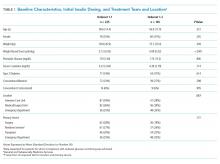
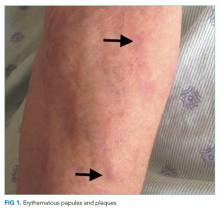
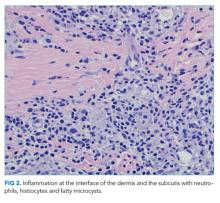
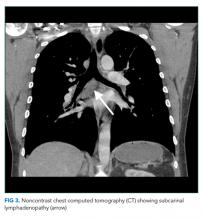
User login
The Importance of Emotional Intelligence When Leading in a Time of Crisis
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
The coronavirus disease of 2019 (COVID-19) pandemic has created innumerable challenges on scales both global and personal while straining health systems and their personnel. Hospitalists and hospital medicine groups are experiencing unique burdens as they confront the pandemic on the frontlines. Hospital medicine groups are being challenged by the rapid operational changes necessary in preparing for and caring for patients with COVID-19. These challenges include drafting new diagnostic and management algorithms, establishing and enacting policies on personal protective equipment (PPE) and patient and provider testing, modifying staffing protocols including deploying staff to new roles or integrating non-hospitalists into hospital medicine roles, and developing capacity for patient surges1—all in the setting of uncertainty about how the pandemic may affect individual hospitals or health systems and how long these repercussions may last. In this perspective, we describe key lessons we have learned in leading our hospital medicine group during the COVID-19 pandemic: how to apply emotional intelligence to proactively address the emotional effects of the crisis.
LEARNING FROM EARLY MISSTEPS
In the early days of the COVID-19 pandemic, the evolving knowledge of the disease process, changing national and local public health guidelines, and instability of the PPE supply chain necessitated rapid change. This pace no longer allowed for our typical time frame of weeks to months for implementation of large-scale operational changes; instead, it demanded adaptation in hours to days. We operated under a strategy of developing new workflows and policies that were logical and reflected the best available information at the time.
For instance, our hospital medicine service cared for some of the earliest-identified COVID-19 patients in the United States in early February 2020. Our initial operational plan for caring for patients with COVID-19 involved grouping these patients on a limited number of direct-care hospitalist teams. The advantages of this approach, which benefitted from low numbers of initial patients, were clear: consolidation of clinical and operational knowledge (including optimal PPE practices) in a few individuals, streamlining communication with infectious diseases specialists and public health departments, and requiring change on only a couple of teams while allowing others to continue their usual workflow. However, we soon learned that providers caring for COVID-19 patients were experiencing an onslaught of negative emotions: fear of contracting the virus themselves or carrying it home to infect loved ones, anxiety of not understanding the clinical disease or having treatments to offer, resentment of having been randomly assigned to the team that would care for these patients, and loneliness of being a sole provider experiencing these emotions. We found ourselves in the position of managing these emotional responses reactively.
APPLYING EMOTIONAL INTELLIGENCE TO LEADING IN A CRISIS
To reduce the distress experienced by our hospitalists and to lead more effectively, we realized the need to proactively address the emotional effects that the pandemic was having. Several authors who have written about valuable leadership lessons during this time have noted the importance of acknowledging the emotional tolls of such a crisis and creating venues for hospitalists to share their experiences.1-4 However, solely adding “wellness” as a checklist item for leaders to address fails to capture the nuances of the complex human emotions that hospitalists may endure at this time and how these emotions influence frontline hospitalists’ responses to operational changes. It is critically important for hospital medicine leaders to employ emotional intelligence, defined as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and to use this information to guide one’s thinking and actions.”5-7 Integrating emotional intelligence allows hospital medicine leaders to anticipate, identify, articulate, and manage the emotional responses to necessary changes and stresses that occur during a crisis such as the COVID-19 pandemic.
As we applied principles of emotional intelligence to our leadership response to the COVID crisis, we found the following seven techniques effective (Appendix Table):
1. ASK. Leaders should ask individual hospitalists “How are you feeling?” instead of “How are you doing?” or “How can I help?” This question may feel too intimate for some, or leaders may worry that the question feels patronizing; however, in our experience, hospitalists respond positively to this prompt, welcome the opportunity to communicate their feelings, and value being heard. Moreover, when hospitalists feel overwhelmed, they may not be able to determine what help they do or do not need. By understanding the emotions of frontline hospitalists, leaders may be better able to address those emotions directly, find solutions to problems, and anticipate reactions to future policies.4
2. SHARE. Leaders should model what they ask of frontline hospitalists and share their own feelings, even if they are experiencing mixed or negative emotions. For instance, a leader who is feeling saddened about the death of a patient can begin a meeting by sharing this sentiment. By allowing themselves to display vulnerability, leaders demonstrate courage and promote a culture of openness, honesty, and mutual trust.
3. INITIATE. Leaders should embrace difficult conversations and be transparent about uncertainty, although they may not have the answers and may need to take local responsibility for consequences of decisions made externally, such as those made by the health system or government. Confronting difficult discussions and being transparent about “unknowns” provides acknowledgement, reassurance, and shared experience that expresses to the hospitalist group that, while the future may be unsettled, they will face it together.
4. ANTICIPATE. Leaders should anticipate the emotional responses to operational changes while designing them and rolling them out. While negative emotions may heavily outweigh positive emotions in times of crisis, we have also found that harnessing positive emotions when designing operational initiatives can assist with successful implementation. For example, by surveying our hospitalists, we found that many felt enthusiastic about caring for patients with COVID-19, curious about new skill sets, and passionate about helping in a time of crisis. By generating a list of these hospitalists up front, we were able to preferentially staff COVID-19 teams with providers who were eager to care for those patients and, thereby, minimize anxiety among those who were more apprehensive.
5. ENCOURAGE. Leaders should provide time and space (including virtually) for hospitalists to discuss their emotions.8 We found that creating multiple layers of opportunity for expression allows for engagement with a wider range of hospitalists, some of whom may be reluctant to share feelings openly or to a group, whereas others may enjoy the opportunity to reveal their feelings publicly. These varied venues for emotional expression may range from brief individual check-ins to open “office hours” to dedicated meetings such as “Hospitalist Town Halls.” For instance, spending the first few minutes of a meeting with a smaller group by encouraging each participant to share something personal can build community and mutual understanding, as well as cue leaders in to where participants may be on the emotional landscape.
6. NURTURE. Beyond inviting the expression of emotions, leaders should ensure that hospitalists have access to more formal systems of support, especially for hospitalists who may be experiencing more intense negative emotions. Support may be provided through unit- or team-based debriefing sessions, health-system sponsored support programs, or individual counseling sessions.4,8
7. APPRECIATE. Leaders should deliberately foster gratitude by sincerely and frequently expressing their appreciation. Because expressing gratitude builds resiliency,9 cultivating a culture of gratitude may bolster resilience in the entire hospital medicine group. Opportunities for thankfulness abound as hospitalists volunteer for extra shifts, cover for ill colleagues, participate in new working groups and task forces, and sacrifice their personal safety on the front lines. We often incorporate statements of appreciation into one-on-one conversations with hospitalists, during operational and divisional meetings, and in email. We also built gratitude expressions into the daily work on the Respiratory Isolation Unit at our hospital via daily interdisciplinary huddles for frontline providers to share their experiences and emotions. During huddles, providers are asked to pair negative emotions with suggestions for improvement and to share a moment of gratitude. This helps to engender a spirit of camaraderie, shared mission, and collective optimism.
CONCLUSION
Hospitalists are experiencing a wide range of emotions related to the COVID-19 pandemic. Hospital medicine leaders must have strategies to understand the emotions providers are experiencing. Being aware of and acknowledging these emotions up front can help leaders plan and implement the operational changes necessary to manage the crisis. Because our health system and city have fortunately been spared the worst of the pandemic so far without large volumes of patients with COVID-19, we recognize that the strategies above may be challenging for leaders in overwhelmed health systems. However, we hope that leaders at all levels can apply the lessons we have learned: to ask hospitalists how they are feeling, share their own feelings, initiate difficult conversations when needed, anticipate the emotional effects of operational changes, encourage expressions of emotion in multiple venues, nurture hospitalists who need more formal support, and appreciate frontline hospitalists. While the emotional needs of hospitalists will undoubtedly change over time as the pandemic evolves, we suspect that these strategies will continue to be important over the coming weeks, months, and longer as we settle into the postpandemic world.
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
1. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
2. Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med. 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
3. Hertling M. Ten tips for a crisis : lessons from a soldier. J Hosp Med. 2020;15(5):275-276. https://doi.org/10.12788/jhm.3424
4. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. Published online April 7, 2020. https://doi.org/10.1001/jama.2020.5893
5. Mintz LJ, Stoller JK. A systematic review of physician leadership and emotional intelligence. J Grad Med Educ. 2014;6(1):21-31. https://doi.org/10.4300/jgme-d-13-00012.1
6. Goleman D, Boyatzis R. Emotional intelligence has 12 elements. Which do you need to work on? Harvard Business Review. February 6, 2017. Accessed April 16, 2020. https://hbr.org/2017/02/emotional-intelligence-has-12-elements-which-do-you-need-to-work-on
7. Salovey P, Mayer JD. Emotional intelligence. Imagin Cogn Pers. 1990;9(3):185-211. https://doi.org/10.2190/DUGG-P24E-52WK-6CDG
8. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. https://doi.org/10.1136/bmj.m1642
9. Kopans D. How to evaluate, manage, and strengthen your resilience. Harvard Business Review. June 14, 2016. Accessed April 21, 2020. https://hbr.org/2016/06/how-to-evaluate-manage-and-strengthen-your-resilience
© 2020 Society of Hospital Medicine
Understanding the Singapore COVID-19 Experience: Implications for Hospital Medicine
One of the worst public health threats of our generation, coronavirus disease 2019 (COVID-19), first emerged in Wuhan, China, in December 2019 and quickly spread to Singapore, Hong Kong, and Taiwan. These three countries have been praised for their control of the pandemic,1,2 while the number of cases worldwide, including those in the United States, has soared. Political alignment, centralized and integrated healthcare systems, small size, effective technology deployment, widespread testing combined with contact tracing and isolation, and personal protective equipment (PPE) availability underscore their successes.1,3-5 Although these factors differ starkly from those currently employed in the United States, a better understanding their experience may positively influence the myriad US responses. We describe some salient features of Singapore’s infection preparedness, provide examples of how these features guided the National University Hospital (NUH) Singapore COVID-19 response, and illustrate how one facet of the NUH response was translated to develop a new care model at the University of California, San Francisco (UCSF).
THE SINGAPORE EXPERIENCE OVER TIME
Singapore, a small island country (278 square miles) city-state in Southeast Asia has a population of 5.8 million people. Most Singaporeans receive their inpatient care in the public hospitals that are organized and resourced through the Singapore Ministry of Health (MOH). In 2003, severe acute respiratory syndrome (SARS) infected 238 people and killed 33 over 3 months in Singapore, which led to a significant economic downturn. Singapore’s initial SARS experience unveiled limitations in infrastructure, staff preparedness, virus control methodology, and centralized crisis systems. Lessons gleaned from the SARS experience laid the foundation for Singapore’s subsequent disaster preparedness.6
Post-SARS, the MOH created structures and systems to prepare Singapore for future epidemics. All public hospitals expanded isolation capacity by constructing new units or repurposing existing ones and creating colocated Emergency Department (ED) isolation facilities. Additionally, the MOH commissioned the National Centre for Infectious Diseases, a 330-bed high-level isolation hospital.7 They also mandated hospital systems to regularly practice mass casualty and infectious (including respiratory) crisis responses through externally evaluated simulation.8 These are orchestrated down to the smallest detail and involve staff at all levels. For example, healthcare workers (HCW) being “deployed” outside of their specialty, housekeepers practicing novel hazardous waste disposal, and security guards managing crowds interact throughout the exercise.
The testing and viral spread control challenges during SARS spawned hospital-system epidemiology capacity building. Infectious diseases reporting guidelines were refined, and communication channels enhanced to include cross-hospital information sharing and direct lines of communication for epidemiology groups to and from the MOH. Enhanced contact tracing methodologies were adopted and practiced regularly. In addition, material stockpiles, supplies, and supply chains were recalibrated.
The Singapore government also adopted the Disease Outbreak Response System Condition (DORSCON) system,9 a color-coded framework for pandemic response that guides activation of crisis interventions broadly (such as temperature screening at airports and restrictions to travel and internal movements), as well as within the healthcare setting.
In addition to prompting these notable preparedness efforts, SARS had a palpable impact on Singaporeans’ collective psychology both within and outside of the hospital system. The very close-knit medical community lost colleagues during the crisis, and the larger community deeply felt the health and economic costs of this crisis.10 The resulting “respect” or “healthy fear” for infectious crises continues to the present day.
THE SINGAPORE COVID-19 RESPONSE: NATIONAL UNIVERSITY HOSPITAL EXPERIENCE
The NUH is a 1,200-bed public tertiary care academic health center in Singapore. Before the first COVID-19 case was diagnosed in Singapore, NUH joined forces with its broader health system, university resources (schools of medicine and public health), and international partners to refine the existing structures and systems in response to this new infectious threat.
One of these structures included the existing NUH ED negative-pressure “fever facility.” In the ED triage, patients are routinely screened for infectious diseases such as H1N1, MERS-CoV, and measles. In early January, these screening criteria were evolved to adapt to COVID-19. High-risk patients bypass common waiting areas and are sent directly to the fever facility for management. From there, patients requiring admission are sent to one of the inpatient isolation wards, each with over 21 negative-pressure isolation rooms. To expand isolation capacity, lower-priority patients were relocated, and the existing negative- and neutral-pressure rooms were converted into COVID-19 pandemic wards.
The pandemic wards are staffed by nurses with previous isolation experience and Internal Medicine and Subspecialty Medicine physicians and trainees working closely with Infectious Diseases experts. Pandemic Ward teams are sequestered from other clinical and administrative teams, wear hospital-laundered scrubs, and use PPE-conserving practices. These strategies, implemented at the outset, are based on international guidelines contextualized to local needs and include extended use (up to 6 hours) of N95 respirators for the pandemic wards, and surgical masks in all other clinical areas. Notably, there have been no documented transmissions to HCW or patients at NUH. The workforce was maximized by limiting nonurgent clinical, administrative, research, and teaching activities.
In February, COVID-19 testing was initiated internally and deployed widely. NUH, at the time of this writing, has performed more than 6,000 swabs with up to 200 tests run per day (with 80 confirmed cases). Testing at this scale has allowed NUH to ensure: (a) prompt isolation of patients, even those with mild symptoms, (b) deisolation of those testing negative thus conserving PPE and isolation facilities, (c) a better understanding of the epidemiology and the wide range of clinical manifestations of COVID-19, and (d) early comprehensive contact tracing including mildly symptomatic patients.
The MOH plays a central role in coordinating COVID-19 activities and supports individual hospital systems such as NUH. Some of their crisis leadership strategies include daily text messages distributed countrywide, two-way communication channels that ensure feedback loops with hospital executives, epidemiology specialists, and operational workgroups, and engendering interhospital collaboration.11
A US HOSPITAL MEDICINE RESPONSE: UC SAN FRANCISCO
In the United States, the Joint Commission provides structures, tools, and processes for hospital systems to prepare for disasters.12 Many hospital systems have experience with natural disasters which, similar to Singapore’s planning, ensures structures and systems are in place during a crisis. Although these are transferable to multiple types of disasters, the US healthcare system’s direct experience with infectious crises is limited. A fairly distinctive facet—and an asset of US healthcare—is the role of hospitalists.
Hospitalists care for the majority of medical inpatients across the United States,13 and as such, they currently, and will increasingly, play a major role in the US COVID-19 response. This is the case at the UCSF Helen Diller Medical Center at Parnassus Heights (UCSFMC), a 600-bed academic medical center. To learn from other’s early experiences with COVID-19, UCSF Health System leadership connected with many outside health systems including NUH. As one of its multiple pandemic responses, they engaged the UCSFMC Division of Hospital Medicine (DHM), a division that includes 117 hospitalists, to work with hospital and health system leadership and launch a respiratory isolation unit (RIU) modeled after the NUH pandemic ward. The aim of the RIU is to group inpatients with either confirmed or suspected COVID-19 patients who do not require critical care.
An interdisciplinary work group comprising hospitalists, infectious disease specialists, emergency department clinicians, nursing, rehabilitation experts, hospital epidemiology and infection-prevention leaders, safety specialists, and systems engineers was assembled to repurpose an existing medical unit and establish new care models for the RIU. This workgroup created clinical guidelines and workflows, and RIU leaders actively solicit feedback from the staff to advance these standards.
Hospitalists and nurses who volunteered to work on the UCSF attending-staffed RIU received extensive training, including online and widely available in-person PPE training delivered by infection-prevention experts. The RIU hospitalists engage with hospitalists nationwide through ongoing conference calls to share best practices and clinical cases. Patients are admitted by hospitalists to the RIU via the emergency department or directly from ambulatory sites. All RIU providers and staff are screened daily for symptoms prior to starting their shifts, wear hospital-laundered scrubs on the unit, and remain on the unit for the duration of their shift. Hospitalists and nurses communicate regularly to cluster their patient visits and interventions while specialists provide virtual consults (as deemed safe and appropriate) to optimize PPE conservation and decrease overall exposure. The Health System establishes and revises PPE protocols based on CDC guidelines, best available evidence, and supply chain realities. These guidelines are evolving and currently include surgical mask, gown, gloves, and eye protection for all patient interactions with suspected or confirmed COVID-19 and respirator use during aerosol-generating procedures. Research studies (eg, clinical trials and evaluations), informatics efforts (eg, patient flow dashboards), and healthcare technology innovations (eg, tablets for telehealth and video visits) are continually integrated into the RIU infrastructure. Robust attention to the well-being of everyone working on the unit includes chaplain visits, daily debriefs, meal delivery, and palliative care service support, which enrich the unit experience and instill a culture of unity.
MOVING FORWARD
The structures and systems born out of the 2003 SARS experience and the “test, trace, and isolate” strategy were arguably key drivers to flatten Singapore’s epidemic curve early in the pandemic.3 Even with these in place, Singapore is now experiencing a second wave with a significantly higher caseload.14 In response, the government instituted strict social distancing measures on April 3, closing schools and most workplaces. This suggests that the COVID-19 pandemic may fluctuate over time and that varying types and levels of interventions will be required to maintain long-term control. The NUH team describes experiencing cognitive overload given the ever-changing nature and volume of information and fatigue due to the effort required and duration of this crisis. New programs addressing these challenges are being developed and rapidly deployed.
Despite early testing limitations and newly minted systems, San Francisco is cautiously optimistic about its epidemic curve. Since the March 17, 2020, “shelter in place” order, COVID-19 hospitalizations have remained manageable and constant.15 This has afforded healthcare systems including UCSF critical time to evolve its clinical operations (eg, the RIU) and to leverage its academic culture coordinating its bench research, global health, epidemiology, clinical research, informatics, and clinical enterprise scholars and experts to advance COVID-19 science and inform pandemic solutions. Although the UCSF frontline teams are challenged by the stresses of being in the throes of the pandemic amidst a rapidly changing landscape (including changes in PPE and testing recommendations specifically), they are working together to build team resilience for what may come.
CONCLUSION
The world is facing a pandemic of tremendous proportions, and the United States is in the midst of a wave the height of which is yet to be seen. As Fisher and colleagues wrote in 2011, “Our response to infectious disease outbreaks is born out of past experience.”4 Singapore and NUH’s structures and systems that were put into place demonstrate this—they are timely, have been effective thus far, and will be tested in this next wave. “However, no two outbreaks are the same,” the authors wrote, “so an understanding of the infectious agent as well as the environment confronting it is fundamental to the response.”4 In the United States, hospitalists are a key asset in our environment to confront this virus. The UCSF experience exemplifies that, by combining new ideas from another system with on-the-ground expertise while working hand-in-hand with the hospital and health system, hospitalists can be a critical facet of the pandemic response. Hospitalists’ intrinsic abilities to collaborate, learn, and innovate will enable them to not only meet this challenge now but also to transform practices and capacities to respond to crises into the future.
Acknowledgment
Bradley Sharpe, MD, Division Chief, Division of Hospital Medicine, University of California, San Francisco, California, for his input on conception and critical review of this manuscript.
1. Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020. https://doi.org/10.1001/jama.2020.3151.
2. Legido-Quigley H, Asgari N, Teo YY, et al. Are high-performing health systems resilient against the COVID-19 epidemic? Lancet. 2020;395(10227):848-850. https://doi.org/10.1016/S0140-6736(20)30551-1.
3. Wong JEL, Leo YS, Tan CC. COVID-19 in Singapore—current experience: critical global issues that require attention and action. JAMA. 2020;323(13):1243-1244. https://doi.org/10.1001/jama.2020.2467.
4. Fisher D, Hui DS, Gao Z, et al. Pandemic response lessons from influenza H1N1 2009 in Asia. Respirology. 2011;16(6):876-882. https://doi.org/ 10.1111/j.1440-1843.2011.02003.x.
5. Wong ATY, Chen H, Liu SH, et al. From SARS to avian influenza preparedness in Hong Kong. Clin Infect Dis. 2017;64(suppl_2):S98-S104. https://doi.org/ 10.1093/cid/cix123.
6. Tan CC. SARS in Singapore--key lessons from an epidemic. Ann Acad Med Singapore. 2006;35(5):345-349.
7. National Centre for Infectious Diseases. About NCID. https://www.ncid.sg/About-NCID/Pages/default.aspx. Accessed April 5, 2020.
8. Cutter J. Preparing for an influenza pandemic in Singapore. Ann Acad Med Singapore. 2008;37(6):497-503.
9. Singapore Ministry of Health. What do the different DORSCON levels mean. http://www.gov.sg/article/what-do-the-different-dorscon-levels-mean. Accessed April 5, 2020.
10. Lee J-W, McKibbin WJ. Estimating the global economic costs of SARS. In: Knobler S, Mahmoud A, Lemon S, et al, eds. Institute of Medicine (US) Forum on Microbial Threats. Washington, DC: National Academies Press (US); 2004.
11. James EH, Wooten L. Leadership as (un)usual: how to display competence in times of crisis. Organ Dyn. 2005;34(2):141-152. https://doi.org/10.1016/j.orgdyn.2005.03.005
12. The Joint Commission. Emergency Management: Coronavirus Resources. 2020. https://www.jointcommission.org/covid-19/. Accessed April 4, 2020.
13. Wachter RM, Goldman L. Zero to 50,000 – the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
14. Singapore Ministry of Health. Official Update of COVID-19 Situation in Singapore. 2020. https://experience.arcgis.com/experience/7e30edc490a5441a874f9efe67bd8b89. Accessed April 5, 2020.
15. Chronicle Digital Team. Coronavirus tracker. San Francisco Chronicle. https://projects.sfchronicle.com/2020/coronavirus-map/. Accessed April 5, 2020.
One of the worst public health threats of our generation, coronavirus disease 2019 (COVID-19), first emerged in Wuhan, China, in December 2019 and quickly spread to Singapore, Hong Kong, and Taiwan. These three countries have been praised for their control of the pandemic,1,2 while the number of cases worldwide, including those in the United States, has soared. Political alignment, centralized and integrated healthcare systems, small size, effective technology deployment, widespread testing combined with contact tracing and isolation, and personal protective equipment (PPE) availability underscore their successes.1,3-5 Although these factors differ starkly from those currently employed in the United States, a better understanding their experience may positively influence the myriad US responses. We describe some salient features of Singapore’s infection preparedness, provide examples of how these features guided the National University Hospital (NUH) Singapore COVID-19 response, and illustrate how one facet of the NUH response was translated to develop a new care model at the University of California, San Francisco (UCSF).
THE SINGAPORE EXPERIENCE OVER TIME
Singapore, a small island country (278 square miles) city-state in Southeast Asia has a population of 5.8 million people. Most Singaporeans receive their inpatient care in the public hospitals that are organized and resourced through the Singapore Ministry of Health (MOH). In 2003, severe acute respiratory syndrome (SARS) infected 238 people and killed 33 over 3 months in Singapore, which led to a significant economic downturn. Singapore’s initial SARS experience unveiled limitations in infrastructure, staff preparedness, virus control methodology, and centralized crisis systems. Lessons gleaned from the SARS experience laid the foundation for Singapore’s subsequent disaster preparedness.6
Post-SARS, the MOH created structures and systems to prepare Singapore for future epidemics. All public hospitals expanded isolation capacity by constructing new units or repurposing existing ones and creating colocated Emergency Department (ED) isolation facilities. Additionally, the MOH commissioned the National Centre for Infectious Diseases, a 330-bed high-level isolation hospital.7 They also mandated hospital systems to regularly practice mass casualty and infectious (including respiratory) crisis responses through externally evaluated simulation.8 These are orchestrated down to the smallest detail and involve staff at all levels. For example, healthcare workers (HCW) being “deployed” outside of their specialty, housekeepers practicing novel hazardous waste disposal, and security guards managing crowds interact throughout the exercise.
The testing and viral spread control challenges during SARS spawned hospital-system epidemiology capacity building. Infectious diseases reporting guidelines were refined, and communication channels enhanced to include cross-hospital information sharing and direct lines of communication for epidemiology groups to and from the MOH. Enhanced contact tracing methodologies were adopted and practiced regularly. In addition, material stockpiles, supplies, and supply chains were recalibrated.
The Singapore government also adopted the Disease Outbreak Response System Condition (DORSCON) system,9 a color-coded framework for pandemic response that guides activation of crisis interventions broadly (such as temperature screening at airports and restrictions to travel and internal movements), as well as within the healthcare setting.
In addition to prompting these notable preparedness efforts, SARS had a palpable impact on Singaporeans’ collective psychology both within and outside of the hospital system. The very close-knit medical community lost colleagues during the crisis, and the larger community deeply felt the health and economic costs of this crisis.10 The resulting “respect” or “healthy fear” for infectious crises continues to the present day.
THE SINGAPORE COVID-19 RESPONSE: NATIONAL UNIVERSITY HOSPITAL EXPERIENCE
The NUH is a 1,200-bed public tertiary care academic health center in Singapore. Before the first COVID-19 case was diagnosed in Singapore, NUH joined forces with its broader health system, university resources (schools of medicine and public health), and international partners to refine the existing structures and systems in response to this new infectious threat.
One of these structures included the existing NUH ED negative-pressure “fever facility.” In the ED triage, patients are routinely screened for infectious diseases such as H1N1, MERS-CoV, and measles. In early January, these screening criteria were evolved to adapt to COVID-19. High-risk patients bypass common waiting areas and are sent directly to the fever facility for management. From there, patients requiring admission are sent to one of the inpatient isolation wards, each with over 21 negative-pressure isolation rooms. To expand isolation capacity, lower-priority patients were relocated, and the existing negative- and neutral-pressure rooms were converted into COVID-19 pandemic wards.
The pandemic wards are staffed by nurses with previous isolation experience and Internal Medicine and Subspecialty Medicine physicians and trainees working closely with Infectious Diseases experts. Pandemic Ward teams are sequestered from other clinical and administrative teams, wear hospital-laundered scrubs, and use PPE-conserving practices. These strategies, implemented at the outset, are based on international guidelines contextualized to local needs and include extended use (up to 6 hours) of N95 respirators for the pandemic wards, and surgical masks in all other clinical areas. Notably, there have been no documented transmissions to HCW or patients at NUH. The workforce was maximized by limiting nonurgent clinical, administrative, research, and teaching activities.
In February, COVID-19 testing was initiated internally and deployed widely. NUH, at the time of this writing, has performed more than 6,000 swabs with up to 200 tests run per day (with 80 confirmed cases). Testing at this scale has allowed NUH to ensure: (a) prompt isolation of patients, even those with mild symptoms, (b) deisolation of those testing negative thus conserving PPE and isolation facilities, (c) a better understanding of the epidemiology and the wide range of clinical manifestations of COVID-19, and (d) early comprehensive contact tracing including mildly symptomatic patients.
The MOH plays a central role in coordinating COVID-19 activities and supports individual hospital systems such as NUH. Some of their crisis leadership strategies include daily text messages distributed countrywide, two-way communication channels that ensure feedback loops with hospital executives, epidemiology specialists, and operational workgroups, and engendering interhospital collaboration.11
A US HOSPITAL MEDICINE RESPONSE: UC SAN FRANCISCO
In the United States, the Joint Commission provides structures, tools, and processes for hospital systems to prepare for disasters.12 Many hospital systems have experience with natural disasters which, similar to Singapore’s planning, ensures structures and systems are in place during a crisis. Although these are transferable to multiple types of disasters, the US healthcare system’s direct experience with infectious crises is limited. A fairly distinctive facet—and an asset of US healthcare—is the role of hospitalists.
Hospitalists care for the majority of medical inpatients across the United States,13 and as such, they currently, and will increasingly, play a major role in the US COVID-19 response. This is the case at the UCSF Helen Diller Medical Center at Parnassus Heights (UCSFMC), a 600-bed academic medical center. To learn from other’s early experiences with COVID-19, UCSF Health System leadership connected with many outside health systems including NUH. As one of its multiple pandemic responses, they engaged the UCSFMC Division of Hospital Medicine (DHM), a division that includes 117 hospitalists, to work with hospital and health system leadership and launch a respiratory isolation unit (RIU) modeled after the NUH pandemic ward. The aim of the RIU is to group inpatients with either confirmed or suspected COVID-19 patients who do not require critical care.
An interdisciplinary work group comprising hospitalists, infectious disease specialists, emergency department clinicians, nursing, rehabilitation experts, hospital epidemiology and infection-prevention leaders, safety specialists, and systems engineers was assembled to repurpose an existing medical unit and establish new care models for the RIU. This workgroup created clinical guidelines and workflows, and RIU leaders actively solicit feedback from the staff to advance these standards.
Hospitalists and nurses who volunteered to work on the UCSF attending-staffed RIU received extensive training, including online and widely available in-person PPE training delivered by infection-prevention experts. The RIU hospitalists engage with hospitalists nationwide through ongoing conference calls to share best practices and clinical cases. Patients are admitted by hospitalists to the RIU via the emergency department or directly from ambulatory sites. All RIU providers and staff are screened daily for symptoms prior to starting their shifts, wear hospital-laundered scrubs on the unit, and remain on the unit for the duration of their shift. Hospitalists and nurses communicate regularly to cluster their patient visits and interventions while specialists provide virtual consults (as deemed safe and appropriate) to optimize PPE conservation and decrease overall exposure. The Health System establishes and revises PPE protocols based on CDC guidelines, best available evidence, and supply chain realities. These guidelines are evolving and currently include surgical mask, gown, gloves, and eye protection for all patient interactions with suspected or confirmed COVID-19 and respirator use during aerosol-generating procedures. Research studies (eg, clinical trials and evaluations), informatics efforts (eg, patient flow dashboards), and healthcare technology innovations (eg, tablets for telehealth and video visits) are continually integrated into the RIU infrastructure. Robust attention to the well-being of everyone working on the unit includes chaplain visits, daily debriefs, meal delivery, and palliative care service support, which enrich the unit experience and instill a culture of unity.
MOVING FORWARD
The structures and systems born out of the 2003 SARS experience and the “test, trace, and isolate” strategy were arguably key drivers to flatten Singapore’s epidemic curve early in the pandemic.3 Even with these in place, Singapore is now experiencing a second wave with a significantly higher caseload.14 In response, the government instituted strict social distancing measures on April 3, closing schools and most workplaces. This suggests that the COVID-19 pandemic may fluctuate over time and that varying types and levels of interventions will be required to maintain long-term control. The NUH team describes experiencing cognitive overload given the ever-changing nature and volume of information and fatigue due to the effort required and duration of this crisis. New programs addressing these challenges are being developed and rapidly deployed.
Despite early testing limitations and newly minted systems, San Francisco is cautiously optimistic about its epidemic curve. Since the March 17, 2020, “shelter in place” order, COVID-19 hospitalizations have remained manageable and constant.15 This has afforded healthcare systems including UCSF critical time to evolve its clinical operations (eg, the RIU) and to leverage its academic culture coordinating its bench research, global health, epidemiology, clinical research, informatics, and clinical enterprise scholars and experts to advance COVID-19 science and inform pandemic solutions. Although the UCSF frontline teams are challenged by the stresses of being in the throes of the pandemic amidst a rapidly changing landscape (including changes in PPE and testing recommendations specifically), they are working together to build team resilience for what may come.
CONCLUSION
The world is facing a pandemic of tremendous proportions, and the United States is in the midst of a wave the height of which is yet to be seen. As Fisher and colleagues wrote in 2011, “Our response to infectious disease outbreaks is born out of past experience.”4 Singapore and NUH’s structures and systems that were put into place demonstrate this—they are timely, have been effective thus far, and will be tested in this next wave. “However, no two outbreaks are the same,” the authors wrote, “so an understanding of the infectious agent as well as the environment confronting it is fundamental to the response.”4 In the United States, hospitalists are a key asset in our environment to confront this virus. The UCSF experience exemplifies that, by combining new ideas from another system with on-the-ground expertise while working hand-in-hand with the hospital and health system, hospitalists can be a critical facet of the pandemic response. Hospitalists’ intrinsic abilities to collaborate, learn, and innovate will enable them to not only meet this challenge now but also to transform practices and capacities to respond to crises into the future.
Acknowledgment
Bradley Sharpe, MD, Division Chief, Division of Hospital Medicine, University of California, San Francisco, California, for his input on conception and critical review of this manuscript.
One of the worst public health threats of our generation, coronavirus disease 2019 (COVID-19), first emerged in Wuhan, China, in December 2019 and quickly spread to Singapore, Hong Kong, and Taiwan. These three countries have been praised for their control of the pandemic,1,2 while the number of cases worldwide, including those in the United States, has soared. Political alignment, centralized and integrated healthcare systems, small size, effective technology deployment, widespread testing combined with contact tracing and isolation, and personal protective equipment (PPE) availability underscore their successes.1,3-5 Although these factors differ starkly from those currently employed in the United States, a better understanding their experience may positively influence the myriad US responses. We describe some salient features of Singapore’s infection preparedness, provide examples of how these features guided the National University Hospital (NUH) Singapore COVID-19 response, and illustrate how one facet of the NUH response was translated to develop a new care model at the University of California, San Francisco (UCSF).
THE SINGAPORE EXPERIENCE OVER TIME
Singapore, a small island country (278 square miles) city-state in Southeast Asia has a population of 5.8 million people. Most Singaporeans receive their inpatient care in the public hospitals that are organized and resourced through the Singapore Ministry of Health (MOH). In 2003, severe acute respiratory syndrome (SARS) infected 238 people and killed 33 over 3 months in Singapore, which led to a significant economic downturn. Singapore’s initial SARS experience unveiled limitations in infrastructure, staff preparedness, virus control methodology, and centralized crisis systems. Lessons gleaned from the SARS experience laid the foundation for Singapore’s subsequent disaster preparedness.6
Post-SARS, the MOH created structures and systems to prepare Singapore for future epidemics. All public hospitals expanded isolation capacity by constructing new units or repurposing existing ones and creating colocated Emergency Department (ED) isolation facilities. Additionally, the MOH commissioned the National Centre for Infectious Diseases, a 330-bed high-level isolation hospital.7 They also mandated hospital systems to regularly practice mass casualty and infectious (including respiratory) crisis responses through externally evaluated simulation.8 These are orchestrated down to the smallest detail and involve staff at all levels. For example, healthcare workers (HCW) being “deployed” outside of their specialty, housekeepers practicing novel hazardous waste disposal, and security guards managing crowds interact throughout the exercise.
The testing and viral spread control challenges during SARS spawned hospital-system epidemiology capacity building. Infectious diseases reporting guidelines were refined, and communication channels enhanced to include cross-hospital information sharing and direct lines of communication for epidemiology groups to and from the MOH. Enhanced contact tracing methodologies were adopted and practiced regularly. In addition, material stockpiles, supplies, and supply chains were recalibrated.
The Singapore government also adopted the Disease Outbreak Response System Condition (DORSCON) system,9 a color-coded framework for pandemic response that guides activation of crisis interventions broadly (such as temperature screening at airports and restrictions to travel and internal movements), as well as within the healthcare setting.
In addition to prompting these notable preparedness efforts, SARS had a palpable impact on Singaporeans’ collective psychology both within and outside of the hospital system. The very close-knit medical community lost colleagues during the crisis, and the larger community deeply felt the health and economic costs of this crisis.10 The resulting “respect” or “healthy fear” for infectious crises continues to the present day.
THE SINGAPORE COVID-19 RESPONSE: NATIONAL UNIVERSITY HOSPITAL EXPERIENCE
The NUH is a 1,200-bed public tertiary care academic health center in Singapore. Before the first COVID-19 case was diagnosed in Singapore, NUH joined forces with its broader health system, university resources (schools of medicine and public health), and international partners to refine the existing structures and systems in response to this new infectious threat.
One of these structures included the existing NUH ED negative-pressure “fever facility.” In the ED triage, patients are routinely screened for infectious diseases such as H1N1, MERS-CoV, and measles. In early January, these screening criteria were evolved to adapt to COVID-19. High-risk patients bypass common waiting areas and are sent directly to the fever facility for management. From there, patients requiring admission are sent to one of the inpatient isolation wards, each with over 21 negative-pressure isolation rooms. To expand isolation capacity, lower-priority patients were relocated, and the existing negative- and neutral-pressure rooms were converted into COVID-19 pandemic wards.
The pandemic wards are staffed by nurses with previous isolation experience and Internal Medicine and Subspecialty Medicine physicians and trainees working closely with Infectious Diseases experts. Pandemic Ward teams are sequestered from other clinical and administrative teams, wear hospital-laundered scrubs, and use PPE-conserving practices. These strategies, implemented at the outset, are based on international guidelines contextualized to local needs and include extended use (up to 6 hours) of N95 respirators for the pandemic wards, and surgical masks in all other clinical areas. Notably, there have been no documented transmissions to HCW or patients at NUH. The workforce was maximized by limiting nonurgent clinical, administrative, research, and teaching activities.
In February, COVID-19 testing was initiated internally and deployed widely. NUH, at the time of this writing, has performed more than 6,000 swabs with up to 200 tests run per day (with 80 confirmed cases). Testing at this scale has allowed NUH to ensure: (a) prompt isolation of patients, even those with mild symptoms, (b) deisolation of those testing negative thus conserving PPE and isolation facilities, (c) a better understanding of the epidemiology and the wide range of clinical manifestations of COVID-19, and (d) early comprehensive contact tracing including mildly symptomatic patients.
The MOH plays a central role in coordinating COVID-19 activities and supports individual hospital systems such as NUH. Some of their crisis leadership strategies include daily text messages distributed countrywide, two-way communication channels that ensure feedback loops with hospital executives, epidemiology specialists, and operational workgroups, and engendering interhospital collaboration.11
A US HOSPITAL MEDICINE RESPONSE: UC SAN FRANCISCO
In the United States, the Joint Commission provides structures, tools, and processes for hospital systems to prepare for disasters.12 Many hospital systems have experience with natural disasters which, similar to Singapore’s planning, ensures structures and systems are in place during a crisis. Although these are transferable to multiple types of disasters, the US healthcare system’s direct experience with infectious crises is limited. A fairly distinctive facet—and an asset of US healthcare—is the role of hospitalists.
Hospitalists care for the majority of medical inpatients across the United States,13 and as such, they currently, and will increasingly, play a major role in the US COVID-19 response. This is the case at the UCSF Helen Diller Medical Center at Parnassus Heights (UCSFMC), a 600-bed academic medical center. To learn from other’s early experiences with COVID-19, UCSF Health System leadership connected with many outside health systems including NUH. As one of its multiple pandemic responses, they engaged the UCSFMC Division of Hospital Medicine (DHM), a division that includes 117 hospitalists, to work with hospital and health system leadership and launch a respiratory isolation unit (RIU) modeled after the NUH pandemic ward. The aim of the RIU is to group inpatients with either confirmed or suspected COVID-19 patients who do not require critical care.
An interdisciplinary work group comprising hospitalists, infectious disease specialists, emergency department clinicians, nursing, rehabilitation experts, hospital epidemiology and infection-prevention leaders, safety specialists, and systems engineers was assembled to repurpose an existing medical unit and establish new care models for the RIU. This workgroup created clinical guidelines and workflows, and RIU leaders actively solicit feedback from the staff to advance these standards.
Hospitalists and nurses who volunteered to work on the UCSF attending-staffed RIU received extensive training, including online and widely available in-person PPE training delivered by infection-prevention experts. The RIU hospitalists engage with hospitalists nationwide through ongoing conference calls to share best practices and clinical cases. Patients are admitted by hospitalists to the RIU via the emergency department or directly from ambulatory sites. All RIU providers and staff are screened daily for symptoms prior to starting their shifts, wear hospital-laundered scrubs on the unit, and remain on the unit for the duration of their shift. Hospitalists and nurses communicate regularly to cluster their patient visits and interventions while specialists provide virtual consults (as deemed safe and appropriate) to optimize PPE conservation and decrease overall exposure. The Health System establishes and revises PPE protocols based on CDC guidelines, best available evidence, and supply chain realities. These guidelines are evolving and currently include surgical mask, gown, gloves, and eye protection for all patient interactions with suspected or confirmed COVID-19 and respirator use during aerosol-generating procedures. Research studies (eg, clinical trials and evaluations), informatics efforts (eg, patient flow dashboards), and healthcare technology innovations (eg, tablets for telehealth and video visits) are continually integrated into the RIU infrastructure. Robust attention to the well-being of everyone working on the unit includes chaplain visits, daily debriefs, meal delivery, and palliative care service support, which enrich the unit experience and instill a culture of unity.
