User login
Improving Healthcare Value: Effectiveness of a Program to Reduce Laboratory Testing for Non-Critically-Ill Patients With COVID-19
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
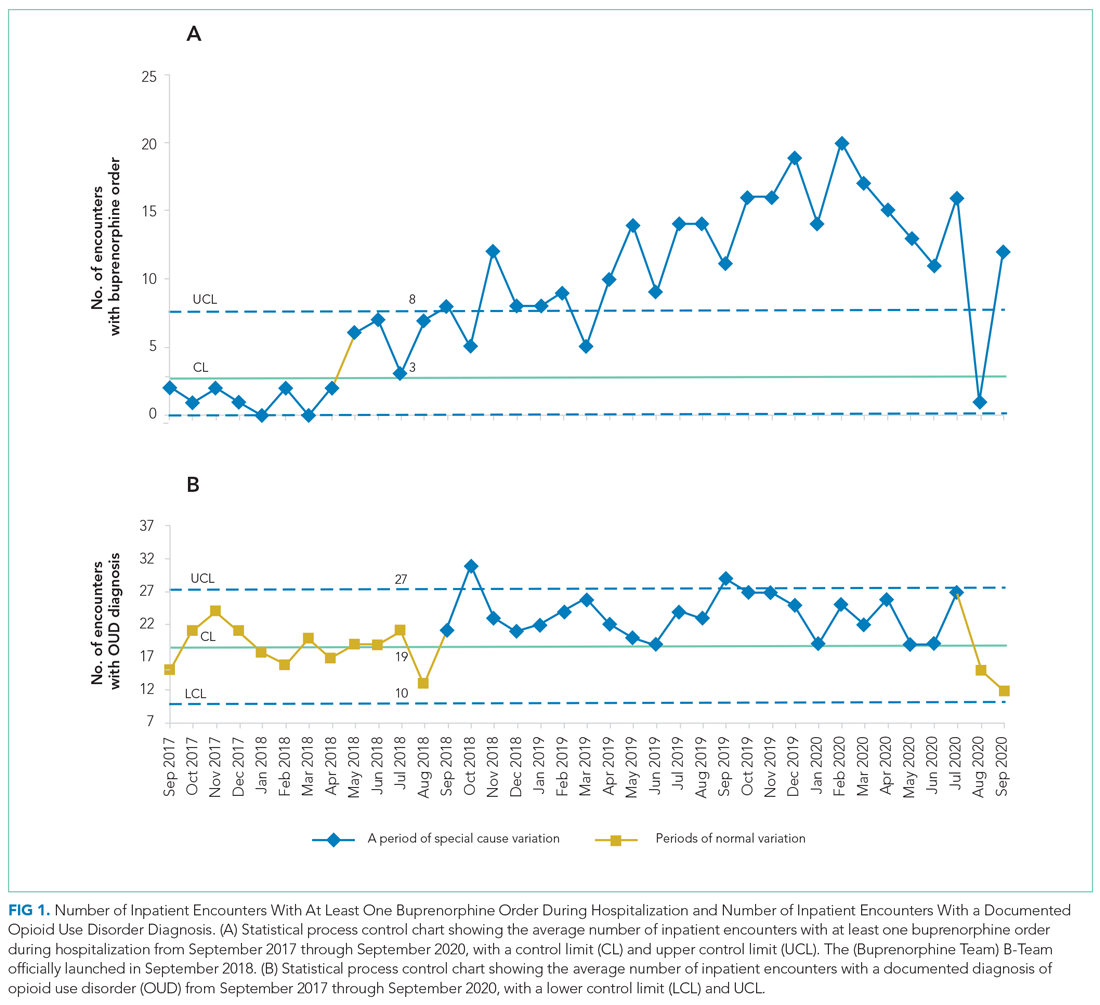
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
© 2021 Society of Hospital Medicine
Hospital Buprenorphine Program for Opioid Use Disorder Is Associated With Increased Inpatient and Outpatient Addiction Treatment
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
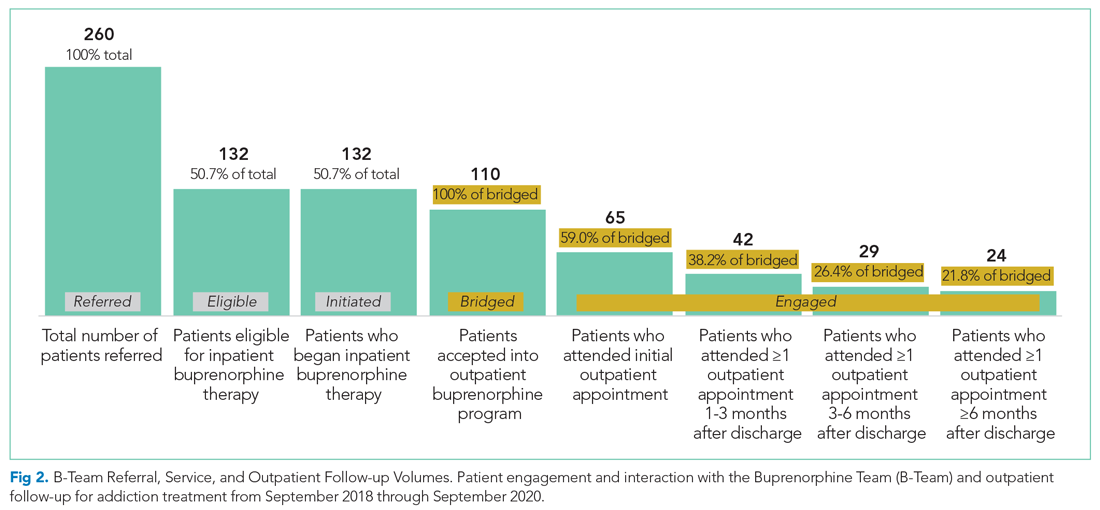
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased
The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).
Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased

The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).

Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased

The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).

Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
© 2021 Society of Hospital Medicine
Caring Wisely: A Program to Support Frontline Clinicians and Staff in Improving Healthcare Delivery and Reducing Costs
© 2017 Society of Hospital Medicine
Strategies are needed to empower frontline clinicians to work with organizational leadership to reduce healthcare costs and improve high-value care. Caring Wisely® is a program developed by the University of California, San Francisco’s (UCSF) Center for Healthcare Value (CHV), aimed at engaging frontline clinicians and staff, connecting them with implementation experts, and supporting the development of targeted interventions to improve value. Financial savings from the program more than cover program costs. Caring Wisely® provides an institutional model for implementing robust interventions to address areas of low-value care.
Launched in 2013, the annual Caring Wisely® program consists of 3 stages for identifying projects that meet the following criteria:
- Potential to measurably reduce UCSF Health’s costs of care without transferring costs to patients, insurers, or other providers
- Plan for ensuring that health outcomes are maintained or improved
- Envision disseminating the intervention within and beyond UCSF
- Demonstrate commitment and engagement of clinical leadership and frontline staff.
The first stage is the Ideas Contest, a UCSF Health-wide call (to learn more about UCSF Health: https://www.ucsf.edu/sites/default/files/052516_About_UCSF.pdf) to identify areas that may be targeted to reduce unnecessary services, inefficiencies, and healthcare costs. We use a crowdsourcing platform—Open Proposals—to solicit the best ideas from frontline clinicians and staff.1 Open Proposals is a secure, web-based platform for transparent and collaborative proposal development that displays threads of comments, responses, and revisions, and allows submissions to be “liked.” Open Proposals is managed by the UCSF Clinical and Translational Science Institute, funded by the National Center for Advancing Translational Sciences (Grant Number UL1 TR000004) at the National Institutes of Health. Using institutional e-mail lists for faculty, staff and residents, as well as described at monthly managers and directors meetings, the Ideas Contest is announced each year by the Chief Medical Officer and the CHV leadership. The Caring Wisely® Executive Steering Committee, which consists of CHV and senior UCSF Health system leaders, selects the top 5-10 ideas based on the above criteria. Each winning idea receives a $100 gift certificate for a popular restaurant in San Francisco, and the list of winners is announced to the entire UCSF community.
The second stage is the Request for Proposals. The Caring Wisely® program solicits proposals that outline implementation plans to target specific areas identified through the Ideas Contest. Finalists from the Ideas Contest are encouraged to submit proposals that address the problem they identified, but anyone affiliated with UCSF Health may submit a proposal on a winning idea. There is an approximately 4-week open submission period during which applicants submit brief 2-page proposals on the Open Proposal platform. This is followed by a period of optimization that leverages the social media aspect of the Open Proposals platform in which the UCSF Health community asks clarifying questions, make suggestions, and modifications can be made to the proposals. All submissions receive written feedback from at least one Steering Committee member. In addition, the Caring Wisely® Director directly invites relevant UCSF colleagues, administrators, or program leaders to comment on proposals and make suggestions for improvement. Plans for assessing financial and health care delivery impacts are developed in collaboration with the UCSF Health Finance department. UCSF Health managers and leaders who are stakeholders in project proposal areas are consulted to provide input and finalize proposal plans, including the identification of existing personnel who can support and drive the project forward. Proposers use this feedback to revise their applications throughout this stage.
The third stage is Project Implementation. The Caring Wisely® Executive Steering Committee selects up to 3 winners from the submitted proposals. Using the program criteria above, each project is scored independently, discussed in committee, and rescored to identify the top proposals. Each selected project receives a maximum budget of $50,000 that can be used for project materials, activities, and salary support for project leaders or staff. In addition to funding, each project team receives input from the implementation science team to co-develop and implement the intervention with a goal of creating a first-test-of-change within 3-6 months. A key feature of Caring Wisely® is the partnership between project teams and the Caring Wisely® implementation team, which includes a director, program manager, data analysts, and implementation scientists (Table 1).
