User login
FDA‐warning for IV Haloperidol: A Review
Haloperidol is Food and Drug Administration (FDA)‐approved in the United States for the management of acute and chronic psychotic disorders and widely used in the management of delirium‐associated agitation in hospitalized patients.1 Delirium in the hospital is an acute confusional state that frequently arises from multiple complex factors and may affect up to 30% of hospitalized patients.2 Although the first step in the management of delirium involves identification and treatment of underlying causes and offering supportive behavioral care; medications may be needed to control severe agitation.2 Low dose intravenous (IV) haloperidol (ie, 0.250.5 mg every 4 hours) is a commonly used medication in this setting as recommended by expert‐groups including the Cochrane Collaboration and the American Psychiatric Association.2, 3
Although injectable haloperidol, a butyrophenone‐derived antipsychotic agent pharmacologically related to the piperazine phenothiazines,4 is approved for IV use in many countries (Table 1), parenteral use is approved only for intramuscular (IM) administration in the US. Thus, IV administration of the drug in the US is considered an off‐label use.5
| Indication | Country | |||||
|---|---|---|---|---|---|---|
| Canada24 | France29 | Germany25 | Great Britain37 | Italy30 | Switzerland31 | |
| ||||||
| Mainly delirium (schizophrenia, other psychosis, short‐term management of psychomotor agitation, excitement, violent or dangerously impulsive behavior, vomiting, hiccup) | Short term treatment of agitation and aggressiveness during an acute or chronic psychotic episode, vomiting along with antimitotic post‐radiotherapy treatment | Acute and chronic schizophrenia, psycho‐motorical agitation of psychotic genesis | Schizophrenia, other psychosis, short‐term adjunctive management if psychomotor agitation, violent or dangerous impulsive behavior | Resistant forms of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis' chronic psychosis High doses restrictions: syndrome of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis, chronic psychosis | Acute schizophrenic episode, mania, vomiting | |
| IV dosing in adults | 12 mg every 24 hours | The use is limited to adult patients and the drug can be administered IM or IV. The IV route is restricted to the treatment of vomiting. | 510 mg/day, daily max.: 30(100) mg | 210 mg initially, PRN every 48 hours, daily max. 18 mg | 510 mg initially, PRN every hour, daily max. 60 mg | 5 mg PRN every 30 minutes |
| IV dosing in geriatric care | 0.250.5 mg | Single dose of 0.51.5 mg, daily max. 5 mg | Half adult dose | Adjust to appropriate dose | 0.5 mg, than PRN | |
| Risk factors for the development of cardiac adverse events | QT prolonging drugs, diabetes, obesity, hypokalemia, congenital long QT syndrome | Bradycardia <55 beats per minute, hypokalemia, congenital QT prolongation, other medications provoking bradycardia, deceleration of the intra‐cardiac transition or prolonged QT interval | QT syndrome, hypokalemia, other electrolyte imbalance, cardiovascular diseases, QT prolongation in the family history | Cardiovalscular disease, drugs that can prolong the QTc, diabetes, obesity, hypokalemia, congenital long QT syndrome | Contraindications: recent cardiac infarction, uncompensated cardiac insufficiency, cardiac arrhythmias, antiarrhythmic drugs, pre‐existing QT prolongation, cases of arrhythmia or torsades de pointes in the family history, untreated potassium imbalance, QTc prolonging drugs | QT syndrome, hypokalemia, hypomagnesemia, other electrolyte imbalances, cardiovascular diseases, hypothyreosis, QT prolongation in the family history |
| Monitoring recommendations | Electrolytes | ECG monitoring at admission time, electrolytes | ECG monitoring, electrolytes | Metabolic parameters | ECG at baseline and regular ECG monitoring, electrolytes | Close ECG monitoring, electrolytes |
| General recommendations | Regular reevaluation in long‐term use | Apply the lowest effective dose | Apply the lowest effective dose | Application per mouth is the route of choice | Decrease dose if QTc >500 msec | Switch to PO as soon as possible |
Haloperidol is often preferred over other antipsychotics as a result of its effectiveness, low rate of anticholinergic side effects, familiarity with dosing and usage, and minimal respiratory or sedative properties.6 Use of the IV route in patients with acute delirium has several advantages over the IM or oral route,7 including rapid onset, immediate bioavailability, and ease and safety of administration.
Prior to September 2007, the package insert for haloperidol alerted healthcare professionals to the risk of cardiovascular side effects. Based on case reports of potentially fatal cardiac events, the FDA revised the label, warning that the QT prolongation (QTP) and risk of torsades de pointes (TdP) were increased with IV administration of haloperidol or administration of the drug at greater than recommended doses. Unfortunately, neither the typical dosing range nor the minimum dose associated with these cardiac side effects were specified in this recommendation.5
It is well‐established that haloperidol may prolong the QT interval by blocking the repolarizing potassium IKr current.8 Although drugs that block the IKr channel can produce arrhythmia in healthy individuals, additional risk factors, such as underlying heart conditions, electrolyte imbalances (ie, hypokalemia and hypomagnesemia), concomitant proarrhythmic drug use, and mechanical ventilation may increase this risk.9 Prolongation of the QT interval has been associated with subsequent malignant cardiac arrhythmias including ventricular fibrillation and TdP.10 Prolongation of the QT interval is considered the strongest risk factor for TdP, particularly with a baseline QTc > 450 msec.9
Based on the increased risk for QTP and TdP and the case reports of cardiac events, the FDA advisory recommended continuous electrocardiogram (ECG) monitoring in patients receiving IV haloperidol.5 However, such monitoring may be impractical and costly in hospitalized patients who require low doses of IV haloperidol to manage acute delirium and who are not in telemetry or intensive care units.
The aim of this review was to evaluate the case reports leading to the recent FDA warning for IV haloperidol, specifically focusing on the presence of risk factors for arrhythmias. Based upon the evidence, an additional aim was to provide an institutional response to this warning toward the optimal use of this agent.
Method
Two search pathways were used to evaluate reports of haloperidol‐associated TdP and/or QT prolongation:
Literature Review
We searched for published literature in humans indexed in Pubmed (1966April 2009), EMBASE (1972April 2009), and Scopus (1823April 2009) using the search terms haloperidol or Haldol combined with intravenous or infusion and at least one of the following terms: QT prolongation, TdP, torsades de pointes, torsades with a specific focus on case reports.
References from the retrieved articles were also reviewed to search for additional case reports.
In addition to cases reported in English journals, several of our reports originated from Japan11 (translation provided by the FDA), Spain12 and Germany13 (translated by the primary author).
Search of the FDA Database
We reviewed all adverse drug events reported through MedWatch or those submitted by the manufacturer from November 1997 to April 2008 through the Freedom of Information Act (FOIA) request. The FDA provided a full‐text summary of 5944 reports involving oral, intramuscular and IV use of haloperidol. The FDA data were transferred to a Microsoft Access database and screened for the key terms torsade, QT, prolongation, wave. Incident report number, date of report, age, gender, origin of report, medication name, role of drug as categorized by the FDA (suspect, concomitant, primary suspect, secondary suspect), route, dose, units, duration, symptoms and FDA outcome category (death, life‐threatening, hospitalization initial or prolonged, disability, congenital anomaly, required intervention to prevent permanent damage, other) were recorded. Only those reports in which IV haloperidol was considered by the reporter to be the primary causative agent for the adverse event were reviewed. Available information included diagnosis, laboratory parameters, QTc measurement, cardiac symptoms, outcomes and a description of recovery. No peer review was applied to the MedWatch reports and the data reported in this publication reflect the original information from the FDA MedWatch database. Baseline QTc was either the value defined as such in the original report or the lowest QTc reported. Haloperidol doses administered were defined as cumulative dose at event, encompassing all doses administered during the hospital stay until the occurrence of the adverse cardiac event.
The drugs listed in the case reports were assessed for proarrhythmic potential using 2 references: the individual package insert and the website of the Arizona Center for Education and Research on Therapeutics.14
The drugs were only considered proarrhythmic when the 2 resources were in agreement.
Duplicates and/or previously published cases, as well as reports involving adverse cardiac effects not associated with QTP or TdP, were identified and excluded.
In their advisory, the FDA does not state the exact origin of the reports, their specific search strategy to identify haloperidol‐associated adverse events, or the role IV haloperidol played in the individual events included in the extended warning. Consequently, the number of events identified in this review may differ from that published in the FDA extended warning.
Results
A total of 70 reported cases of IV haloperidol associated TdP and/or QTP were identified. Of these 70, 41 were identified through the PubMed/EMBASE/Scopus review, while an additional 29 cases were identified through the FDA database search.
Of the 29 cases in the FDA database, 21 were reported by health care professionals and 8 by manufacturers.
A total of 35 publications described cases originating from the US. Three cases took place in Japan and 1 case each in Canada, Germany and Spain. Several cases in the MedWatch database were reported outside the US: 1 case each originated from Austria, Canada, France, Japan, Spain, Switzerland and the United Kingdom. A summary of the published case reports is displayed in Table 2 and the FDA cases are summarized in Table 3.
| Case | Source (reference#) | Date | Age, Years | Gender | Drugs Pro‐arrhyth. | Venti‐ lated | Max. Daily Dose (mg) | Total Dose at Event (mg) | Time to Event | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | ECG Normalization, Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
| 1 | 35 | 1991 | 56 | m | No | Yes | 1200 | 1540 | NR | Yes | 584 (400) | 184 | NR | NR, uneventful |
| 2 | 13 | 1992 | 36 | m | Yes | No | 11.5 | 11.5 | 20 hours after start | Yes | 714 (428) | 286 | Yes | QTc normalization (440 msec), NR |
| 3 | 38 | 1993 | 39 | f | Yes | Yes | NR | 580 | Max. QTc 72 hours after start | Yes | 650 (420) | 230 | Yes | QTc normalization after 6 days, uneventful |
| 4 | 38 | 1993 | 19 | f | Yes | No | 170 | 170 | Max. QT 12 hours after start | Yes | 600 (480) | 120 | Yes | QTc normalization after 8 days, uneventful |
| 5 | 38 | 1993 | 63 | f | Yes | No | NR | 489 | Max. QT 48 hours after start | Yes | 670 (520) | 150 | Yes | QTc normalization after 8 days, uneventful |
| 6 | 38 | 1993 | 74 | f | Yes | Yes | NR | 10 | NR | No | 430 (410) | 20 | Yes | QTc unchanged after 8 days, uneventful |
| 7 | 17 | 1993 | 39 | m | Yes | Yes | NR | >490 | NR | Yes | 457 (348) | 109 | Yes | QTc normalization within 2 to 3 days, no further TdP, NR |
| 8 | 17 | 1993 | 61 | m | Yes | Yes | 115 | 211 | NR | Yes | 500 (390) | 110 | NR | QTc normalization within 2 days, death |
