User login
Shared Decision-Making During Inpatient Rounds: Opportunities for Improvement in Patient Engagement and Communication
The ethos of medicine has shifted from paternalistic, physician-driven care to patient autonomy and engagement, in which the physician shares information and advises.1-3 Although there are ethical, legal, and practical reasons to respect patient preferences,1-4 patient engagement also fosters quality and safety5 and may improve clinical outcomes.5-8 Patients whose preferences are respected are more likely to trust their doctor, feel empowered, and adhere to treatments.9
Providers may partner with patients through shared decision-making (SDM).10,11 Several SDM models describe the process of providers and patients balancing evidence, preferences and context to arrive at a clinical decision.12-15 The National Academy of Medicine and the American Academy of Pediatrics has called for more SDM,16,17 including when clinical evidence is limited,2 equally beneficial options exist,18 clinical stakes are high,19 and even with deferential patients.20 Despite its value, SDM does not reliably occur21,22 and SDM training is often unavailable.4 Clinical decision tools, patient education aids, and various training interventions have shown promising, although inconsistent results.23, 24
Little is known about SDM in inpatient settings where unique patient, clinician, and environmental factors may influence SDM. This study describes the quality and possible predictors of inpatient SDM during attending rounds in 4 academic training settings. Although SDM may occur anytime during a hospitalization, attending rounds present a valuable opportunity for SDM observation given their centrality to inpatient care and teaching.25,26 Because attending physicians bear ultimate responsibility for patient management, we examined whether SDM performance varies among attendings within each service. In addition, we tested the hypothesis that service-level, team-level, and patient-level features explain variation in SDM quality more than individual attending physicians. Finally, we compared peer-observer perspectives of SDM behaviors with patient and/or guardian perspectives.
METHODS
Study Design and Setting
This cross-sectional, observational study examined the diversity of SDM practice within and between 4 inpatient services during attending rounds, including the internal medicine and pediatrics services at Stanford University and the University of California, San Francisco (UCSF). Both institutions provide quaternary care to diverse patient populations with approximately half enrolled in Medicare and/or Medicaid.
One institution had 42 internal medicine (Med-1) and 15 pediatric hospitalists (Peds-1) compared to 8 internal medicine (Med-2) and 12 pediatric hospitalists (Peds-2) at the second location. Both pediatric services used family-centered rounds that included discussions between the patients’ families and the whole team. One medicine service used a similar rounding model that did not necessarily involve the patients’ families. In contrast, the smaller medicine service typically began rounds by discussing all patients in a conference room and then visiting select patients afterwards.
From August 2014 to November 2014, peer observers gathered data on team SDM behaviors during attending rounds. After the rounding team departed, nonphysician interviewers surveyed consenting patients’ (or guardians’) views of the SDM experience, yielding paired evaluations for a subset of SDM encounters. Institutional review board approval was obtained from Stanford University and UCSF.
Participants and Inclusion Criteria
Attending physicians were hospitalists who supervised rounds at least 1 month per year, and did not include those conducting the study. All provided verbal assent to be observed on 3 days within a 7-day period. While team composition varied as needed (eg, to include the nurse, pharmacist, interpreter, etc), we restricted study observations to those teams with an attending and at least one learner (eg, resident, intern, medical student) to capture the influence of attending physicians in their training role. Because services vary in number of attendings on staff, rounds assigned per attending, and patients per round, it was not possible to enroll equal sample sizes per service in the study.
Nonintensive care unit patients who were deemed medically stable by the team were eligible for peer observation and participation in a subsequent patient interview once during the study period. Pediatric patients were invited for an interview if they were between 13 and 21 years old and had the option of having a parent or guardian present; if the pediatric patients were less than 13 years old or they were not interested in being interviewed, then their parents or guardians were invited to be interviewed. Interpreters were on rounds, and thus, non-English participants were able to participate in the peer observations, but could not participate in patient interviews because interpreters were not available during afternoons for study purposes. Consent was obtained from all participating patients and/or guardians.
Data Collection
Round and Patient Characteristics
Peer observers recorded rounding, team, and patient characteristics using a standardized form. Rounding data included date, attending name, duration of rounds, and patient census. Patient level data included the decision(s) discussed, the seniority of the clinician leading the discussion, team composition, minutes spent discussing the patient (both with the patient and/or guardian and total time), hospitalization week, and patient’s primary language. Additional patient data obtained from electronic health records included age, gender, race, ethnicity, date of admission, and admitting diagnosis.
SDM Measures
Peer-observed SDM behaviors were quantified per patient encounter using the 9-item Rochester Participatory Decision-Making Scale (RPAD), with credit given for SDM behaviors exhibited by anyone on the rounding team (team-level metric).27 Each item was scored on a 3-point scale (0 = absent, 0.5 = partial, and 1 = present) for a maximum of 9 points, with higher scores indicating higher-quality SDM (Peer-RPAD Score). We created semistructured patient interview guides by adapting each RPAD item into layperson language (Patient-RPAD Score) and adding open-ended questions to assess the patient experience.
Peer-Observer Training
Eight peer-observers (7 hospitalists and 1 palliative care physician) were trained to perform RPAD ratings using videos of patient encounters. Initially, raters viewed videos together and discussed ratings for each RPAD item. The observers incorporated behavioral anchors and clinical examples into the development of an RPAD rating guide, which they subsequently used to independently score 4 videos from an online medical communication library.28 These scores were discussed to resolve any differences before 4 additional videos were independently viewed, scored, and compared. Interrater reliability was achieved when the standard deviation of summed SDM scores across raters was less than 1 for all 4 videos.
Patient Interviewers
Interviewers were English-speaking volunteers without formal medical training. They were educated in hospital etiquette by a physician and in administering patient interviews through peer-to-peer role playing and an observation and feedback interview with at least 1 patient.
Data Analysis
The analysis set included every unique patient with whom a medical decision was made by an eligible clinical team. To account for the nested study design (patient-level scores within rounds, rounds within attending, and attendings within service), we used mixed-effects models to estimate mean (summary or item) RPAD score by levels of fixed covariate(s). The models included random effects accounting for attending-level and round-level correlations among scores via variance components, and allowing the attending-level random effect to differ by service. Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). We used descriptive statistics to summarize round- and patient-level characteristics.
SDM Variation by Attending and Service
Box plots were used to summarize raw patient-level, Peer-RPAD scores by service and attending. By using the methods described above, we estimated the mean score overall and by service. In both models, we examined the statistical significance of service-specific variation in attending-level random effects by using likelihood-ratio test (LRT) to compare models.
SDM Variation by Round and Patient Characteristics
We used the models described above to identify covariates associated with Peer-RPAD scores. We fit univariate models separately for each covariate, then fit 2 multivariable models, including (1) all covariates and (2) all effects significant in either model at P ≤ .20 according to F tests. For uniformity of presentation, we express continuous covariates categorically; however, we report P values based on continuous versions. Means generated by the multivariable models were calculated at the mean values of all other covariates in model.
Patient-Level RPAD Data
A subsample of patients completed semistructured interviews with analogous RPAD questions. To identify possible selection bias in the full sample, we summarized response rates by service and patient language and modeled Peer-RPAD scores by interview response status. Among responders, we estimated the mean Peer-RPAD and Patient-RPAD scores and their paired differences and correlations, testing for non-zero correlations via the Spearman rank test.
RESULTS
All Patient Encounters
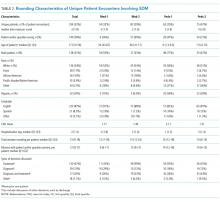
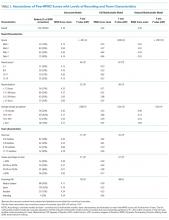
The median duration of rounding sessions was 1.8 hours, median patient census was 9, and median patient encounter was 13 minutes. The duration of rounds and minutes per patient were longest at Med-2 and shortest at Peds-1. See Table 1 for other team characteristics.
Peer Evaluations of SDM Encounters
Characteristics of Patients
Teams spent a median of 13 minutes per SDM encounter, which was not higher than the round median. SDM topics discussed included 47% treatment, 15% diagnostic, 30% both treatment and diagnostic, and 7% other.
Variation in SDM Quality Among Attending Physicians
Overall Peer-RPAD Scores were normally distributed. After adjusting for the nested study design, the overall mean (standard error) score was 4.16 (0.11). Score variability among attendings differed significantly by service (LRT P = .0067). For example, raw scores were lower and more variable among attending physicians at Med-2 than other among attendings in other services (see Appendix Figure in Supporting Information). However, when service was included in the model as a fixed effect, mean scores varied significantly, from 3.0 at Med-2 to 4.7 at Med-1 (P < .0001), but the random variation among attendings no longer differed significantly by service (P = .13). This finding supports the hypothesis that service-level influences are stronger than influences of individual attending physicians, that is, that variation between services exceeded variation among attendings within service.
Aspects of SDM That Are More Prevalent on Rounds
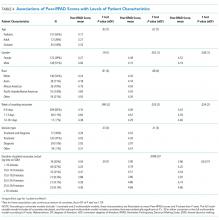
Rounds and Patient Characteristics Associated With Peer-RPAD Scores
Patient-RPAD Results: Dissimilar Perspectives of Patients and/or Guardians and Physician Observers
Among responders, mean Patient-RPAD scores were 6.8 to 7.1 for medicine services and 7.6 to 7.8 for pediatric services (P = .01). The overall mean Patient-RPAD score, 7.46, was significantly greater than the paired Peer-RPAD score by 3.5 (P = .011); however, correlations were not statistically significantly different from 0 (by service, each P > .12).
To understand drivers of the differences between Peer-RPAD and Patient-RPAD scores, we analyzed findings by item. Each mean patient-item score exceeded its peer counterpart (P ≤ .01; panel B of Figure). Peer-item scores fell below 33% on 2 items (Items 9 and 4) and only exceeded 67% on 2 items (Items 1 and 6), whereas patient-item scores ranged from 60% (Item 8) to 97% (Item 7). Three paired differences exceeded 50% (Items 9, 4, and 7) and 3 were below 20% (Items 6, 8 and 1), underlying the lack of correlation between peer and patient scores.
DISCUSSION
In this multisite study of SDM during inpatient attending rounds, SDM quality, specific SDM behaviors, and factors contributing to SDM were identified. Our study found an adjusted overall Peer-RPAD Score of 4.4 out of 9, and found the following 3 SDM elements most needing improvement according to trained peer observers: (1) “Checking understanding of the patient’s perspective”, (2) “Examining barriers to follow-through with the treatment plan”, and (3) “Asking if the patient has questions.” Areas of strength included explaining the clinical issue or nature of the decision and matching medical language to the patient’s level of understanding, with each rated highly by both peer-observers and patients. Broadly speaking, physicians were skillful in delivering information to patients but failed to solicit input from patients. Characteristics associated with increased SDM in the multivariate analysis included the following: service, patient gender, timing of rounds during patient’s hospital stay, and amount of time rounding with each patient.
Patients similarly found that physicians could improve their abilities to elicit information from patients and families, noting the 3 lowest patient-rated SDM elements were as follows: (1) asking open-ended questions, (2) discussing alternatives or uncertainties, and (3) discussing barriers to treatment plan follow through. Overall, patients and guardians perceived the quantity and quality of SDM on rounds more favorably than peer observers, which is consistent with other studies of patient perceptions of communication. 29-31 It is possible that patient ratings are more influenced by demand characteristics, fear of negatively impacting their patient-provider relationships, and conflation of overall satisfaction with quality of communication.32 This difference in patient perception of SDM is worthy of further study.
Prior work has revealed that SDM may occur infrequently during inpatient rounds.11 This study further elucidates specific SDM behaviors used along with univariate and multivariate modeling to explore possible contributing factors. The strengths and weaknesses found were similar at all 4 services and the influence of the service was more important than variability across attendings. This study’s findings are similar to a study by Shields et al.,33 in which the findings in a geographically different outpatient setting 10 years earlier suggesting global and enduring challenges to SDM. To our knowledge, this is the first published study to characterize inpatient SDM behaviors and may serve as the basis for future interventions.
Although the item-level components were ranked similarly across services, on average the summary Peer-RPAD score was lowest at Med-2, where we observed high variability within and between attendings, and was highest at Med-1, where variability was low. Med-2 carried the highest caseload and held the longest rounds, while Med-1 carried the lowest caseload, suggesting that modifiable burdens may hamper SDM performance. Prior studies suggest that patients are often selected based on teaching opportunities, immediate medical need and being newly admitted.34 The high scores at Med-1 may reflect that service’s prediscussion of patients during card-flipping rounds or their selection of which patients to round on as a team. Consistent with prior studies29,35 of SDM and the family-centered rounding model, which includes the involvement of nurses, respiratory therapists, pharmacists, case managers, social workers, and interpreters on rounds, both pediatrics services showed higher SDM scores.
In contrast to prior studies,34,36 team size and number of learners did not affect SDM performance, nor did decision type. Despite teams having up to 17 members, 8 learners, and 14 complex patients, SDM scores did not vary significantly by team. Nonetheless, trends were in the directions expected: Scores tended to decrease as the team size or the percentage of trainees grew, and increased with the seniority of the presenting physician. Interestingly, SDM performance decreased with round-average minutes per patient, which may be measuring on-going intensity across cases that leads to exhaustion. Statistically significant patient factors for increased SDM included longer duration of patient encounters, second week of hospital stay, and female patient gender. Although we anticipated that the high number of decisions made early in hospitalization would facilitate higher SDM scores, continuity and stronger patient-provider relationships may enhance SDM.36 We report service-specific team and patient characteristics, in addition to SDM findings in anticipation that some readers will identify with 1 service more than others.
This study has several important limitations. First, our peer observers were not blinded and primarily observed encounters at their own site. To minimize bias, observers periodically rated videos to recalibrate RPAD scoring. Second, additional SDM conversations with a patient and/or guardian may have occurred outside of rounds and were not captured, and poor patient recall may have affected Patient-RPAD scores despite interviewer prompts and timeliness of interviews within 12 hours of rounds. Third, there might have been a selection bias for the one service who selected a smaller number of patients to see, compared with the three other services that performed bedside rounds on all patients. It is possible that attending physicians selected patients who were deemed most able to have SDM conversations, thus affecting RPAD scores on that service. Fourth, study services had fewer patients on average than other academic hospitals (median 9, range 3-14), which might limit its generalizability. Last, as in any observational study, there is always the possibility of the Hawthorne effect. However, neither teams nor patients knew the study objectives.
