User login
Utilizing Telesimulation for Advanced Skills Training in Consultation and Handoff Communication: A Post-COVID-19 GME Bootcamp Experience
Events requiring communication among and within teams are vulnerable points in patient care in hospital medicine, with communication failures representing important contributors to adverse events.1-4 Consultations and handoffs are exceptionally common inpatient practices, yet training in these practices is variable across educational and practice domains.5,6 Advanced inpatient communication-skills training requires an effective, feasible, and scalable format. Simulation-based bootcamps can effectively support clinical skills training, often in procedural domains, and have been increasingly utilized for communication skills.7,8 We previously described the development and implementation of an in-person bootcamp for training and feedback in consultation and handoff communication.5,8
As hospitalist leaders grapple with how to systematically support and assess essential clinical skills, the COVID-19 pandemic has presented another impetus to rethink current processes. The rapid shift to virtual activities met immediate needs of the pandemic, but also inspired creativity in applying new methodologies to improve teaching strategies and implementation long-term.9,10 One such strategy, telesimulation, offers a way to continue simulation-based training limited by the need for physical distancing.10 Furthermore, recent calls to study the efficacy of virtual bootcamp structures have acknowledged potential benefits, even outside of the pandemic.11
The primary objective of this feasibility study was to convert our previously described consultation and handoff bootcamp to a telesimulation bootcamp (TBC), preserving rigorous performance evaluation and opportunities for skills-based feedback. We additionally compared evaluation between virtual and in-person formats to understand the utility of telesimulation for bootcamp-based clinical education moving forward.
METHODS
Setting and Participants
The TBC occurred in June 2020 during the University of Chicago institution-wide graduate medical education (GME) orientation; 130 interns entering 13 residency programs participated. The comparison group was 128 interns who underwent the traditional University of Chicago GME orientation “Advanced Communication Skills Bootcamp” (ACSBC) in 2019.5,8
Program Description
To develop TBC, we adapted observed structured clinical experiences (OSCEs) created for ACSBC. Until 2020, ACSBC included three in-person OSCEs: (1) requesting a consultation; (2) conducting handoffs; and (3) acquiring informed consent. COVID-19 necessitated conversion of ACSBC to virtual in June 2020. For this, we selected the consultation and handoff OSCEs, as these skills require near-universal and immediate application in clinical practice. Additionally, they required only trained facilitators (TFs), whereas informed consent required standardized patients. Hospitalist and emergency medicine faculty were recruited as TFs; 7 of 12 TFs were hospitalists. Each OSCE had two parts: an asynchronous, mandatory training module and a clinical simulation. For TBC, we adapted the simulations, previously separate experiences, into a 20-minute combined handoff/consultation telesimulation using the Zoom® video platform. Interns were paired with one TF who served as both standardized consultant (for one mock case) and handoff receiver (for three mock cases, including the consultation case). TFs rated intern performance and provided feedback.
TBC occurred on June 17 and 18, 2020. Interns were emailed asynchronous modules on June 1, and mock cases and instructions on June 12. When TBC began, GME staff proctors oriented interns in the Zoom® platform. Proctors placed TFs into private breakout rooms into which interns rotated through 20-minute timeslots. Faculty received copies of all TBC materials for review (Appendix 1) and underwent Zoom®-based training 1 to 2 weeks prior.
We evaluated TBC using several methods: (1) consultation and handoff skills performance measured by two validated checklists5,8; (2) survey of intern self-reported preparedness to practice consultations and handoffs; and (3) survey of intern satisfaction. Surveys were administered both immediately post bootcamp (Appendix 2) and 8 weeks into internship (Appendix 3). Skills performance checklists were a 12-item consultation checklist5 and 6-item handoff checklist.8 The handoff checklist was modified to remove activities impossible to assess virtually (ie, orienting sign-outs in a shared space) and to add a three-level rating scale of “outstanding,” “satisfactory,” and “needs improvement.” This was done based on feedback from ACSBC to allow more nuanced feedback for interns. A rating of “outstanding” was used to define successful completion of the item (Appendix 1). Interns rated preparedness and satisfaction on 5-point Likert-type items. All measures were compared to the 2019 in-person ACSBC cohort.
Data Analysis
Stata 16.1 (StataCorp LP) was used for analysis. We dichotomized preparedness and satisfaction scores, defining ratings of “4” or “5” as “prepared” or “satisfied.” As previously described,5 we created a composite score averaging both checklist scores for each intern. We normalized this score by rater to a z score (mean, 0; SD, 1) to account for rater differences. “Poor” and “outstanding” performances were defined as z scores below and above 1 SD, respectively. Fisher’s exact test was used to compare proportions, and Pearson correlation test to correlate z scores. The University of Chicago Institutional Review Board granted exemption.
RESULTS
All 130 entering interns participated in TBC. Internal medicine (IM) was the largest specialty (n = 37), followed by pediatrics (n = 22), emergency medicine (EM) (n = 16), and anesthesiology (n = 12). The remaining 9 programs ranged from 2 to 10 interns per program. The 128 interns in ACSBC were similar, including 40 IM, 23 pediatrics, 14 EM, and 12 anesthesia interns, with 2 to 10 interns in remaining programs.
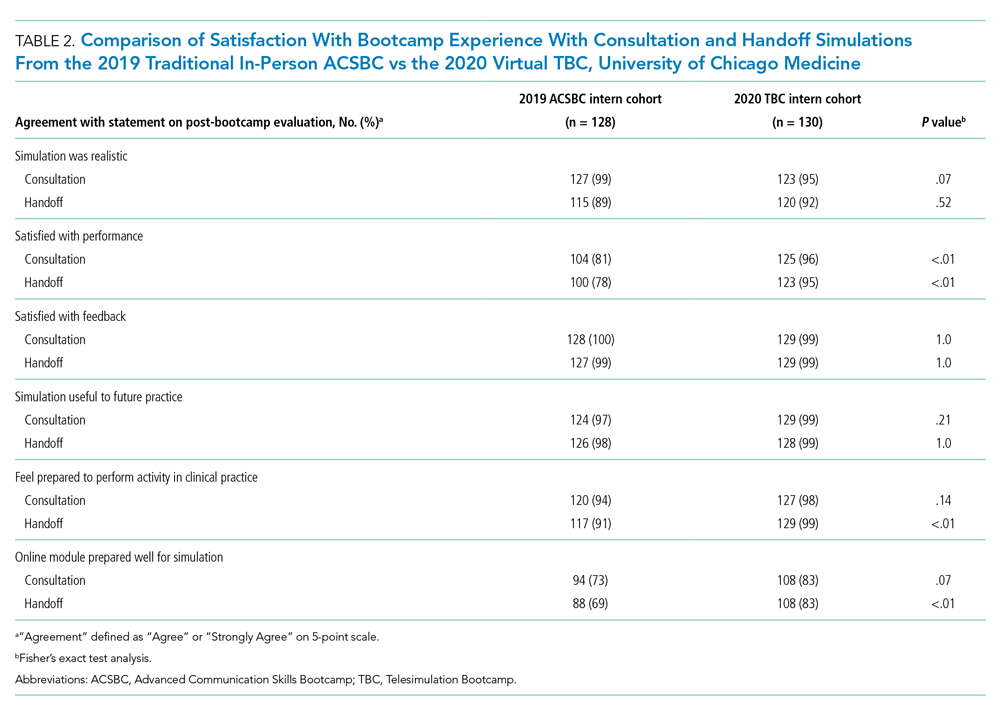
TBC skills performance evaluations were compared to ACSBC (Table 1). The TBC intern cohort’s consultation performance was the same or better than the ACSBC intern cohort’s. For handoffs, TBC interns completed significantly fewer checklist items compared to ACSBC. Performance in each exercise was moderately correlated (r = 0.39, P < .05). For z scores, 14 TBC interns (10.8%) had “outstanding” and 15 (11.6%) had “poor” performances, compared to ACSBC interns with 7 (5.5%) “outstanding” and 10 (7.81%) “poor” performances (P = .15).
All 130 interns (100%) completed the immediate post-TBC survey. Overall, TBC satisfaction was comparable to ACSBC, and significantly improved for satisfaction with performance (Table 2). Compared to ACSBC, TBC interns felt more prepared for simulation and handoff clinical practice. Nearly all interns would recommend TBC (99% vs 96% of ACSBC interns, P = 0.28), and 99% felt the software used for the simulation ran smoothly.
The 8-week post-TBC survey had a response rate of 88% (115/130); 69% of interns reported conducting more effective handoffs due to TBC, and 79% felt confident in handoff skills. Similarly, 73% felt more effective at calling consultations, and 75% reported retained knowledge of consultation frameworks taught during TBC. Additionally, 71% of interns reported that TBC helped identify areas for self-directed improvement. There were no significant differences in 8-week postsurvey ratings between ACSBC and TBC.
DISCUSSION
In converting the advanced communication skills bootcamp from an in-person to a virtual format, telesimulation was well-received by interns and rated similarly to in-person bootcamp in most respects. Nearly all interns agreed the experience was realistic, provided useful feedback, and prepared them for clinical practice. Although we shifted to virtual out of necessity, our results demonstrate a high-quality, streamlined bootcamp experience that was less labor-intensive for interns, staff, and faculty. Telesimulation may represent an effective strategy beyond the COVID-19 pandemic to increase ease of administration and scale the use of bootcamps in supporting advanced clinical skill training for hospital-based practice.
TBC interns felt better prepared for simulation and more satisfied with their performance than ACSBC interns, potentially due to the revised format. The mock cases were adapted and consolidated for TBC, such that the handoff and consultation simulations shared a common case, whereas previously they were separate. Thus, intern preparation for TBC required familiarity with fewer overall cases. Ultimately, TBC maintained the quality of training but required review of less information.
In comparing performance, TBC interns were rated as well or better during consultation simulation compared to ASCBC, but handoffs were rated lower. This was likely due to the change in the handoff checklist from a dichotomous to a three-level rating scale. This change was made after receiving feedback from ACSBC TFs that a rating scale allowing for more nuance was needed to provide adequate feedback to interns. Although we defined handoff item completion for TBC interns as being rated “outstanding,” if the top two rankings, “outstanding” and “satisfactory,” are dichotomized to reflect completion, TBC handoff performance is equivalent or better than ACSBC. TF recruitment additionally differed between TBC and ACSBC cohorts. In ACSBC, resident physicians served as handoff TFs, whereas only faculty were recruited for TBC. Faculty were primarily clinically active hospitalists, whose expertise in handoffs may resulted in more stringent performance ratings, contributing to differences seen.
Hospitalist groups require clinicians to be immediately proficient in essential communication skills like consultation and handoffs, potentially requiring just-in-time training and feedback for large cohorts.12 Bootcamps can meet this need but require participation and time investment by many faculty members, staff, and administrators.5,8 Combining TBC into one virtual handoff/consultation simulation required recruitment and training of 50% fewer TFs and reduced administrative burden. ACSBC consultation simulations were high-fidelity but resource-heavy, requiring reliable two-way telephones with reliable connections and separate spaces for simulation and feedback.5 Conversely, TBC only required consultations to be “called” via audio-only Zoom® discussion, then both individuals turned on cameras for feedback. The slight decrease in perceived fidelity was certainly outweighed by ease of administration. TBC’s more efficient and less labor-intensive format is an appealing strategy for hospitalist groups looking to train up clinicians, including those operating across multiple or geographically distant sites.
Our study has limitations. It occurred with one group of learners at a single site with consistent consultation and handoff communication practices, which may not be the case elsewhere. Our comparison group was a separate cohort, and groups were not randomized; thus, differences seen may reflect inherent dissimilarities in these groups. Changes to the handoff checklist rating scale between 2019 and 2020 additionally may limit the direct comparison of handoff performance between cohorts. While overall fewer resources were required, TBC implementation did require time and institutional support, along with full virtual platform capability without user or time limitations. Our preparedness outcomes were self-reported without direct measurement of clinical performance, which is an area for future work.
We describe a feasible implementation of an adapted telesimulation communication bootcamp, with comparison to a previous in-person cohort’s skills performance and satisfaction. While COVID-19 has made the future of in-person training activities uncertain, it also served as a catalyst for educational innovation that may be sustained beyond the pandemic. Although developed out of necessity, the telesimulation communication bootcamp was effective and well-received. Telesimulation represents an opportunity for hospital medicine groups to implement advanced communication skills training and assessment in a more efficient, flexible, and potentially preferable way, even after the pandemic ends.
Acknowledgments
The authors thank the staff at the University of Chicago Office of Graduate Medical Education and the UChicago Medicine Simulation Center.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/ 10.1097/00001888-200402000-00019
2. Inadequate hand-off communication. Sentinel Event Alert. 2017;(58):1-6.
3. Horwitz LI, Meredith T, Schuur JD, Shah NR, Kulkarni RG, Jenq JY. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701-710. https://doi.org/ 10.1016/j.annemergmed.2008.05.007
4. Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hutter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607-2613. https://doi.org/10.1001/archinte.165.22.2607
5. Martin SK, Carter K, Hellerman N, et al. The consultation observed simulated clinical experience: training, assessment, and feedback for incoming interns on requesting consultations. Acad Med. 2018; 93(12):1814-1820. https://doi.org/10.1097/ACM.0000000000002337
6. Lopez MA, Campbell J. Developing a communication curriculum for primary and consulting services. Med Educ Online. 2020;25(1):1794341. https://doi.org/10.1080/10872981.2020
7. Cohen, ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern bootcamp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a
8. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The Modified, Multi-patient Observed Simulated Handoff Experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
9. Woolliscroft, J. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402.
10. Anderson ML, Turbow S, Willgerodt MA, Ruhnke G. Education in a crisis: the opportunity of our lives. J Hosp. Med 2020;5;287-291. https://doi.org/10.12788/jhm.3431
11. Farr DE, Zeh HJ, Abdelfattah KR. Virtual bootcamps—an emerging solution to the undergraduate medical education-graduate medical education transition. JAMA Surg. 2021;156(3):282-283. https://doi.org/10.1001/jamasurg.2020.6162
12. Hepps JH, Yu CE, Calaman S. Simulation in medical education for the hospitalist: moving beyond the mock code. Pediatr Clin North Am. 2019;66(4):855-866. https://doi.org/10.1016/j.pcl.2019.03.014
Events requiring communication among and within teams are vulnerable points in patient care in hospital medicine, with communication failures representing important contributors to adverse events.1-4 Consultations and handoffs are exceptionally common inpatient practices, yet training in these practices is variable across educational and practice domains.5,6 Advanced inpatient communication-skills training requires an effective, feasible, and scalable format. Simulation-based bootcamps can effectively support clinical skills training, often in procedural domains, and have been increasingly utilized for communication skills.7,8 We previously described the development and implementation of an in-person bootcamp for training and feedback in consultation and handoff communication.5,8
As hospitalist leaders grapple with how to systematically support and assess essential clinical skills, the COVID-19 pandemic has presented another impetus to rethink current processes. The rapid shift to virtual activities met immediate needs of the pandemic, but also inspired creativity in applying new methodologies to improve teaching strategies and implementation long-term.9,10 One such strategy, telesimulation, offers a way to continue simulation-based training limited by the need for physical distancing.10 Furthermore, recent calls to study the efficacy of virtual bootcamp structures have acknowledged potential benefits, even outside of the pandemic.11
The primary objective of this feasibility study was to convert our previously described consultation and handoff bootcamp to a telesimulation bootcamp (TBC), preserving rigorous performance evaluation and opportunities for skills-based feedback. We additionally compared evaluation between virtual and in-person formats to understand the utility of telesimulation for bootcamp-based clinical education moving forward.
METHODS
Setting and Participants
The TBC occurred in June 2020 during the University of Chicago institution-wide graduate medical education (GME) orientation; 130 interns entering 13 residency programs participated. The comparison group was 128 interns who underwent the traditional University of Chicago GME orientation “Advanced Communication Skills Bootcamp” (ACSBC) in 2019.5,8
Program Description
To develop TBC, we adapted observed structured clinical experiences (OSCEs) created for ACSBC. Until 2020, ACSBC included three in-person OSCEs: (1) requesting a consultation; (2) conducting handoffs; and (3) acquiring informed consent. COVID-19 necessitated conversion of ACSBC to virtual in June 2020. For this, we selected the consultation and handoff OSCEs, as these skills require near-universal and immediate application in clinical practice. Additionally, they required only trained facilitators (TFs), whereas informed consent required standardized patients. Hospitalist and emergency medicine faculty were recruited as TFs; 7 of 12 TFs were hospitalists. Each OSCE had two parts: an asynchronous, mandatory training module and a clinical simulation. For TBC, we adapted the simulations, previously separate experiences, into a 20-minute combined handoff/consultation telesimulation using the Zoom® video platform. Interns were paired with one TF who served as both standardized consultant (for one mock case) and handoff receiver (for three mock cases, including the consultation case). TFs rated intern performance and provided feedback.
TBC occurred on June 17 and 18, 2020. Interns were emailed asynchronous modules on June 1, and mock cases and instructions on June 12. When TBC began, GME staff proctors oriented interns in the Zoom® platform. Proctors placed TFs into private breakout rooms into which interns rotated through 20-minute timeslots. Faculty received copies of all TBC materials for review (Appendix 1) and underwent Zoom®-based training 1 to 2 weeks prior.
We evaluated TBC using several methods: (1) consultation and handoff skills performance measured by two validated checklists5,8; (2) survey of intern self-reported preparedness to practice consultations and handoffs; and (3) survey of intern satisfaction. Surveys were administered both immediately post bootcamp (Appendix 2) and 8 weeks into internship (Appendix 3). Skills performance checklists were a 12-item consultation checklist5 and 6-item handoff checklist.8 The handoff checklist was modified to remove activities impossible to assess virtually (ie, orienting sign-outs in a shared space) and to add a three-level rating scale of “outstanding,” “satisfactory,” and “needs improvement.” This was done based on feedback from ACSBC to allow more nuanced feedback for interns. A rating of “outstanding” was used to define successful completion of the item (Appendix 1). Interns rated preparedness and satisfaction on 5-point Likert-type items. All measures were compared to the 2019 in-person ACSBC cohort.
Data Analysis
Stata 16.1 (StataCorp LP) was used for analysis. We dichotomized preparedness and satisfaction scores, defining ratings of “4” or “5” as “prepared” or “satisfied.” As previously described,5 we created a composite score averaging both checklist scores for each intern. We normalized this score by rater to a z score (mean, 0; SD, 1) to account for rater differences. “Poor” and “outstanding” performances were defined as z scores below and above 1 SD, respectively. Fisher’s exact test was used to compare proportions, and Pearson correlation test to correlate z scores. The University of Chicago Institutional Review Board granted exemption.
RESULTS
All 130 entering interns participated in TBC. Internal medicine (IM) was the largest specialty (n = 37), followed by pediatrics (n = 22), emergency medicine (EM) (n = 16), and anesthesiology (n = 12). The remaining 9 programs ranged from 2 to 10 interns per program. The 128 interns in ACSBC were similar, including 40 IM, 23 pediatrics, 14 EM, and 12 anesthesia interns, with 2 to 10 interns in remaining programs.
TBC skills performance evaluations were compared to ACSBC (Table 1). The TBC intern cohort’s consultation performance was the same or better than the ACSBC intern cohort’s. For handoffs, TBC interns completed significantly fewer checklist items compared to ACSBC. Performance in each exercise was moderately correlated (r = 0.39, P < .05). For z scores, 14 TBC interns (10.8%) had “outstanding” and 15 (11.6%) had “poor” performances, compared to ACSBC interns with 7 (5.5%) “outstanding” and 10 (7.81%) “poor” performances (P = .15).
All 130 interns (100%) completed the immediate post-TBC survey. Overall, TBC satisfaction was comparable to ACSBC, and significantly improved for satisfaction with performance (Table 2). Compared to ACSBC, TBC interns felt more prepared for simulation and handoff clinical practice. Nearly all interns would recommend TBC (99% vs 96% of ACSBC interns, P = 0.28), and 99% felt the software used for the simulation ran smoothly.
The 8-week post-TBC survey had a response rate of 88% (115/130); 69% of interns reported conducting more effective handoffs due to TBC, and 79% felt confident in handoff skills. Similarly, 73% felt more effective at calling consultations, and 75% reported retained knowledge of consultation frameworks taught during TBC. Additionally, 71% of interns reported that TBC helped identify areas for self-directed improvement. There were no significant differences in 8-week postsurvey ratings between ACSBC and TBC.
DISCUSSION
In converting the advanced communication skills bootcamp from an in-person to a virtual format, telesimulation was well-received by interns and rated similarly to in-person bootcamp in most respects. Nearly all interns agreed the experience was realistic, provided useful feedback, and prepared them for clinical practice. Although we shifted to virtual out of necessity, our results demonstrate a high-quality, streamlined bootcamp experience that was less labor-intensive for interns, staff, and faculty. Telesimulation may represent an effective strategy beyond the COVID-19 pandemic to increase ease of administration and scale the use of bootcamps in supporting advanced clinical skill training for hospital-based practice.
TBC interns felt better prepared for simulation and more satisfied with their performance than ACSBC interns, potentially due to the revised format. The mock cases were adapted and consolidated for TBC, such that the handoff and consultation simulations shared a common case, whereas previously they were separate. Thus, intern preparation for TBC required familiarity with fewer overall cases. Ultimately, TBC maintained the quality of training but required review of less information.
In comparing performance, TBC interns were rated as well or better during consultation simulation compared to ASCBC, but handoffs were rated lower. This was likely due to the change in the handoff checklist from a dichotomous to a three-level rating scale. This change was made after receiving feedback from ACSBC TFs that a rating scale allowing for more nuance was needed to provide adequate feedback to interns. Although we defined handoff item completion for TBC interns as being rated “outstanding,” if the top two rankings, “outstanding” and “satisfactory,” are dichotomized to reflect completion, TBC handoff performance is equivalent or better than ACSBC. TF recruitment additionally differed between TBC and ACSBC cohorts. In ACSBC, resident physicians served as handoff TFs, whereas only faculty were recruited for TBC. Faculty were primarily clinically active hospitalists, whose expertise in handoffs may resulted in more stringent performance ratings, contributing to differences seen.
Hospitalist groups require clinicians to be immediately proficient in essential communication skills like consultation and handoffs, potentially requiring just-in-time training and feedback for large cohorts.12 Bootcamps can meet this need but require participation and time investment by many faculty members, staff, and administrators.5,8 Combining TBC into one virtual handoff/consultation simulation required recruitment and training of 50% fewer TFs and reduced administrative burden. ACSBC consultation simulations were high-fidelity but resource-heavy, requiring reliable two-way telephones with reliable connections and separate spaces for simulation and feedback.5 Conversely, TBC only required consultations to be “called” via audio-only Zoom® discussion, then both individuals turned on cameras for feedback. The slight decrease in perceived fidelity was certainly outweighed by ease of administration. TBC’s more efficient and less labor-intensive format is an appealing strategy for hospitalist groups looking to train up clinicians, including those operating across multiple or geographically distant sites.
Our study has limitations. It occurred with one group of learners at a single site with consistent consultation and handoff communication practices, which may not be the case elsewhere. Our comparison group was a separate cohort, and groups were not randomized; thus, differences seen may reflect inherent dissimilarities in these groups. Changes to the handoff checklist rating scale between 2019 and 2020 additionally may limit the direct comparison of handoff performance between cohorts. While overall fewer resources were required, TBC implementation did require time and institutional support, along with full virtual platform capability without user or time limitations. Our preparedness outcomes were self-reported without direct measurement of clinical performance, which is an area for future work.
We describe a feasible implementation of an adapted telesimulation communication bootcamp, with comparison to a previous in-person cohort’s skills performance and satisfaction. While COVID-19 has made the future of in-person training activities uncertain, it also served as a catalyst for educational innovation that may be sustained beyond the pandemic. Although developed out of necessity, the telesimulation communication bootcamp was effective and well-received. Telesimulation represents an opportunity for hospital medicine groups to implement advanced communication skills training and assessment in a more efficient, flexible, and potentially preferable way, even after the pandemic ends.
Acknowledgments
The authors thank the staff at the University of Chicago Office of Graduate Medical Education and the UChicago Medicine Simulation Center.
Events requiring communication among and within teams are vulnerable points in patient care in hospital medicine, with communication failures representing important contributors to adverse events.1-4 Consultations and handoffs are exceptionally common inpatient practices, yet training in these practices is variable across educational and practice domains.5,6 Advanced inpatient communication-skills training requires an effective, feasible, and scalable format. Simulation-based bootcamps can effectively support clinical skills training, often in procedural domains, and have been increasingly utilized for communication skills.7,8 We previously described the development and implementation of an in-person bootcamp for training and feedback in consultation and handoff communication.5,8
As hospitalist leaders grapple with how to systematically support and assess essential clinical skills, the COVID-19 pandemic has presented another impetus to rethink current processes. The rapid shift to virtual activities met immediate needs of the pandemic, but also inspired creativity in applying new methodologies to improve teaching strategies and implementation long-term.9,10 One such strategy, telesimulation, offers a way to continue simulation-based training limited by the need for physical distancing.10 Furthermore, recent calls to study the efficacy of virtual bootcamp structures have acknowledged potential benefits, even outside of the pandemic.11
The primary objective of this feasibility study was to convert our previously described consultation and handoff bootcamp to a telesimulation bootcamp (TBC), preserving rigorous performance evaluation and opportunities for skills-based feedback. We additionally compared evaluation between virtual and in-person formats to understand the utility of telesimulation for bootcamp-based clinical education moving forward.
METHODS
Setting and Participants
The TBC occurred in June 2020 during the University of Chicago institution-wide graduate medical education (GME) orientation; 130 interns entering 13 residency programs participated. The comparison group was 128 interns who underwent the traditional University of Chicago GME orientation “Advanced Communication Skills Bootcamp” (ACSBC) in 2019.5,8
Program Description
To develop TBC, we adapted observed structured clinical experiences (OSCEs) created for ACSBC. Until 2020, ACSBC included three in-person OSCEs: (1) requesting a consultation; (2) conducting handoffs; and (3) acquiring informed consent. COVID-19 necessitated conversion of ACSBC to virtual in June 2020. For this, we selected the consultation and handoff OSCEs, as these skills require near-universal and immediate application in clinical practice. Additionally, they required only trained facilitators (TFs), whereas informed consent required standardized patients. Hospitalist and emergency medicine faculty were recruited as TFs; 7 of 12 TFs were hospitalists. Each OSCE had two parts: an asynchronous, mandatory training module and a clinical simulation. For TBC, we adapted the simulations, previously separate experiences, into a 20-minute combined handoff/consultation telesimulation using the Zoom® video platform. Interns were paired with one TF who served as both standardized consultant (for one mock case) and handoff receiver (for three mock cases, including the consultation case). TFs rated intern performance and provided feedback.
TBC occurred on June 17 and 18, 2020. Interns were emailed asynchronous modules on June 1, and mock cases and instructions on June 12. When TBC began, GME staff proctors oriented interns in the Zoom® platform. Proctors placed TFs into private breakout rooms into which interns rotated through 20-minute timeslots. Faculty received copies of all TBC materials for review (Appendix 1) and underwent Zoom®-based training 1 to 2 weeks prior.
We evaluated TBC using several methods: (1) consultation and handoff skills performance measured by two validated checklists5,8; (2) survey of intern self-reported preparedness to practice consultations and handoffs; and (3) survey of intern satisfaction. Surveys were administered both immediately post bootcamp (Appendix 2) and 8 weeks into internship (Appendix 3). Skills performance checklists were a 12-item consultation checklist5 and 6-item handoff checklist.8 The handoff checklist was modified to remove activities impossible to assess virtually (ie, orienting sign-outs in a shared space) and to add a three-level rating scale of “outstanding,” “satisfactory,” and “needs improvement.” This was done based on feedback from ACSBC to allow more nuanced feedback for interns. A rating of “outstanding” was used to define successful completion of the item (Appendix 1). Interns rated preparedness and satisfaction on 5-point Likert-type items. All measures were compared to the 2019 in-person ACSBC cohort.
Data Analysis
Stata 16.1 (StataCorp LP) was used for analysis. We dichotomized preparedness and satisfaction scores, defining ratings of “4” or “5” as “prepared” or “satisfied.” As previously described,5 we created a composite score averaging both checklist scores for each intern. We normalized this score by rater to a z score (mean, 0; SD, 1) to account for rater differences. “Poor” and “outstanding” performances were defined as z scores below and above 1 SD, respectively. Fisher’s exact test was used to compare proportions, and Pearson correlation test to correlate z scores. The University of Chicago Institutional Review Board granted exemption.
RESULTS
All 130 entering interns participated in TBC. Internal medicine (IM) was the largest specialty (n = 37), followed by pediatrics (n = 22), emergency medicine (EM) (n = 16), and anesthesiology (n = 12). The remaining 9 programs ranged from 2 to 10 interns per program. The 128 interns in ACSBC were similar, including 40 IM, 23 pediatrics, 14 EM, and 12 anesthesia interns, with 2 to 10 interns in remaining programs.
TBC skills performance evaluations were compared to ACSBC (Table 1). The TBC intern cohort’s consultation performance was the same or better than the ACSBC intern cohort’s. For handoffs, TBC interns completed significantly fewer checklist items compared to ACSBC. Performance in each exercise was moderately correlated (r = 0.39, P < .05). For z scores, 14 TBC interns (10.8%) had “outstanding” and 15 (11.6%) had “poor” performances, compared to ACSBC interns with 7 (5.5%) “outstanding” and 10 (7.81%) “poor” performances (P = .15).
All 130 interns (100%) completed the immediate post-TBC survey. Overall, TBC satisfaction was comparable to ACSBC, and significantly improved for satisfaction with performance (Table 2). Compared to ACSBC, TBC interns felt more prepared for simulation and handoff clinical practice. Nearly all interns would recommend TBC (99% vs 96% of ACSBC interns, P = 0.28), and 99% felt the software used for the simulation ran smoothly.
The 8-week post-TBC survey had a response rate of 88% (115/130); 69% of interns reported conducting more effective handoffs due to TBC, and 79% felt confident in handoff skills. Similarly, 73% felt more effective at calling consultations, and 75% reported retained knowledge of consultation frameworks taught during TBC. Additionally, 71% of interns reported that TBC helped identify areas for self-directed improvement. There were no significant differences in 8-week postsurvey ratings between ACSBC and TBC.
DISCUSSION
In converting the advanced communication skills bootcamp from an in-person to a virtual format, telesimulation was well-received by interns and rated similarly to in-person bootcamp in most respects. Nearly all interns agreed the experience was realistic, provided useful feedback, and prepared them for clinical practice. Although we shifted to virtual out of necessity, our results demonstrate a high-quality, streamlined bootcamp experience that was less labor-intensive for interns, staff, and faculty. Telesimulation may represent an effective strategy beyond the COVID-19 pandemic to increase ease of administration and scale the use of bootcamps in supporting advanced clinical skill training for hospital-based practice.
TBC interns felt better prepared for simulation and more satisfied with their performance than ACSBC interns, potentially due to the revised format. The mock cases were adapted and consolidated for TBC, such that the handoff and consultation simulations shared a common case, whereas previously they were separate. Thus, intern preparation for TBC required familiarity with fewer overall cases. Ultimately, TBC maintained the quality of training but required review of less information.
In comparing performance, TBC interns were rated as well or better during consultation simulation compared to ASCBC, but handoffs were rated lower. This was likely due to the change in the handoff checklist from a dichotomous to a three-level rating scale. This change was made after receiving feedback from ACSBC TFs that a rating scale allowing for more nuance was needed to provide adequate feedback to interns. Although we defined handoff item completion for TBC interns as being rated “outstanding,” if the top two rankings, “outstanding” and “satisfactory,” are dichotomized to reflect completion, TBC handoff performance is equivalent or better than ACSBC. TF recruitment additionally differed between TBC and ACSBC cohorts. In ACSBC, resident physicians served as handoff TFs, whereas only faculty were recruited for TBC. Faculty were primarily clinically active hospitalists, whose expertise in handoffs may resulted in more stringent performance ratings, contributing to differences seen.
Hospitalist groups require clinicians to be immediately proficient in essential communication skills like consultation and handoffs, potentially requiring just-in-time training and feedback for large cohorts.12 Bootcamps can meet this need but require participation and time investment by many faculty members, staff, and administrators.5,8 Combining TBC into one virtual handoff/consultation simulation required recruitment and training of 50% fewer TFs and reduced administrative burden. ACSBC consultation simulations were high-fidelity but resource-heavy, requiring reliable two-way telephones with reliable connections and separate spaces for simulation and feedback.5 Conversely, TBC only required consultations to be “called” via audio-only Zoom® discussion, then both individuals turned on cameras for feedback. The slight decrease in perceived fidelity was certainly outweighed by ease of administration. TBC’s more efficient and less labor-intensive format is an appealing strategy for hospitalist groups looking to train up clinicians, including those operating across multiple or geographically distant sites.
Our study has limitations. It occurred with one group of learners at a single site with consistent consultation and handoff communication practices, which may not be the case elsewhere. Our comparison group was a separate cohort, and groups were not randomized; thus, differences seen may reflect inherent dissimilarities in these groups. Changes to the handoff checklist rating scale between 2019 and 2020 additionally may limit the direct comparison of handoff performance between cohorts. While overall fewer resources were required, TBC implementation did require time and institutional support, along with full virtual platform capability without user or time limitations. Our preparedness outcomes were self-reported without direct measurement of clinical performance, which is an area for future work.
