
The usual “why” for reducing LOS includes improving constraints on hospital capacity, strains on resources, and deleterious outcomes. In our experience, an evidence-based approach to LOS management should focus on: (1) reduction in patient hospital days through decreased care variation; (2) stabilizing hospitalist workloads; (3) minimizing the fragmentation inherent to the hospitalist care delivery model; and (4) developing service lines to manage patients hospitalized in an observation status and for those patients undergoing procedures deemed medically complex. The literature is mixed on the impact of LOS reductions on other clinical end points, such as readmissions or mortality, with the preponderance indicating no deleterious impact.20-22 Managing LOS using an evidence-based approach that addresses the variability of individual patients is essential to the LOS strategies employed. These strategies should focus on process improvements to drive LOS reduction and utilize metrics under the individual hospitalist control to support their contribution to the hospitalist groups’ overall LOS.23
IMPROVING HOSPITALIST VALUE AROUND LOS MANAGEMENT
Intrinsic factors such as hospitalist staffing fragmentation, high rounding census, failing to prioritize patients ready to be discharged, variability in practice, number of consultants per patient, and hospitalist behaviors contribute to increased LOS.2,6,8 A first precept to management of LOS at the group level is to recognize all hospitalist services are not created equal, and “lumping” hospitalists into a single efficiency metric would not yield actionable information.
The literature is rife with examples of the significant variation in practice styles among hospitalists. A large study including more than 1000 hospitalists identified practice variation as the strongest predictor of variations in mean LOS.7 While Goodwin et al7 identified significant variation among hospitalists’ LOS and the discharge destination of patients, much of the variation could be attributable to the hospitals where they practice. These findings ostensibly highlight the importance of LOS strategies being developed collaboratively among hospitalist groups and the healthcare systems they serve. Similar variation exists among hospitalists on teaching services versus nonteaching services. Our experience parallels that of other studies with regard to teaching services that have found that hospitalists on teaching services often have additional responsibilities and are less able to gain the efficiency of nonresident hospitalists services.3 The impact of teaching services on hospitalist efficiencies is an important component when setting expectations at the hospitalist group level for providers on academic services.
Workload and staffing models for hospitalists have a significant impact on hospitalist efficiency and LOS management. As workload increased, Elliot and colleagues2 identified a proportional increase in LOS. For occupancies of 75% to 85%, LOS increased exponentially above a daily relative value unit of approximately 25 and a census value of approximately 15. The magnitude of this difference in LOS and cost across the range of hospitalist workloads was $262, with an average increase in LOS of 2 days for every unit increase in census. Higher workloads contributed to inferior discussion of treatment options with patients; delays in discharges; delays in placing discharge orders; and unnecessary testing, procedures, and consults.14 To mitigate inefficiency and adverse impacts of higher workloads, hospitalist groups should develop mechanisms to absorb surges in census and unanticipated changes to staffing maintaining the workload within a range appropriate to the patient population.
Decreasing fragmentation, when multiple hospitalists care for the patient during hospitalization, is a necessary component of any LOS-reduction strategy. Studies of pneumonia and heart failure have demonstrated that a 10% increase in hosptialist fragmentation is associated with significant increases in LOS.24 Schedules with hospitalists on 7-day rotating rounding blocks have the intuitive advantage of improving care continuity for patients compared with schedules with a shorter number of consecutive rounding days, resulting in fewer hospitalists caring for each patient and decreased “fragmentation.” Additional value drivers for LOS reduction strategies for hospitalists are listed in the Table.
The 2018 State of Hospital Medicine Report highlighted that, among patients discharged by hospitalist groups, 80.8% were inpatient and 19.2% were outpatient. With nearly one in five patients discharged in observation status, it behooves hospitalist programs to work to effectively manage these patients. Indeed, hospitalist-run observation units have been shown to decrease LOS significantly without an increase in return rates to the emergency department or hospital compared with patients managed prior to the introduction of a dedicated observation unit.9
Although an in-depth discussion is beyond the scope of the present article, it is worth noting the value of hospitalist comanagement (HCoM) strategies. The impact of HCoM teams is demonstrated by reductions in LOS and cost of care resulting from decreases in medical complications, number of consultants per patient, and a decrease in 30-day readmsissions.12 The Society of Hospital Medicine Perioperative Care Work Group has outlined a collaborative framework for hospitalists and healthcare systems to draw from.15
THE CLEVELAND CLINIC INDIAN RIVER HOSPITAL EXPERIENCE
Within the Cleveland Clinic Indian River Hospital (CCIRH) medicine department, many of the aforementioned strategies and tactics were standardized among hospitalist providers. Hospitalists at CCIRH are scheduled on 7-day rotating blocks to reduce fragmentation. In 2019, we targeted a range of 15 to 18 patient contacts per rounding hospitalist per day and utilized a back-up call system to stabilize the hospitalist census. The hospitalist service lines are enhanced through HCoM services with patients cohorted on dedicated HCoM teams. The follow-up to discharge ratio is used to provide feedback at the provider level as both a management and assessment tool.23 The rounding and admitting teams are dedicated to their responsibility (with the occasional exception necessitating the rounding team assist with admissions when the volumes are high). Direct admissions and transfers from outside hospitals are managed by a dedicated hospital medicine “quarterback” to minimize disruption of the admitting and rounding teams. Barriers to discharge are identified at the time of admission by care management and aggressively managed. Prolonged LOS reports are generated daily and disseminated to care managers and physician leadership. In January 2019, the average LOS for inpatients at CCIRH was 4.4 days. In December 2019, the average LOS for the calendar year to-date at CCIRH was 3.9 days (Appendix Figure).
The value proposition for managing LOS should be viewed in the context of the total cost of care over an extended period of time and not viewed in isolation. Readmission rates serve as a counterbalance to LOS-reduction strategies and contribute to higher costs of care when increased. The 30-day readmission rate for this cohort over this same time period was down slightly compared with the previous year to 12.1%. In addition, observation patients at CCIRH are managed in a closed, geographically cohorted unit, staffed by dedicated advanced-practice providers and physicians dedicated to observation medicine. Over this same time period, more than 5500 patients were managed in the observation unit. These patients had an average LOS of 19.2 hours, with approximately four out of every five patients being discharged to home from an observation status.
The impact of COVID-19 and higher hospital volumes are best visualized in the Appendix Figure. Increases in LOS were observed during periods of COVID-19–related “surges” in hospital volume. These reversals in LOS trends during periods of high occupancy echo earlier findings by Elliot et al2 showing that external factors that are not directly under the control of the hospitalist drive LOS and must be considered when developing LOS reduction strategies.
CONCLUSION
The shift toward value-based payment models provides a strong tailwind for healthcare systems to manage LOS. Hospitalists are well positioned to drive LOS-reduction strategies for the healthcare systems they serve and provide value by driving both quality and efficiency. A complete realization of the value proposition of hospitalist programs in driving LOS-reduction initiatives requires the healthcare systems they serve to provide these teams with the appropriate resources and tools.
1. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guise J-M. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
2. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. https://doi.org/10.1001/jamainternmed.2014.300
3. Rachoin JS, Skaf J, Cerceo E, et al. The impact of hospitalists on length of stay and costs: systematic review and meta-analysis. Am J Manag Care. 2012;18(1):e23-30.