| 9 | 17 | 1993 | 48 | m | Yes | Yes | 825 | 825 | NR | Yes | 538 (441) | 97 | NR | QTc normalization in 3 days, rehabilitation |
| 10 | 39 | 1994 | 23 | f | Yes | Yes | 120 | 300 | 12 hours after dose increase | Yes | NR (550) | NR | Yes | NR, uneventful, extubation after 5 days, discharge after 10 days |
| 11 | 39 | 1994 | 28 | m | Yes | Yes | 300 | >300 | 24 hours after dose increase | Yes | NR (>520) | NR | Yes | No recurrence of arrhythmia, patient death (multi‐organ failure) |
| 12 | 40 | 1994 | 65 | m | Yes | NR | 230 | 410 | Worsening from day 2 to day 5 | Yes | 594 (490) | 104 | Yes | QTc normalization (406 msec), no cardiac problems at discharge |
| 13 | 40 | 1994 | 65 | f | Yes | NR | 500 | 980 | After the last 60mg | Yes | 628 (403) | 225 | Yes | QTc normalization (<400 msec), recurrence with oral haloperidol, rehabilitation |
| 14 | 40 | 1994 | 76 | f | Yes | NR | 21 | 26 | Day 2 after several boluses | Yes | 670 (450) | 220 | Yes | QTc normalization within several days (412 msec), rehabilitation |
| 15 | 41 | 1994 | 59 | m | NR | Yes | 865 | 1013 | NR | Yes | 640 (480) | 160 | NR | QTc normalization in 24 hours, survived |
| 16 | 16 | 1995 | 76 | f | Yes | No | NR | 44.5 plus 1 PO | 15 minutes | Yes | 670 (409) | 261 | Yes | ECG normalized the next morning, no further events |
| 17 | 16 | 1995 | 49 | m | Yes | No | NR | 1150 plus 20 IM | 45 minutes | Yes | 648 (380) | 268 | Yes | QTc normalization in 24 hours, anoxic brain insult/rehabilitation |
| 18 | 16 | 1995 | 65 | f | Yes | No | 600 | 965 | 30 minutes | Yes | 628 (403) | 225 | Yes | 3 more episodes of TdP in 3 hours, QTc normalization in 2 days, no recurrence with further haloperidol, NR |
| 19 | 42 | 1995 | 42 | m | Yes | No | 28 | 28 | 20 minutes | Yes | 610 (533) | 77 | Yes | QTc normalization in 5 days, uneventful, ECG normal |
| 20 | 42 | 1995 | 39 | m | Yes | No | 45 | 45 | 5 minutes | Yes | 654 (NR) | NR | Yes | QTc normalization after 24 hours, uneventful |
| 21 | 11 | 1997 | 56 | f | No | No | 10 | 10 | Shortly after | NR | NR (NR) | NR | Yes | TdP resolved after 8 hours, NR |
| 22 | 11 | 1997 | 82 | f | NR | No | 10 | 10 | Shortly after | Yes | 680 (NR) | NR | Yes | QTc normalization on day 6 after admission (470 msec), NR |
| 23 | 11 | 1997 | 35 | m | NR | No | NR | 90 | After 20 mg | Yes | 520 (NR) | NR | Yes | TdP disappeared 12 hours later, NR |
| 24 | 43,44 | 1998 | 45 | m | NR | Yes* | NR | 9 | 203 minutes | Yes | 638 (560) | 78 | Yes | NR, overall survival 100%, significantly prolonged hospital stay |
| 25 | 43,44 | 1998 | 64 | f | NR | NR | 115 | 220 minutes | Yes | 605 (424) | 181 | Yes | ||
| 26 | 43,44 | 1998 | 75 | f | NR | NR | 85 | 60 minutes | Yes | 567 (508) | 59 | Yes | ||
| 27 | 43,44 | 1998 | 71 | f | NR | NR | 55 | 120 minutes | Paced | Paced | Paced | Yes | ||
| 28 | 43,44 | 1998 | 58 | f | NR | NR | 75 | 38 minutes | Yes | 657 (542) | 115 | Yes | ||
| 29 | 43,44 | 1998 | 40 | m | NR | NR | 35 | 15 minutes | Yes | 679 (475) | 204 | Yes | ||
| 30 | 43,44 | 1998 | 71 | m | NR | NR | 70 | 58 minutes | Yes | 521 (478) | 43 | Yes | ||
| 31 | 43,44 | 1998 | 47 | m | NR | 400 | 400 | 79 minutes | Yes | 574 (444) | 130 | Yes | ||
| 32 | 21 | 1999 | 41 | f | Yes | Yes | 320 | 915 | 55 minutes | Yes | 610 (426) | 184 | Yes | QTc normalization after 5 day, uneventful |
| 33 | 21 | 1999 | 31 | m | Yes | Yes | 480 | 1700 | 40 minutes | Yes | 599 (491) | 108 | Yes | QTc normalized in 4 days, NR |
| 34 | 18 | 2000 | 64 | f | Yes | Yes | 175 | 175 | NR | No | 413 (418) | (‐5) | Yes | QTc remained unchanged, uneventful |
| 35 | 8 | 2000 | 75 | m | No | NR | >2 | >2 | NR | Yes | 615 (435) | 180 | No | QTc normalization in 48 hours, uneventful |
| 36 | 8 | 2000 | 68 | m | Yes | Yes | >2 | >2 | NR | Yes | 650 (407) | 243 | No | QTc normalization after 4 day, uneventful after extubation |
| 37 | 8 | 2000 | 77 | m | NR | NR | (4) | 2 | NR | Yes | 550 (393) | 157 | No | QTc normalization in 24 to 36 hours, NR |
| 38 | 12 | 2004 | 34 | m | Yes | NR | 24.5 | 24.5 | 20 minutes | Yes | 560 (420) | 140 | Yes | QTc normalization (440 msec), ECG normal |
| 39 | 23 | 2004 | 58 | f | Yes | NR | 340 | 1010 | NR | Yes | 533 (460) | 73 | Yes | QTc normalization 7 days later discharge after 27days |
| 40 | 45 | 2008 | 86 | f | Yes | No | 2 mg | 2 mg | 8 hours after last dose | Yes | 524 (NR) | Probably 79 | No | QTc normalization (445 msec), NR |
| 41 | 46 | 2009 | 74 | m | Yes | No | 2 | 2 | Shortly after | Yes | NR (579) | NR | Yes | Pre‐existing heart block and fibrillation resolved, nursing home/rehabilitation |
| Report | MedWatch Identifier | Report Date | Age, Years | Gender | Drugs Pro‐arrh. | Maximum Daily Dose (mg) | Total Dose at Event (mg) | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | Outcome; Recovery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
| 1 | 3122988‐1 | 1998 | 61 | m | No | 48 | 48 | Yes | NR | NR | Yes | Intervention; NR |
| 2 | 3157827‐6 | 1998 | 44 | f | No | 160 | 160 | Yes | 550 (440) | 110 | Yes | Intervention; uneventful |
| 3 | 3178715‐5 | 1999 | 60 | m | NR | 415 | 645 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization in 1 day, no recurrence |
| 4 | 3271261‐X | 1999 | 56 | m | NR | NR | 20 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization |
| 5 | 3271080‐4 | 1999 | 35 | m | Yes | 7 | 7 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced, hospitalization prolonged |
| 6 | 3325391‐4 | 1999 | 55 | f | Yes | 75 | 75 | NR | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 7 | 3381921‐8 | 1999 | 52 | m | No | 320 | 634 | Yes | 458 (430) | 28 | Yes | Death; NA |
| 8 | 3483869‐7 | 2000 | 18 | m | No | >200 | >310 | Yes | NR | NR | Yes | Intervention; no recurrence after haloperidol reinstitution |
| 9 | 3516342‐8 | 2000 | NR | NR | NR | NR | NR | NR | NR | NR | Yes | NR; NR |
| 10 | 3516320‐9 | 2000 | 34 | m | Yes | 5 | 5 | Yes | NR | NR | No | Life‐threatening; event abated after dose stopped |
| 11 | 3552263‐2 | 2000 | 46 | f | Yes | NR | 97.5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 12 | 3574705‐9 | 2000 | 78 | m | Yes | NR | 160 | Yes | 603 (453) | 50 | Yes | Intervention; event abated after dose stopped/reduced |
| 13 | 3703871‐7 | 2001 | 27 | m | NR | 530 | 530 | Yes | NR | NR | Yes | Death, NA |
| 14 | 3724567‐1 | 2001 | 31 | m | Yes | 6 | 6 | Yes | 496 (449) | 47 | No | Life‐threatening; ECG returned to baseline |
| 15 | 3851984‐1 | 2002 | 72 | f | NR | 18 | 18 | NR | NR | NR | Yes | Hospitalization; NR |
| 16 | 3942407‐2 | 2002 | 51 | m | Yes | 14 | 14 | Yes | 461 (444) | 17 | Yes | Life‐threatening; no recurrence |
| 17 | 4066580‐3 | 2003 | >60 | f | NR | 50 | 50 | Yes | >600 (480) | >120 | No | Hospitalization; QTc normalization, patient recovered |
| 18 | 4126280‐8 | 2003 | 47 | f | NR | 60 | 180 | Yes | 550 (450) | 100 | No (bradycardia) | Hospitalization; patient recovered |
| 19 | 4150700‐6 | 2003 | NR | m | NR | 5 | 5 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced |
| 20 | 4340092‐1 | 2004 | 52 | m | Yes | 5 | 5 | Yes | >500 (490) | >10 | NR (polymorphous VT) | Life‐threatening; NR |
| 21 | 4714692‐0 | 2005 | NR | m | NR | NR | NR | Yes | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 22 | 4881813‐9 | 2006 | NR | m | NR | NR | 40 | NR | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 23 | 4892225‐6 | 2006 | NR | f | Yes | 10 | >10 | Yes | 493 (300) | 193 | No | Hospitalization; QTc normalization (403 msec) |
| 24 | 4911873‐8 | 2006 | 69 | m | Yes | 6 | 6 | NR | NR | NR | Yes | Cardiac arrest, death; NA |
| 25 | 5366448‐6 | 2007 | 53 | m | Yes | NR | 35 | Yes | NR | NR | NR | Cardiac arrest, life‐threatening; patient recovered |
| 26 | 5563440‐3 | 2007 | 58 | m | Possible | 5 | 5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 27 | 5642929‐2 | 2008 | 42 | m | Yes | 165 | 165 | Yes | 640 (350) | 290 | Yes | Death; NA |
| 28 | 5697758‐0 | 2008 | 38 | m | Yes | NR | 620 | NR | NR | NR | Yes | Hospitalization; patient recovered |
| 29 | 5254840‐X | 2008 | 19 | f | Possible | 15 | 25 | Yes | 461 | NR | NR | Cardiac arrest, hospitalization; patient recovered |
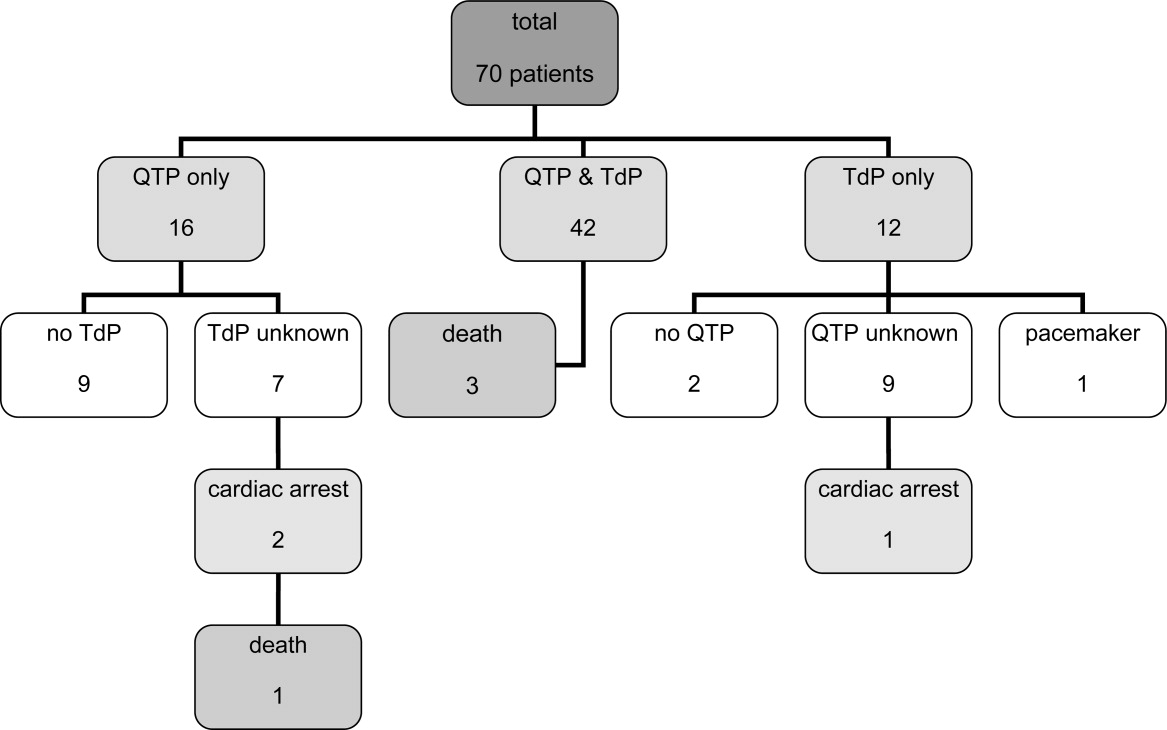
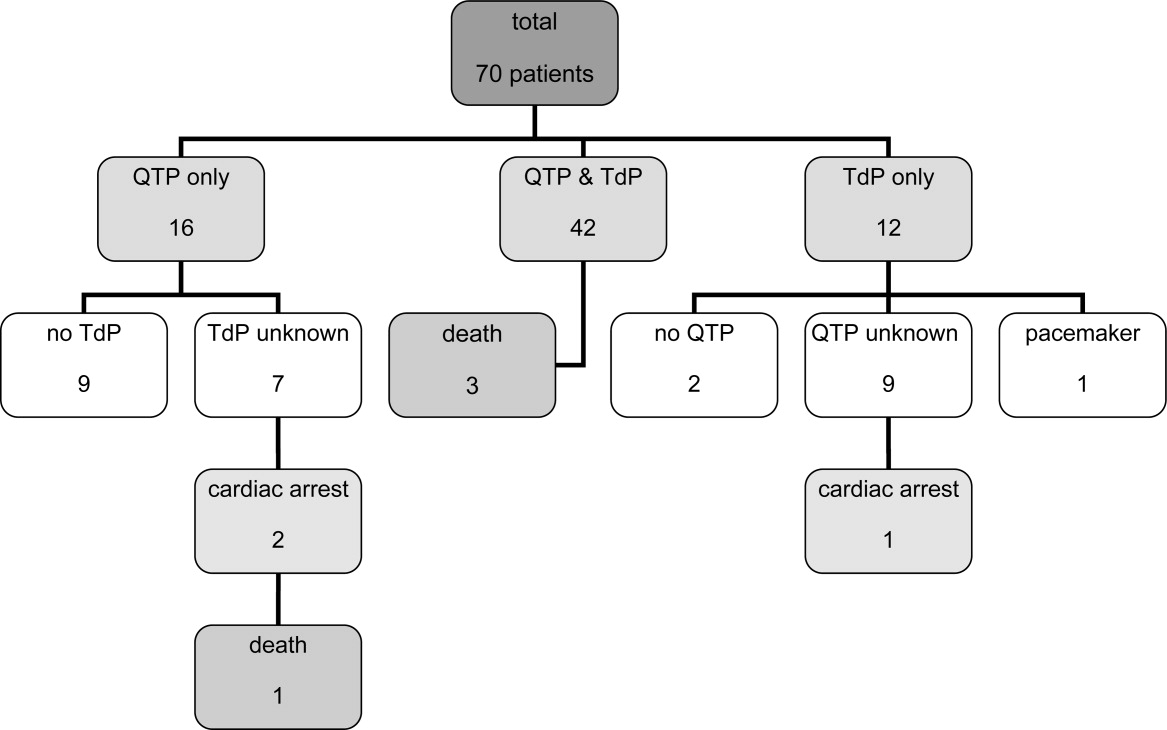
Of the 70 cases, 54 cases of TdP were reported. The remaining 16 of 70 cases involved cases of QTP, 9 of which did not progress to TdP and 7 of which the progression to TdP was unclear. Of note, 42 of 54 of the cases of TdP were reported as preceded by documented QTP. Presence of QTP was unknown in the other 12 original reports. Three out of 70 patients experienced sudden cardiac arrest, 1 of which was fatal. One arrest was preceded by TdP and 2 by QTP (Figure 1).
The patient ages ranged from 18 years to 86 years. Of note, 17 patients experiencing TdP and/or QTP were <40 years old, and 2 of those patients were <30 years old.
Haloperidol‐associated QTP and/or TdP were observed in 27 female and 42 male patients; the gender was not stated in one report. Of the 54 patients experiencing TdP (with or without report of previous QTP), 22 were female and 31 were male (1 gender unknown).
A total of 68 of 70 patients were determined to have associated risk factors15 for QTP/TdP (see Table 4). The circumstances of the remaining 2 patients were not described in sufficient detail to identify associated risk factors.
| Risk Factor | Patients, n (%) |
|---|---|
| |
| Any risk factor | 68/70 (97) |
| Unknown | 2/70 (3) |
| Specific risk factors | |
| Electrolyte imbalance | 27/68 (40) |
| Underlying cardiac disease | 32/68 (47) |
| Concomitant proarrhythmic agents | 39/68 (57) |
| Other drugs influencing cardiac function | 23/68 (34) |
| Baseline QTc >450 msec | 18/68 (26) |
| QTc known: 44 patients | 18/44 (41) |
Overall, 32 patients had underlying heart conditions. Electrolyte imbalances, including hypokalemia, hypomagnesemia, and hypocalcemia, were present in 17 patients. At least 39 patients were receiving potentially proarrhythmic agents (1‐8 proarrhythmic drugs per patient) in addition to IV haloperidol. At least 23 patients were receiving additional drugs with a potential for other cardiac adverse events than QTP and TdP.
A wide range of other disease states previously reported to be associated with QTP15 were identified in these patients: asthma (5 patients), diabetes (5 patients), obesity (3 patients), impaired renal and/or liver function (3 patients each), human immunodeficiency virus (HIV) (2 patients); chronic obstructive pulmonary disease (COPD), pancreatitis and hypothyroidism (1 patient each). A total of 22 patients had a history of substance abuse (alcohol and/or drugs), and 4 patients were smokers.
The administered doses of IV haloperidol varied widely. Considering that information regarding the maximal daily dose was missing in 22 reports and ambiguous in another 20 cases, the results have been presented using cumulative IV haloperidol doses. Patients experiencing TdP without preceding QTP received a cumulative dose (= total dose at event) ranging from 5 mg to 645 mg. Patients with both confirmed QTP and TdP were administered a cumulative dose of 2 mg to 1700 mg. Patients who experienced QTP without TdP received a cumulative dose of 2 mg to 1540 mg of IV haloperidol.
Sudden cardiac arrest following administration of IV haloperidol was observed in cumulative doses ranging from 6 mg to 35 mg. The cardiac arrest leading to a fatal outcome was preceded by an administration of at least 6 mg of IV haloperidol. Overall, 14 out of 70 patients received cumulative doses of 10 mg IV haloperidol.
The time from administration to documentation of QTP and/or TdP ranged from immediately post administration to 8 hours after administration of the last dose of IV haloperidol.
Baseline QTc was known in 44 patients. Baseline QTc was >450 msec in 18 of these 44 patients.
The change from baseline QTc varied widely from 20 msec to 286 msec; 36 patients demonstrated a prolongation of >50 msec.