MOVING FORWARD
The structures and systems born out of the 2003 SARS experience and the “test, trace, and isolate” strategy were arguably key drivers to flatten Singapore’s epidemic curve early in the pandemic.3 Even with these in place, Singapore is now experiencing a second wave with a significantly higher caseload.14 In response, the government instituted strict social distancing measures on April 3, closing schools and most workplaces. This suggests that the COVID-19 pandemic may fluctuate over time and that varying types and levels of interventions will be required to maintain long-term control. The NUH team describes experiencing cognitive overload given the ever-changing nature and volume of information and fatigue due to the effort required and duration of this crisis. New programs addressing these challenges are being developed and rapidly deployed.
Despite early testing limitations and newly minted systems, San Francisco is cautiously optimistic about its epidemic curve. Since the March 17, 2020, “shelter in place” order, COVID-19 hospitalizations have remained manageable and constant.15 This has afforded healthcare systems including UCSF critical time to evolve its clinical operations (eg, the RIU) and to leverage its academic culture coordinating its bench research, global health, epidemiology, clinical research, informatics, and clinical enterprise scholars and experts to advance COVID-19 science and inform pandemic solutions. Although the UCSF frontline teams are challenged by the stresses of being in the throes of the pandemic amidst a rapidly changing landscape (including changes in PPE and testing recommendations specifically), they are working together to build team resilience for what may come.
CONCLUSION
The world is facing a pandemic of tremendous proportions, and the United States is in the midst of a wave the height of which is yet to be seen. As Fisher and colleagues wrote in 2011, “Our response to infectious disease outbreaks is born out of past experience.”4 Singapore and NUH’s structures and systems that were put into place demonstrate this—they are timely, have been effective thus far, and will be tested in this next wave. “However, no two outbreaks are the same,” the authors wrote, “so an understanding of the infectious agent as well as the environment confronting it is fundamental to the response.”4 In the United States, hospitalists are a key asset in our environment to confront this virus. The UCSF experience exemplifies that, by combining new ideas from another system with on-the-ground expertise while working hand-in-hand with the hospital and health system, hospitalists can be a critical facet of the pandemic response. Hospitalists’ intrinsic abilities to collaborate, learn, and innovate will enable them to not only meet this challenge now but also to transform practices and capacities to respond to crises into the future.
Acknowledgment
Bradley Sharpe, MD, Division Chief, Division of Hospital Medicine, University of California, San Francisco, California, for his input on conception and critical review of this manuscript.
1. Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020. https://doi.org/10.1001/jama.2020.3151.
2. Legido-Quigley H, Asgari N, Teo YY, et al. Are high-performing health systems resilient against the COVID-19 epidemic? Lancet. 2020;395(10227):848-850. https://doi.org/10.1016/S0140-6736(20)30551-1.
3. Wong JEL, Leo YS, Tan CC. COVID-19 in Singapore—current experience: critical global issues that require attention and action. JAMA. 2020;323(13):1243-1244. https://doi.org/10.1001/jama.2020.2467.
4. Fisher D, Hui DS, Gao Z, et al. Pandemic response lessons from influenza H1N1 2009 in Asia. Respirology. 2011;16(6):876-882. https://doi.org/ 10.1111/j.1440-1843.2011.02003.x.
5. Wong ATY, Chen H, Liu SH, et al. From SARS to avian influenza preparedness in Hong Kong. Clin Infect Dis. 2017;64(suppl_2):S98-S104. https://doi.org/ 10.1093/cid/cix123.
6. Tan CC. SARS in Singapore--key lessons from an epidemic. Ann Acad Med Singapore. 2006;35(5):345-349.
7. National Centre for Infectious Diseases. About NCID. https://www.ncid.sg/About-NCID/Pages/default.aspx. Accessed April 5, 2020.
8. Cutter J. Preparing for an influenza pandemic in Singapore. Ann Acad Med Singapore. 2008;37(6):497-503.
9. Singapore Ministry of Health. What do the different DORSCON levels mean. http://www.gov.sg/article/what-do-the-different-dorscon-levels-mean. Accessed April 5, 2020.
10. Lee J-W, McKibbin WJ. Estimating the global economic costs of SARS. In: Knobler S, Mahmoud A, Lemon S, et al, eds. Institute of Medicine (US) Forum on Microbial Threats. Washington, DC: National Academies Press (US); 2004.
11. James EH, Wooten L. Leadership as (un)usual: how to display competence in times of crisis. Organ Dyn. 2005;34(2):141-152. https://doi.org/10.1016/j.orgdyn.2005.03.005
12. The Joint Commission. Emergency Management: Coronavirus Resources. 2020. https://www.jointcommission.org/covid-19/. Accessed April 4, 2020.
13. Wachter RM, Goldman L. Zero to 50,000 – the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
14. Singapore Ministry of Health. Official Update of COVID-19 Situation in Singapore. 2020. https://experience.arcgis.com/experience/7e30edc490a5441a874f9efe67bd8b89. Accessed April 5, 2020.
15. Chronicle Digital Team. Coronavirus tracker. San Francisco Chronicle. https://projects.sfchronicle.com/2020/coronavirus-map/. Accessed April 5, 2020.
1. Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020. https://doi.org/10.1001/jama.2020.3151.
2. Legido-Quigley H, Asgari N, Teo YY, et al. Are high-performing health systems resilient against the COVID-19 epidemic? Lancet. 2020;395(10227):848-850. https://doi.org/10.1016/S0140-6736(20)30551-1.
3. Wong JEL, Leo YS, Tan CC. COVID-19 in Singapore—current experience: critical global issues that require attention and action. JAMA. 2020;323(13):1243-1244. https://doi.org/10.1001/jama.2020.2467.
4. Fisher D, Hui DS, Gao Z, et al. Pandemic response lessons from influenza H1N1 2009 in Asia. Respirology. 2011;16(6):876-882. https://doi.org/ 10.1111/j.1440-1843.2011.02003.x.
5. Wong ATY, Chen H, Liu SH, et al. From SARS to avian influenza preparedness in Hong Kong. Clin Infect Dis. 2017;64(suppl_2):S98-S104. https://doi.org/ 10.1093/cid/cix123.
6. Tan CC. SARS in Singapore--key lessons from an epidemic. Ann Acad Med Singapore. 2006;35(5):345-349.
7. National Centre for Infectious Diseases. About NCID. https://www.ncid.sg/About-NCID/Pages/default.aspx. Accessed April 5, 2020.
8. Cutter J. Preparing for an influenza pandemic in Singapore. Ann Acad Med Singapore. 2008;37(6):497-503.
9. Singapore Ministry of Health. What do the different DORSCON levels mean. http://www.gov.sg/article/what-do-the-different-dorscon-levels-mean. Accessed April 5, 2020.
10. Lee J-W, McKibbin WJ. Estimating the global economic costs of SARS. In: Knobler S, Mahmoud A, Lemon S, et al, eds. Institute of Medicine (US) Forum on Microbial Threats. Washington, DC: National Academies Press (US); 2004.
11. James EH, Wooten L. Leadership as (un)usual: how to display competence in times of crisis. Organ Dyn. 2005;34(2):141-152. https://doi.org/10.1016/j.orgdyn.2005.03.005
12. The Joint Commission. Emergency Management: Coronavirus Resources. 2020. https://www.jointcommission.org/covid-19/. Accessed April 4, 2020.
13. Wachter RM, Goldman L. Zero to 50,000 – the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
14. Singapore Ministry of Health. Official Update of COVID-19 Situation in Singapore. 2020. https://experience.arcgis.com/experience/7e30edc490a5441a874f9efe67bd8b89. Accessed April 5, 2020.
15. Chronicle Digital Team. Coronavirus tracker. San Francisco Chronicle. https://projects.sfchronicle.com/2020/coronavirus-map/. Accessed April 5, 2020.
© 2020 Society of Hospital Medicine
ERRATUM: Decreasing Hypoglycemia following Insulin Administration for Inpatient Hyperkalemia
A correction has been made to the Figure. A dosage was incorrect in the Orderset 1.1 (1/1/16-3/19/17) box. The figure listed Insulin 19 Units IV x 1 and should have been Insulin 10 Units IV x 1. Below is the corrected figure..

A correction has been made to the Figure. A dosage was incorrect in the Orderset 1.1 (1/1/16-3/19/17) box. The figure listed Insulin 19 Units IV x 1 and should have been Insulin 10 Units IV x 1. Below is the corrected figure..

A correction has been made to the Figure. A dosage was incorrect in the Orderset 1.1 (1/1/16-3/19/17) box. The figure listed Insulin 19 Units IV x 1 and should have been Insulin 10 Units IV x 1. Below is the corrected figure..

Decreasing Hypoglycemia following Insulin Administration for Inpatient Hyperkalemia
Hyperkalemia (serum potassium ≥5.1 mEq/L), if left untreated, may result in cardiac arrhythmias, severe muscle weakness, or paralysis.1,2 Insulin administration can rapidly correct hyperkalemia by shifting serum potassiufm intracellularly.3 Treatment of hyperkalemia with insulin may lead to hypoglycemia, which, when severe, can cause confusion, seizures, loss of consciousness, and death. The use of regular and short-acting insulins to correct hyperkalemia quickly in hospitalized patients results in the greatest risk of hypoglycemia within three hours of treatment.4 Nonetheless, monitoring blood glucose levels within six hours of postinsulin administration is not a standard part of hyperkalemia treatment guidelines,3 leaving the rates of hypoglycemia in this setting poorly characterized.
Without standardized blood glucose measurement protocols, retrospective studies have reported posttreatment hypoglycemia rates of 8.7%-17.5% among all patients with hyperkalemia,5,6 and 13% among patients with end-stage renal disease.4 These estimates likely underestimate the true hypoglycemia rates as they measure blood glucose sporadically and are often outside the three-hour window of highest risk after insulin administration.
At the University of California, San Francisco Medical Center (UCSFMC), we faced similar issues in measuring the true hypoglycemia rates associated with hyperkalemia treatment. In December 2015, a 12-month retrospective review revealed a 12% hypoglycemia rate among patients treated with insulin for hyperkalemia. This review was limited by the inclusion of only patients treated for hyperkalemia using the standard orderset supplied with the electronic health record system (EHR; EPIC Systems, Verona, Wisconsin) and the absence of specific orders for glucose monitoring. As a result, more than 40% of these inpatients had no documented glucose within six hours of postinsulin administration.
We subsequently designed and implemented an adult inpatient hyperkalemia treatment orderset aimed at reducing iatrogenic hypoglycemia by promoting appropriate insulin use and blood glucose monitoring during the treatment of hyperkalemia. Through rapid improvement cycles, we iteratively revised the orderset to optimally mitigate the risk of hypoglycemia that was associated with insulin use. We describe implementation and outcomes of weight-based insulin dosing,7 automated alerts to identify patients at greatest risk for hypoglycemia, and clinical decision support based on the preinsulin blood glucose level. We report the rates of iatrogenic hypoglycemia after the implementation of these order-set changes.
METHODS
Design Overview
EHR data were extracted from Epic Clarity. We analyzed data following Orderset 1.1 implementation (January 1, 2016-March 19, 2017) when hypoglycemia rates were reliably quantifiable and following orderset revision 1.2 (March 20, 2017-September 30, 2017) to evaluate the impact of the orderset intervention. The data collection was approved by the Institutional Review Board at the University of California, San Francisco.
Additionally, we explored the frequency in which providers ordered insulin through the hyperkalemia orderset for each version of the orderset via two-month baseline reviews. Investigation for Orderset 1.1 was from January 1, 2017 to February 28, 2017 and for Orderset 1.2 was from August 1, 2017 to September 30, 2017. Insulin ordering frequency through the hyperkalemia orderset was defined as ordering insulin through the adult inpatient hyperkalemia orderset versus ordering insulin in and outside of the hyperkalemia orderset.
Last, we measured the nursing point of care testing (POCT) blood glucose measurement compliance with the hyperkalemia orderset. Nursing utilization acceptance of the hyperkalemia orderset was defined as adequate POCT blood glucose levels monitored in comparison to all insulin treatments via the hyperkalemia orderset.
Setting and Participants
We evaluated nonobstetric adult inpatients admitted to UCSF Medical Center between January 2016 and September 2017. All medical and surgical wards and intensive care units were included in the analysis.
Intervention
In June 2012, an EHR developed by Epic Systems was introduced at UCSFMC. In January 2016, we designed a new EHR-based hyperkalemia treatment orderset (Orderset 1.1), which added standard POCT blood glucose checks before and at one, two, four, and six hours after insulin injection (Appendix 1). In March 2017, a newly designed orderset (Orderset 1.2) replaced the previous hyperkalemia treatment orderset (Appendix 2). Orderset 1.2 included three updates. First, providers were now presented the option of ordering insulin as a
CORRECTED FIGURE PER ERRATUM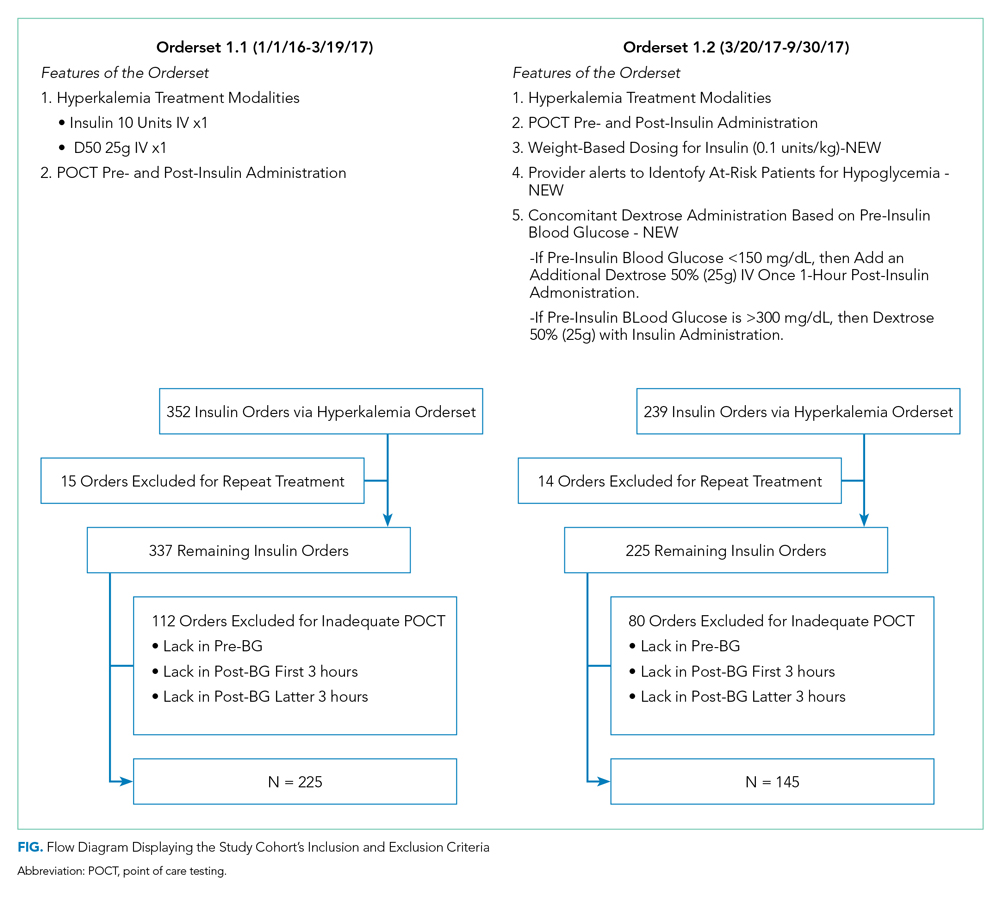
Inclusion and exclusion criteria are shown in the Figure. All patients who had insulin ordered via a hyperkalemia orderset were included in an intention-to-treat analysis. A further analysis was performed for patients for whom orderset compliance was achieved (ie, insulin ordered through the ordersets with adequate blood glucose monitoring). These patients were required to have a POCT blood glucose check preinsulin administration and postinsulin administration as follows: (1) between 30 to 180 minutes (0.5 to three hours) after insulin administration, and (2) between 180 and 360 minutes (three to six hours) after insulin administration. For patients receiving repeated insulin treatments for hyperkalemia within six hours, the first treatment data points were excluded to prevent duplication.
Outcomes
We extracted data on all nonobstetric adult patients admitted to UCSFMC between January 1, 2016 and March 19, 2017 (Orderset 1.1) and between March 20, 2017 and September 30, 2017 (Orderset 1.2).
We measured unique insulin administrations given that each insulin injection poses a risk of iatrogenic hypoglycemia. Hypoglycemia was defined as glucose <70 mg/dL and severe hypoglycemia was defined as glucose <40 mg/dL. Covariates included time and date of insulin administration; blood glucose levels before and at one, two, four, and six hours after insulin injection (if available); sex; weight; dose of insulin given for hyperkalemia treatment; creatinine; known diagnosis of diabetes; concomitant use of albuterol; and concomitant use of corticosteroids. Hyperglycemia was defined as glucose >180 mg/dL. We collected potassium levels pre- and postinsulin treatment. The responsible team’s discipline and the location of the patient (eg, medical/surgical unit, intensive care unit, emergency department) where the insulin orderset was used were recorded.
Statistical Analysis
Statistical analysis for our data included the χ2 test for categorical data and Student t test for continuous data. The bivariate analysis identified potential risk factors and protective factors for hypoglycemia, and logistic regression was used to determine independent predictors of hypoglycemia. Through bivariate analyses, any factor with a P value below .05 was included in the multivariable analyses to investigate a significant contribution to hypoglycemia outcomes. Analyses for hypoglycemia and severe hypoglycemia rates, potassium levels pre- and postinsulin treatment, and hyperglycemia rates were done for both the intention-to-treat group and the group with all criteria met. All analyses were rendered utilizing Stata version 14 (Stata Corp LLC, College Station, Texas).
RESULTS
Baseline patient characteristics, initial insulin dosing, the treatment team, and the location are shown in Table 1. With the implementation of weight-based dosing, a lower dose of insulin was administered with Orderset 1.2 compared with Orderset 1.1.
Orderset adherence rates for Orderset 1.1 and 1.2 were as follows: Acute Care Floor 65% (70%), Intensive Care Unit 63% (66%), and Emergency Department 60% (55%). A two-month audit of orderset usage and compliance revealed that 73% (70 of 96) of insulin treatments were ordered through Orderset 1.1, and 77% (71 of 92) of insulin treatments were ordered through Orderset 1.2. The distribution of orderset usage across location and primary service are shown in Table 1.
The patient distribution is shown in the Figure. In the Orderset 1.1 period, there were 352 total insulin treatments utilizing the newly revised UCSFMC adult inpatient hyperkalemia orderset that were used for the intention-to-treat analysis, and there were 225 patients for whom compliance with orderset monitoring was achieved. Notably, 112 treatments were excluded for the lack of adequate blood glucose monitoring. In the Orderset 1.2 period, there were 239 total insulin treatments utilizing the newly revised UCSFMC adult inpatient hyperkalemia orderset that were used for the intention-to-treat analysis, and there were 145 patients for whom compliance with orderset monitoring was achieved. During this phase, 80 treatments were excluded for inadequate blood glucose monitoring.
Predictors of hypoglycemia following the implementation of Orderset 1.1 are shown in Table 2, and the logistic regression model of these risks is shown in Appendix Table 1. Female gender, weight-based dosing of insulin exceeding 0.14 units/kg, preinsulin blood glucose less than 140 mg/dL, and serum creatinine greater than 2.5 mg/dl were associated with an increased risk of hypoglycemia. A known diagnosis of Type 2 diabetes, concomitant albuterol within one hour of insulin administration, and corticosteroid administration within two hours of insulin administration were associated with a decreased risk of hypoglycemia.
The rates of hypoglycemia (<70 mg/dl) and severe hypoglycemia (<40 mg/dl) are shown in Table 3. During the Orderset 1.1 period,
During the Orderset 1.2 period, for patients with all criteria met, 14 of 145 (10%) had hypoglycemia, and three of 145 (2%) had severe hypoglycemia. Ten of 14 (72%) of these hypoglycemic events occurred in the first three hours, with the remaining four hypoglycemic events (28%) occurring in the last three hours.
An intention-to-treat analysis for hyperglycemia, defined as glucose >180 mg/dl, revealed that during the Orderset 1.1 period, 80 of 352 (23%) had hyperglycemia before insulin administration, and 38 of 352 (11%) had hyperglycemia after insulin administration. During the Orderset 1.2 period, 52 of 239 (22%) had hyperglycemia before insulin administration, and 15 of 239 (6%) had hyperglycemia after insulin administration. Results can be found in Table 3.
Pre- and posttreatment potassium levels are shown in Table 3. An intention-to-treat analysis for potassium reduction postinsulin administration revealed that during the Orderset 1.1 period, there was an absolute reduction of 0.73 mmol/L, while during the Orderset 1.2 period, there was an absolute reduction of 0.95 mmol/L.
DISCUSSION
Treatment of hyperkalemia with insulin may result in significant iatrogenic hypoglycemia. Prior studies have likely underestimated the incidence of hyperkalemia treatment-associated hypoglycemia as glucose levels are rarely checked within three hours of insulin administration.8 In our study, which was designed to ensure appropriate blood glucose measurement, 21% of insulin treatments for hyperkalemia resulted in hypoglycemia, with 92% of hypoglycemic events occurring within the first three hours.
For the Orderset 1.1 period, patient risk factors identified for iatrogenic hypoglycemia postinsulin administration were female sex, doses of regular insulin greater than 0.14 units/kg, preinsulin blood glucose less than 140 mg/dL, and serum creatinine greater than 2.5 mg/dL. These results are consistent with studies suggesting that preinsulin blood glucose levels less than 140 mg/dL and the standard 10 units of insulin for hyperkalemia treatment may increase the risk of hypoglycemia.4,7,9
To decrease the risk of iatrogenic hypoglycemia, we redesigned our hyperkalemia insulin orderset to address the strongest predictors of hypoglycemia (doses of regular insulin greater than 0.14 units/kg and preinsulin blood glucose less than 140 mg/dL). The main changes were weight-based insulin dosing (based on previously published data)10 and adjustment of glucose administration based on the patient’s glucose levels.11 Following these changes, the rates of both hypoglycemia and severe hypoglycemia were statistically significantly reduced. In addition, of the 14 hypoglycemia events identified after the introduction of Orderset 1.2, five could have been prevented (36%) had the protocol been strictly followed. These five hypoglycemia events occurred later than one-hour postinsulin administration in patients with blood sugars < 150 mg/dL prior to insulin administration. In each of these cases, Orderset 1.2 called for an additional dextrose 50% (50 mL) IV bolus, which likely would have prevented the subsequently recorded hypoglycemia. In other words, our orderset indicated that these patients received an additional bolus of dextrose. However, they did not receive their glucose at the appropriate time, contributing to the hypoglycemia events. The orderset did not include a best practice alert (BPA) to remind providers about the extra dextrose bolus. In the future, we plan to add this BPA.
The hypoglycemia rate identified by Orderset 1.1 was 21% and the hypoglycemia rate identified by the Orderset 1.2 was 10%. The severe hypoglycemia rate identified by Orderset 1.1 was 5% and the severe hypoglycemia rate identified by Orderset 1.2 was 2%. The hypoglycemia and severe hypoglycemia rates significantly decreased after the introduction of Orderset 1.2. To mimic a real-world clinical setting, where monitoring of blood glucose is not always achieved multiple times within a six-hour timeframe of postinsulin treatment for hyperkalemia, we conducted an intention-to-treat analysis. Even when including patients for whom full blood glucose monitoring was not achieved, the introduction of Orderset 1.2 was associated with a significant decrease in the hypoglycemia rate.
To demonstrate whether weight-based dosing of insulin was as effective as a standard dose for hyperkalemia treatment, we compared the impact of Orderset 1.1, which only had the option for single standard doses of insulin, with the impact of Orderset 1.2, which included weight-based dosing options. With the introduction of Orderset 1.2, there was a significant decrease in serum potassium, indicating that weight-based dosing options may not only prevent hypoglycemia but may potentially provide more effective hyperkalemia treatment.
We also compared the rate of hyperglycemia (a glucose >180 mg/dl) pre- and posttreatment (Table 3). Although not statistically significant, the rate of hyperglycemia decreased from 11% to 6%, suggesting a trend toward decreased hyperglycemia with orderset usage.
As orderset usage for hyperkalemia management only occurred approximately 75% of the time, likely, forcing the use of these ordersets would further reduce the incidence of treatment-associated hypoglycemia. To encourage the use of ordersets for hyperkalemia management, our medical center has largely restricted insulin ordering so that it can only be done through ordersets with the proper precautions in place, regardless of the indication. Furthermore, adherence to all the blood glucose monitoring orders embedded in the ordersets remained suboptimal irrespective of managing the service or clinical setting
Finally, development and implementation of these hyperkalemia treatment ordersets required an experienced multidisciplinary team, including pharmacists, nurses, hospitalists, endocrinologists, and EHR system programmers.12,13 We, therefore, encourage interprofessional collaboration for any institutions seeking to establish innovative clinical protocols.
This analysis was limited to the insulin administration meeting our inclusion criteria. Thus, we could not identify a true hypoglycemia rate for treatments that were not followed by adequate blood glucose monitoring postinsulin administration, or for insulin administration ordered outside of the hyperkalemia ordersets.
CONCLUSION
The use of a comprehensive EHR orderset for the treatment of hyperkalemia with predefined times for blood glucose monitoring, weight-based insulin dosing, and prompts to warn providers of an individual patient’s risk for hypoglycemia may significantly reduce the incidence of iatrogenic hypoglycemia.
1. Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med. 1998;158(8):917-924. https://doi.org/10.1001/archinte.158.8.917.
2. Fordjour KN, Walton T, Doran JJ. Management of hyperkalemia in hospitalized patients. Am J Med Sci. 2014;347(2):93-100. https://doi.org/10.1097/MAJ.0b013e318279b105.
3. Part-10-Special-Circumstances-of-Resuscitation.pdf. https://eccguidelines.heart.org/wp-content/themes/eccstaging/dompdf-master/pdffiles/part-10-special-circumstances-of-resuscitation.pdf. Accessed December 16, 2017.
4. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. https://doi.org/10.1093/ckj/sfu026.
5. Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypoglycemia following insulin-based acute stabilization of hyperkalemia treatment. J Hosp Med. 2012;7(3):239-242. https://doi.org/10.1002/jhm.977.
6. Boughton CK, Dixon D, Goble E, et al. Preventing hypoglycemia following treatment of hyperkalemia in hospitalized patients. J Hosp Med. 2019;14:E1-E4. https://doi.org/10.12788/jhm.3145.
7. Wheeler DT, Schafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia. J Hosp Med. 2016;11(5):355-357. https://doi.org/10.1002/jhm.2545.
8. Coca A, Valencia AL, Bustamante J, Mendiluce A, Floege J. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS ONE. 2017;12(2):e0172961. https://doi.org/10.1371/journal.pone.0172961.
9. LaRue HA, Peksa GD, Shah SC. A comparison of insulin doses for the treatment of hyperkalemia in patients with renal insufficiency. Pharmacotherapy. 2017;37(12):1516-1522. https://doi.org/10.1002/phar.2038.
10. Brown K, Setji TL, Hale SL, et al. Assessing the impact of an order panel utilizing weight-based insulin and standardized monitoring of blood glucose for patients with hyperkalemia. Am J Med Qual. 2018;33(6):598-603. https://doi.org/10.1177/1062860618764610.
11. Farina N, Anderson C. Impact of dextrose dose on hypoglycemia development following treatment of hyperkalemia. Ther Adv Drug Saf. 2018;9(6):323-329. https://doi.org/10.1177/2042098618768725.
12. Neinstein A, MacMaster HW, Sullivan MM, Rushakoff R. A detailed description of the implementation of inpatient insulin orders with a commercial electronic health record system. J Diabetes Sci Technol. 2014;8(4):641-651. https://doi.org/10.1177/1932296814536290.
13. MacMaster HW, Gonzalez S, Maruoka A, et al. Development and implementation of a subcutaneous Insulin pen label bar code scanning protocol to prevent wrong-patient insulin pen errors. Jt Comm J Qual Patient Saf. 2019;45(5):380-386. https://doi.org/10.1016/j.jcjq.2018.08.006.
Hyperkalemia (serum potassium ≥5.1 mEq/L), if left untreated, may result in cardiac arrhythmias, severe muscle weakness, or paralysis.1,2 Insulin administration can rapidly correct hyperkalemia by shifting serum potassiufm intracellularly.3 Treatment of hyperkalemia with insulin may lead to hypoglycemia, which, when severe, can cause confusion, seizures, loss of consciousness, and death. The use of regular and short-acting insulins to correct hyperkalemia quickly in hospitalized patients results in the greatest risk of hypoglycemia within three hours of treatment.4 Nonetheless, monitoring blood glucose levels within six hours of postinsulin administration is not a standard part of hyperkalemia treatment guidelines,3 leaving the rates of hypoglycemia in this setting poorly characterized.
Without standardized blood glucose measurement protocols, retrospective studies have reported posttreatment hypoglycemia rates of 8.7%-17.5% among all patients with hyperkalemia,5,6 and 13% among patients with end-stage renal disease.4 These estimates likely underestimate the true hypoglycemia rates as they measure blood glucose sporadically and are often outside the three-hour window of highest risk after insulin administration.
At the University of California, San Francisco Medical Center (UCSFMC), we faced similar issues in measuring the true hypoglycemia rates associated with hyperkalemia treatment. In December 2015, a 12-month retrospective review revealed a 12% hypoglycemia rate among patients treated with insulin for hyperkalemia. This review was limited by the inclusion of only patients treated for hyperkalemia using the standard orderset supplied with the electronic health record system (EHR; EPIC Systems, Verona, Wisconsin) and the absence of specific orders for glucose monitoring. As a result, more than 40% of these inpatients had no documented glucose within six hours of postinsulin administration.
We subsequently designed and implemented an adult inpatient hyperkalemia treatment orderset aimed at reducing iatrogenic hypoglycemia by promoting appropriate insulin use and blood glucose monitoring during the treatment of hyperkalemia. Through rapid improvement cycles, we iteratively revised the orderset to optimally mitigate the risk of hypoglycemia that was associated with insulin use. We describe implementation and outcomes of weight-based insulin dosing,7 automated alerts to identify patients at greatest risk for hypoglycemia, and clinical decision support based on the preinsulin blood glucose level. We report the rates of iatrogenic hypoglycemia after the implementation of these order-set changes.
METHODS
Design Overview
EHR data were extracted from Epic Clarity. We analyzed data following Orderset 1.1 implementation (January 1, 2016-March 19, 2017) when hypoglycemia rates were reliably quantifiable and following orderset revision 1.2 (March 20, 2017-September 30, 2017) to evaluate the impact of the orderset intervention. The data collection was approved by the Institutional Review Board at the University of California, San Francisco.
Additionally, we explored the frequency in which providers ordered insulin through the hyperkalemia orderset for each version of the orderset via two-month baseline reviews. Investigation for Orderset 1.1 was from January 1, 2017 to February 28, 2017 and for Orderset 1.2 was from August 1, 2017 to September 30, 2017. Insulin ordering frequency through the hyperkalemia orderset was defined as ordering insulin through the adult inpatient hyperkalemia orderset versus ordering insulin in and outside of the hyperkalemia orderset.
Last, we measured the nursing point of care testing (POCT) blood glucose measurement compliance with the hyperkalemia orderset. Nursing utilization acceptance of the hyperkalemia orderset was defined as adequate POCT blood glucose levels monitored in comparison to all insulin treatments via the hyperkalemia orderset.
Setting and Participants
We evaluated nonobstetric adult inpatients admitted to UCSF Medical Center between January 2016 and September 2017. All medical and surgical wards and intensive care units were included in the analysis.
Intervention
In June 2012, an EHR developed by Epic Systems was introduced at UCSFMC. In January 2016, we designed a new EHR-based hyperkalemia treatment orderset (Orderset 1.1), which added standard POCT blood glucose checks before and at one, two, four, and six hours after insulin injection (Appendix 1). In March 2017, a newly designed orderset (Orderset 1.2) replaced the previous hyperkalemia treatment orderset (Appendix 2). Orderset 1.2 included three updates. First, providers were now presented the option of ordering insulin as a
CORRECTED FIGURE PER ERRATUM
Inclusion and exclusion criteria are shown in the Figure. All patients who had insulin ordered via a hyperkalemia orderset were included in an intention-to-treat analysis. A further analysis was performed for patients for whom orderset compliance was achieved (ie, insulin ordered through the ordersets with adequate blood glucose monitoring). These patients were required to have a POCT blood glucose check preinsulin administration and postinsulin administration as follows: (1) between 30 to 180 minutes (0.5 to three hours) after insulin administration, and (2) between 180 and 360 minutes (three to six hours) after insulin administration. For patients receiving repeated insulin treatments for hyperkalemia within six hours, the first treatment data points were excluded to prevent duplication.
Outcomes
We extracted data on all nonobstetric adult patients admitted to UCSFMC between January 1, 2016 and March 19, 2017 (Orderset 1.1) and between March 20, 2017 and September 30, 2017 (Orderset 1.2).
We measured unique insulin administrations given that each insulin injection poses a risk of iatrogenic hypoglycemia. Hypoglycemia was defined as glucose <70 mg/dL and severe hypoglycemia was defined as glucose <40 mg/dL. Covariates included time and date of insulin administration; blood glucose levels before and at one, two, four, and six hours after insulin injection (if available); sex; weight; dose of insulin given for hyperkalemia treatment; creatinine; known diagnosis of diabetes; concomitant use of albuterol; and concomitant use of corticosteroids. Hyperglycemia was defined as glucose >180 mg/dL. We collected potassium levels pre- and postinsulin treatment. The responsible team’s discipline and the location of the patient (eg, medical/surgical unit, intensive care unit, emergency department) where the insulin orderset was used were recorded.
Statistical Analysis
Statistical analysis for our data included the χ2 test for categorical data and Student t test for continuous data. The bivariate analysis identified potential risk factors and protective factors for hypoglycemia, and logistic regression was used to determine independent predictors of hypoglycemia. Through bivariate analyses, any factor with a P value below .05 was included in the multivariable analyses to investigate a significant contribution to hypoglycemia outcomes. Analyses for hypoglycemia and severe hypoglycemia rates, potassium levels pre- and postinsulin treatment, and hyperglycemia rates were done for both the intention-to-treat group and the group with all criteria met. All analyses were rendered utilizing Stata version 14 (Stata Corp LLC, College Station, Texas).
RESULTS
Baseline patient characteristics, initial insulin dosing, the treatment team, and the location are shown in Table 1. With the implementation of weight-based dosing, a lower dose of insulin was administered with Orderset 1.2 compared with Orderset 1.1.
Orderset adherence rates for Orderset 1.1 and 1.2 were as follows: Acute Care Floor 65% (70%), Intensive Care Unit 63% (66%), and Emergency Department 60% (55%). A two-month audit of orderset usage and compliance revealed that 73% (70 of 96) of insulin treatments were ordered through Orderset 1.1, and 77% (71 of 92) of insulin treatments were ordered through Orderset 1.2. The distribution of orderset usage across location and primary service are shown in Table 1.
The patient distribution is shown in the Figure. In the Orderset 1.1 period, there were 352 total insulin treatments utilizing the newly revised UCSFMC adult inpatient hyperkalemia orderset that were used for the intention-to-treat analysis, and there were 225 patients for whom compliance with orderset monitoring was achieved. Notably, 112 treatments were excluded for the lack of adequate blood glucose monitoring. In the Orderset 1.2 period, there were 239 total insulin treatments utilizing the newly revised UCSFMC adult inpatient hyperkalemia orderset that were used for the intention-to-treat analysis, and there were 145 patients for whom compliance with orderset monitoring was achieved. During this phase, 80 treatments were excluded for inadequate blood glucose monitoring.
Predictors of hypoglycemia following the implementation of Orderset 1.1 are shown in Table 2, and the logistic regression model of these risks is shown in Appendix Table 1. Female gender, weight-based dosing of insulin exceeding 0.14 units/kg, preinsulin blood glucose less than 140 mg/dL, and serum creatinine greater than 2.5 mg/dl were associated with an increased risk of hypoglycemia. A known diagnosis of Type 2 diabetes, concomitant albuterol within one hour of insulin administration, and corticosteroid administration within two hours of insulin administration were associated with a decreased risk of hypoglycemia.
The rates of hypoglycemia (<70 mg/dl) and severe hypoglycemia (<40 mg/dl) are shown in Table 3. During the Orderset 1.1 period,
During the Orderset 1.2 period, for patients with all criteria met, 14 of 145 (10%) had hypoglycemia, and three of 145 (2%) had severe hypoglycemia. Ten of 14 (72%) of these hypoglycemic events occurred in the first three hours, with the remaining four hypoglycemic events (28%) occurring in the last three hours.
An intention-to-treat analysis for hyperglycemia, defined as glucose >180 mg/dl, revealed that during the Orderset 1.1 period, 80 of 352 (23%) had hyperglycemia before insulin administration, and 38 of 352 (11%) had hyperglycemia after insulin administration. During the Orderset 1.2 period, 52 of 239 (22%) had hyperglycemia before insulin administration, and 15 of 239 (6%) had hyperglycemia after insulin administration. Results can be found in Table 3.
Pre- and posttreatment potassium levels are shown in Table 3. An intention-to-treat analysis for potassium reduction postinsulin administration revealed that during the Orderset 1.1 period, there was an absolute reduction of 0.73 mmol/L, while during the Orderset 1.2 period, there was an absolute reduction of 0.95 mmol/L.
DISCUSSION
Treatment of hyperkalemia with insulin may result in significant iatrogenic hypoglycemia. Prior studies have likely underestimated the incidence of hyperkalemia treatment-associated hypoglycemia as glucose levels are rarely checked within three hours of insulin administration.8 In our study, which was designed to ensure appropriate blood glucose measurement, 21% of insulin treatments for hyperkalemia resulted in hypoglycemia, with 92% of hypoglycemic events occurring within the first three hours.
For the Orderset 1.1 period, patient risk factors identified for iatrogenic hypoglycemia postinsulin administration were female sex, doses of regular insulin greater than 0.14 units/kg, preinsulin blood glucose less than 140 mg/dL, and serum creatinine greater than 2.5 mg/dL. These results are consistent with studies suggesting that preinsulin blood glucose levels less than 140 mg/dL and the standard 10 units of insulin for hyperkalemia treatment may increase the risk of hypoglycemia.4,7,9
To decrease the risk of iatrogenic hypoglycemia, we redesigned our hyperkalemia insulin orderset to address the strongest predictors of hypoglycemia (doses of regular insulin greater than 0.14 units/kg and preinsulin blood glucose less than 140 mg/dL). The main changes were weight-based insulin dosing (based on previously published data)10 and adjustment of glucose administration based on the patient’s glucose levels.11 Following these changes, the rates of both hypoglycemia and severe hypoglycemia were statistically significantly reduced. In addition, of the 14 hypoglycemia events identified after the introduction of Orderset 1.2, five could have been prevented (36%) had the protocol been strictly followed. These five hypoglycemia events occurred later than one-hour postinsulin administration in patients with blood sugars < 150 mg/dL prior to insulin administration. In each of these cases, Orderset 1.2 called for an additional dextrose 50% (50 mL) IV bolus, which likely would have prevented the subsequently recorded hypoglycemia. In other words, our orderset indicated that these patients received an additional bolus of dextrose. However, they did not receive their glucose at the appropriate time, contributing to the hypoglycemia events. The orderset did not include a best practice alert (BPA) to remind providers about the extra dextrose bolus. In the future, we plan to add this BPA.
The hypoglycemia rate identified by Orderset 1.1 was 21% and the hypoglycemia rate identified by the Orderset 1.2 was 10%. The severe hypoglycemia rate identified by Orderset 1.1 was 5% and the severe hypoglycemia rate identified by Orderset 1.2 was 2%. The hypoglycemia and severe hypoglycemia rates significantly decreased after the introduction of Orderset 1.2. To mimic a real-world clinical setting, where monitoring of blood glucose is not always achieved multiple times within a six-hour timeframe of postinsulin treatment for hyperkalemia, we conducted an intention-to-treat analysis. Even when including patients for whom full blood glucose monitoring was not achieved, the introduction of Orderset 1.2 was associated with a significant decrease in the hypoglycemia rate.
To demonstrate whether weight-based dosing of insulin was as effective as a standard dose for hyperkalemia treatment, we compared the impact of Orderset 1.1, which only had the option for single standard doses of insulin, with the impact of Orderset 1.2, which included weight-based dosing options. With the introduction of Orderset 1.2, there was a significant decrease in serum potassium, indicating that weight-based dosing options may not only prevent hypoglycemia but may potentially provide more effective hyperkalemia treatment.
We also compared the rate of hyperglycemia (a glucose >180 mg/dl) pre- and posttreatment (Table 3). Although not statistically significant, the rate of hyperglycemia decreased from 11% to 6%, suggesting a trend toward decreased hyperglycemia with orderset usage.
As orderset usage for hyperkalemia management only occurred approximately 75% of the time, likely, forcing the use of these ordersets would further reduce the incidence of treatment-associated hypoglycemia. To encourage the use of ordersets for hyperkalemia management, our medical center has largely restricted insulin ordering so that it can only be done through ordersets with the proper precautions in place, regardless of the indication. Furthermore, adherence to all the blood glucose monitoring orders embedded in the ordersets remained suboptimal irrespective of managing the service or clinical setting
Finally, development and implementation of these hyperkalemia treatment ordersets required an experienced multidisciplinary team, including pharmacists, nurses, hospitalists, endocrinologists, and EHR system programmers.12,13 We, therefore, encourage interprofessional collaboration for any institutions seeking to establish innovative clinical protocols.
This analysis was limited to the insulin administration meeting our inclusion criteria. Thus, we could not identify a true hypoglycemia rate for treatments that were not followed by adequate blood glucose monitoring postinsulin administration, or for insulin administration ordered outside of the hyperkalemia ordersets.