The $150,000 administrative budget for the Caring Wisely® program provides 20% support of the medical director, 50% support of a program manager/analyst, and 10% support of an implementation scientist. Approximately 5% support is donated from additional senior implementation scientists and various UCSF Health experts based on project needs. To make most efficient use of the Caring Wisely® program staff time with the project teams, there is a weekly 60-90 minute works-in-progress session attended by all 3 teams with a rotating schedule for lead presenter during the first 6 months; these meetings occur every 2-3 weeks during the second 6 months. Caring Wisely® program staff and the implementation scientist are also available for 1:1 meetings as needed. The Caring Wisely® Executive Steering Committee is not paid and meets for 90 minutes quarterly. Custom reports and modifications of the electronic health record are provided by the UCSF Health clinical informatics department as part of their operating budget.
The collaboration between the project teams and the implementation science team is guided by the Consolidated Framework for Implementation Research (CFIR)2 and PRECEDE-PROCEED model—a logic model and evaluation tool that is based on a composite of individual behavior change theory and social ecology.3 Table 2 illustrates how we weave PRECEDE-PROCEED and Plan-Do-Study-Act frameworks into project design and strategy. Each funded team is required to submit an end-of-year progress report.
Cost and cost savings estimates were based on administrative financial data obtained through the assistance of the Decision Support Services unit of the Finance Department of UCSF Health. All costs reflect direct institutional costs, rather than charges. For some projects, costs are directly available through computerized dashboards that provide year-to-year comparisons of specific costs of materials, supplies, and services (eg, blood transfusion reduction, surgical supplies project, OR efficiency program). This same dashboard also allows calculation of CMI-adjusted direct costs of hospital care by service line, as used in the perioperative pathways program evaluation. In other cases, the Decision Support Services and/or Caring Wisely® program manager created custom cost reports based on the key performance indicator (eg, nebulizer therapy costs consist of medication costs plus respiratory therapist time; CT scan utilization for suspected pulmonary embolus in emergency department; and antimicrobial utilization for suspected neonatal sepsis).
Ongoing monitoring and sustainability of Caring Wisely® projects is supported by the Caring Wisely® program leaders. Monitoring of ongoing cost savings is based on automated service-line level dashboards related to cost, utilization, and quality outcomes with quarterly updates provided to the Caring Wisely® Steering Committee. Depending on the project or program, appropriate UCSF Health senior leaders determine the level of support within their departments that is required to sustain the program(s). Ongoing monitoring of each program is also included in the strategic deployment visibility room with regular rounding by senior health system executives.
Since 2013, there have been 3 complete Caring Wisely® cycles. The Ideas Contest generated more than 75 ideas in each of the past 3 cycles, ranging from eliminating redundant laboratory or radiological studies to reducing linen and food waste. We received between 13-20 full proposals in each of the request for proposal stages, and 9 projects have been implemented, 3 in each year. Funded projects have been led by a variety of individuals including physicians, nurses, pharmacists, administrators and residents, and topics have ranged from reducing overutilization of tests, supplies and treatments, to improving patient throughput during the perioperative period (Table 3). Estimated cumulative savings to date from Caring Wisely® projects has exceeded $4 million, based on the four projects shown in Table 4. The IV-to-PO switch program and the neonatal sepsis risk prediction project (Table 3) have been successful in reducing unnecessary utilization, but cost and savings estimates are not yet finalized. Three funded projects were equivocal in cost savings but were successful in their primary aims: (1) increasing the appropriateness of CT scan ordering for suspected pulmonary embolus; (2) shortening operating room turnover times; and (3) implementing a postoperative debrief program for the systematic documentation of safety events, waste, and inefficiencies related to surgery.
We developed an innovative program that reduces hospital costs through crowdsourcing of ideas from frontline clinicians and staff, and by connecting these ideas to project and implementation science teams. At a time when healthcare costs have reached unsustainable levels, the Caring Wisely® program provides a process for healthcare personnel to make a positive impact on healthcare costs in areas under their direct control. Through the Open Proposals platform, we have tapped a growing desire among frontline providers to reduce medical waste.
A key criterion for the Caring Wisely® program is to propose changes that reduce cost without adversely affect healthcare quality or outcomes. While this is an important consideration in selecting projects, there is limited power to detect many of the most clinically relevant outcomes. We find this acceptable because many of the sponsored Caring Wisely® project goals were to increase compliance with evidence-based practice guidelines and reduce harms associated with unnecessary treatments (eg, blood transfusion, nebulizer therapy, CT scan, antimicrobial therapy). Selected balancing metrics for each project are reported by established quality and safety programs at UCSF Health, but we acknowledge that many factors that can affect these clinical outcomes are not related to the cost-reduction intervention and are not possible to control outside of a clinical research study. Therefore, any response to changes in these outcome and balancing measures requires further analysis beyond the Caring Wisely® project alone.
We believe one of the key factors in the success of the Caring Wisely® program is the application of implementation science principles to the intervention design strategies (Table 1). These principles included stakeholder engagement, behavior change theory, market (target audience) segmentation, and process measurement and feedback. Because we are conducting this program in an academic health center, resident and fellow education and engagement are also critical to success. In each project, we utilize the PRECEDE model as a guide to ensure that each intervention design includes complementary elements of effective behavior change, intended to increase awareness and motivation to change, to make change “easy,” and to reinforce change(Table 2).3
The Caring Wisely® program—itself a multifaceted intervention—embodies the same PRECEDE dimensions we apply to each specific project. The Ideas Contest serves as a tool for increasing awareness, attitudes, and motivation across the clinical enterprise for reducing healthcare costs. The support provided to the project teams by the Caring Wisely® program is an enabling factor that makes it “easier” for frontline teams to design and implement interventions with a greater likelihood of achieving early success. Timely measurement and feedback of results to the hospital leadership and broadcasting to the larger community reinforces the support of the program at both the leadership and frontline levels.
Collaboration between project teams and the Caring Wisely® program also provides frontline clinicians and staff with practical experience and lessons that they can apply to future improvement work. Project teams learn implementation science principles such as constructing a pragmatic theoretical framework to guide implementation design using CFIR model.2 Incorporating multiple, rapid-cycle tests of change allows teams to modify and adapt final interventions as they learn how the target audience and environment responds to specific intervention components. Access to real-time, actionable data and a data analyst is essential to rapid cycle adaptation that allows teams to focus on specific units or providers. We also find that cross-fertilization between project teams working in different areas helps to share resources and minimize duplication of efforts from the clinical and staff champions. Partnering with UCSF Health system leaders at every phase of project development—from proposal selection, development, and final evaluation of results—enhances sustainable transition of successful projects into clinical operations.
The costs and coordination for the first cycle of Caring Wisely® were supported by the UCSF Center for Healthcare Value. Upon completion of the evaluation of the first cycle, UCSF Health agreed to fund the program going forward, with the expectation that Caring Wisely would continue to achieve direct cost-savings for the organization. The Caring Wisely team provides a final report each year detailing the impact of each project on utilization and associated costs. Currently, program costs are approximately $150,000 for the Caring Wisely program leaders, staff, and other resources, and $50,000 for each of 3 projects for a total program cost of $300,000 per year. Projects included in the first three cycles have already saved more than $4 million, representing a strong return on investment. This program could be a model for other academic health centers to engage frontline clinicians and staff in addressing healthcare costs, and lends itself to being scaled-up into a multi-system collaborative.
LIST OF ABBREVIATIONS
UCSF—University of California, San Francisco; PRECEDE—Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation; PROCEED—Policy, Regulatory and Organizational Constructs in Educational and Environmental Development
Acknowledgments
Other participants in blood transfusion reduction project (D. Johnson, K. Curcione); IV-to-PO Switch (C. Tsourounis, A. Pollock); Surgical Supply Cost Reduction (C. Zygourakis); Perioperative Efficiency (L. Hampson); CT for PE Risk Prediction (E. Weber); ERAS Pathways (L. Chen); Neonatal Sepsis Risk Prediction (T. Newman); Post-Operative Debrief (S. Imershein). Caring Wisely Executive Steering Committee (J. Adler, S. Antrum, A Auerbach, J. Bennan, M. Blum, C. Ritchie, C. Tsourounis). This Center for Healthcare Value is funded in part by a grant from the Grove Foundation. We appreciate additional review and comments to the manuscript provided by George Sawaya and Adams Dudley.
Disclosures
Christopher Moriates has accepted royalties from McGraw-Hill for textbook, Understanding Value-Based Healthcare. Alvin Rajkomar has received fees as a research adviser from Google, Inc.
1. Kahlon M, Yuan L, Gologorskaya O, Johnston SC. Crowdsourcing the CTSA innovation mission. Clin Transl Sci. 2014;7:89-92. PubMed
2. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. PubMed
3. Green LW and Kreuter. Health Program Planning: An Educational and Ecological Approach. 4th Ed. McGraw-Hill. New York, NY. 2005.
4. Zygourakis CC, Valencia V, Moriates C et al. Association between surgeon scorecard use and operating room costs. JAMA Surg. 2016 Dec 7. doi: 10.1001/jamasurg.2016.4674. [Epub ahead of print] PubMed
© 2017 Society of Hospital Medicine
Strategies are needed to empower frontline clinicians to work with organizational leadership to reduce healthcare costs and improve high-value care. Caring Wisely® is a program developed by the University of California, San Francisco’s (UCSF) Center for Healthcare Value (CHV), aimed at engaging frontline clinicians and staff, connecting them with implementation experts, and supporting the development of targeted interventions to improve value. Financial savings from the program more than cover program costs. Caring Wisely® provides an institutional model for implementing robust interventions to address areas of low-value care.
Launched in 2013, the annual Caring Wisely® program consists of 3 stages for identifying projects that meet the following criteria:
- Potential to measurably reduce UCSF Health’s costs of care without transferring costs to patients, insurers, or other providers
- Plan for ensuring that health outcomes are maintained or improved
- Envision disseminating the intervention within and beyond UCSF
- Demonstrate commitment and engagement of clinical leadership and frontline staff.
