User login
Improving Healthcare Value: Effectiveness of a Program to Reduce Laboratory Testing for Non-Critically-Ill Patients With COVID-19
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
© 2021 Society of Hospital Medicine
Hospital Buprenorphine Program for Opioid Use Disorder Is Associated With Increased Inpatient and Outpatient Addiction Treatment
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
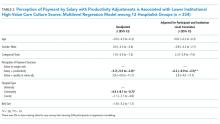
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased
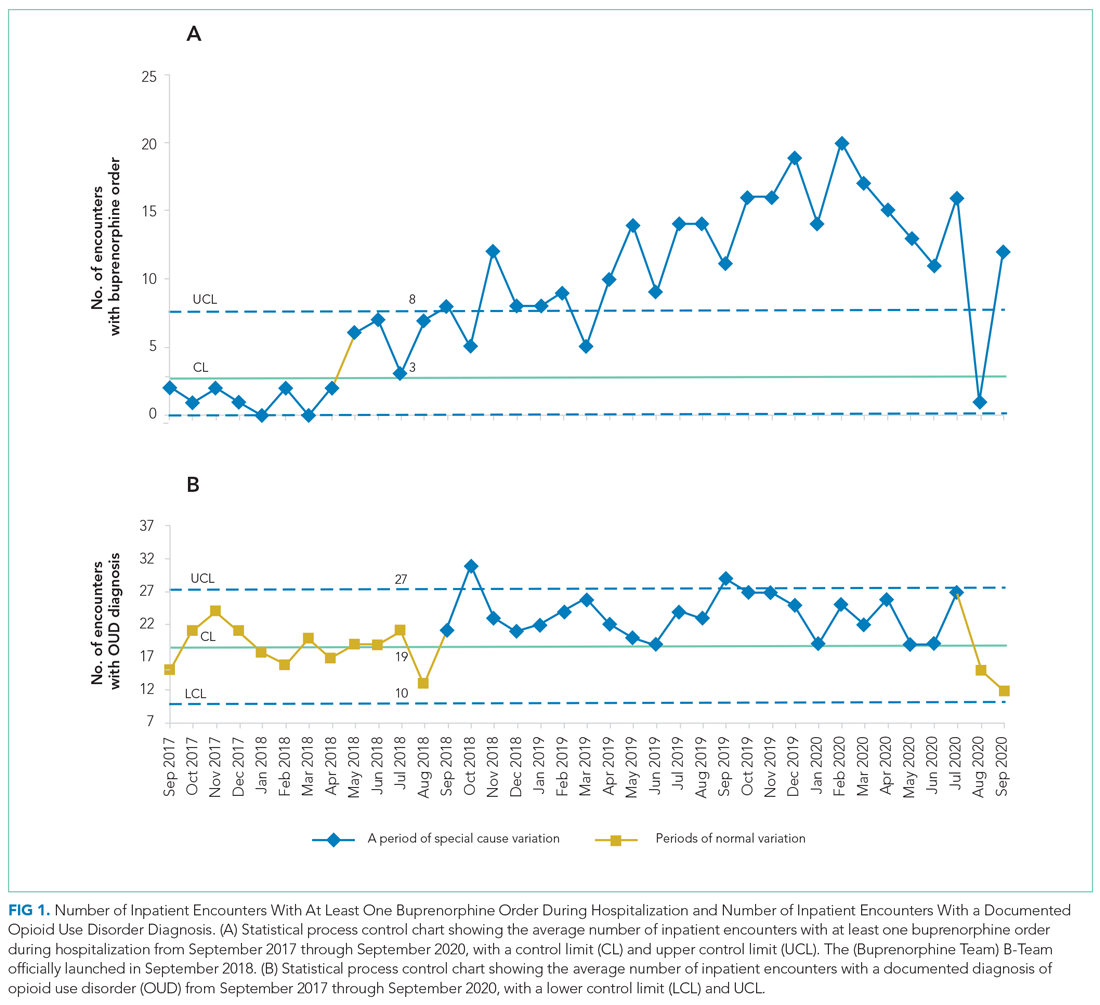
The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).
Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased

The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).

Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased

The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).

Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
© 2021 Society of Hospital Medicine
Stop checking routine lipid panels every year
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
Is COVID-19 accelerating progress toward high-value care?
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
Deimplementation: Discontinuing Low-Value, Potentially Harmful Hospital Care
Nearly 30% of healthcare spending may relate to overuse of unnecessary medical interventions.1 Deimplementation of such practices can reduce negative outcomes and unnecessary costs.2 Nonetheless, changing practice is difficult. Why is it so hard to stop doing things that don’t work? A variety of factors influences deimplementation, and research aiming to identify and understand these factors can promote the delivery of more appropriate care.2
In this issue, Wolk et al describe barriers and facilitators in deimplementing non-guideline adherent use of continuous pulse oximetry (CPO) in pediatric patients with bronchiolitis not requiring supplemental oxygen.3 Unnecessary CPO use for these patients is associated with increased hospitalization rates, length of stay, alarm fatigue, and costs, without evidence of improved clinical outcomes. Despite these data, many hospitals participating in the multicenter Eliminating Monitor Overuse study struggled to decrease CPO usage. The authors conducted semistructured interviews with a broad range of stakeholders from 12 hospitals, representing a variety of institutions with low and high CPO utilization rates.
Specific barriers to deimplementation included institutional factors, eg, unclear or missing guidelines, a culture of high utilization, and challenges educating medical staff. Perceived parental discomfort with stopping CPO was also observed. Four key facilitators were noted: strong institutional leadership, evidence-based guidelines, electronic health record order sets or reminders, and clear institutional policy. These results are similar to other deimplementation studies.
A commonality to deimplementation studies is the difficulty of changing practice. Much like implementation, deimplementation requires multipronged approaches that are sensitive to contextual factors. Interventions must account for local conditions, such as resource availability, practice norms, current workflows and processes of care, relationships among clinicians, and leadership, to create feasible and sustainable change.
Deimplementation may be even more challenging than implementation of new practices, however, because of loss aversion—the tendency to prefer avoiding losses to acquiring equivalent gains. “Taking away” something that clinicians are used to, even when proven to not be helpful, can feel uncomfortable, hindering adoption. Rather than simply discontinuing a practice, replacing it with a better option may help to overcome behavioral inertia and motivate change.
Underscoring the importance of local influences, clinicians often respond more to their close colleagues’ practices than to knowledge of national guidelines. Leveraging existing peer networks can facilitate collaboration, learning, and behavior change.4 Nudge strategies, in which local contexts are primed to promote desired behaviors, are also increasingly used.4 Priming has been effective in deimplementation efforts in medication prescribing and diagnostic testing.4
Including patients’ and families’ perspectives in deimplementation research is critical to practice change. Because diagnostic and treatment plans occur in the context of collaborative decision-making with patients, caregivers, and families, these groups are critical to engage in deimplementation efforts.
Hospitalists’ efforts at the front line of improvement require us to become more proficient in not only adopting evidence-based practices, but also in discontinuing ineffective ones. Identifying what we should stop doing is only the first step. Deimplementation is critical to this effort. Wolk et al provide insights into factors that influence deimplementation success. However, more work is needed, particularly regarding adapting approaches to local contexts, minimizing perceived loss, leveraging local conditions to shape behavior, and partnering with patients and families to achieve higher-value care.
1. Brownlee S, Chalkidou K, Doust J, at al. Evidence for overuse of medical services around the world. Lancet. 2017;390(10090):156-168. https://doi.org/10.1016/S0140-6736(16)32585-5
2. Norton WE, Chambers DA. Unpacking the complexities of de-implementing inappropriate health interventions. Implement Sci. 2020;15(1):2. https://doi.org/10.1186/s13012-019-0960-9
3. Wolk CB, Schondelmeyer AC, Barg FK, et al. Barriers and facilitators to guideline-adherent pulse oximetry use in bronchiolitis. J Hosp Med. 2021;16:23-30. https://doi.org/10.12788/jhm.3535
4 Yoong SL, Hall A, Stacey F, et al. Nudge strategies to improve healthcare providers’ implementation of evidence-based guidelines, policies and practices: a systematic review of trials included within Cochrane systematic reviews. Implement Sci. 2020;15(1):50. https://doi.org/10.1186/s13012-020-01011-0
Nearly 30% of healthcare spending may relate to overuse of unnecessary medical interventions.1 Deimplementation of such practices can reduce negative outcomes and unnecessary costs.2 Nonetheless, changing practice is difficult. Why is it so hard to stop doing things that don’t work? A variety of factors influences deimplementation, and research aiming to identify and understand these factors can promote the delivery of more appropriate care.2
In this issue, Wolk et al describe barriers and facilitators in deimplementing non-guideline adherent use of continuous pulse oximetry (CPO) in pediatric patients with bronchiolitis not requiring supplemental oxygen.3 Unnecessary CPO use for these patients is associated with increased hospitalization rates, length of stay, alarm fatigue, and costs, without evidence of improved clinical outcomes. Despite these data, many hospitals participating in the multicenter Eliminating Monitor Overuse study struggled to decrease CPO usage. The authors conducted semistructured interviews with a broad range of stakeholders from 12 hospitals, representing a variety of institutions with low and high CPO utilization rates.
Specific barriers to deimplementation included institutional factors, eg, unclear or missing guidelines, a culture of high utilization, and challenges educating medical staff. Perceived parental discomfort with stopping CPO was also observed. Four key facilitators were noted: strong institutional leadership, evidence-based guidelines, electronic health record order sets or reminders, and clear institutional policy. These results are similar to other deimplementation studies.
A commonality to deimplementation studies is the difficulty of changing practice. Much like implementation, deimplementation requires multipronged approaches that are sensitive to contextual factors. Interventions must account for local conditions, such as resource availability, practice norms, current workflows and processes of care, relationships among clinicians, and leadership, to create feasible and sustainable change.
Deimplementation may be even more challenging than implementation of new practices, however, because of loss aversion—the tendency to prefer avoiding losses to acquiring equivalent gains. “Taking away” something that clinicians are used to, even when proven to not be helpful, can feel uncomfortable, hindering adoption. Rather than simply discontinuing a practice, replacing it with a better option may help to overcome behavioral inertia and motivate change.
Underscoring the importance of local influences, clinicians often respond more to their close colleagues’ practices than to knowledge of national guidelines. Leveraging existing peer networks can facilitate collaboration, learning, and behavior change.4 Nudge strategies, in which local contexts are primed to promote desired behaviors, are also increasingly used.4 Priming has been effective in deimplementation efforts in medication prescribing and diagnostic testing.4
Including patients’ and families’ perspectives in deimplementation research is critical to practice change. Because diagnostic and treatment plans occur in the context of collaborative decision-making with patients, caregivers, and families, these groups are critical to engage in deimplementation efforts.
Hospitalists’ efforts at the front line of improvement require us to become more proficient in not only adopting evidence-based practices, but also in discontinuing ineffective ones. Identifying what we should stop doing is only the first step. Deimplementation is critical to this effort. Wolk et al provide insights into factors that influence deimplementation success. However, more work is needed, particularly regarding adapting approaches to local contexts, minimizing perceived loss, leveraging local conditions to shape behavior, and partnering with patients and families to achieve higher-value care.
Nearly 30% of healthcare spending may relate to overuse of unnecessary medical interventions.1 Deimplementation of such practices can reduce negative outcomes and unnecessary costs.2 Nonetheless, changing practice is difficult. Why is it so hard to stop doing things that don’t work? A variety of factors influences deimplementation, and research aiming to identify and understand these factors can promote the delivery of more appropriate care.2
In this issue, Wolk et al describe barriers and facilitators in deimplementing non-guideline adherent use of continuous pulse oximetry (CPO) in pediatric patients with bronchiolitis not requiring supplemental oxygen.3 Unnecessary CPO use for these patients is associated with increased hospitalization rates, length of stay, alarm fatigue, and costs, without evidence of improved clinical outcomes. Despite these data, many hospitals participating in the multicenter Eliminating Monitor Overuse study struggled to decrease CPO usage. The authors conducted semistructured interviews with a broad range of stakeholders from 12 hospitals, representing a variety of institutions with low and high CPO utilization rates.
Specific barriers to deimplementation included institutional factors, eg, unclear or missing guidelines, a culture of high utilization, and challenges educating medical staff. Perceived parental discomfort with stopping CPO was also observed. Four key facilitators were noted: strong institutional leadership, evidence-based guidelines, electronic health record order sets or reminders, and clear institutional policy. These results are similar to other deimplementation studies.
A commonality to deimplementation studies is the difficulty of changing practice. Much like implementation, deimplementation requires multipronged approaches that are sensitive to contextual factors. Interventions must account for local conditions, such as resource availability, practice norms, current workflows and processes of care, relationships among clinicians, and leadership, to create feasible and sustainable change.
Deimplementation may be even more challenging than implementation of new practices, however, because of loss aversion—the tendency to prefer avoiding losses to acquiring equivalent gains. “Taking away” something that clinicians are used to, even when proven to not be helpful, can feel uncomfortable, hindering adoption. Rather than simply discontinuing a practice, replacing it with a better option may help to overcome behavioral inertia and motivate change.
Underscoring the importance of local influences, clinicians often respond more to their close colleagues’ practices than to knowledge of national guidelines. Leveraging existing peer networks can facilitate collaboration, learning, and behavior change.4 Nudge strategies, in which local contexts are primed to promote desired behaviors, are also increasingly used.4 Priming has been effective in deimplementation efforts in medication prescribing and diagnostic testing.4
Including patients’ and families’ perspectives in deimplementation research is critical to practice change. Because diagnostic and treatment plans occur in the context of collaborative decision-making with patients, caregivers, and families, these groups are critical to engage in deimplementation efforts.
Hospitalists’ efforts at the front line of improvement require us to become more proficient in not only adopting evidence-based practices, but also in discontinuing ineffective ones. Identifying what we should stop doing is only the first step. Deimplementation is critical to this effort. Wolk et al provide insights into factors that influence deimplementation success. However, more work is needed, particularly regarding adapting approaches to local contexts, minimizing perceived loss, leveraging local conditions to shape behavior, and partnering with patients and families to achieve higher-value care.