Nevertheless, important findings emerged through the use of RPAD Scores to evaluate inpatient SDM practices. In particular, we found that to increase SDM quality in inpatient settings, practitioners should (1) check their understanding of the patient’s perspective, (2) examine barriers to follow-through with the treatment plan, and (3) ask if the patient has questions. Variation among services remained very influential after adjusting for team and patient characteristics, which suggests that “climate” or service culture should be targeted by an intervention, rather than individual attendings or subgroups defined by team or patient characteristics. Notably, team size, number of learners, patient census, and type of decision being made did not affect SDM performance, suggesting that even large, busy services can perform SDM if properly trained.
Acknowledgments
The authors thank the patients, families, pediatric and internal medicine residents, and hospitalists at Stanford School of Medicine and University of California, San Francisco School of Medicine for their participation in this study. We would also like to thank the student volunteers who collected patient perspectives on the encounters.
Disclosure
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an NIH/NCCIH grant R25 AT006573.
1. Braddock CH. The emerging importance and relevance of shared decision making to clinical practice. Med Decis Mak. 2010;30(5 Suppl):5S-7S. doi:10.1177/0272989X10381344. PubMed
2. Braddock CH. Supporting shared decision making when clinical evidence is low. Med Care Res Rev MCRR. 2013;70(1 Suppl):129S-140S. doi:10.1177/1077558712460280. PubMed
3. Elwyn G, Tilburt J, Montori V. The ethical imperative for shared decision-making. Eur J Pers Centered Healthc. 2013;1(1):129-131. doi:10.5750/ejpch.v1i1.645.
4. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
5. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(10):CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
6. Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566-577. doi:10.1164/rccm.200906-0907OC. PubMed
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410-417. doi:10.1370/afm.1161. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
10. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781. doi:10.1056/NEJMp1109283. PubMed
11. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med J Assoc Am Med Coll. 2014;89(11):1548-1557. doi:10.1097/ACM.0000000000000483. PubMed
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692. PubMed
13. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
14. Légaré F, St-Jacques S, Gagnon S, et al. Prenatal screening for Down syndrome: a survey of willingness in women and family physicians to engage in shared decision-making. Prenat Diagn. 2011;31(4):319-326. doi:10.1002/pd.2624. PubMed
15. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. Milbank Q. 2009;87(2):368-390. PubMed
16. National Academy of Medicine. Crossing the quality chasm: a new health system for the 21st century. https://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed on November 30, 2016.
17. Adams RC, Levy SE, Council on Children with Disabilities. Shared Decision-Making and Children with Disabilities: Pathways to Consensus. Pediatrics. 2017; 139(6):1-9. PubMed
18. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
19. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
20. Kehl KL, Landrum MB, Arora NK, et al. Association of Actual and Preferred Decision Roles With Patient-Reported Quality of Care: Shared Decision Making in Cancer Care. JAMA Oncol. 2015;1(1):50-58. doi:10.1001/jamaoncol.2014.112. PubMed
21. Couët N, Desroches S, Robitaille H, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect Int J Public Particip Health Care Health Policy. 2015;18(4):542-561. doi:10.1111/hex.12054. PubMed
22. Fowler FJ, Gerstein BS, Barry MJ. How patient centered are medical decisions?: Results of a national survey. JAMA Intern Med. 2013;173(13):1215-1221. doi:10.1001/jamainternmed.2013.6172. PubMed
23. Légaré F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2014;(9):CD006732. doi:10.1002/14651858.CD006732.pub3. PubMed
24. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;(10):CD001431. doi:10.1002/14651858.CD001431.pub3. PubMed
25. Di Francesco L, Pistoria MJ, Auerbach AD, Nardino RJ, Holmboe ES. Internal medicine training in the inpatient setting. A review of published educational interventions. J Gen Intern Med. 2005;20(12):1173-1180. doi:10.1111/j.1525-1497.2005.00250.x. PubMed
26. Janicik RW, Fletcher KE. Teaching at the bedside: a new model. Med Teach. 2003;25(2):127-130. PubMed
27. Shields CG, Franks P, Fiscella K, Meldrum S, Epstein RM. Rochester Participatory Decision-Making Scale (RPAD): reliability and validity. Ann Fam Med. 2005;3(5):436-442. doi:10.1370/afm.305. PubMed
28. DocCom - enhancing competence in healthcare communication. https://webcampus.drexelmed.edu/doccom/user/. Accessed on November 30, 2016.
29. Bailey SM, Hendricks-Muñoz KD, Mally P. Parental influence on clinical management during neonatal intensive care: a survey of US neonatologists. J Matern Fetal Neonatal Med. 2013;26(12):1239-1244. doi:10.3109/14767058.2013.776531. PubMed
30. Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091-3098. doi:10.1200/JCO.2004.09.069. PubMed
31. Schoenborn NL, Cayea D, McNabney M, Ray A, Boyd C. Prognosis communication with older patients with multimorbidity: Assessment after an educational intervention. Gerontol Geriatr Educ. 2016;38(4):471-481. doi:10.1080/02701960.2015.1115983. PubMed
32. Lipkin M. Shared decision making. JAMA Intern Med. 2013;173(13):1204-1205. doi:10.1001/jamainternmed.2013.6248. PubMed
33. Gonzalo JD, Heist BS, Duffy BL, et al. The art of bedside rounds: a multi-center qualitative study of strategies used by experienced bedside teachers. J Gen Intern Med. 2013;28(3):412-420. doi:10.1007/s11606-012-2259-2. PubMed
34. Rosen P, Stenger E, Bochkoris M, Hannon MJ, Kwoh CK. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics. 2009;123(4):e603-e608. doi:10.1542/peds.2008-2238. PubMed
35. Harrison R, Allen E. Teaching internal medicine residents in the new era. Inpatient attending with duty-hour regulations. J Gen Intern Med. 2006;21(5):447-452. doi:10.1111/j.1525-1497.2006.00425.x. PubMed
36. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med 1982. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056. PubMed
The ethos of medicine has shifted from paternalistic, physician-driven care to patient autonomy and engagement, in which the physician shares information and advises.1-3 Although there are ethical, legal, and practical reasons to respect patient preferences,1-4 patient engagement also fosters quality and safety5 and may improve clinical outcomes.5-8 Patients whose preferences are respected are more likely to trust their doctor, feel empowered, and adhere to treatments.9
Providers may partner with patients through shared decision-making (SDM).10,11 Several SDM models describe the process of providers and patients balancing evidence, preferences and context to arrive at a clinical decision.12-15 The National Academy of Medicine and the American Academy of Pediatrics has called for more SDM,16,17 including when clinical evidence is limited,2 equally beneficial options exist,18 clinical stakes are high,19 and even with deferential patients.20 Despite its value, SDM does not reliably occur21,22 and SDM training is often unavailable.4 Clinical decision tools, patient education aids, and various training interventions have shown promising, although inconsistent results.23, 24
Little is known about SDM in inpatient settings where unique patient, clinician, and environmental factors may influence SDM. This study describes the quality and possible predictors of inpatient SDM during attending rounds in 4 academic training settings. Although SDM may occur anytime during a hospitalization, attending rounds present a valuable opportunity for SDM observation given their centrality to inpatient care and teaching.25,26 Because attending physicians bear ultimate responsibility for patient management, we examined whether SDM performance varies among attendings within each service. In addition, we tested the hypothesis that service-level, team-level, and patient-level features explain variation in SDM quality more than individual attending physicians. Finally, we compared peer-observer perspectives of SDM behaviors with patient and/or guardian perspectives.
METHODS
Study Design and Setting
This cross-sectional, observational study examined the diversity of SDM practice within and between 4 inpatient services during attending rounds, including the internal medicine and pediatrics services at Stanford University and the University of California, San Francisco (UCSF). Both institutions provide quaternary care to diverse patient populations with approximately half enrolled in Medicare and/or Medicaid.
One institution had 42 internal medicine (Med-1) and 15 pediatric hospitalists (Peds-1) compared to 8 internal medicine (Med-2) and 12 pediatric hospitalists (Peds-2) at the second location. Both pediatric services used family-centered rounds that included discussions between the patients’ families and the whole team. One medicine service used a similar rounding model that did not necessarily involve the patients’ families. In contrast, the smaller medicine service typically began rounds by discussing all patients in a conference room and then visiting select patients afterwards.
From August 2014 to November 2014, peer observers gathered data on team SDM behaviors during attending rounds. After the rounding team departed, nonphysician interviewers surveyed consenting patients’ (or guardians’) views of the SDM experience, yielding paired evaluations for a subset of SDM encounters. Institutional review board approval was obtained from Stanford University and UCSF.
Participants and Inclusion Criteria
Attending physicians were hospitalists who supervised rounds at least 1 month per year, and did not include those conducting the study. All provided verbal assent to be observed on 3 days within a 7-day period. While team composition varied as needed (eg, to include the nurse, pharmacist, interpreter, etc), we restricted study observations to those teams with an attending and at least one learner (eg, resident, intern, medical student) to capture the influence of attending physicians in their training role. Because services vary in number of attendings on staff, rounds assigned per attending, and patients per round, it was not possible to enroll equal sample sizes per service in the study.
Nonintensive care unit patients who were deemed medically stable by the team were eligible for peer observation and participation in a subsequent patient interview once during the study period. Pediatric patients were invited for an interview if they were between 13 and 21 years old and had the option of having a parent or guardian present; if the pediatric patients were less than 13 years old or they were not interested in being interviewed, then their parents or guardians were invited to be interviewed. Interpreters were on rounds, and thus, non-English participants were able to participate in the peer observations, but could not participate in patient interviews because interpreters were not available during afternoons for study purposes. Consent was obtained from all participating patients and/or guardians.
Data Collection
Round and Patient Characteristics
Peer observers recorded rounding, team, and patient characteristics using a standardized form. Rounding data included date, attending name, duration of rounds, and patient census. Patient level data included the decision(s) discussed, the seniority of the clinician leading the discussion, team composition, minutes spent discussing the patient (both with the patient and/or guardian and total time), hospitalization week, and patient’s primary language. Additional patient data obtained from electronic health records included age, gender, race, ethnicity, date of admission, and admitting diagnosis.
SDM Measures
Peer-observed SDM behaviors were quantified per patient encounter using the 9-item Rochester Participatory Decision-Making Scale (RPAD), with credit given for SDM behaviors exhibited by anyone on the rounding team (team-level metric).27 Each item was scored on a 3-point scale (0 = absent, 0.5 = partial, and 1 = present) for a maximum of 9 points, with higher scores indicating higher-quality SDM (Peer-RPAD Score). We created semistructured patient interview guides by adapting each RPAD item into layperson language (Patient-RPAD Score) and adding open-ended questions to assess the patient experience.
Peer-Observer Training
Eight peer-observers (7 hospitalists and 1 palliative care physician) were trained to perform RPAD ratings using videos of patient encounters. Initially, raters viewed videos together and discussed ratings for each RPAD item. The observers incorporated behavioral anchors and clinical examples into the development of an RPAD rating guide, which they subsequently used to independently score 4 videos from an online medical communication library.28 These scores were discussed to resolve any differences before 4 additional videos were independently viewed, scored, and compared. Interrater reliability was achieved when the standard deviation of summed SDM scores across raters was less than 1 for all 4 videos.
Patient Interviewers
Interviewers were English-speaking volunteers without formal medical training. They were educated in hospital etiquette by a physician and in administering patient interviews through peer-to-peer role playing and an observation and feedback interview with at least 1 patient.
Data Analysis
The analysis set included every unique patient with whom a medical decision was made by an eligible clinical team. To account for the nested study design (patient-level scores within rounds, rounds within attending, and attendings within service), we used mixed-effects models to estimate mean (summary or item) RPAD score by levels of fixed covariate(s). The models included random effects accounting for attending-level and round-level correlations among scores via variance components, and allowing the attending-level random effect to differ by service. Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). We used descriptive statistics to summarize round- and patient-level characteristics.
SDM Variation by Attending and Service
Box plots were used to summarize raw patient-level, Peer-RPAD scores by service and attending. By using the methods described above, we estimated the mean score overall and by service. In both models, we examined the statistical significance of service-specific variation in attending-level random effects by using likelihood-ratio test (LRT) to compare models.
SDM Variation by Round and Patient Characteristics
We used the models described above to identify covariates associated with Peer-RPAD scores. We fit univariate models separately for each covariate, then fit 2 multivariable models, including (1) all covariates and (2) all effects significant in either model at P ≤ .20 according to F tests. For uniformity of presentation, we express continuous covariates categorically; however, we report P values based on continuous versions. Means generated by the multivariable models were calculated at the mean values of all other covariates in model.
Patient-Level RPAD Data
A subsample of patients completed semistructured interviews with analogous RPAD questions. To identify possible selection bias in the full sample, we summarized response rates by service and patient language and modeled Peer-RPAD scores by interview response status. Among responders, we estimated the mean Peer-RPAD and Patient-RPAD scores and their paired differences and correlations, testing for non-zero correlations via the Spearman rank test.
RESULTS
All Patient Encounters
The median duration of rounding sessions was 1.8 hours, median patient census was 9, and median patient encounter was 13 minutes. The duration of rounds and minutes per patient were longest at Med-2 and shortest at Peds-1. See Table 1 for other team characteristics.
Peer Evaluations of SDM Encounters
Characteristics of Patients
Teams spent a median of 13 minutes per SDM encounter, which was not higher than the round median. SDM topics discussed included 47% treatment, 15% diagnostic, 30% both treatment and diagnostic, and 7% other.
Variation in SDM Quality Among Attending Physicians
Overall Peer-RPAD Scores were normally distributed. After adjusting for the nested study design, the overall mean (standard error) score was 4.16 (0.11). Score variability among attendings differed significantly by service (LRT P = .0067). For example, raw scores were lower and more variable among attending physicians at Med-2 than other among attendings in other services (see Appendix Figure in Supporting Information). However, when service was included in the model as a fixed effect, mean scores varied significantly, from 3.0 at Med-2 to 4.7 at Med-1 (P < .0001), but the random variation among attendings no longer differed significantly by service (P = .13). This finding supports the hypothesis that service-level influences are stronger than influences of individual attending physicians, that is, that variation between services exceeded variation among attendings within service.