CONCLUSION
The use of a comprehensive EHR orderset for the treatment of hyperkalemia with predefined times for blood glucose monitoring, weight-based insulin dosing, and prompts to warn providers of an individual patient’s risk for hypoglycemia may significantly reduce the incidence of iatrogenic hypoglycemia.
Hyperkalemia (serum potassium ≥5.1 mEq/L), if left untreated, may result in cardiac arrhythmias, severe muscle weakness, or paralysis.1,2 Insulin administration can rapidly correct hyperkalemia by shifting serum potassiufm intracellularly.3 Treatment of hyperkalemia with insulin may lead to hypoglycemia, which, when severe, can cause confusion, seizures, loss of consciousness, and death. The use of regular and short-acting insulins to correct hyperkalemia quickly in hospitalized patients results in the greatest risk of hypoglycemia within three hours of treatment.4 Nonetheless, monitoring blood glucose levels within six hours of postinsulin administration is not a standard part of hyperkalemia treatment guidelines,3 leaving the rates of hypoglycemia in this setting poorly characterized.
Without standardized blood glucose measurement protocols, retrospective studies have reported posttreatment hypoglycemia rates of 8.7%-17.5% among all patients with hyperkalemia,5,6 and 13% among patients with end-stage renal disease.4 These estimates likely underestimate the true hypoglycemia rates as they measure blood glucose sporadically and are often outside the three-hour window of highest risk after insulin administration.
At the University of California, San Francisco Medical Center (UCSFMC), we faced similar issues in measuring the true hypoglycemia rates associated with hyperkalemia treatment. In December 2015, a 12-month retrospective review revealed a 12% hypoglycemia rate among patients treated with insulin for hyperkalemia. This review was limited by the inclusion of only patients treated for hyperkalemia using the standard orderset supplied with the electronic health record system (EHR; EPIC Systems, Verona, Wisconsin) and the absence of specific orders for glucose monitoring. As a result, more than 40% of these inpatients had no documented glucose within six hours of postinsulin administration.
We subsequently designed and implemented an adult inpatient hyperkalemia treatment orderset aimed at reducing iatrogenic hypoglycemia by promoting appropriate insulin use and blood glucose monitoring during the treatment of hyperkalemia. Through rapid improvement cycles, we iteratively revised the orderset to optimally mitigate the risk of hypoglycemia that was associated with insulin use. We describe implementation and outcomes of weight-based insulin dosing,7 automated alerts to identify patients at greatest risk for hypoglycemia, and clinical decision support based on the preinsulin blood glucose level. We report the rates of iatrogenic hypoglycemia after the implementation of these order-set changes.
METHODS
Design Overview
EHR data were extracted from Epic Clarity. We analyzed data following Orderset 1.1 implementation (January 1, 2016-March 19, 2017) when hypoglycemia rates were reliably quantifiable and following orderset revision 1.2 (March 20, 2017-September 30, 2017) to evaluate the impact of the orderset intervention. The data collection was approved by the Institutional Review Board at the University of California, San Francisco.
Additionally, we explored the frequency in which providers ordered insulin through the hyperkalemia orderset for each version of the orderset via two-month baseline reviews. Investigation for Orderset 1.1 was from January 1, 2017 to February 28, 2017 and for Orderset 1.2 was from August 1, 2017 to September 30, 2017. Insulin ordering frequency through the hyperkalemia orderset was defined as ordering insulin through the adult inpatient hyperkalemia orderset versus ordering insulin in and outside of the hyperkalemia orderset.
Last, we measured the nursing point of care testing (POCT) blood glucose measurement compliance with the hyperkalemia orderset. Nursing utilization acceptance of the hyperkalemia orderset was defined as adequate POCT blood glucose levels monitored in comparison to all insulin treatments via the hyperkalemia orderset.
Setting and Participants
We evaluated nonobstetric adult inpatients admitted to UCSF Medical Center between January 2016 and September 2017. All medical and surgical wards and intensive care units were included in the analysis.
Intervention
In June 2012, an EHR developed by Epic Systems was introduced at UCSFMC. In January 2016, we designed a new EHR-based hyperkalemia treatment orderset (Orderset 1.1), which added standard POCT blood glucose checks before and at one, two, four, and six hours after insulin injection (Appendix 1). In March 2017, a newly designed orderset (Orderset 1.2) replaced the previous hyperkalemia treatment orderset (Appendix 2). Orderset 1.2 included three updates. First, providers were now presented the option of ordering insulin as a
CORRECTED FIGURE PER ERRATUM
Inclusion and exclusion criteria are shown in the Figure. All patients who had insulin ordered via a hyperkalemia orderset were included in an intention-to-treat analysis. A further analysis was performed for patients for whom orderset compliance was achieved (ie, insulin ordered through the ordersets with adequate blood glucose monitoring). These patients were required to have a POCT blood glucose check preinsulin administration and postinsulin administration as follows: (1) between 30 to 180 minutes (0.5 to three hours) after insulin administration, and (2) between 180 and 360 minutes (three to six hours) after insulin administration. For patients receiving repeated insulin treatments for hyperkalemia within six hours, the first treatment data points were excluded to prevent duplication.
Outcomes
We extracted data on all nonobstetric adult patients admitted to UCSFMC between January 1, 2016 and March 19, 2017 (Orderset 1.1) and between March 20, 2017 and September 30, 2017 (Orderset 1.2).
We measured unique insulin administrations given that each insulin injection poses a risk of iatrogenic hypoglycemia. Hypoglycemia was defined as glucose <70 mg/dL and severe hypoglycemia was defined as glucose <40 mg/dL. Covariates included time and date of insulin administration; blood glucose levels before and at one, two, four, and six hours after insulin injection (if available); sex; weight; dose of insulin given for hyperkalemia treatment; creatinine; known diagnosis of diabetes; concomitant use of albuterol; and concomitant use of corticosteroids. Hyperglycemia was defined as glucose >180 mg/dL. We collected potassium levels pre- and postinsulin treatment. The responsible team’s discipline and the location of the patient (eg, medical/surgical unit, intensive care unit, emergency department) where the insulin orderset was used were recorded.
Statistical Analysis
Statistical analysis for our data included the χ2 test for categorical data and Student t test for continuous data. The bivariate analysis identified potential risk factors and protective factors for hypoglycemia, and logistic regression was used to determine independent predictors of hypoglycemia. Through bivariate analyses, any factor with a P value below .05 was included in the multivariable analyses to investigate a significant contribution to hypoglycemia outcomes. Analyses for hypoglycemia and severe hypoglycemia rates, potassium levels pre- and postinsulin treatment, and hyperglycemia rates were done for both the intention-to-treat group and the group with all criteria met. All analyses were rendered utilizing Stata version 14 (Stata Corp LLC, College Station, Texas).
RESULTS
Baseline patient characteristics, initial insulin dosing, the treatment team, and the location are shown in Table 1. With the implementation of weight-based dosing, a lower dose of insulin was administered with Orderset 1.2 compared with Orderset 1.1.
Orderset adherence rates for Orderset 1.1 and 1.2 were as follows: Acute Care Floor 65% (70%), Intensive Care Unit 63% (66%), and Emergency Department 60% (55%). A two-month audit of orderset usage and compliance revealed that 73% (70 of 96) of insulin treatments were ordered through Orderset 1.1, and 77% (71 of 92) of insulin treatments were ordered through Orderset 1.2. The distribution of orderset usage across location and primary service are shown in Table 1.
The patient distribution is shown in the Figure. In the Orderset 1.1 period, there were 352 total insulin treatments utilizing the newly revised UCSFMC adult inpatient hyperkalemia orderset that were used for the intention-to-treat analysis, and there were 225 patients for whom compliance with orderset monitoring was achieved. Notably, 112 treatments were excluded for the lack of adequate blood glucose monitoring. In the Orderset 1.2 period, there were 239 total insulin treatments utilizing the newly revised UCSFMC adult inpatient hyperkalemia orderset that were used for the intention-to-treat analysis, and there were 145 patients for whom compliance with orderset monitoring was achieved. During this phase, 80 treatments were excluded for inadequate blood glucose monitoring.
Predictors of hypoglycemia following the implementation of Orderset 1.1 are shown in Table 2, and the logistic regression model of these risks is shown in Appendix Table 1. Female gender, weight-based dosing of insulin exceeding 0.14 units/kg, preinsulin blood glucose less than 140 mg/dL, and serum creatinine greater than 2.5 mg/dl were associated with an increased risk of hypoglycemia. A known diagnosis of Type 2 diabetes, concomitant albuterol within one hour of insulin administration, and corticosteroid administration within two hours of insulin administration were associated with a decreased risk of hypoglycemia.
The rates of hypoglycemia (<70 mg/dl) and severe hypoglycemia (<40 mg/dl) are shown in Table 3. During the Orderset 1.1 period,
During the Orderset 1.2 period, for patients with all criteria met, 14 of 145 (10%) had hypoglycemia, and three of 145 (2%) had severe hypoglycemia. Ten of 14 (72%) of these hypoglycemic events occurred in the first three hours, with the remaining four hypoglycemic events (28%) occurring in the last three hours.
An intention-to-treat analysis for hyperglycemia, defined as glucose >180 mg/dl, revealed that during the Orderset 1.1 period, 80 of 352 (23%) had hyperglycemia before insulin administration, and 38 of 352 (11%) had hyperglycemia after insulin administration. During the Orderset 1.2 period, 52 of 239 (22%) had hyperglycemia before insulin administration, and 15 of 239 (6%) had hyperglycemia after insulin administration. Results can be found in Table 3.
Pre- and posttreatment potassium levels are shown in Table 3. An intention-to-treat analysis for potassium reduction postinsulin administration revealed that during the Orderset 1.1 period, there was an absolute reduction of 0.73 mmol/L, while during the Orderset 1.2 period, there was an absolute reduction of 0.95 mmol/L.
DISCUSSION
Treatment of hyperkalemia with insulin may result in significant iatrogenic hypoglycemia. Prior studies have likely underestimated the incidence of hyperkalemia treatment-associated hypoglycemia as glucose levels are rarely checked within three hours of insulin administration.8 In our study, which was designed to ensure appropriate blood glucose measurement, 21% of insulin treatments for hyperkalemia resulted in hypoglycemia, with 92% of hypoglycemic events occurring within the first three hours.
For the Orderset 1.1 period, patient risk factors identified for iatrogenic hypoglycemia postinsulin administration were female sex, doses of regular insulin greater than 0.14 units/kg, preinsulin blood glucose less than 140 mg/dL, and serum creatinine greater than 2.5 mg/dL. These results are consistent with studies suggesting that preinsulin blood glucose levels less than 140 mg/dL and the standard 10 units of insulin for hyperkalemia treatment may increase the risk of hypoglycemia.4,7,9
To decrease the risk of iatrogenic hypoglycemia, we redesigned our hyperkalemia insulin orderset to address the strongest predictors of hypoglycemia (doses of regular insulin greater than 0.14 units/kg and preinsulin blood glucose less than 140 mg/dL). The main changes were weight-based insulin dosing (based on previously published data)10 and adjustment of glucose administration based on the patient’s glucose levels.11 Following these changes, the rates of both hypoglycemia and severe hypoglycemia were statistically significantly reduced. In addition, of the 14 hypoglycemia events identified after the introduction of Orderset 1.2, five could have been prevented (36%) had the protocol been strictly followed. These five hypoglycemia events occurred later than one-hour postinsulin administration in patients with blood sugars < 150 mg/dL prior to insulin administration. In each of these cases, Orderset 1.2 called for an additional dextrose 50% (50 mL) IV bolus, which likely would have prevented the subsequently recorded hypoglycemia. In other words, our orderset indicated that these patients received an additional bolus of dextrose. However, they did not receive their glucose at the appropriate time, contributing to the hypoglycemia events. The orderset did not include a best practice alert (BPA) to remind providers about the extra dextrose bolus. In the future, we plan to add this BPA.
The hypoglycemia rate identified by Orderset 1.1 was 21% and the hypoglycemia rate identified by the Orderset 1.2 was 10%. The severe hypoglycemia rate identified by Orderset 1.1 was 5% and the severe hypoglycemia rate identified by Orderset 1.2 was 2%. The hypoglycemia and severe hypoglycemia rates significantly decreased after the introduction of Orderset 1.2. To mimic a real-world clinical setting, where monitoring of blood glucose is not always achieved multiple times within a six-hour timeframe of postinsulin treatment for hyperkalemia, we conducted an intention-to-treat analysis. Even when including patients for whom full blood glucose monitoring was not achieved, the introduction of Orderset 1.2 was associated with a significant decrease in the hypoglycemia rate.
To demonstrate whether weight-based dosing of insulin was as effective as a standard dose for hyperkalemia treatment, we compared the impact of Orderset 1.1, which only had the option for single standard doses of insulin, with the impact of Orderset 1.2, which included weight-based dosing options. With the introduction of Orderset 1.2, there was a significant decrease in serum potassium, indicating that weight-based dosing options may not only prevent hypoglycemia but may potentially provide more effective hyperkalemia treatment.
We also compared the rate of hyperglycemia (a glucose >180 mg/dl) pre- and posttreatment (Table 3). Although not statistically significant, the rate of hyperglycemia decreased from 11% to 6%, suggesting a trend toward decreased hyperglycemia with orderset usage.
As orderset usage for hyperkalemia management only occurred approximately 75% of the time, likely, forcing the use of these ordersets would further reduce the incidence of treatment-associated hypoglycemia. To encourage the use of ordersets for hyperkalemia management, our medical center has largely restricted insulin ordering so that it can only be done through ordersets with the proper precautions in place, regardless of the indication. Furthermore, adherence to all the blood glucose monitoring orders embedded in the ordersets remained suboptimal irrespective of managing the service or clinical setting
Finally, development and implementation of these hyperkalemia treatment ordersets required an experienced multidisciplinary team, including pharmacists, nurses, hospitalists, endocrinologists, and EHR system programmers.12,13 We, therefore, encourage interprofessional collaboration for any institutions seeking to establish innovative clinical protocols.
This analysis was limited to the insulin administration meeting our inclusion criteria. Thus, we could not identify a true hypoglycemia rate for treatments that were not followed by adequate blood glucose monitoring postinsulin administration, or for insulin administration ordered outside of the hyperkalemia ordersets.
CONCLUSION
The use of a comprehensive EHR orderset for the treatment of hyperkalemia with predefined times for blood glucose monitoring, weight-based insulin dosing, and prompts to warn providers of an individual patient’s risk for hypoglycemia may significantly reduce the incidence of iatrogenic hypoglycemia.
1. Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med. 1998;158(8):917-924. https://doi.org/10.1001/archinte.158.8.917.
2. Fordjour KN, Walton T, Doran JJ. Management of hyperkalemia in hospitalized patients. Am J Med Sci. 2014;347(2):93-100. https://doi.org/10.1097/MAJ.0b013e318279b105.
3. Part-10-Special-Circumstances-of-Resuscitation.pdf. https://eccguidelines.heart.org/wp-content/themes/eccstaging/dompdf-master/pdffiles/part-10-special-circumstances-of-resuscitation.pdf. Accessed December 16, 2017.
4. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. https://doi.org/10.1093/ckj/sfu026.
5. Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypoglycemia following insulin-based acute stabilization of hyperkalemia treatment. J Hosp Med. 2012;7(3):239-242. https://doi.org/10.1002/jhm.977.
6. Boughton CK, Dixon D, Goble E, et al. Preventing hypoglycemia following treatment of hyperkalemia in hospitalized patients. J Hosp Med. 2019;14:E1-E4. https://doi.org/10.12788/jhm.3145.
7. Wheeler DT, Schafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia. J Hosp Med. 2016;11(5):355-357. https://doi.org/10.1002/jhm.2545.
8. Coca A, Valencia AL, Bustamante J, Mendiluce A, Floege J. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS ONE. 2017;12(2):e0172961. https://doi.org/10.1371/journal.pone.0172961.
9. LaRue HA, Peksa GD, Shah SC. A comparison of insulin doses for the treatment of hyperkalemia in patients with renal insufficiency. Pharmacotherapy. 2017;37(12):1516-1522. https://doi.org/10.1002/phar.2038.
10. Brown K, Setji TL, Hale SL, et al. Assessing the impact of an order panel utilizing weight-based insulin and standardized monitoring of blood glucose for patients with hyperkalemia. Am J Med Qual. 2018;33(6):598-603. https://doi.org/10.1177/1062860618764610.
11. Farina N, Anderson C. Impact of dextrose dose on hypoglycemia development following treatment of hyperkalemia. Ther Adv Drug Saf. 2018;9(6):323-329. https://doi.org/10.1177/2042098618768725.
12. Neinstein A, MacMaster HW, Sullivan MM, Rushakoff R. A detailed description of the implementation of inpatient insulin orders with a commercial electronic health record system. J Diabetes Sci Technol. 2014;8(4):641-651. https://doi.org/10.1177/1932296814536290.
13. MacMaster HW, Gonzalez S, Maruoka A, et al. Development and implementation of a subcutaneous Insulin pen label bar code scanning protocol to prevent wrong-patient insulin pen errors. Jt Comm J Qual Patient Saf. 2019;45(5):380-386. https://doi.org/10.1016/j.jcjq.2018.08.006.
1. Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med. 1998;158(8):917-924. https://doi.org/10.1001/archinte.158.8.917.
2. Fordjour KN, Walton T, Doran JJ. Management of hyperkalemia in hospitalized patients. Am J Med Sci. 2014;347(2):93-100. https://doi.org/10.1097/MAJ.0b013e318279b105.
3. Part-10-Special-Circumstances-of-Resuscitation.pdf. https://eccguidelines.heart.org/wp-content/themes/eccstaging/dompdf-master/pdffiles/part-10-special-circumstances-of-resuscitation.pdf. Accessed December 16, 2017.
4. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. https://doi.org/10.1093/ckj/sfu026.
5. Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypoglycemia following insulin-based acute stabilization of hyperkalemia treatment. J Hosp Med. 2012;7(3):239-242. https://doi.org/10.1002/jhm.977.
6. Boughton CK, Dixon D, Goble E, et al. Preventing hypoglycemia following treatment of hyperkalemia in hospitalized patients. J Hosp Med. 2019;14:E1-E4. https://doi.org/10.12788/jhm.3145.
7. Wheeler DT, Schafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia. J Hosp Med. 2016;11(5):355-357. https://doi.org/10.1002/jhm.2545.
8. Coca A, Valencia AL, Bustamante J, Mendiluce A, Floege J. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS ONE. 2017;12(2):e0172961. https://doi.org/10.1371/journal.pone.0172961.
9. LaRue HA, Peksa GD, Shah SC. A comparison of insulin doses for the treatment of hyperkalemia in patients with renal insufficiency. Pharmacotherapy. 2017;37(12):1516-1522. https://doi.org/10.1002/phar.2038.
10. Brown K, Setji TL, Hale SL, et al. Assessing the impact of an order panel utilizing weight-based insulin and standardized monitoring of blood glucose for patients with hyperkalemia. Am J Med Qual. 2018;33(6):598-603. https://doi.org/10.1177/1062860618764610.
11. Farina N, Anderson C. Impact of dextrose dose on hypoglycemia development following treatment of hyperkalemia. Ther Adv Drug Saf. 2018;9(6):323-329. https://doi.org/10.1177/2042098618768725.
12. Neinstein A, MacMaster HW, Sullivan MM, Rushakoff R. A detailed description of the implementation of inpatient insulin orders with a commercial electronic health record system. J Diabetes Sci Technol. 2014;8(4):641-651. https://doi.org/10.1177/1932296814536290.
13. MacMaster HW, Gonzalez S, Maruoka A, et al. Development and implementation of a subcutaneous Insulin pen label bar code scanning protocol to prevent wrong-patient insulin pen errors. Jt Comm J Qual Patient Saf. 2019;45(5):380-386. https://doi.org/10.1016/j.jcjq.2018.08.006.
© 2020 Society of Hospital Medicine
Tissue Isn’t the Issue
A 43-year-old man with a history of asplenia, hepatitis C, and nephrolithiasis reported right-flank pain. He described severe, sharp pain that came in waves and radiated to the right groin, associated with nausea and nonbloody emesis. He noted “pink urine” but no dysuria. He had 4prior similar episodes during which he had passed kidney stones, although stone analysis had never been performed. He denied having fevers or chills.
The patient had been involved in a remote motor vehicle accident complicated by splenic laceration, for which he underwent splenectomy. He was appropriately immunized. The patient also suffered from bipolar affective disorder and untreated chronic hepatitis C infection with no evidence of cirrhosis. He smoked one pack of tobacco per day for the last 10 years and reported distant alcohol and methamphetamine use.
Right-flank pain can arise from conditions affecting the lower thorax (effusion, pneumonia, pulmonary embolism), abdomen (hepatobiliary or intestinal disease), retroperitoneum (hemorrhage or infection), musculoskeletal system, peripheral nerves (herpes zoster), or the genitourinary system (pyelonephritis). Pain radiating to the groin, discolored urine (suggesting hematuria), and history of kidney stones increase the likelihood of renal colic from nephrolithiasis.
Less commonly, flank pain and hematuria may present as initial symptoms of renal cell carcinoma, renal infarction, or aortic dissection. The patient’s immunosuppression from asplenia and active injection drug use could predispose him to septic emboli to his kidneys. Prior trauma causing aortic injury could predispose himto subsequent dissection.
The patient appeared well with a heart rate of 100 beats per minute, blood pressure 122/76 mmHg, temperature 36.8°C, respiratory rate 16 breaths per minute, and oxygen saturation 96% on room air. His cardiopulmonary and abdominal examinations were normal, and he had no costovertebral angle tenderness. His skin was warm and dry without rashes. His white blood cell (WBC) count was 26,000/μL; absolute neutrophil count was 22,000/μL. Serum chemistries were normal, including creatinine 0.63 mg/dL, calcium 8.8 mg/dL, and phosphorus 3.1 mg/dL. Lactate was 0.8 mmol/L (reference range: 0-2.0 mmol/L). Urinalysis revealed large ketones, >50 red blood cells (RBC) per high power field (HPF), <5 WBC per HPF, 1+ calcium oxalate crystals and pH 6.0. A bedside ultrasound showed mild right hydronephrosis. Computed tomography (CT) with intravenous contrast of his abdomen and pelvis demonstrated diffuse, mildly prominent subcentimeter mesenteric lymphadenopathy and no kidney stones. He was treated with intravenous fluids and pain control, and was discharged with a presumptive diagnosis of a passed kidney stone.
A passed stone would not explain this degree of leukocytosis. The CT results reduce the likelihood of a renal neoplasm, renal infarction, or pyelonephritis. Mesenteric lymphadenopathy is nonspecific, but it may signal underlying infection or malignancy with spread to lymph nodes, or it may be part of a systemic disorder causing generalized lymphadenopathy. Malignant causes of mesenteric lymphadenopathy (with no apparent primary tumor) include testicular cancer, lymphoma, and primary urogenital neoplasms.
The lower extremity nodules are consistent with erythema nodosum, which may be observed in numerous infectious and noninfectious illnesses. The rapid tempo of this febrile illness mandates early consideration of infection. Splenectomized patients are at risk for overwhelming post-splenectomy infection from encapsulated organisms, although this risk is significantly mitigated with appropriate immunization. The patient is at risk of bacterial endocarditis, which could explain his fevers and polyarthritis, although plaques, pustules, and oral ulcers would be unusual. Disseminated gonococcal infection causes fevers, oral lesions, polyarthritis and pustular skin lesions, but plaques are uncommon. Disseminated mycobacterial and fungal infections may cause oral ulcers, but affected patients tend to be severely ill and have profound immunosuppression. Secondary syphilis may account for many of the findings; however, oral ulcers would be unusual, and the rash tends to be more widespread, with a predilection for the palms and soles. Human immunodeficiency virus (HIV) can cause oral ulcers and is the chief viral etiology to consider.
Noninfectious illnesses to consider include neoplasms and connective tissue diseases. Malignancy would be unlikely to manifest this abruptly or produce a paraneoplastic disorder with these features.
The patient described severe fatigue and drenching night sweats for two months prior to admission. He denied dyspnea or cough. He was born in the southwestern United States and had lived in California for almost a decade. He had been incarcerated for a few years and released three years prior. He had intermittently lived in homeless shelters, but currently lived alone in downtown San Francisco. He had traveled remotely to the Caribbean, and more recently traveled frequently to the Central Valley in California. The patient formerly worked as a pipe-fitter and welder. He denied animal exposure or recent sick contacts. He was sexually active with women, and intermittently used barrier protection.
His years in the southwestern United States may have exposed the patient to blastomycosis or histoplasmosis; both can mimic mycobacterial disease. Blastomycosis demonstrates a slightly stronger predilection for spreading to the bones, genitourinary tract, and central nervous system, whereas histoplasmosis is a more frequent cause of polyarthrtitis and mesenteric adenopathy. The patient’s travel to the Central Valley, California raises the possibility of coccidioidomycosis, which typically starts with pulmonary disease prior to dissemination to bones, skin, and other less common sites. Pipe-fitters are predisposed to asbestos-related illnesses, including lung cancer and mesothelioma, which would not explain this patient’s presentation. Incarceration and high-risk sexual practices increase his risk for tuberculosis, HIV, and syphilis. Widespread skin involvement is more characteristic of syphilis or primary HIV infection than of disseminated fungal or mycobacterial infection.
WBC measured 29,000/uL with a neutrophilic predominance. His peripheral blood smear was unremarkable. A comprehensive metabolic panel was normal. Lactate dehydrogenase (LDH) was 317 U/L (reference range 140-280 U/L). Erythrocyte sedimentation rate (ESR) was 39 mm/hr (reference range < 20 mm/hr) and C-reactive protein (CRP) was 66 mg/L (reference range <6.3 mg/L). Blood, urine, and throat cultures were sent. Chest radiograph showed clear lungs without adenopathy. Ankle and knee radiographs identified small effusions bilaterally without bony abnormalities. CT of his brain showed a small, hypodense lesion in the right lacrimal gland. A lumbar puncture with cerebrospinal fluid (CSF) analysis showed absence of RBCs; WBC, 2/µL; protein, 35 mg/dL; glucose, 62 mg/dL; negative gram stain. CSF bacterial and fungal cultures, venereal disease research laboratory (VDRL), herpes simplex virus polymerase chain reaction (HSV PCR), and cryptococcal antigen were sent for laboratory analysis. The patient was started on vancomycin and aztreonam.
Lesions of the lacrimal gland feature multiple causes, including autoimmune diseases (Sjögren’s, Behçet’s disease), granulomatous diseases (sarcoidosis, granulomatosis with polyangiitis), neoplasms (salivary gland tumors, lymphoma), and infections. Initiating broad-spectrum antibiotics is reasonable while awaiting additional information from blood and urine cultures, serologies for HIV and syphilis, and purified protein derivative or interferon-gamma release assay (IGRA).
If these tests fail to reveal a diagnosis, the search for atypical infections and noninfectious possibilities should expand.
The patient continued to have intermittent fevers, sweats, and malaise over the next 3 days. All bacterial and fungal cultures remained negative, and antibiotics were discontinued. Rheumatoid factor, anticyclic citrullinated peptide, antinuclear antibody, and cryoglobulins were negative. Serum C3, C4, and angiotensin-converting enzyme (ACE) levels were normal. A rapid plasma reagin (RPR), HIV antibody, IGRA, and serum antibodies for Coccidioides, histoplasmosis, and West Nile virus were negative. Urine nucleic acid amplification testing for gonorrhea and chlamydia was negative. CSF VDRL, HSV PCR and cryptococcal antigen were negative. HSV culture from an oral ulcer showed no growth. The patient had a reactive hepatitis C antibody with a viral load of 3 million virus equivalents/mL.
The additional test results lower
The most likely diagnosis is Löfgren’s syndrome, a variant of sarcoidosis characterized by erythema nodosum, bilateral hilar lymphadenopathy, and polyarthralgias or polyarthritis. Löfgren’s syndrome may include fevers, uveitis, widespread skin lesions and other systemic manifestations. Sarcoidosis could explain the lacrimal gland lesion, and could manifest with recurrent kidney stones. Oral lesions may occur in sarcoidosis. A normal serum ACE level may be observed in up to half of patients. The lack of visualized granulomas on the submental node FNA may reflect sampling error, lower likelihood of visualizing granulomas on FNA (compared with excisional biopsy), or biopsy location (hilar nodes are more likely to demonstrate sarcoid granulomas).
Although Löfgren’s syndrome is often self-limited, treatment can ameliorate symptoms. Nonsteroidal anti-inflammatory medication can be tried first, with prednisone reserved for refractory cases.
The constellation of bilateral hilar adenopathy, arthritis, and erythema nodosum was consistent with Löfgren’s syndrome, further supported by granulomatous infiltrates on biopsy. The patient’s symptoms resolved with naproxen. He was scheduled for follow-up in dermatology and rheumatology clinics and was referred to hepatology for management of hepatitis C.
COMMENTARY
Sarcoidosis is a multisystem granulomatous disease of unclear etiology. The disease derives its name from Boeck’s 1899 report describing benign cutaneous lesions that resembled sarcomas.1 Sarcoidosis most commonly manifests as bilateral hilar adenopathy and pulmonary infiltrates, but may impact any tissue or organ, including the eyes, nonhilar lymph nodes, liver, spleen, joints, mucous membranes, and skin. Nephrolithiasis may result from hypercalcemia and/or hypercalciuria (related to granulomatous production of 1,25 vitamin D) and can be the presenting feature of sarcoidosis.2 Less common presentations include neurologic sarcoidosis (which can present with seizures, aseptic meningitis, encephalopathy, neuroendocrine dysfunction, myelopathy and peripheral neuropathies), cardiac sarcoidosis (which may present with arrhythmias, valvular dysfunction, heart failure, ischemia, or pericardial disease), and Heerfordt syndrome (the constellation of parotid gland enlargement, facial palsy, anterior uveitis, and fever). Sarcoidosis may mimic other diseases, including malignancy, idiopathic pulmonary fibrosis, and infiltrative tuberculosis.3 Sarcoidosis-like reactions have occurred in response to malignancy and medications.4
The patient’s rash demonstrated a predilection for areas of prior scarring, which has a limited differential diagnosis. Keloids and hypertrophic scars occur at sites of former surgical wounds, lacerations, or areas of inflammation. Pruritic urticarial papules and plaques of pregnancy (PUPPP) is a benign inflammatory condition where papules cluster in areas of prior striae. Cutaneous lesions of Behçet’s syndrome display pathergy, where pustular response is observed at sites of injury. Granulomatous infiltration in sarcoidosis may demonstrate a predilection for scars and tattoos (ie, scar or tattoo sarcoidosis).5 Sarcoidosis can have other cutaneous manifestations, including psoriaform, ulcerative, or erythrodermic lesions; subcutaneous nodules; scarring or nonscarring alopecia; and lupus pernio – violaceous, nodular and plaque-like lesions on the nose, earlobes, cheeks, and digits.5
Löfgren’s syndrome is a distinct variant of sarcoidosis.In 1952, Dr. Löfgren described a case series of patients with bilateral hilar lymphadenopathy and coexisting erythema nodosum and polyarthralgia.6 The epidemiology favors young women.7 Patients with Löfgren’s syndrome present acutely (as in this case), which differs from the typical subacute course observed with sarcoidosis. In addition to the classic presentation described above, patients with Löfgren’s syndrome may demonstrate additional manifestations of sarcoidosis, including fevers, peripheral adenopathy, arthritis, and granulomatous skin lesions. Painful symptoms may require short-term anti-inflammatory treatments. Most patients do not require systemic immunosuppression. Symptoms usually decrease over several months, and the majority of patients experience complete remission within years. Rare recurrences have been described up to several years.8
In confirming the diagnosis of sarcoidosis, current guidelines recommend exclusion of other diseases that present similarly, a work-up that generally includes compatible laboratory tests and imaging, and histologic demonstration of noncaseating granulomas.9 However, Löfgren’s syndrome is a notable exception. The constellation of fever, bilateral hilar adenopathy, polyarthralgia, and erythema nodosum suffices to diagnose Löfgren’s syndrome as long as the disease remits rapidly and spontaneously.9 Thus, in this case, although granulomatous infiltrates were confirmed on biopsy, the diagnosis of Löfgren’s syndrome could have been based on clinical and radiologic features alone.
KEY LEARNING POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly presents with bilateral hilar adenopathy and pulmonary infiltrates but can also present atypically, including with nephrolithiasis from hypercalcemia, neurologic syndromes, and cardiac involvement.
- Löfgren’s syndrome, a variant of sarcoidosis, is characterized by relatively acute onset of fevers, erythema nodosum, bilateral hilar adenopathy, and polyarthralgia or polyarthritis. Most patients recover and manifest complete remission.
- A limited differential exists for rashes with a predilection for areas of tattoos and prior scarring, including keloids, PUPPP, Behçet’s disease, and granulomatous infiltration.
Disclosure
There are no conflicts of interest or financial disclosures to report.
1. Multiple Benign Sarcoids of the Skin. JAMA. 1899;XXXIII(26):1620-1621.
2. Rizzato G, Fraioli P, Montemurro L. Nephrolithiasis as a presenting feature of chronic sarcoidosis. Thorax. 1995;50(5):555-559. PubMed
3. Romanov V. Atypical variants of clinical course of sarcoidosis. Eur Respir J. 2014;44(58):3782. PubMed
4. Arish N, Kuint R, Sapir E, et al. Characteristics of Sarcoidosis in Patients with Previous Malignancy: Causality or Coincidence? Respiration. 2017;93(4):247-252. PubMed
5. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25(3):295-302. PubMed
6. Löfgren S. The Bilateral Hilar Lymphoma Syndrome. Acta Med Scand. 1952;142(4):265-273. PubMed
7. Mañá J, Gómez-Vaquero C, Montero A et al. Löfgren’s syndrome revisited: a study of 186 patients. Am J Med. 1999;107(3):240-245. PubMed
8. Gran J, Bohmer E. Acute Sarcoid Arthritis: A Favourable Outcome? Scand J Rheumatol. 1996;25(2):70-73. PubMed
9. American Thoracic Society. Statement on Sarcoidosis. Am J Respir Crit Care Med. 1999;160:736-755.Otate voluptiatia qui aut iur, utendi quiae incipis m PubMed
A 43-year-old man with a history of asplenia, hepatitis C, and nephrolithiasis reported right-flank pain. He described severe, sharp pain that came in waves and radiated to the right groin, associated with nausea and nonbloody emesis. He noted “pink urine” but no dysuria. He had 4prior similar episodes during which he had passed kidney stones, although stone analysis had never been performed. He denied having fevers or chills.
The patient had been involved in a remote motor vehicle accident complicated by splenic laceration, for which he underwent splenectomy. He was appropriately immunized. The patient also suffered from bipolar affective disorder and untreated chronic hepatitis C infection with no evidence of cirrhosis. He smoked one pack of tobacco per day for the last 10 years and reported distant alcohol and methamphetamine use.
Right-flank pain can arise from conditions affecting the lower thorax (effusion, pneumonia, pulmonary embolism), abdomen (hepatobiliary or intestinal disease), retroperitoneum (hemorrhage or infection), musculoskeletal system, peripheral nerves (herpes zoster), or the genitourinary system (pyelonephritis). Pain radiating to the groin, discolored urine (suggesting hematuria), and history of kidney stones increase the likelihood of renal colic from nephrolithiasis.
Less commonly, flank pain and hematuria may present as initial symptoms of renal cell carcinoma, renal infarction, or aortic dissection. The patient’s immunosuppression from asplenia and active injection drug use could predispose him to septic emboli to his kidneys. Prior trauma causing aortic injury could predispose himto subsequent dissection.
The patient appeared well with a heart rate of 100 beats per minute, blood pressure 122/76 mmHg, temperature 36.8°C, respiratory rate 16 breaths per minute, and oxygen saturation 96% on room air. His cardiopulmonary and abdominal examinations were normal, and he had no costovertebral angle tenderness. His skin was warm and dry without rashes. His white blood cell (WBC) count was 26,000/μL; absolute neutrophil count was 22,000/μL. Serum chemistries were normal, including creatinine 0.63 mg/dL, calcium 8.8 mg/dL, and phosphorus 3.1 mg/dL. Lactate was 0.8 mmol/L (reference range: 0-2.0 mmol/L). Urinalysis revealed large ketones, >50 red blood cells (RBC) per high power field (HPF), <5 WBC per HPF, 1+ calcium oxalate crystals and pH 6.0. A bedside ultrasound showed mild right hydronephrosis. Computed tomography (CT) with intravenous contrast of his abdomen and pelvis demonstrated diffuse, mildly prominent subcentimeter mesenteric lymphadenopathy and no kidney stones. He was treated with intravenous fluids and pain control, and was discharged with a presumptive diagnosis of a passed kidney stone.
A passed stone would not explain this degree of leukocytosis. The CT results reduce the likelihood of a renal neoplasm, renal infarction, or pyelonephritis. Mesenteric lymphadenopathy is nonspecific, but it may signal underlying infection or malignancy with spread to lymph nodes, or it may be part of a systemic disorder causing generalized lymphadenopathy. Malignant causes of mesenteric lymphadenopathy (with no apparent primary tumor) include testicular cancer, lymphoma, and primary urogenital neoplasms.
The lower extremity nodules are consistent with erythema nodosum, which may be observed in numerous infectious and noninfectious illnesses. The rapid tempo of this febrile illness mandates early consideration of infection. Splenectomized patients are at risk for overwhelming post-splenectomy infection from encapsulated organisms, although this risk is significantly mitigated with appropriate immunization. The patient is at risk of bacterial endocarditis, which could explain his fevers and polyarthritis, although plaques, pustules, and oral ulcers would be unusual. Disseminated gonococcal infection causes fevers, oral lesions, polyarthritis and pustular skin lesions, but plaques are uncommon. Disseminated mycobacterial and fungal infections may cause oral ulcers, but affected patients tend to be severely ill and have profound immunosuppression. Secondary syphilis may account for many of the findings; however, oral ulcers would be unusual, and the rash tends to be more widespread, with a predilection for the palms and soles. Human immunodeficiency virus (HIV) can cause oral ulcers and is the chief viral etiology to consider.
Noninfectious illnesses to consider include neoplasms and connective tissue diseases. Malignancy would be unlikely to manifest this abruptly or produce a paraneoplastic disorder with these features.
The patient described severe fatigue and drenching night sweats for two months prior to admission. He denied dyspnea or cough. He was born in the southwestern United States and had lived in California for almost a decade. He had been incarcerated for a few years and released three years prior. He had intermittently lived in homeless shelters, but currently lived alone in downtown San Francisco. He had traveled remotely to the Caribbean, and more recently traveled frequently to the Central Valley in California. The patient formerly worked as a pipe-fitter and welder. He denied animal exposure or recent sick contacts. He was sexually active with women, and intermittently used barrier protection.
His years in the southwestern United States may have exposed the patient to blastomycosis or histoplasmosis; both can mimic mycobacterial disease. Blastomycosis demonstrates a slightly stronger predilection for spreading to the bones, genitourinary tract, and central nervous system, whereas histoplasmosis is a more frequent cause of polyarthrtitis and mesenteric adenopathy. The patient’s travel to the Central Valley, California raises the possibility of coccidioidomycosis, which typically starts with pulmonary disease prior to dissemination to bones, skin, and other less common sites. Pipe-fitters are predisposed to asbestos-related illnesses, including lung cancer and mesothelioma, which would not explain this patient’s presentation. Incarceration and high-risk sexual practices increase his risk for tuberculosis, HIV, and syphilis. Widespread skin involvement is more characteristic of syphilis or primary HIV infection than of disseminated fungal or mycobacterial infection.
WBC measured 29,000/uL with a neutrophilic predominance. His peripheral blood smear was unremarkable. A comprehensive metabolic panel was normal. Lactate dehydrogenase (LDH) was 317 U/L (reference range 140-280 U/L). Erythrocyte sedimentation rate (ESR) was 39 mm/hr (reference range < 20 mm/hr) and C-reactive protein (CRP) was 66 mg/L (reference range <6.3 mg/L). Blood, urine, and throat cultures were sent. Chest radiograph showed clear lungs without adenopathy. Ankle and knee radiographs identified small effusions bilaterally without bony abnormalities. CT of his brain showed a small, hypodense lesion in the right lacrimal gland. A lumbar puncture with cerebrospinal fluid (CSF) analysis showed absence of RBCs; WBC, 2/µL; protein, 35 mg/dL; glucose, 62 mg/dL; negative gram stain. CSF bacterial and fungal cultures, venereal disease research laboratory (VDRL), herpes simplex virus polymerase chain reaction (HSV PCR), and cryptococcal antigen were sent for laboratory analysis. The patient was started on vancomycin and aztreonam.
Lesions of the lacrimal gland feature multiple causes, including autoimmune diseases (Sjögren’s, Behçet’s disease), granulomatous diseases (sarcoidosis, granulomatosis with polyangiitis), neoplasms (salivary gland tumors, lymphoma), and infections. Initiating broad-spectrum antibiotics is reasonable while awaiting additional information from blood and urine cultures, serologies for HIV and syphilis, and purified protein derivative or interferon-gamma release assay (IGRA).
If these tests fail to reveal a diagnosis, the search for atypical infections and noninfectious possibilities should expand.
The patient continued to have intermittent fevers, sweats, and malaise over the next 3 days. All bacterial and fungal cultures remained negative, and antibiotics were discontinued. Rheumatoid factor, anticyclic citrullinated peptide, antinuclear antibody, and cryoglobulins were negative. Serum C3, C4, and angiotensin-converting enzyme (ACE) levels were normal. A rapid plasma reagin (RPR), HIV antibody, IGRA, and serum antibodies for Coccidioides, histoplasmosis, and West Nile virus were negative. Urine nucleic acid amplification testing for gonorrhea and chlamydia was negative. CSF VDRL, HSV PCR and cryptococcal antigen were negative. HSV culture from an oral ulcer showed no growth. The patient had a reactive hepatitis C antibody with a viral load of 3 million virus equivalents/mL.