In those patients with reported haloperidol‐associated QTP, 25 patients demonstrated a QTc >600 msec and 38 patients >520 msec.9 Of the cases with known specific QTc values, the QTc was prolonged >450 msec in 48 out of 50 cases. The lowest reported QTc leading to TdP was 413 msec.
A total of 20 patients were reported as having a normalization of QTc (as defined by the original reports) within several hours to 8 days; minimal QTP was reported as persisting in 2 patients. The specifics of the other patients were unknown, although 25 patients were categorized as recovered, 13 were stated as having an uneventful remainder of hospitalization, and 5 patients were discharged to a rehabilitation facility or a nursing home.
Discussion
The current review was performed in response to the FDA warning recommending the use of continuous ECG monitoring associated with the administration of intravenous haloperiodol.5 This warning has resulted in substantial dilemmas for health care organizations, additional resource allocation, and increased scrutiny from regulatory agencies. The results of our review reveal that intravenous haloperidol‐associated QTP and TdP almost uniformly take place in patients with concomitant risk factors and with cumulative doses 2 mg. In light of these findings, it is possible that hospitals may be able to administer intravenous haloperidol in patients without risk factors without continuous ECG monitoring. In reviewing these published reports, it is important to note that the FDA identified 28 published cases of haloperidol‐associated QTP and TdP, while our review yielded a total of 41 published case reports.
The FDA database included 73 cases of haloperidol‐associated TdP in their database. However, these cases included both oral as well as IV administration; using our methodology, we identified 29 additional case reports associated with intravenous haloperidol from this database. Consequently, our review included 41 published case reports and 29 FDA database cases, resulting in the total of 70 patients.
Our review revealed a number of practical findings. First, our summary demonstrated that neither QTP nor TdP has been documented with a cumulative dose of IV haloperidol of <2 mg. The majority of patients (80%) received cumulative IV doses 10 mg. The lowest dose associated with sudden cardiac arrest was 6 mg and this took place in a 69‐year‐old male patient. Second, the majority (97%) of our patients had additional risk factors for QTP and/or TdP. Pre‐existing heart disease,1619 electrolyte imbalance,17, 1921 concomitant proarrhythmic drugs16, 17, 1922 and mechanical ventilation17, 23 were identified as the most commonly observed risk factors (Table 4). Lastly, in those cases in which the data were reported, baseline QTc was >450 msec in 41% of the patients, and 96% had a QTc at the time of the event >450 msec. Therefore, we conclude that patients: (1) receiving low cumulative doses (<2 mg) with (2) no risk factors for prolonged QTc or TdP, and (3) with a normal QTc on baseline EKG can safely be given IV haloperidol in the hospital setting.
This dosage range is consistent with the labelling for IV haloperidol dosing in Canada24 and Germany25 (Table 1), where single doses of 0.25 mg to 1.5 mg are recommended for the treatment of delirium or acute agitation in the geriatric population.24, 25
In a recent Cochrane review, low‐dose IV haloperidol (<3 mg per day) was concluded to be as safe and effective as atypical antipsychotics in the treatment of acute delirium with respect to extrapyramidal adverse effects.2
The American Psychiatric Association recommends an initial IV dose of 12 mg every 24 hours as needed (0.250.50 mg every 4 hours as needed for elderly patients), with titration to higher doses for patients who continue to be agitated for the treatment of patients with delirium (issued 1999, updated 2004).3
While several expert‐groups and investigators currently consider IV haloperidol as an important therapeutic option for treating acute delirium and agitation in the dose range presented above, less consensus exists regarding monitoring requirements.2, 3, 26, 27
The American Psychiatric Association recommends IV haloperidol only after a baseline ECG is obtained. These guidelines have not been updated since the release of the FDA extended warning.3 In their recent review, Morandi et al.28 support the dosage recommendation of the 1999 American Psychiatric Association's practice guidelines for treatment of delirium,3 ie, administration of IV haloperidol in single doses of 0.5 mg to 2 mg in elderly patients, however, only after a baseline ECG is obtained.28 While the package insert of IV haloperidol in France29 recommends a baseline ECG, Germany,25 Italy30 and Switzerland's31 package information states the need for regular ECG monitoring. Guidelines for the treatment of delirium in the intensive care unit published by the American College of Critical Care Medicine and the Society of Critical Care Medicine in collaboration with the American Society of Health‐System Pharmacists consider IV haloperidol as the preferred agent for the treatment of delirium in critically ill patients (grade of recommendation = C). These expert groups recommend that patients should be monitored for electrocardiographic changes (QT interval prolongation and arrhythmias) when receiving haloperidol (Grade of recommendation = B).32
Nevertheless, continuous ECG monitoring (ie, telemetry) is expensive, labor‐intensive and, potentially overutilized.33, 34 Requiring clinicians to place all patients receiving intravenous haloperidol on telemetry is impractical and potentially costly. Mandating telemetry could also lead to unintended harm, ie, use of a less effective or less safe drug to avoid compliance with the telemetry mandate.
Based on our findings and the current recommendations in the literature, inpatient providers should be thoughtful and deliberate in the use of haloperidol to treat acute delirium with agitation. Patients requiring pharmacologic management of their delirium should be screened for risk factors for QTP and TdP (Table 4) and a baseline ECG should be obtained prior to haloperidol administration. If significant risk factors exist or the baseline ECG reveals a prolonged QTc, then the patient should receive continuous ECG monitoring. Similarly, if cumulative doses of 2 mg are needed, the patient should be placed on telemetry.
There are some limitations to our study design. Our findings are based upon previously published case reports or data submitted to the FDA MedWatch. While the content of the FDA's MedWatch database is accessible to the public via the Freedom of Information Act (FOIA), the events are neither categorized nor peer‐reviewed upon entry into the database. Consequently, information may be incomplete or inaccurate. In addition, the denominator representing the overall use of IV haloperidol is unknown, thus a rate of event cannot be assigned and the true scope of the problem cannot be determined. Despite these limitations, this summary represents the most comprehensive review of the literature to date, expanding on the analysis performed by the FDA. Of note, in our review of the FDA database, we noted several cases of haloperidol‐associated QTP or TdP associated with other routes of administration. Thus, it is unknown whether this complication is any greater with IV vs. the IM or per os (PO) routes of administration.
Conclusion
Although the proarrhythmic potential of haloperidol and other antipsychotics has been well established in the literature, IV haloperidol has been considered relatively safe with respect to this complication from the time of its approval in 1967.5, 1722, 35, 36 In reviewing all reported cases of cardiac complications associated with IV haloperidol, as well as the current literature, an association with QTP and TdP is likely. However, the case reports reveal that QTP and TdP generally occur in the setting of concomitant risk factors, and no cases have been reported utilizing a cumulative IV dose of <2 mg. It may therefore be safe to administer a cumulative dose of IV haloperidol of <2 mg without ECG monitoring in patients without risk factors for QTP. However, ECG monitoring should take place with IV haloperidol doses 2 mg and/or in those patients with additional risk factors of developing QTP and/or TdP.
Based on the findings of this review complemented by the guidelines of various expert‐groups and the official labelling information of different countries, the Pharmacy & Therapeutics Committee of the UCSF Medical Center revised the IV haloperidol policy: administration of a total dose of <2 mg IV haloperidol without concurrent telemetry is allowed in a noncritical care setting in patients without risk factors for QTP and TdP.
Acknowledgements
The authors acknowledge Gloria Won of the Fishbon Library at UCSF Medical Center at Mount Zion for her support.
- Haldol® injection (for immediate release) Package Insert.Raritan, NJ:Ortho‐McNeil Pharmaceutical Inc.;2005;rev. 05.2007.
- ,,,. Antipsychotics for delirium (review), the Cochrane collaboration2008;2. Available at: www. cochrane.org. Accessed February 2010.
- American Psychiatric Association: practice guideline for the treatment of patients with delirium.Am J Psychiatry.1999;156(5 suppl):1–20, updated 2004.
- Thomson Micromedex.2008. Micromedex healthcare series: “haloperidol” Thomson Micromedex, Greenwood Village.
- FDA alert: haloperidol (marketed as Haldol, Haldol Decanoate and Haldol Lactate).2007. This alert highlights revisions to the labeling for haloperidol. Available at: www.fda.gov. Accessed February 2010.
- ,,.Use of high‐dose intravenous haloperidol in the treatment of agitated cardiac patients,J Clin Psychopharmacol.1985;5(6):344–347.
- ,,, et al.Postoperative delirium,Am J Psychiatry.2008;165:7.
- ,.Corrected QT interval prolongation associated with intravenous haloperidol in acute coronary syndromes,Catheter Cardiovasc Interv.2000;50(3):352–355.
- ,.Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death.Am J Psych.2001;158(11):1774–1782.
- ,,, et al.Accuracy of uncorrected versus corrected QT Interval for Prediction of torsade de pointes associated with intravenous haloperidol.Pharmacotherapy.2007;27(2):175–182.
- ,,.Three cases of ventricular tachycardia and torsades de pointes induced by antipsychotic drugs.Shinzo.1997;29(1):68–74.
- ,,,.Haloperidol por via intravenosa y torsade de pointes.Medicina intensive.2004;28(2):89.
- ,,, et al.QT‐Verlängerung und Kammerflimmern unter Haloperidol‐ und Clonidin‐Therapie des Alkoholentzugssyndroms.Intensivmedizin und Notfallmedizin.1992;29(4):178–183.
- ARIZONA CERT, Arizona Center for Education and Research on Therapeutics. Available at: www.azcert.org. Accessed February 2010.
- ,.Cardiac arrhythmias—a clinical approach.Edinburgh:Mosby;2003.
- ,.The association between intravenous haloperidol and torsades de pointes—three cases and a literature review.Psychosomatics.1995;36:541–549.
- ,.Prolongation of the corrected QT and torsades de pointes cardiac arrhythmia associated with intravenous haloperidol in the medically ill.J Clin Psychopharmacol.1993;13(2):128–132.
- ,,,,.Torsades de pointes secondary to intravenous haloperidol after coronary bypass grafting surgery.Can J Anesth.2000;47(3):251–254.
- ,.Torsade de pointes associated with the administration of intravenous haloperidol: a review of the literature and practical guidelines for use,Expert Opin Drug Saf.2003;2(6):543–547.
- ,.Conduction disturbances associated with administration of butyrophenone antipsychotics in the critically ill: a review of the literature,Pharmacotherapy.1997;17(3):531–537.
- ,,.Haloperidol‐induced torsades de pointes.Ann Pharacother.1999;33(10):1046–1050.
- ,,, et al.Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary,Crit Care Med.1995;23(9):1596–1600.
- ,,,,,.Prolonged cardiac repolarization after tacrolimus and haloperidol administration in the critically ill patient.Pharmacotherapy.2004;24(3):404–408.
- CPS Compendium of Pharmaceuticals and Specialties, the Canadian drug reference for health professionals, 2007, Canadian pharmacists association.
- Rote Liste Deutschland2008, Rote Liste Service GmbH Frankfurt am Main. Available at: www.rote‐liste.de. Accessed February 2010.
- ,.Delirium in the hospitalized patient: a primer for the pharmacist clinician.J Pharm Pract.2007;20(5):368–372.
- ,,, et al.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,.The pharmacological management of delirium in critical illness.Current Drug Therapy.2008,3:148–157.
- VIDAL‐l'information sur les produits de santé2008, Issy les Moulineaux Cedex. Available at: www.vidal.fr. Accessed February 2010.
- Haldol iniettabile—ufficiale monografia italiana. Available at: www. informatorefarmaceutico.it. Accessed February 2010.
- Arzneimittelkompendium der Schweiz2008, documed Verlag Basel. Availabla at: www.kompendium.ch. Accessed February 2010.
- ,,, et al.Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill, American College of Critical Care Medicine ACCM, Society of Critical Care Medicine SCCM, American Society of Health‐System Pharmacists ASHP.Crit Care Med.2002;30(1):119–141.
- ,,,,.Is telemetry overused? Is it as helpful as thought?Cleve Clin J Med.2009;76(6):368–372.
- ,,.Telemetry outside critical care units: patterns of utilization and influence on management decisions.Clin Cardiol.1998;21(7):503–505.
- ,,.High‐dose intravenous haloperidol for agitated delirium in a cardiac patient on intra‐aortic balloon pump.J Clin Psychopharmacol.1991;11(2):146–147.
- .Haloperidol, midazolam and intravenous sedation.Aust NZ J Psychiatry.1999;33(6):942–943.
- BNF British National Formulary, compendium of pharmaceuticals and specialties of the UK.2007. Available at: www.bnf.org. Accessed February 2010.
- ,,,.Torsade de pointes associated with the use of intravenous haloperidol.Ann Intern Med.1993;119(5):391–394.
- ,.Torsades de pointes: potential consequence of intravenous haloperidol in the intensive care unit.Intensive Care World.1994;11(3):109–112.
- ,.Torsade de pointes caused by high‐dose intravenous haloperidol in cardiac patients.Clin Cardiol.1995;18:285–290.
- ,,.Continuous infusion of haloperidol controls agitation in critically ill patients.Crit Care Med.1994:22(3):433–440.
- ,,.Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalances.Am J Gastroenterol.1995;90(5):822–824.
- ,,,.Torsades de pointes associated with intravenous haloperidol in critically ill patients.Am J Cardiol.1998;81(2):238–240.
- ,,,,.The effect of intravenous haloperidol on QT interval dispersion in critically ill patients: comparison with QT interval prolongation for assessment of risk of torsades de pointes.J Clin Pharmacol.2001;41:1310–1318.
- ,,.Low‐dose haloperidol associated QTc prolongation.J Am Geriatr Soc.2008;56(10):1963–1964.
- ,,.Torsade de pointes following intravenous haloperidol administration in a patient with complete heart block.WMJ.2009;108(1):48–50.