The first stage is the Ideas Contest, a UCSF Health-wide call (to learn more about UCSF Health: https://www.ucsf.edu/sites/default/files/052516_About_UCSF.pdf) to identify areas that may be targeted to reduce unnecessary services, inefficiencies, and healthcare costs. We use a crowdsourcing platform—Open Proposals—to solicit the best ideas from frontline clinicians and staff.1 Open Proposals is a secure, web-based platform for transparent and collaborative proposal development that displays threads of comments, responses, and revisions, and allows submissions to be “liked.” Open Proposals is managed by the UCSF Clinical and Translational Science Institute, funded by the National Center for Advancing Translational Sciences (Grant Number UL1 TR000004) at the National Institutes of Health. Using institutional e-mail lists for faculty, staff and residents, as well as described at monthly managers and directors meetings, the Ideas Contest is announced each year by the Chief Medical Officer and the CHV leadership. The Caring Wisely® Executive Steering Committee, which consists of CHV and senior UCSF Health system leaders, selects the top 5-10 ideas based on the above criteria. Each winning idea receives a $100 gift certificate for a popular restaurant in San Francisco, and the list of winners is announced to the entire UCSF community.
The second stage is the Request for Proposals. The Caring Wisely® program solicits proposals that outline implementation plans to target specific areas identified through the Ideas Contest. Finalists from the Ideas Contest are encouraged to submit proposals that address the problem they identified, but anyone affiliated with UCSF Health may submit a proposal on a winning idea. There is an approximately 4-week open submission period during which applicants submit brief 2-page proposals on the Open Proposal platform. This is followed by a period of optimization that leverages the social media aspect of the Open Proposals platform in which the UCSF Health community asks clarifying questions, make suggestions, and modifications can be made to the proposals. All submissions receive written feedback from at least one Steering Committee member. In addition, the Caring Wisely® Director directly invites relevant UCSF colleagues, administrators, or program leaders to comment on proposals and make suggestions for improvement. Plans for assessing financial and health care delivery impacts are developed in collaboration with the UCSF Health Finance department. UCSF Health managers and leaders who are stakeholders in project proposal areas are consulted to provide input and finalize proposal plans, including the identification of existing personnel who can support and drive the project forward. Proposers use this feedback to revise their applications throughout this stage.
The third stage is Project Implementation. The Caring Wisely® Executive Steering Committee selects up to 3 winners from the submitted proposals. Using the program criteria above, each project is scored independently, discussed in committee, and rescored to identify the top proposals. Each selected project receives a maximum budget of $50,000 that can be used for project materials, activities, and salary support for project leaders or staff. In addition to funding, each project team receives input from the implementation science team to co-develop and implement the intervention with a goal of creating a first-test-of-change within 3-6 months. A key feature of Caring Wisely® is the partnership between project teams and the Caring Wisely® implementation team, which includes a director, program manager, data analysts, and implementation scientists (Table 1).
The $150,000 administrative budget for the Caring Wisely® program provides 20% support of the medical director, 50% support of a program manager/analyst, and 10% support of an implementation scientist. Approximately 5% support is donated from additional senior implementation scientists and various UCSF Health experts based on project needs. To make most efficient use of the Caring Wisely® program staff time with the project teams, there is a weekly 60-90 minute works-in-progress session attended by all 3 teams with a rotating schedule for lead presenter during the first 6 months; these meetings occur every 2-3 weeks during the second 6 months. Caring Wisely® program staff and the implementation scientist are also available for 1:1 meetings as needed. The Caring Wisely® Executive Steering Committee is not paid and meets for 90 minutes quarterly. Custom reports and modifications of the electronic health record are provided by the UCSF Health clinical informatics department as part of their operating budget.
The collaboration between the project teams and the implementation science team is guided by the Consolidated Framework for Implementation Research (CFIR)2 and PRECEDE-PROCEED model—a logic model and evaluation tool that is based on a composite of individual behavior change theory and social ecology.3 Table 2 illustrates how we weave PRECEDE-PROCEED and Plan-Do-Study-Act frameworks into project design and strategy. Each funded team is required to submit an end-of-year progress report.
Cost and cost savings estimates were based on administrative financial data obtained through the assistance of the Decision Support Services unit of the Finance Department of UCSF Health. All costs reflect direct institutional costs, rather than charges. For some projects, costs are directly available through computerized dashboards that provide year-to-year comparisons of specific costs of materials, supplies, and services (eg, blood transfusion reduction, surgical supplies project, OR efficiency program). This same dashboard also allows calculation of CMI-adjusted direct costs of hospital care by service line, as used in the perioperative pathways program evaluation. In other cases, the Decision Support Services and/or Caring Wisely® program manager created custom cost reports based on the key performance indicator (eg, nebulizer therapy costs consist of medication costs plus respiratory therapist time; CT scan utilization for suspected pulmonary embolus in emergency department; and antimicrobial utilization for suspected neonatal sepsis).
Ongoing monitoring and sustainability of Caring Wisely® projects is supported by the Caring Wisely® program leaders. Monitoring of ongoing cost savings is based on automated service-line level dashboards related to cost, utilization, and quality outcomes with quarterly updates provided to the Caring Wisely® Steering Committee. Depending on the project or program, appropriate UCSF Health senior leaders determine the level of support within their departments that is required to sustain the program(s). Ongoing monitoring of each program is also included in the strategic deployment visibility room with regular rounding by senior health system executives.
Since 2013, there have been 3 complete Caring Wisely® cycles. The Ideas Contest generated more than 75 ideas in each of the past 3 cycles, ranging from eliminating redundant laboratory or radiological studies to reducing linen and food waste. We received between 13-20 full proposals in each of the request for proposal stages, and 9 projects have been implemented, 3 in each year. Funded projects have been led by a variety of individuals including physicians, nurses, pharmacists, administrators and residents, and topics have ranged from reducing overutilization of tests, supplies and treatments, to improving patient throughput during the perioperative period (Table 3). Estimated cumulative savings to date from Caring Wisely® projects has exceeded $4 million, based on the four projects shown in Table 4. The IV-to-PO switch program and the neonatal sepsis risk prediction project (Table 3) have been successful in reducing unnecessary utilization, but cost and savings estimates are not yet finalized. Three funded projects were equivocal in cost savings but were successful in their primary aims: (1) increasing the appropriateness of CT scan ordering for suspected pulmonary embolus; (2) shortening operating room turnover times; and (3) implementing a postoperative debrief program for the systematic documentation of safety events, waste, and inefficiencies related to surgery.
We developed an innovative program that reduces hospital costs through crowdsourcing of ideas from frontline clinicians and staff, and by connecting these ideas to project and implementation science teams. At a time when healthcare costs have reached unsustainable levels, the Caring Wisely® program provides a process for healthcare personnel to make a positive impact on healthcare costs in areas under their direct control. Through the Open Proposals platform, we have tapped a growing desire among frontline providers to reduce medical waste.
A key criterion for the Caring Wisely® program is to propose changes that reduce cost without adversely affect healthcare quality or outcomes. While this is an important consideration in selecting projects, there is limited power to detect many of the most clinically relevant outcomes. We find this acceptable because many of the sponsored Caring Wisely® project goals were to increase compliance with evidence-based practice guidelines and reduce harms associated with unnecessary treatments (eg, blood transfusion, nebulizer therapy, CT scan, antimicrobial therapy). Selected balancing metrics for each project are reported by established quality and safety programs at UCSF Health, but we acknowledge that many factors that can affect these clinical outcomes are not related to the cost-reduction intervention and are not possible to control outside of a clinical research study. Therefore, any response to changes in these outcome and balancing measures requires further analysis beyond the Caring Wisely® project alone.
We believe one of the key factors in the success of the Caring Wisely® program is the application of implementation science principles to the intervention design strategies (Table 1). These principles included stakeholder engagement, behavior change theory, market (target audience) segmentation, and process measurement and feedback. Because we are conducting this program in an academic health center, resident and fellow education and engagement are also critical to success. In each project, we utilize the PRECEDE model as a guide to ensure that each intervention design includes complementary elements of effective behavior change, intended to increase awareness and motivation to change, to make change “easy,” and to reinforce change(Table 2).3
The Caring Wisely® program—itself a multifaceted intervention—embodies the same PRECEDE dimensions we apply to each specific project. The Ideas Contest serves as a tool for increasing awareness, attitudes, and motivation across the clinical enterprise for reducing healthcare costs. The support provided to the project teams by the Caring Wisely® program is an enabling factor that makes it “easier” for frontline teams to design and implement interventions with a greater likelihood of achieving early success. Timely measurement and feedback of results to the hospital leadership and broadcasting to the larger community reinforces the support of the program at both the leadership and frontline levels.
Collaboration between project teams and the Caring Wisely® program also provides frontline clinicians and staff with practical experience and lessons that they can apply to future improvement work. Project teams learn implementation science principles such as constructing a pragmatic theoretical framework to guide implementation design using CFIR model.2 Incorporating multiple, rapid-cycle tests of change allows teams to modify and adapt final interventions as they learn how the target audience and environment responds to specific intervention components. Access to real-time, actionable data and a data analyst is essential to rapid cycle adaptation that allows teams to focus on specific units or providers. We also find that cross-fertilization between project teams working in different areas helps to share resources and minimize duplication of efforts from the clinical and staff champions. Partnering with UCSF Health system leaders at every phase of project development—from proposal selection, development, and final evaluation of results—enhances sustainable transition of successful projects into clinical operations.
The costs and coordination for the first cycle of Caring Wisely® were supported by the UCSF Center for Healthcare Value. Upon completion of the evaluation of the first cycle, UCSF Health agreed to fund the program going forward, with the expectation that Caring Wisely would continue to achieve direct cost-savings for the organization. The Caring Wisely team provides a final report each year detailing the impact of each project on utilization and associated costs. Currently, program costs are approximately $150,000 for the Caring Wisely program leaders, staff, and other resources, and $50,000 for each of 3 projects for a total program cost of $300,000 per year. Projects included in the first three cycles have already saved more than $4 million, representing a strong return on investment. This program could be a model for other academic health centers to engage frontline clinicians and staff in addressing healthcare costs, and lends itself to being scaled-up into a multi-system collaborative.
