User login
The Importance of Emotional Intelligence When Leading in a Time of Crisis
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
© 2020 Society of Hospital Medicine
Hospital Medicine Update: High-Impact Literature from March 2018 to April 2019
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
© 2019 Society of Hospital Medicine
Update in Hospital Medicine: Practical Lessons from Current Literature
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
© 2019 Society of Hospital Medicine
FDA‐warning for IV Haloperidol: A Review
Haloperidol is Food and Drug Administration (FDA)‐approved in the United States for the management of acute and chronic psychotic disorders and widely used in the management of delirium‐associated agitation in hospitalized patients.1 Delirium in the hospital is an acute confusional state that frequently arises from multiple complex factors and may affect up to 30% of hospitalized patients.2 Although the first step in the management of delirium involves identification and treatment of underlying causes and offering supportive behavioral care; medications may be needed to control severe agitation.2 Low dose intravenous (IV) haloperidol (ie, 0.250.5 mg every 4 hours) is a commonly used medication in this setting as recommended by expert‐groups including the Cochrane Collaboration and the American Psychiatric Association.2, 3
Although injectable haloperidol, a butyrophenone‐derived antipsychotic agent pharmacologically related to the piperazine phenothiazines,4 is approved for IV use in many countries (Table 1), parenteral use is approved only for intramuscular (IM) administration in the US. Thus, IV administration of the drug in the US is considered an off‐label use.5
| Indication | Country | |||||
|---|---|---|---|---|---|---|
| Canada24 | France29 | Germany25 | Great Britain37 | Italy30 | Switzerland31 | |
| ||||||
| Mainly delirium (schizophrenia, other psychosis, short‐term management of psychomotor agitation, excitement, violent or dangerously impulsive behavior, vomiting, hiccup) | Short term treatment of agitation and aggressiveness during an acute or chronic psychotic episode, vomiting along with antimitotic post‐radiotherapy treatment | Acute and chronic schizophrenia, psycho‐motorical agitation of psychotic genesis | Schizophrenia, other psychosis, short‐term adjunctive management if psychomotor agitation, violent or dangerous impulsive behavior | Resistant forms of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis' chronic psychosis High doses restrictions: syndrome of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis, chronic psychosis | Acute schizophrenic episode, mania, vomiting | |
| IV dosing in adults | 12 mg every 24 hours | The use is limited to adult patients and the drug can be administered IM or IV. The IV route is restricted to the treatment of vomiting. | 510 mg/day, daily max.: 30(100) mg | 210 mg initially, PRN every 48 hours, daily max. 18 mg | 510 mg initially, PRN every hour, daily max. 60 mg | 5 mg PRN every 30 minutes |
| IV dosing in geriatric care | 0.250.5 mg | Single dose of 0.51.5 mg, daily max. 5 mg | Half adult dose | Adjust to appropriate dose | 0.5 mg, than PRN | |
| Risk factors for the development of cardiac adverse events | QT prolonging drugs, diabetes, obesity, hypokalemia, congenital long QT syndrome | Bradycardia <55 beats per minute, hypokalemia, congenital QT prolongation, other medications provoking bradycardia, deceleration of the intra‐cardiac transition or prolonged QT interval | QT syndrome, hypokalemia, other electrolyte imbalance, cardiovascular diseases, QT prolongation in the family history | Cardiovalscular disease, drugs that can prolong the QTc, diabetes, obesity, hypokalemia, congenital long QT syndrome | Contraindications: recent cardiac infarction, uncompensated cardiac insufficiency, cardiac arrhythmias, antiarrhythmic drugs, pre‐existing QT prolongation, cases of arrhythmia or torsades de pointes in the family history, untreated potassium imbalance, QTc prolonging drugs | QT syndrome, hypokalemia, hypomagnesemia, other electrolyte imbalances, cardiovascular diseases, hypothyreosis, QT prolongation in the family history |
| Monitoring recommendations | Electrolytes | ECG monitoring at admission time, electrolytes | ECG monitoring, electrolytes | Metabolic parameters | ECG at baseline and regular ECG monitoring, electrolytes | Close ECG monitoring, electrolytes |
| General recommendations | Regular reevaluation in long‐term use | Apply the lowest effective dose | Apply the lowest effective dose | Application per mouth is the route of choice | Decrease dose if QTc >500 msec | Switch to PO as soon as possible |
Haloperidol is often preferred over other antipsychotics as a result of its effectiveness, low rate of anticholinergic side effects, familiarity with dosing and usage, and minimal respiratory or sedative properties.6 Use of the IV route in patients with acute delirium has several advantages over the IM or oral route,7 including rapid onset, immediate bioavailability, and ease and safety of administration.
Prior to September 2007, the package insert for haloperidol alerted healthcare professionals to the risk of cardiovascular side effects. Based on case reports of potentially fatal cardiac events, the FDA revised the label, warning that the QT prolongation (QTP) and risk of torsades de pointes (TdP) were increased with IV administration of haloperidol or administration of the drug at greater than recommended doses. Unfortunately, neither the typical dosing range nor the minimum dose associated with these cardiac side effects were specified in this recommendation.5
It is well‐established that haloperidol may prolong the QT interval by blocking the repolarizing potassium IKr current.8 Although drugs that block the IKr channel can produce arrhythmia in healthy individuals, additional risk factors, such as underlying heart conditions, electrolyte imbalances (ie, hypokalemia and hypomagnesemia), concomitant proarrhythmic drug use, and mechanical ventilation may increase this risk.9 Prolongation of the QT interval has been associated with subsequent malignant cardiac arrhythmias including ventricular fibrillation and TdP.10 Prolongation of the QT interval is considered the strongest risk factor for TdP, particularly with a baseline QTc > 450 msec.9
Based on the increased risk for QTP and TdP and the case reports of cardiac events, the FDA advisory recommended continuous electrocardiogram (ECG) monitoring in patients receiving IV haloperidol.5 However, such monitoring may be impractical and costly in hospitalized patients who require low doses of IV haloperidol to manage acute delirium and who are not in telemetry or intensive care units.
The aim of this review was to evaluate the case reports leading to the recent FDA warning for IV haloperidol, specifically focusing on the presence of risk factors for arrhythmias. Based upon the evidence, an additional aim was to provide an institutional response to this warning toward the optimal use of this agent.
Method
Two search pathways were used to evaluate reports of haloperidol‐associated TdP and/or QT prolongation:
Literature Review
We searched for published literature in humans indexed in Pubmed (1966April 2009), EMBASE (1972April 2009), and Scopus (1823April 2009) using the search terms haloperidol or Haldol combined with intravenous or infusion and at least one of the following terms: QT prolongation, TdP, torsades de pointes, torsades with a specific focus on case reports.
References from the retrieved articles were also reviewed to search for additional case reports.
In addition to cases reported in English journals, several of our reports originated from Japan11 (translation provided by the FDA), Spain12 and Germany13 (translated by the primary author).
Search of the FDA Database
We reviewed all adverse drug events reported through MedWatch or those submitted by the manufacturer from November 1997 to April 2008 through the Freedom of Information Act (FOIA) request. The FDA provided a full‐text summary of 5944 reports involving oral, intramuscular and IV use of haloperidol. The FDA data were transferred to a Microsoft Access database and screened for the key terms torsade, QT, prolongation, wave. Incident report number, date of report, age, gender, origin of report, medication name, role of drug as categorized by the FDA (suspect, concomitant, primary suspect, secondary suspect), route, dose, units, duration, symptoms and FDA outcome category (death, life‐threatening, hospitalization initial or prolonged, disability, congenital anomaly, required intervention to prevent permanent damage, other) were recorded. Only those reports in which IV haloperidol was considered by the reporter to be the primary causative agent for the adverse event were reviewed. Available information included diagnosis, laboratory parameters, QTc measurement, cardiac symptoms, outcomes and a description of recovery. No peer review was applied to the MedWatch reports and the data reported in this publication reflect the original information from the FDA MedWatch database. Baseline QTc was either the value defined as such in the original report or the lowest QTc reported. Haloperidol doses administered were defined as cumulative dose at event, encompassing all doses administered during the hospital stay until the occurrence of the adverse cardiac event.
The drugs listed in the case reports were assessed for proarrhythmic potential using 2 references: the individual package insert and the website of the Arizona Center for Education and Research on Therapeutics.14
The drugs were only considered proarrhythmic when the 2 resources were in agreement.
Duplicates and/or previously published cases, as well as reports involving adverse cardiac effects not associated with QTP or TdP, were identified and excluded.
In their advisory, the FDA does not state the exact origin of the reports, their specific search strategy to identify haloperidol‐associated adverse events, or the role IV haloperidol played in the individual events included in the extended warning. Consequently, the number of events identified in this review may differ from that published in the FDA extended warning.
Results
A total of 70 reported cases of IV haloperidol associated TdP and/or QTP were identified. Of these 70, 41 were identified through the PubMed/EMBASE/Scopus review, while an additional 29 cases were identified through the FDA database search.
Of the 29 cases in the FDA database, 21 were reported by health care professionals and 8 by manufacturers.
A total of 35 publications described cases originating from the US. Three cases took place in Japan and 1 case each in Canada, Germany and Spain. Several cases in the MedWatch database were reported outside the US: 1 case each originated from Austria, Canada, France, Japan, Spain, Switzerland and the United Kingdom. A summary of the published case reports is displayed in Table 2 and the FDA cases are summarized in Table 3.
| Case | Source (reference#) | Date | Age, Years | Gender | Drugs Pro‐arrhyth. | Venti‐ lated | Max. Daily Dose (mg) | Total Dose at Event (mg) | Time to Event | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | ECG Normalization, Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
| 1 | 35 | 1991 | 56 | m | No | Yes | 1200 | 1540 | NR | Yes | 584 (400) | 184 | NR | NR, uneventful |
| 2 | 13 | 1992 | 36 | m | Yes | No | 11.5 | 11.5 | 20 hours after start | Yes | 714 (428) | 286 | Yes | QTc normalization (440 msec), NR |
| 3 | 38 | 1993 | 39 | f | Yes | Yes | NR | 580 | Max. QTc 72 hours after start | Yes | 650 (420) | 230 | Yes | QTc normalization after 6 days, uneventful |
| 4 | 38 | 1993 | 19 | f | Yes | No | 170 | 170 | Max. QT 12 hours after start | Yes | 600 (480) | 120 | Yes | QTc normalization after 8 days, uneventful |
| 5 | 38 | 1993 | 63 | f | Yes | No | NR | 489 | Max. QT 48 hours after start | Yes | 670 (520) | 150 | Yes | QTc normalization after 8 days, uneventful |
| 6 | 38 | 1993 | 74 | f | Yes | Yes | NR | 10 | NR | No | 430 (410) | 20 | Yes | QTc unchanged after 8 days, uneventful |
| 7 | 17 | 1993 | 39 | m | Yes | Yes | NR | >490 | NR | Yes | 457 (348) | 109 | Yes | QTc normalization within 2 to 3 days, no further TdP, NR |
| 8 | 17 | 1993 | 61 | m | Yes | Yes | 115 | 211 | NR | Yes | 500 (390) | 110 | NR | QTc normalization within 2 days, death |
| 9 | 17 | 1993 | 48 | m | Yes | Yes | 825 | 825 | NR | Yes | 538 (441) | 97 | NR | QTc normalization in 3 days, rehabilitation |
| 10 | 39 | 1994 | 23 | f | Yes | Yes | 120 | 300 | 12 hours after dose increase | Yes | NR (550) | NR | Yes | NR, uneventful, extubation after 5 days, discharge after 10 days |
| 11 | 39 | 1994 | 28 | m | Yes | Yes | 300 | >300 | 24 hours after dose increase | Yes | NR (>520) | NR | Yes | No recurrence of arrhythmia, patient death (multi‐organ failure) |
| 12 | 40 | 1994 | 65 | m | Yes | NR | 230 | 410 | Worsening from day 2 to day 5 | Yes | 594 (490) | 104 | Yes | QTc normalization (406 msec), no cardiac problems at discharge |