1. Brownlee S, Chalkidou K, Doust J, at al. Evidence for overuse of medical services around the world. Lancet. 2017;390(10090):156-168. https://doi.org/10.1016/S0140-6736(16)32585-5
2. Norton WE, Chambers DA. Unpacking the complexities of de-implementing inappropriate health interventions. Implement Sci. 2020;15(1):2. https://doi.org/10.1186/s13012-019-0960-9
3. Wolk CB, Schondelmeyer AC, Barg FK, et al. Barriers and facilitators to guideline-adherent pulse oximetry use in bronchiolitis. J Hosp Med. 2021;16:23-30. https://doi.org/10.12788/jhm.3535
4 Yoong SL, Hall A, Stacey F, et al. Nudge strategies to improve healthcare providers’ implementation of evidence-based guidelines, policies and practices: a systematic review of trials included within Cochrane systematic reviews. Implement Sci. 2020;15(1):50. https://doi.org/10.1186/s13012-020-01011-0
1. Brownlee S, Chalkidou K, Doust J, at al. Evidence for overuse of medical services around the world. Lancet. 2017;390(10090):156-168. https://doi.org/10.1016/S0140-6736(16)32585-5
2. Norton WE, Chambers DA. Unpacking the complexities of de-implementing inappropriate health interventions. Implement Sci. 2020;15(1):2. https://doi.org/10.1186/s13012-019-0960-9
3. Wolk CB, Schondelmeyer AC, Barg FK, et al. Barriers and facilitators to guideline-adherent pulse oximetry use in bronchiolitis. J Hosp Med. 2021;16:23-30. https://doi.org/10.12788/jhm.3535
4 Yoong SL, Hall A, Stacey F, et al. Nudge strategies to improve healthcare providers’ implementation of evidence-based guidelines, policies and practices: a systematic review of trials included within Cochrane systematic reviews. Implement Sci. 2020;15(1):50. https://doi.org/10.1186/s13012-020-01011-0
© 2021 Society of Hospital Medicine
Email: shradhakulk@gmail.com;Telephone: 415-476-1742; Twitter: @shradhakulk.
Hospital Medicine Update: High-Impact Literature from March 2018 to April 2019
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
© 2019 Society of Hospital Medicine
Tackling the Minimizers Hiding Behind High-Value Care
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
© 2019 Society of Hospital Medicine
Association between Hospitalist Productivity Payments and High-Value Care Culture
The Centers of Medicare and Medicaid Services (CMS) has introduced new payment models that tie quality and value incentives to 90% of fee-for-service payments and provide 50% of Medicare payments through alternative payment models.1 The push toward value comes after productivity-based physician reimbursement (ie, fee for service) has been associated with poor quality care, including delayed diagnoses, complications, readmissions, increased length of stay, and high costs of care.2-5 The method of physician payment is widely believed to affect clinical behavior by incentivizing doing more, coding for more, and billing for more.6-7 Although payment systems may be used to achieve policy objectives,8 little is known about the association of different payment systems with the culture of delivering value-based care among frontline clinicians.
Culture is defined as a system of shared assumptions, values, beliefs, and norms within an environment and has a powerful role in shaping clinician practice patterns.9-12 The culture within medicine currently contributes to the overuse of resources,11,13 and a culture for improvement is correlated with clinical outcomes. A systematic review found a consistent association between positive organization culture and improved outcomes including mortality.14 Across health systems, institutions with high scores on patient safety culture surveys have shown improvements in clinical behaviors and patient outcomes.15-18
In this study, we aim to describe high-value care culture among internal medicine hospitalists across diverse hospitals and evaluate the relationship between physician reimbursement and high-value care culture.
METHODS
Study Design
This study is an observational, cross-sectional survey-based study of hospitalists from 12 hospitals in California between January and June 2016.
Study Population
A total of 12 hospitals with hospitalist programs in California were chosen to represent three types of hospitals (ie, four university, four community, and four safety net). Safety-net hospitals, which traditionally serve low-income and medically and socially vulnerable patients were defined as those in the top quartile (ie, greater than 0.5) of their Disproportionate Share Index (DSH), which measures Medicaid patient load.19-20
To select hospitals with varying value-based care performance, we stratified using CMS value-based purchasing (VBP) scores from fiscal year 2015; these scores have been used to adjust reimbursement for just over 3,000 hospitals in the VBP program of CMS.22,23 CMS calculates the VBP total performance score as a composite of four domains: (1) clinical processes of care (20% of total performance); (2) patient satisfaction (30%); (3) patient outcomes, including mortality and complications (30%); and (4) cost defined by Medicare payment per beneficiary (20%).21 Established quality measures are based on data reported by participating hospitals and chart abstraction during 2011-2014.22 Although other clinical measures of care intensity have been used as proxies of value-based care,23,24 we used the measure of value that has been publically reported by the CMS VBP given its wide use and effects on reimbursements for 80% of hospitals in the CMS VBP program in 2015.25 We obtained institution-level data from the CMS VBP Program and Hospital Compare files. Each of the three types of hospitals represented institutions with low, middle, and high VBP performance (split in tertiles) as reported by the CMS VBP program. To increase the number of participants in tertiles with fewer hospitalists, a fourth hospital was selected for each hospital type.
We excluded individual hospitalists who primarily identified as working in subspecialty divisions and those who spent less than eight weeks during the last year providing direct patient care on inpatient internal medicine services at the studied institution.
Measurement
Hospitalists were asked to complete the High-Value Care Culture Survey (HVCCSTM), which measures the culture of value-based decision making among frontline clinicians.26 Similar to other validated surveys for the assessment of patient safety culture,27,28 the HVCCS can be used to identify target areas for improvement. The survey includes four domains: (1) leadership and health system messaging, (2) data transparency and access, (3) comfort with cost conversations, and (4) blame-free environment. This tool was developed by using a two-phase national modified Delphi process. It was evaluated at two academic centers to complete factor analysis and assess internal consistency, reliability, and validity among internal medicine hospitalists and residents. Validation included estimating product-moment correlation of overall HVCCS scores and domain scores with the CMS institutional VBP scores. HVCCS scores are standardized to a 0-100 point scale for each of the four domains and are then averaged to obtain an overall score.26
In the survey, value was defined as the quality of care provided to patients in relation to the costs required to deliver that care, and high-value care was defined as care that tried to maximize quality while minimizing costs. Quality was defined as the degree to which health services increased the likelihood of desired health outcomes that are safe, effective, patient centered, timely, equitable, and consistent with current professional knowledge. Cost was defined as the negative financial, physical, and emotional effects on patients and the health system.26
Data Analysis
We described the overall institutional mean high-value care culture and domain scores measured by the HVCCS, hospitalist demographics and training experiences, and hospital characteristics. We also described individual survey items. Descriptive statistics were stratified and compared on the basis of hospital type (ie, safety net, community, or university). We assessed the relationship between the clinician perception of reimbursement structure within their divisions and individually reported high-value care culture scores using bivariate and multilevel linear regression. We hypothesized that compared with hospitalists who were paid with salaries or wages, those who reported reimbursement with productivity adjustments may report lower HVCCS scores and those who reported reimbursement with quality or value adjustments may report higher HVCCS scores. We adjusted for physician- and hospital-level characteristics, including age, gender, and training track, and considered hospital type and size as random effects.
This study was approved by the Institutional Review Board at all 12 sites. All analyses were conducted using STATA® 13.0 (College Station, Texas).
RESULTS
Hospitalist Characteristics
A total of 255 (68.9%, 255/370) hospitalists across all sites completed the survey. Of these respondents, 135 were female (50.6%). On average, hospitalists were 39 years of age (SD 6.8), trained in categorical tracks (221; 86.7%), and had previously trained for 14.3 months at a safety-net hospital (SD 14.2). In total, 166 hospitalists (65.1%) reported being paid with salary or wages, 77 (30.2%) with salary plus productivity adjustments, and 12 (4.7%) with salary plus quality or value adjustments. Moreover, 123 (48.6%) hospitalists agreed that funding for their group depended on the volume of services they delivered. Community-based hospitalists reported higher rates of reimbursement with salary plus productivity (47; 32.0%) compared with their counterparts from university-based (24; 28.2%) and safety-net based programs (6; 26.1%). Among the three different hospital types, significant differences exist in hospitalist mean age (P < .001), gender (P = .01), and the number of months training in a safety-net hospital (P = .02; Table 1).
Hospital Characteristics
Of the 12 study sites, four from each type of hospital (ie, safety-net based, community based, and university based) and four representing each value-based purchasing performance tertile (ie, high, middle, and low) were included. Eleven (91.7%) sites were located in urban areas with an average DSH index of 0.40 (SD 0.23), case mix index of 1.97 (SD 0.28), and bed size of 435.5 (SD 146.0; Table 1).
In multilevel regression modeling across all 12 sites, hospitalists from community-based hospitalist programs reported lower mean HVCCS scores (β = −4.4, 95% CI −8.1 to −0.7; Table 2) than those from other hospital types.
High-Value Care Culture Survey Scores
The mean HVCCS score was 50.2 (SD 13.6), and mean domain scores across all sites were 65.4 (SD 15.6) for leadership and health system messaging, 32.4 (SD 22.8) for data transparency and access, 52.1 (SD 19.7) for comfort with cost conversations, and 50.7 (SD 21.4) for blame-free environment (Table 1). For the majority (two-thirds) of individual HVCCS items, more than 30% of hospitalists across all sites agreed or strongly agreed that components of a low-value care culture exist within their institutions. For example, over 80% of hospitalists reported low transparency and limited access to data (see Appendix I for complete survey responses).
Hospitalists reported different HVCCS domains as strengths or weaknesses within their institutions in accordance with hospital type. Compared with university-based and safety-net-based hospitalists, community-based hospitalists reported lower scores in having a blame-free environment (466, SD 21.8). Nearly 50% reported that the clinicians’ fear of legal repercussions affects their frequency of ordering unneeded tests or procedures, and 30% reported that individual clinicians are blamed for complications. Nearly 40% reported that clinicians are uncomfortable discussing the costs of tests or treatments with patients and reported that clinicians do not feel that physicians should discuss costs with patients. Notably, community-based hospitalists uniquely differed in how they reported components of leadership and health system messaging. Over 60% reported a work climate or role modeling supportive of delivering quality care at lower costs. Only 48%, however, reported success seen from implemented efforts, and 45% reported weighing costs in clinical decision making (Table 1, Appendix I).
University-based hospitalists had significantly higher scores in leadership and health system messaging (67.4, SD 16.9) than community-based and safety-net-based hospitalists. They reported that their institutions consider their suggestions to improve quality care at low cost (75%), openly discuss ways to deliver this care (64%), and are actively implementing projects (73%). However, only 54% reported seeing success from implemented high-value care efforts (Table 1, Appendix I).
Safety-net hospitalists reported lower scores in leadership and health system messaging (56.8, SD 10.5) than university-based and community-based hospitalists. Few hospitalists reported a work climate (26%) or role modeling (30%) that is supportive of delivering quality care at low costs, openly discusses ways to deliver this care (35%), encourages frontline clinicians to pursue improvement projects (57%), or actively implements projects (26%). They also reported higher scores in the blame-free environment domain (59.8, SD 22.3; Table 1; Appendix 1).
Productivity Adjustments and High-Value Care Culture
In multilevel regression modeling, hospitalists who reported reimbursement with salary plus productivity adjustments had a lower mean HVCCS score (β = −6.2, 95% CI −9.9 to –2.5) than those who reported payment with salary or wages alone. Further multilevel regression modeling for each HVCCS domain revealed that hospitalists who reported reimbursement with salary plus productivity adjustments had lower scores in the leadership and health system messaging domain (β = −4.9, 95% CI −9.3 to −0.6) and data transparency and access domain (β = −10.7, 95% CI −16.7 to −4.6). No statistically significant difference was found between hospitalists who reported reimbursement with quality or value adjustments.
DISCUSSION
Understanding the drivers that are associated with a high-value care culture is necessary as payment models for hospitals transition from volume-based to value-based care. In this study, we found a meaningful association (β = −6.2) between clinician reimbursement schemes and measures of high-value care culture. A six-point change in the HVCCS score would correspond with a hospital moving from the top quartile to the median, which represents a significant change in performance. The relationship between clinician reimbursement schemes and high-value care culture may be a bidirectional relationship. Fee for service, the predominant payment scheme, places pressure on clinicians to maximize volume, focus on billing, and provide reactive care.7,29 Conversely, payment schemes that avoid these incentives (ie, salary, wages, and adjustments for quality or value), especially if incentives are felt by frontline clinicians, may better align with goals for long-term health outcomes for patient populations and reduce excess visits and services.2-6,8,30-34 At the same time, hospitals with a strong high-value care culture may be more likely to introduce shared savings programs and alternative payment models than those without. Through these decisions, the leadership can play an important role in creating an environment for change.34 Similar to the study sites, hospitals in California have a higher percentage of risk-based payments than hospitals in other states (>22%)35 and may also provide incentives to promote a high-value care culture or affect local physician compensation models.
Hospitals have options in how they choose to pay their clinicians, and these decisions may have downstream effects, such as building or eroding high-value care culture among clinicians or staff. A dose-response relationship between physician compensation models and value culture is plausible (salary with productivity < salary only < salary with value incentive). However, we did not find a statistically significant difference for salary with value incentive. This result may be attributed to the relatively small sample size in this study.
Hospitals can also improve their internal processes, organizational structure, and align their institutional payment contracts with those that emphasize value over fee-for-service-based incentives to increase value in care delivery.36 The operation of hospitals is challenging when competing payment incentives are used at the same time,7 and leadership will likely achieve more success in improving a high-value care culture and value performance when all efforts, including clinician and institutional payment, are aligned.37-38
Enduring large systems redesign will require directing attention to local organizational culture. For the majority of individual HVCCS items, 30% or more hospitalists across all sites agreed or strongly agreed that components of low-value care culture exist within their institutions. This response demonstrates a lack of focus on culture to address high-value care improvement among the study sites. Division and program leaders can begin measuring culture within their groups to develop new interventions that target culture change and improve value.34 No single panacea exists for the value improvement of hospitalist programs in California across all hospital types and sites.
Unique trends, however, emerge among each hospital type that could direct future improvements. In addition to all sites requiring increased transparency and access to data, community-based hospitalists identified the need for improvement in the creation of a blame-free environment, comfort with cost conversations, and aspects of leadership and health system messaging. While a high proportion of these hospitalists reported a work culture and role modeling that support the delivery of quality care at low costs, opportunities to create open discussion and frontline involvement in improvement efforts, weigh costs into clinical decision making, and cost conversations with patients exist. We hypothesize that these opportunities exist because community-based hospitals create infrastructure and technology to drive improvement that is often unseen by frontline providers. University-based hospitalists performed higher on three of the four domains compared with their counterparts but may have opportunities to promote a blame-free environment. A great proportion of these hospitalists reported the occurrence of open discussion and active projects within their institutions but also identified opportunities for the improvement of project implementation. Safety-net hospitalists reported the need to improve leadership and health system messaging across most domain items. Further study is required to evaluate reasons for safety-net hospitalists’ responses. We hypothesize that these responses may be related to having limited institutional resources to provide data and coordinated care and different institutional payment models. Each of these sites could identify trends in specific questions identified by the HVCCS for improvement in the high-value care culture.25
Our study evaluated 12 hospitalist programs in California that represent hospitals of different sizes and institutional VBP performance. A large multisite study that evaluates HVCCS across other specialties and disciplines in medicine, all regions of the country, and ambulatory care settings may be conducted in the future. Community-based hospitalist programs also reported low mean HVCCS scores, and further studies could better understand this relationship.