We describe a feasible implementation of an adapted telesimulation communication bootcamp, with comparison to a previous in-person cohort’s skills performance and satisfaction. While COVID-19 has made the future of in-person training activities uncertain, it also served as a catalyst for educational innovation that may be sustained beyond the pandemic. Although developed out of necessity, the telesimulation communication bootcamp was effective and well-received. Telesimulation represents an opportunity for hospital medicine groups to implement advanced communication skills training and assessment in a more efficient, flexible, and potentially preferable way, even after the pandemic ends.
Acknowledgments
The authors thank the staff at the University of Chicago Office of Graduate Medical Education and the UChicago Medicine Simulation Center.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/ 10.1097/00001888-200402000-00019
2. Inadequate hand-off communication. Sentinel Event Alert. 2017;(58):1-6.
3. Horwitz LI, Meredith T, Schuur JD, Shah NR, Kulkarni RG, Jenq JY. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701-710. https://doi.org/ 10.1016/j.annemergmed.2008.05.007
4. Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hutter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607-2613. https://doi.org/10.1001/archinte.165.22.2607
5. Martin SK, Carter K, Hellerman N, et al. The consultation observed simulated clinical experience: training, assessment, and feedback for incoming interns on requesting consultations. Acad Med. 2018; 93(12):1814-1820. https://doi.org/10.1097/ACM.0000000000002337
6. Lopez MA, Campbell J. Developing a communication curriculum for primary and consulting services. Med Educ Online. 2020;25(1):1794341. https://doi.org/10.1080/10872981.2020
7. Cohen, ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern bootcamp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a
8. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The Modified, Multi-patient Observed Simulated Handoff Experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
9. Woolliscroft, J. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402.
10. Anderson ML, Turbow S, Willgerodt MA, Ruhnke G. Education in a crisis: the opportunity of our lives. J Hosp. Med 2020;5;287-291. https://doi.org/10.12788/jhm.3431
11. Farr DE, Zeh HJ, Abdelfattah KR. Virtual bootcamps—an emerging solution to the undergraduate medical education-graduate medical education transition. JAMA Surg. 2021;156(3):282-283. https://doi.org/10.1001/jamasurg.2020.6162
12. Hepps JH, Yu CE, Calaman S. Simulation in medical education for the hospitalist: moving beyond the mock code. Pediatr Clin North Am. 2019;66(4):855-866. https://doi.org/10.1016/j.pcl.2019.03.014
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/ 10.1097/00001888-200402000-00019
2. Inadequate hand-off communication. Sentinel Event Alert. 2017;(58):1-6.
3. Horwitz LI, Meredith T, Schuur JD, Shah NR, Kulkarni RG, Jenq JY. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701-710. https://doi.org/ 10.1016/j.annemergmed.2008.05.007
4. Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hutter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607-2613. https://doi.org/10.1001/archinte.165.22.2607
5. Martin SK, Carter K, Hellerman N, et al. The consultation observed simulated clinical experience: training, assessment, and feedback for incoming interns on requesting consultations. Acad Med. 2018; 93(12):1814-1820. https://doi.org/10.1097/ACM.0000000000002337
6. Lopez MA, Campbell J. Developing a communication curriculum for primary and consulting services. Med Educ Online. 2020;25(1):1794341. https://doi.org/10.1080/10872981.2020
7. Cohen, ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern bootcamp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a
8. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The Modified, Multi-patient Observed Simulated Handoff Experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
9. Woolliscroft, J. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402.
10. Anderson ML, Turbow S, Willgerodt MA, Ruhnke G. Education in a crisis: the opportunity of our lives. J Hosp. Med 2020;5;287-291. https://doi.org/10.12788/jhm.3431
11. Farr DE, Zeh HJ, Abdelfattah KR. Virtual bootcamps—an emerging solution to the undergraduate medical education-graduate medical education transition. JAMA Surg. 2021;156(3):282-283. https://doi.org/10.1001/jamasurg.2020.6162
12. Hepps JH, Yu CE, Calaman S. Simulation in medical education for the hospitalist: moving beyond the mock code. Pediatr Clin North Am. 2019;66(4):855-866. https://doi.org/10.1016/j.pcl.2019.03.014
© 2021 Society of Hospital Medicine
Defining Potential Overutilization of Physical Therapy Consults on Hospital Medicine Services
During hospitalization, patients spend 87% to 100% of their time in bed.1 This prolonged immobilization is a key contributor to the development of hospital-associated disability (HAD), defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge. HAD can lead to readmissions, institutionalization, and death and occurs in approximately one-third of all hospitalized patients.2,3 The most effective way to prevent HAD is by mobilizing patients early and throughout their hospitalization.4 Typically, physical therapists are the primary team members responsible for mobilizing patients, but they are a constrained resource in most inpatient settings.
The Activity Measure-Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a validated tool for measuring physical function.5 The AM-PAC score has been used to predict discharge destination within 48 hours of admission6 and as a guide to allocate inpatient therapy referrals on a medical and a neurosurgical service.7,8 To date, however, no studies have used AM-PAC scores to evaluate overutilization of physical therapy consults on direct care hospital medicine services. In this study, we aimed to assess the potential overutilization of physical therapy consults on direct care hospital medicine services using validated AM-PAC score cutoffs.
METHODS
Study Design and Setting
We analyzed a retrospective cohort of admissions from September 30, 2018, through September 29, 2019, on all direct care hospital medicine services at the University of Chicago Medical Center (UC), Illinois. These services included general medicine, oncology, transplant (renal, lung, and liver), cardiology, and cirrhotic populations at the medical-surgical and telemetry level of care. All patients were hospitalized for longer than 48 hours. Patients who left against medical advice; died; were discharged to hospice, another hospital, or an inpatient psychiatric facility; or received no physical therapy referral during admission were excluded. For the remaining patients, we obtained age, sex, admission and discharge dates, admission and discharge AM-PAC scores, and discharge disposition.
Mobility Measure
At UC, the inpatient mobility protocol requires nursing staff to assess and document AM-PAC mobility scores for each patient at the time of admission and every nursing shift thereafter. They utilize the original version of the AM-PAC “6-Clicks” Basic Mobility score, which includes three questions assessing difficulty with mobility and three questions assessing help needed with mobility activities. It has high interrater reliability, with an intraclass correlation coefficient of 0.85.9
Outcomes and Predictors
The primary outcome was “potential overutilization.” Secondary outcomes were discharge disposition and change in mobility. Our predictors included admission AM-PAC score, age, and sex. Based on previous studies that validated an AM-PAC score of 42.9 (raw score, 17) as a cutoff for predicting discharge to home,6 we defined physical therapy consults as “potentially inappropriate” in patients with admission AM-PAC scores >43.63 (raw score, 18) who were discharged to home. Likewise, in the UC mobility protocol, nursing staff independently mobilize patients with AM-PAC scores >18, another rationale to use this cutoff for defining physical therapy consult inappropriateness. “Discharge to home” was defined as going home with no additional needs or services, going home with outpatient physical therapy, or going home with home health physical therapy services, since none of these require inpatient physical therapy assessment for the order to be placed. Discharge to long-term acute care, skilled nursing facility, subacute rehabilitation facility, or acute rehabilitation facility were considered “discharge to post–acute care.” Loss of mobility was calculated as: discharge AM-PAC − admission AM-PAC, termed delta AM-PAC.
Statistical Analysis
Descriptive statistics were used to summarize age (mean and SD) and age categorized as <65 years or ≥65 years, sex (male or female), admission AM-PAC score (mean and SD) and categorization (≤43.63 or >43.63), discharge AM-PAC score (mean and SD), and discharge destination (home vs post–acute care). Chi-square analysis was used to test for associations between admission AM-PAC score and delta AM-PAC. Two-sample t-test was used to test for difference in mean delta AM-PAC between admission AM-PAC groups. Multivariable logistic regression was used to test for independent associations between age, sex, and admission AM-PAC score and odds of being discharged to home, controlling for length of stay. P values of <.05 were considered statistically significant for all tests. Analyses were performed using Stata statistical software, release 16 (StataCorp LLC).
RESULTS
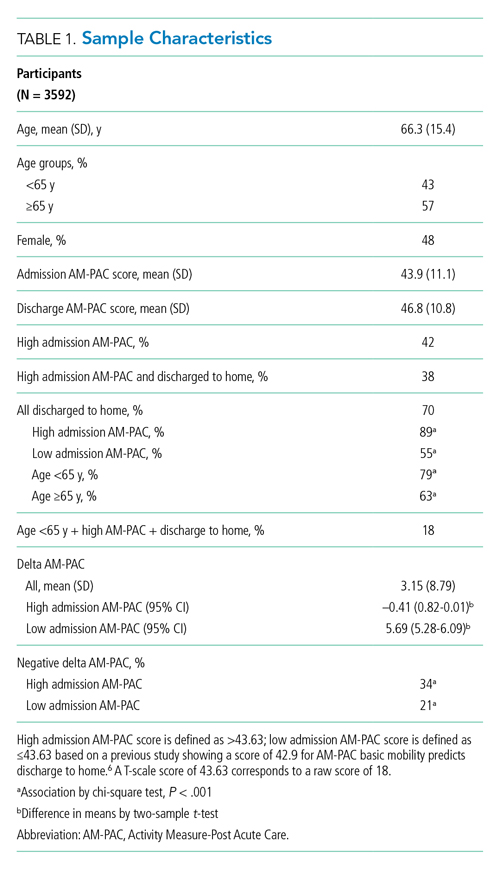
During the 1-year study period, 3592 admissions with physical therapy consults occurred on the direct care hospital medicine services (58% of all admissions). Mean age was 66.3 years (SD, 15.4 years), and 48% of patients were female. The mean admission AM-PAC score was 43.9 (SD, 11.1), and the mean discharge AM-PAC score was 46.8 (SD, 10.8). In our sample, 38% of physical therapy consults were for patients with an AM-PAC score >43.63 who were discharged to home and were therefore deemed “potential overutilization.” Of those, 40% were for patients who were 65 years or younger (18% of all physical therapy consults) (Table 1).
A higher proportion of patients with AM-PAC scores >43.63 were discharged to home compared with those with AM-PAC scores ≤43.63 (89% vs 55%; χ2 [1, N = 3099], 396.5; P < .001). More patients younger than 65 years were discharged to home compared with those 65 years and older (79% vs 63%; χ2 [1, N = 3099], 113.6; P < .001). Additionally, for all patients younger than 65 years, those with AM-PAC score >43.63 were discharged to home more frequently than those with AM-PAC ≤43.63 (92% vs 66%, χ2 [1, N = 1,354], 134.4; P < .001). For 11% (n = 147) of the high-mobility group, the patient was not discharged home but was sent to post–acute care. Reviewing these patient charts showed the reasons for discharge to post–acute care were predominantly personal or social needs (eg, homelessness, need for 24-hour supervision with no family support, patient request) or medical needs (eg, intravenous antibiotics or new tubes, lines, drains, or medications requiring extra nursing support or management). Only 16% of patients in this group (n = 23) experienced deconditioning necessitating physical therapy consult during hospitalization, per their record.
Compared with patients with admission AM-PAC score >43.63, patients with admission AM-PAC ≤43.63 had significantly different changes in mobility as measured by mean delta AM-PAC score (delta AM-PAC, –0.41 for AM-PAC >43.63 vs +5.69 for AM-PAC ≤43.63; t (3097) = –20.3; P < .001) (Table 1).
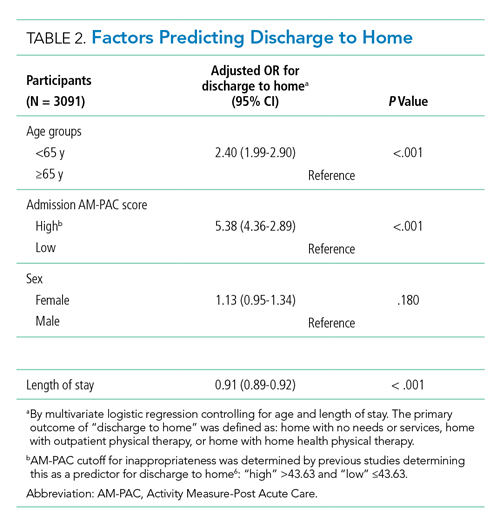
In multivariate logistic regression, AM-PAC >43.63 (OR, 5.38; 95% CI, 4.36-2.89; P < .001) and age younger than 65 years (OR, 2.40; 95% CI, 1.99-2.90; P < .001) were associated with increased odds of discharge to home (Table 2).
DISCUSSION
In this study, we found that physical therapists may be unnecessarily consulted on direct care hospitalist services as much as 38% of the time based on AM-PAC score. We also demonstrated that patients admitted with high mobility by AM-PAC score are more than five times as likely to be discharged to home. When admitted with high AM-PAC scores, patients had virtually no change in mobility during hospitalization, whereas patients with low AM-PAC scores gained mobility during hospitalization, underscoring the benefit of physical therapy referrals for this group.
Given resource scarcity and cost, achieving optimal physical therapy utilization is an important goal for healthcare systems.10 Appropriate allocation of physical therapy has the potential to improve outcomes from the patient to the payor level. While it may be necessary to consult physical therapy for reasons other than mobility later in the hospitalization, identifying patients who will benefit from skilled physical therapy at the time of admission can help prevent disability and institutionalization and shorten length of stay.5,6 Likewise, decreasing physical therapy referrals for low-risk patients can increase the amount of time spent rehabilitating at-risk patients.
There are limitations of our study worth considering. First, our analyses did not consider whether physical therapy contributed to patients’ ability to return home after discharge. However, in our hospital, patients with AM-PAC >43.63 who cannot safely ambulate independently do progressive mobility with nursing staff. Our physical therapy leadership has also observed that the vast majority of highly mobile patients who are referred for physical therapy ultimately receive no treatment. Second, we did not consider discharge diagnosis, but our patient populations present with a wide variety of conditions, and it is impossible to predict their discharge diagnosis. By not including discharge diagnosis, we assess how AM-PAC performs on admission regardless of the medical condition for which someone is treated. Our hospital treats a high proportion of African American and a low proportion of White, Hispanic, and Asian American patients, limiting the generalizability of our findings. Although the AM-PAC “6-Clicks” score has been shown to have high interrater reliability among physical therapists, our AM-PAC scores are assessed and documented by our nursing staff, which might decrease accuracy. However, one single-center study noted an intraclass correlation coefficient of 0.96 between nurses and physical therapists for the AM-PAC “6-Clicks.”11Despite these limitations, this study underscores the need to be more judicious in the decision to refer a patient for inpatient physical therapy, especially at the time of admission, and demonstrates the utility of using standardized mobility assessment to help in that decision-making process.
1. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? A systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
2. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x
3. Brown C.J, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263-1270. https://doi.org/10.1111/j.1532-5415.2004.52354.x
4. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63:55-62. https://doi.org/10.1111/jgs.13193
5. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379-391. https://doi.org/10.2522/ptj.20130199
6. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. https://doi.org/10.2522/ptj.20130359
7. Probasco JC, Lavezza A, Cassell A, et al. Choosing wisely together: physical and occupational therapy consultation for acute neurology inpatients. Neurohospitalist. 2018;8(2):53-59. https://doi.org/10.1177/1941874417729981
8. Young DL, Colantuoni E, Friedman LA, et al. Prediction of disposition within 48 hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9);540-543. https://doi.org/10.12788/jhm.3332
9. Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater reliability of AM-PAC “6-Clicks” basic mobility and daily activity short forms. Phys Ther. 2015;95(5):758-766. https://doi.org/10.2522/ptj.20140174
10. Juneau A, Bolduc A, Nguyen P, et al. Feasibility of implementing an exercise program in a geriatric assessment unit: the SPRINT program. Can Geriatr J. 2018;21(3):284-289. https://doi.org/10.5770/cgj.21.311
11. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110
During hospitalization, patients spend 87% to 100% of their time in bed.1 This prolonged immobilization is a key contributor to the development of hospital-associated disability (HAD), defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge. HAD can lead to readmissions, institutionalization, and death and occurs in approximately one-third of all hospitalized patients.2,3 The most effective way to prevent HAD is by mobilizing patients early and throughout their hospitalization.4 Typically, physical therapists are the primary team members responsible for mobilizing patients, but they are a constrained resource in most inpatient settings.
The Activity Measure-Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a validated tool for measuring physical function.5 The AM-PAC score has been used to predict discharge destination within 48 hours of admission6 and as a guide to allocate inpatient therapy referrals on a medical and a neurosurgical service.7,8 To date, however, no studies have used AM-PAC scores to evaluate overutilization of physical therapy consults on direct care hospital medicine services. In this study, we aimed to assess the potential overutilization of physical therapy consults on direct care hospital medicine services using validated AM-PAC score cutoffs.
METHODS
Study Design and Setting
We analyzed a retrospective cohort of admissions from September 30, 2018, through September 29, 2019, on all direct care hospital medicine services at the University of Chicago Medical Center (UC), Illinois. These services included general medicine, oncology, transplant (renal, lung, and liver), cardiology, and cirrhotic populations at the medical-surgical and telemetry level of care. All patients were hospitalized for longer than 48 hours. Patients who left against medical advice; died; were discharged to hospice, another hospital, or an inpatient psychiatric facility; or received no physical therapy referral during admission were excluded. For the remaining patients, we obtained age, sex, admission and discharge dates, admission and discharge AM-PAC scores, and discharge disposition.
Mobility Measure
At UC, the inpatient mobility protocol requires nursing staff to assess and document AM-PAC mobility scores for each patient at the time of admission and every nursing shift thereafter. They utilize the original version of the AM-PAC “6-Clicks” Basic Mobility score, which includes three questions assessing difficulty with mobility and three questions assessing help needed with mobility activities. It has high interrater reliability, with an intraclass correlation coefficient of 0.85.9
Outcomes and Predictors
The primary outcome was “potential overutilization.” Secondary outcomes were discharge disposition and change in mobility. Our predictors included admission AM-PAC score, age, and sex. Based on previous studies that validated an AM-PAC score of 42.9 (raw score, 17) as a cutoff for predicting discharge to home,6 we defined physical therapy consults as “potentially inappropriate” in patients with admission AM-PAC scores >43.63 (raw score, 18) who were discharged to home. Likewise, in the UC mobility protocol, nursing staff independently mobilize patients with AM-PAC scores >18, another rationale to use this cutoff for defining physical therapy consult inappropriateness. “Discharge to home” was defined as going home with no additional needs or services, going home with outpatient physical therapy, or going home with home health physical therapy services, since none of these require inpatient physical therapy assessment for the order to be placed. Discharge to long-term acute care, skilled nursing facility, subacute rehabilitation facility, or acute rehabilitation facility were considered “discharge to post–acute care.” Loss of mobility was calculated as: discharge AM-PAC − admission AM-PAC, termed delta AM-PAC.
Statistical Analysis
Descriptive statistics were used to summarize age (mean and SD) and age categorized as <65 years or ≥65 years, sex (male or female), admission AM-PAC score (mean and SD) and categorization (≤43.63 or >43.63), discharge AM-PAC score (mean and SD), and discharge destination (home vs post–acute care). Chi-square analysis was used to test for associations between admission AM-PAC score and delta AM-PAC. Two-sample t-test was used to test for difference in mean delta AM-PAC between admission AM-PAC groups. Multivariable logistic regression was used to test for independent associations between age, sex, and admission AM-PAC score and odds of being discharged to home, controlling for length of stay. P values of <.05 were considered statistically significant for all tests. Analyses were performed using Stata statistical software, release 16 (StataCorp LLC).
RESULTS
During the 1-year study period, 3592 admissions with physical therapy consults occurred on the direct care hospital medicine services (58% of all admissions). Mean age was 66.3 years (SD, 15.4 years), and 48% of patients were female. The mean admission AM-PAC score was 43.9 (SD, 11.1), and the mean discharge AM-PAC score was 46.8 (SD, 10.8). In our sample, 38% of physical therapy consults were for patients with an AM-PAC score >43.63 who were discharged to home and were therefore deemed “potential overutilization.” Of those, 40% were for patients who were 65 years or younger (18% of all physical therapy consults) (Table 1).
A higher proportion of patients with AM-PAC scores >43.63 were discharged to home compared with those with AM-PAC scores ≤43.63 (89% vs 55%; χ2 [1, N = 3099], 396.5; P < .001). More patients younger than 65 years were discharged to home compared with those 65 years and older (79% vs 63%; χ2 [1, N = 3099], 113.6; P < .001). Additionally, for all patients younger than 65 years, those with AM-PAC score >43.63 were discharged to home more frequently than those with AM-PAC ≤43.63 (92% vs 66%, χ2 [1, N = 1,354], 134.4; P < .001). For 11% (n = 147) of the high-mobility group, the patient was not discharged home but was sent to post–acute care. Reviewing these patient charts showed the reasons for discharge to post–acute care were predominantly personal or social needs (eg, homelessness, need for 24-hour supervision with no family support, patient request) or medical needs (eg, intravenous antibiotics or new tubes, lines, drains, or medications requiring extra nursing support or management). Only 16% of patients in this group (n = 23) experienced deconditioning necessitating physical therapy consult during hospitalization, per their record.
Compared with patients with admission AM-PAC score >43.63, patients with admission AM-PAC ≤43.63 had significantly different changes in mobility as measured by mean delta AM-PAC score (delta AM-PAC, –0.41 for AM-PAC >43.63 vs +5.69 for AM-PAC ≤43.63; t (3097) = –20.3; P < .001) (Table 1).
In multivariate logistic regression, AM-PAC >43.63 (OR, 5.38; 95% CI, 4.36-2.89; P < .001) and age younger than 65 years (OR, 2.40; 95% CI, 1.99-2.90; P < .001) were associated with increased odds of discharge to home (Table 2).
DISCUSSION
In this study, we found that physical therapists may be unnecessarily consulted on direct care hospitalist services as much as 38% of the time based on AM-PAC score. We also demonstrated that patients admitted with high mobility by AM-PAC score are more than five times as likely to be discharged to home. When admitted with high AM-PAC scores, patients had virtually no change in mobility during hospitalization, whereas patients with low AM-PAC scores gained mobility during hospitalization, underscoring the benefit of physical therapy referrals for this group.
Given resource scarcity and cost, achieving optimal physical therapy utilization is an important goal for healthcare systems.10 Appropriate allocation of physical therapy has the potential to improve outcomes from the patient to the payor level. While it may be necessary to consult physical therapy for reasons other than mobility later in the hospitalization, identifying patients who will benefit from skilled physical therapy at the time of admission can help prevent disability and institutionalization and shorten length of stay.5,6 Likewise, decreasing physical therapy referrals for low-risk patients can increase the amount of time spent rehabilitating at-risk patients.
There are limitations of our study worth considering. First, our analyses did not consider whether physical therapy contributed to patients’ ability to return home after discharge. However, in our hospital, patients with AM-PAC >43.63 who cannot safely ambulate independently do progressive mobility with nursing staff. Our physical therapy leadership has also observed that the vast majority of highly mobile patients who are referred for physical therapy ultimately receive no treatment. Second, we did not consider discharge diagnosis, but our patient populations present with a wide variety of conditions, and it is impossible to predict their discharge diagnosis. By not including discharge diagnosis, we assess how AM-PAC performs on admission regardless of the medical condition for which someone is treated. Our hospital treats a high proportion of African American and a low proportion of White, Hispanic, and Asian American patients, limiting the generalizability of our findings. Although the AM-PAC “6-Clicks” score has been shown to have high interrater reliability among physical therapists, our AM-PAC scores are assessed and documented by our nursing staff, which might decrease accuracy. However, one single-center study noted an intraclass correlation coefficient of 0.96 between nurses and physical therapists for the AM-PAC “6-Clicks.”11Despite these limitations, this study underscores the need to be more judicious in the decision to refer a patient for inpatient physical therapy, especially at the time of admission, and demonstrates the utility of using standardized mobility assessment to help in that decision-making process.
During hospitalization, patients spend 87% to 100% of their time in bed.1 This prolonged immobilization is a key contributor to the development of hospital-associated disability (HAD), defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge. HAD can lead to readmissions, institutionalization, and death and occurs in approximately one-third of all hospitalized patients.2,3 The most effective way to prevent HAD is by mobilizing patients early and throughout their hospitalization.4 Typically, physical therapists are the primary team members responsible for mobilizing patients, but they are a constrained resource in most inpatient settings.
The Activity Measure-Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a validated tool for measuring physical function.5 The AM-PAC score has been used to predict discharge destination within 48 hours of admission6 and as a guide to allocate inpatient therapy referrals on a medical and a neurosurgical service.7,8 To date, however, no studies have used AM-PAC scores to evaluate overutilization of physical therapy consults on direct care hospital medicine services. In this study, we aimed to assess the potential overutilization of physical therapy consults on direct care hospital medicine services using validated AM-PAC score cutoffs.
METHODS
Study Design and Setting
We analyzed a retrospective cohort of admissions from September 30, 2018, through September 29, 2019, on all direct care hospital medicine services at the University of Chicago Medical Center (UC), Illinois. These services included general medicine, oncology, transplant (renal, lung, and liver), cardiology, and cirrhotic populations at the medical-surgical and telemetry level of care. All patients were hospitalized for longer than 48 hours. Patients who left against medical advice; died; were discharged to hospice, another hospital, or an inpatient psychiatric facility; or received no physical therapy referral during admission were excluded. For the remaining patients, we obtained age, sex, admission and discharge dates, admission and discharge AM-PAC scores, and discharge disposition.
Mobility Measure
At UC, the inpatient mobility protocol requires nursing staff to assess and document AM-PAC mobility scores for each patient at the time of admission and every nursing shift thereafter. They utilize the original version of the AM-PAC “6-Clicks” Basic Mobility score, which includes three questions assessing difficulty with mobility and three questions assessing help needed with mobility activities. It has high interrater reliability, with an intraclass correlation coefficient of 0.85.9
Outcomes and Predictors
The primary outcome was “potential overutilization.” Secondary outcomes were discharge disposition and change in mobility. Our predictors included admission AM-PAC score, age, and sex. Based on previous studies that validated an AM-PAC score of 42.9 (raw score, 17) as a cutoff for predicting discharge to home,6 we defined physical therapy consults as “potentially inappropriate” in patients with admission AM-PAC scores >43.63 (raw score, 18) who were discharged to home. Likewise, in the UC mobility protocol, nursing staff independently mobilize patients with AM-PAC scores >18, another rationale to use this cutoff for defining physical therapy consult inappropriateness. “Discharge to home” was defined as going home with no additional needs or services, going home with outpatient physical therapy, or going home with home health physical therapy services, since none of these require inpatient physical therapy assessment for the order to be placed. Discharge to long-term acute care, skilled nursing facility, subacute rehabilitation facility, or acute rehabilitation facility were considered “discharge to post–acute care.” Loss of mobility was calculated as: discharge AM-PAC − admission AM-PAC, termed delta AM-PAC.
Statistical Analysis
Descriptive statistics were used to summarize age (mean and SD) and age categorized as <65 years or ≥65 years, sex (male or female), admission AM-PAC score (mean and SD) and categorization (≤43.63 or >43.63), discharge AM-PAC score (mean and SD), and discharge destination (home vs post–acute care). Chi-square analysis was used to test for associations between admission AM-PAC score and delta AM-PAC. Two-sample t-test was used to test for difference in mean delta AM-PAC between admission AM-PAC groups. Multivariable logistic regression was used to test for independent associations between age, sex, and admission AM-PAC score and odds of being discharged to home, controlling for length of stay. P values of <.05 were considered statistically significant for all tests. Analyses were performed using Stata statistical software, release 16 (StataCorp LLC).
RESULTS
During the 1-year study period, 3592 admissions with physical therapy consults occurred on the direct care hospital medicine services (58% of all admissions). Mean age was 66.3 years (SD, 15.4 years), and 48% of patients were female. The mean admission AM-PAC score was 43.9 (SD, 11.1), and the mean discharge AM-PAC score was 46.8 (SD, 10.8). In our sample, 38% of physical therapy consults were for patients with an AM-PAC score >43.63 who were discharged to home and were therefore deemed “potential overutilization.” Of those, 40% were for patients who were 65 years or younger (18% of all physical therapy consults) (Table 1).
A higher proportion of patients with AM-PAC scores >43.63 were discharged to home compared with those with AM-PAC scores ≤43.63 (89% vs 55%; χ2 [1, N = 3099], 396.5; P < .001). More patients younger than 65 years were discharged to home compared with those 65 years and older (79% vs 63%; χ2 [1, N = 3099], 113.6; P < .001). Additionally, for all patients younger than 65 years, those with AM-PAC score >43.63 were discharged to home more frequently than those with AM-PAC ≤43.63 (92% vs 66%, χ2 [1, N = 1,354], 134.4; P < .001). For 11% (n = 147) of the high-mobility group, the patient was not discharged home but was sent to post–acute care. Reviewing these patient charts showed the reasons for discharge to post–acute care were predominantly personal or social needs (eg, homelessness, need for 24-hour supervision with no family support, patient request) or medical needs (eg, intravenous antibiotics or new tubes, lines, drains, or medications requiring extra nursing support or management). Only 16% of patients in this group (n = 23) experienced deconditioning necessitating physical therapy consult during hospitalization, per their record.
Compared with patients with admission AM-PAC score >43.63, patients with admission AM-PAC ≤43.63 had significantly different changes in mobility as measured by mean delta AM-PAC score (delta AM-PAC, –0.41 for AM-PAC >43.63 vs +5.69 for AM-PAC ≤43.63; t (3097) = –20.3; P < .001) (Table 1).
In multivariate logistic regression, AM-PAC >43.63 (OR, 5.38; 95% CI, 4.36-2.89; P < .001) and age younger than 65 years (OR, 2.40; 95% CI, 1.99-2.90; P < .001) were associated with increased odds of discharge to home (Table 2).
DISCUSSION
In this study, we found that physical therapists may be unnecessarily consulted on direct care hospitalist services as much as 38% of the time based on AM-PAC score. We also demonstrated that patients admitted with high mobility by AM-PAC score are more than five times as likely to be discharged to home. When admitted with high AM-PAC scores, patients had virtually no change in mobility during hospitalization, whereas patients with low AM-PAC scores gained mobility during hospitalization, underscoring the benefit of physical therapy referrals for this group.
Given resource scarcity and cost, achieving optimal physical therapy utilization is an important goal for healthcare systems.10 Appropriate allocation of physical therapy has the potential to improve outcomes from the patient to the payor level. While it may be necessary to consult physical therapy for reasons other than mobility later in the hospitalization, identifying patients who will benefit from skilled physical therapy at the time of admission can help prevent disability and institutionalization and shorten length of stay.5,6 Likewise, decreasing physical therapy referrals for low-risk patients can increase the amount of time spent rehabilitating at-risk patients.
There are limitations of our study worth considering. First, our analyses did not consider whether physical therapy contributed to patients’ ability to return home after discharge. However, in our hospital, patients with AM-PAC >43.63 who cannot safely ambulate independently do progressive mobility with nursing staff. Our physical therapy leadership has also observed that the vast majority of highly mobile patients who are referred for physical therapy ultimately receive no treatment. Second, we did not consider discharge diagnosis, but our patient populations present with a wide variety of conditions, and it is impossible to predict their discharge diagnosis. By not including discharge diagnosis, we assess how AM-PAC performs on admission regardless of the medical condition for which someone is treated. Our hospital treats a high proportion of African American and a low proportion of White, Hispanic, and Asian American patients, limiting the generalizability of our findings. Although the AM-PAC “6-Clicks” score has been shown to have high interrater reliability among physical therapists, our AM-PAC scores are assessed and documented by our nursing staff, which might decrease accuracy. However, one single-center study noted an intraclass correlation coefficient of 0.96 between nurses and physical therapists for the AM-PAC “6-Clicks.”11Despite these limitations, this study underscores the need to be more judicious in the decision to refer a patient for inpatient physical therapy, especially at the time of admission, and demonstrates the utility of using standardized mobility assessment to help in that decision-making process.
1. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? A systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
2. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x
3. Brown C.J, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263-1270. https://doi.org/10.1111/j.1532-5415.2004.52354.x
4. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63:55-62. https://doi.org/10.1111/jgs.13193
5. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379-391. https://doi.org/10.2522/ptj.20130199
6. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. https://doi.org/10.2522/ptj.20130359
7. Probasco JC, Lavezza A, Cassell A, et al. Choosing wisely together: physical and occupational therapy consultation for acute neurology inpatients. Neurohospitalist. 2018;8(2):53-59. https://doi.org/10.1177/1941874417729981
8. Young DL, Colantuoni E, Friedman LA, et al. Prediction of disposition within 48 hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9);540-543. https://doi.org/10.12788/jhm.3332
9. Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater reliability of AM-PAC “6-Clicks” basic mobility and daily activity short forms. Phys Ther. 2015;95(5):758-766. https://doi.org/10.2522/ptj.20140174
10. Juneau A, Bolduc A, Nguyen P, et al. Feasibility of implementing an exercise program in a geriatric assessment unit: the SPRINT program. Can Geriatr J. 2018;21(3):284-289. https://doi.org/10.5770/cgj.21.311
11. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110
1. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? A systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
2. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x
3. Brown C.J, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263-1270. https://doi.org/10.1111/j.1532-5415.2004.52354.x
4. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63:55-62. https://doi.org/10.1111/jgs.13193
5. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379-391. https://doi.org/10.2522/ptj.20130199
6. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. https://doi.org/10.2522/ptj.20130359
7. Probasco JC, Lavezza A, Cassell A, et al. Choosing wisely together: physical and occupational therapy consultation for acute neurology inpatients. Neurohospitalist. 2018;8(2):53-59. https://doi.org/10.1177/1941874417729981
8. Young DL, Colantuoni E, Friedman LA, et al. Prediction of disposition within 48 hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9);540-543. https://doi.org/10.12788/jhm.3332
9. Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater reliability of AM-PAC “6-Clicks” basic mobility and daily activity short forms. Phys Ther. 2015;95(5):758-766. https://doi.org/10.2522/ptj.20140174
10. Juneau A, Bolduc A, Nguyen P, et al. Feasibility of implementing an exercise program in a geriatric assessment unit: the SPRINT program. Can Geriatr J. 2018;21(3):284-289. https://doi.org/10.5770/cgj.21.311
11. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110
© 2021 Society of Hospital Medicine
Objective Measures of Physical Distancing in the Hospital During the COVID-19 Pandemic
The COVID-19 pandemic dramatically altered how healthcare providers care for hospitalized patients. Many hospitals provided physical-distancing guidance to minimize viral transmission and preserve personal protective equipment. This guidance informed clinician behavior on rounds and in workspaces.1 One study reported that clinicians maintained distance from patients by grouping medical interventions, utilizing telemedicine for rounding and consultations, and implementing respiratory isolation units (RIUs) to cohort patients with COVID-19.2
Although physical distancing is recommended during inpatient care, no study to date has used objective measures to quantify the degree to which clinical practice was influenced. We aimed to objectively quantify changes in 24-hour patient room–entries before and during the COVID-19 pandemic using data from existing heat sensors to assess differences in physical distancing in RIUs and general medicine units.