Haloperidol is Food and Drug Administration (FDA)‐approved in the United States for the management of acute and chronic psychotic disorders and widely used in the management of delirium‐associated agitation in hospitalized patients.1 Delirium in the hospital is an acute confusional state that frequently arises from multiple complex factors and may affect up to 30% of hospitalized patients.2 Although the first step in the management of delirium involves identification and treatment of underlying causes and offering supportive behavioral care; medications may be needed to control severe agitation.2 Low dose intravenous (IV) haloperidol (ie, 0.250.5 mg every 4 hours) is a commonly used medication in this setting as recommended by expert‐groups including the Cochrane Collaboration and the American Psychiatric Association.2, 3
Although injectable haloperidol, a butyrophenone‐derived antipsychotic agent pharmacologically related to the piperazine phenothiazines,4 is approved for IV use in many countries (Table 1), parenteral use is approved only for intramuscular (IM) administration in the US. Thus, IV administration of the drug in the US is considered an off‐label use.5
| Indication | Country | |||||
|---|---|---|---|---|---|---|
| Canada24 | France29 | Germany25 | Great Britain37 | Italy30 | Switzerland31 | |
| ||||||
| Mainly delirium (schizophrenia, other psychosis, short‐term management of psychomotor agitation, excitement, violent or dangerously impulsive behavior, vomiting, hiccup) | Short term treatment of agitation and aggressiveness during an acute or chronic psychotic episode, vomiting along with antimitotic post‐radiotherapy treatment | Acute and chronic schizophrenia, psycho‐motorical agitation of psychotic genesis | Schizophrenia, other psychosis, short‐term adjunctive management if psychomotor agitation, violent or dangerous impulsive behavior | Resistant forms of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis' chronic psychosis High doses restrictions: syndrome of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis, chronic psychosis | Acute schizophrenic episode, mania, vomiting | |
| IV dosing in adults | 12 mg every 24 hours | The use is limited to adult patients and the drug can be administered IM or IV. The IV route is restricted to the treatment of vomiting. | 510 mg/day, daily max.: 30(100) mg | 210 mg initially, PRN every 48 hours, daily max. 18 mg | 510 mg initially, PRN every hour, daily max. 60 mg | 5 mg PRN every 30 minutes |
| IV dosing in geriatric care | 0.250.5 mg | Single dose of 0.51.5 mg, daily max. 5 mg | Half adult dose | Adjust to appropriate dose | 0.5 mg, than PRN | |
| Risk factors for the development of cardiac adverse events | QT prolonging drugs, diabetes, obesity, hypokalemia, congenital long QT syndrome | Bradycardia <55 beats per minute, hypokalemia, congenital QT prolongation, other medications provoking bradycardia, deceleration of the intra‐cardiac transition or prolonged QT interval | QT syndrome, hypokalemia, other electrolyte imbalance, cardiovascular diseases, QT prolongation in the family history | Cardiovalscular disease, drugs that can prolong the QTc, diabetes, obesity, hypokalemia, congenital long QT syndrome | Contraindications: recent cardiac infarction, uncompensated cardiac insufficiency, cardiac arrhythmias, antiarrhythmic drugs, pre‐existing QT prolongation, cases of arrhythmia or torsades de pointes in the family history, untreated potassium imbalance, QTc prolonging drugs | QT syndrome, hypokalemia, hypomagnesemia, other electrolyte imbalances, cardiovascular diseases, hypothyreosis, QT prolongation in the family history |
| Monitoring recommendations | Electrolytes | ECG monitoring at admission time, electrolytes | ECG monitoring, electrolytes | Metabolic parameters | ECG at baseline and regular ECG monitoring, electrolytes | Close ECG monitoring, electrolytes |
| General recommendations | Regular reevaluation in long‐term use | Apply the lowest effective dose | Apply the lowest effective dose | Application per mouth is the route of choice | Decrease dose if QTc >500 msec | Switch to PO as soon as possible |
Haloperidol is often preferred over other antipsychotics as a result of its effectiveness, low rate of anticholinergic side effects, familiarity with dosing and usage, and minimal respiratory or sedative properties.6 Use of the IV route in patients with acute delirium has several advantages over the IM or oral route,7 including rapid onset, immediate bioavailability, and ease and safety of administration.
Prior to September 2007, the package insert for haloperidol alerted healthcare professionals to the risk of cardiovascular side effects. Based on case reports of potentially fatal cardiac events, the FDA revised the label, warning that the QT prolongation (QTP) and risk of torsades de pointes (TdP) were increased with IV administration of haloperidol or administration of the drug at greater than recommended doses. Unfortunately, neither the typical dosing range nor the minimum dose associated with these cardiac side effects were specified in this recommendation.5
It is well‐established that haloperidol may prolong the QT interval by blocking the repolarizing potassium IKr current.8 Although drugs that block the IKr channel can produce arrhythmia in healthy individuals, additional risk factors, such as underlying heart conditions, electrolyte imbalances (ie, hypokalemia and hypomagnesemia), concomitant proarrhythmic drug use, and mechanical ventilation may increase this risk.9 Prolongation of the QT interval has been associated with subsequent malignant cardiac arrhythmias including ventricular fibrillation and TdP.10 Prolongation of the QT interval is considered the strongest risk factor for TdP, particularly with a baseline QTc > 450 msec.9
Based on the increased risk for QTP and TdP and the case reports of cardiac events, the FDA advisory recommended continuous electrocardiogram (ECG) monitoring in patients receiving IV haloperidol.5 However, such monitoring may be impractical and costly in hospitalized patients who require low doses of IV haloperidol to manage acute delirium and who are not in telemetry or intensive care units.
The aim of this review was to evaluate the case reports leading to the recent FDA warning for IV haloperidol, specifically focusing on the presence of risk factors for arrhythmias. Based upon the evidence, an additional aim was to provide an institutional response to this warning toward the optimal use of this agent.
Method
Two search pathways were used to evaluate reports of haloperidol‐associated TdP and/or QT prolongation:
Literature Review
We searched for published literature in humans indexed in Pubmed (1966April 2009), EMBASE (1972April 2009), and Scopus (1823April 2009) using the search terms haloperidol or Haldol combined with intravenous or infusion and at least one of the following terms: QT prolongation, TdP, torsades de pointes, torsades with a specific focus on case reports.
References from the retrieved articles were also reviewed to search for additional case reports.
In addition to cases reported in English journals, several of our reports originated from Japan11 (translation provided by the FDA), Spain12 and Germany13 (translated by the primary author).
Search of the FDA Database
We reviewed all adverse drug events reported through MedWatch or those submitted by the manufacturer from November 1997 to April 2008 through the Freedom of Information Act (FOIA) request. The FDA provided a full‐text summary of 5944 reports involving oral, intramuscular and IV use of haloperidol. The FDA data were transferred to a Microsoft Access database and screened for the key terms torsade, QT, prolongation, wave. Incident report number, date of report, age, gender, origin of report, medication name, role of drug as categorized by the FDA (suspect, concomitant, primary suspect, secondary suspect), route, dose, units, duration, symptoms and FDA outcome category (death, life‐threatening, hospitalization initial or prolonged, disability, congenital anomaly, required intervention to prevent permanent damage, other) were recorded. Only those reports in which IV haloperidol was considered by the reporter to be the primary causative agent for the adverse event were reviewed. Available information included diagnosis, laboratory parameters, QTc measurement, cardiac symptoms, outcomes and a description of recovery. No peer review was applied to the MedWatch reports and the data reported in this publication reflect the original information from the FDA MedWatch database. Baseline QTc was either the value defined as such in the original report or the lowest QTc reported. Haloperidol doses administered were defined as cumulative dose at event, encompassing all doses administered during the hospital stay until the occurrence of the adverse cardiac event.
The drugs listed in the case reports were assessed for proarrhythmic potential using 2 references: the individual package insert and the website of the Arizona Center for Education and Research on Therapeutics.14
The drugs were only considered proarrhythmic when the 2 resources were in agreement.
Duplicates and/or previously published cases, as well as reports involving adverse cardiac effects not associated with QTP or TdP, were identified and excluded.
In their advisory, the FDA does not state the exact origin of the reports, their specific search strategy to identify haloperidol‐associated adverse events, or the role IV haloperidol played in the individual events included in the extended warning. Consequently, the number of events identified in this review may differ from that published in the FDA extended warning.
Results
A total of 70 reported cases of IV haloperidol associated TdP and/or QTP were identified. Of these 70, 41 were identified through the PubMed/EMBASE/Scopus review, while an additional 29 cases were identified through the FDA database search.
Of the 29 cases in the FDA database, 21 were reported by health care professionals and 8 by manufacturers.
A total of 35 publications described cases originating from the US. Three cases took place in Japan and 1 case each in Canada, Germany and Spain. Several cases in the MedWatch database were reported outside the US: 1 case each originated from Austria, Canada, France, Japan, Spain, Switzerland and the United Kingdom. A summary of the published case reports is displayed in Table 2 and the FDA cases are summarized in Table 3.
| Case | Source (reference#) | Date | Age, Years | Gender | Drugs Pro‐arrhyth. | Venti‐ lated | Max. Daily Dose (mg) | Total Dose at Event (mg) | Time to Event | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | ECG Normalization, Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
| 1 | 35 | 1991 | 56 | m | No | Yes | 1200 | 1540 | NR | Yes | 584 (400) | 184 | NR | NR, uneventful |
| 2 | 13 | 1992 | 36 | m | Yes | No | 11.5 | 11.5 | 20 hours after start | Yes | 714 (428) | 286 | Yes | QTc normalization (440 msec), NR |
| 3 | 38 | 1993 | 39 | f | Yes | Yes | NR | 580 | Max. QTc 72 hours after start | Yes | 650 (420) | 230 | Yes | QTc normalization after 6 days, uneventful |
| 4 | 38 | 1993 | 19 | f | Yes | No | 170 | 170 | Max. QT 12 hours after start | Yes | 600 (480) | 120 | Yes | QTc normalization after 8 days, uneventful |
| 5 | 38 | 1993 | 63 | f | Yes | No | NR | 489 | Max. QT 48 hours after start | Yes | 670 (520) | 150 | Yes | QTc normalization after 8 days, uneventful |
| 6 | 38 | 1993 | 74 | f | Yes | Yes | NR | 10 | NR | No | 430 (410) | 20 | Yes | QTc unchanged after 8 days, uneventful |
| 7 | 17 | 1993 | 39 | m | Yes | Yes | NR | >490 | NR | Yes | 457 (348) | 109 | Yes | QTc normalization within 2 to 3 days, no further TdP, NR |
| 8 | 17 | 1993 | 61 | m | Yes | Yes | 115 | 211 | NR | Yes | 500 (390) | 110 | NR | QTc normalization within 2 days, death |
| 9 | 17 | 1993 | 48 | m | Yes | Yes | 825 | 825 | NR | Yes | 538 (441) | 97 | NR | QTc normalization in 3 days, rehabilitation |
| 10 | 39 | 1994 | 23 | f | Yes | Yes | 120 | 300 | 12 hours after dose increase | Yes | NR (550) | NR | Yes | NR, uneventful, extubation after 5 days, discharge after 10 days |
| 11 | 39 | 1994 | 28 | m | Yes | Yes | 300 | >300 | 24 hours after dose increase | Yes | NR (>520) | NR | Yes | No recurrence of arrhythmia, patient death (multi‐organ failure) |
| 12 | 40 | 1994 | 65 | m | Yes | NR | 230 | 410 | Worsening from day 2 to day 5 | Yes | 594 (490) | 104 | Yes | QTc normalization (406 msec), no cardiac problems at discharge |
| 13 | 40 | 1994 | 65 | f | Yes | NR | 500 | 980 | After the last 60mg | Yes | 628 (403) | 225 | Yes | QTc normalization (<400 msec), recurrence with oral haloperidol, rehabilitation |
| 14 | 40 | 1994 | 76 | f | Yes | NR | 21 | 26 | Day 2 after several boluses | Yes | 670 (450) | 220 | Yes | QTc normalization within several days (412 msec), rehabilitation |
| 15 | 41 | 1994 | 59 | m | NR | Yes | 865 | 1013 | NR | Yes | 640 (480) | 160 | NR | QTc normalization in 24 hours, survived |
| 16 | 16 | 1995 | 76 | f | Yes | No | NR | 44.5 plus 1 PO | 15 minutes | Yes | 670 (409) | 261 | Yes | ECG normalized the next morning, no further events |
| 17 | 16 | 1995 | 49 | m | Yes | No | NR | 1150 plus 20 IM | 45 minutes | Yes | 648 (380) | 268 | Yes | QTc normalization in 24 hours, anoxic brain insult/rehabilitation |
| 18 | 16 | 1995 | 65 | f | Yes | No | 600 | 965 | 30 minutes | Yes | 628 (403) | 225 | Yes | 3 more episodes of TdP in 3 hours, QTc normalization in 2 days, no recurrence with further haloperidol, NR |
| 19 | 42 | 1995 | 42 | m | Yes | No | 28 | 28 | 20 minutes | Yes | 610 (533) | 77 | Yes | QTc normalization in 5 days, uneventful, ECG normal |
| 20 | 42 | 1995 | 39 | m | Yes | No | 45 | 45 | 5 minutes | Yes | 654 (NR) | NR | Yes | QTc normalization after 24 hours, uneventful |
| 21 | 11 | 1997 | 56 | f | No | No | 10 | 10 | Shortly after | NR | NR (NR) | NR | Yes | TdP resolved after 8 hours, NR |
| 22 | 11 | 1997 | 82 | f | NR | No | 10 | 10 | Shortly after | Yes | 680 (NR) | NR | Yes | QTc normalization on day 6 after admission (470 msec), NR |
| 23 | 11 | 1997 | 35 | m | NR | No | NR | 90 | After 20 mg | Yes | 520 (NR) | NR | Yes | TdP disappeared 12 hours later, NR |
| 24 | 43,44 | 1998 | 45 | m | NR | Yes* | NR | 9 | 203 minutes | Yes | 638 (560) | 78 | Yes | NR, overall survival 100%, significantly prolonged hospital stay |
| 25 | 43,44 | 1998 | 64 | f | NR | NR | 115 | 220 minutes | Yes | 605 (424) | 181 | Yes | ||
| 26 | 43,44 | 1998 | 75 | f | NR | NR | 85 | 60 minutes | Yes | 567 (508) | 59 | Yes | ||
| 27 | 43,44 | 1998 | 71 | f | NR | NR | 55 | 120 minutes | Paced | Paced | Paced | Yes | ||
| 28 | 43,44 | 1998 | 58 | f | NR | NR | 75 | 38 minutes | Yes | 657 (542) | 115 | Yes | ||
| 29 | 43,44 | 1998 | 40 | m | NR | NR | 35 | 15 minutes | Yes | 679 (475) | 204 | Yes | ||
| 30 | 43,44 | 1998 | 71 | m | NR | NR | 70 | 58 minutes | Yes | 521 (478) | 43 | Yes | ||
| 31 | 43,44 | 1998 | 47 | m | NR | 400 | 400 | 79 minutes | Yes | 574 (444) | 130 | Yes | ||
| 32 | 21 | 1999 | 41 | f | Yes | Yes | 320 | 915 | 55 minutes | Yes | 610 (426) | 184 | Yes | QTc normalization after 5 day, uneventful |
| 33 | 21 | 1999 | 31 | m | Yes | Yes | 480 | 1700 | 40 minutes | Yes | 599 (491) | 108 | Yes | QTc normalized in 4 days, NR |
| 34 | 18 | 2000 | 64 | f | Yes | Yes | 175 | 175 | NR | No | 413 (418) | (‐5) | Yes | QTc remained unchanged, uneventful |
| 35 | 8 | 2000 | 75 | m | No | NR | >2 | >2 | NR | Yes | 615 (435) | 180 | No | QTc normalization in 48 hours, uneventful |
| 36 | 8 | 2000 | 68 | m | Yes | Yes | >2 | >2 | NR | Yes | 650 (407) | 243 | No | QTc normalization after 4 day, uneventful after extubation |
| 37 | 8 | 2000 | 77 | m | NR | NR | (4) | 2 | NR | Yes | 550 (393) | 157 | No | QTc normalization in 24 to 36 hours, NR |
| 38 | 12 | 2004 | 34 | m | Yes | NR | 24.5 | 24.5 | 20 minutes | Yes | 560 (420) | 140 | Yes | QTc normalization (440 msec), ECG normal |
| 39 | 23 | 2004 | 58 | f | Yes | NR | 340 | 1010 | NR | Yes | 533 (460) | 73 | Yes | QTc normalization 7 days later discharge after 27days |
| 40 | 45 | 2008 | 86 | f | Yes | No | 2 mg | 2 mg | 8 hours after last dose | Yes | 524 (NR) | Probably 79 | No | QTc normalization (445 msec), NR |