The limitations of the study include its small subgroup sample size and the lack of a gold standard for the measurement of high-value care. As expected, hospitalist groups among safety-net hospitals in California are small, and we may have been underpowered to determine some correlations presented by safety-net sites when stratifying by hospital type. Other correlations also may have been limited by sample size, including differences in HVCCS scores based on reimbursement and hospital type and the correlation between a blame-free environment and reimbursement type. Additionally, the field lacks a gold standard for the measurement of high-value care to help stratify institutional value performance for site selection. The VBP measure presents policy implications and is currently the best available measure with recent value data for over 3,000 hospitals nationally and representing various types of hospitals. This study is also cross-sectional and may benefit from the further evaluation of organizational culture over time and across other settings.
CONCLUSION
The HVCCS can identify clear targets for improvement and has been evaluated among internal medicine hospitalists. Hospitalists who are paid partly based on productivity reported low measures of high-value care culture at their institutions. As the nation moves toward increasingly value-based payment models, hospitals can strive to improve their understanding of their individual culture for value and begin addressing gaps.
Acknowledgments
The authors wish to thank Michael Lazarus, MD from the University of California Los Angeles; Robert Wachter, MD, James Harrison, PhD; Victoria Valencia, MPH from Dell Medical School at the University of Texas at Austin; Mithu Molla, MD from University of California Davis; Gregory Seymann, MD from the University of California San Diego; Bindu Swaroop, MD and Alpesh Amin, MD from University of California Irvine; Jessica Murphy, DO and Danny Sam, MD from Kaiser Permanente Santa Clara; Thomas Baudendistel, MD and Rajeeva Ranga, MD from Kaiser Permanente Oakland; Yile Ding, MD from California Pacific Medical Center; Soma Wali, MD from Los Angeles County/ OliveView UCLA Medical Center; Anshu Abhat, MD, MPH from the LA BioMed Institute at Los Angeles County/ Harbor-UCLA Medical Center; Steve Tringali, MD from Community Regional Medical Center Fresno; and Dan Dworsky, MD from Scripps Green Hospital for their site leadership and participation with the study.
Disclosures
Dr. Gupta is the Director of the Teaching Value in Healthcare Learning Network at Costs of Care. Dr. Moriates receives royalties from McGraw Hill for the textbook “Understanding Value-based Healthcare” outside of the submitted work and is the Director of Implementation at Costs of Care.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13. Colla CH. Swimming against the current—what might work to reduce low-value care? N Engl J Med. 2014;371(14):1280-1283. doi: 10.1056/NEJMp1404503. PubMed
14.
15.
16. Singer S, Lin S, Falwell A, Gaba D, Baker L. Relationship of safety climate and safety performance in hospitals. Health Serv Res. 2009;44(2 Pt 1):399-421. doi: 10.1111/j.1475-6773.2008.00918.x. PubMed
17.
18. Berry JC, Davis JT, Bartman T, et al. Improved safety culture and teamwork climate are associated with decreases in patient harm and hospital mortality across a hospital system. J Patient Saf. 2016. doi: 10.1097/PTS.0000000000000251. PubMed
19. Chatterjee P, Joynt KE, Orav EJ, Jha AK. Patient experience in safety-net hospitals: implications for improving care and value-based purchasing. Arch Intern Med. 2012;172(16):1204-1210. doi: 10.1001/archinternmed.2012.3158. PubMed
20. Centers for Medicare and Medicaid Services, Disproportionate Share Hospital (DSH). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/dsh.html. Accessed May 1, 2018.
21.
22. Center for Medicare and Medicaid Services, Medicare Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html?redirect=/Hospital-Value-Based-Purchasing/. Accessed May 1, 2018.
23. Sexton JB, Helmreich RL, Neilands TB, et al. The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. BMC Health Serv Res. 2006;6:44. doi: 10.1186/1472-6963-6-44. PubMed
24. Singla AK, Kitch BT, Weissman JS, Campbell EG. Assessing patient safety culture. J Patient Saf. 2006;2(3):105-115. doi: 10.1097/01.jps.0000235388.39149.5a.
25. Centers for Medicare and Medicaid Services, HHS, Medicare Program. Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(26):490-547.
26. Gupta R, Moriates C, Clarke R, et al. Development of a high-value care culture survey: a modified Delphi process and psychometric evaluation. BMJ Qual Saf. 2016:1-9. http://dx.doi.org/10.1136/bmjqs-2016-005612 PubMed
27. Centers for Medicare and Medicaid Services. Medicare program; Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(88):26490-26547.
28. Arora A, True A, Dartmouth Atlas of Health Care. What Kind of Physician Will You Be? Variation in Health Care and Its Importance for Residency Training. Dartmouth Institute for Health Policy and Clinical Practice; 2012.
29. Berenson RA, Rich EC. US approaches to physician payment: the deconstruction of primary care. J Gen Intern Med. 2010;25(6):613-618. doi: 10.1007/s11606-010-1295-z. PubMed
30. Rosenthal MB, Dudley RA. Pay-for-performance: will the latest payment trend improve care? JAMA: the Journal of the American Medical Association. 1997;297(7):740-744. doi: 10.1001/jama.297.7.740 PubMed
31. Smith M, Saunders SM, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: the Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; May 10, 2013. PubMed
32. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: Back to the Future? JAMA. 2016;315(1):23-24. doi: 10.1001/jama.2015.17029. PubMed
33. Sinsky CA, Sinsk TA. Lessons from CareMore: A stepping stone to stronger primary care of frail elderly patients. Am J Manag Care. 2015;3(2):2-3.
34. Gupta R, Moriates C. Swimming upstream: creating a culture of high value care. Acad Med. 2016:1-4. doi: 10.1097/ACM.0000000000001485 PubMed
35. Berkeley Forum. California’s delivery system integration and payment system. http://berkeleyhealthcareforum.berkeley.edu/wp-content/uploads/Appendix-II.-California%E2%80%99s-Delivery-System-Integration-and-Payment-System-Methodology.pdf. Accessed July 15, 2018; April 2013.
36. Miller HD. From volume to value: better ways to pay for health care. Health Aff. 2009;28(5):1418-1428. doi: 10.1377/hlthaff.28.5.1418. PubMed
37. Kahn CN, III. Payment reform alone will not transform health care delivery. Health Aff. 2009;28(2):w216-w218. doi: 10.1377/hlthaff.28.2.w216. PubMed
38.
The Centers of Medicare and Medicaid Services (CMS) has introduced new payment models that tie quality and value incentives to 90% of fee-for-service payments and provide 50% of Medicare payments through alternative payment models.1 The push toward value comes after productivity-based physician reimbursement (ie, fee for service) has been associated with poor quality care, including delayed diagnoses, complications, readmissions, increased length of stay, and high costs of care.2-5 The method of physician payment is widely believed to affect clinical behavior by incentivizing doing more, coding for more, and billing for more.6-7 Although payment systems may be used to achieve policy objectives,8 little is known about the association of different payment systems with the culture of delivering value-based care among frontline clinicians.
Culture is defined as a system of shared assumptions, values, beliefs, and norms within an environment and has a powerful role in shaping clinician practice patterns.9-12 The culture within medicine currently contributes to the overuse of resources,11,13 and a culture for improvement is correlated with clinical outcomes. A systematic review found a consistent association between positive organization culture and improved outcomes including mortality.14 Across health systems, institutions with high scores on patient safety culture surveys have shown improvements in clinical behaviors and patient outcomes.15-18
In this study, we aim to describe high-value care culture among internal medicine hospitalists across diverse hospitals and evaluate the relationship between physician reimbursement and high-value care culture.
METHODS
Study Design
This study is an observational, cross-sectional survey-based study of hospitalists from 12 hospitals in California between January and June 2016.
Study Population
A total of 12 hospitals with hospitalist programs in California were chosen to represent three types of hospitals (ie, four university, four community, and four safety net). Safety-net hospitals, which traditionally serve low-income and medically and socially vulnerable patients were defined as those in the top quartile (ie, greater than 0.5) of their Disproportionate Share Index (DSH), which measures Medicaid patient load.19-20
To select hospitals with varying value-based care performance, we stratified using CMS value-based purchasing (VBP) scores from fiscal year 2015; these scores have been used to adjust reimbursement for just over 3,000 hospitals in the VBP program of CMS.22,23 CMS calculates the VBP total performance score as a composite of four domains: (1) clinical processes of care (20% of total performance); (2) patient satisfaction (30%); (3) patient outcomes, including mortality and complications (30%); and (4) cost defined by Medicare payment per beneficiary (20%).21 Established quality measures are based on data reported by participating hospitals and chart abstraction during 2011-2014.22 Although other clinical measures of care intensity have been used as proxies of value-based care,23,24 we used the measure of value that has been publically reported by the CMS VBP given its wide use and effects on reimbursements for 80% of hospitals in the CMS VBP program in 2015.25 We obtained institution-level data from the CMS VBP Program and Hospital Compare files. Each of the three types of hospitals represented institutions with low, middle, and high VBP performance (split in tertiles) as reported by the CMS VBP program. To increase the number of participants in tertiles with fewer hospitalists, a fourth hospital was selected for each hospital type.
We excluded individual hospitalists who primarily identified as working in subspecialty divisions and those who spent less than eight weeks during the last year providing direct patient care on inpatient internal medicine services at the studied institution.
Measurement
Hospitalists were asked to complete the High-Value Care Culture Survey (HVCCSTM), which measures the culture of value-based decision making among frontline clinicians.26 Similar to other validated surveys for the assessment of patient safety culture,27,28 the HVCCS can be used to identify target areas for improvement. The survey includes four domains: (1) leadership and health system messaging, (2) data transparency and access, (3) comfort with cost conversations, and (4) blame-free environment. This tool was developed by using a two-phase national modified Delphi process. It was evaluated at two academic centers to complete factor analysis and assess internal consistency, reliability, and validity among internal medicine hospitalists and residents. Validation included estimating product-moment correlation of overall HVCCS scores and domain scores with the CMS institutional VBP scores. HVCCS scores are standardized to a 0-100 point scale for each of the four domains and are then averaged to obtain an overall score.26
In the survey, value was defined as the quality of care provided to patients in relation to the costs required to deliver that care, and high-value care was defined as care that tried to maximize quality while minimizing costs. Quality was defined as the degree to which health services increased the likelihood of desired health outcomes that are safe, effective, patient centered, timely, equitable, and consistent with current professional knowledge. Cost was defined as the negative financial, physical, and emotional effects on patients and the health system.26
Data Analysis
We described the overall institutional mean high-value care culture and domain scores measured by the HVCCS, hospitalist demographics and training experiences, and hospital characteristics. We also described individual survey items. Descriptive statistics were stratified and compared on the basis of hospital type (ie, safety net, community, or university). We assessed the relationship between the clinician perception of reimbursement structure within their divisions and individually reported high-value care culture scores using bivariate and multilevel linear regression. We hypothesized that compared with hospitalists who were paid with salaries or wages, those who reported reimbursement with productivity adjustments may report lower HVCCS scores and those who reported reimbursement with quality or value adjustments may report higher HVCCS scores. We adjusted for physician- and hospital-level characteristics, including age, gender, and training track, and considered hospital type and size as random effects.
This study was approved by the Institutional Review Board at all 12 sites. All analyses were conducted using STATA® 13.0 (College Station, Texas).
RESULTS
Hospitalist Characteristics
A total of 255 (68.9%, 255/370) hospitalists across all sites completed the survey. Of these respondents, 135 were female (50.6%). On average, hospitalists were 39 years of age (SD 6.8), trained in categorical tracks (221; 86.7%), and had previously trained for 14.3 months at a safety-net hospital (SD 14.2). In total, 166 hospitalists (65.1%) reported being paid with salary or wages, 77 (30.2%) with salary plus productivity adjustments, and 12 (4.7%) with salary plus quality or value adjustments. Moreover, 123 (48.6%) hospitalists agreed that funding for their group depended on the volume of services they delivered. Community-based hospitalists reported higher rates of reimbursement with salary plus productivity (47; 32.0%) compared with their counterparts from university-based (24; 28.2%) and safety-net based programs (6; 26.1%). Among the three different hospital types, significant differences exist in hospitalist mean age (P < .001), gender (P = .01), and the number of months training in a safety-net hospital (P = .02; Table 1).
Hospital Characteristics
Of the 12 study sites, four from each type of hospital (ie, safety-net based, community based, and university based) and four representing each value-based purchasing performance tertile (ie, high, middle, and low) were included. Eleven (91.7%) sites were located in urban areas with an average DSH index of 0.40 (SD 0.23), case mix index of 1.97 (SD 0.28), and bed size of 435.5 (SD 146.0; Table 1).
In multilevel regression modeling across all 12 sites, hospitalists from community-based hospitalist programs reported lower mean HVCCS scores (β = −4.4, 95% CI −8.1 to −0.7; Table 2) than those from other hospital types.
High-Value Care Culture Survey Scores
The mean HVCCS score was 50.2 (SD 13.6), and mean domain scores across all sites were 65.4 (SD 15.6) for leadership and health system messaging, 32.4 (SD 22.8) for data transparency and access, 52.1 (SD 19.7) for comfort with cost conversations, and 50.7 (SD 21.4) for blame-free environment (Table 1). For the majority (two-thirds) of individual HVCCS items, more than 30% of hospitalists across all sites agreed or strongly agreed that components of a low-value care culture exist within their institutions. For example, over 80% of hospitalists reported low transparency and limited access to data (see Appendix I for complete survey responses).
Hospitalists reported different HVCCS domains as strengths or weaknesses within their institutions in accordance with hospital type. Compared with university-based and safety-net-based hospitalists, community-based hospitalists reported lower scores in having a blame-free environment (466, SD 21.8). Nearly 50% reported that the clinicians’ fear of legal repercussions affects their frequency of ordering unneeded tests or procedures, and 30% reported that individual clinicians are blamed for complications. Nearly 40% reported that clinicians are uncomfortable discussing the costs of tests or treatments with patients and reported that clinicians do not feel that physicians should discuss costs with patients. Notably, community-based hospitalists uniquely differed in how they reported components of leadership and health system messaging. Over 60% reported a work climate or role modeling supportive of delivering quality care at lower costs. Only 48%, however, reported success seen from implemented efforts, and 45% reported weighing costs in clinical decision making (Table 1, Appendix I).
