User login
The Importance of Emotional Intelligence When Leading in a Time of Crisis
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
© 2020 Society of Hospital Medicine
Shared Decision-Making During Inpatient Rounds: Opportunities for Improvement in Patient Engagement and Communication
The ethos of medicine has shifted from paternalistic, physician-driven care to patient autonomy and engagement, in which the physician shares information and advises.1-3 Although there are ethical, legal, and practical reasons to respect patient preferences,1-4 patient engagement also fosters quality and safety5 and may improve clinical outcomes.5-8 Patients whose preferences are respected are more likely to trust their doctor, feel empowered, and adhere to treatments.9
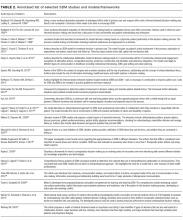
Providers may partner with patients through shared decision-making (SDM).10,11 Several SDM models describe the process of providers and patients balancing evidence, preferences and context to arrive at a clinical decision.12-15 The National Academy of Medicine and the American Academy of Pediatrics has called for more SDM,16,17 including when clinical evidence is limited,2 equally beneficial options exist,18 clinical stakes are high,19 and even with deferential patients.20 Despite its value, SDM does not reliably occur21,22 and SDM training is often unavailable.4 Clinical decision tools, patient education aids, and various training interventions have shown promising, although inconsistent results.23, 24
Little is known about SDM in inpatient settings where unique patient, clinician, and environmental factors may influence SDM. This study describes the quality and possible predictors of inpatient SDM during attending rounds in 4 academic training settings. Although SDM may occur anytime during a hospitalization, attending rounds present a valuable opportunity for SDM observation given their centrality to inpatient care and teaching.25,26 Because attending physicians bear ultimate responsibility for patient management, we examined whether SDM performance varies among attendings within each service. In addition, we tested the hypothesis that service-level, team-level, and patient-level features explain variation in SDM quality more than individual attending physicians. Finally, we compared peer-observer perspectives of SDM behaviors with patient and/or guardian perspectives.
METHODS
Study Design and Setting
This cross-sectional, observational study examined the diversity of SDM practice within and between 4 inpatient services during attending rounds, including the internal medicine and pediatrics services at Stanford University and the University of California, San Francisco (UCSF). Both institutions provide quaternary care to diverse patient populations with approximately half enrolled in Medicare and/or Medicaid.
One institution had 42 internal medicine (Med-1) and 15 pediatric hospitalists (Peds-1) compared to 8 internal medicine (Med-2) and 12 pediatric hospitalists (Peds-2) at the second location. Both pediatric services used family-centered rounds that included discussions between the patients’ families and the whole team. One medicine service used a similar rounding model that did not necessarily involve the patients’ families. In contrast, the smaller medicine service typically began rounds by discussing all patients in a conference room and then visiting select patients afterwards.
From August 2014 to November 2014, peer observers gathered data on team SDM behaviors during attending rounds. After the rounding team departed, nonphysician interviewers surveyed consenting patients’ (or guardians’) views of the SDM experience, yielding paired evaluations for a subset of SDM encounters. Institutional review board approval was obtained from Stanford University and UCSF.
Participants and Inclusion Criteria
Attending physicians were hospitalists who supervised rounds at least 1 month per year, and did not include those conducting the study. All provided verbal assent to be observed on 3 days within a 7-day period. While team composition varied as needed (eg, to include the nurse, pharmacist, interpreter, etc), we restricted study observations to those teams with an attending and at least one learner (eg, resident, intern, medical student) to capture the influence of attending physicians in their training role. Because services vary in number of attendings on staff, rounds assigned per attending, and patients per round, it was not possible to enroll equal sample sizes per service in the study.
Nonintensive care unit patients who were deemed medically stable by the team were eligible for peer observation and participation in a subsequent patient interview once during the study period. Pediatric patients were invited for an interview if they were between 13 and 21 years old and had the option of having a parent or guardian present; if the pediatric patients were less than 13 years old or they were not interested in being interviewed, then their parents or guardians were invited to be interviewed. Interpreters were on rounds, and thus, non-English participants were able to participate in the peer observations, but could not participate in patient interviews because interpreters were not available during afternoons for study purposes. Consent was obtained from all participating patients and/or guardians.
Data Collection
Round and Patient Characteristics
Peer observers recorded rounding, team, and patient characteristics using a standardized form. Rounding data included date, attending name, duration of rounds, and patient census. Patient level data included the decision(s) discussed, the seniority of the clinician leading the discussion, team composition, minutes spent discussing the patient (both with the patient and/or guardian and total time), hospitalization week, and patient’s primary language. Additional patient data obtained from electronic health records included age, gender, race, ethnicity, date of admission, and admitting diagnosis.
SDM Measures
Peer-observed SDM behaviors were quantified per patient encounter using the 9-item Rochester Participatory Decision-Making Scale (RPAD), with credit given for SDM behaviors exhibited by anyone on the rounding team (team-level metric).27 Each item was scored on a 3-point scale (0 = absent, 0.5 = partial, and 1 = present) for a maximum of 9 points, with higher scores indicating higher-quality SDM (Peer-RPAD Score). We created semistructured patient interview guides by adapting each RPAD item into layperson language (Patient-RPAD Score) and adding open-ended questions to assess the patient experience.
Peer-Observer Training
Eight peer-observers (7 hospitalists and 1 palliative care physician) were trained to perform RPAD ratings using videos of patient encounters. Initially, raters viewed videos together and discussed ratings for each RPAD item. The observers incorporated behavioral anchors and clinical examples into the development of an RPAD rating guide, which they subsequently used to independently score 4 videos from an online medical communication library.28 These scores were discussed to resolve any differences before 4 additional videos were independently viewed, scored, and compared. Interrater reliability was achieved when the standard deviation of summed SDM scores across raters was less than 1 for all 4 videos.
Patient Interviewers
Interviewers were English-speaking volunteers without formal medical training. They were educated in hospital etiquette by a physician and in administering patient interviews through peer-to-peer role playing and an observation and feedback interview with at least 1 patient.
Data Analysis
The analysis set included every unique patient with whom a medical decision was made by an eligible clinical team. To account for the nested study design (patient-level scores within rounds, rounds within attending, and attendings within service), we used mixed-effects models to estimate mean (summary or item) RPAD score by levels of fixed covariate(s). The models included random effects accounting for attending-level and round-level correlations among scores via variance components, and allowing the attending-level random effect to differ by service. Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). We used descriptive statistics to summarize round- and patient-level characteristics.
SDM Variation by Attending and Service
Box plots were used to summarize raw patient-level, Peer-RPAD scores by service and attending. By using the methods described above, we estimated the mean score overall and by service. In both models, we examined the statistical significance of service-specific variation in attending-level random effects by using likelihood-ratio test (LRT) to compare models.
SDM Variation by Round and Patient Characteristics
We used the models described above to identify covariates associated with Peer-RPAD scores. We fit univariate models separately for each covariate, then fit 2 multivariable models, including (1) all covariates and (2) all effects significant in either model at P ≤ .20 according to F tests. For uniformity of presentation, we express continuous covariates categorically; however, we report P values based on continuous versions. Means generated by the multivariable models were calculated at the mean values of all other covariates in model.
Patient-Level RPAD Data
A subsample of patients completed semistructured interviews with analogous RPAD questions. To identify possible selection bias in the full sample, we summarized response rates by service and patient language and modeled Peer-RPAD scores by interview response status. Among responders, we estimated the mean Peer-RPAD and Patient-RPAD scores and their paired differences and correlations, testing for non-zero correlations via the Spearman rank test.
RESULTS
All Patient Encounters
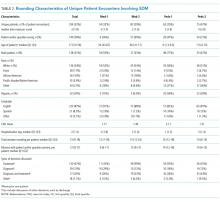
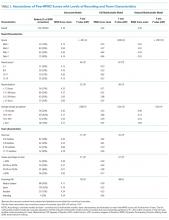
The median duration of rounding sessions was 1.8 hours, median patient census was 9, and median patient encounter was 13 minutes. The duration of rounds and minutes per patient were longest at Med-2 and shortest at Peds-1. See Table 1 for other team characteristics.
Peer Evaluations of SDM Encounters
Characteristics of Patients
Teams spent a median of 13 minutes per SDM encounter, which was not higher than the round median. SDM topics discussed included 47% treatment, 15% diagnostic, 30% both treatment and diagnostic, and 7% other.
Variation in SDM Quality Among Attending Physicians
Overall Peer-RPAD Scores were normally distributed. After adjusting for the nested study design, the overall mean (standard error) score was 4.16 (0.11). Score variability among attendings differed significantly by service (LRT P = .0067). For example, raw scores were lower and more variable among attending physicians at Med-2 than other among attendings in other services (see Appendix Figure in Supporting Information). However, when service was included in the model as a fixed effect, mean scores varied significantly, from 3.0 at Med-2 to 4.7 at Med-1 (P < .0001), but the random variation among attendings no longer differed significantly by service (P = .13). This finding supports the hypothesis that service-level influences are stronger than influences of individual attending physicians, that is, that variation between services exceeded variation among attendings within service.
Aspects of SDM That Are More Prevalent on Rounds
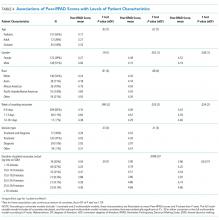
Rounds and Patient Characteristics Associated With Peer-RPAD Scores
Patient-RPAD Results: Dissimilar Perspectives of Patients and/or Guardians and Physician Observers
Among responders, mean Patient-RPAD scores were 6.8 to 7.1 for medicine services and 7.6 to 7.8 for pediatric services (P = .01). The overall mean Patient-RPAD score, 7.46, was significantly greater than the paired Peer-RPAD score by 3.5 (P = .011); however, correlations were not statistically significantly different from 0 (by service, each P > .12).
To understand drivers of the differences between Peer-RPAD and Patient-RPAD scores, we analyzed findings by item. Each mean patient-item score exceeded its peer counterpart (P ≤ .01; panel B of Figure). Peer-item scores fell below 33% on 2 items (Items 9 and 4) and only exceeded 67% on 2 items (Items 1 and 6), whereas patient-item scores ranged from 60% (Item 8) to 97% (Item 7). Three paired differences exceeded 50% (Items 9, 4, and 7) and 3 were below 20% (Items 6, 8 and 1), underlying the lack of correlation between peer and patient scores.
DISCUSSION
In this multisite study of SDM during inpatient attending rounds, SDM quality, specific SDM behaviors, and factors contributing to SDM were identified. Our study found an adjusted overall Peer-RPAD Score of 4.4 out of 9, and found the following 3 SDM elements most needing improvement according to trained peer observers: (1) “Checking understanding of the patient’s perspective”, (2) “Examining barriers to follow-through with the treatment plan”, and (3) “Asking if the patient has questions.” Areas of strength included explaining the clinical issue or nature of the decision and matching medical language to the patient’s level of understanding, with each rated highly by both peer-observers and patients. Broadly speaking, physicians were skillful in delivering information to patients but failed to solicit input from patients. Characteristics associated with increased SDM in the multivariate analysis included the following: service, patient gender, timing of rounds during patient’s hospital stay, and amount of time rounding with each patient.
Patients similarly found that physicians could improve their abilities to elicit information from patients and families, noting the 3 lowest patient-rated SDM elements were as follows: (1) asking open-ended questions, (2) discussing alternatives or uncertainties, and (3) discussing barriers to treatment plan follow through. Overall, patients and guardians perceived the quantity and quality of SDM on rounds more favorably than peer observers, which is consistent with other studies of patient perceptions of communication. 29-31 It is possible that patient ratings are more influenced by demand characteristics, fear of negatively impacting their patient-provider relationships, and conflation of overall satisfaction with quality of communication.32 This difference in patient perception of SDM is worthy of further study.
Prior work has revealed that SDM may occur infrequently during inpatient rounds.11 This study further elucidates specific SDM behaviors used along with univariate and multivariate modeling to explore possible contributing factors. The strengths and weaknesses found were similar at all 4 services and the influence of the service was more important than variability across attendings. This study’s findings are similar to a study by Shields et al.,33 in which the findings in a geographically different outpatient setting 10 years earlier suggesting global and enduring challenges to SDM. To our knowledge, this is the first published study to characterize inpatient SDM behaviors and may serve as the basis for future interventions.
Although the item-level components were ranked similarly across services, on average the summary Peer-RPAD score was lowest at Med-2, where we observed high variability within and between attendings, and was highest at Med-1, where variability was low. Med-2 carried the highest caseload and held the longest rounds, while Med-1 carried the lowest caseload, suggesting that modifiable burdens may hamper SDM performance. Prior studies suggest that patients are often selected based on teaching opportunities, immediate medical need and being newly admitted.34 The high scores at Med-1 may reflect that service’s prediscussion of patients during card-flipping rounds or their selection of which patients to round on as a team. Consistent with prior studies29,35 of SDM and the family-centered rounding model, which includes the involvement of nurses, respiratory therapists, pharmacists, case managers, social workers, and interpreters on rounds, both pediatrics services showed higher SDM scores.
In contrast to prior studies,34,36 team size and number of learners did not affect SDM performance, nor did decision type. Despite teams having up to 17 members, 8 learners, and 14 complex patients, SDM scores did not vary significantly by team. Nonetheless, trends were in the directions expected: Scores tended to decrease as the team size or the percentage of trainees grew, and increased with the seniority of the presenting physician. Interestingly, SDM performance decreased with round-average minutes per patient, which may be measuring on-going intensity across cases that leads to exhaustion. Statistically significant patient factors for increased SDM included longer duration of patient encounters, second week of hospital stay, and female patient gender. Although we anticipated that the high number of decisions made early in hospitalization would facilitate higher SDM scores, continuity and stronger patient-provider relationships may enhance SDM.36 We report service-specific team and patient characteristics, in addition to SDM findings in anticipation that some readers will identify with 1 service more than others.
This study has several important limitations. First, our peer observers were not blinded and primarily observed encounters at their own site. To minimize bias, observers periodically rated videos to recalibrate RPAD scoring. Second, additional SDM conversations with a patient and/or guardian may have occurred outside of rounds and were not captured, and poor patient recall may have affected Patient-RPAD scores despite interviewer prompts and timeliness of interviews within 12 hours of rounds. Third, there might have been a selection bias for the one service who selected a smaller number of patients to see, compared with the three other services that performed bedside rounds on all patients. It is possible that attending physicians selected patients who were deemed most able to have SDM conversations, thus affecting RPAD scores on that service. Fourth, study services had fewer patients on average than other academic hospitals (median 9, range 3-14), which might limit its generalizability. Last, as in any observational study, there is always the possibility of the Hawthorne effect. However, neither teams nor patients knew the study objectives.
Nevertheless, important findings emerged through the use of RPAD Scores to evaluate inpatient SDM practices. In particular, we found that to increase SDM quality in inpatient settings, practitioners should (1) check their understanding of the patient’s perspective, (2) examine barriers to follow-through with the treatment plan, and (3) ask if the patient has questions. Variation among services remained very influential after adjusting for team and patient characteristics, which suggests that “climate” or service culture should be targeted by an intervention, rather than individual attendings or subgroups defined by team or patient characteristics. Notably, team size, number of learners, patient census, and type of decision being made did not affect SDM performance, suggesting that even large, busy services can perform SDM if properly trained.
Acknowledgments
The authors thank the patients, families, pediatric and internal medicine residents, and hospitalists at Stanford School of Medicine and University of California, San Francisco School of Medicine for their participation in this study. We would also like to thank the student volunteers who collected patient perspectives on the encounters.
Disclosure
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an NIH/NCCIH grant R25 AT006573.
1. Braddock CH. The emerging importance and relevance of shared decision making to clinical practice. Med Decis Mak. 2010;30(5 Suppl):5S-7S. doi:10.1177/0272989X10381344. PubMed
2. Braddock CH. Supporting shared decision making when clinical evidence is low. Med Care Res Rev MCRR. 2013;70(1 Suppl):129S-140S. doi:10.1177/1077558712460280. PubMed
3. Elwyn G, Tilburt J, Montori V. The ethical imperative for shared decision-making. Eur J Pers Centered Healthc. 2013;1(1):129-131. doi:10.5750/ejpch.v1i1.645.
4. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
5. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(10):CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
6. Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566-577. doi:10.1164/rccm.200906-0907OC. PubMed
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410-417. doi:10.1370/afm.1161. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
10. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781. doi:10.1056/NEJMp1109283. PubMed
11. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med J Assoc Am Med Coll. 2014;89(11):1548-1557. doi:10.1097/ACM.0000000000000483. PubMed
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692. PubMed
13. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
14. Légaré F, St-Jacques S, Gagnon S, et al. Prenatal screening for Down syndrome: a survey of willingness in women and family physicians to engage in shared decision-making. Prenat Diagn. 2011;31(4):319-326. doi:10.1002/pd.2624. PubMed
15. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. Milbank Q. 2009;87(2):368-390. PubMed
16. National Academy of Medicine. Crossing the quality chasm: a new health system for the 21st century. https://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed on November 30, 2016.
17. Adams RC, Levy SE, Council on Children with Disabilities. Shared Decision-Making and Children with Disabilities: Pathways to Consensus. Pediatrics. 2017; 139(6):1-9. PubMed
18. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
19. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
20. Kehl KL, Landrum MB, Arora NK, et al. Association of Actual and Preferred Decision Roles With Patient-Reported Quality of Care: Shared Decision Making in Cancer Care. JAMA Oncol. 2015;1(1):50-58. doi:10.1001/jamaoncol.2014.112. PubMed
21. Couët N, Desroches S, Robitaille H, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect Int J Public Particip Health Care Health Policy. 2015;18(4):542-561. doi:10.1111/hex.12054. PubMed
22. Fowler FJ, Gerstein BS, Barry MJ. How patient centered are medical decisions?: Results of a national survey. JAMA Intern Med. 2013;173(13):1215-1221. doi:10.1001/jamainternmed.2013.6172. PubMed
23. Légaré F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2014;(9):CD006732. doi:10.1002/14651858.CD006732.pub3. PubMed
24. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;(10):CD001431. doi:10.1002/14651858.CD001431.pub3. PubMed
25. Di Francesco L, Pistoria MJ, Auerbach AD, Nardino RJ, Holmboe ES. Internal medicine training in the inpatient setting. A review of published educational interventions. J Gen Intern Med. 2005;20(12):1173-1180. doi:10.1111/j.1525-1497.2005.00250.x. PubMed
26. Janicik RW, Fletcher KE. Teaching at the bedside: a new model. Med Teach. 2003;25(2):127-130. PubMed
27. Shields CG, Franks P, Fiscella K, Meldrum S, Epstein RM. Rochester Participatory Decision-Making Scale (RPAD): reliability and validity. Ann Fam Med. 2005;3(5):436-442. doi:10.1370/afm.305. PubMed
28. DocCom - enhancing competence in healthcare communication. https://webcampus.drexelmed.edu/doccom/user/. Accessed on November 30, 2016.
29. Bailey SM, Hendricks-Muñoz KD, Mally P. Parental influence on clinical management during neonatal intensive care: a survey of US neonatologists. J Matern Fetal Neonatal Med. 2013;26(12):1239-1244. doi:10.3109/14767058.2013.776531. PubMed
30. Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091-3098. doi:10.1200/JCO.2004.09.069. PubMed
31. Schoenborn NL, Cayea D, McNabney M, Ray A, Boyd C. Prognosis communication with older patients with multimorbidity: Assessment after an educational intervention. Gerontol Geriatr Educ. 2016;38(4):471-481. doi:10.1080/02701960.2015.1115983. PubMed
32. Lipkin M. Shared decision making. JAMA Intern Med. 2013;173(13):1204-1205. doi:10.1001/jamainternmed.2013.6248. PubMed
33. Gonzalo JD, Heist BS, Duffy BL, et al. The art of bedside rounds: a multi-center qualitative study of strategies used by experienced bedside teachers. J Gen Intern Med. 2013;28(3):412-420. doi:10.1007/s11606-012-2259-2. PubMed
34. Rosen P, Stenger E, Bochkoris M, Hannon MJ, Kwoh CK. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics. 2009;123(4):e603-e608. doi:10.1542/peds.2008-2238. PubMed
35. Harrison R, Allen E. Teaching internal medicine residents in the new era. Inpatient attending with duty-hour regulations. J Gen Intern Med. 2006;21(5):447-452. doi:10.1111/j.1525-1497.2006.00425.x. PubMed
36. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med 1982. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056. PubMed
The ethos of medicine has shifted from paternalistic, physician-driven care to patient autonomy and engagement, in which the physician shares information and advises.1-3 Although there are ethical, legal, and practical reasons to respect patient preferences,1-4 patient engagement also fosters quality and safety5 and may improve clinical outcomes.5-8 Patients whose preferences are respected are more likely to trust their doctor, feel empowered, and adhere to treatments.9
Providers may partner with patients through shared decision-making (SDM).10,11 Several SDM models describe the process of providers and patients balancing evidence, preferences and context to arrive at a clinical decision.12-15 The National Academy of Medicine and the American Academy of Pediatrics has called for more SDM,16,17 including when clinical evidence is limited,2 equally beneficial options exist,18 clinical stakes are high,19 and even with deferential patients.20 Despite its value, SDM does not reliably occur21,22 and SDM training is often unavailable.4 Clinical decision tools, patient education aids, and various training interventions have shown promising, although inconsistent results.23, 24
Little is known about SDM in inpatient settings where unique patient, clinician, and environmental factors may influence SDM. This study describes the quality and possible predictors of inpatient SDM during attending rounds in 4 academic training settings. Although SDM may occur anytime during a hospitalization, attending rounds present a valuable opportunity for SDM observation given their centrality to inpatient care and teaching.25,26 Because attending physicians bear ultimate responsibility for patient management, we examined whether SDM performance varies among attendings within each service. In addition, we tested the hypothesis that service-level, team-level, and patient-level features explain variation in SDM quality more than individual attending physicians. Finally, we compared peer-observer perspectives of SDM behaviors with patient and/or guardian perspectives.
METHODS
Study Design and Setting
This cross-sectional, observational study examined the diversity of SDM practice within and between 4 inpatient services during attending rounds, including the internal medicine and pediatrics services at Stanford University and the University of California, San Francisco (UCSF). Both institutions provide quaternary care to diverse patient populations with approximately half enrolled in Medicare and/or Medicaid.
One institution had 42 internal medicine (Med-1) and 15 pediatric hospitalists (Peds-1) compared to 8 internal medicine (Med-2) and 12 pediatric hospitalists (Peds-2) at the second location. Both pediatric services used family-centered rounds that included discussions between the patients’ families and the whole team. One medicine service used a similar rounding model that did not necessarily involve the patients’ families. In contrast, the smaller medicine service typically began rounds by discussing all patients in a conference room and then visiting select patients afterwards.
From August 2014 to November 2014, peer observers gathered data on team SDM behaviors during attending rounds. After the rounding team departed, nonphysician interviewers surveyed consenting patients’ (or guardians’) views of the SDM experience, yielding paired evaluations for a subset of SDM encounters. Institutional review board approval was obtained from Stanford University and UCSF.
Participants and Inclusion Criteria
Attending physicians were hospitalists who supervised rounds at least 1 month per year, and did not include those conducting the study. All provided verbal assent to be observed on 3 days within a 7-day period. While team composition varied as needed (eg, to include the nurse, pharmacist, interpreter, etc), we restricted study observations to those teams with an attending and at least one learner (eg, resident, intern, medical student) to capture the influence of attending physicians in their training role. Because services vary in number of attendings on staff, rounds assigned per attending, and patients per round, it was not possible to enroll equal sample sizes per service in the study.
Nonintensive care unit patients who were deemed medically stable by the team were eligible for peer observation and participation in a subsequent patient interview once during the study period. Pediatric patients were invited for an interview if they were between 13 and 21 years old and had the option of having a parent or guardian present; if the pediatric patients were less than 13 years old or they were not interested in being interviewed, then their parents or guardians were invited to be interviewed. Interpreters were on rounds, and thus, non-English participants were able to participate in the peer observations, but could not participate in patient interviews because interpreters were not available during afternoons for study purposes. Consent was obtained from all participating patients and/or guardians.
Data Collection
Round and Patient Characteristics
Peer observers recorded rounding, team, and patient characteristics using a standardized form. Rounding data included date, attending name, duration of rounds, and patient census. Patient level data included the decision(s) discussed, the seniority of the clinician leading the discussion, team composition, minutes spent discussing the patient (both with the patient and/or guardian and total time), hospitalization week, and patient’s primary language. Additional patient data obtained from electronic health records included age, gender, race, ethnicity, date of admission, and admitting diagnosis.
SDM Measures
Peer-observed SDM behaviors were quantified per patient encounter using the 9-item Rochester Participatory Decision-Making Scale (RPAD), with credit given for SDM behaviors exhibited by anyone on the rounding team (team-level metric).27 Each item was scored on a 3-point scale (0 = absent, 0.5 = partial, and 1 = present) for a maximum of 9 points, with higher scores indicating higher-quality SDM (Peer-RPAD Score). We created semistructured patient interview guides by adapting each RPAD item into layperson language (Patient-RPAD Score) and adding open-ended questions to assess the patient experience.
Peer-Observer Training
Eight peer-observers (7 hospitalists and 1 palliative care physician) were trained to perform RPAD ratings using videos of patient encounters. Initially, raters viewed videos together and discussed ratings for each RPAD item. The observers incorporated behavioral anchors and clinical examples into the development of an RPAD rating guide, which they subsequently used to independently score 4 videos from an online medical communication library.28 These scores were discussed to resolve any differences before 4 additional videos were independently viewed, scored, and compared. Interrater reliability was achieved when the standard deviation of summed SDM scores across raters was less than 1 for all 4 videos.
Patient Interviewers
Interviewers were English-speaking volunteers without formal medical training. They were educated in hospital etiquette by a physician and in administering patient interviews through peer-to-peer role playing and an observation and feedback interview with at least 1 patient.
Data Analysis
The analysis set included every unique patient with whom a medical decision was made by an eligible clinical team. To account for the nested study design (patient-level scores within rounds, rounds within attending, and attendings within service), we used mixed-effects models to estimate mean (summary or item) RPAD score by levels of fixed covariate(s). The models included random effects accounting for attending-level and round-level correlations among scores via variance components, and allowing the attending-level random effect to differ by service. Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). We used descriptive statistics to summarize round- and patient-level characteristics.
SDM Variation by Attending and Service
Box plots were used to summarize raw patient-level, Peer-RPAD scores by service and attending. By using the methods described above, we estimated the mean score overall and by service. In both models, we examined the statistical significance of service-specific variation in attending-level random effects by using likelihood-ratio test (LRT) to compare models.
SDM Variation by Round and Patient Characteristics
We used the models described above to identify covariates associated with Peer-RPAD scores. We fit univariate models separately for each covariate, then fit 2 multivariable models, including (1) all covariates and (2) all effects significant in either model at P ≤ .20 according to F tests. For uniformity of presentation, we express continuous covariates categorically; however, we report P values based on continuous versions. Means generated by the multivariable models were calculated at the mean values of all other covariates in model.
Patient-Level RPAD Data
A subsample of patients completed semistructured interviews with analogous RPAD questions. To identify possible selection bias in the full sample, we summarized response rates by service and patient language and modeled Peer-RPAD scores by interview response status. Among responders, we estimated the mean Peer-RPAD and Patient-RPAD scores and their paired differences and correlations, testing for non-zero correlations via the Spearman rank test.
RESULTS
All Patient Encounters
The median duration of rounding sessions was 1.8 hours, median patient census was 9, and median patient encounter was 13 minutes. The duration of rounds and minutes per patient were longest at Med-2 and shortest at Peds-1. See Table 1 for other team characteristics.
Peer Evaluations of SDM Encounters
Characteristics of Patients
Teams spent a median of 13 minutes per SDM encounter, which was not higher than the round median. SDM topics discussed included 47% treatment, 15% diagnostic, 30% both treatment and diagnostic, and 7% other.
Variation in SDM Quality Among Attending Physicians
Overall Peer-RPAD Scores were normally distributed. After adjusting for the nested study design, the overall mean (standard error) score was 4.16 (0.11). Score variability among attendings differed significantly by service (LRT P = .0067). For example, raw scores were lower and more variable among attending physicians at Med-2 than other among attendings in other services (see Appendix Figure in Supporting Information). However, when service was included in the model as a fixed effect, mean scores varied significantly, from 3.0 at Med-2 to 4.7 at Med-1 (P < .0001), but the random variation among attendings no longer differed significantly by service (P = .13). This finding supports the hypothesis that service-level influences are stronger than influences of individual attending physicians, that is, that variation between services exceeded variation among attendings within service.
Aspects of SDM That Are More Prevalent on Rounds
Rounds and Patient Characteristics Associated With Peer-RPAD Scores
Patient-RPAD Results: Dissimilar Perspectives of Patients and/or Guardians and Physician Observers
Among responders, mean Patient-RPAD scores were 6.8 to 7.1 for medicine services and 7.6 to 7.8 for pediatric services (P = .01). The overall mean Patient-RPAD score, 7.46, was significantly greater than the paired Peer-RPAD score by 3.5 (P = .011); however, correlations were not statistically significantly different from 0 (by service, each P > .12).
To understand drivers of the differences between Peer-RPAD and Patient-RPAD scores, we analyzed findings by item. Each mean patient-item score exceeded its peer counterpart (P ≤ .01; panel B of Figure). Peer-item scores fell below 33% on 2 items (Items 9 and 4) and only exceeded 67% on 2 items (Items 1 and 6), whereas patient-item scores ranged from 60% (Item 8) to 97% (Item 7). Three paired differences exceeded 50% (Items 9, 4, and 7) and 3 were below 20% (Items 6, 8 and 1), underlying the lack of correlation between peer and patient scores.
DISCUSSION
In this multisite study of SDM during inpatient attending rounds, SDM quality, specific SDM behaviors, and factors contributing to SDM were identified. Our study found an adjusted overall Peer-RPAD Score of 4.4 out of 9, and found the following 3 SDM elements most needing improvement according to trained peer observers: (1) “Checking understanding of the patient’s perspective”, (2) “Examining barriers to follow-through with the treatment plan”, and (3) “Asking if the patient has questions.” Areas of strength included explaining the clinical issue or nature of the decision and matching medical language to the patient’s level of understanding, with each rated highly by both peer-observers and patients. Broadly speaking, physicians were skillful in delivering information to patients but failed to solicit input from patients. Characteristics associated with increased SDM in the multivariate analysis included the following: service, patient gender, timing of rounds during patient’s hospital stay, and amount of time rounding with each patient.
Patients similarly found that physicians could improve their abilities to elicit information from patients and families, noting the 3 lowest patient-rated SDM elements were as follows: (1) asking open-ended questions, (2) discussing alternatives or uncertainties, and (3) discussing barriers to treatment plan follow through. Overall, patients and guardians perceived the quantity and quality of SDM on rounds more favorably than peer observers, which is consistent with other studies of patient perceptions of communication. 29-31 It is possible that patient ratings are more influenced by demand characteristics, fear of negatively impacting their patient-provider relationships, and conflation of overall satisfaction with quality of communication.32 This difference in patient perception of SDM is worthy of further study.
Prior work has revealed that SDM may occur infrequently during inpatient rounds.11 This study further elucidates specific SDM behaviors used along with univariate and multivariate modeling to explore possible contributing factors. The strengths and weaknesses found were similar at all 4 services and the influence of the service was more important than variability across attendings. This study’s findings are similar to a study by Shields et al.,33 in which the findings in a geographically different outpatient setting 10 years earlier suggesting global and enduring challenges to SDM. To our knowledge, this is the first published study to characterize inpatient SDM behaviors and may serve as the basis for future interventions.