The additional test results lower
The most likely diagnosis is Löfgren’s syndrome, a variant of sarcoidosis characterized by erythema nodosum, bilateral hilar lymphadenopathy, and polyarthralgias or polyarthritis. Löfgren’s syndrome may include fevers, uveitis, widespread skin lesions and other systemic manifestations. Sarcoidosis could explain the lacrimal gland lesion, and could manifest with recurrent kidney stones. Oral lesions may occur in sarcoidosis. A normal serum ACE level may be observed in up to half of patients. The lack of visualized granulomas on the submental node FNA may reflect sampling error, lower likelihood of visualizing granulomas on FNA (compared with excisional biopsy), or biopsy location (hilar nodes are more likely to demonstrate sarcoid granulomas).
Although Löfgren’s syndrome is often self-limited, treatment can ameliorate symptoms. Nonsteroidal anti-inflammatory medication can be tried first, with prednisone reserved for refractory cases.
The constellation of bilateral hilar adenopathy, arthritis, and erythema nodosum was consistent with Löfgren’s syndrome, further supported by granulomatous infiltrates on biopsy. The patient’s symptoms resolved with naproxen. He was scheduled for follow-up in dermatology and rheumatology clinics and was referred to hepatology for management of hepatitis C.
COMMENTARY
Sarcoidosis is a multisystem granulomatous disease of unclear etiology. The disease derives its name from Boeck’s 1899 report describing benign cutaneous lesions that resembled sarcomas.1 Sarcoidosis most commonly manifests as bilateral hilar adenopathy and pulmonary infiltrates, but may impact any tissue or organ, including the eyes, nonhilar lymph nodes, liver, spleen, joints, mucous membranes, and skin. Nephrolithiasis may result from hypercalcemia and/or hypercalciuria (related to granulomatous production of 1,25 vitamin D) and can be the presenting feature of sarcoidosis.2 Less common presentations include neurologic sarcoidosis (which can present with seizures, aseptic meningitis, encephalopathy, neuroendocrine dysfunction, myelopathy and peripheral neuropathies), cardiac sarcoidosis (which may present with arrhythmias, valvular dysfunction, heart failure, ischemia, or pericardial disease), and Heerfordt syndrome (the constellation of parotid gland enlargement, facial palsy, anterior uveitis, and fever). Sarcoidosis may mimic other diseases, including malignancy, idiopathic pulmonary fibrosis, and infiltrative tuberculosis.3 Sarcoidosis-like reactions have occurred in response to malignancy and medications.4
The patient’s rash demonstrated a predilection for areas of prior scarring, which has a limited differential diagnosis. Keloids and hypertrophic scars occur at sites of former surgical wounds, lacerations, or areas of inflammation. Pruritic urticarial papules and plaques of pregnancy (PUPPP) is a benign inflammatory condition where papules cluster in areas of prior striae. Cutaneous lesions of Behçet’s syndrome display pathergy, where pustular response is observed at sites of injury. Granulomatous infiltration in sarcoidosis may demonstrate a predilection for scars and tattoos (ie, scar or tattoo sarcoidosis).5 Sarcoidosis can have other cutaneous manifestations, including psoriaform, ulcerative, or erythrodermic lesions; subcutaneous nodules; scarring or nonscarring alopecia; and lupus pernio – violaceous, nodular and plaque-like lesions on the nose, earlobes, cheeks, and digits.5
Löfgren’s syndrome is a distinct variant of sarcoidosis.In 1952, Dr. Löfgren described a case series of patients with bilateral hilar lymphadenopathy and coexisting erythema nodosum and polyarthralgia.6 The epidemiology favors young women.7 Patients with Löfgren’s syndrome present acutely (as in this case), which differs from the typical subacute course observed with sarcoidosis. In addition to the classic presentation described above, patients with Löfgren’s syndrome may demonstrate additional manifestations of sarcoidosis, including fevers, peripheral adenopathy, arthritis, and granulomatous skin lesions. Painful symptoms may require short-term anti-inflammatory treatments. Most patients do not require systemic immunosuppression. Symptoms usually decrease over several months, and the majority of patients experience complete remission within years. Rare recurrences have been described up to several years.8
In confirming the diagnosis of sarcoidosis, current guidelines recommend exclusion of other diseases that present similarly, a work-up that generally includes compatible laboratory tests and imaging, and histologic demonstration of noncaseating granulomas.9 However, Löfgren’s syndrome is a notable exception. The constellation of fever, bilateral hilar adenopathy, polyarthralgia, and erythema nodosum suffices to diagnose Löfgren’s syndrome as long as the disease remits rapidly and spontaneously.9 Thus, in this case, although granulomatous infiltrates were confirmed on biopsy, the diagnosis of Löfgren’s syndrome could have been based on clinical and radiologic features alone.
KEY LEARNING POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly presents with bilateral hilar adenopathy and pulmonary infiltrates but can also present atypically, including with nephrolithiasis from hypercalcemia, neurologic syndromes, and cardiac involvement.
- Löfgren’s syndrome, a variant of sarcoidosis, is characterized by relatively acute onset of fevers, erythema nodosum, bilateral hilar adenopathy, and polyarthralgia or polyarthritis. Most patients recover and manifest complete remission.
- A limited differential exists for rashes with a predilection for areas of tattoos and prior scarring, including keloids, PUPPP, Behçet’s disease, and granulomatous infiltration.
Disclosure
There are no conflicts of interest or financial disclosures to report.
A 43-year-old man with a history of asplenia, hepatitis C, and nephrolithiasis reported right-flank pain. He described severe, sharp pain that came in waves and radiated to the right groin, associated with nausea and nonbloody emesis. He noted “pink urine” but no dysuria. He had 4prior similar episodes during which he had passed kidney stones, although stone analysis had never been performed. He denied having fevers or chills.
The patient had been involved in a remote motor vehicle accident complicated by splenic laceration, for which he underwent splenectomy. He was appropriately immunized. The patient also suffered from bipolar affective disorder and untreated chronic hepatitis C infection with no evidence of cirrhosis. He smoked one pack of tobacco per day for the last 10 years and reported distant alcohol and methamphetamine use.
Right-flank pain can arise from conditions affecting the lower thorax (effusion, pneumonia, pulmonary embolism), abdomen (hepatobiliary or intestinal disease), retroperitoneum (hemorrhage or infection), musculoskeletal system, peripheral nerves (herpes zoster), or the genitourinary system (pyelonephritis). Pain radiating to the groin, discolored urine (suggesting hematuria), and history of kidney stones increase the likelihood of renal colic from nephrolithiasis.
Less commonly, flank pain and hematuria may present as initial symptoms of renal cell carcinoma, renal infarction, or aortic dissection. The patient’s immunosuppression from asplenia and active injection drug use could predispose him to septic emboli to his kidneys. Prior trauma causing aortic injury could predispose himto subsequent dissection.
The patient appeared well with a heart rate of 100 beats per minute, blood pressure 122/76 mmHg, temperature 36.8°C, respiratory rate 16 breaths per minute, and oxygen saturation 96% on room air. His cardiopulmonary and abdominal examinations were normal, and he had no costovertebral angle tenderness. His skin was warm and dry without rashes. His white blood cell (WBC) count was 26,000/μL; absolute neutrophil count was 22,000/μL. Serum chemistries were normal, including creatinine 0.63 mg/dL, calcium 8.8 mg/dL, and phosphorus 3.1 mg/dL. Lactate was 0.8 mmol/L (reference range: 0-2.0 mmol/L). Urinalysis revealed large ketones, >50 red blood cells (RBC) per high power field (HPF), <5 WBC per HPF, 1+ calcium oxalate crystals and pH 6.0. A bedside ultrasound showed mild right hydronephrosis. Computed tomography (CT) with intravenous contrast of his abdomen and pelvis demonstrated diffuse, mildly prominent subcentimeter mesenteric lymphadenopathy and no kidney stones. He was treated with intravenous fluids and pain control, and was discharged with a presumptive diagnosis of a passed kidney stone.
A passed stone would not explain this degree of leukocytosis. The CT results reduce the likelihood of a renal neoplasm, renal infarction, or pyelonephritis. Mesenteric lymphadenopathy is nonspecific, but it may signal underlying infection or malignancy with spread to lymph nodes, or it may be part of a systemic disorder causing generalized lymphadenopathy. Malignant causes of mesenteric lymphadenopathy (with no apparent primary tumor) include testicular cancer, lymphoma, and primary urogenital neoplasms.
The lower extremity nodules are consistent with erythema nodosum, which may be observed in numerous infectious and noninfectious illnesses. The rapid tempo of this febrile illness mandates early consideration of infection. Splenectomized patients are at risk for overwhelming post-splenectomy infection from encapsulated organisms, although this risk is significantly mitigated with appropriate immunization. The patient is at risk of bacterial endocarditis, which could explain his fevers and polyarthritis, although plaques, pustules, and oral ulcers would be unusual. Disseminated gonococcal infection causes fevers, oral lesions, polyarthritis and pustular skin lesions, but plaques are uncommon. Disseminated mycobacterial and fungal infections may cause oral ulcers, but affected patients tend to be severely ill and have profound immunosuppression. Secondary syphilis may account for many of the findings; however, oral ulcers would be unusual, and the rash tends to be more widespread, with a predilection for the palms and soles. Human immunodeficiency virus (HIV) can cause oral ulcers and is the chief viral etiology to consider.
Noninfectious illnesses to consider include neoplasms and connective tissue diseases. Malignancy would be unlikely to manifest this abruptly or produce a paraneoplastic disorder with these features.
The patient described severe fatigue and drenching night sweats for two months prior to admission. He denied dyspnea or cough. He was born in the southwestern United States and had lived in California for almost a decade. He had been incarcerated for a few years and released three years prior. He had intermittently lived in homeless shelters, but currently lived alone in downtown San Francisco. He had traveled remotely to the Caribbean, and more recently traveled frequently to the Central Valley in California. The patient formerly worked as a pipe-fitter and welder. He denied animal exposure or recent sick contacts. He was sexually active with women, and intermittently used barrier protection.
His years in the southwestern United States may have exposed the patient to blastomycosis or histoplasmosis; both can mimic mycobacterial disease. Blastomycosis demonstrates a slightly stronger predilection for spreading to the bones, genitourinary tract, and central nervous system, whereas histoplasmosis is a more frequent cause of polyarthrtitis and mesenteric adenopathy. The patient’s travel to the Central Valley, California raises the possibility of coccidioidomycosis, which typically starts with pulmonary disease prior to dissemination to bones, skin, and other less common sites. Pipe-fitters are predisposed to asbestos-related illnesses, including lung cancer and mesothelioma, which would not explain this patient’s presentation. Incarceration and high-risk sexual practices increase his risk for tuberculosis, HIV, and syphilis. Widespread skin involvement is more characteristic of syphilis or primary HIV infection than of disseminated fungal or mycobacterial infection.
WBC measured 29,000/uL with a neutrophilic predominance. His peripheral blood smear was unremarkable. A comprehensive metabolic panel was normal. Lactate dehydrogenase (LDH) was 317 U/L (reference range 140-280 U/L). Erythrocyte sedimentation rate (ESR) was 39 mm/hr (reference range < 20 mm/hr) and C-reactive protein (CRP) was 66 mg/L (reference range <6.3 mg/L). Blood, urine, and throat cultures were sent. Chest radiograph showed clear lungs without adenopathy. Ankle and knee radiographs identified small effusions bilaterally without bony abnormalities. CT of his brain showed a small, hypodense lesion in the right lacrimal gland. A lumbar puncture with cerebrospinal fluid (CSF) analysis showed absence of RBCs; WBC, 2/µL; protein, 35 mg/dL; glucose, 62 mg/dL; negative gram stain. CSF bacterial and fungal cultures, venereal disease research laboratory (VDRL), herpes simplex virus polymerase chain reaction (HSV PCR), and cryptococcal antigen were sent for laboratory analysis. The patient was started on vancomycin and aztreonam.
Lesions of the lacrimal gland feature multiple causes, including autoimmune diseases (Sjögren’s, Behçet’s disease), granulomatous diseases (sarcoidosis, granulomatosis with polyangiitis), neoplasms (salivary gland tumors, lymphoma), and infections. Initiating broad-spectrum antibiotics is reasonable while awaiting additional information from blood and urine cultures, serologies for HIV and syphilis, and purified protein derivative or interferon-gamma release assay (IGRA).
If these tests fail to reveal a diagnosis, the search for atypical infections and noninfectious possibilities should expand.
The patient continued to have intermittent fevers, sweats, and malaise over the next 3 days. All bacterial and fungal cultures remained negative, and antibiotics were discontinued. Rheumatoid factor, anticyclic citrullinated peptide, antinuclear antibody, and cryoglobulins were negative. Serum C3, C4, and angiotensin-converting enzyme (ACE) levels were normal. A rapid plasma reagin (RPR), HIV antibody, IGRA, and serum antibodies for Coccidioides, histoplasmosis, and West Nile virus were negative. Urine nucleic acid amplification testing for gonorrhea and chlamydia was negative. CSF VDRL, HSV PCR and cryptococcal antigen were negative. HSV culture from an oral ulcer showed no growth. The patient had a reactive hepatitis C antibody with a viral load of 3 million virus equivalents/mL.
The additional test results lower
The most likely diagnosis is Löfgren’s syndrome, a variant of sarcoidosis characterized by erythema nodosum, bilateral hilar lymphadenopathy, and polyarthralgias or polyarthritis. Löfgren’s syndrome may include fevers, uveitis, widespread skin lesions and other systemic manifestations. Sarcoidosis could explain the lacrimal gland lesion, and could manifest with recurrent kidney stones. Oral lesions may occur in sarcoidosis. A normal serum ACE level may be observed in up to half of patients. The lack of visualized granulomas on the submental node FNA may reflect sampling error, lower likelihood of visualizing granulomas on FNA (compared with excisional biopsy), or biopsy location (hilar nodes are more likely to demonstrate sarcoid granulomas).
Although Löfgren’s syndrome is often self-limited, treatment can ameliorate symptoms. Nonsteroidal anti-inflammatory medication can be tried first, with prednisone reserved for refractory cases.
The constellation of bilateral hilar adenopathy, arthritis, and erythema nodosum was consistent with Löfgren’s syndrome, further supported by granulomatous infiltrates on biopsy. The patient’s symptoms resolved with naproxen. He was scheduled for follow-up in dermatology and rheumatology clinics and was referred to hepatology for management of hepatitis C.
COMMENTARY
Sarcoidosis is a multisystem granulomatous disease of unclear etiology. The disease derives its name from Boeck’s 1899 report describing benign cutaneous lesions that resembled sarcomas.1 Sarcoidosis most commonly manifests as bilateral hilar adenopathy and pulmonary infiltrates, but may impact any tissue or organ, including the eyes, nonhilar lymph nodes, liver, spleen, joints, mucous membranes, and skin. Nephrolithiasis may result from hypercalcemia and/or hypercalciuria (related to granulomatous production of 1,25 vitamin D) and can be the presenting feature of sarcoidosis.2 Less common presentations include neurologic sarcoidosis (which can present with seizures, aseptic meningitis, encephalopathy, neuroendocrine dysfunction, myelopathy and peripheral neuropathies), cardiac sarcoidosis (which may present with arrhythmias, valvular dysfunction, heart failure, ischemia, or pericardial disease), and Heerfordt syndrome (the constellation of parotid gland enlargement, facial palsy, anterior uveitis, and fever). Sarcoidosis may mimic other diseases, including malignancy, idiopathic pulmonary fibrosis, and infiltrative tuberculosis.3 Sarcoidosis-like reactions have occurred in response to malignancy and medications.4
The patient’s rash demonstrated a predilection for areas of prior scarring, which has a limited differential diagnosis. Keloids and hypertrophic scars occur at sites of former surgical wounds, lacerations, or areas of inflammation. Pruritic urticarial papules and plaques of pregnancy (PUPPP) is a benign inflammatory condition where papules cluster in areas of prior striae. Cutaneous lesions of Behçet’s syndrome display pathergy, where pustular response is observed at sites of injury. Granulomatous infiltration in sarcoidosis may demonstrate a predilection for scars and tattoos (ie, scar or tattoo sarcoidosis).5 Sarcoidosis can have other cutaneous manifestations, including psoriaform, ulcerative, or erythrodermic lesions; subcutaneous nodules; scarring or nonscarring alopecia; and lupus pernio – violaceous, nodular and plaque-like lesions on the nose, earlobes, cheeks, and digits.5
Löfgren’s syndrome is a distinct variant of sarcoidosis.In 1952, Dr. Löfgren described a case series of patients with bilateral hilar lymphadenopathy and coexisting erythema nodosum and polyarthralgia.6 The epidemiology favors young women.7 Patients with Löfgren’s syndrome present acutely (as in this case), which differs from the typical subacute course observed with sarcoidosis. In addition to the classic presentation described above, patients with Löfgren’s syndrome may demonstrate additional manifestations of sarcoidosis, including fevers, peripheral adenopathy, arthritis, and granulomatous skin lesions. Painful symptoms may require short-term anti-inflammatory treatments. Most patients do not require systemic immunosuppression. Symptoms usually decrease over several months, and the majority of patients experience complete remission within years. Rare recurrences have been described up to several years.8
In confirming the diagnosis of sarcoidosis, current guidelines recommend exclusion of other diseases that present similarly, a work-up that generally includes compatible laboratory tests and imaging, and histologic demonstration of noncaseating granulomas.9 However, Löfgren’s syndrome is a notable exception. The constellation of fever, bilateral hilar adenopathy, polyarthralgia, and erythema nodosum suffices to diagnose Löfgren’s syndrome as long as the disease remits rapidly and spontaneously.9 Thus, in this case, although granulomatous infiltrates were confirmed on biopsy, the diagnosis of Löfgren’s syndrome could have been based on clinical and radiologic features alone.
KEY LEARNING POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly presents with bilateral hilar adenopathy and pulmonary infiltrates but can also present atypically, including with nephrolithiasis from hypercalcemia, neurologic syndromes, and cardiac involvement.
- Löfgren’s syndrome, a variant of sarcoidosis, is characterized by relatively acute onset of fevers, erythema nodosum, bilateral hilar adenopathy, and polyarthralgia or polyarthritis. Most patients recover and manifest complete remission.
- A limited differential exists for rashes with a predilection for areas of tattoos and prior scarring, including keloids, PUPPP, Behçet’s disease, and granulomatous infiltration.
Disclosure
There are no conflicts of interest or financial disclosures to report.
1. Multiple Benign Sarcoids of the Skin. JAMA. 1899;XXXIII(26):1620-1621.
2. Rizzato G, Fraioli P, Montemurro L. Nephrolithiasis as a presenting feature of chronic sarcoidosis. Thorax. 1995;50(5):555-559. PubMed
3. Romanov V. Atypical variants of clinical course of sarcoidosis. Eur Respir J. 2014;44(58):3782. PubMed
4. Arish N, Kuint R, Sapir E, et al. Characteristics of Sarcoidosis in Patients with Previous Malignancy: Causality or Coincidence? Respiration. 2017;93(4):247-252. PubMed
5. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25(3):295-302. PubMed
6. Löfgren S. The Bilateral Hilar Lymphoma Syndrome. Acta Med Scand. 1952;142(4):265-273. PubMed
7. Mañá J, Gómez-Vaquero C, Montero A et al. Löfgren’s syndrome revisited: a study of 186 patients. Am J Med. 1999;107(3):240-245. PubMed
8. Gran J, Bohmer E. Acute Sarcoid Arthritis: A Favourable Outcome? Scand J Rheumatol. 1996;25(2):70-73. PubMed
9. American Thoracic Society. Statement on Sarcoidosis. Am J Respir Crit Care Med. 1999;160:736-755.Otate voluptiatia qui aut iur, utendi quiae incipis m PubMed
1. Multiple Benign Sarcoids of the Skin. JAMA. 1899;XXXIII(26):1620-1621.
2. Rizzato G, Fraioli P, Montemurro L. Nephrolithiasis as a presenting feature of chronic sarcoidosis. Thorax. 1995;50(5):555-559. PubMed
3. Romanov V. Atypical variants of clinical course of sarcoidosis. Eur Respir J. 2014;44(58):3782. PubMed
4. Arish N, Kuint R, Sapir E, et al. Characteristics of Sarcoidosis in Patients with Previous Malignancy: Causality or Coincidence? Respiration. 2017;93(4):247-252. PubMed
5. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25(3):295-302. PubMed
6. Löfgren S. The Bilateral Hilar Lymphoma Syndrome. Acta Med Scand. 1952;142(4):265-273. PubMed
7. Mañá J, Gómez-Vaquero C, Montero A et al. Löfgren’s syndrome revisited: a study of 186 patients. Am J Med. 1999;107(3):240-245. PubMed
8. Gran J, Bohmer E. Acute Sarcoid Arthritis: A Favourable Outcome? Scand J Rheumatol. 1996;25(2):70-73. PubMed
9. American Thoracic Society. Statement on Sarcoidosis. Am J Respir Crit Care Med. 1999;160:736-755.Otate voluptiatia qui aut iur, utendi quiae incipis m PubMed
© 2018 Society of Hospital Medicine
What’s the Purpose of Rounds? A Qualitative Study Examining the Perceptions of Faculty and Students
For more than a century, medical rounds have been a cornerstone of patient care and medical education in teaching hospitals. They remain critical activities for exposing generations of trainees to clinical decision making, coordination of care, and patient communication.1
Despite this established importance within medical education and patient care, there is a relative paucity of research addressing the purpose of medical rounds in the 21st century. Medicine has evolved significantly since Osler’s day, and it is unclear whether the purpose of rounds has evolved along with it. Rounds, to Osler, were an important opportunity for future physicians to learn at the bedside from an attending physician. Increased duty hour restrictions, mandatory adoption of electronic medical records, and increasingly complex care have changed how rounds are performed, making it more difficult to achieve Osler’s ideals.2,3 While several studies have aimed to quantify the changes to rounds and have demonstrated a significant decline in bedside teaching,4-6 few studies have explored the purpose of rounds from the perspective of pertinent stakeholders, students, residents, and faculty. The authors have published the results of focus groups of resident stakeholders recently.7 We made the decision to combine the student/faculty data and describe it separately from the resident data to allow the most accurate and relevant discussion as it pertained to each group.
The aim of this study was to explore the perceptions of faculty and students of general inpatient rounds on internal medicine and pediatric rotations, and to identify any notable differences between these key stakeholders.
METHODS
Between April 2014 and June 2014, we conducted 10 semistructured focus groups at 4 teaching hospitals: The University of Chicago Medical Center, Children’s National Health System, Georgetown University Medical Center, and the University of California, San Francisco Medical Center. A sample of eligible 3rd-year medical students and residents on pediatrics and internal medicine hospitalist services as well as hospitalist attendings in pediatrics and internal medicine were invited by e-mail to participate voluntarily without compensation. Identical semistructured focus groups were also conducted with pediatric and internal medicine interns (postgraduate year [PGY1]) and senior residents (PGY2 and PGY3), and those data have been published previously.7
Data Collection
Most focus groups had 6 to 8 participants, with 2 groups of 3 and 4. The groups were interviewed separately by training and specialty: 3rd-year medical students who had completed internal medicine and/or pediatrics rotations, hospitalist attendings in pediatrics, and hospitalist attendings in internal medicine. Attendings with training in medicine-pediatrics were included in the department in which they worked most frequently. The focus group script was informed by a literature review and expert input, and we used open-ended questions to explore perspectives on current and ideal purposes of rounds. Interviews were digitally recorded, transcribed, and names of speakers or references to specific patients were removed to preserve confidentiality and anonymity. The focus groups lasted between 30 and 60 minutes. The author (OH) conducted focus groups at 1 site, and trained facilitators conducted focus groups at the remaining 3 sites. The protocol was determined to be exempt by the institutional review boards at all participating sites. Prior to the focus groups, the definition of family-centered rounds was read aloud; after which, participants were asked to fill out a demographic survey.
Data Analysis
The authors employed a grounded theory approach to data collection and analysis,8 and data were analyzed by using the constant-comparative method.9 There was no a priori hypothesis. Four transcripts were independently reviewed by 2 authors (OH and RR) by using sentences and phrases as the units of data, which were coded with an identifier. The authors discussed initial codes and resolved discrepancies through deliberation and consensus to create codebooks. Themes, made up of multiple codes, were identified inductively and iteratively and were refined to reflect the evolving dataset. One author (OH) independently coded the remaining transcripts by using a revised codebook as a guide. A faculty author (JF) assessed the interrater reliability of the final codebook by reviewing 2 previously coded, randomly selected transcripts with no new codes emerging in the process, with a kappa coefficient of >0.8 indicating significant agreement.
RESULTS
What Do You Perceive the Purpose of Rounds to Be?
With respect to this prompt, we identified 4 themes, which represent 16 codes describing what attendings and medical students believed to be the purpose of rounds (Table 2). These themes are communication, medical education, patient care, and assessment.
Communication
Communication includes all comments addressing the role of rounds as it relates to communication between team members, patients, family members, and all those involved in patient care. There were 4 main codes, including coordination of patient care team, patient/family communication, establishing rapport with patients and/or family, and establishment of roles.
Coordination of patient care team identified rounds as a time “to make sure everyone is on the same page” and “to come together whenever possible,” so that everyone “had the same information of what was going on.” It also included comments related to interdisciplinary communication, with 1 participant describing rounds as “a time when your consulting team, or people with outside expertise, can weigh in on some medical issues.”
Medical Education
The theme of medical education is made up of 6 codes that encompass comments related to teaching and learning during rounds. These 6 codes include delivery of clinical education, exposure to clinical decision making, role modeling, student presentations, establishment of trainee autonomy, and providing a safe learning environment.
Delivery of clinical education included comments identifying rounds as a time for didactic teaching, teachable moments, “clinical pearls,” and bedside teaching of physical exam skills. Exposure to clinical decision making included comments by both medical students and attendings who described the purpose of rounds as a time for learning and teaching, specifically about how best to approach problems and decision making in a systematic manner, with 1 medical student explaining it as a time to “expose [trainees] to the way that people think about problems and how they decided to go about addressing them.”
Role modeling includes comments addressing rounds as a time for attendings to demonstrate appropriate behaviors and skills to trainees. One attending explained that “everybody learns from watching other people present and interact…so everybody has a chance to pick up things that they think, ‘Oh, this works well.’” Student presentations include comments, predominantly from students, that described rounds as an opportunity to practice presentations and receive feedback, with 1 student explaining it was a time “to learn how to present but also to be questioned and challenged.”
Establishing trainee autonomy is a code that identifies rounds as a time to encourage resident and student autonomy in order to achieve rounds that function with minimal input from the attending, with 1 attending describing how they “put resident leadership first as far as priorities… [and] fostering that because I usually let them decide what we’re going to do.”
Providing a safe learning environment identifies the purpose of rounds as being a space in which trainees can feel comfortable learning from their mistakes. One student described rounds as, “…a setting where it’s okay to be wrong and feel comfortable enough to know that it’s about a learning process.”
Assessment
Assessment is a theme composed of comments identifying the purpose of rounds as being related to observation, assessment, and feedback, and it includes 2 codes: attending observation, assessment, and feedback and establishment of expectations. Attending observation, assessment, and feedback includes comments from attendings and students alike who described rounds as a place for observation, evaluation, and provision of feedback regarding the skills and abilities of trainees. One attending explained that rounds gave him an “opportunity to observe trainees interacting with each other, with the patient, the patient’s family, and ancillary staff,” with another commenting it was time used “to assess how med students are gathering information, presenting information, and eventually their assessment and plan.” Establishment of expectations captures comments that describe rounds as a time for the establishment of expectations and goals of the team.
Patient Care
Patient care is a theme comprised of comments identifying the purpose of rounds as being directly related to the formation and delivery of the patient care plan, and it includes 2 codes: formation of the patient care plan and delivery of patient care. Formation of the patient care plan includes comments, which identified rounds as a time for discussing and forming the plan for the day, with an attending stating, “The purpose [of rounds] was to make a plan, a treatment plan, and to include the parents in making the treatment plan.” Delivery of patient care included comments identifying rounds as a means of ensuring timely, safe, and appropriate delivery of patient care occurred. One attending explained, “It can’t be undersold that the priority of rounds is patient care and the more eyes that look over information the less likely there are to be mistakes.”
What Do You Believe the Ideal Purpose of RoundsShould Be?
This study originally sought to compare responses to 2 different questions: “What do you perceive the purpose of rounds to be?” and “What do you believe the ideal purpose of rounds should be?” What became clear during the focus groups was that these were often interpreted to be the same question, and as such, responses to the latter question were truncated or were reiterations of what was previously said: “I think we’ve already discussed that, I think it’s no different than what we already kind of said, patient care, education, and communication,” explained 1 attending. Fifty-four responses to the question regarding the ideal purpose of rounds were coded and did not differ significantly from the previously noted results in terms of the domains represented and the frequency of representation.
Variation Among Respondents
Overall, there is a high level of concordance between the comments from medical students and attendings regarding the purpose of rounds, particularly in the medical education theme. However, medicine and pediatric attendings differ in their comments relating to the theme of communication, with 2 codes primarily accounting for this difference: pediatric attendings place more emphasis on time for patient/family communication and establishing rapport with patients than their internal medicine colleagues. Of note, all of the pediatric attendings involved in the study answered that they conducted family-centered rounds (FCR), compared with 22% of internal medicine attendings.10
Another notable discrepancy came up during focus groups involving comments from medical students who reiterated that the purpose of rounds was not fixed, but rather dependent on the attending that was running rounds. This theme was only identified in focus groups involving medical students. One student explained, “I think that it depends on the attending and if they actually want to teach,” and another commented that “it’s incredibly dependent on what the attending… is willing to invest.” No attendings identified student or attending variability as an important factor influencing the purpose of rounds.
DISCUSSION
This qualitative study is one of the first to explore the purpose of rounds from the perspective of both medical students and attendings. Reassuringly, our results indicate that medical student and attending perceptions are largely concordant. The 4 themes of communication, medical education, assessment, and patient care are in line with the findings of previous observational studies of internal medicine and pediatrics rounds.1,11 The themes are similar to the findings of resident focus groups done at these same sites.7
Our results support that both medical students and attendings identify the importance of medical education during rounds. This is in contrast with findings in previous observational time-motion research by Stickrath that describes the focus on patient care related activities and the relative scarcity of education during rounds.1 This stresses a divide between how medical students and attendings define the purpose of rounds and what other research suggests actually occurs on rounds. This distinction is an important one. It is possible that the way we, and others, define “medical education” and “patient care” may be at least partially responsible for these findings. This is supported by the ambiguous distinction between formal and informal educational activities on rounds and the challenges in characterizing the hidden curriculum and its role in medical student and resident education.11 Attendings role modeling effective patient communication strategies, for example, highlights that patient care, medical education, and communication are frequently indistinguishable.12 This hybridization of activities and dedication to diverse types of learning is an essential quality of rounds and is suggestive of why they have survived as a preeminent tool within the arsenal of medical education for the past century.
Yet, this finding does not excuse or adequately explain a well-documented disappearance of more formal educational activities during rounds. Recent observational studies have shown that the percentage of rounds dedicated to educational activities fell from 25% to 10% after the implementation of duty hour restrictions,1,13,14 and a recent ethnographic study of pediatric attending rounds confirmed teaching during rounds, though seen as a pedagogical ideal, occurred infrequently and inconsistently in large part because of time pressures.15 In our attending focus groups, duty hours and time pressures were frequently cited as actively working against the purpose of rounds, specifically opportunities for teaching, with 1 attending explaining, “I just don’t think we achieve our [teaching] goals like we used to.” Another attending mentioned that, because of time pressures, “I often find myself apologizing. ‘I’m so sorry. I can’t resist. Can I just tell you this one thing? I’m so sorry to do teaching.’” This tension between time pressures and education on rounds is well documented in the literature.4,16,17
Our results highlight that attendings and medical students still believe that medical education is a primary and important purpose of rounds even in the face of increasing time pressures. As such, efforts should be made to better align the many purposes of rounds with the realities of the modern day rounding environment. Increasing the presence of medical education on rounds need not be at the expense of time given that techniques like the 1-minute preceptor have been rated as both efficient and effective methods of teaching and delivering feedback.18 This is echoed in research that has found that faculty development with a focus on teaching significantly increased the rate of clinical education and interdisciplinary communication during rounds.1 Opportunities for faculty development are increasingly accessible,19 including programs like the Advancing Pediatric Excellence Teaching Program, sponsored by the American Academy of Pediatrics Section on Hospital Medicine and the Academic Pediatric Association, and the Teaching Educators Across the Continuum of Healthcare program, sponsored by the Society for General Internal Medicine.20,21
A testament to the adaptability of rounds can be seen in our findings that expose the increased emphasis with which pediatric attendings identify communication as a purpose of rounds, particularly within the themes of patient/family communication and establishing rapport with patients. This is likely due to the practice of FCR by 100% of the pediatric attendings in our focus groups, and is supported elsewhere in the literature.22 A key to family-centered rounds is communication, with active participation in the care discussion by patients and families as described and endorsed by a 2012 American Academy of Pediatrics (AAP) policy.10,23
This emphasis could explain the increased frequency of comments made by pediatric attendings within the themes of patient/family communication and establishing rapport with patients. Furthermore, the AAP policy statement stresses the need to share information in a way that patients and families “effectively participate in care and decision making,” which could explain why pediatric attendings placed greater emphasis on the formation of the patient care plan in the theme of patient care.
As noted, the authors published a related study focusing on resident perceptions regarding the purpose of rounds. We initially undertook a separate analysis of the 3 groups: faculty, residents, and medical students. From that analysis, it became apparent that residents (PGY1-PGY3) viewed rounds differently than faculty and medical students. Where faculty and medical students were more focused on communication and medical education, the residents were more focused on the practical aspects of rounds (eg, “getting work done”). It was also noted that the residents’ focus aligned with the graduate medical education
Our study has a number of limitations. Only 4 university-based hospitals were included in the focus groups. This has the potential to limit the generalizability to the community hospital setting. Within the focus groups, the number of participants varied, and this may have had an impact on the flow and content of conversation. Facilitators were chosen to minimize potential bias and prior relationships with participants; however, this was not always possible, and as such, may have influenced responses. There may be a discrepancy between how people perceive rounds and how rounds actually function. Rounds were not standardized between institutions, departments, or attendings.
CONCLUSION
Rounds are an appropriate metaphor for medical education at large: they are time consuming, complex, and vary in quality, but are nevertheless essential to the goals of patients and learners alike because of their adaptability and hybridization of purpose. Our results highlight that rounds serve 4 critical purposes, including communication, medical education, patient care, and assessment. Importantly, both attendings and students agree on what they perceive to be the many purposes of rounds. Despite this agreement, a disconnect appears to exist between what people believe are the purposes of rounds and what is perceived to be happening during rounds. The causes of this gap are not well defined, and further efforts should be made to better understand the obstacles facing effective rounding. To improve rounds and adapt them to the needs of 21st century learners, it is critical that we better define the scope of medical education, both formal and informal, that occurs during rounds. In doing so, it will be possible to identify areas of development and training for faculty, residents, and medical students, which will ensure that rounds remain useful and critical tools for the development and education of future physicians.
Acknowledgments
The authors would like to acknowledge the following people who assisted on this project: Meghan Daly from The University of Chicago Pritzker School of Medicine, Shannon Martin, MD, MS, Assistant Professor of Medicine from the Department of Medicine at The University of Chicago, Joyce Campbell, BSN, MS, Senior Quality Manager at the Children’s National Medical Center, Benjamin Colburn from the University of California, San Francisco School of Medicine, Kelly Sanders from the University of California, San Francisco School of Medicine, and Alekist Quach from the University of California, San Francisco School of Medicine.
Disclosure
The authors report no external funding source for this study. The authors declare no conflict of interest. The protocol was approved by the institutional review board at all participating institutions.
1. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. doi:10.1001/jamainternmed.2013.6041 PubMed
2. Osler SW. Osler’s “A Way of Life” and Other Addresses, with Commentary and Annotations. Durham: Duke University Press; 2001.
3. Peters M, Ten Cate O. Bedside teaching in medical education: a literature review. Perspect Med Educ. 2014;3(2):76-88. doi:10.1007/s40037-013-0083-y PubMed
4. Gonzalo JD, Heist BS, Duffy BL, et al. Identifying and Overcoming the Barriers to Bedside Rounds: A Multicenter Qualitative Study. Acad Med. 2014;89(2):326-334. doi:10.1097/ACM.0000000000000100 PubMed
5. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending Rounds and Bedside Case Presentations: Medical Student and Medicine Resident Experiences and Attitudes. Teach Learn Med. 2009;21(2):105-110. doi:10.1080/10401330902791156 PubMed
6. Payson HE, Barchas JD. A Time Study of Medical Teaching Rounds. N Engl J Med. 1965;273(27):1468-1471. doi:10.1056/NEJM196512302732706 PubMed
7. Rabinowitz R, Farnan J, Hulland O, et al. Rounds Today: A Qualitative Study of Internal Medicine and Pediatrics Resident Perceptions. J Grad Med Educ. 2016;8(4):523-531. doi:10.4300/JGME-D-15-00106.1 PubMed
8. Charmaz K. Constructing Grounded Theory: A Practical Guide through Qualitative Analysis. London: Sage Publications; 2006. PubMed
9. Starks H, Trinidad SB. Choose Your Method: A Comparison of Phenomenology, Discourse Analysis, and Grounded Theory. Qual Health Res. 2007;17(10):1372-1380. doi:10.1177/1049732307307031 PubMed
10. Sisterhen LL, Blaszak RT, Woods MB, Smith CE. Defining Family-Centered Rounds. Teach Learn Med. 2007;19(3):319-322. doi:10.1080/10401330701366812 PubMed
11. Witman Y. What do we transfer in case discussions? The hidden curriculum in medicine…. Perspect Med Educ. 2014;3(2):113-123. doi:10.1007/s40037-013-0101-0 PubMed
12. Benbassat J. Role Modeling in Medical Education: The Importance of a Reflective Imitation. Acad Med. 2014;89(4):550-554. doi:10.1097/ACM.0000000000000189 PubMed
13. Miller M, Johnson B, Greene DHL, Baier M, Nowlin S. An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646-648. doi:10.1007/BF02599208 PubMed
14. Priest JR, Bereknyei S, Hooper K, Braddock CH III. Relationships of the Location and Content of Rounds to Specialty, Institution, Patient-Census, and Team Size. PLoS One. 2010;5(6):e11246. doi:10.1371/journal.pone.0011246 PubMed
15. Balmer DF, Master CL, Richards BF, Serwint JR, Giardino AP. An ethnographic study of attending rounds in general paediatrics: understanding the ritual. Med Educ. 2010;44(11):1105-1116. doi:10.1111/j.1365-2923.2010.03767.x PubMed
16. Bhansali P, Birch S, Campbell JK, et al. A Time-Motion Study of Inpatient Rounds Using a Family-Centered Rounds Model. Hosp Pediatr. 2013;3(1):31-38. doi:10.1542/hpeds.2012-0021 PubMed
17. Reed DA, Levine RB, Miller RG, et al. Impact of Duty Hour Regulations on Medical Students’ Education: Views of Key Clinical Faculty. J Gen Intern Med. 2008;23(7):1084-1089. doi:10.1007/s11606-008-0532-1 PubMed
18. Aagaard E, Teherani A, Irby DM. Effectiveness of the One-Minute Preceptor Model for Diagnosing the Patient and the Learner: Proof of Concept. Acad Med Spec Theme Teach Clin Ski. 2004;79(1):42-49. PubMed
19. Swanwick T. See one, do one, then what? Faculty development in postgraduate medical education. Postgrad Med J. 2008;84(993):339-343. doi:10.1136/pgmj.2008.068288 PubMed
20. Advancing Pediatric Educator Excellence (APEX) Teaching Program. The American Academy of Pediatrics. https://www.aap.org/en-us/about-the-aap/Committees-Councils-Sections/Section-on-Hospital-Medicine/Pages/Advancing-Pediatric-Educator-Excellence.aspx?nfstatus=401&nftoken=00000000-0000-0000-0000-000000000000&nfstatusdescription=ERROR:+No+local+token. Accessed August 22, 2016.
21. TEACH: Teaching Educators Across the Continuum of Healthcare. Society of General Internal Medicine. http://www.sgim.org/communities/education/sgim-teach-program. Accessed August 22, 2016.
22. Mittal V, Krieger E, Lee BC, et al. Pediatrics Residents’ Perspectives on Family-Centered Rounds: A Qualitative Study at 2 Children’s Hospitals. J Grad Med Educ. 2013;5(1):81-87. doi:10.4300/JGME-D-11-00314.1 PubMed
23. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and Family-Centered Care and the Pediatrician’s Role. Pediatrics. 2012;129(2):394-404. doi:10.1542/peds.2011-3084 PubMed
For more than a century, medical rounds have been a cornerstone of patient care and medical education in teaching hospitals. They remain critical activities for exposing generations of trainees to clinical decision making, coordination of care, and patient communication.1
Despite this established importance within medical education and patient care, there is a relative paucity of research addressing the purpose of medical rounds in the 21st century. Medicine has evolved significantly since Osler’s day, and it is unclear whether the purpose of rounds has evolved along with it. Rounds, to Osler, were an important opportunity for future physicians to learn at the bedside from an attending physician. Increased duty hour restrictions, mandatory adoption of electronic medical records, and increasingly complex care have changed how rounds are performed, making it more difficult to achieve Osler’s ideals.2,3 While several studies have aimed to quantify the changes to rounds and have demonstrated a significant decline in bedside teaching,4-6 few studies have explored the purpose of rounds from the perspective of pertinent stakeholders, students, residents, and faculty. The authors have published the results of focus groups of resident stakeholders recently.7 We made the decision to combine the student/faculty data and describe it separately from the resident data to allow the most accurate and relevant discussion as it pertained to each group.