University-based hospitalists had significantly higher scores in leadership and health system messaging (67.4, SD 16.9) than community-based and safety-net-based hospitalists. They reported that their institutions consider their suggestions to improve quality care at low cost (75%), openly discuss ways to deliver this care (64%), and are actively implementing projects (73%). However, only 54% reported seeing success from implemented high-value care efforts (Table 1, Appendix I).
Safety-net hospitalists reported lower scores in leadership and health system messaging (56.8, SD 10.5) than university-based and community-based hospitalists. Few hospitalists reported a work climate (26%) or role modeling (30%) that is supportive of delivering quality care at low costs, openly discusses ways to deliver this care (35%), encourages frontline clinicians to pursue improvement projects (57%), or actively implements projects (26%). They also reported higher scores in the blame-free environment domain (59.8, SD 22.3; Table 1; Appendix 1).
Productivity Adjustments and High-Value Care Culture
In multilevel regression modeling, hospitalists who reported reimbursement with salary plus productivity adjustments had a lower mean HVCCS score (β = −6.2, 95% CI −9.9 to –2.5) than those who reported payment with salary or wages alone. Further multilevel regression modeling for each HVCCS domain revealed that hospitalists who reported reimbursement with salary plus productivity adjustments had lower scores in the leadership and health system messaging domain (β = −4.9, 95% CI −9.3 to −0.6) and data transparency and access domain (β = −10.7, 95% CI −16.7 to −4.6). No statistically significant difference was found between hospitalists who reported reimbursement with quality or value adjustments.
DISCUSSION
Understanding the drivers that are associated with a high-value care culture is necessary as payment models for hospitals transition from volume-based to value-based care. In this study, we found a meaningful association (β = −6.2) between clinician reimbursement schemes and measures of high-value care culture. A six-point change in the HVCCS score would correspond with a hospital moving from the top quartile to the median, which represents a significant change in performance. The relationship between clinician reimbursement schemes and high-value care culture may be a bidirectional relationship. Fee for service, the predominant payment scheme, places pressure on clinicians to maximize volume, focus on billing, and provide reactive care.7,29 Conversely, payment schemes that avoid these incentives (ie, salary, wages, and adjustments for quality or value), especially if incentives are felt by frontline clinicians, may better align with goals for long-term health outcomes for patient populations and reduce excess visits and services.2-6,8,30-34 At the same time, hospitals with a strong high-value care culture may be more likely to introduce shared savings programs and alternative payment models than those without. Through these decisions, the leadership can play an important role in creating an environment for change.34 Similar to the study sites, hospitals in California have a higher percentage of risk-based payments than hospitals in other states (>22%)35 and may also provide incentives to promote a high-value care culture or affect local physician compensation models.
Hospitals have options in how they choose to pay their clinicians, and these decisions may have downstream effects, such as building or eroding high-value care culture among clinicians or staff. A dose-response relationship between physician compensation models and value culture is plausible (salary with productivity < salary only < salary with value incentive). However, we did not find a statistically significant difference for salary with value incentive. This result may be attributed to the relatively small sample size in this study.
Hospitals can also improve their internal processes, organizational structure, and align their institutional payment contracts with those that emphasize value over fee-for-service-based incentives to increase value in care delivery.36 The operation of hospitals is challenging when competing payment incentives are used at the same time,7 and leadership will likely achieve more success in improving a high-value care culture and value performance when all efforts, including clinician and institutional payment, are aligned.37-38
Enduring large systems redesign will require directing attention to local organizational culture. For the majority of individual HVCCS items, 30% or more hospitalists across all sites agreed or strongly agreed that components of low-value care culture exist within their institutions. This response demonstrates a lack of focus on culture to address high-value care improvement among the study sites. Division and program leaders can begin measuring culture within their groups to develop new interventions that target culture change and improve value.34 No single panacea exists for the value improvement of hospitalist programs in California across all hospital types and sites.
Unique trends, however, emerge among each hospital type that could direct future improvements. In addition to all sites requiring increased transparency and access to data, community-based hospitalists identified the need for improvement in the creation of a blame-free environment, comfort with cost conversations, and aspects of leadership and health system messaging. While a high proportion of these hospitalists reported a work culture and role modeling that support the delivery of quality care at low costs, opportunities to create open discussion and frontline involvement in improvement efforts, weigh costs into clinical decision making, and cost conversations with patients exist. We hypothesize that these opportunities exist because community-based hospitals create infrastructure and technology to drive improvement that is often unseen by frontline providers. University-based hospitalists performed higher on three of the four domains compared with their counterparts but may have opportunities to promote a blame-free environment. A great proportion of these hospitalists reported the occurrence of open discussion and active projects within their institutions but also identified opportunities for the improvement of project implementation. Safety-net hospitalists reported the need to improve leadership and health system messaging across most domain items. Further study is required to evaluate reasons for safety-net hospitalists’ responses. We hypothesize that these responses may be related to having limited institutional resources to provide data and coordinated care and different institutional payment models. Each of these sites could identify trends in specific questions identified by the HVCCS for improvement in the high-value care culture.25
Our study evaluated 12 hospitalist programs in California that represent hospitals of different sizes and institutional VBP performance. A large multisite study that evaluates HVCCS across other specialties and disciplines in medicine, all regions of the country, and ambulatory care settings may be conducted in the future. Community-based hospitalist programs also reported low mean HVCCS scores, and further studies could better understand this relationship.
The limitations of the study include its small subgroup sample size and the lack of a gold standard for the measurement of high-value care. As expected, hospitalist groups among safety-net hospitals in California are small, and we may have been underpowered to determine some correlations presented by safety-net sites when stratifying by hospital type. Other correlations also may have been limited by sample size, including differences in HVCCS scores based on reimbursement and hospital type and the correlation between a blame-free environment and reimbursement type. Additionally, the field lacks a gold standard for the measurement of high-value care to help stratify institutional value performance for site selection. The VBP measure presents policy implications and is currently the best available measure with recent value data for over 3,000 hospitals nationally and representing various types of hospitals. This study is also cross-sectional and may benefit from the further evaluation of organizational culture over time and across other settings.
CONCLUSION
The HVCCS can identify clear targets for improvement and has been evaluated among internal medicine hospitalists. Hospitalists who are paid partly based on productivity reported low measures of high-value care culture at their institutions. As the nation moves toward increasingly value-based payment models, hospitals can strive to improve their understanding of their individual culture for value and begin addressing gaps.
Acknowledgments
The authors wish to thank Michael Lazarus, MD from the University of California Los Angeles; Robert Wachter, MD, James Harrison, PhD; Victoria Valencia, MPH from Dell Medical School at the University of Texas at Austin; Mithu Molla, MD from University of California Davis; Gregory Seymann, MD from the University of California San Diego; Bindu Swaroop, MD and Alpesh Amin, MD from University of California Irvine; Jessica Murphy, DO and Danny Sam, MD from Kaiser Permanente Santa Clara; Thomas Baudendistel, MD and Rajeeva Ranga, MD from Kaiser Permanente Oakland; Yile Ding, MD from California Pacific Medical Center; Soma Wali, MD from Los Angeles County/ OliveView UCLA Medical Center; Anshu Abhat, MD, MPH from the LA BioMed Institute at Los Angeles County/ Harbor-UCLA Medical Center; Steve Tringali, MD from Community Regional Medical Center Fresno; and Dan Dworsky, MD from Scripps Green Hospital for their site leadership and participation with the study.
Disclosures
Dr. Gupta is the Director of the Teaching Value in Healthcare Learning Network at Costs of Care. Dr. Moriates receives royalties from McGraw Hill for the textbook “Understanding Value-based Healthcare” outside of the submitted work and is the Director of Implementation at Costs of Care.
The Centers of Medicare and Medicaid Services (CMS) has introduced new payment models that tie quality and value incentives to 90% of fee-for-service payments and provide 50% of Medicare payments through alternative payment models.1 The push toward value comes after productivity-based physician reimbursement (ie, fee for service) has been associated with poor quality care, including delayed diagnoses, complications, readmissions, increased length of stay, and high costs of care.2-5 The method of physician payment is widely believed to affect clinical behavior by incentivizing doing more, coding for more, and billing for more.6-7 Although payment systems may be used to achieve policy objectives,8 little is known about the association of different payment systems with the culture of delivering value-based care among frontline clinicians.
Culture is defined as a system of shared assumptions, values, beliefs, and norms within an environment and has a powerful role in shaping clinician practice patterns.9-12 The culture within medicine currently contributes to the overuse of resources,11,13 and a culture for improvement is correlated with clinical outcomes. A systematic review found a consistent association between positive organization culture and improved outcomes including mortality.14 Across health systems, institutions with high scores on patient safety culture surveys have shown improvements in clinical behaviors and patient outcomes.15-18
In this study, we aim to describe high-value care culture among internal medicine hospitalists across diverse hospitals and evaluate the relationship between physician reimbursement and high-value care culture.
METHODS
Study Design
This study is an observational, cross-sectional survey-based study of hospitalists from 12 hospitals in California between January and June 2016.
Study Population
A total of 12 hospitals with hospitalist programs in California were chosen to represent three types of hospitals (ie, four university, four community, and four safety net). Safety-net hospitals, which traditionally serve low-income and medically and socially vulnerable patients were defined as those in the top quartile (ie, greater than 0.5) of their Disproportionate Share Index (DSH), which measures Medicaid patient load.19-20
To select hospitals with varying value-based care performance, we stratified using CMS value-based purchasing (VBP) scores from fiscal year 2015; these scores have been used to adjust reimbursement for just over 3,000 hospitals in the VBP program of CMS.22,23 CMS calculates the VBP total performance score as a composite of four domains: (1) clinical processes of care (20% of total performance); (2) patient satisfaction (30%); (3) patient outcomes, including mortality and complications (30%); and (4) cost defined by Medicare payment per beneficiary (20%).21 Established quality measures are based on data reported by participating hospitals and chart abstraction during 2011-2014.22 Although other clinical measures of care intensity have been used as proxies of value-based care,23,24 we used the measure of value that has been publically reported by the CMS VBP given its wide use and effects on reimbursements for 80% of hospitals in the CMS VBP program in 2015.25 We obtained institution-level data from the CMS VBP Program and Hospital Compare files. Each of the three types of hospitals represented institutions with low, middle, and high VBP performance (split in tertiles) as reported by the CMS VBP program. To increase the number of participants in tertiles with fewer hospitalists, a fourth hospital was selected for each hospital type.
We excluded individual hospitalists who primarily identified as working in subspecialty divisions and those who spent less than eight weeks during the last year providing direct patient care on inpatient internal medicine services at the studied institution.
Measurement
Hospitalists were asked to complete the High-Value Care Culture Survey (HVCCSTM), which measures the culture of value-based decision making among frontline clinicians.26 Similar to other validated surveys for the assessment of patient safety culture,27,28 the HVCCS can be used to identify target areas for improvement. The survey includes four domains: (1) leadership and health system messaging, (2) data transparency and access, (3) comfort with cost conversations, and (4) blame-free environment. This tool was developed by using a two-phase national modified Delphi process. It was evaluated at two academic centers to complete factor analysis and assess internal consistency, reliability, and validity among internal medicine hospitalists and residents. Validation included estimating product-moment correlation of overall HVCCS scores and domain scores with the CMS institutional VBP scores. HVCCS scores are standardized to a 0-100 point scale for each of the four domains and are then averaged to obtain an overall score.26
In the survey, value was defined as the quality of care provided to patients in relation to the costs required to deliver that care, and high-value care was defined as care that tried to maximize quality while minimizing costs. Quality was defined as the degree to which health services increased the likelihood of desired health outcomes that are safe, effective, patient centered, timely, equitable, and consistent with current professional knowledge. Cost was defined as the negative financial, physical, and emotional effects on patients and the health system.26
Data Analysis
We described the overall institutional mean high-value care culture and domain scores measured by the HVCCS, hospitalist demographics and training experiences, and hospital characteristics. We also described individual survey items. Descriptive statistics were stratified and compared on the basis of hospital type (ie, safety net, community, or university). We assessed the relationship between the clinician perception of reimbursement structure within their divisions and individually reported high-value care culture scores using bivariate and multilevel linear regression. We hypothesized that compared with hospitalists who were paid with salaries or wages, those who reported reimbursement with productivity adjustments may report lower HVCCS scores and those who reported reimbursement with quality or value adjustments may report higher HVCCS scores. We adjusted for physician- and hospital-level characteristics, including age, gender, and training track, and considered hospital type and size as random effects.
This study was approved by the Institutional Review Board at all 12 sites. All analyses were conducted using STATA® 13.0 (College Station, Texas).
RESULTS
Hospitalist Characteristics
A total of 255 (68.9%, 255/370) hospitalists across all sites completed the survey. Of these respondents, 135 were female (50.6%). On average, hospitalists were 39 years of age (SD 6.8), trained in categorical tracks (221; 86.7%), and had previously trained for 14.3 months at a safety-net hospital (SD 14.2). In total, 166 hospitalists (65.1%) reported being paid with salary or wages, 77 (30.2%) with salary plus productivity adjustments, and 12 (4.7%) with salary plus quality or value adjustments. Moreover, 123 (48.6%) hospitalists agreed that funding for their group depended on the volume of services they delivered. Community-based hospitalists reported higher rates of reimbursement with salary plus productivity (47; 32.0%) compared with their counterparts from university-based (24; 28.2%) and safety-net based programs (6; 26.1%). Among the three different hospital types, significant differences exist in hospitalist mean age (P < .001), gender (P = .01), and the number of months training in a safety-net hospital (P = .02; Table 1).
Hospital Characteristics
Of the 12 study sites, four from each type of hospital (ie, safety-net based, community based, and university based) and four representing each value-based purchasing performance tertile (ie, high, middle, and low) were included. Eleven (91.7%) sites were located in urban areas with an average DSH index of 0.40 (SD 0.23), case mix index of 1.97 (SD 0.28), and bed size of 435.5 (SD 146.0; Table 1).
In multilevel regression modeling across all 12 sites, hospitalists from community-based hospitalist programs reported lower mean HVCCS scores (β = −4.4, 95% CI −8.1 to −0.7; Table 2) than those from other hospital types.
High-Value Care Culture Survey Scores
The mean HVCCS score was 50.2 (SD 13.6), and mean domain scores across all sites were 65.4 (SD 15.6) for leadership and health system messaging, 32.4 (SD 22.8) for data transparency and access, 52.1 (SD 19.7) for comfort with cost conversations, and 50.7 (SD 21.4) for blame-free environment (Table 1). For the majority (two-thirds) of individual HVCCS items, more than 30% of hospitalists across all sites agreed or strongly agreed that components of a low-value care culture exist within their institutions. For example, over 80% of hospitalists reported low transparency and limited access to data (see Appendix I for complete survey responses).