4. Kuo YF, Goodwin JS. Effect of hospitalists on length of stay in the medicare population: variation according to hospital and patient characteristics. J Am Geriatr Soc. 2010;58(9):1649-1657. https://doi.org/10.1111/j.1532-5415.2010.03007.x
5. Lindenauer PK, Rothberg MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589-2600. https://doi.org/10.1056/NEJMsa067735
6. Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338. https://doi.org/10.1002/jhm.675
7. Goodwin JS, Lin Y-L, Singh S, Kuo Y-F. Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370-376. https://doi.org/10.1007/s11606-012-2255-6
8. Johnson T, McNutt R, Odwazny R, Patel D, Baker S. Discrepancy between admission and discharge diagnoses as a predictor of hospital length of stay. J Hosp Med. 2009;4(4):234-239. https://doi.org/10.1002/jhm.453
9. Aplin KS, Coutinho McAllister S, Kupersmith E, Rachoin JS. Caring for patients in a hospitalist-run clinical decision unit is associated with decreased length of stay without increasing revisit rates. J Hosp Med. 2014;9(6):391-395. https://doi.org/10.1002/jhm.2188
10. Selker HP, Beshansky JR, Pauker SG, Kassirer JP. The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days. Med Care. 1989;27(2):112-129. https://doi.org/10.1097/00005650-198902000-00003
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. https://doi.org/10.1111/j.1525-1497.2005.40269.x
12. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
13. Chen LM, Freitag MH, Franco M, Sullivan CD, Dickson C, Brancati FL. Natural history of late discharges from a general medical ward. J Hosp Med. 2009;4(4):226-233. https://doi.org/10.1002/jhm.413
14. Zoucha J, Hull M, Keniston A, et al. Barriers to early hospital discharge: a cross-sectional study at five academic hospitals. J Hosp Med. 2018;13(12):816-822. https://doi.org/10.12788/jhm.3074
15. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. https://doi.org/10.12788/jhm.2717
16. Fail RE, Meier DE. Improving quality of care for seriously ill patients: opportunities for hospitalists. J Hosp Med. 2018;13(3):194-197. https://doi.org/10.12788/jhm.2896
17. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: a quality-improvement project. J Hosp Med. 2016;11(5):341-347. https://doi.org/10.1002/jhm.2546
18. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. https://doi.org/10.1016/0168-8510(88)90029-2
19. Rothberg M, Lee N. Reducing readmissions or length of stay-Which is more important? J Hosp Med. 2017;12(8):685-686. https://doi.org/10.12788/jhm.2790
20. Kaboli PJ, Go JT, Hockenberry J, et al. Associations between reduced hospital length of stay and 30-day readmission rate and mortality: 14-year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837-845. https://doi.org/10.7326/0003-4819-157-12-201212180-00003
21. Rinne ST, Graves MC, Bastian LA, et al. Association between length of stay and readmission for COPD. Am J Manag Care. 2017;23(8):e253-e258.
22. Sud M, Yu B, Wijeysundera HC, et al. Associations between short or long length of stay and 30-day readmission and mortality in hospitalized patients with heart failure. JACC Heart Fail. 2017;5(8):578-588. https://doi.org/10.1016/j.jchf.2017.03.012
23. Rothman RD, Whinney CM, Pappas MA, Zoller DM, Rosencrance JG, Peter DJ. The relationship between the follow-up to discharge ratio and length of stay. Am J Manag Care. 2020;26(9):396-399. https://doi.org/10.37765/ajmc.2020.88490
24. Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338. https://doi.org/10.1002/jhm.675
Healthcare payment model reform has increased pressure on healthcare systems and hospitalists to improve efficiency and reduce the cost of care. These pressures on the healthcare system have been exacerbated by a global pandemic and an aging patient population straining hospital capacity and resources. Hospital capacity constraints may contribute to hospital crowding and can compromise patient outcomes.1 Increasing hospital capacity also contributes to an increase in hospitalist census. This increase in census is accompanied by proportional increases in hospitalist burnout, cost of care, and prolonged length of stay (LOS).2 Managing LOS reduces “waste” (or non–value-added inpatient days) and can improve outcomes and efficiency within the hospital system.
The benefits for LOS reduction when patients are managed by hospitalists compared with primary care practitioners are well described and are associated with decreases in average LOS and cost.3-5 The shorter LOS with hospitalist care is most pronounced in older patients with more complex disease processes, which has temporal importance. The Department of Health and Human Services expects the number of American adults aged >65 years to approach 72 million (20% of the US population) by 2030. Hospitalists are positioned to drive evidence-based care pathways and improve the quality of patient care in this growing patient population. We examine the reasons for managing LOS, summarize factors that contribute to an increased LOS (“waste”), and propose a list of evidence-based value drivers for LOS reduction (Table).2,6-17 Our experience utilizing this approach within Cleveland Clinic Florida following implementation of many of these evidence-based strategies to reduce non–value-added hospital days is also described in the Appendix Figure.
WHY MANAGE LOS?
Barriers to sustainable LOS-reduction strategies have evolved, in part, since the introduction of the Medicare Prospective Payment System, which moved hospital Medicare payments to a predetermined fixed rate for each diagnosis-related group. This led to financial pressures on healthcare systems to identify methods to reduce cost and, in turn, contributed to an increase in postacute facility utilization, with alternative payment models developing in parallel.18,19 These changes along with disaggregated payments between hospitals and postacute facilities have created a formidable challenge to LOS and cost-reduction plans.19
The usual “why” for reducing LOS includes improving constraints on hospital capacity, strains on resources, and deleterious outcomes. In our experience, an evidence-based approach to LOS management should focus on: (1) reduction in patient hospital days through decreased care variation; (2) stabilizing hospitalist workloads; (3) minimizing the fragmentation inherent to the hospitalist care delivery model; and (4) developing service lines to manage patients hospitalized in an observation status and for those patients undergoing procedures deemed medically complex. The literature is mixed on the impact of LOS reductions on other clinical end points, such as readmissions or mortality, with the preponderance indicating no deleterious impact.20-22 Managing LOS using an evidence-based approach that addresses the variability of individual patients is essential to the LOS strategies employed. These strategies should focus on process improvements to drive LOS reduction and utilize metrics under the individual hospitalist control to support their contribution to the hospitalist groups’ overall LOS.23
IMPROVING HOSPITALIST VALUE AROUND LOS MANAGEMENT
Intrinsic factors such as hospitalist staffing fragmentation, high rounding census, failing to prioritize patients ready to be discharged, variability in practice, number of consultants per patient, and hospitalist behaviors contribute to increased LOS.2,6,8 A first precept to management of LOS at the group level is to recognize all hospitalist services are not created equal, and “lumping” hospitalists into a single efficiency metric would not yield actionable information.
The literature is rife with examples of the significant variation in practice styles among hospitalists. A large study including more than 1000 hospitalists identified practice variation as the strongest predictor of variations in mean LOS.7 While Goodwin et al7 identified significant variation among hospitalists’ LOS and the discharge destination of patients, much of the variation could be attributable to the hospitals where they practice. These findings ostensibly highlight the importance of LOS strategies being developed collaboratively among hospitalist groups and the healthcare systems they serve. Similar variation exists among hospitalists on teaching services versus nonteaching services. Our experience parallels that of other studies with regard to teaching services that have found that hospitalists on teaching services often have additional responsibilities and are less able to gain the efficiency of nonresident hospitalists services.3 The impact of teaching services on hospitalist efficiencies is an important component when setting expectations at the hospitalist group level for providers on academic services.
Workload and staffing models for hospitalists have a significant impact on hospitalist efficiency and LOS management. As workload increased, Elliot and colleagues2 identified a proportional increase in LOS. For occupancies of 75% to 85%, LOS increased exponentially above a daily relative value unit of approximately 25 and a census value of approximately 15. The magnitude of this difference in LOS and cost across the range of hospitalist workloads was $262, with an average increase in LOS of 2 days for every unit increase in census. Higher workloads contributed to inferior discussion of treatment options with patients; delays in discharges; delays in placing discharge orders; and unnecessary testing, procedures, and consults.14 To mitigate inefficiency and adverse impacts of higher workloads, hospitalist groups should develop mechanisms to absorb surges in census and unanticipated changes to staffing maintaining the workload within a range appropriate to the patient population.
Decreasing fragmentation, when multiple hospitalists care for the patient during hospitalization, is a necessary component of any LOS-reduction strategy. Studies of pneumonia and heart failure have demonstrated that a 10% increase in hosptialist fragmentation is associated with significant increases in LOS.24 Schedules with hospitalists on 7-day rotating rounding blocks have the intuitive advantage of improving care continuity for patients compared with schedules with a shorter number of consecutive rounding days, resulting in fewer hospitalists caring for each patient and decreased “fragmentation.” Additional value drivers for LOS reduction strategies for hospitalists are listed in the Table.