Aspects of SDM That Are More Prevalent on Rounds
Rounds and Patient Characteristics Associated With Peer-RPAD Scores
Patient-RPAD Results: Dissimilar Perspectives of Patients and/or Guardians and Physician Observers
Among responders, mean Patient-RPAD scores were 6.8 to 7.1 for medicine services and 7.6 to 7.8 for pediatric services (P = .01). The overall mean Patient-RPAD score, 7.46, was significantly greater than the paired Peer-RPAD score by 3.5 (P = .011); however, correlations were not statistically significantly different from 0 (by service, each P > .12).
To understand drivers of the differences between Peer-RPAD and Patient-RPAD scores, we analyzed findings by item. Each mean patient-item score exceeded its peer counterpart (P ≤ .01; panel B of Figure). Peer-item scores fell below 33% on 2 items (Items 9 and 4) and only exceeded 67% on 2 items (Items 1 and 6), whereas patient-item scores ranged from 60% (Item 8) to 97% (Item 7). Three paired differences exceeded 50% (Items 9, 4, and 7) and 3 were below 20% (Items 6, 8 and 1), underlying the lack of correlation between peer and patient scores.
DISCUSSION
In this multisite study of SDM during inpatient attending rounds, SDM quality, specific SDM behaviors, and factors contributing to SDM were identified. Our study found an adjusted overall Peer-RPAD Score of 4.4 out of 9, and found the following 3 SDM elements most needing improvement according to trained peer observers: (1) “Checking understanding of the patient’s perspective”, (2) “Examining barriers to follow-through with the treatment plan”, and (3) “Asking if the patient has questions.” Areas of strength included explaining the clinical issue or nature of the decision and matching medical language to the patient’s level of understanding, with each rated highly by both peer-observers and patients. Broadly speaking, physicians were skillful in delivering information to patients but failed to solicit input from patients. Characteristics associated with increased SDM in the multivariate analysis included the following: service, patient gender, timing of rounds during patient’s hospital stay, and amount of time rounding with each patient.
Patients similarly found that physicians could improve their abilities to elicit information from patients and families, noting the 3 lowest patient-rated SDM elements were as follows: (1) asking open-ended questions, (2) discussing alternatives or uncertainties, and (3) discussing barriers to treatment plan follow through. Overall, patients and guardians perceived the quantity and quality of SDM on rounds more favorably than peer observers, which is consistent with other studies of patient perceptions of communication. 29-31 It is possible that patient ratings are more influenced by demand characteristics, fear of negatively impacting their patient-provider relationships, and conflation of overall satisfaction with quality of communication.32 This difference in patient perception of SDM is worthy of further study.
Prior work has revealed that SDM may occur infrequently during inpatient rounds.11 This study further elucidates specific SDM behaviors used along with univariate and multivariate modeling to explore possible contributing factors. The strengths and weaknesses found were similar at all 4 services and the influence of the service was more important than variability across attendings. This study’s findings are similar to a study by Shields et al.,33 in which the findings in a geographically different outpatient setting 10 years earlier suggesting global and enduring challenges to SDM. To our knowledge, this is the first published study to characterize inpatient SDM behaviors and may serve as the basis for future interventions.
Although the item-level components were ranked similarly across services, on average the summary Peer-RPAD score was lowest at Med-2, where we observed high variability within and between attendings, and was highest at Med-1, where variability was low. Med-2 carried the highest caseload and held the longest rounds, while Med-1 carried the lowest caseload, suggesting that modifiable burdens may hamper SDM performance. Prior studies suggest that patients are often selected based on teaching opportunities, immediate medical need and being newly admitted.34 The high scores at Med-1 may reflect that service’s prediscussion of patients during card-flipping rounds or their selection of which patients to round on as a team. Consistent with prior studies29,35 of SDM and the family-centered rounding model, which includes the involvement of nurses, respiratory therapists, pharmacists, case managers, social workers, and interpreters on rounds, both pediatrics services showed higher SDM scores.
In contrast to prior studies,34,36 team size and number of learners did not affect SDM performance, nor did decision type. Despite teams having up to 17 members, 8 learners, and 14 complex patients, SDM scores did not vary significantly by team. Nonetheless, trends were in the directions expected: Scores tended to decrease as the team size or the percentage of trainees grew, and increased with the seniority of the presenting physician. Interestingly, SDM performance decreased with round-average minutes per patient, which may be measuring on-going intensity across cases that leads to exhaustion. Statistically significant patient factors for increased SDM included longer duration of patient encounters, second week of hospital stay, and female patient gender. Although we anticipated that the high number of decisions made early in hospitalization would facilitate higher SDM scores, continuity and stronger patient-provider relationships may enhance SDM.36 We report service-specific team and patient characteristics, in addition to SDM findings in anticipation that some readers will identify with 1 service more than others.
This study has several important limitations. First, our peer observers were not blinded and primarily observed encounters at their own site. To minimize bias, observers periodically rated videos to recalibrate RPAD scoring. Second, additional SDM conversations with a patient and/or guardian may have occurred outside of rounds and were not captured, and poor patient recall may have affected Patient-RPAD scores despite interviewer prompts and timeliness of interviews within 12 hours of rounds. Third, there might have been a selection bias for the one service who selected a smaller number of patients to see, compared with the three other services that performed bedside rounds on all patients. It is possible that attending physicians selected patients who were deemed most able to have SDM conversations, thus affecting RPAD scores on that service. Fourth, study services had fewer patients on average than other academic hospitals (median 9, range 3-14), which might limit its generalizability. Last, as in any observational study, there is always the possibility of the Hawthorne effect. However, neither teams nor patients knew the study objectives.
Nevertheless, important findings emerged through the use of RPAD Scores to evaluate inpatient SDM practices. In particular, we found that to increase SDM quality in inpatient settings, practitioners should (1) check their understanding of the patient’s perspective, (2) examine barriers to follow-through with the treatment plan, and (3) ask if the patient has questions. Variation among services remained very influential after adjusting for team and patient characteristics, which suggests that “climate” or service culture should be targeted by an intervention, rather than individual attendings or subgroups defined by team or patient characteristics. Notably, team size, number of learners, patient census, and type of decision being made did not affect SDM performance, suggesting that even large, busy services can perform SDM if properly trained.
Acknowledgments
The authors thank the patients, families, pediatric and internal medicine residents, and hospitalists at Stanford School of Medicine and University of California, San Francisco School of Medicine for their participation in this study. We would also like to thank the student volunteers who collected patient perspectives on the encounters.
Disclosure
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an NIH/NCCIH grant R25 AT006573.
The ethos of medicine has shifted from paternalistic, physician-driven care to patient autonomy and engagement, in which the physician shares information and advises.1-3 Although there are ethical, legal, and practical reasons to respect patient preferences,1-4 patient engagement also fosters quality and safety5 and may improve clinical outcomes.5-8 Patients whose preferences are respected are more likely to trust their doctor, feel empowered, and adhere to treatments.9
Providers may partner with patients through shared decision-making (SDM).10,11 Several SDM models describe the process of providers and patients balancing evidence, preferences and context to arrive at a clinical decision.12-15 The National Academy of Medicine and the American Academy of Pediatrics has called for more SDM,16,17 including when clinical evidence is limited,2 equally beneficial options exist,18 clinical stakes are high,19 and even with deferential patients.20 Despite its value, SDM does not reliably occur21,22 and SDM training is often unavailable.4 Clinical decision tools, patient education aids, and various training interventions have shown promising, although inconsistent results.23, 24
Little is known about SDM in inpatient settings where unique patient, clinician, and environmental factors may influence SDM. This study describes the quality and possible predictors of inpatient SDM during attending rounds in 4 academic training settings. Although SDM may occur anytime during a hospitalization, attending rounds present a valuable opportunity for SDM observation given their centrality to inpatient care and teaching.25,26 Because attending physicians bear ultimate responsibility for patient management, we examined whether SDM performance varies among attendings within each service. In addition, we tested the hypothesis that service-level, team-level, and patient-level features explain variation in SDM quality more than individual attending physicians. Finally, we compared peer-observer perspectives of SDM behaviors with patient and/or guardian perspectives.
METHODS
Study Design and Setting
This cross-sectional, observational study examined the diversity of SDM practice within and between 4 inpatient services during attending rounds, including the internal medicine and pediatrics services at Stanford University and the University of California, San Francisco (UCSF). Both institutions provide quaternary care to diverse patient populations with approximately half enrolled in Medicare and/or Medicaid.
One institution had 42 internal medicine (Med-1) and 15 pediatric hospitalists (Peds-1) compared to 8 internal medicine (Med-2) and 12 pediatric hospitalists (Peds-2) at the second location. Both pediatric services used family-centered rounds that included discussions between the patients’ families and the whole team. One medicine service used a similar rounding model that did not necessarily involve the patients’ families. In contrast, the smaller medicine service typically began rounds by discussing all patients in a conference room and then visiting select patients afterwards.
From August 2014 to November 2014, peer observers gathered data on team SDM behaviors during attending rounds. After the rounding team departed, nonphysician interviewers surveyed consenting patients’ (or guardians’) views of the SDM experience, yielding paired evaluations for a subset of SDM encounters. Institutional review board approval was obtained from Stanford University and UCSF.
Participants and Inclusion Criteria
Attending physicians were hospitalists who supervised rounds at least 1 month per year, and did not include those conducting the study. All provided verbal assent to be observed on 3 days within a 7-day period. While team composition varied as needed (eg, to include the nurse, pharmacist, interpreter, etc), we restricted study observations to those teams with an attending and at least one learner (eg, resident, intern, medical student) to capture the influence of attending physicians in their training role. Because services vary in number of attendings on staff, rounds assigned per attending, and patients per round, it was not possible to enroll equal sample sizes per service in the study.
Nonintensive care unit patients who were deemed medically stable by the team were eligible for peer observation and participation in a subsequent patient interview once during the study period. Pediatric patients were invited for an interview if they were between 13 and 21 years old and had the option of having a parent or guardian present; if the pediatric patients were less than 13 years old or they were not interested in being interviewed, then their parents or guardians were invited to be interviewed. Interpreters were on rounds, and thus, non-English participants were able to participate in the peer observations, but could not participate in patient interviews because interpreters were not available during afternoons for study purposes. Consent was obtained from all participating patients and/or guardians.
Data Collection
Round and Patient Characteristics
Peer observers recorded rounding, team, and patient characteristics using a standardized form. Rounding data included date, attending name, duration of rounds, and patient census. Patient level data included the decision(s) discussed, the seniority of the clinician leading the discussion, team composition, minutes spent discussing the patient (both with the patient and/or guardian and total time), hospitalization week, and patient’s primary language. Additional patient data obtained from electronic health records included age, gender, race, ethnicity, date of admission, and admitting diagnosis.
SDM Measures
Peer-observed SDM behaviors were quantified per patient encounter using the 9-item Rochester Participatory Decision-Making Scale (RPAD), with credit given for SDM behaviors exhibited by anyone on the rounding team (team-level metric).27 Each item was scored on a 3-point scale (0 = absent, 0.5 = partial, and 1 = present) for a maximum of 9 points, with higher scores indicating higher-quality SDM (Peer-RPAD Score). We created semistructured patient interview guides by adapting each RPAD item into layperson language (Patient-RPAD Score) and adding open-ended questions to assess the patient experience.
Peer-Observer Training
Eight peer-observers (7 hospitalists and 1 palliative care physician) were trained to perform RPAD ratings using videos of patient encounters. Initially, raters viewed videos together and discussed ratings for each RPAD item. The observers incorporated behavioral anchors and clinical examples into the development of an RPAD rating guide, which they subsequently used to independently score 4 videos from an online medical communication library.28 These scores were discussed to resolve any differences before 4 additional videos were independently viewed, scored, and compared. Interrater reliability was achieved when the standard deviation of summed SDM scores across raters was less than 1 for all 4 videos.
Patient Interviewers
Interviewers were English-speaking volunteers without formal medical training. They were educated in hospital etiquette by a physician and in administering patient interviews through peer-to-peer role playing and an observation and feedback interview with at least 1 patient.
Data Analysis
The analysis set included every unique patient with whom a medical decision was made by an eligible clinical team. To account for the nested study design (patient-level scores within rounds, rounds within attending, and attendings within service), we used mixed-effects models to estimate mean (summary or item) RPAD score by levels of fixed covariate(s). The models included random effects accounting for attending-level and round-level correlations among scores via variance components, and allowing the attending-level random effect to differ by service. Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). We used descriptive statistics to summarize round- and patient-level characteristics.
SDM Variation by Attending and Service
Box plots were used to summarize raw patient-level, Peer-RPAD scores by service and attending. By using the methods described above, we estimated the mean score overall and by service. In both models, we examined the statistical significance of service-specific variation in attending-level random effects by using likelihood-ratio test (LRT) to compare models.
SDM Variation by Round and Patient Characteristics
We used the models described above to identify covariates associated with Peer-RPAD scores. We fit univariate models separately for each covariate, then fit 2 multivariable models, including (1) all covariates and (2) all effects significant in either model at P ≤ .20 according to F tests. For uniformity of presentation, we express continuous covariates categorically; however, we report P values based on continuous versions. Means generated by the multivariable models were calculated at the mean values of all other covariates in model.
Patient-Level RPAD Data
A subsample of patients completed semistructured interviews with analogous RPAD questions. To identify possible selection bias in the full sample, we summarized response rates by service and patient language and modeled Peer-RPAD scores by interview response status. Among responders, we estimated the mean Peer-RPAD and Patient-RPAD scores and their paired differences and correlations, testing for non-zero correlations via the Spearman rank test.
RESULTS
All Patient Encounters
The median duration of rounding sessions was 1.8 hours, median patient census was 9, and median patient encounter was 13 minutes. The duration of rounds and minutes per patient were longest at Med-2 and shortest at Peds-1. See Table 1 for other team characteristics.