METHODS
Study Design
A single-institution study was conducted at the University of Chicago Medicine, Illinois. Room entries were compared between a general medicine unit that transitioned to an RIU (unit A/RIU) and four general medicine units (unit B) using 24-hour patient room–entry data. Unit A was commissioned as an RIU to care exclusively for patients with confirmed COVID-19 on March 25, 2020, and decommissioned on June 23, 2020. Unit B cared for patients under investigation (PUIs) for COVID-19 and patients admitted for other reasons. PUIs were transferred to the RIU if positive for COVID-19. Hospital visitor restrictions were implemented on March 14, 2020, and lifted on June 29, 2020. The University of Chicago Institutional Review Board granted this project an exempt determination.
Data Collection
From January 1, 2020, to August 10, 2020, room-entry data were collected using the PURELL SMARTLINK hand-hygiene system (GOJO Industries, Inc.). This hand-hygiene compliance system tracks unit-level sanitizer dispenses and total room entries and exits via body heat sensors. Similar to our prior studies, this study extracted heat-sensor data to monitor room entries.3,4
Data Analysis
Objective 24-hour room-entry data were analyzed for all units. Rooms with less than two daily entries were assumed to be unoccupied and excluded from the analysis. Hospital-wide physical-distancing guidance published on March 10, 2020, was used to delineate “prepandemic” and “pandemic” periods. Each department adopted these recommendations (eg, physical distancing, conducting prerounds virtually, limiting the number of people seeing patients, using iPads for virtual patient visits) as appropriate.
Interrupted time series analyses were used to examine room-entry changes before and during the pandemic. The segmented function in R 4.0.2 (R Core Team) was used to create a model and estimate final fitting parameters, uncertainties, and data breakpoints using a bootstrap restarting algorithm.5 The Davies test was used to determine statistical significance of breakpoints, which was defined as P < .05.
RESULTS
We examined data from January 1, 2020, to August 10, 2020, from 3283 patients who collectively experienced 655,615 room entries. Unit A/RIU cared for 395 patients during the prepandemic period and 542 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were more likely to be Black (73.7% vs 77.9%) and less likely to be White (17.0% vs 8.7%) (P = .002); were less likely to have respiratory (19.8% vs 8.0%, P < .001) or gastrointestinal (12.4% vs 6.5%, P = .008) primary diagnoses; and had a higher mean case-mix index (1.74 vs 2.38, P < .001) (Table).
Unit B had 718 patients during the prepandemic period and 1628 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were less likely to be female (58.8% vs 53.4%, P = .018); less likely to be Black (77.7% vs 74.7%) and Hispanic (5.7% vs 3.4%) (P < .001); more likely to have circulatory (10.1% vs 14.8%, P = .009) primary diagnoses; less likely to have respiratory (14.4% vs 6.8%, P < .001) primary diagnoses; and had a higher mean case-mix index (1.58 vs 1.82, P = .014) (Table).
During the prepandemic period, unit A/RIU averaged 27 occupied rooms per day. These rooms averaged 85.0 entries per room per day, with no statistically significant change over time. During the pandemic period, this unit averaged 24 daily occupied rooms, and these rooms averaged 44.4 entries per room per day. At the start of the pandemic, daily entries per room decreased by 51.9 (95% CI, 51.1-52.7). This equated to a 60.6% reduction from baseline (95% CI, 59.6%-61.5%; P < .001), with the lowest average occurring after RIU conversion on March 25, 2020 (letter F in Figure, A). Entries remained constant through the end of statewide stay-at-home orders (letter G in Figure, A) until RIU decommission on June 23, 2020 (letter H in Figure, A). Entries then increased by an average of 0.150 entries per room per day (95% CI, 0.097-0.202; P < .001), reaching 52.5 daily entries on August 10, 2020. This equated to 61.3% of prepandemic levels (95% CI, 61.3%-61.6%; P < .001) (Figure, A).
During the prepandemic period, Unit B averaged 63 daily occupied rooms, and these rooms averaged 76.9 entries per room per day, with no statistically significant change over time. During the pandemic period, these units averaged 64 daily occupied rooms, and these rooms averaged 72.4 entries per room per day. Briefly, at the start of the pandemic, daily entries per room decreased by 11.8 (95% CI, 11.6-12), equating to a 14.7% reduction from baseline (95% CI, 14.4%-14.9%; P < .001). Entries then increased by an average of 0.052 entries per room per day (95% CI, –0.01 to 0.115; P = .051), stabilizing in early August 2020 at an average of 74.1 daily entries. This equated to 92.2% of prepandemic levels (95% CI, 92%-92.3%; P < .001) (Figure, B).
Unit A/RIU experienced significantly greater average daily room entries during the prepandemic period (P < .001) and significantly fewer average daily room entries during the pandemic period (P < .001) than unit B. Although unit A and unit B cared for similar patient populations prior to the pandemic, unit B was located in a different building from the resident work room. This likely resulted in batched visits to patients, leading to fewer total room entries per day.
DISCUSSION
This is the first study to measure 24-hour patient room– entries as an objective proxy for physical distancing during the pandemic. Unit A/RIU saw an initial 60.6% decrease in room entries. In contrast, unit B, which cared for PUIs, saw a brief 14.7% decrease in room entries before returning to baseline. In all units, room entries increased over time, although this increase was greater in unit B.
Despite the institutional recommendation of physical distancing, only unit A/RIU saw a large and sustained decrease in room entries. The presence of patients with COVID-19 within this unit likely reminded clinicians of the ongoing need to physically distance. Clinicians may have been fearful of contracting COVID-19 and therefore more stringently followed physical-distancing guidance.
Changes in unit A/RIU room entries tracked with RIU conversion and decommission timeline (letters F and H in Figure, A, respectively) rather than statewide stay-at-home orders (letters E and G in Figure, A). Caring for patients with COVID-19 within the unit might have influenced clinician physical distancing more than state policy. Correspondingly, as the number of hospitalized patients with COVID-19 decreased, room entries trended toward baseline. The difficulty of sustaining behavioral changes has been demonstrated in healthcare settings, including at our own institution.6-8 This gradual extinction in physical distancing could be due to several factors, such as fewer patients with COVID-19 or staff fatigue. Physical distancing may have been more extreme and suboptimal for care at the beginning of the pandemic owing to uncertainty or fear.
This work has implications for how to monitor physical distancing in healthcare facilities. Our study shows that behaviors can change rapidly, but sustaining change is difficult. This suggests the need for regular reinforcement of physical distancing with all staff. Additionally, cohorting patients on RIUs may result in greater physical distancing. It also highlights that PUIs serve as less of a cue to promote physical distancing, possibly due to increased confidence in and availability of COVID-19 tests and/or precautions fatigue.9 Objective room-entry monitoring systems, such as the one used in this study, can provide hospital leaders with crucial real-time feedback to monitor physical distancing practices and determine when and where re-education may be needed.
This study was conducted at a single urban, academic medical center, limiting its generalizability. Many other hospital policies implemented at the beginning of the pandemic may have influenced our results. We are unable to examine the type of clinician entering each room and for how long as well as entries in workrooms and breakrooms. Clinicians were not given real-time or retrospective feedback on room entries during the pandemic period. These data would be important to understand staff responses to physical distancing. Finally, while clinicians responded differently as the pandemic progressed and depending on which unit they were in, the ideal degree of physical distancing remains unknown. Although minimizing patient contact limits nosocomial viral spread, too little contact can also cause harm.
Conclusion
At the onset of the COVID-19 pandemic, 24-hour patient room–entries fell significantly in all units before increasing. This decrease was more pronounced in unit A/RIU. As the pandemic continues, hospitals could consider utilizing novel room-entry monitoring systems to guide physical-distancing implementation and staff education.
Acknowledgment
The authors thank Vera Chu for her support with data requests.
1. Auerbach A, O’Leary KJ, Greysen SR, et al. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Arora VM, Chivu M, Schram A, Meltzer D. Implementing physical distancing in the hospital: a key strategy to prevent nosocomial transmission of COVID-19. J Hosp Med. 2020;15(5):290-291. https://doi.org/10.12788/jhm.3434
3. Erondu AI, Orlov NM, Peirce LB, et al. Characterizing pediatric inpatient sleep duration and disruptions. Sleep Med. 2019;57:87-91. https://doi.org/10.1016/j.sleep.2019.01.030
4. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
5. Muggeo VMR. Segmented: an R package to fit regression models with broken-line relationships. R News. 2008;8:20-25.
6. Cook DJ, Arora VM, Chamberlain M, et al. Improving hospitalized children’s sleep by reducing excessive overnight blood pressure monitoring. Pediatrics. 2020;146(3):e20192217. https://doi.org/10.1542/peds.2019-2217
7. Bernstein M, Hou JK, Weizman AV, et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2016;14(10):1371-1375. https://doi.org/10.1016/j.cgh.2016.05.019
8. Makhni S, Umscheid CA, Soo J, et al. Hand hygiene compliance rate during the COVID-19 pandemic. JAMA Intern Med. 2021;181(7):1006-1008. https://doi.org/10.1001/jamainternmed.2021.1429
9. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
The COVID-19 pandemic dramatically altered how healthcare providers care for hospitalized patients. Many hospitals provided physical-distancing guidance to minimize viral transmission and preserve personal protective equipment. This guidance informed clinician behavior on rounds and in workspaces.1 One study reported that clinicians maintained distance from patients by grouping medical interventions, utilizing telemedicine for rounding and consultations, and implementing respiratory isolation units (RIUs) to cohort patients with COVID-19.2
Although physical distancing is recommended during inpatient care, no study to date has used objective measures to quantify the degree to which clinical practice was influenced. We aimed to objectively quantify changes in 24-hour patient room–entries before and during the COVID-19 pandemic using data from existing heat sensors to assess differences in physical distancing in RIUs and general medicine units.
METHODS
Study Design
A single-institution study was conducted at the University of Chicago Medicine, Illinois. Room entries were compared between a general medicine unit that transitioned to an RIU (unit A/RIU) and four general medicine units (unit B) using 24-hour patient room–entry data. Unit A was commissioned as an RIU to care exclusively for patients with confirmed COVID-19 on March 25, 2020, and decommissioned on June 23, 2020. Unit B cared for patients under investigation (PUIs) for COVID-19 and patients admitted for other reasons. PUIs were transferred to the RIU if positive for COVID-19. Hospital visitor restrictions were implemented on March 14, 2020, and lifted on June 29, 2020. The University of Chicago Institutional Review Board granted this project an exempt determination.
Data Collection
From January 1, 2020, to August 10, 2020, room-entry data were collected using the PURELL SMARTLINK hand-hygiene system (GOJO Industries, Inc.). This hand-hygiene compliance system tracks unit-level sanitizer dispenses and total room entries and exits via body heat sensors. Similar to our prior studies, this study extracted heat-sensor data to monitor room entries.3,4
Data Analysis
Objective 24-hour room-entry data were analyzed for all units. Rooms with less than two daily entries were assumed to be unoccupied and excluded from the analysis. Hospital-wide physical-distancing guidance published on March 10, 2020, was used to delineate “prepandemic” and “pandemic” periods. Each department adopted these recommendations (eg, physical distancing, conducting prerounds virtually, limiting the number of people seeing patients, using iPads for virtual patient visits) as appropriate.
Interrupted time series analyses were used to examine room-entry changes before and during the pandemic. The segmented function in R 4.0.2 (R Core Team) was used to create a model and estimate final fitting parameters, uncertainties, and data breakpoints using a bootstrap restarting algorithm.5 The Davies test was used to determine statistical significance of breakpoints, which was defined as P < .05.
RESULTS
We examined data from January 1, 2020, to August 10, 2020, from 3283 patients who collectively experienced 655,615 room entries. Unit A/RIU cared for 395 patients during the prepandemic period and 542 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were more likely to be Black (73.7% vs 77.9%) and less likely to be White (17.0% vs 8.7%) (P = .002); were less likely to have respiratory (19.8% vs 8.0%, P < .001) or gastrointestinal (12.4% vs 6.5%, P = .008) primary diagnoses; and had a higher mean case-mix index (1.74 vs 2.38, P < .001) (Table).
Unit B had 718 patients during the prepandemic period and 1628 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were less likely to be female (58.8% vs 53.4%, P = .018); less likely to be Black (77.7% vs 74.7%) and Hispanic (5.7% vs 3.4%) (P < .001); more likely to have circulatory (10.1% vs 14.8%, P = .009) primary diagnoses; less likely to have respiratory (14.4% vs 6.8%, P < .001) primary diagnoses; and had a higher mean case-mix index (1.58 vs 1.82, P = .014) (Table).
During the prepandemic period, unit A/RIU averaged 27 occupied rooms per day. These rooms averaged 85.0 entries per room per day, with no statistically significant change over time. During the pandemic period, this unit averaged 24 daily occupied rooms, and these rooms averaged 44.4 entries per room per day. At the start of the pandemic, daily entries per room decreased by 51.9 (95% CI, 51.1-52.7). This equated to a 60.6% reduction from baseline (95% CI, 59.6%-61.5%; P < .001), with the lowest average occurring after RIU conversion on March 25, 2020 (letter F in Figure, A). Entries remained constant through the end of statewide stay-at-home orders (letter G in Figure, A) until RIU decommission on June 23, 2020 (letter H in Figure, A). Entries then increased by an average of 0.150 entries per room per day (95% CI, 0.097-0.202; P < .001), reaching 52.5 daily entries on August 10, 2020. This equated to 61.3% of prepandemic levels (95% CI, 61.3%-61.6%; P < .001) (Figure, A).
During the prepandemic period, Unit B averaged 63 daily occupied rooms, and these rooms averaged 76.9 entries per room per day, with no statistically significant change over time. During the pandemic period, these units averaged 64 daily occupied rooms, and these rooms averaged 72.4 entries per room per day. Briefly, at the start of the pandemic, daily entries per room decreased by 11.8 (95% CI, 11.6-12), equating to a 14.7% reduction from baseline (95% CI, 14.4%-14.9%; P < .001). Entries then increased by an average of 0.052 entries per room per day (95% CI, –0.01 to 0.115; P = .051), stabilizing in early August 2020 at an average of 74.1 daily entries. This equated to 92.2% of prepandemic levels (95% CI, 92%-92.3%; P < .001) (Figure, B).
Unit A/RIU experienced significantly greater average daily room entries during the prepandemic period (P < .001) and significantly fewer average daily room entries during the pandemic period (P < .001) than unit B. Although unit A and unit B cared for similar patient populations prior to the pandemic, unit B was located in a different building from the resident work room. This likely resulted in batched visits to patients, leading to fewer total room entries per day.
DISCUSSION
This is the first study to measure 24-hour patient room– entries as an objective proxy for physical distancing during the pandemic. Unit A/RIU saw an initial 60.6% decrease in room entries. In contrast, unit B, which cared for PUIs, saw a brief 14.7% decrease in room entries before returning to baseline. In all units, room entries increased over time, although this increase was greater in unit B.
Despite the institutional recommendation of physical distancing, only unit A/RIU saw a large and sustained decrease in room entries. The presence of patients with COVID-19 within this unit likely reminded clinicians of the ongoing need to physically distance. Clinicians may have been fearful of contracting COVID-19 and therefore more stringently followed physical-distancing guidance.
Changes in unit A/RIU room entries tracked with RIU conversion and decommission timeline (letters F and H in Figure, A, respectively) rather than statewide stay-at-home orders (letters E and G in Figure, A). Caring for patients with COVID-19 within the unit might have influenced clinician physical distancing more than state policy. Correspondingly, as the number of hospitalized patients with COVID-19 decreased, room entries trended toward baseline. The difficulty of sustaining behavioral changes has been demonstrated in healthcare settings, including at our own institution.6-8 This gradual extinction in physical distancing could be due to several factors, such as fewer patients with COVID-19 or staff fatigue. Physical distancing may have been more extreme and suboptimal for care at the beginning of the pandemic owing to uncertainty or fear.
This work has implications for how to monitor physical distancing in healthcare facilities. Our study shows that behaviors can change rapidly, but sustaining change is difficult. This suggests the need for regular reinforcement of physical distancing with all staff. Additionally, cohorting patients on RIUs may result in greater physical distancing. It also highlights that PUIs serve as less of a cue to promote physical distancing, possibly due to increased confidence in and availability of COVID-19 tests and/or precautions fatigue.9 Objective room-entry monitoring systems, such as the one used in this study, can provide hospital leaders with crucial real-time feedback to monitor physical distancing practices and determine when and where re-education may be needed.
This study was conducted at a single urban, academic medical center, limiting its generalizability. Many other hospital policies implemented at the beginning of the pandemic may have influenced our results. We are unable to examine the type of clinician entering each room and for how long as well as entries in workrooms and breakrooms. Clinicians were not given real-time or retrospective feedback on room entries during the pandemic period. These data would be important to understand staff responses to physical distancing. Finally, while clinicians responded differently as the pandemic progressed and depending on which unit they were in, the ideal degree of physical distancing remains unknown. Although minimizing patient contact limits nosocomial viral spread, too little contact can also cause harm.
Conclusion
At the onset of the COVID-19 pandemic, 24-hour patient room–entries fell significantly in all units before increasing. This decrease was more pronounced in unit A/RIU. As the pandemic continues, hospitals could consider utilizing novel room-entry monitoring systems to guide physical-distancing implementation and staff education.
Acknowledgment
The authors thank Vera Chu for her support with data requests.
The COVID-19 pandemic dramatically altered how healthcare providers care for hospitalized patients. Many hospitals provided physical-distancing guidance to minimize viral transmission and preserve personal protective equipment. This guidance informed clinician behavior on rounds and in workspaces.1 One study reported that clinicians maintained distance from patients by grouping medical interventions, utilizing telemedicine for rounding and consultations, and implementing respiratory isolation units (RIUs) to cohort patients with COVID-19.2
Although physical distancing is recommended during inpatient care, no study to date has used objective measures to quantify the degree to which clinical practice was influenced. We aimed to objectively quantify changes in 24-hour patient room–entries before and during the COVID-19 pandemic using data from existing heat sensors to assess differences in physical distancing in RIUs and general medicine units.
METHODS
Study Design
A single-institution study was conducted at the University of Chicago Medicine, Illinois. Room entries were compared between a general medicine unit that transitioned to an RIU (unit A/RIU) and four general medicine units (unit B) using 24-hour patient room–entry data. Unit A was commissioned as an RIU to care exclusively for patients with confirmed COVID-19 on March 25, 2020, and decommissioned on June 23, 2020. Unit B cared for patients under investigation (PUIs) for COVID-19 and patients admitted for other reasons. PUIs were transferred to the RIU if positive for COVID-19. Hospital visitor restrictions were implemented on March 14, 2020, and lifted on June 29, 2020. The University of Chicago Institutional Review Board granted this project an exempt determination.
Data Collection
From January 1, 2020, to August 10, 2020, room-entry data were collected using the PURELL SMARTLINK hand-hygiene system (GOJO Industries, Inc.). This hand-hygiene compliance system tracks unit-level sanitizer dispenses and total room entries and exits via body heat sensors. Similar to our prior studies, this study extracted heat-sensor data to monitor room entries.3,4
Data Analysis
Objective 24-hour room-entry data were analyzed for all units. Rooms with less than two daily entries were assumed to be unoccupied and excluded from the analysis. Hospital-wide physical-distancing guidance published on March 10, 2020, was used to delineate “prepandemic” and “pandemic” periods. Each department adopted these recommendations (eg, physical distancing, conducting prerounds virtually, limiting the number of people seeing patients, using iPads for virtual patient visits) as appropriate.
Interrupted time series analyses were used to examine room-entry changes before and during the pandemic. The segmented function in R 4.0.2 (R Core Team) was used to create a model and estimate final fitting parameters, uncertainties, and data breakpoints using a bootstrap restarting algorithm.5 The Davies test was used to determine statistical significance of breakpoints, which was defined as P < .05.
RESULTS
We examined data from January 1, 2020, to August 10, 2020, from 3283 patients who collectively experienced 655,615 room entries. Unit A/RIU cared for 395 patients during the prepandemic period and 542 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were more likely to be Black (73.7% vs 77.9%) and less likely to be White (17.0% vs 8.7%) (P = .002); were less likely to have respiratory (19.8% vs 8.0%, P < .001) or gastrointestinal (12.4% vs 6.5%, P = .008) primary diagnoses; and had a higher mean case-mix index (1.74 vs 2.38, P < .001) (Table).
Unit B had 718 patients during the prepandemic period and 1628 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were less likely to be female (58.8% vs 53.4%, P = .018); less likely to be Black (77.7% vs 74.7%) and Hispanic (5.7% vs 3.4%) (P < .001); more likely to have circulatory (10.1% vs 14.8%, P = .009) primary diagnoses; less likely to have respiratory (14.4% vs 6.8%, P < .001) primary diagnoses; and had a higher mean case-mix index (1.58 vs 1.82, P = .014) (Table).
During the prepandemic period, unit A/RIU averaged 27 occupied rooms per day. These rooms averaged 85.0 entries per room per day, with no statistically significant change over time. During the pandemic period, this unit averaged 24 daily occupied rooms, and these rooms averaged 44.4 entries per room per day. At the start of the pandemic, daily entries per room decreased by 51.9 (95% CI, 51.1-52.7). This equated to a 60.6% reduction from baseline (95% CI, 59.6%-61.5%; P < .001), with the lowest average occurring after RIU conversion on March 25, 2020 (letter F in Figure, A). Entries remained constant through the end of statewide stay-at-home orders (letter G in Figure, A) until RIU decommission on June 23, 2020 (letter H in Figure, A). Entries then increased by an average of 0.150 entries per room per day (95% CI, 0.097-0.202; P < .001), reaching 52.5 daily entries on August 10, 2020. This equated to 61.3% of prepandemic levels (95% CI, 61.3%-61.6%; P < .001) (Figure, A).
During the prepandemic period, Unit B averaged 63 daily occupied rooms, and these rooms averaged 76.9 entries per room per day, with no statistically significant change over time. During the pandemic period, these units averaged 64 daily occupied rooms, and these rooms averaged 72.4 entries per room per day. Briefly, at the start of the pandemic, daily entries per room decreased by 11.8 (95% CI, 11.6-12), equating to a 14.7% reduction from baseline (95% CI, 14.4%-14.9%; P < .001). Entries then increased by an average of 0.052 entries per room per day (95% CI, –0.01 to 0.115; P = .051), stabilizing in early August 2020 at an average of 74.1 daily entries. This equated to 92.2% of prepandemic levels (95% CI, 92%-92.3%; P < .001) (Figure, B).
Unit A/RIU experienced significantly greater average daily room entries during the prepandemic period (P < .001) and significantly fewer average daily room entries during the pandemic period (P < .001) than unit B. Although unit A and unit B cared for similar patient populations prior to the pandemic, unit B was located in a different building from the resident work room. This likely resulted in batched visits to patients, leading to fewer total room entries per day.
DISCUSSION
This is the first study to measure 24-hour patient room– entries as an objective proxy for physical distancing during the pandemic. Unit A/RIU saw an initial 60.6% decrease in room entries. In contrast, unit B, which cared for PUIs, saw a brief 14.7% decrease in room entries before returning to baseline. In all units, room entries increased over time, although this increase was greater in unit B.
Despite the institutional recommendation of physical distancing, only unit A/RIU saw a large and sustained decrease in room entries. The presence of patients with COVID-19 within this unit likely reminded clinicians of the ongoing need to physically distance. Clinicians may have been fearful of contracting COVID-19 and therefore more stringently followed physical-distancing guidance.
Changes in unit A/RIU room entries tracked with RIU conversion and decommission timeline (letters F and H in Figure, A, respectively) rather than statewide stay-at-home orders (letters E and G in Figure, A). Caring for patients with COVID-19 within the unit might have influenced clinician physical distancing more than state policy. Correspondingly, as the number of hospitalized patients with COVID-19 decreased, room entries trended toward baseline. The difficulty of sustaining behavioral changes has been demonstrated in healthcare settings, including at our own institution.6-8 This gradual extinction in physical distancing could be due to several factors, such as fewer patients with COVID-19 or staff fatigue. Physical distancing may have been more extreme and suboptimal for care at the beginning of the pandemic owing to uncertainty or fear.
This work has implications for how to monitor physical distancing in healthcare facilities. Our study shows that behaviors can change rapidly, but sustaining change is difficult. This suggests the need for regular reinforcement of physical distancing with all staff. Additionally, cohorting patients on RIUs may result in greater physical distancing. It also highlights that PUIs serve as less of a cue to promote physical distancing, possibly due to increased confidence in and availability of COVID-19 tests and/or precautions fatigue.9 Objective room-entry monitoring systems, such as the one used in this study, can provide hospital leaders with crucial real-time feedback to monitor physical distancing practices and determine when and where re-education may be needed.
This study was conducted at a single urban, academic medical center, limiting its generalizability. Many other hospital policies implemented at the beginning of the pandemic may have influenced our results. We are unable to examine the type of clinician entering each room and for how long as well as entries in workrooms and breakrooms. Clinicians were not given real-time or retrospective feedback on room entries during the pandemic period. These data would be important to understand staff responses to physical distancing. Finally, while clinicians responded differently as the pandemic progressed and depending on which unit they were in, the ideal degree of physical distancing remains unknown. Although minimizing patient contact limits nosocomial viral spread, too little contact can also cause harm.
Conclusion
At the onset of the COVID-19 pandemic, 24-hour patient room–entries fell significantly in all units before increasing. This decrease was more pronounced in unit A/RIU. As the pandemic continues, hospitals could consider utilizing novel room-entry monitoring systems to guide physical-distancing implementation and staff education.
Acknowledgment
The authors thank Vera Chu for her support with data requests.
1. Auerbach A, O’Leary KJ, Greysen SR, et al. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Arora VM, Chivu M, Schram A, Meltzer D. Implementing physical distancing in the hospital: a key strategy to prevent nosocomial transmission of COVID-19. J Hosp Med. 2020;15(5):290-291. https://doi.org/10.12788/jhm.3434
3. Erondu AI, Orlov NM, Peirce LB, et al. Characterizing pediatric inpatient sleep duration and disruptions. Sleep Med. 2019;57:87-91. https://doi.org/10.1016/j.sleep.2019.01.030
4. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
5. Muggeo VMR. Segmented: an R package to fit regression models with broken-line relationships. R News. 2008;8:20-25.
6. Cook DJ, Arora VM, Chamberlain M, et al. Improving hospitalized children’s sleep by reducing excessive overnight blood pressure monitoring. Pediatrics. 2020;146(3):e20192217. https://doi.org/10.1542/peds.2019-2217
7. Bernstein M, Hou JK, Weizman AV, et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2016;14(10):1371-1375. https://doi.org/10.1016/j.cgh.2016.05.019
8. Makhni S, Umscheid CA, Soo J, et al. Hand hygiene compliance rate during the COVID-19 pandemic. JAMA Intern Med. 2021;181(7):1006-1008. https://doi.org/10.1001/jamainternmed.2021.1429
9. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
1. Auerbach A, O’Leary KJ, Greysen SR, et al. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Arora VM, Chivu M, Schram A, Meltzer D. Implementing physical distancing in the hospital: a key strategy to prevent nosocomial transmission of COVID-19. J Hosp Med. 2020;15(5):290-291. https://doi.org/10.12788/jhm.3434
3. Erondu AI, Orlov NM, Peirce LB, et al. Characterizing pediatric inpatient sleep duration and disruptions. Sleep Med. 2019;57:87-91. https://doi.org/10.1016/j.sleep.2019.01.030
4. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
5. Muggeo VMR. Segmented: an R package to fit regression models with broken-line relationships. R News. 2008;8:20-25.
6. Cook DJ, Arora VM, Chamberlain M, et al. Improving hospitalized children’s sleep by reducing excessive overnight blood pressure monitoring. Pediatrics. 2020;146(3):e20192217. https://doi.org/10.1542/peds.2019-2217
7. Bernstein M, Hou JK, Weizman AV, et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2016;14(10):1371-1375. https://doi.org/10.1016/j.cgh.2016.05.019
8. Makhni S, Umscheid CA, Soo J, et al. Hand hygiene compliance rate during the COVID-19 pandemic. JAMA Intern Med. 2021;181(7):1006-1008. https://doi.org/10.1001/jamainternmed.2021.1429
9. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
© 2021 Society of Hospital Medicine
Leveling the Playing Field: Accounting for Academic Productivity During the COVID-19 Pandemic
Professional upheavals caused by the coronavirus disease 2019 (COVID-19) pandemic have affected the academic productivity of many physicians. This is due in part to rapid changes in clinical care and medical education: physician-researchers have been redeployed to frontline clinical care; clinician-educators have been forced to rapidly transition in-person curricula to virtual platforms; and primary care physicians and subspecialists have been forced to transition to telehealth-based practices. In addition to these changes in clinical and educational responsibilities, the COVID-19 pandemic has substantially altered the personal lives of physicians. During the height of the pandemic, clinicians simultaneously wrestled with a lack of available childcare, unexpected home-schooling responsibilities, decreased income, and many other COVID-19-related stresses.1 Additionally, the ever-present “second pandemic” of structural racism, persistent health disparities, and racial inequity has further increased the personal and professional demands facing academic faculty.2
In particular, the pandemic has placed personal and professional pressure on female and minority faculty members. In spite of these pressures, however, the academic promotions process still requires rigid accounting of scholarly productivity. As the focus of academic practices has shifted to support clinical care during the pandemic, scholarly productivity has suffered for clinicians on the frontline. As a result, academic clinical faculty have expressed significant stress and concerns about failing to meet benchmarks for promotion (eg, publications, curricula development, national presentations). To counter these shifts (and the inherent inequity that they create for female clinicians and for men and women who are Black, Indigenous, and/or of color), academic institutions should not only recognize the effects the COVID-19 pandemic has had on faculty, but also adopt immediate solutions to more equitably account for such disruptions to academic portfolios. In this paper, we explore populations whose career trajectories are most at-risk and propose a framework to capture novel and nontraditional contributions while also acknowledging the rapid changes the COVID-19 pandemic has brought to academic medicine.
POPULATIONS AT RISK FOR CAREER DISRUPTION
Even before the COVID-19 pandemic, physician mothers, underrepresented racial/ethnic minority groups, and junior faculty were most at-risk for career disruptions. The closure of daycare facilities and schools and shift to online learning resulting from the pandemic, along with the common challenges of parenting, have taken a significant toll on the lives of working parents. Because women tend to carry a disproportionate share of childcare and household responsibilities, these changes have inequitably leveraged themselves as a “mommy tax” on working women.3,4
As underrepresented medicine faculty (particularly Black, Hispanic, Latino, and Native American clinicians) comprise only 8% of the academic medical workforce,they currently face a variety of personal and professional challenges.5 This is especially true for Black and Latinx physicians who have been experiencing an increased COVID-19 burden in their communities, while concurrently fighting entrenched structural racism and police violence. In academia, these challenges have worsened because of the “minority tax”—the toll of often uncompensated extra responsibilities (time or money) placed on minority faculty in the name of achieving diversity. The unintended consequences of these responsibilities result in having fewer mentors,6 caring for underserved populations,7 and performing more clinical care8 than non-underrepresented minority faculty. Because minority faculty are unlikely to be in leadership positions, it is reasonable to conclude they have been shouldering heavier clinical obligations and facing greater career disruption of scholarly work due to the COVID-19 pandemic.
Junior faculty (eg, instructors and assistant professors) also remain professionally vulnerable during the COVID-19 pandemic. Because junior faculty are often more clinically focused and less likely to hold leadership positions than senior faculty, they are more likely to have assumed frontline clinical positions, which come at the expense of academic work. Junior faculty are also at a critical building phase in their academic career—a time when they benefit from the opportunity to share their scholarly work and network at conferences. Unfortunately, many conferences have been canceled or moved to a virtual platform. Given that some institutions may be freezing academic funding for conferences due to budgetary shortfalls from the pandemic, junior faculty may be particularly at risk if they are not able to present their work. In addition, junior faculty often face disproportionate struggles at home, trying to balance demands of work and caring for young children. Considering the unique needs of each of these groups, it is especially important to consider intersectionality, or the compounded issues for individuals who exist in multiple disproportionately affected groups (eg, a Black female junior faculty member who is also a mother).