The 2018 State of Hospital Medicine Report highlighted that, among patients discharged by hospitalist groups, 80.8% were inpatient and 19.2% were outpatient. With nearly one in five patients discharged in observation status, it behooves hospitalist programs to work to effectively manage these patients. Indeed, hospitalist-run observation units have been shown to decrease LOS significantly without an increase in return rates to the emergency department or hospital compared with patients managed prior to the introduction of a dedicated observation unit.9
Although an in-depth discussion is beyond the scope of the present article, it is worth noting the value of hospitalist comanagement (HCoM) strategies. The impact of HCoM teams is demonstrated by reductions in LOS and cost of care resulting from decreases in medical complications, number of consultants per patient, and a decrease in 30-day readmsissions.12 The Society of Hospital Medicine Perioperative Care Work Group has outlined a collaborative framework for hospitalists and healthcare systems to draw from.15
THE CLEVELAND CLINIC INDIAN RIVER HOSPITAL EXPERIENCE
Within the Cleveland Clinic Indian River Hospital (CCIRH) medicine department, many of the aforementioned strategies and tactics were standardized among hospitalist providers. Hospitalists at CCIRH are scheduled on 7-day rotating blocks to reduce fragmentation. In 2019, we targeted a range of 15 to 18 patient contacts per rounding hospitalist per day and utilized a back-up call system to stabilize the hospitalist census. The hospitalist service lines are enhanced through HCoM services with patients cohorted on dedicated HCoM teams. The follow-up to discharge ratio is used to provide feedback at the provider level as both a management and assessment tool.23 The rounding and admitting teams are dedicated to their responsibility (with the occasional exception necessitating the rounding team assist with admissions when the volumes are high). Direct admissions and transfers from outside hospitals are managed by a dedicated hospital medicine “quarterback” to minimize disruption of the admitting and rounding teams. Barriers to discharge are identified at the time of admission by care management and aggressively managed. Prolonged LOS reports are generated daily and disseminated to care managers and physician leadership. In January 2019, the average LOS for inpatients at CCIRH was 4.4 days. In December 2019, the average LOS for the calendar year to-date at CCIRH was 3.9 days (Appendix Figure).
The value proposition for managing LOS should be viewed in the context of the total cost of care over an extended period of time and not viewed in isolation. Readmission rates serve as a counterbalance to LOS-reduction strategies and contribute to higher costs of care when increased. The 30-day readmission rate for this cohort over this same time period was down slightly compared with the previous year to 12.1%. In addition, observation patients at CCIRH are managed in a closed, geographically cohorted unit, staffed by dedicated advanced-practice providers and physicians dedicated to observation medicine. Over this same time period, more than 5500 patients were managed in the observation unit. These patients had an average LOS of 19.2 hours, with approximately four out of every five patients being discharged to home from an observation status.
The impact of COVID-19 and higher hospital volumes are best visualized in the Appendix Figure. Increases in LOS were observed during periods of COVID-19–related “surges” in hospital volume. These reversals in LOS trends during periods of high occupancy echo earlier findings by Elliot et al2 showing that external factors that are not directly under the control of the hospitalist drive LOS and must be considered when developing LOS reduction strategies.
CONCLUSION
The shift toward value-based payment models provides a strong tailwind for healthcare systems to manage LOS. Hospitalists are well positioned to drive LOS-reduction strategies for the healthcare systems they serve and provide value by driving both quality and efficiency. A complete realization of the value proposition of hospitalist programs in driving LOS-reduction initiatives requires the healthcare systems they serve to provide these teams with the appropriate resources and tools.
Healthcare payment model reform has increased pressure on healthcare systems and hospitalists to improve efficiency and reduce the cost of care. These pressures on the healthcare system have been exacerbated by a global pandemic and an aging patient population straining hospital capacity and resources. Hospital capacity constraints may contribute to hospital crowding and can compromise patient outcomes.1 Increasing hospital capacity also contributes to an increase in hospitalist census. This increase in census is accompanied by proportional increases in hospitalist burnout, cost of care, and prolonged length of stay (LOS).2 Managing LOS reduces “waste” (or non–value-added inpatient days) and can improve outcomes and efficiency within the hospital system.
The benefits for LOS reduction when patients are managed by hospitalists compared with primary care practitioners are well described and are associated with decreases in average LOS and cost.3-5 The shorter LOS with hospitalist care is most pronounced in older patients with more complex disease processes, which has temporal importance. The Department of Health and Human Services expects the number of American adults aged >65 years to approach 72 million (20% of the US population) by 2030. Hospitalists are positioned to drive evidence-based care pathways and improve the quality of patient care in this growing patient population. We examine the reasons for managing LOS, summarize factors that contribute to an increased LOS (“waste”), and propose a list of evidence-based value drivers for LOS reduction (Table).2,6-17 Our experience utilizing this approach within Cleveland Clinic Florida following implementation of many of these evidence-based strategies to reduce non–value-added hospital days is also described in the Appendix Figure.
WHY MANAGE LOS?
Barriers to sustainable LOS-reduction strategies have evolved, in part, since the introduction of the Medicare Prospective Payment System, which moved hospital Medicare payments to a predetermined fixed rate for each diagnosis-related group. This led to financial pressures on healthcare systems to identify methods to reduce cost and, in turn, contributed to an increase in postacute facility utilization, with alternative payment models developing in parallel.18,19 These changes along with disaggregated payments between hospitals and postacute facilities have created a formidable challenge to LOS and cost-reduction plans.19
The usual “why” for reducing LOS includes improving constraints on hospital capacity, strains on resources, and deleterious outcomes. In our experience, an evidence-based approach to LOS management should focus on: (1) reduction in patient hospital days through decreased care variation; (2) stabilizing hospitalist workloads; (3) minimizing the fragmentation inherent to the hospitalist care delivery model; and (4) developing service lines to manage patients hospitalized in an observation status and for those patients undergoing procedures deemed medically complex. The literature is mixed on the impact of LOS reductions on other clinical end points, such as readmissions or mortality, with the preponderance indicating no deleterious impact.20-22 Managing LOS using an evidence-based approach that addresses the variability of individual patients is essential to the LOS strategies employed. These strategies should focus on process improvements to drive LOS reduction and utilize metrics under the individual hospitalist control to support their contribution to the hospitalist groups’ overall LOS.23
IMPROVING HOSPITALIST VALUE AROUND LOS MANAGEMENT
Intrinsic factors such as hospitalist staffing fragmentation, high rounding census, failing to prioritize patients ready to be discharged, variability in practice, number of consultants per patient, and hospitalist behaviors contribute to increased LOS.2,6,8 A first precept to management of LOS at the group level is to recognize all hospitalist services are not created equal, and “lumping” hospitalists into a single efficiency metric would not yield actionable information.
The literature is rife with examples of the significant variation in practice styles among hospitalists. A large study including more than 1000 hospitalists identified practice variation as the strongest predictor of variations in mean LOS.7 While Goodwin et al7 identified significant variation among hospitalists’ LOS and the discharge destination of patients, much of the variation could be attributable to the hospitals where they practice. These findings ostensibly highlight the importance of LOS strategies being developed collaboratively among hospitalist groups and the healthcare systems they serve. Similar variation exists among hospitalists on teaching services versus nonteaching services. Our experience parallels that of other studies with regard to teaching services that have found that hospitalists on teaching services often have additional responsibilities and are less able to gain the efficiency of nonresident hospitalists services.3 The impact of teaching services on hospitalist efficiencies is an important component when setting expectations at the hospitalist group level for providers on academic services.
Workload and staffing models for hospitalists have a significant impact on hospitalist efficiency and LOS management. As workload increased, Elliot and colleagues2 identified a proportional increase in LOS. For occupancies of 75% to 85%, LOS increased exponentially above a daily relative value unit of approximately 25 and a census value of approximately 15. The magnitude of this difference in LOS and cost across the range of hospitalist workloads was $262, with an average increase in LOS of 2 days for every unit increase in census. Higher workloads contributed to inferior discussion of treatment options with patients; delays in discharges; delays in placing discharge orders; and unnecessary testing, procedures, and consults.14 To mitigate inefficiency and adverse impacts of higher workloads, hospitalist groups should develop mechanisms to absorb surges in census and unanticipated changes to staffing maintaining the workload within a range appropriate to the patient population.
Decreasing fragmentation, when multiple hospitalists care for the patient during hospitalization, is a necessary component of any LOS-reduction strategy. Studies of pneumonia and heart failure have demonstrated that a 10% increase in hosptialist fragmentation is associated with significant increases in LOS.24 Schedules with hospitalists on 7-day rotating rounding blocks have the intuitive advantage of improving care continuity for patients compared with schedules with a shorter number of consecutive rounding days, resulting in fewer hospitalists caring for each patient and decreased “fragmentation.” Additional value drivers for LOS reduction strategies for hospitalists are listed in the Table.