Although the item-level components were ranked similarly across services, on average the summary Peer-RPAD score was lowest at Med-2, where we observed high variability within and between attendings, and was highest at Med-1, where variability was low. Med-2 carried the highest caseload and held the longest rounds, while Med-1 carried the lowest caseload, suggesting that modifiable burdens may hamper SDM performance. Prior studies suggest that patients are often selected based on teaching opportunities, immediate medical need and being newly admitted.34 The high scores at Med-1 may reflect that service’s prediscussion of patients during card-flipping rounds or their selection of which patients to round on as a team. Consistent with prior studies29,35 of SDM and the family-centered rounding model, which includes the involvement of nurses, respiratory therapists, pharmacists, case managers, social workers, and interpreters on rounds, both pediatrics services showed higher SDM scores.
In contrast to prior studies,34,36 team size and number of learners did not affect SDM performance, nor did decision type. Despite teams having up to 17 members, 8 learners, and 14 complex patients, SDM scores did not vary significantly by team. Nonetheless, trends were in the directions expected: Scores tended to decrease as the team size or the percentage of trainees grew, and increased with the seniority of the presenting physician. Interestingly, SDM performance decreased with round-average minutes per patient, which may be measuring on-going intensity across cases that leads to exhaustion. Statistically significant patient factors for increased SDM included longer duration of patient encounters, second week of hospital stay, and female patient gender. Although we anticipated that the high number of decisions made early in hospitalization would facilitate higher SDM scores, continuity and stronger patient-provider relationships may enhance SDM.36 We report service-specific team and patient characteristics, in addition to SDM findings in anticipation that some readers will identify with 1 service more than others.
This study has several important limitations. First, our peer observers were not blinded and primarily observed encounters at their own site. To minimize bias, observers periodically rated videos to recalibrate RPAD scoring. Second, additional SDM conversations with a patient and/or guardian may have occurred outside of rounds and were not captured, and poor patient recall may have affected Patient-RPAD scores despite interviewer prompts and timeliness of interviews within 12 hours of rounds. Third, there might have been a selection bias for the one service who selected a smaller number of patients to see, compared with the three other services that performed bedside rounds on all patients. It is possible that attending physicians selected patients who were deemed most able to have SDM conversations, thus affecting RPAD scores on that service. Fourth, study services had fewer patients on average than other academic hospitals (median 9, range 3-14), which might limit its generalizability. Last, as in any observational study, there is always the possibility of the Hawthorne effect. However, neither teams nor patients knew the study objectives.
Nevertheless, important findings emerged through the use of RPAD Scores to evaluate inpatient SDM practices. In particular, we found that to increase SDM quality in inpatient settings, practitioners should (1) check their understanding of the patient’s perspective, (2) examine barriers to follow-through with the treatment plan, and (3) ask if the patient has questions. Variation among services remained very influential after adjusting for team and patient characteristics, which suggests that “climate” or service culture should be targeted by an intervention, rather than individual attendings or subgroups defined by team or patient characteristics. Notably, team size, number of learners, patient census, and type of decision being made did not affect SDM performance, suggesting that even large, busy services can perform SDM if properly trained.
Acknowledgments
The authors thank the patients, families, pediatric and internal medicine residents, and hospitalists at Stanford School of Medicine and University of California, San Francisco School of Medicine for their participation in this study. We would also like to thank the student volunteers who collected patient perspectives on the encounters.
Disclosure
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an NIH/NCCIH grant R25 AT006573.
The ethos of medicine has shifted from paternalistic, physician-driven care to patient autonomy and engagement, in which the physician shares information and advises.1-3 Although there are ethical, legal, and practical reasons to respect patient preferences,1-4 patient engagement also fosters quality and safety5 and may improve clinical outcomes.5-8 Patients whose preferences are respected are more likely to trust their doctor, feel empowered, and adhere to treatments.9
Providers may partner with patients through shared decision-making (SDM).10,11 Several SDM models describe the process of providers and patients balancing evidence, preferences and context to arrive at a clinical decision.12-15 The National Academy of Medicine and the American Academy of Pediatrics has called for more SDM,16,17 including when clinical evidence is limited,2 equally beneficial options exist,18 clinical stakes are high,19 and even with deferential patients.20 Despite its value, SDM does not reliably occur21,22 and SDM training is often unavailable.4 Clinical decision tools, patient education aids, and various training interventions have shown promising, although inconsistent results.23, 24
Little is known about SDM in inpatient settings where unique patient, clinician, and environmental factors may influence SDM. This study describes the quality and possible predictors of inpatient SDM during attending rounds in 4 academic training settings. Although SDM may occur anytime during a hospitalization, attending rounds present a valuable opportunity for SDM observation given their centrality to inpatient care and teaching.25,26 Because attending physicians bear ultimate responsibility for patient management, we examined whether SDM performance varies among attendings within each service. In addition, we tested the hypothesis that service-level, team-level, and patient-level features explain variation in SDM quality more than individual attending physicians. Finally, we compared peer-observer perspectives of SDM behaviors with patient and/or guardian perspectives.
METHODS
Study Design and Setting
This cross-sectional, observational study examined the diversity of SDM practice within and between 4 inpatient services during attending rounds, including the internal medicine and pediatrics services at Stanford University and the University of California, San Francisco (UCSF). Both institutions provide quaternary care to diverse patient populations with approximately half enrolled in Medicare and/or Medicaid.
One institution had 42 internal medicine (Med-1) and 15 pediatric hospitalists (Peds-1) compared to 8 internal medicine (Med-2) and 12 pediatric hospitalists (Peds-2) at the second location. Both pediatric services used family-centered rounds that included discussions between the patients’ families and the whole team. One medicine service used a similar rounding model that did not necessarily involve the patients’ families. In contrast, the smaller medicine service typically began rounds by discussing all patients in a conference room and then visiting select patients afterwards.
From August 2014 to November 2014, peer observers gathered data on team SDM behaviors during attending rounds. After the rounding team departed, nonphysician interviewers surveyed consenting patients’ (or guardians’) views of the SDM experience, yielding paired evaluations for a subset of SDM encounters. Institutional review board approval was obtained from Stanford University and UCSF.
Participants and Inclusion Criteria
Attending physicians were hospitalists who supervised rounds at least 1 month per year, and did not include those conducting the study. All provided verbal assent to be observed on 3 days within a 7-day period. While team composition varied as needed (eg, to include the nurse, pharmacist, interpreter, etc), we restricted study observations to those teams with an attending and at least one learner (eg, resident, intern, medical student) to capture the influence of attending physicians in their training role. Because services vary in number of attendings on staff, rounds assigned per attending, and patients per round, it was not possible to enroll equal sample sizes per service in the study.
Nonintensive care unit patients who were deemed medically stable by the team were eligible for peer observation and participation in a subsequent patient interview once during the study period. Pediatric patients were invited for an interview if they were between 13 and 21 years old and had the option of having a parent or guardian present; if the pediatric patients were less than 13 years old or they were not interested in being interviewed, then their parents or guardians were invited to be interviewed. Interpreters were on rounds, and thus, non-English participants were able to participate in the peer observations, but could not participate in patient interviews because interpreters were not available during afternoons for study purposes. Consent was obtained from all participating patients and/or guardians.
Data Collection
Round and Patient Characteristics
Peer observers recorded rounding, team, and patient characteristics using a standardized form. Rounding data included date, attending name, duration of rounds, and patient census. Patient level data included the decision(s) discussed, the seniority of the clinician leading the discussion, team composition, minutes spent discussing the patient (both with the patient and/or guardian and total time), hospitalization week, and patient’s primary language. Additional patient data obtained from electronic health records included age, gender, race, ethnicity, date of admission, and admitting diagnosis.
SDM Measures
Peer-observed SDM behaviors were quantified per patient encounter using the 9-item Rochester Participatory Decision-Making Scale (RPAD), with credit given for SDM behaviors exhibited by anyone on the rounding team (team-level metric).27 Each item was scored on a 3-point scale (0 = absent, 0.5 = partial, and 1 = present) for a maximum of 9 points, with higher scores indicating higher-quality SDM (Peer-RPAD Score). We created semistructured patient interview guides by adapting each RPAD item into layperson language (Patient-RPAD Score) and adding open-ended questions to assess the patient experience.
Peer-Observer Training
Eight peer-observers (7 hospitalists and 1 palliative care physician) were trained to perform RPAD ratings using videos of patient encounters. Initially, raters viewed videos together and discussed ratings for each RPAD item. The observers incorporated behavioral anchors and clinical examples into the development of an RPAD rating guide, which they subsequently used to independently score 4 videos from an online medical communication library.28 These scores were discussed to resolve any differences before 4 additional videos were independently viewed, scored, and compared. Interrater reliability was achieved when the standard deviation of summed SDM scores across raters was less than 1 for all 4 videos.
Patient Interviewers
Interviewers were English-speaking volunteers without formal medical training. They were educated in hospital etiquette by a physician and in administering patient interviews through peer-to-peer role playing and an observation and feedback interview with at least 1 patient.
Data Analysis
The analysis set included every unique patient with whom a medical decision was made by an eligible clinical team. To account for the nested study design (patient-level scores within rounds, rounds within attending, and attendings within service), we used mixed-effects models to estimate mean (summary or item) RPAD score by levels of fixed covariate(s). The models included random effects accounting for attending-level and round-level correlations among scores via variance components, and allowing the attending-level random effect to differ by service. Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). We used descriptive statistics to summarize round- and patient-level characteristics.
SDM Variation by Attending and Service
Box plots were used to summarize raw patient-level, Peer-RPAD scores by service and attending. By using the methods described above, we estimated the mean score overall and by service. In both models, we examined the statistical significance of service-specific variation in attending-level random effects by using likelihood-ratio test (LRT) to compare models.
SDM Variation by Round and Patient Characteristics
We used the models described above to identify covariates associated with Peer-RPAD scores. We fit univariate models separately for each covariate, then fit 2 multivariable models, including (1) all covariates and (2) all effects significant in either model at P ≤ .20 according to F tests. For uniformity of presentation, we express continuous covariates categorically; however, we report P values based on continuous versions. Means generated by the multivariable models were calculated at the mean values of all other covariates in model.
Patient-Level RPAD Data
A subsample of patients completed semistructured interviews with analogous RPAD questions. To identify possible selection bias in the full sample, we summarized response rates by service and patient language and modeled Peer-RPAD scores by interview response status. Among responders, we estimated the mean Peer-RPAD and Patient-RPAD scores and their paired differences and correlations, testing for non-zero correlations via the Spearman rank test.
RESULTS
All Patient Encounters
The median duration of rounding sessions was 1.8 hours, median patient census was 9, and median patient encounter was 13 minutes. The duration of rounds and minutes per patient were longest at Med-2 and shortest at Peds-1. See Table 1 for other team characteristics.
Peer Evaluations of SDM Encounters
Characteristics of Patients
Teams spent a median of 13 minutes per SDM encounter, which was not higher than the round median. SDM topics discussed included 47% treatment, 15% diagnostic, 30% both treatment and diagnostic, and 7% other.
Variation in SDM Quality Among Attending Physicians
Overall Peer-RPAD Scores were normally distributed. After adjusting for the nested study design, the overall mean (standard error) score was 4.16 (0.11). Score variability among attendings differed significantly by service (LRT P = .0067). For example, raw scores were lower and more variable among attending physicians at Med-2 than other among attendings in other services (see Appendix Figure in Supporting Information). However, when service was included in the model as a fixed effect, mean scores varied significantly, from 3.0 at Med-2 to 4.7 at Med-1 (P < .0001), but the random variation among attendings no longer differed significantly by service (P = .13). This finding supports the hypothesis that service-level influences are stronger than influences of individual attending physicians, that is, that variation between services exceeded variation among attendings within service.
Aspects of SDM That Are More Prevalent on Rounds
Rounds and Patient Characteristics Associated With Peer-RPAD Scores
Patient-RPAD Results: Dissimilar Perspectives of Patients and/or Guardians and Physician Observers
Among responders, mean Patient-RPAD scores were 6.8 to 7.1 for medicine services and 7.6 to 7.8 for pediatric services (P = .01). The overall mean Patient-RPAD score, 7.46, was significantly greater than the paired Peer-RPAD score by 3.5 (P = .011); however, correlations were not statistically significantly different from 0 (by service, each P > .12).
To understand drivers of the differences between Peer-RPAD and Patient-RPAD scores, we analyzed findings by item. Each mean patient-item score exceeded its peer counterpart (P ≤ .01; panel B of Figure). Peer-item scores fell below 33% on 2 items (Items 9 and 4) and only exceeded 67% on 2 items (Items 1 and 6), whereas patient-item scores ranged from 60% (Item 8) to 97% (Item 7). Three paired differences exceeded 50% (Items 9, 4, and 7) and 3 were below 20% (Items 6, 8 and 1), underlying the lack of correlation between peer and patient scores.
DISCUSSION
In this multisite study of SDM during inpatient attending rounds, SDM quality, specific SDM behaviors, and factors contributing to SDM were identified. Our study found an adjusted overall Peer-RPAD Score of 4.4 out of 9, and found the following 3 SDM elements most needing improvement according to trained peer observers: (1) “Checking understanding of the patient’s perspective”, (2) “Examining barriers to follow-through with the treatment plan”, and (3) “Asking if the patient has questions.” Areas of strength included explaining the clinical issue or nature of the decision and matching medical language to the patient’s level of understanding, with each rated highly by both peer-observers and patients. Broadly speaking, physicians were skillful in delivering information to patients but failed to solicit input from patients. Characteristics associated with increased SDM in the multivariate analysis included the following: service, patient gender, timing of rounds during patient’s hospital stay, and amount of time rounding with each patient.
Patients similarly found that physicians could improve their abilities to elicit information from patients and families, noting the 3 lowest patient-rated SDM elements were as follows: (1) asking open-ended questions, (2) discussing alternatives or uncertainties, and (3) discussing barriers to treatment plan follow through. Overall, patients and guardians perceived the quantity and quality of SDM on rounds more favorably than peer observers, which is consistent with other studies of patient perceptions of communication. 29-31 It is possible that patient ratings are more influenced by demand characteristics, fear of negatively impacting their patient-provider relationships, and conflation of overall satisfaction with quality of communication.32 This difference in patient perception of SDM is worthy of further study.
Prior work has revealed that SDM may occur infrequently during inpatient rounds.11 This study further elucidates specific SDM behaviors used along with univariate and multivariate modeling to explore possible contributing factors. The strengths and weaknesses found were similar at all 4 services and the influence of the service was more important than variability across attendings. This study’s findings are similar to a study by Shields et al.,33 in which the findings in a geographically different outpatient setting 10 years earlier suggesting global and enduring challenges to SDM. To our knowledge, this is the first published study to characterize inpatient SDM behaviors and may serve as the basis for future interventions.
Although the item-level components were ranked similarly across services, on average the summary Peer-RPAD score was lowest at Med-2, where we observed high variability within and between attendings, and was highest at Med-1, where variability was low. Med-2 carried the highest caseload and held the longest rounds, while Med-1 carried the lowest caseload, suggesting that modifiable burdens may hamper SDM performance. Prior studies suggest that patients are often selected based on teaching opportunities, immediate medical need and being newly admitted.34 The high scores at Med-1 may reflect that service’s prediscussion of patients during card-flipping rounds or their selection of which patients to round on as a team. Consistent with prior studies29,35 of SDM and the family-centered rounding model, which includes the involvement of nurses, respiratory therapists, pharmacists, case managers, social workers, and interpreters on rounds, both pediatrics services showed higher SDM scores.