| 41 | 46 | 2009 | 74 | m | Yes | No | 2 | 2 | Shortly after | Yes | NR (579) | NR | Yes | Pre‐existing heart block and fibrillation resolved, nursing home/rehabilitation |
| Report | MedWatch Identifier | Report Date | Age, Years | Gender | Drugs Pro‐arrh. | Maximum Daily Dose (mg) | Total Dose at Event (mg) | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | Outcome; Recovery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
| 1 | 3122988‐1 | 1998 | 61 | m | No | 48 | 48 | Yes | NR | NR | Yes | Intervention; NR |
| 2 | 3157827‐6 | 1998 | 44 | f | No | 160 | 160 | Yes | 550 (440) | 110 | Yes | Intervention; uneventful |
| 3 | 3178715‐5 | 1999 | 60 | m | NR | 415 | 645 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization in 1 day, no recurrence |
| 4 | 3271261‐X | 1999 | 56 | m | NR | NR | 20 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization |
| 5 | 3271080‐4 | 1999 | 35 | m | Yes | 7 | 7 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced, hospitalization prolonged |
| 6 | 3325391‐4 | 1999 | 55 | f | Yes | 75 | 75 | NR | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 7 | 3381921‐8 | 1999 | 52 | m | No | 320 | 634 | Yes | 458 (430) | 28 | Yes | Death; NA |
| 8 | 3483869‐7 | 2000 | 18 | m | No | >200 | >310 | Yes | NR | NR | Yes | Intervention; no recurrence after haloperidol reinstitution |
| 9 | 3516342‐8 | 2000 | NR | NR | NR | NR | NR | NR | NR | NR | Yes | NR; NR |
| 10 | 3516320‐9 | 2000 | 34 | m | Yes | 5 | 5 | Yes | NR | NR | No | Life‐threatening; event abated after dose stopped |
| 11 | 3552263‐2 | 2000 | 46 | f | Yes | NR | 97.5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 12 | 3574705‐9 | 2000 | 78 | m | Yes | NR | 160 | Yes | 603 (453) | 50 | Yes | Intervention; event abated after dose stopped/reduced |
| 13 | 3703871‐7 | 2001 | 27 | m | NR | 530 | 530 | Yes | NR | NR | Yes | Death, NA |
| 14 | 3724567‐1 | 2001 | 31 | m | Yes | 6 | 6 | Yes | 496 (449) | 47 | No | Life‐threatening; ECG returned to baseline |
| 15 | 3851984‐1 | 2002 | 72 | f | NR | 18 | 18 | NR | NR | NR | Yes | Hospitalization; NR |
| 16 | 3942407‐2 | 2002 | 51 | m | Yes | 14 | 14 | Yes | 461 (444) | 17 | Yes | Life‐threatening; no recurrence |
| 17 | 4066580‐3 | 2003 | >60 | f | NR | 50 | 50 | Yes | >600 (480) | >120 | No | Hospitalization; QTc normalization, patient recovered |
| 18 | 4126280‐8 | 2003 | 47 | f | NR | 60 | 180 | Yes | 550 (450) | 100 | No (bradycardia) | Hospitalization; patient recovered |
| 19 | 4150700‐6 | 2003 | NR | m | NR | 5 | 5 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced |
| 20 | 4340092‐1 | 2004 | 52 | m | Yes | 5 | 5 | Yes | >500 (490) | >10 | NR (polymorphous VT) | Life‐threatening; NR |
| 21 | 4714692‐0 | 2005 | NR | m | NR | NR | NR | Yes | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 22 | 4881813‐9 | 2006 | NR | m | NR | NR | 40 | NR | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 23 | 4892225‐6 | 2006 | NR | f | Yes | 10 | >10 | Yes | 493 (300) | 193 | No | Hospitalization; QTc normalization (403 msec) |
| 24 | 4911873‐8 | 2006 | 69 | m | Yes | 6 | 6 | NR | NR | NR | Yes | Cardiac arrest, death; NA |
| 25 | 5366448‐6 | 2007 | 53 | m | Yes | NR | 35 | Yes | NR | NR | NR | Cardiac arrest, life‐threatening; patient recovered |
| 26 | 5563440‐3 | 2007 | 58 | m | Possible | 5 | 5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 27 | 5642929‐2 | 2008 | 42 | m | Yes | 165 | 165 | Yes | 640 (350) | 290 | Yes | Death; NA |
| 28 | 5697758‐0 | 2008 | 38 | m | Yes | NR | 620 | NR | NR | NR | Yes | Hospitalization; patient recovered |
| 29 | 5254840‐X | 2008 | 19 | f | Possible | 15 | 25 | Yes | 461 | NR | NR | Cardiac arrest, hospitalization; patient recovered |
Of the 70 cases, 54 cases of TdP were reported. The remaining 16 of 70 cases involved cases of QTP, 9 of which did not progress to TdP and 7 of which the progression to TdP was unclear. Of note, 42 of 54 of the cases of TdP were reported as preceded by documented QTP. Presence of QTP was unknown in the other 12 original reports. Three out of 70 patients experienced sudden cardiac arrest, 1 of which was fatal. One arrest was preceded by TdP and 2 by QTP (Figure 1).

The patient ages ranged from 18 years to 86 years. Of note, 17 patients experiencing TdP and/or QTP were <40 years old, and 2 of those patients were <30 years old.
Haloperidol‐associated QTP and/or TdP were observed in 27 female and 42 male patients; the gender was not stated in one report. Of the 54 patients experiencing TdP (with or without report of previous QTP), 22 were female and 31 were male (1 gender unknown).
A total of 68 of 70 patients were determined to have associated risk factors15 for QTP/TdP (see Table 4). The circumstances of the remaining 2 patients were not described in sufficient detail to identify associated risk factors.
| Risk Factor | Patients, n (%) |
|---|---|
| |
| Any risk factor | 68/70 (97) |
| Unknown | 2/70 (3) |
| Specific risk factors | |
| Electrolyte imbalance | 27/68 (40) |
| Underlying cardiac disease | 32/68 (47) |
| Concomitant proarrhythmic agents | 39/68 (57) |
| Other drugs influencing cardiac function | 23/68 (34) |
| Baseline QTc >450 msec | 18/68 (26) |
| QTc known: 44 patients | 18/44 (41) |
Overall, 32 patients had underlying heart conditions. Electrolyte imbalances, including hypokalemia, hypomagnesemia, and hypocalcemia, were present in 17 patients. At least 39 patients were receiving potentially proarrhythmic agents (1‐8 proarrhythmic drugs per patient) in addition to IV haloperidol. At least 23 patients were receiving additional drugs with a potential for other cardiac adverse events than QTP and TdP.
A wide range of other disease states previously reported to be associated with QTP15 were identified in these patients: asthma (5 patients), diabetes (5 patients), obesity (3 patients), impaired renal and/or liver function (3 patients each), human immunodeficiency virus (HIV) (2 patients); chronic obstructive pulmonary disease (COPD), pancreatitis and hypothyroidism (1 patient each). A total of 22 patients had a history of substance abuse (alcohol and/or drugs), and 4 patients were smokers.
The administered doses of IV haloperidol varied widely. Considering that information regarding the maximal daily dose was missing in 22 reports and ambiguous in another 20 cases, the results have been presented using cumulative IV haloperidol doses. Patients experiencing TdP without preceding QTP received a cumulative dose (= total dose at event) ranging from 5 mg to 645 mg. Patients with both confirmed QTP and TdP were administered a cumulative dose of 2 mg to 1700 mg. Patients who experienced QTP without TdP received a cumulative dose of 2 mg to 1540 mg of IV haloperidol.
Sudden cardiac arrest following administration of IV haloperidol was observed in cumulative doses ranging from 6 mg to 35 mg. The cardiac arrest leading to a fatal outcome was preceded by an administration of at least 6 mg of IV haloperidol. Overall, 14 out of 70 patients received cumulative doses of 10 mg IV haloperidol.
The time from administration to documentation of QTP and/or TdP ranged from immediately post administration to 8 hours after administration of the last dose of IV haloperidol.
Baseline QTc was known in 44 patients. Baseline QTc was >450 msec in 18 of these 44 patients.
The change from baseline QTc varied widely from 20 msec to 286 msec; 36 patients demonstrated a prolongation of >50 msec.
In those patients with reported haloperidol‐associated QTP, 25 patients demonstrated a QTc >600 msec and 38 patients >520 msec.9 Of the cases with known specific QTc values, the QTc was prolonged >450 msec in 48 out of 50 cases. The lowest reported QTc leading to TdP was 413 msec.
A total of 20 patients were reported as having a normalization of QTc (as defined by the original reports) within several hours to 8 days; minimal QTP was reported as persisting in 2 patients. The specifics of the other patients were unknown, although 25 patients were categorized as recovered, 13 were stated as having an uneventful remainder of hospitalization, and 5 patients were discharged to a rehabilitation facility or a nursing home.
Discussion
The current review was performed in response to the FDA warning recommending the use of continuous ECG monitoring associated with the administration of intravenous haloperiodol.5 This warning has resulted in substantial dilemmas for health care organizations, additional resource allocation, and increased scrutiny from regulatory agencies. The results of our review reveal that intravenous haloperidol‐associated QTP and TdP almost uniformly take place in patients with concomitant risk factors and with cumulative doses 2 mg. In light of these findings, it is possible that hospitals may be able to administer intravenous haloperidol in patients without risk factors without continuous ECG monitoring. In reviewing these published reports, it is important to note that the FDA identified 28 published cases of haloperidol‐associated QTP and TdP, while our review yielded a total of 41 published case reports.
The FDA database included 73 cases of haloperidol‐associated TdP in their database. However, these cases included both oral as well as IV administration; using our methodology, we identified 29 additional case reports associated with intravenous haloperidol from this database. Consequently, our review included 41 published case reports and 29 FDA database cases, resulting in the total of 70 patients.
Our review revealed a number of practical findings. First, our summary demonstrated that neither QTP nor TdP has been documented with a cumulative dose of IV haloperidol of <2 mg. The majority of patients (80%) received cumulative IV doses 10 mg. The lowest dose associated with sudden cardiac arrest was 6 mg and this took place in a 69‐year‐old male patient. Second, the majority (97%) of our patients had additional risk factors for QTP and/or TdP. Pre‐existing heart disease,1619 electrolyte imbalance,17, 1921 concomitant proarrhythmic drugs16, 17, 1922 and mechanical ventilation17, 23 were identified as the most commonly observed risk factors (Table 4). Lastly, in those cases in which the data were reported, baseline QTc was >450 msec in 41% of the patients, and 96% had a QTc at the time of the event >450 msec. Therefore, we conclude that patients: (1) receiving low cumulative doses (<2 mg) with (2) no risk factors for prolonged QTc or TdP, and (3) with a normal QTc on baseline EKG can safely be given IV haloperidol in the hospital setting.
This dosage range is consistent with the labelling for IV haloperidol dosing in Canada24 and Germany25 (Table 1), where single doses of 0.25 mg to 1.5 mg are recommended for the treatment of delirium or acute agitation in the geriatric population.24, 25
In a recent Cochrane review, low‐dose IV haloperidol (<3 mg per day) was concluded to be as safe and effective as atypical antipsychotics in the treatment of acute delirium with respect to extrapyramidal adverse effects.2
The American Psychiatric Association recommends an initial IV dose of 12 mg every 24 hours as needed (0.250.50 mg every 4 hours as needed for elderly patients), with titration to higher doses for patients who continue to be agitated for the treatment of patients with delirium (issued 1999, updated 2004).3
While several expert‐groups and investigators currently consider IV haloperidol as an important therapeutic option for treating acute delirium and agitation in the dose range presented above, less consensus exists regarding monitoring requirements.2, 3, 26, 27
The American Psychiatric Association recommends IV haloperidol only after a baseline ECG is obtained. These guidelines have not been updated since the release of the FDA extended warning.3 In their recent review, Morandi et al.28 support the dosage recommendation of the 1999 American Psychiatric Association's practice guidelines for treatment of delirium,3 ie, administration of IV haloperidol in single doses of 0.5 mg to 2 mg in elderly patients, however, only after a baseline ECG is obtained.28 While the package insert of IV haloperidol in France29 recommends a baseline ECG, Germany,25 Italy30 and Switzerland's31 package information states the need for regular ECG monitoring. Guidelines for the treatment of delirium in the intensive care unit published by the American College of Critical Care Medicine and the Society of Critical Care Medicine in collaboration with the American Society of Health‐System Pharmacists consider IV haloperidol as the preferred agent for the treatment of delirium in critically ill patients (grade of recommendation = C). These expert groups recommend that patients should be monitored for electrocardiographic changes (QT interval prolongation and arrhythmias) when receiving haloperidol (Grade of recommendation = B).32
Nevertheless, continuous ECG monitoring (ie, telemetry) is expensive, labor‐intensive and, potentially overutilized.33, 34 Requiring clinicians to place all patients receiving intravenous haloperidol on telemetry is impractical and potentially costly. Mandating telemetry could also lead to unintended harm, ie, use of a less effective or less safe drug to avoid compliance with the telemetry mandate.
Based on our findings and the current recommendations in the literature, inpatient providers should be thoughtful and deliberate in the use of haloperidol to treat acute delirium with agitation. Patients requiring pharmacologic management of their delirium should be screened for risk factors for QTP and TdP (Table 4) and a baseline ECG should be obtained prior to haloperidol administration. If significant risk factors exist or the baseline ECG reveals a prolonged QTc, then the patient should receive continuous ECG monitoring. Similarly, if cumulative doses of 2 mg are needed, the patient should be placed on telemetry.
There are some limitations to our study design. Our findings are based upon previously published case reports or data submitted to the FDA MedWatch. While the content of the FDA's MedWatch database is accessible to the public via the Freedom of Information Act (FOIA), the events are neither categorized nor peer‐reviewed upon entry into the database. Consequently, information may be incomplete or inaccurate. In addition, the denominator representing the overall use of IV haloperidol is unknown, thus a rate of event cannot be assigned and the true scope of the problem cannot be determined. Despite these limitations, this summary represents the most comprehensive review of the literature to date, expanding on the analysis performed by the FDA. Of note, in our review of the FDA database, we noted several cases of haloperidol‐associated QTP or TdP associated with other routes of administration. Thus, it is unknown whether this complication is any greater with IV vs. the IM or per os (PO) routes of administration.
Conclusion
Although the proarrhythmic potential of haloperidol and other antipsychotics has been well established in the literature, IV haloperidol has been considered relatively safe with respect to this complication from the time of its approval in 1967.5, 1722, 35, 36 In reviewing all reported cases of cardiac complications associated with IV haloperidol, as well as the current literature, an association with QTP and TdP is likely. However, the case reports reveal that QTP and TdP generally occur in the setting of concomitant risk factors, and no cases have been reported utilizing a cumulative IV dose of <2 mg. It may therefore be safe to administer a cumulative dose of IV haloperidol of <2 mg without ECG monitoring in patients without risk factors for QTP. However, ECG monitoring should take place with IV haloperidol doses 2 mg and/or in those patients with additional risk factors of developing QTP and/or TdP.
Based on the findings of this review complemented by the guidelines of various expert‐groups and the official labelling information of different countries, the Pharmacy & Therapeutics Committee of the UCSF Medical Center revised the IV haloperidol policy: administration of a total dose of <2 mg IV haloperidol without concurrent telemetry is allowed in a noncritical care setting in patients without risk factors for QTP and TdP.