The 2018 State of Hospital Medicine Report highlighted that, among patients discharged by hospitalist groups, 80.8% were inpatient and 19.2% were outpatient. With nearly one in five patients discharged in observation status, it behooves hospitalist programs to work to effectively manage these patients. Indeed, hospitalist-run observation units have been shown to decrease LOS significantly without an increase in return rates to the emergency department or hospital compared with patients managed prior to the introduction of a dedicated observation unit.9
Although an in-depth discussion is beyond the scope of the present article, it is worth noting the value of hospitalist comanagement (HCoM) strategies. The impact of HCoM teams is demonstrated by reductions in LOS and cost of care resulting from decreases in medical complications, number of consultants per patient, and a decrease in 30-day readmsissions.12 The Society of Hospital Medicine Perioperative Care Work Group has outlined a collaborative framework for hospitalists and healthcare systems to draw from.15
THE CLEVELAND CLINIC INDIAN RIVER HOSPITAL EXPERIENCE
Within the Cleveland Clinic Indian River Hospital (CCIRH) medicine department, many of the aforementioned strategies and tactics were standardized among hospitalist providers. Hospitalists at CCIRH are scheduled on 7-day rotating blocks to reduce fragmentation. In 2019, we targeted a range of 15 to 18 patient contacts per rounding hospitalist per day and utilized a back-up call system to stabilize the hospitalist census. The hospitalist service lines are enhanced through HCoM services with patients cohorted on dedicated HCoM teams. The follow-up to discharge ratio is used to provide feedback at the provider level as both a management and assessment tool.23 The rounding and admitting teams are dedicated to their responsibility (with the occasional exception necessitating the rounding team assist with admissions when the volumes are high). Direct admissions and transfers from outside hospitals are managed by a dedicated hospital medicine “quarterback” to minimize disruption of the admitting and rounding teams. Barriers to discharge are identified at the time of admission by care management and aggressively managed. Prolonged LOS reports are generated daily and disseminated to care managers and physician leadership. In January 2019, the average LOS for inpatients at CCIRH was 4.4 days. In December 2019, the average LOS for the calendar year to-date at CCIRH was 3.9 days (Appendix Figure).
The value proposition for managing LOS should be viewed in the context of the total cost of care over an extended period of time and not viewed in isolation. Readmission rates serve as a counterbalance to LOS-reduction strategies and contribute to higher costs of care when increased. The 30-day readmission rate for this cohort over this same time period was down slightly compared with the previous year to 12.1%. In addition, observation patients at CCIRH are managed in a closed, geographically cohorted unit, staffed by dedicated advanced-practice providers and physicians dedicated to observation medicine. Over this same time period, more than 5500 patients were managed in the observation unit. These patients had an average LOS of 19.2 hours, with approximately four out of every five patients being discharged to home from an observation status.
The impact of COVID-19 and higher hospital volumes are best visualized in the Appendix Figure. Increases in LOS were observed during periods of COVID-19–related “surges” in hospital volume. These reversals in LOS trends during periods of high occupancy echo earlier findings by Elliot et al2 showing that external factors that are not directly under the control of the hospitalist drive LOS and must be considered when developing LOS reduction strategies.
CONCLUSION
The shift toward value-based payment models provides a strong tailwind for healthcare systems to manage LOS. Hospitalists are well positioned to drive LOS-reduction strategies for the healthcare systems they serve and provide value by driving both quality and efficiency. A complete realization of the value proposition of hospitalist programs in driving LOS-reduction initiatives requires the healthcare systems they serve to provide these teams with the appropriate resources and tools.
1. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guise J-M. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
2. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. https://doi.org/10.1001/jamainternmed.2014.300
3. Rachoin JS, Skaf J, Cerceo E, et al. The impact of hospitalists on length of stay and costs: systematic review and meta-analysis. Am J Manag Care. 2012;18(1):e23-30.
4. Kuo YF, Goodwin JS. Effect of hospitalists on length of stay in the medicare population: variation according to hospital and patient characteristics. J Am Geriatr Soc. 2010;58(9):1649-1657. https://doi.org/10.1111/j.1532-5415.2010.03007.x
5. Lindenauer PK, Rothberg MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589-2600. https://doi.org/10.1056/NEJMsa067735
6. Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338. https://doi.org/10.1002/jhm.675
7. Goodwin JS, Lin Y-L, Singh S, Kuo Y-F. Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370-376. https://doi.org/10.1007/s11606-012-2255-6
8. Johnson T, McNutt R, Odwazny R, Patel D, Baker S. Discrepancy between admission and discharge diagnoses as a predictor of hospital length of stay. J Hosp Med. 2009;4(4):234-239. https://doi.org/10.1002/jhm.453
9. Aplin KS, Coutinho McAllister S, Kupersmith E, Rachoin JS. Caring for patients in a hospitalist-run clinical decision unit is associated with decreased length of stay without increasing revisit rates. J Hosp Med. 2014;9(6):391-395. https://doi.org/10.1002/jhm.2188
10. Selker HP, Beshansky JR, Pauker SG, Kassirer JP. The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days. Med Care. 1989;27(2):112-129. https://doi.org/10.1097/00005650-198902000-00003
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. https://doi.org/10.1111/j.1525-1497.2005.40269.x
12. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
13. Chen LM, Freitag MH, Franco M, Sullivan CD, Dickson C, Brancati FL. Natural history of late discharges from a general medical ward. J Hosp Med. 2009;4(4):226-233. https://doi.org/10.1002/jhm.413
14. Zoucha J, Hull M, Keniston A, et al. Barriers to early hospital discharge: a cross-sectional study at five academic hospitals. J Hosp Med. 2018;13(12):816-822. https://doi.org/10.12788/jhm.3074
15. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. https://doi.org/10.12788/jhm.2717
16. Fail RE, Meier DE. Improving quality of care for seriously ill patients: opportunities for hospitalists. J Hosp Med. 2018;13(3):194-197. https://doi.org/10.12788/jhm.2896
17. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: a quality-improvement project. J Hosp Med. 2016;11(5):341-347. https://doi.org/10.1002/jhm.2546
18. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. https://doi.org/10.1016/0168-8510(88)90029-2
19. Rothberg M, Lee N. Reducing readmissions or length of stay-Which is more important? J Hosp Med. 2017;12(8):685-686. https://doi.org/10.12788/jhm.2790
20. Kaboli PJ, Go JT, Hockenberry J, et al. Associations between reduced hospital length of stay and 30-day readmission rate and mortality: 14-year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837-845. https://doi.org/10.7326/0003-4819-157-12-201212180-00003
21. Rinne ST, Graves MC, Bastian LA, et al. Association between length of stay and readmission for COPD. Am J Manag Care. 2017;23(8):e253-e258.
22. Sud M, Yu B, Wijeysundera HC, et al. Associations between short or long length of stay and 30-day readmission and mortality in hospitalized patients with heart failure. JACC Heart Fail. 2017;5(8):578-588. https://doi.org/10.1016/j.jchf.2017.03.012
23. Rothman RD, Whinney CM, Pappas MA, Zoller DM, Rosencrance JG, Peter DJ. The relationship between the follow-up to discharge ratio and length of stay. Am J Manag Care. 2020;26(9):396-399. https://doi.org/10.37765/ajmc.2020.88490
24. Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338. https://doi.org/10.1002/jhm.675
1. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guise J-M. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
2. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. https://doi.org/10.1001/jamainternmed.2014.300
3. Rachoin JS, Skaf J, Cerceo E, et al. The impact of hospitalists on length of stay and costs: systematic review and meta-analysis. Am J Manag Care. 2012;18(1):e23-30.