THE COVID-19-CURRICULUM VITAE MATRIX
The typical format of a professional curriculum vitae (CV) at most academic institutions does not allow one to document potential disruptions or novel contributions, including those that occurred during the COVID-19 pandemic. As a group of academic clinicians, educators, and researchers whose careers have been affected by the pandemic, we created a COVID-19 CV matrix, a potential framework to serve as a supplement for faculty. In this matrix, faculty members may document their contributions, disruptions that affected their work, and caregiving responsibilities during this time period, while also providing a rubric for promotions and tenure committees to equitably evaluate the pandemic period on an academic CV. Our COVID-19 CV matrix consists of six domains: (1) clinical care, (2) research, (3) education, (4) service, (5) advocacy/media, and (6) social media. These domains encompass traditional and nontraditional contributions made by healthcare professionals during the pandemic (Table). This matrix broadens the ability of both faculty and institutions to determine the actual impact of individuals during the pandemic.
ACCOUNT FOR YOUR (NEW) IMPACT
Throughout the COVID-19 pandemic, academic faculty have been innovative, contributing in novel ways not routinely captured by promotions committees—eg, the digital health researcher who now directs the telemedicine response for their institution and the health disparities researcher who now leads daily webinar sessions on structural racism to medical students. Other novel contributions include advancing COVID-19 innovations and engaging in media and community advocacy (eg, organizing large-scale donations of equipment and funds to support organizations in need). While such nontraditional contributions may not have been readily captured or thought “CV worthy” in the past, faculty should now account for them. More importantly, promotions committees need to recognize that these pivots or alterations in career paths are not signals of professional failure, but rather evidence of a shifting landscape and the respective response of the individual. Furthermore, because these pivots often help fulfill an institutional mission, they are impactful.
ACKNOWLEDGE THE DISRUPTION
It is important for promotions and tenure committees to recognize the impact and disruption COVID-19 has had on traditional academic work, acknowledging the time and energy required for a faculty member to make needed work adjustments. This enables a leader to better assess how a faculty member’s academic portfolio has been affected. For example, researchers have had to halt studies, medical educators have had to redevelop and transition curricula to virtual platforms, and physicians have had to discontinue clinician quality improvement initiatives due to competing hospital priorities. Faculty members who document such unintentional alterations in their academic career path can explain to their institution how they have continued to positively influence their field and the community during the pandemic. This approach is analogous to the current model of accounting for clinical time when judging faculty members’ contributions in scholarly achievement.
The COVID-19 CV matrix has the potential to be annotated to explain the burden of one’s personal situation, which is often “invisible” in the professional environment. For example, many physicians have had to assume additional childcare responsibilities, tend to sick family members, friends, and even themselves. It is also possible that a faculty member has a partner who is also an essential worker, one who had to self-isolate due to COVID-19 exposure or illness, or who has been working overtime due to high patient volumes.
INSTITUTIONAL RESPONSE
How can institutions respond to the altered academic landscape caused by the COVID-19 pandemic? Promotions committees typically have two main tools at their disposal: adjusting the tenure clock or the benchmarks. Extending the period of time available to qualify for tenure is commonplace in the “publish-or-perish” academic tracks of university research professors. Clock adjustments are typically granted to faculty following the birth of a child or for other specific family- or health-related hardships, in accordance with the Family and Medical Leave Act. Unfortunately, tenure-clock extensions for female faculty members can exacerbate gender inequity: Data on tenure-clock extensions show a higher rate of tenure granted to male faculty compared to female faculty.9 For this reason, it is also important to explore adjustments or modifications to benchmark criteria. This could be accomplished by broadening the criteria for promotion, recognizing that impact occurs in many forms, thereby enabling meeting a benchmark. It can also occur by examining the trajectory of an individual within a promotion pathway before it was disrupted to determine impact. To avoid exacerbating social and gender inequities within academia, institutions should use these professional levers and create new ones to provide parity and equality across the promotional playing field. While the CV matrix openly acknowledges the disruptions and tangents the COVID-19 pandemic has had on academic careers, it remains important for academic institutions to recognize these disruptions and innovate the manner in which they acknowledge scholarly contributions.
Conclusion
While academic rigidity and known social taxes (minority and mommy taxes) are particularly problematic in the current climate, these issues have always been at play in evaluating academic success. Improved documentation of novel contributions, disruptions, caregiving, and other challenges can enable more holistic and timely professional advancement for all faculty, regardless of their sex, race, ethnicity, or social background. Ultimately, we hope this framework initiates further conversations among academic institutions on how to define productivity in an age where journal impact factor or number of publications is not the fullest measure of one’s impact in their field.
1. Jones Y, Durand V, Morton K, et al; ADVANCE PHM Steering Committee. Collateral damage: how covid-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
2. Manning KD. When grief and crises intersect: perspectives of a black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
3. Cohen P, Hsu T. Pandemic could scar a generation of working mothers. New York Times. Published June 3, 2020. Updated June 30, 2020. Accessed November 11, 2020. https://www.nytimes.com/2020/06/03/business/economy/coronavirus-working-women.html
4. Cain Miller C. Nearly half of men say they do most of the home schooling. 3 percent of women agree. Published May 6, 2020. Updated May 8, 2020. Accessed November 11, 2020. New York Times. https://www.nytimes.com/2020/05/06/upshot/pandemic-chores-homeschooling-gender.html
5. Rodríguez JE, Campbell KM, Pololi LH. Addressing disparities in academic medicine: what of the minority tax? BMC Med Educ. 2015;15:6. https://doi.org/10.1186/s12909-015-0290-9
6. Lewellen-Williams C, Johnson VA, Deloney LA, Thomas BR, Goyol A, Henry-Tillman R. The POD: a new model for mentoring underrepresented minority faculty. Acad Med. 2006;81(3):275-279. https://doi.org/10.1097/00001888-200603000-00020
7. Pololi LH, Evans AT, Gibbs BK, Krupat E, Brennan RT, Civian JT. The experience of minority faculty who are underrepresented in medicine, at 26 representative U.S. medical schools. Acad Med. 2013;88(9):1308-1314. https://doi.org/10.1097/acm.0b013e31829eefff
8. Richert A, Campbell K, Rodríguez J, Borowsky IW, Parikh R, Colwell A. ACU workforce column: expanding and supporting the health care workforce. J Health Care Poor Underserved. 2013;24(4):1423-1431. https://doi.org/10.1353/hpu.2013.0162
9. Woitowich NC, Jain S, Arora VM, Joffe H. COVID-19 threatens progress toward gender equity within academic medicine. Acad Med. 2020;29:10.1097/ACM.0000000000003782. https://doi.org/10.1097/acm.0000000000003782
Professional upheavals caused by the coronavirus disease 2019 (COVID-19) pandemic have affected the academic productivity of many physicians. This is due in part to rapid changes in clinical care and medical education: physician-researchers have been redeployed to frontline clinical care; clinician-educators have been forced to rapidly transition in-person curricula to virtual platforms; and primary care physicians and subspecialists have been forced to transition to telehealth-based practices. In addition to these changes in clinical and educational responsibilities, the COVID-19 pandemic has substantially altered the personal lives of physicians. During the height of the pandemic, clinicians simultaneously wrestled with a lack of available childcare, unexpected home-schooling responsibilities, decreased income, and many other COVID-19-related stresses.1 Additionally, the ever-present “second pandemic” of structural racism, persistent health disparities, and racial inequity has further increased the personal and professional demands facing academic faculty.2
In particular, the pandemic has placed personal and professional pressure on female and minority faculty members. In spite of these pressures, however, the academic promotions process still requires rigid accounting of scholarly productivity. As the focus of academic practices has shifted to support clinical care during the pandemic, scholarly productivity has suffered for clinicians on the frontline. As a result, academic clinical faculty have expressed significant stress and concerns about failing to meet benchmarks for promotion (eg, publications, curricula development, national presentations). To counter these shifts (and the inherent inequity that they create for female clinicians and for men and women who are Black, Indigenous, and/or of color), academic institutions should not only recognize the effects the COVID-19 pandemic has had on faculty, but also adopt immediate solutions to more equitably account for such disruptions to academic portfolios. In this paper, we explore populations whose career trajectories are most at-risk and propose a framework to capture novel and nontraditional contributions while also acknowledging the rapid changes the COVID-19 pandemic has brought to academic medicine.
POPULATIONS AT RISK FOR CAREER DISRUPTION
Even before the COVID-19 pandemic, physician mothers, underrepresented racial/ethnic minority groups, and junior faculty were most at-risk for career disruptions. The closure of daycare facilities and schools and shift to online learning resulting from the pandemic, along with the common challenges of parenting, have taken a significant toll on the lives of working parents. Because women tend to carry a disproportionate share of childcare and household responsibilities, these changes have inequitably leveraged themselves as a “mommy tax” on working women.3,4
As underrepresented medicine faculty (particularly Black, Hispanic, Latino, and Native American clinicians) comprise only 8% of the academic medical workforce,they currently face a variety of personal and professional challenges.5 This is especially true for Black and Latinx physicians who have been experiencing an increased COVID-19 burden in their communities, while concurrently fighting entrenched structural racism and police violence. In academia, these challenges have worsened because of the “minority tax”—the toll of often uncompensated extra responsibilities (time or money) placed on minority faculty in the name of achieving diversity. The unintended consequences of these responsibilities result in having fewer mentors,6 caring for underserved populations,7 and performing more clinical care8 than non-underrepresented minority faculty. Because minority faculty are unlikely to be in leadership positions, it is reasonable to conclude they have been shouldering heavier clinical obligations and facing greater career disruption of scholarly work due to the COVID-19 pandemic.
Junior faculty (eg, instructors and assistant professors) also remain professionally vulnerable during the COVID-19 pandemic. Because junior faculty are often more clinically focused and less likely to hold leadership positions than senior faculty, they are more likely to have assumed frontline clinical positions, which come at the expense of academic work. Junior faculty are also at a critical building phase in their academic career—a time when they benefit from the opportunity to share their scholarly work and network at conferences. Unfortunately, many conferences have been canceled or moved to a virtual platform. Given that some institutions may be freezing academic funding for conferences due to budgetary shortfalls from the pandemic, junior faculty may be particularly at risk if they are not able to present their work. In addition, junior faculty often face disproportionate struggles at home, trying to balance demands of work and caring for young children. Considering the unique needs of each of these groups, it is especially important to consider intersectionality, or the compounded issues for individuals who exist in multiple disproportionately affected groups (eg, a Black female junior faculty member who is also a mother).
THE COVID-19-CURRICULUM VITAE MATRIX
The typical format of a professional curriculum vitae (CV) at most academic institutions does not allow one to document potential disruptions or novel contributions, including those that occurred during the COVID-19 pandemic. As a group of academic clinicians, educators, and researchers whose careers have been affected by the pandemic, we created a COVID-19 CV matrix, a potential framework to serve as a supplement for faculty. In this matrix, faculty members may document their contributions, disruptions that affected their work, and caregiving responsibilities during this time period, while also providing a rubric for promotions and tenure committees to equitably evaluate the pandemic period on an academic CV. Our COVID-19 CV matrix consists of six domains: (1) clinical care, (2) research, (3) education, (4) service, (5) advocacy/media, and (6) social media. These domains encompass traditional and nontraditional contributions made by healthcare professionals during the pandemic (Table). This matrix broadens the ability of both faculty and institutions to determine the actual impact of individuals during the pandemic.

ACCOUNT FOR YOUR (NEW) IMPACT
Throughout the COVID-19 pandemic, academic faculty have been innovative, contributing in novel ways not routinely captured by promotions committees—eg, the digital health researcher who now directs the telemedicine response for their institution and the health disparities researcher who now leads daily webinar sessions on structural racism to medical students. Other novel contributions include advancing COVID-19 innovations and engaging in media and community advocacy (eg, organizing large-scale donations of equipment and funds to support organizations in need). While such nontraditional contributions may not have been readily captured or thought “CV worthy” in the past, faculty should now account for them. More importantly, promotions committees need to recognize that these pivots or alterations in career paths are not signals of professional failure, but rather evidence of a shifting landscape and the respective response of the individual. Furthermore, because these pivots often help fulfill an institutional mission, they are impactful.
ACKNOWLEDGE THE DISRUPTION
It is important for promotions and tenure committees to recognize the impact and disruption COVID-19 has had on traditional academic work, acknowledging the time and energy required for a faculty member to make needed work adjustments. This enables a leader to better assess how a faculty member’s academic portfolio has been affected. For example, researchers have had to halt studies, medical educators have had to redevelop and transition curricula to virtual platforms, and physicians have had to discontinue clinician quality improvement initiatives due to competing hospital priorities. Faculty members who document such unintentional alterations in their academic career path can explain to their institution how they have continued to positively influence their field and the community during the pandemic. This approach is analogous to the current model of accounting for clinical time when judging faculty members’ contributions in scholarly achievement.
The COVID-19 CV matrix has the potential to be annotated to explain the burden of one’s personal situation, which is often “invisible” in the professional environment. For example, many physicians have had to assume additional childcare responsibilities, tend to sick family members, friends, and even themselves. It is also possible that a faculty member has a partner who is also an essential worker, one who had to self-isolate due to COVID-19 exposure or illness, or who has been working overtime due to high patient volumes.
INSTITUTIONAL RESPONSE
How can institutions respond to the altered academic landscape caused by the COVID-19 pandemic? Promotions committees typically have two main tools at their disposal: adjusting the tenure clock or the benchmarks. Extending the period of time available to qualify for tenure is commonplace in the “publish-or-perish” academic tracks of university research professors. Clock adjustments are typically granted to faculty following the birth of a child or for other specific family- or health-related hardships, in accordance with the Family and Medical Leave Act. Unfortunately, tenure-clock extensions for female faculty members can exacerbate gender inequity: Data on tenure-clock extensions show a higher rate of tenure granted to male faculty compared to female faculty.9 For this reason, it is also important to explore adjustments or modifications to benchmark criteria. This could be accomplished by broadening the criteria for promotion, recognizing that impact occurs in many forms, thereby enabling meeting a benchmark. It can also occur by examining the trajectory of an individual within a promotion pathway before it was disrupted to determine impact. To avoid exacerbating social and gender inequities within academia, institutions should use these professional levers and create new ones to provide parity and equality across the promotional playing field. While the CV matrix openly acknowledges the disruptions and tangents the COVID-19 pandemic has had on academic careers, it remains important for academic institutions to recognize these disruptions and innovate the manner in which they acknowledge scholarly contributions.
Conclusion
While academic rigidity and known social taxes (minority and mommy taxes) are particularly problematic in the current climate, these issues have always been at play in evaluating academic success. Improved documentation of novel contributions, disruptions, caregiving, and other challenges can enable more holistic and timely professional advancement for all faculty, regardless of their sex, race, ethnicity, or social background. Ultimately, we hope this framework initiates further conversations among academic institutions on how to define productivity in an age where journal impact factor or number of publications is not the fullest measure of one’s impact in their field.
Professional upheavals caused by the coronavirus disease 2019 (COVID-19) pandemic have affected the academic productivity of many physicians. This is due in part to rapid changes in clinical care and medical education: physician-researchers have been redeployed to frontline clinical care; clinician-educators have been forced to rapidly transition in-person curricula to virtual platforms; and primary care physicians and subspecialists have been forced to transition to telehealth-based practices. In addition to these changes in clinical and educational responsibilities, the COVID-19 pandemic has substantially altered the personal lives of physicians. During the height of the pandemic, clinicians simultaneously wrestled with a lack of available childcare, unexpected home-schooling responsibilities, decreased income, and many other COVID-19-related stresses.1 Additionally, the ever-present “second pandemic” of structural racism, persistent health disparities, and racial inequity has further increased the personal and professional demands facing academic faculty.2
In particular, the pandemic has placed personal and professional pressure on female and minority faculty members. In spite of these pressures, however, the academic promotions process still requires rigid accounting of scholarly productivity. As the focus of academic practices has shifted to support clinical care during the pandemic, scholarly productivity has suffered for clinicians on the frontline. As a result, academic clinical faculty have expressed significant stress and concerns about failing to meet benchmarks for promotion (eg, publications, curricula development, national presentations). To counter these shifts (and the inherent inequity that they create for female clinicians and for men and women who are Black, Indigenous, and/or of color), academic institutions should not only recognize the effects the COVID-19 pandemic has had on faculty, but also adopt immediate solutions to more equitably account for such disruptions to academic portfolios. In this paper, we explore populations whose career trajectories are most at-risk and propose a framework to capture novel and nontraditional contributions while also acknowledging the rapid changes the COVID-19 pandemic has brought to academic medicine.
POPULATIONS AT RISK FOR CAREER DISRUPTION
Even before the COVID-19 pandemic, physician mothers, underrepresented racial/ethnic minority groups, and junior faculty were most at-risk for career disruptions. The closure of daycare facilities and schools and shift to online learning resulting from the pandemic, along with the common challenges of parenting, have taken a significant toll on the lives of working parents. Because women tend to carry a disproportionate share of childcare and household responsibilities, these changes have inequitably leveraged themselves as a “mommy tax” on working women.3,4
As underrepresented medicine faculty (particularly Black, Hispanic, Latino, and Native American clinicians) comprise only 8% of the academic medical workforce,they currently face a variety of personal and professional challenges.5 This is especially true for Black and Latinx physicians who have been experiencing an increased COVID-19 burden in their communities, while concurrently fighting entrenched structural racism and police violence. In academia, these challenges have worsened because of the “minority tax”—the toll of often uncompensated extra responsibilities (time or money) placed on minority faculty in the name of achieving diversity. The unintended consequences of these responsibilities result in having fewer mentors,6 caring for underserved populations,7 and performing more clinical care8 than non-underrepresented minority faculty. Because minority faculty are unlikely to be in leadership positions, it is reasonable to conclude they have been shouldering heavier clinical obligations and facing greater career disruption of scholarly work due to the COVID-19 pandemic.
Junior faculty (eg, instructors and assistant professors) also remain professionally vulnerable during the COVID-19 pandemic. Because junior faculty are often more clinically focused and less likely to hold leadership positions than senior faculty, they are more likely to have assumed frontline clinical positions, which come at the expense of academic work. Junior faculty are also at a critical building phase in their academic career—a time when they benefit from the opportunity to share their scholarly work and network at conferences. Unfortunately, many conferences have been canceled or moved to a virtual platform. Given that some institutions may be freezing academic funding for conferences due to budgetary shortfalls from the pandemic, junior faculty may be particularly at risk if they are not able to present their work. In addition, junior faculty often face disproportionate struggles at home, trying to balance demands of work and caring for young children. Considering the unique needs of each of these groups, it is especially important to consider intersectionality, or the compounded issues for individuals who exist in multiple disproportionately affected groups (eg, a Black female junior faculty member who is also a mother).
THE COVID-19-CURRICULUM VITAE MATRIX
The typical format of a professional curriculum vitae (CV) at most academic institutions does not allow one to document potential disruptions or novel contributions, including those that occurred during the COVID-19 pandemic. As a group of academic clinicians, educators, and researchers whose careers have been affected by the pandemic, we created a COVID-19 CV matrix, a potential framework to serve as a supplement for faculty. In this matrix, faculty members may document their contributions, disruptions that affected their work, and caregiving responsibilities during this time period, while also providing a rubric for promotions and tenure committees to equitably evaluate the pandemic period on an academic CV. Our COVID-19 CV matrix consists of six domains: (1) clinical care, (2) research, (3) education, (4) service, (5) advocacy/media, and (6) social media. These domains encompass traditional and nontraditional contributions made by healthcare professionals during the pandemic (Table). This matrix broadens the ability of both faculty and institutions to determine the actual impact of individuals during the pandemic.

ACCOUNT FOR YOUR (NEW) IMPACT
Throughout the COVID-19 pandemic, academic faculty have been innovative, contributing in novel ways not routinely captured by promotions committees—eg, the digital health researcher who now directs the telemedicine response for their institution and the health disparities researcher who now leads daily webinar sessions on structural racism to medical students. Other novel contributions include advancing COVID-19 innovations and engaging in media and community advocacy (eg, organizing large-scale donations of equipment and funds to support organizations in need). While such nontraditional contributions may not have been readily captured or thought “CV worthy” in the past, faculty should now account for them. More importantly, promotions committees need to recognize that these pivots or alterations in career paths are not signals of professional failure, but rather evidence of a shifting landscape and the respective response of the individual. Furthermore, because these pivots often help fulfill an institutional mission, they are impactful.
ACKNOWLEDGE THE DISRUPTION
It is important for promotions and tenure committees to recognize the impact and disruption COVID-19 has had on traditional academic work, acknowledging the time and energy required for a faculty member to make needed work adjustments. This enables a leader to better assess how a faculty member’s academic portfolio has been affected. For example, researchers have had to halt studies, medical educators have had to redevelop and transition curricula to virtual platforms, and physicians have had to discontinue clinician quality improvement initiatives due to competing hospital priorities. Faculty members who document such unintentional alterations in their academic career path can explain to their institution how they have continued to positively influence their field and the community during the pandemic. This approach is analogous to the current model of accounting for clinical time when judging faculty members’ contributions in scholarly achievement.
The COVID-19 CV matrix has the potential to be annotated to explain the burden of one’s personal situation, which is often “invisible” in the professional environment. For example, many physicians have had to assume additional childcare responsibilities, tend to sick family members, friends, and even themselves. It is also possible that a faculty member has a partner who is also an essential worker, one who had to self-isolate due to COVID-19 exposure or illness, or who has been working overtime due to high patient volumes.
INSTITUTIONAL RESPONSE
How can institutions respond to the altered academic landscape caused by the COVID-19 pandemic? Promotions committees typically have two main tools at their disposal: adjusting the tenure clock or the benchmarks. Extending the period of time available to qualify for tenure is commonplace in the “publish-or-perish” academic tracks of university research professors. Clock adjustments are typically granted to faculty following the birth of a child or for other specific family- or health-related hardships, in accordance with the Family and Medical Leave Act. Unfortunately, tenure-clock extensions for female faculty members can exacerbate gender inequity: Data on tenure-clock extensions show a higher rate of tenure granted to male faculty compared to female faculty.9 For this reason, it is also important to explore adjustments or modifications to benchmark criteria. This could be accomplished by broadening the criteria for promotion, recognizing that impact occurs in many forms, thereby enabling meeting a benchmark. It can also occur by examining the trajectory of an individual within a promotion pathway before it was disrupted to determine impact. To avoid exacerbating social and gender inequities within academia, institutions should use these professional levers and create new ones to provide parity and equality across the promotional playing field. While the CV matrix openly acknowledges the disruptions and tangents the COVID-19 pandemic has had on academic careers, it remains important for academic institutions to recognize these disruptions and innovate the manner in which they acknowledge scholarly contributions.
Conclusion
While academic rigidity and known social taxes (minority and mommy taxes) are particularly problematic in the current climate, these issues have always been at play in evaluating academic success. Improved documentation of novel contributions, disruptions, caregiving, and other challenges can enable more holistic and timely professional advancement for all faculty, regardless of their sex, race, ethnicity, or social background. Ultimately, we hope this framework initiates further conversations among academic institutions on how to define productivity in an age where journal impact factor or number of publications is not the fullest measure of one’s impact in their field.
1. Jones Y, Durand V, Morton K, et al; ADVANCE PHM Steering Committee. Collateral damage: how covid-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
2. Manning KD. When grief and crises intersect: perspectives of a black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
3. Cohen P, Hsu T. Pandemic could scar a generation of working mothers. New York Times. Published June 3, 2020. Updated June 30, 2020. Accessed November 11, 2020. https://www.nytimes.com/2020/06/03/business/economy/coronavirus-working-women.html
4. Cain Miller C. Nearly half of men say they do most of the home schooling. 3 percent of women agree. Published May 6, 2020. Updated May 8, 2020. Accessed November 11, 2020. New York Times. https://www.nytimes.com/2020/05/06/upshot/pandemic-chores-homeschooling-gender.html
5. Rodríguez JE, Campbell KM, Pololi LH. Addressing disparities in academic medicine: what of the minority tax? BMC Med Educ. 2015;15:6. https://doi.org/10.1186/s12909-015-0290-9
6. Lewellen-Williams C, Johnson VA, Deloney LA, Thomas BR, Goyol A, Henry-Tillman R. The POD: a new model for mentoring underrepresented minority faculty. Acad Med. 2006;81(3):275-279. https://doi.org/10.1097/00001888-200603000-00020
7. Pololi LH, Evans AT, Gibbs BK, Krupat E, Brennan RT, Civian JT. The experience of minority faculty who are underrepresented in medicine, at 26 representative U.S. medical schools. Acad Med. 2013;88(9):1308-1314. https://doi.org/10.1097/acm.0b013e31829eefff
8. Richert A, Campbell K, Rodríguez J, Borowsky IW, Parikh R, Colwell A. ACU workforce column: expanding and supporting the health care workforce. J Health Care Poor Underserved. 2013;24(4):1423-1431. https://doi.org/10.1353/hpu.2013.0162
9. Woitowich NC, Jain S, Arora VM, Joffe H. COVID-19 threatens progress toward gender equity within academic medicine. Acad Med. 2020;29:10.1097/ACM.0000000000003782. https://doi.org/10.1097/acm.0000000000003782
1. Jones Y, Durand V, Morton K, et al; ADVANCE PHM Steering Committee. Collateral damage: how covid-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
2. Manning KD. When grief and crises intersect: perspectives of a black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
3. Cohen P, Hsu T. Pandemic could scar a generation of working mothers. New York Times. Published June 3, 2020. Updated June 30, 2020. Accessed November 11, 2020. https://www.nytimes.com/2020/06/03/business/economy/coronavirus-working-women.html
4. Cain Miller C. Nearly half of men say they do most of the home schooling. 3 percent of women agree. Published May 6, 2020. Updated May 8, 2020. Accessed November 11, 2020. New York Times. https://www.nytimes.com/2020/05/06/upshot/pandemic-chores-homeschooling-gender.html
5. Rodríguez JE, Campbell KM, Pololi LH. Addressing disparities in academic medicine: what of the minority tax? BMC Med Educ. 2015;15:6. https://doi.org/10.1186/s12909-015-0290-9
6. Lewellen-Williams C, Johnson VA, Deloney LA, Thomas BR, Goyol A, Henry-Tillman R. The POD: a new model for mentoring underrepresented minority faculty. Acad Med. 2006;81(3):275-279. https://doi.org/10.1097/00001888-200603000-00020
7. Pololi LH, Evans AT, Gibbs BK, Krupat E, Brennan RT, Civian JT. The experience of minority faculty who are underrepresented in medicine, at 26 representative U.S. medical schools. Acad Med. 2013;88(9):1308-1314. https://doi.org/10.1097/acm.0b013e31829eefff
8. Richert A, Campbell K, Rodríguez J, Borowsky IW, Parikh R, Colwell A. ACU workforce column: expanding and supporting the health care workforce. J Health Care Poor Underserved. 2013;24(4):1423-1431. https://doi.org/10.1353/hpu.2013.0162
9. Woitowich NC, Jain S, Arora VM, Joffe H. COVID-19 threatens progress toward gender equity within academic medicine. Acad Med. 2020;29:10.1097/ACM.0000000000003782. https://doi.org/10.1097/acm.0000000000003782
© 2021 Society of Hospital Medicine
Rethinking Hospital-Associated Disability for Patients With COVID-19
Between February 1 and July 1, 2020, SARS-CoV-2 killed over 120,000 people in the United States alone. Nearly 80% of deaths occurred in those 65 years and older; by contrast, this age group constituted only 65% of deaths from influenza during the same time period.1 Though the reasons for these differences have not been completely elucidated, one thing is abundantly clear: Our nation’s oldest and most frail have been among the most likely to die of COVID-19. With an estimated mortality rate of 4.7% in the United States, we are fortunate that most infected patients survive2,3; however, many survivors require an exceptionally long hospital stay in isolation. Hospitalizations for patients with COVID-19 are distinct and confer a high risk for hospital-associated disability (HAD). HAD, defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge, occurs in approximately one-third of all hospitalized patients.4 In this perspective, we explore why HAD might be worse in patients with COVID-19 and offer new models for delivery of physical and occupational therapy to help them with functional recovery during and after hospitalization.
HOSPITAL-ASSOCIATED DISABILITY BEFORE COVID-19
Functional decline, a life-altering condition that patients experience as part of posthospital syndrome,5 is characterized by loss of mobility, cognitive decline, and HAD. The effects of functional decline can lead to a cascade of readmissions, institutionalization, and even death. During hospitalization, patients spend 87% to 100% of their time in bed. This immobilization is a major contributor to the development of HAD.6,7 The $58.5 billion dollars in yearly Medicare spending that is attributed to post–acute care also highlights the financial toll arising from such disability.8 Early mobilization with physical and occupational therapy is important to prevent HAD. However, even under normal conditions, care teams face innumerable barriers to mobilizing patients: symptomatic patients can be resistant to mobilizing during illness, providers have fears of worsening symptoms or falls, and some providers are unaware of the importance of mobilization. In patients with COVID-19, the barriers are only magnified.
HOSPITAL-ASSOCIATED DISABILITY DURING COVID-19
Given the increasing numbers of COVID-19 survivors discharged from the hospital, it is critical to consider why HAD could be an even larger problem in these patients. Consider their age, symptom burden, and illness severity: Among 5,700 patients who were admitted for COVID-19 in the New York City area, most were elderly (median age, 63 years), many were tachypneic (17%), and many required supplemental oxygen (28%).9 Fourteen percent of these patients required care in the intensive care unit (ICU), most of whom required mechanical ventilation (86%), which independently places them at higher risk of HAD. Given these severe respiratory issues in COVID-19, mobilization may cause significant discomfort. Being symptomatic is, by far, the most common reason hospitalized patients refuse to ambulate.10 As a result, this could make early mobilization for these COVID-19 patients exceptionally difficult.
Patients with COVID-19 also experience prolonged hospitalization. The median hospital length of stay (LOS) is 9.3 days for survivors of SARS-CoV-2 infection compared with the 7-day average LOS for patients with pneumonia requiring ICU admission and 5-day average LOS for patients with influenza.11-13 Complications of COVID, such as cardiac injury, critical illness polyneuropathy or myopathy, or cognitive impairment, also contribute to the significant need for rehabilitation long after recovery from the acute illness.14
Physical and occupational therapy involve prolonged close contact with patients, a known risk factor for contracting SARS-CoV-2.15 For staff, mobilizing a patient with COVID-19 takes longer due to intricate PPE donning and doffing procedures and patients requiring rest breaks because of weakness and respiratory-related recovery time. For patients who are mobilized, their activity is constrained by isolation restrictions that prohibit patients from leaving the confines of their hospital rooms. On March 23, 2020, the World Confederation for Physical Therapy (WCPT) endorsed guidelines created by the Australian Physiotherapy Association (APA) on caring for patients with COVID-19 acknowledging this risk16. The guidelines suggested that personal protective equipment (PPE) required for reducing risk of droplet transmission is appropriate for some scenarios, but they noted that exercising may induce coughing or expectoration, which could make physical therapy an aerosol-generating procedure. Therefore, the guidelines recommended that therapists wear N95 masks and recommend that direct face-to-face physical therapy should be limited to patients with certain functional limitations, including frailty, multiple comorbidities, and advanced age.
Patients with COVID-19 face additional barriers to accessing therapy services following hospital discharge. Post–acute care placement may be difficult due to limited availability of isolation rooms for patients with COVID-19 and the requirement of negative results for recovering patients. For those who manage to secure a bed, PPE shortages in nursing facilities could lead to lower prioritization of therapy interventions among staff and more bedridden days for the patients. Given social distancing restrictions, home health and outpatient therapy may not be possible for similar reasons.
The confluence of often highly symptomatic and even fragile patients, time-consuming visits with high concern for contagion, limited space to freely mobilize, and barriers to post–acute care illustrates why it is likely that COVID-19 admissions will be associated with a higher degree of HAD than admissions for other illnesses.
COVID-19: INNOVATION IN THERAPY SERVICES
The entire healthcare system has had to evolve and innovate rapidly to combat the morbidity and mortality of COVID-19. In the case of HAD, nursing staff, new billing guidelines, hospital redesign, and telemedicine are all facilitating novel ways to mobilize patients during and after hospitalization.
To limit the numbers of staff exposed to patients with COVID-19, the APA recommends engaging nursing staff in initial therapy evaluations and simple exercises that can be performed in a hospital room. Meaningful in-room exercise for some patients may include getting out of bed and walking to the bathroom to brush their teeth or complete other ADLs. Assessment of cognition should be carefully considered for discharge planning given its effects on the patient’s ability to independently participate in exercises and ADLs. For this reason, treatment and prevention of delirium or cognitive changes with interventions targeting environmental modifications, maintenance of healthy sleep-wake cycles, and orientation strategies are vital.
Therapy evaluations can also be administered remotely via phone call or video. To help facilitate telehealth visits, the Centers for Medicaid & Medicare Services has released new guidelines under the Coronavirus Aid, Relief, and Economic Security (CARES) Act. Physical and occupational therapists have been historically excluded from the list of providers able to bill for telehealth services, but the CARES Act allows physical and occupational therapists who accept Medicare part B to bill for telehealth services and e-visits. The new rule applies to patients in healthcare facilities or patients at home.17 Transitioning some physical and occupational therapy to telehealth could prove to be a critical resource for patients with COVID-19 trying to regain strength and independence during and after hospitalization.