The aim of this study was to explore the perceptions of faculty and students of general inpatient rounds on internal medicine and pediatric rotations, and to identify any notable differences between these key stakeholders.
METHODS
Between April 2014 and June 2014, we conducted 10 semistructured focus groups at 4 teaching hospitals: The University of Chicago Medical Center, Children’s National Health System, Georgetown University Medical Center, and the University of California, San Francisco Medical Center. A sample of eligible 3rd-year medical students and residents on pediatrics and internal medicine hospitalist services as well as hospitalist attendings in pediatrics and internal medicine were invited by e-mail to participate voluntarily without compensation. Identical semistructured focus groups were also conducted with pediatric and internal medicine interns (postgraduate year [PGY1]) and senior residents (PGY2 and PGY3), and those data have been published previously.7
Data Collection
Most focus groups had 6 to 8 participants, with 2 groups of 3 and 4. The groups were interviewed separately by training and specialty: 3rd-year medical students who had completed internal medicine and/or pediatrics rotations, hospitalist attendings in pediatrics, and hospitalist attendings in internal medicine. Attendings with training in medicine-pediatrics were included in the department in which they worked most frequently. The focus group script was informed by a literature review and expert input, and we used open-ended questions to explore perspectives on current and ideal purposes of rounds. Interviews were digitally recorded, transcribed, and names of speakers or references to specific patients were removed to preserve confidentiality and anonymity. The focus groups lasted between 30 and 60 minutes. The author (OH) conducted focus groups at 1 site, and trained facilitators conducted focus groups at the remaining 3 sites. The protocol was determined to be exempt by the institutional review boards at all participating sites. Prior to the focus groups, the definition of family-centered rounds was read aloud; after which, participants were asked to fill out a demographic survey.
Data Analysis
The authors employed a grounded theory approach to data collection and analysis,8 and data were analyzed by using the constant-comparative method.9 There was no a priori hypothesis. Four transcripts were independently reviewed by 2 authors (OH and RR) by using sentences and phrases as the units of data, which were coded with an identifier. The authors discussed initial codes and resolved discrepancies through deliberation and consensus to create codebooks. Themes, made up of multiple codes, were identified inductively and iteratively and were refined to reflect the evolving dataset. One author (OH) independently coded the remaining transcripts by using a revised codebook as a guide. A faculty author (JF) assessed the interrater reliability of the final codebook by reviewing 2 previously coded, randomly selected transcripts with no new codes emerging in the process, with a kappa coefficient of >0.8 indicating significant agreement.
RESULTS
What Do You Perceive the Purpose of Rounds to Be?
With respect to this prompt, we identified 4 themes, which represent 16 codes describing what attendings and medical students believed to be the purpose of rounds (Table 2). These themes are communication, medical education, patient care, and assessment.
Communication
Communication includes all comments addressing the role of rounds as it relates to communication between team members, patients, family members, and all those involved in patient care. There were 4 main codes, including coordination of patient care team, patient/family communication, establishing rapport with patients and/or family, and establishment of roles.
Coordination of patient care team identified rounds as a time “to make sure everyone is on the same page” and “to come together whenever possible,” so that everyone “had the same information of what was going on.” It also included comments related to interdisciplinary communication, with 1 participant describing rounds as “a time when your consulting team, or people with outside expertise, can weigh in on some medical issues.”
Medical Education
The theme of medical education is made up of 6 codes that encompass comments related to teaching and learning during rounds. These 6 codes include delivery of clinical education, exposure to clinical decision making, role modeling, student presentations, establishment of trainee autonomy, and providing a safe learning environment.
Delivery of clinical education included comments identifying rounds as a time for didactic teaching, teachable moments, “clinical pearls,” and bedside teaching of physical exam skills. Exposure to clinical decision making included comments by both medical students and attendings who described the purpose of rounds as a time for learning and teaching, specifically about how best to approach problems and decision making in a systematic manner, with 1 medical student explaining it as a time to “expose [trainees] to the way that people think about problems and how they decided to go about addressing them.”
Role modeling includes comments addressing rounds as a time for attendings to demonstrate appropriate behaviors and skills to trainees. One attending explained that “everybody learns from watching other people present and interact…so everybody has a chance to pick up things that they think, ‘Oh, this works well.’” Student presentations include comments, predominantly from students, that described rounds as an opportunity to practice presentations and receive feedback, with 1 student explaining it was a time “to learn how to present but also to be questioned and challenged.”
Establishing trainee autonomy is a code that identifies rounds as a time to encourage resident and student autonomy in order to achieve rounds that function with minimal input from the attending, with 1 attending describing how they “put resident leadership first as far as priorities… [and] fostering that because I usually let them decide what we’re going to do.”
Providing a safe learning environment identifies the purpose of rounds as being a space in which trainees can feel comfortable learning from their mistakes. One student described rounds as, “…a setting where it’s okay to be wrong and feel comfortable enough to know that it’s about a learning process.”
Assessment
Assessment is a theme composed of comments identifying the purpose of rounds as being related to observation, assessment, and feedback, and it includes 2 codes: attending observation, assessment, and feedback and establishment of expectations. Attending observation, assessment, and feedback includes comments from attendings and students alike who described rounds as a place for observation, evaluation, and provision of feedback regarding the skills and abilities of trainees. One attending explained that rounds gave him an “opportunity to observe trainees interacting with each other, with the patient, the patient’s family, and ancillary staff,” with another commenting it was time used “to assess how med students are gathering information, presenting information, and eventually their assessment and plan.” Establishment of expectations captures comments that describe rounds as a time for the establishment of expectations and goals of the team.
Patient Care
Patient care is a theme comprised of comments identifying the purpose of rounds as being directly related to the formation and delivery of the patient care plan, and it includes 2 codes: formation of the patient care plan and delivery of patient care. Formation of the patient care plan includes comments, which identified rounds as a time for discussing and forming the plan for the day, with an attending stating, “The purpose [of rounds] was to make a plan, a treatment plan, and to include the parents in making the treatment plan.” Delivery of patient care included comments identifying rounds as a means of ensuring timely, safe, and appropriate delivery of patient care occurred. One attending explained, “It can’t be undersold that the priority of rounds is patient care and the more eyes that look over information the less likely there are to be mistakes.”
What Do You Believe the Ideal Purpose of RoundsShould Be?
This study originally sought to compare responses to 2 different questions: “What do you perceive the purpose of rounds to be?” and “What do you believe the ideal purpose of rounds should be?” What became clear during the focus groups was that these were often interpreted to be the same question, and as such, responses to the latter question were truncated or were reiterations of what was previously said: “I think we’ve already discussed that, I think it’s no different than what we already kind of said, patient care, education, and communication,” explained 1 attending. Fifty-four responses to the question regarding the ideal purpose of rounds were coded and did not differ significantly from the previously noted results in terms of the domains represented and the frequency of representation.
Variation Among Respondents
Overall, there is a high level of concordance between the comments from medical students and attendings regarding the purpose of rounds, particularly in the medical education theme. However, medicine and pediatric attendings differ in their comments relating to the theme of communication, with 2 codes primarily accounting for this difference: pediatric attendings place more emphasis on time for patient/family communication and establishing rapport with patients than their internal medicine colleagues. Of note, all of the pediatric attendings involved in the study answered that they conducted family-centered rounds (FCR), compared with 22% of internal medicine attendings.10
Another notable discrepancy came up during focus groups involving comments from medical students who reiterated that the purpose of rounds was not fixed, but rather dependent on the attending that was running rounds. This theme was only identified in focus groups involving medical students. One student explained, “I think that it depends on the attending and if they actually want to teach,” and another commented that “it’s incredibly dependent on what the attending… is willing to invest.” No attendings identified student or attending variability as an important factor influencing the purpose of rounds.
DISCUSSION
This qualitative study is one of the first to explore the purpose of rounds from the perspective of both medical students and attendings. Reassuringly, our results indicate that medical student and attending perceptions are largely concordant. The 4 themes of communication, medical education, assessment, and patient care are in line with the findings of previous observational studies of internal medicine and pediatrics rounds.1,11 The themes are similar to the findings of resident focus groups done at these same sites.7
Our results support that both medical students and attendings identify the importance of medical education during rounds. This is in contrast with findings in previous observational time-motion research by Stickrath that describes the focus on patient care related activities and the relative scarcity of education during rounds.1 This stresses a divide between how medical students and attendings define the purpose of rounds and what other research suggests actually occurs on rounds. This distinction is an important one. It is possible that the way we, and others, define “medical education” and “patient care” may be at least partially responsible for these findings. This is supported by the ambiguous distinction between formal and informal educational activities on rounds and the challenges in characterizing the hidden curriculum and its role in medical student and resident education.11 Attendings role modeling effective patient communication strategies, for example, highlights that patient care, medical education, and communication are frequently indistinguishable.12 This hybridization of activities and dedication to diverse types of learning is an essential quality of rounds and is suggestive of why they have survived as a preeminent tool within the arsenal of medical education for the past century.
Yet, this finding does not excuse or adequately explain a well-documented disappearance of more formal educational activities during rounds. Recent observational studies have shown that the percentage of rounds dedicated to educational activities fell from 25% to 10% after the implementation of duty hour restrictions,1,13,14 and a recent ethnographic study of pediatric attending rounds confirmed teaching during rounds, though seen as a pedagogical ideal, occurred infrequently and inconsistently in large part because of time pressures.15 In our attending focus groups, duty hours and time pressures were frequently cited as actively working against the purpose of rounds, specifically opportunities for teaching, with 1 attending explaining, “I just don’t think we achieve our [teaching] goals like we used to.” Another attending mentioned that, because of time pressures, “I often find myself apologizing. ‘I’m so sorry. I can’t resist. Can I just tell you this one thing? I’m so sorry to do teaching.’” This tension between time pressures and education on rounds is well documented in the literature.4,16,17
Our results highlight that attendings and medical students still believe that medical education is a primary and important purpose of rounds even in the face of increasing time pressures. As such, efforts should be made to better align the many purposes of rounds with the realities of the modern day rounding environment. Increasing the presence of medical education on rounds need not be at the expense of time given that techniques like the 1-minute preceptor have been rated as both efficient and effective methods of teaching and delivering feedback.18 This is echoed in research that has found that faculty development with a focus on teaching significantly increased the rate of clinical education and interdisciplinary communication during rounds.1 Opportunities for faculty development are increasingly accessible,19 including programs like the Advancing Pediatric Excellence Teaching Program, sponsored by the American Academy of Pediatrics Section on Hospital Medicine and the Academic Pediatric Association, and the Teaching Educators Across the Continuum of Healthcare program, sponsored by the Society for General Internal Medicine.20,21
A testament to the adaptability of rounds can be seen in our findings that expose the increased emphasis with which pediatric attendings identify communication as a purpose of rounds, particularly within the themes of patient/family communication and establishing rapport with patients. This is likely due to the practice of FCR by 100% of the pediatric attendings in our focus groups, and is supported elsewhere in the literature.22 A key to family-centered rounds is communication, with active participation in the care discussion by patients and families as described and endorsed by a 2012 American Academy of Pediatrics (AAP) policy.10,23
This emphasis could explain the increased frequency of comments made by pediatric attendings within the themes of patient/family communication and establishing rapport with patients. Furthermore, the AAP policy statement stresses the need to share information in a way that patients and families “effectively participate in care and decision making,” which could explain why pediatric attendings placed greater emphasis on the formation of the patient care plan in the theme of patient care.
As noted, the authors published a related study focusing on resident perceptions regarding the purpose of rounds. We initially undertook a separate analysis of the 3 groups: faculty, residents, and medical students. From that analysis, it became apparent that residents (PGY1-PGY3) viewed rounds differently than faculty and medical students. Where faculty and medical students were more focused on communication and medical education, the residents were more focused on the practical aspects of rounds (eg, “getting work done”). It was also noted that the residents’ focus aligned with the graduate medical education
Our study has a number of limitations. Only 4 university-based hospitals were included in the focus groups. This has the potential to limit the generalizability to the community hospital setting. Within the focus groups, the number of participants varied, and this may have had an impact on the flow and content of conversation. Facilitators were chosen to minimize potential bias and prior relationships with participants; however, this was not always possible, and as such, may have influenced responses. There may be a discrepancy between how people perceive rounds and how rounds actually function. Rounds were not standardized between institutions, departments, or attendings.
CONCLUSION
Rounds are an appropriate metaphor for medical education at large: they are time consuming, complex, and vary in quality, but are nevertheless essential to the goals of patients and learners alike because of their adaptability and hybridization of purpose. Our results highlight that rounds serve 4 critical purposes, including communication, medical education, patient care, and assessment. Importantly, both attendings and students agree on what they perceive to be the many purposes of rounds. Despite this agreement, a disconnect appears to exist between what people believe are the purposes of rounds and what is perceived to be happening during rounds. The causes of this gap are not well defined, and further efforts should be made to better understand the obstacles facing effective rounding. To improve rounds and adapt them to the needs of 21st century learners, it is critical that we better define the scope of medical education, both formal and informal, that occurs during rounds. In doing so, it will be possible to identify areas of development and training for faculty, residents, and medical students, which will ensure that rounds remain useful and critical tools for the development and education of future physicians.
Acknowledgments
The authors would like to acknowledge the following people who assisted on this project: Meghan Daly from The University of Chicago Pritzker School of Medicine, Shannon Martin, MD, MS, Assistant Professor of Medicine from the Department of Medicine at The University of Chicago, Joyce Campbell, BSN, MS, Senior Quality Manager at the Children’s National Medical Center, Benjamin Colburn from the University of California, San Francisco School of Medicine, Kelly Sanders from the University of California, San Francisco School of Medicine, and Alekist Quach from the University of California, San Francisco School of Medicine.
Disclosure
The authors report no external funding source for this study. The authors declare no conflict of interest. The protocol was approved by the institutional review board at all participating institutions.
For more than a century, medical rounds have been a cornerstone of patient care and medical education in teaching hospitals. They remain critical activities for exposing generations of trainees to clinical decision making, coordination of care, and patient communication.1
Despite this established importance within medical education and patient care, there is a relative paucity of research addressing the purpose of medical rounds in the 21st century. Medicine has evolved significantly since Osler’s day, and it is unclear whether the purpose of rounds has evolved along with it. Rounds, to Osler, were an important opportunity for future physicians to learn at the bedside from an attending physician. Increased duty hour restrictions, mandatory adoption of electronic medical records, and increasingly complex care have changed how rounds are performed, making it more difficult to achieve Osler’s ideals.2,3 While several studies have aimed to quantify the changes to rounds and have demonstrated a significant decline in bedside teaching,4-6 few studies have explored the purpose of rounds from the perspective of pertinent stakeholders, students, residents, and faculty. The authors have published the results of focus groups of resident stakeholders recently.7 We made the decision to combine the student/faculty data and describe it separately from the resident data to allow the most accurate and relevant discussion as it pertained to each group.
The aim of this study was to explore the perceptions of faculty and students of general inpatient rounds on internal medicine and pediatric rotations, and to identify any notable differences between these key stakeholders.
METHODS
Between April 2014 and June 2014, we conducted 10 semistructured focus groups at 4 teaching hospitals: The University of Chicago Medical Center, Children’s National Health System, Georgetown University Medical Center, and the University of California, San Francisco Medical Center. A sample of eligible 3rd-year medical students and residents on pediatrics and internal medicine hospitalist services as well as hospitalist attendings in pediatrics and internal medicine were invited by e-mail to participate voluntarily without compensation. Identical semistructured focus groups were also conducted with pediatric and internal medicine interns (postgraduate year [PGY1]) and senior residents (PGY2 and PGY3), and those data have been published previously.7
Data Collection
Most focus groups had 6 to 8 participants, with 2 groups of 3 and 4. The groups were interviewed separately by training and specialty: 3rd-year medical students who had completed internal medicine and/or pediatrics rotations, hospitalist attendings in pediatrics, and hospitalist attendings in internal medicine. Attendings with training in medicine-pediatrics were included in the department in which they worked most frequently. The focus group script was informed by a literature review and expert input, and we used open-ended questions to explore perspectives on current and ideal purposes of rounds. Interviews were digitally recorded, transcribed, and names of speakers or references to specific patients were removed to preserve confidentiality and anonymity. The focus groups lasted between 30 and 60 minutes. The author (OH) conducted focus groups at 1 site, and trained facilitators conducted focus groups at the remaining 3 sites. The protocol was determined to be exempt by the institutional review boards at all participating sites. Prior to the focus groups, the definition of family-centered rounds was read aloud; after which, participants were asked to fill out a demographic survey.
Data Analysis
The authors employed a grounded theory approach to data collection and analysis,8 and data were analyzed by using the constant-comparative method.9 There was no a priori hypothesis. Four transcripts were independently reviewed by 2 authors (OH and RR) by using sentences and phrases as the units of data, which were coded with an identifier. The authors discussed initial codes and resolved discrepancies through deliberation and consensus to create codebooks. Themes, made up of multiple codes, were identified inductively and iteratively and were refined to reflect the evolving dataset. One author (OH) independently coded the remaining transcripts by using a revised codebook as a guide. A faculty author (JF) assessed the interrater reliability of the final codebook by reviewing 2 previously coded, randomly selected transcripts with no new codes emerging in the process, with a kappa coefficient of >0.8 indicating significant agreement.
RESULTS
What Do You Perceive the Purpose of Rounds to Be?
With respect to this prompt, we identified 4 themes, which represent 16 codes describing what attendings and medical students believed to be the purpose of rounds (Table 2). These themes are communication, medical education, patient care, and assessment.
Communication
Communication includes all comments addressing the role of rounds as it relates to communication between team members, patients, family members, and all those involved in patient care. There were 4 main codes, including coordination of patient care team, patient/family communication, establishing rapport with patients and/or family, and establishment of roles.
Coordination of patient care team identified rounds as a time “to make sure everyone is on the same page” and “to come together whenever possible,” so that everyone “had the same information of what was going on.” It also included comments related to interdisciplinary communication, with 1 participant describing rounds as “a time when your consulting team, or people with outside expertise, can weigh in on some medical issues.”
Medical Education
The theme of medical education is made up of 6 codes that encompass comments related to teaching and learning during rounds. These 6 codes include delivery of clinical education, exposure to clinical decision making, role modeling, student presentations, establishment of trainee autonomy, and providing a safe learning environment.
Delivery of clinical education included comments identifying rounds as a time for didactic teaching, teachable moments, “clinical pearls,” and bedside teaching of physical exam skills. Exposure to clinical decision making included comments by both medical students and attendings who described the purpose of rounds as a time for learning and teaching, specifically about how best to approach problems and decision making in a systematic manner, with 1 medical student explaining it as a time to “expose [trainees] to the way that people think about problems and how they decided to go about addressing them.”
Role modeling includes comments addressing rounds as a time for attendings to demonstrate appropriate behaviors and skills to trainees. One attending explained that “everybody learns from watching other people present and interact…so everybody has a chance to pick up things that they think, ‘Oh, this works well.’” Student presentations include comments, predominantly from students, that described rounds as an opportunity to practice presentations and receive feedback, with 1 student explaining it was a time “to learn how to present but also to be questioned and challenged.”
Establishing trainee autonomy is a code that identifies rounds as a time to encourage resident and student autonomy in order to achieve rounds that function with minimal input from the attending, with 1 attending describing how they “put resident leadership first as far as priorities… [and] fostering that because I usually let them decide what we’re going to do.”
Providing a safe learning environment identifies the purpose of rounds as being a space in which trainees can feel comfortable learning from their mistakes. One student described rounds as, “…a setting where it’s okay to be wrong and feel comfortable enough to know that it’s about a learning process.”
Assessment
Assessment is a theme composed of comments identifying the purpose of rounds as being related to observation, assessment, and feedback, and it includes 2 codes: attending observation, assessment, and feedback and establishment of expectations. Attending observation, assessment, and feedback includes comments from attendings and students alike who described rounds as a place for observation, evaluation, and provision of feedback regarding the skills and abilities of trainees. One attending explained that rounds gave him an “opportunity to observe trainees interacting with each other, with the patient, the patient’s family, and ancillary staff,” with another commenting it was time used “to assess how med students are gathering information, presenting information, and eventually their assessment and plan.” Establishment of expectations captures comments that describe rounds as a time for the establishment of expectations and goals of the team.
Patient Care
Patient care is a theme comprised of comments identifying the purpose of rounds as being directly related to the formation and delivery of the patient care plan, and it includes 2 codes: formation of the patient care plan and delivery of patient care. Formation of the patient care plan includes comments, which identified rounds as a time for discussing and forming the plan for the day, with an attending stating, “The purpose [of rounds] was to make a plan, a treatment plan, and to include the parents in making the treatment plan.” Delivery of patient care included comments identifying rounds as a means of ensuring timely, safe, and appropriate delivery of patient care occurred. One attending explained, “It can’t be undersold that the priority of rounds is patient care and the more eyes that look over information the less likely there are to be mistakes.”
What Do You Believe the Ideal Purpose of RoundsShould Be?
This study originally sought to compare responses to 2 different questions: “What do you perceive the purpose of rounds to be?” and “What do you believe the ideal purpose of rounds should be?” What became clear during the focus groups was that these were often interpreted to be the same question, and as such, responses to the latter question were truncated or were reiterations of what was previously said: “I think we’ve already discussed that, I think it’s no different than what we already kind of said, patient care, education, and communication,” explained 1 attending. Fifty-four responses to the question regarding the ideal purpose of rounds were coded and did not differ significantly from the previously noted results in terms of the domains represented and the frequency of representation.
Variation Among Respondents
Overall, there is a high level of concordance between the comments from medical students and attendings regarding the purpose of rounds, particularly in the medical education theme. However, medicine and pediatric attendings differ in their comments relating to the theme of communication, with 2 codes primarily accounting for this difference: pediatric attendings place more emphasis on time for patient/family communication and establishing rapport with patients than their internal medicine colleagues. Of note, all of the pediatric attendings involved in the study answered that they conducted family-centered rounds (FCR), compared with 22% of internal medicine attendings.10
Another notable discrepancy came up during focus groups involving comments from medical students who reiterated that the purpose of rounds was not fixed, but rather dependent on the attending that was running rounds. This theme was only identified in focus groups involving medical students. One student explained, “I think that it depends on the attending and if they actually want to teach,” and another commented that “it’s incredibly dependent on what the attending… is willing to invest.” No attendings identified student or attending variability as an important factor influencing the purpose of rounds.
DISCUSSION
This qualitative study is one of the first to explore the purpose of rounds from the perspective of both medical students and attendings. Reassuringly, our results indicate that medical student and attending perceptions are largely concordant. The 4 themes of communication, medical education, assessment, and patient care are in line with the findings of previous observational studies of internal medicine and pediatrics rounds.1,11 The themes are similar to the findings of resident focus groups done at these same sites.7
Our results support that both medical students and attendings identify the importance of medical education during rounds. This is in contrast with findings in previous observational time-motion research by Stickrath that describes the focus on patient care related activities and the relative scarcity of education during rounds.1 This stresses a divide between how medical students and attendings define the purpose of rounds and what other research suggests actually occurs on rounds. This distinction is an important one. It is possible that the way we, and others, define “medical education” and “patient care” may be at least partially responsible for these findings. This is supported by the ambiguous distinction between formal and informal educational activities on rounds and the challenges in characterizing the hidden curriculum and its role in medical student and resident education.11 Attendings role modeling effective patient communication strategies, for example, highlights that patient care, medical education, and communication are frequently indistinguishable.12 This hybridization of activities and dedication to diverse types of learning is an essential quality of rounds and is suggestive of why they have survived as a preeminent tool within the arsenal of medical education for the past century.
Yet, this finding does not excuse or adequately explain a well-documented disappearance of more formal educational activities during rounds. Recent observational studies have shown that the percentage of rounds dedicated to educational activities fell from 25% to 10% after the implementation of duty hour restrictions,1,13,14 and a recent ethnographic study of pediatric attending rounds confirmed teaching during rounds, though seen as a pedagogical ideal, occurred infrequently and inconsistently in large part because of time pressures.15 In our attending focus groups, duty hours and time pressures were frequently cited as actively working against the purpose of rounds, specifically opportunities for teaching, with 1 attending explaining, “I just don’t think we achieve our [teaching] goals like we used to.” Another attending mentioned that, because of time pressures, “I often find myself apologizing. ‘I’m so sorry. I can’t resist. Can I just tell you this one thing? I’m so sorry to do teaching.’” This tension between time pressures and education on rounds is well documented in the literature.4,16,17
Our results highlight that attendings and medical students still believe that medical education is a primary and important purpose of rounds even in the face of increasing time pressures. As such, efforts should be made to better align the many purposes of rounds with the realities of the modern day rounding environment. Increasing the presence of medical education on rounds need not be at the expense of time given that techniques like the 1-minute preceptor have been rated as both efficient and effective methods of teaching and delivering feedback.18 This is echoed in research that has found that faculty development with a focus on teaching significantly increased the rate of clinical education and interdisciplinary communication during rounds.1 Opportunities for faculty development are increasingly accessible,19 including programs like the Advancing Pediatric Excellence Teaching Program, sponsored by the American Academy of Pediatrics Section on Hospital Medicine and the Academic Pediatric Association, and the Teaching Educators Across the Continuum of Healthcare program, sponsored by the Society for General Internal Medicine.20,21
A testament to the adaptability of rounds can be seen in our findings that expose the increased emphasis with which pediatric attendings identify communication as a purpose of rounds, particularly within the themes of patient/family communication and establishing rapport with patients. This is likely due to the practice of FCR by 100% of the pediatric attendings in our focus groups, and is supported elsewhere in the literature.22 A key to family-centered rounds is communication, with active participation in the care discussion by patients and families as described and endorsed by a 2012 American Academy of Pediatrics (AAP) policy.10,23
This emphasis could explain the increased frequency of comments made by pediatric attendings within the themes of patient/family communication and establishing rapport with patients. Furthermore, the AAP policy statement stresses the need to share information in a way that patients and families “effectively participate in care and decision making,” which could explain why pediatric attendings placed greater emphasis on the formation of the patient care plan in the theme of patient care.
As noted, the authors published a related study focusing on resident perceptions regarding the purpose of rounds. We initially undertook a separate analysis of the 3 groups: faculty, residents, and medical students. From that analysis, it became apparent that residents (PGY1-PGY3) viewed rounds differently than faculty and medical students. Where faculty and medical students were more focused on communication and medical education, the residents were more focused on the practical aspects of rounds (eg, “getting work done”). It was also noted that the residents’ focus aligned with the graduate medical education
Our study has a number of limitations. Only 4 university-based hospitals were included in the focus groups. This has the potential to limit the generalizability to the community hospital setting. Within the focus groups, the number of participants varied, and this may have had an impact on the flow and content of conversation. Facilitators were chosen to minimize potential bias and prior relationships with participants; however, this was not always possible, and as such, may have influenced responses. There may be a discrepancy between how people perceive rounds and how rounds actually function. Rounds were not standardized between institutions, departments, or attendings.
CONCLUSION
Rounds are an appropriate metaphor for medical education at large: they are time consuming, complex, and vary in quality, but are nevertheless essential to the goals of patients and learners alike because of their adaptability and hybridization of purpose. Our results highlight that rounds serve 4 critical purposes, including communication, medical education, patient care, and assessment. Importantly, both attendings and students agree on what they perceive to be the many purposes of rounds. Despite this agreement, a disconnect appears to exist between what people believe are the purposes of rounds and what is perceived to be happening during rounds. The causes of this gap are not well defined, and further efforts should be made to better understand the obstacles facing effective rounding. To improve rounds and adapt them to the needs of 21st century learners, it is critical that we better define the scope of medical education, both formal and informal, that occurs during rounds. In doing so, it will be possible to identify areas of development and training for faculty, residents, and medical students, which will ensure that rounds remain useful and critical tools for the development and education of future physicians.
Acknowledgments
The authors would like to acknowledge the following people who assisted on this project: Meghan Daly from The University of Chicago Pritzker School of Medicine, Shannon Martin, MD, MS, Assistant Professor of Medicine from the Department of Medicine at The University of Chicago, Joyce Campbell, BSN, MS, Senior Quality Manager at the Children’s National Medical Center, Benjamin Colburn from the University of California, San Francisco School of Medicine, Kelly Sanders from the University of California, San Francisco School of Medicine, and Alekist Quach from the University of California, San Francisco School of Medicine.
Disclosure
The authors report no external funding source for this study. The authors declare no conflict of interest. The protocol was approved by the institutional review board at all participating institutions.
1. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. doi:10.1001/jamainternmed.2013.6041 PubMed
2. Osler SW. Osler’s “A Way of Life” and Other Addresses, with Commentary and Annotations. Durham: Duke University Press; 2001.
3. Peters M, Ten Cate O. Bedside teaching in medical education: a literature review. Perspect Med Educ. 2014;3(2):76-88. doi:10.1007/s40037-013-0083-y PubMed
4. Gonzalo JD, Heist BS, Duffy BL, et al. Identifying and Overcoming the Barriers to Bedside Rounds: A Multicenter Qualitative Study. Acad Med. 2014;89(2):326-334. doi:10.1097/ACM.0000000000000100 PubMed
5. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending Rounds and Bedside Case Presentations: Medical Student and Medicine Resident Experiences and Attitudes. Teach Learn Med. 2009;21(2):105-110. doi:10.1080/10401330902791156 PubMed
6. Payson HE, Barchas JD. A Time Study of Medical Teaching Rounds. N Engl J Med. 1965;273(27):1468-1471. doi:10.1056/NEJM196512302732706 PubMed
7. Rabinowitz R, Farnan J, Hulland O, et al. Rounds Today: A Qualitative Study of Internal Medicine and Pediatrics Resident Perceptions. J Grad Med Educ. 2016;8(4):523-531. doi:10.4300/JGME-D-15-00106.1 PubMed
8. Charmaz K. Constructing Grounded Theory: A Practical Guide through Qualitative Analysis. London: Sage Publications; 2006. PubMed
9. Starks H, Trinidad SB. Choose Your Method: A Comparison of Phenomenology, Discourse Analysis, and Grounded Theory. Qual Health Res. 2007;17(10):1372-1380. doi:10.1177/1049732307307031 PubMed
10. Sisterhen LL, Blaszak RT, Woods MB, Smith CE. Defining Family-Centered Rounds. Teach Learn Med. 2007;19(3):319-322. doi:10.1080/10401330701366812 PubMed
11. Witman Y. What do we transfer in case discussions? The hidden curriculum in medicine…. Perspect Med Educ. 2014;3(2):113-123. doi:10.1007/s40037-013-0101-0 PubMed
12. Benbassat J. Role Modeling in Medical Education: The Importance of a Reflective Imitation. Acad Med. 2014;89(4):550-554. doi:10.1097/ACM.0000000000000189 PubMed
13. Miller M, Johnson B, Greene DHL, Baier M, Nowlin S. An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646-648. doi:10.1007/BF02599208 PubMed
14. Priest JR, Bereknyei S, Hooper K, Braddock CH III. Relationships of the Location and Content of Rounds to Specialty, Institution, Patient-Census, and Team Size. PLoS One. 2010;5(6):e11246. doi:10.1371/journal.pone.0011246 PubMed
15. Balmer DF, Master CL, Richards BF, Serwint JR, Giardino AP. An ethnographic study of attending rounds in general paediatrics: understanding the ritual. Med Educ. 2010;44(11):1105-1116. doi:10.1111/j.1365-2923.2010.03767.x PubMed
16. Bhansali P, Birch S, Campbell JK, et al. A Time-Motion Study of Inpatient Rounds Using a Family-Centered Rounds Model. Hosp Pediatr. 2013;3(1):31-38. doi:10.1542/hpeds.2012-0021 PubMed
17. Reed DA, Levine RB, Miller RG, et al. Impact of Duty Hour Regulations on Medical Students’ Education: Views of Key Clinical Faculty. J Gen Intern Med. 2008;23(7):1084-1089. doi:10.1007/s11606-008-0532-1 PubMed
18. Aagaard E, Teherani A, Irby DM. Effectiveness of the One-Minute Preceptor Model for Diagnosing the Patient and the Learner: Proof of Concept. Acad Med Spec Theme Teach Clin Ski. 2004;79(1):42-49. PubMed
19. Swanwick T. See one, do one, then what? Faculty development in postgraduate medical education. Postgrad Med J. 2008;84(993):339-343. doi:10.1136/pgmj.2008.068288 PubMed
20. Advancing Pediatric Educator Excellence (APEX) Teaching Program. The American Academy of Pediatrics. https://www.aap.org/en-us/about-the-aap/Committees-Councils-Sections/Section-on-Hospital-Medicine/Pages/Advancing-Pediatric-Educator-Excellence.aspx?nfstatus=401&nftoken=00000000-0000-0000-0000-000000000000&nfstatusdescription=ERROR:+No+local+token. Accessed August 22, 2016.
21. TEACH: Teaching Educators Across the Continuum of Healthcare. Society of General Internal Medicine. http://www.sgim.org/communities/education/sgim-teach-program. Accessed August 22, 2016.
22. Mittal V, Krieger E, Lee BC, et al. Pediatrics Residents’ Perspectives on Family-Centered Rounds: A Qualitative Study at 2 Children’s Hospitals. J Grad Med Educ. 2013;5(1):81-87. doi:10.4300/JGME-D-11-00314.1 PubMed
23. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and Family-Centered Care and the Pediatrician’s Role. Pediatrics. 2012;129(2):394-404. doi:10.1542/peds.2011-3084 PubMed
1. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. doi:10.1001/jamainternmed.2013.6041 PubMed
2. Osler SW. Osler’s “A Way of Life” and Other Addresses, with Commentary and Annotations. Durham: Duke University Press; 2001.
3. Peters M, Ten Cate O. Bedside teaching in medical education: a literature review. Perspect Med Educ. 2014;3(2):76-88. doi:10.1007/s40037-013-0083-y PubMed
4. Gonzalo JD, Heist BS, Duffy BL, et al. Identifying and Overcoming the Barriers to Bedside Rounds: A Multicenter Qualitative Study. Acad Med. 2014;89(2):326-334. doi:10.1097/ACM.0000000000000100 PubMed
5. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending Rounds and Bedside Case Presentations: Medical Student and Medicine Resident Experiences and Attitudes. Teach Learn Med. 2009;21(2):105-110. doi:10.1080/10401330902791156 PubMed
6. Payson HE, Barchas JD. A Time Study of Medical Teaching Rounds. N Engl J Med. 1965;273(27):1468-1471. doi:10.1056/NEJM196512302732706 PubMed
7. Rabinowitz R, Farnan J, Hulland O, et al. Rounds Today: A Qualitative Study of Internal Medicine and Pediatrics Resident Perceptions. J Grad Med Educ. 2016;8(4):523-531. doi:10.4300/JGME-D-15-00106.1 PubMed
8. Charmaz K. Constructing Grounded Theory: A Practical Guide through Qualitative Analysis. London: Sage Publications; 2006. PubMed
9. Starks H, Trinidad SB. Choose Your Method: A Comparison of Phenomenology, Discourse Analysis, and Grounded Theory. Qual Health Res. 2007;17(10):1372-1380. doi:10.1177/1049732307307031 PubMed
10. Sisterhen LL, Blaszak RT, Woods MB, Smith CE. Defining Family-Centered Rounds. Teach Learn Med. 2007;19(3):319-322. doi:10.1080/10401330701366812 PubMed
11. Witman Y. What do we transfer in case discussions? The hidden curriculum in medicine…. Perspect Med Educ. 2014;3(2):113-123. doi:10.1007/s40037-013-0101-0 PubMed
12. Benbassat J. Role Modeling in Medical Education: The Importance of a Reflective Imitation. Acad Med. 2014;89(4):550-554. doi:10.1097/ACM.0000000000000189 PubMed
13. Miller M, Johnson B, Greene DHL, Baier M, Nowlin S. An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646-648. doi:10.1007/BF02599208 PubMed
14. Priest JR, Bereknyei S, Hooper K, Braddock CH III. Relationships of the Location and Content of Rounds to Specialty, Institution, Patient-Census, and Team Size. PLoS One. 2010;5(6):e11246. doi:10.1371/journal.pone.0011246 PubMed
15. Balmer DF, Master CL, Richards BF, Serwint JR, Giardino AP. An ethnographic study of attending rounds in general paediatrics: understanding the ritual. Med Educ. 2010;44(11):1105-1116. doi:10.1111/j.1365-2923.2010.03767.x PubMed
16. Bhansali P, Birch S, Campbell JK, et al. A Time-Motion Study of Inpatient Rounds Using a Family-Centered Rounds Model. Hosp Pediatr. 2013;3(1):31-38. doi:10.1542/hpeds.2012-0021 PubMed
17. Reed DA, Levine RB, Miller RG, et al. Impact of Duty Hour Regulations on Medical Students’ Education: Views of Key Clinical Faculty. J Gen Intern Med. 2008;23(7):1084-1089. doi:10.1007/s11606-008-0532-1 PubMed
18. Aagaard E, Teherani A, Irby DM. Effectiveness of the One-Minute Preceptor Model for Diagnosing the Patient and the Learner: Proof of Concept. Acad Med Spec Theme Teach Clin Ski. 2004;79(1):42-49. PubMed
19. Swanwick T. See one, do one, then what? Faculty development in postgraduate medical education. Postgrad Med J. 2008;84(993):339-343. doi:10.1136/pgmj.2008.068288 PubMed
20. Advancing Pediatric Educator Excellence (APEX) Teaching Program. The American Academy of Pediatrics. https://www.aap.org/en-us/about-the-aap/Committees-Councils-Sections/Section-on-Hospital-Medicine/Pages/Advancing-Pediatric-Educator-Excellence.aspx?nfstatus=401&nftoken=00000000-0000-0000-0000-000000000000&nfstatusdescription=ERROR:+No+local+token. Accessed August 22, 2016.
21. TEACH: Teaching Educators Across the Continuum of Healthcare. Society of General Internal Medicine. http://www.sgim.org/communities/education/sgim-teach-program. Accessed August 22, 2016.
22. Mittal V, Krieger E, Lee BC, et al. Pediatrics Residents’ Perspectives on Family-Centered Rounds: A Qualitative Study at 2 Children’s Hospitals. J Grad Med Educ. 2013;5(1):81-87. doi:10.4300/JGME-D-11-00314.1 PubMed
23. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and Family-Centered Care and the Pediatrician’s Role. Pediatrics. 2012;129(2):394-404. doi:10.1542/peds.2011-3084 PubMed
© 2017 Society of Hospital Medicine
Thinking Outside the Checkbox
A 34-year-old, previously healthy Japanese man developed a dry cough. He did not have dyspnea, nasal discharge, sore throat, facial pain, nasal congestion, or postnasal drip. His symptoms persisted despite several courses of antibiotics (from different physicians), including clarithromycin, minocycline, and levofloxacin. A chest x-ray after 2 months of symptoms and a noncontrast chest computed tomography (CT) after 4 months of symptoms were normal, and bacterial and mycobacterial sputum cultures were sterile. Treatment with salmeterol and fluticasone was ineffective.
The persistence of a cough for longer than 8 weeks constitutes chronic cough. The initial negative review of systems argues against several of the usual etiologies. The lack of nasal discharge, sore throat, facial pain, nasal congestion, and postnasal drip lessens the probability of upper airway cough syndrome. The absence of dyspnea decreases the likelihood of congestive heart failure, asthma, or chronic obstructive pulmonary disease. Additional history should include whether the patient has orthopnea, paroxysmal nocturnal dyspnea, or a reduced exercise tolerance.
The persistence of symptoms despite multiple courses of antibiotics suggests that the process is inflammatory but not infectious, that the infection is not susceptible to the selected antibiotics, that the antibiotics cannot penetrate the site of infection, or that the ongoing symptoms are related to the antibiotics themselves. Pathogens that may cause chronic cough for months include mycobacteria, fungi (eg, Aspergillus , endemic mycoses), and parasites (eg, Strongyloides , Paragonimus ). Even when appropriately treated, many infections may result in a prolonged cough (eg, pertussis). The fluoroquinolone and macrolide exposure may have suppressed the mycobacterial cultures. The lack of response to salmeterol and fluticasone lessens the probability of asthma.
After 4 months of symptoms, his cough worsened, and he developed dysphagia and odynophagia, particularly when he initiated swallowing. He experienced daily fevers with temperatures between 38.0°C and 38.5°C. A repeat chest x-ray was normal. His white blood cell count was 14,200 per μL, and the C-reactive protein (CRP) was 12.91 mg/dL (normal <0.24 mg/dL). His symptoms did not improve with additional courses of clarithromycin, levofloxacin, or moxifloxacin. After 5 months of symptoms, he was referred to the internal medicine clinic of a teaching hospital in Japan.
The patient’s fevers, leukocytosis, and elevated CRP signal an inflammatory process, but whether it is infectious or not remains uncertain. The normal repeat chest x-ray lessens the likelihood of a pulmonary infection. Difficulty with initiating a swallow characterizes oropharyngeal dysphagia which features coughing or choking with oral intake and is typically caused by neuromuscular conditions like stroke, amyotrophic lateral sclerosis, or myasthenia gravis. The coexistence of oropharyngeal dysphagia and odynophagia may indicate pharyngitis, a retropharyngeal or parapharyngeal abscess, or oropharyngeal cancer.
Esophageal dysphagia occurs several seconds following swallow initiation and may arise with mucosal, smooth muscle, or neuromuscular diseases of the esophagus. Concomitant dysphagia and odynophagia may indicate esophageal spasm or esophagitis. Causes of esophagitis include infection (eg, candidiasis, herpes simplex virus [HSV], cytomegalovirus [CMV], or human immunodeficiency virus [HIV]), infiltration (eg, eosinophilic esophagitis), or irritation (eg, from medication, caustic ingestion, or gastroesophageal reflux). He is at risk for esophageal candidiasis following multiple courses of antibiotics. Esophageal dysphagia occurring with liquids and solids may indicate disordered motility, as opposed to dysphagia with solids alone, which may signal endoluminal obstruction.