| 13 | 40 | 1994 | 65 | f | Yes | NR | 500 | 980 | After the last 60mg | Yes | 628 (403) | 225 | Yes | QTc normalization (<400 msec), recurrence with oral haloperidol, rehabilitation |
| 14 | 40 | 1994 | 76 | f | Yes | NR | 21 | 26 | Day 2 after several boluses | Yes | 670 (450) | 220 | Yes | QTc normalization within several days (412 msec), rehabilitation |
| 15 | 41 | 1994 | 59 | m | NR | Yes | 865 | 1013 | NR | Yes | 640 (480) | 160 | NR | QTc normalization in 24 hours, survived |
| 16 | 16 | 1995 | 76 | f | Yes | No | NR | 44.5 plus 1 PO | 15 minutes | Yes | 670 (409) | 261 | Yes | ECG normalized the next morning, no further events |
| 17 | 16 | 1995 | 49 | m | Yes | No | NR | 1150 plus 20 IM | 45 minutes | Yes | 648 (380) | 268 | Yes | QTc normalization in 24 hours, anoxic brain insult/rehabilitation |
| 18 | 16 | 1995 | 65 | f | Yes | No | 600 | 965 | 30 minutes | Yes | 628 (403) | 225 | Yes | 3 more episodes of TdP in 3 hours, QTc normalization in 2 days, no recurrence with further haloperidol, NR |
| 19 | 42 | 1995 | 42 | m | Yes | No | 28 | 28 | 20 minutes | Yes | 610 (533) | 77 | Yes | QTc normalization in 5 days, uneventful, ECG normal |
| 20 | 42 | 1995 | 39 | m | Yes | No | 45 | 45 | 5 minutes | Yes | 654 (NR) | NR | Yes | QTc normalization after 24 hours, uneventful |
| 21 | 11 | 1997 | 56 | f | No | No | 10 | 10 | Shortly after | NR | NR (NR) | NR | Yes | TdP resolved after 8 hours, NR |
| 22 | 11 | 1997 | 82 | f | NR | No | 10 | 10 | Shortly after | Yes | 680 (NR) | NR | Yes | QTc normalization on day 6 after admission (470 msec), NR |
| 23 | 11 | 1997 | 35 | m | NR | No | NR | 90 | After 20 mg | Yes | 520 (NR) | NR | Yes | TdP disappeared 12 hours later, NR |
| 24 | 43,44 | 1998 | 45 | m | NR | Yes* | NR | 9 | 203 minutes | Yes | 638 (560) | 78 | Yes | NR, overall survival 100%, significantly prolonged hospital stay |
| 25 | 43,44 | 1998 | 64 | f | NR | NR | 115 | 220 minutes | Yes | 605 (424) | 181 | Yes | ||
| 26 | 43,44 | 1998 | 75 | f | NR | NR | 85 | 60 minutes | Yes | 567 (508) | 59 | Yes | ||
| 27 | 43,44 | 1998 | 71 | f | NR | NR | 55 | 120 minutes | Paced | Paced | Paced | Yes | ||
| 28 | 43,44 | 1998 | 58 | f | NR | NR | 75 | 38 minutes | Yes | 657 (542) | 115 | Yes | ||
| 29 | 43,44 | 1998 | 40 | m | NR | NR | 35 | 15 minutes | Yes | 679 (475) | 204 | Yes | ||
| 30 | 43,44 | 1998 | 71 | m | NR | NR | 70 | 58 minutes | Yes | 521 (478) | 43 | Yes | ||
| 31 | 43,44 | 1998 | 47 | m | NR | 400 | 400 | 79 minutes | Yes | 574 (444) | 130 | Yes | ||
| 32 | 21 | 1999 | 41 | f | Yes | Yes | 320 | 915 | 55 minutes | Yes | 610 (426) | 184 | Yes | QTc normalization after 5 day, uneventful |
| 33 | 21 | 1999 | 31 | m | Yes | Yes | 480 | 1700 | 40 minutes | Yes | 599 (491) | 108 | Yes | QTc normalized in 4 days, NR |
| 34 | 18 | 2000 | 64 | f | Yes | Yes | 175 | 175 | NR | No | 413 (418) | (‐5) | Yes | QTc remained unchanged, uneventful |
| 35 | 8 | 2000 | 75 | m | No | NR | >2 | >2 | NR | Yes | 615 (435) | 180 | No | QTc normalization in 48 hours, uneventful |
| 36 | 8 | 2000 | 68 | m | Yes | Yes | >2 | >2 | NR | Yes | 650 (407) | 243 | No | QTc normalization after 4 day, uneventful after extubation |
| 37 | 8 | 2000 | 77 | m | NR | NR | (4) | 2 | NR | Yes | 550 (393) | 157 | No | QTc normalization in 24 to 36 hours, NR |
| 38 | 12 | 2004 | 34 | m | Yes | NR | 24.5 | 24.5 | 20 minutes | Yes | 560 (420) | 140 | Yes | QTc normalization (440 msec), ECG normal |
| 39 | 23 | 2004 | 58 | f | Yes | NR | 340 | 1010 | NR | Yes | 533 (460) | 73 | Yes | QTc normalization 7 days later discharge after 27days |
| 40 | 45 | 2008 | 86 | f | Yes | No | 2 mg | 2 mg | 8 hours after last dose | Yes | 524 (NR) | Probably 79 | No | QTc normalization (445 msec), NR |
| 41 | 46 | 2009 | 74 | m | Yes | No | 2 | 2 | Shortly after | Yes | NR (579) | NR | Yes | Pre‐existing heart block and fibrillation resolved, nursing home/rehabilitation |
| Report | MedWatch Identifier | Report Date | Age, Years | Gender | Drugs Pro‐arrh. | Maximum Daily Dose (mg) | Total Dose at Event (mg) | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | Outcome; Recovery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
| 1 | 3122988‐1 | 1998 | 61 | m | No | 48 | 48 | Yes | NR | NR | Yes | Intervention; NR |
| 2 | 3157827‐6 | 1998 | 44 | f | No | 160 | 160 | Yes | 550 (440) | 110 | Yes | Intervention; uneventful |
| 3 | 3178715‐5 | 1999 | 60 | m | NR | 415 | 645 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization in 1 day, no recurrence |
| 4 | 3271261‐X | 1999 | 56 | m | NR | NR | 20 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization |
| 5 | 3271080‐4 | 1999 | 35 | m | Yes | 7 | 7 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced, hospitalization prolonged |
| 6 | 3325391‐4 | 1999 | 55 | f | Yes | 75 | 75 | NR | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 7 | 3381921‐8 | 1999 | 52 | m | No | 320 | 634 | Yes | 458 (430) | 28 | Yes | Death; NA |
| 8 | 3483869‐7 | 2000 | 18 | m | No | >200 | >310 | Yes | NR | NR | Yes | Intervention; no recurrence after haloperidol reinstitution |
| 9 | 3516342‐8 | 2000 | NR | NR | NR | NR | NR | NR | NR | NR | Yes | NR; NR |
| 10 | 3516320‐9 | 2000 | 34 | m | Yes | 5 | 5 | Yes | NR | NR | No | Life‐threatening; event abated after dose stopped |
| 11 | 3552263‐2 | 2000 | 46 | f | Yes | NR | 97.5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 12 | 3574705‐9 | 2000 | 78 | m | Yes | NR | 160 | Yes | 603 (453) | 50 | Yes | Intervention; event abated after dose stopped/reduced |
| 13 | 3703871‐7 | 2001 | 27 | m | NR | 530 | 530 | Yes | NR | NR | Yes | Death, NA |
| 14 | 3724567‐1 | 2001 | 31 | m | Yes | 6 | 6 | Yes | 496 (449) | 47 | No | Life‐threatening; ECG returned to baseline |
| 15 | 3851984‐1 | 2002 | 72 | f | NR | 18 | 18 | NR | NR | NR | Yes | Hospitalization; NR |
| 16 | 3942407‐2 | 2002 | 51 | m | Yes | 14 | 14 | Yes | 461 (444) | 17 | Yes | Life‐threatening; no recurrence |
| 17 | 4066580‐3 | 2003 | >60 | f | NR | 50 | 50 | Yes | >600 (480) | >120 | No | Hospitalization; QTc normalization, patient recovered |
| 18 | 4126280‐8 | 2003 | 47 | f | NR | 60 | 180 | Yes | 550 (450) | 100 | No (bradycardia) | Hospitalization; patient recovered |
| 19 | 4150700‐6 | 2003 | NR | m | NR | 5 | 5 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced |
| 20 | 4340092‐1 | 2004 | 52 | m | Yes | 5 | 5 | Yes | >500 (490) | >10 | NR (polymorphous VT) | Life‐threatening; NR |
| 21 | 4714692‐0 | 2005 | NR | m | NR | NR | NR | Yes | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 22 | 4881813‐9 | 2006 | NR | m | NR | NR | 40 | NR | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 23 | 4892225‐6 | 2006 | NR | f | Yes | 10 | >10 | Yes | 493 (300) | 193 | No | Hospitalization; QTc normalization (403 msec) |
| 24 | 4911873‐8 | 2006 | 69 | m | Yes | 6 | 6 | NR | NR | NR | Yes | Cardiac arrest, death; NA |
| 25 | 5366448‐6 | 2007 | 53 | m | Yes | NR | 35 | Yes | NR | NR | NR | Cardiac arrest, life‐threatening; patient recovered |
| 26 | 5563440‐3 | 2007 | 58 | m | Possible | 5 | 5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 27 | 5642929‐2 | 2008 | 42 | m | Yes | 165 | 165 | Yes | 640 (350) | 290 | Yes | Death; NA |
| 28 | 5697758‐0 | 2008 | 38 | m | Yes | NR | 620 | NR | NR | NR | Yes | Hospitalization; patient recovered |
| 29 | 5254840‐X | 2008 | 19 | f | Possible | 15 | 25 | Yes | 461 | NR | NR | Cardiac arrest, hospitalization; patient recovered |
Of the 70 cases, 54 cases of TdP were reported. The remaining 16 of 70 cases involved cases of QTP, 9 of which did not progress to TdP and 7 of which the progression to TdP was unclear. Of note, 42 of 54 of the cases of TdP were reported as preceded by documented QTP. Presence of QTP was unknown in the other 12 original reports. Three out of 70 patients experienced sudden cardiac arrest, 1 of which was fatal. One arrest was preceded by TdP and 2 by QTP (Figure 1).
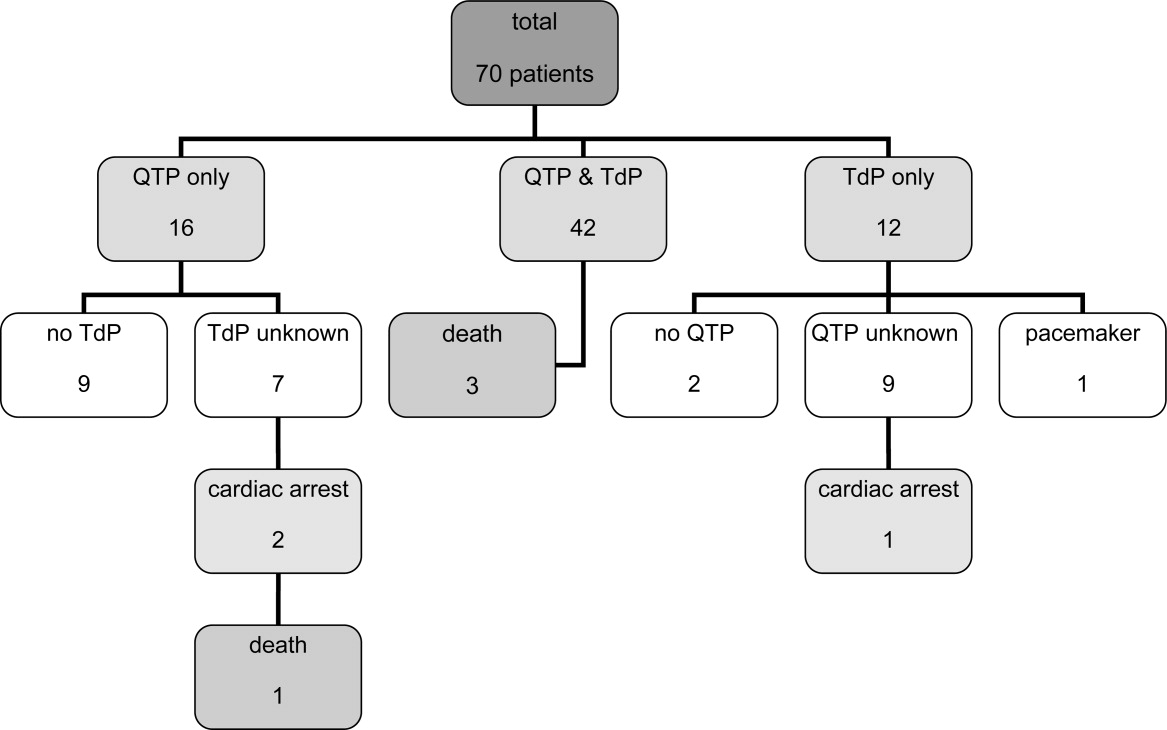
The patient ages ranged from 18 years to 86 years. Of note, 17 patients experiencing TdP and/or QTP were <40 years old, and 2 of those patients were <30 years old.
Haloperidol‐associated QTP and/or TdP were observed in 27 female and 42 male patients; the gender was not stated in one report. Of the 54 patients experiencing TdP (with or without report of previous QTP), 22 were female and 31 were male (1 gender unknown).
A total of 68 of 70 patients were determined to have associated risk factors15 for QTP/TdP (see Table 4). The circumstances of the remaining 2 patients were not described in sufficient detail to identify associated risk factors.
| Risk Factor | Patients, n (%) |
|---|---|
| |
| Any risk factor | 68/70 (97) |
| Unknown | 2/70 (3) |
| Specific risk factors | |
| Electrolyte imbalance | 27/68 (40) |
| Underlying cardiac disease | 32/68 (47) |
| Concomitant proarrhythmic agents | 39/68 (57) |
| Other drugs influencing cardiac function | 23/68 (34) |
| Baseline QTc >450 msec | 18/68 (26) |
| QTc known: 44 patients | 18/44 (41) |
Overall, 32 patients had underlying heart conditions. Electrolyte imbalances, including hypokalemia, hypomagnesemia, and hypocalcemia, were present in 17 patients. At least 39 patients were receiving potentially proarrhythmic agents (1‐8 proarrhythmic drugs per patient) in addition to IV haloperidol. At least 23 patients were receiving additional drugs with a potential for other cardiac adverse events than QTP and TdP.
A wide range of other disease states previously reported to be associated with QTP15 were identified in these patients: asthma (5 patients), diabetes (5 patients), obesity (3 patients), impaired renal and/or liver function (3 patients each), human immunodeficiency virus (HIV) (2 patients); chronic obstructive pulmonary disease (COPD), pancreatitis and hypothyroidism (1 patient each). A total of 22 patients had a history of substance abuse (alcohol and/or drugs), and 4 patients were smokers.
The administered doses of IV haloperidol varied widely. Considering that information regarding the maximal daily dose was missing in 22 reports and ambiguous in another 20 cases, the results have been presented using cumulative IV haloperidol doses. Patients experiencing TdP without preceding QTP received a cumulative dose (= total dose at event) ranging from 5 mg to 645 mg. Patients with both confirmed QTP and TdP were administered a cumulative dose of 2 mg to 1700 mg. Patients who experienced QTP without TdP received a cumulative dose of 2 mg to 1540 mg of IV haloperidol.
Sudden cardiac arrest following administration of IV haloperidol was observed in cumulative doses ranging from 6 mg to 35 mg. The cardiac arrest leading to a fatal outcome was preceded by an administration of at least 6 mg of IV haloperidol. Overall, 14 out of 70 patients received cumulative doses of 10 mg IV haloperidol.
The time from administration to documentation of QTP and/or TdP ranged from immediately post administration to 8 hours after administration of the last dose of IV haloperidol.
Baseline QTc was known in 44 patients. Baseline QTc was >450 msec in 18 of these 44 patients.
The change from baseline QTc varied widely from 20 msec to 286 msec; 36 patients demonstrated a prolongation of >50 msec.
In those patients with reported haloperidol‐associated QTP, 25 patients demonstrated a QTc >600 msec and 38 patients >520 msec.9 Of the cases with known specific QTc values, the QTc was prolonged >450 msec in 48 out of 50 cases. The lowest reported QTc leading to TdP was 413 msec.
A total of 20 patients were reported as having a normalization of QTc (as defined by the original reports) within several hours to 8 days; minimal QTP was reported as persisting in 2 patients. The specifics of the other patients were unknown, although 25 patients were categorized as recovered, 13 were stated as having an uneventful remainder of hospitalization, and 5 patients were discharged to a rehabilitation facility or a nursing home.
Discussion
The current review was performed in response to the FDA warning recommending the use of continuous ECG monitoring associated with the administration of intravenous haloperiodol.5 This warning has resulted in substantial dilemmas for health care organizations, additional resource allocation, and increased scrutiny from regulatory agencies. The results of our review reveal that intravenous haloperidol‐associated QTP and TdP almost uniformly take place in patients with concomitant risk factors and with cumulative doses 2 mg. In light of these findings, it is possible that hospitals may be able to administer intravenous haloperidol in patients without risk factors without continuous ECG monitoring. In reviewing these published reports, it is important to note that the FDA identified 28 published cases of haloperidol‐associated QTP and TdP, while our review yielded a total of 41 published case reports.
The FDA database included 73 cases of haloperidol‐associated TdP in their database. However, these cases included both oral as well as IV administration; using our methodology, we identified 29 additional case reports associated with intravenous haloperidol from this database. Consequently, our review included 41 published case reports and 29 FDA database cases, resulting in the total of 70 patients.