Other solutions include converting areas of a hospital into rehabilitation units solely for patients recovering from COVID-19. Alternatively, rural hospitals, which usually run below capacity, or certain post–acute care facilities that are already prepared to manage infectious patients could serve as dedicated COVID-19 rehabilitation facilities, which can offer novel ways to continue therapy services after discharge while decreasing new exposures to COVID-19.18
Given the social isolation patients with COVID-19 experience during hospitalization, virtual group exercise classes may help for overall recovery. Most therapy companies already offer this service, and several include an app that allows therapists to monitor the patient’s exercises and progress. However, when transitioning to telemedicine, it is also important to consider the needs of those who may not be able to navigate technology effectively. For example, some elderly patients can be limited by a range of issues from poor computer skills and “technophobia” to visual and cognitive impairments. Having a friend or family member available to assist with technology should be considered. Additionally, being elderly, having lower income, or having a lower level of education makes it less likely that a patient will have access to internet or smartphones. Therefore, patients with these limitations may be poor candidates for telehealth and require post–acute care for their therapy services.19,20
CONCLUSION
With all the devastation that COVID-19 has created, it might be easy to forget the importance of physical and occupational therapy. But without this focus, the disability resulting from COVID-19 hospitalizations could inflict considerable long-lasting effects on our patients at great cost to an already strained healthcare system. Immediate changes in how we adapt and innovate these services for patients with COVID-19 are critical. It may prove to have enormous impact on patients and the healthcare system long after the worst of the virus is forgotten.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Arora is funded by National Heart, Lung and Blood Institute (NHLBI Grant K24HL136859).
1. Provisional COVID-19 Death Counts by Sex, Age, and State. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
2. Rajgor DD, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776-777. https://dx.doi.org/10.1016/S1473-3099(20)30244-9
3. Coronavirus Resource Center: Maps & Trends: Mortality Analyses. Johns Hopkins University & Medicine. Accessed April 26, 2020. https://coronavirus.jhu.edu/data/mortality
4. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461.e5. https://doi.org/10.1016/j.jamda.2019.09.015
5. Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/nejmp1212324
6. Summary Health Statistics: National Health Interview Survey, 2017. Tables P10a-P10c; p. 1-9. Centers for Disease Control and Prevention. Accessed April 26,2020. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_P-10.pdf
7. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? a systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
8. Fact Sheet: Post-Acute Care. American Hospital Association. July 2019. Accessed April 26, 2020. https://www.aha.org/system/files/media/file/2019/07/fact-sheet-post-acute-care-0719.pdf
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
10. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305-313. https://doi.org/10.1002/jhm.209
11. Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ 2020;369:m1923. https://doi.org/10.1136/bmj.m1923
12. Williams S, Gousen S, DeFrances C. National Hospital Care Survey Demonstration Projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report. 2018;(116):1-11.
13. Milenkovic M, Russo CA, Elixhauser A. Hospital Stays for Influenza, 2004: Statistical Brief #16. 2006 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed April 26, 2020 https://www.ncbi.nlm.nih.gov/books/NBK63484/
14. Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. Am J Phys Med Rehabil. 2020;99(6):470-474. https://doi.org/10.1097/phm.0000000000001443
15. Coronavirus Disease 2019 (COVID-19): Social Distancing. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
16. Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73-82. https://doi.org/10.1016/j.jphys.2020.03.011
17. COVID1-9 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services. Accessed April 23, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
18. Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. 2020;323(20):2007-2008. https://doi.org/10.1001/jama.2020.4686
19. Eung-Hun K, Stolvar A, Lober WB, et al. Challenges to using an electronic health record by a low-income elderly population. J Med Internet Res. 2009;11(4):e44. https://doi.org/10.2196/jmir.1256
20. Rajasekaran K. Access to telemedicine-are we doing all that we can during the COVID-19 pandemic? Otolaryngol Head Neck Surg. 2020;163(1):104-106. https://doi.org/10.1177/0194599820925049
Between February 1 and July 1, 2020, SARS-CoV-2 killed over 120,000 people in the United States alone. Nearly 80% of deaths occurred in those 65 years and older; by contrast, this age group constituted only 65% of deaths from influenza during the same time period.1 Though the reasons for these differences have not been completely elucidated, one thing is abundantly clear: Our nation’s oldest and most frail have been among the most likely to die of COVID-19. With an estimated mortality rate of 4.7% in the United States, we are fortunate that most infected patients survive2,3; however, many survivors require an exceptionally long hospital stay in isolation. Hospitalizations for patients with COVID-19 are distinct and confer a high risk for hospital-associated disability (HAD). HAD, defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge, occurs in approximately one-third of all hospitalized patients.4 In this perspective, we explore why HAD might be worse in patients with COVID-19 and offer new models for delivery of physical and occupational therapy to help them with functional recovery during and after hospitalization.
HOSPITAL-ASSOCIATED DISABILITY BEFORE COVID-19
Functional decline, a life-altering condition that patients experience as part of posthospital syndrome,5 is characterized by loss of mobility, cognitive decline, and HAD. The effects of functional decline can lead to a cascade of readmissions, institutionalization, and even death. During hospitalization, patients spend 87% to 100% of their time in bed. This immobilization is a major contributor to the development of HAD.6,7 The $58.5 billion dollars in yearly Medicare spending that is attributed to post–acute care also highlights the financial toll arising from such disability.8 Early mobilization with physical and occupational therapy is important to prevent HAD. However, even under normal conditions, care teams face innumerable barriers to mobilizing patients: symptomatic patients can be resistant to mobilizing during illness, providers have fears of worsening symptoms or falls, and some providers are unaware of the importance of mobilization. In patients with COVID-19, the barriers are only magnified.
HOSPITAL-ASSOCIATED DISABILITY DURING COVID-19
Given the increasing numbers of COVID-19 survivors discharged from the hospital, it is critical to consider why HAD could be an even larger problem in these patients. Consider their age, symptom burden, and illness severity: Among 5,700 patients who were admitted for COVID-19 in the New York City area, most were elderly (median age, 63 years), many were tachypneic (17%), and many required supplemental oxygen (28%).9 Fourteen percent of these patients required care in the intensive care unit (ICU), most of whom required mechanical ventilation (86%), which independently places them at higher risk of HAD. Given these severe respiratory issues in COVID-19, mobilization may cause significant discomfort. Being symptomatic is, by far, the most common reason hospitalized patients refuse to ambulate.10 As a result, this could make early mobilization for these COVID-19 patients exceptionally difficult.
Patients with COVID-19 also experience prolonged hospitalization. The median hospital length of stay (LOS) is 9.3 days for survivors of SARS-CoV-2 infection compared with the 7-day average LOS for patients with pneumonia requiring ICU admission and 5-day average LOS for patients with influenza.11-13 Complications of COVID, such as cardiac injury, critical illness polyneuropathy or myopathy, or cognitive impairment, also contribute to the significant need for rehabilitation long after recovery from the acute illness.14
Physical and occupational therapy involve prolonged close contact with patients, a known risk factor for contracting SARS-CoV-2.15 For staff, mobilizing a patient with COVID-19 takes longer due to intricate PPE donning and doffing procedures and patients requiring rest breaks because of weakness and respiratory-related recovery time. For patients who are mobilized, their activity is constrained by isolation restrictions that prohibit patients from leaving the confines of their hospital rooms. On March 23, 2020, the World Confederation for Physical Therapy (WCPT) endorsed guidelines created by the Australian Physiotherapy Association (APA) on caring for patients with COVID-19 acknowledging this risk16. The guidelines suggested that personal protective equipment (PPE) required for reducing risk of droplet transmission is appropriate for some scenarios, but they noted that exercising may induce coughing or expectoration, which could make physical therapy an aerosol-generating procedure. Therefore, the guidelines recommended that therapists wear N95 masks and recommend that direct face-to-face physical therapy should be limited to patients with certain functional limitations, including frailty, multiple comorbidities, and advanced age.
Patients with COVID-19 face additional barriers to accessing therapy services following hospital discharge. Post–acute care placement may be difficult due to limited availability of isolation rooms for patients with COVID-19 and the requirement of negative results for recovering patients. For those who manage to secure a bed, PPE shortages in nursing facilities could lead to lower prioritization of therapy interventions among staff and more bedridden days for the patients. Given social distancing restrictions, home health and outpatient therapy may not be possible for similar reasons.
The confluence of often highly symptomatic and even fragile patients, time-consuming visits with high concern for contagion, limited space to freely mobilize, and barriers to post–acute care illustrates why it is likely that COVID-19 admissions will be associated with a higher degree of HAD than admissions for other illnesses.
COVID-19: INNOVATION IN THERAPY SERVICES
The entire healthcare system has had to evolve and innovate rapidly to combat the morbidity and mortality of COVID-19. In the case of HAD, nursing staff, new billing guidelines, hospital redesign, and telemedicine are all facilitating novel ways to mobilize patients during and after hospitalization.
To limit the numbers of staff exposed to patients with COVID-19, the APA recommends engaging nursing staff in initial therapy evaluations and simple exercises that can be performed in a hospital room. Meaningful in-room exercise for some patients may include getting out of bed and walking to the bathroom to brush their teeth or complete other ADLs. Assessment of cognition should be carefully considered for discharge planning given its effects on the patient’s ability to independently participate in exercises and ADLs. For this reason, treatment and prevention of delirium or cognitive changes with interventions targeting environmental modifications, maintenance of healthy sleep-wake cycles, and orientation strategies are vital.
Therapy evaluations can also be administered remotely via phone call or video. To help facilitate telehealth visits, the Centers for Medicaid & Medicare Services has released new guidelines under the Coronavirus Aid, Relief, and Economic Security (CARES) Act. Physical and occupational therapists have been historically excluded from the list of providers able to bill for telehealth services, but the CARES Act allows physical and occupational therapists who accept Medicare part B to bill for telehealth services and e-visits. The new rule applies to patients in healthcare facilities or patients at home.17 Transitioning some physical and occupational therapy to telehealth could prove to be a critical resource for patients with COVID-19 trying to regain strength and independence during and after hospitalization.
Other solutions include converting areas of a hospital into rehabilitation units solely for patients recovering from COVID-19. Alternatively, rural hospitals, which usually run below capacity, or certain post–acute care facilities that are already prepared to manage infectious patients could serve as dedicated COVID-19 rehabilitation facilities, which can offer novel ways to continue therapy services after discharge while decreasing new exposures to COVID-19.18
Given the social isolation patients with COVID-19 experience during hospitalization, virtual group exercise classes may help for overall recovery. Most therapy companies already offer this service, and several include an app that allows therapists to monitor the patient’s exercises and progress. However, when transitioning to telemedicine, it is also important to consider the needs of those who may not be able to navigate technology effectively. For example, some elderly patients can be limited by a range of issues from poor computer skills and “technophobia” to visual and cognitive impairments. Having a friend or family member available to assist with technology should be considered. Additionally, being elderly, having lower income, or having a lower level of education makes it less likely that a patient will have access to internet or smartphones. Therefore, patients with these limitations may be poor candidates for telehealth and require post–acute care for their therapy services.19,20
CONCLUSION
With all the devastation that COVID-19 has created, it might be easy to forget the importance of physical and occupational therapy. But without this focus, the disability resulting from COVID-19 hospitalizations could inflict considerable long-lasting effects on our patients at great cost to an already strained healthcare system. Immediate changes in how we adapt and innovate these services for patients with COVID-19 are critical. It may prove to have enormous impact on patients and the healthcare system long after the worst of the virus is forgotten.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Arora is funded by National Heart, Lung and Blood Institute (NHLBI Grant K24HL136859).
Between February 1 and July 1, 2020, SARS-CoV-2 killed over 120,000 people in the United States alone. Nearly 80% of deaths occurred in those 65 years and older; by contrast, this age group constituted only 65% of deaths from influenza during the same time period.1 Though the reasons for these differences have not been completely elucidated, one thing is abundantly clear: Our nation’s oldest and most frail have been among the most likely to die of COVID-19. With an estimated mortality rate of 4.7% in the United States, we are fortunate that most infected patients survive2,3; however, many survivors require an exceptionally long hospital stay in isolation. Hospitalizations for patients with COVID-19 are distinct and confer a high risk for hospital-associated disability (HAD). HAD, defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge, occurs in approximately one-third of all hospitalized patients.4 In this perspective, we explore why HAD might be worse in patients with COVID-19 and offer new models for delivery of physical and occupational therapy to help them with functional recovery during and after hospitalization.
HOSPITAL-ASSOCIATED DISABILITY BEFORE COVID-19
Functional decline, a life-altering condition that patients experience as part of posthospital syndrome,5 is characterized by loss of mobility, cognitive decline, and HAD. The effects of functional decline can lead to a cascade of readmissions, institutionalization, and even death. During hospitalization, patients spend 87% to 100% of their time in bed. This immobilization is a major contributor to the development of HAD.6,7 The $58.5 billion dollars in yearly Medicare spending that is attributed to post–acute care also highlights the financial toll arising from such disability.8 Early mobilization with physical and occupational therapy is important to prevent HAD. However, even under normal conditions, care teams face innumerable barriers to mobilizing patients: symptomatic patients can be resistant to mobilizing during illness, providers have fears of worsening symptoms or falls, and some providers are unaware of the importance of mobilization. In patients with COVID-19, the barriers are only magnified.
HOSPITAL-ASSOCIATED DISABILITY DURING COVID-19
Given the increasing numbers of COVID-19 survivors discharged from the hospital, it is critical to consider why HAD could be an even larger problem in these patients. Consider their age, symptom burden, and illness severity: Among 5,700 patients who were admitted for COVID-19 in the New York City area, most were elderly (median age, 63 years), many were tachypneic (17%), and many required supplemental oxygen (28%).9 Fourteen percent of these patients required care in the intensive care unit (ICU), most of whom required mechanical ventilation (86%), which independently places them at higher risk of HAD. Given these severe respiratory issues in COVID-19, mobilization may cause significant discomfort. Being symptomatic is, by far, the most common reason hospitalized patients refuse to ambulate.10 As a result, this could make early mobilization for these COVID-19 patients exceptionally difficult.
Patients with COVID-19 also experience prolonged hospitalization. The median hospital length of stay (LOS) is 9.3 days for survivors of SARS-CoV-2 infection compared with the 7-day average LOS for patients with pneumonia requiring ICU admission and 5-day average LOS for patients with influenza.11-13 Complications of COVID, such as cardiac injury, critical illness polyneuropathy or myopathy, or cognitive impairment, also contribute to the significant need for rehabilitation long after recovery from the acute illness.14
Physical and occupational therapy involve prolonged close contact with patients, a known risk factor for contracting SARS-CoV-2.15 For staff, mobilizing a patient with COVID-19 takes longer due to intricate PPE donning and doffing procedures and patients requiring rest breaks because of weakness and respiratory-related recovery time. For patients who are mobilized, their activity is constrained by isolation restrictions that prohibit patients from leaving the confines of their hospital rooms. On March 23, 2020, the World Confederation for Physical Therapy (WCPT) endorsed guidelines created by the Australian Physiotherapy Association (APA) on caring for patients with COVID-19 acknowledging this risk16. The guidelines suggested that personal protective equipment (PPE) required for reducing risk of droplet transmission is appropriate for some scenarios, but they noted that exercising may induce coughing or expectoration, which could make physical therapy an aerosol-generating procedure. Therefore, the guidelines recommended that therapists wear N95 masks and recommend that direct face-to-face physical therapy should be limited to patients with certain functional limitations, including frailty, multiple comorbidities, and advanced age.
Patients with COVID-19 face additional barriers to accessing therapy services following hospital discharge. Post–acute care placement may be difficult due to limited availability of isolation rooms for patients with COVID-19 and the requirement of negative results for recovering patients. For those who manage to secure a bed, PPE shortages in nursing facilities could lead to lower prioritization of therapy interventions among staff and more bedridden days for the patients. Given social distancing restrictions, home health and outpatient therapy may not be possible for similar reasons.
The confluence of often highly symptomatic and even fragile patients, time-consuming visits with high concern for contagion, limited space to freely mobilize, and barriers to post–acute care illustrates why it is likely that COVID-19 admissions will be associated with a higher degree of HAD than admissions for other illnesses.
COVID-19: INNOVATION IN THERAPY SERVICES
The entire healthcare system has had to evolve and innovate rapidly to combat the morbidity and mortality of COVID-19. In the case of HAD, nursing staff, new billing guidelines, hospital redesign, and telemedicine are all facilitating novel ways to mobilize patients during and after hospitalization.
To limit the numbers of staff exposed to patients with COVID-19, the APA recommends engaging nursing staff in initial therapy evaluations and simple exercises that can be performed in a hospital room. Meaningful in-room exercise for some patients may include getting out of bed and walking to the bathroom to brush their teeth or complete other ADLs. Assessment of cognition should be carefully considered for discharge planning given its effects on the patient’s ability to independently participate in exercises and ADLs. For this reason, treatment and prevention of delirium or cognitive changes with interventions targeting environmental modifications, maintenance of healthy sleep-wake cycles, and orientation strategies are vital.
Therapy evaluations can also be administered remotely via phone call or video. To help facilitate telehealth visits, the Centers for Medicaid & Medicare Services has released new guidelines under the Coronavirus Aid, Relief, and Economic Security (CARES) Act. Physical and occupational therapists have been historically excluded from the list of providers able to bill for telehealth services, but the CARES Act allows physical and occupational therapists who accept Medicare part B to bill for telehealth services and e-visits. The new rule applies to patients in healthcare facilities or patients at home.17 Transitioning some physical and occupational therapy to telehealth could prove to be a critical resource for patients with COVID-19 trying to regain strength and independence during and after hospitalization.
Other solutions include converting areas of a hospital into rehabilitation units solely for patients recovering from COVID-19. Alternatively, rural hospitals, which usually run below capacity, or certain post–acute care facilities that are already prepared to manage infectious patients could serve as dedicated COVID-19 rehabilitation facilities, which can offer novel ways to continue therapy services after discharge while decreasing new exposures to COVID-19.18
Given the social isolation patients with COVID-19 experience during hospitalization, virtual group exercise classes may help for overall recovery. Most therapy companies already offer this service, and several include an app that allows therapists to monitor the patient’s exercises and progress. However, when transitioning to telemedicine, it is also important to consider the needs of those who may not be able to navigate technology effectively. For example, some elderly patients can be limited by a range of issues from poor computer skills and “technophobia” to visual and cognitive impairments. Having a friend or family member available to assist with technology should be considered. Additionally, being elderly, having lower income, or having a lower level of education makes it less likely that a patient will have access to internet or smartphones. Therefore, patients with these limitations may be poor candidates for telehealth and require post–acute care for their therapy services.19,20
CONCLUSION
With all the devastation that COVID-19 has created, it might be easy to forget the importance of physical and occupational therapy. But without this focus, the disability resulting from COVID-19 hospitalizations could inflict considerable long-lasting effects on our patients at great cost to an already strained healthcare system. Immediate changes in how we adapt and innovate these services for patients with COVID-19 are critical. It may prove to have enormous impact on patients and the healthcare system long after the worst of the virus is forgotten.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Arora is funded by National Heart, Lung and Blood Institute (NHLBI Grant K24HL136859).
1. Provisional COVID-19 Death Counts by Sex, Age, and State. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
2. Rajgor DD, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776-777. https://dx.doi.org/10.1016/S1473-3099(20)30244-9
3. Coronavirus Resource Center: Maps & Trends: Mortality Analyses. Johns Hopkins University & Medicine. Accessed April 26, 2020. https://coronavirus.jhu.edu/data/mortality
4. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461.e5. https://doi.org/10.1016/j.jamda.2019.09.015
5. Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/nejmp1212324
6. Summary Health Statistics: National Health Interview Survey, 2017. Tables P10a-P10c; p. 1-9. Centers for Disease Control and Prevention. Accessed April 26,2020. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_P-10.pdf
7. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? a systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
8. Fact Sheet: Post-Acute Care. American Hospital Association. July 2019. Accessed April 26, 2020. https://www.aha.org/system/files/media/file/2019/07/fact-sheet-post-acute-care-0719.pdf
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
10. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305-313. https://doi.org/10.1002/jhm.209
11. Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ 2020;369:m1923. https://doi.org/10.1136/bmj.m1923
12. Williams S, Gousen S, DeFrances C. National Hospital Care Survey Demonstration Projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report. 2018;(116):1-11.
13. Milenkovic M, Russo CA, Elixhauser A. Hospital Stays for Influenza, 2004: Statistical Brief #16. 2006 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed April 26, 2020 https://www.ncbi.nlm.nih.gov/books/NBK63484/
14. Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. Am J Phys Med Rehabil. 2020;99(6):470-474. https://doi.org/10.1097/phm.0000000000001443
15. Coronavirus Disease 2019 (COVID-19): Social Distancing. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
16. Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73-82. https://doi.org/10.1016/j.jphys.2020.03.011
17. COVID1-9 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services. Accessed April 23, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
18. Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. 2020;323(20):2007-2008. https://doi.org/10.1001/jama.2020.4686
19. Eung-Hun K, Stolvar A, Lober WB, et al. Challenges to using an electronic health record by a low-income elderly population. J Med Internet Res. 2009;11(4):e44. https://doi.org/10.2196/jmir.1256
20. Rajasekaran K. Access to telemedicine-are we doing all that we can during the COVID-19 pandemic? Otolaryngol Head Neck Surg. 2020;163(1):104-106. https://doi.org/10.1177/0194599820925049
1. Provisional COVID-19 Death Counts by Sex, Age, and State. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
2. Rajgor DD, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776-777. https://dx.doi.org/10.1016/S1473-3099(20)30244-9
3. Coronavirus Resource Center: Maps & Trends: Mortality Analyses. Johns Hopkins University & Medicine. Accessed April 26, 2020. https://coronavirus.jhu.edu/data/mortality
4. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461.e5. https://doi.org/10.1016/j.jamda.2019.09.015
5. Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/nejmp1212324
6. Summary Health Statistics: National Health Interview Survey, 2017. Tables P10a-P10c; p. 1-9. Centers for Disease Control and Prevention. Accessed April 26,2020. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_P-10.pdf
7. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? a systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
8. Fact Sheet: Post-Acute Care. American Hospital Association. July 2019. Accessed April 26, 2020. https://www.aha.org/system/files/media/file/2019/07/fact-sheet-post-acute-care-0719.pdf
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
10. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305-313. https://doi.org/10.1002/jhm.209
11. Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ 2020;369:m1923. https://doi.org/10.1136/bmj.m1923
12. Williams S, Gousen S, DeFrances C. National Hospital Care Survey Demonstration Projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report. 2018;(116):1-11.
13. Milenkovic M, Russo CA, Elixhauser A. Hospital Stays for Influenza, 2004: Statistical Brief #16. 2006 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed April 26, 2020 https://www.ncbi.nlm.nih.gov/books/NBK63484/
14. Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. Am J Phys Med Rehabil. 2020;99(6):470-474. https://doi.org/10.1097/phm.0000000000001443
15. Coronavirus Disease 2019 (COVID-19): Social Distancing. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
16. Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73-82. https://doi.org/10.1016/j.jphys.2020.03.011
17. COVID1-9 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services. Accessed April 23, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
18. Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. 2020;323(20):2007-2008. https://doi.org/10.1001/jama.2020.4686
19. Eung-Hun K, Stolvar A, Lober WB, et al. Challenges to using an electronic health record by a low-income elderly population. J Med Internet Res. 2009;11(4):e44. https://doi.org/10.2196/jmir.1256
20. Rajasekaran K. Access to telemedicine-are we doing all that we can during the COVID-19 pandemic? Otolaryngol Head Neck Surg. 2020;163(1):104-106. https://doi.org/10.1177/0194599820925049
© 2020 Society of Hospital Medicine
Evaluation of the Order SMARTT: An Initiative to Reduce Phlebotomy and Improve Sleep-Friendly Labs on General Medicine Services
Frequent daily laboratory testing for inpatients contributes to excessive costs,1 anemia,2 and unnecessary testing.3 The ABIM Foundation’s Choosing Wisely® campaign recommends avoiding routine labs, like complete blood counts (CBCs) and basic metabolic panels (BMP), in the face of clinical and laboratory stability.4,5 Prior interventions have reduced unnecessary labs without adverse outcomes.6-8
In addition to lab frequency, hospitalized patients face suboptimal lab timing. Labs are often ordered as early as 4
METHODS
Setting
This study was conducted on the University of Chicago Medicine (UCM) general medicine services, which consisted of a resident-covered service supervised by general medicine, subspecialist, or hospitalist attendings and a hospitalist service staffed by hospitalists and advanced practice providers.
Development of Order SMARTT
To inform intervention development, we surveyed providers about lab-ordering preferences with use of questions from a prior survey to provide a benchmark (Appendix Table 2).15 While reducing lab frequency was supported, the modal response for how frequently a stable patient should receive routine labs was every 48 hours (Appendix Table 2). Therefore, we hypothesized that labs ordered every 48 hours may be popular. Taking labs every 48 hours would not require an urgent 4
Physician Education
We created a 20-minute presentation on the harms of excessive labs and the benefits of sleep-friendly ordering. Instructional Order SMARTT posters were posted in clinician workrooms that emphasized forgoing labs on stable patients and using the “Order Sleep” shortcut when nonurgent labs were needed.
Labs Utilization Data
We used Epic Systems software (Verona, Wisconsin) and our institutional Tableau scorecard to obtain data on CBC and BMP ordering, patient census, and demographics for medical inpatients between July 1, 2017, and November 1, 2018.
Cost Analysis
Costs of lab tests (actual cost to our institution) were obtained from our institutional phlebotomy services’ estimates of direct variable labor and benefits costs and direct variable supplies cost.
Statistical Analysis
Data analysis was performed with SAS version 9.4 statistical software (Cary, North Carolina, USA) and R version 3.6.2 (Vienna, Austria). Descriptive statistics were used to summarize data. Surveys were analyzed using chi-square tests for categorical variables and two-sample t tests for continuous variables. For lab ordering data, interrupted time series analyses (ITSA) were used to determine the changes in ordering practices with the implementation of the two interventions controlling for service lines (resident vs hospitalist service). ITSA enables examination of changes in lab ordering while controlling for time. The AUTOREG function in SAS was used to build the model and estimate final parameters. This function automatically tests for autocorrelation, heteroscedasticity, and estimates any autoregressive parameters required in the model. Our main model tested the association between our two separate interventions on ordering practices, controlling for service (hospitalist or resident).16
RESULTS
Of 125 residents, 82 (65.6%) attended the session and completed the survey. Attendance and response rate for hospitalists was 80% (16 of 20). Similar to a prior study, many residents (73.1%) reported they would be comfortable if patients received less daily laboratory testing (Appendix Table 2).
We reviewed data from 7,045 total patients over 50,951 total patient days between July1, 2017, and November 1, 2018 (Appendix Table 3).
Total Lab Draws
After accounting for total patient days, we saw 26.3% reduction on average in total lab draws per patient-day per week postintervention (4.68 before vs 3.45 after; difference, 1.23; 95% CI, 0.82-1.63; P < .05; Appendix Table 3). When total lab draws were stratified by service, we saw 28% reduction on average in total lab draws per patient-day per week on resident services (4.67 before vs 3.36 after; difference, 1.31; 95% CI, 0.88-1.74; P < .05) and 23.9% reduction on average in lab draws/patient-day per week on the hospitalist service (4.73 before vs 3.60 after; difference, 1.13; 95% CI, 0.61-1.64; P < .05; Appendix Table 3).
Sleep-Friendly Labs by Intervention
For patients with routine labs, the proportion of sleep-friendly labs drawn per patient-day increased from 6% preintervention to 21% postintervention (P < .001). ITSA demonstrated both interventions were associated with improving lab timing. There was a statistically significant increase in sleep-friendly labs ordered per patient encounter per week immediately after the launch of “Order Sleep” (intercept, 0.49; standard error (SE), 0.14; P = .001) and the “4

Sleep-Friendly Lab Orders by Service
Over the study period, there was no significant difference in total sleep-friendly labs ordered/month between resident and hospitalist services (84.88 vs 86.19; P = .95).
In ITSA, “Order Sleep” was associated with a statistically significant immediate increase in sleep-friendly lab orders per patient encounter per week on resident services (intercept, 1.03; SE, 0.29; P < .001). However, this initial increase was followed by a decrease over time in sleep-friendly lab orders per week (slope change, –0.1; SE, 0.04; P = .02; Table, Figure B). There was no statistically significant change observed on the hospitalist service with “Order Sleep.”
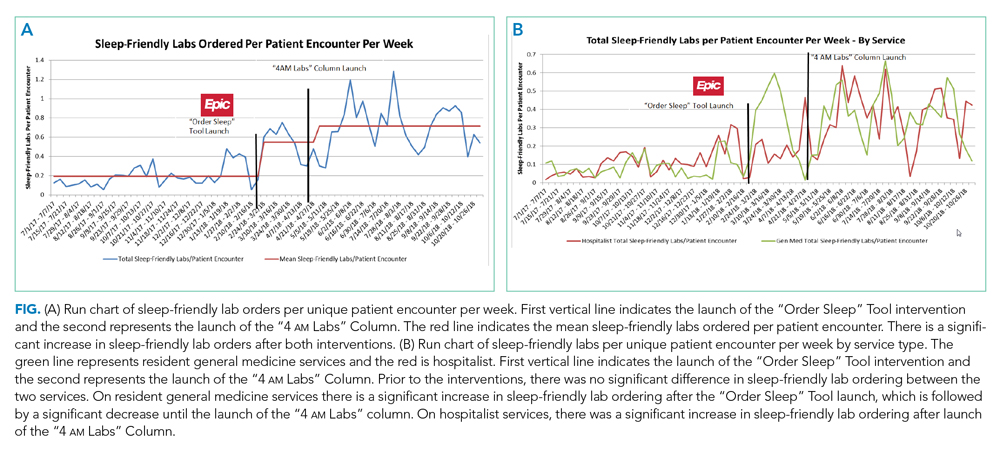
In contrast, the “4
Cost Savings
Using an estimated cost of $7.70 for CBCs and $8.01 for BMPs from our laboratory, our intervention saved an estimated $60,278 in lab costs alone over the 16-month study period (Appendix Table 4).
DISCUSSION
To our knowledge, this is the first study showing a multicomponent intervention using EHR tools can both reduce frequency and optimize timing of routine lab ordering. Our project had two interventions implemented at two different times: First, an “Order Sleep” shortcut was introduced to select sleep-friendly lab timing, including a 6
While the “Order Sleep” tool was initially associated with significant increases in sleep-friendly orders on resident services, this change was not sustained. This could have been caused by the short-lived effect of education more than sustained adoption of the tool. In contrast, the “4
The “4
While other institutions have attempted to shift lab-timing by altering phlebotomy workflows10 or via conscious decision-making on rounds,9 our study differs in several ways. We avoided default options and allowed clinicians to select sleep-friendly labs to promote buy-in. It is sometimes necessary to order 4
Our study had several limitations. First, this was a single center study on adult medicine services, which limits generalizability. Although we considered surgical services, their early rounds made deviations from 4
In conclusion, a multicomponent intervention using EHR tools can reduce inpatient daily lab frequency and optimize lab timing to help promote patient sleep.
Acknowledgments
The authors would like to thank The University of Chicago Center for Healthcare Delivery Science and Innovation for sponsoring their annual Choosing Wisely Challenge, which allowed for access to institutional support and resources for this study. We would also like to thank Mary Kate Springman, MHA, and John Fahrenbach, PhD, for their assistance with this project. Dr Tapaskar also received mentorship through the Future Leader Program for the High Value Practice Academic Alliance.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. https://doi.org/10.1001/jamainternmed.2017.5152
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
3. Korenstein D, Husain S, Gennarelli RL, White C, Masciale JN, Roman BR. Impact of clinical specialty on attitudes regarding overuse of inpatient laboratory testing. J Hosp Med. 2018;13(12):844-847. https://doi.org/10.12788/jhm.2978
4. Choosing Wisely. 2020. Accessed January 10, 2020. http://www.choosingwisely.org/getting-started/
5. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
6. Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146(5):524-527. https://doi.org/10.1001/archsurg.2011.103
7. Attali M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73(5):787-794.
8. Vidyarthi AR, Hamill T, Green AL, Rosenbluth G, Baron RB. Changing resident test ordering behavior: a multilevel intervention to decrease laboratory utilization at an academic medical center. Am J Med Qual. 2015;30(1):81-87. https://doi.org/10.1177/1062860613517502
9. Krafft CA, Biondi EA, Leonard MS, et al. Ending the 4 AM Blood Draw. Presented at: American Academy of Pediatrics Experience; October 25, 2015, Washington, DC. Accessed January 10, 2020. https://aap.confex.com/aap/2015/webprogrampress/Paper31640.html
10. Ramarajan V, Chima HS, Young L. Implementation of later morning specimen draws to improve patient health and satisfaction. Lab Med. 2016;47(1):e1-e4. https://doi.org/10.1093/labmed/lmv013
11. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients - a clinical review. Ann Intensive Care. 2015;5:3. https://doi.org/10.1186/s13613-015-0043-2
12. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163-178. https://doi.org/10.1016/j.smrv.2007.01.002
13. Ho A, Raja B, Waldhorn R, Baez V, Mohammed I. New onset of insomnia in hospitalized patients in general medical wards: incidence, causes, and resolution rate. J Community Hosp Int. 2017;7(5):309-313. https://doi.org/10.1080/20009666.2017.1374108
14. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
15. Roman BR, Yang A, Masciale J, Korenstein D. Association of Attitudes Regarding Overuse of Inpatient Laboratory Testing With Health Care Provider Type. JAMA Intern Med. 2017;177(8):1205-1207. https://doi.org/10.1001/jamainternmed.2017.1634
16. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
Frequent daily laboratory testing for inpatients contributes to excessive costs,1 anemia,2 and unnecessary testing.3 The ABIM Foundation’s Choosing Wisely® campaign recommends avoiding routine labs, like complete blood counts (CBCs) and basic metabolic panels (BMP), in the face of clinical and laboratory stability.4,5 Prior interventions have reduced unnecessary labs without adverse outcomes.6-8
In addition to lab frequency, hospitalized patients face suboptimal lab timing. Labs are often ordered as early as 4
METHODS
Setting
This study was conducted on the University of Chicago Medicine (UCM) general medicine services, which consisted of a resident-covered service supervised by general medicine, subspecialist, or hospitalist attendings and a hospitalist service staffed by hospitalists and advanced practice providers.