In contrast to prior studies,34,36 team size and number of learners did not affect SDM performance, nor did decision type. Despite teams having up to 17 members, 8 learners, and 14 complex patients, SDM scores did not vary significantly by team. Nonetheless, trends were in the directions expected: Scores tended to decrease as the team size or the percentage of trainees grew, and increased with the seniority of the presenting physician. Interestingly, SDM performance decreased with round-average minutes per patient, which may be measuring on-going intensity across cases that leads to exhaustion. Statistically significant patient factors for increased SDM included longer duration of patient encounters, second week of hospital stay, and female patient gender. Although we anticipated that the high number of decisions made early in hospitalization would facilitate higher SDM scores, continuity and stronger patient-provider relationships may enhance SDM.36 We report service-specific team and patient characteristics, in addition to SDM findings in anticipation that some readers will identify with 1 service more than others.
This study has several important limitations. First, our peer observers were not blinded and primarily observed encounters at their own site. To minimize bias, observers periodically rated videos to recalibrate RPAD scoring. Second, additional SDM conversations with a patient and/or guardian may have occurred outside of rounds and were not captured, and poor patient recall may have affected Patient-RPAD scores despite interviewer prompts and timeliness of interviews within 12 hours of rounds. Third, there might have been a selection bias for the one service who selected a smaller number of patients to see, compared with the three other services that performed bedside rounds on all patients. It is possible that attending physicians selected patients who were deemed most able to have SDM conversations, thus affecting RPAD scores on that service. Fourth, study services had fewer patients on average than other academic hospitals (median 9, range 3-14), which might limit its generalizability. Last, as in any observational study, there is always the possibility of the Hawthorne effect. However, neither teams nor patients knew the study objectives.
Nevertheless, important findings emerged through the use of RPAD Scores to evaluate inpatient SDM practices. In particular, we found that to increase SDM quality in inpatient settings, practitioners should (1) check their understanding of the patient’s perspective, (2) examine barriers to follow-through with the treatment plan, and (3) ask if the patient has questions. Variation among services remained very influential after adjusting for team and patient characteristics, which suggests that “climate” or service culture should be targeted by an intervention, rather than individual attendings or subgroups defined by team or patient characteristics. Notably, team size, number of learners, patient census, and type of decision being made did not affect SDM performance, suggesting that even large, busy services can perform SDM if properly trained.
Acknowledgments
The authors thank the patients, families, pediatric and internal medicine residents, and hospitalists at Stanford School of Medicine and University of California, San Francisco School of Medicine for their participation in this study. We would also like to thank the student volunteers who collected patient perspectives on the encounters.
Disclosure
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an NIH/NCCIH grant R25 AT006573.
1. Braddock CH. The emerging importance and relevance of shared decision making to clinical practice. Med Decis Mak. 2010;30(5 Suppl):5S-7S. doi:10.1177/0272989X10381344. PubMed
2. Braddock CH. Supporting shared decision making when clinical evidence is low. Med Care Res Rev MCRR. 2013;70(1 Suppl):129S-140S. doi:10.1177/1077558712460280. PubMed
3. Elwyn G, Tilburt J, Montori V. The ethical imperative for shared decision-making. Eur J Pers Centered Healthc. 2013;1(1):129-131. doi:10.5750/ejpch.v1i1.645.
4. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
5. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(10):CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
6. Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566-577. doi:10.1164/rccm.200906-0907OC. PubMed
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410-417. doi:10.1370/afm.1161. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
10. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781. doi:10.1056/NEJMp1109283. PubMed
11. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med J Assoc Am Med Coll. 2014;89(11):1548-1557. doi:10.1097/ACM.0000000000000483. PubMed
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692. PubMed
13. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
14. Légaré F, St-Jacques S, Gagnon S, et al. Prenatal screening for Down syndrome: a survey of willingness in women and family physicians to engage in shared decision-making. Prenat Diagn. 2011;31(4):319-326. doi:10.1002/pd.2624. PubMed
15. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. Milbank Q. 2009;87(2):368-390. PubMed
16. National Academy of Medicine. Crossing the quality chasm: a new health system for the 21st century. https://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed on November 30, 2016.
17. Adams RC, Levy SE, Council on Children with Disabilities. Shared Decision-Making and Children with Disabilities: Pathways to Consensus. Pediatrics. 2017; 139(6):1-9. PubMed
18. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
19. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
20. Kehl KL, Landrum MB, Arora NK, et al. Association of Actual and Preferred Decision Roles With Patient-Reported Quality of Care: Shared Decision Making in Cancer Care. JAMA Oncol. 2015;1(1):50-58. doi:10.1001/jamaoncol.2014.112. PubMed
21. Couët N, Desroches S, Robitaille H, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect Int J Public Particip Health Care Health Policy. 2015;18(4):542-561. doi:10.1111/hex.12054. PubMed
22. Fowler FJ, Gerstein BS, Barry MJ. How patient centered are medical decisions?: Results of a national survey. JAMA Intern Med. 2013;173(13):1215-1221. doi:10.1001/jamainternmed.2013.6172. PubMed
23. Légaré F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2014;(9):CD006732. doi:10.1002/14651858.CD006732.pub3. PubMed
24. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;(10):CD001431. doi:10.1002/14651858.CD001431.pub3. PubMed
25. Di Francesco L, Pistoria MJ, Auerbach AD, Nardino RJ, Holmboe ES. Internal medicine training in the inpatient setting. A review of published educational interventions. J Gen Intern Med. 2005;20(12):1173-1180. doi:10.1111/j.1525-1497.2005.00250.x. PubMed
26. Janicik RW, Fletcher KE. Teaching at the bedside: a new model. Med Teach. 2003;25(2):127-130. PubMed
27. Shields CG, Franks P, Fiscella K, Meldrum S, Epstein RM. Rochester Participatory Decision-Making Scale (RPAD): reliability and validity. Ann Fam Med. 2005;3(5):436-442. doi:10.1370/afm.305. PubMed
28. DocCom - enhancing competence in healthcare communication. https://webcampus.drexelmed.edu/doccom/user/. Accessed on November 30, 2016.
29. Bailey SM, Hendricks-Muñoz KD, Mally P. Parental influence on clinical management during neonatal intensive care: a survey of US neonatologists. J Matern Fetal Neonatal Med. 2013;26(12):1239-1244. doi:10.3109/14767058.2013.776531. PubMed
30. Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091-3098. doi:10.1200/JCO.2004.09.069. PubMed
31. Schoenborn NL, Cayea D, McNabney M, Ray A, Boyd C. Prognosis communication with older patients with multimorbidity: Assessment after an educational intervention. Gerontol Geriatr Educ. 2016;38(4):471-481. doi:10.1080/02701960.2015.1115983. PubMed
32. Lipkin M. Shared decision making. JAMA Intern Med. 2013;173(13):1204-1205. doi:10.1001/jamainternmed.2013.6248. PubMed
33. Gonzalo JD, Heist BS, Duffy BL, et al. The art of bedside rounds: a multi-center qualitative study of strategies used by experienced bedside teachers. J Gen Intern Med. 2013;28(3):412-420. doi:10.1007/s11606-012-2259-2. PubMed
34. Rosen P, Stenger E, Bochkoris M, Hannon MJ, Kwoh CK. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics. 2009;123(4):e603-e608. doi:10.1542/peds.2008-2238. PubMed
35. Harrison R, Allen E. Teaching internal medicine residents in the new era. Inpatient attending with duty-hour regulations. J Gen Intern Med. 2006;21(5):447-452. doi:10.1111/j.1525-1497.2006.00425.x. PubMed
36. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med 1982. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056. PubMed
1. Braddock CH. The emerging importance and relevance of shared decision making to clinical practice. Med Decis Mak. 2010;30(5 Suppl):5S-7S. doi:10.1177/0272989X10381344. PubMed
2. Braddock CH. Supporting shared decision making when clinical evidence is low. Med Care Res Rev MCRR. 2013;70(1 Suppl):129S-140S. doi:10.1177/1077558712460280. PubMed
3. Elwyn G, Tilburt J, Montori V. The ethical imperative for shared decision-making. Eur J Pers Centered Healthc. 2013;1(1):129-131. doi:10.5750/ejpch.v1i1.645.
4. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
5. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(10):CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
6. Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566-577. doi:10.1164/rccm.200906-0907OC. PubMed
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410-417. doi:10.1370/afm.1161. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
10. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781. doi:10.1056/NEJMp1109283. PubMed
11. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med J Assoc Am Med Coll. 2014;89(11):1548-1557. doi:10.1097/ACM.0000000000000483. PubMed
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692. PubMed
13. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
14. Légaré F, St-Jacques S, Gagnon S, et al. Prenatal screening for Down syndrome: a survey of willingness in women and family physicians to engage in shared decision-making. Prenat Diagn. 2011;31(4):319-326. doi:10.1002/pd.2624. PubMed
15. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. Milbank Q. 2009;87(2):368-390. PubMed
16. National Academy of Medicine. Crossing the quality chasm: a new health system for the 21st century. https://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed on November 30, 2016.
17. Adams RC, Levy SE, Council on Children with Disabilities. Shared Decision-Making and Children with Disabilities: Pathways to Consensus. Pediatrics. 2017; 139(6):1-9. PubMed
18. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
19. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
20. Kehl KL, Landrum MB, Arora NK, et al. Association of Actual and Preferred Decision Roles With Patient-Reported Quality of Care: Shared Decision Making in Cancer Care. JAMA Oncol. 2015;1(1):50-58. doi:10.1001/jamaoncol.2014.112. PubMed
21. Couët N, Desroches S, Robitaille H, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect Int J Public Particip Health Care Health Policy. 2015;18(4):542-561. doi:10.1111/hex.12054. PubMed
22. Fowler FJ, Gerstein BS, Barry MJ. How patient centered are medical decisions?: Results of a national survey. JAMA Intern Med. 2013;173(13):1215-1221. doi:10.1001/jamainternmed.2013.6172. PubMed
23. Légaré F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2014;(9):CD006732. doi:10.1002/14651858.CD006732.pub3. PubMed
24. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;(10):CD001431. doi:10.1002/14651858.CD001431.pub3. PubMed
25. Di Francesco L, Pistoria MJ, Auerbach AD, Nardino RJ, Holmboe ES. Internal medicine training in the inpatient setting. A review of published educational interventions. J Gen Intern Med. 2005;20(12):1173-1180. doi:10.1111/j.1525-1497.2005.00250.x. PubMed
26. Janicik RW, Fletcher KE. Teaching at the bedside: a new model. Med Teach. 2003;25(2):127-130. PubMed
27. Shields CG, Franks P, Fiscella K, Meldrum S, Epstein RM. Rochester Participatory Decision-Making Scale (RPAD): reliability and validity. Ann Fam Med. 2005;3(5):436-442. doi:10.1370/afm.305. PubMed
28. DocCom - enhancing competence in healthcare communication. https://webcampus.drexelmed.edu/doccom/user/. Accessed on November 30, 2016.
29. Bailey SM, Hendricks-Muñoz KD, Mally P. Parental influence on clinical management during neonatal intensive care: a survey of US neonatologists. J Matern Fetal Neonatal Med. 2013;26(12):1239-1244. doi:10.3109/14767058.2013.776531. PubMed
30. Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091-3098. doi:10.1200/JCO.2004.09.069. PubMed
31. Schoenborn NL, Cayea D, McNabney M, Ray A, Boyd C. Prognosis communication with older patients with multimorbidity: Assessment after an educational intervention. Gerontol Geriatr Educ. 2016;38(4):471-481. doi:10.1080/02701960.2015.1115983. PubMed
32. Lipkin M. Shared decision making. JAMA Intern Med. 2013;173(13):1204-1205. doi:10.1001/jamainternmed.2013.6248. PubMed
33. Gonzalo JD, Heist BS, Duffy BL, et al. The art of bedside rounds: a multi-center qualitative study of strategies used by experienced bedside teachers. J Gen Intern Med. 2013;28(3):412-420. doi:10.1007/s11606-012-2259-2. PubMed
34. Rosen P, Stenger E, Bochkoris M, Hannon MJ, Kwoh CK. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics. 2009;123(4):e603-e608. doi:10.1542/peds.2008-2238. PubMed
35. Harrison R, Allen E. Teaching internal medicine residents in the new era. Inpatient attending with duty-hour regulations. J Gen Intern Med. 2006;21(5):447-452. doi:10.1111/j.1525-1497.2006.00425.x. PubMed
36. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med 1982. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056. PubMed
© 2018 Society of Hospital Medicine
The SDM 3 Circle Model: A Literature Synthesis and Adaptation for Shared Decision Making in the Hospital
Evolving models of medical care emphasize the importance of shared decision-making (SDM) on practical and ethical grounds.1-3 SDM is a cognitive, emotional, and relational process in which provider and patient collaborate in a decision after discussing the options, evidence, and potential benefits and harms, while considering the patient’s values, preferences, and circumstances.4 Categories of decisions include information gathering, pharmacotherapy, therapeutic procedures, consultations and referrals, counseling and precautions (eg, behavior modification, goals of care, end-of-life care), and care transitions (eg, transfer or discharge to home).5 Decisions span the continuum of urgency and may be anticipatory or reactive.6 The patient’s environment7,8 and the provider-patient relationship9 have been explicitly incorporated into the ideal SDM process.
SDM has been conceptually and empirically linked with evidence-based practice,1 although the relationship between SDM and clinical outcomes is less clear.10,11 SDM is desired by patients12 and may bolster patient satisfaction, trust, and adherence.13,14 Limited evidence suggests SDM could reduce inappropriate treatments and testing,15 decrease adverse events,16 and promote greater patient safety,17-19 but more well-designed studies are needed.
Provider, patient, and contextual factors influence the extent to which SDM occurs. Providers commonly cite time constraints and perceived lack of applicability to certain clinical scenarios or settings.19 Providers may also lack training and competency in SDM skills.2 Patients may be reluctant to disagree with their provider or fear being mislabeled as “difficult.”20 When faced with high stakes or emotionally charged decisions, patients’ surrogates may prefer to have the provider serve as the sole decision-maker.21 Contextually, there may be limited evidence, high clinical stake, or a number of equally beneficial (or harmful) options.22,23
Current SDM models guide clinicians in determining when and how to engage in SDM, yet models vary widely. For example, Elwyn’s model emphasizes the ethical imperative for SDM and outlines 3 SDM steps: introduce choice, describe options, and help patients explore preferences and make decisions.3 Using a multimodal review and clinician-driven feedback, Legaré’s “IP-SDM” (Interprofessional Shared Decision Making) model illustrates the roles of the interprofessional team and emphasizes the influence of environmental factors on decision-making.24 Recent systematic reviews of SDM models have attempted to identify common elements, language, and processes.2,25,26
This study reviews leading SDM models to construct a more environmentally and contextually sensitive model that is appropriate for the hospital setting. Although developed with hospital medicine in mind, a synthesized model that attends to environmental and systems context, provider/team factors, patient factors, and disease/medical variables is highly relevant in any setting where SDM occurs.
METHODS
We constructed a model that is appropriate for SDM across the care continuum through the following 3-part, iterative group process: (1) a comprehensive literature review of existing SDM models, (2) synthesis and inductive development of a new draft model, and (3) modification of the new model using feedback from SDM experts.
Narrative Literature Review
We performed a structured, comprehensive literature review 29 to compare and contrast existing SDM models and frameworks. Leading models and key concepts were first identified using 2 systematic reviews 25,26 and a comprehensive review.2 In order to extend the search to 2016 and include any overlooked articles, a PubMed search was performed using the terms “shared decision-making” or “medical decision-making” AND “model” or “theory” or “framework” for English-language articles from inception to 2016. The search was repeated using Google Scholar to verify results and obtain the number of citations per article as a proxy for impact and saturation. In order to minimize possible search error or selection bias, reference lists in high-impact publications were hand searched to identify additional articles. All abstracts were manually reviewed by 2 independent authors for relevance and later inclusion in our group iterative process. A priori inclusion criteria were limited to provider-patient SDM (ie, not clinical reasoning or making decisions in general) and complete descriptions of a conceptual model or framework. Additional publications suggested by experts (eg, perspective pieces or terminology summaries) were also reviewed.