Peer Evaluations of SDM Encounters
Characteristics of Patients
Teams spent a median of 13 minutes per SDM encounter, which was not higher than the round median. SDM topics discussed included 47% treatment, 15% diagnostic, 30% both treatment and diagnostic, and 7% other.
Variation in SDM Quality Among Attending Physicians
Overall Peer-RPAD Scores were normally distributed. After adjusting for the nested study design, the overall mean (standard error) score was 4.16 (0.11). Score variability among attendings differed significantly by service (LRT P = .0067). For example, raw scores were lower and more variable among attending physicians at Med-2 than other among attendings in other services (see Appendix Figure in Supporting Information). However, when service was included in the model as a fixed effect, mean scores varied significantly, from 3.0 at Med-2 to 4.7 at Med-1 (P < .0001), but the random variation among attendings no longer differed significantly by service (P = .13). This finding supports the hypothesis that service-level influences are stronger than influences of individual attending physicians, that is, that variation between services exceeded variation among attendings within service.
Aspects of SDM That Are More Prevalent on Rounds
Rounds and Patient Characteristics Associated With Peer-RPAD Scores
Patient-RPAD Results: Dissimilar Perspectives of Patients and/or Guardians and Physician Observers
Among responders, mean Patient-RPAD scores were 6.8 to 7.1 for medicine services and 7.6 to 7.8 for pediatric services (P = .01). The overall mean Patient-RPAD score, 7.46, was significantly greater than the paired Peer-RPAD score by 3.5 (P = .011); however, correlations were not statistically significantly different from 0 (by service, each P > .12).
To understand drivers of the differences between Peer-RPAD and Patient-RPAD scores, we analyzed findings by item. Each mean patient-item score exceeded its peer counterpart (P ≤ .01; panel B of Figure). Peer-item scores fell below 33% on 2 items (Items 9 and 4) and only exceeded 67% on 2 items (Items 1 and 6), whereas patient-item scores ranged from 60% (Item 8) to 97% (Item 7). Three paired differences exceeded 50% (Items 9, 4, and 7) and 3 were below 20% (Items 6, 8 and 1), underlying the lack of correlation between peer and patient scores.
DISCUSSION
In this multisite study of SDM during inpatient attending rounds, SDM quality, specific SDM behaviors, and factors contributing to SDM were identified. Our study found an adjusted overall Peer-RPAD Score of 4.4 out of 9, and found the following 3 SDM elements most needing improvement according to trained peer observers: (1) “Checking understanding of the patient’s perspective”, (2) “Examining barriers to follow-through with the treatment plan”, and (3) “Asking if the patient has questions.” Areas of strength included explaining the clinical issue or nature of the decision and matching medical language to the patient’s level of understanding, with each rated highly by both peer-observers and patients. Broadly speaking, physicians were skillful in delivering information to patients but failed to solicit input from patients. Characteristics associated with increased SDM in the multivariate analysis included the following: service, patient gender, timing of rounds during patient’s hospital stay, and amount of time rounding with each patient.
Patients similarly found that physicians could improve their abilities to elicit information from patients and families, noting the 3 lowest patient-rated SDM elements were as follows: (1) asking open-ended questions, (2) discussing alternatives or uncertainties, and (3) discussing barriers to treatment plan follow through. Overall, patients and guardians perceived the quantity and quality of SDM on rounds more favorably than peer observers, which is consistent with other studies of patient perceptions of communication. 29-31 It is possible that patient ratings are more influenced by demand characteristics, fear of negatively impacting their patient-provider relationships, and conflation of overall satisfaction with quality of communication.32 This difference in patient perception of SDM is worthy of further study.
Prior work has revealed that SDM may occur infrequently during inpatient rounds.11 This study further elucidates specific SDM behaviors used along with univariate and multivariate modeling to explore possible contributing factors. The strengths and weaknesses found were similar at all 4 services and the influence of the service was more important than variability across attendings. This study’s findings are similar to a study by Shields et al.,33 in which the findings in a geographically different outpatient setting 10 years earlier suggesting global and enduring challenges to SDM. To our knowledge, this is the first published study to characterize inpatient SDM behaviors and may serve as the basis for future interventions.
Although the item-level components were ranked similarly across services, on average the summary Peer-RPAD score was lowest at Med-2, where we observed high variability within and between attendings, and was highest at Med-1, where variability was low. Med-2 carried the highest caseload and held the longest rounds, while Med-1 carried the lowest caseload, suggesting that modifiable burdens may hamper SDM performance. Prior studies suggest that patients are often selected based on teaching opportunities, immediate medical need and being newly admitted.34 The high scores at Med-1 may reflect that service’s prediscussion of patients during card-flipping rounds or their selection of which patients to round on as a team. Consistent with prior studies29,35 of SDM and the family-centered rounding model, which includes the involvement of nurses, respiratory therapists, pharmacists, case managers, social workers, and interpreters on rounds, both pediatrics services showed higher SDM scores.
In contrast to prior studies,34,36 team size and number of learners did not affect SDM performance, nor did decision type. Despite teams having up to 17 members, 8 learners, and 14 complex patients, SDM scores did not vary significantly by team. Nonetheless, trends were in the directions expected: Scores tended to decrease as the team size or the percentage of trainees grew, and increased with the seniority of the presenting physician. Interestingly, SDM performance decreased with round-average minutes per patient, which may be measuring on-going intensity across cases that leads to exhaustion. Statistically significant patient factors for increased SDM included longer duration of patient encounters, second week of hospital stay, and female patient gender. Although we anticipated that the high number of decisions made early in hospitalization would facilitate higher SDM scores, continuity and stronger patient-provider relationships may enhance SDM.36 We report service-specific team and patient characteristics, in addition to SDM findings in anticipation that some readers will identify with 1 service more than others.
This study has several important limitations. First, our peer observers were not blinded and primarily observed encounters at their own site. To minimize bias, observers periodically rated videos to recalibrate RPAD scoring. Second, additional SDM conversations with a patient and/or guardian may have occurred outside of rounds and were not captured, and poor patient recall may have affected Patient-RPAD scores despite interviewer prompts and timeliness of interviews within 12 hours of rounds. Third, there might have been a selection bias for the one service who selected a smaller number of patients to see, compared with the three other services that performed bedside rounds on all patients. It is possible that attending physicians selected patients who were deemed most able to have SDM conversations, thus affecting RPAD scores on that service. Fourth, study services had fewer patients on average than other academic hospitals (median 9, range 3-14), which might limit its generalizability. Last, as in any observational study, there is always the possibility of the Hawthorne effect. However, neither teams nor patients knew the study objectives.
Nevertheless, important findings emerged through the use of RPAD Scores to evaluate inpatient SDM practices. In particular, we found that to increase SDM quality in inpatient settings, practitioners should (1) check their understanding of the patient’s perspective, (2) examine barriers to follow-through with the treatment plan, and (3) ask if the patient has questions. Variation among services remained very influential after adjusting for team and patient characteristics, which suggests that “climate” or service culture should be targeted by an intervention, rather than individual attendings or subgroups defined by team or patient characteristics. Notably, team size, number of learners, patient census, and type of decision being made did not affect SDM performance, suggesting that even large, busy services can perform SDM if properly trained.
Acknowledgments
The authors thank the patients, families, pediatric and internal medicine residents, and hospitalists at Stanford School of Medicine and University of California, San Francisco School of Medicine for their participation in this study. We would also like to thank the student volunteers who collected patient perspectives on the encounters.
Disclosure
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an NIH/NCCIH grant R25 AT006573.
1. Braddock CH. The emerging importance and relevance of shared decision making to clinical practice. Med Decis Mak. 2010;30(5 Suppl):5S-7S. doi:10.1177/0272989X10381344. PubMed
2. Braddock CH. Supporting shared decision making when clinical evidence is low. Med Care Res Rev MCRR. 2013;70(1 Suppl):129S-140S. doi:10.1177/1077558712460280. PubMed
3. Elwyn G, Tilburt J, Montori V. The ethical imperative for shared decision-making. Eur J Pers Centered Healthc. 2013;1(1):129-131. doi:10.5750/ejpch.v1i1.645.
4. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
5. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(10):CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
6. Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566-577. doi:10.1164/rccm.200906-0907OC. PubMed
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410-417. doi:10.1370/afm.1161. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
10. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781. doi:10.1056/NEJMp1109283. PubMed
11. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med J Assoc Am Med Coll. 2014;89(11):1548-1557. doi:10.1097/ACM.0000000000000483. PubMed
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692. PubMed
13. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
14. Légaré F, St-Jacques S, Gagnon S, et al. Prenatal screening for Down syndrome: a survey of willingness in women and family physicians to engage in shared decision-making. Prenat Diagn. 2011;31(4):319-326. doi:10.1002/pd.2624. PubMed
15. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. Milbank Q. 2009;87(2):368-390. PubMed
16. National Academy of Medicine. Crossing the quality chasm: a new health system for the 21st century. https://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed on November 30, 2016.
17. Adams RC, Levy SE, Council on Children with Disabilities. Shared Decision-Making and Children with Disabilities: Pathways to Consensus. Pediatrics. 2017; 139(6):1-9. PubMed
18. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
19. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
20. Kehl KL, Landrum MB, Arora NK, et al. Association of Actual and Preferred Decision Roles With Patient-Reported Quality of Care: Shared Decision Making in Cancer Care. JAMA Oncol. 2015;1(1):50-58. doi:10.1001/jamaoncol.2014.112. PubMed
21. Couët N, Desroches S, Robitaille H, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect Int J Public Particip Health Care Health Policy. 2015;18(4):542-561. doi:10.1111/hex.12054. PubMed
22. Fowler FJ, Gerstein BS, Barry MJ. How patient centered are medical decisions?: Results of a national survey. JAMA Intern Med. 2013;173(13):1215-1221. doi:10.1001/jamainternmed.2013.6172. PubMed
23. Légaré F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2014;(9):CD006732. doi:10.1002/14651858.CD006732.pub3. PubMed
24. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;(10):CD001431. doi:10.1002/14651858.CD001431.pub3. PubMed
25. Di Francesco L, Pistoria MJ, Auerbach AD, Nardino RJ, Holmboe ES. Internal medicine training in the inpatient setting. A review of published educational interventions. J Gen Intern Med. 2005;20(12):1173-1180. doi:10.1111/j.1525-1497.2005.00250.x. PubMed
26. Janicik RW, Fletcher KE. Teaching at the bedside: a new model. Med Teach. 2003;25(2):127-130. PubMed
27. Shields CG, Franks P, Fiscella K, Meldrum S, Epstein RM. Rochester Participatory Decision-Making Scale (RPAD): reliability and validity. Ann Fam Med. 2005;3(5):436-442. doi:10.1370/afm.305. PubMed
28. DocCom - enhancing competence in healthcare communication. https://webcampus.drexelmed.edu/doccom/user/. Accessed on November 30, 2016.
29. Bailey SM, Hendricks-Muñoz KD, Mally P. Parental influence on clinical management during neonatal intensive care: a survey of US neonatologists. J Matern Fetal Neonatal Med. 2013;26(12):1239-1244. doi:10.3109/14767058.2013.776531. PubMed
30. Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091-3098. doi:10.1200/JCO.2004.09.069. PubMed
31. Schoenborn NL, Cayea D, McNabney M, Ray A, Boyd C. Prognosis communication with older patients with multimorbidity: Assessment after an educational intervention. Gerontol Geriatr Educ. 2016;38(4):471-481. doi:10.1080/02701960.2015.1115983. PubMed
32. Lipkin M. Shared decision making. JAMA Intern Med. 2013;173(13):1204-1205. doi:10.1001/jamainternmed.2013.6248. PubMed
33. Gonzalo JD, Heist BS, Duffy BL, et al. The art of bedside rounds: a multi-center qualitative study of strategies used by experienced bedside teachers. J Gen Intern Med. 2013;28(3):412-420. doi:10.1007/s11606-012-2259-2. PubMed
34. Rosen P, Stenger E, Bochkoris M, Hannon MJ, Kwoh CK. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics. 2009;123(4):e603-e608. doi:10.1542/peds.2008-2238. PubMed
35. Harrison R, Allen E. Teaching internal medicine residents in the new era. Inpatient attending with duty-hour regulations. J Gen Intern Med. 2006;21(5):447-452. doi:10.1111/j.1525-1497.2006.00425.x. PubMed
36. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med 1982. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056. PubMed
1. Braddock CH. The emerging importance and relevance of shared decision making to clinical practice. Med Decis Mak. 2010;30(5 Suppl):5S-7S. doi:10.1177/0272989X10381344. PubMed
2. Braddock CH. Supporting shared decision making when clinical evidence is low. Med Care Res Rev MCRR. 2013;70(1 Suppl):129S-140S. doi:10.1177/1077558712460280. PubMed
3. Elwyn G, Tilburt J, Montori V. The ethical imperative for shared decision-making. Eur J Pers Centered Healthc. 2013;1(1):129-131. doi:10.5750/ejpch.v1i1.645.
4. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
5. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(10):CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
6. Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566-577. doi:10.1164/rccm.200906-0907OC. PubMed
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410-417. doi:10.1370/afm.1161. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
10. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781. doi:10.1056/NEJMp1109283. PubMed
11. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med J Assoc Am Med Coll. 2014;89(11):1548-1557. doi:10.1097/ACM.0000000000000483. PubMed
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692. PubMed
13. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
14. Légaré F, St-Jacques S, Gagnon S, et al. Prenatal screening for Down syndrome: a survey of willingness in women and family physicians to engage in shared decision-making. Prenat Diagn. 2011;31(4):319-326. doi:10.1002/pd.2624. PubMed
15. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. Milbank Q. 2009;87(2):368-390. PubMed
16. National Academy of Medicine. Crossing the quality chasm: a new health system for the 21st century. https://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed on November 30, 2016.