Our review revealed a number of practical findings. First, our summary demonstrated that neither QTP nor TdP has been documented with a cumulative dose of IV haloperidol of <2 mg. The majority of patients (80%) received cumulative IV doses 10 mg. The lowest dose associated with sudden cardiac arrest was 6 mg and this took place in a 69‐year‐old male patient. Second, the majority (97%) of our patients had additional risk factors for QTP and/or TdP. Pre‐existing heart disease,1619 electrolyte imbalance,17, 1921 concomitant proarrhythmic drugs16, 17, 1922 and mechanical ventilation17, 23 were identified as the most commonly observed risk factors (Table 4). Lastly, in those cases in which the data were reported, baseline QTc was >450 msec in 41% of the patients, and 96% had a QTc at the time of the event >450 msec. Therefore, we conclude that patients: (1) receiving low cumulative doses (<2 mg) with (2) no risk factors for prolonged QTc or TdP, and (3) with a normal QTc on baseline EKG can safely be given IV haloperidol in the hospital setting.
This dosage range is consistent with the labelling for IV haloperidol dosing in Canada24 and Germany25 (Table 1), where single doses of 0.25 mg to 1.5 mg are recommended for the treatment of delirium or acute agitation in the geriatric population.24, 25
In a recent Cochrane review, low‐dose IV haloperidol (<3 mg per day) was concluded to be as safe and effective as atypical antipsychotics in the treatment of acute delirium with respect to extrapyramidal adverse effects.2
The American Psychiatric Association recommends an initial IV dose of 12 mg every 24 hours as needed (0.250.50 mg every 4 hours as needed for elderly patients), with titration to higher doses for patients who continue to be agitated for the treatment of patients with delirium (issued 1999, updated 2004).3
While several expert‐groups and investigators currently consider IV haloperidol as an important therapeutic option for treating acute delirium and agitation in the dose range presented above, less consensus exists regarding monitoring requirements.2, 3, 26, 27
The American Psychiatric Association recommends IV haloperidol only after a baseline ECG is obtained. These guidelines have not been updated since the release of the FDA extended warning.3 In their recent review, Morandi et al.28 support the dosage recommendation of the 1999 American Psychiatric Association's practice guidelines for treatment of delirium,3 ie, administration of IV haloperidol in single doses of 0.5 mg to 2 mg in elderly patients, however, only after a baseline ECG is obtained.28 While the package insert of IV haloperidol in France29 recommends a baseline ECG, Germany,25 Italy30 and Switzerland's31 package information states the need for regular ECG monitoring. Guidelines for the treatment of delirium in the intensive care unit published by the American College of Critical Care Medicine and the Society of Critical Care Medicine in collaboration with the American Society of Health‐System Pharmacists consider IV haloperidol as the preferred agent for the treatment of delirium in critically ill patients (grade of recommendation = C). These expert groups recommend that patients should be monitored for electrocardiographic changes (QT interval prolongation and arrhythmias) when receiving haloperidol (Grade of recommendation = B).32
Nevertheless, continuous ECG monitoring (ie, telemetry) is expensive, labor‐intensive and, potentially overutilized.33, 34 Requiring clinicians to place all patients receiving intravenous haloperidol on telemetry is impractical and potentially costly. Mandating telemetry could also lead to unintended harm, ie, use of a less effective or less safe drug to avoid compliance with the telemetry mandate.
Based on our findings and the current recommendations in the literature, inpatient providers should be thoughtful and deliberate in the use of haloperidol to treat acute delirium with agitation. Patients requiring pharmacologic management of their delirium should be screened for risk factors for QTP and TdP (Table 4) and a baseline ECG should be obtained prior to haloperidol administration. If significant risk factors exist or the baseline ECG reveals a prolonged QTc, then the patient should receive continuous ECG monitoring. Similarly, if cumulative doses of 2 mg are needed, the patient should be placed on telemetry.
There are some limitations to our study design. Our findings are based upon previously published case reports or data submitted to the FDA MedWatch. While the content of the FDA's MedWatch database is accessible to the public via the Freedom of Information Act (FOIA), the events are neither categorized nor peer‐reviewed upon entry into the database. Consequently, information may be incomplete or inaccurate. In addition, the denominator representing the overall use of IV haloperidol is unknown, thus a rate of event cannot be assigned and the true scope of the problem cannot be determined. Despite these limitations, this summary represents the most comprehensive review of the literature to date, expanding on the analysis performed by the FDA. Of note, in our review of the FDA database, we noted several cases of haloperidol‐associated QTP or TdP associated with other routes of administration. Thus, it is unknown whether this complication is any greater with IV vs. the IM or per os (PO) routes of administration.
Conclusion
Although the proarrhythmic potential of haloperidol and other antipsychotics has been well established in the literature, IV haloperidol has been considered relatively safe with respect to this complication from the time of its approval in 1967.5, 1722, 35, 36 In reviewing all reported cases of cardiac complications associated with IV haloperidol, as well as the current literature, an association with QTP and TdP is likely. However, the case reports reveal that QTP and TdP generally occur in the setting of concomitant risk factors, and no cases have been reported utilizing a cumulative IV dose of <2 mg. It may therefore be safe to administer a cumulative dose of IV haloperidol of <2 mg without ECG monitoring in patients without risk factors for QTP. However, ECG monitoring should take place with IV haloperidol doses 2 mg and/or in those patients with additional risk factors of developing QTP and/or TdP.
Based on the findings of this review complemented by the guidelines of various expert‐groups and the official labelling information of different countries, the Pharmacy & Therapeutics Committee of the UCSF Medical Center revised the IV haloperidol policy: administration of a total dose of <2 mg IV haloperidol without concurrent telemetry is allowed in a noncritical care setting in patients without risk factors for QTP and TdP.
Acknowledgements
The authors acknowledge Gloria Won of the Fishbon Library at UCSF Medical Center at Mount Zion for her support.
- Haldol® injection (for immediate release) Package Insert.Raritan, NJ:Ortho‐McNeil Pharmaceutical Inc.;2005;rev. 05.2007.
- ,,,. Antipsychotics for delirium (review), the Cochrane collaboration2008;2. Available at: www. cochrane.org. Accessed February 2010.
- American Psychiatric Association: practice guideline for the treatment of patients with delirium.Am J Psychiatry.1999;156(5 suppl):1–20, updated 2004.
- Thomson Micromedex.2008. Micromedex healthcare series: “haloperidol” Thomson Micromedex, Greenwood Village.
- FDA alert: haloperidol (marketed as Haldol, Haldol Decanoate and Haldol Lactate).2007. This alert highlights revisions to the labeling for haloperidol. Available at: www.fda.gov. Accessed February 2010.
- ,,.Use of high‐dose intravenous haloperidol in the treatment of agitated cardiac patients,J Clin Psychopharmacol.1985;5(6):344–347.
- ,,, et al.Postoperative delirium,Am J Psychiatry.2008;165:7.
- ,.Corrected QT interval prolongation associated with intravenous haloperidol in acute coronary syndromes,Catheter Cardiovasc Interv.2000;50(3):352–355.
- ,.Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death.Am J Psych.2001;158(11):1774–1782.
- ,,, et al.Accuracy of uncorrected versus corrected QT Interval for Prediction of torsade de pointes associated with intravenous haloperidol.Pharmacotherapy.2007;27(2):175–182.
- ,,.Three cases of ventricular tachycardia and torsades de pointes induced by antipsychotic drugs.Shinzo.1997;29(1):68–74.
- ,,,.Haloperidol por via intravenosa y torsade de pointes.Medicina intensive.2004;28(2):89.
- ,,, et al.QT‐Verlängerung und Kammerflimmern unter Haloperidol‐ und Clonidin‐Therapie des Alkoholentzugssyndroms.Intensivmedizin und Notfallmedizin.1992;29(4):178–183.
- ARIZONA CERT, Arizona Center for Education and Research on Therapeutics. Available at: www.azcert.org. Accessed February 2010.
- ,.Cardiac arrhythmias—a clinical approach.Edinburgh:Mosby;2003.
- ,.The association between intravenous haloperidol and torsades de pointes—three cases and a literature review.Psychosomatics.1995;36:541–549.
- ,.Prolongation of the corrected QT and torsades de pointes cardiac arrhythmia associated with intravenous haloperidol in the medically ill.J Clin Psychopharmacol.1993;13(2):128–132.
- ,,,,.Torsades de pointes secondary to intravenous haloperidol after coronary bypass grafting surgery.Can J Anesth.2000;47(3):251–254.
- ,.Torsade de pointes associated with the administration of intravenous haloperidol: a review of the literature and practical guidelines for use,Expert Opin Drug Saf.2003;2(6):543–547.
- ,.Conduction disturbances associated with administration of butyrophenone antipsychotics in the critically ill: a review of the literature,Pharmacotherapy.1997;17(3):531–537.
- ,,.Haloperidol‐induced torsades de pointes.Ann Pharacother.1999;33(10):1046–1050.
- ,,, et al.Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary,Crit Care Med.1995;23(9):1596–1600.
- ,,,,,.Prolonged cardiac repolarization after tacrolimus and haloperidol administration in the critically ill patient.Pharmacotherapy.2004;24(3):404–408.
- CPS Compendium of Pharmaceuticals and Specialties, the Canadian drug reference for health professionals, 2007, Canadian pharmacists association.
- Rote Liste Deutschland2008, Rote Liste Service GmbH Frankfurt am Main. Available at: www.rote‐liste.de. Accessed February 2010.
- ,.Delirium in the hospitalized patient: a primer for the pharmacist clinician.J Pharm Pract.2007;20(5):368–372.
- ,,, et al.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,.The pharmacological management of delirium in critical illness.Current Drug Therapy.2008,3:148–157.
- VIDAL‐l'information sur les produits de santé2008, Issy les Moulineaux Cedex. Available at: www.vidal.fr. Accessed February 2010.
- Haldol iniettabile—ufficiale monografia italiana. Available at: www. informatorefarmaceutico.it. Accessed February 2010.
- Arzneimittelkompendium der Schweiz2008, documed Verlag Basel. Availabla at: www.kompendium.ch. Accessed February 2010.
- ,,, et al.Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill, American College of Critical Care Medicine ACCM, Society of Critical Care Medicine SCCM, American Society of Health‐System Pharmacists ASHP.Crit Care Med.2002;30(1):119–141.
- ,,,,.Is telemetry overused? Is it as helpful as thought?Cleve Clin J Med.2009;76(6):368–372.
- ,,.Telemetry outside critical care units: patterns of utilization and influence on management decisions.Clin Cardiol.1998;21(7):503–505.
- ,,.High‐dose intravenous haloperidol for agitated delirium in a cardiac patient on intra‐aortic balloon pump.J Clin Psychopharmacol.1991;11(2):146–147.
- .Haloperidol, midazolam and intravenous sedation.Aust NZ J Psychiatry.1999;33(6):942–943.
- BNF British National Formulary, compendium of pharmaceuticals and specialties of the UK.2007. Available at: www.bnf.org. Accessed February 2010.
- ,,,.Torsade de pointes associated with the use of intravenous haloperidol.Ann Intern Med.1993;119(5):391–394.
- ,.Torsades de pointes: potential consequence of intravenous haloperidol in the intensive care unit.Intensive Care World.1994;11(3):109–112.
- ,.Torsade de pointes caused by high‐dose intravenous haloperidol in cardiac patients.Clin Cardiol.1995;18:285–290.
- ,,.Continuous infusion of haloperidol controls agitation in critically ill patients.Crit Care Med.1994:22(3):433–440.
- ,,.Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalances.Am J Gastroenterol.1995;90(5):822–824.
- ,,,.Torsades de pointes associated with intravenous haloperidol in critically ill patients.Am J Cardiol.1998;81(2):238–240.
- ,,,,.The effect of intravenous haloperidol on QT interval dispersion in critically ill patients: comparison with QT interval prolongation for assessment of risk of torsades de pointes.J Clin Pharmacol.2001;41:1310–1318.
- ,,.Low‐dose haloperidol associated QTc prolongation.J Am Geriatr Soc.2008;56(10):1963–1964.
- ,,.Torsade de pointes following intravenous haloperidol administration in a patient with complete heart block.WMJ.2009;108(1):48–50.
Haloperidol is Food and Drug Administration (FDA)‐approved in the United States for the management of acute and chronic psychotic disorders and widely used in the management of delirium‐associated agitation in hospitalized patients.1 Delirium in the hospital is an acute confusional state that frequently arises from multiple complex factors and may affect up to 30% of hospitalized patients.2 Although the first step in the management of delirium involves identification and treatment of underlying causes and offering supportive behavioral care; medications may be needed to control severe agitation.2 Low dose intravenous (IV) haloperidol (ie, 0.250.5 mg every 4 hours) is a commonly used medication in this setting as recommended by expert‐groups including the Cochrane Collaboration and the American Psychiatric Association.2, 3
Although injectable haloperidol, a butyrophenone‐derived antipsychotic agent pharmacologically related to the piperazine phenothiazines,4 is approved for IV use in many countries (Table 1), parenteral use is approved only for intramuscular (IM) administration in the US. Thus, IV administration of the drug in the US is considered an off‐label use.5
| Indication | Country | |||||
|---|---|---|---|---|---|---|
| Canada24 | France29 | Germany25 | Great Britain37 | Italy30 | Switzerland31 | |
| ||||||
| Mainly delirium (schizophrenia, other psychosis, short‐term management of psychomotor agitation, excitement, violent or dangerously impulsive behavior, vomiting, hiccup) | Short term treatment of agitation and aggressiveness during an acute or chronic psychotic episode, vomiting along with antimitotic post‐radiotherapy treatment | Acute and chronic schizophrenia, psycho‐motorical agitation of psychotic genesis | Schizophrenia, other psychosis, short‐term adjunctive management if psychomotor agitation, violent or dangerous impulsive behavior | Resistant forms of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis' chronic psychosis High doses restrictions: syndrome of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis, chronic psychosis | Acute schizophrenic episode, mania, vomiting | |
| IV dosing in adults | 12 mg every 24 hours | The use is limited to adult patients and the drug can be administered IM or IV. The IV route is restricted to the treatment of vomiting. | 510 mg/day, daily max.: 30(100) mg | 210 mg initially, PRN every 48 hours, daily max. 18 mg | 510 mg initially, PRN every hour, daily max. 60 mg | 5 mg PRN every 30 minutes |
| IV dosing in geriatric care | 0.250.5 mg | Single dose of 0.51.5 mg, daily max. 5 mg | Half adult dose | Adjust to appropriate dose | 0.5 mg, than PRN | |
| Risk factors for the development of cardiac adverse events | QT prolonging drugs, diabetes, obesity, hypokalemia, congenital long QT syndrome | Bradycardia <55 beats per minute, hypokalemia, congenital QT prolongation, other medications provoking bradycardia, deceleration of the intra‐cardiac transition or prolonged QT interval | QT syndrome, hypokalemia, other electrolyte imbalance, cardiovascular diseases, QT prolongation in the family history | Cardiovalscular disease, drugs that can prolong the QTc, diabetes, obesity, hypokalemia, congenital long QT syndrome | Contraindications: recent cardiac infarction, uncompensated cardiac insufficiency, cardiac arrhythmias, antiarrhythmic drugs, pre‐existing QT prolongation, cases of arrhythmia or torsades de pointes in the family history, untreated potassium imbalance, QTc prolonging drugs | QT syndrome, hypokalemia, hypomagnesemia, other electrolyte imbalances, cardiovascular diseases, hypothyreosis, QT prolongation in the family history |
| Monitoring recommendations | Electrolytes | ECG monitoring at admission time, electrolytes | ECG monitoring, electrolytes | Metabolic parameters | ECG at baseline and regular ECG monitoring, electrolytes | Close ECG monitoring, electrolytes |
| General recommendations | Regular reevaluation in long‐term use | Apply the lowest effective dose | Apply the lowest effective dose | Application per mouth is the route of choice | Decrease dose if QTc >500 msec | Switch to PO as soon as possible |
Haloperidol is often preferred over other antipsychotics as a result of its effectiveness, low rate of anticholinergic side effects, familiarity with dosing and usage, and minimal respiratory or sedative properties.6 Use of the IV route in patients with acute delirium has several advantages over the IM or oral route,7 including rapid onset, immediate bioavailability, and ease and safety of administration.