At his outpatient evaluation, he denied headache, vision changes, chest pain, hemoptysis, palpitations, abdominal pain, dysuria, musculoskeletal symptoms, anorexia, or symptoms of gastroesophageal reflux. He did not have chills, rigors, or night sweats, but he had lost 3.4 kg in 5 months. He had not traveled within or outside of Japan in many years and was not involved in outdoor activities. He was engaged to and monogamous with his female partner of 5 years. He smoked 10 cigarettes per day for 14 years but stopped smoking during the last 2 months on account of his symptoms. He drank 6 beers per month and worked as a researcher at a chemical company but did not have any inhalational exposures.
His weight loss could be from reduced caloric intake due to dysphagia and odynophagia or may reflect an energy deficit related to chronic illness and inflammatory state. His smoking history increases his risk of bronchopulmonary infection and malignancy. Bronchogenic carcinoma may present with chronic cough, fevers, weight loss, or dysphagia from external compression by lymphadenopathy or mediastinal disease; however, his young age and recent chest CT results make lung cancer unlikely.
The white coating on his tongue could reflect oral leukoplakia, a reactive and potentially precancerous process that typically manifests as patches or plaques on oral mucosa. It can be distinguished from candidiasis, which scrapes off using a tongue blade. The extensive tongue coating is consistent with oral candidiasis. Potential predispositions include inhaled corticosteroids, antibiotic exposure, and/or an undiagnosed immunodeficiency syndrome (eg, HIV).
The initial diagnostic branch point for nontraumatic oral ulcers is infectious versus noninfectious. Infections that cause oral ulcers include HSV, CMV, and syphilis. The appearance and occurrence of the ulcers on freely moveable mucosa are consistent with aphthous stomatitis. Recurrent aphthous ulcers may occur in autoimmune diseases, including Behçet disease, Crohn disease, celiac sprue, and reactive arthritis. An endoscopy should be considered to detect esophageal ulcerations or esophageal candidiasis.
The rash may indicate folliculitis, usually attributable to Staphylococcus aureus or to Pseudomonas in the setting of recreational water exposure. Broad-spectrum antibiotics or immunodeficiency predisposes to candida folliculitis, while systemic candidiasis may cause metastatic skin lesions. The most common cutaneous manifestation of Behçet disease is erythema nodosum, but follicular and papulopustular lesions are also characteristic.
Pulmonary nodules are caused by infections, noninfectious inflammation, and malignancy. Infectious causes of pulmonary nodules include septic emboli, bacterial abscesses, and mycobacterial and fungal infection; noninfectious inflammatory causes include vasculitis (eg, granulomatosis with polyangiitis), rheumatoid arthritis, sarcoidosis, and lymphomatoid granulomatosis. Although additional culture data, serologic testing, and tuberculin skin testing or an interferon-gamma release assay may help to exclude these infections, the chronicity of symptoms, and lack of response to multiple antibiotic courses favor a noninfectious etiology.
Thickening of the aorta and left pulmonary artery may arise from an infectious, infiltrative, or inflammatory process. Arterial infections arise from direct inoculation, such as catheterization, trauma, or a contiguous site of infection, or from embolic seeding of atherosclerotic plaques or aneurysms. Malignant and nonmalignant processes, including sarcomas, lymphomas, histiocytoses (eg, Erdheim–Chester disease), and IgG4-related disease, may infiltrate the vascular walls. He has no evidence of visceral organ involvement to suggest these multisystem diagnoses.
The combined involvement of the aorta and pulmonary artery suggest a large-vessel vasculitis. Giant cell arteritis is exceedingly rare in patients younger than 50. Takayasu arteritis is a large-vessel vasculitis that predominantly affects women and may present with hypertension, arterial bruits, or discrepant blood pressure between arms, none of which were reported in this case. Behçet disease affects blood vessels of all sizes, including the aorta and pulmonary vasculature. His fevers, oral ulcers, perifollicular rash, and lymphadenopathy are consistent with this diagnosis, although he lacks the genital ulcers that occur in the majority of patients. Pulmonary nodules in Behçet disease arise from pulmonary or pleural vasculitis, resulting in focal inflammation, hemorrhage, or infarction. An ophthalmologic examination for uveitis and a pathergy test would support this diagnosis.
FDG accumulation in the aorta and pulmonary arteries signals large-vessel inflammation. The lack of FDG-avidity of the ground-glass opacities and nodular lesion suggests that these are not metabolically active tumors or infections but may be sequelae of the underlying disease, such as a hemorrhage or infarction from vasculitis. Sarcoidosis could account for the lung findings, but large-vessel vasculopathy would be exceedingly uncommon. Microscopic polyangiitis and granulomatosis with polyangiitis also cause pulmonary and vascular inflammation, but the nonreactive ANCA, absence of sinus disease, and normal urinalysis and kidney function make pauci-immune vasculitis unlikely. While the large-vessel involvement is consistent with Takayasu arteritis, the oral ulcers and rash are not.
Despite the absence of uveitis and the negative pathergy test, his oral aphthosis, papulopustular rash, and large-vessel vasculitis make Behçet disease the likely diagnosis. Behçet disease is most strongly associated with HLA B51, although other HLA haplotypes (including HLA A26 and HLA B52) are frequent in Behçet disease as well. As aortitis and pulmonary vasculitis can be associated with substantial morbidity and mortality, an urgent consultation with a rheumatologist regarding the initiation of immunosuppression is warranted.
Based on the mucocutaneous lesions, radiologic findings consistent with large-vessel vasculitis, and positive HLA A26 and HLA B52, he was diagnosed with Behçet disease. After 1 week of treatment with prednisolone 60 mg daily, his cough resolved and the oral aphthous ulcers and papulopustular rash improved. One month later, a chest CT showed significant reduction of the wall thickening of the aorta, its branches, and of the left pulmonary artery. The nodular lesion in the left lower lobe was unchanged, but the ground-glass opacities in the left upper lobe had disappeared.
When prednisolone was tapered down to 17.5 mg, his dry cough and low-grade fevers recurred, along with a slight elevation of inflammatory markers, and a ground-glass opacity appeared on the periphery of the left upper lobe. A sputum culture and fungal antigens were negative. His cough improved with the resumption of the previous dose of prednisolone. He remained symptom-free after 2 years of treatment with azathioprine 150 mg daily and prednisolone 2 mg daily and is now only treated with azathioprine.
DISCUSSION
Behçet disease is a multisystem vasculitis involving blood vessels of all sizes in the arterial and venous circulation that presents with oral and genital ulcers, ocular abnormalities (uveitis, retinitis), skin lesions (erythema nodosum, nonfollicular papulopustular lesions, or “pseudofolliculitis”), pathergy, and vascular lesions (thrombophlebitis, thrombosis, and aneurysm).
This patient presented with a chronic cough from pulmonary involvement by Behçet disease. The most common presenting symptom in a study of 47 patients with Behçet disease with pulmonary arteriopathy was hemoptysis followed by a nonbloody cough.2 Among these patients with pulmonary artery aneurysm, thrombosis, or both, 40 (85%) had nodules caused by infarction or inflammation and 21 (45%) had ground-glass opacities attributed to intraparenchymal hemorrhage. There are several case reports of chronic cough attributed to large-vessel vasculitis.3-5 Although the pathology of vasculitis-related cough is not fully understood, the inflammation of large vessels (aorta and pulmonary arteries) adjacent to the tracheobronchial tree may irritate regional cough receptors.3
Disease classification criteria are common in rheumatologic diseases; these criteria are developed to categorize patients for research studies and are not intended to diagnose individual patients.6 The classification criteria favor increased specificity at the expense of sensitivity to avoid misclassifying patients as having a disease, which would compromise the results of research studies. For instance, a study assessing a treatment for Behçet disease must exclude patients with inflammatory bowel disease, as these distinct patient populations may demonstrate discrepant responses to the investigative therapy. The specificity and homogeneity favored by classification criteria make those criteria inappropriate to rely on exclusively for the diagnosis of individual patients.7 The symptoms of many autoimmune diseases develop sequentially over time. Waiting for a patient with active, multisystem vasculitis to fulfill all of the Behçet disease classification criteria can lead to the harmful withholding of disease-modifying treatment.
The diagnosis of Behçet disease is made on clinical grounds; there is no gold standard test or histopathologic finding, and classification criteria remain imperfect. Although classification criteria help clinicians understand cardinal disease features, they cannot substitute for the more complex clinical reasoning required to establish a working diagnosis. The clinician must understand the pretest probability of disease, consider the presence or absence of characteristic features, exclude competing diagnoses, and decipher the risk-to-benefit ratio of therapeutic options and the urgency of treatment when assigning a diagnostic label. This patient’s pneumonitis, mucocutaneous changes, aortopathy, and compatible HLA typing (coupled with the exclusion of infectious diseases) were sufficient to diagnose Behçet disease. This case reminds us that classification criteria serve as a starting point, not as an end point, and that clinicians must ultimately make diagnoses and initiate treatment by thinking outside the checkbox.
TEACHING POINTS
- Large-vessel vasculitis is a rare cause of chronic cough.
- Although the most well-recognized signs of Behçet disease include genital and oral ulcers and uveitis, patients may also present with less common manifestations such as skin lesions (erythema nodosum, nonfollicular papulopustular lesions, or “pseudofolliculitis”) and vascular lesions of the artery (arteritis and aneurysm) and veins (thrombophlebitis and thrombosis).
- Classification criteria capture cardinal features of a disease but favor specificity over sensitivity and should not serve as a checklist for diagnosing a patient.
Acknowledgment
A brief version of this case was published as a case report in the Journal of Integrated Medicine 2013;23(12):1014-1017. Images from that publication were republished here with the permission of the publisher (Igaku-Shoin Ltd).
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. All other authors have nothing to disclose.
1. Kanamori M, Kubo T, Sakemi H. What’s your diagnosis? [in Japanese] J Integrated Med. 2013; 23 (12):1014-1017.
2. Seyahi E, Melikoglu M, Akman C, et al. Pulmonary artery involvement and associated lung disease in Behçet disease: a series of 47 patients. Medicine (Baltimore). 2012;91(1):35-48. PubMed
3. Olopade CO, Sekosan M, Schraufnagel DE. Giant cell arteritis manifesting as chronic cough and fever of unknown origin. Mayo Clin Proc. 1997;72(11):1048-1050. PubMed
4. Hellmann DB. Temporal arteritis: a cough, toothache, and tongue infarction. JAMA. 2002;287(22):2996-3000. PubMed
5. Karagiannis A, Mathiopoulou L, Tziomalos K, et al. Dry cough as first manifestation of giant-cell arteritis. J Am Geriatr Soc. 2006;54(12):1957-1958. PubMed
6. Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken). 2015;67(7):891-897. PubMed
7. Rao JK, Allen NB, Pincus T. Limitations of the 1990 American College of Rheumatology classification criteria in the diagnosis of vasculitis. Ann Intern Med. 1998;129(5):345-352. PubMed
8. Davatchi F, Sadeghi Abdollahi B, Shahram F, Chams-Davatchi C, Shams H, Nadji A. Classification and Diagnosis Criteria for Behçet’s Disease. In: Emmi L, ed. Behçet’s Syndrome. From Pathogenesis to Treatment. Milan, Italy: Springer; 2014:189-198.
9. Criteria for diagnosis of Behcet’s disease. International Study Group for Behçet’s Disease. Lancet. 1990;335(8697):1078-1080. PubMed
10. Davatchi F, Assaad-Khalil S, Calamia KT, et al. The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28(3):338–347. PubMed
11. Suzuki Kurokawa M, Suzuki N. Behçet’s disease. Clin Exp Med. 2004;4(1):10-20. PubMed
A 34-year-old, previously healthy Japanese man developed a dry cough. He did not have dyspnea, nasal discharge, sore throat, facial pain, nasal congestion, or postnasal drip. His symptoms persisted despite several courses of antibiotics (from different physicians), including clarithromycin, minocycline, and levofloxacin. A chest x-ray after 2 months of symptoms and a noncontrast chest computed tomography (CT) after 4 months of symptoms were normal, and bacterial and mycobacterial sputum cultures were sterile. Treatment with salmeterol and fluticasone was ineffective.
The persistence of a cough for longer than 8 weeks constitutes chronic cough. The initial negative review of systems argues against several of the usual etiologies. The lack of nasal discharge, sore throat, facial pain, nasal congestion, and postnasal drip lessens the probability of upper airway cough syndrome. The absence of dyspnea decreases the likelihood of congestive heart failure, asthma, or chronic obstructive pulmonary disease. Additional history should include whether the patient has orthopnea, paroxysmal nocturnal dyspnea, or a reduced exercise tolerance.
The persistence of symptoms despite multiple courses of antibiotics suggests that the process is inflammatory but not infectious, that the infection is not susceptible to the selected antibiotics, that the antibiotics cannot penetrate the site of infection, or that the ongoing symptoms are related to the antibiotics themselves. Pathogens that may cause chronic cough for months include mycobacteria, fungi (eg, Aspergillus , endemic mycoses), and parasites (eg, Strongyloides , Paragonimus ). Even when appropriately treated, many infections may result in a prolonged cough (eg, pertussis). The fluoroquinolone and macrolide exposure may have suppressed the mycobacterial cultures. The lack of response to salmeterol and fluticasone lessens the probability of asthma.
After 4 months of symptoms, his cough worsened, and he developed dysphagia and odynophagia, particularly when he initiated swallowing. He experienced daily fevers with temperatures between 38.0°C and 38.5°C. A repeat chest x-ray was normal. His white blood cell count was 14,200 per μL, and the C-reactive protein (CRP) was 12.91 mg/dL (normal <0.24 mg/dL). His symptoms did not improve with additional courses of clarithromycin, levofloxacin, or moxifloxacin. After 5 months of symptoms, he was referred to the internal medicine clinic of a teaching hospital in Japan.
The patient’s fevers, leukocytosis, and elevated CRP signal an inflammatory process, but whether it is infectious or not remains uncertain. The normal repeat chest x-ray lessens the likelihood of a pulmonary infection. Difficulty with initiating a swallow characterizes oropharyngeal dysphagia which features coughing or choking with oral intake and is typically caused by neuromuscular conditions like stroke, amyotrophic lateral sclerosis, or myasthenia gravis. The coexistence of oropharyngeal dysphagia and odynophagia may indicate pharyngitis, a retropharyngeal or parapharyngeal abscess, or oropharyngeal cancer.
Esophageal dysphagia occurs several seconds following swallow initiation and may arise with mucosal, smooth muscle, or neuromuscular diseases of the esophagus. Concomitant dysphagia and odynophagia may indicate esophageal spasm or esophagitis. Causes of esophagitis include infection (eg, candidiasis, herpes simplex virus [HSV], cytomegalovirus [CMV], or human immunodeficiency virus [HIV]), infiltration (eg, eosinophilic esophagitis), or irritation (eg, from medication, caustic ingestion, or gastroesophageal reflux). He is at risk for esophageal candidiasis following multiple courses of antibiotics. Esophageal dysphagia occurring with liquids and solids may indicate disordered motility, as opposed to dysphagia with solids alone, which may signal endoluminal obstruction.
At his outpatient evaluation, he denied headache, vision changes, chest pain, hemoptysis, palpitations, abdominal pain, dysuria, musculoskeletal symptoms, anorexia, or symptoms of gastroesophageal reflux. He did not have chills, rigors, or night sweats, but he had lost 3.4 kg in 5 months. He had not traveled within or outside of Japan in many years and was not involved in outdoor activities. He was engaged to and monogamous with his female partner of 5 years. He smoked 10 cigarettes per day for 14 years but stopped smoking during the last 2 months on account of his symptoms. He drank 6 beers per month and worked as a researcher at a chemical company but did not have any inhalational exposures.
His weight loss could be from reduced caloric intake due to dysphagia and odynophagia or may reflect an energy deficit related to chronic illness and inflammatory state. His smoking history increases his risk of bronchopulmonary infection and malignancy. Bronchogenic carcinoma may present with chronic cough, fevers, weight loss, or dysphagia from external compression by lymphadenopathy or mediastinal disease; however, his young age and recent chest CT results make lung cancer unlikely.
The white coating on his tongue could reflect oral leukoplakia, a reactive and potentially precancerous process that typically manifests as patches or plaques on oral mucosa. It can be distinguished from candidiasis, which scrapes off using a tongue blade. The extensive tongue coating is consistent with oral candidiasis. Potential predispositions include inhaled corticosteroids, antibiotic exposure, and/or an undiagnosed immunodeficiency syndrome (eg, HIV).
The initial diagnostic branch point for nontraumatic oral ulcers is infectious versus noninfectious. Infections that cause oral ulcers include HSV, CMV, and syphilis. The appearance and occurrence of the ulcers on freely moveable mucosa are consistent with aphthous stomatitis. Recurrent aphthous ulcers may occur in autoimmune diseases, including Behçet disease, Crohn disease, celiac sprue, and reactive arthritis. An endoscopy should be considered to detect esophageal ulcerations or esophageal candidiasis.
The rash may indicate folliculitis, usually attributable to Staphylococcus aureus or to Pseudomonas in the setting of recreational water exposure. Broad-spectrum antibiotics or immunodeficiency predisposes to candida folliculitis, while systemic candidiasis may cause metastatic skin lesions. The most common cutaneous manifestation of Behçet disease is erythema nodosum, but follicular and papulopustular lesions are also characteristic.
Pulmonary nodules are caused by infections, noninfectious inflammation, and malignancy. Infectious causes of pulmonary nodules include septic emboli, bacterial abscesses, and mycobacterial and fungal infection; noninfectious inflammatory causes include vasculitis (eg, granulomatosis with polyangiitis), rheumatoid arthritis, sarcoidosis, and lymphomatoid granulomatosis. Although additional culture data, serologic testing, and tuberculin skin testing or an interferon-gamma release assay may help to exclude these infections, the chronicity of symptoms, and lack of response to multiple antibiotic courses favor a noninfectious etiology.
Thickening of the aorta and left pulmonary artery may arise from an infectious, infiltrative, or inflammatory process. Arterial infections arise from direct inoculation, such as catheterization, trauma, or a contiguous site of infection, or from embolic seeding of atherosclerotic plaques or aneurysms. Malignant and nonmalignant processes, including sarcomas, lymphomas, histiocytoses (eg, Erdheim–Chester disease), and IgG4-related disease, may infiltrate the vascular walls. He has no evidence of visceral organ involvement to suggest these multisystem diagnoses.
The combined involvement of the aorta and pulmonary artery suggest a large-vessel vasculitis. Giant cell arteritis is exceedingly rare in patients younger than 50. Takayasu arteritis is a large-vessel vasculitis that predominantly affects women and may present with hypertension, arterial bruits, or discrepant blood pressure between arms, none of which were reported in this case. Behçet disease affects blood vessels of all sizes, including the aorta and pulmonary vasculature. His fevers, oral ulcers, perifollicular rash, and lymphadenopathy are consistent with this diagnosis, although he lacks the genital ulcers that occur in the majority of patients. Pulmonary nodules in Behçet disease arise from pulmonary or pleural vasculitis, resulting in focal inflammation, hemorrhage, or infarction. An ophthalmologic examination for uveitis and a pathergy test would support this diagnosis.
FDG accumulation in the aorta and pulmonary arteries signals large-vessel inflammation. The lack of FDG-avidity of the ground-glass opacities and nodular lesion suggests that these are not metabolically active tumors or infections but may be sequelae of the underlying disease, such as a hemorrhage or infarction from vasculitis. Sarcoidosis could account for the lung findings, but large-vessel vasculopathy would be exceedingly uncommon. Microscopic polyangiitis and granulomatosis with polyangiitis also cause pulmonary and vascular inflammation, but the nonreactive ANCA, absence of sinus disease, and normal urinalysis and kidney function make pauci-immune vasculitis unlikely. While the large-vessel involvement is consistent with Takayasu arteritis, the oral ulcers and rash are not.
Despite the absence of uveitis and the negative pathergy test, his oral aphthosis, papulopustular rash, and large-vessel vasculitis make Behçet disease the likely diagnosis. Behçet disease is most strongly associated with HLA B51, although other HLA haplotypes (including HLA A26 and HLA B52) are frequent in Behçet disease as well. As aortitis and pulmonary vasculitis can be associated with substantial morbidity and mortality, an urgent consultation with a rheumatologist regarding the initiation of immunosuppression is warranted.
Based on the mucocutaneous lesions, radiologic findings consistent with large-vessel vasculitis, and positive HLA A26 and HLA B52, he was diagnosed with Behçet disease. After 1 week of treatment with prednisolone 60 mg daily, his cough resolved and the oral aphthous ulcers and papulopustular rash improved. One month later, a chest CT showed significant reduction of the wall thickening of the aorta, its branches, and of the left pulmonary artery. The nodular lesion in the left lower lobe was unchanged, but the ground-glass opacities in the left upper lobe had disappeared.
When prednisolone was tapered down to 17.5 mg, his dry cough and low-grade fevers recurred, along with a slight elevation of inflammatory markers, and a ground-glass opacity appeared on the periphery of the left upper lobe. A sputum culture and fungal antigens were negative. His cough improved with the resumption of the previous dose of prednisolone. He remained symptom-free after 2 years of treatment with azathioprine 150 mg daily and prednisolone 2 mg daily and is now only treated with azathioprine.
DISCUSSION
Behçet disease is a multisystem vasculitis involving blood vessels of all sizes in the arterial and venous circulation that presents with oral and genital ulcers, ocular abnormalities (uveitis, retinitis), skin lesions (erythema nodosum, nonfollicular papulopustular lesions, or “pseudofolliculitis”), pathergy, and vascular lesions (thrombophlebitis, thrombosis, and aneurysm).
This patient presented with a chronic cough from pulmonary involvement by Behçet disease. The most common presenting symptom in a study of 47 patients with Behçet disease with pulmonary arteriopathy was hemoptysis followed by a nonbloody cough.2 Among these patients with pulmonary artery aneurysm, thrombosis, or both, 40 (85%) had nodules caused by infarction or inflammation and 21 (45%) had ground-glass opacities attributed to intraparenchymal hemorrhage. There are several case reports of chronic cough attributed to large-vessel vasculitis.3-5 Although the pathology of vasculitis-related cough is not fully understood, the inflammation of large vessels (aorta and pulmonary arteries) adjacent to the tracheobronchial tree may irritate regional cough receptors.3
Disease classification criteria are common in rheumatologic diseases; these criteria are developed to categorize patients for research studies and are not intended to diagnose individual patients.6 The classification criteria favor increased specificity at the expense of sensitivity to avoid misclassifying patients as having a disease, which would compromise the results of research studies. For instance, a study assessing a treatment for Behçet disease must exclude patients with inflammatory bowel disease, as these distinct patient populations may demonstrate discrepant responses to the investigative therapy. The specificity and homogeneity favored by classification criteria make those criteria inappropriate to rely on exclusively for the diagnosis of individual patients.7 The symptoms of many autoimmune diseases develop sequentially over time. Waiting for a patient with active, multisystem vasculitis to fulfill all of the Behçet disease classification criteria can lead to the harmful withholding of disease-modifying treatment.
The diagnosis of Behçet disease is made on clinical grounds; there is no gold standard test or histopathologic finding, and classification criteria remain imperfect. Although classification criteria help clinicians understand cardinal disease features, they cannot substitute for the more complex clinical reasoning required to establish a working diagnosis. The clinician must understand the pretest probability of disease, consider the presence or absence of characteristic features, exclude competing diagnoses, and decipher the risk-to-benefit ratio of therapeutic options and the urgency of treatment when assigning a diagnostic label. This patient’s pneumonitis, mucocutaneous changes, aortopathy, and compatible HLA typing (coupled with the exclusion of infectious diseases) were sufficient to diagnose Behçet disease. This case reminds us that classification criteria serve as a starting point, not as an end point, and that clinicians must ultimately make diagnoses and initiate treatment by thinking outside the checkbox.
TEACHING POINTS
- Large-vessel vasculitis is a rare cause of chronic cough.
- Although the most well-recognized signs of Behçet disease include genital and oral ulcers and uveitis, patients may also present with less common manifestations such as skin lesions (erythema nodosum, nonfollicular papulopustular lesions, or “pseudofolliculitis”) and vascular lesions of the artery (arteritis and aneurysm) and veins (thrombophlebitis and thrombosis).
- Classification criteria capture cardinal features of a disease but favor specificity over sensitivity and should not serve as a checklist for diagnosing a patient.
Acknowledgment
A brief version of this case was published as a case report in the Journal of Integrated Medicine 2013;23(12):1014-1017. Images from that publication were republished here with the permission of the publisher (Igaku-Shoin Ltd).
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. All other authors have nothing to disclose.
A 34-year-old, previously healthy Japanese man developed a dry cough. He did not have dyspnea, nasal discharge, sore throat, facial pain, nasal congestion, or postnasal drip. His symptoms persisted despite several courses of antibiotics (from different physicians), including clarithromycin, minocycline, and levofloxacin. A chest x-ray after 2 months of symptoms and a noncontrast chest computed tomography (CT) after 4 months of symptoms were normal, and bacterial and mycobacterial sputum cultures were sterile. Treatment with salmeterol and fluticasone was ineffective.
The persistence of a cough for longer than 8 weeks constitutes chronic cough. The initial negative review of systems argues against several of the usual etiologies. The lack of nasal discharge, sore throat, facial pain, nasal congestion, and postnasal drip lessens the probability of upper airway cough syndrome. The absence of dyspnea decreases the likelihood of congestive heart failure, asthma, or chronic obstructive pulmonary disease. Additional history should include whether the patient has orthopnea, paroxysmal nocturnal dyspnea, or a reduced exercise tolerance.
The persistence of symptoms despite multiple courses of antibiotics suggests that the process is inflammatory but not infectious, that the infection is not susceptible to the selected antibiotics, that the antibiotics cannot penetrate the site of infection, or that the ongoing symptoms are related to the antibiotics themselves. Pathogens that may cause chronic cough for months include mycobacteria, fungi (eg, Aspergillus , endemic mycoses), and parasites (eg, Strongyloides , Paragonimus ). Even when appropriately treated, many infections may result in a prolonged cough (eg, pertussis). The fluoroquinolone and macrolide exposure may have suppressed the mycobacterial cultures. The lack of response to salmeterol and fluticasone lessens the probability of asthma.
After 4 months of symptoms, his cough worsened, and he developed dysphagia and odynophagia, particularly when he initiated swallowing. He experienced daily fevers with temperatures between 38.0°C and 38.5°C. A repeat chest x-ray was normal. His white blood cell count was 14,200 per μL, and the C-reactive protein (CRP) was 12.91 mg/dL (normal <0.24 mg/dL). His symptoms did not improve with additional courses of clarithromycin, levofloxacin, or moxifloxacin. After 5 months of symptoms, he was referred to the internal medicine clinic of a teaching hospital in Japan.
The patient’s fevers, leukocytosis, and elevated CRP signal an inflammatory process, but whether it is infectious or not remains uncertain. The normal repeat chest x-ray lessens the likelihood of a pulmonary infection. Difficulty with initiating a swallow characterizes oropharyngeal dysphagia which features coughing or choking with oral intake and is typically caused by neuromuscular conditions like stroke, amyotrophic lateral sclerosis, or myasthenia gravis. The coexistence of oropharyngeal dysphagia and odynophagia may indicate pharyngitis, a retropharyngeal or parapharyngeal abscess, or oropharyngeal cancer.
Esophageal dysphagia occurs several seconds following swallow initiation and may arise with mucosal, smooth muscle, or neuromuscular diseases of the esophagus. Concomitant dysphagia and odynophagia may indicate esophageal spasm or esophagitis. Causes of esophagitis include infection (eg, candidiasis, herpes simplex virus [HSV], cytomegalovirus [CMV], or human immunodeficiency virus [HIV]), infiltration (eg, eosinophilic esophagitis), or irritation (eg, from medication, caustic ingestion, or gastroesophageal reflux). He is at risk for esophageal candidiasis following multiple courses of antibiotics. Esophageal dysphagia occurring with liquids and solids may indicate disordered motility, as opposed to dysphagia with solids alone, which may signal endoluminal obstruction.
At his outpatient evaluation, he denied headache, vision changes, chest pain, hemoptysis, palpitations, abdominal pain, dysuria, musculoskeletal symptoms, anorexia, or symptoms of gastroesophageal reflux. He did not have chills, rigors, or night sweats, but he had lost 3.4 kg in 5 months. He had not traveled within or outside of Japan in many years and was not involved in outdoor activities. He was engaged to and monogamous with his female partner of 5 years. He smoked 10 cigarettes per day for 14 years but stopped smoking during the last 2 months on account of his symptoms. He drank 6 beers per month and worked as a researcher at a chemical company but did not have any inhalational exposures.
His weight loss could be from reduced caloric intake due to dysphagia and odynophagia or may reflect an energy deficit related to chronic illness and inflammatory state. His smoking history increases his risk of bronchopulmonary infection and malignancy. Bronchogenic carcinoma may present with chronic cough, fevers, weight loss, or dysphagia from external compression by lymphadenopathy or mediastinal disease; however, his young age and recent chest CT results make lung cancer unlikely.
The white coating on his tongue could reflect oral leukoplakia, a reactive and potentially precancerous process that typically manifests as patches or plaques on oral mucosa. It can be distinguished from candidiasis, which scrapes off using a tongue blade. The extensive tongue coating is consistent with oral candidiasis. Potential predispositions include inhaled corticosteroids, antibiotic exposure, and/or an undiagnosed immunodeficiency syndrome (eg, HIV).
The initial diagnostic branch point for nontraumatic oral ulcers is infectious versus noninfectious. Infections that cause oral ulcers include HSV, CMV, and syphilis. The appearance and occurrence of the ulcers on freely moveable mucosa are consistent with aphthous stomatitis. Recurrent aphthous ulcers may occur in autoimmune diseases, including Behçet disease, Crohn disease, celiac sprue, and reactive arthritis. An endoscopy should be considered to detect esophageal ulcerations or esophageal candidiasis.
The rash may indicate folliculitis, usually attributable to Staphylococcus aureus or to Pseudomonas in the setting of recreational water exposure. Broad-spectrum antibiotics or immunodeficiency predisposes to candida folliculitis, while systemic candidiasis may cause metastatic skin lesions. The most common cutaneous manifestation of Behçet disease is erythema nodosum, but follicular and papulopustular lesions are also characteristic.
Pulmonary nodules are caused by infections, noninfectious inflammation, and malignancy. Infectious causes of pulmonary nodules include septic emboli, bacterial abscesses, and mycobacterial and fungal infection; noninfectious inflammatory causes include vasculitis (eg, granulomatosis with polyangiitis), rheumatoid arthritis, sarcoidosis, and lymphomatoid granulomatosis. Although additional culture data, serologic testing, and tuberculin skin testing or an interferon-gamma release assay may help to exclude these infections, the chronicity of symptoms, and lack of response to multiple antibiotic courses favor a noninfectious etiology.
Thickening of the aorta and left pulmonary artery may arise from an infectious, infiltrative, or inflammatory process. Arterial infections arise from direct inoculation, such as catheterization, trauma, or a contiguous site of infection, or from embolic seeding of atherosclerotic plaques or aneurysms. Malignant and nonmalignant processes, including sarcomas, lymphomas, histiocytoses (eg, Erdheim–Chester disease), and IgG4-related disease, may infiltrate the vascular walls. He has no evidence of visceral organ involvement to suggest these multisystem diagnoses.
The combined involvement of the aorta and pulmonary artery suggest a large-vessel vasculitis. Giant cell arteritis is exceedingly rare in patients younger than 50. Takayasu arteritis is a large-vessel vasculitis that predominantly affects women and may present with hypertension, arterial bruits, or discrepant blood pressure between arms, none of which were reported in this case. Behçet disease affects blood vessels of all sizes, including the aorta and pulmonary vasculature. His fevers, oral ulcers, perifollicular rash, and lymphadenopathy are consistent with this diagnosis, although he lacks the genital ulcers that occur in the majority of patients. Pulmonary nodules in Behçet disease arise from pulmonary or pleural vasculitis, resulting in focal inflammation, hemorrhage, or infarction. An ophthalmologic examination for uveitis and a pathergy test would support this diagnosis.
FDG accumulation in the aorta and pulmonary arteries signals large-vessel inflammation. The lack of FDG-avidity of the ground-glass opacities and nodular lesion suggests that these are not metabolically active tumors or infections but may be sequelae of the underlying disease, such as a hemorrhage or infarction from vasculitis. Sarcoidosis could account for the lung findings, but large-vessel vasculopathy would be exceedingly uncommon. Microscopic polyangiitis and granulomatosis with polyangiitis also cause pulmonary and vascular inflammation, but the nonreactive ANCA, absence of sinus disease, and normal urinalysis and kidney function make pauci-immune vasculitis unlikely. While the large-vessel involvement is consistent with Takayasu arteritis, the oral ulcers and rash are not.
Despite the absence of uveitis and the negative pathergy test, his oral aphthosis, papulopustular rash, and large-vessel vasculitis make Behçet disease the likely diagnosis. Behçet disease is most strongly associated with HLA B51, although other HLA haplotypes (including HLA A26 and HLA B52) are frequent in Behçet disease as well. As aortitis and pulmonary vasculitis can be associated with substantial morbidity and mortality, an urgent consultation with a rheumatologist regarding the initiation of immunosuppression is warranted.
Based on the mucocutaneous lesions, radiologic findings consistent with large-vessel vasculitis, and positive HLA A26 and HLA B52, he was diagnosed with Behçet disease. After 1 week of treatment with prednisolone 60 mg daily, his cough resolved and the oral aphthous ulcers and papulopustular rash improved. One month later, a chest CT showed significant reduction of the wall thickening of the aorta, its branches, and of the left pulmonary artery. The nodular lesion in the left lower lobe was unchanged, but the ground-glass opacities in the left upper lobe had disappeared.
When prednisolone was tapered down to 17.5 mg, his dry cough and low-grade fevers recurred, along with a slight elevation of inflammatory markers, and a ground-glass opacity appeared on the periphery of the left upper lobe. A sputum culture and fungal antigens were negative. His cough improved with the resumption of the previous dose of prednisolone. He remained symptom-free after 2 years of treatment with azathioprine 150 mg daily and prednisolone 2 mg daily and is now only treated with azathioprine.
DISCUSSION
Behçet disease is a multisystem vasculitis involving blood vessels of all sizes in the arterial and venous circulation that presents with oral and genital ulcers, ocular abnormalities (uveitis, retinitis), skin lesions (erythema nodosum, nonfollicular papulopustular lesions, or “pseudofolliculitis”), pathergy, and vascular lesions (thrombophlebitis, thrombosis, and aneurysm).
This patient presented with a chronic cough from pulmonary involvement by Behçet disease. The most common presenting symptom in a study of 47 patients with Behçet disease with pulmonary arteriopathy was hemoptysis followed by a nonbloody cough.2 Among these patients with pulmonary artery aneurysm, thrombosis, or both, 40 (85%) had nodules caused by infarction or inflammation and 21 (45%) had ground-glass opacities attributed to intraparenchymal hemorrhage. There are several case reports of chronic cough attributed to large-vessel vasculitis.3-5 Although the pathology of vasculitis-related cough is not fully understood, the inflammation of large vessels (aorta and pulmonary arteries) adjacent to the tracheobronchial tree may irritate regional cough receptors.3
Disease classification criteria are common in rheumatologic diseases; these criteria are developed to categorize patients for research studies and are not intended to diagnose individual patients.6 The classification criteria favor increased specificity at the expense of sensitivity to avoid misclassifying patients as having a disease, which would compromise the results of research studies. For instance, a study assessing a treatment for Behçet disease must exclude patients with inflammatory bowel disease, as these distinct patient populations may demonstrate discrepant responses to the investigative therapy. The specificity and homogeneity favored by classification criteria make those criteria inappropriate to rely on exclusively for the diagnosis of individual patients.7 The symptoms of many autoimmune diseases develop sequentially over time. Waiting for a patient with active, multisystem vasculitis to fulfill all of the Behçet disease classification criteria can lead to the harmful withholding of disease-modifying treatment.
The diagnosis of Behçet disease is made on clinical grounds; there is no gold standard test or histopathologic finding, and classification criteria remain imperfect. Although classification criteria help clinicians understand cardinal disease features, they cannot substitute for the more complex clinical reasoning required to establish a working diagnosis. The clinician must understand the pretest probability of disease, consider the presence or absence of characteristic features, exclude competing diagnoses, and decipher the risk-to-benefit ratio of therapeutic options and the urgency of treatment when assigning a diagnostic label. This patient’s pneumonitis, mucocutaneous changes, aortopathy, and compatible HLA typing (coupled with the exclusion of infectious diseases) were sufficient to diagnose Behçet disease. This case reminds us that classification criteria serve as a starting point, not as an end point, and that clinicians must ultimately make diagnoses and initiate treatment by thinking outside the checkbox.
TEACHING POINTS
- Large-vessel vasculitis is a rare cause of chronic cough.
- Although the most well-recognized signs of Behçet disease include genital and oral ulcers and uveitis, patients may also present with less common manifestations such as skin lesions (erythema nodosum, nonfollicular papulopustular lesions, or “pseudofolliculitis”) and vascular lesions of the artery (arteritis and aneurysm) and veins (thrombophlebitis and thrombosis).
- Classification criteria capture cardinal features of a disease but favor specificity over sensitivity and should not serve as a checklist for diagnosing a patient.
Acknowledgment
A brief version of this case was published as a case report in the Journal of Integrated Medicine 2013;23(12):1014-1017. Images from that publication were republished here with the permission of the publisher (Igaku-Shoin Ltd).
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. All other authors have nothing to disclose.
1. Kanamori M, Kubo T, Sakemi H. What’s your diagnosis? [in Japanese] J Integrated Med. 2013; 23 (12):1014-1017.
2. Seyahi E, Melikoglu M, Akman C, et al. Pulmonary artery involvement and associated lung disease in Behçet disease: a series of 47 patients. Medicine (Baltimore). 2012;91(1):35-48. PubMed
3. Olopade CO, Sekosan M, Schraufnagel DE. Giant cell arteritis manifesting as chronic cough and fever of unknown origin. Mayo Clin Proc. 1997;72(11):1048-1050. PubMed
4. Hellmann DB. Temporal arteritis: a cough, toothache, and tongue infarction. JAMA. 2002;287(22):2996-3000. PubMed
5. Karagiannis A, Mathiopoulou L, Tziomalos K, et al. Dry cough as first manifestation of giant-cell arteritis. J Am Geriatr Soc. 2006;54(12):1957-1958. PubMed
6. Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken). 2015;67(7):891-897. PubMed
7. Rao JK, Allen NB, Pincus T. Limitations of the 1990 American College of Rheumatology classification criteria in the diagnosis of vasculitis. Ann Intern Med. 1998;129(5):345-352. PubMed
8. Davatchi F, Sadeghi Abdollahi B, Shahram F, Chams-Davatchi C, Shams H, Nadji A. Classification and Diagnosis Criteria for Behçet’s Disease. In: Emmi L, ed. Behçet’s Syndrome. From Pathogenesis to Treatment. Milan, Italy: Springer; 2014:189-198.
9. Criteria for diagnosis of Behcet’s disease. International Study Group for Behçet’s Disease. Lancet. 1990;335(8697):1078-1080. PubMed
10. Davatchi F, Assaad-Khalil S, Calamia KT, et al. The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28(3):338–347. PubMed
11. Suzuki Kurokawa M, Suzuki N. Behçet’s disease. Clin Exp Med. 2004;4(1):10-20. PubMed
1. Kanamori M, Kubo T, Sakemi H. What’s your diagnosis? [in Japanese] J Integrated Med. 2013; 23 (12):1014-1017.
2. Seyahi E, Melikoglu M, Akman C, et al. Pulmonary artery involvement and associated lung disease in Behçet disease: a series of 47 patients. Medicine (Baltimore). 2012;91(1):35-48. PubMed
3. Olopade CO, Sekosan M, Schraufnagel DE. Giant cell arteritis manifesting as chronic cough and fever of unknown origin. Mayo Clin Proc. 1997;72(11):1048-1050. PubMed
4. Hellmann DB. Temporal arteritis: a cough, toothache, and tongue infarction. JAMA. 2002;287(22):2996-3000. PubMed
5. Karagiannis A, Mathiopoulou L, Tziomalos K, et al. Dry cough as first manifestation of giant-cell arteritis. J Am Geriatr Soc. 2006;54(12):1957-1958. PubMed
6. Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken). 2015;67(7):891-897. PubMed
7. Rao JK, Allen NB, Pincus T. Limitations of the 1990 American College of Rheumatology classification criteria in the diagnosis of vasculitis. Ann Intern Med. 1998;129(5):345-352. PubMed
8. Davatchi F, Sadeghi Abdollahi B, Shahram F, Chams-Davatchi C, Shams H, Nadji A. Classification and Diagnosis Criteria for Behçet’s Disease. In: Emmi L, ed. Behçet’s Syndrome. From Pathogenesis to Treatment. Milan, Italy: Springer; 2014:189-198.
9. Criteria for diagnosis of Behcet’s disease. International Study Group for Behçet’s Disease. Lancet. 1990;335(8697):1078-1080. PubMed
10. Davatchi F, Assaad-Khalil S, Calamia KT, et al. The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28(3):338–347. PubMed
11. Suzuki Kurokawa M, Suzuki N. Behçet’s disease. Clin Exp Med. 2004;4(1):10-20. PubMed
© 2018 Society of Hospital Medicine
The Authors Reply, “What Can Be Done to Maintain Positive Patient Experience and Improve Residents’ Satisfaction?” and “Standardized Attending Rounds to Improve the Patient Experience: A Pragmatic Cluster Randomized Controlled Trial”
We thank Talari et al. for their comments in response to our randomized controlled trial evaluating the impact of standardized rounds on patient, attending, and trainee satisfaction. We agree that many factors beyond rounding structure contribute to resident satisfaction, including those highlighted by the authors, and would enthusiastically welcome additional research in this realm.