17. Adams RC, Levy SE, Council on Children with Disabilities. Shared Decision-Making and Children with Disabilities: Pathways to Consensus. Pediatrics. 2017; 139(6):1-9. PubMed
18. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
19. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
20. Kehl KL, Landrum MB, Arora NK, et al. Association of Actual and Preferred Decision Roles With Patient-Reported Quality of Care: Shared Decision Making in Cancer Care. JAMA Oncol. 2015;1(1):50-58. doi:10.1001/jamaoncol.2014.112. PubMed
21. Couët N, Desroches S, Robitaille H, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect Int J Public Particip Health Care Health Policy. 2015;18(4):542-561. doi:10.1111/hex.12054. PubMed
22. Fowler FJ, Gerstein BS, Barry MJ. How patient centered are medical decisions?: Results of a national survey. JAMA Intern Med. 2013;173(13):1215-1221. doi:10.1001/jamainternmed.2013.6172. PubMed
23. Légaré F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2014;(9):CD006732. doi:10.1002/14651858.CD006732.pub3. PubMed
24. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;(10):CD001431. doi:10.1002/14651858.CD001431.pub3. PubMed
25. Di Francesco L, Pistoria MJ, Auerbach AD, Nardino RJ, Holmboe ES. Internal medicine training in the inpatient setting. A review of published educational interventions. J Gen Intern Med. 2005;20(12):1173-1180. doi:10.1111/j.1525-1497.2005.00250.x. PubMed
26. Janicik RW, Fletcher KE. Teaching at the bedside: a new model. Med Teach. 2003;25(2):127-130. PubMed
27. Shields CG, Franks P, Fiscella K, Meldrum S, Epstein RM. Rochester Participatory Decision-Making Scale (RPAD): reliability and validity. Ann Fam Med. 2005;3(5):436-442. doi:10.1370/afm.305. PubMed
28. DocCom - enhancing competence in healthcare communication. https://webcampus.drexelmed.edu/doccom/user/. Accessed on November 30, 2016.
29. Bailey SM, Hendricks-Muñoz KD, Mally P. Parental influence on clinical management during neonatal intensive care: a survey of US neonatologists. J Matern Fetal Neonatal Med. 2013;26(12):1239-1244. doi:10.3109/14767058.2013.776531. PubMed
30. Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091-3098. doi:10.1200/JCO.2004.09.069. PubMed
31. Schoenborn NL, Cayea D, McNabney M, Ray A, Boyd C. Prognosis communication with older patients with multimorbidity: Assessment after an educational intervention. Gerontol Geriatr Educ. 2016;38(4):471-481. doi:10.1080/02701960.2015.1115983. PubMed
32. Lipkin M. Shared decision making. JAMA Intern Med. 2013;173(13):1204-1205. doi:10.1001/jamainternmed.2013.6248. PubMed
33. Gonzalo JD, Heist BS, Duffy BL, et al. The art of bedside rounds: a multi-center qualitative study of strategies used by experienced bedside teachers. J Gen Intern Med. 2013;28(3):412-420. doi:10.1007/s11606-012-2259-2. PubMed
34. Rosen P, Stenger E, Bochkoris M, Hannon MJ, Kwoh CK. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics. 2009;123(4):e603-e608. doi:10.1542/peds.2008-2238. PubMed
35. Harrison R, Allen E. Teaching internal medicine residents in the new era. Inpatient attending with duty-hour regulations. J Gen Intern Med. 2006;21(5):447-452. doi:10.1111/j.1525-1497.2006.00425.x. PubMed
36. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med 1982. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056. PubMed
© 2018 Society of Hospital Medicine
Update in Hospital Palliative Care: Symptom Management, Communication, Caregiver Outcomes, and Moral Distress
The aim of palliative care (PC) is to improve quality of life for patients facing serious, life-threatening illness and their families.1 Due to insufficient numbers of PC specialists to meet the PC needs for every hospitalized patient,2 all hospitalists should maintain basic PC skills as recognized by PC being a core competency for hospitalists.3,4
We summarize and critique PC research articles published between January 1, 2016, and December 31, 2016, that have a high likelihood of impacting the practice of hospital medicine. We hand searched 15 journals and conducted a MEDLINE keyword search of PC terms (see Table). All titles and/or abstracts were reviewed and selected for full review based on the following factors: palliative medicine content, scientific rigor, impact on practice, and relevance to hospital medicine. Fifty-five articles were individually reviewed and scored by all authors according to rigor, impact, and relevance. Articles were ranked according to their mean scores, and 9 articles were chosen for inclusion through consensus discussion.
SYMPTOM MANAGEMENT
Antipsychotics Were Inferior to a Placebo in Treating Nonterminal Delirium
Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34-42.
Background
Delirium is highly prevalent in PC and is associated with significant distress.5 Antipsychotics are widely used for symptoms of delirium, although current evidence does not support this practice in hospitalized adults.6,7
Findings
This was a double-blind, parallel-arm, placebo randomized controlled trial (RCT) of 247 patients with delirium with an estimated life expectancy of ≥7 days in 11 PC or hospice units across Australia. Patients were randomized to receive risperidone, haloperidol, or a placebo in addition to nonpharmacological management of delirium. Delirium symptom scores after 3 days of treatment, the use of midazolam as a rescue medication, and the presence of extrapyramidal symptoms (EPS) were measured. The risperidone and haloperidol arms had significantly higher delirium symptom scores (P = .02 and P = .009, respectively), mean EPS symptoms (P < .001), and more use of rescue midazolam than the placebo arm. Mortality was higher for antipsychotics, with a hazard ratio of 1.73 for haloperidol (P = .003), 1.29 for risperidone (P = .14), and 1.47 for any antipsychotic (P = .01).
Cautions
The study population was elderly (mean age >70 years) with mild delirium scores. The use of antipsychotics was associated with more benzodiazepine use, which could itself worsen delirium. As patients with clinician-predicted life expectancy of <7 days were excluded, findings cannot be extrapolated to the treatment of terminal delirium, which can often be more symptomatic and difficult to treat.
Implications
Avoid scheduled antipsychotics in patients with nonterminal delirium, as they can increase risk of harm without advantages, over nonpharmacologic interventions.
Low-Dose Morphine Was Superior to Weak Opioids in the Treatment of Moderate Cancer Pain
Bandieri E, Romero M, Ripamonti CI, et al. Randomized trial of low-dose morphine versus weak opioids in moderate cancer pain. J Clin Oncol. 2016;34(5):436-442.
Background
The World Health Organization guidelines recommend the use of weak opioids (WOs), such as codeine or tramadol, as a sequential step in the management of cancer pain.8 This strategy has not been tested against low doses of stronger opioids.
Findings
In this multicenter, open-label RCT, 240 patients in Italy were randomized and stratified by age (<75 years or ≥75 years) to either the WO group or low-dose morphine (M) group. The primary outcome measure was a reduction in pain intensity by 20% or more. Secondary outcomes included an improvement in symptom scores, a ≥30% and ≥50% reduction in pain, increased opioid dosage, and adverse side effects. Compared with the WO group, the M group had more patients with a 20% reduction in pain (88.2% vs 54.7%; P < .001), more evidence of pain control in the first week (80.9% vs 43.6%; P < .001), more patients with a ≥30% and ≥50% reduction in pain, and less need to switch to a stronger opioid (15.5% vs 35.0%; P = .001) or require dose increases. Adverse effects were similar in both groups.
Cautions
Patients with chronic kidney disease (CKD) were excluded due to concerns about the accumulation of morphine metabolites. Additionally, this study was open label, increasing the risk of bias.
Implications
Low-dose morphine should be considered over the use of WOs to achieve better and more rapid pain control in patients without CKD.
The Use of Methadone as a Coanalgesic May Improve Moderate Cancer Pain
Courtemanche F, Dao D, Gagné F, et al. Methadone as a coanalgesic for palliative care cancer patients. J Palliat Med. 2016;19(9):972-978.
Background
Methadone is effective at treating cancer pain and is often utilized when patients have neuropathic pain, fail to respond to traditional opioids, or have renal failure.9,10 However, its long half-life and many drug interactions make methadone challenging to use.
Findings
This cohort study looked at 153 inpatient or outpatient PC patients in Montreal who received methadone as a coanalgesic for cancer pain. The patients’ median morphine equivalent dose was 120 mg when initiating methadone. The median starting dose of methadone was 3 mg per day. Of patients, 49.3% had a significant response (≥30% pain reduction), with a median response time of 7 days, and 30.1% achieved a substantial response (≥50% pain reduction), with a median response time of 3 days. Patients with higher initial pain scores were more likely to respond to adjuvant methadone. Those who had not responded after a week of methadone were unlikely to respond despite dose escalations. Adverse effects included drowsiness (51.4%), confusion (27.4%), constipation (24.7%), nausea (19.9%), and myoclonia (16.4%).
Cautions
This was an observational study with retrospective data, leading to higher levels of missing data. A high rate of adverse side effects was reported (90.4%). Further study is needed to validate and reproduce the findings.
Implications
The use of adjuvant low-dose methadone may be considered in patients with moderate pain despite high-dose opioids. If a response is not seen within 7 days, then methadone use should be reconsidered.
ANTIBIOTIC STEWARDSHIP
Many Hospitalized Patients on Comfort Care Still Receive Antimicrobials
Merel SE, Meier CA, McKinney CM, Pottinger PS. Antimicrobial use in patients on a comfort care protocol: a retrospective cohort study. J Palliat Med. 2016;19(11):1210-1214.
Background
It is unknown how often patients who are hospitalized at the end of life continue to receive antimicrobials and what factors are associated with antimicrobial use.
Findings
This retrospective cohort study of 1881 hospitalized adults transitioned to a comfort care order (CCO) set at 2 academic medical centers found that 77% of these patients received antimicrobials during their hospital stay (62.4% at 24 hours prior to CCO). Of the 711 still alive at ≥24 hours after CCO, 111 (15.6%) were still on antimicrobials, with that proportion remaining stable for the remainder of hospitalization. In comparing those who did and did not receive antimicrobials after 24 hours of CCO, the presence of a documented infection was not significantly different after adjusting for age. Those with a cancer diagnosis (adjusted risk ratio [ARR] = 1.44: P = .04), a longer length of stay (≥7 days vs <7 days; ARR = 1.49; P = .05), and those discharged home (ARR 2.93; P < .001) or to a facility (ARR 3.63; P < .001) versus dying in the hospital were more likely to be on antimicrobials 24 hours after CCO. Compared with those on a medicine service, patients in the medical and surgical intensive care units (ICUs) were less likely to receive antimicrobials (medical ICU ARR = 0.32; P = .01; surgical ICU and/or neuro-ICU ARR = 0.32; P = .02). The most commonly administered antimicrobials were fluoroquinolones and vancomycin.
Cautions
Only 111 patients were still on antimicrobials at 24 hours, which limited analysis. Investigators relied on retrospective data for medication administration and diagnoses.
Implications
Further work is needed to understand and address the expectations of clinicians, patients, and families regarding the role of antimicrobials at the end of life.
COMMUNICATION AND DECISION MAKING
Video Decision Aids Improved Rates of Advance Care Planning and Hospice Use and Decreased Costs
Volandes, AE, Paasche-Orlow MK, Davis AD et al. Use of video decision aids to promote advance care planning in Hilo, Hawai‘i. J Gen Intern Med. 2016;31(9):1035-1040.
Background
Advance care planning (ACP) can be enhanced with the use of video decision aids, which may help address scalability and cost.11 The Hawaii Medical Service Association began an initiative to improve ACP rates, which included a financial incentive. Clinician training and patient access to ACP videos were implemented 1 year into this campaign, which was intended for patients with late-stage disease.
Findings
This study tested the impact of the video intervention on the rates of ACP documentation in Hilo, Hawaii, along with secondary outcomes of hospice use, hospital deaths, and costs. The intervention was sequentially rolled out to Hilo Medical Center (HMC), followed by hospice and primary care practices. Following the video introduction, the proportion of patients discharged from HMC with ACP documentation markedly increased (3.2% to 39.9%; P < .001). The percentage of hospital patients discharged to hospice increased from 5.7% to 13.8% (P < .001). Overall admissions to the Hospice of Hilo increased at a greater rate than in other parts of Hawaii. After the intervention in Hilo, the in-hospital death rate among patients >65 years old declined slightly (P = .14), while in the rest of the state, the rate remained essentially unchanged. ACP planning did not reduce healthcare costs at the end of life, but costs seemed to increase more slowly in Hilo after the intervention than they did in the rest of Hawaii (P < .05).
Cautions
This report relies on before-and-after comparisons, with potential confounding by a background pay-for-quality initiative; however, the timing of the changes in outcomes correlates well with the introduction of the videos. ACP videos have been studied in other settings, so the intervention is likely generalizable to other states.
Implications
A widespread distribution of ACP videos and training for physicians in their use may lead to significant increases in ACP documentation and other beneficial clinical outcomes for patients and health systems.
A Standardized Palliative Care-Led Intervention Did Not Improve Psychological Outcomes in Families of Patients with Chronic Critical Illness
Carson SS, Cox CE, Wallenstein S, et al. Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA. 2016;316(1):51-62.
Background
Chronic critical illness (CCI) occurs when a patient neither recovers nor dies for days to weeks after an acute illness requiring aggressive intensive care. CCI is associated with poor patient and family outcomes.12 Does a protocol-driven support and information meeting led by PC providers improve these outcomes?
Findings
This multicenter RCT compared 130 CCI patients (184 surrogates) who received a structured intervention to 126 patients (181 surrogates) with usual care. The structured intervention was led by PC clinicians in order to provide supportive conversations and information about CCI and prognosis compared with the usual intensivist communication. The support and information team met with the families of patients in the intervention group after day 7 of mechanical ventilation (MV) and again 10 days later. Both the intervention and control groups received validated information about CCI, and all were eligible for specialty PC consultation, as indicated. The primary outcome of the study was the Hospital Anxiety and Depression Scale (HADS) at 90-day follow-up with the surrogates. Secondary endpoints included posttraumatic stress disorder (PTSD) assessment and other communication measures as well as patient outcomes (hospital mortality, 90-day survival, length of stay, and days of MV). At least 1 meeting took place for 89% of patients (82% of surrogates) in the intervention arm. Fewer patients in the intervention arm had nonstudy PC consultations (13% vs 22%). Ninety-day HADS results were similar in the 2 groups. PTSD symptoms, however, were higher in the intervention group (Impact of Event Scale-Revised score: 25.9 for intervention and 21.3 for control; intergroup difference 4.6 [95% confidence interval, 0.01-9.10]). There were no statistically significant differences among the patient-focused measures, including survival.