LIST OF ABBREVIATIONS
UCSF—University of California, San Francisco; PRECEDE—Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation; PROCEED—Policy, Regulatory and Organizational Constructs in Educational and Environmental Development
Acknowledgments
Other participants in blood transfusion reduction project (D. Johnson, K. Curcione); IV-to-PO Switch (C. Tsourounis, A. Pollock); Surgical Supply Cost Reduction (C. Zygourakis); Perioperative Efficiency (L. Hampson); CT for PE Risk Prediction (E. Weber); ERAS Pathways (L. Chen); Neonatal Sepsis Risk Prediction (T. Newman); Post-Operative Debrief (S. Imershein). Caring Wisely Executive Steering Committee (J. Adler, S. Antrum, A Auerbach, J. Bennan, M. Blum, C. Ritchie, C. Tsourounis). This Center for Healthcare Value is funded in part by a grant from the Grove Foundation. We appreciate additional review and comments to the manuscript provided by George Sawaya and Adams Dudley.
Disclosures
Christopher Moriates has accepted royalties from McGraw-Hill for textbook, Understanding Value-Based Healthcare. Alvin Rajkomar has received fees as a research adviser from Google, Inc.
© 2017 Society of Hospital Medicine
Strategies are needed to empower frontline clinicians to work with organizational leadership to reduce healthcare costs and improve high-value care. Caring Wisely® is a program developed by the University of California, San Francisco’s (UCSF) Center for Healthcare Value (CHV), aimed at engaging frontline clinicians and staff, connecting them with implementation experts, and supporting the development of targeted interventions to improve value. Financial savings from the program more than cover program costs. Caring Wisely® provides an institutional model for implementing robust interventions to address areas of low-value care.
Launched in 2013, the annual Caring Wisely® program consists of 3 stages for identifying projects that meet the following criteria:
- Potential to measurably reduce UCSF Health’s costs of care without transferring costs to patients, insurers, or other providers
- Plan for ensuring that health outcomes are maintained or improved
- Envision disseminating the intervention within and beyond UCSF
- Demonstrate commitment and engagement of clinical leadership and frontline staff.
The first stage is the Ideas Contest, a UCSF Health-wide call (to learn more about UCSF Health: https://www.ucsf.edu/sites/default/files/052516_About_UCSF.pdf) to identify areas that may be targeted to reduce unnecessary services, inefficiencies, and healthcare costs. We use a crowdsourcing platform—Open Proposals—to solicit the best ideas from frontline clinicians and staff.1 Open Proposals is a secure, web-based platform for transparent and collaborative proposal development that displays threads of comments, responses, and revisions, and allows submissions to be “liked.” Open Proposals is managed by the UCSF Clinical and Translational Science Institute, funded by the National Center for Advancing Translational Sciences (Grant Number UL1 TR000004) at the National Institutes of Health. Using institutional e-mail lists for faculty, staff and residents, as well as described at monthly managers and directors meetings, the Ideas Contest is announced each year by the Chief Medical Officer and the CHV leadership. The Caring Wisely® Executive Steering Committee, which consists of CHV and senior UCSF Health system leaders, selects the top 5-10 ideas based on the above criteria. Each winning idea receives a $100 gift certificate for a popular restaurant in San Francisco, and the list of winners is announced to the entire UCSF community.
The second stage is the Request for Proposals. The Caring Wisely® program solicits proposals that outline implementation plans to target specific areas identified through the Ideas Contest. Finalists from the Ideas Contest are encouraged to submit proposals that address the problem they identified, but anyone affiliated with UCSF Health may submit a proposal on a winning idea. There is an approximately 4-week open submission period during which applicants submit brief 2-page proposals on the Open Proposal platform. This is followed by a period of optimization that leverages the social media aspect of the Open Proposals platform in which the UCSF Health community asks clarifying questions, make suggestions, and modifications can be made to the proposals. All submissions receive written feedback from at least one Steering Committee member. In addition, the Caring Wisely® Director directly invites relevant UCSF colleagues, administrators, or program leaders to comment on proposals and make suggestions for improvement. Plans for assessing financial and health care delivery impacts are developed in collaboration with the UCSF Health Finance department. UCSF Health managers and leaders who are stakeholders in project proposal areas are consulted to provide input and finalize proposal plans, including the identification of existing personnel who can support and drive the project forward. Proposers use this feedback to revise their applications throughout this stage.
The third stage is Project Implementation. The Caring Wisely® Executive Steering Committee selects up to 3 winners from the submitted proposals. Using the program criteria above, each project is scored independently, discussed in committee, and rescored to identify the top proposals. Each selected project receives a maximum budget of $50,000 that can be used for project materials, activities, and salary support for project leaders or staff. In addition to funding, each project team receives input from the implementation science team to co-develop and implement the intervention with a goal of creating a first-test-of-change within 3-6 months. A key feature of Caring Wisely® is the partnership between project teams and the Caring Wisely® implementation team, which includes a director, program manager, data analysts, and implementation scientists (Table 1).
The $150,000 administrative budget for the Caring Wisely® program provides 20% support of the medical director, 50% support of a program manager/analyst, and 10% support of an implementation scientist. Approximately 5% support is donated from additional senior implementation scientists and various UCSF Health experts based on project needs. To make most efficient use of the Caring Wisely® program staff time with the project teams, there is a weekly 60-90 minute works-in-progress session attended by all 3 teams with a rotating schedule for lead presenter during the first 6 months; these meetings occur every 2-3 weeks during the second 6 months. Caring Wisely® program staff and the implementation scientist are also available for 1:1 meetings as needed. The Caring Wisely® Executive Steering Committee is not paid and meets for 90 minutes quarterly. Custom reports and modifications of the electronic health record are provided by the UCSF Health clinical informatics department as part of their operating budget.
The collaboration between the project teams and the implementation science team is guided by the Consolidated Framework for Implementation Research (CFIR)2 and PRECEDE-PROCEED model—a logic model and evaluation tool that is based on a composite of individual behavior change theory and social ecology.3 Table 2 illustrates how we weave PRECEDE-PROCEED and Plan-Do-Study-Act frameworks into project design and strategy. Each funded team is required to submit an end-of-year progress report.
Cost and cost savings estimates were based on administrative financial data obtained through the assistance of the Decision Support Services unit of the Finance Department of UCSF Health. All costs reflect direct institutional costs, rather than charges. For some projects, costs are directly available through computerized dashboards that provide year-to-year comparisons of specific costs of materials, supplies, and services (eg, blood transfusion reduction, surgical supplies project, OR efficiency program). This same dashboard also allows calculation of CMI-adjusted direct costs of hospital care by service line, as used in the perioperative pathways program evaluation. In other cases, the Decision Support Services and/or Caring Wisely® program manager created custom cost reports based on the key performance indicator (eg, nebulizer therapy costs consist of medication costs plus respiratory therapist time; CT scan utilization for suspected pulmonary embolus in emergency department; and antimicrobial utilization for suspected neonatal sepsis).
Ongoing monitoring and sustainability of Caring Wisely® projects is supported by the Caring Wisely® program leaders. Monitoring of ongoing cost savings is based on automated service-line level dashboards related to cost, utilization, and quality outcomes with quarterly updates provided to the Caring Wisely® Steering Committee. Depending on the project or program, appropriate UCSF Health senior leaders determine the level of support within their departments that is required to sustain the program(s). Ongoing monitoring of each program is also included in the strategic deployment visibility room with regular rounding by senior health system executives.
Since 2013, there have been 3 complete Caring Wisely® cycles. The Ideas Contest generated more than 75 ideas in each of the past 3 cycles, ranging from eliminating redundant laboratory or radiological studies to reducing linen and food waste. We received between 13-20 full proposals in each of the request for proposal stages, and 9 projects have been implemented, 3 in each year. Funded projects have been led by a variety of individuals including physicians, nurses, pharmacists, administrators and residents, and topics have ranged from reducing overutilization of tests, supplies and treatments, to improving patient throughput during the perioperative period (Table 3). Estimated cumulative savings to date from Caring Wisely® projects has exceeded $4 million, based on the four projects shown in Table 4. The IV-to-PO switch program and the neonatal sepsis risk prediction project (Table 3) have been successful in reducing unnecessary utilization, but cost and savings estimates are not yet finalized. Three funded projects were equivocal in cost savings but were successful in their primary aims: (1) increasing the appropriateness of CT scan ordering for suspected pulmonary embolus; (2) shortening operating room turnover times; and (3) implementing a postoperative debrief program for the systematic documentation of safety events, waste, and inefficiencies related to surgery.
We developed an innovative program that reduces hospital costs through crowdsourcing of ideas from frontline clinicians and staff, and by connecting these ideas to project and implementation science teams. At a time when healthcare costs have reached unsustainable levels, the Caring Wisely® program provides a process for healthcare personnel to make a positive impact on healthcare costs in areas under their direct control. Through the Open Proposals platform, we have tapped a growing desire among frontline providers to reduce medical waste.
A key criterion for the Caring Wisely® program is to propose changes that reduce cost without adversely affect healthcare quality or outcomes. While this is an important consideration in selecting projects, there is limited power to detect many of the most clinically relevant outcomes. We find this acceptable because many of the sponsored Caring Wisely® project goals were to increase compliance with evidence-based practice guidelines and reduce harms associated with unnecessary treatments (eg, blood transfusion, nebulizer therapy, CT scan, antimicrobial therapy). Selected balancing metrics for each project are reported by established quality and safety programs at UCSF Health, but we acknowledge that many factors that can affect these clinical outcomes are not related to the cost-reduction intervention and are not possible to control outside of a clinical research study. Therefore, any response to changes in these outcome and balancing measures requires further analysis beyond the Caring Wisely® project alone.