Prior to September 2007, the package insert for haloperidol alerted healthcare professionals to the risk of cardiovascular side effects. Based on case reports of potentially fatal cardiac events, the FDA revised the label, warning that the QT prolongation (QTP) and risk of torsades de pointes (TdP) were increased with IV administration of haloperidol or administration of the drug at greater than recommended doses. Unfortunately, neither the typical dosing range nor the minimum dose associated with these cardiac side effects were specified in this recommendation.5
It is well‐established that haloperidol may prolong the QT interval by blocking the repolarizing potassium IKr current.8 Although drugs that block the IKr channel can produce arrhythmia in healthy individuals, additional risk factors, such as underlying heart conditions, electrolyte imbalances (ie, hypokalemia and hypomagnesemia), concomitant proarrhythmic drug use, and mechanical ventilation may increase this risk.9 Prolongation of the QT interval has been associated with subsequent malignant cardiac arrhythmias including ventricular fibrillation and TdP.10 Prolongation of the QT interval is considered the strongest risk factor for TdP, particularly with a baseline QTc > 450 msec.9
Based on the increased risk for QTP and TdP and the case reports of cardiac events, the FDA advisory recommended continuous electrocardiogram (ECG) monitoring in patients receiving IV haloperidol.5 However, such monitoring may be impractical and costly in hospitalized patients who require low doses of IV haloperidol to manage acute delirium and who are not in telemetry or intensive care units.
The aim of this review was to evaluate the case reports leading to the recent FDA warning for IV haloperidol, specifically focusing on the presence of risk factors for arrhythmias. Based upon the evidence, an additional aim was to provide an institutional response to this warning toward the optimal use of this agent.
Method
Two search pathways were used to evaluate reports of haloperidol‐associated TdP and/or QT prolongation:
Literature Review
We searched for published literature in humans indexed in Pubmed (1966April 2009), EMBASE (1972April 2009), and Scopus (1823April 2009) using the search terms haloperidol or Haldol combined with intravenous or infusion and at least one of the following terms: QT prolongation, TdP, torsades de pointes, torsades with a specific focus on case reports.
References from the retrieved articles were also reviewed to search for additional case reports.
In addition to cases reported in English journals, several of our reports originated from Japan11 (translation provided by the FDA), Spain12 and Germany13 (translated by the primary author).
Search of the FDA Database
We reviewed all adverse drug events reported through MedWatch or those submitted by the manufacturer from November 1997 to April 2008 through the Freedom of Information Act (FOIA) request. The FDA provided a full‐text summary of 5944 reports involving oral, intramuscular and IV use of haloperidol. The FDA data were transferred to a Microsoft Access database and screened for the key terms torsade, QT, prolongation, wave. Incident report number, date of report, age, gender, origin of report, medication name, role of drug as categorized by the FDA (suspect, concomitant, primary suspect, secondary suspect), route, dose, units, duration, symptoms and FDA outcome category (death, life‐threatening, hospitalization initial or prolonged, disability, congenital anomaly, required intervention to prevent permanent damage, other) were recorded. Only those reports in which IV haloperidol was considered by the reporter to be the primary causative agent for the adverse event were reviewed. Available information included diagnosis, laboratory parameters, QTc measurement, cardiac symptoms, outcomes and a description of recovery. No peer review was applied to the MedWatch reports and the data reported in this publication reflect the original information from the FDA MedWatch database. Baseline QTc was either the value defined as such in the original report or the lowest QTc reported. Haloperidol doses administered were defined as cumulative dose at event, encompassing all doses administered during the hospital stay until the occurrence of the adverse cardiac event.
The drugs listed in the case reports were assessed for proarrhythmic potential using 2 references: the individual package insert and the website of the Arizona Center for Education and Research on Therapeutics.14
The drugs were only considered proarrhythmic when the 2 resources were in agreement.
Duplicates and/or previously published cases, as well as reports involving adverse cardiac effects not associated with QTP or TdP, were identified and excluded.
In their advisory, the FDA does not state the exact origin of the reports, their specific search strategy to identify haloperidol‐associated adverse events, or the role IV haloperidol played in the individual events included in the extended warning. Consequently, the number of events identified in this review may differ from that published in the FDA extended warning.
Results
A total of 70 reported cases of IV haloperidol associated TdP and/or QTP were identified. Of these 70, 41 were identified through the PubMed/EMBASE/Scopus review, while an additional 29 cases were identified through the FDA database search.
Of the 29 cases in the FDA database, 21 were reported by health care professionals and 8 by manufacturers.
A total of 35 publications described cases originating from the US. Three cases took place in Japan and 1 case each in Canada, Germany and Spain. Several cases in the MedWatch database were reported outside the US: 1 case each originated from Austria, Canada, France, Japan, Spain, Switzerland and the United Kingdom. A summary of the published case reports is displayed in Table 2 and the FDA cases are summarized in Table 3.
| Case | Source (reference#) | Date | Age, Years | Gender | Drugs Pro‐arrhyth. | Venti‐ lated | Max. Daily Dose (mg) | Total Dose at Event (mg) | Time to Event | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | ECG Normalization, Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
| 1 | 35 | 1991 | 56 | m | No | Yes | 1200 | 1540 | NR | Yes | 584 (400) | 184 | NR | NR, uneventful |
| 2 | 13 | 1992 | 36 | m | Yes | No | 11.5 | 11.5 | 20 hours after start | Yes | 714 (428) | 286 | Yes | QTc normalization (440 msec), NR |
| 3 | 38 | 1993 | 39 | f | Yes | Yes | NR | 580 | Max. QTc 72 hours after start | Yes | 650 (420) | 230 | Yes | QTc normalization after 6 days, uneventful |
| 4 | 38 | 1993 | 19 | f | Yes | No | 170 | 170 | Max. QT 12 hours after start | Yes | 600 (480) | 120 | Yes | QTc normalization after 8 days, uneventful |
| 5 | 38 | 1993 | 63 | f | Yes | No | NR | 489 | Max. QT 48 hours after start | Yes | 670 (520) | 150 | Yes | QTc normalization after 8 days, uneventful |
| 6 | 38 | 1993 | 74 | f | Yes | Yes | NR | 10 | NR | No | 430 (410) | 20 | Yes | QTc unchanged after 8 days, uneventful |
| 7 | 17 | 1993 | 39 | m | Yes | Yes | NR | >490 | NR | Yes | 457 (348) | 109 | Yes | QTc normalization within 2 to 3 days, no further TdP, NR |
| 8 | 17 | 1993 | 61 | m | Yes | Yes | 115 | 211 | NR | Yes | 500 (390) | 110 | NR | QTc normalization within 2 days, death |
| 9 | 17 | 1993 | 48 | m | Yes | Yes | 825 | 825 | NR | Yes | 538 (441) | 97 | NR | QTc normalization in 3 days, rehabilitation |
| 10 | 39 | 1994 | 23 | f | Yes | Yes | 120 | 300 | 12 hours after dose increase | Yes | NR (550) | NR | Yes | NR, uneventful, extubation after 5 days, discharge after 10 days |
| 11 | 39 | 1994 | 28 | m | Yes | Yes | 300 | >300 | 24 hours after dose increase | Yes | NR (>520) | NR | Yes | No recurrence of arrhythmia, patient death (multi‐organ failure) |
| 12 | 40 | 1994 | 65 | m | Yes | NR | 230 | 410 | Worsening from day 2 to day 5 | Yes | 594 (490) | 104 | Yes | QTc normalization (406 msec), no cardiac problems at discharge |
| 13 | 40 | 1994 | 65 | f | Yes | NR | 500 | 980 | After the last 60mg | Yes | 628 (403) | 225 | Yes | QTc normalization (<400 msec), recurrence with oral haloperidol, rehabilitation |
| 14 | 40 | 1994 | 76 | f | Yes | NR | 21 | 26 | Day 2 after several boluses | Yes | 670 (450) | 220 | Yes | QTc normalization within several days (412 msec), rehabilitation |
| 15 | 41 | 1994 | 59 | m | NR | Yes | 865 | 1013 | NR | Yes | 640 (480) | 160 | NR | QTc normalization in 24 hours, survived |
| 16 | 16 | 1995 | 76 | f | Yes | No | NR | 44.5 plus 1 PO | 15 minutes | Yes | 670 (409) | 261 | Yes | ECG normalized the next morning, no further events |
| 17 | 16 | 1995 | 49 | m | Yes | No | NR | 1150 plus 20 IM | 45 minutes | Yes | 648 (380) | 268 | Yes | QTc normalization in 24 hours, anoxic brain insult/rehabilitation |
| 18 | 16 | 1995 | 65 | f | Yes | No | 600 | 965 | 30 minutes | Yes | 628 (403) | 225 | Yes | 3 more episodes of TdP in 3 hours, QTc normalization in 2 days, no recurrence with further haloperidol, NR |
| 19 | 42 | 1995 | 42 | m | Yes | No | 28 | 28 | 20 minutes | Yes | 610 (533) | 77 | Yes | QTc normalization in 5 days, uneventful, ECG normal |
| 20 | 42 | 1995 | 39 | m | Yes | No | 45 | 45 | 5 minutes | Yes | 654 (NR) | NR | Yes | QTc normalization after 24 hours, uneventful |
| 21 | 11 | 1997 | 56 | f | No | No | 10 | 10 | Shortly after | NR | NR (NR) | NR | Yes | TdP resolved after 8 hours, NR |
| 22 | 11 | 1997 | 82 | f | NR | No | 10 | 10 | Shortly after | Yes | 680 (NR) | NR | Yes | QTc normalization on day 6 after admission (470 msec), NR |
| 23 | 11 | 1997 | 35 | m | NR | No | NR | 90 | After 20 mg | Yes | 520 (NR) | NR | Yes | TdP disappeared 12 hours later, NR |
| 24 | 43,44 | 1998 | 45 | m | NR | Yes* | NR | 9 | 203 minutes | Yes | 638 (560) | 78 | Yes | NR, overall survival 100%, significantly prolonged hospital stay |
| 25 | 43,44 | 1998 | 64 | f | NR | NR | 115 | 220 minutes | Yes | 605 (424) | 181 | Yes | ||
| 26 | 43,44 | 1998 | 75 | f | NR | NR | 85 | 60 minutes | Yes | 567 (508) | 59 | Yes | ||
| 27 | 43,44 | 1998 | 71 | f | NR | NR | 55 | 120 minutes | Paced | Paced | Paced | Yes | ||
| 28 | 43,44 | 1998 | 58 | f | NR | NR | 75 | 38 minutes | Yes | 657 (542) | 115 | Yes | ||
| 29 | 43,44 | 1998 | 40 | m | NR | NR | 35 | 15 minutes | Yes | 679 (475) | 204 | Yes | ||
| 30 | 43,44 | 1998 | 71 | m | NR | NR | 70 | 58 minutes | Yes | 521 (478) | 43 | Yes | ||
| 31 | 43,44 | 1998 | 47 | m | NR | 400 | 400 | 79 minutes | Yes | 574 (444) | 130 | Yes | ||
| 32 | 21 | 1999 | 41 | f | Yes | Yes | 320 | 915 | 55 minutes | Yes | 610 (426) | 184 | Yes | QTc normalization after 5 day, uneventful |
| 33 | 21 | 1999 | 31 | m | Yes | Yes | 480 | 1700 | 40 minutes | Yes | 599 (491) | 108 | Yes | QTc normalized in 4 days, NR |
| 34 | 18 | 2000 | 64 | f | Yes | Yes | 175 | 175 | NR | No | 413 (418) | (‐5) | Yes | QTc remained unchanged, uneventful |
| 35 | 8 | 2000 | 75 | m | No | NR | >2 | >2 | NR | Yes | 615 (435) | 180 | No | QTc normalization in 48 hours, uneventful |
| 36 | 8 | 2000 | 68 | m | Yes | Yes | >2 | >2 | NR | Yes | 650 (407) | 243 | No | QTc normalization after 4 day, uneventful after extubation |
| 37 | 8 | 2000 | 77 | m | NR | NR | (4) | 2 | NR | Yes | 550 (393) | 157 | No | QTc normalization in 24 to 36 hours, NR |
| 38 | 12 | 2004 | 34 | m | Yes | NR | 24.5 | 24.5 | 20 minutes | Yes | 560 (420) | 140 | Yes | QTc normalization (440 msec), ECG normal |
| 39 | 23 | 2004 | 58 | f | Yes | NR | 340 | 1010 | NR | Yes | 533 (460) | 73 | Yes | QTc normalization 7 days later discharge after 27days |
| 40 | 45 | 2008 | 86 | f | Yes | No | 2 mg | 2 mg | 8 hours after last dose | Yes | 524 (NR) | Probably 79 | No | QTc normalization (445 msec), NR |
| 41 | 46 | 2009 | 74 | m | Yes | No | 2 | 2 | Shortly after | Yes | NR (579) | NR | Yes | Pre‐existing heart block and fibrillation resolved, nursing home/rehabilitation |
| Report | MedWatch Identifier | Report Date | Age, Years | Gender | Drugs Pro‐arrh. | Maximum Daily Dose (mg) | Total Dose at Event (mg) | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | Outcome; Recovery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
| 1 | 3122988‐1 | 1998 | 61 | m | No | 48 | 48 | Yes | NR | NR | Yes | Intervention; NR |
| 2 | 3157827‐6 | 1998 | 44 | f | No | 160 | 160 | Yes | 550 (440) | 110 | Yes | Intervention; uneventful |
| 3 | 3178715‐5 | 1999 | 60 | m | NR | 415 | 645 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization in 1 day, no recurrence |
| 4 | 3271261‐X | 1999 | 56 | m | NR | NR | 20 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization |
| 5 | 3271080‐4 | 1999 | 35 | m | Yes | 7 | 7 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced, hospitalization prolonged |
| 6 | 3325391‐4 | 1999 | 55 | f | Yes | 75 | 75 | NR | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 7 | 3381921‐8 | 1999 | 52 | m | No | 320 | 634 | Yes | 458 (430) | 28 | Yes | Death; NA |
| 8 | 3483869‐7 | 2000 | 18 | m | No | >200 | >310 | Yes | NR | NR | Yes | Intervention; no recurrence after haloperidol reinstitution |
| 9 | 3516342‐8 | 2000 | NR | NR | NR | NR | NR | NR | NR | NR | Yes | NR; NR |
| 10 | 3516320‐9 | 2000 | 34 | m | Yes | 5 | 5 | Yes | NR | NR | No | Life‐threatening; event abated after dose stopped |
| 11 | 3552263‐2 | 2000 | 46 | f | Yes | NR | 97.5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 12 | 3574705‐9 | 2000 | 78 | m | Yes | NR | 160 | Yes | 603 (453) | 50 | Yes | Intervention; event abated after dose stopped/reduced |
| 13 | 3703871‐7 | 2001 | 27 | m | NR | 530 | 530 | Yes | NR | NR | Yes | Death, NA |
| 14 | 3724567‐1 | 2001 | 31 | m | Yes | 6 | 6 | Yes | 496 (449) | 47 | No | Life‐threatening; ECG returned to baseline |
| 15 | 3851984‐1 | 2002 | 72 | f | NR | 18 | 18 | NR | NR | NR | Yes | Hospitalization; NR |
| 16 | 3942407‐2 | 2002 | 51 | m | Yes | 14 | 14 | Yes | 461 (444) | 17 | Yes | Life‐threatening; no recurrence |
| 17 | 4066580‐3 | 2003 | >60 | f | NR | 50 | 50 | Yes | >600 (480) | >120 | No | Hospitalization; QTc normalization, patient recovered |
| 18 | 4126280‐8 | 2003 | 47 | f | NR | 60 | 180 | Yes | 550 (450) | 100 | No (bradycardia) | Hospitalization; patient recovered |
| 19 | 4150700‐6 | 2003 | NR | m | NR | 5 | 5 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced |
| 20 | 4340092‐1 | 2004 | 52 | m | Yes | 5 | 5 | Yes | >500 (490) | >10 | NR (polymorphous VT) | Life‐threatening; NR |
| 21 | 4714692‐0 | 2005 | NR | m | NR | NR | NR | Yes | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 22 | 4881813‐9 | 2006 | NR | m | NR | NR | 40 | NR | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 23 | 4892225‐6 | 2006 | NR | f | Yes | 10 | >10 | Yes | 493 (300) | 193 | No | Hospitalization; QTc normalization (403 msec) |
| 24 | 4911873‐8 | 2006 | 69 | m | Yes | 6 | 6 | NR | NR | NR | Yes | Cardiac arrest, death; NA |
| 25 | 5366448‐6 | 2007 | 53 | m | Yes | NR | 35 | Yes | NR | NR | NR | Cardiac arrest, life‐threatening; patient recovered |
| 26 | 5563440‐3 | 2007 | 58 | m | Possible | 5 | 5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 27 | 5642929‐2 | 2008 | 42 | m | Yes | 165 | 165 | Yes | 640 (350) | 290 | Yes | Death; NA |
| 28 | 5697758‐0 | 2008 | 38 | m | Yes | NR | 620 | NR | NR | NR | Yes | Hospitalization; patient recovered |
| 29 | 5254840‐X | 2008 | 19 | f | Possible | 15 | 25 | Yes | 461 | NR | NR | Cardiac arrest, hospitalization; patient recovered |
Of the 70 cases, 54 cases of TdP were reported. The remaining 16 of 70 cases involved cases of QTP, 9 of which did not progress to TdP and 7 of which the progression to TdP was unclear. Of note, 42 of 54 of the cases of TdP were reported as preceded by documented QTP. Presence of QTP was unknown in the other 12 original reports. Three out of 70 patients experienced sudden cardiac arrest, 1 of which was fatal. One arrest was preceded by TdP and 2 by QTP (Figure 1).

