User login
Buried Deep
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
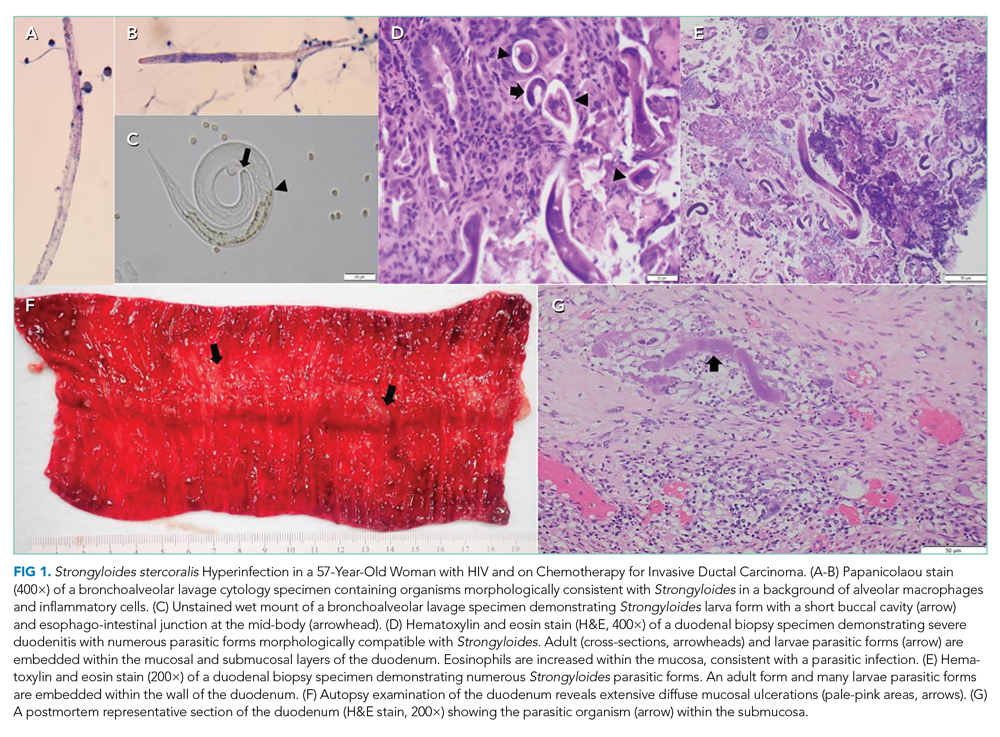
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
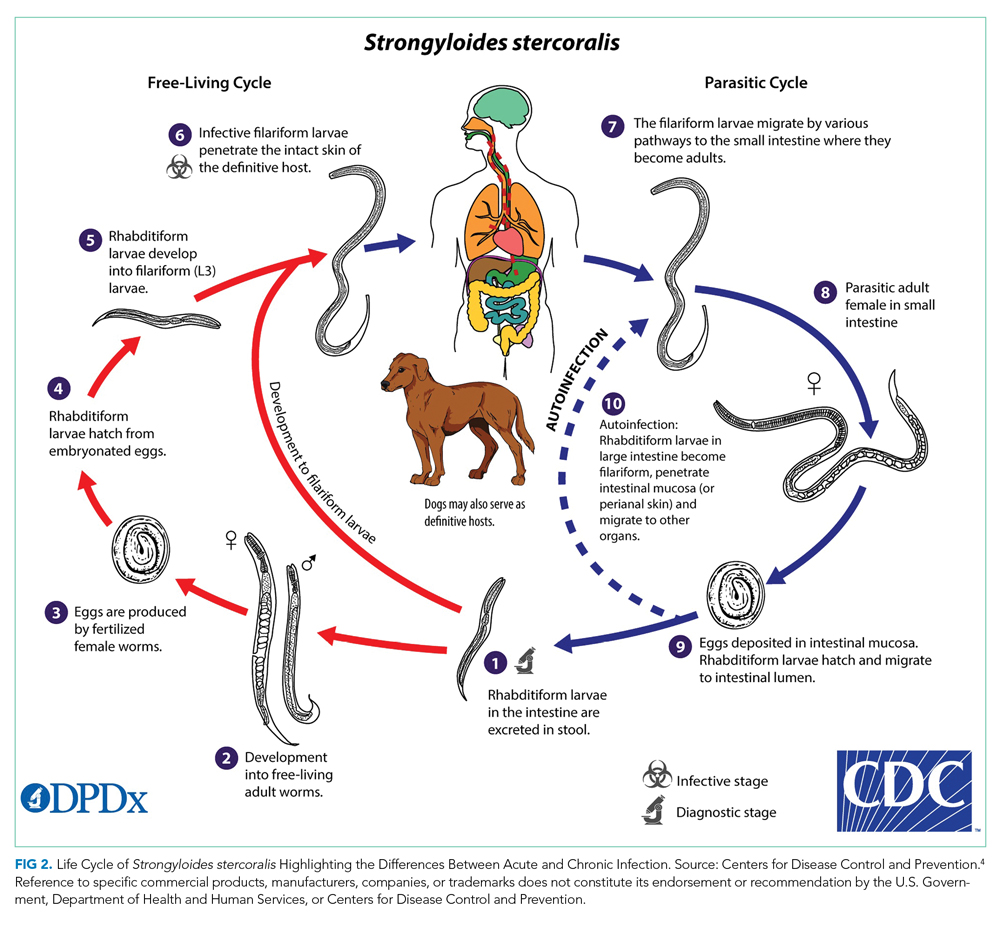
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
© 2021 Society of Hospital Medicine