4. Kuo YF, Goodwin JS. Effect of hospitalists on length of stay in the medicare population: variation according to hospital and patient characteristics. J Am Geriatr Soc. 2010;58(9):1649-1657. https://doi.org/10.1111/j.1532-5415.2010.03007.x
5. Lindenauer PK, Rothberg MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589-2600. https://doi.org/10.1056/NEJMsa067735
6. Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338. https://doi.org/10.1002/jhm.675
7. Goodwin JS, Lin Y-L, Singh S, Kuo Y-F. Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370-376. https://doi.org/10.1007/s11606-012-2255-6
8. Johnson T, McNutt R, Odwazny R, Patel D, Baker S. Discrepancy between admission and discharge diagnoses as a predictor of hospital length of stay. J Hosp Med. 2009;4(4):234-239. https://doi.org/10.1002/jhm.453
9. Aplin KS, Coutinho McAllister S, Kupersmith E, Rachoin JS. Caring for patients in a hospitalist-run clinical decision unit is associated with decreased length of stay without increasing revisit rates. J Hosp Med. 2014;9(6):391-395. https://doi.org/10.1002/jhm.2188
10. Selker HP, Beshansky JR, Pauker SG, Kassirer JP. The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days. Med Care. 1989;27(2):112-129. https://doi.org/10.1097/00005650-198902000-00003
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. https://doi.org/10.1111/j.1525-1497.2005.40269.x
12. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
13. Chen LM, Freitag MH, Franco M, Sullivan CD, Dickson C, Brancati FL. Natural history of late discharges from a general medical ward. J Hosp Med. 2009;4(4):226-233. https://doi.org/10.1002/jhm.413
14. Zoucha J, Hull M, Keniston A, et al. Barriers to early hospital discharge: a cross-sectional study at five academic hospitals. J Hosp Med. 2018;13(12):816-822. https://doi.org/10.12788/jhm.3074
15. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. https://doi.org/10.12788/jhm.2717
16. Fail RE, Meier DE. Improving quality of care for seriously ill patients: opportunities for hospitalists. J Hosp Med. 2018;13(3):194-197. https://doi.org/10.12788/jhm.2896
17. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: a quality-improvement project. J Hosp Med. 2016;11(5):341-347. https://doi.org/10.1002/jhm.2546
18. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. https://doi.org/10.1016/0168-8510(88)90029-2
19. Rothberg M, Lee N. Reducing readmissions or length of stay-Which is more important? J Hosp Med. 2017;12(8):685-686. https://doi.org/10.12788/jhm.2790
20. Kaboli PJ, Go JT, Hockenberry J, et al. Associations between reduced hospital length of stay and 30-day readmission rate and mortality: 14-year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837-845. https://doi.org/10.7326/0003-4819-157-12-201212180-00003
21. Rinne ST, Graves MC, Bastian LA, et al. Association between length of stay and readmission for COPD. Am J Manag Care. 2017;23(8):e253-e258.
22. Sud M, Yu B, Wijeysundera HC, et al. Associations between short or long length of stay and 30-day readmission and mortality in hospitalized patients with heart failure. JACC Heart Fail. 2017;5(8):578-588. https://doi.org/10.1016/j.jchf.2017.03.012
23. Rothman RD, Whinney CM, Pappas MA, Zoller DM, Rosencrance JG, Peter DJ. The relationship between the follow-up to discharge ratio and length of stay. Am J Manag Care. 2020;26(9):396-399. https://doi.org/10.37765/ajmc.2020.88490
24. Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338. https://doi.org/10.1002/jhm.675
© 2021 Society of Hospital Medicine
Improving Healthcare Value: Effectiveness of a Program to Reduce Laboratory Testing for Non-Critically-Ill Patients With COVID-19
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
© 2021 Society of Hospital Medicine
Policy in Clinical Practice: Choosing Post-Acute Care in the New Decade
CLINICAL SCENARIO
A 70-year-old woman with Medicare insurance and a history of mild dementia and chronic bronchiectasis was hospitalized for acute respiratory failure due to influenza. She was treated in the intensive care unit (ICU) for 2 days, received mechanical ventilation, and was subsequently extubated and weaned to high-flow nasal cannula (HFNC) at 8 liters of oxygen per minute and noninvasive ventilation at bedtime. She had otherwise stable cognition and required no other medical or nursing therapies. For recovery, she was referred to a
BACKGROUND AND HISTORY
In 2018, 44% of hospitalized patients with fee-for-service Medicare (herein referred to as Medicare) were discharged to PAC, accounting for nearly $60 billion in annual Medicare spending.1 PAC includes four levels of care—home health agencies (HHAs), SNFs, inpatient rehabilitation facilities (IRFs), and LTACHs—which vary in intensity and complexity of the medical, skilled nursing, and rehabilitative services they provide; use separate reimbursement systems; employ different quality metrics; and have different regulatory requirements (Table 1). Because hospitalists care for the majority of these patients and commonly serve in leadership roles for transitions of care and PAC use, PAC policy is important, as it has direct implications on discharge patterns and the quality and nature of patient care after discharge.
HHAs, the most commonly used PAC setting, provide skilled nursing or therapy to homebound beneficiaries.1 HHAs were historically reimbursed a standardized 60-day episode payment based on casemix, which was highly dependent on the number of therapy visits provided, with extremely little contribution from nontherapy services, such as skilled nursing and home health aide visits.2
SNFs, which comprise nearly half of PAC spending, provide short-term skilled nursing and rehabilitative services following hospitalization. SNFs are reimbursed on a per diem basis by Medicare, with reimbursement historically determined by the intensity of the dominant service furnished to the patient—either nursing, ancillary care (which includes medications, supplies/equipment, and diagnostic testing), or rehabilitation.3 Due to strong financial incentives, payment for more than 90% of SNF days was based solely on rehabilitation therapy furnished, with 33% of SNF patients receiving ultra-high rehabilitation (>720 minutes/week),3
IRFs provide intensive rehabilitation to patients who are able to participate in at least 3 hours of multidisciplinary therapy per day.1 IRF admissions are paid a bundled rate by Medicare based on the patient’s primary reason for rehabilitation, their age, and their level of functioning and cognition.
LTACHs, the most intensive and expensive PAC setting, care for patients with a range of complex hospital-level care needs, including intravenous (IV) infusions, complex wound care, and respiratory support. Since 2002, the only requirements for LTACHs have been to meet Medicare’s requirements for hospital accreditation and maintain an average length of stay of 25 days for their population.5 LTACH stays are paid a bundled rate by Medicare based on diagnosis.
POLICIES IN CLINICAL PRACTICE
Due to considerable variation in PAC use, with concerns that similar patients can be treated in different PAC settings,6,7 the
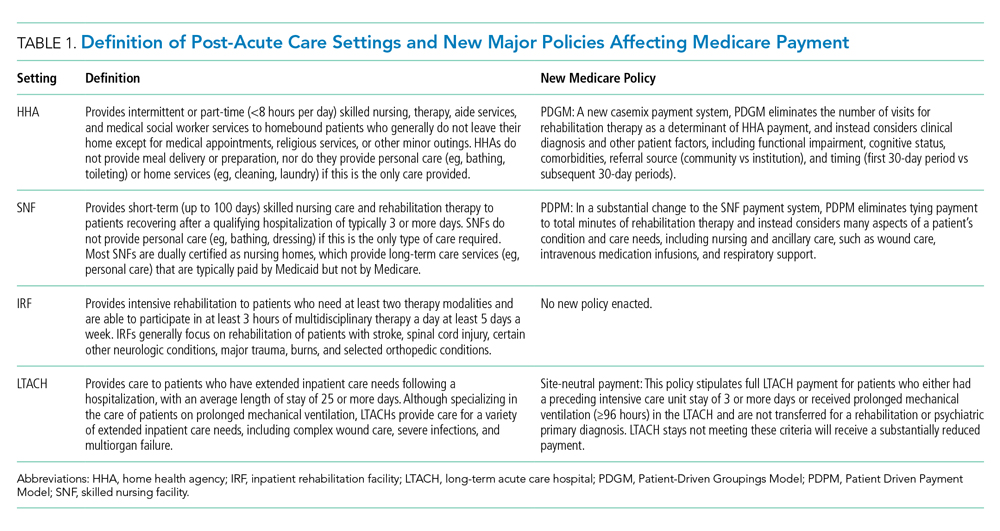
For HHAs and SNFs, CMS implemented new payment models to better align payment with patients’ care needs rather than the provision of rehabilitation therapy.1 For SNFs, the Patient
Driven Payment Model (PDPM) was implemented October 1, 2019, and for HHAs, the Patient-Driven Groupings Model (PDGM) was implemented January 1, 2020. These policies increase payment for patients who have nursing or ancillary care needs, such as IV medications, wound care, and respiratory support. For example, the per diem payment to SNFs is projected to increase 10% to 30% for patients needing dialysis, IV medications, wound care, and respiratory support, such as tracheostomy care.8 These policies also increase payment for patients with greater severity and complexity, such as patients with severe cognitive impairment and multimorbidity. Importantly, these policies pay HHAs and SNFs based on patients’ clinical needs and not solely based on the amount of rehabilitation therapy delivered, which could increase both the number and complexity of patients that SNFs accept.