Model Development and Expert Review
The draft model and a standardized set of questions (supplementary Appendix A) were then emailed to all first and last authors of the reviewed studies (Table 2). Expert responses were compiled, coded, and analyzed independently by 3 coauthors. Inductive coding techniques and a constant comparative approach were used to code the qualitative data.32 Preliminary findings were shared among the 3 reviewers and discussed until consensus was reached on emerging themes and implications for the new SDM model and multistep SDM pathway. A master list of suggested revisions was shared with the larger authorship team and the model was refined accordingly.
RESULTS
Two previously published systematic reviews25,26 identified 494 articles, 161 conceptual definitions of SDM, and over 30 separate key concepts. The additional PubMed search garnered 1957 publications (with many overlapping from the systematic reviews). A manual search of the systematic reviews and PubMed abstracts identified 16 unique and complete decision-making models for further review. Hand searches of their citations yielded an additional 6 models for a total of 22 models.3,4,13,23,33-51 The majority of excluded articles described specific decision aids and small clinical studies, focused on only one step of the decision-making process, or were not otherwise relevant. The first (SR) and senior authors (JS) reviewed the 22 models for SDM relevance, generalizability, and content saturation, yielding a final sample of 9 SDM models. A subsequent Google Scholar search did not identify any new SDM models but 2 SDM theory papers1,52 and 2 commentaries53,54 were selected based on influence (ie, number of citations), expert recommendation, or coverage of a novel aspect of SDM. A total of 15 studies (9 SDM models + 6 reviews; Table 2) were used by our development team to create a synthesized SDM model. A 10th SDM model55 and 3 additional descriptive and normative studies8,56,57 were later added based on expert feedback and incorporated into our final SDM 3 Circle Model.
Expert Feedback
Twenty-one of 27 (78%) SDM expert authors responded to our e-mail request for feedback. The majority (62%) agreed with the basic elements of the model, including the environmental frame and the 3 domains. Some respondents viewed SDM as strictly a process between patient and provider independent of the disease, leading to refinement of the medical context category. Several experts emphasized the importance of SDM “set-up,” which includes the elicitation of patient preferences in how decisions are made and the extent of patient and/or surrogate involvement.
Several respondents identified time constraints (N = 2), acuity of disease (N = 3), and presence of multiple teams (N = 6) to be the significant factors distinguishing inpatient from outpatient SDM. For some experts, “team” referred to the interprofessional care team, whereas others referred to it as the collaboration among attending physicians and trainees. Experts noted that although the intensity and frequency of inpatient interactions could promote SDM, higher patient acuity and the urgency of decisions could negatively influence SDM and/or the patient’s ability to participate. Similarly, the presence of other team members may either impede or promote SDM by either contributing to miscommunication or bringing well-trained SDM experts to the bedside. Financial impact on patients and resource constraints were also noted as relevant. All of these elements have been incorporated into the final SDM 3 Circle Model and multistep SDM Pathway (Supplemental Appendix A and B).
The SDM 3 Circle Model
The SDM 3 Circle Model comprises 3 categories of SDM barriers and facilitators that intersect within the environmental frame of an inpatient ward or other setting: (1) provider/team, (2) patient/family, and (3) medical context. A Venn diagram visually represents the conceptual overlaps and distinctions among these categories that are all affected by the environment in which they occur (Supplemental Appendix A).
The patient/family circle mirrors prior SDM models that address the role of patient preferences in making decisions,3,4,12 with the explicit addition of the roles of families and surrogates as either decision-makers or influencers. This circle includes personal characteristics, such as cognitions (eg, beliefs, attitudes), emotions (eg, anxiety, hope), behaviors (eg, adherence, assertiveness), illness history (ie, subjective experience and understanding of one’s own medical history), and related social features (eg, culture, education, literacy, social supports).
Patient factors are not static over time or context. They occur within an environmental setting and are likely to be influenced by concurrent provider and medical variables (the second and third circles). Disease exacerbation leading to hospitalization or transfer to a subacute facility could dramatically shift the calculus a patient uses to determine preferences or activate dormant family dynamics. Strong provider-patient rapport (the overlap of patient and provider factors) may influence the development of trust and subsequent decisions.9 The type of disease or symptom presentation (circle 3–medical context) may further influence patient factors due to stigma, perceived vulnerability, or assumed prognosis.
The provider/team circle includes both individual and team-based factors falling into similar categories as the patient/family domain, such as cognitions, behavior, and social features; however, these factors include both personal (eg, the provider’s personal history, values, and beliefs) and professional (eg, past medical training, decision-making style, past experiences treating a disease) characteristics. Decisions may involve an interprofessional team representing a broad range of personalities and professional values. Decisions and decision-making processes may change over time as team composition changes, as level of provider expertise varies, or as environmental, patient, or disease/illness factors influence providers and teams.
Medical context includes factors related to the disease and the potential ways to evaluate or manage it. Examples of disease factors include acuity, symptoms, course, and prognosis. Most obviously, disease factors will influence the content of risk-benefit discussions but may also affect the SDM process through disease stigma or cultural assumptions about etiology. Disease evaluation factors include the psychometrics of a diagnostic screen, invasive and noninvasive testing, or a range of different preventive or therapeutic interventions. Treatment variables include the available options, costs, and risk of complications. Medical context variables evolve as evidence-based medicine and biomedical knowledge increase and new treatment options emerge.
Each of the 3 circles operates within the same environmental frame, such as an inpatient medicine ward, which itself operates within a hospital and the broader healthcare system. This frame exerts overt and subtle influences on providers, patients, and even the medical context. Features of the environmental frame include culture (eg, values, preferences, social norms), university versus community setting, incentives, formularies, quality improvement campaigns, regulations, and technology use.
The dynamic interactivity of the environmental frame and the 3 circles inform the process of SDM and highlight key differences that may occur between care settings. Certain features may predominate in different situations, but all will influence and be influenced by features of other circles during the course of SDM.
Application of the SDM 3 Circle Model
Although the SDM process is similar across clinical settings, its operationalization varies in important ways for hospital decision-making. In some situations, patients may defer all decisions to their providers or decisions may be considered with multiple providers concurrently. In the hospital, SDM may not be possible, such as in emergency surgery for an obtunded patient or when the patient and surrogate are not available or able to participate in the decision. Therefore, providers may bypass the steps of information sharing and discussion of the decision (big arrow in the Figure and supplemental
DISCUSSION
The SDM 3 Circle Model provides a concise, ecologically valid, contextually sensitive representation of SDM that synthesizes and extends beyond recent SDM models.3,7,40 Each circle represents the forces that influence SDM across settings. Although the multistep SDM pathway occurs similarly in outpatient and inpatient settings, how each step is operationalized and how each “circle” exerts its influence may differ and warrants further consideration throughout the SDM process. For example, hospitalized patients may have greater stress and anxiety, have more family involvement, be more motivated to adhere to treatment, and may be under greater financial and social pressures. Unlike outpatient primary care, patients are less likely to have an existing relationship with their inpatient providers, potentially compromising patient confidence in the provider, and necessitating expeditious trust building.
The SDM 3 Circle Model captures “setting” in both the broader environmental frame and within the provider/team category of variables. The frame also captures health system and broader community variables that may influence the practicality of some medical decisions. Within this essential frame, all 3 categories of patient, provider, and medical context are included as part of the SDM process. A better understanding of their interplay may be of great value for clinicians, researchers, administrators, and policy makers who wish to further study and promote SDM. Both the SDM 3 Circle Model and its accompanying pathway (Figures 1 and 2) highlight opportunities for intervention and research, and may drive quality improvement initiatives to improve clinical outcomes.
Limitations
We did not perform a new systematic review, potentially omitting lesser-known publications. We mitigated this risk by using recent systematic reviews, searching multiple databases, hand searching citation lists, and making inquiries to SDM experts. Our selection of models used as a foundation for the synthesized model was based on consensus, which included an element of subjective, clinical judgment. Our SDM expert sample was small and limited to authors of the papers we reviewed, potentially restricting the range of viewpoints received. Lastly, the SDM 3 Circle Model highlights key concept areas rather than all possible factors that influence SDM.
CONCLUSIONS
We present a peer-reviewed, literature-based SDM model capable of accounting for the unique circumstances and challenges of SDM in the hospital. The SDM 3 Circle Model identifies the primary categories of variables thought to influence SDM, places them in a shared environmental frame, and visually represents their interactive nature. A multistep representation of the SDM process further illustrates how the unique features and challenges of hospitalization might exert influence at various points as patients and providers reach a shared decision. As the interrelationships of patient and provider/team, medical context, and the environmental frame in which they occur are better understood, more effective and targeted interventions to promote SDM can be developed and evaluated.
Acknowledgments
The authors would like to thank Evans Whitaker for his assistance with the literature review and the Patient Engagement Project volunteers for their support and assistance with data collection.
Disclosure
Financial support for this study was provided entirely by a grant from NIH/NCCIH (grant #R25 AT006573, awarded to Dr. Jason Satterfield). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The following authors are employed by the sponsor: Stephanie Rennke, MD, Patrick Yuan, BA, Brad Monash, MD, Rebecca Blankenburg, MD, MPH, Ian Chua, MD, Stephanie Harman, MD, Debbie S. Sakai, MD, Joan F. Hilton, DSc, MPH., and Jason Satterfield, PhD.
1. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312(13):1295-1296. doi:10.1001/jama.2014.10186. PubMed
2. Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
3. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
4. Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651-661. PubMed
5. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. What is a medical decision? A units. Am J Respir Crit Care Med. 2011;183(7):915-921. doi:10.1164/rccm.201008-1214OC. PubMed
22. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
23. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
24. Légaré F, Bekker H, Desroches S, et al. How can continuing professional development better promote shared decision-making? Perspectives from an international collaboration. Implement Sci. 2011;6:68. doi:10.1186/1748-5908-6-68. PubMed
25. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301-312. doi:10.1016/j.pec.2005.06.010. PubMed
26. Moumjid N, Gafni A, Brémond A, Carrère MO. Shared decision making in the medical taxonomy based on physician statements in hospital encounters: a qualitative study. BMJ Open. 2016;6(2):e010098. doi:10.1136/bmjopen-2015-010098. PubMed
6. Fowler FJ, Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff (Millwood). 2011;30(4):699-706. doi:10.1377/hlthaff.2011.0003. PubMed
7. Weiner SJ, Kelly B, Ashley N, et al. Content coding for contextualization of care: evaluating physician performance at patient-centered decision making. Med Decis Making. 2014;34(1):97-106. doi:10.1177/0272989X13493146. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Matthias MS, Salyers MP, Frankel RM. Re-thinking shared decision-making: context matters. Patient Educ Couns. 2013;91(2):176-179. doi:10.1016/j.pec.2013.01.006 PubMed
10. Clayman ML, Bylund CL, Chewning B, Makoul G. The Impact of Patient Participation in Health Decisions Within Medical Encounters: A Systematic Review. Med Decis Making. 2016;36(4):427-452. doi:10.1177/0272989X15613530. PubMed
11. Shay LA, Lafata JE. Understanding patient perceptions of shared decision making. Patient Educ Couns. 2014;96(3):295-301. doi:10.1016/j.pec.2014.07.017. PubMed
12. Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18. doi:10.1016/j.pec.2011.02.004. PubMed
13. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
14. Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219-226. doi:10.1159/000126073. PubMed
15. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
16. Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269-277. doi:10.1093/intqhc/mzr002. PubMed
17. Mohammed K, Nolan MB, Rajjo T, et al. Creating a Patient-Centered Health Care Delivery System: A Systematic Review of Health Care Quality From the Patient Perspective. Am J Med Qual. 2014;31(1):12-21. doi:10.1177/1062860614545124. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. doi:10.1136/bmjqs-2012-001769. PubMed
19. Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526-535. doi:10.1016/j.pec.2008.07.018. PubMed
20. Frosch DL, May SG, Rendle KAS, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled “difficult” among key obstacles to shared decision making. Health Aff (Millwood). 2012;31(5):1030-1038. doi:10.1377/hlthaff.2011.0576. PubMed
21. Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care encounter: are we all talking about the same thing? Med Decis Making. 2007;27(5):539-546. doi:10.1177/0272989X07306779. PubMed
27. Hallström I, Elander G. Decision-making during hospitalization: parents’ and children’s involvement. J Clin Nurs. 2004;13(3):367-375. PubMed
28. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. Temporal characteristics of decisions in hospital encounters: a threshold for shared decision making? A qualitative study. Patient Educ Couns. 2014;97(2):216-222. doi:10.1016/j.pec.2014.08.005. PubMed
29. Baumeister RF, Leary MR. Writing narrative literature reviews. Rev Gen Psychol. 1997;1(3):311.
30. Moody DL. Theoretical and practical issues in evaluating the quality of conceptual models: current state and future directions. Data Knowl Eng. 2005;55(3):243-276. doi:10.1016/j.datak.2004.12.005.
31. McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351-377. PubMed
32. Basics of Qualitative Research | SAGE Publications Inc. https://us.sagepub.com/en-us/nam/basics-of-qualitative-research/book235578. Accessed on September 13, 2016. PubMed
33. 2013;2(4):421-433. doi:10.2217/cer.13.46.J Comp Eff Res33. Halley MC, Rendle KA, Frosch DL. A conceptual model of the multiple stages of communication necessary to support patient-centered care. PubMed
34. 2012;87(1):54-61. doi:10.1016/j.pec.2011.07.027.Patient Educ Couns34. Torke AM, Petronio S, Sachs GA, Helft PR, Purnell C. A conceptual model of the role of communication in surrogate decision making for hospitalized adults. PubMed
35. 2009;15(6):1142-1151. doi:10.1111/j.1365-2753.2009.01315.x.J Eval Clin Pract35. Falzer PR, Garman MD. A conditional model of evidence-based decision making: Model of evidence-based decision making. PubMed
36. 2012;8(4):161-164. doi:10.1097/PTS.0b013e318267c56e.J Patient Saf36. Holzmueller CG, Wu AW, Pronovost PJ. A framework for encouraging patient engagement in medical decision making. PubMed
37. 2014;97(2):158-164. doi:10.1016/j.pec.2014.07.027.Patient Educ Couns37. Elwyn G, Lloyd A, May C, et al. Collaborative deliberation: a model for patient care. PubMed
38. 2002;35(5-6):313-321. doi:10.1016/S1532-0464(03)00037-6.J Biomed Inform38. Ruland CM, Bakken S. Developing, implementing, and evaluating decision support systems for shared decision making in patient care: a conceptual model and case illustration. PubMed
39. 1999;319(7212):764.BMJ39. Shepperd S, Charnock D, Gann B. Helping patients access high quality health information. PubMed
40. 2011;25(1):18-25. doi:10.3109/13561820.2010.490502.J Interprof Care40. Légaré F, Stacey D, Pouliot S, et al. Interprofessionalism and shared decision-making in primary care: a stepwise approach towards a new model. PubMed
41. 2015;25(1):141-152. doi:10.1007/s10926-014-9532-7.J Occup Rehabil41. Coutu MF, Légaré F, Durand MJ, et al. Operationalizing a Shared Decision Making Model for Work Rehabilitation Programs: A Consensus Process. PubMed
42. 2013;13:231.BMC Health Serv Res42. Hölzel LP, Kriston L, Härter M. Patient preference for involvement, experienced involvement, decisional conflict, and satisfaction with physician: a structural equation model test. PubMed
43. 2008;134(4):835-843. doi:10.1378/chest.08-0235.Chest43. Curtis JR, White DB. Practical guidance for evidence-based ICU family conferences. PubMed
44. 2013;8:29-36. doi:10.4137/IMI.S12783.Integr Med Insights44. Brooks AT, Silverman L, Wallen G. Shared Decision Making: A Fundamental Tenet in a Conceptual Framework of Integrative Healthcare Delivery. PubMed
45. 2013;33(1):37-47. doi:10.1177/0272989X12458159.Med Decis Making45. Müller-Engelmann M, Donner-Banzhoff N, Keller H, et al. When decisions should be shared: a study of social norms in medical decision making using a factorial survey approach. PubMed
46. 2007;101(4):205-211.Z Arztl Fortbild Qualitatssich46. Mccaffery KJ, Shepherd HL, Trevena L, et al. Shared decision-making in Australia. PubMed
47. 2014;20(2):311-318. doi:10.1007/s12028-013-9922-2.Neurocrit Care
47. Rubin MA. The Collaborative Autonomy Model of Medical Decision-Making. 48. 2013;70(1 Suppl):141S-158S. doi:10.1177/1077558712461952.Med Care Res Rev PubMed
48. McCullough LB. The professional medical ethics model of decision making under conditions of clinical uncertainty. PubMed
49. 2009;87(2):368–390.Milbank Q49. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. PubMed
50. 2015;25(3):276-282. doi:10.1016/j.whi.2015.02.002.Womens Health Issues50. Moore JE, Titler MG, Kane Low L, Dalton VK, Sampselle CM. Transforming Patient-Centered Care: Development of the Evidence Informed Decision Making through Engagement Model. PubMed
51. 1997;44(5):681-692.Soc Sci Med51. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). PubMed
52. 2010;80(2):164-172. doi:10.1016/j.pec.2009.10.015.Patient Educ Couns52. Stacey D, Légaré F, Pouliot S, Kryworuchko J, Dunn S. Shared decision making models to inform an interprofessional perspective on decision making: a theory analysis. PubMed
53. 2013;70(1 Suppl):94S-112S. doi:10.1177/1077558712459216.Med Care Res Rev53. Epstein RM, Gramling RE. What is shared in shared decision making? Complex decisions when the evidence is unclear. PubMed
54. 2010;304(8):903-904. doi:10.1001/jama.2010.1208.JAMA54. Kon AA. The shared decision-making continuum. PubMed
55. 2008;30(3):429-444. doi:10.1111/j.1467-9566.2007.01064.x.Sociol Health Illn55. Rapley T. Distributed decision making: the anatomy of decisions-in-action. PubMed
56. 1997;12(6):339-345.J Gen Intern Med56. Braddock CH 3rd, Fihn SD, Levinson W, Jonsen AR, Pearlman RA. How doctors and patients discuss routine clinical decisions. Informed decision making in the outpatient setting. PubMed
57. 1999;282(24):2313-2320.JAMA57. Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. PubMed
58. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056.Soc Sci Med58. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups.