The patient ages ranged from 18 years to 86 years. Of note, 17 patients experiencing TdP and/or QTP were <40 years old, and 2 of those patients were <30 years old.
Haloperidol‐associated QTP and/or TdP were observed in 27 female and 42 male patients; the gender was not stated in one report. Of the 54 patients experiencing TdP (with or without report of previous QTP), 22 were female and 31 were male (1 gender unknown).
A total of 68 of 70 patients were determined to have associated risk factors15 for QTP/TdP (see Table 4). The circumstances of the remaining 2 patients were not described in sufficient detail to identify associated risk factors.
| Risk Factor | Patients, n (%) |
|---|---|
| |
| Any risk factor | 68/70 (97) |
| Unknown | 2/70 (3) |
| Specific risk factors | |
| Electrolyte imbalance | 27/68 (40) |
| Underlying cardiac disease | 32/68 (47) |
| Concomitant proarrhythmic agents | 39/68 (57) |
| Other drugs influencing cardiac function | 23/68 (34) |
| Baseline QTc >450 msec | 18/68 (26) |
| QTc known: 44 patients | 18/44 (41) |
Overall, 32 patients had underlying heart conditions. Electrolyte imbalances, including hypokalemia, hypomagnesemia, and hypocalcemia, were present in 17 patients. At least 39 patients were receiving potentially proarrhythmic agents (1‐8 proarrhythmic drugs per patient) in addition to IV haloperidol. At least 23 patients were receiving additional drugs with a potential for other cardiac adverse events than QTP and TdP.
A wide range of other disease states previously reported to be associated with QTP15 were identified in these patients: asthma (5 patients), diabetes (5 patients), obesity (3 patients), impaired renal and/or liver function (3 patients each), human immunodeficiency virus (HIV) (2 patients); chronic obstructive pulmonary disease (COPD), pancreatitis and hypothyroidism (1 patient each). A total of 22 patients had a history of substance abuse (alcohol and/or drugs), and 4 patients were smokers.
The administered doses of IV haloperidol varied widely. Considering that information regarding the maximal daily dose was missing in 22 reports and ambiguous in another 20 cases, the results have been presented using cumulative IV haloperidol doses. Patients experiencing TdP without preceding QTP received a cumulative dose (= total dose at event) ranging from 5 mg to 645 mg. Patients with both confirmed QTP and TdP were administered a cumulative dose of 2 mg to 1700 mg. Patients who experienced QTP without TdP received a cumulative dose of 2 mg to 1540 mg of IV haloperidol.
Sudden cardiac arrest following administration of IV haloperidol was observed in cumulative doses ranging from 6 mg to 35 mg. The cardiac arrest leading to a fatal outcome was preceded by an administration of at least 6 mg of IV haloperidol. Overall, 14 out of 70 patients received cumulative doses of 10 mg IV haloperidol.
The time from administration to documentation of QTP and/or TdP ranged from immediately post administration to 8 hours after administration of the last dose of IV haloperidol.
Baseline QTc was known in 44 patients. Baseline QTc was >450 msec in 18 of these 44 patients.
The change from baseline QTc varied widely from 20 msec to 286 msec; 36 patients demonstrated a prolongation of >50 msec.
In those patients with reported haloperidol‐associated QTP, 25 patients demonstrated a QTc >600 msec and 38 patients >520 msec.9 Of the cases with known specific QTc values, the QTc was prolonged >450 msec in 48 out of 50 cases. The lowest reported QTc leading to TdP was 413 msec.
A total of 20 patients were reported as having a normalization of QTc (as defined by the original reports) within several hours to 8 days; minimal QTP was reported as persisting in 2 patients. The specifics of the other patients were unknown, although 25 patients were categorized as recovered, 13 were stated as having an uneventful remainder of hospitalization, and 5 patients were discharged to a rehabilitation facility or a nursing home.
Discussion
The current review was performed in response to the FDA warning recommending the use of continuous ECG monitoring associated with the administration of intravenous haloperiodol.5 This warning has resulted in substantial dilemmas for health care organizations, additional resource allocation, and increased scrutiny from regulatory agencies. The results of our review reveal that intravenous haloperidol‐associated QTP and TdP almost uniformly take place in patients with concomitant risk factors and with cumulative doses 2 mg. In light of these findings, it is possible that hospitals may be able to administer intravenous haloperidol in patients without risk factors without continuous ECG monitoring. In reviewing these published reports, it is important to note that the FDA identified 28 published cases of haloperidol‐associated QTP and TdP, while our review yielded a total of 41 published case reports.
The FDA database included 73 cases of haloperidol‐associated TdP in their database. However, these cases included both oral as well as IV administration; using our methodology, we identified 29 additional case reports associated with intravenous haloperidol from this database. Consequently, our review included 41 published case reports and 29 FDA database cases, resulting in the total of 70 patients.
Our review revealed a number of practical findings. First, our summary demonstrated that neither QTP nor TdP has been documented with a cumulative dose of IV haloperidol of <2 mg. The majority of patients (80%) received cumulative IV doses 10 mg. The lowest dose associated with sudden cardiac arrest was 6 mg and this took place in a 69‐year‐old male patient. Second, the majority (97%) of our patients had additional risk factors for QTP and/or TdP. Pre‐existing heart disease,1619 electrolyte imbalance,17, 1921 concomitant proarrhythmic drugs16, 17, 1922 and mechanical ventilation17, 23 were identified as the most commonly observed risk factors (Table 4). Lastly, in those cases in which the data were reported, baseline QTc was >450 msec in 41% of the patients, and 96% had a QTc at the time of the event >450 msec. Therefore, we conclude that patients: (1) receiving low cumulative doses (<2 mg) with (2) no risk factors for prolonged QTc or TdP, and (3) with a normal QTc on baseline EKG can safely be given IV haloperidol in the hospital setting.
This dosage range is consistent with the labelling for IV haloperidol dosing in Canada24 and Germany25 (Table 1), where single doses of 0.25 mg to 1.5 mg are recommended for the treatment of delirium or acute agitation in the geriatric population.24, 25
In a recent Cochrane review, low‐dose IV haloperidol (<3 mg per day) was concluded to be as safe and effective as atypical antipsychotics in the treatment of acute delirium with respect to extrapyramidal adverse effects.2
The American Psychiatric Association recommends an initial IV dose of 12 mg every 24 hours as needed (0.250.50 mg every 4 hours as needed for elderly patients), with titration to higher doses for patients who continue to be agitated for the treatment of patients with delirium (issued 1999, updated 2004).3
While several expert‐groups and investigators currently consider IV haloperidol as an important therapeutic option for treating acute delirium and agitation in the dose range presented above, less consensus exists regarding monitoring requirements.2, 3, 26, 27
The American Psychiatric Association recommends IV haloperidol only after a baseline ECG is obtained. These guidelines have not been updated since the release of the FDA extended warning.3 In their recent review, Morandi et al.28 support the dosage recommendation of the 1999 American Psychiatric Association's practice guidelines for treatment of delirium,3 ie, administration of IV haloperidol in single doses of 0.5 mg to 2 mg in elderly patients, however, only after a baseline ECG is obtained.28 While the package insert of IV haloperidol in France29 recommends a baseline ECG, Germany,25 Italy30 and Switzerland's31 package information states the need for regular ECG monitoring. Guidelines for the treatment of delirium in the intensive care unit published by the American College of Critical Care Medicine and the Society of Critical Care Medicine in collaboration with the American Society of Health‐System Pharmacists consider IV haloperidol as the preferred agent for the treatment of delirium in critically ill patients (grade of recommendation = C). These expert groups recommend that patients should be monitored for electrocardiographic changes (QT interval prolongation and arrhythmias) when receiving haloperidol (Grade of recommendation = B).32
Nevertheless, continuous ECG monitoring (ie, telemetry) is expensive, labor‐intensive and, potentially overutilized.33, 34 Requiring clinicians to place all patients receiving intravenous haloperidol on telemetry is impractical and potentially costly. Mandating telemetry could also lead to unintended harm, ie, use of a less effective or less safe drug to avoid compliance with the telemetry mandate.
Based on our findings and the current recommendations in the literature, inpatient providers should be thoughtful and deliberate in the use of haloperidol to treat acute delirium with agitation. Patients requiring pharmacologic management of their delirium should be screened for risk factors for QTP and TdP (Table 4) and a baseline ECG should be obtained prior to haloperidol administration. If significant risk factors exist or the baseline ECG reveals a prolonged QTc, then the patient should receive continuous ECG monitoring. Similarly, if cumulative doses of 2 mg are needed, the patient should be placed on telemetry.
There are some limitations to our study design. Our findings are based upon previously published case reports or data submitted to the FDA MedWatch. While the content of the FDA's MedWatch database is accessible to the public via the Freedom of Information Act (FOIA), the events are neither categorized nor peer‐reviewed upon entry into the database. Consequently, information may be incomplete or inaccurate. In addition, the denominator representing the overall use of IV haloperidol is unknown, thus a rate of event cannot be assigned and the true scope of the problem cannot be determined. Despite these limitations, this summary represents the most comprehensive review of the literature to date, expanding on the analysis performed by the FDA. Of note, in our review of the FDA database, we noted several cases of haloperidol‐associated QTP or TdP associated with other routes of administration. Thus, it is unknown whether this complication is any greater with IV vs. the IM or per os (PO) routes of administration.
Conclusion
Although the proarrhythmic potential of haloperidol and other antipsychotics has been well established in the literature, IV haloperidol has been considered relatively safe with respect to this complication from the time of its approval in 1967.5, 1722, 35, 36 In reviewing all reported cases of cardiac complications associated with IV haloperidol, as well as the current literature, an association with QTP and TdP is likely. However, the case reports reveal that QTP and TdP generally occur in the setting of concomitant risk factors, and no cases have been reported utilizing a cumulative IV dose of <2 mg. It may therefore be safe to administer a cumulative dose of IV haloperidol of <2 mg without ECG monitoring in patients without risk factors for QTP. However, ECG monitoring should take place with IV haloperidol doses 2 mg and/or in those patients with additional risk factors of developing QTP and/or TdP.
Based on the findings of this review complemented by the guidelines of various expert‐groups and the official labelling information of different countries, the Pharmacy & Therapeutics Committee of the UCSF Medical Center revised the IV haloperidol policy: administration of a total dose of <2 mg IV haloperidol without concurrent telemetry is allowed in a noncritical care setting in patients without risk factors for QTP and TdP.
Acknowledgements
The authors acknowledge Gloria Won of the Fishbon Library at UCSF Medical Center at Mount Zion for her support.