To discourage LTACH use by patients who are unlikely to benefit from this level of care, CMS fully implemented the
COMMENTARY AND RECOMMENDATIONS
Historically, PAC payment policy has not properly incentivized the appropriate amount of care to be delivered in the appropriate setting.9 The recent HHA, SNF, and LTACH policy changes not only shift the discharge of patients across PAC settings, but also change the amount and type of care that occurs at each PAC site (Table 2). The potential benefit of these new policies is that they will help to align the right level of PAC with patients’ needs by discouraging inappropriate use and unnecessary services.
In terms of broader payment reform, the four PAC settings are still fragmented, with little effort to unify payment, regulation, and quality across the PAC continuum. As required by the Improving Medicare Post-Acute Care Transformation (IMPACT) Act of 2014, we would encourage the adoption of a unified PAC payment system that spans the four settings, with payments based on patient characteristics and needs rather than site of service.12 This type of reform would also harmonize regulation and quality measurement and reward payments across settings. Currently, CMS is standardizing patient assessment data and quality metrics across the four PAC settings. Given the COVID-19 pandemic, the transition to a unified PAC payment system is likely several years away.
WHAT SHOULD I TELL MY PATIENT?
For our patient who was transferred to an LTACH after referrals to SNFs were denied, PAC options now differ following these major PAC policy reforms, and SNF transfer would be an option. This is because SNFs will receive higher payment for providing respiratory support under the PDPM, and LTACHs will receive considerably lower reimbursement because the patient did not have a qualifying ICU stay or require prolonged mechanical ventilation. Furthermore, hospitals participating in accountable care organizations would achieve greater savings, given that LTACHs cost at least three times as much as SNFs for comparable diagnoses.
Instead of referring this patient to a LTACH, the care team (hospitalist, discharge navigator, and case manager) should inform and educate the patient about discharge options to SNFs for weaning from respiratory support. To help patients and caregivers choose a facility, the discharge planning team should provide data about the quality of SNFs (eg, CMS Star Ratings scores) instead of simply providing a list of names and locations.13,14
CONCLUSION
Recent major PAC policy changes will change where hospitals discharge medically complex patients and the services they will receive at these PAC settings. Historically, reduction in PAC use has been a key source for savings in alternative payment models that encourage value over volume, such as accountable care organizations and episode-based (“bundled”) payment models.15 We anticipate these PAC policy changes are a step in the right direction to further enable hospitals to achieve value by more closely aligning PAC incentives with patients’ needs.
1. Report to the Congress: Medicare Payment Policy. Medicare Payment Advisory Commision; 2020. http://www.medpac.gov/docs/default-source/reports/mar20_entirereport_sec.pdf?sfvrsn=0
2. Medicare and Medicaid Programs; CY 2020 Home Health Prospective Payment System Rate Update; Home Health Value-Based Purchasing Model; Home Heatlh Quality Reporting Requirements; and Home Infusion Therapy Requirements. Fed Regist. 2019;84(217):60478-60646. To be codified at 42 CFR Parts 409, 414, 484, and 486. https://www.govinfo.gov/content/pkg/FR-2019-11-08/pdf/2019-24026.pdf
3. Medicare Program; Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities (SNF) Final Rule for FY 2019, SNF Value-Based Purchasing Program, and SNF Quality Reporting Program. Fed Regist. 2018;83(153):39162-39290. To be codified at 42 CFR Parts 411, 413, and 424. https://www.govinfo.gov/content/pkg/FR-2018-08-08/pdf/2018-16570.pdf
4. Weaver C, Mathews AW, McGinty T. How Medicare rewards copious nursing-home therapy. Wall Street Journal. Updated August 16, 2015. Accessed October 13, 2020. https://www.wsj.com/articles/how-medicare-rewards-copious-nursing-home-therapy-1439778701
5. Eskildsen MA. Long-term acute care: a review of the literature. J Am Geriatr Soc. 2007;55(5):775-779. https://doi.org/10.1111/j.1532-5415.2007.01162.x
6. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227-1228. https://doi.org/10.1001/jama.2013.278139
7. Makam AN, Nguyen OK, Xuan L, Miller ME, Goodwin JS, Halm EA. Factors associated with variation in long-term acute care hospital vs skilled nursing facility use among hospitalized older adults. JAMA Intern Med. 2018;178(3):399-405. https://doi.org/10.1001/jamainternmed.2017.8467
8. Skilled Nursing Facilities Payment Models Research Technical Report. Acumen; 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/Downloads/SNF_Payment_Models_Research_Technical_Report201704.pdf
9. Ackerly DC, Grabowski DC. Post-acute care reform—beyond the ACA. New Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
10. Span P. A change in Medicare has therapists alarmed. New York Times. November 29, 2019. Accessed September 16, 2020. https://www.nytimes.com/2019/11/29/health/new-old-age-medicare-physical-therapy.html
11. Graham J. Why home health care is suddenly harder to come by for Medicare patients. Kaiser Health News (KHN). February 3, 2020. Accessed September 16, 2020. https://khn.org/news/why-home-health-care-is-suddenly-harder-to-come-by-for-medicare-patients/
12. Medicare Payment Advisory Commision. Implementing a unified payment system for post-acute care. In: Report to the Congress: Medicare and the Health Care Delivery System. Medicare Payment Advisory Commision; 2017:chap 1. http://www.medpac.gov/docs/default-source/reports/jun17_ch1.pdf?sfvrsn=0
13. Nazir A, Little MO, Arling GW. More than just location: helping patients and families select an appropriate skilled nursing facility. Ann Long Term Care: Clin Care Aging. 2014;22(11):30-34. Published online August 12, 2014. https://www.managedhealthcareconnect.com/articles/more-just-location-helping-patients-and-families-select-appropriate-skilled-nursing
14. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff (Millwood). 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155
15. Barnett ML, Mehrotra A, Grabowski DC. Postacute care—the piggy bank for savings in alternative payment models? New Engl J Med. 2019;381(4):302-303. https://doi.org/10.1056/NEJMp1901896
CLINICAL SCENARIO
A 70-year-old woman with Medicare insurance and a history of mild dementia and chronic bronchiectasis was hospitalized for acute respiratory failure due to influenza. She was treated in the intensive care unit (ICU) for 2 days, received mechanical ventilation, and was subsequently extubated and weaned to high-flow nasal cannula (HFNC) at 8 liters of oxygen per minute and noninvasive ventilation at bedtime. She had otherwise stable cognition and required no other medical or nursing therapies. For recovery, she was referred to a
BACKGROUND AND HISTORY
In 2018, 44% of hospitalized patients with fee-for-service Medicare (herein referred to as Medicare) were discharged to PAC, accounting for nearly $60 billion in annual Medicare spending.1 PAC includes four levels of care—home health agencies (HHAs), SNFs, inpatient rehabilitation facilities (IRFs), and LTACHs—which vary in intensity and complexity of the medical, skilled nursing, and rehabilitative services they provide; use separate reimbursement systems; employ different quality metrics; and have different regulatory requirements (Table 1). Because hospitalists care for the majority of these patients and commonly serve in leadership roles for transitions of care and PAC use, PAC policy is important, as it has direct implications on discharge patterns and the quality and nature of patient care after discharge.
HHAs, the most commonly used PAC setting, provide skilled nursing or therapy to homebound beneficiaries.1 HHAs were historically reimbursed a standardized 60-day episode payment based on casemix, which was highly dependent on the number of therapy visits provided, with extremely little contribution from nontherapy services, such as skilled nursing and home health aide visits.2
SNFs, which comprise nearly half of PAC spending, provide short-term skilled nursing and rehabilitative services following hospitalization. SNFs are reimbursed on a per diem basis by Medicare, with reimbursement historically determined by the intensity of the dominant service furnished to the patient—either nursing, ancillary care (which includes medications, supplies/equipment, and diagnostic testing), or rehabilitation.3 Due to strong financial incentives, payment for more than 90% of SNF days was based solely on rehabilitation therapy furnished, with 33% of SNF patients receiving ultra-high rehabilitation (>720 minutes/week),3
IRFs provide intensive rehabilitation to patients who are able to participate in at least 3 hours of multidisciplinary therapy per day.1 IRF admissions are paid a bundled rate by Medicare based on the patient’s primary reason for rehabilitation, their age, and their level of functioning and cognition.
LTACHs, the most intensive and expensive PAC setting, care for patients with a range of complex hospital-level care needs, including intravenous (IV) infusions, complex wound care, and respiratory support. Since 2002, the only requirements for LTACHs have been to meet Medicare’s requirements for hospital accreditation and maintain an average length of stay of 25 days for their population.5 LTACH stays are paid a bundled rate by Medicare based on diagnosis.