Because our study intervention addressed rounding structure, we elected to specifically focus on satisfaction with rounds, both from the physician and patient perspectives. We chose to ask about patient satisfaction with attending rounds, as opposed to more generic measures of patient satisfaction, to allow for more direct comparison between attending/resident responses and patient responses. Certainly, there are many other factors that affect overall patient experience. Surveys such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) and Press Ganey do not specifically address rounds, are often completed several weeks following hospitalization, and may have low response rates. Relying on such global assessments of patient experience may also reduce the power of the study. Although patient responses to our survey may be higher than scores seen with HCAHPS and Press Ganey, the randomized nature of our study helps control for other differences in the hospitalization experience unrelated to rounding structure. Similarly, because physician teams were randomly assigned, differences in census were not a major factor in the study. Physician blinding was not possible due to the nature of the intervention, which may have affected the satisfaction reports from attendings and residents. For our primary outcome (patient satisfaction with rounds), patients were blinded to the nature of our intervention, and all study team members involved in data collection and statistical analyses were blinded to study arm allocation.
In summary, we feel that evaluating the trade-offs and consequences of interventions should be examined from multiple perspectives, and we welcome additional investigations in this area.
We thank Talari et al. for their comments in response to our randomized controlled trial evaluating the impact of standardized rounds on patient, attending, and trainee satisfaction. We agree that many factors beyond rounding structure contribute to resident satisfaction, including those highlighted by the authors, and would enthusiastically welcome additional research in this realm.
Because our study intervention addressed rounding structure, we elected to specifically focus on satisfaction with rounds, both from the physician and patient perspectives. We chose to ask about patient satisfaction with attending rounds, as opposed to more generic measures of patient satisfaction, to allow for more direct comparison between attending/resident responses and patient responses. Certainly, there are many other factors that affect overall patient experience. Surveys such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) and Press Ganey do not specifically address rounds, are often completed several weeks following hospitalization, and may have low response rates. Relying on such global assessments of patient experience may also reduce the power of the study. Although patient responses to our survey may be higher than scores seen with HCAHPS and Press Ganey, the randomized nature of our study helps control for other differences in the hospitalization experience unrelated to rounding structure. Similarly, because physician teams were randomly assigned, differences in census were not a major factor in the study. Physician blinding was not possible due to the nature of the intervention, which may have affected the satisfaction reports from attendings and residents. For our primary outcome (patient satisfaction with rounds), patients were blinded to the nature of our intervention, and all study team members involved in data collection and statistical analyses were blinded to study arm allocation.
In summary, we feel that evaluating the trade-offs and consequences of interventions should be examined from multiple perspectives, and we welcome additional investigations in this area.
We thank Talari et al. for their comments in response to our randomized controlled trial evaluating the impact of standardized rounds on patient, attending, and trainee satisfaction. We agree that many factors beyond rounding structure contribute to resident satisfaction, including those highlighted by the authors, and would enthusiastically welcome additional research in this realm.
Because our study intervention addressed rounding structure, we elected to specifically focus on satisfaction with rounds, both from the physician and patient perspectives. We chose to ask about patient satisfaction with attending rounds, as opposed to more generic measures of patient satisfaction, to allow for more direct comparison between attending/resident responses and patient responses. Certainly, there are many other factors that affect overall patient experience. Surveys such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) and Press Ganey do not specifically address rounds, are often completed several weeks following hospitalization, and may have low response rates. Relying on such global assessments of patient experience may also reduce the power of the study. Although patient responses to our survey may be higher than scores seen with HCAHPS and Press Ganey, the randomized nature of our study helps control for other differences in the hospitalization experience unrelated to rounding structure. Similarly, because physician teams were randomly assigned, differences in census were not a major factor in the study. Physician blinding was not possible due to the nature of the intervention, which may have affected the satisfaction reports from attendings and residents. For our primary outcome (patient satisfaction with rounds), patients were blinded to the nature of our intervention, and all study team members involved in data collection and statistical analyses were blinded to study arm allocation.
In summary, we feel that evaluating the trade-offs and consequences of interventions should be examined from multiple perspectives, and we welcome additional investigations in this area.
© 2017 Society of Hospital Medicine
Fecal occult blood testing in hospitalized patients with upper gastrointestinal bleeding
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 47-year-old man with a history of alcohol abuse, cirrhosis, and grade II esophageal varices is admitted for treatment of alcohol withdrawal. He reports having some dark-colored stools a week prior to admission, but his stools since then have been normal in color. A repeat hemoglobin is stable, but a fecal occult blood test is positive. What should be done next?
BACKGROUND
The US Preventive Services Task Force and the American College of Gastroenterology recommend fecal occult blood testing (FOBT) as one method for colorectal cancer (CRC) screening in average risk populations.1,2 FOBTs can be divided into guaiac-based tests (gFOBTs), which measure heme, and fecal immunochemical tests (FITs), which measure the globin portion of human hemoglobin (Hb). In gFOBTs, heme present in the sample reacts with a hydrogen peroxide developer to oxidize guaiac, producing a blue color.3 Screening gFOBT was shown to decrease mortality from CRC in several landmark studies in the 1990s, but its sensitivity is poor, ranging from 30% to 57%.4 Because the guaiac-induced color change is determined visually, interpretation of gFOBT results are subject to error. In a survey of 173 medical providers, 12% did not accurately interpret gFOBT results.5 In light of these limitations, recent guidelines support the use of newer FITs for CRC screening. FITs utilize antibodies directed against the human globin moiety and demonstrate an increased sensitivity when compared with gFOBTs (by 32% to 62%) for detecting neoplasm.6 While evidence supports the use of FOBTs in CRC screening, providers use these tests for nonvalidated purposes, including the evaluation of suspected acute upper gastrointestinal bleeding (UGIB).
WHY YOU MIGHT THINK FOBT is HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
Given the incidence (up to 100 per 100,000 persons per year) and high mortality of UGIB (up to 20,000 deaths annually in the United States),7 there would ideally be a noninvasive test available to help guide management. In evaluating a patient with possible acute UGIB, FOBT affords several theoretical benefits. FOBT is quick, inexpensive, and can be performed by any health professional. In contrast, the primary diagnostic procedure for UGIB, esophagogastroduodenoscopy (EGD), carries procedural and sedation-related risks, can be costly and time-consuming, and requires consultation from subspecialty providers.
WHY FOBT is NOT HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
While FOBTs are valuable as screening tests for CRC in the outpatient setting, their use has been extended to diagnose gastrointestinal (GI) bleeding in the inpatient setting without supporting data. As is true for many screening tests, FOBT is associated with a high incidence of false-positive results, or type I errors.8,9 False-positive FOBT results can occur from ingested blood via extra-intestinal sources (eg, epistaxis, gingival bleeding, pharyngitis, hemoptysis), or in medical conditions with intestinal mucosal inflammation (eg, esophagitis, gastritis, inflammatory bowel disease). False-positive results can also be due to clinically insignificant GI blood loss induced by medications (eg, aspirin, nonsteroidal anti-inflammatory drugs), alcohol,10 or by ingestion of meats, fruits, or vegetables containing peroxidase (eg, broccoli, cauliflower).11
Outpatients using FOBTs for cancer screening are advised to hold medications and avoid foods that may lead to false-positive results. Despite institution of these restrictions, false-positive rates are still high, as 37% to 53% of CRC screening patients with a positive FOBT have a subsequent negative colonoscopy, and only 11% to 21% of these patients have a source of bleeding identified on subsequent EGD.12 False-positive results might be even higher in the inpatient setting, where patients typically do not adhere to these restrictions. A review of FOBTs performed in 3 acute care hospitals revealed that 65% of patients tested were on at least one medication that impacted the validity of gFOBT results, and 98% had no evidence of dietary restriction prior to testing.13
The use of FOBTs (particularly FITs) is also subject to false-negative results, or type II errors. While FITs have increased specificity for lower GI bleeding, their ability to detect UGIB is limited, because most Hb is digested in the small intestine and not present in rectal stool.14 In a study of more than 2,700 patients, FIT results were not correlated with the presence of upper GI pathology.15 False-negative results are less common with gFOBTs, although these may occur with low volume, slow or intermittent bleeding,16 or with ingestion of substances that inhibit oxidation, such as vitamin C.17
Beyond these test limitations, studies suggest that the majority of inpatient FOBT results do not impact immediate medical decision-making or management. In one study, only 34% of hospitalized patients with a positive FOBT underwent further GI studies, with the majority of those patients (60%) receiving endoscopy before the results of the FOBT were known.18 In another study of 201 FOBTs performed on hospitalized patients, those with negative results underwent further GI evaluation at a higher rate than those with positive results (41% vs 38%).8 This aligns with a study that revealed the majority of patients suspected of having a GI bleed underwent endoscopic evaluation regardless of the FOBT result.9
WHEN MIGHT FOBT BE HELPFUL?
FOBT currently has a role in CRC screening and
WHAT WE SHOULD DO INSTEAD
A careful history, physical examination, and visual inspection of the stool remain the foundation of establishing UGIB as the etiology of anemia. Observed melena (either by passed stool or a rectal examination) has a likelihood ratio (LR) of 25 for UGIB; a patient’s self-report of stools that sounds melenic (black or tarry) has an LR of 5-6.19 An upper GI source may be further supported by an elevated blood urea nitrogen (BUN) to creatinine ratio, as blood is absorbed through the small bowel and patients may have concomitant decreased renal perfusion. A BUN to creatinine ratio of >30 is associated with a positive LR (LR+) of 7.5 for UGIB.19 Recall that the higher the LR+, and the lower the negative LR (LR-), the better the test is at ruling in and out the diagnosis, respectively. LR+ of 2–10 and LR– of 0.1–0.5 represent a modestly helpful diagnostic test, whereas LR+ >10 and LR- <0.1 are considered robust. These are generalizations only, as value of LR+/LR- depends on pretest probability.
Although Gastroccult23 may be considered for the detection of occult blood in gastric juice, its package insert states: “As with any occult blood test, results with the Gastroccult test cannot be considered conclusive evidence of the presence or absence of upper gastrointestinal bleeding or pathology.” As with any diagnostic evaluation, we would only recommend this test if it would change management.
- FOBT should not be performed to diagnose UGIB.
- When there is clinical suspicion of acute GI bleeding, the best diagnostic tools are a good history, physical examination, and visual inspection of the stool by the clinician to determine the presence of hematochezia or melena.
- Deferring FOBT to the ambulatory setting may improve test performance characteristics.
CONCLUSION
FOBT is validated as an outpatient colon cancer screening tool in asymptomatic patients, not for inpatient evaluation of acute GIB. Given the poor positive predictive value for a positive FOBT in an acute GIB scenario, the potential risk for unnecessary treatments or procedures is real. Conversely, a negative FOBT (particularly FIT) does not rule out GI bleeding and risks a false sense of security that may result in under-treatment. In most scenarios in which FOBT is performed, clinicians can make decisions based on a composite of history, physical exam, visual inspection of the stool, and laboratory investigation. Until further research substantiates the utility of FOBT for this purpose, we would recommend against the routine use of FOBT for evaluating UGIB in hospitalized patients.
Acknowledgment
Disclosure: The authors do not have any relevant financial disclosures to report. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailingTWDFNR@hospitalmedicine.org.
1. U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129-131. PubMed
2. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. PubMed
3. Carroll MRR, Seaman HE, Halloran HP. Tests and investigations for colorectal cancer screening. Clinical Biochemistry. 2014;47:921-939. PubMed
4. Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64(8):1327-1337. PubMed
5. Selinger RR, et al. Failure of health care professionals to interpret fecal occult blood tests accurately. Am J Med. 2003;114(1):64-67. PubMed
6. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104(3):739-750. PubMed
7. El-Tawil AM. Trends on gastrointestinal bleeding and mortality: Where are we standing? World J Gastroenterol. 2012;18(11):1154. PubMed
8. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269. PubMed
9. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426. PubMed
10. Fleming, JL, Ahlquist DA, McGill DB, Zinsmeister AR, Ellefson RD, Schwartz S. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163. PubMed
11. Macrae FA, St John DJB. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82:891-898. PubMed
12. Allard J, et al. Gastroscopy following a positive fecal occult blood test and negative colonoscopy: systematic review and guideline. Can J Gastroenterol. 2010;24(2):113-120. PubMed
13. Friedman A, Chan A, Chin LC, Deen A, Hammerschlag G, Lee M, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111. PubMed
14. Allison JE, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99(19):1462-1470. PubMed
15. Chiang TH, Lee YC, Tu CH, Chiu HM, Wu MS. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481. PubMed
16. Bassett ML, Goulston KJ. False positive and negative hemoccult reactions on a normal diet and effect of diet restriction. Aust N Z J Med. 1980;10(1):1-4. PubMed
17. Jaffe, RM, Kasten B, Young DS, MacLowry JD. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med. 1975;83(6):824-826. PubMed
18. Ip S, Sokoro AAH, Kaita L, Ruiz C, McIntyre E, Singh H. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494. PubMed
19. Srygley FD, Gerardo CJ, Trun T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. PubMed
20. Logue KA. Data Request - FOBT. June 2016. Regions Hospital, HealthPartners Laboratory, Saint Paul, Minnesota.
21. Population Clock. http://www.census.gov/popclock/. Accessed July 8, 2016.
22. Mosadeghi S, Ren H, Yen I, Bhuket T. Evaluation of fecal occult blood testing in the acute hospital setting. Gastrointestinal Endoscopy. 2015;81(5).
23. Gastroccult [package insert]. Beckman Coulter, Brea, CA. https://www.beckmancoulter.com/wsrportal/wsr/diagnostics/clinical-products/rapid-diagnostics/gas troccult/index.htm. Accessed March 18, 2008.
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 47-year-old man with a history of alcohol abuse, cirrhosis, and grade II esophageal varices is admitted for treatment of alcohol withdrawal. He reports having some dark-colored stools a week prior to admission, but his stools since then have been normal in color. A repeat hemoglobin is stable, but a fecal occult blood test is positive. What should be done next?
BACKGROUND
The US Preventive Services Task Force and the American College of Gastroenterology recommend fecal occult blood testing (FOBT) as one method for colorectal cancer (CRC) screening in average risk populations.1,2 FOBTs can be divided into guaiac-based tests (gFOBTs), which measure heme, and fecal immunochemical tests (FITs), which measure the globin portion of human hemoglobin (Hb). In gFOBTs, heme present in the sample reacts with a hydrogen peroxide developer to oxidize guaiac, producing a blue color.3 Screening gFOBT was shown to decrease mortality from CRC in several landmark studies in the 1990s, but its sensitivity is poor, ranging from 30% to 57%.4 Because the guaiac-induced color change is determined visually, interpretation of gFOBT results are subject to error. In a survey of 173 medical providers, 12% did not accurately interpret gFOBT results.5 In light of these limitations, recent guidelines support the use of newer FITs for CRC screening. FITs utilize antibodies directed against the human globin moiety and demonstrate an increased sensitivity when compared with gFOBTs (by 32% to 62%) for detecting neoplasm.6 While evidence supports the use of FOBTs in CRC screening, providers use these tests for nonvalidated purposes, including the evaluation of suspected acute upper gastrointestinal bleeding (UGIB).
WHY YOU MIGHT THINK FOBT is HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
Given the incidence (up to 100 per 100,000 persons per year) and high mortality of UGIB (up to 20,000 deaths annually in the United States),7 there would ideally be a noninvasive test available to help guide management. In evaluating a patient with possible acute UGIB, FOBT affords several theoretical benefits. FOBT is quick, inexpensive, and can be performed by any health professional. In contrast, the primary diagnostic procedure for UGIB, esophagogastroduodenoscopy (EGD), carries procedural and sedation-related risks, can be costly and time-consuming, and requires consultation from subspecialty providers.
WHY FOBT is NOT HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
While FOBTs are valuable as screening tests for CRC in the outpatient setting, their use has been extended to diagnose gastrointestinal (GI) bleeding in the inpatient setting without supporting data. As is true for many screening tests, FOBT is associated with a high incidence of false-positive results, or type I errors.8,9 False-positive FOBT results can occur from ingested blood via extra-intestinal sources (eg, epistaxis, gingival bleeding, pharyngitis, hemoptysis), or in medical conditions with intestinal mucosal inflammation (eg, esophagitis, gastritis, inflammatory bowel disease). False-positive results can also be due to clinically insignificant GI blood loss induced by medications (eg, aspirin, nonsteroidal anti-inflammatory drugs), alcohol,10 or by ingestion of meats, fruits, or vegetables containing peroxidase (eg, broccoli, cauliflower).11
Outpatients using FOBTs for cancer screening are advised to hold medications and avoid foods that may lead to false-positive results. Despite institution of these restrictions, false-positive rates are still high, as 37% to 53% of CRC screening patients with a positive FOBT have a subsequent negative colonoscopy, and only 11% to 21% of these patients have a source of bleeding identified on subsequent EGD.12 False-positive results might be even higher in the inpatient setting, where patients typically do not adhere to these restrictions. A review of FOBTs performed in 3 acute care hospitals revealed that 65% of patients tested were on at least one medication that impacted the validity of gFOBT results, and 98% had no evidence of dietary restriction prior to testing.13
The use of FOBTs (particularly FITs) is also subject to false-negative results, or type II errors. While FITs have increased specificity for lower GI bleeding, their ability to detect UGIB is limited, because most Hb is digested in the small intestine and not present in rectal stool.14 In a study of more than 2,700 patients, FIT results were not correlated with the presence of upper GI pathology.15 False-negative results are less common with gFOBTs, although these may occur with low volume, slow or intermittent bleeding,16 or with ingestion of substances that inhibit oxidation, such as vitamin C.17
Beyond these test limitations, studies suggest that the majority of inpatient FOBT results do not impact immediate medical decision-making or management. In one study, only 34% of hospitalized patients with a positive FOBT underwent further GI studies, with the majority of those patients (60%) receiving endoscopy before the results of the FOBT were known.18 In another study of 201 FOBTs performed on hospitalized patients, those with negative results underwent further GI evaluation at a higher rate than those with positive results (41% vs 38%).8 This aligns with a study that revealed the majority of patients suspected of having a GI bleed underwent endoscopic evaluation regardless of the FOBT result.9
WHEN MIGHT FOBT BE HELPFUL?
FOBT currently has a role in CRC screening and
WHAT WE SHOULD DO INSTEAD
A careful history, physical examination, and visual inspection of the stool remain the foundation of establishing UGIB as the etiology of anemia. Observed melena (either by passed stool or a rectal examination) has a likelihood ratio (LR) of 25 for UGIB; a patient’s self-report of stools that sounds melenic (black or tarry) has an LR of 5-6.19 An upper GI source may be further supported by an elevated blood urea nitrogen (BUN) to creatinine ratio, as blood is absorbed through the small bowel and patients may have concomitant decreased renal perfusion. A BUN to creatinine ratio of >30 is associated with a positive LR (LR+) of 7.5 for UGIB.19 Recall that the higher the LR+, and the lower the negative LR (LR-), the better the test is at ruling in and out the diagnosis, respectively. LR+ of 2–10 and LR– of 0.1–0.5 represent a modestly helpful diagnostic test, whereas LR+ >10 and LR- <0.1 are considered robust. These are generalizations only, as value of LR+/LR- depends on pretest probability.
Although Gastroccult23 may be considered for the detection of occult blood in gastric juice, its package insert states: “As with any occult blood test, results with the Gastroccult test cannot be considered conclusive evidence of the presence or absence of upper gastrointestinal bleeding or pathology.” As with any diagnostic evaluation, we would only recommend this test if it would change management.
- FOBT should not be performed to diagnose UGIB.
- When there is clinical suspicion of acute GI bleeding, the best diagnostic tools are a good history, physical examination, and visual inspection of the stool by the clinician to determine the presence of hematochezia or melena.
- Deferring FOBT to the ambulatory setting may improve test performance characteristics.
CONCLUSION
FOBT is validated as an outpatient colon cancer screening tool in asymptomatic patients, not for inpatient evaluation of acute GIB. Given the poor positive predictive value for a positive FOBT in an acute GIB scenario, the potential risk for unnecessary treatments or procedures is real. Conversely, a negative FOBT (particularly FIT) does not rule out GI bleeding and risks a false sense of security that may result in under-treatment. In most scenarios in which FOBT is performed, clinicians can make decisions based on a composite of history, physical exam, visual inspection of the stool, and laboratory investigation. Until further research substantiates the utility of FOBT for this purpose, we would recommend against the routine use of FOBT for evaluating UGIB in hospitalized patients.
Acknowledgment
Disclosure: The authors do not have any relevant financial disclosures to report. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailingTWDFNR@hospitalmedicine.org.
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 47-year-old man with a history of alcohol abuse, cirrhosis, and grade II esophageal varices is admitted for treatment of alcohol withdrawal. He reports having some dark-colored stools a week prior to admission, but his stools since then have been normal in color. A repeat hemoglobin is stable, but a fecal occult blood test is positive. What should be done next?
BACKGROUND
The US Preventive Services Task Force and the American College of Gastroenterology recommend fecal occult blood testing (FOBT) as one method for colorectal cancer (CRC) screening in average risk populations.1,2 FOBTs can be divided into guaiac-based tests (gFOBTs), which measure heme, and fecal immunochemical tests (FITs), which measure the globin portion of human hemoglobin (Hb). In gFOBTs, heme present in the sample reacts with a hydrogen peroxide developer to oxidize guaiac, producing a blue color.3 Screening gFOBT was shown to decrease mortality from CRC in several landmark studies in the 1990s, but its sensitivity is poor, ranging from 30% to 57%.4 Because the guaiac-induced color change is determined visually, interpretation of gFOBT results are subject to error. In a survey of 173 medical providers, 12% did not accurately interpret gFOBT results.5 In light of these limitations, recent guidelines support the use of newer FITs for CRC screening. FITs utilize antibodies directed against the human globin moiety and demonstrate an increased sensitivity when compared with gFOBTs (by 32% to 62%) for detecting neoplasm.6 While evidence supports the use of FOBTs in CRC screening, providers use these tests for nonvalidated purposes, including the evaluation of suspected acute upper gastrointestinal bleeding (UGIB).
WHY YOU MIGHT THINK FOBT is HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
Given the incidence (up to 100 per 100,000 persons per year) and high mortality of UGIB (up to 20,000 deaths annually in the United States),7 there would ideally be a noninvasive test available to help guide management. In evaluating a patient with possible acute UGIB, FOBT affords several theoretical benefits. FOBT is quick, inexpensive, and can be performed by any health professional. In contrast, the primary diagnostic procedure for UGIB, esophagogastroduodenoscopy (EGD), carries procedural and sedation-related risks, can be costly and time-consuming, and requires consultation from subspecialty providers.
WHY FOBT is NOT HELPFUL FOR EVALUATION OF INPATIENTS WITH SUSPECTED ACUTE UGIB
While FOBTs are valuable as screening tests for CRC in the outpatient setting, their use has been extended to diagnose gastrointestinal (GI) bleeding in the inpatient setting without supporting data. As is true for many screening tests, FOBT is associated with a high incidence of false-positive results, or type I errors.8,9 False-positive FOBT results can occur from ingested blood via extra-intestinal sources (eg, epistaxis, gingival bleeding, pharyngitis, hemoptysis), or in medical conditions with intestinal mucosal inflammation (eg, esophagitis, gastritis, inflammatory bowel disease). False-positive results can also be due to clinically insignificant GI blood loss induced by medications (eg, aspirin, nonsteroidal anti-inflammatory drugs), alcohol,10 or by ingestion of meats, fruits, or vegetables containing peroxidase (eg, broccoli, cauliflower).11
Outpatients using FOBTs for cancer screening are advised to hold medications and avoid foods that may lead to false-positive results. Despite institution of these restrictions, false-positive rates are still high, as 37% to 53% of CRC screening patients with a positive FOBT have a subsequent negative colonoscopy, and only 11% to 21% of these patients have a source of bleeding identified on subsequent EGD.12 False-positive results might be even higher in the inpatient setting, where patients typically do not adhere to these restrictions. A review of FOBTs performed in 3 acute care hospitals revealed that 65% of patients tested were on at least one medication that impacted the validity of gFOBT results, and 98% had no evidence of dietary restriction prior to testing.13
The use of FOBTs (particularly FITs) is also subject to false-negative results, or type II errors. While FITs have increased specificity for lower GI bleeding, their ability to detect UGIB is limited, because most Hb is digested in the small intestine and not present in rectal stool.14 In a study of more than 2,700 patients, FIT results were not correlated with the presence of upper GI pathology.15 False-negative results are less common with gFOBTs, although these may occur with low volume, slow or intermittent bleeding,16 or with ingestion of substances that inhibit oxidation, such as vitamin C.17
Beyond these test limitations, studies suggest that the majority of inpatient FOBT results do not impact immediate medical decision-making or management. In one study, only 34% of hospitalized patients with a positive FOBT underwent further GI studies, with the majority of those patients (60%) receiving endoscopy before the results of the FOBT were known.18 In another study of 201 FOBTs performed on hospitalized patients, those with negative results underwent further GI evaluation at a higher rate than those with positive results (41% vs 38%).8 This aligns with a study that revealed the majority of patients suspected of having a GI bleed underwent endoscopic evaluation regardless of the FOBT result.9
WHEN MIGHT FOBT BE HELPFUL?
FOBT currently has a role in CRC screening and
WHAT WE SHOULD DO INSTEAD
A careful history, physical examination, and visual inspection of the stool remain the foundation of establishing UGIB as the etiology of anemia. Observed melena (either by passed stool or a rectal examination) has a likelihood ratio (LR) of 25 for UGIB; a patient’s self-report of stools that sounds melenic (black or tarry) has an LR of 5-6.19 An upper GI source may be further supported by an elevated blood urea nitrogen (BUN) to creatinine ratio, as blood is absorbed through the small bowel and patients may have concomitant decreased renal perfusion. A BUN to creatinine ratio of >30 is associated with a positive LR (LR+) of 7.5 for UGIB.19 Recall that the higher the LR+, and the lower the negative LR (LR-), the better the test is at ruling in and out the diagnosis, respectively. LR+ of 2–10 and LR– of 0.1–0.5 represent a modestly helpful diagnostic test, whereas LR+ >10 and LR- <0.1 are considered robust. These are generalizations only, as value of LR+/LR- depends on pretest probability.
Although Gastroccult23 may be considered for the detection of occult blood in gastric juice, its package insert states: “As with any occult blood test, results with the Gastroccult test cannot be considered conclusive evidence of the presence or absence of upper gastrointestinal bleeding or pathology.” As with any diagnostic evaluation, we would only recommend this test if it would change management.
- FOBT should not be performed to diagnose UGIB.
- When there is clinical suspicion of acute GI bleeding, the best diagnostic tools are a good history, physical examination, and visual inspection of the stool by the clinician to determine the presence of hematochezia or melena.
- Deferring FOBT to the ambulatory setting may improve test performance characteristics.
CONCLUSION
FOBT is validated as an outpatient colon cancer screening tool in asymptomatic patients, not for inpatient evaluation of acute GIB. Given the poor positive predictive value for a positive FOBT in an acute GIB scenario, the potential risk for unnecessary treatments or procedures is real. Conversely, a negative FOBT (particularly FIT) does not rule out GI bleeding and risks a false sense of security that may result in under-treatment. In most scenarios in which FOBT is performed, clinicians can make decisions based on a composite of history, physical exam, visual inspection of the stool, and laboratory investigation. Until further research substantiates the utility of FOBT for this purpose, we would recommend against the routine use of FOBT for evaluating UGIB in hospitalized patients.
Acknowledgment
Disclosure: The authors do not have any relevant financial disclosures to report. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailingTWDFNR@hospitalmedicine.org.
1. U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129-131. PubMed
2. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. PubMed
3. Carroll MRR, Seaman HE, Halloran HP. Tests and investigations for colorectal cancer screening. Clinical Biochemistry. 2014;47:921-939. PubMed
4. Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64(8):1327-1337. PubMed
5. Selinger RR, et al. Failure of health care professionals to interpret fecal occult blood tests accurately. Am J Med. 2003;114(1):64-67. PubMed
6. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104(3):739-750. PubMed
7. El-Tawil AM. Trends on gastrointestinal bleeding and mortality: Where are we standing? World J Gastroenterol. 2012;18(11):1154. PubMed
8. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269. PubMed
9. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426. PubMed
10. Fleming, JL, Ahlquist DA, McGill DB, Zinsmeister AR, Ellefson RD, Schwartz S. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163. PubMed
11. Macrae FA, St John DJB. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82:891-898. PubMed
12. Allard J, et al. Gastroscopy following a positive fecal occult blood test and negative colonoscopy: systematic review and guideline. Can J Gastroenterol. 2010;24(2):113-120. PubMed
13. Friedman A, Chan A, Chin LC, Deen A, Hammerschlag G, Lee M, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111. PubMed
14. Allison JE, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99(19):1462-1470. PubMed
15. Chiang TH, Lee YC, Tu CH, Chiu HM, Wu MS. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481. PubMed
16. Bassett ML, Goulston KJ. False positive and negative hemoccult reactions on a normal diet and effect of diet restriction. Aust N Z J Med. 1980;10(1):1-4. PubMed
17. Jaffe, RM, Kasten B, Young DS, MacLowry JD. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med. 1975;83(6):824-826. PubMed
18. Ip S, Sokoro AAH, Kaita L, Ruiz C, McIntyre E, Singh H. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494. PubMed
19. Srygley FD, Gerardo CJ, Trun T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. PubMed
20. Logue KA. Data Request - FOBT. June 2016. Regions Hospital, HealthPartners Laboratory, Saint Paul, Minnesota.
21. Population Clock. http://www.census.gov/popclock/. Accessed July 8, 2016.
22. Mosadeghi S, Ren H, Yen I, Bhuket T. Evaluation of fecal occult blood testing in the acute hospital setting. Gastrointestinal Endoscopy. 2015;81(5).
23. Gastroccult [package insert]. Beckman Coulter, Brea, CA. https://www.beckmancoulter.com/wsrportal/wsr/diagnostics/clinical-products/rapid-diagnostics/gas troccult/index.htm. Accessed March 18, 2008.
1. U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129-131. PubMed
2. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. PubMed
3. Carroll MRR, Seaman HE, Halloran HP. Tests and investigations for colorectal cancer screening. Clinical Biochemistry. 2014;47:921-939. PubMed
4. Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64(8):1327-1337. PubMed
5. Selinger RR, et al. Failure of health care professionals to interpret fecal occult blood tests accurately. Am J Med. 2003;114(1):64-67. PubMed
6. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104(3):739-750. PubMed
7. El-Tawil AM. Trends on gastrointestinal bleeding and mortality: Where are we standing? World J Gastroenterol. 2012;18(11):1154. PubMed
8. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269. PubMed
9. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426. PubMed
10. Fleming, JL, Ahlquist DA, McGill DB, Zinsmeister AR, Ellefson RD, Schwartz S. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163. PubMed
11. Macrae FA, St John DJB. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82:891-898. PubMed
12. Allard J, et al. Gastroscopy following a positive fecal occult blood test and negative colonoscopy: systematic review and guideline. Can J Gastroenterol. 2010;24(2):113-120. PubMed
13. Friedman A, Chan A, Chin LC, Deen A, Hammerschlag G, Lee M, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111. PubMed
14. Allison JE, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99(19):1462-1470. PubMed
15. Chiang TH, Lee YC, Tu CH, Chiu HM, Wu MS. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481. PubMed
16. Bassett ML, Goulston KJ. False positive and negative hemoccult reactions on a normal diet and effect of diet restriction. Aust N Z J Med. 1980;10(1):1-4. PubMed
17. Jaffe, RM, Kasten B, Young DS, MacLowry JD. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med. 1975;83(6):824-826. PubMed
18. Ip S, Sokoro AAH, Kaita L, Ruiz C, McIntyre E, Singh H. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494. PubMed
19. Srygley FD, Gerardo CJ, Trun T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. PubMed
20. Logue KA. Data Request - FOBT. June 2016. Regions Hospital, HealthPartners Laboratory, Saint Paul, Minnesota.
21. Population Clock. http://www.census.gov/popclock/. Accessed July 8, 2016.
22. Mosadeghi S, Ren H, Yen I, Bhuket T. Evaluation of fecal occult blood testing in the acute hospital setting. Gastrointestinal Endoscopy. 2015;81(5).
23. Gastroccult [package insert]. Beckman Coulter, Brea, CA. https://www.beckmancoulter.com/wsrportal/wsr/diagnostics/clinical-products/rapid-diagnostics/gas troccult/index.htm. Accessed March 18, 2008.
© 2017 Society of Hospital Medicine
Hot in the tropics
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 42-year-old Malaysian construction worker with subjective fevers of 4 days’ duration presented to an emergency department in Singapore. He reported nonproductive cough, chills without rigors, sore throat, and body aches. He denied sick contacts. Past medical history included chronic hepatitis B virus (HBV) infection. The patient was not taking any medications.
For this patient presenting acutely with subjective fevers, nonproductive cough, chills, aches, and lethargy, initial considerations include infection with a common virus (influenza virus, adenovirus, Epstein-Barr virus [EBV]), acute human immunodeficiency virus (HIV) infection, emerging infection (severe acute respiratory syndrome [SARS], Middle Eastern respiratory syndrome coronavirus [MERS-CoV] infection, avian influenza), and tropical infection (dengue, chikungunya). Also possible are bacterial infections (eg, with Salmonella typhi or Rickettsia or Mycoplasma species), parasitic infections (eg, malaria), and noninfectious illnesses (eg, autoimmune diseases, thyroiditis, acute leukemia, environmental exposures).
The patient’s temperature was 38.5°C; blood pressure, 133/73 mm Hg; heart rate, 95 beats per minute; respiratory rate, 18 breaths per minute; and oxygen saturation, 100% on ambient air. On physical examination, he appeared comfortable, and heart, lung, abdomen, skin, and extremities were normal. Laboratory test results included white blood cell (WBC) count, 4400/μL (with normal differential); hemoglobin, 16.1 g/dL; and platelet count, 207,000/μL. Serum chemistries were normal. C-reactive protein (CRP) level was 44.6 mg/L (reference range, 0.2-9.1 mg/L), and procalcitonin level was 0.13 ng/mL (reference range, <0.50 ng/mL). Chest radiograph was normal. Dengue antibodies (immunoglobulin M, immunoglobulin G [IgG]) and dengue NS1 antigen were negative. The patient was discharged with a presumptive diagnosis of viral upper respiratory tract infection.
There is no left shift characteristic of bacterial infection or lymphopenia characteristic of rickettsial disease or acute HIV infection. The serologic testing and the patient’s overall appearance make dengue unlikely. The low procalcitonin supports a nonbacterial cause of illness. CRP elevation may indicate an inflammatory process and is relatively nonspecific.
Myalgias, pharyngitis, and cough improved over several days, but fevers persisted, and a rash developed over the lower abdomen. The patient returned to the emergency department and was admitted. He denied weight loss and night sweats. He had multiple female sexual partners, including commercial sex workers, within the previous 6 months. Temperature was 38.5°C. The posterior oropharynx was slightly erythematous. There was no lymphadenopathy. Firm, mildly erythematous macules were present on the anterior abdominal wall (Figure 1). The rest of the physical examination was normal.
Laboratory testing revealed WBC count, 5800/μL (75% neutrophils, 19% lymphocytes, 3% monocytes, 2% atypical mononuclear cells); hemoglobin, 16.3 g/dL; platelet count, 185,000/μL; sodium, 131 mmol/L; potassium, 3.4 mmol/L; creatinine, 0.9 mg/dL; albumin, 3.2 g/dL; alanine aminotransferase (ALT), 99 U/L; aspartate aminotransferase (AST), 137 U/L; alkaline phosphatase (ALP), 63 U/L; and total bilirubin, 1.9 mg/dL. Prothrombin time was 11.1 seconds; partial thromboplastin time, 36.1 seconds; erythrocyte sedimentation rate, 14 mm/h; and CRP, 62.2 mg/L.
EBV, acute HIV, and cytomegalovirus infections often present with adenopathy, which is absent here. Disseminated gonococcal infection can manifest with fever, body aches, and rash, but his rash and the absence of penile discharge, migratory arthritis, and enthesitis are not characteristic. Mycoplasma infection can present with macules, urticaria, or erythema multiforme. Rickettsia illnesses typically cause vasculitis with progression to petechiae or purpura resulting from endothelial damage. Patients with secondary syphilis may have widespread macular lesions, and the accompanying syphilitic hepatitis often manifests with elevations in ALP instead of ALT and AST. The mild elevation in ALT and AST can occur with many systemic viral infections. Sweet syndrome may manifest with febrile illness and rash, but the acuity of this patient’s illness and the rapid evolution favor infection.
The patient’s fevers (35°-40°C) continued without pattern over the next 3 days. Blood and urine cultures were negative. Polymerase chain reaction (PCR) test of the nasal mucosa was negative for respiratory viruses. PCR blood tests for EBV, HIV-1, and cytomegalovirus were also negative. Antistreptolysin O (ASO) titer was 400 IU/mm (reference range, <200 IU/mm). Antinuclear antibodies were negative, and rheumatoid factor was 12.4 U/mL (reference range, <10.3 U/mL). Computed tomography (CT) of the thorax, abdomen, and pelvis was normal. Results of a biopsy of an anterior abdominal wall skin lesion showed perivascular and periadnexal lymphocytic inflammation. Amoxicillin was started for the treatment of possible group A streptococcal infection.
PCR for HIV would be positive at a high level in acute HIV. The skin biopsy is not characteristic of Sweet syndrome, which typically shows neutrophilic infiltrate without leukocytoclastic vasculitis, or of syphilis, which typically shows a plasma cell infiltrate.
The patient’s erythematous oropharynx may indicate recent streptococcal pharyngitis. The fevers, elevated ASO titer, and CRP level are consistent with acute rheumatic fever, but arthritis, carditis, and neurologic manifestations are lacking. Erythema marginatum manifests on the trunk and limbs as macules or papules with central clearing as the lesions spread outward—and differs from the patient’s rash, which is firm and restricted to the abdominal wall.
Fevers persisted through hospital day 7. The WBC count was 1100/μL (75.7% neutrophils, 22.5% lymphocytes), hemoglobin was 10.3 g/dL, and platelet count was 52,000/μL. Additional laboratory test results included ALP, 234 U/L; ALT, 250 U/L; AST, 459 U/L; lactate dehydrogenase, 2303 U/L (reference range, 222-454 U/L); and ferritin, 14,964 ng/mL (reference range, 47-452 ng/mL).
The duration of illness and negative diagnostic tests for infections increases suspicion for a noninfectious illness. Conditions commonly associated with marked hyperferritinemia include adult-onset Still disease (AOSD) and hemophagocytic lymphohistiocytosis (HLH). Of the 9 AOSD diagnostic (Yamaguchi) criteria, 5 are met in this case: fever, rash, sore throat, abnormal liver function tests, and negative rheumatologic tests. However, the patient lacks arthritis, leukocytosis, lymphadenopathy, and hepatosplenomegaly. Except for the elevated ferritin, the AOSD criteria overlap substantially with the criteria for acute rheumatic fever, and still require that infections be adequately excluded. HLH, a state of abnormal immune activation with resultant organ dysfunction, can be a primary disorder, but in adults more often is secondary to underlying infectious, autoimmune, or malignant (often lymphoma) conditions. Elevated ferritin, cytopenias, elevated ALT and AST, elevated CRP and erythrocyte sedimentation rate, and elevated lactate dehydrogenase are consistent with HLH. The HLH diagnosis can be more firmly established with the more specific findings of hypertriglyceridemia, hypofibrinogenemia, and elevated soluble CD25 level. The histopathologic finding of hemophagocytosis in the bone marrow, lymph nodes, or liver may further support the diagnosis of HLH.
Rash and fevers persisted. Hepatitis A, hepatitis C, Rickettsia IgG, Burkholderia pseudomallei (the causative organism of melioidosis), and Leptospira serologies, as well as PCR for herpes simplex virus and parvovirus, were all negative. Hepatitis B viral load was 962 IU/mL (2.98 log), hepatitis B envelope antigen was negative, and hepatitis B envelope antibody was positive. Orientia tsutsugamushi (organism responsible for scrub typhus) IgG titer was elevated at 1:128. Antiliver kidney microsomal antibodies and antineutrophil cytoplasmic antibodies were negative. Fibrinogen level was 0.69 g/L (reference range, 1.8-4.8 g/L), and beta-2 microglobulin level was 5078 ng/mL (reference range, 878-2000 ng/mL). Bone marrow biopsy results showed hypocellular marrow with suppressed myelopoiesis, few atypical lymphoid cells, and few hemophagocytes. Flow cytometry was negative for clonal B lymphocytes and aberrant expression of T lymphocytes. Bone marrow myobacterial PCR and fungal cultures were negative.
The patient’s chronic HBV infection is unlikely to be related to his presentation given his low viral load and absence of signs of hepatic dysfunction. Excluding rickettsial disease requires paired acute and convalescent serologies. O tsutsugamushi, the causative agent of the rickettsial disease scrub typhus, is endemic in Malaysia; thus, his positive O tsutsugamushi IgG may indicate past exposure. His fevers, myalgias, truncal rash, and hepatitis are consistent with scrub typhus, but he lacks the characteristic severe headache and generalized lymphadenopathy. Although eschar formation with evolution of a papular rash is common in scrub typhus, it is often absent in the variant found in Southeast Asia. Although elevated β2 microglobulin level is used as a prognostic marker in multiple myeloma and Waldenström macroglobulinemia, it can be elevated in many immune-active states. The patient likely has HLH, which is supported by the hemophagocytosis seen on bone marrow biopsy, and the hypofibrinogenemia. Potential HLH triggers include O tsutsugamushi infection or recent streptococcal pharyngitis.