Hospitalists reported different HVCCS domains as strengths or weaknesses within their institutions in accordance with hospital type. Compared with university-based and safety-net-based hospitalists, community-based hospitalists reported lower scores in having a blame-free environment (466, SD 21.8). Nearly 50% reported that the clinicians’ fear of legal repercussions affects their frequency of ordering unneeded tests or procedures, and 30% reported that individual clinicians are blamed for complications. Nearly 40% reported that clinicians are uncomfortable discussing the costs of tests or treatments with patients and reported that clinicians do not feel that physicians should discuss costs with patients. Notably, community-based hospitalists uniquely differed in how they reported components of leadership and health system messaging. Over 60% reported a work climate or role modeling supportive of delivering quality care at lower costs. Only 48%, however, reported success seen from implemented efforts, and 45% reported weighing costs in clinical decision making (Table 1, Appendix I).
University-based hospitalists had significantly higher scores in leadership and health system messaging (67.4, SD 16.9) than community-based and safety-net-based hospitalists. They reported that their institutions consider their suggestions to improve quality care at low cost (75%), openly discuss ways to deliver this care (64%), and are actively implementing projects (73%). However, only 54% reported seeing success from implemented high-value care efforts (Table 1, Appendix I).
Safety-net hospitalists reported lower scores in leadership and health system messaging (56.8, SD 10.5) than university-based and community-based hospitalists. Few hospitalists reported a work climate (26%) or role modeling (30%) that is supportive of delivering quality care at low costs, openly discusses ways to deliver this care (35%), encourages frontline clinicians to pursue improvement projects (57%), or actively implements projects (26%). They also reported higher scores in the blame-free environment domain (59.8, SD 22.3; Table 1; Appendix 1).
Productivity Adjustments and High-Value Care Culture
In multilevel regression modeling, hospitalists who reported reimbursement with salary plus productivity adjustments had a lower mean HVCCS score (β = −6.2, 95% CI −9.9 to –2.5) than those who reported payment with salary or wages alone. Further multilevel regression modeling for each HVCCS domain revealed that hospitalists who reported reimbursement with salary plus productivity adjustments had lower scores in the leadership and health system messaging domain (β = −4.9, 95% CI −9.3 to −0.6) and data transparency and access domain (β = −10.7, 95% CI −16.7 to −4.6). No statistically significant difference was found between hospitalists who reported reimbursement with quality or value adjustments.
DISCUSSION
Understanding the drivers that are associated with a high-value care culture is necessary as payment models for hospitals transition from volume-based to value-based care. In this study, we found a meaningful association (β = −6.2) between clinician reimbursement schemes and measures of high-value care culture. A six-point change in the HVCCS score would correspond with a hospital moving from the top quartile to the median, which represents a significant change in performance. The relationship between clinician reimbursement schemes and high-value care culture may be a bidirectional relationship. Fee for service, the predominant payment scheme, places pressure on clinicians to maximize volume, focus on billing, and provide reactive care.7,29 Conversely, payment schemes that avoid these incentives (ie, salary, wages, and adjustments for quality or value), especially if incentives are felt by frontline clinicians, may better align with goals for long-term health outcomes for patient populations and reduce excess visits and services.2-6,8,30-34 At the same time, hospitals with a strong high-value care culture may be more likely to introduce shared savings programs and alternative payment models than those without. Through these decisions, the leadership can play an important role in creating an environment for change.34 Similar to the study sites, hospitals in California have a higher percentage of risk-based payments than hospitals in other states (>22%)35 and may also provide incentives to promote a high-value care culture or affect local physician compensation models.
Hospitals have options in how they choose to pay their clinicians, and these decisions may have downstream effects, such as building or eroding high-value care culture among clinicians or staff. A dose-response relationship between physician compensation models and value culture is plausible (salary with productivity < salary only < salary with value incentive). However, we did not find a statistically significant difference for salary with value incentive. This result may be attributed to the relatively small sample size in this study.
Hospitals can also improve their internal processes, organizational structure, and align their institutional payment contracts with those that emphasize value over fee-for-service-based incentives to increase value in care delivery.36 The operation of hospitals is challenging when competing payment incentives are used at the same time,7 and leadership will likely achieve more success in improving a high-value care culture and value performance when all efforts, including clinician and institutional payment, are aligned.37-38
Enduring large systems redesign will require directing attention to local organizational culture. For the majority of individual HVCCS items, 30% or more hospitalists across all sites agreed or strongly agreed that components of low-value care culture exist within their institutions. This response demonstrates a lack of focus on culture to address high-value care improvement among the study sites. Division and program leaders can begin measuring culture within their groups to develop new interventions that target culture change and improve value.34 No single panacea exists for the value improvement of hospitalist programs in California across all hospital types and sites.
Unique trends, however, emerge among each hospital type that could direct future improvements. In addition to all sites requiring increased transparency and access to data, community-based hospitalists identified the need for improvement in the creation of a blame-free environment, comfort with cost conversations, and aspects of leadership and health system messaging. While a high proportion of these hospitalists reported a work culture and role modeling that support the delivery of quality care at low costs, opportunities to create open discussion and frontline involvement in improvement efforts, weigh costs into clinical decision making, and cost conversations with patients exist. We hypothesize that these opportunities exist because community-based hospitals create infrastructure and technology to drive improvement that is often unseen by frontline providers. University-based hospitalists performed higher on three of the four domains compared with their counterparts but may have opportunities to promote a blame-free environment. A great proportion of these hospitalists reported the occurrence of open discussion and active projects within their institutions but also identified opportunities for the improvement of project implementation. Safety-net hospitalists reported the need to improve leadership and health system messaging across most domain items. Further study is required to evaluate reasons for safety-net hospitalists’ responses. We hypothesize that these responses may be related to having limited institutional resources to provide data and coordinated care and different institutional payment models. Each of these sites could identify trends in specific questions identified by the HVCCS for improvement in the high-value care culture.25
Our study evaluated 12 hospitalist programs in California that represent hospitals of different sizes and institutional VBP performance. A large multisite study that evaluates HVCCS across other specialties and disciplines in medicine, all regions of the country, and ambulatory care settings may be conducted in the future. Community-based hospitalist programs also reported low mean HVCCS scores, and further studies could better understand this relationship.
The limitations of the study include its small subgroup sample size and the lack of a gold standard for the measurement of high-value care. As expected, hospitalist groups among safety-net hospitals in California are small, and we may have been underpowered to determine some correlations presented by safety-net sites when stratifying by hospital type. Other correlations also may have been limited by sample size, including differences in HVCCS scores based on reimbursement and hospital type and the correlation between a blame-free environment and reimbursement type. Additionally, the field lacks a gold standard for the measurement of high-value care to help stratify institutional value performance for site selection. The VBP measure presents policy implications and is currently the best available measure with recent value data for over 3,000 hospitals nationally and representing various types of hospitals. This study is also cross-sectional and may benefit from the further evaluation of organizational culture over time and across other settings.
CONCLUSION
The HVCCS can identify clear targets for improvement and has been evaluated among internal medicine hospitalists. Hospitalists who are paid partly based on productivity reported low measures of high-value care culture at their institutions. As the nation moves toward increasingly value-based payment models, hospitals can strive to improve their understanding of their individual culture for value and begin addressing gaps.
Acknowledgments
The authors wish to thank Michael Lazarus, MD from the University of California Los Angeles; Robert Wachter, MD, James Harrison, PhD; Victoria Valencia, MPH from Dell Medical School at the University of Texas at Austin; Mithu Molla, MD from University of California Davis; Gregory Seymann, MD from the University of California San Diego; Bindu Swaroop, MD and Alpesh Amin, MD from University of California Irvine; Jessica Murphy, DO and Danny Sam, MD from Kaiser Permanente Santa Clara; Thomas Baudendistel, MD and Rajeeva Ranga, MD from Kaiser Permanente Oakland; Yile Ding, MD from California Pacific Medical Center; Soma Wali, MD from Los Angeles County/ OliveView UCLA Medical Center; Anshu Abhat, MD, MPH from the LA BioMed Institute at Los Angeles County/ Harbor-UCLA Medical Center; Steve Tringali, MD from Community Regional Medical Center Fresno; and Dan Dworsky, MD from Scripps Green Hospital for their site leadership and participation with the study.
Disclosures
Dr. Gupta is the Director of the Teaching Value in Healthcare Learning Network at Costs of Care. Dr. Moriates receives royalties from McGraw Hill for the textbook “Understanding Value-based Healthcare” outside of the submitted work and is the Director of Implementation at Costs of Care.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13. Colla CH. Swimming against the current—what might work to reduce low-value care? N Engl J Med. 2014;371(14):1280-1283. doi: 10.1056/NEJMp1404503. PubMed
14.
15.
16. Singer S, Lin S, Falwell A, Gaba D, Baker L. Relationship of safety climate and safety performance in hospitals. Health Serv Res. 2009;44(2 Pt 1):399-421. doi: 10.1111/j.1475-6773.2008.00918.x. PubMed
17.
18. Berry JC, Davis JT, Bartman T, et al. Improved safety culture and teamwork climate are associated with decreases in patient harm and hospital mortality across a hospital system. J Patient Saf. 2016. doi: 10.1097/PTS.0000000000000251. PubMed
19. Chatterjee P, Joynt KE, Orav EJ, Jha AK. Patient experience in safety-net hospitals: implications for improving care and value-based purchasing. Arch Intern Med. 2012;172(16):1204-1210. doi: 10.1001/archinternmed.2012.3158. PubMed
20. Centers for Medicare and Medicaid Services, Disproportionate Share Hospital (DSH). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/dsh.html. Accessed May 1, 2018.
21.
22. Center for Medicare and Medicaid Services, Medicare Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html?redirect=/Hospital-Value-Based-Purchasing/. Accessed May 1, 2018.
23. Sexton JB, Helmreich RL, Neilands TB, et al. The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. BMC Health Serv Res. 2006;6:44. doi: 10.1186/1472-6963-6-44. PubMed
24. Singla AK, Kitch BT, Weissman JS, Campbell EG. Assessing patient safety culture. J Patient Saf. 2006;2(3):105-115. doi: 10.1097/01.jps.0000235388.39149.5a.
25. Centers for Medicare and Medicaid Services, HHS, Medicare Program. Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(26):490-547.
26. Gupta R, Moriates C, Clarke R, et al. Development of a high-value care culture survey: a modified Delphi process and psychometric evaluation. BMJ Qual Saf. 2016:1-9. http://dx.doi.org/10.1136/bmjqs-2016-005612 PubMed
27. Centers for Medicare and Medicaid Services. Medicare program; Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(88):26490-26547.
28. Arora A, True A, Dartmouth Atlas of Health Care. What Kind of Physician Will You Be? Variation in Health Care and Its Importance for Residency Training. Dartmouth Institute for Health Policy and Clinical Practice; 2012.
29. Berenson RA, Rich EC. US approaches to physician payment: the deconstruction of primary care. J Gen Intern Med. 2010;25(6):613-618. doi: 10.1007/s11606-010-1295-z. PubMed
30. Rosenthal MB, Dudley RA. Pay-for-performance: will the latest payment trend improve care? JAMA: the Journal of the American Medical Association. 1997;297(7):740-744. doi: 10.1001/jama.297.7.740 PubMed
31. Smith M, Saunders SM, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: the Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; May 10, 2013. PubMed
32. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: Back to the Future? JAMA. 2016;315(1):23-24. doi: 10.1001/jama.2015.17029. PubMed
33. Sinsky CA, Sinsk TA. Lessons from CareMore: A stepping stone to stronger primary care of frail elderly patients. Am J Manag Care. 2015;3(2):2-3.
34. Gupta R, Moriates C. Swimming upstream: creating a culture of high value care. Acad Med. 2016:1-4. doi: 10.1097/ACM.0000000000001485 PubMed
35. Berkeley Forum. California’s delivery system integration and payment system. http://berkeleyhealthcareforum.berkeley.edu/wp-content/uploads/Appendix-II.-California%E2%80%99s-Delivery-System-Integration-and-Payment-System-Methodology.pdf. Accessed July 15, 2018; April 2013.
36. Miller HD. From volume to value: better ways to pay for health care. Health Aff. 2009;28(5):1418-1428. doi: 10.1377/hlthaff.28.5.1418. PubMed
37. Kahn CN, III. Payment reform alone will not transform health care delivery. Health Aff. 2009;28(2):w216-w218. doi: 10.1377/hlthaff.28.2.w216. PubMed
38.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13. Colla CH. Swimming against the current—what might work to reduce low-value care? N Engl J Med. 2014;371(14):1280-1283. doi: 10.1056/NEJMp1404503. PubMed
14.
15.
16. Singer S, Lin S, Falwell A, Gaba D, Baker L. Relationship of safety climate and safety performance in hospitals. Health Serv Res. 2009;44(2 Pt 1):399-421. doi: 10.1111/j.1475-6773.2008.00918.x. PubMed
17.
18. Berry JC, Davis JT, Bartman T, et al. Improved safety culture and teamwork climate are associated with decreases in patient harm and hospital mortality across a hospital system. J Patient Saf. 2016. doi: 10.1097/PTS.0000000000000251. PubMed
19. Chatterjee P, Joynt KE, Orav EJ, Jha AK. Patient experience in safety-net hospitals: implications for improving care and value-based purchasing. Arch Intern Med. 2012;172(16):1204-1210. doi: 10.1001/archinternmed.2012.3158. PubMed
20. Centers for Medicare and Medicaid Services, Disproportionate Share Hospital (DSH). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/dsh.html. Accessed May 1, 2018.
21.
22. Center for Medicare and Medicaid Services, Medicare Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html?redirect=/Hospital-Value-Based-Purchasing/. Accessed May 1, 2018.
23. Sexton JB, Helmreich RL, Neilands TB, et al. The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. BMC Health Serv Res. 2006;6:44. doi: 10.1186/1472-6963-6-44. PubMed
24. Singla AK, Kitch BT, Weissman JS, Campbell EG. Assessing patient safety culture. J Patient Saf. 2006;2(3):105-115. doi: 10.1097/01.jps.0000235388.39149.5a.
25. Centers for Medicare and Medicaid Services, HHS, Medicare Program. Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(26):490-547.
26. Gupta R, Moriates C, Clarke R, et al. Development of a high-value care culture survey: a modified Delphi process and psychometric evaluation. BMJ Qual Saf. 2016:1-9. http://dx.doi.org/10.1136/bmjqs-2016-005612 PubMed
27. Centers for Medicare and Medicaid Services. Medicare program; Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(88):26490-26547.