POLICIES IN CLINICAL PRACTICE
Due to considerable variation in PAC use, with concerns that similar patients can be treated in different PAC settings,6,7 the

For HHAs and SNFs, CMS implemented new payment models to better align payment with patients’ care needs rather than the provision of rehabilitation therapy.1 For SNFs, the Patient
Driven Payment Model (PDPM) was implemented October 1, 2019, and for HHAs, the Patient-Driven Groupings Model (PDGM) was implemented January 1, 2020. These policies increase payment for patients who have nursing or ancillary care needs, such as IV medications, wound care, and respiratory support. For example, the per diem payment to SNFs is projected to increase 10% to 30% for patients needing dialysis, IV medications, wound care, and respiratory support, such as tracheostomy care.8 These policies also increase payment for patients with greater severity and complexity, such as patients with severe cognitive impairment and multimorbidity. Importantly, these policies pay HHAs and SNFs based on patients’ clinical needs and not solely based on the amount of rehabilitation therapy delivered, which could increase both the number and complexity of patients that SNFs accept.
To discourage LTACH use by patients who are unlikely to benefit from this level of care, CMS fully implemented the
COMMENTARY AND RECOMMENDATIONS
Historically, PAC payment policy has not properly incentivized the appropriate amount of care to be delivered in the appropriate setting.9 The recent HHA, SNF, and LTACH policy changes not only shift the discharge of patients across PAC settings, but also change the amount and type of care that occurs at each PAC site (Table 2). The potential benefit of these new policies is that they will help to align the right level of PAC with patients’ needs by discouraging inappropriate use and unnecessary services.

In terms of broader payment reform, the four PAC settings are still fragmented, with little effort to unify payment, regulation, and quality across the PAC continuum. As required by the Improving Medicare Post-Acute Care Transformation (IMPACT) Act of 2014, we would encourage the adoption of a unified PAC payment system that spans the four settings, with payments based on patient characteristics and needs rather than site of service.12 This type of reform would also harmonize regulation and quality measurement and reward payments across settings. Currently, CMS is standardizing patient assessment data and quality metrics across the four PAC settings. Given the COVID-19 pandemic, the transition to a unified PAC payment system is likely several years away.
WHAT SHOULD I TELL MY PATIENT?
For our patient who was transferred to an LTACH after referrals to SNFs were denied, PAC options now differ following these major PAC policy reforms, and SNF transfer would be an option. This is because SNFs will receive higher payment for providing respiratory support under the PDPM, and LTACHs will receive considerably lower reimbursement because the patient did not have a qualifying ICU stay or require prolonged mechanical ventilation. Furthermore, hospitals participating in accountable care organizations would achieve greater savings, given that LTACHs cost at least three times as much as SNFs for comparable diagnoses.
Instead of referring this patient to a LTACH, the care team (hospitalist, discharge navigator, and case manager) should inform and educate the patient about discharge options to SNFs for weaning from respiratory support. To help patients and caregivers choose a facility, the discharge planning team should provide data about the quality of SNFs (eg, CMS Star Ratings scores) instead of simply providing a list of names and locations.13,14
CONCLUSION
Recent major PAC policy changes will change where hospitals discharge medically complex patients and the services they will receive at these PAC settings. Historically, reduction in PAC use has been a key source for savings in alternative payment models that encourage value over volume, such as accountable care organizations and episode-based (“bundled”) payment models.15 We anticipate these PAC policy changes are a step in the right direction to further enable hospitals to achieve value by more closely aligning PAC incentives with patients’ needs.
CLINICAL SCENARIO
A 70-year-old woman with Medicare insurance and a history of mild dementia and chronic bronchiectasis was hospitalized for acute respiratory failure due to influenza. She was treated in the intensive care unit (ICU) for 2 days, received mechanical ventilation, and was subsequently extubated and weaned to high-flow nasal cannula (HFNC) at 8 liters of oxygen per minute and noninvasive ventilation at bedtime. She had otherwise stable cognition and required no other medical or nursing therapies. For recovery, she was referred to a
BACKGROUND AND HISTORY
In 2018, 44% of hospitalized patients with fee-for-service Medicare (herein referred to as Medicare) were discharged to PAC, accounting for nearly $60 billion in annual Medicare spending.1 PAC includes four levels of care—home health agencies (HHAs), SNFs, inpatient rehabilitation facilities (IRFs), and LTACHs—which vary in intensity and complexity of the medical, skilled nursing, and rehabilitative services they provide; use separate reimbursement systems; employ different quality metrics; and have different regulatory requirements (Table 1). Because hospitalists care for the majority of these patients and commonly serve in leadership roles for transitions of care and PAC use, PAC policy is important, as it has direct implications on discharge patterns and the quality and nature of patient care after discharge.
HHAs, the most commonly used PAC setting, provide skilled nursing or therapy to homebound beneficiaries.1 HHAs were historically reimbursed a standardized 60-day episode payment based on casemix, which was highly dependent on the number of therapy visits provided, with extremely little contribution from nontherapy services, such as skilled nursing and home health aide visits.2
SNFs, which comprise nearly half of PAC spending, provide short-term skilled nursing and rehabilitative services following hospitalization. SNFs are reimbursed on a per diem basis by Medicare, with reimbursement historically determined by the intensity of the dominant service furnished to the patient—either nursing, ancillary care (which includes medications, supplies/equipment, and diagnostic testing), or rehabilitation.3 Due to strong financial incentives, payment for more than 90% of SNF days was based solely on rehabilitation therapy furnished, with 33% of SNF patients receiving ultra-high rehabilitation (>720 minutes/week),3
IRFs provide intensive rehabilitation to patients who are able to participate in at least 3 hours of multidisciplinary therapy per day.1 IRF admissions are paid a bundled rate by Medicare based on the patient’s primary reason for rehabilitation, their age, and their level of functioning and cognition.
LTACHs, the most intensive and expensive PAC setting, care for patients with a range of complex hospital-level care needs, including intravenous (IV) infusions, complex wound care, and respiratory support. Since 2002, the only requirements for LTACHs have been to meet Medicare’s requirements for hospital accreditation and maintain an average length of stay of 25 days for their population.5 LTACH stays are paid a bundled rate by Medicare based on diagnosis.
POLICIES IN CLINICAL PRACTICE
Due to considerable variation in PAC use, with concerns that similar patients can be treated in different PAC settings,6,7 the

For HHAs and SNFs, CMS implemented new payment models to better align payment with patients’ care needs rather than the provision of rehabilitation therapy.1 For SNFs, the Patient
Driven Payment Model (PDPM) was implemented October 1, 2019, and for HHAs, the Patient-Driven Groupings Model (PDGM) was implemented January 1, 2020. These policies increase payment for patients who have nursing or ancillary care needs, such as IV medications, wound care, and respiratory support. For example, the per diem payment to SNFs is projected to increase 10% to 30% for patients needing dialysis, IV medications, wound care, and respiratory support, such as tracheostomy care.8 These policies also increase payment for patients with greater severity and complexity, such as patients with severe cognitive impairment and multimorbidity. Importantly, these policies pay HHAs and SNFs based on patients’ clinical needs and not solely based on the amount of rehabilitation therapy delivered, which could increase both the number and complexity of patients that SNFs accept.
To discourage LTACH use by patients who are unlikely to benefit from this level of care, CMS fully implemented the
COMMENTARY AND RECOMMENDATIONS
Historically, PAC payment policy has not properly incentivized the appropriate amount of care to be delivered in the appropriate setting.9 The recent HHA, SNF, and LTACH policy changes not only shift the discharge of patients across PAC settings, but also change the amount and type of care that occurs at each PAC site (Table 2). The potential benefit of these new policies is that they will help to align the right level of PAC with patients’ needs by discouraging inappropriate use and unnecessary services.

In terms of broader payment reform, the four PAC settings are still fragmented, with little effort to unify payment, regulation, and quality across the PAC continuum. As required by the Improving Medicare Post-Acute Care Transformation (IMPACT) Act of 2014, we would encourage the adoption of a unified PAC payment system that spans the four settings, with payments based on patient characteristics and needs rather than site of service.12 This type of reform would also harmonize regulation and quality measurement and reward payments across settings. Currently, CMS is standardizing patient assessment data and quality metrics across the four PAC settings. Given the COVID-19 pandemic, the transition to a unified PAC payment system is likely several years away.