2006;9(4):321-332. doi:10.1111/j.1369-7625.2006.00404.x.Health Expect. PubMed
59. Towle A, Godolphin W, Grams G, Lamarre A. Putting informed and shared decision making into practice. PubMed
60. 2011;17(4):554-564. doi: 10.1111/j.1365-2753.2010.01515.x.J Eval Clin Pract60. Légaré F, Stacey D, Gagnon S, et al. Validating a conceptual model for an interprofessional approach to shared decision making: a mixed methods study. PubMed
Evolving models of medical care emphasize the importance of shared decision-making (SDM) on practical and ethical grounds.1-3 SDM is a cognitive, emotional, and relational process in which provider and patient collaborate in a decision after discussing the options, evidence, and potential benefits and harms, while considering the patient’s values, preferences, and circumstances.4 Categories of decisions include information gathering, pharmacotherapy, therapeutic procedures, consultations and referrals, counseling and precautions (eg, behavior modification, goals of care, end-of-life care), and care transitions (eg, transfer or discharge to home).5 Decisions span the continuum of urgency and may be anticipatory or reactive.6 The patient’s environment7,8 and the provider-patient relationship9 have been explicitly incorporated into the ideal SDM process.
SDM has been conceptually and empirically linked with evidence-based practice,1 although the relationship between SDM and clinical outcomes is less clear.10,11 SDM is desired by patients12 and may bolster patient satisfaction, trust, and adherence.13,14 Limited evidence suggests SDM could reduce inappropriate treatments and testing,15 decrease adverse events,16 and promote greater patient safety,17-19 but more well-designed studies are needed.
Provider, patient, and contextual factors influence the extent to which SDM occurs. Providers commonly cite time constraints and perceived lack of applicability to certain clinical scenarios or settings.19 Providers may also lack training and competency in SDM skills.2 Patients may be reluctant to disagree with their provider or fear being mislabeled as “difficult.”20 When faced with high stakes or emotionally charged decisions, patients’ surrogates may prefer to have the provider serve as the sole decision-maker.21 Contextually, there may be limited evidence, high clinical stake, or a number of equally beneficial (or harmful) options.22,23
Current SDM models guide clinicians in determining when and how to engage in SDM, yet models vary widely. For example, Elwyn’s model emphasizes the ethical imperative for SDM and outlines 3 SDM steps: introduce choice, describe options, and help patients explore preferences and make decisions.3 Using a multimodal review and clinician-driven feedback, Legaré’s “IP-SDM” (Interprofessional Shared Decision Making) model illustrates the roles of the interprofessional team and emphasizes the influence of environmental factors on decision-making.24 Recent systematic reviews of SDM models have attempted to identify common elements, language, and processes.2,25,26
This study reviews leading SDM models to construct a more environmentally and contextually sensitive model that is appropriate for the hospital setting. Although developed with hospital medicine in mind, a synthesized model that attends to environmental and systems context, provider/team factors, patient factors, and disease/medical variables is highly relevant in any setting where SDM occurs.
METHODS
We constructed a model that is appropriate for SDM across the care continuum through the following 3-part, iterative group process: (1) a comprehensive literature review of existing SDM models, (2) synthesis and inductive development of a new draft model, and (3) modification of the new model using feedback from SDM experts.
Narrative Literature Review
We performed a structured, comprehensive literature review 29 to compare and contrast existing SDM models and frameworks. Leading models and key concepts were first identified using 2 systematic reviews 25,26 and a comprehensive review.2 In order to extend the search to 2016 and include any overlooked articles, a PubMed search was performed using the terms “shared decision-making” or “medical decision-making” AND “model” or “theory” or “framework” for English-language articles from inception to 2016. The search was repeated using Google Scholar to verify results and obtain the number of citations per article as a proxy for impact and saturation. In order to minimize possible search error or selection bias, reference lists in high-impact publications were hand searched to identify additional articles. All abstracts were manually reviewed by 2 independent authors for relevance and later inclusion in our group iterative process. A priori inclusion criteria were limited to provider-patient SDM (ie, not clinical reasoning or making decisions in general) and complete descriptions of a conceptual model or framework. Additional publications suggested by experts (eg, perspective pieces or terminology summaries) were also reviewed.
Model Development and Expert Review
The draft model and a standardized set of questions (supplementary Appendix A) were then emailed to all first and last authors of the reviewed studies (Table 2). Expert responses were compiled, coded, and analyzed independently by 3 coauthors. Inductive coding techniques and a constant comparative approach were used to code the qualitative data.32 Preliminary findings were shared among the 3 reviewers and discussed until consensus was reached on emerging themes and implications for the new SDM model and multistep SDM pathway. A master list of suggested revisions was shared with the larger authorship team and the model was refined accordingly.
RESULTS
Two previously published systematic reviews25,26 identified 494 articles, 161 conceptual definitions of SDM, and over 30 separate key concepts. The additional PubMed search garnered 1957 publications (with many overlapping from the systematic reviews). A manual search of the systematic reviews and PubMed abstracts identified 16 unique and complete decision-making models for further review. Hand searches of their citations yielded an additional 6 models for a total of 22 models.3,4,13,23,33-51 The majority of excluded articles described specific decision aids and small clinical studies, focused on only one step of the decision-making process, or were not otherwise relevant. The first (SR) and senior authors (JS) reviewed the 22 models for SDM relevance, generalizability, and content saturation, yielding a final sample of 9 SDM models. A subsequent Google Scholar search did not identify any new SDM models but 2 SDM theory papers1,52 and 2 commentaries53,54 were selected based on influence (ie, number of citations), expert recommendation, or coverage of a novel aspect of SDM. A total of 15 studies (9 SDM models + 6 reviews; Table 2) were used by our development team to create a synthesized SDM model. A 10th SDM model55 and 3 additional descriptive and normative studies8,56,57 were later added based on expert feedback and incorporated into our final SDM 3 Circle Model.
Expert Feedback
Twenty-one of 27 (78%) SDM expert authors responded to our e-mail request for feedback. The majority (62%) agreed with the basic elements of the model, including the environmental frame and the 3 domains. Some respondents viewed SDM as strictly a process between patient and provider independent of the disease, leading to refinement of the medical context category. Several experts emphasized the importance of SDM “set-up,” which includes the elicitation of patient preferences in how decisions are made and the extent of patient and/or surrogate involvement.
Several respondents identified time constraints (N = 2), acuity of disease (N = 3), and presence of multiple teams (N = 6) to be the significant factors distinguishing inpatient from outpatient SDM. For some experts, “team” referred to the interprofessional care team, whereas others referred to it as the collaboration among attending physicians and trainees. Experts noted that although the intensity and frequency of inpatient interactions could promote SDM, higher patient acuity and the urgency of decisions could negatively influence SDM and/or the patient’s ability to participate. Similarly, the presence of other team members may either impede or promote SDM by either contributing to miscommunication or bringing well-trained SDM experts to the bedside. Financial impact on patients and resource constraints were also noted as relevant. All of these elements have been incorporated into the final SDM 3 Circle Model and multistep SDM Pathway (Supplemental Appendix A and B).
The SDM 3 Circle Model
The SDM 3 Circle Model comprises 3 categories of SDM barriers and facilitators that intersect within the environmental frame of an inpatient ward or other setting: (1) provider/team, (2) patient/family, and (3) medical context. A Venn diagram visually represents the conceptual overlaps and distinctions among these categories that are all affected by the environment in which they occur (Supplemental Appendix A).
The patient/family circle mirrors prior SDM models that address the role of patient preferences in making decisions,3,4,12 with the explicit addition of the roles of families and surrogates as either decision-makers or influencers. This circle includes personal characteristics, such as cognitions (eg, beliefs, attitudes), emotions (eg, anxiety, hope), behaviors (eg, adherence, assertiveness), illness history (ie, subjective experience and understanding of one’s own medical history), and related social features (eg, culture, education, literacy, social supports).
Patient factors are not static over time or context. They occur within an environmental setting and are likely to be influenced by concurrent provider and medical variables (the second and third circles). Disease exacerbation leading to hospitalization or transfer to a subacute facility could dramatically shift the calculus a patient uses to determine preferences or activate dormant family dynamics. Strong provider-patient rapport (the overlap of patient and provider factors) may influence the development of trust and subsequent decisions.9 The type of disease or symptom presentation (circle 3–medical context) may further influence patient factors due to stigma, perceived vulnerability, or assumed prognosis.
The provider/team circle includes both individual and team-based factors falling into similar categories as the patient/family domain, such as cognitions, behavior, and social features; however, these factors include both personal (eg, the provider’s personal history, values, and beliefs) and professional (eg, past medical training, decision-making style, past experiences treating a disease) characteristics. Decisions may involve an interprofessional team representing a broad range of personalities and professional values. Decisions and decision-making processes may change over time as team composition changes, as level of provider expertise varies, or as environmental, patient, or disease/illness factors influence providers and teams.
Medical context includes factors related to the disease and the potential ways to evaluate or manage it. Examples of disease factors include acuity, symptoms, course, and prognosis. Most obviously, disease factors will influence the content of risk-benefit discussions but may also affect the SDM process through disease stigma or cultural assumptions about etiology. Disease evaluation factors include the psychometrics of a diagnostic screen, invasive and noninvasive testing, or a range of different preventive or therapeutic interventions. Treatment variables include the available options, costs, and risk of complications. Medical context variables evolve as evidence-based medicine and biomedical knowledge increase and new treatment options emerge.
Each of the 3 circles operates within the same environmental frame, such as an inpatient medicine ward, which itself operates within a hospital and the broader healthcare system. This frame exerts overt and subtle influences on providers, patients, and even the medical context. Features of the environmental frame include culture (eg, values, preferences, social norms), university versus community setting, incentives, formularies, quality improvement campaigns, regulations, and technology use.
The dynamic interactivity of the environmental frame and the 3 circles inform the process of SDM and highlight key differences that may occur between care settings. Certain features may predominate in different situations, but all will influence and be influenced by features of other circles during the course of SDM.
Application of the SDM 3 Circle Model
Although the SDM process is similar across clinical settings, its operationalization varies in important ways for hospital decision-making. In some situations, patients may defer all decisions to their providers or decisions may be considered with multiple providers concurrently. In the hospital, SDM may not be possible, such as in emergency surgery for an obtunded patient or when the patient and surrogate are not available or able to participate in the decision. Therefore, providers may bypass the steps of information sharing and discussion of the decision (big arrow in the Figure and supplemental
DISCUSSION
The SDM 3 Circle Model provides a concise, ecologically valid, contextually sensitive representation of SDM that synthesizes and extends beyond recent SDM models.3,7,40 Each circle represents the forces that influence SDM across settings. Although the multistep SDM pathway occurs similarly in outpatient and inpatient settings, how each step is operationalized and how each “circle” exerts its influence may differ and warrants further consideration throughout the SDM process. For example, hospitalized patients may have greater stress and anxiety, have more family involvement, be more motivated to adhere to treatment, and may be under greater financial and social pressures. Unlike outpatient primary care, patients are less likely to have an existing relationship with their inpatient providers, potentially compromising patient confidence in the provider, and necessitating expeditious trust building.
The SDM 3 Circle Model captures “setting” in both the broader environmental frame and within the provider/team category of variables. The frame also captures health system and broader community variables that may influence the practicality of some medical decisions. Within this essential frame, all 3 categories of patient, provider, and medical context are included as part of the SDM process. A better understanding of their interplay may be of great value for clinicians, researchers, administrators, and policy makers who wish to further study and promote SDM. Both the SDM 3 Circle Model and its accompanying pathway (Figures 1 and 2) highlight opportunities for intervention and research, and may drive quality improvement initiatives to improve clinical outcomes.
Limitations
We did not perform a new systematic review, potentially omitting lesser-known publications. We mitigated this risk by using recent systematic reviews, searching multiple databases, hand searching citation lists, and making inquiries to SDM experts. Our selection of models used as a foundation for the synthesized model was based on consensus, which included an element of subjective, clinical judgment. Our SDM expert sample was small and limited to authors of the papers we reviewed, potentially restricting the range of viewpoints received. Lastly, the SDM 3 Circle Model highlights key concept areas rather than all possible factors that influence SDM.
CONCLUSIONS
We present a peer-reviewed, literature-based SDM model capable of accounting for the unique circumstances and challenges of SDM in the hospital. The SDM 3 Circle Model identifies the primary categories of variables thought to influence SDM, places them in a shared environmental frame, and visually represents their interactive nature. A multistep representation of the SDM process further illustrates how the unique features and challenges of hospitalization might exert influence at various points as patients and providers reach a shared decision. As the interrelationships of patient and provider/team, medical context, and the environmental frame in which they occur are better understood, more effective and targeted interventions to promote SDM can be developed and evaluated.
Acknowledgments
The authors would like to thank Evans Whitaker for his assistance with the literature review and the Patient Engagement Project volunteers for their support and assistance with data collection.
Disclosure
Financial support for this study was provided entirely by a grant from NIH/NCCIH (grant #R25 AT006573, awarded to Dr. Jason Satterfield). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The following authors are employed by the sponsor: Stephanie Rennke, MD, Patrick Yuan, BA, Brad Monash, MD, Rebecca Blankenburg, MD, MPH, Ian Chua, MD, Stephanie Harman, MD, Debbie S. Sakai, MD, Joan F. Hilton, DSc, MPH., and Jason Satterfield, PhD.