We believe one of the key factors in the success of the Caring Wisely® program is the application of implementation science principles to the intervention design strategies (Table 1). These principles included stakeholder engagement, behavior change theory, market (target audience) segmentation, and process measurement and feedback. Because we are conducting this program in an academic health center, resident and fellow education and engagement are also critical to success. In each project, we utilize the PRECEDE model as a guide to ensure that each intervention design includes complementary elements of effective behavior change, intended to increase awareness and motivation to change, to make change “easy,” and to reinforce change(Table 2).3
The Caring Wisely® program—itself a multifaceted intervention—embodies the same PRECEDE dimensions we apply to each specific project. The Ideas Contest serves as a tool for increasing awareness, attitudes, and motivation across the clinical enterprise for reducing healthcare costs. The support provided to the project teams by the Caring Wisely® program is an enabling factor that makes it “easier” for frontline teams to design and implement interventions with a greater likelihood of achieving early success. Timely measurement and feedback of results to the hospital leadership and broadcasting to the larger community reinforces the support of the program at both the leadership and frontline levels.
Collaboration between project teams and the Caring Wisely® program also provides frontline clinicians and staff with practical experience and lessons that they can apply to future improvement work. Project teams learn implementation science principles such as constructing a pragmatic theoretical framework to guide implementation design using CFIR model.2 Incorporating multiple, rapid-cycle tests of change allows teams to modify and adapt final interventions as they learn how the target audience and environment responds to specific intervention components. Access to real-time, actionable data and a data analyst is essential to rapid cycle adaptation that allows teams to focus on specific units or providers. We also find that cross-fertilization between project teams working in different areas helps to share resources and minimize duplication of efforts from the clinical and staff champions. Partnering with UCSF Health system leaders at every phase of project development—from proposal selection, development, and final evaluation of results—enhances sustainable transition of successful projects into clinical operations.
The costs and coordination for the first cycle of Caring Wisely® were supported by the UCSF Center for Healthcare Value. Upon completion of the evaluation of the first cycle, UCSF Health agreed to fund the program going forward, with the expectation that Caring Wisely would continue to achieve direct cost-savings for the organization. The Caring Wisely team provides a final report each year detailing the impact of each project on utilization and associated costs. Currently, program costs are approximately $150,000 for the Caring Wisely program leaders, staff, and other resources, and $50,000 for each of 3 projects for a total program cost of $300,000 per year. Projects included in the first three cycles have already saved more than $4 million, representing a strong return on investment. This program could be a model for other academic health centers to engage frontline clinicians and staff in addressing healthcare costs, and lends itself to being scaled-up into a multi-system collaborative.
LIST OF ABBREVIATIONS
UCSF—University of California, San Francisco; PRECEDE—Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation; PROCEED—Policy, Regulatory and Organizational Constructs in Educational and Environmental Development
Acknowledgments
Other participants in blood transfusion reduction project (D. Johnson, K. Curcione); IV-to-PO Switch (C. Tsourounis, A. Pollock); Surgical Supply Cost Reduction (C. Zygourakis); Perioperative Efficiency (L. Hampson); CT for PE Risk Prediction (E. Weber); ERAS Pathways (L. Chen); Neonatal Sepsis Risk Prediction (T. Newman); Post-Operative Debrief (S. Imershein). Caring Wisely Executive Steering Committee (J. Adler, S. Antrum, A Auerbach, J. Bennan, M. Blum, C. Ritchie, C. Tsourounis). This Center for Healthcare Value is funded in part by a grant from the Grove Foundation. We appreciate additional review and comments to the manuscript provided by George Sawaya and Adams Dudley.
Disclosures
Christopher Moriates has accepted royalties from McGraw-Hill for textbook, Understanding Value-Based Healthcare. Alvin Rajkomar has received fees as a research adviser from Google, Inc.
1. Kahlon M, Yuan L, Gologorskaya O, Johnston SC. Crowdsourcing the CTSA innovation mission. Clin Transl Sci. 2014;7:89-92. PubMed
2. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. PubMed
3. Green LW and Kreuter. Health Program Planning: An Educational and Ecological Approach. 4th Ed. McGraw-Hill. New York, NY. 2005.
4. Zygourakis CC, Valencia V, Moriates C et al. Association between surgeon scorecard use and operating room costs. JAMA Surg. 2016 Dec 7. doi: 10.1001/jamasurg.2016.4674. [Epub ahead of print] PubMed
1. Kahlon M, Yuan L, Gologorskaya O, Johnston SC. Crowdsourcing the CTSA innovation mission. Clin Transl Sci. 2014;7:89-92. PubMed
2. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. PubMed
3. Green LW and Kreuter. Health Program Planning: An Educational and Ecological Approach. 4th Ed. McGraw-Hill. New York, NY. 2005.
4. Zygourakis CC, Valencia V, Moriates C et al. Association between surgeon scorecard use and operating room costs. JAMA Surg. 2016 Dec 7. doi: 10.1001/jamasurg.2016.4674. [Epub ahead of print] PubMed
Association Between DCBN and LOS
Slow hospital throughputthe process whereby a patient is admitted, placed in a room, and eventually dischargedcan worsen outcomes if admitted patients are boarded in emergency rooms or postanesthesia units.[1] One potential method to improve throughput is to discharge patients earlier in the day,[2] freeing up available beds and conceivably reducing hospital length of stay (LOS).
To quantify throughput, hospitals are beginning to measure the proportion of patients discharged before noon (DCBN). One study, looking at discharges on a single medical floor in an urban academic medical center, suggested that increasing the percentage of patients discharged by noon decreased observed‐to‐expected LOS in hospitalized medicine patients,[3] and a follow‐up study demonstrated that it was associated with admissions from the emergency department occurring earlier in the day.[4] However, these studies did not adjust for changes in case mix index (CMI) and other patient‐level characteristics that may also have affected these outcomes. Concerns persist that more efforts to discharge patients by noon could inadvertently increase LOS if staff chose to keep patients overnight for an early discharge the following day.
We undertook a retrospective analysis of data from patients discharged from a large academic medical center where an institution‐wide emphasis was placed on discharging more patients by noon. Using these data, we examined the association between discharges before noon and LOS in medical and surgical inpatients.
METHODS
Site and Subjects
Our study was based at the University of California, San Francisco (UCSF) Medical Center, a 400‐bed academic hospital located in San Francisco, California. We examined adult medical and surgical discharges from July 2012 through April 2015. Patients who stayed less than 24 hours or more than 20 days were excluded. Discharges from the hospital medicine service and the following surgical services were included in the analysis: cardiac surgery, colorectal surgery, cardiothoracic surgery, general surgery, gynecologic oncology, gynecology, neurosurgery, orthopedics, otolaryngology, head and neck surgery, plastic surgery, thoracic surgery, urology, and vascular surgery. No exclusions were made based on patient status (eg, observation vs inpatient). UCSF's institutional review board approved our study.
During the time of our study, discharges before noon time became an institutional priority. To this end, rates of DCBN were tracked using retrospective data, and various units undertook efforts such as informal afternoon meetings to prompt planning for the next morning's discharges. These efforts did not differentially affect medical or surgical units or emergent or nonemergent admissions, and no financial incentives or other changes in workflow were in place to increase DCBN rates.
Data Sources
We used the cost accounting system at UCSF (Enterprise Performance System Inc. [EPSI], Chicago, IL) to collect demographic information about each patient, including age, sex, primary race, and primary ethnicity. This system was also used to collect characteristics of each hospitalization including LOS (calculated from admission date time and discharge date time), hospital service at discharge, the discharge attending, discharge disposition of the patient, and the CMI, a marker of the severity of illness of the patient during that hospitalization. EPSI was also used to collect data on the admission type of all patients, either emergent, urgent, or routine, and the insurance status of the patient during that hospitalization.
Data on time of discharge were entered by the discharging nurse or unit assistant to reflect the time the patient left the hospital. Using these data, we defined a before‐noon discharge as one taking place between 8:00 am and 12:00 pm.
Statistical Analysis
Wilcoxon rank sum test and 2 statistics were used to compare baseline characteristics of hospitalizations of patients discharged before and after noon.
We used generalized linear models to assess the association of a discharge before noon on the LOS with gamma models. We accounted for clustering of discharge attendings using generalized estimating equations with exchangeable working correlation and robust standard errors. After the initial unadjusted analyses, covariates were included in the adjusted analysis if they were associated with an LOS at P < 0.05 or reasons of face validity. These variables are shown in Table 1. Because an effort to increase the discharges before noon was started in the 2014 academic year, we added an interaction term between the date of discharge and whether a discharge occurred before noon. The interaction term was included by dividing the study period into time periods corresponding to sequential 6‐month intervals. A new variable was defined by a categorical variable that indicated in which of these time periods a discharge occurred.