Acknowledgements
The authors acknowledge Gloria Won of the Fishbon Library at UCSF Medical Center at Mount Zion for her support.
Haloperidol is Food and Drug Administration (FDA)‐approved in the United States for the management of acute and chronic psychotic disorders and widely used in the management of delirium‐associated agitation in hospitalized patients.1 Delirium in the hospital is an acute confusional state that frequently arises from multiple complex factors and may affect up to 30% of hospitalized patients.2 Although the first step in the management of delirium involves identification and treatment of underlying causes and offering supportive behavioral care; medications may be needed to control severe agitation.2 Low dose intravenous (IV) haloperidol (ie, 0.250.5 mg every 4 hours) is a commonly used medication in this setting as recommended by expert‐groups including the Cochrane Collaboration and the American Psychiatric Association.2, 3
Although injectable haloperidol, a butyrophenone‐derived antipsychotic agent pharmacologically related to the piperazine phenothiazines,4 is approved for IV use in many countries (Table 1), parenteral use is approved only for intramuscular (IM) administration in the US. Thus, IV administration of the drug in the US is considered an off‐label use.5
| Indication | Country | |||||
|---|---|---|---|---|---|---|
| Canada24 | France29 | Germany25 | Great Britain37 | Italy30 | Switzerland31 | |
| ||||||
| Mainly delirium (schizophrenia, other psychosis, short‐term management of psychomotor agitation, excitement, violent or dangerously impulsive behavior, vomiting, hiccup) | Short term treatment of agitation and aggressiveness during an acute or chronic psychotic episode, vomiting along with antimitotic post‐radiotherapy treatment | Acute and chronic schizophrenia, psycho‐motorical agitation of psychotic genesis | Schizophrenia, other psychosis, short‐term adjunctive management if psychomotor agitation, violent or dangerous impulsive behavior | Resistant forms of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis' chronic psychosis High doses restrictions: syndrome of psycho‐motorical excitement, acute delirious and/or hallucinatory psychosis, chronic psychosis | Acute schizophrenic episode, mania, vomiting | |
| IV dosing in adults | 12 mg every 24 hours | The use is limited to adult patients and the drug can be administered IM or IV. The IV route is restricted to the treatment of vomiting. | 510 mg/day, daily max.: 30(100) mg | 210 mg initially, PRN every 48 hours, daily max. 18 mg | 510 mg initially, PRN every hour, daily max. 60 mg | 5 mg PRN every 30 minutes |
| IV dosing in geriatric care | 0.250.5 mg | Single dose of 0.51.5 mg, daily max. 5 mg | Half adult dose | Adjust to appropriate dose | 0.5 mg, than PRN | |
| Risk factors for the development of cardiac adverse events | QT prolonging drugs, diabetes, obesity, hypokalemia, congenital long QT syndrome | Bradycardia <55 beats per minute, hypokalemia, congenital QT prolongation, other medications provoking bradycardia, deceleration of the intra‐cardiac transition or prolonged QT interval | QT syndrome, hypokalemia, other electrolyte imbalance, cardiovascular diseases, QT prolongation in the family history | Cardiovalscular disease, drugs that can prolong the QTc, diabetes, obesity, hypokalemia, congenital long QT syndrome | Contraindications: recent cardiac infarction, uncompensated cardiac insufficiency, cardiac arrhythmias, antiarrhythmic drugs, pre‐existing QT prolongation, cases of arrhythmia or torsades de pointes in the family history, untreated potassium imbalance, QTc prolonging drugs | QT syndrome, hypokalemia, hypomagnesemia, other electrolyte imbalances, cardiovascular diseases, hypothyreosis, QT prolongation in the family history |
| Monitoring recommendations | Electrolytes | ECG monitoring at admission time, electrolytes | ECG monitoring, electrolytes | Metabolic parameters | ECG at baseline and regular ECG monitoring, electrolytes | Close ECG monitoring, electrolytes |
| General recommendations | Regular reevaluation in long‐term use | Apply the lowest effective dose | Apply the lowest effective dose | Application per mouth is the route of choice | Decrease dose if QTc >500 msec | Switch to PO as soon as possible |
Haloperidol is often preferred over other antipsychotics as a result of its effectiveness, low rate of anticholinergic side effects, familiarity with dosing and usage, and minimal respiratory or sedative properties.6 Use of the IV route in patients with acute delirium has several advantages over the IM or oral route,7 including rapid onset, immediate bioavailability, and ease and safety of administration.
Prior to September 2007, the package insert for haloperidol alerted healthcare professionals to the risk of cardiovascular side effects. Based on case reports of potentially fatal cardiac events, the FDA revised the label, warning that the QT prolongation (QTP) and risk of torsades de pointes (TdP) were increased with IV administration of haloperidol or administration of the drug at greater than recommended doses. Unfortunately, neither the typical dosing range nor the minimum dose associated with these cardiac side effects were specified in this recommendation.5
It is well‐established that haloperidol may prolong the QT interval by blocking the repolarizing potassium IKr current.8 Although drugs that block the IKr channel can produce arrhythmia in healthy individuals, additional risk factors, such as underlying heart conditions, electrolyte imbalances (ie, hypokalemia and hypomagnesemia), concomitant proarrhythmic drug use, and mechanical ventilation may increase this risk.9 Prolongation of the QT interval has been associated with subsequent malignant cardiac arrhythmias including ventricular fibrillation and TdP.10 Prolongation of the QT interval is considered the strongest risk factor for TdP, particularly with a baseline QTc > 450 msec.9
Based on the increased risk for QTP and TdP and the case reports of cardiac events, the FDA advisory recommended continuous electrocardiogram (ECG) monitoring in patients receiving IV haloperidol.5 However, such monitoring may be impractical and costly in hospitalized patients who require low doses of IV haloperidol to manage acute delirium and who are not in telemetry or intensive care units.
The aim of this review was to evaluate the case reports leading to the recent FDA warning for IV haloperidol, specifically focusing on the presence of risk factors for arrhythmias. Based upon the evidence, an additional aim was to provide an institutional response to this warning toward the optimal use of this agent.
Method
Two search pathways were used to evaluate reports of haloperidol‐associated TdP and/or QT prolongation:
Literature Review
We searched for published literature in humans indexed in Pubmed (1966April 2009), EMBASE (1972April 2009), and Scopus (1823April 2009) using the search terms haloperidol or Haldol combined with intravenous or infusion and at least one of the following terms: QT prolongation, TdP, torsades de pointes, torsades with a specific focus on case reports.
References from the retrieved articles were also reviewed to search for additional case reports.
In addition to cases reported in English journals, several of our reports originated from Japan11 (translation provided by the FDA), Spain12 and Germany13 (translated by the primary author).
Search of the FDA Database
We reviewed all adverse drug events reported through MedWatch or those submitted by the manufacturer from November 1997 to April 2008 through the Freedom of Information Act (FOIA) request. The FDA provided a full‐text summary of 5944 reports involving oral, intramuscular and IV use of haloperidol. The FDA data were transferred to a Microsoft Access database and screened for the key terms torsade, QT, prolongation, wave. Incident report number, date of report, age, gender, origin of report, medication name, role of drug as categorized by the FDA (suspect, concomitant, primary suspect, secondary suspect), route, dose, units, duration, symptoms and FDA outcome category (death, life‐threatening, hospitalization initial or prolonged, disability, congenital anomaly, required intervention to prevent permanent damage, other) were recorded. Only those reports in which IV haloperidol was considered by the reporter to be the primary causative agent for the adverse event were reviewed. Available information included diagnosis, laboratory parameters, QTc measurement, cardiac symptoms, outcomes and a description of recovery. No peer review was applied to the MedWatch reports and the data reported in this publication reflect the original information from the FDA MedWatch database. Baseline QTc was either the value defined as such in the original report or the lowest QTc reported. Haloperidol doses administered were defined as cumulative dose at event, encompassing all doses administered during the hospital stay until the occurrence of the adverse cardiac event.
The drugs listed in the case reports were assessed for proarrhythmic potential using 2 references: the individual package insert and the website of the Arizona Center for Education and Research on Therapeutics.14
The drugs were only considered proarrhythmic when the 2 resources were in agreement.
Duplicates and/or previously published cases, as well as reports involving adverse cardiac effects not associated with QTP or TdP, were identified and excluded.
In their advisory, the FDA does not state the exact origin of the reports, their specific search strategy to identify haloperidol‐associated adverse events, or the role IV haloperidol played in the individual events included in the extended warning. Consequently, the number of events identified in this review may differ from that published in the FDA extended warning.
Results
A total of 70 reported cases of IV haloperidol associated TdP and/or QTP were identified. Of these 70, 41 were identified through the PubMed/EMBASE/Scopus review, while an additional 29 cases were identified through the FDA database search.
Of the 29 cases in the FDA database, 21 were reported by health care professionals and 8 by manufacturers.
A total of 35 publications described cases originating from the US. Three cases took place in Japan and 1 case each in Canada, Germany and Spain. Several cases in the MedWatch database were reported outside the US: 1 case each originated from Austria, Canada, France, Japan, Spain, Switzerland and the United Kingdom. A summary of the published case reports is displayed in Table 2 and the FDA cases are summarized in Table 3.
| Case | Source (reference#) | Date | Age, Years | Gender | Drugs Pro‐arrhyth. | Venti‐ lated | Max. Daily Dose (mg) | Total Dose at Event (mg) | Time to Event | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | ECG Normalization, Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
| 1 | 35 | 1991 | 56 | m | No | Yes | 1200 | 1540 | NR | Yes | 584 (400) | 184 | NR | NR, uneventful |
| 2 | 13 | 1992 | 36 | m | Yes | No | 11.5 | 11.5 | 20 hours after start | Yes | 714 (428) | 286 | Yes | QTc normalization (440 msec), NR |
| 3 | 38 | 1993 | 39 | f | Yes | Yes | NR | 580 | Max. QTc 72 hours after start | Yes | 650 (420) | 230 | Yes | QTc normalization after 6 days, uneventful |
| 4 | 38 | 1993 | 19 | f | Yes | No | 170 | 170 | Max. QT 12 hours after start | Yes | 600 (480) | 120 | Yes | QTc normalization after 8 days, uneventful |
| 5 | 38 | 1993 | 63 | f | Yes | No | NR | 489 | Max. QT 48 hours after start | Yes | 670 (520) | 150 | Yes | QTc normalization after 8 days, uneventful |
| 6 | 38 | 1993 | 74 | f | Yes | Yes | NR | 10 | NR | No | 430 (410) | 20 | Yes | QTc unchanged after 8 days, uneventful |
| 7 | 17 | 1993 | 39 | m | Yes | Yes | NR | >490 | NR | Yes | 457 (348) | 109 | Yes | QTc normalization within 2 to 3 days, no further TdP, NR |
| 8 | 17 | 1993 | 61 | m | Yes | Yes | 115 | 211 | NR | Yes | 500 (390) | 110 | NR | QTc normalization within 2 days, death |
| 9 | 17 | 1993 | 48 | m | Yes | Yes | 825 | 825 | NR | Yes | 538 (441) | 97 | NR | QTc normalization in 3 days, rehabilitation |
| 10 | 39 | 1994 | 23 | f | Yes | Yes | 120 | 300 | 12 hours after dose increase | Yes | NR (550) | NR | Yes | NR, uneventful, extubation after 5 days, discharge after 10 days |
| 11 | 39 | 1994 | 28 | m | Yes | Yes | 300 | >300 | 24 hours after dose increase | Yes | NR (>520) | NR | Yes | No recurrence of arrhythmia, patient death (multi‐organ failure) |
| 12 | 40 | 1994 | 65 | m | Yes | NR | 230 | 410 | Worsening from day 2 to day 5 | Yes | 594 (490) | 104 | Yes | QTc normalization (406 msec), no cardiac problems at discharge |
| 13 | 40 | 1994 | 65 | f | Yes | NR | 500 | 980 | After the last 60mg | Yes | 628 (403) | 225 | Yes | QTc normalization (<400 msec), recurrence with oral haloperidol, rehabilitation |
| 14 | 40 | 1994 | 76 | f | Yes | NR | 21 | 26 | Day 2 after several boluses | Yes | 670 (450) | 220 | Yes | QTc normalization within several days (412 msec), rehabilitation |
| 15 | 41 | 1994 | 59 | m | NR | Yes | 865 | 1013 | NR | Yes | 640 (480) | 160 | NR | QTc normalization in 24 hours, survived |
| 16 | 16 | 1995 | 76 | f | Yes | No | NR | 44.5 plus 1 PO | 15 minutes | Yes | 670 (409) | 261 | Yes | ECG normalized the next morning, no further events |
| 17 | 16 | 1995 | 49 | m | Yes | No | NR | 1150 plus 20 IM | 45 minutes | Yes | 648 (380) | 268 | Yes | QTc normalization in 24 hours, anoxic brain insult/rehabilitation |
| 18 | 16 | 1995 | 65 | f | Yes | No | 600 | 965 | 30 minutes | Yes | 628 (403) | 225 | Yes | 3 more episodes of TdP in 3 hours, QTc normalization in 2 days, no recurrence with further haloperidol, NR |
| 19 | 42 | 1995 | 42 | m | Yes | No | 28 | 28 | 20 minutes | Yes | 610 (533) | 77 | Yes | QTc normalization in 5 days, uneventful, ECG normal |
| 20 | 42 | 1995 | 39 | m | Yes | No | 45 | 45 | 5 minutes | Yes | 654 (NR) | NR | Yes | QTc normalization after 24 hours, uneventful |
| 21 | 11 | 1997 | 56 | f | No | No | 10 | 10 | Shortly after | NR | NR (NR) | NR | Yes | TdP resolved after 8 hours, NR |
| 22 | 11 | 1997 | 82 | f | NR | No | 10 | 10 | Shortly after | Yes | 680 (NR) | NR | Yes | QTc normalization on day 6 after admission (470 msec), NR |
| 23 | 11 | 1997 | 35 | m | NR | No | NR | 90 | After 20 mg | Yes | 520 (NR) | NR | Yes | TdP disappeared 12 hours later, NR |
| 24 | 43,44 | 1998 | 45 | m | NR | Yes* | NR | 9 | 203 minutes | Yes | 638 (560) | 78 | Yes | NR, overall survival 100%, significantly prolonged hospital stay |
| 25 | 43,44 | 1998 | 64 | f | NR | NR | 115 | 220 minutes | Yes | 605 (424) | 181 | Yes | ||
| 26 | 43,44 | 1998 | 75 | f | NR | NR | 85 | 60 minutes | Yes | 567 (508) | 59 | Yes | ||
| 27 | 43,44 | 1998 | 71 | f | NR | NR | 55 | 120 minutes | Paced | Paced | Paced | Yes | ||