Cautions
Although the teams contained skilled clinicians led by PC practitioners, this was not an ordinary PC intervention. The intervention included information and emotional support meetings alone rather than support from a PC team driven by clinical considerations. This study included surrogates of patients with CCI but not other conditions.
Implications
Protocol-driven support and information meetings may not improve, and may slightly worsen, outcomes in families of patients with CCI. This study did not evaluate and should not be applied to clinically indicated, specialty PC consultation in the ICU.
CAREGIVER OUTCOMES
Caregivers of Patients Surviving Prolonged Critical Illness Experience High and Persistent Rates of Depression
Cameron JI, Chu LM, Matte A, et al. One-year outcomes in caregivers of critically ill patients. N Engl J Med. 2016;374(19):1831-1841.
Background
More than half of patients with a CCI require caregiver support 1 year after hospitalization.13 Caregivers provide tremendous physical and psychosocial support to their loved ones, but that care is often associated with significant burden.14
Findings
This prospective parallel cohort study followed caregivers of surviving patients ventilated for at least 7 days from 10 academic hospitals in Canada. The prevalence of depression (Center for Epidemiologic Studies–Depression scale ≥16) in this cohort of 280 caregivers (70% were women) was 67%, 49%, 43%, and 43% at the survey intervals of 7 days, 3 months, 6 months, and 12 months after ICU discharge, respectively. Using latent-class linear mixed models, the investigators identified 2 groups of caregivers: those whose depressive symptoms decreased over time (84%) and those whose depressive symptoms persisted at a high level for the year (16%). Patient characteristics (such as age, comorbidity, sex, and functional status) were not associated with caregiver outcomes. Younger caregiver age, greater effect of patient care on other activities, less social support, less mastery (sense of control), and less personal growth were associated with worse caregiver mental health outcomes.
Cautions
Although this is a high-quality prospective study, causality of caregiving on the high rates of depressive symptoms cannot be confirmed without a control group or knowledge of the caregivers’ mental health status prior to the episode of prolonged critical illness.
Implications
Patient critical illness may have serious impacts on caregiver health and well-being. Hospitalists should be attentive to factors associated with caregiver vulnerability and offer support. Improving caregivers’ sense of control and social support may be targets for interventions.
People with Non-normative Sexuality or Gender Face Additional Barriers and Stressors with Partner Loss
Bristowe K, Marshall S, Harding R. The bereavement experiences of lesbian, gay, bisexual and/or trans* people who have lost a partner: A systematic review, thematic synthesis and modelling of the literature. Palliat Med. 2016;30(8):730-744.
Background
Grief and bereavement impact individuals differently as they adjust to a death. Increasingly, it is recognized that lesbian, gay, bisexual, and/or transgender (LGBT) communities may face additional barriers when interacting with the healthcare system. This review sought to identify and appraise the evidence of the bereavement experiences among LGBT communities.
Findings
This systematic review summarized quantitative and qualitative data from 23 articles (13 studies). The synthesis noted that the pain associated with the loss of a partner was a universal experience regardless of sexual identity or gender history. Additional barriers and stressors of bereavement were reported for LGBT people, including homophobia, failure to acknowledge the relationship, additional legal and financial issues, and the shadow of human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS). LGBT people turned to additional resources for bereavement help: professional support, social and familial support, and societal and community support. Caregiver bereavement support experiences were shaped by whether the relationships were disclosed and accepted (acceptance-disclosure model).
Cautions
The quantitative data was mostly from the 1990s and described the context of HIV/AIDS. The qualitative studies, however, were done in the last decade. Very little research was available for transgender or bisexual caregivers.
Implications
People who identify as LGBT face additional barriers and stressors with the loss of a partner. The described acceptance-disclosure model may help providers be mindful of the additional barriers to LGBT bereavement support.
MORAL DISTRESS AND RESILIENCY
Physician Trainees Experience Significant Moral Distress with Futile Treatments
Dzeng E, Colaianni A, Roland M, et al. Moral distress amongst American physician trainees regarding futile treatments at the end of life: a qualitative study. J Gen Intern Med. 2016;31(1):93-99.
Background
Physician trainees are often faced with ethical challenges in providing end-of-life care. These ethical challenges can create confusion and conflict about the balance between the benefits and burdens experienced by patients.
Findings
The authors used semistructured, in-depth, qualitative interviews of 22 internal medicine trainees from 3 academic medical centers. An analysis of these interviews revealed several themes. Trainees reported moral distress when (1) many of the treatments provided in end-of-life care (ie, feeding tubes in advanced dementia) were perceived to be futile; (2) they felt obligated to provide end-of-life care that was not in the patient’s best interest, leading to “torture” or “suffering” for the patient; (3) they provided care they felt not to be in the patient’s best interest; (4) they perceived themselves to be powerless to affect change in these dilemmas; (5) they attributed some of their powerlessness to the hierarchy of their academic institutions; and (6) they feared that dehumanization and cynicism would be required to endure this distress.
Cautions
Resident recruitment occurred by solicitation, which may invite bias. Generalizability of qualitative studies to other settings can be limited.
Implications
Trainees may experience several dimensions of moral distress in end-of-life care. These findings challenge training programs to find ways to reduce the dehumanization, sense of powerlessness, and cynicism that this distress may cause.
Disclosure
The authors declare that they have no relevant financial conflicts of interest.
1. Morrison RS, Meier DE. Palliative care. N Engl J Med. 2004;350:2582-2590. PubMed
2. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. PubMed
3. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1:21-28. PubMed
4. Society of Hospital Medicine. Palliative care. J Hosp Med. 2006;1,S1:80-81.
5. Hosie A, Davidson PM, Agar M, Sanderson CR, Phillips J. Delirium prevalence, incidence, and implications for screening in specialist palliative care inpatient settings: a systematic review. Palliat Med. 2013;27(6):486-493. PubMed
6. Carnes M, Howell T, Rosenberg M, Francis J, Hildebrand C, Knuppel J. Physicians vary in approaches to the clinical management of delirium. J Am Geriatr Soc. 2003;51(2):234-239. PubMed
7. Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705-14. PubMed
8. WHO. Cancer Pain Relief. 2nd ed. Geneva, Switzerland: WHO; 1996.
9. Leppert W. The role of methadone in cancer pain treatment—a review. Int J Clin Pract. 2009;63(7):1095-1109. PubMed
10. Morley JS, Bridson J, Nash TP, et al. Low-dose methadone has an analgesic effect in neuropathic pain: a double-blind randomized controlled crossover trial. Palliat Med. 2003;17(7):576-587. PubMed
11. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2014.
12. Nelson JE, Cox CE, Hope AA, Carson SS. Chronic Critical Illness. Am J Respir Crit Care Med. 2010;182(4):446-454. PubMed
13. Chelluri L, Im KA, Belle SH, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32(1):61-9. PubMed
14. Van Beusekom I, Bakhshi-Raiez F, deKeizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care. 2015;20:16. PubMed
The aim of palliative care (PC) is to improve quality of life for patients facing serious, life-threatening illness and their families.1 Due to insufficient numbers of PC specialists to meet the PC needs for every hospitalized patient,2 all hospitalists should maintain basic PC skills as recognized by PC being a core competency for hospitalists.3,4
We summarize and critique PC research articles published between January 1, 2016, and December 31, 2016, that have a high likelihood of impacting the practice of hospital medicine. We hand searched 15 journals and conducted a MEDLINE keyword search of PC terms (see Table). All titles and/or abstracts were reviewed and selected for full review based on the following factors: palliative medicine content, scientific rigor, impact on practice, and relevance to hospital medicine. Fifty-five articles were individually reviewed and scored by all authors according to rigor, impact, and relevance. Articles were ranked according to their mean scores, and 9 articles were chosen for inclusion through consensus discussion.
SYMPTOM MANAGEMENT
Antipsychotics Were Inferior to a Placebo in Treating Nonterminal Delirium
Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34-42.
Background
Delirium is highly prevalent in PC and is associated with significant distress.5 Antipsychotics are widely used for symptoms of delirium, although current evidence does not support this practice in hospitalized adults.6,7
Findings
This was a double-blind, parallel-arm, placebo randomized controlled trial (RCT) of 247 patients with delirium with an estimated life expectancy of ≥7 days in 11 PC or hospice units across Australia. Patients were randomized to receive risperidone, haloperidol, or a placebo in addition to nonpharmacological management of delirium. Delirium symptom scores after 3 days of treatment, the use of midazolam as a rescue medication, and the presence of extrapyramidal symptoms (EPS) were measured. The risperidone and haloperidol arms had significantly higher delirium symptom scores (P = .02 and P = .009, respectively), mean EPS symptoms (P < .001), and more use of rescue midazolam than the placebo arm. Mortality was higher for antipsychotics, with a hazard ratio of 1.73 for haloperidol (P = .003), 1.29 for risperidone (P = .14), and 1.47 for any antipsychotic (P = .01).
Cautions
The study population was elderly (mean age >70 years) with mild delirium scores. The use of antipsychotics was associated with more benzodiazepine use, which could itself worsen delirium. As patients with clinician-predicted life expectancy of <7 days were excluded, findings cannot be extrapolated to the treatment of terminal delirium, which can often be more symptomatic and difficult to treat.
Implications
Avoid scheduled antipsychotics in patients with nonterminal delirium, as they can increase risk of harm without advantages, over nonpharmacologic interventions.
Low-Dose Morphine Was Superior to Weak Opioids in the Treatment of Moderate Cancer Pain
Bandieri E, Romero M, Ripamonti CI, et al. Randomized trial of low-dose morphine versus weak opioids in moderate cancer pain. J Clin Oncol. 2016;34(5):436-442.
Background
The World Health Organization guidelines recommend the use of weak opioids (WOs), such as codeine or tramadol, as a sequential step in the management of cancer pain.8 This strategy has not been tested against low doses of stronger opioids.
Findings
In this multicenter, open-label RCT, 240 patients in Italy were randomized and stratified by age (<75 years or ≥75 years) to either the WO group or low-dose morphine (M) group. The primary outcome measure was a reduction in pain intensity by 20% or more. Secondary outcomes included an improvement in symptom scores, a ≥30% and ≥50% reduction in pain, increased opioid dosage, and adverse side effects. Compared with the WO group, the M group had more patients with a 20% reduction in pain (88.2% vs 54.7%; P < .001), more evidence of pain control in the first week (80.9% vs 43.6%; P < .001), more patients with a ≥30% and ≥50% reduction in pain, and less need to switch to a stronger opioid (15.5% vs 35.0%; P = .001) or require dose increases. Adverse effects were similar in both groups.
Cautions
Patients with chronic kidney disease (CKD) were excluded due to concerns about the accumulation of morphine metabolites. Additionally, this study was open label, increasing the risk of bias.
Implications
Low-dose morphine should be considered over the use of WOs to achieve better and more rapid pain control in patients without CKD.
The Use of Methadone as a Coanalgesic May Improve Moderate Cancer Pain
Courtemanche F, Dao D, Gagné F, et al. Methadone as a coanalgesic for palliative care cancer patients. J Palliat Med. 2016;19(9):972-978.
Background
Methadone is effective at treating cancer pain and is often utilized when patients have neuropathic pain, fail to respond to traditional opioids, or have renal failure.9,10 However, its long half-life and many drug interactions make methadone challenging to use.
Findings
This cohort study looked at 153 inpatient or outpatient PC patients in Montreal who received methadone as a coanalgesic for cancer pain. The patients’ median morphine equivalent dose was 120 mg when initiating methadone. The median starting dose of methadone was 3 mg per day. Of patients, 49.3% had a significant response (≥30% pain reduction), with a median response time of 7 days, and 30.1% achieved a substantial response (≥50% pain reduction), with a median response time of 3 days. Patients with higher initial pain scores were more likely to respond to adjuvant methadone. Those who had not responded after a week of methadone were unlikely to respond despite dose escalations. Adverse effects included drowsiness (51.4%), confusion (27.4%), constipation (24.7%), nausea (19.9%), and myoclonia (16.4%).
Cautions
This was an observational study with retrospective data, leading to higher levels of missing data. A high rate of adverse side effects was reported (90.4%). Further study is needed to validate and reproduce the findings.
Implications
The use of adjuvant low-dose methadone may be considered in patients with moderate pain despite high-dose opioids. If a response is not seen within 7 days, then methadone use should be reconsidered.
ANTIBIOTIC STEWARDSHIP
Many Hospitalized Patients on Comfort Care Still Receive Antimicrobials
Merel SE, Meier CA, McKinney CM, Pottinger PS. Antimicrobial use in patients on a comfort care protocol: a retrospective cohort study. J Palliat Med. 2016;19(11):1210-1214.
Background
It is unknown how often patients who are hospitalized at the end of life continue to receive antimicrobials and what factors are associated with antimicrobial use.
Findings
This retrospective cohort study of 1881 hospitalized adults transitioned to a comfort care order (CCO) set at 2 academic medical centers found that 77% of these patients received antimicrobials during their hospital stay (62.4% at 24 hours prior to CCO). Of the 711 still alive at ≥24 hours after CCO, 111 (15.6%) were still on antimicrobials, with that proportion remaining stable for the remainder of hospitalization. In comparing those who did and did not receive antimicrobials after 24 hours of CCO, the presence of a documented infection was not significantly different after adjusting for age. Those with a cancer diagnosis (adjusted risk ratio [ARR] = 1.44: P = .04), a longer length of stay (≥7 days vs <7 days; ARR = 1.49; P = .05), and those discharged home (ARR 2.93; P < .001) or to a facility (ARR 3.63; P < .001) versus dying in the hospital were more likely to be on antimicrobials 24 hours after CCO. Compared with those on a medicine service, patients in the medical and surgical intensive care units (ICUs) were less likely to receive antimicrobials (medical ICU ARR = 0.32; P = .01; surgical ICU and/or neuro-ICU ARR = 0.32; P = .02). The most commonly administered antimicrobials were fluoroquinolones and vancomycin.
Cautions
Only 111 patients were still on antimicrobials at 24 hours, which limited analysis. Investigators relied on retrospective data for medication administration and diagnoses.
Implications
Further work is needed to understand and address the expectations of clinicians, patients, and families regarding the role of antimicrobials at the end of life.