| Discharged Before Noon | Discharged After Noon | P Value | |
|---|---|---|---|
| |||
| Median LOS (IQR) | 3.4 (2.25.9) | 3.7 (2.36.3) | <0.0005 |
| Median CMI (IQR) | 1.8 (1.12.4) | 1.7 (1.12.5) | 0.006 |
| Service type, N (%) | |||
| Hospital medicine | 1,919 (29.6) | 11,290 (35.4) | |
| Surgical services | 4,565 (70.4) | 20,591 (64.6) | <0.0005 |
| Discharged before noon, N (%) | 6,484 (16.9) | 31,881 (83.1) | |
| Discharged on weekend, N (%) | |||
| Yes | 1,543 (23.8) | 7,411 (23.3) | |
| No | 4,941 (76.2) | 24,470 (76.8) | 0.34 |
| Discharge disposition, N (%) | |||
| Home with home health | 748 (11.5) | 5,774 (18.1) | |
| Home without home health | 3,997 (61.6) | 17,862 (56.0) | |
| SNF | 837 (12.9) | 3,082 (9.7) | |
| Other | 902 (13.9) | 5,163 (16.2) | <0.0005 |
| 6‐month interval, N (%) | |||
| JulyDecember 2012 | 993 (15.3) | 5,596 (17.6) | |
| JanuaryJune 2013 | 980 (15.1) | 5,721 (17.9) | |
| JulyDecember 2013 | 1,088 (16.8) | 5,690 (17.9) | |
| JanuaryJune 2014 | 1,288 (19.9) | 5,441 (17.1) | |
| JulyDecember 2014 | 1,275 (19.7) | 5,656 (17.7) | |
| JanuaryApril 2015 | 860 (13.3) | 3,777 (11.9) | <0.0005 |
| Age category, N (%) | |||
| 1864 years | 4,177 (64.4) | 20,044 (62.9) | |
| 65+ years | 2,307 (35.6) | 11,837 (37.1) | 0.02 |
| Male, N (%) | 3,274 (50.5) | 15,596 (48.9) | |
| Female, N (%) | 3,210 (49.5) | 16,284 (51.1) | 0.06 |
| Race, N (%) | |||
| White or Caucasian | 4,133 (63.7) | 18,798 (59.0) | |
| African American | 518 (8.0) | 3,020 (9.5) | |
| Asian | 703 (10.8) | 4,052 (12.7) | |
| Other | 1,130 (17.4) | 6,011 (18.9) | <0.0005 |
| Ethnicity, N (%) | |||
| Hispanic or Latino | 691 (10.7) | 3,713 (11.7) | |
| Not Hispanic or Latino | 5,597 (86.3) | 27,209 (85.4) | |
| Unknown/declined | 196 (3.0) | 959 (3.0) | 0.07 |
| Admission type, N (%) | |||
| Elective | 3,494 (53.9) | 13,881 (43.5) | |
| Emergency | 2,047 (31.6) | 12,145 (38.1) | |
| Urgent | 889 (13.7) | 5,459 (17.1) | |
| Other | 54 (0.8) | 396 (1.2) | <0.0005 |
| Payor class, N (%) | |||
| Medicare | 2,648 (40.8) | 13,808 (43.3) | |
| Medi‐Cal | 1,060 (16.4) | 5,913 (18.6) | |
| Commercial | 2,633 (40.6) | 11,242 (35.3) | |
| Other | 143 (2.2) | 918 (2.9) | <0.0005 |
We conducted a sensitivity analysis using propensity scores. The propensity score was based on demographic and clinical variables (as listed in Table 1) that exhibited P < 0.2 in bivariate analysis between the variable and being discharged before noon. We then used the propensity score as a covariate in a generalized linear model of the LOS with a gamma distribution and with generalized estimating equations as described above.
Finally, we performed prespecified secondary subset analyses of patients admitted emergently and nonemergently.
Statistical modeling and analysis was completed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
Patient Demographics and Discharge Before Noon
Our study population comprised 27,983 patients for a total of 38,365 hospitalizations with a median LOS of 3.7 days. We observed 6484 discharges before noon (16.9%) and 31,881 discharges after noon (83.1%). The characteristics of the hospitalizations are shown in Table 1.
Patients who were discharged before noon tended to be younger, white, and discharged with a disposition to home without home health. The median CMI was slightly higher in discharges before noon (1.81, P = 0.006), and elective admissions were more likely than emergent to be discharged before noon (53.9% vs 31.6%, P < 0.0005).
Multivariable Analysis
A discharge before noon was associated with a 4.3% increase in LOS (adjusted odds ratio [OR]: 1.043, 95% confidence interval [CI]: 1.003‐1.086), adjusting for CMI, the service type, discharge on the weekend, discharge disposition, age, sex, ethnicity, race, urgency of admission, payor class, and a full interaction with the date of discharge (in 6‐month intervals). In preplanned subset analyses, the association between longer LOS and DCBN was more pronounced in patients admitted emergently (adjusted OR: 1.14, 95% CI: 1.033‐1.249) and less pronounced for patients not admitted emergently (adjusted OR: 1.03, 95% CI: 0.988‐1.074), although the latter did not meet statistical significance. In patients admitted emergently, this corresponds to approximately a 12‐hour increase in LOS. The interaction term of discharge date and DCBN was significant in the model. In further subset analyses, the association between longer LOS and DCBN was more pronounced in medicine patients (adjusted OR: 1.116, 95% CI: 1.014‐1.228) than in surgical patients (adjusted OR: 1.030, 95% CI: 0.989‐1.074), although the relationship in surgical patients did not meet statistical significance.
We also undertook sensitivity analyses utilizing propensity scores as a covariate in our base multivariable models. Results from these analyses did not differ from the base models and are not presented here. Results also did not differ when comparing discharges before and after the initiation of an attending only service.
DISCUSSION AND CONCLUSION
In our retrospective study of patients discharged from an academic medical center, discharge before noon was associated with a longer LOS, with the effect more pronounced in patients admitted emergently in the hospital. Our results suggest that efforts to discharge patients earlier in the day may have varying degrees of success depending on patient characteristics. Conceivably, elective admissions recover according to predictable plans, allowing for discharges earlier in the day. In contrast, patients discharged from emergent hospitalizations may have ongoing evolution of their care plan, making plans for discharging before noon more challenging.
Our results differ from a previous study,[3] which suggested that increasing the proportion of before‐noon discharges was associated with a fall in observed‐to‐expected LOS. However, observational studies of DCBN are challenging, because the association between early discharge and LOS is potentially bidirectional. One interpretation, for example, is that patients were kept longer in order to be discharged by noon the following day, which for the subgroups of patients admitted emergently corresponded to a roughly 12‐hour increase in LOS. However, it is also plausible that patients who stayed longer also had more time to plan for an early discharge. In either scenario, the ability of managers to utilize LOS as a key metric of throughput efforts may be flawed, and suggests that alternatives (eg, number of patients waiting for beds off unit) may be a more reasonable measure of throughput. Our results have several limitations. As in any observational study, our results are vulnerable to biases from unmeasured covariates that confound the analysis. We caution that a causal relationship between a discharge before noon and LOS cannot be determined from the nature of the study. Our results are also limited in that we were unable to adjust for day‐to‐day hospital capacity and other variables that affect LOS including caregiver and transportation availability, bed capacity at receiving care facilities, and patient consent to discharge. Finally, as a single‐site study, our findings may not be applicable to nonacademic settings.
In conclusion, our observational study discerned an association between discharging patients before noon and longer LOS. We believe our findings suggest a rationale for alternate approaches to measuring an early discharge program's effectiveness, namely, that the evaluation of the success of an early discharge initiative should consider multiple evaluation metrics including the effect on emergency department wait times, intensive care unit or postanesthesia transitions, and on patient reported experiences of care transitions.
Disclosures
Andrew Auerbach, MD, is supported by a K24 grant from the National Heart, Lung, and Blood Institute: K24HL098372. The authors report no conflicts of interest.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- Centers for Medicare 2013.
- , , , et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210–214.
- , , , et al. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664–669.
Slow hospital throughputthe process whereby a patient is admitted, placed in a room, and eventually dischargedcan worsen outcomes if admitted patients are boarded in emergency rooms or postanesthesia units.[1] One potential method to improve throughput is to discharge patients earlier in the day,[2] freeing up available beds and conceivably reducing hospital length of stay (LOS).
To quantify throughput, hospitals are beginning to measure the proportion of patients discharged before noon (DCBN). One study, looking at discharges on a single medical floor in an urban academic medical center, suggested that increasing the percentage of patients discharged by noon decreased observed‐to‐expected LOS in hospitalized medicine patients,[3] and a follow‐up study demonstrated that it was associated with admissions from the emergency department occurring earlier in the day.[4] However, these studies did not adjust for changes in case mix index (CMI) and other patient‐level characteristics that may also have affected these outcomes. Concerns persist that more efforts to discharge patients by noon could inadvertently increase LOS if staff chose to keep patients overnight for an early discharge the following day.
We undertook a retrospective analysis of data from patients discharged from a large academic medical center where an institution‐wide emphasis was placed on discharging more patients by noon. Using these data, we examined the association between discharges before noon and LOS in medical and surgical inpatients.
METHODS
Site and Subjects
Our study was based at the University of California, San Francisco (UCSF) Medical Center, a 400‐bed academic hospital located in San Francisco, California. We examined adult medical and surgical discharges from July 2012 through April 2015. Patients who stayed less than 24 hours or more than 20 days were excluded. Discharges from the hospital medicine service and the following surgical services were included in the analysis: cardiac surgery, colorectal surgery, cardiothoracic surgery, general surgery, gynecologic oncology, gynecology, neurosurgery, orthopedics, otolaryngology, head and neck surgery, plastic surgery, thoracic surgery, urology, and vascular surgery. No exclusions were made based on patient status (eg, observation vs inpatient). UCSF's institutional review board approved our study.
During the time of our study, discharges before noon time became an institutional priority. To this end, rates of DCBN were tracked using retrospective data, and various units undertook efforts such as informal afternoon meetings to prompt planning for the next morning's discharges. These efforts did not differentially affect medical or surgical units or emergent or nonemergent admissions, and no financial incentives or other changes in workflow were in place to increase DCBN rates.
Data Sources
We used the cost accounting system at UCSF (Enterprise Performance System Inc. [EPSI], Chicago, IL) to collect demographic information about each patient, including age, sex, primary race, and primary ethnicity. This system was also used to collect characteristics of each hospitalization including LOS (calculated from admission date time and discharge date time), hospital service at discharge, the discharge attending, discharge disposition of the patient, and the CMI, a marker of the severity of illness of the patient during that hospitalization. EPSI was also used to collect data on the admission type of all patients, either emergent, urgent, or routine, and the insurance status of the patient during that hospitalization.
Data on time of discharge were entered by the discharging nurse or unit assistant to reflect the time the patient left the hospital. Using these data, we defined a before‐noon discharge as one taking place between 8:00 am and 12:00 pm.
Statistical Analysis
Wilcoxon rank sum test and 2 statistics were used to compare baseline characteristics of hospitalizations of patients discharged before and after noon.