Haloperidol is Food and Drug Administration (FDA)‐approved in the United States for the management of acute and chronic psychotic disorders and widely used in the management of delirium‐associated agitation in hospitalized patients.1 Delirium in the hospital is an acute confusional state that frequently arises from multiple complex factors and may affect up to 30% of hospitalized patients.2 Although the first step in the management of delirium involves identification and treatment of underlying causes and offering supportive behavioral care; medications may be needed to control severe agitation.2 Low dose intravenous (IV) haloperidol (ie, 0.250.5 mg every 4 hours) is a commonly used medication in this setting as recommended by expert‐groups including the Cochrane Collaboration and the American Psychiatric Association.2, 3
Although injectable haloperidol, a butyrophenone‐derived antipsychotic agent pharmacologically related to the piperazine phenothiazines,4 is approved for IV use in many countries (Table 1), parenteral use is approved only for intramuscular (IM) administration in the US. Thus, IV administration of the drug in the US is considered an off‐label use.5
| Indication | Country | |||||
|---|---|---|---|---|---|---|
| Canada24 | France29 | Germany25 | Great Britain37 | Italy30 | Switzerland31 | |
| ||||||
| Mainly delirium (schizophrenia, other psychosis, short‐term management of psychomotor agitation, excitement, violent or dangerously impulsive behavior, vomiting, hiccup) | Short term treatment of agitation and aggressiveness during an acute or chronic psychotic episode, vomiting along with antimitotic post‐radiotherapy treatment | Acute and chronic schizophrenia, psycho‐motorical agitation of psychotic genesis | Schizophrenia, other psychosis, short‐term adjunctive management if psychomotor agitation, violent or dangerous impulsive behavior | Resistant forms of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis' chronic psychosis High doses restrictions: syndrome of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis, chronic psychosis | Acute schizophrenic episode, mania, vomiting | |
| IV dosing in adults | 12 mg every 24 hours | The use is limited to adult patients and the drug can be administered IM or IV. The IV route is restricted to the treatment of vomiting. | 510 mg/day, daily max.: 30(100) mg | 210 mg initially, PRN every 48 hours, daily max. 18 mg | 510 mg initially, PRN every hour, daily max. 60 mg | 5 mg PRN every 30 minutes |
| IV dosing in geriatric care | 0.250.5 mg | Single dose of 0.51.5 mg, daily max. 5 mg | Half adult dose | Adjust to appropriate dose | 0.5 mg, than PRN | |
| Risk factors for the development of cardiac adverse events | QT prolonging drugs, diabetes, obesity, hypokalemia, congenital long QT syndrome | Bradycardia <55 beats per minute, hypokalemia, congenital QT prolongation, other medications provoking bradycardia, deceleration of the intra‐cardiac transition or prolonged QT interval | QT syndrome, hypokalemia, other electrolyte imbalance, cardiovascular diseases, QT prolongation in the family history | Cardiovalscular disease, drugs that can prolong the QTc, diabetes, obesity, hypokalemia, congenital long QT syndrome | Contraindications: recent cardiac infarction, uncompensated cardiac insufficiency, cardiac arrhythmias, antiarrhythmic drugs, pre‐existing QT prolongation, cases of arrhythmia or torsades de pointes in the family history, untreated potassium imbalance, QTc prolonging drugs | QT syndrome, hypokalemia, hypomagnesemia, other electrolyte imbalances, cardiovascular diseases, hypothyreosis, QT prolongation in the family history |
| Monitoring recommendations | Electrolytes | ECG monitoring at admission time, electrolytes | ECG monitoring, electrolytes | Metabolic parameters | ECG at baseline and regular ECG monitoring, electrolytes | Close ECG monitoring, electrolytes |
| General recommendations | Regular reevaluation in long‐term use | Apply the lowest effective dose | Apply the lowest effective dose | Application per mouth is the route of choice | Decrease dose if QTc >500 msec | Switch to PO as soon as possible |
Haloperidol is often preferred over other antipsychotics as a result of its effectiveness, low rate of anticholinergic side effects, familiarity with dosing and usage, and minimal respiratory or sedative properties.6 Use of the IV route in patients with acute delirium has several advantages over the IM or oral route,7 including rapid onset, immediate bioavailability, and ease and safety of administration.
Prior to September 2007, the package insert for haloperidol alerted healthcare professionals to the risk of cardiovascular side effects. Based on case reports of potentially fatal cardiac events, the FDA revised the label, warning that the QT prolongation (QTP) and risk of torsades de pointes (TdP) were increased with IV administration of haloperidol or administration of the drug at greater than recommended doses. Unfortunately, neither the typical dosing range nor the minimum dose associated with these cardiac side effects were specified in this recommendation.5
It is well‐established that haloperidol may prolong the QT interval by blocking the repolarizing potassium IKr current.8 Although drugs that block the IKr channel can produce arrhythmia in healthy individuals, additional risk factors, such as underlying heart conditions, electrolyte imbalances (ie, hypokalemia and hypomagnesemia), concomitant proarrhythmic drug use, and mechanical ventilation may increase this risk.9 Prolongation of the QT interval has been associated with subsequent malignant cardiac arrhythmias including ventricular fibrillation and TdP.10 Prolongation of the QT interval is considered the strongest risk factor for TdP, particularly with a baseline QTc > 450 msec.9
Based on the increased risk for QTP and TdP and the case reports of cardiac events, the FDA advisory recommended continuous electrocardiogram (ECG) monitoring in patients receiving IV haloperidol.5 However, such monitoring may be impractical and costly in hospitalized patients who require low doses of IV haloperidol to manage acute delirium and who are not in telemetry or intensive care units.
The aim of this review was to evaluate the case reports leading to the recent FDA warning for IV haloperidol, specifically focusing on the presence of risk factors for arrhythmias. Based upon the evidence, an additional aim was to provide an institutional response to this warning toward the optimal use of this agent.
Method
Two search pathways were used to evaluate reports of haloperidol‐associated TdP and/or QT prolongation:
Literature Review
We searched for published literature in humans indexed in Pubmed (1966April 2009), EMBASE (1972April 2009), and Scopus (1823April 2009) using the search terms haloperidol or Haldol combined with intravenous or infusion and at least one of the following terms: QT prolongation, TdP, torsades de pointes, torsades with a specific focus on case reports.
References from the retrieved articles were also reviewed to search for additional case reports.
In addition to cases reported in English journals, several of our reports originated from Japan11 (translation provided by the FDA), Spain12 and Germany13 (translated by the primary author).
Search of the FDA Database
We reviewed all adverse drug events reported through MedWatch or those submitted by the manufacturer from November 1997 to April 2008 through the Freedom of Information Act (FOIA) request. The FDA provided a full‐text summary of 5944 reports involving oral, intramuscular and IV use of haloperidol. The FDA data were transferred to a Microsoft Access database and screened for the key terms torsade, QT, prolongation, wave. Incident report number, date of report, age, gender, origin of report, medication name, role of drug as categorized by the FDA (suspect, concomitant, primary suspect, secondary suspect), route, dose, units, duration, symptoms and FDA outcome category (death, life‐threatening, hospitalization initial or prolonged, disability, congenital anomaly, required intervention to prevent permanent damage, other) were recorded. Only those reports in which IV haloperidol was considered by the reporter to be the primary causative agent for the adverse event were reviewed. Available information included diagnosis, laboratory parameters, QTc measurement, cardiac symptoms, outcomes and a description of recovery. No peer review was applied to the MedWatch reports and the data reported in this publication reflect the original information from the FDA MedWatch database. Baseline QTc was either the value defined as such in the original report or the lowest QTc reported. Haloperidol doses administered were defined as cumulative dose at event, encompassing all doses administered during the hospital stay until the occurrence of the adverse cardiac event.
The drugs listed in the case reports were assessed for proarrhythmic potential using 2 references: the individual package insert and the website of the Arizona Center for Education and Research on Therapeutics.14
The drugs were only considered proarrhythmic when the 2 resources were in agreement.
Duplicates and/or previously published cases, as well as reports involving adverse cardiac effects not associated with QTP or TdP, were identified and excluded.
In their advisory, the FDA does not state the exact origin of the reports, their specific search strategy to identify haloperidol‐associated adverse events, or the role IV haloperidol played in the individual events included in the extended warning. Consequently, the number of events identified in this review may differ from that published in the FDA extended warning.
Results
A total of 70 reported cases of IV haloperidol associated TdP and/or QTP were identified. Of these 70, 41 were identified through the PubMed/EMBASE/Scopus review, while an additional 29 cases were identified through the FDA database search.
Of the 29 cases in the FDA database, 21 were reported by health care professionals and 8 by manufacturers.
A total of 35 publications described cases originating from the US. Three cases took place in Japan and 1 case each in Canada, Germany and Spain. Several cases in the MedWatch database were reported outside the US: 1 case each originated from Austria, Canada, France, Japan, Spain, Switzerland and the United Kingdom. A summary of the published case reports is displayed in Table 2 and the FDA cases are summarized in Table 3.
| Case | Source (reference#) | Date | Age, Years | Gender | Drugs Pro‐arrhyth. | Venti‐ lated | Max. Daily Dose (mg) | Total Dose at Event (mg) | Time to Event | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | ECG Normalization, Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
| 1 | 35 | 1991 | 56 | m | No | Yes | 1200 | 1540 | NR | Yes | 584 (400) | 184 | NR | NR, uneventful |
| 2 | 13 | 1992 | 36 | m | Yes | No | 11.5 | 11.5 | 20 hours after start | Yes | 714 (428) | 286 | Yes | QTc normalization (440 msec), NR |
| 3 | 38 | 1993 | 39 | f | Yes | Yes | NR | 580 | Max. QTc 72 hours after start | Yes | 650 (420) | 230 | Yes | QTc normalization after 6 days, uneventful |
| 4 | 38 | 1993 | 19 | f | Yes | No | 170 | 170 | Max. QT 12 hours after start | Yes | 600 (480) | 120 | Yes | QTc normalization after 8 days, uneventful |
| 5 | 38 | 1993 | 63 | f | Yes | No | NR | 489 | Max. QT 48 hours after start | Yes | 670 (520) | 150 | Yes | QTc normalization after 8 days, uneventful |
| 6 | 38 | 1993 | 74 | f | Yes | Yes | NR | 10 | NR | No | 430 (410) | 20 | Yes | QTc unchanged after 8 days, uneventful |
| 7 | 17 | 1993 | 39 | m | Yes | Yes | NR | >490 | NR | Yes | 457 (348) | 109 | Yes | QTc normalization within 2 to 3 days, no further TdP, NR |
| 8 | 17 | 1993 | 61 | m | Yes | Yes | 115 | 211 | NR | Yes | 500 (390) | 110 | NR | QTc normalization within 2 days, death |
| 9 | 17 | 1993 | 48 | m | Yes | Yes | 825 | 825 | NR | Yes | 538 (441) | 97 | NR | QTc normalization in 3 days, rehabilitation |
| 10 | 39 | 1994 | 23 | f | Yes | Yes | 120 | 300 | 12 hours after dose increase | Yes | NR (550) | NR | Yes | NR, uneventful, extubation after 5 days, discharge after 10 days |
| 11 | 39 | 1994 | 28 | m | Yes | Yes | 300 | >300 | 24 hours after dose increase | Yes | NR (>520) | NR | Yes | No recurrence of arrhythmia, patient death (multi‐organ failure) |
| 12 | 40 | 1994 | 65 | m | Yes | NR | 230 | 410 | Worsening from day 2 to day 5 | Yes | 594 (490) | 104 | Yes | QTc normalization (406 msec), no cardiac problems at discharge |
| 13 | 40 | 1994 | 65 | f | Yes | NR | 500 | 980 | After the last 60mg | Yes | 628 (403) | 225 | Yes | QTc normalization (<400 msec), recurrence with oral haloperidol, rehabilitation |
| 14 | 40 | 1994 | 76 | f | Yes | NR | 21 | 26 | Day 2 after several boluses | Yes | 670 (450) | 220 | Yes | QTc normalization within several days (412 msec), rehabilitation |
| 15 | 41 | 1994 | 59 | m | NR | Yes | 865 | 1013 | NR | Yes | 640 (480) | 160 | NR | QTc normalization in 24 hours, survived |
| 16 | 16 | 1995 | 76 | f | Yes | No | NR | 44.5 plus 1 PO | 15 minutes | Yes | 670 (409) | 261 | Yes | ECG normalized the next morning, no further events |
| 17 | 16 | 1995 | 49 | m | Yes | No | NR | 1150 plus 20 IM | 45 minutes | Yes | 648 (380) | 268 | Yes | QTc normalization in 24 hours, anoxic brain insult/rehabilitation |
| 18 | 16 | 1995 | 65 | f | Yes | No | 600 | 965 | 30 minutes | Yes | 628 (403) | 225 | Yes | 3 more episodes of TdP in 3 hours, QTc normalization in 2 days, no recurrence with further haloperidol, NR |
| 19 | 42 | 1995 | 42 | m | Yes | No | 28 | 28 | 20 minutes | Yes | 610 (533) | 77 | Yes | QTc normalization in 5 days, uneventful, ECG normal |
| 20 | 42 | 1995 | 39 | m | Yes | No | 45 | 45 | 5 minutes | Yes | 654 (NR) | NR | Yes | QTc normalization after 24 hours, uneventful |
| 21 | 11 | 1997 | 56 | f | No | No | 10 | 10 | Shortly after | NR | NR (NR) | NR | Yes | TdP resolved after 8 hours, NR |
| 22 | 11 | 1997 | 82 | f | NR | No | 10 | 10 | Shortly after | Yes | 680 (NR) | NR | Yes | QTc normalization on day 6 after admission (470 msec), NR |
| 23 | 11 | 1997 | 35 | m | NR | No | NR | 90 | After 20 mg | Yes | 520 (NR) | NR | Yes | TdP disappeared 12 hours later, NR |
| 24 | 43,44 | 1998 | 45 | m | NR | Yes* | NR | 9 | 203 minutes | Yes | 638 (560) | 78 | Yes | NR, overall survival 100%, significantly prolonged hospital stay |
| 25 | 43,44 | 1998 | 64 | f | NR | NR | 115 | 220 minutes | Yes | 605 (424) | 181 | Yes | ||
| 26 | 43,44 | 1998 | 75 | f | NR | NR | 85 | 60 minutes | Yes | 567 (508) | 59 | Yes | ||
| 27 | 43,44 | 1998 | 71 | f | NR | NR | 55 | 120 minutes | Paced | Paced | Paced | Yes | ||
| 28 | 43,44 | 1998 | 58 | f | NR | NR | 75 | 38 minutes | Yes | 657 (542) | 115 | Yes | ||
| 29 | 43,44 | 1998 | 40 | m | NR | NR | 35 | 15 minutes | Yes | 679 (475) | 204 | Yes | ||
| 30 | 43,44 | 1998 | 71 | m | NR | NR | 70 | 58 minutes | Yes | 521 (478) | 43 | Yes | ||
| 31 | 43,44 | 1998 | 47 | m | NR | 400 | 400 | 79 minutes | Yes | 574 (444) | 130 | Yes | ||