COMMUNICATION AND DECISION MAKING
Video Decision Aids Improved Rates of Advance Care Planning and Hospice Use and Decreased Costs
Volandes, AE, Paasche-Orlow MK, Davis AD et al. Use of video decision aids to promote advance care planning in Hilo, Hawai‘i. J Gen Intern Med. 2016;31(9):1035-1040.
Background
Advance care planning (ACP) can be enhanced with the use of video decision aids, which may help address scalability and cost.11 The Hawaii Medical Service Association began an initiative to improve ACP rates, which included a financial incentive. Clinician training and patient access to ACP videos were implemented 1 year into this campaign, which was intended for patients with late-stage disease.
Findings
This study tested the impact of the video intervention on the rates of ACP documentation in Hilo, Hawaii, along with secondary outcomes of hospice use, hospital deaths, and costs. The intervention was sequentially rolled out to Hilo Medical Center (HMC), followed by hospice and primary care practices. Following the video introduction, the proportion of patients discharged from HMC with ACP documentation markedly increased (3.2% to 39.9%; P < .001). The percentage of hospital patients discharged to hospice increased from 5.7% to 13.8% (P < .001). Overall admissions to the Hospice of Hilo increased at a greater rate than in other parts of Hawaii. After the intervention in Hilo, the in-hospital death rate among patients >65 years old declined slightly (P = .14), while in the rest of the state, the rate remained essentially unchanged. ACP planning did not reduce healthcare costs at the end of life, but costs seemed to increase more slowly in Hilo after the intervention than they did in the rest of Hawaii (P < .05).
Cautions
This report relies on before-and-after comparisons, with potential confounding by a background pay-for-quality initiative; however, the timing of the changes in outcomes correlates well with the introduction of the videos. ACP videos have been studied in other settings, so the intervention is likely generalizable to other states.
Implications
A widespread distribution of ACP videos and training for physicians in their use may lead to significant increases in ACP documentation and other beneficial clinical outcomes for patients and health systems.
A Standardized Palliative Care-Led Intervention Did Not Improve Psychological Outcomes in Families of Patients with Chronic Critical Illness
Carson SS, Cox CE, Wallenstein S, et al. Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA. 2016;316(1):51-62.
Background
Chronic critical illness (CCI) occurs when a patient neither recovers nor dies for days to weeks after an acute illness requiring aggressive intensive care. CCI is associated with poor patient and family outcomes.12 Does a protocol-driven support and information meeting led by PC providers improve these outcomes?
Findings
This multicenter RCT compared 130 CCI patients (184 surrogates) who received a structured intervention to 126 patients (181 surrogates) with usual care. The structured intervention was led by PC clinicians in order to provide supportive conversations and information about CCI and prognosis compared with the usual intensivist communication. The support and information team met with the families of patients in the intervention group after day 7 of mechanical ventilation (MV) and again 10 days later. Both the intervention and control groups received validated information about CCI, and all were eligible for specialty PC consultation, as indicated. The primary outcome of the study was the Hospital Anxiety and Depression Scale (HADS) at 90-day follow-up with the surrogates. Secondary endpoints included posttraumatic stress disorder (PTSD) assessment and other communication measures as well as patient outcomes (hospital mortality, 90-day survival, length of stay, and days of MV). At least 1 meeting took place for 89% of patients (82% of surrogates) in the intervention arm. Fewer patients in the intervention arm had nonstudy PC consultations (13% vs 22%). Ninety-day HADS results were similar in the 2 groups. PTSD symptoms, however, were higher in the intervention group (Impact of Event Scale-Revised score: 25.9 for intervention and 21.3 for control; intergroup difference 4.6 [95% confidence interval, 0.01-9.10]). There were no statistically significant differences among the patient-focused measures, including survival.
Cautions
Although the teams contained skilled clinicians led by PC practitioners, this was not an ordinary PC intervention. The intervention included information and emotional support meetings alone rather than support from a PC team driven by clinical considerations. This study included surrogates of patients with CCI but not other conditions.
Implications
Protocol-driven support and information meetings may not improve, and may slightly worsen, outcomes in families of patients with CCI. This study did not evaluate and should not be applied to clinically indicated, specialty PC consultation in the ICU.
CAREGIVER OUTCOMES
Caregivers of Patients Surviving Prolonged Critical Illness Experience High and Persistent Rates of Depression
Cameron JI, Chu LM, Matte A, et al. One-year outcomes in caregivers of critically ill patients. N Engl J Med. 2016;374(19):1831-1841.
Background
More than half of patients with a CCI require caregiver support 1 year after hospitalization.13 Caregivers provide tremendous physical and psychosocial support to their loved ones, but that care is often associated with significant burden.14
Findings
This prospective parallel cohort study followed caregivers of surviving patients ventilated for at least 7 days from 10 academic hospitals in Canada. The prevalence of depression (Center for Epidemiologic Studies–Depression scale ≥16) in this cohort of 280 caregivers (70% were women) was 67%, 49%, 43%, and 43% at the survey intervals of 7 days, 3 months, 6 months, and 12 months after ICU discharge, respectively. Using latent-class linear mixed models, the investigators identified 2 groups of caregivers: those whose depressive symptoms decreased over time (84%) and those whose depressive symptoms persisted at a high level for the year (16%). Patient characteristics (such as age, comorbidity, sex, and functional status) were not associated with caregiver outcomes. Younger caregiver age, greater effect of patient care on other activities, less social support, less mastery (sense of control), and less personal growth were associated with worse caregiver mental health outcomes.
Cautions
Although this is a high-quality prospective study, causality of caregiving on the high rates of depressive symptoms cannot be confirmed without a control group or knowledge of the caregivers’ mental health status prior to the episode of prolonged critical illness.
Implications
Patient critical illness may have serious impacts on caregiver health and well-being. Hospitalists should be attentive to factors associated with caregiver vulnerability and offer support. Improving caregivers’ sense of control and social support may be targets for interventions.
People with Non-normative Sexuality or Gender Face Additional Barriers and Stressors with Partner Loss
Bristowe K, Marshall S, Harding R. The bereavement experiences of lesbian, gay, bisexual and/or trans* people who have lost a partner: A systematic review, thematic synthesis and modelling of the literature. Palliat Med. 2016;30(8):730-744.
Background
Grief and bereavement impact individuals differently as they adjust to a death. Increasingly, it is recognized that lesbian, gay, bisexual, and/or transgender (LGBT) communities may face additional barriers when interacting with the healthcare system. This review sought to identify and appraise the evidence of the bereavement experiences among LGBT communities.
Findings
This systematic review summarized quantitative and qualitative data from 23 articles (13 studies). The synthesis noted that the pain associated with the loss of a partner was a universal experience regardless of sexual identity or gender history. Additional barriers and stressors of bereavement were reported for LGBT people, including homophobia, failure to acknowledge the relationship, additional legal and financial issues, and the shadow of human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS). LGBT people turned to additional resources for bereavement help: professional support, social and familial support, and societal and community support. Caregiver bereavement support experiences were shaped by whether the relationships were disclosed and accepted (acceptance-disclosure model).
Cautions
The quantitative data was mostly from the 1990s and described the context of HIV/AIDS. The qualitative studies, however, were done in the last decade. Very little research was available for transgender or bisexual caregivers.
Implications
People who identify as LGBT face additional barriers and stressors with the loss of a partner. The described acceptance-disclosure model may help providers be mindful of the additional barriers to LGBT bereavement support.
MORAL DISTRESS AND RESILIENCY
Physician Trainees Experience Significant Moral Distress with Futile Treatments
Dzeng E, Colaianni A, Roland M, et al. Moral distress amongst American physician trainees regarding futile treatments at the end of life: a qualitative study. J Gen Intern Med. 2016;31(1):93-99.
Background
Physician trainees are often faced with ethical challenges in providing end-of-life care. These ethical challenges can create confusion and conflict about the balance between the benefits and burdens experienced by patients.
Findings
The authors used semistructured, in-depth, qualitative interviews of 22 internal medicine trainees from 3 academic medical centers. An analysis of these interviews revealed several themes. Trainees reported moral distress when (1) many of the treatments provided in end-of-life care (ie, feeding tubes in advanced dementia) were perceived to be futile; (2) they felt obligated to provide end-of-life care that was not in the patient’s best interest, leading to “torture” or “suffering” for the patient; (3) they provided care they felt not to be in the patient’s best interest; (4) they perceived themselves to be powerless to affect change in these dilemmas; (5) they attributed some of their powerlessness to the hierarchy of their academic institutions; and (6) they feared that dehumanization and cynicism would be required to endure this distress.
Cautions
Resident recruitment occurred by solicitation, which may invite bias. Generalizability of qualitative studies to other settings can be limited.
Implications
Trainees may experience several dimensions of moral distress in end-of-life care. These findings challenge training programs to find ways to reduce the dehumanization, sense of powerlessness, and cynicism that this distress may cause.
Disclosure
The authors declare that they have no relevant financial conflicts of interest.
The aim of palliative care (PC) is to improve quality of life for patients facing serious, life-threatening illness and their families.1 Due to insufficient numbers of PC specialists to meet the PC needs for every hospitalized patient,2 all hospitalists should maintain basic PC skills as recognized by PC being a core competency for hospitalists.3,4
We summarize and critique PC research articles published between January 1, 2016, and December 31, 2016, that have a high likelihood of impacting the practice of hospital medicine. We hand searched 15 journals and conducted a MEDLINE keyword search of PC terms (see Table). All titles and/or abstracts were reviewed and selected for full review based on the following factors: palliative medicine content, scientific rigor, impact on practice, and relevance to hospital medicine. Fifty-five articles were individually reviewed and scored by all authors according to rigor, impact, and relevance. Articles were ranked according to their mean scores, and 9 articles were chosen for inclusion through consensus discussion.
SYMPTOM MANAGEMENT
Antipsychotics Were Inferior to a Placebo in Treating Nonterminal Delirium
Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34-42.
Background
Delirium is highly prevalent in PC and is associated with significant distress.5 Antipsychotics are widely used for symptoms of delirium, although current evidence does not support this practice in hospitalized adults.6,7
Findings
This was a double-blind, parallel-arm, placebo randomized controlled trial (RCT) of 247 patients with delirium with an estimated life expectancy of ≥7 days in 11 PC or hospice units across Australia. Patients were randomized to receive risperidone, haloperidol, or a placebo in addition to nonpharmacological management of delirium. Delirium symptom scores after 3 days of treatment, the use of midazolam as a rescue medication, and the presence of extrapyramidal symptoms (EPS) were measured. The risperidone and haloperidol arms had significantly higher delirium symptom scores (P = .02 and P = .009, respectively), mean EPS symptoms (P < .001), and more use of rescue midazolam than the placebo arm. Mortality was higher for antipsychotics, with a hazard ratio of 1.73 for haloperidol (P = .003), 1.29 for risperidone (P = .14), and 1.47 for any antipsychotic (P = .01).
Cautions
The study population was elderly (mean age >70 years) with mild delirium scores. The use of antipsychotics was associated with more benzodiazepine use, which could itself worsen delirium. As patients with clinician-predicted life expectancy of <7 days were excluded, findings cannot be extrapolated to the treatment of terminal delirium, which can often be more symptomatic and difficult to treat.
Implications
Avoid scheduled antipsychotics in patients with nonterminal delirium, as they can increase risk of harm without advantages, over nonpharmacologic interventions.
Low-Dose Morphine Was Superior to Weak Opioids in the Treatment of Moderate Cancer Pain
Bandieri E, Romero M, Ripamonti CI, et al. Randomized trial of low-dose morphine versus weak opioids in moderate cancer pain. J Clin Oncol. 2016;34(5):436-442.
Background
The World Health Organization guidelines recommend the use of weak opioids (WOs), such as codeine or tramadol, as a sequential step in the management of cancer pain.8 This strategy has not been tested against low doses of stronger opioids.
Findings
In this multicenter, open-label RCT, 240 patients in Italy were randomized and stratified by age (<75 years or ≥75 years) to either the WO group or low-dose morphine (M) group. The primary outcome measure was a reduction in pain intensity by 20% or more. Secondary outcomes included an improvement in symptom scores, a ≥30% and ≥50% reduction in pain, increased opioid dosage, and adverse side effects. Compared with the WO group, the M group had more patients with a 20% reduction in pain (88.2% vs 54.7%; P < .001), more evidence of pain control in the first week (80.9% vs 43.6%; P < .001), more patients with a ≥30% and ≥50% reduction in pain, and less need to switch to a stronger opioid (15.5% vs 35.0%; P = .001) or require dose increases. Adverse effects were similar in both groups.
Cautions
Patients with chronic kidney disease (CKD) were excluded due to concerns about the accumulation of morphine metabolites. Additionally, this study was open label, increasing the risk of bias.
Implications
Low-dose morphine should be considered over the use of WOs to achieve better and more rapid pain control in patients without CKD.
The Use of Methadone as a Coanalgesic May Improve Moderate Cancer Pain
Courtemanche F, Dao D, Gagné F, et al. Methadone as a coanalgesic for palliative care cancer patients. J Palliat Med. 2016;19(9):972-978.
Background
Methadone is effective at treating cancer pain and is often utilized when patients have neuropathic pain, fail to respond to traditional opioids, or have renal failure.9,10 However, its long half-life and many drug interactions make methadone challenging to use.
Findings
This cohort study looked at 153 inpatient or outpatient PC patients in Montreal who received methadone as a coanalgesic for cancer pain. The patients’ median morphine equivalent dose was 120 mg when initiating methadone. The median starting dose of methadone was 3 mg per day. Of patients, 49.3% had a significant response (≥30% pain reduction), with a median response time of 7 days, and 30.1% achieved a substantial response (≥50% pain reduction), with a median response time of 3 days. Patients with higher initial pain scores were more likely to respond to adjuvant methadone. Those who had not responded after a week of methadone were unlikely to respond despite dose escalations. Adverse effects included drowsiness (51.4%), confusion (27.4%), constipation (24.7%), nausea (19.9%), and myoclonia (16.4%).
Cautions
This was an observational study with retrospective data, leading to higher levels of missing data. A high rate of adverse side effects was reported (90.4%). Further study is needed to validate and reproduce the findings.