Development of Order SMARTT
To inform intervention development, we surveyed providers about lab-ordering preferences with use of questions from a prior survey to provide a benchmark (Appendix Table 2).15 While reducing lab frequency was supported, the modal response for how frequently a stable patient should receive routine labs was every 48 hours (Appendix Table 2). Therefore, we hypothesized that labs ordered every 48 hours may be popular. Taking labs every 48 hours would not require an urgent 4
Physician Education
We created a 20-minute presentation on the harms of excessive labs and the benefits of sleep-friendly ordering. Instructional Order SMARTT posters were posted in clinician workrooms that emphasized forgoing labs on stable patients and using the “Order Sleep” shortcut when nonurgent labs were needed.
Labs Utilization Data
We used Epic Systems software (Verona, Wisconsin) and our institutional Tableau scorecard to obtain data on CBC and BMP ordering, patient census, and demographics for medical inpatients between July 1, 2017, and November 1, 2018.
Cost Analysis
Costs of lab tests (actual cost to our institution) were obtained from our institutional phlebotomy services’ estimates of direct variable labor and benefits costs and direct variable supplies cost.
Statistical Analysis
Data analysis was performed with SAS version 9.4 statistical software (Cary, North Carolina, USA) and R version 3.6.2 (Vienna, Austria). Descriptive statistics were used to summarize data. Surveys were analyzed using chi-square tests for categorical variables and two-sample t tests for continuous variables. For lab ordering data, interrupted time series analyses (ITSA) were used to determine the changes in ordering practices with the implementation of the two interventions controlling for service lines (resident vs hospitalist service). ITSA enables examination of changes in lab ordering while controlling for time. The AUTOREG function in SAS was used to build the model and estimate final parameters. This function automatically tests for autocorrelation, heteroscedasticity, and estimates any autoregressive parameters required in the model. Our main model tested the association between our two separate interventions on ordering practices, controlling for service (hospitalist or resident).16
RESULTS
Of 125 residents, 82 (65.6%) attended the session and completed the survey. Attendance and response rate for hospitalists was 80% (16 of 20). Similar to a prior study, many residents (73.1%) reported they would be comfortable if patients received less daily laboratory testing (Appendix Table 2).
We reviewed data from 7,045 total patients over 50,951 total patient days between July1, 2017, and November 1, 2018 (Appendix Table 3).
Total Lab Draws
After accounting for total patient days, we saw 26.3% reduction on average in total lab draws per patient-day per week postintervention (4.68 before vs 3.45 after; difference, 1.23; 95% CI, 0.82-1.63; P < .05; Appendix Table 3). When total lab draws were stratified by service, we saw 28% reduction on average in total lab draws per patient-day per week on resident services (4.67 before vs 3.36 after; difference, 1.31; 95% CI, 0.88-1.74; P < .05) and 23.9% reduction on average in lab draws/patient-day per week on the hospitalist service (4.73 before vs 3.60 after; difference, 1.13; 95% CI, 0.61-1.64; P < .05; Appendix Table 3).
Sleep-Friendly Labs by Intervention
For patients with routine labs, the proportion of sleep-friendly labs drawn per patient-day increased from 6% preintervention to 21% postintervention (P < .001). ITSA demonstrated both interventions were associated with improving lab timing. There was a statistically significant increase in sleep-friendly labs ordered per patient encounter per week immediately after the launch of “Order Sleep” (intercept, 0.49; standard error (SE), 0.14; P = .001) and the “4

Sleep-Friendly Lab Orders by Service
Over the study period, there was no significant difference in total sleep-friendly labs ordered/month between resident and hospitalist services (84.88 vs 86.19; P = .95).
In ITSA, “Order Sleep” was associated with a statistically significant immediate increase in sleep-friendly lab orders per patient encounter per week on resident services (intercept, 1.03; SE, 0.29; P < .001). However, this initial increase was followed by a decrease over time in sleep-friendly lab orders per week (slope change, –0.1; SE, 0.04; P = .02; Table, Figure B). There was no statistically significant change observed on the hospitalist service with “Order Sleep.”

In contrast, the “4
Cost Savings
Using an estimated cost of $7.70 for CBCs and $8.01 for BMPs from our laboratory, our intervention saved an estimated $60,278 in lab costs alone over the 16-month study period (Appendix Table 4).
DISCUSSION
To our knowledge, this is the first study showing a multicomponent intervention using EHR tools can both reduce frequency and optimize timing of routine lab ordering. Our project had two interventions implemented at two different times: First, an “Order Sleep” shortcut was introduced to select sleep-friendly lab timing, including a 6
While the “Order Sleep” tool was initially associated with significant increases in sleep-friendly orders on resident services, this change was not sustained. This could have been caused by the short-lived effect of education more than sustained adoption of the tool. In contrast, the “4
The “4
While other institutions have attempted to shift lab-timing by altering phlebotomy workflows10 or via conscious decision-making on rounds,9 our study differs in several ways. We avoided default options and allowed clinicians to select sleep-friendly labs to promote buy-in. It is sometimes necessary to order 4
Our study had several limitations. First, this was a single center study on adult medicine services, which limits generalizability. Although we considered surgical services, their early rounds made deviations from 4
In conclusion, a multicomponent intervention using EHR tools can reduce inpatient daily lab frequency and optimize lab timing to help promote patient sleep.
Acknowledgments
The authors would like to thank The University of Chicago Center for Healthcare Delivery Science and Innovation for sponsoring their annual Choosing Wisely Challenge, which allowed for access to institutional support and resources for this study. We would also like to thank Mary Kate Springman, MHA, and John Fahrenbach, PhD, for their assistance with this project. Dr Tapaskar also received mentorship through the Future Leader Program for the High Value Practice Academic Alliance.
Frequent daily laboratory testing for inpatients contributes to excessive costs,1 anemia,2 and unnecessary testing.3 The ABIM Foundation’s Choosing Wisely® campaign recommends avoiding routine labs, like complete blood counts (CBCs) and basic metabolic panels (BMP), in the face of clinical and laboratory stability.4,5 Prior interventions have reduced unnecessary labs without adverse outcomes.6-8
In addition to lab frequency, hospitalized patients face suboptimal lab timing. Labs are often ordered as early as 4
METHODS
Setting
This study was conducted on the University of Chicago Medicine (UCM) general medicine services, which consisted of a resident-covered service supervised by general medicine, subspecialist, or hospitalist attendings and a hospitalist service staffed by hospitalists and advanced practice providers.
Development of Order SMARTT
To inform intervention development, we surveyed providers about lab-ordering preferences with use of questions from a prior survey to provide a benchmark (Appendix Table 2).15 While reducing lab frequency was supported, the modal response for how frequently a stable patient should receive routine labs was every 48 hours (Appendix Table 2). Therefore, we hypothesized that labs ordered every 48 hours may be popular. Taking labs every 48 hours would not require an urgent 4
Physician Education
We created a 20-minute presentation on the harms of excessive labs and the benefits of sleep-friendly ordering. Instructional Order SMARTT posters were posted in clinician workrooms that emphasized forgoing labs on stable patients and using the “Order Sleep” shortcut when nonurgent labs were needed.
Labs Utilization Data
We used Epic Systems software (Verona, Wisconsin) and our institutional Tableau scorecard to obtain data on CBC and BMP ordering, patient census, and demographics for medical inpatients between July 1, 2017, and November 1, 2018.
Cost Analysis
Costs of lab tests (actual cost to our institution) were obtained from our institutional phlebotomy services’ estimates of direct variable labor and benefits costs and direct variable supplies cost.
Statistical Analysis
Data analysis was performed with SAS version 9.4 statistical software (Cary, North Carolina, USA) and R version 3.6.2 (Vienna, Austria). Descriptive statistics were used to summarize data. Surveys were analyzed using chi-square tests for categorical variables and two-sample t tests for continuous variables. For lab ordering data, interrupted time series analyses (ITSA) were used to determine the changes in ordering practices with the implementation of the two interventions controlling for service lines (resident vs hospitalist service). ITSA enables examination of changes in lab ordering while controlling for time. The AUTOREG function in SAS was used to build the model and estimate final parameters. This function automatically tests for autocorrelation, heteroscedasticity, and estimates any autoregressive parameters required in the model. Our main model tested the association between our two separate interventions on ordering practices, controlling for service (hospitalist or resident).16
RESULTS
Of 125 residents, 82 (65.6%) attended the session and completed the survey. Attendance and response rate for hospitalists was 80% (16 of 20). Similar to a prior study, many residents (73.1%) reported they would be comfortable if patients received less daily laboratory testing (Appendix Table 2).
We reviewed data from 7,045 total patients over 50,951 total patient days between July1, 2017, and November 1, 2018 (Appendix Table 3).
Total Lab Draws
After accounting for total patient days, we saw 26.3% reduction on average in total lab draws per patient-day per week postintervention (4.68 before vs 3.45 after; difference, 1.23; 95% CI, 0.82-1.63; P < .05; Appendix Table 3). When total lab draws were stratified by service, we saw 28% reduction on average in total lab draws per patient-day per week on resident services (4.67 before vs 3.36 after; difference, 1.31; 95% CI, 0.88-1.74; P < .05) and 23.9% reduction on average in lab draws/patient-day per week on the hospitalist service (4.73 before vs 3.60 after; difference, 1.13; 95% CI, 0.61-1.64; P < .05; Appendix Table 3).
Sleep-Friendly Labs by Intervention
For patients with routine labs, the proportion of sleep-friendly labs drawn per patient-day increased from 6% preintervention to 21% postintervention (P < .001). ITSA demonstrated both interventions were associated with improving lab timing. There was a statistically significant increase in sleep-friendly labs ordered per patient encounter per week immediately after the launch of “Order Sleep” (intercept, 0.49; standard error (SE), 0.14; P = .001) and the “4

Sleep-Friendly Lab Orders by Service
Over the study period, there was no significant difference in total sleep-friendly labs ordered/month between resident and hospitalist services (84.88 vs 86.19; P = .95).
In ITSA, “Order Sleep” was associated with a statistically significant immediate increase in sleep-friendly lab orders per patient encounter per week on resident services (intercept, 1.03; SE, 0.29; P < .001). However, this initial increase was followed by a decrease over time in sleep-friendly lab orders per week (slope change, –0.1; SE, 0.04; P = .02; Table, Figure B). There was no statistically significant change observed on the hospitalist service with “Order Sleep.”

In contrast, the “4
Cost Savings
Using an estimated cost of $7.70 for CBCs and $8.01 for BMPs from our laboratory, our intervention saved an estimated $60,278 in lab costs alone over the 16-month study period (Appendix Table 4).
DISCUSSION
To our knowledge, this is the first study showing a multicomponent intervention using EHR tools can both reduce frequency and optimize timing of routine lab ordering. Our project had two interventions implemented at two different times: First, an “Order Sleep” shortcut was introduced to select sleep-friendly lab timing, including a 6
While the “Order Sleep” tool was initially associated with significant increases in sleep-friendly orders on resident services, this change was not sustained. This could have been caused by the short-lived effect of education more than sustained adoption of the tool. In contrast, the “4
The “4
While other institutions have attempted to shift lab-timing by altering phlebotomy workflows10 or via conscious decision-making on rounds,9 our study differs in several ways. We avoided default options and allowed clinicians to select sleep-friendly labs to promote buy-in. It is sometimes necessary to order 4
Our study had several limitations. First, this was a single center study on adult medicine services, which limits generalizability. Although we considered surgical services, their early rounds made deviations from 4
In conclusion, a multicomponent intervention using EHR tools can reduce inpatient daily lab frequency and optimize lab timing to help promote patient sleep.
Acknowledgments
The authors would like to thank The University of Chicago Center for Healthcare Delivery Science and Innovation for sponsoring their annual Choosing Wisely Challenge, which allowed for access to institutional support and resources for this study. We would also like to thank Mary Kate Springman, MHA, and John Fahrenbach, PhD, for their assistance with this project. Dr Tapaskar also received mentorship through the Future Leader Program for the High Value Practice Academic Alliance.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. https://doi.org/10.1001/jamainternmed.2017.5152
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
3. Korenstein D, Husain S, Gennarelli RL, White C, Masciale JN, Roman BR. Impact of clinical specialty on attitudes regarding overuse of inpatient laboratory testing. J Hosp Med. 2018;13(12):844-847. https://doi.org/10.12788/jhm.2978
4. Choosing Wisely. 2020. Accessed January 10, 2020. http://www.choosingwisely.org/getting-started/
5. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
6. Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146(5):524-527. https://doi.org/10.1001/archsurg.2011.103
7. Attali M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73(5):787-794.
8. Vidyarthi AR, Hamill T, Green AL, Rosenbluth G, Baron RB. Changing resident test ordering behavior: a multilevel intervention to decrease laboratory utilization at an academic medical center. Am J Med Qual. 2015;30(1):81-87. https://doi.org/10.1177/1062860613517502
9. Krafft CA, Biondi EA, Leonard MS, et al. Ending the 4 AM Blood Draw. Presented at: American Academy of Pediatrics Experience; October 25, 2015, Washington, DC. Accessed January 10, 2020. https://aap.confex.com/aap/2015/webprogrampress/Paper31640.html
10. Ramarajan V, Chima HS, Young L. Implementation of later morning specimen draws to improve patient health and satisfaction. Lab Med. 2016;47(1):e1-e4. https://doi.org/10.1093/labmed/lmv013
11. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients - a clinical review. Ann Intensive Care. 2015;5:3. https://doi.org/10.1186/s13613-015-0043-2
12. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163-178. https://doi.org/10.1016/j.smrv.2007.01.002
13. Ho A, Raja B, Waldhorn R, Baez V, Mohammed I. New onset of insomnia in hospitalized patients in general medical wards: incidence, causes, and resolution rate. J Community Hosp Int. 2017;7(5):309-313. https://doi.org/10.1080/20009666.2017.1374108
14. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
15. Roman BR, Yang A, Masciale J, Korenstein D. Association of Attitudes Regarding Overuse of Inpatient Laboratory Testing With Health Care Provider Type. JAMA Intern Med. 2017;177(8):1205-1207. https://doi.org/10.1001/jamainternmed.2017.1634
16. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. https://doi.org/10.1001/jamainternmed.2017.5152
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
3. Korenstein D, Husain S, Gennarelli RL, White C, Masciale JN, Roman BR. Impact of clinical specialty on attitudes regarding overuse of inpatient laboratory testing. J Hosp Med. 2018;13(12):844-847. https://doi.org/10.12788/jhm.2978
4. Choosing Wisely. 2020. Accessed January 10, 2020. http://www.choosingwisely.org/getting-started/
5. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
6. Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146(5):524-527. https://doi.org/10.1001/archsurg.2011.103
7. Attali M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73(5):787-794.
8. Vidyarthi AR, Hamill T, Green AL, Rosenbluth G, Baron RB. Changing resident test ordering behavior: a multilevel intervention to decrease laboratory utilization at an academic medical center. Am J Med Qual. 2015;30(1):81-87. https://doi.org/10.1177/1062860613517502
9. Krafft CA, Biondi EA, Leonard MS, et al. Ending the 4 AM Blood Draw. Presented at: American Academy of Pediatrics Experience; October 25, 2015, Washington, DC. Accessed January 10, 2020. https://aap.confex.com/aap/2015/webprogrampress/Paper31640.html
10. Ramarajan V, Chima HS, Young L. Implementation of later morning specimen draws to improve patient health and satisfaction. Lab Med. 2016;47(1):e1-e4. https://doi.org/10.1093/labmed/lmv013
11. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients - a clinical review. Ann Intensive Care. 2015;5:3. https://doi.org/10.1186/s13613-015-0043-2
12. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163-178. https://doi.org/10.1016/j.smrv.2007.01.002
13. Ho A, Raja B, Waldhorn R, Baez V, Mohammed I. New onset of insomnia in hospitalized patients in general medical wards: incidence, causes, and resolution rate. J Community Hosp Int. 2017;7(5):309-313. https://doi.org/10.1080/20009666.2017.1374108
14. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
15. Roman BR, Yang A, Masciale J, Korenstein D. Association of Attitudes Regarding Overuse of Inpatient Laboratory Testing With Health Care Provider Type. JAMA Intern Med. 2017;177(8):1205-1207. https://doi.org/10.1001/jamainternmed.2017.1634
16. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
© 2020 Society of Hospital Medicine
Things We Do For No Reason™: Routine Overnight Vital Sign Checks

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™”(TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 73-year-old man with non–insulin dependent diabetes and essential hypertension to the general medicine ward for lower extremity cellulitis. The hospitalist uses standard admission orders, encourages him to elevate his leg above his heart, starts intravenous antibiotics, and monitors him throughout the day and night with regular vital signs. On his second day of admission, the patient’s cellulitis clinically improves, and the team prepares for discharge. However, the nurse notes that the patient did not sleep well and has not slept since his 4
WHY YOU MIGHT THINK Q4 VITAL SIGNS OVERNIGHT ARE HELPFUL
General medicine floors commonly default frequency for measuring vital signs to every 4 hours (Q4), a practice that dates back more than a century to the time of Florence Nightingale.This custom remains in place to ensure the ability to identify and intervene for those at risk for clinical deterioration and preventable death. Research supports the notion that frequent and consistent vital sign checks can minimize mortality and morbidity in the hospital. In fact, validated scoring systems incorporate vital signs with other clinical findings as a way of quickly identifying a patient with worsening clinical status.1 Further, trends and trajectories in vital signs may enable us to identify those with impending decompensation.2 A 2008 consensus statement made by experts in patient safety encouraged hospitals to use frequent vital sign monitoring of patients when available and affordable.3 These interventions aim to help identify and treat patients with early clinical deterioration to prevent poor outcomes.
WHY Q4 VITAL SIGNS OVERNIGHT MIGHT NOT BE NECESSARY
The practice of checking vital signs every 4 hours throughout the night dates to long before the modern era of evidence-based medicine. Research thus far has not focused on the necessity of vital sign checks every 4 hours throughout the night, despite affecting almost every hospitalized patient. Further, patient acuity or need for monitoring does not drive the frequency of overnight vital signs; instead habit and defaults do. We often monitor patients at high risk for clinical deterioration just as frequently as patients at low risk.4
While evidence-based medicine influences much of clinical care, “real-world” needs encountered at the bedside often drive early adapters to innovate. Nurses, who spend the most time at the bedside and conduct the most regular patient assessments, have recognized that not all patients need vital signs checked every 4 hours throughout the night. In 2013, Hands et al conducted a chart review of hospital patterns and found that nurses obtained complete vital sign checks on patients less frequently throughout the night than during the day.5 Their work further showed that nurses used their clinical judgment to make decisions about risk: Those patients deemed low risk by the nurses received fewer vital sign checks while the sicker patients received monitoring every 4 hours throughout the night.
Few researchers have quantitatively identified reasons why nurses may choose to not conduct frequent observations for some patients, beyond the providers’ own experience and judgment. In one study, Hope et al conducted a qualitative analysis of nurses to better understand their reasoning behind who should and should not receive overnight monitoring.6 The results of the analysis revealed that nurses recognize the importance of sleep in support of health and healing and use their clinical judgement when deciding which patients and conditions can forgo frequent observations.Stiver et al conducted trailblazing work that examines the outcomes of decreasing overnight vital sign checks for low-risk hospitalized patients through a randomized pilot study.7 In order to ensure patient safety, their group employed regular nurse observations throughout the night without waking the patient. Those patients assigned to less monitoring overnight reported a trend toward better sleep during hospitalization without the occurrence of any adverse events or escalation in care.
Most important, evidence indicates that sleep disruptions in the hospital worsen health and impede healing; further supporting nurses’ instincts and practices. Hospitalized adults without comorbidities who experience inadequate sleep during hospitalization have a higher perception of pain.8 Similarly, research has associated hospital-induced sleep deprivation and a higher odds of elevated blood glucose in those without diabetes, or “hyperglycemia of hospitalization.” 9 Furthermore, national organizations have recognized the importance of sleep. The American Academy of Nursing, as part of its Choosing Wisely™ campaign, states that, in the hospital, nurses should not disturb a patient’s sleep “unless the patient’s condition or care specifically requires it.”10
Finally, in the era of COVID-19, any opportunity to support physical distancing and to limit face-to-face interaction could protect our patients and staff from acquiring SARS-CoV-2.
WHAT WE SHOULD DO INSTEAD
While consistent vital sign checks allow for early identification of those trending toward clinical deterioration, risk stratification of ward patients can identify those who may benefit from overnight Q4 vital sign checks. While clinicians often use their judgment to identify a subset of low-risk patients for de-escalation of overnight care, artificial intelligence such as Modified Early Warning Score (MEWS) and Pediatric Early Warning Signs (PEWS) may have a role to play. These validated systems use physiologic symptoms that present prior to significant vital sign alterations to identify patients at risk for clinical deterioration.11 As an example, one randomized, controlled trial used a risk stratification tool to eliminate overnight monitoring for low-risk patients. Patients slept more soundly and reported fewer noise disruptions and higher satisfaction with the nursing staff. No adverse events were reported for those who were electronically stratified as low risk.12Further, forcing clinicians to decide on the need for overnight vitals by removing the Q4 vital sign default in the electronic health records (EHR) may minimize overnight disruptions. The University of Chicago in Illinois has implemented “sleep-friendly” options for vital sign ordering in the EHR for both children and adults. Enhanced order sets force providers to consider whether patients qualify for fewer overnight interventions. This change, alongside staff education and empowerment, reduced interruptions overnight for both populations and improved patient experience.13 This patient-centered practice mirrors a recent recommendation from the American Academy of Nursing to minimize sleep disruptions for hospitalized patients by letting low-risk patients sleep.10
RECOMMENDATIONS
- Use clinical judgment or an existing risk stratification system, such as MEWS or PEWS, to identify patients who may benefit from more or less monitoring.
- Forgo overnight vital sign checks for low-risk patients.
- Check overnight vitals for low-risk patients at 10
pm and 6am. - Use pulse oximetry or regular nurse checks as a balancing measure, especially in the pediatric population.
CONCLUSION
Minimizing unnecessary sleep disruptors for hospitalized patients is essential for healing and health. The patient in the clinical scenario had iatrogenic comorbidities added during his hospitalization and an increase in length of stay that resulted from sleep-associated delirium. Hospitalists should take the lead in developing sleep protocols that can leverage current technology to “nudge” clinicians to improve patient sleep. We can modify the frequency of checking vital signs for low-acuity patients and alter environmental factors that may impair sleep, such as noise, light, and temperature, for high-risk patients who cannot forgo overnight vital sign checks. In addition to clinical judgment, artificial intelligence can enable hospitalists and nurses to determine which patients may benefit least from overnight vital sign checks. Finally, if we stop disrupting low-risk patients’ sleep, we can better target resources to patients at high risk for clinical deterioration. Let’s start improving inpatient sleep by eliminating the disruptive things we do for no reason.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Moon A, Cosgrove JF, Lea D, Fairs A, Cressey DM. An eight-year audit before and after the introduction of Modified Early Warning Score (MEWS) charts, of patients admitted to a tertiary referral intensive care unit after CPR. Resuscitation. 2011;82(2):150-154. https://doi.org/10.1016/j.resuscitation.2010.09.480.
2. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102(5):1-5. https://doi.org/10.1016/j.resuscitation.2016.02.005.
3. DeVita MA, Smith GB, Adam SK, et al. ‘‘Identifying the hospitalized patient in crisis’’—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. https://doi.org/10.1016/j.resuscitation.2009.12.008.
4. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. https://doi.org/10.1001/jamainternmed.2013.7791
5. Hands C, Reid E, Meredith P, et al. Patterns in the recording of vital sign and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf. 2013;22(9):719-726. https://doi.org/10.1136/bmjqs-2013-001954
6. Hope J, Recio-Saucedo A, Fogg C, et al. A fundamental conflict of care: nurses’ accounts of balancing patients’ sleep with taking vital sign observations at night. J Clin Nurs. 2018;27:1860-1871. https://doi.org/10.1111/jocn.14234.
7. Stiver K, Sharma N, Geller K, Smith L, Stephens J. “Quiet at night”: reduced overnight vital sign monitoring linked to both safety and improvements in patients’ perception of hospital sleep quality. Patient Exp J. 2017;4(1):Article 10. https://doi.org/10.35680/2372-0247.1185.
8. Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;93(3):381-388. https://doi.org/10.1016/s0304-3959(01)00282-2.
9. DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188-193. https://doi.org/10.2337/dc16-1683.
10. American Academy of Nursing. Choosing Wisely. Twenty-Five Things Nurses and Patients Should Question. July 2018. https://www.choosingwisely.org/wp-content/uploads/2015/02/AANursing-Choosing-Wisely-List.pdf.
11. van Galen LS, Dijkstra CC, Ludikhuize J, Kramer MHH, Nanayakkara PWB. A protocolised once a day Modified Early Warning Score (MEWS) measurement is an appropriate screening tool for major adverse events in a general hospital population. PLoS One. 2016;11(8):e0160811. https://doi.org/10.1371/journal.pone.0160811.
12. Edelson DP, Carey K, Twu NM, et al. Acuity-based nighttime vital sign assessments: a randomized controlled trial. Abstract presented at: Hospital Medicine 2019; March 24-27, 2019; National Harbor, Maryland. https://www.shmabstracts.com/abstract/acuity-based-nighttime-vital-sign-assessments-a-randomized-controlled-trial/. Accessed March 20, 2020
13. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™”(TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 73-year-old man with non–insulin dependent diabetes and essential hypertension to the general medicine ward for lower extremity cellulitis. The hospitalist uses standard admission orders, encourages him to elevate his leg above his heart, starts intravenous antibiotics, and monitors him throughout the day and night with regular vital signs. On his second day of admission, the patient’s cellulitis clinically improves, and the team prepares for discharge. However, the nurse notes that the patient did not sleep well and has not slept since his 4
WHY YOU MIGHT THINK Q4 VITAL SIGNS OVERNIGHT ARE HELPFUL
General medicine floors commonly default frequency for measuring vital signs to every 4 hours (Q4), a practice that dates back more than a century to the time of Florence Nightingale.This custom remains in place to ensure the ability to identify and intervene for those at risk for clinical deterioration and preventable death. Research supports the notion that frequent and consistent vital sign checks can minimize mortality and morbidity in the hospital. In fact, validated scoring systems incorporate vital signs with other clinical findings as a way of quickly identifying a patient with worsening clinical status.1 Further, trends and trajectories in vital signs may enable us to identify those with impending decompensation.2 A 2008 consensus statement made by experts in patient safety encouraged hospitals to use frequent vital sign monitoring of patients when available and affordable.3 These interventions aim to help identify and treat patients with early clinical deterioration to prevent poor outcomes.
WHY Q4 VITAL SIGNS OVERNIGHT MIGHT NOT BE NECESSARY
The practice of checking vital signs every 4 hours throughout the night dates to long before the modern era of evidence-based medicine. Research thus far has not focused on the necessity of vital sign checks every 4 hours throughout the night, despite affecting almost every hospitalized patient. Further, patient acuity or need for monitoring does not drive the frequency of overnight vital signs; instead habit and defaults do. We often monitor patients at high risk for clinical deterioration just as frequently as patients at low risk.4
While evidence-based medicine influences much of clinical care, “real-world” needs encountered at the bedside often drive early adapters to innovate. Nurses, who spend the most time at the bedside and conduct the most regular patient assessments, have recognized that not all patients need vital signs checked every 4 hours throughout the night. In 2013, Hands et al conducted a chart review of hospital patterns and found that nurses obtained complete vital sign checks on patients less frequently throughout the night than during the day.5 Their work further showed that nurses used their clinical judgment to make decisions about risk: Those patients deemed low risk by the nurses received fewer vital sign checks while the sicker patients received monitoring every 4 hours throughout the night.
Few researchers have quantitatively identified reasons why nurses may choose to not conduct frequent observations for some patients, beyond the providers’ own experience and judgment. In one study, Hope et al conducted a qualitative analysis of nurses to better understand their reasoning behind who should and should not receive overnight monitoring.6 The results of the analysis revealed that nurses recognize the importance of sleep in support of health and healing and use their clinical judgement when deciding which patients and conditions can forgo frequent observations.Stiver et al conducted trailblazing work that examines the outcomes of decreasing overnight vital sign checks for low-risk hospitalized patients through a randomized pilot study.7 In order to ensure patient safety, their group employed regular nurse observations throughout the night without waking the patient. Those patients assigned to less monitoring overnight reported a trend toward better sleep during hospitalization without the occurrence of any adverse events or escalation in care.
Most important, evidence indicates that sleep disruptions in the hospital worsen health and impede healing; further supporting nurses’ instincts and practices. Hospitalized adults without comorbidities who experience inadequate sleep during hospitalization have a higher perception of pain.8 Similarly, research has associated hospital-induced sleep deprivation and a higher odds of elevated blood glucose in those without diabetes, or “hyperglycemia of hospitalization.” 9 Furthermore, national organizations have recognized the importance of sleep. The American Academy of Nursing, as part of its Choosing Wisely™ campaign, states that, in the hospital, nurses should not disturb a patient’s sleep “unless the patient’s condition or care specifically requires it.”10
Finally, in the era of COVID-19, any opportunity to support physical distancing and to limit face-to-face interaction could protect our patients and staff from acquiring SARS-CoV-2.
WHAT WE SHOULD DO INSTEAD
While consistent vital sign checks allow for early identification of those trending toward clinical deterioration, risk stratification of ward patients can identify those who may benefit from overnight Q4 vital sign checks. While clinicians often use their judgment to identify a subset of low-risk patients for de-escalation of overnight care, artificial intelligence such as Modified Early Warning Score (MEWS) and Pediatric Early Warning Signs (PEWS) may have a role to play. These validated systems use physiologic symptoms that present prior to significant vital sign alterations to identify patients at risk for clinical deterioration.11 As an example, one randomized, controlled trial used a risk stratification tool to eliminate overnight monitoring for low-risk patients. Patients slept more soundly and reported fewer noise disruptions and higher satisfaction with the nursing staff. No adverse events were reported for those who were electronically stratified as low risk.12Further, forcing clinicians to decide on the need for overnight vitals by removing the Q4 vital sign default in the electronic health records (EHR) may minimize overnight disruptions. The University of Chicago in Illinois has implemented “sleep-friendly” options for vital sign ordering in the EHR for both children and adults. Enhanced order sets force providers to consider whether patients qualify for fewer overnight interventions. This change, alongside staff education and empowerment, reduced interruptions overnight for both populations and improved patient experience.13 This patient-centered practice mirrors a recent recommendation from the American Academy of Nursing to minimize sleep disruptions for hospitalized patients by letting low-risk patients sleep.10
RECOMMENDATIONS
- Use clinical judgment or an existing risk stratification system, such as MEWS or PEWS, to identify patients who may benefit from more or less monitoring.
- Forgo overnight vital sign checks for low-risk patients.
- Check overnight vitals for low-risk patients at 10
pm and 6am. - Use pulse oximetry or regular nurse checks as a balancing measure, especially in the pediatric population.
CONCLUSION
Minimizing unnecessary sleep disruptors for hospitalized patients is essential for healing and health. The patient in the clinical scenario had iatrogenic comorbidities added during his hospitalization and an increase in length of stay that resulted from sleep-associated delirium. Hospitalists should take the lead in developing sleep protocols that can leverage current technology to “nudge” clinicians to improve patient sleep. We can modify the frequency of checking vital signs for low-acuity patients and alter environmental factors that may impair sleep, such as noise, light, and temperature, for high-risk patients who cannot forgo overnight vital sign checks. In addition to clinical judgment, artificial intelligence can enable hospitalists and nurses to determine which patients may benefit least from overnight vital sign checks. Finally, if we stop disrupting low-risk patients’ sleep, we can better target resources to patients at high risk for clinical deterioration. Let’s start improving inpatient sleep by eliminating the disruptive things we do for no reason.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™”(TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 73-year-old man with non–insulin dependent diabetes and essential hypertension to the general medicine ward for lower extremity cellulitis. The hospitalist uses standard admission orders, encourages him to elevate his leg above his heart, starts intravenous antibiotics, and monitors him throughout the day and night with regular vital signs. On his second day of admission, the patient’s cellulitis clinically improves, and the team prepares for discharge. However, the nurse notes that the patient did not sleep well and has not slept since his 4
WHY YOU MIGHT THINK Q4 VITAL SIGNS OVERNIGHT ARE HELPFUL
General medicine floors commonly default frequency for measuring vital signs to every 4 hours (Q4), a practice that dates back more than a century to the time of Florence Nightingale.This custom remains in place to ensure the ability to identify and intervene for those at risk for clinical deterioration and preventable death. Research supports the notion that frequent and consistent vital sign checks can minimize mortality and morbidity in the hospital. In fact, validated scoring systems incorporate vital signs with other clinical findings as a way of quickly identifying a patient with worsening clinical status.1 Further, trends and trajectories in vital signs may enable us to identify those with impending decompensation.2 A 2008 consensus statement made by experts in patient safety encouraged hospitals to use frequent vital sign monitoring of patients when available and affordable.3 These interventions aim to help identify and treat patients with early clinical deterioration to prevent poor outcomes.