28. Arora A, True A, Dartmouth Atlas of Health Care. What Kind of Physician Will You Be? Variation in Health Care and Its Importance for Residency Training. Dartmouth Institute for Health Policy and Clinical Practice; 2012.
29. Berenson RA, Rich EC. US approaches to physician payment: the deconstruction of primary care. J Gen Intern Med. 2010;25(6):613-618. doi: 10.1007/s11606-010-1295-z. PubMed
30. Rosenthal MB, Dudley RA. Pay-for-performance: will the latest payment trend improve care? JAMA: the Journal of the American Medical Association. 1997;297(7):740-744. doi: 10.1001/jama.297.7.740 PubMed
31. Smith M, Saunders SM, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: the Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; May 10, 2013. PubMed
32. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: Back to the Future? JAMA. 2016;315(1):23-24. doi: 10.1001/jama.2015.17029. PubMed
33. Sinsky CA, Sinsk TA. Lessons from CareMore: A stepping stone to stronger primary care of frail elderly patients. Am J Manag Care. 2015;3(2):2-3.
34. Gupta R, Moriates C. Swimming upstream: creating a culture of high value care. Acad Med. 2016:1-4. doi: 10.1097/ACM.0000000000001485 PubMed
35. Berkeley Forum. California’s delivery system integration and payment system. http://berkeleyhealthcareforum.berkeley.edu/wp-content/uploads/Appendix-II.-California%E2%80%99s-Delivery-System-Integration-and-Payment-System-Methodology.pdf. Accessed July 15, 2018; April 2013.
36. Miller HD. From volume to value: better ways to pay for health care. Health Aff. 2009;28(5):1418-1428. doi: 10.1377/hlthaff.28.5.1418. PubMed
37. Kahn CN, III. Payment reform alone will not transform health care delivery. Health Aff. 2009;28(2):w216-w218. doi: 10.1377/hlthaff.28.2.w216. PubMed
38.
© 2019 Society of Hospital Medicine
Caring Wisely: A Program to Support Frontline Clinicians and Staff in Improving Healthcare Delivery and Reducing Costs
© 2017 Society of Hospital Medicine
Strategies are needed to empower frontline clinicians to work with organizational leadership to reduce healthcare costs and improve high-value care. Caring Wisely® is a program developed by the University of California, San Francisco’s (UCSF) Center for Healthcare Value (CHV), aimed at engaging frontline clinicians and staff, connecting them with implementation experts, and supporting the development of targeted interventions to improve value. Financial savings from the program more than cover program costs. Caring Wisely® provides an institutional model for implementing robust interventions to address areas of low-value care.
Launched in 2013, the annual Caring Wisely® program consists of 3 stages for identifying projects that meet the following criteria:
- Potential to measurably reduce UCSF Health’s costs of care without transferring costs to patients, insurers, or other providers
- Plan for ensuring that health outcomes are maintained or improved
- Envision disseminating the intervention within and beyond UCSF
- Demonstrate commitment and engagement of clinical leadership and frontline staff.
The first stage is the Ideas Contest, a UCSF Health-wide call (to learn more about UCSF Health: https://www.ucsf.edu/sites/default/files/052516_About_UCSF.pdf) to identify areas that may be targeted to reduce unnecessary services, inefficiencies, and healthcare costs. We use a crowdsourcing platform—Open Proposals—to solicit the best ideas from frontline clinicians and staff.1 Open Proposals is a secure, web-based platform for transparent and collaborative proposal development that displays threads of comments, responses, and revisions, and allows submissions to be “liked.” Open Proposals is managed by the UCSF Clinical and Translational Science Institute, funded by the National Center for Advancing Translational Sciences (Grant Number UL1 TR000004) at the National Institutes of Health. Using institutional e-mail lists for faculty, staff and residents, as well as described at monthly managers and directors meetings, the Ideas Contest is announced each year by the Chief Medical Officer and the CHV leadership. The Caring Wisely® Executive Steering Committee, which consists of CHV and senior UCSF Health system leaders, selects the top 5-10 ideas based on the above criteria. Each winning idea receives a $100 gift certificate for a popular restaurant in San Francisco, and the list of winners is announced to the entire UCSF community.
The second stage is the Request for Proposals. The Caring Wisely® program solicits proposals that outline implementation plans to target specific areas identified through the Ideas Contest. Finalists from the Ideas Contest are encouraged to submit proposals that address the problem they identified, but anyone affiliated with UCSF Health may submit a proposal on a winning idea. There is an approximately 4-week open submission period during which applicants submit brief 2-page proposals on the Open Proposal platform. This is followed by a period of optimization that leverages the social media aspect of the Open Proposals platform in which the UCSF Health community asks clarifying questions, make suggestions, and modifications can be made to the proposals. All submissions receive written feedback from at least one Steering Committee member. In addition, the Caring Wisely® Director directly invites relevant UCSF colleagues, administrators, or program leaders to comment on proposals and make suggestions for improvement. Plans for assessing financial and health care delivery impacts are developed in collaboration with the UCSF Health Finance department. UCSF Health managers and leaders who are stakeholders in project proposal areas are consulted to provide input and finalize proposal plans, including the identification of existing personnel who can support and drive the project forward. Proposers use this feedback to revise their applications throughout this stage.
The third stage is Project Implementation. The Caring Wisely® Executive Steering Committee selects up to 3 winners from the submitted proposals. Using the program criteria above, each project is scored independently, discussed in committee, and rescored to identify the top proposals. Each selected project receives a maximum budget of $50,000 that can be used for project materials, activities, and salary support for project leaders or staff. In addition to funding, each project team receives input from the implementation science team to co-develop and implement the intervention with a goal of creating a first-test-of-change within 3-6 months. A key feature of Caring Wisely® is the partnership between project teams and the Caring Wisely® implementation team, which includes a director, program manager, data analysts, and implementation scientists (Table 1).
The $150,000 administrative budget for the Caring Wisely® program provides 20% support of the medical director, 50% support of a program manager/analyst, and 10% support of an implementation scientist. Approximately 5% support is donated from additional senior implementation scientists and various UCSF Health experts based on project needs. To make most efficient use of the Caring Wisely® program staff time with the project teams, there is a weekly 60-90 minute works-in-progress session attended by all 3 teams with a rotating schedule for lead presenter during the first 6 months; these meetings occur every 2-3 weeks during the second 6 months. Caring Wisely® program staff and the implementation scientist are also available for 1:1 meetings as needed. The Caring Wisely® Executive Steering Committee is not paid and meets for 90 minutes quarterly. Custom reports and modifications of the electronic health record are provided by the UCSF Health clinical informatics department as part of their operating budget.
The collaboration between the project teams and the implementation science team is guided by the Consolidated Framework for Implementation Research (CFIR)2 and PRECEDE-PROCEED model—a logic model and evaluation tool that is based on a composite of individual behavior change theory and social ecology.3 Table 2 illustrates how we weave PRECEDE-PROCEED and Plan-Do-Study-Act frameworks into project design and strategy. Each funded team is required to submit an end-of-year progress report.
Cost and cost savings estimates were based on administrative financial data obtained through the assistance of the Decision Support Services unit of the Finance Department of UCSF Health. All costs reflect direct institutional costs, rather than charges. For some projects, costs are directly available through computerized dashboards that provide year-to-year comparisons of specific costs of materials, supplies, and services (eg, blood transfusion reduction, surgical supplies project, OR efficiency program). This same dashboard also allows calculation of CMI-adjusted direct costs of hospital care by service line, as used in the perioperative pathways program evaluation. In other cases, the Decision Support Services and/or Caring Wisely® program manager created custom cost reports based on the key performance indicator (eg, nebulizer therapy costs consist of medication costs plus respiratory therapist time; CT scan utilization for suspected pulmonary embolus in emergency department; and antimicrobial utilization for suspected neonatal sepsis).
Ongoing monitoring and sustainability of Caring Wisely® projects is supported by the Caring Wisely® program leaders. Monitoring of ongoing cost savings is based on automated service-line level dashboards related to cost, utilization, and quality outcomes with quarterly updates provided to the Caring Wisely® Steering Committee. Depending on the project or program, appropriate UCSF Health senior leaders determine the level of support within their departments that is required to sustain the program(s). Ongoing monitoring of each program is also included in the strategic deployment visibility room with regular rounding by senior health system executives.
Since 2013, there have been 3 complete Caring Wisely® cycles. The Ideas Contest generated more than 75 ideas in each of the past 3 cycles, ranging from eliminating redundant laboratory or radiological studies to reducing linen and food waste. We received between 13-20 full proposals in each of the request for proposal stages, and 9 projects have been implemented, 3 in each year. Funded projects have been led by a variety of individuals including physicians, nurses, pharmacists, administrators and residents, and topics have ranged from reducing overutilization of tests, supplies and treatments, to improving patient throughput during the perioperative period (Table 3). Estimated cumulative savings to date from Caring Wisely® projects has exceeded $4 million, based on the four projects shown in Table 4. The IV-to-PO switch program and the neonatal sepsis risk prediction project (Table 3) have been successful in reducing unnecessary utilization, but cost and savings estimates are not yet finalized. Three funded projects were equivocal in cost savings but were successful in their primary aims: (1) increasing the appropriateness of CT scan ordering for suspected pulmonary embolus; (2) shortening operating room turnover times; and (3) implementing a postoperative debrief program for the systematic documentation of safety events, waste, and inefficiencies related to surgery.
We developed an innovative program that reduces hospital costs through crowdsourcing of ideas from frontline clinicians and staff, and by connecting these ideas to project and implementation science teams. At a time when healthcare costs have reached unsustainable levels, the Caring Wisely® program provides a process for healthcare personnel to make a positive impact on healthcare costs in areas under their direct control. Through the Open Proposals platform, we have tapped a growing desire among frontline providers to reduce medical waste.
A key criterion for the Caring Wisely® program is to propose changes that reduce cost without adversely affect healthcare quality or outcomes. While this is an important consideration in selecting projects, there is limited power to detect many of the most clinically relevant outcomes. We find this acceptable because many of the sponsored Caring Wisely® project goals were to increase compliance with evidence-based practice guidelines and reduce harms associated with unnecessary treatments (eg, blood transfusion, nebulizer therapy, CT scan, antimicrobial therapy). Selected balancing metrics for each project are reported by established quality and safety programs at UCSF Health, but we acknowledge that many factors that can affect these clinical outcomes are not related to the cost-reduction intervention and are not possible to control outside of a clinical research study. Therefore, any response to changes in these outcome and balancing measures requires further analysis beyond the Caring Wisely® project alone.
We believe one of the key factors in the success of the Caring Wisely® program is the application of implementation science principles to the intervention design strategies (Table 1). These principles included stakeholder engagement, behavior change theory, market (target audience) segmentation, and process measurement and feedback. Because we are conducting this program in an academic health center, resident and fellow education and engagement are also critical to success. In each project, we utilize the PRECEDE model as a guide to ensure that each intervention design includes complementary elements of effective behavior change, intended to increase awareness and motivation to change, to make change “easy,” and to reinforce change(Table 2).3
The Caring Wisely® program—itself a multifaceted intervention—embodies the same PRECEDE dimensions we apply to each specific project. The Ideas Contest serves as a tool for increasing awareness, attitudes, and motivation across the clinical enterprise for reducing healthcare costs. The support provided to the project teams by the Caring Wisely® program is an enabling factor that makes it “easier” for frontline teams to design and implement interventions with a greater likelihood of achieving early success. Timely measurement and feedback of results to the hospital leadership and broadcasting to the larger community reinforces the support of the program at both the leadership and frontline levels.
Collaboration between project teams and the Caring Wisely® program also provides frontline clinicians and staff with practical experience and lessons that they can apply to future improvement work. Project teams learn implementation science principles such as constructing a pragmatic theoretical framework to guide implementation design using CFIR model.2 Incorporating multiple, rapid-cycle tests of change allows teams to modify and adapt final interventions as they learn how the target audience and environment responds to specific intervention components. Access to real-time, actionable data and a data analyst is essential to rapid cycle adaptation that allows teams to focus on specific units or providers. We also find that cross-fertilization between project teams working in different areas helps to share resources and minimize duplication of efforts from the clinical and staff champions. Partnering with UCSF Health system leaders at every phase of project development—from proposal selection, development, and final evaluation of results—enhances sustainable transition of successful projects into clinical operations.
The costs and coordination for the first cycle of Caring Wisely® were supported by the UCSF Center for Healthcare Value. Upon completion of the evaluation of the first cycle, UCSF Health agreed to fund the program going forward, with the expectation that Caring Wisely would continue to achieve direct cost-savings for the organization. The Caring Wisely team provides a final report each year detailing the impact of each project on utilization and associated costs. Currently, program costs are approximately $150,000 for the Caring Wisely program leaders, staff, and other resources, and $50,000 for each of 3 projects for a total program cost of $300,000 per year. Projects included in the first three cycles have already saved more than $4 million, representing a strong return on investment. This program could be a model for other academic health centers to engage frontline clinicians and staff in addressing healthcare costs, and lends itself to being scaled-up into a multi-system collaborative.
LIST OF ABBREVIATIONS
UCSF—University of California, San Francisco; PRECEDE—Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation; PROCEED—Policy, Regulatory and Organizational Constructs in Educational and Environmental Development
Acknowledgments
Other participants in blood transfusion reduction project (D. Johnson, K. Curcione); IV-to-PO Switch (C. Tsourounis, A. Pollock); Surgical Supply Cost Reduction (C. Zygourakis); Perioperative Efficiency (L. Hampson); CT for PE Risk Prediction (E. Weber); ERAS Pathways (L. Chen); Neonatal Sepsis Risk Prediction (T. Newman); Post-Operative Debrief (S. Imershein). Caring Wisely Executive Steering Committee (J. Adler, S. Antrum, A Auerbach, J. Bennan, M. Blum, C. Ritchie, C. Tsourounis). This Center for Healthcare Value is funded in part by a grant from the Grove Foundation. We appreciate additional review and comments to the manuscript provided by George Sawaya and Adams Dudley.
Disclosures
Christopher Moriates has accepted royalties from McGraw-Hill for textbook, Understanding Value-Based Healthcare. Alvin Rajkomar has received fees as a research adviser from Google, Inc.
1. Kahlon M, Yuan L, Gologorskaya O, Johnston SC. Crowdsourcing the CTSA innovation mission. Clin Transl Sci. 2014;7:89-92. PubMed
2. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. PubMed
3. Green LW and Kreuter. Health Program Planning: An Educational and Ecological Approach. 4th Ed. McGraw-Hill. New York, NY. 2005.