Implications
The use of adjuvant low-dose methadone may be considered in patients with moderate pain despite high-dose opioids. If a response is not seen within 7 days, then methadone use should be reconsidered.
ANTIBIOTIC STEWARDSHIP
Many Hospitalized Patients on Comfort Care Still Receive Antimicrobials
Merel SE, Meier CA, McKinney CM, Pottinger PS. Antimicrobial use in patients on a comfort care protocol: a retrospective cohort study. J Palliat Med. 2016;19(11):1210-1214.
Background
It is unknown how often patients who are hospitalized at the end of life continue to receive antimicrobials and what factors are associated with antimicrobial use.
Findings
This retrospective cohort study of 1881 hospitalized adults transitioned to a comfort care order (CCO) set at 2 academic medical centers found that 77% of these patients received antimicrobials during their hospital stay (62.4% at 24 hours prior to CCO). Of the 711 still alive at ≥24 hours after CCO, 111 (15.6%) were still on antimicrobials, with that proportion remaining stable for the remainder of hospitalization. In comparing those who did and did not receive antimicrobials after 24 hours of CCO, the presence of a documented infection was not significantly different after adjusting for age. Those with a cancer diagnosis (adjusted risk ratio [ARR] = 1.44: P = .04), a longer length of stay (≥7 days vs <7 days; ARR = 1.49; P = .05), and those discharged home (ARR 2.93; P < .001) or to a facility (ARR 3.63; P < .001) versus dying in the hospital were more likely to be on antimicrobials 24 hours after CCO. Compared with those on a medicine service, patients in the medical and surgical intensive care units (ICUs) were less likely to receive antimicrobials (medical ICU ARR = 0.32; P = .01; surgical ICU and/or neuro-ICU ARR = 0.32; P = .02). The most commonly administered antimicrobials were fluoroquinolones and vancomycin.
Cautions
Only 111 patients were still on antimicrobials at 24 hours, which limited analysis. Investigators relied on retrospective data for medication administration and diagnoses.
Implications
Further work is needed to understand and address the expectations of clinicians, patients, and families regarding the role of antimicrobials at the end of life.
COMMUNICATION AND DECISION MAKING
Video Decision Aids Improved Rates of Advance Care Planning and Hospice Use and Decreased Costs
Volandes, AE, Paasche-Orlow MK, Davis AD et al. Use of video decision aids to promote advance care planning in Hilo, Hawai‘i. J Gen Intern Med. 2016;31(9):1035-1040.
Background
Advance care planning (ACP) can be enhanced with the use of video decision aids, which may help address scalability and cost.11 The Hawaii Medical Service Association began an initiative to improve ACP rates, which included a financial incentive. Clinician training and patient access to ACP videos were implemented 1 year into this campaign, which was intended for patients with late-stage disease.
Findings
This study tested the impact of the video intervention on the rates of ACP documentation in Hilo, Hawaii, along with secondary outcomes of hospice use, hospital deaths, and costs. The intervention was sequentially rolled out to Hilo Medical Center (HMC), followed by hospice and primary care practices. Following the video introduction, the proportion of patients discharged from HMC with ACP documentation markedly increased (3.2% to 39.9%; P < .001). The percentage of hospital patients discharged to hospice increased from 5.7% to 13.8% (P < .001). Overall admissions to the Hospice of Hilo increased at a greater rate than in other parts of Hawaii. After the intervention in Hilo, the in-hospital death rate among patients >65 years old declined slightly (P = .14), while in the rest of the state, the rate remained essentially unchanged. ACP planning did not reduce healthcare costs at the end of life, but costs seemed to increase more slowly in Hilo after the intervention than they did in the rest of Hawaii (P < .05).
Cautions
This report relies on before-and-after comparisons, with potential confounding by a background pay-for-quality initiative; however, the timing of the changes in outcomes correlates well with the introduction of the videos. ACP videos have been studied in other settings, so the intervention is likely generalizable to other states.
Implications
A widespread distribution of ACP videos and training for physicians in their use may lead to significant increases in ACP documentation and other beneficial clinical outcomes for patients and health systems.
A Standardized Palliative Care-Led Intervention Did Not Improve Psychological Outcomes in Families of Patients with Chronic Critical Illness
Carson SS, Cox CE, Wallenstein S, et al. Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA. 2016;316(1):51-62.
Background
Chronic critical illness (CCI) occurs when a patient neither recovers nor dies for days to weeks after an acute illness requiring aggressive intensive care. CCI is associated with poor patient and family outcomes.12 Does a protocol-driven support and information meeting led by PC providers improve these outcomes?
Findings
This multicenter RCT compared 130 CCI patients (184 surrogates) who received a structured intervention to 126 patients (181 surrogates) with usual care. The structured intervention was led by PC clinicians in order to provide supportive conversations and information about CCI and prognosis compared with the usual intensivist communication. The support and information team met with the families of patients in the intervention group after day 7 of mechanical ventilation (MV) and again 10 days later. Both the intervention and control groups received validated information about CCI, and all were eligible for specialty PC consultation, as indicated. The primary outcome of the study was the Hospital Anxiety and Depression Scale (HADS) at 90-day follow-up with the surrogates. Secondary endpoints included posttraumatic stress disorder (PTSD) assessment and other communication measures as well as patient outcomes (hospital mortality, 90-day survival, length of stay, and days of MV). At least 1 meeting took place for 89% of patients (82% of surrogates) in the intervention arm. Fewer patients in the intervention arm had nonstudy PC consultations (13% vs 22%). Ninety-day HADS results were similar in the 2 groups. PTSD symptoms, however, were higher in the intervention group (Impact of Event Scale-Revised score: 25.9 for intervention and 21.3 for control; intergroup difference 4.6 [95% confidence interval, 0.01-9.10]). There were no statistically significant differences among the patient-focused measures, including survival.
Cautions
Although the teams contained skilled clinicians led by PC practitioners, this was not an ordinary PC intervention. The intervention included information and emotional support meetings alone rather than support from a PC team driven by clinical considerations. This study included surrogates of patients with CCI but not other conditions.
Implications
Protocol-driven support and information meetings may not improve, and may slightly worsen, outcomes in families of patients with CCI. This study did not evaluate and should not be applied to clinically indicated, specialty PC consultation in the ICU.
CAREGIVER OUTCOMES
Caregivers of Patients Surviving Prolonged Critical Illness Experience High and Persistent Rates of Depression
Cameron JI, Chu LM, Matte A, et al. One-year outcomes in caregivers of critically ill patients. N Engl J Med. 2016;374(19):1831-1841.
Background
More than half of patients with a CCI require caregiver support 1 year after hospitalization.13 Caregivers provide tremendous physical and psychosocial support to their loved ones, but that care is often associated with significant burden.14
Findings
This prospective parallel cohort study followed caregivers of surviving patients ventilated for at least 7 days from 10 academic hospitals in Canada. The prevalence of depression (Center for Epidemiologic Studies–Depression scale ≥16) in this cohort of 280 caregivers (70% were women) was 67%, 49%, 43%, and 43% at the survey intervals of 7 days, 3 months, 6 months, and 12 months after ICU discharge, respectively. Using latent-class linear mixed models, the investigators identified 2 groups of caregivers: those whose depressive symptoms decreased over time (84%) and those whose depressive symptoms persisted at a high level for the year (16%). Patient characteristics (such as age, comorbidity, sex, and functional status) were not associated with caregiver outcomes. Younger caregiver age, greater effect of patient care on other activities, less social support, less mastery (sense of control), and less personal growth were associated with worse caregiver mental health outcomes.
Cautions
Although this is a high-quality prospective study, causality of caregiving on the high rates of depressive symptoms cannot be confirmed without a control group or knowledge of the caregivers’ mental health status prior to the episode of prolonged critical illness.
Implications
Patient critical illness may have serious impacts on caregiver health and well-being. Hospitalists should be attentive to factors associated with caregiver vulnerability and offer support. Improving caregivers’ sense of control and social support may be targets for interventions.
People with Non-normative Sexuality or Gender Face Additional Barriers and Stressors with Partner Loss
Bristowe K, Marshall S, Harding R. The bereavement experiences of lesbian, gay, bisexual and/or trans* people who have lost a partner: A systematic review, thematic synthesis and modelling of the literature. Palliat Med. 2016;30(8):730-744.
Background
Grief and bereavement impact individuals differently as they adjust to a death. Increasingly, it is recognized that lesbian, gay, bisexual, and/or transgender (LGBT) communities may face additional barriers when interacting with the healthcare system. This review sought to identify and appraise the evidence of the bereavement experiences among LGBT communities.
Findings
This systematic review summarized quantitative and qualitative data from 23 articles (13 studies). The synthesis noted that the pain associated with the loss of a partner was a universal experience regardless of sexual identity or gender history. Additional barriers and stressors of bereavement were reported for LGBT people, including homophobia, failure to acknowledge the relationship, additional legal and financial issues, and the shadow of human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS). LGBT people turned to additional resources for bereavement help: professional support, social and familial support, and societal and community support. Caregiver bereavement support experiences were shaped by whether the relationships were disclosed and accepted (acceptance-disclosure model).
Cautions
The quantitative data was mostly from the 1990s and described the context of HIV/AIDS. The qualitative studies, however, were done in the last decade. Very little research was available for transgender or bisexual caregivers.
Implications
People who identify as LGBT face additional barriers and stressors with the loss of a partner. The described acceptance-disclosure model may help providers be mindful of the additional barriers to LGBT bereavement support.
MORAL DISTRESS AND RESILIENCY
Physician Trainees Experience Significant Moral Distress with Futile Treatments
Dzeng E, Colaianni A, Roland M, et al. Moral distress amongst American physician trainees regarding futile treatments at the end of life: a qualitative study. J Gen Intern Med. 2016;31(1):93-99.
Background
Physician trainees are often faced with ethical challenges in providing end-of-life care. These ethical challenges can create confusion and conflict about the balance between the benefits and burdens experienced by patients.
Findings
The authors used semistructured, in-depth, qualitative interviews of 22 internal medicine trainees from 3 academic medical centers. An analysis of these interviews revealed several themes. Trainees reported moral distress when (1) many of the treatments provided in end-of-life care (ie, feeding tubes in advanced dementia) were perceived to be futile; (2) they felt obligated to provide end-of-life care that was not in the patient’s best interest, leading to “torture” or “suffering” for the patient; (3) they provided care they felt not to be in the patient’s best interest; (4) they perceived themselves to be powerless to affect change in these dilemmas; (5) they attributed some of their powerlessness to the hierarchy of their academic institutions; and (6) they feared that dehumanization and cynicism would be required to endure this distress.
Cautions
Resident recruitment occurred by solicitation, which may invite bias. Generalizability of qualitative studies to other settings can be limited.
Implications
Trainees may experience several dimensions of moral distress in end-of-life care. These findings challenge training programs to find ways to reduce the dehumanization, sense of powerlessness, and cynicism that this distress may cause.
Disclosure
The authors declare that they have no relevant financial conflicts of interest.
1. Morrison RS, Meier DE. Palliative care. N Engl J Med. 2004;350:2582-2590. PubMed
2. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. PubMed
3. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1:21-28. PubMed
4. Society of Hospital Medicine. Palliative care. J Hosp Med. 2006;1,S1:80-81.
5. Hosie A, Davidson PM, Agar M, Sanderson CR, Phillips J. Delirium prevalence, incidence, and implications for screening in specialist palliative care inpatient settings: a systematic review. Palliat Med. 2013;27(6):486-493. PubMed
6. Carnes M, Howell T, Rosenberg M, Francis J, Hildebrand C, Knuppel J. Physicians vary in approaches to the clinical management of delirium. J Am Geriatr Soc. 2003;51(2):234-239. PubMed
7. Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705-14. PubMed
8. WHO. Cancer Pain Relief. 2nd ed. Geneva, Switzerland: WHO; 1996.
9. Leppert W. The role of methadone in cancer pain treatment—a review. Int J Clin Pract. 2009;63(7):1095-1109. PubMed
10. Morley JS, Bridson J, Nash TP, et al. Low-dose methadone has an analgesic effect in neuropathic pain: a double-blind randomized controlled crossover trial. Palliat Med. 2003;17(7):576-587. PubMed
11. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2014.
12. Nelson JE, Cox CE, Hope AA, Carson SS. Chronic Critical Illness. Am J Respir Crit Care Med. 2010;182(4):446-454. PubMed
13. Chelluri L, Im KA, Belle SH, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32(1):61-9. PubMed
14. Van Beusekom I, Bakhshi-Raiez F, deKeizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care. 2015;20:16. PubMed
1. Morrison RS, Meier DE. Palliative care. N Engl J Med. 2004;350:2582-2590. PubMed
2. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. PubMed
3. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1:21-28. PubMed
4. Society of Hospital Medicine. Palliative care. J Hosp Med. 2006;1,S1:80-81.
5. Hosie A, Davidson PM, Agar M, Sanderson CR, Phillips J. Delirium prevalence, incidence, and implications for screening in specialist palliative care inpatient settings: a systematic review. Palliat Med. 2013;27(6):486-493. PubMed
6. Carnes M, Howell T, Rosenberg M, Francis J, Hildebrand C, Knuppel J. Physicians vary in approaches to the clinical management of delirium. J Am Geriatr Soc. 2003;51(2):234-239. PubMed
7. Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705-14. PubMed
8. WHO. Cancer Pain Relief. 2nd ed. Geneva, Switzerland: WHO; 1996.
9. Leppert W. The role of methadone in cancer pain treatment—a review. Int J Clin Pract. 2009;63(7):1095-1109. PubMed
10. Morley JS, Bridson J, Nash TP, et al. Low-dose methadone has an analgesic effect in neuropathic pain: a double-blind randomized controlled crossover trial. Palliat Med. 2003;17(7):576-587. PubMed
11. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2014.
12. Nelson JE, Cox CE, Hope AA, Carson SS. Chronic Critical Illness. Am J Respir Crit Care Med. 2010;182(4):446-454. PubMed
13. Chelluri L, Im KA, Belle SH, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32(1):61-9. PubMed
14. Van Beusekom I, Bakhshi-Raiez F, deKeizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care. 2015;20:16. PubMed
© 2017 Society of Hospital Medicine