| 28 | 43,44 | 1998 | 58 | f | NR | NR | 75 | 38 minutes | Yes | 657 (542) | 115 | Yes | ||
| 29 | 43,44 | 1998 | 40 | m | NR | NR | 35 | 15 minutes | Yes | 679 (475) | 204 | Yes | ||
| 30 | 43,44 | 1998 | 71 | m | NR | NR | 70 | 58 minutes | Yes | 521 (478) | 43 | Yes | ||
| 31 | 43,44 | 1998 | 47 | m | NR | 400 | 400 | 79 minutes | Yes | 574 (444) | 130 | Yes | ||
| 32 | 21 | 1999 | 41 | f | Yes | Yes | 320 | 915 | 55 minutes | Yes | 610 (426) | 184 | Yes | QTc normalization after 5 day, uneventful |
| 33 | 21 | 1999 | 31 | m | Yes | Yes | 480 | 1700 | 40 minutes | Yes | 599 (491) | 108 | Yes | QTc normalized in 4 days, NR |
| 34 | 18 | 2000 | 64 | f | Yes | Yes | 175 | 175 | NR | No | 413 (418) | (‐5) | Yes | QTc remained unchanged, uneventful |
| 35 | 8 | 2000 | 75 | m | No | NR | >2 | >2 | NR | Yes | 615 (435) | 180 | No | QTc normalization in 48 hours, uneventful |
| 36 | 8 | 2000 | 68 | m | Yes | Yes | >2 | >2 | NR | Yes | 650 (407) | 243 | No | QTc normalization after 4 day, uneventful after extubation |
| 37 | 8 | 2000 | 77 | m | NR | NR | (4) | 2 | NR | Yes | 550 (393) | 157 | No | QTc normalization in 24 to 36 hours, NR |
| 38 | 12 | 2004 | 34 | m | Yes | NR | 24.5 | 24.5 | 20 minutes | Yes | 560 (420) | 140 | Yes | QTc normalization (440 msec), ECG normal |
| 39 | 23 | 2004 | 58 | f | Yes | NR | 340 | 1010 | NR | Yes | 533 (460) | 73 | Yes | QTc normalization 7 days later discharge after 27days |
| 40 | 45 | 2008 | 86 | f | Yes | No | 2 mg | 2 mg | 8 hours after last dose | Yes | 524 (NR) | Probably 79 | No | QTc normalization (445 msec), NR |
| 41 | 46 | 2009 | 74 | m | Yes | No | 2 | 2 | Shortly after | Yes | NR (579) | NR | Yes | Pre‐existing heart block and fibrillation resolved, nursing home/rehabilitation |
| Report | MedWatch Identifier | Report Date | Age, Years | Gender | Drugs Pro‐arrh. | Maximum Daily Dose (mg) | Total Dose at Event (mg) | Prolonged QT | QTc Maximal (baseline), msec | Change in QTc (msec) | TdP | Outcome; Recovery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
| 1 | 3122988‐1 | 1998 | 61 | m | No | 48 | 48 | Yes | NR | NR | Yes | Intervention; NR |
| 2 | 3157827‐6 | 1998 | 44 | f | No | 160 | 160 | Yes | 550 (440) | 110 | Yes | Intervention; uneventful |
| 3 | 3178715‐5 | 1999 | 60 | m | NR | 415 | 645 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization in 1 day, no recurrence |
| 4 | 3271261‐X | 1999 | 56 | m | NR | NR | 20 | Yes | NR | NR | Yes | Life‐threatening; QTc normalization |
| 5 | 3271080‐4 | 1999 | 35 | m | Yes | 7 | 7 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced, hospitalization prolonged |
| 6 | 3325391‐4 | 1999 | 55 | f | Yes | 75 | 75 | NR | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 7 | 3381921‐8 | 1999 | 52 | m | No | 320 | 634 | Yes | 458 (430) | 28 | Yes | Death; NA |
| 8 | 3483869‐7 | 2000 | 18 | m | No | >200 | >310 | Yes | NR | NR | Yes | Intervention; no recurrence after haloperidol reinstitution |
| 9 | 3516342‐8 | 2000 | NR | NR | NR | NR | NR | NR | NR | NR | Yes | NR; NR |
| 10 | 3516320‐9 | 2000 | 34 | m | Yes | 5 | 5 | Yes | NR | NR | No | Life‐threatening; event abated after dose stopped |
| 11 | 3552263‐2 | 2000 | 46 | f | Yes | NR | 97.5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 12 | 3574705‐9 | 2000 | 78 | m | Yes | NR | 160 | Yes | 603 (453) | 50 | Yes | Intervention; event abated after dose stopped/reduced |
| 13 | 3703871‐7 | 2001 | 27 | m | NR | 530 | 530 | Yes | NR | NR | Yes | Death, NA |
| 14 | 3724567‐1 | 2001 | 31 | m | Yes | 6 | 6 | Yes | 496 (449) | 47 | No | Life‐threatening; ECG returned to baseline |
| 15 | 3851984‐1 | 2002 | 72 | f | NR | 18 | 18 | NR | NR | NR | Yes | Hospitalization; NR |
| 16 | 3942407‐2 | 2002 | 51 | m | Yes | 14 | 14 | Yes | 461 (444) | 17 | Yes | Life‐threatening; no recurrence |
| 17 | 4066580‐3 | 2003 | >60 | f | NR | 50 | 50 | Yes | >600 (480) | >120 | No | Hospitalization; QTc normalization, patient recovered |
| 18 | 4126280‐8 | 2003 | 47 | f | NR | 60 | 180 | Yes | 550 (450) | 100 | No (bradycardia) | Hospitalization; patient recovered |
| 19 | 4150700‐6 | 2003 | NR | m | NR | 5 | 5 | NR | NR | NR | Yes | NR; event abated after dose stopped/reduced |
| 20 | 4340092‐1 | 2004 | 52 | m | Yes | 5 | 5 | Yes | >500 (490) | >10 | NR (polymorphous VT) | Life‐threatening; NR |
| 21 | 4714692‐0 | 2005 | NR | m | NR | NR | NR | Yes | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 22 | 4881813‐9 | 2006 | NR | m | NR | NR | 40 | NR | NR | NR | Yes | Hospitalization; event abated after dose stopped/reduced |
| 23 | 4892225‐6 | 2006 | NR | f | Yes | 10 | >10 | Yes | 493 (300) | 193 | No | Hospitalization; QTc normalization (403 msec) |
| 24 | 4911873‐8 | 2006 | 69 | m | Yes | 6 | 6 | NR | NR | NR | Yes | Cardiac arrest, death; NA |
| 25 | 5366448‐6 | 2007 | 53 | m | Yes | NR | 35 | Yes | NR | NR | NR | Cardiac arrest, life‐threatening; patient recovered |
| 26 | 5563440‐3 | 2007 | 58 | m | Possible | 5 | 5 | Yes | NR | NR | Yes | Life‐threatening; event abated after dose stopped/reduced |
| 27 | 5642929‐2 | 2008 | 42 | m | Yes | 165 | 165 | Yes | 640 (350) | 290 | Yes | Death; NA |
| 28 | 5697758‐0 | 2008 | 38 | m | Yes | NR | 620 | NR | NR | NR | Yes | Hospitalization; patient recovered |
| 29 | 5254840‐X | 2008 | 19 | f | Possible | 15 | 25 | Yes | 461 | NR | NR | Cardiac arrest, hospitalization; patient recovered |
Of the 70 cases, 54 cases of TdP were reported. The remaining 16 of 70 cases involved cases of QTP, 9 of which did not progress to TdP and 7 of which the progression to TdP was unclear. Of note, 42 of 54 of the cases of TdP were reported as preceded by documented QTP. Presence of QTP was unknown in the other 12 original reports. Three out of 70 patients experienced sudden cardiac arrest, 1 of which was fatal. One arrest was preceded by TdP and 2 by QTP (Figure 1).

The patient ages ranged from 18 years to 86 years. Of note, 17 patients experiencing TdP and/or QTP were <40 years old, and 2 of those patients were <30 years old.
Haloperidol‐associated QTP and/or TdP were observed in 27 female and 42 male patients; the gender was not stated in one report. Of the 54 patients experiencing TdP (with or without report of previous QTP), 22 were female and 31 were male (1 gender unknown).
A total of 68 of 70 patients were determined to have associated risk factors15 for QTP/TdP (see Table 4). The circumstances of the remaining 2 patients were not described in sufficient detail to identify associated risk factors.
| Risk Factor | Patients, n (%) |
|---|---|
| |
| Any risk factor | 68/70 (97) |
| Unknown | 2/70 (3) |
| Specific risk factors | |
| Electrolyte imbalance | 27/68 (40) |
| Underlying cardiac disease | 32/68 (47) |
| Concomitant proarrhythmic agents | 39/68 (57) |
| Other drugs influencing cardiac function | 23/68 (34) |
| Baseline QTc >450 msec | 18/68 (26) |
| QTc known: 44 patients | 18/44 (41) |
Overall, 32 patients had underlying heart conditions. Electrolyte imbalances, including hypokalemia, hypomagnesemia, and hypocalcemia, were present in 17 patients. At least 39 patients were receiving potentially proarrhythmic agents (1‐8 proarrhythmic drugs per patient) in addition to IV haloperidol. At least 23 patients were receiving additional drugs with a potential for other cardiac adverse events than QTP and TdP.
A wide range of other disease states previously reported to be associated with QTP15 were identified in these patients: asthma (5 patients), diabetes (5 patients), obesity (3 patients), impaired renal and/or liver function (3 patients each), human immunodeficiency virus (HIV) (2 patients); chronic obstructive pulmonary disease (COPD), pancreatitis and hypothyroidism (1 patient each). A total of 22 patients had a history of substance abuse (alcohol and/or drugs), and 4 patients were smokers.
The administered doses of IV haloperidol varied widely. Considering that information regarding the maximal daily dose was missing in 22 reports and ambiguous in another 20 cases, the results have been presented using cumulative IV haloperidol doses. Patients experiencing TdP without preceding QTP received a cumulative dose (= total dose at event) ranging from 5 mg to 645 mg. Patients with both confirmed QTP and TdP were administered a cumulative dose of 2 mg to 1700 mg. Patients who experienced QTP without TdP received a cumulative dose of 2 mg to 1540 mg of IV haloperidol.
Sudden cardiac arrest following administration of IV haloperidol was observed in cumulative doses ranging from 6 mg to 35 mg. The cardiac arrest leading to a fatal outcome was preceded by an administration of at least 6 mg of IV haloperidol. Overall, 14 out of 70 patients received cumulative doses of 10 mg IV haloperidol.
The time from administration to documentation of QTP and/or TdP ranged from immediately post administration to 8 hours after administration of the last dose of IV haloperidol.
Baseline QTc was known in 44 patients. Baseline QTc was >450 msec in 18 of these 44 patients.
The change from baseline QTc varied widely from 20 msec to 286 msec; 36 patients demonstrated a prolongation of >50 msec.
In those patients with reported haloperidol‐associated QTP, 25 patients demonstrated a QTc >600 msec and 38 patients >520 msec.9 Of the cases with known specific QTc values, the QTc was prolonged >450 msec in 48 out of 50 cases. The lowest reported QTc leading to TdP was 413 msec.
A total of 20 patients were reported as having a normalization of QTc (as defined by the original reports) within several hours to 8 days; minimal QTP was reported as persisting in 2 patients. The specifics of the other patients were unknown, although 25 patients were categorized as recovered, 13 were stated as having an uneventful remainder of hospitalization, and 5 patients were discharged to a rehabilitation facility or a nursing home.
Discussion
The current review was performed in response to the FDA warning recommending the use of continuous ECG monitoring associated with the administration of intravenous haloperiodol.5 This warning has resulted in substantial dilemmas for health care organizations, additional resource allocation, and increased scrutiny from regulatory agencies. The results of our review reveal that intravenous haloperidol‐associated QTP and TdP almost uniformly take place in patients with concomitant risk factors and with cumulative doses 2 mg. In light of these findings, it is possible that hospitals may be able to administer intravenous haloperidol in patients without risk factors without continuous ECG monitoring. In reviewing these published reports, it is important to note that the FDA identified 28 published cases of haloperidol‐associated QTP and TdP, while our review yielded a total of 41 published case reports.
The FDA database included 73 cases of haloperidol‐associated TdP in their database. However, these cases included both oral as well as IV administration; using our methodology, we identified 29 additional case reports associated with intravenous haloperidol from this database. Consequently, our review included 41 published case reports and 29 FDA database cases, resulting in the total of 70 patients.
Our review revealed a number of practical findings. First, our summary demonstrated that neither QTP nor TdP has been documented with a cumulative dose of IV haloperidol of <2 mg. The majority of patients (80%) received cumulative IV doses 10 mg. The lowest dose associated with sudden cardiac arrest was 6 mg and this took place in a 69‐year‐old male patient. Second, the majority (97%) of our patients had additional risk factors for QTP and/or TdP. Pre‐existing heart disease,1619 electrolyte imbalance,17, 1921 concomitant proarrhythmic drugs16, 17, 1922 and mechanical ventilation17, 23 were identified as the most commonly observed risk factors (Table 4). Lastly, in those cases in which the data were reported, baseline QTc was >450 msec in 41% of the patients, and 96% had a QTc at the time of the event >450 msec. Therefore, we conclude that patients: (1) receiving low cumulative doses (<2 mg) with (2) no risk factors for prolonged QTc or TdP, and (3) with a normal QTc on baseline EKG can safely be given IV haloperidol in the hospital setting.
This dosage range is consistent with the labelling for IV haloperidol dosing in Canada24 and Germany25 (Table 1), where single doses of 0.25 mg to 1.5 mg are recommended for the treatment of delirium or acute agitation in the geriatric population.24, 25
In a recent Cochrane review, low‐dose IV haloperidol (<3 mg per day) was concluded to be as safe and effective as atypical antipsychotics in the treatment of acute delirium with respect to extrapyramidal adverse effects.2
The American Psychiatric Association recommends an initial IV dose of 12 mg every 24 hours as needed (0.250.50 mg every 4 hours as needed for elderly patients), with titration to higher doses for patients who continue to be agitated for the treatment of patients with delirium (issued 1999, updated 2004).3
While several expert‐groups and investigators currently consider IV haloperidol as an important therapeutic option for treating acute delirium and agitation in the dose range presented above, less consensus exists regarding monitoring requirements.2, 3, 26, 27
The American Psychiatric Association recommends IV haloperidol only after a baseline ECG is obtained. These guidelines have not been updated since the release of the FDA extended warning.3 In their recent review, Morandi et al.28 support the dosage recommendation of the 1999 American Psychiatric Association's practice guidelines for treatment of delirium,3 ie, administration of IV haloperidol in single doses of 0.5 mg to 2 mg in elderly patients, however, only after a baseline ECG is obtained.28 While the package insert of IV haloperidol in France29 recommends a baseline ECG, Germany,25 Italy30 and Switzerland's31 package information states the need for regular ECG monitoring. Guidelines for the treatment of delirium in the intensive care unit published by the American College of Critical Care Medicine and the Society of Critical Care Medicine in collaboration with the American Society of Health‐System Pharmacists consider IV haloperidol as the preferred agent for the treatment of delirium in critically ill patients (grade of recommendation = C). These expert groups recommend that patients should be monitored for electrocardiographic changes (QT interval prolongation and arrhythmias) when receiving haloperidol (Grade of recommendation = B).32
Nevertheless, continuous ECG monitoring (ie, telemetry) is expensive, labor‐intensive and, potentially overutilized.33, 34 Requiring clinicians to place all patients receiving intravenous haloperidol on telemetry is impractical and potentially costly. Mandating telemetry could also lead to unintended harm, ie, use of a less effective or less safe drug to avoid compliance with the telemetry mandate.