4. Zygourakis CC, Valencia V, Moriates C et al. Association between surgeon scorecard use and operating room costs. JAMA Surg. 2016 Dec 7. doi: 10.1001/jamasurg.2016.4674. [Epub ahead of print] PubMed
© 2017 Society of Hospital Medicine
Strategies are needed to empower frontline clinicians to work with organizational leadership to reduce healthcare costs and improve high-value care. Caring Wisely® is a program developed by the University of California, San Francisco’s (UCSF) Center for Healthcare Value (CHV), aimed at engaging frontline clinicians and staff, connecting them with implementation experts, and supporting the development of targeted interventions to improve value. Financial savings from the program more than cover program costs. Caring Wisely® provides an institutional model for implementing robust interventions to address areas of low-value care.
Launched in 2013, the annual Caring Wisely® program consists of 3 stages for identifying projects that meet the following criteria:
- Potential to measurably reduce UCSF Health’s costs of care without transferring costs to patients, insurers, or other providers
- Plan for ensuring that health outcomes are maintained or improved
- Envision disseminating the intervention within and beyond UCSF
- Demonstrate commitment and engagement of clinical leadership and frontline staff.
The first stage is the Ideas Contest, a UCSF Health-wide call (to learn more about UCSF Health: https://www.ucsf.edu/sites/default/files/052516_About_UCSF.pdf) to identify areas that may be targeted to reduce unnecessary services, inefficiencies, and healthcare costs. We use a crowdsourcing platform—Open Proposals—to solicit the best ideas from frontline clinicians and staff.1 Open Proposals is a secure, web-based platform for transparent and collaborative proposal development that displays threads of comments, responses, and revisions, and allows submissions to be “liked.” Open Proposals is managed by the UCSF Clinical and Translational Science Institute, funded by the National Center for Advancing Translational Sciences (Grant Number UL1 TR000004) at the National Institutes of Health. Using institutional e-mail lists for faculty, staff and residents, as well as described at monthly managers and directors meetings, the Ideas Contest is announced each year by the Chief Medical Officer and the CHV leadership. The Caring Wisely® Executive Steering Committee, which consists of CHV and senior UCSF Health system leaders, selects the top 5-10 ideas based on the above criteria. Each winning idea receives a $100 gift certificate for a popular restaurant in San Francisco, and the list of winners is announced to the entire UCSF community.
The second stage is the Request for Proposals. The Caring Wisely® program solicits proposals that outline implementation plans to target specific areas identified through the Ideas Contest. Finalists from the Ideas Contest are encouraged to submit proposals that address the problem they identified, but anyone affiliated with UCSF Health may submit a proposal on a winning idea. There is an approximately 4-week open submission period during which applicants submit brief 2-page proposals on the Open Proposal platform. This is followed by a period of optimization that leverages the social media aspect of the Open Proposals platform in which the UCSF Health community asks clarifying questions, make suggestions, and modifications can be made to the proposals. All submissions receive written feedback from at least one Steering Committee member. In addition, the Caring Wisely® Director directly invites relevant UCSF colleagues, administrators, or program leaders to comment on proposals and make suggestions for improvement. Plans for assessing financial and health care delivery impacts are developed in collaboration with the UCSF Health Finance department. UCSF Health managers and leaders who are stakeholders in project proposal areas are consulted to provide input and finalize proposal plans, including the identification of existing personnel who can support and drive the project forward. Proposers use this feedback to revise their applications throughout this stage.
The third stage is Project Implementation. The Caring Wisely® Executive Steering Committee selects up to 3 winners from the submitted proposals. Using the program criteria above, each project is scored independently, discussed in committee, and rescored to identify the top proposals. Each selected project receives a maximum budget of $50,000 that can be used for project materials, activities, and salary support for project leaders or staff. In addition to funding, each project team receives input from the implementation science team to co-develop and implement the intervention with a goal of creating a first-test-of-change within 3-6 months. A key feature of Caring Wisely® is the partnership between project teams and the Caring Wisely® implementation team, which includes a director, program manager, data analysts, and implementation scientists (Table 1).
The $150,000 administrative budget for the Caring Wisely® program provides 20% support of the medical director, 50% support of a program manager/analyst, and 10% support of an implementation scientist. Approximately 5% support is donated from additional senior implementation scientists and various UCSF Health experts based on project needs. To make most efficient use of the Caring Wisely® program staff time with the project teams, there is a weekly 60-90 minute works-in-progress session attended by all 3 teams with a rotating schedule for lead presenter during the first 6 months; these meetings occur every 2-3 weeks during the second 6 months. Caring Wisely® program staff and the implementation scientist are also available for 1:1 meetings as needed. The Caring Wisely® Executive Steering Committee is not paid and meets for 90 minutes quarterly. Custom reports and modifications of the electronic health record are provided by the UCSF Health clinical informatics department as part of their operating budget.
The collaboration between the project teams and the implementation science team is guided by the Consolidated Framework for Implementation Research (CFIR)2 and PRECEDE-PROCEED model—a logic model and evaluation tool that is based on a composite of individual behavior change theory and social ecology.3 Table 2 illustrates how we weave PRECEDE-PROCEED and Plan-Do-Study-Act frameworks into project design and strategy. Each funded team is required to submit an end-of-year progress report.
Cost and cost savings estimates were based on administrative financial data obtained through the assistance of the Decision Support Services unit of the Finance Department of UCSF Health. All costs reflect direct institutional costs, rather than charges. For some projects, costs are directly available through computerized dashboards that provide year-to-year comparisons of specific costs of materials, supplies, and services (eg, blood transfusion reduction, surgical supplies project, OR efficiency program). This same dashboard also allows calculation of CMI-adjusted direct costs of hospital care by service line, as used in the perioperative pathways program evaluation. In other cases, the Decision Support Services and/or Caring Wisely® program manager created custom cost reports based on the key performance indicator (eg, nebulizer therapy costs consist of medication costs plus respiratory therapist time; CT scan utilization for suspected pulmonary embolus in emergency department; and antimicrobial utilization for suspected neonatal sepsis).
Ongoing monitoring and sustainability of Caring Wisely® projects is supported by the Caring Wisely® program leaders. Monitoring of ongoing cost savings is based on automated service-line level dashboards related to cost, utilization, and quality outcomes with quarterly updates provided to the Caring Wisely® Steering Committee. Depending on the project or program, appropriate UCSF Health senior leaders determine the level of support within their departments that is required to sustain the program(s). Ongoing monitoring of each program is also included in the strategic deployment visibility room with regular rounding by senior health system executives.
Since 2013, there have been 3 complete Caring Wisely® cycles. The Ideas Contest generated more than 75 ideas in each of the past 3 cycles, ranging from eliminating redundant laboratory or radiological studies to reducing linen and food waste. We received between 13-20 full proposals in each of the request for proposal stages, and 9 projects have been implemented, 3 in each year. Funded projects have been led by a variety of individuals including physicians, nurses, pharmacists, administrators and residents, and topics have ranged from reducing overutilization of tests, supplies and treatments, to improving patient throughput during the perioperative period (Table 3). Estimated cumulative savings to date from Caring Wisely® projects has exceeded $4 million, based on the four projects shown in Table 4. The IV-to-PO switch program and the neonatal sepsis risk prediction project (Table 3) have been successful in reducing unnecessary utilization, but cost and savings estimates are not yet finalized. Three funded projects were equivocal in cost savings but were successful in their primary aims: (1) increasing the appropriateness of CT scan ordering for suspected pulmonary embolus; (2) shortening operating room turnover times; and (3) implementing a postoperative debrief program for the systematic documentation of safety events, waste, and inefficiencies related to surgery.
We developed an innovative program that reduces hospital costs through crowdsourcing of ideas from frontline clinicians and staff, and by connecting these ideas to project and implementation science teams. At a time when healthcare costs have reached unsustainable levels, the Caring Wisely® program provides a process for healthcare personnel to make a positive impact on healthcare costs in areas under their direct control. Through the Open Proposals platform, we have tapped a growing desire among frontline providers to reduce medical waste.
A key criterion for the Caring Wisely® program is to propose changes that reduce cost without adversely affect healthcare quality or outcomes. While this is an important consideration in selecting projects, there is limited power to detect many of the most clinically relevant outcomes. We find this acceptable because many of the sponsored Caring Wisely® project goals were to increase compliance with evidence-based practice guidelines and reduce harms associated with unnecessary treatments (eg, blood transfusion, nebulizer therapy, CT scan, antimicrobial therapy). Selected balancing metrics for each project are reported by established quality and safety programs at UCSF Health, but we acknowledge that many factors that can affect these clinical outcomes are not related to the cost-reduction intervention and are not possible to control outside of a clinical research study. Therefore, any response to changes in these outcome and balancing measures requires further analysis beyond the Caring Wisely® project alone.
We believe one of the key factors in the success of the Caring Wisely® program is the application of implementation science principles to the intervention design strategies (Table 1). These principles included stakeholder engagement, behavior change theory, market (target audience) segmentation, and process measurement and feedback. Because we are conducting this program in an academic health center, resident and fellow education and engagement are also critical to success. In each project, we utilize the PRECEDE model as a guide to ensure that each intervention design includes complementary elements of effective behavior change, intended to increase awareness and motivation to change, to make change “easy,” and to reinforce change(Table 2).3
The Caring Wisely® program—itself a multifaceted intervention—embodies the same PRECEDE dimensions we apply to each specific project. The Ideas Contest serves as a tool for increasing awareness, attitudes, and motivation across the clinical enterprise for reducing healthcare costs. The support provided to the project teams by the Caring Wisely® program is an enabling factor that makes it “easier” for frontline teams to design and implement interventions with a greater likelihood of achieving early success. Timely measurement and feedback of results to the hospital leadership and broadcasting to the larger community reinforces the support of the program at both the leadership and frontline levels.
Collaboration between project teams and the Caring Wisely® program also provides frontline clinicians and staff with practical experience and lessons that they can apply to future improvement work. Project teams learn implementation science principles such as constructing a pragmatic theoretical framework to guide implementation design using CFIR model.2 Incorporating multiple, rapid-cycle tests of change allows teams to modify and adapt final interventions as they learn how the target audience and environment responds to specific intervention components. Access to real-time, actionable data and a data analyst is essential to rapid cycle adaptation that allows teams to focus on specific units or providers. We also find that cross-fertilization between project teams working in different areas helps to share resources and minimize duplication of efforts from the clinical and staff champions. Partnering with UCSF Health system leaders at every phase of project development—from proposal selection, development, and final evaluation of results—enhances sustainable transition of successful projects into clinical operations.
The costs and coordination for the first cycle of Caring Wisely® were supported by the UCSF Center for Healthcare Value. Upon completion of the evaluation of the first cycle, UCSF Health agreed to fund the program going forward, with the expectation that Caring Wisely would continue to achieve direct cost-savings for the organization. The Caring Wisely team provides a final report each year detailing the impact of each project on utilization and associated costs. Currently, program costs are approximately $150,000 for the Caring Wisely program leaders, staff, and other resources, and $50,000 for each of 3 projects for a total program cost of $300,000 per year. Projects included in the first three cycles have already saved more than $4 million, representing a strong return on investment. This program could be a model for other academic health centers to engage frontline clinicians and staff in addressing healthcare costs, and lends itself to being scaled-up into a multi-system collaborative.
LIST OF ABBREVIATIONS
UCSF—University of California, San Francisco; PRECEDE—Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation; PROCEED—Policy, Regulatory and Organizational Constructs in Educational and Environmental Development
Acknowledgments
Other participants in blood transfusion reduction project (D. Johnson, K. Curcione); IV-to-PO Switch (C. Tsourounis, A. Pollock); Surgical Supply Cost Reduction (C. Zygourakis); Perioperative Efficiency (L. Hampson); CT for PE Risk Prediction (E. Weber); ERAS Pathways (L. Chen); Neonatal Sepsis Risk Prediction (T. Newman); Post-Operative Debrief (S. Imershein). Caring Wisely Executive Steering Committee (J. Adler, S. Antrum, A Auerbach, J. Bennan, M. Blum, C. Ritchie, C. Tsourounis). This Center for Healthcare Value is funded in part by a grant from the Grove Foundation. We appreciate additional review and comments to the manuscript provided by George Sawaya and Adams Dudley.
Disclosures
Christopher Moriates has accepted royalties from McGraw-Hill for textbook, Understanding Value-Based Healthcare. Alvin Rajkomar has received fees as a research adviser from Google, Inc.
© 2017 Society of Hospital Medicine
Strategies are needed to empower frontline clinicians to work with organizational leadership to reduce healthcare costs and improve high-value care. Caring Wisely® is a program developed by the University of California, San Francisco’s (UCSF) Center for Healthcare Value (CHV), aimed at engaging frontline clinicians and staff, connecting them with implementation experts, and supporting the development of targeted interventions to improve value. Financial savings from the program more than cover program costs. Caring Wisely® provides an institutional model for implementing robust interventions to address areas of low-value care.
Launched in 2013, the annual Caring Wisely® program consists of 3 stages for identifying projects that meet the following criteria:
- Potential to measurably reduce UCSF Health’s costs of care without transferring costs to patients, insurers, or other providers
- Plan for ensuring that health outcomes are maintained or improved
- Envision disseminating the intervention within and beyond UCSF
- Demonstrate commitment and engagement of clinical leadership and frontline staff.
The first stage is the Ideas Contest, a UCSF Health-wide call (to learn more about UCSF Health: https://www.ucsf.edu/sites/default/files/052516_About_UCSF.pdf) to identify areas that may be targeted to reduce unnecessary services, inefficiencies, and healthcare costs. We use a crowdsourcing platform—Open Proposals—to solicit the best ideas from frontline clinicians and staff.1 Open Proposals is a secure, web-based platform for transparent and collaborative proposal development that displays threads of comments, responses, and revisions, and allows submissions to be “liked.” Open Proposals is managed by the UCSF Clinical and Translational Science Institute, funded by the National Center for Advancing Translational Sciences (Grant Number UL1 TR000004) at the National Institutes of Health. Using institutional e-mail lists for faculty, staff and residents, as well as described at monthly managers and directors meetings, the Ideas Contest is announced each year by the Chief Medical Officer and the CHV leadership. The Caring Wisely® Executive Steering Committee, which consists of CHV and senior UCSF Health system leaders, selects the top 5-10 ideas based on the above criteria. Each winning idea receives a $100 gift certificate for a popular restaurant in San Francisco, and the list of winners is announced to the entire UCSF community.