WHY Q4 VITAL SIGNS OVERNIGHT MIGHT NOT BE NECESSARY
The practice of checking vital signs every 4 hours throughout the night dates to long before the modern era of evidence-based medicine. Research thus far has not focused on the necessity of vital sign checks every 4 hours throughout the night, despite affecting almost every hospitalized patient. Further, patient acuity or need for monitoring does not drive the frequency of overnight vital signs; instead habit and defaults do. We often monitor patients at high risk for clinical deterioration just as frequently as patients at low risk.4
While evidence-based medicine influences much of clinical care, “real-world” needs encountered at the bedside often drive early adapters to innovate. Nurses, who spend the most time at the bedside and conduct the most regular patient assessments, have recognized that not all patients need vital signs checked every 4 hours throughout the night. In 2013, Hands et al conducted a chart review of hospital patterns and found that nurses obtained complete vital sign checks on patients less frequently throughout the night than during the day.5 Their work further showed that nurses used their clinical judgment to make decisions about risk: Those patients deemed low risk by the nurses received fewer vital sign checks while the sicker patients received monitoring every 4 hours throughout the night.
Few researchers have quantitatively identified reasons why nurses may choose to not conduct frequent observations for some patients, beyond the providers’ own experience and judgment. In one study, Hope et al conducted a qualitative analysis of nurses to better understand their reasoning behind who should and should not receive overnight monitoring.6 The results of the analysis revealed that nurses recognize the importance of sleep in support of health and healing and use their clinical judgement when deciding which patients and conditions can forgo frequent observations.Stiver et al conducted trailblazing work that examines the outcomes of decreasing overnight vital sign checks for low-risk hospitalized patients through a randomized pilot study.7 In order to ensure patient safety, their group employed regular nurse observations throughout the night without waking the patient. Those patients assigned to less monitoring overnight reported a trend toward better sleep during hospitalization without the occurrence of any adverse events or escalation in care.
Most important, evidence indicates that sleep disruptions in the hospital worsen health and impede healing; further supporting nurses’ instincts and practices. Hospitalized adults without comorbidities who experience inadequate sleep during hospitalization have a higher perception of pain.8 Similarly, research has associated hospital-induced sleep deprivation and a higher odds of elevated blood glucose in those without diabetes, or “hyperglycemia of hospitalization.” 9 Furthermore, national organizations have recognized the importance of sleep. The American Academy of Nursing, as part of its Choosing Wisely™ campaign, states that, in the hospital, nurses should not disturb a patient’s sleep “unless the patient’s condition or care specifically requires it.”10
Finally, in the era of COVID-19, any opportunity to support physical distancing and to limit face-to-face interaction could protect our patients and staff from acquiring SARS-CoV-2.
WHAT WE SHOULD DO INSTEAD
While consistent vital sign checks allow for early identification of those trending toward clinical deterioration, risk stratification of ward patients can identify those who may benefit from overnight Q4 vital sign checks. While clinicians often use their judgment to identify a subset of low-risk patients for de-escalation of overnight care, artificial intelligence such as Modified Early Warning Score (MEWS) and Pediatric Early Warning Signs (PEWS) may have a role to play. These validated systems use physiologic symptoms that present prior to significant vital sign alterations to identify patients at risk for clinical deterioration.11 As an example, one randomized, controlled trial used a risk stratification tool to eliminate overnight monitoring for low-risk patients. Patients slept more soundly and reported fewer noise disruptions and higher satisfaction with the nursing staff. No adverse events were reported for those who were electronically stratified as low risk.12Further, forcing clinicians to decide on the need for overnight vitals by removing the Q4 vital sign default in the electronic health records (EHR) may minimize overnight disruptions. The University of Chicago in Illinois has implemented “sleep-friendly” options for vital sign ordering in the EHR for both children and adults. Enhanced order sets force providers to consider whether patients qualify for fewer overnight interventions. This change, alongside staff education and empowerment, reduced interruptions overnight for both populations and improved patient experience.13 This patient-centered practice mirrors a recent recommendation from the American Academy of Nursing to minimize sleep disruptions for hospitalized patients by letting low-risk patients sleep.10
RECOMMENDATIONS
- Use clinical judgment or an existing risk stratification system, such as MEWS or PEWS, to identify patients who may benefit from more or less monitoring.
- Forgo overnight vital sign checks for low-risk patients.
- Check overnight vitals for low-risk patients at 10
pm and 6am. - Use pulse oximetry or regular nurse checks as a balancing measure, especially in the pediatric population.
CONCLUSION
Minimizing unnecessary sleep disruptors for hospitalized patients is essential for healing and health. The patient in the clinical scenario had iatrogenic comorbidities added during his hospitalization and an increase in length of stay that resulted from sleep-associated delirium. Hospitalists should take the lead in developing sleep protocols that can leverage current technology to “nudge” clinicians to improve patient sleep. We can modify the frequency of checking vital signs for low-acuity patients and alter environmental factors that may impair sleep, such as noise, light, and temperature, for high-risk patients who cannot forgo overnight vital sign checks. In addition to clinical judgment, artificial intelligence can enable hospitalists and nurses to determine which patients may benefit least from overnight vital sign checks. Finally, if we stop disrupting low-risk patients’ sleep, we can better target resources to patients at high risk for clinical deterioration. Let’s start improving inpatient sleep by eliminating the disruptive things we do for no reason.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Moon A, Cosgrove JF, Lea D, Fairs A, Cressey DM. An eight-year audit before and after the introduction of Modified Early Warning Score (MEWS) charts, of patients admitted to a tertiary referral intensive care unit after CPR. Resuscitation. 2011;82(2):150-154. https://doi.org/10.1016/j.resuscitation.2010.09.480.
2. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102(5):1-5. https://doi.org/10.1016/j.resuscitation.2016.02.005.
3. DeVita MA, Smith GB, Adam SK, et al. ‘‘Identifying the hospitalized patient in crisis’’—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. https://doi.org/10.1016/j.resuscitation.2009.12.008.
4. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. https://doi.org/10.1001/jamainternmed.2013.7791
5. Hands C, Reid E, Meredith P, et al. Patterns in the recording of vital sign and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf. 2013;22(9):719-726. https://doi.org/10.1136/bmjqs-2013-001954
6. Hope J, Recio-Saucedo A, Fogg C, et al. A fundamental conflict of care: nurses’ accounts of balancing patients’ sleep with taking vital sign observations at night. J Clin Nurs. 2018;27:1860-1871. https://doi.org/10.1111/jocn.14234.
7. Stiver K, Sharma N, Geller K, Smith L, Stephens J. “Quiet at night”: reduced overnight vital sign monitoring linked to both safety and improvements in patients’ perception of hospital sleep quality. Patient Exp J. 2017;4(1):Article 10. https://doi.org/10.35680/2372-0247.1185.
8. Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;93(3):381-388. https://doi.org/10.1016/s0304-3959(01)00282-2.
9. DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188-193. https://doi.org/10.2337/dc16-1683.
10. American Academy of Nursing. Choosing Wisely. Twenty-Five Things Nurses and Patients Should Question. July 2018. https://www.choosingwisely.org/wp-content/uploads/2015/02/AANursing-Choosing-Wisely-List.pdf.
11. van Galen LS, Dijkstra CC, Ludikhuize J, Kramer MHH, Nanayakkara PWB. A protocolised once a day Modified Early Warning Score (MEWS) measurement is an appropriate screening tool for major adverse events in a general hospital population. PLoS One. 2016;11(8):e0160811. https://doi.org/10.1371/journal.pone.0160811.
12. Edelson DP, Carey K, Twu NM, et al. Acuity-based nighttime vital sign assessments: a randomized controlled trial. Abstract presented at: Hospital Medicine 2019; March 24-27, 2019; National Harbor, Maryland. https://www.shmabstracts.com/abstract/acuity-based-nighttime-vital-sign-assessments-a-randomized-controlled-trial/. Accessed March 20, 2020
13. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091.
1. Moon A, Cosgrove JF, Lea D, Fairs A, Cressey DM. An eight-year audit before and after the introduction of Modified Early Warning Score (MEWS) charts, of patients admitted to a tertiary referral intensive care unit after CPR. Resuscitation. 2011;82(2):150-154. https://doi.org/10.1016/j.resuscitation.2010.09.480.
2. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102(5):1-5. https://doi.org/10.1016/j.resuscitation.2016.02.005.
3. DeVita MA, Smith GB, Adam SK, et al. ‘‘Identifying the hospitalized patient in crisis’’—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. https://doi.org/10.1016/j.resuscitation.2009.12.008.
4. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. https://doi.org/10.1001/jamainternmed.2013.7791
5. Hands C, Reid E, Meredith P, et al. Patterns in the recording of vital sign and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf. 2013;22(9):719-726. https://doi.org/10.1136/bmjqs-2013-001954
6. Hope J, Recio-Saucedo A, Fogg C, et al. A fundamental conflict of care: nurses’ accounts of balancing patients’ sleep with taking vital sign observations at night. J Clin Nurs. 2018;27:1860-1871. https://doi.org/10.1111/jocn.14234.
7. Stiver K, Sharma N, Geller K, Smith L, Stephens J. “Quiet at night”: reduced overnight vital sign monitoring linked to both safety and improvements in patients’ perception of hospital sleep quality. Patient Exp J. 2017;4(1):Article 10. https://doi.org/10.35680/2372-0247.1185.
8. Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;93(3):381-388. https://doi.org/10.1016/s0304-3959(01)00282-2.
9. DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188-193. https://doi.org/10.2337/dc16-1683.
10. American Academy of Nursing. Choosing Wisely. Twenty-Five Things Nurses and Patients Should Question. July 2018. https://www.choosingwisely.org/wp-content/uploads/2015/02/AANursing-Choosing-Wisely-List.pdf.
11. van Galen LS, Dijkstra CC, Ludikhuize J, Kramer MHH, Nanayakkara PWB. A protocolised once a day Modified Early Warning Score (MEWS) measurement is an appropriate screening tool for major adverse events in a general hospital population. PLoS One. 2016;11(8):e0160811. https://doi.org/10.1371/journal.pone.0160811.
12. Edelson DP, Carey K, Twu NM, et al. Acuity-based nighttime vital sign assessments: a randomized controlled trial. Abstract presented at: Hospital Medicine 2019; March 24-27, 2019; National Harbor, Maryland. https://www.shmabstracts.com/abstract/acuity-based-nighttime-vital-sign-assessments-a-randomized-controlled-trial/. Accessed March 20, 2020
13. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091.
© 2020 Society of Hospital Medicine
Implementing Physical Distancing in the Hospital: A Key Strategy to Prevent Nosocomial Transmission of COVID-19
Hospitalists serve as frontline healthcare professionals caring for the increasing number of COVID-19 patients in the United States. The safety of hospitalists and other frontline healthcare workers is paramount to preventing high nosocomial transmission as has been reported in several other countries. Much effort to date has rightly focused on ensuring healthcare workers have appropriate personal protective equipment (PPE) given the known increased risk of nosocomial infection to healthcare workers. However, another important strategy to prevent nosocomial transmission is to implement “social distancing,” or avoiding close contact with others. While this approach has received considerable press with regards to implementation in communities, social, or physical, distancing in the hospital is also a critical way to prevent nosocomial transmission and ensure the health and welfare of our workforce to meet the challenge. The Centers for Disease Control and Prevention (CDC) defines close contact as less than 6 feet away for over 10 minutes.1 Given the myriad clinical interactions that occur within teams in the hospital, such distancing can prove challenging.
At the University of Chicago Medicine in Illinois, our hospitalist group was an early adopter of implementing several strategies to facilitate physical distancing in the context of clinical care to minimize community transmission of COVID-19 among healthcare professionals. We describe how to implement physical distancing effectively in specific hospital settings, including some challenges and strategies to surmount them.
EDUCATIONAL CONFERENCES AND ADMINISTRATIVE MEETINGS
Educational conferences and administrative meetings need to be transitioned to virtual meetings. While it may be easy to broadcast a conference in lieu of meeting in a conference room, it is critical that hospital clinicians do not “huddle close together” in front of a computer, which would defeat the purpose of physical distancing. While “flipping the classroom” in preclinical and higher education is common, this method can be effective to deliver standard education followed by a virtual question and answer session or chat room.2
Educational discussions can also occur asynchronously through learning management systems, such as Canvas, or even closed social media channels, such as Slack, that enable discussions. These tools require training to work, so it is important to invest in education on the chosen platform to ensure that it functions smoothly. It is equally important that administrators become familiar with these tools while working remotely and can facilitate administrative meetings without difficulty. We created a one-page tip sheet to help ease the transition for department administrators. The tip sheet highlighted how to start a virtual meeting and meeting etiquette (eg, mute upon entry into the meeting, mute when not talking, announce yourself when talking) as well as ensuring that dial-ins could easily access the meeting by including one-touch options, when available, on calendar invites in addition to the weblink. A daily email update can be an important adjunct to administrative meetings to ensure critical updates are reaching all clinicians in a group and also preserves meeting time for clarifying questions.
CLINICAL WORKROOMS
Perhaps the biggest challenge is how many clinical workrooms in hospitals today are crowded with computers next to each other. Ventilation can also be poor, making conditions riskier. This makes implemention of social distancing extremely challenging, but also critical, given how much time hospital-based clinicians spend on computers and in their workrooms. The first step to achieving social distancing in the workroom is to take an inventory of how many people work there and get a log of the number of computers. Consider whether existing computers can be rearranged with a goal of keeping people 6 feet apart. For particularly cramped workrooms, this may require assigning computer spaces to physicians across a floor or several floors, using computers out on a unit, or using mobile computers to limit the number of people in the workroom at one time. We suggest working with physical plant leaders and Information Technology to reallocate mobile workstations, laptops, or desktops to conference rooms, patient visiting areas, and offices that are not being used. Because coronavirus can survive on surfaces for several hours, it is also important to stock work rooms with disinfectants to clean surfaces such as keyboards and desktops frequently. One other important thing to consider is whether computers can be assigned to specific teams or people to limit the use of a computer by multiple people.
ROUNDING, SIGN-OUT, AND MULTIDISCIPLINARY ROUNDS
Rounding
Perhaps one of the most fundamental hardships with physical distancing is how to conduct routine clinical care such as rounds, sign-out, or multidisciplinary rounds. Rounds on teaching services are particularly challenging given the number of people. At many teaching institutions, medical students are no longer on clinical rotations, which immediately reduces the number of people on teaching teams. The other thing to consider is how rounds are conducted. As opposed to a large team walking together, assign one person from the team as the liaison for the patient, which also has the added benefit of conserving precious PPE. Virtual rounding enables clinicians, including residents and attendings, to work together and decide the plan for the day without first crowding into a patient room. This is perhaps the most important cultural hurdle that one may face.
Another administrative hurdle and common concern is how to bill for such interactions. While federal guidance evolves, our institution created smartphrases for this type of virtual rounding whereby attendings attest to resident notes even if they did not physically see the patient. Additional information may be obtained from patients by calling them on their patient-room phones or by using telemedicine as some hospitals are implementing.3 For large “mega” teams, split the team into smaller groups to facilitate continuity and easier conversations.
Sign-out
When feasible, it is important to transition to phone sign-out supplemented with viewing an updated shared sign-out, ideally electronically, for shift change. When using phone sign-out, it is ideal to implement a verbal read-back to ensure understanding and to keep your sign-out updated. Because using the telephone is not the most effective communication channel for sign-out, it is key to be vigilant with other sign-out best practices, such as using a standard template like IPASS4 or another framework, prioritizing sick patients, and ensuring a focus on to-do and if/then items that are critical for the receiver to ensure understanding.5
Multidisciplinary Rounds
As multidisciplinary rounds typically occur either at the bedside or in a conference room, it is key to ensure that these occur virtually whenever possible. One option is to use conference calls or video chat (eg, Zoom) for multidisciplinary rounds whenever possible. Calendar invites or paging reminders can be used to prompt teams when to call in to discuss patients. Because multiple people are entering a virtual room at once, it is important to establish an order or have a leader orchestrate who is next. In addition, given the importance of multiple people contributing to the discussion, it is also equally important for those speaking always to announce who they are and their role (eg, social worker, case manager, physical therapist) since it may not be possible to recognize people’s voices alone. This is where visual recognition can be helpful through use of institutional video conferencing that enables hearing and seeing someone. Further, it is important to ensure that the platform being used is HIPAA compliant.
CALL ROOMS
Call rooms in hospitals can be particularly challenging if they are shared. Finding additional call rooms may require use of cots or reallocation of patient rooms. It is also possible for hospitalists to consider air mattresses in their offices or other private spaces to avoid sharing call rooms. Consider assigning the same call room to the same few people over the course of a rotation or period to avoid many people sharing one room. If a hospital is converting units to group patients under investigation or those who are COVID-19 positive, reallocating call rooms may be necessary to accommodate new teams. Lastly, it is important to communicate proactively with environmental services staff to make sure all call rooms are equipped with cleaning supplies and hand sanitizer and are cleaned daily to avoid nosocomial transmission.
CONCLUSION
/section>Containing nosocomial spread of coronavirus is particularly challenging for hospitals because of how contagious the virus is, the extreme shortage of PPE, and lack of mass testing to identify those who are sick. Therefore, physical distancing in the hospital is critical to ensure the health and well-being of the health professional workforce during the pandemic.
1. Centers for Disease Control and Prevention. Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 2, 2020.
2. Stephenson CR, Wang AT, Szostek JH, et al. Flipping the continuing medical education classroom: validating a measure of attendees’ perceptions. J Contin Educ Health Prof. 2016;36(4):256-262. https://doi.org/10.1097/CEH.0000000000000113.
3. Doshi A, Platt Y, Dressen JR, K Mathews BK, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(4):xxx-xxxx. https://doi.org/10.12788/jhm.3419.
4. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
5. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The modified, multi-patient observed simulated handoff experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
Hospitalists serve as frontline healthcare professionals caring for the increasing number of COVID-19 patients in the United States. The safety of hospitalists and other frontline healthcare workers is paramount to preventing high nosocomial transmission as has been reported in several other countries. Much effort to date has rightly focused on ensuring healthcare workers have appropriate personal protective equipment (PPE) given the known increased risk of nosocomial infection to healthcare workers. However, another important strategy to prevent nosocomial transmission is to implement “social distancing,” or avoiding close contact with others. While this approach has received considerable press with regards to implementation in communities, social, or physical, distancing in the hospital is also a critical way to prevent nosocomial transmission and ensure the health and welfare of our workforce to meet the challenge. The Centers for Disease Control and Prevention (CDC) defines close contact as less than 6 feet away for over 10 minutes.1 Given the myriad clinical interactions that occur within teams in the hospital, such distancing can prove challenging.
At the University of Chicago Medicine in Illinois, our hospitalist group was an early adopter of implementing several strategies to facilitate physical distancing in the context of clinical care to minimize community transmission of COVID-19 among healthcare professionals. We describe how to implement physical distancing effectively in specific hospital settings, including some challenges and strategies to surmount them.
EDUCATIONAL CONFERENCES AND ADMINISTRATIVE MEETINGS
Educational conferences and administrative meetings need to be transitioned to virtual meetings. While it may be easy to broadcast a conference in lieu of meeting in a conference room, it is critical that hospital clinicians do not “huddle close together” in front of a computer, which would defeat the purpose of physical distancing. While “flipping the classroom” in preclinical and higher education is common, this method can be effective to deliver standard education followed by a virtual question and answer session or chat room.2
Educational discussions can also occur asynchronously through learning management systems, such as Canvas, or even closed social media channels, such as Slack, that enable discussions. These tools require training to work, so it is important to invest in education on the chosen platform to ensure that it functions smoothly. It is equally important that administrators become familiar with these tools while working remotely and can facilitate administrative meetings without difficulty. We created a one-page tip sheet to help ease the transition for department administrators. The tip sheet highlighted how to start a virtual meeting and meeting etiquette (eg, mute upon entry into the meeting, mute when not talking, announce yourself when talking) as well as ensuring that dial-ins could easily access the meeting by including one-touch options, when available, on calendar invites in addition to the weblink. A daily email update can be an important adjunct to administrative meetings to ensure critical updates are reaching all clinicians in a group and also preserves meeting time for clarifying questions.
CLINICAL WORKROOMS
Perhaps the biggest challenge is how many clinical workrooms in hospitals today are crowded with computers next to each other. Ventilation can also be poor, making conditions riskier. This makes implemention of social distancing extremely challenging, but also critical, given how much time hospital-based clinicians spend on computers and in their workrooms. The first step to achieving social distancing in the workroom is to take an inventory of how many people work there and get a log of the number of computers. Consider whether existing computers can be rearranged with a goal of keeping people 6 feet apart. For particularly cramped workrooms, this may require assigning computer spaces to physicians across a floor or several floors, using computers out on a unit, or using mobile computers to limit the number of people in the workroom at one time. We suggest working with physical plant leaders and Information Technology to reallocate mobile workstations, laptops, or desktops to conference rooms, patient visiting areas, and offices that are not being used. Because coronavirus can survive on surfaces for several hours, it is also important to stock work rooms with disinfectants to clean surfaces such as keyboards and desktops frequently. One other important thing to consider is whether computers can be assigned to specific teams or people to limit the use of a computer by multiple people.
ROUNDING, SIGN-OUT, AND MULTIDISCIPLINARY ROUNDS
Rounding
Perhaps one of the most fundamental hardships with physical distancing is how to conduct routine clinical care such as rounds, sign-out, or multidisciplinary rounds. Rounds on teaching services are particularly challenging given the number of people. At many teaching institutions, medical students are no longer on clinical rotations, which immediately reduces the number of people on teaching teams. The other thing to consider is how rounds are conducted. As opposed to a large team walking together, assign one person from the team as the liaison for the patient, which also has the added benefit of conserving precious PPE. Virtual rounding enables clinicians, including residents and attendings, to work together and decide the plan for the day without first crowding into a patient room. This is perhaps the most important cultural hurdle that one may face.
Another administrative hurdle and common concern is how to bill for such interactions. While federal guidance evolves, our institution created smartphrases for this type of virtual rounding whereby attendings attest to resident notes even if they did not physically see the patient. Additional information may be obtained from patients by calling them on their patient-room phones or by using telemedicine as some hospitals are implementing.3 For large “mega” teams, split the team into smaller groups to facilitate continuity and easier conversations.
Sign-out
When feasible, it is important to transition to phone sign-out supplemented with viewing an updated shared sign-out, ideally electronically, for shift change. When using phone sign-out, it is ideal to implement a verbal read-back to ensure understanding and to keep your sign-out updated. Because using the telephone is not the most effective communication channel for sign-out, it is key to be vigilant with other sign-out best practices, such as using a standard template like IPASS4 or another framework, prioritizing sick patients, and ensuring a focus on to-do and if/then items that are critical for the receiver to ensure understanding.5
Multidisciplinary Rounds
As multidisciplinary rounds typically occur either at the bedside or in a conference room, it is key to ensure that these occur virtually whenever possible. One option is to use conference calls or video chat (eg, Zoom) for multidisciplinary rounds whenever possible. Calendar invites or paging reminders can be used to prompt teams when to call in to discuss patients. Because multiple people are entering a virtual room at once, it is important to establish an order or have a leader orchestrate who is next. In addition, given the importance of multiple people contributing to the discussion, it is also equally important for those speaking always to announce who they are and their role (eg, social worker, case manager, physical therapist) since it may not be possible to recognize people’s voices alone. This is where visual recognition can be helpful through use of institutional video conferencing that enables hearing and seeing someone. Further, it is important to ensure that the platform being used is HIPAA compliant.
CALL ROOMS
Call rooms in hospitals can be particularly challenging if they are shared. Finding additional call rooms may require use of cots or reallocation of patient rooms. It is also possible for hospitalists to consider air mattresses in their offices or other private spaces to avoid sharing call rooms. Consider assigning the same call room to the same few people over the course of a rotation or period to avoid many people sharing one room. If a hospital is converting units to group patients under investigation or those who are COVID-19 positive, reallocating call rooms may be necessary to accommodate new teams. Lastly, it is important to communicate proactively with environmental services staff to make sure all call rooms are equipped with cleaning supplies and hand sanitizer and are cleaned daily to avoid nosocomial transmission.
CONCLUSION
/section>Containing nosocomial spread of coronavirus is particularly challenging for hospitals because of how contagious the virus is, the extreme shortage of PPE, and lack of mass testing to identify those who are sick. Therefore, physical distancing in the hospital is critical to ensure the health and well-being of the health professional workforce during the pandemic.
Hospitalists serve as frontline healthcare professionals caring for the increasing number of COVID-19 patients in the United States. The safety of hospitalists and other frontline healthcare workers is paramount to preventing high nosocomial transmission as has been reported in several other countries. Much effort to date has rightly focused on ensuring healthcare workers have appropriate personal protective equipment (PPE) given the known increased risk of nosocomial infection to healthcare workers. However, another important strategy to prevent nosocomial transmission is to implement “social distancing,” or avoiding close contact with others. While this approach has received considerable press with regards to implementation in communities, social, or physical, distancing in the hospital is also a critical way to prevent nosocomial transmission and ensure the health and welfare of our workforce to meet the challenge. The Centers for Disease Control and Prevention (CDC) defines close contact as less than 6 feet away for over 10 minutes.1 Given the myriad clinical interactions that occur within teams in the hospital, such distancing can prove challenging.
At the University of Chicago Medicine in Illinois, our hospitalist group was an early adopter of implementing several strategies to facilitate physical distancing in the context of clinical care to minimize community transmission of COVID-19 among healthcare professionals. We describe how to implement physical distancing effectively in specific hospital settings, including some challenges and strategies to surmount them.
EDUCATIONAL CONFERENCES AND ADMINISTRATIVE MEETINGS
Educational conferences and administrative meetings need to be transitioned to virtual meetings. While it may be easy to broadcast a conference in lieu of meeting in a conference room, it is critical that hospital clinicians do not “huddle close together” in front of a computer, which would defeat the purpose of physical distancing. While “flipping the classroom” in preclinical and higher education is common, this method can be effective to deliver standard education followed by a virtual question and answer session or chat room.2
Educational discussions can also occur asynchronously through learning management systems, such as Canvas, or even closed social media channels, such as Slack, that enable discussions. These tools require training to work, so it is important to invest in education on the chosen platform to ensure that it functions smoothly. It is equally important that administrators become familiar with these tools while working remotely and can facilitate administrative meetings without difficulty. We created a one-page tip sheet to help ease the transition for department administrators. The tip sheet highlighted how to start a virtual meeting and meeting etiquette (eg, mute upon entry into the meeting, mute when not talking, announce yourself when talking) as well as ensuring that dial-ins could easily access the meeting by including one-touch options, when available, on calendar invites in addition to the weblink. A daily email update can be an important adjunct to administrative meetings to ensure critical updates are reaching all clinicians in a group and also preserves meeting time for clarifying questions.
CLINICAL WORKROOMS
Perhaps the biggest challenge is how many clinical workrooms in hospitals today are crowded with computers next to each other. Ventilation can also be poor, making conditions riskier. This makes implemention of social distancing extremely challenging, but also critical, given how much time hospital-based clinicians spend on computers and in their workrooms. The first step to achieving social distancing in the workroom is to take an inventory of how many people work there and get a log of the number of computers. Consider whether existing computers can be rearranged with a goal of keeping people 6 feet apart. For particularly cramped workrooms, this may require assigning computer spaces to physicians across a floor or several floors, using computers out on a unit, or using mobile computers to limit the number of people in the workroom at one time. We suggest working with physical plant leaders and Information Technology to reallocate mobile workstations, laptops, or desktops to conference rooms, patient visiting areas, and offices that are not being used. Because coronavirus can survive on surfaces for several hours, it is also important to stock work rooms with disinfectants to clean surfaces such as keyboards and desktops frequently. One other important thing to consider is whether computers can be assigned to specific teams or people to limit the use of a computer by multiple people.
ROUNDING, SIGN-OUT, AND MULTIDISCIPLINARY ROUNDS
Rounding
Perhaps one of the most fundamental hardships with physical distancing is how to conduct routine clinical care such as rounds, sign-out, or multidisciplinary rounds. Rounds on teaching services are particularly challenging given the number of people. At many teaching institutions, medical students are no longer on clinical rotations, which immediately reduces the number of people on teaching teams. The other thing to consider is how rounds are conducted. As opposed to a large team walking together, assign one person from the team as the liaison for the patient, which also has the added benefit of conserving precious PPE. Virtual rounding enables clinicians, including residents and attendings, to work together and decide the plan for the day without first crowding into a patient room. This is perhaps the most important cultural hurdle that one may face.
Another administrative hurdle and common concern is how to bill for such interactions. While federal guidance evolves, our institution created smartphrases for this type of virtual rounding whereby attendings attest to resident notes even if they did not physically see the patient. Additional information may be obtained from patients by calling them on their patient-room phones or by using telemedicine as some hospitals are implementing.3 For large “mega” teams, split the team into smaller groups to facilitate continuity and easier conversations.
Sign-out
When feasible, it is important to transition to phone sign-out supplemented with viewing an updated shared sign-out, ideally electronically, for shift change. When using phone sign-out, it is ideal to implement a verbal read-back to ensure understanding and to keep your sign-out updated. Because using the telephone is not the most effective communication channel for sign-out, it is key to be vigilant with other sign-out best practices, such as using a standard template like IPASS4 or another framework, prioritizing sick patients, and ensuring a focus on to-do and if/then items that are critical for the receiver to ensure understanding.5
Multidisciplinary Rounds
As multidisciplinary rounds typically occur either at the bedside or in a conference room, it is key to ensure that these occur virtually whenever possible. One option is to use conference calls or video chat (eg, Zoom) for multidisciplinary rounds whenever possible. Calendar invites or paging reminders can be used to prompt teams when to call in to discuss patients. Because multiple people are entering a virtual room at once, it is important to establish an order or have a leader orchestrate who is next. In addition, given the importance of multiple people contributing to the discussion, it is also equally important for those speaking always to announce who they are and their role (eg, social worker, case manager, physical therapist) since it may not be possible to recognize people’s voices alone. This is where visual recognition can be helpful through use of institutional video conferencing that enables hearing and seeing someone. Further, it is important to ensure that the platform being used is HIPAA compliant.
CALL ROOMS
Call rooms in hospitals can be particularly challenging if they are shared. Finding additional call rooms may require use of cots or reallocation of patient rooms. It is also possible for hospitalists to consider air mattresses in their offices or other private spaces to avoid sharing call rooms. Consider assigning the same call room to the same few people over the course of a rotation or period to avoid many people sharing one room. If a hospital is converting units to group patients under investigation or those who are COVID-19 positive, reallocating call rooms may be necessary to accommodate new teams. Lastly, it is important to communicate proactively with environmental services staff to make sure all call rooms are equipped with cleaning supplies and hand sanitizer and are cleaned daily to avoid nosocomial transmission.
CONCLUSION
/section>Containing nosocomial spread of coronavirus is particularly challenging for hospitals because of how contagious the virus is, the extreme shortage of PPE, and lack of mass testing to identify those who are sick. Therefore, physical distancing in the hospital is critical to ensure the health and well-being of the health professional workforce during the pandemic.
1. Centers for Disease Control and Prevention. Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 2, 2020.
2. Stephenson CR, Wang AT, Szostek JH, et al. Flipping the continuing medical education classroom: validating a measure of attendees’ perceptions. J Contin Educ Health Prof. 2016;36(4):256-262. https://doi.org/10.1097/CEH.0000000000000113.
3. Doshi A, Platt Y, Dressen JR, K Mathews BK, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(4):xxx-xxxx. https://doi.org/10.12788/jhm.3419.
4. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
5. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The modified, multi-patient observed simulated handoff experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
1. Centers for Disease Control and Prevention. Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 2, 2020.
2. Stephenson CR, Wang AT, Szostek JH, et al. Flipping the continuing medical education classroom: validating a measure of attendees’ perceptions. J Contin Educ Health Prof. 2016;36(4):256-262. https://doi.org/10.1097/CEH.0000000000000113.
3. Doshi A, Platt Y, Dressen JR, K Mathews BK, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(4):xxx-xxxx. https://doi.org/10.12788/jhm.3419.
4. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
5. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The modified, multi-patient observed simulated handoff experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
© 2020 Society of Hospital Medicine
Describing Variability of Inpatient Consultation Practices: Physician, Patient, and Admission Factors
Inpatient consultation is an extremely common practice with the potential to improve patient outcomes significantly.1-3 However, variability in consultation practices may be risky for patients. In addition to underuse when the benefit is clear, the overuse of consultation may lead to additional testing and therapies, increased length of stay (LOS) and costs, conflicting recommendations, and opportunities for communication breakdown.
Consultation use is often at the discretion of individual providers. While this decision is frequently driven by patient needs, significant variation in consultation practices not fully explained by patient factors exists.1 Prior work has described hospital-level variation1 and that primary care physicians use more consultation than hospitalists.4 However, other factors affecting consultation remain unknown. We sought to explore physician-, patient-, and admission-level factors associated with consultation use on inpatient general medicine services.