Based on our findings and the current recommendations in the literature, inpatient providers should be thoughtful and deliberate in the use of haloperidol to treat acute delirium with agitation. Patients requiring pharmacologic management of their delirium should be screened for risk factors for QTP and TdP (Table 4) and a baseline ECG should be obtained prior to haloperidol administration. If significant risk factors exist or the baseline ECG reveals a prolonged QTc, then the patient should receive continuous ECG monitoring. Similarly, if cumulative doses of 2 mg are needed, the patient should be placed on telemetry.
There are some limitations to our study design. Our findings are based upon previously published case reports or data submitted to the FDA MedWatch. While the content of the FDA's MedWatch database is accessible to the public via the Freedom of Information Act (FOIA), the events are neither categorized nor peer‐reviewed upon entry into the database. Consequently, information may be incomplete or inaccurate. In addition, the denominator representing the overall use of IV haloperidol is unknown, thus a rate of event cannot be assigned and the true scope of the problem cannot be determined. Despite these limitations, this summary represents the most comprehensive review of the literature to date, expanding on the analysis performed by the FDA. Of note, in our review of the FDA database, we noted several cases of haloperidol‐associated QTP or TdP associated with other routes of administration. Thus, it is unknown whether this complication is any greater with IV vs. the IM or per os (PO) routes of administration.
Conclusion
Although the proarrhythmic potential of haloperidol and other antipsychotics has been well established in the literature, IV haloperidol has been considered relatively safe with respect to this complication from the time of its approval in 1967.5, 1722, 35, 36 In reviewing all reported cases of cardiac complications associated with IV haloperidol, as well as the current literature, an association with QTP and TdP is likely. However, the case reports reveal that QTP and TdP generally occur in the setting of concomitant risk factors, and no cases have been reported utilizing a cumulative IV dose of <2 mg. It may therefore be safe to administer a cumulative dose of IV haloperidol of <2 mg without ECG monitoring in patients without risk factors for QTP. However, ECG monitoring should take place with IV haloperidol doses 2 mg and/or in those patients with additional risk factors of developing QTP and/or TdP.
Based on the findings of this review complemented by the guidelines of various expert‐groups and the official labelling information of different countries, the Pharmacy & Therapeutics Committee of the UCSF Medical Center revised the IV haloperidol policy: administration of a total dose of <2 mg IV haloperidol without concurrent telemetry is allowed in a noncritical care setting in patients without risk factors for QTP and TdP.
Acknowledgements
The authors acknowledge Gloria Won of the Fishbon Library at UCSF Medical Center at Mount Zion for her support.
- Haldol® injection (for immediate release) Package Insert.Raritan, NJ:Ortho‐McNeil Pharmaceutical Inc.;2005;rev. 05.2007.
- ,,,. Antipsychotics for delirium (review), the Cochrane collaboration2008;2. Available at: www. cochrane.org. Accessed February 2010.
- American Psychiatric Association: practice guideline for the treatment of patients with delirium.Am J Psychiatry.1999;156(5 suppl):1–20, updated 2004.
- Thomson Micromedex.2008. Micromedex healthcare series: “haloperidol” Thomson Micromedex, Greenwood Village.
- FDA alert: haloperidol (marketed as Haldol, Haldol Decanoate and Haldol Lactate).2007. This alert highlights revisions to the labeling for haloperidol. Available at: www.fda.gov. Accessed February 2010.
- ,,.Use of high‐dose intravenous haloperidol in the treatment of agitated cardiac patients,J Clin Psychopharmacol.1985;5(6):344–347.
- ,,, et al.Postoperative delirium,Am J Psychiatry.2008;165:7.
- ,.Corrected QT interval prolongation associated with intravenous haloperidol in acute coronary syndromes,Catheter Cardiovasc Interv.2000;50(3):352–355.
- ,.Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death.Am J Psych.2001;158(11):1774–1782.
- ,,, et al.Accuracy of uncorrected versus corrected QT Interval for Prediction of torsade de pointes associated with intravenous haloperidol.Pharmacotherapy.2007;27(2):175–182.
- ,,.Three cases of ventricular tachycardia and torsades de pointes induced by antipsychotic drugs.Shinzo.1997;29(1):68–74.
- ,,,.Haloperidol por via intravenosa y torsade de pointes.Medicina intensive.2004;28(2):89.
- ,,, et al.QT‐Verlängerung und Kammerflimmern unter Haloperidol‐ und Clonidin‐Therapie des Alkoholentzugssyndroms.Intensivmedizin und Notfallmedizin.1992;29(4):178–183.
- ARIZONA CERT, Arizona Center for Education and Research on Therapeutics. Available at: www.azcert.org. Accessed February 2010.
- ,.Cardiac arrhythmias—a clinical approach.Edinburgh:Mosby;2003.
- ,.The association between intravenous haloperidol and torsades de pointes—three cases and a literature review.Psychosomatics.1995;36:541–549.
- ,.Prolongation of the corrected QT and torsades de pointes cardiac arrhythmia associated with intravenous haloperidol in the medically ill.J Clin Psychopharmacol.1993;13(2):128–132.
- ,,,,.Torsades de pointes secondary to intravenous haloperidol after coronary bypass grafting surgery.Can J Anesth.2000;47(3):251–254.
- ,.Torsade de pointes associated with the administration of intravenous haloperidol: a review of the literature and practical guidelines for use,Expert Opin Drug Saf.2003;2(6):543–547.
- ,.Conduction disturbances associated with administration of butyrophenone antipsychotics in the critically ill: a review of the literature,Pharmacotherapy.1997;17(3):531–537.
- ,,.Haloperidol‐induced torsades de pointes.Ann Pharacother.1999;33(10):1046–1050.
- ,,, et al.Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary,Crit Care Med.1995;23(9):1596–1600.
- ,,,,,.Prolonged cardiac repolarization after tacrolimus and haloperidol administration in the critically ill patient.Pharmacotherapy.2004;24(3):404–408.
- CPS Compendium of Pharmaceuticals and Specialties, the Canadian drug reference for health professionals, 2007, Canadian pharmacists association.
- Rote Liste Deutschland2008, Rote Liste Service GmbH Frankfurt am Main. Available at: www.rote‐liste.de. Accessed February 2010.
- ,.Delirium in the hospitalized patient: a primer for the pharmacist clinician.J Pharm Pract.2007;20(5):368–372.
- ,,, et al.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,.The pharmacological management of delirium in critical illness.Current Drug Therapy.2008,3:148–157.
- VIDAL‐l'information sur les produits de santé2008, Issy les Moulineaux Cedex. Available at: www.vidal.fr. Accessed February 2010.
- Haldol iniettabile—ufficiale monografia italiana. Available at: www. informatorefarmaceutico.it. Accessed February 2010.
- Arzneimittelkompendium der Schweiz2008, documed Verlag Basel. Availabla at: www.kompendium.ch. Accessed February 2010.
- ,,, et al.Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill, American College of Critical Care Medicine ACCM, Society of Critical Care Medicine SCCM, American Society of Health‐System Pharmacists ASHP.Crit Care Med.2002;30(1):119–141.
- ,,,,.Is telemetry overused? Is it as helpful as thought?Cleve Clin J Med.2009;76(6):368–372.
- ,,.Telemetry outside critical care units: patterns of utilization and influence on management decisions.Clin Cardiol.1998;21(7):503–505.
- ,,.High‐dose intravenous haloperidol for agitated delirium in a cardiac patient on intra‐aortic balloon pump.J Clin Psychopharmacol.1991;11(2):146–147.
- .Haloperidol, midazolam and intravenous sedation.Aust NZ J Psychiatry.1999;33(6):942–943.
- BNF British National Formulary, compendium of pharmaceuticals and specialties of the UK.2007. Available at: www.bnf.org. Accessed February 2010.
- ,,,.Torsade de pointes associated with the use of intravenous haloperidol.Ann Intern Med.1993;119(5):391–394.
- ,.Torsades de pointes: potential consequence of intravenous haloperidol in the intensive care unit.Intensive Care World.1994;11(3):109–112.
- ,.Torsade de pointes caused by high‐dose intravenous haloperidol in cardiac patients.Clin Cardiol.1995;18:285–290.
- ,,.Continuous infusion of haloperidol controls agitation in critically ill patients.Crit Care Med.1994:22(3):433–440.
- ,,.Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalances.Am J Gastroenterol.1995;90(5):822–824.
- ,,,.Torsades de pointes associated with intravenous haloperidol in critically ill patients.Am J Cardiol.1998;81(2):238–240.
- ,,,,.The effect of intravenous haloperidol on QT interval dispersion in critically ill patients: comparison with QT interval prolongation for assessment of risk of torsades de pointes.J Clin Pharmacol.2001;41:1310–1318.
- ,,.Low‐dose haloperidol associated QTc prolongation.J Am Geriatr Soc.2008;56(10):1963–1964.
- ,,.Torsade de pointes following intravenous haloperidol administration in a patient with complete heart block.WMJ.2009;108(1):48–50.
- Haldol® injection (for immediate release) Package Insert.Raritan, NJ:Ortho‐McNeil Pharmaceutical Inc.;2005;rev. 05.2007.
- ,,,. Antipsychotics for delirium (review), the Cochrane collaboration2008;2. Available at: www. cochrane.org. Accessed February 2010.
- American Psychiatric Association: practice guideline for the treatment of patients with delirium.Am J Psychiatry.1999;156(5 suppl):1–20, updated 2004.
- Thomson Micromedex.2008. Micromedex healthcare series: “haloperidol” Thomson Micromedex, Greenwood Village.
- FDA alert: haloperidol (marketed as Haldol, Haldol Decanoate and Haldol Lactate).2007. This alert highlights revisions to the labeling for haloperidol. Available at: www.fda.gov. Accessed February 2010.
- ,,.Use of high‐dose intravenous haloperidol in the treatment of agitated cardiac patients,J Clin Psychopharmacol.1985;5(6):344–347.
- ,,, et al.Postoperative delirium,Am J Psychiatry.2008;165:7.
- ,.Corrected QT interval prolongation associated with intravenous haloperidol in acute coronary syndromes,Catheter Cardiovasc Interv.2000;50(3):352–355.
- ,.Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death.Am J Psych.2001;158(11):1774–1782.
- ,,, et al.Accuracy of uncorrected versus corrected QT Interval for Prediction of torsade de pointes associated with intravenous haloperidol.Pharmacotherapy.2007;27(2):175–182.
- ,,.Three cases of ventricular tachycardia and torsades de pointes induced by antipsychotic drugs.Shinzo.1997;29(1):68–74.
- ,,,.Haloperidol por via intravenosa y torsade de pointes.Medicina intensive.2004;28(2):89.
- ,,, et al.QT‐Verlängerung und Kammerflimmern unter Haloperidol‐ und Clonidin‐Therapie des Alkoholentzugssyndroms.Intensivmedizin und Notfallmedizin.1992;29(4):178–183.
- ARIZONA CERT, Arizona Center for Education and Research on Therapeutics. Available at: www.azcert.org. Accessed February 2010.
- ,.Cardiac arrhythmias—a clinical approach.Edinburgh:Mosby;2003.
- ,.The association between intravenous haloperidol and torsades de pointes—three cases and a literature review.Psychosomatics.1995;36:541–549.
- ,.Prolongation of the corrected QT and torsades de pointes cardiac arrhythmia associated with intravenous haloperidol in the medically ill.J Clin Psychopharmacol.1993;13(2):128–132.
- ,,,,.Torsades de pointes secondary to intravenous haloperidol after coronary bypass grafting surgery.Can J Anesth.2000;47(3):251–254.
- ,.Torsade de pointes associated with the administration of intravenous haloperidol: a review of the literature and practical guidelines for use,Expert Opin Drug Saf.2003;2(6):543–547.
- ,.Conduction disturbances associated with administration of butyrophenone antipsychotics in the critically ill: a review of the literature,Pharmacotherapy.1997;17(3):531–537.
- ,,.Haloperidol‐induced torsades de pointes.Ann Pharacother.1999;33(10):1046–1050.
- ,,, et al.Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary,Crit Care Med.1995;23(9):1596–1600.
- ,,,,,.Prolonged cardiac repolarization after tacrolimus and haloperidol administration in the critically ill patient.Pharmacotherapy.2004;24(3):404–408.
- CPS Compendium of Pharmaceuticals and Specialties, the Canadian drug reference for health professionals, 2007, Canadian pharmacists association.
- Rote Liste Deutschland2008, Rote Liste Service GmbH Frankfurt am Main. Available at: www.rote‐liste.de. Accessed February 2010.
- ,.Delirium in the hospitalized patient: a primer for the pharmacist clinician.J Pharm Pract.2007;20(5):368–372.
- ,,, et al.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,.The pharmacological management of delirium in critical illness.Current Drug Therapy.2008,3:148–157.
- VIDAL‐l'information sur les produits de santé2008, Issy les Moulineaux Cedex. Available at: www.vidal.fr. Accessed February 2010.
- Haldol iniettabile—ufficiale monografia italiana. Available at: www. informatorefarmaceutico.it. Accessed February 2010.
- Arzneimittelkompendium der Schweiz2008, documed Verlag Basel. Availabla at: www.kompendium.ch. Accessed February 2010.
- ,,, et al.Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill, American College of Critical Care Medicine ACCM, Society of Critical Care Medicine SCCM, American Society of Health‐System Pharmacists ASHP.Crit Care Med.2002;30(1):119–141.
- ,,,,.Is telemetry overused? Is it as helpful as thought?Cleve Clin J Med.2009;76(6):368–372.
- ,,.Telemetry outside critical care units: patterns of utilization and influence on management decisions.Clin Cardiol.1998;21(7):503–505.
- ,,.High‐dose intravenous haloperidol for agitated delirium in a cardiac patient on intra‐aortic balloon pump.J Clin Psychopharmacol.1991;11(2):146–147.
- .Haloperidol, midazolam and intravenous sedation.Aust NZ J Psychiatry.1999;33(6):942–943.
- BNF British National Formulary, compendium of pharmaceuticals and specialties of the UK.2007. Available at: www.bnf.org. Accessed February 2010.
- ,,,.Torsade de pointes associated with the use of intravenous haloperidol.Ann Intern Med.1993;119(5):391–394.
- ,.Torsades de pointes: potential consequence of intravenous haloperidol in the intensive care unit.Intensive Care World.1994;11(3):109–112.
- ,.Torsade de pointes caused by high‐dose intravenous haloperidol in cardiac patients.Clin Cardiol.1995;18:285–290.
- ,,.Continuous infusion of haloperidol controls agitation in critically ill patients.Crit Care Med.1994:22(3):433–440.
- ,,.Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalances.Am J Gastroenterol.1995;90(5):822–824.
- ,,,.Torsades de pointes associated with intravenous haloperidol in critically ill patients.Am J Cardiol.1998;81(2):238–240.
- ,,,,.The effect of intravenous haloperidol on QT interval dispersion in critically ill patients: comparison with QT interval prolongation for assessment of risk of torsades de pointes.J Clin Pharmacol.2001;41:1310–1318.
- ,,.Low‐dose haloperidol associated QTc prolongation.J Am Geriatr Soc.2008;56(10):1963–1964.
- ,,.Torsade de pointes following intravenous haloperidol administration in a patient with complete heart block.WMJ.2009;108(1):48–50.