The second stage is the Request for Proposals. The Caring Wisely® program solicits proposals that outline implementation plans to target specific areas identified through the Ideas Contest. Finalists from the Ideas Contest are encouraged to submit proposals that address the problem they identified, but anyone affiliated with UCSF Health may submit a proposal on a winning idea. There is an approximately 4-week open submission period during which applicants submit brief 2-page proposals on the Open Proposal platform. This is followed by a period of optimization that leverages the social media aspect of the Open Proposals platform in which the UCSF Health community asks clarifying questions, make suggestions, and modifications can be made to the proposals. All submissions receive written feedback from at least one Steering Committee member. In addition, the Caring Wisely® Director directly invites relevant UCSF colleagues, administrators, or program leaders to comment on proposals and make suggestions for improvement. Plans for assessing financial and health care delivery impacts are developed in collaboration with the UCSF Health Finance department. UCSF Health managers and leaders who are stakeholders in project proposal areas are consulted to provide input and finalize proposal plans, including the identification of existing personnel who can support and drive the project forward. Proposers use this feedback to revise their applications throughout this stage.
The third stage is Project Implementation. The Caring Wisely® Executive Steering Committee selects up to 3 winners from the submitted proposals. Using the program criteria above, each project is scored independently, discussed in committee, and rescored to identify the top proposals. Each selected project receives a maximum budget of $50,000 that can be used for project materials, activities, and salary support for project leaders or staff. In addition to funding, each project team receives input from the implementation science team to co-develop and implement the intervention with a goal of creating a first-test-of-change within 3-6 months. A key feature of Caring Wisely® is the partnership between project teams and the Caring Wisely® implementation team, which includes a director, program manager, data analysts, and implementation scientists (Table 1).
The $150,000 administrative budget for the Caring Wisely® program provides 20% support of the medical director, 50% support of a program manager/analyst, and 10% support of an implementation scientist. Approximately 5% support is donated from additional senior implementation scientists and various UCSF Health experts based on project needs. To make most efficient use of the Caring Wisely® program staff time with the project teams, there is a weekly 60-90 minute works-in-progress session attended by all 3 teams with a rotating schedule for lead presenter during the first 6 months; these meetings occur every 2-3 weeks during the second 6 months. Caring Wisely® program staff and the implementation scientist are also available for 1:1 meetings as needed. The Caring Wisely® Executive Steering Committee is not paid and meets for 90 minutes quarterly. Custom reports and modifications of the electronic health record are provided by the UCSF Health clinical informatics department as part of their operating budget.
The collaboration between the project teams and the implementation science team is guided by the Consolidated Framework for Implementation Research (CFIR)2 and PRECEDE-PROCEED model—a logic model and evaluation tool that is based on a composite of individual behavior change theory and social ecology.3 Table 2 illustrates how we weave PRECEDE-PROCEED and Plan-Do-Study-Act frameworks into project design and strategy. Each funded team is required to submit an end-of-year progress report.
Cost and cost savings estimates were based on administrative financial data obtained through the assistance of the Decision Support Services unit of the Finance Department of UCSF Health. All costs reflect direct institutional costs, rather than charges. For some projects, costs are directly available through computerized dashboards that provide year-to-year comparisons of specific costs of materials, supplies, and services (eg, blood transfusion reduction, surgical supplies project, OR efficiency program). This same dashboard also allows calculation of CMI-adjusted direct costs of hospital care by service line, as used in the perioperative pathways program evaluation. In other cases, the Decision Support Services and/or Caring Wisely® program manager created custom cost reports based on the key performance indicator (eg, nebulizer therapy costs consist of medication costs plus respiratory therapist time; CT scan utilization for suspected pulmonary embolus in emergency department; and antimicrobial utilization for suspected neonatal sepsis).
Ongoing monitoring and sustainability of Caring Wisely® projects is supported by the Caring Wisely® program leaders. Monitoring of ongoing cost savings is based on automated service-line level dashboards related to cost, utilization, and quality outcomes with quarterly updates provided to the Caring Wisely® Steering Committee. Depending on the project or program, appropriate UCSF Health senior leaders determine the level of support within their departments that is required to sustain the program(s). Ongoing monitoring of each program is also included in the strategic deployment visibility room with regular rounding by senior health system executives.
Since 2013, there have been 3 complete Caring Wisely® cycles. The Ideas Contest generated more than 75 ideas in each of the past 3 cycles, ranging from eliminating redundant laboratory or radiological studies to reducing linen and food waste. We received between 13-20 full proposals in each of the request for proposal stages, and 9 projects have been implemented, 3 in each year. Funded projects have been led by a variety of individuals including physicians, nurses, pharmacists, administrators and residents, and topics have ranged from reducing overutilization of tests, supplies and treatments, to improving patient throughput during the perioperative period (Table 3). Estimated cumulative savings to date from Caring Wisely® projects has exceeded $4 million, based on the four projects shown in Table 4. The IV-to-PO switch program and the neonatal sepsis risk prediction project (Table 3) have been successful in reducing unnecessary utilization, but cost and savings estimates are not yet finalized. Three funded projects were equivocal in cost savings but were successful in their primary aims: (1) increasing the appropriateness of CT scan ordering for suspected pulmonary embolus; (2) shortening operating room turnover times; and (3) implementing a postoperative debrief program for the systematic documentation of safety events, waste, and inefficiencies related to surgery.
We developed an innovative program that reduces hospital costs through crowdsourcing of ideas from frontline clinicians and staff, and by connecting these ideas to project and implementation science teams. At a time when healthcare costs have reached unsustainable levels, the Caring Wisely® program provides a process for healthcare personnel to make a positive impact on healthcare costs in areas under their direct control. Through the Open Proposals platform, we have tapped a growing desire among frontline providers to reduce medical waste.
A key criterion for the Caring Wisely® program is to propose changes that reduce cost without adversely affect healthcare quality or outcomes. While this is an important consideration in selecting projects, there is limited power to detect many of the most clinically relevant outcomes. We find this acceptable because many of the sponsored Caring Wisely® project goals were to increase compliance with evidence-based practice guidelines and reduce harms associated with unnecessary treatments (eg, blood transfusion, nebulizer therapy, CT scan, antimicrobial therapy). Selected balancing metrics for each project are reported by established quality and safety programs at UCSF Health, but we acknowledge that many factors that can affect these clinical outcomes are not related to the cost-reduction intervention and are not possible to control outside of a clinical research study. Therefore, any response to changes in these outcome and balancing measures requires further analysis beyond the Caring Wisely® project alone.
We believe one of the key factors in the success of the Caring Wisely® program is the application of implementation science principles to the intervention design strategies (Table 1). These principles included stakeholder engagement, behavior change theory, market (target audience) segmentation, and process measurement and feedback. Because we are conducting this program in an academic health center, resident and fellow education and engagement are also critical to success. In each project, we utilize the PRECEDE model as a guide to ensure that each intervention design includes complementary elements of effective behavior change, intended to increase awareness and motivation to change, to make change “easy,” and to reinforce change(Table 2).3
The Caring Wisely® program—itself a multifaceted intervention—embodies the same PRECEDE dimensions we apply to each specific project. The Ideas Contest serves as a tool for increasing awareness, attitudes, and motivation across the clinical enterprise for reducing healthcare costs. The support provided to the project teams by the Caring Wisely® program is an enabling factor that makes it “easier” for frontline teams to design and implement interventions with a greater likelihood of achieving early success. Timely measurement and feedback of results to the hospital leadership and broadcasting to the larger community reinforces the support of the program at both the leadership and frontline levels.
Collaboration between project teams and the Caring Wisely® program also provides frontline clinicians and staff with practical experience and lessons that they can apply to future improvement work. Project teams learn implementation science principles such as constructing a pragmatic theoretical framework to guide implementation design using CFIR model.2 Incorporating multiple, rapid-cycle tests of change allows teams to modify and adapt final interventions as they learn how the target audience and environment responds to specific intervention components. Access to real-time, actionable data and a data analyst is essential to rapid cycle adaptation that allows teams to focus on specific units or providers. We also find that cross-fertilization between project teams working in different areas helps to share resources and minimize duplication of efforts from the clinical and staff champions. Partnering with UCSF Health system leaders at every phase of project development—from proposal selection, development, and final evaluation of results—enhances sustainable transition of successful projects into clinical operations.
The costs and coordination for the first cycle of Caring Wisely® were supported by the UCSF Center for Healthcare Value. Upon completion of the evaluation of the first cycle, UCSF Health agreed to fund the program going forward, with the expectation that Caring Wisely would continue to achieve direct cost-savings for the organization. The Caring Wisely team provides a final report each year detailing the impact of each project on utilization and associated costs. Currently, program costs are approximately $150,000 for the Caring Wisely program leaders, staff, and other resources, and $50,000 for each of 3 projects for a total program cost of $300,000 per year. Projects included in the first three cycles have already saved more than $4 million, representing a strong return on investment. This program could be a model for other academic health centers to engage frontline clinicians and staff in addressing healthcare costs, and lends itself to being scaled-up into a multi-system collaborative.
LIST OF ABBREVIATIONS
UCSF—University of California, San Francisco; PRECEDE—Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation; PROCEED—Policy, Regulatory and Organizational Constructs in Educational and Environmental Development
Acknowledgments
Other participants in blood transfusion reduction project (D. Johnson, K. Curcione); IV-to-PO Switch (C. Tsourounis, A. Pollock); Surgical Supply Cost Reduction (C. Zygourakis); Perioperative Efficiency (L. Hampson); CT for PE Risk Prediction (E. Weber); ERAS Pathways (L. Chen); Neonatal Sepsis Risk Prediction (T. Newman); Post-Operative Debrief (S. Imershein). Caring Wisely Executive Steering Committee (J. Adler, S. Antrum, A Auerbach, J. Bennan, M. Blum, C. Ritchie, C. Tsourounis). This Center for Healthcare Value is funded in part by a grant from the Grove Foundation. We appreciate additional review and comments to the manuscript provided by George Sawaya and Adams Dudley.
Disclosures
Christopher Moriates has accepted royalties from McGraw-Hill for textbook, Understanding Value-Based Healthcare. Alvin Rajkomar has received fees as a research adviser from Google, Inc.
1. Kahlon M, Yuan L, Gologorskaya O, Johnston SC. Crowdsourcing the CTSA innovation mission. Clin Transl Sci. 2014;7:89-92. PubMed
2. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. PubMed
3. Green LW and Kreuter. Health Program Planning: An Educational and Ecological Approach. 4th Ed. McGraw-Hill. New York, NY. 2005.
4. Zygourakis CC, Valencia V, Moriates C et al. Association between surgeon scorecard use and operating room costs. JAMA Surg. 2016 Dec 7. doi: 10.1001/jamasurg.2016.4674. [Epub ahead of print] PubMed
1. Kahlon M, Yuan L, Gologorskaya O, Johnston SC. Crowdsourcing the CTSA innovation mission. Clin Transl Sci. 2014;7:89-92. PubMed
2. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. PubMed
3. Green LW and Kreuter. Health Program Planning: An Educational and Ecological Approach. 4th Ed. McGraw-Hill. New York, NY. 2005.
4. Zygourakis CC, Valencia V, Moriates C et al. Association between surgeon scorecard use and operating room costs. JAMA Surg. 2016 Dec 7. doi: 10.1001/jamasurg.2016.4674. [Epub ahead of print] PubMed
Letter to the Editor/
We certainly agree with Dr. LaBrin that there are a minority of inpatients and outpatients who might benefit from nebulizer therapy. In our review article,[1] we attempted not to make a sweeping generalization, even if we did not explicitly mention some chronic obstructive pulmonary disease patients with suboptimal peak inspiratory flow rate (PIFR) or those with neuromuscular disease as populations where nebulizer therapy may be preferred. Our recommendation included this statement: Inpatient use of nebulizers may be more appropriate than metered‐dose inhalers (MDIs) for patients with dementia or altered mental status, as well as those in extreme distress resulting in an inability to coordinate inhaler usage. Very low health literacy may be an additional barrier to appropriate MDI teaching and usage.[1] Our list was not all‐inclusive, and patients with suboptimal PIFR or with neuromuscular disease are good additions to this recommendation.
As for proper MDI technique, it is unclear whether MDI teaching will result in long‐term mastery of the skill.[2] The only way to master a skill is to practice it. Thus, by prescribing MDIs and training patients on their proper usage during every admission, we will provide medically appropriate patients with many opportunities to practice the skill and reinforce effective techniques.
- , . Nebulized bronchodilators instead of metered‐dose inhalers for obstructive pulmonary symptoms. J Hosp Med. 2015;10(10):691–693.
- , , , et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011;26(6):635–642.
We certainly agree with Dr. LaBrin that there are a minority of inpatients and outpatients who might benefit from nebulizer therapy. In our review article,[1] we attempted not to make a sweeping generalization, even if we did not explicitly mention some chronic obstructive pulmonary disease patients with suboptimal peak inspiratory flow rate (PIFR) or those with neuromuscular disease as populations where nebulizer therapy may be preferred. Our recommendation included this statement: Inpatient use of nebulizers may be more appropriate than metered‐dose inhalers (MDIs) for patients with dementia or altered mental status, as well as those in extreme distress resulting in an inability to coordinate inhaler usage. Very low health literacy may be an additional barrier to appropriate MDI teaching and usage.[1] Our list was not all‐inclusive, and patients with suboptimal PIFR or with neuromuscular disease are good additions to this recommendation.
As for proper MDI technique, it is unclear whether MDI teaching will result in long‐term mastery of the skill.[2] The only way to master a skill is to practice it. Thus, by prescribing MDIs and training patients on their proper usage during every admission, we will provide medically appropriate patients with many opportunities to practice the skill and reinforce effective techniques.
We certainly agree with Dr. LaBrin that there are a minority of inpatients and outpatients who might benefit from nebulizer therapy. In our review article,[1] we attempted not to make a sweeping generalization, even if we did not explicitly mention some chronic obstructive pulmonary disease patients with suboptimal peak inspiratory flow rate (PIFR) or those with neuromuscular disease as populations where nebulizer therapy may be preferred. Our recommendation included this statement: Inpatient use of nebulizers may be more appropriate than metered‐dose inhalers (MDIs) for patients with dementia or altered mental status, as well as those in extreme distress resulting in an inability to coordinate inhaler usage. Very low health literacy may be an additional barrier to appropriate MDI teaching and usage.[1] Our list was not all‐inclusive, and patients with suboptimal PIFR or with neuromuscular disease are good additions to this recommendation.
As for proper MDI technique, it is unclear whether MDI teaching will result in long‐term mastery of the skill.[2] The only way to master a skill is to practice it. Thus, by prescribing MDIs and training patients on their proper usage during every admission, we will provide medically appropriate patients with many opportunities to practice the skill and reinforce effective techniques.
- , . Nebulized bronchodilators instead of metered‐dose inhalers for obstructive pulmonary symptoms. J Hosp Med. 2015;10(10):691–693.
- , , , et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011;26(6):635–642.
- , . Nebulized bronchodilators instead of metered‐dose inhalers for obstructive pulmonary symptoms. J Hosp Med. 2015;10(10):691–693.
- , , , et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011;26(6):635–642.