Evolving models of medical care emphasize the importance of shared decision-making (SDM) on practical and ethical grounds.1-3 SDM is a cognitive, emotional, and relational process in which provider and patient collaborate in a decision after discussing the options, evidence, and potential benefits and harms, while considering the patient’s values, preferences, and circumstances.4 Categories of decisions include information gathering, pharmacotherapy, therapeutic procedures, consultations and referrals, counseling and precautions (eg, behavior modification, goals of care, end-of-life care), and care transitions (eg, transfer or discharge to home).5 Decisions span the continuum of urgency and may be anticipatory or reactive.6 The patient’s environment7,8 and the provider-patient relationship9 have been explicitly incorporated into the ideal SDM process.
SDM has been conceptually and empirically linked with evidence-based practice,1 although the relationship between SDM and clinical outcomes is less clear.10,11 SDM is desired by patients12 and may bolster patient satisfaction, trust, and adherence.13,14 Limited evidence suggests SDM could reduce inappropriate treatments and testing,15 decrease adverse events,16 and promote greater patient safety,17-19 but more well-designed studies are needed.
Provider, patient, and contextual factors influence the extent to which SDM occurs. Providers commonly cite time constraints and perceived lack of applicability to certain clinical scenarios or settings.19 Providers may also lack training and competency in SDM skills.2 Patients may be reluctant to disagree with their provider or fear being mislabeled as “difficult.”20 When faced with high stakes or emotionally charged decisions, patients’ surrogates may prefer to have the provider serve as the sole decision-maker.21 Contextually, there may be limited evidence, high clinical stake, or a number of equally beneficial (or harmful) options.22,23
Current SDM models guide clinicians in determining when and how to engage in SDM, yet models vary widely. For example, Elwyn’s model emphasizes the ethical imperative for SDM and outlines 3 SDM steps: introduce choice, describe options, and help patients explore preferences and make decisions.3 Using a multimodal review and clinician-driven feedback, Legaré’s “IP-SDM” (Interprofessional Shared Decision Making) model illustrates the roles of the interprofessional team and emphasizes the influence of environmental factors on decision-making.24 Recent systematic reviews of SDM models have attempted to identify common elements, language, and processes.2,25,26
This study reviews leading SDM models to construct a more environmentally and contextually sensitive model that is appropriate for the hospital setting. Although developed with hospital medicine in mind, a synthesized model that attends to environmental and systems context, provider/team factors, patient factors, and disease/medical variables is highly relevant in any setting where SDM occurs.
METHODS
We constructed a model that is appropriate for SDM across the care continuum through the following 3-part, iterative group process: (1) a comprehensive literature review of existing SDM models, (2) synthesis and inductive development of a new draft model, and (3) modification of the new model using feedback from SDM experts.
Narrative Literature Review
We performed a structured, comprehensive literature review 29 to compare and contrast existing SDM models and frameworks. Leading models and key concepts were first identified using 2 systematic reviews 25,26 and a comprehensive review.2 In order to extend the search to 2016 and include any overlooked articles, a PubMed search was performed using the terms “shared decision-making” or “medical decision-making” AND “model” or “theory” or “framework” for English-language articles from inception to 2016. The search was repeated using Google Scholar to verify results and obtain the number of citations per article as a proxy for impact and saturation. In order to minimize possible search error or selection bias, reference lists in high-impact publications were hand searched to identify additional articles. All abstracts were manually reviewed by 2 independent authors for relevance and later inclusion in our group iterative process. A priori inclusion criteria were limited to provider-patient SDM (ie, not clinical reasoning or making decisions in general) and complete descriptions of a conceptual model or framework. Additional publications suggested by experts (eg, perspective pieces or terminology summaries) were also reviewed.
Model Development and Expert Review
The draft model and a standardized set of questions (supplementary Appendix A) were then emailed to all first and last authors of the reviewed studies (Table 2). Expert responses were compiled, coded, and analyzed independently by 3 coauthors. Inductive coding techniques and a constant comparative approach were used to code the qualitative data.32 Preliminary findings were shared among the 3 reviewers and discussed until consensus was reached on emerging themes and implications for the new SDM model and multistep SDM pathway. A master list of suggested revisions was shared with the larger authorship team and the model was refined accordingly.
RESULTS
Two previously published systematic reviews25,26 identified 494 articles, 161 conceptual definitions of SDM, and over 30 separate key concepts. The additional PubMed search garnered 1957 publications (with many overlapping from the systematic reviews). A manual search of the systematic reviews and PubMed abstracts identified 16 unique and complete decision-making models for further review. Hand searches of their citations yielded an additional 6 models for a total of 22 models.3,4,13,23,33-51 The majority of excluded articles described specific decision aids and small clinical studies, focused on only one step of the decision-making process, or were not otherwise relevant. The first (SR) and senior authors (JS) reviewed the 22 models for SDM relevance, generalizability, and content saturation, yielding a final sample of 9 SDM models. A subsequent Google Scholar search did not identify any new SDM models but 2 SDM theory papers1,52 and 2 commentaries53,54 were selected based on influence (ie, number of citations), expert recommendation, or coverage of a novel aspect of SDM. A total of 15 studies (9 SDM models + 6 reviews; Table 2) were used by our development team to create a synthesized SDM model. A 10th SDM model55 and 3 additional descriptive and normative studies8,56,57 were later added based on expert feedback and incorporated into our final SDM 3 Circle Model.
Expert Feedback
Twenty-one of 27 (78%) SDM expert authors responded to our e-mail request for feedback. The majority (62%) agreed with the basic elements of the model, including the environmental frame and the 3 domains. Some respondents viewed SDM as strictly a process between patient and provider independent of the disease, leading to refinement of the medical context category. Several experts emphasized the importance of SDM “set-up,” which includes the elicitation of patient preferences in how decisions are made and the extent of patient and/or surrogate involvement.
Several respondents identified time constraints (N = 2), acuity of disease (N = 3), and presence of multiple teams (N = 6) to be the significant factors distinguishing inpatient from outpatient SDM. For some experts, “team” referred to the interprofessional care team, whereas others referred to it as the collaboration among attending physicians and trainees. Experts noted that although the intensity and frequency of inpatient interactions could promote SDM, higher patient acuity and the urgency of decisions could negatively influence SDM and/or the patient’s ability to participate. Similarly, the presence of other team members may either impede or promote SDM by either contributing to miscommunication or bringing well-trained SDM experts to the bedside. Financial impact on patients and resource constraints were also noted as relevant. All of these elements have been incorporated into the final SDM 3 Circle Model and multistep SDM Pathway (Supplemental Appendix A and B).
The SDM 3 Circle Model
The SDM 3 Circle Model comprises 3 categories of SDM barriers and facilitators that intersect within the environmental frame of an inpatient ward or other setting: (1) provider/team, (2) patient/family, and (3) medical context. A Venn diagram visually represents the conceptual overlaps and distinctions among these categories that are all affected by the environment in which they occur (Supplemental Appendix A).
The patient/family circle mirrors prior SDM models that address the role of patient preferences in making decisions,3,4,12 with the explicit addition of the roles of families and surrogates as either decision-makers or influencers. This circle includes personal characteristics, such as cognitions (eg, beliefs, attitudes), emotions (eg, anxiety, hope), behaviors (eg, adherence, assertiveness), illness history (ie, subjective experience and understanding of one’s own medical history), and related social features (eg, culture, education, literacy, social supports).
Patient factors are not static over time or context. They occur within an environmental setting and are likely to be influenced by concurrent provider and medical variables (the second and third circles). Disease exacerbation leading to hospitalization or transfer to a subacute facility could dramatically shift the calculus a patient uses to determine preferences or activate dormant family dynamics. Strong provider-patient rapport (the overlap of patient and provider factors) may influence the development of trust and subsequent decisions.9 The type of disease or symptom presentation (circle 3–medical context) may further influence patient factors due to stigma, perceived vulnerability, or assumed prognosis.
The provider/team circle includes both individual and team-based factors falling into similar categories as the patient/family domain, such as cognitions, behavior, and social features; however, these factors include both personal (eg, the provider’s personal history, values, and beliefs) and professional (eg, past medical training, decision-making style, past experiences treating a disease) characteristics. Decisions may involve an interprofessional team representing a broad range of personalities and professional values. Decisions and decision-making processes may change over time as team composition changes, as level of provider expertise varies, or as environmental, patient, or disease/illness factors influence providers and teams.
Medical context includes factors related to the disease and the potential ways to evaluate or manage it. Examples of disease factors include acuity, symptoms, course, and prognosis. Most obviously, disease factors will influence the content of risk-benefit discussions but may also affect the SDM process through disease stigma or cultural assumptions about etiology. Disease evaluation factors include the psychometrics of a diagnostic screen, invasive and noninvasive testing, or a range of different preventive or therapeutic interventions. Treatment variables include the available options, costs, and risk of complications. Medical context variables evolve as evidence-based medicine and biomedical knowledge increase and new treatment options emerge.
Each of the 3 circles operates within the same environmental frame, such as an inpatient medicine ward, which itself operates within a hospital and the broader healthcare system. This frame exerts overt and subtle influences on providers, patients, and even the medical context. Features of the environmental frame include culture (eg, values, preferences, social norms), university versus community setting, incentives, formularies, quality improvement campaigns, regulations, and technology use.
The dynamic interactivity of the environmental frame and the 3 circles inform the process of SDM and highlight key differences that may occur between care settings. Certain features may predominate in different situations, but all will influence and be influenced by features of other circles during the course of SDM.
Application of the SDM 3 Circle Model
Although the SDM process is similar across clinical settings, its operationalization varies in important ways for hospital decision-making. In some situations, patients may defer all decisions to their providers or decisions may be considered with multiple providers concurrently. In the hospital, SDM may not be possible, such as in emergency surgery for an obtunded patient or when the patient and surrogate are not available or able to participate in the decision. Therefore, providers may bypass the steps of information sharing and discussion of the decision (big arrow in the Figure and supplemental
DISCUSSION
The SDM 3 Circle Model provides a concise, ecologically valid, contextually sensitive representation of SDM that synthesizes and extends beyond recent SDM models.3,7,40 Each circle represents the forces that influence SDM across settings. Although the multistep SDM pathway occurs similarly in outpatient and inpatient settings, how each step is operationalized and how each “circle” exerts its influence may differ and warrants further consideration throughout the SDM process. For example, hospitalized patients may have greater stress and anxiety, have more family involvement, be more motivated to adhere to treatment, and may be under greater financial and social pressures. Unlike outpatient primary care, patients are less likely to have an existing relationship with their inpatient providers, potentially compromising patient confidence in the provider, and necessitating expeditious trust building.
The SDM 3 Circle Model captures “setting” in both the broader environmental frame and within the provider/team category of variables. The frame also captures health system and broader community variables that may influence the practicality of some medical decisions. Within this essential frame, all 3 categories of patient, provider, and medical context are included as part of the SDM process. A better understanding of their interplay may be of great value for clinicians, researchers, administrators, and policy makers who wish to further study and promote SDM. Both the SDM 3 Circle Model and its accompanying pathway (Figures 1 and 2) highlight opportunities for intervention and research, and may drive quality improvement initiatives to improve clinical outcomes.
Limitations
We did not perform a new systematic review, potentially omitting lesser-known publications. We mitigated this risk by using recent systematic reviews, searching multiple databases, hand searching citation lists, and making inquiries to SDM experts. Our selection of models used as a foundation for the synthesized model was based on consensus, which included an element of subjective, clinical judgment. Our SDM expert sample was small and limited to authors of the papers we reviewed, potentially restricting the range of viewpoints received. Lastly, the SDM 3 Circle Model highlights key concept areas rather than all possible factors that influence SDM.
CONCLUSIONS
We present a peer-reviewed, literature-based SDM model capable of accounting for the unique circumstances and challenges of SDM in the hospital. The SDM 3 Circle Model identifies the primary categories of variables thought to influence SDM, places them in a shared environmental frame, and visually represents their interactive nature. A multistep representation of the SDM process further illustrates how the unique features and challenges of hospitalization might exert influence at various points as patients and providers reach a shared decision. As the interrelationships of patient and provider/team, medical context, and the environmental frame in which they occur are better understood, more effective and targeted interventions to promote SDM can be developed and evaluated.
Acknowledgments
The authors would like to thank Evans Whitaker for his assistance with the literature review and the Patient Engagement Project volunteers for their support and assistance with data collection.
Disclosure
Financial support for this study was provided entirely by a grant from NIH/NCCIH (grant #R25 AT006573, awarded to Dr. Jason Satterfield). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The following authors are employed by the sponsor: Stephanie Rennke, MD, Patrick Yuan, BA, Brad Monash, MD, Rebecca Blankenburg, MD, MPH, Ian Chua, MD, Stephanie Harman, MD, Debbie S. Sakai, MD, Joan F. Hilton, DSc, MPH., and Jason Satterfield, PhD.
1. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312(13):1295-1296. doi:10.1001/jama.2014.10186. PubMed
2. Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
3. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
4. Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651-661. PubMed
5. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. What is a medical decision? A units. Am J Respir Crit Care Med. 2011;183(7):915-921. doi:10.1164/rccm.201008-1214OC. PubMed
22. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
23. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
24. Légaré F, Bekker H, Desroches S, et al. How can continuing professional development better promote shared decision-making? Perspectives from an international collaboration. Implement Sci. 2011;6:68. doi:10.1186/1748-5908-6-68. PubMed
25. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301-312. doi:10.1016/j.pec.2005.06.010. PubMed
26. Moumjid N, Gafni A, Brémond A, Carrère MO. Shared decision making in the medical taxonomy based on physician statements in hospital encounters: a qualitative study. BMJ Open. 2016;6(2):e010098. doi:10.1136/bmjopen-2015-010098. PubMed
6. Fowler FJ, Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff (Millwood). 2011;30(4):699-706. doi:10.1377/hlthaff.2011.0003. PubMed
7. Weiner SJ, Kelly B, Ashley N, et al. Content coding for contextualization of care: evaluating physician performance at patient-centered decision making. Med Decis Making. 2014;34(1):97-106. doi:10.1177/0272989X13493146. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Matthias MS, Salyers MP, Frankel RM. Re-thinking shared decision-making: context matters. Patient Educ Couns. 2013;91(2):176-179. doi:10.1016/j.pec.2013.01.006 PubMed
10. Clayman ML, Bylund CL, Chewning B, Makoul G. The Impact of Patient Participation in Health Decisions Within Medical Encounters: A Systematic Review. Med Decis Making. 2016;36(4):427-452. doi:10.1177/0272989X15613530. PubMed
11. Shay LA, Lafata JE. Understanding patient perceptions of shared decision making. Patient Educ Couns. 2014;96(3):295-301. doi:10.1016/j.pec.2014.07.017. PubMed
12. Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18. doi:10.1016/j.pec.2011.02.004. PubMed
13. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
14. Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219-226. doi:10.1159/000126073. PubMed
15. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
16. Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269-277. doi:10.1093/intqhc/mzr002. PubMed
17. Mohammed K, Nolan MB, Rajjo T, et al. Creating a Patient-Centered Health Care Delivery System: A Systematic Review of Health Care Quality From the Patient Perspective. Am J Med Qual. 2014;31(1):12-21. doi:10.1177/1062860614545124. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. doi:10.1136/bmjqs-2012-001769. PubMed
19. Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526-535. doi:10.1016/j.pec.2008.07.018. PubMed
20. Frosch DL, May SG, Rendle KAS, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled “difficult” among key obstacles to shared decision making. Health Aff (Millwood). 2012;31(5):1030-1038. doi:10.1377/hlthaff.2011.0576. PubMed
21. Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care encounter: are we all talking about the same thing? Med Decis Making. 2007;27(5):539-546. doi:10.1177/0272989X07306779. PubMed
27. Hallström I, Elander G. Decision-making during hospitalization: parents’ and children’s involvement. J Clin Nurs. 2004;13(3):367-375. PubMed
28. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. Temporal characteristics of decisions in hospital encounters: a threshold for shared decision making? A qualitative study. Patient Educ Couns. 2014;97(2):216-222. doi:10.1016/j.pec.2014.08.005. PubMed
29. Baumeister RF, Leary MR. Writing narrative literature reviews. Rev Gen Psychol. 1997;1(3):311.
30. Moody DL. Theoretical and practical issues in evaluating the quality of conceptual models: current state and future directions. Data Knowl Eng. 2005;55(3):243-276. doi:10.1016/j.datak.2004.12.005.
31. McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351-377. PubMed
32. Basics of Qualitative Research | SAGE Publications Inc. https://us.sagepub.com/en-us/nam/basics-of-qualitative-research/book235578. Accessed on September 13, 2016. PubMed
33. 2013;2(4):421-433. doi:10.2217/cer.13.46.J Comp Eff Res33. Halley MC, Rendle KA, Frosch DL. A conceptual model of the multiple stages of communication necessary to support patient-centered care. PubMed
34. 2012;87(1):54-61. doi:10.1016/j.pec.2011.07.027.Patient Educ Couns34. Torke AM, Petronio S, Sachs GA, Helft PR, Purnell C. A conceptual model of the role of communication in surrogate decision making for hospitalized adults. PubMed
35. 2009;15(6):1142-1151. doi:10.1111/j.1365-2753.2009.01315.x.J Eval Clin Pract35. Falzer PR, Garman MD. A conditional model of evidence-based decision making: Model of evidence-based decision making. PubMed
36. 2012;8(4):161-164. doi:10.1097/PTS.0b013e318267c56e.J Patient Saf36. Holzmueller CG, Wu AW, Pronovost PJ. A framework for encouraging patient engagement in medical decision making. PubMed
37. 2014;97(2):158-164. doi:10.1016/j.pec.2014.07.027.Patient Educ Couns37. Elwyn G, Lloyd A, May C, et al. Collaborative deliberation: a model for patient care. PubMed
38. 2002;35(5-6):313-321. doi:10.1016/S1532-0464(03)00037-6.J Biomed Inform38. Ruland CM, Bakken S. Developing, implementing, and evaluating decision support systems for shared decision making in patient care: a conceptual model and case illustration. PubMed
39. 1999;319(7212):764.BMJ39. Shepperd S, Charnock D, Gann B. Helping patients access high quality health information. PubMed
40. 2011;25(1):18-25. doi:10.3109/13561820.2010.490502.J Interprof Care40. Légaré F, Stacey D, Pouliot S, et al. Interprofessionalism and shared decision-making in primary care: a stepwise approach towards a new model. PubMed
41. 2015;25(1):141-152. doi:10.1007/s10926-014-9532-7.J Occup Rehabil41. Coutu MF, Légaré F, Durand MJ, et al. Operationalizing a Shared Decision Making Model for Work Rehabilitation Programs: A Consensus Process. PubMed
42. 2013;13:231.BMC Health Serv Res42. Hölzel LP, Kriston L, Härter M. Patient preference for involvement, experienced involvement, decisional conflict, and satisfaction with physician: a structural equation model test. PubMed
43. 2008;134(4):835-843. doi:10.1378/chest.08-0235.Chest43. Curtis JR, White DB. Practical guidance for evidence-based ICU family conferences. PubMed
44. 2013;8:29-36. doi:10.4137/IMI.S12783.Integr Med Insights44. Brooks AT, Silverman L, Wallen G. Shared Decision Making: A Fundamental Tenet in a Conceptual Framework of Integrative Healthcare Delivery. PubMed
45. 2013;33(1):37-47. doi:10.1177/0272989X12458159.Med Decis Making45. Müller-Engelmann M, Donner-Banzhoff N, Keller H, et al. When decisions should be shared: a study of social norms in medical decision making using a factorial survey approach. PubMed
46. 2007;101(4):205-211.Z Arztl Fortbild Qualitatssich46. Mccaffery KJ, Shepherd HL, Trevena L, et al. Shared decision-making in Australia. PubMed
47. 2014;20(2):311-318. doi:10.1007/s12028-013-9922-2.Neurocrit Care
47. Rubin MA. The Collaborative Autonomy Model of Medical Decision-Making. 48. 2013;70(1 Suppl):141S-158S. doi:10.1177/1077558712461952.Med Care Res Rev PubMed
48. McCullough LB. The professional medical ethics model of decision making under conditions of clinical uncertainty. PubMed
49. 2009;87(2):368–390.Milbank Q49. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. PubMed
50. 2015;25(3):276-282. doi:10.1016/j.whi.2015.02.002.Womens Health Issues50. Moore JE, Titler MG, Kane Low L, Dalton VK, Sampselle CM. Transforming Patient-Centered Care: Development of the Evidence Informed Decision Making through Engagement Model. PubMed
51. 1997;44(5):681-692.Soc Sci Med51. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). PubMed
52. 2010;80(2):164-172. doi:10.1016/j.pec.2009.10.015.Patient Educ Couns52. Stacey D, Légaré F, Pouliot S, Kryworuchko J, Dunn S. Shared decision making models to inform an interprofessional perspective on decision making: a theory analysis. PubMed
53. 2013;70(1 Suppl):94S-112S. doi:10.1177/1077558712459216.Med Care Res Rev53. Epstein RM, Gramling RE. What is shared in shared decision making? Complex decisions when the evidence is unclear. PubMed
54. 2010;304(8):903-904. doi:10.1001/jama.2010.1208.JAMA54. Kon AA. The shared decision-making continuum. PubMed
55. 2008;30(3):429-444. doi:10.1111/j.1467-9566.2007.01064.x.Sociol Health Illn55. Rapley T. Distributed decision making: the anatomy of decisions-in-action. PubMed
56. 1997;12(6):339-345.J Gen Intern Med56. Braddock CH 3rd, Fihn SD, Levinson W, Jonsen AR, Pearlman RA. How doctors and patients discuss routine clinical decisions. Informed decision making in the outpatient setting. PubMed
57. 1999;282(24):2313-2320.JAMA57. Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. PubMed
58. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056.Soc Sci Med58. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups.
2006;9(4):321-332. doi:10.1111/j.1369-7625.2006.00404.x.Health Expect. PubMed
59. Towle A, Godolphin W, Grams G, Lamarre A. Putting informed and shared decision making into practice. PubMed
60. 2011;17(4):554-564. doi: 10.1111/j.1365-2753.2010.01515.x.J Eval Clin Pract60. Légaré F, Stacey D, Gagnon S, et al. Validating a conceptual model for an interprofessional approach to shared decision making: a mixed methods study. PubMed
1. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312(13):1295-1296. doi:10.1001/jama.2014.10186. PubMed
2. Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
3. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
4. Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651-661. PubMed
5. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. What is a medical decision? A units. Am J Respir Crit Care Med. 2011;183(7):915-921. doi:10.1164/rccm.201008-1214OC. PubMed
22. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
23. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
24. Légaré F, Bekker H, Desroches S, et al. How can continuing professional development better promote shared decision-making? Perspectives from an international collaboration. Implement Sci. 2011;6:68. doi:10.1186/1748-5908-6-68. PubMed
25. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301-312. doi:10.1016/j.pec.2005.06.010. PubMed
26. Moumjid N, Gafni A, Brémond A, Carrère MO. Shared decision making in the medical taxonomy based on physician statements in hospital encounters: a qualitative study. BMJ Open. 2016;6(2):e010098. doi:10.1136/bmjopen-2015-010098. PubMed
6. Fowler FJ, Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff (Millwood). 2011;30(4):699-706. doi:10.1377/hlthaff.2011.0003. PubMed
7. Weiner SJ, Kelly B, Ashley N, et al. Content coding for contextualization of care: evaluating physician performance at patient-centered decision making. Med Decis Making. 2014;34(1):97-106. doi:10.1177/0272989X13493146. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Matthias MS, Salyers MP, Frankel RM. Re-thinking shared decision-making: context matters. Patient Educ Couns. 2013;91(2):176-179. doi:10.1016/j.pec.2013.01.006 PubMed
10. Clayman ML, Bylund CL, Chewning B, Makoul G. The Impact of Patient Participation in Health Decisions Within Medical Encounters: A Systematic Review. Med Decis Making. 2016;36(4):427-452. doi:10.1177/0272989X15613530. PubMed
11. Shay LA, Lafata JE. Understanding patient perceptions of shared decision making. Patient Educ Couns. 2014;96(3):295-301. doi:10.1016/j.pec.2014.07.017. PubMed
12. Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18. doi:10.1016/j.pec.2011.02.004. PubMed
13. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
14. Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219-226. doi:10.1159/000126073. PubMed
15. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
16. Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269-277. doi:10.1093/intqhc/mzr002. PubMed
17. Mohammed K, Nolan MB, Rajjo T, et al. Creating a Patient-Centered Health Care Delivery System: A Systematic Review of Health Care Quality From the Patient Perspective. Am J Med Qual. 2014;31(1):12-21. doi:10.1177/1062860614545124. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. doi:10.1136/bmjqs-2012-001769. PubMed
19. Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526-535. doi:10.1016/j.pec.2008.07.018. PubMed
20. Frosch DL, May SG, Rendle KAS, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled “difficult” among key obstacles to shared decision making. Health Aff (Millwood). 2012;31(5):1030-1038. doi:10.1377/hlthaff.2011.0576. PubMed
21. Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care encounter: are we all talking about the same thing? Med Decis Making. 2007;27(5):539-546. doi:10.1177/0272989X07306779. PubMed
27. Hallström I, Elander G. Decision-making during hospitalization: parents’ and children’s involvement. J Clin Nurs. 2004;13(3):367-375. PubMed
28. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. Temporal characteristics of decisions in hospital encounters: a threshold for shared decision making? A qualitative study. Patient Educ Couns. 2014;97(2):216-222. doi:10.1016/j.pec.2014.08.005. PubMed
29. Baumeister RF, Leary MR. Writing narrative literature reviews. Rev Gen Psychol. 1997;1(3):311.
30. Moody DL. Theoretical and practical issues in evaluating the quality of conceptual models: current state and future directions. Data Knowl Eng. 2005;55(3):243-276. doi:10.1016/j.datak.2004.12.005.
31. McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351-377. PubMed
32. Basics of Qualitative Research | SAGE Publications Inc. https://us.sagepub.com/en-us/nam/basics-of-qualitative-research/book235578. Accessed on September 13, 2016. PubMed
33. 2013;2(4):421-433. doi:10.2217/cer.13.46.J Comp Eff Res33. Halley MC, Rendle KA, Frosch DL. A conceptual model of the multiple stages of communication necessary to support patient-centered care. PubMed
34. 2012;87(1):54-61. doi:10.1016/j.pec.2011.07.027.Patient Educ Couns34. Torke AM, Petronio S, Sachs GA, Helft PR, Purnell C. A conceptual model of the role of communication in surrogate decision making for hospitalized adults. PubMed
35. 2009;15(6):1142-1151. doi:10.1111/j.1365-2753.2009.01315.x.J Eval Clin Pract35. Falzer PR, Garman MD. A conditional model of evidence-based decision making: Model of evidence-based decision making. PubMed
36. 2012;8(4):161-164. doi:10.1097/PTS.0b013e318267c56e.J Patient Saf36. Holzmueller CG, Wu AW, Pronovost PJ. A framework for encouraging patient engagement in medical decision making. PubMed
37. 2014;97(2):158-164. doi:10.1016/j.pec.2014.07.027.Patient Educ Couns37. Elwyn G, Lloyd A, May C, et al. Collaborative deliberation: a model for patient care. PubMed
38. 2002;35(5-6):313-321. doi:10.1016/S1532-0464(03)00037-6.J Biomed Inform38. Ruland CM, Bakken S. Developing, implementing, and evaluating decision support systems for shared decision making in patient care: a conceptual model and case illustration. PubMed
39. 1999;319(7212):764.BMJ39. Shepperd S, Charnock D, Gann B. Helping patients access high quality health information. PubMed
40. 2011;25(1):18-25. doi:10.3109/13561820.2010.490502.J Interprof Care40. Légaré F, Stacey D, Pouliot S, et al. Interprofessionalism and shared decision-making in primary care: a stepwise approach towards a new model. PubMed
41. 2015;25(1):141-152. doi:10.1007/s10926-014-9532-7.J Occup Rehabil41. Coutu MF, Légaré F, Durand MJ, et al. Operationalizing a Shared Decision Making Model for Work Rehabilitation Programs: A Consensus Process. PubMed
42. 2013;13:231.BMC Health Serv Res42. Hölzel LP, Kriston L, Härter M. Patient preference for involvement, experienced involvement, decisional conflict, and satisfaction with physician: a structural equation model test. PubMed
43. 2008;134(4):835-843. doi:10.1378/chest.08-0235.Chest43. Curtis JR, White DB. Practical guidance for evidence-based ICU family conferences. PubMed
44. 2013;8:29-36. doi:10.4137/IMI.S12783.Integr Med Insights44. Brooks AT, Silverman L, Wallen G. Shared Decision Making: A Fundamental Tenet in a Conceptual Framework of Integrative Healthcare Delivery. PubMed
45. 2013;33(1):37-47. doi:10.1177/0272989X12458159.Med Decis Making45. Müller-Engelmann M, Donner-Banzhoff N, Keller H, et al. When decisions should be shared: a study of social norms in medical decision making using a factorial survey approach. PubMed
46. 2007;101(4):205-211.Z Arztl Fortbild Qualitatssich46. Mccaffery KJ, Shepherd HL, Trevena L, et al. Shared decision-making in Australia. PubMed
47. 2014;20(2):311-318. doi:10.1007/s12028-013-9922-2.Neurocrit Care
47. Rubin MA. The Collaborative Autonomy Model of Medical Decision-Making. 48. 2013;70(1 Suppl):141S-158S. doi:10.1177/1077558712461952.Med Care Res Rev PubMed
48. McCullough LB. The professional medical ethics model of decision making under conditions of clinical uncertainty. PubMed
49. 2009;87(2):368–390.Milbank Q49. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. PubMed
50. 2015;25(3):276-282. doi:10.1016/j.whi.2015.02.002.Womens Health Issues50. Moore JE, Titler MG, Kane Low L, Dalton VK, Sampselle CM. Transforming Patient-Centered Care: Development of the Evidence Informed Decision Making through Engagement Model. PubMed
51. 1997;44(5):681-692.Soc Sci Med51. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). PubMed
52. 2010;80(2):164-172. doi:10.1016/j.pec.2009.10.015.Patient Educ Couns52. Stacey D, Légaré F, Pouliot S, Kryworuchko J, Dunn S. Shared decision making models to inform an interprofessional perspective on decision making: a theory analysis. PubMed
53. 2013;70(1 Suppl):94S-112S. doi:10.1177/1077558712459216.Med Care Res Rev53. Epstein RM, Gramling RE. What is shared in shared decision making? Complex decisions when the evidence is unclear. PubMed
54. 2010;304(8):903-904. doi:10.1001/jama.2010.1208.JAMA54. Kon AA. The shared decision-making continuum. PubMed
55. 2008;30(3):429-444. doi:10.1111/j.1467-9566.2007.01064.x.Sociol Health Illn55. Rapley T. Distributed decision making: the anatomy of decisions-in-action. PubMed
56. 1997;12(6):339-345.J Gen Intern Med56. Braddock CH 3rd, Fihn SD, Levinson W, Jonsen AR, Pearlman RA. How doctors and patients discuss routine clinical decisions. Informed decision making in the outpatient setting. PubMed
57. 1999;282(24):2313-2320.JAMA57. Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. PubMed
58. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056.Soc Sci Med58. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups.
2006;9(4):321-332. doi:10.1111/j.1369-7625.2006.00404.x.Health Expect. PubMed
59. Towle A, Godolphin W, Grams G, Lamarre A. Putting informed and shared decision making into practice. PubMed
60. 2011;17(4):554-564. doi: 10.1111/j.1365-2753.2010.01515.x.J Eval Clin Pract60. Légaré F, Stacey D, Gagnon S, et al. Validating a conceptual model for an interprofessional approach to shared decision making: a mixed methods study. PubMed
© 2017 Society of Hospital Medicine