| 32 | 21 | 1999 | 41 | f | Yes | Yes | 320 | 915 | 55 minutes | Yes | 610 (426) | 184 | Yes | QTc normalization after 5 day, uneventful |
| 33 | 21 | 1999 | 31 | m | Yes | Yes | 480 | 1700 | 40 minutes | Yes | 599 (491) | 108 | Yes | QTc normalized in 4 days, NR |
| 34 | 18 | 2000 | 64 | f | Yes | Yes | 175 | 175 | NR | No | 413 (418) | (‐5) | Yes | QTc remained unchanged, uneventful |
| 35 | 8 | 2000 | 75 | m | No | NR | >2 | >2 | NR | Yes | 615 (435) | 180 | No | QTc normalization in 48 hours, uneventful |
| 36 | 8 | 2000 | 68 | m | Yes | Yes | >2 | >2 | NR | Yes | 650 (407) | 243 | No | QTc normalization after 4 day, uneventful after extubation |
| 37 | 8 | 2000 | 77 | m | NR | NR | (4) | 2 | NR | Yes | 550 (393) | 157 | No | QTc normalization in 24 to 36 hours, NR |
| 38 | 12 | 2004 | 34 | m | Yes | NR | 24.5 | 24.5 | 20 minutes | Yes | 560 (420) | 140 | Yes | QTc normalization (440 msec), ECG normal |
| 39 | 23 | 2004 | 58 | f | Yes | NR | 340 | 1010 | NR | Yes | 533 (460) | 73 | Yes | QTc normalization 7 days later discharge after 27days |
| 40 | 45 | 2008 | 86 | f | Yes | No | 2 mg | 2 mg | 8 hours after last dose | Yes | 524 (NR) | Probably 79 | No | QTc normalization (445 msec), NR |
| 41 | 46 | 2009 | 74 | m | Yes | No | 2 | 2 | Shortly after | Yes | NR (579) | NR | Yes | Pre‐existing heart block and fibrillation resolved, nursing home/rehabilitation |
| Report | MedWatch Identifier | Report Date | Age, Years | Gender | Drugs Pro‐arrh. | Maximum Daily Dose (mg) | Total Dose at Event (mg) | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | Outcome; Recovery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
| 1 | 3122988‐1 | 1998 | 61 | m | No | 48 | 48 | Yes | NR | NR | Yes | Intervention; NR |
| 2 | 3157827‐6 | 1998 | 44 | f | No | 160 | 160 | Yes | 550 (440) | 110 | Yes | Intervention; uneventful |
| 3 | 3178715‐5 | 1999 | 60 | m | NR | 415 | 645 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization in 1 day, no recurrence |
| 4 | 3271261‐X | 1999 | 56 | m | NR | NR | 20 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization |
| 5 | 3271080‐4 | 1999 | 35 | m | Yes | 7 | 7 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced, hospitalization prolonged |
| 6 | 3325391‐4 | 1999 | 55 | f | Yes | 75 | 75 | NR | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 7 | 3381921‐8 | 1999 | 52 | m | No | 320 | 634 | Yes | 458 (430) | 28 | Yes | Death; NA |
| 8 | 3483869‐7 | 2000 | 18 | m | No | >200 | >310 | Yes | NR | NR | Yes | Intervention; no recurrence after haloperidol reinstitution |
| 9 | 3516342‐8 | 2000 | NR | NR | NR | NR | NR | NR | NR | NR | Yes | NR; NR |
| 10 | 3516320‐9 | 2000 | 34 | m | Yes | 5 | 5 | Yes | NR | NR | No | Life‐threatening; event abated after dose stopped |
| 11 | 3552263‐2 | 2000 | 46 | f | Yes | NR | 97.5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 12 | 3574705‐9 | 2000 | 78 | m | Yes | NR | 160 | Yes | 603 (453) | 50 | Yes | Intervention; event abated after dose stopped/reduced |
| 13 | 3703871‐7 | 2001 | 27 | m | NR | 530 | 530 | Yes | NR | NR | Yes | Death, NA |
| 14 | 3724567‐1 | 2001 | 31 | m | Yes | 6 | 6 | Yes | 496 (449) | 47 | No | Life‐threatening; ECG returned to baseline |
| 15 | 3851984‐1 | 2002 | 72 | f | NR | 18 | 18 | NR | NR | NR | Yes | Hospitalization; NR |
| 16 | 3942407‐2 | 2002 | 51 | m | Yes | 14 | 14 | Yes | 461 (444) | 17 | Yes | Life‐threatening; no recurrence |
| 17 | 4066580‐3 | 2003 | >60 | f | NR | 50 | 50 | Yes | >600 (480) | >120 | No | Hospitalization; QTc normalization, patient recovered |
| 18 | 4126280‐8 | 2003 | 47 | f | NR | 60 | 180 | Yes | 550 (450) | 100 | No (bradycardia) | Hospitalization; patient recovered |
| 19 | 4150700‐6 | 2003 | NR | m | NR | 5 | 5 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced |
| 20 | 4340092‐1 | 2004 | 52 | m | Yes | 5 | 5 | Yes | >500 (490) | >10 | NR (polymorphous VT) | Life‐threatening; NR |
| 21 | 4714692‐0 | 2005 | NR | m | NR | NR | NR | Yes | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 22 | 4881813‐9 | 2006 | NR | m | NR | NR | 40 | NR | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 23 | 4892225‐6 | 2006 | NR | f | Yes | 10 | >10 | Yes | 493 (300) | 193 | No | Hospitalization; QTc normalization (403 msec) |
| 24 | 4911873‐8 | 2006 | 69 | m | Yes | 6 | 6 | NR | NR | NR | Yes | Cardiac arrest, death; NA |
| 25 | 5366448‐6 | 2007 | 53 | m | Yes | NR | 35 | Yes | NR | NR | NR | Cardiac arrest, life‐threatening; patient recovered |
| 26 | 5563440‐3 | 2007 | 58 | m | Possible | 5 | 5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 27 | 5642929‐2 | 2008 | 42 | m | Yes | 165 | 165 | Yes | 640 (350) | 290 | Yes | Death; NA |
| 28 | 5697758‐0 | 2008 | 38 | m | Yes | NR | 620 | NR | NR | NR | Yes | Hospitalization; patient recovered |
| 29 | 5254840‐X | 2008 | 19 | f | Possible | 15 | 25 | Yes | 461 | NR | NR | Cardiac arrest, hospitalization; patient recovered |
Of the 70 cases, 54 cases of TdP were reported. The remaining 16 of 70 cases involved cases of QTP, 9 of which did not progress to TdP and 7 of which the progression to TdP was unclear. Of note, 42 of 54 of the cases of TdP were reported as preceded by documented QTP. Presence of QTP was unknown in the other 12 original reports. Three out of 70 patients experienced sudden cardiac arrest, 1 of which was fatal. One arrest was preceded by TdP and 2 by QTP (Figure 1).

The patient ages ranged from 18 years to 86 years. Of note, 17 patients experiencing TdP and/or QTP were <40 years old, and 2 of those patients were <30 years old.
Haloperidol‐associated QTP and/or TdP were observed in 27 female and 42 male patients; the gender was not stated in one report. Of the 54 patients experiencing TdP (with or without report of previous QTP), 22 were female and 31 were male (1 gender unknown).
A total of 68 of 70 patients were determined to have associated risk factors15 for QTP/TdP (see Table 4). The circumstances of the remaining 2 patients were not described in sufficient detail to identify associated risk factors.
| Risk Factor | Patients, n (%) |
|---|---|
| |
| Any risk factor | 68/70 (97) |
| Unknown | 2/70 (3) |
| Specific risk factors | |
| Electrolyte imbalance | 27/68 (40) |
| Underlying cardiac disease | 32/68 (47) |
| Concomitant proarrhythmic agents | 39/68 (57) |
| Other drugs influencing cardiac function | 23/68 (34) |
| Baseline QTc >450 msec | 18/68 (26) |
| QTc known: 44 patients | 18/44 (41) |
Overall, 32 patients had underlying heart conditions. Electrolyte imbalances, including hypokalemia, hypomagnesemia, and hypocalcemia, were present in 17 patients. At least 39 patients were receiving potentially proarrhythmic agents (1‐8 proarrhythmic drugs per patient) in addition to IV haloperidol. At least 23 patients were receiving additional drugs with a potential for other cardiac adverse events than QTP and TdP.
A wide range of other disease states previously reported to be associated with QTP15 were identified in these patients: asthma (5 patients), diabetes (5 patients), obesity (3 patients), impaired renal and/or liver function (3 patients each), human immunodeficiency virus (HIV) (2 patients); chronic obstructive pulmonary disease (COPD), pancreatitis and hypothyroidism (1 patient each). A total of 22 patients had a history of substance abuse (alcohol and/or drugs), and 4 patients were smokers.
The administered doses of IV haloperidol varied widely. Considering that information regarding the maximal daily dose was missing in 22 reports and ambiguous in another 20 cases, the results have been presented using cumulative IV haloperidol doses. Patients experiencing TdP without preceding QTP received a cumulative dose (= total dose at event) ranging from 5 mg to 645 mg. Patients with both confirmed QTP and TdP were administered a cumulative dose of 2 mg to 1700 mg. Patients who experienced QTP without TdP received a cumulative dose of 2 mg to 1540 mg of IV haloperidol.
Sudden cardiac arrest following administration of IV haloperidol was observed in cumulative doses ranging from 6 mg to 35 mg. The cardiac arrest leading to a fatal outcome was preceded by an administration of at least 6 mg of IV haloperidol. Overall, 14 out of 70 patients received cumulative doses of 10 mg IV haloperidol.
The time from administration to documentation of QTP and/or TdP ranged from immediately post administration to 8 hours after administration of the last dose of IV haloperidol.
Baseline QTc was known in 44 patients. Baseline QTc was >450 msec in 18 of these 44 patients.
The change from baseline QTc varied widely from 20 msec to 286 msec; 36 patients demonstrated a prolongation of >50 msec.
In those patients with reported haloperidol‐associated QTP, 25 patients demonstrated a QTc >600 msec and 38 patients >520 msec.9 Of the cases with known specific QTc values, the QTc was prolonged >450 msec in 48 out of 50 cases. The lowest reported QTc leading to TdP was 413 msec.
A total of 20 patients were reported as having a normalization of QTc (as defined by the original reports) within several hours to 8 days; minimal QTP was reported as persisting in 2 patients. The specifics of the other patients were unknown, although 25 patients were categorized as recovered, 13 were stated as having an uneventful remainder of hospitalization, and 5 patients were discharged to a rehabilitation facility or a nursing home.
Discussion
The current review was performed in response to the FDA warning recommending the use of continuous ECG monitoring associated with the administration of intravenous haloperiodol.5 This warning has resulted in substantial dilemmas for health care organizations, additional resource allocation, and increased scrutiny from regulatory agencies. The results of our review reveal that intravenous haloperidol‐associated QTP and TdP almost uniformly take place in patients with concomitant risk factors and with cumulative doses 2 mg. In light of these findings, it is possible that hospitals may be able to administer intravenous haloperidol in patients without risk factors without continuous ECG monitoring. In reviewing these published reports, it is important to note that the FDA identified 28 published cases of haloperidol‐associated QTP and TdP, while our review yielded a total of 41 published case reports.
The FDA database included 73 cases of haloperidol‐associated TdP in their database. However, these cases included both oral as well as IV administration; using our methodology, we identified 29 additional case reports associated with intravenous haloperidol from this database. Consequently, our review included 41 published case reports and 29 FDA database cases, resulting in the total of 70 patients.
Our review revealed a number of practical findings. First, our summary demonstrated that neither QTP nor TdP has been documented with a cumulative dose of IV haloperidol of <2 mg. The majority of patients (80%) received cumulative IV doses 10 mg. The lowest dose associated with sudden cardiac arrest was 6 mg and this took place in a 69‐year‐old male patient. Second, the majority (97%) of our patients had additional risk factors for QTP and/or TdP. Pre‐existing heart disease,1619 electrolyte imbalance,17, 1921 concomitant proarrhythmic drugs16, 17, 1922 and mechanical ventilation17, 23 were identified as the most commonly observed risk factors (Table 4). Lastly, in those cases in which the data were reported, baseline QTc was >450 msec in 41% of the patients, and 96% had a QTc at the time of the event >450 msec. Therefore, we conclude that patients: (1) receiving low cumulative doses (<2 mg) with (2) no risk factors for prolonged QTc or TdP, and (3) with a normal QTc on baseline EKG can safely be given IV haloperidol in the hospital setting.
This dosage range is consistent with the labelling for IV haloperidol dosing in Canada24 and Germany25 (Table 1), where single doses of 0.25 mg to 1.5 mg are recommended for the treatment of delirium or acute agitation in the geriatric population.24, 25
In a recent Cochrane review, low‐dose IV haloperidol (<3 mg per day) was concluded to be as safe and effective as atypical antipsychotics in the treatment of acute delirium with respect to extrapyramidal adverse effects.2
The American Psychiatric Association recommends an initial IV dose of 12 mg every 24 hours as needed (0.250.50 mg every 4 hours as needed for elderly patients), with titration to higher doses for patients who continue to be agitated for the treatment of patients with delirium (issued 1999, updated 2004).3
While several expert‐groups and investigators currently consider IV haloperidol as an important therapeutic option for treating acute delirium and agitation in the dose range presented above, less consensus exists regarding monitoring requirements.2, 3, 26, 27
The American Psychiatric Association recommends IV haloperidol only after a baseline ECG is obtained. These guidelines have not been updated since the release of the FDA extended warning.3 In their recent review, Morandi et al.28 support the dosage recommendation of the 1999 American Psychiatric Association's practice guidelines for treatment of delirium,3 ie, administration of IV haloperidol in single doses of 0.5 mg to 2 mg in elderly patients, however, only after a baseline ECG is obtained.28 While the package insert of IV haloperidol in France29 recommends a baseline ECG, Germany,25 Italy30 and Switzerland's31 package information states the need for regular ECG monitoring. Guidelines for the treatment of delirium in the intensive care unit published by the American College of Critical Care Medicine and the Society of Critical Care Medicine in collaboration with the American Society of Health‐System Pharmacists consider IV haloperidol as the preferred agent for the treatment of delirium in critically ill patients (grade of recommendation = C). These expert groups recommend that patients should be monitored for electrocardiographic changes (QT interval prolongation and arrhythmias) when receiving haloperidol (Grade of recommendation = B).32
Nevertheless, continuous ECG monitoring (ie, telemetry) is expensive, labor‐intensive and, potentially overutilized.33, 34 Requiring clinicians to place all patients receiving intravenous haloperidol on telemetry is impractical and potentially costly. Mandating telemetry could also lead to unintended harm, ie, use of a less effective or less safe drug to avoid compliance with the telemetry mandate.
Based on our findings and the current recommendations in the literature, inpatient providers should be thoughtful and deliberate in the use of haloperidol to treat acute delirium with agitation. Patients requiring pharmacologic management of their delirium should be screened for risk factors for QTP and TdP (Table 4) and a baseline ECG should be obtained prior to haloperidol administration. If significant risk factors exist or the baseline ECG reveals a prolonged QTc, then the patient should receive continuous ECG monitoring. Similarly, if cumulative doses of 2 mg are needed, the patient should be placed on telemetry.