We used generalized linear models to assess the association of a discharge before noon on the LOS with gamma models. We accounted for clustering of discharge attendings using generalized estimating equations with exchangeable working correlation and robust standard errors. After the initial unadjusted analyses, covariates were included in the adjusted analysis if they were associated with an LOS at P < 0.05 or reasons of face validity. These variables are shown in Table 1. Because an effort to increase the discharges before noon was started in the 2014 academic year, we added an interaction term between the date of discharge and whether a discharge occurred before noon. The interaction term was included by dividing the study period into time periods corresponding to sequential 6‐month intervals. A new variable was defined by a categorical variable that indicated in which of these time periods a discharge occurred.
| Discharged Before Noon | Discharged After Noon | P Value | |
|---|---|---|---|
| |||
| Median LOS (IQR) | 3.4 (2.25.9) | 3.7 (2.36.3) | <0.0005 |
| Median CMI (IQR) | 1.8 (1.12.4) | 1.7 (1.12.5) | 0.006 |
| Service type, N (%) | |||
| Hospital medicine | 1,919 (29.6) | 11,290 (35.4) | |
| Surgical services | 4,565 (70.4) | 20,591 (64.6) | <0.0005 |
| Discharged before noon, N (%) | 6,484 (16.9) | 31,881 (83.1) | |
| Discharged on weekend, N (%) | |||
| Yes | 1,543 (23.8) | 7,411 (23.3) | |
| No | 4,941 (76.2) | 24,470 (76.8) | 0.34 |
| Discharge disposition, N (%) | |||
| Home with home health | 748 (11.5) | 5,774 (18.1) | |
| Home without home health | 3,997 (61.6) | 17,862 (56.0) | |
| SNF | 837 (12.9) | 3,082 (9.7) | |
| Other | 902 (13.9) | 5,163 (16.2) | <0.0005 |
| 6‐month interval, N (%) | |||
| JulyDecember 2012 | 993 (15.3) | 5,596 (17.6) | |
| JanuaryJune 2013 | 980 (15.1) | 5,721 (17.9) | |
| JulyDecember 2013 | 1,088 (16.8) | 5,690 (17.9) | |
| JanuaryJune 2014 | 1,288 (19.9) | 5,441 (17.1) | |
| JulyDecember 2014 | 1,275 (19.7) | 5,656 (17.7) | |
| JanuaryApril 2015 | 860 (13.3) | 3,777 (11.9) | <0.0005 |
| Age category, N (%) | |||
| 1864 years | 4,177 (64.4) | 20,044 (62.9) | |
| 65+ years | 2,307 (35.6) | 11,837 (37.1) | 0.02 |
| Male, N (%) | 3,274 (50.5) | 15,596 (48.9) | |
| Female, N (%) | 3,210 (49.5) | 16,284 (51.1) | 0.06 |
| Race, N (%) | |||
| White or Caucasian | 4,133 (63.7) | 18,798 (59.0) | |
| African American | 518 (8.0) | 3,020 (9.5) | |
| Asian | 703 (10.8) | 4,052 (12.7) | |
| Other | 1,130 (17.4) | 6,011 (18.9) | <0.0005 |
| Ethnicity, N (%) | |||
| Hispanic or Latino | 691 (10.7) | 3,713 (11.7) | |
| Not Hispanic or Latino | 5,597 (86.3) | 27,209 (85.4) | |
| Unknown/declined | 196 (3.0) | 959 (3.0) | 0.07 |
| Admission type, N (%) | |||
| Elective | 3,494 (53.9) | 13,881 (43.5) | |
| Emergency | 2,047 (31.6) | 12,145 (38.1) | |
| Urgent | 889 (13.7) | 5,459 (17.1) | |
| Other | 54 (0.8) | 396 (1.2) | <0.0005 |
| Payor class, N (%) | |||
| Medicare | 2,648 (40.8) | 13,808 (43.3) | |
| Medi‐Cal | 1,060 (16.4) | 5,913 (18.6) | |
| Commercial | 2,633 (40.6) | 11,242 (35.3) | |
| Other | 143 (2.2) | 918 (2.9) | <0.0005 |
We conducted a sensitivity analysis using propensity scores. The propensity score was based on demographic and clinical variables (as listed in Table 1) that exhibited P < 0.2 in bivariate analysis between the variable and being discharged before noon. We then used the propensity score as a covariate in a generalized linear model of the LOS with a gamma distribution and with generalized estimating equations as described above.
Finally, we performed prespecified secondary subset analyses of patients admitted emergently and nonemergently.
Statistical modeling and analysis was completed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
Patient Demographics and Discharge Before Noon
Our study population comprised 27,983 patients for a total of 38,365 hospitalizations with a median LOS of 3.7 days. We observed 6484 discharges before noon (16.9%) and 31,881 discharges after noon (83.1%). The characteristics of the hospitalizations are shown in Table 1.
Patients who were discharged before noon tended to be younger, white, and discharged with a disposition to home without home health. The median CMI was slightly higher in discharges before noon (1.81, P = 0.006), and elective admissions were more likely than emergent to be discharged before noon (53.9% vs 31.6%, P < 0.0005).
Multivariable Analysis
A discharge before noon was associated with a 4.3% increase in LOS (adjusted odds ratio [OR]: 1.043, 95% confidence interval [CI]: 1.003‐1.086), adjusting for CMI, the service type, discharge on the weekend, discharge disposition, age, sex, ethnicity, race, urgency of admission, payor class, and a full interaction with the date of discharge (in 6‐month intervals). In preplanned subset analyses, the association between longer LOS and DCBN was more pronounced in patients admitted emergently (adjusted OR: 1.14, 95% CI: 1.033‐1.249) and less pronounced for patients not admitted emergently (adjusted OR: 1.03, 95% CI: 0.988‐1.074), although the latter did not meet statistical significance. In patients admitted emergently, this corresponds to approximately a 12‐hour increase in LOS. The interaction term of discharge date and DCBN was significant in the model. In further subset analyses, the association between longer LOS and DCBN was more pronounced in medicine patients (adjusted OR: 1.116, 95% CI: 1.014‐1.228) than in surgical patients (adjusted OR: 1.030, 95% CI: 0.989‐1.074), although the relationship in surgical patients did not meet statistical significance.
We also undertook sensitivity analyses utilizing propensity scores as a covariate in our base multivariable models. Results from these analyses did not differ from the base models and are not presented here. Results also did not differ when comparing discharges before and after the initiation of an attending only service.
DISCUSSION AND CONCLUSION
In our retrospective study of patients discharged from an academic medical center, discharge before noon was associated with a longer LOS, with the effect more pronounced in patients admitted emergently in the hospital. Our results suggest that efforts to discharge patients earlier in the day may have varying degrees of success depending on patient characteristics. Conceivably, elective admissions recover according to predictable plans, allowing for discharges earlier in the day. In contrast, patients discharged from emergent hospitalizations may have ongoing evolution of their care plan, making plans for discharging before noon more challenging.
Our results differ from a previous study,[3] which suggested that increasing the proportion of before‐noon discharges was associated with a fall in observed‐to‐expected LOS. However, observational studies of DCBN are challenging, because the association between early discharge and LOS is potentially bidirectional. One interpretation, for example, is that patients were kept longer in order to be discharged by noon the following day, which for the subgroups of patients admitted emergently corresponded to a roughly 12‐hour increase in LOS. However, it is also plausible that patients who stayed longer also had more time to plan for an early discharge. In either scenario, the ability of managers to utilize LOS as a key metric of throughput efforts may be flawed, and suggests that alternatives (eg, number of patients waiting for beds off unit) may be a more reasonable measure of throughput. Our results have several limitations. As in any observational study, our results are vulnerable to biases from unmeasured covariates that confound the analysis. We caution that a causal relationship between a discharge before noon and LOS cannot be determined from the nature of the study. Our results are also limited in that we were unable to adjust for day‐to‐day hospital capacity and other variables that affect LOS including caregiver and transportation availability, bed capacity at receiving care facilities, and patient consent to discharge. Finally, as a single‐site study, our findings may not be applicable to nonacademic settings.
In conclusion, our observational study discerned an association between discharging patients before noon and longer LOS. We believe our findings suggest a rationale for alternate approaches to measuring an early discharge program's effectiveness, namely, that the evaluation of the success of an early discharge initiative should consider multiple evaluation metrics including the effect on emergency department wait times, intensive care unit or postanesthesia transitions, and on patient reported experiences of care transitions.
Disclosures
Andrew Auerbach, MD, is supported by a K24 grant from the National Heart, Lung, and Blood Institute: K24HL098372. The authors report no conflicts of interest.
Slow hospital throughputthe process whereby a patient is admitted, placed in a room, and eventually dischargedcan worsen outcomes if admitted patients are boarded in emergency rooms or postanesthesia units.[1] One potential method to improve throughput is to discharge patients earlier in the day,[2] freeing up available beds and conceivably reducing hospital length of stay (LOS).
To quantify throughput, hospitals are beginning to measure the proportion of patients discharged before noon (DCBN). One study, looking at discharges on a single medical floor in an urban academic medical center, suggested that increasing the percentage of patients discharged by noon decreased observed‐to‐expected LOS in hospitalized medicine patients,[3] and a follow‐up study demonstrated that it was associated with admissions from the emergency department occurring earlier in the day.[4] However, these studies did not adjust for changes in case mix index (CMI) and other patient‐level characteristics that may also have affected these outcomes. Concerns persist that more efforts to discharge patients by noon could inadvertently increase LOS if staff chose to keep patients overnight for an early discharge the following day.
We undertook a retrospective analysis of data from patients discharged from a large academic medical center where an institution‐wide emphasis was placed on discharging more patients by noon. Using these data, we examined the association between discharges before noon and LOS in medical and surgical inpatients.
METHODS
Site and Subjects
Our study was based at the University of California, San Francisco (UCSF) Medical Center, a 400‐bed academic hospital located in San Francisco, California. We examined adult medical and surgical discharges from July 2012 through April 2015. Patients who stayed less than 24 hours or more than 20 days were excluded. Discharges from the hospital medicine service and the following surgical services were included in the analysis: cardiac surgery, colorectal surgery, cardiothoracic surgery, general surgery, gynecologic oncology, gynecology, neurosurgery, orthopedics, otolaryngology, head and neck surgery, plastic surgery, thoracic surgery, urology, and vascular surgery. No exclusions were made based on patient status (eg, observation vs inpatient). UCSF's institutional review board approved our study.