WHAT SHOULD I TELL MY PATIENT?
For our patient who was transferred to an LTACH after referrals to SNFs were denied, PAC options now differ following these major PAC policy reforms, and SNF transfer would be an option. This is because SNFs will receive higher payment for providing respiratory support under the PDPM, and LTACHs will receive considerably lower reimbursement because the patient did not have a qualifying ICU stay or require prolonged mechanical ventilation. Furthermore, hospitals participating in accountable care organizations would achieve greater savings, given that LTACHs cost at least three times as much as SNFs for comparable diagnoses.
Instead of referring this patient to a LTACH, the care team (hospitalist, discharge navigator, and case manager) should inform and educate the patient about discharge options to SNFs for weaning from respiratory support. To help patients and caregivers choose a facility, the discharge planning team should provide data about the quality of SNFs (eg, CMS Star Ratings scores) instead of simply providing a list of names and locations.13,14
CONCLUSION
Recent major PAC policy changes will change where hospitals discharge medically complex patients and the services they will receive at these PAC settings. Historically, reduction in PAC use has been a key source for savings in alternative payment models that encourage value over volume, such as accountable care organizations and episode-based (“bundled”) payment models.15 We anticipate these PAC policy changes are a step in the right direction to further enable hospitals to achieve value by more closely aligning PAC incentives with patients’ needs.
1. Report to the Congress: Medicare Payment Policy. Medicare Payment Advisory Commision; 2020. http://www.medpac.gov/docs/default-source/reports/mar20_entirereport_sec.pdf?sfvrsn=0
2. Medicare and Medicaid Programs; CY 2020 Home Health Prospective Payment System Rate Update; Home Health Value-Based Purchasing Model; Home Heatlh Quality Reporting Requirements; and Home Infusion Therapy Requirements. Fed Regist. 2019;84(217):60478-60646. To be codified at 42 CFR Parts 409, 414, 484, and 486. https://www.govinfo.gov/content/pkg/FR-2019-11-08/pdf/2019-24026.pdf
3. Medicare Program; Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities (SNF) Final Rule for FY 2019, SNF Value-Based Purchasing Program, and SNF Quality Reporting Program. Fed Regist. 2018;83(153):39162-39290. To be codified at 42 CFR Parts 411, 413, and 424. https://www.govinfo.gov/content/pkg/FR-2018-08-08/pdf/2018-16570.pdf
4. Weaver C, Mathews AW, McGinty T. How Medicare rewards copious nursing-home therapy. Wall Street Journal. Updated August 16, 2015. Accessed October 13, 2020. https://www.wsj.com/articles/how-medicare-rewards-copious-nursing-home-therapy-1439778701
5. Eskildsen MA. Long-term acute care: a review of the literature. J Am Geriatr Soc. 2007;55(5):775-779. https://doi.org/10.1111/j.1532-5415.2007.01162.x
6. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227-1228. https://doi.org/10.1001/jama.2013.278139
7. Makam AN, Nguyen OK, Xuan L, Miller ME, Goodwin JS, Halm EA. Factors associated with variation in long-term acute care hospital vs skilled nursing facility use among hospitalized older adults. JAMA Intern Med. 2018;178(3):399-405. https://doi.org/10.1001/jamainternmed.2017.8467
8. Skilled Nursing Facilities Payment Models Research Technical Report. Acumen; 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/Downloads/SNF_Payment_Models_Research_Technical_Report201704.pdf
9. Ackerly DC, Grabowski DC. Post-acute care reform—beyond the ACA. New Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
10. Span P. A change in Medicare has therapists alarmed. New York Times. November 29, 2019. Accessed September 16, 2020. https://www.nytimes.com/2019/11/29/health/new-old-age-medicare-physical-therapy.html
11. Graham J. Why home health care is suddenly harder to come by for Medicare patients. Kaiser Health News (KHN). February 3, 2020. Accessed September 16, 2020. https://khn.org/news/why-home-health-care-is-suddenly-harder-to-come-by-for-medicare-patients/
12. Medicare Payment Advisory Commision. Implementing a unified payment system for post-acute care. In: Report to the Congress: Medicare and the Health Care Delivery System. Medicare Payment Advisory Commision; 2017:chap 1. http://www.medpac.gov/docs/default-source/reports/jun17_ch1.pdf?sfvrsn=0
13. Nazir A, Little MO, Arling GW. More than just location: helping patients and families select an appropriate skilled nursing facility. Ann Long Term Care: Clin Care Aging. 2014;22(11):30-34. Published online August 12, 2014. https://www.managedhealthcareconnect.com/articles/more-just-location-helping-patients-and-families-select-appropriate-skilled-nursing
14. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff (Millwood). 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155
15. Barnett ML, Mehrotra A, Grabowski DC. Postacute care—the piggy bank for savings in alternative payment models? New Engl J Med. 2019;381(4):302-303. https://doi.org/10.1056/NEJMp1901896
1. Report to the Congress: Medicare Payment Policy. Medicare Payment Advisory Commision; 2020. http://www.medpac.gov/docs/default-source/reports/mar20_entirereport_sec.pdf?sfvrsn=0
2. Medicare and Medicaid Programs; CY 2020 Home Health Prospective Payment System Rate Update; Home Health Value-Based Purchasing Model; Home Heatlh Quality Reporting Requirements; and Home Infusion Therapy Requirements. Fed Regist. 2019;84(217):60478-60646. To be codified at 42 CFR Parts 409, 414, 484, and 486. https://www.govinfo.gov/content/pkg/FR-2019-11-08/pdf/2019-24026.pdf
3. Medicare Program; Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities (SNF) Final Rule for FY 2019, SNF Value-Based Purchasing Program, and SNF Quality Reporting Program. Fed Regist. 2018;83(153):39162-39290. To be codified at 42 CFR Parts 411, 413, and 424. https://www.govinfo.gov/content/pkg/FR-2018-08-08/pdf/2018-16570.pdf
4. Weaver C, Mathews AW, McGinty T. How Medicare rewards copious nursing-home therapy. Wall Street Journal. Updated August 16, 2015. Accessed October 13, 2020. https://www.wsj.com/articles/how-medicare-rewards-copious-nursing-home-therapy-1439778701
5. Eskildsen MA. Long-term acute care: a review of the literature. J Am Geriatr Soc. 2007;55(5):775-779. https://doi.org/10.1111/j.1532-5415.2007.01162.x
6. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227-1228. https://doi.org/10.1001/jama.2013.278139
7. Makam AN, Nguyen OK, Xuan L, Miller ME, Goodwin JS, Halm EA. Factors associated with variation in long-term acute care hospital vs skilled nursing facility use among hospitalized older adults. JAMA Intern Med. 2018;178(3):399-405. https://doi.org/10.1001/jamainternmed.2017.8467
8. Skilled Nursing Facilities Payment Models Research Technical Report. Acumen; 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/Downloads/SNF_Payment_Models_Research_Technical_Report201704.pdf
9. Ackerly DC, Grabowski DC. Post-acute care reform—beyond the ACA. New Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
10. Span P. A change in Medicare has therapists alarmed. New York Times. November 29, 2019. Accessed September 16, 2020. https://www.nytimes.com/2019/11/29/health/new-old-age-medicare-physical-therapy.html
11. Graham J. Why home health care is suddenly harder to come by for Medicare patients. Kaiser Health News (KHN). February 3, 2020. Accessed September 16, 2020. https://khn.org/news/why-home-health-care-is-suddenly-harder-to-come-by-for-medicare-patients/
12. Medicare Payment Advisory Commision. Implementing a unified payment system for post-acute care. In: Report to the Congress: Medicare and the Health Care Delivery System. Medicare Payment Advisory Commision; 2017:chap 1. http://www.medpac.gov/docs/default-source/reports/jun17_ch1.pdf?sfvrsn=0
13. Nazir A, Little MO, Arling GW. More than just location: helping patients and families select an appropriate skilled nursing facility. Ann Long Term Care: Clin Care Aging. 2014;22(11):30-34. Published online August 12, 2014. https://www.managedhealthcareconnect.com/articles/more-just-location-helping-patients-and-families-select-appropriate-skilled-nursing
14. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff (Millwood). 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155
15. Barnett ML, Mehrotra A, Grabowski DC. Postacute care—the piggy bank for savings in alternative payment models? New Engl J Med. 2019;381(4):302-303. https://doi.org/10.1056/NEJMp1901896
© 2021 Society of Hospital Medicine