There are some limitations to our study design. Our findings are based upon previously published case reports or data submitted to the FDA MedWatch. While the content of the FDA's MedWatch database is accessible to the public via the Freedom of Information Act (FOIA), the events are neither categorized nor peer‐reviewed upon entry into the database. Consequently, information may be incomplete or inaccurate. In addition, the denominator representing the overall use of IV haloperidol is unknown, thus a rate of event cannot be assigned and the true scope of the problem cannot be determined. Despite these limitations, this summary represents the most comprehensive review of the literature to date, expanding on the analysis performed by the FDA. Of note, in our review of the FDA database, we noted several cases of haloperidol‐associated QTP or TdP associated with other routes of administration. Thus, it is unknown whether this complication is any greater with IV vs. the IM or per os (PO) routes of administration.
Conclusion
Although the proarrhythmic potential of haloperidol and other antipsychotics has been well established in the literature, IV haloperidol has been considered relatively safe with respect to this complication from the time of its approval in 1967.5, 1722, 35, 36 In reviewing all reported cases of cardiac complications associated with IV haloperidol, as well as the current literature, an association with QTP and TdP is likely. However, the case reports reveal that QTP and TdP generally occur in the setting of concomitant risk factors, and no cases have been reported utilizing a cumulative IV dose of <2 mg. It may therefore be safe to administer a cumulative dose of IV haloperidol of <2 mg without ECG monitoring in patients without risk factors for QTP. However, ECG monitoring should take place with IV haloperidol doses 2 mg and/or in those patients with additional risk factors of developing QTP and/or TdP.
Based on the findings of this review complemented by the guidelines of various expert‐groups and the official labelling information of different countries, the Pharmacy & Therapeutics Committee of the UCSF Medical Center revised the IV haloperidol policy: administration of a total dose of <2 mg IV haloperidol without concurrent telemetry is allowed in a noncritical care setting in patients without risk factors for QTP and TdP.
Acknowledgements
The authors acknowledge Gloria Won of the Fishbon Library at UCSF Medical Center at Mount Zion for her support.
- Haldol® injection (for immediate release) Package Insert.Raritan, NJ:Ortho‐McNeil Pharmaceutical Inc.;2005;rev. 05.2007.
- ,,,. Antipsychotics for delirium (review), the Cochrane collaboration2008;2. Available at: www. cochrane.org. Accessed February 2010.
- American Psychiatric Association: practice guideline for the treatment of patients with delirium.Am J Psychiatry.1999;156(5 suppl):1–20, updated 2004.
- Thomson Micromedex.2008. Micromedex healthcare series: “haloperidol” Thomson Micromedex, Greenwood Village.
- FDA alert: haloperidol (marketed as Haldol, Haldol Decanoate and Haldol Lactate).2007. This alert highlights revisions to the labeling for haloperidol. Available at: www.fda.gov. Accessed February 2010.
- ,,.Use of high‐dose intravenous haloperidol in the treatment of agitated cardiac patients,J Clin Psychopharmacol.1985;5(6):344–347.
- ,,, et al.Postoperative delirium,Am J Psychiatry.2008;165:7.
- ,.Corrected QT interval prolongation associated with intravenous haloperidol in acute coronary syndromes,Catheter Cardiovasc Interv.2000;50(3):352–355.
- ,.Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death.Am J Psych.2001;158(11):1774–1782.
- ,,, et al.Accuracy of uncorrected versus corrected QT Interval for Prediction of torsade de pointes associated with intravenous haloperidol.Pharmacotherapy.2007;27(2):175–182.
- ,,.Three cases of ventricular tachycardia and torsades de pointes induced by antipsychotic drugs.Shinzo.1997;29(1):68–74.
- ,,,.Haloperidol por via intravenosa y torsade de pointes.Medicina intensive.2004;28(2):89.
- ,,, et al.QT‐Verlängerung und Kammerflimmern unter Haloperidol‐ und Clonidin‐Therapie des Alkoholentzugssyndroms.Intensivmedizin und Notfallmedizin.1992;29(4):178–183.
- ARIZONA CERT, Arizona Center for Education and Research on Therapeutics. Available at: www.azcert.org. Accessed February 2010.
- ,.Cardiac arrhythmias—a clinical approach.Edinburgh:Mosby;2003.
- ,.The association between intravenous haloperidol and torsades de pointes—three cases and a literature review.Psychosomatics.1995;36:541–549.
- ,.Prolongation of the corrected QT and torsades de pointes cardiac arrhythmia associated with intravenous haloperidol in the medically ill.J Clin Psychopharmacol.1993;13(2):128–132.
- ,,,,.Torsades de pointes secondary to intravenous haloperidol after coronary bypass grafting surgery.Can J Anesth.2000;47(3):251–254.
- ,.Torsade de pointes associated with the administration of intravenous haloperidol: a review of the literature and practical guidelines for use,Expert Opin Drug Saf.2003;2(6):543–547.
- ,.Conduction disturbances associated with administration of butyrophenone antipsychotics in the critically ill: a review of the literature,Pharmacotherapy.1997;17(3):531–537.
- ,,.Haloperidol‐induced torsades de pointes.Ann Pharacother.1999;33(10):1046–1050.
- ,,, et al.Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary,Crit Care Med.1995;23(9):1596–1600.
- ,,,,,.Prolonged cardiac repolarization after tacrolimus and haloperidol administration in the critically ill patient.Pharmacotherapy.2004;24(3):404–408.
- CPS Compendium of Pharmaceuticals and Specialties, the Canadian drug reference for health professionals, 2007, Canadian pharmacists association.
- Rote Liste Deutschland2008, Rote Liste Service GmbH Frankfurt am Main. Available at: www.rote‐liste.de. Accessed February 2010.
- ,.Delirium in the hospitalized patient: a primer for the pharmacist clinician.J Pharm Pract.2007;20(5):368–372.
- ,,, et al.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,.The pharmacological management of delirium in critical illness.Current Drug Therapy.2008,3:148–157.
- VIDAL‐l'information sur les produits de santé2008, Issy les Moulineaux Cedex. Available at: www.vidal.fr. Accessed February 2010.
- Haldol iniettabile—ufficiale monografia italiana. Available at: www. informatorefarmaceutico.it. Accessed February 2010.
- Arzneimittelkompendium der Schweiz2008, documed Verlag Basel. Availabla at: www.kompendium.ch. Accessed February 2010.
- ,,, et al.Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill, American College of Critical Care Medicine ACCM, Society of Critical Care Medicine SCCM, American Society of Health‐System Pharmacists ASHP.Crit Care Med.2002;30(1):119–141.
- ,,,,.Is telemetry overused? Is it as helpful as thought?Cleve Clin J Med.2009;76(6):368–372.
- ,,.Telemetry outside critical care units: patterns of utilization and influence on management decisions.Clin Cardiol.1998;21(7):503–505.
- ,,.High‐dose intravenous haloperidol for agitated delirium in a cardiac patient on intra‐aortic balloon pump.J Clin Psychopharmacol.1991;11(2):146–147.
- .Haloperidol, midazolam and intravenous sedation.Aust NZ J Psychiatry.1999;33(6):942–943.
- BNF British National Formulary, compendium of pharmaceuticals and specialties of the UK.2007. Available at: www.bnf.org. Accessed February 2010.
- ,,,.Torsade de pointes associated with the use of intravenous haloperidol.Ann Intern Med.1993;119(5):391–394.
- ,.Torsades de pointes: potential consequence of intravenous haloperidol in the intensive care unit.Intensive Care World.1994;11(3):109–112.
- ,.Torsade de pointes caused by high‐dose intravenous haloperidol in cardiac patients.Clin Cardiol.1995;18:285–290.
- ,,.Continuous infusion of haloperidol controls agitation in critically ill patients.Crit Care Med.1994:22(3):433–440.
- ,,.Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalances.Am J Gastroenterol.1995;90(5):822–824.
- ,,,.Torsades de pointes associated with intravenous haloperidol in critically ill patients.Am J Cardiol.1998;81(2):238–240.
- ,,,,.The effect of intravenous haloperidol on QT interval dispersion in critically ill patients: comparison with QT interval prolongation for assessment of risk of torsades de pointes.J Clin Pharmacol.2001;41:1310–1318.
- ,,.Low‐dose haloperidol associated QTc prolongation.J Am Geriatr Soc.2008;56(10):1963–1964.
- ,,.Torsade de pointes following intravenous haloperidol administration in a patient with complete heart block.WMJ.2009;108(1):48–50.
- Haldol® injection (for immediate release) Package Insert.Raritan, NJ:Ortho‐McNeil Pharmaceutical Inc.;2005;rev. 05.2007.
- ,,,. Antipsychotics for delirium (review), the Cochrane collaboration2008;2. Available at: www. cochrane.org. Accessed February 2010.
- American Psychiatric Association: practice guideline for the treatment of patients with delirium.Am J Psychiatry.1999;156(5 suppl):1–20, updated 2004.
- Thomson Micromedex.2008. Micromedex healthcare series: “haloperidol” Thomson Micromedex, Greenwood Village.
- FDA alert: haloperidol (marketed as Haldol, Haldol Decanoate and Haldol Lactate).2007. This alert highlights revisions to the labeling for haloperidol. Available at: www.fda.gov. Accessed February 2010.
- ,,.Use of high‐dose intravenous haloperidol in the treatment of agitated cardiac patients,J Clin Psychopharmacol.1985;5(6):344–347.
- ,,, et al.Postoperative delirium,Am J Psychiatry.2008;165:7.
- ,.Corrected QT interval prolongation associated with intravenous haloperidol in acute coronary syndromes,Catheter Cardiovasc Interv.2000;50(3):352–355.
- ,.Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death.Am J Psych.2001;158(11):1774–1782.
- ,,, et al.Accuracy of uncorrected versus corrected QT Interval for Prediction of torsade de pointes associated with intravenous haloperidol.Pharmacotherapy.2007;27(2):175–182.
- ,,.Three cases of ventricular tachycardia and torsades de pointes induced by antipsychotic drugs.Shinzo.1997;29(1):68–74.
- ,,,.Haloperidol por via intravenosa y torsade de pointes.Medicina intensive.2004;28(2):89.
- ,,, et al.QT‐Verlängerung und Kammerflimmern unter Haloperidol‐ und Clonidin‐Therapie des Alkoholentzugssyndroms.Intensivmedizin und Notfallmedizin.1992;29(4):178–183.
- ARIZONA CERT, Arizona Center for Education and Research on Therapeutics. Available at: www.azcert.org. Accessed February 2010.
- ,.Cardiac arrhythmias—a clinical approach.Edinburgh:Mosby;2003.
- ,.The association between intravenous haloperidol and torsades de pointes—three cases and a literature review.Psychosomatics.1995;36:541–549.
- ,.Prolongation of the corrected QT and torsades de pointes cardiac arrhythmia associated with intravenous haloperidol in the medically ill.J Clin Psychopharmacol.1993;13(2):128–132.
- ,,,,.Torsades de pointes secondary to intravenous haloperidol after coronary bypass grafting surgery.Can J Anesth.2000;47(3):251–254.
- ,.Torsade de pointes associated with the administration of intravenous haloperidol: a review of the literature and practical guidelines for use,Expert Opin Drug Saf.2003;2(6):543–547.
- ,.Conduction disturbances associated with administration of butyrophenone antipsychotics in the critically ill: a review of the literature,Pharmacotherapy.1997;17(3):531–537.
- ,,.Haloperidol‐induced torsades de pointes.Ann Pharacother.1999;33(10):1046–1050.
- ,,, et al.Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary,Crit Care Med.1995;23(9):1596–1600.
- ,,,,,.Prolonged cardiac repolarization after tacrolimus and haloperidol administration in the critically ill patient.Pharmacotherapy.2004;24(3):404–408.
- CPS Compendium of Pharmaceuticals and Specialties, the Canadian drug reference for health professionals, 2007, Canadian pharmacists association.
- Rote Liste Deutschland2008, Rote Liste Service GmbH Frankfurt am Main. Available at: www.rote‐liste.de. Accessed February 2010.
- ,.Delirium in the hospitalized patient: a primer for the pharmacist clinician.J Pharm Pract.2007;20(5):368–372.
- ,,, et al.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,.The pharmacological management of delirium in critical illness.Current Drug Therapy.2008,3:148–157.
- VIDAL‐l'information sur les produits de santé2008, Issy les Moulineaux Cedex. Available at: www.vidal.fr. Accessed February 2010.
- Haldol iniettabile—ufficiale monografia italiana. Available at: www. informatorefarmaceutico.it. Accessed February 2010.
- Arzneimittelkompendium der Schweiz2008, documed Verlag Basel. Availabla at: www.kompendium.ch. Accessed February 2010.
- ,,, et al.Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill, American College of Critical Care Medicine ACCM, Society of Critical Care Medicine SCCM, American Society of Health‐System Pharmacists ASHP.Crit Care Med.2002;30(1):119–141.
- ,,,,.Is telemetry overused? Is it as helpful as thought?Cleve Clin J Med.2009;76(6):368–372.
- ,,.Telemetry outside critical care units: patterns of utilization and influence on management decisions.Clin Cardiol.1998;21(7):503–505.
- ,,.High‐dose intravenous haloperidol for agitated delirium in a cardiac patient on intra‐aortic balloon pump.J Clin Psychopharmacol.1991;11(2):146–147.
- .Haloperidol, midazolam and intravenous sedation.Aust NZ J Psychiatry.1999;33(6):942–943.
- BNF British National Formulary, compendium of pharmaceuticals and specialties of the UK.2007. Available at: www.bnf.org. Accessed February 2010.
- ,,,.Torsade de pointes associated with the use of intravenous haloperidol.Ann Intern Med.1993;119(5):391–394.
- ,.Torsades de pointes: potential consequence of intravenous haloperidol in the intensive care unit.Intensive Care World.1994;11(3):109–112.
- ,.Torsade de pointes caused by high‐dose intravenous haloperidol in cardiac patients.Clin Cardiol.1995;18:285–290.
- ,,.Continuous infusion of haloperidol controls agitation in critically ill patients.Crit Care Med.1994:22(3):433–440.
- ,,.Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalances.Am J Gastroenterol.1995;90(5):822–824.
- ,,,.Torsades de pointes associated with intravenous haloperidol in critically ill patients.Am J Cardiol.1998;81(2):238–240.
- ,,,,.The effect of intravenous haloperidol on QT interval dispersion in critically ill patients: comparison with QT interval prolongation for assessment of risk of torsades de pointes.J Clin Pharmacol.2001;41:1310–1318.
- ,,.Low‐dose haloperidol associated QTc prolongation.J Am Geriatr Soc.2008;56(10):1963–1964.
- ,,.Torsade de pointes following intravenous haloperidol administration in a patient with complete heart block.WMJ.2009;108(1):48–50.