A deep-punch skin biopsy of the anterior abdominal wall skin lesion was performed because of the absence of subcutaneous fat in the first biopsy specimen. The latest biopsy results showed irregular interstitial expansion of medium-size lymphocytes in a lobular panniculated pattern. The lymphocytes contained enlarged, irregularly contoured nucleoli and were positive for T-cell markers CD2 and CD3 with reduction in CD5 expression. The lymphomatous cells were of CD8+ with uniform expression of activated cytotoxic granule protein granzyme B and were positive for T-cell hemireceptor β.
Positron emission tomography (PET) CT, obtained for staging purposes, showed multiple hypermetabolic subcutaneous and cutaneous lesions over the torso and upper and lower limbs—compatible with lymphomatous infiltrates (Figure 2). Examination, pathology, and imaging findings suggested a rare neoplasm: subcutaneous panniculitis-like T-cell lymphoma (SPTCL). SPTCL was confirmed by T-cell receptor gene rearrangements studies.
HLH was diagnosed on the basis of the fevers, cytopenias, hypofibrinogenemia, elevated ferritin level, and evidence of hemophagocytosis. SPTCL was suspected as the HLH trigger.
The patient was treated with cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone. While on this regimen, he developed new skin lesions, and his ferritin level was persistently elevated. He was switched to romidepsin, a histone deacetylase inhibitor that specifically targets cutaneous T-cell lymphoma, but the lesions continued to progress. The patient then was treated with gemcitabine, dexamethasone, and cisplatin, and the rashes resolved. The most recent PET-CT showed nearly complete resolution of the subcutaneous lesions.
DISCUSSION
When residents or visitors to tropical or sub-tropical regions, those located near or between the Tropics of Cancer and Capricorn, present with fever, physicians usually first think of infectious diseases. This patient’s case is a reminder that these important first considerations should not be the last.
Generating a differential diagnosis for tropical illnesses begins with the patient’s history. Factors to be considered include location (regional disease prevalence), exposures (food/water ingestion, outdoor work/recreation, sexual contact, animal contact), and timing (temporal relationship of symptom development to possible exposure). Common tropical infections are malaria, dengue, typhoid, and emerging infections such as chikungunya, avian influenza, and Zika virus infection.1This case underscores the need to analyze diagnostic tests critically. Interpreting tests as simply positive or negative, irrespective of disease features, epidemiology, and test characteristics, can contribute to diagnostic error. For example, the patient’s positive ASO titer requires an understanding of disease features and a nuanced interpretation based on the clinical presentation. The erythematous posterior oropharynx prompted concern for postinfectious sequelae of streptococcal pharyngitis, but his illness was more severe and more prolonged than is typical of that condition. The isolated elevated O tsutsugamushi IgG titer provides an example of the role of epidemiology in test interpretation. Although a single positive value might indicate a new exposure for a visitor to an endemic region, IgG seropositivity in Singapore, where scrub typhus is endemic, likely reflects prior exposure to the organism. Diagnosing an acute scrub typhus infection in a patient in an endemic region requires PCR testing. The skin biopsy results highlight the importance of understanding test characteristics. A skin biopsy specimen must be adequate in order to draw valid and accurate conclusions. In this case, the initial skin biopsy was superficial, and the specimen inadequate, but the test was not “negative.” In the diagnostic skin biopsy, deeper tissue was sampled, and panniculitis (inflammation of subcutaneous fat), which arises in inflammatory, infectious, traumatic, enzymatic, and malignant conditions, was identified. An adequate biopsy specimen that contains subcutaneous fat is essential in making this diagnosis.2This patient eventually manifested several elements of hemophagocytic lymphohistiocytosis (HLH), a syndrome of excessive inflammation and resultant organ injury relating to abnormal immune activation and excessive inflammation. HLH results from deficient down-regulation of activated macrophages and lymphocytes.3 It was initially described in pediatric patients but is now recognized in adults, and associated with mortality as high as 50%.3 A high ferritin level (>2000 ng/mL) has 70% sensitivity and 68% specificity for pediatric HLH and should trigger consideration of HLH in any age group.4 The diagnostic criteria for HLH initially proposed in 2004 by the Histiocyte Society to identify patients for recruitment into a clinical trial included molecular testing consistent with HLH and/or 5 of 8 clinical, laboratory, or histopathologic features (Table 1).5 HScore is a more recent validated scoring system that predicts the probability of HLH (Table 2). A score above 169 signifies diagnostic sensitivity of 93% and specificity of 86%.6
The diagnosis of HLH warrants a search for its underlying cause. Common triggers are viral infections (eg, EBV), autoimmune diseases (eg, systemic lupus erythematosus), and hematologic malignancies. These triggers typically stimulate or suppress the immune system. Initial management involves treatment of the underlying trigger and, potentially, immunosuppression with high
In this case, SPTCL triggered HLH. SPTCL is a rare non-Hodgkin lymphoma characterized by painless subcutaneous nodules or indurated plaques (panniculitis-like) on the trunk or extremities, constitutional symptoms, and, in some cases, HLH.7-10 SPTCL is diagnosed by deep skin biopsy, with immunohistochemistry showing CD8-positive pathologic T cells expressing cytotoxic proteins (eg, granzyme B).9,11 SPTCL can either have an alpha/beta T-cell phenotype (SPTCL-AB) or gamma/delta T-cell phenotype (SPTCL-GD). Seventeen percent of patients with SPTCL-AB and 45% of patients with SPTCL-GD have HLH on diagnosis. Concomitant HLH is associated with decreased 5-year survival.12This patient presented with fevers and was ultimately diagnosed with HLH secondary to SPLTCL. His case is a reminder that not all diseases in the tropics are tropical diseases. In the diagnosis of a febrile illness, a broad evaluative framework and rigorous test results evaluation are essential—no matter where a patient lives or visits.
KEY TEACHING POINTS
- A febrile illness acquired in the tropics is not always attributable to a tropical infection.
- To avoid diagnostic error, weigh positive or negative test results against disease features, patient epidemiology, and test characteristics.
- HLH is characterized by fevers, cytopenias, hepatosplenomegaly, hyperferritinemia, hypertriglyceridemia, and hypofibrinogenemia. In tissue specimens, hemophagocytosis may help differentiate HLH from competing conditions.
- After HLH is diagnosed, try to determine its underlying cause, which may be an infection, autoimmunity, or a malignancy (commonly, a lymphoma).
Disclosure
Nothing to report.
1. Centers for Disease Control and Prevention. Destinations [list]. http://wwwnc.cdc.gov/travel/destinations/list/. Accessed April 22, 2016.
2. Diaz Cascajo C, Borghi S, Weyers W. Panniculitis: definition of terms and diagnostic strategy. Am J Dermatopathol. 2000;22(6):530-549. PubMed
3. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503-1516. PubMed
4. Lehmberg K, McClain KL, Janka GE, Allen CE. Determination of an appropriate cut-off value for ferritin in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2014;61(11):2101-2103. PubMed
5. Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. PubMed
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. PubMed
7. Aronson IK, Worobed CM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23(4):389-402. PubMed
8. Willemze R, Jansen PM, Cerroni L, et al; EORTC Cutaneous Lymphoma Group. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group study of 83 cases. Blood. 2008;111(2):838-845. PubMed
9. Kumar S, Krenacs L, Medeiros J, et al. Subcutaneous panniculitic T-cell lymphoma is a tumor of cytotoxic T lymphocytes. Hum Pathol. 1998;29(4):397-403. PubMed
10. Salhany KE, Macon WR, Choi JK, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinicopathologic, immunophenotypic, and genotypic analysis of alpha/beta and gamma/delta subtypes. Am J Surg Pathol. 1998;22(7):881-893. PubMed
11. Jaffe ES, Nicolae A, Pittaluga S. Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol. 2013;26(suppl 1):S71-S87. PubMed
12. Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M; ESMO Guidelines Working Group. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi149-vi154. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 42-year-old Malaysian construction worker with subjective fevers of 4 days’ duration presented to an emergency department in Singapore. He reported nonproductive cough, chills without rigors, sore throat, and body aches. He denied sick contacts. Past medical history included chronic hepatitis B virus (HBV) infection. The patient was not taking any medications.
For this patient presenting acutely with subjective fevers, nonproductive cough, chills, aches, and lethargy, initial considerations include infection with a common virus (influenza virus, adenovirus, Epstein-Barr virus [EBV]), acute human immunodeficiency virus (HIV) infection, emerging infection (severe acute respiratory syndrome [SARS], Middle Eastern respiratory syndrome coronavirus [MERS-CoV] infection, avian influenza), and tropical infection (dengue, chikungunya). Also possible are bacterial infections (eg, with Salmonella typhi or Rickettsia or Mycoplasma species), parasitic infections (eg, malaria), and noninfectious illnesses (eg, autoimmune diseases, thyroiditis, acute leukemia, environmental exposures).
The patient’s temperature was 38.5°C; blood pressure, 133/73 mm Hg; heart rate, 95 beats per minute; respiratory rate, 18 breaths per minute; and oxygen saturation, 100% on ambient air. On physical examination, he appeared comfortable, and heart, lung, abdomen, skin, and extremities were normal. Laboratory test results included white blood cell (WBC) count, 4400/μL (with normal differential); hemoglobin, 16.1 g/dL; and platelet count, 207,000/μL. Serum chemistries were normal. C-reactive protein (CRP) level was 44.6 mg/L (reference range, 0.2-9.1 mg/L), and procalcitonin level was 0.13 ng/mL (reference range, <0.50 ng/mL). Chest radiograph was normal. Dengue antibodies (immunoglobulin M, immunoglobulin G [IgG]) and dengue NS1 antigen were negative. The patient was discharged with a presumptive diagnosis of viral upper respiratory tract infection.
There is no left shift characteristic of bacterial infection or lymphopenia characteristic of rickettsial disease or acute HIV infection. The serologic testing and the patient’s overall appearance make dengue unlikely. The low procalcitonin supports a nonbacterial cause of illness. CRP elevation may indicate an inflammatory process and is relatively nonspecific.
Myalgias, pharyngitis, and cough improved over several days, but fevers persisted, and a rash developed over the lower abdomen. The patient returned to the emergency department and was admitted. He denied weight loss and night sweats. He had multiple female sexual partners, including commercial sex workers, within the previous 6 months. Temperature was 38.5°C. The posterior oropharynx was slightly erythematous. There was no lymphadenopathy. Firm, mildly erythematous macules were present on the anterior abdominal wall (Figure 1). The rest of the physical examination was normal.
Laboratory testing revealed WBC count, 5800/μL (75% neutrophils, 19% lymphocytes, 3% monocytes, 2% atypical mononuclear cells); hemoglobin, 16.3 g/dL; platelet count, 185,000/μL; sodium, 131 mmol/L; potassium, 3.4 mmol/L; creatinine, 0.9 mg/dL; albumin, 3.2 g/dL; alanine aminotransferase (ALT), 99 U/L; aspartate aminotransferase (AST), 137 U/L; alkaline phosphatase (ALP), 63 U/L; and total bilirubin, 1.9 mg/dL. Prothrombin time was 11.1 seconds; partial thromboplastin time, 36.1 seconds; erythrocyte sedimentation rate, 14 mm/h; and CRP, 62.2 mg/L.
EBV, acute HIV, and cytomegalovirus infections often present with adenopathy, which is absent here. Disseminated gonococcal infection can manifest with fever, body aches, and rash, but his rash and the absence of penile discharge, migratory arthritis, and enthesitis are not characteristic. Mycoplasma infection can present with macules, urticaria, or erythema multiforme. Rickettsia illnesses typically cause vasculitis with progression to petechiae or purpura resulting from endothelial damage. Patients with secondary syphilis may have widespread macular lesions, and the accompanying syphilitic hepatitis often manifests with elevations in ALP instead of ALT and AST. The mild elevation in ALT and AST can occur with many systemic viral infections. Sweet syndrome may manifest with febrile illness and rash, but the acuity of this patient’s illness and the rapid evolution favor infection.
The patient’s fevers (35°-40°C) continued without pattern over the next 3 days. Blood and urine cultures were negative. Polymerase chain reaction (PCR) test of the nasal mucosa was negative for respiratory viruses. PCR blood tests for EBV, HIV-1, and cytomegalovirus were also negative. Antistreptolysin O (ASO) titer was 400 IU/mm (reference range, <200 IU/mm). Antinuclear antibodies were negative, and rheumatoid factor was 12.4 U/mL (reference range, <10.3 U/mL). Computed tomography (CT) of the thorax, abdomen, and pelvis was normal. Results of a biopsy of an anterior abdominal wall skin lesion showed perivascular and periadnexal lymphocytic inflammation. Amoxicillin was started for the treatment of possible group A streptococcal infection.
PCR for HIV would be positive at a high level in acute HIV. The skin biopsy is not characteristic of Sweet syndrome, which typically shows neutrophilic infiltrate without leukocytoclastic vasculitis, or of syphilis, which typically shows a plasma cell infiltrate.
The patient’s erythematous oropharynx may indicate recent streptococcal pharyngitis. The fevers, elevated ASO titer, and CRP level are consistent with acute rheumatic fever, but arthritis, carditis, and neurologic manifestations are lacking. Erythema marginatum manifests on the trunk and limbs as macules or papules with central clearing as the lesions spread outward—and differs from the patient’s rash, which is firm and restricted to the abdominal wall.
Fevers persisted through hospital day 7. The WBC count was 1100/μL (75.7% neutrophils, 22.5% lymphocytes), hemoglobin was 10.3 g/dL, and platelet count was 52,000/μL. Additional laboratory test results included ALP, 234 U/L; ALT, 250 U/L; AST, 459 U/L; lactate dehydrogenase, 2303 U/L (reference range, 222-454 U/L); and ferritin, 14,964 ng/mL (reference range, 47-452 ng/mL).
The duration of illness and negative diagnostic tests for infections increases suspicion for a noninfectious illness. Conditions commonly associated with marked hyperferritinemia include adult-onset Still disease (AOSD) and hemophagocytic lymphohistiocytosis (HLH). Of the 9 AOSD diagnostic (Yamaguchi) criteria, 5 are met in this case: fever, rash, sore throat, abnormal liver function tests, and negative rheumatologic tests. However, the patient lacks arthritis, leukocytosis, lymphadenopathy, and hepatosplenomegaly. Except for the elevated ferritin, the AOSD criteria overlap substantially with the criteria for acute rheumatic fever, and still require that infections be adequately excluded. HLH, a state of abnormal immune activation with resultant organ dysfunction, can be a primary disorder, but in adults more often is secondary to underlying infectious, autoimmune, or malignant (often lymphoma) conditions. Elevated ferritin, cytopenias, elevated ALT and AST, elevated CRP and erythrocyte sedimentation rate, and elevated lactate dehydrogenase are consistent with HLH. The HLH diagnosis can be more firmly established with the more specific findings of hypertriglyceridemia, hypofibrinogenemia, and elevated soluble CD25 level. The histopathologic finding of hemophagocytosis in the bone marrow, lymph nodes, or liver may further support the diagnosis of HLH.
Rash and fevers persisted. Hepatitis A, hepatitis C, Rickettsia IgG, Burkholderia pseudomallei (the causative organism of melioidosis), and Leptospira serologies, as well as PCR for herpes simplex virus and parvovirus, were all negative. Hepatitis B viral load was 962 IU/mL (2.98 log), hepatitis B envelope antigen was negative, and hepatitis B envelope antibody was positive. Orientia tsutsugamushi (organism responsible for scrub typhus) IgG titer was elevated at 1:128. Antiliver kidney microsomal antibodies and antineutrophil cytoplasmic antibodies were negative. Fibrinogen level was 0.69 g/L (reference range, 1.8-4.8 g/L), and beta-2 microglobulin level was 5078 ng/mL (reference range, 878-2000 ng/mL). Bone marrow biopsy results showed hypocellular marrow with suppressed myelopoiesis, few atypical lymphoid cells, and few hemophagocytes. Flow cytometry was negative for clonal B lymphocytes and aberrant expression of T lymphocytes. Bone marrow myobacterial PCR and fungal cultures were negative.
The patient’s chronic HBV infection is unlikely to be related to his presentation given his low viral load and absence of signs of hepatic dysfunction. Excluding rickettsial disease requires paired acute and convalescent serologies. O tsutsugamushi, the causative agent of the rickettsial disease scrub typhus, is endemic in Malaysia; thus, his positive O tsutsugamushi IgG may indicate past exposure. His fevers, myalgias, truncal rash, and hepatitis are consistent with scrub typhus, but he lacks the characteristic severe headache and generalized lymphadenopathy. Although eschar formation with evolution of a papular rash is common in scrub typhus, it is often absent in the variant found in Southeast Asia. Although elevated β2 microglobulin level is used as a prognostic marker in multiple myeloma and Waldenström macroglobulinemia, it can be elevated in many immune-active states. The patient likely has HLH, which is supported by the hemophagocytosis seen on bone marrow biopsy, and the hypofibrinogenemia. Potential HLH triggers include O tsutsugamushi infection or recent streptococcal pharyngitis.
A deep-punch skin biopsy of the anterior abdominal wall skin lesion was performed because of the absence of subcutaneous fat in the first biopsy specimen. The latest biopsy results showed irregular interstitial expansion of medium-size lymphocytes in a lobular panniculated pattern. The lymphocytes contained enlarged, irregularly contoured nucleoli and were positive for T-cell markers CD2 and CD3 with reduction in CD5 expression. The lymphomatous cells were of CD8+ with uniform expression of activated cytotoxic granule protein granzyme B and were positive for T-cell hemireceptor β.
Positron emission tomography (PET) CT, obtained for staging purposes, showed multiple hypermetabolic subcutaneous and cutaneous lesions over the torso and upper and lower limbs—compatible with lymphomatous infiltrates (Figure 2). Examination, pathology, and imaging findings suggested a rare neoplasm: subcutaneous panniculitis-like T-cell lymphoma (SPTCL). SPTCL was confirmed by T-cell receptor gene rearrangements studies.
HLH was diagnosed on the basis of the fevers, cytopenias, hypofibrinogenemia, elevated ferritin level, and evidence of hemophagocytosis. SPTCL was suspected as the HLH trigger.
The patient was treated with cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone. While on this regimen, he developed new skin lesions, and his ferritin level was persistently elevated. He was switched to romidepsin, a histone deacetylase inhibitor that specifically targets cutaneous T-cell lymphoma, but the lesions continued to progress. The patient then was treated with gemcitabine, dexamethasone, and cisplatin, and the rashes resolved. The most recent PET-CT showed nearly complete resolution of the subcutaneous lesions.
DISCUSSION
When residents or visitors to tropical or sub-tropical regions, those located near or between the Tropics of Cancer and Capricorn, present with fever, physicians usually first think of infectious diseases. This patient’s case is a reminder that these important first considerations should not be the last.
Generating a differential diagnosis for tropical illnesses begins with the patient’s history. Factors to be considered include location (regional disease prevalence), exposures (food/water ingestion, outdoor work/recreation, sexual contact, animal contact), and timing (temporal relationship of symptom development to possible exposure). Common tropical infections are malaria, dengue, typhoid, and emerging infections such as chikungunya, avian influenza, and Zika virus infection.1This case underscores the need to analyze diagnostic tests critically. Interpreting tests as simply positive or negative, irrespective of disease features, epidemiology, and test characteristics, can contribute to diagnostic error. For example, the patient’s positive ASO titer requires an understanding of disease features and a nuanced interpretation based on the clinical presentation. The erythematous posterior oropharynx prompted concern for postinfectious sequelae of streptococcal pharyngitis, but his illness was more severe and more prolonged than is typical of that condition. The isolated elevated O tsutsugamushi IgG titer provides an example of the role of epidemiology in test interpretation. Although a single positive value might indicate a new exposure for a visitor to an endemic region, IgG seropositivity in Singapore, where scrub typhus is endemic, likely reflects prior exposure to the organism. Diagnosing an acute scrub typhus infection in a patient in an endemic region requires PCR testing. The skin biopsy results highlight the importance of understanding test characteristics. A skin biopsy specimen must be adequate in order to draw valid and accurate conclusions. In this case, the initial skin biopsy was superficial, and the specimen inadequate, but the test was not “negative.” In the diagnostic skin biopsy, deeper tissue was sampled, and panniculitis (inflammation of subcutaneous fat), which arises in inflammatory, infectious, traumatic, enzymatic, and malignant conditions, was identified. An adequate biopsy specimen that contains subcutaneous fat is essential in making this diagnosis.2This patient eventually manifested several elements of hemophagocytic lymphohistiocytosis (HLH), a syndrome of excessive inflammation and resultant organ injury relating to abnormal immune activation and excessive inflammation. HLH results from deficient down-regulation of activated macrophages and lymphocytes.3 It was initially described in pediatric patients but is now recognized in adults, and associated with mortality as high as 50%.3 A high ferritin level (>2000 ng/mL) has 70% sensitivity and 68% specificity for pediatric HLH and should trigger consideration of HLH in any age group.4 The diagnostic criteria for HLH initially proposed in 2004 by the Histiocyte Society to identify patients for recruitment into a clinical trial included molecular testing consistent with HLH and/or 5 of 8 clinical, laboratory, or histopathologic features (Table 1).5 HScore is a more recent validated scoring system that predicts the probability of HLH (Table 2). A score above 169 signifies diagnostic sensitivity of 93% and specificity of 86%.6
The diagnosis of HLH warrants a search for its underlying cause. Common triggers are viral infections (eg, EBV), autoimmune diseases (eg, systemic lupus erythematosus), and hematologic malignancies. These triggers typically stimulate or suppress the immune system. Initial management involves treatment of the underlying trigger and, potentially, immunosuppression with high
In this case, SPTCL triggered HLH. SPTCL is a rare non-Hodgkin lymphoma characterized by painless subcutaneous nodules or indurated plaques (panniculitis-like) on the trunk or extremities, constitutional symptoms, and, in some cases, HLH.7-10 SPTCL is diagnosed by deep skin biopsy, with immunohistochemistry showing CD8-positive pathologic T cells expressing cytotoxic proteins (eg, granzyme B).9,11 SPTCL can either have an alpha/beta T-cell phenotype (SPTCL-AB) or gamma/delta T-cell phenotype (SPTCL-GD). Seventeen percent of patients with SPTCL-AB and 45% of patients with SPTCL-GD have HLH on diagnosis. Concomitant HLH is associated with decreased 5-year survival.12This patient presented with fevers and was ultimately diagnosed with HLH secondary to SPLTCL. His case is a reminder that not all diseases in the tropics are tropical diseases. In the diagnosis of a febrile illness, a broad evaluative framework and rigorous test results evaluation are essential—no matter where a patient lives or visits.
KEY TEACHING POINTS
- A febrile illness acquired in the tropics is not always attributable to a tropical infection.
- To avoid diagnostic error, weigh positive or negative test results against disease features, patient epidemiology, and test characteristics.
- HLH is characterized by fevers, cytopenias, hepatosplenomegaly, hyperferritinemia, hypertriglyceridemia, and hypofibrinogenemia. In tissue specimens, hemophagocytosis may help differentiate HLH from competing conditions.
- After HLH is diagnosed, try to determine its underlying cause, which may be an infection, autoimmunity, or a malignancy (commonly, a lymphoma).
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 42-year-old Malaysian construction worker with subjective fevers of 4 days’ duration presented to an emergency department in Singapore. He reported nonproductive cough, chills without rigors, sore throat, and body aches. He denied sick contacts. Past medical history included chronic hepatitis B virus (HBV) infection. The patient was not taking any medications.
For this patient presenting acutely with subjective fevers, nonproductive cough, chills, aches, and lethargy, initial considerations include infection with a common virus (influenza virus, adenovirus, Epstein-Barr virus [EBV]), acute human immunodeficiency virus (HIV) infection, emerging infection (severe acute respiratory syndrome [SARS], Middle Eastern respiratory syndrome coronavirus [MERS-CoV] infection, avian influenza), and tropical infection (dengue, chikungunya). Also possible are bacterial infections (eg, with Salmonella typhi or Rickettsia or Mycoplasma species), parasitic infections (eg, malaria), and noninfectious illnesses (eg, autoimmune diseases, thyroiditis, acute leukemia, environmental exposures).
The patient’s temperature was 38.5°C; blood pressure, 133/73 mm Hg; heart rate, 95 beats per minute; respiratory rate, 18 breaths per minute; and oxygen saturation, 100% on ambient air. On physical examination, he appeared comfortable, and heart, lung, abdomen, skin, and extremities were normal. Laboratory test results included white blood cell (WBC) count, 4400/μL (with normal differential); hemoglobin, 16.1 g/dL; and platelet count, 207,000/μL. Serum chemistries were normal. C-reactive protein (CRP) level was 44.6 mg/L (reference range, 0.2-9.1 mg/L), and procalcitonin level was 0.13 ng/mL (reference range, <0.50 ng/mL). Chest radiograph was normal. Dengue antibodies (immunoglobulin M, immunoglobulin G [IgG]) and dengue NS1 antigen were negative. The patient was discharged with a presumptive diagnosis of viral upper respiratory tract infection.
There is no left shift characteristic of bacterial infection or lymphopenia characteristic of rickettsial disease or acute HIV infection. The serologic testing and the patient’s overall appearance make dengue unlikely. The low procalcitonin supports a nonbacterial cause of illness. CRP elevation may indicate an inflammatory process and is relatively nonspecific.
Myalgias, pharyngitis, and cough improved over several days, but fevers persisted, and a rash developed over the lower abdomen. The patient returned to the emergency department and was admitted. He denied weight loss and night sweats. He had multiple female sexual partners, including commercial sex workers, within the previous 6 months. Temperature was 38.5°C. The posterior oropharynx was slightly erythematous. There was no lymphadenopathy. Firm, mildly erythematous macules were present on the anterior abdominal wall (Figure 1). The rest of the physical examination was normal.
Laboratory testing revealed WBC count, 5800/μL (75% neutrophils, 19% lymphocytes, 3% monocytes, 2% atypical mononuclear cells); hemoglobin, 16.3 g/dL; platelet count, 185,000/μL; sodium, 131 mmol/L; potassium, 3.4 mmol/L; creatinine, 0.9 mg/dL; albumin, 3.2 g/dL; alanine aminotransferase (ALT), 99 U/L; aspartate aminotransferase (AST), 137 U/L; alkaline phosphatase (ALP), 63 U/L; and total bilirubin, 1.9 mg/dL. Prothrombin time was 11.1 seconds; partial thromboplastin time, 36.1 seconds; erythrocyte sedimentation rate, 14 mm/h; and CRP, 62.2 mg/L.
EBV, acute HIV, and cytomegalovirus infections often present with adenopathy, which is absent here. Disseminated gonococcal infection can manifest with fever, body aches, and rash, but his rash and the absence of penile discharge, migratory arthritis, and enthesitis are not characteristic. Mycoplasma infection can present with macules, urticaria, or erythema multiforme. Rickettsia illnesses typically cause vasculitis with progression to petechiae or purpura resulting from endothelial damage. Patients with secondary syphilis may have widespread macular lesions, and the accompanying syphilitic hepatitis often manifests with elevations in ALP instead of ALT and AST. The mild elevation in ALT and AST can occur with many systemic viral infections. Sweet syndrome may manifest with febrile illness and rash, but the acuity of this patient’s illness and the rapid evolution favor infection.
The patient’s fevers (35°-40°C) continued without pattern over the next 3 days. Blood and urine cultures were negative. Polymerase chain reaction (PCR) test of the nasal mucosa was negative for respiratory viruses. PCR blood tests for EBV, HIV-1, and cytomegalovirus were also negative. Antistreptolysin O (ASO) titer was 400 IU/mm (reference range, <200 IU/mm). Antinuclear antibodies were negative, and rheumatoid factor was 12.4 U/mL (reference range, <10.3 U/mL). Computed tomography (CT) of the thorax, abdomen, and pelvis was normal. Results of a biopsy of an anterior abdominal wall skin lesion showed perivascular and periadnexal lymphocytic inflammation. Amoxicillin was started for the treatment of possible group A streptococcal infection.
PCR for HIV would be positive at a high level in acute HIV. The skin biopsy is not characteristic of Sweet syndrome, which typically shows neutrophilic infiltrate without leukocytoclastic vasculitis, or of syphilis, which typically shows a plasma cell infiltrate.
The patient’s erythematous oropharynx may indicate recent streptococcal pharyngitis. The fevers, elevated ASO titer, and CRP level are consistent with acute rheumatic fever, but arthritis, carditis, and neurologic manifestations are lacking. Erythema marginatum manifests on the trunk and limbs as macules or papules with central clearing as the lesions spread outward—and differs from the patient’s rash, which is firm and restricted to the abdominal wall.
Fevers persisted through hospital day 7. The WBC count was 1100/μL (75.7% neutrophils, 22.5% lymphocytes), hemoglobin was 10.3 g/dL, and platelet count was 52,000/μL. Additional laboratory test results included ALP, 234 U/L; ALT, 250 U/L; AST, 459 U/L; lactate dehydrogenase, 2303 U/L (reference range, 222-454 U/L); and ferritin, 14,964 ng/mL (reference range, 47-452 ng/mL).
The duration of illness and negative diagnostic tests for infections increases suspicion for a noninfectious illness. Conditions commonly associated with marked hyperferritinemia include adult-onset Still disease (AOSD) and hemophagocytic lymphohistiocytosis (HLH). Of the 9 AOSD diagnostic (Yamaguchi) criteria, 5 are met in this case: fever, rash, sore throat, abnormal liver function tests, and negative rheumatologic tests. However, the patient lacks arthritis, leukocytosis, lymphadenopathy, and hepatosplenomegaly. Except for the elevated ferritin, the AOSD criteria overlap substantially with the criteria for acute rheumatic fever, and still require that infections be adequately excluded. HLH, a state of abnormal immune activation with resultant organ dysfunction, can be a primary disorder, but in adults more often is secondary to underlying infectious, autoimmune, or malignant (often lymphoma) conditions. Elevated ferritin, cytopenias, elevated ALT and AST, elevated CRP and erythrocyte sedimentation rate, and elevated lactate dehydrogenase are consistent with HLH. The HLH diagnosis can be more firmly established with the more specific findings of hypertriglyceridemia, hypofibrinogenemia, and elevated soluble CD25 level. The histopathologic finding of hemophagocytosis in the bone marrow, lymph nodes, or liver may further support the diagnosis of HLH.
Rash and fevers persisted. Hepatitis A, hepatitis C, Rickettsia IgG, Burkholderia pseudomallei (the causative organism of melioidosis), and Leptospira serologies, as well as PCR for herpes simplex virus and parvovirus, were all negative. Hepatitis B viral load was 962 IU/mL (2.98 log), hepatitis B envelope antigen was negative, and hepatitis B envelope antibody was positive. Orientia tsutsugamushi (organism responsible for scrub typhus) IgG titer was elevated at 1:128. Antiliver kidney microsomal antibodies and antineutrophil cytoplasmic antibodies were negative. Fibrinogen level was 0.69 g/L (reference range, 1.8-4.8 g/L), and beta-2 microglobulin level was 5078 ng/mL (reference range, 878-2000 ng/mL). Bone marrow biopsy results showed hypocellular marrow with suppressed myelopoiesis, few atypical lymphoid cells, and few hemophagocytes. Flow cytometry was negative for clonal B lymphocytes and aberrant expression of T lymphocytes. Bone marrow myobacterial PCR and fungal cultures were negative.
The patient’s chronic HBV infection is unlikely to be related to his presentation given his low viral load and absence of signs of hepatic dysfunction. Excluding rickettsial disease requires paired acute and convalescent serologies. O tsutsugamushi, the causative agent of the rickettsial disease scrub typhus, is endemic in Malaysia; thus, his positive O tsutsugamushi IgG may indicate past exposure. His fevers, myalgias, truncal rash, and hepatitis are consistent with scrub typhus, but he lacks the characteristic severe headache and generalized lymphadenopathy. Although eschar formation with evolution of a papular rash is common in scrub typhus, it is often absent in the variant found in Southeast Asia. Although elevated β2 microglobulin level is used as a prognostic marker in multiple myeloma and Waldenström macroglobulinemia, it can be elevated in many immune-active states. The patient likely has HLH, which is supported by the hemophagocytosis seen on bone marrow biopsy, and the hypofibrinogenemia. Potential HLH triggers include O tsutsugamushi infection or recent streptococcal pharyngitis.
A deep-punch skin biopsy of the anterior abdominal wall skin lesion was performed because of the absence of subcutaneous fat in the first biopsy specimen. The latest biopsy results showed irregular interstitial expansion of medium-size lymphocytes in a lobular panniculated pattern. The lymphocytes contained enlarged, irregularly contoured nucleoli and were positive for T-cell markers CD2 and CD3 with reduction in CD5 expression. The lymphomatous cells were of CD8+ with uniform expression of activated cytotoxic granule protein granzyme B and were positive for T-cell hemireceptor β.
Positron emission tomography (PET) CT, obtained for staging purposes, showed multiple hypermetabolic subcutaneous and cutaneous lesions over the torso and upper and lower limbs—compatible with lymphomatous infiltrates (Figure 2). Examination, pathology, and imaging findings suggested a rare neoplasm: subcutaneous panniculitis-like T-cell lymphoma (SPTCL). SPTCL was confirmed by T-cell receptor gene rearrangements studies.
HLH was diagnosed on the basis of the fevers, cytopenias, hypofibrinogenemia, elevated ferritin level, and evidence of hemophagocytosis. SPTCL was suspected as the HLH trigger.
The patient was treated with cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone. While on this regimen, he developed new skin lesions, and his ferritin level was persistently elevated. He was switched to romidepsin, a histone deacetylase inhibitor that specifically targets cutaneous T-cell lymphoma, but the lesions continued to progress. The patient then was treated with gemcitabine, dexamethasone, and cisplatin, and the rashes resolved. The most recent PET-CT showed nearly complete resolution of the subcutaneous lesions.
DISCUSSION
When residents or visitors to tropical or sub-tropical regions, those located near or between the Tropics of Cancer and Capricorn, present with fever, physicians usually first think of infectious diseases. This patient’s case is a reminder that these important first considerations should not be the last.
Generating a differential diagnosis for tropical illnesses begins with the patient’s history. Factors to be considered include location (regional disease prevalence), exposures (food/water ingestion, outdoor work/recreation, sexual contact, animal contact), and timing (temporal relationship of symptom development to possible exposure). Common tropical infections are malaria, dengue, typhoid, and emerging infections such as chikungunya, avian influenza, and Zika virus infection.1This case underscores the need to analyze diagnostic tests critically. Interpreting tests as simply positive or negative, irrespective of disease features, epidemiology, and test characteristics, can contribute to diagnostic error. For example, the patient’s positive ASO titer requires an understanding of disease features and a nuanced interpretation based on the clinical presentation. The erythematous posterior oropharynx prompted concern for postinfectious sequelae of streptococcal pharyngitis, but his illness was more severe and more prolonged than is typical of that condition. The isolated elevated O tsutsugamushi IgG titer provides an example of the role of epidemiology in test interpretation. Although a single positive value might indicate a new exposure for a visitor to an endemic region, IgG seropositivity in Singapore, where scrub typhus is endemic, likely reflects prior exposure to the organism. Diagnosing an acute scrub typhus infection in a patient in an endemic region requires PCR testing. The skin biopsy results highlight the importance of understanding test characteristics. A skin biopsy specimen must be adequate in order to draw valid and accurate conclusions. In this case, the initial skin biopsy was superficial, and the specimen inadequate, but the test was not “negative.” In the diagnostic skin biopsy, deeper tissue was sampled, and panniculitis (inflammation of subcutaneous fat), which arises in inflammatory, infectious, traumatic, enzymatic, and malignant conditions, was identified. An adequate biopsy specimen that contains subcutaneous fat is essential in making this diagnosis.2This patient eventually manifested several elements of hemophagocytic lymphohistiocytosis (HLH), a syndrome of excessive inflammation and resultant organ injury relating to abnormal immune activation and excessive inflammation. HLH results from deficient down-regulation of activated macrophages and lymphocytes.3 It was initially described in pediatric patients but is now recognized in adults, and associated with mortality as high as 50%.3 A high ferritin level (>2000 ng/mL) has 70% sensitivity and 68% specificity for pediatric HLH and should trigger consideration of HLH in any age group.4 The diagnostic criteria for HLH initially proposed in 2004 by the Histiocyte Society to identify patients for recruitment into a clinical trial included molecular testing consistent with HLH and/or 5 of 8 clinical, laboratory, or histopathologic features (Table 1).5 HScore is a more recent validated scoring system that predicts the probability of HLH (Table 2). A score above 169 signifies diagnostic sensitivity of 93% and specificity of 86%.6
The diagnosis of HLH warrants a search for its underlying cause. Common triggers are viral infections (eg, EBV), autoimmune diseases (eg, systemic lupus erythematosus), and hematologic malignancies. These triggers typically stimulate or suppress the immune system. Initial management involves treatment of the underlying trigger and, potentially, immunosuppression with high
In this case, SPTCL triggered HLH. SPTCL is a rare non-Hodgkin lymphoma characterized by painless subcutaneous nodules or indurated plaques (panniculitis-like) on the trunk or extremities, constitutional symptoms, and, in some cases, HLH.7-10 SPTCL is diagnosed by deep skin biopsy, with immunohistochemistry showing CD8-positive pathologic T cells expressing cytotoxic proteins (eg, granzyme B).9,11 SPTCL can either have an alpha/beta T-cell phenotype (SPTCL-AB) or gamma/delta T-cell phenotype (SPTCL-GD). Seventeen percent of patients with SPTCL-AB and 45% of patients with SPTCL-GD have HLH on diagnosis. Concomitant HLH is associated with decreased 5-year survival.12This patient presented with fevers and was ultimately diagnosed with HLH secondary to SPLTCL. His case is a reminder that not all diseases in the tropics are tropical diseases. In the diagnosis of a febrile illness, a broad evaluative framework and rigorous test results evaluation are essential—no matter where a patient lives or visits.
KEY TEACHING POINTS
- A febrile illness acquired in the tropics is not always attributable to a tropical infection.
- To avoid diagnostic error, weigh positive or negative test results against disease features, patient epidemiology, and test characteristics.
- HLH is characterized by fevers, cytopenias, hepatosplenomegaly, hyperferritinemia, hypertriglyceridemia, and hypofibrinogenemia. In tissue specimens, hemophagocytosis may help differentiate HLH from competing conditions.
- After HLH is diagnosed, try to determine its underlying cause, which may be an infection, autoimmunity, or a malignancy (commonly, a lymphoma).
Disclosure
Nothing to report.
1. Centers for Disease Control and Prevention. Destinations [list]. http://wwwnc.cdc.gov/travel/destinations/list/. Accessed April 22, 2016.
2. Diaz Cascajo C, Borghi S, Weyers W. Panniculitis: definition of terms and diagnostic strategy. Am J Dermatopathol. 2000;22(6):530-549. PubMed
3. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503-1516. PubMed
4. Lehmberg K, McClain KL, Janka GE, Allen CE. Determination of an appropriate cut-off value for ferritin in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2014;61(11):2101-2103. PubMed
5. Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. PubMed
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. PubMed
7. Aronson IK, Worobed CM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23(4):389-402. PubMed
8. Willemze R, Jansen PM, Cerroni L, et al; EORTC Cutaneous Lymphoma Group. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group study of 83 cases. Blood. 2008;111(2):838-845. PubMed
9. Kumar S, Krenacs L, Medeiros J, et al. Subcutaneous panniculitic T-cell lymphoma is a tumor of cytotoxic T lymphocytes. Hum Pathol. 1998;29(4):397-403. PubMed
10. Salhany KE, Macon WR, Choi JK, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinicopathologic, immunophenotypic, and genotypic analysis of alpha/beta and gamma/delta subtypes. Am J Surg Pathol. 1998;22(7):881-893. PubMed
11. Jaffe ES, Nicolae A, Pittaluga S. Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol. 2013;26(suppl 1):S71-S87. PubMed
12. Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M; ESMO Guidelines Working Group. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi149-vi154. PubMed
1. Centers for Disease Control and Prevention. Destinations [list]. http://wwwnc.cdc.gov/travel/destinations/list/. Accessed April 22, 2016.
2. Diaz Cascajo C, Borghi S, Weyers W. Panniculitis: definition of terms and diagnostic strategy. Am J Dermatopathol. 2000;22(6):530-549. PubMed
3. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503-1516. PubMed
4. Lehmberg K, McClain KL, Janka GE, Allen CE. Determination of an appropriate cut-off value for ferritin in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2014;61(11):2101-2103. PubMed
5. Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. PubMed
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. PubMed
7. Aronson IK, Worobed CM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23(4):389-402. PubMed
8. Willemze R, Jansen PM, Cerroni L, et al; EORTC Cutaneous Lymphoma Group. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group study of 83 cases. Blood. 2008;111(2):838-845. PubMed
9. Kumar S, Krenacs L, Medeiros J, et al. Subcutaneous panniculitic T-cell lymphoma is a tumor of cytotoxic T lymphocytes. Hum Pathol. 1998;29(4):397-403. PubMed
10. Salhany KE, Macon WR, Choi JK, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinicopathologic, immunophenotypic, and genotypic analysis of alpha/beta and gamma/delta subtypes. Am J Surg Pathol. 1998;22(7):881-893. PubMed
11. Jaffe ES, Nicolae A, Pittaluga S. Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol. 2013;26(suppl 1):S71-S87. PubMed
12. Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M; ESMO Guidelines Working Group. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi149-vi154. PubMed
© 2017 Society of Hospital Medicine