METHODS
Study Design
We conducted a retrospective analysis of data from the University of Chicago Hospitalist Project (UCHP). UCHP is a longstanding study of the care of hospitalized patients admitted to the University of Chicago general medicine services, involving both patient data collection and physician experience surveys.5 Data were obtained for enrolled UCHP patients between 2011-2016 from the Center for Research Informatics (CRI). The University of Chicago Institutional Review Board approved this study.
Data Collection
Attendings and patients consented to UCHP participation. Data collection details are described elsewhere.5,6 Data from EpicCare (EpicSystems Corp, Wisconsin) and Centricity Billing (GE Healthcare, Illinois) were obtained via CRI for all encounters of enrolled UCHP patients during the study period (N = 218,591).
Attending Attribution
We determined attending attribution for admissions as follows: the attending author of the first history and physical (H&P) was assigned. If this was unavailable, the attending author of the first progress note (PN) was assigned. For patients admitted by hospitalists on admitting shifts to nonteaching services (ie, service without residents/students), the author of the first PN was assigned if different from H&P. Where available, attribution was corroborated with call schedules.
Sample and Variables
All encounters containing inpatient admissions to the University of Chicago from May 10, 2011 (Electronic Health Record activation date), through December 31, 2016, were considered for inclusion (N = 51,171, Appendix 1). Admissions including only documentation from ancillary services were excluded (eg, encounters for hemodialysis or physical therapy). Admissions were limited to a length of stay (LOS) ≤ 5 days, corresponding to the average US inpatient LOS of 4.6 days,7 to minimize the likelihood of attending handoffs (N = 31,592). If attending attribution was not possible via the above-described methods, the admission was eliminated (N = 3,103; 10.9% of admissions with LOS ≤ 5 days). Finally, the sample was restricted to general medicine service admissions under attendings enrolled in UCHP who completed surveys. After the application of all criteria, 6,153 admissions remained for analysis.
The outcome variable was the number of consultations per admission, determined by counting the unique number of services creating clinical documentation, and subtracting one for the primary team. If the Medical/Surgical intensive care unit (ICU) was a service, then two were subtracted to account for the ICU transfer.
Attending years in practice (ie, years since medical school graduation) and gender were determined from public resources. Practice characteristics were determined from UCHP attending surveys, which address perceptions of workload and satisfaction (Appendix 2).
Patient characteristics (gender, age, Elixhauser Indices) and admission characteristics (LOS, season of admission, payor) were determined from UCHP and CRI data. The Elixhauser Index uses a well-validated system combining the presence/absence of 31 comorbidities to predict mortality and 30-day readmission.8 Elixhauser Indices were calculated using the “Creation of Elixhauser Comorbidity Index Scores 1.0” software.9 For admissions under hospitalist attendings, teaching/nonteaching team was ascertained via internal teaching service calendars.
Analysis
We used descriptive statistics to examine demographic characteristics. The difference between the lowest and highest quartile consultation use was determined via a two-sample t test. Given the multilevel nature of our count data, we used a mixed-effects Poisson model accounting for within-group variation by clustering on attending and patient (3-level random-effects model). The analysis was done using Stata 15 (StataCorp, Texas).
RESULTS
From 2011 to 2016, 14,848 patients and 88 attendings were enrolled in UCHP; 4,772 patients (32%) and 69 attendings (59.4%) had data available and were included. Mean LOS was 3.0 days (SD = 1.3). Table 1 describes the characteristics of attendings, patients, and admissions.
Seventy-six percent of admissions included at least one consultation. Consultation use varied widely, ranging from 0 to 10 per admission (mean = 1.39, median = 1; standard deviation [SD] = 1.17). The number of consultations per admission in the highest quartile of consultation frequency (mean = 3.47, median = 3) was 5.7-fold that of the lowest quartile (mean = 0.613, median = 1; P <.001).
In multivariable regression, physician-, patient-, and admission-level characteristics were associated with the differential use of consultation (Table 2). On teaching services, consultations called by hospitalist vs nonhospitalist generalists did not differ (P =.361). However, hospitalists on nonteaching services called 8.6% more consultations than hospitalists on teaching services (P =.02). Attending agreement with survey item “The interruption of my personal life by work is a problem” was associated with 8.2% fewer consultations per admission (P =.002).
Patients older than 75 years received 19% fewer consultations compared with patients younger than 49 years (P <.001). Compared with Medicare, Medicaid admissions had 12.2% fewer consultations (P <.001), whereas privately insured admissions had 10.7% more (P =.001). The number of consultations per admission decreased every year, with 45.3% fewer consultations in 2015 than 2011 (P <.001). Consultations increased by each 22% per day increase in LOS (P <.001).
DISCUSSION
Our analysis described several physician-, patient-, and admission-level characteristics associated with the use of inpatient consultation. Our results strengthen prior work demonstrating that patient-level factors alone are insufficient to explain consultation variability.1
Hospitalists on nonteaching services called more consultations, which may reflect a higher workload on these services. Busy hospitalists on nonteaching teams may lack time to delve deeply into clinical problems and require more consultations, especially for work with heavy cognitive loads such as diagnosis. “Outsourcing” tasks when workload increases occurs in other cognitive activities such as teaching.10 The association between work interrupting personal life and fewer consultations may also implicate the effects of time. Attendings who are experiencing work encroaching on their personal lives may be those spending more time with patients and consulting less. This finding merits further study, especially with increasing concern about balancing time spent in meaningful patient care activities with risk of physician burnout.
This finding could also indicate that trainee participation modifies consultation use for hospitalists. Teaching service teams with more individual members may allow a greater pool of collective knowledge, decreasing the need for consultation to answer clinical questions.11 Interestingly, there was no difference in consultation use between generalists or subspecialists and hospitalists on teaching services, possibly suggesting a unique effect in hospitalists who vary clinical practice depending on team structure. These differences deserve further investigation, with implications for education and resource utilization.
We were surprised by the finding that consultations decreased each year, despite increasing patient complexity and availability of consultation services. This could be explained by a growing emphasis on shortening LOS in our institution, thus shifting consultative care to outpatient settings. Understanding these effects is critically important with growing evidence that consultation improves patient outcomes because these external pressures could lead to unintended consequences for quality or access to care.
Several findings related to patient factors additionally emerged, including age and insurance status. Although related to medical complexity, these effects persist despite adjustment, which raises the question of whether they contribute to the decision to seek consultation. Older patients received fewer consultations, which could reflect the use of more conservative practice models in the elderly,12 or ageism, which is associated with undertreatment.13 With respect to insurance status, Medicaid patients were associated with fewer consultations. This finding is consistent with previous work showing the decreased intensity of hospital services used for Medicaid patients.14Our study has limitations. Our data were from one large urban academic center that limits generalizability. Although systematic and redundant, attending attribution may have been flawed: incomplete or erroneous documentation could have led to attribution error, and we cannot rule out the possibility of service handoffs. We used a LOS ≤ 5 days to minimize this possibility, but this limits the applicability of our findings to longer admissions. Unsurprisingly, longer LOS correlated with the increased use of consultation even within our restricted sample, and future work should examine the effects of prolonged LOS. As a retrospective analysis, unmeasured confounders due to our limited adjustment will likely explain some findings, although we took steps to address this in our statistical design. Finally, we could not measure patient outcomes and, therefore, cannot determine the value of more or fewer consultations for specific patients or illnesses. Positive and negative outcomes of increased consultation are described, and understanding the impact of consultation is critical for further study.2,3
CONCLUSION
We found that the use of consultation on general medicine services varies widely between admissions, with large differences between the highest and lowest frequencies of use. This variation can be partially explained by several physician-, patient-, and admission-level characteristics. Our work may help identify patient and attending groups at high risk for under- or overuse of consultation and guide the subsequent development of interventions to improve value in consultation. One additional consultation over the average LOS of 4.6 days adds $420 per admission or $4.8 billion to the 11.5 million annual Medicare admissions.15 Increasing research, guidelines, and education on the judicious use of inpatient consultation will be key in maximizing high-value care and improving patient outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable support and assistance of the University of Chicago Hospitalist Project, the Pritzker School of Medicine Summer Research Program, the University of Chicago Center for Quality, and the University of Chicago Center for Health and the Social Sciences (CHeSS). The authors would additionally like to thank John Cursio, PhD, for his support and guidance in statistical analysis for this project.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. Preliminary results of this analysis were presented at the 2018 Society of Hospital Medicine Annual Meeting in Orlando, Florida. All coauthors have seen and agree with the contents of the manuscript. The submission is not under review by any other publication.
1. Stevens JP, Nyweide D, Maresh S, et al. Variation in inpatient consultation among older adults in the United States. J Gen Intern Med. 2015;30(7):992-999. https://doi.org/10.1007/s11606-015-3216-7.
2. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. https://doi.org/10.1097/MD.0b013e3181b8fccb.
3. Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff Proj Hope. 2011;30(3):454-463. https://doi.org/10.1377/hlthaff.2010.0929.
4. Stevens JP, Nyweide DJ, Maresh S, Hatfield LA, Howell MD, Landon BE. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781. https://doi.org/10.1001/jamainternmed.2017.5824.
5. Meltzer D. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866. https://doi.org/10.7326/0003-4819-137-11-200212030-00007.
6. Martin SK, Farnan JM, Flores A, Kurina LM, Meltzer DO, Arora VM. Exploring entrustment: Housestaff autonomy and patient readmission. Am J Med. 2014;127(8):791-797. https://doi.org/10.1016/j.amjmed.2014.04.013.
7. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed July 7, 2017.
8. Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65-e72. https://doi.org/10.1097/MLR.0b013e318297429c.
9. Elixhauser Comorbidity Software. Elixhauser Comorbidity Software. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#references. Accessed May 13, 2019.
10. Roshetsky LM, Coltri A, Flores A, et al. No time for teaching? Inpatient attending physicians’ workload and teaching before and after the implementation of the 2003 duty hours regulations. Acad Med J Assoc Am Med Coll. 2013;88(9):1293-1298. https://doi.org/10.1097/ACM.0b013e31829eb795.
11. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
12. Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type A aortic dissection in elderly patients. J Cardiothorac Surg. 2018;13(1):129. https://doi.org/10.1186/s13019-018-0814-6.
13. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774. https://doi.org/10.1056/NEJMoa067423.
14. Yergan J, Flood AB, Diehr P, LoGerfo JP. Relationship between patient source of payment and the intensity of hospital services. Med Care. 1988;26(11):1111-1114. https://doi.org/10.1097/00005650-198811000-00009.
15. Center for Medicare and Medicaid Services. MDCR INPT HOSP 1.; 2008. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2013/Downloads/MDCR_UTIL/CPS_MDCR_INPT_HOSP_1.pdf. Accessed April 15, 2018.
Inpatient consultation is an extremely common practice with the potential to improve patient outcomes significantly.1-3 However, variability in consultation practices may be risky for patients. In addition to underuse when the benefit is clear, the overuse of consultation may lead to additional testing and therapies, increased length of stay (LOS) and costs, conflicting recommendations, and opportunities for communication breakdown.
Consultation use is often at the discretion of individual providers. While this decision is frequently driven by patient needs, significant variation in consultation practices not fully explained by patient factors exists.1 Prior work has described hospital-level variation1 and that primary care physicians use more consultation than hospitalists.4 However, other factors affecting consultation remain unknown. We sought to explore physician-, patient-, and admission-level factors associated with consultation use on inpatient general medicine services.
METHODS
Study Design
We conducted a retrospective analysis of data from the University of Chicago Hospitalist Project (UCHP). UCHP is a longstanding study of the care of hospitalized patients admitted to the University of Chicago general medicine services, involving both patient data collection and physician experience surveys.5 Data were obtained for enrolled UCHP patients between 2011-2016 from the Center for Research Informatics (CRI). The University of Chicago Institutional Review Board approved this study.
Data Collection
Attendings and patients consented to UCHP participation. Data collection details are described elsewhere.5,6 Data from EpicCare (EpicSystems Corp, Wisconsin) and Centricity Billing (GE Healthcare, Illinois) were obtained via CRI for all encounters of enrolled UCHP patients during the study period (N = 218,591).
Attending Attribution
We determined attending attribution for admissions as follows: the attending author of the first history and physical (H&P) was assigned. If this was unavailable, the attending author of the first progress note (PN) was assigned. For patients admitted by hospitalists on admitting shifts to nonteaching services (ie, service without residents/students), the author of the first PN was assigned if different from H&P. Where available, attribution was corroborated with call schedules.
Sample and Variables
All encounters containing inpatient admissions to the University of Chicago from May 10, 2011 (Electronic Health Record activation date), through December 31, 2016, were considered for inclusion (N = 51,171, Appendix 1). Admissions including only documentation from ancillary services were excluded (eg, encounters for hemodialysis or physical therapy). Admissions were limited to a length of stay (LOS) ≤ 5 days, corresponding to the average US inpatient LOS of 4.6 days,7 to minimize the likelihood of attending handoffs (N = 31,592). If attending attribution was not possible via the above-described methods, the admission was eliminated (N = 3,103; 10.9% of admissions with LOS ≤ 5 days). Finally, the sample was restricted to general medicine service admissions under attendings enrolled in UCHP who completed surveys. After the application of all criteria, 6,153 admissions remained for analysis.
The outcome variable was the number of consultations per admission, determined by counting the unique number of services creating clinical documentation, and subtracting one for the primary team. If the Medical/Surgical intensive care unit (ICU) was a service, then two were subtracted to account for the ICU transfer.
Attending years in practice (ie, years since medical school graduation) and gender were determined from public resources. Practice characteristics were determined from UCHP attending surveys, which address perceptions of workload and satisfaction (Appendix 2).
Patient characteristics (gender, age, Elixhauser Indices) and admission characteristics (LOS, season of admission, payor) were determined from UCHP and CRI data. The Elixhauser Index uses a well-validated system combining the presence/absence of 31 comorbidities to predict mortality and 30-day readmission.8 Elixhauser Indices were calculated using the “Creation of Elixhauser Comorbidity Index Scores 1.0” software.9 For admissions under hospitalist attendings, teaching/nonteaching team was ascertained via internal teaching service calendars.
Analysis
We used descriptive statistics to examine demographic characteristics. The difference between the lowest and highest quartile consultation use was determined via a two-sample t test. Given the multilevel nature of our count data, we used a mixed-effects Poisson model accounting for within-group variation by clustering on attending and patient (3-level random-effects model). The analysis was done using Stata 15 (StataCorp, Texas).
RESULTS
From 2011 to 2016, 14,848 patients and 88 attendings were enrolled in UCHP; 4,772 patients (32%) and 69 attendings (59.4%) had data available and were included. Mean LOS was 3.0 days (SD = 1.3). Table 1 describes the characteristics of attendings, patients, and admissions.
Seventy-six percent of admissions included at least one consultation. Consultation use varied widely, ranging from 0 to 10 per admission (mean = 1.39, median = 1; standard deviation [SD] = 1.17). The number of consultations per admission in the highest quartile of consultation frequency (mean = 3.47, median = 3) was 5.7-fold that of the lowest quartile (mean = 0.613, median = 1; P <.001).
In multivariable regression, physician-, patient-, and admission-level characteristics were associated with the differential use of consultation (Table 2). On teaching services, consultations called by hospitalist vs nonhospitalist generalists did not differ (P =.361). However, hospitalists on nonteaching services called 8.6% more consultations than hospitalists on teaching services (P =.02). Attending agreement with survey item “The interruption of my personal life by work is a problem” was associated with 8.2% fewer consultations per admission (P =.002).
Patients older than 75 years received 19% fewer consultations compared with patients younger than 49 years (P <.001). Compared with Medicare, Medicaid admissions had 12.2% fewer consultations (P <.001), whereas privately insured admissions had 10.7% more (P =.001). The number of consultations per admission decreased every year, with 45.3% fewer consultations in 2015 than 2011 (P <.001). Consultations increased by each 22% per day increase in LOS (P <.001).
DISCUSSION
Our analysis described several physician-, patient-, and admission-level characteristics associated with the use of inpatient consultation. Our results strengthen prior work demonstrating that patient-level factors alone are insufficient to explain consultation variability.1
Hospitalists on nonteaching services called more consultations, which may reflect a higher workload on these services. Busy hospitalists on nonteaching teams may lack time to delve deeply into clinical problems and require more consultations, especially for work with heavy cognitive loads such as diagnosis. “Outsourcing” tasks when workload increases occurs in other cognitive activities such as teaching.10 The association between work interrupting personal life and fewer consultations may also implicate the effects of time. Attendings who are experiencing work encroaching on their personal lives may be those spending more time with patients and consulting less. This finding merits further study, especially with increasing concern about balancing time spent in meaningful patient care activities with risk of physician burnout.
This finding could also indicate that trainee participation modifies consultation use for hospitalists. Teaching service teams with more individual members may allow a greater pool of collective knowledge, decreasing the need for consultation to answer clinical questions.11 Interestingly, there was no difference in consultation use between generalists or subspecialists and hospitalists on teaching services, possibly suggesting a unique effect in hospitalists who vary clinical practice depending on team structure. These differences deserve further investigation, with implications for education and resource utilization.
We were surprised by the finding that consultations decreased each year, despite increasing patient complexity and availability of consultation services. This could be explained by a growing emphasis on shortening LOS in our institution, thus shifting consultative care to outpatient settings. Understanding these effects is critically important with growing evidence that consultation improves patient outcomes because these external pressures could lead to unintended consequences for quality or access to care.
Several findings related to patient factors additionally emerged, including age and insurance status. Although related to medical complexity, these effects persist despite adjustment, which raises the question of whether they contribute to the decision to seek consultation. Older patients received fewer consultations, which could reflect the use of more conservative practice models in the elderly,12 or ageism, which is associated with undertreatment.13 With respect to insurance status, Medicaid patients were associated with fewer consultations. This finding is consistent with previous work showing the decreased intensity of hospital services used for Medicaid patients.14Our study has limitations. Our data were from one large urban academic center that limits generalizability. Although systematic and redundant, attending attribution may have been flawed: incomplete or erroneous documentation could have led to attribution error, and we cannot rule out the possibility of service handoffs. We used a LOS ≤ 5 days to minimize this possibility, but this limits the applicability of our findings to longer admissions. Unsurprisingly, longer LOS correlated with the increased use of consultation even within our restricted sample, and future work should examine the effects of prolonged LOS. As a retrospective analysis, unmeasured confounders due to our limited adjustment will likely explain some findings, although we took steps to address this in our statistical design. Finally, we could not measure patient outcomes and, therefore, cannot determine the value of more or fewer consultations for specific patients or illnesses. Positive and negative outcomes of increased consultation are described, and understanding the impact of consultation is critical for further study.2,3
CONCLUSION
We found that the use of consultation on general medicine services varies widely between admissions, with large differences between the highest and lowest frequencies of use. This variation can be partially explained by several physician-, patient-, and admission-level characteristics. Our work may help identify patient and attending groups at high risk for under- or overuse of consultation and guide the subsequent development of interventions to improve value in consultation. One additional consultation over the average LOS of 4.6 days adds $420 per admission or $4.8 billion to the 11.5 million annual Medicare admissions.15 Increasing research, guidelines, and education on the judicious use of inpatient consultation will be key in maximizing high-value care and improving patient outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable support and assistance of the University of Chicago Hospitalist Project, the Pritzker School of Medicine Summer Research Program, the University of Chicago Center for Quality, and the University of Chicago Center for Health and the Social Sciences (CHeSS). The authors would additionally like to thank John Cursio, PhD, for his support and guidance in statistical analysis for this project.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. Preliminary results of this analysis were presented at the 2018 Society of Hospital Medicine Annual Meeting in Orlando, Florida. All coauthors have seen and agree with the contents of the manuscript. The submission is not under review by any other publication.
Inpatient consultation is an extremely common practice with the potential to improve patient outcomes significantly.1-3 However, variability in consultation practices may be risky for patients. In addition to underuse when the benefit is clear, the overuse of consultation may lead to additional testing and therapies, increased length of stay (LOS) and costs, conflicting recommendations, and opportunities for communication breakdown.
Consultation use is often at the discretion of individual providers. While this decision is frequently driven by patient needs, significant variation in consultation practices not fully explained by patient factors exists.1 Prior work has described hospital-level variation1 and that primary care physicians use more consultation than hospitalists.4 However, other factors affecting consultation remain unknown. We sought to explore physician-, patient-, and admission-level factors associated with consultation use on inpatient general medicine services.
METHODS
Study Design
We conducted a retrospective analysis of data from the University of Chicago Hospitalist Project (UCHP). UCHP is a longstanding study of the care of hospitalized patients admitted to the University of Chicago general medicine services, involving both patient data collection and physician experience surveys.5 Data were obtained for enrolled UCHP patients between 2011-2016 from the Center for Research Informatics (CRI). The University of Chicago Institutional Review Board approved this study.
Data Collection
Attendings and patients consented to UCHP participation. Data collection details are described elsewhere.5,6 Data from EpicCare (EpicSystems Corp, Wisconsin) and Centricity Billing (GE Healthcare, Illinois) were obtained via CRI for all encounters of enrolled UCHP patients during the study period (N = 218,591).
Attending Attribution
We determined attending attribution for admissions as follows: the attending author of the first history and physical (H&P) was assigned. If this was unavailable, the attending author of the first progress note (PN) was assigned. For patients admitted by hospitalists on admitting shifts to nonteaching services (ie, service without residents/students), the author of the first PN was assigned if different from H&P. Where available, attribution was corroborated with call schedules.
Sample and Variables
All encounters containing inpatient admissions to the University of Chicago from May 10, 2011 (Electronic Health Record activation date), through December 31, 2016, were considered for inclusion (N = 51,171, Appendix 1). Admissions including only documentation from ancillary services were excluded (eg, encounters for hemodialysis or physical therapy). Admissions were limited to a length of stay (LOS) ≤ 5 days, corresponding to the average US inpatient LOS of 4.6 days,7 to minimize the likelihood of attending handoffs (N = 31,592). If attending attribution was not possible via the above-described methods, the admission was eliminated (N = 3,103; 10.9% of admissions with LOS ≤ 5 days). Finally, the sample was restricted to general medicine service admissions under attendings enrolled in UCHP who completed surveys. After the application of all criteria, 6,153 admissions remained for analysis.
The outcome variable was the number of consultations per admission, determined by counting the unique number of services creating clinical documentation, and subtracting one for the primary team. If the Medical/Surgical intensive care unit (ICU) was a service, then two were subtracted to account for the ICU transfer.
Attending years in practice (ie, years since medical school graduation) and gender were determined from public resources. Practice characteristics were determined from UCHP attending surveys, which address perceptions of workload and satisfaction (Appendix 2).
Patient characteristics (gender, age, Elixhauser Indices) and admission characteristics (LOS, season of admission, payor) were determined from UCHP and CRI data. The Elixhauser Index uses a well-validated system combining the presence/absence of 31 comorbidities to predict mortality and 30-day readmission.8 Elixhauser Indices were calculated using the “Creation of Elixhauser Comorbidity Index Scores 1.0” software.9 For admissions under hospitalist attendings, teaching/nonteaching team was ascertained via internal teaching service calendars.
Analysis
We used descriptive statistics to examine demographic characteristics. The difference between the lowest and highest quartile consultation use was determined via a two-sample t test. Given the multilevel nature of our count data, we used a mixed-effects Poisson model accounting for within-group variation by clustering on attending and patient (3-level random-effects model). The analysis was done using Stata 15 (StataCorp, Texas).
RESULTS
From 2011 to 2016, 14,848 patients and 88 attendings were enrolled in UCHP; 4,772 patients (32%) and 69 attendings (59.4%) had data available and were included. Mean LOS was 3.0 days (SD = 1.3). Table 1 describes the characteristics of attendings, patients, and admissions.
Seventy-six percent of admissions included at least one consultation. Consultation use varied widely, ranging from 0 to 10 per admission (mean = 1.39, median = 1; standard deviation [SD] = 1.17). The number of consultations per admission in the highest quartile of consultation frequency (mean = 3.47, median = 3) was 5.7-fold that of the lowest quartile (mean = 0.613, median = 1; P <.001).
In multivariable regression, physician-, patient-, and admission-level characteristics were associated with the differential use of consultation (Table 2). On teaching services, consultations called by hospitalist vs nonhospitalist generalists did not differ (P =.361). However, hospitalists on nonteaching services called 8.6% more consultations than hospitalists on teaching services (P =.02). Attending agreement with survey item “The interruption of my personal life by work is a problem” was associated with 8.2% fewer consultations per admission (P =.002).
Patients older than 75 years received 19% fewer consultations compared with patients younger than 49 years (P <.001). Compared with Medicare, Medicaid admissions had 12.2% fewer consultations (P <.001), whereas privately insured admissions had 10.7% more (P =.001). The number of consultations per admission decreased every year, with 45.3% fewer consultations in 2015 than 2011 (P <.001). Consultations increased by each 22% per day increase in LOS (P <.001).
DISCUSSION
Our analysis described several physician-, patient-, and admission-level characteristics associated with the use of inpatient consultation. Our results strengthen prior work demonstrating that patient-level factors alone are insufficient to explain consultation variability.1
Hospitalists on nonteaching services called more consultations, which may reflect a higher workload on these services. Busy hospitalists on nonteaching teams may lack time to delve deeply into clinical problems and require more consultations, especially for work with heavy cognitive loads such as diagnosis. “Outsourcing” tasks when workload increases occurs in other cognitive activities such as teaching.10 The association between work interrupting personal life and fewer consultations may also implicate the effects of time. Attendings who are experiencing work encroaching on their personal lives may be those spending more time with patients and consulting less. This finding merits further study, especially with increasing concern about balancing time spent in meaningful patient care activities with risk of physician burnout.
This finding could also indicate that trainee participation modifies consultation use for hospitalists. Teaching service teams with more individual members may allow a greater pool of collective knowledge, decreasing the need for consultation to answer clinical questions.11 Interestingly, there was no difference in consultation use between generalists or subspecialists and hospitalists on teaching services, possibly suggesting a unique effect in hospitalists who vary clinical practice depending on team structure. These differences deserve further investigation, with implications for education and resource utilization.
We were surprised by the finding that consultations decreased each year, despite increasing patient complexity and availability of consultation services. This could be explained by a growing emphasis on shortening LOS in our institution, thus shifting consultative care to outpatient settings. Understanding these effects is critically important with growing evidence that consultation improves patient outcomes because these external pressures could lead to unintended consequences for quality or access to care.
Several findings related to patient factors additionally emerged, including age and insurance status. Although related to medical complexity, these effects persist despite adjustment, which raises the question of whether they contribute to the decision to seek consultation. Older patients received fewer consultations, which could reflect the use of more conservative practice models in the elderly,12 or ageism, which is associated with undertreatment.13 With respect to insurance status, Medicaid patients were associated with fewer consultations. This finding is consistent with previous work showing the decreased intensity of hospital services used for Medicaid patients.14Our study has limitations. Our data were from one large urban academic center that limits generalizability. Although systematic and redundant, attending attribution may have been flawed: incomplete or erroneous documentation could have led to attribution error, and we cannot rule out the possibility of service handoffs. We used a LOS ≤ 5 days to minimize this possibility, but this limits the applicability of our findings to longer admissions. Unsurprisingly, longer LOS correlated with the increased use of consultation even within our restricted sample, and future work should examine the effects of prolonged LOS. As a retrospective analysis, unmeasured confounders due to our limited adjustment will likely explain some findings, although we took steps to address this in our statistical design. Finally, we could not measure patient outcomes and, therefore, cannot determine the value of more or fewer consultations for specific patients or illnesses. Positive and negative outcomes of increased consultation are described, and understanding the impact of consultation is critical for further study.2,3
CONCLUSION
We found that the use of consultation on general medicine services varies widely between admissions, with large differences between the highest and lowest frequencies of use. This variation can be partially explained by several physician-, patient-, and admission-level characteristics. Our work may help identify patient and attending groups at high risk for under- or overuse of consultation and guide the subsequent development of interventions to improve value in consultation. One additional consultation over the average LOS of 4.6 days adds $420 per admission or $4.8 billion to the 11.5 million annual Medicare admissions.15 Increasing research, guidelines, and education on the judicious use of inpatient consultation will be key in maximizing high-value care and improving patient outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable support and assistance of the University of Chicago Hospitalist Project, the Pritzker School of Medicine Summer Research Program, the University of Chicago Center for Quality, and the University of Chicago Center for Health and the Social Sciences (CHeSS). The authors would additionally like to thank John Cursio, PhD, for his support and guidance in statistical analysis for this project.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. Preliminary results of this analysis were presented at the 2018 Society of Hospital Medicine Annual Meeting in Orlando, Florida. All coauthors have seen and agree with the contents of the manuscript. The submission is not under review by any other publication.
1. Stevens JP, Nyweide D, Maresh S, et al. Variation in inpatient consultation among older adults in the United States. J Gen Intern Med. 2015;30(7):992-999. https://doi.org/10.1007/s11606-015-3216-7.
2. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. https://doi.org/10.1097/MD.0b013e3181b8fccb.
3. Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff Proj Hope. 2011;30(3):454-463. https://doi.org/10.1377/hlthaff.2010.0929.
4. Stevens JP, Nyweide DJ, Maresh S, Hatfield LA, Howell MD, Landon BE. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781. https://doi.org/10.1001/jamainternmed.2017.5824.
5. Meltzer D. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866. https://doi.org/10.7326/0003-4819-137-11-200212030-00007.
6. Martin SK, Farnan JM, Flores A, Kurina LM, Meltzer DO, Arora VM. Exploring entrustment: Housestaff autonomy and patient readmission. Am J Med. 2014;127(8):791-797. https://doi.org/10.1016/j.amjmed.2014.04.013.
7. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed July 7, 2017.
8. Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65-e72. https://doi.org/10.1097/MLR.0b013e318297429c.
9. Elixhauser Comorbidity Software. Elixhauser Comorbidity Software. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#references. Accessed May 13, 2019.
10. Roshetsky LM, Coltri A, Flores A, et al. No time for teaching? Inpatient attending physicians’ workload and teaching before and after the implementation of the 2003 duty hours regulations. Acad Med J Assoc Am Med Coll. 2013;88(9):1293-1298. https://doi.org/10.1097/ACM.0b013e31829eb795.
11. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
12. Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type A aortic dissection in elderly patients. J Cardiothorac Surg. 2018;13(1):129. https://doi.org/10.1186/s13019-018-0814-6.
13. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774. https://doi.org/10.1056/NEJMoa067423.
14. Yergan J, Flood AB, Diehr P, LoGerfo JP. Relationship between patient source of payment and the intensity of hospital services. Med Care. 1988;26(11):1111-1114. https://doi.org/10.1097/00005650-198811000-00009.
15. Center for Medicare and Medicaid Services. MDCR INPT HOSP 1.; 2008. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2013/Downloads/MDCR_UTIL/CPS_MDCR_INPT_HOSP_1.pdf. Accessed April 15, 2018.
1. Stevens JP, Nyweide D, Maresh S, et al. Variation in inpatient consultation among older adults in the United States. J Gen Intern Med. 2015;30(7):992-999. https://doi.org/10.1007/s11606-015-3216-7.
2. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. https://doi.org/10.1097/MD.0b013e3181b8fccb.
3. Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff Proj Hope. 2011;30(3):454-463. https://doi.org/10.1377/hlthaff.2010.0929.
4. Stevens JP, Nyweide DJ, Maresh S, Hatfield LA, Howell MD, Landon BE. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781. https://doi.org/10.1001/jamainternmed.2017.5824.
5. Meltzer D. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866. https://doi.org/10.7326/0003-4819-137-11-200212030-00007.
6. Martin SK, Farnan JM, Flores A, Kurina LM, Meltzer DO, Arora VM. Exploring entrustment: Housestaff autonomy and patient readmission. Am J Med. 2014;127(8):791-797. https://doi.org/10.1016/j.amjmed.2014.04.013.
7. HCUP-US NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed July 7, 2017.
8. Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65-e72. https://doi.org/10.1097/MLR.0b013e318297429c.
9. Elixhauser Comorbidity Software. Elixhauser Comorbidity Software. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#references. Accessed May 13, 2019.
10. Roshetsky LM, Coltri A, Flores A, et al. No time for teaching? Inpatient attending physicians’ workload and teaching before and after the implementation of the 2003 duty hours regulations. Acad Med J Assoc Am Med Coll. 2013;88(9):1293-1298. https://doi.org/10.1097/ACM.0b013e31829eb795.
11. Barnett ML, Boddupalli D, Nundy S, Bates DW. Comparative accuracy of diagnosis by collective intelligence of multiple physicians vs individual physicians. JAMA Netw Open. 2019;2(3):e190096. https://doi.org/10.1001/jamanetworkopen.2019.0096.
12. Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type A aortic dissection in elderly patients. J Cardiothorac Surg. 2018;13(1):129. https://doi.org/10.1186/s13019-018-0814-6.
13. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774. https://doi.org/10.1056/NEJMoa067423.
14. Yergan J, Flood AB, Diehr P, LoGerfo JP. Relationship between patient source of payment and the intensity of hospital services. Med Care. 1988;26(11):1111-1114. https://doi.org/10.1097/00005650-198811000-00009.
15. Center for Medicare and Medicaid Services. MDCR INPT HOSP 1.; 2008. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2013/Downloads/MDCR_UTIL/CPS_MDCR_INPT_HOSP_1.pdf. Accessed April 15, 2018.
© 2020 Society of Hospital Medicine
Tackling the Minimizers Hiding Behind High-Value Care
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
© 2019 Society of Hospital Medicine