During the time of our study, discharges before noon time became an institutional priority. To this end, rates of DCBN were tracked using retrospective data, and various units undertook efforts such as informal afternoon meetings to prompt planning for the next morning's discharges. These efforts did not differentially affect medical or surgical units or emergent or nonemergent admissions, and no financial incentives or other changes in workflow were in place to increase DCBN rates.
Data Sources
We used the cost accounting system at UCSF (Enterprise Performance System Inc. [EPSI], Chicago, IL) to collect demographic information about each patient, including age, sex, primary race, and primary ethnicity. This system was also used to collect characteristics of each hospitalization including LOS (calculated from admission date time and discharge date time), hospital service at discharge, the discharge attending, discharge disposition of the patient, and the CMI, a marker of the severity of illness of the patient during that hospitalization. EPSI was also used to collect data on the admission type of all patients, either emergent, urgent, or routine, and the insurance status of the patient during that hospitalization.
Data on time of discharge were entered by the discharging nurse or unit assistant to reflect the time the patient left the hospital. Using these data, we defined a before‐noon discharge as one taking place between 8:00 am and 12:00 pm.
Statistical Analysis
Wilcoxon rank sum test and 2 statistics were used to compare baseline characteristics of hospitalizations of patients discharged before and after noon.
We used generalized linear models to assess the association of a discharge before noon on the LOS with gamma models. We accounted for clustering of discharge attendings using generalized estimating equations with exchangeable working correlation and robust standard errors. After the initial unadjusted analyses, covariates were included in the adjusted analysis if they were associated with an LOS at P < 0.05 or reasons of face validity. These variables are shown in Table 1. Because an effort to increase the discharges before noon was started in the 2014 academic year, we added an interaction term between the date of discharge and whether a discharge occurred before noon. The interaction term was included by dividing the study period into time periods corresponding to sequential 6‐month intervals. A new variable was defined by a categorical variable that indicated in which of these time periods a discharge occurred.
| Discharged Before Noon | Discharged After Noon | P Value | |
|---|---|---|---|
| |||
| Median LOS (IQR) | 3.4 (2.25.9) | 3.7 (2.36.3) | <0.0005 |
| Median CMI (IQR) | 1.8 (1.12.4) | 1.7 (1.12.5) | 0.006 |
| Service type, N (%) | |||
| Hospital medicine | 1,919 (29.6) | 11,290 (35.4) | |
| Surgical services | 4,565 (70.4) | 20,591 (64.6) | <0.0005 |
| Discharged before noon, N (%) | 6,484 (16.9) | 31,881 (83.1) | |
| Discharged on weekend, N (%) | |||
| Yes | 1,543 (23.8) | 7,411 (23.3) | |
| No | 4,941 (76.2) | 24,470 (76.8) | 0.34 |
| Discharge disposition, N (%) | |||
| Home with home health | 748 (11.5) | 5,774 (18.1) | |
| Home without home health | 3,997 (61.6) | 17,862 (56.0) | |
| SNF | 837 (12.9) | 3,082 (9.7) | |
| Other | 902 (13.9) | 5,163 (16.2) | <0.0005 |
| 6‐month interval, N (%) | |||
| JulyDecember 2012 | 993 (15.3) | 5,596 (17.6) | |
| JanuaryJune 2013 | 980 (15.1) | 5,721 (17.9) | |
| JulyDecember 2013 | 1,088 (16.8) | 5,690 (17.9) | |
| JanuaryJune 2014 | 1,288 (19.9) | 5,441 (17.1) | |
| JulyDecember 2014 | 1,275 (19.7) | 5,656 (17.7) | |
| JanuaryApril 2015 | 860 (13.3) | 3,777 (11.9) | <0.0005 |
| Age category, N (%) | |||
| 1864 years | 4,177 (64.4) | 20,044 (62.9) | |
| 65+ years | 2,307 (35.6) | 11,837 (37.1) | 0.02 |
| Male, N (%) | 3,274 (50.5) | 15,596 (48.9) | |
| Female, N (%) | 3,210 (49.5) | 16,284 (51.1) | 0.06 |
| Race, N (%) | |||
| White or Caucasian | 4,133 (63.7) | 18,798 (59.0) | |
| African American | 518 (8.0) | 3,020 (9.5) | |
| Asian | 703 (10.8) | 4,052 (12.7) | |
| Other | 1,130 (17.4) | 6,011 (18.9) | <0.0005 |
| Ethnicity, N (%) | |||
| Hispanic or Latino | 691 (10.7) | 3,713 (11.7) | |
| Not Hispanic or Latino | 5,597 (86.3) | 27,209 (85.4) | |
| Unknown/declined | 196 (3.0) | 959 (3.0) | 0.07 |
| Admission type, N (%) | |||
| Elective | 3,494 (53.9) | 13,881 (43.5) | |
| Emergency | 2,047 (31.6) | 12,145 (38.1) | |
| Urgent | 889 (13.7) | 5,459 (17.1) | |
| Other | 54 (0.8) | 396 (1.2) | <0.0005 |
| Payor class, N (%) | |||
| Medicare | 2,648 (40.8) | 13,808 (43.3) | |
| Medi‐Cal | 1,060 (16.4) | 5,913 (18.6) | |
| Commercial | 2,633 (40.6) | 11,242 (35.3) | |
| Other | 143 (2.2) | 918 (2.9) | <0.0005 |
We conducted a sensitivity analysis using propensity scores. The propensity score was based on demographic and clinical variables (as listed in Table 1) that exhibited P < 0.2 in bivariate analysis between the variable and being discharged before noon. We then used the propensity score as a covariate in a generalized linear model of the LOS with a gamma distribution and with generalized estimating equations as described above.
Finally, we performed prespecified secondary subset analyses of patients admitted emergently and nonemergently.
Statistical modeling and analysis was completed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
Patient Demographics and Discharge Before Noon
Our study population comprised 27,983 patients for a total of 38,365 hospitalizations with a median LOS of 3.7 days. We observed 6484 discharges before noon (16.9%) and 31,881 discharges after noon (83.1%). The characteristics of the hospitalizations are shown in Table 1.
Patients who were discharged before noon tended to be younger, white, and discharged with a disposition to home without home health. The median CMI was slightly higher in discharges before noon (1.81, P = 0.006), and elective admissions were more likely than emergent to be discharged before noon (53.9% vs 31.6%, P < 0.0005).
Multivariable Analysis
A discharge before noon was associated with a 4.3% increase in LOS (adjusted odds ratio [OR]: 1.043, 95% confidence interval [CI]: 1.003‐1.086), adjusting for CMI, the service type, discharge on the weekend, discharge disposition, age, sex, ethnicity, race, urgency of admission, payor class, and a full interaction with the date of discharge (in 6‐month intervals). In preplanned subset analyses, the association between longer LOS and DCBN was more pronounced in patients admitted emergently (adjusted OR: 1.14, 95% CI: 1.033‐1.249) and less pronounced for patients not admitted emergently (adjusted OR: 1.03, 95% CI: 0.988‐1.074), although the latter did not meet statistical significance. In patients admitted emergently, this corresponds to approximately a 12‐hour increase in LOS. The interaction term of discharge date and DCBN was significant in the model. In further subset analyses, the association between longer LOS and DCBN was more pronounced in medicine patients (adjusted OR: 1.116, 95% CI: 1.014‐1.228) than in surgical patients (adjusted OR: 1.030, 95% CI: 0.989‐1.074), although the relationship in surgical patients did not meet statistical significance.
We also undertook sensitivity analyses utilizing propensity scores as a covariate in our base multivariable models. Results from these analyses did not differ from the base models and are not presented here. Results also did not differ when comparing discharges before and after the initiation of an attending only service.
DISCUSSION AND CONCLUSION
In our retrospective study of patients discharged from an academic medical center, discharge before noon was associated with a longer LOS, with the effect more pronounced in patients admitted emergently in the hospital. Our results suggest that efforts to discharge patients earlier in the day may have varying degrees of success depending on patient characteristics. Conceivably, elective admissions recover according to predictable plans, allowing for discharges earlier in the day. In contrast, patients discharged from emergent hospitalizations may have ongoing evolution of their care plan, making plans for discharging before noon more challenging.
Our results differ from a previous study,[3] which suggested that increasing the proportion of before‐noon discharges was associated with a fall in observed‐to‐expected LOS. However, observational studies of DCBN are challenging, because the association between early discharge and LOS is potentially bidirectional. One interpretation, for example, is that patients were kept longer in order to be discharged by noon the following day, which for the subgroups of patients admitted emergently corresponded to a roughly 12‐hour increase in LOS. However, it is also plausible that patients who stayed longer also had more time to plan for an early discharge. In either scenario, the ability of managers to utilize LOS as a key metric of throughput efforts may be flawed, and suggests that alternatives (eg, number of patients waiting for beds off unit) may be a more reasonable measure of throughput. Our results have several limitations. As in any observational study, our results are vulnerable to biases from unmeasured covariates that confound the analysis. We caution that a causal relationship between a discharge before noon and LOS cannot be determined from the nature of the study. Our results are also limited in that we were unable to adjust for day‐to‐day hospital capacity and other variables that affect LOS including caregiver and transportation availability, bed capacity at receiving care facilities, and patient consent to discharge. Finally, as a single‐site study, our findings may not be applicable to nonacademic settings.
In conclusion, our observational study discerned an association between discharging patients before noon and longer LOS. We believe our findings suggest a rationale for alternate approaches to measuring an early discharge program's effectiveness, namely, that the evaluation of the success of an early discharge initiative should consider multiple evaluation metrics including the effect on emergency department wait times, intensive care unit or postanesthesia transitions, and on patient reported experiences of care transitions.
Disclosures
Andrew Auerbach, MD, is supported by a K24 grant from the National Heart, Lung, and Blood Institute: K24HL098372. The authors report no conflicts of interest.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- Centers for Medicare 2013.
- , , , et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210–214.
- , , , et al. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664–669.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- Centers for Medicare 2013.
- , , , et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210–214.
- , , , et al. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664–669.
© 2015 Society of Hospital Medicine




